
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol. , 05 April 2024
Sec. Microbe and Virus Interactions with Plants
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1362722
 Ameni Ben Zineb1*
Ameni Ben Zineb1* Mariem Lamine2
Mariem Lamine2 Ahlem Khallef2,3
Ahlem Khallef2,3 Helmi Hamdi1
Helmi Hamdi1 Talaat Ahmed4
Talaat Ahmed4 Hareb Al-Jabri1,5
Hareb Al-Jabri1,5 Mohammed Alsafran6
Mohammed Alsafran6 Ahmed Mliki2
Ahmed Mliki2 Sami Sayadi1*
Sami Sayadi1* Mahmoud Gargouri2*†
Mahmoud Gargouri2*†Date palm cultivation has thrived in the Gulf Cooperation Council region since ancient times, where it represents a vital sector in agricultural and socio-economic development. However, climate change conditions prevailing for decades in this area, next to rarefication of rain, hot temperatures, intense evapotranspiration, rise of sea level, salinization of groundwater, and intensification of cultivation, contributed to increase salinity in the soil as well as in irrigation water and to seriously threaten date palm cultivation sustainability. There are also growing concerns about soil erosion and its repercussions on date palm oases. While several reviews have reported on solutions to sustain date productivity, including genetic selection of suitable cultivars for the local harsh environmental conditions and the implementation of efficient management practices, no systematic review of the desertic plants’ below-ground microbial communities and their potential contributions to date palm adaptation to climate change has been reported yet. Indeed, desert microorganisms are expected to address critical agricultural challenges and economic issues. Therefore, the primary objectives of the present critical review are to (1) analyze and synthesize current knowledge and scientific advances on desert plant-associated microorganisms, (2) review and summarize the impacts of their application on date palm, and (3) identify possible gaps and suggest relevant guidance for desert plant microbes’ inoculation approach to sustain date palm cultivation within the Gulf Cooperation Council in general and in Qatar in particular.
Among the arid zones of the globe, which occupy a third of the Earth’s surface (Neilson et al., 2017), the Gulf Cooperation Council (GCC) region, known for its large sandy deserts (Alsharif et al., 2020), has experienced the most intense climate changes over the last two decades, inducing profound disruptions that seriously threaten the sustainability of existing agrosystems (Fernández-López et al., 2022).
Within this area, the cultivation of date palm (Phoenix dactylifera L.), or phœniciculture, is the flagship activity, the symbol of life, and the cornerstone of oasis agrosystems. It is considered as a “holy tree” due to its vital nutritional (fruits and by-products) and socio-economic importance (Almadini et al., 2021; Naqvi et al., 2021). In addition to its agronomic and economic importance, date palm is known to tolerate harsh environmental conditions such as extreme temperature, drought, and high salinity of soil (Abumaali et al., 2023b). Since the Middle East has sandy soils and a dry climate, this tree contributes efficiently to mitigating desertification and erosion and preserving the oasis microclimate (Jain et al., 2011). Nevertheless, and despite this robustness, date palm encounters diverse constraints that, if no intelligent strategies are urgently implemented, would progressively compromise its sustainability and, in the long term, its extinction from this geographical area (El-juhany, 2014; Almadini et al., 2021). Among these structural and climatic constraints are: low organic soil matter content (Darwish and Fadel, 2017), limited and reduced groundwater levels (Sherif et al., 2023), growing salinity (Al Kharusi et al., 2019), resurgence and spread of pests and diseases (Alotaibi et al., 2023), and a very limited survival rate of newly planted plantlets due to their low potential to adapt to the harsh conditions of the environment (Saadaoui et al., 2019). Indeed, date palms are adapted to arid and semi-arid regions. They withstand moderately alkaline soils, with a pH ranging from 7 to 8.5, but extremely high alkalinity can be detrimental to their growth (Alotaibi et al., 2023; Sanka Loganathachetti and Mundra, 2023). They are adapted to soils with low organic matter levels, ranging from 0.1 to 1 (Mlih et al., 2016, 2019), while proper fertilization is essential to ensure their optimal growth. Furthermore, date palms exhibit a notable tolerance to salinity levels, commonly ranging from 4 to 8 dS/m or even higher under specific conditions (Muller et al., 2017; Alotaibi et al., 2023). Nevertheless, the irrigation of date palm with saline groundwater, due to water scarcity, is a common practice in the MENA regions, which threatens the sustainability of date palm cultivation (Shamim et al., 2022; Sanka Loganathachetti and Mundra, 2023).
To overcome these constraints, several useful solutions have been developed (Cai and Liu, 2015), among them, biotechnological approaches involve innovative breeding programs (Hazzouri et al., 2020). However, the efficiency of these conventional approaches in combating various stresses in plants was limited on one hand, and the non-conventional approaches, such as tissue in vitro technology, require more labor and may produce less resilient plants, particularly in woody trees (Al-Khateeb et al., 2020). Moreover, the excess of chemical fertilizer amendments and agricultural practices (e.g., tillage) has dramatically affected the diversity of the beneficial soil microbiota. This situation is worsened by intense climate changes, which impart severe impacts on plant–soil–microorganism interactions by altering the structure, abundance, composition, and functional activity of the rhizosphere microbiome (Sabir et al., 2021). Indeed, plants’ endosphere, rhizosphere, leaves, and other tissues are home to a multitude of microorganisms, known as the microbiome (Bonatelli et al., 2021). The rhizosphere microbiome interacts with and affects, often positively, the adaptation of its host plants to their environment (Bais et al., 2006; Kumar and Dubey, 2020). Indeed, the influence of plant roots on microbes is governed by the root exudates (Williams and de Vries, 2020), which include low-molecular-weight primary metabolites, like organic acids, amino acids, and sugars, and secondary metabolites, such as phenols, flavonoids, and terpenoids (De Vries et al., 2019). A plant’s exudate may be affected by climate change as a result of alterations in the plant’s photosynthetic apparatus, which will indirectly affect the root microbes by changing the carbon sources available to them and leading to their cell lysis (Chen et al., 2022). In their study, Naylor et al. (2017) compared the root rhizosphere of 18 species of monocot plants under drought stress and found that Actinobacteria are more abundant during a water deficit. Rice root-associated microbiota were also found to be enriched in Actinobacteria and Chloroflexi under drought stress, while several Acidobacteria and Deltaproteobacteria were depleted (Andreo-Jimenez et al., 2019).
Several ecosystem processes are directly or indirectly influenced by soil microorganisms, which play a vital role in enhancing ecosystem resilience and complexity (Robinson et al., 2023). These microorganisms are known to have beneficial attributes promoting nutrient cycling (e.g., solubilizing or decomposing soil’s below-ground complexed phosphorus, Ben Zineb et al., 2019b), plant health (e.g., systemic tolerance can be induced by plant growth-promoting microorganisms through biochemical mechanisms) (De Zelicourt et al., 2013), and climate regulation (e.g., CO2, CH4, and N2O producing or consuming). Therefore, their use in the restoration, conservation, and maintenance of the date palm ecology and production is becoming more challenging. There is an urgent need for more integrated research to improve simultaneously the productivity of the low-cost date palm system and its sustainability and to develop technologies favoring/restoring its microbial diversity.
Microbes play crucial roles in the rhizosphere of date palm, contributing to its overall health and nutrient availability. Indeed, several investigations have uncovered a wide spectrum of interactions between plant growth-promoting (PGP) microbes and date palm, including the promotion of shoot and root growth (Cherif et al., 2015), inducing systemic tolerance against abiotic stresses (Harkousse et al., 2021), as well as the inhibition of some pathogenic fungi (Siala et al., 2016). In light of this, date palm sustainability can be met through the intelligent use of native plant microbiomes, which boost plant potential to adapt and survive under intense abiotic stresses (Koziol et al., 2018; Qiu et al., 2019; Alsharif et al., 2020). Among these, desert indigenous microorganisms are increasingly recognized as a long-term environmental and ecological potential solution to sustain agriculture in the oasis ecosystem (Almutawa, 2022). Their application benefits have been documented to: (i) promote date palm growth and survival rate of seedlings in the nursery (Shabbir et al., 2011); (ii) improve nutrient uptake by maintaining metabolic processes (Van Oosten et al., 2017; Alsharif et al., 2020); (iii) improve resistance to harmful pathogens (Mefteh et al., 2018); and (iv) induce better tolerance to complex abiotic stresses, drought, and salinity at a priority level (Benhiba et al., 2015; Akensous et al., 2022b). Consequently, focusing on beneficial microorganisms from arid and desertic lands would represent potential and innovating biotechnological tools to restore and promote agricultural activity in desertic areas in general and of date palm in oases in particular (Symanczik et al., 2014; Ferjani et al., 2015).
Alotaibi et al. (2023) reported on date palm biotechnology, including overviews of soil and environmental conditions of date palm cultivation, and Jain et al. (2011) reported on research progress and applications in this domain. Nevertheless, no holistic and comprehensive review has yet been conducted on the arid land’s microbiome, including prokaryotic and fungal communities, and its contribution to phœniciculture sustainability. Therefore, a deep study of the diversity and role of the date palm microbiome, including bacteria, fungi, archaea, viruses, and other microbes, is particularly relevant. Decades of research have demonstrated the importance of gathering information from the genetic repertoires of microbial communities from various hosts (Lucaciu et al., 2019). The recent advent of high-throughput sequencing technologies coupled with a variety of “omics” techniques has marked the beginning of a new green era in agriculture (Pantigoso et al., 2023). Modern sequencing techniques, such as next-generation sequencing, 16S rRNA gene sequencing, internal transcribed spacer sequencing, or the combination of these methods, provide in-depth information about plant microbial partners. They were able to better characterize the structure and function of these communities (Mitter et al., 2019; Satam et al., 2023). Still, the cultivation of those microbial partners is needed. Indeed, engineering date palm cultivable microbes might involve two approaches, either by re-introducing in situ enriched indigenous beneficial microorganisms or by inoculating them with exogenous beneficial microorganisms. In situ direct inoculation of microbes with PGP activities is the most commonly used strategy to enhance date palm growth (Yaish et al., 2015; Hazzouri et al., 2020). Although inoculating date palm with exogenous microorganisms may not directly promote their growth, they could still benefit by recruiting other microbial species able to enhance their resilience against abiotic stress (Alsharif et al., 2020).
