- State Key Laboratory of Tree Genetics and Breeding, Key Open Laboratory of Forest Genetics and Gene Engineering of National Forestry and Grassland Administration, Co-Innovation Center for Sustainable Forestry in Southern China, Nanjing Forestry University, Nanjing, China
Introduction: Rhizosphere microorganisms are influenced by vegetation. Meanwhile, they respond to vegetation through their own changes, developing an interactive feedback system between microorganisms and vegetation. However, it is still unclear whether the functional diversity of rhizosphere soil microorganisms varies with different carbon storage levels and what factors affect the functional diversity of rhizosphere soil microorganisms.
Methods: In this study, the Biolog-Eco microplate technique was used to analyze the metabolic diversity of carbon source of rhizosphere soil microorganisms from 6 Pinus massoniana provenances with three levels of high, medium and low carbon storage.
Results: The results showed that the average well color development(AWCD) value of rhizosphere microorganisms was significantly positive correlated with carbon storage level of Pinus massoniana (p < 0.05). The AWCD value, Simpson and Shannon diversity of high carbon sequestrance provenances were 1.40 (144h incubation) 0.96 and 3.24, respectively, which were significantly higher (p < 0.05) than those of other P. massoniana provenances. The rhizosphere microbial AWCD, Shannon and Simpson diversity of the 6 provenances showed the same variation trend (SM>AY>QJ>SX>HF>SW). Similarly, microbial biomass carbon (MBC) content was positively correlated with carbon storage level, and there were significant differences among high, medium and low carbon storage provenances. The PCA results showed that the differences in the carbon source metabolism of rhizosphere microorganisms were mainly reflected in the utilization of amino acids, carboxylic acids and carbohydrates. Pearson correlation analysis showed that soil organic carbon (SOC), total nitrogen (TN) and pH were significantly correlated with rhizosphere AWCD (p < 0.05).
Conclusion: Soil properties are important factors affecting rhizosphere microbial carbon source metabolism. The study confirmed that the microorganisms of high carbon storage provenances had relatively high carbon metabolic activity. Among them, the carbon metabolic activity of rhizosphere microorganisms of SM provenance was the highest, which was the preferred provenances in effective ecological service function.
1 Introduction
As the largest carbon reservoir in the terrestrial ecosystem, forests play a vital role in mitigating greenhouse gas emissions and slowing down global warming (Chen et al., 2022). Forest ecosystems effectively regulate CO2 accumulation by converting it into organic carbon through plant photosynthesis and sequestering it in plants or soil, a process known as forest carbon storage (Cai et al., 2023). According to statistics, the total global forest carbon storage is 662PgC, with approximately 45% stored in above-ground biomass carbon (Li et al., 2020). Recently, with the increasing problems of climate change and carbon emissions, forest carbon storage research has become a global priority. More and more studies have focused on the correlation between forest above-ground biological carbon storage and environmental factors such as climatic conditions (Labriere et al., 2023), soil moisture and temperature (Mercado et al., 2009), total carbon content (Jiao et al., 2016), and total nitrogen (TN) content (Heinrich et al., 2021).
Pinus massoniana, with its substantial forest carbon storage, has become a primary afforestation species in barren mountains in China due to its adaptability, wide planting range, and ease of survival. It is the most widely distributed species of Pinus in China (Yao Z. et al., 2023). The distribution area of P. massoniana covers 1.4 × 107 hm2, and its total area and stock volume rank first among conifer species in China (Hu et al., 2022). Furthermore, the total carbon stock of P. massoniana forest is approximately 908.21 million m3, making it the third most abundant tree species in China after Chinese fir and poplar (Ji et al., 2022). In southern China, P. massoniana forests provide more significant ecological service benefits compared to other forest types, playing a key role in carbon sink ecosystems and subtropical forests in China (He et al., 2022). Studies have shown that there is a certain pattern of change in the average carbon storage of different P. massoniana families or provenances, with spatial distribution displaying regularity (Pan et al., 2017). Soil microorganisms are widely involved in various soil biochemical processes within forest ecosystems, such as organic matter decomposition, mineralization, and degradation (Zhou et al., 2023). They play a pivotal role in soil nutrient cycling and biogeochemical processes (Delgado-Baquerizo et al., 2016). Previous research has demonstrated significant differences in rhizosphere soil microbial community structure among P. massoniana provenances with different levels of carbon storage, exhibiting certain correlations with the organic matter content of the rhizosphere soil (Huang et al., 2023).
The rhizosphere refers to the surface of roots and the microzone of the soil layer in close proximity to the roots. Rhizosphere soil is defined as the soil adhering to the roots within a thickness of approximately 1 mm, serving as an important site for communication between plants and rhizosphere microorganisms (Schweinsberg Mickan et al., 2012). Rhizosphere microorganisms are not only influenced by plants (Lucas Borja et al., 2012) but also establish an interactive feedback system with plants through their own modifications (Zinn et al., 2005). Within the rhizosphere, microorganisms are involved in nutrient cycling and metabolic processes. They indirectly impact plant growth and health by improving soil organic matter content (Delgado-Baquerizo et al., 2016; Deng et al., 2019). Microbial metabolic function diversity can be used to assess soil processes and ecological functions (Xia et al., 2022). The functional diversity of soil microorganisms is affected by various factors such as vegetation cover, elevation gradient, and soil physical and chemical properties (Wei et al., 2009; Burton et al., 2010; Ibell et al., 2010). Researchers have found that land cover conversion significantly influences surface soil carbon and nitrogen storage as well as soil microbial functional diversity in alpine meadows on the Qinghai-Tibet (Zhu et al., 2015). Additionally, different altitude gradients have significant effects on the metabolic diversity of microbial communities in alpine meadows (Wang et al., 2018). Forest ecosystems play a pivotal role in maintaining the functional diversity of soil microorganisms. There are differences in the maintenance of functional diversity among soil microorganisms across different forest stand types (Yao Z. W. et al., 2023). However, there have been limited studies on the effects of plant carbon storage levels on the functional diversity of soil microorganisms.
The main methods for assessing the structural composition and functional diversity of soil microbial communities are plate colony counting, cell morphology, and the 16S/ITS amplification technique (Ge et al., 2018). However, these methods often have disadvantages, including complex operation, time-consuming analysis, and poor repeatability. Researchers have developed the Biolog Eco microplate technology as a simpler and faster method to evaluate microbial metabolic functional diversity (Feigl et al., 2017). The Biolog Eco microplates can characterize microbial functions based on metabolic activity and were specifically designed for analyzing bacterial communities in environmental samples (Li et al., 2021). They provide comprehensive information on the function of six types of soil microorganisms, including 31 carbon sources, for microbial community analysis (Zhang et al., 2023). Studies have shown that plant diversity (Zhu et al., 2015) and forest type are closely linked to soil microbial functional diversity (Yao Z. W. et al., 2023), influencing soil microbial structure, activity, and biomass. Although these studies have focused on the correlation between above-ground vegetation and soil microorganisms, few have investigated the impact of woody plants with different levels of carbon storage on soil microbial functional diversity using the Biolog Eco microplates.
In this study, we compared the functional diversity of rhizosphere soil microorganisms at high, medium, and low carbon storage levels of P. massoniana provenances. Our research addresses two questions: (1) Is there a significant difference in the microbial functional diversity of rhizosphere soil among P. massoniana provenances at different carbon storage levels? and (2) What are the key factors that influence microbial functional diversity in the rhizosphere soil of P. massoniana plantations? Analyzing the microbial functional diversity in the rhizosphere soil of P. massoniana provenances with different carbon storage levels and identifying its influencing factors hold significant importance for the research on the carbon cycle system of P. massoniana plantation forests.
2 Materials and methods
2.1 Study site and experimental design
Our sampling location was the state-owned forest seed farm in Yu‘an District, Lu‘an City, Anhui Province, China. The farm is situated at an elevation between 80 and 100 m, with slopes ranging from 5°to 10°. The region experiences a north subtropical monsoon climate, with an annual rainfall of 1239.8 mm and an average annual temperature of 15°C. The soil predominantly consists of clay disks of yellow-brown soil derived from loess, with a slightly acidic pH. The soil depth is considerable, ranging mainly from 70 to 150 cm.
