
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 30 May 2024
Sec. Terrestrial Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1405115
This article is part of the Research Topic Soilborne Pathogenic Fungi: Systematics, Pathogenesis and Disease Control View all 8 articles
Fusarium crown rot (FCR) is one of the most important soilborne diseases affecting wheat production. To investigate the diversity of the pathogens causing this disease, 199 diseased wheat samples were collected from 13 cities in Shandong province. In total, 468 isolates were obtained, and from these isolates, 11 Fusarium species were identified based on phylogenetic analyses with the translation elongation factor-1α (TEF-1α), RNA polymerase II largest subunit (RPB1), and RNA polymerase II second largest subunit (RPB2) gene sequences. Of these Fusarium isolates, 283 were identified as Fusarium pseudograminearum and the remaining isolates were identified as Fusarium graminearum (n = 113), Fusarium sinensis (n = 28), Fusarium acuminatum (n = 18), Fusarium incarnatum (n = 13), Fusarium ipomoeae (n = 5), Fusarium flocciferum (n = 3), Fusarium proliferatum (n = 2), Fusarium asiaticum (n = 1), Fusarium culmorum (n = 1), and Fusarium oxysporum (n = 1), suggesting that F. pseudograminearum is the dominant pathogen of FCR of wheat in Shandong province. Pathogenicity tests demonstrated that all 11 Fusarium species could cause typical symptoms of FCR on wheat seedlings. The results of the study indicate that a greater diversity of Fusarium species can cause FCR of wheat in Shandong province than that has been previously reported. This is the first report in the world of Fusarium incarnatum, Fusarium ipomoeae, and Fusarium flocciferum as pathogens causing FCR in wheat.
Wheat (Triticum aestivum L.) is the second most important grain crop and is grown in diverse areas worldwide (Singh et al., 2016). Fusarium crown rot (FCR) of wheat is one of the most destructive soil−/residue-borne diseases in many arid and semi-arid cropping regions of the world (Kazan and Gardiner, 2018). This disease was causing damage to the wheat plant in China but only in a limited way before 2010. In recent years, it has become highly prevalent in the Huanghuai wheat-growing area, in part due to the adoption of moisture-preserving cultural practices, such as minimum tillage and stubble retention (Deng et al., 2020).
FCR occurs in the seedling stage, causing the death of seedlings before or after emergence. Brown discoloration appears on the coleoptile, subcrown internode, lower leaf sheaths and adjacent stems, and nodal tissues of the survived seedlings. The browning of the lower stems occurs with an occasional pink coloration of the nodes or stems under the leaf sheaths (Kazan and Gardiner, 2018). This disease process culminates with premature senescence of heads, called whiteheads, with no or shriveled grains (Kazan and Gardiner, 2018; Zhou et al., 2019). The incidence of FCR and its severity are negatively correlated with grain yield, tiller height, and straw weight (Smiley et al., 2005). Smiley et al. (2005) reported that FCR can cause up to 35% reduction in wheat grain yield under natural inoculum in the Pacific Northwest of the United States. In addition, FCR may lead to the contamination of wheat grains by mycotoxins (Mudge et al., 2006).
FCR of wheat is caused by a number of Fusarium species, and the composition of Fusarium species varies among regions. In the UK, Fusarium avenaceum and Fusarium culmorum were the pathogens causing FCR (Pettitt et al., 2003). In Queensland and northern New South Wales, Fusarium acuminatum, Fusarium avenaceum, Fusarium babinda, Fusarium crookwellense, Fusarium graminearum, Fusarium subglutinans, Fusarium torulosum, Fusarium tricinctum, Fusarium proliferatum, and Fusarium pseudograminearum were aggressive in causing FCR (Akinsanmi et al., 2004). In Turkey, six Fusarium species, such as F. avenaceum, F. culmorum, F. graminearum, Fusarium hostae, F. pseudograminearum, and Fusarium redolens, could cause crown rot with different levels of severity (Shikur Gebremariam et al., 2018). Among 12 Fusarium species isolated from diseased wheat samples in Azerbaijan, Fusarium algeriense, F. avenaceum, F. culmorum, F. graminearum, F. hostae, and F. pseudograminearum were pathogenic to wheat (Özer et al., 2020).
With ongoing research, a greater number of Fusarium species have been identified to cause FCR in a certain wheat-growing area. In China, a previous survey on agents causing FCR in Anhui, Jiangsu, Henan, Shandong, and Hebei provinces revealed that F. acuminatum, F. asiaticum, F. avenaceum, F. graminearum, and F. pseudograminearum were the pathogens responsible for the disease (Zhang et al., 2015). F. acuminatum, F. asiaticum, F. culmorum, F. equiseti, F. graminearum, F. oxysporum, F. proliferatum, F. pseudograminearum, and F. sinensis were the pathogens causing FCR in the Huanghuai wheat-growing region (including Anhui, Jiangsu, Henan, Shanxi, Shaanxi, Shandong, and Hebei provinces) (Zhou et al., 2019).
Information on species complexity is essential for designing effective management strategies, especially since different species of Fusarium exhibit varying degrees of sensitivity to fungicides. Previous research reported that Fusarium verticillioides was sensitive to tebuconazole, with inhibition values of 94%, while F. proliferatum and F. graminearum showed lower inhibition values of 77 and 67%, respectively (Masiello et al., 2019). Therefore, the objectives of this study were to isolate and identify the Fusarium species causing FCR of wheat in Shandong province and evaluate the pathogenic diversity of different Fusarium species on wheat seedlings so that suitable strategies could be developed for disease management.
The stems of the diseased wheat plants exhibiting crown rot symptoms were collected from Shandong province. The wheat fields were selected randomly, and the selected fields were at least 3 km apart. The area of each field was more than 667 m2. At least six wheat fields in each city were selected for sample collection. The samples were collected from five sites in the field in a zigzag pattern (Fang, 1998). Each sampling site was approximately 1 m2 and at least 10 m apart. Three diseased wheat plants were collected from each sampling site, meaning that one sample consisted of 15 diseased plants. In total, 199 samples were collected from 13 cities (Figure 1). Small tissue pieces (approximately 3–6 mm in length) were cut from healthy to diseased margins, surface-sterilized with 70% ethanol for 40 s and 0.5% sodium hypochlorite (NaClO) solution for 2 min, rinsed with sterilized water three times, and then air dried on sterilized filter papers. The pieces were placed on potato dextrose agar (PDA) (Abate et al., 2018) plates containing 50 μg/mL streptomycin sulfate and incubated at 25°C in the dark for 48–72 h. Suspected Fusarium colonies were transferred to fresh PDA plates, and pure cultures were obtained from hyphal tips. Then, the Fusarium-like isolates were obtained and stored at −4°C for further studies.
