- 1Key Laboratory of Tropical Medicinal Resource Chemistry of Ministry of Education, College of Chemistry and Chemical Engineering, Hainan Normal University, Haikou, China
- 2Key Laboratory of Tropical Medicinal Plant Chemistry of Hainan Province, Haikou, China
Bacterial infections pose a significant clinical burden on global health. The growing incidence of drug-resistant pathogens highlights the critical necessity to identify and isolate bioactive compounds from marine resources. Marine-derived fungi could provide novel lead compounds against pathogenic bacteria. Due to the particularity of the marine environment, Aspergillus species derived from marine sources have proven to be potent producers of bioactive secondary metabolites and have played a considerable role in advancing drug development. This study reviews the structural diversity and activities against pathogenic bacteria of secondary metabolites isolated from marine-derived Aspergillus species over the past 14 years (January 2010–June 2024), and 337 natural products (including 145 new compounds) were described. The structures were divided into five major categories—terpenoids, nitrogen-containing compounds, polyketides, steroids, and other classes. These antimicrobial metabolites will offer lead compounds to the development and innovation of antimicrobial agents.
1 Introduction
Bacterial infections pose a significant clinical burden on global health (Xuan et al., 2023; Wallis et al., 2023). An estimated 7.7 million deaths are attributed to bacterial infections each year (Okeke et al., 2024; Ikuta et al., 2022). For example, Staphylococcus aureus, a frequent colonizer of the human population and one of the foremost opportunistic bacterial pathogens of humans, was associated with more than 1 million deaths in 2019. Staphylococcus aureus caused significant morbidity and mortality globally (Howden et al., 2023). Additionally, four additional pathogens (Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa) were also associated with more than 0.5 million deaths each in 2019 (Ikuta et al., 2022). Deaths related to bacteria would rank as the second leading cause of death globally. Furthermore, antimicrobial resistance (AMR) remains a global threat. AMR posed a significant global public health threat owing to the rapid global acceleration of resistance in microorganisms. This trend limited the effectiveness of preventing and treating infections caused by viruses, bacteria, and parasites (Charani et al., 2023; Haenni et al., 2022; de Alcântara Rodrigues et al., 2020). A global surveillance report by the World Health Organization (WHO) identified the severe economic effects of AMR (de Alcântara Rodrigues et al., 2020). For instance, the estimated annual expense for the US healthcare system alone ranges from $21 to $34 billion. Beyond the health sector, AMR was projected to cause a decline in actual gross domestic product (GDP) of 0.4 to 1.6% (Gow et al., 2022; Jin et al., 2023). Consequently, the lack of new antimicrobial drugs to replace those that become ineffective underscored the urgent need to preserve the efficacy of existing drugs (Prestinaci et al., 2015). The increasing challenge of AMR highlighted the importance of marine microbial resources as crucial assets in developing new antimicrobial drugs (Alahmari et al., 2022; Carroll et al., 2024). Marine microorganisms, through long-term adaptation to extreme environments, have evolved unique metabolic pathways capable of synthesizing various structurally diverse antimicrobial compounds (Pinedo-Rivilla et al., 2022; Hai et al., 2021), such as marine sponge-derived terpenoid 13-(E)-geoditin A (Chen B. et al., 2022), marine coral-derived steroid lobocaloid B (Zhu et al., 2024), ascidian lactone prunolide C (Holland et al., 2022), mangrove sediments polyketone stemphone C (Cai et al., 2023). Thus, marine microorganism resources emerged as an essential source of structurally novel and antimicrobial natural products (Jeewon et al., 2023; Yurchenko et al., 2021; Han et al., 2023; Xu et al., 2022).
Genus Aspergillus has been considered one of the most significant general fungi, and representatives have been found in almost all aerobic environments, such as plants, soil, marine life, and submarine sediments (Ibrahim et al., 2023; Sun et al., 2022). Several metabolites of Aspergillus have been proven to possess valuable activities, such as aspergillomarasmine A from Aspergillus versicolor surmount metallo-β-lactamase antibiotic resistance, and Simvastatin, from Aspergillus terreus with a critical blood-lipid-lowering medicine, as a potential drug against S. aureus biofilm (King et al., 2014; Graziano et al., 2015). Furthermore, marine-derived Aspergillus fungi, which lived the diverse and hostile environments, produced a variety of structurally novel and antibacterial chemical compounds, and a significant proportion of these compounds were secondary metabolites with antimicrobial activity (Orfali et al., 2021; Li H. H. et al., 2023; Wang and Ding, 2018; Lee et al., 2013), such as marine-derived fungus Aspergillus ustus polyketone stromemycin B (Xue et al., 2024), marine gorgonian-derived fungus Aspergillus sclerotiorum alkaloid sclerotiamide L (Meng et al., 2022), marine coral-derived fungus Aspergillus hiratsukae terpene chevalone H (Chen X. Y. et al., 2022), marine sediment-derived fungus A. terreus lactone butyrolactone I (Bao et al., 2021). Moreover, a series of outstanding reviews on marine-derived Aspergillus fungi has been published. In 2013, Lee et al. reviewed the bioactive secondary metabolites of Aspergillus derived from marine sources. In 2018, Wang et al. conducted a review of 232 new bioactive metabolites of Aspergillus in the marine environment from 2006 to 2016 and categorized their bioactivity and chemical structures (Wang and Ding, 2018). In 2020, Xu et al. summarized the structural diversity and biological activity of 130 heterocyclic alkaloids produced by Aspergillus of marine origin from 2014 to 2018 (Xu K. et al., 2020). In 2021, Orfali et al. highlight secondary metabolites from various marine-derived Aspergillus species reported between 2015 and 2020 along with their biological potential and structural aspects whenever applicable (Orfali et al., 2021). In 2023, Li et al. summarized the antimicrobial compounds from marine Aspergillus from January 2021 to March 2023 (Li H. H. et al., 2023). However, no studies have been carried out on the antimicrobial compounds from marine Aspergillus from 2010 to 2024. It is believed that the study of Aspergillus living in marine environments will facilitate the discovery of drug lead compounds. Consequently, this review discussed the antibacterial substances derived from Aspergillus species in the marine environment from January 2010 to June 2024. A total of 117 cited references were presented in the review. It comprehensively covered the chemical diversity and antimicrobial properties of 337 reported compounds, including 145 new compounds isolated from marine-derived Aspergillus fungi. These compounds were structurally categorized into terpenoids (32 compounds), nitrogen-containing compounds (98 compounds), polyketides (139 compounds), steroids (18 compounds), and other compounds (50 compounds). Some potential compounds’ relevant biological and pharmacological activities are also highlighted, which will benefit future drug development and innovation. Notably, some antimicrobial compounds against human pathogenic bacteria produced by Aspergillus fungi also showed activities against agriculture and fish pathogenic bacteria and so on (Zhang et al., 2024; Xue et al., 2024), which might be suggested as one of the probable candidate drugs for “One Health” in the utilization in healthcare, agriculture, and fishery.
2 Structural and antibacterial activity studies
2.1 Terpenoids
Terpenoids were generally composed of structural units derived from isoprene or isopentane. A total of 32 antibacterial terpenoids (including 13 new compounds) were found in the marine-derived fungal genus Aspergillus sp., comprising 18 sesquiterpenes, four diterpenes, and 10 triterpenoids. The structures and the absolute configurations of the new compounds and novel skeleton compounds were elucidated by a detailed spectroscopic analysis of nuclear magnetic resonance (NMR) spectroscopy and mass spectrometry (MS) data, electronic circular dichroism (ECD) calculations, and single-crystal X-ray diffraction.
2.1.1 Sesquiterpenes
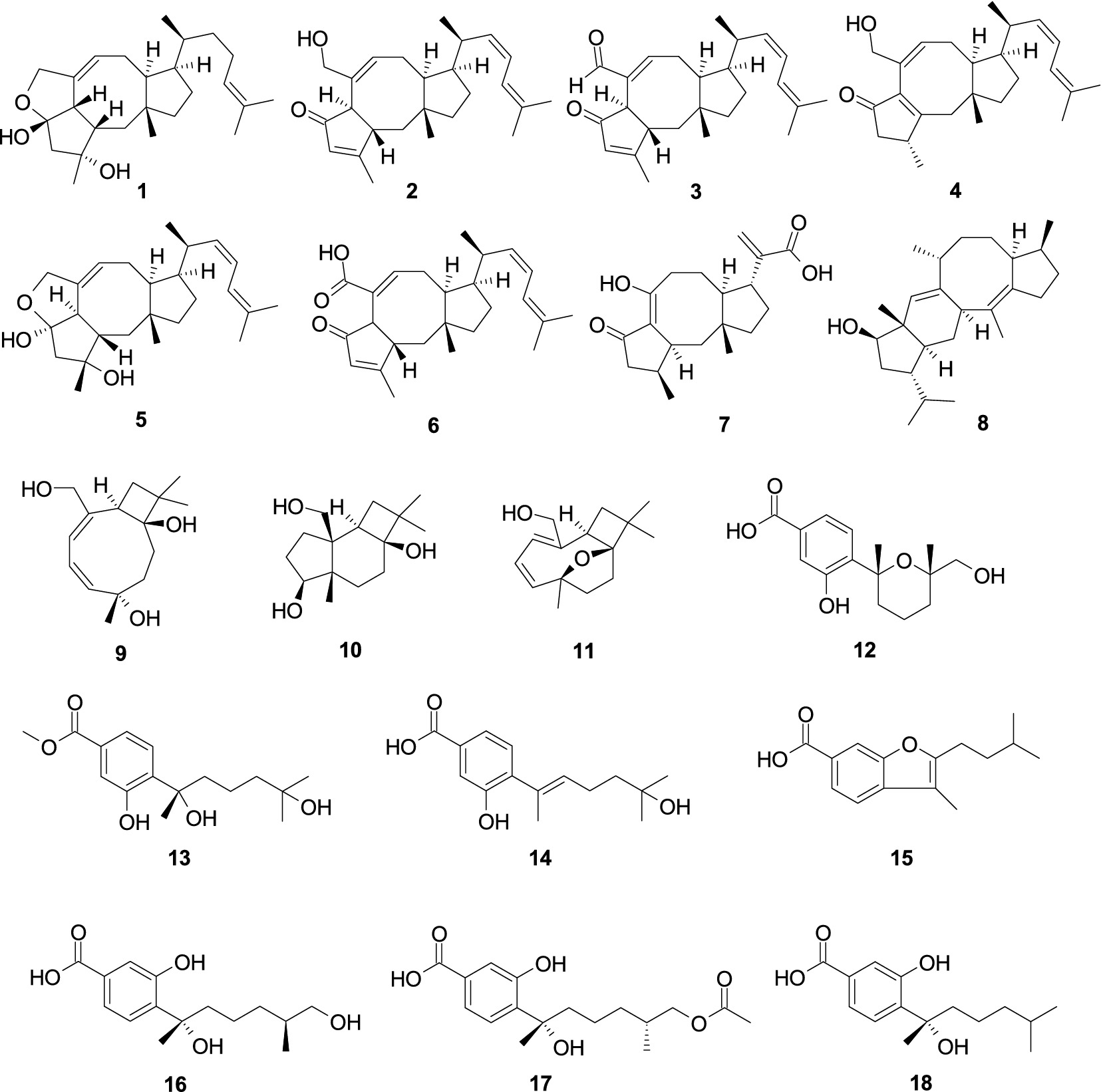
One new ophiobolin sesterterpenoid, (5S,6S)-16,17-dihydroophiobolin H (1), together with two known analogs, (6α)-21,21-O-dihydroophiobolin G (2) and 6-epi-ophiobolin G (3), were isolated from the cold-seep-derived fungus A. insuetus SD-512 (Chi et al., 2020). Compound 1–3 exhibited broad-spectrum antibacterial efficacy against eight tested bacterial strains (Escherichia coli, P. aeruginosa, Aeromonas hydrophilia, Edwardsiella tarda, Vibrio alginolyticus, Vibrio anguillarum, Vibrio Parahemolyticus, and Vibrio vulnificus) with the minimum inhibitory concentration (MIC) values from 4.0 to 32.0 μg/mL. A novel ophiobolin sesterterpenoid ophiobolin U (4) and a known analog (5ɑ,6ɑ)-ophiobolin H (5) were obtained from alga-derived fungus A. ustus cf-42 (Liu et al., 2013). Compounds 4–5 showed inhibitory effects against E. coli, demonstrating inhibition zones of 15.0 and 10.0 mm at a concentration of 30 μg/disk, respectively. Asperophiobolin E (6) was obtained from the coral-derived fungus A. hiratsukae SCSIO 5Bn1003 (Zeng et al., 2022a). Compound 6 demonstrated strong antibacterial efficacy against Bacillus subtilis (MIC, 17.0 μg/mL), which exhibited weak activity against S. aureus, with the MIC value of 102.86 μg/mL. One new sesterterpenoid, asperbrunneo acid (7), was obtained from the marine-derived fungus Aspergillus brunneoviolaceus MF180246 (Xu et al., 2024). Compound 7 showed weak antibacterial efficacy against S. aureus with the MIC value of 200 μg/mL. Aspergilol C (8) was obtained from the marine-derived fungus Aspergillus sp. ZZ1861 (Ha et al., 2024). Compound 8 exhibited potent antibacterial activity against E. coli, with the MIC value of 6.25 μg/mL. Punctaporonins B (9), D (10), and G (11), were obtained from the fungus A. terreus SCSIO 41202 (Zhang et al., 2024). Compounds 9–11 showed a strong antibacterial effect against Xanthomonas citri subsp. citri with the MIC values of 0.625, 0.625, and 0.3125 mg/mL, respectively. One novel bisabolene-type sesquiterpenoid, 12-hydroxysydowic acid (12), along with two known analogs, aspergoterpenin C (13) and engyodontiumone I (14), were extracted from the fungus A. versicolor SD-330 (Li et al., 2021). Compounds 12–14 exhibited selective inhibitory activity against A. hydrophilia, E. coli, E. tarda, and Vibrio harveyi, with the MIC values ranging 1.0–8.0 μg/mL. Aspergillusene B (15), (7S,11S)-(+)-12-hydroxysydonic acid (16), expansol G (17), and (S)-sydonic acid (18), were isolated from the fungus Aspergillus. sydowii LW09 (Yang et al., 2023). Compounds 15, 17, and 18 demonstrated weak antibacterial efficacy against Ralstonia solanacarum (the same MIC, 32.0 μg/mL). Compound 16 demonstrated weak antibacterial activity against P. syringae, exhibiting the MIC value of 32.0 μg/mL (Figure 1).
2.1.2 Diterpenoids
A new tetranorlabdane diterpenoid asperolide D (19), along with one known analog asperolide A (20), was isolated from the fungus Aspergillus wentii SD-310 (Li et al., 2016). Compounds 19 and 20 exhibited antibacterial activity against E. tarda, with the same MIC value of 16.0 μg/mL. Two pimarane diterpenes, sphaeropsidin A (21) and aspergiloid E (22), were obtained from the algal-derived fungus Aspergillus porosus G23 (Neuhaus et al., 2019). Compounds 21 and 22 showed activity against S. aureus ATCC 25923 and ATCC BAA-41, with the MIC values ranging 32.6–77.8 μM (Figure 2).
2.1.3 Meroterpenoids
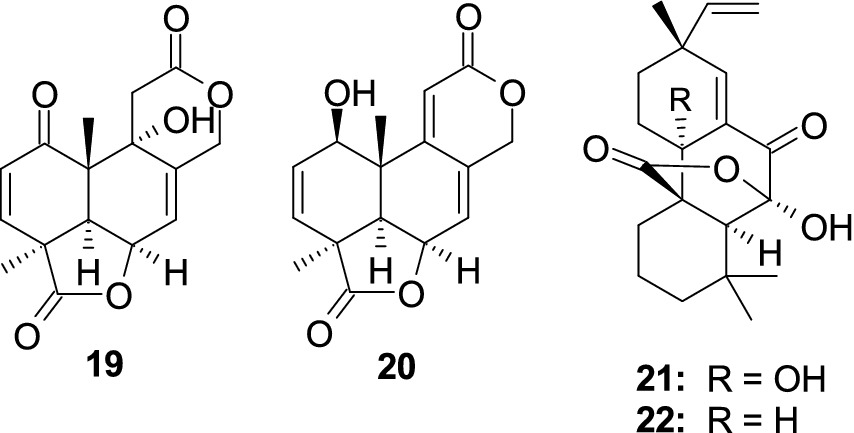
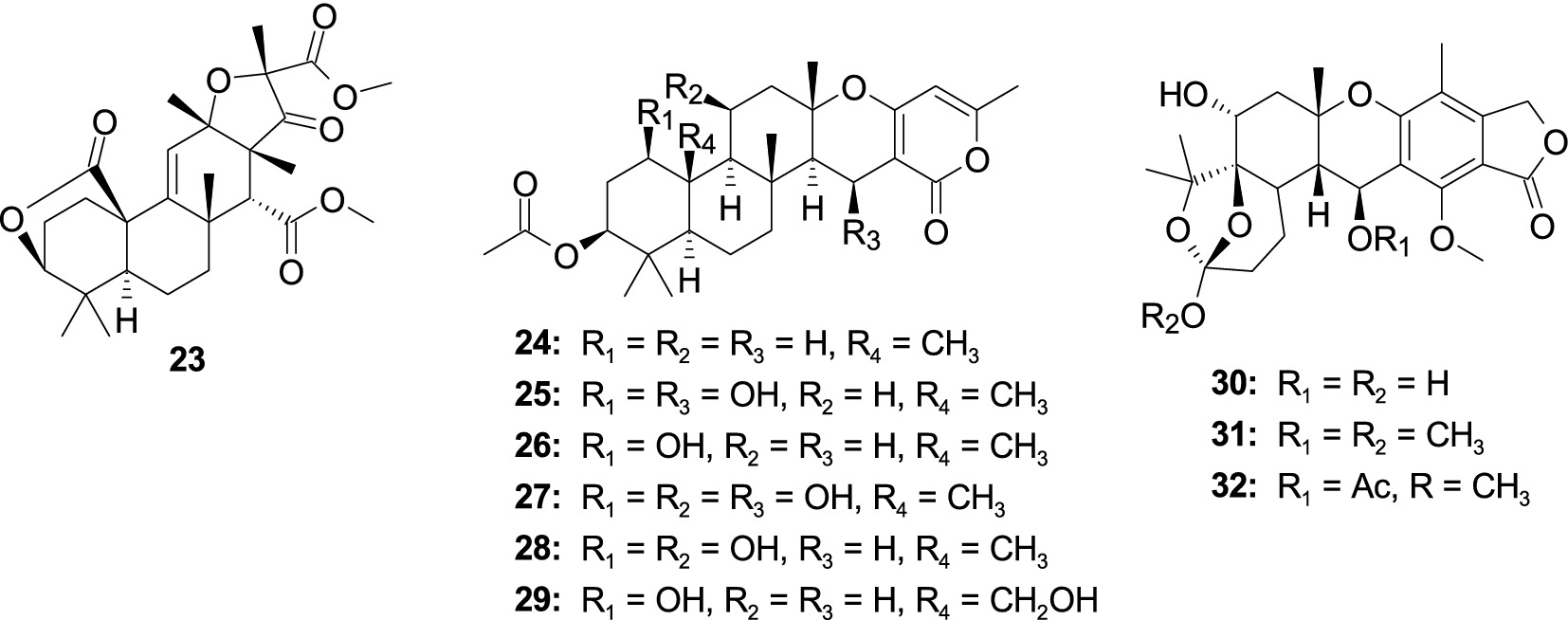
A new 3,5-dimethylor-sellinic acid-based meroterpenoid, aspergillactone (23), from the marine-derived fungus Aspergillus sp. CSYZ-1 (Cen et al., 2021), exhibited potent antimicrobial activity against Helicobacter pylori (ATCC 43504, G27, Hp159, and BY583) and S. aureus (ATCC 25923, USA300, BKS231, BKS233) with the MIC values of 1.0–4.0 and 2.0–16.0 μg/mL. A new meroterpenoid, chevalone B (24), was obtained from the marine-derived fungus Aspergillus sp. H30 (Hu et al., 2019). Compound 24 showed weak antimicrobial activity against S. aureus with the MIC value of 50 μg/mL. Five new α-pyrone meroterpenoids, chevalones H–L (25–29), isolated from the gorgonian-derived fungus A. hiratsukae SCSIO 7S2001 (Chen X. Y. et al., 2022), showed antibacterial activities against Micrococcus lutea, K. pneumoniae, methicillin-resistant Staphylococcus aureus (MRSA) and Streptococcus faecalis, with the MIC values of 6.25–100 μg/mL. A new meroterpenoid, austalide R (30), and two known compounds, austalides M (31) and N (32), were isolated from the sponge-derived fungus Aspergillus sp. (Zhou et al., 2014). Compounds 30 and 31 displayed broad-spectrum inhibitory activity against eight tested strains (Halomonas aquamarine, Pseudoalteromonas elyakovii, V. harveyi, Roseobacter litoralis, Polaribacter irgensii, and Shewanella putrefaciens) with the MIC values range from 0.01 to 0.1 μg/mL, whereas 32 displayed inhibitory activity against V. natriegens and R. litoralis with the same MIC value of 0.01 μg/mL (Figure 3).
2.2 Nitrogen-containing compounds
Nitrogenous secondary metabolites were ubiquitous in nature with a wide range of biological activities. A total of 98 nitrogen-containing antimicrobial compounds (including 53 new compounds) were discovered from the genus Aspergillus sp., including 39 indole alkaloids, 11 quinazolinone alkaloids, four cytochalasan alkaloids, 13 peptides, and 31 other nitrogen-containing metabolites. The structures and the absolute configurations of the new compounds and novel skeleton compounds were elucidated by a detailed spectroscopic analysis of NMR and MS data, ECD calculations, and single-crystal X-ray diffraction. The absolute configurations of the amino acid residues of the peptides were determined by Marfey’s method.
