
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Microbiol. , 05 January 2024
Sec. Virology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1321531
This article is part of the Research Topic Viral Interactions with the Nucleus, Volume II View all 6 articles
Human Rhinoviruses (RV) are a major cause of common colds and infections in early childhood and can lead to subsequent development of asthma via an as yet unknown mechanism. Asthma is a chronic inflammatory pulmonary disease characterized by significant airway remodeling. A key component of airway remodeling is the transdifferentiation of airway epithelial and fibroblast cells into cells with a more contractile phenotype. Interestingly, transforming growth factor-beta (TGF-β), a well characterized inducer of transdifferentiation, is significantly higher in airways of asthmatics compared to non-asthmatics. RV infection induces TGF-β signaling, at the same time nucleoporins (Nups), including Nup153, are cleaved by RV proteases disrupting nucleocytoplasmic transport. As Nup153 regulates nuclear export of SMAD2, a key intermediate in the TGF-β transdifferentiation pathway, its loss of function would result in nuclear retention of SMAD2 and dysregulated TGF-β signaling. We hypothesize that RV infection leads to increased nuclear SMAD2, resulting in sustained TGF-β induced gene expression, priming the airway for subsequent development of asthma. Our hypothesis brings together disparate studies on RV, asthma and Nup153 with the aim to prompt new research into the role of RV infection in development of asthma.
Asthma is a chronic inflammatory lung disease characterized by episodes of bronchoconstriction (exacerbations or attacks) caused by many different stimuli, including virus infections (AIHW, 2023). Rhinovirus (RV) infections are the most common viral cause of exacerbations in children and adults with asthma (Nicholson et al., 1993; Johnston et al., 1995). Additionally, RV infection may predispose children to developing asthma (Brouard et al., 2016; Toivonen et al., 2016; Jartti and Gern, 2017). The thickened airway wall with increased mucus secretion characteristic of asthma is attributed to airway remodeling (AR). There appears to be an association of severe asthma with repeated RV infections. Persistent and repeated RV infections are observed in people with asthma (Kling et al., 2005; Zlateva et al., 2014), while repeated RV infections may lead to an injury-repair loop that induces AR. In this perspectives article, we hypothesize that RV induced disruption of nucleocytoplasmic transport results in sustained AR related gene expression, priming the airway for asthma development. This could be the mechanism whereby RV infection predisposes children to developing asthma.
The many strains of rhinovirus (RV) are the main etiologic cause of upper respiratory tract infections (URTIs), commonly referred to as the “common cold” (Pappas, 2018). The incidence of RV infections tends to decrease with increasing age, with infants and children experiencing an average of seven to 10 episodes of the common cold annually, while adults experience an average of only two to five episodes each year (Gwaltney et al., 1967; D'Alessio et al., 1976). In addition to causing approximately half of all URTIs, RV can cause asymptomatic infections (Makela et al., 1998; Juven et al., 2000) or, more seriously, severe lower respiratory tract infections (LRTIs), and exacerbations of asthma and chronic obstructive pulmonary disease (COPD) that may lead to death (see Supplementary Figure S1A; Mosser et al., 2002; Self et al., 2016; Kerr et al., 2021).
Asthma is a chronic inflammatory pulmonary disease of the conducting airways, usually caused by an immunological reaction. Asthma causes episodes of bronchoconstriction, which result in coughing, wheezing, breathlessness, and tightness of the chest. These symptoms result from increased sensitivity of the airways, bronchial wall inflammation and increased mucus secretions (see Supplementary Figure S1B; Kumar et al., 2015). Airway narrowing is a classic phenotypic sign of asthma and can occur as a result of airway remodeling (AR; Carroll et al., 1993) discussed in the next section.
RV infection is the major cause of viral induced asthmatic attacks in adults and children (Mak et al., 2011). In a cross-sectional study of hospitalized children, 85% of asthma exacerbations were a result of respiratory viral infections, two thirds of which were caused by RV infection (Mak et al., 2011).
Some studies have shown links between RV infections in childhood and the development of asthma later in life (Brouard et al., 2016; Toivonen et al., 2016; Jartti et al., 2020). Exposure to RV during infancy can predispose children to asthma, potentially leading to the development of the condition (AIHW, 2023). Infants affected by RV-associated wheezing during the first 3 years of life experience a 10-fold increased risk of developing asthma by age six; with nearly 90% of the affected infants developing asthma (Jackson et al., 2008). Only 16% of children who were not affected by wheezing developed asthma by age six (Jackson et al., 2008).
AR refers to a series of physical and structural changes to the airway wall that increases wall thickness and reduces the passage of air through the airway as shown in Supplementary Figures S1B,C (Breton et al., 2018; Michalik et al., 2018). These structural changes are indicative of repetitive airway injuries and are found in nearly all asthmatic airways (Elias et al., 1999; Breton et al., 2018; Hsieh et al., 2023). The result of these injuries can include subepithelial fibrosis, increased smooth muscle mass (thickening), gland enlargement, neovascularization and epithelial alterations as shown in Supplementary Figures S1B,C (Bergeron et al., 2010). One of the main structural changes observed during AR includes the transdifferentiation of epithelial cells to mesenchymal cells (EMT) and fibroblasts into myofibroblasts (FMT). Since myofibroblasts are practically absent in normal airways, FMT is one of the key events contributing to the chronic sequelae of asthma (Hackett et al., 2009), ultimately leading to permanently impaired pulmonary function (Pascual and Peters, 2005; Breton et al., 2018; Michalik et al., 2018). FMT can occur as part of a normal response to injury and, when no longer required, myofibroblasts undergo apoptosis or transition back into fibroblasts (Breton et al., 2018; Michalik et al., 2018). However, this does not appear to be the case in asthmatic airways (AA) where myofibroblasts remain after their initial purpose has finished, contributing to AR and chronic impairment (Breton et al., 2018; Michalik et al., 2018). Transforming Growth Factor-β (TGF-β) is a well characterized profibrotic cytokine that is elevated in the asthmatic lung. Importantly, TGF-β is a major inducer of both EMT and FMT (Breton et al., 2018; Walker et al., 2019) and a key contributor to AR.
