- 1Department of Pediatrics, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 2Clinical Research Service Center, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
- 3Department of Orthopedics, Affiliated Hospital of Guangdong Medical University, Zhanjiang, China
Objective: The aim of this study is to comprehensively investigate the temporal dynamics of faecal gut microbiota and metabonomics in early postnatal with a focus on very low or extremely low birth weight (VLBW/ELBW) infants.
Methods: We collected faecal samples from 157 VLBW/ELBW infants at three time points: days 1, 14, and 28 in a prospective cohort study. The faecal microbial diversity, abundance, composition, and metabolomic analyses were determined using 16S rRNA sequencing and liquid chromatography tandem mass spectrometry (LC-MS/MS). Microbiome functional analyses were conducted utilizing PICRUSt2. The ecological association networks were employed to investigate the interactions between gut microbiota and identify the core genus within 28 days of birth, as well as to unveil correlations between taxa and metabolites.
Result: (1) The alpha diversity of gut microbiota significantly decreased from D1 to D28, accompanied by an interrupted trajectory lacking obligate anaerobes. At the phylum level, the 16S RNA sequencing results showed an increase in Proteobacteria and a decrease in Firmicutes and Bacteroidota from D1 to D28. At the genus level, there was a decrease in the relative abundance of Staphylococcus, Acinetobacter and Ureaplasma, with Klebsiella and Enterococcus emerging as the most abundant genera. (2) The analysis revealed a total of 561 metabolic markers that exhibited significant and distinct alterations between D1 and D14. (3) Ecological association networks revealed that the gut microbiota in D1 exhibited a significantly higher degree of microbial interactions compared to those in D14 and D28. Additionally, Enterococcus, Klebsiella, and Enterobacter were major contributors to the co-occurring network at these three time points. (4) Steroid hormone biosynthesis, including tetrahydrocortisone, androsterone glucuronide, androstenedione and etiocholanolone glucuronide, decreased within 28 days after birth.
Conclusion: We have successfully demonstrated a significant dysbiosis in the gut microbiota and a subsequent decrease in its diversity within 4 weeks postpartum in VLBW/ELBW infants. Monitoring the gut microbiota of VLBW/ELBW infants and promptly rectifying dysbiosis in the early stages may represent a potential therapeutic strategy.
Introduction
The gut microbiome, referred to as the “second genome” of humans, play a pivotal role in maintaining immune system function and overall health (Wan et al., 2020). Numerous studies have indicated that the gut microbiota actively participated in various physiological processes such as regulating host energy balance, supporting immune responses, managing energy metabolism, controlling inflammation, and promoting epithelial development (Han et al., 2022; Zhu et al., 2023). The stability and resilience of gut microbiota, however, can be influenced by various factors including dietary choices, living conditions, medication usage, illnesses, particularly the administration of antibiotics (Ho et al., 2020).
Numerous research studies consistently demonstrate a robust correlation between gut microbiota and diseases commonly observed in premature infants. Several investigations underscore the pivotal role of intestinal bacteria in the development of conditions such as necrotizing enterocolitis (NEC), early onset neonatal sepsis (EONS), feeding intolerance, and bronchopulmonary dysplasia (Collins et al., 2017; Hong et al., 2023; Jia et al., 2022). Our previous study findings indicated a significant imbalance in the composition of gut microbiota among premature infants with white matter injury. Specifically, certain groups of microorganisms such as Bacteroidetes, Staphylococcus, and Acinetobacter potentially contributes to impairing the brain’s white matter through downregulation of the bile acid biosynthesis pathway (Liu et al., 2023). A considerable proportion of premature infants in the neonatal intensive care unit (NICU) are administered antibiotics shortly after birth, potentially leading to alterations in the early-life development of their gut microbiome and impacting various metabolic pathways (Bender et al., 2021; Russell et al., 2021). The gut microbial composition of preterm babies may exhibit increased vulnerability to disruption caused by external factors such as postnatal antibiotic administration, mode of delivery, and feeding (Wang et al., 2020).
While previous longitudinal studies had documented the dynamic changes in gut microbiota among preterm infants of varying gestational age, conflicting findings had emerged (Hong et al., 2023; Jia et al., 2022). Our study also identified distinct characteristics in the faecal microbiome and metabolome of preterm infants compared to term infants, particularly those born before 32 weeks. The early postnatal period for VLBW/ELBW infants represents a unique biological process wherein disruptions in microbial colonization can have long-lasting and potentially irreversible effects on ecological succession events. However, there has been limited research conducted on the dynamic changes of gut microbiome and critical metabolites derived from gut microbiota in VLBW/ELBW infants during the early postnatal period. In this study, we aimed to investigate the sequential alterations in gut microbiota and associated metabolites of VLBW/ELBW infants within 28 days after birth. Additionally, we analyzed changes in the diversity of gut microbiota and investigate the crosstalk between gut microbiota and faecal metabolomic dynamics. Monitoring the gut microbiota of VLBW/ELBW infants and promptly rectifying dysbiosis in the early stages may represent a potential therapeutic strategy.
Methods
Participants and sample collection
The present observational cohort study was conducted at Affiliated Hospital of Guangdong Medical University between January 2022 and December 2023. Prior to sample collection, written informed consent was obtained from the guardians of each infant. The study included infants who met the following criteria: (1) gestational age at birth <32 weeks; (2) birth weight <1.5 kg; (3) admission within 24 h after birth. Participants were excluded if they met any of the following criteria: (1) death; (2) patients with congenital chromosomal abnormalities or genetic metabolic diseases; (3) use of microecological preparations such as probiotics, prebiotics and biostime.
The meconium was collected within 24 h after birth, and faecal samples were also collected on days 14 and 28 postpartum, named as D1, D14 and D28, respectively. The specific details can be found in Table 1. Faecal samples were obtained from the diapers of three different location using sterile tubes, with each tube containing approximately 0.2 g (similar in size to a soybean). All collected samples were promptly sealed, labeled, and subsequently stored at −80°C in the laboratory for genetic analysis.
DNA extraction, library preparation and sequencing
The E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, United States) was utilized to extract genomic DNA from the samples following the manufacturer’s instructions. The ABI GeneAmp® 9700 PCR thermocycler (ABI, CA, United States) was employed for amplifying the hypervariable region V3–V4 of the bacterial 16S rRNA gene using primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The amplification process involved an initial denaturation step at 95°C for 3 min, followed by a series of cycles consisting of denaturing at 95°C for 30 s, annealing at 55°C for 30 s and extension at 72°Cfor 45 s. A final single extension step was performed at 72°C for a duration of 10 min before concluding the process with cooling down to 4°C.
The amplicons were purified and combined in equal amounts before undergoing paired-end sequencing on an Illumina MiSeq PE300 platform (Illumina, San Diego, United States) following standard protocols provided by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China). The resulting raw reads have been deposited into the NCBI Sequence Read Archive (SRA) database under the BioProject ID PRJNA1101213.
Species annotation
The taxonomic classification of each Operational Taxonomic Unit (OTU) representative sequence was assessed using RDP Classifier version 2.2, against the 16S rRNA gene database (e.g., Silva v138), with a confidence threshold set at 0.7. The analysis of gut microbiota’s bioinformatics was conducted on the Majorbio Cloud platform.1 Utilizing information from OTUs, we calculated rarefaction curves and alpha diversity indices such as observed OTUs, Chao1 richness, and Shannon index using Mothur v1.30.1 (Schloss et al., 2009). To examine beta diversity, principal coordinate analysis (PCoA) based on Bray–Curtis dissimilarity measure was performed employing the Vegan v2.5-3 package. The Linear discriminant analysis Effect Size (LEfSE) method was employed with an LDA score threshold of 4.0 to identify significant biomarkers across different taxonomic levels, ranging from phylum to genus level. Additionally, we assessed the gut microbiome health index (GMHI) and gut microbiota health disorder index (MDI). The network diagram was constructed based on Spearman correlation analysis, considering species interactions in the sample with a correlation coefficient value ≥0.5 and p < 0.05. Moreover, function prediction was performed using PICRUSt2.0 software (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States). Furthermore, the microbial ecological network was analyzed using Networkx (version v1.11) through Spearman correlation analysis.
