
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 24 July 2024
Sec. Microbial Symbioses
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1415329
This article is part of the Research TopicExploring Processes and Applications of Metal-Microbe InteractionsView all 11 articles
Some plant-associated microorganisms could improve host plants biotic and abiotic stress tolerance. Imperata cylindrica is a dominant pioneer plant in some abandoned mine lands with higher concentrations of heavy metal (HM). To discover the specific microbiome of I. cylindrica in this extreme environment and evaluate its role, the microbiome of I. cylindrica’s seeds and rhizosphere soils from HM heavily contaminated (H) and lightly contaminated (L) sites were studied. It was found that HM-contamination significantly reduced the richness of endophytic bacteria in seeds, but increased the abundance of resistant species, such as Massilia sp. and Duganella sp. Spearman’s rank correlation coefficient analysis showed that both Massilia sp. and Duganella sp. showed a significant positive correlation with Zn concentration, indicating that it may have a strong tolerance to Zn. A comparison of the microbiome of rhizosphere soils (RS) and adjacent bare soils (BS) of site H showed that I. cylindrica colonization significantly increased the diversity of fungi in rhizosphere soil and the abundance of Ascomycota associated with soil nutrient cycling. Spearman’s rank correlation coefficient analysis showed that Ascomycota was positively correlated with the total nitrogen. Combined with the fact that the total nitrogen content of RS was significantly higher than that of BS, we suppose that Ascomycota may enhance the nitrogen fixation of I. cylindrica, thereby promoting its growth in such an extreme environment. In conclusion, the concentration of HM and nutrient contents in the soil significantly affected the microbial community of rhizosphere soils and seeds of I. cylindrica, in turn, the different microbiomes further affected soil HM concentration and nutrient contents. The survival of I. cylindrica in HM severely contaminated environment may mainly be through recruiting more microorganisms that can enhance its nutrition supply.
In the past 100 years, with the continuous progress and development of human society, the scale and intensity of mineral resources have increased (Gutierrez, 2020). However, during resource extraction, a large quantity of mineral waste was produced, which contains many toxic and harmful substances and poses a potential threat to the environment (Trannum et al., 2020; Xie and van Zyl, 2020). Revegetation is a plant-based technology for in situ restoration (Zhou et al., 2020). Vegetation restoration is environmentally sustainable and less costly than traditional physicochemical techniques (Mendez and Maier, 2008). Due to the characteristics of high heavy metal (HM) content, barren soil, and low microbial activity in the tailing area, it is generally difficult for plants to survive (De la Iglesia et al., 2006; Ginocchio et al., 2017). A previous study found that the role of the dominant plant species (Imperata cylindrica) was found to be important in the restoration of the plant community in the mining district (Jia et al., 2020). I. cylindrica, a gramineous genus, is a common plant in the wasteland and exposed tailing areas. Its rhizomes are well-developed, which can maximize the nutrients in the soil and have strong adaptability to extreme environments (Song et al., 2019). I. cylindrica has been shown to grow as an HMs accumulator plant in the mining district (Shaltout et al., 2016; Mahdavian et al., 2017; Li et al., 2018; Vidal et al., 2021). Previous studies showed that microbial colonization could enhance nutrient uptake and HM resistance of I. cylindrica, thereby promoting its growth in HM-stress environment (Jia et al., 2019; Liang et al., 2023).
Seeds are carriers of many endophytes. Seed endophytes are mostly passed down from generation to generation by vertical transmission, so they become the basis for the establishment of the plant endophyte community (Mao et al., 2023). Seed endophytes not only improve the viability and germination rate of seeds but also promote the growth and development of plants, thus enhancing the biotic and abiotic stress resistance of the host plant (Santoyo et al., 2016; Shahzad et al., 2016). For example, Parmar et al. (2022) found that seed endophyte Epicoccum nigrum (FZT214) can directly affect the reclaimed plants by utilizing hormone regulation and antioxidant stress, to alleviate HM stress in the mine environment and promote plant growth and “site fitness.” In addition, when the pioneer plant seeds migrated to the exposed tailing area, the endophytic bacteria could provide some nutrients for the pioneer plant by producing plant hormones, phosphorus solubilizing, potassium solubilizing, and nitrogen-fixing (Islam et al., 2021). Wang et al. (2020) and Mahmud et al. (2021) found that Epichloë can give plants stronger environmental tolerance by adjusting soil physical and chemical properties and changing the soil microbiome. Therefore, in the process of vegetation reclamation in the exposed tailing area, seed endophytes can significantly promote seed colonization, germination, and seedling growth of pioneer plants, enhance their heavy metal resistance, and facilitate vegetation reclamation in the tailing area (Ultra and Manyiwa, 2021).
Soil microorganisms can change the soil pH value, the physical, and chemical properties, and soil respiration rate, etc. by producing various active ingredients and play an extremely important role in soil nutrient cycling, organic matter content, biogeochemical cycling, and plant biomass (Zhao et al., 2021; Naz et al., 2022). Zhao et al. (2021) found that different vegetation reclamation modes in mining areas have different soil microbial communities, which in turn feed on the development of the plant community. Gazitúa et al. (2021) confirmed the important role of the soil microbiome in mining adaptability, early colonization, and pioneer plant growth. Therefore, soil biomes play a key role in the restoration of degraded terrestrial ecosystems (Dangi et al., 2012; Cheng et al., 2022).
However, the role of microorganisms on pioneer plants’ survival in the tailing area with higher concentrations of HM is still unknown. It was supposed that the pioneer plant growing in HM heavily contaminated sites may contain some special seed endophytes, which benefited them to grow in these extreme environments. And the colonization of these pioneer plants may further change the rhizosphere microbial community, which benefit the other plants growth by colonization of these special endophytes in heavy metals contaminated areas. Therefore, in the present study, culture-independent technology was used to analyze the microbial communities of seeds and rhizosphere soils of I. cylindrica, a pioneer plant of an abandoned Pb-Zn tailing area.
The study site located in Puxiong town, Jianshui county, Yunnan province, Southwest China. One sampling site is the Pb-Zn tailing area (site H-HM heavily contaminated) (23°30′26″ N, 103°1′13″ E), with an altitude of 1924.9 m, and the vegetation was sparse, I. cylindrica was a dominant pioneer plant there. The other sampling site L (site L-HM lightly contaminated) is 5 km away from site H (23°30′21″ N, 103°2′18″ E), with an altitude of 1896.5 m. Soil contents of total potassium (TK) and total phosphorus (TP) were significantly lower at L site than at H site. And the concentrations of Pb, Zn, and Cd at L site were significantly lower than those at H site (p < 0.05, t-test) (Table 1). The sample was collected on November 25, 2020.
The S-type sampling method was used to randomly select 15 healthy plants from sites H and L respectively, about 40 m2 area of each site were chosen for sampling and the rhizosphere soil was collected by shaking root method (Teixeira et al., 2010). Simultaneously, the background soil (without plants growing) at a depth of 5–10 cm adjacent to the sampling plants was collected. Each sample was placed separately into a sterile plastic bag, labeled, and transported to the laboratory, and the seeds were surface sterilized within 24 h. I. cylindrica seeds from site H and L were mixed, respectively, and evenly divided into three portions, and 0.25 ± 0.023 g was taken from each portion. Then, the seeds were surface sterilized by immersing in 75% (v/v) ethanol for 2.5 min, and were extensively rinsed with sterile distilled water five times, followed by repeating the above procedures one time (Li et al., 2012). The efficacy of the surface sterilization was checked by following the imprint method. Meanwhile, 20 ± 0.75 g soils were taken from BS and RS of site H. Thereafter, the samples were homogenized in liquid nitrogen. The seeds and soil samplings were then stored to −80°C, respectively.
The soils were ground using a high-speed blender, and the content of Pb, Zn, total nitrogen, total phosphorus, and total potassium were determined by Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) (Li et al., 2016), and the concentration of Cd was analyzed by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) (Chen et al., 2022).
The total genomic DNA was extracted taking approximately 0.2 mg of homogenized powdered samples using the MoBio PowerSoil® DNA Isolation Kit (MO BIO Laboratories, Inc., Carlsbad, CA, United States) following the manufacturer’s protocol. Extracted DNA wasverified by electrophoresis on a 1.5% (w/v) agarose gel. The qualified DNA samples were stored at −20°C for subsequent analyses.
