- 1Department of Medical Parasitology, Faculty of Medicine, Al-Azhar University (Assiut Branch), Assiut, Egypt
- 2Department of Medical Parasitology, Faculty of Medicine, Assiut University, Assiut, Egypt
- 3Department of Parasitology, School of Veterinary Medicine, Badr University in Assiut, Assiut, Egypt
- 4Department of Biology, Faculty of Science, Taibah University, Alula, Saudi Arabia
- 5Department of Medical Laboratory, College of Applied Medical Sciences, Prince Sattam Bin Abdulaziz University, AlKharj, Saudi Arabia
- 6Department of Parasitology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt
- 7Department of Medical Parasitology, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
- 8Department of Biology, College of Science, Princess Nourah Bint Abdulrahman University, Riyadh, Saudi Arabia
- 9Departamento de Sanidad Animal, Grupo de Investigación en Sanidad Animal y Zoonosis (GISAZ), UIC Zoonosis y Enfermedades Emergentes ENZOEM, Universidad de Córdoba, Córdoba, Spain
- 10Department of Zoonoses, Faculty of Veterinary Medicine, Sohag University, Sohag, Egypt
Introduction: Trichomoniasis remains one of the most significant sexually transmitted disease (STDs) for public health. The disease is caused by parasitic protozoa, Trichomonas vaginalis (T. vaginalis), which is often underestimated in tropical medicine. Despite its public health importance, the epidemiology and molecular characteristics of trichomoniasis in Egypt remains poorly understood, particularly in the southern part of the country (Upper Egypt). This study targeted exploring the genetic variability of T. vaginalis infections in Egyptian women living in Upper Egypt using restriction fragment length polymorphism (RFLP).
Patient and techniques: This cross-sectional study included 150 female patients, who visited the gynaecology and obstetrics outpatient clinics at Sohag General Hospital between 2019 and 2022, exhibiting symptoms of trichomoniasis. Vaginal washout samples were collected from each patient and analyzed using three diagnostic techniques: direct wet mount microscopy, culture on TYM Diamond’s medium, and PCR amplification and Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) targeting the actin gene, which was applied to all 16 samples that tested positive in culture. The PCR-RFLP results were then visualized through agarose gels electrophoresis to detect DNA fragments.
Results: Out of 150 vaginal washout samples, 12 cases (8%) tested positive for T. vaginalis trophozoites via direct wet mount microscopy, while 16 samples (10.6%) were positive in culture. Additionally, PCR-RFLP analysis of the 16 culture-positive samples revealed that 13 samples were confirmed positive using this molecular method. The amplified products were digested with the restriction enzyme Hind II, yielding three DNA fragments of 60, 213, and 827 bp, which were then detected by agarose gel electrophoresis. Digestion with RsaI produced five fragments measuring 87, 103/106, 236, and 568 bp, while MseI digestion resulted in three distinct fragments of 204, 315, and 581 bp.
Conclusion: This study provides robust baseline data on the prevalence and microscopic characteristics of T. vaginalis in Upper Egypt, while also presenting, for the first time, molecular detection and genotyping and revealed that genotype E is the only prevalent genotype in the region.
1 Introduction
Trichomoniasis is a widespread sexually transmitted infection caused by the parasite Trichomonas vaginalis, which impacts both men and women. The infection by this parasite typically causes symptoms like vaginal discharge and itching in women, though many individuals may experience no symptoms. The World Health Organization (WHO) estimates that approximately 143 million new cases of trichomoniasis occur annually (WHO, 2016), with prevalence rates varying worldwide due to factors such as geographic location, age, race, community, and diagnostic methods. In 2016, WHO estimated the global prevalence of trichomoniasis at 5.3% in women and 0.6% in men, resulting in a total of 156 million cases (Rowley et al., 2019). Regarding its clinical impact, trichomoniasis is linked to serious reproductive complications, including infertility, ectopic pregnancy, pelvic inflammatory disease, premature rupture of membranes, preterm birth, and low birth weight (Hamouda et al., 2022). It also plays a critical role in the transmission and acquisition of human immunodeficiency virus (HIV) (Alves et al., 2020) and human papillomavirus (Heikal et al., 2023). Taken into consideration, the variation in clinical outcomes, such as differences in virulence, pathogenicity, and drug resistance, highlights the need to connect these phenotypic differences to specific genotypes (Hawksworth et al., 2015). The genome of T. vaginalis comprises over 60,000 protein-coding genes within a large 160 Mb genome, predominantly consisting of transposable elements and repetitive sequences (Carlton et al., 2007). The primary cytoskeleton of T. vaginalis is composed of actin proteins, which are essential for cellular motility and adhesion (Crucitti et al., 2008). Due to its extensive conservation across the species, actin is a promising target for molecular identification within T. vaginalis (Drouin et al., 1995). It should be also stressed that PCR and related techniques are widely used for genetic research on various organisms. The random amplified polymorphic DNA (RAPD) method has been instrumental in linking metronidazole resistance to specific genotypes of T. vaginalis (Meade and Carlton, 2013).
Recent advancements in the genetic analysis of T. vaginalis isolates revealed a strong link between the organism’s genetic diversity and the wide range of clinical outcomes in trichomoniasis, as well as its disease-related complications (Leitsch, 2016). Several methods of genotyping of the parasite revealed extensive genetic variation among T. vaginalis strains (Meade and Carlton, 2013). In this regard, molecular studies consistently identified two predominant genotypes of T. vaginalis. Genotype I is believed to be evolutionarily older than genotype II, as it displays greater genetic variation. Interestingly, both genotypes are found in similar proportions globally, although genotype I is more prevalent in South Africa, whereas genotype II is more common in Mexico. Clinically, genotype I tends to be associated with more pathogenic infections, while genotype II has been linked to resistance to metronidazole (van der Veer et al., 2016). Additionally, previous research (Meade et al., 2009) employed PCR-RFLP techniques to reveal the genetic diversity among clinical isolates of T. vaginalis. Currently, the PCR-RFLP technique, based on the amplification of the actin gene, is considered a sensitive and reliable method for typing T. vaginalis isolates (Tavakoli Oliaee et al., 2017). Studies employing this technique have been conducted in Iran (Orujzadeh et al., 2019), China (Zhang et al., 2018), and Turkey (Demirağ et al., 2017) and showed that genotype E is the most prevalent among twenty T. vaginalis isolates from symptomatic females, followed by genotype G. Only one isolate was identified as genotype H, and two isolates were mixed, containing both genotypes. Similarly, Zhang et al. (2018) reported three mixed genotypes and two isolates with E and H genotypes out of 68 isolates. Recent research in Iran identified three genotypes—E, G, and I—in T. vaginalis isolates infected with dsRNA viruses (Orujzadeh et al., 2019). Understanding the genetic diversity of T. vaginalis populations is crucial for the effective prevention and management of trichomoniasis.
