
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 December 2024
Sec. Microbial Symbioses
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1497987
This article is part of the Research Topic Plant Microbiome: Interactions, Mechanisms of Action, and Applications, Volume III View all 22 articles
Introduction: The biological activities of osthole have been widely reported in recent years. However, few studies have been conducted on osthole in agriculture, and its effects on plant growth have little been reported.
Methods: Three experimental treatments were set up in this experiment: blank control (CK), osthole (CLS), and B. amyloliquefaciens (LKWS). In this study, the effects of osthole and Bacillus amyloliquefaciens on the growth parameters, photosynthesis, antioxidant enzyme activities, disease incidence, and microbiome of forested P. quinquefolius were tested.
Results: This study demonstrates that the use of osthole and B. amyloliquefaciens significantly improved the growth of Panax quinquefolius in a forest compared to that in the control treatment, increased the total chlorophyll and carotenoid content of P. quinquefolius, significantly increased its net photosynthetic rate, and decreased the stomatal conductance and intercellular CO2 levels. In addition, the use of osthole and B. amyloliquefaciens significantly improved ascorbate peroxidase and peroxidase (POD) activities, enhanced antioxidant activities of the P. quinquefolius POD, and reduced the disease incidence and index of American ginseng anthracnose. Based on the American ginseng microbiome analysis, the use of osthole and B. amyloliquefaciens could change the structure of the American ginseng microbial community, significantly increase the diversity of American ginseng bacteria, significantly decrease the diversity of American ginseng fungi, stimulate the recruitment of more growth-promoting microorganisms to American ginseng, and build a more stable microbial network in American ginseng.
Discussion: In conclusion, we found that the application of osthole had a positive effect on the growth of American ginseng, providing a theoretical basis for its subsequent application in agriculture.
Osthole, also known as osthol (7-methoxy-8-(3-methyl-2-butenyl)-2H-1-benzopyran-2-one; C15H16O3), is a natural coumarin first derived from Cnidium plant. The mature fruits of Cnidium contain high levels of osthole, which is commonly used in the clinical practice of traditional Chinese medicine, and osthole is also widely found in other medicinal plants (Zhang et al., 2015). Previous studies have shown that osthole has many pharmacological properties, including anti-inflammatory (Liao et al., 2010) and anti-osteoporosis (Kuo et al., 2005) effects, and can inhibit systolic blood pressure (Ogawa et al., 2007), Alzheimer’s disease (Kuo et al., 2005) and cancer (Shokoohinia et al., 2018). Osthole has long been used as an antifungal agent and insecticide against plant diseases and pests in China due to it exhibiting less harmful effects on humans and the environment (Ren Z. et al., 2020; Zhang et al., 2016). In addition, Bacillus amyloliquefaciens, as a biofertilizer, provides significant help in increasing agricultural yields and plant resistance.
Bacillus amyloliquefaciens is a gram-positive spore-forming bacterium found in the soil that can colonize the inter-roots of plants and grow under stressful conditions. It has been defined as a non-toxic and environmentally friendly plant growth-promoting agent (Chen et al., 2007; Qiao et al., 2014). As a plant growth-promoting inter-root bacterium, B. amyloliquefaciens is considered an excellent agent for exploring biofertilizers and biocontrols in agriculture, and is used to improve plant tolerance to biotic and abiotic stresses (Dimopoulou et al., 2021; Gamez et al., 2019; Kazerooni et al., 2021).
Panax quinquefolius is native to the eastern temperate forest regions of North America and was first discovered in Quebec, Canada. American ginseng has been cultivated in China since the 1980s (McGraw et al., 2013). After more than 40 years of development, China has become the third largest country for P. quinquefolius cultivation (Huang et al., 2013). American ginseng is a well-known medicinal plant with a high market demand as dietary supplements and functional foods (Pang et al., 2023). American ginseng is known for its wide range of pharmacological effects, including anticancer, antioxidant, anti-aging, anti-fatigue, memory-enhancing, and immune-enhancing effects (Cheong et al., 2014; Hwang et al., 2014; Kim et al., 2018; Riaz et al., 2019; Tan et al., 2013). Various chemical components, including ginsenosides, lipids, polysaccharides, organic acids, amino acids, phenolic acids, and vitamins, have been identified in P. quinquefolius that exert various effects (Guo et al., 2015; Lin et al., 2019; Wang et al., 2015). In addition to specific advantages over field farming, such as lower costs and often higher prices, American ginseng forest plantations can improve forest health and increase biodiversity (Sheban et al., 2022).
In order to better utilize the growth-promoting effects of osthole and Bacillus amyloliquefaciens in the cultivation of Panax quinquefolius, the present study aimed to (1) investigate the effects of osthole and Bacillus amyloliquefaciens on the growth, photosynthesis, and antioxidant systems of Panax quinquefolius in the understory, and (2) to determine the regulatory effects of osthole and Bacillus amyloliquefaciens on the endophytic bacterial communities in Panax quinquefolius, and to explore the potential mechanisms of its role in the promotion of plant growth. The results of this study are expected to provide an important scientific basis for further application of osthole in agriculture and practical suggestions for optimizing the cultivation and management of American ginseng.
Osthole was purchased from Chengdu New Sunrise Crop Science Co., Ltd. (Chengdu, China), with the main ingredient of 0.4% osthole used in subsequent tests. A B. amyloliquefaciens strain was purchased from Zhongnong Lukang (Beijing) Biotechnology Co. (Beijing, China).
The experiment was conducted in 2022 at a forested American ginseng base in Luquan County, Kunming, Yunnan Province, China. The site is located at 25°38′ N latitude and 102°80′ E longitude, covering an area of approximately 50,000 m2, with an elevation of 2,700 m. The average annual temperature is approximately 7.0–10.9°C, ≥10°C effective cumulative temperature is 3,300°C, and annual precipitation is 1,000 mm. Precipitation in the months of June–August accounts for approximately 70% of the annual precipitation. The test material comprised 2-year-old understory western ginseng, with a plant spacing of 7 × 20 cm in a north–south direction.
The randomized group method was used, and three treatments set up: blank control (CK), osthole (CLS), and B. amyloliquefaciens (LKWS). Three replications and 2-year-old American ginseng seedlings were used, isolation boards set up between the treatments to prevent mutual influences, and the treatments initiated from June 1. Treatments were performed every 15 days for a total of five treatments and processed up until July 30; all treatment concentrations used were in accordance with instructions and product manuals of the corresponding companies. CLS (7.720 mL) was diluted 400 times, and LKWS (21.45 g) diluted 700 times.
The agronomic traits of American ginseng were measured at the leaf spreading stage. The main metrics measured were plant height, stem thickness, root thickness, and root dry weight. Plant height was measured by placing a tape measure from the bottom of the ginseng to the top of the first leaf. Stem and root thicknesses were measured at the thickest point using a vernier caliper. At harvest, the roots were uprooted and washed with distilled water, where after they were dried at 70°C for 1 week. When the weight was almost unchanged, the dry weight of the roots was weighed with an electronic balance of one ten-thousandth of a millimeter.
Stomatal conductance (Gs), intercellular CO2 concentration (Ci), net photosynthetic rate (Pn), and transpiration rate (Tr) of P. quinquefolius were measured during its flowering period using an LI-6400 portable photosynthesis assay and photosynthesizer under sunny and cloudless weather conditions and between 10:00 and 12:00 Beijing time.
Leaves were collected on the same day, immediately placed into liquid nitrogen, and returned to the laboratory to be stored in a − 80°C refrigerator until the chlorophyll content was measured. The chlorophyll content of P. quinquefolius was determined in strict accordance with the instructions of a chlorophyll kit (G0601F) supplied by Suzhou Grace Biotechnology Co. (Suzhou, China). The absorbance value A was read at wavelengths of 665, 649, and 470 nm, and the chlorophyll a (Chla), chlorophyll b (Chlb), total chlorophyll (Chl), and carotenoid (Car) contents determined according to Equations (1–5) below:
The collected leaves of American ginseng were immediately transported back to the laboratory with liquid nitrogen preservation and stored in a refrigerator at −80°C before the determination of peroxidase (POD) and ascorbate peroxidase (APX) activities in the leaves. POD and APX activities in the ginseng leaves were measured via visible spectrophotometry using the corresponding enzyme activity kits, including the POD assay (G0107F) and APX activity assay (G0203F) kits (Suzhou Grace Biotechnology Co.).
Each treatment was performed with three replicates, and the average distribution randomly taken for each replicate of 100 plants (a total of 300 plants). The number of diseased plants were counted for every 10 days of the investigation until the wilting stage, and the incidence rate, disease index, and other indices also observed. The disease grade was divided into six levels, and the specific grading standards used are shown in Table 1. The incidence rate, disease index, and effect of the control were calculated according to Equations (6–8) below, respectively:
Fifteen days after the final treatment, P. quinquefolius was collected using a five-point sampling method with three replications. The ginseng was then divided into roots and above-ground parts, and all samples surface-sterilized with 75% alcohol for 2 min, washed twice with distilled water, and then quickly frozen in liquid nitrogen, followed by storage at −80°C prior to detecting its microbial diversity. High-throughput Illumina sequencing was used to characterize the microbial community structure in the plant samples (Majorbio Bio-Pharm Technology Co., Shanghai, China). Using primers 799F (5′-AACMGGATTAGTACCCKG-3′) and 1193R (5′-ACGTCATCC CCACCTTCC-3′) (Bulgarelli et al., 2015), the V3–V4 region of the bacterial 16S rRNA gene from American ginseng was amplified. Using primers ITS1F (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and ITS2R (5′-GCT) (GCGTTCTTCATCGATGC-3′) (Sun et al., 2018), the ITS1F–ITS2R region of the fungal gene from American ginseng was amplified. Specific primers with barcodes were synthesized according to the indicated sequencing regions, and the samples then amplified using a thermal cycler (GeneAmp 9700; ABI, USA).
