
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CORRECTION article
Front. Microbiol. , 12 December 2023
Sec. Virology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1343080
This article is part of the Research Topic Recent highlights in the development of therapeutic antiviral strategies View all 12 articles
This article is a correction to:
Hantavirus: an overview and advancements in therapeutic approaches for infection
 Samia Afzal1
Samia Afzal1 Liaqat Ali2*
Liaqat Ali2* Anum Batool2
Anum Batool2 Momina Afzal1
Momina Afzal1 Nida Kanwal2
Nida Kanwal2 Muhammad Hassan1
Muhammad Hassan1 Muhammad Safdar1
Muhammad Safdar1 Atif Ahmad1
Atif Ahmad1 Jing Yang3*
Jing Yang3*A corrigendum on
Hantavirus: an overview and advancements in therapeutic approaches for infection
by Afzal, S., Ali, L., Batool, A., Afzal, M., Kanwal, N., Hassan, M., Safdar, M., Ahmad, A., and Yang, J. (2023). Front. Microbiol. 14:1233433. doi: 10.3389/fmicb.2023.1233433
In the published article, there were errors in Table 1, Table 3, and Table 4.
The caption for Table 1 only listed North and South America, but Table 1 contains countries from North, Central, and South America.
In Table 1, the reference for the row “Canada” was incorrectly listed as “Jonsson et al. (2010)”. The correct reference is “Warner et al. (2020)”.
In Table 1, the reference for the row “Panama” was incorrectly listed as “Martinez-Valdebenito et al. (2014)”. The correct reference is “Armién et al. (2023)”. The corrected Table 1 and its caption “Reported HTNV cases across North, Central, and South America.” appear below.
In Table 3, the reference for the row “Lactoferrin” was incorrectly listed as “Gorbunova et al. (2010) and Arikawa et al. (1989)”. The correct reference is “Murphy et al. (2000, 2001)”.
In Table 3, the reference for the row “Ribavirin” was incorrectly listed as “Schmaljohn et al. (1990) and Liang et al. (1996)”. The correct reference is “Chung et al. (2013) and Ogg et al. (2013)”.
In Table 3, the reference for the row “Favipiravir” was incorrectly listed as “Arikawa et al. (1992)”. The correct reference is “Safronetz et al. (2013)”.
In Table 3, the reference for the row “Vandenatib” was incorrectly listed as “Garrido et al. (2018)”. The correct reference is “Bird et al. (2016)”.
In Table 3, the reference for the row “ETAR” was incorrectly listed as “Golias et al. (2007)”. The correct reference is “Chung et al. (2008)”.
In Table 3, the reference for the row “Coritcosteroids” was incorrectly listed as “Mills et al. (1999) and Xu et al. (2009)”. The correct reference is “Vial et al. (2013) and Brocato and Hooper (2019)”.
In Table 3, the reference for the row “Human Immune Sera” was incorrectly listed as “Tian et al. (2021)”. The correct reference is “Vial et al. (2015)”.
In Table 3, the reference for the row “JL16 and MIB22” was incorrectly listed as “Weinberg and Arbuthnot (2010)”. The correct reference is “Garrido et al. (2018)”.
In Table 3, the reference for the row “Domain III and Stem Peptides” was incorrectly listed as “Taylor et al. (2013)”. The correct reference is “Barriga et al. (2016)”.
In Table 3, the reference for the row “CLVRNLAWC and CQATTARNC” was incorrectly listed as “Cicardi et al. (2010)”. The correct reference is “Hall et al. (2008)”.
In Table 3, the reference for the row “Incatibant” was incorrectly listed as “Avižiniene et al. (2023) and Mittler et al. (2023)”. The correct reference is “Antonen et al. (2013) and Laine et al. (2015)”.
In Table 3, the reference for the row “TNF-α” was incorrectly listed as “Brocato et al. (2012), Manigold and Vial (2014), and Vial et al. (2015)”. The correct reference is “Vilcek (1991), Sundstrom et al. (2001), and Maes et al. (2004)”.
In Table 3, the reference for the row “RANTES/IP-10/MCP-1” was incorrectly listed as “Manigold and Vial (2014), and Malley et al. (2004)”. The correct reference is “Sundstrom et al. (2001) and Glass et al. (2003)”. The corrected Table 3 and its caption “Lists some examples of potential antiviral therapies against Hantavirus.” appear below.
In Table 4, the reference for the row “Inactivated Vaccine” was incorrectly listed as “Sroga et al. (2021)”. The correct reference is “Khan et al. (2019)”.
In Table 4, the reference for the row “Virus-like Particles 1” was incorrectly listed as “Jonsson et al. (2005)”. The correct reference is “Dong et al. (2019)”.
