- Key Laboratory of North China Water-Saving Agriculture, Ministry of Agriculture and Rural Affairs, Hebei Key Laboratory of Vegetable Germplasm Innovation and Utilization, Collaborative Innovation Center of Vegetable Industry in Hebei, College of Horticulture, Hebei Agricultural University, Baoding, China
Soil borne diseases are one of the most serious diseases which often results the decline of vegetables quality and loss of production. Moreover, it is difficult for plants to exhibit disease symptoms in the early stages attributing to strong concealment of soil borne pathogens. Therefore, early detection of pathogens and their physiological races plays an important role in reducing the harm of pathogens associated with diseases of vegetable crops. The traditional diagnostic techniques relied on the time consuming and less accurate methods like disease symptom observation, microscopic diagnosis, and culture techniques etc. The development of molecular biology technology has brought revolutionary changes to the diagnosis of vegetable soil borne diseases, improving the accuracy and efficiency of diagnosis. This paper reviews the various molecular detection techniques for vegetable soil borne pathogens (PCR, nested-PCR, multiplex PCR, etc.) and their physiological races (host identification, DNA molecular markers, transposon detection, etc.), explains the advantages and disadvantages of each detection technique. Furthermore, the paper comprehensively introduces the application of molecular detection technology for soil borne pathogen detection in soil, plants, and seeds. Finally, we put forward important perspectives for the future development of rapid detection methods, aiming to promote rapid diagnosis of soil pathogenic microorganisms and provide guidance for the control of biological risks.
1 Introduction
Vegetables are among the essential foods in people’s daily diet and are also used in food, nutrition, healthcare, etc. (Willer et al., 2023). As per reports of the Food and Agriculture Organization (FAO), China is the first largest global producer of vegetables, with a planting area of 23.42 million·hm2 (FAO, 2023). However, with the continuous cultivation of vegetables for several years, soil borne pathogens have accumulated and cropping obstacles have become seriously increasing. The typical soil borne pathogens commonly found including fungi (Fusarium sp., Rhizoctonia sp., Phytophthora sp., etc) and bacteria (Ralstonia sp., Pectobacterium sp., Clavibacter sp., etc). The infected plants developed slowly, their leaves defoliated and wilted, their roots turned brown, with decayed cortical tissues that shriveled. Due to the long incubation period, complexity and rapid spread of soil borne pathogens, soil borne diseases cause huge economic losses to vegetables (Xie et al., 2024).
The early detection of vegetable soil borne pathogens is arguably the most challenging task, mainly due to unclear disease symptoms in the early stages, their complex and diverse physiological races, and limitations of different detection methods. Notably, physiological races of vegetable soil borne pathogens are particularly difficult to detect due to their high similarity. Traditional techniques for detecting soil-borne pathogens, such as the use of selective media, are of great value because they are relatively inexpensive and not technically demanding. Microscopic diagnosis is a fast technique to identify the spores of soil borne fungal pathogens in vegetables. Traditional detection methods such as symptom observation, microscopic diagnosis, pathogen plate culture technology, are time consuming, laborious, low sensitivity and not suitable for the rapid as well as early diagnosis of soil borne diseases. New technologies, such as molecular biology detection technology, provide better means for the diagnosis as well as study of vegetable diseases causing pathogens and physiological race with high reliability, precision and accuracy.
This review focused on the various molecular detection techniques for vegetable soil borne pathogens (conventional PCR, nested-PCR, multiplex PCR, RT-qPCR, PMA-qPCR, LAMP, RPA/CRISPR-Cas12a, genomics) and their physiological races (host identification, conventional PCR, DNA molecular markers, transposon, pathogenicity-related genes, genomics), also explained the advantages and disadvantages of each detection technique. Moreover, the new technology, both currently in use and under development were also described, for diagnosing soil borne diseases of vegetables, with an emphasis on the application of different detection techniques in different tissues (soil, plants, and seeds). This review is the comprehensive summary about the progress and application of recent molecular detection techniques for vegetable soil borne diseases. Moreover, providing the references for the further development and application of biology detection technology in the detection of pathogenic microorganisms for disease prevention and control.
2 Molecular detection techniques for soil borne pathogens in vegetables
2.1 Conventional PCR based detection technology
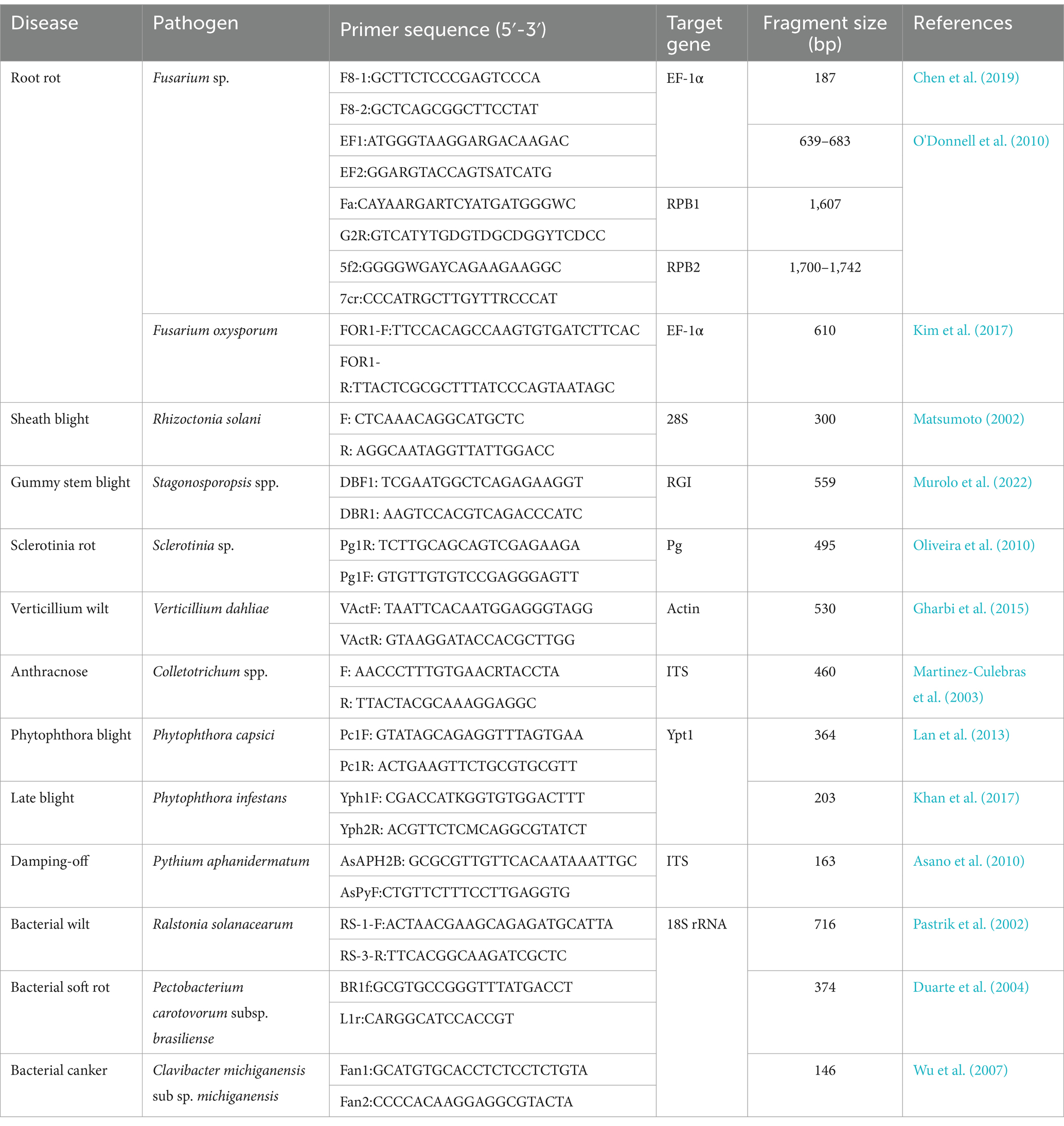
With the rapid development of modern biotechnology, detection methods based on molecular biology have widely adopted. At present, 16S rDNA, ITS sequences and other genes (RBP1, RBP2, TEF-1α, gyrB) are mainly used as templates to design specific primers for PCR amplification. The use of PCR based detection technology has been widely reported for vegetable soil borne pathogens (Fusarium oxysporum, R. solani, Verticillium dahliae, Phytophthora capsici, etc.) (Table 1). The advantages of PCR technology include the ability to detect single pathogens and non-culturable pathogens in complex mixed soil/plant samples (Shneyder et al., 2022). Although the detection speed of conventional PCR is fast at low cost and sensitivity, thus, it can only be used for qualitative analysis. Moreover, the distribution of pathogens in soil is uneven, which can lead to false negatives in conventional PCR detection.
2.2 Nested-PCR based detection technology
The nested PCR technique uses two pairs of PCR primers to amplify the complete fragment (Koentjoro et al., 2023). The main advantage is that the results of the second amplification can change the erroneous fragments produced by the first amplification. Nested PCR techniques have been developed to detect latent infections caused by fungi (Mutasa et al., 1996; Grote et al., 2002; Mudiyanselage et al., 2021), bacteria (Liop et al., 2000) and viruses (Nair and Manimekalai, 2021) in host plants. A nested PCR assay developed for rapid detection of F. oxysporum f. sp. lactucae in lettuce seeds permitted the detection of the pathogen in seed lots with an infestation rate as low as 0.1% (Mbofung and Pryor, 2010). Klemsdal and Elen developed a nested PCR detection technique for Fusarium culmorum with a detection limit of 5–50 fg of purified target DNA, and its sensitivity is 100 times higher than that of conventional PCR (Klemsdal and Elen, 2006). Li et al. (2014) established a nested PCR detection system for Phytophthora with detection limits of 100 fg of genomic DNA per 25-μL reaction. Qin et al. (2011) established a quick and accurate technique to detect infection of rape oil seed by S. sclerotiorum via a nested PCR technique, which can detect 50 fg of genomic DNA in approximately 6 h. Jesús et al. (2002) designed specific primers based on the DNA (RAPD) marker sequence with a band size of 1958 bp and established a nested PCR technique for the detection of non-defoliating (ND) V. dahliae. However, detection technology based on nested PCR still has some shortcomings. First, this technique is time consuming, as it requires two PCRs followed by confirmation of the positive result by agarose gel electrophoresis. Second, in open environments where multiple samples are processed, nested PCR is more prone to contamination (Cordova et al., 2014). The above shortcomings often lead to false positives, thus reducing the efficiency of molecular diagnosis of pathogens associated with vegetable diseases (Youssef et al., 2017).
2.3 Multiplex PCR based detection technology
Multiplex PCR (M-PCR) is a variant of PCR in which two or more target sequences are simultaneously amplified in the same reaction, combining the advantages of conventional PCR and nested PCR (Israa et al., 2023). M-PCR is more practical in diagnostics and research in the vegetable crops thus, saves time and cost, as many pathogens often infect the same vegetable (Panno et al., 2014). Notably, construction of the M-PCR system requires evaluation of the compatibility of specific primers of multiple pathogens. Ozdemir (2005) used dual PCR to detect tomato canker (Cmm) and scab (Xav). Gao et al. (2010) established an M-PCR detection technique for the pathogens Cladosporium cucumerinum, F. oxysporum and Mycosphaerella melonis in infected plant tissues. The M-PCR detection technology established by Quinterovásquez et al. (2010) can simultaneously detect Clavibacter and Fusarium in tomato which has high application value. Umesha and Avinash (2015) developed a dual PCR technology that can be used to detect bacterial wilt and scab in vegetables. Gao et al. (2016) established an M-PCR detection technology to successfully measure the levels of Corynespora cassiicola, Colletotrichum orbiculare, and Pseudomonas syringae pv. lachrymans. Kang et al. (2018) designed specific primers for quadruple PCR detection of tomato soil borne pathogens. It is rapid and stable technique, including the detection of Pseudomonas syringae, C. michiganensis subsp. michiganensis, R. solanacearum, and Xanthomonas campestris (Kang et al., 2018). Liu et al. (2019) established M-PCR technology for simultaneous detection of P. aphanidermatum, F. oxysporum and V. dahliae in vegetable fields, with improved efficiency and reduction in the time to detect each pathogen. Moreover, the three soil borne pathogens R. solanacearum, V. dahliae and Sclerotium rolfsii were identified in eggplant, using this M-PCR technology with the detection rate (Zou et al., 2023). The disadvantage is that multiple primer pairs, templates, etc. are prone to non-specific amplification in the same reaction, leading to false positive detection results. Other obvious limitations are if the length difference of amplified fragments is limited by the resolution of agarose gel electrophoresis, it may affect the detection sensitivity. It was unable to ascertain the pathogenicity and infectivity of the organism detected using M-PCR technology. Furthermore, it was unable to infer data on microbial cell integrity, which has an impact on epidemiological assessment. Therefore, in designing primers, it is necessary to choose a consistent PCR amplification system, especially the annealing temperature. Only in this way can the efficiency of soil borne pathogens detection be improved.
