
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 09 January 2024
Sec. Microbiotechnology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1330538
This article is part of the Research TopicFermentation of Silage in Cold and Tropical Regions: Effects of Phyllosphere MicrobiotaView all 6 articles
Whole-plant corn silage (WPCS) has been widely used as the main roughage for ruminant, which promoted the utilization of corn stover for animal feed production. However, rigid cell wall structure of corn stover limits the fiber digestion and nutrients adsorption of WPCS. This study investigated the effect of adding cellulase on improving the fermentation quality of WPCS ensiling with a Bacillus complex inoculant. With the Bacillus (BA), the lactic acid accumulation in the WPCS was significantly higher than that in control (CK). The additive cellulase (BC) increased the lactic acid content to the highest of 8.2% DW at 60 days, which was significantly higher than that in the CK and BA groups, and it reduced the neutral detergent fiber (NDF) and acid detergent fiber (ADF) contents from 42.5 to 31.7% DW and 28.4 to 20.3% DW, respectively, which were significantly lower than that in the CK and BA groups. The crude protein and starch were not obviously lost. Dynamic microbial community analysis showed that the Bacillus inoculant promoted the lactic acid bacteria (LAB) fermentation, because higher abundance of Lactobacillus as the dominant bacteria was observed in BA group. Although the addition of cellulase slowed the Lactobacillus fermentation, it increased the bacterial community, where potential lignocellulolytic microorganisms and more functional enzymes were observed, thus leading to the significant degradation of NDF and ADF. The results revealed the mechanism behind the degradation of NDF and ADF in corn stover, and also suggested the potential of cellulase for improving the nutritional quality of WPCS.
In recent years, the development of whole-plant corn silage (WPCS) on a large scale has greatly promoted the utilization of corn stover for animal feed production (Ferraretto et al., 2018; Cui et al., 2022). To produce high quality WPCS, silage inoculants are usually applied to enhance the fermentation process, thus leading to a change in palatability, inhibition of aerobic spoilage, and nutrient preservation (Muck et al., 2018). The silage inoculants have been divided into 6 categories: homofermentative lactic acid bacteria (LAB), obligate heterofermentative LAB, combination inoculants containing obligate heterofermentative LAB plus homofermentative LAB, and other inoculants, chemicals, and enzymes (Muck et al., 2018).
LAB are the most commonly used additives during fermentation to improve the ensiling process of silages (Muck et al., 2018). However, LAB are probably insufficient to improve the nutritional quality of silages and animal performance. The corn stover in WPCS has a rigid cell wall structure formed by cellulose, hemicellulose and lignin, thus limiting the fiber digestion and nutrients adsorption in ruminants in vivo (Guo et al., 2022). As reported earlier, the digestibility of WPCS in the rumen of ruminants is usually less than 60% and is even lower when the WPCS is harvested late (Raffrenato et al., 2017). To improve the nutritional value of WPCS, microbes or enzymes that can destroy the cell wall of corn stover are necessary.
Bacillus, as a kind of silage inoculant, has the potential to improve the nutritional quality of silages since it can produce a broad spectrum of lignocellulolytic enzymes, thus leading to the degradation of lignocellulose, and it also has the characteristics of strong resistance to adverse environments (Guo et al., 2022). Bacillus has also been proven to improve the fermentation quality of silages and inhibit their aerobic spoilage (Lara et al., 2015; Bai et al., 2020). In addition, as a biological control agent, it could effectively protect plants against plant pathogens (Arrebola et al., 2010; Zhao et al., 2014). It could also be used as a feed supplement directly or as a bacterial inoculant in biological feeds for monogastric animals, such as poultry and pigs (Shi et al., 2017; Zhang et al., 2018; Li et al., 2020).
Compared with microbial fermentation, exogenous cellulase can also degrade structural carbohydrates of the cell wall to soluble sugars, which are substrates for LAB fermentation (Wang et al., 2002; Colombatto et al., 2004). Thus, the interaction of cellulase with LAB to improve the quality of silages has been extensively studied, and the advantages in increasing dry matter, crude protein and lactic acid contents, and decreasing acid detergent fiber (ADF) and neutral detergent fiber (NDF) contents have been verified. The silages include fresh rice straw silage (Sarwono et al., 2021), oat silage (Xu et al., 2022), Caragana korshinskii silage (Bai et al., 2023), mulberry leaf silage (Lactobacillus) (He et al., 2019), a mixture of amaranth and rice straw (Lactobacillus plantarum) (Mu et al., 2020), and mixed silage of whole-plant corn and peanut Vines (Wang et al., 2022).
As far as we understand, cellulase is not sufficient for the effective degradation of lignocellulose, and the synergy of cellulase with other enzymes, such as xylanase and feruloyl esterase (Wang et al., 2022), or with the corresponding enzyme-producing bacteria could be more effective (Zhang et al., 2022). To date, few studies have focused on improving the fermentation quality of WPCS by cooperation of Bacillus and cellulase inoculants. We hypothesize that they are effective for the degradation of lignocellulose, decreasing the contents of neutral detergent fiber (NDF) and acid detergent fiber (ADF), thus leading to the improvement of the nutritional quality of WPCS. In this study, we investigated the effect of adding cellulase with a Bacillus complex inoculant on the degradation of NDF and ADF, the accumulation of organic acids, and the nutrient contents in WPCS. Furthermore, the bacterial community was also analyzed to provide insight into the improvement of fermentation by the Bacillus complex inoculant and cellulase.
Bacillus complex inoculant, composed of B. amyloliquefaciens and B. subtilis, was provided by Zhongbang Biotechnology Co. LTD (Baoding, China). The commercial cellulase Cellic® CTec2 (Novozymes A/S, Bagsværd, Denmark) was used as the cellulase preparation.
Whole-plant corn was harvested on the 10th of September 2021 in the area surrounding Baoding, China. The harvested whole-corn plant was sliced off and transported to the feed processing site of the Hebei Province Feed Microorganism Technology Innovation Center and then chopped to 1–2 cm in size with a forage cutter (Baoding Golden Land Ecological Engineering Co., Ltd., Baoding, China).
Bacillus silage inoculant was added at a dosage of 9 log10 CFU/kg (fresh weight) to chopped whole-plant corn, which was used as the treatment group (BA). The same amount of distilled water was added to the whole-plant corn and designated as the control group (CK). The cellulase was added with the dosage of 500 FPU/kg on the basis of the ensiling by the Bacillus silage inoculant (BC group).
Whole-plant corn of the CK, BA, and BC groups was put into 30 cm × 40 cm polyethylene plastic bags in 1.0 kg amounts, and the bags were vacuum-sealed. There were 20 silos for each treatment, stored at room temperature (25 ± 2°C), and 3 silos were opened and sampled after 2, 15, 30, and 60 days of ensiling. The WPCS taken from each treatment was divided into two portions. One portion was used to evaluate the quality of the silage by measuring the fermentation parameters including the pH, organic acids, nutrients, and the NDF and ADF contents. The other portion was stored in a −80°C refrigerator immediately and used for microbial diversity analysis.
For the pH and organic acid analyses, a total of 20 g samples were homogenized in 180 mL of distilled water, stirred for 30 min and then filtered through 4 layers of medical gauze. The pH value of the filtrate was measured using a pH meter (E-201-D, Shanghai Yidian Scientific Instrument Co., Ltd., China). The lactic acid, acetic acid, and butyric acid in the filtrate were analyzed using a high-performance liquid chromatography machine (Agilent 1260, Agilent Technologies Inc., United States). Experimental conditions: SB-AQ C18 column (4.6 mm × 250 mm); mobile phase A (methanol): mobile phase B (0.01 mol/L (NH4)2HPO4, pH = 2.70) = 3:97; flow rate was 1.0 mL/min; injection volume was 20 μL; detection wavelength was 210 nm; and column temperature was 25°C.
The dry matter (DM) contents of the WPCS were calculated based on the fresh weight and dry weight after oven-drying at 65°C for 72 h. After drying, the samples were ground through a 1 mm screen using a waring blender for nutrient analyses. The nitrogen content was determined using a Kjeldahl apparatus (K9860, Automated Analyzer, Hanon Instruments, China), and the crude protein (CP) was calculated by multiplying the nitrogen content by 6.25. The NDF and ADF were determined according to the methods using a full-automatic fiber meter (A2000i, ANKOM, United States) (Van Soest et al., 1991). The starch was determined by means of polarimetry (Polax 2 L, Atago®, Tokyo, Japan).
The lignin content was measured according to the NREL method (Sluiter et al., 2012). Materials weighing 300 mg were treated with 72% H2SO4 for 1 h at 30°C in a 100-mL triangular flask and then diluted to 4% H2SO4 with deionized water and autoclaved for 1 h at 121°C. The slurry was vacuum filtered through filtering crucibles. The filtrate and residue were used for acid soluble lignin and acid insoluble lignin determination, respectively. The total lignin content was calculated. The monosaccharide (ESC) content was analyzed with the 3,5-dinitrosalicylic acid method (Miller, 1959).