Metabolite extraction and LC-MS/MS analysis
A 50 mg sample was introduced into a centrifuge tube with a capacity of 2 mL, followed by the addition of a grinding bead measuring 6 mm in diameter. For metabolite extraction, an extraction solution consisting of methanol and water in a ratio of 4:1 (v:v) was used, containing an internal standard (L-2-chlorophenylalanine) at a concentration of 0.02 mg/mL. The samples were subjected to freezing at −20°C for a duration of 30 min, followed by centrifugation at 13,000 g and 4°C for 15 min. Subsequently, the supernatant was transferred to an injection vial for LC-MS/MS analysis.
The LC-MS/MS analysis of the sample was conducted using a Thermo UHPLC-Q Exactive HF-X system equipped with an ACQUITY HSS T3 column (100 mm × 2.1 mm i.d., 1.8 μm, Waters, United States) at Majorbio Bio-Pharm Technology Co., Ltd. in Shanghai, China. The Progenesis QI software was utilized for pretreating the LC/MS raw data, resulting in a three-dimensional data matrix exported in CSV format. Additionally, metabolites were identified through database searches primarily utilizing HMDB,2 Metlin,3 and Majorbio Database resources. Subsequently, the R package “ropls” (Version 1.6.2) was employed to conduct principal component analysis (PCA) and orthogonal least partial squares discriminant analysis (OPLS-DA). Furthermore, a 7-cycle interactive validation approach was implemented to evaluate the model’s stability.
The significantly different metabolites were identified based on their Variable Importance in the Projection (VIP) values >1 and p < 0.05. Metabolic enrichment analysis, followed by Benjamini–Hochberg correction and pathway analysis using the KEGG database,4 was performed to classify these differential metabolites into their respective biochemical pathways. Enrichment analysis using the Python package “scipy.stats”5 was conducted to identify the most relevant biological pathways associated with the experimental treatments. Spearman correlation coefficient (≥0.6, p < 0.5) was utilized for network analysis to establish connections between metabolites and species variables.
Bioinformatics and statistical analysis
The data analysis was performed using IBM SPSS Statistics 26 and R software (Version 2.15.3). Categorical variables were expressed as frequencies and percentages [n (%)], while continuous variables, whether normally or non-normally distributed, were presented as means ± standard deviation or medians with interquartile ranges (IQR). The normality of distribution comparisons between groups was assessed using the Student’s t-test. For groups with non-normal distributions, such as alpha diversity, relative abundance of bacteria, GMHI, and MDI, either the Mann–Whitney U test or the Wilcoxon rank sum test was employed for analysis. The differences in categorical data were assessed using statistical tests such as chi-squared or Fisher’s exact test. Furthermore, the association between gut microbiota at the genus level and metabolite parameters was evaluated through the Spearman correlation coefficient test. Statistical significance was determined based on a two-sided p-value threshold of less than 0.05. *p < 0.05, **p < 0.01, and ***p < 0.001.
Results
Study cohort description
During the period from January 2022 to December 2023, a total of 173 eligible subjects were enrolled at the Affiliated Hospital of Guangdong Medical University. After excluding cases involving 16 deaths or discontinuation of treatment by their families, the study ultimately included 157 individuals. The baseline characteristics of these patients are summarized in Table 1. Among them, there were 87 (55.4%) male participants with an average gestational age of 30.4 ± 0.18 weeks and an average birth weight of 1.27 ± 0.018 kg upon admission.
Alterations in the gut microbiota’s diversity and composition over a period of time
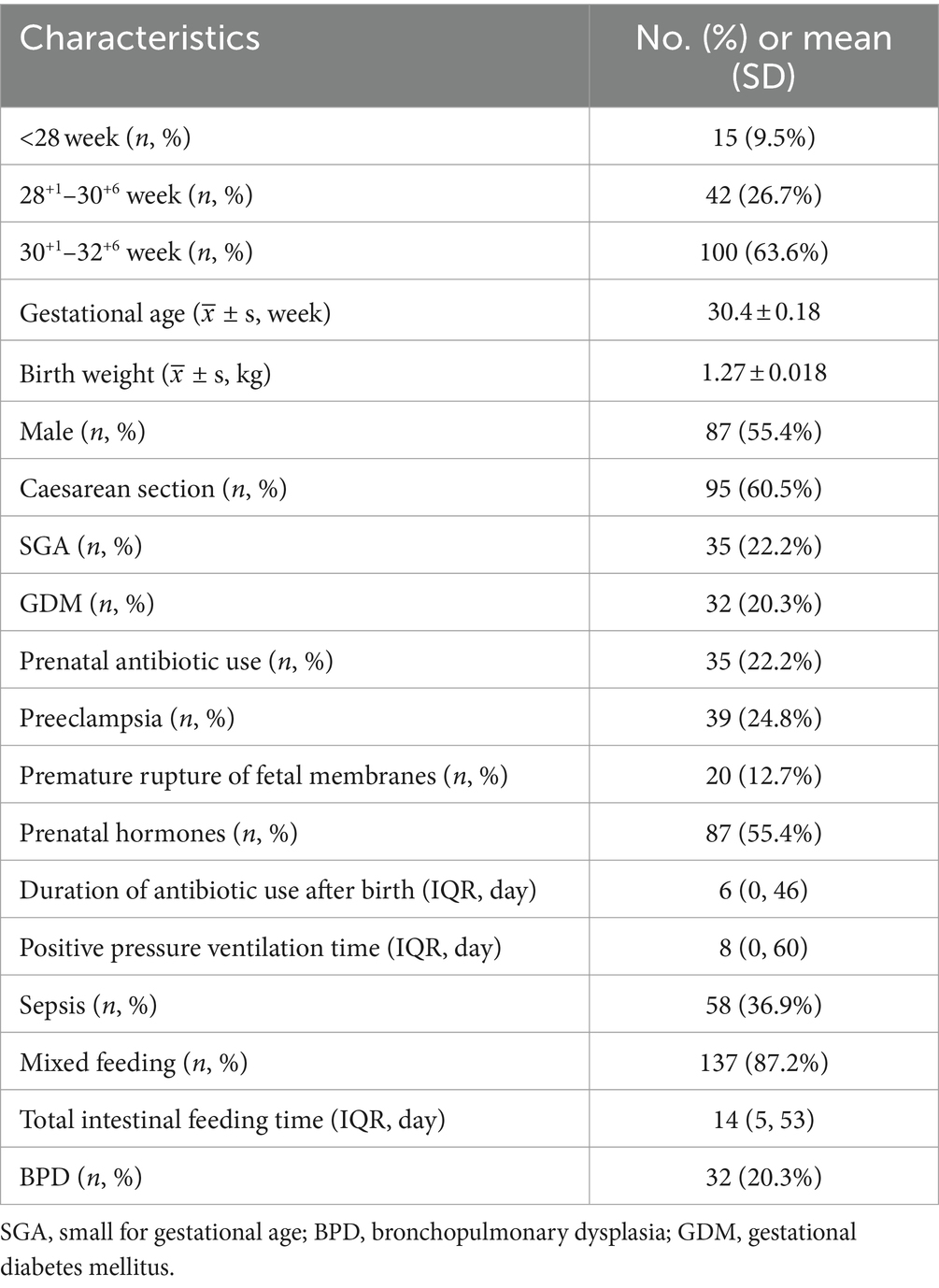
The alpha diversity indices, including Chao, Shannon, and ACE indices, exhibited a significant distinction (Wilcoxon test, p < 0.05; Figure 1A; Supplementary Figure S1A), indicating that the abundance and diversity of gut microbiota were notably higher in D1 compared to D14 and D28. The alpha diversity metrics did not show any significant variation at D14 and D28. The PCoA analysis based on Bray–Curtis distances revealed distinct segregation at the genus level over time, specifically at sampling timepoints D1, D14, and D28 (R2 = 0.056, p = 0.001; Figure 1B). The ANOSIM/Adonis test demonstrated a significant and substantial divergence in the overall community structure between time points D1 versus both D14 and D28 (p < 0.001; Supplementary Figures S1B–D), indicating that microbial composition at D1 differs from days 14 and 28. The diversity of VLBW/ELBW exhibited significant fluctuations, gradually decreasing postnatally.