2.4 RT–qPCR based detection technology
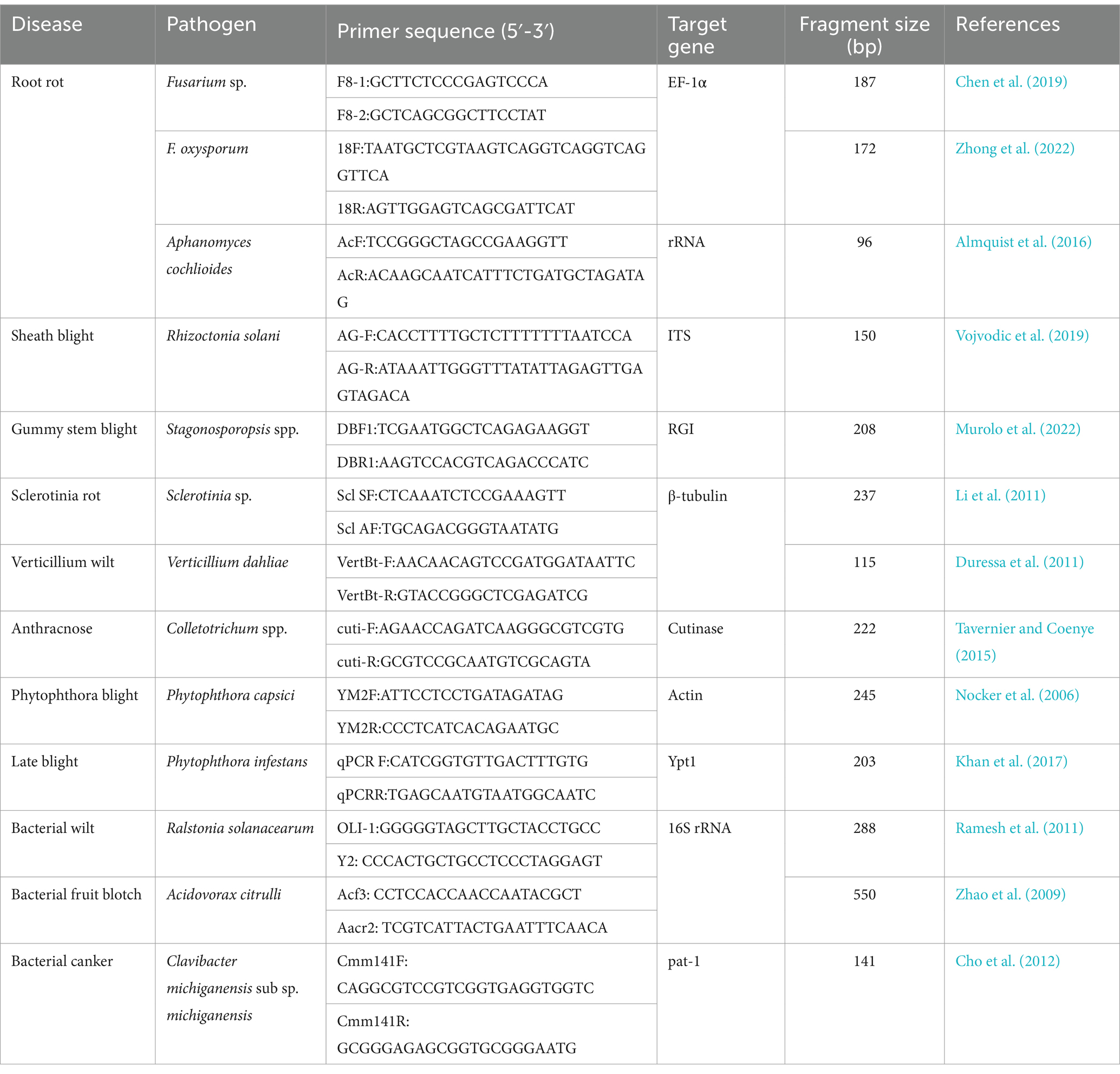
Real-time quantitative PCR (RT–qPCR) is well developed detection technology. This technique uses fluorescence signals to monitor the amplification products of each cycle in the DNA replication process in real time in-vitro conditions. This technique allowing the quantitative and qualitative analysis of DNA templates, thus, has become the gold standard for vegetable disease diagnosis compared with conventional PCR technology (Chen et al., 2023). In particular, RT–qPCR can quantitatively detect non-culturable pathogens in vegetable tissues and pathogens that cannot be extracted from host tissue (Mirmajlessi et al., 2015; Abou-Jawdah et al., 2019). The use of RT–qPCR detection technology has been widely reported in research on soil borne vegetable diseases (Table 2). Vojvodic et al. (2019) designed specific primers and developed an RT–qPCR technique for R. solani that was 100 times more sensitive than the conventional PCR technique. Chen et al. (2019) designed Fusarium-specific primers for the TEF gene and constructed an RT–qPCR detection system with the sensitivity 10,000 times higher than that of conventional PCR. Yang et al. (2022) designed specific primers and established an RT–qPCR technique for the detection of Colletotrichum spp. in strawberry with the detection limit of 10–105 copies. Cheng et al. (2018) established an RT–qPCR detection system for P. capsici using the YM2F/YM2R primers, with a sensitivity of 10−1 pg.·μL−1, 100 times more than that of conventional PCR. The advantages of RT–qPCR are as follows: (i) speed: compared with conventional PCR, the main advantage of RT–qPCR is that the whole RT–qPCR runs can be performed in 1 to 2 h without complicated steps; (ii) sensitivity: RT–qPCR is 10 ~ 104 times more sensitive than conventional PCR; (iii) specificity: evaluation of specificity can be done by melting curve analysis during operation; (iv) quantification: compared with conventional PCR, fluorescence PCR can be used to quantitatively measure the levels of vegetable pathogens through comparison with a standard curve. RT–qPCR technique is its inability to differentiate between live and dead cells of pathogens but identify their presence. Although this technology is efficient and applicable, there are limitations in the quantitative detection of soil borne pathogens. It is used mainly for research in the field of soil borne disease management. With the recognition of RT–qPCR detection technology by farmers, the demand for commercial detection is likely to increase.
2.5 PMA-qPCR based detection technology
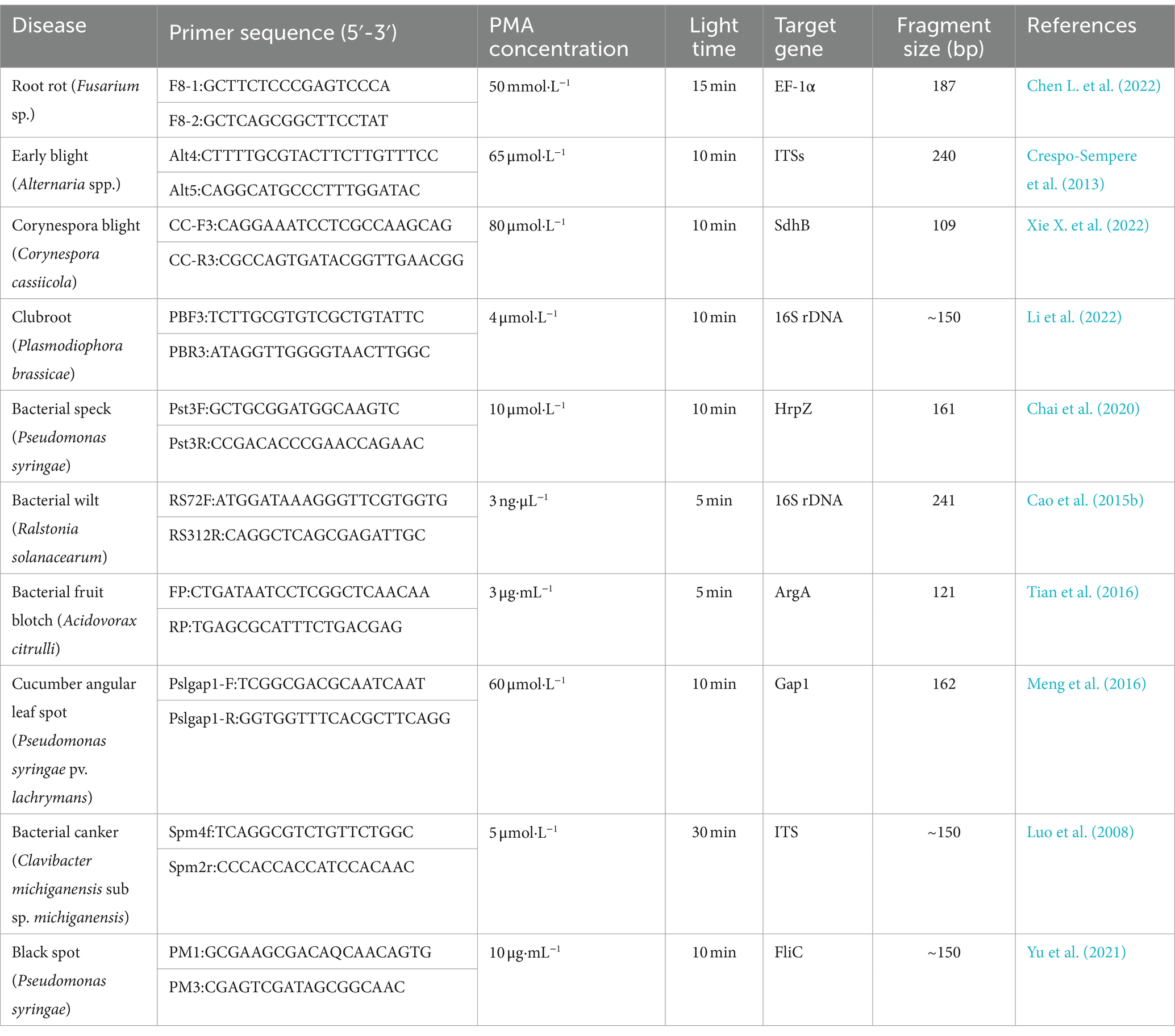
PMA (Propidium Monoazide) is a special membrane impermeable dye so that can penetrate damaged cell membranes to emit fluorescent signals without direct impact on intact cells (Foteini et al., 2023). Following a specific duration of light reaction, PMA, after entering dead/ damaged cells, combines with their DNA. It causes the loss of fluorescence signals during bacterial DNA amplification and ignores the level of dead cells. The process of constructing a PMA-qPCR detection system for vegetable diseases includes screening the concentration of PMA and illumination time. PMA inhibit the PCR amplification of dead-cell DNA reducing the overestimation of cell count caused by dead-cell DNA in qPCR detection (Tavernier and Coenye, 2015). Since the first description by Nocker et al. (2006), PMA has been applied to a wide variety of microorganisms, including bacteria, viruses and fungi (Table 3). Chen L. et al. (2022) designed the specific primers F8-1/F8-2 based on the translation elongation factor (TEF) gene, screened PMA concentration (50 mmol·L−1) and illumination time (15 min). With this they established a PMA-qPCR technique to amplify and quantify living cells of Fusarium in soil. Xie X. W. et al. (2022) designed PMA-qPCR primers based on the SdhB sequence of the Corynespora blight pathogen, which effectively detect C. cassiicola in soil. Compared with its use to study vegetable-infecting, the application of PMA-qPCR detection technology in fungal research is relatively rare compared with studies on bacterial pathogens. This technology differentiate the dead and live cells of pathogens hence it is highly applicable in field disease control and drug screening. However, PMA is expensive and only suitable for professional laboratory personnel, not for field operation.
2.6 LAMP based detection technology
LAMP (loop-mediated isothermal amplification), an isothermal nucleic acid amplification platform devised by Notomi, has emerged as a popular tool for phytoplasma molecular detection (Khat et al., 2024). The basic principle of this technique involves the design of four specific primers for six different regions on the target sequence, including a pair of internal primers (FIP and BIP), a pair of external primers (F3 and B3) and a pair of ring primers (LF and LB). The LAMP reaction can be simply carried out under isothermal conditions by using BstDNA polymerase with high displacement activity. LAMP technology has been widely used in the detection of soil borne pathogens in vegetables, such as bacteria (Gieroń et al., 2023), viruses (Supakitthanakorn et al., 2022), and oomycetes (Htun et al., 2020), because of its applicability in open field operations. In addition, there have also been widespread reports of the use of LAMP technology to detect soil borne pathogenic fungi in vegetables (Zeng et al., 2017; Achari et al., 2023; Xie X. W. et al., 2022). Peng et al. (2013) tested 8 artificially inoculated samples and 85 field soil samples with the LAMP technique, and the detection limit for F. oxysporum was 103 spores. Huang W. et al. (2016) established LAMP technology for the detection of R. solanacearum in vegetables, and its sensitivity was 10 times higher than that of conventional PCR. Yao et al. (2016) constructed a LAMP detection system for Didymella bryoniae; its sensitivity was 1,000 times that of conventional PCR, and the detection limit was 0.1 fg·μL−1. Almasi (2019) used LAMP to detect the DNA of F. oxysporum, and the detection efficiency was 100 times greater than that of conventional PCR. For Colletotrichum species, LAMP detection exhibits accuracy and strong sensitivity, for the detection of pathogen DNA at 100 pg.·μL−1 (Liu Y. et al., 2021). Lu et al. (2015) designed and screened specific primers based on the ITS sequence of R. solani for establishing the LAMP detection system which allowed successful as well as rapid diagnosis for this species. The advantages of LAMP are as follows: (1) high amplification efficiency, 10–1,000 times higher than that of conventional PCR; (2) high speed, the whole reaction can be completed within 30–60 min; (3) strong specificity, the four specific primers for LAMP are for six conserved sites in the target gene sequence, and DNA amplification cannot be performed if any site does not match; (4) simple operation and (5) low cost. The disadvantage of LAMP is that it can only be used for qualitative analysis, not for quantitative detection. In addition, LAMP is prone to producing false positives due to its open operation, which affects the results.
