- 1Key Laboratory of Basic Pharmacology and Joint International Research Laboratory of Ethnomedicine of Ministry of Education, Zunyi Medical University, Zunyi, China
- 2Bioresource Institute for Healthy Utilization, Zunyi Medical University, Zunyi, China
- 3Department of Microbiology, Faculty of Science, Dr. Rammanohar Lohia Avadh University, Ayodhya, Uttar Pradesh, India
Plant-microbe interactions are pivotal for ecosystem dynamics and sustainable agriculture, and are influenced by various factors, such as host characteristics, environmental conditions, and human activities. Omics technologies, including genomics, transcriptomics, proteomics, and metabolomics, have revolutionized our understanding of these interactions. Genomics elucidates key genes, transcriptomics reveals gene expression dynamics, proteomics identifies essential proteins, and metabolomics profiles small molecules, thereby offering a holistic perspective. This review synthesizes diverse microbial-plant interactions, showcasing the application of omics in understanding mechanisms, such as nitrogen fixation, systemic resistance induction, mycorrhizal association, and pathogen-host interactions. Despite the challenges of data integration and ethical considerations, omics approaches promise advancements in precision intervention and resilient agricultural practices. Future research should address data integration challenges, enhance omics technology resolution, explore epigenomics, and understand plant-microbe dynamics under diverse conditions. In conclusion, omics technologies hold immense promise for optimizing agricultural strategies and fortifying resilient plant-microbe alliances, paving the way for sustainable agriculture and environmental stewardship.
1 Introduction
The profound effects of climate change, including shifting precipitation patterns, rising temperatures, and extreme weather, pose significant threats to agricultural practices and food security (Olanrewaju et al., 2024). Agriculture serves as a linchpin for economic growth, laying the foundation for secondary and tertiary industries (Shah et al., 2024). With global food production losses estimated at 352 million tons by 2070 owing to population growth (Sartori et al., 2024), there is an urgent need to accelerate food production over the next 30 years (Kimotho and Maina, 2024). Although crop improvement programs are underway, insufficient attention has been paid to modern techniques and balanced fertilization, leading to nutritional insecurity in staple crops (Jalal et al., 2024). The World Health Organization underscores the importance of food safety and urging measures to prevent foodborne diseases across various stages of food processing, production, storage, transportation, and consumption (Su et al., 2024). Sustainability of these challenges is crucial for global food systems, environmental stability, and climate resilience.
Recent advancements in omics approaches offer a promising avenue to address the challenges facing crop productivity by elucidating the benefits of plant-microbe interactions (Olanrewaju et al., 2024). Breakthroughs in analytical methodologies have provided comprehensive insights into the intricate dynamics of these interactions (Sharma et al., 2024). Omics techniques, including genomics, transcriptomics, and metabolomics, have proven invaluable in exploring the biochemical, physiological, and molecular aspects of plant-microbe interactions across various conditions (Tiwari et al., 2024). The integration of multi-omics data from different databases is essential for effective utilization of omics technologies, offering a comprehensive understanding of biological processes and interactions (Chao et al., 2024). These advancements have resulted in the accumulation of vast amounts of information at all levels, enabling deep insight into mechanisms under stressful conditions (Ahmed et al., 2024). The integrated use of multi-omics approaches enhances data analysis, visualization, and interpretation, facilitating a deeper understanding of biological processes.
The intricate relationship between plants and microbes plays a vital role in ecosystem dynamics and sustainable agriculture and is influenced by host-related elements, edaphic conditions, environmental dynamics, and anthropogenic factors (Rane et al., 2022). Understanding these factors is essential for unraveling the complexity of symbiotic relationships. Omics approaches, including genomics, transcriptomics, proteomics, and metabolomics, illuminate the molecular intricacies of plant-microbe symbiosis (Sarim et al., 2020). Genomics identifies key genes, transcriptomics reveals gene expression dynamics, proteomics identifies essential proteins, and metabolomics profiles small molecules, thereby offering a comprehensive perspective. Multi-omics techniques enable the study of various interactions, including obligate symbiotic relationships, such as those involving arbuscular mycorrhizal fungi and plants, extending to interactions with non-mycorrhizal microbes such as biofilms (Mishra et al., 2022). These insights underscore the transformative potential of omics technologies in advancing sustainable agriculture through a deeper understanding of beneficial plant-microbe relationships.
The integration of multi-omics and bioinformatics has revealed that plant-microbe interactions in polluted rhizospheres trigger the release of antioxidants and phytohormones, thereby activating plant defense mechanisms (Sengupta and Pal, 2021). Phytohormones serve as vital messengers that regulate plant growth and synthesize secondary metabolites (De Palma et al., 2022). Omics tools such as metagenomics and metatranscriptomics aid in the detection and analysis of phytohormones, deepening our understanding of their roles in these interactions. Understanding secretory metabolites can help in the design of next-generation microbial inoculants to enhance plant growth (Mishra et al., 2022). Advancements in sequencing technologies have facilitated the analysis of trace chemical substances, aiding the investigation of allelochemical biosynthesis and plant responses, thereby enhancing our understanding of rhizosphere chemistry (Weidenhamer et al., 2023). Penicillium and Aspergillus release phosphorus and potassium during mineral degradation, benefiting plant growth and defense in beneficial plant-microbe interactions (Paul et al., 2024). The presence of CAZymes from the GT4 and GT2 families suggests potential tripartite symbiotic relationships among plants, rhizospheric bacteriomes, and fungiomes, thereby guiding the application of omics tools to enhance plant resilience to environmental stress (Alshareef, 2024). Salvia miltiorrhiza boosts stress resistance through signals transmitted via AM (arbuscular mycorrhiza) hyphal networks post-pathogen stress from Fusarium solani, with metagenomics tools aiding in understanding and enhancing its resilience (Han et al., 2023).
There are limitations to each omics strategy that may affect the sensitivity or specificity of the technique. However, some of these limitations can be overcome by integrating different approaches. The objective of this review article is to explore the transformative impact of omics technologies on understanding plant-microbe interactions, which play a pivotal role in shaping ecosystems and promoting sustainable agriculture. This review explores the complexities of these interactions, which are influenced by various factors, such as host-related traits, environmental conditions, and anthropogenic activities. This highlights the emergence of omics approaches, including genomics, transcriptomics, proteomics, metabolomics, and epigenomics, which elucidate their roles in deciphering the molecular mechanisms underlying beneficial plant-microbe associations. By synthesizing findings from diverse microbial-plant interactions, this review showcases the application of omics technologies in unraveling mechanisms, such as nitrogen fixation, induction of systemic resistance, mycorrhizal association, and pathogen-host interactions. Furthermore, we discuss modern web-based omics tools that facilitate data analysis and interpretation. Ultimately, the review underscores the potential of omics approaches to revolutionize agricultural practices by offering insights into crop improvement, disease management, and sustainable agriculture, while addressing challenges in data integration and ethical considerations.
2 Molecular mechanisms underlying plant-microbe interactions
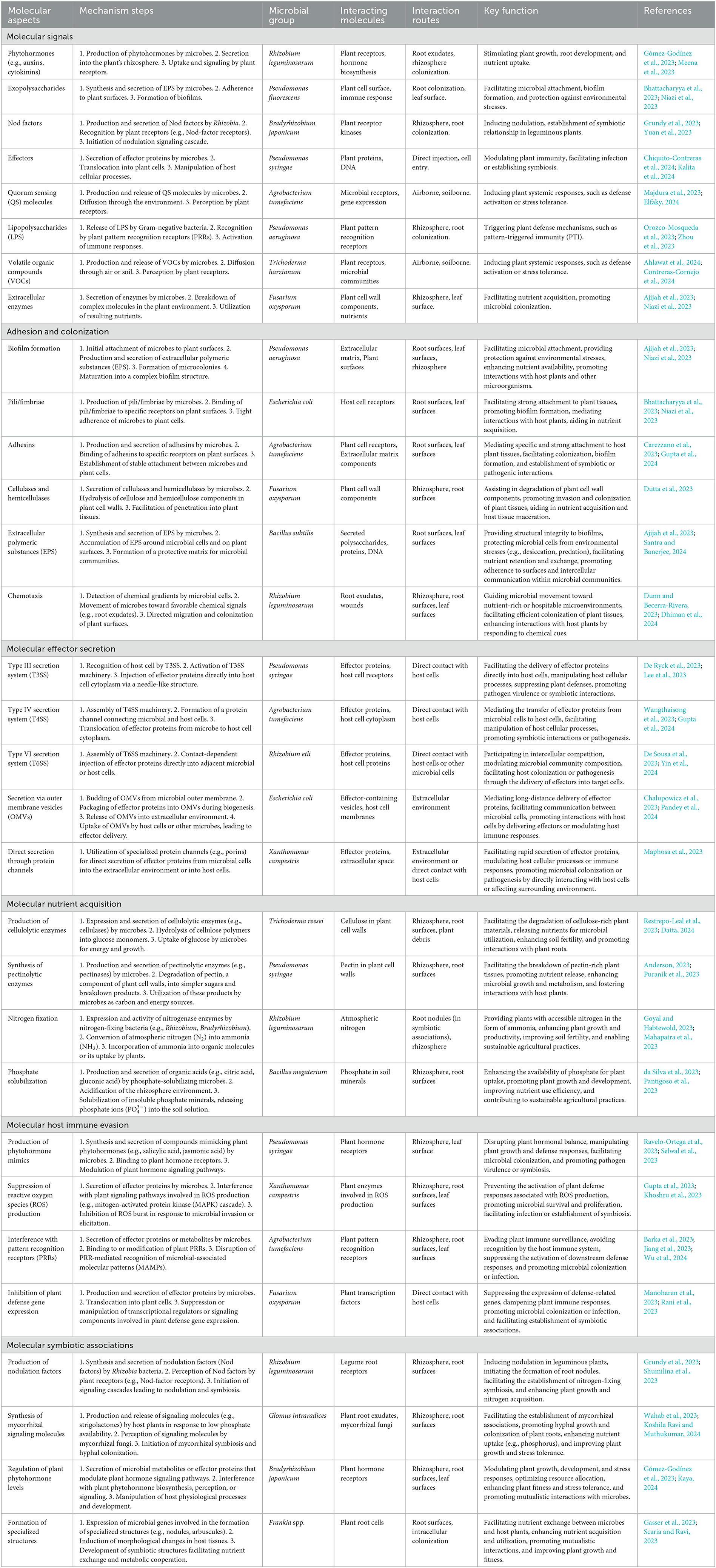
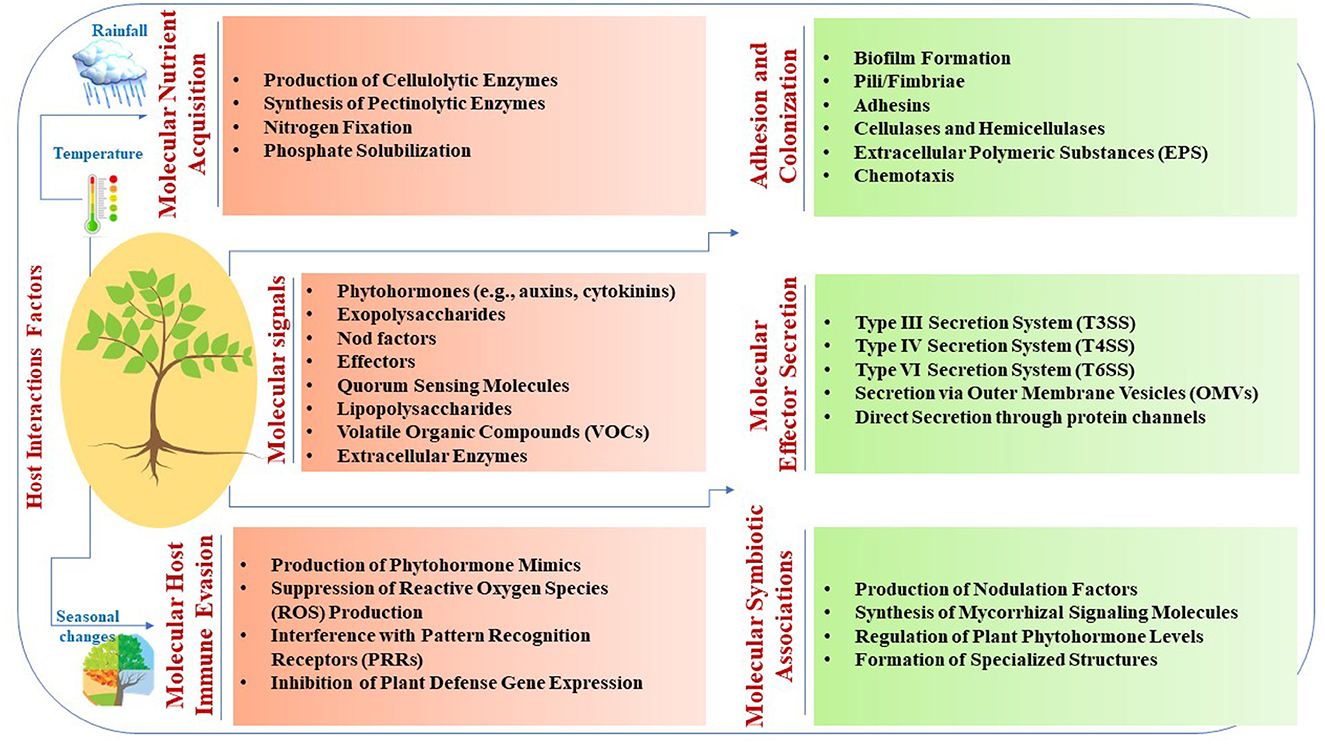
Microbial interactions with host plants involve intricate molecular signaling mechanisms that are crucial for symbiosis, pathogenesis, and environmental adaptation (Table 1 and Figure 1). Phytohormones, such as auxins and cytokinins, produced by microbes such as Rhizobium leguminosarum stimulate plant growth and nutrient uptake through signaling by plant receptors (Meena et al., 2023). Exopolysaccharides synthesized by microbes such as Pseudomonas fluorescens facilitate attachment to plant surfaces, biofilm formation, and protection against environmental stress (Niazi et al., 2023). Nod factors secreted by rhizobia such as Bradyrhizobium japonicum induce nodulation signaling cascades and establish symbiotic relationships in leguminous plants (Grundy et al., 2023; Yuan et al., 2023). Effectors from pathogens, such as Pseudomonas syringae, manipulate host cellular processes to modulate plant immunity and facilitate infection (Kalita et al., 2024). Quorum-sensing molecules produced by microbes such as Agrobacterium tumefaciens induce systemic plant responses, whereas lipopolysaccharides from bacteria such as Pseudomonas aeruginosa trigger plant defense mechanisms (Majdura et al., 2023; Orozco-Mosqueda et al., 2023; Zhou et al., 2023; Elfaky, 2024). Volatile organic compounds produced by microbes such as Trichoderma harzianum also elicit plant systemic responses (Contreras-Cornejo et al., 2024). Furthermore, extracellular enzymes such as Fusarium oxysporum facilitate nutrient acquisition and microbial colonization (Niazi et al., 2023). These molecular mechanisms play pivotal roles in microbial interactions with host plants, influencing symbiosis, pathogenesis, and plant-microbe-environment interactions.