TGF-β is part of a family of growth factors responsible for cell proliferation, tissue regulation, differentiation, and apoptosis (Kubiczkova et al., 2012; Walton et al., 2017). A ubiquitously expressed, secreted cytokine, TGF-β plays important roles in many physiological and pathological processes during development and in carcinogenesis (Chaudhury and Howe, 2009; Baba et al., 2022). TGF-β is induced in response to a variety of stimuli including RV infections (Dosanjh, 2006; Xia et al., 2017; Wieczfinska et al., 2022). TGF-β was not induced when primary bronchial epithelial cells were infected with low levels of RV (Bedke et al., 2012) and the authors concluded that basal endogenous production of TGF-β contributed to the observed effect on RV infection in cells from asthmatic airways. A recent study found upregulation of TGF-βR (TGF-β receptor) activity in RV infection in vitro and in vivo, implying increased TGF-β production (Dy et al., 2023).
TGF-β signals through two receptor classes (Finnson et al., 2008; Miller and Hill, 2016) resulting in signaling cascades dependent on SMAD proteins. SMADs are intracellular transcription factors and key intermediates in TGF-β signaling (Bedke et al., 2012; Miller and Hill, 2016). A well-studied pathway associated with fibrosis is the TGF-β1/Activin receptor like kinase 5 (ALK5) pathway (Figure 1A, image labeled “non-infected cells”), which transduces intracellular signals through SMAD2/3 in most cell types (Finnson et al., 2008). Upstream receptor dependent interactions result in the phosphorylation of SMAD2/3, promoting their binding to SMAD4 to form a cytosolic complex (Kamato et al., 2013). The SMAD2/3/4 complex translocates to the nucleus where transcription of target genes is activated or repressed (Xu et al., 2012) inducing fibrosis (Walton et al., 2017). For details of the signaling pathway please refer to excellent published reviews (Kubiczkova et al., 2012; Massague and Sheppard, 2023). SMAD2/3 subsequently become dephosphorylated and Ran-binding protein 3 (RanBP3) exports them back to the cytoplasm for recycling or termination of TGF-β1 signaling (Dai et al., 2009; Figure 1B, image labeled “non-infected cells”).
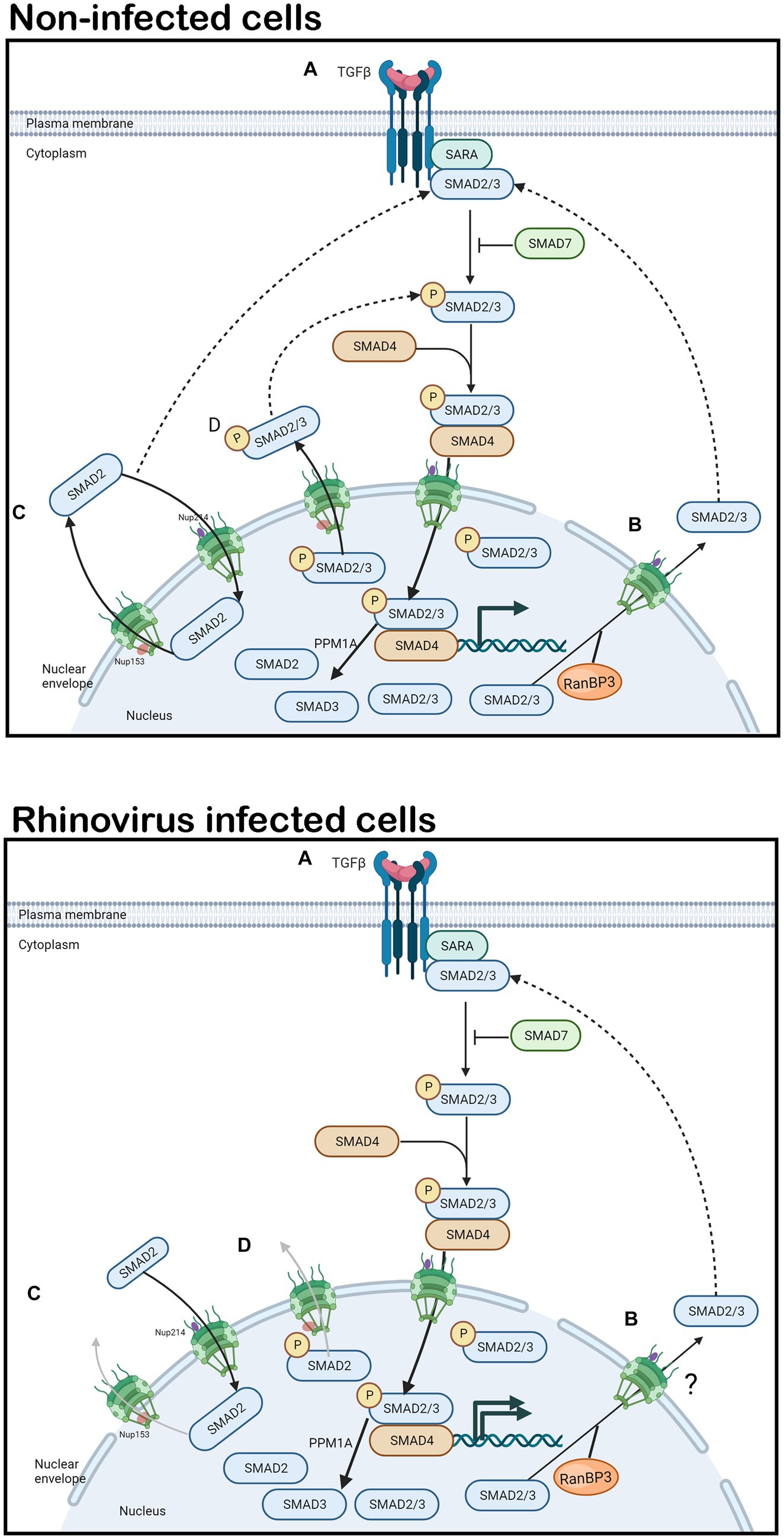
Figure 1. TGF-β1 signaling and SMAD2 shuttling in non-infected and rhinovirus infected cells. In non-infected or rhinovirus infected cells (A) TGF-β1 binds to the constitutively phosphorylated receptor TβRII. TβRII and TβRI receptors dimerize, the TβRI receptor becomes phosphorylated. Activated TβRI in turn phosphorylates SMAD2/3. SMAD7 can inhibit SMAD2/3 phosphorylation, thereby terminating TGF-β1 signaling. SMAD2/3 heterodimerize and bind to SMAD4, forming a cytosolic complex that translocates to the nucleus in an importin-dependent manner where it initiates airway remodeling associated gene expression. PPM1A acts to dephosphorylate SMAD2/3. (B) RanBP3 mediates nuclear export of dephosphorylated SMAD2/3 in a CRM1 independent manner that is not fully elucidated. (C,D) SMAD2 is continually imported into the nucleus by binding to Nup214 and exported by binding to Nup153, in stimulated and unstimulated cells in non-infected cells. In rhinovirus infected cells, Nup153 is cleaved, leading to inhibition of SMAD2 shuttling. SMAD2 and its phosphorylated form accumulate in the nucleus resulting in continued gene expression. Solid arrows denote signaling/transport direction, dotted arrows denote feedback mechanisms, faded arrows denote inhibition.
