
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 July 2024
Sec. Infectious Agents and Disease
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1427327
 Shima Mahmoudi1,2
Shima Mahmoudi1,2 Babak Pourakbari2
Babak Pourakbari2 Mohammad Ali Shahbabaie3
Mohammad Ali Shahbabaie3 Maryam Sotoudeh4
Maryam Sotoudeh4 Erfaneh Jafari2
Erfaneh Jafari2 Reihaneh Hosseinpour Sadeghi2*
Reihaneh Hosseinpour Sadeghi2* Setareh Mamishi2,4*
Setareh Mamishi2,4*Introduction: Limited data are available regarding SARS-CoV-2 serological response dynamics in pediatric patients with COVID-19, contributing to gaps in our understanding of the immune response in this population. This study aimed to investigate SARS-CoV-2 IgG seropositivity in patients diagnosed with COVID-19 during hospitalization and 2–4 weeks after discharge.
Methods: A cohort of patients, consisting of 31 individuals with confirmed acute COVID-19 infection and 27 diagnosed with Multisystem Inflammatory Syndrome in Children (MIS-C), was enrolled in the study. Follow-up clinic appointments were scheduled for 2–4 weeks post-discharge. During admission and follow-up, blood samples were collected from each patient for laboratory analysis. Anti-nucleoprotein SARS-CoV-2 IgG levels were determined using the Enzyme-Linked Immunosorbent Assay (ELISA) method.
Results: In this study, a cohort of 58 patients was examined. At admission, 52% (n = 14) of MIS-C patients and 10% (n = 3) of acute COVID-19 patients had positive SARS-CoV-2 IgG test. Only 48 cases were referred to the hospital, and follow-up data was available for 20 cases with MIS-C and 28 cases with acute COVID-19. All patients (n = 15) who initially tested positive for SARS-CoV-2 IgG at admission remained positive serology during follow-up (100%). Among the 33 patients who initially tested negative, 12 (37.5%) showed a positive serology result during follow-up, while 21 (62.5%) remained negative. Within this subgroup, 11 cases (44%) were diagnosed with acute COVID-19, and one patient (12.5%) presented with MIS-C. Fourteen cases with acute COVID-19 infection (56%) and seven cases with MIS-C (87.5%) consistently showed negative serology results throughout the study. During follow-up, the median lymphocyte count demonstrated a significant difference, with 0.96 × 109 cells per L (IQR: 0.75–3.0 × 109 cells per L) in the SARS-CoV-2 IgG-negative group and 2.9 × 109 cells per L (IQR = 1.33–7.22 × 109 cells per L) in the SARS-CoV-2 IgG-positive group (p-value = 0.03). Patients who demonstrated seropositivity during the follow-up were associated with a notably severe disease (p-value = 0.028).
Conclusion: Our study highlights the dynamic nature of SARS-CoV-2 IgG antibody responses in pediatric patients with COVID-19 infection. We observed a notable increase in seropositivity rates during follow-up. Furthermore, patients who were seropositive at follow-up demonstrated a severe disease course and lower lymphocyte counts compared to those with persistently negative serology. Our findings underscore the importance of longitudinal serological monitoring in understanding disease progression and immune response dynamics in pediatric COVID-19 cases.
In the early stages of the Coronavirus disease 2019 (COVID-19) pandemic, the proportion of confirmed cases among children was relatively low, and children were thought to be rarely affected by SARS-CoV-2 (Mahmoudi et al., 2020; Wu and McGoogan, 2020; Mohammadpour et al., 2022). Subsequent studies have consistently shown that children and adolescents are susceptible to SARS-CoV-2 infection, however, a large percentage of children are either asymptomatic or mildly symptomatic, and the true incidence of infection is underestimated due to low testing rates in children (Mahmoudi et al., 2023). Despite generally milder symptoms, children with COVID-19 are shown to be at a lower risk of hospitalization and severe complications (Vosoughi et al., 2022; Sedik, 2023). However, children with comorbidities may manifest more severe symptoms (Ekbatani et al., 2021; Mahmoudi et al., 2021). Moreover, in the course of the COVID-19 pandemic, a distinct phenomenon known as a multisystem inflammatory syndrome in children (MIS-C) has emerged, showing similarities to Kawasaki Disease (Mamishi et al., 2020, 2022a,b; Sancho-Shimizu et al., 2021). Both MIS-C and Kawasaki Disease present with mucocutaneous involvement, conjunctivitis, lymphadenopathy, and elevated inflammatory markers. However, the differentiation between MIS-C and Kawasaki Disease remains a topic of ongoing debate among medical professionals (Rostami-Maskopaee et al., 2022).
To complement the diagnosis, tests such as real-time reverse transcription PCR (RT-PCR) for SARS-CoV-2 and serology have been used. Serology relies on the detection of antibodies, specifically IgM and IgG, to offer valuable insights into an individual’s exposure to the SARS-CoV-2 virus. IgM antibodies, emerging early post-infection, act as initial indicators but exhibit a swift decline, limiting their reliability in later stages. On the contrary, IgG antibodies, appearing later in the infection timeline, persist for an extended period, offering a more stable marker of past infection or vaccination (Lippi et al., 2023). Limited data are available regarding SARS-CoV-2 serological response dynamics in pediatric patients with COVID-19, and there are substantial gaps in our understanding of the immune response in children (Bohn et al., 2023). A recent meta-analysis estimated the combined prevalence of positive SARS-CoV-2 IgM and IgG antibody tests in MIS-C to be 39% and 81%, respectively (Ghazizadeh Esslami et al., 2023). However, there is a scarcity of reports in pediatrics, particularly those employing a prospective or longitudinal design regarding SARS-CoV-2 serological response dynamics. This could be attributed to the challenges associated with accessing patient data and acquiring blood samples (Hoste et al., 2023).
This study aimed to investigate SARS-CoV-2 IgG seropositivity in patients diagnosed with COVID-19 during hospitalization and 2–4 weeks after discharge. This is a valuable approach to understanding the dynamics of antibody response over time, which can provide insights into the duration and persistence of immunity following infection.
In this study, patients referred to Children’s Medical Center between September 2020 and April 2021, age ≤ 18 with a confirmed diagnosis of acute COVID-19 or MIS-C were enrolled. The sampling protocol was approved by the Ethical Committee of Tehran University of Medical Sciences, Tehran, Iran (IR.TUMS.CHMC.REC1399.069), and written consent was obtained from parents/guardians of patients before data collection.