The geographical provenance test forest of P. massoniana was established in 1981, covering a total area of 11.67 hm2. It consists of 64 provenances, representing geographical locations in 14 provinces and autonomous regions in China. Each provenance was arranged in a completely randomized block experiment, divided into six cell groups separated by trails. There were 64 provenances in each plot, with 8 provenances in each row and column and 9 duplicate provenances within each plot, with a plant spacing of 1 m x 1 m. The experimental forest has not undergone thinning for the past 41 years. Due to insect pests, diseases, and growth competition among individual trees, some provenances did not meet the requirements for statistical analysis. Biomass estimation for different provenances was carried out using a binary model based on diameter at breast height (DBH) and tree height measurements (Hu et al., 2022) according to the Chinese Forestry Industry Standard (LY/T2263-2014). We multiplied the biomass of different organs estimated by the model by the corresponding carbon content coefficient (Zeng and Xiao, 2011). The carbon storage of each organ was obtained. The carbon storage of each plant from various sources was obtained by summing. According to the order of carbon storage from high to low, six provenances representing high, medium, and low carbon storage levels of P. massoniana were selected: AnYuan (AY) and SanMing (SM) for high carbon storage, ShaoWu (SW) and QingJiang (QJ) for medium carbon storage, and ShengXian (SX) and HeFeng (HF) for low carbon storage (Supplementary Table S1).
2.2 Soil sampling
For each P. massoniana provenance, we selected three trees with DBH and height measurements that were close to the mean value, serving as three replicates. A total of three 20-cm sections were taken in three directions (120°) from each tree, allowing for multiple transverse sections of thin roots with varying diameters. We specifically focused on fine roots with a diameter smaller than 2 mm and collected the rhizosphere soil adhering to their surfaces. The rhizosphere soil mixture of the three sections is mixed as a repetition. The collected soil was divided into two parts. One part was used for the determination of soil properties, while the other part was used for the assessment of soil microbial biomass carbon (MBC), nitrogen content, and the Biolog Eco microplate analysis.
2.3 Soil properties and microbial biomass
We used a glass composite electrode to determine the pH value with a ratio of water to soil of 1:2.5. Soil organic carbon (SOC) and TN were determined by an external heating method with potassium dichromate (Benbi, 2018) and a flow analyzer (Bunch and Bernot, 2012), respectively. Soil MBC and microbial biomass nitrogen (MBN) were determined by chloroform fumigation (Setia et al., 2012).
2.4 Biolog Eco microplate analysis
To analyze the differential microbial utilization of carbon sources, we used the Biolog Eco microplate method (Garland and Mills, 1991). The information on 31 carbon sources is shown in Supplementary Figure S1. A volume of 45 mL of sterile deionized water was added to a 100-ml sterilized triangle bottle containing 5 g of wet soil. The bottle was sealed and shaken at room temperature for 30 min. Next, 1 ml of soil suspension was transferred to a sterile centrifuge tube using a pipette and centrifuged at 8,000 rpm for 20 min. The supernatant was then discarded. A volume of 1 ml of sterile saline was added and thoroughly mixed. The tube was centrifuged at 8,000 rpm for 20 min. We added 1.2 ml of sterile normal saline after discarding the supernatant and thoroughly mixed it. Then, we centrifuged it at 2000 rpm for 1 min. Finally, 0.8 ml of the supernatant was transferred to a sterile test tube containing 20 ml of normal saline and mixed well. This mixture served as the reaction liquid. The Biolog Eco microplate was preheated to 25°C before use. A pipette was used to take 150 μl of the diluent into each well, adding double steaming water as a control. The microplate was then incubated at 28°C. At time intervals of 0, 24, 48, 72, 96, 120, 144, 168, 192, 216, and 240 h, the absorption value was measured at 590 nm.
The AWCD and its change over time serve as indicators of overall microbial metabolic activity. It is generally recognized that samples with a higher AWCD value exhibit greater carbon source metabolic activity, indicating higher microbial abundance characteristics. AWCD is calculated as follows:
Ci is the absorption value of the well, except for the control well. R is the absorption value of the control well.
The utilization values (OD595) of 31 carbon sources formed a multivariate vector of the microbial metabolic characteristics of each sample. Shannon diversity, richness index, and Simpson diversity were further used to assess the diversity of bacterial community metabolic functions. The diversity function in the R language’s vegan package was used to calculate the diversity index. SPSS 19.0 software was used to carry out principal component analysis (PCA). The remaining charts were created with Origin2022.
3 Results
3.1 Soil properties
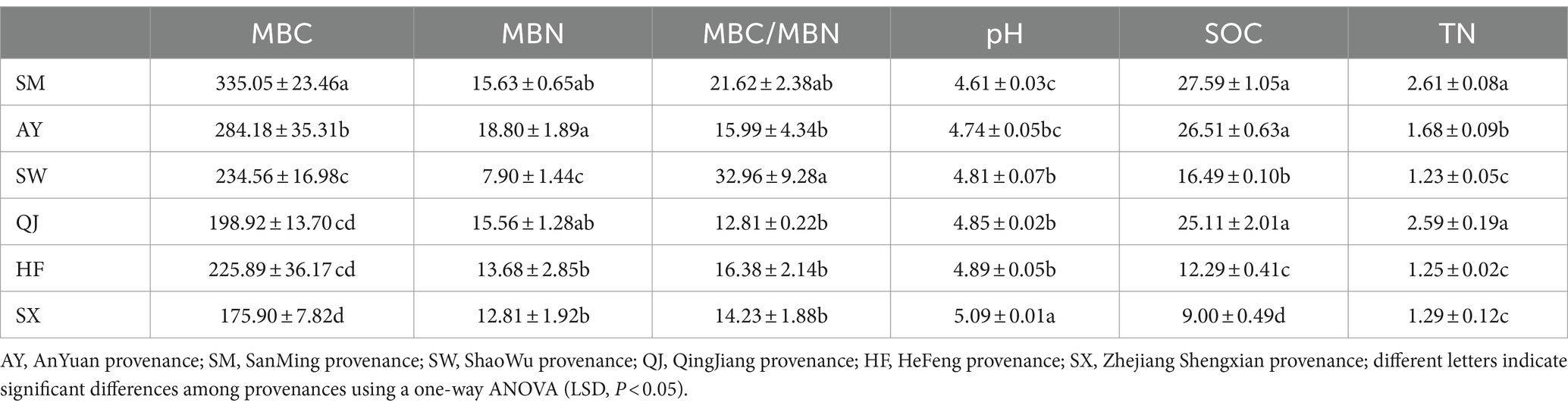
The SOC content exhibited significant differences among the six provenances (p < 0.05), with the order of SM > AY > QJ> > SW > HF > SX (Table 1). SM and AY, which were high carbon storage provenances, had significantly higher SOC content compared to other provenances. Meanwhile, the SOC content of HF provenance was significantly higher than that of SX provenance. Similarly, SM showed the highest TN content, followed by QJ. The TN content of SM and QJ was significantly higher than that of other provenances (p < 0.05). The descending order of TN content was SM > QJ > AY > SX > HF > SW. The TN content of SW, HF, and SX provenances was significantly lower than that of other provenances (p < 0.05). The pH values demonstrated significant variation among the six provenances. The descending order of pH values was SX > HF > QJ > SW > AY > SM. The trend observed was low carbon storage > medium carbon storage > high carbon storage. The pH value of the SM provenance was significantly lower than that of other provenances (p < 0.05).
There were significant differences in MBC content among the six provenances (Table 1). The MBC content followed the order of SM > AY > SW > HF > QJ > SX from high to low. The MBC content of high carbon storage provenances, AY and SM, was significantly higher than that of the medium and low carbon storage provenances (p < 0.05). Conversely, the MBC content of SX was significantly lower compared to other provenances (p < 0.05). The MBN content of the AY provenance (high carbon storage) was significantly higher than that of the medium and low carbon storage provenances (SW, HF, and SX), followed by the SM and QJ provenances (p < 0.05). Furthermore, the MBN content of SW was significantly lower than that of HF and SX (p < 0.05). The descending order of MBC/MBN was SM > QJ > AY > SW > SX > HF. The MBC/MBN ratio of SW provenances was significantly higher than that of other provenances (p < 0.05).
