- Key Laboratory of Edible Fungal Resources and Utilization (North), Ministry of Agriculture and Rural Affairs, Jilin Agricultural University, Changchun, China
Cystolepiota is a tiny lepiotaceous fungi. During our 3 years fieldwork, we found four new species of Cystolepiota from northeastern China. A phylogenetic study of a combined dataset of ITS+nrLSU+rpb2+tef1-α revealed that Cystolepiota changbaishanensis and Cystolepiota hetieri are sister clades; Cystolepiota hongshiensis belongs to Cystolepiota seminuda complex; Cystolepiota luteosquamulosa formed a clade not closely related with any other; Cystolepiota nivalis and Cystolepiota sp. (HMJAU68235) formed a sister clade. All new species are provided with descriptions, photos of the basidiomata, and colored illustrations of the microstructures. A key for the identification of Cystolepiota species from China is also presented.
1 Introduction
The humus layer of the forest harbors a myriad of tiny mushrooms that often go unnoticed, including Cystolepiota Singer. The genus Cystolepiota was erected by Singer in Singer and Digilio (1952) to accommodate small lepiotaceous fungi species with epithelioid squamules and inamyloid, non-dextrinoid basidiospores. Then Singer and Clémençon (1972) divided this genus into two sections: C. sect. Pseudoamyloideae Singer and Clémençon for species showing basidiospores with dextrinoid reactions in Melzer’s reagent (e.g., Cystolepiota icterina F. H. Møller ex Knudsen), and C. sect. Cystolepiota Singer with non-reactive basidiospores in Melzer’s reagent [e.g., Cystolepiota fumosifolia (Murrill) Vellinga]. In addition, Bon (1993) established a new genus, Pulverolepiota Bon, which includes species with the pileus covered by squamules formed by elongated and inflated cells, lacking clamp connections, and basidiospores slowly turning red brown in Melzer’s reagent [e.g., C. petasiformis (Murrill) Vellinga = Pulverolepiota petasiformis (Murrill) H. Qu, Damm and Z. W. Ge]. However, Vellinga treated this genus as a section of Cystolepiota (Vellinga and Huijser, 1998). Recently, Qu et al. (2023) found that Pulverolepiota formed a unique branch independent of the core members of Cystolepiota, and revived Pulverolepiota as a genus. Nevertheless, much controversy remains in the academic community regarding the boundaries of Cystolepiota.
Like Cystolepiota, the Melanophyllum Velen. (Velenovský, 1921) basidiomata pileus is also composed of loosely arranged spherical cells and hyphae. However, Melanophyllum has basidiomata with lamellae of a distinctive color, reddish or greenish, and it has ornamented basidiospores. Vellinga (2003) observed that Melanophyllum, although it has colored spores, belonged to the same evolutionary branch as Cystolepiota, instead of being related to Agaricus L., as proposed by Singer (1986). Qu et al. (2023) confirmed that Melanophyllum and Cystolepiota form a monophyletic group and that some species in the C. seminuda complex also have basidiospore ornamentation. Therefore, the relationship between these two genera is difficult to define.
In addition to the well-recognized Cystolepiota species, several species assumed to be Lepiota (Pers.) Gray have pileus surface squamules composed of chains of sphaerocyst cells. Knudsen (1978) transferred sect. Echinatae from Lepiota to Cystolepiota because of the presence of sphaerocysts on their pileus. However, he revised this view and later treated it as Lepiota sect. Echinatae (Knudsen, 1980). Bon (1991) included these species in Echinoderma (Locq. ex Bon) Bon. Then, phylogenetic studies (Vellinga, 2003; Hou and Ge, 2020) have shown that Echinoderma is polyphyletic, species with globose to ellipsoid basidiospores are members of Lepiota (e.g., Lepiota omninoflava Y. J. Hou and Z. W. Ge), whereas those with subcylindrical spores should be placed under Echinoderma [e.g., Echinoderma asperum (Pers.) Bon].
According to the Index Fungorum (http://www.indexfungorum.org/, accessed on December 19, 2023), more than 40 Cystolepiota species have been described. However, several species have rarely been found since publication (e.g., C. constricta Singer, the type species of Cystolepiota). Nine Cystolepiota species have been recorded in China: C. adulterina F. H. Møller ex Knudsen, C. fumosifolia, C. hetieri (Boud.) Singer, C. pseudofumosifolia M. L. Xu and R. L. Zhao, C. pseudogranulosa (Berk. and Broome) Pegler, C. pseudoseminuda Y. J. Hou, H. Qu and Z. W. Ge, C. pyramidosquamulosa H. Qu and Z. W. Ge, C. seminuda (Lasch) Bon, and C. squamulosa (T. Bau and Yu Li) Zhu L. Yang (Mao et al., 1997; Bau and Li, 2004; Chou, 2010; Xu et al., 2016; Yang and Ge, 2017; Yang et al., 2019; Qu et al., 2023). Of these, C. squamulosa was a species previously discovered by our research team during a survey of species in northeastern China (Bau and Li, 2004). Before this survey, only three Cystolepiota species (C. pseudoseminuda, C. seminuda, and C. squamulosa) had been reported in northeastern China.
Through morphological and phylogenetic analyses, we identified four additional Cystolepiota species from northeastern China. Since the type species of Cystolepiota, C. constricta, has no available sequence in GenBank. The concept of Cystolepiota s.l. was used in this study to include all the species of Cystolepiota and Melanophyllum aforementioned.
2 Materials and methods
2.1 Morphological studies
Specimens were collected from northeastern China between June and September of 2021–2023. Photos of the basidiomata were taken during field collection and the macroscopic characteristics of the basidiomata were recorded, with color descriptions based on Kornerup and Wanscher (1963). Then specimens were dried using silica gel, and the specimens are currently stored in the Herbarium of Jilin Agricultural University (HMJAU). The colored illustrations are based on photos of the basidiomata in the field collection. Light microscopy (LM: Olympus CX33) was used to observe the microstructure, the samples were rehydrated in 5% KOH, and OPLENIC Pro v1.92 was utilized to measure the microstructure. Among them, in basidiospores, in the notation [n, m, p], n represents the number of basidiospores measured, of m basidiomata of p specimens, and a − b × c − d represents the minimum − maximum value of the length × width of the basidiospores, and Q = a − b represents the minimum − maximum value of the length/width of the basidiospores, Qv = represents the average of the length/width of the basidiospores. Descriptive terminology follows terms proposed by Vellinga (1988) and Clémençon (2012).
To know accurately whether the basidiospore’s surface is ornamented or not, we treated the lamellae with gold spray after placing them on a carrier stage and observed the basidiospore surface under a scanning electron microscope (SEM: Zeiss MERLIN, EHT1-5Kv).
In addition, Congo red was used to stain the structures for better observation. To determine whether the basidiospores wall was amyloid or not, Melzer’s reagent was employed. Cresyl blue was used to detect the metachromatic reaction, while cotton blue revealed whether the basidiospores were cyanophilous.
2.2 Phylogenetic studies
DNA was extracted from dried specimens using the NuClean PlantGen DNA kit (CWBIO, Beijing, China). In PCR amplification, the primer pairs ITS1F/ITS (White et al., 1990; Gardes and Bruns, 1993), LR0R/LR5 (Vilgalys and Hester, 1990; Rehner and Samuels, 1994), 6F/RPB2-7.1R (Matheny, 2005), and EF1-983F/EF1-1567R (Rehner and Buckley, 2005) were used to amplify the sequences of four DNA regions, ITS, nrLSU, rpb2, and tef1-α, respectively. The PCR procedures followed Hou and Ge (2020): pre-denaturation at 94°C for 5 min, followed by 94°C for 50 s, annealing for 50 s, LSU and tef1-α at 50°C, ITS at 52°C, rpb2 at 55°C, extension at 72°C for 1 min, and 35 cycles. The PCR products were purified and sequenced by Sangon Biotech Co., Ltd. (Shanghai, China). The newly generated sequences were deposited in GenBank.1
The phylogenetic analysis included the available sequences of Cystolepiota and its closely related genera Melanophyllum, Pulverolepiota, Echinoderma, and Lepiota, according to Sánchez-García et al. (2020) study, Coprinus comatus (O. F. Müll.) Pers. and Cop. sterquilinus (Fr.) Fr. were selected as the outgroups. Finally, the analyzed matrix contains 179 ITS sequences, 54 nrLSU sequences, 30 rpb2 sequences, and 26 tef1-α sequences, which are listed in Table 1. Multiple sequences were compared using MAFFT v7.110 (Katoh et al., 2019), and the resulting alignments were manually checked and optimized in MEGA v7.0.26 (Kumar et al., 2016). Gap sites were removed with trimAl (Capella-Gutiérrez et al., 2009) using “-automated1” command. ModelFinder (Kalyaanamoorthy et al., 2017) was used to select the best-fit model using AIC criterion. A maximum-likelihood (ML) analysis was performed using raxmlGUI v2.0 with GTRGAMMAI as the model of evolution, and branch support was estimated over 1,000 bootstrap partitions (BP) with the rapid bootstrap option (Edler et al., 2021). Bayesian Inference phylogenies were inferred using MrBayes v3.2.6 (Ronquist et al., 2012) under partition model (2 parallel runs, 21,772,200 generations), in which the initial 25% of sampled data were discarded as burn-in. All phylogenetic graph results are exported for viewing in Figtree v1.4.3 (Rambaut, 2016).
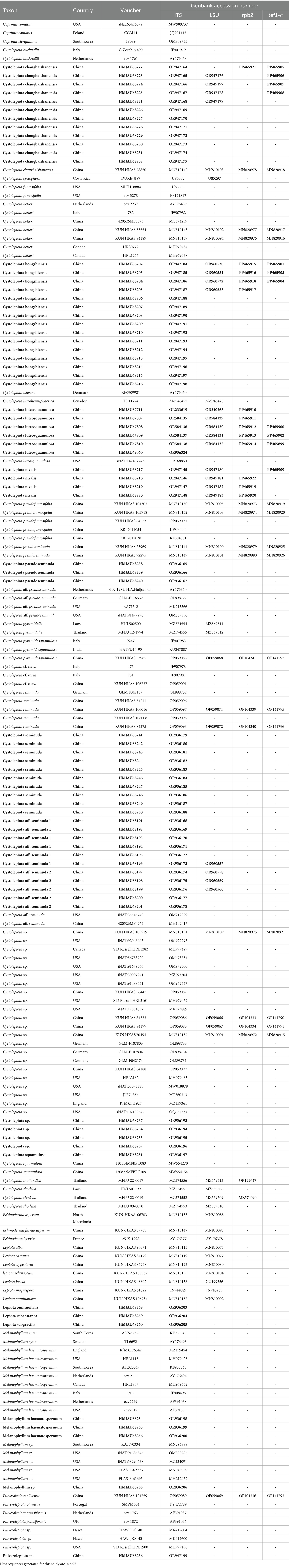
Table 1. GenBank accession numbers, geographical origins, and voucher numbers of taxa used for the phylogenetic analyses.