The adhesion and colonization of microbes on plant surfaces involve intricate molecular processes that are essential for establishing symbiotic or pathogenic relationships. Microbes utilize various strategies such as EPS (exopolysaccharides) production, pili/fimbriae binding, adhesin secretion, and enzymatic degradation of plant cell wall components (Bhattacharyya et al., 2023; Carezzano et al., 2023; Dutta et al., 2023; Niazi et al., 2023). Additionally, chemical gradients, such as root exudates, guide microbial migration and colonization of plant surfaces (Dunn and Becerra-Rivera, 2023; Dhiman et al., 2024). Molecular effector secretion by microbial pathogens involves sophisticated mechanisms for manipulating host cellular processes. Pathogens utilize various secretion systems, including T3SS, T4SS, T6SS, outer membrane vesicles (OMVs), and direct secretion systems to deliver effector proteins into host cells (Chalupowicz et al., 2023; De Ryck et al., 2023; De Sousa et al., 2023; Maphosa et al., 2023; Wangthaisong et al., 2023). These effectors manipulate the host cellular processes to promote infection and pathogenesis. The T4SS system in rhizobial species, akin to Agrobacterium tumefaciens Vir proteins, shares similarities in key components, such as trbD (virB3), trbI (virB10), and trbL (virB6). These elements facilitate genetic exchange through tra genes, such as traD, traR, and traG, which are analogous to virD4 and are essential for conjugative transfer within rhizobial populations. Additionally, Mesorhizobium loti strain R7A utilizes its T4SS to transfer specific proteins, such as Msi059 (a protease) and Msi061 (involved in ubiquitinylation), contributing to various cellular processes and interactions with host plants (Gupta et al., 2024). Furthermore, T6SS genes are prevalent in plant-associated bacteria, including rhizobial species, and play a crucial role in interbacterial competition, providing advantages in multimicrobial plant environments (De Sousa et al., 2023). Moreover, mutations in immune receptors, such as EFR, FLS2, and BAK1, minimally affect OMV-induced immune priming (Chalupowicz et al., 2023).
Microbial nutrient acquisition involves intricate mechanisms for acquiring essential nutrients from the plant environment. Microbes produce enzymes and metabolites that degrade complex plant molecules and enhance nutrient availability (Anderson, 2023; Puranik et al., 2023). Nitrogen-fixing bacteria such as Rhizobium leguminosarum convert atmospheric nitrogen to ammonia, thereby enhancing plant growth and soil fertility (Goyal and Habtewold, 2023). Phosphate-solubilizing microbes produce organic acids to solubilize phosphate minerals and promote plant growth (da Silva et al., 2023). Microbial immune evasion strategies involve sophisticated mechanisms for subverting plant defense responses. Pathogens mimic plant hormones, interfere with signaling pathways, and suppress immune surveillance to promote colonization and infection (Barka et al., 2023; Khoshru et al., 2023; Ravelo-Ortega et al., 2023). These mechanisms highlight the ability of microbes to circumvent plant immune defenses. Symbiotic associations involve intricate mechanisms that establish mutualistic relationships. Microbes synthesize signaling molecules, respond to host signals, regulate hormone pathways, and form specialized structures for nutrient exchange (Scaria and Ravi, 2023; Shumilina et al., 2023; Kaya, 2024; Koshila Ravi and Muthukumar, 2024). These mechanisms underscore the complexity of the symbiotic interactions between microbes and host plants. These symbiotic associations not only contribute to plant health and vigor but also foster ecosystem resilience and sustainability by enhancing soil fertility and nutrient cycling (Scaria and Ravi, 2023; Wahab et al., 2023). These examples highlight the complexity and versatility of the molecular mechanisms underlying plant-microbe interactions, underscoring the importance of understanding these processes for elucidating host-microbe dynamics and developing strategies for sustainable agriculture and disease management.
3 Omics approaches mechanism in beneficial plant-microbe interaction
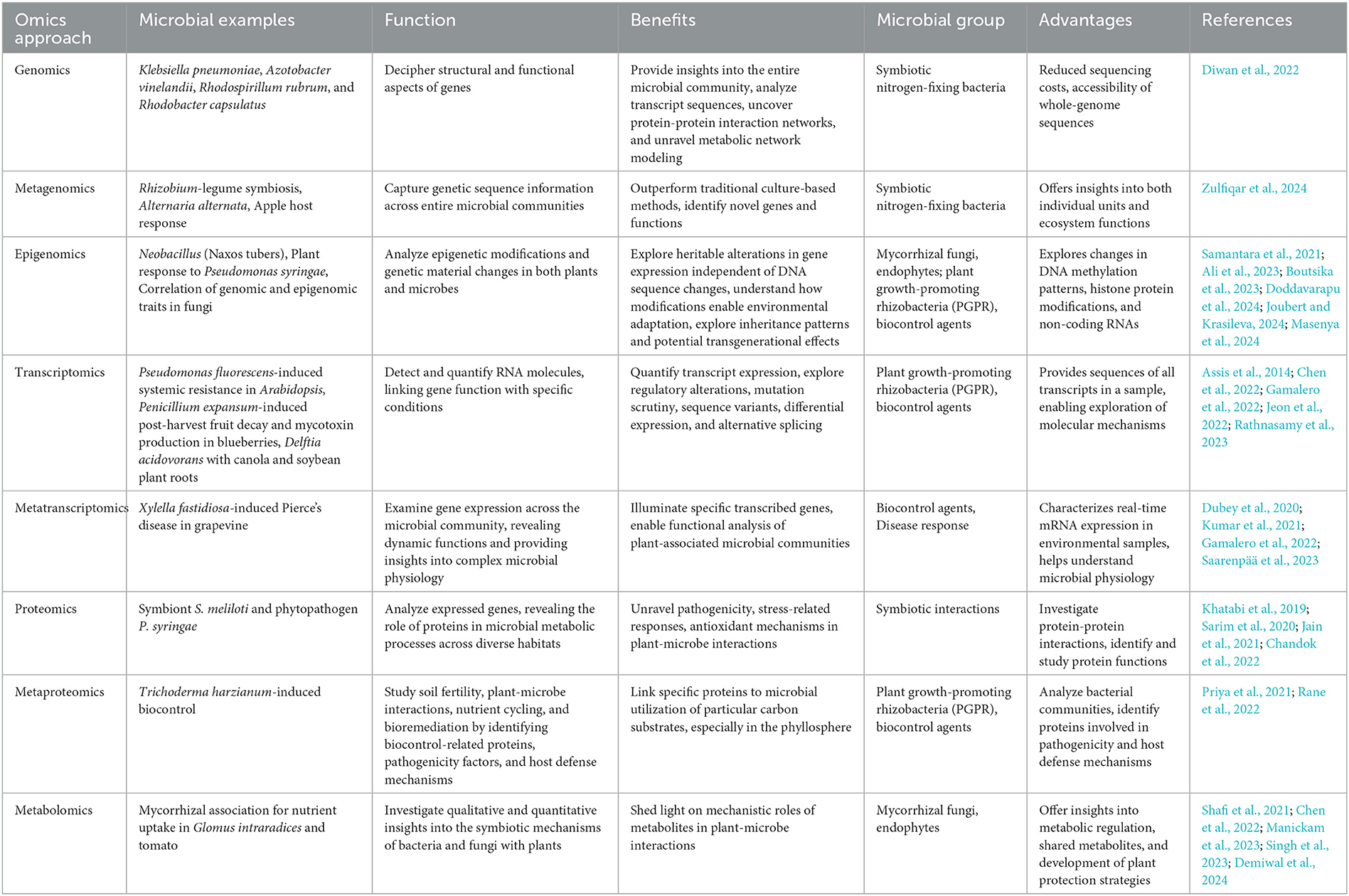
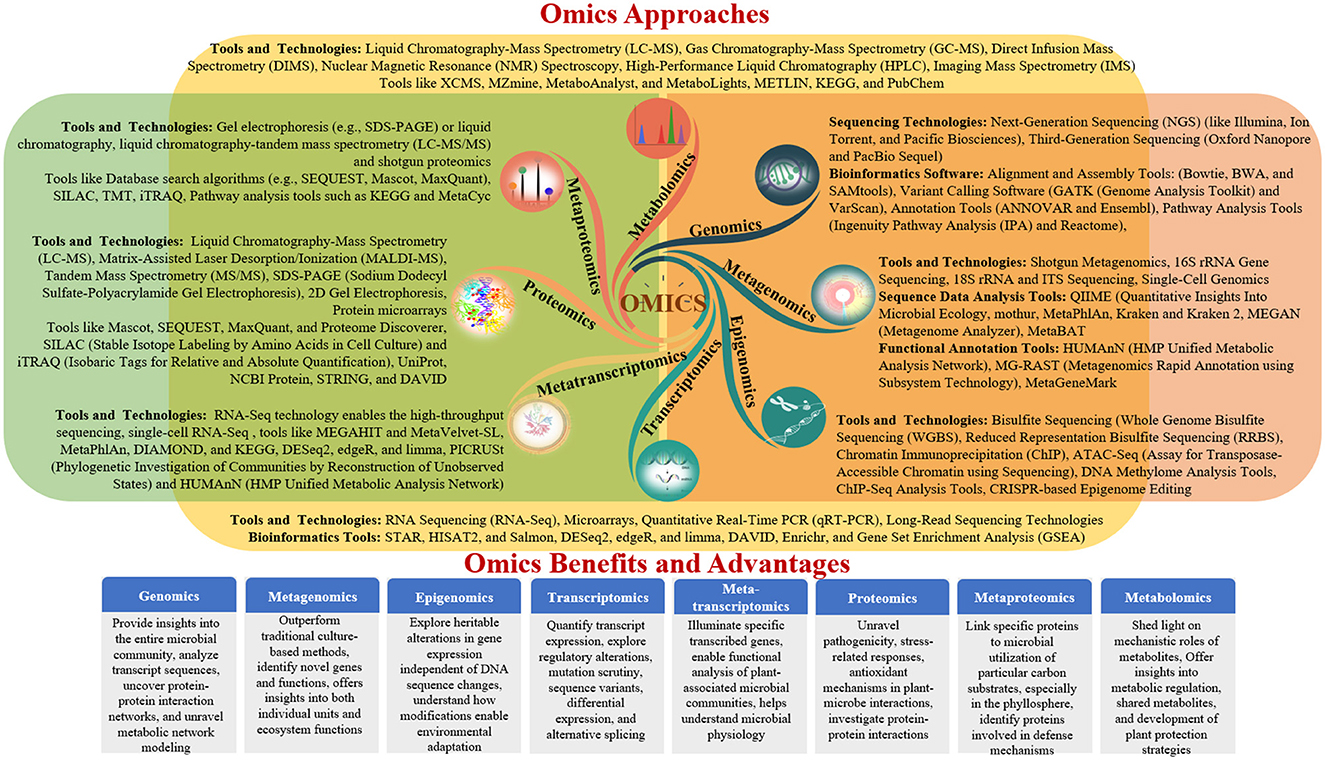
Advances in molecular biology and high-resolution analytical technologies have enabled the comprehensive investigation of plant-microbe interactions through genomics, metagenomics, transcriptomics, proteomics, and metabolomics (Diwan et al., 2022). These omics approaches decipher the functional and structural aspects of genes, provide insights into the entire microbial community, analyse transcript sequences, uncover protein-protein interaction networks, and unravel metabolic network modeling (Zulfiqar et al., 2024). Together, these findings enhance our understanding of the effects of omics on interactions between plants and microbes. This review provides a comprehensive overview of each omics approach and its specific focus areas in elucidating beneficial plant-microbe interactions, ultimately contributing to the development of sustainable agricultural practices (Table 2 and Figure 2).