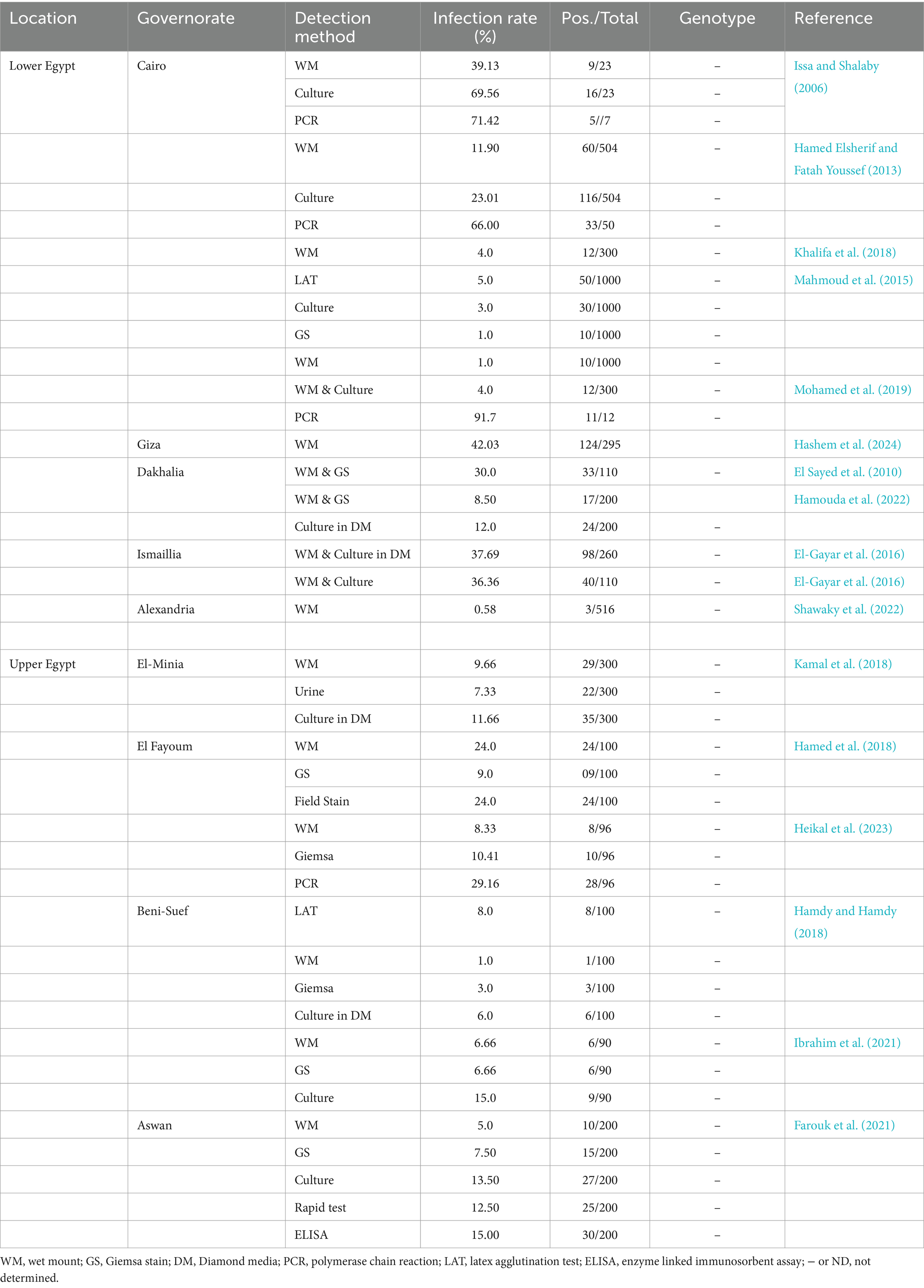
In Egypt, various studies performed in several Egyptian governorates, including Beni Suef, Qalyubia and Cairo governorates, and reported a prevalence rate which is ranged from 3 to 11% (Hamdy and Hamdy, 2018; Hussein et al., 2015; Mahmoud et al., 2015). Earlier efforts to investigate the diversity of T. vaginalis in Egypt included isomeric pattern analysis (Salem et al., 1992), serotyping (Salem et al., 1992), immunoblotting (El-Okbi et al., 2005), biological variability studies (Laila et al., 2012), HSP70-RFLP (Hussein et al., 2015), and multilocus sequence typing (Mohamed et al., 2019). These studies concluded that clinical isolates of T. vaginalis might exhibit both unique and shared patterns in terms of antigen levels, immunogens, pathogenicity, and metronidazole (MTZ) resistance. However, upon reviewing the available literature, there is a notable lack of data on the molecular characteristics of T. vaginalis among women in Upper Egypt. In addition to summarizing previous studies conducted on T. vaginalis at the national level (Table 1; references: Hamouda et al., 2022; Hamdy and Hamdy, 2018; Mahmoud et al., 2015; Mohamed et al., 2019; Issa and Shalaby, 2006; Hamed Elsherif and Fatah Youssef, 2013; Khalifa et al., 2018; El Sayed et al., 2010; El-Gayar et al., 2016; Shawaky et al., 2022; Kamal et al., 2018; Hamed et al., 2018; Heikal et al., 2023; Ibrahim et al., 2021; Farouk et al., 2021; Hashem et al., 2024), this study aims to investigate the genetic diversity of T. vaginalis in women from Upper Egypt, employing PCR-RFLP methods alongside morphological and microscopic techniques.
