- 1West China Hospital, Sichuan University, Chengdu, China
- 2Department of Pharmacy, West China Hospital, Sichuan University, Chengdu, China
- 3Chengdu Medical College, Chengdu, China
- 4Department of Outpatient, West China Hospital, Sichuan University, Chengdu, China
- 5Department of Psychiatry and National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University, Chengdu, China
- 6Mental Health Center, West China Hospital, Sichuan University, Chengdu, China
The role of the gut microbiota in the pathophysiology of depression has been explored in numerous studies, which have confirmed that the baseline gut microbial profiles of patients with depression differ from those of healthy individuals. The gut microbiome affects metabolic activity in the immune and central nervous systems and regulates intestinal ecology through the neuroendocrine system. Additionally, baseline changes in the gut microbiota differed among patients with depression who demonstrated varying treatment response. Currently, probiotics are an emerging treatment for depression; however, the efficacy of modulating the gut microbiota in the treatment of depression remains uncertain. Additionally, the mechanisms by which changes in the gut microbiota affect treatment response in patients with depression remain unclear. In this review, we aimed to summarize the differences in the baseline gut microbiota between the remission and non-remission groups after antidepressant therapy. Additionally, we summarized the possible mechanisms that may contribute to antidepressant resistance through the effects of the gut microbiome on the immune and nervous systems, various enzymes, bioaccumulation, and blood–brain barrier, and provide a basis for treating depression by targeting the gut microbiota.
1 Introduction
Depression is a psychiatric disorder characterized by various symptoms, including depressed mood, anhedonia, appetite changes, sleep disturbances, psychomotor retardation and/or agitation, fatigue, feelings of guilt, poor concentration, suicidal ideation, and cognitive impairment (GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018; Burrows et al., 2020). The etiology of depression involves abnormal neuroendocrine (Farzi et al., 2018), neuroimmune (Birmann et al., 2021), metabolic (Lukić et al., 2022), and neurotransmitter (Cammisuli et al., 2022) functioning. Epidemiological studies have estimated the global prevalence of depression to be 4.4% (Ferrari et al., 2013), affecting approximately 350 million people worldwide (Joca et al., 2015). According to the World Health Organization World Mental Health survey, the estimated lifetime prevalence of depression is 14.6% in high-income countries and 11.1% in low- and middle-income countries (Kessler and Bromet, 2013). In China, the prevalence of depression is 6.87%, affecting approximately 90 million people (Liang et al., 2018). Epidemiological surveys have shown that a substantial proportion of patients with major depressive disorder (MDD) fail to respond to current first-line antidepressants, imposing a substantial healthcare burden on society (Zhdanava et al., 2021). According to a meta-analysis, only 46% of individuals achieved remission by the end of treatment, even when a combination of psychotherapy and pharmacotherapy was used (de Maat et al., 2007). In addition, the adverse effects of antidepressants cause many patients to avoid these treatment options. The clinical treatment of depression includes drug therapy, cognitive behavioral therapy, physical therapy, exercise therapy, and acupuncture (Guideline Development Panel for the Treatment of Depressive Disorders, 2022; Qaseem et al., 2023), with antidepressants being the most common treatment method (Cipriani et al., 2018).
Patients with depression may not respond to treatment due to the inherent environmental and biological aspects of the disease (Minelli et al., 2022), including genetic predisposition (Pettai et al., 2016; Fabbri et al., 2018; Minelli et al., 2022), inflammatory factors (Schmidt et al., 2016; Szałach et al., 2019), thyroid autoimmunity (Dwyer et al., 2020), neurotrophic factors (Pettai et al., 2016), and dietary influences (Molendijk et al., 2018; Chakraborti et al., 2021; Marx et al., 2021; Herselman et al., 2022; Wang et al., 2022). In recent years, numerous studies have shown an association between the gut microbiota and patient response to depression treatment. Some studies have analyzed the fecal microbiota of patients undergoing treatment for depression, and found that the baseline composition of the gut microbiota differed between the two groups (Liskiewicz et al., 2021; Lee et al., 2022). Here, the findings of studies investigating the relationship between the gut microbiota and prognosis of depression are summarized to provide a basis for the treatment of depression by targeting the gut microbiota.
2 The gut–brain axis
The gut microbiota in adult humans includes bacteria, viruses, fungi, archaea, and protozoa (Thursby and Juge, 2017). The gut microbiota is predominantly composed of six phyla (Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Fusobacteria, and Verrucomicrobia), with Firmicutes and Bacteroidetes constituting the major phyla (Laterza et al., 2016; Sherwin et al., 2016). Individuals have unique gut microbiome profiles which depend on various factors, including the intestinal environment, hormonal changes, immunity, lifestyle, dietary habits, and drug use (Wang and Liu, 2020; Perler et al., 2023).
Some studies have suggested that the gut microbiota interacts with the central nervous system (CNS) through the gut–brain axis (Góralczyk-Bińkowska et al., 2022; Kim et al., 2023; Borrego-Ruiz and Borrego, 2024). Evidence supports the idea that the gut microbiota may regulate higher-level CNS functions, such as behavior and mood, through bidirectional signal transmission (Sherwin et al., 2016).
Numerous studies have observed two-way communication between the microbiota and the brain (Kunugi, 2021; Sharma et al., 2021; Trzeciak and Herbet, 2021; Ye et al., 2021; Hou et al., 2022; Dziedzic et al., 2024) through the gut–brain axis, which encompasses multiple components, including the CNS, spinal cord, autonomic nervous system, enteric nervous system, immune system, and hypothalamic–pituitary–adrenal axis (Carabotti et al., 2015). The gut microbiota can produce various molecules that act at distal sites to imitate the function of endocrine organs (Tsigos et al., 2000; Clarke et al., 2014), such as short-chain fatty acids (SCFAs), neurotransmitters (including serotonin, dopamine, norepinephrine, and γ-aminobutyric acid), cholic acids, tryptophan, L-dopa, adipokines, and hormones (Clarke et al., 2014). The mechanisms of action of the gut–brain axis are shown in Figure 1.
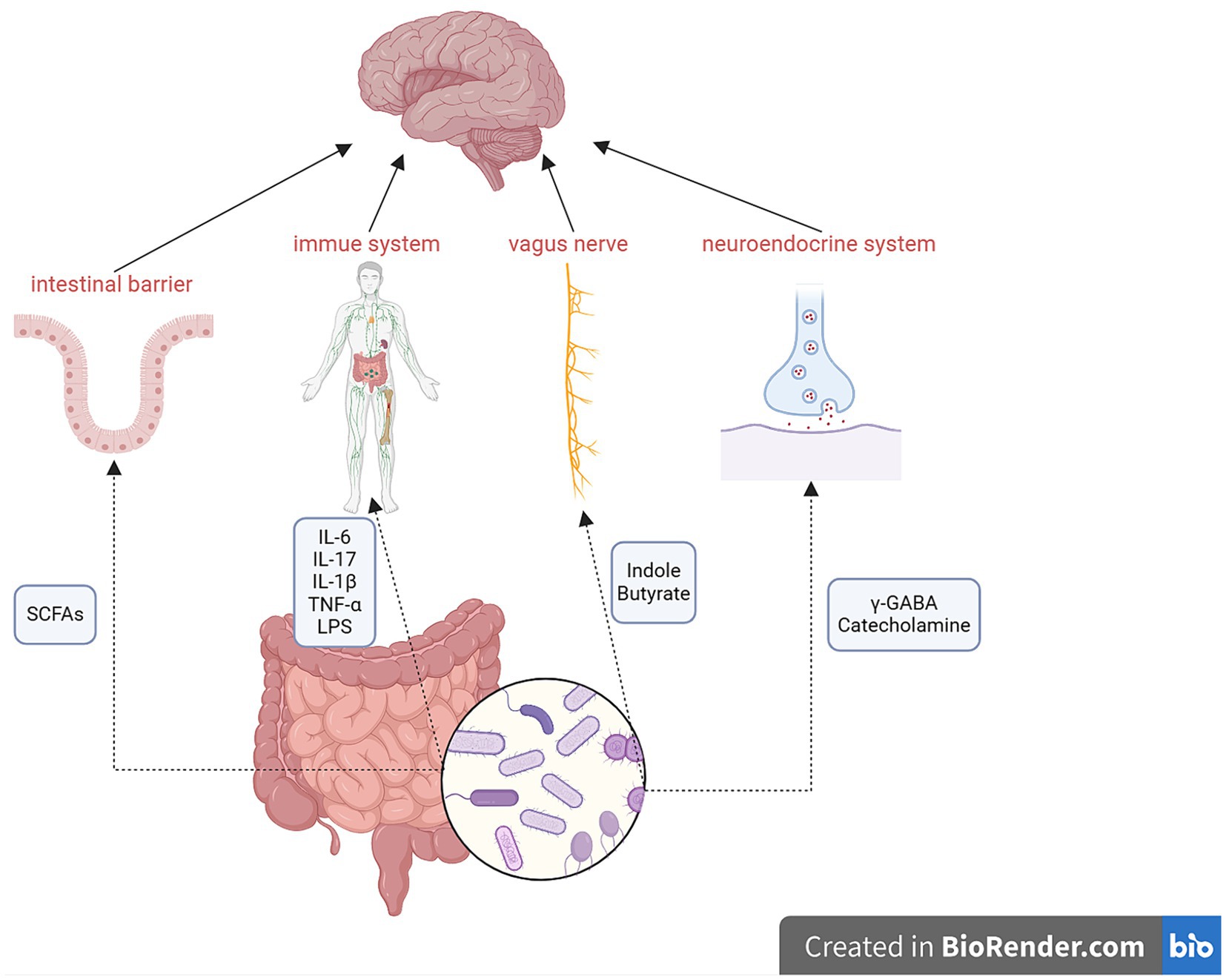
Figure 1. Mechanisms of the gut–brain axis. Metabolites of the gut microbiota can affect the intestinal barrier, immune system, vagus nerve, and neuroendocrine system to regulate the function of the brain via the gut–brain axis. Conversely, the brain can also influence the gut microbiota. SCFAs, short-chain fatty acids; γ-GABA, γ-aminobutyric acid.