3.1 Genomics
Recent advances in genomics have revealed that plants have adapted to a wide range of biotic interactions that extend beyond their relationships with beneficial symbionts (Carper et al., 2022; Chiquito-Contreras et al., 2024). Advancements in next-generation sequencing (NGS) technologies have not only reduced sequencing costs but also accelerated access to whole-genome sequences, de novo assemblies, and resequencing of multiple strains within species (Crandall et al., 2020). Through these analyses, researchers have uncovered the genetic mechanisms responsible for crucial functions such as nitrogen fixation and synthesis of growth-promoting compounds. Metagenomic approaches further reveal the diversity and functional potential of microbial communities, shedding light on their contribution to plant health. Transcriptomic and proteomic analyses complement genomic insights by elucidating gene expression and protein profiles during interactions, thereby revealing the molecular underpinnings. Leveraging this genomic knowledge, synthetic biology strategies enable the engineering of beneficial traits in plants and microbes, whereas microbiome engineering endeavors to optimize microbial communities to bolster plant vigor and productivity (Chandok et al., 2022).
3.1.1 Metagenomics
Metagenomics systematically captures genetic sequence information across entire microbial communities, outperforming traditional culture-based methods (Ghosh et al., 2019). It encompasses structural and functional approaches, and offers insights into both individual units and ecosystem functions. Initially, using Sanger sequencing and later transitioning to next-generation sequencing, metagenomics employed 16S rRNA (ribosomal ribonucleic acid) and random shotgun sequencing to identify novel genes (Regalado et al., 2020). Metagenomics has been applied to diverse areas, from identifying novel nitrogen-fixing genes in Rhizobium-legume symbiosis (Regalado et al., 2020) to detecting mycotoxin biosynthetic genes in Alternaria alternata and its host response in apples (Bhargava et al., 2019). This involves sequencing of environmental models comprising various life forms, many of which are unculturable. The sequencing of universal genomic regions, such as ribosomal RNA genes, from diverse microbial species has proven successful (Zhang et al., 2021). Metagenomic analysis can be performed through amplicon targeting or shotgun sequencing to provide valuable insights into plant-associated microbial communities.
Nif operons, such as nifHDK, nifRLA, nifENB, nifUSVM, nifJ, and nifWF, constitute a core genetic component of diazotrophs, which are free-living anaerobic bacteria capable of nitrogen fixation. Notable examples include Azotobacter vinelandii, Klebsiella pneumoniae, Rhodobacter capsulatus, and Rhodospirillum rubrum (Idris Usman and Muazu Wali, 2024). These genes are pivotal in nitrogen fixation, as they synthesize essential components and regulate enzymes crucial for the process. Advancements in omics technologies, such as genomics and gene manipulation, have tremendous potential for bolstering crop yield. Additionally, the rhizosphere microbiome synthesizes ACC (1-aminocyclopropane-1-carboxylate) deaminase, which facilitates the breakdown of ACC into α-ketobutyrate and ammonia. This breakdown aids in plant nutrition and reduces the concentration of ethylene, thus mitigating its adverse effects. The production of ACC deaminase (ACCD) by plant growth-promoting rhizobacteria significantly enhances plant resilience to various abiotic stressors (Kumari and Kumawat, 2024). The ACCD structural gene (AcdS) is found in the genomes of rhizospheric bacteria, symbiotic rhizobia, and endophytes. In nitrogen-fixing bacteria, such as Rhizobia and Mesorhizobium, acdS expression is regulated differently. Specifically, the NifA2 gene and σ54 sigma factor control acdS expression in N-fixing bacteria (Larekeng et al., 2024). The genetic makeup of Rhizobium sp. and Hydrogenophaga sp. was comprehensively characterized using Illumina and Nanopore sequencers coupled with MaSuRCA assembly, revealing genes involved in metabolic functions and compound synthesis that contribute to plant growth stimulation. These findings underscore the symbiotic relationship between rhizobacteria and plants, potentially implicating processes such as nitrogen fixation and production of growth-promoting compounds (Ilangumaran et al., 2024). Notably, under non-sterile conditions, only Pseudomonas sivasensis exhibited notable promotion of canola growth, possibly because of the presence of additional genes in its genome, including those responsible for ACC deaminase (acdA), indole-3-acetic acid (IAA) production (trpF and trpG), and siderophore production (fbpA, mbtH, and acrB), which enhances its capacity to stimulate plant growth (Swiatczak et al., 2024). Moreover, rhizobacteria trigger defense responses through the expression of pathogenesis-related proteins (PR-proteins), such as chitinases, which are pivotal for defense mechanisms. A genome-wide examination of soybean chitinases identified GmChi01, GmChi02, and GmChi16, whose defense contributions were verified against Fusarium oxysporum in Arabidopsis transgenic lines, with GmChi02 and GmChi16 enhancing defense against F. oxysporum, whereas GmChi02 was significantly induced by Burkholderia ambifaria (Chen et al., 2024). Furthermore, transgenic expression of entomocidal and antimicrobial proteins from Bacillus thuringiensis (Bt) in maize is an alternative to host resistance against Fusarium ear rot (FER), and the recruitment of beneficial microbes is dependent on the genetic background of the host, highlighting the importance of microbe-microbe interactions in modulating FER severity (Adams et al., 2024).
3.1.2 Epigenomics
Epigenomics, as a tool for studying plant-microbe interactions, involves a thorough analysis of epigenetic modifications and genetic material changes in both plants and microbes. This approach explores heritable alterations in gene expression independent of DNA (deoxyribonucleic acid) sequence changes (Samantara et al., 2021). Epigenomics in plant-microbe interactions involves studying changes in DNA methylation patterns, histone protein modifications such as acetylation and methylation, and the roles of non-coding RNAs such as microRNAs. It also includes analyzing chromatin remodeling, understanding how these modifications enable environmental adaptation, and exploring their inheritance patterns and potential transgenerational effects on plant and microbial traits (Ali et al., 2023). Furthermore, epigenetics plays a crucial role in plant responses to diseases, encompassing both biotic and abiotic stresses. EpiEffectors, such as zinc finger (ZF), transcription activator-like effector (TALE), or modified CRISPR/Cas9 complexes, such as dead Cas9 (dCas9), are used for targeted epigenome editing, and are often fused with the catalytic domain of epigenetic enzymes for precise modifications (Doddavarapu et al., 2024). Active DNA demethylation has been shown to positively affect plant resistance to pathogens such as Pseudomonas syringae (Masenya et al., 2024). The proline-alanine-valine (PAV) gene may be associated with specific genomic and epigenomic traits in fungi, offering potential predictive insights into fungal plant pathogen adaptation to hosts and aiding in the development of more effective disease prevention strategies (Joubert and Krasileva, 2024). Epigenomic analysis revealed that hypermethylation of xyloglucan endotransglycosylase protein could be influenced by Neobacillus, a dominant and highly abundant species found exclusively in Naxos tubers (Boutsika et al., 2023). This integrated approach identifies pivotal microbial taxa, their pathways, and epigenetic markers shaping host-microbe interactions, and offers novel insights into agriculture (Masenya et al., 2024).
3.2 Transcriptomics and metatranscriptomics
Transcriptomics employs next-generation sequencing (NGS) to detect and quantify RNA molecules in biological samples, linking gene functions under specific conditions. It uses methods such as microarray analysis, SOLiD-SAGE, and RNA sequencing (RNA-seq) for transcriptional profiling (Chen et al., 2022). Transcriptome analysis quantifies the expression and provides sequences of all samples, enabling the exploration of regulatory alterations, mutation scrutiny, sequence variants, differential expression, and alternative splicing (Weidemüller et al., 2021; Jeon et al., 2022). Transcriptome analyses have identified genes crucial in transitioning plant-microbe interactions from mutualistic to pathogenic, shedding light on disrupted associations (Rathnasamy et al., 2023). Assis et al. (2014) employed transcriptomics to uncover plant defense-related genes during Pseudomonas fluorescens-induced systemic resistance in Arabidopsis, while Jeon et al. (2022) utilized transcriptomics to unveil host pathways responding to pathogen infection in Penicillium expansum-induced post-harvest fruit decay and mycotoxin production in blueberries. RNA-seq transcriptomic analysis elucidated the mechanisms underlying the interactions of the plant growth-promoting bacteria (PGPB) Delftia acidovorans RAY209 with canola and soybean plant roots, focusing on the colonization process (Gamalero et al., 2022). Metatranscriptomics examines gene expression across the microbial community, revealing dynamic functions and providing insights into the complex microbial physiology (Dubey et al., 2020). It is a powerful tool for analyzing functional profiles and understanding the structure of microbial communities. Metatranscriptomics, which investigates total mRNA, provides real-time insights into mRNA expression in environmental samples (Dubey et al., 2020). This technique aids in identifying specific transcribed genes and enables the functional analysis of plant-associated microbial communities (Kumar et al., 2021; Gamalero et al., 2022). Comparative metatranscriptomic analyses of the microbial expression levels in uncontaminated and contaminated samples will enhance phytoremediation strategies. Dubey et al. (2020) utilized metatranscriptomics to examine transcriptome-wide changes in gene expression during Xylella fastidiosa-induced Pierce's disease in grapevines.
3.3 Proteomics and metaproteomics
Proteomic studies have analyzed expressed genes, revealing the role of proteins in microbial metabolic processes across diverse habitats. During plant-microbe interactions, proteins play a crucial role in cellular homeostasis, signaling networks, and defense responses. Proteomic analysis offers practical insights into these interactions, particularly in unculturable microbes. Khatabi et al. (2019) used proteomics to study pathogen effectors and host proteins in Phytophthora infestans-induced disease in potatoes, while Jain et al. (2021) employed proteomics to identify secreted proteins in Erwinia amylovora-induced fire blight disease in apples. Proteomic techniques are instrumental in unraveling the pathogenicity, stress-related responses, and antioxidant mechanisms involved in plant-microbe interactions, providing valuable insights into physiological and cellular processes. Functional proteomics utilizes the yeast two-hybrid system (Y2H) to investigate protein-protein interactions (PPIs) by employing “Prey” and “Bait” proteins. Mass spectrometric data analysis tools, such as PeptIdent, MultiIdent, MASCOT, SEQUEST, and Sherpa, facilitate proteomic analysis, enabling researchers to identify and study protein interactions and functions. Additionally, programs such as ProFound, MS-Fit, MOWSE, PepSea, PepFrag, and MS-Tag offer specialized analyses of MALDI-TOF and MS/MS spectra, enhancing the depth of proteomic investigation (Chandok et al., 2022). Gel-based resolution methods, such as two-dimensional gel electrophoresis (2DE) and differential gel electrophoresis (DIGE) coupled with mass spectrometry (MS), enable the identification and quantification of proteins. Studies have identified plant-related proteins using 2D-LC/MS/MS in M. truncatula nodules and revealed differential protein expression in rice tissues treated with S. meliloti. Additionally, comparative proteomic analyses have elucidated the protein secretion patterns of the symbiont S. meliloti and phytopathogen P. syringae DC3000 in response to root exudates from different host plants. Investigations into near-isogenic line alleles of Fhb1 have provided insights into Fusarium graminearum tolerance, while studies on beneficial microbes in the pea rhizosphere have highlighted differential proteomic responses upon infection by the necrotrophic fungus, Sclerotinia sclerotiorum. These findings underscore the importance of proteomic approaches in understanding the complex interactions between plants and microbes (Jain et al., 2021). Comparative proteomics of the B. cinerea-secreted proteome identified altered proteins, primarily pectinases, that are responsible for cell wall degradation. The secreted proteome also features a predominance of serine proteases, followed by metalloproteases, and threonine proteases (Sarim et al., 2020). iTRAQ proteomics revealed the regulatory mechanism in both resistant and susceptible rice cultivars against Magnaporthe oryzae. Metaproteomic investigations are pivotal for studying soil fertility, plant-microbe interactions, nutrient cycling, and bioremediation. Priya et al. (2021) conducted the first metaproteogenomic study analyzing bacterial communities in the phyllosphere of various plants, revealing consistency in dominant bacterial taxa and identified proteins across different species. Rane et al. (2022) used metaproteomics to identify biocontrol-related proteins in Trichoderma harzianum-induced biocontrol, whereas Priya et al. (2021) employed metaproteomics to identify proteins involved in the pathogenicity of Fusarium oxysporum-induced vascular wilt and host defense modulation in watermelon. Metaproteomics can link specific proteins to microbial utilization of particular carbon substrates, especially in the phyllosphere.
3.4 Metabolomics
Over the past decade, metabolomics has emerged as a well-established technique for investigating plant-microbe interactions, providing both qualitative and quantitative insights into the symbiotic mechanisms of bacteria and fungi with plants (Chouchani, 2022). Shafi et al. (2021) used metabolomics to profile the changes in metabolite levels associated with mycorrhizal associations for nutrient uptake in Glomus intraradices and tomatoes (Chen et al., 2022). Untargeted metabolomics has identified lipid indicators of Plasmopara viticola inoculation in grapevines, whereas a study of maize genotypes interacting with nitrogen-fixing PGPB species revealed alterations in plant metabolites owing to bacterial nitrogen fixation (Diwan et al., 2022). Despite being underutilized in root-pathogen interaction studies compared to other omics methods, untargeted metabolomics provides valuable insights into plant-microbial interactions across various tissues, aiding the development of more effective crop and plant protection strategies (Demiwal et al., 2024). Detailed metabolomic analysis revealed Pseudomonas syringae-triggered hyperaccumulation of dihydrocamalexic acid (DHCA) in the apoplastic space of pad3 but not in cyp71a12/a13 plants, and infiltration of DHCA into cyp71a12/a13 mutant leaves restored resistance, indicating its role in restricting P. syringae growth in plants (Singh et al., 2023). Metabolomic profiling of rice infested with Magnaporthe grisea using liquid chromatography–mass spectrometry (LC-MS), gas chromatography-mass spectrometry (GC-MS), and nuclear magnetic resonance (NMR) methods revealed a varied metabolomic profile. Metabolomic profiling of Dendrobium nobile co-cultured with Trichoderma longibrachiatum using LC-MS revealed the metabolomic profile of dendrobine (Sarsaiya et al., 2024). Additionally, in maize, the resistance mechanism against Fusarium graminearum uncovered two metabolites, smiglaside and smilaside, whereas analysis of resistance to southern corn leaf blight identified polyphenols, lignin, and flavonoids through metabolite profiling using Fourier transform infrared (FT-IR) and NMR resonance (Manickam et al., 2023).