Silage samples weighing 10.0 g were put into sterilized eluent (0.9%NaCl +0.1%Tween-80) and blended at 100 rpm for 2 h, and then 4 layers of gauze were used to filter the supernatant. The precipitate was obtained by centrifugation at 5000 rpm for 15 min. The total genomic DNA of the microbes was extracted from the precipitate by using a DNA extraction kit (TIANGEN, Beijing). The quality and concentration of the DNA were detected using a Nanodrop 2000 spectrophotometer (Thermo Scientific, Wilmington, SC, United States).
The bacterial 16S rRNA genes were amplified via PCR using the primers 340F (5′-CCTACGGGNBGCASCAG-3′) and 805R (5′-GACTACNVGGGTAT CTAATCC-3′). The bacterial 16S amplicon was sequenced with a Hiseq2500 (Illumina, San Diego, CA, United States) according to a previous description (Guo et al., 2019). Quality-filter and cluster analyses were performed. The 16S rRNA sequencing data were analyzed using the online Majorbio Cloud Platform (Shanghai Majorbio Bio-Pharm Technology Co., Ltd., China).
All sequences were homologously clustered into operational taxonomic units (OTUs) based on 97% sequence similarity by using the QIIME UCluster method. To determine the species classification information corresponding to each OTU, the RDP classifier Bayesian algorithm was used for the taxonomic analysis of representative OTU sequences with 97% similarity, and they were then compared to the 16S rRNA database. The community species composition of each sample was measured at the Domain, Kingdom, Phylum, Class, Order, Family, Genus, and Species levels. The microbial diversity in the silage was analyzed according to the α diversity index, including the Shannon index, Chao1 index, Simpson index, Ace index and community coverage index. The differences in bacterial proportion between the groups at genus level were analyzed using one-way ANOVA. Bacterial function prediction was verified from the Kyoto Encyclopedia of Genes and Genomes (KEGG) database using Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt2), which predicts the functional abundance of samples based on the abundance of marker gene sequences in the sample.
The effects of the treatments on fermentation parameters, chemical composition were analyzed by means of a mixed linear model (procedure MIXED in SPSS 15.0.1, SPSS Inc., Chicago, IL). The model used was y = μ + treatment+time + treatment×time + ε, where μ is a constant. The “treatment” (i.e., BA and BC) and “time” (i.e., ensiling days) were regarded as fixed factors and ε as a random term. The significance of the difference between the treatments at different ensiling days was tested by contrasts using Bonferroni-corrected significance levels. The number of replicate samples was three.
The pH values of WPCS in both the control (CK) and treatment groups (BA and BC) started to decrease after 2 days of ensiling and then decreased rapidly after 30 days. At 60 days, the pH of the samples in the BC group decreased to 3.1, which was slightly lower than that in the CK and BA groups (Figure 1A). The decrease in pH values was due to the accumulation of organic acids, including lactic acid and acetic acid (Figures 1B,C). Butyric acid was not observed in the three groups. The percentages of lactic acid increased with the extension of ensiling time (Figure 1B). In CK, 6.4% lactic acid in dry weight (DW) was observed after 30 days, and then it increased slightly to 6.8% until 60 days. The content of lactic acid in BA was significantly higher (p ≤ 0.05) starting from 15 days than that in CK, and 7.1% was accumulated after 30 days. In the BC groups, the lactic acid content was lower than that in the CK and BA groups within 30 days, but it was significantly higher (p ≤ 0.05), reaching 8.2% after 60 days. There were no significant differences in the accumulation of acetic acid between CK and the treatment groups within 30 days, but at 60 days, the contents of acetic acid in BA and BC were slightly higher than that in CK (Figure 1C).
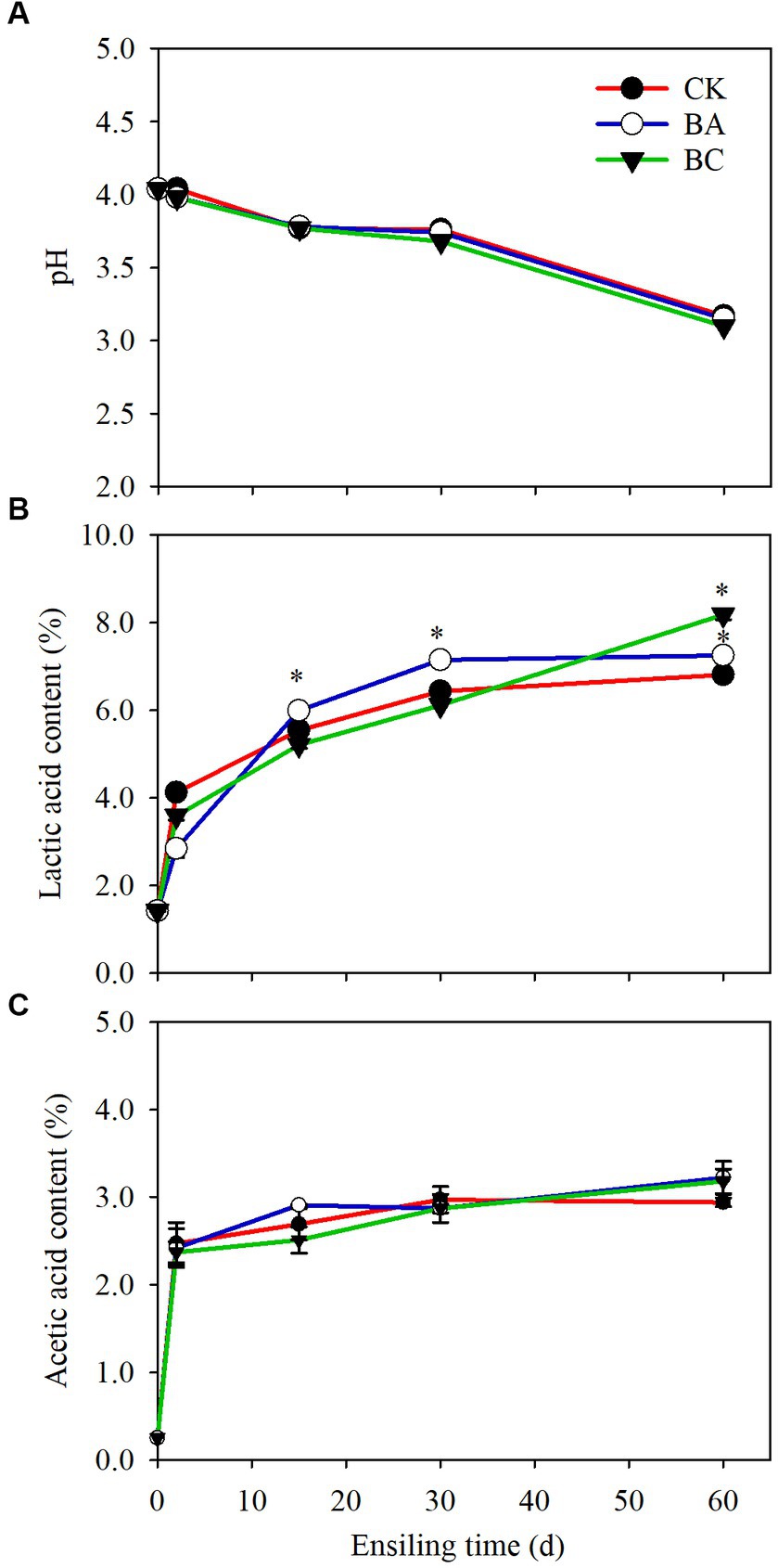
Figure 1. The pH (A), lactic acid content (B) and acetic acid content (C) in WPCS in CK, BA and BC groups during the ensiling process. The asterisks indicate the significant differences level of the samples compared to CK, *p ≤ 0.05.
The results suggested that considerable organic acids accumulated in the CK group without using the silage inoculants. As reported earlier, anaerobic environment is conducive to the fermentation of LAB that are attached to the plant surface (Bai et al., 2020). The lactic acid content in the BA group was higher than that in the CK group, indicating that the Bacillus inoculant probably helped to consume the remaining oxygen during the early stages of ensiling, thus creating a better anaerobic environment for Lactobacillus fermentation (Bai et al., 2020). Interestingly, the lactic acid content in the BC group exceeded that in the CK and BA groups after 60 days, which could be due to the effect of additive cellulase. The cellulase possibly increased the degradation of lignocellulose to the fermentable sugars for the growth of multiple bacteria, which slightly suppressed the LAB growth within 30 days, and with the time extension to 60 days, the LAB still maintained fermentation. Thus, the investigation of the microbial community could possibly explain the dynamic changes in lactic acid contents. Acetic acid can be produced by heterofermentative LAB, that improving the aerobic stability of silages (Xu et al., 2019), but also can be formed by the degradation of acetyl groups from hemicellulose (Jönsson and Martín, 2016), because the pH values in these three groups were all low, and acidic conditions led to the degradation of hemicellulose and further to acetic acid.