2.7 RPA/CRISPR-Cas12a based detection technology
The clustered regularly inter spaced palindromic repeats/CRISPR-associated proteins system technology is currently an emerging nucleic acid detection technology. The principle is that RPA rapidly amplify the nucleic acid fragment to be detected at 37°C to achieve the minimum detection limit of CRISPR/Cas12a (Kim et al., 2023). After activation of the RuvC cleavage site of Cas12a, it will exert its non-specific nuclease activity (Zetsche et al., 2015; Chen et al., 2018). Cas12a can release fluorescent signals released by Cas12a captured by qPCR equipment, and the presence of nucleic acid fragments can be observed with the naked eye under ultraviolet/blue light (Ding et al., 2020; Wang et al., 2020). Currently, The RPA-CRISPR/Cas12a detection system has been used in the research of mycoplasma (Wang et al., 2019), COVID-19 (Ding et al., 2020), transgenic crops (Liu H. et al., 2021), rice diseases (Kang et al., 2021), etc., but there is no relevant report on the detection of vegetable soil borne diseases. Kuang et al. (2022) established a rapid detection method of RPA/CRISPR-Cas12a for specifically detecting black stem fungus by RPA reaction for 30 min under constant temperature 37°C and CRISPR-Cas12a reaction for 20 min. Lei et al. (2022) designed specific primers based on the Ypt1 gene of Phytophthora syringae to established rapid detection methods using fluorescence and lateral flow chromatography strips. In this method gene was amplified at 37°C for 40 min and could specifically detect P. syringae with a sensitivity of 133 fg, which is equivalent to fluorescence quantitative PCR. Wei et al. (2024) established a rapid detection system for F. pseudograminearum using RPA/CRISPR-Cas12a, which can detect the target pathogen within 40 min under constant temperature conditions of 37°C and sensitivity can reach 10−3 pg.·μL−1. The detection results can be intuitively read through the color reaction of nucleic acid test strips. This system has the advantages of specificity, sensitivity, and speed (Wei et al., 2024). It is also simple operation, fast and flexible to operation, high throughput and automated, and does not require complex temperature control equipment. The test strip detection does not require fluorescence equipment such as excitation light sources, making it very suitable for developing on-site rapid detection platforms. However, the cost of RPA/CRISPR-Cas12a reagents is higher than PCR. Hence in the future, the cost can be reduced through innovation and optimization conditions, to have a broader application.
2.8 Genomics based detection technology
With the advent of high-throughput sequencing technology, the detection of soil borne pathogens in vegetables has advanced greatly. High-throughput second-generation sequencing and single-molecule long-read third-generation sequencing improved the accuracy of bacterial detection. Also this technique identify a variety of specific microbial populations, such as unknown bacteria, viruses and viroids. This technique does not require the design of primers for specific sequences of microorganisms or plate culture of microorganisms (Zhang X. et al., 2023; Zhang Y. et al., 2023), which is important because only approximately 10% of bacteria are culturable (Pace, 1997). Next-generation sequencing (NGS, for which various platforms exist, such as Solexa, 454 Roche, Illumina and Ion Torrent), pyrosequencing and metagenomics have been widely used in research on soil borne vegetable diseases (Hopkins et al., 2013). At present, the use of sequencing technology to identify unknown pathogens costs $850 and takes approximately 2 weeks or longer (Olmos et al., 2013). Yuan et al. (2020) detected high levels of F. oxysporum, Gibberella, Bacillaceae, and Xanthomonadaceae in diseased soil and Streptomyces, Bradyrhizobiaceae, Comamonadaceae, and Mortierella in healthy soil using high-throughput sequencing technology. Previous reports have detected the oomycetes and fungi P. ultimum, P. irregulare, P. aphanidermatum, P. nicotianae, P. capsici, P. cinnamomi, R. solani, and F. oxysporum in soil via genomics technology (Jambhulkar et al., 2015; Huang C. H. et al., 2016). Genomic technology can be used to detect unknown soil bacteria, which is not suitable for common disease diagnosis. Metagenomics sequencing is the sequencing of all DNA in the environment. The cost of genome sequencing is relatively high, and the computational resources required for subsequent data analysis are also relatively high. With further research and over time, the cost of this technology will gradually decrease. In contrast, amplicon technology has become the main means of environmental microbiome research due to its low cost advantage. Amplicon sequencing mainly targets ribosomal RNA genes and functional genes. The former amplifies molecular markers such as bacteria, archaea, fungi, and ITS sequences, while the latter amplifies specific functional genes in microorganisms (such as those involved in carbon and nitrogen cycling).
3 Molecular detection techniques for physiological races of soil borne pathogens in vegetables
3.1 Host based identification technology
Each strain of pathogen normally infects one or a few host species, and the host-specific form called forma specialis which is further divided into physiological races depending on their cultivar specificity. The traditional biological identification techniques for specialized forms of pathogens (e.g., F. oxysporum, P. capsici, R. solanacearum, P. brassicae) mainly rely on the pathogenicity of the pathogen on different species, while the identification of physiological races mainly relies on the pathogenicity on different varieties of the same host, which is time-consuming and labor-intensive (Martyn and Netzer, 1991; Geng et al., 2010; Ji et al., 2007; Williams, 1966; Buczack et al., 1975; Jones et al., 1982). There are some differences between routine pathogen identification and physiological race identification in vegetables. The former only requires pathogenicity testing on plants of the same genus, while the latter requires pathogenicity testing on different cultivars of same hosts, which is time-consuming and labor-intensive, and easily affected by different environmental conditions.
3.2 Conventional PCR based detection technology
Molecular biology identification techniques have the advantages of speed, high sensitivity, and high accuracy and thus have been applied in the identification of most physiological races of pathogenic bacteria (Rui et al., 2022; Fu et al., 2020). The ITS has been proposed as the barcode for fungal species identification. The TEF and the DNA-directed RNA polymerase II with their subumits via., largest subunit (RPBI) and second largest subunit (RPB2) are phylogenetically informative loci in Fusarium allowing for species identification (O'Donnell et al., 2015). The TEF locus is also informative at the intraspecific level and can be combined with others, such as the ribosomal intergenic spacer, to reveal the complex genetic diversity within F. oxysporum (Lecomte et al., 2016; Ortu et al., 2018). Generally, using ITS, TEF, β-tubulin, Actin, and RPB genes to design specific primers can effectively distinguish between genera or species of pathogens but cannot distinguish physiological races (Chang et al., 2018). To compensate for the above shortcomings, four pairs of specific primers, uni, sp13, sp23, and sprl, were used to amplify the DNA of the tomato wilt pathogen, which can effectively identify Fol 1, Fol 2, Fol 3, and FORL of F. oxysporum (Hirano and Arie, 2006).
3.3 DNA based molecular markers based detection technology
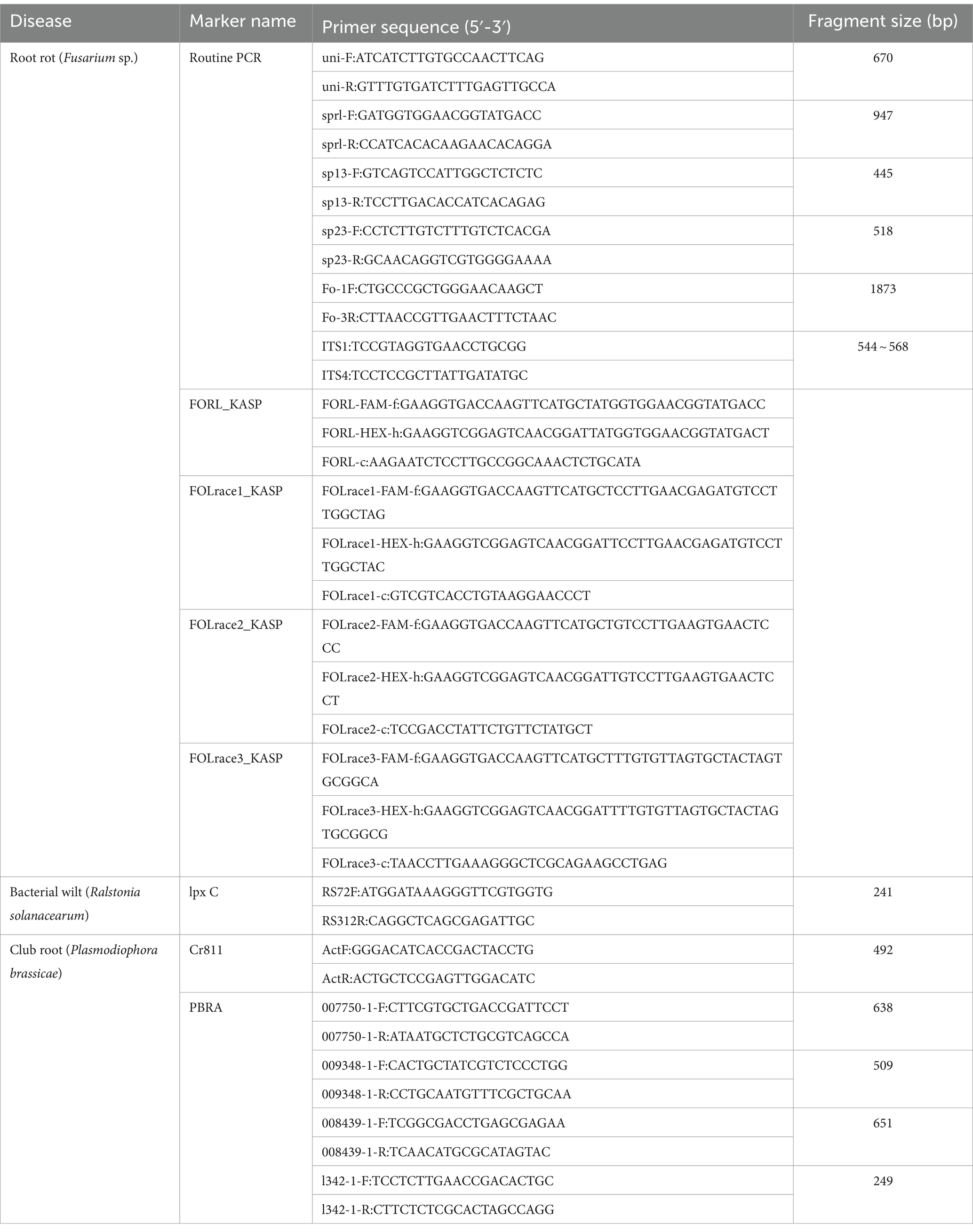
At present, molecular detection techniques based on specific primers have been successfully applied to the identification of many physiological races of pathogens (Lin et al., 2008; Cabanás et al., 2011; Aruga et al., 2012; Manzanares-dauleux et al., 2000) (Table 4). Cao et al. (2015a) screened a pair of primers, RS72F/RS312R, from a subtractive gene library of R. solanacearum and used qPCR technology to specifically amplify its physiological race 5. Zhang et al. (2015) utilized the nonhousekeeping genes reported by NCBI to screen and obtain Cr811, which can specifically identify race 5 of P. brassicae. In 2018, Zheng et al. (2018) screened molecular marker genes that could identify several physiological races of P. brassicae. The molecular markers PBRA_007750 and PBRA_009348 used for distinguishing P11 from P4, P7, and P9; PBRA_009348 and Novel342 used for distinguishing P9 from P4, P7, and P11; and PBRA_008439 and Novel342 could represent P4. Yi et al. (2020) screened two molecular marker genes, PBRA, based on previous transcriptome sequencing data_000030, and Novel00510, which can specifically identify the dominant physiological race 4 of P. brassicae. The polygalacturonase gene (pgx) is conserved, with high species specificity, has been widely used for the detection of physiological races of Fusarium (Lievens et al., 2009a). Ye et al. (2022) designed KASP-SNP primers based on the pgx4 gene locus of F. oxysporum and for the first time developed KASP-SNP molecular markers for physiological races of F. oxysporum. The KASP-SNP technique used to detect Fol 1, Fol 2, Fol 3, and FORL rapidly and accurately. In the early stages of vegetable disease, the following specific primers can be used to identify the pathogen DNA, thus an early disease prevention and control plan can be formulated. The reading of KASP-SNP test data is completely automated, while the routine PCR test needs to be analyzed by agarose gel electrophoresis. The reading of test results are subjective, and errors are prone to occur in the judgment based on the clarity of the target strip. However, the above mentioned molecular marker techniques have drawbacks, such as requiring a large amount of DNA, poor repeatability, and complex operation.
3.4 Transposon based detection technology
Transposons are ubiquitous in all organisms and pathogens (Lievens et al., 2008). Based on the genomic flanking regions, Pasquali et al. (2004) constructed a molecular identification technique for F. oxysporum physiological races using a special insertion fragment (Mg5/Mg6) of the Fot 1 transposon. Pasquali et al. (2007) indicated that inter-retrotranspos on sequence-characterized amplified regions (IR-SCAR) was used to develop a specific set of PCR primers (Ha3F/Ha3R) utilized for differentiating F. oxysporum race 1 from other F. oxysporum isolates. López-Berges et al. (2009) demonstrated that the artificially modified Impala transposon has a decisive effect on the toxicity of F. oxysporum on tomato by inserting it. Research has shown that the Fot 1 transposon can serve as a target sequence resource library for identifying specific physiological races (1, 2, 8) of F. oxysporum, while the Impala transposon can be used for identifying physiological race 4. Currently, the majority of their genomes have not been annotated for soil borne pathogens, and there is relatively little research on using transposon technology to identify physiological races (Table 5). The problem encountered when using transposons for identification is that they can move back and forth across the genome after complete cleavage, which poses difficulties in finding stable molecular markers. The number of reads at the transposon insertion site can intuitively reflect the necessity of a gene. Therefore, read counts are an important parameter in Tn seq analysis. When read counts equals 0, it indicates that the gene is an essential gene for bacterial growth. The larger the read counts value, the smaller the impact of the gene on bacterial growth. For example, the transposon insertion density of gene A is relatively high, and most insertion sites have high read counts, while under condition B, the results are opposite, indicating that this type of gene is called a conditionally necessary gene (McDonald,1993). Designing primers based on essential genetic differences can effectively identify different types of pathogens.