2 Patient and methods
2.1 Ethical considerations
The study design was reviewed and approved by the Research Ethics Committee of the Faculty of Medicine at Assiut University, adhering to the Declaration of Helsinki and regulations set forth by the Egyptian Ministry of Higher Education. Informed consent was obtained from each participant after a comprehensive explanation of the study’s objectives. Appropriate treatment was prescribed by a gynaecologist for participants who tested positive for trichomoniasis (Ethical No. 1720024/27-8-2018).
2.2 Study population
This observational study involved 150 women suspected of trichomoniasis, attending the obstetrics and gynaecology outpatient clinics at Sohag General Hospital between 2019 and 2022.
2.3 Inclusion criteria
Women were included if they were not menstruating and presented with gynecological symptoms suggestive of trichomoniasis, such as vaginal discharge, dyspareunia, dysuria, or vulvar pruritus.
2.4 Exclusion criteria
Women were excluded if they had engaged in sexual activity or douching within the past 2 days or had used antiprotozoal or antibiotic treatments in the previous 2 weeks. Menstruating women and virgins were also excluded. Each participant completed a detailed medical history and provided demographic information. After a thorough explanation of the study, all participants signed informed consent forms.
2.5 Sample collection
Vaginal washout samples were collected from participants according to the method described by McMillan et al. (1979). A sterile Cusco speculum was inserted into the vagina and adjusted to a 90-degree angle to enhance visibility of the cervix. Subsequently, 5 mL of sterile isotonic PBS solution were injected intravaginally using a needle-free sterile syringe. The fluid was then aspirated from the posterior fornix with a plastic pipette and transferred into sterile tubes. The samples were analyzed using direct wet mount microscopy and cultured on modified Diamond’s medium for the detection of T. vaginalis. Additionally, a portion of each sample was aliquoted into 1.5 mL microcentrifuge tubes and stored at −20°C for further PCR analysis.
2.6 Examination of vaginal washout
Following sample collection, a direct wet mount microscopic examination was performed using a light microscope with ×10, ×40, and ×100 objectives. This procedure aimed to promptly identify motile T. vaginalis trophozoites by observing their characteristic flagellate movement (Radonjic et al., 2006). The modified TYM Diamond culture medium was prepared in the parasitology laboratory of the Faculty of Medicine, Assiut University, following the protocol described by Diamond (1957). The medium consisted of the following ingredients: 24 g of Tryptone (Oxoid, United Kingdom), 12 g of Yeast Extract Powder (Oxoid, United Kingdom), 6 g of Maltose (Arabic Laboratory Equipment Co., Egypt), 1.2 g of L-Cysteine Hydrochloride (WINLAB, Egypt), 0.24 g of L-Ascorbic Acid (Arabic Laboratory Equipment Co., Egypt), and 900 mL of distilled water. The pH was adjusted to 6.3 using the pH meter and by adding 1 N HCL. The medium, contained in a Pyrex tube, was autoclaved at 121°C for 25 min and then allowed to cool. After cooling to 50°C, 100 mL of sterile inactivated horse serum (VACSERA, Dokki, Egypt) and an antibiotic mixture—prepared by adding 2 mL of sterile distilled water to each vial of penicillin and streptomycin and mixing thoroughly—were introduced into the Pyrex bottle. The final solution was labeled as modified Diamond medium. Then, a drop of the vaginal washout was inoculated into a culture tube containing Diamond TYM medium, which was then sealed and incubated at 37°C. Daily microscopic examinations were performed to check for the presence of T. vaginalis trophozoites. This process continued for up to 7 days. Specimens that did not show any trophozoites under the microscope by the end of this period were considered negative for T. vaginalis using the culture method (Garcia and Alderete, 2007; García, 2016).
2.7 Molecular detection of Trichomonas vaginalis
Extraction of DNA was performed from 100 μL of vaginal washout samples tested positive by culture methods using the MagMAX™ CORE Nucleic Acid Purification Kit (Cat. No. A32700, Thermo Fisher Scientific, United States). Nested PCR was performed using outer primers Tv8S and Tv9R and inner primers Tv10S and Tv11R (see Table 2), targeting the T. vaginalis actin gene (Crucitti et al., 2008; Crucitti et al., 2003). The outer primers targeted amplification of a 1,260 bp fragment, while the inner primers amplified a 1,100 bp fragment. The PCR reaction mixture (25 μL) included 12.5 μL of 2x MyTaq™ Red Mix Master Mix (Cat. No. BIO-25043, Meridian Bioscience, United Kingdom), 0.75 μL (10 μM) of each primer, 5 μL of the DNA template, and 6 μL of deionized water. PCR amplification was performed in two stages using a SimpliAmp™ Thermal Cycler (Cat. No. A24811, Applied Biosystems, United States). The first stage consisted of an initial denaturation step at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 1 min, followed by a final extension at 72°C for 10 min. In the second stage, the process began with an initial denaturation at 95°C for 3 min, followed by 40 cycles of denaturation at 95°C for 30 s, annealing at 50°C for 30 s, and extension at 72°C for 1 min, finishing with a final extension at 72°C for 10 min. After amplification, 7 μL of the PCR product was analyzed via electrophoresis on a 1.5% agarose gel in Tris-acetate-EDTA (TAE) buffer (pH 8.5) and visualized using the InGenius3 gel documentation system (Syngene Bio Imaging, United Kingdom) with 0.5 μg/mL ethidium bromide (Cat. No. E1510, Sigma-Aldrich, Darmstadt, Germany).