SMAD2/3 and SMAD4 continuously shuttle between the cytoplasm and the nucleus in unstimulated as well as stimulated cells, providing a dynamic pool that is competitively drawn by cytoplasmic and nuclear signal transduction partners. While nuclear export of SMAD4 is dependent on the nuclear exporter CRM1, SMAD2/3 are exported via CRM1 independent mechanisms (Inman et al., 2002). SMAD3 is exported into the cytoplasm by Exportin 4 in a Ran GTPase dependent manner (Kurisaki et al., 2006). In unstimulated cells, SMAD2 is imported into the nucleus by its direct interaction with CAN/Nup (Nucleoporin) 214; it is exported to the cytoplasm in stimulated and unstimulated cells by direct binding to Nup153 (Xu et al., 2002; Figures 1C,D, image labeled “non-infected cells”). Significantly, TGF-β receptor-mediated phosphorylation does not alter the affinity of SMAD2 for Nup153. SMAD shuttling during active signaling involves continuous (but low level) dephosphorylation. Importantly, dominant-negative CAN/Nup214 or Nup153 constructs interfere with TGF-β activation of SMAD-dependent transcription. Exactly how or even if RanBP3 and Nup153 dependent nuclear export of SMAD2 synergize, compete, or compensate for each other is not clear. Cleavage of Nup153 and the subsequent nuclear accumulation of SMAD2 (Figure 1, image labeled “rhinovirus-infected cells”) could result in continual TGF-β stimulation as is observed when SMAD2 interaction with RanBP3 is inhibited (Dai et al., 2009).
The RV proteases 2A and 3C are responsible for the cleavage of several host proteins, in addition to their roles in proteolytic self-cleavage of RV polyprotein (Gustin, 2003; Amineva et al., 2004; Finnson et al., 2008; Castello et al., 2009; Caly et al., 2015; Jensen et al., 2015; Walker et al., 2016). RV proteases target the nuclear pore complex (NPC), cleaving several nucleoporins including Nup153, that make up the structure of the pore and enable transport through it with the result that nuclear transport in the infected cell is disrupted. The role of the NPC is to facilitate bidirectional nucleo-cytoplasmic shuttling of macromolecules through the nuclear membrane (Hoelz et al., 2011). While smaller molecules (<40 kDa) and ions can diffuse through the nuclear envelope freely, Nups, such as Nup153, are required to escort larger molecules (40-60 kDa) into and out of the nucleus in a cyclic fashion (Nofrini et al., 2016). Although 2A protease is capable of cleaving Nup153, 3C protease is thought to be the main protease responsible for cleavage in infected cells, as 3C protease activity correlates temporally with observed cleavage of Nup153 (Walker et al., 2013). The cleavage of Nup153 clearly contributes to the disruption of nuclear transport observed in infected cells (Gustin and Sarnow, 2002; Ghildyal et al., 2009).
TGF-β produced by RV infected cells could auto induce the signaling pathway resulting in nuclear import of SMAD2 (Figure 1A, image labeled “rhinovirus-infected cells”). In the context of cleaved Nup153, SMAD2/3 would not be able to be exported out of the nucleus (Figures 1C,D, image labeled “rhinovirus-infected cells”) resulting in continuous induction of TGF-β dependent genes, inducing AR. Indeed, a 2017 study by Minor and Proud found that RV or TGF-β alone caused 6 (+/− 3)% or 2 (+/− 1.1)% EMT in Beas-2B cells respectively, but together, resulted in 23.3 (+/− 7.6)% EMT (Minor and Proud, 2017).