3.5 Pathogenicity-related genes based detection technology
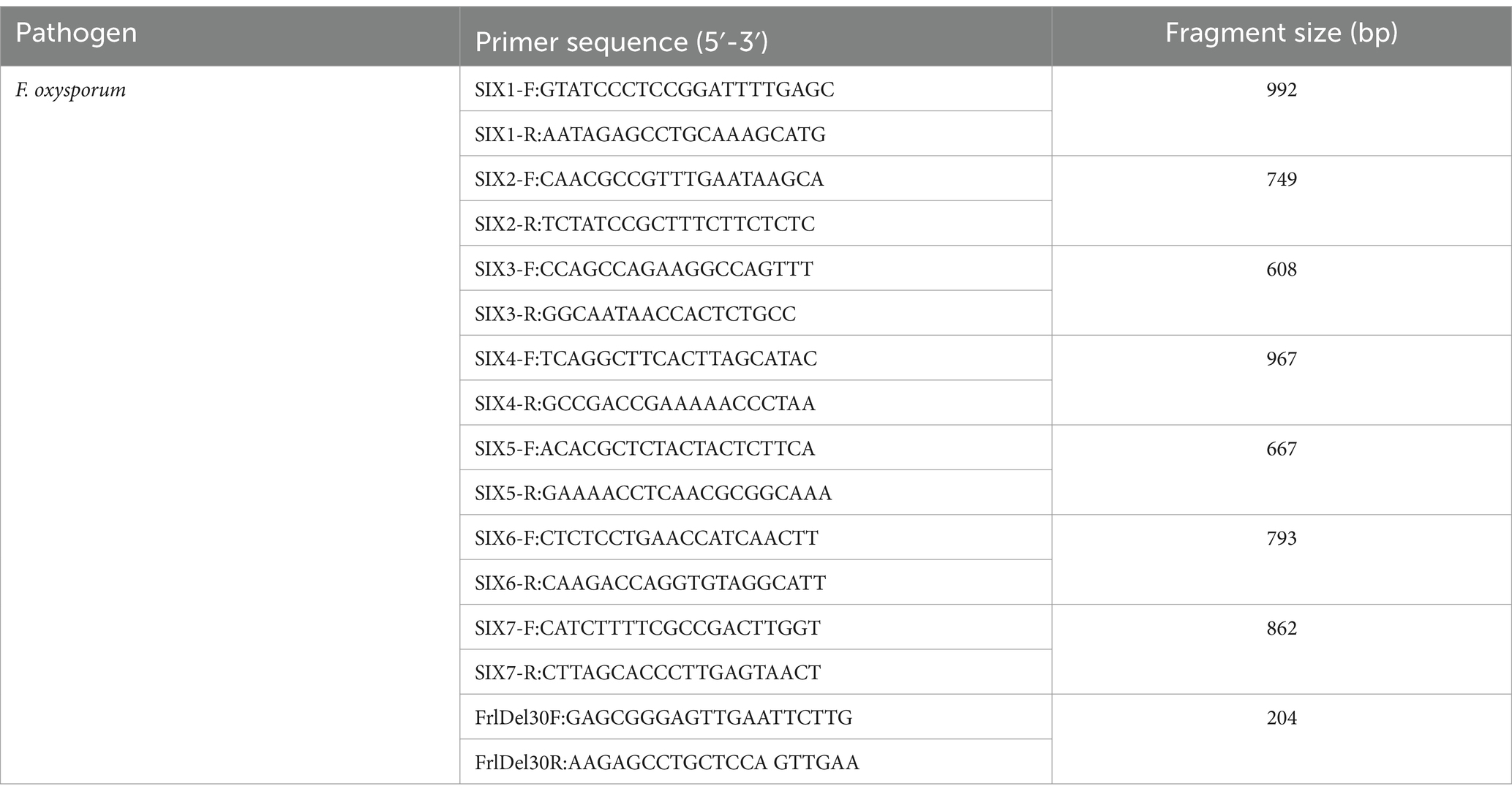
Usually, the ability of fungi to infect specific vegetables depends on the unique genes encoding the host, which play important roles in the process of fungal infection. The differential factors that determine the toxicity of pathogenic bacteria include small mutations in a specific gene in the genome that controls the production of toxins by pathogenic bacteria. One of the first such tools developed for the forma special is lycopersici, based on a host-specific virulence gene. The gene encodes a small protein secreted in the xylem (SIX) that confers virulence to the fungus. Fourteen SIX genes are currently known, and a few homologs have been found in other forma speciales, such as cepae, cubense, and conglutinans (Li et al., 2016; Taylor et al., 2016) (Table 6). PCR primers were designed from SIX sequences to discriminate forma speciales cubense and lycopersici from other forma speciales (Fraser-Smith et al., 2014). Molecular markers based on other virulence factors were also designed for forma specialis phaseoli and for race 4 of forma specialis cubense (Aguayo et al., 2017). Lievens et al. (2009b) designed 7 pairs of tomato wilt pathogen-specific primers (SIX1 ~ SIX7) based on nontoxic genes, and all of them could amplify specific fragments in Fol 1 ~ 3 (excluding SIX4 primers). In FORL, none of the 7 pairs of primers were amplified. Zhang X. et al. (2023) and Zhang Y. et al. (2023) designed a specific primer FrlDel30 F/R based on the mutation site in the first chromosome of the nontoxic genes (SIX1 ~ SIX7), which can effectively distinguish between root rot pathogens and it physiological races. Poueymiro et al. (2009) induced dual mutations in the non-toxic genes avrA and popP1, resulting different in pathogenic abilities of the bacterial strain (race 1), which is beneficial for distinguishing physiological races. Among them, popP1 and popP2 are located on the pathogenic island of 79 kb, while popP3 is located on the pathogenic island of 83 kb (Xu et al., 2011). Among the analyzed proteomes of different Phytophthora species, Shands et al. (2024) found several orthogonal groups with the highest number of shared proteins (OG7457), and the conserved positive group only existed in the Pc2113 and Pc2109 isolates. Interestingly, a larger proportion of differentially expressed (DE) effectors were found in these orthogonal groups after pathogen infection, indicating that some of these effectors may play a conservative role in the pathogenicity of Phytophthora. The routine pathogen identification designs specific primers based on the differential loci between different genera of the pathogen, which is easy to operate and has obvious differential sequences, while the physiological race identification requires comparing the differential loci of virulence genes of the pathogen to design primers. Although knowledge on these genetic determinants was scarce until recently, it has considerably improved in the last decade.
3.6 Genomics based detection technology
Recently, genomics technology has provided sequence information on dominant pathogenicity for the identification and analysis of physiological races of vegetable soil borne pathogens. A comparison of the entire genome suggests that the effect pool of each template may determine host specificity. However, comparative genomics is the next step to identify host specificity in F oxysporum (Van Dam et al., 2016). We performed whole genome sequencing of 45\u00B0F. oxysporum strains and managed to differentiate forma speciales cucumerinum, niveum, melonis, radicis-cucumerinum, and lycopersici on the basis of their effector pattern. Two years later, Van Dam et al. (2018) designed PCR primers to discriminate the seven forma speciales that affect Cucurbitaceae based on candidate effectors extracted from 82 genome assemblies. Zhang et al. (2014) used comparative genomics technique to design specific primers based on genome sequencing data of physiological races 1 and 2 of Brassica oleracea. They also confirmed the indeed differences in genome sequences between the two physiological races of B. oleracea. There is no doubt that expanding access to whole-genome sequences will continuously improve F. oxysporum host range identification. Therefore, for developing a rapid identification method for physiological races of pathogens is an ideal approach from the the perspective of searching for unique genes or fragments of different physiological races (Lievens et al., 2008). Meanwhile, the classification of physiological races of pathogens at the molecular level will be a major trend in future development.
4 Application of molecular detection techniques to detect pathogen load in soil
4.1 Pathogens load in soil
Soil is the main site for the growth and propagation of pathogenic or nonpathogenic microorganisms, resulting in continuous outbreaks of vegetable diseases (Yuan et al., 2020). Therefore, it is very important to identify and measure the levels of pathogens in soil. The occurrence and severity of diseases are positively related to the population of pathogens in soil; therefore, accurate measurement of the pathogen population in soil is the premise for disease forecasting and effective control. Huang C. H. et al. (2016) reported the design of a TaqMan probe and PCR primers for the DNA sequence of the species-specific virulence gene SIX1. Further results showed there was a significant positive correlation between the severity of soil borne disease and the concentration of F. oxysporum DNA in soil. Almquist et al. (2016) used RT–qPCR to quantitatively detect Aphanomyces cochlioides DNA in field soil samples and found that the bacterial content in clay was lower than that in sandy soil. Lastra et al. (2018) used RT–qPCR to determine that the DNA content of F. solani in diseased strawberry soil was between 16 and 190 pg.·mg−1, which became an effective tool for early warning and prevention of soil disease before plant transplantation. Zhong et al. (2022) developed an RT–qPCR assay for F. oxysporum, revealing that the total DNA of pathogens in soil after CaCN2 (240 and 300 mg·cm3) treatment decreased from 11.26 and 10.55 pg.·ng−1 to 4.21 and 4.01 pg./ng, respectively. Chen L. et al. (2022) determined that the Fusarium content of 8 out of 18 soil samples with Fusarium was 104–106 spores/g via PMA–qPCR, which provided a basis for the prediction of natural soil borne diseases. Chen L. D. et al. (2022) reported that fumigation with calcium cyanamide could lead to a relative reduction in the populations of soil pathogens, such as Acremonium, Alternaria, Fusarium, Penicillium, and Verticillium, using heterotrophic plate counts, PCR and MiSeq high-throughput sequencing. C. cassiicola is a potential pathogen in soil. The C. cassiicola content in soil after CaCN2 and plastic film treatment decreased from 107 to 103 spores·g−1 via PMA-qPCR detection method (Xie X. et al., 2022). Gu et al. (2022) revealed that tomato bacterial wilt was induced by isolation of the tomato rhizosphere microbial community. Disease diagnosis could be performed two weeks earlier based on the abundance of pathogenic bacteria causing tomato wilt in the rhizosphere microbial group using high-throughput sequencing technology.
4.2 Pathogens load in plants
Soil borne pathogen-infected vegetables showed root rot, browning, withering and root damage. The detection of pathogens in plants can be used in molecular biology research, such as for disease prediction, pathogen control and determination of pathogen interaction mechanisms. Gao et al. (2019) detected V. dahliae species and different metabolic substances in soil using macrogenomics technology, revealing the pathogenic mechanism underlying plant diseases. Meng et al. (2016) used RT–qPCR technology to detect P. amygdali pv. lachrymans in cucumber leaves, which allowed rapid and easy early assessment of angular leaf spot disease. Kuang et al. (2017) designed LAMP primers for B. gladioli pv. alliicola based on the ITS gene, which became an effective technique for detecting the pathogen in onion plants. Zhu et al. (2016) reported the development of an RT–qPCR assay based on the mitochondrial small subunit rDNA of F. commune, which will facilitate monitoring of the pathogen and improvised disease management. Kim et al. (2017) designed specific primers for F. oxysporum sp. raphani (FOR2-F/FOR2-R) and confirmed that the markers For610 and For425 could distinguish pathogenic F. oxysporum isolates. Klosterman et al. (2009) quantitatively detected the change in DNA content of Verticillium wilt V. dahliae by RNAseq technology and confirmed that V. dahliae infection was caused by wounds or cracks in the lateral roots of plants. Zhou et al. (2022) revealed that cross-kingdom (fungi and bacteria) synthetic communities (SynComs) were more effective in suppressing soil borne Fusarium wilt disease (FWD) than fungal or bacterial SynComs alone by plate isolation and culturing, RT–qPCR and high-throughput sequencing. Ten putative effectors were identified within FOC, including 7 SIX genes first reported in F. oxysporum f. sp. lycopersici, which can identify the types of pathogens in onion (Taylor et al., 2016). A specific combination of hydrolysis probes/primers has been developed using virulence genes, which can distinguish Foc race 4 (Aguayo et al., 2017). This new detection method can be used for plant regulatory detection applications.
