- 1Key Laboratory of Fruit and Vegetable Function and Health Research of Taizhou, Zhejiang Citrus Research Institute, Zhejiang Academy of Agricultural Sciences, Taizhou, China
- 2The Sainsbury Laboratory, University of East Anglia, Norwich Research Park, Norwich, United Kingdom
The effect of pesticide pollution on environmental microorganisms in soil has become the focus of widespread concern in society today. The response of earthworm gut and surrounding soil microbial functional diversity and enzyme activity to carbendazim (CBD) was studied in a soil-earthworm ecosystem amended with manure. In the experiment, CBD was added to the manured soil (MS). Meanwhile, the pesticide treatment without manure and the control treatment without pesticides were also set up. The activities of catalase (CAT) and acetylcholinesterase (AChE) were measured to evaluate the toxicity of CBD. The Biolog method was used to assess the functional diversity of the microbial community. In the 2 mg/kg CBD treatment, earthworm AChE activity decreased significantly in the MS after 14 d, which occurred earlier than in the un-manured soil (NS). The changes of earthworm CAT activity in the pesticide treatments showed a trend of initially increasing and then maintaining at a high activity level. However, the CAT activities at 28 d in the manured soils were clearly lower than that at 7 d for both the CBD treatments, while they remained stable in the control treatments. The carbon source utilization, Simpson index, Shannon index, and McIntosh index of soil microorganisms in the MS treatments were significantly higher than those in the NS treatments. The overall activity of earthworm gut microorganisms in the MS treated with 2 mg/kg CBD was higher than that in the control. Also, CBD treatment (2 mg/kg) increased significantly the Simpson index and McIntosh index of earthworm gut microorganisms. The results indicated that the enzyme activities in the manured soils increased before 7 d for the pesticide treatments. Furthermore, exposure to CBD at a high concentration in the MS not only led to the earlier inhibition of earthworm enzyme activity but also significantly improved the overall activity of earthworm gut microorganisms and microbial functional diversity. This study revealed the ecotoxicological effects of earthworms in response to pesticide stress following the use of organic fertilizers under facility environmental conditions, which can provide a theoretical basis for the remediation of pesticide pollution in soil in the future.
1 Introduction
Earthworms are the most typical soil animals among temperate, tropical, and subtropical terrestrial ecosystems. They play an irreplaceable role in many key soil processes and are often referred to as “ecosystem engineers” (Datta et al., 2016; Liu et al., 2019). The swallowing and digging behaviors of earthworms play an important ecological role in the formation of soil structure, nutrient cycle, and pollution remediation (Wang et al., 2024; Liu et al., 2025). Many ecological functions of earthworms are related to their gut microorganisms. The digestive function of the earthworm gut can affect the structure and function of the soil microbial community (Blouin et al., 2013; Zhang et al., 2022). Earthworm gut microorganisms and soil microorganisms are important components of the soil ecosystem, which play an important role in element cycling, organic matter decomposition, pollutant degradation, improving soil fertility, and enhancing crop yield (Drake and Horn, 2007; Ma et al., 2016). However, pollutants such as pesticides, fertilizers, and heavy metals in the agricultural environment pose a threat to the ecosystem. As earthworms feed on organic matter in the soil, they come into contact with pollutants through their intestines, allowing these pollutants to enter into their bodies, where they accumulate in tissue and organs. This accumulation can produce certain negative effects on the survival, growth, and reproduction of earthworms (Liu et al., 2018; Qi et al., 2018; Wang et al., 2020).
In recent years, there has been increasing attention to the effect of adding exogenous organic fertilizer on soil fertility. There is a certain relationship between the use of organic fertilizer in soil and the microbial community structure in earthworms. Studies have shown that when various organic wastes in nature are fermented and contain a large number of microorganisms, which enter the digestive system of earthworms after being applied to the soil. Under the action of intestinal protease, lipase, fibrase, and amylase, organic matter is rapidly decomposed and transformed into nutrients that can be easily utilized by the earthworms themselves or other organisms (Van Groenigen et al., 2019; Vidal et al., 2023; Wu et al., 2024). After digestion by earthworms, the number of beneficial bacteria increases exponentially, while pathogen populations dependent on nutrient are controlled. Also, its excrement can play a role in promoting soil microbial structure and pollution remediation (Ferris and Tuomisto, 2015; Sułowicz et al., 2023; Chen et al., 2024).
At present, the effect of carbendazim (CBD) on earthworm enzyme activity and soil microbial functional diversity has been reported (Chuang et al., 2021; Guo et al., 2023; Kenko Nkontcheu et al., 2023; Zhou et al., 2023; Gautam et al., 2024). However, there are few studies focusing on earthworm enzyme activity and microbial functional diversity under the stress of CBD in manured soil (MS). Studies have shown that the stress of pesticides, heavy metals, and other pollutants can induce earthworm to produce reactive oxygen species (ROS) such as NO, H2O2, O2· − , and ·OH − (Yang et al., 2022; Xu et al., 2023; Lee et al., 2024; Yan et al., 2024). These ROS may activate the active oxygen scavenging system and lead to changes in some physiological and biochemical indices in earthworms (such as antioxidant enzyme system and non-antioxidant enzyme system). Such changes in these biochemical indicators can indicate pollutant toxicity and serve as an early warning system for soil pollution (Liu et al., 2017; Li et al., 2018; Zhang et al., 2021). Catalase (CAT) is a key enzyme in the biological antioxidant enzyme system, which defends organisms against antioxidant damage by catalyzing the decomposition of H2O2 into H2O and O2, thus preventing cell peroxidation. Under the combined action of superoxide dismutase and CAT, O2· − is eventually converted into H2O (Wu et al., 2012; Hu et al., 2016). Acetylcholinesterase (AChE) is a key enzyme in biological nerve conduction and can hydrolyze acetylcholine into choline and acetic acid to ensure the normal transmission of nerve signals (Calisi et al., 2013). CAT and AChE are sensitive to pollutants and are widely used to evaluate the pollutant toxicity.
The Biolog method is widely used in the study of environmental microbial ecology which is simple and does not require isolating and culturing microorganisms (Huang et al., 2024). This technique can obtain the metabolic fingerprint of the microbial community in a short time, characterize differences in physiological characteristics, and reflect the functional diversity of microbial community by measuring the variations in microbial utilization of different carbon sources (Gryta et al., 2014; Koner et al., 2022). This study will be more accurate, intuitive, and scientific in reflecting the effects of organic fertilizers on enzyme activities and microbial functional diversity in earthworms, in order to reveal the ecotoxicological effects of earthworms under pesticide stress from the perspective of microorganisms. Meanwhile, the results can provide reference and theoretical basis for the remediation of pesticide pollution in soil under facility environment.
2 Materials and methods
2.1 Chemicals and reagents
The protein quantitative determination kit (Coomassie brilliant blue method), acetylcholinesterase (AChE) test kit, and catalase (CAT) test kit (ammonium molybdate method) were provided by Nanjing Jiancheng Bioengineering Research Institute (Nanjing, China).
2.2 Experimental design and soil sampling
The sieved soils (3.5 kg dry weight) were weighed and then added 3% manure and stirred evenly. Then CBD standard solution was added to the MS to reach 1 mg/kg and 2 mg/kg corresponding to the recommended doses and the double dose, respectively (Daam et al., 2020). Meanwhile, sterile water was added to reach 60% of the soil’s maximum water holding capacity and the mixture was thoroughly stirred, passed the 2-mm sieve, and then transferred into plastic pots (upper diameter: 95 mm, height: 65 mm, bottom diameter: 70 mm). The mature earthworms (Eisenia fetida) were purchased from Shandong Agricultural University (Taian, China). Each plastic pot was filled with 150 g (dry weight) of soil, and 10 earthworms after clearing intestines were placed on the soil surface until they entered the soil actively. All treatments were performed in triplicate. Meanwhile, the pesticide treatments without manure and the control treatments without pesticide were set up including the un-manured control soil (NS-CK), the un-manured soil with 1 mg/kg CBD (NS-CBD1), the un-manured soil with 2 mg/kg CBD (NS-CBD2), the manured control soil (MS-CK), the manured soil with 1 mg/kg CBD (MS-CBD1), and the manured soil with 2 mg/kg CBD (MS-CBD2). All plastic pots were placed in the biochemical incubator at 20°C, 75% relative humidity, and 400 lux with a light–dark cycle of 12 h each. The soil water content was adjusted by weighing method every 2 d. After exposure to 1 d, 3 d, 7 d, 14 d, 21 d, and 28 d, 1–2 earthworms were taken out for the determination of enzyme activity. After exposure for 28 d, 5.0 g of soil sample and 0.3 g of earthworm gut tissue were collected for microbial functional analysis.
For earthworm gut dissection, several earthworms were taken out from each replicate and placed in a petri dish. The soil adhered to the earthworm surface was carefully removed. The earthworms were immersed in pure ethanol for 10 s to make them die quickly, and then washed with a 75% alcohol solution. In the clean bench, the earthworms were washed with sterile water 3 times and dried the earthworm’s surface water using the filter paper. Then the earthworm was fixed with pins on a foam board placed on filter paper and dissected with sterilized medical eye scissors. The body surface of the earthworm clitellum to the anus was cut off, and the gut tissue of the earthworm was carefully clamped out with sterilized tweezers, which was then weighed and placed into a 1.5 mL sterilized Eppendorf (EP) tube.
