- 1Graduate School, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 2College of Traditional Chinese Medicine, Tianjin University of Traditional Chinese Medicine, Tianjin, China
- 3Tianjin Key Laboratory of Modern Chinese Medicine Theory of Innovation and Application, Tianjin, China
- 4Guo Aichun Institute of Medical History and Literature, Tianjin University of Traditional Chinese Medicine, Tianjin, China
Background: Studies have indicated an association between gut microbiota (GM) and non-Hodgkin lymphoma (NHL). However, the causality between GM and NHL remains unclear. This study aims to investigate the causality between GM and NHL using Mendelian randomization (MR).
Methods: Data on GM is sourced from the MiBioGen consortium, while data on NHL and its subtypes is sourced from the FinnGen consortium R10 version. Inverse variance weighted (IVW) was employed for the primary MR analysis method, with methods such as Bayesian weighted Mendelian randomisation (BWMR) as an adjunct. Sensitivity analyses were conducted using Cochran’s Q test, MR-Egger regression, MR-PRESSO, and the “Leave-one-out” method.
Results: The MR results showed that there is a causality between 27 GMs and NHL. Among them, 20 were negatively associated (OR < 1), and 7 were positively associated (OR > 1) with the corresponding diseases. All 27 MR results passed sensitivity tests, and there was no reverse causal association.
Conclusion: By demonstrating a causal link between GM and NHL, this research offers novel ideas to prevent, monitor, and cure NHL later.
1 Introduction
NHL is a prevalent malignancy tumor in the haematology system, accounting for about 90% of lymphomas overall. It can be classified into three basic types: B-cell type, T-cell type, and NK-cell type (Shankland et al., 2012). The prevalence of NHL is progressively rising on an annual basis. Based on statistical data, the number of new NHL cases in 2020 was 544,000, with approximately 260,000 deaths (Mafra et al., 2022). The number of new cases is projected to reach 778,000 by 2040, an increase of about 43% compared to 2020 (Chu et al., 2023). While the etiology of NHL is not fully understood, infection, immunosuppression, immunodeficiency syndromes, and autoimmune diseases are commonly recognized as significant risk factors for the onset of NHL (Ansell, 2015; Armitage et al., 2017). In terms of treatment, from the anti-CD20 monoclonal antibody (rituximab) in 1982 (Miller et al., 1982), to the current immune checkpoint inhibitors (ICI) and bispecific antibodies (Bock et al., 2022; Abou Dalle et al., 2024), immunotherapy combined with chemotherapy has always been a focus in the treatment of NHL. Despite some progress made in these treatment methods, the therapy of relapsed/refractory NHL is still a major dilemma in the field, with many unmet needs in NHL therapy (Chaudhari et al., 2019).
The gastrointestinal tract, as the most common extranodal site involved in NHL (Hanafy et al., 2020), harbors a large number of microbes, such as bacteria and fungi. This subset of microorganisms is collectively referred to as GM (Costea et al., 2017). Recently, the close connection between GM and NHL has been increasingly confirmed. Research has shown that the abundance of GM in diffuse large B-cell lymphoma (DLBCL) patients is markedly greater than that in healthy individuals, as revealed by 16S rRNA gene sequencing (Yuan et al., 2021). In terms of NHL occurrence, studies have found an association between mucosa-associated lymphoid tissue (MALT) lymphoma and the invasion of GM such as Burkholderia. GM like Burkholderia may influence the mechanism of MALT lymphoma occurrence through the synthesis of Mvin protein (Kuo et al., 2019; Tanaka et al., 2021). Regarding the diagnosis of NHL, some scholars have suggested that GM can serve as a diagnostic marker for NK/T cell lymphoma (Shi et al., 2023). In addition, GM can also modulate the efficacy of immunotherapy. Studies have shown that the treatment response of cancer patients receiving immune checkpoint inhibitors (ICIs) is associated with the composition of GM. For example, GM such as Bacteroides may enhance patients’ anti-tumor capacity by improving the function of effector T cells in the tumor microenvironment (Gopalakrishnan et al., 2018). Furthermore, studies have shown that oral administration of Akkermansia muciniphila and fecal microbiota transplantation can restore the efficacy of immune checkpoint inhibitors (ICI) in drug-resistant tumor mice through an interleukin-12-dependent mechanism (Routy et al., 2018). Therefore, by modulating GM, it is possible to improve the therapeutic effect of immunotherapies such as ICB, lower associated side effects (Park E. M. et al., 2022), and mitigate the development of resistance to ICIs in cancer patients (Routy et al., 2018). Myeloablative conditioning and the use of broad-spectrum antibiotics before hematopoietic stem cell transplantation (HSCT) can damage the intestinal epithelium and mucosal barrier, leading to gastrointestinal mucositis, and consequently increasing the risk of infections in patients (Keefe et al., 2007). Meanwhile, GM can influence the immune system and maintain intestinal homeostasis by regulating cells such as Treg and TH17 (Arpaia et al., 2013; Smith et al., 2013). Based on differences in GM, it is possible to predict and assess pre-transplant risks in NHL patients undergoing HSCT, aiding in the identification and prevention of high-risk individuals (Montassier et al., 2016). For instance, assessing the diversity of gut microbiota (GM) in patients on the day of transplant surgery can predict those at high risk of mortality during HSCT (Taur et al., 2014). In the future, GM may be a novel diagnostic biomarker and therapeutic target for NHL. Therefore, research on the causal relationship between the two is necessary.
MR explores the causality between exposure and outcome by utilizing instrumental variables (IVs) (Davies et al., 2018). Under the principle of random assignment, MR studies could avoid confounding factors or reverse causation interference (Davey Smith and Hemani, 2014), resulting in more stable and reliable research outcomes. For the research, we employ a two-sample MR methodology to investigate the causality between GM and NHL.
2 Methods
2.1 Data sources
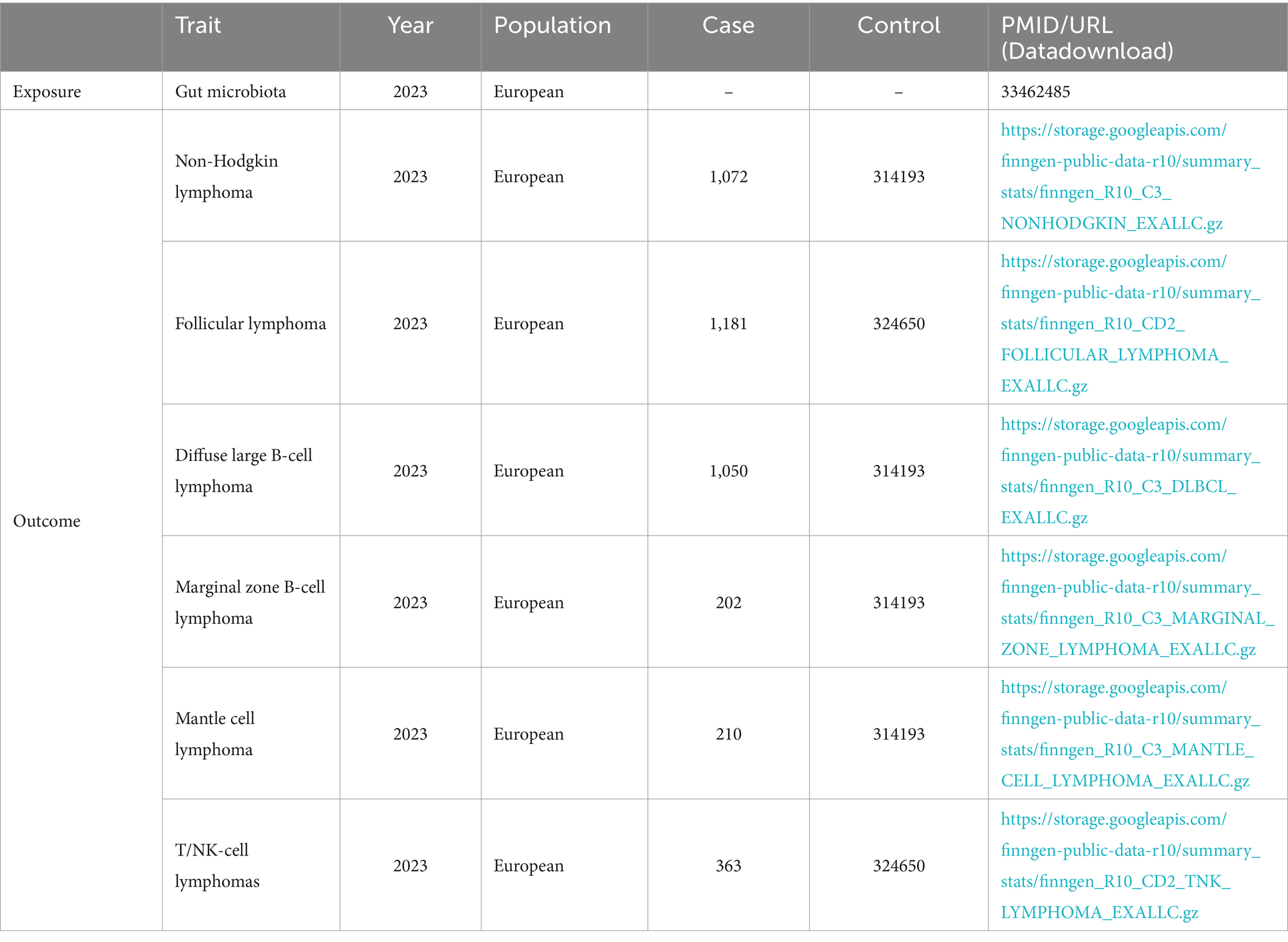
MiBioGen consortium provided genetic variation data on GM (Kurilshikov et al., 2021). This research involved 18, 340 persons and generated corresponding genetic sequencing and genotyping data. It included 211 GMs, classified into five categories: phylum, class, order, family, and genus. Three unknown families and twelve unknown genera were excluded. Eventually, the study included nine phyla, sixteen classes, twenty orders, thirty-two families, and one hundred nineteen genera, totaling 196 GMs. The genetic variation data for NHL originates from the FinnGen consortium R10 version GWAS summary data (Kurki et al., 2023). It includes NHL and its five subtypes: follicular lymphoma (FL), DLBCL, marginal zone B-cell lymphoma (MZBL), T/NK cell lymphoma, and mantle cell lymphoma (MCL) (Table 1). The diagnostic criteria for NHL refer to ICD-10 codes C82, C83, C84, C85; The diagnostic criteria for FL refer to ICD-10 code C82; The diagnostic criteria for DLBCL refer to ICD-10 code C83.3; The diagnostic criteria for MZBL refer to ICD-10 codes C83.80, C83.89; The diagnostic criteria for T/NK cell lymphoma refer to ICD-10 code C84; The diagnostic criteria for MCL refer to ICD-10 code C83.1.