Amplification of the seed endophytic bacterial 16S rRNA V3-V4 region was performed using primer 799F (5’-AACMGGATTA GATACCCKG-3′) and 1193R (5’-ACGTCATCCCCACCTTCC-3′) resulting in amplicons of approximately 394 bp. And amplification of the soil bacterial 16S rRNA V3-V4 region was performed using primer 338F (5’-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5’-GGACTACH VGGGTWTCTAAT-3′) resulting in amplicons of approximately 468 bp. In addition, amplification of the fungal 18S ITS1 (internal transcribed spacer 1) region was performed using primer ITS1-F (5’-CTTGGTCA TTTAGAGGAAGTAA-3′) and ITS2-R (5’-GCTGCGTTCTTCAT CGATGC-3′) resulting in amplicons of approximately 350 bp. PCR reactions were performed in a 25 μL volume and contained: 2.5 μL 10× PCR buffer, 1.5 μL Mg2+ (25 mM MgCl2), 2.5 μL dNTP mixture (4 mM each), 0.5 μL KOD-PlusNeo (1 units μL−1; TOYOBO), 1 μL Template DNA (0.4 ng), 2.5 μL primer (10 μM each) and 14.5 μL sterilized double-distilled H2O. The PCR program consisted of an initial denaturation step at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 20 s, annealing at 50°C for 30 s, elongation at 72°C for 30 s, with a final extension of 5 min at 72°C. The PCR products were purified with an OMEGA Gel Extraction Kit (Omega Bio-Tek, United States) according to the manufacturer’s protocol. The resulting amplicons were subsequently subjected to high-throughput sequencing using the Illumina MiSeq platform (Illumina, 2013). All steps were implemented at Shanghai Majorbio Bio-pharm Technology Company (Shanghai, China). The Illumina sequencing data are available in the NCBI Sequence Read Archive (SRA) repository with the BioProject accession number PRJNA1037316.
The raw Illumina MiSeq sequencing data were obtained in FASTA files along with sequencing quality files. Paired-end reads from the original DNA fragments theoretically were merged using FLASH v.1.2.11, and files were accessed using MOTHUR v.1.30.2 bioinformatics software for further processing and analyses. All sequences were denoised before barcodes, and primers were removed. The cleaned-up sequences were aligned and classified along known sequences in the SILVA v.138 rRNA database. Next, chimeric sequences were detected using the UCHIME algorithm, and the remaining sequences were assigned to OTUs based on a 97% similarity criterion. Rarefaction curves were performed to check the sample adequacy using a 50 sequence increment. Finally, taxonomic information for each OTU was used the RDP Classifier v.2.13 at 0.5 confidence threshold (Wang et al., 2007). To indicate the microbial diversity in seeds, the α-diversity indices (including Shannon’s H′ and Ace indices) were quantified in terms of OTU richness.
Relative abundance differences among different groups were detected by Kruskal-Wallis (KW) sum-rank test. A t-test was used to estimate the difference of α-diversity indices of seed endophytes between sites H and L and soil microbiome between RS and BS. All statistical analyses were performed with SPSS 27.
The OTUs of endophytic bacterial in seeds were more abundant at site L (537) than at site H (313), and 208 OTUs were shared by both two sites (Figure 1A). Rank-Abundance curve showed that the richness of seed endophytic bacteria at site L was relatively higher than that at site H (Figure 1B).
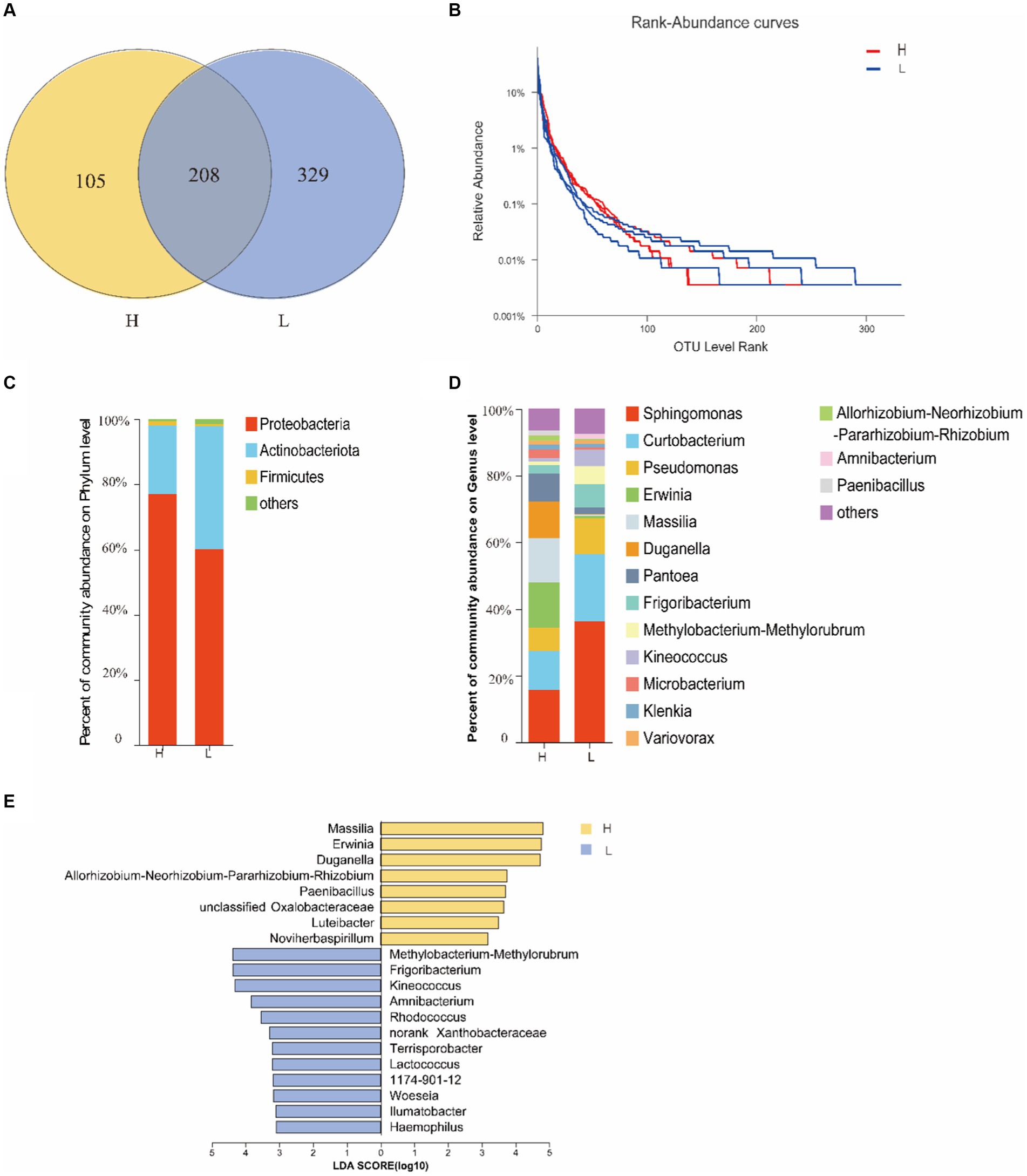
Figure 1. The diversity of seed bacterial endophytes of I. cylindrica from different heavy metal-contaminated environments. (A) The Venn diagram analysis of OTUs. (B) The rank curve based on OTU abundance. (C,D) Relative abundance of seed endophytic bacterial at phylum and genus level. (E) The indicator species of seed endophytic bacterial at genus level. H, Heavy metal heavily contaminated site; L, Heavy metal lightly contaminated site.
The results of 16S rRNA sequencing of seed endophytic bacterial were assigned to 19 phyla, 37 classes, 111 orders, 191 families, and 358 genera. Proteobacteria displayed the most relative abundance with sites H and L accounting for 77.01 and 60.41%, respectively (Figure 1C). At genus level, Sphinomonas sp. was the most dominant genus at both sites, and its relative abundance consists 36.08% at site L and 15.74% at site H. Erwinia sp. (13.51%) (p = 0.0495, Kruskal-Wallis sum-rank test) and Massilia sp. (13.16%) (p = 0.0495, Kruskal-Wallis sum-rank test) were also dominant genera at site H (Figure 1D), and their relative abundance at site H was significantly higher than that at site L (Figure 1E). Meanwhile, Erwinia sp. and Massilia sp. are also the indicator species of seed endophytic bacterial at site H at genus level (Figure 1E). It was found that the soil HM-contamination significantly decreased the richness of seed endophytic bacterial (p = 0.0398, t-test; Ace index; Table 2).