2.3 Determination of earthworm enzyme activity
Preparation of 5% earthworm tissue homogenate: 1–2 earthworms were taken from each treatment and placed in a petri dish padded with wet filter paper to clear the gut overnight. Nineteen times the volume of normal saline was added at the ratio of 1:19, homogenized in an ice water bath, centrifuged at 2500 rpm for 10 min, and the supernatant was taken for detection.
The enzyme activities were determined according to the manufacturer’s instructions for reagent kits (Jiancheng; China). The protein concentration of the sample was determined by the Coomassie brilliant blue method, and the protein content was determined based on the absorbance measured at 595 nm. The activity of CAT was calculated by measuring the absorbance of the light yellow complex formed by residual H2O2 and ammonium molybdate at 405 nm. The activity of AChE was detected by measuring the absorbance at 412 nm according to the color reaction of the TNB (symmetrical trinitrobenzene) yellow compound generated by choline and sulfhydryl chromogenic agents (Lin et al., 2016; Fang et al., 2022).
2.4 Microbial functional diversity in earthworm gut and soil
The overall functional diversity of microorganisms in the soil and gut after manured treatment was determined using Biolog® EcoPlates™ (Biolog Inc., Hayward, CA, United States) by referring to our previously used methods (Han et al., 2020). The metabolic potential of microbial communities was assessed using 31 different carbon sources categorized into six groups, including seven carbohydrates, 10 carboxylic acids, six amino acids, four complex carbon sources, two phosphate carbon sources, and two amines (Preston-Mafham et al., 2002; Ge et al., 2018; Nagata et al., 2023).
0.3 g of earthworm gut tissue was weighed into a 100 mL conical flask, and 30 mL of 0.85% NaCl solution was added and homogenized for 30 s. 5.0 g (dry weight) of soil sample was weighed into a 100 mL conical flask, and 45 mL of the sterilized 0.85% NaCl solution was added, shaken in a shaker under the conditions of 25°C, 150 rpm in the dark, and then placed in a clean bench to stand for 30 min. 1 mL of earthworm gut suspension or soil suspension was diluted to 10−4 diluents by the step-by-step dilution method. 150 μL of the diluent was inoculated into each well of the Biolog ECO plate with an 8-channel pipette. All plates were placed in an incubator at 25°C in the dark. After incubation for 4, 24, 48, 72, 96, 120, 144, and 168 h, all plates were read at 750 nm and 590 nm using a Biolog reader. All treatments were in triplicate.
2.5 Statistical analyses
Earthworm enzyme activity and microbial diversity index were analyzed by one-way analysis of variance (ANOVA) and carried out Duncan’s test with the IBM SPSS Statistics 26. The difference between OD590 and OD750 was used to represent the metabolic activity of microorganisms for subsequent data calculation and analysis. When the value was less than 0.06, it was treated as 0. The changes of microbial functional diversity index of earthworm gut and soil were analyzed by absorbance value of Biolog ECO plate after incubation for 72 h (Gryta et al., 2014; Fang et al., 2016; Urbaniak et al., 2024). The rate of substrate utilization by microorganisms was measured by calculating the average well color development (AWCD) using the following Equation 1:
Where A represents the difference of absorbance value between two bands for each well in Biolog ECO plate, ACK represents absorbance value of blank control well. Functional diversities assessed by the Shannon diversity index (H), Simpson index (1/D), and McIntosh index (U), were calculated using the Equations 2–4:
Where pi represents the ratio of the relative absorbance value (A-ACK) of the well i to the sum of the relative absorbance value of the whole plate, ni represents the relative absorbance value (A-ACK) of the well i, N represents the sum of the relative absorbance value of the whole plate.
3 Results
3.1 Effect of CBD on AChE activity
The changes in acetylcholinesterase (AChE) activity of earthworms under CBD stress in un-manured soil are shown in Figure 1. In the control treatment (NS-CK), the AChE activity of the earthworms remained stable. CBD stimulated the AChE activity of earthworms at the initial stage (0 to 7 days) and significantly inhibited the AChE activity at the later stage (21 to 28 days) with the extension of exposure time. On the 7th day, the AChE activities of earthworms in NS-CBD1 and NS-CBD2 were at the highest, which were significantly higher than that of the NS-CK. On the 21th-28th days, the AChE activities of earthworms were significantly lower than those of the control. The changes in earthworm AChE activity in the manured soil are shown in Figure 2. CBD initially increased earthworm enzyme activity, which then subsequently decreased. MS-CBD2 was significantly higher than MS-CK on the 3rd and 7th days, while MS-CBD1 was significantly higher than MS-CK on the 7th and 14th days. In the later stage of exposure (21 to 28 d), however, earthworm AChE activities were significantly lower than those in MS-CK. The response trend of earthworm AChE activity under CBD stress in manured soil (MS) and un-manured soil (NS) is generally similar. The difference was that earthworm enzyme activity in MS-CBD2 treatment peaked on the 3rd day and decreased significantly on the 14th day, while in the NS-CBD2 treatment, it peaked on the 7th day and was significantly inhibited on the 21st day.
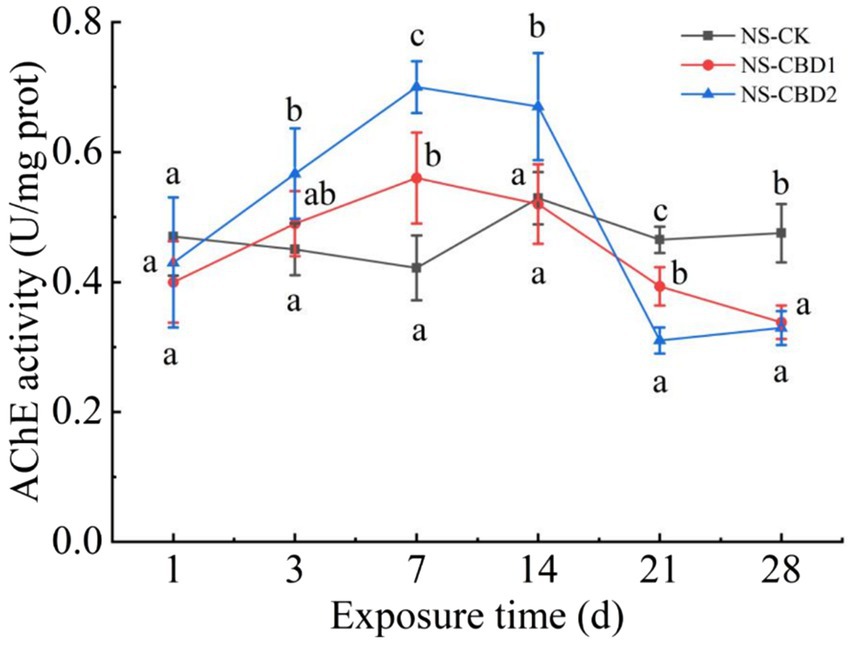
Figure 1. Effects of carbendazim on acetylcholinesterase activity of earthworm in the un-manured soil. NS-CK, NS-CBD1 and NS-CBD2 indicated 0, 1 and 2 mg/kg carbendazim treatment in the un-manured soil, respectively. The different letters above the curves indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
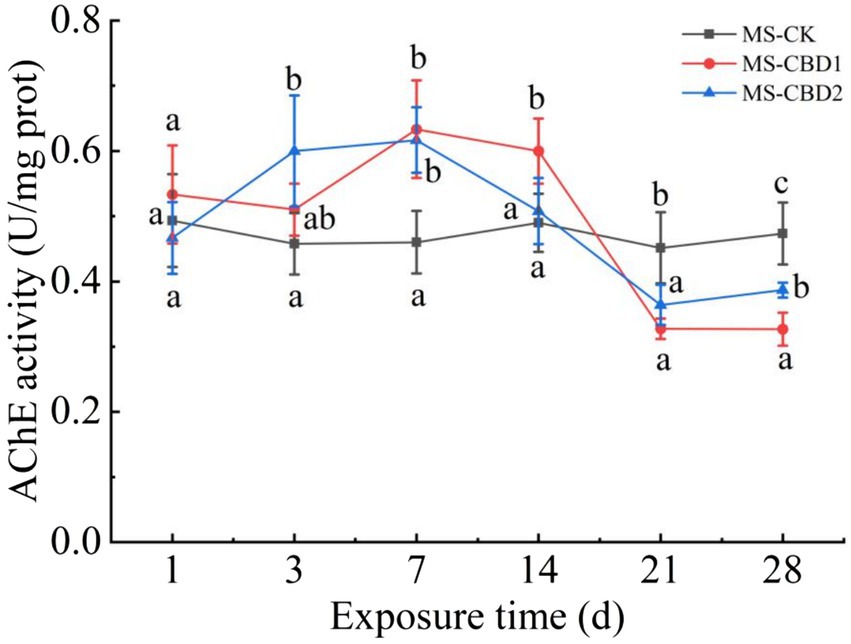
Figure 2. Effects of carbendazim on acetylcholinesterase activity of earthworm in the manured soil. MS-CK, MS-CBD1 and MS-CBD2 indicated 0, 1 and 2 mg/kg carbendazim treatment in the manured soil, respectively. The different letters above the curves indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
3.2 Effect of CBD on CAT activity
The change of earthworm CAT activity under CBD stress in NS is shown in Figure 3. The CAT activities of earthworm in NS-CBD1 and NS-CBD2 were significantly higher than those in the NS-CK after 3 days. With the extension of exposure time, the CAT activity reached the highest level on the 7th day, and then decreased slowly within 7–28 days, but still maintained at a high level. The change of earthworm CAT activity in the MS is shown in Figure 4. The CAT activity of earthworms in the MS-CBD1 and MS-CBD2 increased significantly after 3 days, gradually reached to its peak after 7 days, and then slowly decreased between 14 to 28 days. Compared with MS-CK, CAT activity remained at a high level. In general, a similar trend was observed in both MS and NS: CBD treatment initially increased CAT activity and then maintained a high level. Furthermore, there were no significant difference in enzyme activity among different concentrations of treatment.