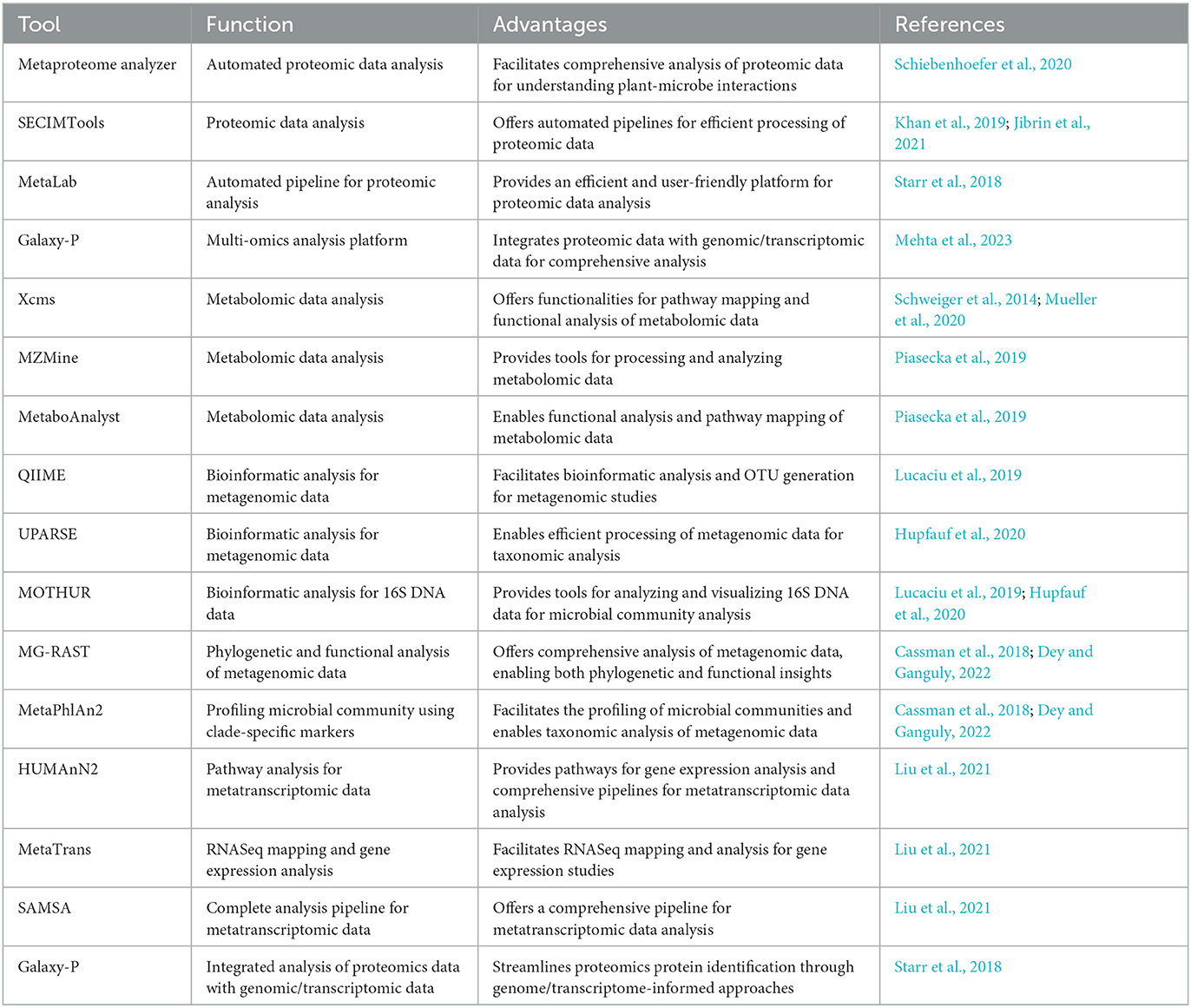
4 Modern web-based omics tools for plant-microbe interaction
Web-based tools have revolutionized the analysis of modern “omics”-generated data, providing researchers with accessible and efficient platforms for data processing and interpretation. A plethora of tools cater to various omics disciplines, including automated pipelines, functional analysis, and pathway mapping (Table 3 and Figure 3). For proteomic analysis, tools like Metaproteome Analyzer (Schiebenhoefer et al., 2020), SECIMTools (Khan et al., 2019; Jibrin et al., 2021), and MetaLab (Starr et al., 2018) provide automated pipelines for proteomic data analysis, while Galaxy-P (Mehta et al., 2023) offers a multi-omics analysis platform. Metabolomic data analysis is facilitated by tools such as Xcms (Schweiger et al., 2014; Mueller et al., 2020), MZMine, and Metabolome MetaboAnalyst (Piasecka et al., 2019), which offer functionalities for pathway mapping and analysis. For metagenomic studies, tools such as QIIME, UPARSE, and MOTHUR aid in bioinformatics data analysis and operational taxonomic unit (OTU) generation (Lucaciu et al., 2019; Hupfauf et al., 2020), whereas MG-RAST and MetaPhlAn2 (Cassman et al., 2018; Dey and Ganguly, 2022) enable phylogenetic and functional analysis of metagenomic data. Metatranscriptomic analysis tools such as HUMAnN2, MetaTrans, and SAMSA (Liu et al., 2021) provide pathways for gene expression analysis and comprehensive pipelines for metatranscriptomic data analysis. Starr et al. (2018) introduced the Galaxy Integrated Omics (GIO) platform, aiming to streamline proteomics protein identification through genome/transcriptome-informed approaches. Galaxy-P (Galaxy for Proteomics) facilitates the integrative analysis of proteomic data alongside genomic or transcriptomic data. Its application in metaproteomics offers a comprehensive solution, encompassing database generation from sequencing data, iterative database searches, and subsequent taxonomic and functional analyses using external tools, such as Unipept and MEGAN (Shah et al., 2023). These web-based tools serve as invaluable resources for researchers to decipher complex biological datasets and to advance our understanding of omics-driven research across various disciplines.
5 Omics advancement and applications
Genomic, transcriptomic, proteomic, and metabolomic technologies have transformed agriculture, medicine, food science, and life science. New insights from plant-microbe interaction omics illuminate the complex interactions between plants and microbes and their effects on crop productivity, sustainability, and environmental resilience (Sindelar, 2024). These advances have revealed a rich tapestry of plant-associated microbial species and their roles in nutrient cycling, disease control, stress tolerance, and plant-microbe signaling networks. The omics data revealed microbial traits that boosted plant growth, stress resistance, and nutrient uptake (Figure 4). Using these insights, omics-driven strategies have been developed to exploit beneficial plant-microbe symbioses, such as biofertilisation, pest management, and plant health and productivity. These interventions aim to improve soil fertility, chemical dependence, and agricultural sustainability (Ramlal et al., 2023). Precision agriculture uses plant-microbe interaction omics to optimize crop performance in diverse environments by fine-tuning microbial communities. Integrated with advanced computational tools, omics data enables predictive modeling, microbial consortia design, and agricultural management, boosting productivity and resilience (Shoaib et al., 2023).
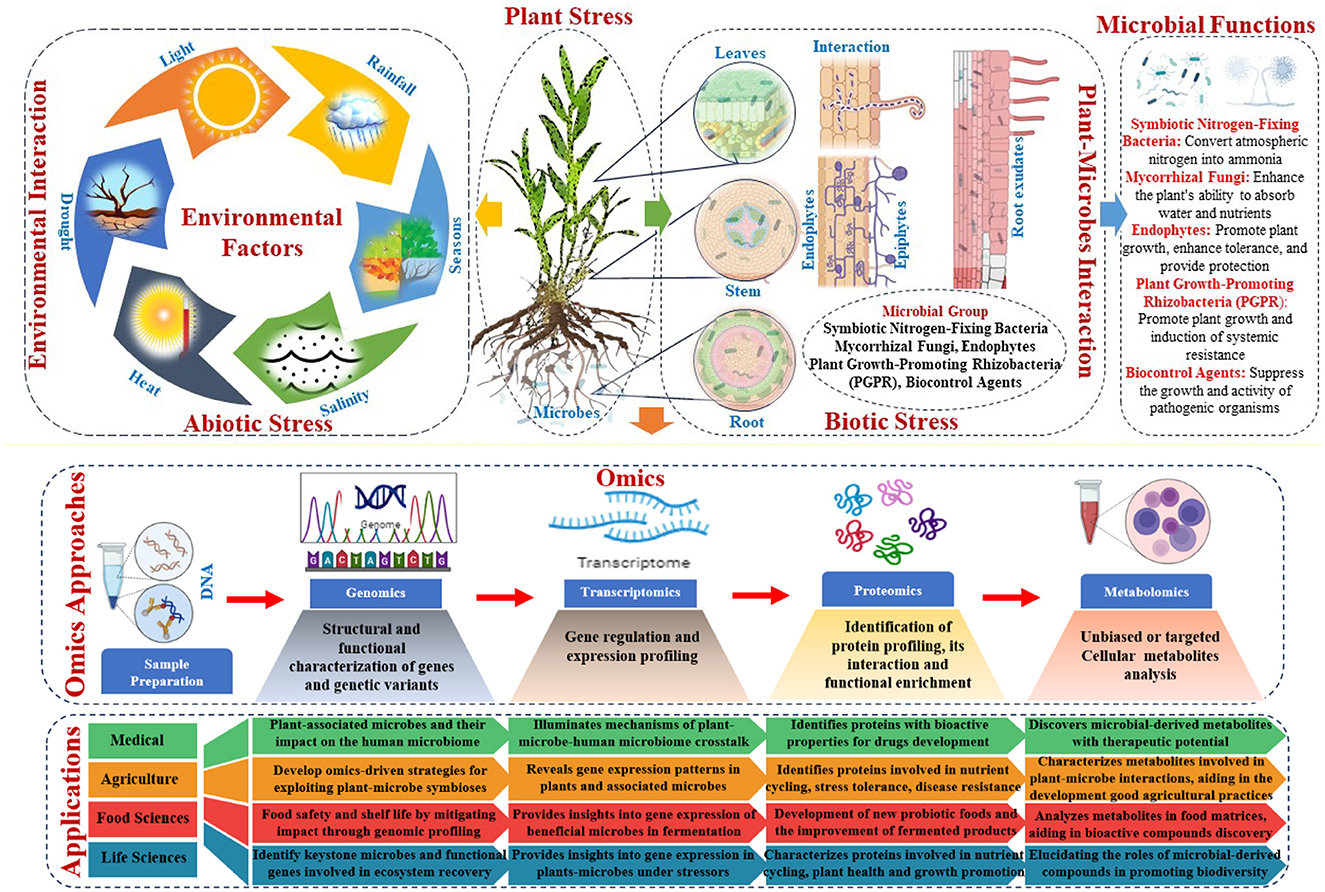
Figure 4. Omics approaches to understand host factors, microbial trait interactions, and their applications.
Studies have shown that plant-associated microbes such as those present in the diet may affect the human microbiome, immune function, metabolism, and disease susceptibility. Omics-based approaches can illuminate the molecular mechanisms of plant-associated microbe-human microbiome crosstalk (Bashiardes et al., 2016). Researchers have identified microbial-derived metabolites, proteins, and signaling molecules that modulate host physiology and immune responses by characterizing the genomic, transcriptomic, proteomic, and metabolomic profiles of plants and human-associated microbes (Saravanakumar et al., 2022). Additionally, plant-microbe interaction omics may offer novel therapeutic approaches for human health. Plant-derived antimicrobial, anti-inflammatory, or immunomodulatory compounds can be used to develop drugs or dietary supplements for microbiome diseases or dysfunction (Mitropoulou et al., 2023). Some plant-associated microbes produce bioactive metabolites that kill human pathogens and promote the growth of commensal bacteria (Kandasamy and Kathirvel, 2023). Omics-based screening can identify these microbial-derived compounds and reveal their mechanisms of action, enabling the development of new antimicrobials or probiotics for infectious diseases, inflammatory disorders, and metabolic syndromes (Garia et al., 2024). Plant-microbe interaction omics can also help personalize medicine by revealing inter-individual microbial community variability and dietary component interactions (Wright et al., 2023; Speckmann et al., 2024).
Understanding and controlling foodborne pathogens and spoilage microorganisms are important. Researchers can identify potential pathogens and spoilage organisms, predict their behavior under different conditions, and develop strategies to mitigate their impact on food safety and shelf life by analyzing the genomic and proteomic profiles of microbial communities in food matrices. Using omics approaches, beneficial microbes used in fermentation processes, such as yogurt, cheese, and kimchi, can be studied to optimize fermentation conditions and develop new probiotic foods with improved nutritional and health benefits. In addition, plant-microbe interaction omics helps to create environmentally friendly food production methods (Joshi et al., 2023; Kumari et al., 2023). By understanding the role of plant-associated microbes in nutrient cycling, soil health, and plant growth promotion, researchers can identify strains that can be used as biofertilizers, biocontrol agents against plant pathogens, and biostimulants to boost crop productivity and resilience to environmental stressors. Microbial alternatives to conventional agrochemicals can reduce pollution and promote eco-friendly agriculture (Hussain et al., 2023; Rai et al., 2023).