The OTUs of endophytic fungal in seeds were more abundant at site H (402) than at site L (343), and 240 OTUs were shared by both two sites (Figure 2A). Rank-Abundance curve indicated that the richness of seed endophytic fungi at site H was relatively higher than that at site L (Figure 2B).
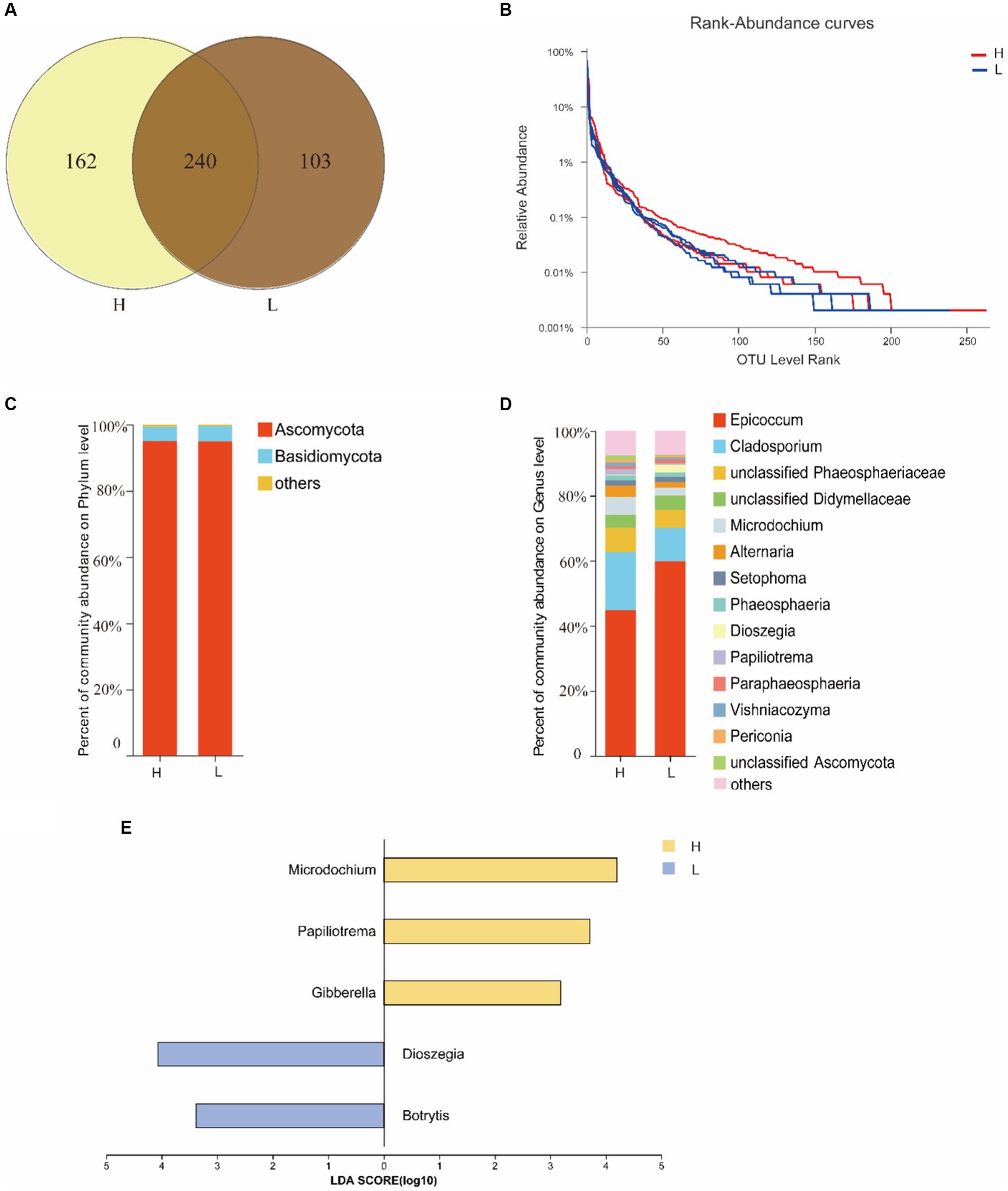
Figure 2. The diversity of seed fungal endophytes of I. cylindrica from different heavy metal-contaminated environments. (A) The Venn diagram analysis of OTUs. (B) The rank curve based on OTU abundance. (C,D) Relative abundance of seed endophytic fungal at phylum and genus level. (E) The indicator species of seed endophytic fungal at genus level. H, Heavy metal heavily contaminated site; L, Heavy metal lightly contaminated site.
The results of ITS sequencing of seed endophytic fungal were assigned to 6 phyla, 323 classes, 56 orders, 123 families, and 222 genera. Ascomycota was the most dominant fungal phylum (Figure 2C). At genus level, Epicoccum sp. displayed the most relative abundance in both sites H and L, with 44.78 and 55.91%, respectively. Other dominant genera in site H followed by Cladosporium sp. (17.87%) and unclassified Phaeosphaeriaceae (7.57%), with higher relative abundance than site L (Figure 2D), but neither of them was a indicator species at site H at genus level (Figure 2E). According to α-diversity analysis, it was found that I. cylindrica seed endophytic fungal diversity and richness of site H were higher than site L, but the difference was not significant (Table 2).
The contents of HMs (Cd, Pb, Zn) at site H were significantly higher than those at site L, while the contents of nutrients (TN and TK) at site L were significantly higher than those at site H (Table 1). Massilia sp. had the highest relative abundance of seed endophytic bacteria of I. cylindrica in site H. Spearman’s rank correlation coefficient analysis showed a significant positive correlation between Massilia sp. and Zn (Figure 3A). In addition, the result showed that Cladosporium sp. was negatively correlated with pH. Similarly, Microdochium sp. was a relatively dominant fungus at site H, and its abundance was significantly different from that at site L. Spearman’s rank correlation coefficient analysis indicated that Microdochium sp. was significantly positively correlated with TP and Pb (Figure 3B).
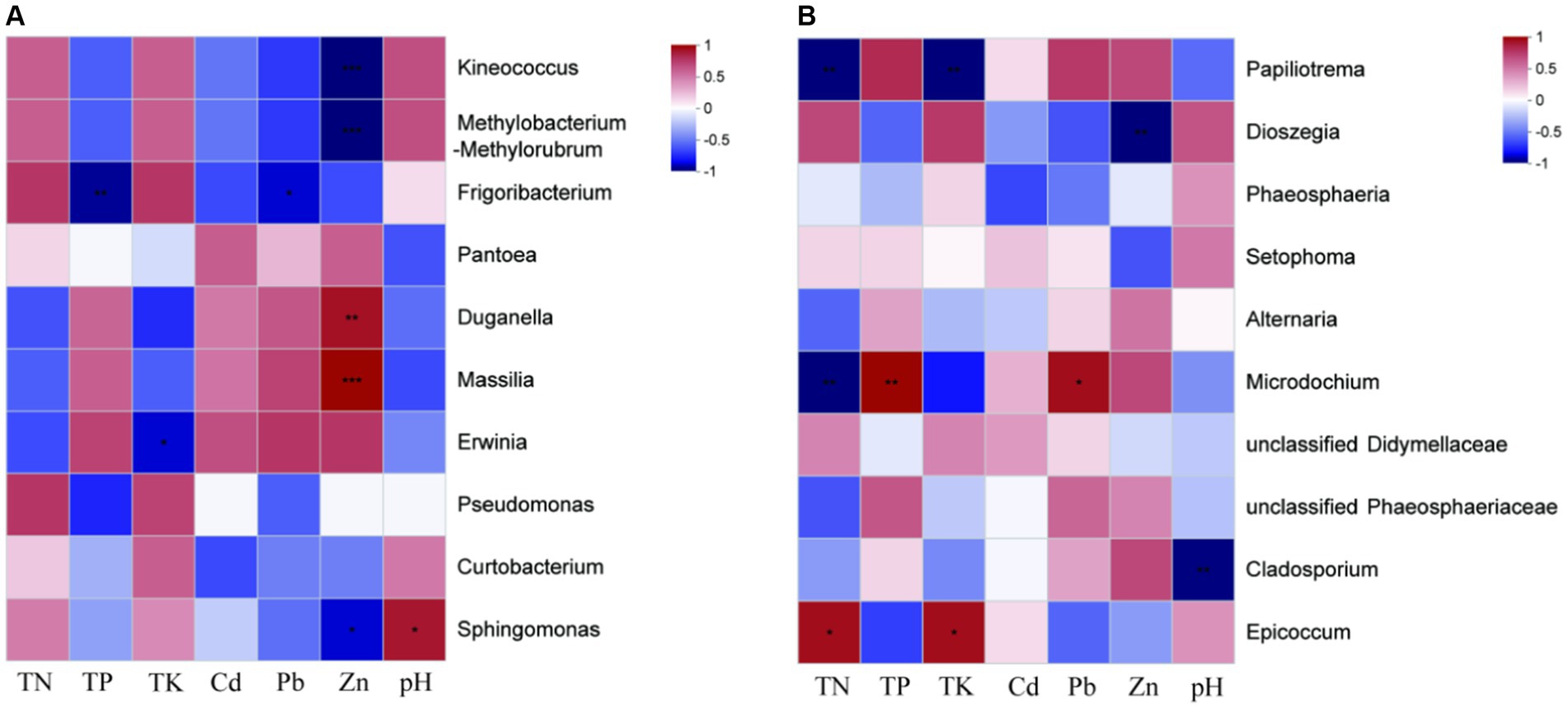
Figure 3. Spearman correlation heatmap of I. cylindrica seed endophytes at genus level. (A) Seed endophytic bacteria. (B) Seed endophytic fungi. “*” indicates statistically significant difference (*p < 0.05; **p < 0.01; ***p < 0.001).