3.2 Functional diversity of soil microorganisms
The AWCDs of rhizosphere soil microorganisms from different P. massoniana provenances with varying carbon storage levels are shown in Figure 1. The results showed that AWCD values among the rhizosphere microorganisms of different P. massoniana provenances were significantly different (p < 0.05). Furthermore, the AWCD values of all rhizosphere microorganisms exhibited a distinct hysteresis effect after 24 h of culture. Then, with the increase in culture time, the AWCD value gradually increased. Notably, the AWCD dynamics of SM were significantly higher than those of other provenances (p < 0.05), followed by AY and QJ provenance. The results indicated that rhizosphere microorganisms from high carbon storage provenances exhibited the highest metabolic activity toward soil carbon sources. At the end of the culture period, the AWCD values for SM and AY were 1.39 and 0.78, respectively. Compared with SM and AY, the AWCD values for medium and low carbon storage provenances increased at a slower rate. The metabolic rate of SW was the lowest, with AWCD values reaching only 0.03 after 240 h of culture.
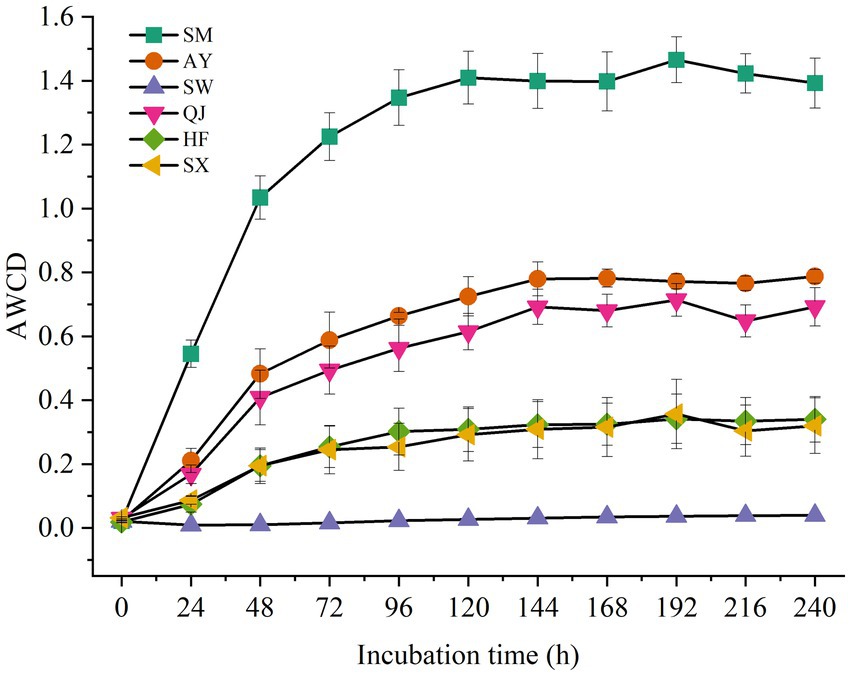
Figure 1. AWCD of carbon sources in the rhizosphere soil microbial communities of P. massoniana provenances within the incubation time.
The metabolic function diversity of rhizosphere microorganisms was evaluated using the Shannon diversity index (H), Simpson diversity index (D), and richness indexes after incubation for 192 h (Figure 2). Higher diversity index values indicate a greater variety of carbon sources available to microbes (Xia et al., 2015). The Shannon diversity index exhibited the following descending order: SM > AY > QJ > SX > HF > SW. Among them, the Shannon diversity of SM provenances was significantly higher than that of medium and low carbon storage provenances, including QJ, SW, HF, and SX (p < 0.05). Overall, the Shannon diversity displayed the trend of high carbon storage>medium carbon storage > low carbon storage. The Simpson diversity index followed the order of SM > AY > QJ > HF > SX > SW from high to low. The Simpson diversity of high carbon storage provenances SM and AY and medium carbon storage provenance QJ was significantly higher than that of other provenances. Richness showed the descending order of AY > HF > QJ > SM > SX > SW. The richness of AY was significantly higher than SW (p < 0.05).
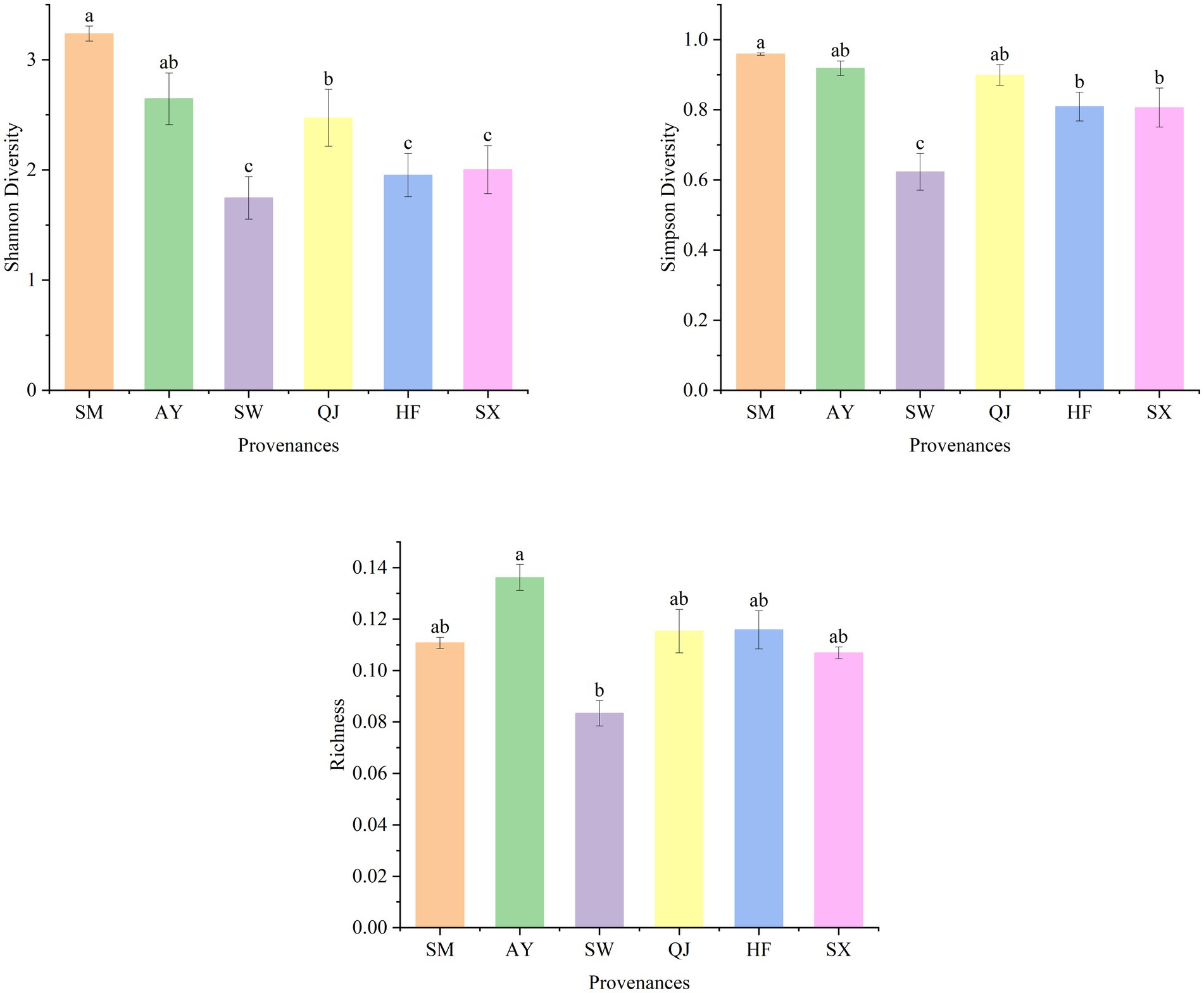
Figure 2. Shannon diversity, Simpson diversity, and richness index of soil microorganisms in different P. massoniana provenances. Data are means ± standard errors, and different lower-case letters indicate significant differences among provenances (p < 0.05).
3.3 PCA of carbon source metabolization
PCA was conducted on absorbance values incubated for 144 h (Figure 3). The first and second principal components accounted for variance contribution rates of 64.35 and 11.60%, respectively. Figure 3 shows the distribution range of the six provenances, which were differentiated along the PC1 axis and PC2 axis. SM, AY, and QJ were located in the direction of PC1, while SW, HF, and SX were positioned in the negative direction of the PC1 axis. The distribution of high, medium, and low carbon storage provenances in different quadrants indicated that there were significant differences in the utilization of carbon sources among the various provenances.