16S rRNA and ITS gene sequencing reads were demultiplexed using Fastp (version 0.19.6), merged with FLASH (version 1.2.11), and operational taxonomic units with 97% similarity clustered using UPARSE (version 11). Taxonomic assignments were made using the bacterial SILVA reference (version 138) and fungal UNITE (version 8.0) databases. Alpha diversity was determined using Mothur v.1.30.2. The basic R package “stats” (version 3.3.1.) was used to perform a two-tailed Wilcoxon rank-sum test (wilcox.test function). For high-throughput Illumina sequencing data analysis, the Majorbio Cloud online platform was used.
Data for this trial were organized using Microsoft Excel 2019. SPSS 19.0 was used to test for significant differences in the means (p < 0.05) and for correlation analysis. Origin 2021 was used for graphing.
To determine the effects of osthole and B. amyloliquefaciens on P. quinquefolius, plant height, stem thickness, root dry weight, and root thickness were measured (Table 2). Treatment with CLS and LKWS significantly increased the plant height, stem thickness, root dry weight, and root thickness of P. quinquefolius compared to those after treatment with CK. Specifically, CLS significantly (p < 0.05) increased the plant height, stem thickness, root dry weight, and root thickness of P. quinquefolius by 9.03, 10.63, 61.67, and 25.57%, respectively, and LKWS significantly (p < 0.05) increased these by 4.51, 12.94, 62.81, and 22.57%, respectively.
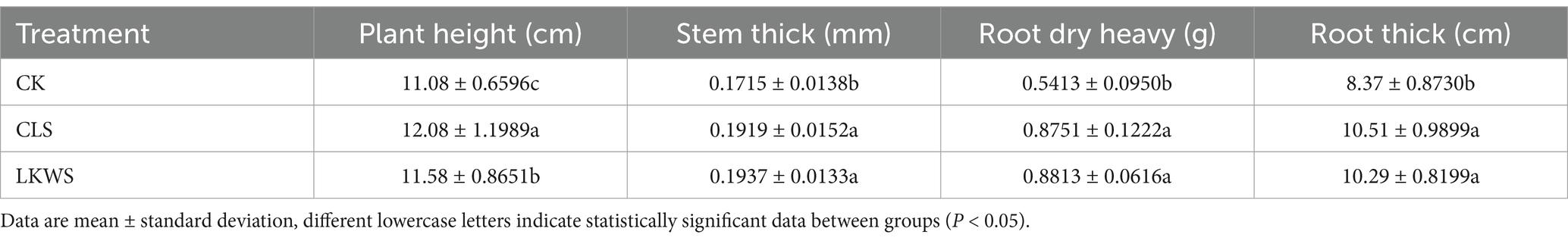
Table 2. Effect of Osthole and Bacillus amyloliquefaciens on morphological indexes of American ginseng.
As shown in Table 3, the control treatment (CK) had the highest disease incidence of P. quinquefolius anthracnose (16.67%), whereas CLS and LKWS treatment significantly (p < 0.05) reduced this, showing lower disease incidence (3.67 and 2.13%, respectively). Consequently, both CLS and LKWS showed good control of western ginseng anthracnose, with CLS showing better control by 76.52%.
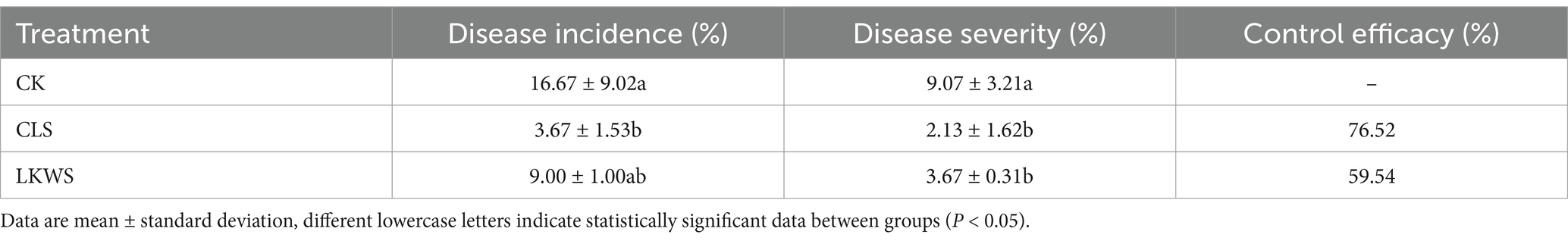
Table 3. Preventive effect of Osthole and Bacillus amyloliquefaciens against the occurrence of American ginseng anthrax.
The CLS and LKWS treatments had significant effects on the chlorophyll content of P. quinquefolius in the forest (Figure 1). CLS significantly (p < 0.05) increased the content of Chla and Chlb in P. quinquefolius by 43.13 and 35.33%, respectively, compared to that of CK. LKWS did not significantly (p < 0.05) increase the content of Chla and Chlb in P. quinquefolius; however, they were increased by 15.63 and 6.45%, respectively, compared to that of CK (Figures 1A,B). CLS and LKWS significantly (p < 0.05) increased the Chl content of P. quinquefolius by 40.57 and 12.61%, respectively, compared to that of CK (Figure 1C). Compared to that of CK, CLS and LKWS significantly (p < 0.05) increased the Car content of P. quinquefolius by 31.04 and 24.61%, respectively (Figure 1D).
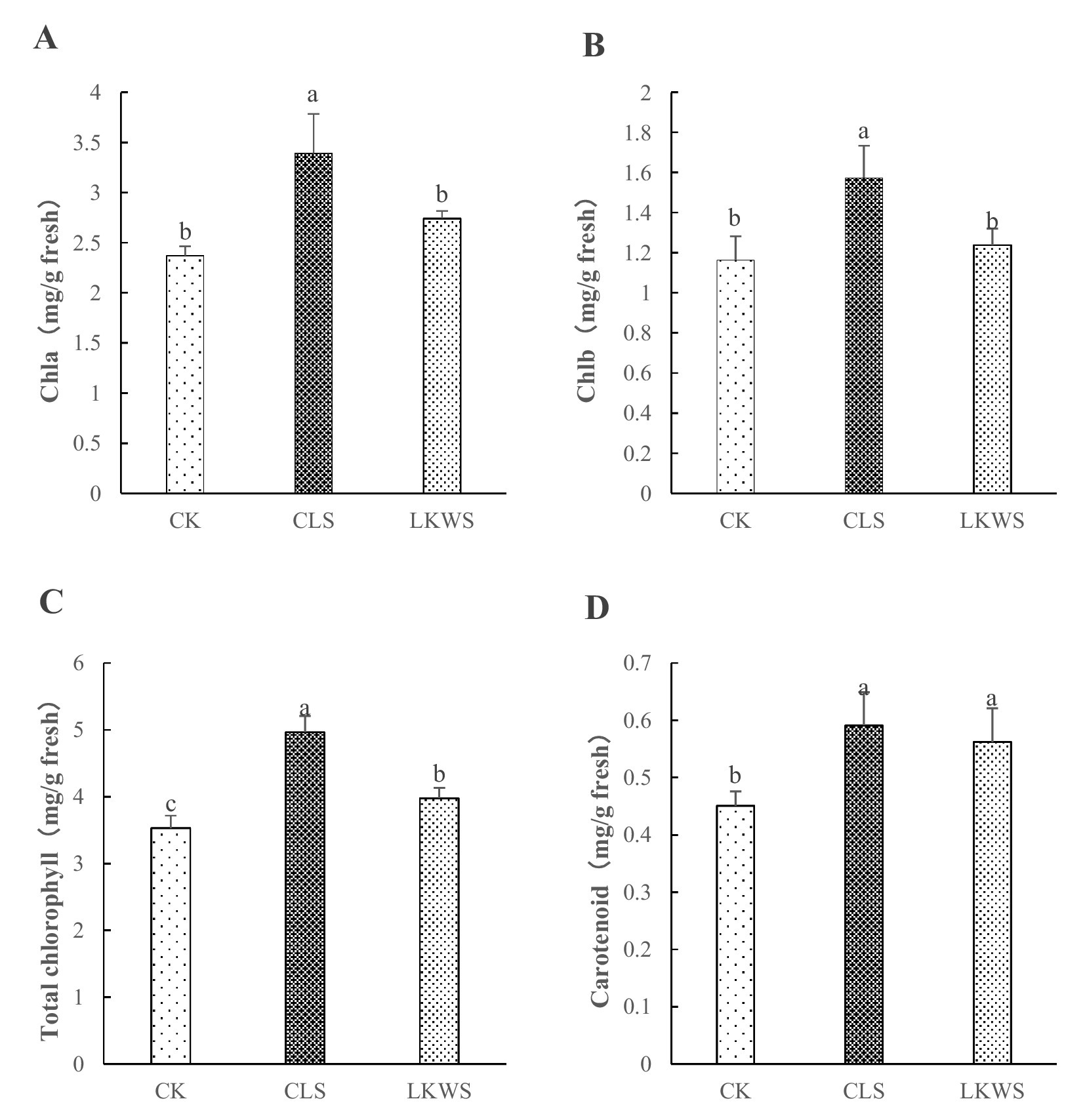
Figure 1. Effect of osthole and Bacillus amyloliquefaciens chlorophyll content. Effect of osthole and Bacillus amyloliquefaciens on (A) Chlorophyll A, (B) Chlorophyll B, (C) Total chlorophyll and (D) Carotenoid. Different lowercase letters indicate significant difference (p < 0.05).
