- 1College of Life Sciences, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China
- 2College of Science, Inner Mongolia Agricultural University, Hohhot, Inner Mongolia, China
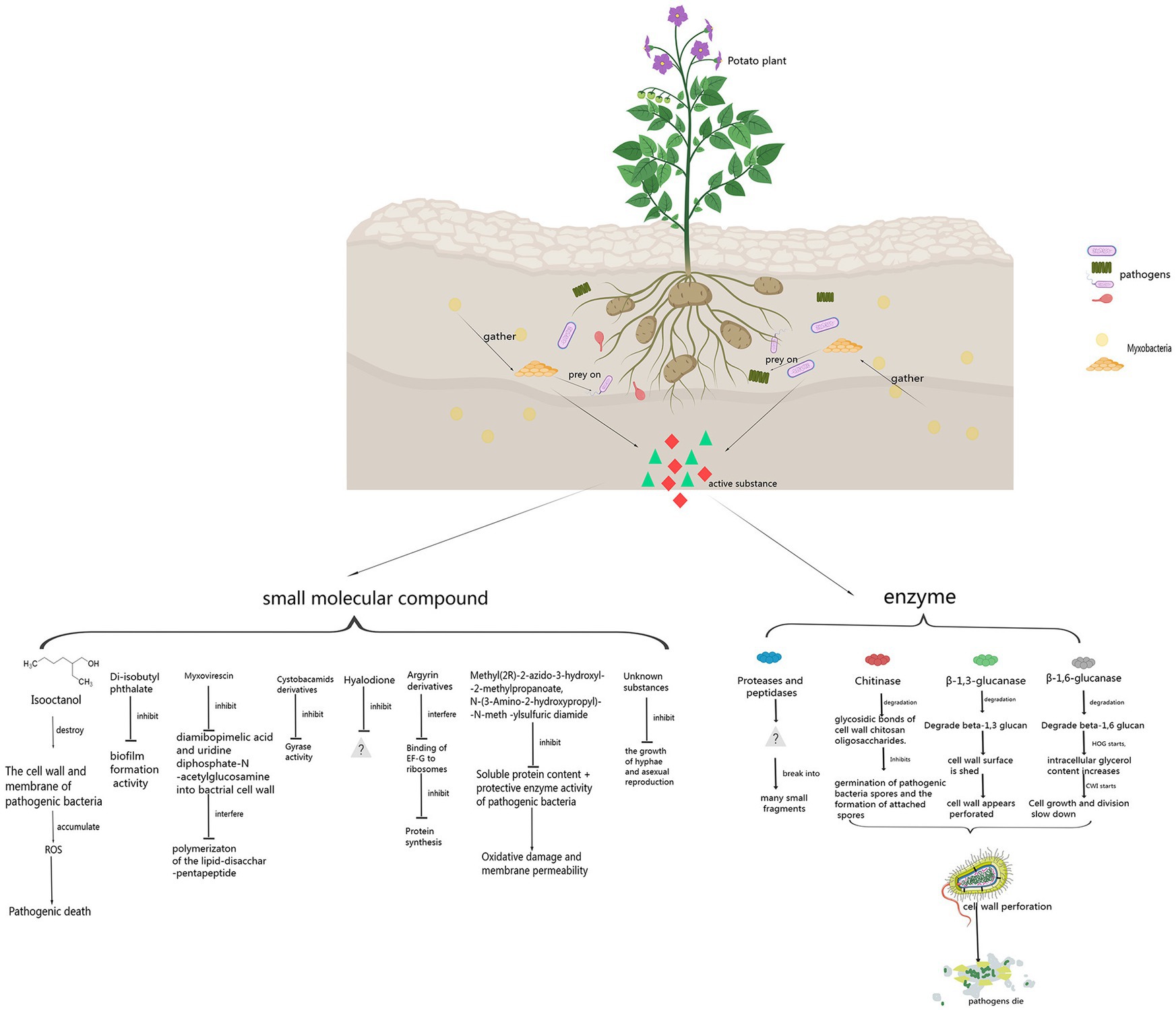
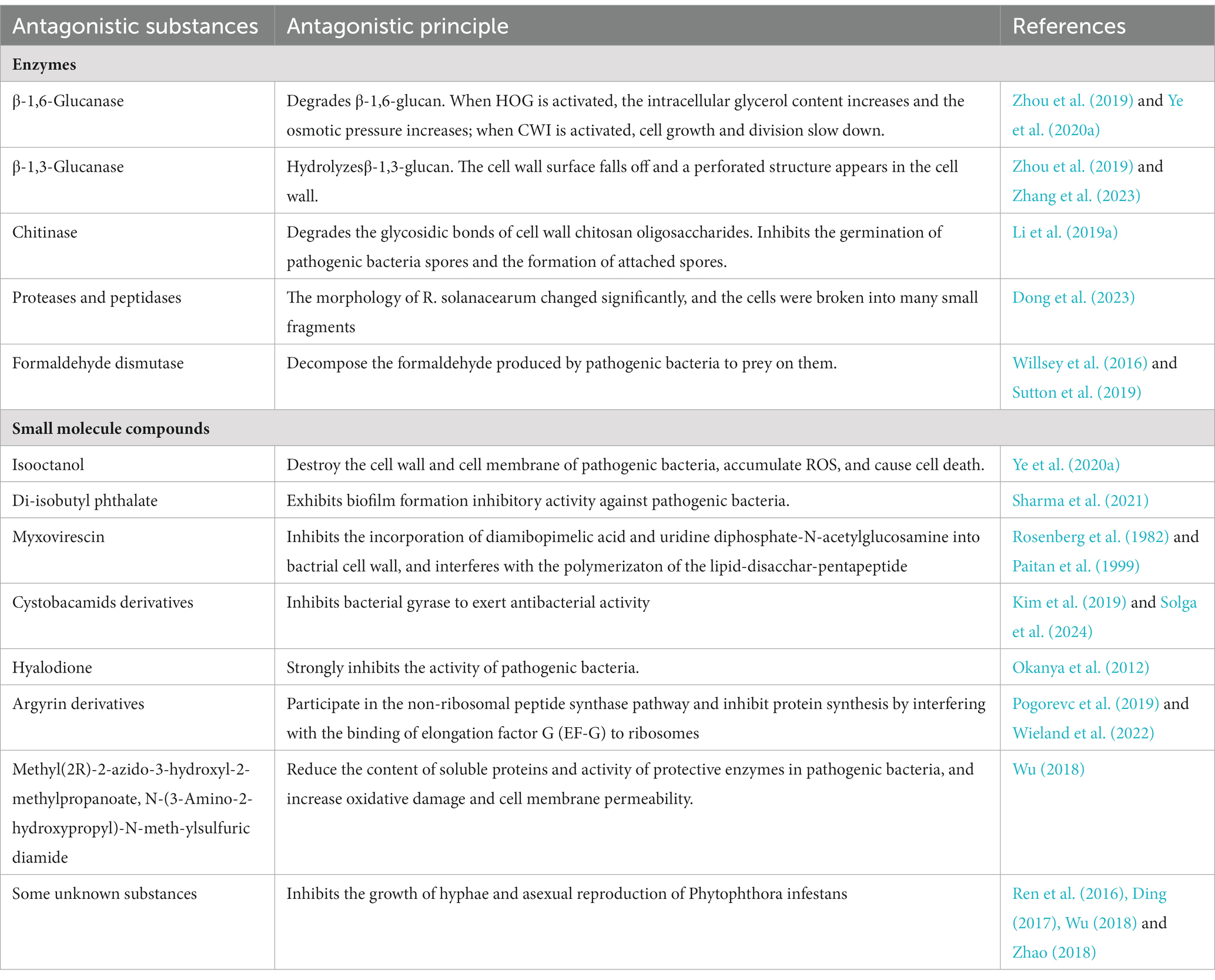
Myxobacteria have a complex life cycle and unique social behavior. They can prey on plant pathogenic fungi, bacteria, and oomycetes in the soil by producing some enzymes and small molecule compounds. The enzymes mainly include β-1,6-glucanase, β-1,3-glucanase, chitinase, protease, peptidase, and formaldehyde dismutase. β-1,6-glucanase, β-1,3-glucanase, and chitinase can degrade the glycosidic bonds in the cell wall of plant pathogen, causing some holes to form on the cell walls of the plant pathogen. Proteases and peptidases can break plant pathogenic cells into many small fragments and facilitate extracellular digestion of proteins during myxobacterial predation. Formaldehyde dismutase converts formaldehyde to formate and methanol, it can help myxobactria protect themselves in the process of predation. Small molecule substances produced by myxobacteria include isooctanol, di-isobutyl phthalate, myxovirescin, cystobactamid derivatives, hyalodione, argyrin derivatives, Methyl (2R)-2-azido-3-hydroxyl-2-methylpropanoate and N-(3-Amino-2-hydroxypropyl)-N-meth-ylsulfuric diamide, etc. Isooctanol destroyed the cell wall and cell membrane of plant pathogen, causing intracellular reactive oxygen species (ROS) to accumulate, leading to apoptosis and cell death. Di-isobutyl phthalate had biofilm inhibitory activity against bacteria. Myxovirescin could inhibit the incorporation of diamibopimelic acid and uridine diphosphate-Nacetylglucosamine intobacterial cell wall and interfered with the polymerizaton of