
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 04 April 2024
Sec. Food Microbiology
Volume 15 - 2024 | https://doi.org/10.3389/fmicb.2024.1353015
This article is part of the Research TopicInteractions between Bioactive Food Ingredients and Intestinal Microbiota, volume IIView all 16 articles
Constipation is a common gastrointestinal disease that seriously affects human physical and mental health. Studies have reported that hemp seeds can improve constipation, however the specific mechanism is still unclear. This study investigates that hemp seed (HS) and its water-ethanol extract (HSE) attenuates loperamide-induced constipation in mice. The research results show that: the fecal water content and small intestinal transit rate of mice in the hemp seed group and hemp seed hydroalcoholic extract group were significantly increased compared with MC group, and the first red feces defecation time was significantly shortened; HS and HSE significantly influence serum levels of Gastrin (Gas), motilin (MTL), substance P (SP), and endothelin (ET), potentially mediating their effects on gastrointestinal motility. HS and HSE can improve colon inflammation in constipated mice with H&E staining. Compared with the model of constipation group, the content of short-chain fatty acids in the HS group and HSE group increased significantly. Gut microbiome studies have shown that the structure and abundance of intestinal flora are altered. HS and HSE changed the abundance of Odoribacter, Bacteroide, Lactobacillus and Prevotella. Together, these results suggest that HS have the potential to stimulate the proliferation of beneficial gut microbes and promote intestinal motility, thereby improving gut health and relieving symptoms of constipation.
Constipation, a term used to describe a variety of symptoms, including hard stools, over-stress, frequent bowel movements, abdominal distension and abdominal pain, is one of the most common gastrointestinal disorders worldwide (Camilleri et al., 2017). According to epidemiological data, the global prevalence of constipation in adults is 18.9% (Salari et al., 2023). It is well known that the occurrence of constipation imposes a significant economic burden and serious psychological impact on the patients. It is estimated that the cost of purchasing laxatives exceeds $800 million per year in the United States (Wang et al., 2022). Chronic constipation can induce the accumulation of pathogenic bacteria in the colon and is associated with an increased risk of gastrointestinal disorders, such as irritable bowel syndrome and colorectal cancer (Heidelbaugh et al., 2015). Currently, laxatives are the first choice for constipation. However, prolonged use of these laxatives will cause some adverse effects, such as abdominal cramps, rashes, excessive flatulence and dizziness (Ding et al., 2016), more than 50% of patients are not completely satisfied with the treatment of constipation (Huang et al., 2022). Therefore, it is of practical significance to develop a simple and affordable method to improve constipation.
Hemp seeds (HS) are the dried ripe fruit of Cannabis sativa L. (Moraceae). Hemp seeds have been utilized both as a food and medicinal ingredient in traditional Chinese medicine for at least 3,000 years (Yan et al., 2015). They have the effects of preventing constipation, promoting cardiovascular health, regulating immunity, and treating skin and gastrointestinal disorders. Hemp seeds have been listed as a Chinese herbal medicine of affinal drug and diet by the National Health Commission of the People's Republic of China. Hemp seeds are rich in nutrients and natural active ingredients. As one of the most commonly used traditional Chinese medicine for the treatment of constipation, hemp seeds are rich in plant proteins, unsaturated fatty acids, vitamins and other nutrients, containing up to 28% of dietary fiber (Opyd et al., 2020). Modern pharmacology has confirmed that hemp seeds contain fatty oil, which can stimulate the intestinal mucosa to increase secretion, promote peristalsis and reduce water absorption in the large intestine, thereby exerting a laxative effect. In a population-based cohort study of 216 patients by Zheng et al., Chinese patent drug Dama Wan (literally Cannabis pills) prepared from hemp seeds and other raw materials was shown to have a reliable and safe efficacy in functional constipation at a dose of 7.5 g bid (Cheng et al., 2011). Huang L. S. et al. (2018) reported the efficacy of Maziren Wan (literally hemp seed pills) in stimulating intestinal mucosa, reducing water absorption in the intestine, softening feces, restoring gastrointestinal homeostasis to increase intestinal peristalsis and relieve constipation. A study by Cheng et al. concluded that hemp seeds improved colonic transit, increased the bowel movement frequency, and reduced the severity of constipation in patients with functional constipation (Zhong et al., 2019).
Currently, hemp seeds are primarily used in the form of hemp seed oil (HSE) or traditional Chinese decoction, which leads to a low utility of many beneficial ingredients in hemp seeds and production of waste residue. Moreover, there are few research reports on utilization of complete hemp seed to treat constipation. Therefore, we aimed to study the protective effect of HS and HSE in mice with loperamide-induced constipation.
Hemp seeds were purchased from Nanjing Traditional Chinese Medicine Market. Loperamide hydrochloride were purchased from Yifeng Pharmacy in Nanchang. Gastrin (Gas), motilin (MTL), substance P (SP), acetylcholinesterase (AchE), endothelin (ET), vasoactive intestinal peptide (VIP) enzyme-linked immunosorbent assay (ELISA) kit had been purchased from Shanghai Yuanju Biotechnology Center. Hematoxylin and eosin (H&E) dye, paraformaldehyde (Wuhan Qian Baidu Biotechnology Co., Ltd.). Short-chain fatty acids (SCFAs) standards: acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, and isovaleric acid standards were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd.
5804R refrigerated centrifuge, 5424R refrigerated centrifuge [Ebende (Shanghai) International Trade Co., Ltd.]; SB-02 high-speed multifunctional crusher (Shanghai Puheng Information Technology Co., Ltd.); RT-6000 microplate reader {Leica DM500 upright microscope [Leica Microsystems (Shanghai) Trading Co., Ltd.]; Agilent 7890B gas chromatograph [Agilent Technologies (China) Co., Ltd.]}.
Hemp seeds were added to the grinder and crushed into uniform particles for 3 min. Grind the maintenance feed for 3 min into powder. In every 90 g of maintenance feed, 10 g of crushed hemp seeds were evenly mixed to crush particles, then put into the mold for plasticity. The oven was heated at 60 degrees Celsius for 8 h to remove excess water and fix the shape, and 10% hemp seeds were made into added feed.
We accurately weighed 20.0 g of hemp seed, ground them, and added 200 mL distilled water according to the solvent ratio of 1:10, vortexed and mixed it, ultrasonic at 40°C for 1 h, centrifuged at 4,500 rpm/min for 5 min, and pour the supernatant into a clean vial. Ethanol solution (200 mL) was added 1:10 to the residue, vortexed and mixed, and the above steps were repeated. Water extraction and alcohol extraction were repeated three times, respectively, and the above extracts were combined. Rotary evaporation was carried out at the temperature of 50°C and the to a constant volume of 30 mL at a speed of 25 and extract concentrate (30 ml) was obtained and stored at −20°C for subsequent experiments.
Forty specific pathogen-free (SPF) female BALB/c mice, 6–7 weeks old, weighing 16–19 g, were purchased from Spefford (Beijing) Biotechnology Co., Ltd. [license number: SCXK (Beijing) 2019–0010]; mouse maintenance feed was purchased from Jiangsu Synergy Biotechnology Co., Ltd. (Nanjing, China). The experimental animals were housed in a specific pathogen-free (SPF) facility, with unrestricted access to food and water, with a 12-h light-dark cycle, and the growth environment temperature was 23°C ± 2°C. After adaptive feeding for 3 days, the mice were randomly divided into four groups (n = 10): blank control group (CN: normal saline + maintenance feed), model of constipation group (MC: loperamide 10 mg/kg + maintenance feed), hemp seed group (HS: normal saline + 10% hemp seed powder added to feed), hemp seed extract group (HSE: hemp seed extract + maintenance feed). During the entire experiment, mice in the CN group were fed standard mouse chow without any treatment. After the adaptation period, mice in MC, HS, and HSE groups were intragastrically administered loperamide 10 mg/kg at 9:00 a.m. every day for 2 weeks (Huang et al., 2022). One week after modeling, the HSE group was intragastrically administered with the test substance (0.2 mL). The Hemp seed group were given free access to the maintenance feed containing with 10% hemp seed supplemented. When the number of defecation pellets of the mice in the constipation group is significantly different from the number of defecation pellets of the mice in the blank control group, it means that the constipation model has been successfully established. During the experiment, the body weight and food intake of the mice were recorded twice a week; the physiological and fecal status of the mice were observed every day.