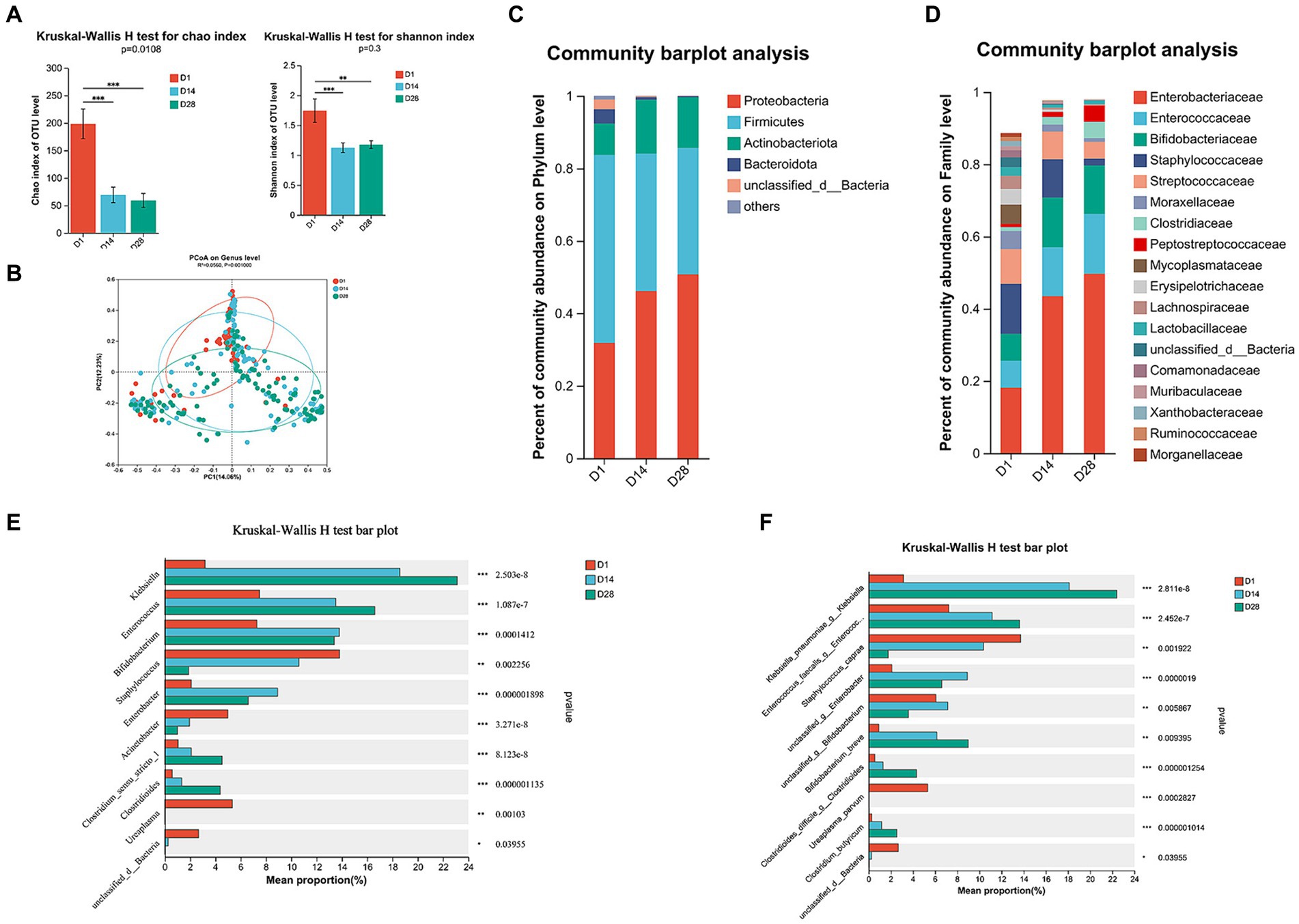
Figure 1. Temporal dynamics of gut microbiota composition through 16S rRNA gene sequencing. (A) The alpha diversity of gut microbiota was assessed using the Chao1 and Shannon indices. (B) The beta diversity of the gut microbiota was visualized by principal coordinates analysis (PCoA) plot based on Bray–Curtis analysis. (C) Relative abundance of microbial composition at the phylum level. (D) Family-level relative abundance of microbial composition. (E) Significant differences were observed in the genus-level composition of gut microbiota. (F) Abundance profiles at the species level were determined for all time points. Differences between groups were assessed using the Wilcoxon rank sum test. The p-value was corrected for multiple comparisons using the Benjamini–Hochberg method. *p < 0.05, **p < 0.01, and ***p < 0.001.
The temporal dynamics of gut microbiota composition were investigated across various taxonomic levels, ranging from phylum to genus. VLBW/ELBW infants exhibited a conspicuous absence of obligate anaerobes within the first 28 days after birth. Compared to D14 and D28, the relative abundance of Firmicutes, Bacteroidota, Fusobacteriota, and Patescibacteria at the phylum level was significantly increased in D1 (Figure 1C; Supplementary Figure S2A). Furthermore, there was an increase in the prevalence of Proteobacteria, the most dominant phylum, from D1 to D28. Conversely, Firmicutes, Bacteroidota and Fusobacteriota exhibited a decrease in relative abundances from D1 to D28 (Figure 1C; Supplementary Figures S2B,C). At the family level, temporal changes in the relative increased abundance dominant bacteria of Enterobacteriaceae and Enterococcaceae (≥30% relative abundance) were observed from D1 to D28. Furthermore, there was a tendency towards decreased proportions of Staphylococcaceae and Moraxellaceae over time (Figure 1D; Supplementary Figure S2D). Conversely, Bifidobacteriaceae exhibited a higher abundance in D14 and D28 compared to D1 (Figure 1D; Supplementary Figure S2D).
At the genus level, D1 exhibited a prevalence of Staphylococcus, Acinetobacter, Ureaplasma and Faecalibaculum. In contrast, D14 was characterized by the presence of Enterobacter genera. Additionally, D28 showed a high abundance of Clostridium_sensu_stricto_1 and Clostridioides (Figures 1E, 2B). During the period from D1 to D28, we noted a significant augmentation in the abundance of specific gut microbiota including Klebsiella, Enterococcus, Clostridium_sensu_stricto_1 and Clostridioides. In contrast, there was a notable decline in levels of Staphylococcus, Acinetobacter and Ureaplasma. The LEfSe analysis (Figure 2A) revealed that in D1, the most predominant bacterial taxa were o_Pseudomonadales, g_Acinetobacter, f_Lachnospiraceae, o_Lachnospirales and p_Bacteroidota (Figure 2A, LDA ≥4). Importantly, a higher abundance of g_Enterobacter was observed in D14, while increased abundances of g_Klebsiella, f_Enterococcaceae and g_Enterococcus were found in D28.
