
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Microbiol., 07 February 2025
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 16 - 2025 | https://doi.org/10.3389/fmicb.2025.1466419
 Zhen Wu1†
Zhen Wu1† Zhan-Peng Xie1†
Zhan-Peng Xie1† Xin-Xin Cui1
Xin-Xin Cui1 Xiang-Bin Sun1
Xiang-Bin Sun1 Fang-Yi Zhao1
Fang-Yi Zhao1 Nuo Wang1
Nuo Wang1 Yu Li1,2,3
Yu Li1,2,3 Haixia Wang1,2,3
Haixia Wang1,2,3 Li Zhang1,2,3
Li Zhang1,2,3 Jing Shen4
Jing Shen4 Fulei Chen4
Fulei Chen4 Haogang Sun4
Haogang Sun4 Jia He1,2,3*
Jia He1,2,3*Background: The use of highly active antiretroviral therapy has transformed AIDS into a chronic infectious disease, but issues of chronic inflammation and immune system activation persist. Modulating the gut microbiome of patients may improve this situation, yet the specific association mechanisms between HIV and the gut microbiome remain unclear. This study aims to explore the research hotspots and trends of the HIV and the gut microbiome, providing direction for future research.
Methods: We conducted a search of the Web of Science Core Collection database up to April 30, 2024 to retrieve articles related to the relationship between the HIV and the gut microbiome. The scientific achievements and research frontiers in this field were analyzed using CiteSpace, VOSviewer, and Bibliometrix statistical software.
Results: As of April 30, 2024, a total of 379 articles met the inclusion criteria. The number of publications in this field peaked in 2023, and the number of articles published after 2020 declined. The country with the highest number of publications was the United States (184 articles), and the institution with the most publications was the University of Colorado (USA) (21 articles). The author with the most publications was Routy Jean-Pierre (Canada) (14 articles). High-frequency keywords, aside from the key terms, included “HIV,” “inflammation,” “immune activation,” “gut microbiota,” and “translocation.” Keyword burst results indicated that short-chain fatty acids, T cells and obesity might become the focus of future research.
Conclusion: The research hotspots in this field should prioritize examining the role of the primary gut microbiome metabolite, short-chain fatty acids, in reducing immune system activation and inflammation. Another emerging area of interest could be the investigation into the annual increase in obesity rates within this field. Furthermore, understanding the metabolic mechanisms of short-chain fatty acids in T cells is essential. Additionally, multi-omics analysis holds potential.
Acquired immune deficiency syndrome (AIDS) is caused by HIV infection. It is a chronic infectious disease, which can lead to immune deficiency (Chen and Lai, 2023; du et al., 2023). HIV primarily targets CD4+ T cells, compromising immune function and leading to the development of opportunistic infections and death (Sponaugle et al., 2023; Wang et al., 2023). HAART has increased CD4+ T cell counts in people living with HIV (PLWH), thereby extending their lifespans (Wen et al., 2023). However, approximately 15–30% of patients experience an inability to restore CD4+ T cell counts, termed immunological non-responders (Yang et al., 2020). CD4+ T cell destruction is not the sole factor in HIV progression; it is influenced by multifaceted factors, with sustained immune activation being the most significant, largely driven by gut microbiome dysbiosis (Rb-Silva et al., 2019; Nakanjako et al., 2016; Moretti et al., 2023). Alzahrani et al. (2019) demonstrated that supplementing probiotics to PLWH can reduce abnormal immune system activation and inflammatory responses, thereby improving HIV progression. Additionally, 16S sequencing reveals differences in gut microbiome between PLWH and healthy individuals (Zhang et al., 2023). However, the specific mechanism underlying gut microbiome dysbiosis in PLWH remains unclear. Therefore, investigating research hotspots and trends in HIV and the gut microbiome holds promise for addressing issues such as sustained immune activation and gut microbiome dysbiosis in the progression of HIV/AIDS in this population.
Bibliometrics is a quantitative method for visualizing scientific information that demonstrates research trends and hotspots in a specific field (Fu et al., 2023). To date, no reports on bibliometric studies of the gut microbiome and HIV exist. In this study, we utilized Citespace 6.1.R6, VOSviewer 1.6.19, and Bibliometrix to analyze literature on “HIV and the gut microbiome” published up to April 30, 2024. Our aim was to identify changes in hotspots and trends, providing directions for the future prevention and treatment of HIV.
The Web of Science Core Collection (WoSCC, Clarivate Analytics, Philadelphia, PA, USA) was chosen for literature retrieval in this study. The search included articles from the establishment of the database to April 30, 2024.
To capture as many articles as possible, this study used the MeSH terms from the PubMed database. The search strategy for this study was created following the retrieval rules of WoSCC, using the main subject terms “HIV/AIDS and gut microbiome” along with associated keywords (specific search strategy available in the Supplementary material).
This study included research papers and review articles as the types of literature. The search was limited to publications in the English language. Two independent researchers conducted the screening process, resulting in a total of 2,372 records retrieved. By examining the titles, abstracts, and keywords of the retrieved literature, the researchers determined whether they had clear relevance to both “gut microbiome” and “HIV.” Manual filtering was performed, and if the relevance could not be determined solely based on the title, abstract, and keywords, the full text was consulted. Duplicate records and articles that clearly did not meet the inclusion criteria were excluded. Each researcher identified 353 and 401 articles, respectively. The results of the included analysis were cross-checked, and any disagreements regarding inclusion were resolved by a third researcher. Ultimately, 1,994 articles were excluded, and a total of 379 articles met the inclusion criteria. The flowchart of the study process is presented in Supplementary Figure 1.
A total of 379 articles that met the inclusion criteria were selected for this study. These articles were exported and downloaded in Plain Text File, Tab Delimited File, and BibTeX formats. We used CiteSpace version 6.1.R6 (Drexel University, Pfizer, America) to extract and organize burst keywords, overlay dual maps of journals, and generate keyword timeline graphs based on their frequency of occurrence. Additionally, co-occurrence maps were generated. VOSviewer version 1.6.18 (Leiden University, Leiden, Netherlands) was utilized to identify the countries, institutions, journals, and keywords associated with the articles, and to create co-occurrence maps. The bibliometrix package in R Studio version 4.2.3 was employed for visualizing the analysis of countries and authors. Microsoft Office Excel 2019 (Microsoft, America) was used for data management and analysis of publication trends, including yearly articles and co-citations.