A total of 263,311 and 355,189 sequences high-quality of bacteria and fungi were obtained after demultiplexing and filtration steps. The mean number of valid bacterial sequences was 31,563, whereas the mean number of valid fungal sequences was 32,254. These sequences were divided into 2,434 and 1,933 different OTUs, respectively, with 97% similarity.
The results of 16S rRNA sequencing of soil bacterial were assigned to 32 phyla, 91 classes, 312 orders, 316 families, and 570 genera. Actinobacteriota displayed the most relative abundance in both soil samples (Figure 4A). At genus level, Rhodococcus sp. was the most dominant genus in BS (Bare soil) (9.90%). Contrary to this, the most dominant genus of RS (Rhizosphere soil) was Antrobacter sp. (8.99%). Many OTUs were unclassified at genus level in both soil samples (Figure 4B). The results of α-diversity (Shannon’s H′ and Ace indexes) analysis indicated that BS and RS was not significantly different (Figures 4C,D).
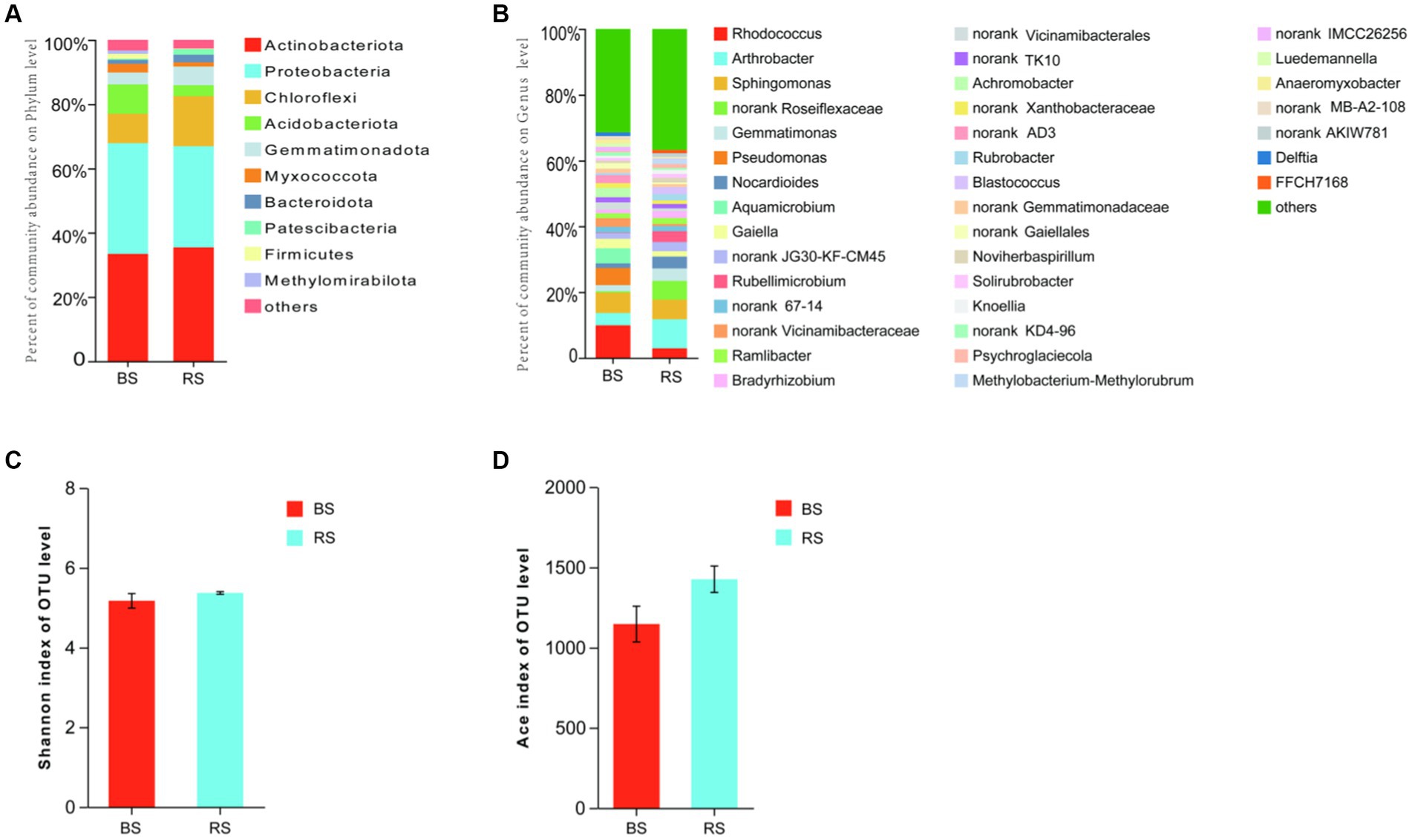
Figure 4. Bacterial diversity in bare soils and rhizosphere soils in HM heavily contaminated site. (A,B) Relative abundance of soil bacterial composition at the level of phylum and genus. (C) Shannon’s H′. (D) Ace index. BS, Bare soils of site H; RS, Rhizosphere soils of site H.
The results of ITS sequencing of soil fungal were assigned to 9 phyla, 37 classes, 98 orders, 233 families, and 497 genera. Ascomycota displayed the most relative abundance in both soil samples (Figure 5A), and the relative abundance of RS was significantly higher than that of BS (p = 0.0495, Kruskal-Wallis sum-rank test) (Figure 5B). At the same time, Ascomycota is also the indicator species of soil fungal at phylum level of RS (Figure 5B). At genus level, unclassified Ascomycota and unclassified Didymellaceae were the most dominant fungi in BS (15.03%) and in RS (10.28%) (Figure 5C). The results of Ace index indicating that the species richness of RS was significantly higher than that of BS (p = 0.0237, t-test) (Figure 5D), while the results of Shannon index showed that the diversity of the two soil samples was not significantly different (Figure 5E).