NDF and ADF are important indicators to evaluate the quality of roughage. It is generally acknowledged that the NDF determines DM intake, and the higher the NDF content is, the lower the intake. The ADF affects the digestibility and energy of roughage. Thus, the lower the ADF is, the better. The NDF and ADF contents in both the control and treatment groups decreased during the whole ensiling process (Figure 2). In CK group, the NDF content decreased to 38.1% DW after 60 days of ensiling. In BA group, the NDF content decreased to 35.9%. However, it is worth noting that the NDF content in the BC group decreased to 31.7%, which was significantly lower (p ≤ 0.05) than that in both the CK and BA groups (Figure 2A). There were no significantly differences in ADF contents in CK and BA groups (Figure 2B). However, in BC group, the ADF contents were significantly lower (p ≤ 0.05) than those in the CK and BA groups after 2 days of ensiling.
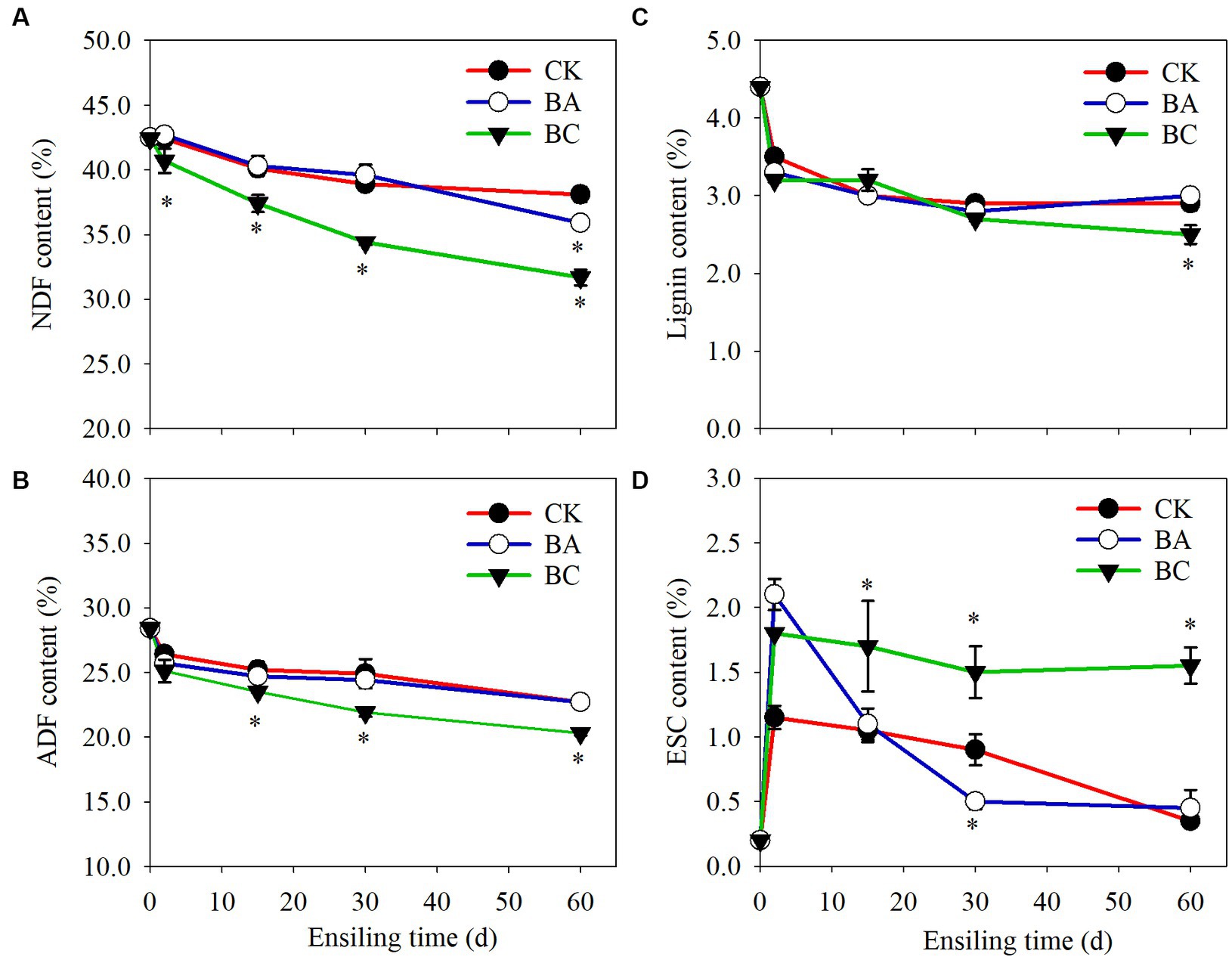
Figure 2. The contents of neutral detergent fiber (NDF) (A), acid detergent fiber (ADF) (B), lignin (C) and monosaccharide (ESC) (D) in WPCS in CK, BA, and BC groups during the ensiling process. The asterisks indicate the significant differences level of the samples compared to CK, *p ≤ 0.05.
The results indicated that the lignocellulose in corn stover degraded during the ensiling process, and the addition of the Bacillus inoculant exhibited the positive effect during the late fermentation period after 30 days, particularly on NDF removal. Significant lignocellulose degradation was observed in the BC group than that that in the BA group, indicating improvements in the fermentation quality of WPCS by the addition of cellulase. The decrease in NDF and ADF contents could be contributed to two processes, including chemical and enzymatic hydrolysis. As described earlier, the accumulated organic acids can degrade lignocellulose (Larsen et al., 2017). On the other hand, cellulase or microbes (Bacillus and other microbes) produce cellulase, hemicellulase or other enzymes such as feruloyl esterase can hydrolyze the structural carbohydrates in plants during silage fermentation, which has been widely reported (Ning et al., 2017). The further decrease in NDF content in the BA group was probably because of the function of Bacillus. In the present study, the Bacillus inoculant contained B. subtilis and B. amyloliquefaciens, which have been reported to be capable of degrading lignocellulose (Li et al., 2014, 2015; Tian et al., 2017). This is also in agreement with the study of both WPCS and alfalfa silage ensiling by Bacillus inoculation (Bai et al., 2020, 2022).
Additive cellulase promoted the greatest degradation degree of WPCS, which is in agreement with studies using cellulase as an additive inoculant (Mu et al., 2020; Bai et al., 2023). Additionally, we found that the lignin content in the CK, BA and BC groups decreased after 2 days of ensiling, and remained stable in the following process, and the lignin content in the BC group was significantly lower than that in CK and BA groups after 60 days (Figure 2C), indicating that the degradation of lignocellulose was not only contributed by cellulase but also by microbes or their synergistic effect. The monosaccharide (ESC) content in CK was maintained at a low level and that in the BA group increased after 2 days but decreased rapidly in the following days (Figure 2D). In the BC group, the ESC increased after 2 days and was maintained in the following days. It is acknowledged that ESC can be carbon source for the growth of microbes. The relatively high level of ESC in the BC group probably promoted the growth of Bacillus or other microbes.
The contents of dry matter (DM), starch and crude protein in the raw WPCS were 41.0, 33.4, and 9.2% DW, respectively. After 2 days of ensiling, the DM contents in the three groups started to decrease (Table 1), and the DM contents in BC groups were significantly lower (p ≤ 0.05) than that in CK group, which confirmed the considerable degradation of NDF and ADF in BC group. The starch contents in the three groups decreased at the early stage of ensiling (2 or 15 days), and then increased during the late stage of the ensiling (30 or 60 days), which was probably due to the relative decrease of NDF and ADF contents. There were no obvious changes in crude protein content. Generally, there were no considerable differences in the contents of such nutrients between the three groups, indicating that the addition of Bacillus and cellulase inoculant did not lead to the loss of nutrient components during the ensiling process.