Confirmation of acute COVID-19 infection relied on clinical symptoms (fever, cough, shortness of breath, fatigue, etc.), along with SARS-COV-2 positive RT-PCR (Mamishi et al., 2022b). MIS-C diagnosis was established based on a history of close contact with SARS-CoV-2, persistent fever (>38°C for more than 24 h), involvement of at least two organs including cardiac, renal, respiratory, hematologic, dermatologic, gastrointestinal or neurological, and laboratory results confirming systemic inflammation (Mamishi et al., 2020).
Patients who met the following criteria were eligible for discharge: they had been afebrile for more than 3 days, showed improved respiratory symptoms, had pulmonary imaging indicating significant resolution of inflammation, and/or tested negative for SARS-CoV-2 RT-PCR twice consecutively (sampling interval ≥ 24 h). After taking blood samples from the patients to perform laboratory and serology tests, the parents were asked to refer to the specialized clinic 2–4 weeks after discharge for follow-up. Moreover, the characteristics data including age, sex, presence of underlying diseases, immunodeficiency, and previous known COVID-19 contact were collected via a questionnaire. A complete blood count (White blood cell (WBC), neutrophil, lymphocyte, and platelet count), D-Dimer, ferritin, fibrinogen, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and CD4+ and CD8+ T cell measurements were performed at admission and during a 2- to 4-week follow-up period after discharge. The cells were examined using a BD FACS Canto II flow cytometer (BD Biosciences). Based on the patients’ age, the lymphocyte subset percentage was divided into three categories: below normal range, within normal range, and above normal range (Mahmoudi et al., 2021; Shearer et al., 2003).
Serum samples were collected at admission and 2–4 weeks after discharge. For serological analysis, enzyme-linked immunoabsorbent assay (ELISA) was done on the serum samples using Pishtaz Teb Anti-nucleoprotein SARS-CoV-2 ELISA (IgG) and according to manufacturer’s instructions to measure antibody titer against SARS-CoV-2. The cut-off value was obtained according to the following formula: Cut-off value = 0.15 + the average light absorbance of the negative control (Cut-off Index (COI) = OD of sample/cut-off value). Values higher than 1.1 were considered positive and values lower than 0.9 were considered negative.
The data was analyzed using SPSS software version 22. Categorical variables were reported as frequencies and percentages, whereas continuous variables were described as median and interquartile range (IQR) values. Chi-square tests were performed to compare variables, and two-sided p-values less than 0.05 were considered statistically significant.
In this study, a cohort of 58 patients were examined, with 31 individuals confirmed to have acute COVID-19 infection and 27 diagnosed with MIS-C.
The sex distribution was 55% males (n = 17) in the acute COVID-19 group and 56% males (n = 15) in the MIS-C group. Participants ranged in age from less than 1 month to 15 years, with a mean age of 71.3 ± 60.0 months. All cases were supposed to have follow-up laboratory tests and SARS-CoV-2 IgG tests 2–4 weeks later. Only 48 cases were referred to the hospital, and follow-up data was available for 20 cases with MIS-C and 28 cases with acute COVID-19. The mean time interval between the two tests was 20.7 ± 11.1 days for patients with MIS-C and 20.3 ± 11.2 days for patients with acute COVID-19. At admission, 52% (n = 14) of MIS-C patients and 10% (n = 3) of acute COVID-19 patients were SARS-CoV-2 IgG positive. During follow-up, the positive SARS-CoV-2 IgG was found in 56.3% (n = 27) of cases with MIS-C and 52% (n = 14) in cases with acute COVID-19 (Figure 1).
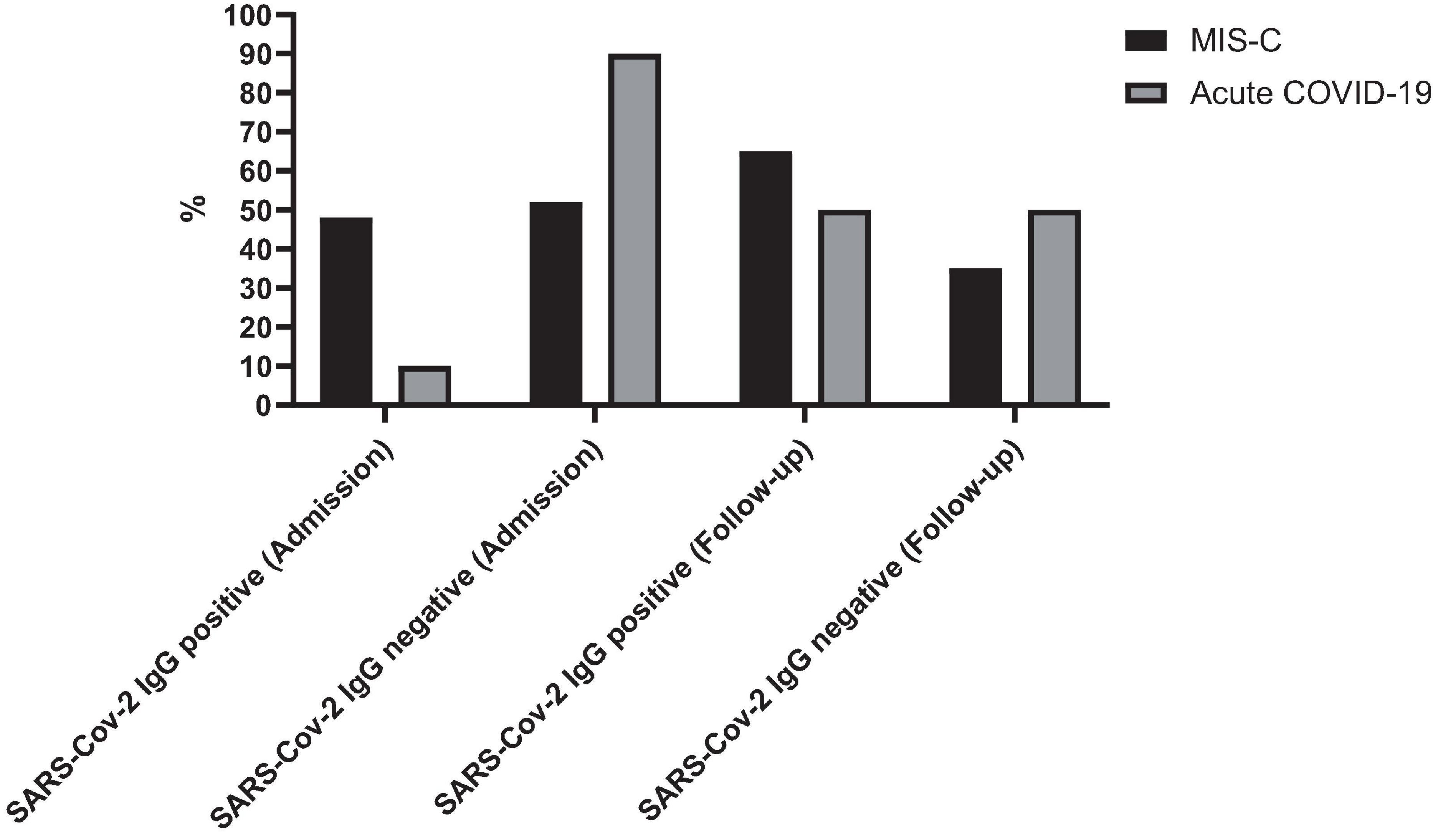
Figure 1. Seropositivity rates of SARS-CoV-2 IgG in patients with MIS-C and acute COVID-19 at admission and during follow-up. The figure illustrates the percentage of patients testing positive for SARS-CoV-2 IgG antibodies at admission and during follow-up.