In Table 4, the reference for the row “Virus-like Particles 2” was incorrectly listed as “Wray et al. (1985)”. The correct reference is “Dong et al. (2019)”.
In Table 4, the reference for the row “Virus-Vector Vaccines 1” was incorrectly listed as “Hopper et al. (1999)”. The correct reference is “Warner et al. (2019)”.
In Table 4, the reference for the row “Virus-Vector Vaccines 2” was incorrectly listed as “Brocato et al. (2013)”. The correct reference is “Prescott et al. (2014)”.
In Table 4, the reference for the row “Virus-Vector Vaccines 3” was incorrectly listed as “Deng et al. (2020)”. The correct reference is “Safronetz et al. (2009)”.
In Table 4, the reference for the row “Recombinant Vaccines 1” was incorrectly listed as “Hopper et al. (2014)”. The correct reference is “Geldmacher et al. (2004)”.
In Table 4, the reference for the row “Recombinant Vaccines 2” was incorrectly listed as “Ogg et al. (2013)”. The correct reference is “de Carvalho et al. (2002)”.
In Table 4, the reference for the row “Recombinant Vaccines 3” was incorrectly listed as “Ogg et al. (2013)”. The correct reference is “Maes et al. (2006)”.
In Table 4, the reference for the row “DNA Vaccines 1” was incorrectly listed as “Vial et al. (2013)”. The correct reference is “Hooper et al. (2013)”.
In Table 4, the reference for the row “DNA Vaccines 2” was incorrectly listed as “Antonen et al. (2013)”. The correct reference is “Hooper et al. (2001)”.
In Table 4, the reference for the row “DNA Vaccines 3” was incorrectly listed as “Laine et al. (2015)”. The correct reference is “Hooper et al. (2006)”.
In Table 4, the reference for the row “DNA Vaccines 4” was incorrectly listed as “Vial et al. (2013)”. The correct reference is “Hooper et al. (2013)”.
In Table 4, the reference for the row “DNA Vaccines 5” was incorrectly listed as “Fire et al. (1998)”. The correct reference is “Brocato et al. (2013)”.
In Table 4, the reference for the row “DNA Vaccines 6” was incorrectly listed as “Safronetz et al. (2013)”. The correct reference is “Jiang et al. (2017)”.
In Table 4, the reference for the row “Subunit Vaccines” was incorrectly listed as “Ye et al. (2019)”. The correct reference is “Maes et al. (2008)”. The corrected Table 4 and its caption “Describing evaluation of Hantavirus vaccines in various animal models and some vaccines currently undergoing clinical trials.” appear below.
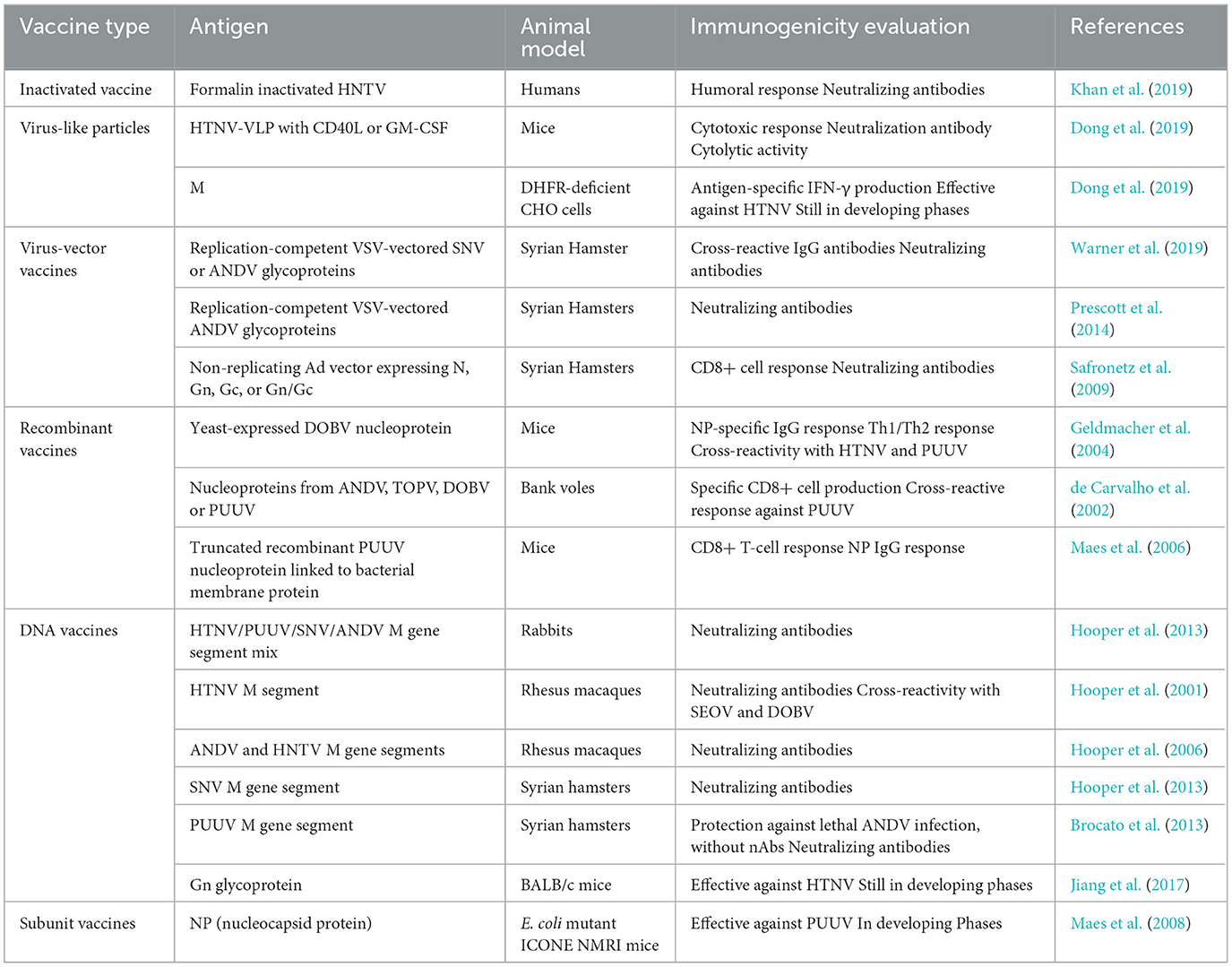
Table 4. Describing evaluation of Hantavirus vaccines in various animal models and some vaccines currently undergoing clinical trials.
The authors apologize for these errors and state that they do not change the scientific conclusions of the article in any way. The original article has been updated.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Antonen, J., Leppänen, I., Tenhunen, J., Arvola, P., Mäkelä, S., Vaheri, A., et al. (2013). A severe case of Puumala Hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Scand. J. Infect. Dis. 45, 494–496. doi: 10.3109/00365548.2012.755268