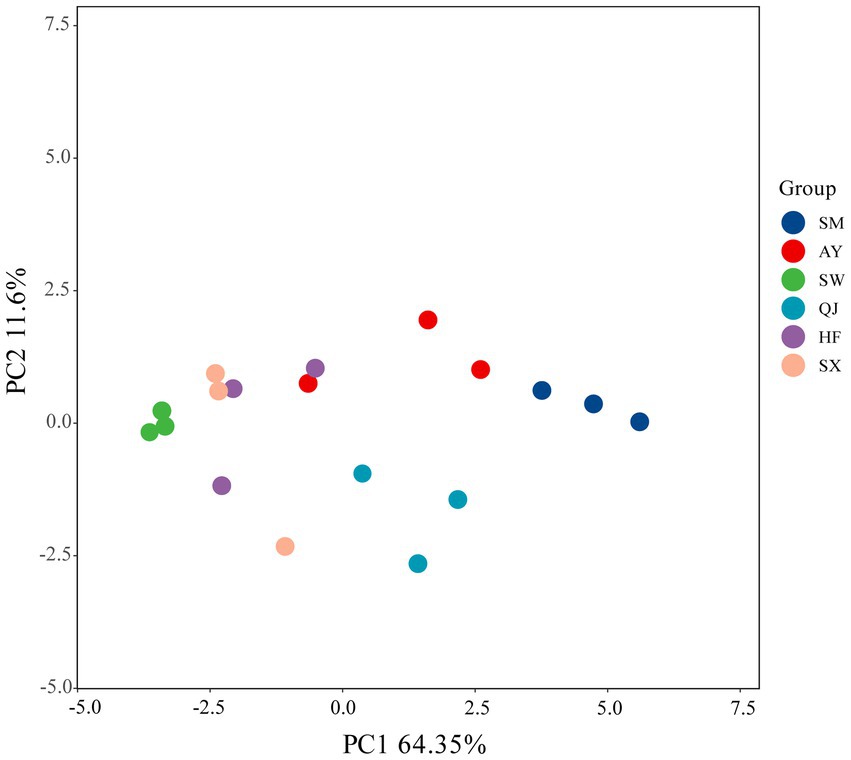
Figure 3. Principal component analysis (PCA) for the carbon source utilization of soil microbial communities in different P. massoniana provenances.
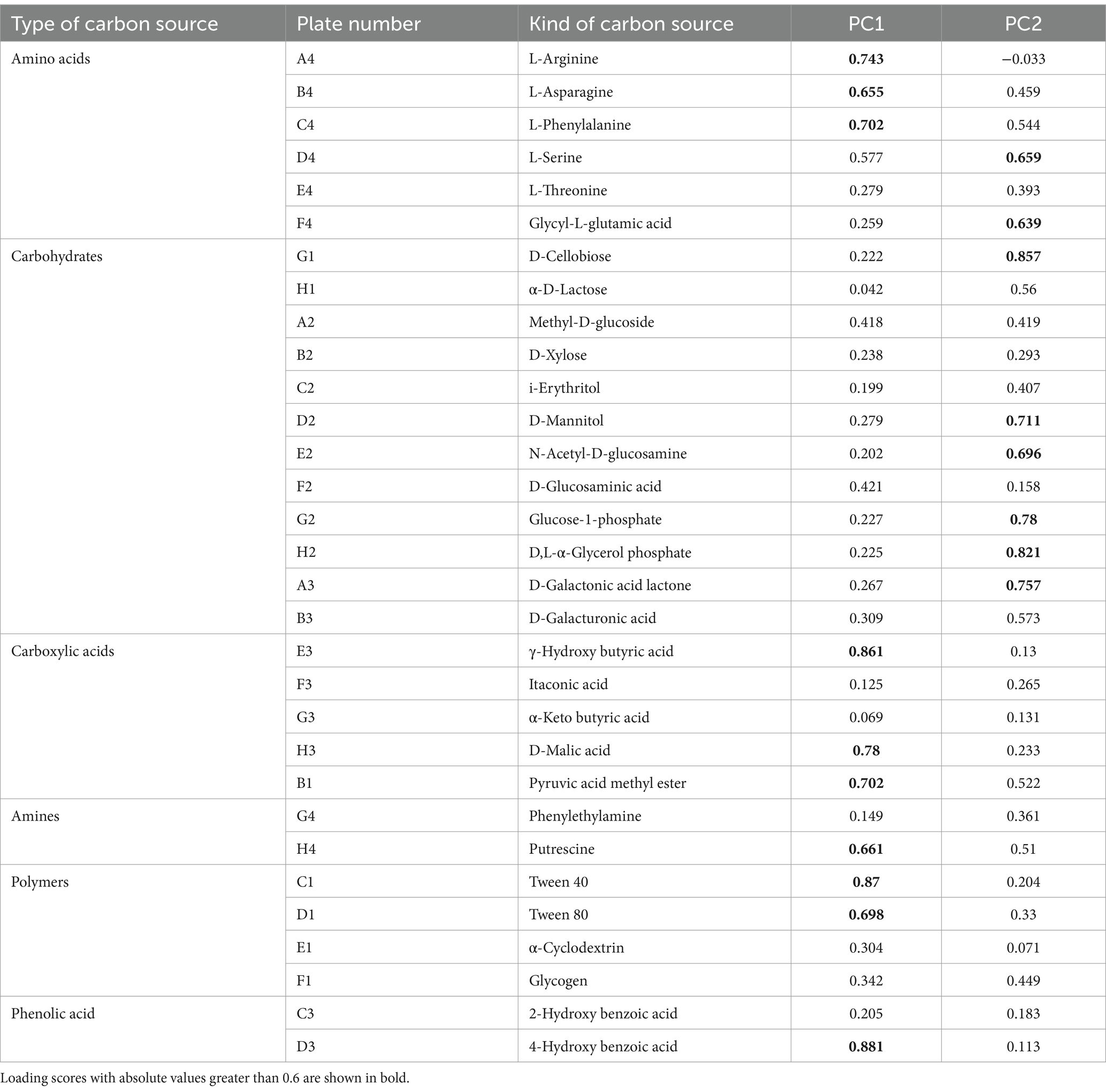
Table 2 shows the loading scores of the 31 carbon sources in the first two principal components. Larger loading scores indicate a stronger influence of the carbon source on the principal component. Carbon sources with an absolute value greater than 0.6 are considered the main types of carbon sources utilized by the soil microbial community (Yao Z. W. et al., 2023). As can be seen from the results in Table 2, there are 10 types of carbon sources that primarily affect PC1. These sources include three types of amino acids, three types of carboxylic acids, one type of amine, two types of polymers, and one type of phenolic acid. Among them, 4-hydroxy benzoic acid is the carbon source most related to PC1 (load score = 0.881), followed by Tween 40 (0.870) and γ-hydroxy butyric acid (0.861). There are seven carbon sources primarily influenced by PC2, consisting of six types of carbohydrates and one type of amino acid. D-Cellobiose had the strongest connection with PC2 (load score = 0.857), followed by D,L-α-glycerol phosphate (0.821) and glucose-1-phosphate (0.780).
3.4 Metabolic utilization of biochemical classification substrates
According to their biochemical properties, the 31 carbon source substrates in the Biolog Eco microporous plates can be divided into six types: amines, amino acids, carbohydrates, carboxylic acids, phenolic acids, and polymers (Table 2). The rhizosphere microbial communities of P. massoniana provenances have different capacities for utilizing the six carbon sources. We summarized the AWCD results for the six types of carbon sources (Figure 4). The results showed significant differences in the utilization of carbohydrates among different P. massoniana provenances. The utilization capacity of the other five carbon sources, except for polymers, was significantly higher for SM than for other provenances (p < 0.05). Among them, AY had the second highest utilization efficiency for amino acids, amines, and carbohydrates, followed by SM. QJ exhibited the second highest utilization rate for carboxylic acids. There was no significant difference in the utilization efficiency of the mentioned four carbon sources for the two low carbon storage provenances (HF and SX). Both of them were lower than the AY and SM provenances. SW had the lowest utilization efficiency of the six carbon sources. Compared with other carbon sources, the utilization rate of polymers varies greatly. AY had the highest utilization rate for polymers, followed by QJ, while SM ranked third.
