
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 06 December 2024
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1457348
This article is part of the Research Topic Animal Models, Gut Microbiota and Brain Diseases View all 21 articles
 Mingxing Tang1,2*†
Mingxing Tang1,2*† Yongliang Wu1,2†
Yongliang Wu1,2† Junyi Liang1,2
Junyi Liang1,2 Shuai Yang1,2
Shuai Yang1,2 Zuofeng Huang1,2
Zuofeng Huang1,2 Jing Hu1
Jing Hu1 Qiong Yang1
Qiong Yang1 Fei Liu1,2*
Fei Liu1,2* Shuo Li1,2*
Shuo Li1,2*Obstructive sleep apnea (OSA) is a state of sleep disorder, characterized by repetitive episodes of apnea and chronic intermittent hypoxia. OSA has an extremely high prevalence worldwide and represents a serious challenge to public health, yet its severity is frequently underestimated. It is now well established that neurocognitive dysfunction, manifested as deficits in attention, memory, and executive functions, is a common complication observed in patients with OSA, whereas the specific pathogenesis remains poorly understood, despite the likelihood of involvement of inflammation. Here, we provide an overview of the current state of the art, demonstrating the intimacy of OSA with inflammation and cognitive impairment. Subsequently, we present the recent findings on the investigation of gut microbiota alteration in the OSA conditions, based on both patients-based clinical studies and animal models of OSA. We present an insightful discussion on the role of changes in the abundance of specific gut microbial members, including short-chain fatty acid (SCFA)-producers and/or microbes with pathogenic potential, in the pathogenesis of inflammation and further cognitive dysfunction. The transplantation of fecal microbiota from the mouse model of OSA can elicit inflammation and neurobehavioral disorders in naïve mice, thereby validating the causal relationship to inflammation and cognitive abnormality. This work calls for greater attention on OSA and the associated inflammation, which require timely and effective therapy to protect the brain from irreversible damage. This work also suggests that modification of the gut microbiota using prebiotics, probiotics or fecal microbiota transplantation may represent a potential adjuvant therapy for OSA.
Obstructive sleep apnea (OSA) is a chronic sleep-related breathing disorder that is characterized by recurrent collapses in the upper airway during sleep, directly causing sleep fragmentation (SF) and chronic intermittent hypoxemia (IH) (Lévy et al., 2015). The development of OSA is largely attributed to a narrow, high-arched hard palate, or midface hypoplasia with retro-positioning of the maxilla and chin, or an enlarged pharynx, in the majority of cases observed in individuals with obesity (Neelapu et al., 2017; Kubota et al., 2005). The structural disproportions would in turn bring the soft palate and tongue closer to the back of the throat, thus leading to partial or complete airway blockage. Apnea hypopnea index (AHI), which is defined as an average number of partial or full breathing stop events within an hour of sleep, is the most common used indicator for the OSA diagnosis and severity determination (Shahar, 2014; Pevernagie et al., 2020). A number of epidemiological studies based on AHI have revealed a high prevalence of OSA globally (Benjafield et al., 2019; Grote, 2019; Wei et al., 2022; Lv et al., 2023). Two consecutive works have demonstrated that the OSA incidence in the adult population of the USA is approximately 33% among males but lower among females (Benjafield et al., 2019). The overall prevalence of OSA among 38,000 Russian citizens is 48.9% (Khokhrina et al., 2020). Notably, studies covering China (Ding et al., 2022), Chile (Peñafiel et al., 2019), Canada (Dosman et al., 2022), Germany (Fietze et al., 2019), Switzerland (Heinzer et al., 2015), Singapore (Tan et al., 2016), and Japan (Nakayama-Ashida et al., 2008) revealed a higher incidence of OSA, all of them exceeding 50%. Therefore, these findings collectively indicate that OSA is the most prevalent disease diagnosed in the department of otorhinolaryngology. The typical symptoms of OSA often include snoring, breathing breaks, excessive daytime sleepiness, and dry mouth and headache upon waking (Veasey and Rosen, 2019; Gottlieb and Punjabi, 2020). More importantly, prolonged exposure of OSA patients to IH could activate systematic inflammation and impact central nervous system (CNS), which ultimately lead to brain structural injury and severe neurocognitive deficits. The precise mechanism by which inflammation is induced in the OSA condition remains poorly understood.
Herein, we provide an overview of the current state of the art, and discuss a hypothetical scenario in which OSA may directly alter the composition of the gut microbiota, elicit inflammatory responses, and consequently lead to neurocognitive impairment. This paper reviews progress from both clinical studies and animal models are included.
Recent decades have borne witness to an increasing clarity regarding the prevalence of neurocognitive dysfunction among patients diagnosed with OSA. This phenomenon is characterized by deficits in attention, memory, and executive functions (Vanek et al., 2020; Xia et al., 2023; Kloepfer et al., 2009; Bawden et al., 2011; Hrubos-Strøm et al., 2012; Shieu et al., 2022). Indeed, clinical studies focusing on the effects of OSA have found that three distinct types of memory, including verbal, procedural and working memory, significantly decayed in the patients with OSA (Cunningham et al., 2023; Teh et al., 2023; Kloepfer et al., 2009; Naëgelé et al., 2006). Furthermore, several studies employed a more comprehensive set of tools to systematically identify cognitive impairment relevant to OSA (Xia et al., 2023; Bawden et al., 2011; Gnoni et al., 2023). Reviews of high quality are recommended to be consulted for a more detailed account of the cognitive impairment caused by OSA. This relatively underdiagnosed syndrome affects approximately 1 billion people globally and represents a significant public health concern (Benjafield et al., 2019).
In alignment with the abnormal cognitive function, structural alterations in brain tissues or regions have been identified through the utilization of diverse imaging technologies such as resting-state functional magnetic resonance imaging (fMRI) and computed tomography (CT). The affected areas across studies are diverse and often involved with multiple subregions (Zimmerman and Aloia, 2006), including the integrity of the gray or white matter (Lee et al., 2022; Castronovo et al., 2014), hippocampus (Kheirandish-Gozal et al., 2018; Macey et al., 2018; Gale and Hopkins, 2004), frontal lobe (Bai et al., 2021; Shu et al., 2022), temporal lobe (Shu et al., 2022; Morrell et al., 2010), cerebellum (Shu et al., 2022, Morrell et al., 2010), corpus callosum (Kheirandish-Gozal et al., 2018), and insular cortex (Kheirandish-Gozal et al., 2018). Despite the complexity and diversity, most of these areas are responsible for neurocognitive performance, indicating that structural alteration is a probable underlying cause of the observed deterioration in neurocognitive performance. For example, changes in the hippocampal volume have been identified in multiple studies through the MRI-based imaging analysis, while the hippocampus apoptosis or atrophy can cause learning, mnemonic, attentional, and executive function deficits. An early study, which involved with 17 newly diagnosed, untreated OSA patients and 15 age-matched healthy control subjects found that neurocognitive impairments were linked with a reduction of gray matter volume in the left hippocampus (entorhinal cortex), left posterior parietal cortex, and right superior frontal gyrus (Canessa et al., 2011). In another similar investigation, researchers identified atrophy of the neocortex and cerebellum, as well as a reduction in the volume of the hippocampal dentate gyrus and cerebellar dentate nucleus in patients with OSA (Kim H. et al., 2016). Of particular interest, after the continuous positive airway pressure (CPAP) treatment was administered to the patient cohorts in both studies, the impaired brain structure was restored to normality together with improved cognitive function, indicative of the reversibility of cognition deficits.
The intimate association of OSA with neurocognitive dysfunction has been also observed in a range of animal models, including pigs (Lonergan et al., 1998), dogs (Hendricks et al., 1993; Hendricks et al., 1987), rabbits (Schiefer et al., 2020; Yu et al., 2014), cats (Neuzeret et al., 2011), rats (Nácher et al., 2007; Farré et al., 2003), and mice (Qiu et al., 2023; Puech et al., 2022; Veasey et al., 2013; Nair et al., 2011a; Zhu et al., 2007; Puech et al., 2023). The disease has been modeled using either sleep fragmentation (SF), or intermittent hypoxia (IH), or both. SF can be induced by sleep disruption with experimental devices (Nair et al., 2011b; Ramesh et al., 2012), while IH is triggered by repeated exposure to lower oxygen levels (Badran et al., 2020; Puech et al., 2022). Although adverse effects of IH and SF on cognitive function may differ, common outcomes include impaired sleep quality, abnormal behavior, reduced learning ability and impaired physical functioning. In a study based on a rat model of OSA, for example, exposure to IH resulted in deficits in spatial memory and learning performance as assessed by Morris water maze tasks, together with the hippocampal apoptosis (Gao et al., 2017).