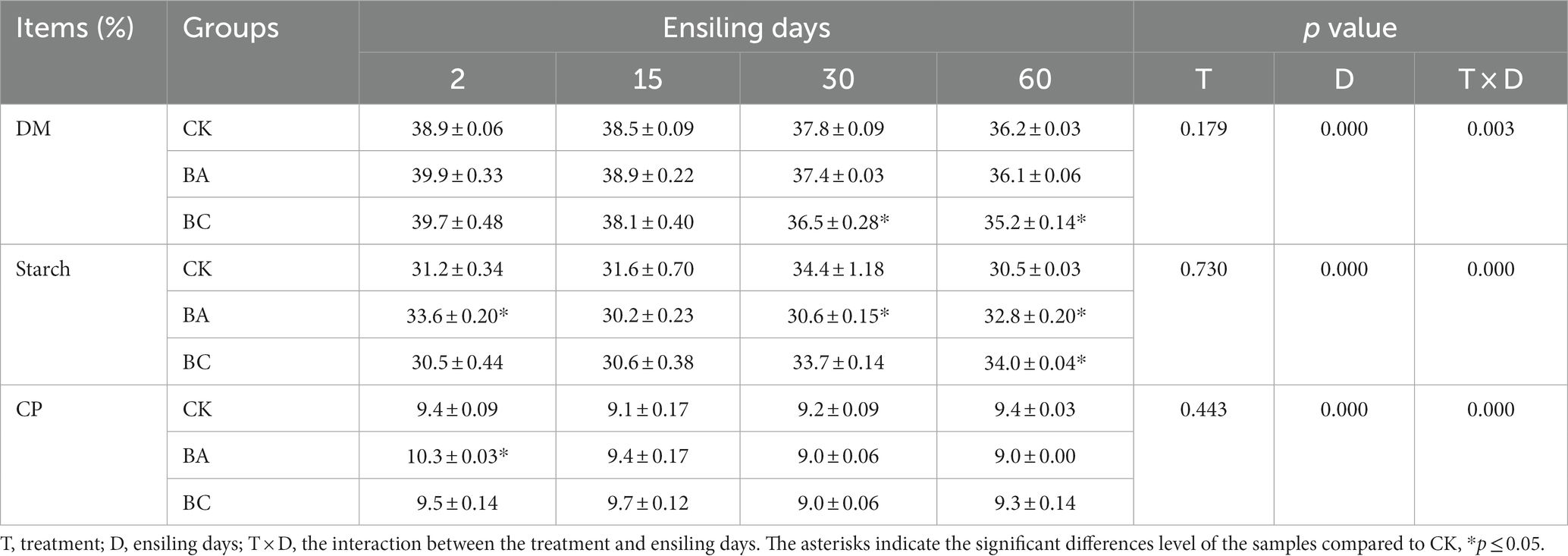
Table 1. Changes in the contents of dry matter (DM), starch and crude protein (CP) in CK, BA, and BC groups during the ensiling process.
To gain insight into the WPCS ensiling process, the dynamic changes in bacterial communities in the CK, BA and BC groups were analyzed. In total, 2,041,555 quality-filtered sequences of 16S rRNA were acquired from 36 samples and clustered into 1,051 OTUs. The Good’s coverage was approximately 0.99 in all treatments (Table 2).
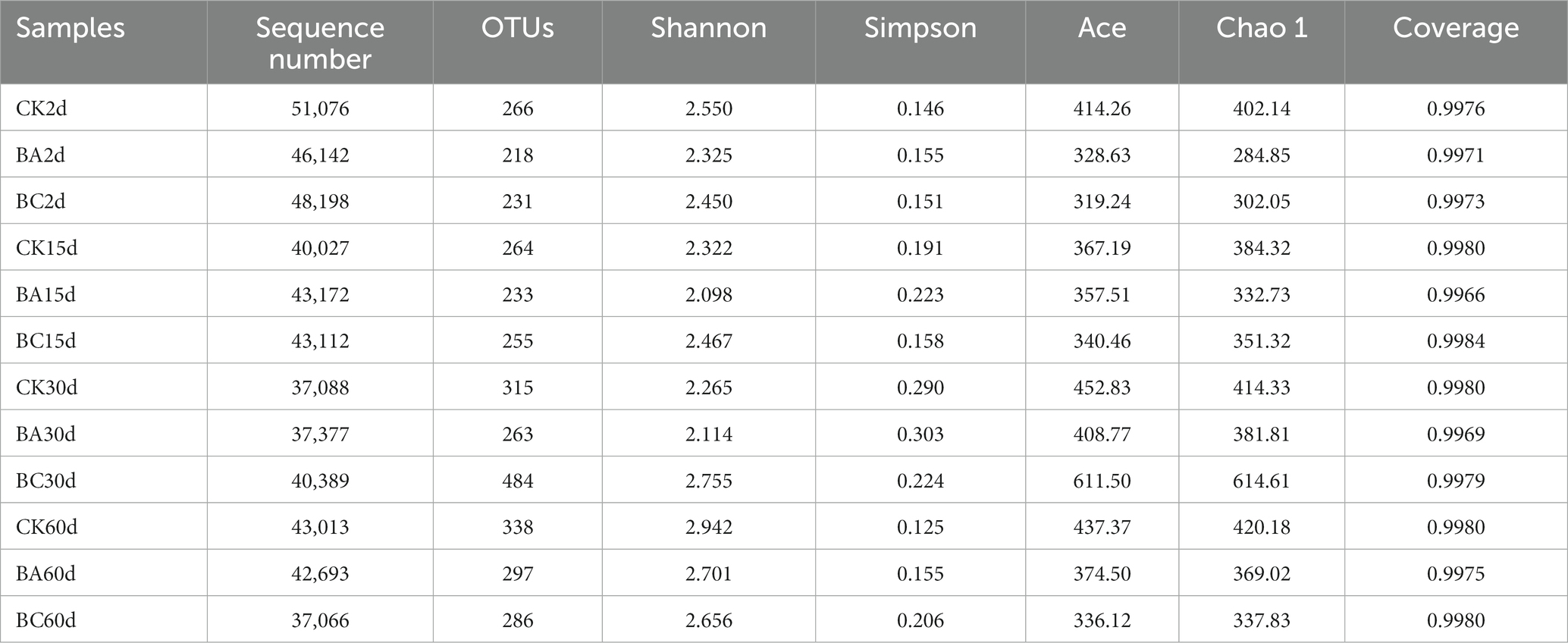
Table 2. Richness and diversity indices of microbial communities in CK, BA, and BC groups during the ensiling process.
According to Chao1 index, the alpha diversity of bacteria during the ensiling process showed no obvious differences in the abundance of the bacterial community in the WPCS after 2 and 15 days of ensiling (Figure 3). However, after 30 days, the abundance of WPCS in the BC group significantly increased, and they were returned back to a consistent level after 60 days. The similar tendency was observed in Ace and Shannon indexes, and the Simpson index showed the contrary tendency (Table 2). It has been reported that when LAB became the dominant bacteria, the alpha diversity decreased (Xu et al., 2020). It was true that the alpha diversity decreased compared to the samples without ensiling (Xu et al., 2020). However, according to our results, it seems that significantly higher alpha diversity in the BC group after 30 days of ensiling was observed. The reason could be explained by the relatively higher amount of ESC for the growth of different bacteria (Figure 2D). Thus, it is interesting to see the bacterial community of the WPCS to explain the effect of Bacillus inoculant and the additive cellulase.
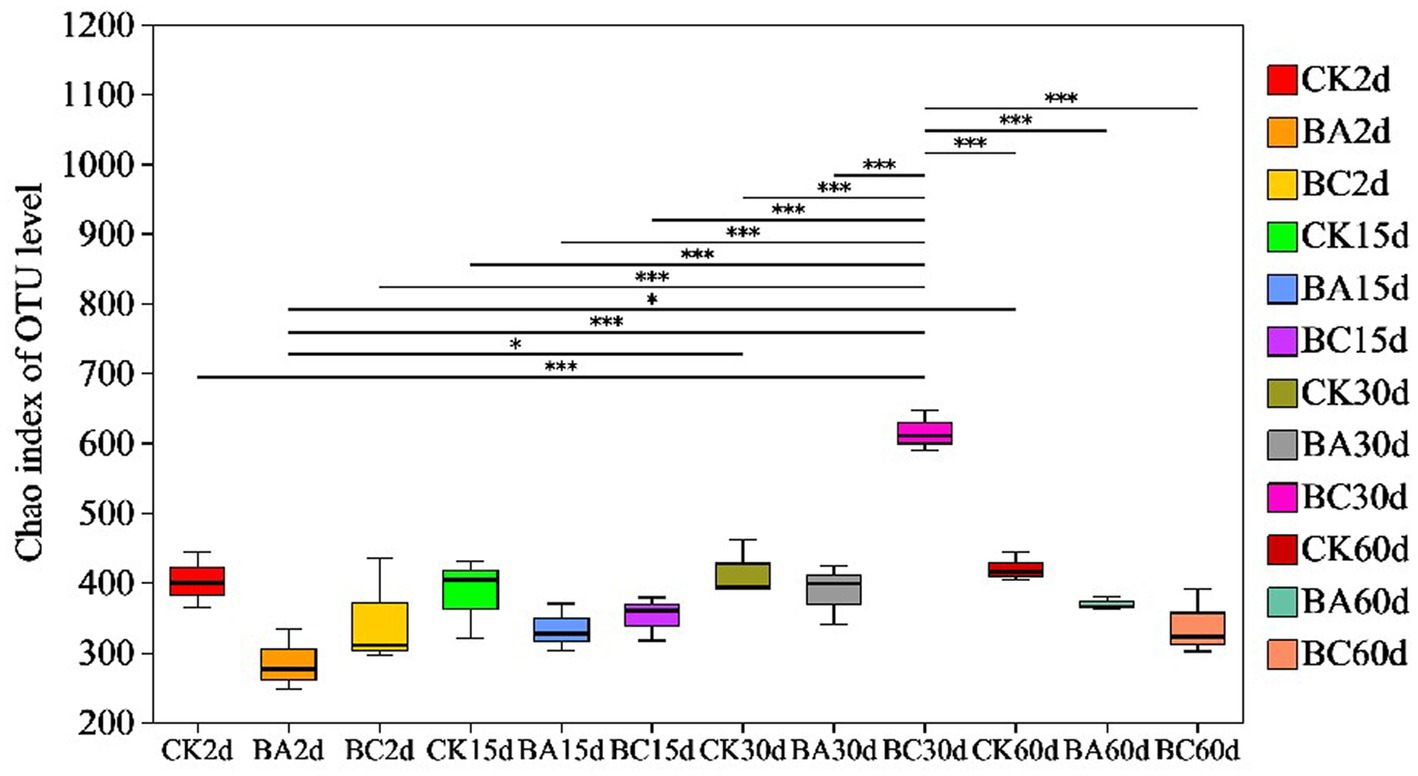
Figure 3. Differences in bacterial community diversity and richness in Chao index in CK, BA, and BC groups during the ensiling process. The asterisks indicate the significant differences level between the samples, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001.
