
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 12 March 2025
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1488732
 An-Qi Deng1,2,3,4†
An-Qi Deng1,2,3,4† Shao-Yu Yue1,2,3
Shao-Yu Yue1,2,3 Di Niu1,2,3
Di Niu1,2,3 Dan-Dan Zhang5
Dan-Dan Zhang5 Bing-Bing Hou1,2,3*
Bing-Bing Hou1,2,3* Li Zhang1,2,3*
Li Zhang1,2,3* Chao-Zhao Liang1,2,3*
Chao-Zhao Liang1,2,3* He-Xi Du1,2,3*†
He-Xi Du1,2,3*†Chronic prostatitis/Chronic pelvis pain syndrome (CP/CPPS), a kind of frequent urinary condition among adult males, has caused a lot of inconvenience to patients in life, whose pathogenesis is unclear. Current evidence suggests that it is most likely to be an autoimmune disease. Symbiotic microbes, a highly diverse biological community that harbors trillions of microbes in each region of the human body, have gradually made people realize their important role in immune regulation, material metabolism, and health maintenance. In recent years, increasing studies have shown a connection between microbiota and CP/CPPS. In view of this, we performed this review to summarize the literature pertaining to microbiota and its association with the pathophysiological mechanism of CP/CPPS. In addition, we gleaned the latest progress in the therapeutic strategy of CP/CPPS that related to microbiota regulation in order to offer new perspectives on the management of CP/CPPS.
Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS), a prevalent urological disorder, demonstrates a substantial incidence rate reaching 8.2% (Krieger et al., 2008). This condition not only poses significant health risks to affected individuals but also imposes substantial socioeconomic burdens (Bartoletti et al., 2007; Suskind et al., 2013). According to the National Institutes of Health (NIH) classification system, prostatitis is categorized into four distinct types, among which CP/CPPS (NIH category III) accounts for over 90% of clinical cases (Rees et al., 2015). The hallmark symptom of CP/CPPS manifests as recurrent and persistent pelvic pain, typically enduring for more than 3 months, frequently accompanied by varying degrees of lower urinary tract symptoms and sexual dysfunction. Characteristically, bacterial cultures from expressed prostatic secretions (EPS), semen samples, or post-prostatic massage urine specimens (VB3) typically yield negative results, rendering CP/CPPS diagnosis particularly challenging and its underlying etiology a subject of ongoing debate (Habermacher et al., 2006; Schaeffer et al., 2002). Emerging evidence, however, increasingly supports the hypothesis that CP/CPPS may represent an autoimmune-mediated condition (Lu et al., 2018).
The human microbiota, constituting a complex ecosystem of microorganisms established at birth, coexists with the host as an essential biological component and undergoes parallel development throughout the host’s lifespan (Dominguez-Bello et al., 2019). From birth to death, dynamic alterations take place spanning the development trajectory of human microbiota and giving rise to the shaping of phenotypes (Ochman et al., 2010). Ascribing to the tremendous reproduction rates compared to humans and the exceptional adaptability of the metagenome to environmental changes, microbiota may play a crucial role in maintaining systemic homeostasis across multiple host systems (Levy et al., 2017). Consequently, microbial communities maintain a symbiotic relationship with host health, while microbial dysbiosis can readily contribute to disease pathogenesis. Tracing back to 2008, the NIH Common Fund Office initiated the Human Microbiome Project (HMP), a landmark endeavor designed to uncover the fundamental relationships between human microbiota and health, thereby highlighting the critical role of microbial communities in human physiology (Integrative HMP (iHMP) Research Network Consortium, 2019).
Over the past few years, investigators have gradually revealed the inseparable association between human microbiota and various diseases (Ruff et al., 2020; Young, 2017). It is noteworthy that microbiota actively engages with the immune system, potentially to be linked to numerous immune-mediated disorders. Scientific evidence demonstrates that an alteration in certain bacteria in the gut may induce the development of rheumatoid arthritis (RA) (Scher et al., 2013; Vaahtovuo et al., 2008). In another research, Zhang et al. (2023) freshly published their study highlighting that gut microbiota influences autoimmune thyroiditis pathogenesis through hydrogen sulfide (H2S) regulation. Collectively, these findings establish microbial involvement in diverse immunological disease processes.
Similar to their involvement in immunological disease pathogenesis, the disturbance of human microbiota also plays a modulating role in prostatic diseases (Porter et al., 2018). What caught our attention was that related studies on CP/CPPS over the past few years have collectively suggested that the microbiota may be able to address the pending pathogenesis of CP/CPPS (Choi et al., 2020; Wu et al., 2020). Some microbiota-cenric interventions emerged as the key crucial elements in prostate biology, which are paramount for establishing prevention and developing therapeutic strategies (Terrisse et al., 2022; Uchugonova et al., 2015). Despite growing evidence linking microbiota to CP/CPPS, the precise mechanisms underlying this interaction remain largely undefined. Based on such conditions, this review aims to: systematically evaluate current evidence regarding microbial involvement in CP/CPPS pathogenesis, with particular emphasis on disease etiology and progression (Figure 1 and Table 1); and (b) critically assess recent advances in both clinical and basic research on CP/CPPS (Table 2), thereby providing a foundation for well-designed studies and innovative therapeutic approaches.
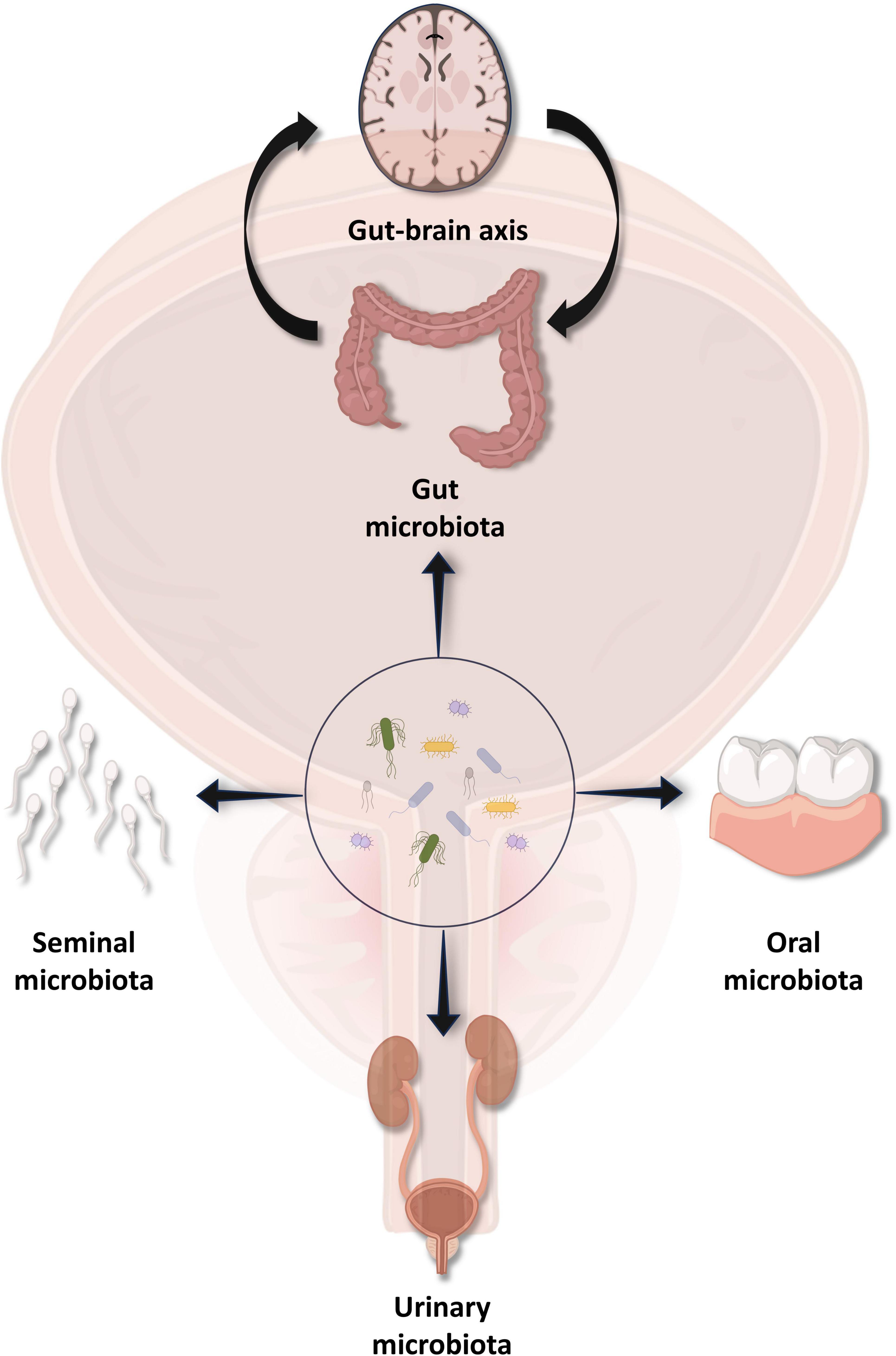
Figure 1. Various microbiota of different regions and chronic prostatitis/chronic pelvis pain syndrome (CP/CPPS). Microbiota present in the gut, oral cavity, urinary system, and semen have proven to correlate with etiopathogenesis as well as the treatment of CP/CPPS.