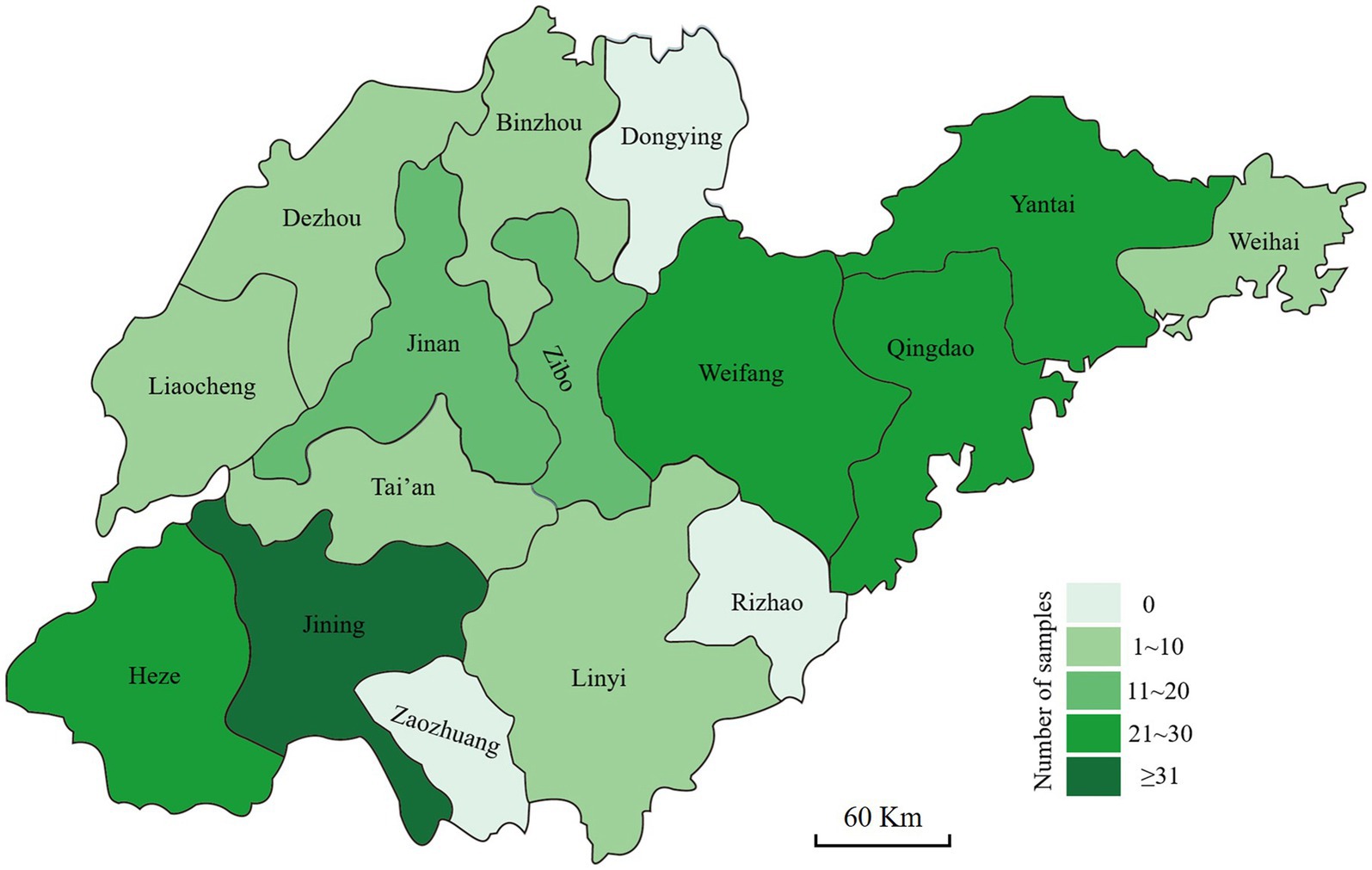
Figure 1. Geographic origins where the diseased wheat samples with symptoms of Fusarium crown rot were collected in Shandong province.
All the Fusarium-like isolates were grown on PDA plates for 4–7 days at 25°C in the dark. A sample of the mycelia (20 mg) of each isolate was carefully collected from the agar medium surface and ground to a fine powder in liquid nitrogen. Genomic DNA was extracted using the cetyltrimethylammonium bromide (CTAB) method, as described by Lee and Taylor (1990). The obtained DNA pellet was dried under vacuum, dissolved in 30 μL ddH2O, and stored at −20°C until use.
The partial translation elongation factor-1α (TEF-1α), RNA polymerase II largest subunit (RPB1), and RNA polymerase II second largest subunit (RPB2) genes were amplified with the primers EF1 and EF2 (O’Donnell et al., 1998), F7 and G2R (O’Donnell et al., 2022), and 5f2 and 7cr (O’Donnell et al., 2022) (Table 1). The PCR reaction mixture consisted of 10.5 μL ddH2O, 12.5 μL 2× F8 FastLong PCR MasterMix (PC80, Aidlab Biotechnologies Co., Ltd., Beijing, China; containing 0.05 units/μL F8 FastLong DNA Polymerase, 0.4 mM dNTPs, and 4 mM MgCl2), 0.5 μL of each primer (10 μM), and 1 μL DNA template (100 μg/mL). Negative controls contained the same reagents but without the DNA template. Amplifications were performed in an Eppendorf Mastercycler gradient thermal cycler (Eppendorf, Hamburg, Germany). All primers and PCR conditions are summarized in Table 1.