3 Results
3.1 Phylogenetic analyses
The ITS phylogenetic tree (Figure 1) included 154 sequences with 693 characters, and the multi-DNA regions phylogenetic tree (Figure 2) 133 sequences with 2,765 characters, including 133 ITS sequences, 54 nrLSU sequences, 30 rpb2 sequences, and 26 tef1-α sequences. BI and ML analysis resulted in a very similar topology, so the ML tree is provided in this study (Figures 1, 2). Bootstrap support (BS) values ≥70%, and Bayesian posterior probability (PP) values ≥0.95 are indicated on branches (BS/PP).
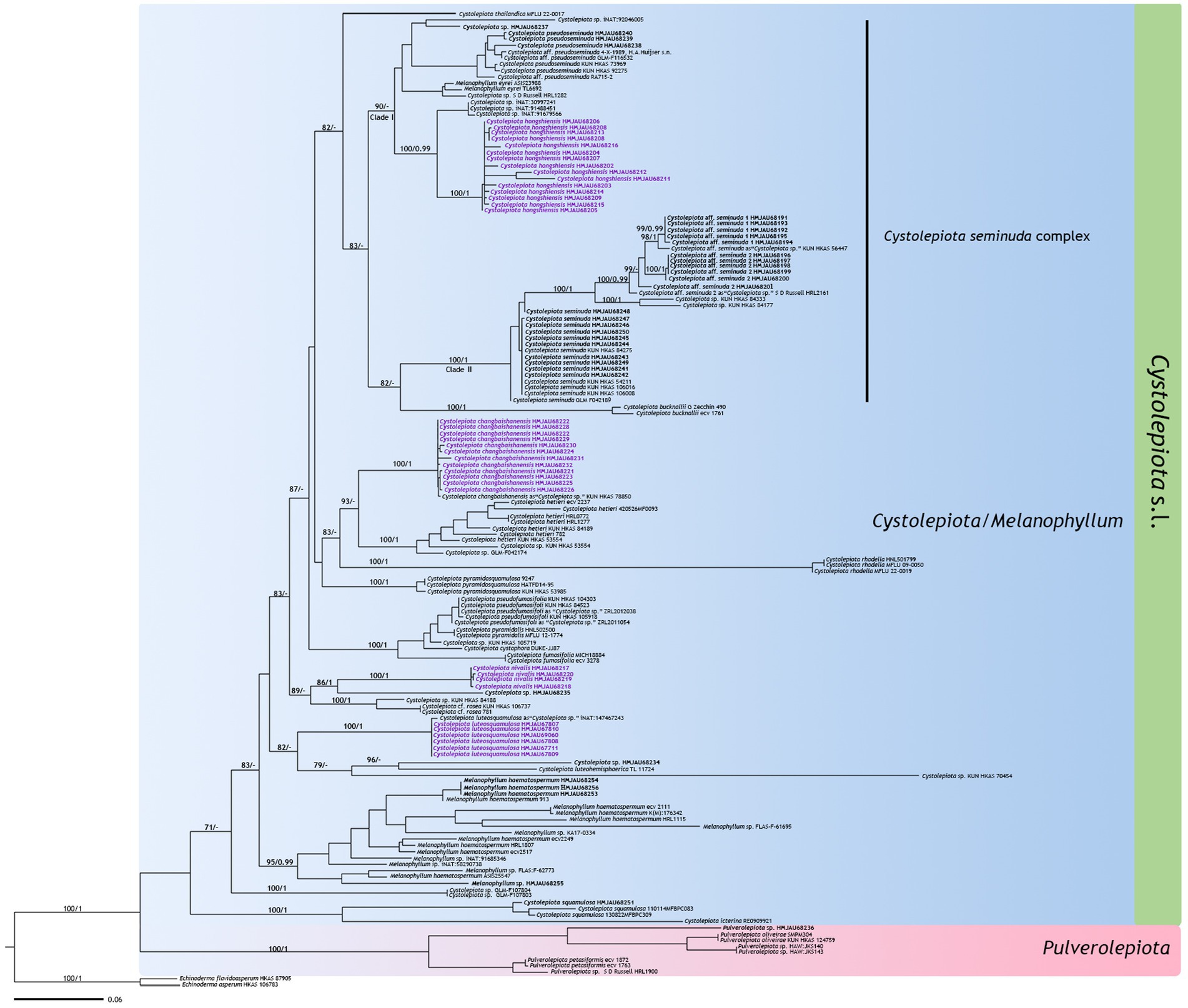
Figure 1. Maximum likelihood tree based on ITS sequences. New sequences generated for this study are in bold, new species sequences generated for this study are in purple bold. Bootstrap support (BS) values ≥70%, and Bayesian posterior probability (PP) values ≥0.95 are indicated on branches (BS/PP). Echinoderma asperum and E. flavidoasperum are used as outgroup.
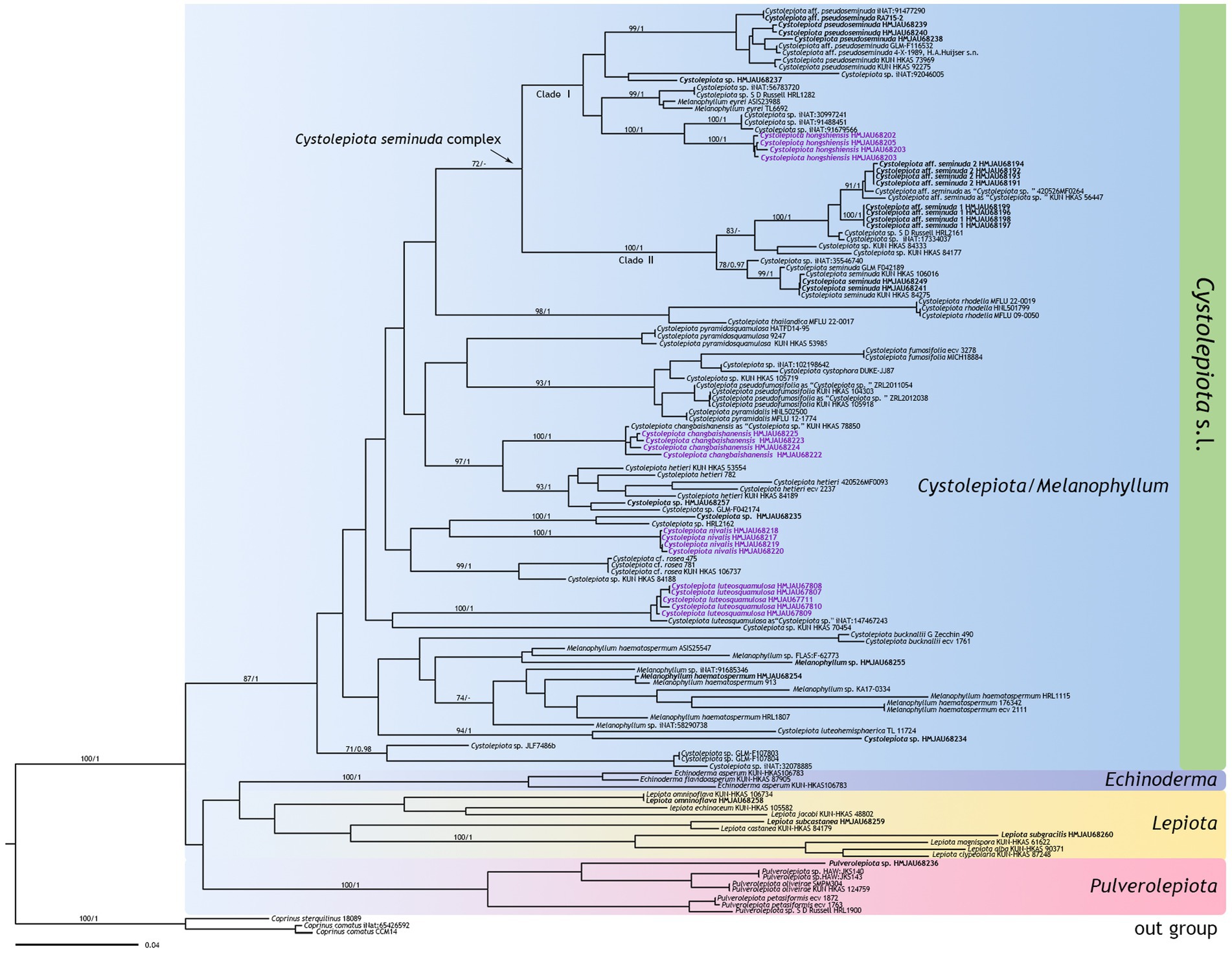
Figure 2. Phylogenetic tree of Cystolepiota obtained from the maximum likelihood analysis (ML) based on ITS, nrLSU, rpb2, and tefl-a sequence data. New sequences generated for this study are in bold, new species sequences generated for this study are in purple bold. Bootstrap support (BS) values ≥70%, and Bayesian posterior probability (PP) values ≥0.95 are indicated on branches (BS/PP). Coprinus comatus and Cop. sterquilinus are used as outgroup.