The bacterial community dynamics during the ensiling process of the WPCS were observed (Figure 4). At the phylum level, the dominant phyla of the CK, BA and BC groups were Firmicutes, Proteobacteria, and Cyanobacteria (Figure 4A). Among them, Firmicutes was the most predominant phylum. Basically, the abundance in the CK group was higher than that in the BA and BC groups after 2 days. However, after 15 days until 30 days, it was higher in the BA group than that in the CK and BC groups. After 60 days, they were at similar levels. During the whole ensiling process, the abundance of Proteobacteria gradually increased, and the abundance of Cyanobacteria decreased in the three groups. Additionally, Bacteroidota and Actionobacteriota were also annotated, and their abundances in BC30d and CK60d were relatively higher than those in the other groups.
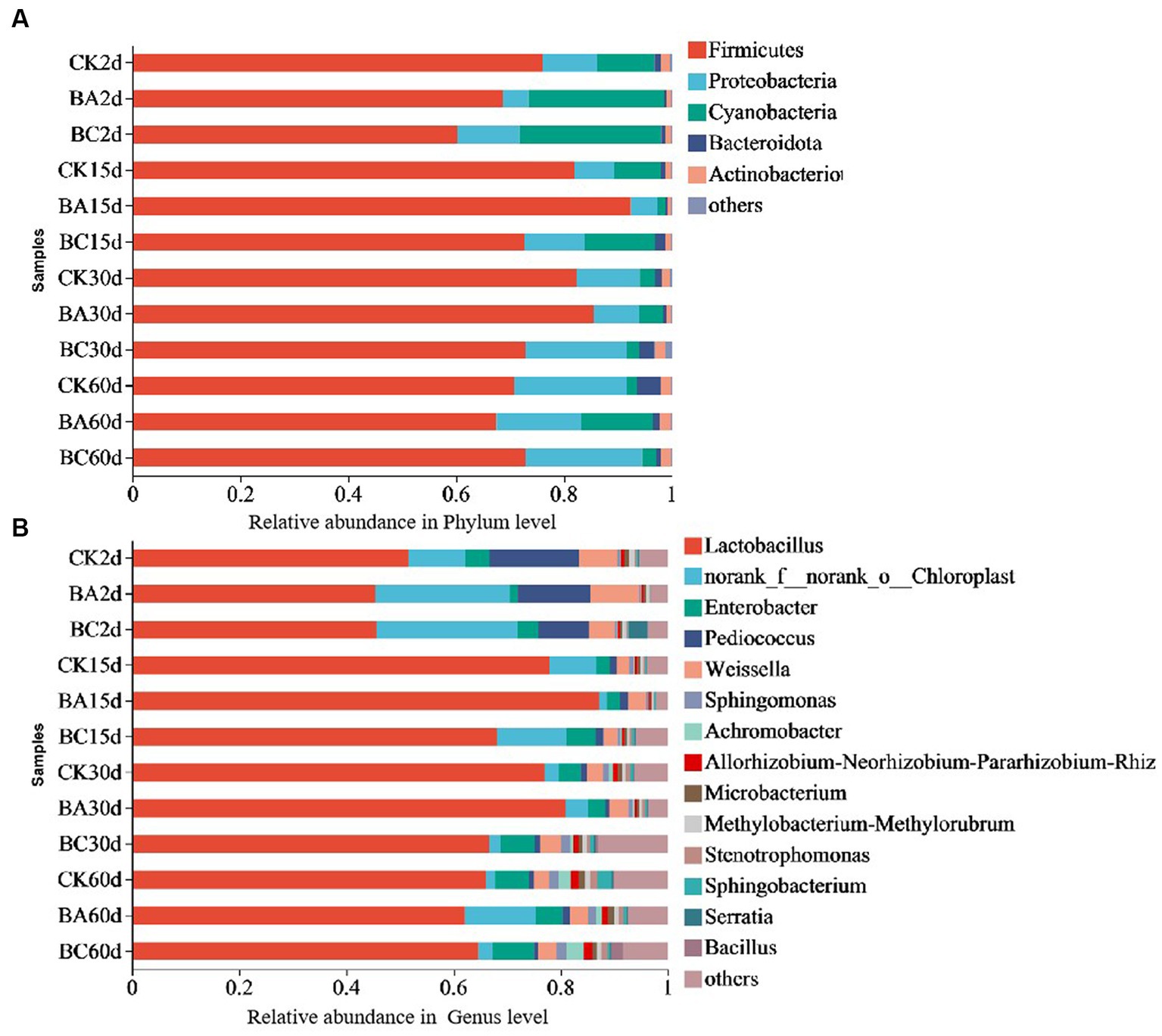
Figure 4. Relative bacteria abundance at phylum (A) and genus (B) levels in CK, BA and BC groups during the ensiling process.
The compositions of bacterial communities in the WPCS at the genus level were identified (Figure 4B). The dominant genus in all samples was Lactobacillus, followed by Enterobacter, Pediococcus, and Weissella. Similar to Firmicutes, the abundance of Lactobacillus increased after 15 days of ensiling, and it was higher in the BA group until 30 days. After 60 days, the levels were similar in the three groups. It is difficult to find a clear pattern of change in the abundances of Enterobacte, Pediococcus, and Weissella in the three groups during the whole ensiling process. The relative abundance of Bacillus was very low in the samples at 2 and 15 days. However, in the BC group, it started to increase after 30 days and to a certain level at 60 days.
The results indicated that Firmicutes became the most predominant phylum as the silage process progressed, and the most effective genus was Lactobacillus, which is consistent with the studies of alfalfa silage fermentation (Yang et al., 2019; Bai et al., 2020). This verified the reason for the increase in the lactic acid content and decrease in the pH values (Guan et al., 2018; Yang et al., 2019) The Lactobacillus abundance increased in the BA group after 15 and 30 days of ensiling, indicating the positive effect of the Bacillus inoculant on creating a better anaerobic environment for Lactobacillus fermentation. This could also explain the higher lactic acid content in BA group than the other groups (Figure 1A). The addition of cellulase did not increase Lactobacillus abundance; however, it increased the growth of Bacillus and other bacteria after 30 days of ensiling, which would be helpful for the degradation of lignocellulose. Considering the effect of Bacillus on lignocellulose degradation, the increased abundance of Bacillus probably played a role in improving silage quality from the point of view of lignocellulose degradation (Guo et al., 2022).
The differences in bacterial proportion among the three groups at genus level were analyzed using one-way ANOVA (Figure 5). After 30 days of ensiling, the proportion of Lactobacillus in the BA group was significantly higher than that in the CK group, and it was much lower in the BC group (Figure 5A). However, the proportions of Enterbacter, Weissella, Sphingomonas, Sphingobacterium, and Chryseobacterium in the BC group were higher than those in the other two groups. In addition, bacteria, including Pseudomonas, Massilia, and Pseudofulvimonas, were also significantly higher in the BC group. After 60 days of ensiling, the abundance of Bacillus, Pseudomonas, and Paenibacillus in the BC group were significantly higher than those in the other two groups (Figure 5B). The proportions of Sphingobacterium, Acinetobacter, Roseomonas, and Flavobacterium in the CK group were relatively higher. It is worth noting that the proportion of Bacillus in the BA group was significantly higher than that in CK but lower than in that in the BC group.