After the constipation model was successfully established, mouse feces were collected every day and photos were taken to record the appearance of the feces. Each group of mice was placed in a clean cage, deprived of food and water, and allowed to defecate freely for 30 min. Fresh feces were collected in a 1.5 ml dry centrifuge tube, and the resolved samples were weighed using an electronic balance. All fecal samples were dried in an electric constant-temperature drying oven at 60°C until the samples had a constant weight. The formula for calculating feces water content is as follows:
Moisture content of feces (%) = (feces weight before drying—feces weight after drying)/feces weight before drying * 100
Preparation of red ink: a solution of 6 g phenol red in 100 ml 1% carboxymethylcellulose, which was stirred continuously and maintained at 37°C (Crowe and Kinsey, 2017).
This experiment was conducted after the 21st day, and all mice were fasted and water-free for 12 h. After the fasting, 0.2 ml of red ink was administered into the stomach and each mouse was individually transferred to a clean empty cage. The time when each mouse first discharged red stool was recorded.
This experiment was conducted after the 22nd day, and all mice were fasted and water-free for 12 h. After fasting, 0.2 ml of red ink was administered into the stomach, and the eyes were anesthetized for 10 min to collect blood and then killed by neck dissection. Measure the distance traveled by the red ink and the total length of the small intestine. The gastrointestinal transit rate is calculated according to the formula:
Gastrointestinal transit rate (%) = red ink advancement distance/total length of small intestine*100
Colon tissue was fixed with 4% paraformaldehyde, embedded in paraffin, and cut into 4 μm thick sections. Fixed sections were dehydrocarbonated with xylene, hydrated with graded ethanol, and rinsed with distilled water. Hematoxylin and eosin (H&E) staining kit was used to detect morphological changes in the colon and ileum.
After the intestinal transport experiment, the mice were anesthetized with isoflurane, and the eyeballs were enucleated and blood was collected. All blood samples were centrifuged at 4°C, 3,500 rpm (5804R desktop refrigerated centrifuge) for 15 min, and serum samples were obtained and stored in a −80°C ultra-low temperature refrigerator. According to the instructions of the enzyme-linked immunosorbent assay (ELISA) kit, the gastric motility protein (MTL), gastrin (Gas), endothelin (ET), substance P (SP), acetylcholinesterase (AChE), and blood vessels in the serum were measured. Viable intestinal peptide (VIP) levels.
Gas chromatography was used to determine the contents of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid and isovaleric acid. Mouse feces were collected, quickly frozen in liquid nitrogen, and placed in an 80°C freezer for testing. Weigh 200 mg of feces into a 1.5 ml centrifuge tube, add 1 ml of ultrapure water, add 10 μl of concentrated hydrochloric acid to acidify, homogenize for 3 min, let stand for 20 min after homogenization, centrifuge at 10,000 rpm for 10 min in a high-speed centrifuge at 4°C, and remove the supernatant The liquid is transferred to the injection bottle for testing.
Before the end of the experiment, the sterile feces of each mouse were collected, quickly frozen in liquid nitrogen, and then stored in an −80°C freezer for later use. Mouse fecal samples were taken out. Magnetic Soil and Stool DNA Kit (TianGen) was used to extract genomic DNA from the samples. The purity and concentration of DNA were detected by 1% agarose gel electrophoresis. Appropriate sample DNA was placed in a centrifuge tube and diluted to 1 ng/μL with sterile water. Then the PCR products were obtained and purified. The Library was constructed using NEB Next? Ultra™ II FS DNA PCR-free Library Prep Kit (New England Biolabs). The constructed library was quantified by Qubit and Q-PCR. Enable NovaSeq6000 to perform PE 250 on-machine sequencing. According to Barcode sequence and PCR amplification primer sequence, the sample data were separated from the disembarkation data. Qiime2 is used to analyze the raw data obtained and generate a graph of the analysis results on the Yun Tutu platform.
SPSS 13.0 statistical software was used for analysis, and GraphPad Prism 9.0 software was used for graphing. Data were expressed as Mean ± standard error of the mean (SEM). One-way analysis of variance was used for comparison between groups. P<0.05 is considered as statistically significant.
The status and fecal morphology of the mice are shown in Figure 1A. Mice in the CN group appeared to be behaviorally active, with shiny hair and normal fecal pellets in size with a smooth and moist surface. In contrast, mice in the MC group were found to be less behaviorally active by curling themselves up, with fluffy hair and dark hard stools with a rough dry surface after induced constipation. After 2 weeks of treatment with HS and HSE, improvement in physiological state was observed in both HS and HSE groups. Mice in the HS group appeared to be behaviorally active, with fecal pellets that were smooth and soft, while mice in the HSE group were in a good mental state, with smaller fecal pellets that were smooth on surface and less hard. As shown in Figures 1B, C, there was no significant difference in weight variation and food consumption across the four groups. As shown in Figure 1D, there was a significant difference in water content of feces between the CN group and the MC group, that is, a significant increase in moisture content of feces was observed for mice in the HS and HSE groups after treatment with HS and HSE, respectively (P < 0.05).
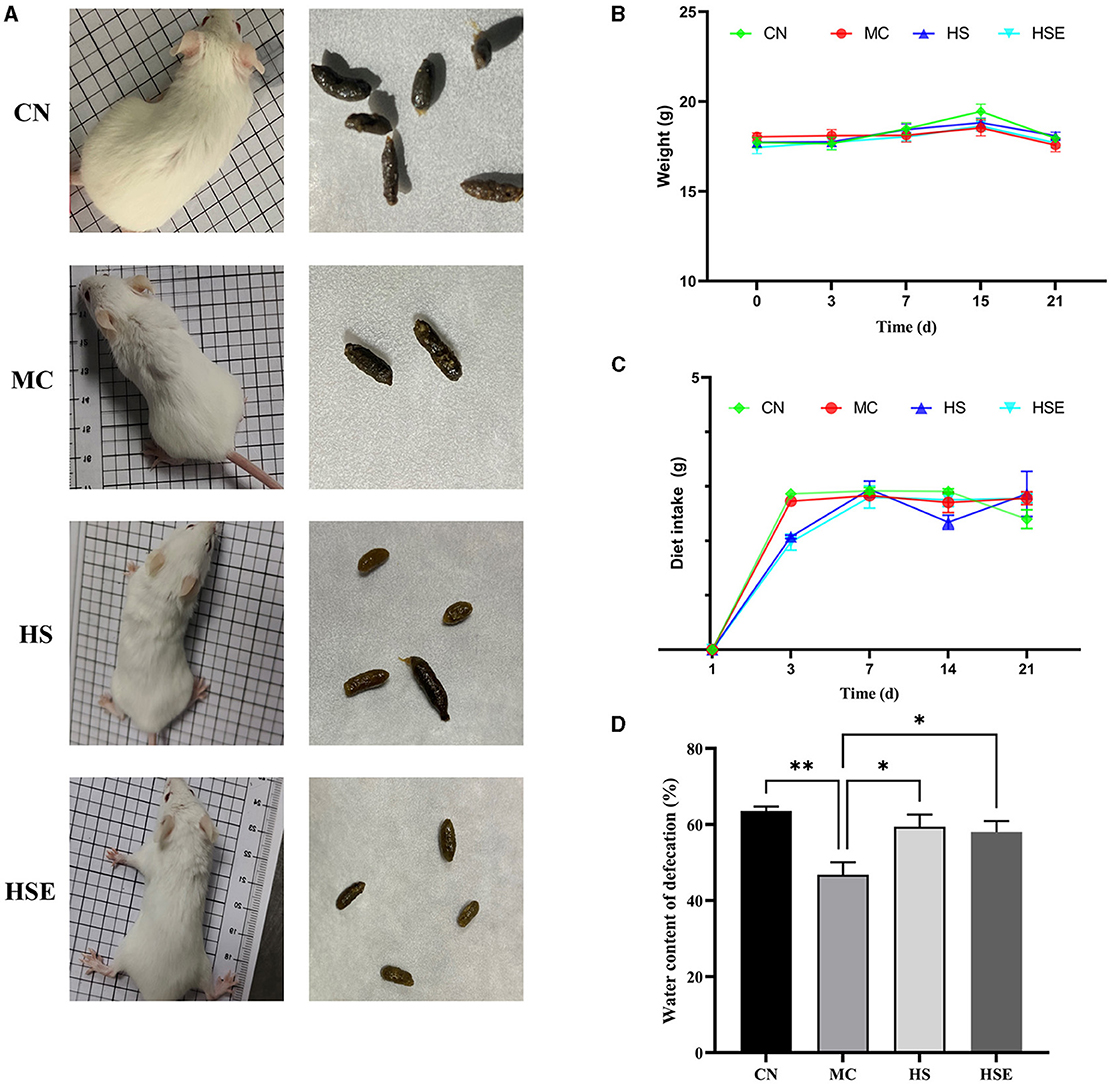
Figure 1. Effects of hemp seeds on the growth parameters and moisture content of feces in mice with constipation (A) Physiological status and feces of mice (B) Weight variation of mice (C) Food consumption of mice, and (D) Moisture content of feces. Results are provided as mean ± SEM (n = 4–6) *P < 0.05, and **P < 0.01 compared to constipation group.