The pathogenesis of cognitive impairment induced by OSA is believed to be complex and remains poorly elucidated (Lv et al., 2023; Liu et al., 2020; Orrù et al., 2020). However, several lines of evidence from both clinical and animal model studies strongly support involvement of inflammation. Firstly, numerous studies have found that OSA can cause a systematic or local inflammation, as evidenced by increased levels of serum inflammatory cytokines are often observed in OSA patients (Liu et al., 2020; Nadeem et al., 2013; Bouloukaki et al., 2017; Bozic et al., 2018; Motamedi et al., 2018; Svatikova et al., 2003; Sozer et al., 2018). Tumor necrosis factor (TNF)-α and interleukin (IL)-6 are two representative biomarkers that are closely linked with OSA (Kheirandish-Gozal and Gozal, 2019), and more intriguingly both are also thought to contribute to neurocognitive dysfunction (Tegeler et al., 2016). Secondly, it is frequently observed that the activation of inflammatory processes and cognitive deficits occur concurrently in a considerable number of rodent models of OSA induced by IH (Liu et al., 2020; Dong et al., 2018; Sapin et al., 2015; Shi et al., 2018; Snyder et al., 2017; Darnall et al., 2017; Kim et al., 2013; Smith S. M. et al., 2013; Deng et al., 2015). Thirdly, in clinical studies, the magnitude of inflammation, as indicated by serum levels of various inflammatory cytokines, is frequently correlated with the severity of OSA (Nadeem et al., 2013; Sozer et al., 2018; Bouloukaki et al., 2017; Bozic et al., 2018; Motamedi et al., 2018). For instance, a recent analysis involving 858 OSA patients and 190 matched controls demonstrated that the serum levels of uric acid and high-sensitivity C-reactive protein (hsCRP), two markers of inflammation, were elevated in the severe group compared to the mild group (Bouloukaki et al., 2017). Fourthly, effective OSA therapy by CPAP can improve neurocognitive performance and also reduce/resolve inflammation (Tichanon et al., 2016; Lu et al., 2017; Ohga et al., 2003; Kuramoto et al., 2009; Wu et al., 2010; Jin et al., 2017; Yokoe et al., 2003; Steiropoulos et al., 2009; Schiza et al., 2010). Altogether, ample evidence from these works substantiates the pivotal role of OSA-induced neuroinflammation in the pathogenesis of neuronal injury and subsequent cognitive deficits.
The precise mechanistic pathway by which OSA triggers inflammation remains poorly understood. However, it has been postulated that HIF-1α, a critical transcription factor responsive to hypoxic conditions, is activated to increase ROS synthesis, which would in turn initiate oxidative stress and the inflammatory process (McGettrick and O’Neill, 2020). Furthermore, there is also evidence to show that chronic IH conditions observed in OSA patients stimulate leptin, an obesity biomarker in white adipose tissue (Pan and Kastin, 2014), while leptin can further promote production of proinflammatory cytokines (Berger and Polotsky, 2018). These changes may further lead to monocyte-endothelial cell adhesion, dysfunction of endothelial cells, breach of the blood–brain barrier, and finally the perfusion of inflammatory cytokines and macrophages into the central nervous system. Consequently, the excessive neuroinflammatory response results in the activation of glial cells, synaptic damage and loss, neuronal necrosis and apoptosis, and ultimately a significant exacerbation of neurocognitive deficits (Liu et al., 2020).
In addition to the aforementioned effects on inflammation and cognitive function, the impact of OSA can even extend to the gastrointestinal tract to modulate the oxygen concentrations and further the ecosystem, where at least 100 trillion bacteria colonize (Honda and Littman, 2016; Rooks and Garrett, 2016; Gomaa, 2020). In light of the existence of an oxygen concentration gradient in the range of 150–200 μm near the gut epithelium (Espey, 2013) and the susceptible responsiveness of gut microbiota to oxygen level change (Albenberg et al., 2014), it seems highly probable that chronic exposure to hypoxia would favor the survival of obligate anaerobes and therefore alter the bacterial diversity. Indeed, several mouse model-based studies have demonstrated that IH intervention can induce a periodic hypoxia pattern in the arterial blood and the lumen of the small intestine, as well as an increased abundance of obligate and facultative anaerobes (Moreno-Indias et al., 2015; Khalyfa et al., 2021; Lucking et al., 2018; Durgan et al., 2016), despite the possibility that IH-induced systemic immune responses may exert a modulatory effect on gut microbiota. Another important feature of OSA is nocturnal arousal due to sleep fragmentation. Interestingly exposure of mice to sleep fragmentation also caused notable change in gut microbiota, characterized by an increase in Firmicutes and a decrease in Bacteroidetes at the phylum level (Poroyko et al., 2016). It is noteworthy that a gut dysbiosis was also a prevalent trait in OSA patients. Two clinical studies investigating the OSA patients from disparate regions in China identified an altered gut microbiota profile (Ko et al., 2019; Wang et al., 2022). Furthermore, the composition of the gut microbiota was found to be significantly altered in pediatric patients with OSA in comparison to their age-matched healthy controls (Valentini et al., 2020; Collado et al., 2019). Consistently, an interesting single-armed study that investigated the responses of nine normal-weight men under two occasions, either with two nights of normal sleep or two nights of partial sleep deprivation. It revealed that sleep loss can directly induce an increased Firmicutes to Bacteroides (F/B) ratio in gut microbiota (Benedict et al., 2016).
In light of the pivotal roles of gut microbiota in regulating human physiology, particularly immunity (Kamada et al., 2012; Donohoe et al., 2011; Zheng et al., 2020; Belkaid and Harrison, 2017; Albhaisi et al., 2020; Morais et al., 2021), a hypothesis was therefore proposed that the OSA-induced gut dysbiosis, often featured with a changed F/B ratio, might contribute to inflammation, and potentially the cognitive dysfunction. In the context of the microbiota-immune system interaction, multiple microbial metabolites and components, including short-chain fatty acids (SCFAs), lipopolysaccharide (LPS) and exotoxins, act as potent effectors, facilitating a bridge between gut microbiota and local or systematic immunity (Rooks and Garrett, 2016; Wang G. et al., 2019). Indeed, the immunological effects of these microbiota-derived molecules are manifold, encompassing both innate and adaptive immunity (Tang et al., 2021). SCFAs are primarily produced from indigestible oligosaccharides by some beneficial members of the Bacteroidetes phylum, including the families Lactobacillaceae, Ruminococcaceae, Erysipelotrichaceae, Bifidobacteriaceae, and Clostridium (den Besten et al., 2013). In addition to serving as a source of energy for intestinal epithelial cells (IEC) (Rivière et al., 2016), SCFAs have pleotropic roles in the fortification of the gut barrier and maintenance of immune homeostasis. More specifically, these beneficial regulatory actions include stimulation of mucus production (Wrzosek et al., 2013), regulation of tight junction (TJ) proteins via multifaceted signaling pathways (Parada Venegas et al., 2019), polarization of anti-inflammatory macrophages (Ji et al., 2016), increased production of antimicrobial peptide (AMP) (Qiu et al., 2012), activation of NLR-family-pyrin-domain-containing-3 (NLRP3) inflammasomes and production of homeostatic cytokine interleukin-18 (IL-18) (Macia et al., 2015), modulation of B cell differentiation and Immunoglobulin A (IgA) secretion (Kim M. et al., 2016), reduced expression of T cell-activating molecules on antigen-presenting cells (Park et al., 2015), and increased number and function of colonic regulatory T (Tregs) cells (Smith P. M. et al., 2013). In contrast, several gut microbial members, including Desulfovibrio, Prevotella, Lachnospiraceae, and Paraprevotella, have been demonstrated to induce inflammatory responses and disrupt the structural integrity of the gut barrier. LPS is a well-known bacterial endotoxin with profound immunostimulatory and inflammatory capacity. Contrary to the beneficial effects of SCFAs, LPS has the potential to bind to its cognate toll-like-receptor (TLR4) and promote the activation of inflammatory macrophage (M1 polarization), which in turn leads to the production of an array of inflammatory cytokines, IL-1β, IL-6, IL-12, and TNF-α (Martinez et al., 2008). Through the same signal pathway, LPS can also compromise the integrity of the intestinal barrier (Guo et al., 2013). Furthermore, the prevalence of Prevotella and Desulfovibrio, which possess mucin-degrading capabilities, was found to be elevated in individuals with OSA, thereby exacerbating the gut permeability. An increase in those bacterial species will therefore cause the leakage of LPS and other bacterial components from the gut into the blood circulation, thus stimulating the release of inflammatory mediators and aggravating systemic inflammation.