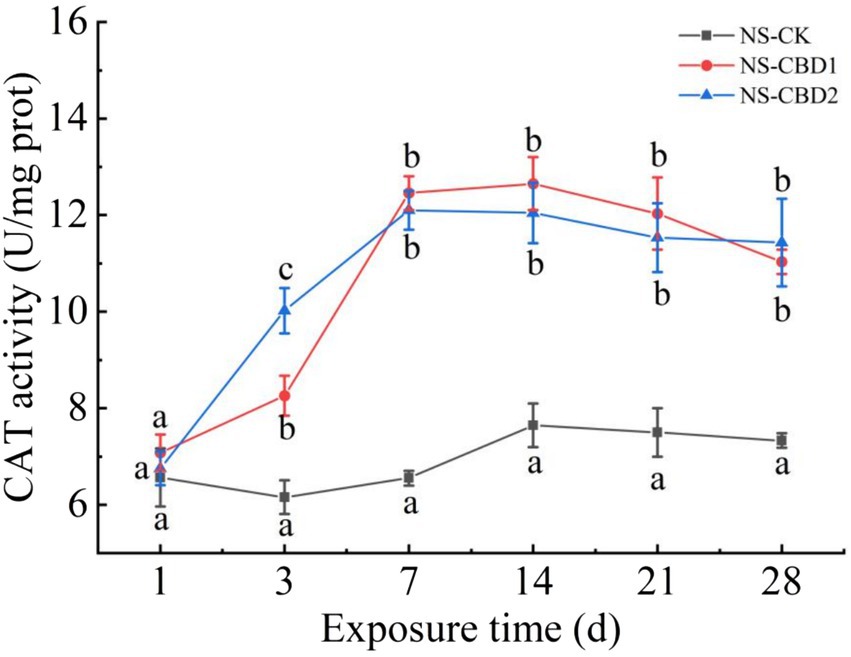
Figure 3. Effects of carbendazim stress on catalase activity of earthworm in the un-manured soil. The different letters above the curves indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
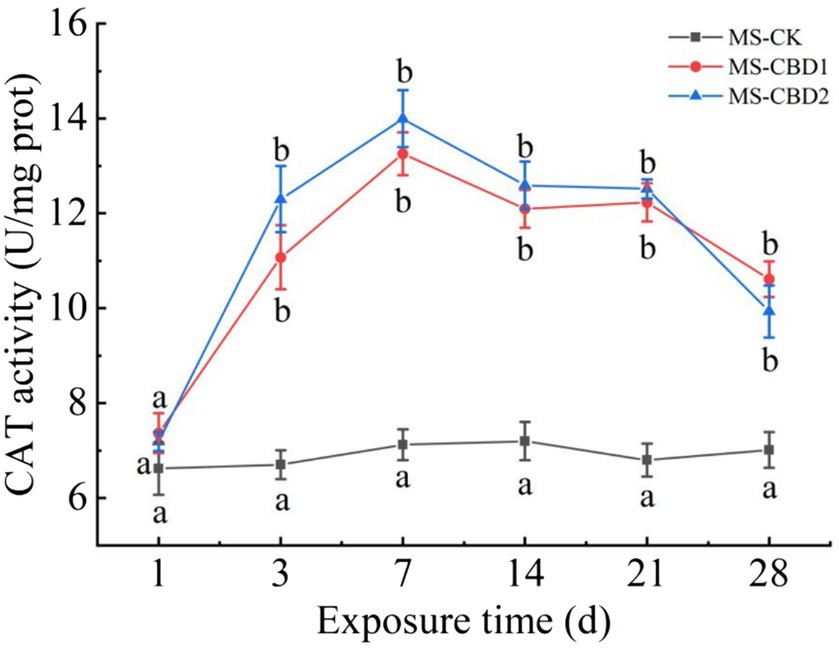
Figure 4. Effects of carbendazim stress on catalase activity of earthworm in the manured soil. The different letters above the curves indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
3.3 Effect of CBD on carbon source utilization diversity
The changes of carbon source utilization diversity in earthworm gut and soil microorganisms under CBD stress are shown in Figure 5. In the MS and NS soils, the AWCD values of soil microorganisms in the 1 mg/kg carbendazim soil (CBD1) treatments were significantly lower than those in the control treatment, and the AWCD values of soil microorganisms in the 2 mg/kg carbendazim soil (CBD2) treatments were either slightly higher than or similar to those in the control soil. The AWCD values of soil microorganisms in all MS treatments were higher than those in the NS treatments. The AWCD values of earthworm gut microorganisms in the NS did not change significantly across all treatment groups, except for the NS-CBD2 where AWCD values were lower than the control at 48–72 h, but then returned to the control level. The AWCD values of earthworm gut microorganisms in the MS-CBD1 were lower than those of the control between 48 and 120 h, and then the activity gradually recovered to the control level. However, the AWCD values of earthworm gut microorganisms in the MS-CBD2 were not significantly different from those of the control at 0–24 h but were significantly higher than those of the control at 48–120 h, and then gradually recovered to the control level.
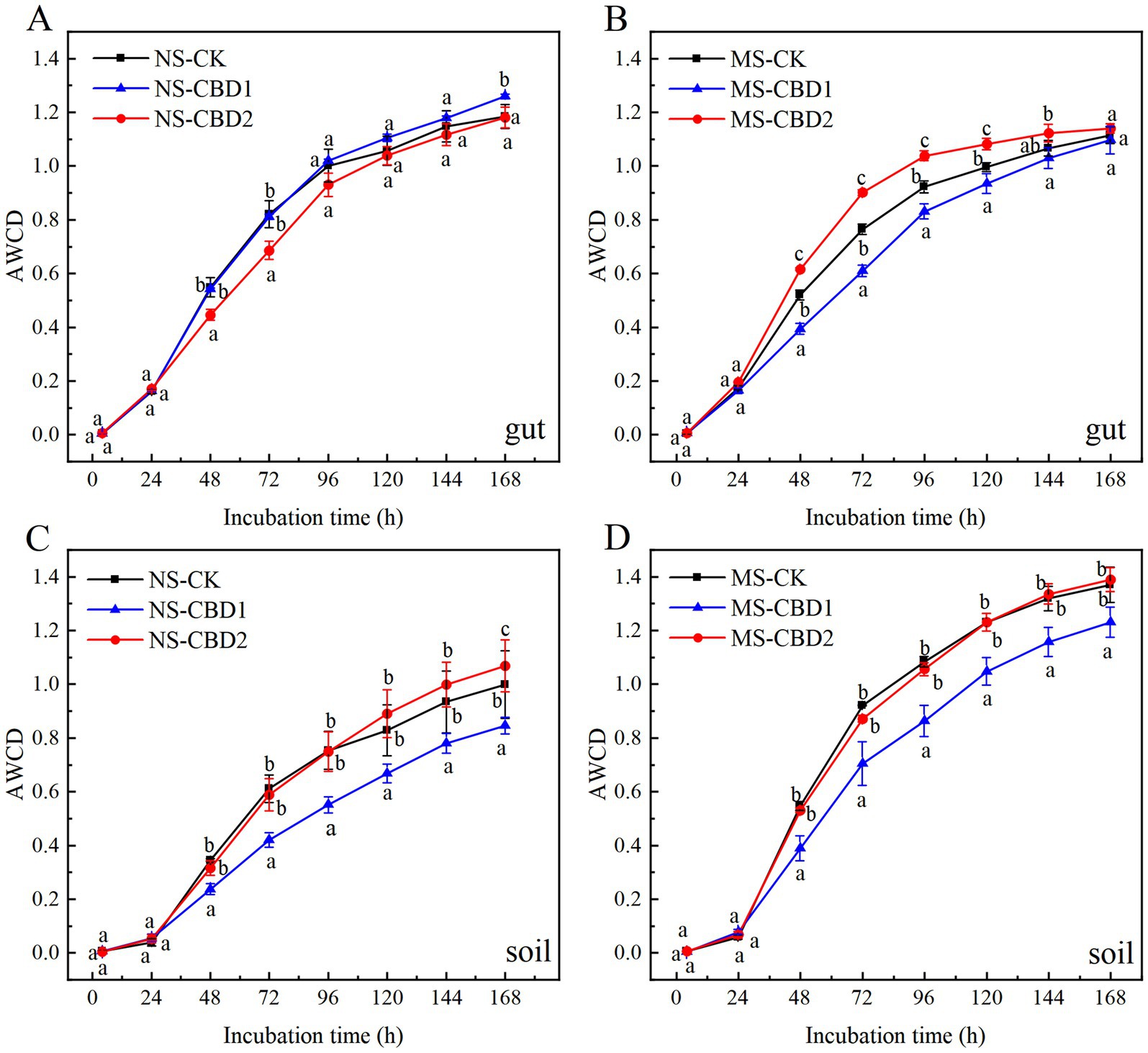
Figure 5. Variations in the average well color development (AWCD) of earthworm gut (A,B) and soil (C,D) microorganisms under different treatments. The different letters above the curves indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
3.4 Changes in the functional diversity indices of microorganisms
The changes in soil microbial functional diversity indices under CBD stress are shown in Figures 6 –8. The 1/D and H of soil microorganisms in the NS-CBD2 were significantly higher than those in the NS-CK, while the U showed no significant difference compared with the NS-CK (p < 0.05). In the NS-CBD1, there was no significant difference between the 1/D and H, while the U was significantly lower than the NS-CK (p < 0.05). In MS, the 1/D and H of soil microorganisms treated with carbendazim (1 mg/kg and 2 mg/kg, MS-CBD) showed no significant difference compared with the MS-CK. The U of soil microorganisms in MS-CBD2 was not significantly different from the MS-CK, while the U in MS-CBD1 was significantly lower than that of the MS-CK (p < 0.05).