The time to first bowel movement and small intestinal transit rates of the mice are provided in Figures 2A, B, D. After induced constipation, the time to first bowel movement was significantly prolonged in the MC group compared with the CN group (P < 0.05), while the small intestinal transit rate was significantly decreased (P < 0.01), conforming to the characteristics of constipation in mice. After treatment with HS and HSE, the time to first bowel movement was significantly reduced in the HS and HSE groups (P < 0.01). In terms of improvement of small intestinal transit rate, the HS group showed an extremely significant change (P < 0.01) whereas the HSE group showed a significant change (P < 0.05). The experimental results showed that HS was superior to HSE in terms of the effects of reducing the time to bowel movement and promoting small intestinal transit. To further investigate the protective effects of HS and HSE on the intestinal tract in mice with constipation, we observed the histomorphological changes of the distal colon by histological staining. As shown in Figure 2C, increased infiltration of inflammatory cells, shorter crypts, fewer goblet cells and greater muscularis-crypt distance were observed in the colon of the MC group compared with the CN group. After treatment with HS and HSE, decreased inflammatory cell infiltration and increased goblet cells were noticed, indicating that constipation induced colitis in the mice and HS/HSE could relieve the inflammatory reaction caused by constipation.
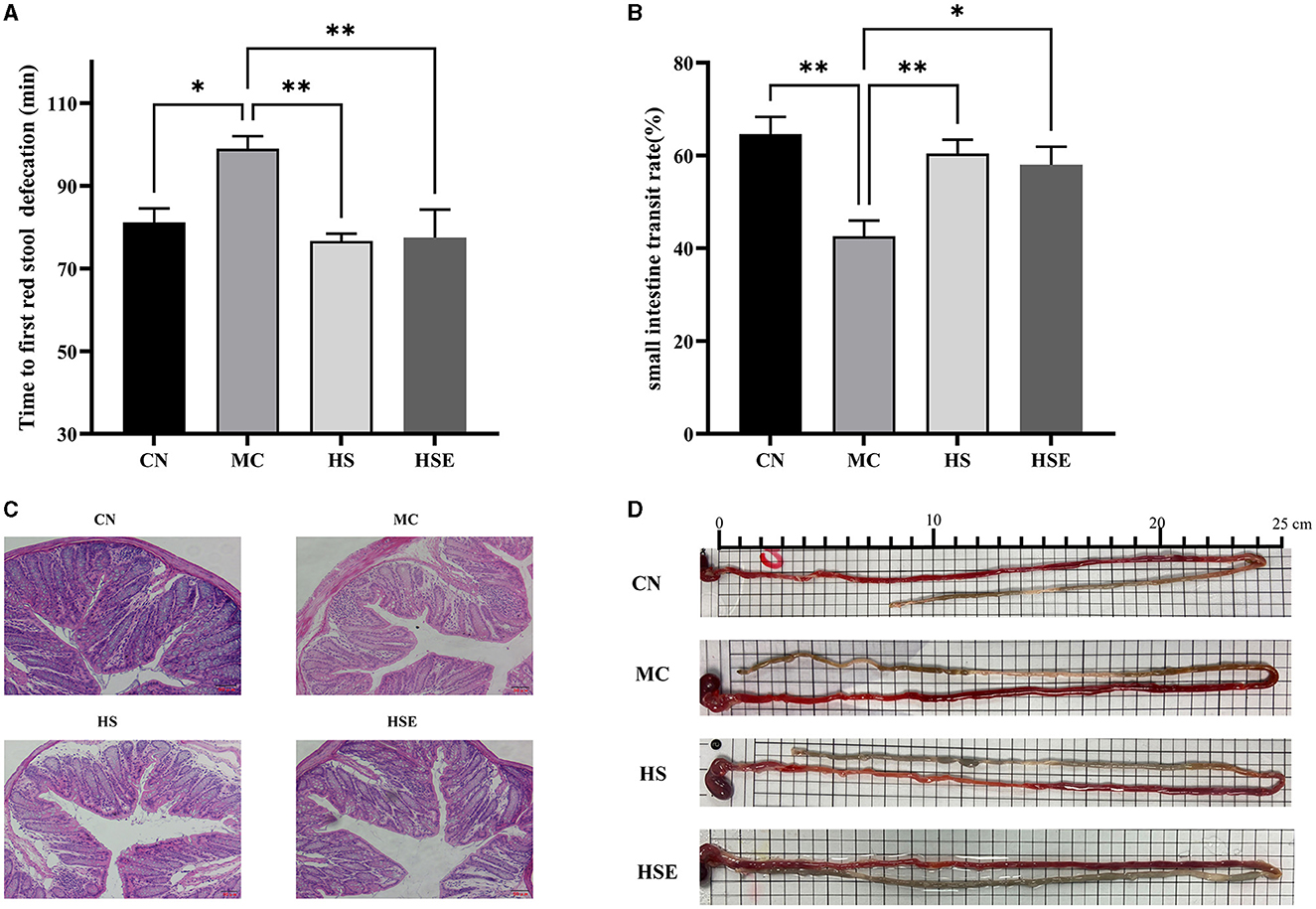
Figure 2. Effects of hemp seeds treatment on intestinal transit and protective effects on colonic tissue in mice with constipation (A) Time to discharge of the first red fecal pellet (B) Small intestinal transit rate (C) HE staining in the colon of mice, and (D) Image of assay on small intestinal transit rate (each cell in the picture is 0.5 cm long). Results are provided as mean ± SEM (n = 5–6) *P < 0.05 and **P < 0.01 compared with constipation group.
To further evaluate the effects of HS and HSE on constipation, serum parameters were measured. As shown in Figure 3, the levels of MTL, GAS and SP in the MC group were significantly lower than those in the CN group (*P < 0.05 for MTL, *P < 0.05 for GAS, and **P < 0.01 for SP), while the levels of ET and VIP increased but were not significantly different from those in the CN group, and the level of AchE decreased but was not significantly different from that in the CN group. After treatment with HS, the levels of MTL and GAS were significantly increased (*P < 0.05), and the level of ET was significantly decreased (**P < 0.01), but there were no significant differences in the levels of SP, VIP and AchE. In contrast, HSE-treated mice were observed with significant increases in the levels of MTL, GAS, and SP (**P < 0.01 for MTL, **P < 0.01 for GAS, and ***P < 0.001 for SP), and a significant decrease in the level of ET (*P < 0.05), whereas there were no significant differences in the levels of VIP and AchE.