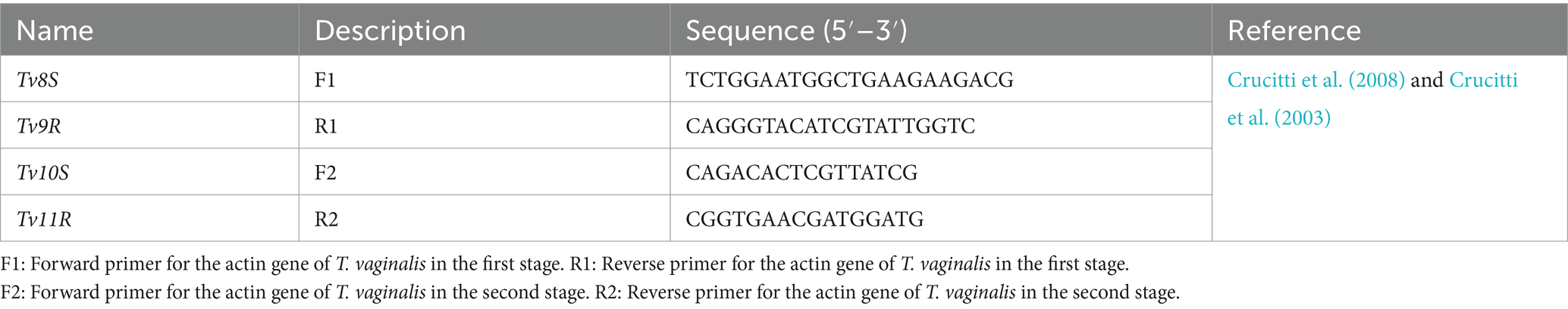
Table 2. Oligonucleotides were used in Nested PCR for the molecular identification of the T. vaginalis in this study.
2.8 Genotyping by PCR-RFLP
The β-actin gene was amplified using standard PCR protocols as previously described (Crucitti et al., 2008). Following amplification, the PCR products, which had a target length of 1,100 bp, were digested with three restriction enzymes. Each digestion reaction was prepared according to the manufacturer’s instructions for the specific enzyme. Ten microliters of the amplified product were treated with 0.5 μL of each restriction enzyme: HindII (10 U/μl, 500 units, Cat. No. RE1274), MseI (10 U/μl, 300 units, Cat. No. RE1350), and RsaI (10 U/μl, 1,000 units, Cat. No. RE1324) (Vivantis, Malaysia). Reactions were incubated for 4 h at 37°C for HindII and RsaI, and at 65°C for MseI. The resulting RFLPs were analyzed by electrophoresis on 2.5–3% (w/v) agarose gels. The gels were then visualized and documented using the InGenius3 gel documentation system (Syngene Bio Imaging, United Kingdom). Fragment sizes were determined using the VC 100 bp Plus DNA Ladder (Cat. No. NL1407, Vivantis, Malaysia). The actin genotypes, fragment lengths, and pattern groupings of the T. vaginalis isolates are detailed in Table 3.
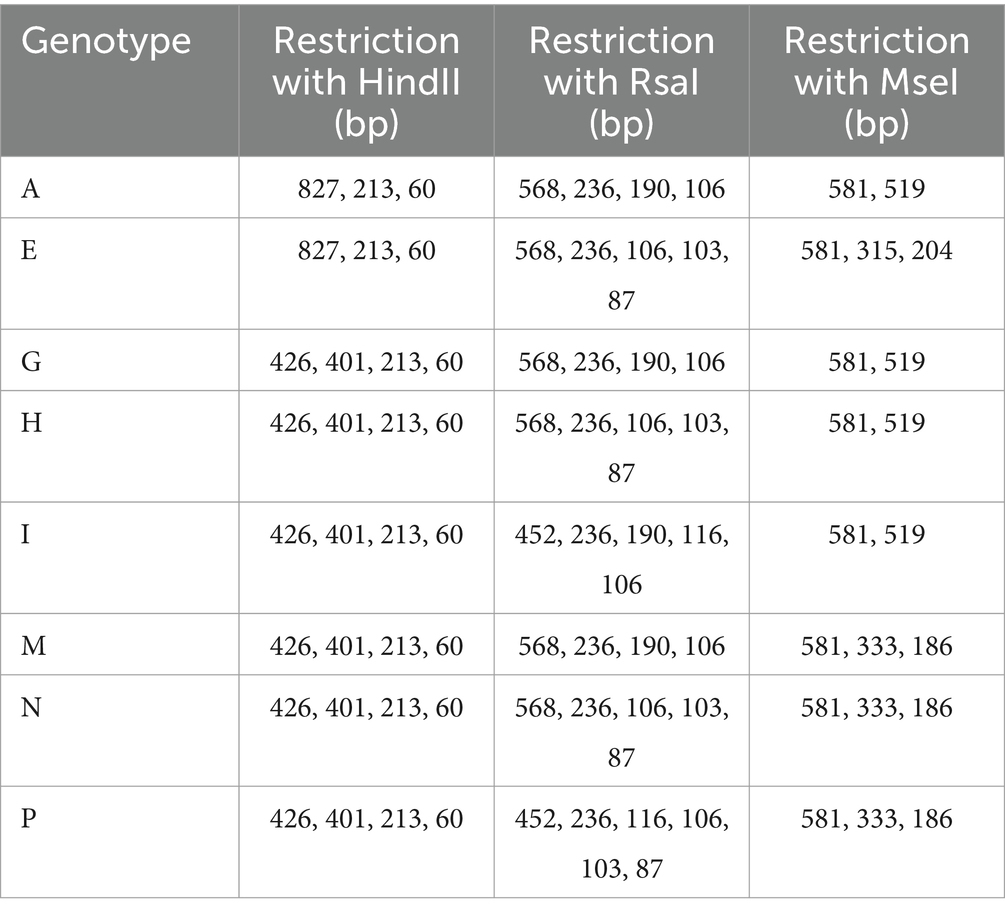
Table 3. Size of fragments, pattern groups and actin genotypes of the T. vaginalis (Crucitti et al., 2008).
2.9 Statistical analysis
Data were systematically organized and analyzed using SPSS version 16 (SPSS Inc., Chicago, IL). Results were presented as counts and percentages. To assess statistical significance, the chi-square test (X2), also known as Fisher’s exact test, was employed. The student’s t-test was used to calculate confidence intervals for the detection rate. A result was considered statistically significant if the two-sided p value was less than 0.05.
