
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 27 March 2024
Sec. Microbiotechnology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1383813
This article is part of the Research TopicFuturistic Plant Microbes Biotechnology and BioengineeringView all 6 articles
Phosphorus (P) is an important nutrient for plants, and a lack of available P greatly limits plant growth and development. Phosphate-solubilizing microorganisms (PSMs) significantly enhance the ability of plants to absorb and utilize P, which is important for improving plant nutrient turnover and yield. This article summarizes and analyzes how PSMs promote the absorption and utilization of P nutrients by plants from four perspectives: the types and functions of PSMs, phosphate-solubilizing mechanisms, main functional genes, and the impact of complex inoculation of PSMs on plant P acquisition. This article reviews the physiological and molecular mechanisms of phosphorus solubilization and growth promotion by PSMs, with a focus on analyzing the impact of PSMs on soil microbial communities and its interaction with root exudates. In order to better understand the ability of PSMs and their role in soil P transformation and to provide prospects for research on PSMs promoting plant P absorption. PSMs mainly activate insoluble P through the secretion of organic acids, phosphatase production, and mycorrhizal symbiosis, mycorrhizal symbiosis indirectly activates P via carbon exchange. PSMs can secrete organic acids and produce phosphatase, which plays a crucial role in soil P cycling, and related genes are involved in regulating the P-solubilization ability. This article reviews the mechanisms by which microorganisms promote plant uptake of soil P, which is of great significance for a deeper understanding of PSM-mediated soil P cycling, plant P uptake and utilization, and for improving the efficiency of P utilization in agriculture.
Phosphorus (P) is an essential nutrient for plant growth and development, playing an important role in the synthesis of DNA, cell membrane components (phospholipids), adenosine triphosphate (ATP), respiration, and photosynthesis (Kafle et al., 2019; Bai et al., 2020). P in soil includes two forms: organic and inorganic P. Although soil contains a large amount of phosphorus, it usually exists in a form that cannot be directly utilized by plants (Ikhajiagbe et al., 2020; Divjot et al., 2021). P combines with Ca, Fe, and Al metals to form minerals, and P is adsorbed by iron/aluminum (hydrogen) oxides, leading to P fixation in the soil (Ma J. et al., 2021; Zhou J. et al., 2021). The mobility of P is poor in soil, and plants cannot directly absorb and utilize P, leading to the widespread phenomenon of low P in ecosystems, which limits plant growth and yield. A large amount of P fertilizer is applied during production to meet the P demand of plants. Because of the adsorption and fixation effects of soil on P, the applied P fertilizer rapidly becomes fixed, resulting in a P fertilizer utilization efficiency of only 10–25% (Dejene et al., 2023; Dong et al., 2023). Moreover, fixed P in the soil can lead to non-point source pollution, resulting in large amounts of P fertilizer flowing into water bodies, in turn leading to groundwater eutrophication, which is not conducive to the sustainable development of the ecological environment (Lyu et al., 2023; Wang et al., 2023). Phosphate ore is a nonrenewable resource. Half of the world’s existing P reserves are predicted to be depleted within 50–100 years (Zhu et al., 2018). Therefore, improving the utilization efficiency of P in soil is crucial for promoting plant growth, reducing environmental pollution, and improving resource management.
Phosphate-solubilizing microorganisms (PSMs) can convert soil P into a form that plants can absorb and utilize, and the application of PSMs is currently an important measure for increasing the available P content in soil (Yadav et al., 2017; Yadav, 2020). Many types of microorganisms dissolve P, which plays an important role in P cycling processes such as organic P mineralization, insoluble inorganic P dissolution, and P absorption (Zhu et al., 2018; Wise et al., 2021). Inoculating PSMs is an environmentally friendly method to promote crop productivity and understanding the mechanism of P solubilization by PSMs is of great significance for plants to adapt to low P stress and improve P utilization efficiency (Billah et al., 2019). This article discusses the types and functions of PSMs, P-solubilization mechanisms, main functional genes, and the impact of composite inoculation of PSMs on plant P partitioning, emphasizing the role of PSMs in plant P acquisition and utilization, and—based on this—proposes issues and corresponding measures that need to be considered in future research and applications of PSMs.
PSMs are widely distributed in nature, and microorganisms with P-solubilizing functions include bacteria, fungi, actinomycetes, and cyanobacteria, among which P-solubilizing fungi account for 0.1–0.5% of PSMs, and P-solubilizing bacteria account for 1–50% of the total (Fatima et al., 2022). P-solubilizing bacteria included 34 genera, including Bacillus, Pseudomonas, Escherichia, and Burkholderia. Of these, Bacillus, Pseudomonas, and Acinetobacter have been studied extensively (Divjot et al., 2021; Timofeeva et al., 2022). P-solubilizing fungi include Arbuscularmy sp., Aspergillus, Penicillium, among which Aspergillus is the most reported, followed by Penicillium (Jiang et al., 2020; Divjot et al., 2021; Etesami et al., 2021). P-solubilizing fungi produce 10 times more organic acids than P-solubilizing bacteria and can increase the contact area with the soil through the mycelium, thereby increasing the application potential of P-solubilizing fungi (Jiang et al., 2020). The main P-solubilizing actinomycetes are Streptomyces and Micromonospora (Aallam et al., 2021; De Zutter et al., 2022). Microorganisms not only promote the conversion of difficult-to-utilize P to available P but also assist plants with absorbing P outside the rhizosphere, thus playing an important role in the process of plant P acquisition (Castagno et al., 2021).
PSMs not only have P-solubilizing effects but can also produce organic acids and iron carriers, regulate plant hormone levels, and fix nitrogen to promote the acquisition and growth of rice nutrients (Ribeiro et al., 2018; Unnikrishnan and Binitha, 2024). PSMs can secrete plant hormones such as auxins, cytokinins, and gibberellins, produce antifungal compounds and volatile bactericidal metabolites, and synthesize 1-aminocyclopropane-1-carboxylate (ACC) deaminase to improve phosphorus absorption and disease resistance, thereby increasing plant growth and yield (Hakim et al., 2021; Rawat et al., 2021). PSMs can also secrete antibiotics, iron carriers, and lyases to protect plants from various soil-borne pathogens and promote plant growth (Toscano-Verduzco et al., 2020; Kumawat et al., 2021). Moreover, inoculation with PSMs can significantly affect the diversity and abundance of soil microbial communities and enhance the interactions between microorganisms, ultimately resulting in improved organic matter degradation and soil nutrient quality (Zhang X. et al., 2021). PSMs also have different functions in different ecological environments and can enhance crop resistance to certain abiotic stresses, including cold, salt, heavy metals, and drought (Table 1). The P-solubilizing bacterium Bacillus atrophaeus GQJK17 S8 can tolerate 11% NaCl, which can improve the germination rate, seedling biomass, and growth vitality index of quinoa plants (Mahdi et al., 2021). In addition, there are strains with different abiotic stress tolerance abilities, Such as Pseudomonas PGERs17, which is resistant to cold stress (Rizvi et al., 2021), Bacillus YMX5, which is resistant to high salt stress (Jiang et al., 2020), and Streptomyces laurentii EU-LWT3-69, which is resistant to drought stress (Toscano-Verduzco et al., 2020). This type of PSMs not only promotes plant P absorption but also helps plants grow in extreme environments.
P in soil includes two forms: inorganic and organic P. Inorganic P usually exists as phosphates, divided into soluble and insoluble P. Insoluble P mainly includes phosphates such as aluminum phosphate, iron phosphate, magnesium phosphate, and calcium phosphate (Aliyat et al., 2022), while soluble P mainly exists as hydrogen phosphate and dihydrogen phosphate ions (HPO42− and H2PO4−) (Hao et al., 2020; Divjot et al., 2021; Li et al., 2021). Organic P mainly includes P-containing organic compounds, such as orthophosphate monoesters, orthophosphate diesters, organic polyphosphates, and phosphonates (Li C. et al., 2019). Organic acids and phosphatases produced by microorganisms are crucial for the cycling of inorganic and organic phosphorus in soil (Rasul et al., 2021). Insoluble inorganic P is mainly dissolved by organic acids, and enzymatic hydrolysis is the main method used to dissolve the organic forms of P (Figure 1). The mechanisms of microbial P solubilization can be divided into the following types:
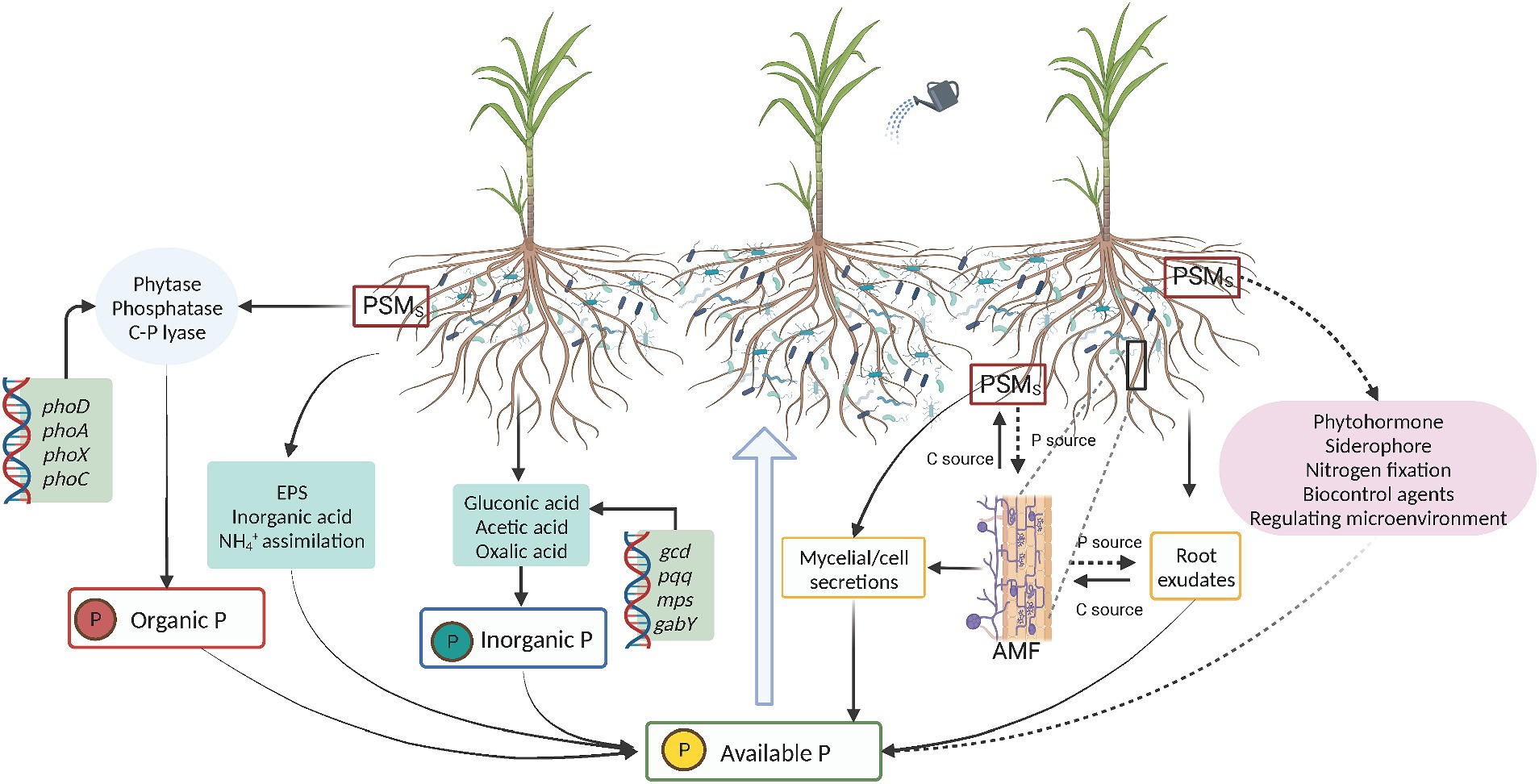
Figure 1. PSMs promote the production of available phosphorus and plant absorption through multiple pathways. AMF, arbuscular mycorrhizal fungi; PSMs, phosphate solubilizing microorganisms; EPS, extracellular polysaccharides.