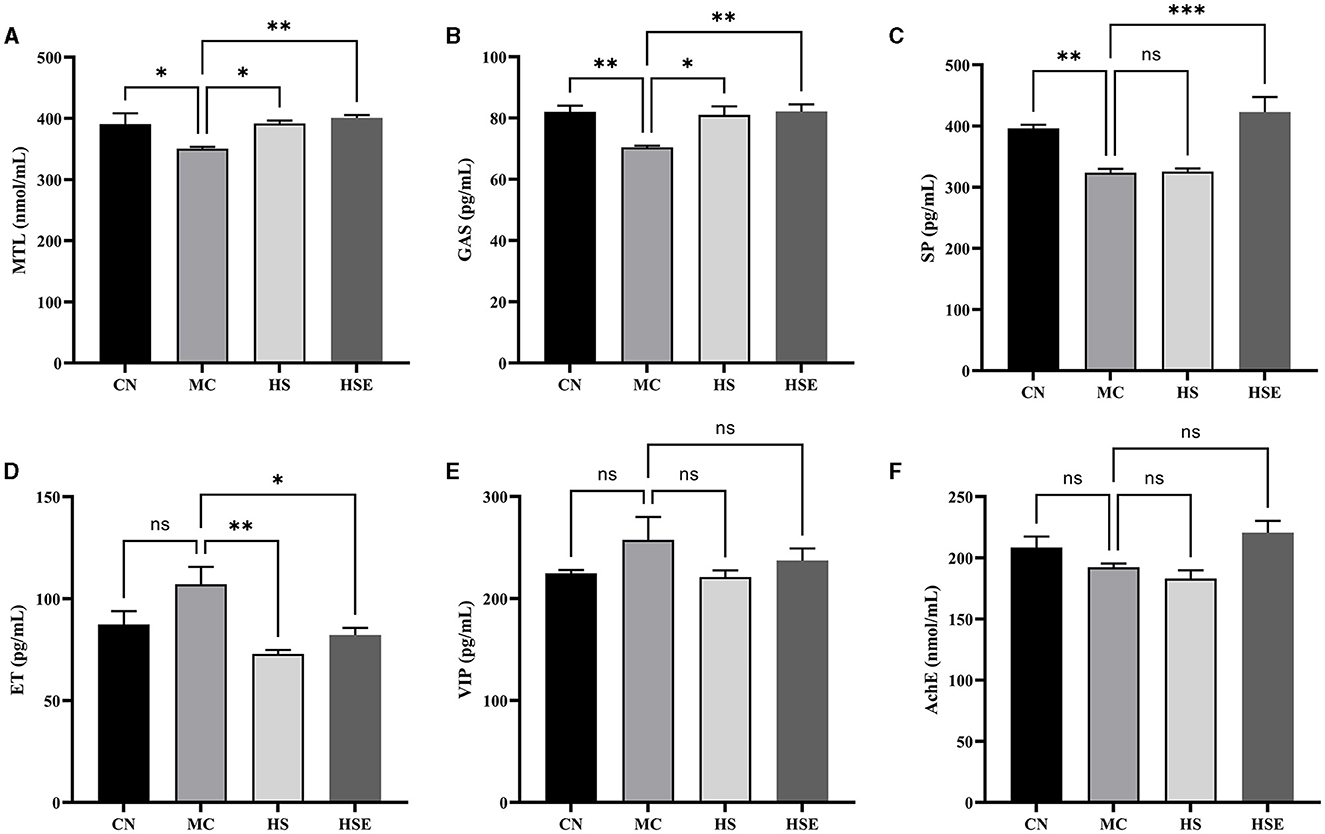
Figure 3. Effects of hemp seeds treatment on serum levels of neurotransmitters relating to gastrointestinal regulation in mice with constipation. (A–F) represents MTL, GAS, SP, ET, VIP, and AchE, respectively. Results are provided as mean ± SEM (n = 4) *P < 0.05, **P < 0.01, and ***P < 0.001 compared with constipation group.
SCFAs are the main products from the fermentation of indigestible carbohydrates by symbiotic bacteria. In addition to the effects of inhibiting the growth of pathogenic microorganisms and increasing the absorption of certain nutrients, SCFAs were also reported to regulate intestinal neurons and affect gastrointestinal motility. The fecal concentrations of SCFAs in this study are provided in Figure 4. The fecal concentrations of acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid and total acids were significantly lower in the MC group than in the CN group. After HS treatment, the HS group was observed with a significant increase in the fecal concentrations of the six short-chain fatty acids, while the HSE group was noticed with a significant increase in the fecal concentrations of propionic acid, butyric acid, valeric acid and total acids compared with the MC group, without no significant change in the fecal concentrations of acetic acid, isobutyric acid and isovaleric acid. The results showed that HS/HOS treatment led to a change in the content of SCFAs, which was possibly the protective mechanism of HS and HSE on the mice with constipation.
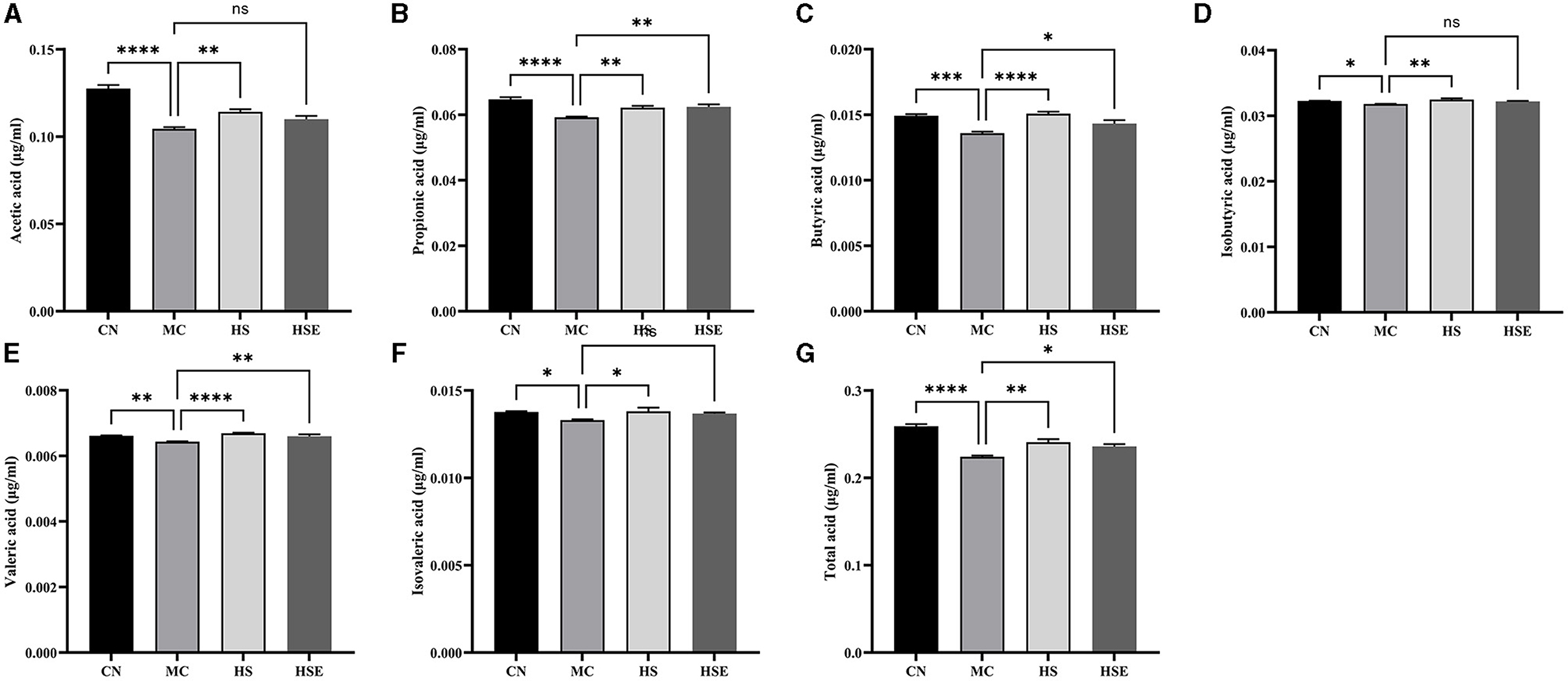
Figure 4. Effects of hemp seeds treatment on short-chain fatty acids (SCFAs) in the mice with constipation. (A–G) represents acetic acid, propionic acid, butyric acid, isobutyric acid, valeric acid, isovaleric acid and total acids, respectively. Results are provided as mean ± SEM (n = 6) *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 compared with constipation group.
To assess the effects of hemp seeds on the microbiota in intestinal feces, we performed metagenomic analysis of 16SrRNA gene sequences to examine changes in intestinal microorganisms after prophylaxis with hemp seeds (HS, HSE) and to characterize the effects on intestinal microbiota in mice after loperamide treatment. Alpha diversity (encompassing multiple indexes) is used as a measure of microbiota diversity (Cao et al., 2021). Totally 24 samples from four groups were evaluated. After sequence optimization, a total of 22,711,313 readable sequences were generated, with an average of 112,972 readable sequences per group. The results and the calculated α-diversity indexes of microbiota are shown in Figures 5A, B. At the OUT level, after Loperamide induced constipation, the Shannon and pielou_evenness indexes of intestinal microbiota in the MC group were higher than those in other groups, but there was no significant difference between groups as compared, indicating that HS and HSE treatment had no significant effect on the α-diversity of microbiota. As shown by the results of β-diversity (as measured by the Bray-Curtis distance) in Figure 5C, a trend toward segregation was observed with mice in the CN and MC group, whereas the HS and HSE groups were largely segregated from the MC group.