Compared to those of CK, treatment with CLS significantly (p < 0.05) increased the Pn by 67.96% and significantly decreased the Gs, Ci, and Tr by 28.57, 9.83, and 18.18%, respectively (Table 4). Compared to those of CK, LKWS significantly (p < 0.05) increased the Pn and Tr by 13.02 and 14.97%, respectively, and significantly (p < 0.05) decreased the Gs by 14.29%.
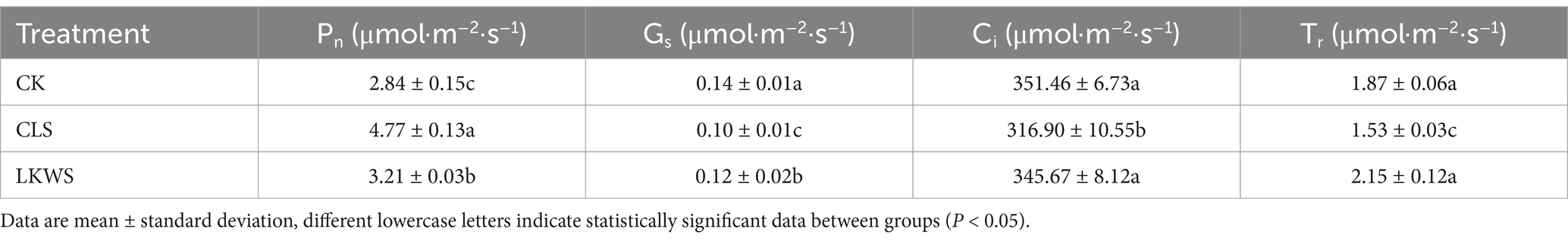
Table 4. Effect of Osthole and Bacillus amyloliquefaciens on photosynthetic parameters of American ginseng.
The application of CLS and LKWS significantly (p < 0.05) increased the activity of antioxidant enzymes in P. quinquefolius (Figure 2). Compared to that of CK, CLS and LKWS significantly (p < 0.05) increased the activity of APX enzymes in P. quinquefolius by 151.79 and 115.41%, respectively. Moreover, the use of CLS and LKWS significantly (p < 0.05) increased POD activity in P. quinquefolius by 36.78 and 102.93%, respectively, compared to that of CK.
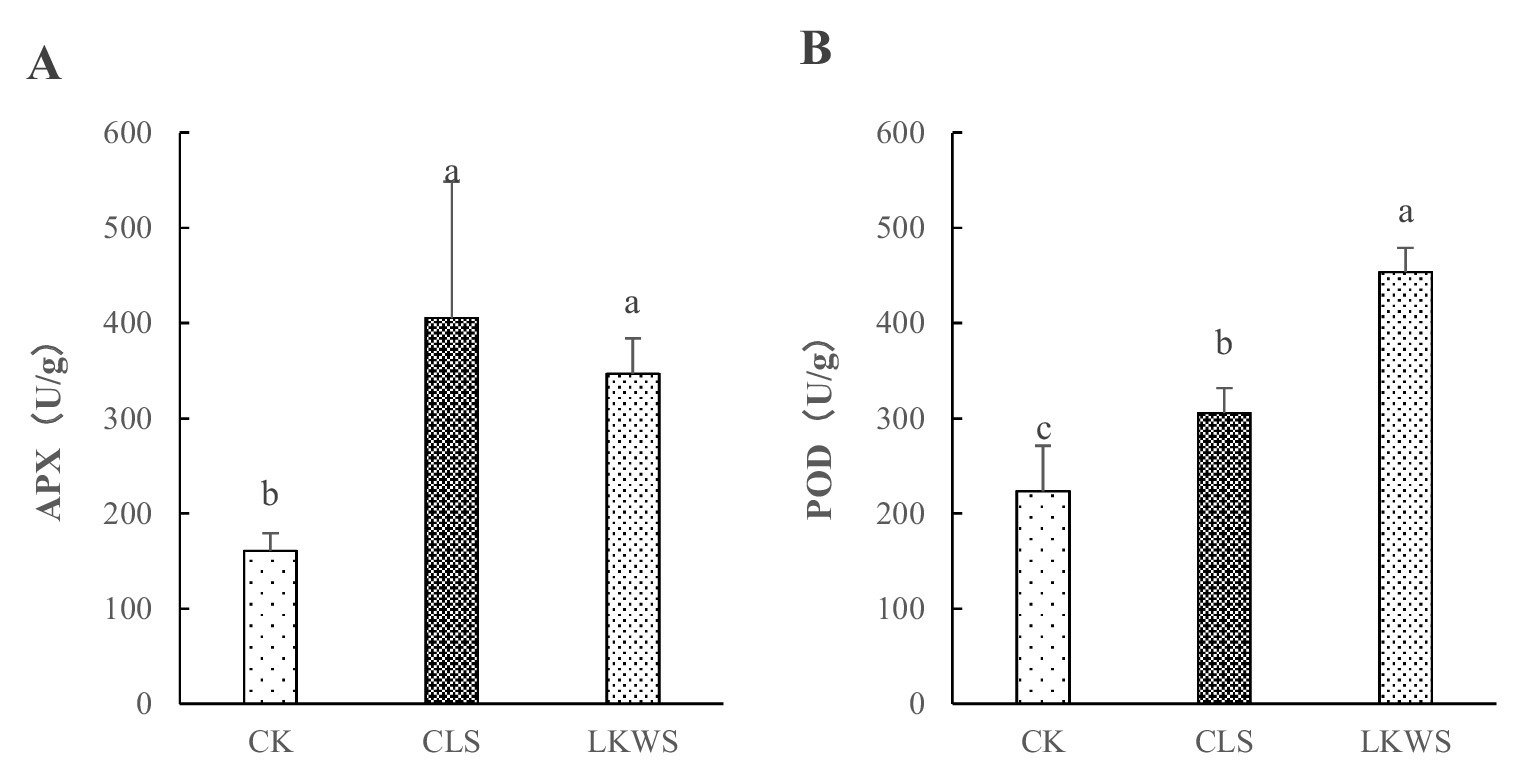
Figure 2. Effects of osthole and Bacillus amyloliquefaciens against oxidase activity. Effect of Osthole and Bacillus on (A) APX and (B) POD enzymes. Different lowercase letters indicate significant difference (p < 0.05).
Shannon’s index was used to assess microbial diversity of the aboveground and root parts of P. quinquefolius under the different treatment conditions (Figure 3). Compared with that of CK, CLS increased the bacterial diversity in aboveground and root parts of P. quinquefolius by 52.29 and 29.11%, respectively, and LKWS increased these by 61.25 and 19.92%, respectively. Compared with that of CK, CLS significantly decreased fungal diversity in the aboveground and root parts of P. quinquefolius by 25.07 and 40.34%, respectively, and LKWS significantly decreased these by 32.70 and 28.04%, respectively.
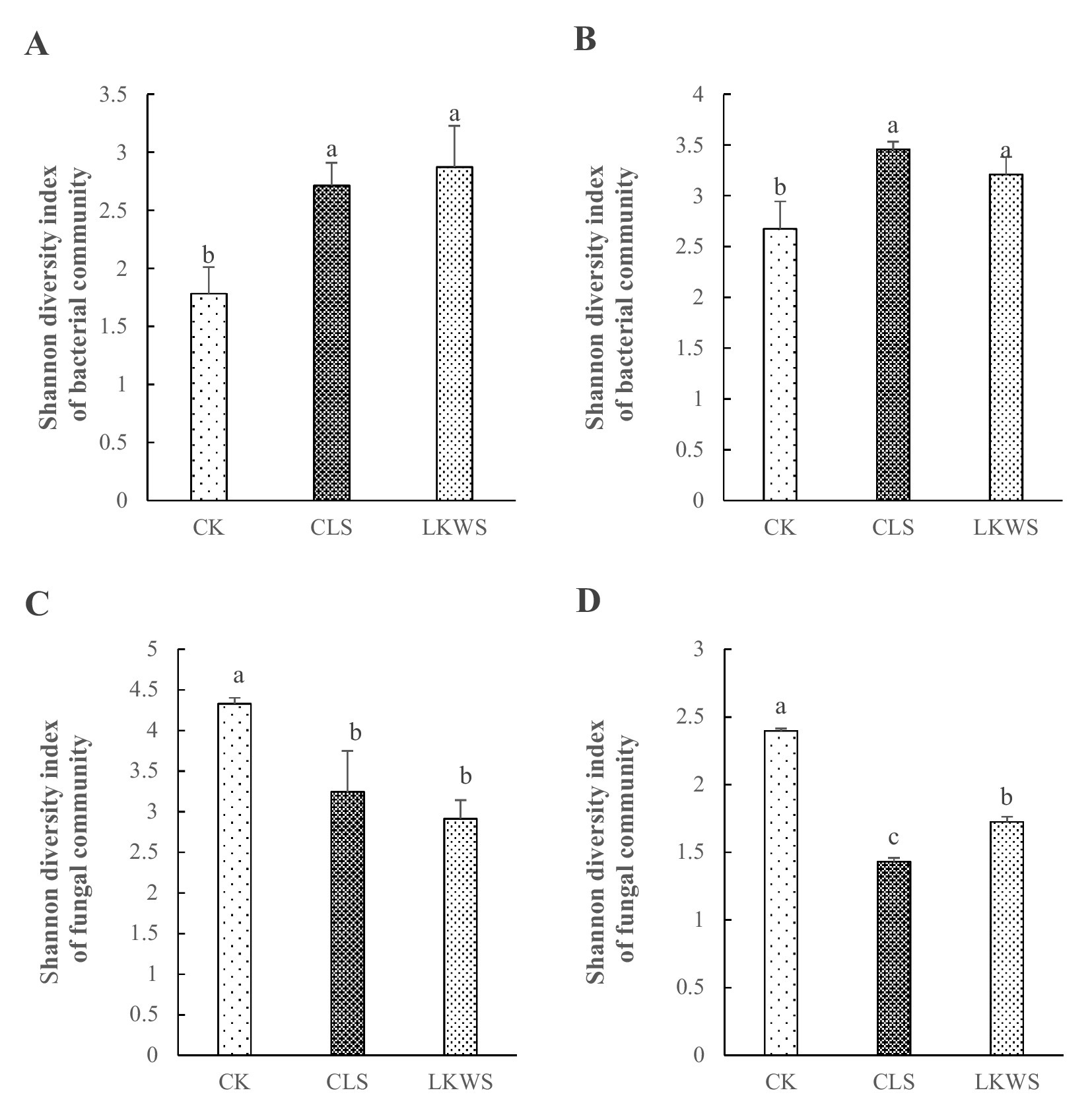
Figure 3. Shannon diversity index of the bacterial community structure of the (A) aboveground and (B) root of American ginseng and Shannon diversity index of the fungal community structure of the (C) aboveground and (D) root of American ginseng. Different lowercase letters indicate significant difference (p < 0.05).