2.2 Selection of IVs
IVs were screened based on the following criteria (Figure 1): (1) In order to obtain IVs that are strongly correlated with GM and have robust relationships, we set the significance threshold at p < 1.0 × 10−8. However, the number of obtained IVs was small and difficult to meet the requirements of this study. Therefore, we referred to previous research (Sanna et al., 2019) and set the significance threshold at p < 1.0 × 10−5. At the same time, to ensure the mutual independence of the selected IVs, we removed linkage disequilibrium in the IVs (r2 < 0.001, kb = 10,000); (2) To ensure the independence of the IVs, the IVs are unrelated to any confounding factors; (3) IVs can only influence the occurrence of NHL through GM and cannot affect NHL through other pathways. Additionally, to avoid bias from weak IVs, we excluded weak IVs using the F-statistic (F > 10). We also removed palindromic sequences from the IVs.
2.3 Positive MR analysis and sensitivity analysis
We utilized six methods, including IVW, MR Egger, weighted median, simple mode, weighted mode, and BWMR, to investigate the causality between GM and NHL. However, IVW was the primary evaluation method (Hemani et al., 2018). Given the uniqueness of GWAS data, the IVW method is widely recognized as the primary method for exploring causal relationships in MR analysis. By conducting a meta-analysis of each Wald ratio of the included valid IVs, it can provide the most accurate estimate of the effect. This approach is also commonly seen in other literature (Legason et al., 2017; Lu et al., 2023; Martín-Masot et al., 2023; Ruan et al., 2023; Li et al., 2024; Zheng et al., 2024). The IVW method is divided into random-effects IVW and fixed-effects IVW, with the selection based on heterogeneity in MR results (Greco et al., 2015). Since no single method can perfectly suit all situations, additional methods such as MR Egger and weighted median are used as supplements (Bowden et al., 2015, 2016). For instance, when there is pleiotropy present, the MR Egger method is more suitable for inferring causal relationships. Finally, to mitigate the effects of multi-genic structure and pleiotropy, we utilized the BWMR method to further validate the obtained causal relationships (Zhao et al., 2020).
Sensitivity analysis includes heterogeneity testing, leave-one-out testing, and multivariate testing (Hemani et al., 2018). We evaluated the potential bias in the results by examining the pleiotropy of genes and the heterogeneity of the data. Cochran’s Q test assesses heterogeneity, based on whether the p-value in Cochran’s IVW is less than 0.05. MR-Egger regression detects horizontal pleiotropy, determined by the difference between its intercept and 0. MR-PRESSO can detect and lower horizontal pleiotropy (Burgess et al., 2020). Additionally, “leave-one-out” analysis can identify outlier SNPs within the SNPs, thus avoiding bias introduced by individual outlier SNPs on the overall MR results and enhancing the stability of the results.
2.4 Reverse MR analysis
In order to avoid interference from reverse causal relationships on the positive MR results, we conducted a reverse MR analysis with NHL and its five subtypes as exposure and GM as the outcome.
2.5 Statistical analysis
The statistical analyses in R 4.3.0 used the “TwoSampleMR” package.
3 Results
3.1 Obtained IVs
412 IVs related to non-Hodgkin lymphoma and its subtypes were obtained through screening (Supplementary Table S1). Among them, there were no palindromic sequences, and the F > 10 (range 17.421–88.429). The included GMs were divided into five categories, so there may be overlaps among SNPs under each GM.
3.2 Results of positive MR analysis
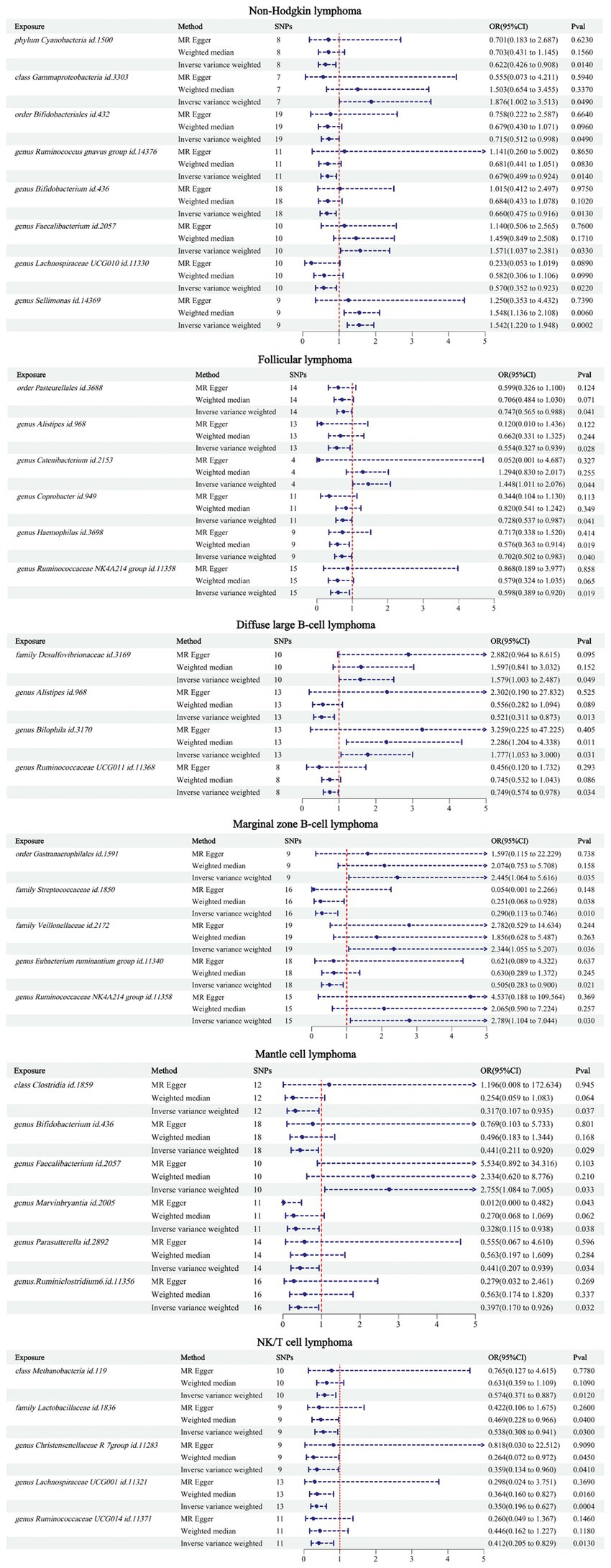
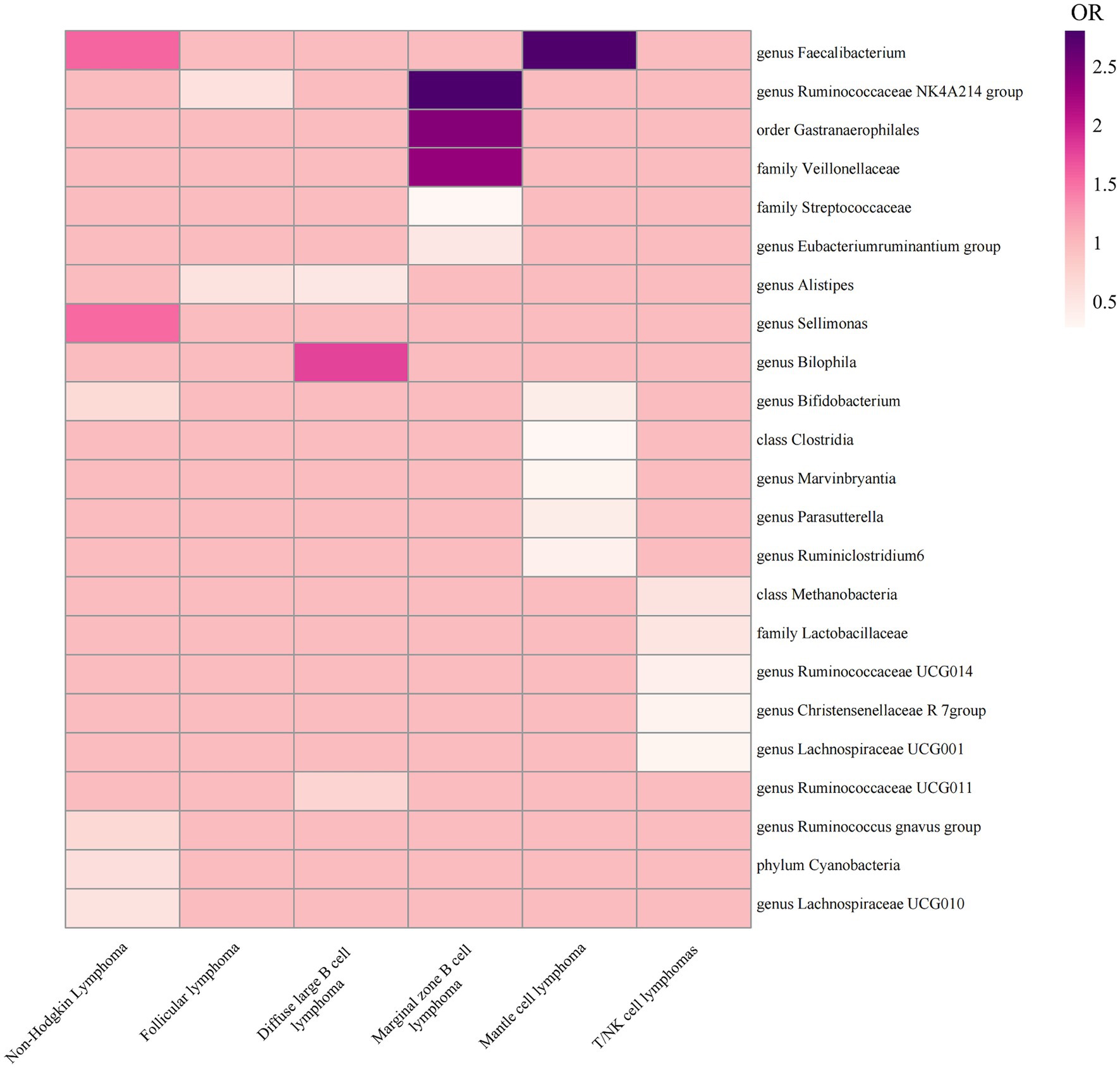
The IVW results showed that there were 34 GMs associated with NHL and its subtypes. Among them, 8 were associated with NHL, 6 with FL, 4 with DLBCL, 5 with MZBL, 6 with MCL, and 5 with T/NK cell lymphoma (Figure 2).