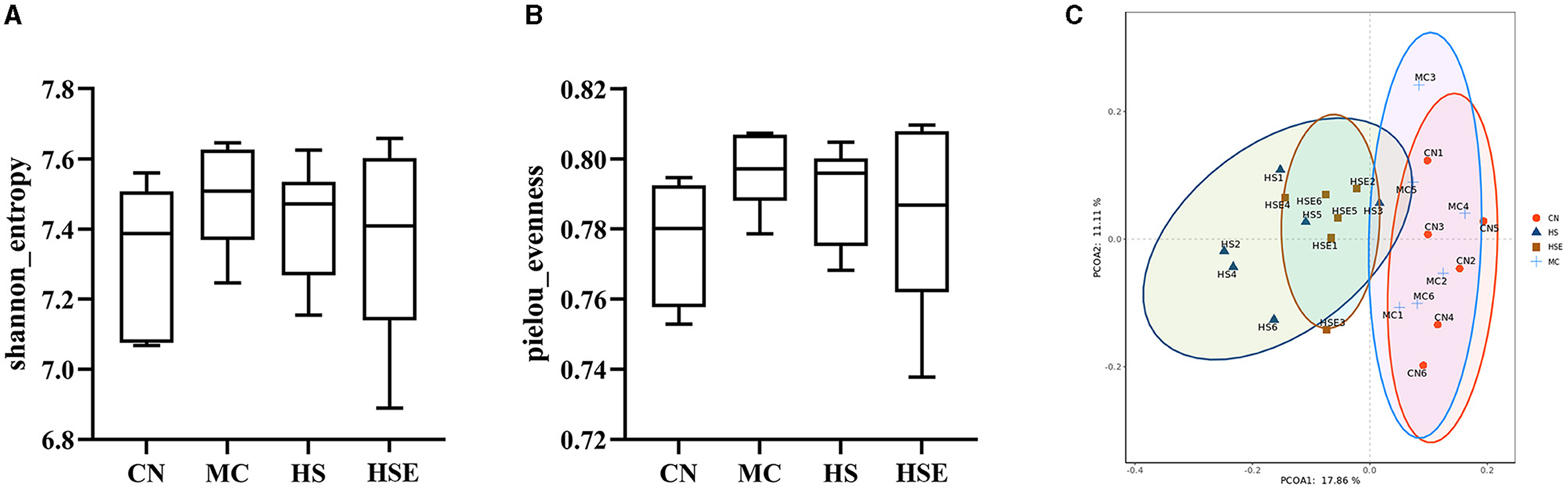
Figure 5. Review on the composition and diversity of intestinal microbiota in mice treated with hemp seeds for constipation. (A) Shannon Index, (B) Evenness Index, and (C) Plot of Principal Coordinate Analysis (PCoA) Score. Data are provided as mean ± SEM (n = 6).
Taxonomically, Figure 6A presents the distribution of fecal flora at the phylum level. Although a total of 11 phyla were identified in the feces from all mice, the fecal flora was mostly composed of Firmicutes/Bacteroides. Therefore, we further conducted a discussion on changes in the proportion of the two phyla. Compared with the CN group, the proportion of Bacteroides was decreased and that of Firmicute was increased in the MC group, and the Firmicutes/Bacteroides ratio was 1.14 in the MC group, being higher than the ratio of 0.99 in the CN group, without a significant difference. After treatment with HS/HSE, the proportion of Firmicutes increased in the HS group, and interestingly, the number of Bacteroides also showed a trend toward increasing in the HS group compared with the MC group, but the Firmicutes/Bacteroides ratio in the HS group was almost equal to that in the MC group. On the other hand, the proportion of firmicutes decreased but that of Bacteroides increased in the HSE group compared with the MC group, and therefore the Firmicutes/Bacteroides ratio in the HSE group was lower than that in the MC group, but there was not a significant difference, which was consistent with the study findings (Lin et al., 2022). At the genus level, as shown in Figures 6B–F, the proportions of Bacteroides and Odoribacter were significantly lower in the MC group than in the CN group. Interestingly, the microbial change trend was basically identical between HS and HSE group. Compared with the MC group, an increased proportion of Odoribacter and of Bacteroides was observed with HS and HSE group. On the other hand, the proportion of Lactobacillus genus increased significantly in the HSE group, but there was no significant increase in HS group. Compared with the MC group, the abundance of Precotella in HS and HSE groups decreased significantly. Finally, we inferred that HS and HSE exerted effects on constipation by affecting different genera of bacteria to influence intestinal microbiota.
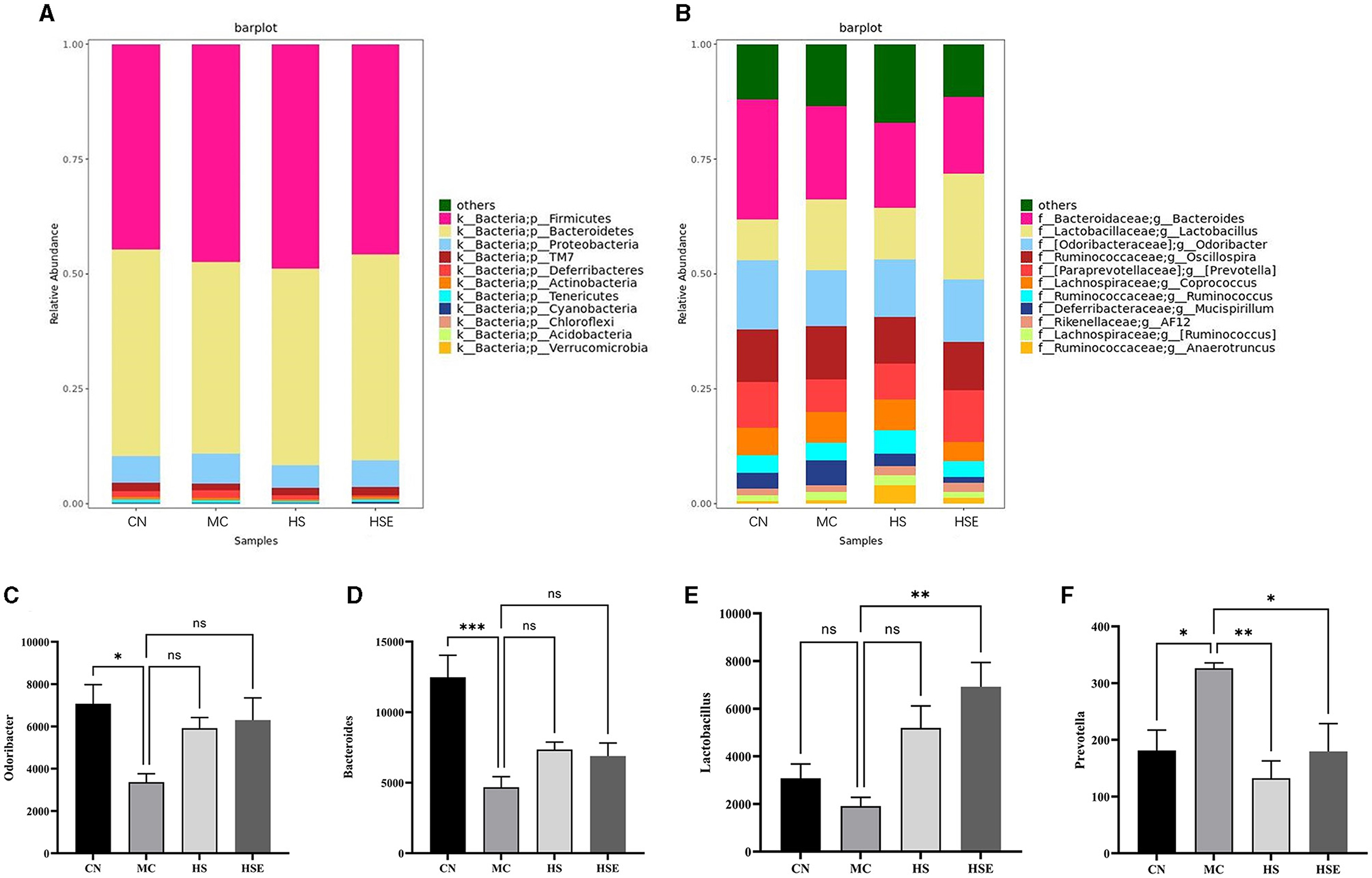
Figure 6. review on relative abundance of intestinal microbiota in mice treated with hemp seeds for constipation- composition of the microbiota. (A) phylum and (B) genus levels, (C) Relative abundance of Odoribacter, (D) Relative abundance of Bacteroides, (E) Relative abundance of Lactobacillus, (F) Relative abundance of Prevotella. Data are provided as mean ± SEM *P < 0.05, **P < 0.01, and ***P < 0.001 compared with constipation group.