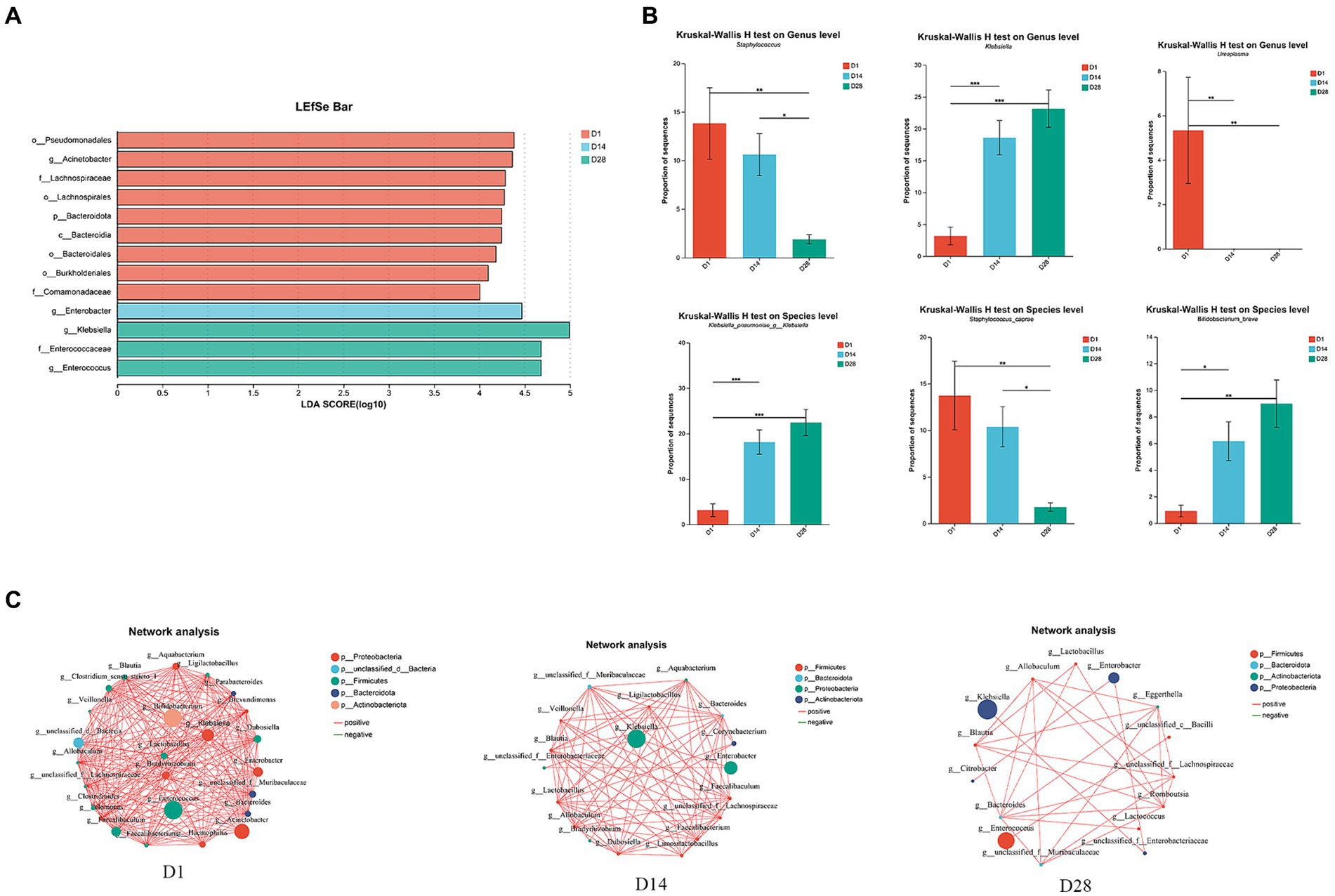
Figure 2. Overall variations in the gut microbiota across time points D1, D14, and D28. (A) LEfSe analysis was conducted to identify the highly significant microbiota, ranging from phylum to genus level, using a threshold of LDA (LDA ≥4). (B) The relative abundance of g__Staphylococcus, g__Klebsiella, Ureaplasma, Klebsiella_pneumoniae_g__Klebsiella, Staphylococcus_caprae, and Bifidobacterium breve varied significantly. (C) Ecological association networks were constructed to analyze the gut microbiota at the genus level on days 1, 14, and 28. Only p-values <0.05 and |r| ≥ 0.5 are displayed in the network. Each node represented a genus. Red line: positive correlation; green line: negative correlation. The number of connecting lines increases as the relationship between genera becomes closer. Each node is assigned a color according to its corresponding phylum.
Analysis of ecological association networks in gut microbiota
Ecological association networks primarily reflect species correlations across various taxonomic levels. Therefore, we conducted Spearman’s correlation analysis and constructed ecological association networks for the top 30 most abundant genera to elucidate the interactions between gut microbiota and identify the core genera within 28 days of birth (Figure 2C). The results revealed significant variations in the gut microbiota, with D1 exhibiting the highest level of microbial interactions compared to D14 and D28. Within the microbial community, Enterococcus, Bifidobacterium, Acinetobacter and Klebsiella emerged as dominant species in D1. Interestingly, both Klebsiella and Enterobacter demonstrated a substantial advantage in D14 and D28. During the early postnatal period, time-dependent changes were observed in the ecological association networks among gut microbiota. The co-occurrence network was primarily influenced by Enterococcus, Klebsiella and Enterobacter at three distinct timepoints (Figure 2C).
Temporal dynamics of faecal metabolomic profiles
To elucidate the metabolomic profile within 1 month postpartum, untargeted metabolomics analysis was performed on faecal samples in conjunction with 16S rRNA profiling. A total of 1,570 identifiable metabolites of positive iron and 1,086 identifiable metabolites of negative iron were determined based on a combination of statistically significant parameters from univariate analysis (p < 0.05) and multivariate analysis (VIP >1) with advancing gestation. The OPLS-DA analysis revealed a significant perturbation in the dynamic distributions of metabolomic data (p < 0.001), resulting in discernible variations in the overall faecal metabolic profiles (Figures 3A,B). A total of 561 faecal metabolites exhibited significant changes between D1 and D14, while 623 showed considerable alterations between D1 and D28. Furthermore, there were 201 metabolites that displayed notable differences between D14 and D28 (Figure 3C).
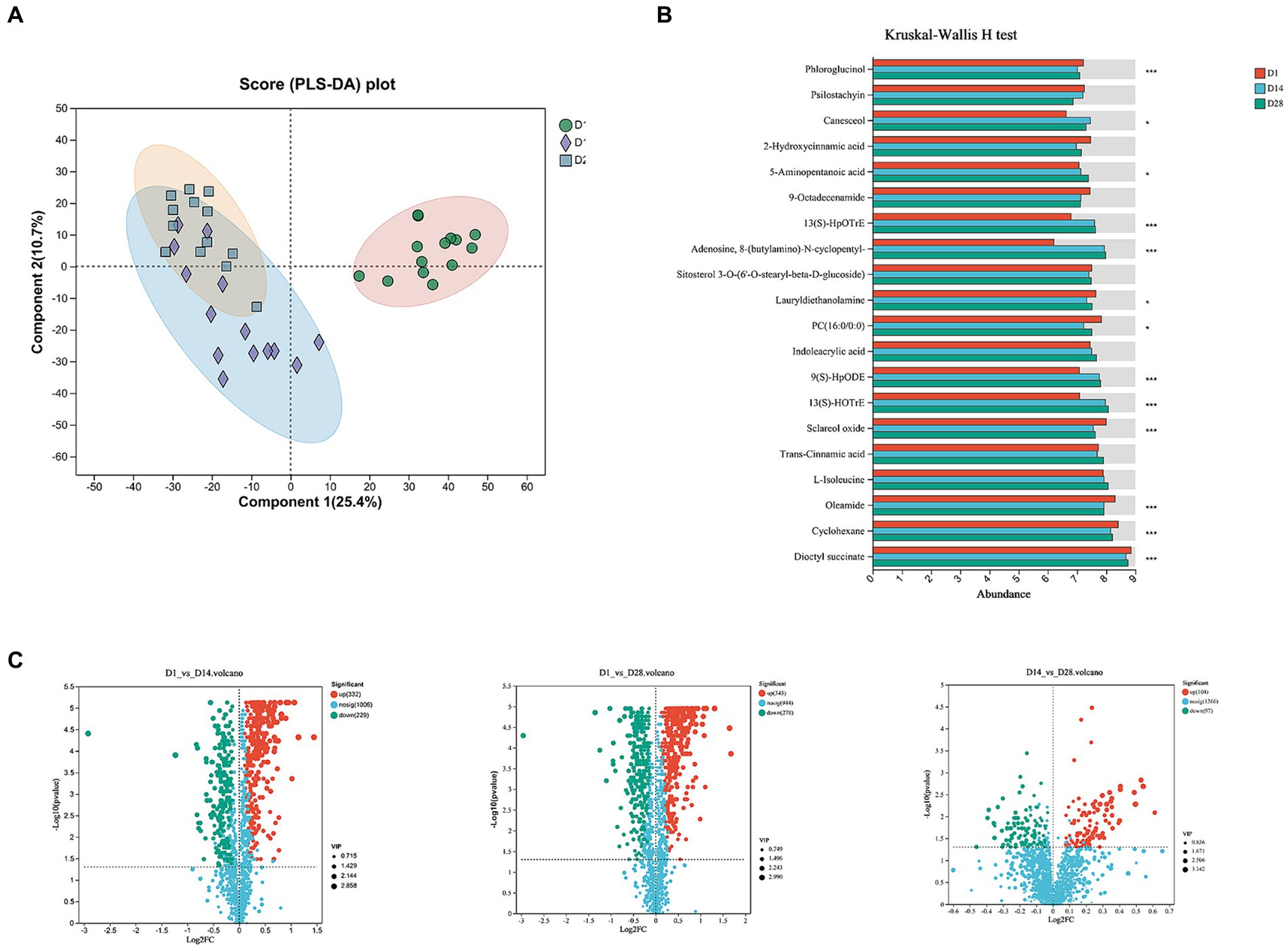
Figure 3. Temporal changes in faecal metabolites. (A) OPLS-DA score plot. (B) The histogram displayed the top 20 most abundant metabolites of significant importance. (C) The volcano plot illustrated different metabolites in positive ion compound: green indicates the down-regulated differential metabolite, while red represents the up-regulated differential metabolite.