The mean age of patients with positive SARS-CoV-2 IgG did not significantly differ from those with negative SARS-CoV-2 IgG at admission (82 ± 56 months vs. 66.8 ± 61.4 months) (p-value = 0.39). A notable association was observed between the presence of underlying disease and the positivity of IgG at admission (p-value = 0.016). Among cases with negative SARS-CoV-2 IgG at admission, 46% (19 out of 41) had underlying disease, whereas only 12% of cases (2 out of 17) with positive SARS-CoV-2 IgG had underlying disease. Immune deficiency did not show a significant association with IgG positivity either at admission (p-value = 0.41) or during follow-up (p-value = 0.71). Conversely, the severity of the disease, classified as mild, moderate, or severe, was significantly associated with IgG positivity at admission. Remarkably, this association persisted and remained significant during follow-up (p-value = 0.008).
The examination of CD4+ and CD8+ T cell markers’ normality or impairment upon admission and subsequent follow-up revealed significant trends. In MIS-C patients, 60% (three out of five cases) with subnormal CD4+ T cell levels at admission normalized during follow-up. Conversely, among patients with acute COVID-19, only 20% (n = 2) with elevated CD4+ T cell levels experienced normalization during follow-up, while 70% (n = 7) maintained elevated levels at follow-up.
Regarding CD8+ T cell markers, there was an increase during follow-up in 71% of MIS-C cases (5 out of 7) and 27% (3 cases) of patients with acute COVID-19. Notably, half of the patients (n = 3) with elevated CD8+ T cell levels at admission demonstrated normal levels during follow-up (p-value = 0.026).
Figure 2 presents the serology results of 48 patients who underwent SARS-CoV-2 IgG testing both at the time of admission and during a follow-up period 2–4 weeks later. All patients (n = 15) who initially tested positive for SARS-CoV-2 IgG at admission remained positive serology during follow-up (100%). Among the 33 patients who initially tested negative, 12 (37.5%) showed a positive serology result during follow-up, while 21 (62.5%) remained negative (p-value < 0.001). Within this subgroup, 11 cases (44%) were diagnosed with acute COVID-19, and one patient (12.5%) presented with MIS-C. Fourteen cases with acute COVID-19 infection (56%) and seven cases with MIS-C (87.5%) consistently showed negative serology results throughout the study. Figure 3 shows the anti-nucleoprotein SARS-CoV-2 IgG levels in patients at admission and follow-up in patients with MIS-C and acute COVID-19. The median SARS-CoV-2 IgG COI level in patients with acute COVID-19 did not significantly differ between admission (0.2 [IQR: 0.2-0.3]) and follow-up (0.5 [IQR: 0.4–7.47]). On the other hand, patients with MIS-C exhibited a markedly higher median SARS-CoV-2 IgG COI level during follow-up (15.1 [IQR: 0.6–35.9]) compared to admission (5.85 [IQR: 0.4–11.6]) (p-value < 0.001) (Figure 4). Due to the limited sample size in the MIS-C group, which comprised only 8 patients, the examination of factors influencing seropositivity was exclusively conducted within the cohort diagnosed with acute COVID-19 infection. Table 1 provides a comparison of data for patients who initially tested negative for SARS-CoV-2 IgG antibodies at admission. It categorizes them based on subsequent negative or positive SARS-CoV-2 IgG test results during follow-up. The median WBC count was 5.16 × 109 cells per L (IQR = 3.77–9.3 × 109 cells per L) in the group that remained negative for SARS-CoV-2 IgG and 9.47 × 109 cells per L (IQR = 3.88–11.47 × 109 cells per L) in the subset that converted to SARS-CoV-2 IgG-positive status at follow-up, showing no significant difference (p-value = 0.2). During follow-up, the median lymphocyte count demonstrated a significant difference, with 0.96 × 109 cells per L (IQR: 0.75–3.0 × 109 cells per L) in the SARS-CoV-2 IgG-negative group and 2.9 × 109 cells per L (IQR = 1.33-7.22 × 109 cells per L) in the SARS-CoV-2 IgG-positive group (p-value = 0.03). Other parameters, including neutrophil count, platelet count, ferritin level, fibrinogen level, D-dimer level, CRP level, ESR, and CD4+ and CD8+ T cell percentage, did not exhibit significant differences between the two groups.