Organic acids secreted by PSMs transform insoluble inorganic P into plant-usable P. Microorganisms produce organic acids in two ways: physiological secretion and decomposition of organic matter (Schneider et al., 2019). The organic acids secreted by PSMs include gluconic, lactic, citric, and oxalic acids (Kalayu, 2019; Azaroual et al., 2020). Organic acids mainly dissolve insoluble inorganic P as follows: organic acid anions compete with phosphate ions for binding sites on soil particles, reducing soil adsorption of phosphate ions; complex metal ions such as iron, aluminum, and calcium in soil to release bound phosphate ions; and reduction of the pH of the medium promotes the dissolution of insoluble inorganic P (Adeleke et al., 2017; Rawat et al., 2021). The organic acids secreted by PSMs not only enhance the solubility of insoluble P, such as apatite and calcium phosphate, but also chelate with cations such as Ca2+, Fe3+, Al3+, and Mg2+, and organic acid anions compete with inorganic P physically or electrostatically for the same adsorption sites in the soil, releasing phosphate ions and increasing the effective P content (Rawat et al., 2021). The organic acids produced by microorganisms chelate with cations through hydroxyl and carboxyl groups, transforming phosphates into soluble forms and increasing the effective P content (Bhattacharyya et al., 2016). Many bacteria secrete organic acids (carboxylic acids) that can increase the solubility of calcium phosphate (Jayakumar et al., 2019). In addition, organic acids can promote the dissolution of insoluble inorganic phosphate compounds, such as tricalcium phosphate, dicalcium phosphate, hydroxyapatite, and phosphate rock, thereby improving the utilization rate of phosphate fertilizers (Oteino et al., 2015; Cheng et al., 2017). PSMs secrete various organic acids to convert insoluble inorganic phosphorus into soluble orthophosphates that are easily absorbed by plants (Venkiteshwaran et al., 2021; Campos et al., 2023). The types, contents, and phosphate solubility of organic acids produced by PSM vary, such as of Enterobacter sp. strain 15S, can produce organic acids such as citric, fumaric, ketoglutaric, malic, and oxalic acids (Zuluaga et al., 2023). However, Trichoderma sp. produce different types of organic acids, including lactic acid, fuzzy acid, ascorbic acid, isocitric acid, malic acid, citric acid, and phytic acid (Bononi et al., 2020).
Gluconic acid is considered a common and important organic acid, and is one of the many organic acids produced by microorganisms that have been extensively studied (Kaur et al., 2021). Glucose can form gluconic acid through the synergistic effects of pyrroloquinoline quinine (PQQ) and glucose dehydrogenase (GDH), which dissolves insoluble phosphate (Jaiswal et al., 2021). Pseudomonas produces gluconic acid to increase phosphate solubility, which has become an important technology for improving phosphate fertilizer management in modern agriculture (Wang et al., 2022; Rai et al., 2023). Inoculation of Pseudomonas fluorescens and Pseudomonas putida under soluble phosphate-restricted conditions can produce a large amount of gluconic acid, which promotes plant growth (Jin et al., 2022).
Organic P cannot be directly absorbed by plants but needs to be mineralized into inorganic P before it can be utilized by plants. Enzymatic hydrolysis is the main way to mineralize organic P under conditions of low available P content, PSMs can hydrolyze organic P through biological enzymes, such as phosphatase, phytase, and C-P lyase (Stefanoni Rubio et al., 2016; Prabhu et al., 2019). Two hydrolytic enzymes, phytase and phosphatase, play important roles in PSM mineralization (Liu et al., 2022). Phytase is an extracellular enzyme involved in the mineralization process of soil P, and phytase produced by microorganisms can release orthophosphate from phytate organic compounds, converting P into a form that can be utilized by plants (Ortega-Torres et al., 2021; Timofeeva et al., 2022). Microbial phytase activity is closely related to its ability to dissolve phosphorus (Ben Zineb et al., 2020).
PSMs not only secrete phytase, but also produce phosphatase to mineralize organic P. Phosphatases are divided into acid phosphatase (ACP) and alkaline phosphatase (ALP), and their existence is greatly influenced by the acidity and alkalinity of the environment. ACP is more abundant in acidic soils, while ALP dominates in neutral and alkaline soils (Borges et al., 2021; Cheng et al., 2023). The activity of ALP is inhibited by inorganic phosphates in the environment, while ACP activity is not inhibited by high levels of phosphates (Li et al., 2021; Xie et al., 2021). In addition, temperature can affect phosphatase activity, and an increase in temperature can enhance the activity of phosphatases secreted by PSMs (Hessen et al., 2017; Jiang et al., 2018). Phosphatases are responsible for mineralizing approximately 90% of the organic P in soils, except phytates (Alori et al., 2017; Chen and Arai, 2023). Many microorganisms, including Aspergillus, Bacillus, and Pseudomonas, produce phosphatases (Shrivastava et al., 2018; Kaur and Chatli, 2019; Zaborowska et al., 2020). Purified ALP from Bacillus licheniformis MTCC 2312 has been added to sterilized soil, which improved the phosphate content in the roots and stems of maize (Singh and Banik, 2019).
Mycorrhizal fungi can form mutualistic symbioses with plant roots and help plants absorb mineral elements and water from the soil, while plants provide carbohydrates to the fungi (Chiu and Paszkowski, 2019; Genre et al., 2020). Arbuscular mycorrhizal fungi (AMF) can form mycorrhizal fungi in symbiosis with 70–80% of terrestrial plants, which is an effective way for plants to obtain P (Shi et al., 2021). The symbiotic interface between AMF and plants is an important site for material exchange between plants and fungi, which can increase the range of plant P absorption and the transport of P to root cells (Bao et al., 2022). Plants absorb P through a series of morphological changes to expand the surface area of the roots and improve the exchange interface between the roots and soil when subjected to low P stress. Examples of this include increasing the number and length of root hairs; increasing the root-to-shoot ratio; adjusting the angle of root growth; increasing the number of lateral roots, adventitious roots, and young roots; increasing root length, shallow roots; and increasing root length and density in the soil surface layer (Hammelehle et al., 2018; Lynch, 2019; Zhang Z. et al., 2021). Mycorrhizal plants can obtain P from the soil through the root pathway absorbed by root hairs and root epidermal cells, as well as the hyphal pathway absorbed by arbuscular mycorrhizal fungi, which synergistically promote nutrient absorption (Ferrol et al., 2019; Chu et al., 2020; Zhou J. et al., 2021). In the hyphal pathway, arbuscular mycorrhizal fungi improve plant P nutrient status through the hyphae. Hyphae not only penetrate soil pores smaller than root hairs but also extend further from the root surface to obtain a larger range of P in the soil (Ma X. et al., 2021; Gregory, 2022). While expanding the absorption range, AMF can stimulate the secretion of organic acids and ACP by host plant roots; their own mycelia can also secrete organic acids and ACP, reduce the pH of the surrounding soil, and convert insoluble phosphates into available P, which has the similar function as phosphate-solubilizing bacteria (Zhang L. et al., 2022; Xing et al., 2023). Organic acids, carbohydrates, amino acids, plant hormones, and other substances have been found in the mycelial secretions of AMF (Rhizophagus clarius and Rhizophagus irregularis) (Luthfiana et al., 2021). Plants are likely to form mycorrhizal symbioses with mycorrhizal fungi to enhance their ability to obtain P under P-deficient conditions (Raven et al., 2018). The absorption of soil P through mycorrhizal fungi is an effective way for plants to supplement P (Figure 2).
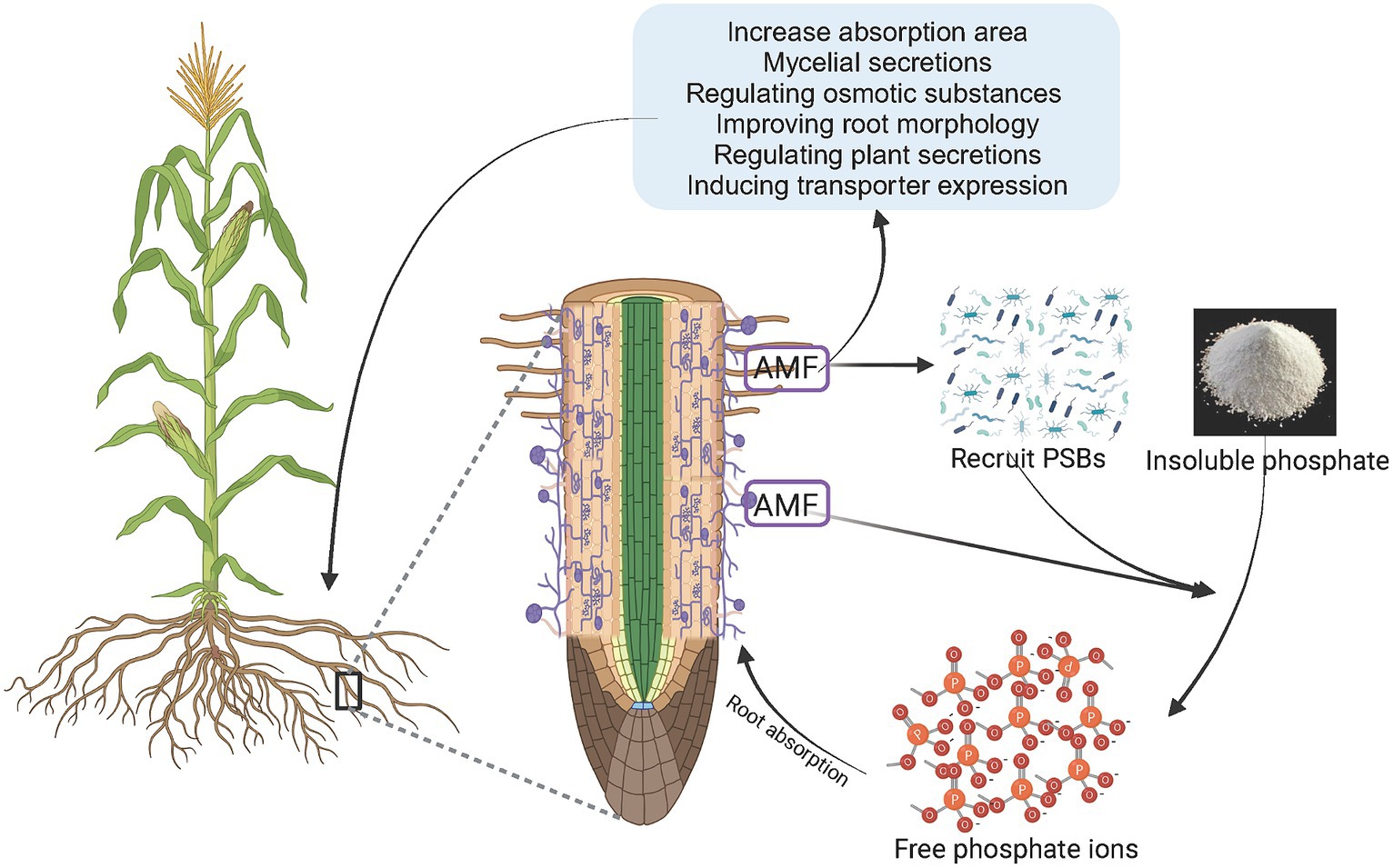
Figure 2. AMF recruiting PSBs or directly activating phosphorus elements to enhance plant phosphorus absorption. AMF, arbuscular mycorrhizal fungi; PSB, phosphate solubilizing bacteria.