3 Results
3.1 Wet-mount microscopic examination and culture
Overall, 8.0% [12/150; 95% Confidence Interval (CI): 3.66–12.34] of female patients with suggestive clinical symptoms tested positive for T. vaginalis through microscopy (Supplementary Table S1). On the other hand, T. vaginalis trophozoites were isolated from 10.67% [16/150; 95% Confidence Interval (CI): 5.73–15.61] of washout samples from female patients with suggestive clinical symptoms, using cultivation on TYM Diamond’s medium (Supplementary Table S1). The morphology of T. vaginalis trophozoites was observed using wet mount microscopic analysis using an oil immersion lens (100x) and following culture on TYM Diamond’s media. The main indicator of a trophozoite’s presence is its distinctive motility, particularly observed through wet mount analysis. Under the microscope, these trophozoites display a nucleus and can take on various shapes, including pear, spherical, or oval. They possess five flagella, with four located in the anterior region and the fifth integrated into the undulating membrane. Regarding motility, trophozoites demonstrate more vigorous movement in TYM Diamond’s medium than in vaginal wet mount samples, where their movement can be influenced by the dynamics of the surrounding fluids and the presence of various components in the sample.
3.2 Molecular detection of Trichomonas vaginalis
PCR-RFLP analysis of the 16 culture-positive samples revealed that 13 samples were confirmed positive using this molecular method (Table 3). The amplified DNA products were digested by the HindII restriction enzyme, resulting in three distinct fragments: 827 bp, 213 bp, and 60 bp. Further digestion with RsaI yielded five fragments, approximately 87 bp, 103–106 bp, 236 bp, and 568 bp in size. MseI digestion of the amplified product produced three fragments measuring 204 bp, 315 bp, and 581 bp. Analysis of the DNA fragment patterns across all isolates consistently revealed the presence of actin genotype E. Notably, none of the isolates exhibited the actin genotypes A, G, I, M, N, or P. Nested PCR amplification of the T. vaginalis actin gene revealed a 1,100 bp fragment in all isolates. Agarose gel electrophoresis confirmed that the length of the amplicons was consistent across all samples, showing no variations.
4 Discussion
Trichomoniasis remains one of the most widespread protozoans sexually transmitted infections globally, affecting individuals across all age groups, ethnicities, and socioeconomic backgrounds (Schumann and Plasner, 2024). Rapid and sensitive diagnostic methods are crucial for timely treatment, which helps prevent the transmission of the infection (Shipitsyna et al., 2013) and protects against adverse gynecological and obstetric outcomes (Muzny et al., 2012). In this study, T. vaginalis infection was identified in 12 cases (8%) using wet mount preparation of vaginal washouts. This detection rate is lower than the 12.7% positive cases observed through wet microscopic analysis of vaginal swabs from the same cohort. This variation in detection rates is consistent with previous study at Aswan University Hospital outpatient clinic (Hassan et al., 2019) reported a 5% prevalence (10/200) using wet mount techniques, while Mahmoud et al. (2015) found a lower prevalence of 1% (10/1000) at Kasr Al-Ainy Cairo University Hospitals. In our study, T. vaginalis was cultured using TYM Diamond’s medium in 150 cases and identified higher detection rate of 10.6% (16 cases) for T. vaginalis. Our findings are consistent with those of a previous work (Al-Saeed, 2011) reported a 5.4% detection rate through culture compared to 2.4% with the wet mount method. Similar detection rates were also found in studies by Matini et al. (2012) and Farouk et al. (2021), which reported rates of 1.7 and 2.1%, respectively. Notably, after cultivation, trophozoites of the parasite exhibited significant morphological and motility changes compared to those initially observed through wet mount preparation. These changes included the trophozoites becoming rounded, losing their flagella, and displaying a much slower, barely noticeable motility.
In our study, of the 16 samples that tested positive in culture, 13 yielded results when analyzed using PCR-RFLP. The amplified DNA products from these samples were successfully digested by the restriction enzyme HindII producing three fragments of 60, 213, and 827 bp. Similarly, digestion with RsaI produced five fragments measuring 87, 103, 106, 236, and 568 bp, while digestion with MseI resulted in three fragments of 204, 315, and 581 bp. These DNA fragment patterns were consistent with the presence of the actin genotype E in all the isolates tested. Notably, genotypes A, G, I, M, and P were not detected in any of the samples. The present findings are consistent with several previous molecular studies that reported genotypes G and E as highly prevalent in the African continent. Notably, research on female populations in Kenya and the Democratic Republic of Congo revealed that genotype E was the most common strain of T. vaginalis, identified through actin gene analysis, while genotype G was most prevalent in Zambia (Crucitti et al., 2008; Masha et al., 2017). The present finding contrasts with a study (Chetty et al., 2020) reported that PCR-RFLP analysis of the actin gene identified three distinct T. vaginalis genotypes, with genotype G being the most prevalent. In research conducted outside of Africa, Momeni et al. (2015) identified five distinct T. vaginalis genotypes, with genotype G being the most prevalent among both men and women in Iran. However, more recent studies (Khalili et al., 2017; Tavakoli Oliaee et al., 2017; Matini et al., 2012) revealed different genotype distributions compared to those reported by Momeni et al. (2015) and the patterns observed in Africa (Crucitti et al., 2008; Masha et al., 2017). In this context a previous work (Matini et al., 2012) examined symptomatic and asymptomatic women visiting gynaecology clinics in western Iran and found genotype A to be the most dominant based on actin gene digestion profiles. Similarly, several previous works conducted in Iran reported that genotype H was the most frequent among women attending general healthcare services (Khalili et al., 2017; Tavakoli Oliaee et al., 2017). This diversity indicates potential variability in the genetic makeup of T. vaginalis in certain populations. The genetic differences observed in these studies might be explained by factors such as larger sample sizes or sampling from regions with higher infection rates (Abou-kamar et al., 2017).