Along with the incessant search for sustainable processes to produce dates in hyper-arid ecosystems, like the GCC area, there is a need to collect updated information, encouraging researchers to engage in new eco-friendly, insightful studies. Therefore, this review critically reports knowledge and pertinent scientific achievements on desert plant-associated microorganisms and their applications on date palms. We also targeted the knowledge available, the gaps, and what would be recommended for the desert plant microbes’ inoculation approaches to sustain the GCC phœniciculture, with a particular emphasis on Qatar.
Decades of empirical and theoretical research have revealed that plants are not standalone entities. They are influenced by their association with microbiota, named “holobiont” (Vandenkoornhuyse et al., 2015; Wagg et al., 2022). The holobiont of desert plants is the center of interest regarding its performance under severe environmental constraints (Araya et al., 2020). Nowadays, several projects have reported promising results for the improvement of agricultural production systems sustainability, owing to inoculation with microbial rhizospheres deriving from plants surviving in arid and desertic areas (Köberl et al., 2011, 2013; Eida et al., 2018; Ha et al., 2021; Procter et al., 2022).
This high-performance potential was reported for the holobiont of the desert plant cassava (Manihot esculenta Crantz) (Ha et al., 2021). The African desert grass Stipagrostis pungens, grown under severe drought conditions, revealed harboring beneficial bacteria that produce extracellular polymeric substances (e.g., exopolysaccharide), which form a hydrophilic biofilm around plant roots (Rolli et al., 2015). Thus, protecting the roots from desiccation as well as amending the soil structure and its aggregation properties result in increased soil water holding capacity and improve the overall resilience of the holobiont (Huang et al., 2022; Marasco et al., 2022). For the Atacama’s desert plants, Cistanthe longiscapa and Citrullus colocynthis, it has been reported that their survival strategy was forged through intimate interactions with associated soil bacteria and fungi (Araya et al., 2020; Procter et al., 2022). Citrullus colocynthis was reported to develop symbiotic interactions with plant growth-promoting bacteria such as Acidobacteria, Bacterioidetes, and Actinobacteria for nitrogen, sulfur, and carbon cycles, as well as for the solubilization of phosphate and the synthesis of indole-2-acetic acid and siderophores (Procter et al., 2022). The four native Saudi Arabian desert plants, Zygophyllum simplex, Panicum turgidum, Euphorbia granulate, and Tribulus terrestris, harbor bacterial strains that exhibit distinct biochemical pathways regarding nutrient uptake and survival under stress conditions (Eida et al., 2018). Recently, Abumaali et al. (2023a,b) stated that Qatari wild date palm (Phoenix sylvestris) displayed specific and unique bacterial operational taxonomic units (OTUs) that could improve date palm tolerance to salinity and drought. Indeed, the rhizospheric core microbiome from arid regions may improve the ability of date palm to withstand harsh environmental conditions by promoting microbe-induced systemic tolerance. To cope with abiotic stress and low organic carbon, microbes engage a multitude of direct and indirect mechanisms to support plants (Figure 1; Mohanty et al., 2021). Direct mechanisms involve the increase of vital nutrient acquisition (e.g., N, P, and Fe) (Anli et al., 2020), the accumulation of osmolytes that impart drought tolerance in plants (e.g., soluble sugars, proline, glycine, organic acids, Huang et al., 2014), the production of exopolysaccharide (Rolli et al., 2015), the regulation of phytohormone levels including auxin, gibberellin, and cytokinin, and particularly the 1-aminocyclopropane-1-carboxylate (ACC) deaminase to reduce the ethylene level in roots (Lau et al., 2022), and the induction of stress-responsive genes (e.g., NCED, P5CS) (Poudel et al., 2021). Indirect mechanisms encompass actions where microbes enhance plants’ resilience by improving soil characteristics (Ait-El-Mokhtar et al., 2020), maximizing the total area of the root, resulting in improved nutrient and water absorption (Ngumbi and Kloepper, 2016), or suppressing pathogens that may exacerbate stress conditions (Poudel et al., 2021). Through these intricate interactions, microbes play a key role in bolstering plant resilience and enabling them to thrive in challenging environments.
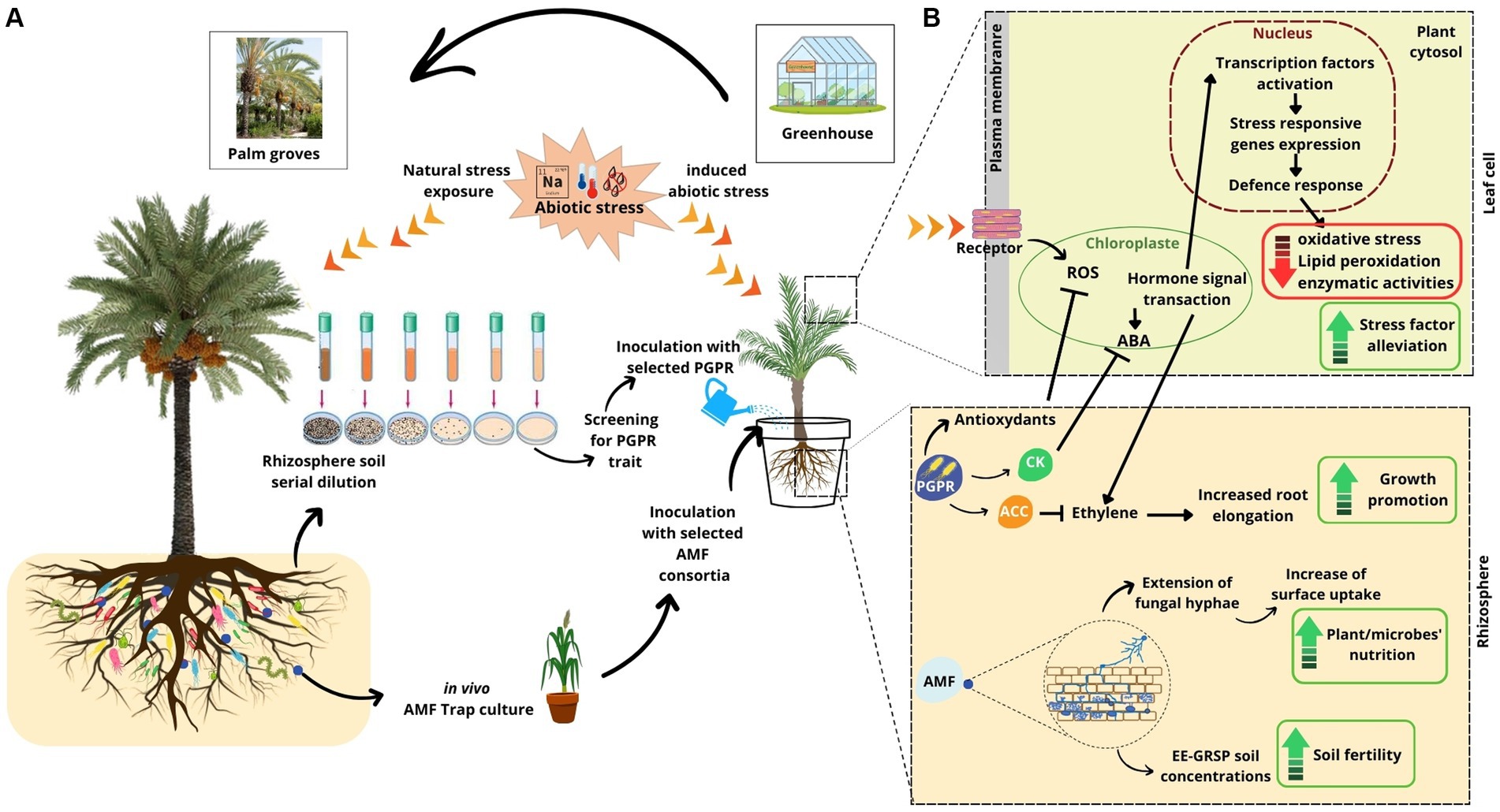
Figure 1. (A) An overview of the date palm close-up inoculation system through re-introduction in situ of enriched indigenous beneficial microorganisms. (B) Date palm perception of external stimuli and the activation of both direct and indirect defense mechanisms to support the plant in dealing with abiotic stresses. Arrows indicate promotion; blunt-ended lines indicate inhibition. All the stress response mechanisms of PGP bacteria and AMF shown in this figure are synthesized from the studies listed in Table 1. AMF, arbuscular mycorrhizal fungi; PGP, plant growth-promoting; IAA, indole-3-acetic acid; ABA, abscisic acid; ACC, 1-aminocyclopropane-1-carboxylic acid; CK, cytokinin; ROS, reactive oxygen species.
Next, to the advantageous ecological and protective services offered by desert plant-associated microorganisms (Köberl et al., 2016; Shilev et al., 2019; Karray et al., 2020; Gargouri et al., 2021b), few microbial profiling studies have been carried out on the rhizospheres and root systems of date palm, the iconic oasis keystone (Chebaane et al., 2020; Gagou et al., 2023; Abumaali et al., 2023a). Moreover, although high-throughput sequencing technology provides excellent opportunities for the investigation of microbiomes, studies on date palm microbiomes remain scarce.
In accordance with the available data, date palm rhizosphere soil and root systems shelter a reservoir of beneficial symbiotic microorganisms that positively regulate its homeostasis (Ferjani et al., 2015). Mosqueira et al. (2019) carried out a broad survey of bacterial diversity associated with date palm grown across the Sahara desert in Tunisia. They identified two major endophytic bacterial phyla, Gammaproteobacteria and Alphaproteobacteria, known to perform ecological functions of biopromotion and biofertilization in harsh environments. Shamim et al. (2022) demonstrated that Micromonospora and Mycobacterium bacterial taxa were effective in alleviating salinity stress when date palms were irrigated with saline water. Cherif et al. (2015) further showed that Gammaproteobacteria, a class of endophytic bacteria isolated from date palm, was also effective in improving plant drought tolerance. Using pyrosequencing, Yaish et al. (2016) revealed that the composition of endophytic bacterial and fungal communities in P. dactylifera differs according to the concentration of salt in the irrigation water.