Compared with organic acids, the efficiency of inorganic acids (such as sulfuric acid and nitric acid) produced by PSMs in dissolving phosphates is lower. Nitrobacter and Thiobacillus spp. produce inorganic acids, such as nitric acid and sulfuric acid, to dissolve P and increase the available P content in the soil (Shrivastava et al., 2018; Dipta et al., 2019). PSMs also produce extracellular polysaccharides (EPS) that can form complexes with metal ions and enhance the solubilization of P (Naseem et al., 2018; Thampi et al., 2023). The combined action of EPS and organic acids can dissolve Ca3(PO4)2, which adds EPS to the culture medium and increases the solubilization of tricalcium phosphate by organic acids (Mendoza-Arroyo et al., 2020; Liu et al., 2024). Ammonium (NH4+) present in soil is absorbed by PSMs to synthesize amino acids. Proton efflux caused by ammonium ion assimilation is another P-solubilization mechanism in microorganisms. Bacillus marisflavi FA7 is accompanied by ammonium ion assimilation, which decreases the pH of the culture medium and dissolves insoluble phosphates (Prabhu et al., 2018). PSMs can also promote P absorption by increasing root weight, root length, projection area, surface area, tip, and branch number (Liu X. et al., 2019; Galindo et al., 2022).
With the continuous development of molecular biology, researchers have explored the mechanism of P solubilization from a genetic perspective. The specific molecular genetic mechanisms underlying mineral phosphate dissolution have not been clearly elucidated to date (Timofeeva et al., 2022). Research on the functional genes of PSMs has mainly focused on genes related to the microbial secretion of organic acids and phosphatase production.
Gluconic acid is the main organic acid secreted by PSMs (Zhang et al., 2023). Genes related to gluconic acid synthesis are key for the regulation of P-solubilization ability. Gluconic acid is synthesized by the oxidation of glucose by GDH, which requires pyroquinoline quinone (PQQ) as a cofactor to participate in the reaction (Karagoz et al., 2020; Wu et al., 2022). PQQ synthesis involves six core genes (pqqA, pqqB, pqqC, pqqD, pqqE, and pqqF) associated with dehydrogenase activity and mineral phosphate dissolution in microorganisms (Wan et al., 2020; Dudeja et al., 2021; Joshi et al., 2023). The pqqA gene plays an important role in PQQ biosynthesis and P solubilization. Mutations in pqqA in Rahnella aquatilis HX2H significantly reduce the gluconic acid content in the culture medium, leading to a significant decrease in the solubility of mineral phosphates (Li et al., 2014). In addition, pqqE is highly conserved and crucial for the biosynthesis of PQQ (Ludueña et al., 2017; Lo et al., 2023). Pantoea sp. and Pseudomonas sp. carrying pqqE can solubilize P and increase crop yields (Tahir et al., 2020). The expression level of the pqq gene in Serratia sp. S119 increases under P-deficient growth conditions, catalyzing the oxidation of glucose to gluconic acid and alleviating P deficiency (Ludueña et al., 2017).
The membrane-bound quinoprotein glucose dehydrogenase (PQQGDH) is an important enzyme that regulates the synthesis of gluconic acid and dissolution of insoluble phosphate and is encoded by the gcd gene (Jha et al., 2019; Wu et al., 2022). The genes related to gluconic acid production include gabY and mps (Zhao et al., 2014; Rawat et al., 2021). Pseudomonas sp. MS16 was isolated from the rhizosphere soil of wheat and its P-solubilization activity was further validated through amplification, sequencing, and phylogenetic analysis of gcd gene (Suleman et al., 2018). The abundances of gcd genes were significantly correlated with environmental factors such as dissolved oxygen, phosphorus hydrochloride, and dissolved total phosphorus (Li Y. et al., 2019). The gcd gene can serve as a genetic marker to evaluate the potential of microorganisms to dissolve inorganic phosphorus. Acinetobacter sp. MR5 and Pseudomonas sp. MR7 carrying the gcd gene have the effect of promoting plant P absorption and growth, and rice plants treated with bacteria exhibited an increase in P content and grain yield of approximately 67 and 55%, respectively, compared with control plants (Rasul et al., 2019). However, the expression of gcd is inhibited by an increase in the soluble phosphate concentration (Zeng et al., 2016).
Phosphatases are important enzymes for mineralizing organic P and include ACP and ALP. ACP is mainly secreted by plants and fungi, whereas ALP is mainly produced by bacteria (Fraser et al., 2017). ALP and gluconic acid are important factors that affect the availability of P in soil (Liang et al., 2020; Wang et al., 2021). Among the enzymes involved in organic P mineralization, bacterial ALP has been extensively studied in terms of its biosynthesis, genetic control, and catalytic properties (Drozd et al., 2011; Kageyama et al., 2011; Sebastián and Ammerman, 2011; Park et al., 2022; Wijeratne et al., 2022). ALP is primarily encoded by PhoA, PhoD, and PhoX (Liu et al., 2018; Wang et al., 2021; Zhou Y. et al., 2021). PhoA hydrolyzes phosphate monoesters, whereas phoD and phoX decompose phosphate monoesters and phosphate diesters (Chen et al., 2019; Srivastava et al., 2021; Yuan et al., 2023). Among the genes encoding ALP, phoD is a key gene in soil microorganisms (Tan et al., 2013; Sun et al., 2019; Huang et al., 2020). The abundance of phoD in soil correlated positively with ALP activity and the available P concentration (Fraser et al., 2015; Wang et al., 2021; Xu et al., 2022). The phoD gene is used as a marker gene to estimate the abundance and community composition of organic P-mineralization microorganisms, thereby allowing investigation of the microbial regulatory mechanisms of phosphorus cycling (Hu et al., 2020; Azene et al., 2023). In addition to the ALP genes, the ACP genes mainly include phoC, whereas the phytase genes include phyA, appA, etc. The phoC gene is an important gene encoding acid phosphatase (Apel et al., 2007; Fraser et al., 2017). In neutral or low-pH soils, the phoC gene is more dominant than the phoD gene (Fraser et al., 2017). After genetic transformation of maize using the phytase gene (phyA2) of Aspergillus ficuum, the growth and ability to obtain P from phytates were significantly improved (Jiao et al., 2021). In addition, a large number of studies have reported the isolation of various genes with P-solubilization ability from different species, such as mMDH from Penicillium oxalicum C2 (Lü et al., 2012), vgb from Vitreoscilla hemoglobin (Yadav et al., 2014), Eno from Burkholderia cenococcia 71-2 (Liu C. et al., 2019), Zymomonas mobility (invB), and Saccharomyces cerevisiae (suc2) (Kumar et al., 2016).
The use of metagenomic methods to analyze soil microbial P-cycling functional genes lays a solid foundation for the study of P-cycling genes, which helps us explore the potential functions of PSMs from a more comprehensive perspective. The P-cycling functional genes are mainly divided into three categories and seven functional groups, including those involved in P activation (including phosphate ester mineralization and inorganic phosphate dissolution), P absorption (phosphate ester transport and inorganic phosphate transport), and regulation of P-deficiency-induced responses (Liu et al., 2018; Dai et al., 2020; Siles et al., 2022). With the development of omics technologies and improvement of functional gene reference databases, researchers will more comprehensively reveal the functions and molecular mechanisms of microorganisms involved in plant soil P cycling.
P-solubilizing bacteria and fungi on their own have a limited ability to mineralize organic P and solubilize inorganic P. Co-inoculation of plants with two or more strains promotes P absorption and plant growth (Table 2). The interaction between AMF and phosphate-solubilizing bacteria is more effective than inoculation alone for promoting P absorption and plant growth (El Maaloum et al., 2020; Wahid et al., 2020). The combination of bacteria and fungi has a synergistic effect, and mixed inoculation of AMF and P-solubilizing bacteria increases the root dry weight of plants by up to 58% compared with a single inoculation (Sharma et al., 2020). Mixed inoculation of AMF and PSMs not only improves soil fertility but also significantly increases crop yield. Inoculation with Azospirillum brasilense and Bacillus subtilis can improve the efficiency of P fertilizer utilization in sugarcane and positively affect the quality and yield of sugarcane crops (Rosa et al., 2020). Co-inoculation with Trichoderma viride, Humicola spp., Paecilomyces lilacinus, Gluconacetobater diazotropicus, Azospiriillum brasilense, and Bacillus subtilis can improve nutrient cycling and soil fertility, thereby promoting sugarcane root development (Tayade et al., 2019). Co-inoculation with phosphate-solubilizing bacteria and AMF significantly increases the soil enzyme activity and rhizosphere microbial count, and both synergistically promote nitrogen and P nutrient uptake (Varinderpal-Singh et al., 2020; Cozzolino et al., 2021). The composite inoculation of Bradyrhizobium japonicum 5038 and Paenibacillus mucilaginosus 3016 on soybeans resulted in a significant increase in the abundance of phosphorus cycle genes, as well as an increase in soil available phosphorus and phosphatase activity (Xing et al., 2022).
The interactions between microorganisms to form complexes help plants absorb P. Fructose secreted by AMF can stimulate the expression of PSMs phosphatase genes, promote phosphatase synthesis and secretion, and increase the mineralization of organic P (Zhang et al., 2018). The mycelia of AMF can secrete compounds such as sugars, carboxylates, and amino acids, which can be utilized by phosphate-solubilizing bacteria (Cartabia et al., 2021; Weng et al., 2022). The exudate of AM fungi can serve as a source of carbon for bacteria as well as a signal and effector molecule that stimulates bacterial growth and activity (Zhang et al., 2016; Zeng et al., 2018; Zhang et al., 2018). In addition, bacteria on the surface of the mycelium can move along the mycelium and migrate to nutrient patches to activate organic P, thereby improving the utilization efficiency of the P in the soil by plants (Jiang et al., 2021). Bacteria colonize the surface of AMF mycelia to facilitate the acquisition of mycelial exudates (Emmett et al., 2021). Cooperation between AMF and bacteria is a manifestation of the symbiotic relationship between AMF and plants.
The symbiotic relationship between AMF and terrestrial plants is one of the most representative examples of microbial–plant cooperation (Brundrett and Tedersoo, 2018). The interaction between AMF and phosphate-solubilizing bacteria can affect the P exchange between plants and mycorrhizal fungi because the interaction between AMF and phosphate-solubilizing bacteria increases the secretion of phosphatase and gluconic acid, promoting the absorption and transport of P by extraradicular hyphae. P is transferred to plant roots by AMF, and plants, in turn, provide carbon sources to AMF, improving C-P exchange between plants and AMF (Duan et al., 2023). Under natural conditions, close cooperation between microorganisms is scientifically more effective for completing ecological functions than the independent actions of a single microorganism. Suillus grevillea synergistically mineralizes phytic acid by recruiting Cedecea lapeti and stimulates the upregulation of its own P-solubilization-related gene expression and growth of Cedecea lapeti, thereby promoting plant uptake of organic P (Mei et al., 2024). Ectomycorrhizal fungi recruit specific bacterial colonies by providing carbon sources, such as trehalose, mannitol, and organic acids (Deveau et al., 2010; Haq et al., 2017). These recruited bacteria can perform various ecological functions, such as promoting mycelial growth and assisting ectomycorrhizal fungi in absorbing nutrients (Tarkka et al., 2018; Pent et al., 2020). The co-inoculation of phosphate-solubilizing bacteria and fungi has shown good results in promoting plant growth, nutrient absorption, mycorrhizal symbiosis, and microbial biomass. In addition, the co-inoculation of PSMs with other functional microorganisms can achieve various goals to promote plant growth. For example, the co-inoculation of PSMs with nitrogen-fixing bacteria can increase the utilization of phosphorus and the fixation of nitrogen in the atmosphere, thereby improving soil fertility and crop yield, and promoting the development of sustainable agriculture (Zveushe et al., 2023). The co-inoculation of PSMs with biocontrol bacteria not only improves plant absorption of phosphorus, but also significantly reduces the incidence and severity of diseases, which is more effective than the single inoculation of PSMs (Nepomuceno et al., 2019).