A total of 379 articles were included based on the search strategy and selection criteria, covering the period from January 1, 1995, to April 30, 2024. The articles’ output and their co-citation network are depicted in Figure 1. From 1995 to 2012, there was relatively low publication output, with a peak of 5 articles in 2011. The volume of publications surged from 2013 to 2019, indicating widespread interest in research on the relationship between gut microbiome and HIV. The year 2023 marked the peak of publication output, followed by a decline in articles after 2020. Articles related to gut microbiome and HIV were published in 149 different journals, as shown in Supplementary Figure 2. The top 10 journals are listed in Supplementary Table 1, with MICROBIOME having the highest impact factor and the AIDS having the highest publication output. Furthermore, we conducted a dual-map overlay of journals for HIV and gut microbiome, as shown in Supplementary Figure 3, to further explore the thematic distribution of journals and the transfer path of interdisciplinary knowledge. The cited journals mainly belong to clinical, molecular biology, and medical disciplines, while the citing journals are primarily from molecular, biological, and genetic fields.
As depicted in Figure 2, the top 10 authors in terms of publication volume include Routy Jean-Pierre, who has published 14 papers, ranking first (seven articles, seven reviews). Routy Jean-Pierre’s research agenda is predominantly centered on intervention methodologies encompassing probiotics, cannabinoids, Metformin, and terbinafine, within the framework of HIV and the gut microbiome (Costiniuk et al., 2019; Isnard et al., 2020; el-Far et al., 2021; Ouyang et al., 2023). Notably, all publications by these authors have surfaced within the last decade.
A total of 51 countries are engaged in research on HIV and gut microbiome, visualized in Figure 3. The United States has the highest number of publications, with 184 papers, followed by China with 59. Thirteen countries have published more than 10 papers. Collaboration among countries is primarily driven by high-output nations, with significant interactions among them. The country outbreak graph is shown in Supplementary Figure 4, revealing that the primary publishing countries in the future will be Ghana, Belgium, Switzerland and Nigerla with two of these being developing nations.
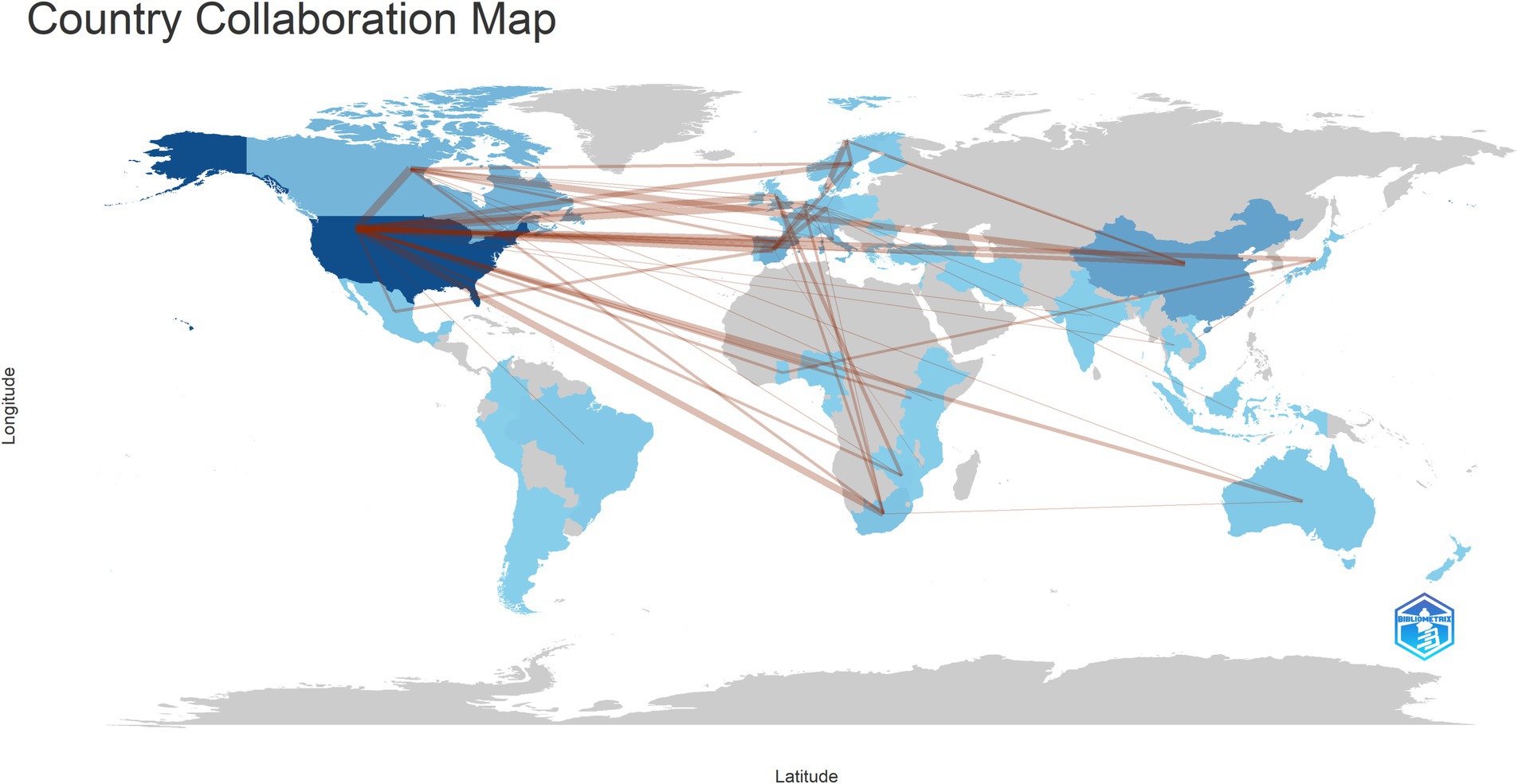
Figure 3. The visualization of national output collaboration. With darker colors representing higher output and thicker lines indicating closer collaboration.
A total of 817 institutions have published papers, as depicted in the visualization in Figure 4. The University of Colorado is the leading institution in terms of publication volume, with 21 papers. The top 10 institutions are predominantly from developed countries, with eight based in the United States, indicating the leading position of the United States in this field. The institution outbreak graph is shown in Supplementary Figure 5, with Capital Medical University being the sole institution from a developing country.

Figure 4. The institution produces visual analyses. Where the size of the nodes represents the volume of publications, and the thickness of the connections represents the level of collaboration.