We next addressed differential changes in the relative content of microorganisms. As shown in Figure 7A, Clostridium was the most significantly enriched genus in the CN group, Roseburia and Lachnospiraceae were significantly enriched in the MC group, whilst Rikenella, Ruminococcus, Anaerotruncus, Allobaculum and Desulfovibrio were the significantly enriched genus in the HS group.
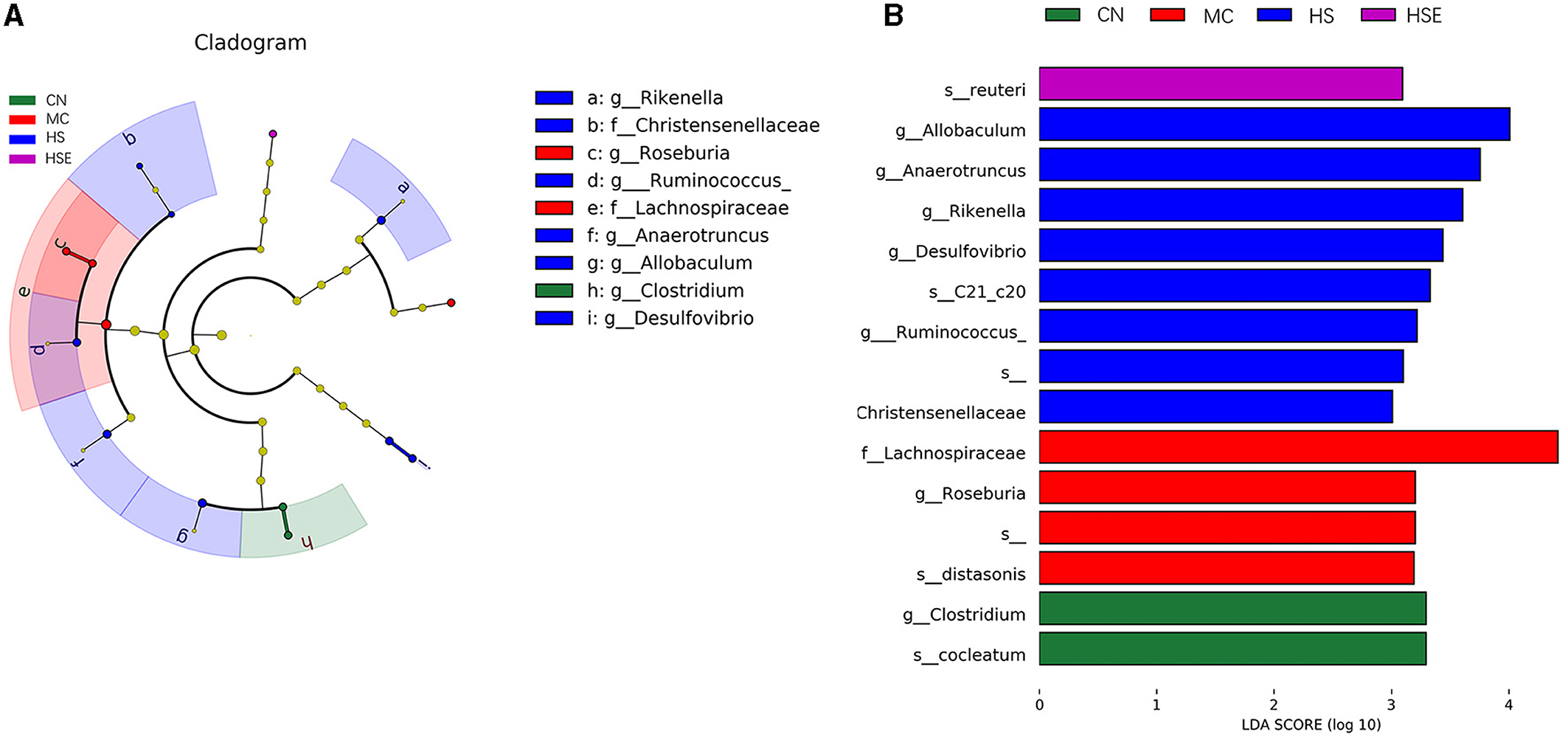
Figure 7. Differential classification of microorganisms in mice treated with hemp seeds for constipation. (A) Linear discriminant analysis effect size (LEfSe), and (B) Linear discriminant analysis (LDA). Data are provided as mean ± SEM (n = 6).
The results of linear discriminant analysis (LDA) provided in Figure 7B showed that Clostridium was the dominant genus in the NC group, Roseburia was the genus enriched in the MC group, and interestingly, Allobaculum was the genus enriched significantly in the HS group after HS treatment.
There is a steady rise in the number of people with constipation in China with improvement of the living standards. Constipation, a functional gastrointestinal disorder that involves altered bowel movements, will seriously affect daily living and work and has become a common concern in the medical field (Rao et al., 2016). Loperamide is a drug intended for diarrhea control approved by the US Food and Drug Administration. It is often used as a model inducer for the treatment of constipation, by prolonging the duration of emptying, inhibiting peristalsis and reducing mucus in the colon. The feasibility and stability of loperamide in constipation model have been widely acknowledged (Wang et al., 2017). Fecal moisture content, time to first bowel movement, and intestinal charcoal propulsion rate are important measures used to assess gastrointestinal function (Fernández-Bañares, 2006). As one of traditional Chinese medicine with a long history in China, hemp seeds are one of the resources of affinal drug and diet, Meanwhile, hemp seeds have demonstrated potential for treating constipation, but it is still limited in popularization as a human food. This study discusses the effect of hemp seeds and water/ethanol extracts from hemp seeds in improving constipation in mice. The results showed that both hemp seeds and hemp seed extracts significantly improved the defecation parameters in mice, including fecal moisture content and time to first bowel movement. Parameters reflecting intestinal peristalsis are related to multiple aspects, such as fecal weight, fecal moisture content, time to fecal discharge, gastrointestinal transit time, small intestinal propulsion rate, and intestinal contractile activity (Gu et al., 2022). The improvement in defecation parameters indicates, to some extent, the effect of HS and HSE on intestinal peristalsis. In addition, the results of colon staining showed that HS/HSE improved to some extent the inflammatory reactions caused by constipation, which was possibly one of the reasons why hemp seeds have the effect of improving intestinal peristalsis.
We also studied the effects of hemp seeds on the serum levels of neurotransmitters and gastrointestinal hormones. MTL and GAS play an important role in regulating gastrointestinal peristalsis by stimulating the secretion of gastric acid and pepsin, promoting pyloric sphincter relaxation, gastrointestinal peristalsis and gastric emptying (Iijima et al., 2015). SP is an excitatory peptide neurotransmitter that stimulates intestinal peristalsis (Suo et al., 2014). Some studies have reported that SP stimulates interstitial cells of Cajal, induces contraction of gastrointestinal smooth muscles, and promotes gastrointestinal peristalsis (Faussone-Pellegrini, 2006). ET plays an important role in maintaining basal vascular tone and basic cardiovascular system. Constipation not only causes disorders (including intestinal obstruction and other serious diseases), but also induces or aggravates cardiovascular and cerebrovascular diseases in the elderly (Fevang et al., 2001).
In our study, after loperamide induced constipation, the levels of MTL, GAS and SP in the serum of mice in MC group were significantly lower than those in CN group, indicating that the occurrence of constipation is related to these neurotransmitters. After preventive treatment with hemp seeds and water/ethanol extracts from hemp seeds, hemp seed significantly increased the levels of MTL and GAS in serum of mice in HS group, and decreased the levels of ET, while hemp seed extract significantly increased the levels of MTL, GAS and SP in serum of mice in HSE group, and decreased the levels of ET. Extracts from hemp seeds demonstrated a stronger effect in regulating serum levels of neurotransmitters and gastrointestinal hormones. The differences observed were possibly a result of increased utility of the active ingredients in the extracts following extraction from hemp seeds. Variation in the levels of these gastrointestinal hormones would lead to a change in gastrointestinal peristalsis. We inferred that the improved intestinal propulsion rate and the shortened time to bowel movement were closely related to variation in the serum levels of such gastrointestinal hormones and neurotransmitters, suggesting that hemp seeds may alleviate the symptoms of constipation by regulating neurotransmitters and gastrointestinal hormones to stimulate intestinal peristalsis.