Genes, socioeconomic status, diet, medications, and environmental factors can influence the gut–brain axis. Lifestyle factors, especially diet, are crucial in regulating the gut–brain axis. Additionally, drugs, especially antibiotics, can directly affect the gut microbiota, which, in turn, can impact the gut–brain axis (Hou et al., 2022).
3 Baseline changes in gut microbiota during different responses to depression
Several studies have reported the differences in the gut microbiota profiles of patients with depression and healthy individuals (Jiang et al., 2015; Trzeciak and Herbet, 2021). Recent studies have shown that, after treatment, the gut microbiota differs between patients who achieve clinical remission and those who do not (Liskiewicz et al., 2021; Ye et al., 2021; Lee et al., 2022). Individuals who achieved remission had a higher baseline abundance of their gut microbiota compared to non-remitters (Wang et al., 2023). The baseline gut microbiota was defined as the first gut microbiota measurement after enrollment and before initiation of antidepressant treatment.
3.1 Methods for evaluating response to depression treatment
The treatment response to depression is primarily evaluated using clinical scales, with treatment effectiveness evaluated by changes in the scores after treatment. Therefore, reliable and valid instruments are necessary for diagnosing and treating depression.
Over the past few decades, various instruments have been developed to assess the severity of depressive symptoms, including the Hamilton Depression Rating Scale (HAMD) (Hamilton, 1960), Montgomery–Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), and Beck Depression Inventory (Beck et al., 1961). The first two are observer rating instruments, while the latter is a self-rating measure.
The HAMD is the most commonly used scale for clinically assessing depression (Hamilton, 1960). The original version included 17 questions related to depression; however, the number of items gradually increased to 24 as the understanding of the disease improved; currently, there are three versions of the HAMD, with 17 (HAMD-17), 21 (HAMD-21), and 24 (HAMD-24) questions. The HAMD-24 comprises 24 questions related to depression, with most items scored on a five-point scale ranging from zero to four points. A total score of >35 indicates MDD. Remission from depression is defined as HAMD-24 score ≤ 8 at the end-point (Schramm et al., 2020). The MADRS consists of 10 questions, each scored from zero to six (Montgomery and Asberg, 1979). A total score of ≥30 indicates MDD, and a total score of ≥12 suggests remission (Bharwani et al., 2020).
Treatment response can also be defined as a reduction of 50% or more from the baseline score on standardized scales, such as HAMD and MADRS, after treatment for depression (Keller, 2003). Some studies have evaluated treatment response by observing animal behavior before and after treatment as well as through specific experiments, such as open-field, tail suspension, and forced swimming tests (Ding et al., 2021; Song et al., 2022).
3.2 Comparison of baseline changes in the gut microbiota between patients with varying response to treatment
3.2.1 Changes in the level of alpha and beta diversity
Alpha diversity, which considers richness and the relative abundances of species, can be used to compare sample groups. Specifically, low alpha diversity is a sign of dysbiosis. Gut microbiota can be divided into different operational taxonomic units (OTUs) using alpha diversity (Knight et al., 2018). Beta diversity calculates the mean value of species divergence between the focal and neighboring samples. It captures the dissimilarity between a pair of samples or communities, thereby generating a distance matrix based on the presence or absence of species abundance data (Nguyen et al., 2021). Some studies have revealed no significant community-level alpha or beta diversity changes between remission and non-remission groups (Sanada et al., 2020; Liskiewicz et al., 2021; Lee et al., 2022). However, other clinical studies have found a negative association between microbial alpha diversity and depression severity (Madan et al., 2020; Shen et al., 2021).
Some studies have revealed that remitters exhibit greater baseline diversity than non-remitters (Jiang et al., 2015; Bharwani et al., 2020; Duan et al., 2021). In one study, patients with MDD were divided into the remitter and non-remitter groups based on their MADRS scores after treatment with escitalopram. OTU levels were examined after 3 and 6 months of treatment, and it was found that OTUs were not altered in non-remitters. Overall, 35 OTUs differed between remitters and non-remitters at 3 months; however, 16 OTUs were no longer different after 6 months. At 6 months, 42 OTUs differed, of which 23 were unique, and 19 were the remaining OTUs at 3 months. The results indicated that antidepressants could affect the gut microbiota of patients with MDD at the OTU level (Bharwani et al., 2020). Another study revealed that 125 and 87 OTUs were uniquely present in non-remission and remission groups, respectively (Duan et al., 2021).
3.2.2 Changes in the phylum Firmicutes
Another study revealed that 125 and 87 OTUs were uniquely present in non-remission and remission groups, respectively (Duan et al., 2021). Clostridiales was strongly negatively correlated with the severity of depression (Liskiewicz et al., 2021; Xiong et al., 2022; Wang et al., 2023). An increase in the abundance of the order Clostridiales was found in the remission group after 6 months of escitalopram treatment (Bharwani et al., 2020). The abundance of Faecalibacterium negatively correlated with the severity of depressive symptoms (Jiang et al., 2015), and lower levels of Faecalibacterium were associated with MDD development (Kelly et al., 2016; Zheng et al., 2016; Cheung et al., 2019). Animal studies showed that the ingestion of Faecalibacterium prausnitzii improved anxiety- and depression-like behaviors in chronic unpredictable mild stress (CUMS) mice (Hao et al., 2019). In one study, the enrichment of baseline Faecalibacterium levels in levomilnacipran-treated patients with MDD aged >60 years was associated with remission outcomes (Lee et al., 2022). The abundance of Faecalibacterium was lower in MDD patients than in healthy controls, but was restored after sertraline treatment (Zhou et al., 2023). Higher abundances of Faecalibacterium were also associated with higher quality of life indicators (Valles-Colomer et al., 2019).
In addition, enrichment of Agathobacter (Lee et al., 2022), Coprococcus (Valles-Colomer et al., 2019; Gao et al., 2023), Roseburia (Zhou et al., 2023), and Eubacterium (Xiong et al., 2022; Wang et al., 2023) was associated with the treatment outcome of remission. The increased relative abundances of the family Christensenellaceae (Dong et al., 2022) and decreased relative abundances of the families Ruminococcaceae, Lactobacillaceae, and Peptostreptococcaceae and the genera Coprococcus (Sanada et al., 2020), Oscillibacter (Xiong et al., 2022), Tyzzerella, and Butyricicoccus (Shen et al., 2023) were related to non-remission (Fontana et al., 2020; Duan et al., 2021).
Firmicutes are important butyrate-producing bacteria (Kim et al., 2020). Butyrate was reported to reduce depression-like behaviors, produce antidepressant effects, and relieve symptoms of depression (Zhou et al., 2018).
3.2.3 Changes in the phylum Bacteroidetes
The severity and treatment response of depression are strongly correlated with Paraprevotella abundance (Lin et al., 2017; Liskiewicz et al., 2021; Hu et al., 2023). An experiment using CUMS mice showed an increase in the relative abundance of Prevotellaceae_UCG-003 in the remission group but not in the non-remission group (Duan et al., 2021). Clinical studies showed that the abundance of Bacteroidota, especially Odoribacter, was higher in responders (Matsuzaki et al., 2024). By contrast, another study found that the baseline abundance of Bacteroidetes gradually decreased after treatment with vortioxetine hydrobromide, consistent with the remission of depressive symptoms (Ye et al., 2021).