The four new species are distributed in different clades as follows: Cystolepiota changbaishanensis and C. hetieri are sister clades (Figure 1: BS/PP = 93/-; Figure 2: BS/PP = 97/1). Cystolepiota hongshiensis belongs to C. seminuda complex clade I, with a highly supported sister relationship with the clade formed by three specimens of Cystolepiota sp. (iNAT:30997241, iNAT:91488451, iNAT:91679566) (Figure 1: BS/PP = 100/0.99; Figure 2: BS/PP = 100/1). Cystolepiota nivalis and Cystolepiota sp. (HMJAU68235) also formed a sister clade (Figure 1: BS/PP = 86/1); and Cystolepiota luteosquamulosa formed a clade not closely related with any other (Figure 1: BS/PP = 82/-). In addition, Cystolepiota sp. (HMJAU68234, HMJAU68235, HMJAU68257), Melanophyllum sp. (HMJAU68255), and Pulverolepiota sp. (HMJAU68236) each form an independent clade on the phylogenetic trees (Figures 1, 2), which is not described here for the moment because only one specimen is available for observation.
3.2 Taxonomy
3.2.1 Cystolepiota changbaishanensis T. Bau and X. Y. Zhou, sp. nov.
MycoBank number: MB 851389 (Figures 3, 4).
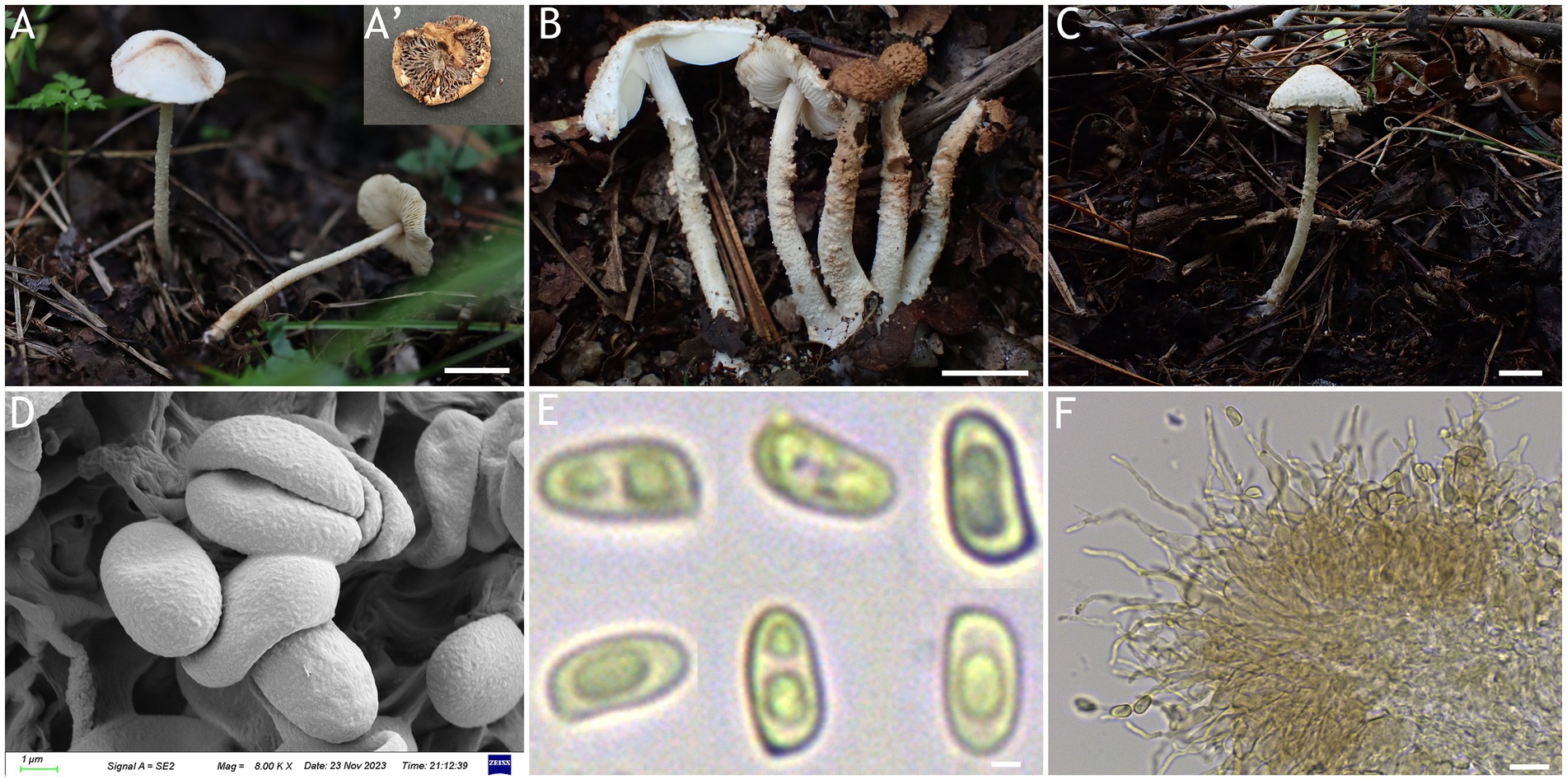
Figure 3. Cystolepiota changbaishanensis. (A–C) Basidiomata, (A′) dried specimen, (D) basidiospores under SEM, (E) basidiospores under LM, (F) cheilocystidia; (A,D–F) HMJAU68224 (holotype), (B) HMJAU68223, (C) HMJAU68222; bars: A–C =1 cm, E = 1 μm, F = 10 μm.
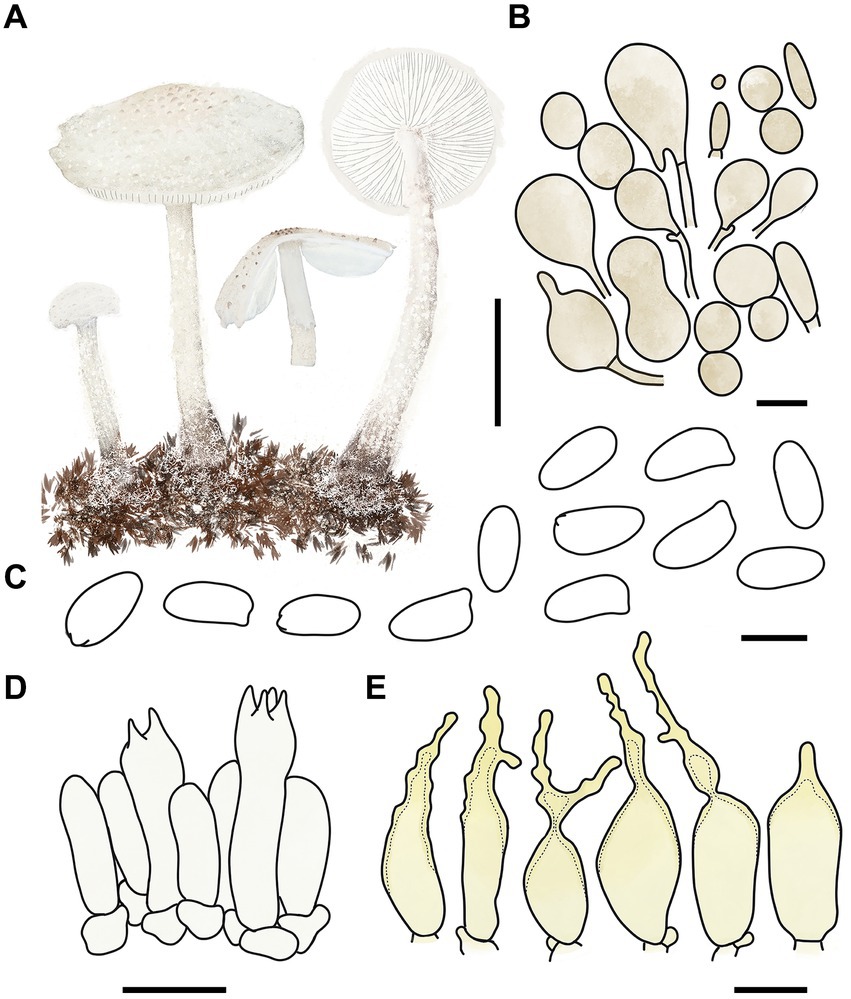
Figure 4. Cystolepiota changbaishanensis. (A) Basidiomata, (B) cells of the squamules, (C) basidiospores under LM, (D) basidia, (E) cheilocystidia; bars: A = 2 cm, B = 30 μm, C = 5 μm, D,E = 10 μm.
Diagnosis: The identifying features of C. changbaishanensis are that the pileus is dirty white to cream, with pulverulent, granulose or subpyramidal squamules, cream, greyish orange, light brown, brown; pileus and pileus context becoming greyish orange to brown after drying; lamellae white to cream, turn grayish orange to light brown when drying; basidiospores obscure small warts visible under SEM; and cheilocystidia lageniform to broadly lageniform.
Etymology: The species epithet “changbaishanensis” is derived from the name of the mountain where the material was collected.
Type: China, Jilin Province, Jiaohe City, Qianjin forest farm, July 23, 2022, coll. T. Bau and H. B. Song (HMJAU68224), Holotype!
Description: Basidiomata small. Pileus 0.8–2.2 cm, hemispherical when young, expanding to plano-convex or applanate, slightly subumbonate with age, dirty white to cream; with pulverulent, granulose or subpyramidal squamules, dirty white to cream, greyish orange (6B2–B8), light brown (7D5–D8), brown (7E5–E8); pileus context whitish, pileus and pileus context becoming greyish orange (6B5–B7) to brown (7E6–E8) after drying. Lamellae free, white to cream, crowded, up to 0.4 cm broad, with 1–3 tiers of lamellulae, turning grayish orange (6B2–B8) to light brown (6D2–D8) when drying. Stipe 3.2–6.2 × 0.1–0.5 cm, subcylindrical, occasionally downward thickened; white to cream on the upper portion, subsmooth, with granulose squamules from the annular area downwards, concolorous with pileus, fragile and fugacious. Annulus white, fugacious. Odor and taste not recorded (Figures 3A–C, 4A).
Basidiospores [150,5,5] 4.6–6.0 (−6.4) × 2.1–3.0 (−3.4) μm, Q = 1.63–2.54, Qv = 2.08, long ellipsoid to cylindrical, hyaline, slightly thick-walled, smooth-walled under the LM, small warts visible under SEM, inamyloid, non-dextrinoid, metachromatic in cresyl blue, cyanophilous. Basidia13–20 × 4–7 μm, clavate, 4-spored, sometimes 2-spored, greyish yellow (4C4–C6). Lamellar trama regular, greyish yellow (4C3–C7). Cheilocystidia 32–56 × 6–12 μm, lageniform to broadly lageniform, greyish yellow (4C4–C7) to golden yellow (1B4–B8), with a long cylindrical-tortuous apex, slightly thick-walled. Pleurocystidia absent. Pileus and stipe covering an irregular epithelium composed of globose, subglobose, spheropedunculate, 10–21 μm in diam., usually 2–5 cells in a string, brownish orange (5C2–C5). Clamp connections present in all structures (Figures 3D–F, 4B–E).