Disturbance of the intestinal microbiota is one of the characteristics of patients with constipation (Tian et al., 2020), It was revealed in the study by Huang et al. that patients with chronic functional constipation (CFC) had abnormalities in the number and composition of intestinal microbiota (Huang T. et al., 2018). Some studies suggested that changes in the intestinal microorganisms may alleviate or worsen constipation (Dimidi et al., 2017). In a study of pentose extracted from human milk to improve constipation in mice, it was shown that constipation was improved by increasing beneficial bacteria such as Lactobacillus, Ruminococcaceae_UCG-014 and Bacteroidales_S24-7 to improve the composition of intestinal microorganisms (Huang et al., 2020). In addition, hemp seeds have been widely reported to have effects on the intestinal microbiota. Changes in the intestinal microbiota in mice are primarily detected by changes in bacteria in the feces (Wang et al., 2017). Therefore, this study was of research significance by investigating whether hemp seeds could improve constipation by improving the intestinal microbiota. The results showed that after loperamide-induced constipation, significant differences in intestinal microbiota were observed between the CN and MC groups at the genus level. Our results showed that the intestinal microbiota was primarily composed of Firmicutes and Bacteroides and secondarily of Proteobacteria, and there was not a significant difference in the proportion of Firmicutes or Bacteroides at the genus level across the four groups, which was consistent with the study findings by (Gu et al., 2022). At the genus level, HS and HSE groups were observed with an increase in the proportion of Odoribacter, Bacteroides and Lactobacillus, and interestingly, the HS group and the HSE group were observed with a lower proportion of Precotella compared with the MC group. These results suggest that hemp seeds regulate the symptoms of constipation by increasing the proportion of beneficial bacteria at the genus level. Lactobacillus works to decompose oligosaccharides in the intestinal tract, produce organic acids (including butyric acid, acetic acid, and propionic acid), improve intestinal peristalsis, and reduce the time to bowel movement. In addition, lactobacillus produces a great amount of volatile fatty acids and inhibit the excessive production of aerobic harmful bacteria, thereby maintaining the balance of intestinal microbiota (Cao et al., 2018). It has been found that butyric acid can be used as a carbon source for intestinal microbiota to produce acetic acid (Zhuang et al., 2019).
Hemp seeds are able to regulate the intestinal microbiota, one ability of intestinal bacteria is to produce SCFAs, but this process is influenced by the number of bacteria, pH, and substrate in the intestinal tract (Liu and Zhi, 2021). SCFAs are the metabolites of dietary fiber fermented by intestinal microorganisms. There is also a strong association between SCFAs and constipation. They affect the intestinal secretion function and enhance the intestinal mucosal barrier by regulating the functions of cell subsets like enterocytes (proliferation and differentiation) and enteroendocrine cells through different mechanisms. Organic acids act on the intestinal wall to lower pH in the intestinal tract and regulate gastrointestinal peristalsis and gastrointestinal function (Malagelada et al., 2017). Acetic acid acts to up-regulate the barrier function of enterocytes of the host (Fukuda et al., 2011), whilst propionate reduces fat production, serum cholesterol levels, and carcinogenesis in other tissues (Hosseini et al., 2011). Butyrate, which is a major source of metabolic energy in the large intestine, is the most important SCFAs and help to maintain the integrity of the large intestine, control intestinal inflammation, and support the genomic stability (Jiang et al., 2020). In a study reported on hemp seed, hemp seed increased the concentration of short-chain fatty acids in intestinal contents to improve intestinal health (Jurgoński et al., 2020). SCFAs can promote the secretion of intestinal fluids, accelerate colonic motility and are even considered as candidates for the treatment of constipation (Nicholson et al., 2012). In our study, the HS group was found to have significantly higher concentrations of the six SCFAs compared with the MC group, and the HSE group was observed with higher levels of propionic acid, butyric acid and valeric acid than the MC group, but HSE (an extract from hemp seeds) appeared inferior to HS in terms of the effect of increasing the levels of SCFAs. This was possibly because that production of SCFAs is affected by the quantity of microorganisms, the ambient pH value and the substrate concentration. The amounts and proportions of SCFAs produced by different substrate vary (Ritzhaupt et al., 1998). After extraction, HSE was inferior to HS in terms of both the type and concentration of substrate, and this was possibly one of the reasons for differences in the levels of SCFAs between the HS and HSE groups.
This study investigated the effects of hemp seeds and hemp seed extracts in the mice with constipation. The results showed that both hemp seeds and hemp seed extracts significantly improved the moisture content of feces, shortened the time to bowel movement, and promoted intestinal peristalsis in the mice with constipation. Hemp seeds were superior to hemp seed extracts in terms of the above effects. Both affected the levels of gastrointestinal hormones in the serum, whilst hemp seed extracts exhibited a stronger effect than hemp seeds. In contrast, hemp seed extracts had less effects on the levels of SCFAs than hemp seeds. In conclusion, both hemp seeds and hemp seed extracts provide protective effects in the mice with constipation to some extent.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
The animal studies were approved by Animal Ethics Committee of Changshu Hospital Affiliated to Nanjing University of Chinese Medicine. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
HH: Writing – original draft, Conceptualization, Formal analysis, Investigation, Methodology, Resources, Software. WY: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. DX: Conceptualization, Data curation, Methodology, Writing – review & editing. LF: Data curation, Software, Writing – original draft. GJ: Methodology, Formal analysis, Writing – review & editing. JJ: Formal analysis, Supervision, Writing – review & editing. ZF: Data curation, Formal analysis, Methodology, Writing – original draft, Writing – review & editing. JL: Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by Changshu Municipal Science and Technology Bureau Supporting Project (Grant Number: CS202227), Changshu Health Commission Science and Technology Plan Project (Grant Number: CSWS202103), Jiangsu Traditional Chinese Medicine Science and Technology Development Project (Grant Number: MS2021058), and Open Project of Zhenjiang Traditional Chinese Medicine Spleen and Stomach Diseases Clinical Medicine Research Center (Grant Number: SSPW2022-KF08).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Camilleri, M., Ford, A. C., Mawe, G. M., Dinning, P. G., Rao, S. S., Chey, W. D., et al. (2017). Chronic constipation. Nat. Rev. Dis. Primers. 3, 17095. doi: 10.1038/nrdp.2017.95
Cao, P. Q., Li, X. P., Ou-Yang, J., Jiang, R. G., Huang, F. F., Wen, B. B., et al. (2021). The protective effects of yellow tea extract against loperamide-induced constipation in mice. Food Funct. 12, 5621–5636. doi: 10.1039/d0fo02969f
Cao, Y. N., Feng, L. J., Wang, B. M., Jiang, K., Li, S., Xu, X., et al. (2018). Lactobacillus acidophilus and Bifidobacterium longum supernatants upregulate the serotonin transporter expression in intestinal epithelial cells. Saudi J. Gastroenterol. 24, 59–66. doi: 10.4103/sjg.SJG_333_17
Cheng, C. W., Bian, Z. X., Zhu, L. X., Wu, J. C., and Sung, J. J. (2011). Efficacy of a Chinese herbal proprietary medicine (Hemp Seed Pill) for functional constipation. Am. J. Gastroenterol. 106, 120–129. doi: 10.1038/ajg.2010.305