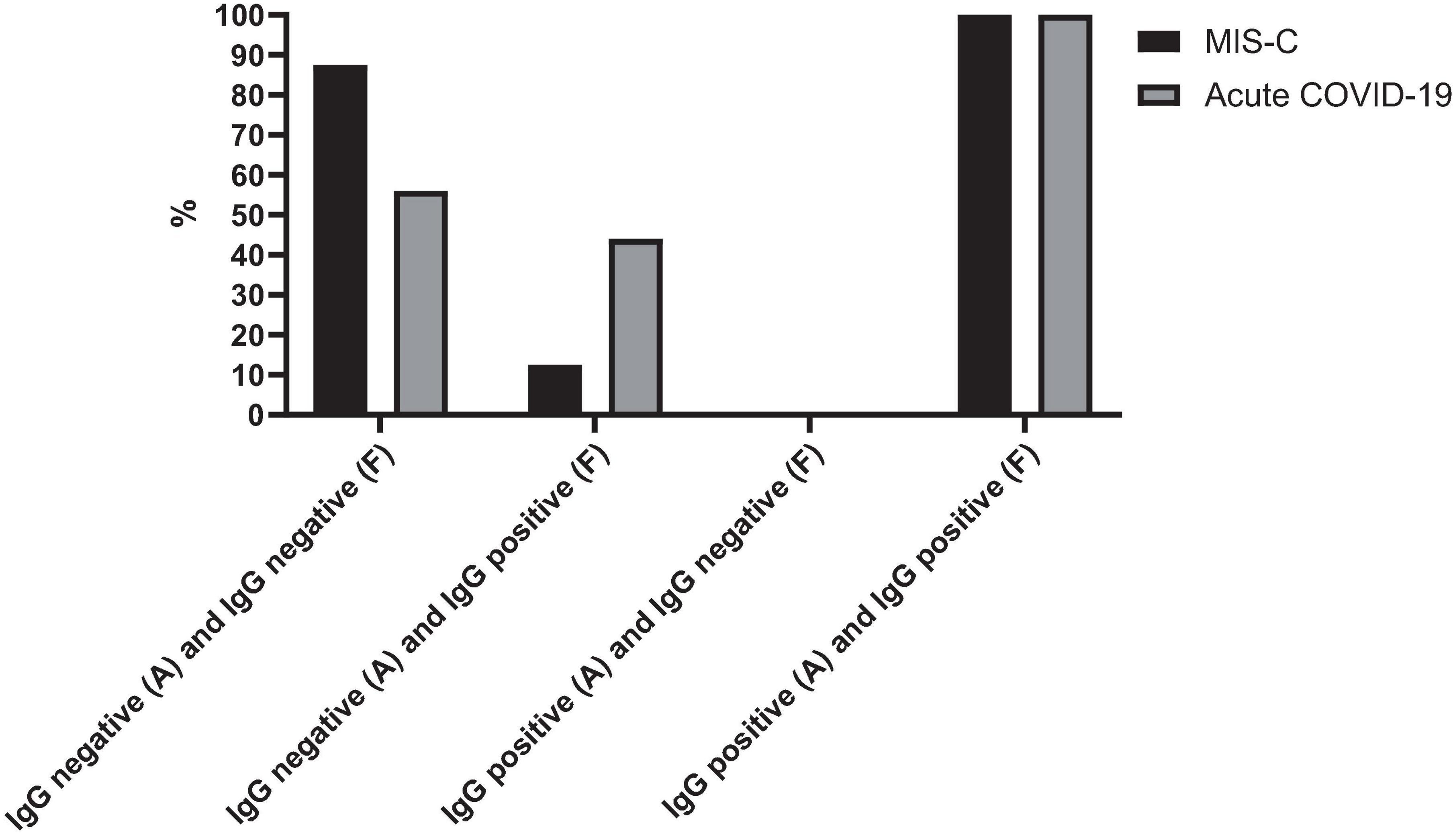
Figure 2. SARS-CoV-2 IgG test changes over time in cases with MIS-C and acute COVID-19. The figure presents the serology outcomes of 48 patients who underwent both admission and follow-up SARS-CoV-2 IgG testing. A, admission; F, follow-up.

Figure 3. Anti-nucleoprotein SARS-CoV-2 IgG level in patients at admission and follow-up in panel (A) MIS-C group, (B) acute COVID-19 group. SARS-CoV-2 IgG was calculated based on the cut-off index (COI). Among the 8 patients with MIS-C who had a negative SARS-CoV-2 IgG level at admission, 7 (87.5%) remained negative during the follow-up, while 1 (12.5%) became positive in the follow-up. Among patients with acute COVID-19, 3 patients had a positive SARS-CoV-2 IgG level at both admission and follow-up. Among the 25 patients who had a negative SARS-CoV-2 IgG level at admission, 14 (56%) remained negative, while 11 (44%) showed a positive SARS-CoV-2 IgG level at follow-up.
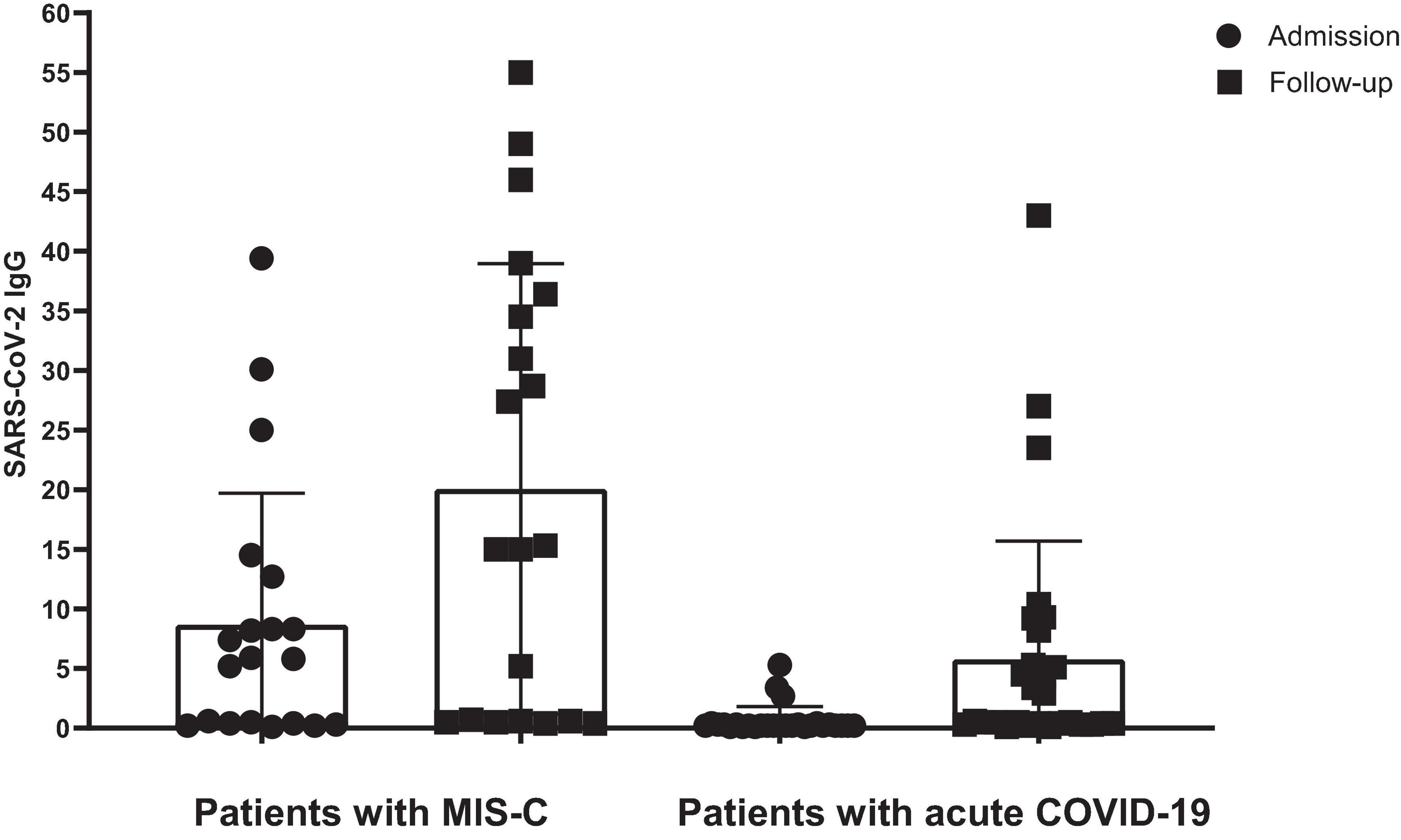
Figure 4. The level of SARS-CoV-2 IgG in patients with MIS-C and acute COVID-19. SARS-CoV-2 IgG was calculated based on the cut-off index (COI). Lines represent medians and interquartile ranges.