5 Conclusion
Our study showed that the culture method for detecting T. vaginalis seems to be more sensitive than direct microscopic examination. Phosphate-buffered vaginal washout samples were utilized for direct wet mount microscopic examination, with the goal of rapidly detecting motile T. vaginalis trophozoites by identifying their distinctive flagellar movement. Additionally, our genetic analysis confirmed the molecular identification of T. vaginalis in Upper Egypt, indicating that only genotype E was present in this study. Collectively, these findings suggest that while culture techniques and specimen collection methods are crucial for accurate diagnosis, understanding genetic variations of the parasite is essential for regional epidemiological assessments and targeted interventions. Future research is recommended to investigate the prevalence and molecular characteristics of T. vaginalis on a larger scale within the Egyptian population. Additionally, there is a need to raise public health awareness about the importance of adopting strict hygiene measures to control the spread of this sexually transmitted infection.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Research Ethics Committee of the Faculty of Medicine at Assiut University granted approval for this study [Approval number 1720024/27-8-2018], adhering to the Declaration of Helsinki and regulations set forth by the Egyptian Ministry of Higher Education. Participants provided written informed consent to participate in the study. Informed consent was obtained from each participant after a comprehensive explanation of the study’s objectives. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
NE-k: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. AD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. NA: Funding acquisition, Resources, Software, Writing – review & editing, Data curation, Validation. AS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. HaA: Data curation, Formal analysis, Funding acquisition, Software, Validation, Visualization, Writing – review & editing, Resources. AG: Data curation, Formal analysis, Software, Validation, Writing – review & editing, Funding acquisition, Resources, Visualization. EH: Data curation, Formal analysis, Software, Validation, Writing – review & editing, Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – original draft. HiA: Data curation, Formal analysis, Funding acquisition, Resources, Software, Validation, Writing – review & editing. EE: Data curation, Formal analysis, Funding acquisition, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. EE was supported by a postdoctoral contract María Zambrano (University of Córdoba) from the Program of Requalification of the Spanish University System (Spanish Ministry of Universities) financed by the European Union-NextGenerationEU.
Acknowledgments
This study was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project No. (PNURSP2024R401), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1499270/full#supplementary-material
References
Abou-kamar, W., Abdel-Mageid, A., El-Nahas, H., Atia, R., and El-Tantawy, N. (2017). Genetic relatedness of trichomonas vaginalis isolates to the clinical variability. J. Mol. Microbiol. 11, 1–4.
Al-Saeed, W. M. (2011). Detection of trichomonas vaginalis by different methods in women from Dohok province, Iraq. East Mediterr. Health J. 17, 706–709. doi: 10.26719/2011.17.9.706
Alves, M. S. D., Das Neves, R. N., Sena-Lopes, Â., Domingues, M., Casaril, A. M., Segatto, N. V., et al. (2020). Antiparasitic activity of furanyl N-acylhydrazone derivatives against trichomonas vaginalis: in vitro and in silico analyses. Parasit. Vectors 13:59. doi: 10.1186/s13071-020-3923-8
Carlton, J. M., Hirt, R. P., Silva, J. C., Delcher, A. L., Schatz, M., Zhao, Q., et al. (2007). Draft genome sequence of the sexually transmitted pathogen trichomonas vaginalis. Science 315, 207–212. doi: 10.1126/science.1132894
Chetty, R., Mabaso, N., and Abbai, N. (2020). Genotypic variation in trichomonas vaginalis detected in south African pregnant women. Infect. Dis. Obstet. Gynecol. 2020:1687427. doi: 10.1155/2020/1687427
Crucitti, T., Abdellati, S., Van Dyck, E., and Buvé, A. (2008). Molecular typing of the actin gene of trichomonas vaginalis isolates by PCR-restriction fragment length polymorphism. Clin. Microbiol. Infect. 14, 844–852. doi: 10.1111/j.1469-0691.2008.02034.x
Crucitti, T., Van Dyck, E., Tehe, A., Abdellati, S., Vuylsteke, B., Buve, A., et al. (2003). Comparison of culture and different PCR assays for detection of trichomonas vaginalis in self-collected vaginal swab specimens. Sex. Transm. Infect. 79, 393–398. doi: 10.1136/sti.79.5.393
Demirağ, S., Malatyalı, E., Ertuğ, S., and Ertabaklar, H. (2017). Determination of trichomonas vaginalis genotypes using PCR-restriction fragment length polymorphism (RFLP). Turkiye Parazitol. Derg. 41, 188–191. doi: 10.5152/tpd.2017.5496
Diamond, L. (1957). The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43, 488–490. doi: 10.2307/3274682
Drouin, G., Moniz de Sá, M., and Zuker, M. (1995). The Giardia lamblia actin gene and the phylogeny of eukaryotes. J. Mol. Evol. 41, 841–849. doi: 10.1007/BF00173163
El Sayed, Z. M., Raafat, D., El Emshaty, W., Azab, M. S., and Goda, H. (2010). Correlation of trichomonas vaginalis to bacterial vaginosis: a laboratory-based study. J. Infect. Dev. Ctries. 4, 156–163. doi: 10.3855/jidc.434
El-Gayar, E., Mokhtar, A., Awad, S., Soliman, R., and Hassan, W. (2016). The endosymbiotic relationship between trichomonas vaginalis and Mycoplasma hominis in Egyptian women and its correlation with pathogenicity. Parasitol. United J. 9:80. doi: 10.4103/1687-7942.205169
El-Gayar, E. K., Mokhtar, A. B., and Hassan, W. A. (2016). Molecular characterization of double-stranded RNA virus in trichomonas vaginalis Egyptian isolates and its association with pathogenicity. Parasitol. Res. 115, 4027–4036. doi: 10.1007/s00436-016-5174-3
El-Okbi, L. M., Khalifa, K. E., Elwakil, H. S., Mohamed, A. A., Abdel-Hameed, D. M., and Tawfik, R. A. (2005). Characterization of specific trichomonas vaginalis target antigens from different isolates by immunoblotting against hyperimmune rabbit serum. J. Egypt. Soc. Parasitol. 35, 891–898.