4.3 Pathogens load in seeds
At present, there is a high demand for commercial vegetable seeds, leading to a higher probability of seeds carrying pathogens or long-distance transmission. Unlike the diagnosis of plant diseases, it is difficult to determine whether seeds carry pathogens because in most cases, infected seeds have no obvious symptoms of disease. In addition, the proportion of diseased seeds is small, and the distribution is uneven. All quarantine seeds do not conform to the actual situation. PCR technology is usually used for qualitative testing of most vegetable seed pathogens because it can detect even low DNA content of pathogens in diseased seeds and being discarded by the farm. Regarding, the occurrence of disease outbreaks and epidemics depends mainly on the number of seed carriers, suitability of the environment and host plant type (Lievens et al., 2008). Therefore, a sensitive, accurate and quantitative detection technology is needed to determine whether vegetable seeds carry pathogens to control the infection and spread of diseases from the root (Glynn and Edwards, 2010). The detection of vegetable seed-borne disease carriers requires a real-time detection technique with high sensitivity. The nested PCR-based technique could detect 32 conidia in 100 seeds within 4 h, which is suitable for commercial seed quarantine technology (Chiocchetti et al., 2001). Konstantinova et al. (2002) designed primers and confirmed that pathogens in carrot seeds contained pathogens such as A. radicina, A. dauci, and A. alternata using a PCR detection technique, which provided a favorable basis for the control of seed-borne vegetable diseases. Recently, some researchers have confirmed the existence of A. brassicae in cabbage and radish seeds by PCR and RT–qPCR techniques, where the pathogen DNA content was relatively high (Guillemette et al., 2004). The quantitative detection of V. dahliae in spinach seeds based on RT–qPCR allows the evaluation of seed infection rate up to 1.3%, providing a good technology for seed carrier quarantine and improved seed screening (Duressa et al., 2011). Sensitivity is very important in the detection of vegetable seed borne diseases. A new detection technique developed by Webb et al. (2014) has a sensitivity of 10 fg of pathogen DNA. Sousa et al. (2015) used RT–qPCR to detect and quantify the content of F. oxysporum f. sp. phaseoli in bean seeds, adding value to research on the spread of seed-borne pathogens. Tomato canker is a widespread and serious disease in vegetable production, especially due to the long-distance transmission of seed carriers that infect healthy plants. Wang et al. (2014) detected CMM in tomato seeds based on the RT–qPCR technique with high specificity and sensitivity. Ahmed et al. (2017) successfully isolated Cladosporium spp., F. semitectum, F. oxysporum, Rhizoctonia spp., and Alternaria from vegetable seeds by using standard blotter paper and the agar plate technique. This will be helpful for seed treatment with appropriate fungicides before sowing to overcome the loss caused by seed-borne fungi. A specific set of PCR primers was developed using IR-SCAR, which could uniquely amplify F. oxysporum race 1 from lettuce seeds in Italy, Portugal, the United States, Japan, and Taiwan (Sousa et al., 2015).
5 Conclusions and perspectives
Herein, we reviewed the recent progress of various molecular detection techniques for vegetable soil borne pathogens (PCR, nested-PCR, multiplex PCR, etc.) and their physiological races (host identification, DNA molecular markers, transposon detection, etc.), explaining the advantages and disadvantages of each detection technique. Furthermore, the paper comprehensively introduces the application of molecular detection technology in soil borne pathogen detection of soil, plants, and seeds. This paper will provide a value reference for future detection technique development for disease prevention and management of vegetable soil borne pathogen.
However, the applicability of soil borne pathogen and their physiological races detection is determined by technique sensitivity, planting variety, actual local conditions, etc. If possible, comparative genomics technology will be used in the further to analyze the entire genome data of various physiological races of soil borne pathogens, identify specific fragments and design specific primers to identify target strains, which will overcome the difficulty of identifying different physiological races of soil borne pathogens. Moreover, nowadays most of vegetables usually only have resistance to a certain physiological race of the pathogen, and this resistance may be overcome by more virulent physiological races, leading to more severe disease symptoms in the host crop. In the future more hosts will be used for pathogenicity assessment to screen vegetable insensitive or less-sensitive varieties to local dominant physiological race pathogens, thereby reducing economic losses and also provide important materials for disease resistance breeding (Pang et al., 2020; Schwelm and Ludwig-Muller, 2021). Furthermore, the objects polymorphism and adaptability of complex detection environments still need to be improved. It is necessary to focus on the ability of various detection methods to adapt to multi-objective and complex environments, improve the accuracy and efficiency. For researchers, the CRISPR/Cas12a method has its own advantages and good detection efficiency in detecting soil borne pathogens. For farmers, microscopic detection methods are more common and cost-effective. Therefore, precise detection of pathogens is not a single method. In practical applications, multiple methods need to be combined to improve the accuracy and reliability of detection. For example, preliminary detection can be conducted through a microscope, followed by validation and confirmation using the CRISPR/Cas12a method. The rapid progress of molecular detection technique for vegetables soil borne diseases will continuously promote the improvement of vegetable yield and quality, providing new vitality for green and sustainable development of the vegetable industry.
Author contributions
LC: Writing – original draft, Writing – review & editing. GL: Writing – original draft, Writing – review & editing. SY: Conceptualization, Data curation, Supervision, Writing – review & editing. BG: Conceptualization, Data curation, Project administration, Supervision, Writing – review & editing. YL: Conceptualization, Supervision, Writing – review & editing. XW: Data curation, Investigation, Software, Supervision, Writing – review & editing. JL: Data curation, Formal analysis, Investigation, Supervision, Visualization, Writing – review & editing. HG: Conceptualization, Funding acquisition, Resources, Software, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hebei Facility Vegetables Innovation Team of Modern Agro-industry Technology (No. HBCT2023100211) and Hebei Provincial Natural Science Foundation of China (C2024204182).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abou-Jawdah, Y., Aknadibossian, V., Jawhari, M., Tawidian, P., and Abrahamian, P. (2019). Real-time PCR protocol for phytoplasma detection and quantifcation. Methods Mol. Biol. 1875, 117–130. doi: 10.1007/978-1-4939-8837-2_9
Achari, S. R., Mann, R. C., Sharma, M., and Edwards, J. (2023). Diagnosis of Fusarium oxysporum f. sp. ciceris causing Fusarium wilt of chickpea using loop-mediated isothermal amplification (LAMP) and conventional end-point PCR. Sci. Rep. 13:2640. doi: 10.1038/s41598-023-29730-6
Aguayo, J., Mostert, D., Fourrier-Jeandeal, C., Cerf-Wendling, I., Hostachy, B., Viljoen, A., et al. (2017). Development of hydrolysis probe-based real-time assay for the detection of tropical strains of Fusarium oxysporum f.sp.cubense race 4. PLoS One 12:e0171767. doi: 10.1371/journal.pone.0171767
Ahmed, B., Khan, B., Ghazanfar, M. U., Rajput, N. A., and Walait, M. (2017). Occurrence and distribution of vegetables seed-borne mycoflora in Punjab Pakistan. Pak. J. Phytopathol. 29, 265–271. doi: 10.33866/phytopathol.029.02.0405
Almasi, M. A. (2019). Development of a colorimetric loop-mediated isothermal amplification assay for the visual detection of fusarium oxysporum f.sp. melonis. Horticult. Plant J. 5, 41–48. doi: 10.1016/j.hpj.2019.01.004
Almquist, C., Persson, L., Olsson, A., Sundstro, M. J., and Jonsson, A. (2016). Disease risk assessment of sugar beet root rot using quantitative real-time PCR analysis of aphanomyces cochlioides in naturally infested soil samples. Eur. J. Plant Pathol. 145, 731–742. doi: 10.1007/s10658-016-0862-5
Aruga, D., Tsuchiya, N., Matsumura, H., Matsumoto, E., and Hayashida, N. (2012). Analysis of RAPD and AFLP markers linked to resistance to Fusarium oxysporum f.sp. lactucae race 2 in lettuce (Lactuca sativa L.). Euphytica 187, 1–9. doi: 10.1007/s10681-012-0665-5
Asano, T., Senda, M., Suga, H., and Kageyama, K. (2010). Development of multiplex PCR to detect five Pythium species related to Turfgrass diseases. J. Phytopathol. 158, 609–615. doi: 10.1111/j.1439-0434.2009.01660.x
Buczack, S. T., Toxopeus, H., Mattusch, P., Johnston, T. D., and Hobolth, L. A. (1975). Study of physiologic specialization in Plasmodiophora brassicae: proposals for attempted rationalization through an international approach. Trans. Br. Mycol. Soc. 65, 295–303. doi: 10.1016/S0007-1536(75)80013-1
Cabanás, C. G. L., Valverde Corredor, A., and Pérez Artés, E. (2011). Molecular analysis of Spanish populations of Fusarium oxysporum f. sp. dianthi demonstrates a high genetic diversity and identifies virulence groups in races 1 and 2 of the pathogen. Eur. J. Plant Pathol. 132, 561–576. doi: 10.1007/s10658-011-9901-4
Cao, M. Q., Bao, Q., Yang, C. F., Wang, J., Sheng, S., and Wu, F. A. (2015a). Establishment of a qPCR method for rapid detect ion of ralstonia solanacearum race 5. Sci. Sericult. 41, 807–814. doi: 10.13441/j.cnki.cykx.2015.05.005
Cao, M. Q., Wang, X. D., Wang, J., Sheng, S., and Wu, F. (2015b). Detection of living cells of Ralstonia solanacearum race 5 by PMA-qPCR method. Sci. Sericult. 41, 1004–1010. doi: 10.13441/j.cnki.cykx.2015.06.005
Chai, A. L., Ben, H. Y., Guo, W. T., Shi, Y. X., Xie, X. W., Li, L., et al. (2020). Quantification of viable cells of Pseudomonas syringae pv. Tomato in tomato seed using propidium monoazide and a real-time PCR assay. Plant Dis. 104, 2225–2232. doi: 10.1094/PDIS-11-19-2397-RE
Chang, Y. D., Du, B., Wang, L., Ji, P., Xie, Y. J., Li, X. F., et al. (2018). A study on the pathogen species and physiological races of tomato Fusarium wilt in Shanxi, China. J. Integr. Agricult. 17, 1380–1390. doi: 10.1016/S2095-3119(18)61983-5
Chen, Z., Halford, N. G., and Liu, C. (2023). Real-time quantitative PCR: primer design, reference gene selection, calculations and statistics. Meta 13:806. doi: 10.3390/METABO13070806
Chen, L., Li, L., Xie, X., Chai, A., Shi, Y., Fan, T., et al. (2022). An improved method for quantification of viable Fusarium cells in infected soil products by Propidium Monoazide coupled with real-time PCR. Microorganisms 10:1037. doi: 10.3390/MICROORGANISMS10051037
Chen, J., Ma, E., Lucas, B., Da, C., Tian, X., Palefsky, J., et al. (2018). CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 360, 436–439. doi: 10.1126/science.aar6245
Chen, L. D., Xie, X. W., Kang, H. J., Liu, R. C., Shi, Y. X., Li, L., et al. (2022). Efficiency of calcium cyanamide on the control of tomato soil borne disease and their impacts on the soil microbial community. Appl. Soil Ecol. 176:104522. doi: 10.1016/J.APSOIL.2022.104522
Chen, L. D., Yuan, J. H., Li, L., Shi, Y. X., Chai, A. L., Xie, X. W., et al. (2019). Development and application of quantitative PCR for detection of Fusarium. Acta Agricult. Boreali-Sinica 34, 296–301. doi: 10.7668/hbnxb.20190656 (in Chinese)
Cheng, Y. C., Kang, H. J., Shi, Y. X., Chai, A. L., Zhang, H., Xie, X. W., et al. (2018). Development and application of real-time fluorescent quantitative PCR for detection of Phytophthora capsici. Acta Horticult. Sin. 45, 997–1006. doi: 10.16420/j.issn.0513-353x.2017-0862
Chiocchetti, A., Sciaudone, I., Durando, F., Garibaldi, A., and Migheli, Q. (2001). PCR detection of Fusarium oxysporum f. sp. basilici on basil. Plant Dis. 85, 607–611. doi: 10.1094/PDIS.2001.85.6.607
Cho, M. S., Lee, J. H., Her, N. H., Kim, C. K., Seol, Y. J., Hahn, J. H., et al. (2012). A quantitative and direct PCR assay for the subspecies-specific detection of Clavibacter michiganensis subsp. Michiganensis based on a ferredoxin reductase gene. J. Microbiol. 50, 496–501. doi: 10.1007/s12275-012-1611-x
Cordova, I., Oropeza, C., Puch-Hau, C., Harrison, N., Colli-Rodríguez, A., and Narvaez, M. A. (2014). Real-time PCR assay for detection of coconut lethal yellowing phytoplasmas of group 16SrIV subgroups a, D and E found in the Americas. J. Plant Pathol. 96, 343–352. doi: 10.4454/JPP.V96I2.031
Crespo-Sempere, A., Estiarte, N., Marín, S., Sanchis, V., and Ramos, A. (2013). Propidium monoazide combined with real-time quantitative PCR to quantify viable Alternaria spp. contamination in tomato products. Int. J. Food Microbiol. 165, 214–220. doi: 10.1016/j.ijfoodmicro.2013.05.017
Ding, X., Yin, K., Li, Z., Lalla, R. V., Ballesteros, E., Sfeir, M. M., et al. (2020). Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 11:4711. doi: 10.1038/s41467-020-18575-6
Duarte, V., De Boer, S. H., Ward, L. J., and de Oliveira, A. M. R. (2004). Characterization of atypical Erwinia carotovora strains causing blackleg of potato in Brazil. J. Appl. Microbiol. 96, 535–545. doi: 10.1111/j.1365-2672.2004.02173.x
Duressa, D., Rauscher, G., Koike, S. T., Mou, B., Hayes, R. J., Marutchachalam, K., et al. (2011). A real-time PCR assay for detection and quantification of Verticillium dahliae in spinach seed. Phytopathology 102, 443–451. doi: 10.1094/PHYTO-10-11-0280
FAO (2023). Food and agricultural commodities production for 2023: Production of vegetable by countries [database.]. Rome: FAO Available at: http://faostat3.fao.org/browse/Q/QC/E.