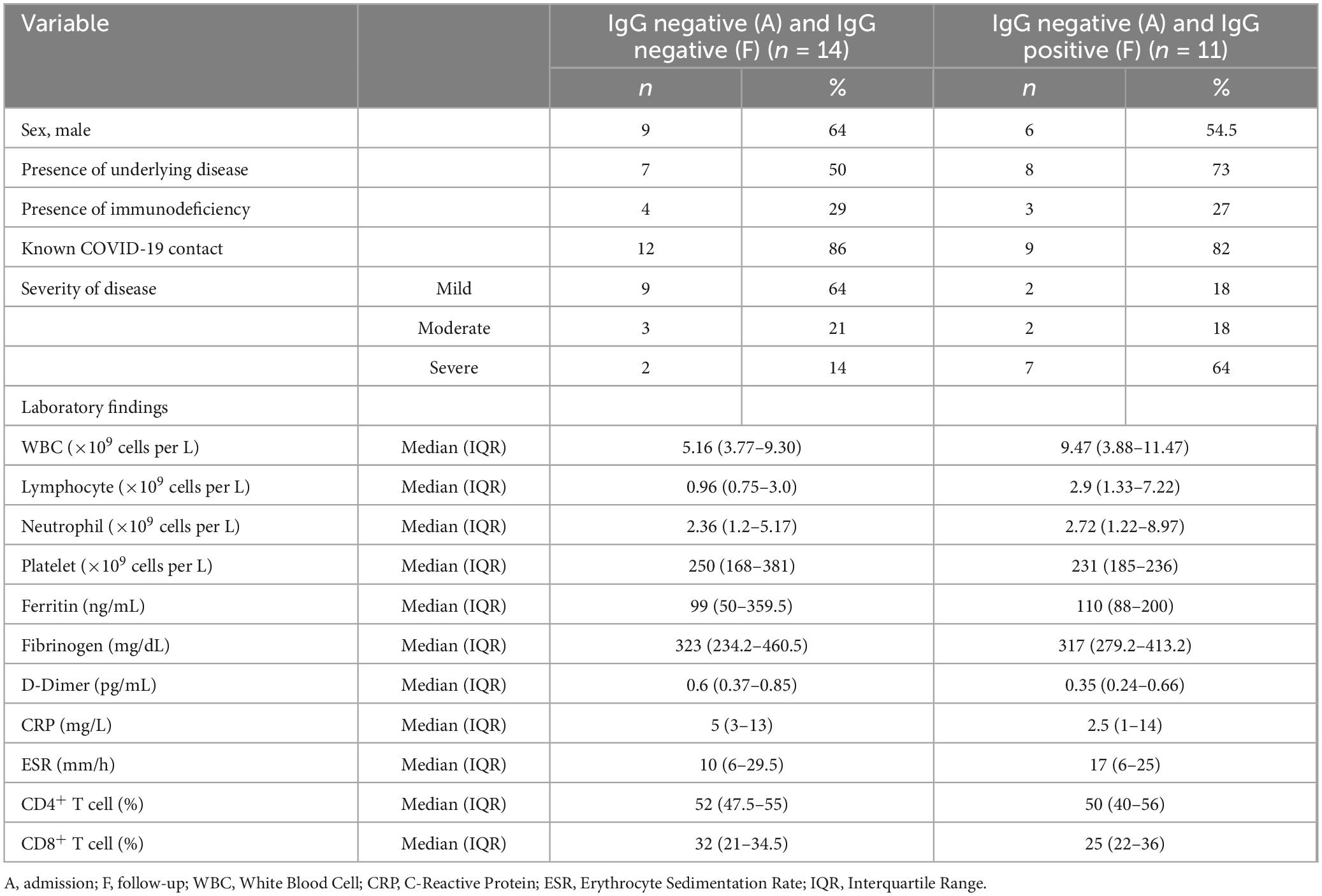
Table 1. Comparison of laboratory findings and clinical characteristics of pediatric patients with acute COVID-19, initially negative for SARS-CoV-2 IgG antibodies, stratified by subsequent SARS-CoV-2 IgG test results in follow-up.
Among patients who tested negative for SARS-CoV-2 IgG at admission, 40.5% (15 out of 37) had CD4+ T cell counts higher than the normal range, 54% (20 out of 37) had counts within the normal range, and 5 (2 out of 37) had counts lower than the normal range. In comparison, among cases with positive SARS-CoV-2 IgG at admission, 33% (4 out of 12), 58% (7 out of 12), and 8% (1 out of 12) had lower, within the normal range, and higher than normal range CD4+ T cell counts, respectively (p-value = 0.013). Conversely, there was no significant difference observed in seropositivity concerning CD8+ T cells (p-value = 0.955).