The top 10 cited articles are presented in Supplementary Table 2. Among them, nine were authored by institutions based in the United States. Eight of these articles concentrate on 16S sequencing and genome sequencing and investigate differences in gut bacterial and viral populations among PLWH, non-HIV-infected individuals, and PLWH under HAART treatment (Vujkovic-Cvijin et al., 2013; Dillon et al., 2014; Dinh et al., 2015; Lozupone et al., 2013; Mutlu et al., 2014; Monaco et al., 2016; Noguera-Julian et al., 2016; Handley et al., 2012). These investigations did not incorporate proteomics or metabolomics methodologies, and the sample sizes were relatively modest, with only one study encompassing over 100 participants and a total sample size of 240 (Noguera-Julian et al., 2016). Please refer to Supplementary Figure 6 for a comprehensive overview of the seminal reference. Vujkovic-Cvijin and Somsouk (2019) deliver a thorough review delineating the mechanisms underlying alterations in the gut microbiome during HIV disease progression, while also proposing intervention strategies for enhancing gut microbiome health. Furthermore, they investigate the influence of lifestyle and behavioral factors on the microbiome. Additionally, they explored through cohort studies that the dysbiosis of the microbiome in PLWH is unrelated to gender and sexual behavior, but rather correlates with nadir CD4 levels, inflammatory markers, and comorbidities (Vujkovic-Cvijin et al., 2020). Armstrong et al. (2018) discussed the factors contributing to intestinal flora imbalance in individuals with AIDS.
A total of 1,712 keywords were included and incorporated into the keyword network, as shown in Figure 5. Among these, inflammation, immune activation and translocation were the most frequently occurring and closely interconnected keywords, Clustering analysis primarily focused on three domains: first (Red clustering), The mechanism of microbial dysbiosis in PLWH; second (Green clustering), interventions in this field (Blue clustering); Clinical manifestations and classification in this field. The 20 most significant keywords in this domain are presented in Supplementary Figure 7. The earliest appearance is associated with microbiome, spanning from 2007 to 2013, with an intensity rating of 2.49. Future research should continue to pay attention to short-chain fatty acids, T cells, and obesity.
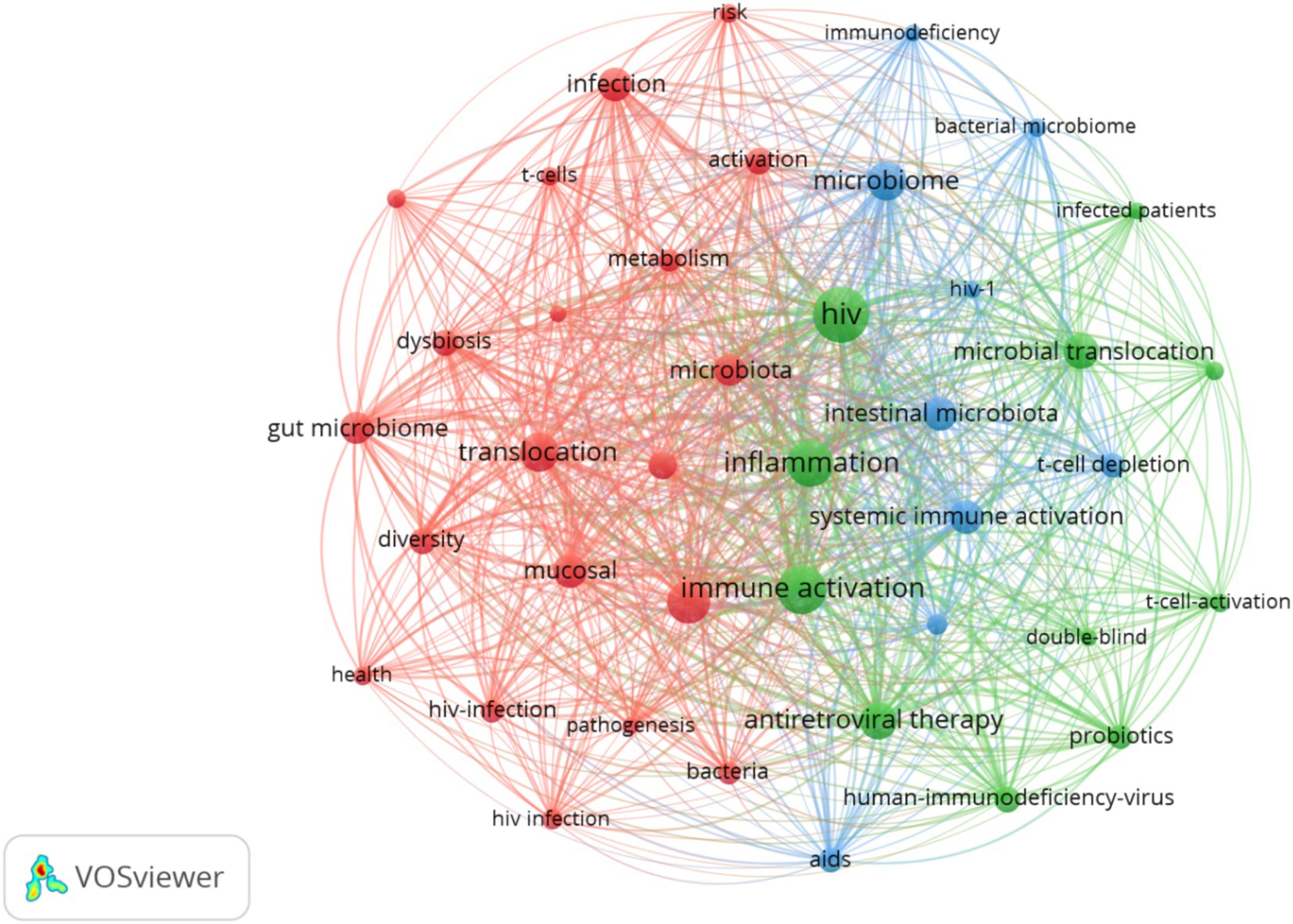
Figure 5. Keyword visual analysis. Node size represents keyword frequency, thickness represents the strength of connection.
The fluctuation in the volume of academic publications serves as a crucial indicator for the development trend of a field. Prior to 2019, the publication volume exhibited a yearly increasing trend, while after 2019, the publication volume began to decline. This timing coincides with the outbreak of COVID-19, leading to speculation that the global research focus shifted toward the prevention and treatment of COVID-19, consequently resulting in fewer studies on HIV and other infectious diseases. The decrease in PLWH1 may also be related to the Joint United Nations Programme on HIV/AIDS target of zero new PLWH by 2030 (Xue et al., 2023), which may have affected the publication volume on HIV and gut microbiome. However, with the end of the pandemic and the ongoing publication of longitudinal research papers, the number of articles in this field is expected to rise. As a result, there is anticipation for additional clarification on the mechanisms that underlie the relationship between gut microbiome and HIV. At the same time, we found that the number of articles published in this field began to increase in 2013, and we also found that nine of the top 10 cited articles were published after 2012. The research of Handley et al. (2012) found that enterovirus is the main reason for HIV progress through genome analysis. Later, various studies studied the difference and role of flora in PLWH and healthy people through 16 s sequencing and genome sequencing. In addition, The United Nations Programme on HIV/AIDS said in 2014 that 90% of people living with HIV should reduce the viral load to zero, while 10–40% of PLWH have poor immune cell recovery after receiving highly effective antiretroviral reduction disease, which becomes poor immune reconstitution, and intestinal flora plays an important role in this mechanism (Wan et al., 2023). Therefore, we believe that the above two reasons are the reasons for the increase in publication volume in 2013. Analysis of the visualized journal citation network revealed that the AIDS and Frontiers in Immunology were the top journals for publication volume, while Microbiome has the highest impact factor, thus providing high-quality frontier information for HIV and gut microbiome research.