Parabacteroides protect the nervous system by altering the levels of neurotransmitters, including glutamate and γ-aminobutyric acid, in the hippocampus(Liskiewicz et al., 2021). Odoribacter might generate butyrate by accelerating L-lysine degradation, which can promote the barrier function of gut epithelium (Matsuzaki et al., 2024; Vasileva et al., 2024). The level of L-lysine was proved to be lower in responders after antidepressive treatment(Matsuzaki et al., 2024).
3.2.4 Changes in the phylum Actinobacteria
The relative abundances of Actinobacteria and Eggerthellaceae are higher in non-responders than in responders (Fontana et al., 2020; Alexander et al., 2022; Dong et al., 2022). A significant increase in the proportion of Bifidobacterium was found after treatment with vortioxetine hydrobromide (Ye et al., 2021; Gao et al., 2023). In another study, the abundance of Enterorhabdus was higher in CUMS mice than in the control group. However, this increase was reversed after venlafaxine treatment (Shen et al., 2023).
3.2.5 Changes in the phylum Proteobacteria
Studies demonstrated that with the remission of depressive symptoms, the abundance of Proteobacteria significantly decreases after treatment with vortioxetine hydrobromide (Ye et al., 2021). Responders demonstrated reduced Proteobacteria abundance when compared with non-responders (Fontana et al., 2020). Another study showed that puerarin relieved CUMS-induced depression-like behavior in rats and reduced the abundance of Desulfovibrio (Song et al., 2022). Changes in the gut microbiota of patient with depression with varying responses to treatment are shown in Table 1.
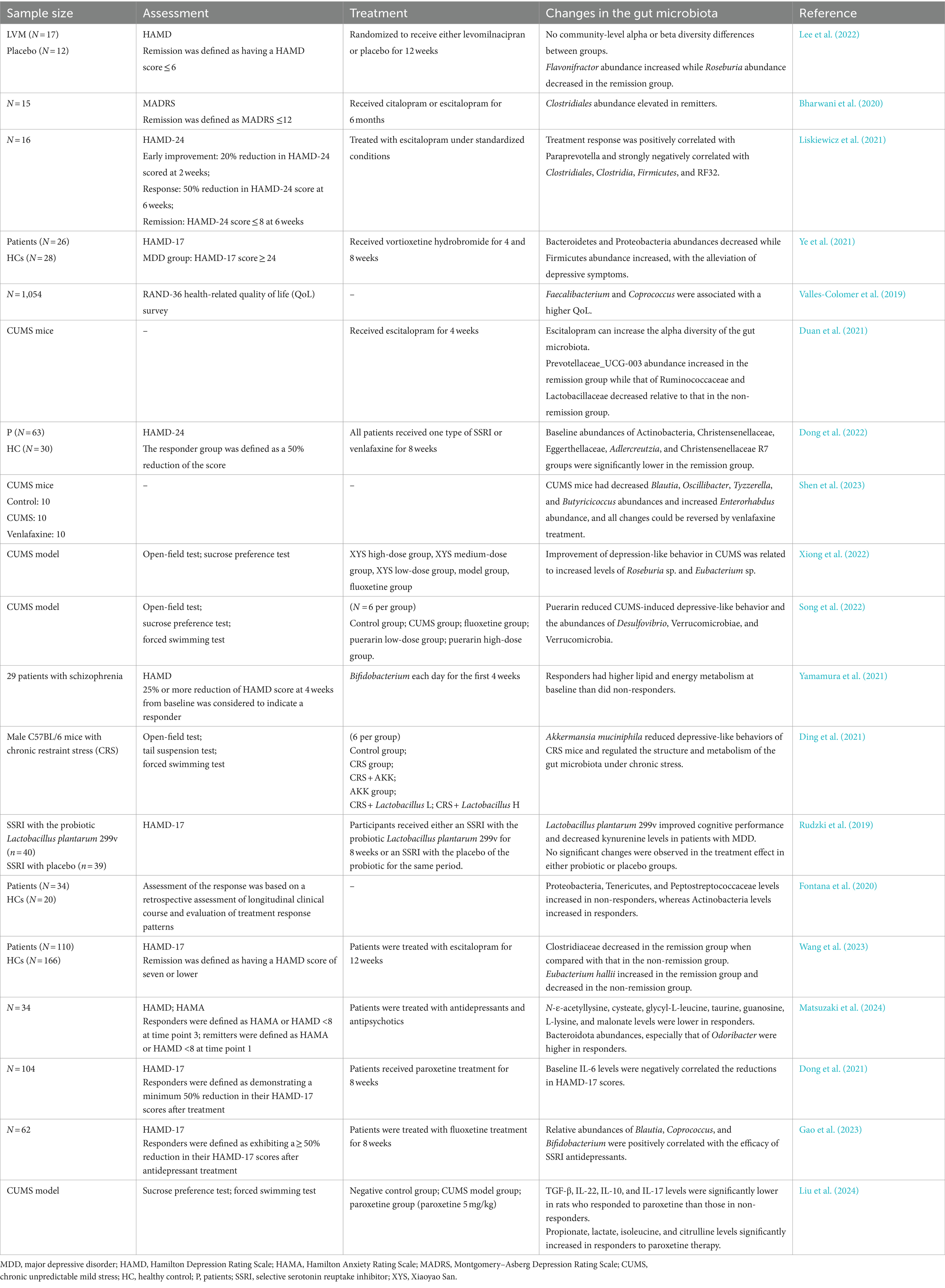
Table 1. Summary of the articles on changes in the baseline of gut microbiota associated with the response to treatment.
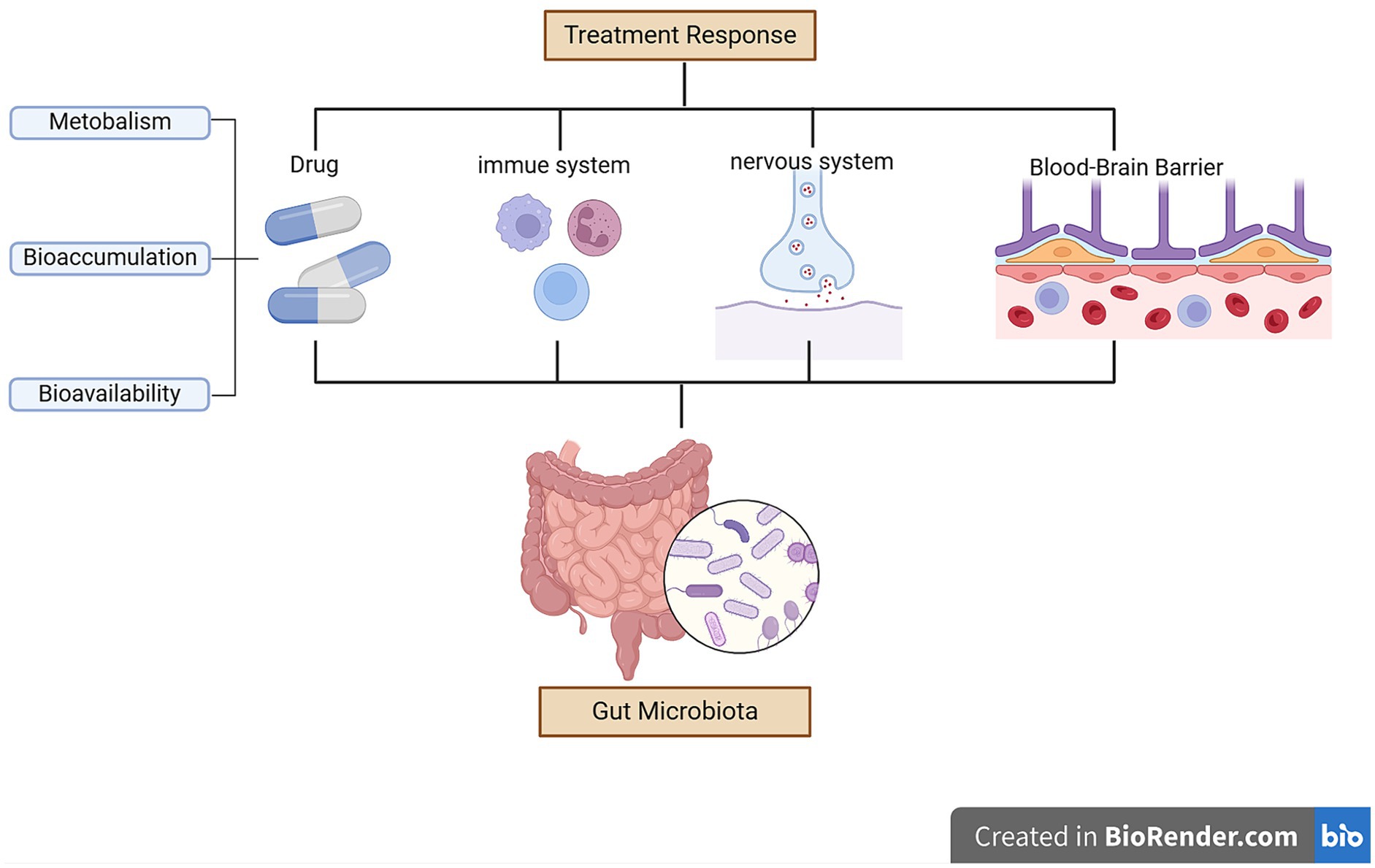
3.3 Possible mechanisms of how the gut microbiota affect treatment response in depression
Although the interactions between drugs and gut microbiota exist, the underlying mechanisms remain unclear.