Up-to-date, there are few reports addressing date palm microbial profiling (Ferjani et al., 2015; Yaish et al., 2016; Mosqueira et al., 2019; Chebaane et al., 2020; Al-busaidi et al., 2022; Shamim et al., 2022). Few of them go deeper beyond the species identification level. Consequently, advanced technologies, such as high-throughput sequencing, have become highly recommended to be able to characterize in depth the rhizosphere and endophytic microbiota of P. dactylifera, which would further contribute to dissecting more beneficial microbial taxa and better understanding their role in enhancing date palm stress mitigation.
The rhizosphere and endosphere of arid land habitats offer a valuable reservoir of biomolecules with fertilizing and biocontrol properties against a large spectrum of biotic and abiotic constraints (Alsharif et al., 2020). They feature a wide diversity of plant growth-promoting (PGP) microbial communities involved in vital processes, exchanging services for niches and nutrients, ultimately resulting in a win–win and high-performance partnership with the plant partner (Vacheron et al., 2013; Soussi et al., 2016). Consequently, they are regarded as potential and pertinent candidates to substitute conventional fertilizers and pesticides. This would promote food security and the sustainability of food production systems (Gargouri et al., 2021b; Ben Zineb et al., 2022).
The recent overview by Alsharif et al. (2020) on the diversity of desert plant rhizosphere microbiomes, including the latest findings and applications, reported that desert PGP microorganisms are genetically better equipped to adapt to harsh environments than those evolving in non-arid soils. Furthermore, many research teams were focusing on studying arid land-associated microbial communities to explore their beneficial agronomical contributions following their inoculation with cash crops, such as wheat (Singh and Jha, 2016), cowpea (Minaxi et al., 2012), Salicornia (Marasco et al., 2016), and date palm (Anli et al., 2020; Chebaane et al., 2020). Among the available data, a consensus emerges on the advantageous contribution provided by the inoculation of PGP bacteria on date palm to better adapt to abiotic stresses (Figure 1; Table 1).
Producing dates with an economically profitable yield and competitive quality under the constraints of continuously increasing salinity remains a difficult challenge to overcome. Selection of tolerant date palms to salinity was addressed by means of in vitro culture (Roy et al., 2014), given that working directly in the soil makes the task difficult in arid and hyper-arid regions affected by salinity (Al Kharusi et al., 2019). Studies indicate that close inoculation of date palm with PGP bacteria (Figure 1) could reduce oxidative stress, directly or indirectly, for example, via the accumulation of osmolytes or by the production of hormones capable of modulating the plant’s response (De Zelicourt et al., 2013; Hazzouri et al., 2020). Yaish et al. (2015) reported that date palm endophytic bacteria synthesize 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase capable of cleaving part of ACC (a precursor in the ethylene biosynthesis pathway) induced by salt stress and causing inhibition of root elongation (Lau et al., 2022). This molecule can thus play a role in promoting the growth and development of the date palm in saline environments.
Furthermore, date palm nutrient utilization under salinity constraints can be improved when associated with mycorrhiza. This symbiotic relationship may enhance their yield by improving absorption of soil nutrients through fungal hyphae extension in the rhizosphere area, thereby increasing surface uptake (Akensous et al., 2022b). Accordingly, Meddich et al. (2018) noticed a consistent increase of Ca, P, K, Mg, and Mn when date palm seedlings, inoculated with arbuscular mycorrhizal fungi (AMF), were subjected to different stresses. Moreover, the inoculation of date palm plantlets with a consortium made of AMF and a mixture of Glomus sp., Sclerocystis sp., and Acaulospora sp. improved the physiological responses of the stressed plants (photosynthetic efficiency, leaf water potential, and stomata conductance). The enhancement of photosynthetic capacity resulted in a higher capacity of gas exchange, a better efficiency of the photosystem II (PS II), and a more efficient regulation of the energy flow between the photochemical reactions and the non-photochemical reactions (Ait-El-Mokhtar et al., 2019, 2022). More interestingly, the strains of AMF from Tunisian oasis ecosystems increased the fraction of easily extractable glomalin-related soil protein (EE-GRSP), suggesting that the AMF undergo a survival mode to mitigate the negative effects of salt stress for themselves as well as for their date palm host plants (Chebaane et al., 2020).
In addition to strengthening date palm trees’ resilience to salinity, arid land’s microorganisms employ different pathways to counter the negative consequences of drought, mainly in young date palm plantations (Hazzouri et al., 2020). They induce systemic tolerance by triggering a series of biochemical and physiological responses. In this respect, Harkousse et al. (2021) reported that inoculation with a consortium composed of 28 species of rhizosphere AMF, collected from an oasis palm grove, improved the relative water content of the leaves of stressed date palm plants and increased their proline content, a fundamental osmoregulation solute (Liang et al., 2013). Next to this, Anli et al. (2020) reported that the co-inoculation with plant growth-promoting rhizobacteria and AMF (composed of Sclerocystis sp., Acaulospora sp., and Glomus sp.) increased date palm protein and soluble sugar contents and boosted the antioxidant defense activity. Date palm plants inoculated and subjected to water stress responded with an increase in their water potential and water content, which ensured the maintenance of physiological turgor levels. It should be noted that the accumulation of osmolytes and the strengthening of antioxidant power can contribute to osmotic regulation, the maintenance of cellular turgor, the preservation of cellular structures, and the traps of reactive oxygen species [e.g., hydrogen peroxide, H2O2, and malondialdehyde (MDA)]. This likely resembles a primary avoidance strategy developed by date palms inoculated in response to water stress. Furthermore, the inoculation of date palm roots, indoor and outdoor, activates hormonal biosynthesis (ABA, for example) to ensure acceptable levels of stomatal and photosynthetic activities (Meddich et al., 2021). Indeed, inoculated date palms responded with an increase in the elasticity of their leaf cell walls and modified the redistribution of water between the symplastic and the apoplastic compartments (Baslam et al., 2014). Therefore, this could serve as an alternative strategy to survive water stress through corrective responses at the level of vital physiological attributes such as relative water content (RWC), electrolyte loss/leakage (EL), and stomatal conductance.
Overall, the inoculation with microorganisms from the date palm rhizosphere could serve as an integrated approach to improve date palm defenses and mitigate the negative effects imposed by salt and water stresses and by pathogens. Such inoculation could serve as an effective means to improve their growth and productivity under future climate change scenarios.
Cultivated date palm microorganisms’ inoculum has been used to boost date palm resistance to abiotic and biotic stresses and would represent potential substitutes for conventional pest control products (Meddich et al., 2018; Omomowo et al., 2023). Nevertheless, they remain facing efficiency, technical, and sustainability challenges (Qiu et al., 2019). To reach an acceptable efficiency level on date palm, these beneficial microbes must overcome key steps: establishment, survival, colonization, and interaction with the host tree.
Date palms are typically grown in arid regions where soils are often coarse-textured and calcareous, deficient in nutrients and organic matter, and where the pH is rather alkaline (Alotaibi et al., 2023). Therefore, the availability of nutrients and the effectiveness of fertilizers, particularly phosphorus, can affect their development. Indeed, phosphorus represents the macronutrient most sensitive to soil pH, and its availability in alkaline and calcareous soils is made low in particular by the presence of Ca2+, whose precipitation and retention power are rather high (Ben Zineb et al., 2019b). The use of slow-release phosphorus fertilizer, such as rock phosphate, might be a potential solution (Ben Zineb et al., 2022). Moreover, to reduce the adverse effects of limestone, phosphate-solubilizing microorganisms’ amendments are recommended. Yet, interactions between phosphate-solubilizing microbes with intrinsic soil properties (humidity, water and nutrient availability, temperature, pH, etc.) and date palm exudates must also be taken into account (Uroz et al., 2019; Fitzpatrick et al., 2020). Additionally, current strategies implemented in soil management are rather inappropriate (Almadini et al., 2021), as they are heavily dependent on fertilizers produced by the chemical industry, which are often harmful to soil microorganisms. Indeed, chemical fertilizers might be partially immobilized right after their application, which induces lower root colonization and thereby limited inoculation efficiency. In this respect, El Hilali et al. (2022) found reduced levels of mycorrhizal root colonization in date palms receiving synthetic fertilizers.
In the date palm multiplication process, the transition from the laboratory to the field, acclimation, is one of the most critical stages, as it represents the transition from an assisted or autotrophic lifestyle to an autonomous/self-sufficient or heterotrophic mode (Nazir et al., 2015; Solangi et al., 2022). Indeed, after in vitro cultivation, the plantlets are extracted from their synthetic cultivation medium and transferred into soil and acclimatized in a less controlled environment, where they have to adapt to survive (different light, less humidity, different atmosphere, different nutrients, and substrate) (Hassan, 2017; Saadaoui et al., 2019). In addition, plants can modify, directly or indirectly, the habitat of the rhizosphere, notably by rhizodeposits, which end up modifying the surrounding conditions of the roots. Therefore, research work remains necessary to identify the optimal physiological stage of date palm for inoculation with PGP microorganisms in order to obtain maximum benefit.
Inoculant formulation is a critical aspect and should be optimized to allow high competition and survivability of the inoculum under severe environmental conditions. The exogenous microorganisms have to overcome colonization issues and establish a symbiotic and beneficial environment for both entities (Ferjani et al., 2015). Indeed, the persistence of the inoculated microorganisms can be promoted via the use of consortia composed of resistant PGP microorganisms (e.g., engineered microbial communities, SynComs) rather than monocultures containing single selected strains in order to improve the survival rates and the physiological activity of the microbial inoculum (Uroz et al., 2019; Fitzpatrick et al., 2020). In this respect, Anli et al. (2020) reported that a close-up inoculation of date palm with indigenous PGP bacteria increased the AMF root system infection under drought stress, probably because the inoculated bacteria enhanced the AMF multiplication and activity (Ben Zineb et al., 2022).