The low available P content in the soil and the low efficiency of P fertilizer utilization limit plant growth and yield. PSMs increase the soil available P content, improve the P fertilizer utilization efficiency, and promote plant growth. Therefore, the use of biological pathways to improve the utilization efficiency of P in soil has attracted the attention of scientists in various countries. Domestic and foreign scholars have identified many microorganisms with P-solubilization abilities through screening and conducted research on them. However, owing to factors such as the microbial P-solubilization ability, the colonization ability in the plant rhizosphere, and stability, relatively few strains have been applied in production practices to date. Many studies have focused on the effects of PSMs on plant growth under conventional cultivation conditions; however, little attention has been paid to their effects on plant growth under abiotic stress conditions. With the continuous development of molecular technologies and genomics, related P-solubilizing genes are constantly being explored; however, gene research mainly focuses on functional verification, and the interaction mechanism between P-solubilizing genes is still unclear. AMF and phosphate-solubilizing bacteria originate from different sources, and combining the two is not a natural correlation and may result in an unstable synergistic effect. The mechanism by which compound inoculation with PSMs causes microbial community changes in plant roots is not yet fully understood. Unlike the extensive research on the interactions between microorganisms and plant roots, there is currently limited research on the impact of microbial interactions on plant P-uptake efficiency.
Regarding the research direction of inoculating PSMs to improve the utilization efficiency of plant P, the current focus is to (1) expand the application scope of PSMs, combine functional research such as nitrogen fixation and soil remediation, and increase the application of composite microbial fertilizers to better serve agricultural development. (2) Continuously explore P-solubilization-related genes using a combination of genomics, proteomics, and metabolomics to further explain the mechanisms of soil P dissolution. Single-cell Raman D2O technology and high-throughput P-cycling functional gene chips can determine the corresponding functional genes, groups, and activities of PSMs, thereby improving our understanding of the types and functions of PSMs. (3) Strengthening research on microbial interactions and mixed inoculation, which combines AMF with naturally related bacteria, may provide advantages over artificially combined microorganisms. Moreover, by using molecular biology techniques and isotope labeling methods to explore microbial interactions at a deeper level, the optimal effect of microbial interactions in the soil can be achieved, thereby playing an important role in improving soil fertility. (4) Further research is being conducted on the effects of specific chemical substances related to different stages of microbial interactions through a combination of transcriptomics and metabolomics. (5) More attention should be paid to the isolation of indigenous microorganisms, screening for higher-quality and multifunctional PSMs, enhancing plant uptake of soil P, and enhancing their resistance to different stress conditions, which is of great significance for promoting plant growth.
FP: Writing – original draft, Writing – review & editing. QL: Writing – original draft. MS: Conceptualization, Writing – original draft. ZW: Conceptualization, Funding acquisition, Writing – review & editing. Y-XX: Conceptualization, Writing – review & editing. D-FD: Funding acquisition, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The present work was supported by the Guangxi Natural Science Foundation (CN) (2022GXNSFDA035074; 2022GXNSFBA035542; and 2023GXNSFAA026182) and National Natural Science Foundation of China (32101836).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aallam, Y., Dhiba, D., Lemriss, S., Souiri, A., Karray, F., Rasafi, T. E., et al. (2021). Isolation and characterization of phosphate solubilizing Streptomyces sp. endemic from sugar beet fields of the Beni-Mellal region in Morocco. Microorganisms 9:914. doi: 10.3390/microorganisms9050914
Adeleke, R., Nwangburuka, C., and Oboirien, B. (2017). Origins, roles and fate of organic acids in soils: a review. S. Afr. J. Bot. 108, 393–406. doi: 10.1016/j.sajb.2016.09.002
Adhikari, P., Jain, R., Sharma, A., and Pandey, A. (2021). Plant growth promotion at low temperature by phosphate-solubilizing Pseudomonas spp. isolated from high-altitude Himalayan soil. Microb. Ecol. 82, 677–687. doi: 10.1007/s00248-021-01702-1
Ahmad, I., Ahmad, M., Hussain, A., and Jamil, M. (2021). Integrated use of phosphate-solubilizing Bacillus subtilis strain IA6 and zinc-solubilizing Bacillus sp. strain IA16: a promising approach for improving cotton growth. Folia Microbiol. 66, 115–125. doi: 10.1007/s12223-020-00831-3
Aliyat, F. Z., Maldani, M., El Guilli, M., Nassiri, L., and Ibijbijen, J. (2022). Phosphate-solubilizing bacteria isolated from phosphate solid sludge and their ability to solubilize three inorganic phosphate forms: calcium, iron, and aluminum phosphates. Microorganisms 10:980. doi: 10.3390/microorganisms10050980
Alori, E. T., Glick, B. R., and Babalola, O. O. (2017). Microbial phosphorus solubilization and its potential for use in sustainable agriculture. Front. Microbiol. 8:971. doi: 10.3389/fmicb.2017.00971
Apel, A. K., Sola-Landa, A., Rodríguez-García, A., and Martín, J. F. (2007). Phosphate control of phoA, phoC and phoD gene expression in Streptomyces coelicolor reveals significant differences in binding of PhoP to their promoter regions. Microbiology 153, 3527–3537. doi: 10.1099/mic.0.2007/007070-0
Azaroual, S. E., Hazzoumi, Z., Mernissi, N. E., Aasfar, A., Meftah Kadmiri, I., and Bouizgarne, B. (2020). Role of inorganic phosphate solubilizing Bacilli isolated from moroccan phosphate rock mine and rhizosphere soils in wheat (Triticum aestivum L) phosphorus uptake. Curr. Microbiol. 77, 2391–2404. doi: 10.1007/s00284-020-02046-8
Azene, B., Zhu, R., Pan, K., Sun, X., Nigussie, Y., Gruba, P., et al. (2023). Land use change alters phosphatase enzyme activity and phosphatase-harboring microbial abundance in the subalpine ecosystem of southeastern Qinghai-Tibet Plateau, China. Ecol. Indic. 153:110416. doi: 10.1016/j.ecolind.2023.110416
Bai, Y.-C., Chang, Y.-Y., Hussain, M., Lu, B., Zhang, J.-P., Song, X.-B., et al. (2020). Soil chemical and microbiological properties are changed by long-term chemical fertilizers that limit ecosystem functioning. Microorganisms 8:694. doi: 10.3390/microorganisms8050694
Bao, X., Zou, J., Zhang, B., Wu, L., Yang, T., and Huang, Q. (2022). Arbuscular mycorrhizal fungi and microbes interaction in rice mycorrhizosphere. Agronomy 12:1277. doi: 10.3390/agronomy12061277
Belkebla, N., Bessai, S. A., Melo, J., Caeiro, M. F., Cruz, C., and Nabti, E.-H. (2022). Restoration of Triticum aestivum growth under salt stress by phosphate-solubilizing bacterium isolated from Southern Algeria. Agronomy 12:2050. doi: 10.3390/agronomy12092050
Ben Zineb, A., Trabelsi, D., Ayachi, I., Barhoumi, F., Aroca, R., and Mhamdi, R. (2020). Inoculation with elite strains of phosphate-solubilizing bacteria enhances the effectiveness of fertilization with rock phosphates. Geomicrobiol J. 37, 22–30. doi: 10.1080/01490451.2019.1658826
Bhattacharyya, P., Goswami, M., and Bhattacharyya, L. (2016). Perspective of beneficial microbes in agriculture under changing climatic scenario: a review. J. Phytology 8, 26–41. doi: 10.19071/jp.2016.v8.3022
Billah, M., Khan, M., Bano, A., Hassan, T. U., Munir, A., and Gurmani, A. R. (2019). Phosphorus and phosphate solubilizing bacteria: keys for sustainable agriculture. Geomicrobiol J. 36, 904–916. doi: 10.1080/01490451.2019.1654043
Biswas, J. K., Banerjee, A., Rai, M., Naidu, R., Biswas, B., Vithanage, M., et al. (2018). Potential application of selected metal resistant phosphate solubilizing bacteria isolated from the gut of earthworm (Metaphire posthuma) in plant growth promotion. Geoderma 330, 117–124. doi: 10.1016/j.geoderma.2018.05.034
Bononi, L., Chiaramonte, J. B., Pansa, C. C., Moitinho, M. A., and Melo, I. S. (2020). Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 10:2858. doi: 10.1038/s41598-020-59793-8
Borges, B., Gallo, G., Coelho, C., Negri, N., Maiello, F., Hardy, L., et al. (2021). Dynamic cross correlation analysis of Thermus thermophilus alkaline phosphatase and determinants of thermostability. Biochim. Biophys. Acta 1865:129895. doi: 10.1016/j.bbagen.2021.129895
Brundrett, M. C., and Tedersoo, L. (2018). Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 220, 1108–1115. doi: 10.1111/nph.14976
Campos, A. L., Fronza, B. M., Rodrigues, M. C., Souza Chiari, M. D. E., and Braga, R. R. (2023). Influence of the calcium orthophosphate: glass ratio and calcium orthophosphate functionalization on the degree of conversion and mechanical properties of resin-based composites. J. Biomed. Mater. Res. B 111, 95–102. doi: 10.1002/jbm.b.35136
Cartabia, A., Tsiokanos, E., Tsafantakis, N., Lalaymia, I., Termentzi, A., Miguel, M., et al. (2021). The arbuscular mycorrhizal fungus Rhizophagus irregularis MUCL 41833 modulates metabolites production of Anchusa officinalis L. under semi-hydroponic cultivation. Front. Plant Sci. 12:724352. doi: 10.3389/fpls.2021.724352
Castagno, L. N., Sannazzaro, A. I., Gonzalez, M. E., Pieckenstain, F. L., and Estrella, M. J. (2021). Phosphobacteria as key actors to overcome phosphorus deficiency in plants. Ann. Appl. Biol. 178, 256–267. doi: 10.1111/aab.12673
Chen, A., and Arai, Y. (2023). A review of the reactivity of phosphatase controlled by clays and clay minerals: implications for understanding phosphorus mineralization in soils. Clay Clay Miner. 71, 119–142. doi: 10.1007/s42860-023-00243-7
Chen, X., Jiang, N., Condron, L. M., Dunfield, K. E., Chen, Z., Wang, J., et al. (2019). Soil alkaline phosphatase activity and bacterial phoD gene abundance and diversity under long-term nitrogen and manure inputs. Geoderma 349, 36–44. doi: 10.1016/j.geoderma.2019.04.039
Cheng, Y., Narayanan, M., Shi, X., Chen, X., Li, Z., and Ma, Y. (2023). Phosphate-solubilizing bacteria: their agroecological function and optimistic application for enhancing agro-productivity. Sci. Total Environ. 901:166468. doi: 10.1016/j.scitotenv.2023.166468
Cheng, J., Zhuang, W., Li, N., Tang, C., and Ying, H. (2017). Efficient biosynthesis of d-ribose using a novel co-feeding strategy in Bacillus subtilis without acid formation. Lett. Appl. Microbiol. 64, 73–78. doi: 10.1111/lam.12685
Chiu, C. H., and Paszkowski, U. (2019). Mechanisms and impact of symbiotic phosphate acquisition. Cold Spring Harb. Perspect. Biol. 11:a034603. doi: 10.1101/cshperspect.a034603
Chu, Q., Zhang, L., Zhou, J., Yuan, L., Chen, F., Zhang, F., et al. (2020). Soil plant-available phosphorus levels and maize genotypes determine the phosphorus acquisition efficiency and contribution of mycorrhizal pathway. Plant Soil 449, 357–371. doi: 10.1007/s11104-020-04494-4