Altered metabolic pathways and their biological significance
To investigate the alterations in metabolic pathways associated with differentially expressed metabolites and their biological functions, we performed an enrichment analysis using KEGG. The most prominent metabolic pathways identified included steroid hormone biosynthesis, caffeine metabolism, Pathways in cancer, prostate cancer, alpha-Linolenic acid metabolism, Linoleic acid metabolism, and GnRH secretion pathway (Figure 4A). KEGG enrichment analysis revealed significant alterations in 28 metabolic pathways between D1 and D14, with adjusted p-value <0.05. Compared to D14, downregulation of the GnRH signaling pathway, plant hormone signal transduction, endocrine resistance, linoleic acid metabolism, caffeine metabolism, and alpha-linolenic acid metabolism was observed in D1 (Figure 4B). Conversely, an upregulation of steroid hormone biosynthesis was evident in D1. The comparison between D1 and D28 revealed 45 perturbed metabolic pathways with significantly lower p-values and higher pathway impact (Figure 4C). These included down-regulated endocrine resistance, linoleic acid metabolism, alpha-linolenic acid metabolism, and caffeine metabolism; as well as up-regulated ovarian steroidogenesis and steroid hormone biosynthesis in D1 compared to D28. The metabolic pathways, including down-regulated tyrosine metabolism, butanoate metabolism, GABAergic synapse and arginine and proline metabolism, showed lower p-values and higher pathway impact in D14 compared to D28. Conversely, phototransduction and drug metabolism - other enzymes were up-regulated in D14 compared to D28 (Figure 4D). Overall, the metabolomics analysis revealed significant and programmed changes in both microbial community functions as gestation progressed.
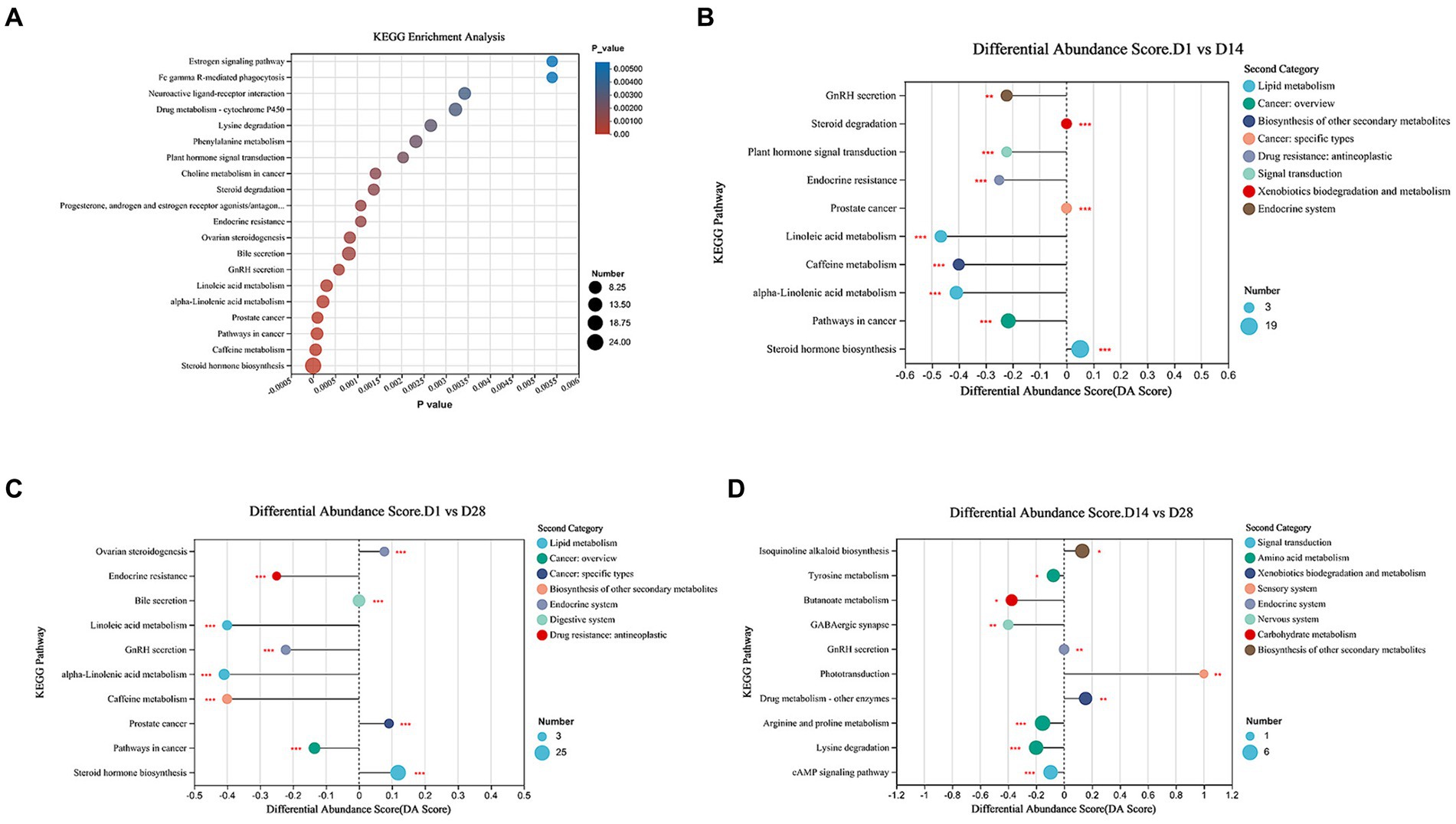
Figure 4. Metabolic pathways enrichment analysis. (A) Overview of metabolites that exhibited enrichment based on the KEGG database during early postnatal period. (B) Comparison of the top 10 significantly different metabolic pathways between D1 and D14. (C) Comparison of the top 10 significantly different metabolic pathways between D1 and D28 was conducted. (D) Comparison of top 10 significantly differential metabolic pathways between D14 and D28. The differential abundance (DA) score is represented on the X-axis, and the KEGG metabolic pathways are depicted on the Y-axis. A score of 1 signifies an up-regulated expression trend, whereas −1 indicates a down-regulated expression trend.