2.2.1 Indole alkaloids
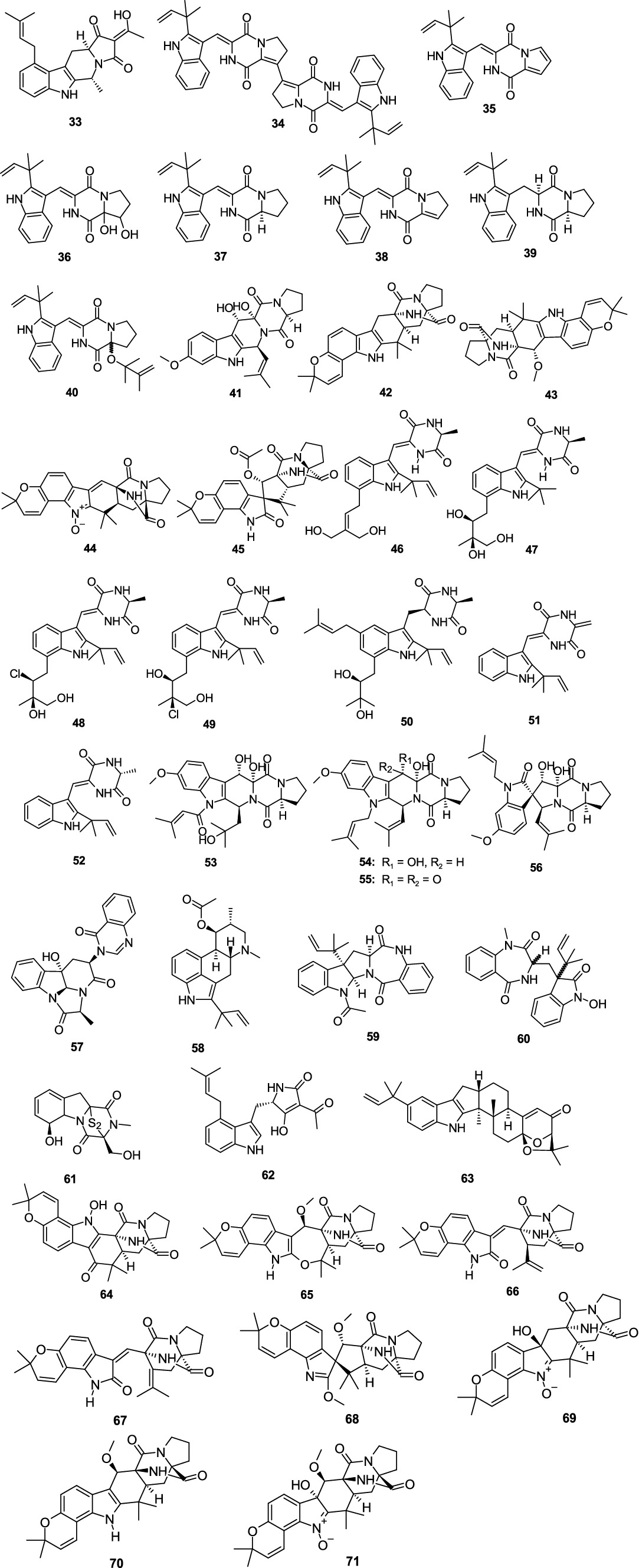
Griseofamine A (33), isolated from the deep-sea derived fungus Aspergillus sp. SCSIO 41024 (Chen et al., 2020), exhibited weak antibacterial activity against E. coli with the MIC value of 64.0 μg/mL. Four new indole alkaloids brevianamides S–V (34–37), together with two known analogs brevianamide K (38) and deoxybrevianamide E (39), were isolated from the fungus A. versicolor MF030 (Song F. H. et al., 2021). Compounds 34–39 displayed antibacterial effects against Bacille Calmette-Guérin (BCG), with the MIC values of 6.25, 50, 25, 100, 50, and 100 μg/mL, respectively. Compound 39 also showed antibacterial effects against S. aureus and B. subtilis with the MIC values of 100 and 50 μg/mL, respectively. A new alkaloid, 9ξ-O-2(2,3-dimethylbut-3-enyl)brevianamide Q (40), was isolated from the alga-derived fungus A versicolor pt20 (Miao et al., 2012). Compound 40 exhibited a weak inhibitory effect on E. coli and S. aureus, with the same inhibition zone of 7.0 mm at a disk concentration of 30 μg/mL, respectively. 12,13-Dihydroxy-fumitremorgin C (41), separated from the fungus Aspergillus sp. SCSIO Ind09F01 demonstrated potent inhibitory activity against Mycobacterium tuberculosis, with the MIC value of 2.41 μM (Luo et al., 2017). (−)-stephacidin A (42) was separated from a gorgonian-derived fungus Aspergillus sp. XS-20090066 revealed a selective antibacterial effect against Staphylococcus epidermidis (MIC, 14.5 μM) (Chen et al., 2013). Notoamide F (43) was obtained from the fungus A. sclerotiorum GDST-2013-0501 (Wang C. Y. et al., 2022). Compound 43 exhibited a moderate antibacterial effect against S. epidermidis, with the MIC value of 12.5 μM. Two new indole alkaloids, asperthrins A (44) and E (45), were obtained from the fungus Aspergillus sp. YJ191021 (Yang et al., 2021). Compound 44 displayed antibacterial effects against E. tarda, V. anguillarum, A. hydrophilia and Vibrio parahaemolyticus (MIC, 16, 8, 32, and 16 μg/mL, respectively). Compound 45 displayed an inhibitory effect against Rhizoctonia solani with the MIC value of 25 μg/mL. Five new indole alkaloids, 24,25-dihydroxyvariecolorin G (46), 25-hydroxy-rubrumazine B (47), 22-chloro-25-hydroxyrubrumazine B (48), 25-hydroxy-variecolorin F (49), and 27-epi-aspechinulin D (50), along with the known analog neoechinulin B (51) were isolated from the fungus Aspergillus Chevalieri CS-122 (Yan et al., 2023). Compound 46 displayed significant inhibitory activity against E. coli (MIC, 4.0 μg/mL), while compound 48 displayed an inhibitory effect against Vibrio harveyi (MIC, 8.0 μg/mL). Moreover, compounds 47 and 50 exhibited broad-spectrum antibacterial effects against five evaluated bacterial strains (V. harveyi, E. tarda, Aeromonas hydrophila, E. coli, and Micrococcus luteus) with the MIC values ranging 16.0–32.0 μg/mL. Compound 51 showed significant activities against A. hydrophila (MIC, 4.0 μg/mL) and E. coli (MIC, 8.0 μg/mL). A known compound, neoechinulin A (52), was separated from the coral-derived fungus A. hiratsukae SCSIO 7S2001 (Chen X. Y. et al., 2022). Compound 52 showed weak antibacterial activities against K. pneumoniae and S. faecalis with MIC values of 50.0 and 12.5 μg/mL, respectively. Compound 52 also had an antibacterial effect against H. pylori Hp159 with the MIC value of 16 μg/mL (Yu et al., 2022). Asperfumigatin (53), 12,13-dihydroxyfumitremorgin C (41), fumitremorgin B (54), 13-oxofumitremorgin B (55), spirotryprostatin C (56), (−)-chaetominine (57), and fumigaclavine C (58) were isolated from the fungus Aspergillus fumigatus H22 (Zhang R. et al., 2022). Compounds 41 and 53–58 showed antibacterial activity against MRSA, with the MIC values from 1.25 to 25.0 μM. Epi-aszonalenin A (59) were isolated from the fungus A. fumigatus SCSIO 41012 (Limbadri et al., 2018). Compound 59 displayed antibacterial effect against A. baumanii ATCC19606 (MIC, 50 μg/mL) and ATCC 15122 (MIC, 6.25 μg/mL). A new tryptophan-derived alkaloid, 3-((1-hydroxy-3-(2-methylbut-3-en-2-yl)-2-oxoindolin-3-yl)methyl)-1-methyl-3,4-dihydrobenzo[e]-[1,4]-diazepine-2,5-dione (60), was separated from the sponge-associated fungus Aspergillus sp. (Zhou et al., 2014). Compound 60 selectively inhibited V. harveyi and Vibrio natriegens, with the same MIC value of 1.0 μg/mL. Gliotoxin (61), separated from the fungus Aspergillus sp. SCSIO Ind09F01, strongly inhibited M. tuberculosis (MIC, 0.03 μM) (Luo et al., 2017). β-Cyclopiazonic acid (62), isolated from sponge-derived fungus Aspergillus felis FM324, showed antibacterial effects on S. aureus, MRSA, and B. subtilis—all exhibiting the same MIC value of 59.2 μM (Wang et al., 2021). One new indole-diterpenoid, (2R,4bR,6aS,12bS,12cS,14aS)-4b-deoxy-β-aflatrem (63), was isolated from the marine-derived fungus Aspergillus flavus OUCMDZ-2205 (Sun et al., 2014). Compound 63 exhibited antibacterial activity against S. aureus with the MIC value of 20.5 μM. Eight new notoamide-type alkaloids, sclerotiamides K–R (64–71), were isolated from a marine gorgonian-derived fungus A. sclerotiorum LZDX-33-4 (Meng et al., 2022). Compounds 64–71 showed antibacterial activity against S. aureus ATCC29213 with MIC values ranging 4–64 μM (Figure 4).
2.2.2 Quinazolinone alkaloids
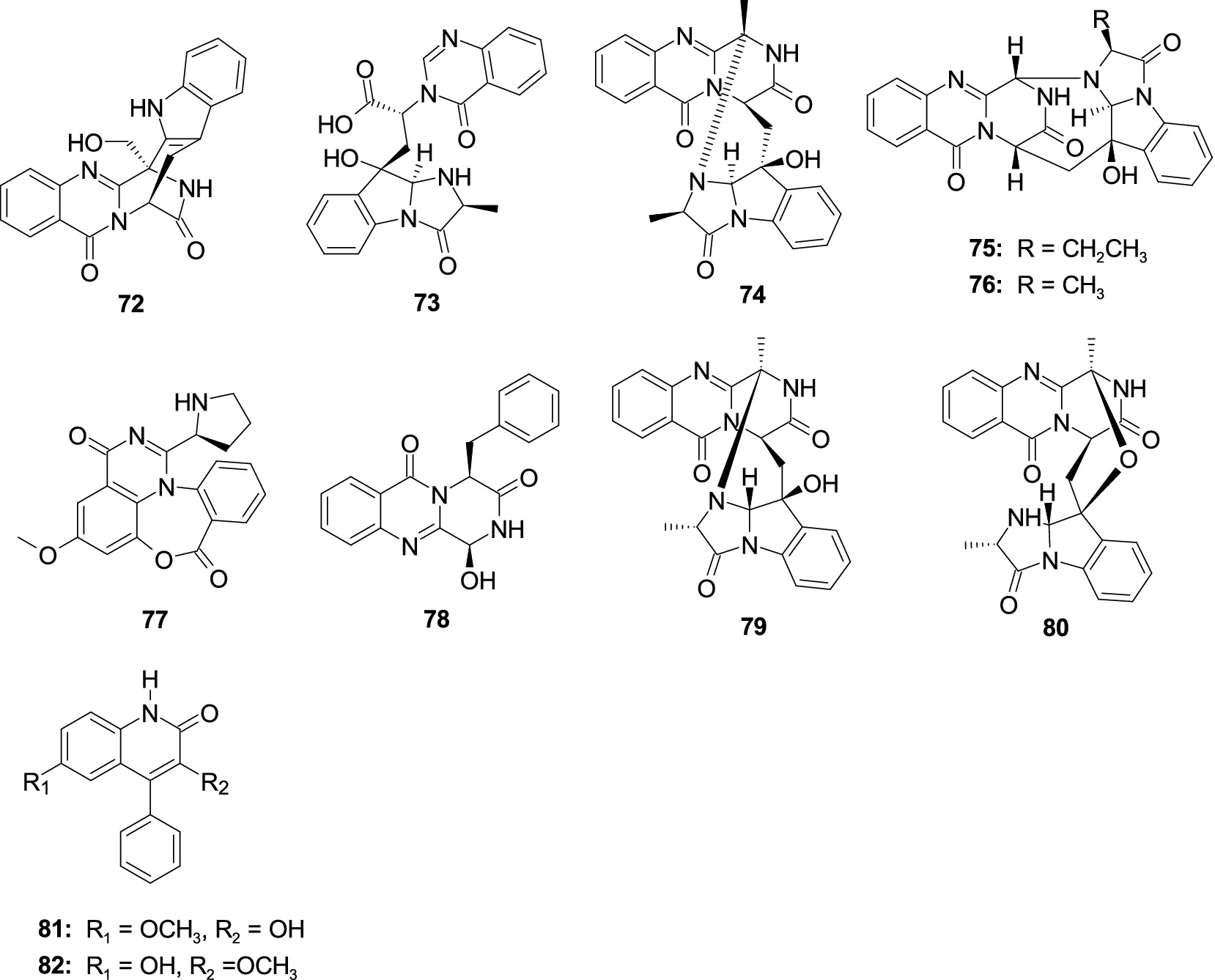
Two novel alkaloids fumigatosides E–F (72–73), along with a known alkaloid fumiquinazoline G (74), were isolated from A. fumigatus SCSIO 41012 (Limbadri et al., 2018). Compound 72 showed activities against Acinetobacter baumanii ATCC 19606, A. baumanii ATCC 15122, S. aureus ATCC 16339, and K. pneumonia ATCC 14578 with the MIC values of 12.5, 6.25, 6.25, and 12.5 μg/mL, respectively. Compound 73 exhibited activity against A. baumanii ATCC 19606 with the MIC value of 6.25 μg/mL. Compound 73 exhibited significant activity against S. aureus ATCC16339 and 29,213, (MIC, 1.56 and 0.78 μg/mL). Compound 74 showed activities against A. baumanii ATCC 15122, S. aureus ATCC 16339, S. aureus ATCC29213, and K. pneumonia ATCC 14578 with the MIC values of 6.25, 12.5, 12.5, and 25 μg/mL, respectively. One new alkaloid cottoquinazoline H (75) and a known analog cottoquinazoline A (76) were separated from the coral-associated fungus A. versicolor AS-212 (Dong et al., 2023a). Compound 75 showed potent inhibitory effects against the aquatic pathogenic bacterium Vibrio harvryi (MIC, 18.1 μM) and V. parahemolyticus (MIC, 9.0 μM). Compound 76 exhibited moderate activity against A. hydrophila with an MIC value of 18.6 μM. Compound 76 also showed strong antibacterial effect against E. coli with the MIC value of 5.0 μM (Zhang L. et al., 2020; Zhang Y. H. et al., 2020). A new alkaloid, aspergicin (77), was separated from the mixed cultivation of two mangrove-associated mangrove fungi Aspergillus sp. (Zhu et al., 2011). Compound 77 exhibited a moderate antibacterial effect against B. subtilis and B. dysenteriae, with consistent MIC values of 15.6 μg/mL. Brevianamide M (70) was separated from the alga-associated fungus A. versicolor pt20 (Miao et al., 2012). Compound 78 exhibited antibacterial activity against E. coli and S. aureus, with inhibition zones of 11.0 and 10.0 mm observed at a concentration of 30 μg/disk, respectively. Fumiquinazolines D (79) and C (80), were separated from the sea cucumber-associated fungus A. fumigatus M580 (Tuan et al., 2022). Compounds 79 and 80 exhibited antibacterial activity against Gram-positive Enterococcus faecalis with the same MIC value of 32.0 μg/mL. 3-Hydroxy-6-methoxy-4-phenylquinolin-2(1H)-one (81) and 3-methoxy-6-hydroxy-4-phenylquinolin-2(1H)-one (82) were separated from a coral-derived fungus A. versicolor AS-212 (Dong et al., 2023b). Compounds 81 and 82 demonstrated an antibacterial effect against aquatic pathogenic bacteria V. harveyi and V. alginolyticus, with the MIC values from 8 to 32 μg/mL (Figure 5).
2.2.3 Cytochalasan alkaloids
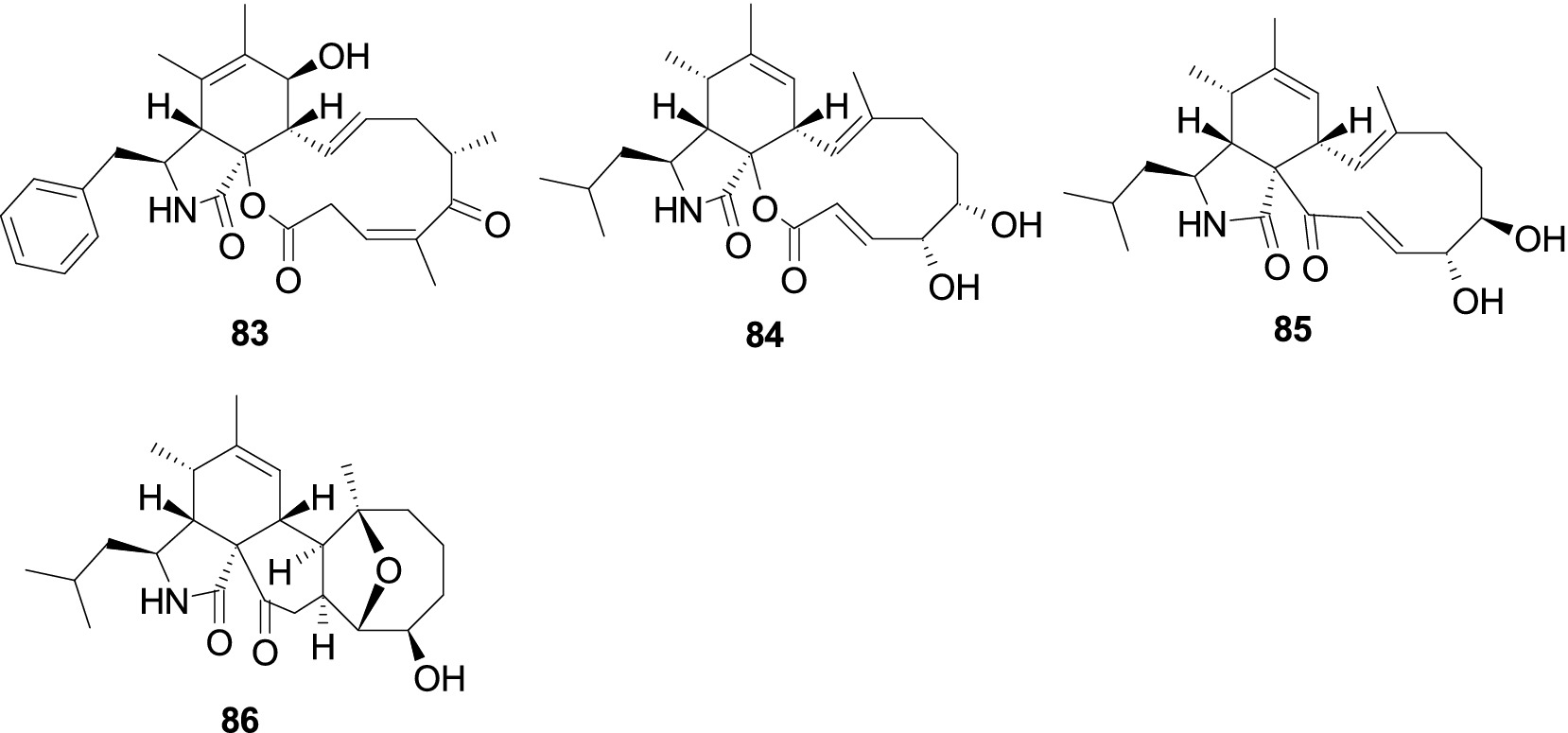
Cytochalasin Z17 (83) was isolated from the sponge-derived fungus Aspergillus sp., and it showed selective and pronounced activity effect R. litoralis with the MIC value of 0.0001 μg/mL (Zhou et al., 2014). Aspochalasins I (84), D (85), and PZ (86), were separated from the coral-associated fungus Aspergillus elegans (Zheng et al., 2013). Compound 84 showed moderate antibacterial activity against S. epidermidis (MIC, 20 μM) and S. aureus (MIC, 10 μM). Compound 85 exhibited extensive antibacterial effects against four pathogenic bacteria (S. albus, S. aureus, E. coli, and Bacillus cereus) with a consistent MIC value of 10 μM. Compound 86 displayed an antibacterial effect against S. epidermidis with the same MIC value of 20 μM (Figure 6).
2.2.4 Peptides
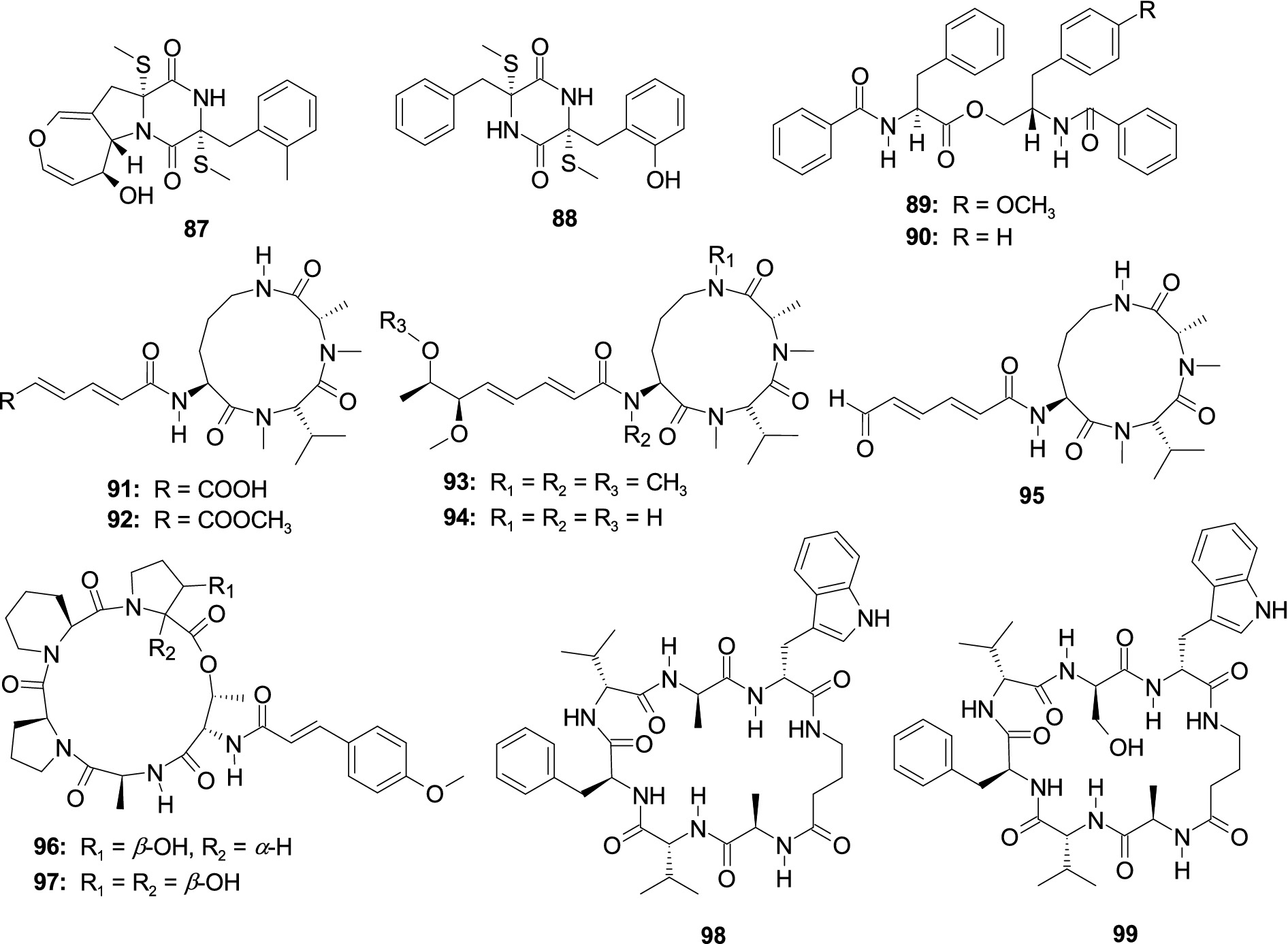
One novel thiodiketopiperazine, emestrin M (87), and a known monomer compound, emethacin C (88), were separated from the fungus A. terreus RA2905 (Wu et al., 2020a). Compounds 87 and 88 displayed antibacterial activity against P. aeruginosa ATCC 27853 with the MIC values of 64 and 32 μg/mL, respectively. One novel phenylalanine derivative 4′-OMe-asperphenamate (89) and another known phenylalanine derivative asperphenamate (90) were separated from the coral-associated fungus A. elegans ZJ-2008010 (Zheng et al., 2013). Compounds 89 and 90 showed an antibacterial effect against S. epidermidis with the same MIC value of 10.0 μM. Three novel aspochracin-type cyclic tripeptides, sclerotiotides M–O (91–93), together with two previously identified analogs, sclerotiotides L (94) and F (95), were originated from the fungus Aspergillu insulicola HDN151418 (Sun et al., 2020). Compounds 91 and 92 dispalyed a broad antibacterial effect on eight pathogenic strains (B. cereus, Proteusspecies, Mycobacterium phlei, B. subtilis, V. parahemolyticus, E. tarda, MRCNS, and MRSA) with the MIC values ranging 1.56–25.0 μM. Compound 93 showed an antibacterial effect on E. tarda and V. parahemolyticus with consistent MIC values of 25 μM. Compounds 94 and 95 showed antibacterial activity effects on four bacterial strains (B. cereus, Proteus species, E. tarda, and V. parahemolyticus) with consistent MIC values of 25 μM. Two new pentadepsipeptides, aspertides D (96) and E (97), were originated from the multistrain fermentation of two marine-associated fungi Aspergillus tamarii MA-21 and Aspergillus insuetus SD-512 (Chi et al., 2023). Compound 96 exhibited an antibacterial effect on four aquatic bacterial pathogens (E. tarda, V. alginolyticus, V. anguillarum, and V. vulnificus) with the MIC values of 8.0–32.0 μg/mL. Compound 97 had an antibacterial effect on E. tarda and S. aureus with the MIC values of 16.0 and 8.0 μg/mL, respectively (Figure 7). Unguisins A (98) and B (99) were isolated from marine sponge-derived fungus Aspergillus nidulans M256, displayed antibacterial activity against E. faecalis with the MIC values of 32 and 128, respectively.