Consistent with the above analysis, an investigation into the alteration of the gut microbiota in OSA patients indeed validated the abundance reduction in the abundance of bacteria associated with SCFA production, or alternatively an increase in the abundance of pathogenic ones, despite the fact that only a limited number of clinical studies in this direction existed. One study involving 93 OSA patients revealed that alterations in the gut microbiota were characterized by a decrease in the abundance of SCFA producers (including Faecalibacterium, Bifidobacterium, Lactobacillus, and Bacteroides), an increase for the pathogenic Prevotella, and concomitantly a reduction in the serum levels of IL-6 (Ko et al., 2019). Interestingly, this work did not identify a profound change in the F/B ratio between the OSA patients and healthy controls (Ko et al., 2019), suggesting that the F/B ratio alteration may not be a general OSA-associated rule, and that a more detailed analysis of specific bacterial taxa level should be necessary. In another observational study, the inflammation-related bacteria (Megamonas, Lactobacillus, Megasphaera, and Coprococcus at the genus level) were also enriched in OSA patients, while Alistipes, Eubacterium coprostanoligenes, Blautia, Roseburia, Fusobacteria, and Ruminococcus gnavus were found to be depleted. The pro-inflammatory cytokine IL-1β and TNF-α were elevated with OSA (Lu et al., 2022). A comparable pattern was identified among the pediatric OSA patients, whereby the relative abundances of several well-documented SCFA producers, including Bacteroides, Bifidobacterium, Ruminococcus, Collinsella, and Faecalibacterium, exhibited a decline (Valentini et al., 2020). The hypothesis was further validated in the two clinical trials, in which healthy human subjects were exposed to either a short-term or a long-term period of sleep deprivation, directly resulted in a reduction of the abovementioned species with SCFA-production capacity (Gao et al., 2023; Benedict et al., 2016).
Moreover, a comparable pattern was observed in multiple OSA animal model-based gut microfloral analyses, with an increase in pathogenic microbes being a particularly prevalent finding. For example, Prevotella, Paraprevotella, Desulfovibrio, and Lachnospiraceae were found to be enriched in a mouse model of OSA, induced by either SF or IH (Gao et al., 2023; Poroyko et al., 2016; Badran et al., 2020; Khalyfa et al., 2021; Badran et al., 2023; Yan et al., 2024). Interestingly, treatment of the sleep-deprived mice with butyrate, a type of SCFA, significantly ameliorated intestinal mucosa injury and inflammation response through the suppression of the HDAC3–GSK-3β–Nrf2–NF-κB signaling cascade (Gao et al., 2023). In another study, neonatal brain immaturity, white matter injury (WMI), reduced abundance of beneficial gut microbes Bacteroides thetaiotaomicron and Parabacteroides distasonis and accumulation of microbiota-derived cholic acid were identified in chronically hypoxic rats, whereas administration of B. thetaiotaomicron and P. distasonis reverted the cholic acid concentration and rescued the chronic hypoxia-induced WMI and inflammation. These findings suggest that the administration of SCFAs or probiotics may represent a potential strategy for alleviating the inflammation and neurocognitive deficits associated with OSA (Yan et al., 2024).
Fecal microbiota transplant (FMT) simply implies the transfer of stool samples from a donor’s colon to a recipient’s colon (Gupta and Khanna, 2017). FMT has demonstrated considerable promise in the treatment of intestinal infection, inflammatory bowel disease, hypertension, obesity, and diabetes mellitus (Kelly et al., 2015; Turnbaugh et al., 2006; Adnan et al., 2017; Wang H. et al., 2019). Although only a few studies have employed FMT in analyzing the basis of OSA, two recent works showed that FMT from the IH-mice can elicit inflammation and neurobehavioral disorders in naïve mice (Badran et al., 2020; Poroyko et al., 2016). This provides further robust evidence that gut microbiota dysbiosis is, at least partially, a causal basis of cognitive dysfunction.
The prevalence of OSA is markedly high across countries and regions, and it is frequently complexed with a variety of comorbidities. With firm evidence from both clinical studies and animal models, the present review reveals a clear and compelling link of OSA with alterations in the gut microbiota, systematic inflammation, brain substructural changes and neurocognitive impairment, respectively. A hypothesis is therefore proposed to explain the pathogenesis of OSA that chronic IH or SF in OSA patients would trigger gut microbiota dysbiosis, which is often characterized by depletion of producers of beneficial microbial metabolites, or/and enrichment of microbes with potentials to impair mucosa and activate inflammation (Figure 1). This in turn results in the activation of systematic inflammation, with inflammatory cytokines breaching the blood–brain barrier, activating microglial cells, and ultimately causing necrosis and apoptosis of neural cells and neurocognitive deficits. It is imperative to emphasize the importance of timely diagnosis and treatment of OSA and its associated inflammation, in order to prevent or alleviate the irreversible neurocognitive damage. Moreover, the modulation of gut microbiota using prebiotics (such as butyrate), or probiotics (SCFA producers) may represent a potential and effective adjuvant therapy for OSA. More future works in this direction are still needed.