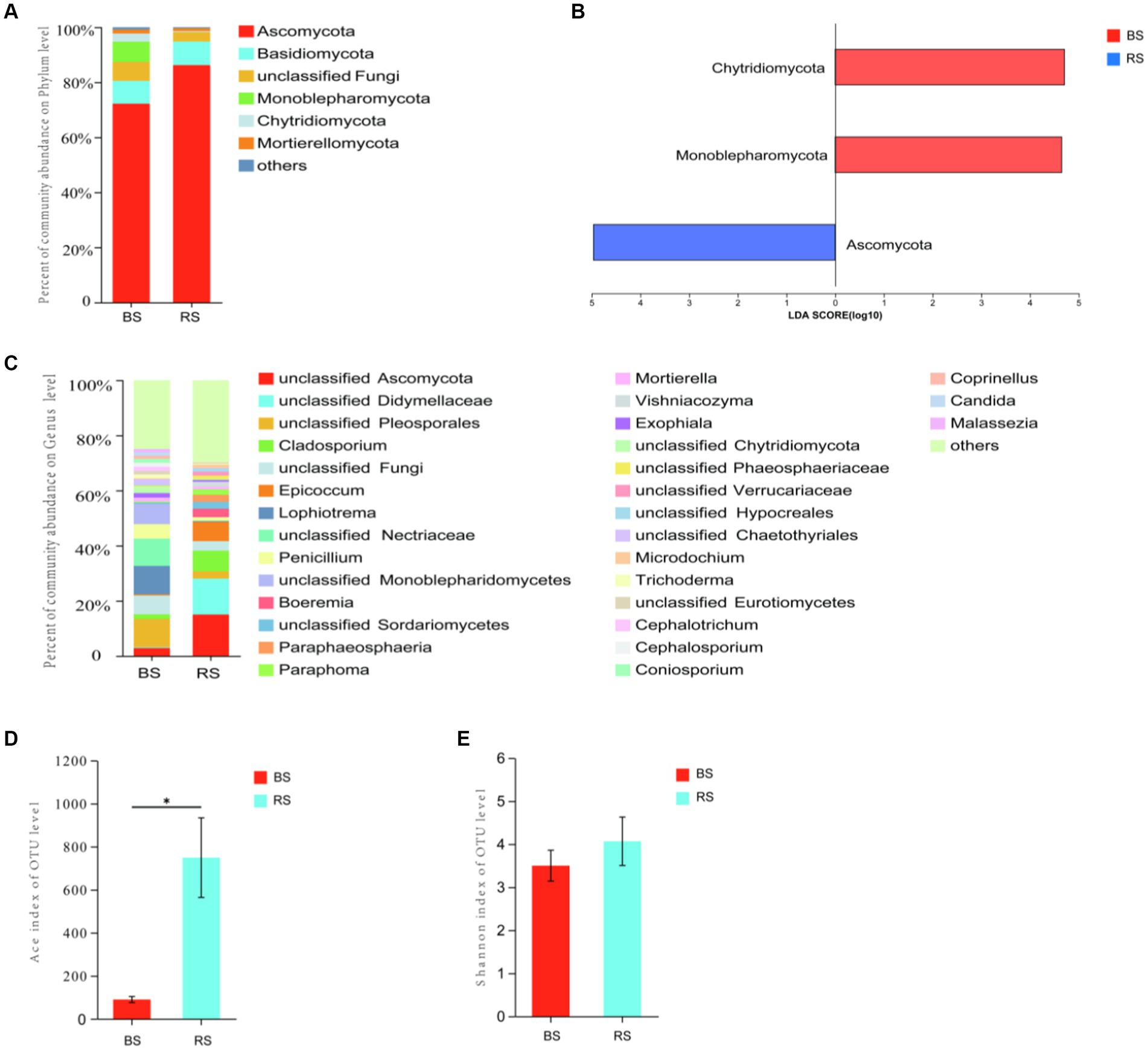
Figure 5. Fungal diversity in bare soils and rhizosphere soils in HM heavily contaminated site. (A) Relative abundance of soil fungal composition at the level of phylum. (B) The indicator species of soil fungal at phylum level. (C) Relative abundance of soil fungal composition at the level of genus. (D) Ace index. (E) Shannon’s H′. “*” indicates statistically significant difference (*p < 0.05, t-test). BS, Bare soils of site H; RS, Rhizosphere soils of site H.
The contents of HMs (Pb and Zn) and nutrients (TP and TK) of BS were significantly higher than that of RS (Table 1). Mantel-test Network heatmap showed that at the phylum level, bacterial communities in BS were negatively correlated with Pb, while bacterial communities in RS were only positively correlated with TP and Cd, but the differences were not significant (Figure 6A). For fungi, at phylum level, Mantel-test Network heatmap shown that fungal communities in BS were positively correlated with TN, Cd, and pH, while fungal communities in RS were positively correlated with TN, Cd, Zn, and pH, but the differences were not significant (Figure 6B). Spearman’s rank correlation coefficient analysis showed that Rhodococcus sp. was significantly positively correlated with Pb, Zn, and TK, and Arthrobacter sp. was significantly negatively correlated with TP at genus level (Figure 6C). For fungi, Spearman’s rank correlation coefficient analysis indicated that Ascomycota was significantly positively correlated with TN, but significantly negatively correlated with TP (Figure 6D).
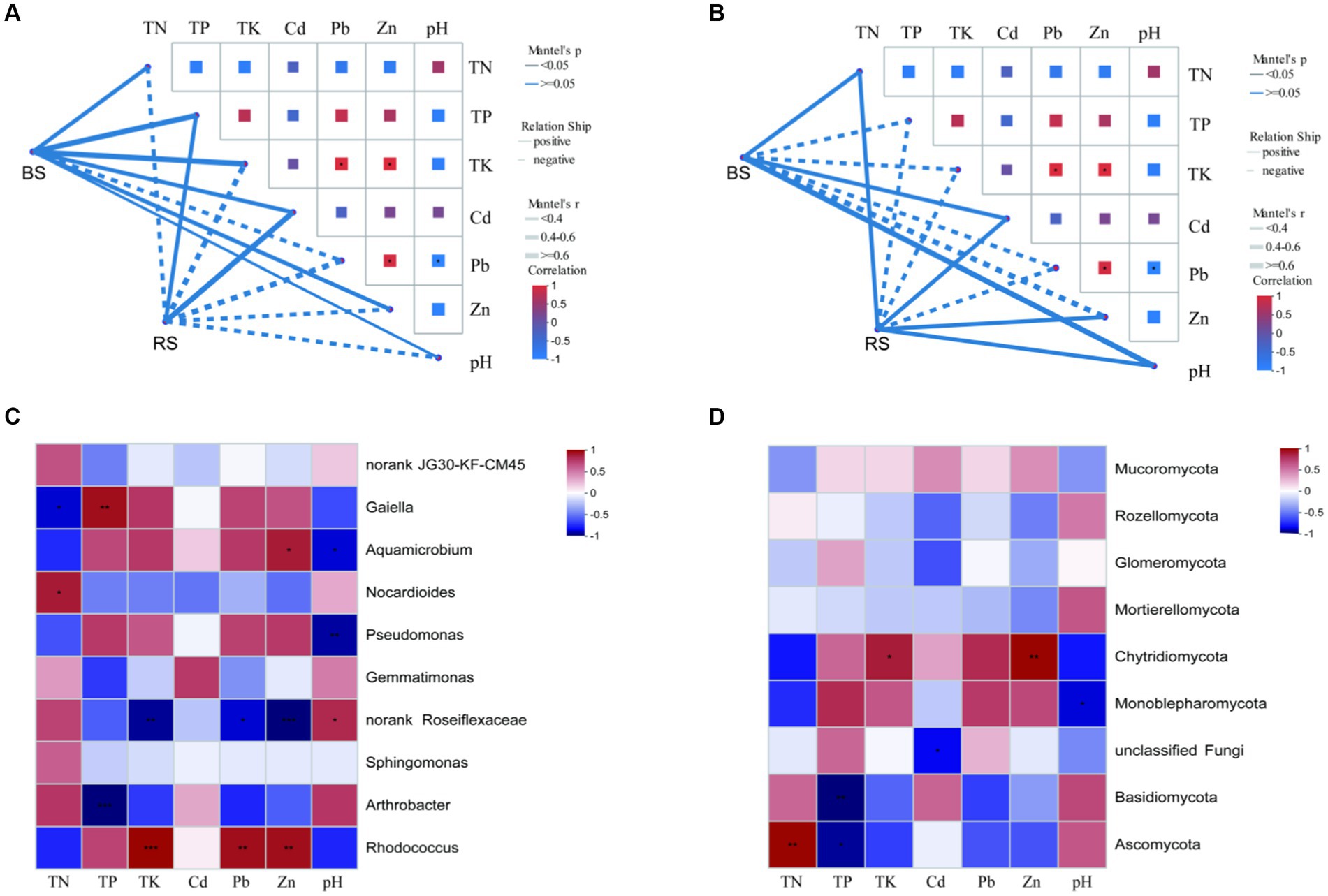
Figure 6. Heat map of bacterial and fungal correlation with environmental factors in bare soils and rhizosphere soils of HM heavily contaminated site. (A,B) Mantel-test Network heatmap of bacteria and fungi at phylum level. (C) Spearman’s rank correlation coefficient heatmap of bacteria at genus level. (D) Spearman’s rank correlation coefficient heatmap of fungi at phylum level. “*” indicates statistically significant difference (*p < 0.05; **p < 0.01). BS, Bare soils of site H; RS, Rhizosphere soils of site H.