Symbiotic networks of bacterial and fungal communities varied among the different parts of P. quinquefolius and among treatments (Figure 4, Tables 5, 6). With the exception of the CLS treatment, the structure of the bacterial network in the roots of P. quinquefolius was typically more complex than that in the aboveground parts, based on the number of edges and nodes, as well as the average extent. Among all the bacterial networks, that of the CLS treatment was the simplest in the roots of P. quinquefolius (nodes: 352; edges: 10721: average degree: 60.915). In the aboveground parts and roots of P. quinquefolius, both CLS and LKWS treatments had higher positive and lower negative correlations than those of CK.
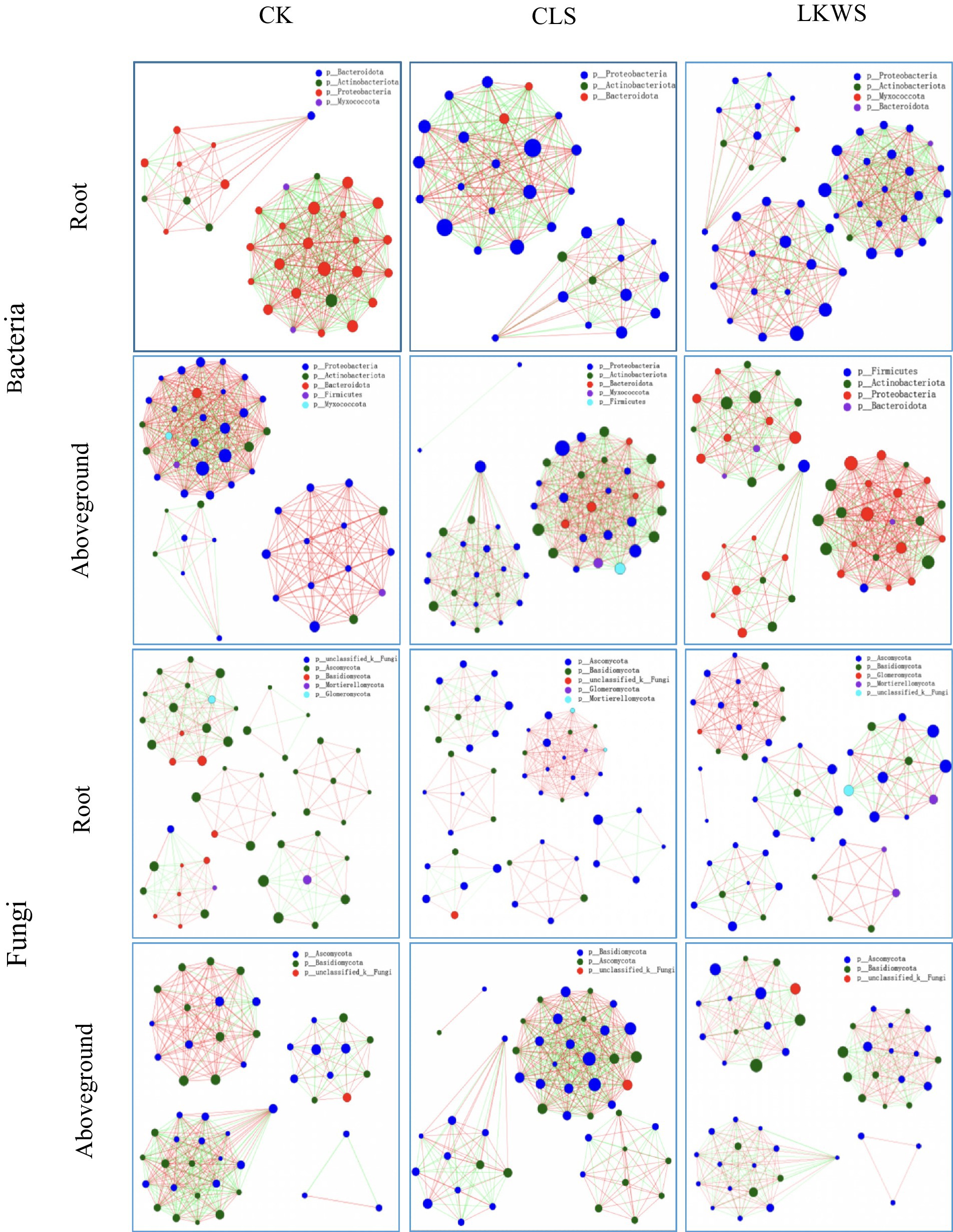
Figure 4. Network analysis under different treatments of American ginseng. The size of the nodes in the figure indicates the abundance of the species, different colors indicate different species; the color of the line indicates positive and negative correlation, red indicates positive correlation, green indicates negative correlation; the thickness of the line indicates the size of the correlation coefficient, the thicker the line, the higher the correlation between species; the more lines, the closer the connection between the species and other species.
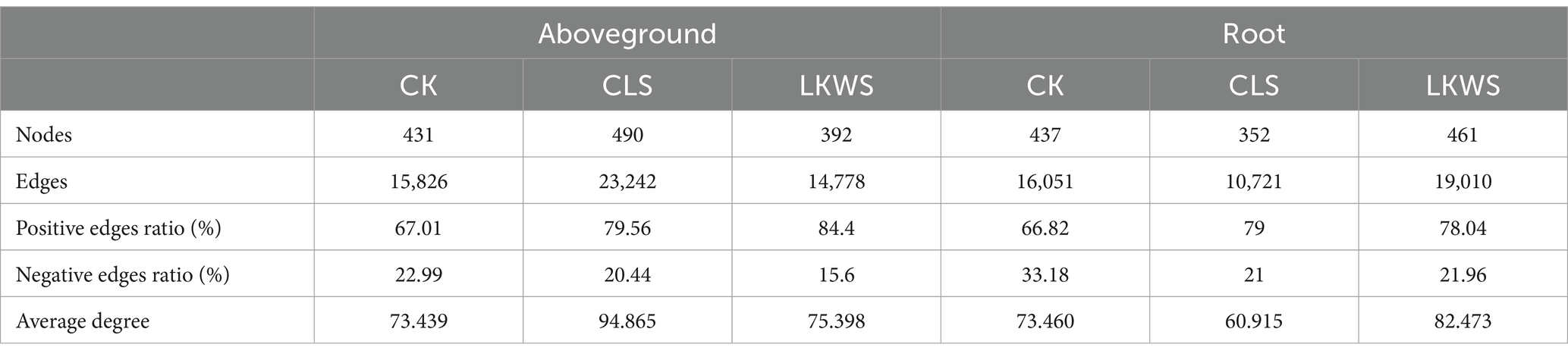
Table 5. Key topological characteristics of bacterial networks in ground and roots of American ginseng.