Habitat: Solitary, scattered or clustered on dead leaves and soil of mixed coniferous forests.
Distribution: Found only in Jilin Province, northwestern China.
Additional specimens examined: China, Jilin Province, Helong City, Xianfeng National Forest Park, August 22, 2021, coll. T. Bau and X. Wang (HMJAU68221); Jiaohe City, Qianjin forest farm, July 23, 2022, coll. T. Bau, L. Y. Zhu, W. N. Hou and H. B. Song, (HMJAU68228, HMJAU68229, HMJAU68230); Dunhua City, State forest farm, July 27, 2022, coll. T. Bau and W. N. Hou (HMJAU68225); Baishan City, Jingyu National White Bear Reserve, July 29, 2022, coll. T. Bau and L. Y. Zhu (HMJAU682310); Tonghua City, Baijifeng National Forest Park, July 8, 2023, coll. T. Bau, Q. R. Liu, Z. Q. Cheng, M. Liu and J. L. Wei (HMJAU68226, HMJAU68227, HMJAU68232, HMJAU68222, HMJAU68223).
Notes: Macromorphologically, this species is similar to C. fumosifolia, C. pyramidalis and C. pyramidosquamulosa, because all of them present subpyramidal squamules on the pileus and stipe surface. But the lamellae of Cystolepiota fumosifolia usually have brown spots, and it has pleurocystidia (Vellinga, 2006). Cystolepiota pyramidalis has orange white to pale orange pileus, pale yellow lamellae, which turn brownish orange when touched or mature, and ellipsoid-ovoid basidiospores (Sysouphanthong et al., 2022). The lamellae of Cystolepiota pyramidosquamulosa are yellowish white, do not change color after drying, and do not have cystidia (Qu et al., 2023).
In the phylogenetic trees (Figures 1, 2), Cystolepiota changbaishanensis and C. hetieri are sister clades, but the lamellae of the latter’s basidiomata did not change color after drying and exhibited pleurocystidia.
3.2.2 Cystolepiota hongshiensis T. Bau and X. Y. Zhou, sp. nov.
MycoBank number: MB 851390 (Figures 5, 6).
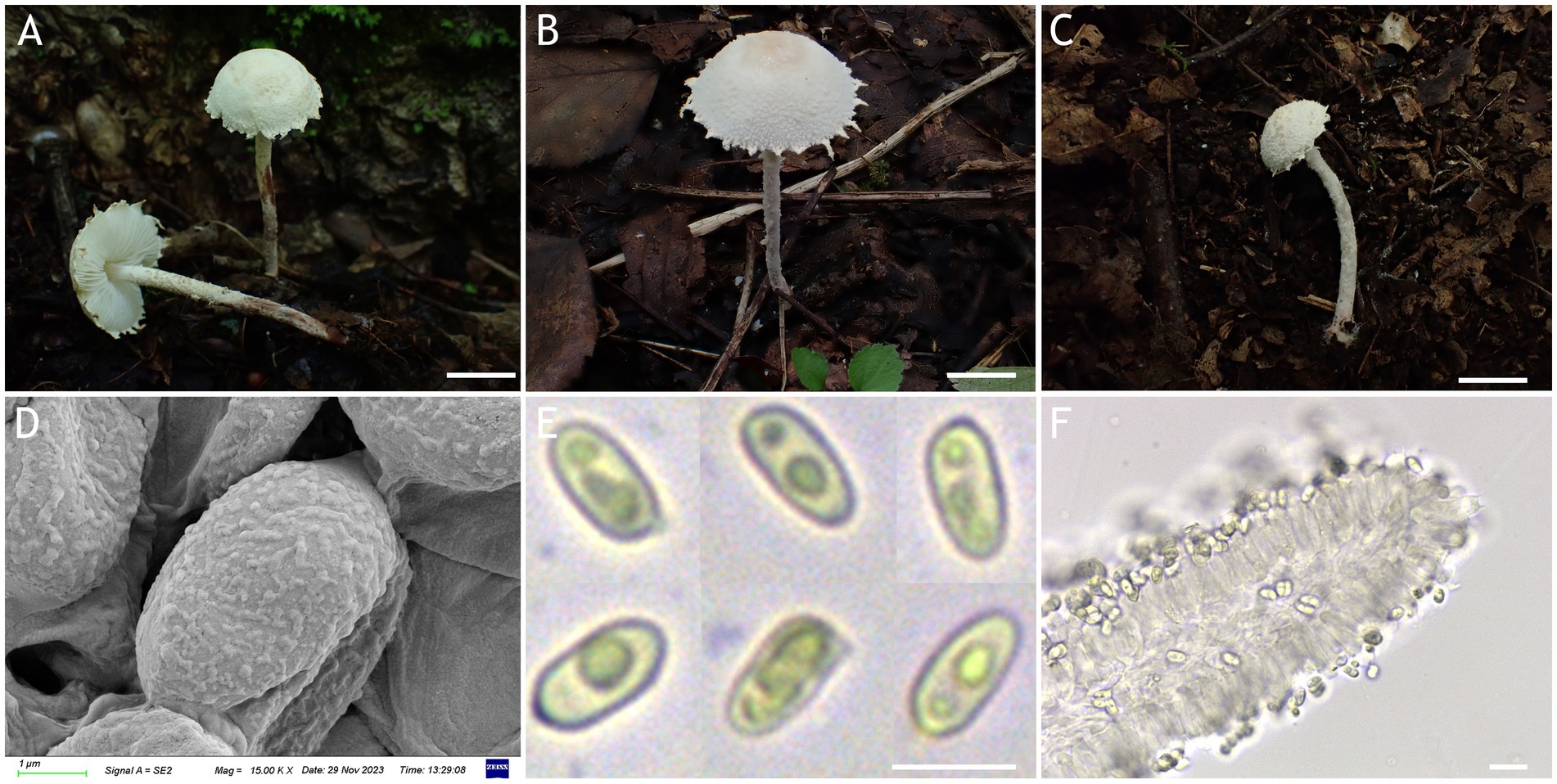
Figure 5. Cystolepiota hongshiensis. (A–C) Basidiomata, (D) basidiospores under SEM, (E) basidiospores under LM, (F) hyphae ends on the hymenium; (A) HMJAU68204 (holytype), (B) HMJAU68202, (C) HMJAU68207; bars: A–C = 1 cm, E = 5 μm, F = 10 μm.
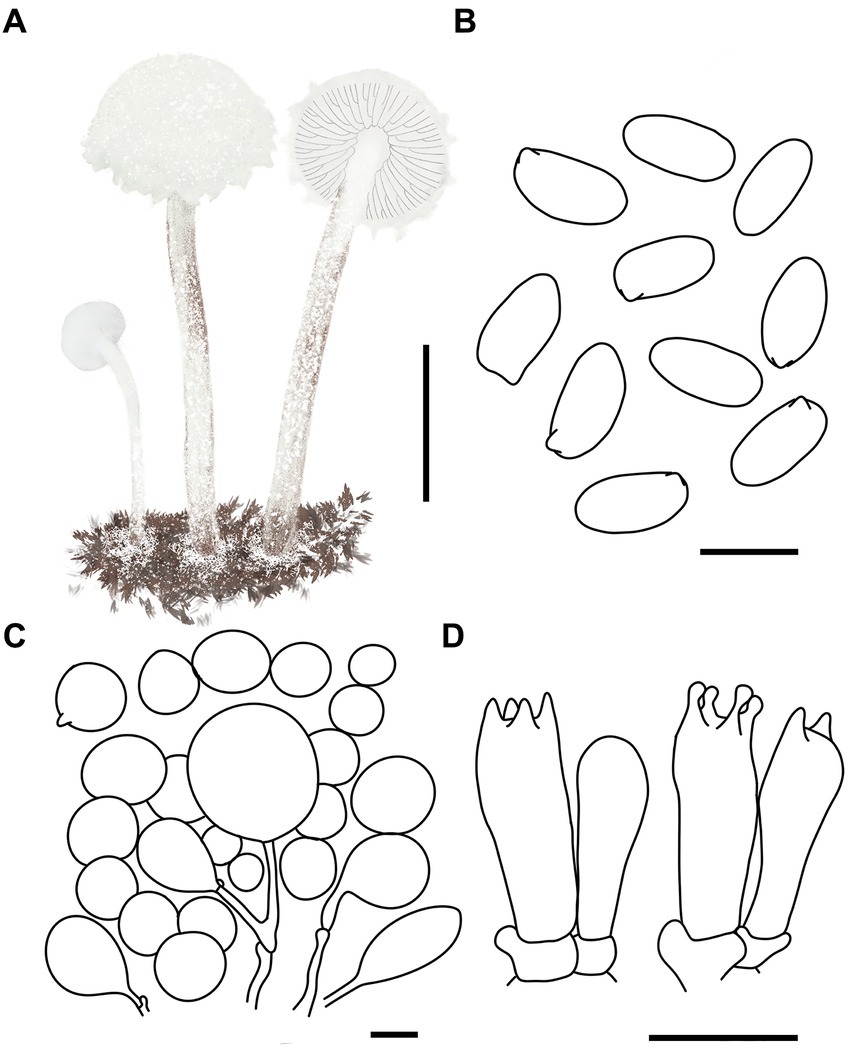
Figure 6. Cystolepiota hongshiensis. (A) Basidiomata, (B) basidiospores under LM, (C) cells of the squamules, (D) basidia; bars: A = 2 cm; B = 5 μm; C = 30 μm; D = 10 μm.
Diagnosis: C. hongshiensis is distinguished from other Cystolepiota species by its hemispherical to convex pileus, with granulose to warty squamules, white to cream, and rough basidiospores under SEM. Its ITS, LSU, rpb2, and tef1-α sequences are different from those of other species.