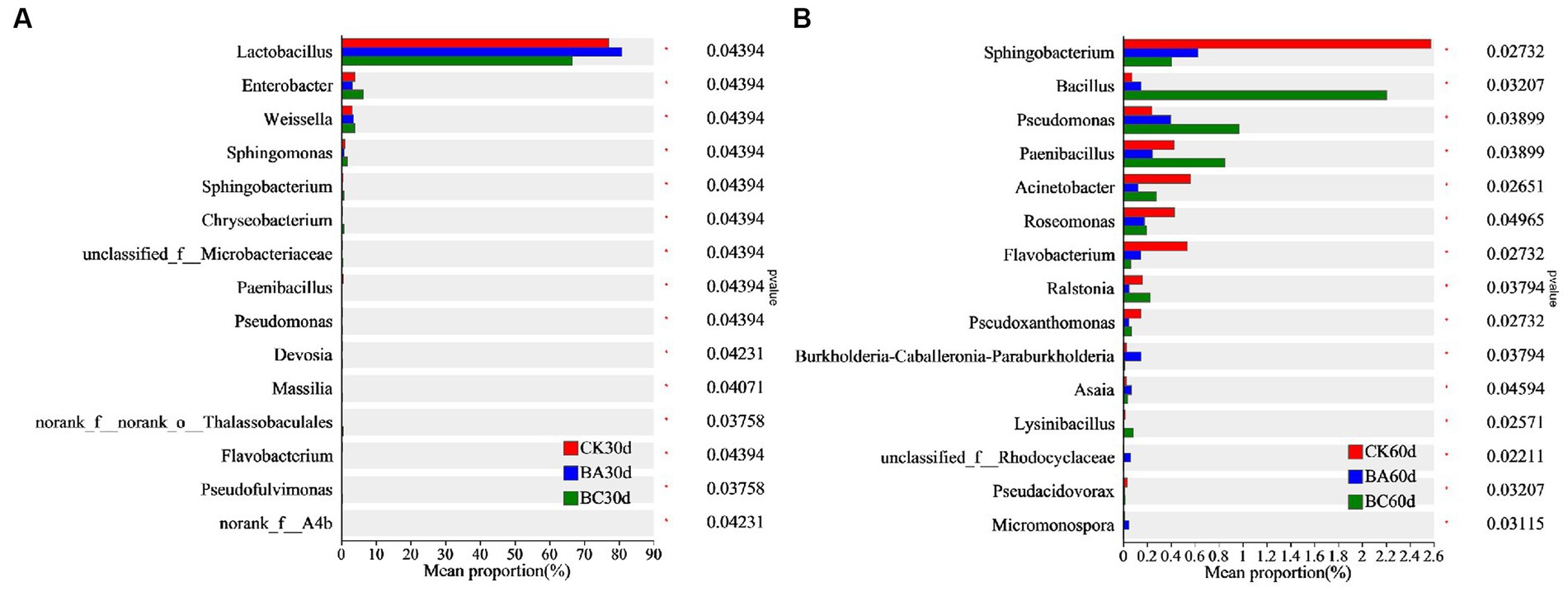
Figure 5. Comparison of different bacteria among the CK, BA, and BC groups after 30 days (A) and 60 days (B) ensiling. The asterisks indicate the significant differences level of between the samples, *p ≤ 0.05.
The results showed that in addition to LAB, more other bacterial genera were observed in the BC group after 30 and 60 days of ensiling, indicating the promotion effect of additive cellulase on bacterial growth. In these bacterial genera, some bacteria have been reported to be capable of degrading lignocellulose. Enterobacter sp. SUK-Bio produced cellulolytic and hemicellulolytic enzymes, β-glucosidase, endoglucanase, exoglucanase, xylanase and glucoamylase by using sorghum husk as a substrate, thus leading to a reducing sugar production rate of 3.84 mg/h/L (Pankajkumar et al., 2018). Sphingobacterium athyrii sp. nov. isolated from a decaying fern (Athyrium wallichianum Ching) was also observed as gram-stain-negative, rod-shaped, nonmotile and aerobic with cellulose and xylan degradation abilities (Cheng et al., 2019). Chryseobacterium culicis Bp16 isolated from the Bandipur forest area, Karnataka, has also been reported as a potent cellulase producer (Kumar et al., 2023). The dominant bacterial in CK, such as Paenibacillus, has also been reported to produce glucanases, chitinases, cellulases, and proteases that are implicated in the destruction of eukaryotic cell walls (Grady et al., 2016).
The results confirmed that the Bacillus inoculant promoted Lactobacillus fermentation within 30 days of ensiling, which is why the lactic acid content was generally higher in the BA group. However, there were no significant differences in Bacillus proportions in the three groups at 30 days, indicating the slow growth of added Bacillus at the beginning of the ensiling process. Interestingly, at 60 days, the proportion of Bacillus in the BA group was significantly higher than that in the CK, which was probably related to the significant decrease of in NDF in the BA group at 60 days because Bacillus has the ability to degrade lignocellulose. In the BC group, the proportion of not only Bacillus but also the other bacteria were highest, and the synergy of these bacteria with cellulase possibly contributed to the higher NDF and ADF removal in the BC group.
The enzymes of the bacterial communities of the three groups at different ensiling stages were predicted by PICRUSt2 software (Figure 6). The cellulase (EC:3.2.1.4), endo-1,4-beta-xylanase (EC:3.2.1.37), feruloyl esterase (EC:3.1.1.73), and α-amylase (EC:3.2.1.1) that are closely related to silage fermentation were chosen in the enzyme classification (EC) database. Overall, the abundances of cellulase, xylanase, and feruloyl esterase, which are regarded as the functional enzymes for lignocellulose degradation, decreased at 15 days of ensiling compared to those at 2 days (Figures 6A–C). However, their abundances increased afterward until 60 days. Basically, within 30 days, the abundances in the BC group were higher than those in the CK group, followed by the BA group. Interestingly, at 60 days, their abundances in the CK group exceeded the BC group. The abundances in BA groups consistently lower than the other two groups. The abundance of α-amylase, which is related to the starch degradation, were higher in BC groups at 2 days than in the CK and BA groups, and they all decreased in the following days of ensiling (Figure 6D).
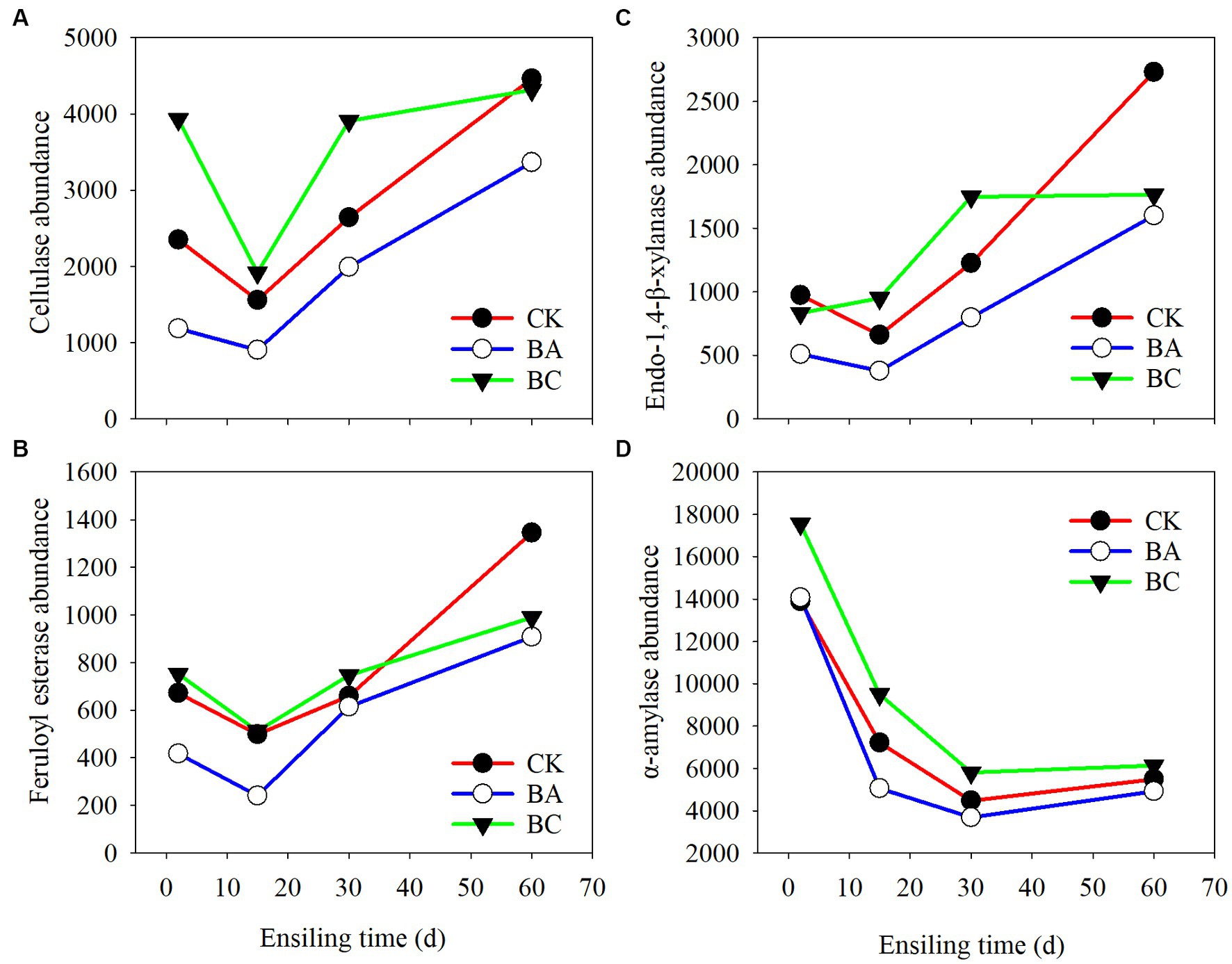
Figure 6. Bacterial alterations that contribute to abundance of enzyme shifts during the ensiling process. (A) cellulase; (B) endo-1,4-beta-xylanase; (C) feruloyl esterase; (D) α-amylase.