3.3.1 Gut microbiota affects drug pharmacokinetics
Hepatic metabolites of drugs are secreted into the gut for further metabolism. Bacterial metabolites of a drug can, in turn, be absorbed and transported to the liver. During this process, drugs interact with the gut microbiota. However, drugs can alter the intestinal microenvironment and/or directly affect the growth, composition, and function of bacteria (Weersma et al., 2020). Some antidepressant drugs, such as sertraline, fluoxetine, and escitalopram, exert antibacterial effects, disrupting the integrity and stability of the gut microbiota (Macedo et al., 2017; Karine de Sousa et al., 2018). Moreover, changes in the gut microbiota can affect drug metabolism and influence the efficacy of drugs.
A systematic analysis tested the ability of 76 gut microbial strains to metabolize 271 drugs administered orally and found that 176 drugs (66%) were metabolized by at least one microbial strain (Zimmermann et al., 2019b). The gut microbiota can alter the structure, bioavailability, biological activity, and toxicity of drugs via enzymes, directly affecting individual responses to a particular drug (Maini Rekdal et al., 2019; Zimmermann et al., 2019a,b; Weersma et al., 2020). For example, the gut microbiota can transform the chemical structure of drugs and modulate xenobiotic metabolism, including drug metabolic pathways (Wilson and Nicholson, 2017). The gut microbiota can enhance the activity of indoleamine 2,3-dioxygenase 1, a rate-limiting enzyme which transforms tryptophan into kynurenine and its derivatives, thereby affecting the bioavailability of antidepressants (Agus et al., 2018; Wu et al., 2022). Changes in the gut microbiota can also impact intestinal permeability and the function of the intestinal barrier, which affects drug absorption (Kelly et al., 2015). Firmicutes break down carbohydrates into SCFAs (Stilling et al., 2016; Deleu et al., 2021), which have been shown to enhance the integrity of the blood–brain and intestinal barriers (Unger et al., 2016). Reduced levels of Firmicutes results in decreased SCFA production (Huang et al., 2018). Additionally, gut microbiota possess tryptophanase, which can produce indole to regulate intestinal barrier permeability (Trzeciak and Herbet, 2021). Significant increases in lactate levels were observed in CUMS rats that responded to treatment (Liu et al., 2024). Supplementation with either Bifidobacterium or Lactobacillus can enhance the integrity of the intestinal barrier and alleviate the symptoms of stress-induced intestinal leakage (Couto et al., 2020; Lenoir et al., 2020).
3.3.2 Gut microbiota influences immune regulation
Evidence suggests that systemic inflammation, mediated by intestinal dysbiosis, could play a crucial role in the development of therapy resistance in patients with depression (Vitetta et al., 2014). TGF-β, IL-22, IL-10, and IL-17 levels were significantly higher in CUMS rats who responded to paroxetine therapy than those in non-responders (Liu et al., 2024). Baseline IL-6 levels were negatively correlated with reduced HAMD-17 scores in clinical studies (Dong et al., 2021). Faecalibacterium prausnitzii produces butyrate in the human colon, and appropriate levels of butyrate production can improve mucin secretion, prevent intestinal leakage, and suppress inflammation (Furusawa et al., 2013; Samara et al., 2022). It can also regulate intestinal epithelial cells to decrease pro-inflammatory cytokine levels and increase anti-inflammatory factor levels (Couto et al., 2020; Lenoir et al., 2020). Increased F. prausnitzii levels can lead to increased SCFA production and higher levels of inflammatory factors, such as IL-10, in the plasma (Hao et al., 2019). Microbiota metabolites can also modulate the proportions of T helper 17 and regulatory T cells to promote resilience to stress-induced depressive-like behaviors (Westfall et al., 2021). High levels of Prevotellaceae UCG-003 may regulate intestinal inflammation by producing succinate, which, in turn, activates dendritic cells (Koh et al., 2016). Bifidobacteria increase butyrate levels by altering the relative abundances of other microbiota involved in lipid metabolism, therefore possessing anti-inflammatory properties (Sugahara et al., 2015). Moreover, Bifidobacteria regulate the levels of pro-inflammatory cytokines and anti-inflammatory factors (Couto et al., 2020; Lenoir et al., 2020; Samara et al., 2022). Furthermore, members of the genus Eggerthella, including Eggarthella lenta, induce intestinal inflammation by activating Th17 cells (Alexander et al., 2022).
3.3.3 Gut microbiota affects the nervous system
The gut microbiota is essential for nervous system communication via the vagus nerve. The vagus nerve plays an important role in behavioral abnormalities in antibiotic-treated mice after ingesting Lactobacillus (Wang et al., 2020). In animal models, dysbiosis impairs vagus signaling, affects brain structure, regulates brain-derived neurotrophic factors, and reduces hippocampal protein synthesis (Fond et al., 2015; Dehghani et al., 2022). The relative abundance of Proteobacteria is correlated with stress-induced behavioral changes (Wong et al., 2016; Werbner et al., 2019). Members of the genus Bifidobacteria play crucial roles in maintaining the balance of the gastrointestinal tract by reducing oxidative stress (Kant et al., 2015). Additionally, Oscillibacter is beneficial in protecting brain modulatory functions, ultimately leading to increased amygdala and hippocampal volume (Lee et al., 2022). Parabacteroides can alter neurotransmitter levels in the brain, including those of glutamate and aminobutyric acid (Olson et al., 2018). Furthermore, Bifidobacterium and Lactobacillus stimulate γ-aminobutyric acid production by metabolizing indigestible fibers (Couto et al., 2020; Lenoir et al., 2020).
3.3.4 Other effects of the gut microbiota
The gut microbiota may affect the therapeutic outcomes of antidepressants by altering the permeability of the blood–brain barrier permeability during treatment (Xu et al., 2023). Higher abundances of Firmicutes were associated with higher levels of SCFAs (Stilling et al., 2016; Deleu et al., 2021). SCFAs can enhance the integrity of the blood–brain barrier (Unger et al., 2016). The gut microbiota can modulate the availability of antidepressants through bioaccumulation (Doestzada et al., 2018; Klünemann et al., 2021), resulting in direct reductions in drug availability and changes in the secretion of metabolites (Wu et al., 2017; Klünemann et al., 2021). Streptococcus salivarius, Bacteroides uniformis, Bacteroides thetaiotaomicron, and Escherichia coli increase duloxetine bioaccumulation, thereby decreasing its bioavailability (Klünemann et al., 2021). Figure 2 summarizes the possible mechanisms by which the gut microbiota affects treatment response in patients with depression.
3.4 Current progress in microbiota-targeting therapies
Some studies using adjuvant microbial supplements for the treatment of depression have achieved preliminary success (Rieder et al., 2017); Current research findings indicate that probiotic therapy may have moderate efficacy in alleviating depressive symptoms (Nikolova et al., 2021; Tian et al., 2022). Most studies have utilized the genera Lactobacilli and Bifidobacteria as probiotics for treating depression. However, the effectiveness of probiotic monotherapy is limited (Nikolova et al., 2021). Since most antidepressants exhibit antimicrobial activity (Nikolova et al., 2021), probiotics can promote therapeutic benefits by restoring the balance of the gut microbiota and minimizing gastrointestinal discomfort. However, the complexity and variability of the gut microbiota, as well as its susceptibility to various influencing factors, lead to heterogenous trial results (Tian et al., 2022).
Current preclinical research findings indicate that fecal microbiota transplantation has significant potential in the treatment of MDD, but there are still limited number of studies on humans, and several issues remain, including resistance to microbiota colonization, potential pathogen transmission, and ethical considerations related to donor-recipient matching (Meng et al., 2024). Further studies on the treatment of MDD using gut microbiota are needed.