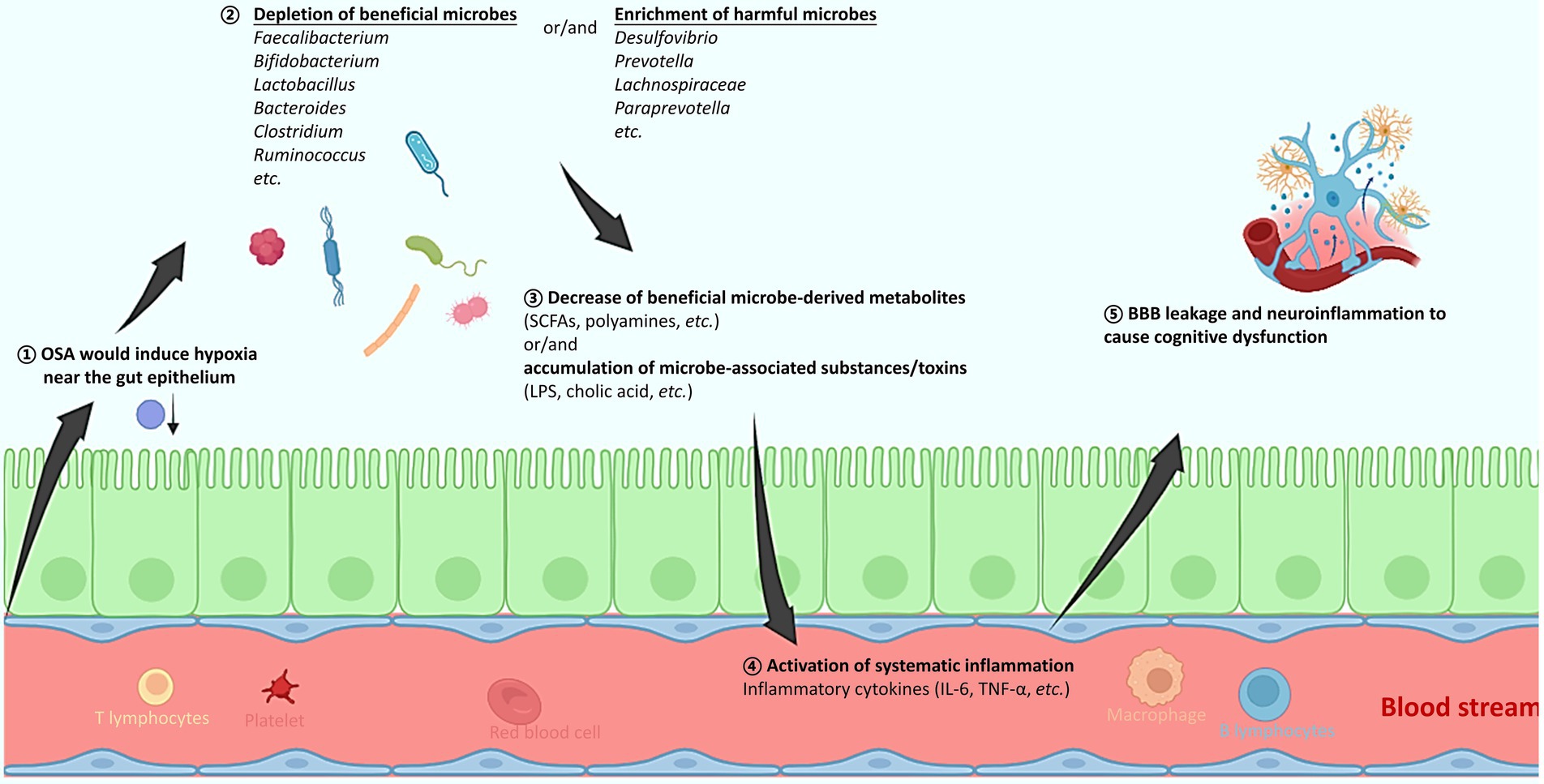
Figure 1. A schematic diagram explaining how obstructive sleep apnea (OSA) would alter the gut microbiota, activate systematic inflammation, and consequently induce brain tissue injury and cognitive dysfunction. (1) The OSA-induced hypoxia might alter the oxygen concentration near the gut epithelium. (2) The depleted oxygen radiation thus favors the increased abundance of obligate and facultative anaerobes, resulting in the gut microbiota change that is mostly featured with an increased ratio of Firmicutes to Bacteroides (F/B). Specifically, producers of beneficial metabolites such as SCFAs and polyamines decrease, while microbial members with pathogenic potentials increase. (3) The gut microbiota composition change further leads to accumulation of microbial toxins, and/or decrease of a wide spectrum of beneficial metabolites. (4) The gut barrier function might be compromised, and levels of various inflammatory cytokines are elevated, resulting in a systematic inflammation. (5) The inflammatory cytokines can breach the BBB, activate microglial cells, cause neuroinflammation, and consequently result in cognitive deficits. OSA, obstructive sleep apnea; SCFAs, short chain fatty acids; LPS, lipopolysaccharide; IL-6, interleukin-6; TNF-α, tumor necrosis factor-α; BBB, blood brain barrier.
MT: Conceptualization, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing. YW: Data curation, Formal analysis, Writing – original draft. JL: Writing – original draft. SY: Data curation, Writing – original draft. ZH: Writing – original draft. JH: Writing – review & editing. QY: Writing – review & editing. FL: Writing – original draft. SL: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was funded by the Medical Research Foundation of GuangDong Province (A2022046 and B2022097), the Outstanding Young Researcher program (NSZD2023011), the general project (2020072) and the key program (NS2022005) granted by the Science and Technology Key Research Program in Nanshan District Health Care System, the Shenzhen Science and Technology Innovation Commission for Research and Development Projects (JCYJ20210324112607020, JCYJ20220530141616037, and JCYJ20230807115827057), Shenzhen University ENT Discipline Development Project sponsored by Shenzhen Huaqiang Holdings Co., Ltd., and the GuangDong Basic and Applied Basic Research Foundation (2023A1515110855).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adnan, S., Nelson, J. W., Ajami, N. J., Venna, V. R., Petrosino, J. F., Bryan, R. M. Jr., et al. (2017). Alterations in the gut microbiota can elicit hypertension in rats. Physiol. Genomics 49, 96–104. doi: 10.1152/physiolgenomics.00081.2016
Albenberg, L., Esipova, T. V., Judge, C. P., Bittinger, K., Chen, J., Laughlin, A., et al. (2014). Correlation between intraluminal oxygen gradient and radial partitioning of intestinal microbiota. Gastroenterology 147, 1055–1063.e8. doi: 10.1053/j.gastro.2014.07.020
Albhaisi, S. A. M., Bajaj, J. S., and Sanyal, A. J. (2020). Role of gut microbiota in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 318, G84–G98. doi: 10.1152/ajpgi.00118.2019
Badran, M., Khalyfa, A., Ericsson, A., and Gozal, D. (2020). Fecal microbiota transplantation from mice exposed to chronic intermittent hypoxia elicits sleep disturbances in naïve mice. Exp. Neurol. 334:113439. doi: 10.1016/j.expneurol.2020.113439
Badran, M., Khalyfa, A., Ericsson, A. C., Puech, C., Mcadams, Z., Bender, S. B., et al. (2023). Gut microbiota mediate vascular dysfunction in a murine model of sleep apnoea: effect of probiotics. Eur. Respir. J. 61:2200002. doi: 10.1183/13993003.00002-2022
Bai, J., Wen, H., Tai, J., Peng, Y., Li, H., Mei, L., et al. (2021). Altered spontaneous brain activity related to neurologic and sleep dysfunction in children with obstructive sleep apnea syndrome. Front. Neurosci. 15:595412. doi: 10.3389/fnins.2021.595412
Bawden, F. C., Oliveira, C. A., and Caramelli, P. (2011). Impact of obstructive sleep apnea on cognitive performance. Arq. Neuropsiquiatr. 69, 585–589. doi: 10.1590/s0004-282x2011000500003
Belkaid, Y., and Harrison, O. J. (2017). Homeostatic immunity and the microbiota. Immunity 46, 562–576. doi: 10.1016/j.immuni.2017.04.008
Benedict, C., Vogel, H., Jonas, W., Woting, A., Blaut, M., Schürmann, A., et al. (2016). Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol. Metab. 5, 1175–1186. doi: 10.1016/j.molmet.2016.10.003
Benjafield, A. V., Ayas, N. T., Eastwood, P. R., Heinzer, R., Ip, M. S. M., Morrell, M. J., et al. (2019). Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir. Med. 7, 687–698. doi: 10.1016/s2213-2600(19)30198-5
Berger, S., and Polotsky, V. Y. (2018). Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: a possible link to oxidative stress and cardiovascular complications. Oxidative Med. Cell. Longev. 2018:5137947. doi: 10.1155/2018/5137947
Bouloukaki, I., Mermigkis, C., Tzanakis, N., Kallergis, E., Moniaki, V., Mauroudi, E., et al. (2017). Evaluation of inflammatory markers in a large sample of obstructive sleep apnea patients without comorbidities. Mediat. Inflamm. 2017:4573756. doi: 10.1155/2017/4573756
Bozic, J., Borovac, J. A., Galic, T., Kurir, T. T., Supe-Domic, D., and Dogas, Z. (2018). Adropin and inflammation biomarker levels in male patients with obstructive sleep apnea: a link with glucose metabolism and sleep parameters. J. Clin. Sleep Med. 14, 1109–1118. doi: 10.5664/jcsm.7204
Canessa, N., Castronovo, V., Cappa, S. F., Aloia, M. S., Marelli, S., Falini, A., et al. (2011). Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am. J. Respir. Crit. Care Med. 183, 1419–1426. doi: 10.1164/rccm.201005-0693OC
Castronovo, V., Scifo, P., Castellano, A., Aloia, M. S., Iadanza, A., Marelli, S., et al. (2014). White matter integrity in obstructive sleep apnea before and after treatment. Sleep 37, 1465–1475. doi: 10.5665/sleep.3994
Collado, M. C., Katila, M. K., Vuorela, N. M., Saarenpää-Heikkilä, O., Salminen, S., and Isolauri, E. (2019). Dysbiosis in snoring children: An interlink to comorbidities? J. Pediatr. Gastroenterol. Nutr. 68, 272–277. doi: 10.1097/mpg.0000000000002161
Cunningham, T. J., Kishore, D., Guo, M., Igue, M., Malhotra, A., Stickgold, R., et al. (2023). The effect of obstructive sleep apnea on sleep-dependent emotional memory consolidation. Ann. Am. Thorac. Soc. 20, 296–306. doi: 10.1513/AnnalsATS.202204-315OC
Darnall, R. A., Chen, X., Nemani, K. V., Sirieix, C. M., Gimi, B., Knoblach, S., et al. (2017). Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism. Pediatr. Res. 82, 164–172. doi: 10.1038/pr.2017.102