The visualization of countries, institutions, and authors indicates that the publication volume and influence of developed countries far surpass those of developing countries for HIV and gut microbiome research. However, compared to developed countries, there are inconsistencies in terms of race, dietary habits, lifestyle customs, and the misuse of antibiotics in developing countries (Batool et al., 2023). Parbie et al. (2021) utilized high-throughput macroeconomic tools for the first time to characterize the fecal microbiome of HIV-1-infected Ghanaian adults. A Dutch cohort was selected as a representative non-African cohort for comparison. The HIV- and HIV+ groups in their study exhibited significantly lower alpha diversity and significantly different beta diversity compared to their Dutch counterparts (Parbie et al., 2021). As a result, research findings from developed countries may not be applicable to developing countries. In terms of the number of PLWH, developing countries greatly exceed developed countries, with sub-Saharan Africa (SSA) accounting for two-thirds of the total (Sadler et al., 2023). Burkhart Colorado et al. discovered that, in comparison to urban areas in Zimbabwe, successful antiretroviral therapy (ART) in rural PLWH exhibited inferior outcomes in terms of reducing intestinal microbial composition and T-cell activation (Burkhart Colorado et al., 2024). This article, published in Microbiome, suggests that there is considerable potential for research in HIV and gut flora related research in SSA.
Institutions and countries that rank high in publication volume demonstrated closer interconnections, suggesting that cooperation leads to more fruitful results. Hence, developing countries should actively collaborate with developed countries to jointly explore the specific mechanisms of gut microbiome and HIV for the cure of HIV or immunological reconstruction for HIV. The top five institutions in terms of publication volume are all from the United States, with very few research institutions from developing countries, particularly from SSA. However, observations from the visualized outbreak maps of countries and institutions indicate that future publications from developing countries and institutions will gradually increase. In Figure 2, Routy Jean-Pierre emerges as the most prolific contributor, as evidenced by the highest publication volume. His scholarly endeavors center around the investigation of influential determinants and intervention strategies within the designated field. Initially, his research delved into examining the correlation between PLWH and alterations in the gut microbiome, positing a potential linkage to immune activation and T cell dynamics (Lu et al., 2018). Subsequently, adopting a gut microbiome-centric approach, he explored various intervention modalities, encompassing probiotics, metformin interventions, and terbinafine interventions (Costiniuk et al., 2019; Isnard et al., 2020; el-Far et al., 2021; Ouyang et al., 2023; Lu et al., 2018).
Keywords can indicate the evolution and research hotspots in this field. Based on Figures 5 and Supplementary Figure 7. Future research endeavors are anticipated to predominantly revolve around three focal areas: fundamental inquiries, intervention methodologies, and the exploration of adverse repercussions. Insights gleaned from keyword burst analyses suggest a burgeoning interest in topics such as short-chain fatty acids, T cells, and obesity, signaling a progressive trajectory. These areas hold promising potential as viable treatment modalities for HIV, underscoring their significance in advancing therapeutic strategies.
Current research indicates that the disruption of the gut microbiome caused by HIV had been confirmed (Li et al., 2024). The dysregulation of the gut microbiome leading to immune system activation and chronic inflammation is an important factor in the development of HIV (Munjoma et al., 2023). However there have been few reports on the metabolic products of the gut microbiome and the metabolic pathways in the progression and pathogenesis of HIV. Existing studies on the metabolic products of the gut microbiome indicate that probiotics can produce short-chain fatty acids (SCFAs) (e.g., butyrate) which can induce regulatory T cells to reduce immune activation and alleviate gut inflammatory responses thus slowing down the progression of HIV (Enriquez et al., 2023). Furthermore they can also promote the production of CD4+ T cells interleukin-10 and interleukin-22 maintaining gut immunity and promoting immune reconstitution in the body (Kibbie et al., 2021). The dysregulation of the gut microbiome elicited by HIV encompasses a diminution in beneficial bacteria alongside an augmentation of pathogenic counterparts. Concurrently it fosters intestinal barrier impairment exacerbating immune system activation and precipitating dysregulated immune responses. This immune system activation precipitates a perturbation in immune homeostasis. Moreover the dysregulation engenders an imbalance in the differentiation between Treg and Th17 cells precipitating an elevation in pro-inflammatory cytokines coupled with a reduction in anti-inflammatory counterparts thereby instigating chronic inflammation.
Notably, short-chain fatty acids play a pivotal role in ameliorating intestinal barrier integrity and bolstering anti-inflammatory cytokine levels (Sereti et al., 2023). The gut microbiome and its metabolites hold potential sway over the development and differentiation of bone marrow cells and B cells, potentially influencing aberrant antibody differentiation (Geng et al., 2020). Jiang et al. (2023) corroborate the notion that short-chain fatty acids can enhance the specific immune response elicited by HIV vaccines, aligning with their immunomodulatory role and capacity to foster antibody responses. However, there is limited research on medium-chain and long-chain fatty acids in relation to HIV. In addition to their anti-inflammatory properties, long chain fatty acid esters significantly inhibit the proliferation and cytokine secretion of CD4 + and CD8 + T cells. Treatment of T cells with mitochondrial targeted antioxidant mito tembo can restore T cell function damage caused by eicosahexaenoic acid. Therefore, inhibiting the action of long-chain fatty acids can restore T cell function damage (Li et al., 2023). Furthermore, the majority of research on SCFAs had been cross-sectional, indicating the need for large-scale prospective studies to elucidate the specific mechanisms and causal relationships of the metabolic products of the gut microbiome in the development of HIV. While the mechanism of action of SCFAs in HIV is not yet clear, it is currently possible to increase the content of SCFAs by supplementing beneficial bacteria to improve the gut microbiome (Ma et al., 2023).