Patients who were seropositive during the follow-up were associated with a notably severe disease (p-value = 0.028) (Figure 5). Specifically, among cases of acute COVID-19 with negative SARS-CoV-2 IgG tests upon admission but positive results during follow-up, 64% (n = 7) experienced severe COVID-19. In contrast, only 14% (n = 2) of cases with negative SARS-CoV-2 IgG tests both at admission and follow-up had severe COVID-19.
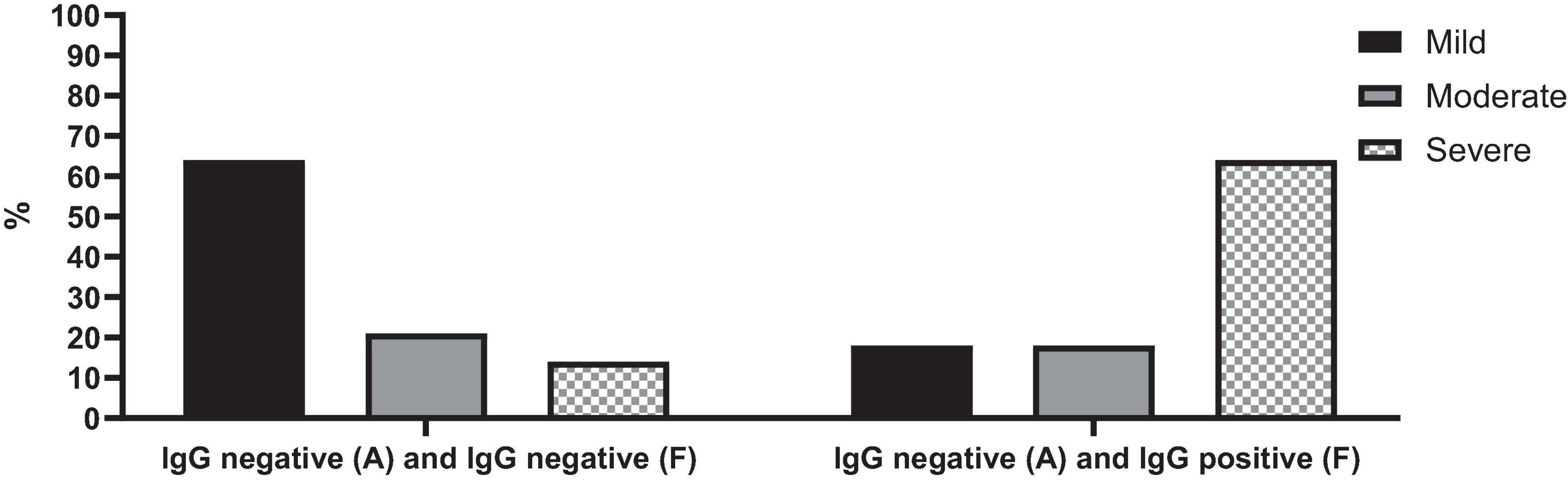
Figure 5. SARS-CoV-2 IgG serology test changes over time in cases with acute COVID-19 based on the severity of the disease.
Our study contributes valuable insights into the serological response dynamics of pediatric patients diagnosed with acute COVID-19 and MIS-C. We observed a higher proportion of MIS-C patients testing positive for SARS-CoV-2 IgG at admission compared to acute COVID-19 patients, with 52% of MIS-C patients and only 10% of acute COVID-19 patients exhibiting seropositivity. Moreover, our longitudinal analysis revealed interesting trends in seropositivity rates during follow-up. All patients who initially tested positive for SARS-CoV-2 IgG at admission maintained positive serology throughout the follow-up period, indicating stable immune responses over time. In contrast, among patients initially testing negative, 37.5% exhibited seropositivity during follow-up. This observation underscores the dynamic nature of immune responses in pediatric COVID-19 cases and highlights the importance of longitudinal serological monitoring to capture changes in serostatus over time. This finding aligns with previous research indicating that antibody responses may take time to reach detectable levels, underscoring the importance of continued monitoring (Mamishi et al., 2021; Oygar et al., 2021).
In the previous study investigating the long-term antibody response to SARS-CoV-2 in children, detectable levels of anti-spike IgG and anti-nucleocapsid IgA/IgG/IgM antibodies were found for up to 270 days after the onset of symptoms (Dunay et al., 2023). Additionally, in longitudinal monitoring of antibody responses in pediatric populations, 95.8% of individuals maintained positive antibody status for a duration of up to 9 months post-infection (Oygar et al., 2021). In the study of Luo et al. (2020), during follow-up, SARS-CoV-2 IgG antibody was positive in 178 cases (10.7%), which was much lower than our report. In the study of Isoldi et al. (2021), an increase in SARS-CoV-2 IgG level was recorded in all patients after 1 month (mean IgG level 84.9 ± 24.7, median = 89.4, IQR = 32.3). In our study, patients who exhibited positive SARS-CoV-2 IgG during follow-up were associated with more severe disease manifestations and lower lymphocyte counts. This suggests a potential link between the strength of the immune response, disease severity, and the likelihood of seropositivity.
In adult patients, it is common to observe complete blood count abnormalities, especially lymphopenia, which has not been confirmed in children (Cohen et al., 2020; Rezaei et al., 2021). In our study, the median lymphocyte count in the SARS-CoV-2 IgG-negative group was 2.9 × 109 cells per L (IQR: 1.33–7.22 × 109 cells per L), while in the group with SARS-CoV-2 IgG-positive, it was 0.96 × 109 cells per L (IQR: 0.75–3 × 109 cells per L). In simpler terms, individuals with acute COVID-19 infection who were seropositive during the follow-up period had a lower lymphocyte count compared to those who remained negative serology.
All patients who tested positive for SARS-Cov-2 IgG at admission consistently maintained positive serology during follow-up (15 cases, 100%). In contrast, among the cohort of 33 patients who were negative for SARS-Cov-2 IgG at admission, 12 cases demonstrated seropositivity during follow-up (37.5%), while the remaining 21 patients remained seronegative (62.5%) (p-value < 0.001).
In a study by Zinszer et al. (2023) conducted during the early Omicron-dominant period from May 2022 to October 2022, a significant increase in seroconversion rates among seronegative children was observed. Specifically, children in this study were approximately 9–12 times more likely to seroconvert during this period compared to previous rounds of data collection conducted from May to September 2021 and November 2021 to February 2022 (Zinszer et al., 2023).