the lipid-disacchar-pentapeptide. Cystobactamid derivatives exerted their natural antibacterial properties by inhibition of bacterial gyrases. Hyalodione had broad antibacterial and antifungal activity. Argyrin derivatives inhibited protein synthesis by interfering with the binding of elongation factor G (EF-G) to ribosomes. Methyl (2R)-2-azido-3-hydroxyl-2-methylpropanoate and N-(3-Amino-2-hydroxypropyl)-N-meth-ylsulfuric diamide reduced the content of soluble proteins and the activity of protective enzymes (PPO, POD, PAL, and SOD) in plant pathogen, increased oxidative damage and cell membrane permeability. Myxobacteria, as a new natural compound resource bank, can control plant pathogenic fungi, oomycetes and bacteria by producing some enzymes and small molecule compounds, so it has great potential in plant disease control.
1 Introduction
Myxobacteria are microorganisms of the phylum Myxococcota (Waite et al., 2020; Oren and Garrity, 2021), which are well known for their complex life cycles and unique social behaviors. Myxobacteria have a wide range of habitats, including soil rich in organic matter, rotting wood, animal dung and marine environment (Saggu et al., 2023). They can survive in high-salinity environments (Gemperlein et al., 2018). Some halophilic myxobacteria i.e., Haliangium spp. (Ryosuke et al., 2002), Plesiocystis pacifica (Iizuka et al., 2003a) and Enhygromyxa salina (Iizuka et al., 2003b) had been isolated from marine environment. Research over the past few decades has proven that myxobacteria have become a resource library of new natural compounds, ranking second only to Actinomycetes and Bacillus among prokaryotes (Arguelles-Arias et al., 2009; Weissman and Müller, 2010). Metabolites produced by myxobacteria often have structures that