Nup153 has been shown to be a key component in a variety of different processes which are independent of its role in transportation (Jacinto et al., 2015; Kitazawa and Rijli, 2017; Khan et al., 2020). While the exact mechanisms are not well defined, Nup153 is known to play a significant role in gene regulation and chromatin re-structuring (Kadota et al., 2020) by itself or in tandem with Sox2. Sox2 is a significant transcription factor responsible for a variety of regulatory processes in a range of cell types. The knock-down of either Sox2 or Nup153 results in significantly decreased levels of co-occupied genes in various models (Zhang and Cui, 2014; Kitazawa and Rijli, 2017; Kuo et al., 2020). Interestingly, Nup153 was the only nuclear structural protein enriched in a genome-wide analysis of 654 Sox2-enriched genes (Kitazawa and Rijli, 2017). Nup153 works with Sox2 to regulate cell type-specific transcriptional programs for the maintenance of neuronal progenitor cells and significantly, knock down of Nup153 leads to differentiation (Toda et al., 2017). An increasing number of studies show that Sox2 plays a vital role in EMT processes with TGF-β and Nup153 (Kuo et al., 2020).
The cleavage of Nup153 in RV infected cells could have significant impacts on gene expression directly, in addition to effects via nuclear retention of SMAD2.
Previous work on RV biology from our group (Ghildyal et al., 2009; Walker et al., 2013, 2015, 2016; Caly et al., 2015; Jensen et al., 2015) and that from other groups (Gustin and Sarnow, 2002; Amineva et al., 2004; Castello et al., 2009; Watters and Palmenberg, 2011) has shown that RV proteases cleave several Nups, including Nup153, resulting in disruption of nucleocytoplasmic transport in infected cells. Our recent work on AR cell culture models (Breton et al., 2018; Walker et al., 2019) has demonstrated that increased TGF-β in the cellular milieu induces AR related pathways. Data from cell neuroscience (Zhang and Cui, 2014) and stem cell biology (Jacinto et al., 2015) has shown that Nup153 has an important role in regulation of transcription related to differentiation. We hypothesize that the cleavage of Nup153 in RV infection leads to accumulation of TGF-β induced SMAD2 in the nucleus and sustained AR associated gene expression, essentially priming the airway to increased risk of asthma in later years.
RV infection induces the production of TGF-β (Dosanjh, 2006; Xia et al., 2017; Wieczfinska et al., 2022), which binds to its cell surface receptors and induces an intracellular signaling cascade leading to nuclear localization of SMAD2/3/4. SMADs bind to specific gene loci and induce gene expression that drives AR. In uninfected cells, SMAD2 released from chromatin would translocate to the cytoplasm with the help of Nup153 (Xu et al., 2002) or RanBP3 (Dai et al., 2009). However, Nup153 is cleaved in RV infected cells (Walker et al., 2013), and we hypothesize that in that context, SMAD2 will be retained in the nucleus with consequent sustained AR associated signaling. We also hypothesize that the cleaved Nup153 is unable to continue its transcription functions in association with Sox2, further pushing the gene expression toward EMT/FMT and increased AR.
If the above is true, RV infected cells should have decreased expression of Nup153/Sox2 associated genes that have a role in cellular differentiation. We performed a first such analysis on the GEO Profiles dataset GDS4832 that represents microarray expression profiling using cultured bronchial epithelial cells (four donors) after RV infection (Proud et al., 2012). TUBB3, SOX12 and ALDH1L1, that are downregulated by Nup153 (Toda et al., 2017), showed a trend for increased expression in RV infected samples (Figure 2A). BMi1, CCND1 and CCND2, that are upregulated by Nup153, showed a trend for decreased expression (Figure 2B). Although the changes are not statistically significant, this analysis provides preliminary support for our hypothesis. Future research could investigate the relationship between Nup153 function, TGF-β signaling and development of asthma in models where Nup153 is either downregulated or knocked out.