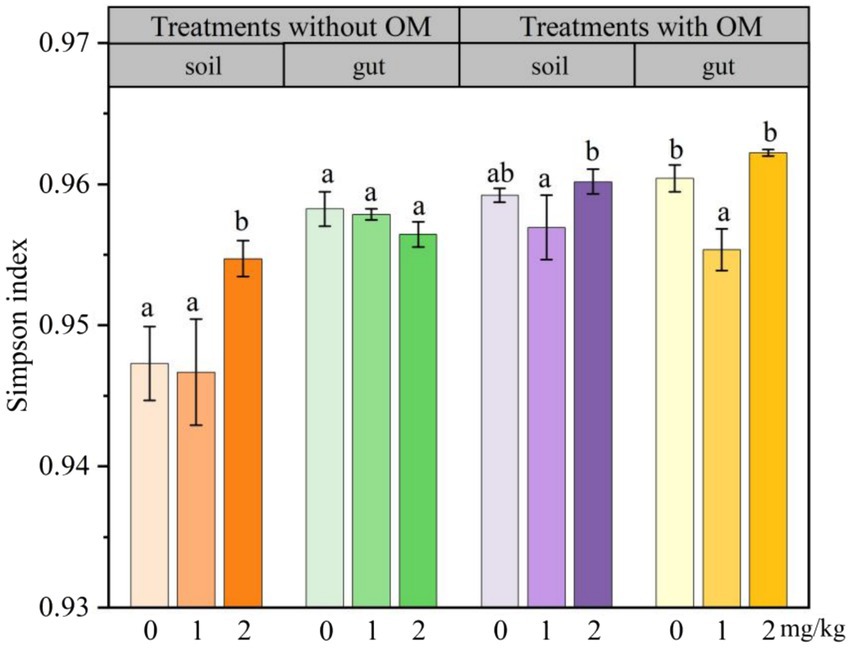
Figure 6. Changes in Simpson index of earthworm gut and soil microorganisms in different treatments. The different letters above the columns indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
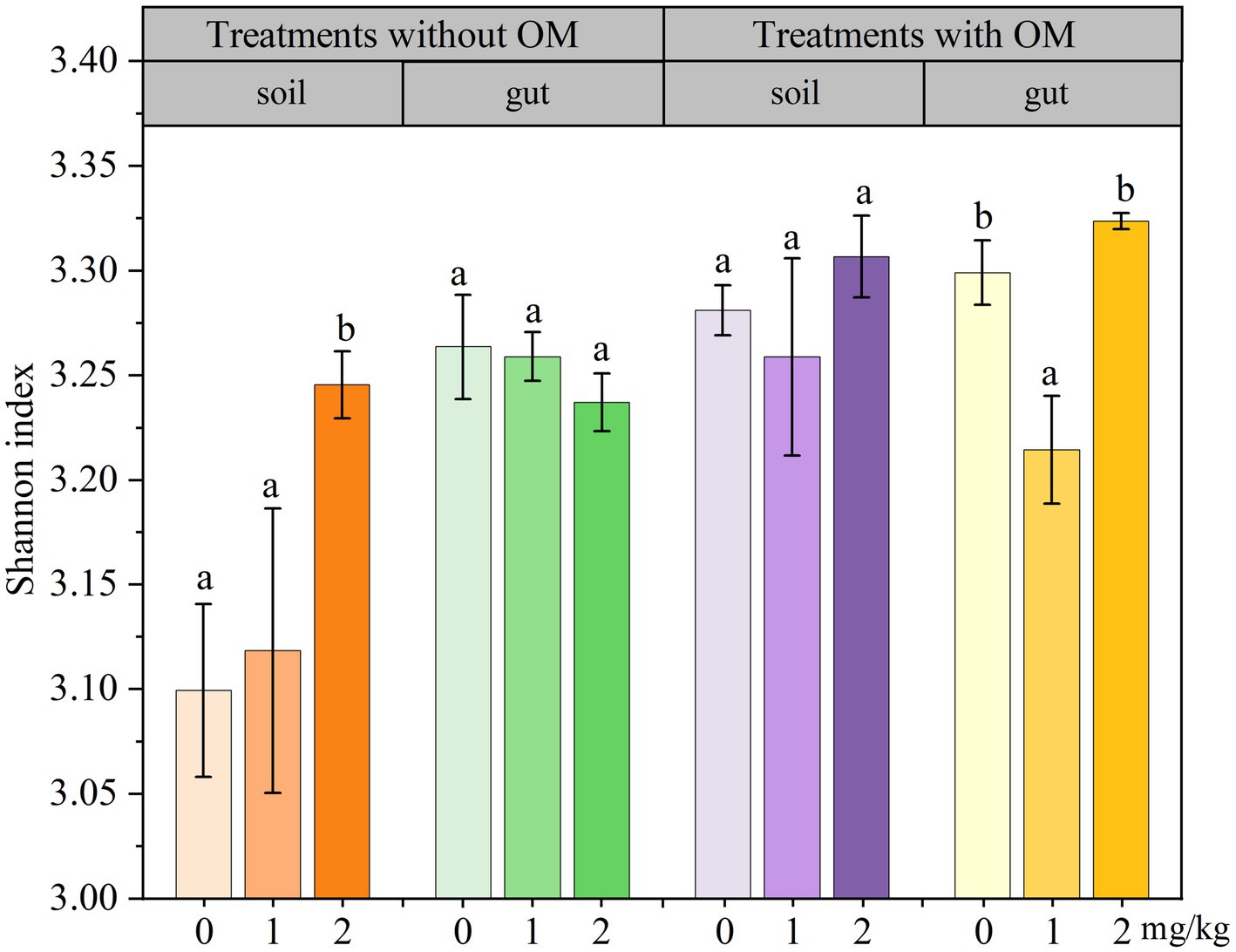
Figure 7. Changes in Shannon index of earthworm gut and soil microorganisms in different treatments. The different letters above the columns indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
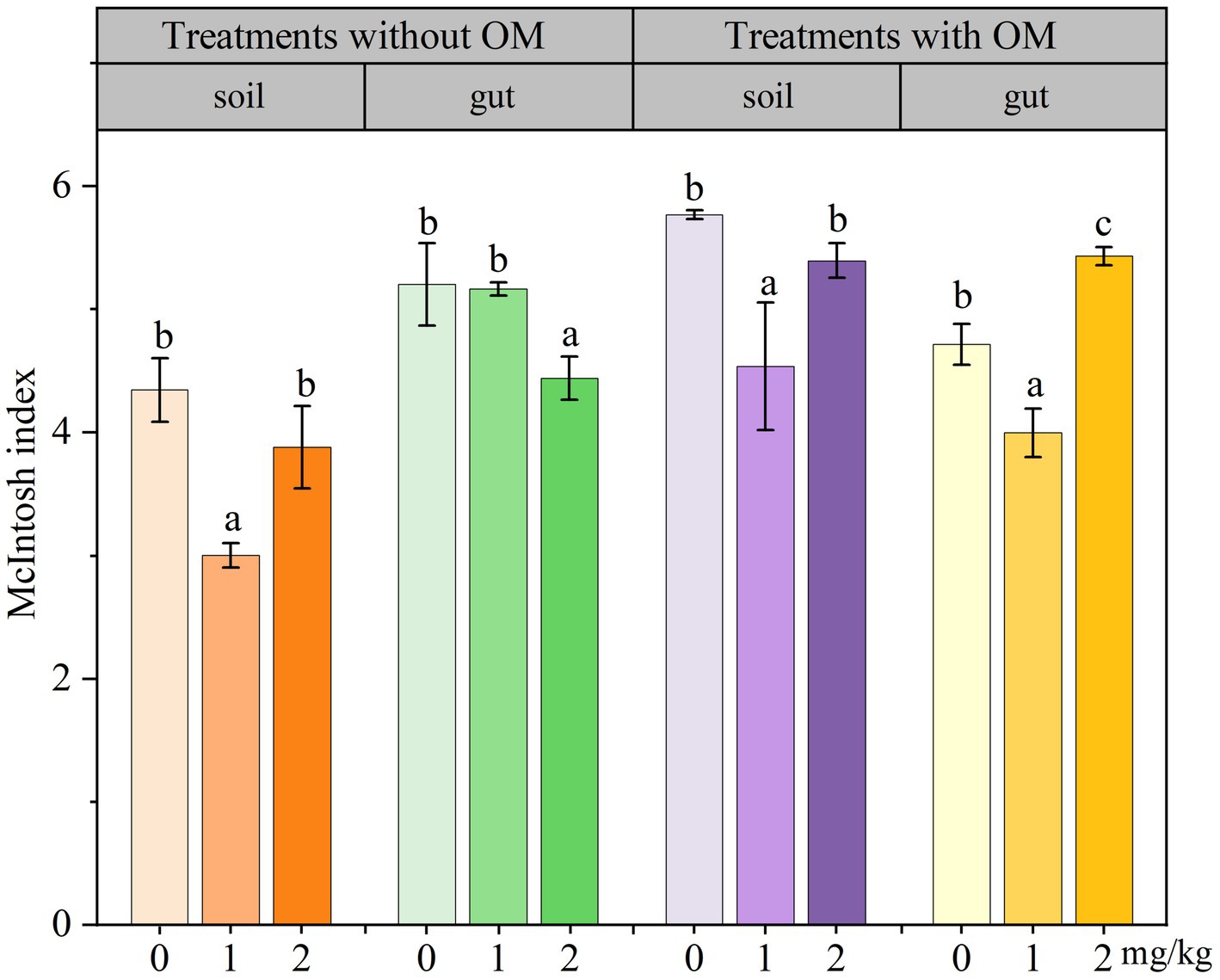
Figure 8. Changes in McIntosh index of earthworm gut and soil microorganisms in different treatments. The different letters above the columns indicate a significant difference (p < 0.05) based on variance analysis. The error bars represent the standard errors of the mean of triplicate samples.