Etymology: The species epithet “hongshiensis” is derived from the name of the park where the material was collected.
Type: China, Jilin Province, Huadian City, Hongshi Township, Red Rock National Forest Park, August 27, 2023, coll. T. Bau and X. Wang (HMJAU68204), Holotype!
Description: Basidiomata small. Pileus 0.3–2.2 cm, hemispherical when young, hemispherical to convex with age, white to cream; with granulose to warty squamules, white to cream, yellowish white (4A2–A3), orange white (6A2–A3); occasionally pinkish orange (6A2–A3) on center, margin appendiculate with veil remnants when young, concolorous with pileus; pileus context white to cream. Lamellae free, white to cream, crowded, up to 0.3 cm broad, with 1–3 tiers of lamellulae. Stipe 2.1–5.3 × 0.1–0.2 cm, subcylindrical, slightly enlarged at base, surface white to cream on the upper portion, greyish orange (5B2–B3) to reddish brown (8E4–E8) at base, with age gradually turning to reddish brown (8E4–E8) towards the middle and lower portion, with pulverulent to granulose squamules, concolorous with pileus, fugacious; context reddish brown (8E4–E8) at stipe base. Annulus white, fugacious. Odor and taste not recorded (Figures 5A–C, 6A).
Basidiospores [120,4,4] (−3.7) 4.4–5.9 (−6.1) × 2.0–3.5 μm, Q = 1.53–2.30, Qv = 1.89, long ellipsoid, hyaline, thin-walled, smooth-walled under the LM, distinct warts visible under SEM, inamyloid, non-dextrinoid, metachromatic in cresyl blue, cyanophilous. Basidia 15–22 × 5–7 μm, clavate, 4(2)-spored. Lamellar trama regular. Pleurocystidia and cheilocystidia absent. Pileus and stipe covering an irregular epithelium composed of globose to subglobose elements, 10–43 μm in diam., usually 2–5 cells forming loosely arranged chains, hyphae 1–4 μm in diam., slightly thick-walled, Clamp connections present in all structures (Figures 5D–F, 6B–D).
Habitat: Solitary to scattered on dead branches and rotten leaves of mixed forest.
Distribution: Found only in Jilin Province, northwestern China.
Additional specimens examined: China, Jilin Province, Jiaohe City, Qianjin forest farm, August 25, 2022, coll. T. Bau and H. Cheng (HMJAU68205); Jiaohe City, Hongyegu, July 31, 2023, coll. T. Bau and S. Y. Li (HMJAU68216); Huadian City, Zhaodaji Mountain National Forest Park, August 21, 2023, coll. T. Bau and X. Wang (HMJAU68207); Huadian City, Red Rock National Forest Park, August 27, 2023, coll. T. Bau, M. L. and X. Y. Zhou (HMJAU68203, HMJAU68206); August 28, 2023, coll. T. Bau, H. Cheng and X. Y. Zhou (HMJAU68202, HMJAU68212, HMJAU68215).
Notes: Macromorphologically, Cystolepiota hongshiensis and C. pseudoseminuda, with similar pileus surface squamules. But the latter pileus is plano-convex or applanate slightly umbonate, basidiospores (−3) 3.5–4.5 (−5) × 2–3 (−3.5) μm, Q = (−1.21) 1.24–1.85 (−2.20), Qm = 1.55 ± 0.19, ovoid to ellipsoid, the basidiospores of C. hongshiensis are more elongated than those of C. pseudoseminuda (Qu et al., 2023). In addition, there are 65 (out of 706) nucleotide differences between the ITS sequences of the holotype of C. hongshiensis and that of the holotype of C. pseudoseminuda.
3.2.3 Cystolepiota luteosquamulosa T. Bau and X. Y. Zhou, sp. nov.
MycoBank number: MB 849380 (Figures 7, 8).
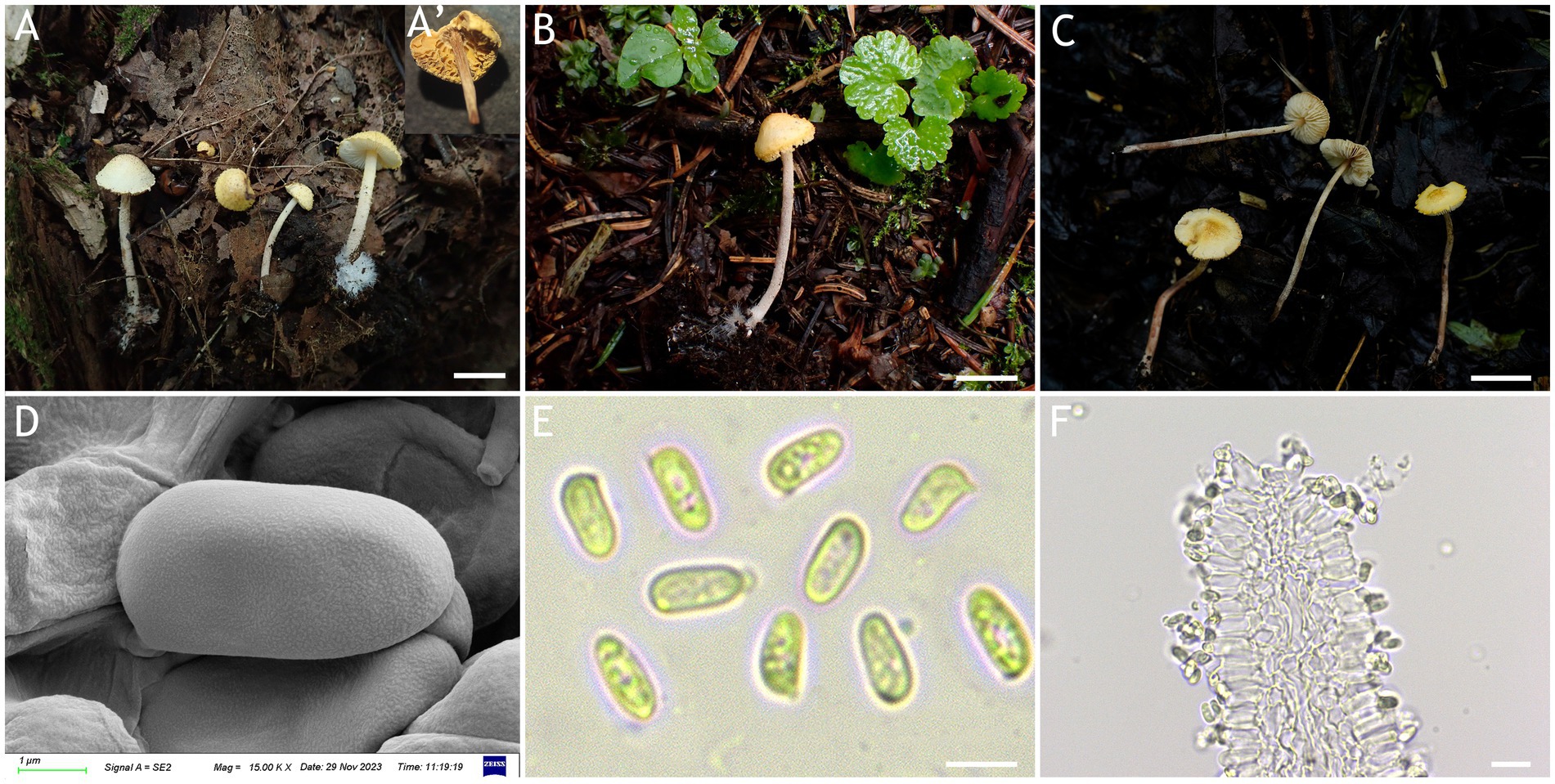
Figure 7. Cystolepiota luteosquamulosa. (A–C) Basidiomata, (A′) dried specimen, (D) basidiospores under SEM, (E) basidiospores under LM, (F) hyphae ends on the hymenium; (A,D–F) HMJAU67711 (holotype), (B) HMJAU67809, (C) HMJAU67810; bars: A–C = 1 cm.
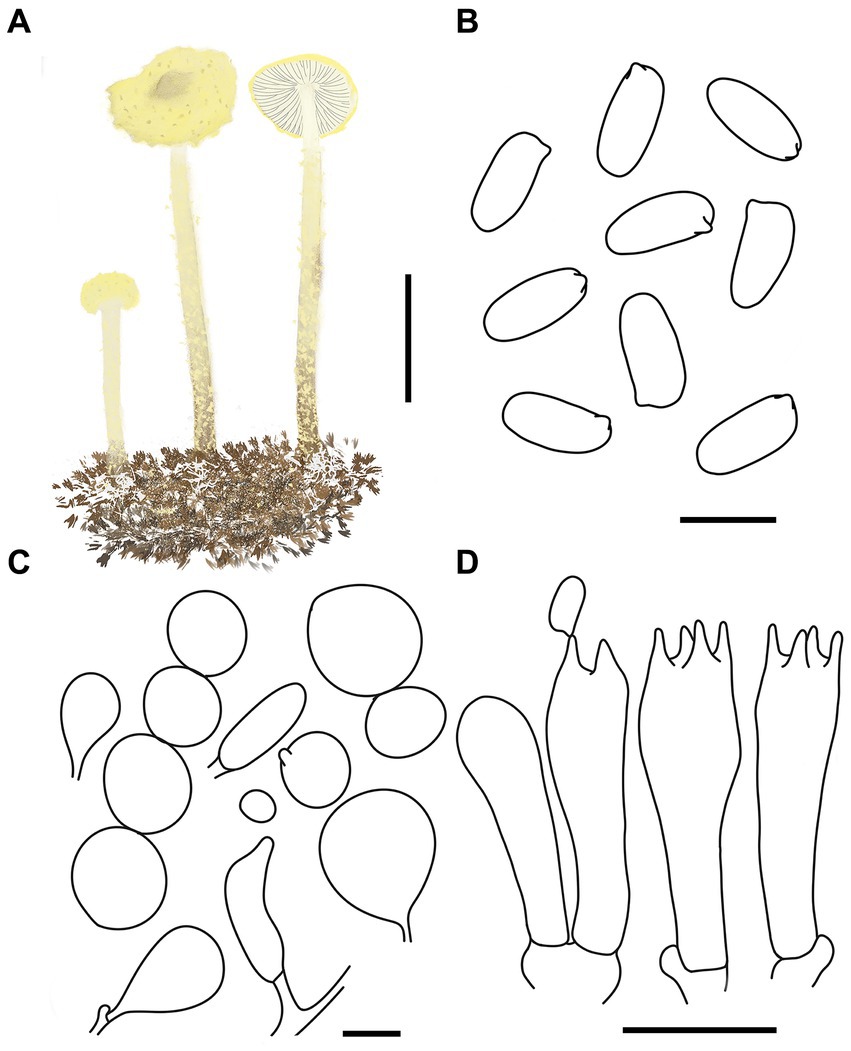
Figure 8. Cystolepiota luteosquamulosa. (A) Basidiomata, (B) basidiospores under LM, (C) cells of the squamules, (D) basidia; bars: A = 2 cm; B = 5 μm; C = 30 μm; D = 10 μm.