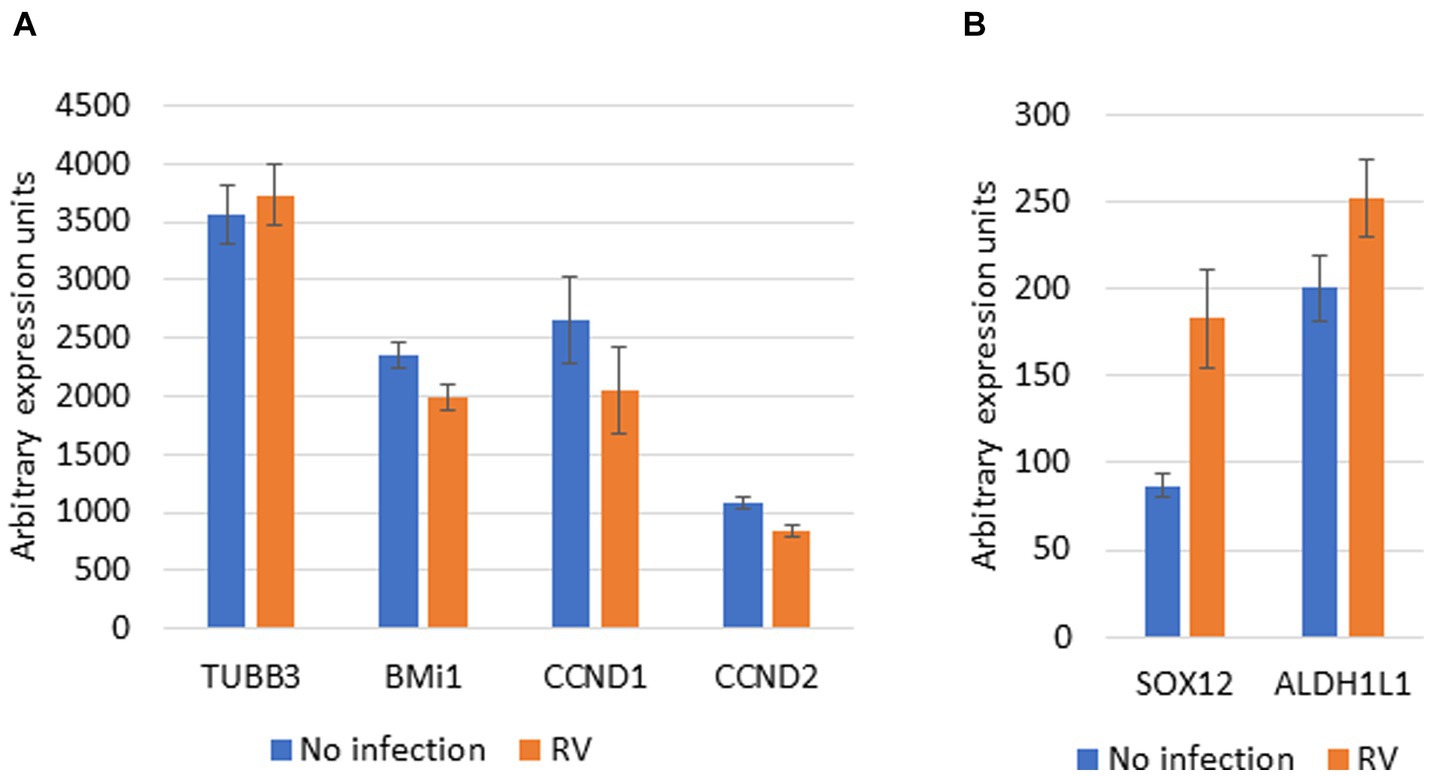
Figure 2. Effect of RV infection on Nup153 dependent genes. Microarray data for expression of Nup153 genes was downloaded from GEO Profiles Accension number GDS4832. Data on the gene expression levels (arbitrary units) for selected genes in presence and absence of infection with RV was extracted and is presented here. (A) Selected genes upregulated by Nup153. TUBB3, tubulin beta 3; BMi1, B cell-specific Moloney murine leukemia virus integration site 1; CCND1, cyclin D1; CCND2, cyclin D2. (B) Selected genes downregulated by Nup153. SOX12, SRY-Box Transcription Factor 12; ALDH1L1, Aldehyde Dehydrogenase 1 Family Member L1. The source data was generated by array expression profiling and used cells from four donors (Proud et al., 2012). Data are presented as Mean +/− SEM for data from all 4 donors.
Our hypothesis predicts that RV infected cells will have increased levels of SMAD2 in the nucleus; this has not yet been tested. If our hypothesis holds true in the clinic, repeated RV infections would increase the risk of later development of asthma. Our hypothesis also predicts that allergen injury following on initial RV infection has increased risk of asthma development compared to allergen injury alone. Interrogation of large longitudinal clinical datasets should clarify these and other clinical predictions of our hypothesis.
Publicly available datasets were analyzed in this study. This data can be found at: GEO Profiles dataset GDS4832.
JM: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. NV: Visualization, Writing – original draft, Writing – review & editing. SS: Supervision, Writing – review & editing. MN: Writing – review & editing. RA: Methodology, Supervision, Visualization, Writing – review & editing. RG: Conceptualization, Supervision, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors acknowledge the help from Timothy Veldre, Canberra Health Directorate, in preparing some background material and the Figure 1.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1321531/full#supplementary-material
Amineva, S. P., Aminev, A. G., Palmenberg, A. C., and Gern, J. E. (2004). Rhinovirus 3C protease precursors 3CD and 3CD' localize to the nuclei of infected cells. J. Gen. Virol. 85, 2969–2979. doi: 10.1099/vir.0.80164-0
Baba, A. B., Rah, B., Bhat, G. R., Mushtaq, I., Parveen, S., Hassan, R., et al. (2022). Transforming growth factor-Beta (TGF-beta) signaling in Cancer-a betrayal within. Front. Pharmacol. 13:791272. doi: 10.3389/fphar.2022.791272
Bedke, N., Sammut, D., Green, B., Kehagia, V., Dennison, P., Jenkins, G., et al. (2012). Transforming growth factor-beta promotes rhinovirus replication in bronchial epithelial cells by suppressing the innate immune response. PloS One 7:e44580. doi: 10.1371/journal.pone.0044580
Bergeron, C., Tulic, M. K., and Hamid, Q. (2010). Airway remodelling in asthma: from benchside to clinical practice. Can. Respir. J. 17, e85–e93. doi: 10.1155/2010/318029
Breton, J. D., Heydet, D., Starrs, L. M., Veldre, T., and Ghildyal, R. (2018). Molecular changes during TGFbeta-mediated lung fibroblast-myofibroblast differentiation: implication for glucocorticoid resistance. Physiol. Rep. 6:e13669. doi: 10.14814/phy2.13669
Brouard, J., Dupont, C., Tran, L., Ribault, M., and Vabret, A. (2016). Rhinovirus during childhood: asthma at adolescence? The chicken or the egg causality dilemma. Arch. Pediatr. 23, 557–560. doi: 10.1016/j.arcped.2016.02.022
Caly, L., Ghildyal, R., and Jans, D. A. (2015). Respiratory virus modulation of host nucleocytoplasmic transport; target for therapeutic intervention? Front. Microbiol. 6:848. doi: 10.3389/fmicb.2015.00848
Carroll, N., Elliot, J., Morton, A., and James, A. (1993). The structure of large and small airways in nonfatal and fatal asthma. Am. Rev. Respir. Dis. 147, 405–410. doi: 10.1164/ajrccm/147.2.405
Castello, A., Izquierdo, J. M., Welnowska, E., and Carrasco, L. (2009). RNA nuclear export is blocked by poliovirus 2A protease and is concomitant with nucleoporin cleavage. J. Cell Sci. 122, 3799–3809. doi: 10.1242/jcs.055988
Chaudhury, A., and Howe, P. H. (2009). The tale of transforming growth factor-beta (TGFbeta) signaling: a soigne enigma. IUBMB Life 61, 929–939. doi: 10.1002/iub.239