Foteini, R., Jorge, B., Alejandro, G., and Marta, P. (2023). Real-time PCR, and recombinase polymerase amplification combined with SYBR green I for naked-eye detection, along with Propidium Monoazide (PMA) for the detection of viable patulin-producing fungi in apples and by-products. Food Control 144:109347. doi: 10.1016/J.FOODCONT.2022.109347
Fraser-Smith, S., Czislowski, E., Meldrum, R. A., Zander, M., O'Neill, W., Balali, G. R., et al. (2014). Sequence variation in the putative effector gene SIX8 facilitates molecular differentiation of Fusarium oxysporum f.sp.cubense. Plant Pathol. 63, 1044–1052. doi: 10.1111/ppa.12184
Fu, H. T., Yang, Y. L., Mishra, V., Zhou, Q. X., Zuzak, K., Feindel, D., et al. (2020). Most Plasmodiophora brassicae populations in single canola root galls from Alberta fields are mixtures of multiple strains. Plant Dis. 104, 116–120. doi: 10.1094/PDIS-06-19-1235-RE
Gao, Y. Y., Wang, N., Gao, G. P., and Wang, W. (2010). Triplex PCR detection of Cladosporium cucumerinum, Fusarium oxysporum fsp. Niveum and Mycosphaerella melonis in infected plant tissues. Acta Phytopathol. Sin. 40, 343–350. doi: 10.13926/i.cnki.apps.2010.04016 (in Chinese)
Gao, S. G., Zeng, R., Xu, L. H., Luo, J. Y., Chen, L., and Dai, F. M. (2016). Triplex PCR detection for Corynespora cassiicola, Colletotrichum orbiculare and Pseudomonas syringae pv. Lachrymans. Sci. Agric. Sin. 49, 3119–3129. doi: 10.3864/j.issn.0578-1752.2016.16.006
Gao, F., Zhang, B. S., Zhao, J. H., Huang, J. F., Jia, P. S., Wang, S., et al. (2019). Deacetylation of chitin oligomers increases virulence in soil borne fungal pathogens. Nat. Plants 5, 1167–1176. doi: 10.1038/s41477-019-0527-4
Geng, L. H., Guo, S. G., Lv, G. Y., Zhang, H. Y., Gong, G. Y., and Xu, Y. (2010). Establishment and identification system for physiological races of Fusarium oxyporum f. sp. niveum. China Vegetables 1, 52–56. doi: 10.19928/j.cnki.1000-6346.2010.20.010 (in Chinese)
Gharbi, Y., Triki, M. A., Trabelsi, R., Fendri, I., Daayf, F., and Gdoura, R. (2015). Genetic structure of Verticillium dahliae isolates infecting olive trees in Tunisia using AFLP, pathogenicity and PCR markers. Plant Pathol. 64, 871–879. doi: 10.1111/ppa.12323
Gieroń, M., Żarnowiec, P., Zegadło, K., Gmiter, D., Czerwonka, G., Kaca, W., et al. (2023). Loop-mediated isothermal amplification of DNA (LAMP) as an alternative method for determining Bacteria in wound infections. Int. J. Mol. Sci. 25:411. doi: 10.3390/IJMS25010411
Glynn, N. C., and Edwards, S. G. (2010). Evaluation of PCR assays for quantifying seed-borne infection by Fusarium and Microdochium seedling blight pathogens. J. Appl. Microbiol. 108, 81–87. doi: 10.1111/j.1365-2672.2009.04410.x
Grote, D., Olmos, A., Kofoet, A., Tuset, J. J., Bertolini, E., and Cambra, M. (2002). Specific and sensitive detection of Phytophthora nicotianae by simple and nested-PCR. Eur. J. Plant Pathol. 108, 197–207. doi: 10.1023/A:1015139410793
Gu, Y., Samiran, B., Francisco, D. A., Xu, Y. C., Shen, Q. R., Alexandre, J., et al. (2022). Small changes in rhizosphere microbiome composition predict disease outcomes earlier than pathogen density variations. ISME 16, 2448–2456. doi: 10.1038/s41396-022-01290-z
Guillemette, T., Iacomi-Vasilescu, B., and Simoneau, P. (2004). Conventional and real-time PCR-based assay for detecting pathogenic Alternaria brassicae in cruciferous seed. Plant Dis. 88, 490–496. doi: 10.1094/PDIS.2004.88.5.490
Hirano, Y., and Arie, T. (2006). PCR-based differentiation of Fusarium oxysporum f. sp. lycopersici and radicis-lycopersici and races of F. Oxysporum f. sp. lycopersici. J. Gen. Plant Pathol. 72, 273–283. doi: 10.1007/s10327-006-0287-7
Hopkins, A. J. M., Castanyo, C., Boberg, J. B., and Stenlid, J. (2013). Methods for the early detection of new invasive forest pathogens. Acta Phytopathol. Sin. 43:85.
Htun, M. Z., Rotchanapreeda, T., Rujirawat, T., Lohnoo, T., Yingyong, W., Kumsang, Y., et al. (2020). Loop-mediated isothermal amplification (LAMP) for identification of Pythium insidiosum. Int. J. Infect. Dis. 101, 149–159. doi: 10.1016/j.ijid.2020.09.1430
Huang, C. H., Tsai, R. T. E., and Vallad, G. (2016). Development of a TaqMan real-time polymerase chain reaction assay for detection and quantification of Fusarium oxysporum f. sp. lycopersici in soil. J. Phytopathol. 164, 455–463. doi: 10.1111/jph.12471
Huang, W., Xu, J., Zhang, H., Xu, J. S., Ding, W., and Feng, J. (2016). Development of a LAMP approach for detection of Ralstonia solanacearum. Sci. Agric. Sin. 49, 2093–2102. doi: 10.3864/j.issn.0578-1752.2016.11.006
Israa, A. H., Hayder, H. I., and Aleem, M. K. (2023). A review of the development in the multiplex PCR technique for the detection of Bacillus cereus. ESS Open Arch. 29:2023. doi: 10.22541/au.168006062.20963062/v1
Jambhulkar, P. P., Sharma, M., Lakshman, D., and Sharma, P. (2015). “Natural mechanisms of soil suppressiveness against diseases caused by Fusarium, Rhizoctonia, Pythium, and Phytophthora” in Organic amendments and soil suppressiveness in plant disease management. eds. M. K. Meghvansi and A. Varma (New York: Springer), 95–124.
Jesús, M. B., Dolores, R. J., Encarnación, P.-A., and Rafael, M. J. D. (2002). Detection of the defoliating Pathotype of Verticillium dahliae in infected olive plants by nested PCR. Eur. J. Plant Pathol. 50, 609–619. doi: 10.1046/j.1365-3059.2001.00601.x
Ji, P. S., Allen, C., Sanchez-Perez, A., Yao, J., Elphinstone, J. G., and Jones, J. B. (2007). New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Dis. 91, 195–203. doi: 10.1094/PDIS-91-2-0195
Jones, D. R., Ingram, D. S., and Dixon, G. R. (1982). Factors affecting tests for differential pathogenicity in populations of Plasmodiophora brassicae. Plant Pathol. 31, 229–238. doi: 10.1111/j.1365-3059.1982.tb01273.x
Kang, H. J., Chai, A. L., Shi, Y. X., Xie, X. W., Yuan, J. H., and Li, B. J. (2018). Quadruple PCR detection of Pseudomonas syringae pv. Tomato, Clavibacter michiganensis subsp. michiganensis, Ralstonia solanacearum and Xanthomonas campestris pv. Vesicatoria in infected tomato tissues. Acta Horticult. Sin. 45, 2255–2264. doi: 10.16420/j.issn.0513-353x.2018-0153
Kang, H. X., Peng, Y., Hua, K. Y., Deng, Y. F., Bellizzi, M., Gupta, D. R., et al. (2021). Rapid detection of wheat blast pathogen Magnaporthe oryzae triticum pathotype using genome-specific primers and Cas12a-mediated technology. Engineering 7, 1326–1335. doi: 10.1016/j.eng.2020.07.016
Khan, M., Li, B., Jiang, Y., Weng, Q., and Chen, Q. (2017). Evaluation of different PCR-based assays and LAMP method for rapid detection of Phytophthora infestans by targeting the Ypt1 gene. Front. Microbiol. 8:1920. doi: 10.3389/fmicb.2017.01920
Khat, M. N., Nisachon, J., and Lapatrada, T. (2024). Evaluation of molecular inhibitors of loop-mediated isothermal amplification (LAMP). Sci. Rep. 14:5916. doi: 10.1038/S41598-024-55241-Z
Kim, H., Hwang, S. M., Lee, J. H., Oh, M., Han, J. W., and Choi, G. J. (2017). Specific PCR detection of Fusarium oxysporum f. sp. raphani: a causal agent of Fusarium wilt on radish plants. Lett. Appl. Microbiol. 65, 133–140. doi: 10.1111/lam.12761
Kim, D., Jeong, R. D., Choi, S., Ju, H. J., and Yoon, J. Y. (2023). Application of rapid and reliable detection of Cymbidium mosaic virus by reverse transcription recombinase polymerase amplification combined with lateral flow immunoassay. Plant Pathol. J. 39:158. doi: 10.5423/PPJ.ER.10.2022.0147
Klemsdal, S. S., and Elen, O. (2006). Development of a highly sensitive nested-PCR method using a single closed tube for detection of Fusarium culmorum in cereal samples. Lett. Appl. Microbiol. 42, 544–548. doi: 10.1111/j.1472-765X.2006.01880.x
Klosterman, S. J., Atallah, Z. K., Vallad, G. E., and Subbarao, K. V. (2009). Diversity, pathogenicity, and management of verticillium species. Annu. Rev. Phytopathol. 47, 39–62. doi: 10.1146/annurev-phyto-080508-081748
Koentjoro, M. P., Hidayat, T., Maat, S., Fasya, A. H., and Prasetyo, E. N. (2023). Development of nested-PCR assay for the rapid detection of Aspergillus fumigatus. In AIP conference. 2634, AIP publishing.