As depicted in Figures 5. Probiotics and prebiotics are important intervention measures for increasing SCFAs and restoring the stability of the gut microbiome. The results of the random effects meta-analysis showed that probiotic supplementation resulted in a moderate increase in CD4 lymphocyte counts in ART-untreated individuals. In contrast, no significant changes were observed in ART-experienced individuals (Oyadiran et al., 2024; Sachdeva et al., 2022). However, if probiotics are used as an adjunctive treatment for HIV, they could help to improve PLWH’s life Quality. Studies have shown that the use of probiotics may be therapeutically helpful in preventing diarrhea and re-establishing normal gut microbiota in PLWH (Irvine et al., 2010; Carter et al., 2016). Subgroup analyses conducted for intervention time showed that the incidence of AIDS-related diarrhea decreased when probiotics were given for more than 30 days. When probiotics were given for <30 days, there was no change in the incidence of AIDS-related diarrhea. Where the type and geographical location of the probiotic did not affect the results (Zhang et al., 2021). This hints to researchers to design experiments on the effectiveness of probiotic-assisted HIV treatment with adequate intervention times. Of course, other confounding factors such as probiotic dose and treatment modality (monobacteria or mixed bacteria), mode of administration, and diet need to be taken into account. In conclusion, we emphasize that probiotics can only be used as adjunctive therapy in the treatment of HIV and that conducting large-scale cohort studies over a long period is strong evidence to validate the efficacy of probiotic-assisted treatment of HIV. We look forward to the exploration and additions of future researchers in this direction.
T cells had become a hot topic in this field, as the HIV infects cells expressing the CD4 molecule, such as CD4+ T cells, macrophages, and dendritic cells. The gut contains a large number of CD4+ T cells and macrophages, serving as a reservoir for the HIV. Therefore, the elimination of infected cells in the gut is crucial for the treatment of HIV. Shytaj, IL and others had found that down- regulation of glycolysis is a metabolic feature of the latent period of HIV-infected cells. It is still not clear what role glycolysis plays in the latency of the virus (Shytaj et al., 2021). Studies had shown an increase in glycolysis in immune cells during infection, and Zhang, JH and others had found that blocking glycolysis can inhibit the M1 polarization of macrophages, which is associated with chronic inflammation (Zhang et al., 2023). Furthermore, bacterial lipopolysaccharides can promote the activation of immune cells and the production of pro-inflammatory factors, while SCFAs in the gut microbiome can be metabolized by immune cells, providing anti-inflammatory effects by supplying energy to immune cells and inhibiting glycolysis. In the future, immunometabolism may become a treatment for HIV. Researchers should focus on the metabolic effects of gut microbiome metabolites in immune cells.
The incidence of obesity among PLWH is progressively rising, a trend attributed to several factors. Firstly, the utilization of HAART has extended the lifespan of PLWH, consequently aligning their obesity rates with those of the general populace (Cook et al., 2020). Secondly, research by Baltazar-Díaz et al. suggests that different HAART medications exert varying effects on the gut microbiota, thereby influencing weight gain differentially (Baltazar-Díaz et al., 2023). Furthermore, obese PLWH individuals are prone to immune cell activation and heightened production of inflammatory cytokines like IL-6. The confluence of obesity and HIV fosters dysbiosis of the intestinal flora, exacerbating both AIDS and obesity—a phenomenon perpetuating a deleterious cycle necessitating prompt intervention. However, findings from Gogokhia et al. contend that irrespective of the HAART regimen employed, the obesity incidence consistently rises. Consequently (Gogokhia et al., 2020), the etiology likely encompasses multifaceted factors, including lifestyle choices. Future investigations should delve into elucidating the specific determinants to inform targeted interventions effectively.
Upon analysis of the top 10 cited articles, it is noted that the sample size in these studies is generally small, with limited capacity to control confounding factors. Currently, a large amount of literature pertains to cross-sectional studies on the gut microbiome and HIV. Future efforts should address the specific causal relationships and mechanisms through longitudinal studies, as well as expanding the sample size to obtain more reliable results. In terms of research methodology, 16S sequencing and genomic studies are predominant, while metabolomics, proteomics, and transcriptomics are less prevalent. Genomic studies often focus on the classification differences of the microbiome, lacking functional information, which can be complemented by metabolomics and proteomics. Metabolomics can evaluate the role of gut microbiome metabolites, opening new avenues for the prevention and treatment of HIV. Proteomics can use SCFAs as a starting point to investigate their effects on PLWH, exploring the pathways and mechanisms of the gut microbiome in HIV.
The efficacy of the HIV eradication strategy hinges upon a comprehensive comprehension of HIV-1 infected cells, which exhibit significant heterogeneity. The advent of single-cell multi-group methodologies offers a solution to address the challenges posed by this heterogeneity (Wong et al., 2023). For instance, Wu et al. used a single-cell assay for transposase accessible chromatin (scATACseq) and employed a human-virus comparison strategy developed in a chimeric antigen receptor (CAR) T-cell model, incorporating the presence of integrated provirus as a molecular beacon to identify PLWH-infected cells from treated and untreated PLWH directly (Wu et al., 2023). They provided the first direct and unbiased identification of experimentally and in vivo infected memory CD4 T cells at single-cell resolution, thereby accelerating our understanding of HIV reservoirs to target therapies. Multi-omic studies of the microbiome can provide insight into the mechanisms underlying host–microbe interactions (Chetty and Blekhman, 2024). Bailin et al. used multiomics factor analysis (MOFA) results to suggest that SAT dysregulation and dyslipidemia were major contributors to phenotypic variation in metrics associated with cardiometabolic disease in a contemporary ART virus-suppressed PLWH cohort (Bailin et al., 2024). A relatively small number of multi-omics studies have been conducted in the area of HIV and gut flora. This may be related to the high cost of sample collection and data generation.
In this study, we employed CiteSpace, VOSviewer, and Bibliometrix to evaluate 379 articles focusing on the research of HIV and the gut microbiome up to April 30, 2024. Our analysis reveals a burgeoning field, with contributions emanating from 51 countries, 817 institutions, and involving 1,712 authors. Moving forward, we recommend longitudinal research for sustained observational insights, emphasizing the exploration of causal mechanisms linking the gut microbiome and HIV, particularly in relation to T cells and chain fatty acids. It is recommended that researchers explore the potential of probiotics as an adjunctive treatment for HIV. Additionally, it is imperative to investigate the underlying factors driving the increasing incidence of obesity and implement targeted intervention measures. Furthermore, we advocate for supplementing our understanding of gut microbiome metabolites through multi-omics analyses, with the aim of unveiling novel avenues for the prevention and treatment of HIV.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
ZW: Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing. Z-PX: Project administration, Supervision, Writing – review & editing. X-XC: Project administration, Supervision, Writing – review & editing. X-BS: Methodology, Supervision, Writing – review & editing. F-YZ: Supervision, Writing – review & editing. NW: Writing – review & editing. YL: Methodology, Project administration, Supervision, Writing – review & editing. HW: Investigation, Methodology, Project administration, Software, Supervision, Writing – review & editing. LZ: Investigation, Software, Supervision, Writing – review & editing. JS: Formal analysis, Methodology, Supervision, Writing – review & editing. FC: Methodology, Software, Supervision, Writing – review & editing. HS: Methodology, Supervision, Writing – review & editing. JH: Funding acquisition, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (no. 81560551), Shihezi University High Level Talent Research Launch Project (no. RCZK202367), and the Shihezi University Innovation Outstanding Young Talents Program (Natural Science) (no. CXPY202004).