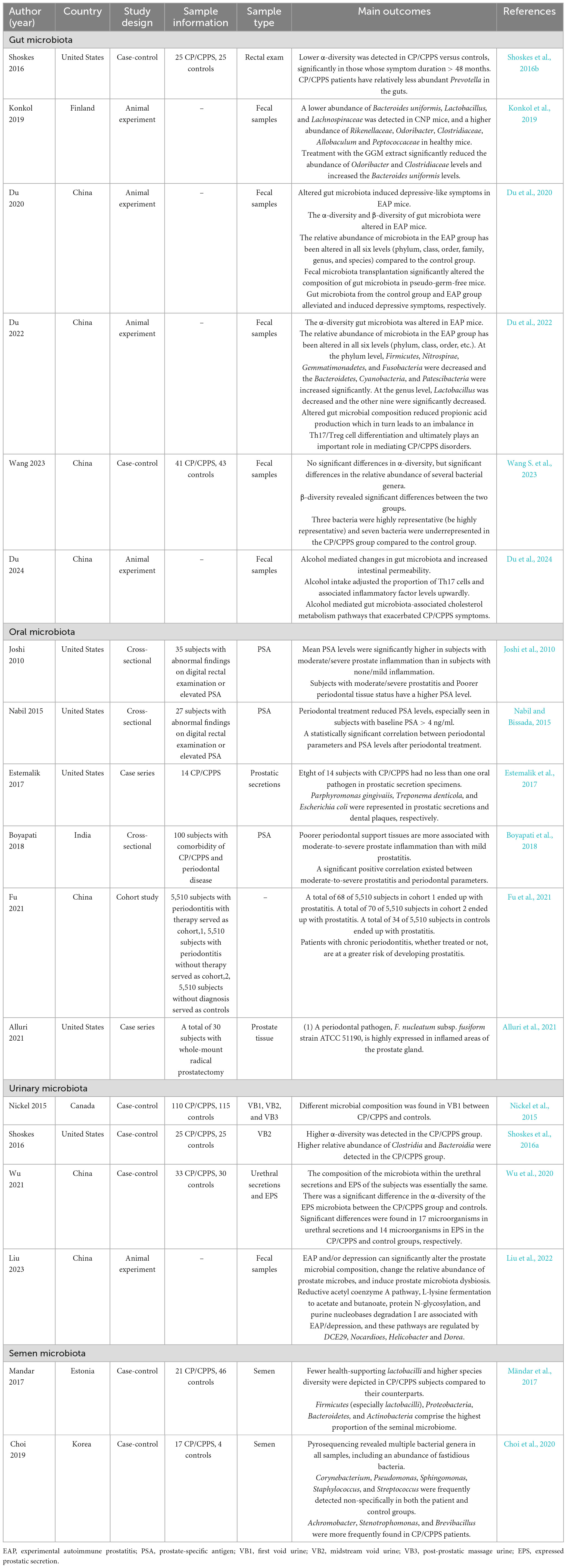
Table 1. Overviewing of select studies investigating the various categories of microbiota in chronic prostatitis/chronic pelvis pain syndrome (CP/CPPS).
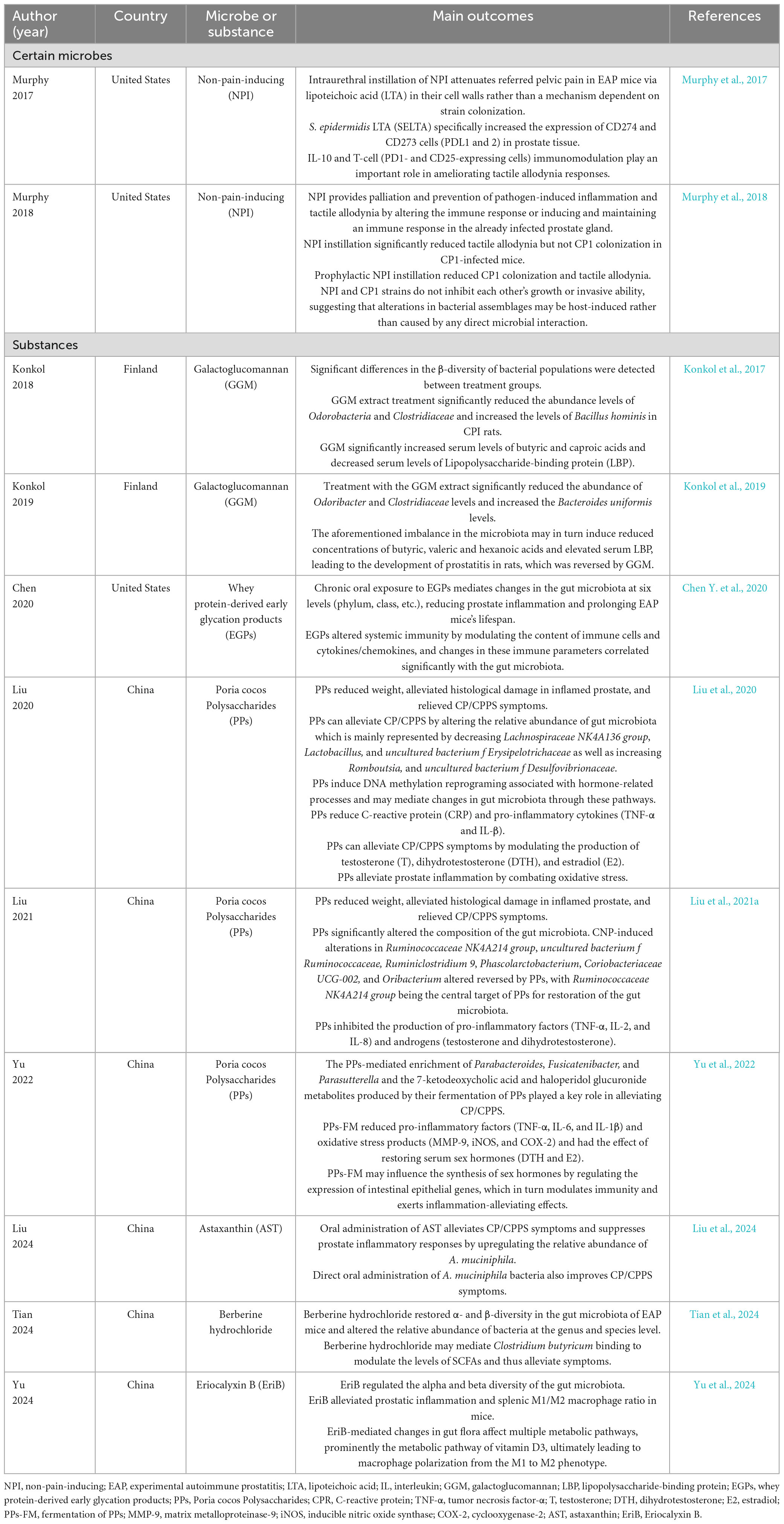
Table 2. Novel microbiota-centric therapeutic strategies for chronic prostatitis/chronic pelvis pain syndrome (CP/CPPS).
The human gut harbors an extensive microbial ecosystem comprising over 10 trillion diverse microorganisms (Zmora et al., 2019). These coevolved microorganisms establish a symbiotic relationship with their host, thriving in the nutrient-dense intestinal environment. They perform crucial physiological functions while simultaneously being involved in disease processes by synthesizing a myriad of bioactive metabolites (Erny et al., 2021; Jugder et al., 2021). Applying the specific mode of producing small molecules, gut microbiota acts as a fundamental factor in the execution of protective, metabolic, and structural homeostasis (Adak and Khan, 2019; Gomaa, 2020). For instance, compelling evidence suggests that gut microbiota comprising its metabolic products significantly influence antitumor immune response, modulate immune checkpoint inhibitor efficacy, and impact cancer progression across multiple tumor types (Lu et al., 2022). In a like manner, gut microbiota affects prostatic pathophysiology by means of the above modes (Matsushita et al., 2021; Zhong et al., 2022).
Specific inflammatory conditions like CP/CPPS are also relevant to gut microbiota dysbiosis. In 2016, Shoskes et al. (2016b) conducted comparative analyses of gut microbiota composition between CP/CPPS patients and healthy controls. Their findings showed great interindividual variability in the gut microbiota and a lower α-diversity in CP/CPPS subjects, particularly in cases with symptom duration exceeding 48 months. In addition, several specific bacterial taxa were over- or under-represented, a case in point with the abundance of Prevotella (Shoskes et al., 2016b). Not limited to studies of the human microbiota, analogous results have been corroborated in animal models. Experimental autoimmune prostatitis (EAP) mouse model, established through subcutaneous injection of prostate antigens (PAg) along with adjuvants, remains the most widely utilized preclinical system for CP/CPPS research. The model recapitulates symptoms and immunologic features resembling human CP/CPPS (Breser et al., 2017). In 2019, Konkol et al. (2019) identified distinct microbial signatures in CP/CPPS rats, with five taxa showing increased abundance and four demonstrating decreased levels compared to healthy controls. Not coming solely but in pairs, Du et al. (2020) also noted comprehensive microbial alterations across all taxonomic levels (phylum, class, order, family, genus, and species) in the EAP models. Supporting these findings, subsequent investigations by the same team identified specific microbial alterations across multiple taxonomic hierarchies. It warrants mentioning that the EAP group showed a decrease in all nine bacterial groups at the genus level, except for Lactobacillus, which increased (Du et al., 2022), enhancing the synthesis of short-chain fatty acids (SCFAs) (Markowiak-Kopeć and Śliewska, 2020). Parallel research revealed distinct β-diversity patterns and identified three enriched and seven depleted bacterial taxa in CP/CPPS cohorts (Wang S. et al., 2023). Taken together, the findings above provide a compelling association between gut microbiota alterations and CP/CPPS pathogenesis. Interestingly, recurrent observation in α-diversity alterations might suggest its potential utility as a diagnostic biomarker for CP/CPPS. Whereas, such abnormal α-diversity may also reflect confounding factors including prolonged antibiotic exposure, aging, or systemic physiological changes (Haak et al., 2019; Odamaki et al., 2016). Furthermore, specific microbial alterations might represent either protective or etiological features. Prevotella, a dietary fiber-responsive bacterial genus that predominates in individuals consuming plant-rich diets, has been implicated in energy metabolism optimization and anti-inflammatory processes. Its established health-promoting properties and observed depletion in CP/CPPS patients suggest its potential dual role as both a diagnostic biomarker and therapeutic target for this condition (Ley, 2016).