Den Besten, G., Van Eunen, K., Groen, A. K., Venema, K., Reijngoud, D.-J., and Bakker, B. M. (2013). The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 54, 2325–2340. doi: 10.1194/jlr.R036012
Deng, Y., Yuan, X., Guo, X. L., Zhu, D., Pan, Y. Y., and Liu, H. G. (2015). Efficacy of atorvastatin on hippocampal neuronal damage caused by chronic intermittent hypoxia: involving tlr4 and its downstream signaling pathway. Respir. Physiol. Neurobiol. 218, 57–63. doi: 10.1016/j.resp.2015.07.006
Ding, S., Zhang, P., Wang, L., Wang, D., Sun, K., Ma, Y., et al. (2022). Prevalence of obstructive sleep apnea syndrome in hospitalized patients with type 2 diabetes in Beijing, China. J. Diabetes Investig. 13, 1889–1896. doi: 10.1111/jdi.13868
Dong, P., Zhao, J., Li, N., Lu, L., Li, L., Zhang, X., et al. (2018). Sevoflurane exaggerates cognitive decline in a rat model of chronic intermittent hypoxia by aggravating microglia-mediated neuroinflammation via downregulation of ppar-γ in the hippocampus. Behav. Brain Res. 347, 325–331. doi: 10.1016/j.bbr.2018.03.031
Donohoe, D. R., Garge, N., Zhang, X., Sun, W., O'connell, T. M., Bunger, M. K., et al. (2011). The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 13, 517–526. doi: 10.1016/j.cmet.2011.02.018
Dosman, J. A., Karunanayake, C. P., Fenton, M., Ramsden, V. R., Seeseequasis, J., Mike, D., et al. (2022). Stop-bang score and prediction of severity of obstructive sleep apnea in a first nation community in Saskatchewan, Canada. Clocks Sleep 4, 535–548. doi: 10.3390/clockssleep4040042
Durgan, D. J., Ganesh, B. P., Cope, J. L., Ajami, N. J., Phillips, S. C., Petrosino, J. F., et al. (2016). Role of the gut microbiome in obstructive sleep apnea-induced hypertension. Hypertension 67, 469–474. doi: 10.1161/hypertensionaha.115.06672
Espey, M. G. (2013). Role of oxygen gradients in shaping redox relationships between the human intestine and its microbiota. Free Radic. Biol. Med. 55, 130–140. doi: 10.1016/j.freeradbiomed.2012.10.554
Farré, R., Rotger, M., Montserrat, J. M., Calero, G., and Navajas, D. (2003). Collapsible upper airway segment to study the obstructive sleep apnea/hypopnea syndrome in rats. Respir. Physiol. Neurobiol. 136, 199–209. doi: 10.1016/S1569-9048(03)00082-X
Fietze, I., Laharnar, N., Obst, A., Ewert, R., Felix, S. B., Garcia, C., et al. (2019). Prevalence and association analysis of obstructive sleep apnea with gender and age differences – results of ship-trend. J. Sleep Res. 28:e12770. doi: 10.1111/jsr.12770
Gale, S. D., and Hopkins, R. O. (2004). Effects of hypoxia on the brain: neuroimaging and neuropsychological findings following carbon monoxide poisoning and obstructive sleep apnea. J. Int. Neuropsychol. Soc. 10, 60–71. doi: 10.1017/S1355617704101082
Gao, H., Han, Z., Huang, S., Bai, R., Ge, X., Chen, F., et al. (2017). Intermittent hypoxia caused cognitive dysfunction relate to mirnas dysregulation in hippocampus. Behav. Brain Res. 335, 80–87. doi: 10.1016/j.bbr.2017.06.025
Gao, T., Wang, Z., Dong, Y., Cao, J., and Chen, Y. (2023). Butyrate ameliorates insufficient sleep-induced intestinal mucosal damage in humans and mice. Microbiol. Spectr. 11, e02000–e02022. doi: 10.1128/spectrum.02000-22
Gnoni, V., Mesquita, M., O'regan, D., Delogu, A., Chakalov, I., Antal, A., et al. (2023). Distinct cognitive changes in male patients with obstructive sleep apnoea without co-morbidities. Front. Sleep 2:1097946. doi: 10.3389/frsle.2023.1097946
Gomaa, E. Z. (2020). Human gut microbiota/microbiome in health and diseases: a review. Antonie Van Leeuwenhoek 113, 2019–2040. doi: 10.1007/s10482-020-01474-7
Gottlieb, D. J., and Punjabi, N. M. (2020). Diagnosis and management of obstructive sleep apnea: a review. JAMA 323, 1389–1400. doi: 10.1001/jama.2020.3514
Grote, L. (2019). The global burden of sleep apnoea. Lancet Respir. Med. 7, 645–647. doi: 10.1016/s2213-2600(19)30226-7
Guo, S., Al-Sadi, R., Said, H. M., and Ma, T. Y. (2013). Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of tlr-4 and cd14. Am. J. Pathol. 182, 375–387. doi: 10.1016/j.ajpath.2012.10.014
Gupta, A., and Khanna, S. (2017). Fecal microbiota transplantation. JAMA 318:102. doi: 10.1001/jama.2017.6466
Heinzer, R., Vat, S., Marques-Vidal, P., Marti-Soler, H., Andries, D., Tobback, N., et al. (2015). Prevalence of sleep-disordered breathing in the general population: the hypnolaus study. Lancet Respir. Med. 3, 310–318. doi: 10.1016/s2213-2600(15)00043-0
Hendricks, J. C., Kline, L. R., Kovalski, R. J., O'Brien, J. A., Morrison, A. R., and Pack, A. I. (1987). The english bulldog: a natural model of sleep-disordered breathing. J. Appl. Physiol. 63, 1344–1350. doi: 10.1152/jappl.1987.63.4.1344
Hendricks, J. C., Petrof, B. J., Panckeri, K., and Pack, A. I. (1993). Upper airway dilating muscle hyperactivity during non-rapid eye movement sleep in english bulldogs. Am. Rev. Respir. Dis. 148, 185–194. doi: 10.1164/ajrccm/148.1.185
Honda, K., and Littman, D. R. (2016). The microbiota in adaptive immune homeostasis and disease. Nature 535, 75–84. doi: 10.1038/nature18848
Hrubos-Strøm, H., Nordhus, I. H., Einvik, G., Randby, A., Omland, T., Sundet, K., et al. (2012). Obstructive sleep apnea, verbal memory, and executive function in a community-based high-risk population identified by the berlin questionnaire Akershus sleep apnea project. Sleep Breath. 16, 223–231. doi: 10.1007/s11325-011-0493-1
Ji, J., Shu, D., Zheng, M., Wang, J., Luo, C., Wang, Y., et al. (2016). Microbial metabolite butyrate facilitates M2 macrophage polarization and function. Sci. Rep. 6:24838. doi: 10.1038/srep24838
Jin, F., Liu, J., Zhang, X., Cai, W., Zhang, Y., Zhang, W., et al. (2017). Effect of continuous positive airway pressure therapy on inflammatory cytokines and atherosclerosis in patients with obstructive sleep apnea syndrome. Mol. Med. Rep. 16, 6334–6339. doi: 10.3892/mmr.2017.7399
Kamada, N., Kim, Y. G., Sham, H. P., Vallance, B. A., Puente, J. L., Martens, E. C., et al. (2012). Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329. doi: 10.1126/science.1222195
Kelly, C. R., Kahn, S., Kashyap, P., Laine, L., Rubin, D., Atreja, A., et al. (2015). Update on fecal microbiota transplantation 2015: indications, methodologies, mechanisms, and outlook. Gastroenterology 149, 223–237. doi: 10.1053/j.gastro.2015.05.008
Khalyfa, A., Ericsson, A., Qiao, Z., Almendros, I., Farré, R., and Gozal, D. (2021). Circulating exosomes and gut microbiome induced insulin resistance in mice exposed to intermittent hypoxia: effects of physical activity. EBioMedicine 64:103208. doi: 10.1016/j.ebiom.2021.103208