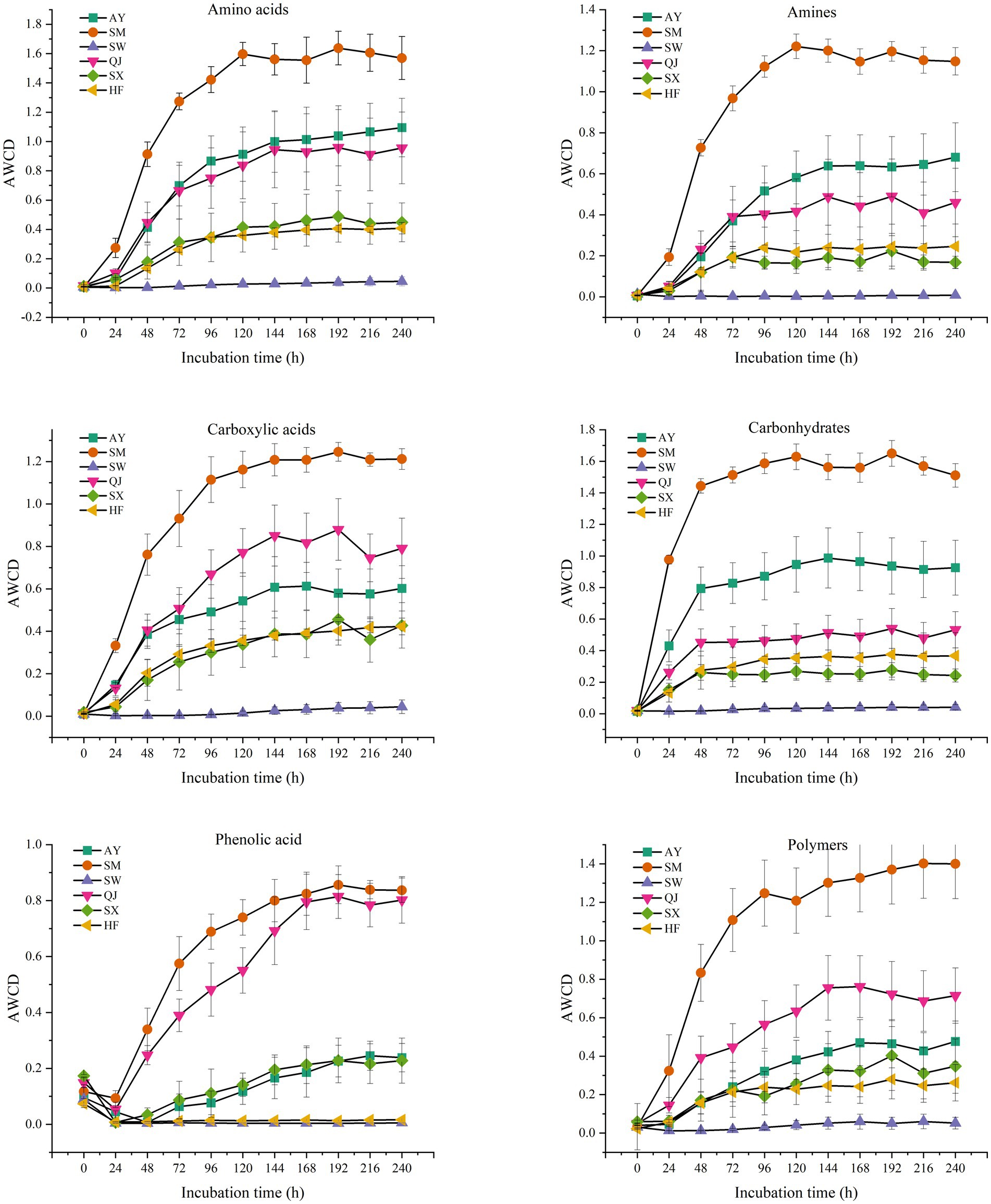
Figure 4. Average well-color development of six types of carbon source microbial communities from different P. massoniana provenances after inoculation for 240 h, including amines, amino acids, carboxylic acids, carbohydrates, phenolic acid, and polymers.
3.5 Correlation analysis between the utilization of C sources and environmental factors
We performed Pearson’s correlation analysis to examine the correlation between rhizosphere microbial carbon source utilization and carbon storage levels, as well as the soil properties of P. massoniana provenances (Figure 5). The results revealed a significant positive correlation between the utilization rates of each carbon source (PM, CH, PA, CA, AA, and AM) (p < 0.05). The rates were significantly positively correlated with SOC and TN (p < 0.05). The carbon storage levels of the provenances were also positively correlated with the utilization rates of these carbon sources (p < 0.05). Moreover, the soil MBC content had positive correlations with the utilization rates of PM, CH, and AM. Soil pH had a negative influence on the utilization rates of CH, AA, and AM. MBN content displayed positive correlations with the utilization rates of PM, CH, CA, AA, and AM (p < 0.05). In addition, the MBC content was significantly positively correlated with the SOC content and negatively correlated with the pH value (p < 0.05). The MBN content was significantly positively correlated with SOC and TN contents (p < 0.05). Moreover, MBC/N was significantly negatively correlated with the pH value (p < 0.05).
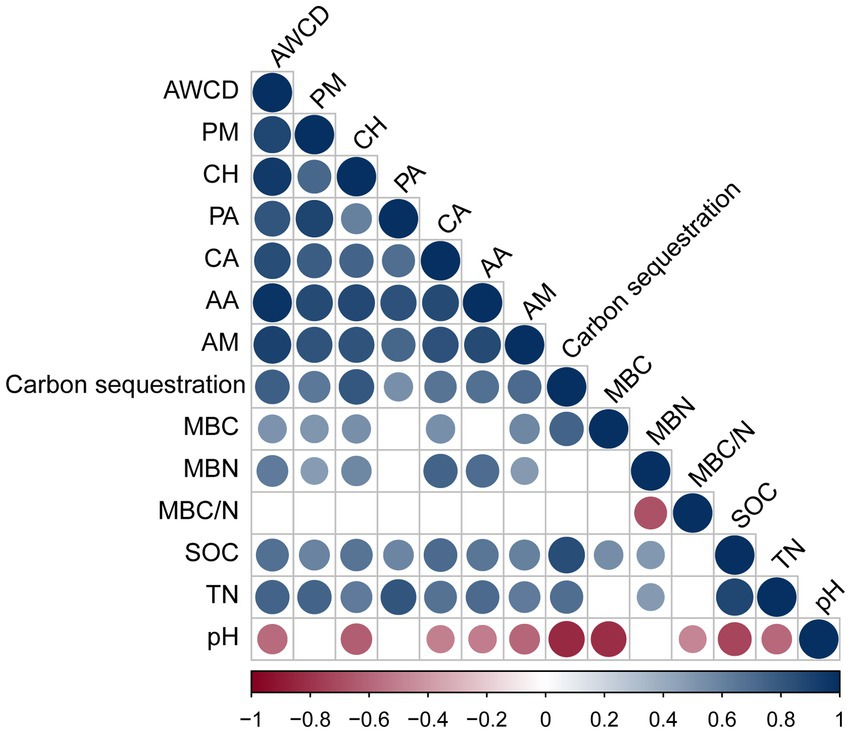
Figure 5. Correlation analysis between the utilization of carbon sources, carbon storage levels, and soil properties. Those with significant correlations are circled. If there is no significant correlation, it is blank. Soil properties: pH, soil pH; SOC, soil organic carbon; TN, total nitrogen; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; MBC/N, microbial biomass carbon/nitrogen. Six carbon source types: CH, carbohydrates; CA, carboxylic acids; AA, amino acids; PM, polymers; PA, phenolic compounds; AM, amines.