2.2.5 Other nitrogen-containing metabolites
Ochratoxin A methyl ester (100) was separated from the fungus A. elegans KUFA0015 (Kumla et al., 2021). Compound 100 showed a broad spectrum of antibacterial effect against E. faecalis ATCC29212, E. faecalis B3/101, S. aureus ATCC29213, and MRSA S. aureus 66/1 with the MIC values of 16, 16, 8, and 16 μg/mL, respectively. A new chlorinated amino acid derivative, aspergamide A (101), was obtained from the sponge-associated fungus Aspergillus sp. LS53 (Zhang L. et al., 2020; Zhang Y. H. et al., 2020). Compound 101 had a weak antibacterial effect on V. harveyi, with the MIC value of 16 μg/mL. 11-O-methylpseurotin A (102), azaspirofurans B (103), and A (104) were separated from the marine-associated fungus A. fumigatus H22 (Zhang R. et al., 2022). Compounds 102–104 showed a strong antibacterial effect against MRSA (MIC, 10.0, 5.0, and 5.0 μM, respectively). A new benzofuran derivative, dibetanide (105), was separated from the sponge-derived fungus Aspergillus sp. LS57 (Li W. H. et al., 2023). Compound 105 displayed inhibitory activity against Botrytis cinerea with the MIC value of 256 μg/mL. Ochratoxin B (106) was separated from the sponge-associated fungus A. elegans KUFA0015 (Duraes et al., 2021). Compound 106 had a weak antibacterial effect against S. aureus 272,123 with the MIC value of 50.0 μM. Dihydroisoflavipucine (107) was separated from the sponge-associated fungus Aspergillus sp. and showed strong activity against R. litoralis with the MIC value of 0.0001 μg/mL (Zhou et al., 2014). A racemate of benzyl furanone, (+)-asperfuranone (108) and (−)-asperfuranone (109), were separated from coral-associated fungus A. terreus RA2905 (Wu et al., 2020b). Compounds 108–109 displayed an antibacterial effect against P. aeruginosa ATCC 27853 with the MIC values of 32 and 128 μg/mL, respectively. A novel compound, carneusin B (110), was separated from the fungus Aspergillus carneus GXIMD00519 (Lu et al., 2023). Compound 110 displayed weak antibacterial activities against Vibrio rotiferianus and Alteromonas macleodii with the consistent MIC value of 64.0 μg/mL. Seven novel benzoic acid-containing alkaloids, asperalins A–F (111–116) and N-(3-acetamidopropyl)-3,4-dihydroxybenzamide (117), were separated from a seagrass-associated fungus Aspergillus alabamensis SYSU-6778 (Hu et al., 2023). Compounds 111–116 revealed moderate-to-potent activities against Streptococcu iniae and Streptococcus parauberis with the MIC values ranging 2.2–87.3 μM, respectively. Compound 117 showed weak antibacterial effect on Edwardsiella ictaluri with MIC value of 79.3 μM. Two new compounds, sclerotiamides I (118) and J (119), were isolated from a marine gorgonian-derived fungus A. sclerotiorum LZDX-33-4 (Meng et al., 2022). Compounds 118 and 119 displayed antibacterial activity against S. aureus ATCC29213 with the same MIC value of 16 μM. Two novel nucleoside derivatives, kipukasins H (120) and I (121), together with two known analogs, kipukasins E (122) and D (123), originated from the fungus A. versicolor (Chen et al., 2014). Compounds 120–123 exhibited antibacterial effects on S. epidermidis with the MIC values of 12.5, 12.5, 50.0, and 50.0 μM, respectively. Two rare tetracyclic skeleton alkaloids, perinadines B (124) and C (125), were originated from the fungus Aspergillus sp. LS116 (Liu Y. et al., 2022). Compounds 124–125 exhibited moderate antibacterial effects on B. subtilis (MIC, 32.0 and 64.0 μg/mL, respectively). Neoaspergillic (126), isolated from coral-associated fungus Aspergillus sp. CF07002 showed a weak antibacterial effect on three tested bacterial strains (B. cereus, K. pneumoniae, and E. coli) with MIC values ranging 30.0–40.0 μg/mL (Cardoso-Martinez et al., 2015). A novel dimer of a zinc complex, dizinchydroxyneoaspergillin (128), and a known compound hydroxyneoaspergillic acid (127), originated from the fungus Aspergillus ochraceopetaliformis SCSIO 41018 (Guo et al., 2021). Compound 127 exhibited potent inhibitory effects against A. baumannii with the MIC value of 0.45 μg/mL. Compound 128 showed significant bactericide effects against MRSA, S. aureus, E. faecalis, A. baumannii, and K. pneumonia with the MIC values from 0.45 to 7.8 μg/mL. A racemic mixture alkaloid, (±)-puniceusine N (129), was isolated from the fungus Aspergillus puniceus SCSIO z021 (Liu C. M. et al., 2022). Compound (±)-129 had medium antibacterial activities against S. aureus, MRSA, and E. coli with a consistent MIC value of 100 μg/mL. Preussin (130), separated from the fungus Aspergillus candidus KUFA0062, displayed inhibitory activity against S. aureus ATCC 29213, E. faecalis ATCC 29212, MRSA, and vancomycin-resistant enterococci with consistent MIC value of 32.0 μg/mL (Buttachon et al., 2018) (Figure 8).
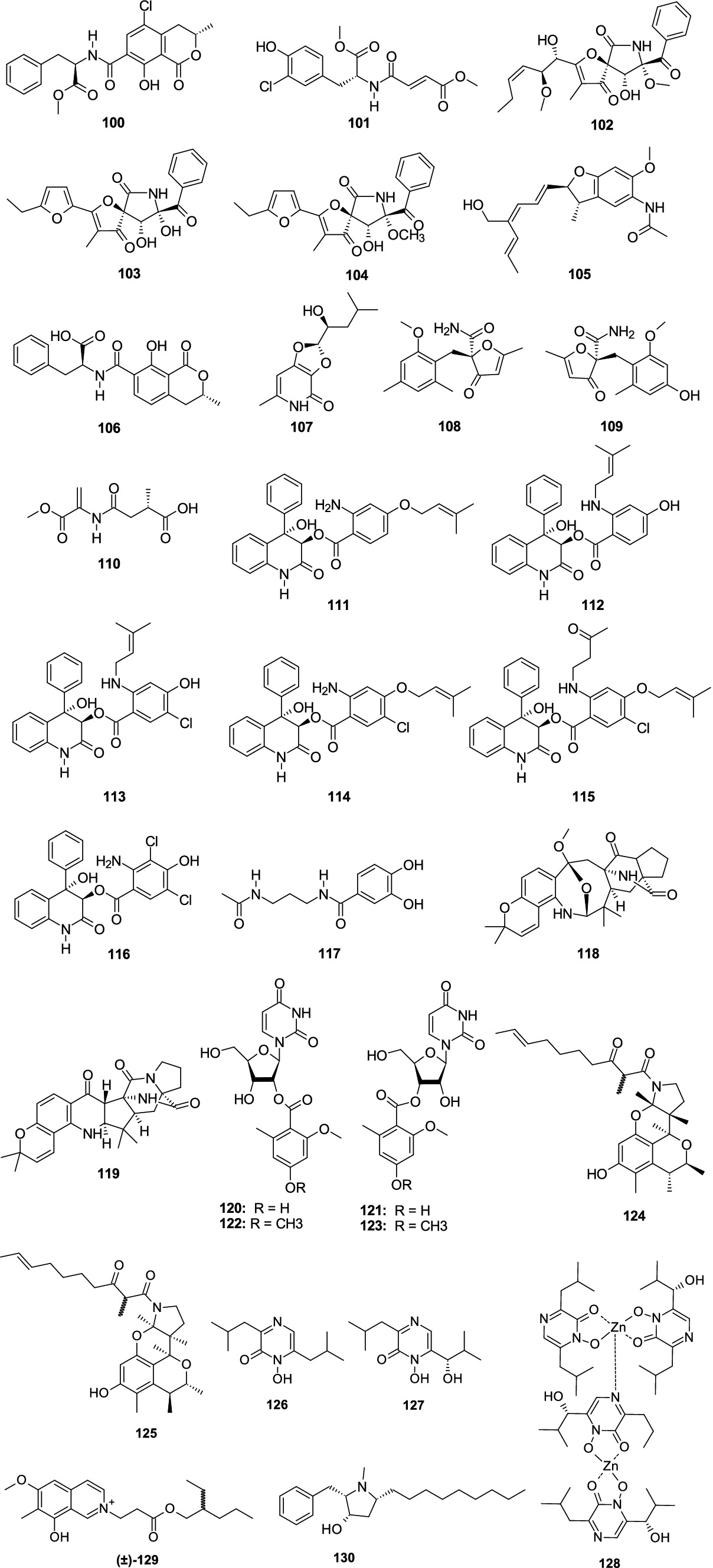
Figure 8. Chemical structures of other nitrogen-containing antibacterial metabolites 100–130 from Aspergillus spp.
2.3 Polyketides
Polyketides were a group of compounds recognized for their wide range of structures and biological activities. These compounds were produced through a series of Claisen condensation reactions, usually utilizing acetyl-coenzyme A (acetyl-CoA), malonyl-coenzyme A (malonyl-CoA), and other substrates. A total of 139 antibacterial polyketides (including 54 new compounds) were separated from the genus of Aspergillus sp., including 20 anthraquinones, 31 xanthones, 59 lactones, and 29 other polyketide metabolites. The structures and the absolute configurations of the new compounds were elucidated by a detailed spectroscopic analysis of NMR and MS data, ECD calculations, as well as single-crystal X-ray diffraction.
2.3.1 Anthraquinones
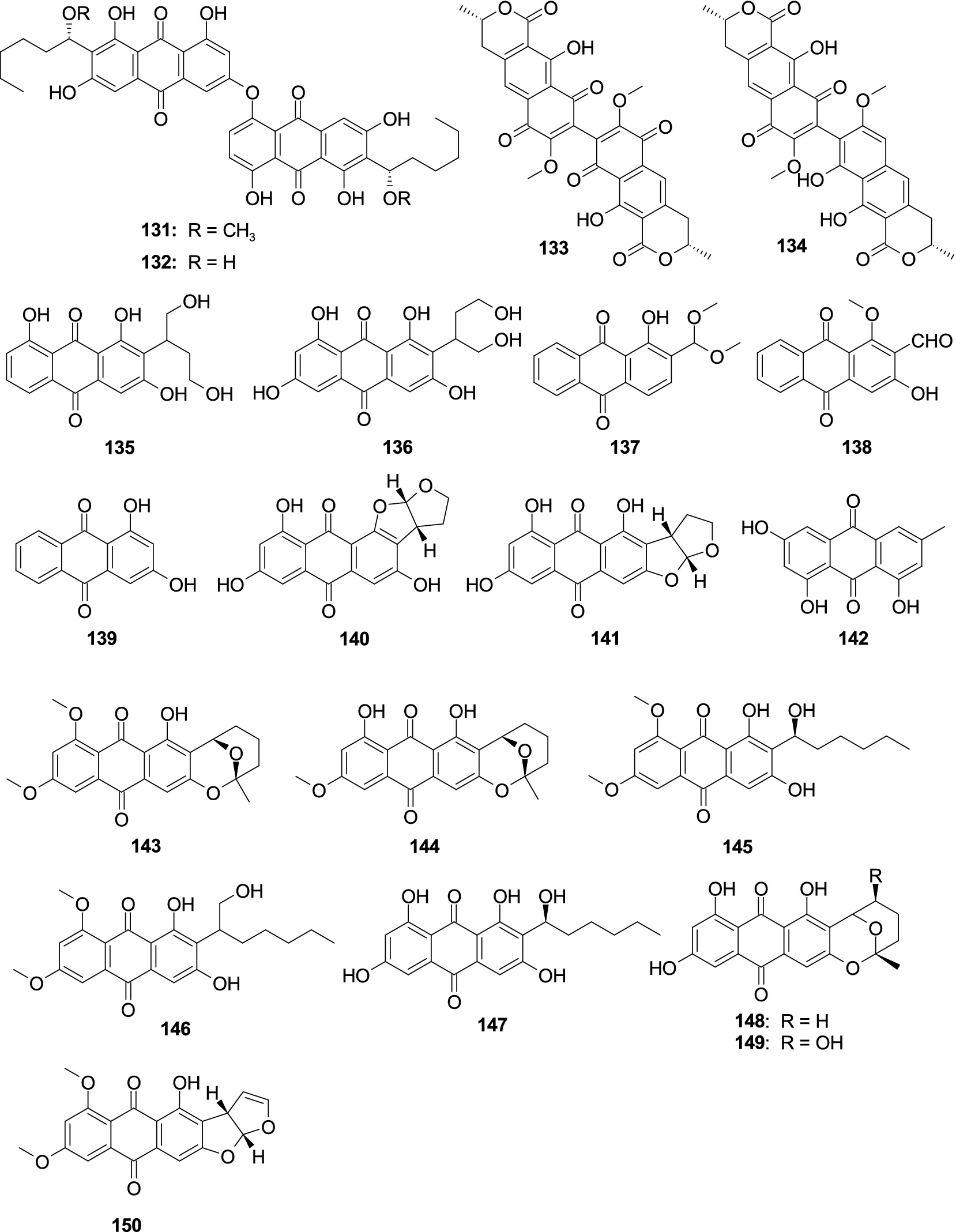
Two new anthraquinone dimers, 6,6′-oxybis(1,3,8-trihydroxy-2-((S)-1-methoxyhexyl)anthracene-9,10-dione) (131) and 6,6′-oxybis(1,3,8-trihydroxy-2-((S)-1-hydroxyhexyl)anthracene-9,10-dione) (132) were originated from the fungus A. versicolor INF16-17 (Li et al., 2019). Compounds 131–132 demonstrated a selective antibacterial effect on S. aureus at a concentration of 30.0 μg/well. Xanthomegnin (133) and viomellein (134) were separated from the sponge-associated fungus A. elegans KUFA0015 (Kumla et al., 2021). Compounds 133–134 had a moderate antibacterial effect on E. faecalis ATCC29212, S. aureus ATCC29213, and S. aureus 66/1 (MRSA), with the MIC values ranging 2.0–32.0 μg/mL. One new anthraquinone versiconol B (135) and a known compound versiconol (136) were originated from the fungus Aspergillus sp. F40 (Tian et al., 2018). Compounds 135–136 exhibited weak antibacterial activity against S. aureus and V. parahaemolyticus with the MIC values of 12–48 μg/mL. One novel anthraquinone derivative, 2-(dimethoxymethyl)-1-hydroxyanthracene-9,10-dione (137), along with two previously reported analogs, damnacanthal (138) and xanthopurpurin (139), were separated from the fungus A. versicolor 3A00029 (Wang et al., 2018). Compound 137 displayed a potent inhibitory effect on MRSA (ATCC 43300 and CGMCC 1.12409), with the MIC values of 3.9 and 7.8 μg/mL, respectively. Compound 138–139 showed a weak antibacterial effect on V. vulnificus MCCC E1758, V. rotiferianus MCCC E385, and Vibrio campbellii MCCC E333, with the MIC values ranging 62.5–125 μg/mL. One novel anthraquinone isoversicolorin C (140) and one known anthraquinone derivative versicolorin C (141) were separated from the fungus A. nidulans MA-143 (Yang et al., 2018a). Compound 140 demonstrated a remarkable antibacterial effect on V. alginolyticus (MIC, 1.0 μg/mL) and E. ictaluri (MIC, 4.0 μg/mL). Compound 141 exhibited an antibacterial effect against five tested bacterial strains (E. coli, M. luteus, V. alginolyticus, V. parahaemolyticus, and E. ictaluri), with the MIC values ranging 1.0–8.0 μg/mL. Emodin (142) was separated from the fungus A. fumigatus MF029 (Song Z. J. et al., 2021). Compound 142 showed potent activity against BCG with the MIC value of 1.25 μg/mL, along with 142 demonstrated moderate antibacterial activities effect on MRSA and S. aureus with the same MIC value of 50.0 μg/mL. 6,8-Di-O-methylaverufin (143) and 6-O-methylaverufin (144) were separated from the alga-associated fungus A. versicolor pt20 (Miao et al., 2012). Compounds 143–144 displayed an antibacterial effect against E. coli and S. aureus, showing the same inhibition zone of 10.0 mm at 30 μg/disk. The new anthraquinone, 6,8-di-O-methylaverantin (145), together with one known congener 6,8-di-O-methylversiconol (146), was separated from the fungus A. versicolor EN-7 (Zhang et al., 2012). Compounds 145 and 146 showed weak inhibition against E. coli, with the inhibition zones 7.0 and 6.5 mm at 20 μg/disk, respectively. Averantin (147), averufin (148), and nidurufin (149) were originated from the fungus A. versicolor PF10M (Lee et al., 2010). Compounds 147–149 showed a better antibacterial effect on Streptococcus pyogenes and S. aureus with the MIC values from 0.78 to 6.25 μg/mL. 6,8-Di-O-methylversicolorin A (150) was originated from the fungus Aspergillus sp. WHUF05236 (Lv et al., 2022). Compound 150 displayed an antibacterial effect against H. pylori, with the MIC values from 20.00 to 43.47 μM (Figure 9).