According to the IVW analysis results, phylum Cyanobacteria (OR: 0.622, CI: 0.426–0.908, p = 0.014), order Bifidobacteriales (OR: 0.715, CI: 0.512–0.998, p = 0.049), genus Ruminococcus gnavus group (OR: 0.679, CI: 0.499–0.924, p = 0.014), genus Bifidobacterium (OR: 0.660, CI: 0.475–0.916, p = 0.013), and genus Lachnospiraceae UCG010 (OR: 0.570, CI: 0.352–0.923, p = 0.022) were negatively associated with an increased risk of NHL. Class Gammaproteobacteria (OR: 1.876, CI: 1.002–3.513, p = 0.049), genus Faecalibacterium (OR: 1.571, CI: 1.037–2.381, p = 0.033), and genus Sellimonas (OR: 1.542, CI: 1.220–1.948, p = 0.0002) were positively associated with an increased risk of NHL.
According to the IVW analysis results, order Pasteurellales (OR: 0.747, CI: 0.565–0.988, p = 0.041), genus Alistipes (OR: 0.554, CI: 0.327–0.939, p = 0.028), genus Coprobacter (OR: 0.728, CI: 0.537–0.987, p = 0.041), genus Haemophilus (OR: 0.702, CI: 0.502–0.983, p = 0.040), and genus Ruminococcaceae NK4A214 group (OR: 0.598, CI: 0.389–0.920, p = 0.019) were negatively associated with an increased risk of FL. Genus Catenibacterium (OR: 1.448, CI: 1.011–2.076, p = 0.044) was positively associated with an increased risk of FL.
According to the IVW analysis results, genus Alistipes (OR: 0.521, CI: 0.311–0.873, p = 0.013), genus Ruminococcaceae UCG011 (OR: 0.749, CI: 0.574–0.978, p = 0.034) were negatively associated with an increased risk of DLBCL. Family Desulfovibrionaceae (OR: 1.579, CI: 1.033–2.487, p = 0.049), genus Bilophila (OR: 1.777, CI: 1.053–3.000, p = 0.031) were positively associated with an increased risk of DLBCL.
According to the IVW analysis results, family Streptococcaceae (OR: 0.290, CI: 0.113–0.746, p = 0.010), genus Eubacterium ruminantium group (OR: 0.505, CI: 0.283–0.900, p = 0.021) were negatively associated with an increased risk of MZBL. Order Gastranaerophilales (OR: 2.445, CI: 1.064–5.616, p = 0.035), family Veillonellaceae (OR: 2.344, CI: 1.055–5.207, p = 0.036), genus Ruminococcaceae NK4A214 group (OR: 2.789, CI: 1.104–7.044, p = 0.030) were positively associated with an increased risk of MZBL.
According to the IVW analysis results, class Clostridia (OR: 0.317, CI: 0.107–0.935, p = 0.037), genus Bifidobacterium (OR: 0.441, CI: 0.211–0.920, p = 0.029), genus Marvinbryantia (OR: 0.328, CI: 0.115–0.938, p = 0.038), genus Parasutterella (OR: 0.441, CI: 0.207–0.939, p = 0.034), genus Ruminiclostridium 6 (OR: 0.397, CI: 0.70–0.926, p = 0.032) were negatively associated with an increased risk of MCL. Genus Faecalibacterium (OR: 2.755, CI: 1.084–7.005, p = 0.033) was positively associated with an increased risk of MCL.