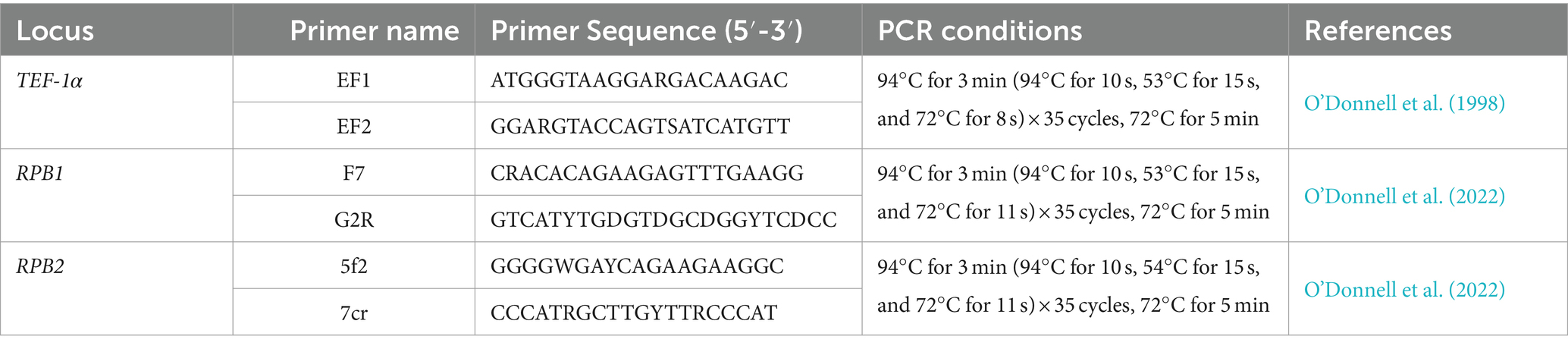
Table 1. Amplification sites, primer names, primer sequences, PCR conditions, and references used in this study.
The PCR products were purified with an Aidlab DNA Gel Extraction Kit (Aidlab Biotechnologies) and cloned into a pTOPO-T Simple Vector (CV15, Zero Background pTOPO-TA Simple Cloning Kit, Aidlab Biotechnologies) according to manufacturer’s instructions. The ligation reaction mixture was transformed into competent cells of Escherichia coli TreliefTM 5α (TSC-C01, Qingdao Tsingke Biotechnology Co., Ltd., Qingdao, China), and transformants were cultured on Luria-Bertani (LB) agar plates containing ampicillin (50 μg/mL), 5-bromo-4-chloro-3-indolyl-β-D-galactoside (X-gal, 100 μg/mL), and isopropyl-b-D-thiogalactopyranoside (IPTG, 100 μg/mL). White colonies with the target DNA insertion verified by PCR were sent to Qingdao Tsingke Biotechnology for sequencing.
All the isolates were initially examined molecularly by the sequence analysis of the TEF-1α gene. The putative identifications were made based on the percent shared identity of consensus sequences to related Fusarium species in the NCBI GenBank database using BLASTn searches. To further verify the accuracy of characterization using the TEF-1α gene, 53 isolates representing 11 different Fusarium species according to the TEF-1α gene sequence analysis were then examined for the RPB1 and RPB2 gene regions. Multiple sequence alignments were constructed using an online version of MAFFT v.7 (https://mafft.cbrc.jp/alignment/server/) (Katoh and Standley, 2013). The aligned sequences were edited using BioEdit software and completed by manual adjustments. The maximum likelihood (ML) analyses of independent (TEF-1α) and concatenated (TEF-1α, RPB1, and RPB2) gene datasets were performed using RAxML-HPC BlackBox v.8.2.10 (Stamatakis, 2006) within the Cyberinfrastructure for Phylogenetic Research (CIPRES) portal (https://www.phylo.org/portal2/) (Miller et al., 2010). Branch stability was estimated with 1,000 bootstrap replicates. Sequences of Stemphylium vesicarium or Fusarium solani served as the outgroup taxon in the analyses. The phylogenetic trees were viewed in MEGA v.7.0, and bootstrap values ≥70% were considered as significant and indicated in the phylogenetic trees. The basic information of 53 representative isolates in this study, 22 representative isolates of the 11 Fusarium species, and outgroup S. vesicarium strain CBS 191.86 and two strains of F. solani (NRRL 23244 and 32,810) are shown in Table 2.
Based on the pathogen identification results, 418 representative Fusarium isolates, obtained from different cities or counties and representing different Fusarium species, were selected to determine the pathogenicity. The experiment was performed with minor modifications of a method described by Zhang et al. (2015). Briefly, tests were conducted on the ‘Jimai 22’ variety of wheat seedlings, and the length of seedlings were approximately 3 cm after pre-germination at 28°C for 3 days. The selected Fusarium isolates were incubated on PDA plates at 25°C in the dark for 4 days, and agar plugs (5 mm in diameter) were cut from the edge of the colonies. Ten wheat seedlings were equably arranged on the absorbent gauze strip (approximately 20 × 3 cm [length × width]), and one agar plug was inoculated at the base of each wheat seedling stem. The absorbent gauze strip was then rolled up and placed vertically in an empty Petri dish. Sterile water was added to the dish to keep the gauze moist. The controls consisted of seedlings that were inoculated with sterile plugs of PDA. The dishes were placed in plastic boxes, covered with clear plastic to maintain high humidity, and incubated in a growth chamber at 25°C and 90% relative humidity (RH) with a 12 h photoperiod per day for 7 days. After incubation, disease severity (DS) was scored on a six-point rating system modified from Smiley et al. (2005): 0 = apparently healthy plant with no discoloration of any tissue; 1 = browning of the coleoptile and the browning area < 50%; 2 = browning of the coleoptile and the browning area of 50 ~ 100%; 3 = the browning area exceeded the coleoptile from bottom to top, but the euphylla are still green; 4 = the browning area exceeded the coleoptile from bottom to top, and the euphylla appear to have partial chlorosis; and 5 = whole plant turns yellow or withered and died. Disease index (DI) was calculated using the following formula: DI = [100 × ∑ (n × corresponding DS)]/(N × 5), where n is the number of the infected seedlings corresponding to each disease rating, and N is the total number of inoculation seedlings. Re-isolations from the inoculated seedlings were attempted, and the resulting isolates were confirmed as the corresponding Fusarium species based on the molecular characteristics described above to fulfill Koch’s postulates. The experiment was conducted three times. Statistical significance was determined with SPSS (v. 20.0; SPSS Inc.) using a least significant difference (LSD) test at a significance level of P of <0.05.