Of note, mounting lines of evidence enlightened that there are evident interrelationships between gut microbiota composition and psychological status in CP/CPPS patients (Huang et al., 2019; Kelly et al., 2019). In clinical diagnosis and treatment, psychological dysfunction may be concomitant with physical discomfort in patients suffering from CP/CPPS. Up to 78% of patients showed comorbid depression when compared with the general male population (Egan and Krieger, 1994). Analogous to CP/CPPS, depression exhibits complex etiology, and while the causal relationship remains undefined, their parallel progression is elucidated (Stamatiou et al., 2023). It has been verified, at least in animal models, that prostate inflammation induces depressive symptomatic behavior and cognitive declines (Šutulović et al., 2023). Extensive gut-brain axis-related studies have emerged to provide supporting evidence for the development of depression, and one of the contributors appears to be tied to the gut microbiota (Stower, 2019). Given the fact that altered gut microbiota exists in patients with CP/CPPS, researchers have actively explored the hypothesis that CP/CPPS primarily mediates the gut microbiota leading to depression. Concentrating on this area, Du and his colleagues utilizing the EAP model successively conducted animal experiments that showed much support for such theory that CP/CPPS may lead to the alteration of gut microbiota and induce depressive symptoms (Du et al., 2020; Du et al., 2022).
The underlying mechanism of CP/CPPS remains incompletely characterized. One of the mechanisms, by which the gut microbiota exerts their protective effects, is to occupy the intestine surfaces and maintain intestinal barrier integrity to block the invasion of foreign pathogenic microbes (Karczewski et al., 2010). This mechanism provides substantial support for the hypothesis that CP/CPPS pathogenesis may involve compromised microbial barrier function, permitting pathogen translocation (Song et al., 2023).
Aside from the direct pathway, CP/CPPS bears a relation to disrupting the homeostasis of gut microbiota and the secondary changes it engenders in metabolites resulting from compositional changes (Konkol et al., 2019). In the study of Liu et al. (2021b), they used multi-omics analysis to explore the alteration in the composition of gut microbiota, gene expression, and DNA methylation in the CP/CPPS rat model. Their analysis displayed 185 differentially expressed genes in the intestinal epithelium and identified 73,232 differentially methylated sites (DMSs). In another study by Du et al. (2022), a significant imbalance in the ratio of Th17 cells to Treg cells in the EAP group drew extensive concerns from researchers. Thus, they proposed that the propionate might modulate T cell differentiation through the GPR43-HDAC6 axis, restoring the balance of Th17 and Treg cell ratios. Their updated findings indicated alcohol-mediated gut microbiota alterations stimulate Th17 differentiation and response via the cholesterol biosynthesis metabolic pathway, which ultimately exacerbated CP/CPPS symptoms. The cholesterol biosynthesis regulator SREBP2 played an integral function in this pathway (Du et al., 2024). Taking all results into account, the changes in gut microbiota might alter epithelial gene expression, successively modulating the immunocytes such as Th17/Treg cells balance and their respective pro-/anti-inflammatory cytokine profiles, thus ultimately exerting systemic effects (Wang J. et al., 2023). In addition, the outstanding performance of propionate captured our attention. SCFAs, particularly propionate, domain a leading part of metabolites generated by the gut microbiota through colonic fermentation of undigested carbohydrates, with well-established roles in inflammatory regulation (Cummings et al., 1987; Hamer et al., 2008; Trompette et al., 2014). They exert their effects in a manner of binding to the corresponding G protein-coupled receptors (GPR) such as GPR43 or by interacting with histone deacetylase (HDACs), initiating downstream signaling cascades thus resulting in the corresponding cellular effects (Rooks and Garrett, 2016; Sivaprakasam et al., 2016). Collectively, these findings position gut microbiota as a crucial agent in driving the regulation of host immunity, primarily through microbial metabolites including SCFAs (Schluter et al., 2020; Smith et al., 2013). These mechanistic insights into gut microbiota affecting CP/CPPS have led to the proposal of a gut-prostate axis, which resembles the gut-brain axis, gut-lung axis, and gut-liver axis, etc., (Cryan et al., 2019; Du et al., 2022). The gut-prostate axis builds bidirectional communication between intestinal and prostatic systems which paved the way for researchers to increasingly recognize various domains (Figure 2), but it still warrants further investigation.
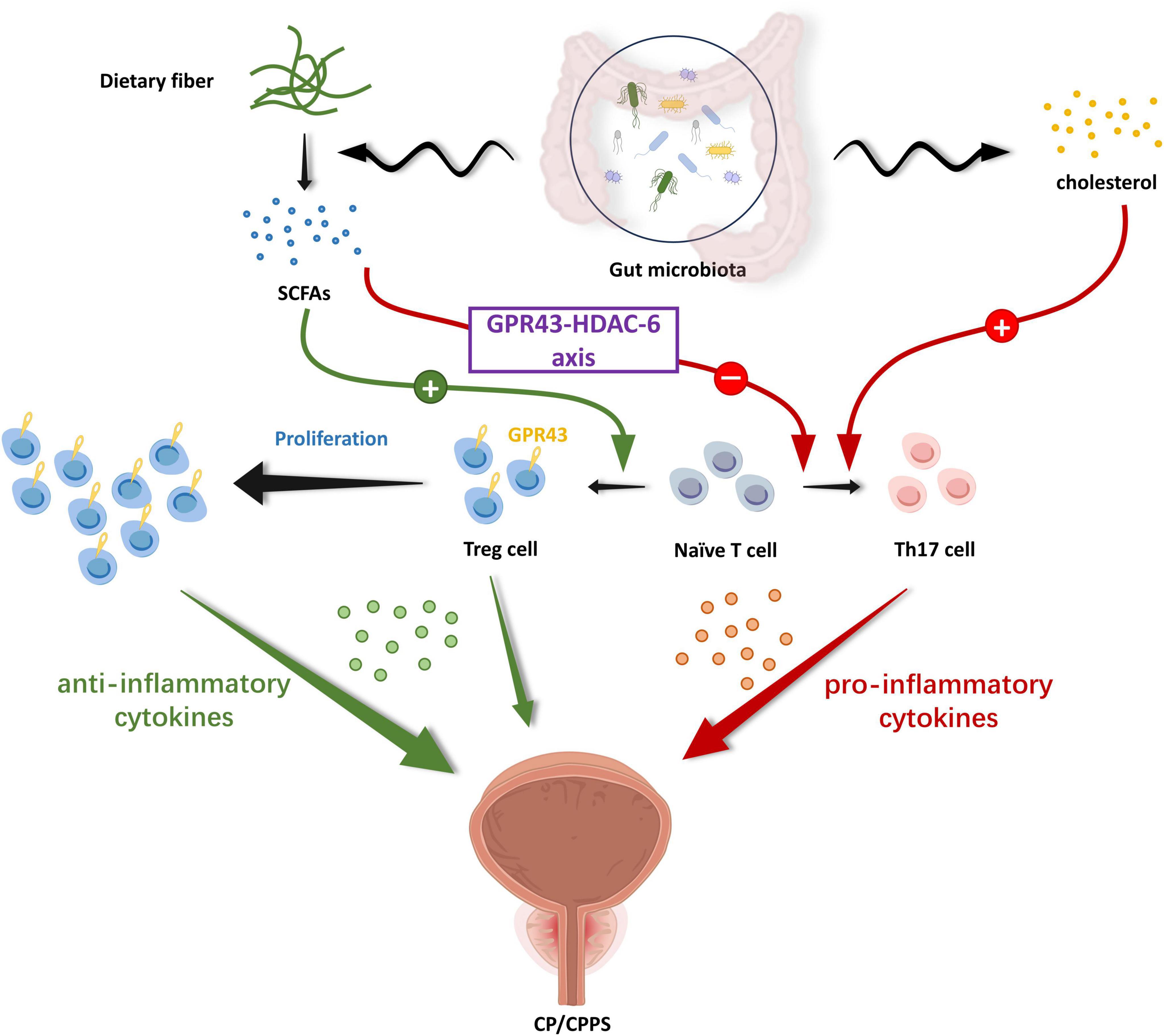
Figure 2. The gut-prostate axis: immune mechanism. SCFAs produced by gut microbiota decomposing dietary fibers in food possess the ability to regulate the proliferation and differentiation of immune cells via the corresponding G protein-coupled receptor pathway. Gut microbiota-derived SCFAs propionate can promote the differentiation of initial T cells to Treg cells and inhibit their differentiation to Th17 cells via the GPR43-HDAC-6 axis, with the former secreting anti-inflammatory cytokines and the latter secreting pro-inflammatory cytokines. Furthermore, gut microbiota up-regulate Th17 cell ratios and their responses through microbial-associated cholesterol metabolism pathways, exacerbating the inflammatory response. The relative balance of Th17/Treg is critical in the inflammatory environment of the prostate. This immune mechanism by which the gut microbiota and its metabolites drive host immune regulation has further deepened researchers’ understanding of the gut-prostate axis. (SCFAs, short chain fat acids; GPR, G protein-coupled receptor; HDAC, histone deacetylase).