Konstantinova, P., Bonants, P. J. M., Gent-Pelzer, M. P. E. V., Zouwen, P. V. D., and Bulk, R. V. D. (2002). Development of specific primers for detection and identification of Alternaria spp. in carrot material by PCR and comparison with blotter and plating assays. Mycol. Res. 106, 23–33. doi: 10.1017/S0953756201005160
Kuang, R. R., Lei, R., Jiang, L., Duan, W. J., Li, X. L., Fu, N., et al. (2022). Establishment of RPA/CRISPR-Cas12a rapid detection for Leptosphaeria lindquistii. Plant Prot. 48, 69–76. doi: 10.16688/j.zwbh.2022401 (In Chinese)
Kuang, W. L., and Luo, L., W. N. Gao, Lei, Y. H., Lv, Q. Y., and Li, J. Q. (2017). Development of a real-time fluorescence loop-mediated isothermal amplification assay for detection of Burkholderia gladioli pv. Alliicola. J. Phytopathol. 165, 82–90. doi: 10.1111/jph.12539
Lan, C. Z., Liu, P. Q., Li, B. J., Chen, Q. H., and Weng, Q. Y. (2013). Development of a specific pcr assay for the rapid and sensitive detection of Phytophthora capsici Australas. Plant Pathol. 42, 379–384. doi: 10.1007/s13313-012-0185-8
Lastra, E., Basallote-Ureba, M. J., Santos, B., Miranda, L., Vela-Delgado, M. D., and Capote, N. (2018). A TaqMan real-time polymerase chain reaction assay for accurate detection and quantification of Fusarium solani in strawberry plants and soil. Sci. Hortic. 237, 128–134. doi: 10.1016/j.scienta.2018.04.007
Lecomte, C., Edel-Hermann, V., Cannesan, M. A., Gautheron, N., Langlois, A., Alabouvette, C., et al. (2016). Fusarium oxysporum f.sp.cyclaminis: underestimated genetic diversity. Eur. J. Plant Pathol. 145, 421–431. doi: 10.1007/s10658-016-0856-3
Lei, R., Sun, X. W., Jiang, L., Wang, Z. H., Li, G. Q., Li, Y., et al. (2022). Development of rapid detection for Phytophthora syringae based on RPA/CRISPR-Cas12a. Plant Quarantine 36, 31–38. doi: 10.19662/j.cnki.issn1005-2755.2022.03.006 (In Chinese)
Li, B. J., Liu, P. Q., Xie, S. Y., Yin, R. M., Weng, Q. Y., and Chen, Q. H. (2014). Specific and sensitive detection of phytophthora nicotianae by nested pcr and loop-mediated isothermal amplification assays. J. Phytopathol. 163, 185–193. doi: 10.1111/jph.12305
Li, E., Wang, G., Xiao, J., Ling, J., Yang, Y., and Xie, B. (2016). A SIXI homolog in Fusarium oxysporum f.sp. conglutinans is required for full virulence on cabbage. PLoS One 11:e0152273. doi: 10.1371/journal.pone.0152273
Li, X. J., Zhang, S. Y., Liu, D., Yuan, X. W., Li, X. S., Shi, Y. X., et al. (2022). Establishment and application of rapid quantitative detection of viable Plasmodiophora brassicae by PMAxx-qPCR method. Sci. Agric. Sin. 55, 1938–1948. doi: 10.3864/j.issn.0578-1752.2022.10.005
Li, J., Zhao, W., Wang, J. X., and Zhou, M. G. (2011). Detection of Sclerotinia sclerotiorum by a quantitativereal-time PCR. Acta Phytopathol. Sin. 185, 5828–5834. doi: 10.4049/jimmunol.0903636
Lievens, B., Houterman, P., and Rep, M. (2009a). Effector gene screening allows unambiguous identification of Fusarium oxysporum f.sp lycopersici races and discrimination from other formae speciales. FEMS Microbiol. Lett. 300, 201–215. doi: 10.1111/j.1574-6968.2009.01783.x
Lievens, B., Rep, M., and Thomma, B. P. (2008). Recent developments in the molecular discrimination of formae speciales of Fusarium oxysporum. Pest Manag. Sci. 64, 781–788. doi: 10.1002/ps.1564
Lievens, B., van Baarlen, P., Verreth, C., van Kerckhove, S., Rep, M., and Thomma, B. P. H. J. (2009b). Evolutionary relationships between Fusarium oxysporum f. sp. lycopersici and F. Oxysporum f. sp. radicis-lycopersici isolates inferred from mating type, elongation factor-1α and exopolygalacturonase sequences. Mycol. Res. 113, 1181–1191. doi: 10.1016/j.mycres.2009.07.019
Lin, Y. H., Chang, J. Y., Liu, E. T., Chao, C. P., Huang, J. W., and Chang, P. F. L. (2008). Development of a molecular marker for specific detection of Fusarium oxysporum f.sp. cubense race 4. Eur. J. Plant Pathol. 123, 353–365. doi: 10.1007/s10658-008-9372-4
Liop, P., Bonaterra, A., Peñalver, J., and López, M. M. (2000). Development of a highly sensitive nested-PCR procedure using a single closed tube for detection of Erwinia amylovora in asymptomatic plant material. Appl. Environ. Microbiol. 66, 2071–2078. doi: 10.1128/AEM.66.5.2071-2078.2000
Liu, R. C., Cheng, Y. P., Chai, A. L., Shi, Y. X., Xie, X. W., and Patiguli, L. B. J. (2019). Establishment and application of a triplex PCR detection system for vegetable soil borne pathogens. Sci. Agric. Sin. 52, 2069–2078. doi: 10.3864/j.issn.0578-1752.2019.12.005
Liu, Y., Ji, Y., Han, Y. C., Song, L. L., and Duan, K. (2021). Loop-mediated isothermal amplification and PCR combined assay to detect and distinguish latent Colletotrichum spp. infection on strawberry. J. Plant Pathol. 103, 1–13. doi: 10.1007/S42161-021-00873-7
Liu, H., Wang, J. B., Zeng, H. J., Liu, X. F., Jiang, W., Wang, Y., et al. (2021). RPA-Cas12a-FS: a frontline nucleic acid rapid detection system for food safety based on CRISPR-Cas12a combined with recombinase polymerase amplification. Food Chem. 334:127608. doi: 10.1016/j.foodchem.2020.127608
López-Berges, M. S., di Pietro, A., Daboussi, M. J., Wahab, H. A., Vasnier, C., Roncero, M. I., et al. (2009). Identification of virulence genes in Fusarium oxysporum f.sp. lycopersici by large-scale transposon tagging. Mol. Plant Pathol. 10, 95–107. doi: 10.1111/j.1364-3703.2008.00512.x
Lu, C., Song, B., Zhang, H. F., Wang, Y. C., and Zheng, X. B. (2015). Rapid diagnosis of soybean seedling blight caused by rhizoctonia solani and soybean charcoal rot caused by macrophomina phaseolina using lamp assays. Phytopathology 105, 1612–1617. doi: 10.1094/PHYTO-01-15-0023-R
Luo, L. X., Walters, C., Bolkan, H., Liu, X. L., and Li, J. Q. (2008). Quantification of viable cells of Clavibacter michiganensis subsp. michiganensis using a DNA binding dye and a real-time PCR assay. Plant Pathol. 57, 332–337. doi: 10.1111/j.1365-3059.2007.01736.x
Manzanares-dauleux, M. J., Barret, P., and Thomas, G. (2000). Development of a pathotype specific SCAR marker in Plasmodiophora brassicae. Eur. J. Plant Pathol. 106, 781–787. doi: 10.1023/A:1026586803761
Martinez-Culebras, P. V., Querol, A., Suarez-Fernandez, M. B., Garcia-Lopez, M. D., and Barrio, E. (2003). Phylogenetic relationships among Colletotrichum pathogens of strawberry and design of PCR primer for their identification. Phytopathology 151, 135–143. doi: 10.1046/j.1439-0434.2003.00694.x
Martyn, R. D., and Netzer, D. (1991). Resistance to races 0, 1, and 2 of Fusarium wilt of watermelon in Citrullus sp. PI296341-FR. HortScience 26, 429–432. doi: 10.21273/HORTSCI.26.4.429
Matsumoto, M. (2002). Trials of direct detection and identification of Rhizoctonia solani AG1 and AG2 subgroups using specifically primed PCR analysis. Mycoscience 43, 185–189. doi: 10.1007/s102670200026
Mbofung, G. C. Y., and Pryor, B. M. (2010). A PCR-based assay for detection of Fusarium oxysporum f. sp. lactucae in lettuce seed. Plant Dis. 94, 860–866. doi: 10.1094/PDIS-94-7-0860
McDonald, J. F. (1993). Evolution and consequences of transposable elements. Curr. Opin. Genet. Dev. 3, 855–864. doi: 10.1016/0959-437X(93)90005-A
Meng, X. L., Chai, A. L., Chen, L., Shi, Y. X., Xie, X. W., Ma, Z. H., et al. (2016). Rapid detection and quantification of viable Pseudomonassyringae pv. Lachrymans cells in contaminated cucumber seeds using propidium monoazide and a real-time PCR assay. Can. J. Plant Pathol. 38, 296–306. doi: 10.1080/07060661.2016.1216897
Mirmajlessi, S. M., Loit, E., Maend, M., and Mansouripour, S. M. (2015). Real time PCR applied to study on plant pathogens: potential applications in diagnosis-a review. Plant Prot. Sci. 51, 177–190. doi: 10.17221/104/2014-PPS
Mudiyanselage, A. M. H., Jones, E. E., Jaspers, M. V., Walter, M., and Ridgway, H. J. (2021). A one step nested pcr method for detection of peronospora sparsa, the downy mildew pathogen, in boysenberry (rubus ursinus). Eur. J. Plant Pathol. 160, 973–981. doi: 10.1007/s10658-021-02283-y
Murolo, S., Moumni, M., Mancini, V., Allagui, M. B., Landi, L., and Romanazzi, G. (2022). Detection and quantification of Stagonosporopsis cucurbitacearum in seeds of Cucurbita maxima using droplet digital polymerase chain reaction. Front. Microbiol. 12:764447. doi: 10.3389/FMICB.2021.764447
Mutasa, E. S., Chwarszcynska, D. M., and Asher, M. J. C. (1996). Single-tube, nested PCR for the diagnosis of Polymyxa betae infection in sugar beet roots and colorimetric analysis of amplified products. Phytopathology 86, 493–497. doi: 10.1094/Phyto-86-493
Nair, S., and Manimekalai, R. (2021). Phytoplasma diseases of plants: molecular diagnostics and way forward. World J. Microbiol. Biotechnol. 37:102. doi: 10.1007/S11274-021-03061-Y
Nocker, A., Cheung, C. Y., and Camper, A. K. (2006). Comparison of propidium monoazide with ethidium monoazide for differentiation of live vs. dead bacteria by selective removal of DNA from dead cells. J. Microbiol. Methods 67, 310–320. doi: 10.1016/j.mimet.2006.04.015
O'Donnell, K., Sutton, D. A., Rinaldi, M. G., Sarver, B. A. J., and Geiser, D. M. (2010). Internet-accessible DNA sequence database for identifying Fusaria from human and animal infections. J. Clin. Microbiol. 48, 3708–3718. doi: 10.1128/jcm.00989-10
O'Donnell, K., Ward, T. J., Robert, V. A. R. G., Crous, P. W., Geiser, D. M., and Kang, S. (2015). DNA sequence-based identification of Fusarium: current status and future directions. Phytoparasitica 43, 583–595. doi: 10.1007/s12600-015-0484-z
Oliveira, M. B., Nascimento, L. B., Junior, M. L., and Petrofeza, S. (2010). Characterization of the dry bean polygalacturonase-inhibiting protein (pgip) gene family during Sclerotinia sclerotiorum (sclerotiniaceae) infection. GMR 9, 994–1004. doi: 10.4238/vol9-2gmr776
Olmos, A., Bertolini, E., Martınez, M. C., Candresse, T., Glasa, M., Pina, J. A., et al. (2013). Deep sequencing: a powerful tool for detection and characterization of known and new plant viruses and viroids. Acta Phytopathol. Sin. 43:280.
Ortu, G., Bertetti, D., Gullino, M. L., and Garibaldi, A. (2018). Fusarium oxysporum f. sp. lavandulae, a novel forma specialis causing wilt on Lavandula × allardii. J. Plant Pathol. 100, 391–397. doi: 10.1007/s42161-018-0084-0
Ozdemir, Z. (2005). Development of a multiplex PCR assay for concurrent detection of Clavibacter michiganensis ssp. michiganensis and Xanthomonas axonopodis pv. Vesicatoria. Plant Pathol. J. 4, 133–137. doi: 10.3923/ppj.2005.133.137
Pace, N. R. (1997). A molecular view of microbial diversity and the biosphere. Science 276, 734–740. doi: 10.1126/science.276.5313.734
Pang, W. X., Liang, Y., Zhan, Z. X., Li, X. N., and Piao, Z. Y. (2020). Development of a Sinitic clubroot differential set for the pathotype classification of Plasmodiophora brassicae. Front. Plant Sci. 11:568771. doi: 10.3389/fpls.2020.568771
Panno, S., Ferriol, I., Rangel, E., Olmos, A., Han, C. G., Martinelli, F., et al. (2014). Detection and identification of Fabavirus species by one-step RT-PCR and multiplex RT-PCR. J. Virol. Methods 197, 77–82. doi: 10.1016/j.jviromet.2013.12.002
Pasquali, M., Dematheis, F., Gullino, M. L., and Garibaldi, A. (2007). Identification of race 1 of Fusarium oxysporum f. sp. lactucae on lettuce by inter-retrotransposon sequence-characterized amplified region technique. Phytopathology 97, 987–996. doi: 10.1094/PHYTO-97-8-0987
Pasquali, M., Marena, L., Fiora, E., Piatti, P., Gullino, M. L., and Garibaldi, A. (2004). Real time PCR for the identification of a highly pathogenic group of Fusarium oxysporum f. sp. chrysanthemi on Argyranthemum frutescens L. J. Plant Pathol. 86, 51–57. doi: 10.2307/41998167
Pastrik, K. H., Elphinstone, J. G., and Pukall, R. (2002). Sequence analysis and detection of Ralstonia solanacearum by multiplex PCR amplification of 16S-23S ribosomal intergenic spacer region with internal positive control. Eur. J. Plant Pathol. 108, 831–842. doi: 10.1023/A:1021218201771
Peng, J., Zhan, Y. F., Zeng, F. Y., Long, H. B., Pei, Y. L., and Guo, J. R. (2013). Development of a real-time fluorescence loop-mediated isothermal amplification assay for rapid and quantitative detection of Fusarium oxysporum f. sp. niveum in soil. FEMS Microbiol. Lett. 349, 127–134. doi: 10.1111/1574-6968.12305