A total of 199 FCR samples resulted in the isolation of a total of 468 Fusarium isolates (Table 3). The TEF-1α partial gene from all 468 isolates were amplified and sequenced to confirm their identities. The RPB1 and RPB2 gene sequences of 53 representative isolates were also analyzed. The basic local alignment search tool (BLASTn) searches using TEF-1α partial gene sequence of each isolate showed that all 468 isolates represented the 11 species of F. pseudograminearum, F. graminearum, F. sinensis, F. acuminatum, F. incarnatum, F. ipomoeae, F. flocciferum, F. proliferatum, F. asiaticum, F. culmorum, and F. oxysporum. This represented an isolate ratio of 60.47, 24.15, 5.98, 3.85, 2.78, 1.07, 0.64, 0.43, 0.21, 0.21, and 0.21%, respectively (Table 3).
Of the analyzed wheat samples, 83.42% were infected by individual Fusarium species, including 43.72% of the samples infected by F. pseudograminearum, 23.12% infected by F. graminearum, 9.05% infected by F. sinensis, 3.02% infected by F. acuminatum, 2.51% infected by F. incarnatum, and 0.50% infected by F. ipomoeae, F. flocciferum, F. asiaticum, and F. culmorum, respectively; two or three Fusarium species were found in 16.58% of the samples isolated from the diseased tissues, and F. pseudograminearum or F. graminearum combined with other Fusarium species infected the vast majority of the samples (Supplementary Table S1).
Tree topology resulting from an ML analysis of the independent alignment of TEF-1α partial gene sequences divided the 53 representative isolates into 11 clades (F. pseudograminearum, F. graminearum, F. sinensis, F. acuminatum, F. incarnatum, F. ipomoeae, F. flocciferum, F. proliferatum, F. asiaticum, F. culmorum, and F. oxysporum) (Figure 2; Supplementary Figure S1), which is consistent with the result of BLASTn comparison. The phylogenetic tree based on the concatenated sequences of three loci (TEF-1α, RPB1, and RPB2) using the ML method divided the 53 representative isolates into 11 clades (Figure 3), which is congruent with the tree of independent data of the TEF-1α partial gene. These results indicated that using the TEF-1α partial gene to identify Fusarium species is rapid, effective, and accurate.
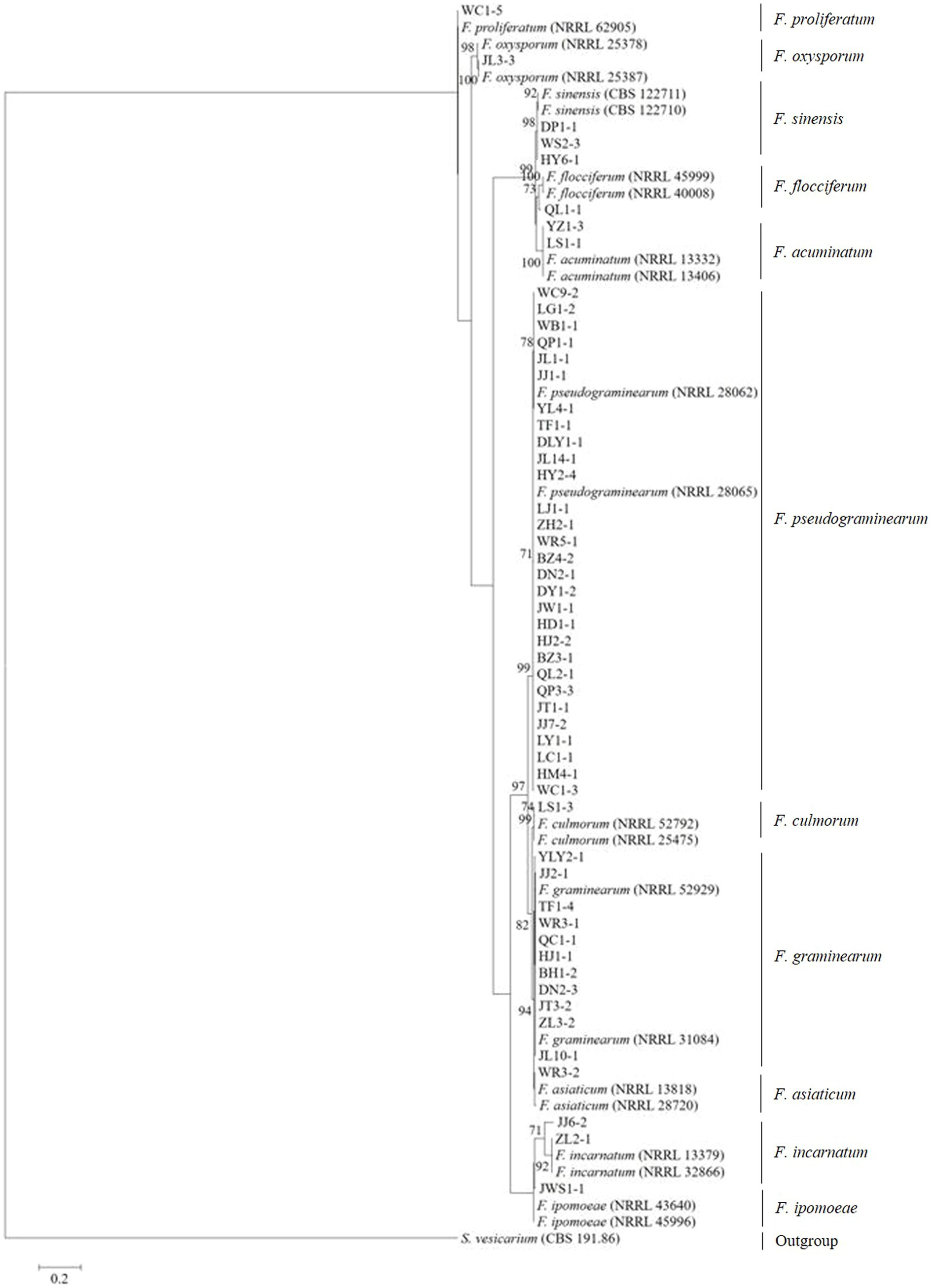
Figure 2. Maximum likelihood phylogenetic analysis of 11 Fusarium species based on TEF-1α partial gene sequences. The tree was rooted with sequences of Stemphylium vesicarium. The number of bootstrap replications was set to 1,000. Support values at nodes represent bootstrap percentages with values ≥70% are shown above the branches.