Previous researches has demonstrated that I. cylindrica plays an important role in plant community restoration in the mining district (Zheng et al., 2019; Jia et al., 2020). Meanwhile, various research indicated that plant-associated microorganisms can enhance host plants’ stress tolerance (Trivedi et al., 2020). However, the microbiome of pioneer plants growing in the bare mine tailing with higher concentrations of HMs as well as its role are still unknown. The present study indicated that HM contamination reduced the endophytic bacterial richness of I. cylindrica seeds. Proteobacteria was the most dominant of I. cylindrica seeds in site H. Some studies have established that Proteobacteria is relatively more abundant in plants growing in HM-contamination environments, as it is composed of many facultative anaerobic members that can survive in extreme pH environments, proving to be taxa most tolerant to HMs (Sun et al., 2010; Sánchez-López et al., 2018; Kasemodel et al., 2019). Simultaneously, some Proteobacteria are plant growth-promoting bacteria that have been proven to fix nitrogen symbiotically with host plants (Wu et al., 2022). Massilia sp. is a genus of Proteobacteria. It was found to be a dominant genus in site H, and its relative abundance was significantly higher than that in site L. At the same time, it is an indicator species for seed endophytic bacterial in site H. Moreover, Spearman’s rank correlation coefficient analysis demonstrated that Massilia sp. had a significant positive correlation with Zn concentration in soils. Wang et al. (2021) found that Massilia was a potential Zinc-mobilizing species in soil and that it was significantly positively correlated with Zn concentration in wheat grains. Analogously, Wang et al. (2022) found that Massilia sp. had a significant positive relationship with DTPA-Pb (diethylene-triamine-pentaacetic-acid-Pb) in soils and Pb accumulation in roots. Massilia sp. was isolated from sludge, farmland, and mining soils contaminated by heavy metals, suggesting that Massilia sp. is an important microorganism in HM-contaminated soils (Bensidhoum and Nabti, 2019). In addition, it has also been reported as Massilia sp. with strong phosphate solubilizing ability (Zheng et al., 2017). In terms of fungi, the diversity and richness of seed endophytes of I. cylindrica in site H were higher than those in site L, but the difference was not significant. The results indicated that HM contamination had no significant effect on seed endophytic fungal diversity and richness. However, relative abundance of Microdochium sp. in site H was significantly higher than that in site L. At the same time, it is an indicator species for endophytic fungi in seeds in site H. It was found that Microdochium sp. could promote the growth of Hordeum vulgare L. under Cd stress, and significantly increase Cd accumulation in barley roots (Shadmani et al., 2021). In addition, it has been reported that Microdochium sp. is well tolerant to Pb and it has also been found that the accumulation of Pb in Microdochium sp. increases with the concentration of Pb in the growth media (Parada et al., 2022). Similarly, our earlier study found that Microdochium sp. was tolerant to 2000 mg/L Pb. This was consistent with the results of correlation analysis of soil environmental factors, which showed a significant positive correlation between Microdochium sp. and Pb, indicating that its large presence in the seeds of I. cylindrica in the tailing area may improve the host’s Pb-tolerance. At the same time, a previous study found that Microdochium sp. is capable of synthesizing IAA in vitro (Rothen et al., 2018). Based on the above results, we supposed that Massilia sp. and Microdochium sp. may play an important role in the tailing area plant community restoration. For this reason, a series of pot experiments used to investigate the effects of Massilia sp. and Microdochium sp. on seed germination, plant growth, and rhizosphere microbiome reshaping of I. cylindrica is already in progress.
It is well known that in addition to endophytes, rhizosphere microorganisms also play an important role in the process of vegetation community restoration in the tailing area (Wang et al., 2023). Plant growth has been reported to alter the abundance of specific functional microorganisms (Sun et al., 2018; Zhu et al., 2023). In the present study, it was found that the microbial diversity and richness in rhizosphere soils of I. cylindrica were higher than those in bare soils, and there was a significant difference in fungal richness between the two soil samples. Ascomycota displayed the most relative abundance in rhizosphere soils, and it was significantly higher than that in bare soils. The same phenomenon was observed in other studies (Bourceret et al., 2016; Rosatto et al., 2019). We suggested that one of the reason for the higher relative abundance of Ascomycota in rhizosphere soils of I. cylindrica may be its ability to efflux complex spores providing extra-resistant against the toxicities of HMs (López-González et al., 2015; Liu et al., 2022). Simultaneously, Spearman correlation analysis showed that Ascomycota was positively correlated with total nitrogen content. Regarding bacteria, Arthrobacter sp. is the most dominant genus in rhizosphere soils, and its relative abundance in rhizosphere soils was significantly higher than that in bare soils. It has been demonstrated that Arthrobacter sp. not only contained HM-resistance genes but also strongly correlated with higher siderophore and IAA production (Rosatto et al., 2019; Senthil Kumar et al., 2023). Meanwhile, Wan et al. (2020) found that Arthrobacter sp. could utilize multiple phosphorus sources. Therefore, we suggested that to adapt HM-contamination environment, I. cylindrica recruits many beneficial microorganisms to colonize in the rhizosphere, which helps it enhance HM resistance and nutrient uptake.
Some studies have shown that endophytes can help host plants resist stressful environments by regulating root exudates and remodeling rhizosphere microbiome structure (Li et al., 2019; Wang et al., 2023). Jin et al. (2022) found that under Cd stress, inoculation of Epichloe gansuensis could increase the contents of organic acids and amino acids in root exudates of Achnatherum inebrians, thereby recruiting different rhizosphere microbe and enhancing the host plant resistance to Cd stress. We suggested that the endophytic consortium in the seeds of I. cylindrica in the HM heavily contaminated site might increase the relative abundance of microorganisms in rhizosphere soils related to HM-fixation and soil nutrient cycling by regulating root exudates during plant growth, which would help plants adapt to HM stress (Liu et al., 2022). However, the functional mechanisms by which the interactions among plants, endophytes, and soils enhance the “site fitness” of host plants remain unclear. Studying the combined effects of endophytes and rhizosphere soil microorganisms can better reveal the reasons for the restoration of natural vegetation in the tailing area.
HM-contamination significantly reduced the richness of endophytic bacteria in seeds, but increased the abundance of resistant species. The colonization of I. cylindrica significantly increased the richness of fungi in rhizosphere soils. Ascomycota was positively correlated with the total nitrogen, and it may enhance the nitrogen fixation of I. cylindrica, thus promoting its growth in extreme environment. The survival of I. cylindrica in HM severely contaminated environment may mainly be through recruiting more microorganisms that can enhance its nutrition supply. The study indicated that the seed endophytic community of I. cylindrica from HM heavily contaminated site differed from that of uncontaminated site, therefore, the future work will focus on discovering the function and mechanism of the special endophytes of pioneer plants from HM-contaminated site, and further exploring them in the tailing area restoration.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1037316.
WM: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. YW: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. QL: Formal analysis, Investigation, Writing – review & editing. YX: Formal analysis, Investigation, Writing – review & editing. WT: Formal analysis, Investigation, Supervision, Writing – review & editing. HH: Supervision, Writing – review & editing. XJ: Supervision, Writing – review & editing. HL: Conceptualization, Funding acquisition, Methodology, Writing – review & editing, Project administration, Resources.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was financially supported by the National Natural Science Foundation of China (42267059), Yunnan International Joint Laboratory of Research and Development of Crop Safety Production on Heavy Metal Pollution Areas.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Bensidhoum, L., and Nabti, E. (2019). “Plant growth-promoting bacteria for improving crops under saline conditions” in Microorganisms in saline environments: strategies and functions. eds. B. Giri and A. Varma (Cham: Springer International Publishing), 329–352.