Ecological restoration and conservation biology are increasingly being applied in the life sciences. Plant-microbe interaction omics allows researchers to study how habitat degradation, climate change, and pollution affect plant-microbe interactions and ecosystem resilience (Ge et al., 2023; Nadarajah and Abdul Rahman, 2023). Scientists can develop targeted restoration strategies to promote beneficial plant-microbe associations, ecosystem stability, and biodiversity by identifying keystone microbial species and functional genes involved in ecosystem recovery (Jansson et al., 2023; Timmusk et al., 2023). Plant-microbe interaction omics also helps us understand plant and microbial evolution and adaptation (Sa, 2024). Researchers can determine the genetics of trait variation, speciation events, and host-microbe co-evolutionary dynamics by comparing plant and microbe genomic and transcriptomic data across environments and evolutionary time scales (Kwak and Hansen, 2023; Mandal et al., 2023). These findings illuminate the evolutionary forces that shape biodiversity patterns and species interactions, informing conservation and ecosystem management strategies in the face of global environmental change. Omics technologies continue to innovate and discover diverse fields, solve complex problems, and reshape our understanding of biological systems and their applications.
6 Challenges, limitation, and future perspectives
Intensive agricultural practices on limited land with reduced fertilizer and agrochemical inputs present a significant global challenge. Plants such as humans interact with a diverse range of microorganisms that can have both beneficial and harmful effects. Despite ongoing advancements in omics-based approaches, experimental and computational validation procedures lack standardized protocols (Diwan et al., 2022). Discovering complex microbial communities in diverse environments remains a significant challenge, compounded by the fact that only a small fraction of microbes has been thoroughly characterized to date. The primary obstacle lies in the selection of the most suitable technology and methods to effectively address specific problems. Additionally, limitations in available databases present another hurdle that must be overcome to advance microbial research comprehensively (Kumar et al., 2023). Despite these advancements, the development of analytical methods to integrate multiple datasets remains a challenge in the field. To address this, there is a growing need for more multi-omics studies that incorporate classical approaches, such as metabolomics, transcriptomics, proteomics, and metagenomics, and embrace new and emerging techniques, such as genomics, epigenomics, and lipidomics (Kimotho and Maina, 2024).
The complexity of these interactions, encompassing various signaling molecules, pathways, and regulatory networks, makes deciphering their dynamics and outcomes challenging. Additionally, elucidating the precise mechanisms and kinetics of molecular signal recognition, transduction, and response in different plant-microbe systems requires sophisticated experimental approaches and analytical techniques. Furthermore, the diversity of microbial species and their adaptation strategies add another layer of complexity, necessitating comprehensive studies across diverse microbial taxa and ecological niches. Integrating multi-omics data from genomics, transcriptomics, proteomics, and metabolomics poses computational and analytical challenges, including data integration, standardization, and interpretation. Metabolomics, transcriptomics, proteomics, and metagenomics are invaluable tools for deciphering the complexities of plant-microbe interactions, but they pose distinct challenges. Metabolomics applies to the analytical complexity of diverse metabolites, identification hurdles, and dynamic nature of metabolite levels. Transcriptomics faces issues with RNA stability, the complexity of regulatory networks, and the need for robust bioinformatics tools. Proteomics encounters challenges in dealing with the complexity of the proteome, sensitivity in detecting low-abundance proteins, and in ensuring quantitative accuracy. Metagenomics struggles with data integration, sample contamination, and functional annotation.
Developing modern web-based omics tools for studying plant-microbe interactions presents several challenges. First, ensuring the integration and compatibility of diverse omics data types, including genomics, transcriptomics, proteomics, metabolomics, and metagenomics, within a single platform is crucial. This requires addressing issues related to data standardization, normalization, and interoperability across omics datasets. Second, incorporating advanced analytical algorithms and computational methods to handle the complexity and volume of omics data while maintaining scalability and efficiency poses significant technical hurdles. Additionally, ensuring user-friendly interfaces and intuitive designs to cater to users with varying levels of computational expertise are essential for the widespread adoption and usability of these tools. Moreover, ensuring data privacy, security, and compliance with ethical standards while facilitating data sharing and collaboration among researchers remains a challenge. Addressing these challenges requires interdisciplinary collaboration among bioinformaticians, biologists, and web developers, along with continuous updates and improvements to keep pace with advancements in omics technology and research.
While omics techniques have revolutionized our understanding of plant-microbe interactions, they are not without limitations. One significant challenge is the complexity and variability of biological systems, which can lead to issues such as ambiguity in data interpretation and difficulty distinguishing causative factors from correlations. In addition, omics approaches often generate vast amounts of data, necessitating sophisticated computational tools and expertise in analysis and interpretation. Moreover, the dynamic nature of plant-microbe interactions across different environmental conditions and developmental stages poses challenges in capturing the full spectrum of interactions. Another limitation is the dependency of omics techniques on high-quality reference genomes and databases, which may be lacking for non-model organisms or poorly characterized microbial taxa. Furthermore, although omics techniques provide insights into molecular mechanisms, they may not fully capture the spatial and temporal dynamics of the interactions occurring in complex ecosystems. Ethical considerations related to data sharing, privacy, and potential misuse also need to be addressed to ensure the responsible application of omics technologies in plant-microbe interaction studies. Despite these limitations, continued advancements in omics methodologies and interdisciplinary collaborations hold promise for overcoming these challenges and further enhancing our understanding of plant-microbe interactions for sustainable agriculture and environmental stewardship.
Future perspectives for omics approaches in understanding the benefits of plant-microbe interactions hold immense promise for unraveling the intricate dynamics of these relationships. Utilizing genomics, transcriptomics, proteomics, metabolomics, metagenomics, and other omics tools offers opportunities to delve deeper into the molecular mechanisms underlying various interactions, from mutualistic symbiosis to pathogenicity. By integrating multi-omics data and leveraging modern web-based tools, researchers can gain comprehensive insights into the genetic, transcriptional, proteomic, and metabolic landscapes of plant-microbe associations. This holistic understanding will not only advance fundamental knowledge but also pave the way for innovative agricultural practices, including the development of tailored microbial inoculants for sustainable crop production, identification of novel biocontrol strategies, and enhancement of plant resilience to biotic and abiotic stresses. Moreover, elucidating microbial-mediated molecular mechanisms, such as phytohormone modulation, effector secretion, and nutrient acquisition, holds promise for engineering beneficial plant-microbe interactions to improve crop productivity and environmental sustainability. As omics technologies continue to evolve and become more accessible, their integration with traditional approaches will undoubtedly revolutionize our understanding of plant-microbe interactions and shape the future of agriculture and ecosystem management.
7 Conclusion
In conclusion, the integration of omics technologies, including genomics, transcriptomics, proteomics, and metabolomics, represents a revolutionary leap in unraveling the complexities of plant-microbe interactions. By navigating the intricate factors that shape these associations, this review highlights the indispensable role of omics approaches in elucidating molecular nuances. From genomic insights into genetic variations in the metagenomic revelation of microbial communities and functions, each omics facet adds depth to our understanding. Transcriptomics unveils gene expression dynamics, whereas proteomics and metabolomics have shed light on protein functions and metabolic landscapes. Despite persistent challenges, such as standardization and data integration, omics technologies hold immense promise for optimizing agricultural strategies and fortifying resilient plant-microbe alliances. Future research should prioritize enhancing omics technology resolution and throughput, addressing challenges in data integration, and fostering interdisciplinary collaborations. Moreover, exploring the dynamics of plant-microbe interactions under diverse environmental conditions and elucidating the role of epigenomics will be pivotal. Overall, continued advancements in omics technologies offer exciting opportunities to deepen our understanding and harness the benefits of interactions for sustainable agriculture, environmental preservation, and plant health.
Author contributions
AJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Visualization, Writing—original draft, Writing—review & editing. SS: Data curation, Formal analysis, Funding acquisition, Investigation, Visualization, Writing—original draft, Writing—review & editing. QG: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing—review & editing. QW: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing—review & editing. JS: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—review & editing. RS: Writing—review & editing, Resources, Visualization, Software.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Guizhou Science and Technology Corporation Platform Talents Fund [Grant Nos.: [2017]5733-001 and CK-1130-002] and the National Natural Science Foundation of China (U1812403 and 82373981).
Acknowledgments
The authors express their gratitude for the financial support received through the Distinguished High-Level Talents Research Grant from the Guizhou Science and Technology Corporation Platform Talents Fund [Grant Nos.: [2017]5733-001 and CK-1130-002], the National Natural Science Foundation of China (U1812403 and 82373981), and the support provided by Zunyi Medical University, China. Special appreciation was extended to all laboratory colleagues and research staff members for their valuable insights, constructive guidance, and assistance throughout this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adams, A. K., Landry, D., Sykes, V. R., Rickman, T., Cham, A., Timling, A., et al. (2024). Maize Kernel-associated metagenomes reveal potential microbe-microbe interactions that underlie fusarium ear rot disease. Phytobiomes J. 23:74. doi: 10.1094/PBIOMES-07-23-0074-R
Ahlawat, O. P., Yadav, D., Walia, N., Kashyap, P. L., Sharma, P., Tiwari, R., et al. (2024). Root exudates and their significance in abiotic stress amelioration in plants: a review. J. Plant Growth Regul. 24:7. doi: 10.1007/s00344-024-11237-7
Ahmed, S., Khan, M. S. S., Xue, S., Islam, F., Ikram, A. U., Abdullah, M., et al. (2024). A comprehensive overview of omics-based approaches to enhance biotic and abiotic stress tolerance in sweet potato. Hortic Res. 11:uhae014. doi: 10.1093/hr/uhae014