The lignocellulose-degrading enzymes with high abundances in the three groups during the first 2 days of the ensiling probably acted on the decrease in NDF and ADF contents (Figure 2). The decrease in the abundances of these enzymes at 15 days was probably due to the significant increase in the abundance of Lactobacillus. As reported earlier, the dominance of LAB decreased alpha diversity (Xu et al., 2020). The enzyme abundances increased in the following days, which was due to the decrease in Lactobacillus and increase in Bacillus and other bacteria. However, in CK, no Bacillus was added as an inoculant; thus other bacteria, such as the observed Paenibacillus, could probably contributed to the increased enzyme activities. Correspondingly, there were more different bacteria in the BC group, which resulted in relatively higher enzyme activities for the degradation of NDF and ADF (Figure 5A). At 60 days, the abundances in CK increased rapidly to the highest, which could be due to the growth of bacteria, such as Sphingobacterium (Figure 5B).
The a-amylase during the first 2 days could probably act on starch degradation, as decreased starch contents were determined in the three groups. However, the abundances decreased in the following days, which can be explained by the increased abundances of bacteria for lignocellulose degradation. Of course, the chemical process for the degradation of NDF and ADF or starch cannot be ruled out because acidic conditions can also degrade carbohydrates (Larsen et al., 2017). As reported in an earlier study, the decrease in fiber content in Bacillus subtilis inoculated-silage might not be related to the abundance of cellulase (Bai et al., 2022).
Overall, it seems that there was a dynamic balance in the abundance of Lactobacillus and other bacteria, including the lignocellulolytic bacteria. The higher the abundance of Lactobacillus was, the lower abundance of the other bacteria. The Bacillus inoculant promoted the fast growth of Lactobacillus and suppressed the growth of other bacteria, leading to the rapid accumulation of lactic acid and little effect on NDF and ADF. Interestingly, the addition of cellulase helped the growth of lignocellulolytic bacteria, leading to the removal of NAD and ADF; and meanwhile, the degradation of NDF and ADF to ESC maintained the Lactobacillus fermentation until 60 days, leading to the significant accumulation of Lactic acid.
The Bacillus inoculant promoted fast lactic acid accumulation during the ensiling process of the WPCS. Although the addition of cellulase slowed lactic acid accumulation, it increased to highest level late. More importantly, cellulase significantly increased the degradation of NDF and ADF. The dynamic microbial community analysis suggested that the Bacillus inoculant seemed to have little effect on lignocellulose degradation. However, the addition of cellulase promoted microbial diversity, in which some potential lignocellulolytic microorganisms were observed, thus leading to more degradation of NDF and ADF. The results suggested that the addition of cellulase has potential to improve the nutritional quality of WPCS.
The data of 16S RNA sequences presented in this study are deposited in NCBI Bioproject repository, accession number PRJNA1035803.
XL: Data curation, Formal analysis, Investigation, Writing – review & editing. AW: Data curation, Software, Validation, Writing – review & editing. LZ: Formal analysis, Resources, Writing – review & editing. WG: Methodology, Visualization, Writing – review & editing. XG: Conceptualization, Writing – review & editing. BZ: Supervision, Writing – review & editing. MY: Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Key Research and Development Program in Hebei Province (21322917D), the Hebei Natural Science Foundation (B2021204034), the Central Government Guides Local Funds for Science and Technology Development (226Z2904G), and Start-up Fund for Introduced Talents of Hebei Agricultural University (YJ201910).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Arrebola, E., Jacobs, R., and Korsten, L. (2010). Iturin a is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J. Appl. Microbiol. 108, 386–395. doi: 10.1111/j.1365-2672.2009.04438.x
Bai, B., Qiu, R., Wang, Z., Liu, Y., Bao, J., and Sun, L. (2023). Effects of cellulase and lactic acid bacteria on ensiling performance and bacterial community of Caragana korshinskii silage. Microorganisms 11:337. doi: 10.3390/microorganisms11020337
Bai, J., Xu, D. M., Xie, D. M., Wang, M., Li, Z. Q., and Guo, X. S. (2020). Effects of antibacterial peptide-producing Bacillus subtilis and Lactobacillus buchneri on fermentation, aerobic stability, and microbial community of alfalfa silage. Bioresour. Technol. 315:123881. doi: 10.1016/j.biortech.2020.123881
Bai, J., Franco, M., Ding, Z. T., Hao, L., Ke, W. C., and Wang, M. S. (2022). Effect of Bacillus amyloliquefaciens and Bacillus subtilis on fermentation, dynamics of bacterial community and their functional shifts of whole-plant corn silage. J. Anim. Sci. Biotechnol. 13:7. doi: 10.1186/s40104-021-00649-0
Cheng, J. F., Guo, J. X., Bian, Y. N., Chen, Z. L., Li, C. L., and Li, X. D. (2019). Sphingobacterium athyrii sp. nov., a cellulose- and xylan-degrading bacterium isolated from a decaying fern (Athyrium wallichianum Ching). Int. J. Syst. Evol. Microbiol. 69, 752–760. doi: 10.1099/ijsem.0.003231
Colombatto, D., Mould, F. L., Bhat, M. K., Phipps, R. H., and Owen, E. (2004). In vitro evaluation of fibrolytic enzymes as additives for maize (Zea mays L.) silage: II. Effects on rate of acidification, fibre degradation during ensiling and rumen fermentation. Anim. Feed Sci. Technol. 111, 129–143. doi: 10.1016/j.anifeedsci.2003.08.011
Cui, Y. L., Liu, H., Gao, Z. M., Xu, J. Y., Liu, B. S., Guo, M., et al. (2022). Whole-plant corn silage improves rumen fermentation and growth performance of beef cattle by altering rumen microbiota. Appl. Microbiol. Biotechnol. 106, 4187–4198. doi: 10.1007/s00253-022-11956-5
Ferraretto, L. F., Shaver, R. D., and Luck, B. D. (2018). Silage review: recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 101, 3937–3951. doi: 10.3168/jds.2017-13728
Grady, E. N., MacDonald, J., Liu, L., Richman, A., and Yuan, Z. C. (2016). Current knowledge and perspectives of Paenibacillus: a review. Microb. Cell Factories 15:203. doi: 10.1186/s12934-016-0603-7
Guan, H., Yan, Y. H., Li, X. L., Li, X. M., Shuai, Y., and Feng, G. Y. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. doi: 10.1016/j.biortech.2018.06.018
Guo, W., Guo, X. J., Zhu, B. C., Guo, Y. Y., and Zhou, X. (2019). In situ degradation, ruminal fermentation, and the rumen bacterial community of cattles fed corn Stover fermented by lignocellulolytic microorganisms. Anim. Feed Sci. Technol. 248, 10–19. doi: 10.1016/j.anifeedsci.2018.07.007
Guo, X., Guo, W., Yang, M., Sun, Y., Wang, Y., Yan, Y., et al. (2022). Effect of Bacillus additives on fermentation quality and bacterial community during the ensiling process of whole-plant corn silage. PRO 10:978. doi: 10.3390/pr10050978
He, L. W., Zhou, W., Wang, C., Yang, F. Y., Chen, X. Y., and Zhang, Q. (2019). Effect of cellulase and Lactobacillus casei on ensiling characteristics, chemical composition, antioxidant activity, and digestibility of mulberry leaf silage. J. Dairy Sci. 102, 9919–9931. doi: 10.3168/jds.2019-16468
Jönsson, L. J., and Martín, C. (2016). Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 199, 103–112. doi: 10.1016/j.biortech.2015.10.009