4 Conclusion
Multiple studies have confirmed that differences exist in the gut microbiota of patients with MDD and healthy subjects; however, they have not conclusively shown that these differences are correlated with disease severity. This review summarized the existing studies that compared the baseline gut microbiota between remission and non-remission groups and found that changes in the abundances of specific microbiota are associated with treatment response in MDD.
However, most of these studies have certain limitations. Most notably, the sample sizes are often too small to confirm significant differences between the two groups. Some trials have not included healthy controls, and some have lost a high proportion of participants to follow-up, leading to greater selection bias. Recent studies suggest that baseline changes in specific gut microbiota are related to MDD remission; however, the provided evidence is insufficient to confirm that these specific organisms can be used as predictors for the treatment response of depression. In addition, the detection of the gut microbiota is greatly affected by individual differences and environmental factors, which warrants further research.
This review provides supporting evidence for microbiota therapy and indicates novel research directions for using the gut microbiota as a target in prognosticating and treating depression. Continued research is crucial for understanding the relationship between the gut microbiota and depression, which can offer new prospects in treating this complex condition. Therapies targeting the gut microbiota are expected to be widely utilized as a treatment option for depression in the future.
Author contributions
YX: Writing – original draft, Writing – review & editing. HZ: Methodology, Writing – review & editing, Conceptualization. YY: Methodology, Writing – review & editing, Supervision. XG: Writing – review & editing, Methodology, Validation. QX: Funding acquisition, Writing – review & editing. ZD: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the National Natural Science Foundation of China [Grant No. 81801350], the Key R&D Projects of Science and Technology Department of Sichuan Province [Grant No. 2023YFS0292], and Fujian provincial health technology project [Grant No. 2023CXB010].
Acknowledgments
We would like to thank all participants for their contributions, Editage for English language editing, and BioRender for the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agus, A., Planchais, J., and Sokol, H. (2018). Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724. doi: 10.1016/j.chom.2018.05.003
Alexander, M., Ang, Q. Y., Nayak, R. R., Bustion, A. E., Sandy, M., Zhang, B., et al. (2022). Human gut bacterial metabolism drives Th17 activation and colitis. Cell Host Microbe 30, 17–30.e9. doi: 10.1016/j.chom.2021.11.001
Beck, A. T., Ward, C. H., Mendelson, M., Mock, J., and Erbaugh, J. (1961). An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571. doi: 10.1001/archpsyc.1961.01710120031004
Bharwani, A., Bala, A., Surette, M., Bienenstock, J., Vigod, S. N., and Taylor, V. H. (2020). Gut microbiome patterns associated with treatment response in patients with major depressive disorder. Can. J. Psychiatry-Revue Can. Psychiatrie 65, 278–280. doi: 10.1177/0706743719900464
Birmann, P. T., Casaril, A. M., Pesarico, A. P., Caballero, P. S., Smaniotto, T., Rodrigues, R. R., et al. (2021). Komagataella pastoris Km71h modulates Neuroimmune and oxidative stress parameters in animal models of depression: a proposal for a new probiotic with antidepressant-like effect. Pharmacol. Res. 171:105740. doi: 10.1016/j.phrs.2021.105740
Borrego-Ruiz, A., and Borrego, J. J. (2024). An updated overview on the relationship between human gut microbiome Dysbiosis and psychiatric and psychological disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 128:110861. doi: 10.1016/j.pnpbp.2023.110861
Burrows, K., Stewart, J. L., Antonacci, C., Kuplicki, R., Thompson, K., Taylor, A., et al. (2020). Association of Poorer Dietary Quality and Higher Dietary Inflammation with greater symptom severity in depressed individuals with appetite loss. J. Affect. Disord. 263, 99–106. doi: 10.1016/j.jad.2019.11.160
Cammisuli, D. M., Fusi, J., Scarfò, G., Daniele, S., Castelnuovo, G., and Franzoni, F. (2022). A Minireview exploring the interplay of the muscle-gut-brain (Mgb) Axis to improve knowledge on mental disorders: implications for clinical neuroscience research and therapeutics. Oxidative Med. Cell. Longev. 2022:8806009. doi: 10.1155/2022/8806009
Carabotti, M., Scirocco, A., Maselli, M. A., and Severi, C. (2015). The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 28, 203–209
Chakraborti, A., Graham, C., Chehade, S., Vashi, B., Umfress, A., Kurup, P., et al. (2021). High fructose corn syrup-moderate fat diet potentiates Anxio-depressive behavior and alters ventral striatal neuronal signaling. Front. Neurosci. 15:669410. doi: 10.3389/fnins.2021.669410
Cheung, S. G., Goldenthal, A. R., Uhlemann, A. C., Mann, J. J., Miller, J. M., and Sublette, M. E. (2019). Systematic review of gut microbiota and major depression. Front. Psych. 10:34. doi: 10.3389/fpsyt.2019.00034
Cipriani, A., Furukawa, T. A., Salanti, G., Chaimani, A., Atkinson, L. Z., Ogawa, Y., et al. (2018). Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network Meta-analysis. Lancet (London, England) 391, 1357–1366. doi: 10.1016/s0140-6736(17)32802-7
Clarke, G., Stilling, R. M., Kennedy, P. J., Stanton, C., Cryan, J. F., and Dinan, T. G. (2014). Minireview: gut microbiota: the neglected endocrine organ. Molecular Endocrinol. 28, 1221–1238. doi: 10.1210/me.2014-1108
Couto, M. R., Gonçalves, P., Magro, F., and Martel, F. (2020). Microbiota-derived butyrate regulates intestinal inflammation: focus on inflammatory bowel disease. Pharmacol. Res. 159:104947. doi: 10.1016/j.phrs.2020.104947
de Maat, S. M., Dekker, J., Schoevers, R. A., and de Jonghe, F. (2007). Relative efficacy of psychotherapy and combined therapy in the treatment of depression: a Meta-analysis. Eur. Psychiatry 22, 1–8. doi: 10.1016/j.eurpsy.2006.10.008
Dehghani, F., Abdollahi, S., Shidfar, F., Clark, C. C. T., and Soltani, S. (2022). Probiotics supplementation and brain-derived neurotrophic factor (Bdnf): a systematic review and Meta-analysis of randomized controlled trials. Nutr. Neurosci. 26, 942–952. doi: 10.1080/1028415x.2022.2110664
Deleu, S., Machiels, K., Raes, J., Verbeke, K., and Vermeire, S. (2021). Short Chain fatty acids and its producing organisms: an overlooked therapy for Ibd? EBioMedicine 66:103293. doi: 10.1016/j.ebiom.2021.103293
Ding, Y., Bu, F., Chen, T., Shi, G., Yuan, X., Feng, Z., et al. (2021). A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 105, 8411–8426. doi: 10.1007/s00253-021-11622-2
Doestzada, M., Vila, A. V., Zhernakova, A., Koonen, D. P. Y., Weersma, R. K., Touw, D. J., et al. (2018). Pharmacomicrobiomics: a novel route towards personalized medicine? Protein Cell 9, 432–445. doi: 10.1007/s13238-018-0547-2
Dong, Z., Kuang, W., Shen, X., and Tian, L. (2021). Plasma levels of Interleukin-6 and antidepressant response to paroxetine in Chinese depressive patients. Psychiatry Res. 297:113723. doi: 10.1016/j.psychres.2021.113723
Dong, Z., Shen, X., Hao, Y., Li, J., Xu, H., Yin, L., et al. (2022). Gut microbiome: a potential Indicator for predicting treatment outcomes in major depressive disorder. Front. Neurosci. 16:813075. doi: 10.3389/fnins.2022.813075
Duan, J., Huang, Y., Tan, X., Chai, T., Wu, J., Zhang, H., et al. (2021). Characterization of gut microbiome in mice model of depression with divergent response to escitalopram treatment. Transl. Psychiatry 11:303. doi: 10.1038/s41398-021-01428-1
Dwyer, J. B., Aftab, A., Radhakrishnan, R., Widge, A., Rodriguez, C. I., Carpenter, L. L., et al. (2020). Hormonal treatments for major depressive disorder: state of the art. Am. J. Psychiatry 177, 686–705. doi: 10.1176/appi.ajp.2020.19080848