The gut microbiota correlates with faecal metabolites and inferred metabolic pathways
We conducted Spearman’s correlation analysis to investigate the potential association between gut bacteria and relative concentrations of faecal metabolites (Figure 5). The phylum-level analysis revealed that p_Bacteroidota and p_Verrucomicrobiota were the primary drivers of alterations in faecal metabolite concentrations. The abundances of p_Bacteroidota exhibited inverse correlations with the concentrations of faecal succinic acid, adenosine, 8-(butylamino)-N-cyclopentyl-, 12-hydroxyoctadecanoic acid and dodecylbenzenesulfonic acid (Figures 5A,C). The abundances of p_Bacteroidota exhibited positive correlations with the concentrations of faecal Taurochenodeoxycholate-3-sulfate, cyclohexane, oleamide and sapacitabine (Figures 5A,C). The abundances of p_Verrucomicrobiota exhibited inverse correlations with the concentrations of faecal adenosine, 8-(butylamino)-N-cyclopentyl-, (10Z,12E)-tetradecadienoylcarnitine and hydroxytetradecadienyl-l-carnitine (Figures 5A,C). Significantly, the genera Faecalibaculum and Dubosiella emerged as the primary drivers of alterations in faecal metabolite concentrations. Notably, variations in the microbiota exhibited a robust correlation with changes in lipid and lipid-like molecule levels. We investigated the associations between taxa and metabolites, revealing a negative correlation between the abundance of Faecalibaculum, Faecalibacterium and Dubosiella with the concentration of (10Z,12E)-tetradecadienoylcarnitine and 13-hydroxyhexadecanoylcarnitine. Additionally, we observed a negative correlation between the abundance of Faecalibaculum and adenosine, 8-(butylamino)-N-cyclopentyl- (Figures 5B,D).
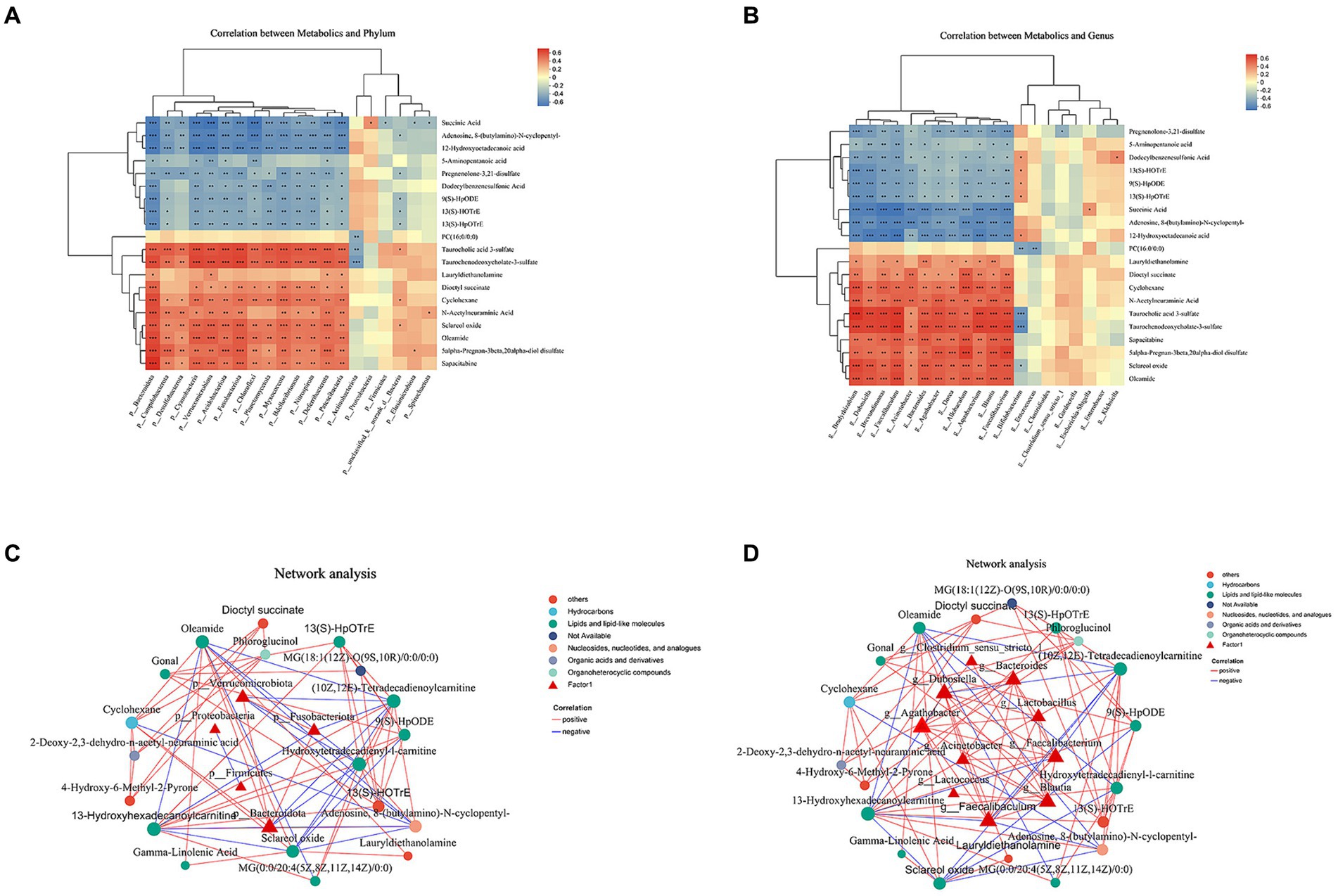
Figure 5. Interactions between the abundance of gut microbiota and faecal metabolites. (A) Heatmap illustrating the correlation coefficients between the top 20 identified metabolites in stool and the top 10 differentially abundant phyla. (B) Heatmap illustrating the repeated measures correlation coefficients between the top 20 identified metabolites in stool and the top 10 differentially abundant genera. Relevant correlations are denoted by a “+” symbol, accompanied by FDR-corrected p < 0.05. (C) Correlation network analysis of gut microbiota and faecal metabolites at the phylum level. (D) Correlation network analysis of gut microbiota and faecal metabolites at genus level.
Discussion
This finding holded great significance as it established a fundamental understanding of the pivotal role played by the overall gut ecosystem in VLBW/ELBW infants. By integrating 16S rRNA gene sequencing with LC-MS/MS metabolomics, we observed substantial fluctuations in both the alpha diversity and overall composition and metabolic function from day 1 to day 28. Furthermore, the ecological networks effectively captured the developmental trajectory of gut microbes from day 1 to day 28. The identification of these early indicators within the gut microbiota had provided innovative perspectives on tailoring treatment approaches for VLBW/ELBW infants.
The gut microbiota of preterm infants exhibits significant dissimilarities compared to those full-term, characterized by reduced microbial diversity and increased colonization of pathogenic microorganisms (Westaway et al., 2022; Zhang et al., 2022). Our study has demonstrated that the gut microbial richness and diversity of D1 were significantly higher than those of D14 and D28, and the diversity of VLBW/ELBW gradually decreased after birth. Based on these findings, we hypothesized that most VLBW/ELBW infants may have initiated prophylactic antimicrobial treatment within 14 days. The microbial diversity did not revert to the baseline levels within 28 days postpartum. Jia et al. (2022) discovered a higher alpha diversity of meconium in the extremely preterm group compared to the very preterm, moderate to late preterm, and term groups. Furthermore, our previous research also found that both ACE and Chao indices were significantly elevated in in less than 32 weeks gestational age group compared to term infant. The neonatal gut microbiota exhibits remarkable dynamism and undergoes evolutionary changes throughout the first 3 years of life (Hui et al., 2021).
All the analyses conducted at multiple levels consistently supported the hypothesis that there were significant shifts in signatures from D1 to D28. The analysis of our data revealed an observed increase in the prevalence of Proteobacteria within the gut microbiome of VLBW/ELBW infants from D1 to D28. In contrast, there was a decline in the relative abundances of Firmicutes, Bacteroidota, and Fusobacteriota during this time period. Furthermore, the high abundance of Proteobacteria had been proposed to signify dysbiosis-related microbial characteristics and reflected the unstable structure of the intestinal microbial community (Shin et al., 2015). Previous studies had demonstrated that the gut microbiota of all infants is initially dominated by Proteobacteria, which gradually decreased from birth up to 24 months of age, while Bacteroidetes and Firmicutes exhibited a gradual increase (Yap et al., 2021). The findings of our research, however, contradicted the prevailing notion. Our study necessitates an extended follow-up period to further validate the enduring alterations in the gut microbiota. Our previous study found that increased relative abundances of γ-proteobacteria and Escherichia-Shigella and decreased abundance of Bacteroides in meconium were associated with an increased risk of feeding intolerance in VLBW/ELBW infants (Liu et al., 2022).