The authors express their gratitude to Jia He for reviewing the bibliometric methodology utilized in this publication. We also extend our thanks to the collaborators from the Department of Public Health and the Key Laboratory of Xinjiang Endemic and Ethnic Diseases of the Ministry of Education, as well as the Key Laboratory for Prevention and Control of Emerging Infectious Diseases and Public Health Security, and the First Affiliated Hospital for their assistance in revising the complete manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2025.1466419/full#supplementary-material
Alzahrani, J., Hussain, T., Simar, D., Palchaudhuri, R., Abdel-Mohsen, M., Crowe, S. M., et al. (2019). Inflammatory and immunometabolic consequences of gut dysfunction in HIV: parallels with IBD and implications for reservoir persistence and non-AIDS comorbidities. EBioMedicine 46, 522–531. doi: 10.1016/j.ebiom.2019.07.027
Armstrong, A., Shaffer, M., Nusbacher, N. M., Griesmer, C., Fiorillo, S., Schneider, J. M., et al. (2018). An exploration of Prevotella-rich microbiomes in HIV and men who have sex with men. Microbiome 6:198. doi: 10.1186/s40168-018-0580-7
Bailin, S. S., Ma, S., Perry, A. S., Terry, J. G., Carr, J. J., Nair, S., et al. (2024). The primacy of adipose tissue gene expression and plasma Lipidome in Cardiometabolic disease in persons with HIV. J. Infect. Dis. 9:jiae532. doi: 10.1093/infdis/jiae532
Baltazar-Díaz, T. A., Amador-Lara, F., Andrade-Villanueva, J. F., González-Hernández, L. A., Cabrera-Silva, R. I., Sánchez-Reyes, K., et al. (2023). Gut bacterial communities in HIV-infected individuals with metabolic syndrome: effects of the therapy with integrase Strand transfer inhibitor-based and protease inhibitor-based regimens. Microorganisms 11:40951. doi: 10.3390/microorganisms11040951
Batool, M., Keating, C., Javed, S., Nasir, A., Muddassar, M., and Ijaz, U. Z. (2023). A cross-sectional study of potential antimicrobial resistance and ecology in gastrointestinal and Oral microbial communities of young Normoweight Pakistani individuals. Microorganisms 11:279. doi: 10.3390/microorganisms11020279
Burkhart Colorado, A. S., Lazzaro, A., Neff, C. P., Nusbacher, N., Boyd, K., Fiorillo, S., et al. (2024). Differential effects of antiretroviral treatment on immunity and gut microbiome composition in people living with HIV in rural versus urban Zimbabwe. Microbiome. 12:18. doi: 10.1186/s40168-023-01718-4
Carter, G. M., Esmaeili, A., Shah, H., Indyk, D., Johnson, M., Andreae, M., et al. (2016). Probiotics in human immunodeficiency virus infection: A systematic review and evidence synthesis of benefits and risks. Open forum. Infect. Dis. 3:ofw164. doi: 10.1093/ofid/ofw164
Chen, X., and Lai, Y. (2023). Knowledge domain and emerging trends in HIV pre-exposure prophylaxis: a visualization analysis via CiteSpace. Front. Microbiol. 14:1099132. doi: 10.3389/fmicb.2023.1099132
Chetty, A., and Blekhman, R. (2024). Multi-omic approaches for host-microbiome data integration. Gut Microbes 16:860. doi: 10.1080/19490976.2023.2297860
Cook, R. R., Fulcher, J. A., Tobin, N. H., Li, F., Lee, D., Woodward, C., et al. (2020). Combined effects of HIV and obesity on the gastrointestinal microbiome of young men who have sex with men. HIV Med. 21, 365–377. doi: 10.1111/hiv.12838
Costiniuk, C. T., Saneei, Z., Routy, J. P., Margolese, S., Mandarino, E., Singer, J., et al. (2019). Oral cannabinoids in people living with HIV on effective antiretroviral therapy: CTN PT028-study protocol for a pilot randomised trial to assess safety, tolerability and effect on immune activation. BMJ Open 9:e24793. doi: 10.1136/bmjopen-2018-024793
Dillon, S. M., Lee, E. J., Kotter, C. V., Austin, G. L., Dong, Z., Hecht, D. K., et al. (2014). An altered intestinal mucosal microbiome in HIV-1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 7, 983–994. doi: 10.1038/mi.2013.116
Dinh, D. M., Volpe, G. E., Duffalo, C., Bhalchandra, S., Tai, A. K., Kane, A. V., et al. (2015). Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 211, 19–27. doi: 10.1093/infdis/jiu409
du, X., Zhang, Q., Hao, J., Gong, X., Liu, J., and Chen, J. (2023). Global trends in depression among patients living with HIV: A bibliometric analysis. Front. Psychol. 14:1125300. doi: 10.3389/fpsyg.2023.1125300
el-Far, M., Durand, M., Turcotte, I., Larouche-Anctil, E., Sylla, M., Zaidan, S., et al. (2021). Upregulated IL-32 expression and reduced gut short chain fatty acid Caproic acid in people living with HIV with subclinical atherosclerosis. Front. Immunol. 12:664371. doi: 10.3389/fimmu.2021.664371
Enriquez, A. B., Ten, C. F., Ghneim, K., Sekaly, R-P., and Sharma, A. (2023). Regulation of immune homeostasis, inflammation, and HIV persistence by the microbiome, short-chain fatty acids, and bile acids. Annu. Rev. Virol 10, 397–422. doi: 10.1146/annurev-virology-040323-082822
Fu, X., Tan, H., Huang, L., Chen, W., Ren, X., and Chen, D. (2023). Gut microbiota and eye diseases: a bibliometric study and visualization analysis. Front. Cell. Infect. Microbiol. 13:1225859. doi: 10.3389/fcimb.2023.1225859
Geng, S. T., Zhang, Z. Y., Wang, Y. X., Lu, D., Yu, J., Zhang, J. B., et al. (2020). Regulation of gut microbiota on immune reconstitution in patients with Acquired immunodeficiency syndrome. Front. Microbiol. 11:594820. doi: 10.3389/fmicb.2020.594820