Farouk, H. A., Ahmed, K., Tasneem, M. H., and Mohammed, F. M. (2021). Parasitological studies on trichomonas vaginalis on female patients presented with vaginal discharge at Aswan university hospital. Med. J. Cairo Univ. 89, 1147–1154. doi: 10.21608/mjcu.2021.185002
Garcia, A. F., and Alderete, J. (2007). Characterization of the trichomonas vaginalis surface-associated AP65 and binding domain interacting with trichomonads and host cells. BMC Microbiol. 7:116. doi: 10.1186/1471-2180-7-116
Hamdy, D., and Hamdy, H. (2018). Prevalence, sociodemographic factors and clinical criteria of trichomonas vaginalis infection among symptomatic women in Beni-Suef governorate, Egypt. J. Egypt. Soc. Parasitol. 48, 109–117. doi: 10.21608/jesp.2018.77471
Hamed Elsherif, R., and Fatah Youssef, M. A. (2013). Real-time PCR improve detection of trichomonas vaginalis compared to conventional techniques. Comp. Clin. Pathol. 22, 295–300. doi: 10.1007/s00580-011-1402-5
Hamed, H., Esmail, N., Aldardiry, M., and Mostafa, R. (2018). The use of modified Field’s stain in diagnosis of trichomonas vaginalis. Hum. Androl. 8, 19–29. doi: 10.21608/ha.2018.1368.1011
Hamouda, M., Mohamed, S., Elgendy, S., Esam Eldeen, N., and EL-Zayady, W. (2022). Is trichomoniasis associated with adverse preganancy outcome? Parasitol. United J. 15, 202–209. doi: 10.21608/puj.2022.139781.1169
Hashem, H. E., Ibrahim, Z. H., Nada, A. M., and Ahmed, W. O. (2024). The valuable microbiological role of vaginal and cervical swabs in the Management of Persistent and Recurrent Reproductive Tract Infections (RTIs). Egypt J. Hosp. Med. 95, 1352–1358. doi: 10.21608/ejhm.2024.348921
Hassan, F. A., Al-Marsomy, H. D., and Mustafa, S. A. (2019). Diagnosis of trichomonas vaginalis infection by detection of Glutaminase (Glu) gene by nested PCR. Indian J. Public Health Res. Dev. 10:496. doi: 10.5958/0976-5506.2019.01619.X
Hawksworth, J., Levy, M., Smale, C., Cheung, D., Whittle, A., Longhurst, D., et al. (2015). Population structure and genetic diversity of the parasite trichomonas vaginalis in Bristol, UK. Infect. Genet. Evol. 34, 36–43. doi: 10.1016/j.meegid.2015.06.006
Heikal, E. A., Elamir, A. M., Hegazi, M. A., Salem, H. S., Tawfeik, A. M., Bosilah, A. H., et al. (2023). Signature of real-time PCR in detection of trichomonas vaginalis infection and its association with human papillomavirus genotype 16. Eur. Rev. Med. Pharmacol. Sci. 27, 501–510. doi: 10.26355/eurrev_202301_31050
Hussein, A. H., Saleh, M. H., Nagaty, I. M., Aghieth, K., and El-Azab, N. A. (2015). Prevalence, clinical criteria and sociodemographic predictors of trichomonas vaginalis infection in suspected Egyptian women, using direct diagnostic techniques. Iran. J. Parasitol. 10, 432–440.
Ibrahim, S., Ismail, M., Elaskary, H., Khalil, E., Khalil, D., and Raafat, A. (2021). Potential role of trichomonas vaginalis in women with primary and secondary infertility in Beni-suef, Egypt. J. Egypt. Soc. Parasitol. 51, 119–126. doi: 10.21608/jesp.2021.165951
Issa, R. M., and Shalaby, A. S. (2006). Diagnosis of trichomonas vaginalis infection by PCR. Iran. J. Clin. Infect. Dis. 1, 171–175.
Kamal, A. M., Ahmed, A. K., Mowafy, N. M. E.-S., Shawki, H. E., Sanad, A. S., and Hassan, E. E. (2018). Incidence of antenatal Trichomoniasis and evaluation of its role as a cause of preterm birth in pregnant women referring to Minia university hospital, Egypt. Iran. J. Parasitol. 13, 58–66.
Khalifa, K. E., Mohammed, B. O., Elleboudy, N. A., Hussein, H. M., and Azab, M. E. (2018). Growth kinetics of Egyptian isolates of trichomonas vaginalis: possible correlation to clinical presentation. Egypt J. Hosp. Med. 72, 4428–4433. doi: 10.21608/ejhm.2018.9493
Khalili, B., Ghasemi-Dehkordi, P., Pourshahbazi, G., Yousofi-Darani, H., Hashemzadeh-Chaleshtori, M., and Doosti, A. (2017). Genotyping of trichomonas vaginalis isolates from women in Shahrekord city (southwestern Iran). Genetika 49, 1059–1070. doi: 10.2298/GENSR1703059K
Laila, M. B., Safia, M. A., El Sayed, I. E. A., and Eglal, I. A. (2012). Biological and biochemical studies for characterization of some Egyptian T. vaginalis isolates. Parasitol. United J. 5, 175–188.
Leitsch, D. (2016). Recent advances in the trichomonas vaginalis field. F1000Res 5:162. doi: 10.12688/f1000research.7594.1
Mahmoud, A., Sherif, N. A., Abdella, R., El-Genedy, A. R., El Kateb, A. Y., and Askalani, A. N. (2015). Prevalence of trichomonas vaginalis infection among Egyptian women using culture and latex agglutination: cross-sectional study. BMC Womens Health 15:7. doi: 10.1186/s12905-015-0169-2
Masha, S. C., Cools, P., Crucitti, T., Sanders, E. J., and Vaneechoutte, M. (2017). Molecular typing of trichomonas vaginalis isolates by actin gene sequence analysis and carriage of T. Vaginalis viruses. Parasit. Vectors 10:537. doi: 10.1186/s13071-017-2496-7
Matini, M., Rezaie, S., Mohebali, M., Maghsood, A., Rabiee, S., Fallah, M., et al. (2012). Prevalence of trichomonas vaginalis infection in Hamadan City, Western Iran. Iran. J. Parasitol. 7, 67–72.