Diagnosis: C. luteosquamulosa is distinguished from other Cystolepiota species by its light yellow to greyish yellow pileus, with greyish yellow to dark yellow warty to subpyramidal squamules, light reddish brown stipe with white to light yellow floccose squamules, and pleurocystidia and cheilocystidia absent.
Etymology: “luteo-” means yellow, and “luteosquamulosa” refers to the yellow squamules on the pileus.
Type: China, Jilin province, Jiaohe City, Hongyegu, September 6, 2021, coll. T. Bau and X. Wang (HMJAU67711), Holotype!
Description: Basidiomata small. Pileus 0.8–1.4 cm, hemispherical to obtusely conical when young, expanding to plano-convex or applanate with a slightly umbonate center with age, light yellow (1A4–A8) to greyish yellow (2B5–B8), with greyish yellow (2C7–C8) to dark yellow (3C5–C8) warty to subpyramidal squamules; margin appendiculate with veil remnants when young, and then finely appendiculate, concolorous with pileus; context white, thin. Lamellae free, white to cream, crowded, 0.1–0.3 cm wide, with 1–3 tiers of lamellulae, drying brownish orange (5C2–C6). Stipe 2.5–5.6 × 0.1–0.2 cm, subcylindrical, light reddish brown (7E5–E8), with some floccose squamules, white to light yellow (1A2–A8), with conspicuous white mycelia on the base. Annulus not visible. Odor and taste not recorded (Figures 7A–C, 8A).
Basidiospores [120,4,4] 4.9–6.3 (−6.6) × (−2.0) 2.4–3.0 (−3.3) μm, Q = 1.70–2.63, Qv = 2.10, long ellipsoid to cylindrical, hyaline, smooth-walled under the LM, finely punctate under SEM, inamyloid, non-dextrinoid, metachromatic in cresyl blue, cyanophilous. Basidia 15–23 × 4–7 μm, clavate, 4-spored, sometimes 2-spored. Lamellar trama regular. Pleurocystidia and cheilocystidia absent. Squamules composed of loosely-arranged globose, subglobose, ovoid, 13–62 μm in diam., rarely gourd-shaped or fusiform, 17–42 × 7–16 μm, sometimes 2–4 cells are connected in a string, smooth-walled, slightly thick-walled, hyaline, or orange white (5A2–A3). Clamp connections present in all structures (Figures 7D–F, 8B–D).
Habitat: Solitary or scattered on dead leaves or soil of mixed forest.
Distribution: Northeastern China.
Additional specimens examined: China, Jilin Province, Jiaohe City, Qianjin forest farm, July 24, 2022, coll. T. Bau and L. Y. Zhu (HMJAU67810); Dunhua City, State Forest farm, July 27, 2022, coll. T. Bau, W. N. Hou and F. Guo (HMJAU67808, HMJAU69060); Huadian City, Red Rock National Forest Park, August 28, 2023, coll. T. Bau and H. Cheng (HMJAU67807). Heilongjiang Province, Yichun City, Xing’an National Forest Park, July 25, 2023, coll. T. Bau and W. N. Hou (HMJAU67809).
Notes: Macromorphologically, both C. luteosquamulosa and C. luteohemisphaerica have yellow pileus. But in the latter, the pileus is radially veined and micaceous-mealy, with broadly elliptical to elliptical basidiospores (Saar and Laessoe, 2008). C. icterina also has a yellow pileus, but it is easy to distinguish from C. lutesquamulosa by its pileus surface with finely floccose-farinose squamules, by its smaller (3.5–4.5 × 2.5 μm) and dextrinoid basidiospores and by the presence of cheilocystidia (Knudsen, 1978).
3.2.4 Cystolepiota nivalis T. Bau and X. Y. Zhou, sp. nov.
MycoBank number: MB 851388 (Figures 9, 10).
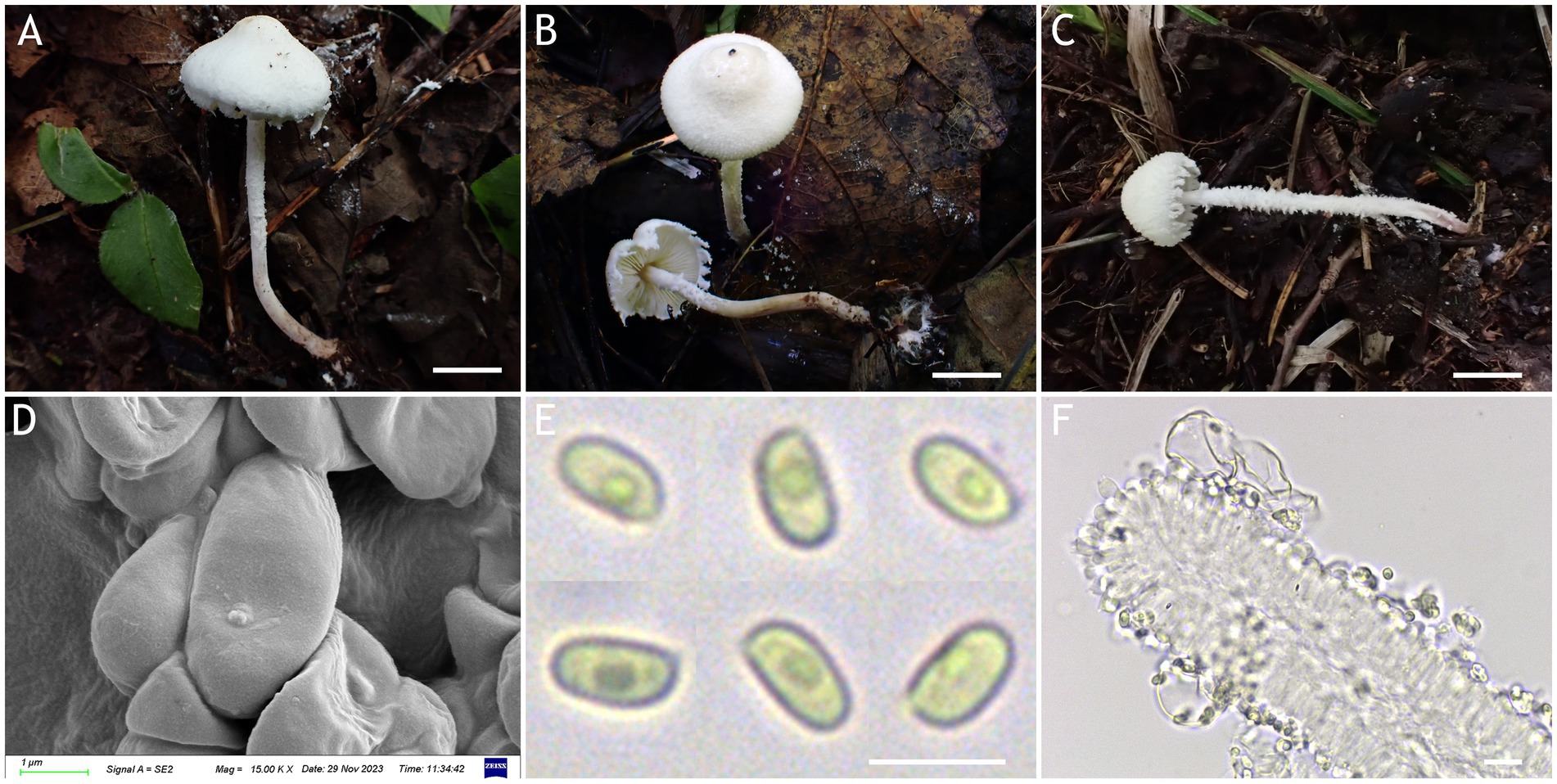
Figure 9. Cystolepiota nivalis. (A–C) Basidiomata, (D) basidiospores under SEM, (E) basidiospores under LM, (F) hyphae ends on the hymenium; (A,D–F) HMJAU68220 (holytype), (B) HMJAU68218, (C) HMJAU68219; bars: A–C = 1 cm, E = 5 μm, F = 10 μm.