Poueymiro, M., Cunnac, S., Barberis, P., Deslandes, L., Peeters, N., Cazale-Noel, A. C., et al. (2009). Two type III secretion system effectors from Ralstonia solanacearum GMI1000 determine host range specificity on tobacco. Mol. Plant Microbe Interact. 22, 538–550. doi: 10.1094/MPMI-22-5-0538
Qin, L., Fu, Y., Xie, J., Cheng, J., Jiang, D., Li, G., et al. (2011). A nested-PCR method for rapid detection of Sclerotinia sclerotiorum on petals of oilseed rape (Brassica napus). Plant Pathol. 60, 271–277. doi: 10.1111/j.1365-3059.2010.02372.x
Quinterovásquez, G. A., Bazántejeda, M. L., Martínezpeñafiel, E., Kameyamakawabe, L., and Bermúdez-Cruz, R. M. (2010). Multiplex PCR to detect four different tomato-infecting pathogens. Folia Microbiol. 58, 269–276. doi: 10.1007/s12223-012-0206-6
Ramesh, R., Anthony, J., Jaxon, T. C. D., Gaitonde, S., and Achari, G. (2011). PCR-based sensitive detection of Ralstonia solanacearum from soil, eggplant, seeds and weeds. Arch. Phytopathol. Plant Protect. 44, 1908–1919. doi: 10.1080/03235408.2010.516087
Rui, T. T., Gao, Q. Y., Li, X. J., Shi, Y. X., Xie, X. W., Li, L., et al. (2022). Methylene blue combined agarose assay for single-spore isolation of Plasmodiophora brassicae and pathotype differentiation. Acta Horticult. Sin. 49, 1290–1300. doi: 10.16420/j.issn.0513-353x.2021-0421
Schwelm, A., and Ludwig-Muller, J. (2021). Molecular pathotyping of Plasmodiophora brassicae-genomes, marker genes, and obstacles. Pathogens 10:259. doi: 10.3390/pathogens10030259
Shands, A. C., Xu, G., Belisle, R. J., Seifbarghi, S., Jackson, N., Bombarely, A., et al. (2024). Genomic and transcriptomic analyses of Phytophthora cinnamomi reveal complex genome architecture, expansion of pathogenicity factors, and host-dependent gene expression profiles. Front. Microbiol. 15:1341803. doi: 10.3389/fmicb.2024.1341803
Shneyder, Y., Karimova, E., Zhivaeva, T., Lozovaya, E., Bashkirova, I., and Prikhodko, Y. (2022). Comparison of express-diagnostic kit and PCR tests for detection of tomato brown rugose fruit virus. Acta Hortic. 1351, 113–118. doi: 10.17660/ActaHortic.2022.1351.18
Sousa, M., Machado, J., Simmons, H. E., and Munkvold, G. P. (2015). Real-time quantitative pcr assays for the rapid detection and quantification of fusarium oxysporum f. sp. Phaseoli in phaseolus vulgaris (common bean) seeds. Plant Pathol. 64, 478–488. doi: 10.1111/ppa.12257
Supakitthanakorn, S., Vichittragoontavorn, K., Sunpapao, A., Kunasakdakul, K., Thapanapongworakul, P., and Ruangwong, O. U. (2022). Tobacco mosaic virus infection of chrysanthemums in Thailand: development of colorimetric reverse-transcription loop-mediated isothermal amplification (RT–LAMP) technique for sensitive and rapid detection. Plan. Theory 11:1788. doi: 10.3390/PLANTS11141788
Tavernier, S., and Coenye, T. (2015). Quantification of Pseudomonas aeruginosa in multispecies biofilms using PMA-qPCR. PeerJ 3:e787. doi: 10.7717/peerj.787
Taylor, A., Vagany, V., Jackson, A. C., Harrison, R. J., Rainoni, A., and Clarkson, J. P. (2016). Identification of pathogenicity-related genes in Fusarium oxysporum f.sp.cepae. Mol. Plant Pathol. 17, 1032–1047. doi: 10.1111/mpp.12346
Tian, Q., Feng, J. J., Hu, J., and Zhao, W. J. (2016). Selective detection of viable seed-borne Acidovorax citrulli by real-time PCR with propidium monoazide. Sci. Rep. UK 6:35457. doi: 10.1038/srep35457
Umesha, S., and Avinash, P. (2015). Multiplex PCR for simultaneous identification of Ralstonia solanacearum and Xanthomonas perforans. Biotech 5, 245–252. doi: 10.1007/s13205-014-0223-z
Van Dam, P., de Sain, M., ter Horst, A., van der Gragt, M., and Rep, M. (2018). Use of comparative genomics-based markers for discrimination of host specificity in Fusarium oxysporum. Appl. Environ. Microbiol. 84, e01868–e01817. doi: 10.1128/AEM.01868-17
Van Dam, P., Fokkens, L., Schmidt, S. M., Linmans, J. H. J., Kistler, H. C., Ma, L. J., et al. (2016). Effect or profiles distinguish formae speciales of Fusarium oxysporum. Environ. Microbiol. 18, 4087–4102. doi: 10.1111/1462-2920.13445
Vojvodic, M., Lazic, D., Mitrovic, P., Tanovic, B., and Bulajic, A. (2019). Conventional and real-time PCR assays for detection and identification of Rhizoctonia solani AG-2-2, the causal agent of root rot of sugar beet. Pesticidi i Fitomedicina 34, 19–29. doi: 10.2298/PIF1901019V
Wang, N. W., Wang, T., Shen, J. G., and Hu, F. P. (2014). Rapid detection for Clavibacter michiganensis subsp. michiganensis using real-time PCR based on padlock probe. Sci. Agric. Sin. 47, 903–911. doi: 10.3864/j.issn.0578-1752.2014.05.007
Wang, B., Wang, R., Wang, D. Q., Wu, J., Li, J. X., Wang, J., et al. (2019). Cas12aVDet: a CRISPR/Cas12a-based platform for rapid and visual nucleic acid detection. Anal. Chem. 91, 12156–12161. doi: 10.1021/acs.analchem.9b01526
Wang, X., Zhong, M., Liu, Y., Ma, P. X., and Liu, M. (2020). Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a- based detection with naked eye readout, CRISPR/Cas12a-NER. Sci. Bull. 65, 1436–1439. doi: 10.1016/j.scib.2020.04.041
Webb, K., Gilardi, G., Matic, S., Gullino, M. L., and Garibaldi, A. (2014). Development of a rapid in planta detection method for identifying Plectosphaerella cucumerina on wild rocket by real-time PCR. Eur. J. Plant Pathol. 47, 903–911. doi: 10.14601/PhytopatholMediterr-15108
Wei, Y. K., Wang, Q., Xu, X. M., Hu, X. P., and Shang, W. J. (2024). Development and application of rapid detection system for Fusarium pseudograminearum based on RPA-CRISPR/Cas12a. Mycosystema 43:240061. doi: 10.13346/j.mycosystema.240061
Willer, H., Meier, C., Schlatter, B., and Travnicek, J. (2023). The state and evolution of organic fruit and vegetables: production and market at a world scale. Acta Hortic. 1367, 1–12. doi: 10.17660/ActaHortic.2023.1367.1
Williams, P. H. (1966). A system for the determination of races of Plasmodiophorae brassicae that infect cabbage and rutabaga. Phytopathology 56, 624–626.
Wu, X. H., Shao, X. L., Deng, M. J., Chen, C. F., Li, Y., and Liang, C. Z. (2007). Study on rapid detection of Clavibacter michiganensis subsp.michiganensis by real-time fluorescent PCR. Acta Agricult. Jiangxi 19, 34–36. doi: 10.19386/j.cnki.jxnyxb.2007.03.012 (in Chinese)
Xie, X., Chen, L., Shi, Y., Chai, A., Fan, T., Li, L., et al. (2022). The calcium cyanamide and polyethylene blocks the secondary transmission and infection of vegetable leaf diseases. Front. Plant Sci. 13:1027584. doi: 10.3389/FPLS.2022.1027584
Xie, X., Chen, L., Shi, Y., Chai, A., Fan, T., Li, B., et al. (2024). Response of microbial recovery rate to straw return after calcium Cyanamide soil disinfection. Horticulturae 10:2. doi: 10.3390/horticulturae10010002
Xie, X. W., Liu, S. C., Shi, Y. X., Zhang, S. F., Chen, L. D., Chai, A. L., et al. (2022). Establishment and application of LAMP rapid detection assay for stephylium solani. J. Agricult. Biotechnol. 30, 2045–2052. doi: 10.3969/j.issn.1674-7968.2022.10.018
Xu, J., Zheng, H. J., Liu, L., Pan, Z. C., Prior, P., Tang, B., et al. (2011). Complete genome sequence of the plant pathogen Ralstonia solanacearum strain Po82. J. Bacteriol. 193, 4261–4262. doi: 10.1128/JB.05384-11
Yang, J., Duan, K., Liu, Y., Song, L., and Gao, Q. (2022). Method to detect and quantify colonization of anthracnose causal agent Colletotrichum gloeosporioides species complex in strawberry by real-time PCR. J. Phytopathol. 170, 326–336. doi: 10.1111/jph.13082
Yao, X., Li, P., Xu, J., Zhang, M., Ren, R., Liu, G., et al. (2016). Rapid and sensitive detection of Didymella bryoniae by visual loop-mediated isothermal amplification assay. Front. Microbiol. 7:1372. doi: 10.3389/fmicb.2016.01372
Ye, Q. J., Wang, R. Q., Ruan, M. Y., Cheng, Y., Wan, H. J., Li, Z. M., et al. (2022). Establishment of a KASP-SNP detection method for filamentous fungi Fusarium oxysporum f. sp. lycopersici and Fusarium oxysporum f. sp. radicis lycopersici. J. Plant Prot. 49, 879–889. doi: 10.13802/j.cnki.zwbhxb.2022.2020180
Yi, C. L., Zheng, J., Fu, Y. D., Sun, Q. Y., Jing, C., Fang, X. Y., et al. (2020). The specific molecular marker for identification of Plasmodiophora brassicae pathotype 4. J. Sichuan Agricult. Univ. 38:6. doi: 10.16036/j.issn.1000-2650.2020.03.008 (in Chinese)
Youssef, S. A., Sayed, Y., Hassan, O. S., Safwat, G., and Shalaby, A. A. (2017). Universal and specifc 16S-23Sr RNA PCR primers for identification of phytoplasma associated with sesame in Egypt. Int. J. Adv. Res. Biol. Sci. 4, 191–200. doi: 10.22192/ijarbs.2017.04.07.024
Yu, X., Wang, W. F., Li, X. F., Feng, L. X., Qian, Y. K., Zhang, C. J., et al. (2021). PMA-qPCR assay for the detection of viable Pseudomonas syringae pv. Maculicola. Plant Quarantine 35, 49–54. doi: 10.19662/j.cnki.issn1005-2755.2021.04.009
Yuan, J., Wen, T., Zhang, H., Zhao, M. L., Penton, C., Ryan, S., et al. (2020). Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME 14, 2936–2950. doi: 10.1038/s41396-020-0720-5
Zeng, D. D., Ye, W. W., Xu, M., Lu, C. C., and Tian, Q. (2017). Rapid diagnosis of soybean root rot caused by Fusarium culmorum using a loop-Mediat ed isothermal amplification assay. J. Phytopathol. 165, 249–256. doi: 10.1111/jph.12556
Zetsche, B., Gootenberg, J. S., Abudayyeh, O. O., Slaymaker, I. M., Makarova, K. S., Essletzbichler, P., et al. (2015). Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163, 759–771. doi: 10.1016/j.cell.2015.09.038
Zhang, Y., Fang, W., Yan, D., Ji, Y., Chen, X., Guo, A., et al. (2023). Comparison of drip-irrigated or injected allyl isothiocyanate against key soil-borne pathogens and weeds. Pest Manag. Sci. 79, 3860–3870. doi: 10.1002/ps.7590
Zhang, H., Feng, J., Manolii, V., Strelkov, S. E., and Hwang, S. F. (2015). Characterization of a gene identified in pathotype 5 of the clubroot pathogen Plasmodiaphora brassicae. Phytopathology 105, 764–770. doi: 10.1094/PHYTO-10-14-0270-R
Zhang, X., Li, F., Wang, H., and Wang, F. (2023). Identification of tomato fusarium crown and root rot pathogen in Shandong province. J. Qingdao Agricult. Univ. 40, 24–29. doi: 10.3969/J.ISSN.1674-148X (In Chinese)
Zhang, J. X., Ling, J., Xie, B. Y., and Yang, Y. H. (2014). Rapid detection and identification of Fusarium oxysporum f.sp. conglutinans race 1 and race 2. Acta Phytopathol. Sin. 44, 586–594. doi: 10.13926/j.cnki.apps.2014.06.004
Zhao, T., Feng, J., Sechler, A., Randhawa, P., Li, J., and Schaad, N. W. (2009). An improved assay for detection of Acidovorax citrulli in watermelon and melon seed. Seed Sci. Technol. 37, 337–349. doi: 10.15258/sst.2009.37.2.08
Zheng, J., Wang, X. L., Li, Q., Yuan, S., Wei, S. Q., Tian, X. Y., et al. (2018). Characterization of five molecular markers for pathotype identification of the clubroot pathogen Plasmodiophora brassicae. Phytopathology 108, 1486–1492. doi: 10.1094/PHYTO-11-17-0362-R
Zhong, X., Yang, Y., Zhao, J., Gong, B. B., Li, J. R., Wu, X. L., et al. (2022). Detection and quantification of Fusarium oxysporum f. sp. niveum race 1 in plants and soil by real-time PCR. Plant Pathol. J. 38, 229–238. doi: 10.5423/PPJ.OA.03.2022.0039
Zhou, X., Wang, J. T., Liu, F., Liang, J. M., Zhao, P., Tsui, C. K. M., et al. (2022). Cross-kingdom synthetic microbiota supports tomato suppression of Fusarium wilt disease. Nat. Commun. 13:7890. doi: 10.1038/s41467-022-35452-6
Zhu, Z. X., Zheng, L., Hsiang, T., Yang, G. L., Zhao, D. L., Lv, B., et al. (2016). Detection and quantification of Fusarium commune in host tissue and infested soil using real-time PCR. Plant Pathol. 65, 218–226. doi: 10.1111/ppa.12412
Keywords: vegetable, soil borne pathogens, detection techniques, primer information, physiological races
Citation: Chen L, Lü G, Yang S, Gong B, Lu Y, Wu X, Li J and Gao H (2024) Advances in the detection technology of vegetable soil borne fungi and bacteria. Front. Microbiol. 15:1460729. doi: 10.3389/fmicb.2024.1460729
Edited by:
Islam Hamim, Bangladesh Agricultural University, BangladeshReviewed by:
Malkhan Singh Gurjar, Indian Agricultural Research Institute (ICAR), IndiaVikram Poria, Central University of Haryana, India
Saratha Mohana Sundaram M., Central Silk Board, India
Copyright © 2024 Chen, Lü, Yang, Gong, Lu, Wu, Li and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongbo Gao, aG9uZ2JvZ2FvQGhlYmF1LmVkdS5jbg==
†These authors have contributed equally to this work
 Lida Chen
Lida Chen Guiyun Lü†
Guiyun Lü† Jingrui Li
Jingrui Li Hongbo Gao
Hongbo Gao