Cozzolino, V., Monda, H., Savy, D., Di Meo, V., Vinci, G., and Smalla, K. (2021). Cooperation among phosphate-solubilizing bacteria, humic acids and arbuscular mycorrhizal fungi induces soil microbiome shifts and enhances plant nutrient uptake. Chem. Biol. Technol. Agric. 8:31. doi: 10.1186/s40538-021-00230-x
Dai, Z., Liu, G., Chen, H., Chen, C., Wang, J., Ai, S., et al. (2020). Long-term nutrient inputs shift soil microbial functional profiles of phosphorus cycling in diverse agroecosystems. ISME J. 14, 757–770. doi: 10.1038/s41396-019-0567-9
De Zutter, N., Ameye, M., Vermeir, P., Verwaeren, J., De Gelder, L., and Audenaert, K. (2022). Innovative rhizosphere-based enrichment under P-limitation selects for bacterial isolates with high-performance P-solubilizing traits. Microbiol. Spectr. 10:e0205222. doi: 10.1128/spectrum.02052-22
Dejene, M., Abera, G., and Desalegn, T. (2023). The effect of phosphorus fertilizer sources and lime on acidic soil properties of mollic rhodic nitisol in Welmera District, Central Ethiopia. Appl. Environ. Soil Sci. 2023:7002816. doi: 10.1155/2023/7002816
Della Mónica, I. F., Godeas, A. M., and Scervino, J. M. (2020). In vivo modulation of arbuscular mycorrhizal symbiosis and soil quality by fungal P solubilizers. Microb. Ecol. 79, 21–29. doi: 10.1007/s00248-019-01396-6
Deveau, A., Brulé, C., Palin, B., Champmartin, D., Rubini, P., Garbaye, J., et al. (2010). Role of fungal trehalose and bacterial thiamine in the improved survival and growth of the ectomycorrhizal fungus Laccaria bicolor S238N and the helper bacterium Pseudomonas fluorescens BBc6R8. Environ. Microbiol. Rep. 2, 560–568. doi: 10.1111/j.1758-2229.2010.00145.x
Dipta, B., Bhardwaj, S., Kaushal, M., Kirti, S., and Sharma, R. (2019). Obliteration of phosphorus deficiency in plants by microbial interceded approach. Symbiosis 78, 163–176. doi: 10.1007/s13199-019-00600-y
Divjot, K., Rana, K. L., Tanvir, K., Yadav, N., Yadav, A. N., Kumar, M., et al. (2021). Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and-mobilizing microbes: a review. Pedosphere 31, 43–75. doi: 10.1016/S1002-0160(20)60057-1
Dong, Z., Liu, Y., Li, M., Ci, B., Lu, X., Feng, X., et al. (2023). Effect of different NPK fertilization timing sequences management on soil-petiole system nutrient uptake and fertilizer utilization efficiency of drip irrigation cotton. Sci. Rep. 13:14287. doi: 10.1038/s41598-023-40620-9
Drozd, M., Gangaiah, D., Liu, Z., and Rajashekara, G. (2011). Contribution of TAT system translocated PhoX to Campylobacter jejuni phosphate metabolism and resilience to environmental stresses. PLoS One 6:e26336. doi: 10.1371/journal.pone.0026336
Duan, S., Declerck, S., Feng, G., and Zhang, L. (2023). Hyphosphere interactions between Rhizophagus irregularis and Rahnella aquatilis promote carbon–phosphorus exchange at the peri-arbuscular space in Medicago truncatula. Environ. Microbiol. 25, 867–879. doi: 10.1111/1462-2920.16333
Dudeja, S. S., Suneja-Madan, P., Paul, M., Maheswari, R., and Kothe, E. (2021). Bacterial endophytes: molecular interactions with their hosts. J. Basic Microbiol. 61, 475–505. doi: 10.1002/jobm.202000657
El Maaloum, S., Elabed, A., Alaoui-Talibi, Z. E., Meddich, A., Filali-Maltouf, A., Douira, A., et al. (2020). Effect of arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria consortia associated with phospho-compost on phosphorus solubilization and growth of tomato seedlings (Solanum lycopersicum L.). Commun. Soil Sci. Plant Anal. 51, 622–634. doi: 10.1080/00103624.2020.1729376
Emmett, B. D., Lévesque-Tremblay, V., and Harrison, M. J. (2021). Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J. 15, 2276–2288. doi: 10.1038/s41396-021-00920-2
Etesami, H., Jeong, B. R., and Glick, B. R. (2021). Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci. 12:699618. doi: 10.3389/fpls.2021.699618
Fatima, F., Ahmad, M., Verma, S., and Pathak, N. (2022). Relevance of phosphate solubilizing microbes in sustainable crop production: a review. Int. J. Environ. Sci. Technol. 19, 9283–9296. doi: 10.1007/s13762-021-03425-9
Fernandes, G. C., Rosa, P. A. L., Jalal, A., Oliveira, C. E. S., Galindo, F. S., Viana, R. S., et al. (2023). Technological quality of sugarcane inoculated with plant-growth-promoting bacteria and residual effect of phosphorus rates. Plan. Theory 12:2699. doi: 10.3390/plants12142699
Ferrol, N., Azcón-Aguilar, C., and Pérez-Tienda, J. (2019). Arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: an overview on the mechanisms involved. Plant Sci. 280, 441–447. doi: 10.1016/j.plantsci.2018.11.011
Fraser, T., Lynch, D. H., Entz, M. H., and Dunfield, K. E. (2015). Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 257–258, 115–122. doi: 10.1016/j.geoderma.2014.10.016
Fraser, T. D., Lynch, D. H., Gaiero, J., Khosla, K., and Dunfield, K. E. (2017). Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 111, 48–56. doi: 10.1016/j.apsoil.2016.11.013
Galindo, F. S., Pagliari, P. H., Fernandes, G. C., Rodrigues, W. L., Boleta, E. H. M., Jalal, A., et al. (2022). Improving sustainable field-grown wheat production with Azospirillum brasilense under tropical conditions: a potential tool for improving nitrogen management. Front. Environ. Sci. 10:821628. doi: 10.3389/fenvs.2022.821628
Genre, A., Lanfranco, L., Perotto, S., and Bonfante, P. (2020). Unique and common traits in mycorrhizal symbioses. Nat. Rev. Microbiol. 18, 649–660. doi: 10.1038/s41579-020-0402-3
Gregory, P. J. (2022). Russell review: are plant roots only “in” soil or are they “of” it? Roots, soil formation and function. Eur. J. Soil Sci. 73:e13219. doi: 10.1111/ejss.13219
Hakim, S., Naqqash, T., Nawaz, M. S., Laraib, I., Siddique, M. J., Zia, R., et al. (2021). Rhizosphere engineering with plant growth-promoting microorganisms for agriculture and ecological sustainability. Front. Sustain. Food Syst. 5:617157. doi: 10.3389/fsufs.2021.617157
Hammelehle, A., Oberson, A., Lüscher, A., Mäder, P., and Mayer, J. (2018). Above-and belowground nitrogen distribution of a red clover-perennial ryegrass sward along a soil nutrient availability gradient established by organic and conventional cropping systems. Plant Soil 425, 507–525. doi: 10.1007/s11104-018-3559-z
Hao, J., Knoll, A. H., Huang, F., Schieber, J., Hazen, R. M., and Daniel, I. (2020). Cycling phosphorus on the Archean earth: part II. Phosphorus limitation on primary production in Archean ecosystems. Geochim. Cosmochim. Acta 280, 360–377. doi: 10.1016/j.gca.2020.04.005
Haq, I. U., Dini-Andreote, F., and Van Elsas, J. D. (2017). Transcriptional responses of the bacterium Burkholderia terrae BS001 to the fungal host Lyophyllum sp. strain Karsten under soil-mimicking conditions. Microb. Ecol. 73, 236–252. doi: 10.1007/s00248-016-0885-7
Hessen, D. O., Hafslund, O. T., Andersen, T., Broch, C., Shala, N. K., and Wojewodzic, M. W. (2017). Changes in stoichiometry, cellular RNA, and alkaline phosphatase activity of Chlamydomonas in response to temperature and nutrients. Front. Microbiol. 8:18. doi: 10.3389/fmicb.2017.00018
Hu, M., Penuelas, J., Sardans, J., Tong, C., Chang, C. T., and Cao, W. (2020). Dynamics of phosphorus speciation and the phoD phosphatase gene community in the rhizosphere and bulk soil along an estuarine freshwater-oligohaline gradient. Geoderma 365:114236. doi: 10.1016/j.geoderma.2020.114236
Huang, B., Yan, D., Ouyang, C., Zhang, D., Zhu, J., Liu, J., et al. (2020). Chloropicrin fumigation alters the soil phosphorus and the composition of the encoding alkaline phosphatase PhoD gene microbial community. Sci. Total Environ. 711:135080. doi: 10.1016/j.scitotenv.2019.135080
Ikhajiagbe, B., Anoliefo, G. O., Olise, O. F., Rackelmann, F., Sommer, M., and Adekunle, I. J. (2020). Major phosphorus in soils is unavailable, yet critical for plant development. Not. Sci. Biol. 12, 500–535. doi: 10.15835/nsb12310672
Jaiswal, S. K., Mohammed, M., Ibny, F. Y., and Dakora, F. D. (2021). Rhizobia as a source of plant growth-promoting molecules: potential applications and possible operational mechanisms. Front. Sustain. Food Syst. 4:619676. doi: 10.3389/fsufs.2020.619676
Jayakumar, N., Paulraj, P., Sajeesh, P., Sajna, K., and Zinneera, A. (2019). Application of native phosphate solubilizing bacteria for the use of cheap organic and inorganic phosphate source in agricultural practise of Capsicum annum (chili)-a pilot scale field study. Mater. Today: Proc. 16, 1630–1639. doi: 10.1016/j.matpr.2019.06.028
Jha, V., Dafale, N. A., and Purohit, H. J. (2019). Regulatory rewiring through global gene regulations by PhoB and alarmone (p) ppGpp under various stress conditions. Microbiol. Res. 227:126309. doi: 10.1016/j.micres.2019.126309
Jha, Y., and Mohamed, H. I. (2023). Inoculation with Lysinibacillus fusiformis strain YJ4 and Lysinibacillus sphaericus strain YJ5 alleviates the effects of cold stress in maize plants. Gesunde Pflanzen 75, 77–95. doi: 10.1007/s10343-022-00666-7
Jiang, S., Lu, H., Liu, J., Lin, Y., Dai, M., and Yan, C. (2018). Influence of seasonal variation and anthropogenic activity on phosphorus cycling and retention in mangrove sediments: a case study in China. Estuar. Coast. Shelf Sci. 202, 134–144. doi: 10.1016/j.ecss.2017.12.011
Jiang, Y., Tian, J., and Ge, F. (2020). New insight into carboxylic acid metabolisms and pH regulations during insoluble phosphate solubilisation process by Penicillium oxalicum PSF-4. Curr. Microbiol. 77, 4095–4103. doi: 10.1007/s00284-020-02238-2
Jiang, F., Zhang, L., Zhou, J., George, T. S., and Feng, G. (2021). Arbuscular mycorrhizal fungi enhance mineralisation of organic phosphorus by carrying bacteria along their extraradical hyphae. New Phytol. 230, 304–315. doi: 10.1111/nph.17081
Jiao, P., Yuan, W.-Y., Zhao, H.-D., Qu, J., Wang, P.-W., Guan, S.-Y., et al. (2021). Construction of a new plant expression vector and the development of maize germplasm expressing the Aspergillus ficuum phytase gene PhyA2. Genet. Resour. Crop. Evol. 68, 1103–1115. doi: 10.1007/s10722-020-01052-w
Jin, T., Ren, J., Li, Y., Bai, B., Liu, R., and Wang, Y. (2022). Plant growth-promoting effect and genomic analysis of the P. putida LWPZF isolated from C. japonicum rhizosphere. AMB Express 12:101. doi: 10.1186/s13568-022-01445-3
Joshi, S., Gangola, S., Jaggi, V., and Sahgal, M. (2023). Functional characterization and molecular fingerprinting of potential phosphate solubilizing bacterial candidates from Shisham rhizosphere. Sci. Rep. 13:7003. doi: 10.1038/s41598-023-33217-9
Kafle, A., Cope, K. R., Raths, R., Krishna Yakha, J., Subramanian, S., Bücking, H., et al. (2019). Harnessing soil microbes to improve plant phosphate efficiency in cropping systems. Agronomy 9:127. doi: 10.3390/agronomy9030127
Kageyama, H., Tripathi, K., Rai, A. K., Cha-Um, S., Waditee-Sirisattha, R., and Takabe, T. (2011). An alkaline phosphatase/phosphodiesterase, PhoD, induced by salt stress and secreted out of the cells of Aphanothece halophytica, a halotolerant cyanobacterium. Appl. Environ. Microbiol. 77, 5178–5183. doi: 10.1128/AEM.00667-11
Kalayu, G. (2019). Phosphate solubilizing microorganisms: promising approach as biofertilizers. Int. J. Agron. 2019:4917256. doi: 10.1155/2019/4917256