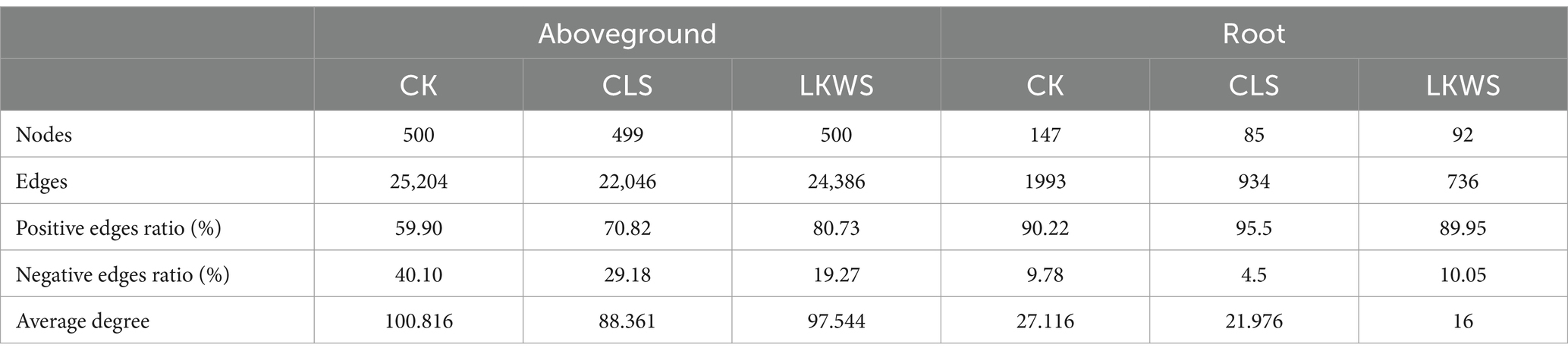
Table 6. Key topological characteristics of fungal networks in ground and roots of American ginseng.
The structure of fungal networks in the aboveground parts of P. quinquefolius was generally more complex than that in the roots, based on the number of edges and nodes, as well as the average degree when compared to the bacterial networks (Figure 4, Tables 5, 6). Nodes, edges, and the average extent of fungal networks were lower in the aboveground parts and roots in the CLS treatment than in the CK. Edges and the average extent of fungal networks were lower in aboveground parts and roots of P. quinquefolius in LKWS treatments compared to those of CK, but no differences were observed in the nodes of the aboveground parts.
Microorganisms of the aboveground parts and roots of P. quinquefolius were observed and constructed using bacterial and fungal genera with relative abundances greater than 1%, respectively, to assess the relationship between microorganisms in different parts of P. quinquefolius and under different treatments (Figure 5). The relationship between bacteria and different treatments in the aboveground parts of P. quinquefolius is shown in Figure 5A. Pseudomonas (40.94%) and Bordetella (25.87%) were the main bacterial genera present in the CK treatment in the aboveground parts of P. quinquefolius. Similarly, the main bacterial genera in the aboveground parts of P. quinquefolius in the CLS treatment were Pseudomonas (36.99%) and Bordetella (22.48%), whereas those in the LKWS treatment were Bacillus (18.11%), Nocardioides (12.97%), and Pseudomonas (12.31%). The relationship between the bacteria in the roots of ginseng and the different treatments is shown in Figure 5B. Pseudomonas (27.65%), Variovorax (14.44%), and Bordetella (12.30%) were the dominant bacterial genera in the CK treatment in the roots of P. quinquefolius. The dominant bacterial genera in the CLS treatment in the roots of P. quinquefolius were Variovorax (17.91%), Pseudomonas (12.56%) and Tardiphaga (11.20%), whereas those in the LKWS treatment were Pseudomonas (33.79%) and Bordetella (16.52%). The relationship between fungi in the aboveground parts of P. quinquefolius and the different treatments is shown in Figure 5C. The main fungal genera present in aboveground parts of P. quinquefolius in the CK treatment were Devriesia (23.12%) and Capnodiales (13.65%). The main fungal genera in aboveground parts of P. quinquefolius in the CLS treatment were Vishniacozyma (31.82%) and Sporidiobolaceae (15.53%), whereas those of the LKWS treatment were Vishniacozyma (19.23%) and Bullera (17.64%). The relationship between fungi in the roots of P. quinquefolius and the different treatments is shown in Figure 5D. The main fungal genera in the roots of P. quinquefolius in the CK treatment were Cadophora (33.42%) and Cistella (11.21%). The main fungal genera in the roots of P. quinquefolius in the CLS treatment were Cadophora (46.57%), Ilyonectria (25.85%), and Fusidium (11.18%), whereas those of the LKWS were Cadophora (36.18%), Alatospora (30.44%), and Fusidium (12.96%).
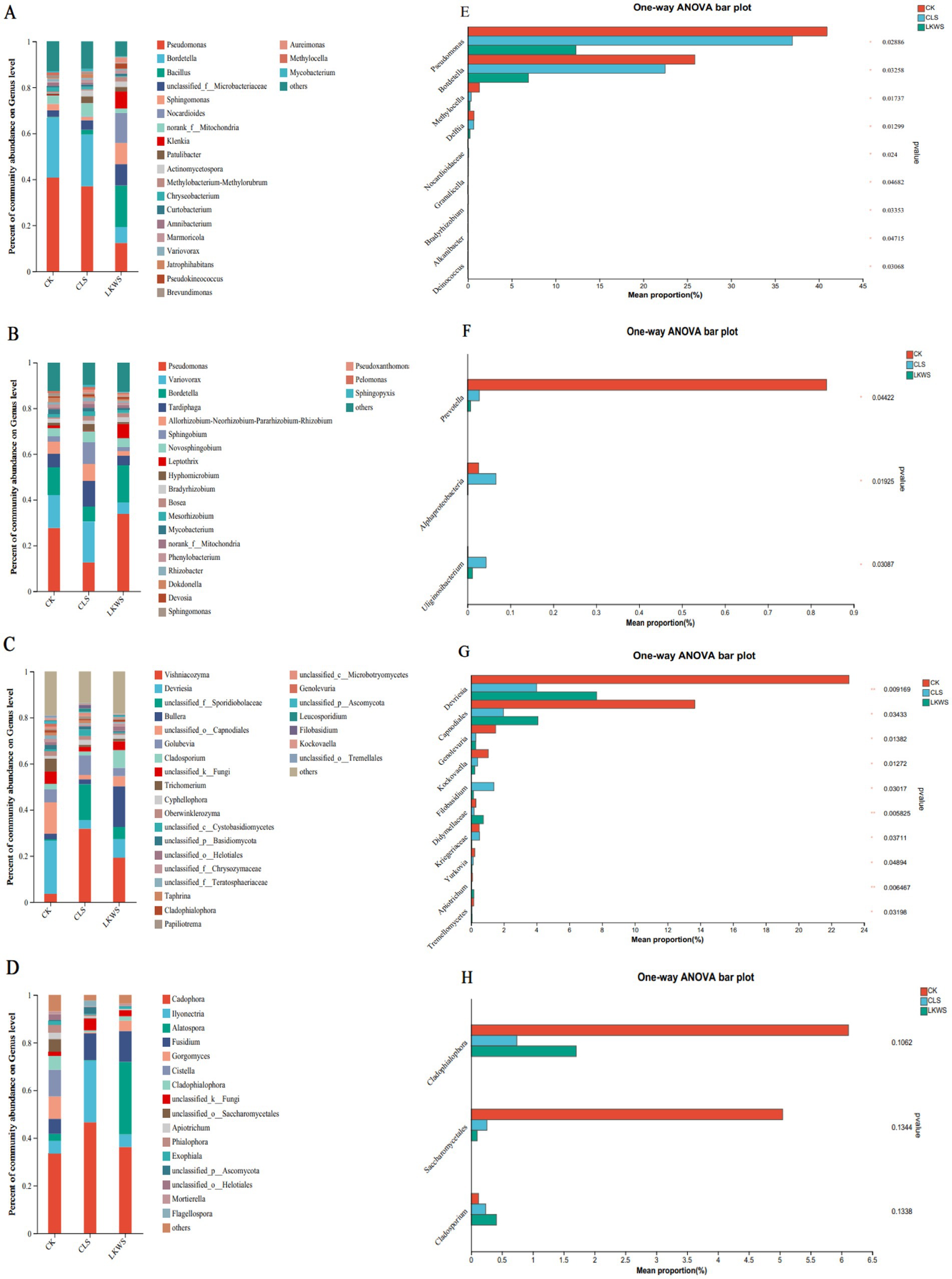
Figure 5. Microbial composition and difference analysis of aerial parts and roots of American ginseng. Relative abundance at the bacterial level of aerial parts (A) and root (B); relative abundance of aerial parts (C) and root (D). The abscissa is the treatment, the ordinate is the proportion of the genus in the sample, the columns of different colors represent different species, and the length of the pillars represents the size of the proportion of the species. One-way analysis of variance (ANOVA) was used to test differences between the bacterial levels of the ground (E) and root (F) and the ground (G) and root (H). The y-axis represents species names at the genus level, the x-axis represents the mean relative abundance of different species groups, and columns of different colors represent different groups. On the far right is the value of p, * p < 0.05 * * p < 0.01 * * * p < 0.001.
Microorganisms in American ginseng differed significantly among the different treatments. In the aboveground parts of P. quinquefolius, the bacterial genera, Pseudomonas and Bordetella, were significantly higher in the CK treatment than in the CLS and LKWS treatments (Figure 5E). In the roots of American ginseng, the bacterial genus, Prevotella, was significantly higher in the CK treatment than in the CLS and LKWS treatments, and the bacterial genera, Alphaproteobacteria and Uliginosibacterium, significantly more abundant in the CLS treatment than in the other treatments (Figure 5F). In the aboveground portion of C. occidentalis, the fungal genera, Devriesia and Capnodiales, were significantly more abundant in the CK treatment than in the other treatments (Figure 5G).