Dai, F., Lin, X., Chang, C., and Feng, X. H. (2009). Nuclear export of Smad2 and Smad3 by RanBP3 facilitates termination of TGF-beta signaling. Dev. Cell 16, 345–357. doi: 10.1016/j.devcel.2009.01.022
D'Alessio, D. J., Peterson, J. A., Dick, C. R., and Dick, E. C. (1976). Transmission of experimental rhinovirus colds in volunteer married couples. J Infect Dis 133, 28–36. doi: 10.1093/infdis/133.1.28
Dosanjh, A. (2006). Transforming growth factor-beta expression induced by rhinovirus infection in respiratory epithelial cells. Acta Biochim. Biophys. Sin. Shanghai 38, 911–914. doi: 10.1111/j.1745-7270.2006.00234.x
Dy, A. B. C., Girkin, J., Marrocco, A., Collison, A., Mwase, C., O'Sullivan, M. J., et al. (2023). Rhinovirus infection induces secretion of endothelin-1 from airway epithelial cells in both in vitro and in vivo models. Respir. Res. 24:205. doi: 10.1186/s12931-023-02510-6
Elias, J. A., Zhu, Z., Chupp, G., and Homer, R. J. (1999). Airway remodeling in asthma. J. Clin. Invest. 104, 1001–1006. doi: 10.1172/JCI8124
Finnson, K. W., Parker, W. L., ten Dijke, P., Thorikay, M., and Philip, A. (2008). ALK1 opposes ALK5/Smad3 signaling and expression of extracellular matrix components in human chondrocytes. J. Bone Miner. Res. 23, 896–906. doi: 10.1359/jbmr.080209
Ghildyal, R., Jordan, B., Li, D., Dagher, H., Bardin, P. G., Gern, J. E., et al. (2009). Rhinovirus 3C protease can localize in the nucleus and alter active and passive nucleocytoplasmic transport. J. Virol. 83, 7349–7352. doi: 10.1128/JVI.01748-08
Gustin, K. E. (2003). Inhibition of nucleo-cytoplasmic trafficking by RNA viruses: targeting the nuclear pore complex. Virus Res. 95, 35–44. doi: 10.1016/s0168-1702(03)00165-5
Gustin, K. E., and Sarnow, P. (2002). Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 76, 8787–8796. doi: 10.1128/jvi.76.17.8787-8796.2002
Gwaltney, J. M., Hendley, J. O., Simon, G., and Jordan, W. S. (1967). Rhinovirus infections in an industrial population. II. Characteristics of illness and antibody response. JAMA 202, 494–500. doi: 10.1001/jama.1967.03130190100014
Hackett, T. L., Warner, S. M., Stefanowicz, D., Shaheen, F., Pechkovsky, D. V., Murray, L. A., et al. (2009). Induction of epithelial-mesenchymal transition in primary airway epithelial cells from patients with asthma by transforming growth factor-beta1. Am. J. Respir. Crit. Care Med. 180, 122–133. doi: 10.1164/rccm.200811-1730OC
Hoelz, A., Debler, E. W., and Blobel, G. (2011). The structure of the nuclear pore complex. Annu. Rev. Biochem. 80, 613–643. doi: 10.1146/annurev-biochem-060109-151030
Hsieh, A., Assadinia, N., and Hackett, T. L. (2023). Airway remodeling heterogeneity in asthma and its relationship to disease outcomes. Front. Physiol. 14:1113100. doi: 10.3389/fphys.2023.1113100
Inman, G. J., Nicolas, F. J., and Hill, C. S. (2002). Nucleocytoplasmic shuttling of Smads 2, 3, and 4 permits sensing of TGF-beta receptor activity. Mol. Cell 10, 283–294. doi: 10.1016/s1097-2765(02)00585-3
Jacinto, F. V., Benner, C., and Hetzer, M. W. (2015). The nucleoporin Nup153 regulates embryonic stem cell pluripotency through gene silencing. Genes Dev. 29, 1224–1238. doi: 10.1101/gad.260919.115
Jackson, D. J., Gangnon, R. E., Evans, M. D., Roberg, K. A., Anderson, E. L., Pappas, T. E., et al. (2008). Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am. J. Respir. Crit. Care Med. 178, 667–672. doi: 10.1164/rccm.200802-309OC
Jartti, T., and Gern, J. E. (2017). Role of viral infections in the development and exacerbation of asthma in children. J. Allergy Clin. Immunol. 140, 895–906. doi: 10.1016/j.jaci.2017.08.003
Jartti, T., Papadopoulos, N. G., and Feleszko, W., EAACI Task Force on Clinical Practice Recommendations on Preschool Wheeze (2020). Reply to: medical algorithm: diagnosis and treatment of preschool asthma. Allergy 75, 2716–2717. doi: 10.1111/all.14285
Jensen, L. M., Walker, E. J., Jans, D. A., and Ghildyal, R. (2015). Proteases of human rhinovirus: role in infection. Methods Mol. Biol. 1221, 129–141. doi: 10.1007/978-1-4939-1571-2_10
Johnston, S. L., Pattemore, P. K., Sanderson, G., Smith, S., Lampe, F., Josephs, L., et al. (1995). Community study of role of viral infections in exacerbations of asthma in 9-11 year old children. BMJ 310, 1225–1229. doi: 10.1136/bmj.310.6989.1225