Dziedzic, A., Maciak, K., Bliźniewska-Kowalska, K., Gałecka, M., Kobierecka, W., and Saluk, J. (2024). The power of Psychobiotics in depression: a modern approach through the microbiota-gut-brain Axis: a literature review. Nutrients 16:1504. doi: 10.3390/nu16071054
Fabbri, C., Tansey, K. E., Perlis, R. H., Hauser, J., Henigsberg, N., Maier, W., et al. (2018). New insights into the pharmacogenomics of antidepressant response from the Gendep and star*D studies: rare variant analysis and high-density imputation. Pharmacogenomics J. 18, 413–421. doi: 10.1038/tpj.2017.44
Farzi, A., Fröhlich, E. E., and Holzer, P. (2018). Gut microbiota and the neuroendocrine system. Neurotherapeutics 15, 5–22. doi: 10.1007/s13311-017-0600-5
Ferrari, A. J., Charlson, F. J., Norman, R. E., Patten, S. B., Freedman, G., Murray, C. J., et al. (2013). Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 10:e1001547. doi: 10.1371/journal.pmed.1001547
Fond, G., Boukouaci, W., Chevalier, G., Regnault, A., Eberl, G., Hamdani, N., et al. (2015). The "Psychomicrobiotic": targeting microbiota in major psychiatric disorders: a systematic review. Pathol. Biol. 63, 35–42. doi: 10.1016/j.patbio.2014.10.003
Fontana, A., Manchia, M., Panebianco, C., Paribello, P., Arzedi, C., Cossu, E., et al. (2020). Exploring the role of gut microbiota in major depressive disorder and in treatment resistance to antidepressants. Biomedicines 8:311. doi: 10.3390/biomedicines8090311
Furusawa, Y., Obata, Y., Fukuda, S., Endo, T. A., Nakato, G., Takahashi, D., et al. (2013). Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450. doi: 10.1038/nature12721
Gao, M., Tu, H., Liu, P., Zhang, Y., Zhang, R., Jing, L., et al. (2023). Association analysis of gut microbiota and efficacy of Ssris antidepressants in patients with major depressive disorder. J. Affect. Disord. 330, 40–47. doi: 10.1016/j.jad.2023.02.143
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. doi: 10.1016/s0140-6736(18)32279-7
Góralczyk-Bińkowska, A., Szmajda-Krygier, D., and Kozłowska, E. (2022). The microbiota-gut-brain Axis in psychiatric disorders. Int. J. Mol. Sci. 23:11245. doi: 10.3390/ijms231911245
Guideline Development Panel for the Treatment of Depressive Disorders (2022). Summary of the clinical practice guideline for the treatment of depression across three age cohorts. Am. Psychol. 77, 770–780. doi: 10.1037/amp0000904
Hamilton, M. (1960). A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. doi: 10.1136/jnnp.23.1.56
Hao, Z., Wang, W., Guo, R., and Liu, H. (2019). Faecalibacterium Prausnitzii (Atcc 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology 104, 132–142. doi: 10.1016/j.psyneuen.2019.02.025
Herselman, M. F., Bailey, S., and Bobrovskaya, L. (2022). The effects of stress and diet on the "brain-gut" and "gut-brain" pathways in animal models of stress and depression. Int. J. Mol. Sci. 23:2013. doi: 10.3390/ijms23042013
Hou, K., Wu, Z. X., Chen, X. Y., Wang, J. Q., Zhang, D., Xiao, C., et al. (2022). Microbiota in health and diseases. Signal Transduct. Target. Ther. 7:135. doi: 10.1038/s41392-022-00974-4
Hu, X., Li, Y., Wu, J., Zhang, H., Huang, Y., Tan, X., et al. (2023). Changes of gut microbiota reflect the severity of major depressive disorder: a Cross sectional study. Transl. Psychiatry 13:137. doi: 10.1038/s41398-023-02436-z
Huang, Y., Shi, X., Li, Z., Shen, Y., Shi, X., Wang, L., et al. (2018). Possible Association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr. Dis. Treat. 14, 3329–3337. doi: 10.2147/ndt.S188340
Jiang, H., Ling, Z., Zhang, Y., Mao, H., Ma, Z., Yin, Y., et al. (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 48, 186–194. doi: 10.1016/j.bbi.2015.03.016
Joca, S. R., Moreira, F. A., and Wegener, G. (2015). Atypical neurotransmitters and the neurobiology of depression. CNS Neurol. Disord. Drug Targets 14, 1001–1011. doi: 10.2174/1871527314666150909114804
Kant, R., Rasinkangas, P., Satokari, R., Pietilä, T. E., and Palva, A. (2015). Genome sequence of the butyrate-producing anaerobic bacterium Anaerostipes Hadrus Pel 85. Genome Announc. 3:e00224–15. doi: 10.1128/genomeA.00224-15
Karine de Sousa, A., Rocha, J. E., Gonçalves de Souza, T., Sampaio de Freitas, T., Ribeiro-Filho, J., and Melo Coutinho, H. D. (2018). New roles of fluoxetine in pharmacology: antibacterial effect and modulation of antibiotic activity. Microb. Pathog. 123, 368–371. doi: 10.1016/j.micpath.2018.07.040
Keller, M. B. (2003). Past, present, and future directions for defining optimal treatment outcome in depression: remission and beyond. JAMA 289, 3152–3160. doi: 10.1001/jama.289.23.3152
Kelly, J. R., Borre, Y., OB, C., Patterson, E., El Aidy, S., Deane, J., et al. (2016). Transferring the blues: depression-associated gut microbiota induces Neurobehavioural changes in the rat. J. Psychiatr. Res. 82, 109–118. doi: 10.1016/j.jpsychires.2016.07.019
Kelly, J. R., Kennedy, P. J., Cryan, J. F., Dinan, T. G., Clarke, G., and Hyland, N. P. (2015). Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 9:392. doi: 10.3389/fncel.2015.00392
Kessler, R. C., and Bromet, E. J. (2013). The epidemiology of depression across cultures. Annu. Rev. Public Health 34, 119–138. doi: 10.1146/annurev-publhealth-031912-114409
Kim, J., Choi, J. H., Ko, G., Jo, H., Oh, T., Ahn, B., et al. (2020). Anti-inflammatory properties and gut microbiota modulation of Porphyra Tenera extracts in dextran sodium sulfate-induced colitis in mice. Antioxidants 9:988. doi: 10.3390/antiox9100988
Kim, I. B., Park, S. C., and Kim, Y. K. (2023). Microbiota-gut-brain Axis in major depression: a new therapeutic approach. Adv. Exp. Med. Biol. 1411, 209–224. doi: 10.1007/978-981-19-7376-5_10
Klünemann, M., Andrejev, S., Blasche, S., Mateus, A., Phapale, P., Devendran, S., et al. (2021). Bioaccumulation of therapeutic drugs by human gut Bacteria. Nature 597, 533–538. doi: 10.1038/s41586-021-03891-8
Knight, R., Vrbanac, A., Taylor, B. C., Aksenov, A., Callewaert, C., Debelius, J., et al. (2018). Best practices for Analysing microbiomes. Nat. Rev. Microbiol. 16, 410–422. doi: 10.1038/s41579-018-0029-9
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary Fiber to host physiology: short-Chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Kunugi, H. (2021). Gut microbiota and pathophysiology of depressive disorder. Ann. Nutr. Metab. 77, 11–20. doi: 10.1159/000518274
Laterza, L., Rizzatti, G., Gaetani, E., Chiusolo, P., and Gasbarrini, A. (2016). The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterranean J. Hematol. Infect. Diseases 8:e2016025. doi: 10.4084/mjhid.2016.025
Lee, S. M., Dong, T. S., Krause-Sorio, B., Siddarth, P., Milillo, M. M., Lagishetty, V., et al. (2022). The intestinal microbiota as a predictor for antidepressant treatment outcome in geriatric depression: a prospective pilot study. Int. Psychogeriatr. 34, 33–45. doi: 10.1017/s1041610221000120
Lee, S. M., Milillo, M. M., Krause-Sorio, B., Siddarth, P., Kilpatrick, L., Narr, K. L., et al. (2022). Gut microbiome diversity and abundance correlate with gray matter volume (Gmv) in older adults with depression. Int. J. Environ. Res. Public Health 19:2405. doi: 10.3390/ijerph19042405
Lenoir, M., Martín, R., Torres-Maravilla, E., Chadi, S., González-Dávila, P., Sokol, H., et al. (2020). Butyrate mediates anti-inflammatory effects of Faecalibacterium Prausnitzii in intestinal epithelial cells through Dact3. Gut Microbes 12, 1–16. doi: 10.1080/19490976.2020.1826748
Liang, D., Mays, V. M., and Hwang, W. C. (2018). Integrated mental health Services in China: challenges and planning for the future. Health Policy Plan. 33, 107–122. doi: 10.1093/heapol/czx137
Lin, P., Ding, B., Feng, C., Yin, S., Zhang, T., Qi, X., et al. (2017). Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J. Affect. Disord. 207, 300–304. doi: 10.1016/j.jad.2016.09.051
Liskiewicz, P., Kaczmarczyk, M., Misiak, B., Wronski, M., Baba-Kubis, A., Skonieczna-Zydecka, K., et al. (2021). Analysis of gut microbiota and intestinal integrity markers of inpatients with major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 106:106. doi: 10.1016/j.pnpbp.2020.110076
Liu, X., Li, M., Jian, C., and Qin, X. (2024). Characterization of "microbiome-metabolome-immunity" in depressed rats with divergent responses to paroxetine. J. Affect. Disord. 352, 201–213. doi: 10.1016/j.jad.2024.02.017