Kumar, H. K. N., Mohana, N. C., Rakshith, D., Abhilash, M. R., Harini, B. P., and Satish, S. (2023). Bioprocessing of cellulosic waste biomass for ethanol production by Chryseobacterium culicis Bp16. Sustain. Chem. Pharm. 33:101081. doi: 10.1016/j.scp.2023.101081
Lara, E. C., Basso, F. C., De Assis, F. B., Souza, F. A., Berchielli, T. T., and Reis, R. A. (2015). Changes in the nutritive value and aerobic stability of corn silages inoculated with Bacillus subtilis alone or combined with Lactobacillus plantarum. Anim. Prod. Sci. 56:1867. doi: 10.1071/AN14686
Larsen, S. U., Hjort-Gregersen, K., Vazifehkhoran, A. H., and Triolo, J. M. (2017). Co-ensiling of straw with sugar beet leaves increases the methane yield from straw. Bioresour. Technol. 245, 106–115. doi: 10.1016/j.biortech.2017.08.117
Li, H., Li, S., Wang, S., Wang, Q., and Zhu, B. (2014). Screening, identification of lignin-degradating Bacillus MN-8 and its characteristics in degradation of maize straw lignin. Sci. Agric. Sin. 47, 324–333. (In Chinese). doi: 10.3864/j.issn.0578-1752.2014.02.012
Li, H., Li, S., Wang, S., Wang, Q., Xue, Y., and Zhu, B. (2015). Degradation of lignocellulose in the corn straw by Bacillus amyloliquefaciens MN-8. Chin. J. Appl. Ecol. 26, 1404–1410. (In Chinese). doi: 10.13287/j.1001-9332.20150302.013
Li, Y., Guo, B. Z., Wu, Z. K., Wang, W. W., Li, C., and Liu, G. H. (2020). Effects of fermented soybean meal supplementation on the growth performance and cecal microbiota community of broiler chickens. Animals 10:1098. doi: 10.3390/ani10061098
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31, 426–428. doi: 10.1021/ac60147a030
Mu, L., Xie, Z., Hu, L., Chen, G., and Zhang, Z. (2020). Cellulase interacts with Lactobacillus plantarum to affect chemical composition, bacterial communities, and aerobic stability in mixed silage of high-moisture amaranth and rice straw. Bioresour. Technol. 315:123772. doi: 10.1016/j.biortech.2020.123772
Muck, R. E., Nadeau, E., Mcallister, T. A., Contreras-Govea, F. E., and Kung, L. J. (2018). Silage review: recent advances and future uses of silage additives. J. Dairy Sci. 101, 3980–4000. doi: 10.3168/jds.2017-13839
Ning, T., Wang, H., Zheng, M., Niu, D., Zuo, S., and Xu, C. (2017). Effects of microbial enzymes on starch and hemicellulose degradation in total mixed ration silages. Asian Aust. J. Anim. Sci. 30, 171–180. doi: 10.5731/ajas.16.0046
Pankajkumar, R., Waghmare, S. M., Patil, S. L., Jadhav, B. J., and Govindwar, S. P. (2018). Utilization of agricultural waste biomass by cellulolytic isolate Enterobacter sp. SUK-Bio. Agricult. Nat. Resour. 52, 399–406. doi: 10.1016/j.anres.2018.10.019
Raffrenato, E., Fievisohn, R., Cotanch, K. W., Grant, R. J., Chase, L. E., and Van Amburgh, M. E. (2017). Effect of lignin linkages with other plant cell wall components on in vitro and in vivo neutral detergent fiber digestibility and rate of digestion of grass forages. J. Dairy Sci. 100, 8119–8131. doi: 10.3168/jds.2016-12364
Sarwono, K. A., Rohmatussolihat, R., Watman, M., Ratnakomala, S., Astuti, D. W., Fidriyanto, R., et al. (2021). Characteristics of fresh rice straw silage quality prepared with addition of lactic acid bacteria and crude cellulase. AIMS Agricult. Food 7, 481–499. doi: 10.3934/agrfood.2022030
Shi, C., Zhang, Y., Yin, Y., Wang, C., Lu, Z., and Wang, F. (2017). Amino acid and phosphorus digestibility of fermented corn–soybean meal mixed feed with and fed to pigs. J. Anim. Sci. 95, 3996–4004. doi: 10.2527/jas2017.1516
Sluiter, A., Hames, B., Ruiz, R., Scarlata, C., Sluiter, J., and Templeton, D. (2012). Determination of structural carbohydrates and lignin in biomass. Laboratory analytical procedure of National Renewable Energy Laboratory (NREL), Golden, Colorado.
Tian, X. Y., Song, F. P., Zhang, J., Liu, R. M., Zhang, X. P., and Duan, J. Y. (2017). Diversity of gut bacteria in larval Protaetia brevitarsis (Coleoptera:Scarabaedia) fed on corn stalk. Acta Entomol. Sin. 60, 632–641. doi: 10.16380/j.kcxb.2017.06.003
Van Soest, P. J., Robertson, J. B., and Lewis, B. A. (1991). Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74, 3583–3597. doi: 10.3168/jds.S0022-0302(91)78551-2
Wang, H., Qi, X., Gao, S., Zhang, Y., and An, Y. (2022). Biochemical characterization of an engineered bifunctional xylanase/feruloyl esterase and its synergistic effects with cellulase on lignocellulose hydrolysis. Bioresour. Technol. 355:127244. doi: 10.1016/j.biortech.2022.127244
Wang, Q. D., Wang, R. X., Wang, C. Y., Dong, W. D., Zhang, Z. G., and Zhao, L. P. (2022). Effects of cellulase and Lactobacillus plantarum on fermentation quality, chemical composition, and microbial community of mixed silage of whole-plant corn and peanut vines. Appl. Biochem. Biotechnol. A Enzyme Eng. Biotechnol. 194, 2465–2480. doi: 10.1007/s12010-022-03821-y
Wang, Y. X., McAllister, T., Rode, L., Beauchemin, K., Morgavi, D., and Nsereko, V. (2002). Effects of exogenous fibrolytic enzymes on epiphytic microbial populations and in vitro digestion of silage. J. Sci. Food Agric. 82, 760–768. doi: 10.1002/jsfa.1103
Xu, D. M., Wang, N., Rinne, M., Ke, W. C., Weinberg, Z. G., and Da, M. (2020). The bacterial community and metabolome dynamics and their interactions modulate fermentation process of whole crop corn silage prepared with or without inoculants. Microb. Biotechnol. 14, 561–576. doi: 10.1111/1751-7915.13623
Xu, J. Y., Zhang, K. Y., Lin, Y. F., Li, M. X., Wang, X. K., and Yu, Q. (2022). Effect of cellulase and lactic acid bacteria on the fermentation quality, carbohydrate conversion, and microbial community of ensiling oat with different moisture contents. Front. Microbiol. 13:1013258. doi: 10.3389/fmicb.2022.1013258
Xu, S. W., Yang, J. L., Meng, Q., Smiley, B., Rutherford, W., and Wang, Y. X. (2019). Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage. J. Anim. Sci. 97, 1273–1285. doi: 10.1093/jas/skz021
Yang, L. L., Yuan, X. J., Li, J. F., Dong, Z. H., and Shao, T. (2019). Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 275, 280–287. doi: 10.1016/j.biortech.2018.12.067
Zhang, X., Ke, W., Ding, Z., Xu, D., Wang, M., and Chen, M. (2022). Microbial mechanisms of using feruloyl esterase-producing Lactobacillus plantarum A1 and grape pomace to improve fermentation quality and mitigate ruminal methane emission of ensiled alfalfa for cleaner animal production. J. Environ. Manag. 308:114637. doi: 10.1016/j.jenvman.2022.114637
Zhang, Y., Shi, C. Y., Wang, C., Lu, Z. Q., Wang, F. Q., and Feng, J. (2018). Effect of soybean meal fermented with Bacillus subtilis BS12 on growth performance and small intestinal immune status of piglets. Food Agric. Immunol. 29, 133–146. doi: 10.1080/09540105.2017.1360258
Keywords: whole-plant corn silage, Bacillus inoculant, cellulase, fermentation, microbial community
Citation: Liu X, Wang A, Zhu L, Guo W, Guo X, Zhu B and Yang M (2024) Effect of additive cellulase on fermentation quality of whole-plant corn silage ensiling by a Bacillus inoculant and dynamic microbial community analysis. Front. Microbiol. 14:1330538. doi: 10.3389/fmicb.2023.1330538
Received: 31 October 2023; Accepted: 18 December 2023;
Published: 09 January 2024.
Edited by:
Zhihao Dong, Nanjing Agricultural University, ChinaReviewed by:
Ping Li, Guizhou University, ChinaCopyright © 2024 Liu, Wang, Zhu, Guo, Guo, Zhu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ming Yang, c2hteW1AaGViYXUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.