Kheirandish-Gozal, L., and Gozal, D. (2019). Obstructive sleep apnea and inflammation: proof of concept based on two illustrative cytokines. Int. J. Mol. Sci. 20:459. doi: 10.3390/ijms20030459
Kheirandish-Gozal, L., Sahib, A. K., Macey, P. M., Philby, M. F., Gozal, D., and Kumar, R. (2018). Regional brain tissue integrity in pediatric obstructive sleep apnea. Neurosci. Lett. 682, 118–123. doi: 10.1016/j.neulet.2018.06.002
Khokhrina, A., Andreeva, E., and Degryse, J. M. (2020). The prevalence of sleep-disordered breathing in Northwest Russia: the arkhsleep study. Chron. Respir. Dis. 17:1479973120928103. doi: 10.1177/1479973120928103
Kim, H., Joo, E., Suh, S., Kim, J. H., Kim, S. T., and Hong, S. B. (2016). Effects of long-term treatment on brain volume in patients with obstructive sleep apnea syndrome. Hum. Brain Mapp. 37, 395–409. doi: 10.1002/hbm.23038
Kim, S. M., Kim, H., Lee, J. S., Park, K. S., Jeon, G. S., Shon, J., et al. (2013). Intermittent hypoxia can aggravate motor neuronal loss and cognitive dysfunction in als mice. PLoS One 8:e81808. doi: 10.1371/journal.pone.0081808
Kim, M., Qie, Y., Park, J., and Kim, C. H. (2016). Gut microbial metabolites fuel host antibody responses. Cell Host Microbe 20, 202–214. doi: 10.1016/j.chom.2016.07.001
Kloepfer, C., Riemann, D., Nofzinger, E. A., Feige, B., Unterrainer, J., O'Hara, R., et al. (2009). Memory before and after sleep in patients with moderate obstructive sleep apnea. J. Clin. Sleep Med. 5, 540–548. doi: 10.5664/jcsm.27655
Ko, C. Y., Liu, Q. Q., Su, H. Z., Zhang, H. P., Fan, J. M., Yang, J. H., et al. (2019). Gut microbiota in obstructive sleep apnea-hypopnea syndrome: disease-related dysbiosis and metabolic comorbidities. Clin. Sci. 133, 905–917. doi: 10.1042/cs20180891
Kubota, Y., Nakayama, H., Takada, T., Matsuyama, N., Sakai, K., Yoshizawa, H., et al. (2005). Facial axis angle as a risk factor for obstructive sleep apnea. Intern. Med. 44, 805–810. doi: 10.2169/internalmedicine.44.805
Kuramoto, E., Kinami, S., Ishida, Y., Shiotani, H., and Nishimura, Y. (2009). Continuous positive nasal airway pressure decreases levels of serum amyloid a and improves autonomic function in obstructive sleep apnea syndrome. Int. J. Cardiol. 135, 338–345. doi: 10.1016/j.ijcard.2008.03.078
Lee, M. H., Lee, S. K., Kim, S., Kim, R. E. Y., Nam, H. R., Siddiquee, A. T., et al. (2022). Association of obstructive sleep apnea with white matter integrity and cognitive performance over a 4-year period in middle to late adulthood. JAMA Netw. Open 5, –e2222999. doi: 10.1001/jamanetworkopen.2022.22999
Lévy, P., Kohler, M., Mcnicholas, W. T., Barbé, F., Mcevoy, R. D., Somers, V. K., et al. (2015). Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 1:15015. doi: 10.1038/nrdp.2015.15
Liu, X., Ma, Y., Ouyang, R., Zeng, Z., Zhan, Z., Lu, H., et al. (2020). The relationship between inflammation and neurocognitive dysfunction in obstructive sleep apnea syndrome. J. Neuroinflammation 17:229. doi: 10.1186/s12974-020-01905-2
Lonergan, R. P., Ware, J. C., Atkinson, R. L., Winter, W. C., and Suratt, P. M. (1998). Sleep apnea in obese miniature pigs. J. Appl. Physiol. 84, 531–536. doi: 10.1152/jappl.1998.84.2.531
Lu, D., Li, N., Yao, X., and Zhou, L. (2017). Potential inflammatory markers in obstructive sleep apnea-hypopnea syndrome. Bosn. J. Basic Med. Sci. 17, 47–53. doi: 10.17305/bjbms.2016.1579
Lu, D., Xu, S., Dai, P., Wu, L., Zhang, H., and Zhou, B. (2022). Gut microbiota in hypertensive patients with versus without obstructive sleep apnea. J. Clin. Hypertens. 24, 1598–1605. doi: 10.1111/jch.14598
Lucking, E. F., O'connor, K. M., Strain, C. R., Fouhy, F., Bastiaanssen, T. F. S., Burns, D. P., et al. (2018). Chronic intermittent hypoxia disrupts cardiorespiratory homeostasis and gut microbiota composition in adult male guinea-pigs. EBioMedicine 38, 191–205. doi: 10.1016/j.ebiom.2018.11.010
Lv, R., Liu, X., Zhang, Y., Dong, N., Wang, X., He, Y., et al. (2023). Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 8:218. doi: 10.1038/s41392-023-01496-3
Macey, P. M., Prasad, J. P., Ogren, J. A., Moiyadi, A. S., Aysola, R. S., Kumar, R., et al. (2018). Sex-specific hippocampus volume changes in obstructive sleep apnea. Neuroimage Clin. 20, 305–317. doi: 10.1016/j.nicl.2018.07.027
Macia, L., Tan, J., Vieira, A. T., Leach, K., Stanley, D., Luong, S., et al. (2015). Metabolite-sensing receptors gpr43 and gpr109a facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat. Commun. 6:6734. doi: 10.1038/ncomms7734
Martinez, F. O., Sica, A., Mantovani, A., and Locati, M. (2008). Macrophage activation and polarization. Front. Biosci. 13, 453–461. doi: 10.2741/2692
Mcgettrick, A. F., and O’Neill, L. A. J. (2020). The role of hif in immunity and inflammation. Cell Metab. 32, 524–536. doi: 10.1016/j.cmet.2020.08.002
Morais, L. H., Schreiber, H. L., and Mazmanian, S. K. (2021). The gut microbiota–brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 19, 241–255. doi: 10.1038/s41579-020-00460-0
Moreno-Indias, I., Torres, M., Montserrat, J. M., Sanchez-Alcoholado, L., Cardona, F., Tinahones, F. J., et al. (2015). Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur. Respir. J. 45, 1055–1065. doi: 10.1183/09031936.00184314
Morrell, M. J., Jackson, M. L., Twigg, G. L., Ghiassi, R., Mcrobbie, D. W., Quest, R. A., et al. (2010). Changes in brain morphology in patients with obstructive sleep apnoea. Thorax 65, 908–914. doi: 10.1136/thx.2009.126730
Motamedi, V., Kanefsky, R., Matsangas, P., Mithani, S., Jeromin, A., Brock, M. S., et al. (2018). Elevated tau and interleukin-6 concentrations in adults with obstructive sleep apnea. Sleep Med. 43, 71–76. doi: 10.1016/j.sleep.2017.11.1121
Nácher, M., Serrano-Mollar, A., Farré, R., Panés, J., Seguí, J., and Montserrat, J. M. (2007). Recurrent obstructive apneas trigger early systemic inflammation in a rat model of sleep apnea. Respir. Physiol. Neurobiol. 155, 93–96. doi: 10.1016/j.resp.2006.06.004
Nadeem, R., Molnar, J., Madbouly, E. M., Nida, M., Aggarwal, S., Sajid, H., et al. (2013). Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J. Clin. Sleep Med. 9, 1003–1012. doi: 10.5664/jcsm.3070
Naëgelé, B., Launois, S. H., Mazza, S., Feuerstein, C., Pépin, J.-L., and Lévy, P. (2006). Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep 29, 533–544. doi: 10.1093/sleep/29.4.533
Nair, D., Dayyat, E. A., Zhang, S. X., Wang, Y., and Gozal, D. (2011a). Intermittent hypoxia-induced cognitive deficits are mediated by NADPH oxidase activity in a murine model of sleep apnea. PLoS One 6:e19847. doi: 10.1371/journal.pone.0019847