Lukić, I., Ivković, S., Mitić, M., and Adžić, M. (2022). Tryptophan metabolites in depression: modulation by gut microbiota. Front. Behav. Neurosci. 16:987697. doi: 10.3389/fnbeh.2022.987697
Macedo, D., Filho, A., Soares de Sousa, C. N., Quevedo, J., Barichello, T., Júnior, H. V. N., et al. (2017). Antidepressants, antimicrobials or both? Gut microbiota Dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 208, 22–32. doi: 10.1016/j.jad.2016.09.012
Madan, A., Thompson, D., Fowler, J. C., Ajami, N. J., Salas, R., Frueh, B. C., et al. (2020). The gut microbiota is associated with psychiatric symptom severity and treatment outcome among individuals with serious mental illness. J. Affect. Disord. 264, 98–106. doi: 10.1016/j.jad.2019.12.020
Maini Rekdal, V., Bess, E. N., Bisanz, J. E., Turnbaugh, P. J., and Balskus, E. P. (2019). Discovery and inhibition of an interspecies gut bacterial pathway for levodopa metabolism. Science 364:eaau6323. doi: 10.1126/science.aau6323
Marx, W., Lane, M., Hockey, M., Aslam, H., Berk, M., Walder, K., et al. (2021). Diet and depression: exploring the biological mechanisms of action. Mol. Psychiatry 26, 134–150. doi: 10.1038/s41380-020-00925-x
Matsuzaki, J., Kurokawa, S., Iwamoto, C., Miyaho, K., Takamiya, A., Ishii, C., et al. (2024). Intestinal metabolites predict treatment resistance of patients with depression and anxiety. Gut Pathogens 16:8. doi: 10.1186/s13099-024-00601-3
Meng, Y., Sun, J., and Zhang, G. (2024). Pick fecal microbiota transplantation to enhance therapy for major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 128:110860. doi: 10.1016/j.pnpbp.2023.110860
Minelli, A., Barlati, S., and Baune, B. T. (2022). Evaluating study designs and treatment outcomes of antidepressant pharmacogenetic clinical trials - challenges and future perspectives. A critical review. Eur. Neuropsychopharmacol. 59, 68–81. doi: 10.1016/j.euroneuro.2022.04.007
Molendijk, M., Molero, P., Ortuño Sánchez-Pedreño, F., Van der Does, W., and Angel, M.-G. M. (2018). Diet quality and depression risk: a systematic review and dose-response Meta-analysis of prospective studies. J. Affect. Disord. 226, 346–354. doi: 10.1016/j.jad.2017.09.022
Montgomery, S. A., and Asberg, M. (1979). A new depression scale designed to be sensitive to change. Br. J. Psychiatry 134, 382–389. doi: 10.1192/bjp.134.4.382
Nguyen, T. T., Hathaway, H., Kosciolek, T., Knight, R., and Jeste, D. V. (2021). Gut microbiome in serious mental illnesses: a systematic review and critical evaluation. Schizophr. Res. 234, 24–40. doi: 10.1016/j.schres.2019.08.026
Nikolova, V. L., Cleare, A. J., Young, A. H., and Stone, J. M. (2021). Updated review and Meta-analysis of probiotics for the treatment of clinical depression: adjunctive vs. stand-alone treatment. J. Clin. Med. 10:647. doi: 10.3390/jcm10040647
Olson, C. A., Vuong, H. E., Yano, J. M., Liang, Q. Y., Nusbaum, D. J., and Hsiao, E. Y. (2018). The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell 173, 1728–41.e13. doi: 10.1016/j.cell.2018.04.027
Perler, B. K., Friedman, E. S., and Wu, G. D. (2023). The role of the gut microbiota in the relationship between diet and human health. Annu. Rev. Physiol. 85, 449–468. doi: 10.1146/annurev-physiol-031522-092054
Pettai, K., Milani, L., Tammiste, A., Võsa, U., Kolde, R., Eller, T., et al. (2016). Whole-genome expression analysis reveals genes associated with treatment response to escitalopram in major depression. Eur. Neuropsychopharmacol. 26, 1475–1483. doi: 10.1016/j.euroneuro.2016.06.007
Qaseem, A., Owens, D. K., Etxeandia-Ikobaltzeta, I., Tufte, J., Cross, J. T. Jr., Wilt, T. J., et al. (2023). Nonpharmacologic and pharmacologic treatments of adults in the acute phase of major depressive disorder: a living clinical guideline from the American College of Physicians. Ann. Intern. Med. 176, 239–252. doi: 10.7326/m22-2056
Rieder, R., Wisniewski, P. J., Alderman, B. L., and Campbell, S. C. (2017). Microbes and mental health: a review. Brain Behav. Immun. 66, 9–17. doi: 10.1016/j.bbi.2017.01.016
Rudzki, L., Ostrowska, L., Pawlak, D., Małus, A., Pawlak, K., Waszkiewicz, N., et al. (2019). Probiotic Lactobacillus Plantarum 299v decreases kynurenine concentration and improves cognitive functions in patients with major depression: a double-blind, randomized, placebo controlled study. Psychoneuroendocrinology 100, 213–222. doi: 10.1016/j.psyneuen.2018.10.010
Samara, J., Moossavi, S., Alshaikh, B., Ortega, V. A., Pettersen, V. K., Ferdous, T., et al. (2022). Supplementation with a probiotic mixture accelerates gut microbiome maturation and reduces intestinal inflammation in extremely preterm infants. Cell Host Microbe 30, 696–711.e5. doi: 10.1016/j.chom.2022.04.005
Sanada, K., Nakajima, S., Kurokawa, S., Barceló-Soler, A., Ikuse, D., Hirata, A., et al. (2020). Gut microbiota and major depressive disorder: a systematic review and Meta-analysis. J. Affect. Disord. 266, 1–13. doi: 10.1016/j.jad.2020.01.102
Schmidt, F. M., Kirkby, K. C., and Lichtblau, N. (2016). Inflammation and immune regulation as potential drug targets in antidepressant treatment. Curr. Neuropharmacol. 14, 674–687. doi: 10.2174/1570159x14666160115130414
Schramm, E., Mack, S., Thiel, N., Jenkner, C., Elsaesser, M., and Fangmeier, T. (2020). Interpersonal psychotherapy vs. treatment as usual for major depression related to work stress: a pilot randomized controlled study. Front. Psych. 11:193. doi: 10.3389/fpsyt.2020.00193
Sharma, R., Gupta, D., Mehrotra, R., and Mago, P. (2021). Psychobiotics: the next-generation probiotics for the brain. Curr. Microbiol. 78, 449–463. doi: 10.1007/s00284-020-02289-5
Shen, W., Tao, Y., Zheng, F., Zhou, H., Wu, H., Shi, H., et al. (2023). The alteration of gut microbiota in venlafaxine-ameliorated chronic unpredictable mild stress-induced depression in mice. Behav. Brain Res. 446:114399. doi: 10.1016/j.bbr.2023.114399
Shen, Y., Yang, X., Li, G., Gao, J., and Liang, Y. (2021). The change of gut microbiota in Mdd patients under Ssris treatment. Sci. Rep. 11:14918. doi: 10.1038/s41598-021-94481-1
Sherwin, E., Sandhu, K. V., Dinan, T. G., and Cryan, J. F. (2016). May the force be with you: the light and dark sides of the microbiota-gut-brain Axis in neuropsychiatry. CNS Drugs 30, 1019–1041. doi: 10.1007/s40263-016-0370-3
Song, X., Wang, W., Ding, S., Wang, Y., Ye, L., Chen, X., et al. (2022). Exploring the potential antidepressant mechanisms of Puerarin: anti-inflammatory response via the gut-brain Axis. J. Affect. Disord. 310, 459–471. doi: 10.1016/j.jad.2022.05.044
Stilling, R. M., van de Wouw, M., Clarke, G., Stanton, C., Dinan, T. G., and Cryan, J. F. (2016). The neuropharmacology of butyrate: the bread and butter of the microbiota-gut-brain Axis? Neurochem. Int. 99, 110–132. doi: 10.1016/j.neuint.2016.06.011
Sugahara, H., Odamaki, T., Fukuda, S., Kato, T., Xiao, J. Z., Abe, F., et al. (2015). Probiotic Bifidobacterium Longum alters gut luminal metabolism through modification of the gut microbial community. Sci. Rep. 5:13548. doi: 10.1038/srep13548
Szałach, Ł. P., Lisowska, K. A., and Cubała, W. J. (2019). The influence of antidepressants on the immune system. Arch. Immunol. Ther. Exp. 67, 143–151. doi: 10.1007/s00005-019-00543-8
Thursby, E., and Juge, N. (2017). Introduction to the human gut microbiota. Biochem. J. 474, 1823–1836. doi: 10.1042/bcj20160510
Tian, P., Chen, Y., Zhu, H., Wang, L., Qian, X., Zou, R., et al. (2022). Bifidobacterium Breve Ccfm1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: a randomized clinical trial. Brain Behav. Immun. 100, 233–241. doi: 10.1016/j.bbi.2021.11.023
Trzeciak, P., and Herbet, M. (2021). Role of the intestinal microbiome, intestinal barrier and Psychobiotics in depression. Nutrients 13:927. doi: 10.3390/nu13030927
Tsigos, C., Kyrou, I., Kassi, E., and Chrousos, G. P. (2000). “Stress: endocrine physiology and pathophysiology” in Endotext. eds. K. R. Feingold, B. Anawalt, M. R. Blackman, A. Boyce, G. Chrousos, and E. Corpas, et al. (South Dartmouth, MA: MDText.com, Inc).