4 Discussion
4.1 Effects of different Pinus massoniana provenances on the contents of MBC, MBN, and MBC/N in rhizosphere soil
In this study, the MBC content of different P. massoniana provenances significantly varied. Studies have shown that different pine genotypes have differences in productivity resource allocation, leading to variations in underground root turnover rate and fine-root standing crop, which can impact microbial biomass (microbial activity) (Tyree et al., 2014). The MBC content of SM provenances was significantly higher than that of other provenances in this study. Therefore, we speculate that SM provenances possess a higher turnover rate and a standing crop of fine roots. In this study, there was a significant positive correlation between MBC content and carbon storage of P. massoniana (p < 0.05). Plants sequester carbon through photosynthesis, and the products of photosynthesis are transported into the soil as root exudates and litter (Terrer et al., 2019). Provenances with high carbon storage exhibit greater input of soil carbon into the rhizosphere soil, thereby increasing the MBC content. In this study, compared to other provenances, SW provenances demonstrated a significant reduction in MBN content, while MBC/N was relatively high. MBN represents the most active organic nitrogen component in soil, reflecting soil microbial nitrogen retention and mineralization dynamics (Dou et al., 2023). The soil MBC/N is also a reflection of the nitrogen supply capacity of the soil. Soil nitrogen shows higher bioavailability when the microbial carbon-to-nitrogen ratio is low (Fu et al., 2012). Therefore, we concluded that SW provenances exhibit lower rates of rhizosphere nitrogen mineralization. As a medium carbon storage provenance, the content of MBN and TN in the QJ provenance was similar to that of the SM provenance from the high carbon storage provenance. The MBC/N was lower in the QJ provenance, which suggested that the rhizosphere soil of the QJ provenance exhibited higher nitrogen bioavailability. This difference is likely attributed to changes in root exudation as well as the rate of fine-root turnover (Tyree et al., 2014).
4.2 Soil microbial functional diversity
The AWCD values of the high carbon storage provenances SM and AY were significantly higher than those of other provenances at each period of the 240 h culture in this study. These differences can be attributed to plant species, root exudates, litter decomposition, and plant residues (Yao Z. W. et al., 2023). Both Shannon diversity and Simpson diversity of rhizosphere microorganisms with high carbon storage provenances (SM and AY) were significantly higher than those from medium and low carbon storage (p < 0.05). The results showed that the microbial functional diversity of rhizosphere soil in high carbon storage provenances was significantly higher than that in medium and low carbon storage provenances. Rhizosphere microorganisms from high carbon storage provenance exhibited a greater ability to utilize various carbon sources (Yao Z. W. et al., 2023). On the whole, high carbon storage provenances exhibited enhanced forest ecological functions. Plant root secretions provide diverse nutrients to rhizosphere microorganisms, thereby improving soil microbial metabolic activity and increasing the utilization of carbon sources by soil microorganisms (Yao Z. W. et al., 2023). Studies have shown that the amount of carbohydrates secreted by plants into the rhizosphere soil positively correlates with the ability of rhizosphere microorganisms to utilize carbon substrates (Wu et al., 2014). In this study, high carbon storage provenances exhibited a high level of rhizosphere microbial carbon source metabolism, indicating that within the P. massoniana forest ecosystem, higher carbon storage levels corresponded to greater soil microbial functional diversity. In addition, the microbial carbon source metabolic diversity was also influenced by provenance differences. Variations in rhizosphere exudates among different provenances can lead to diversification in substrate utilization by rhizosphere microorganisms, thereby affecting their functional diversity and community structure (Doornbos et al., 2012). It has been reported that rhizosphere soil microbial diversity differs among plants with different genotypes (Kaiser et al., 2001). Our study showed that the functional diversity of rhizosphere soil microorganisms was significantly different among P. massoniana provenances with varying carbon storage levels. The microbial functional diversity of high carbon storage provenances was significantly higher than that of medium and low carbon storage provenances. Among them, the soil microbial function diversity and MBC of the SM provenance were significantly higher than in other provenances. Thus, the SM provenance is favored for its effective ecological service function. The results of PCA analysis showed that the six P. massoniana provenances were positioned in different quadrants, indicating that there were differences in their utilization of different carbon sources (Yao Z. W. et al., 2023). Amino acids, carbohydrates, and carboxylic acids were the key carbon sources that distinguished the metabolic characteristics of soil microbial communities. In other words, the differences in carbon source metabolism of rhizosphere microorganisms among different P. massoniana provenances primarily involved the utilization of amino acids, carbohydrates, and carboxylic acids.
4.3 Environmental regulation mechanism
Previous studies have shown that different genotypes of pine affect rhizosphere microbial activity (Tyree et al., 2014). However, the effects of different carbon storage genotypes (provenances) on the metabolic capacity of rhizosphere microorganisms have not been reported. In this study, the carbon storage of P. massoniana provenances was significantly positively correlated with the total microbial carbon source AWCD and the AWCD of each carbon source species (p < 0.05). This indicates a significant positive correlation between the carbon storage function of P. massoniana and the metabolic capacity of microbial carbon sources. Furthermore, an increase in rhizosphere organic carbon input had a positive effect on the metabolic capacity of soil microbial carbon sources (Xia et al., 2015). Soil physical and chemical properties and nutrients are widely recognized as critical drivers of soil bacterial communities (Nguyen et al., 2018; Rath et al., 2019; Yang et al., 2020). In this study, the rhizosphere SOC and TN contents of high carbon storage provenances were generally high, providing necessary nutrients and energy for soil microorganisms and promoting microbial activity. In addition, the pH value was significantly negatively correlated with the AWCD of CA, CH, PM, PA, and AM. These results indicate that pH value is another key factor affecting the carbon source metabolism ability of rhizosphere soil microorganisms in P. massoniana plantations. Multiple studies have demonstrated the influence of soil pH on microbial communities’ structure and distribution. For example, the study by Liu et al. (2014) showed that the composition and diversity of the bacterial community were significantly correlated with soil pH in black soil farmlands in northeast China. The rhizosphere soil of P. massoniana forest tends to be weakly acidic. As the carbon storage of P. massoniana provenances increases, the rhizosphere soil pH value decreases. Weakly acidic conditions can stimulate the growth of certain eosinophilic bacteria (Wang et al., 2014). The bacteria adapted to the long-term weak acidic conditions through their own regulatory system. Under the dual influence of organic matter and pH value, rhizosphere microorganisms from different P. massoniana provenances performed different metabolic characteristics (Liu et al., 2017).
In this study, there was a significant positive correlation between soil MBC and the metabolic diversity of microbial carbon sources. MBC content is an important indicator of soil microbial activity (Mendoza et al., 2020). A higher MBC content indicates greater microbial activity (Waldrop and Firestone, 2004). Accordingly, the metabolic activity of carbon sources also increases. Yao et al. also came to a similar conclusion that soil microbial biomass is an important indicator of the metabolic activity of microbial communities (Yao Z. W. et al., 2023). This relationship is primarily influenced by litter quantity and quality, as well as differences in fine-root turnover among different provenances (Brown and Markewitz, 2018). The diversity index of the SM provenance is significantly higher than that of other provenances, suggesting a relatively high rate of fine-root turnover. The results of the study confirmed that P. massoniana provenances with high carbon storage had a higher metabolic level in the rhizosphere microorganism, indicating their superior ability to maintain the diversity of the soil microbiotic community (Yao Z. W. et al., 2023). Among these provenances, the SM provenances showed a higher level of rhizosphere microbial metabolism compared to others, making it a preferable choice for establishing high carbon sequestration P. massoniana plantations in the future. These findings hold certain guiding significance for the scientific management of carbon storage forestry.
5 Conclusion
In this study, the Biolog Eco microplate method was used to study the rhizosphere microorganisms of different P. massoniana provenances. The results showed that there were significant differences in the carbon metabolism of rhizosphere microorganisms among the various P. massoniana provenances. Carbon metabolic activity increased with an increase in carbon storage levels, with the SM provenance exhibiting the highest carbon metabolic activity. The differences in carbon source metabolism among the rhizosphere microorganisms of different P. massoniana provenances were primarily reflected in the utilization of amino acids, carboxylic acids, and carbohydrates. The soil environmental factors that contributed to the variation in carbon metabolism utilization included pH, SOC, and TN.