Armién, B., Muñoz, C., Cedeño, H., Salazar, J. R., Salinas, T. P., González, P., et al. (2023). Hantavirus in Panama: twenty years of epidemiological surveillance experience. Viruses 15:1395. doi: 10.3390/v15061395
Barriga, G. P., Villalón-Letelier, F., Márquez, C. L., Bignon, E. A., Acuña, R., Ross, B. H., et al. (2016). Inhibition of the Hantavirus fusion process by predicted domain III and stem peptides from glycoprotein Gc. PLoS Negl. Trop. Dis. 10:e0004799. doi: 10.1371/journal.pntd.0004799
Bird, B. H., Shrivastava-Ranjan, P., Dodd, K. A., Erickson, B. R., and Spiropoulou, C. F. (2016). Effect of Vandetanib on Andes virus survival in the hamster model of Hantavirus pulmonary syndrome. Antivir. Res. 132, 66–69. doi: 10.1016/j.antiviral.2016.05.014
Brocato, R., Josleyn, M., Wahl-Jensen, V., Schmaljohn, C., and Hooper, J. (2013). Construction and nonclinical testing of a Puumala virus synthetic M gene-based DNA vaccine. Clin. Vaccine Immunol. 20, 218–226. doi: 10.1128/CVI.00546-12
Brocato, R. L., and Hooper, J. W. (2019). Progress on the prevention and treatment of Hantavirus disease. Viruses 11:610. doi: 10.3390/v11070610
Chung, D.-H., Kumarapperuma, S. C., Sun, Y., Li, Q., Chu, Y.-K., Arterburn, J. B., et al. (2008). Synthesis of 1-β-d-ribofuranosyl-3-ethynyl-[1, 2, 4] triazole and its in vitro and in vivo efficacy against Hantavirus. Antivir. Res. 79, 19–27. doi: 10.1016/j.antiviral.2008.02.003
Chung, D.-H., Västermark, Å., Camp, J. V., McAllister, R., Remold, S. K., Chu, Y.-K., et al. (2013). The murine model for Hantaan virus-induced lethal disease shows two distinct paths in viral evolutionary trajectory with and without ribavirin treatment. J. Virol. 87, 10997–11007. doi: 10.1128/JVI.01394-13
de Carvalho, N., Gonzalez Delia Valle, M., Padula, P., Bjorling, E., Plyusnin, A., and Lundkvist, A. (2002). Cross-protection against challenge with Puumala virus after immunization with nucleocapsid proteins from different hantaviruses. J. Virol. 76, 6669–6677. doi: 10.1128/jvi.76.13.6669-6677.2002
Dong, Y., Ma, T., Zhang, X., Ying, Q., Han, M., Zhang, M., et al. (2019). Incorporation of CD40 ligand or granulocyte-macrophage colony stimulating factor into Hantaan virus (HTNV) virus-like particles significantly enhances the long-term immunity potency against HTNV infection. J. Med. Microbiol. 68, 480–492. doi: 10.1099/jmm.0.000897
Garrido, J. L., Prescott, J., Calvo, M., Bravo, F., Alvarez, R., Salas, A., et al. (2018). Two recombinant human monoclonal antibodies that protect against lethal Andes Hantavirus infection in vivo. Sci. Transl. Med. 10:eaat6420. doi: 10.1126/scitranslmed.aat6420
Geldmacher, A., Skrastina, D., Petrovskis, I., Borisova, G., Berriman, J. A., Roseman, A. M., et al. (2004). An amino-terminal segment of Hantavirus nucleocapsid protein presented on hepatitis B virus core particles induces a strong and highly cross-reactive antibody response in mice. Virology 323, 108–119. doi: 10.1016/j.virol.2004.02.022