The changes in the functional diversity index of earthworm gut microorganisms under CBD stress are shown in Figures 6–8. The 1/D and H of earthworm gut microorganisms were suppressed in NS-CBD, but the differences were not significant (p < 0.05) when compared with the NS-CK. The U of earthworm gut microorganisms in NS-CBD2 was significantly lower than that in the NS-CK (p < 0.05), but there was no significant difference between NS-CBD1 and NS-CK. MS-CBD1 resulted in a significant reduction in the 1/D, H, and U indices of earthworm gut microorganisms compared to MS-CK (p < 0.05). The MS-CBD2 showed no significant difference in 1/D and H, while the U was significantly higher than that of the MS-CK (p < 0.05). The diversity indices of earthworm gut microorganisms in the MS-CBD2 were higher than those in the NS-CBD2.
4 Discussion
The acetylcholine receptor is the target of the triazole fungicide carbendazim, which can interfere with the signal transmission of the central nervous system of the body, causing paralysis or even death. Whereas AChE can hydrolyze acetylcholine to ensure normal neurotransmission and maintain normal function of the nervous system (Goulson and Kleijn, 2013). After exposure to carbendazim, the AChE activity in earthworms initially increased with contamination concentration but gradually decreased over time. Similar to our results, Yang et al. (2018) found that the AChE activity of earthworms increased significantly on the 3rd and 7th d of exposure to 3-(2-chloroethyl) phosphate (1 mg/kg and 10 mg/kg), and decreased significantly on the 14th d. Hackenberger et al. (2018) found that the AChE activity of earthworm (Dendrobaenaveneta) increased significantly after 7 d of exposure to 2,160 μg/kg glyphosate, and returned to the control level after 28 d. This is due to the fact that AChE activity gradually recovers in the later stages of staining because the pesticide has a lower comprehensive toxic effect with the extension of exposure time and the cells develop an anti-stress repair mechanism. In the MS, stress response and recovery of AChE activity were more rapid under the high concentration of pollution, because organic matter increased the nutrition of earthworms and promoted the growth and development of earthworms. Moreover, the intake of organic fertilizer increased the types and quantities of beneficial microorganisms in the earthworm gut of earthworms, enhanced the symbiotic network between microorganisms and earthworms, and improved the ability to resist external risks and stress (Markad et al., 2015; Chen et al., 2017). However, some studies have reported different results that the AChE activity of earthworms can be inhibited by some insecticides, such as triazophos, chlorpyrifos, deltamethrin, and so on (Bednarska et al., 2017; Singh et al., 2019). When earthworms were exposed to the neonicotinoid guadipyr initially, AChE activity was significantly reduced, but during subsequent tests, AChE activity returned to the control level without significant difference (Wang et al., 2015). In addition, Yang et al. (2018) found that trimethylphenyl phosphate (0.1 mg/kg and 10 mg/kg) significantly inhibited AChE activity on the 3rd d of exposure, and then AChE activity gradually recovered with the extension of exposure time. Zhao et al. (2019) found that N-ethyl perfluorooctane sulfonamide ethanol treatment had no significant effect on AChE activity of earthworms. Due to the various mechanisms of action and complex effects of pesticides, their effects on AChE activity are also different. Therefore, when using AChE as an environmental biomarker, especially in environments contaminated with multiple classes of chemicals, it is important to assess the effect of contaminants on AChE activity (Frasco et al., 2005).
CAT is an important antioxidant enzyme and a major defense enzyme against ROS in the body. CBD exposure significantly increased the CAT activity of earthworms, indicating that the antioxidant enzyme system of earthworms was activated in response to oxidative stress. Among them, SOD discriminates superoxide anion into H2O2, and CAT in turn catalyzes H2O2 into O2 and H2O (Lushchak, 2016), thus actively resisting oxidative damage caused by exogenous pollution. Similar to our results, different concentrations of fluoxastrobin (0.1, 1.0, and 2.5 mg/kg) in soil induced the changes in earthworm CAT activity, and the values for CAT were lower on days 7, 14, and 28 and greater on day 21 compared to those of the controls (Zhang et al., 2018). Xu et al. (2021) found that azoxystrobin (1.0 mg/kg and 2.5 mg/kg) in black soil and red clay soil induced a significant increase in earthworm CAT, which remained significantly higher than the control treatment after 56 d. Our results also revealed that CAT activity slowly decreased on the 14th day after pollutant exposure in earthworms but remained significantly higher than the control, indicating that the oxidation of the body stimulates the antioxidant capacity of CAT. The increase of hydroxyl free radicals caused by exposure to pollutants would enhance CAT activity, leading to oxidative stress (Malev et al., 2012). Zhu et al. (2020) found that the CAT activity of earthworms in red clay soil with 0.1 mg/kg and 1.0 mg/kg chlorpyrifos was higher than that in the control treatment during the whole exposure period (56 d). This may be because the dynamic balance of SOD-H2O2 did not negatively affect CAT activity. Liu et al. (2021) also found that the CAT activity of earthworms exposed to 20 mg/kg R-acetochlor was significantly higher than that in the control treatment within 7–42 d. In contrast to our findings, there was a trend of stimulation followed by recovery or inhibition of earthworm CAT activity under some pollutant stresses with longer exposure times. Yin et al. (2021) found that the CAT activity of M.guillelmi increased and then decreased significantly after 7 d of exposure to 0.1–50 mg/kg tetracycline. Under the stress of 10 mg/kg ciprofloxacin, the CAT activity of earthworms was higher on the 7th and 14th d and returned to the control level with the extension of treatment time (Yang et al., 2020).
The AWCD in the Biolog-ECO disk reflects the overall capacity of soil microorganisms to utilize carbon sources and microbial activity, and can be used as an effective indicator of soil microbial activity, which is sensitive to soil environmental stress (O’Donnell et al., 2001; Han et al., 2020). The AWCD values of microorganisms in the MS were higher than that in the NS, which may be due to the fact that manure not only contains a large number of microorganisms, but also provides a large amount of carbon, nitrogen, and other nutrients for the growth and propagation of soil microorganisms (Fang et al., 2014; Han et al., 2019; Zeb et al., 2020). Similarly, Lin et al. (2016) reported that two earthworm species, Eisenia fetida and Amynthas robustus E. Perrier, stimulated the soil microbial utilization of amines, amino acids, carbohydrates, and carboxyl acids in pentachlorophenol-contaminated soils. The AWCD values of the CBD2 were close to those of the control soil, which may be attributed to the fact that the higher concentration of carbendazim (2 mg/kg) stimulated the proliferation of more versatile microbial populations in the gut of the earthworms. This, in turn, affects the community structure of soil microorganisms around the earthworms and promoting the overall activity of soil microorganisms to maintain a high level (Han et al., 2023). The NS-CBD2 can stimulate the adaptation of some microorganisms in soil at the absence of nutrients. The change rule of AWCD values in earthworm gut microorganisms was more complicated, but the AWCD values of earthworm gut microorganisms under the MS-CBD2 were significantly increased. This increase is because organic fertilizer can provide essential nutrients for earthworms, and the CBD-enriched residues in the body stimulate more functional bacteria in the gut, which can enhance the activity in the intestinal tract and optimize the earthworms’ ecological service function (Lin et al., 2018; Xiao et al., 2020; Han et al., 2023). Ning et al. (2019) studied the changes in the functional diversity of earthworm gut and soil microbial communities under cadmium stress by the Biolog method. It was found that earthworms not only regulate their physiological functions (such as microbial community structure and stress mechanism), but also influence the external soil microbial community structure to obtain the substances required for growth.