A majority of investigators have predominantly attempted to explain the causative efficacy in CP/CPPS of the microbiota which is located in the urogenital system, yet they seldom notice that oral microbiota has unique interest in CP/CPPS. Existing research suggests that oral health status is firmly associated with the development of several systemic diseases (Babaev et al., 2017; Suresh et al., 2016). Accumulating oral microbiota-centric research has substantiated this phenomenon, with a wealth of evidence that oral microbiota may act as suspicious etiologic agents in the onset of prostatic diseases (Boland et al., 2013; Wu et al., 2019). More critically, a couple of important studies have also exemplified both direct and indirect mechanistic links between the oral microbiota and CP/CPPS.
To start with, several studies have provided indirect evidence of the association of oral microbes with CP/CPPS. Elevated serum prostate-specific antigen (PSA), a well-established prostate cancer biomarker, showed a statistical association with CP/CPPS (Nadler et al., 2006). It is indicated that prostatic inflammation contributes greatly to elevating serum PSA concentrations (Nickel et al., 1999; Yaman et al., 2003). Zooming into previous epidemiological studies, PSA level also seems to be statistically associated with periodontal disease (Boyapati et al., 2018; Joshi et al., 2010). Merging epidemiological studies have shown an indirect correlation between CP/CPPS and periodontal disease through the intermediary of PSA. Interestingly, periodontal treatment has been shown to reduce serum PSA level and alleviate CP/CPPS symptoms (Nabil and Bissada, 2015). Herein, there may be potential PSA-mediated connections between periodontal health and CP/CPPS.
Beyond indirect associations, some evidence also objectively suggests a relatively direct potential relationship between CP/CPPS and oral microbiota. Initial small-scale case analyses isolated exact oral pathogens from EPS of comorbid patients with periodontal disease and CP/CPPS. The investigators found that a total of 8 of 14 (57.1%) subjects had no less than one oral pathogen in their samples (Estemalik et al., 2017). In another cross-sectional study by Boyapati et al. (2018), they found the mean clinical attachment level, a standard parameter that reflects the extent of periodontal tissue destruction, notably increasing in moderate-to-severe CP/CPPS patients in spite of mild CP/CPPS patients (Sanz et al., 2020). A cohort study selected periodontitis patients who were excluded from prior CP/CPPS diagnosis into two cohorts of patients with and without periodontitis treatment, and a matched control cohort without periodontitis was set. At the endpoint, the characteristic patterned increased CP/CPPS susceptibility among periodontitis patients, regardless of treatment status (Fu et al., 2021). Alluri et al. (2021) isolated Fusobacterium nucleatum subsp. fusiform strain ATCC 51190, a periodontal pathogen, in histologically abnormal prostate tissues, suggesting its potential role in linking periodontal disease and prostatic inflammation. As noted above, we can hypothesize that there is a correlation between oral microbiota and CP/CPPS. However, cross-sectional studies are limited to probing causality, and further randomized controlled trials (RCTs) and experimental validations remain necessary to elucidate oral microbiota’s role in CP/CPPS pathogenesis.
The precise mechanism through which oral microbiota affects CP/CPPS pathogenesis remains elusive, with the available evidence principally supporting two primary direct pathways, microbial translocation and indirect systemic inflammation. For the direct pathway, previously mentioned results suggest the hypothesis that hematogenous dissemination of oral microorganisms to the prostate triggers histological changes that contribute to the occurrence of CP/CPPS (Alluri et al., 2021; Estemalik et al., 2017). For the indirect pathway, increasingly circulating levels of periodontal-derived pro-inflammatory factors, including interleukins (IL-1β, IL-2, IL-6, IL-8), tumor necrosis factor (TNF-α), and C-reactive protein (CRP), may act as indirect agents in the onset of CP/CPPS (Boyapati et al., 2018; Hegde and Awan, 2019). These locally produced, periodontitis-derived inflammatory mediators can trigger a systemic inflammatory response through the circulatory system, which in turn causes or exacerbates CP/CPPS (Figure 3).
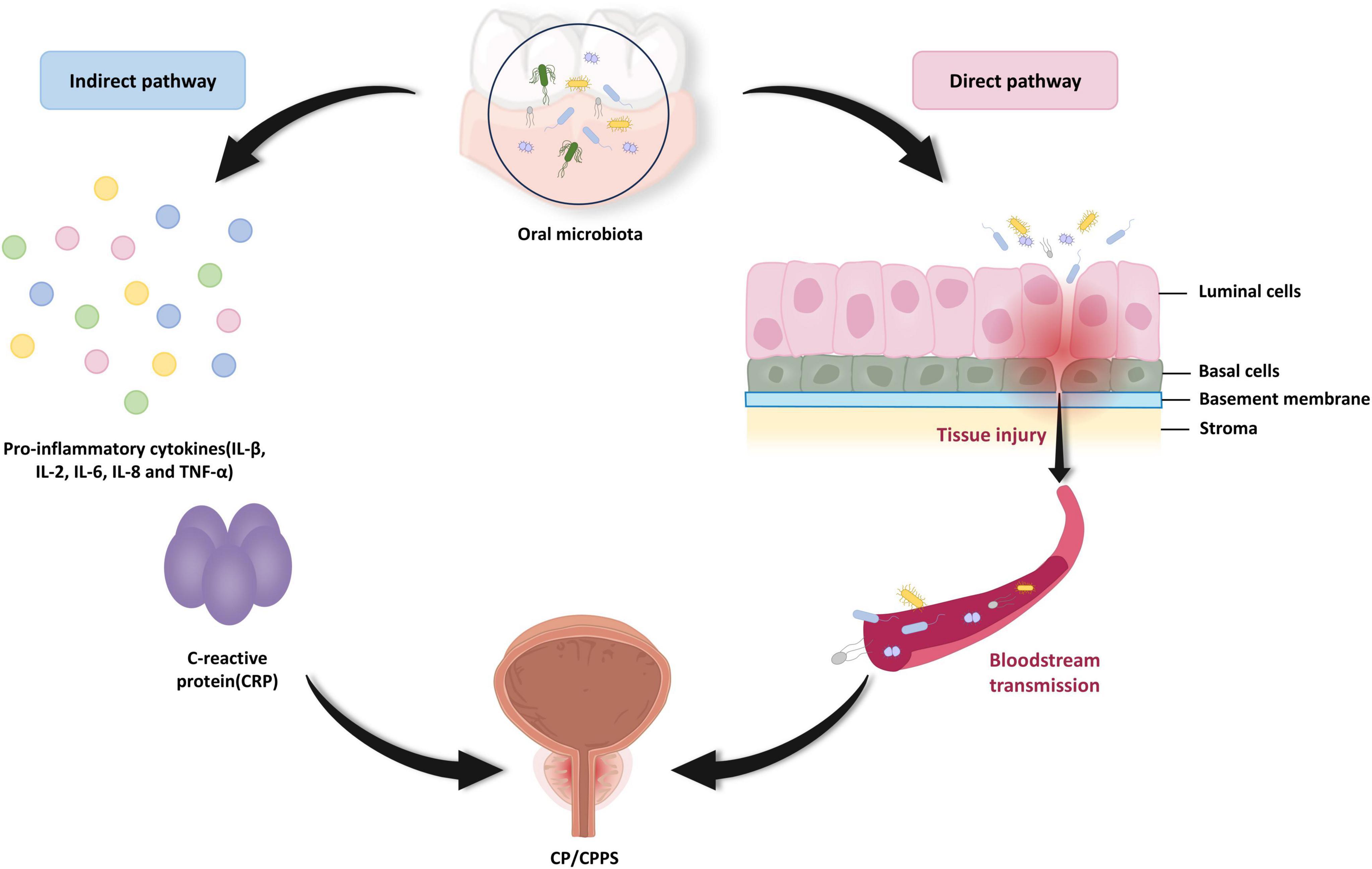
Figure 3. The possible mechanisms of oral microbiota-induced chronic prostatitis/chronic pelvis pain syndrome (CP/CPPS). Oral pathogens may reach the prostate via hematogenous spread, thereby directly injuring prostate tissue. In addition to this, oral pathogens may mediate the production and dissemination of pro-inflammatory factors, such as IL-1β, IL-2, IL-6, IL-8, TNF-α, and CRP that indirectly trigger CP/CPPS onset. This hypothesis of an indirect pathogenic pathway provides an argument for systemic immune disorders to lead to CP/CPPS. (IL, interleukin; TNF-α, tumor necrosis factor-α; CRP, C-reactive protein).
In an extraordinarily long period, the longstanding paradigm of urinary sterility has dominated throughout the entire research process owing to conventional culture methods. However, next-generation sequencing (NGS) has revolutionized this concept, revealing complex microbial ecosystems within the urinary tract (Wolfe et al., 2012). Given the anatomical proximity and shared urothelium, urinary microbiota might be of particular interest to prostatic diseases (Porter et al., 2018). Accordingly, a tremendous amount of energy was invested into the domain of how urinary microbiota affects human health with all sorts of evidence denoting that urinary microbiota plays a role in urinary diseases (Shrestha et al., 2018).
Nickel et al. (2015) yielded the first glimpse into urinary microbiota and CP/CPPS and, identified an excess of Burkholderia cenocepacia in first-void urine (VB1) specimens from patients. Correspondingly, a study by Shoskes et al. (2016a) subsequently demonstrated increased Clostridia and Bacteroidia and elevated α-diversity in CP/CPPS patient samples. Their findings revealed a statistical correlation between the severity of patient symptoms and the composition of the urinary microbiota, in addition to a significant increase in the bacterial diversity of the urinary microbiota as the symptom score increased (Shoskes et al., 2016a). Recently, Wu et al. (2020) employed high-throughput NGS to demonstrate a similarity in the composition of microbial communities between urethral secretions and EPS samples in CP/CPPS patients and healthy populations, respectively. Meanwhile, seventeen bacterial species were differentially represented in the urethral secretions in cases versus controls, though no typical pathogens were detected in CP/CPPS patients. As noted above, B. cenocepacia, an opportunistic human pathogen, was described as potentially involved in the etiology of CP/CPPS (Organ et al., 2010; Schwager et al., 2013). The abnormally richer α-diversity and the specific class-level alterations may represent promising clinical diagnostic biomarkers. To clarify, the discovery of Wu et al. implies that non-cellular microbes in CP/CPPS patients provide new insights into CP/CPPS etiology and mechanisms.