Gogokhia, L., Taur, Y., Juluru, K., Yagan, N., Zhu, Y. S., Pamer, E., et al. (2020). Intestinal Dysbiosis and markers of systemic inflammation in viscerally and generally obese persons living with HIV. JAIDS 83, 81–89. doi: 10.1097/QAI.0000000000002229
Handley, S. A., Thackray, L. B., Zhao, G., Presti, R., Miller, A. D., Droit, L., et al. (2012). Pathogenic simian immunodeficiency virus infection is associated with expansion of the enteric virome. Cell 151, 253–266. doi: 10.1016/j.cell.2012.09.024
Irvine, S. L., Hummelen, R., Hekmat, S., Looman, C. W., Habbema, J. D., and Reid, G. (2010). Probiotic yogurt consumption is associated with an increase of CD4 count among people living with HIV/AIDS. J. Clin. Gastroenterol. 44, e201–e205. doi: 10.1097/MCG.0b013e3181d8fba8
Isnard, S., Lin, J., Fombuena, B., Ouyang, J., Varin, T. V., Richard, C., et al. (2020). Repurposing metformin in nondiabetic people with HIV: influence on weight and gut microbiota. Open Forum Infect. Dis. 7:a338. doi: 10.1093/ofid/ofaa338
Jiang, D., Goswami, R., Dennis, M., Heimsath, H., Kozlowski, P. A., Ardeshir, A., et al. (2023). Sutterella and its metabolic pathways positively correlate with vaccine-elicited antibody responses in infant rhesus macaques. Front. Immunol. 14:1283343. doi: 10.3389/fimmu.2023.1283343
Kibbie, J. J., Dillon, S. M., Thompson, T. A., Purba, C. M., McCarter, M. D., and Wilson, C. C. (2021). Butyrate directly decreases human gut lamina propria CD4 T cell function through histone deacetylase (HDAC) inhibition and GPR43 signaling. Immunobiology 226:152126. doi: 10.1016/j.imbio.2021.152126
Li, S. Y., Yin, L. B., Ding, H. B., Liu, M., Lv, J. N., Li, J. Q., et al. (2023). Altered lipid metabolites accelerate early dysfunction of T cells in HIV-infected rapid progressors by impairing mitochondrial function. Front. Immunol. 14:1106881. doi: 10.3389/fimmu.2023.1106881
Li, K., Zhang, C., Deng, J., Zeng, H., Zhang, Y., Lai, G., et al. (2024). Causal effects of gut microbiome on HIV infection: a two-sample mendelian randomization analysis. BMC Infect. Dis. 24:280. doi: 10.1186/s12879-024-09176-5
Lozupone, C. A., Li, M., Campbell, T. B., Flores, S. C., Linderman, D., Gebert, M. J., et al. (2013). Alterations in the gut microbiota associated with HIV-1 infection. Cell Host Microbe 14, 329–339. doi: 10.1016/j.chom.2013.08.006
Lu, W., Feng, Y., Jing, F., Han, Y., Lyu, N., Liu, F., et al. (2018). Association between gut microbiota and CD4 recovery in HIV-1 infected patients. Front. Microbiol. 9:1451. doi: 10.3389/fmicb.2018.01451
Ma, J., Wen, S., Dong, A., Fan, W., and Kang, Y. (2023). Gut microbiome (Bacteria, Fungi, and viruses) and HIV infection: revealing novel treatment strategies. Mol. Nutr. Food Res. 67:e2300566. doi: 10.1002/mnfr.202300566
Monaco, C. L., Gootenberg, D. B., Zhao, G., Handley, S. A., Ghebremichael, M. S., Lim, E. S., et al. (2016). Altered Virome and bacterial microbiome in human immunodeficiency virus-associated Acquired immunodeficiency syndrome. Cell Host Microbe 19, 311–322. doi: 10.1016/j.chom.2016.02.011
Moretti, S., Schietroma, I., Sberna, G., Maggiorella, M. T., Sernicola, L., Farcomeni, S., et al. (2023). HIV-1-host interaction in gut-associated lymphoid tissue (GALT): effects on local environment and comorbidities. Int. J. Mol. Sci. 24:193. doi: 10.3390/ijms241512193
Munjoma, P. T., Chandiwana, P., Wyss, J., Mazhandu, A. J., Jordi, S. B. U., Gutsire, R., et al. (2023). Immune activation and inflammation in lactating women on combination antiretroviral therapy: role of gut dysfunction and gut microbiota imbalance. Front. Immunol. 14:1280262. doi: 10.3389/fimmu.2023.1280262
Mutlu, E. A., Keshavarzian, A., Losurdo, J., Swanson, G., Siewe, B., Forsyth, C., et al. (2014). A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 10:e1003829. doi: 10.1371/journal.ppat.1003829
Nakanjako, D., Kiragga, A. N., Musick, B. S., Yiannoutsos, C. T., Wools-Kaloustian, K., Diero, L., et al. (2016). Frequency and impact of suboptimal immune recovery on first-line antiretroviral therapy within the international epidemiologic databases to evaluate AIDS in East Africa. AIDS 30, 1913–1922. doi: 10.1097/QAD.0000000000001085
Noguera-Julian, M., Rocafort, M., Guillén, Y., Rivera, J., Casadellà, M., Nowak, P., et al. (2016). Gut microbiota linked to sexual preference and HIV infection. EBioMedicine 5, 135–146. doi: 10.1016/j.ebiom.2016.01.032
Ouyang, J., Yan, J., Zhou, X., Isnard, S., Tang, S., Costiniuk, C. T., et al. (2023). The influence of Oral Terbinafine on gut fungal microbiome composition and microbial translocation in people living with HIV treated for onychomycosis. J. Fungi 9:963. doi: 10.3390/jof9100963
Oyadiran, O. T., Ogunlade, S. B., Okusanya, T. R., Okoka, E. M., Kuyebi, M. A., Omotayo, M. O., et al. (2024). Effect of intake of probiotics and probiotic fermented foods on clinical outcomes among people living with HIV: A systematic review and meta-analysis. Trop. Med. Int. Health 29, 113–127. doi: 10.1111/tmi.13955
Parbie, P. K., Mizutani, T., Ishizaka, A., Kawana-Tachikawa, A., Runtuwene, L. R., Seki, S., et al. (2021). Dysbiotic fecal microbiome in HIV-1 infected individuals in Ghana. Front. Cell. Infect. Microbiol. 11:646467. doi: 10.3389/fcimb.2021.646467
Rb-Silva, R., Goios, A., Kelly, C., Teixeira, P., João, C., Horta, A., et al. (2019). Definition of immunological nonresponse to antiretroviral therapy: a systematic review. JAIDS 82, 452–461. doi: 10.1097/QAI.0000000000002157