Generally, inoculant development mainly focuses on genetic and PGP traits and often neglects ecological traits that are critical to the success of inoculations (Kaminsky et al., 2019). The release of exotic species into the rhizosphere of date palms risks disrupting their ecological balance, which would make indigenous communities less competitive and therefore more vulnerable compared to exogenous species. Therefore, the isolation and screening of pre-adapted dryland microorganisms should take into consideration both PGP and ecological criteria, which means strains that are both functional and have increased environmental adaptation potentials (Mefteh et al., 2018; Ben Zineb et al., 2019a; Toubali et al., 2020; Harkousse et al., 2021). Furthermore, due to sampling constraints, extensive niche specialization, and the low adaptability of conventional cultural practices, many date palm microorganisms were neglected in terms of cultivation and characterization in the laboratory (Bull et al., 2016). Consequently, the creation of complete collections of strains via systematic culturomics, diversifying culture conditions, and taking advantage of high-throughput sequencing should improve our understanding of the diversity of cultivable rhizosphere microbiomes of the date palm (Matar and Bilen, 2022; Li et al., 2023).
Additionally, the formulation of the inoculant has to support, at the same time, microbial growth and the protection of viable cells in order to trigger an efficient response in date palm (Bashan et al., 2016). An inoculum formulation using varied and innovative technologies should be tested (microencapsulation, nanotechnology, etc.) to increase the efficiency of inoculum application (Kragh et al., 2018). Furthermore, it is recommended that date palm pre-adapted inoculum and/or selected compounds of prebiotics, such as phytoalexin and triterpenes, be used in order to favor microbes of interest (Pantigoso et al., 2023). Finally, a multiple approach combining conventional pathogenicity tests, targeting non-target organisms, and genomics should be implemented before field dissemination of the inoculum (Qiu et al., 2019).
AMF counts among the most represented, oldest, most widespread, and most important symbioses on Earth that contribute to feeding the world. They associate with an estimate of 72% of land plants and can deliver up to 90% of the total plant phosphorus, making these microorganisms a focal point of attention for many biotech companies. AMF inoculum production remains mainly limited to in vivo systems (e.g., greenhouse), which are often inexpensive and suitable for large-scale production with densities reaching 80–100 propagules per cm3 of substrate (Gianinazzi et al., 2002). Yet, these cultivation systems are not exempt from contaminants and may require large spaces. Their cultivation under in vitro conditions using whole plants or their organs is a promising alternative to producing high-quality inoculum that is free of any contaminants and requires limited space. Still, in vitro production yields do not reach the level of in vivo systems; they are costly and restricted to a few species (Ijdo et al., 2011). Therefore, there is an urgent need to propose novel in vitro cultivation systems providing clean, safe, and robust spore date palm inoculum, produced at high densities and with reduced costs (Gargouri et al., 2021a).
At the application level, administration practices must favor the protection of the inoculated microbes against environmental stresses. Thus, the inoculation technique is decisive for the success of the inoculation.
The date palm is in constant challenge against lack of water, rising salinity, extreme temperatures, degradation and loss of soil fertility, pests, and diseases. The combination of multiple stresses of biotic and abiotic nature (high salinity, accentuated drought, high temperatures, pathogens, etc.) or abiotic stresses alone (salinity stress, water stress, or thermal stress) could have more harmful effects on the survival of the date palm (Safronov et al., 2017; Khan et al., 2020). In this respect, Meddich et al. (2021) reported that the effect of drought induces more severe damage when the plant is at the same time infected with Fusarium oxysporum f. sp. albedinis (Foa). High temperatures also make plants more vulnerable to diseases and contribute to the emergence of more virulent pathogens (Ali et al., 2023). Khan et al. (2020) revealed a more significant diminution of shoot elongation when date palms were simultaneously exposed to a combined stress composed of salt and cadmium than when they were exposed to a single stress (NaCl or Cd). In fact, when cadmium (Cd) interacts with NaCl, there is the formation of Cd-Cl complexes, which act as stimulators of Cd uptake by the plant. However, studies dealing with the effect of multiple stresses (more than two) on tolerant microbiota to monitor date palm cultivar responses are still missing. Consequently, systematic studies are pivotal to understanding the tripartite interactions involving date palm trees, stress-tolerant microbiota, and multi-abiotic stresses (Anderegg et al., 2015). Ultimately, the development of an approach for beneficial microbiota tolerant to multiple stresses will make it possible to better understand the behavior of the date palm with respect to climate change.
We collected and analyzed the available literature (mainly international peer-reviewed studies) published in recent years from 2010 up to May 2023 based on the search engines of Web of Science, ScienceDirect, and Google Scholar, using the following keywords: “Qatar and date palm,” “Qatar and microbiome,” “Qatar and soil microbial communities,” “Qatar and soil bacterial communities,” “Qatar and soil fungal communities,” “Qatar and soil mycorrhizae.” This query led to insufficient results because of the lack of studies on the investigated topics, particularly those studying microbial ecology, community structure, and their interactions with date palm plants.
In the GCC, where water scarcity and desert conditions pose significant challenges to agriculture (Al-Khateeb et al., 2020), date palm trees have been of keen research interest as they are among the main agricultural sectors concerned by the sustainability issue (Al Nabil, 2021; Karanisa et al., 2021). Despite multiple programs aiming at the rehabilitation and rescue of palm groves (Meddich et al., 2018), the soil microbiota associated with the date palm is poorly represented in global microbial databases. Moreover, there are still no detailed reports based on modern science on the microbiome of wild plant species in the GCC soils (Al-Thani and Yasseen, 2021; Abumaali et al., 2023a).
Although ancestors of wild date palm populations exist in remote areas of the GCC region and have been shown to be quite different from those found in Africa and the Middle East (Gros-Balthazard et al., 2018), only a limited number of publications report on wild date palm and desert plants or the inoculation of cultivated date palm (Abumaali et al., 2023a). It is obvious that the restoration of any ecosystem needs to integrate different components and data available on the ecosystem in question, in particular the microorganisms associated with the local plants (Anli et al., 2020).
Accordingly, in order to enhance date palm resilience to climate change and promote sustainable cultivation and production of dates in Qatar, a number of research priorities were identified: (i) To aid in understanding how date palm–microbe interactions would help them resist in the desert environmental stresses, further investigation is required to have a more holistic perception regarding date palm root-associated microbiome, especially wild date palm, as suggested in Figure 2. The use of modern high-throughput sequencing should improve the characterization of the composition and performance of the rhizospheric and endophytic microbiota of the date palm to be able to exploit and valorize it optimally. (ii) Given the scarcity of data on desert microbiome and the effect of their application in date palm oases in Qatar, it’s important to note that research and experimentation specific to autochthone microbes would be necessary to determine their most effective synthetic combination and optimal screening and application methods for a profitable cultivation of date palm in the area (Figure 2), paving the path for beneficial agricultural applications (Al-Yahya’ei et al., 2011). (iii) Future research programs using agro-ecological approaches should prioritize the maintenance and improvement of soil fertility and structure. Practices like cover plants and adapted halophytes for ecological or agricultural purposes can enhance soil organic matter and reduce erosion, leading to healthier and more productive soils. In addition, it makes sense to provide a microclimate favorable to the development of complementary underlying crops, particularly of a fodder nature (Toubali et al., 2020). Overall, recognizing the importance of integrating different ecosystem components, particularly microorganisms, for ecosystem restoration, research priorities have been identified to enhance date palm resilience in the GCC. These priorities include investigating date palm–microbe interactions, optimizing high-throughput sequencing for characterizing the microbiota, studying desert microbiomes for application in date palm cultivation, and implementing agro-ecological approaches to improve soil fertility. A holistic model, suggested in Figure 2, should be developed, involving modern and environmentally friendly agricultural technologies, to serve as a lever and catalyst for establishing sustainable agriculture and economy.
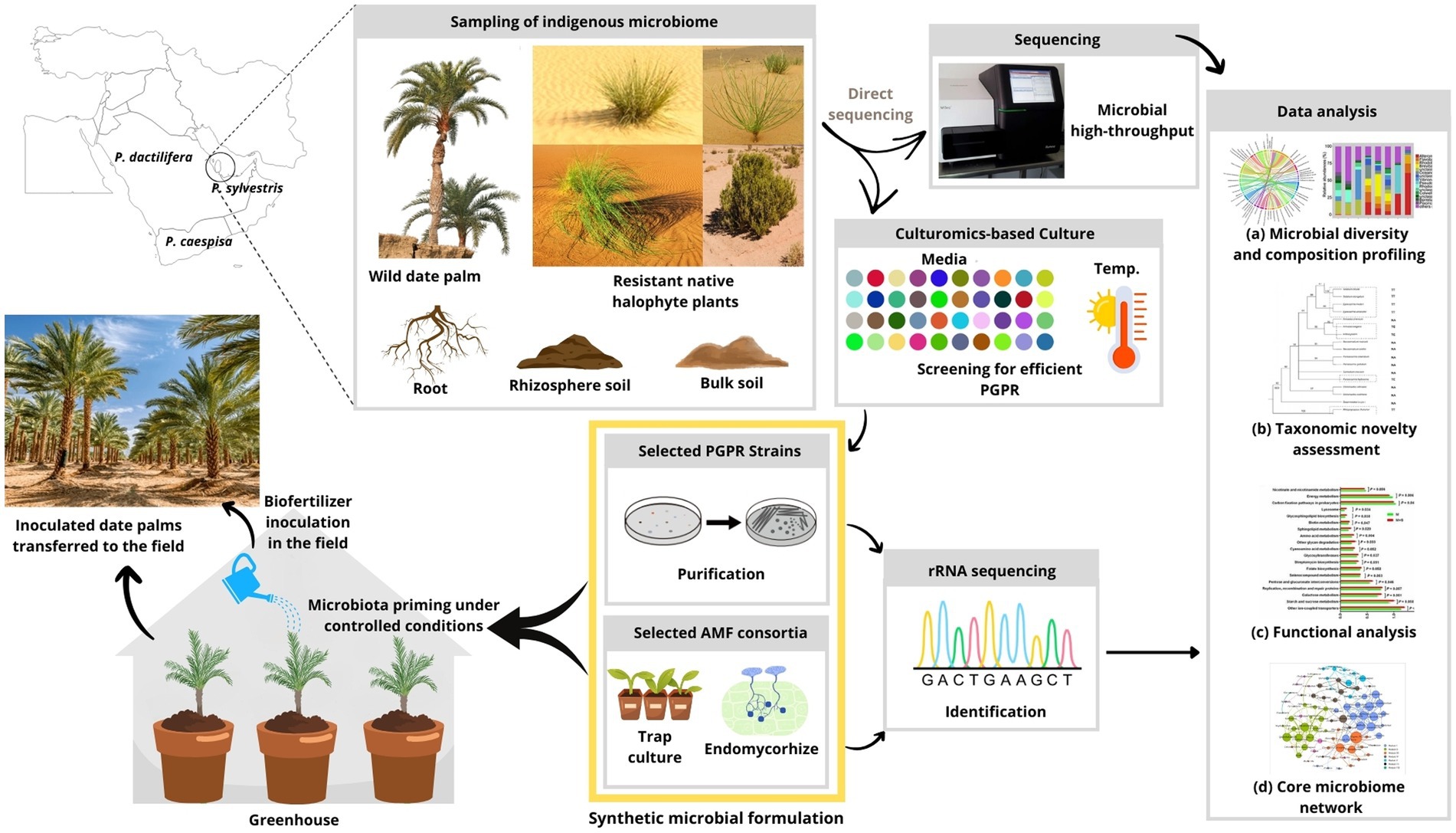
Figure 2. Road map of future studies that will further promote the sustainability of date palm in the GCC. Based on the research question of interest, the concept of a synthetic microbial community generated from indigenous microbes screened from the autochthone wild date palm populations (P. sylvestris) and native desertic plants is necessary to pave the path for beneficial agriculture practices. The application of culturomics technology and microbial high-throughput sequencing to determine (a) the microbial diversity and composition profiling, (b) the taxonomic novelty assessment, (c) the microbial functional analysis, and (d) the core microbiome network of the native plants. This knowledge should help guide next-generation field applications to promote the sustainability of date palm cultivation.