According to the IVW analysis results, class Methanobacteria (OR: 0.574, CI: 0.371–0.887, p = 0.012), family Lactobacillaceae (OR: 0.538, CI: 0.308–0.941, p = 0.030), genus Christensenellaceae R 7group (OR: 0.359, CI: 0.134–0.960, p = 0.041), genus Lachnospiraceae UCG001 (OR: 0.350, CI: 0.196–0.627, p = 0.0004), genus Ruminococcaceae UCG014 (OR: 0.412, CI: 0.205–0.829, p = 0.013) were negatively associated with an increased risk of T/NK cell lymphoma.
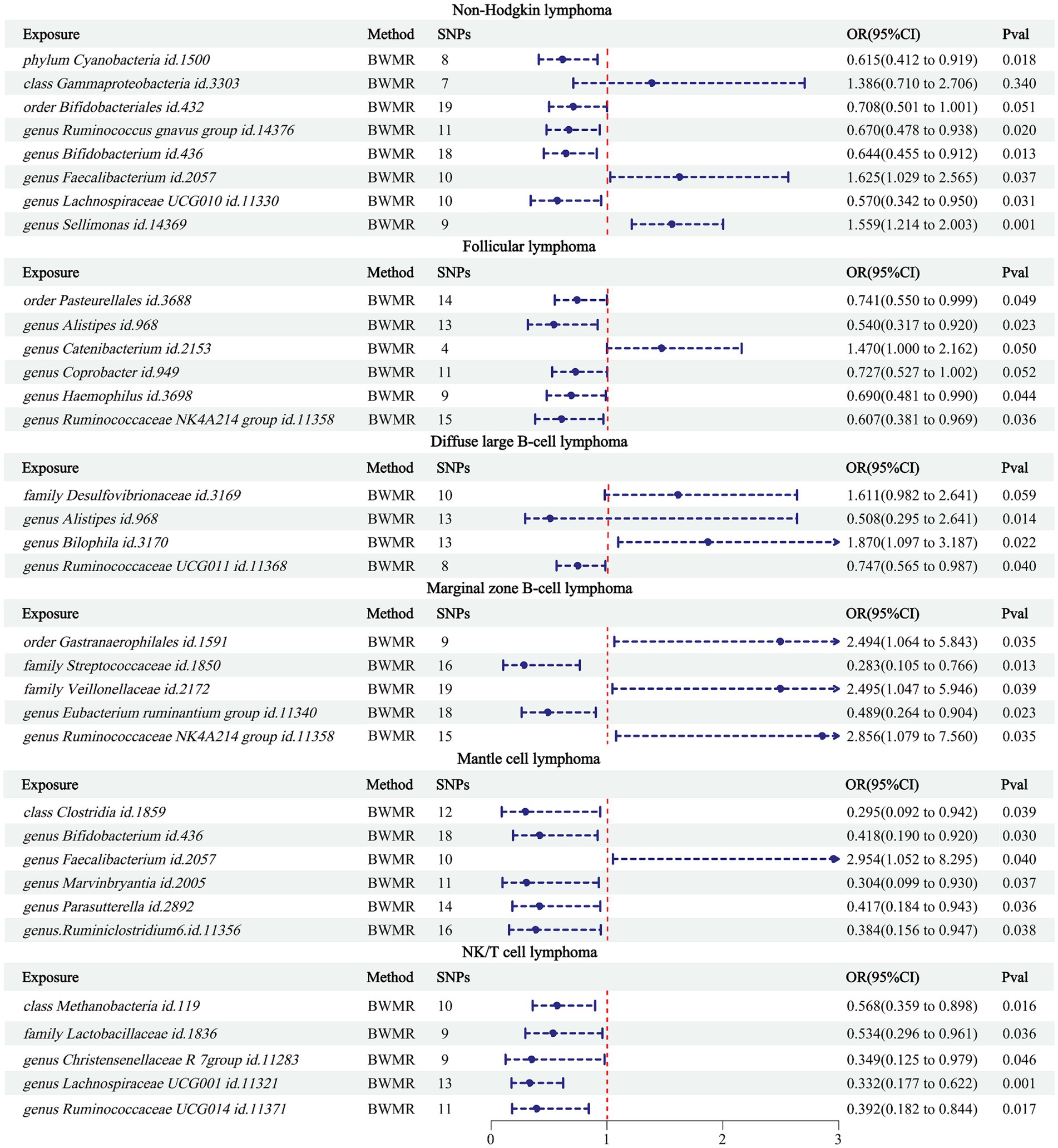
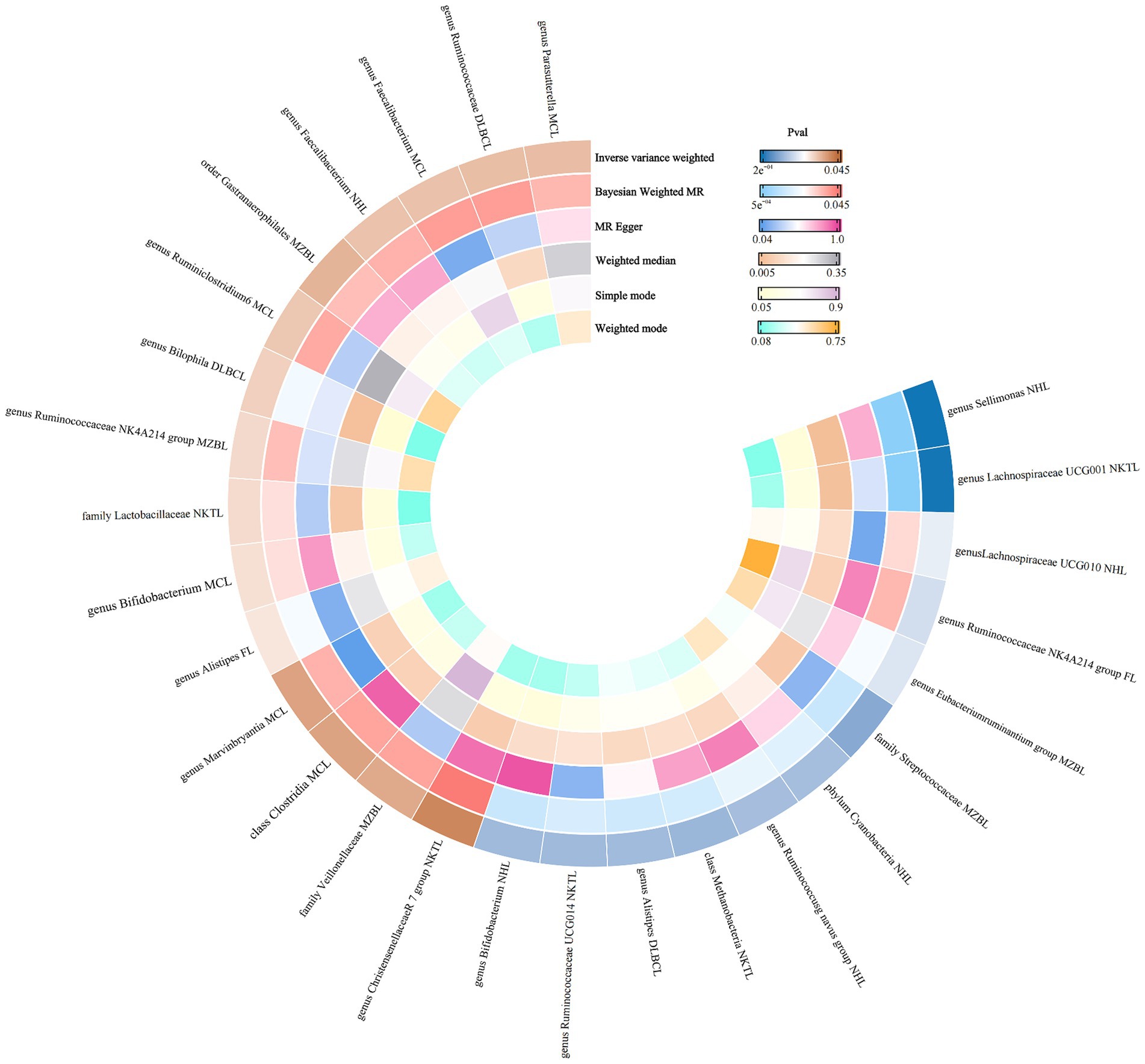
Using BWMR to validate the 34 pairs of causalities between the obtained GMs and NHL (Figure 3), the results showed that class Gammaproteobacteria (p = 0.340) and order Bifidobacteriales (p = 0.051) were not causally related to NHL; genus Catenibacterium (p = 0.050) and genus Coprobacter (p = 0.052) were not causally related to FL; family Desulfovibrionaceae was not causally related to DLBCL. Comparing these 5 pairs of relationships between the results of BWMR and IVW (Table 2), it was found that although causal relationships existed in the IVW results, their p-values were close to 0.05. Therefore, these 5 pairs of relationships were excluded from our study.
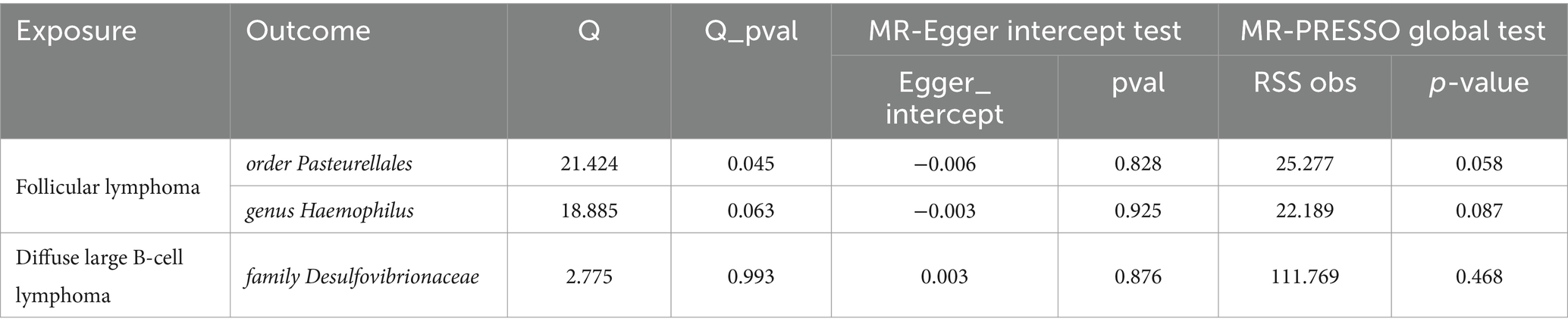
Finally, sensitivity analysis of the MR results was conducted (Supplementary Table S1). The p-values of Cochran’s Q test were all >0.05, indicating no heterogeneity. The p-values of the MR-Egger intercept (Supplementary Figures S1, S2) and MR-PRESSO results were all >0.05, indicating no horizontal pleiotropy. Results from the “leave-one-out” method (Supplementary Figures S3, S4) showed that removing any single SNP would not significantly affect the MR results.
3.3 Results of reverse MR analysis
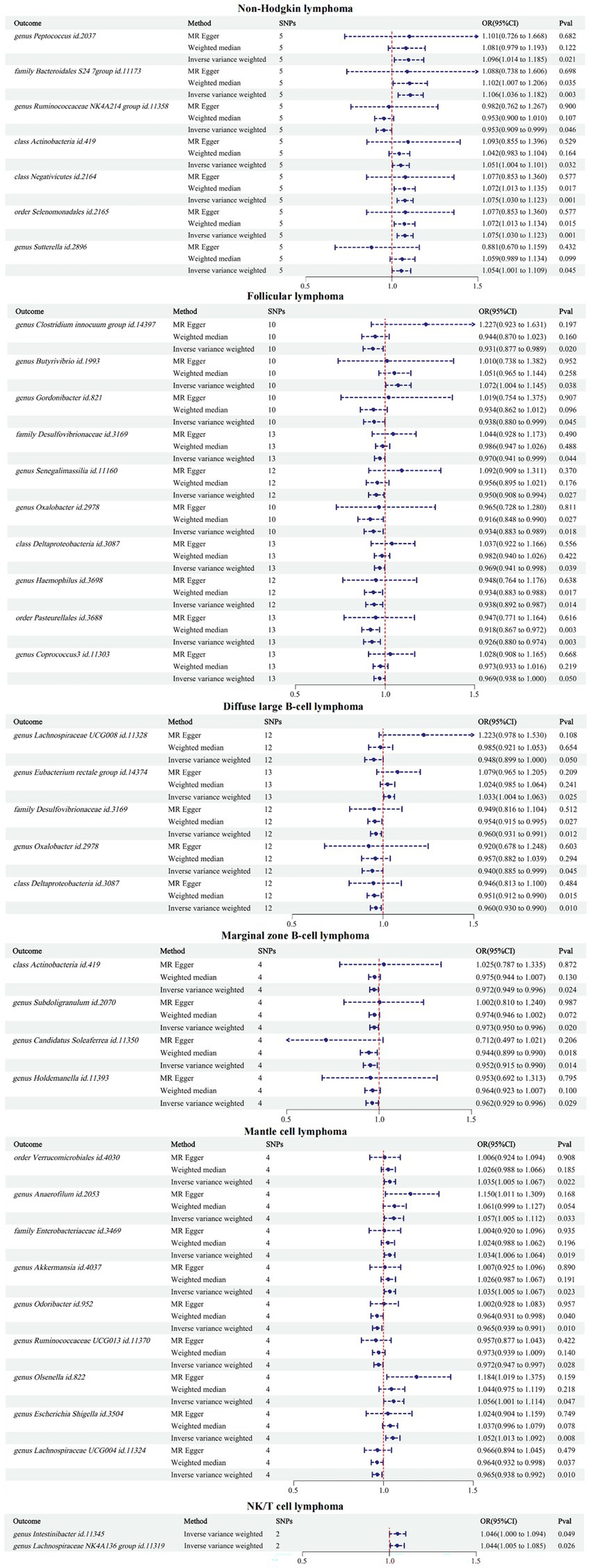
The reverse MR results showed that NHL and its subtypes were associated with 37 GMs (Supplementary Table S3). Among them, there were 7 associated with NHL, 10 with FL, 5 with DLBCL, 4 with MZBL, 9 with MCL, and 2 with T/NK cell lymphoma. Mapping the forest, see Figure 4. After comparing with the results of the forward MR, among the 34 forward MR results, FL was inversely associated with order Pasteurellales and genus Haemophilus, DLBCL was inversely associated with family Desulfovibrionaceae, and no other reverse causal associations were found in the remaining forward MR results. Sensitivity analysis was conducted for the three reverse causal associations mentioned above (Table 3). Except for the presence of heterogeneity in the MR results between FL and order Pasteurellales (without horizontal pleiotropy), the remaining two MR results showed no heterogeneity or horizontal pleiotropy.
Therefore, we finally identified 27 GMs with clear causal relationships with NHL and its subtypes, and presented them in the form of a heatmap (Figures 5, 6).