Crowe, M. S., and Kinsey, S. G. (2017). MAGL inhibition modulates gastric secretion and motility following NSAID exposure in mice. Eur. J. Pharmacol. 807, 198–204. doi: 10.1016/j.ejphar.2017.05.006
Dimidi, E., Christodoulides, S., Scott, S. M., and Whelan, K. (2017). Mechanisms of action of probiotics and the gastrointestinal microbiota on gut motility and constipation. Adv. Nutrit. 8, 484–494. doi: 10.3945/an.116.014407
Ding, C., Ge, X., Zhang, X., Tian, H., Wang, H., Gu, L., et al. (2016). Efficacy of synbiotics in patients with slow transit constipation: a prospective randomized trial. Nutrients 8, 605. doi: 10.3390/nu8100605
Faussone-Pellegrini, M. S. (2006). Relationships between neurokinin receptor-expressing interstitial cells of Cajal and tachykininergic nerves in the gut. J. Cell. Mol. Med. 10, 20–32. doi: 10.1111/j.1582-4934.2006.tb00288.x
Fernández-Bañares, F. (2006). Nutritional care of the patient with constipation. Best Pract. Res. Clin. Gastroenterol. 20, 575–587. doi: 10.1016/j.bpg.2005.11.002
Fevang, J., Ovreb,ø, K., Myking, O., Grong, K., and Svanes, K. (2001). Role of endothelin in the circulatory changes associated with small bowel strangulation obstruction in pigs: effects of the endothelin receptor antagonist bosentan. J. Surg. Res. 96, 224–232. doi: 10.1006/jsre.2000.6066
Fukuda, S., Toh, H., Hase, K., Oshima, K., Nakanishi, Y., Yoshimura, K., et al. (2011). Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 469, 543–547. doi: 10.38/nature09646
Gu, Y., Qin, X., Zhou, G., Wang, C., Mu, C., Liu, X., et al. (2022). Lactobacillus rhamnosus GG supernatant promotes intestinal mucin production through regulating 5-HT4R and gut microbiota. Food Funct. 13, 12144–12155. doi: 10.1039/d2fo01900k
Heidelbaugh, J. J., Stelwagon, M., Miller, S. A., Shea, E. P., and Chey, W. D. (2015). The spectrum of constipation-predominant irritable bowel syndrome and chronic idiopathic constipation: US survey assessing symptoms, care seeking, and disease burden. Am. J. Gastroenterol. 110, 580–587. doi: 10.1038/ajg.2015.67
Hosseini, E., Grootaert, C., Verstraete, W., and Van de Wiele, T. (2011). Propionate as a health-promoting microbial metabolite in the human gut. Nutr. Rev. 69, 245–258. doi: 10.1111/j.1753-4887.2011.00388.x
Huang, J., Li, S., Wang, Q., Guan, X., Qian, L., Li, J., et al. (2020). Pediococcus pentosaceus B49 from human colostrum ameliorates constipation in mice. Food Funct. 11, 5607–5620. doi: 10.1039/d0fo00208a
Huang, J., Lin, B., Zhang, Y., Xie, Z., Zheng, Y., Wang, Q., et al. (2022). Bamboo shavings derived O-acetylated xylan alleviates loperamide-induced constipation in mice. Carbohydr. Polym. 276, 118761. doi: 10.1016/j.carbpol.2021.118761
Huang, L. S., Kong, C., Gao, R. Y., Yan, X., Yu, H. J., Wen, B., et al. (2018). Analysis of fecal microbiota in patients with functional constipation undergoing treatment with synbiotics. Eur. J. Clini. Microbiol. Infect. Dis. 37, 555–563. doi: 10.1007/s10096-017-3149-7
Huang, T., Ning, Z., Hu, D., Zhang, M., Zhao, L., Lin, C., et al. (2018). Uncovering the mechanisms of chinese herbal medicine (MaZiRenWan) for functional constipation by focused network pharmacology approach. Front. Pharmacol. 9, 270. doi: 10.3389/fphar.2018.00270
Iijima, K., Koike, T., Ara, N., Nakagawa, K., Kondo, Y., Uno, K., et al. (2015). Identification of a high-risk group for low-dose aspirin-induced gastropathy by measuring serum pepsinogen in H. pylori-infected subjects. J. Gastroenterol. 50, 305–312. doi: 10.1007/s00535-014-0976-5
Jiang, H., Dong, J., Jiang, S., Liang, Q., Zhang, Y., Liu, Z., et al. (2020). Effect of Durio zibethinus rind polysaccharide on functional constipation and intestinal microbiota in rats. Food Res. Int. 136, 109316. doi: 10.1016/j.foodres.2020.109316
Jurgoński, A., Opyd, P. M., and Fotschki, B. (2020). Effects of native or partially defatted hemp seeds on hindgut function, antioxidant status and lipid metabolism in diet-induced obese rats. J. Funct. Foods. 72, 104071. doi: 10.1016/j.jff.2020.104071
Lin, Q., Liu, M., Erhunmwunsee, F., Li, B., Mou, Y., Wang, S., et al. (2022). Chinese patent medicine shouhui tongbian capsule attenuated loperamide-induced constipation through modulating the gut microbiota in rat. J. Ethnopharmacol. 298, 115575. doi: 10.1016/j.jep.2022.115575
Liu, W., and Zhi, A. (2021). The potential of Quercetin to protect against loperamide-induced constipation in rats. Food Sci. Nutrit. 9, 3297–3307. doi: 10.1002/fsn3.2296
Malagelada, C., Nieto, A., Mendez, S., Accarino, A., Santos, J., Malagelada, J. R., et al. (2017). Effect of prucalopride on intestinal gas tolerance in patients with functional bowel disorders and constipation. J. Gastroenterol. Hepatol. 32, 1457–1462. doi: 10.1111/jgh.13733
Nicholson, J. K., Holmes, E., Kinross, J., Burcelin, R., Gibson, G., Jia, W., et al. (2012). Host-gut microbiota metabolic interactions. Science 336, 1262–1267. doi: 10.1126/science.1223813
Opyd, P. M., Jurgoński, A., Fotschki, B., and Juśkiewicz, J. (2020). Dietary hemp seeds more effectively attenuate disorders in genetically obese rats than their lipid fraction. J. Nutr. 150, 1425–1433. doi: 10.1093/jn/nxaa081
Rao, S. S., Rattanakovit, K., and Patcharatrakul, T. (2016). Diagnosis and management of chronic constipation in adults. Nat. Rev. Gastroenterol. Hepatol. 13, 295–305. doi: 10.1038/nrgastro.2016.53
Ritzhaupt, A., Wood, I. S., Ellis, A., Hosie, K. B., and Shirazi-Beechey, S. P. (1998). Identification and characterization of a monocarboxylate transporter (MCT1) in pig and human colon: its potential to transport L-lactate as well as butyrate. J. Physiol. 513, 719–732. doi: 10.1111/j.1469-7793.1998.719ba.x
Salari, N., Ghasemianrad, M., Ammari-Allahyari, M., Rasoulpoor, S., Shohaimi, S., and Mohammadi, M. (2023). Global prevalence of constipation in older adults: a systematic review and meta-analysis. Wien. Klin. Wochenschr. 135, 389–398. doi: 10.1007/s00508-023-02156-w
Suo, H., Zhao, X., Qian, Y., Li, G., Liu, Z., Xie, J., et al. (2014). Therapeutic effect of activated carbon-induced constipation mice with Lactobacillus fermentum Suo on treatment. Int. J. Mol. Sci. 15, 21875–21895. doi: 10.3390/ijms151221875
Tian, Y., Zuo, L., Guo, Q., Li, J., Hu, Z., Zhao, K., et al. (2020). Potential role of fecal microbiota in patients with constipation. Therap. Adv. Gastroenterol. 13, 1756284820968423. doi: 10.1177/1756284820968423
Wang, L., Hu, L., Xu, Q., Jiang, T., Fang, S., Wang, G., et al. (2017). Bifidobacteria exert species-specific effects on constipation in BALB/c mice. Food Funct. 8, 3587–3600. doi: 10.1039/c6fo01641c
Wang, L., Wu, F., Hong, Y., Shen, L., Zhao, L., and Lin, X. (2022). Research progress in the treatment of slow transit constipation by traditional Chinese medicine. J. Ethnopharmacol. 290, 115075. doi: 10.1016/j.jep.2022.115075
Yan, X., Tang, J., dos Santos Passos, C., Nurisso, A., Simões-Pires, C. A., Ji, M., et al. (2015). Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 63, 10611–10619. doi: 10.1021/acs.jafc.5b05282
Zhong, L. L. D., Cheng, C. W., Kun, W., Dai, L., Hu, D. D., Ning, Z. W., et al. (2019). Efficacy of MaZiRenWan, a Chinese herbal medicine, in patients with functional constipation in a randomized controlled trial. Clini. Gastroenterol. Hepatol. 17, 1303–1310.e18. doi: 10.1016/j.cgh.2018.04.005
Keywords: hemp seed, hemp seed extract, constipation, intestinal flora, short-chain fatty acids
Citation: Hua H, Yongtong W, Xufeng D, Fang L, Jing G, Fumao Z, Jie J and Lijiang J (2024) Hemp seeds attenuate loperamide-induced constipation in mice. Front. Microbiol. 15:1353015. doi: 10.3389/fmicb.2024.1353015
Received: 09 December 2023; Accepted: 19 February 2024;
Published: 04 April 2024.
Edited by:
Fengjie Sun, Georgia Gwinnett College, United StatesReviewed by:
Jun-Xia Xiao, Qingdao Agricultural University, ChinaCopyright © 2024 Hua, Yongtong, Xufeng, Fang, Jing, Fumao, Jie and Lijiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ji Lijiang, ZnN5eTAxMDQxQG5qdWNtLmVkdS5jbg==; Jiang Jie, Y2hldW5nY2hpQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.