To verify the infection hypothesis of CP/CPPS-associated chronic pain is clinically relevant to infection, Rudick et al. (2011) inoculated murine models with CP1, an atypical isolate distinct obtained from EPS and VB3 of a CP/CPPS patient. They showed CP1’s ability to induce and maintain chronic pelvic pain in murine models. Aside from this specific Gram-negative bacterial isolate, the same laboratory subsequently demonstrated that multiple Gram-positive patient-derived bacteria were up to trigger CP/CPPS-like symptoms in murine models (Murphy et al., 2019). Its induction mechanism appears to involve STAT6-mediated regulation of IL-4 and IL-13 secretion (Bell-Cohn et al., 2019). These findings establish a foundation for developing more representative animal models and may address gaps in the etiology of CP/CPPS.
As is presented psychiatric comorbidities (particularly depression and somatization) were found to have negative impacts on CP/CPPS symptom severity, and some adequately powered studies were designed concerning prostate microbiota to address this clinical challenge (Koh et al., 2014). Liu et al. (2022) demonstrated a multitude of prostate microbiota composition was aberrant in their assay of EAP/depression mice. In tandem, several bacteria-related metabolic pathways were confirmed to be correlated with both EAP and depression. These findings provide novel therapeutic targets for addressing CP/CPPS-depression comorbidity.
One of the classifications of CP/CPPS is the presence of leukocytes and bacteria in the EPS, the composition of which provides insights into prostate health status (Krieger et al., 1999). Song et al. (2024) detected characteristic microbes in the EPS from CP/CPPS patients, with significant compositional changes in patients who underwent effective low-intensity pulsed ultrasound therapy. Taking credit for the constituent of semen and EPS shares high similarity, semen analysis serves as an alternative diagnostic approach when EPS collection is challenging (Motrich et al., 2018). However, this alternative has shortcomings, as semen-derived microbes and inflammatory markers may originate from non-prostatic sources, which can lead to clinical diagnosis bias (Kermes et al., 2003). Nonetheless, some microbes are significantly enriched in male semen with urinary diseases, and quantitative microbial parameters also show salient correlations with inflammation (Korrovits et al., 2006).
Mändar et al. (2017) analyzed the semen microbiota of CP/CPPS patients and healthy controls by applying 16S rRNA gene sequencing. They found that fewer health-supporting Lactobacillus and higher species diversity were depicted in CP/CPPS subjects compared to their counterparts. The predominant phyla detected in semen samples are Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria. In another pilot study, Choi et al. (2020) displayed the discrepancy that Achromobacter, Stenotrophomonas, Brevibacillus were more commonly present in the semen of CP/CPPS patients. Both of the results showed the association between semen microbiota and CP/CPPS, suggesting the possible protective effects of Lactobacillus.
As the etiology of CP/CPPS is undetermined, empirical antibiotic therapy remains a common clinical approach (Rees et al., 2015). Abusing antibiotics entails several undue toxicities, which can’t be underestimated (Singh et al., 2017). Promisingly, several substances that can modulate microbial abundance and specific commensal isolates have shown initial symptom-relieving efficacy in preclinical studies.
Some studies in recent years have shown that bacteria isolated from healthy populations appear to ameliorate symptoms of CP/CPPS. The non-pain-inducing (NPI) strain of Staphylococcus epidermidis, isolated from the prostatic secretions, has been detected to lessen the symptoms in murine models with CP/CPPS and increase CD4+IL-17A+T-cell populations (Murphy et al., 2017), known to be salient in disease orchestration and pelvic tactile allodynia development (Murphy et al., 2015). Further on, the team also found that prophylactic instillation of NPI palliated colonization, pain response, and immune activation of urinary pathogens (Murphy et al., 2018). The discoveries highlight the therapeutic potential of human commensal bacteria and warrant further investigation into their clinical applications for CP/CPPS management.
Meanwhile, some compounds are competent to regulate the relative abundance of microbes upstream, thereby improving the symptoms in animal models.
Chen Y. et al. (2020) administered whey protein-derived early glycation products (EGPs) at 600 mg/kg/day to non-obese diabetic (NOD) mice with concurrent spontaneous autoimmune prostatitis. After undergoing 6 months of rearing, EGP-treated mice exhibited significantly improved survival rates compared to controls. Moreover, attenuated prostate inflammation, decreased splenic M1 macrophage and lymphocyte populations, as well as increased systemic anti-inflammatory factor levels were exhibited in the group with EGPs. Changes in the relative abundance of gut microbiota and increased Bacteroides acidifaciens counts paralleled the alleviation of inflammation, perhaps correlating the intrinsic mechanism of EGP in regulating the systemic immune status in a manner.
In Konkol et al. (2017) study, galactoglucomannan (GGM) was clarified to possess the ability to improve lower urinary tract symptoms (LUTS) associated with chronic prostatic inflammation in rat models. In subsequent experiments, they established a non-bacterial prostatitis model with subcutaneous testosterone and 17β-estradiol hormone pellets on adult male Wister rats, administering water containing 2% GGM orally for feeding whilst the control group was given tap water. Following 5 weeks of treatment, GGM normalized prostate inflammation-induced elevations in Odoribacter and Clostridiaceae levels while increasing Bacteroides uniformis abundance. In addition, GGM was demonstrated to significantly increase the serum levels of butyric acid and caproic acid while lipopolysaccharide binding protein (LBP) concentrations, thereby contributing to relieving the LUTS of prostatitis (Konkol et al., 2019).
Liu et al. (2020, 2021a) found that Poria cocos Polysaccharides (PPs) pre-treated with 250 mg/kg per day by gavage for 7 days alleviated inflammation in a non-bacterial prostatitis model induced by intraperitoneal injections of 1% λ-carrageenan in male Sprague Dawley (SD) rats, with gut microbiota modulation, particularly targeting the Ruminococcaceae NK4A214 group to ameliorate symptom. In addition to the blank control and the untreated group, the researchers installed a finasteride-treated group, the outcome showed that PPs could target different genera compared to the finasteride group, thus exerting therapeutic effects. Research thereafter clarified that PPs may modulate several key bacteria and metabolic outputs, including 7-keto-deoxycholic acid and haloperidol glucuronide, resulting in palliative effects (Yu et al., 2022).
Astaxanthin (AST), a compound with potent antioxidant, anti-inflammatory, and immunomodulatory properties (Chen Z. et al., 2020), has recently been the subject of related studies that have demonstrated its value in upregulating the relative abundance of Akkermansia muciniphila, which in turn ameliorating CP/CPPS symptoms. NOD mice were stratified into four groups: control, EAP, EAP + AST 50 mg/kg group, and EAP + AST 100 mg/kg group. All mice excluding the control group received oral AST administration every other day for 6 weeks starting from the first subcutaneous immunization until the end of the experiment, following a second immunization to obtain the EAP model at the fourth week. Feces from CP/CPPS patients exhibited a reduction of A. muciniphila in the gut microbiota compared to the healthy population the same as in EAP mice versus the normal mice, whereas AST treatment restored A. muciniphila levels in EAP models. A. muciniphila transplantation to EAP mice mirrored similar benefits of AST intervention in elevating the concentration of SCFAs, especially acetic acid which ultimately eases prostate inflammation (Liu et al., 2024).
Berberine hydrochloride is known to work in the therapeutic area across various diseases. In EAP models, daily administration of berberine hydrochloride 200 mg/kg for 4 weeks restored the α- and β-diversity of the gut microbiota, reduced prostate inflammation, and mitigated alterations in the relative abundance of the bacterial species at the genus and species level. Strikingly, Clostridium butyricum increased SCFA production in the course that berberine hydrochloride regulates the intestinal microbiota, representing a candidate target of the berberine hydrochloride effect. Fecal microbiome transplantation (FMT) based on pseudo-germ-free mice (PGFR) revealed that recipient mice receiving microbiota from berberine hydrochloride-treated EAP donors exhibited more severe inflammatory reactions than those receiving untreated EAP microbiota, reaffirming the mitigating effect of berberine hydrochloride (Tian et al., 2024).
In the work of Yu et al. (2024), prostate inflammation was significantly alleviated in EAP mice receiving daily oral gavage of EriB 10 mg/kg for 14 consecutive days, as well as altered gut microbiota α- and β-diversity in EAP mice compared to untreated controls. Such changes in microbiota composition altered the metabolic pathway of vitamin D3, promoting macrophage polarization from pro-inflammatory M1 to anti-inflammatory M2. Subsequent FMT in PGFR confirmed these findings, showing reduced M1/M2 ratios in recipients of EriB-treated donor microbiota compared to the EAP group without EriB, further substantiating the above mechanism.
While promising, the mutual deficiency of these studies is the boundedness of murine models. Their pharmaceutical validity and safety for human application are unverified, necessitating human-appropriate formulations and large-scale clinical trials. Nevertheless, these compounds represent promising candidates to help develop rational alternative treatment scheme regimens of CP/CPPS at this stage.
Overwhelming evidence demonstrates that alterations in human microbiota are intimately associated with CP/CPPS, which raises informed conjectures about the pathogenesis of this clinically intractable dilemma. With respect to the advances in sequencing technologies, microbiota and their metabolic products were proven to play an important role in CP/CPPS pathophysiology. The utilization of gut microbiota and microbiota-related metabolites as novel promising biomarkers for CP/CPPS prediction and diagnosis, as well as targets for disease management, has shown immense research interest. Future research directions will focus on microbiota-targeted interventions such as probiotics, prebiotics, and fecal microbiota transplantation, offering safer and more effective clinical alternative treatment schemes to current therapies with fewer adverse effects.