4 Discussion
In the 2020 cancer diagnosis statistics, NHL ranked 11th, and its incidence has been increasing year by year (Sung et al., 2021). Although GMs play important roles in the occurrence, development, diagnosis, and treatment of NHL (Upadhyay Banskota et al., 2023), the specific causality between the two is unknown. Previous studies have investigated the causal relationship between lipids (Kleinstern et al., 2020) and diet (Zhou et al., 2024), among other factors (Shi et al., 2024), and NHL through MR. Our research identified 27 GMs with causal relationships with NHL and its subtypes through forward and reverse MR analyses, as well as sensitivity analysis. Among them, phylum Cyanobacteria, genus Ruminococcus gnavus group, genus Bifidobacterium, genus Lachnospiraceae UCG010, genus Alistipes, genus Ruminococcaceae NK4A214 group, genus Ruminococcaceae UCG011, family Streptococcaceae, genus Eubacterium ruminantium group, class Clostridia, genus Marvinbryantia, genus Parasutterella, genus Ruminiclostridium 6, class Methanobacteria, family Lactobacillaceae, genus Christensenellaceae R 7group, genus Lachnospiraceae UCG001, and genus Ruminococcaceae UCG014 were negatively associated with the disease (OR < 1), indicating a protective effect against the corresponding types of NHL. Genus Faecalibacterium, genus Sellimonas, genus Bilophila, order Gastranaerophilales, family Veillonellaceae, genus Ruminococcaceae NK4A214 group, and genus Faecalibacterium were positively associated with the disease (OR > 1), serving as risk factors for the corresponding types of NHL. It is worth mentioning that in our positive MR analysis between GM and NHL, we observed that the absence of order Pasteurellales and genus Haemophilus might play a promoting role in FL occurrence. However, in the reverse MR analysis, we found that the occurrence of FL could inhibit the production of order Pasteurellales and genus Haemophilus. Therefore, we cannot ascertain whether the lack of order Pasteurellales and genus Haemophilus is the cause or the consequence of FL occurrence. To avoid interference from reverse causal relationships, we excluded the portion of results that exhibited reverse causal associations from the positive MR results.
The relationship between GM and NHL is complex. With the development of technologies in fields like 16S rRNA sequencing or shotgun metagenomics sequencing, researchers have gained a clearer understanding of the specific taxonomic groups in the GM and their relationship with diseases. Due to the involvement of numerous GM species in NHL and its subtypes in this study, we focused our discussion on the MR results related to NHL.
The phylum Cyanobacteria is a group of ancient and diverse prokaryotes (Schirrmeister et al., 2011) that can be divided into different genera such as Aphanothece, Leptolyngbya, and Spirulina (Walter et al., 2017). Research has found that Cyanobacteria can synthesize 1,600 types of compounds (Bohlin et al., 2010; Nagarajan et al., 2012), which play positive roles in antiviral, antibacterial, and immunomodulatory aspects (Sieber and Marahiel, 2005; De Morais et al., 2015; Sathasivam et al., 2019), thus they are widely applied in various fields. In addition, Cyanobacteria have significant anti-cancer effects, for example, Somocystinamide A (ScA), a lipopeptide compound isolated from Cyanobacteria, can inhibit tumor cell proliferation by inducing programmed cell death (Wrasidlo et al., 2008, p. 8). Curacin A, produced by Cyanobacteria, is a hybrid polyketide-peptide compound and an effective anticancer agent (Catassi et al., 2006). It can induce cancer cell death by inhibiting the activity of microtubule protein polymerization (Blokhin et al., 1995). In the results of this study, the phylum Cyanobacteria was found to decrease the risk of NHL, which is similar to the aforementioned previous research findings. Based on previous studies, we speculate that Cyanobacteria may also exhibit similar anticancer effects in NHL. Furthermore, most cancer-related chemotherapy drugs are derived from natural products in nature (Sithranga Boopathy and Kathiresan, 2010), and Cyanobacteria not only exist in the human gut but can also be obtained from marine (Mondal et al., 2020), soil, and agricultural runoff (Senousy et al., 2020). Hence, future research could delve into the anticancer mechanisms of Cyanobacteria in NHL, thereby laying the groundwork for the extraction and development of novel drugs related to NHL from natural products.
Ruminococcus gnavus is a Gram-positive anaerobic bacterium found primarily within the gastrointestinal tract of humans and animals (Qin et al., 2010). In terms of human health, Ruminococcus gnavus constitutes a significant proportion of the infant GM (Sagheddu et al., 2016), correlating with infant nutrition absorption (Yatsunenko et al., 2012) and growth development (Mennella et al., 2022), with these effects persisting into adulthood. In terms of disease, Ruminococcus gnavus is closely associated with gastrointestinal diseases and immune regulation. Research revealed that the relative abundance of Ruminococcus gnavus in normal humans is usually below 1%, while in some inflammatory bowel disease patients, it can reach around 70% (Zhang et al., 2023). However, some researchers found that after transferring Ruminococcus gnavus and other microbes into colorectal cancer mice, Ruminococcus gnavus could inhibit tumor growth and activate the immune surveillance function of CD8+ T cells (Zhang et al., 2023). Therefore, the role of Ruminococcus gnavus in the human body is complex, and its effects on disease are influenced by multiple factors. In this study, the genus Ruminococcus gnavus group was identified as a beneficial bacterium for NHL, which can reduce the risk of its occurrence. Lachnospiraceae and Ruminococcus gnavus belong to the phylum Firmicutes. Lachnospiraceae is a family of gut bacteria that is widely present in the gastrointestinal tract of fauna (Gosalbes et al., 2011; Meehan and Beiko, 2014). It is an important member of the human GM, accounting for approximately 10 to 45% of the total bacterial population (Liu et al., 2021). Lachnospiraceae can be divided into different genera, such as Lachnospira, Oribacterium, and Dorea (Vacca et al., 2020), which are the primary contributors to short-chain fatty acids that are beneficial to human health (Vital et al., 2014; Chambers et al., 2015; Bui et al., 2021). Meloxicam, a nonsteroidal anti-inflammatory drug, is associated with reducing the risk of cancer, while Lachnospiraceae can produce meloxicam by altering the heterocyclic structure of flavonoids (Sugiyama et al., 2013; Braune and Blaut, 2016). Although there is limited research on the association between Lachnospiraceae and NHL, and it is not commonly found in other hematological tumors (Guevara-Ramírez et al., 2023), the viewpoint of Lachnospiraceae being considered beneficial bacteria in previous literature is similar to the results of this study. Therefore, more attention should be paid to the study of the association between the phylum Firmicutes and NHL as well as blood tumors, to clearly elucidate the specific mechanisms by which Lachnospiraceae may contribute to the treatment of NHL, thereby providing new insights into the prevention and treatment of NHL.
Bifidobacterium is a well-recognized beneficial microorganism for human health (Hidalgo-Cantabrana et al., 2017; Leser and Baker, 2023), with functions such as inhibiting intestinal pathogens (Moreno Muñoz et al., 2011), preventing gastrointestinal infections (Weizman et al., 2005), improving gastrointestinal symptoms (Waller et al., 2011), and regulating the immune system (Roller et al., 2007), thus it is widely used in the food and pharmaceutical industries. Studies have shown that GM such as Bifidobacterium can influence the therapeutic effects of immunotherapy on tumors (Matson et al., 2018). Bifidobacterium can also enhance the efficacy of ICIs in cancer mice by producing adenosine (Mager et al., 2020). Chimeric antigen receptor T-cell immunotherapy (CAR-T) is a novel precision-targeted therapy for treating malignant tumors of the hematopoietic system. Studies have found that GM such as Bifidobacterium are associated with the efficacy of Chimeric antigen receptor T-cell therapy in BCL and can influence the progression of BCL (Stein-Thoeringer et al., 2023). In addition, researchers have observed that the diversity of Bifidobacterium in multiple myeloma people after receiving CAR-T therapy vary depending on the efficacy of the treatment (Hu et al., 2022). Therefore, the differences in the diversity and abundance of Bifidobacterium are important indicators for predicting the therapeutic effects of lymphoma and other malignant tumors of the hematopoietic system. This study found that Bifidobacterium can effectively reduce the risk of NHL, while previous literature has not addressed this aspect of research. Therefore, we hope that this study can provide valuable reference for future exploration. Moreover, existing studies indicate an association between Bifidobacterium and the immunotherapeutic effects on tumors such as BCL and multiple myeloma, yet they do not directly establish a connection between Bifidobacterium and BCL. Therefore, subsequent research can use this as a starting point.
In this study, we found that genus Faecalibacterium and genus Sellimonas are the only two intestinal microbiota that can increase the risk of NHL. However, it is worth noting that genus Faecalibacterium is commonly found in the population and is generally considered beneficial to health, with the potential to become the next generation of probiotics (Langella et al., 2019). For example, Faecalibacterium prausnitzii, an important member of the genus Faecalibacterium, constitutes more than 5 percent of the overall fecal microbiome of healthy individuals. It can maintain the stability of the healthy gut environment (Miquel et al., 2013) and also act as a probiotic to regulate the intestinal environment of Crohn’s disease patients (Sokol et al., 2008). Additionally, some species within the genus Faecalibacterium can produce significant amounts of fructose, providing energy for human colonic epithelial cells and supporting epithelial cell growth (Fagundes et al., 2021; Park J.-H. et al., 2022). Therefore, the results regarding genus Faecalibacterium in this study differ somewhat from previous related research. However, some researchers suggest that the interaction between Faecalibacterium and its host is not always constant (Martín et al., 2023). Since the discovery of Faecalibacterium, with the continuous advancement of techniques such as 16S rRNA gene sequence as well as whole-genome sequencing, the taxonomy of this genus has been evolving. In 2021, two new species were added: Faecalibacterium butyricigenerans and Faecalibacterium longum (Zou et al., 2021); and in 2022, three more new species were discovered, namely: Faecalibacterium duncaniae, Faecalibacterium hattorii, and Faecalibacterium gallinarum (Sakamoto et al., 2022). Therefore, the interactions between the genus Faecalibacterium and the host are continually being updated. Further research is needed to explore the impact of Faecalibacterium on NHL.