other microbial metabolites do not have, and40% of myxobacterial metabolites have novel chemical structures. For example, in contrast to Actinomycetes derivatives, most small molecules of myxobacteria are not glycosylated (Rix et al., 2002). It is currently unclear why myxobacteria produce large amounts of metabolites, but researchers generally believe that metabolites play an important role in regulating cell-to-cell interactions within a population (Davies et al., 2006) and in prey hunting (Xiao et al., 2011).
Myxobacteria can prey on plant pathogen and destroy pathogen’s cell morphology and structure. When myxobacteria prey on pathogen, they can kill microorganisms and lyse cells by producing metabolites such as antibiotics, cell wall degrading enzymes, lipases, nucleases, polysaccharases, and proteases, thereby clearing the pathogens. The destroyed pathogenic cells are surrounded by many filamentous substances. The cell structure become loose and irregular, and the cell contents overflow, and eventually the pathogen lyse and die.
Therefore, myxobacteria can serve as biological control agents (BCAs) of plant diseases (Ye et al., 2020b). The BCAs in agricultural planting can reduce the use of pesticides, reduce the adverse effects caused by excessive use of chemicals and achieve the purpose of controlling soil-borne plant diseases. The BCAs are very effective in preventing and managing plant diseases and achieving ecological and economic benefits such as increasing agricultural output and reducing environmental pollution. Research on myxobacteria can provide new potential ways for biological control of plant diseases. This paper reviews the research progress on the active substances of myxobacteria against plant diseases and their action mechanisms.