2.3.2 Xanthones
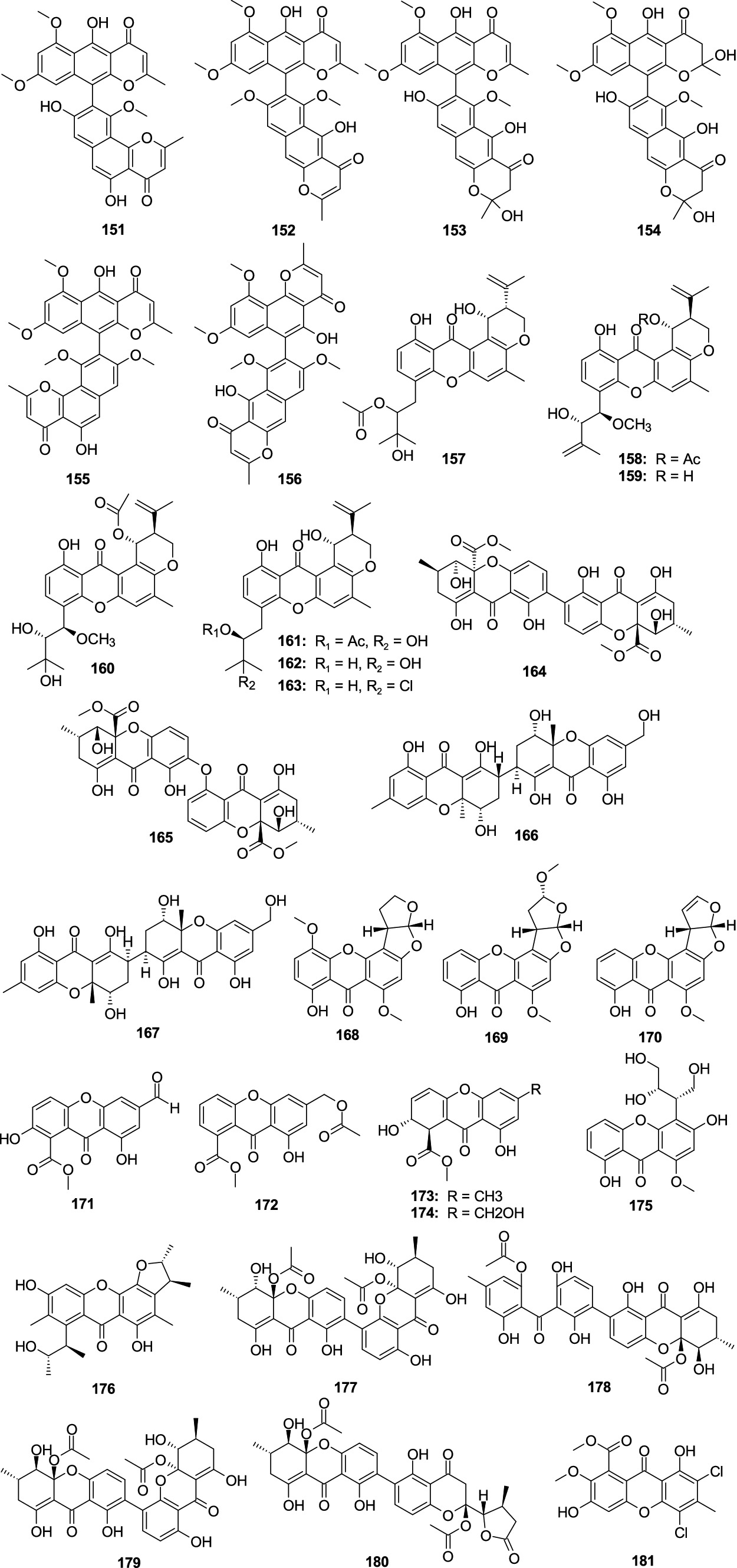
Asperpyrone A (151), aurasperones A (152), F (153), and B (154), were separated from the mangrove-associated fungus Aspergillus sp. DM94 (Gou et al., 2020). Compound 151–154 displayed an obvious antibacterial effect on H. pylori with the MIC values ranging 4.0–32.0 μg/mL. Fonsecinone A (155) and asperpyrone C (156) were separated from the fungus A. welwitschiae CUGBMF180262 (Han et al., 2022). Compounds 155 and 156 showed moderate antibacterial activities against H. pylori with the same MIC value of 16 μg/mL. Three novel prenylxanthone derivatives, aspergixanthones I–K (157–159), and four known analogss aspergixanthone A (160), 15-acetyl tajixanthone hydrate (161), tajixanthone hydrate (162), and 16-chlorotajixanthone (163), were originated from the fungus Aspergillus sp. ZA-01 (Zhu et al., 2018). Compounds 157–163 displayed anti-Vibrio activities to three pathogenic Vibrio spp. (V. parahemolyticus, V. anguillarum, and V. alginolyticus), with the MIC values between 1.56 and 25.0 μM. Among them, 157 exhibited significant anti-Vibrio activity, suggesting that the propenyl group at C-20 with α-stereoconfiguration might be crucial for the anti-Vibrio activity. Homodimeric tetrahydroxanthone secalonic acid D (164) was isolated from A. aculeatinus WHUF0198 and 164 performed activities against H. pylori G27, H. pylori 26,695, H. pylori 129, H. pylori 159, S. aureus USA300, and B. subtilis 168 with MIC values of 4.0, 4.0, 2.0, 2.0, 2.0, and 1.0 μg/mL, respectively (Wu et al., 2023). A new tetrahydroxanthone dimer, 5-epi-asperdichrome (165), was originated from the mangrove-associated fungus A. versicolor HDN1009 (Yu et al., 2018). Compound 165 exhibited weak activity against four tested bacterial strains (V. parahemolyticus, B. subtilis, M. phlei, and P. aeruginosa), with the MIC values ranging 100.0–200.0 μg/mL. Two new heterodimeric tetrahydroxanthones, aflaxanthones A (166) and B (167), were separated from mangrove-associated fungus A. flavus QQYZ (Zang et al., 2022). Compound 166 possessed a moderate inhibitory effect on MRSA (MIC, 12.5 μM), and compounds 166 and 167 showed a weak inhibitory effect on B. subtilis with the same MIC value of 25 μM. A new sterigmatocystin, 5-methoxydihydrosterigmatocystin (168), was originated from the sponge-associated fungus A. versicolor MF359 (Song et al., 2014). Compound 168 exhibited a significant antibacterial effect against B. subtilis (MIC, 3.125 μg/mL) and S. aureus (MIC, 12.5 μg/mL). Oxisterigmatocystin C (169) was separated from the fungus Aspergillus sp. F40 (Tian et al., 2018). Compound 169 displayed weak antibacterial activity against S. aureus (MIC, 48.0 μg/mL). Sterigmatocystin (170) originated from a sponge-derived fungus A. sydowii DC08 (Handayani et al., 2022). Compound 170 showed activities against MRSA, Multidrug-resistant P. aeruginosa (MDRPA), E. coli, S. aureus, and P. aeruginosa with the MIC values of 64.0, 128.0, 16.0, 32.0, and 32.0 μg/mL, respectively. Two new anthrone derivatives, 2-hydroxy-6-formyl-vertixanthone (171) and 12-O-acetyl-sydowinin A (172), together with two known analogs aspergillusone A (173) and AGI-B4 (174), were originated from the fungus A. sydowii C1-S01-A7 (Wang et al., 2019). Compounds 171–174 showed weak activities to MRSA with the MIC values ranging 15.0–32.0 μg/mL. A new xanthone, isosecosterigmatocystin (175) was separated from the fungus A. nidulans MA-143 (Yang et al., 2018a). Compound 175 showed weak activity against E. ictaluri (MIC, 16.0 μg/mL). A new citrinin dimer, seco-penicitrinol A (176), was separated from the algal-associated fungal A. sydowii EN-534 (Yang et al., 2018b). Compound 176 showed weak inhibitory activity against four bacterial strains (M. luteus, E. ictaluri, V. alginolyticus, and V. c), with the MIC values ranging 16.0–32.0 μg/mL. Secalonic acid F1 (177), secalonic acid H (178), penicillixanthone A (179), and chrysoxanthone C (180) showed weak antibacterial activity against S. aureus with the MIC values 25.0, 50.0, 6.25, and 50.0 μg/mL, respectively, which were separated from the fungus A. brunneoviolaceus MF180246 (Xu et al., 2024). A new chlorinated biphenyl, aspergetherin A (181), displayed weak activity against MRSA 05–72 and MRSA USA300, with the same MIC value of 128.0 μg/mL, which was separated from the sponge-associated fungus A. terreus 164,018 (Li J. X. et al., 2023) (Figure 10).
2.3.3 Lactones
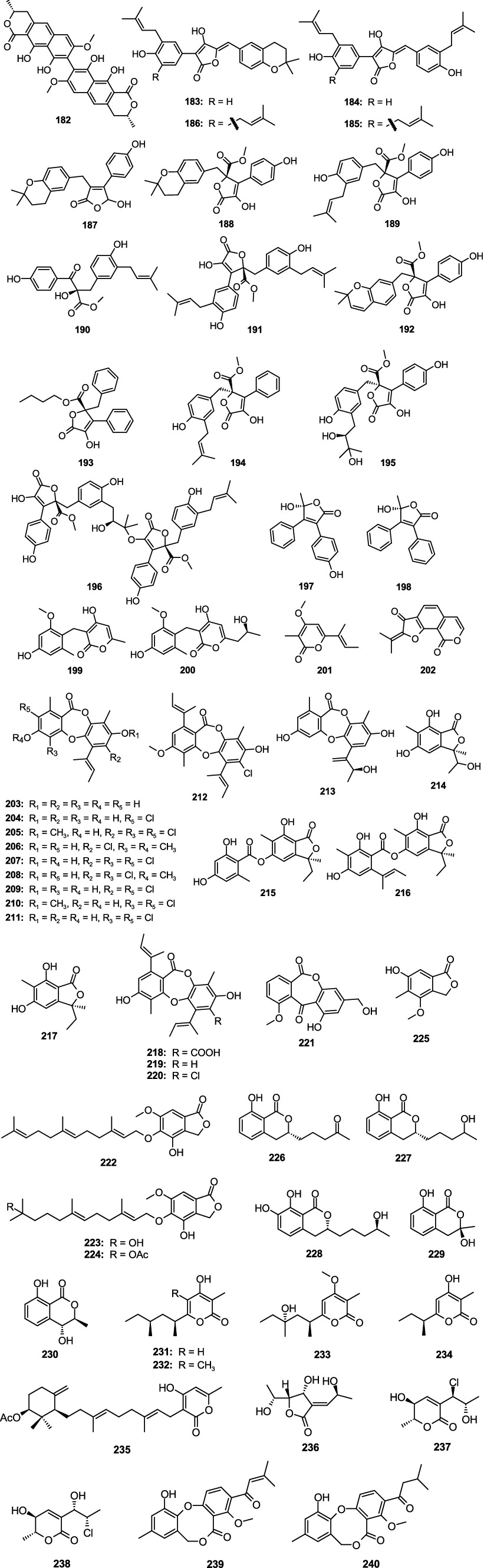
Vioxanthin (182) showed significant antibacterial effect on E. faecalis ATCC29212, E. faecalis (VRE) B3/101, S. aureus ATCC29213, and S. aureus (MRSA) 66/1 with the MIC values 2.0, 1.0, 2.0 and 0.5, respectively, which was separated from the sponge-associated fungus A. elegans KUFA0015 (Kumla et al., 2021). Two new prenylated phenylbutyrolactones, aspulvinones R–S (185–186), together with two known compounds aspulvinones B′ (183) and H (184) were separated from the fungus Aspergillus flavipes KUFA1152 (Machado et al., 2021). Compounds 183–186 displayed strong activities against E. faecalis and S. aureus with the MIC values ranging 8.0–16.0 μg/mL. Asperteretal E (187) and aspernolide A (188) were originated from the fungus A. terreus SCSIO FZQ028 (Zeng et al., 2020b), and they showed moderate antimicrobial activities against S. aureus ATCC 29213 and Bacillus thuringiensis ATCC 10792, with inhibitory diameters from 7.49 to 8.94 mm at 30 μg/disk, respectively. Butyrolactone I (189) displayed significant antibacterial against S. aureus with the MIC value of 0.78 μg/mL, which was collected from the fungus Aspergillus sp. SCSIO 41029 (Chen et al., 2021). A new aromatic butanolide, asperbutenolide D (190), along with two known analogs (+)-3′,3′-di-(dimethylallyl)-butyrolactone II (191) and aspernolide E (192), displayed moderate antibacterial against S. aureus with the MIC values of 21.3, 17.4, and 26.1 μM, respectively, which were separated from sediment-associated fungus A. terreus SCAU011 (Bao et al., 2021). A novel butyrolactone derivative, flavipesin A (193), demonstrated obvious antibacterial activities against S. aureus (MIC, 8.0 μg/mL) and B. subtillis (MIC, 0.25 μg/mL), and the fungus was separated from the mangrove-associated fungus A. flavipes AIL8 (Bai et al., 2014). Versicolactone B (194) and butyrolactone VI (195) were separated from the coral-derived fungus A. terreus SCSIO41404 (Peng et al., 2022). Compound 194 demonstrated weak antibacterial against E. faecalis (MIC, 5 μg/mL). Compound 195 demonstrated weak antibacterial against K. pneumoniae (MIC, 50 μg/mL). A novel aromatic butanolide, asperbutenolide A (196), with strong inhibition activity against S. aureus (MIC, 1.30 μg/mL) and V. splendidus (MIC, 3.70 μg/mL), was separated from the mangrove sediment-derived fungus A. terreus SCAU011 (Bao et al., 2020). 5R-(+)-9-hydroxymicroperfuranone (197) and 5R-(+)-microperfuranone (198), with weak inhibition activity against E. coli with the MIC values of 50 and 25 μg/mL, respectively, which were separated the fungus Aspergillus sp. ZZ1861 (Ha et al., 2024). Two new benzyl pyrones, asperpyranones A–B (199–200), exhibited weak antibacterial against P. aeruginosa ATCC 27853 with the MIC values of 32 and 128 μg/mL, respectively, which were separated from a marine-derived fungus A. terreus RA2905 (Wu et al., 2020b). Nectriapyrone (201) and asperisocoumarin A (202), displayed a weak antibacterial effect on V. harveyi with MIC values of 64.0 and 32.0 μg/mL, respectively, which were separated from the fungus Aspergillus sp. LS53 (Zhang L. et al., 2020; Zhang Y. H. et al., 2020). Unguinol (203), 2-chlorounguinol (204), and nidulin (205) showed strong antibacterial activity against E. coli, P. aeruginosa, S. aureus, E. faecalis, B. subtilis, Salmonella. typosa, Vibrio cholera Inaba, and M. luteus, with MIC values ranging 0.78–3.12 μg/disk, which were separated from the fungus Aspergillus unguis WR8 (Handayani et al., 2020). One novel depsidone derivative, aspergillusidone H (206), together with three known compounds nornidulin (207), aspergillusidones B (208), and C (209), were separated from the fungus A. unguis GXIMD02505 (Zhang Y. T. et al., 2022). Compounds 207 and 209 had antibacterial activity against MRSA, Mylabris variabilis, and Methanocaldococcus jannaschii, with MIC values from 2 to 32 μg/mL. Compound 208 displayed antibacterial activity against M. variabilis (MIC, 128 μg/mL). One new depsidone 7-dechloronidulin (210), together with two known compounds 2,4-dichlorounguinol (211) and emeguisin B (212) were separated from the fungus A. unguis GXIMD02505 (Thi et al., 2023). Compound 210 was selectively bioactive on three Gram-positive bacteria (B. cereus, E. faecalis, S. aureus) (MICs: 2–4 μg/mL). Compound 211 had broad-spectrum antimicrobial activity against six bacteria (B. cereus, E. faecalis, S. aureus, E. coli, P. aeruginosa, and S. enterica), with the MIC values ranging 16–64 μg/mL. Compound 212 showed weak activity against E. faecalis with the MIC value of 256 μg/mL. One new depsidone asperunguissidone A (213), one new phthalide asperunguislide A (214), and six known compounds asperlide (215), aspergiside C (216), (3S)-3-ethyl-5,7-dihydroxy-3,6-dimethylphthalide (217), aspergisidone (218), folipastatin (219), emeguisins A (220), were separated from the fungus A. unguis PSU-MF16 (Saetang et al., 2021). Compounds 213–220 showed activity against S. aureus and MRSA with the MIC values from 1.0 to 200.0 μg/mL. 8-Demethoxy-10-methoxy-wentiquinone C (221) was separated from the fungus A. sydowii C1-S01-A7, and showed a weak antibacterial activity against MRSA with an MIC value of 32.4 μg/mL (Wang et al., 2019). Three new farnesylated phthalide derivatives farnesylemefuranones D–F (222–224) were isolated from the cold-seep-derived fungus A. insuetus SD-512, and they exhibited inhibitory effects against V. vulnificus with the same MIC value of 4.0 μg/mL, while 221 and 223 also inhibited V. alginolyticus with the same MIC value of 4.0 μg/mL (Chi et al., 2020). Silvaticol (225) was separated from the fungus Aspergillus sp. ZZ1861, and 225 displayed inhibitory activity against E. coli with the MIC value of 12.5 μg/mL (Ha et al., 2024). Two novel dihydroisocoumarin derivatives, aspergillumarins A (226) and B (227), were separated from the marine-associated fungus Aspergillus sp. (Li et al., 2012). Compounds 226 and 227 demonstrated weak antibacterial against S. aureus and B. subtilis at a concentration of 50 μg/mL. A new dihydroisocoumarin, aspergimarin G (228), was separated from the sponge-associated fungus Aspergillus sp. NBUF87 (Lin S. X. et al., 2023), and showed a moderate activity against S. aureus and S. enteritidis with MIC values from 16.0 to 64.0 μg/mL. (R)-3-Hydroxymellein (229) and (3R,4S)-trans-4-hydroxymellein (230) were separated from the fungus Aspergillus sp. SCSCIO41405 (Peng et al., 2021). Compound 229 demonstrated a weak antibacterial effect on MRSA (MIC, 100.0 μg/mL). Compound 230 displayed a weak antibacterial effect on E. faecalis (MIC, 100.0 μg/mL). Three new 4-hydroxy-α-pyrones nipyrones A–C (231–233) and one known analog germicidin C (234) were separated from the sponge-associated fungus A. niger LS24 (Ding et al., 2019). Compound 233 demonstrated a significant inhibitory effect on S. aureus and B. subtilis with the MIC values of 8.0 and 16.0 μg/mL, respectively. Sartorypyrone A (235) was separated from the fungus Aspergillus sp. WHUF03110 and displayed a strong inhibitory activity against B. subtilis, S. aureus ATCC25923, S. aureus NEWMAN, S. aureus USA300, and S. aureus NRS 271 with MIC values ranging 1.0–2.0 μg/mL (Lv et al., 2021). Asperochrin A (236), chlorohydroaspyrones A (237) and B (238), were separated from the mangrove-associated fungus spergillus ochraceus MA-15 (Liu et al., 2015). Compound 236 showed an inhibitory activity against A. hydrophila, V. anguillarum, and V. harveyi with the MIC values of 8.0, 16.0, and 8.0 μg/mL, respectively. 237 and 238 showed weak inhibitory activity against the above three pathogenic bacterial (MIC, 16–32 μg/mL). One novel penicillide analog, ∆2′-1′-dehydropenicillide (239) and a known analog dehydropenicillide (240), were separated from the fungus Aspergillus sp. IMCASMFI80035 (Song F. H. et al., 2021), which demonstrated significant antibacterial activities against H. pylori (MIC, 21.73 and 21.61 μM, respectively) (Figure 11).
2.3.4 Other polyketide metabolites
The novel compound aspergiloxathene A (241), separated from the marine-associated fungus Aspergillus sp. IMCASMF180035, exhibited significant antibacterial activities against S. aureus (MIC, 5.60 μM) and MRSA (MIC, 22.40 μM) (Song F. H. et al., 2021). A new compound, cowabenzophenone A (242), was separated from the mangrove-associated fungus A. terreus (Ukwatta et al., 2020). Compound 242 showed strong antibacterial activity against B. subtilis (MIC, 1.0 μg/mL) and S. aureus (MIC, 2.0 μg/mL). Penicitrinone A (243), penicitrinone F (244), and citrinin (245) showed weak activity against E. ictaluri and V. alginolyticus with the MIC values from 16.0 to 32.0 μg/mL, were separated from the fungal A. sydowii EN-534 (Yang et al., 2018b). Two new compounds 25S-O-methylarugosin A (246), 25R-O-methylarugosin A (247) were separated from the fungus Aspergillus sp. ZZ1861 (Ha et al., 2024). Compound 247 showed weak activities against MRSA (MIC, 50.0 μg/mL). The new compound 12S-aspertetranone D (248), separated from sea trench-derived fungus Aspergillus sp. SY2601 (Sun et al., 2024), exhibited antibacterial effects on MRSA and E. coli with the MIC values of 3.75 and 5.0 μg/mL, respectively. Four new anthraquinone derivatives, (10S,12S)-chevalierone, (10S,12R)-chevalierone, (10R,12S)-chevalierone, and (10R,12R)-chevalierone (249–252), were isolated from the fungus A. chevalieri HP-5 (Wang Q. Y. et al., 2022). Compounds 250–252 showed significant inhibition against the opportunistic pathogenic bacterium P. aeruginosa (inhibition rate: 81.0–91.5%) and MRSA (inhibition rate: 74.0–88.5%) at the concentration of 200 μM, while the structural congener compound 249 only showed weak inhibition (inhibition rate: 38.2%) against the P. aeruginosa at 200 μM. Two novel phenome compounds, asperphenones A (253) and B (254), were separated from the mangrove-derived fungus Aspergillus sp. YHZ-1 (Guo et al., 2018). Compounds 253 and 254 demonstrated weak antibacterial effects on four Gram-positive bacteria, S. aureus, S. pyogenes, B. subtilis, and M. luteus, with the MIC values from 32.0 to 64.0 μg/mL. One new compound penibenzophenone E (255) and a known compound sulochrin (256) were originated from the fungus A. fumigatus H22 (Zhang R. et al., 2022). Compounds 255 and 256 demonstrated activity against MRSA with the same MIC value of 1.25 μM. Aspergisides A–B (257–258), together with agonodepsides A–B (259–260), were separated from sponge-derived fungus A. unguis PSU-MF16 (Saetang et al., 2021). Compounds 257, 259, and 260 had strong antibacterial activity against S. aureus and MRSA with the MIC values from 2.0 to 16.0 μg/mL. Compound 258 displayed a weak activity against S. aureus and MRSA with the same MIC value of 200.0 μg/mL. Guisinol (261) was separated from the fungus A. unguis GXIMD 02505 (Zhang Y. T. et al., 2022). Compound 261 showed antibacterial activities against MRSA (MIC, 16.0 μg/mL) and M. variabilis (MIC, 64.0 μg/mL). Two new phenolic polyketides, unguidepside C (262) and agonodepside C (263), were isolated from two marine-associated fungal strains of A. unguis (Anh et al., 2022). Compounds 262 and 263 demonstrated inhibitory effects against S. aureus, M. luteus, and B. subtilis, with the MIC values from 8.0 to 22.1 μM. One new chromone, aspergilluone A (264), was separated from the fungus Aspergillus sp. LS57, which displayed an antibacterial effect on M. tuberculosis (MIC, 32.0 μg/mL) and S. aureus (MIC, 64.0 μg/mL) (Liu et al., 2021). Phomaligol A (265), separated from the fungus A. flavus MFA500, displayed a weak activity against S. aureus with MIC value of 31.2 μg/mL (Yang et al., 2011). Trypacidin (266) showed significant antitubercular activity with the MIC value of 1.25 μg/mL, which was separated from the fungus A. fumigatus MF029 (Song Z. J. et al., 2021). (+)-Geodin (267) and chlorotrypacidin (268) showed a weak antibacterial effect on Staphylococcus albus, S. aureus, and V. anguillarum with the same MIC value of 25.0 μM, and they were separated from the fungi of A. versicolor TA01-14 (Zhang et al., 2019). Eugenitol (269) demonstrated weak inhibitory activity against MRSA with the MIC value of 485.4 μM, which was separated from the mangrove sediment-associated fungus Aspergillus sp. SCSIO41407 (Cai et al., 2021) (Figure 12).
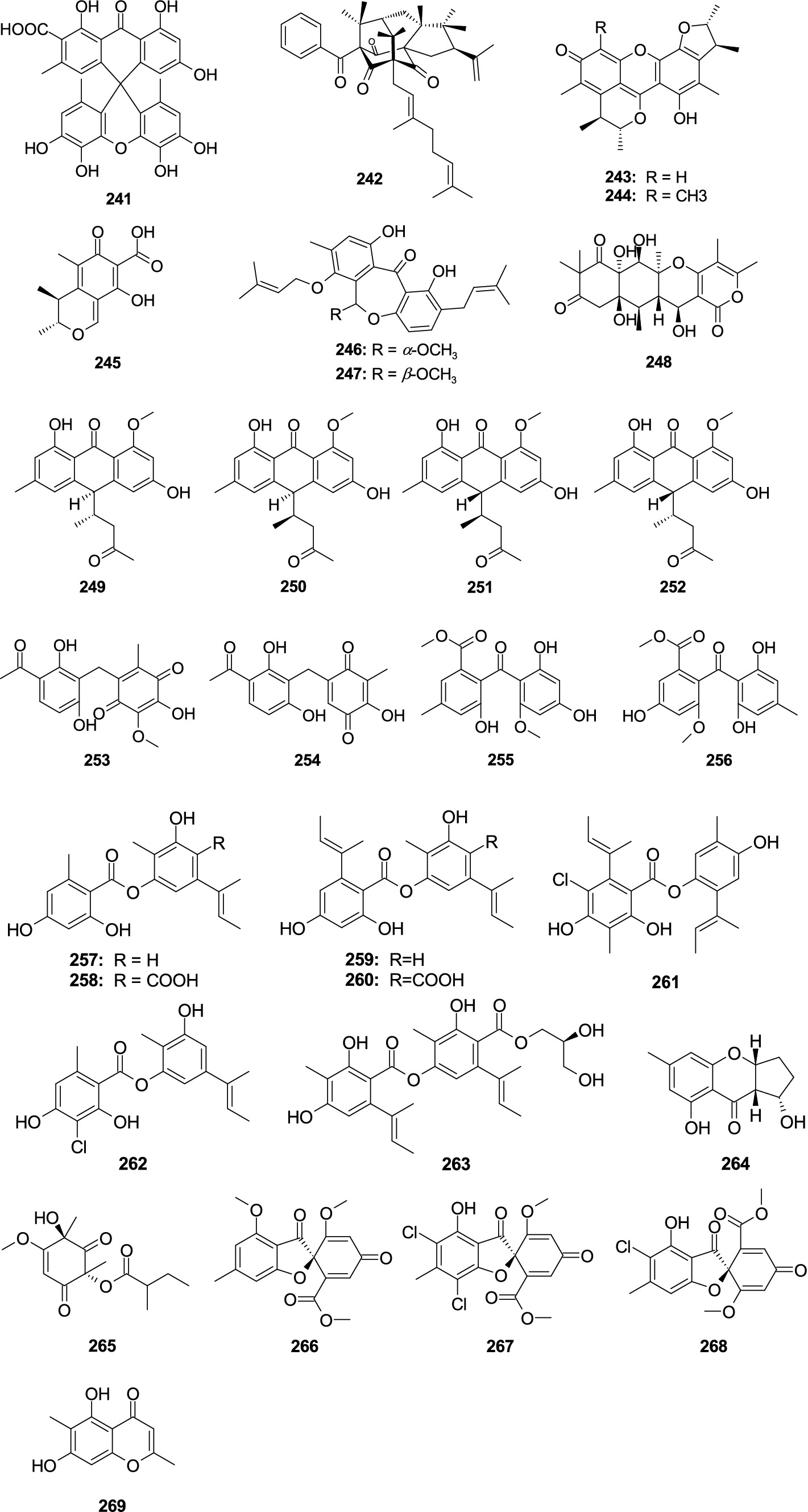
Figure 12. Chemical structures of other antibacterial polyketide metabolites 241–269 from Aspergillus spp.