The diversity indices actually reflect different aspects of the functional diversity of soil and gut microbial communities, with 1/D representing the most common dominant species in the community, H evaluating the community species richness, and U being a measure of community species homogeneity. The microbial 1/D, H, and U of MS treatments in soil were higher than those of NS treatments as a whole, indicating that organic fertilizer additions increased soil microbial dominance, abundance and homogeneity. Similar to our results, Urbaniak et al. (2024) showed that the implementation of NBS (Wild flower Meadow) had a positive influence on the values of Shannon-Weaver diversity (H′) in spring, H′ increased by 63% compared to pre-implementation stage. The substrate richness index (S) increased by 53% after NBS implementation in the spring season, while S values were lower in other locations. The change rule of earthworm gut microbial diversity indices in the MS was similar to that of soil microbial diversity index. With the increase of carbendazim concentration, microbial activity showed inhibition first and then increased, which is consistent with the previous research results (Fang et al., 2014; Han et al., 2019). Meanwhile, the microbial diversity indices in both gut and soil of the MS-CBD2 were higher than those in the NS-CBD2. This may be attributed to the fact that most of the microorganisms carried by manure were able to colonize the gut of earthworms, resulting in microbial community compositions of the gut microorganisms that were similar to those of the surrounding soil microorganisms to a certain extent (Gao et al., 2022; Lejoly et al., 2023; Ferlian et al., 2024), and such an effect has also been reported for CBD and glyphosate (Ratcliff et al., 2006; Tortella et al., 2013). Zhou et al. (2020) found that the Simpson index and Shannon Wiener index of earthworm gut microorganisms initially increased and then decreased with the increase of cadmium concentration, indicating that the richness and dominance of earthworm gut microbial community were significantly enhanced by pollutant within a certain concentration range. However, there was a significant difference between the gut microbial diversity index of earthworm and the soil microbial diversity index in the NS, indicating that the addition of organic fertilizers provides nutrients for microbial growth and reproduction, promotes the proliferation of functional gut bacteria, increases the diversity and function of soil microorganisms when they enter the soil, and promotes the remediation of pollutants together with the soil microorganisms, making the soil environment more stable (Vivas et al., 2009; Yakushev et al., 2009; Doan et al., 2013). At the same time, earthworms and their companion animals produce more secretions accompanied by humus production, which enriches the food chain in the ecosystem (Hale et al., 2005; Han et al., 2023).
5 Conclusion
The study focused on the enzyme activities and gut microbial of earthworms response to CBD under the treatment of manure. The results showed that the AChE activities of earthworms in NS-CBD were stimulated at the initial stage and significantly inhibited at the later stage with the extension of exposure time. The trends of earthworm AChE activity under MS-CBD were generally similar to those in NS-CBD, except that the stimulatory and inhibitory effects on earthworm AChE activity were advanced by the treatment of MS-CBD2. The trend of CAT activity of earthworms in MS was similar to that in NS, and the CBD treatment resulted in a tendency for CAT activity to initially increase and then remain at a higher activity. The AWCD values, 1/D, and H of earthworm gut microorganisms did not change significantly in the NS, but MS-CBD2 resulted in significant increase in AWCD values, 1/D, H, and U of earthworm gut microorganisms. The dominance, abundance, and homogeneity of earthworm gut microorganisms in the MS-CBD2 were significantly higher than those in the NS-CBD2. The results of this study can provide a data reference and theoretical basis for monitoring and remediation of pesticide pollution in soil environment.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
TW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing – review & editing. ZY: Data curation, Methodology, Software, Writing – original draft. LJ: Data curation, Methodology, Writing – original draft. WZ: Resources, Software, Supervision, Writing – review & editing. XF: Data curation, Resources, Visualization, Writing – review & editing. WM: Formal analysis, Resources, Visualization, Writing – review & editing. ML: Formal analysis, Funding acquisition, Investigation, Project administration, Resources, Supervision, Validation, Visualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Taizhou Municipal Science and Technology Bureau (22nyb08), and the Chinese Ministry of Agriculture project (PJ2023-013).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bednarska, A. J., Choczynski, M., Laskowski, R., and Walczak, M. (2017). Combined effects of chlorpyriphos, copper and temperature on acetylcholinesterase activity and toxicokinetics of the chemicals in the earthworm Eisenia fetida. Environ. Pollut. 220, 567–576. doi: 10.1016/j.envpol.2016.10.004
Blouin, M., Hodson, M. E., Delgado, E. A., Baker, G., Brussaard, L., Butt, K. R., et al. (2013). A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 64, 161–182. doi: 10.1111/ejss.12025
Calisi, A., Zaccarelli, N., Lionetto, M. G., and Schettino, T. (2013). Integrated biomarker analysis in the earthworm Lumbricus terrestris: application to the monitoring of soil heavy metal pollution. Chemosphere 90, 2637–2644. doi: 10.1016/j.chemosphere.2012.11.040
Chen, X., Wang, X., Gu, X., Jiang, Y., and Ji, R. (2017). Oxidative stress responses and insights into the sensitivity of the earthworms Metaphire guillelmi and Eisenia fetida to soil cadmium. Sci. Total Environ. 574, 300–306. doi: 10.1016/j.scitotenv.2016.09.059
Chen, H., Yang, L., Zhao, S., Xu, H., and Zhang, Z. (2024). Long-term toxic effects of iron-based metal-organic framework nanopesticides on earthworm-soil microorganism interactions in the soil environment. Sci. Total Environ. 917:170146. doi: 10.1016/j.scitotenv.2024.170146
Chuang, S., Yang, H., Wang, X., Xue, C., Jiang, J., and Hong, Q. (2021). Potential effects of Rhodococcus qingshengii strain djl-6 on the bioremediation of carbendazim-contaminated soil and the assembly of its microbiome. J. Hazard. Mater. 414:125496. doi: 10.1016/j.jhazmat.2021.125496
Daam, M. A., Garcia, M. V., Scheffczyk, A., and Rombke, J. (2020). Acute and chronic toxicity of the fungicide carbendazim to the earthworm Eisenia fetida under tropical versus temperate laboratory conditions. Chemosphere 255:126871. doi: 10.1016/j.chemosphere.2020.126871
Datta, S., Singh, J., Singh, S., and Singh, J. (2016). Earthworms, pesticides and sustainable agriculture: a review. Environ. Sci. Pollut. Res. Int. 23, 8227–8243. doi: 10.1007/s11356-016-6375-0
Doan, T. T., Jusselme, D. M., Lata, J.-C., Van Nguyen, B., and Jouquet, P. (2013). The earthworm species Metaphire posthuma modulates the effect of organic amendments (compost vs. vermicompost from buffalo manure) on soil microbial properties. A laboratory experiment. Eur. J.Soil Biol. 59, 15–21. doi: 10.1016/j.ejsobi.2013.08.005
Drake, H. L., and Horn, M. A. (2007). As the worm turns: the earthworm gut as a transient habitat for soil microbial biomes. Ann. Rev. Microbiol. 61, 169–189. doi: 10.1146/annurev.micro.61.080706.093139
Fang, H., Han, L., Cui, Y., Xue, Y., Cai, L., and Yu, Y. (2016). Changes in soil microbial community structure and function associated with degradation and resistance of carbendazim and chlortetracycline during repeated treatments. Sci. Total Environ. 572, 1203–1212. doi: 10.1016/j.scitotenv.2016.08.038
Fang, H., Han, Y. L., Yin, Y. M., Jin, X. X., Wang, S. Y., Tang, F. F., et al. (2014). Microbial response to repeated treatments of manure containing sulfadiazine and chlortetracycline in soil. J. Environ. Sci. Health B 49, 609–615. doi: 10.1080/03601234.2014.911592
Fang, J., Wang, B., Fang, K., Liu, T., Yan, S., and Wang, X. (2022). Assessing the bioavailability and biotoxicity of spiromesifen and its main metabolite spiromesifen-enol (M01) reveals the defense mechanisms of earthworms (Eisenia fetida). Sci. Total Environ. 813:151910. doi: 10.1016/j.scitotenv.2021.151910