Several immunological hypotheses have been proposed to elucidate why children may exhibit lower rates of seroconversion (Selva et al., 2021). Firstly, differences in antibody profiles, including antibody isotypes and subclasses, along with variations in memory B-cell populations, may contribute to this phenomenon. Secondly, disparities in T-cell responses have been noted between SARS-CoV-2-infected children and adults. However, these findings are predominantly associated with disease severity (Toh et al., 2022).
SARS-CoV2 antibody responses have been observed to correlate with several factors, including age, viral load, sex, comorbidities (such as diabetes, cancer, and immunosuppression), as well as the severity of the disease (Toh et al., 2022). In this study, we did not find a significant relationship between the serological results of the patients or seropositivity with the age and sex of the patients, and the presence of underlying diseases. However, upon comparing lymphocyte counts between the two groups, those whose SARS-CoV-2 IgG became positive during follow-up and those who remained negative, we observed a significant difference.
Our study encountered several limitations, notably regarding the acquisition of follow-up blood samples from all enrolled children. Some families expressed concerns about returning to the hospital due to fears of potential reinfection, leading to incomplete follow-up data. Additionally, the study was restricted by a relatively small sample size, potentially limiting the generalizability of the findings to a broader population. Furthermore, we did not assess the long-term persistence of antibodies, and the duration of immunity post-infection remains an area requiring further investigation.
In conclusion, our study highlights the dynamic nature of SARS-CoV-2 IgG antibody responses in pediatric patients with COVID-19 infection. We observed a notable increase in seropositivity rates during follow-up. Furthermore, patients who were seropositive during follow-up demonstrated a more severe disease course and lower lymphocyte counts compared to those with persistently negative serology. Our findings underscore the importance of longitudinal serological monitoring in understanding disease progression and immune response dynamics in pediatric COVID-19 cases. Continuous follow-up of COVID-19 patients can provide valuable insights into their clinical conditions post-discharge, aiding in better patient management and healthcare decision-making.
The data that support the findings of this study are available from the corresponding authors upon reasonable request.
The studies involving humans were approved by the Tehran University of Medical Sciences. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
ShM: Conceptualization, Supervision, Writing – review and editing, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. BP: Investigation, Validation, Writing – review and editing, Conceptualization, Supervision. MAS: Investigation, Validation, Writing – review and editing, Formal analysis, Methodology. MS: Conceptualization, Methodology, Writing – review and editing, Investigation. EJ: Formal analysis, Visualization, Writing – original draft. RH: Investigation, Methodology, Writing – review and editing. SeM: Conceptualization, Funding acquisition, Investigation, Project administration, Supervision, Writing – review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by a grant (grant number: 99-1-149-48200) from Tehran University of Medical Sciences to SeM.
We extend our sincere acknowledgment to MAS, whose thesis served as the cornerstone of this study. The work of ShM received partial support from the European Commission-European Research Executive Agency (REA) under grant agreement No. 101130873.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
COVID-19, coronavirus disease 19; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real-time reverse transcription polymerase chain reaction; MIS-C, multisystem inflammatory syndrome in children; ELISA, enzyme-linked immunoabsorbent assay.
Bohn, M., Steele, S., and Adeli, K. (2023). SARS-CoV-2 serology in pediatrics: seroprevalence studies in unvaccinated children and humoral antibody response post vaccination. Clin. Biochem. 119:110630. doi: 10.1016/j.clinbiochem.2023.110630
Cohen, R., Jung, C., Ouldali, N., Sellam, A., Batard, C., Cahn-Sellem, F., et al. (2020). Assessment of spread of SARS-CoV-2 by RT-PCR and concomitant serology in children in a region heavily affected by COVID-19 pandemic. medRxiv [Preprint] doi: 10.1136/bmjpo-2020-000887
Dunay, G., Barroso, M., Woidy, M., Danecka, M., Engels, G., and Hermann, K. (2023). Long-term antibody response to SARS-CoV-2 in children. J. Clin. Immunol. 43, 46–56.
Ekbatani, M., Hassani, S., Tahernia, L., Yaghmaei, B., Mahmoudi, S., Navaeian, A., et al. (2021). Atypical and novel presentations of Coronavirus disease 2019: a case series of three children. Br. J. Biomed. Sci. 78, 47–52. doi: 10.1080/09674845.2020.1785102
Ghazizadeh Esslami, G., Mamishi, S., Pourakbari, B., and Mahmoudi, S. (2023). Systematic review and meta-analysis on the serological, immunological, and cardiac parameters of the multisystem inflammatory syndrome (MIS-C) associated with SARS-CoV-2 infection. J. Med. Virol. 95:e28927. doi: 10.1002/jmv.28927
Hoste, L., Prytula, A., Dehoorne, J., De Bruyne, R., Van Biervliet, S., and De Waele, K. (2023). Comparison of SARS-CoV-2 seroconversion in children with chronic diseases with healthy children and adults during the first waves of the COVID-19 pandemic. Front. Pediatr. 11:1210181. doi: 10.3389/fped.2023.1210181
Isoldi, S., Mallardo, S., Marcellino, A., Bloise, S., Dilillo, A., Iorfida, D., et al. (2021). The comprehensive clinic, laboratory, and instrumental evaluation of children with COVID-19: a 6-months prospective study. J. Med. Virol. 93, 3122–3132. doi: 10.1002/jmv.26871
Lippi, G., Henry, B., Pighi, L., De Nitto, S., and Salvagno, G. (2023). Are anti-SARS-CoV-2 S/N IgG/IgM antibodies always predictive of previous SARS-CoV-2 infection? Adv. Lab. Med. 4, 175–184. doi: 10.1515/almed-2023-0008
Luo, S., Guo, Y., Zhang, X., and Xu, H. (2020). A follow-up study of recovered patients with COVID-19 in Wuhan. China. Int. J. Infect. Dis. 99, 408–409.