2.4 Steroids
Steroids were biosynthesized through complex cyclization reactions involving squalene and mevalonate pathways. A total of 18 antibacterial steroids (including 11 new compounds) were identified from marine-derived Aspergillus species. The steroid structures and the absolute configurations of the new compounds were elucidated by a detailed spectroscopic analysis of NMR and MS data, optical rotatory dispersion, ECD calculations, and single-crystal X-ray diffraction.
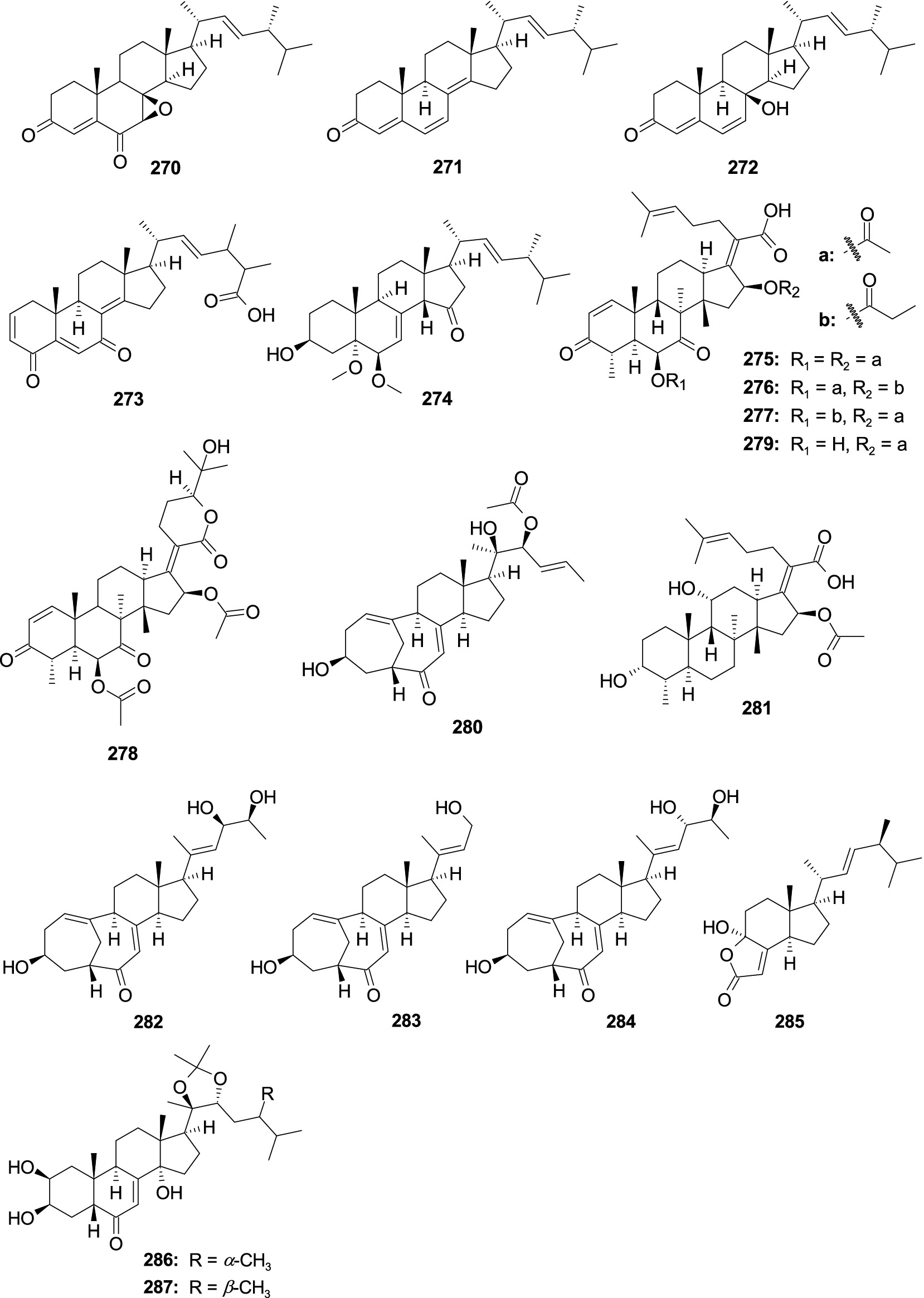
A new steroid 7β,8β-Epoxy-(22E,24R)-24-methylcholesta-4,22-diene-3,6-dione (270) and a known steroid ergosta-4,6,8(14),22-tetraene-3-one (271) were separated from the fungus Aspergillus penicillioides SD-311 (Chi et al., 2021b). Compound 270 showed antibacterial activity against V. anguillarum with the MIC value of 32.0 μg/mL, while 271 displayed inhibitory activity against E. tarda and M. luteus with the same MIC value of 16.0 μg/mL. One new ergosterol derivative, isocyathisterol (272), exhibited a weak antibacterial activity against E. coli and S. aureus, with inhibitory diameters of 6.7 and 5.7 mm at 30 μg/disk, respectively, was originated from the alga-derived fungus A. ustus cf-42 (Liu et al., 2014). One new oxygenated steroid, aspersteroid A (273), was isolated from the marine-derived fungus A. flavus YJ07-1 (Yang M. Y. et al., 2018). Compound 273 showed antibacterial activities against V. anguillarum, V. parahemolyticus, and V. alginolyticus with the same MIC value of 12.5 μM. One new oxygenated ergostane-type steroid, 3β-hydroxy-5ɑ,6β-methoxyergosta-7,22-dien-15-one (274), was isolated from the marine sponge-derived fungus Aspergillus sp. NR151817 (Wen et al., 2024). Compound 274 showed weak inhibitory activity against S. aureus with an MIC value of 64 μg/mL. A known steroid C-21 acid helvolic acid (275) was isolated from the fungus Aspergillus sp. SCS-KFD66 (An et al., 2018). Compound 275 exhibited strong activity against S. aureus ATCC 6538 with an MIC value of 2.0 μg/mL. Three new helvolic acid derivatives, 16-O-propionyl-16-O-deacetylhelvolic acid (276), 6-O-propionyl-6-O-deacetylhelvolic acid (277), and 24-epi-6β,16β-diacetoxy-25-hydroxy-3,7-dioxo-29-nordammara-1,17(20)-diene-21,24-lactone (278), were isolated from the marine-derived fungus A. fumigatus HNMF0047 (Kong et al., 2018). Compounds 276–278 showed antibacterial activities against Streptococcus agalactiae and S. aureus with MIC values ranging 2.0–64.0 μg/mL. A new steroid 3,7-diketo-cephalosporin P1 (279), along with a known analog 22-O-acetylisocyclocitrinol A (280), were isolated from deep sea-derived fungus A. fumigatus SCSIO 41012 (Limbadri et al., 2018). Compound 279 showed weak activity against A. baumanii 19,606 with the MIC value of 50.0 μg/mL. Compound 280 exhibited high antibacterial activity with A. baumanii ATCC15122 and K. pneumonia ATCC14578 with the MIC values of 12.5 and 3.12 μg/mL, respectively. Fusidic acid (281) and neocyclocitrinol D (282) were obtained from the marine-derived fungus A. flavus JK07-1 (Ren et al., 2020). Compound 281 showed significant inhibitory activities against Micrococcus lysodeikticus, B. cereus, Bacillus megaterium, Bacillus Anthracis, and Salmonella typhi, with the MIC values of 0.07, 0.07, 0.07, 0.30, and 0.60 μM, respectively. Compound 282 showed effective inhibitory activity against M. lysodeikticus with an MIC value of 1.30 μM. A new C-23 steroid with bicyclo[4.4.1]A/B ring aspergillsteroid A (283) and a known analog neocyclocitrinol B (284) exhibited antibacterial activity against V. harveyi KP635244 with the MIC values of 16.0 and 128.0 μg/mL, respectively, which were separated from marine-derived fungus Aspergillus sp. LS116 (Xu P. et al., 2020). Demethylincisterol A2 (285) was separated from the coral-derived fungus A. hiratsukae SCSIO 5Bn1003 (Zeng et al., 2022a). Compound 285 displayed strong activity against B. subtilis with the MIC value of 10.26 μg/mL. Two new polyhydroxylated mycoecdysteroids, punicesterones B (286) and C (287), were separated from the deep-sea-derived fungus A. puniceus SCSIO z021 (Huang et al., 2023). Compounds 286 and 287 could show significantly inhibitory activity against S. iniae, S. agalactiae, E. coli, B. subtilis, and S. aureus at a concentration of 0.132 mM (Figure 13).
2.5 Other classes
Additionally, there were also some other classes of antibacterial secondary metabolites isolated from Aspergillus spp., including fatty acids, glycosides, and benzene derivatives. A total of 50 antibacterial compounds (including 14 new compounds) were isolated from the Aspergillus spp. The structures, like three undescribed compounds, carnemycins H − I and stromemycin B, were elucidated by comprehensive spectroscopic data and J-based configurational analysis.
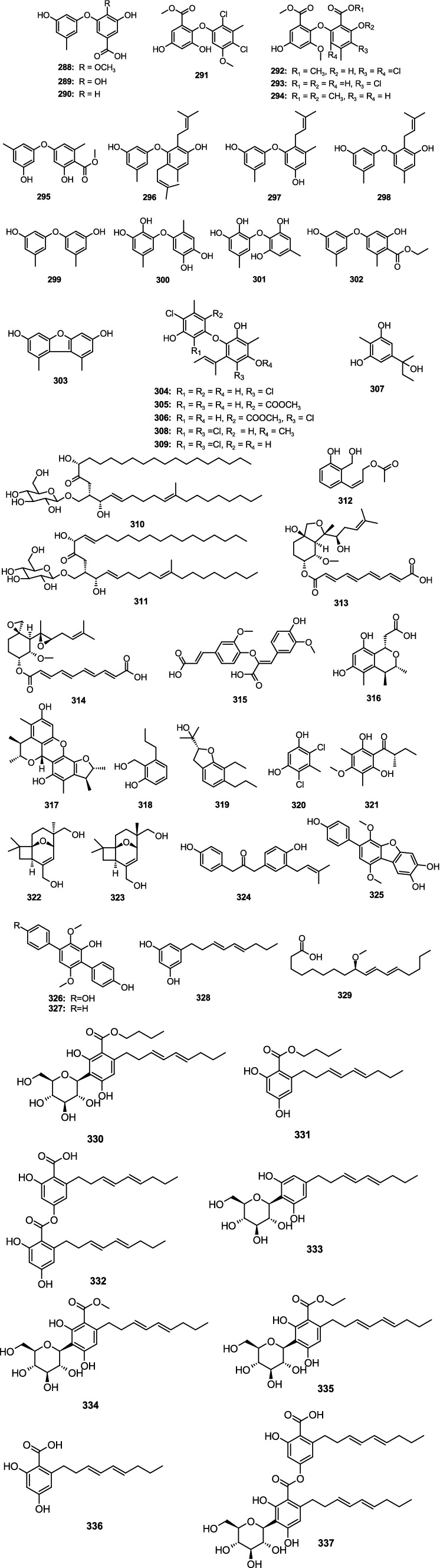
A new phenyl ether derivative, 3-hydroxy-5-(3-hydroxy-5-methylphenoxy)-4-methoxybenzoic acid (288), together with two known analogs 3,4-dihydroxy-5-(3-hydroxy-5-methylphenoxy)benzoic acid (289) and 3-hydroxy-5-(3-hydroxy-5-methylphenoxy)-benzoic acid (290), were separated from the marine-derived fungus A. carneus (Xu et al., 2017). Compounds 288–290 had weak activity against S. aureus, V. anguillarum, and E. coli with the same MIC value of 25 μM. A new compound aspergetherin C (291) and two known analogs, methyl 3,5-dichloroasterric acid (292) and methyl chloroasterrate (293), were isolated from the fungus A. terreus 164,018 (Li J. X. et al., 2023). Compounds 291 and 293 showed weak antibacterial activity against MRSA 05–72 and MRSA USA300 (MIC, 64.0 μg/mL). Compound 292 had strong inhibitory activity against MRSA 05–72 with the MIC value of 1.0 μg/mL. Dimethyl 2,3′-dimethylosoate (294) was isolated from A. fumigatus H22 (Zhang R. et al., 2022). Compound 294 showed strong inhibitory activity against MRSA with the same MIC value of 5.0 μM. 4-Methoxycarbonyldiorcinol (295), showed strong inhibitory activity against P. aeruginosa with the MIC value of 13.9 μM, which was separated from the marine algae-derived fungus A. versicolor OUCMDZ-2738 (Liu et al., 2019). One new diphenyl ether, diorcinol K (296), along with two known analog diorcinols D (297) and I (298), were isolated from a fungus Aspergillus sp. CUGB-F046 (Xu et al., 2018). Compounds 296–298 displayed significant antibacterial activity against S. aureus and MRSA with the MIC values from 3.13 to 6.25 μg/mL. Diorcinol (299) was isolated from the deep-sea-derived A. versicolor 170,217 (Lin S. H. et al., 2023). Compound 299 exhibited weak inhibitory activity against V. parahemolyticus with an MIC value of 128.0 μg/mL. Violaceol-I (300), violaceol-II (301), 4-carbethoxydiorcinal (302), and 1,9-dimethyl-3,7-dibenzofurandiol (303) were isolated from the fungus Aspergillus sp. ZZ1861 (Ha et al., 2024). Compounds 300–303 showed inhibitory activity against MRSA and E. coli with the MIC values from 6.25 to 50.0 μg/mL. Two new diphenyl ethers, aspergillusethers E (304) and F (309), together with three known compounds aspergillusethers C (305) and D (306) and pilobolusate (307), were isolated from sponge-derived fungus Aspergillus sp. PSU-MF16 (Saetang et al., 2021). Compound 304 demonstrated moderate inhibitory activity against S. aureus and MRSA with the same MIC value of 16.0 μg/mL. Compounds 305–307 had weak antibacterial activity against S. aureus and MRSA with MIC values from 64.0 to 128.0 μg/mL. Aspergillusethers J (308) and F (309) showed inhibitory activity against MRSA, M. variabilis, and M. jannaschii with MIC values ranging 2.0–64.0 μg/mL, which were separated from coral-derived fungus A. unguis GXIMD 02505 (Zhang Y. T. et al., 2022). Two new cerebroside derivatives, flavusides A (310) and B (311), were isolated from the marine-derived fungus A. flavus MFA500 (Yang et al., 2011). Compounds 310 and 311 showed moderate inhibitory activity against S. aureus with the same MIC value of 15.6 μg/mL. One new phenol derivative, acetylpeniciphenol (312), showed activity against E. tarda, V. alginolyticus, and V. vulnificus with the MIC values of 4.0, 8.0, and 8.0 μg/mL, respectively, which was separated from the cold-seep-derived fungus A. insuetus SD-512 (Chi et al., 2021a). Fumagiringillin (313) and fumagillin (314) were isolated from the marine-derived fungus A. fumigatus H22 (Zhang R. et al., 2022). Compounds 313 and 314 showed inhibitory activity against MRSA with MIC values of 25.0 and 2.50 μg/mL, respectively. 8-O-4-dehydrodiferulic acid (315) was isolated from the sponge-derived fungus Aspergillus sp. (Zhou et al., 2014). Compound 315 displayed activity against R. litoralis with an MIC value of 1.0 μg/mL. A new citrinin monomer penicitrinol L (316) and a known compound penicitrinol A (317) were separated from the marine algal-derived fungus A. sydowii EN-534 (Yang et al., 2018b). Compound 316 displayed weak inhibitory activity against E. coli, E. ictaluri and V. alginolyticus with the same MIC value of 64.0 μg/mL. Compound 317 showed inhibitory activity against E. coli, M. luteus, E. ictaluri, V. alginolyticus, and V. parahaemolyticus with the MIC values from 4.0 to 32.0 μg/mL. 2-(Hydroxymethyl)-3-propylphenol (318) and (−)-brassicadiol (319) were separated from the mangrove-derived fungus Aspergillus sp. ZJ-68 (Cai et al., 2019). Compounds 318 and 319 showed strong activity against S. aureus, E. coli and B. subtilis (MIC, 4.15–12.5 μg/mL). 4,6-Dichloro-5-methylbenzene-1,3-diol (320) was isolated from deep-sea derived fungus A. terreus CC-S06-18 (Huang et al., 2024). Compound 320 showed inhibitory activity against V. parahaemolyticus ATCC 17802, exhibiting an MIC value of 7.8 μg/mL. 1-(2,6-Dihydroxy-4-methoxy-3,5-dimethylphenyl)-2-methylbutan-1-one (321) was isolated from A. unguis GXIMD 02505 (Zhang Y. T. et al., 2022). Compound 321 showed inhibitory activities against M. variabilis and M. jannaschii with MIC values of 8.0 and 32.0 μg/mL, respectively. Two novel compounds, asperporonins A (322) and B (323), were separated from a marine fungus A. terreus SCSIO 41202 (Zhang et al., 2024). Compounds 322 and 323 showed antibacterial effects against X. citri subsp. citri with the same MIC value of 0.3125 mg/mL. Terrusnolide A (324) was separated from the deep-sea-derived fungus Aspergillus sp. SCSIO 41029 (Chen et al., 2021). Compound 324 displayed inhibitory activity against S. aureus with an MIC value of 6.25 μg/mL. Candidusin A (325), terphenyllin (326), and 4″-deoxyterphenyllin (327) were separated from a coral-derived fungus Aspergillus sp. SCSIO40435 (Ye et al., 2022). Compound 325 showed antibacterial activities against E. coli, A. baumannii, S. aureus, and MRSA with the MIC values of 1.0, 64.0, 32.0, and 16.0 μg/mL, respectively. Compound 326 had strong antibacterial activity against E. coli with an MIC value of 0.5 μg/mL. Compound 327 exhibited weak inhibitory activity against B. subtilis and M. luteus with MIC values of 64.0 and 32.0 μg/mL, respectively. 5[(3E,5E)-nona-3,5-dien-1-yl]benzene (328) was separated from the sponge-associated fungus A. stellatus KUFA2017 (Machado et al., 2022). Compound 328 showed antibacterial activity against E. faecalis ATCC 29212, E. faecalis B3/101 (VRE), S. aureus, and MRSA with the MIC values of 16.0, 16.0, 32.0, and 16.0 μg/mL, respectively (9R,10E,12E)-9-methoxyoctadecadienoic acid (329) was separated from a marine fungus A. terreus SCSIO41202 (Zhang et al., 2024). Compound 329 showed an antibacterial effect against X. citri subsp. citri with an MIC value of 0.078 mg/mL. Three undescribed compounds, carnemycins H–I (330–331) and stromemycin B (332), together with six phenolic compounds carnemycin E (333), carnemycin B (334), carnemycin A (335), 2,4-dihydroxy-6-[(3E,5E)-nona-3,5-dien-1-yl]-benzoic acid (336), and stromemycin (337), were separated from marine-derived fungus A. ustus (Xue et al., 2024). Compounds 330–337 showed different inhibitory activity against R. solanacearum with MIC values from 3 to 35 μg/mL (Figure 14).
3 Comprehensive overview and conclusions
In recent years, marine fungi have become a research hotspot because they can produce bioactive compounds. In conjunction with a series of previous literature, we conducted a comprehensive study focusing on antimicrobial compounds produced by Aspergillus fungi from different marine origins between January 2010 and June 2024 in Table 1.
The structural diversities of the antibacterial secondary metabolites isolated from Aspergillus spp. are shown in Figure 15. The reported numbers of Aspergillus were based on structural classification, including 32 terpenoids, 98 nitrogen-containing compounds, 139 polyketides, 18 steroids, and 50 other derivatives discovered. The number and types of compounds with broad-spectrum antibacterial activity, activity against resistant bacteria, and activity against non-human pathogenic bacteria are shown in Figure 16.