2 Enzymes
Myxobacteria produce some enzymes playing important roles in preying on pathogens. These enzymes include carbohydrate-active enzymes (CAZymes), peptidases, lipases, etc. CAZymes include glycosyltransferases (GTs), glycoside hydrolases (GHs), carbohydrate esterases (CEs), auxiliary activities (AAs), carbohydrate-binding modules (CBMs), and polysaccharide lyases (PLs). CAZymes can modify the glycosidic bonds of carbohydrates and are important basic functional units in carbohydrate metabolism pathways (Dong et al., 2023). GHs hydrolyze glycosidic bonds and play an important role in the hydrolysis and synthesis of sugars and glycoconjugates in organisms (Kaushal and Singh, 2020). The enzymes produced by myxobacteria to control plant pathogens are shown in Figure 1 and Table 1.
2.1 β-1,6-Glucanase
β-1,6-glucan is a component of the fungal cell wall smaller than chitin and β-1,3-glucan. It can cross-link cell wall proteins to the chitin layer and β-1,3-glucan layer. Inhibiting the synthesis of β-1,6-glucan is conducive to the effective disintegration and further degradation of pathogen cell wall during the process of myxobacteria preying on plant pathogen. β-1,6-glucanase can hydrolyze the glycosidic bonds of β-1,6-glucan, thereby destroying the entire cell wall structure of fungi. β-1,6-glucanase GluM from the strain EGB of Coralococcus sp. is a novel family of outer membrane β-barrel proteins that can inhibit fungal embryonic tube development (Li et al., 2019b). β-1,6-glucanase GluM is essential in the initial sensing and efficient decomposition of fungi.
Electron microscopy observation of the hyphae of Magnaporthe oryzae treated with β-1,6-glucanase GluM showed that the hyphae were stretched and partially broken, and the hyphal cell wall changed from a dense structure to a loose structure. The spore folds of the treated M. oryzae were irregular. The density of spore decreased, and the morphology of spore showed a deformed state. The morphological and structural changes of M. oryzae were speculated to be due to the hydrolysis of the cell wall by β-1,6-glucanase GluM, resulting in incomplete cell structure and outflow of contents, ultimately leading to morphological changes. Therefore, β-1,6-glucanase GluM inhibited the infection of M. oryzae in rice by digesting the pathogen’s cell wall (Zhou et al., 2019).
After treated with GluM, the hyphae and spores of Fusarium oxysporum f. sp. cucumerinum (FOC) shrank obviously. The cell wall of FOC appeared to be perforated and damaged, the cell wall structure was loose, and large vacuoles formed in the cells. The High Osmolarity Glycerol (HOG) in FOC cells was activated. The phosphorylation level of Hog1-likemitogenactivated proteinkinase (MAPK) was significantly increased and the glycerol content increased 2.6 times. The osmotic pressure in FOC cells increased, which accelerated cell lysis. When the strain EGB of Corallococcus sp. were inoculated with the potted cucumbers, the strain could adapt well to the soil environment and effectively reduced the abundance of soil-borne F. oxysporum and the occurrence of cucumber wilt disease (Ye et al., 2020b).
In the GluM transgenic experiment, the β-1,6-glucanase gene was transferred into japonica rice variety ZH11 to obtain transgenic japonica rice with overexpression of GluM. In the fungal disease resistance experiment, the rice blast area of GluM transgenic rice was reduced by 82.7%. The sheath blight disease was reduced by 35.76%−43.67% and the incidence of rice smut disease was reduced by 65.79%. The results showed that transgenic rice containing GluM protein could degrade fungal cell walls through specific hydrolysis and enhanced resistance to fungal diseases (Shen et al., 2023).