Nair, D., Zhang, S. X., Ramesh, V., Hakim, F., Kaushal, N., Wang, Y., et al. (2011b). Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase-dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 184, 1305–1312. doi: 10.1164/rccm.201107-1173OC
Nakayama-Ashida, Y., Takegami, M., Chin, K., Sumi, K., Nakamura, T., Takahashi, K.-I., et al. (2008). Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep 31, 419–425. doi: 10.1093/sleep/31.3.419
Neelapu, B. C., Kharbanda, O. P., Sardana, H. K., Balachandran, R., Sardana, V., Kapoor, P., et al. (2017). Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: a systematic review and meta-analysis of cephalometric studies. Sleep Med. Rev. 31, 79–90. doi: 10.1016/j.smrv.2016.01.007
Neuzeret, P.-C., Gormand, F., Reix, P., Parrot, S., Sastre, J.-P., Buda, C., et al. (2011). A new animal model of obstructive sleep apnea responding to continuous positive airway pressure. Sleep 34, 541–548. doi: 10.1093/sleep/34.4.541
Ohga, E., Tomita, T., Wada, H., Yamamoto, H., Nagase, T., and Ouchi, Y. (2003). Effects of obstructive sleep apnea on circulating icam-1, il-8, and mcp-1. J. Appl. Physiol. 94, 179–184. doi: 10.1152/japplphysiol.00177.2002
Orrù, G., Storari, M., Scano, A., Piras, V., Taibi, R., and Viscuso, D. (2020). Obstructive sleep apnea, oxidative stress, inflammation and endothelial dysfunction-an overview of predictive laboratory biomarkers. Eur. Rev. Med. Pharmacol. Sci. 24, 6939–6948. doi: 10.26355/eurrev_202006_21685
Pan, W., and Kastin, A. J. (2014). Leptin: A biomarker for sleep disorders? Sleep Med. Rev. 18, 283–290. doi: 10.1016/j.smrv.2013.07.003
Parada Venegas, D., De La Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10:277. doi: 10.3389/fimmu.2019.00277
Park, J., Kim, M., Kang, S. G., Jannasch, A. H., Cooper, B., Patterson, J., et al. (2015). Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR–S6K pathway. Mucosal Immunol. 8, 80–93. doi: 10.1038/mi.2014.44
Peñafiel, F. S., Brockmann, V. P., Santín Martínez, J., Fuentes-López, E., Leiva, R. I., and Valdivia, C. G. (2019). Performance of sleep questionnaires for the diagnosis of obstructive sleep apnea syndrome. Rev. Med. Chile 147, 1543–1552. doi: 10.4067/S0034-98872019001201543
Pevernagie, D. A., Gnidovec-Strazisar, B., Grote, L., Heinzer, R., Mcnicholas, W. T., Penzel, T., et al. (2020). On the rise and fall of the apnea−hypopnea index: a historical review and critical appraisal. J. Sleep Res. 29:e13066. doi: 10.1111/jsr.13066
Poroyko, V. A., Carreras, A., Khalyfa, A., Khalyfa, A. A., Leone, V., Peris, E., et al. (2016). Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci. Rep. 6:35405. doi: 10.1038/srep35405
Puech, C., Badran, M., Barrow, M. B., Runion, A. R., and Gozal, D. (2023). Solriamfetol improves chronic sleep fragmentation-induced increases in sleep propensity and ameliorates explicit memory in male mice. Sleep 46:zsad057. doi: 10.1093/sleep/zsad057
Puech, C., Badran, M., Runion, A. R., Barrow, M. B., Qiao, Z., Khalyfa, A., et al. (2022). Explicit memory, anxiety and depressive like behavior in mice exposed to chronic intermittent hypoxia, sleep fragmentation, or both during the daylight period. Neurobiol. Sleep Circadian Rhythms 13:100084. doi: 10.1016/j.nbscr.2022.100084
Qiu, J., Heller, J. J., Guo, X., Chen, Z.-M. E., Fish, K., Fu, Y.-X., et al. (2012). The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36, 92–104. doi: 10.1016/j.immuni.2011.11.011
Qiu, X., Li, L., Wei, J., An, X., Ampadu, J. A., Zheng, W., et al. (2023). The protective role of nrf2 on cognitive impairment in chronic intermittent hypoxia and sleep fragmentation mice. Int. Immunopharmacol. 116:109813. doi: 10.1016/j.intimp.2023.109813
Ramesh, V., Nair, D., Zhang, S. X., Hakim, F., Kaushal, N., Kayali, F., et al. (2012). Disrupted sleep without sleep curtailment induces sleepiness and cognitive dysfunction via the tumor necrosis factor-α pathway. J. Neuroinflammation 9:91. doi: 10.1186/1742-2094-9-91
Rivière, A., Selak, M., Lantin, D., Leroy, F., and De Vuyst, L. (2016). Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front. Microbiol. 7:979. doi: 10.3389/fmicb.2016.00979
Rooks, M. G., and Garrett, W. S. (2016). Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 16, 341–352. doi: 10.1038/nri.2016.42
Sapin, E., Peyron, C., Roche, F., Gay, N., Carcenac, C., Savasta, M., et al. (2015). Chronic intermittent hypoxia induces chronic low-grade neuroinflammation in the dorsal hippocampus of mice. Sleep 38, 1537–1546. doi: 10.5665/sleep.5042
Schiefer, M., Gamble, J., Baskin, J., and Strohl, K. (2020). Hypoglossal nerve stimulation in a rabbit model of obstructive sleep apnea reduces apneas and improves oxygenation. J. Appl. Physiol. 129, 442–448. doi: 10.1152/japplphysiol.00828.2019
Schiza, S. E., Mermigkis, C., Panagiotis, P., Bouloukaki, I., Kallergis, E., Tzanakis, N., et al. (2010). C-reactive protein evolution in obstructive sleep apnoea patients under CPAP therapy. Eur. J. Clin. Investig. 40, 968–975. doi: 10.1111/j.1365-2362.2010.02348.x
Shahar, E. (2014). Apnea-hypopnea index: time to wake up. Nat. Sci. Sleep 6, 51–56. doi: 10.2147/nss.S61853
Shi, Y., Guo, X., Zhang, J., Zhou, H., Sun, B., and Feng, J. (2018). DNA binding protein HMGB1 secreted by activated microglia promotes the apoptosis of hippocampal neurons in diabetes complicated with OSA. Brain Behav. Immun. 73, 482–492. doi: 10.1016/j.bbi.2018.06.012
Shieu, M. M., Dunietz, G. L., Paulson, H. L., Chervin, R. D., and Braley, T. J. (2022). The association between obstructive sleep apnea risk and cognitive disorders: a population-based study. J. Clin. Sleep Med. 18, 1177–1185. doi: 10.5664/jcsm.9832
Shu, Y., Liu, X., Yu, P., Li, H., Duan, W., Wei, Z., et al. (2022). Inherent regional brain activity changes in male obstructive sleep apnea with mild cognitive impairment: a resting-state magnetic resonance study. Front. Aging Neurosci. 14:1022628. doi: 10.3389/fnagi.2022.1022628
Smith, S. M., Friedle, S. A., and Watters, J. J. (2013). Chronic intermittent hypoxia exerts CNS region-specific effects on rat microglial inflammatory and TLR4 gene expression. PLoS One 8:e81584. doi: 10.1371/journal.pone.0081584
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly, Y. M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Snyder, B., Shell, B., Cunningham, J. T., and Cunningham, R. L. (2017). Chronic intermittent hypoxia induces oxidative stress and inflammation in brain regions associated with early-stage neurodegeneration. Physiol. Rep. 5:e13258. doi: 10.14814/phy2.13258
Sozer, V., Kutnu, M., Atahan, E., Calıskaner Ozturk, B., Hysi, E., Cabuk, C., et al. (2018). Changes in inflammatory mediators as a result of intermittent hypoxia in obstructive sleep apnea syndrome. Clin. Respir. J. 12, 1615–1622. doi: 10.1111/crj.12718