Kang, S.-M., Khan, M.-A., Hamayun, M., Kim, L.-R., Kwon, E.-H., Kang, Y.-S., et al. (2021). Phosphate-solubilizing Enterobacter ludwigii AFFR02 and Bacillus megaterium Mj1212 rescues alfalfa’s growth under post-drought stress. Agriculture 11:485. doi: 10.3390/agriculture11060485
Karagoz, P., Mandair, R., Manayil, J. C., Lad, J., Chong, K., Kyriakou, G., et al. (2020). Purification and immobilization of engineered glucose dehydrogenase: a new approach to producing gluconic acid from breadwaste. Biotechnol. Biofuels 13:100. doi: 10.1186/s13068-020-01735-7
Karimzadeh, J., Alikhani, H. A., Etesami, H., and Pourbabaei, A. A. (2021). Improved phosphorus uptake by wheat plant (Triticum aestivum L.) with rhizosphere fluorescent pseudomonads strains under water-deficit stress. J. Plant Growth Regul. 40, 162–178. doi: 10.1007/s00344-020-10087-3
Kaur, P., and Chatli, A. S. (2019). P Solubilisers for sustainance of agriculture-a review. Ind. J. Pure App. Biosci. 7, 375–392. doi: 10.18782/2320-7051.7852
Kaur, C., Selvakumar, G., and Upreti, K. K. (2021). Organic acid profiles of phosphate solubilizing bacterial strains in the presence of different insoluble phosphatic sources under. J. Pure Appl. Microbiol. 15, 1006–1015. doi: 10.22207/JPAM.15.2.59
Kour, D., Rana, K. L., Kaur, T., Sheikh, I., Yadav, A. N., Kumar, V., et al. (2020). Microbe-mediated alleviation of drought stress and acquisition of phosphorus in great millet (Sorghum bicolour L.) by drought-adaptive and phosphorus-solubilizing microbes. Biocatal. Agric. Biotechnol. 23:101501. doi: 10.1016/j.bcab.2020.101501
Kour, D., and Yadav, A. N. (2023). Alleviation of cold stress in wheat with psychrotrophic phosphorus solubilizing Acinetobacter rhizosphaerae EU-KL44. Braz. J. Microbiol. 54, 371–383. doi: 10.1007/s42770-023-00913-7
Kumar, C., Wagh, J., Archana, G., and Naresh Kumar, G. (2016). Sucrose dependent mineral phosphate solubilization in Enterobacter asburiae PSI3 by heterologous overexpression of periplasmic invertases. World J. Microbiol. Biotechnol. 32:194. doi: 10.1007/s11274-016-2153-x
Kumawat, K. C., Sharma, P., Nagpal, S., Gupta, R., Sirari, A., Nair, R. M., et al. (2021). Dual microbial inoculation, a game changer?—bacterial biostimulants with multifunctional growth promoting traits to mitigate salinity stress in spring mungbean. Front. Microbiol. 11:600576. doi: 10.3389/fmicb.2020.600576
Li, L., Jiao, Z., Hale, L., Wu, W., and Guo, Y. (2014). Disruption of gene pqq A or pqq B reduces plant growth promotion activity and biocontrol of crown gall disease by Rahnella aquatilis HX2. PLoS One 9:e115010. doi: 10.1371/journal.pone.0115010
Li, C., Li, Q., Wang, Z., Ji, G., Zhao, H., Gao, F., et al. (2019). Environmental fungi and bacteria facilitate lecithin decomposition and the transformation of phosphorus to apatite. Sci. Rep. 9:15291. doi: 10.1038/s41598-019-51804-7
Li, H., Song, C., Yang, L., Qin, H., Cao, X., and Zhou, Y. (2021). Nutrients regeneration pathway, release potential, transformation pattern and algal utilization strategies jointly drove cyanobacterial growth and their succession. J. Environ. Sci. 103, 255–267. doi: 10.1016/j.jes.2020.11.010
Li, X., Sun, P., Zhang, Y., Jin, C., and Guan, C. (2020). A novel PGPR strain Kocuria rhizophila Y1 enhances salt stress tolerance in maize by regulating phytohormone levels, nutrient acquisition, redox potential, ion homeostasis, photosynthetic capacity and stress-responsive genes expression. Environ. Exp. Bot. 174:104023. doi: 10.1016/j.envexpbot.2020.104023
Li, Y., Zhang, J., Gong, Z., Xu, W., and Mou, Z. (2019). Gcd gene diversity of quinoprotein glucose dehydrogenase in the sediment of Sancha lake and its response to the environment. Int. J. Environ. Res. Public Health 16:1. doi: 10.3390/ijerph16010001
Liang, J.-L., Liu, J., Jia, P., Yang, T.-T., Zeng, Q.-W., Zhang, S.-C., et al. (2020). Novel phosphate-solubilizing bacteria enhance soil phosphorus cycling following ecological restoration of land degraded by mining. ISME J. 14, 1600–1613. doi: 10.1038/s41396-020-0632-4
Liu, J., Cade-Menun, B. J., Yang, J., Hu, Y., Liu, C. W., Tremblay, J., et al. (2018). Long-term land use affects phosphorus speciation and the composition of phosphorus cycling genes in agricultural soils. Front. Microbiol. 9:1643. doi: 10.3389/fmicb.2018.01643
Liu, X., Han, R., Cao, Y., Turner, B. L., and Ma, L. Q. (2022). Enhancing phytate availability in soils and phytate-P acquisition by plants: a review. Environ. Sci. Technol. 56, 9196–9219. doi: 10.1021/acs.est.2c00099
Liu, X., Jiang, X., He, X., Zhao, W., Cao, Y., Guo, T., et al. (2019). Phosphate-solubilizing Pseudomonas sp. strain P34-L promotes wheat growth by colonizing the wheat rhizosphere and improving the wheat root system and soil phosphorus nutritional status. J. Plant Growth Regul. 38, 1314–1324. doi: 10.1007/s00344-019-09935-8
Liu, J., Liu, X., Zhang, Q., Li, S., Sun, Y., Lu, W., et al. (2020). Response of alfalfa growth to arbuscular mycorrhizal fungi and phosphate-solubilizing bacteria under different phosphorus application levels. AMB Express 10:200. doi: 10.1186/s13568-020-01137-w
Liu, C., Mou, L., Yi, J., Wang, J., Liu, A., and Yu, J. (2019). The eno gene of Burkholderia cenocepacia strain 71-2 is involved in phosphate solubilization. Curr. Microbiol. 76, 495–502. doi: 10.1007/s00284-019-01642-7
Liu, F., Qian, J., Zhu, Y., Wang, P., Hu, J., Lu, B., et al. (2024). Phosphate solubilizing microorganisms increase soil phosphorus availability: a review. Geomicrobiol J. 41, 1–16. doi: 10.1080/01490451.2023.2272620
Lo, S.-C., Tsai, S.-Y., Chang, W.-H., Wu, I.-C., Sou, N.-L., Hung, S.-H. W., et al. (2023). Characterization of the pyrroloquinoline quinone producing Rhodopseudomonas palustris as a plant growth-promoting bacterium under photoautotrophic and photoheterotrophic culture conditions. Int. J. Mol. Sci. 24:14080. doi: 10.3390/ijms241814080
Lü, J., Gao, X., Dong, Z., and An, L. (2012). Expression of mitochondrial malate dehydrogenase in Escherichia coli improves phosphate solubilization. Ann. Microbiol. 62, 607–614. doi: 10.1007/s13213-011-0297-3
Ludueña, L. M., Anzuay, M. S., Magallanes-Noguera, C., Tonelli, M. L., Ibañez, F. J., Angelini, J. G., et al. (2017). Effects of P limitation and molecules from peanut root exudates on pqqE gene expression and pqq promoter activity in the phosphate-solubilizing strain Serratia sp. S119. Res. Microbiol. 168, 710–721. doi: 10.1016/j.resmic.2017.07.001
Luthfiana, N., Inamura, N., Tantriani, S., Sato, T., Saito, K., Oikawa, A., et al. (2021). Metabolite profiling of the hyphal exudates of Rhizophagus clarus and Rhizophagus irregularis under phosphorus deficiency. Mycorrhiza 31, 403–412. doi: 10.1007/s00572-020-01016-z
Lynch, J. P. (2019). Root phenotypes for improved nutrient capture: an underexploited opportunity for global agriculture. New Phytol. 223, 548–564. doi: 10.1111/nph.15738
Lyu, J., Huang, Y., Nie, Q., Lu, C., Zhang, Y., Fu, X., et al. (2023). Spatiotemporal variations and risk characteristics of potential non-point source pollution driven by LUCC in the loess plateau region, China. Front. Ecol. Evol. 11:1253328. doi: 10.3389/fevo.2023.1253328
Ma, X., Li, X., and Ludewig, U. (2021). Arbuscular mycorrhizal colonization outcompetes root hairs in maize under low phosphorus availability. Ann. Bot. 127, 155–166. doi: 10.1093/aob/mcaa159
Ma, J., Ma, Y., Wei, R., Chen, Y., Weng, L., Ouyang, X., et al. (2021). Phosphorus transport in different soil types and the contribution of control factors to phosphorus retardation. Chemosphere 276:130012. doi: 10.1016/j.chemosphere.2021.130012
Mahdi, I., Fahsi, N., Hafidi, M., Benjelloun, S., Allaoui, A., and Biskri, L. (2021). Rhizospheric phosphate solubilizing Bacillus atrophaeus GQJK17 S8 increases quinoa seedling, withstands heavy metals, and mitigates salt stress. Sustain. For. 13:3307. doi: 10.3390/su13063307
Mei, Y., Zhang, M., Cao, G., Zhu, J., Zhang, A., Bai, H., et al. (2024). Endofungal bacteria and ectomycorrhizal fungi synergistically promote the absorption of organic phosphorus in Pinus massoniana. Plant Cell Environ. 47, 600–610. doi: 10.1111/pce.14742
Mendoza-Arroyo, G. E., Chan-Bacab, M. J., Aguila-Ramírez, R. N., Ortega-Morales, B. O., Canché Solís, R. E., Chab-Ruiz, A. O., et al. (2020). Inorganic phosphate solubilization by a novel isolated bacterial strain Enterobacter sp. ITCB-09 and its application potential as biofertilizer. Agriculture 10:383. doi: 10.3390/agriculture10090383
Murgese, P., Santamaria, P., Leoni, B., and Crecchio, C. (2020). Ameliorative effects of PGPB on yield, physiological parameters, and nutrient transporter genes expression in barattiere (Cucumis melo L.). J. Soil Sci. Plant Nutr. 20, 784–793. doi: 10.1007/s42729-019-00165-1
Nacoon, S., Seemakram, W., Ekprasert, J., Jogloy, S., Kuyper, T. W., Mongkolthanaruk, W., et al. (2022). Promoting growth and production of sunchoke (Helianthus tuberosus) by co-inoculation with phosphate solubilizing bacteria and arbuscular mycorrhizal fungi under drought. Front. Plant Sci. 13:1022319. doi: 10.3389/fpls.2022.1022319
Naseem, H., Ahsan, M., Shahid, M. A., and Khan, N. (2018). Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 58, 1009–1022. doi: 10.1002/jobm.201800309
Nepomuceno, R. A., Brown, C. M. B., Mojica, P. N., and Brown, M. B. (2019). Biological control potential of vesicular arbuscular mycorrhizal root inoculant (VAMRI) and associated phosphate solubilizing bacteria, Pseudochrobactrum asaccharolyticum against soilborne phytopathogens of onion (Allium cepa L. var. red creole). Arch. Phytopathol. Plant Protect. 52, 714–732. doi: 10.1080/03235408.2019.1644058
Ortega-Torres, A. E., Rico-García, E., Guzmán-Cruz, R., Torres-Pacheco, I., Tovar-Pérez, E. G., and Guevara-González, R. G. (2021). Addition of phosphatases and phytases to mature compost to increase available phosphorus: a short study. Agronomy 11:2555. doi: 10.3390/agronomy11122555
Osman, H. S., Rady, A. M., Awadalla, A., Omara, A. E.-D., and Hafez, E. M. (2022). Improving the antioxidants system, growth, and sugar beet quality subjected to long-term osmotic stress by phosphate solubilizing bacteria and compost tea. Int. J. Plant Prod. 16, 119–135. doi: 10.1007/s42106-021-00176-y
Oteino, N., Lally, R. D., Kiwanuka, S., Lloyd, A., Ryan, D., Germaine, K. J., et al. (2015). Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 6:745. doi: 10.3389/fmicb.2015.00745
Park, Y., Solhtalab, M., Thongsomboon, W., and Aristilde, L. (2022). Strategies of organic phosphorus recycling by soil bacteria: acquisition, metabolism, and regulation. Environ. Microbiol. Rep. 14, 3–24. doi: 10.1111/1758-2229.13040
Pent, M., Bahram, M., and Põldmaa, K. (2020). Fruitbody chemistry underlies the structure of endofungal bacterial communities across fungal guilds and phylogenetic groups. ISME J. 14, 2131–2141. doi: 10.1038/s41396-020-0674-7
Prabhu, N., Borkar, S., and Garg, S. (2018). Phosphate solubilization mechanisms in alkaliphilic bacterium Bacillus marisflavi FA7. Curr. Sci. 114, 845–853. doi: 10.18520/cs/v114/i04/845-853
Prabhu, N., Borkar, S., and Garg, S. (2019). “Chapter 11—Phosphate solubilization by microorganisms: overview, mechanisms, applications and advances” in Advances in biological science research. eds. S. N. Meena and M. M. Naik (London: Academic Press), 161–176.