Bourceret, A., Cébron, A., Tisserant, E., Poupin, P., Bauda, P., Beguiristain, T., et al. (2016). The bacterial and fungal diversity of an aged PAH- and heavy metal-contaminated soil is affected by plant cover and edaphic parameters. Microb. Ecol. 71, 711–724. doi: 10.1007/s00248-015-0682-8
Chen, W., Yang, Y., Fu, K., Zhang, D., and Wang, Z. (2022). Progress in ICP-MS analysis of minerals and heavy metals in traditional medicine. Front. Pharmacol. 13:891273. doi: 10.3389/fphar.2022.891273
Cheng, C., Li, Y., Long, M., Gao, M., Zhang, Y., Lin, J., et al. (2022). Moss biocrusts buffer the negative effects of karst rocky desertification on soil properties and soil microbial richness. Plant Soil 475, 153–168. doi: 10.1007/s11104-020-04602-4
Dangi, S. R., Stahl, P. D., Wick, A. F., Ingram, L. J., and Buyer, J. S. (2012). Soil microbial community recovery in reclaimed soils on a surface coal mine site. Soil Sci. Soc. Am. J. 76, 915–924. doi: 10.2136/sssaj2011.0288
De la Iglesia, R., Castro, D., Ginocchio, R., van der Lelie, D., and González, B. (2006). Factors influencing the composition of bacterial communities found at abandoned copper-tailings dumps. J. Appl. Microbiol. 100, 537–544. doi: 10.1111/j.1365-2672.2005.02793.x
Gazitúa, M. C., Morgante, V., Poupin, M. J., Ledger, T., Rodríguez-Valdecantos, G., Herrera, C., et al. (2021). The microbial community from the early-plant colonizer (Baccharis linearis) is required for plant establishment on copper mine tailings. Sci. Rep. 11:10448. doi: 10.1038/s41598-021-89769-1
Ginocchio, R., León-Lobos, P., Arellano, E. C., Anic, A., Ovalle, J. F., and Baker, A. J. M. (2017). Soil physicochemical factors as environmental filters for spontaneous plant colonization of abandoned tailing dumps. Environ. Sci. Pollut. Res. Int. 24, 13484–13496. doi: 10.1007/s11356-017-8894-8
Gutierrez, M. (2020). Editorial for special issue “Sustainable use of abandoned mines.”. Fortschr. Mineral. 10:1015. doi: 10.3390/min10111015
Illumina . (2013). 16S metagenomic sequencing library preparation. Available at: http://support.illumina.com/downloads/16s_metagenomic_sequencing_library_preparation.html
Islam, M. S., Kormoker, T., Idris, A. M., Proshad, R., Kabir, M. H., and Ustaoğlu, F. (2021). Plant–microbe–metal interactions for heavy metal bioremediation: a review. Crop Pasture Sci. 73, 181–201. doi: 10.1071/CP21322
Jia, T., Guo, T., and Chai, B. (2020). Bacterial community characteristics and enzyme activities in Imperata cylindrica litter as phytoremediation progresses in a copper tailings dam. PeerJ 8:e9612. doi: 10.7717/peerj.9612
Jia, T., Wang, R., and Chai, B. (2019). Effects of heavy metal pollution on soil physicochemical properties and microbial diversity over different reclamation years in a copper tailings dam. J. Soil Water Conserv. 74, 439–448. doi: 10.2489/jswc.74.5.439
Jin, J., Huang, R., Wang, J., Wang, C., Liu, R., Zhang, H., et al. (2022). Increase in Cd tolerance through seed-borne endophytic fungus Epichloë gansuensis affected root exudates and rhizosphere bacterial community of Achnatherum inebrians. Int. J. Mol. Sci. 23:13094. doi: 10.3390/ijms232113094
Kasemodel, M. C., Sakamoto, I. K., Varesche, M. B. A., and Rodrigues, V. G. S. (2019). Potentially toxic metal contamination and microbial community analysis in an abandoned Pb and Zn mining waste deposit. Sci. Total Environ. 675, 367–379. doi: 10.1016/j.scitotenv.2019.04.223
Li, B., Li, Y., Jiang, M., Chen, J., Wang, J., Li, T., et al. (2018). Accumulation and distribution of heavy metals in Imperata cylindrica at lead-zinc mining area. IOP Conf. Ser. Earth Environ. Sci. 199:042050. doi: 10.1088/1755-1315/199/4/042050
Li, X., Ma, L., Li, Y., Wang, L., and Zhang, L. (2019). Endophyte infection enhances accumulation of organic acids and minerals in rice under Pb2+ stress conditions. Ecotoxicol. Environ. Saf. 174, 255–262. doi: 10.1016/j.ecoenv.2019.02.072
Li, H.-Y., Shen, M., Zhou, Z.-P., Li, T., Wei, Y., and Lin, L. (2012). Diversity and cold adaptation of endophytic fungi from five dominant plant species collected from the Baima Snow Mountain, Southwest China. Fungal Divers. 54, 79–86. doi: 10.1007/s13225-012-0153-1
Li, L., Zheng, S., Yang, Q., Chen, S., and Huang, L. (2016). Distinguishing astragalus mongholicus and its planting soil samples from different regions by ICP-AES. Molecules 21:482. doi: 10.3390/molecules21040482
Liang, X., Liang, X., Liao, M., and Jia, T. (2023). Effects of Arbuscular Mycorrhizal fungi on the growth of Imperata cylindrica and soil enzyme activies under copper pollution. J. Shanxi Univ. Nat. Sci. Ed. 46, 951–960. doi: 10.13451/j.sxu.ns.2022081
Liu, B., Yao, J., Ma, B., Li, S., and Duran, R. (2022). Disentangling biogeographic and underlying assembly patterns of fungal communities in metalliferous mining and smelting soils. Sci. Total Environ. 845:157151. doi: 10.1016/j.scitotenv.2022.157151
López-González, J. A., Vargas-García, M. D. C., López, M. J., Suárez-Estrella, F., Jurado, M. D. M., and Moreno, J. (2015). Biodiversity and succession of mycobiota associated to agricultural lignocellulosic waste-based composting. Bioresour. Technol. 187, 305–313. doi: 10.1016/j.biortech.2015.03.124
Mahdavian, K., Ghaderian, S. M., and Torkzadeh-Mahani, M. (2017). Accumulation and phytoremediation of Pb, Zn, and Ag by plants growing on Koshk lead–zinc mining area, Iran. J. Soils Sediments 17, 1310–1320. doi: 10.1007/s11368-015-1260-x
Mahmud, K., Lee, K., Hill, N. S., Mergoum, A., and Missaoui, A. (2021). Influence of Tall fescue Epichloë endophytes on rhizosphere soil microbiome. Microorganisms 9:1843. doi: 10.3390/microorganisms9091843
Mao, W., Wu, Y., Li, F., Tang, W., Gong, W., Han, X., et al. (2023). Seed endophytes and their roles in host plant stress resistance. J. Soil Sci. Plant Nutr. 23, 2927–2937. doi: 10.1007/s42729-023-01279-3
Mendez, M. O., and Maier, R. M. (2008). Phytostabilization of mine tailings in arid and semiarid environments--an emerging remediation technology. Environ. Health Perspect. 116, 278–283. doi: 10.1289/ehp.10608
Naz, M., Dai, Z., Hussain, S., Tariq, M., Danish, S., Khan, I. U., et al. (2022). The soil pH and heavy metals revealed their impact on soil microbial community. J. Environ. Manag. 321:115770. doi: 10.1016/j.jenvman.2022.115770
Parada, R., Mendoza, L., Cotoras, M., and Ortiz, C. (2022). Endophytic fungi isolated from plants present in a mine tailing facility show a differential growth response to lead. Lett. Appl. Microbiol. 75, 345–354. doi: 10.1111/lam.13730
Parmar, S., Sharma, V. K., Li, T., Tang, W., and Li, H. (2022). Fungal seed endophyte FZT214 improves Dysphania ambrosioides Cd tolerance throughout different developmental stages. Front. Microbiol. 12:783475. doi: 10.3389/fmicb.2021.783475
Rosatto, S., Roccotiello, E., Di Piazza, S., Cecchi, G., Greco, G., Zotti, M., et al. (2019). Rhizosphere response to nickel in a facultative hyperaccumulator. Chemosphere 232, 243–253. doi: 10.1016/j.chemosphere.2019.05.193
Rothen, C., Miranda, V., Fracchia, S., Godeas, A., and Rodríguez, A. (2018). Microdochium bolleyi (Ascomycota: Xylariales): Physiological characterization and structural features of its association with wheat. Bol. Soc. Argent. Bot. 53, 169–182. doi: 10.31055/1851.2372.v53.n2.20574