The causal relationship between GM and NHL is influenced not only by internal factors but also by external factors such as diet, medication, and delivery type. Dietary fiber is an important nutrient that is difficult for the human body to digest and absorb. However, there is a significant association between a high consumption of fruits, soy, and green vegetables and a reduced risk of NHL (Chiu, 1996; Wei et al., 2016). It is worth noting that certain GM, such as Lachnospiraceae, can ferment dietary fiber and produce substances like short-chain fatty acids, increasing the content of butyrate in the body, thereby promoting apoptosis of lymphoma cells (Wei et al., 2016; Zaplana et al., 2024). Therefore, increasing the intake of dietary fiber in the body appropriately can promote the growth of GM such as Lachnospiraceae. Additionally, certain living biotherapeutic products (LBPs) associated with Lachnospiraceae have been attempted to be developed as probiotics to improve conditions such as metabolic syndrome (Gilijamse et al., 2020). Probiotics are a type of beneficial active microorganisms for the human body. Bifidobacterium, as a crucial member of probiotics, plays an important role in the prevention and treatment of cancers such as colon cancer (Bahmani et al., 2019), gastric cancer (Devi et al., 2021), breast cancer (Shimizu et al., 2020), and lung cancer (An et al., 2020). This study identified a significant number of GM, including Bifidobacterium and Ruminococcus gnavus, that may potentially reduce the risk of NHL. Whether these GM can participate in the prevention and treatment of NHL as probiotics or other forms such as LBPs in the future is worth exploring.
The research has a few restrictions. Firstly, since the data on GM and NHL and its subtypes are all from European populations, we cannot guarantee whether the results are applicable to other populations. The GM dataset included in this study is currently the largest GWAS dataset of GM, but it predominantly focuses on the European population, hence there are limitations in generalizing to other populations. As GWAS databases of GM in various populations continue to be updated, we will continue to monitor research on the causal relationship between GM and NHL in other populations. Secondly, there are fewer cases in certain subtypes of NHL, such as MZBL, MCL, and T/NK cell lymphoma, which limits the scope of the study. We will continue to monitor this aspect of the research as the FinnGen database is continually updated. Lastly, this study only elucidates the causality between GM and NHL, and the underlying mechanisms driving this association are not yet clear, requiring further research for support.
5 Conclusion
Through this study, we have identified the causality between GM and NHL, and determined the beneficial and harmful microbiota for NHL. In the future, it may be considered to selectively alter these GM through measures such as diet, probiotics, and prebiotics to influence NHL. Additionally, the development of targeted and effective GM in clinical settings holds certain reference significance as novel therapeutic modalities and monitoring indicators for NHL. Therefore, this research offers novel ideas to prevent, monitor, and cure NHL later.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JF: Data curation, Writing – original draft, Writing – review & editing, Software. ZH: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the scientific research project of the State Administration of Traditional Chinese Medicine (GZY-KJS-2020-037).
Acknowledgments
We express our sincere gratitude to the FinnGen and GWAS Catalog personnel and volunteers, as well as the MiBioGen consortium, for their invaluable contribution in supplying gut microbiology data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1403825/full#supplementary-material
References
Abou Dalle, I., Dulery, R., Moukalled, N., Ricard, L., Stocker, N., El-Cheikh, J., et al. (2024). Bi- and tri-specific antibodies in non-Hodgkin lymphoma: current data and perspectives. Blood Cancer J. 14:23. doi: 10.1038/s41408-024-00989-w
An, J., Kim, H., and Yang, K. M. (2020). An aqueous extract of a Bifidobacterium species induces apoptosis and inhibits invasiveness of non-small cell lung cancer cells. J. Microbiol. Biotechnol. 30, 885–893. doi: 10.4014/jmb.1912.12054
Ansell, S. M. (2015). Non-Hodgkin lymphoma: diagnosis and treatment. Mayo Clin. Proc. 90, 1152–1163. doi: 10.1016/j.mayocp.2015.04.025
Armitage, J. O., Gascoyne, R. D., Lunning, M. A., and Cavalli, F. (2017). Non-Hodgkin lymphoma. Lancet 390, 298–310. doi: 10.1016/S0140-6736(16)32407-2
Arpaia, N., Campbell, C., Fan, X., Dikiy, S., Van Der Veeken, J., deRoos, P., et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504, 451–455. doi: 10.1038/nature12726
Bahmani, S., Azarpira, N., and Moazamian, E. (2019). Anti-colon cancer activity of Bifidobacterium metabolites on colon cancer cell line SW742. Turk. J. Gastroenterol. 30, 835–842. doi: 10.5152/tjg.2019.18451
Blokhin, A. V., Yoo, H. D., Geralds, R. S., Nagle, D. G., Gerwick, W. H., and Hamel, E. (1995). Characterization of the interaction of the marine cyanobacterial natural product curacin a with the colchicine site of tubulin and initial structure-activity studies with analogues. Mol. Pharmacol. 48, 523–531
Bock, A. M., Nowakowski, G. S., and Wang, Y. (2022). Bispecific antibodies for non-Hodgkin lymphoma treatment. Curr. Treat. Options Oncol. 23, 155–170. doi: 10.1007/s11864-021-00925-1
Bohlin, L., Göransson, U., Alsmark, C., Wedén, C., and Backlund, A. (2010). Natural products in modern life science. Phytochem. Rev. 9, 279–301. doi: 10.1007/s11101-009-9160-6
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Braune, A., and Blaut, M. (2016). Bacterial species involved in the conversion of dietary flavonoids in the human gut. Gut Microbes 7, 216–234. doi: 10.1080/19490976.2016.1158395
Bui, T. P. N., Mannerås-Holm, L., Puschmann, R., Wu, H., Troise, A. D., Nijsse, B., et al. (2021). Conversion of dietary inositol into propionate and acetate by commensal anaerostipes associates with host health. Nat. Commun. 12:4798. doi: 10.1038/s41467-021-25081-w
Burgess, S., Davey Smith, G., Davies, N. M., Dudbridge, F., Gill, D., Glymour, M. M., et al. (2020). Guidelines for performing Mendelian randomization investigations. Wellcome Open Res. 4:186. doi: 10.12688/wellcomeopenres.15555.2
Catassi, A., Cesario, A., Arzani, D., Menichini, P., Alama, A., Bruzzo, C., et al. (2006). Characterization of apoptosis induced by marine natural products in non small cell lung cancer A549 cells. Cell. Mol. Life Sci. 63, 2377–2386. doi: 10.1007/s00018-006-6264-7
Chambers, E. S., Viardot, A., Psichas, A., Morrison, D. J., Murphy, K. G., Zac-Varghese, S. E. K., et al. (2015). Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 64, 1744–1754. doi: 10.1136/gutjnl-2014-307913
Chaudhari, K., Rizvi, S., and Syed, B. A. (2019). Non-Hodgkin lymphoma therapy landscape. Nat. Rev. Drug Discov. 18, 663–664. doi: 10.1038/d41573-019-00051-6
Chiu, B. C.-H. (1996). Diet and risk of non-Hodgkin lymphoma in older women. JAMA 275, 1315–1321. doi: 10.1001/jama.1996.03530410029029
Chu, Y., Liu, Y., Fang, X., Jiang, Y., Ding, M., Ge, X., et al. (2023). The epidemiological patterns of non-Hodgkin lymphoma: global estimates of disease burden, risk factors, and temporal trends. Front. Oncol. 13:1059914. doi: 10.3389/fonc.2023.1059914
Costea, P. I., Coelho, L. P., Sunagawa, S., Munch, R., Huerta-Cepas, J., Forslund, K., et al. (2017). Subspecies in the global human gut microbiome. Mol. Syst. Biol. 13:960. doi: 10.15252/msb.20177589
Davey Smith, G., and Hemani, G. (2014). Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum. Mol. Genet. 23, R89–R98. doi: 10.1093/hmg/ddu328
Davies, N. M., Holmes, M. V., and Davey Smith, G. (2018). Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ 362:k601. doi: 10.1136/bmj.k601
De Morais, M. G., Vaz, B. D. S., De Morais, E. G., and Costa, J. A. V. (2015). Biologically active metabolites synthesized by microalgae. Biomed. Res. Int. 2015, 1–15. doi: 10.1155/2015/835761
Devi, T. B., Devadas, K., George, M., Gandhimathi, A., Chouhan, D., Retnakumar, R. J., et al. (2021). Low Bifidobacterium abundance in the lower gut microbiota is associated with Helicobacter pylori-related gastric ulcer and gastric cancer. Front. Microbiol. 12:631140. doi: 10.3389/fmicb.2021.631140
Fagundes, R. R., Bourgonje, A. R., Saeed, A., Vich Vila, A., Plomp, N., Blokzijl, T., et al. (2021). Inulin-grown Faecalibacterium prausnitzii cross-feeds fructose to the human intestinal epithelium. Gut Microbes 13:1993582. doi: 10.1080/19490976.2021.1993582