The present study showed that the use of osthole and B. amyloliquefaciens significantly (p < 0.05) improved the growth of P. quinquefolius in a forest (Table 2), where their application significantly increased the plant height, stem thickness, root dry weight, and root thickness. The improvement of these growth indicators not only reflects the growth status of the plant, but also closely related to the physiological and ecological adaptations of the plant, which can directly affect the growth, development and yield of the plant. Studies have shown that plant height and stem thickness are important reflections of the competitive ability of plants, and higher plant height helps to obtain more light, thus promoting photosynthesis (Rebitanim et al., 2020). Root dry weight and root thickness are related to the water and nutrient uptake capacity of plants, and the enhancement of root development can improve the adaptability of plants to adverse environments (Comas et al., 2013). Our results are in line with those of previous studies on the promotion of coumarin production in faba beans (Saleh et al., 2015) and the growth promotion of Arabidopsis thaliana by B. amyloliquefaciens (Lu et al., 2021). The growth-promoting effect of osthole, a derivative of coumarin, on Codonopsis may affect plant growth by interfering with phytohormone metabolism (Cheynier et al., 2013). Plant growth can be affected by B. amyloliquefaciens via the secretion of IAA (Ji et al., 2021), which promotes nitrogen fixation by plant roots (Abdallah et al., 2018), solubilizes phosphates (Vinci et al., 2018) and (Jiang et al., 2015), generates iron carriers (Dimopoulou et al., 2021), among others, to promote plant growth. Together, these mechanisms of action promoted the growth of Panax quinquefolius, further validating the importance of growth indicators in assessing plant growth status and production potential.
Chlorophylls are the most abundant pigments in the photosynthetic system of land plants and algae and are indispensable for the absorption of light energy and transfer of electrons in photosynthesis (Hu et al., 2021). Carotenoids are necessary for leaf photosynthesis and photoprotection (Rodriguez-Concepcion and Daròs, 2022). In our study, the use of osthole and Bacillus amyloliquefaciens significantly increased chlorophyll and carotenoid content (Figure 1). Photosynthesis plays a crucial role in the synthesis and accumulation of organic matter, plant growth, nutrient uptake, and responses to abiotic and biotic stress (Bunce, 2008). Our results showed that the use of osthole significantly increased the Pn (Table 4), which may be due to the fact that osthole significantly increased the chlorophyll content of American ginseng. Compared with those of CK, the use of B. amyloliquefaciens also significantly increased the Pn and Tr, which is similar to the results of a previous study on alfalfa seedlings (Han et al., 2022). These results indicate the promising application of osthole and Bacillus amyloliquefaciens and its use in the optimization of photosynthesis efficiency. In the present study, the use of both osthole and B. amyloliquefaciens significantly reduced the Gs, which may be due to the ability of the plant to avoid excessive water loss by closing stomata (Novick et al., 2016). This further illustrates the potential of osthole and Bacillus amyloliquefaciens in water regulation.
Numerous studies have shown that antioxidant enzymes play a great role in alleviating the accumulation of reactive oxygen species (ROS) and reducing oxidative stress in plants (Han et al., 2022). APX and POD are the main antioxidant enzymes that convert H2O2 to H2O (Ren Y. et al., 2020; Rosa et al., 2010). The present study showed that the use of osthole and B. amyloliquefaciens significantly increased the activities of APX and POD in P. quinquefolius. This result suggests that they may be effective in enhancing the antioxidant defense system of Panax quinquefolius, thereby improving its tolerance to oxidative stress. Coumarin has the function of enhancing the antioxidant defense system (Wu et al., 2009), and osthole, a naturally occurring coumarin, has the potential to inhibit the production of active oxidants (Tsai et al., 2015), which may explain its ability to enhance the antioxidant enzyme activity of forest ginseng. The use of B. amyloliquefaciens has previously been shown to enhance APX and POD activities in tomatoes (Wang et al., 2019), which is consistent with our results.
In addition, we investigated the effects of osthole and B. amyloliquefaciens on the P. quinquefolius microbiome. The structure of the plant microbiome is influenced by complex interactions among the host, microorganisms, and other relevant environmental factors (Dastogeer et al., 2020). Our results showed that the use of osthole and B. amyloliquefaciens significantly increased the diversity of bacteria in the aboveground parts and roots of P. quinquefolius, while significantly decreasing the diversity of fungi (Figure 3). The significant decrease in fungal diversity of P. quinquefolius may be due to its strong antifungal activity (Guo et al., 2021). Previously, the use of B. amyloliquefaciens was shown to significantly increase ginseng bacterial diversity and decrease its fungal diversity (Tian et al., 2018), which is consistent with the results of our study. Interactions with microorganisms can affect community stability (Coyte et al., 2015). Our results showed that CLS was more complex than CK was in the bacterial network of the aboveground parts of sago ginseng; LKWS was more complex than CK was in the bacterial network of sago ginseng roots; and CK was more complex than CLS and LKWS were in the fungal network based on edges and nodes (Figure 4, Tables 5, 6). This suggests that the use of osthole and B. amyloliquefaciens can increase the complexity of bacterial communities while decreasing the complexity and improving the stability of fungal communities. The complexity of microbial networks may be related to the alpha diversity (Fan et al., 2018). Previous studies have shown that Pseudomonas and Bordetella are potential pathogens (Hamidou Soumana et al., 2017; Fernández et al., 2015), where our study showed that the use of CLS and LKWS significantly reduced their abundance when compared to that of CK (Figure 5E), which may also explain the reduction observed in disease incidence and severity in C. occidentalis after the use of osthole and deconjugated B. amyloliquefaciens. Previous studies have identified Variovorax as a class of plant growth-promoting inter-root bacteria (Han et al., 2011) that enhances host plant resilience and disease resistance (Belimov et al., 2009; Belimov et al., 2005). Tardiphaga plays an important role in the N cycle (Alves et al., 2014). Our results suggest that Variovorax and Tardiphaga are the main bacterial genera of CLS in the roots of P. quinquefolius (Figure 5B), which may explain why the use of osthole promotes the growth of P. quinquefolius while enhancing stress and disease resistance. Previous studies have shown the potential growth-promoting effects of Alphaproteobacteria (Pini et al., 2011), and that Uliginosibacterium contributes to biofilm formation (Jiao et al., 2021). The bacterial genera, Alphaproteobacteria and Uliginosibacterium, were significantly higher in CLS than in other treatments tested in our study (Figure 5F). Some potential pathogenic bacteria, such as Devriesia and Capnodiales, were significantly lower than those in CK after both CLS and LKWS treatments (Abdollahzadeh et al., 2020; Li et al., 2013) (Figure 5G). The relative abundance of some potential plant growth-promoting microorganisms, such as Vishniacozyma, Cadophora, and Alatospora, was higher than that of CK in the CLS and LKWS treatments (Figures 5C,D) (Artigas et al., 2017; Bizabani and Dames, 2015; Lutz et al., 2020). Therefore, we hypothesized that the application of osthole and B. amyloliquefaciens may recruit plant growth-promoting microorganisms by stimulating C. occidentalis and providing more nutrients for plant growth while inhibiting the invasion and proliferation of potential pathogens (Wang et al., 2022).
In this study, the application of osthole and B. amyloliquefaciens to understory ginseng revealed that their use improved the growth of understory American ginseng by enhancing photosynthetic capacity, stimulating the activity of antioxidant enzymes to increase the tolerance of P. quinquefolius, and promoting the accumulation of plant biomass. In addition, the use of osthole and B. amyloliquefaciens altered the structure of the microbial community of P. quinquefolius, significantly increased the diversity of P. quinquefolius bacteria, significantly decreased the diversity of P. quinquefolius fungi, and stimulated the recruitment of more growth-promoting microorganisms into the American ginseng to build a more stable microbial network, which resulted in a significant decrease in the incidence of P. quinquefolius anthracnose and the index of the disease. Therefore, based on the above results, it was shown that the use of osthole is an effective way to improve the growth of P. quinquefolius in the forest and, at the same time, provides a theoretical basis for its effective application in agriculture. In order to further deepen the research and promote the agricultural dissemination of osthole, future work could focus on (1) analyzing in depth the specific mechanisms by which osthole affects the microbial community of American ginseng and further verifying the relationship between these community changes and disease resistance; (2) evaluating the potential for the application of osthole in other cash crops, especially in terms of reduction of chemical pesticides and enhancement of crop resistance to disease, in order to explore its broader applicability.
The data presented in the study are deposited in the NCBI repository, accession number PRJNA1189344.
JJ: Writing – original draft. GF: Writing – original draft. RW: Writing – review & editing. TL: Writing – review & editing. SH: Writing – review & editing. SY: Writing – review & editing. SZ: Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Yunnan Province Agricultural Joint Special Key Project (No. 202401BD070001-014), and the Yunnan Provincial Association for Science and Technology project of the Farmer Academician Science and Technology Service Station in Xundian County.
The authors thank all participants involved in this research project. The authors would like to thank Yirong QI (College of Agronomy and Biotechnology, Yunnan Agricultural University, Kunming, China) for checking the pictures. The authors would also like to thank Editage (www.editage.com) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdallah, D. B., Frikha-Gargouri, O., and Tounsi, S. (2018). Rizhospheric competence, plant growth promotion and biocontrol efficacy of Bacillus amyloliquefaciens subsp. plantarum strain 32a. Biol. Control 124, 61–67. doi: 10.1016/j.biocontrol.2018.01.013
Abdollahzadeh, J., Groenewald, J. Z., Coetzee, M. P. A., Wingfield, M. J., and Crous, P. W. (2020). Evolution of lifestyles in Capnodiales. Stud. Mycol. 95, 381–414. doi: 10.1016/j.simyco.2020.02.004
Alves, L. M. C., De Souza, J. A. M., de Mello Varani, A., and de Macedo Lemos, E., (2014). The family rhizobiaceae. by E. Rosenberg, E. F. DeLong, and S. Lory, Berlin: Springer, 419–437.