AB: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. ML: Writing – review & editing. AK: Writing – review & editing. HH: Writing – review & editing. TA: Writing – review & editing. HA-J: Writing – review & editing. MA: Writing – review & editing. AM: Writing – review & editing. SS: Funding acquisition, Writing – review & editing. MG: Writing – original draft, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This publication was made possible by the QNRF-MME award (MME03-1120-210024) from the Qatar National Research Fund (a member of the Qatar Foundation). The findings are solely the responsibility of the authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abumaali, D. A., Al-Hadidi, S. H., Ahmed, T., Al-khis, A. F., Al-Malki, S. A., Yaish, M., et al. (2023a). Bacterial community structure and predicted function in the rhizosphere of wild and cultivated date palms: effects of fertilizers on composition and functionality. Ecol. Genet. Genomics 29:100195. doi: 10.1016/j.egg.2023.100195
Abumaali, D. A., Al-Hadidi, S. H., Daly Yahia, M. N., Erfanian, M. B., Ahmed, T. A., and Alatalo, J. M. (2023b). The date palm microbiome: a review. Ecol. Genet. Genomics 29:100212. doi: 10.1016/j.egg.2023.100212
Ait-El-Mokhtar, M., Baslam, M., Ben-Laouane, R., Anli, M., Boutasknit, A., Mitsui, T., et al. (2020). Alleviation of detrimental effects of salt stress on date palm (Phoenix dactylifera L.) by the application of arbuscular mycorrhizal fungi and/or compost. Front. Sustain. Food Syst. 4:131. doi: 10.3389/fsufs.2020.00131
Ait-El-Mokhtar, M., Ben Laouane, R., Anli, M., Boutasknit, A., Wahbi, S., and Meddich, A. (2021). Arbuscular mycorrhizal fungi in saline soil: alleviating date palm under high-salt stress in Bio-stimulants for sustainable agriculture in oasis ecosystem towards improving date palm tolerance to biotic and abiotic stress, eds. A. Meddich and A. Zaid, United Arab Emirates: Khalifa International Award for Date Palm and Agricultural Innovation, 126–147.
Ait-El-Mokhtar, M., Ben-Laouane, R., Boutasknit, A., Anli, M., Fakhech, A., Ait-Rahou, Y., et al. (2022). Evaluation of young date palm tolerance to salinity stress under arbuscular mycorrhizal fungi and compost application. Environ. Sci. Proc. 16:15. doi: 10.3390/environsciproc2022016015
Ait-El-Mokhtar, M., Laouane, R. B., Anli, M., Boutasknit, A., Wahbi, S., and Meddich, A. (2019). Use of mycorrhizal fungi in improving tolerance of the date palm (Phoenix dactylifera L.) seedlings to salt stress. Sci. Hortic. 253, 429–438. doi: 10.1016/j.scienta.2019.04.066
Akensous, F. Z., Anli, M., Boutasknit, A., Ben-Laouane, R., Ait-Rahou, Y., Ahmed, H. B., et al. (2022a). Boosting date palm (Phoenix dactylifera L.) growth under drought dtress: effects of innovative biostimulants. Gesunde Pflanz. 74, 961–982. doi: 10.1007/s10343-022-00651-0
Akensous, F. Z., Anli, M., and Meddich, A. (2022b). Biostimulants as innovative tools to boost date palm (Phoenix dactylifera L.) performance under drought, salinity, and heavy metal(Oid)s’ stresses: a concise review. Sustain. For. 14, 1–30. doi: 10.3390/su142315984
Akensous, F. Z., Anli, M., and Meddich, A. (2023). Arbuscular mycorrhizal fungi as solubilizers of rock phosphate and compost application improve date palm (Phoenix dactylifera L.)‘s resilience to drought. Gesunde Pflanz. 76, 161–179. doi: 10.1007/s10343-023-00927-z
Al Kharusi, L., Sunkar, R., Al-Yahyai, R., and Yaish, M. W. (2019). Comparative water relations of two contrasting date palm genotypes under salinity. Int. J. Agron. 2019, 1–16. doi: 10.1155/2019/4262013
Al-busaidi, A., Glick, B. R., and Yaish, M. W. (2022). The effect of date palm genotypes on rhizobacterial community structures under saline environments. Biology 11:1666. doi: 10.3390/biology11111666
Ali, S., Ali, S., Tyagi, A., and Mir, R. A. (2023). Plant beneficial microbiome a boon for improving multiple stress tolerance in plants. Front. Plant Sci. 14:1266182. doi: 10.3389/fpls.2023.1266182
Al-Khateeb, S. A., Al-Khateeb, A. A., Sattar, M. N., and Mohmand, A. S. (2020). Induced in vitro adaptation for salt tolerance in date palm (Phoenix dactylifera L.) cultivar Khalas. Biol. Res. 53, 37–12. doi: 10.1186/s40659-020-00305-3
Almadini, A. M., Ismail, A. I. H., and Ameen, F. A. (2021). Assessment of farmers practices to date palm soil fertilization and its impact on productivity at Al-Hassa oasis of KSA. Saudi J. Biol. Sci. 28, 1451–1458. doi: 10.1016/j.sjbs.2020.11.084
Almutawa, A. A. (2022). Date production in the Al-Hassa region, Saudi Arabia in the face of climate change. J. Water Clim. Chang. 13, 2627–2647. doi: 10.2166/wcc.2022.461
Alotaibi, K. D., Alharbi, H. A., Yaish, M. W., Ahmed, I., Alharbi, S. A., Alotaibi, F., et al. (2023). Date palm cultivation: A review of soil and environmental conditions and future challenges. L. Degrad. Dev. 34, 2431–2444. doi: 10.1002/ldr.4619
Alsharif, W., Saad, M. M., and Hirt, H. (2020). Desert microbes for boosting sustainable agriculture in extreme environments. Front. Microbiol. 11:1666. doi: 10.3389/fmicb.2020.01666
Al-Thani, R. F., and Yasseen, B. T. (2021). Microbial ecology of Qatar, the arabian gulf: possible roles of microorganisms. Front. Mar. Sci. 8:697269. doi: 10.3389/fmars.2021.697269
Al-Yahya’ei, M. N., Oehl, F., Vallino, M., Lumini, E., Redecker, D., Wiemken, A., et al. (2011). Unique arbuscular mycorrhizal fungal communities uncovered in date palm plantations and surrounding desert habitats of southern Arabia. Mycorrhiza 21, 195–209. doi: 10.1007/s00572-010-0323-5
Anderegg, W. R. L., Hicke, J. A., Fisher, R. A., Allen, C. D., Aukema, J., Bentz, B., et al. (2015). Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 208, 674–683. doi: 10.1111/nph.13477
Andreo-Jimenez, B., Vandenkoornhuyse, P., Lê Van, A., Heutinck, A., Duhamel, M., Kadam, N., et al. (2019). Plant host and drought shape the root associated fungal microbiota in rice. PeerJ 7, e7463–e7423. doi: 10.7717/peerj.7463
Anli, M., Baslam, M., Boutasknit, A., Ait, M., and Mokhtar, E. (2021). Soil inoculation with symbiotic microorganisms (mycorrhizas and Rhizobium) and compost promote date palm performance under drought condition: from controlled-condition to open-field system in Bio-stimulants for sustainable agriculture in oasis ecosystem towards improving date palm tolerance to biotic and abiotic stress, eds. A. Meddich and A. Zaid, United Arab Emirates: Khalifa International Award for Date Palm and Agricultural Innovation.