Glass, W. G., Rosenberg, H. F., and Murphy, P. M. (2003). Chemokine regulation of inflammation during acute viral infection. Curr. Opin. Allergy Clin. Immunol. 3, 467–473. doi: 10.1097/00130832-200312000-00008
Hall, P. R., Hjelle, B., Brown, D. C., Ye, C., Bondu-Hawkins, V., Kilpatrick, K. A., et al. (2008). Multivalent presentation of antihantavirus peptides on nanoparticles enhances infection blockade. Antimicrob. Agents Chemother. 52, 2079–2088. doi: 10.1128/AAC.01415-07
Hooper, J., Custer, D., Thompson, E., and Schmaljohn, C. (2001). DNA vaccination with the Hantaan virus M gene protects hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in Rhesus monkeys. J. Virol. 75, 8469–8477. doi: 10.1128/JVI.75.18.8469-8477.2001
Hooper, J. W., Custer, D. M., Smith, J., and Wahl-Jensen, V. (2006). Hantaan/Andes virus DNA vaccine elicits a broadly cross-reactive neutralizing antibody response in nonhuman primates. Virology 347, 208–216. doi: 10.1016/j.virol.2005.11.035
Hooper, J. W., Josleyn, M., Ballantyne, J., and Brocato, R. (2013). A novel sin Nombre virus DNA vaccine and its inclusion in a candidate pan-Hantavirus vaccine against Hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS). Vaccine 31, 4314–4321. doi: 10.1016/j.vaccine.2013.07.025
Jiang, D.-B., Sun, L.-J., Cheng, L.-F., Zhang, J.-P., Xiao, S.-B., Sun, Y.-J., et al. (2017). Recombinant DNA vaccine of Hantavirus Gn and LAMP1 induced long-term immune protection in mice. Antivir. Res. 138, 32–39. doi: 10.1016/j.antiviral.2016.12.001
Khan, A., Shin, O. S., Na, J., Kim, J. K., Seong, R.-K., Park, M.-S., et al. (2019). A systems vaccinology approach reveals the mechanisms of immunogenic responses to hantavax vaccination in humans. Sci. Rep. 9:4760. doi: 10.1038/s41598-019-41205-1
Laine, O., Leppänen, I., Koskela, S., Antonen, J., Mäkelä, S., Sinisalo, M., et al. (2015). Severe Puumala virus infection in a patient with a lymphoproliferative disease treated with icatibant. Infect. Dis. 47, 107–111. doi: 10.3109/00365548.2014.969304
Maes, P., Clement, J., Cauwe, B., Bonnet, V., Keyaerts, E., Robert, A., et al. (2008). Truncated recombinant puumala virus nucleocapsid proteins protect mice against challenge in vivo. Viral Immunol. 21, 49–60. doi: 10.1089/vim.2007.0059
Maes, P., Clement, J., Gavrilovskaya, I., and Van Ranst, M. (2004). Hantaviruses: immunology, treatment, and prevention. Viral Immunol. 17, 481–497. doi: 10.1089/vim.2004.17.481
Maes, P., Keyaerts, E., Bonnet, V., Clement, J., Avsic-Zupanc, T., Robert, A., et al. (2006). Truncated recombinant Dobrava Hantavirus nucleocapsid proteins induce strong, long-lasting immune responses in mice. Intervirology 49, 253–260. doi: 10.1159/000093454
Murphy, M., Kariwa, H., Mizutani, T., Yoshimatsu, K., Arikawa, J., and Takashima, I. (2000). In vitro antiviral activity of lactoferrin and ribavirin upon Hantavirus. Arch. Virol. 145, 1571–1582. doi: 10.1007/s007050070077
Murphy, M. E., Kariwa, H., Mizutani, T., Tanabe, H., Yoshimatsu, K., Arikawa, J., et al. (2001). Characterization of in vitro and in vivo antiviral activity of lactoferrin and ribavirin upon Hantavirus. J. Vet. Med. Sci. 63, 637–645. doi: 10.1292/jvms.63.637