The results of the study confirmed that P. massoniana provenances with high carbon storage had a higher metabolic level in the rhizosphere microorganism. The SM provenance is identified as a favorable provenance for maintaining soil microbial functional diversity in the P. massoniana ecosystem. The Biolog Eco microplate method can provide a fast and accurate assessment of the metabolic activity of the rhizosphere microbial community in P. massoniana. However, this method does have some limitations as it is only suitable for culturable bacterial communities, potentially missing other specialized functional microbiota. Therefore, to further explore the rhizosphere microbial community structure of different P. massoniana provenances, it will be necessary to combine molecular biology methods to examine the community structure, species richness, molecular ecological network, and other relevant factors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
ZH: Conceptualization, Investigation, Software, Writing – original draft, Writing – review & editing. YQ: Conceptualization, Investigation, Methodology, Software, Writing – review & editing. XH: Data curation, Investigation, Methodology, Writing – review & editing. MZ: Data curation, Investigation, Methodology, Software, Writing – review & editing. XR: Investigation, Software, Visualization, Writing – review & editing. WY: Formal analysis, Software, Visualization, Writing – review & editing. KJ: Funding acquisition, Investigation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the National Key R&D Program of China (2022YFD2200202), and the project was funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2024.1365111/full#supplementary-material
References
Benbi, D. K. (2018). Evaluation of a rapid microwave digestion method for determination of total organic carbon in soil. Commun. Soil Sci. Plant Anal. 49, 2103–2112. doi: 10.1080/00103624.2018.1495732
Brown, R., and Markewitz, D. (2018). Soil heterotrophic respiration: measuring and modeling seasonal variation and silvicultural impacts. For. Ecol. Manag. 430, 594–608. doi: 10.1016/j.foreco.2018.08.018
Bunch, N. D., and Bernot, M. J. (2012). Nitrate and ammonium uptake by natural stream sediment microbial communities in response to nutrient enrichment. Res. Microbiol. 163, 137–141. doi: 10.1016/j.resmic.2011.11.004
Burton, J., Chen, C., Xu, Z., and Ghadiri, H. (2010). Soil microbial biomass, activity and community composition in adjacent native and plantation forests of subtropical Australia. J. Soils Sediments 10, 1267–1277. doi: 10.1007/s11368-010-0238-y
Cai, W., Xu, L., Li, M., Sun, O. J., and He, N. (2023). Imbalance of inter-provincial forest carbon sequestration rate from 2010 to 2060 in China and its regulation strategy. J. Geogr. Sci. 33, 3–17. doi: 10.1007/s11442-023-2071-4
Chen, S., Lu, N., Fu, B., Wang, S., Deng, L., and Wang, L. (2022). Current and future carbon stocks of natural forests in China. For. Ecol. Manag. 511:120137. doi: 10.1016/j.foreco.2022.120137
Delgado-Baquerizo, M., Maestre, F. T., Reich, P. B., Jeffries, T. C., Gaitan, J. J., Encinar, D., et al. (2016). Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 7, 1–8. doi: 10.1038/ncomms10541
Deng, J., Zhou, Y., Bai, X., Luo, J., Yin, Y., and Zhu, W. (2019). Soil microbial functional diversity responses to different revegetation types in Baishilazi nature reserve. Pol. J. Environ. Stud. 28, 3675–3686. doi: 10.15244/pjoes/99100
Doornbos, R. F., van Loon, L. C., and Bakker, P. (2012). Impact of root exudates and plant defense signaling on bacterial communities in the rhizosphere. Agron. Sustain. Develop. 32, 227–243. doi: 10.1007/s13593-011-0028-y
Dou, X., Yu, H. Z., Wang, J. Y., Li, F., Liu, Q., Sun, L., et al. (2023). Effect of prescribed burning on the small-scale spatial heterogeneity of soil microbial biomass in Pinus koraiensis and Quercus mongolica forests of China. J. For. Res. 34, 609–622. doi: 10.1007/s11676-022-01516-y
Feigl, V., Ujaczki, E., Vaszita, E., and Molnar, M. (2017). Influence of red mud on soil microbial communities: application and comprehensive evaluation of the Biolog Eco plate approach as a tool in soil microbiological studies. Sci. Total Environ. 595, 903–911. doi: 10.1016/j.scitotenv.2017.03.266
Fu, G., Shen, Z. X., Zhang, X. Z., and Zhou, Y. T. (2012). Response of soil microbial biomass to short-term experimental warming in alpine meadow on the Tibetan Plateau. Appl. Soil Ecol. 61, 158–160. doi: 10.1016/j.apsoil.2012.05.002
Garland, J., and Mills, A. (1991). Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 57, 2351–2359. doi: 10.1128/aem.57.8.2351-2359.1991
Ge, Z. W., Du, H. J., Gao, Y. L., and Qiu, W. F. (2018). Analysis on metabolic functions of stored Rice microbial communities by BIOLOG ECO microplates. Front. Microbiol. 9:1375. doi: 10.3389/fmicb.2018.01375
He, H. Z., Zhang, Z. M., Zhou, W., and Zhou, Y. C. (2022). Soil aggregates, distribution characteristics and organic carbon protection mechanism of Pinus massoniana forests of different ages. Pak. J. Bot. 54, 1803–1812. doi: 10.30848/pjb2022-5(11)
Heinrich, V., Dalagnol, R., Cassol, H., Rosan, T., de Almeida, C., Silva, J. C., et al. (2021). Large carbon sink potential of secondary forests in the Brazilian Amazon to mitigate climate change. Nat. Commun. 12, 1–11. doi: 10.1038/s41467-021-22050-1
Hu, X. F., Wu, F., Sun, X. B., Chen, H. P., Yin, A. Z., and Ji, K. S. (2022). Joint analysis of growth and wood property of 38-year-old Pinus massoniana from 55 provenances. J. Nanjing Forest. Univ. 46, 203–212. doi: 10.12302/j.issn.1000-2006.202104044
Huang, Z. C., He, X., Zhang, C., Zhang, M. Y., Wang, J. N., Hou, Y. Q., et al. (2023). Microbial communities and functions changed in rhizosphere soil of Pinus massoniana provenances with different carbon storage. Front. Microbiol. 14:1264670. doi: 10.3389/fmicb.2023.1264670
Ibell, P., Xu, Z., and Blumfield, T. (2010). Effects of weed control and fertilization on soil carbon and nutrient pools in an exotic pine plantation of subtropical Australia. J. Soils Sediments 10, 1027–1038. doi: 10.1007/s11368-010-0222-6
Ji, K. S., Xu, L. A., Wang, D. B., Ni, Z. X., and Wang, Z. R. (2022). Progresses and achievements of genetic improvement on Masson pine (Pinus massoniana) in China. J. Nanjing Forest. Univ. 46, 10–22. doi: 10.12303/j.issn.1000-2006.202207020
Jiao, C. C., Yu, G. R., He, N. P., Ma, A. N., Ge, J. P., and Hu, Z. M. (2016). The spatial pattern of grassland aboveground biomass and its environmental controls in the Eurasian steppe. Acta Geograph. Sin. 71, 781–796. doi: 10.11821/dlxb201605007
Kaiser, O., Pühler, A., and Selbitschka, W. (2001). Phylogenetic analysis of microbial diversity in the rhizoplane of oilseed rape (Brassica napus cv. Westar) employing cultivation-dependent and cultivation-independent approaches. Microb. Ecol. 42, 136–149. doi: 10.1007/s002480000121
Labriere, N., Davies, S., Disney, M., Duncanson, L., Herold, M., Lewis, S., et al. (2023). Toward a forest biomass reference measurement system for remote sensing applications. Glob. Chang. Biol. 29, 827–840. doi: 10.1111/gcb.16497
Li, Y. C., Li, M. Y., Li, C., and Liu, Z. Z. (2020). Forest aboveground biomass estimation using Landsat 8 and sentinel-1A data with machine learning algorithms. Sci. Rep. 10:9952. doi: 10.1038/s41598-020-67024-3
Li, C., Liu, X., Meng, M. J., Zhai, L., Zhang, B., Jia, Z. H., et al. (2021). The use of Biolog eco microplates to compare the effects of sulfuric and nitric acid rain on the metabolic functions of soil microbial communities in a subtropical plantation within the Yangtze River Delta region. Catena 198:105039. doi: 10.1016/j.catena.2020.105039