Sánchez-López, A. S., Thijs, S., Beckers, B., González-Chávez, M. C., Weyens, N., Carrillo-González, R., et al. (2018). Community structure and diversity of endophytic bacteria in seeds of three consecutive generations of Crotalaria pumila growing on metal mine residues. Plant Soil 422, 51–66. doi: 10.1007/s11104-017-3176-2
Santoyo, G., Moreno-Hagelsieb, G., Orozco-Mosqueda M del, C., and Glick, B. R. (2016). Plant growth-promoting bacterial endophytes. Microbiol. Res. 183, 92–99. doi: 10.1016/j.micres.2015.11.008
Senthil Kumar, R., Koner, S., Tsai, H., Chen, J., Huang, S., and Hsu, B. (2023). Deciphering endemic rhizosphere microbiome community’s structure towards the host-derived heavy metals tolerance and plant growth promotion functions in serpentine geo-ecosystem. J. Hazard. Mater. 452:131359. doi: 10.1016/j.jhazmat.2023.131359
Shadmani, L., Jamali, S., and Fatemi, A. (2021). Effects of root endophytic fungus, Microdochium bolleyi on cadmium uptake, translocation and tolerance by Hordeum vulgare L. Biologia 76, 711–719. doi: 10.2478/s11756-020-00598-5
Shahzad, R., Waqas, M., Khan, A. L., Asaf, S., Khan, M. A., Kang, S., et al. (2016). Seed-borne endophytic Bacillus amyloliquefaciens RWL-1 produces gibberellins and regulates endogenous phytohormones of Oryza sativa. Plant Physiol. Biochem. 106, 236–243. doi: 10.1016/j.plaphy.2016.05.006
Shaltout, K. H., Galal, T. M., and El-Komi, T. M. (2016). Phenology, biomass and nutrients of Imperata cylindrica and Desmostachya bipinnata along the water courses in Nile Delta, Egypt. Rendiconti Lincei 27, 215–228. doi: 10.1007/s12210-015-0459-5
Song, F., Zhang, X., Liu, J., Pu, D., Zhao, Y., and Qiao, Q. (2019). Absorption and accumulation of heavy metals by Imperata cylindrical in iron tailings. J. Northwest A F Univ. 47, 83–90+100. doi: 10.13207/j.cnki.jnwafu.2019.04.011
Sun, L., Zhang, Y., He, L., Chen, Z., Wang, Q., Qian, M., et al. (2010). Genetic diversity and characterization of heavy metal-resistant-endophytic bacteria from two copper-tolerant plant species on copper mine wasteland. Bioresour. Technol. 101, 501–509. doi: 10.1016/j.biortech.2009.08.011
Sun, X., Zhou, Y., Tan, Y., Wu, Z., Lu, P., Zhang, G., et al. (2018). Restoration with pioneer plants changes soil properties and remodels the diversity and structure of bacterial communities in rhizosphere and bulk soil of copper mine tailings in Jiangxi Province, China. Environ. Sci. Pollut. Res. Int. 25, 22106–22119. doi: 10.1007/s11356-018-2244-3
Teixeira, L. C. R. S., Peixoto, R. S., Cury, J. C., Sul, W. J., Pellizari, V. H., Tiedje, J., et al. (2010). Bacterial diversity in rhizosphere soil from Antarctic vascular plants of Admiralty Bay, maritime Antarctica. ISME J. 4, 989–1001. doi: 10.1038/ismej.2010.35
Trannum, H. C., Næss, R., and Gundersen, H. (2020). Macrofaunal colonization of mine tailings impacted sediments. Sci. Total Environ. 708:134866. doi: 10.1016/j.scitotenv.2019.134866
Trivedi, P., Leach, J. E., Tringe, S. G., Sa, T., and Singh, B. K. (2020). Plant-microbiome interactions: from community assembly to plant health. Nat. Rev. Microbiol. 18, 607–621. doi: 10.1038/s41579-020-0412-1
Ultra, V. U., and Manyiwa, T. (2021). Influence of mycorrhiza and fly ash on the survival, growth and heavy metal accumulation in three Acacia species grown in Cu–Ni mine soil. Environ. Geochem. Health 43, 1337–1353. doi: 10.1007/s10653-020-00627-x
Vidal, C., Larama, G., Riveros, A., Meneses, C., and Cornejo, P. (2021). Main molecular pathways associated with copper tolerance response in Imperata cylindrica by de novo transcriptome assembly. Plants (Basel) 10:357. doi: 10.3390/plants10020357
Wan, W., Qin, Y., Wu, H., Zuo, W., He, H., Tan, J., et al. (2020). Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 11:752. doi: 10.3389/fmicb.2020.00752
Wang, Q., Garrity, G. M., Tiedje, J. M., and Cole, J. R. (2007). Naive Bayesian Classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267. doi: 10.1128/AEM.00062-07
Wang, L., Gong, L., Gan, D., Li, X., Yao, J., Wang, L., et al. (2022). Diversity, function and assembly of the Trifolium repens L. root-associated microbiome under lead stress. J. Hazard. Mater. 438:129510. doi: 10.1016/j.jhazmat.2022.129510
Wang, S., Guo, Z., Wang, L., Zhang, Y., Jiang, F., Wang, X., et al. (2021). Wheat rhizosphere metagenome reveals newfound potential soil Zn-mobilizing bacteria contributing to cultivars’ variation in grain Zn concentration. Front. Microbiol. 12:689855. doi: 10.3389/fmicb.2021.689855
Wang, J., Hou, W., Christensen, M. J., Li, X., Xia, C., Li, C., et al. (2020). Role of Epichloë endophytes in improving host grass resistance ability and soil properties. J. Agric. Food Chem. 68, 6944–6955. doi: 10.1021/acs.jafc.0c01396
Wang, H., Liu, H., Yang, T., Lv, G., Li, W., Chen, Y., et al. (2023). Mechanisms underlying the succession of plant rhizosphere microbial community structure and function in an alpine open-pit coal mining disturbance zone. J. Environ. Manag. 325:116571. doi: 10.1016/j.jenvman.2022.116571
Wu, B., Luo, S., Luo, H., Huang, H., Xu, F., Feng, S., et al. (2022). Improved phytoremediation of heavy metal contaminated soils by Miscanthus floridulus under a varied rhizosphere ecological characteristic. Sci. Total Environ. 808:151995. doi: 10.1016/j.scitotenv.2021.151995
Xie, L., and van Zyl, D. (2020). Distinguishing reclamation, revegetation and phytoremediation, and the importance of geochemical processes in the reclamation of sulfidic mine tailings: A review. Chemosphere 252:126446. doi: 10.1016/j.chemosphere.2020.126446
Zhao, J., Ma, J., Yang, Y., Yu, H., Zhang, S., and Chen, F. (2021). Response of soil microbial community to vegetation reconstruction modes in mining areas of the loess plateau, China. Front. Microbiol. 12:714967. doi: 10.3389/fmicb.2021.714967
Zheng, B., Bi, Q., Hao, X., Zhou, G., and Yang, X. (2017). Massilia phosphatilytica sp. nov., a phosphate solubilizing bacteria isolated from a long-term fertilized soil. Int. J. Syst. Evol. Microbiol. 67, 2514–2519. doi: 10.1099/ijsem.0.001916
Zheng, H., Zhang, Z., Xing, X., Hu, T., Qu, C., Chen, W., et al. (2019). Potentially toxic metals in soil and dominant plants from tonglushan Cu–Fe deposit, central China. Bull. Environ. Contam. Toxicol. 102, 92–97. doi: 10.1007/s00128-018-2501-7
Zhou, W., Wang, Y., Lian, Z., Yang, T., Zeng, Q., Feng, S., et al. (2020). Revegetation approach and plant identity unequally affect structure, ecological network and function of soil microbial community in a highly acidified mine tailings pond. Sci. Total Environ. 744:140793. doi: 10.1016/j.scitotenv.2020.140793
Keywords: heavy metal, Pioneer plant, Imperata cylindrica, seed endophyte, microbiota, revegetation
Citation: Mao W, Wu Y, Li Q, Xiang Y, Tang W, Hu H, Ji X and Li H (2024) Seed endophytes and rhizosphere microbiome of Imperata cylindrica, a pioneer plant of abandoned mine lands. Front. Microbiol. 15:1415329. doi: 10.3389/fmicb.2024.1415329
Received: 10 April 2024; Accepted: 11 July 2024;
Published: 24 July 2024.
Edited by:
Louis S. Tisa, University of New Hampshire, United StatesReviewed by:
Dominika Thiem, Nicolaus Copernicus University, PolandCopyright © 2024 Mao, Wu, Li, Xiang, Tang, Hu, Ji and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Li, bGh5eHJuQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.