Our findings suggested an increase in the abundance of specific gut microbiota, including Klebsiella, Enterococcus, Clostridium_sensu_stricto_1 and Clostridioides, from D1 to D28. Conversely, Staphylococcus, Acinetobacter and Ureaplasma demonstrated a decrease from D1 to D28. Our previous investigation had demonstrated that inhibiting the initial abnormal colonization of Staphylococcus and Acinetobacter in preterm neonates holded potential to enhance the efficacy of interventions aimed at preventing and managing white matter injury in premature infants (Liu et al., 2023). The presence of Klebsiella in faecal samples obtained from premature infants showed a significant increase within the first 2 weeks after birth (Yap et al., 2021). Significantly, the overgrowth of Klebsiella in the gastrointestinal tract exhibits a strong predictive value for brain injury (Seki et al., 2021). Our findings revealed a progressive increase in the abundance of Klebsiella_pneumoniae_g__Klebsiella, Enterococcus_faecalis_g__Enterococcus, Clostridioides_difficile_g__Clostridioides, and Bifidobacterium_breve as gestation advanced. Conversely, Staphylococcus_caprae and Ureaplasma_parvum exhibited a declining trend. The increased abundance of K. pneumoniae was found to be significantly associated with the risk of necrotizing enterocolitis (NEC) in preterm infants (Dobbler et al., 2017).
The ecological association networks exhibited a higher number of microbial correlations in D1 compared to D14 and D28. We further identified three types of facultative anaerobes, namely Enterococcus, Klebsiella and Enterobacter, as the primary contributors to the co-occurrence network at three different time points. Certain dominant genera identified in D1, such as Bifidobacterium and Acinetobacter, were absent at D14 and D28. The observed disparity in ecological networks indicates the instability of microflora in VLBW/ELBW infants. Enterococcus is recognized for its ability to express metalloproteases, thereby compromising the integrity of the intestinal barrier and facilitating translocation into the bloodstream in susceptible hosts, leading to inflammatory responses (Pan et al., 2024). Enterococcus, as a potential pathogen, facilitated infection in various sites such as the urinary tract, wounded epithelium, heart, and bloodstream due to its ability to survive in an antibiotic-rich harsh environment (Zhang R. et al., 2023). Jia et al. (2022) demonstrated variations in the duration of colonization by opportunistic pathogens such as Klebsiella and Enterococcus, with different dominant colonization patterns observed across gestational age groups. Newborn gut is initially characterized by aerobic conditions, which gradually transition to anaerobic environments. This transition facilitates the establishment of strict anaerobes such as Bifidobacterium, Clostridium, and Bacteroides during the process of gut colonization (Arrieta et al., 2014; Matamoros et al., 2013). However, no significant alteration was observed in VLBW/ELBW infants within the first 28 days. During the initial 28 days of life, the gut microbiota in VLBW/ELBW infants exhibited remarkable instability and potential pathogenicity.
The metabolic pathways in VLBW/ELBW infants exhibited a down-regulation of the GnRH signaling pathway, plant hormone signal transduction, endocrine resistance, linoleic acid metabolism, caffeine metabolism and alpha-Linolenic acid metabolism within 28 days of birth. Additionally, there is an up-regulation of steroid hormone biosynthesis. Steroid hormones are crucial for the regulation of hydration and sodium levels, metabolic processes, stress management, as well as the preservation of sexual differentiation and reproductive functions (Zhang Y. et al., 2023). The exposure of PM2.5 to the gastrointestinal tract induces systemic metabolic alterations by perturbing the gut microbiome and disrupting pathways involved in steroid hormone biosynthesis metabolism (Zhang Y. et al., 2023). In a predictive analysis, the potential carcinogenic pathways identified were microbial steroid hormone biosynthesis, mineral absorption, and retinol metabolism (Kalinen et al., 2024). From the perspective of host metabolism, Faecalibaculum and Dubosiella may play pivotal roles within a 28-day timeframe.
We explored correlations between taxa and metabolites, and found the abandunce of the Faecalibaculum, Faecalibacterium and Dubosiella was negatively correlated with the (10Z,12E)-tetradecadienoylcarnitine and 13-hydroxyhexadecanoylcarnitine concentration, and the abandunce of the Faecalibaculum was negatively correlated with Adenosine, 8-(butylamino)-N-cyclopentyl-. The involvement of Faecalibaculum has been implicated in the pathogenesis of diabetes and obesity (Yan et al., 2022). The study conducted by Li et al. (2022) demonstrated a positive correlation between the presence of Enterococcus, and Faecalibaculum species, as well as intestinal inflammation involving NF-κB and STAT3 signaling pathways.
Nevertheless, it is important to acknowledge certain constraints in our study. Firstly, the research was conducted solely at a single center, necessitating the need for validation across multiple cohorts. Secondly, the duration of sample collection in our study was limited to 28 days. Although high-throughput sequencing was utilized in this study, a more comprehensive analysis would require high-resolution shotgun metagenomic sequencing for greater accuracy and depth of information. Another limitation lied in the lack of detailed documentation regarding feeding practices (breastfeeding, formula, or mixed) and specific administration of antibiotics among preterm infants. However, larger cohorts would potentially unveil additional associations between microbes and metabolites. In the future, we aim to further extend both the follow-up duration and sample size.
Conclusion
This study elucidated temporal dynamics in the gut microbiota and faecal metabolites during the first 28 days after birth of VLBW/ELBW infants. In addition to distinct dynamics of gut microbiota, the metabolite profiles exhibited significant alterations from D1 to D28, emphasizing the necessity for tailored microbiota-associated treatments during different postnatal periods.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, BioProject: PRJNA1101213.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Affiliated Hospital of Guangdong Medical University (PJ2021-097). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
LL: Writing – original draft, Funding acquisition, Conceptualization. CC: Writing – original draft, Methodology, Investigation, Formal analysis, Data curation. YL: Writing – original draft, Validation, Supervision, Software, Project administration. DA: Writing – review & editing, Data curation, Conceptualization. JW: Writing – original draft, Methodology, Formal analysis. NC: Writing – original draft, Supervision, Methodology. WL: Writing – original draft, Formal analysis, Data curation, Conceptualization. MX: Writing – review & editing, Resources, Formal analysis, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by Guangdong Medical Research Foundation (A2022441), Affiliated Hospital of Guangdong Medical University Clinical Research Program (LCYJ2020DL01 and LCYJ2020B004).