Rai, A., Sharma, N. K., Singh, V. K., Dwivedi, B. S., Singh, J. S., and Rai, P. K. (2023). Study of phosphate solubilizing fluorescent Pseudomonas recovered from rhizosphere and endorhizosphere of Aloe barbadensis (L.). Geomicrobiol J. 40, 347–359. doi: 10.1080/01490451.2023.2171165
Rasul, M., Yasmin, S., Suleman, M., Zaheer, A., Reitz, T., Tarkka, M. T., et al. (2019). Glucose dehydrogenase gene containing phosphobacteria for biofortification of phosphorus with growth promotion of rice. Microbiol. Res. 223-225, 1–12. doi: 10.1016/j.micres.2019.03.004
Rasul, M., Yasmin, S., Yahya, M., Breitkreuz, C., Tarkka, M., and Reitz, T. (2021). The wheat growth-promoting traits of Ochrobactrum and Pantoea species, responsible for solubilization of different P sources, are ensured by genes encoding enzymes of multiple P-releasing pathways. Microbiol. Res. 246:126703. doi: 10.1016/j.micres.2021.126703
Raven, J. A., Lambers, H., Smith, S. E., and Westoby, M. (2018). Costs of acquiring phosphorus by vascular land plants: patterns and implications for plant coexistence. New Phytol. 217, 1420–1427. doi: 10.1111/nph.14967
Rawat, P., Das, S., Shankhdhar, D., and Shankhdhar, S. (2021). Phosphate-solubilizing microorganisms: mechanism and their role in phosphate solubilization and uptake. J. Soil Sci. Plant Nutr. 21, 49–68. doi: 10.1007/s42729-020-00342-7
Rawat, P., Sharma, A., Shankhdhar, D., and Shankhdhar, S. C. (2022). Improvement of phosphorus uptake, phosphorus use efficiency, and grain yield of upland rice (Oryza sativa L.) in response to phosphate-solubilizing bacteria blended with phosphorus fertilizer. Pedosphere 32, 752–763. doi: 10.1016/j.pedsph.2022.06.005
Ribeiro, V. P., Marriel, I. E., Sousa, S. M. D., Lana, U. G. D. P., Mattos, B. B., Oliveira, C. A., et al. (2018). Endophytic Bacillus strains enhance pearl millet growth and nutrient uptake under low-P. Braz. J. Microbiol. 49, 40–46. doi: 10.1016/j.bjm.2018.06.005
Rizvi, A., Ahmed, B., Khan, M. S., Umar, S., and Lee, J. (2021). Psychrophilic bacterial phosphate-biofertilizers: a novel extremophile for sustainable crop production under cold environment. Microorganisms 9:2451. doi: 10.3390/microorganisms9122451
Rosa, P. A. L., Galindo, F. S., Oliveira, C. E. D. S., Jalal, A., Mortinho, E. S., Fernandes, G. C., et al. (2022). Inoculation with plant growth-promoting bacteria to reduce phosphate fertilization requirement and enhance technological quality and yield of sugarcane. Microorganisms 10:192. doi: 10.3390/microorganisms10010192
Rosa, P. A. L., Mortinho, E. S., Jalal, A., Galindo, F. S., Buzetti, S., Fernandes, G. C., et al. (2020). Inoculation with growth-promoting bacteria associated with the reduction of phosphate fertilization in sugarcane. Front. Environ. Sci. 8:32. doi: 10.3389/fenvs.2020.00032
Schneider, K. D., Thiessen Martens, J. R., Zvomuya, F., Reid, D. K., Fraser, T. D., Lynch, D. H., et al. (2019). Options for improved phosphorus cycling and use in agriculture at the field and regional scales. J. Environ. Qual. 48, 1247–1264. doi: 10.2134/jeq2019.02.0070
Sebastián, M., and Ammerman, J. (2011). Role of the phosphatase PhoX in the phosphorus metabolism of the marine bacterium Ruegeria pomeroyi DSS-3. Environ. Microbiol. Rep. 3, 535–542. doi: 10.1111/j.1758-2229.2011.00253.x
Shaffique, S., Khan, M. A., Wani, S. H., Imran, M., Kang, S.-M., Pande, A., et al. (2022). Biopriming of maize seeds with a novel bacterial strain SH-6 to enhance drought tolerance in South Korea. Plan. Theory 11:1674. doi: 10.3390/plants11131674
Shah, S. H., Hussain, M. B., Zahir, Z. A., Haq, T. U., and Matloob, A. (2022). Thermal plasticity and cotton production enhancing attributes of phosphate-solubilizing bacteria from cotton rhizosphere. J. Soil Sci. Plant Nutr. 22, 3885–3900. doi: 10.1007/s42729-022-00937-2
Sharma, S., Compant, S., Ballhausen, M.-B., Ruppel, S., and Franken, P. (2020). The interaction between Rhizoglomus irregulare and hyphae attached phosphate solubilizing bacteria increases plant biomass of Solanum lycopersicum. Microbiol. Res. 240:126556. doi: 10.1016/j.micres.2020.126556
Shi, J., Zhao, B., Zheng, S., Zhang, X., Wang, X., Dong, W., et al. (2021). A phosphate starvation response-centered network regulates mycorrhizal symbiosis. Cell 184, 5527–5540.e18. doi: 10.1016/j.cell.2021.09.030
Shrivastava, M., Srivastava, P., and D’souza, S. (2018). “Phosphate-solubilizing microbes: diversity and phosphates solubilization mechanism” in Role of rhizospheric microbes in soil. ed. V. Meena (Singapore: Springer), 137–165.
Siles, J. A., Starke, R., Martinovic, T., Fernandes, M. L. P., Orgiazzi, A., and Bastida, F. (2022). Distribution of phosphorus cycling genes across land uses and microbial taxonomic groups based on metagenome and genome mining. Soil Biol. Biochem. 174:108826. doi: 10.1016/j.soilbio.2022.108826
Singh, P., and Banik, R. M. (2019). Effect of purified alkaline phosphatase from Bacillus licheniformis on growth of Zea mays L. Plant Sci. Today 6, 583–589. doi: 10.14719/pst.2019.6.sp1.676
Srivastava, A., Saavedra, D. E., Thomson, B., García, J. A., Zhao, Z., Patrick, W. M., et al. (2021). Enzyme promiscuity in natural environments: alkaline phosphatase in the ocean. ISME J. 15, 3375–3383. doi: 10.1038/s41396-021-01013-w
Stefanoni Rubio, P. J., Godoy, M. S., Della Mónica, I. F., Pettinari, M. J., Godeas, A. M., and Scervino, J. M. (2016). Carbon and nitrogen sources influence tricalcium phosphate solubilization and extracellular phosphatase activity by Talaromyces flavus. Curr. Microbiol. 72, 41–47. doi: 10.1007/s00284-015-0914-7
Suleman, M., Yasmin, S., Rasul, M., Yahya, M., Atta, B. M., and Mirza, M. S. (2018). Phosphate solubilizing bacteria with glucose dehydrogenase gene for phosphorus uptake and beneficial effects on wheat. PLoS One 13:e0204408. doi: 10.1371/journal.pone.0204408
Sun, Q., Qiu, H., Hu, Y., Wei, X., Chen, X., Ge, T., et al. (2019). Cellulose and lignin regulate partitioning of soil phosphorus fractions and alkaline phosphomonoesterase encoding bacterial community in phosphorus-deficient soils. Biol. Fertil. Soils 55, 31–42. doi: 10.1007/s00374-018-1325-2
Tahir, M., Naeem, M. A., Shahid, M., Khalid, U., Farooq, A. U., Ahmad, N., et al. (2020). Inoculation of pqqE gene inhabiting Pantoea and Pseudomonas strains improves the growth and grain yield of wheat with a reduced amount of chemical fertilizer. J. Appl. Microbiol. 129, 575–589. doi: 10.1111/jam.14630
Tan, H., Barret, M., Mooij, M. J., Rice, O., Morrissey, J. P., Dobson, A., et al. (2013). Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soils 49, 661–672. doi: 10.1007/s00374-012-0755-5
Tarkka, M. T., Drigo, B., and Deveau, A. (2018). Mycorrhizal microbiomes. Mycorrhiza 28, 403–409. doi: 10.1007/s00572-018-0865-5
Tayade, A., Geetha, P., Anusha, S., Dhanapal, R., and Hari, K. (2019). Bio-intensive modulation of sugarcane ratoon rhizosphere for enhanced soil health and sugarcane productivity under tropical Indian condition. Sugar Tech 21, 278–288. doi: 10.1007/s12355-018-0669-0
Thampi, M., Dhanraj, N., Prasad, A., Ganga, G., and Jisha, M. (2023). Phosphorus solubilizing microbes (PSM): biological tool to combat salinity stress in crops. Symbiosis 91, 15–32. doi: 10.1007/s13199-023-00947-3
Timofeeva, A., Galyamova, M., and Sedykh, S. (2022). Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plan. Theory 11:2119. doi: 10.3390/plants11162119
Toscano-Verduzco, F. A., Cedeño-Valdivia, P. A., Chan-Cupul, W., Hernández-Ortega, H. A., Ruiz-Sánchez, E., Galindo-Velasco, E., et al. (2020). Phosphates solubilization, indol-3-acetic acid and siderophores production by Beauveria brongniartii and its effect on growth and fruit quality of Capsicum chinense. J. Hortic. Sci. Biotechnol. 95, 235–246. doi: 10.1080/14620316.2019.1662737
Unnikrishnan, B. V., and Binitha, N. K. (2024). Positive effect of inoculation with an Aspergillus strain on phosphorus and iron nutrition plus volatile organic compounds in rice. Folia Microbiol., 1–10. doi: 10.1007/s12223-024-01129-4
Varinderpal-Singh, S., Sharma, S., Kunal,, Gosal, S. K., Choudhary, R., Singh, R., et al. (2020). Synergistic use of plant growth-promoting rhizobacteria, arbuscular mycorrhizal fungi, and spectral properties for improving nutrient use efficiencies in wheat (Triticum aestivum L.). Commun. Soil Sci. Plant Anal. 51, 14–27. doi: 10.1080/00103624.2019.1689259
Venkiteshwaran, K., Kennedy, E., Graeber, C., Mallick, S. P., Mcnamara, P. J., and Mayer, B. K. (2021). Conversion of soluble recalcitrant phosphorus to recoverable orthophosphate form using UV/H2O2. Chemosphere 278:130391. doi: 10.1016/j.chemosphere.2021.130391
Wahid, F., Fahad, S., Danish, S., Adnan, M., Yue, Z., Saud, S., et al. (2020). Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 10:334. doi: 10.3390/agriculture10080334
Wan, W., Qin, Y., Wu, H., Zuo, W., He, H., Tan, J., et al. (2020). Isolation and characterization of phosphorus solubilizing bacteria with multiple phosphorus sources utilizing capability and their potential for lead immobilization in soil. Front. Microbiol. 11:752. doi: 10.3389/fmicb.2020.00752