Liu, J. J., Sui, Y. Y., Yu, Z. H., Shi, Y., Chu, H. Y., Jin, J., et al. (2014). High throughput sequencing analysis of biogeographical distribution of bacterial communities in the black soils of Northeast China. Soil Biol. Biochem. 70, 113–122. doi: 10.1016/j.soilbio.2013.12.014
Liu, X., Zhang, B., Zhao, W. R., Wang, L., Xie, D. J., Huo, W. T., et al. (2017). Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci. Total Environ. 601-602, 669–678. doi: 10.1016/j.scitotenv.2017.05.151
Lucas Borja, M. E., Candel, D., Jindo, K., Moreno, J. L., Andres, M., and Bastida, F. (2012). Soil microbial community structure and activity in monospecific and mixed forest stands, under Mediterranean humid conditions. Plant Soil 354, 359–370. doi: 10.1007/s11104-011-1072-8
Mendoza, B., Bejar, J., Luna, D., Osorio, M., Jimenez, M., and Melendez, J. R. (2020). Differences in the ratio of soil microbial biomass carbon (MBC) and soil organic carbon (SOC) at various altitudes of Hyperalic Alisol in the Amazon region of Ecuador. F1000Research 9:443. doi: 10.12688/f1000research.22922.1
Mercado, L. M., Bellouin, N., Sitch, S., Boucher, O., Huntingford, C., Wild, M., et al. (2009). Impact of changes in diffuse radiation on the global land carbon sink. Nature 458, 1014–1017. doi: 10.1038/nature07949
Nguyen, L. T. T., Osanai, Y., Lai, K., Anderson, I. C., Bange, M. P., Tissue, D. T., et al. (2018). Responses of the soil microbial community to nitrogen fertilizer regimes and historical exposure to extreme weather events: flooding or prolonged-drought. Soil Biol. Biochem. 118, 227–236. doi: 10.1016/j.soilbio.2017.12.016
Pan, P., Han, T. Y., Ouyang, X. Z., Liu, Y. Q., Zang, H., Ning, J. K., et al. (2017). Carbon density distribution characteristics and influencing factors in aerially seeded Pinus massoniana plantations. Chin. J. Appl. Ecol. 28, 3841–3847. doi: 10.13287/j.1001-9332.201712.001
Rath, K. M., Fierer, N., Murphy, D. V., and Rousk, J. (2019). Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 13, 836–846. doi: 10.1038/s41396-018-0313-8
Schweinsberg Mickan, M., Joergensen, R., and Mueller, T. (2012). Rhizodeposition: its contribution to microbial growth and carbon and nitrogen turnover within the rhizosphere. J. Plant Nutr. Soil Sci. 175, 750–760. doi: 10.1002/jpln.201100300
Setia, R., Verma, S. L., and Marschner, P. (2012). Measuring microbial biomass carbon by direct extraction - comparison with chloroform fumigation-extraction. Eur. J. Soil Biol. 53, 103–106. doi: 10.1016/j.ejsobi.2012.09.005
Terrer, C., Jackson, R., Prentice, I., Keenan, T., Kaiser, C., Vicca, S., et al. (2019). Nitrogen and phosphorus constrain the CO2 fertilization of global plant biomass. Nat. Clim. Chang. 9:684. doi: 10.1038/s41558-019-0545-2
Tyree, M. C., Seiler, J. R., and Maier, C. A. (2014). Contrasting genotypes, soil amendments, and their interactive effects on short-term total soil CO2 efflux in a 3-year-old Pinus taeda L. plantation. Soil Biol. Biochem. 69, 93–100. doi: 10.1016/j.soilbio.2013.10.050
Waldrop, M. P., and Firestone, M. K. (2004). Microbial community utilization of recalcitrant and simple carbon compounds: impact of oak-woodland plant communities. Oecologia 138, 275–284. doi: 10.1007/s00442-003-1419-9
Wang, L., Chen, Z., Shang, H., Wang, J., and Zhang, P. Y. (2014). Impact of simulated acid rain on soil microbial community function in Masson pine seedlings. Electron. J. Biotechnol. 17, 199–203. doi: 10.1016/j.ejbt.2014.07.008
Wang, Y., Zong, N., He, N. P., Zhang, J. J., Tian, J., and Li, L. T. (2018). Soil microbial functional diversity patterns and drivers along an elevation gradient on Qinghai-Tibet, China. Acta Geographica Sinica 38, 5837–5845. doi: 10.5846/stxb201707261343
Wei, Y. C., Ouyang, Z. Y., Miao, H., and Zheng, H. (2009). Exotic Pinus carbaea causes soil quality to deteriorate on former abandoned land compared to an indigenous Podocarpus plantation in the tropical forest area of southern China. J. For. Res. 14, 221–228. doi: 10.1007/s10310-009-0130-z
Wu, L. K., Lin, X. M., and Lin, W. X. (2014). Advances and perspective in research on plant-soil-microbe interactions mediated by root exudates. Chinese J. Plant. Ecol. 38, 298–310. doi: 10.3724/SP.J.1258.2014.00027
Xia, P. H., Kou, Y. Z., and Yu, L. F. (2015). Carbon metabolic soil microbial community in Caohai karst plateau degraded wetland: a case study in Southwest China. Acta Sci. Circumst. 35, 2549–2555. doi: 10.13671/j.hjkxxb.2014.1010
Xia, L., Zhao, B. Q., Luo, T., Xu, W. N., Guo, T., and Xia, D. (2022). Microbial functional diversity in rhizosphere and non-rhizosphere soil of different dominant species in a vegetation concrete slope. Biotechnol. Biotechnol. Equip. 36, 379–388. doi: 10.1080/13102818.2022.2082319
Yang, W., Cai, A. D., Wang, J. S., Luo, Y. Q., Cheng, X. L., and An, S. Q. (2020). Exotic Spartina alterniflora Loisel. Invasion significantly shifts soil bacterial communities with the successional gradient of saltmarsh in eastern China. Plant Soil 449, 97–115. doi: 10.1007/s11104-020-04470-y
Yao, Z., Jiao, P., Wu, X., Yan, Q., Liu, X., Hu, Y., et al. (2023). Effect of fire-deposited charcoal on soil organic carbon pools and associated enzyme activities in a recently harvested Pinus massoniana plantation subjected to broadcast burning. Huan Jing Ke Xue 44, 4201–4210. doi: 10.13227/j.hjkx.202209081
Yao, Z. W., Zhang, X. D., Wang, X., Shu, Q., Liu, X. M., Wu, H. L., et al. (2023). Functional diversity of soil microorganisms and influencing factors in three typical water-conservation forests in Danjiangkou reservoir area. Forests 14:67. doi: 10.3390/f14010067
Zeng, W., and Xiao, Q. (2011). Analysis on carbon content factors of different organs on Masson pine in southern China. Central South Forest Invent. Plan. 30, 51–55. doi: 10.16166/j.cnki.cn43-1095.2011.02.013
Zhang, W. J., Wang, J. H., Zhu, L. S., Wang, J., Mao, S. S., Yan, X. J., et al. (2023). New insights into the effects of antibiotics and copper on microbial community diversity and carbon source utilization. Environ. Geochem. Health 45, 4779–4793. doi: 10.1007/s10653-023-01491-1
Zhou, Y., Sha, M. Y., Jin, H. Q., Wang, L. F., Zhang, J., Xu, Z. F., et al. (2023). The expansion of evergreen and deciduous shrubs changed the chemical characteristics and biological community of alpine meadows soil. Eur. J. Soil Biol. 117, 103505–103510. doi: 10.1016/j.ejsobi.2023.103505
Zhu, P., Chen, R., Song, Y., Liu, G., Chen, T., and Zhang, W. (2015). Effects of land cover conversion on soil properties and soil microbial activity in an alpine meadow on the Tibetan plateau. Environ. Earth Sci. 74, 4523–4533. doi: 10.1007/s12665-015-4509-1
Keywords: Pinus massoniana, rhizosphere microorganisms, Biolog Eco, carbon source metabolism, functional diversity
Citation: Huang Z, Qin Y, He X, Zhang M, Ren X, Yu W and Ji K (2024) Analysis on metabolic functions of rhizosphere microbial communities of Pinus massoniana provenances with different carbon storage by Biolog Eco microplates. Front. Microbiol. 15:1365111. doi: 10.3389/fmicb.2024.1365111
Edited by:
Reeta Goel, GLA University, IndiaReviewed by:
Arti Goel, Amity University, IndiaKrishna Giri, Indian Council of Forestry Research and Education (ICFRE), India
Copyright © 2024 Huang, Qin, He, Zhang, Ren, Yu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kongshu Ji, a3NqaUBuamZ1LmVkdS5jbg==
 Zichen Huang
Zichen Huang Yiyun Qin
Yiyun Qin Xin He
Xin He Kongshu Ji
Kongshu Ji