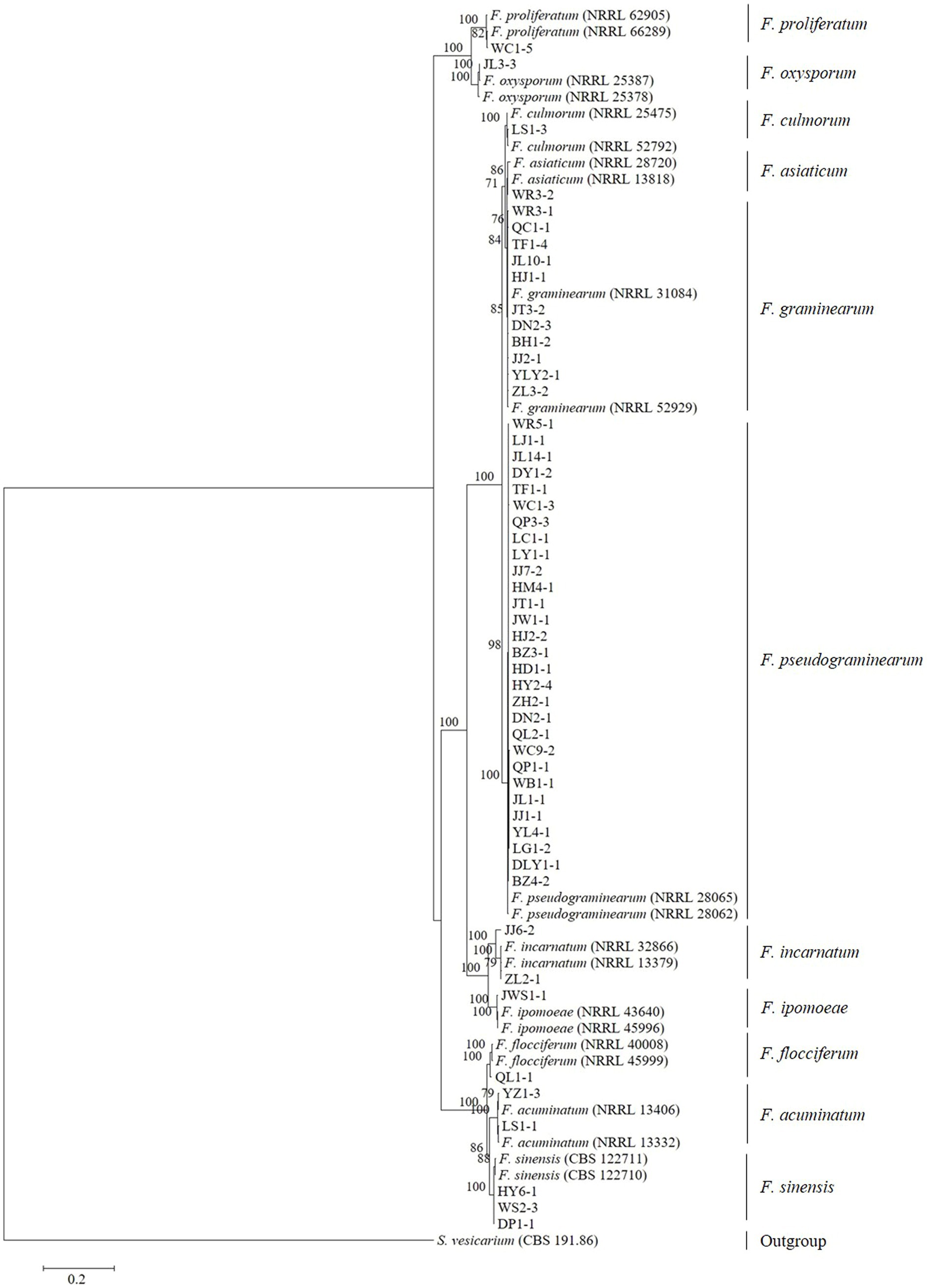
Figure 3. Maximum likelihood phylogenetic tree based on a concatenated alignment of TEF-1α, RPB1, and RPB2 gene sequences. The tree was rooted using sequences of Stemphylium vesicarium. Support values at nodes represent RAxML bootstrap percentages with values ≥70% are shown above the branches.
The 418 tested Fusarium isolates, including 283\u00B0F. pseudograminearum, 75\u00B0F. graminearum, 24\u00B0F. sinensis, 12\u00B0F. acuminatum, 13\u00B0F. incarnatum, four F. ipomoeae, two F. flocciferum, two F. proliferatum, one F. asiaticum, one F. culmorum, and one F. oxysporum, could cause typical symptoms of FCR on wheat seedlings. The symptoms ranged from very faint lesions on the coleoptile only to intense brown necrotic discoloration on the leaf sheaths and finally to plant death resulting from stem rotting, while no symptoms of FCR were observed on control seedlings inoculated with PDA agar plugs not containing Fusarium mycelia (Figure 4). The average disease incidence and average disease index caused by F. pseudograminearum, F. graminearum, F. sinensis, F. acuminatum, F. incarnatum, F. ipomoeae, F. flocciferum, and F. proliferatum on wheat seedlings ranged from 38.3 to 99.1% and from 8.7 to 72.4, respectively. The disease incidence and disease index (98.1% and 72.4, 99.1% and 64.5, respectively) of F. pseudograminearum and F. graminearum were significantly higher than those of F. sinensis (64.2% and 15.6), F. incarnatum (74.9% and 17.9), and F. ipomoeae (60.8% and 12.8). Only one F. asiaticum isolate, one F. culmorum isolate, and one F. oxysporum isolate were identified among all 468 Fusarium isolates, and their disease incidence and disease index were 100.0% and 73.3, 100.0% and 76.7, and 100.0% and 26.0, respectively (Table 4). Isolates of F. pseudograminearum, F. graminearum, F. asiaticum, and F. culmorum generally exhibited a high level of virulence on wheat seedlings, while isolates of F. sinensis, F. acuminatum, F. incarnatum, F. ipomoeae, F. flocciferum, F. proliferatum, and F. oxysporum exhibited a relatively low level of virulence.

Figure 4. Pathogenicity assays of the representative isolates of 11 Fusarium species on wheat seedlings. (A) CK; (B) Fusarium pseudograminearum; (C) F. graminearum; (D) F. sinensis; (E) F. acuminatum; (F) F. incarnatum; (G) F. ipomoeae; (H) F. flocciferum; (I) F. proliferatum; (J) F. asiaticum; (K) F. culmorum; (L) F. oxysporum. The experiment was conducted on wheat seedlings ‘Jimai 22’. Agar plugs (5 mm in diameter) were placed on the base of wheat seedling stems, which were pregerminated at 28°C for 3 days. Disease severity (DS) was scored after 7 days of incubation at 25°C and 90% relative humidity using a six-point rating system.