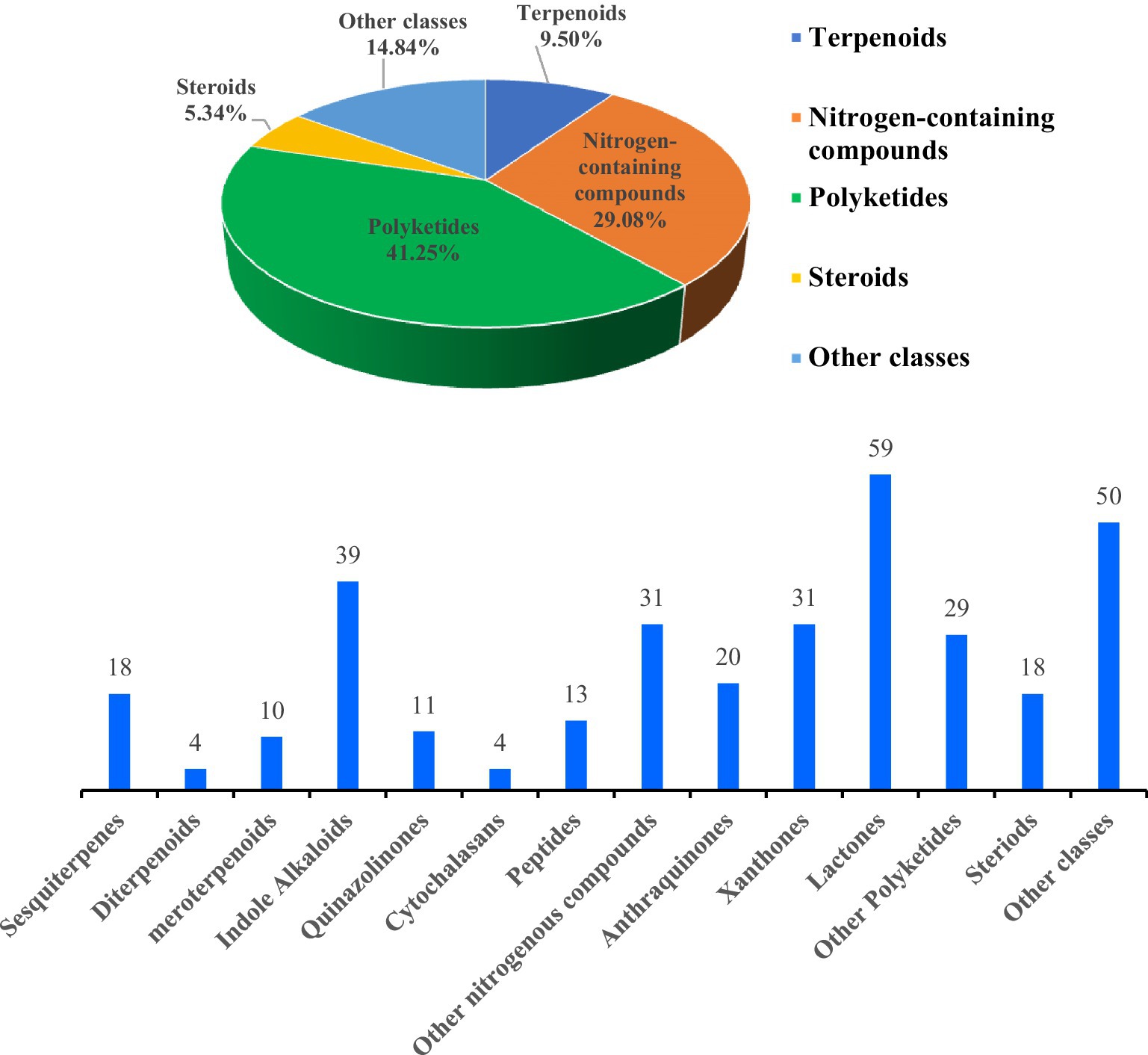
Figure 15. Structural diversity of the antibacterial secondary metabolites from the genus of Aspergillus (January 2010 to June 2024).
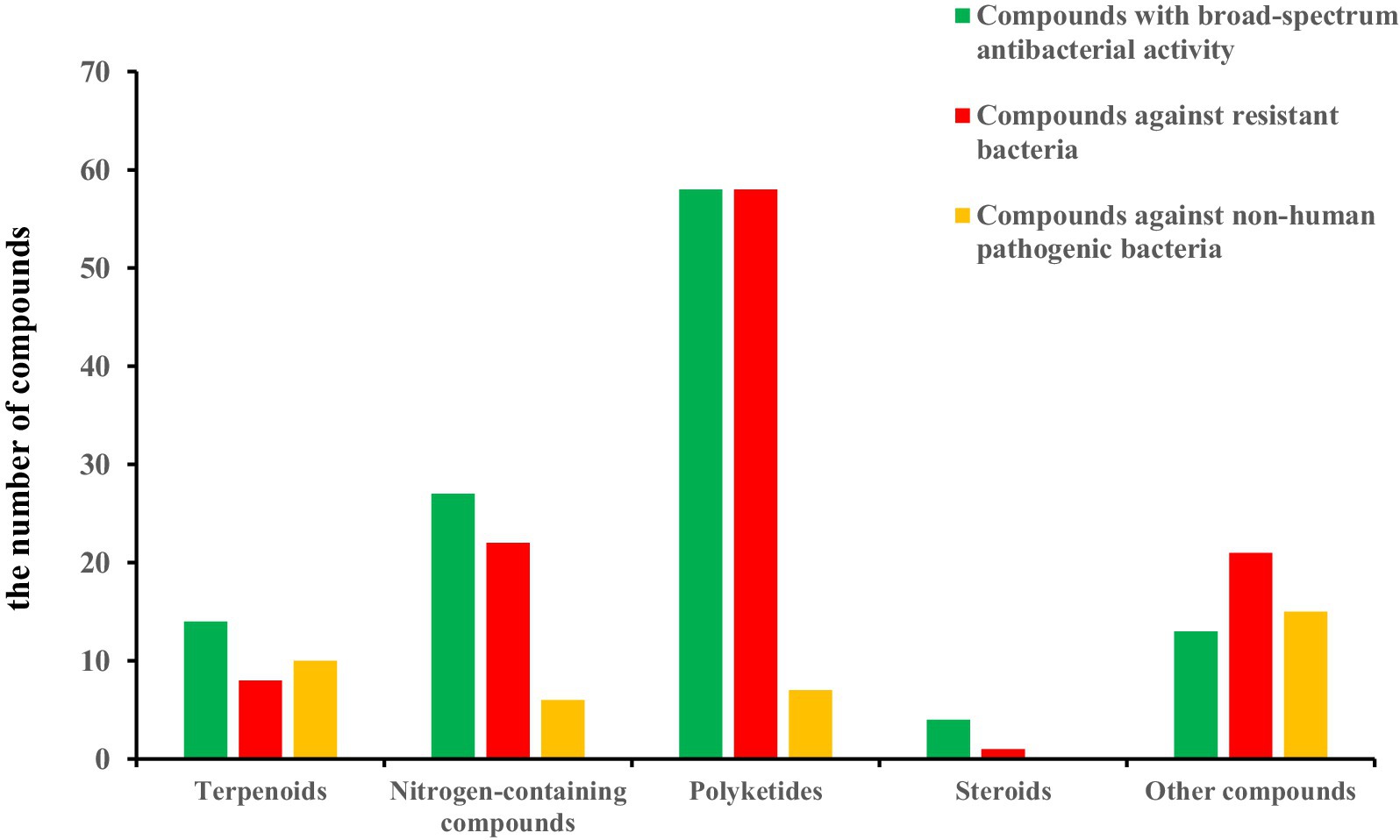
Figure 16. The number and types of compounds with broad-spectrum antibacterial activity, activity against resistant bacteria, and activity against non-human pathogenic bacteria.
Interesting, the conjugated double bonds at C-16 and C-18 are essential for the antibacterial activities of the ophiobolin sesterterpenes when having −CH2OH (2) or −CHO (3) groups positioned at C-7 (Chi et al., 2020). Notoamides (69–71, 118, and 119) are featured by the conserved moieties of a pyranoindole ring and a proline-bearing bicyclo[2.2.2]diazaoctane core. Sclerotiamide L (65) with a 6,6,5,7,6,5-ring system inhibited pathogenic bacteria including methicillin-resistant S. aureus (Meng et al., 2022). Nevertheless, this study provides indole diketopiperazine alkaloids as the undescribed natural scaffolds for the development of antibacterial agents. A large number of depsidone derivatives (203–221) had antibacterial activity against S. aureus and MRSA has been reported in the literature (Handayani et al., 2020; Zhang Y. T. et al., 2022; Thi et al., 2023; Saetang et al., 2021). The possible and preliminary structure–activity relationship was discussed; the phenolic hydroxyl group can improve the activity. Natural polyphenol compounds have significant antimicrobial activity (Chen et al., 2024). The chlorine-substituted group can be beneficial for the activity.
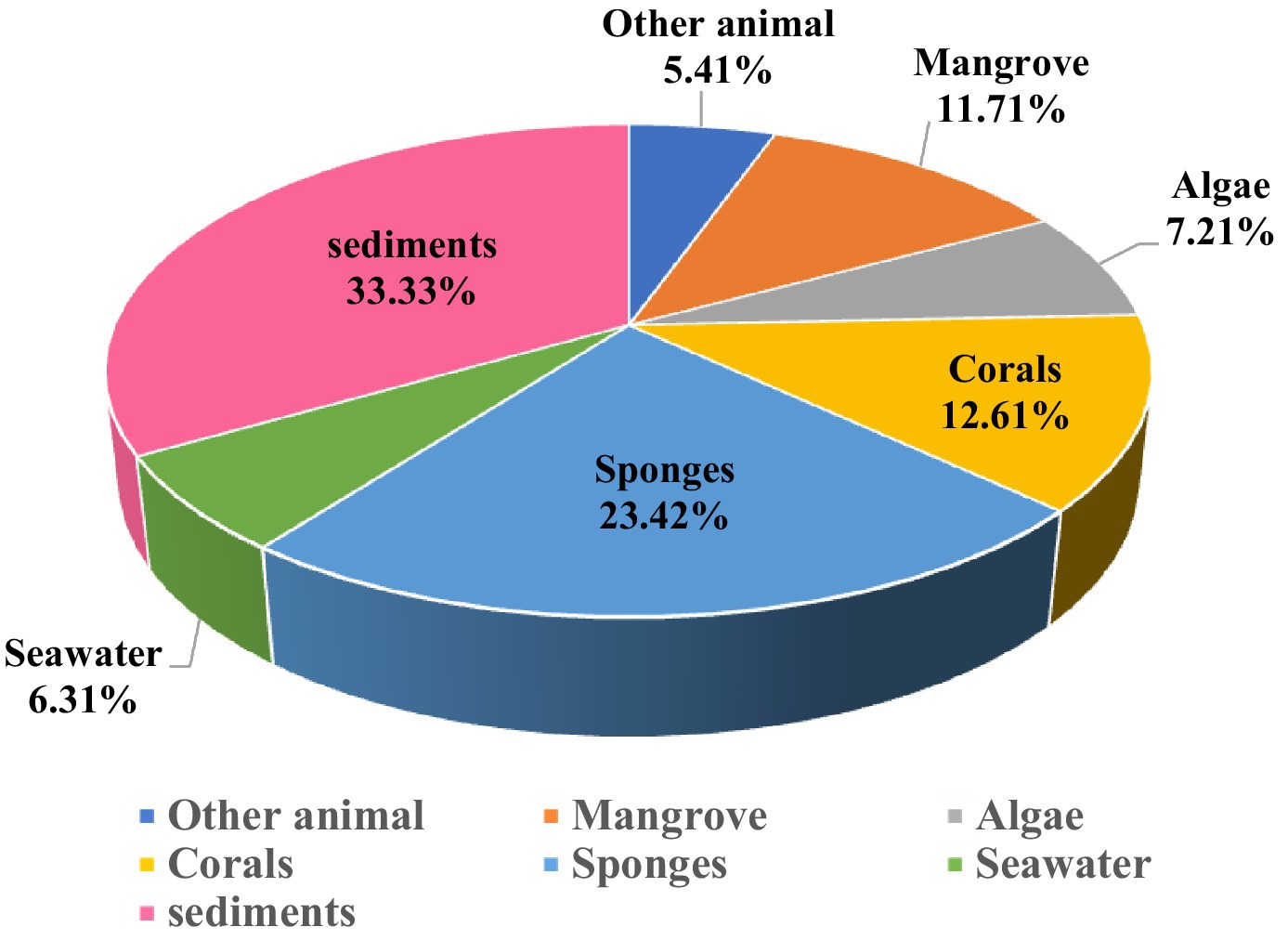
We sorted out the different marine sources of these Aspergillus spp., such as marine algae, corals, sponges, other animals, mangroves, seawater, and marine sediments, are shown in Figure 17. The most Aspergillus spp. were derived from marine sediment, accounting for 33.33%, and from marine sponges ranked second, comprising 23.42% of the total.
The number of antibacterial secondary metabolites from the genus of Aspergillus annually from 2010 to 2023 is shown in Figure 18. The progress of research in antimicrobial compounds from the genus Aspergillus was relatively slow from 2010 to 2017. However, there has been rapid development in antimicrobial research since 2018. These data indicated that research related to antibacterial compounds from Aspergillus spp. is increasingly receiving attention. Many of these compounds show inhibitory effects against S. aureus, while some showed activity against E. coli and B. subtilis. These active compounds hold promise for treating bacterial infections, offering valuable insights for the development of new anti-infective drugs.
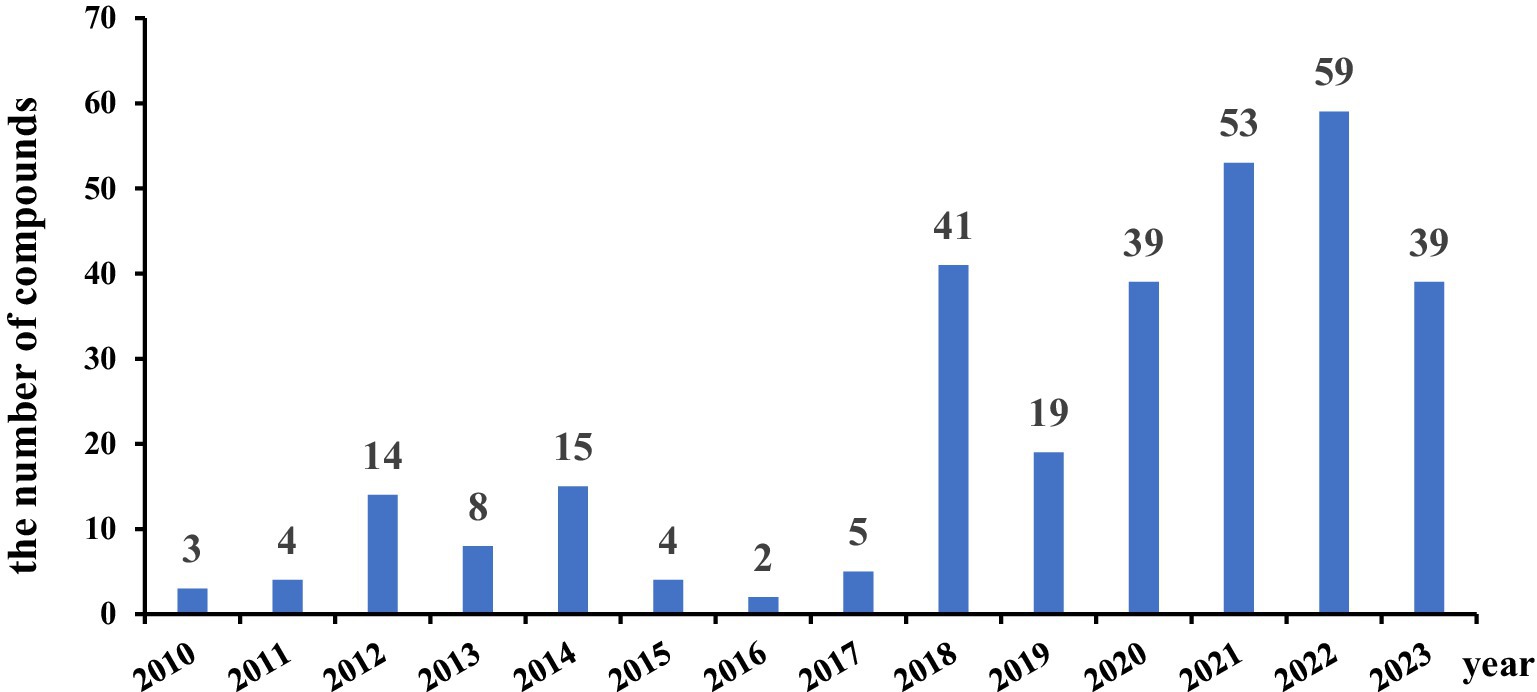
Figure 18. Each year of the antibacterial secondary metabolites from the genus of Aspergillus (2010–2023) (the data for 2024 is not accurate, so it will not be included).
Notably, some antimicrobial compounds produced by Aspergillus fungi also showed activities against agriculture and fish pathogenic bacteria and so on. For example, asperalin E (115), with a rare 4-amino-2-butanone moiety, exhibited the strongest inhibitory effects against fish pathogenic bacterium S. iniae, with potential for development as a new bactericide, and asperalin F (116) showed moderate-to-potent inhibitory activity against three fish pathogenic bacterium among E. ictalurid, S. iniae, and S. parauberis, with potential for development as a new bactericide. (9R,10E,12E)-9-methoxyoctadecadienoic acid (329) exhibited an excellent anti-Xanthomonas citri subsp. citri effect with the MIC value of 0.078 mg/mL, which was significantly more potent than the positive control CuSO4 (MIC, 0.3125 mg/mL). Compound 329 inhibited cell growth by disrupting biofilm formation, destroying the cell membrane, and inducing the accumulation of reactive oxygen species. Compound 6 is highly effective in controlling citrus canker disease in vivo tests, indicating 6 has the potential to lead compound for the development of new environmentally friendly and efficient anti-Xcc pesticides (Zhang et al., 2024). Stromemycin B (332) could effectively control the development of wilting symptoms and considerably minimize the occurrence of bacterial wilt in tomato plants. At 14 days after inoculation, compound 332 exerted a controlled efficacy of over 80% at a concentration of 100 μg/mL, which was better than that of streptomycin sulfate (100 μg/mL), indicating that compound 332 was a significant candidate as an antibacterial agent against Ralstonia solanacearum (Xue et al., 2024). These results suggested that the antibacterial lead compounds might be used as one of the probable candidates’ drugs for “One Health” in the utilization in healthcare, agriculture, and fishery.
4 Conclusion
337 secondary metabolites (including 145 new compounds) were isolated from marine-derived Aspergillus fungi; the compounds were classified into five chemical types: 32 terpenoids, 98 nitrogen-containing compounds, 139 polyketides, 18 steroids, and 50 other derivatives (Figure 15). The distribution of these compounds is as follows: terpenoids (9.50%), nitrogen-containing compounds (29.08%), polyketides (41.25%), steroids (5.34%), and other compounds (14.84%). Polyketides displayed the most substantial proportion of the observed antibacterial compounds, alongside notable contributions from terpenoids and nitrogen-containing compounds. This comprehensive analysis highlights the potential for developing antimicrobial agents from these natural products.
Additionally, the samples were obtained from various environments: 7.21% from algae, 12.61% from corals, 23.42% from sponges, 5.41% from other animals, 11.71% from mangroves, and 6.31% from seawater. Most significantly, 33.33% originated from sediment samples (Figure 18). This extensive environmental sampling underscores the compounds’ efficacy and potential applications in combating antibiotic-resistant bacteria. Specifically, terpenoid compounds were classified as 18 sesquiterpenes, four diterpenes, and 10 meroterpenoids. Nitrogen-containing compounds included 39 indole alkaloids, 11 quinazolinone alkaloids, four cytochalasan alkaloids, 13 peptides, and 31 other nitrogen-containing compounds. Polyketide compounds were identified as 20 anthraquinones, 31 xanthones, 59 lactones, and 29 other polyketide metabolites. 18 steriods and 50 other classes are shown in Figure 15. We observed that research progress in antimicrobial compounds from the genus of Aspergillus was relatively slow from 2010 to 2017. However, there has been rapid development in antimicrobial research since 2018. These data indicated that research related to antibacterial compounds from Aspergillus spp. are increasingly receiving attention. By classifying multiple antibacterial compounds, a foundation is laid for predicting which types may exert more potent pharmacological effects on specific biological targets, guiding drug design and validation through simulation or experimentation.
Among all antibacterial active compounds, some were found to have activity levels approaching or reaching the nanomolar range, such as fumigatoside F (65), cytochalasin Z17 (75), dihydroisoflavipucine (90), emeguisin A (204), and fusidic acid (265). As a first-in-class BCG-selective diketopiperazine dimer antibiotic, brevianamide S (34) was indicative of a possible new mechanism of action that could, if translated to M. tuberculosis, represent a valuable new lead in the search for next-generation antitubercular drugs. These compounds could become promising lead compounds for use as antimicrobial agents in the future. Notably, some antimicrobial compounds produced by Aspergillus fungi also showed activities against agriculture and fish pathogenic bacteria, and so on.
In summary, the chemical diversity and potent antibacterial activities ofsecondary metabolites from marine-derived Aspergillus species indicated their potential in antibiotic drug discovery. The identified metabolites demonstrate a wide range of antimicrobial activities, showing potent effects against various pathogens. Future research aims to elucidate their mechanisms of action and optimize production methods to fully harness their therapeutic potential in fighting infectious diseases. Marine-derived Aspergillus species present a promising frontier for developing novel natural products with applications in medical treatments and agricultural antimicrobial agents.