Juven, T., Mertsola, J., Waris, M., Leinonen, M., Meurman, O., Roivainen, M., et al. (2000). Etiology of community-acquired pneumonia in 254 hospitalized children. Pediatr. Infect. Dis. J. 19, 293–298. doi: 10.1097/00006454-200004000-00006
Kadota, S., Ou, J., Shi, Y., Lee, J. T., Sun, J., and Yildirim, E. (2020). Nucleoporin 153 links nuclear pore complex to chromatin architecture by mediating CTCF and cohesin binding. Nat. Commun. 11:2606. doi: 10.1038/s41467-020-16394-3
Kamato, D., Burch, M. L., Piva, T. J., Rezaei, H. B., Rostam, M. A., Xu, S., et al. (2013). Transforming growth factor-beta signalling: role and consequences of Smad linker region phosphorylation. Cell. Signal. 25, 2017–2024. doi: 10.1016/j.cellsig.2013.06.001
Kerr, S. L., Mathew, C., and Ghildyal, R. (2021). Rhinovirus and cell death. Viruses 13:629. doi: 10.3390/v13040629
Khan, A. U., Qu, R., Ouyang, J., and Dai, J. (2020). Role of nucleoporins and transport receptors in cell differentiation. Front. Physiol. 11:239. doi: 10.3389/fphys.2020.00239
Kitazawa, T., and Rijli, F. M. (2017). Nuclear pore protein meets transcription factor in neural fate. Neuron 96, 259–261. doi: 10.1016/j.neuron.2017.09.059
Kling, S., Donninger, H., Williams, Z., Vermeulen, J., Weinberg, E., Latiff, K., et al. (2005). Persistence of rhinovirus RNA after asthma exacerbation in children. Clin. Exp. Allergy 35, 672–678. doi: 10.1111/j.1365-2222.2005.02244.x
Kubiczkova, L., Sedlarikova, L., Hajek, R., and Sevcikova, S. (2012). TGF-beta—an excellent servant but a bad master. J. Transl. Med. 10:183. doi: 10.1186/1479-5876-10-183
Kumar, V., Abbas, A., and Aster, J. (2015). Robbins and Cotran pathologic basis of disease. Philadelphia, PA, USA: Elsevier/Saunders.
Kuo, M. H., Lee, A. C., Hsiao, S. H., Lin, S. E., Chiu, Y. F., Yang, L. H., et al. (2020). Cross-talk between SOX2 and TGFbeta signaling regulates EGFR-TKI tolerance and lung Cancer dissemination. Cancer Res. 80, 4426–4438. doi: 10.1158/0008-5472.CAN-19-3228
Kurisaki, A., Kurisaki, K., Kowanetz, M., Sugino, H., Yoneda, Y., Heldin, C. H., et al. (2006). The mechanism of nuclear export of Smad3 involves exportin 4 and ran. Mol. Cell. Biol. 26, 1318–1332. doi: 10.1128/MCB.26.4.1318-1332.2006
Mak, R. K., Tse, L. Y., Lam, W. Y., Wong, G. W., Chan, P. K., and Leung, T. F. (2011). Clinical spectrum of human rhinovirus infections in hospitalized Hong Kong children. Pediatr. Infect. Dis. J. 30, 749–753. doi: 10.1097/INF.0b013e31821b8c71
Makela, M. J., Puhakka, T., Ruuskanen, O., Leinonen, M., Saikku, P., Kimpimaki, M., et al. (1998). Viruses and bacteria in the etiology of the common cold. J. Clin. Microbiol. 36, 539–542. doi: 10.1128/JCM.36.2.539-542.1998
Massague, J., and Sheppard, D. (2023). TGF-beta signaling in health and disease. Cells 186, 4007–4037. doi: 10.1016/j.cell.2023.07.036
Michalik, M., Wojcik-Pszczola, K., Paw, M., Wnuk, D., Koczurkiewicz, P., Sanak, M., et al. (2018). Fibroblast-to-myofibroblast transition in bronchial asthma. Cell. Mol. Life Sci. 75, 3943–3961. doi: 10.1007/s00018-018-2899-4
Miller, D., and Hill, C. (2016). “TGF-β superfamily signaling” in Encyclopedia of cell biology. eds. R. Bradshaw and P. Stahl (Cambridge, Massachusetts, United States of America: Academic Press), 37–50.
Minor, D. M., and Proud, D. (2017). Role of human rhinovirus in triggering human airway epithelial-mesenchymal transition. Respir. Res. 18:110. doi: 10.1186/s12931-017-0595-9
Mosser, A. G., Brockman-Schneider, R., Amineva, S., Burchell, L., Sedgwick, J. B., Busse, W. W., et al. (2002). Similar frequency of rhinovirus-infectible cells in upper and lower airway epithelium. J Infect Dis 185, 734–743. doi: 10.1086/339339
Nicholson, K. G., Kent, J., and Ireland, D. C. (1993). Respiratory viruses and exacerbations of asthma in adults. BMJ 307, 982–986. doi: 10.1136/bmj.307.6910.982
Nofrini, V., Di Giacomo, D., and Mecucci, C. (2016). Nucleoporin genes in human diseases. Eur. J. Hum. Genet. 24, 1388–1395. doi: 10.1038/ejhg.2016.25
Pappas, D. (2018). “The common cold” in Principles and practice of pediatric infectious diseases. eds. S. Long, C. Prober, and M. Fischer. 5th ed (Philadelphia, Pennsylvania, United States of America: Elsevier), 199–202.