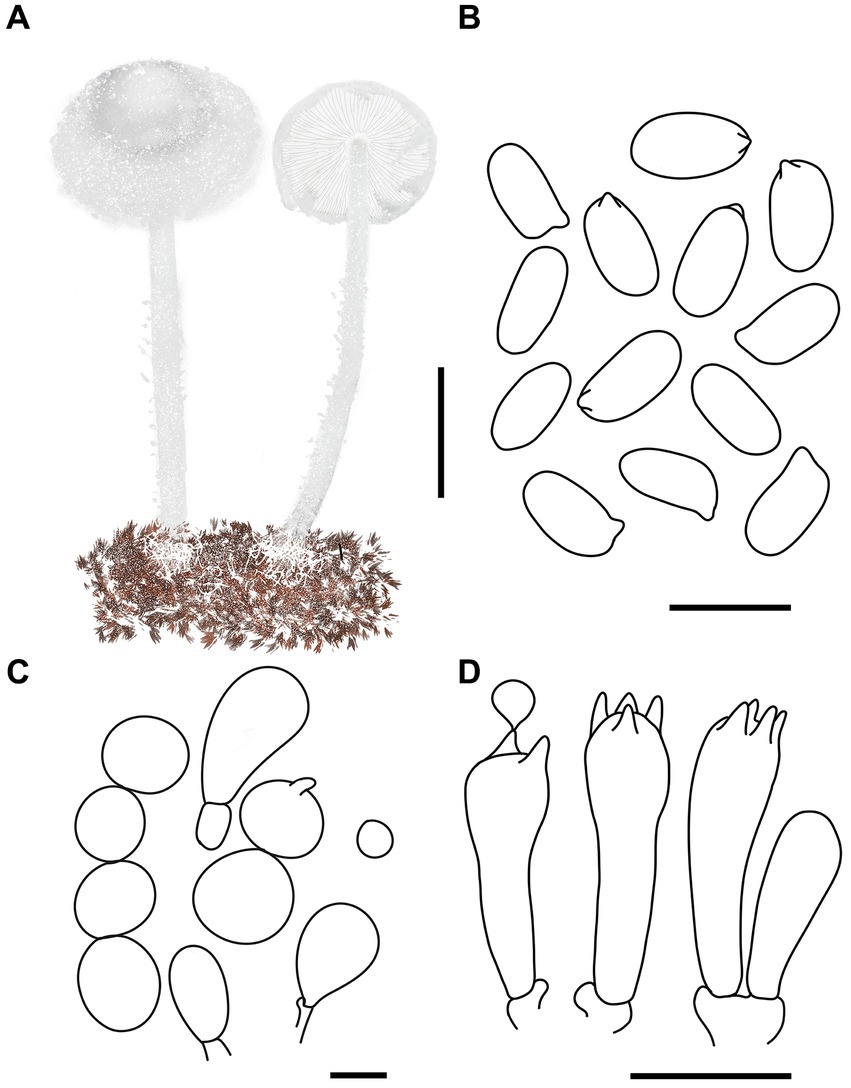
Figure 10. Cystolepiota nivalis. (A) Basidiomata, (B) basidiospores under LM, (C) cells of the squamules, (D) basidia; bars: A = 2 cm; B = 5 μm; C = 30 μm; D = 10 μm.
Diagnosis: The main distinguishing features of C. nivalis are the widely umbonate, white, farinose pileus, with a strongly appendiculate margin, with a farinose stipe and cystidia absent.
Etymology: “nivea” refers to snowy white pileus.
Type: China, Jilin province, Jiaohe City, Qianjin forest farm, August 25, 2023, coll. T. Bau and M. Liu (HMJAU68220), Holytype!
Description: Basidiomata small. Pileus 1.2–1.7 cm, hemispherical, then campanulate, with a broad umbo, with concolorous farinose squamules; margin appendiculate strongly farinose, concolorous with pileus; context thin, whitish. Lamellae free, crowded, white to light cream, unequal, with 1–3 tiers of lamellulae. Stipe 3.7–5.6 × 0.1–0.2 cm, central, subcylindrical to cylindrical, surface strongly farinose, white to cream, light brown (6D5–D8) to reddish brown (7E4–E8) towards the base. Annulus white, fugacious. Odorless, taste not recorded (Figures 9A–C, 10A).
Basidiospores [120,4,4] 3.8–4.7 (−4.9) × 2.0–3.0 μm, Q = 1.47–2.06 (−2.28), Qv = 1.85, ellipsoid to cylindrical, slightly thick-walled, smooth-walled under the LM and SEM, hyaline inamyloid, non-dextrinoid, metachromatic in cresyl blue, cyanophilous. Basidia 12–17 × 3.5–5 (−6) μm, clavate, 4-spored, sometimes 2-spored, hyaline. Lamellar trama regular. Pleurocystidia and cheilocystidia absent. Pileus and stipe covering composed of globose, subglobose, pyriform cells 8–36 μm in diam., or 9–25 × 4–12 μm, sometimes 2–5 cells connected in a string, thin-walled, hyaline. Clamp connections present in all structures (Figures 9D–F, 10B–D).
Habitat: Solitary to scattered in mixed forest.
Distribution: Found only in Jilin Province, northwestern China.
Additional specimens examined: China, Jilin province, Jiaohe City, Qianjin forest farm, August 25, 2023, coll. T. Bau and H. Cheng (HMJAU68219); Huadian City, Red Rock National Forest Park, August 27, 2023, coll. T. Bau and X. Y. Zhou (HMJAU68217, HMJAU68218).
Notes: Morphologically, P. petasiformis also has a white pileus with an obvious umbonate, but it is easy to distinguish from C. nivalis by its context turns pale orange after cut, and lacks clamp connections (Vellinga and Huijser, 1998; Yang et al., 2019).
Key to species of Cystolepiota in China.
1. Pileus surface squamules fluorescent pink or greyish yellow……………………………………………………………………………2
1′. Pileus and pileus surface squamules white, cream, pale pinkish, pale yellow, light yellow brown………………………………………………………………………………………….………………………3
2. Pileus surface squamules fluorescent pink………………………………………………………………………………C. squamulosa
2′. Pileus surface squamules greyish yellow……………………………………………………………………………C. luteosquamulosa
3. Pileus and pileus surface squamules white only……………………………………..…………………………………………C. nivalis
3′. Pileus white to cream, pileus surface squamules white, cream, pale pinkish, pale yellow, light yellow brown……………………………………………………………………………………………………4
4. Lamellulae dry to greyish orange, light brown, greyish brown…………………………………………………………………5
4′. Lamellulae dry unchanged to greyish orange, light brown, greyish brown…………………………………………………………6
5. Pleurocystidia absent…………………….……………………….………………………………………………...C. changbaishanensis
5′. Pleurocystidia present, numerous…………………….……………………………………………………………………C. fumosifolia
6. Cheilocystidia present……………………………………………………………………………………………………………………7
6′. Cheilocystidia absent……………………………………………………………………………………………………………………9
7. Pleurocystidia absent…………………………………………………...……………………………………………………C. adulterina
7′. Pleurocystidia present…………………………………………………………………………………………………………………8
8. Cheilocystidia ventricose-capitate at apex, pleurocystidia rarely, occasionally clavate to fusiform……………………………… ……………………………………………………C. pseudofumosifolia
8′. Cheilocystidia capitate and cylindrical or moniliform excrescence at apex, pleurocystidia similar to cheilocystidia………………………………………………………………………………C. hetieri
9. Basidiospores strongly dextrinoid……………………………………………………………………………………C. pseudogranulosa
9′. Basidiospore inamyloid, non-dextrinoid………………………………………………………………………………………………10
10. Basidiospores surface rough under SEM………………………………………………….………………………………………….....11
10′. Basidiospores surface smooth under SEM………………………………………….………………………………………….12
11. Pileus expanding to plano-convex or applanate slightly umbonate, basidiospores (−3)3.5–4.5(−5) × 2–3 (−3.5) μm, Qm = 1.55 ± 0.19, ovoid to ellipsoid……………………C. pseudoseminuda
11. Pileus hemispherical to convex, without umbonate, basidiospores (−3.7)4.4–5.7(−6.1) × 2–3.5 μm, Qm = 1.89 ± 0.02, long ellipsoid…………………………………….………….C. hongshiensis
12. Pileus surface squamules irregular pyramidal……………………………………………………………………C. pyramidosquamulosa
12′. Pileus surface squamules powdery to granulose…………………………………………………………………………C. seminuda
4 Discussion
In both phylogenetic trees, the two species in Melanophyllum belong to Cystolepiota. Because a Melanophyllum (Velenovský, 1921) description was published earlier than that of Cystolepiota (Singer and Digilio, 1952), Melanophyllum should be used as the legal name for these two genera (Turland et al., 2018). However, the number of species in Cystolepiota is significantly higher than that in Melanophyllum. If merged, numerous synonyms can be produced. We thus applied Cystolepiota s.l. to both genera. We also found that no molecular data are available for many of the species in Cystolepiota. In particular, no molecular data is available for the model species C. constricta. For most species, the available molecular data is limited to ITS sequences. Other DNA regions (LSU, rpb2, tef1-α) have been sequenced for very few species. More detailed and comprehensive sampling is required to facilitate further studies of Cystolepiota s.l.
The macroscopic and microscopic characteristics of many Cystolepiota species overlap. Molecular data and phylogenetic analyses are thus necessary to identify Cystolepiota species with similar morphological features. For example, the species in Cystolepiota seminuda complex are morphologically similar. Cystolepita hongshiensis is a novel species examined in this study. Morphologically, Cystolepita hongshiensis and C. pseudoseminuda are similar, and require further characterization using molecular data and phylogenetic analyses. Among the Cystolepiota seminuda complex, we also examined C. aff. seminuda 1 and C. aff. seminuda 2. We found no morphological differences between them (Table 2). In two phylogenetic trees (Figures 1, 2), C. aff. seminuda 1 and C. aff. seminuda 2 are genetically distant from C. seminuda. We are thus temporarily treating it as a cryptic species.