A-QD: Writing – original draft. S-YY: Writing – review and editing. DN: Writing – review and editing. D-DZ: Writing – review and editing. B-BH: Funding acquisition, Writing – review and editing. LZ: Writing – original draft, Writing – review and editing. C-ZL: Funding acquisition, Writing – review and editing. H-XD: Funding acquisition, Writing – review and editing.
The author(s) declare that financial support was received for the research and/ or publication of this article. This work was supported by the National Natural Science Fund of China (82100815, 82100814, 82170787, and 82370776), Anhui Natural Science Foundation (2108085QH315, 2408085Y038), and Anhui Medical University Funded Project (2023xki132).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adak, A., and Khan, M. (2019). An insight into gut microbiota and its functionalities. Cell. Mol. Life Sci. 76, 473–493. doi: 10.1007/s00018-018-2943-4
Alluri, L., Paes Batista da Silva, A., Verma, S., Fu, P., Shen, D., MacLennan, G., et al. (2021). Presence of specific periodontal pathogens in prostate gland diagnosed with chronic inflammation and adenocarcinoma. Cureus 13:e17742. doi: 10.7759/cureus.17742
Babaev, E., Balmasova, I., Mkrtumyan, A., Kostryukova, S., Vakhitova, E., Il’ina, E., et al. (2017). Metagenomic analysis of gingival sulcus microbiota and pathogenesis of periodontitis associated with Type 2 diabetes mellitus. Bull. Exp. Biol. Med. 163, 718–721. doi: 10.1007/s10517-017-3888-6
Bartoletti, R., Cai, T., Mondaini, N., Dinelli, N., Pinzi, N., Pavone, C., et al. (2007). Prevalence, incidence estimation, risk factors and characterization of chronic prostatitis/chronic pelvic pain syndrome in urological hospital outpatients in Italy: Results of a multicenter case-control observational study. J. Urol. 178, 2411–2415.
Bell-Cohn, A., Mazur, D., Hall, C., Schaeffer, A., and Thumbikat, P. (2019). Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type 2 cytokine signaling. Am. J. Physiol. Renal. Physiol. 316, F682–F692. doi: 10.1152/ajprenal.00222.2018
Boland, M., Hripcsak, G., Albers, D., Wei, Y., Wilcox, A., Wei, J., et al. (2013). Discovering medical conditions associated with periodontitis using linked electronic health records. J. Clin. Periodontol. 40, 474–482. doi: 10.1111/jcpe.12086
Boyapati, R., Swarna, C., Devulapalli, N., Sanivarapu, S., Katuri, K., and Kolaparthy, L. (2018). Unveiling the link between prostatitis and periodontitis. Contemp. Clin. Dent. 9, 524–529. doi: 10.4103/ccd.ccd_746_18
Breser, M., Motrich, R., Sanchez, L., and Rivero, V. (2017). Chronic pelvic pain development and prostate inflammation in strains of mice with different susceptibility to experimental autoimmune prostatitis. Prostate 77, 94–104. doi: 10.1002/pros.23252
Chen, Y., Guo, K., Nagy, T., and Guo, T. (2020). Chronic oral exposure to glycated whey proteins increases survival of aged male NOD mice with autoimmune prostatitis by regulating the gut microbiome and anti-inflammatory responses. Food Functon. 11, 153–162. doi: 10.1039/c9fo01740b
Chen, Z., Xiao, J., Liu, H., Yao, K., Hou, X., Cao, Y., et al. (2020). Astaxanthin attenuates oxidative stress and immune impairment in D-galactose-induced aging in rats by activating the Nrf2/Keap1 pathway and suppressing the NF-κB pathway. Food Funct. 11, 8099–8111. doi: 10.1039/d0fo01663b
Choi, J., Lee, S., Kang, S., Lee, S., and Choe, H. (2020). Analysis of bacterial community using pyrosequencing in semen from patients with chronic pelvic pain syndrome: A pilot study. Transl. Androl. Urol. 9, 398–404. doi: 10.21037/tau.2020.02.05
Cryan, J., O’Riordan, K., Cowan, C., Sandhu, K., Bastiaanssen, T., Boehme, M., et al. (2019). The microbiota-gut-brain axis. Physiol. Rev. 99, 1877–2013. doi: 10.1152/physrev.00018.2018
Cummings, J., Pomare, E., Branch, W., Naylor, C., and Macfarlane, G. (1987). Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 28, 1221–1227.
Dominguez-Bello, M., Godoy-Vitorino, F., Knight, R., and Blaser, M. (2019). Role of the microbiome in human development. Gut 68, 1108–1114. doi: 10.1136/gutjnl-2018-317503
Du, H., Liu, Y., Zhang, L., Zhan, C., Chen, J., Zhang, M., et al. (2020). Abnormal gut microbiota composition is associated with experimental autoimmune prostatitis-induced depressive-like behaviors in mice. Prostate 80, 663–673. doi: 10.1002/pros.23978
Du, H., Yue, S., Niu, D., Liu, C., Zhang, L., Chen, J., et al. (2022). Gut Microflora modulates Th17/Treg cell differentiation in experimental autoimmune prostatitis via the short-chain fatty acid propionate. Front. Immunol. 13:915218. doi: 10.3389/fimmu.2022.915218
Du, H., Yue, S., Niu, D., Liu, X., Li, W., Wang, X., et al. (2024). Alcohol intake exacerbates experimental autoimmune prostatitis through gut microbiota driving cholesterol biosynthesis-mediated Th17 differentiation. Int. Immunopharmacol. 139:112669. doi: 10.1016/j.intimp.2024.112669
Egan, K., and Krieger, J. (1994). Psychological problems in chronic prostatitis patients with pain. Clin. J. Pain 10, 218–226.
Erny, D., Dokalis, N., Mezö, C., Castoldi, A., Mossad, O., Staszewski, O., et al. (2021). Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell. Metab. 33, 2260–2276.e7. doi: 10.1016/j.cmet.2021.10.010
Estemalik, J., Demko, C., Bissada, N., Joshi, N., Bodner, D., Shankar, E., et al. (2017). Simultaneous detection of oral pathogens in subgingival plaque and prostatic fluid of men with periodontal and prostatic diseases. J. Periodontol. 88, 823–829. doi: 10.1902/jop.2017.160477
Fu, E., Cheng, C., Chung, C., Lee, W., Chen, W., Sun, G., et al. (2021). Association of chronic periodontitis with prostatic hyperplasia and prostatitis: A population-based cohort study in Taiwan. J. Periodontol. 92, 72–86. doi: 10.1002/JPER.19-0706
Gomaa, E. (2020). Human gut microbiota/microbiome in health and diseases: A review. Antonie Van Leeuwenhoek 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Haak, B., Lankelma, J., Hugenholtz, F., Belzer, C., de Vos, W., and Wiersinga, W. (2019). Long-term impact of oral vancomycin, ciprofloxacin and metronidazole on the gut microbiota in healthy humans. J. Antimicrob. Chemother. 74, 782–786. doi: 10.1093/jac/dky471
Habermacher, G., Chason, J., and Schaeffer, A. (2006). Prostatitis/chronic pelvic pain syndrome. Annu. Rev. Med. 57, 195–206.
Hamer, H., Jonkers, D., Venema, K., Vanhoutvin, S., Troost, F., and Brummer, R. (2008). Review article: The role of butyrate on colonic function. Alimentary Pharmacol. Therapeutics. 27, 104–119.
Hegde, R., and Awan, K. (2019). Effects of periodontal disease on systemic health. Dis. Mon. 65, 185–192. doi: 10.1016/j.disamonth.2018.09.011
Huang, T., Lai, J., Du, Y., Xu, Y., Ruan, L., and Hu, S. (2019). Current understanding of gut microbiota in mood disorders: An update of human studies. Front. Genet. 10:98. doi: 10.3389/fgene.2019.00098
Integrative HMP (iHMP) Research Network Consortium. (2019). The integrative human microbiome project. Nature 569, 641–648. doi: 10.1038/s41586-019-1238-8
Joshi, N., Bissada, N., Bodner, D., Maclennan, G., Narendran, S., Jurevic, R., et al. (2010). Association between periodontal disease and prostate-specific antigen levels in chronic prostatitis patients. J. Periodontol. 81, 864–869. doi: 10.1902/jop.2010.090646
Jugder, B., Kamareddine, L., and Watnick, P. (2021). Microbiota-derived acetate activates intestinal innate immunity via the Tip60 histone acetyltransferase complex. Immunity 54:17. doi: 10.1016/j.immuni.2021.05.017
Karczewski, J., Troost, F., Konings, I., Dekker, J., Kleerebezem, M., Brummer, R., et al. (2010). Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G851–G859. doi: 10.1152/ajpgi.00327.2009
Kelly, J., Keane, V., Cryan, J., Clarke, G., and Dinan, T. (2019). Mood and microbes: Gut to brain communication in depression. Gastroenterol. Clin. North Am. 48, 389–405. doi: 10.1016/j.gtc.2019.04.006
Kermes, K., Punab, M., Lõivukene, K., and Mändar, R. (2003). Anaerobic seminal fluid micro-flora in chronic prostatitis/chronic pelvic pain syndrome patients. Anaerobe 9, 117–123. doi: 10.1016/s1075-9964(03)00085-4
Koh, J., Ko, H., Wang, S., Cho, K., Kim, J., Lee, S., et al. (2014). Depression and somatic symptoms may influence on chronic prostatitis/chronic pelvic pain syndrome: A preliminary study. Psychiatry Investig. 11, 495–498. doi: 10.4306/pi.2014.11.4.495
Konkol, Y., Keskitalo, A., Vuorikoski, H., Pietilä, S., Elo, L., Munukka, E., et al. (2019). Chronic nonbacterial prostate inflammation in a rat model is associated with changes of gut microbiota that can be modified with a galactoglucomannan-rich hemicellulose extract in the diet. BJU Int. 123, 899–908. doi: 10.1111/bju.14553
Konkol, Y., Vuorikoski, H., Tuomela, J., Holmbom, B., and Bernoulli, J. (2017). Galactoglucomannan-rich hemicellulose extract from Norway spruce (Picea abies) exerts beneficial effects on chronic prostatic inflammation and lower urinary tract symptoms in vivo. Int. J. Biol. Macromol. 101, 222–229. doi: 10.1016/j.ijbiomac.2017.03.079
Korrovits, P., Punab, M., Türk, S., and Mändar, R. (2006). Seminal microflora in asymptomatic inflammatory (NIH IV category) prostatitis. Eur. Urol. 50, 1338–1344. doi: 10.1016/j.eururo.2006.05.013
Krieger, J., Lee, S., Jeon, J., Cheah, P., Liong, M., and Riley, D. (2008). Epidemiology of prostatitis. Int. J. Antimicrob. Agents 31, S85–S90. doi: 10.1016/j.ijantimicag.2007.08.028
Krieger, J., Nyberg, L., and Nickel, J. C. (1999). NIH consensus definition and classification of prostatitis. JAMA 282, 236–237.