McMillan, A., McNeillage, G., Young, H., and Bain, S. S. (1979). Secretory antibody response of the cervix to infection with Neisseria gonorrhoeae. Br. J. Vener. Dis. 55, 265–270. doi: 10.1136/sti.55.4.265
Meade, J. C., and Carlton, J. M. (2013). Genetic diversity in trichomonas vaginalis. Sex. Transm. Infect. 89, 444–448. doi: 10.1136/sextrans-2013-051098
Meade, J. C., de Mestral, J., Stiles, J. K., Secor, W. E., Finley, R. W., Cleary, J. D., et al. (2009). Genetic diversity of trichomonas vaginalis clinical isolates determined by EcoRI restriction fragment length polymorphism of heat-shock protein 70 genes. Am. J. Trop. Med. Hyg. 80, 245–251. doi: 10.4269/ajtmh.2009.80.245
Mohamed, B., Elleboudy, N., Hussein, H., Khalifa, K., and Azab, M. (2019). Genotyping of trichomonas vaginalis isolates from Egypt. Parasitol. United J. 12, 209–220. doi: 10.21608/puj.2019.19332.1054
Momeni, Z., Sadraei, J., Kazemi, B., and Dalimi, A. (2015). Molecular typing of the actin gene of trichomonas vaginalis isolates by PCR-RFLP in Iran. Exp. Parasitol. 159, 259–263. doi: 10.1016/j.exppara.2015.10.011
Muzny, C., Barnes, A., and Mena, L. (2012). Symptomatic trichomonas vaginalis infection in the setting of severe nitroimidazole allergy: successful treatment with boric acid. Sex. Health 9, 389–391. doi: 10.1071/SH11114
Orujzadeh, F., Tabatabaie, F., Khanaliha, K., Akhlaghi, L., Bokharaei-Salim, F., Fallah, S., et al. (2019). Prevalence and genotyping of trichomonas vaginalis infected to dsRNA virus by PCR-restriction fragment length polymorphism (RFLP). Iran. J. Parasitol. 14, 250–257. doi: 10.18502/ijpa.v14i2.1137
Radonjic, I. V., Dzamic, A. M., Mitrovic, S. M., Arsic Arsenijevic, V. S., Popadic, D. M., and Kranjcic Zec, I. F. (2006). Diagnosis of trichomonas vaginalis infection: the sensitivities and specificities of microscopy, culture and PCR assay. Eur. J. Obstet. Gynecol. Reprod. Biol. 126, 116–120. doi: 10.1016/j.ejogrb.2005.07.033
Rowley, J., Vander Hoorn, S., Korenromp, E., Low, N., Unemo, M., Abu-Raddad, L. J., et al. (2019). Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bull. World Health Organ. 97, 548–562P. doi: 10.2471/BLT.18.228486
Salem, S. A., Azab, M. E., Abd el Ghaffar, F. M., el Sherif, E. A., Makled, K. A., and Habib, F. S. (1992). Characterization of Egyptian isolates of trichomonas vaginalis I. Isoenzyme patterns. J. Egypt. Soc. Parasitol. 22, 675–682. doi: 10.21203/rs.3.rs-4811368/v1
Schumann, J. A., and Plasner, S. T. (2024). [Updated 2023 Jun 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available at: https://www.ncbi.nlm.nih.gov/books/NBK534826/
Shawaky, S. M., Al Shammari, M. M. A., Sewelliam, M. S., Ghazal, A. A. E. R., and Amer, A. N. (2022). A study on vaginitis among pregnant and non-pregnant females in Alexandria, Egypt: an unexpected high rate of mixed vaginal infection. AIMS Microbiol. 8, 167–177. doi: 10.3934/microbiol.2022014
Shipitsyna, E., Zolotoverkhaya, E., Chen, C. Y., Chi, K. H., Grigoryev, A., Savicheva, A., et al. (2013). Evaluation of polymerase chain reaction assays for the diagnosis of trichomonas vaginalis infection in Russia. J. Eur. Acad. Dermatol. Venereol. 27, e217–e223. doi: 10.1111/j.1468-3083.2012.04593.x
Tavakoli Oliaee, R., Babaei, Z., Hatam, G. R., Tavakoli Kareshk, A., Mahmoudvand, H., Vafafar, A., et al. (2017). Considerable genetic diversity of trichomonas vaginalis clinical isolates in a targeted population in south of Iran. Iran. J. Parasitol. 12, 251–259.
van der Veer, C., Himschoot, M., and Bruisten, S. M. (2016). Multilocus sequence typing of trichomonas vaginalis clinical samples from Amsterdam, the Netherlands. BMJ Open 6:e013997. doi: 10.1136/bmjopen-2016-013997
WHO. Fact sheet on Sexually Transmitted Infections (STIs). (2016). Available at: http://www.who.int/mediacentre/factsheets/fs110/en/ (accessed August 30, 2024).
Keywords: Trichomonas vaginalis , nested PCR, genotyping, RFLP, Upper Egypt
Citation: El-kareem NMA, Dyab AK, Albalawi NO, El Samea AA, Taha MAA, AlQadeeb H, Gareh A, Hiekal EA, Alzaylaee H and Elmahallawy EK (2024) Microscopic and molecular detection of Trichomonas vaginalis in outpatients seeking medical care in Upper Egypt. Front. Microbiol. 15:1499270. doi: 10.3389/fmicb.2024.1499270
Edited by:
Hosny El-Adawy, Friedrich Loeffler Institut, GermanyReviewed by:
Aman Ullah Khan, University of Veterinary and Animal Sciences, PakistanSarah Abdo, Kafrelsheikh University, Egypt
Copyright © 2024 El-kareem, Dyab, Albalawi, El Samea, Taha, AlQadeeb, Gareh, Hiekal, Alzaylaee and Elmahallawy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ehab Kotb Elmahallawy, c2EyZWxlbGVAdWNvLmVz
 Nasser Mohamed Abd El-kareem1
Nasser Mohamed Abd El-kareem1 Ahmed Kamal Dyab
Ahmed Kamal Dyab Nada Oudah Albalawi
Nada Oudah Albalawi Hajar AlQadeeb
Hajar AlQadeeb Ahmed Gareh
Ahmed Gareh Ehab Kotb Elmahallawy
Ehab Kotb Elmahallawy