Anli, M., Baslam, M., Tahiri, A., Raklami, A., Symanczik, S., Boutasknit, A., et al. (2020). Biofertilizers as strategies to improve photosynthetic apparatus, growth, and drought stress tolerance in the date palm. Front. Plant Sci. 11:516818. doi: 10.3389/fpls.2020.516818
Araya, J. P., González, M., Cardinale, M., Schnell, S., and Stoll, A. (2020). Microbiome dynamics associated with the atacama flowering desert. Front. Microbiol. 10:3160. doi: 10.3389/fmicb.2019.03160
Bais, H. P., Weir, T. L., Perry, L. G., Gilroy, S., and Vivanco, J. M. (2006). The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 57, 233–266. doi: 10.1146/annurev.arplant.57.032905.105159
Bashan, Y., Kloepper, J. W., de-Bashan, L. E., and Nannipieri, P. (2016). A need for disclosure of the identity of microorganisms, constituents, and application methods when reporting tests with microbe-based or pesticide-based products. Biol. Fertil. Soils 52, 283–284. doi: 10.1007/s00374-016-1091-y
Baslam, M., Qaddoury, A., and Goicoechea, N. (2014). Role of native and exotic mycorrhizal symbiosis to develop morphological, physiological and biochemical responses coping with water drought of date palm, Phoenix dactylifera. Trees 28, 161–172. doi: 10.1007/s00468-013-0939-0
Ben Zineb, A., Gargouri, M., López-Ráez, J. A., Trabelsi, D., Aroca, R., and Mhamdi, R. (2022). Interaction between P fertilizers and microbial inoculants at the vegetative and flowering stage of Medicago truncatula. Plant Growth Regul. 98, 511–524. doi: 10.1007/s10725-022-00886-x
Ben Zineb, A., Trabelsi, D., Ayachi, I., Barhoumi, F., Aroca, R., and Mhamdi, R. (2019a). Inoculation with elite strains of phosphate-solubilizing bacteria enhances the effectiveness of fertilization with rock phosphates. Geomicrobiol J. 37, 22–30. doi: 10.1080/01490451.2019.1658826
Ben Zineb, A., Trabelsi, D., Barhoumi, F., Dhane, S. F., and Mhamdi, R. (2019b). Potentialities and soil impact analysis of rock phosphorus fertilization of perennial and annual legume crops. Arch. Agron. Soil Sci. 66, 1074–1088. doi: 10.1080/03650340.2019.1655731
Benhiba, L., Fouad, M. O., Essahibi, A., Ghoulam, C., and Qaddoury, A. (2015). Arbuscular mycorrhizal symbiosis enhanced growth and antioxidant metabolism in date palm subjected to long-term drought. Trees 29, 1725–1733. doi: 10.1007/s00468-015-1253-9
Bonatelli, M. L., Lacerda-Júnior, G. V., dos Reis Junior, F. B., Fernandes-Júnior, P. I., Melo, I. S., and Quecine, M. C. (2021). Beneficial plant-associated microorganisms from semiarid regions and seasonally dry environments: a review. Front. Microbiol. 11:553223. doi: 10.3389/fmicb.2020.553223
Bull, A. T., Asenjo, J. A., Goodfellow, M., and Gómez-Silva, B. (2016). The atacama desert: technical resources and the growing importance of novel microbial diversity. Ann. Rev. Microbiol. 70, 215–234. doi: 10.1146/annurev-micro-102215-095236
Cai, S., and Liu, D. (2015). Detecting change dates from dense satellite time series using a sub-annual change detection algorithm. Remote Sens. 7, 8705–8727. doi: 10.3390/rs70708705
Chebaane, A., Symanczik, S., Oehl, F., Azri, R., Gargouri, M., Mäder, P., et al. (2020). Arbuscular mycorrhizal fungi associated with Phoenix dactylifera L. grown in Tunisian sahara oases of different salinity levels. Symbiosis 81, 173–186. doi: 10.1007/s13199-020-00692-x
Chen, Y., Yao, Z., Sun, Y., Wang, E., Tian, C., Sun, Y., et al. (2022). Current studies of the effects of drought stress on root exudates and rhizosphere microbiomes of crop plant species. Int. J. Mol. Sci. 23:2374. doi: 10.3390/ijms23042374
Cherif, H., Marasco, R., Rolli, E., Ferjani, R., Fusi, M., Soussi, A., et al. (2015). Oasis desert farming selects environment-specific date palm root endophytic communities and cultivable bacteria that promote resistance to drought. Environ. Microbiol. Rep. 7, 668–678. doi: 10.1111/1758-2229.12304
Darwish, T., and Fadel, A. (2017). Mapping of soil organic carbon stock in the Arab countries to mitigate land degradation. Arab. J. Geosci. 10:474. doi: 10.1007/s12517-017-3267-7
De Vries, F. T., Williams, A., Stringer, F., Willcocks, R., McEwing, R., Langridge, H., et al. (2019). Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 224, 132–145. doi: 10.1111/nph.16001
De Zelicourt, A., Al-Yousif, M., and Hirt, H. (2013). Rhizosphere microbes as essential partners for plant stress tolerance. Mol. Plant 6, 242–245. doi: 10.1093/mp/sst028
Eida, A. A., Ziegler, M., Lafi, F. F., Michell, C. T., Voolstra, C. R., Hirt, H., et al. (2018). Desert plant bacteria reveal host influence and beneficial plant growth properties. PLoS One 13:e0208223. doi: 10.1371/journal.pone.0208223
El Hilali, R., Symanczik, S., El Kinany, S., Oehl, F., Ouahmane, L., Bouamri, R., et al. (2022). Cultivation, identification, and application of arbuscular mycorrhizal fungi associated with date palm plants in Drâa-Tafilalet oasis. Rhizosphere. 22.
El-juhany, L. I. (2014). Degradation of date palm trees and date production in Arab countries: causes and potential rehabilitation. Aust. J. Basic Appl. Sci. 4, 3998–4010.
Faghire, M., Samri, S., Baslam, M., Goicoechea, N., Meddich, A., and Qaddoury, A. (2010). Positive effects of arbuscular mycorrhizal fungi on biomass production, nutrient status and water relations in date palm seedlings under water deficiency. Acta Hortic. 1, 833–838. doi: 10.17660/ActaHortic.2010.882.95
Ferjani, R., Marasco, R., Rolli, E., Cherif, H., Cherif, A., Gtari, M., et al. (2015). The date palm tree rhizosphere is a niche for plant growth promoting bacteria in the oasis ecosystem. Biomed. Res. Int. 2015:153851. doi: 10.1155/2015/153851
Fernández-López, J., Viuda-Martos, M., Sayas-Barberá, E., Navarro-Rodríguez de Vera, C., and Pérez-Álvarez, J. Á. (2022). Biological, nutritive, functional and healthy potential of date palm fruit (Phoenix dactylifera L.): current research and future prospects. Agronomy 12:876. doi: 10.3390/agronomy12040876
Fitzpatrick, C. R., Salas-González, I., Conway, J. M., Finkel, O. M., Gilbert, S., Russ, D., et al. (2020). The plant microbiome: from ecology to reductionism and beyond. Ann. Rev. Microbiol. 74, 81–100. doi: 10.1146/annurev-micro-022620-014327
Gagou, E., Chakroune, K., Abbas, M., Lamkami, T., and Hakkou, A. (2023). Evaluation of the mycorrhizal potential of date palm (Phoenix dactylifera L.) rhizosphere soils in the Figuig oasis (southeastern Morocco). J. Fungi 9:931. doi: 10.3390/jof9090931
Gargouri, M., Bates, P. D., and Declerck, S. (2021a). Combinatorial reprogramming of lipid metabolism in plants: a way towards mass-production of bio-fortified arbuscular mycorrhizal fungi inoculants. Microb. Biotechnol. 14, 31–34. doi: 10.1111/1751-7915.13684
Gargouri, M., Karray, F., Chebaane, A., Mhiri, N., Partida-Martínez, L. P., Sayadi, S., et al. (2021b). Increasing aridity shapes beta diversity and the network dynamics of the belowground fungal microbiome associated with Opuntia ficus-indica. Sci. Total Environ. 773:145008. doi: 10.1016/j.scitotenv.2021.145008
Gianinazzi, S., Schuepp, H., Barea, J., and Haselwandter, K. (2002) in Mycorrhizal technology in agriculture, from genes to bioproducts. eds. S. Gianinazzi, H. Schuepp, J. Barea, and K. Haselwandter (Basel, Switzerland: Springer Basel AG).
Gros-Balthazard, M., Hazzouri, K. M., and Flowers, J. M. (2018). Genomic insights into date palm origins. Genes 9:502. doi: 10.3390/genes9100502
Ha, J., Gao, Y., Zhang, R., Li, K., Zhang, Y., Niu, X., et al. (2021). Diversity of the bacterial microbiome associated with the endosphere and rhizosphere of different cassava (Manihot esculenta Crantz) genotypes. Front. Microbiol. 12:729022. doi: 10.3389/fmicb.2021.729022
Harkousse, O., Slimani, A., Jadrane, I., Aitboulahsen, M., Mazri, M. A., Zouahri, A., et al. (2021). Role of local biofertilizer in enhancing the oxidative stress defence systems of date palm seedling (Phoenix dactylifera) against abiotic stress. Appl. Environ. Soil Sci. 2021:6628544. doi: 10.1155/2021/6628544
Hassan, M. M. (2017). “Improvement of in vitro date palm plantlet acclimatization rate with kinetin and hoagland solution” in Date palm biotechnology protocols volume 1: tissue culture applications, methods in molecular biology. eds. J. Al-Khayri, S. Jain, and D. Johnson (New York, NY: Humana Press), 185–200.