Gilijamse, P. W., Hartstra, A. V., Levin, E., Wortelboer, K., Serlie, M. J., Ackermans, M. T., et al. (2020). Treatment with Anaerobutyricum soehngenii: a pilot study of safety and dose–response effects on glucose metabolism in human subjects with metabolic syndrome. NPJ Biofilms Microbiomes 6:16. doi: 10.1038/s41522-020-0127-0
Gopalakrishnan, V., Spencer, C. N., Nezi, L., Reuben, A., Andrews, M. C., Karpinets, T. V., et al. (2018). Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science 359, 97–103. doi: 10.1126/science.aan4236
Gosalbes, M. J., Durbán, A., Pignatelli, M., Abellan, J. J., Jiménez-Hernández, N., Pérez-Cobas, A. E., et al. (2011). Metatranscriptomic approach to analyze the functional human gut microbiota. PLoS One 6:e17447. doi: 10.1371/journal.pone.0017447
Greco, M. F. D., Minelli, C., Sheehan, N. A., and Thompson, J. R. (2015). Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat. Med. 34, 2926–2940. doi: 10.1002/sim.6522
Guevara-Ramírez, P., Cadena-Ullauri, S., Paz-Cruz, E., Tamayo-Trujillo, R., Ruiz-Pozo, V. A., and Zambrano, A. K. (2023). Role of the gut microbiota in hematologic cancer. Front. Microbiol. 14:1185787. doi: 10.3389/fmicb.2023.1185787
Hanafy, A. K., Morani, A. C., Menias, C. O., Pickhardt, P. J., Shaaban, A. M., Mujtaba, B., et al. (2020). Hematologic malignancies of the gastrointestinal luminal tract. Abdom Radiol 45, 3007–3027. doi: 10.1007/s00261-019-02278-8
Hemani, G., Zheng, J., Elsworth, B., Wade, K. H., Haberland, V., Baird, D., et al. (2018). The MR-base platform supports systematic causal inference across the human phenome. eLife 7:e34408. doi: 10.7554/eLife.34408
Hidalgo-Cantabrana, C., Delgado, S., Ruiz, L., Ruas-Madiedo, P., Sánchez, B., and Margolles, A. (2017). Bifidobacteria and their health-promoting effects. Microbiol. Spectr. 5:5.3.21. doi: 10.1128/microbiolspec.BAD-0010-2016
Hu, Y., Li, J., Ni, F., Yang, Z., Gui, X., Bao, Z., et al. (2022). CAR-T cell therapy-related cytokine release syndrome and therapeutic response is modulated by the gut microbiome in hematologic malignancies. Nat. Commun. 13:5313. doi: 10.1038/s41467-022-32960-3
Keefe, D. M., Schubert, M. M., Elting, L. S., Sonis, S. T., Epstein, J. B., Raber-Durlacher, J. E., et al. (2007). Updated clinical practice guidelines for the prevention and treatment of mucositis. Cancer 109, 820–831. doi: 10.1002/cncr.22484
Kleinstern, G., Camp, N. J., Berndt, S. I., Birmann, B. M., Nieters, A., Bracci, P. M., et al. (2020). Lipid trait variants and the risk of non-Hodgkin lymphoma subtypes: a Mendelian randomization study. Cancer Epidemiol. Biomarkers Prev. 29, 1074–1078. doi: 10.1158/1055-9965.EPI-19-0803
Kuo, S.-H., Wu, M.-S., Yeh, K.-H., Lin, C.-W., Hsu, P.-N., Chen, L.-T., et al. (2019). Novel insights of lymphomagenesis of Helicobacter pylori-dependent gastric mucosa-associated lymphoid tissue lymphoma. Cancers 11:547. doi: 10.3390/cancers11040547
Kurilshikov, A., Medina-Gomez, C., Bacigalupe, R., Radjabzadeh, D., Wang, J., Demirkan, A., et al. (2021). Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165. doi: 10.1038/s41588-020-00763-1
Kurki, M. I., Karjalainen, J., Palta, P., Sipilä, T. P., Kristiansson, K., Donner, K. M., et al. (2023). FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518. doi: 10.1038/s41586-022-05473-8
Langella, P., Guarner, F., and Martín, R. (2019). Editorial: next-generation probiotics: from commensal Bacteria to novel drugs and food supplements. Front. Microbiol. 10:1973. doi: 10.3389/fmicb.2019.01973
Legason, I. D., Pfeiffer, R. M., Udquim, K.-I., Bergen, A. W., Gouveia, M. H., Kirimunda, S., et al. (2017). Evaluating the causal link between malaria infection and endemic Burkitt lymphoma in northern Uganda: a Mendelian randomization study. EBioMedicine 25, 58–65. doi: 10.1016/j.ebiom.2017.09.037
Leser, T., and Baker, A. (2023). Bifidobacterium adolescentis – a beneficial microbe. Benef. Microbes 14, 525–551. doi: 10.1163/18762891-20230030
Li, Z., Wang, Q., Huang, X., Wu, Y., Fu, R., Wen, X., et al. (2024). A Mendelian randomisation analysis reveals no relationship between periodontitis and coronary atherosclerosis. Int. Dent. J. :S0020653924000534. doi: 10.1016/j.identj.2024.01.027
Liu, C., Du, M.-X., Abuduaini, R., Yu, H.-Y., Li, D.-H., Wang, Y.-J., et al. (2021). Enlightening the taxonomy darkness of human gut microbiomes with a cultured biobank. Microbiome 9:119. doi: 10.1186/s40168-021-01064-3
Lu, C., Chen, Q., Tao, H., Xu, L., Li, J., Wang, C., et al. (2023). The causal effect of inflammatory bowel disease on diffuse large B-cell lymphoma: two-sample Mendelian randomization study. Front. Immunol. 14:1171446. doi: 10.3389/fimmu.2023.1171446
Mafra, A., Laversanne, M., Gospodarowicz, M., Klinger, P., De Paula Silva, N., Piñeros, M., et al. (2022). Global patterns of non-Hodgkin lymphoma in 2020. Int. J. Cancer 151, 1474–1481. doi: 10.1002/ijc.34163
Mager, L. F., Burkhard, R., Pett, N., Cooke, N. C. A., Brown, K., Ramay, H., et al. (2020). Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 369, 1481–1489. doi: 10.1126/science.abc3421
Martín, R., Rios-Covian, D., Huillet, E., Auger, S., Khazaal, S., Bermúdez-Humarán, L. G., et al. (2023). Faecalibacterium: a bacterial genus with promising human health applications. FEMS Microbiol. Rev. 47:fuad039. doi: 10.1093/femsre/fuad039
Martín-Masot, R., Herrador-López, M., Navas-López, V. M., Carmona, F. D., Nestares, T., and Bossini-Castillo, L. (2023). Celiac disease is a risk factor for mature T and NK cell lymphoma: a Mendelian randomization study. IJMS 24:7216. doi: 10.3390/ijms24087216
Matson, V., Fessler, J., Bao, R., Chongsuwat, T., Zha, Y., Alegre, M.-L., et al. (2018). The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science 359, 104–108. doi: 10.1126/science.aao3290
Meehan, C. J., and Beiko, R. G. (2014). A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated Bacteria. Genome Biol. Evol. 6, 703–713. doi: 10.1093/gbe/evu050
Mennella, J. A., Li, Y., Bittinger, K., Friedman, E. S., Zhao, C., Li, H., et al. (2022). The macronutrient composition of infant formula produces differences in gut microbiota maturation that associate with weight gain velocity and weight status. Nutrients 14:1241. doi: 10.3390/nu14061241
Miller, R. A., Maloney, D. G., Warnke, R., and Levy, R. (1982). Treatment of B-cell lymphoma with monoclonal anti-idiotype antibody. N. Engl. J. Med. 306, 517–522. doi: 10.1056/NEJM198203043060906
Miquel, S., Martín, R., Rossi, O., Bermúdez-Humarán, L., Chatel, J., Sokol, H., et al. (2013). Faecalibacterium prausnitzii and human intestinal health. Curr. Opin. Microbiol. 16, 255–261. doi: 10.1016/j.mib.2013.06.003
Mondal, A., Bose, S., Banerjee, S., Patra, J. K., Malik, J., Mandal, S. K., et al. (2020). Marine cyanobacteria and microalgae metabolites—a rich source of potential anticancer drugs. Mar. Drugs 18:476. doi: 10.3390/md18090476
Montassier, E., Al-Ghalith, G. A., Ward, T., Corvec, S., Gastinne, T., Potel, G., et al. (2016). Pretreatment gut microbiome predicts chemotherapy-related bloodstream infection. Genome Med. 8:49. doi: 10.1186/s13073-016-0301-4
Moreno Muñoz, J. A., Chenoll, E., Casinos, B., Bataller, E., Ramón, D., Genovés, S., et al. (2011). Novel probiotic Bifidobacterium longum subsp. infantis CECT 7210 strain active against rotavirus infections. Appl. Environ. Microbiol. 77, 8775–8783. doi: 10.1128/AEM.05548-11
Nagarajan, M., Maruthanayagam, V., and Sundararaman, M. (2012). A review of pharmacological and toxicological potentials of marine cyanobacterial metabolites. J. Appl. Toxicol. 32, 153–185. doi: 10.1002/jat.1717
Park, E. M., Chelvanambi, M., Bhutiani, N., Kroemer, G., Zitvogel, L., and Wargo, J. A. (2022). Targeting the gut and tumor microbiota in cancer. Nat. Med. 28, 690–703. doi: 10.1038/s41591-022-01779-2
Park, J.-H., Song, W.-S., Lee, J., Jo, S.-H., Lee, J.-S., Jeon, H.-J., et al. (2022). An integrative multiomics approach to characterize prebiotic inulin effects on Faecalibacterium prausnitzii. Front. Bioeng. Biotechnol. 10:825399. doi: 10.3389/fbioe.2022.825399
Qin, J., Li, R., Raes, J., Arumugam, M., Burgdorf, K. S., Manichanh, C., et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature 464, 59–65. doi: 10.1038/nature08821
Roller, M., Clune, Y., Collins, K., Rechkemmer, G., and Watzl, B. (2007). Consumption of prebiotic inulin enriched with oligofructose in combination with the probiotics lactobacillus rhamnosus and Bifidobacterium lactis has minor effects on selected immune parameters in polypectomised and colon cancer patients. Br. J. Nutr. 97, 676–684. doi: 10.1017/S0007114507450292