No Fusarium isolates were re-isolated from the control seedlings, while Fusarium isolates were consistently re-isolated from wheat seedlings with symptoms of FCR. The identities of the re-isolated fungi were confirmed by molecular characterizations as described above, thus fulfilling Koch’s postulates.
In this study, 11 Fusarium species were identified as causal agents of FCR in the main wheat-producing regions of Shandong province in China. The identified species were F. pseudograminearum (60.47%), F. graminearum (24.15%), F. sinensis (5.98%), F. acuminatum (3.85%), F. incarnatum (2.78%), F. ipomoeae (1.07%), F. flocciferum (0.64%), F. proliferatum (0.43%), F. asiaticum (0.21%), F. culmorum (0.21%), and F. oxysporum (0.21%). To our knowledge, this is the first report in the world of F. incarnatum, F. ipomoeae, and F. flocciferum causing crown rot of wheat.
A total of 468 Fusarium isolates were obtained from 199 wheat samples with FCR symptoms, and the isolation ratio of Fusarium species was 2.35 in the study. An earlier research from the Huanghuai wheat-growing region showed that 1,196 Fusarium isolates were isolated from 222 samples with the isolation ratio of 5.39 (Zhou et al., 2019). Another study showed that the isolation ratio of Fusarium species was 8.26, and the wheat samples were collected from central, eastern, and southeastern Kazakhstan (Bozoğlu et al., 2022).
This study revealed a change and diversity of Fusarium species that causes crown rot of wheat in Shandong province. A previous survey by Zhang et al. (2015) in the five major wheat-growing provinces of China, which include Shandong province, revealed that the dominant pathogen was F. asiaticum, followed by F. graminearum. Another study reported that F. pseudograminearum, F. graminearum, F. sinensis, F. acuminatum, F. equiseti, F. proliferatum, and F. oxysporum are the pathogens causing FCR in wheat in Shandong province, and F. pseudograminearum and F. graminearum are both the dominant pathogens and have the same isolation frequency (41%, respectively) (Zhou et al., 2019). Recent report indicated that F. pseudograminearum, F. graminearum, and F. asiaticum were responsible for crown rot of wheat in Shandong province, with F. pseudograminearum being the most prevalent species (Deng et al., 2020). Our results were consistent with those of previous studies, which showed that F. pseudograminearum was the dominant pathogen, but we found more abundant Fusarium species causing crown rot of wheat in the Shandong province, such as F. incarnatum, F. ipomoeae, F. flocciferum, and F. culmorum.
Climate may play a crucial role in determining the prevalence of Fusarium species. Temperature impacts the aggressiveness of F. pseudograminearum, while cooler diurnal temperatures (e.g., 15/15°C vs. 25/15°C) increased the aggressiveness of F. pseudograminearum (Sabburg et al., 2015). Deng et al. (2020) found that the frequency of F. asiaticum was higher than F. graminearum in Jiangsu province, while F. asiaticum was rarely isolated in Shandong province. The bias toward Jiangsu in the distribution of F. asiaticum coincided with the climate envelope modeling, indicating that F. asiaticum occurs in areas with warm and wet summers (Backhouse, 2014), as the year-round climate in Jiangsu is warmer and wetter than that of Shandong. Other reports highlighted that the distribution of F. pseudograminearum was related to low rainfall, raised temperatures in summer, or elevated levels of carbon dioxide (Melloy et al., 2010; Moya-Elizondo et al., 2011; Xu et al., 2018).
For the uniquely reported species, F. incarnatum was isolated from samples collected from Binzhou, Heze, Jining, and Zibo (the inland areas) and Qingdao and Yantai (the coastal areas), F. ipomoeae was isolated from Jining and Weifang (the inland areas), and F. flocciferum was isolated from Heze and Qingdao. Climatic differences may not affect the distribution of F. incarnatum, F. ipomoeae, and F. flocciferum since F. incarnatum and F. flocciferum were found in the inland areas and the coastal areas, respectively, and F. ipomoeae was reported to be the pathogen of peanut leaf spot in Laixi (the coastal areas), China (Xu et al., 2021) and the pathogen of soybean wilt in South Korea (Choi et al., 2023). Naeem et al. (2019) considered that the diversity of Fusarium species on intercropped soybean pods was associated with soybean varieties. Further studies are needed to confirm whether the wheat variety affects the distribution of the three uniquely reported species.
The results of the assessment of pathogenicity show that all Fusarium isolates tested for pathogenicity could cause symptoms of FCR. F. culmorum was the most virulent species, followed by F. asiaticum, F. pseudograminearum, F. graminearum, F. oxysporum, F. acuminatum, F. proliferatum, F. incarnatum, F. sinensis, F. ipomoeae, and F. flocciferum. However, F. culmorum and F. asiaticum had low isolation percentages (each was 0.21%) and were only recovered from the cities of Liaocheng and Weihai, respectively. In contrast, the most prevalent species, F. pseudograminearum, was isolated from the samples collected in all sampled cities. Similarly, F. graminearum was commonly isolated except from the samples collected in Linyi city. Therefore, F. pseudograminearum and F. graminearum should be regarded as the major pathogens when designing and implementing disease management programs.
Maize [Zea mays L.] is an important food and feed crop and often rotated with wheat in Shandong province. Maize seedling blight commonly occurred in the Shandong province, and the disease incidence was up to 50% in some fields. The root system of infected plants displayed poor development. The primary roots were brown and rotted. The leaves at the base of the plants were drying up, and then, the whole plant withered (Jiang et al., 2022). Maize seedling blight is a serious threat to maize yield. Recently, it was first reported that F. pseudograminearum caused maize seedling blight in Zibo city, Shandong province (Jiang et al., 2022), which indicated that the crown rot of wheat caused by F. pseudograminearum may aggravate the occurrence of maize seedling blight. Controlling the occurrence of FCR and changing rotation crops are particularly important for the healthy production of wheat and maize.
Fusarium head blight (FHB) is a devastating disease affecting wheat in many regions throughout the world. It has the capacity to destroy a potentially high-yielding crop within a few weeks of harvest (McMullen et al., 1997). In addition to direct yield losses, FHB reduces grain quality, and the harvested grain is often contaminated with mycotoxins (Seitz et al., 1986). Previous studies reported that F. pseudograminearum and F. graminearum are also the major pathogens of FHB (Xu et al., 2015, 2021). The relationship between FCR and FHB needs further study. As FCR of wheat caused by F. pseudograminearum is an increasing problem in the Shandong province, it is appropriate to monitor the role of F. pseudograminearum in FHB in the future.