Ferlian, O., Goldmann, K., Bonkowski, M., Dumack, K., Wubet, T., and Eisenhauer, N. (2024). Invasive earthworms shift soil microbial community structure in northern North American forest ecosystems. iScience 27:108889. doi: 10.1016/j.isci.2024.108889
Ferris, H., and Tuomisto, H. (2015). Unearthing the role of biological diversity in soil health. Soil Biol. Biochem. 85, 101–109. doi: 10.1016/j.soilbio.2015.02.037
Frasco, M. F., Fournier, D., Carvalho, F., and Guilhermino, L. (2005). Do metals inhibit acetylcholinesterase (AChE)? Implementation of assay conditions for the use of AChE activity as a biomarker of metal toxicity. Biomarkers 10, 360–375. doi: 10.1080/13547500500264660
Gao, F., Fan, H., Chapman, S. J., and Yao, H. (2022). Changes in soil microbial community activity and composition following substrate amendment within the MicroResp™ system. J. Soils Sediments 22, 1242–1251. doi: 10.1007/s11368-022-03143-w
Gautam, K., Dwivedi, S., Verma, R., Vamadevan, B., Patnaik, S., and Anbumani, S. (2024). Combined effects of polyethylene microplastics and carbendazim on Eisenia fetida: a comprehensive ecotoxicological study. Environ. Pollut. 348:123854. doi: 10.1016/j.envpol.2024.123854
Ge, Z., Du, H., Gao, Y., and Qiu, W. (2018). Analysis on metabolic functions of stored Rice microbial communities by BIOLOG ECO microplates. Front. Microbiol. 9:1375. doi: 10.3389/fmicb.2018.01375
Goulson, D., and Kleijn, D. (2013). REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 50, 977–987. doi: 10.1111/1365-2664.12111
Gryta, A., Frąc, M., and Oszust, K. (2014). The application of the Biolog EcoPlate approach in Ecotoxicological evaluation of dairy sewage sludge. Appl. Biochem. Biotechnol. 174, 1434–1443. doi: 10.1007/s12010-014-1131-8
Guo, T., O'Connor, P. J., Zhao, X., Zhou, T., Wang, Y., and Zhang, M. (2023). The win-win effects of nitrification inhibitors on soil-crop systems: decreasing carbendazim residues but promoting soil bacterial community diversities and stabilities and crop yields. J. Hazard. Mater. 451:131175. doi: 10.1016/j.jhazmat.2023.131175
Hackenberger, D. K., Stjepanovic, N., Loncaric, Z., and Hackenberger, B. K. (2018). Acute and subchronic effects of three herbicides on biomarkers and reproduction in earthworm Dendrobaena veneta. Chemosphere 208, 722–730. doi: 10.1016/j.chemosphere.2018.06.047
Hale, C. M., Frelich, L. E., Reich, P. B., and Pastor, J. (2005). Effects of European earthworm invasion on soil characteristics in northern hardwood forests of Minnesota, USA. Ecosystems 8, 911–927. doi: 10.1007/s10021-005-0066-x
Han, L., Cai, L., Zhang, H., Long, Z., Yu, Y., and Fang, H. (2019). Development of antibiotic resistance genes in soils with ten successive treatments of chlortetracycline and ciprofloxacin. Environ. Pollut. 253, 152–160. doi: 10.1016/j.envpol.2019.07.031
Han, L., Fang, K., You, X., Li, Y., Wang, X., and Wang, J. (2023). Earthworms synergize with indigenous soil functional microorganisms to accelerate the preferential degradation of the highly toxic S-enantiomer of the fungicide imazalil in soil. J. Hazard. Mater. 457:131778. doi: 10.1016/j.jhazmat.2023.131778
Han, L., Liu, Y., Fang, K., Zhang, X., Liu, T., Wang, F., et al. (2020). Azoxystrobin dissipation and its effect on soil microbial community structure and function in the presence of chlorothalonil, chlortetracycline and ciprofloxacin. Environ. Pollut. 257:113578. doi: 10.1016/j.envpol.2019.113578
Hu, S., Zhang, W., Li, J., Lin, K., and Ji, R. (2016). Antioxidant and gene expression responses of Eisenia fetida following repeated exposure to BDE209 and Pb in a soil-earthworm system. Sci. Total Environ. 556, 163–168. doi: 10.1016/j.scitotenv.2016.02.194
Huang, Z., Qin, Y., He, X., Zhang, M., Ren, X., Yu, W., et al. (2024). Analysis on metabolic functions of rhizosphere microbial communities of Pinus massoniana provenances with different carbon storage by Biolog eco microplates. Front. Microbiol. 15:1365111. doi: 10.3389/fmicb.2024.1365111
Kenko Nkontcheu, D. B., Fotio, A. L., Kenfack, A. D., Tasse Taboue, G. C., Acha, D. A., and Fokam, E. B. (2023). Ecological risk assessment of pesticides based on earthworms in soils on the southeast slopes of Mount Cameroon. Soil Environ. Health 1:100047. doi: 10.1016/j.seh.2023.100047
Koner, S., Chen, J. S., Hsu, B. M., Rathod, J., Huang, S. W., Chien, H. Y., et al. (2022). Depth-resolved microbial diversity and functional profiles of trichloroethylene-contaminated soils for Biolog EcoPlate-based biostimulation strategy. J. Hazard. Mater. 424:127266. doi: 10.1016/j.jhazmat.2021.127266
Lee, S., Choi, J., Jho, E. H., and Shin, S. (2024). Effects of polyvinyl chloride and low-density polyethylene microplastics on oxidative stress and mitochondria function of earthworm (Eisenia fetida). Ecotox. Environ. Saf. 283:116847. doi: 10.1016/j.ecoenv.2024.116847
Lejoly, J., Quideau, S., Laganière, J., Karst, J., Martineau, C., Swallow, M., et al. (2023). Earthworm-invaded boreal forest soils harbour distinct microbial communities. Soil 9, 461–478. doi: 10.5194/soil-9-461-2023
Li, X., Zhu, L., Du, Z., Li, B., Wang, J., Wang, J., et al. (2018). Mesotrione-induced oxidative stress and DNA damage in earthworms (Eisenia fetida). Ecol. Indic. 95, 436–443. doi: 10.1016/j.ecolind.2018.08.001
Lin, Z., Zhen, Z., Ren, L., Yang, J., Luo, C., Zhong, L., et al. (2018). Effects of two ecological earthworm species on atrazine degradation performance and bacterial community structure in red soil. Chemosphere 196, 467–475. doi: 10.1016/j.chemosphere.2017.12.177
Lin, Z., Zhen, Z., Wu, Z., Yang, J., Zhong, L., Hu, H., et al. (2016). The impact on the soil microbial community and enzyme activity of two earthworm species during the bioremediation of pentachlorophenol-contaminated soils. J. Hazard. Mater. 301, 35–45. doi: 10.1016/j.jhazmat.2015.08.034
Liu, T., Chen, X., Gong, X., Lubbers, I. M., Jiang, Y., Feng, W., et al. (2019). Earthworms coordinate soil biota to improve multiple ecosystem functions. Curr. Biol. 29:e3425, 3420–3429.e5. doi: 10.1016/j.cub.2019.08.045
Liu, Q., Eisenhauer, N., Scheu, S., Reitz, T., and Schädler, M. (2025). Grasslands support more diverse and resilient earthworm communities to climate change than croplands in Central Europe. Agric. Ecosyst. Environ. 377:109259. doi: 10.1016/j.agee.2024.109259
Liu, Y., Fang, K., Zhang, X., Liu, T., and Wang, X. (2021). Enantioselective toxicity and oxidative stress effects of acetochlor on earthworms (Eisenia fetida) by mediating the signaling pathway. Sci. Total Environ. 766:142630. doi: 10.1016/j.scitotenv.2020.142630
Liu, G., Ling, S., Zhan, X., Lin, Z., Zhang, W., and Lin, K. (2017). Interaction effects and mechanism of Pb pollution and soil microorganism in the presence of earthworm. Chemosphere 173, 227–234. doi: 10.1016/j.chemosphere.2017.01.022
Liu, T., Wang, X., Chen, D., Li, Y., and Wang, F. (2018). Growth, reproduction and biochemical toxicity of chlorantraniliprole in soil on earthworms (Eisenia fetida). Ecotoxicol. Environ. Saf. 150, 18–25. doi: 10.1016/j.ecoenv.2017.12.010
Lushchak, V. I. (2016). Contaminant-induced oxidative stress in fish: a mechanistic approach. Fish Physiol. Biochem. 42, 711–747. doi: 10.1007/s10695-015-0171-5
Ma, T., Chen, L., Wu, L., Zhang, H., and Luo, Y. (2016). Oxidative stress, cytotoxicity and genotoxicity in earthworm Eisenia fetida at different Di-n-butyl phthalate exposure levels. PLoS One 11:e0151128. doi: 10.1371/journal.pone.0151128
Malev, O., Klobučar, R. S., Fabbretti, E., and Trebše, P. (2012). Comparative toxicity of imidacloprid and its transformation product 6-chloronicotinic acid to non-target aquatic organisms: microalgae Desmodesmus subspicatus and amphipod Gammarus fossarum. Pestic. Biochem. Phys. 104, 178–186. doi: 10.1016/j.pestbp.2012.07.008
Markad, V. L., Gaupale, T. C., Bhargava, S., Kodam, K. M., and Ghole, V. S. (2015). Biomarker responses in the earthworm, Dichogaster curgensis exposed to fly ash polluted soils. Ecotoxicol. Environ. Saf. 118, 62–70. doi: 10.1016/j.ecoenv.2015.04.011
Nagata, Y., Osono, T., Hasegawa, M., Hobara, S., Hirose, D., Tanabe, Y., et al. (2023). Application of the Biolog EcoPlate™ technique for assessing the microbial metabolic diversity in moss profiles of continental Antarctica. Pol. Sci. 35:100924. doi: 10.1016/j.polar.2023.100924
Ning, Y., Zhou, H., and Zhou, D. (2019). Study on the microbial community in earthworm and soil under cadmium stress based on contour line analysis. Environ. Sci. Pollut. Res. Int. 26, 20989–21000. doi: 10.1007/s11356-019-05207-w
O’Donnell, A. G., Seasman, M., Macrae, A., Waite, I., and Davies, J. T. (2001). Plants and fertilisers as drivers of change in microbial community structure and function in soils. Plant Soil 232, 135–145. doi: 10.1023/A:1010394221729