Sachdeva, M., Sra, H. K., Agarwal, A., Chauhan, A., Pradhan, P., Singh, M., et al. (2022). Effect of probiotics on the frequency of CD4+T-cells in HIV-infected children and adolescents: A systematic review and Meta-analysis of randomized controlled trials. J. Trop. Pediatr. 68:fmac006. doi: 10.1093/tropej/fmac006
Sadler, M., Kuhoga, E., Thumma-Reddy, N., Chuma, E., Said, K., Kaminyoge, M. S., et al. (2023). The role of cognitive reserve in mediating HIV-associated neurocognitive disorders in older adults living with-treated HIV in Mbeya, Tanzania: A cross-sectional observational study. Int. J. Geriatr. Psychiatry 38:e6042. doi: 10.1002/gps.6042
Sereti, I., Verburgh, M. L., Gifford, J., Lo, A., Boyd, A., Verheij, E., et al. (2023). Impaired gut microbiota-mediated short-chain fatty acid production precedes morbidity and mortality in people with HIV. Cell Rep. 42:113336. doi: 10.1016/j.celrep.2023.113336
Shytaj, I. L., Procopio, F. A., Tarek, M., Carlon-Andres, I., Tang, H. Y., Goldman, A. R., et al. (2021). Glycolysis downregulation is a hallmark of HIV-1 latency and sensitizes infected cells to oxidative stress. EMBO Mol. Med. 13:e13901. doi: 10.15252/emmm.202013901
Sponaugle, A., Weideman, A., Ranek, J., Atassi, G., Kuruc, J., Adimora, A. A., et al. (2023). Dominant CD4(+) T cell receptors remain stable throughout antiretroviral therapy-mediated immune restoration in people with HIV. Cell Rep. Med. 4:101268. doi: 10.1016/j.xcrm.2023.101268
Vujkovic-Cvijin, I., Dunham, R. M., Iwai, S., Maher, M. C., Albright, R. G., Broadhurst, M. J., et al. (2013). Dysbiosis of the gut microbiota is associated with HIV disease progression and tryptophan catabolism. Sci. Transl. Med. 5, 191r–193r. doi: 10.1126/scitranslmed.3006438
Vujkovic-Cvijin, I., and Somsouk, M. (2019). HIV and the gut microbiota: composition, consequences, and avenues for amelioration. Curr. HIV/AIDS Rep. 16, 204–213. doi: 10.1007/s11904-019-00441-w
Vujkovic-Cvijin, I., Sortino, O., Verheij, E., Sklar, J., Wit, F. W., Kootstra, N. A., et al. (2020). HIV-associated gut dysbiosis is independent of sexual practice and correlates with noncommunicable diseases. Nat. Commun. 11:2448. doi: 10.1038/s41467-020-16222-8
Wan, L. Y., Huang, H. H., Zhen, C., Chen, S. Y., Song, B., Cao, W. J., et al. (2023). Distinct inflammation-related proteins associated with T cell immune recovery during chronic HIV-1 infection. Emerg. Microbes Inf. 12:2150566. doi: 10.1080/22221751.2022.2150566
Wang, D., Zhou, M., Wang, P., Zhang, J., Mi, Y., Cheng, F., et al. (2023). Treatment-naive people living with HIV aged 50 years or older in Beijing, China, 2010-2020: joinpoint regression model analysis of surveillance data. J. Int. AIDS Soc. 26:e26193. doi: 10.1002/jia2.26193
Wen, L., Shi, L., Wan, S. S., Xu, T., Zhang, L., and Zhou, Z. G. (2023). Changes in the balance of Th17/Treg cells and oxidative stress markers in patients with HIV-associated pulmonary tuberculosis who develop IRIS. Exp. Ther. Med. 25:271. doi: 10.3892/etm.2023.11970
Wong, M., Wei, Y. L., and Ho, Y. C. (2023). Single-cell multiomic understanding of HIV-1 reservoir at epigenetic, transcriptional, and protein levels. Curr. Opin. HIV AIDS 18, 246–256. doi: 10.1097/COH.0000000000000809
Wu, V. H., Nordin, J. M. L., Nguyen, S., Joy, J., Mampe, F., Del Rio Estrada, P. M., et al. (2023). Profound phenotypic and epigenetic heterogeneity of the HIV-1-infected CD4+ T cell reservoir. Nat. Immunol. 24, 359–370. doi: 10.1038/s41590-022-01371-3
Xue, L., Sun, Y., Ren, X., and Sun, W. (2023). Modelling the transmission dynamics and optimal control strategies for HIV infection in China. J. Biol. Dyn. 17:2174275. doi: 10.1080/17513758.2023.2174275
Yang, X., Su, B., Zhang, X., Liu, Y., Wu, H., and Zhang, T. (2020). Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J. Leukoc. Biol. 107, 597–612. doi: 10.1002/JLB.4MR1019-189R
Zhang, X. L., Chen, M. H., Geng, S. T., Yu, J., Kuang, Y. Q., Luo, H. Y., et al. (2021). Effects of probiotics on diarrhea and CD4 cell count in people living with HIV: A systematic review and Meta-analysis. Front. Pharmacol. 12:570520. doi: 10.3389/fphar.2021.570520
Zhang, Y., Xie, Z., Zhou, J., Li, Y., Ning, C., Su, Q., et al. (2023). The altered metabolites contributed by dysbiosis of gut microbiota are associated with microbial translocation and immune activation during HIV infection. Front. Immunol. 13:1020822. doi: 10.3389/fimmu.2022.1020822
Keywords: HIV/AIDS, gut microbiome, bibliometrics, short-chain fatty acids, T cells, obesity
Citation: Wu Z, Xie Z-P, Cui X-X, Sun X-B, Zhao F-Y, Wang N, Li Y, Wang H, Zhang L, Shen J, Chen F, Sun H and He J (2025) HIV and the gut microbiome: future research hotspots and trends. Front. Microbiol. 16:1466419. doi: 10.3389/fmicb.2025.1466419
Received: 18 July 2024; Accepted: 27 January 2025;
Published: 07 February 2025.
Edited by:
Aniefiok John Udoakang, Centre for Molecular Biosciences and Medical Genomics, NigeriaReviewed by:
Gratiela Gradisteanu Pircalabioru, University of Bucharest, RomaniaCopyright © 2025 Wu, Xie, Cui, Sun, Zhao, Wang, Li, Wang, Zhang, Shen, Chen, Sun and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jia He, aGVqaWExMjMuc2hpaGV6aUAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.