Acknowledgments
The authors are grateful to the editor and reviewers for their suggestions to improve the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1438213/full#supplementary-material
Footnotes
References
Arrieta, M. C., Stiemsma, L. T., Amenyogbe, N., Brown, E. M., and Finlay, B. (2014). The intestinal microbiome in early life: health and disease. Front. Immunol. 5:427. doi: 10.3389/fimmu.2014.00427
Bender, J. M., Li, F., Purswani, H., Capretz, T., Cerini, C., Zabih, S., et al. (2021). Early exposure to antibiotics in the neonatal intensive care unit alters the taxonomic and functional infant gut microbiome. J. Matern. Fetal Neonatal Med. 34, 3335–3343. doi: 10.1080/14767058.2019.1684466
Collins, C. T., Makrides, M., McPhee, A. J., Sullivan, T. R., Davis, P. G., Thio, M., et al. (2017). Docosahexaenoic acid and bronchopulmonary dysplasia in preterm infants. N. Engl. J. Med. 376, 1245–1255. doi: 10.1056/NEJMoa1611942
Dobbler, P. T., Procianoy, R. S., Mai, V., Silveira, R. C., Corso, A. L., Rojas, B. S., et al. (2017). Low microbial diversity and abnormal microbial succession is associated with necrotizing enterocolitis in preterm infants. Front. Microbiol. 8:2243. doi: 10.3389/fmicb.2017.02243
Han, Y., Xu, J., Yan, Y., and Zhao, X. (2022). Dynamics of the gut microbiota in rats after hypobaric hypoxia exposure. PeerJ 10:e14090. doi: 10.7717/peerj.14090
Ho, K. M., Kalgudi, S., Corbett, J. M., and Litton, E. (2020). Gut microbiota in surgical and critically ill patients. Anaesth. Intensive Care 48, 179–195. doi: 10.1177/0310057X20903732
Hong, L., Huang, Y., Han, J., Li, S., Zhang, L., Jiang, S., et al. (2023). Dynamics and crosstalk between gut microbiota, metabolome, and fecal calprotectin in very preterm infants: insights into feeding intolerance. Nutrients 15:4849. doi: 10.3390/nu15224849
Hui, Y., Smith, B., Mortensen, M. S., Krych, L., Sorensen, S. J., Greisen, G., et al. (2021). The effect of early probiotic exposure on the preterm infant gut microbiome development. Gut Microbes 13:1951113. doi: 10.1080/19490976.2021.1951113
Jia, Q., Yu, X., Chang, Y., You, Y., Chen, Z., Wang, Y., et al. (2022). Dynamic changes of the gut microbiota in preterm infants with different gestational age. Front. Microbiol. 13:923273. doi: 10.3389/fmicb.2022.923273
Kalinen, S., Kallonen, T., Gunell, M., Ettala, O., Jambor, I., Knaapila, J., et al. (2024). Differences in gut microbiota profiles and microbiota steroid hormone biosynthesis in men with and without prostate cancer. Eur. Urol. Open Sci. 62, 140–150. doi: 10.1016/j.euros.2024.02.004
Li, D., Cui, L., Gao, Y., Li, Y., Tan, X., and Xu, H. (2022). Fecal microbiota transplantation improves intestinal inflammation in mice with ulcerative colitis by modulating intestinal flora composition and down-regulating NF-kB signaling pathway. Microb. Pathog. 173:105803. doi: 10.1016/j.micpath.2022.105803
Liu, L., Ao, D., Cai, X., Huang, P., Cai, N., Lin, S., et al. (2022). Early gut microbiota in very low and extremely low birth weight preterm infants with feeding intolerance: a prospective case-control study. J. Microbiol. 60, 1021–1031. doi: 10.1007/s12275-022-2180-2
Liu, L., Xiang, M., Cai, X., Wu, B., Chen, C., Cai, N., et al. (2023). Multi-omics analyses of gut microbiota via 16S rRNA gene sequencing, LC-MS/MS and diffusion tension imaging reveal aberrant microbiota-gut-brain axis in very low or extremely low birth weight infants with white matter injury. BMC Microbiol. 23:387. doi: 10.1186/s12866-023-03103-5
Matamoros, S., Gras-Leguen, C., Le Vacon, F., Potel, G., and de La Cochetiere, M. F. (2013). Development of intestinal microbiota in infants and its impact on health. Trends Microbiol. 21, 167–173. doi: 10.1016/j.tim.2012.12.001
Pan, R., Guo, M., Chen, Y., Lin, G., Tian, P., Wang, L., et al. (2024). Dynamics of the gut microbiota and faecal and serum metabolomes during pregnancy-a longitudinal study. Nutrients 16:483. doi: 10.3390/nu16040483
Russell, J. T., Lauren Ruoss, J., de la Cruz, D., Li, N., Bazacliu, C., Patton, L., et al. (2021). Antibiotics and the developing intestinal microbiome, metabolome and inflammatory environment in a randomized trial of preterm infants. Sci. Rep. 11:1943. doi: 10.1038/s41598-021-80982-6
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E. B., et al. (2009). Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541. doi: 10.1128/AEM.01541-09
Seki, D., Mayer, M., Hausmann, B., Pjevac, P., Giordano, V., Goeral, K., et al. (2021). Aberrant gut-microbiota-immune-brain axis development in premature neonates with brain damage. Cell Host Microbe 29, 1558–1572.e6. doi: 10.1016/j.chom.2021.08.004
Shin, N. R., Whon, T. W., and Bae, J. W. (2015). Proteobacteria: microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 33, 496–503. doi: 10.1016/j.tibtech.2015.06.011
Wan, L., Ge, W. R., Zhang, S., Sun, Y. L., Wang, B., and Yang, G. (2020). Case-control study of the effects of gut microbiota composition on neurotransmitter metabolic pathways in children with attention deficit hyperactivity disorder. Front. Neurosci. 14:127. doi: 10.3389/fnins.2020.00127
Wang, Z., Neupane, A., Vo, R., White, J., Wang, X., and Marzano, S. L. (2020). Comparing gut microbiome in mothers’ own breast milk- and formula-fed moderate-late preterm infants. Front. Microbiol. 11:891. doi: 10.3389/fmicb.2020.00891
Westaway, J. A. F., Huerlimann, R., Kandasamy, Y., Miller, C. M., Norton, R., Staunton, K. M., et al. (2022). The bacterial gut microbiome of probiotic-treated very-preterm infants: changes from admission to discharge. Pediatr. Res. 92, 142–150. doi: 10.1038/s41390-021-01738-6
Yan, X., Zhai, Y., Zhou, W., Qiao, Y., Guan, L., Liu, H., et al. (2022). Intestinal flora mediates antiobesity effect of rutin in high-fat-diet mice. Mol. Nutr. Food Res. 66:e2100948. doi: 10.1002/mnfr.202100948
Yap, P. S. X., Chong, C. W., Ahmad Kamar, A., Yap, I. K. S., Choo, Y. M., Lai, N. M., et al. (2021). Neonatal intensive care unit (NICU) exposures exert a sustained influence on the progression of gut microbiota and metabolome in the first year of life. Sci. Rep. 11:1353. doi: 10.1038/s41598-020-80278-1
Zhang, Z., Jiang, J., Li, Z., and Wan, W. (2022). The change of cytokines and gut microbiome in preterm infants for bronchopulmonary dysplasia. Front. Microbiol. 13:804887. doi: 10.3389/fmicb.2022.804887
Zhang, Y., Li, M., Pu, Z., Chi, X., and Yang, J. (2023). Multi-omics data reveals the disturbance of glycerophospholipid metabolism and linoleic acid metabolism caused by disordered gut microbiota in PM2.5 gastrointestinal exposed rats. Ecotoxicol. Environ. Saf. 262:115182. doi: 10.1016/j.ecoenv.2023.115182
Zhang, R., Qiu, W., Sun, X., Li, J., Geng, X., Yu, S., et al. (2023). Gut microbiota dynamics in a 1-year follow-up after adult liver transplantation in Northeast China. Front. Physiol. 14:1266635. doi: 10.3389/fphys.2023.1266635
Keywords: gut microbiota, VLBW/ELBW infants, perterm infants, steroid hormone biosynthesis, 16S rRNA sequencing
Citation: Liu L, Chen C, Li Y, Ao D, Wu J, Cai N, Li W and Xiang M (2024) Dynamics alteration of the gut microbiota and faecal metabolomes in very low or extremely low birth weight infants: a Chinese single-center, prospective cohort study. Front. Microbiol. 15:1438213. doi: 10.3389/fmicb.2024.1438213
Edited by:
Silvia Turroni, University of Bologna, ItalyReviewed by:
Beata Łoniewska, Pomeranian Medical University, PolandCaroline Hayley Orr, Teesside University, United Kingdom
Copyright © 2024 Liu, Chen, Li, Ao, Wu, Cai, Li and Xiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Li, bGl3ZW40MTBAMTYzLmNvbQ==; Min Xiang, MzA2NzgyOTEwQHFxLmNvbQ==
†These authors have contributed equally to this work
 Ling Liu
Ling Liu Chaohong Chen
Chaohong Chen YeShan Li1
YeShan Li1 Dang Ao
Dang Ao Jiayuan Wu
Jiayuan Wu Wen Li
Wen Li Min Xiang
Min Xiang