The use of clean and chemically disinfected seeds, adjusting the date of seeding, proper fertilization, crop rotations avoiding other host crops, and use of cultivars with resistance to the pathogens or to water stress have been suggested for the management of FCR of wheat (Cook, 2010). Among these strategies, fungicide seed treatment has always been a primary method for controlling FCR (Moya-Elizondo and Jacobsen, 2016). The accurate identification of Fusarium species is critical to disease management. Among F. avenaceum, F. culmorum, F. graminearum, and F. poae, it was observed that F. graminearum showed the highest sensitivity to prochloraz and F. poae showed lower sensitivity to metconazole compared to F. culmorum (Tini et al., 2020). As F. pseudograminearum and F. graminearum were confirmed as the causal agents of FCR of wheat in the Shandong province, further research should focus on the sensitivity of these two Fusarium species to commonly used fungicides.
The data presented in the study are deposited in the GenBank repository. The accession numbers can be found in the article.
GM: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing. HW: Investigation, Resources, Writing – review & editing. KQ: Resources, Writing – review & editing. LM: Formal analysis, Writing – review & editing. BZ: Methodology, Writing – review & editing. YZ: Writing – review & editing. HJ: Writing – review & editing. XW: Writing – review & editing. JQ: Funding acquisition, Project administration, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was financially supported by the Shandong Provincial Natural Science Foundation (ZR2022QC131), the National Natural Science Foundation of China (32202274), Wheat Industry Technology System of Shandong Province (SDAIT-01-10), and Agricultural Scientific and Technological Innovation Project of Shandong Academy of Agricultural Sciences (CXGC2023F04).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1405115/full#supplementary-material
Supplementary Figure S1 | Maximum likelihood phylogenetic analysis of 11 Fusarium species based on TEF-1α partial gene sequences. Two strains of F. solani (NRRL 23244 and 32810) are the outgroup. The number of bootstrap replications was set to 1000. Support values at nodes represent bootstrap percentages with values ≥ 70% are shown above the branches.
Abate, D., Pastore, C., Gerin, D., De Miccolis Angelini, R. M., Rotolo, C., Pollastro, S., et al. (2018). Characterization of Monilinia spp. populations on stone fruit in South Italy. Plant Dis. 102, 1708–1717. doi: 10.1094/PDIS-08-17-1314-RE
Akinsanmi, O. A., Mitter, V., Simpfendorfer, S., Backhouse, D., and Chakraborty, S. (2004). Identity and pathogenicity of fusarium spp. isolated from wheat fields in Queensland and northern New South Wales. Aust. J. Agric. Res. 55, 97–107. doi: 10.1071/AR03090
Backhouse, D. (2014). Global distribution of fusarium graminearum, F. Asiaticum and F. Boothii from wheat in relation to climate. Eur. J. Plant Pathol. 139, 161–173. doi: 10.1007/s10658-013-0374-5
Bozoğlu, T., Derviş, S., Imren, M., Amer, M., Özdemir, F., Paulitz, T. C., et al. (2022). Fungal pathogens associated with crown and root rot of wheat in central, eastern, and southeastern Kazakhstan. J. Fungi 8:417. doi: 10.3390/jof8050417
Choi, H. W., Ryu, H., Lee, Y., Jang, Y. W., Yi, H., Hong, S. K., et al. (2023). First report of fusarium ipomoeae causing fusarium wilt on Glycine max in South Korea. Plant Dis. 107:575. doi: 10.1094/PDIS-07-21-1499-PDN
Cook, R. J. (2010). “Fusarium root, crown, and foot rots and associated seedling diseases” in Compendium of wheat diseases and pests. eds. W. W. Bockus, R. L. Bowden, R. M. Hunger, W. L. Morrill, T. D. Murray, and R. W. Smiley (Minnesota, USA: The Pennsylvania State University Press), 37–39.
Deng, Y. Y., Li, W., Zhang, P., Sun, H. Y., Zhang, X. X., Zhang, A. X., et al. (2020). Fusarium pseudograminearum as an emerging pathogen of crown rot of wheat in eastern China. Plant Pathol. 69, 240–248. doi: 10.1111/ppa.13122
Fang, Z. D. (1998). “Sampling method” in Research methodology for plant diseases. ed. H. G. Zhang (Beijing: Chinese Agriculture Press), 5–7.
Jiang, H., Ma, L. G., Qi, K., Zhang, Y. L., Zhang, B., Ma, G. P., et al. (2022). First report of maize seedling blight caused by fusarium pseudograminearum in China. Plant Dis. 106:2519. doi: 10.1094/PDIS-01-22-0099-PDN
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kazan, K., and Gardiner, D. M. (2018). Fusarium crown rot caused by fusarium pseudograminearum in cereal crops: recent progress and future prospects. Mol. Plant Pathol. 19, 1547–1562. doi: 10.1111/mpp.12639
Lee, S. B., and Taylor, J. W. (1990). Isolation of DNA from fungal mycelia and single spores. In: M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (Ed.) PCR protocols: A guide to methods and applications. San Diego, CA: Academic Press, pp:282–287.
Masiello, M., Somma, S., Ghionna, V., Logrieco, A. F., and Moretti, A. (2019). In vitro and in field response of different fungicides against aspergillus flavus and fusarium species causing ear rot disease of maize. Toxins 11:11. doi: 10.3390/toxins11010011
McMullen, M. P., Jones, R., and Gallenberg, D. (1997). Scab of wheat and barley: a re-emerging disease of devastating impact. Plant Dis. 81, 1340–1348. doi: 10.1094/PDIS.1997.81.12.1340
Melloy, P., Hollaway, G., Luck, J., Norton, R., Aitken, E., and Chakraborty, S. (2010). Production and fitness of fusarium pseudograminearum inoculum at elevated carbon dioxide in FACE. Glob. Chang. Biol. 16, 3363–3373. doi: 10.1111/j.1365-2486.2010.02178.x
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES science gateway for inference of large phylogenetic trees” in Proceedings of the gateway computing environments workshop (GCE) (LA: New Orleans), 1–8.