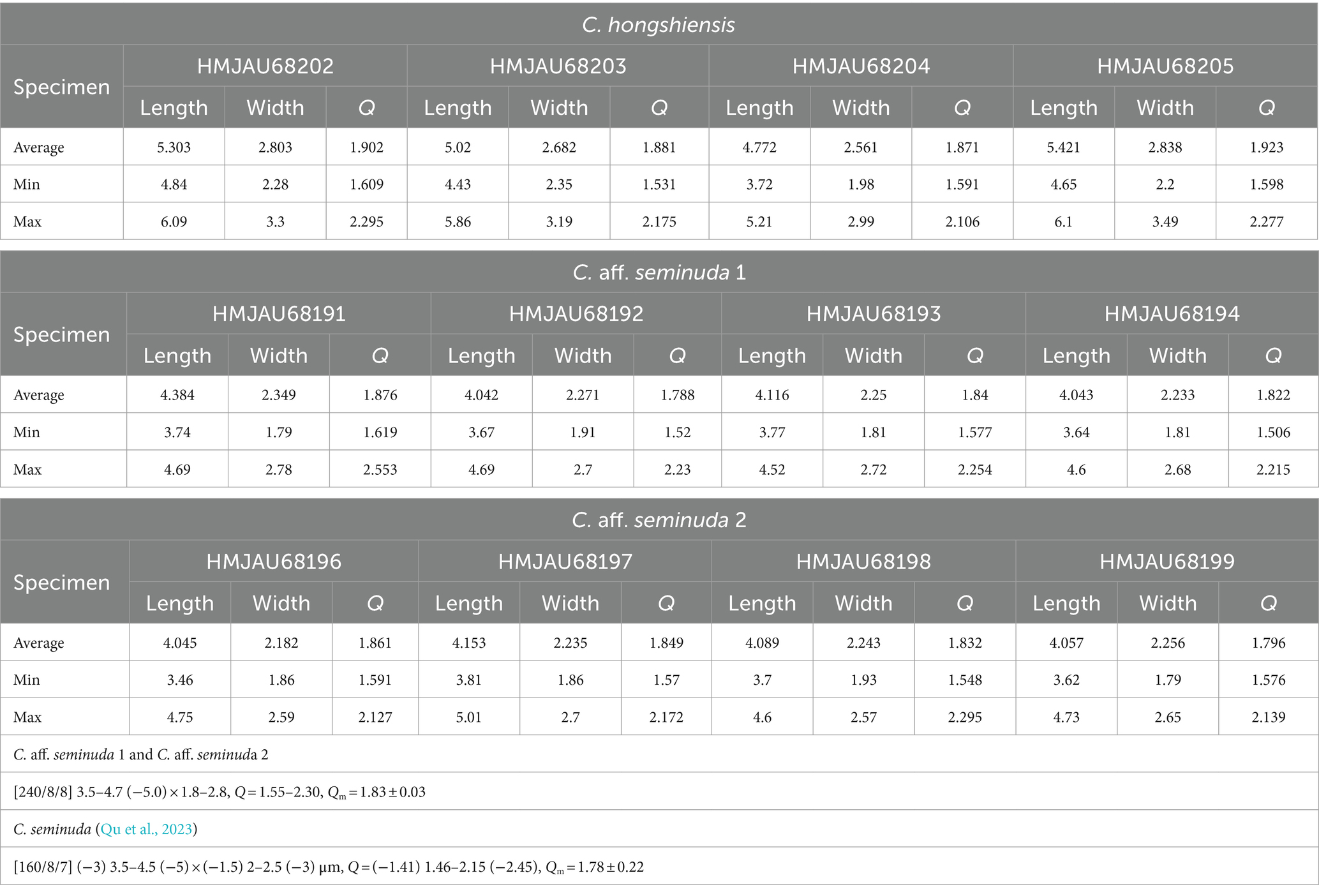
Table 2. Average, minimum, and maximum of 30 mature spore measurements for C. hongshiensis, C. seminuda, C. aff. seminuda.
We also found that Cystolepiota species morphology did not correspond to phylogeny. Cystolepiota bucknallii, C. rhodella, and C. icterina in Cystolepiota sect. Pseudoamyloideae did not form a clade in the phylogenetic tree. They each formed a distinct long clade. Cystolepiota luteosquamulosa with basidiospore ornamentation did not form a clade with other species displaying basidiospore ornamentation. These require further research.
This study describes four new species belonging to Cystolepiota from northeast China. They are well-supported by molecular phylogenetic and morphological evidence. Thereby enriching the species diversity of Cystolepiota in China. In the phylogenetic trees (Figures 1, 2), Cystolepiota sp. (HMJAU68234, HMJAU68235, HMJAU68257), Melanophyllum sp. (HMJAU68255), and Pulverolepiota sp. (HMJAU68236) are just one specimen. The findings of this study indicate the potential existence of undiscovered species in northeast China needs to be studied further.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
X-YZ: Conceptualization, Investigation, Methodology, Writing – original draft, Writing – review & editing. TB: Conceptualization, Investigation, Methodology, Resources, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was financed by the National Natural Science Foundation of China (Project No. 32070010).
Acknowledgments
The authors are very grateful to the National Natural Science Foundation of China (Project No. 32070010) for supporting this research. The authors sincerely thank the teacher and the team for their help. The authors would also like to thank the reviewers and editors whose corrections and suggestions have enabled our work to be published.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
Bau, T., and Li, Y. (2004). Lepiota squamulosa, a new species from China. J. Fungal Res. 2, 49–50. doi: 10.13341/j.jfr.2004.03.011
Bon, M. (1991). Les genres Echinoderma (Locq. ex Bon) st. nov. et Rugosomyces Raithelhuber ss. Lato. Doc. Mycol. 21, 61–66.
Capella-Gutiérrez, S., Silla-Martínez, J. M., and Gabaldón, T. (2009). trimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. doi: 10.1093/bioinformatics/btp348
Chou, W. N. (2010). Nine species of Lepiota sensu lato (Basidiomycotina) new to Taiwan. Collect. Res. 23, 1–7. doi: 10.6693/CAR.2010.23.1
Clémençon, H. (2012). Cytology and Plectology of the Hymenomycetes. 2nd revised edition. Stuttgart: Gebrüder Borntraeger Verlagsbuchhandlung.
Edler, D., Klein, J., Antonelli, A., and Silvestro, D. (2021). raxmlGUI 2.0: a graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 12, 373–377. doi: 10.1111/2041-210x.13512
Gardes, M., and Bruns, T. D. (1993). ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Mol. Ecol. 2, 113–118. doi: 10.1111/j.1365-294x.1993.tb00005.x
Hou, Y. J., and Ge, Z. W. (2020). New species of Echinoderma and Lepiota (Agaricaceae) from China. Phytotaxa 447, 221–236. doi: 10.11646/phytotaxa.447.4.1
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K., Von Haeseler, A., and Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Katoh, K., Rozewicki, J., and Yamada, K. D. (2019). MAFFT online service: multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 20, 1160–1166. doi: 10.1093/bib/bbx108
Knudsen, H. (1980). A revision of Lepiota sect. Echinatae and Amyloideae (Agaricaceae) in Europe. Bot. Tidsskr. 75, 121–155.
Kumar, S., Stecher, G., and Tamura, K. (2016). MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874. doi: 10.1093/molbev/msw054
Mao, X. L., Zhuang, J. Y., Zhuang, W. Y., Guo, Y. L., Guo, L., Zhang, X. Q., et al. (1997). Fungi of the Qinling Mountains. Beijing: China Agricultural Science and Technology Press.
Matheny, P. B. (2005). Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol. Phylogenet. 35, 1–20. doi: 10.1016/j.ympev.2004.11.014
Qu, H., Damm, U., Hou, Y. J., and Ge, Z. W. (2023). Taxonomy and phylogeny of Cystolepiota (Agaricaceae, Agaricales): new species, new combinations and notes on the C. seminuda Complex. J. Fungi 9:537. doi: 10.3390/jof9050537
Rambaut, A. (2016). “FigTree v1.4.3 2006–2016” in Tree figure drawing tool (Institute of Evolutionary Biology University of Edinburgh) Available at: https://vcru.wisc.edu/simonlab/bioinformatics/programs/figtree/README.txt
Rehner, S. A., and Buckley, E. (2005). A Beauveria phylogeny inferred from nuclear ITS and EF1-alpha sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia 97, 84–98. doi: 10.3852/mycologia.97.1.84
Rehner, S. A., and Samuels, G. J. (1994). Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 98, 625–634. doi: 10.1016/S0953-7562(09)80409-7
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Saar, I., and Laessoe, T. (2008). A re-evaluation of Cystoderma luteohemisphaericum. Mycotaxon 104, 313–320.
Sánchez-García, M., Ryberg, M., Khan, F. K., Varga, T., Nagy, L. G., and Hibbett, D. S. (2020). Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi. Proc. Natl. Acad. Sci. U.S.A. 117, 32528–32534. doi: 10.1073/pnas.1922539117
Singer, R. (1986). The Agaricales in modern taxonomy, 4. Koenigstein: Koeltz Scientific Books, Koenigstein.
Singer, R., and Clémençon, H. (1972). Notes on some leucosporous and rhodosporous European agarics. Nova Hedwigia 23, 305–351.
Sysouphanthong, P., Thongklang, N., Liu, Y. S., and Vellinga, E. C. (2022). Three new species of Cystolepiota from Laos and Thailand. Diversity 14:449. doi: 10.3390/d14060449
Turland, N. J., Wiersema, J. H., Barrie, F. R., Greuter, W., Hawksworth, D. L., Herendeen, P. S., et al. (2018). International code of nomenclature for algae, fungi, and plants. Nineteenth International Botanical Congress Shenzhen, China Koeltz botanical books.
Vellinga, E. C. (1988) “Glossary,” in Flora agaricina neerlandica Volume 1. eds. C. Bas, T. W. Kuyper, M. E. Noordeloos and E. C. Vellinga (Rotterdam: AABalkema).
Vellinga, E. C. (2003). Phylogeny of Lepiota (Agaricaceae)—evidence from nrITS and nrLSU sequences. Mycol. Prog. 2, 305–322. doi: 10.1007/s11557-006-0068-x
Vellinga, E. C. (2006). Lepiotaceous fungi California, U.S.A.—4. Type studies of Lepiota fumosifolia and L. petasiformis. Mycotaxon 98, 225–232.
Vellinga, E. C., and Huijser, H. A. (1998). Notes on Cystolepiota: sections Cystolepiota and Pulverolepiota. Pers.: Mol. Phylogeny Evol. Fungi 16, 513–526.
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from severa Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
White, T. J., Bruns, T., Lee, S. J. W. T., and Taylor, J. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Xu, M. L., Li, G. J., Zhou, J. L., Bai, X. M., and Zhao, R. L. (2016). New species of Cystolepiota from China. Mycology 7, 165–170. doi: 10.1080/21501203.2016.1239231
Yang, Z. L., and Ge, Z. (2017). Six new combinations of lepiotaceous fungi from China. Mycosystema 36, 542–551. doi: 10.13346/j.mycosystema.160221
Keywords: Cystolepiota, new species, phylogeny, taxonomy, northeastern China
Citation: Zhou X-Y and Bau T (2024) Four new species of Cystolepiota (Agaricaceae, Agaricales) from northeastern China. Front. Microbiol. 15:1358612. doi: 10.3389/fmicb.2024.1358612
Edited by:
George Tsiamis, University of Patras, GreeceReviewed by:
Laura Guzmán-Dávalos, University of Guadalajara, MexicoXinli Wei, Chinese Academy of Sciences (CAS), China
Copyright © 2024 Zhou and Bau. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tolgor Bau, anVud3VzdW9AMTI2LmNvbQ==
 Xian-Yan Zhou
Xian-Yan Zhou Tolgor Bau
Tolgor Bau