Steiropoulos, P., Kotsianidis, I., Nena, E., Tsara, V., Gounari, E., Hatzizisi, O., et al. (2009). Long-term effect of continuous positive airway pressure therapy on inflammation markers of patients with obstructive sleep apnea syndrome. Sleep 32, 537–543. doi: 10.1093/sleep/32.4.537
Svatikova, A., Wolk, R., Shamsuzzaman, A. S., Kara, T., Olson, E. J., and Somers, V. K. (2003). Serum amyloid a in obstructive sleep apnea. Circulation 108, 1451–1454. doi: 10.1161/01.Cir.0000089091.09527.B8
Tan, A., Cheung, Y. Y., Yin, J., Lim, W.-Y., Tan, L. W. L., and Lee, C. H. (2016). Prevalence of sleep-disordered breathing in a multiethnic asian population in Singapore: a community-based study. Respirology 21, 943–950. doi: 10.1111/resp.12747
Tang, M. X., Li, S., Wei, L., Hou, Z. H., Qu, J., and Li, L. (2021). Do engineered nanomaterials affect immune responses by interacting with gut microbiota? Front. Immunol. 12:11. doi: 10.3389/fimmu.2021.684605
Tegeler, C., O'sullivan, J. L., Bucholtz, N., Goldeck, D., Pawelec, G., Steinhagen-Thiessen, E., et al. (2016). The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the berlin aging study II. Neurobiol. Aging 38, 112–117. doi: 10.1016/j.neurobiolaging.2015.10.039
Teh, J. Z., Grummitt, L., Haroutonian, C., Cross, N. E., Skinner, B., Bartlett, D. J., et al. (2023). Overnight declarative memory consolidation and non-rapid eye movement sleep electroencephalographic oscillations in older adults with obstructive sleep apnea. Sleep 46:zsad087. doi: 10.1093/sleep/zsad087
Tichanon, P., Wilaiwan, K., Sopida, S., Orapin, P., Watchara, B., and Banjamas, I. (2016). Effect of continuous positive airway pressure on airway inflammation and oxidative stress in patients with obstructive sleep apnea. Can. Respir. J. 2016:3107324. doi: 10.1155/2016/3107324
Turnbaugh, P. J., Ley, R. E., Mahowald, M. A., Magrini, V., Mardis, E. R., and Gordon, J. I. (2006). An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031. doi: 10.1038/nature05414
Valentini, F., Evangelisti, M., Arpinelli, M., Di Nardo, G., Borro, M., Simmaco, M., et al. (2020). Gut microbiota composition in children with obstructive sleep apnoea syndrome: a pilot study. Sleep Med. 76, 140–147. doi: 10.1016/j.sleep.2020.10.017
Vanek, J., Prasko, J., Genzor, S., Ociskova, M., Kantor, K., Holubova, M., et al. (2020). Obstructive sleep apnea, depression and cognitive impairment. Sleep Med. 72, 50–58. doi: 10.1016/j.sleep.2020.03.017
Veasey, S. C., Lear, J., Zhu, Y., Grinspan, J. B., Hare, D. J., Wang, S., et al. (2013). Long-term intermittent hypoxia elevates cobalt levels in the brain and injures white matter in adult mice. Sleep 36, 1471–1481. doi: 10.5665/sleep.3038
Veasey, S. C., and Rosen, I. M. (2019). Obstructive sleep apnea in adults. N. Engl. J. Med. 380, 1442–1449. doi: 10.1056/NEJMcp1816152
Wang, G., Huang, S., Wang, Y., Cai, S., Yu, H., Liu, H., et al. (2019). Bridging intestinal immunity and gut microbiota by metabolites. Cell. Mol. Life Sci. 76, 3917–3937. doi: 10.1007/s00018-019-03190-6
Wang, F., Liu, Q., Wu, H., Tang, T., Zhao, T., and Li, Z. (2022). The dysbiosis gut microbiota induces the alternation of metabolism and imbalance of Th17/Treg in OSA patients. Arch. Microbiol. 204:217. doi: 10.1007/s00203-022-02825-w
Wang, H., Lu, Y., Yan, Y., Tian, S., Zheng, D., Leng, D., et al. (2019). Promising treatment for type 2 diabetes: fecal microbiota transplantation reverses insulin resistance and impaired islets. Front. Cell. Infect. Microbiol. 9:455. doi: 10.3389/fcimb.2019.00455
Wei, Y., Liu, Y., Ayas, N., and Laher, I. (2022). A narrative review on obstructive sleep apnea in China: a sleeping giant in disease pathology. Heart Mind 6, 232–241. doi: 10.4103/hm.hm_49_22
Wrzosek, L., Miquel, S., Noordine, M.-L., Bouet, S., Chevalier-Curt, M. J., Robert, V., et al. (2013). Bacteroides thetaiotaomicron and Faecalibacterium prausnitzii influence the production of mucus glycans and the development of goblet cells in the colonic epithelium of a gnotobiotic model rodent. BMC Biol. 11:61. doi: 10.1186/1741-7007-11-61
Wu, K. M., Lin, C. C., Chiu, C. H., and Liaw, S. F. (2010). Effect of treatment by nasal continuous positive airway pressure on serum high mobility group box-1 protein in obstructive sleep apnea. Chest 137, 303–309. doi: 10.1378/chest.09-0936
Xia, W., Jing, Y., and Yuan, C. (2023). Correlation between obstructive sleep apnea syndrome (OSAS) and cognitive dysfunction in elderly patients with hypertension. J. Integr. Neurosci. 22:83. doi: 10.31083/j.jin2204083
Yan, Y., Zheng, X., Liu, G., Shi, G., Li, C., Chen, H., et al. (2024). Gut microbiota-derived cholic acid mediates neonatal brain immaturity and white matter injury under chronic hypoxia. iScience 27:109633. doi: 10.1016/j.isci.2024.109633
Yokoe, T., Minoguchi, K., Matsuo, H., Oda, N., Minoguchi, H., Yoshino, G., et al. (2003). Elevated levels of C-reactive protein and interleukin-6 in patients with obstructive sleep apnea syndrome are decreased by nasal continuous positive airway pressure. Circulation 107, 1129–1134. doi: 10.1161/01.cir.0000052627.99976.18
Yu, M. S., Jung, N. R., Choi, K. H., Choi, K., Lee, B. J., and Chung, Y. S. (2014). An animal model of obstructive sleep apnea in rabbit. Laryngoscope 124, 789–796. doi: 10.1002/lary.24398
Zheng, D., Liwinski, T., and Elinav, E. (2020). Interaction between microbiota and immunity in health and disease. Cell Res. 30, 492–506. doi: 10.1038/s41422-020-0332-7
Zhu, Y., Fenik, P., Zhan, G., Mazza, E., Kelz, M., Aston-Jones, G., et al. (2007). Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J. Neurosci. 27, 10060–10071. doi: 10.1523/jneurosci.0857-07.2007
Keywords: obstructive sleep apnea, intermittent hypoxia, systematic inflammation, neurocognitive dysfunction, gut microbiota
Citation: Tang M, Wu Y, Liang J, Yang S, Huang Z, Hu J, Yang Q, Liu F and Li S (2024) Gut microbiota has important roles in the obstructive sleep apnea-induced inflammation and consequent neurocognitive impairment. Front. Microbiol. 15:1457348. doi: 10.3389/fmicb.2024.1457348
Received: 30 June 2024; Accepted: 13 November 2024;
Published: 06 December 2024.
Edited by:
Jin Song, Capital Medical University, ChinaReviewed by:
Svetlana Tomic, Osijek Clinical Hospital Center, CroatiaCopyright © 2024 Tang, Wu, Liang, Yang, Huang, Hu, Yang, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingxing Tang, VGFuZ3N0YXIyMDEzQDE2My5jb20=; Fei Liu, bGl1ZmVpMjAwNHBoaWxAMTI2LmNvbQ==; Shuo Li, c2h1b2xpQGVtYWlsLnN6dS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.