Routy, B., Le Chatelier, E., Derosa, L., Duong, C. P. M., Alou, M. T., Daillère, R., et al. (2018). Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science 359, 91–97. doi: 10.1126/science.aan3706
Ruan, X., Huang, D., Zhan, Y., Huang, J., Huang, J., Ng, A. T.-L., et al. (2023). Risk of second primary cancers after a diagnosis of first primary cancer: a pan-cancer analysis and Mendelian randomization study. eLife 12:e86379. doi: 10.7554/eLife.86379
Sagheddu, V., Patrone, V., Miragoli, F., Puglisi, E., and Morelli, L. (2016). Infant early gut colonization by Lachnospiraceae: high frequency of Ruminococcus gnavus. Front. Pediatr. 4. doi: 10.3389/fped.2016.00057
Sakamoto, M., Sakurai, N., Tanno, H., Iino, T., Ohkuma, M., and Endo, A. (2022). Genome-based, phenotypic and chemotaxonomic classification of Faecalibacterium strains: proposal of three novel species Faecalibacterium duncaniae sp. nov., Faecalibacterium hattorii sp. nov. and Faecalibacterium gallinarum sp. nov. Int. J. Syst. Evol. Microbiol. 72. doi: 10.1099/ijsem.0.005379
Sanna, S., van Zuydam, N. R., Mahajan, A., Kurilshikov, A., Vich Vila, A., Võsa, U., et al. (2019). Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases. Nat. Genet. 51, 600–605. doi: 10.1038/s41588-019-0350-x
Sathasivam, R., Radhakrishnan, R., Hashem, A., and Abd_Allah, E. F. (2019). Microalgae metabolites: a rich source for food and medicine. Saudi J. Biol. Sci. 26, 709–722. doi: 10.1016/j.sjbs.2017.11.003
Schirrmeister, B. E., Antonelli, A., and Bagheri, H. C. (2011). The origin of multicellularity in cyanobacteria. BMC Evol. Biol. 11:45. doi: 10.1186/1471-2148-11-45
Senousy, H. H., Abd Ellatif, S., and Ali, S. (2020). Assessment of the antioxidant and anticancer potential of different isolated strains of cyanobacteria and microalgae from soil and agriculture drain water. Environ. Sci. Pollut. Res. 27, 18463–18474. doi: 10.1007/s11356-020-08332-z
Shankland, K. R., Armitage, J. O., and Hancock, B. W. (2012). Non-Hodgkin lymphoma. Lancet 380, 848–857. doi: 10.1016/S0140-6736(12)60605-9
Shi, Z., Hu, G., Li, M. W., Zhang, L., Li, X., Li, L., et al. (2023). Gut microbiota as non-invasive diagnostic and prognostic biomarkers for natural killer/T-cell lymphoma. Gut 72, 1999–2002. doi: 10.1136/gutjnl-2022-328256
Shi, X., Wallach, J. D., Ma, X., and Rogne, T. (2024). Autoimmune diseases and risk of non-Hodgkin lymphoma: a Mendelian randomisation study. medRxiv. doi: 10.1101/2024.01.20.24301459
Shimizu, Y., Isoda, K., Taira, Y., Taira, I., Kondoh, M., and Ishida, I. (2020). Anti-tumor effect of a recombinant Bifidobacterium strain secreting a claudin-targeting molecule in a mouse breast cancer model. Eur. J. Pharmacol. 887:173596. doi: 10.1016/j.ejphar.2020.173596
Sieber, S. A., and Marahiel, M. A. (2005). Molecular mechanisms underlying nonribosomal peptide synthesis: approaches to new antibiotics. Chem. Rev. 105, 715–738. doi: 10.1021/cr0301191
Sithranga Boopathy, N., and Kathiresan, K. (2010). Anticancer drugs from marine flora: an overview. J. Oncol. 2010, 1–18. doi: 10.1155/2010/214186
Smith, P. M., Howitt, M. R., Panikov, N., Michaud, M., Gallini, C. A., Bohlooly-Y, M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic T reg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165
Sokol, H., Pigneur, B., Watterlot, L., Lakhdari, O., Bermúdez-Humarán, L. G., Gratadoux, J.-J., et al. (2008). Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 105, 16731–16736. doi: 10.1073/pnas.0804812105
Stein-Thoeringer, C. K., Saini, N. Y., Zamir, E., Blumenberg, V., Schubert, M.-L., Mor, U., et al. (2023). A non-antibiotic-disrupted gut microbiome is associated with clinical responses to CD19-CAR-T cell cancer immunotherapy. Nat. Med. 29, 906–916. doi: 10.1038/s41591-023-02234-6
Sugiyama, Y., Masumori, N., Fukuta, F., Yoneta, A., Hida, T., Yamashita, T., et al. (2013). Influence of isoflavone intake and equol-producing intestinal Flora on prostate cancer risk. Asian Pac. J. Cancer Prev. 14, 1–4. doi: 10.7314/APJCP.2013.14.1.1
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71, 209–249. doi: 10.3322/caac.21660
Tanaka, T., Matsuno, Y., Torisu, T., Shibata, H., Hirano, A., Umeno, J., et al. (2021). Gastric microbiota in patients with Helicobacter pylori-negative gastric MALT lymphoma. Medicine 100:e27287. doi: 10.1097/MD.0000000000027287
Taur, Y., Jenq, R. R., Perales, M.-A., Littmann, E. R., Morjaria, S., Ling, L., et al. (2014). The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood 124, 1174–1182. doi: 10.1182/blood-2014-02-554725
Upadhyay Banskota, S., Skupa, S. A., El-Gamal, D., and D’Angelo, C. R. (2023). Defining the role of the gut microbiome in the pathogenesis and treatment of lymphoid malignancies. IJMS 24:2309. doi: 10.3390/ijms24032309
Vacca, M., Celano, G., Calabrese, F. M., Portincasa, P., Gobbetti, M., and De Angelis, M. (2020). The controversial role of human gut Lachnospiraceae. Microorganisms 8:573. doi: 10.3390/microorganisms8040573
Vital, M., Howe, A. C., and Tiedje, J. M. (2014). Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 5, e00889–e00814. doi: 10.1128/mBio.00889-14
Waller, P. A., Gopal, P. K., Leyer, G. J., Ouwehand, A. C., Reifer, C., Stewart, M. E., et al. (2011). Dose-response effect of Bifidobacterium lactis HN019 on whole gut transit time and functional gastrointestinal symptoms in adults. Scand. J. Gastroenterol. 46, 1057–1064. doi: 10.3109/00365521.2011.584895
Walter, J. M., Coutinho, F. H., Dutilh, B. E., Swings, J., Thompson, F. L., and Thompson, C. C. (2017). Ecogenomics and taxonomy of Cyanobacteria phylum. Front. Microbiol. 8:2132. doi: 10.3389/fmicb.2017.02132
Wei, W., Sun, W., Yu, S., Yang, Y., and Ai, L. (2016). Butyrate production from high-fiber diet protects against lymphoma tumor. Leuk. Lymphoma 57, 2401–2408. doi: 10.3109/10428194.2016.1144879
Weizman, Z., Asli, G., and Alsheikh, A. (2005). Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 115, 5–9. doi: 10.1542/peds.2004-1815
Wrasidlo, W., Mielgo, A., Torres, V. A., Barbero, S., Stoletov, K., Suyama, T. L., et al. (2008). The marine lipopeptide somocystinamide a triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA 105, 2313–2318. doi: 10.1073/pnas.0712198105
Yatsunenko, T., Rey, F. E., Manary, M. J., Trehan, I., Dominguez-Bello, M. G., Contreras, M., et al. (2012). Human gut microbiome viewed across age and geography. Nature 486, 222–227. doi: 10.1038/nature11053
Yuan, L., Wang, W., Zhang, W., Zhang, Y., Wei, C., Li, J., et al. (2021). Gut microbiota in untreated diffuse large B cell lymphoma patients. Front. Microbiol. 12:646361. doi: 10.3389/fmicb.2021.646361
Zaplana, T., Miele, S., and Tolonen, A. C. (2024). Lachnospiraceae are emerging industrial biocatalysts and biotherapeutics. Front. Bioeng. Biotechnol. 11:1324396. doi: 10.3389/fbioe.2023.1324396
Zhang, X., Yu, D., Wu, D., Gao, X., Shao, F., Zhao, M., et al. (2023). Tissue-resident Lachnospiraceae family bacteria protect against colorectal carcinogenesis by promoting tumor immune surveillance. Cell Host Microbe 31, 418–432.e8. doi: 10.1016/j.chom.2023.01.013
Zhao, J., Ming, J., Hu, X., Chen, G., Liu, J., and Yang, C. (2020). Bayesian weighted Mendelian randomization for causal inference based on summary statistics. Bioinformatics 36, 1501–1508. doi: 10.1093/bioinformatics/btz749
Zheng, T., Liu, C., Wang, Y., Zhou, H., Zhou, R., Zhu, X., et al. (2024). Inflammatory cytokines mediating the effect of oral lichen planus on oral cavity cancer risk: a univariable and multivariable Mendelian randomization study. BMC Oral Health 24:375. doi: 10.1186/s12903-024-04104-0
Zhou, M., Xia, J., Chen, X., Wu, T., Xu, K., Zou, Y., et al. (2024). Assessing the causal association between dietary vitamin intake and lymphoma risk: a Mendelian randomisation study. Int. J. Food Sci. Nutr. 75, 92–101. doi: 10.1080/09637486.2023.2278420
Keywords: gut microbiota, non-Hodgkin lymphoma, diffuse large B-cell lymphoma, haematology system, Mendelian randomization
Citation: Fu J and Hao Z (2024) The causality between gut microbiota and non-Hodgkin lymphoma: a two-sample bidirectional Mendelian randomization study. Front. Microbiol. 15:1403825. doi: 10.3389/fmicb.2024.1403825
Edited by:
Lifeng Zhu, Nanjing University of Chinese Medicine, ChinaReviewed by:
Zhengrui Li, Shanghai Jiao Tong University, ChinaHong Liu, Shandong University of Technology, China
Copyright © 2024 Fu and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Hao, MTA2MDI1NjAxMEBxcS5jb20=
 Jinjie Fu
Jinjie Fu Zheng Hao2,3,4*
Zheng Hao2,3,4*