Levy, M., Kolodziejczyk, A., Thaiss, C., and Elinav, E. (2017). Dysbiosis and the immune system. Nat. Rev. Immunol. 17, 219–232. doi: 10.1038/nri.2017.7
Ley, R. (2016). Gut microbiota in 2015: Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 13, 69–70. doi: 10.1038/nrgastro.2016.4
Liu, F., Xu, X., Wang, Z., and Wu, P. (2022). Abnormal prostate microbiota composition is associated with experimental autoimmune prostatitis complicated with depression in rats. Front. Cell. Infect. Microbiol. 12:966004. doi: 10.3389/fcimb.2022.966004
Liu, J., Liu, L., Zhang, G., and Peng, X. (2021a). Poria cocos polysaccharides attenuate chronic nonbacterial prostatitis by targeting the gut microbiota: Comparative study of Poria cocos polysaccharides and finasteride in treating chronic prostatitis. Int. J. Biol. Macromol. 189, 346–355. doi: 10.1016/j.ijbiomac.2021.08.139
Liu, J., Wang, Y., Zhang, G., Liu, L., and Peng, X. (2021b). Multi-omics analysis reveals changes in the intestinal microbiome, transcriptome, and methylome in a rat model of chronic non-bacterial prostatitis: Indications for the existence of the gut-prostate axis. Front. Physiol. 12:753034. doi: 10.3389/fphys.2021.753034
Liu, J., Yu, J., and Peng, X. (2020). Poria cocos polysaccharides alleviates chronic nonbacterial prostatitis by preventing oxidative stress, regulating hormone production, modifying gut microbiota, and remodeling the DNA methylome. J. Agric. Food Chem. 68, 12661–12670. doi: 10.1021/acs.jafc.0c05943
Liu, Y., Xie, W., Xi, P., Zhang, Z., Chen, R., Fu, S., et al. (2024). Astaxanthin alleviates chronic prostatitis/chronic pelvic pain syndrome by increasing colonization of Akkermansia muciniphila in the intestine. Phytomed. Int. J. Phytother. Phytopharmacol. 123:155249. doi: 10.1016/j.phymed.2023.155249
Lu, J., Shen, J., Hu, X., Peng, L., Hong, Z., and Yao, B. (2018). Identification and preliminary study of immunogens involved in autoimmune prostatitis in human males. Prostate doi: 10.1002/pros.23684 Online ahead of print.
Lu, Y., Yuan, X., Wang, M., He, Z., Li, H., Wang, J., et al. (2022). Gut microbiota influence immunotherapy responses: Mechanisms and therapeutic strategies. J. Hematol. Oncol. 15:47. doi: 10.1186/s13045-022-01273-9
Mändar, R., Punab, M., Korrovits, P., Türk, S., Ausmees, K., Lapp, E., et al. (2017). Seminal microbiome in men with and without prostatitis. Int. J. Urol. 24, 211–216. doi: 10.1111/iju.13286
Markowiak-Kopeć, P., and Śliewska, K. (2020). The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 12:1107. doi: 10.3390/nu12041107
Matsushita, M., Fujita, K., Hayashi, T., Kayama, H., Motooka, D., Hase, H., et al. (2021). Gut microbiota-derived short-chain fatty acids promote prostate cancer growth via IGF1 signaling. Cancer Res. 81, 4014–4026. doi: 10.1158/0008-5472.CAN-20-4090
Motrich, R., Salazar, F., Breser, M., Mackern-Oberti, J., Godoy, G., Olivera, C., et al. (2018). Implications of prostate inflammation on male fertility. Andrologia 50:e13093. doi: 10.1111/and.13093
Murphy, S., Anker, J., Mazur, D., Hall, C., Schaeffer, A., and Thumbikat, P. (2019). Role of gram-positive bacteria in chronic pelvic pain syndrome (CPPS). Prostate 79, 160–167. doi: 10.1002/pros.23721
Murphy, S., Hall, C., Done, J., Schaeffer, A., and Thumbikat, P. (2018). A prostate derived commensal Staphylococcus epidermidis strain prevents and ameliorates induction of chronic prostatitis by UPEC infection. Sci. Rep. 8:17420. doi: 10.1038/s41598-018-35818-1
Murphy, S., Schaeffer, A., Done, J., Quick, M., Acar, U., and Thumbikat, P. (2017). Commensal bacterial modulation of the host immune response to ameliorate pain in a murine model of chronic prostatitis. Pain 158, 1517–1527. doi: 10.1097/j.pain.0000000000000944
Murphy, S., Schaeffer, A., Done, J., Wong, L., Bell-Cohn, A., Roman, K., et al. (2015). IL17 mediates pelvic pain in experimental autoimmune prostatitis (EAP). PLoS One 10:e0125623. doi: 10.1371/journal.pone.0125623
Nabil, F., and Bissada, N. A. (2015). Periodontal treatment improves prostate symptoms and lowers serum PSA in men with high PSA and chronic periodontitis. Dentistry 5:3. doi: 10.4172/2161-1122.1000284
Nadler, R., Collins, M., Propert, K., Mikolajczyk, S., Knauss, J., Landis, J., et al. (2006). Prostate-specific antigen test in diagnostic evaluation of chronic prostatitis/chronic pelvic pain syndrome. Urology 67, 337–342.
Nickel, J., Downey, J., Young, I., and Boag, S. (1999). Asymptomatic inflammation and/or infection in benign prostatic hyperplasia. BJU Int. 84, 976–981. doi: 10.1046/j.1464-410x.1999.00352.x
Nickel, J., Stephens, A., Landis, J., Chen, J., Mullins, C., van Bokhoven, A., et al. (2015). Search for microorganisms in men with urologic chronic pelvic pain syndrome: A culture-independent analysis in the MAPP research network. J. Urol. 194, 127–135. doi: 10.1016/j.juro.2015.01.037
Ochman, H., Worobey, M., Kuo, C., Ndjango, J., Peeters, M., Hahn, B., et al. (2010). Evolutionary relationships of wild hominids recapitulated by gut microbial communities. PLoS Biol. 8:e1000546. doi: 10.1371/journal.pbio.1000546
Odamaki, T., Kato, K., Sugahara, H., Hashikura, N., Takahashi, S., Xiao, J., et al. (2016). Age-related changes in gut microbiota composition from newborn to centenarian: A cross-sectional study. BMC Microbiol. 16:90. doi: 10.1186/s12866-016-0708-5
Organ, M., Grantmyre, J., and Hutchinson, J. (2010). Burkholderia cepacia infection of the prostate caused by inoculation of contaminated ultrasound gel during transrectal biopsy of the prostate. Can. Urol. Assoc. J. 4, E58–E60.
Porter, C., Shrestha, E., Peiffer, L., and Sfanos, K. (2018). The microbiome in prostate inflammation and prostate cancer. Prostate Cancer Prostatic Dis. 21, 345–354. doi: 10.1038/s41391-018-0041-1
Rees, J., Abrahams, M., Doble, A., and Cooper, A. (2015). Diagnosis and treatment of chronic bacterial prostatitis and chronic prostatitis/chronic pelvic pain syndrome: A consensus guideline. BJU Int. 116, 509–525. doi: 10.1111/bju.13101
Rooks, M., and Garrett, W. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Rudick, C., Berry, R., Johnson, J., Johnston, B., Klumpp, D., Schaeffer, A., et al. (2011). Uropathogenic Escherichia coli induces chronic pelvic pain. Infect. Immun. 79, 628–635. doi: 10.1128/iai.00910-10
Ruff, W., Greiling, T., and Kriegel, M. (2020). Host-microbiota interactions in immune-mediated diseases. Nat. Rev. Microbiol. 18, 521–538. doi: 10.1038/s41579-020-0367-2
Sanz, M., Herrera, D., Kebschull, M., Chapple, I., Jepsen, S., Beglundh, T., et al. (2020). Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 47:13290. doi: 10.1111/jcpe.13290
Schaeffer, A., Landis, J., Knauss, J., Propert, K., Alexander, R., Litwin, M., et al. (2002). Demographic and clinical characteristics of men with chronic prostatitis: The national institutes of health chronic prostatitis cohort study. J. Urol. 168, 593–598.