Wang, M., Jiang, T., Mao, Y., Wang, F., Yu, J., and Zhu, C. (2023). Current situation of agricultural non-point source pollution and its control. Water Air Soil Pollut. 234:471. doi: 10.1007/s11270-023-06462-x
Wang, C., Xue, L., and Jiao, R. (2021). Soil phosphorus fractions, phosphatase activity, and the abundance of phoC and phoD genes vary with planting density in subtropical Chinese fir plantations. Soil Tillage Res. 209:104946. doi: 10.1016/j.still.2021.104946
Wang, Z., Yu, Z., Solanki, M., Yang, L., Xing, Y., Dong, D., et al. (2020). Diversity of sugarcane root-associated endophytic Bacillus and their activities in enhancing plant growth. J. Appl. Microbiol. 128, 814–827. doi: 10.1111/jam.14512
Wang, L., Zhou, F., Zhou, J., Harvey, P. R., Yu, H., Zhang, G., et al. (2022). Genomic analysis of Pseudomonas asiatica JP233: An efficient phosphate-solubilizing bacterium. Genes 13:2290. doi: 10.3390/genes13122290
Weng, W., Yan, J., Zhou, M., Yao, X., Gao, A., Ma, C., et al. (2022). Roles of arbuscular mycorrhizal fungi as a biocontrol agent in the control of plant diseases. Microorganisms 10:1266. doi: 10.3390/microorganisms10071266
Wijeratne, S., Bakshi, A., and Talbert, J. (2022). Comparative analysis of NanoLuc luciferase and alkaline phosphatase luminescence reporter systems for phage-based detection of bacteria. Bioengineering 9:479. doi: 10.3390/bioengineering9090479
Wise, N. M., Wagner, S. J., Worst, T. J., Sprague, J. E., and Oechsle, C. M. (2021). Comparison of swab types for collection and analysis of microorganisms. MicrobiologyOpen 10:e1244. doi: 10.1002/mbo3.1244
Wu, X., Rensing, C., Han, D., Xiao, K.-Q., Dai, Y., Tang, Z., et al. (2022). Genome-resolved metagenomics reveals distinct phosphorus acquisition strategies between soil microbiomes. mSystems 7, e01107–e01121. doi: 10.1128/msystems.01107-21
Xie, E., Su, Y., Deng, S., Kontopyrgou, M., and Zhang, D. (2021). Significant influence of phosphorus resources on the growth and alkaline phosphatase activities of Microcystis aeruginosa. Environ. Pollut. 268:115807. doi: 10.1016/j.envpol.2020.115807
Xing, Y., Wang, F., Yu, S., Zhu, Y., Ying, Y., and Shi, W. (2023). Enhancing Phyllostachys edulis seedling growth in phosphorus-deficient soil: complementing the role of phosphate-solubilizing microorganisms with arbuscular mycorrhizal fungi. Plant Soil, 1–18. doi: 10.1007/s11104-023-06406-8
Xing, P., Zhao, Y., Guan, D., Li, L., Zhao, B., Ma, M., et al. (2022). Effects of bradyrhizobium co-inoculated with Bacillus and Paenibacillus on the structure and functional genes of soybean rhizobacteria community. Genes 13:1922. doi: 10.3390/genes13111922
Xu, L., Cao, H., Li, C., Wang, C., He, N., Hu, S., et al. (2022). The importance of rare versus abundant phoD-harboring subcommunities in driving soil alkaline phosphatase activity and available P content in Chinese steppe ecosystems. Soil Biol. Biochem. 164:108491. doi: 10.1016/j.soilbio.2021.108491
Yadav, A. N. (2020). “Plant microbiomes for sustainable agriculture: current research and future challenges” in Plant microbiomes for sustainable agriculture. eds. A. Yadav, J. Singh, A. Rastegari, and N. Yadav (Cham: Springer), 475–482.
Yadav, K., Kumar, C., Archana, G., and Kumar, G. N. (2014). Artificial citrate operon and Vitreoscilla hemoglobin gene enhanced mineral phosphate solubilizing ability of Enterobacter hormaechei DHRSS. Appl. Microbiol. Biotechnol. 98, 8327–8336. doi: 10.1007/s00253-014-5912-3
Yadav, A. N., Kumar, R., Kumar, S., Kumar, V., Sugitha, T., Singh, B., et al. (2017). Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J. Appl. Biol. Biotechnol. 5, 45–57. doi: 10.7324/JABB.2017.50607
Yarzábal, L. A., Monserrate, L., Buela, L., and Chica, E. (2018). Antarctic Pseudomonas spp. promote wheat germination and growth at low temperatures. Polar Biol. 41, 2343–2354. doi: 10.1007/s00300-018-2374-6
Yuan, H., Cai, Y., Wang, H., Liu, E., Li, Q., and Zeng, Q. (2023). How phoD-harboring functional microbial populations trigger the release risk of phosphorus in water sediment system of Shijiuhu Lake, China after experiencing the transseasonal shift. Water Res. 240:120107. doi: 10.1016/j.watres.2023.120107
Zaborowska, M., Wyszkowska, J., and Kucharski, J. (2020). Soil enzyme response to bisphenol F contamination in the soil bioaugmented using bacterial and mould fungal consortium. Environ. Monit. Assess. 192:20. doi: 10.1007/s10661-019-7999-6
Zai, X.-M., Fan, J.-J., Hao, Z.-P., Liu, X.-M., and Zhang, W.-X. (2021). Effect of co-inoculation with arbuscular mycorrhizal fungi and phosphate solubilizing fungi on nutrient uptake and photosynthesis of beach palm under salt stress environment. Sci. Rep. 11:5761. doi: 10.1038/s41598-021-84284-9
Zeng, T., Holmer, R., Hontelez, J., Te Lintel-Hekkert, B., Marufu, L., De Zeeuw, T., et al. (2018). Host-and stage-dependent secretome of the arbuscular mycorrhizal fungus Rhizophagus irregularis. Plant J. 94, 411–425. doi: 10.1111/tpj.13908
Zeng, Q., Wu, X., and Wen, X. (2016). Effects of soluble phosphate on phosphate-solubilizing characteristics and expression of gcd gene in Pseudomonas frederiksbergensis JW-SD2. Curr. Microbiol. 72, 198–206. doi: 10.1007/s00284-015-0938-z
Zhang, L., Feng, G., and Declerck, S. (2018). Signal beyond nutrient, fructose, exuded by an arbuscular mycorrhizal fungus triggers phytate mineralization by a phosphate solubilizing bacterium. ISME J. 12, 2339–2351. doi: 10.1038/s41396-018-0171-4
Zhang, J., Han, X., Su, Y., Staehelin, C., and Xu, C. (2023). T-DNA insertion mutagenesis in Penicillium brocae results in identification of an enolase gene mutant impaired in secretion of organic acids and phosphate solubilization. Microbiology 169:001325. doi: 10.1099/mic.0.001325
Zhang, J., Wang, P., Tao, Z., Tian, H., and Guo, T. (2022). Phosphate-solubilizing bacteria abate cadmium absorption and restore the rhizospheric bacterial community composition of grafted watermelon plants. J. Hazard. Mater. 438:129563. doi: 10.1016/j.jhazmat.2022.129563
Zhang, L., Xu, M., Liu, Y., Zhang, F., Hodge, A., and Feng, G. (2016). Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 210, 1022–1032. doi: 10.1111/nph.13838
Zhang, X., Zhan, Y., Zhang, H., Wang, R., Tao, X., Zhang, L., et al. (2021). Inoculation of phosphate-solubilizing bacteria (Bacillus) regulates microbial interaction to improve phosphorus fractions mobilization during kitchen waste composting. Bioresour. Technol. 340:125714. doi: 10.1016/j.biortech.2021.125714
Zhang, L., Zhou, J., George, T. S., Limpens, E., and Feng, G. (2022). Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 27, 402–411. doi: 10.1016/j.tplants.2021.10.008
Zhang, Z., Zhu, L., Li, D., Wang, N., Sun, H., Zhang, Y., et al. (2021). In situ root phenotypes of cotton seedlings under phosphorus stress revealed through RhizoPot. Front. Plant Sci. 12:716691. doi: 10.3389/fpls.2021.716691
Zhao, K., Penttinen, P., Zhang, X., Ao, X., Liu, M., Yu, X., et al. (2014). Maize rhizosphere in Sichuan, China, hosts plant growth promoting Burkholderia cepacia with phosphate solubilizing and antifungal abilities. Microbiol. Res. 169, 76–82. doi: 10.1016/j.micres.2013.07.003
Zhou, Y., Zhang, T., Jin, S., Chen, S., and Zhang, Y. (2021). Effects of Escherichia coli alkaline phosphatase PhoA on the mineralization of dissolved organic phosphorus. Water 13:3315. doi: 10.3390/w13233315
Zhou, J., Zhang, Y., Wu, K., Hu, M., Wu, H., and Chen, D. (2021). National estimates of environmental thresholds for upland soil phosphorus in China based on a meta-analysis. Sci. Total Environ. 780:146677. doi: 10.1016/j.scitotenv.2021.146677
Zhu, J., Li, M., and Whelan, M. (2018). Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: a review. Sci. Total Environ. 612, 522–537. doi: 10.1016/j.scitotenv.2017.08.095
Zolfaghari, R., Rezaei, K., Fayyaz, P., Naghiha, R., and Namvar, Z. (2021). The effect of indigenous phosphate-solubilizing bacteria on Quercus brantii seedlings under water stress. J. Sustain. For. 40, 733–747. doi: 10.1080/10549811.2020.1817757
Zuluaga, M. Y. A., De Oliveira, A. L. M., Valentinuzzi, F., Jayme, N. S., Monterisi, S., Fattorini, R., et al. (2023). An insight into the role of the organic acids produced by Enterobacter sp. strain 15S in solubilizing tricalcium phosphate: in situ study on cucumber. BMC Microbiol. 23:184. doi: 10.1186/s12866-023-02918-6
Keywords: phosphorus-solubilizing microorganisms, phosphorus, organic acids, phosphatase, arbuscular mycorrhizal fungi
Citation: Pang F, Li Q, Solanki MK, Wang Z, Xing Y-X and Dong D-F (2024) Soil phosphorus transformation and plant uptake driven by phosphate-solubilizing microorganisms. Front. Microbiol. 15:1383813. doi: 10.3389/fmicb.2024.1383813
Received: 08 February 2024; Accepted: 14 March 2024;
Published: 27 March 2024.
Edited by:
Surendra Sarsaiya, Zunyi Medical University, ChinaReviewed by:
Zengqiang Li, Henan Institute of Science and Technology, ChinaCopyright © 2024 Pang, Li, Solanki, Wang, Xing and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhen Wang, d2FuZzc5ODExMDUxMEAxNjMuY29t; Yong-Xiu Xing, ZG9jdW1lbnQxMjZAMTI2LmNvbQ==; Deng-Feng Dong, ZG9uZ2RmeHlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.