Author contributions
BW: Writing – original draft, Data curation. JC: Writing – original draft, Data curation. LH: Writing – review & editing. YC: Writing – review & editing. RW: Writing – review & editing. ML: Writing – review & editing. MY: Writing – review & editing. MZ: Writing – review & editing. Nasihat: Writing – review & editing. GC: Project administration, Supervision, Writing – review & editing. GH: Project administration, Supervision, Writing – review & editing, Data curation, Software, Writing – original draft. CZ: Methodology, Project administration, Supervision, Writing – review & editing, Data curation, Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (Nos. 32160108 and 2217702), the Key Research and Development Program of Hainan Province (No. ZDYF2024SHFZ116 and ZDYF2021SHFZ270), the Team Innovation Center for Academicians of Hainan Province, the Specific Research Fund for the Innovation Center of Hainan Province Academicians (No. YSPTZX202309), and the Key Science and Technology Program of Hainan Province (No. ZDKJ202008).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer FC declared a past co-authorship with the author CZ to the handling editor.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alahmari, A. N., Hassoubah, S. A., and Alaidaroos, B. A. (2022). Sponges-associated marine bacteria as sources of antimicrobial compounds. Novel. Res. Microbiol. J. 6, 1742–1767. doi: 10.21608/nrmj.2022.267424
An, C. L., Kong, F. D., Ma, Q. Y., Xie, Q. Y., Yuan, J. Z., Zhou, L. M., et al. (2018). Chemical constituents of the marine-derived fungus Aspergillus sp. SCS-KFD66. Mar. Drugs 16:468. doi: 10.3390/md16120468
Anh, C. V., Kwon, J. H., Kang, J. S., Lee, H. S., Heo, C. S., and Shin, H. J. (2022). Antibacterial and cytotoxic phenolic polyketides from two marine-derived fungal strains of Aspergillus unguis. Pharmaceuticals 15:74. doi: 10.3390/ph15010074
Bai, Z. Q., Lin, X. P., Wang, Y. Z., Wang, J. F., Zhou, X. F., Yang, B., et al. (2014). New phenyl derivatives from endophytic fungus Aspergillus flavipes AIL8 derived of mangrove plant Acanthus ilicifolius. Fitoterapia 95, 194–202. doi: 10.1016/j.fitote.2014.03.021
Bao, J., Li, X. X., He, F., Zhang, X. Y., Zhu, K. K., Tao, H. R., et al. (2020). Asperbutenolide a, an unusual aromatic butenolide dimer with diverse bioactivities from a marine-derived fungus Aspergillus terreus SCAU011. Tetrahedron Lett. 61:152193. doi: 10.1016/j.tetlet.2020.152193
Bao, J., Li, X. X., Zhu, K. K., He, F., Wang, Y. Y., Yu, J. H., et al. (2021). Bioactive aromatic butenolides from a mangrove sediment originated fungal species, Aspergillus terreus SCAU011. Fitoterapia 150:104856. doi: 10.1016/j.fitote.2021.104856
Buttachon, S., Ramos, A. A., Inacio, A., Dethoup, T., Gales, L., Lee, M., et al. (2018). Bis-indolyl benzenoids, hydroxypyrrolidine derivatives and other constituents from cultures of the marine sponge-associated fungus Aspergillus candidus KUFA0062. Mar. Drugs 16:119. doi: 10.3390/md16040119
Cai, J., Chen, C. M., Tan, Y. H., Chen, W. H., Luo, X. W., Luo, L. X., et al. (2021). Bioactive polyketide and diketopiperazine derivatives from the mangrove-sediment-derived fungus Aspergillus sp. SCSIO41407. Molecules 26:4851. doi: 10.3390/molecules26164851
Cai, R. L., Jiang, H. M., Zang, Z. M., Li, C. Y., and She, Z. G. (2019). New benzofuranoids and phenylpropanoids from the mangrove endophytic fungus, Aspergillus sp. ZJ-68. Mar. Drugs 17:478. doi: 10.3390/md17080478
Cai, J., Wang, X. N., Gan, X., Zhou, Q., Luo, X. W., Yang, B., et al. (2023). New chlorinated metabolites and antiproliferative polyketone from the mangrove sediments-derived fungus Mollisia sp. SCSIO41409. Mar. Drugs 21:32. doi: 10.3390/md21010032
Cardoso-Martinez, F., De la Rosa, J. M., Diaz-Marrero, A. R., Darias, J., D'Croz, L., Cerella, C., et al. (2015). Oximoaspergillimide, a fungal derivative from a marine isolate of Aspergillus sp. Eur. J. Org. Chem. 2015, 2256–2261. doi: 10.1002/ejoc.201403668
Carroll, A. R., Copp, B. R., Davis, R. A., Keyzers, R. A., and Prinsep, M. R. (2024). Marine natural products. Nat. Prod. Rep. 41, 162–207. doi: 10.1039/D3NP00061C
Cen, S. Y., Jia, J., Ge, Y. C., Ma, Y. H., Li, X. Y., Wei, J. H., et al. (2021). A new antibacterial 3,5-dimethylorsellinic acid-based meroterpene from the marine fungus Aspergillus sp. CSYZ-1. Fitoterapia 152:104908. doi: 10.1016/j.fitote.2021.104908
Charani, E., Mendelson, M., Pallett, S. J. C., Ahmad, R., Mpundu, M., Mbamalu, O., et al. (2023). An analysis of existing national action plans for antimicrobial resistance-gaps and opportunities in strategies optimising antibiotic use in human populations. Lancet Glob. Health 11, e466–e474. doi: 10.1016/S2214-109X(23)00019-0
Chen, W. H., Chen, C. M., Long, J. Y., Lan, S. J., Lin, X. P., Liao, S. R., et al. (2021). Bioactive secondary metabolites from the deep-sea derived fungus Aspergillus sp. SCSIO 41029. J. Antibiot. 74, 156–159. doi: 10.1038/s41429-020-00378-y
Chen, M., Fu, X. M., Kong, C. J., and Wang, C. Y. (2014). Nucleoside derivatives from the marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 28, 895–900. doi: 10.1080/14786419.2014.891114
Chen, X. N., Lan, W. Q., and Xie, J. (2024). Natural phenolic compounds: antimicrobial properties, antimicrobial mechanisms, and potential utilization in the preservation of aquatic products. Food Chem. 440:138198. doi: 10.1016/j.foodchem.2023.138198
Chen, W. H., Liu, H. Y., Long, J. Y., Tao, H. M., Lin, X. P., Liao, S. R., et al. (2020). Asperpentenone a, a novel polyketide isolated from the deep-sea derived fungus Aspergillus sp. SCSIO 41024. Phytochem. Lett. 35, 99–102. doi: 10.1016/j.phytol.2019.11.009
Chen, B., Qiu, P. J., Xu, B. F., Zhao, Q. M., Gu, Y. C., Fu, L., et al. (2022). Cytotoxic and antibacterial isomalabaricane terpenoids from the sponge Rhabdastrella globostellata. J. Nat. Prod. 85, 1799–1807. doi: 10.1021/acs.jnatprod.2c00348
Chen, M., Shao, C. L., Fu, X. M., Xu, R. F., Zheng, J. J., Zhao, D. L., et al. (2013). Bioactive indole alkaloids and phenyl ether derivatives from a marine-derived Aspergillus sp. fungus. J. Nat. Prod. 76:1229. doi: 10.1021/np400465r
Chen, X. Y., Zeng, Q., Chen, Y. C., Zhong, W. M., Xiang, Y., Wang, J. F., et al. (2022). Chevalones H-M: six new α-pyrone meroterpenoids from the gorgonian coral-drived fungus Aspergillus hiratsukae SCSIO 7S2001. Mar. Drugs 20:71. doi: 10.3390/md20010071
Chi, L. P., Li, X. M., Wan, Y. P., Li, Y. H., Li, X., and Wang, B. G. (2021a). Two new phenol derivatives from the cold seep-derived fungus Aspergillus insuetus SD-512. Chem. Biodivers. 18:e2100512. doi: 10.1002/cbdv.202100512
Chi, L. P., Li, X. M., Wan, Y. P., Li, X., and Wang, B. G. (2020). Ophiobolin sesterterpenoids and farnesylated phthalide derivatives from the deep sea cold-seep-derived fungus Aspergillus insuetus SD-512. J. Nat. Prod. 83, 3652–3660. doi: 10.1021/acs.jnatprod.0c00860
Chi, L. P., Liu, D., Li, X. M., Wan, Y. P., Wang, B. G., and Li, X. (2023). Aspertides A-E: antimicrobial pentadepsipeptides with a unique p-methoxycinnamoyl amide group from the marine isolates Aspergillus tamarii MA-21 and Aspergillus insuetus SD-512. J. Agr. Food. Chem. 71, 13316–13324. doi: 10.1021/acs.jafc.3c02610
Chi, L. P., Yang, S. Q., Li, X. M., Li, X. D., Wang, B. G., and Li, X. (2021b). A new steroid with 7β,8β-epoxidation from the deep sea-derived fungus Aspergillus penicillioides SD-311. J. Asian Nat. Prod. Res. 23, 884–891. doi: 10.1080/10286020.2020.1791096
de Alcântara Rodrigues, I., Ferrari, R. G., Panzenhagen, P. H. N., Mano, S. B., and Conte, C. A. J. (2020). Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 112, 143–183. doi: 10.1016/bs.aambs.2020.03.001
Ding, L. J., Ren, L., Li, S., Song, J. J., Han, Z. W., He, S., et al. (2019). Production of new antibacterial 4-hydroxy-α-pyrones by a marine fungus Aspergillus niger cultivated in solid medium. Mar. Drugs 17:344. doi: 10.3390/md17060344
Dong, Y. L., Li, X. M., Shi, X. S., Wang, Y. R., Wang, B. G., and Meng, L. H. (2023a). Diketopiperazine alkaloids and bisabolene sesquiterpenoids from Aspergillus versicolor AS-212, an endozoic fungus associated with deep-sea coral of magellan seamounts. Mar. Drugs 21:293. doi: 10.3390/md21050293
Dong, Y. L., Li, X. M., Wang, Y. R., Shi, X. S., Wang, B. G., and Meng, L. H. (2023b). Oxepine-containing pyrazinopyrimidine alkaloids and quinolinone derivatives produced by Aspergillus versicolor AS-212, a deep-sea-derived endozoic fungus. Fitoterapia 168:105559. doi: 10.1016/j.fitote.2023.105559
Duraes, F., Szemeredi, N., Kumla, D., Pinto, M., Kijjoa, A., and Spengler, G. (2021). Metabolites from marine-derived fungi as potential antimicrobial adjuvants. Mar. Drugs 19:475. doi: 10.3390/md19090475
Gou, X. S., Jia, J., Xue, Y. X., Ding, W. J., Dong, Z. T., Tian, D. M., et al. (2020). New pyrones and their analogs from the marine mangrove-derived Aspergillus sp. DM94 with antibacterial activity against Helicobacter pylori. Appl. Microbiol. Biotechnol. 104, 7971–7978. doi: 10.1007/s00253-020-10792-9
Gow, N. A. R., Johnson, C., Berman, J., Coste, A. T., Cuomo, C. A., Perlin, D. S., et al. (2022). The importance of antimicrobial resistance in medical mycology. Nat. Commun. 13:5352. doi: 10.1038/s41467-022-32249-5
Graziano, T. S., Cuzzullin, M. C., Franco, G. C., Schwartz-Filho, O., Dias, D. A. E., Groppo, F. C., et al. (2015). Statins and antimicrobial effects: simvastatin as a potential drug against Staphylococcus aureus biofilm. PLoS One 10:e0128098. doi: 10.1371/journal.pone.0128098
Guo, C., Wang, P., Pang, X. Y., Lin, X. P., Liao, S. R., Yang, B., et al. (2021). Discovery of a dimeric zinc complex and five cyclopentenone derivatives from the sponge-associated fungus Aspergillus ochraceopetaliformis. ACS Omega 6, 8942–8949. doi: 10.1021/acsomega.0c06218
Guo, Z. K., Zhou, Y. Q., Han, H., Wang, W., Xiang, L., Deng, X. Z., et al. (2018). New antibacterial phenone derivatives asperphenone A-C from mangrove-derived fungus Aspergillus sp. YHZ-1. Mar. Drugs 16:45. doi: 10.3390/md16020045
Ha, Y. R., Zhou, Y. F., Ma, M. Z., Wang, N., Wang, P. B., and Zhang, Z. Z. (2024). Antimicrobial metabolites from the marine-derived fungus Aspergillus sp. ZZ1861. Phytochemistry 224:114164. doi: 10.1016/j.phytochem.2024.114164
Haenni, M., Dagot, C., Chesneau, O., Bibbal, D., Labanowski, J., Vialette, M., et al. (2022). Environmental contamination in a high-income country (France) by antibiotics, antibiotic-resistant bacteria, and antibiotic resistance genes: status and possible causes. Environ. Int. 159:107047. doi: 10.1016/j.envint.2021.107047
Hai, Y., Wei, M. Y., Wang, C. Y., Gu, Y. C., and Shao, C. L. (2021). The intriguing chemistry and biology of sulfur-containing natural products from marine microorganisms (1987-2020). Mar. Life Sci. Tech. 3, 488–518. doi: 10.1007/s42995-021-00101-2
Han, J. H., Yang, N., Wei, S. Z., Jia, J., Lin, R., Li, J. P., et al. (2022). Dimeric hexylitaconic acids from the marine-derived fungus Aspergillus welwitschiae CUGBMF180262. Nat. Prod. Res. 36, 578–585. doi: 10.1080/14786419.2020.1793152
Han, Y. Q., Zhang, Q., Xu, W. F., Hai, Y., Chao, R., Wang, C. F., et al. (2023). Targeted isolation of antitubercular cycloheptapeptides and an unusual pyrroloindoline-containing new analog, asperpyrroindotide a, using LC-MS/MS-based molecular networking. Mar. Life Sci. Tech. 5, 85–93. doi: 10.1007/s42995-022-00157-8
Handayani, D., Dwinatrana, K., and Rustini, R. (2022). Antibacterial compound from marine sponge derived fungus Aspergillus sydowii DC08. Rasayan J. Chem. 15, 2485–2492. doi: 10.31788/RJC.2022.1546971
Handayani, D., Rendowati, A., Aminah, I., Ariantari, N. P., and Proksch, P. (2020). Bioactive compounds from marine sponge derived fungus Aspergillus unguis WR8. Rasayan. J. Chem. 13, 2633–2638. doi: 10.31788/RJC.2020.1345781
Holland, D. C., Prebble, D. W., Er, S., Hayton, J. B., Robertson, L. P., Avery, V. M., et al. (2022). α-Synuclein aggregation inhibitory prunolides and a dibrominated β-carboline sulfamate from the ascidian Synoicum prunum. J. Nat. Prod. 85, 441–452. doi: 10.1021/acs.jnatprod.1c01172
Howden, B. P., Giulier, S. G., Lung, T. W. F., Baines, S. L., Sharkey, L. K., Lee, J. Y. H., et al. (2023). Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 21, 380–395. doi: 10.1038/s41579-023-00852-y
Hu, Y. Y., Yang, M., Zhao, J., Liao, Z. X., Qi, J., Wang, X. Z., et al. (2019). A meroterpenoid isolated from the fungus Aspergillus sp. Nat. Prod. Commun. 14:1934578X1987893. doi: 10.1177/1934578X19878933
Hu, Z. B., Zhu, Y. J., Chen, J. J., Chen, J., Li, C. Y., Gao, Z. Z., et al. (2023). Discovery of novel bactericides from Aspergillus alabamensis and their antibacterial activity against fish pathogens. J. Agric. Food. Chem. 71, 4298–4305. doi: 10.1021/acs.jafc.2c09141
Huang, Z. H., Liang, X., Gu, Q., Ma, X., and Qi, S. H. (2023). Punicesterones A-G, polyhydroxylated mycoecdysteroids from the deep-sea-derived fungus Aspergillus puniceus SCSIO z021. Phytochemistry 205:113511. doi: 10.1016/j.phytochem.2022.113511
Huang, X. M., Wang, Y. C., Li, G. Y., Shao, Z. Z., Xia, J. M., Qin, J. J., et al. (2024). Secondary metabolites from the deep-sea derived fungus Aspergillus terreus MCCC M28183. Front. Microbiol. 15:1361550. doi: 10.3389/fmicb.2024.1361550
Ibrahim, S. R. M., Mohamed, S. G. A., Alsaadi, B. H., Althubyani, M. M., Awari, Z. I., Hussein, H. G. A., et al. (2023). Secondary metabolites, biological activities, and industrial and biotechnological importance of Aspergillus sydowii. Mar. Drugs 21:441. doi: 10.3390/md21080441
Ikuta, K. S., Swetschinski, L. R., Aguilar, G. R., Sharara, F., Mestrovic, T., Gray, A. P., et al. (2022). Global mortality associated with 33 bacterial pathogens in 2019: a systematic analysis for the global burden of disease study 2019. Lancet 400, 2221–2248. doi: 10.1016/S0140-6736(22)02185-7
Jeewon, R., Aullybux, A. A., Puchooa, D., Nazurally, N., Alrefaei, A. F., and Zhang, Y. (2023). Marine microbial polysaccharides: an untapped resource for biotechnological applications. Mar. Drugs 21:420. doi: 10.3390/md21070420
Jin, M., Osman, M., Green, B. A., Yang, Y. F., Ahuja, A., Lu, Z. Y., et al. (2023). Evidence for the transmission of antimicrobial resistant bacteria between humans and companion animals: a scoping review. One Health 17:100593. doi: 10.1016/j.onehlt.2023.100593
King, A. M., Reid-Yu, S. A., Wang, W., King, D. T., Pascale, G. D., Strynadka, N. C., et al. (2014). Aspergillomarasmine A overcomes metallo-β-lactamase antibiotic resistance. Nature 510, 503–506. doi: 10.1038/nature13445
Kong, F. D., Huang, X. L., Ma, Q. Y., Xie, Q. Y., Wang, P., Chen, P. W., et al. (2018). Helvolic acid derivatives with antibacterial activities against Streptococcus agalactiae from the marine-derived fungus Aspergillus fumigatus HNMF0047. J. Nat. Prod. 81, 1869–1876. doi: 10.1021/acs.jnatprod.8b00382
Kumla, D., Sousa, E., Marengo, A., Dethoup, T., Pereira, J. A., Gales, L., et al. (2021). 1,3-dioxepine and spiropyran derivatives of viomellein and other dimeric naphthopyranones from cultures of Aspergillus elegans KUFA0015 and their antibacterial activity. Phytochemistry 181:112575. doi: 10.1016/j.phytochem.2020.112575
Lee, Y. M., Kim, M. J., Li, H. Y., Zhang, P., Bao, B. Q., Lee, K. J., et al. (2013). Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 15, 499–519. doi: 10.1007/s10126-013-9506-3
Lee, Y. M., Li, H. Y., Hong, J. K., Cho, H. Y., Bae, K. S., Kim, M. A., et al. (2010). Bioactive metabolites from the sponge-derived fungus Aspergillus versicolor. Arch. Pharm. Res. 33, 231–235. doi: 10.1007/s12272-010-0207-4
Li, H. H., Fu, Y. Q., and Song, F. H. (2023). Marine Aspergillus: a treasure trove of antimicrobial compounds. Mar. Drugs 21:277. doi: 10.3390/md21050277
Li, W. H., Gao, Q., Hu, Y. J., Shi, Y. T., Yan, X. J., Ding, L. J., et al. (2023). Dibetanide, a new benzofuran derivative with the rare conjugated triene side chain from a sponge-associated fungus Aspergillus species. J. Mol. Struct. 1271:134082. doi: 10.1016/j.molstruc.2022.134082
Li, J. L., Jiang, X., Liu, X. P., He, C. W., Di, Y. X., Lu, S. J., et al. (2019). Antibacterial anthraquinone dimers from marine derived fungus Aspergillus sp. Fitoterapia 133, 1–4. doi: 10.1016/j.fitote.2018.11.015
Li, X. D., Li, X., Li, X. M., Xu, G. M., Zhang, P., Meng, L. H., et al. (2016). Tetranorlabdane diterpenoids from the deep sea sediment-derived fungus Aspergillus wentii SD-310. Planta Med. 82, 877–881. doi: 10.1055/s-0042-102965
Li, X. D., Li, X., Li, X. M., Yin, X. L., and Wang, B. G. (2021). Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat. Prod. Res. 35, 4265–4271. doi: 10.1080/14786419.2019.1696792
Li, S. D., Wei, M. Y., Chen, G. Y., and Lin, Y. C. (2012). Two new dihydroisocoumarins from the endophytic fungus Aspergillus sp. collected from the South China Sea. Chem. Nat. Compd. 48, 371–373. doi: 10.1007/s10600-012-0254-9
Li, J. X., Xu, Q. H., Shang, R. Y., Liu, Q., Luo, X. C., Lin, H. W., et al. (2023). Aspergetherins A-D, new chlorinated biphenyls with anti-MRSA activity from the marine sponge symbiotic fungus Aspergillus terreus 164018. Chem. Biodivers. 20:e202300010. doi: 10.1002/cbdv.202300010
Limbadri, S., Luo, X. W., Lin, X. P., Liao, S. R., Wang, J. F., Zhou, X. F., et al. (2018). Bioactive novel indole alkaloids and steroids from deep sea-derived fungus Aspergillus fumigatus SCSIO 41012. Molecules 23:2379. doi: 10.3390/molecules23092379
Lin, S. X., Li, J., Chen, W. Z., He, J. X., Shi, Y. T., Jin, H. X., et al. (2023). A new antibacterial dihydroisocoumarin from the marine sponge-associated fungus Aspergillus sp. Chem. Nat. Compd. 59, 246–248. doi: 10.1007/s10600-023-03967-z
Lin, S. H., Yan, Q. X., Zhang, Y., Wu, T. Z., Zou, Z. B., Liu, Q. M., et al. (2023). Citriquinolinones a and B: rare isoquinolinone-embedded citrinin analogues and related metabolites from the deep-sea-derived Aspergillus versicolor 170217. Mar. Drugs 21:504. doi: 10.3390/md21100504
Liu, Y., Ding, L. J., He, J. X., Zhang, Z. M., Deng, Y. T., He, S., et al. (2021). A new antibacterial chromone from a marine sponge-associated fungus Aspergillus sp. LS57. Fitoterapia 154:105004. doi: 10.1016/j.fitote.2021.105004
Liu, Y., Ding, L. J., Shi, Y. T., Yan, X. J., Wu, B., and He, S. (2022). Molecular networking-driven discovery of antibacterial perinadines, new tetracyclic alkaloids from the marine sponge-derived fungus Aspergillus sp. ACS Omega 7, 9909–9916. doi: 10.1021/acsomega.2c00402
Liu, Y., Li, X. M., Meng, L. H., and Wang, B. G. (2015). Polyketides from the marine mangrove-derived fungus Aspergillus ochraceus MA-15 and their activity against aquatic pathogenic bacteria. Phytochem. Lett. 12, 232–236. doi: 10.1016/j.phytol.2015.04.009
Liu, X. H., Miao, F. P., Liang, X. R., and Ji, N. Y. (2014). Ergosteroid derivatives from an algicolous strain of Aspergillus ustus. Nat. Prod. Res. 28, 1182–1186. doi: 10.1080/14786419.2014.923996
Liu, X. H., Miao, F. P., Qiao, M. F., Cichewicz, R. H., and Ji, N. Y. (2013). Terretonin, ophiobolin, and drimane terpenes with absolute configurations from an algicolous Aspergillus ustus. RSC Adv. 3, 588–595. doi: 10.1039/C2RA22701K