Hazzouri, K. M., Flowers, J. M., Nelson, D., Lemansour, A., Masmoudi, K., and Amiri, K. M. A. (2020). Prospects for the study and improvement of abiotic stress tolerance in date palms in the post-genomics era. Front. Plant Sci. 11:293. doi: 10.3389/fpls.2020.00293
Huang, B., DaCosta, M., and Jiang, Y. (2014). Research advances in mechanisms of Turfgrass tolerance to abiotic stresses: from physiology to molecular biology. CRC. Crit. Rev. Plant Sci. 33, 141–189. doi: 10.1080/07352689.2014.870411
Huang, L., Jin, Y., Zhou, D., Liu, L., Huang, S., Zhao, Y., et al. (2022). A review of the role of extracellular polymeric substances (EPS) in wastewater treatment systems. Int. J. Environ. Res. Public Health 19:12191. doi: 10.3390/ijerph191912191
Ijdo, M., Cranenbrouck, S., and Declerck, S. (2011). Methods for large-scale production of AM fungi: past, present, and future. Mycorrhiza 21, 1–16. doi: 10.1007/s00572-010-0337-z
Jain, S. M., Al-Khayri, J. M., and Johnson, D. V. (2011) in Date palm biotechnology. eds. S. M. Jain, J. M. Al-Khayri, and D. V. Johnson (Dordrecht: Springer)
Kaminsky, L. M., Trexler, R. V., Malik, R. J., Hockett, K. L., and Bell, T. H. (2019). The inherent conflicts in developing soil microbial inoculants. Trends Biotechnol. 37, 140–151. doi: 10.1016/j.tibtech.2018.11.011
Karanisa, T., Amato, A., Richer, R., Abdul Majid, S., Skelhorn, C., and Sayadi, S. (2021). Agricultural production in Qatar’s hot arid climate. Sustain. For. 13:4059. doi: 10.3390/su13074059
Karray, F., Gargouri, M., Chebaane, A., Mhiri, N., Mliki, A., and Sayadi, S. (2020). Climatic aridity gradient modulates the diversity of the rhizosphere and endosphere bacterial microbiomes of Opuntia ficus-indica. Front. Microbiol. 11:1622. doi: 10.3389/fmicb.2020.01622
Khan, A., Bilal, S., Khan, A. L., Imran, M., Al-Harrasi, A., Al-Rawahi, A., et al. (2020). Silicon-mediated alleviation of combined salinity and cadmium stress in date palm (Phoenix dactylifera L.) by regulating physio-hormonal alteration. Ecotoxicol. Environ. Saf. 188:109885. doi: 10.1016/j.ecoenv.2019.109885
Köberl, M., Erlacher, A., Ramadan, E. M., El-Arabi, T. F., Müller, H., Bragina, A., et al. (2016). Comparisons of diazotrophic communities in native and agricultural desert ecosystems reveal plants as important drivers in diversity. FEMS Microbiol. Ecol. 92:fiv166. doi: 10.1093/femsec/fiv166
Köberl, M., Müller, H., Ramadan, E. M., and Berg, G. (2011). Desert farming benefits from microbial potential in arid soils and promotes diversity and plant health. PLoS One 6:e24452. doi: 10.1371/journal.pone.0024452
Köberl, M., Schmidt, R., Ramadan, E. M., Bauer, R., and Berg, G. (2013). The microbiome of medicinal plants: diversity and importance for plant growth, quality, and health. Front. Microbiol. 4:400. doi: 10.3389/fmicb.2013.00400
Koziol, L., Schultz, P. A., House, G. L., Bauer, J. T., Middleton, E. L., and Bever, J. D. (2018). The plant microbiome and native plant restoration: the example of native mycorrhizal fungi. Bioscience 68, 996–1006. doi: 10.1093/biosci/biy125
Kragh, K. N., Alhede, M., Rybtke, M., Stavnsberg, C., Jensen, P., Tolker-Nielsen, T., et al. (2018). The inoculation method could impact the outcome of microbiological experiments. Appl. Environ. Microbiol. 84, 1–14. doi: 10.1128/AEM.02264-17
Kumar, A., and Dubey, A. (2020). Rhizosphere microbiome: engineering bacterial competitiveness for enhancing crop production. J. Adv. Res. 24, 337–352. doi: 10.1016/j.jare.2020.04.014
Lau, S.-E., Teo, W. F. A., Teoh, E. Y., and Tan, B. C. (2022). Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discov. Food 2:9. doi: 10.1007/s44187-022-00009-5
Li, S., Lian, W., Han, J., Ali, M., Lin, Z., Liu, Y., et al. (2023). Capturing the microbial dark matter in desert soils using culturomics-based metagenomics and high-resolution analysis. NPJ Biofilms Microbiomes 9:67. doi: 10.1038/s41522-023-00439-8
Liang, X., Zhang, L., Natarajan, S. K., and Becker, D. F. (2013). Proline mechanisms of stress survival. Antioxid. Redox Signal. 19, 998–1011. doi: 10.1089/ars.2012.5074
Lucaciu, R., Pelikan, C., Gerner, S. M., Zioutis, C., Köstlbacher, S., Marx, H., et al. (2019). A bioinformatics guide to plant microbiome analysis. Front. Plant Sci. 10:1313. doi: 10.3389/fpls.2019.01313
Marasco, R., Fusi, M., Mosqueira, M., Booth, J. M., Rossi, F., Cardinale, M., et al. (2022). Rhizosheath–root system changes exopolysaccharide content but stabilizes bacterial community across contrasting seasons in a desert environment. Environ. Microbiomes 17, 14–19. doi: 10.1186/s40793-022-00407-3
Marasco, R., Mapelli, F., Rolli, E., Mosqueira, M. J., Fusi, M., Bariselli, P., et al. (2016). Salicornia strobilacea (synonym of Halocnemum strobilaceum) grown under different tidal regimes selects rhizosphere bacteria capable of promoting plant growth. Front. Microbiol. 7:1286. doi: 10.3389/fmicb.2016.01286
Matar, G., and Bilen, M. (2022). Culturomics, a potential approach paving the way toward bacteriotherapy. Curr. Opin. Microbiol. 69:102194. doi: 10.1016/j.mib.2022.102194
Meddich, A. (2021). Bio-stimulants for sustainable agriculture in oasis ecosystem towards improving date palm tolerance to biotic and abiotic stress. ed. A. Zaid (United Arab Emirates: Khalifa International Award for Date Palm and Agricultural Innovation).
Meddich, A., Ait El Mokhtar, M., Bourzik, W., Mitsui, T., Baslam, M., and Hafidi, M. (2018). “Optimizing growth and tolerance of date palm (Phoenix dactylifera L.) to drought, salinity, and vascular fusarium-induced wilt (Fusarium oxysporum) by application of arbuscular mycorrhizal fungi (AMF)” in Root biology. eds. B. Giri, R. Prasad, and A. Varma (Cham: Springer), 239–258.
Meddich, A., Ait El Mokhtar, M., Bourzik, W., Mitsui, T., Baslam, M., and Hafidi, M. (2021). “Optimizing growth and tolerance of date palm (Phoenix dactylifera L.) to drought and vascular fusarium-induced wilt (Fusarium oxysporum) by application of arbuscular mycorrhizal fungi (AMF)” in Bio-stimulants for sustainable agriculture in oasis ecosystem towards improving date palm tolerance to biotic and abiotic stress, in Soil Biology. eds. A. Giri, B. Prasad, and R. Varma. (Springer, Cham), 239–252. doi: 10.1007/978-3-319-75910-4_9
Mefteh, F. B., Daoud, A., Bouket, A. C., Thissera, B., Kadri, Y., Cherif-Silini, H., et al. (2018). Date palm trees root-derived endophytes as fungal cell factories for diverse bioactive metabolites. Int. J. Mol. Sci. 19:1986. doi: 10.3390/ijms19071986
Minaxi, L. N., Yadav, R. C., and Saxena, J. (2012). Characterization of multifaceted Bacillus sp. RM-2 for its use as plant growth promoting bioinoculant for crops grown in semi arid deserts. Appl. Soil Ecol. 59, 124–135. doi: 10.1016/j.apsoil.2011.08.001
Mitter, B., Brader, G., Pfaffenbichler, N., and Sessitsch, A. (2019). Next generation microbiome applications for crop production—limitations and the need of knowledge-based solutions. Curr. Opin. Microbiol. 49, 59–65. doi: 10.1016/j.mib.2019.10.006
Mlih, R., Bol, R., Amelung, W., and Brahim, N. (2016). Soil organic matter amendments in date palm groves of the middle eastern and north African region: a mini-review. J. Arid. Land 8, 77–92. doi: 10.1007/s40333-015-0054-8
Mlih, R. K., Gocke, M. I., Bol, R., Berns, A. E., Fuhrmann, I., and Brahim, N. (2019). Soil organic matter composition in coastal and continental date palm systems: insights from Tunisian oases. Pedosphere 29, 444–456. doi: 10.1016/S1002-0160(19)60814-3
Mohanty, P., Singh, P. K., Chakraborty, D., Mishra, S., and Pattnaik, R. (2021). Insight into the role of PGPR in sustainable agriculture and environment. Front. Sustain. Food Syst. 5:667150. doi: 10.3389/fsufs.2021.667150
Mosqueira, M. J., Marasco, R., Fusi, M., Michoud, G., Merlino, G., Cherif, A., et al. (2019). Consistent bacterial selection by date palm root system across heterogeneous desert oasis agroecosystems. Sci. Rep. 9, 4033–4012. doi: 10.1038/s41598-019-40551-4
Muller, H. M., Schäfer, N., Bauer, H., Geiger, D., Lautner, S., Fromm, J., et al. (2017). The desert plant Phoenix dactylifera closes stomata via nitrate-regulated SLAC1 anion channel. New Phytol. 216, 150–162. doi: 10.1111/nph.14672
Naqvi, S. A., Shafqat, W., Haider, M. S., Awan, S., Khan, A., and Jaskani, M. J. (2021) in The date palm genome. eds. J. M. Al-Khayri, S. Jain, and D. Johnson (Cham: Springer International Publishing) Available at: http://www.springer.com/series/11805
Naylor, D., Degraaf, S., Purdom, E., and Coleman-Derr, D. (2017). Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 11, 2691–2704. doi: 10.1038/ismej.2017.118
Nazir, H., Osman, E. A., Talaat, A., and Al-Yafa, M. S. (2015). “Date palm status and perspective in Qatar” in Date palm genetic resources and utilization: Asia and Europe. eds. J. M. Al-Khayri, S. M. Jain, and D. V. Johnson (Dordrecht: Springer), 447–485.