Ogg, M., Jonsson, C. B., Camp, J. V., and Hooper, J. W. (2013). Ribavirin protects Syrian hamsters against lethal Hantavirus pulmonary syndrome—after intranasal exposure to Andes virus. Viruses 5, 2704–2720. doi: 10.3390/v5112704
Prescott, J., DeBuysscher, B. L., Brown, K. S., and Feldmann, H. (2014). Long-term single-dose efficacy of a vesicular stomatitis virus-based Andes virus vaccine in Syrian hamsters. Viruses 6, 516–523. doi: 10.3390/v6020516
Safronetz, D., Falzarano, D., Scott, D. P., Furuta, Y., Feldmann, H., and Gowen, B. B. (2013). Antiviral efficacy of favipiravir against two prominent etiological agents of Hantavirus pulmonary syndrome. Antimicrob. Agents Chemother. 57, 4673–4680. doi: 10.1128/AAC.00886-13
Safronetz, D., Hegde, N. R., Ebihara, H., Denton, M., Kobinger, G. P., St. Jeor, S., et al. (2009). Adenovirus vectors expressing Hantavirus proteins protect hamsters against lethal challenge with Andes virus. J. Virol. 83, 7285–7295. doi: 10.1128/JVI.00373-09
Sundstrom, J. B., McMullan, L. K., Spiropoulou, C. F., Hooper, W. C., Ansari, A. A., Peters, C. J., et al. (2001). Hantavirus infection induces the expression of RANTES and IP-10 without causing increased permeability in human lung microvascular endothelial cells. J. Virol. 75, 6070–6085. doi: 10.1128/JVI.75.13.6070-6085.2001
Vial, P., Valdivieso, F., Ferres, M., Riquelme, R., Rioseco, M., Calvo, M., et al. (2013). Hantavirus study Group in Chile High-dose intravenous methylprednisolone for Hantavirus cardiopulmonary syndrome in Chile: a double-blind, randomized controlled clinical trial. Clin. Infect. Dis. 57, 943–951. doi: 10.1093/cid/cit394
Vial, P. A., Valdivieso, F., Calvo, M., Rioseco, M. L., Riquelme, R., Araneda, A., et al. (2015). A non-randomized multicentre trial of human immune plasma for treatment of Hantavirus cardiopulmonary syndrome caused by Andes virus. Antivir. Ther. 20, 377–386. doi: 10.3851/IMP2875
Vilcek, J. (1991). Tumor necrosis factor-new insights into the molecular mechanisms of its multiple actions. J. Biol. Chem. 266:7313. doi: 10.1016/S0021-9258(20)89445-9
Warner, B. M., Dowhanik, S., Audet, J., Grolla, A., Dick, D., Strong, J. E., et al. (2020). Hantavirus cardiopulmonary syndrome in Canada. Emerg. Infect. Dis. 26:3020. doi: 10.3201/eid2612.202808
Keywords: hantavirus, HFRS, HPS, immunotherapy, siRNA
Citation: Afzal S, Ali L, Batool A, Afzal M, Kanwal N, Hassan M, Safdar M, Ahmad A and Yang J (2023) Corrigendum: Hantavirus: an overview and advancements in therapeutic approaches for infection. Front. Microbiol. 14:1343080. doi: 10.3389/fmicb.2023.1343080
Received: 22 November 2023; Accepted: 23 November 2023;
Published: 12 December 2023.
Approved by:
Frontiers Editorial Office, Frontiers Media SA, SwitzerlandCopyright © 2023 Afzal, Ali, Batool, Afzal, Kanwal, Hassan, Safdar, Ahmad and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liaqat Ali, bGlhcWF0YmlvdGVjaEBnbWFpbC5jb20=; Jing Yang, V0lCUEpZYW5nQDEyNi5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.