Mahmoudi, S., Mehdizadeh, M., Shervin Badv, R., Navaeian, A., Pourakbari, B., Rostamyan, M., et al. (2020). The coronavirus disease 2019 (COVID-19) in children: a study in an iranian children’s referral hospital. Infect. Drug Resist. 13, 2649–2655. doi: 10.2147/IDR.S259064
Mahmoudi, S., Pourakbari, B., Benvari, S., Hosseinpour Sadeghi, R., Abdolsalehi, M., and Shahbabaie, M. (2023). Clinical and laboratory features of SARS-CoV-2 variants across multiple rounds of pandemic waves in hospitalized children in an Iranian referral hospital. BMC Pediatr. 23:241. doi: 10.1186/s12887-023-04042-w
Mahmoudi, S., Yaghmaei, B., Sharifzadeh Ekbatani, M., Pourakbari, B., Navaeian, A., Parvaneh, N., et al. (2021). Effects of coronavirus disease 2019 (COVID-19) on peripheral blood lymphocytes and their subsets in children: imbalanced CD4(+)/CD8(+) T cell ratio and disease severity. Front. Pediatr. 9:643299. doi: 10.3389/fped.2021.643299
Mamishi, S., Esslami, G., Mohammadi, M., Abdolsalehi, M., Sadeghi, R., Mahmoudi, S., et al. (2021). Detection of SARS-CoV-2 antibodies in pediatric patients: an Iranian referral hospital-based study. Hum. Antibodies 29, 217–223. doi: 10.3233/HAB-210448
Mamishi, S., Movahedi, Z., Mohammadi, M., Ziaee, V., Khodabandeh, M., and Abdolsalehi, M. (2020). Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: a first report from Iran. Epidemiol. Infect. 148: e196.
Mamishi, S., Olfat, M., Pourakbari, B., Eshaghi, H., Abdolsalehi, M., Shahbabaie, M., et al. (2022a). Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: update and new insights from the second report of an Iranian referral hospital. Epidemiol. Infect. 150:e179. doi: 10.1017/S0950268822001522
Mamishi, S., Pourakbari, B., Mehdizadeh, M., Navaeian, A., Eshaghi, H., and Yaghmaei, B. (2022b). Children with SARS-CoV-2 infection during the novel coronaviral disease (COVID-19) outbreak in Iran: an alarming concern for severity and mortality of the disease. BMC Infect. Dis. 22, 1–10. doi: 10.1186/s12879-022-07200-0
Mohammadpour, M., Hassani, S., Sharifzadeh, M., Tahernia, L., Mamishi, S., Yaghmaie, B., et al. (2022). COVID-19 pandemic experiences in pediatric intensive care unit: an Iranian referral hospital-based study. Int. J. Clin. Pract. 2022:1682986. doi: 10.1155/2022/1682986
Oygar, P., Ozsurekci, Y., Gurlevik, S., Aykac, K., Kukul, M., and Cura Yayla, B. (2021). Longitudinal follow-up of antibody responses in pediatric patients with COVID-19 up to 9 months after infection. Pediatr. Infect. Dis. J. 40, e294–e299. doi: 10.1097/INF.0000000000003199
Rezaei, M., Mahmoudi, S., Mortaz, E., and Marjani, M. (2021). Immune cell profiling and antibody responses in patients with COVID-19. BMC Infect. Dis. 21:646. doi: 10.1186/s12879-021-06278-2
Rostami-Maskopaee, F., Ladomenou, F., Razavi-Amoli, S., Navaeifar, M., Hajialibeig, A., and Shahbaznejad, L. (2022). Clinical characteristics and outcomes of the multisystem inflammatory syndrome in children (MIS-C) following COVID-19 infection in Iran: a multicenter study. PLoS One 17:e0274104. doi: 10.1371/journal.pone.0274104
Sancho-Shimizu, V., Brodin, P., Cobat, A., Biggs, C., Toubiana, J., Lucas, C., et al. (2021). SARS-CoV-2-related MIS-C: a key to the viral and genetic causes of Kawasaki disease? J. Exp. Med. 218:e20210446. doi: 10.1084/jem.20210446
Sedik, R. (2023). The clinical course and outcomes of SARS-CoV-2 virus infection in children: a 24-week follow-up study in Sulaimaniyah, Iraq. BMC Pediatr. 23:303. doi: 10.1186/s12887-023-04111-0
Selva, K., van de Sandt, C., Lemke, M., Lee, C., Shoffner, S., Chua, B., et al. (2021). Systems serology detects functionally distinct coronavirus antibody features in children and elderly. Nat. Commun. 12, 1–14. doi: 10.1038/s41467-021-22236-7
Shearer, W., Rosenblatt, H., Gelman, R., Oyomopito, R., Plaeger, S., and Stiehm, E. (2003). Lymphocyte subsets in healthy children from birth through 18 years of age: the Pediatric AIDS clinical trials group P1009 study. J. Allergy Clin. Immunol. 112, 973–980. doi: 10.1016/j.jaci.2003.07.003
Toh, Z., Anderson, J., Mazarakis, N., Neeland, M., Higgins, R., Rautenbacher, K., et al. (2022). Comparison of seroconversion in children and adults with mild COVID-19. JAMA Netw Open 5:e221313.
Vosoughi, F., Makuku, R., Tantuoyir, M., Yousefi, F., Shobeiri, P., Karimi, A., et al. (2022). A systematic review and meta-analysis of the epidemiological characteristics of COVID-19 in children. BMC Pediatr. 22:613. doi: 10.1186/s12887-022-03624-4
Wu, Z., and McGoogan, J. (2020). Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA 323, 1239–1242. doi: 10.1001/jama.2020.2648
Keywords: COVID-19, SARS-CoV-2, antibody, seropositivity, children
Citation: Mahmoudi S, Pourakbari B, Shahbabaie MA, Sotoudeh M, Jafari E, Hosseinpour Sadeghi R and Mamishi S (2024) Post-discharge follow-up of pediatric COVID-19 patients: insights into serological dynamics. Front. Microbiol. 15:1427327. doi: 10.3389/fmicb.2024.1427327
Received: 03 May 2024; Accepted: 25 June 2024;
Published: 09 July 2024.
Edited by:
Annalisa Ciabattini, University of Siena, ItalyReviewed by:
Israel Parra-Ortega, Federico Gómez Children’s Hospital, MexicoCopyright © 2024 Mahmoudi, Pourakbari, Shahbabaie, Sotoudeh, Jafari, Hosseinpour Sadeghi and Mamishi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reihaneh Hosseinpour Sadeghi, cmVpaGFuZWhzYWRlZ2hpMTk4NkBnbWFpbC5jb20=; Setareh Mamishi, c21hbWlzaGlAc2luYS50dW1zLmFjLmly
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.