Neilson, J. W., Califf, K., Cardona, C., Copeland, A., van Treuren, W., Josephson, K. L., et al. (2017). Significant impacts of increasing aridity on the arid soil microbiome. mSystems 2:e00195-16. doi: 10.1128/msystems.00195-16
Ngumbi, E., and Kloepper, J. (2016). Bacterial-mediated drought tolerance: current and future prospects. Appl. Soil Ecol. 105, 109–125. doi: 10.1016/j.apsoil.2016.04.009
Omomowo, I. O., Amao, J. A., Abubakar, A., Ogundola, A. F., Ezediuno, L. O., and Bamigboye, C. O. (2023). A review on the trends of endophytic fungi bioactivities. Sci. Afr. 20:e01594. doi: 10.1016/j.sciaf.2023.e01594
Outamamat, E., Bourhia, M., Dounas, H., Salamatullah, A. M., Alzahrani, A., Alyahya, H. K., et al. (2021). Application of native or exotic arbuscular mycorrhizal fungi complexes and monospecific isolates from saline semi-arid mediterranean ecosystems improved phoenix dactylifera’s growth and mitigated salt stress negative effects. Plan. Theory 10:2501. doi: 10.3390/plants10112501
Pantigoso, H. A., Newberger, D., and Vivanco, J. M. (2023). The rhizosphere microbiome: plant–microbial interactions for resource acquisition. Front. Soil Sci. 133, 2864–2876. doi: 10.1111/jam.15686
Poudel, M., Mendes, R., Costa, L. A. S., Bueno, C. G., Meng, Y., Folimonova, S. Y., et al. (2021). The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Front. Microbiol. 12:743512. doi: 10.3389/fmicb.2021.743512
Procter, M., Kundu, B., Sudalaimuthuasari, N., AlMaskari, R. S., Saeed, E. E., Hazzouri, K. M., et al. (2022). Microbiome of Citrullus colocynthis (L.) Schrad. Reveals a potential association with non-photosynthetic cyanobacteria. Microorganisms 10:2083. doi: 10.3390/microorganisms10102083
Qiu, Z., Egidi, E., Liu, H., Kaur, S., and Singh, B. K. (2019). New frontiers in agriculture productivity: optimised microbial inoculants and in situ microbiome engineering. Biotechnol. Adv. 37:107371. doi: 10.1016/j.biotechadv.2019.03.010
Robinson, J. M., Hodgson, R., Krauss, S. L., Liddicoat, C., Malik, A. A., Martin, B. C., et al. (2023). Opportunities and challenges for microbiomics in ecosystem restoration. Trends Ecol. Evol. 38, 1189–1202. doi: 10.1016/j.tree.2023.07.009
Rolli, E., Marasco, R., Vigani, G., Ettoumi, B., Mapelli, F., Deangelis, M. L., et al. (2015). Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 17, 316–331. doi: 10.1111/1462-2920.12439
Roy, S. J., Negrão, S., and Tester, M. (2014). Salt resistant crop plants. Curr. Opin. Biotechnol. 26, 115–124. doi: 10.1016/j.copbio.2013.12.004
Saadaoui, I., Sedky, R., Rasheed, R., Bounnit, T., Almahmoud, A., Elshekh, A., et al. (2019). Algae-based biofertilizer for date palm cultivation in Qatar. J. Appl. Phycol. 31, 457–463. doi: 10.29117/quarfe.2020.0034
Sabir, M. S., Shahzadi, F., Ali, F., Shakeela, Q., Niaz, Z., and Ahmed, S. (2021). Comparative effect of fertilization practices on soil microbial diversity and activity: an overview. Curr. Microbiol. 78, 3644–3655. doi: 10.1007/s00284-021-02634-2
Safronov, O., Kreuzwieser, J., Haberer, G., Alyousif, M. S., Schulze, W., Al-Harbi, N., et al. (2017). Detecting early signs of heat and drought stress in Phoenix dactylifera (date palm). PLoS One 12:e0177883. doi: 10.1371/journal.pone.0177883
Sanka Loganathachetti, D., and Mundra, S. (2023). Water pH, not soil pH, alters bacterial community structural pattern and nitrogen cycling pathways in date palm (Phoenix dactylifera L.) roots and bulk soil under freshwater irrigation regime. Front. Ecol. Evol. 11:1142073. doi: 10.3389/fevo.2023.1142073
Satam, H., Joshi, K., Mangrolia, U., Waghoo, S., Zaidi, G., Rawool, S., et al. (2023). Next-generation sequencing technology: current trends and advancements. Biology 12:997. doi: 10.3390/biology12070997
Shabbir, G., Dakheel, A., Brown, G., and Rillig, M. (2011). Potential of arbuscular mycorrhizal technology in date palm production in Date palm biotechnology, eds. S. M. Jain, J. M. Al-Khayri, and D. V. Johnson. Netherland: Springer, 449–476.
Shamim, A., Sanka Loganathachetti, D., Chandran, S., Masmoudi, K., and Mundra, S. (2022). Salinity of irrigation water selects distinct bacterial communities associated with date palm (Phoenix dactylifera L.) root. Sci. Rep. 12, 12733–12711. doi: 10.1038/s41598-022-16869-x
Sherif, M., Abrar, M., Baig, F., and Kabeer, S. (2023). Gulf cooperation council countries’ water and climate research to strengthen UN’s SDGs 6 and 13. Heliyon 9:e14584. doi: 10.1016/j.heliyon.2023.e14584
Shilev, S., Azaizeh, H., Vassilev, N., Georgiev, D., and Babrikova, I. (2019). Microbial interventions in agriculture and environment: research trends, priorities and prospects. eds. D. Singh, V. Gupta, and R. Prabha. Singapore: Springer Nature Singapore.
Siala, R., Ben Chobba, I., Vallaeys, T., Triki, M. A., Jrad, M., Cheffi, M., et al. (2016). Analysis of the cultivable endophytic bacterial diversity in the date palm (Phoenix dactylifera L.) and evaluation of its antagonistic potential against pathogenic fusarium species that cause date palm bayound disease. J. Appl. Environ. Microbiol. 4, 93–104. doi: 10.12691/jaem-4-5-2
Singh, R. P., and Jha, P. N. (2016). The multifarious PGPR Serratia marcescens CDP-13 augments induced systemic resistance and enhanced salinity tolerance of wheat (Triticum aestivum L.). PLoS One 11:e0155026. doi: 10.1371/journal.pone.0155026
Solangi, N., Jatoi, M. A., Markhand, G. S., Abul-Soad, A. A., Solangi, M. A., Jatt, T., et al. (2022). Optimizing tissue culture protocol for in vitro shoot and root development and acclimatization of date palm (Phoenix dactylifera L.) plantlets. Erwerbs-obstbau 64, 97–106. doi: 10.1007/s10341-021-00622-1
Soussi, A., Ferjani, R., Marasco, R., Guesmi, A., Cherif, H., Rolli, E., et al. (2016). Plant-associated microbiomes in arid lands: diversity, ecology and biotechnological potential. Plant Soil 405, 357–370. doi: 10.1007/s11104-015-2650-y
Symanczik, S., Błaszkowski, J., Chwat, G., Boller, T., Wiemken, A., and Al-Yahya’ei, M. N. (2014). Three new species of arbuscular mycorrhizal fungi discovered at one location in a desert of Oman: Diversispora omaniana, Septoglomus nakheelum, and Rhizophagus arabicus. Mycologia 106, 243–259. doi: 10.3852/106.2.243
Toubali, S., Tahiri, A., Anli, M., Symanczik, S., Boutasknit, A., Ait-El-Mokhtar, M., et al. (2020). Physiological and biochemical behaviors of date palm vitroplants treated with microbial consortia and compost in response to salt stress. Appl. Sci. 10, 1–25. doi: 10.3390/app10238665
Uroz, S., Courty, P. E., and Oger, P. (2019). Plant symbionts are engineers of the plant-associated microbiome. Trends Plant Sci. 24, 905–916. doi: 10.1016/j.tplants.2019.06.008
Vacheron, J., Desbrosses, G., Bouffaud, M. L., Touraine, B., Moënne-Loccoz, Y., Muller, D., et al. (2013). Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 4:356. doi: 10.3389/fpls.2013.00356
Van Oosten, M. J., Pepe, O., De Pascale, S., Silletti, S., and Maggio, A. (2017). The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 4:5. doi: 10.1186/s40538-017-0089-5
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Wagg, C., Roscher, C., Ebeling, A., Luca, E.De, Roeder, A., Marquard, E., et al. (2022). Biodiversity – stability relationships strengthen over time in a long-term grassland experiment. Nat. Commun. 13:7752. doi: 10.1038/s41467-022-35189-2
Williams, A., and de Vries, F. T. (2020). Plant root exudation under drought: implications for ecosystem functioning. New Phytol. 225, 1899–1905. doi: 10.1111/nph.16223
Yaish, M. W., Al-Harrasi, I., Alansari, A. S., Al-Yahyai, R., and Glick, B. R. (2016). The use of high throughput DNA sequence analysis to assess the endophytic microbiome of date palm roots grown under different levels of salt stress. Int. Microbiol. 19, 143–155. doi: 10.2436/20.1501.01.272
Keywords: date palm, desert plant microbes, sustainability, biofertilizers, GCC
Citation: Ben Zineb A, Lamine M, Khallef A, Hamdi H, Ahmed T, Al-Jabri H, Alsafran M, Mliki A, Sayadi S and Gargouri M (2024) Harnessing rhizospheric core microbiomes from arid regions for enhancing date palm resilience to climate change effects. Front. Microbiol. 15:1362722. doi: 10.3389/fmicb.2024.1362722
Received: 28 December 2023; Accepted: 11 March 2024;
Published: 05 April 2024.
Edited by:
James T. Tambong, Agriculture and Agri-Food Canada (AAFC), CanadaReviewed by:
Nagaraju Yalavarthi, Central Silk Board, IndiaCopyright © 2024 Ben Zineb, Lamine, Khallef, Hamdi, Ahmed, Al-Jabri, Alsafran, Mliki, Sayadi and Gargouri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ameni Ben Zineb, YW1lbnliZW56aW5lYkBnbWFpbC5jb20=; Mahmoud Gargouri, bWFobW91ZC5nYXJnb3VyaUBjYmJjLnJucnQudG4=; Sami Sayadi, c3NheWFkaUBxdS5lZHUucWE=
†Present address: Mahmoud Gargouri, Institute of Biological Chemistry, Washington State University, Pullman, WA, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.