Scher, J., Sczesnak, A., Longman, R., Segata, N., Ubeda, C., Bielski, C., et al. (2013). Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2:e01202. doi: 10.7554/eLife.01202
Schluter, J., Peled, J., Taylor, B., Markey, K., Smith, M., Taur, Y., et al. (2020). The gut microbiota is associated with immune cell dynamics in humans. Nature 588, 303–307. doi: 10.1038/s41586-020-2971-8
Schwager, S., Agnoli, K., Köthe, M., Feldmann, F., Givskov, M., Carlier, A., et al. (2013). Identification of Burkholderia cenocepacia strain H111 virulence factors using nonmammalian infection hosts. Infect. Immun. 81, 143–153. doi: 10.1128/IAI.00768-12
Shoskes, D., Altemus, J., Polackwich, A., Tucky, B., Wang, H., and Eng, C. (2016a). The urinary microbiome differs significantly between patients with chronic prostatitis/chronic pelvic pain syndrome and controls as well as between patients with different clinical phenotypes. Urology 92, 26–32. doi: 10.1016/j.urology.2016.02.043
Shoskes, D., Wang, H., Polackwich, A., Tucky, B., Altemus, J., and Eng, C. (2016b). Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J. Urol. 196, 435–441. doi: 10.1016/j.juro.2016.02.2959
Shrestha, E., White, J., Yu, S., Kulac, I., Ertunc, O., De Marzo, A., et al. (2018). Profiling the urinary microbiome in men with positive versus negative biopsies for prostate cancer. J. Urol. 199, 161–171. doi: 10.1016/j.juro.2017.08.001
Singh, S., Young, K., and Silver, L. (2017). What is an “ideal” antibiotic? Discovery challenges and path forward. Biochem. Pharmacol. 133, 63–73. doi: 10.1016/j.bcp.2017.01.003
Sivaprakasam, S., Prasad, P., and Singh, N. (2016). Benefits of short-chain fatty acids and their receptors in inflammation and carcinogenesis. Pharmacol. Ther. 164, 144–151. doi: 10.1016/j.pharmthera.2016.04.007
Smith, P., Howitt, M., Panikov, N., Michaud, M., Gallini, C., Bohlooly, Y. M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Song, W., Gao, J., Huang, J., Liu, Y., Long, Z., and He, L. (2023). Is type III prostatitis also associated with bacterial infection? Front. Cell. Infect. Microbiol. 13:1189081. doi: 10.3389/fcimb.2023.1189081
Song, W., Huang, J., Liu, Y., Wang, J., Ding, W., Chen, B., et al. (2024). Effects of low-intensity pulsed ultrasound on the microorganisms of expressed prostatic secretion in patients with IIIB prostatitis. Sci. Rep. 14:15368. doi: 10.1038/s41598-024-66329-x
Stamatiou, K., Trinchieri, M., Trinchieri, M., Perletti, G., and Magri, V. (2023). Chronic prostatitis and related psychological problems. Which came first: The chicken or the egg? A systematic review. Arch. Ital. Urol. Androl. 95:11300. doi: 10.4081/aiua.2023.11300
Stower, H. (2019). Depression linked to the microbiome. Nat. Med. 25:358. doi: 10.1038/s41591-019-0396-4
Suresh, S., Mahendra, J., Sudhakar, U., Pradeep, A., and Singh, G. (2016). Evaluation of plasma reactive oxygen metabolites levels in obese subjects with periodontal disease. Indian J. Dent. Res. 27, 155–159. doi: 10.4103/0970-9290.183117
Suskind, A., Berry, S., Ewing, B., Elliott, M., Suttorp, M., and Clemens, J. (2013). The prevalence and overlap of interstitial cystitis/bladder pain syndrome and chronic prostatitis/chronic pelvic pain syndrome in men: Results of the RAND Interstitial cystitis epidemiology male study. J. Urol. 189, 141–145. doi: 10.1016/j.juro.2012.08.088
Šutulović, N., Veskoviæ, M., Puškaš, N., Zubelić, A., Jerotić, D., Šuvakov, S., et al. (2023). Chronic prostatitis/chronic pelvic pain syndrome induces depression-like behavior and learning-memory impairment: A possible link with decreased hippocampal neurogenesis and astrocyte activation. Oxid. Med. Cell. Longev. 2023:3199988. doi: 10.1155/2023/3199988
Terrisse, S., Goubet, A., Ueda, K., Thomas, A., Quiniou, V., Thelemaque, C., et al. (2022). Immune system and intestinal microbiota determine efficacy of androgen deprivation therapy against prostate cancer. J. Immunother. Cancer 10:e004191. doi: 10.1136/jitc-2021-004191
Tian, Y., Ren, X., Wang, J., Li, X., Yin, Y., Guo, Z., et al. (2024). Berberine hydrochloride alleviates chronic prostatitis/chronic pelvic pain syndrome by modifying gut microbiome signaling. Asian J. Androl. 26, 500–509. doi: 10.4103/aja202427
Trompette, A., Gollwitzer, E., Yadava, K., Sichelstiel, A., Sprenger, N., Ngom-Bru, C., et al. (2014). Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat. Med. 20, 159–166. doi: 10.1038/nm.3444
Uchugonova, A., Zhang, Y., Salz, R., Liu, F., Suetsugu, A., Zhang, L., et al. (2015). Imaging the different mechanisms of prostate cancer cell-killing by tumor-targeting Salmonella typhimurium A1-R. Anticancer Res. 35, 5225–5229.
Vaahtovuo, J., Munukka, E., Korkeamäki, M., Luukkainen, R., and Toivanen, P. (2008). Fecal microbiota in early rheumatoid arthritis. J. Rheumatol. 35, 1500–1505.
Wang, J., Zhao, X., and Wan, Y. (2023). Intricacies of TGF-β signaling in Treg and Th17 cell biology. Cell. Mol. Immunol. 20, 1002–1022. doi: 10.1038/s41423-023-01036-7
Wang, S., Zang, M., Yang, X., Lv, L., Chen, L., Cui, J., et al. (2023). Gut microbiome in men with chronic prostatitis/chronic pelvic pain syndrome: Profiling and its predictive significance. World J. Urol. 41, 3019–3026. doi: 10.1007/s00345-023-04587-6
Wolfe, A., Toh, E., Shibata, N., Rong, R., Kenton, K., Fitzgerald, M., et al. (2012). Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 50, 1376–1383. doi: 10.1128/JCM.05852-11
Wu, L., Li, B., Wang, Y., Wang, C., Zi, H., Weng, H., et al. (2019). Periodontal disease and risk of benign prostate hyperplasia: A cross-sectional study. Mil. Med. Res. 6:34. doi: 10.1186/s40779-019-0223-8
Wu, Y., Jiang, H., Tan, M., and Lu, X. (2020). Screening for chronic prostatitis pathogens using high-throughput next-generation sequencing. Prostate 80, 577–587. doi: 10.1002/pros.23971
Yaman, O., Göğüş, C., Tulunay, O., Tokatli, Z., and Ozden, E. (2003). Increased prostate-specific antigen in subclinical prostatitis: The role of aggressiveness and extension of inflammation. Urol. Int. 71, 160–164.
Young, V. (2017). The role of the microbiome in human health and disease: An introduction for clinicians. BMJ 356, j831. doi: 10.1136/bmj.j831
Yu, J., Hu, Q., Liu, J., Luo, J., Liu, L., and Peng, X. (2022). Metabolites of gut microbiota fermenting Poria cocos polysaccharide alleviates chronic nonbacterial prostatitis in rats. Int. J. Biol. Macromol. 209, 1593–1604. doi: 10.1016/j.ijbiomac.2022.04.029
Yu, Z., Du, H., Gao, S., and Liang, C. (2024). Eriocalyxin B ameliorated experimental autoimmune prostatitis via modulation of macrophage polarization through gut microbiota-mediated vitamin D3 alteration. Phytomed. Int. J. Phytother. Phytopharmacol. 135:156191. doi: 10.1016/j.phymed.2024.156191
Zhang, S., Zhao, X., Wang, X., Jin, H., Chen, L., Ma, Y., et al. (2023). Gut microecology may be involved in pathogenesis of Hashimoto’s thyroiditis by reducing production of hydrogen sulfide. J Clin. Endocrinol. Metab. 109, 792–801. doi: 10.1210/clinem/dgad588
Zhong, W., Wu, K., Long, Z., Zhou, X., Zhong, C., Wang, S., et al. (2022). Gut dysbiosis promotes prostate cancer progression and docetaxel resistance via activating NF-κB-IL6-STAT3 axis. Microbiome 10:94. doi: 10.1186/s40168-022-01289-w
Keywords: microbiota, CP/CPPS, pathogenesis, treatment microbiota, treatment
Citation: Deng A-Q, Yue S-Y, Niu D, Zhang D-D, Hou B-B, Zhang L, Liang C-Z and Du H-X (2025) The role of microbiota in the chronic prostatitis/chronic pelvis pain syndrome: a review. Front. Microbiol. 16:1488732. doi: 10.3389/fmicb.2025.1488732
Received: 30 August 2024; Accepted: 24 February 2025;
Published: 12 March 2025.
Edited by:
Ying Yang, Zhejiang University, ChinaReviewed by:
Megan L. Falsetta, University of Rochester, United StatesCopyright © 2025 Deng, Yue, Niu, Zhang, Hou, Zhang, Liang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He-Xi Du, ZHVoZXhpMTk4OUAxNjMuY29t; Chao-Zhao Liang, YXlmeW13QDE2My5jb20=; Li Zhang, bHpoYW5nQGFobXUuZWR1LmNu; Bing-Bing Hou, YmluZ2dvYXphQDE2My5jb20=
†ORCID: An-Qi Deng, orcid.org/0009-0001-0587-8547; He-Xi Du, orcid.org/0000-0003-3931-2469
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.