Artigas, J., Rossi, F., Gerphagnon, M., and Mallet, C. (2017). Sensitivity of laccase activity to the fungicide tebuconazole in decomposing litter. Sci. Total Environ. 584-585, 1084–1092. doi: 10.1016/j.scitotenv.2017.01.167
Belimov, A. A., Dodd, I. C., Hontzeas, N., Theobald, J. C., Safronova, V. I., and Davies, W. J. (2009). Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytol. 181, 413–423. doi: 10.1111/j.1469-8137.2008.02657.x
Belimov, A. A., Hontzeas, N., Safronova, V. I., Demchinskaya, S. V., Piluzza, G., Bullitta, S., et al. (2005). Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biol. Biochem. 37, 241–250. doi: 10.1016/j.soilbio.2004.07.033
Bizabani, C., and Dames, J. (2015). Effects of inoculating Lachnum and Cadophora isolates on the growth of Vaccinium corymbosum. Microbiol. Res. 181, 68–74. doi: 10.1016/j.micres.2015.08.005
Bulgarelli, D., Garrido-Oter, R., Münch, P. C., Weiman, A., Dröge, J., Pan, Y., et al. (2015). Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17, 392–403. doi: 10.1016/j.chom.2015.01.011
Bunce, J. (2008). Acclimation of photosynthesis to temperature in Arabidopsis thaliana and Brassica oleracea. Photosynthetica 46, 517–524. doi: 10.1007/s11099-008-0088-7
Chen, X. H., Koumoutsi, A., Scholz, R., Eisenreich, A., Schneider, K., Heinemeyer, I., et al. (2007). Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 25, 1007–1014. doi: 10.1038/nbt1325
Cheong, K. L., Wu, D. T., Hu, D. J., Zhao, J., Cao, K. Y., Qiao, C. F., et al. (2014). Comparison and characterization of the glycome of Panax species by high-performance thin-layer chromatography. J. Planar Chromatogr. 27, 449–453. doi: 10.1556/JPC.27.2014.6.8
Cheynier, V., Comte, G., Davies, K. M., Lattanzio, V., and Martens, S. (2013). Plant phenolics: recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 72, 1–20. doi: 10.1016/j.plaphy.2013.05.009
Comas, L. H., Becker, S. R., Cruz, V. M. V., Byrne, P. F., and Dierig, D. A. (2013). Root traits contributing to plant productivity under drought. Front. Plant Sci. 4:442. doi: 10.3389/fpls.2013.00442
Coyte, K. Z., Schluter, J., and Foster, K. R. (2015). The ecology of the microbiome: networks, competition, and stability. Science 350, 663–666. doi: 10.1126/science.aad2602
Dastogeer, K. M., Tumpa, F. H., Sultana, A., Akter, M. A., and Chakraborty, A. (2020). Plant microbiome–an account of the factors that shape community composition and diversity. Curr. Plant Biol. 23:100161. doi: 10.1016/j.cpb.2020.100161
Dimopoulou, A., Theologidis, I., Benaki, D., Koukounia, M., Zervakou, A., Tzima, A., et al. (2021). Direct antibiotic activity of Bacillibactin broadens the biocontrol range of Bacillus amyloliquefaciens MBI600. Msphere 6, 00376–00321. doi: 10.1128/mSphere.00376-21
Fan, K., Weisenhorn, P., Gilbert, J. A., and Chu, H. (2018). Wheat rhizosphere harbors a less complex and more stable microbial co-occurrence pattern than bulk soil. Soil Biol. Biochem. 125, 251–260. doi: 10.1016/j.soilbio.2018.07.022
Fernández, M., Porcel, M., de la Torre, J., Molina-Henares, M. A., Daddaoua, A., Llamas, M. A., et al. (2015). Analysis of the pathogenic potential of nosocomial Pseudomonas putida strains. Front. Microbiol. 6:871.
Gamez, R., Cardinale, M., Montes, M., Ramirez, S., Schnell, S., and Rodriguez, F. (2019). Screening, plant growth promotion and root colonization pattern of two rhizobacteria (Pseudomonas fluorescens Ps006 and Bacillus amyloliquefaciens Bs006) on banana cv. Williams (Musa acuminata Colla). Microbiol. Res. 220, 12–20. doi: 10.1016/j.micres.2018.11.006
Guo, Q., Cui, S. W., Kang, J., Ding, H., Wang, Q., and Wang, C. (2015). Non-starch polysaccharides from American ginseng: physicochemical investigation and structural characterization. Food Hydrocoll. 44, 320–327. doi: 10.1016/j.foodhyd.2014.09.031
Guo, Y., Chen, J., Ren, D., Du, B., Wu, L., Zhang, Y., et al. (2021). Synthesis of osthol-based botanical fungicides and their antifungal application in crop protection. Bioorg. Med. Chem. 40:116184. doi: 10.1016/j.bmc.2021.116184
Hamidou Soumana, I., Linz, B., and Harvill, E. T. (2017). Environmental origin of the genus Bordetella. Front. Microbiol. 8:28. doi: 10.3389/fmicb.2017.00028
Han, J. I., Choi, H. K., Lee, S. W., Orwin, P. M., Kim, J., LaRoe, S. L., et al. (2011). Complete genome sequence of the metabolically versatile plant growth-promoting endophyte Variovorax paradoxus S110. J. Bacteriol. 193, 1183–1190. doi: 10.1128/JB.00925-10
Han, L., Zhang, M., Du, L., Zhang, L., and Li, B. (2022). Effects of Bacillus amyloliquefaciens QST713 on photosynthesis and antioxidant characteristics of alfalfa (Medicago sativa L.) under drought stress. Agronomy 12:2177. doi: 10.3390/agronomy12092177
Huang, L. F., Suo, F. M., Song, J. Y., Wen, M. J., Jia, G. L., Xie, C. X., et al. (2013). Quality variation and ecotype division of Panax quinquefolium in China. Acta Pharma. Sinica 48, 580–589
Hwang, C. R., Lee, S. H., Jang, G. Y., Hwang, I. G., Kim, H. Y., Woo, K. S., et al. (2014). Changes in ginsenoside compositions and antioxidant activities of hydroponic-cultured ginseng roots and leaves with heating temperature. J. Ginseng Res. 38, 180–186. doi: 10.1016/j.jgr.2014.02.002
Ji, C., Zhang, M., Kong, Z., Chen, X., Wang, X., Ding, W., et al. (2021). Genomic analysis reveals potential mechanisms underlying promotion of tomato plant growth and antagonism of soilborne pathogens by Bacillus amyloliquefaciens Ba13. Microbiol. Spectr. 9, e01615–e01621. doi: 10.1128/Spectrum.01615-21
Jiang, C.-H., Wu, F., Yu, Z.-Y., Xie, P., Ke, H.-J., Li, H.-W., et al. (2015). Study on screening and antagonistic mechanisms of Bacillus amyloliquefaciens 54 against bacterial fruit blotch (BFB) caused by Acidovorax avenae subsp. citrulli. Microbiol. Res. 170, 95–104. doi: 10.1016/j.micres.2014.08.009
Jiao, Y., Yuan, Q., Wang, W., Yan, L., Mu, X., Li, H., et al. (2021). Vallisnerian natans tolerance and response of microbial community in wetlands to excess nutrients loading. Ecol. Indic. 131:108179. doi: 10.1016/j.ecolind.2021.108179
Kazerooni, E. A., Maharachchikumbura, S. S., Al-Sadi, A. M., Kang, S.-M., Yun, B.-W., and Lee, I. J. (2021). Biocontrol potential of Bacillus amyloliquefaciens against Botrytis pelargonii and Alternaria alternata on Capsicum annuum. J. Fungi 7:472. doi: 10.3390/jof7060472
Kim, S.-H., Kim, S.-Y., and Choi, H.-K. (2018). Lipids in ginseng (Panax ginseng) and their analysis. Nat. Prod. Sci. 24, 1–12. doi: 10.20307/nps.2018.24.1.1
Kuo, P. L., Hsu, Y. L., Chang, C. H., and Chang, J. K. (2005). Osthole-mediated cell differentiation through bone morphogenetic protein-2/p38 and extracellular signal-regulated kinase 1/2 pathway in human osteoblast cells. J. Pharmacol. Exp. Ther. 314, 1290–1299. doi: 10.1124/jpet.105.085092
Li, W., Xiao, Y., Wang, C., Dang, J., Chen, C., Gao, L., et al. (2013). A new species of Devriesia causing sooty blotch and flyspeck on rubber trees in China. Mycol. Prog. 12, 733–738. doi: 10.1007/s11557-012-0885-z
Liao, P. C., Chien, S. C., Ho, C. L., Wang, E. I. C., Lee, S. C., Kuo, Y. H., et al. (2010). Osthole regulates inflammatory mediator expression through modulating NF-κB, mitogen-activated protein kinases, protein kinase C, and reactive oxygen species. J. Agric. Food Chem. 58, 10445–10451. doi: 10.1021/jf102812t
Lin, H., Zhu, H., Tan, J., Wang, C., Dong, Q., Wu, F., et al. (2019). Comprehensive investigation on metabolites of wild-simulated American ginseng root based on ultra-high-performance liquid chromatography–quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 67, 5801–5819. doi: 10.1021/acs.jafc.9b01581