Ajijah, N., Fiodor, A., Pandey, A. K., Rana, A., and Pranaw, K. (2023). Plant Growth-Promoting Bacteria (PGPB) with biofilm-forming ability: a multifaceted agent for sustainable agriculture. Diversity 15:112. doi: 10.3390/d15010112
Ali, M. H., Mandal, S., Ghorai, M., Lal, M. K., Tiwari, R. K., Kumar, M., et al. (2023). “Chapter 6 - Perspectives of omics and plant microbiome,” in Plant Biology, Sustainability and Climate Change, Genomics, Transcriptomics, Proteomics and Metabolomics of Crop Plants, eds. A. Husen and A. Ahmad (Academic Press), 131–144. doi: 10.1016/B978-0-323-95989-6.00014-0
Alshareef, S. A. (2024). Metabolic analysis of the CAZy class glycosyltransferases in rhizospheric soil fungiome of the plant species Moringa oleifera. Saudi J. Biol. Sci. 31:103956. doi: 10.1016/j.sjbs.2024.103956
Anderson, J. C. (2023). Ill communication: host metabolites as virulence-regulating signals for plant-pathogenic bacteria. Annu. Rev. Phytopathol. 61, 49–71. doi: 10.1146/annurev-phyto-021621-114026
Assis, A., Oliveira, E., Donate, P., Giuliatti, S., Nguyen, C., and Passos, G. (2014). “What is the transcriptome and how it is evaluated?,” in Transcriptomics in Health and Disease, ed. G. Passos (Cham: Springer), 3–48. doi: 10.1007/978-3-319-11985-4_1
Barka, G. D., Castro, I. S. L., Alves, D. R., de Almeida, D. P., and Caixeta, E. T. (2023). “Chapter 4 - The role of receptor-like kinases in fungal/microbial resistance in plants,” in Plant Receptor-Like Kinases, eds. S. K. Upadhyay and Shumayla (Academic Press), 63–85. doi: 10.1016/B978-0-323-90594-7.00019-3
Bashiardes, S., Zilberman-Schapira, G., and Elinav, E. (2016). Use of metatranscriptomics in microbiome research. Bioinform. Biol. Insights 10:BBI.S34610. doi: 10.4137/BBI.S34610
Bhargava, P., Khan, M., Verma, A., Singh, A., Singh, S., Vats, S., et al. (2019). “Metagenomics as a tool to explore new insights from plant-microbe interface,” in Plant Microbe Interface, eds. A. Varma, S. Tripathi, and R. Prasad (Cham: Springer), 271–289. doi: 10.1007/978-3-030-19831-2_12
Bhattacharyya, A., Mavrodi, O., Bhowmik, N., Weller, D., Thomashow, L., Mavrodi, D., et al. (2023). Bacterial biofilms as an essential component of rhizosphere plant-microbe interactions. Methods Microbiol. 53, 3–48. doi: 10.1016/bs.mim.2023.05.006
Boutsika, A., Michailidis, M., Ganopoulou, M., Dalakouras, A., Skodra, C., Xanthopoulou, A., et al. (2023). A wide foodomics approach coupled with metagenomics elucidates the environmental signature of potatoes. iScience 26:105917. doi: 10.1016/j.isci.2022.105917
Carezzano, M. E., Paletti Rovey, M. F., Cappellari, L. d. R., Gallarato, L. A., Bogino, P., Oliva, M. d. l. M., et al. (2023). Biofilm-forming ability of phytopathogenic bacteria: a review of its involvement in plant stress. Plants 12:2207. doi: 10.3390/plants12112207
Carper, D. L., Appidi, M. R., Mudbhari, S., Shrestha, H. K., Hettich, R. L., Abraham, P. E., et al. (2022). The promises, challenges, and opportunities of omics for studying the plant holobiont. Microorganisms 10:2013. doi: 10.3390/microorganisms10102013
Cassman, N. A., Lourenço, K. S., do Carmo, J. B., Cantarella, H., and Kuramae, E. E. (2018). Genome-resolved metagenomics of sugarcane vinasse bacteria. Biotechnol. Biofuels 11:48. doi: 10.1186/s13068-018-1036-9
Chalupowicz, L., Mordukhovich, G., Assoline, N., Katsir, L., Sela, N., Bahar, O., et al. (2023). Bacterial outer membrane vesicles induce a transcriptional shift in arabidopsis towards immune system activation leading to suppression of pathogen growth in planta. J. Extracell. Vesicles 12:12285. doi: 10.1002/jev2.12285
Chandok, I. K., Afreen, H., Afreen, R., Haider, S., Moharana, D. P., Hussain, T., et al. (2022). “Chapter 9 - Functional genomics tools for studying microbe-mediated stress tolerance in plants,” in Mitigation of Plant Abiotic Stress by Microorganisms, eds. G. Santoyo, A. Kumar, M. Aamir, and S. Uthandi (Academic Press), 175–204. doi: 10.1016/B978-0-323-90568-8.00009-2
Chao, H., Zhang, S., Hu, Y., Ni, Q., Xin, S., Zhao, L., et al. (2024). Integrating omics databases for enhanced crop breeding. J. Integr. Bioinform. 20:12. doi: 10.1515/jib-2023-0012
Chen, J. Y., Sang, H., Chilvers, M. I., Wu, C. H., and Chang, H. X. (2024). Characterization of soybean chitinase genes induced by rhizobacteria involved in the defense against Fusarium oxysporum. Front. Plant Sci. 15:1341181. doi: 10.3389/fpls.2024.1341181
Chen, X. L., Sun, M. C., Chong, S. L., Si, J. P., and Wu, L. S. (2022). Transcriptomic and metabolomic approaches deepen our knowledge of plant-endophyte interactions. Front. Plant Sci. 12:700200. doi: 10.3389/fpls.2021.700200
Chiquito-Contreras, C. J., Meza-Menchaca, T., Guzmán-López, O., Vásquez, E. C., and Ricaño-Rodríguez, J. (2024). Molecular insights into plant-microbe interactions: a comprehensive review of key mechanisms. Front. Biosci. Elite 16:9. doi: 10.31083/j.fbe1601009
Chouchani, E. T. (2022). Logic and mechanisms of metabolite signalling. Nat. Rev. Endocrinol. 18, 71–72. doi: 10.1038/s41574-021-00618-7
Contreras-Cornejo, H. A., Schmoll, M., Esquivel-Ayala, B. A., González-Esquivel, C. E., Rocha-Ramírez, V., Larsen, J., et al. (2024). Mechanisms for plant growth promotion activated by Trichoderma in natural and managed terrestrial ecosystems. Microbiol. Res. 281:127621. doi: 10.1016/j.micres.2024.127621
Crandall, S. G., Gold, K. M., Jiménez-Gasco, M. d. M., Filgueiras, C. C., and Willett, D. S. (2020). A multi-omics approach to solving problems in plant disease ecology. PLoS ONE 15:e0237975. doi: 10.1371/journal.pone.0237975
da Silva, L. I., Pereira, M. C., de Carvalho, A. M. X., Buttrós, V. H., Pasqual, M., and Dória, J. (2023). Phosphorus-solubilizing microorganisms: a key to sustainable agriculture. Agriculture 13:462. doi: 10.3390/agriculture13020462
Datta, R. (2024). Enzymatic degradation of cellulose in soil: a review. Heliyon 10:e24022. doi: 10.1016/j.heliyon.2024.e24022
De Palma, M., Scotti, R., D'Agostino, N., Zaccardelli, M., and Tucci, M. (2022). Phyto-friendly soil bacteria and fungi provide beneficial outcomes in the host plant by differently modulating its responses through (in)direct mechanisms. Plants 11:2672. doi: 10.3390/plants11202672
De Ryck, J., Van Damme, P., and Goormachtig, S. (2023). From prediction to function: current practices and challenges towards the functional characterization of type III effectors. Front. Microbiol. 14:1113442. doi: 10.3389/fmicb.2023.1113442
De Sousa, B. F. S., Domingo-Serrano, L., Salinero-Lanzarote, A., Palacios, J. M., and Rey, L. (2023). The T6SS-dependent effector Re78 of Rhizobium etli Mim1 benefits bacterial competition. Biology 12:678. doi: 10.3390/biology12050678
Demiwal, P., Tayade, S., Yadav, S. R., and Sircar, D. (2024). A metabolomics perspective on root-derived plant immunity and phytohormone interaction. Physiol. Plant. 176:14150. doi: 10.1111/ppl.14150
Dey, K. K., and Ganguly, S. (2022). “Plant-microbe interactions in the age of sequencing,” in Plant-Microbe Interactions: Harnessing Next-Generation Molecular Technologies for Sustainable Agriculture, eds. J. Sahu, A. Vaishnav, and H. B. Singh (CRC Press), 113–127. doi: 10.1201/9781003171416
Dhiman, N., Uthoff, J., Scharf, B., and Kumar, V. (2024). “Plant-microbe interaction to improve soil health,” in Advancements in Microbial Biotechnology for Soil Health. Microorganisms for Sustainability, eds. R. K. Bhatia and A. Walia (Singapore: Springer), 113–127. doi: 10.1007/978-981-99-9482-3_10
Diwan, D., Rashid, M. d. M., and Vaishnav, A. (2022). Current understanding of plant-microbe interaction through the lenses of multi-omics approaches and their benefits in sustainable agriculture. Microbiol. Res. 265:127180. doi: 10.1016/j.micres.2022.127180
Doddavarapu, B., Lata, C., and Shah, J. M. (2024). Epigenetic regulation influenced by soil microbiota and nutrients: paving road to epigenome editing in plants. Biochim. Biophys. Acta 1868:130580. doi: 10.1016/j.bbagen.2024.130580
Dubey, R. K., Tripathi, V., Prabha, R., Chaurasia, R., Singh, D. P., Rao, C. S., et al. (2020). “Metatranscriptomics and metaproteomics for microbial communities profiling,” in Unravelling the Soil Microbiome. Springer Briefs in Environmental Science (Cham: Springer), 51–60. doi: 10.1007/978-3-030-15516-2_5
Dunn, M. F., and Becerra-Rivera, V. A. (2023). The biosynthesis and functions of polyamines in the interaction of plant growth-promoting rhizobacteria with plants. Plants 12:2671. doi: 10.3390/plants12142671
Dutta, P., Mahanta, M., Singh, S. B., Thakuria, D., Deb, L., Kumari, A., et al. (2023). Molecular interaction between plants and Trichoderma species against soil-borne plant pathogens. Front. Plant Sci. 14:1145715. doi: 10.3389/fpls.2023.1145715
Elfaky, M. A. (2024). Unveiling the hidden language of bacteria: anti-quorum sensing strategies for gram-negative bacteria infection control. Arch. Microbiol. 206:124. doi: 10.1007/s00203-024-03900-0
Gamalero, E., Bona, E., and Glick, B. R. (2022). Current techniques to study beneficial plant-microbe interactions. Microorganisms 10:1380. doi: 10.3390/microorganisms10071380
Garia, P., Chaubey, K. K., Rawat, H., Sinha, A., Sharma, S., Goyal, U., et al. (2024). “Microbial metabolites and recent advancement,” in Fourth Congress on Intelligent Systems. CIS 2023, Lecture Notes in Networks and Systems, eds. S. Kumar, K. Balachandran, J. H. Kim, J. C. Bansal (Singapore: Springer), 175–194. doi: 10.1007/978-981-99-9037-5_14
Gasser, M., Keller, J., Fournier, P., Pujic, P., Normand, P., Boubakri, H., et al. (2023). Identification and evolution of nsLTPs in the root nodule nitrogen fixation clade and molecular response of Frankia to AgLTP24. Sci. Rep. 13:16020. doi: 10.1038/s41598-023-41117-1
Ge, J., Li, D., Ding, J., Xiao, X., and Liang, Y. (2023). Microbial coexistence in the rhizosphere and the promotion of plant stress resistance: a review. Environ. Res. 222:115298. doi: 10.1016/j.envres.2023.115298
Ghosh, A., Mehta, A., and Khan, A. M. (2019). “Metagenomic analysis and its applications,” in Encyclopedia of Bioinformatics and Computational Biology, eds. S. Ranganathan, M. Gribskov, K. Nakai, and C. Schönbach (Academic Press), 184–193. doi: 10.1016/B978-0-12-809633-8.20178-7
Gómez-Godínez, L. J., Aguirre-Noyola, J. L., Martínez-Romero, E., Arteaga-Garibay, R. I., Ireta-Moreno, J., Ruvalcaba-Gómez, J. M. A., et al. (2023). Look at plant-growth-promoting bacteria. Plants 12:1668. doi: 10.3390/plants12081668
Goyal, R. K., and Habtewold, J. Z. (2023). Evaluation of legume-rhizobial symbiotic interactions beyond nitrogen fixation that help the host survival and diversification in hostile environments. Microorganisms 11:1454. doi: 10.3390/microorganisms11061454
Grundy, E. B., Gresshoff, P. M., Su, H., and Ferguson, B. J. (2023). Legumes regulate symbiosis with rhizobia via their innate immune system. Int. J. Mol. Sci. 24:2800. doi: 10.3390/ijms24032800
Gupta, G., Chauhan, P. S., Jha, P. N., Verma, R. K., Singh, S., Yadav, V. K., et al. (2024). Secretory molecules from secretion systems fine-tune the host-beneficial bacteria (PGPRs) interaction. Front. Microbiol. 15:1355750. doi: 10.3389/fmicb.2024.1355750
Gupta, S., Pandey, S., Nandi, S. P., and Singh, M. (2023). Modulation of ethylene and ROS-scavenging enzymes by multifarious plant growth-promoting endophytes in tomato (Solanum lycopersicum) plants to combat Xanthomonas -induced stress. Plant Physiol. Biochem. 202:107982. doi: 10.1016/j.plaphy.2023.107982
Han, S., Na, L., Rongchao, Z., Xiuqin, H., Wenyu, Z., Bo, Z., et al. (2023). Study on signal transmission mechanism of arbuscular mycorrhizal hyphal network against root rot of Salvia miltiorrhiza. Sci. Rep. 13:16936. doi: 10.1038/s41598-023-43278-5
Hupfauf, S., Etemadi, M., Fernández-Delgado Juárez, M., Gómez-Brandón, M., Insam, H., Podmirseg, S. M., et al. (2020). CoMA—an intuitive and user-friendly pipeline for amplicon-sequencing data analysis. PLoS ONE 15:e0243241. doi: 10.1371/journal.pone.0243241
Hussain, M., Zahra, N., Lang, T., Zain, M., Raza, M., Shakoor, N., et al. (2023). Integrating nanotechnology with plant microbiome for next-generation crop health. Plant Physiol. Biochem. 196, 703–711. doi: 10.1016/j.plaphy.2023.02.022
Idris Usman, N., and Muazu Wali, M. (2024). “Nitrogen fixation by rhizobacterial nif mechanism: an advanced genetic perspective,” in Updates on Rhizobacteria, ed. M. Gull (IntechOpen), 113. doi: 10.5772/intechopen.1004087