Liu, W., Wang, L. P., Wang, B., Xu, Y. C., Zhu, G. L., Lan, M. M., et al. (2019). Diketopiperazine and diphenylether derivatives from marine algae-derived Aspergillus versicolor OUCMDZ-2738 by epigenetic activation. Mar. Drugs 17:6. doi: 10.3390/md17010006
Liu, C. M., Yao, F. H., Lu, X. H., Zhang, X. X., Luo, L. X., Liang, X., et al. (2022). Isoquinoline alkaloids as protein tyrosine phosphatase inhibitors from a deep-sea-derived fungus Aspergillus puniceus. Mar. Drugs 20:78. doi: 10.3390/md20010078
Lu, C. J., Tang, Z. Z., Su, Z. W., Li, H. Y., Zhang, G. S., Gao, C. H., et al. (2023). Secondary metabolites from marine-derived fungus Aspergillus carneus GXIMD00519. Rec. Nat. Prod. 17, 343–351. doi: 10.25135/rnp.355.2207.2518
Luo, X. W., Zhou, X. F., Lin, X. P., Qin, X. C., Zhang, T. Y., Wang, J. F., et al. (2017). Antituberculosis compounds from a deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. Nat. Prod. Res. 31, 1958–1962. doi: 10.1080/14786419.2016.1266353
Lv, H. W., Wang, K. B., Xue, Y. X., Chen, J., Su, H. B., Zhang, J. K., et al. (2021). Three new metabolites from the marine-derived fungus Aspergillus sp. WHUF03110. Nat. Prod. Commun. 16:1934578X2110550. doi: 10.1177/1934578X211055009
Lv, H. W., Zhang, J. K., Xue, Y. X., Li, S. W., Sun, X. Y., Jia, J., et al. (2022). Two new austocystin analogs from the marine-derived fungus Aspergillus sp. WHUF05236. Chem. Biodivers. 19:e202200207. doi: 10.1002/cbdv.202200207
Machado, F. P., Kumla, D., Pereira, J. A., Sousa, E., Dethoup, T., Freitas-Silva, J., et al. (2021). Prenylated phenylbutyrolactones from cultures of a marine sponge-associated fungus Aspergillus flavipes KUFA1152. Phytochemistry 185:112709. doi: 10.1016/j.phytochem.2021.112709
Machado, F. P., Rodrigues, I. C., Gales, L., Pereira, J. A., Costa, P. M., Dethoup, T., et al. (2022). New alkylpyridinium anthraquinone, isocoumarin, c-glucosyl resorcinol derivative and prenylated pyranoxanthones from the culture of a marine sponge-associated fungus, Aspergillus stellatus KUFA 2017. Mar. Drugs 20:672. doi: 10.3390/md20110672
Meng, Q. Y., Guo, X., Wu, J. S., Liu, D., Gu, Y. C., Huang, J., et al. (2022). Prenylated notoamide-type alkaloids isolated from the fungus Aspergillus sclerotiorum and their inhibition of NLRP3 inflammasome activation and antibacterial activities. Phytochemistry 203:113424. doi: 10.1016/j.phytochem.2022.113424
Miao, F. P., Li, X. D., Liu, X. H., Cichewicz, R. H., and Ji, N. Y. (2012). Secondary metabolites from an algicolous Aspergillus versicolor strain. Mar. Drugs 10, 131–139. doi: 10.3390/md10010131
Neuhaus, G. F., Adpressa, D. A., Bruhn, T., and Loesgen, S. (2019). Polyketides from marine-derived Aspergillus porosus: challenges and opportunities for determining absolute configuration. J. Nat. Prod. 82, 2780–2789. doi: 10.1021/acs.jnatprod.9b00416
Okeke, I. N., de Kraker, M. E. A., Van Boeckel, T. P., Kumar, C. K., Schmitt, H., Gales, A. C., et al. (2024). The scope of the antimicrobial resistance challenge. Lancet 403, 2426–2438. doi: 10.1016/S0140-6736(24)00876-6
Orfali, R., Aboseada, M. A., Abdel-Wahab, N. M., Hassan, H. M., Perveen, S., Ameen, F., et al. (2021). Recent updates on the bioactive compounds of the marine-derived genus Aspergillus. RSC Adv. 11, 17116–17150. doi: 10.1039/D1RA01359A
Peng, Q. Y., Cai, J., Long, J. Y., Yang, B., Lin, X. P., Wang, J. F., et al. (2021). New azaphthalide and phthalide derivatives from the marine coral-derived fungus Aspergillus sp. SCSIO41405. Phytochem. Lett. 43, 94–97. doi: 10.1016/J.PHYTOL.2021.03.019
Peng, Q. Y., Chen, W. H., Lin, X. P., Xiao, J., Liu, Y. H., and Zhou, X. F. (2022). Butenolides from the coral-derived fungus Aspergillius terreus SCSIO41404. Mar. Drugs 20:212. doi: 10.3390/md20030212
Pinedo-Rivilla, C., Aleu, J., and Duran-Patron, R. (2022). Cryptic metabolites from marine-derived microorganisms using OSMAC and epigenetic approaches. Mar. Drugs 20:84. doi: 10.3390/md20020084
Prestinaci, F., Pezzotti, P., and Pantosti, A. (2015). Antimicrobial resistance: a global multifaceted phenomenon. Pathog. Glob. Health 109, 309–318. doi: 10.1179/2047773215Y.0000000030
Ren, J. M., Yang, J. K., Zhu, H. J., and Cao, F. (2020). Bioactive steroids from the marine-derived fungus Aspergillus flavus JK07-1. Chem. Nat. Compd. 56, 945–947. doi: 10.1007/s10600-020-03195-9
Saetang, P., Rukachaisirikul, V., Phongpaichit, S., Preedanon, S., Sakayaroj, J., Hadsadee, S., et al. (2021). Antibacterial and antifungal polyketides from the fungus Aspergillus unguis PSU-MF16. J. Nat. Prod. 84, 1498–1506. doi: 10.1021/acs.jnatprod.0c01308
Song, F. H., Lin, R., Yang, N., Jia, J., Wei, S. Z., Han, J. H., et al. (2021). Antibacterial secondary metabolites from marine-derived fungus Aspergillus sp. IMCASMF180035. Antibiotics 10:377. doi: 10.3390/antibiotics10040377
Song, Z. J., Liu, Y., Gao, J. Y., Hu, J. S., He, H. T., Dai, S. W., et al. (2021). Antitubercular metabolites from the marine-derived fungus strain Aspergillus fumigatus MF029. Nat. Prod. Res. 35, 2647–2654. doi: 10.1080/14786419.2019.1660331
Song, F. H., Liu, X. R., Guo, H., Ren, B., Chen, C. X., and Piggott, A. M. (2012). Brevianamides with antitubercular potential from a marine-derived isolate of Aspergillus versicolor. Org. Lett. 14, 4770–4773. doi: 10.1021/ol302051x
Song, F. H., Ren, B., Chen, C. X., Yu, K., Liu, X. R., Zhang, Y. H., et al. (2014). Three new sterigmatocystin analogues from marine-derived fungus Aspergillus versicolor MF359. Appl. Microbiol. Biotechnol. 98, 3753–3758. doi: 10.1007/s00253-013-5409-5
Sun, C. Z., Ha, Y. R., Liu, X., Wang, N., Lian, X. Y., and Zhang, Z. Z. (2024). Isolation and structure elucidation of new metabolites from the mariana-trench-associated fungus Aspergillus sp. SY2601. Molecules 29:459. doi: 10.3390/molecules29020459
Sun, K. L., Li, Y., Guo, L., Wang, L., Liu, P. P., and Zhu, W. M. (2014). Indole diterpenoids and isocoumarin from the fungus, Aspergillus flavus, isolated from the prawn, Penaeus vannamei. Mar. Drugs 12, 3970–3981. doi: 10.3390/md12073970
Sun, L. X., Wang, H. N., Yan, M. C., Sai, C. M., and Zhang, Z. (2022). Research advances of bioactive sesquiterpenoids isolated from marine-derived Aspergillus sp. Molecules 27:7376. doi: 10.3390/molecules27217376
Sun, C. X., Zhang, Z. P., Ren, Z. L., Yu, L., Zhou, H., Han, Y. X., et al. (2020). Antibacterial cyclic tripeptides from Antarctica-sponge-derived fungus Aspergillus insulicola HDN151418. Mar. Drugs 18:532. doi: 10.3390/md18110532
Thi, H. A. N., Mai, A. N., Thi, T. H. V., Thi, M. H. D., Van, C. P., Thanh, X. D., et al. (2023). Antimicrobial activity of depsidones and macrocyclic peptides isolated from marine sponge-derived fungus Aspergillus nidulans M256. Chem. Biodivers. 20:e202301660. doi: 10.1002/cbdv.202301660
Tian, Y. Q., Lin, S. T., Kumaravel, K., Zhou, H., Wang, S. Y., and Liu, Y. H. (2018). Polyketide-derived metabolites from the sponge-derived fungus Aspergillus sp. F40. Phytochem. Lett. 27, 74–77. doi: 10.1016/j.phytol.2018.06.009
Tuan, C. D., Van Hung, N., Minh, L. T. H., Lien, H. T. H., Chae, J. W., Yun, H. Y., et al. (2022). A new indole glucoside and other constituents from the sea cucumber-derived Aspergillus fumigatus M580 and their biological activities. Rec. Nat. Prod. 16, 633–638. doi: 10.25135/rnp.310.2110.2248
Ukwatta, K. M., Lawrence, J. L., and Wijayarathne, C. D. (2020). Antimicrobial, anti-cancer, anti-filarial and anti-inflammatory activities of cowabenzophenone a extracted from the endophytic fungus Aspergillus terreus isolated from a mangrove plant Bruguiera gymnorrhyza. Mycology 11, 297–305. doi: 10.1080/21501203.2019.1707722
Wallis, R. S., O’Garra, A., Sher, A., and Wack, A. (2023). Host-directed immunotherapy of viral and bacterial infections: past, present and future. Nat. Rev. Immunol. 23, 121–133. doi: 10.1038/s41577-022-00734-z
Wang, W. Y., Chen, R. X., Luo, Z. H., Wang, W., and Chen, J. M. (2018). Antimicrobial activity and molecular docking studies of a novel anthraquinone from a marine-derived fungus Aspergillus versicolor. Nat. Prod. Res. 32, 558–563. doi: 10.1080/14786419.2017.1329732
Wang, Q. Y., Chen, H. P., Wu, K. Y., Li, X. Y., and Liu, J. K. (2022). Antibacterial and β-amyloid precursor protein-cleaving enzyme 1 inhibitory polyketides from the fungus Aspergillus chevalieri. Front. Microbiol. 13:1051281. doi: 10.3389/fmicb.2022.1051281
Wang, K. W., and Ding, P. (2018). New bioactive metabolites from the marine-derived fungi Aspergillus. Mini-Rev. Med. Chem. 18, 1072–1094. doi: 10.2174/1389557518666180305160856
Wang, W. Y., Gao, M. L., Luo, Z. H., Liao, Y. Y., Zhang, B. B., Ke, W. Q., et al. (2019). Secondary metabolites isolated from the deep sea-derived fungus Aspergillus sydowii C1-S01-A7. Nat. Prod. Res. 33, 3077–3082. doi: 10.1080/14786419.2018.1519561
Wang, C. Y., Liu, X. H., Zheng, Y. Y., Ning, X. Y., Zhang, Y. H., Fu, X. M., et al. (2022). 2,5-diketopiperazines from a sponge-derived fungus Aspergillus sclerotiorum. Front. Microbiol. 13:808532. doi: 10.3389/fmicb.2022.808532
Wang, C., Sarotti, A. M., Zaman, K. H., Ahammad, U., Wu, X. H., and Cao, S. G. (2021). New alkaloids from a Hawaiian fungal strain Aspergillus felis FM324. Front. Chem. 9:724617. doi: 10.3389/fchem.2021.724617
Wen, H. M., Zhang, Y. W., Feng, F. J., Huang, G. B., Lv, Y. H., Zhang, Z. Y., et al. (2024). Antibacterial oxygenated ergostane-type steroids produced by the marine sponge-derived fungus Aspergillus sp. J. Asian Nat. Prod. Res. 26, 548–554. doi: 10.1080/10286020.2023.2259317
Wu, J. S., Shi, X. H., Yao, G. S., Shao, C. L., Fu, X. M., Zhang, X. L., et al. (2020a). New thiodiketopiperazine and 3,4-dihydroisocoumarin derivatives from the marine-derived fungus Aspergillus terreus. Mar. Drugs 18:132. doi: 10.3390/md18030132
Wu, J. S., Shi, X. H., Zhang, Y. H., Shao, C. L., Fu, X. M., Li, X., et al. (2020b). Benzyl furanones and pyrones from the marine-derived fungus Aspergillus terreus induced by chemical epigenetic modification. Molecules 25:3927. doi: 10.3390/molecules25173927
Wu, J., Shui, H., Zhang, M. K., Zeng, Y. D., Zheng, M. X., Zhu, K. K., et al. (2023). Aculeaxanthones A-E, new xanthones from the marine-derived fungus Aspergillus aculeatinus WHUF0198. Front. Microbiol. 14:1138830. doi: 10.3389/fmicb.2023.1138830
Xu, P., Ding, L. J., Wei, J. X., Li, Q., Gui, M. J., He, X. P., et al. (2020). A new aquatic pathogen inhibitor produced by the marine fungus Aspergillus sp. LS116. Aquaculture 520:734670. doi: 10.1016/j.aquaculture.2019.734670
Xu, X. L., Han, J. H., Zhang, X. W., Xu, W., Yang, J. P., and Song, F. H. (2024). Investigation on the chemical constituents of the marine-derived fungus strain Aspergillus brunneoviolaceus MF180246. Nat. Prod. Res. 38, 1369–1374. doi: 10.1080/14786419.2022.2144300
Xu, W. F., Wu, N. N., Wu, Y. W., Qi, Y. X., Wei, M. Y., Pineda, L. M., et al. (2022). Structure modification, antialgal, antiplasmodial, and toxic evaluations of a series of new marine-derived 14-membered resorcylic acid lactone derivatives. Mar. Life Sci. Tech. 4, 88–97. doi: 10.1007/s42995-021-00103-0
Xu, X. L., Yang, H. J., Xu, H. T., Yin, L. Y., Chen, Z. K., and Shen, H. H. (2018). Diphenyl ethers from a marine-derived isolate of Aspergillus sp. CUGB-F046. Nat. Prod. Res. 32, 821–825. doi: 10.1080/14786419.2017.1363754
Xu, K., Yuan, X. L., Li, C., and Li, A. X. (2020). Recent discovery of heterocyclic alkaloids from marine-derived Aspergillus species. Mar. Drugs 18:54. doi: 10.3390/md18010054
Xu, L. L., Zhang, C. C., Zhu, X. Y., Cao, F., and Zhu, H. J. (2017). Bioactive phenyl ether derivatives from the marine-derived fungus Aspergillus carneus. Nat. Prod. Res. 31, 1875–1879. doi: 10.1080/14786419.2016.1263848
Xuan, J. Q., Feng, W. G., Wang, J. Y., Wang, R. C., Zhang, B. W., Bo, L. T., et al. (2023). Antimicrobial peptides for combating drug-resistant bacterial infections. Drug Resist. Update 68:100954. doi: 10.1016/j.drup.2023.100954
Xue, J. J., Guo, X. P., Xu, G. X., Chen, X., Jiao, L. H., and Tang, X. X. (2024). Discovery, identification, and mode of action of phenolics from marine-derived fungus Aspergillus ustus as antibacterial wilt agents. J. Agr. Food. Chem. 72, 2989–2996. doi: 10.1021/acs.jafc.3c07826
Yan, L. H., Du, F. Y., Li, X. M., Yang, S. Q., Wang, B. G., and Li, X. (2023). Antibacterial indole diketopiperazine alkaloids from the deep-sea cold seep-derived fungus Aspergillus chevalieri. Mar. Drugs 21:195. doi: 10.3390/md21030195
Yang, J., Gong, L. Z., Guo, M. M., Jiang, Y., Ding, Y., Wang, Z. J., et al. (2021). Bioactive indole diketopiperazine alkaloids from the marine endophytic fungus Aspergillus sp. YJ191021. Mar. Drugs 19:157. doi: 10.3390/md19030157
Yang, S. Q., Li, X. M., Li, X., Li, H. L., Meng, L. H., and Wang, B. G. (2018b). New citrinin analogues produced by coculture of the marine algal-derived endophytic fungal strains Aspergillus sydowii EN-534 and Penicillium citrinum EN-535. Phytochem. Lett. 25, 191–195. doi: 10.1016/j.phytol.2018.04.023
Yang, S. Q., Li, X. M., Xu, G. M., Li, X., An, C. Y., and Wang, B. G. (2018a). Antibacterial anthraquinone derivatives isolated from a mangrove-derived endophytic fungus Aspergillus nidulans by ethanol stress strategy. J. Antibiot. 71, 778–784. doi: 10.1038/s41429-018-0063-x
Yang, G. H., Sandjo, L., Yun, K., Leutou, A. S., Kim, G. D., Choi, H. D., et al. (2011). Flavusides a and B, antibacterial cerebrosides from the marine-derived fungus Aspergillus flavus. Chem. Pharm. Bull. 59, 1174–1177. doi: 10.1248/cpb.59.1174
Yang, M. Y., Yang, J. K., Yang, J. K., Hu, L. D., Zhu, H. J., and Cao, F. (2018). New oxygenated steroid from the marine-derived fungus Aspergillus flavus. Nat. Prod. Commun. 13:1934578X1801300. doi: 10.1177/1934578X1801300807
Yang, X., Yu, H. J., Ren, J. W., Cai, L., Xu, L. J., and Liu, L. (2023). Sulfoxide-containing bisabolane sesquiterpenoids with antimicrobial and nematicidal activities from the marine-derived fungus Aspergillus sydowii LW09. J. Fungi 9:347. doi: 10.3390/jof9030347
Ye, W. X., Zhao, M. R., Wang, L., Jiang, X. D., Zhang, W. J., Zhang, C. S., et al. (2022). Isolation, identification, and bioactive metabolites of coral-derived fungus Aspergillus sp. SCSIO 40435 from the South China Sea. Weishengwu Xuebao 62, 1819–1831. doi: 10.13343/j.cnki.wsxb.20210568
Yu, G. H., Wu, G. W., Sun, Z. C., Zhang, X. M., Che, Q., Gu, Q. Q., et al. (2018). Cytotoxic tetrahydroxanthone dimers from the mangrove-associated fungus Aspergillus versicolor HDN1009. Mar. Drugs 16:335. doi: 10.3390/md16090335
Yu, H. J., Xue, Y. X., Hong, K., Jia, J., Bi, H. K., Xu, L. J., et al. (2022). Secondary metabolites from a mangrove-derived fungus Aspergillus sp. WHUF0343. Weishengwu Xuebao 62, 2658–2670. doi: 10.13343/j.cnki.wsxb.20210655
Yurchenko, A. N., Girich, E. V., and Yurchenko, E. A. (2021). Metabolites of marine sediment-derived fungi: actual trends of biological activity studies. Mar. Drugs 19:88. doi: 10.3390/md19020088
Zang, Z. M., Yang, W. C., Cui, H., Cai, R. L., Li, C. Y., Zou, G., et al. (2022). Two antimicrobial heterodimeric tetrahydroxanthones with a 7,7′-linkage from mangrove endophytic fungus Aspergillus flavus QQYZ. Molecules 27:2691. doi: 10.3390/molecules27092691
Zeng, Q., Chen, Y. C., Wang, J. F., Shi, X. F., Che, Y. H., Chen, X. Y., et al. (2022a). Diverse secondary metabolites from the coral-derived fungus Aspergillus hiratsukae SCSIO 5Bn1003. Mar. Drugs 20:150. doi: 10.3390/md20020150
Zeng, Q., Zhong, W. M., Chen, Y. C., Xiang, Y., Chen, X. Y., Tian, X. P., et al. (2020b). A new butenolide derivative from the deep-sea fungus Aspergillus terreus SCSIO FZQ028. Nat. Prod. Res. 34, 1984–1991. doi: 10.1080/14786419.2019.1569658
Zhang, J., Gao, L. L., Lin, H. T., Liang, Y., You, M. N., Ding, L. J., et al. (2024). Discovery of antibacterial compounds against Xanthomonas citri subsp. citri from a marine fungus Aspergillus terreus SCSIO 41202 and the mode of action. J. Agric. Food. Chem. 72, 12596–12606. doi: 10.1021/acs.jafc.4c02769
Zhang, Y. H., Hou, X. M., Yu, M. L., and Wang, C. Y. (2019). Secondary metabolites and their bioactivities from the gorgonian-derived fungus Aspergillus versicolor. Chem. Nat. Compd. 55, 327–330. doi: 10.1007/s10600-019-02680-0
Zhang, Y. T., Li, Z. C., Huang, B. Y., Liu, K., Peng, S., Liu, X. M., et al. (2022). Anti-osteoclastogenic and antibacterial effects of chlorinated polyketides from the beibu gulf coral-derived fungus Aspergillus unguis GXIMD02505. Mar. Drugs 20:178. doi: 10.3390/md20030178
Zhang, Y., Li, X. M., and Wang, B. G. (2012). Anthraquinone derivatives produced by marine-derived fungus Aspergillus versicolor EN-7. Biosci. Biotechnol. Biochem. 76, 1774–1776. doi: 10.1271/bbb.120047
Zhang, L., Qiu, P. P., Ding, L. J., Li, Q., Song, J. J., Han, Z. W., et al. (2020). A new antibacterial chlorinated amino acid derivative from the sponge-derived fungus Aspergillus sp. LS53. Chem. Nat. Compd. 56, 109–111. doi: 10.1007/s10600-020-02955-x
Zhang, R., Wang, H. F., Chen, B. S., Dai, H. Q., Sun, J. Z., Han, J. J., et al. (2022). Discovery of anti-MRSA secondary metabolites from a marine-derived fungus Aspergillus fumigatus. Mar. Drugs 20:302. doi: 10.3390/md20050302
Zhang, Y. H., Xu, Y., Wang, C. Y., and Cao, F. (2020). Alkaloids and sesquiterpenoids from the marine-derived fungus Aspergillus versicolor. Chem. Nat. Compd. 56, 971–973. doi: 10.1007/s10600-020-03205-w
Zheng, C. J., Shao, C. L., Wu, L. Y., Chen, M., Wang, K. L., Zhao, D. L., et al. (2013). Bioactive phenylalanine derivatives and cytochalasins from the soft coral-derived fungus, Aspergillus elegans. Mar. Drugs 11, 2054–2068. doi: 10.3390/md11062054
Zhou, Y. M., Debbab, A., Wray, V., Lin, W. H., Schulz, B., Trepos, R., et al. (2014). Marine bacterial inhibitors from the sponge-derived fungus Aspergillus sp. Tetrahedron Lett. 55, 2789–2792. doi: 10.1016/j.tetlet.2014.02.062
Zhu, S. H., Chang, Y. M., Su, M. Z., Yao, L. G., Li, S. W., Wang, H., et al. (2024). Nine new antibacterial diterpenes and steroids from the South China Sea soft coral Lobophytum catalai Tixier-Durivault. Mar. Drugs 22:50. doi: 10.3390/md22010050
Zhu, F., Chen, G. Y., Chen, X., Huang, M. Z., and Wan, X. Q. (2011). Aspergicin, a new antibacterial alkaloid produced by mixed fermentation of two marine-derived mangrove epiphytic fungi. Chem. Nat. Compd. 47, 767–769. doi: 10.1007/s10600-011-0053-8
Keywords: marine-derived, Aspergillus sp., secondary metabolites, antibacterial activity, antimicrobial resistance
Citation: Wang B, Cai J, Huang L, Chen Y, Wang R, Luo M, Yang M, Zhang M, Nasihat, Chen G, Huang G and Zheng C (2024) Significance of research on natural products from marine-derived Aspergillus species as a source against pathogenic bacteria. Front. Microbiol. 15:1464135. doi: 10.3389/fmicb.2024.1464135
Edited by:
Dany Domínguez Pérez, Zoological Station Anton Dohrn, ItalyReviewed by:
Fei Cao, Hebei University, ChinaJoko Tri Wibowo, National Research and Innovation Agency (BRIN), Indonesia
Muaaz Alajlani, Al-Sham Private University, Syria
Copyright © 2024 Wang, Cai, Huang, Chen, Wang, Luo, Yang, Zhang, Nasihat, Chen, Huang and Zheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guolei Huang, aHVhbmdndW9sZWkxOTgyQDE2My5jb20=; Caijuan Zheng, Y2FpanVhbjIwMDJAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Bin Wang1,2†
Bin Wang1,2† Caijuan Zheng
Caijuan Zheng