Lu, P., Jiang, K., Hao, Y. Q., Chu, W. Y., Xu, Y. D., Yang, J. Y., et al. (2021). Profiles of bacillus spp. isolated from the rhizosphere of Suaeda glauca and their potential to promote plant growth and suppress fungal phytopathogens. J. Microbiol. Biotechnol. 1, 1231–1240. doi: 10.4014/jmb.2105.05010
Lutz, M. C., Lopes, C. A., Sosa, M. C., and Sangorrin, M. P. (2020). Semi-commercial testing of regional yeasts selected from North Patagonia Argentina for the biocontrol of pear postharvest decays. Biol. Control 150:104246. doi: 10.1016/j.biocontrol.2020.104246
McGraw, J. B., Lubbers, A. E., Van der Voort, M., Mooney, E. H., Furedi, M. A., Souther, S., et al. (2013). Ecology and conservation of ginseng (Panax quinquefolius) in a changing world. Ann. N. Y. Acad. Sci. 1286, 62–91. doi: 10.1111/nyas.12032
Novick, K. A., Ficklin, D. L., Stoy, P. C., Williams, C. A., Bohrer, G., Oishi, A. C., et al. (2016). The increasing importance of atmospheric demand for ecosystem water and carbon fluxes. Nat. Clim. Chang. 6, 1023–1027. doi: 10.1038/nclimate3114
Ogawa, H., Sasai, N., Kamisako, T., and Baba, K. (2007). Effects of osthol on blood pressure and lipid metabolism in stroke-prone spontaneously hypertensive rats. J. Ethnopharmacol. 112, 26–31. doi: 10.1016/j.jep.2007.01.028
Pang, S., Piao, X., Zhang, X., Chen, X., Zhang, H., Jin, Y., et al. (2023). Discrimination for geographical origin of Panax quinquefolius L. using UPLC Q-Orbitrap MS-based metabolomics approach. Food Sci. Nutr. 11, 4843–4852. doi: 10.1002/fsn3.3461
Pini, F., Galardini, M., Bazzicalupo, M., and Mengoni, A. (2011). Plant-Bacteria association and Symbiosis: are there common genomic traits in Alphaproteobacteria? Genes 2, 1017–1032. doi: 10.3390/genes2041017
Qiao, J.-Q., Wu, H.-J., Huo, R., Gao, X.-W., and Borriss, R. (2014). Stimulation of plant growth and biocontrol by Bacillus amyloliquefaciens subsp. plantarum FZB42 engineered for improved action. Chem. Biol. Technol. Agric. 1, 1–14. doi: 10.1186/s40538-014-0012-2
Ren, Y., Xue, Y., Tian, D., Zhang, L., Xiao, G., and He, J. (2020). Improvement of postharvest anthracnose resistance in mango fruit by nitric oxide and the possible mechanisms involved. J. Agric. Food Chem. 68, 15460–15467. doi: 10.1021/acs.jafc.0c04270
Ren, Z., Lv, M., Li, T., Hao, M., Li, S., and Xu, H. (2020). Construction of oxime ester derivatives of osthole from Cnidium monnieri, and evaluation of their agricultural activities and control efficiency. Pest Manag. Sci. 76, 3560–3567. doi: 10.1002/ps.6056
Riaz, M., Rahman, N. U., Zia-Ul-Haq, M., Jaffar, H. Z., and Manea, R. (2019). Ginseng: a dietary supplement as immune-modulator in various diseases. Trends Food Sci. Technol. 83, 12–30. doi: 10.1016/j.tifs.2018.11.008
Rosa, S. B., Caverzan, A., Teixeira, F. K., Lazzarotto, F., Silveira, J. A., Ferreira-Silva, S. L., et al. (2010). Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71, 548–558. doi: 10.1016/j.phytochem.2010.01.003
Hu, X., Gu, T., Khan, I., Zada, A., and Jia, T. (2021). Research progress in the interconversion, turnover and degradation of chlorophyll. Cells 10:3134. doi: 10.3390/cells10113134
Rebitanim, N. A., Hanafi, M. M., Idris, A. S., Abdullah, S. N. A., Mohidin, H., and Rebitanim, N. Z. (2020). GanoCare® improves oil palm growth and resistance against Ganoderma basal stem rot disease in nursery and field trials. Biomed. Res. Int. 2020:3063710.
Rodriguez-Concepcion, M., and Daròs, J.-A. (2022). Transient expression systems to rewire plant carotenoid metabolism. Curr. Opin. Plant Biol. 66:102190. doi: 10.1016/j.pbi.2022.102190
Saleh, A. M., Madany, M. M., and González, L. (2015). The effect of coumarin application on early growth and some physiological parameters in faba bean (Vicia faba L.). J. Plant Growth Regul. 34, 233–241. doi: 10.1007/s00344-014-9459-4
Sheban, K. C., Woodbury, D. J., and Duguid, M. C. (2022). Importance of environmental factors on plantings of wild-simulated American ginseng. Agrofor. Syst. 96, 147–160. doi: 10.1007/s10457-021-00705-8
Shokoohinia, Y., Jafari, F., Mohammadi, Z., Bazvandi, L., Hosseinzadeh, L., Chow, N., et al. (2018). Potential anticancer properties of osthol: a comprehensive mechanistic review. Nutrients 10:36. doi: 10.3390/nu10010036
Sun, X., Lyu, G., Luan, Y., Zhao, Z., Yang, H., and Su, D. (2018). Analyses of microbial community of naturally homemade soybean pastes in Liaoning Province of China by Illumina Miseq sequencing. Food Res. Int. 111, 50–57. doi: 10.1016/j.foodres.2018.05.006
Tan, S., Zhou, F., Li, N., Dong, Q., Zhang, X., Ye, X., et al. (2013). Anti-fatigue effect of ginsenoside Rb1 on postoperative fatigue syndrome induced by major small intestinal resection in rat. Biol. Pharm. Bull. 36, 1634–1639. doi: 10.1248/bpb.b13-00522
Tian, L., Shi, S., Ji, L., Nasir, F., Ma, L., and Tian, C. (2018). Effect of the biocontrol bacterium Bacillus amyloliquefaciens on the rhizosphere in ginseng plantings. Int. Microbiol. 21, 153–162. doi: 10.1007/s10123-018-0015-0
Tsai, Y.-F., Yu, H.-P., Chung, P.-J., Leu, Y.-L., Kuo, L.-M., Chen, C.-Y., et al. (2015). Osthol attenuates neutrophilic oxidative stress and hemorrhagic shock-induced lung injury via inhibition of phosphodiesterase 4. Free Radic. Biol. Med. 89, 387–400. doi: 10.1016/j.freeradbiomed.2015.08.008
Vinci, G., Cozzolino, V., Mazzei, P., Monda, H., Savy, D., Drosos, M., et al. (2018). Effects of Bacillus amyloliquefaciens and different phosphorus sources on maize plants as revealed by NMR and GC-MS based metabolomics. Plant Soil 429, 437–450. doi: 10.1007/s11104-018-3701-y
Wang, D.-C., Jiang, C.-H., Zhang, L.-N., Chen, L., Zhang, X.-Y., and Guo, J.-H. (2019). Biofilms positively contribute to Bacillus amyloliquefaciens 54-induced drought tolerance in tomato plants. Int. J. Mol. Sci. 20:6271. doi: 10.3390/ijms20246271
Wang, Y., Choi, H.-K., Brinckmann, J. A., Jiang, X., and Huang, L. (2015). Chemical analysis of Panax quinquefolius (north American ginseng): a review. J. Chromatogr. A 1426, 1–15. doi: 10.1016/j.chroma.2015.11.012
Wang, Y., Wang, L., Suo, M., Qiu, Z., Wu, H., Zhao, M., et al. (2022). Regulating root fungal community using Mortierella alpina for fusarium oxysporum resistance in Panax ginseng. Front. Microbiol. 13:850917. doi: 10.3389/fmicb.2022.850917
Wu, L., Wang, X., Xu, W., Farzaneh, F., and Xu, R. (2009). The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 16, 4236–4260. doi: 10.2174/092986709789578187
Zhang, R.-R., Liu, J., Zhang, Y., Hou, M.-Q., Zhang, M.-Z., Zhou, F., et al. (2016). Microwave-assisted synthesis and antifungal activity of novel coumarin derivatives: Pyrano [3, 2-c] chromene-2, 5-diones. Eur. J. Med. Chem. 116, 76–83. doi: 10.1016/j.ejmech.2016.03.069
Keywords: osthole, Bacillus amyloliquefaciens, Panax quinquefolius, microbiome, resistance
Citation: Jiang J, Fan G, Wen R, Liu T, He S, Yang S and Zi S (2024) Effects of osthole and Bacillus amyloliquefaciens on the physiological growth of Panax quinquefolius in a forest. Front. Microbiol. 15:1497987. doi: 10.3389/fmicb.2024.1497987
Received: 18 September 2024; Accepted: 20 November 2024;
Published: 12 December 2024.
Edited by:
Prem Lal Kashyap, Indian Institute of Wheat and Barley Research (ICAR), IndiaReviewed by:
Swarnalee Dutta, Jeonbuk National University, Republic of KoreaCopyright © 2024 Jiang, Fan, Wen, Liu, He, Yang and Zi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Wen, MjI1NzQzODM2NUBxcS5jb20=; Tao Liu, NTIxMzM0OTBAcXEuY29t; Shuran He, c2h1cmFuQHluYXUuZWR1LmNu; Shengchao Yang, c2hlbmdjaGFveWFuZ0AxNjMuY29t; Shuhui Zi, MjU4NDM3ODg5QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.