Unger, M. M., Spiegel, J., Dillmann, K. U., Grundmann, D., Philippeit, H., Bürmann, J., et al. (2016). Short Chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age-matched controls. Parkinsonism Relat. Disord. 32, 66–72. doi: 10.1016/j.parkreldis.2016.08.019
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
Vasileva, S. S., Yang, Y., Baker, A., Siskind, D., Gratten, J., and Eyles, D. (2024). Associations of the gut microbiome with treatment resistance in schizophrenia. JAMA Psychiatry 81, 292–302. doi: 10.1001/jamapsychiatry.2023.5371
Vitetta, L., Bambling, M., and Alford, H. (2014). The gastrointestinal tract microbiome, probiotics, and mood. Inflammopharmacology 22, 333–339. doi: 10.1007/s10787-014-0216-x
Wang, S., Ishima, T., Zhang, J., Qu, Y., Chang, L., Pu, Y., et al. (2020). Ingestion of Lactobacillus intestinalis and Lactobacillus Reuteri causes depression- and anhedonia-like phenotypes in antibiotic-treated mice via the Vagus nerve. J. Neuroinflammation 17:241. doi: 10.1186/s12974-020-01916-z
Wang, X. W., and Liu, Y. Y. (2020). Comparative study of classifiers for human microbiome data. Med. Microecol. 4:4. doi: 10.1016/j.medmic.2020.100013
Wang, D., Wu, J., Zhu, P., Xie, H., Lu, L., Bai, W., et al. (2022). Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: the potential involvement of gut-brain Axis. Food Res. Int. 157:111289. doi: 10.1016/j.foodres.2022.111289
Wang, Y., Zhou, J., Ye, J., Sun, Z., He, Y., Zhao, Y., et al. (2023). Multi-omics reveal microbial determinants impacting the treatment outcome of antidepressants in major depressive disorder. Microbiome 11:195. doi: 10.1186/s40168-023-01635-6
Weersma, R. K., Zhernakova, A., and Fu, J. (2020). Interaction between drugs and the gut microbiome. Gut 69, 1510–1519. doi: 10.1136/gutjnl-2019-320204
Werbner, M., Barsheshet, Y., Werbner, N., Zigdon, M., Averbuch, I., Ziv, O., et al. (2019). Social-stress-responsive microbiota induces stimulation of self-reactive effector T helper cells. mSystems 4, e00292-18. doi: 10.1128/mSystems.00292-18
Westfall, S., Caracci, F., Zhao, D., Wu, Q.-l., Frolinger, T., Simon, J., et al. (2021). Microbiota metabolites modulate the T helper 17 to regulatory T cell (Th17/Treg) imbalance promoting resilience to stress-induced anxiety- and depressive-like behaviors. Brain Behav. Immun. 91, 350–368. doi: 10.1016/j.bbi.2020.10.013
Wilson, I. D., and Nicholson, J. K. (2017). Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 179, 204–222. doi: 10.1016/j.trsl.2016.08.002
Wong, M. L., Inserra, A., Lewis, M. D., Mastronardi, C. A., Leong, L., Choo, J., et al. (2016). Inflammasome signaling affects anxiety- and depressive-like behavior and gut microbiome composition. Mol. Psychiatry 21, 797–805. doi: 10.1038/mp.2016.46
Wu, H., Esteve, E., Tremaroli, V., Khan, M. T., Caesar, R., Mannerås-Holm, L., et al. (2017). Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858. doi: 10.1038/nm.4345
Wu, L., Ran, L., Wu, Y., Liang, M., Zeng, J., Ke, F., et al. (2022). Oral administration of 5-Hydroxytryptophan restores gut microbiota dysbiosis in a mouse model of depression. Front. Microbiol. 13:864571. doi: 10.3389/fmicb.2022.864571
Xiong, L. L., Mao, M. L., and Shu, Q. L. (2022). A preliminary study on the diversity of butyrate-producing Bacteria in response to the treatment of depression with Xiaoyaosan. Lett. Appl. Microbiol. 75, 844–856. doi: 10.1111/lam.13737
Xu, F., Xie, Q., Kuang, W., and Dong, Z. (2023). Interactions between antidepressants and intestinal microbiota. Neurotherapeutics 20, 359–371. doi: 10.1007/s13311-023-01362-8
Yamamura, R., Okubo, R., Katsumata, N., Odamaki, T., Hashimoto, N., Kusumi, I., et al. (2021). Lipid and energy metabolism of the gut microbiota is associated with the response to probiotic Bifidobacterium Breve strain for anxiety and depressive symptoms in schizophrenia. J. Pers. Med. 11:987. doi: 10.3390/jpm11100987
Ye, X., Wang, D., Zhu, H., Wang, D., Li, J., Tang, Y., et al. (2021). Gut microbiota changes in patients with major depressive disorder treated with vortioxetine. Front. Psych. 12:641491. doi: 10.3389/fpsyt.2021.641491
Zhdanava, M., Pilon, D., Ghelerter, I., Chow, W., Joshi, K., Lefebvre, P., et al. (2021). The prevalence and National Burden of treatment-resistant depression and major depressive disorder in the United States. J. Clin. Psychiatry 82:20m13699. doi: 10.4088/JCP.20m13699
Zheng, P., Zeng, B., Zhou, C., Liu, M., Fang, Z., Xu, X., et al. (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the Host's metabolism. Mol. Psychiatry 21, 786–796. doi: 10.1038/mp.2016.44
Zhou, M., Fan, Y., Xu, L., Yu, Z., Wang, S., Xu, H., et al. (2023). Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 11:145. doi: 10.1186/s40168-023-01589-9
Zhou, L., Zhang, M., Wang, Y., Dorfman, R. G., Liu, H., Yu, T., et al. (2018). Faecalibacterium Prausnitzii produces butyrate to maintain Th17/Treg balance and to ameliorate colorectal colitis by inhibiting histone deacetylase 1. Inflamm. Bowel Dis. 24, 1926–1940. doi: 10.1093/ibd/izy182
Zimmermann, M., Zimmermann-Kogadeeva, M., Wegmann, R., and Goodman, A. L. (2019b). Separating host and microbiome contributions to drug pharmacokinetics and toxicity. Science 363:eaat9931. doi: 10.1126/science.aat9931
Keywords: major depressive disorder, gut microbiota, gut-brain axis, psychobiotic, treatment response
Citation: Xie Y, Zhu H, Yuan Y, Guan X, Xie Q and Dong Z (2024) Baseline gut microbiota profiles affect treatment response in patients with depression. Front. Microbiol. 15:1429116. doi: 10.3389/fmicb.2024.1429116
Edited by:
Jin Song, Capital Medical University, ChinaReviewed by:
Shabnam Nohesara, Iran University of Medical Sciences, IranWenzhi Hao, Jinan University, China
Copyright © 2024 Xie, Zhu, Yuan, Guan, Xie and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinglian Xie, eGllcWluZ2xpYW4xMjNAd2Noc2N1LmNu; Zaiquan Dong, emFpcXVhbmRvbmdAd2Noc2N1LmNu
 Yingjing Xie1
Yingjing Xie1 Zaiquan Dong
Zaiquan Dong