Preston-Mafham, J., Boddy, L., and Randerson, P. F. (2002). Analysis of microbial community functional diversity using sole-carbon-source utilisation profiles - a critique. FEMS Microbiol. Ecol. 42, 1–14. doi: 10.1111/j.1574-6941.2002.tb00990.x
Qi, S., Wang, D., Zhu, L., Teng, M., Wang, C., Xue, X., et al. (2018). Effects of a novel neonicotinoid insecticide cycloxaprid on earthworm, Eisenia fetida. Environ. Sci. Pollut. Res. Int. 25, 14138–14147. doi: 10.1007/s11356-018-1624-z
Ratcliff, A. W., Busse, M. D., and Shestak, C. J. (2006). Changes in microbial community structure following herbicide (glyphosate) additions to forest soils. Appl. Soil Ecol. 34, 114–124. doi: 10.1016/j.apsoil.2006.03.002
Singh, S., Tiwari, R. K., and Pandey, R. S. (2019). Acute toxicity evaluation of triazophos, deltamethrin and their combination on earthworm, Eudrilus eugeniae and its impact on AChE activity. Chem. Ecol. 35, 563–575. doi: 10.1080/02757540.2019.1600679
Sułowicz, S., Markowicz, A., Dulski, M., Nowak, A., Środek, D., and Borymski, S. (2023). Assessment of the ecotoxicological impact of captan@ZnO35–45nm and captan@SiO2 20–30nm nanopesticide on non-target soil microorganisms – a 100-day case study. Appl. Soil Ecol. 184:104789. doi: 10.1016/j.apsoil.2022.104789
Tortella, G. R., Mella-Herrera, R. A., Sousa, D. Z., Rubilar, O., Briceno, G., Parra, L., et al. (2013). Carbendazim dissipation in the biomixture of on-farm biopurification systems and its effect on microbial communities. Chemosphere 93, 1084–1093. doi: 10.1016/j.chemosphere.2013.05.084
Urbaniak, M., Mierzejewska-Sinner, E., Bednarek, A., Krauze, K., and Wlodarczyk-Marciniak, R. (2024). Microbial response to nature-based solutions in urban soils: a comprehensive analysis using Biolog(R) EcoPlates. Sci. Total Environ. 928:172360. doi: 10.1016/j.scitotenv.2024.172360
Van Groenigen, J. W., Van Groenigen, K. J., Koopmans, G. F., Stokkermans, L., Vos, H. M. J., and Lubbers, I. M. (2019). How fertile are earthworm casts? A meta-analysis. Geoderma 338, 525–535. doi: 10.1016/j.geoderma.2018.11.001
Vidal, A., Blouin, M., Lubbers, I., Capowiez, Y., Sanchez-Hernandez, J. C., Calogiuri, T., et al. (2023). Chapter one - the role of earthworms in agronomy: consensus, novel insights and remaining challenges. Adv. Agron. 181, 1–78. doi: 10.1016/bs.agron.2023.05.001
Vivas, A., Moreno, B., Garcia-Rodriguez, S., and Benitez, E. (2009). Assessing the impact of composting and vermicomposting on bacterial community size and structure, and microbial functional diversity of an olive-mill waste. Bioresour. Technol. 100, 1319–1326. doi: 10.1016/j.biortech.2008.08.014
Wang, K., Mu, X., Qi, S., Chai, T., Pang, S., Yang, Y., et al. (2015). Toxicity of a neonicotinoid insecticide, guadipyr, in earthworm (Eisenia fetida). Ecotoxicol. Environ. Saf. 114, 17–22. doi: 10.1016/j.ecoenv.2014.12.037
Wang, G., Xia, X., Yang, J., Tariq, M., Zhao, J., Zhang, M., et al. (2020). Exploring the bioavailability of nickel in a soil system: physiological and histopathological toxicity study to the earthworms (Eisenia fetida). J. Hazard. Mater. 383:121169. doi: 10.1016/j.jhazmat.2019.121169
Wang, J., Yang, Y., Wu, J., Zhao, K., and Zhang, X. (2024). The interaction between biochar and earthworms: revealing the potential ecological risks of biochar application and the feasibility of their co-application. Sci. Total Environ. 950:175240. doi: 10.1016/j.scitotenv.2024.175240
Wu, Y., Deng, S., Hao, P., Tang, H., Xu, Y., Zhang, Y., et al. (2024). Roxarsone reduces earthworm-mediated nutrient cycling by suppressing aggregate formation and enzymic activity in soil with manure application. Environ. Pollut. 360:124777. doi: 10.1016/j.envpol.2024.124777
Wu, S., Zhang, H., Zhao, S., Wang, J., Li, H., and Chen, J. (2012). Biomarker responses of earthworms (Eisenia fetida) exposured to phenanthrene and pyrene both singly and combined in microcosms. Chemosphere 87, 285–293. doi: 10.1016/j.chemosphere.2011.11.055
Xiao, L., Li, M. H., Dai, J., Motelica-Heino, M., Chen, X. F., Wu, J. L., et al. (2020). Assessment of earthworm activity on cu, cd, Pb and Zn bioavailability in contaminated soils using biota to soil accumulation factor and DTPA extraction. Ecotoxicol. Environ. Saf. 195:110513. doi: 10.1016/j.ecoenv.2020.110513
Xu, Y., Li, B., Hou, K., Du, Z., Allen, S. C., Zhu, L., et al. (2021). Ecotoxicity evaluation of azoxystrobin on Eisenia fetida in different soils. Environ. Res. 194:110705. doi: 10.1016/j.envres.2020.110705
Xu, Q., Shi, Y., Ke, L., Qian, L., Zhou, X., and Shao, X. (2023). Ciprofloxacin enhances cadmium toxicity to earthworm Eisenia fetida by altering the gut microorganism composition. Environ. Pollut. 333:122106. doi: 10.1016/j.envpol.2023.122106
Yakushev, A. V., Blagodatsky, S. A., and Byzov, B. A. (2009). The effect of earthworms on the physiological state of the microbial community at vermicomposting. Microbiology 78, 510–519. doi: 10.1134/s002626170904016x
Yan, Y., Zhai, J., Wang, L., and Wang, X. (2024). Response and defense mechanisms of the earthworms Eisenia foetida to natural saline soil stress. Sci. Total Environ. 951:175480. doi: 10.1016/j.scitotenv.2024.175480
Yang, X., Li, Y., and Wang, X. (2020). Effects of ciprofloxacin exposure on the earthworm Eisenia fetida. Environ. Pollut. 262:114287. doi: 10.1016/j.envpol.2020.114287
Yang, X., Shang, G., and Wang, X. (2022). Biochemical, transcriptomic, gut microbiome responses and defense mechanisms of the earthworm Eisenia fetida to salt stress. Ecotox. Environ. Saf. 239:113684. doi: 10.1016/j.ecoenv.2022.113684
Yang, Y., Xiao, Y., Chang, Y., Cui, Y., Klobucar, G., and Li, M. (2018). Intestinal damage, neurotoxicity and biochemical responses caused by tris (2-chloroethyl) phosphate and tricresyl phosphate on earthworm. Ecotoxicol. Environ. Saf. 158, 78–86. doi: 10.1016/j.ecoenv.2018.04.012
Yin, B., Zhang, M., Zeng, Y., Chen, H., Fan, T., Wu, Z., et al. (2021). The changes of antioxidant system and intestinal bacteria in earthworms (Metaphire guillelmi) on the enhanced degradation of tetracycline. Chemosphere 265:129097. doi: 10.1016/j.chemosphere.2020.129097
Zeb, A., Li, S., Wu, J., Lian, J., Liu, W., and Sun, Y. (2020). Insights into the mechanisms underlying the remediation potential of earthworms in contaminated soil: a critical review of research progress and prospects. Sci. Total Environ. 740:140145. doi: 10.1016/j.scitotenv.2020.140145
Zhang, W., Xia, X., Wang, J., Zhu, L., Wang, J., Wang, G., et al. (2021). Oxidative stress and genotoxicity of nitenpyram to earthworms (Eisenia foetida). Chemosphere 264:128493. doi: 10.1016/j.chemosphere.2020.128493
Zhang, Y., Yang, Z., Li, X., Song, P., and Wang, J. (2022). Effects of diisononyl phthalate exposure on the oxidative stress and gut microorganisms in earthworms (Eisenia fetida). Sci. Total Environ. 822:153563. doi: 10.1016/j.scitotenv.2022.153563
Zhang, C., Zhu, L., Wang, J., Wang, J., Du, Z., Li, B., et al. (2018). Evaluating subchronic toxicity of fluoxastrobin using earthworms (Eisenia fetida). Sci. Total Environ. 642, 567–573. doi: 10.1016/j.scitotenv.2018.06.091
Zhao, S., Liu, T., Wang, B., Fu, J., Liang, T., Zhong, Z., et al. (2019). Accumulation, biodegradation and toxicological effects of N-ethyl perfluorooctane sulfonamidoethanol on the earthworms Eisenia fetida exposed to quartz sands. Ecotoxicol. Environ. Saf. 181, 138–145. doi: 10.1016/j.ecoenv.2019.05.062
Zhou, T., Guo, T., Wang, Y., Wang, A., and Zhang, M. (2023). Carbendazim: ecological risks, toxicities, degradation pathways and potential risks to human health. Chemosphere 314:137723. doi: 10.1016/j.chemosphere.2022.137723
Zhou, H., Zhang, T., Zhuang, J., Xu, M., Liu, X., Shi, Q., et al. (2020). Study on the regulation of earthworm physiological function under cadmium stress based on a compound mathematical model. Environ. Toxicol. Phar. 80:103499. doi: 10.1016/j.etap.2020.103499
Keywords: manure, carbendazim, earthworm, enzyme activity, microbial functional diversity
Citation: Wang T, Zhang L, Yao Z, Jin L, Zhang W, Feng X, Ma W and Lin M (2024) Response of earthworm enzyme activity and gut microbial functional diversity to carbendazim in the manured soil. Front. Microbiol. 15:1461880. doi: 10.3389/fmicb.2024.1461880
Edited by:
Marika Pellegrini, University of L’Aquila, ItalyCopyright © 2024 Wang, Zhang, Yao, Jin, Zhang, Feng, Ma and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weibin Ma, Weibin.Ma@tsl.ac.uk; Mei Lin, hylm84712002@126.com
 Tianyu Wang1
Tianyu Wang1