Ilangumaran, G., Subramanian, S., and Smith, D. L. (2024). Complete genome sequences of Rhizobium sp. strain SL42 and Hydrogenophaga sp. strain SL48, microsymbionts of Amphicarpaea bracteata. Front. Microb. 3:1309947. doi: 10.3389/frmbi.2024.1309947
Jain, A., Singh, H. B., and Das, S. (2021). Deciphering plant-microbe crosstalk through proteomics studies. Microbiol. Res. 242:126590. doi: 10.1016/j.micres.2020.126590
Jalal, A., Júnior, E. F., and Teixeira Filho, M. C. M. (2024). Interaction of zinc mineral nutrition and plant growth-promoting bacteria in tropical agricultural systems: a review. Plants 13:571. doi: 10.3390/plants13050571
Jansson, J. K., McClure, R., and Egbert, R. G. (2023). Soil microbiome engineering for sustainability in a changing environment. Nat. Biotechnol. 41, 1716–1728. doi: 10.1038/s41587-023-01932-3
Jeon, J., Kim, K. T., Choi, J., Cheong, K., Ko, J., Choi, G., et al. (2022). Alternative splicing diversifies the transcriptome and proteome of the rice blast fungus during host infection. RNA Biol. 19, 373–386. doi: 10.1080/15476286.2022.2043040
Jiang, C., Li, Z., Zheng, L., Yu, Y., and Niu, D. (2023). Small RNAs: efficient and miraculous effectors that play key roles in plant-microbe interactions. Mol. Plant Pathol. 24, 999–1013. doi: 10.1111/mpp.13329
Jibrin, M. O., Liu, Q., Guingab-Cagmat, J., Jones, J. B., Garrett, T. J., Zhang, S., et al. (2021). Metabolomics insights into chemical convergence in Xanthomonas perforans and metabolic changes following treatment with the small molecule carvacrol. Metabolites 11:879. doi: 10.3390/metabo11120879
Joshi, N., Ruparelia, J. A., Saraf, M., and Jha, C.K. (2023). “Techniques to study plant-microbe interactions that lead to efficient sustainable agriculture,” in Plant Microbiome for Plant Productivity and Sustainable Agriculture, eds. S. Chhabra, R. Prasad, N. R. Maddela, and N. Tuteja (Singapore: Springer), 401–421. doi: 10.1007/978-981-19-5029-2_17
Joubert, P. M., and Krasileva, K. V. (2024). Distinct genomic contexts predict gene presence-absence variation in different pathotypes of Magnaporthe oryzae. Genetics 2024:iyae012. doi: 10.1093/genetics/iyae012
Kalita, P., Mohapatra, B., and Maruthi, M. (2024). “Role of effectors in plant-pathogen interactions,” in Biotechnological Advances for Disease Tolerance in Plants, eds. K. Singh, R. Kaur, and R. Deshmukh (Singapore: Springer), 363–376. doi: 10.1007/978-981-99-8874-7_15
Kandasamy, G. D., and Kathirvel, P. (2023). Insights into bacterial endophytic diversity and isolation with a focus on their potential applications—a review. Microbiol. Res. 266:127256. doi: 10.1016/j.micres.2022.127256
Kaya, C. (2024). Microbial modulation of hormone signaling, proteomic dynamics, and metabolomics in plant drought adaptation. Food Energy Secur. 13:513. doi: 10.1002/fes3.513
Khan, N., Bano, A., and Babar, M. A. (2019). Metabolic and physiological changes induced by plant growth regulators and plant growth promoting rhizobacteria and their impact on drought tolerance in Cicer arietinum L. PLoS ONE 14:e0213040. doi: 10.1371/journal.pone.0213040
Khatabi, B., Gharechahi, J., Ghaffari, M. R., Liu, D., Haynes, P. A., McKay, M. J., et al. (2019). Plant-microbe symbiosis: what has proteomics taught us? Proteomics 19:201800105. doi: 10.1002/pmic.201800105
Khoshru, B., Mitra, D., Joshi, K., Adhikari, P., Rion, M. S. I., Fadiji, A. E., et al. (2023). Decrypting the multi-functional biological activators and inducers of defense responses against biotic stresses in plants. Heliyon 9:e13825. doi: 10.1016/j.heliyon.2023.e13825
Kimotho, R. N., and Maina, S. (2024). Unraveling plant-microbe interactions: can integrated omics approaches offer concrete answers? J. Exp. Bot. 75, 1289–1313. doi: 10.1093/jxb/erad448
Koshila Ravi, R., and Muthukumar, T. (2024). “Root exudates and their importance in arbuscular mycorrhizal symbiosis and nutrients navigation from inaccessible soil: an efficient mediator of mineral acquisition in nutrient deprived soil,” in Mycorrhizal Symbiosis and Agroecosystem Restoration, eds. R. A. Ansari, R. Rizvi, and I. Mahmood (Singapore: Springer), 101–123. doi: 10.1007/978-981-99-5030-0_5
Kumar, G. C., Chaudhary, J., Meena, L. K., Meena, A. L., and Kumar, A. (2021). “Function-driven microbial genomics for ecofriendly agriculture,” in Microbes in Land Use Change Management, eds. J. S. Singh, S. Tiwari, C. Singh, and A. K. Singh (Elsevier), 389–431. doi: 10.1016/B978-0-12-824448-7.00021-8
Kumar, U., Raj, S., Sreenikethanam, A., Maddheshiya, R., Kumari, S., Han, S., et al. (2023). Multi-omics approaches in plant-microbe interactions hold enormous promise for sustainable agriculture. Agronomy 13:1804. doi: 10.3390/agronomy13071804
Kumari, A., Kumari, A., Sharma, H., Sharma, P., Bhattacharya, S., Mishra, T., et al. (2023). “Modern approaches in studying the role of plant-microbial interactions: a way towards the development of sustainable agriculture,” in New Frontiers in Plant-Environment Interactions. Environmental Science and Engineering, ed. T. Aftab (Cham: Springer), 69–91. doi: 10.1007/978-3-031-43729-8_4
Kumari, N., and Kumawat, K. C. (2024). “Chapter 19 - Microbial ACC-deaminase properties, functions and perspectives in climate stressed agriculture,” in Microbiome Research in Plants and Soil, Microbiome Drivers of Ecosystem Function, eds. J. A. Parray, N. Shameem, and D. Egamberdieva (Academic Press), 431–446. doi: 10.1016/B978-0-443-19121-3.00008-9
Kwak, Y., and Hansen, A. K. (2023). Unveiling metabolic integration in psyllids and their nutritional endosymbionts through comparative transcriptomics analysis. iScience 26:107930. doi: 10.1016/j.isci.2023.107930
Larekeng, S. H., Ngadiman, N., Khairina, Y., Simarmata, R., and Christita, M. (2024). Unraveling the potential of ACC Deaminase-producing microbes in various agricultural stresses: current status, limitations, and recommendations. Pak. J. Bot. 56:34. doi: 10.30848/PJB2024-2(34)
Lee, B., Lee, J. I., Kwon, S. K., Ryu, C. M., and Kim, J. F. A. (2023). Marine bacterium with animal-pathogen-like type III secretion elicits the nonhost hypersensitive response in a land plant. Plant Pathol. J. 39, 584–591. doi: 10.5423/PPJ.FT.09.2023.0125
Liu, Z., Ma, A., Mathé, E., Merling, M., Ma, Q., Liu, B., et al. (2021). Network analyses in microbiome based on high-throughput multi-omics data. Brief Bioinform. 22, 1639–1655. doi: 10.1093/bib/bbaa005
Lucaciu, R., Pelikan, C., Gerner, S. M., Zioutis, C., Köstlbacher, S., Marx, H., et al. (2019). A bioinformatics guide to plant microbiome analysis. Front. Plant Sci. 10:1313. doi: 10.3389/fpls.2019.01313
Mahapatra, R., Mishra, P., and Patel, Z. M. (2023). “Chapter 9 - The molecular architecture of rhizobium-plant symbiosis in nitrogen fixation,” in The Chemical Dialogue Between Plants and Beneficial Microorganisms, eds. V. Sharma, R. Salwan, E. Moliszewska, D. Ruano-Rosa, and M. Jȩdryczka (Academic Press), 137–144. doi: 10.1016/B978-0-323-91734-6.00006-5
Majdura, J., Jankiewicz, U., Gałazka, A., and Orzechowski, S. (2023). The role of quorum sensing molecules in bacterial-plant interactions. Metabolites 13:114. doi: 10.3390/metabo13010114
Mandal, M., Das, S., Roy, A., Rakwal, R., Jones, O. A. H., Popek, R., et al. (2023). Interactive relations between plants, the phyllosphere microbial community, and particulate matter pollution. Sci. Tot. Environ. 890:164352. doi: 10.1016/j.scitotenv.2023.164352
Manickam, S., Rajagopalan, V. R., Kambale, R., Rajasekaran, R., Kanagarajan, S., Muthurajan, R., et al. (2023). Plant metabolomics: current initiatives and future prospects. Curr. Iss. Mol. Biol. 45, 8894–8906. doi: 10.3390/cimb45110558
Manoharan, B., Narayanasamy, S., Joshi, J. B., Jegadeesan, S., Qi, S., Dai, Z., et al. (2023). “Molecular events and defence mechanism against biotic stress induced by bio-priming of beneficial microbes,” in Microbial Biocontrol: Molecular Perspective in Plant Disease Management. Microorganisms for Sustainability, eds. K. K. Bastas, A. Kumar, and U. Sivakumar (Singapore: Springer), 61–87. doi: 10.1007/978-981-99-3947-3_3
Maphosa, S., Moleleki, L. N., and Motaung, T. E. (2023). Bacterial secretion system functions: evidence of interactions and downstream implications. Microbiology 169:1326. doi: 10.1099/mic.0.001326
Masenya, K., Manganyi, M. C., and Dikobe, T. B. (2024). Exploring cereal metagenomics: unravelling microbial communities for improved food security. Microorganisms 12:510. doi: 10.3390/microorganisms12030510
Meena, M., Mehta, T., Nagda, A., Yadav, G., and Sonigra, P. (2023). “Chapter 11 - PGPR-mediated synthesis and alteration of different secondary metabolites during plant-microbe interactions,” in Plant-Microbe Interaction - Recent Advances in Molecular and Biochemical Approaches, eds. P. Swapnil, M. Meena, Harish, A. Marwal, S. Vijayalakshmi, A. Zehra (Academic Press), 229–255. doi: 10.1016/B978-0-323-91875-6.00002-5
Mehta, S., Bernt, M., Chambers, M., Fahrner, M., Föll, M. C., Gruening, B., et al. (2023). A Galaxy of informatics resources for MS-based proteomics. Expert Rev. Proteom. 20, 251–266. doi: 10.1080/14789450.2023.2265062
Mishra, A. K., Sudalaimuthuasari, N., Hazzouri, K. M., Saeed, E. E., Shah, I., Amiri, K. M. A., et al. (2022). Tapping into plant-microbiome interactions through the lens of multi-omics techniques. Cells 11:3254. doi: 10.3390/cells11203254
Mitropoulou, G., Stavropoulou, E., Vaou, N., Tsakris, Z., Voidarou, C., Tsiotsias, A., et al. (2023). Insights into antimicrobial and anti-inflammatory applications of plant bioactive compounds. Microorganisms 11:1156. doi: 10.3390/microorganisms11051156
Mueller, L. O., Borstein, S. R., Tague, E. D., Dearth, S. P., Castro, H. F., Campagna, S. R., et al. (2020). Populations of Populus angustifolia have evolved distinct metabolic profiles that influence their surrounding soil. Plant Soil 448, 399–411. doi: 10.1007/s11104-019-04405-2
Nadarajah, K., and Abdul Rahman, N. S. N. (2023). The microbial connection to sustainable agriculture. Plants 12:2307. doi: 10.3390/plants12122307
Niazi, P., Monib, A. W., Ozturk, H., Mansoor, M., Azizi, A., Hassand, M. H., et al. (2023). Review on surface elements and bacterial biofilms in plant-bacterial associations. J. Res. Appl. Sci. Biotechnol. 2, 204–214. doi: 10.55544/jrasb.2.1.30
Olanrewaju, O. S., Glick, B. R., and Babalola, O. O. (2024). Metabolomics-guided utilization of beneficial microbes for climate-resilient crops. Curr. Opin. Chem. Biol. 79:102427. doi: 10.1016/j.cbpa.2024.102427
Orozco-Mosqueda, M. d. C., Fadiji, A. E., Babalola, O. O., and Santoyo, G. (2023). Bacterial elicitors of the plant immune system: an overview and the way forward. Plant Stress 7:100138. doi: 10.1016/j.stress.2023.100138
Pandey, S., Blache, A., and Achouak, W. (2024). Insights into bacterial extracellular vesicle biogenesis, functions, and implications in plant-microbe interactions. Microorganisms 12:532. doi: 10.3390/microorganisms12030532
Pantigoso, H. A., Manter, D. K., Fonte, S. J., and Vivanco, J. M. (2023). Root exudate-derived compounds stimulate the phosphorus solubilizing ability of bacteria. Sci. Rep. 13:4050. doi: 10.1038/s41598-023-30915-2
Paul, S., Parvez, S. S., Goswami, A., and Banik, A. (2024). Exopolysaccharides from agriculturally important microorganisms: conferring soil nutrient status and plant health. Int. J. Biol. Macromol. 262:129954. doi: 10.1016/j.ijbiomac.2024.129954
Piasecka, A., Kachlicki, P., and Stobiecki, M. (2019). Analytical methods for detection of plant metabolomes changes in response to biotic and abiotic stresses. Int. J. Mol. Sci. 20:379. doi: 10.3390/ijms20020379
Priya, P., Aneesh, B., and Harikrishnan, K. (2021). Genomics as a potential tool to unravel the rhizosphere microbiome interactions on plant health. J. Microbiol. Methods 185:106215. doi: 10.1016/j.mimet.2021.106215
Puranik, S., Bundela, V., Shylla, A., Elakkya, M., Shukla, L., Singh, S. K., et al. (2023). “Chapter 10 - Peeking into plant-microbe interactions during plant defense,” in Plant-Microbe Interaction - Recent Advances in Molecular and Biochemical Approaches, eds. P. Swapnil, M. Meena, Harish, A. Marwal, S. Vijayalakshmi, and A. Zehra (Academic Press), 167–200. doi: 10.1016/B978-0-323-91876-3.00012-9
Rai, S., Omar, A. F., Rehan, M., Al-Turki, A., Sagar, A., Ilyas, N., et al. (2023). Crop microbiome: their role and advances in molecular and omic techniques for the sustenance of agriculture. Planta 257:27. doi: 10.1007/s00425-022-04052-5