Moya-Elizondo, E. A., and Jacobsen, B. J. (2016). Integrated management of fusarium crown rot of wheat using fungicide seed treatment, cultivar resistance, and induction of systemic acquired resistance (SAR). Biol. Control 92, 153–163. doi: 10.1016/j.biocontrol.2015.10.006
Moya-Elizondo, E., Rew, L. J., Jacobsen, B. J., Hogg, A. C., and Dyer, A. T. (2011). Distribution and prevalence of fusarium crown rot and common root rot pathogens of wheat in Montana. Plant Dis. 95, 1099–1108. doi: 10.1094/PDIS-11-10-0795
Mudge, A. M., Dill-Macky, R., Dong, Y. H., Gardiner, D. M., White, R. G., and Manners, J. M. (2006). A role for the mycotoxin deoxynivalenol in stem colonisation during crown rot disease of wheat caused by fusarium graminearum and fusarium pseudograminearum. Physiol. Mol. Plant P. 69, 73–85. doi: 10.1016/j.pmpp.2007.01.003
Naeem, M., Li, H., Yan, L., Raza, M. A., Gong, G., Chen, H., et al. (2019). Characterization and pathogenicity of fusarium species associated with soybean pods in maize/soybean strip intercropping. Pathogens 8:245. doi: 10.3390/pathogens8040245
O’Donnell, K., Kistler, H. C., Cigelnik, E., and Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. P. Natl. Acad. Sci. USA 95, 2044–2049. doi: 10.1073/pnas.95.5.2044
O’Donnell, K., Whitaker, B. K., Laraba, I., Proctor, R. H., Brown, D. W., Broders, K., et al. (2022). DNA sequence-based identification of fusarium: a work in progress. Plant Dis. 106, 1597–1609. doi: 10.1094/PDIS-09-21-2035-SR
Özer, G., Paulitz, T. C., Imren, M., Alkan, M., Muminjanov, H., and Dababat, A. A. (2020). Identity and pathogenicity of fungi associated with crown and root rot of dryland winter wheat in Azerbaijan. Plant Dis. 104, 2149–2157. doi: 10.1094/PDIS-08-19-1799-RE
Pettitt, T., Xu, X. M., and Parry, D. (2003). Association of fusarium species in the wheat stem rot complex. Eur. J. Plant Pathol. 109, 769–774. doi: 10.1023/A:1026042711064
Sabburg, R., Obanor, F., Aitken, E., and Chakraborty, S. (2015). Changing fitness of a necrotrophic plant pathogen under increasing temperature. Glob. Chang. Biol. 21, 3126–3137. doi: 10.1111/gcb.12927
Seitz, L. M., Eustace, W. D., Mohr, H. E., Shogren, M. D., and Yamazaki, W. T. (1986). Cleaning, milling, and baking tests with hard red winter wheat containing deoxynivalenol. Cereal Chem. 63, 146–150.
Shikur Gebremariam, E., Sharma-Poudyal, D., Paulitz, T. C., Erginbas-Orakci, G., Karakaya, A., and Dababat, A. A. (2018). Identity and pathogenicity of fusarium species associated with crown rot on wheat (Triticum spp.) in Turkey. Eur. J. Plant Pathol. 150, 387–399. doi: 10.1007/s10658-017-1285-7
Singh, R. P., Singh, P. K., Rutkoski, J., Hodson, D. P., He, X. Y., Jørgensen, L. N., et al. (2016). Disease impact on wheat yield potential and prospects of genetic control. Annu. Rev. Phytopathol. 54, 303–322. doi: 10.1146/annurev-phyto-080615-095835
Smiley, R. W., Gourlie, J. A., Easley, S. A., Patterson, L. M., and Whittaker, R. G. (2005). Crop damage estimates for crown rot of wheat and barley in the Pacific northwest. Plant Dis. 89, 595–604. doi: 10.1094/PD-89-0595
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Tini, F., Beccari, G., Onofri, A., Ciavatta, E., Gardiner, D. M., and Covarelli, L. (2020). Fungicides may have differential efficacies towards the main causal agents of fusarium head blight of wheat. Pest Manag. Sci. 76, 3738–3748. doi: 10.1002/ps.5923
Xu, F., Liu, W., Song, Y. L., Zhou, Y. L., Xu, X. M., Yang, G. Q., et al. (2021). The distribution of fusarium graminearum and fusarium asiaticum causing fusarium head blight of wheat in relation to climate and cropping system. Plant Dis. 105, 2830–2835. doi: 10.1094/PDIS-01-21-0013-RE
Xu, F., Song, Y. L., Yang, G. Q., Wang, J. M., Liu, L. L., and Li, Y. H. (2015). First report of fusarium pseudograminearum from wheat heads with fusarium head blight in North China plain. Plant Dis. 99:156. doi: 10.1094/PDIS-05-14-0543-PDN
Xu, F., Yang, G. Q., Wang, J. M., Song, Y. L., Liu, L. L., Zhao, K., et al. (2018). Spatial distribution of root and crown rot fungi associated with winter wheat in the North China plain and its relationship with climate variables. Front. Microbiol. 9:1054. doi: 10.3389/fmicb.2018.01054
Xu, M., Zhang, X., Yu, J., Guo, Z., Li, Y., Wu, J., et al. (2021). First report of fusarium ipomoeae causing peanut leaf spot in China. Plant Dis. 105:3754. doi: 10.1094/PDIS-01-21-0226-PDN
Zhang, X. X., Sun, H. Y., Shen, C. M., Li, W., Yu, H. S., and Chen, H. G. (2015). Survey of fusarium spp. causing wheat crown rot in major winter wheat growing regions of China. Plant Dis. 99, 1610–1615. doi: 10.1094/PDIS-04-14-0422-RE
Keywords: wheat, Fusarium crown rot, Fusarium spp., characterization, pathogenicity
Citation: Ma G, Wang H, Qi K, Ma L, Zhang B, Zhang Y, Jiang H, Wu X and Qi J (2024) Isolation, characterization, and pathogenicity of Fusarium species causing crown rot of wheat. Front. Microbiol. 15:1405115. doi: 10.3389/fmicb.2024.1405115
Received: 22 March 2024; Accepted: 03 May 2024;
Published: 30 May 2024.
Edited by:
Shitou Xia, Hunan Agricultural University, ChinaReviewed by:
Zhenhui Zhong, Sichuan University, ChinaCopyright © 2024 Ma, Wang, Qi, Ma, Zhang, Zhang, Jiang, Wu and Qi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuehong Wu, d3V4dWVob25nQGNhdS5lZHUuY24=; Junshan Qi, cWk5OTlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.