Pascual, R. M., and Peters, S. P. (2005). Airway remodeling contributes to the progressive loss of lung function in asthma: an overview. J. Allergy Clin. Immunol. 116, 477–486. doi: 10.1016/j.jaci.2005.07.011
Proud, D., Hudy, M. H., Wiehler, S., Zaheer, R. S., Amin, M. A., Pelikan, J. B., et al. (2012). Cigarette smoke modulates expression of human rhinovirus-induced airway epithelial host defense genes. PloS One 7:e40762. doi: 10.1371/journal.pone.0040762
Self, W. H., Williams, D. J., Zhu, Y., Ampofo, K., Pavia, A. T., Chappell, J. D., et al. (2016). Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis 213, 584–591. doi: 10.1093/infdis/jiv323
Toda, T., Hsu, J. Y., Linker, S. B., Hu, L., Schafer, S. T., Mertens, J., et al. (2017). Nup153 interacts with Sox2 to enable bimodal gene regulation and maintenance of neural progenitor cells. Cell Stem Cell 21, 618–634.e7. doi: 10.1016/j.stem.2017.08.012
Toivonen, L., Schuez-Havupalo, L., Karppinen, S., Teros-Jaakkola, T., Rulli, M., Mertsola, J., et al. (2016). Rhinovirus infections in the first 2 years of life. Pediatrics 138:1309. doi: 10.1542/peds.2016-1309
Walker, E. J., Heydet, D., Veldre, T., and Ghildyal, R. (2019). Transcriptomic changes during TGF-beta-mediated differentiation of airway fibroblasts to myofibroblasts. Sci. Rep. 9:20377. doi: 10.1038/s41598-019-56955-1
Walker, E. J., Jensen, L. M., Croft, S., and Ghildyal, R. (2015). Variation in the nuclear effects of infection by different human rhinovirus serotypes. Front. Microbiol. 6:875. doi: 10.3389/fmicb.2015.00875
Walker, E., Jensen, L., Croft, S., Wei, K., Fulcher, A. J., Jans, D. A., et al. (2016). Rhinovirus 16 2A protease affects nuclear localization of 3CD during infection. J. Virol. 90, 11032–11042. doi: 10.1128/JVI.00974-16
Walker, E. J., Younessi, P., Fulcher, A. J., McCuaig, R., Thomas, B. J., Bardin, P. G., et al. (2013). Rhinovirus 3C protease facilitates specific nucleoporin cleavage and mislocalisation of nuclear proteins in infected host cells. PloS One 8:e71316. doi: 10.1371/journal.pone.0071316
Walton, K. L., Johnson, K. E., and Harrison, C. A. (2017). Targeting TGF-beta mediated SMAD signaling for the prevention of fibrosis. Front. Pharmacol. 8:461. doi: 10.3389/fphar.2017.00461
Watters, K., and Palmenberg, A. C. (2011). Differential processing of nuclear pore complex proteins by rhinovirus 2A proteases from different species and serotypes. J. Virol. 85, 10874–10883. doi: 10.1128/JVI.00718-11
Wieczfinska, J., Sitarek, P., Kowalczyk, T., Rieske, P., and Pawliczak, R. (2022). Curcumin modulates airway remodelling-contributing genes-the significance of transcription factors. J. Cell. Mol. Med. 26, 736–749. doi: 10.1111/jcmm.17102
Xia, Y. C., Radwan, A., Keenan, C. R., Langenbach, S. Y., Li, M., Radojicic, D., et al. (2017). Glucocorticoid insensitivity in virally infected airway epithelial cells is dependent on transforming growth factor-beta activity. PLoS Pathog. 13:e1006138. doi: 10.1371/journal.ppat.1006138
Xu, L., Kang, Y., Col, S., and Massague, J. (2002). Smad2 nucleocytoplasmic shuttling by nucleoporins CAN/Nup214 and Nup153 feeds TGFbeta signaling complexes in the cytoplasm and nucleus. Mol. Cell 10, 271–282. doi: 10.1016/s1097-2765(02)00586-5
Xu, P., Liu, J., and Derynck, R. (2012). Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS Lett. 586, 1871–1884. doi: 10.1016/j.febslet.2012.05.010
Zhang, S., and Cui, W. (2014). Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells 6, 305–311. doi: 10.4252/wjsc.v6.i3.305
Keywords: nucleoporin (Nup) 153, rhinovirus, airway remodeling, asthma, transdifferentiation
Citation: Moorhouse J, Val N, Shahriari S, Nelson M, Ashby R and Ghildyal R (2024) Rhinovirus protease cleavage of nucleoporins: perspective on implications for airway remodeling. Front. Microbiol. 14:1321531. doi: 10.3389/fmicb.2023.1321531
Received: 14 October 2023; Accepted: 08 December 2023;
Published: 05 January 2024.
Edited by:
Gualtiero Alvisi, University of Padua, ItalyReviewed by:
Anthony Bosco, University of Arizona, United StatesCopyright © 2024 Moorhouse, Val, Shahriari, Nelson, Ashby and Ghildyal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Reena Ghildyal, cmVlbmEuZ2hpbGR5YWxAY2FuYmVycmEuZWR1LmF1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.