Ramlal, A., Rani, A., Nautiyal, A., Kalra, C., Kumari, R., Kumar, J., et al. (2023). Importance of omics approaches in plant-microbe interaction for plant disease control. Physiol. Mol. Plant Pathol. 128:102153. doi: 10.1016/j.pmpp.2023.102153
Rane, N. R., Tapase, S., Kanojia, A., Watharkar, A., Salama, E. S., Jang, M., et al. (2022). Molecular insights into plant-microbe interactions for sustainable remediation of contaminated environment. Bioresour. Technol. 344:126246. doi: 10.1016/j.biortech.2021.126246
Rani, A., Rana, A., Dhaka, R. K., Singh, A. P., Chahar, M., Singh, S., et al. (2023). Bacterial volatile organic compounds as biopesticides, growth promoters and plant-defense elicitors: current understanding and future scope. Biotechnol. Adv. 63:108078. doi: 10.1016/j.biotechadv.2022.108078
Rathnasamy, S. A., Gothandapani, S., Chellamuthu, S., Chakraborty, A., Gurusamy, D., Roy, A., et al. (2023). “Omics technologies unravelling the plant-pathogen interaction and stress response,” in Genomics of Plant-Pathogen Interaction and the Stress Response, eds. A. Mani and S. Kushwaha (CRC Press), 74–110. doi: 10.1201/9781003153481
Ravelo-Ortega, G., Raya-González, J., and López-Bucio, J. (2023). Compounds from rhizosphere microbes that promote plant growth. Curr. Opin. Plant Biol. 73:102336. doi: 10.1016/j.pbi.2023.102336
Regalado, J., Lundberg, D. S., Deusch, O., Kersten, S., Karasov, T., Poersch, K., et al. (2020). Combining whole-genome shotgun sequencing and rRNA gene amplicon analyses to improve detection of microbe-microbe interaction networks in plant leaves. ISME J. 14, 2116–2130. doi: 10.1038/s41396-020-0665-8
Restrepo-Leal, J. D., Belair, M., Fischer, J., Richet, N., Fontaine, F., Rémond, C., et al. (2023). Differential carbohydrate-active enzymes and secondary metabolite production by the grapevine trunk pathogen Neofusicoccum parvum Bt-67 grown on host and non-host biomass. Mycologia 115, 579–601. doi: 10.1080/00275514.2023.2216122
Sa, T. (ed.)., (2024). “Chapter 1 - Plant-microbe interactions for enhanced plant tolerance to stress,” in Beneficial Microbes for Sustainable Agriculture Under Stress Conditions (Academic Press), 1–24. doi: 10.1016/B978-0-443-13193-6.00001-4
Saarenpää, S., Shalev, O., Ashkenazy, H., Carlos, V., Lundberg, D. S., Weigel, D., et al. (2023). Spatial metatranscriptomics resolves host-bacteria-fungi interactomes. Nat. Biotechnol. 23:2. doi: 10.1038/s41587-023-01979-2
Samantara, K., Shiv, A., de Sousa, L. L., Sandhu, K. S., Priyadarshini, P., Mohapatra, S. R., et al. (2021). A comprehensive review on epigenetic mechanisms and application of epigenetic modifications for crop improvement. Environ. Exp. Bot. 188:104479. doi: 10.1016/j.envexpbot.2021.104479
Santra, H. K., and Banerjee, D. (2024). “Chapter 5 - Microbial extracellular polymeric substance: function and role against environmental stress,” in Nanobiotechnology for Plant Protection, Bacterial Secondary Metabolites, eds. K. A. Abd-Elsalam and H. I. Mohamed (Elsevier), 83–106. doi: 10.1016/B978-0-323-95251-4.00018-1
Saravanakumar, K., Santosh, S. S., Ahamed, M. A., Sathiyaseelan, A., Sultan, G., Irfan, N., et al. (2022). Bioinformatics strategies for studying the molecular mechanisms of fungal extracellular vesicles with a focus on infection and immune responses. Brief Bioinform. 23:bbac250. doi: 10.1093/bib/bbac250
Sarim, K. M., Srivastava, R., and Ramteke, P. W. (2020). “Chapter 9 - Next-generation omics technologies for exploring complex metabolic regulation during plant-microbe interaction,” in Microbial Services in Restoration Ecology, eds. J. S. Singh and S. R. Vimal (Elsevier), 123–138. doi: 10.1016/B978-0-12-819978-7.00009-9
Sarsaiya, S., Jain, A., Shu, F., Yang, M., Pu, M., Jia, Q., et al. (2024). Enhancing dendrobine production in Dendrobium nobile through mono-culturing of endophytic fungi, Trichoderma longibrachiatum (MD33) in a temporary immersion bioreactor system. Front. Plant Sci. 15:1302817. doi: 10.3389/fpls.2024.1302817
Sartori, M., Ferrari, E., M'Barek, R., Philippidis, G., Boysen-Urban, K., Borrelli, P., et al. (2024). Remaining loyal to our soil: a prospective integrated assessment of soil erosion on global food security. Ecol. Econ. 219:108103. doi: 10.1016/j.ecolecon.2023.108103
Scaria, S. S., and Ravi, L. (2023). “Chapter 22 - Symbiotic associations of Frankia in actinorhizal plants,” in Developments in Applied Microbiology and Biotechnology, Microbial Symbionts, ed. D. Dharumadurai (Academic Press), 397–416. doi: 10.1016/B978-0-323-99334-0.00002-5
Schiebenhoefer, H., Schallert, K., Renard, B. Y., Trappe, K., Schmid, E., Benndorf, D., et al. (2020). A complete and flexible workflow for metaproteomics data analysis based on MetaProteomeAnalyzer and Prophane. Nat. Protoc. 15, 3212–3239. doi: 10.1038/s41596-020-0368-7
Schweiger, R., Baier, M. C., and Müller, C. (2014). Arbuscular mycorrhiza-induced shifts in foliar metabolism and photosynthesis mirror the developmental stage of the symbiosis and are only partly driven by improved phosphate uptake. Mol. Plant-Microbe Interact. 27, 1403–1412. doi: 10.1094/MPMI-05-14-0126-R
Selwal, N., Wani, A. K., Akhtar, N., Kaur, M., and Jassal, P. S. (2023). Molecular insights of strigolactone biosynthesis, signalling pathways, regulatory roles, and hormonal crosstalks in plant systems. South Afri. J. Bot. 160, 9–22. doi: 10.1016/j.sajb.2023.06.046
Sengupta, K., and Pal, S. (2021). “Rhizospheric plant-microbe interactions releasing antioxidants and phytostimulating compounds in polluted agroecosystems,” in Antioxidants in Plant-Microbe Interaction, eds. H. B. Singh, A. Vaishnav, and R. Sayyed (Singapore: Springer), 157–179. doi: 10.1007/978-981-16-1350-0_8
Shafi, A., Zahoor, I., and Habib, H. (2021). “Omics technologies to unravel plant-microbe interactions,” in Plant-Microbe Dynamics: Recent Advances for Sustainable Agriculture, 1st Edn, eds. T. B. Pirzadah, B. Malik, and K. R. Hakeem (CRC Press), 201–220. doi: 10.1201/9781003106784
Shah, K., Upadhye, V. J., and Shrivastav, A. (2023). New Developments in Techniques Like Metagenomics and Metaproteomics for Isolation, Identification, and Characterization of Microbes from Varied Environment, 487–496.
Shah, W. U. H., Lu, Y., Liu, J., Rehman, A., and Yasmeen, R. (2024). The impact of climate change and production technology heterogeneity on China's agricultural total factor productivity and production efficiency. Sci. Tot. Environ. 907:168027. doi: 10.1016/j.scitotenv.2023.168027
Sharma, A., Choudhary, P., Chakdar, H., and Shukla, P. (2024). Molecular insights and omics-based understanding of plant-microbe interactions under drought stress. World J. Microbiol. Biotechnol. 40:42. doi: 10.1007/s11274-023-03837-4
Shoaib, M., Shah, B., Sayed, N., Ali, F., Ullah, R., Hussain, I., et al. (2023). Deep learning for plant bioinformatics: an explainable gradient-based approach for disease detection. Front. Plant Sci. 14:1283235. doi: 10.3389/fpls.2023.1283235
Shumilina, J., Soboleva, A., Abakumov, E., Shtark, O. Y., Zhukov, V. A., Frolov, A., et al. (2023). Signaling in legume-rhizobia symbiosis. Int. J. Mol. Sci. 24:17397. doi: 10.3390/ijms242417397
Sindelar, R. D. (2024). “Genomics, other “OMIC” technologies, precision medicine, and additional biotechnology-related techniques,” in Pharmaceutical Biotechnology, eds. D. J. A. Crommelin, R. D. Sindelar, and B. Meibohm (Cham: Springer International Publishing), 179–221. doi: 10.1007/978-1-4614-6486-0_8
Singh, G., Agrawal, H., and Bednarek, P. (2023). Specialized metabolites as versatile tools in shaping plant-microbe associations. Mol. Plant 16, 122–144. doi: 10.1016/j.molp.2022.12.006
Speckmann, B., Ehring, E., Hu, J., and Rodriguez Mateos, A. (2024). Exploring substrate-microbe interactions: a metabiotic approach toward developing targeted synbiotic compositions. Gut Microbes 16:2305167. doi: 10.1080/19490976.2024.2305716
Starr, A. E., Deeke, S. A., Li, L., Zhang, X., Daoud, R., Ryan, J., et al. (2018). Proteomic and metaproteomic approaches to understand host-microbe interactions. Anal. Chem. 90, 86–109. doi: 10.1021/acs.analchem.7b04340
Su, G., Yu, C., Liang, S., Wang, W., and Wang, H. (2024). Multi-omics in food safety and authenticity in terms of food components. Food Chem. 437:137943. doi: 10.1016/j.foodchem.2023.137943
Swiatczak, J., Kalwasińska, A., and Brzezinska, M. S. (2024). Plant growth-promoting rhizobacteria: Peribacillus frigoritolerans 2RO30 and Pseudomonas sivasensis 2RO45 for their effect on canola growth under controlled as well as natural conditions. Front. Plant Sci. 14:1233237. doi: 10.3389/fpls.2023.1233237
Timmusk, S., Pall, T., Raz, S., Fetsiukh, A., and Nevo, E. (2023). The potential for plant growth-promoting bacteria to impact crop productivity in future agricultural systems is linked to understanding the principles of microbial ecology. Front. Microbiol. 14:1141862. doi: 10.3389/fmicb.2023.1141862
Tiwari, P., Bose, S. K., Park, K. I., Dufossé, L., and Fouillaud, M. (2024). Plant-microbe interactions under the extreme habitats and their potential applications. Microorganisms 12:448. doi: 10.3390/microorganisms12030448
Wahab, A., Muhammad, M., Munir, A., Abdi, G., Zaman, W., Ayaz, A., et al. (2023). Role of arbuscular mycorrhizal fungi in regulating growth, enhancing productivity, and potentially influencing ecosystems under abiotic and biotic stresses. Plants 12:3102. doi: 10.3390/plants12173102
Wangthaisong, P., Piromyou, P., Songwattana, P., Wongdee, J., Teamtaisong, K., Tittabutr, P., et al. (2023). The type IV secretion system (T4SS) mediates symbiosis between Bradyrhizobium sp. SUTN9-2 and legumes. Appl. Environ. Microbiol. 89:23. doi: 10.1128/aem.00040-23
Weidemüller, P., Kholmatov, M., Petsalaki, E., and Zaugg, J. B. (2021). Transcription factors: bridge between cell signaling and gene regulation. Proteomics 21, 23–24. doi: 10.1002/pmic.202000034
Weidenhamer, J. D., Cipollini, D., Morris, K., Gurusinghe, S., and Weston, L. A. (2023). Ecological realism and rigor in the study of plant-plant allelopathic interactions. Plant Soil 489, 1–39. doi: 10.1007/s11104-023-06022-6
Wright, A. T., Hudson, L. A., and Garcia, W. L. (2023). Activity-based protein profiling—enabling phenotyping of host-associated and environmental microbiomes. Isr. J. Chem. 63:99. doi: 10.1002/ijch.202200099
Wu, D., Tian, H., Xu, F., Yang, J., Feng, W., Bell, S., et al. (2024). The prodomain of Arabidopsis metacaspase 2 positively regulates immune signaling mediated by pattern-recognition receptors. New Phytol. 241, 430–443. doi: 10.1111/nph.19365
Yin, R., Cheng, J., and Lin, J. (2024). The role of the type VI secretion system in the stress resistance of plant-associated bacteria. Stress Biol. 4:16. doi: 10.1007/s44154-024-00151-3
Yuan, S., Ke, D., Liu, B., Zhang, M., Li, X., Chen, H., et al. (2023). The Bax inhibitor GmBI-1α interacts with a Nod factor receptor and plays a dual role in the legume-rhizobia symbiosis. J. Exp. Bot. 74, 5820–5839. doi: 10.1093/jxb/erad276
Zhang, L., Chen, F., Zeng, Z., Xu, M., Sun, F., Yang, L., et al. (2021). Advances in metagenomics and its application in environmental microorganisms. Front. Microbiol. 12:766364. doi: 10.3389/fmicb.2021.766364
Zhou, D., Chen, X., Chen, X., Xia, Y., Liu, J., Zhou, G., et al. (2023). Plant immune receptors interact with hemibiotrophic pathogens to activate plant immunity. Front. Microbiol. 14:1252039. doi: 10.3389/fmicb.2023.1252039
Keywords: plant-microbe interactions, omics technologies, genomics, transcriptomics, proteomics, metabolomics
Citation: Jain A, Sarsaiya S, Singh R, Gong Q, Wu Q and Shi J (2024) Omics approaches in understanding the benefits of plant-microbe interactions. Front. Microbiol. 15:1391059. doi: 10.3389/fmicb.2024.1391059
Received: 24 February 2024; Accepted: 29 April 2024;
Published: 27 May 2024.
Edited by:
Mao Peng, Westerdijk Fungal Biodiversity Institute, NetherlandsReviewed by:
Manoj Kumar Solanki, University of Silesia in Katowice, PolandPankaj Singh, Dr. Rammanohar Lohia Avadh University, India
Puja Ray, Presidency University, India
Sudisha Jogaiah, Karnatak University, India
Copyright © 2024 Jain, Sarsaiya, Singh, Gong, Wu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingshan Shi, c2hpanNAem11LmVkdS5jbg==
†These authors share first authorship
 Archana Jain
Archana Jain Surendra Sarsaiya
Surendra Sarsaiya Ranjan Singh3
Ranjan Singh3 Qihai Gong
Qihai Gong Jingshan Shi
Jingshan Shi