The β-1,6-glucanase produced by myxobacteria inhibits not only the growth of pathogenic fungus, but also the growth of oomycetes. The fermentation products of C. coralliformis strain CMC0606 had a strong inhibitory effect on Phytophthora capsici, and the diameter of inhibition zone was 16mm (Bader et al., 2022). The strain EGB had a strong inhibitory effect on the growth of P. capsica. The mycelium of P. capsica collapsed and the growth of the pathogen was obviously inhibited. The results showed that β-1,6-glucanase in the fermentation supernation of strain EGB was effective in inhibiting oomycetes (Zhang et al., 2023).
2.2 β-1,3-Glucanase
β-1,3-glucan is a component of the fungal cell wall. Extensive hydrolysis of fungal cell wall polymer chains by β-1,3-glucanase can reduce the mechanical strength of the cell wall, leading to the final lysis of the fungal cell. β-1,3-glucanase IamC from strain EGB can cleave β-1,3- or β-1,6-glucan substrates by exo-hydrolysis. Cu2+, Co2+, Mg2+, and Cr3+ inhibit the activity of IamC, while Mn2+ is an effective activator of IamC, indicating that IamC is a metal ion-dependent hydrolase. After exposure of M. oryzae to IamC, the germ tube and appressorium formation rates were significantly reduced from 94 and 97% to 59 and 51%. The hyphae of M. oryzae was enlarged and deformed, and more granular contents appeared inside the hyphae. There was a large accumulation of reactive oxygen species (ROS) in the spores and hyphae of M. oryzae and the distribution of chitin in the cell wall of pathogen changed. The β-1,3-glucanase IamC derived from strain EGB acted on different β-glycosidic bonds in the cell wall of M. oryzae . β-glycosidic bonds of polysaccharides from different sites of cell wall of pathogen were hydrolyzed, ultimately leading to cell lysis of M. oryzae (Zhou et al., 2019).
Archangium strain AC19 showed strong predatory activity gainst Phytophthora sojae P6497 and protected soybeans from stem rot disease. Strain AC19 was observed to prey on P. sojae, and the hyphal cell wall of P. sojae P6497 showed perforation. The fermentation supernatant of strain AC19 significantly inhibited the growth and infection of P. sojae. The active substances that digested P. sojae were the CAZymes secreted by strain AC19. The cell wall-acting CAZymes in strain AC19 were specialized β-1,3-glucanases (AcGlu13.1,−13.2, and−13.3). These β-1,3-glucanases targeted β-1,3-glucan from the cell wall of Phytophthora. AcGlu13.1 caused cell wall surface shedding through its degradative activity. AcGlu13.2 and AcGlu13.3 could cause perforated structures in the cell wall (Zhang et al., 2023).
2.3 Chitinase
The fungal cell wall is mainly composed of chitin, β-1,3-glucan and β-1,6-glucan. The chitin accounts for 22%−40% of the fungal cell wall (Bowman and Free, 2006). Chitinase ofGHs family can hydrolyze chitin in fungal cell walls, so chitinase is regarded as an antifungal factor for biocontrol of fungal diseases (Shehata et al., 2018; Li et al., 2019a). Strain EGB can synthesize the endo-chitinase CcCti1 which belongs to the GHs family 18 (GH18) and has potential antifungal activity. CcCti1 can not only degrade chitosan oligosaccharide, but also hydrolyze chitin into N-acetylated chitohexaose (GlcNAc)6. CcCti1 had biological control activity against the plant pathogen M. oryzae, inhibiting the germination of conidia and the formation of appressoria of M. oryzae at a concentration of 0.08 mg/mL (Li et al., 2019a). Rice blast caused by M. oryzae is the main limiting factor in global rice production and is one of the most destructive diseases in cultivated rice in the world (Talbot, 2003). The transgenic plants with chitinase genes showed strong resistance to rice blast. The reason may be that the cell wall integrity of pathogen changed, leading to significant internal expansion pressure and lysis of fungal cells (Selitrennikoff, 2001). Therefore, transgenic plants with chitinase genes were more resistant to the M. oryzae.
2.4 Proteases and peptidases
Tomato bacterial wilt (TBW) caused by Ralstonia solanacearum is one of the most destructive soilborne diseases, and tomato production has suffered huge losses due to the epidemic of TBW (Mansfield et al., 2012). M. xanthus R31 had good biological control potential against TBW, and the biocontrol efficiency against TBW in pot experiments was as high as 81.9%. The MEROPS database of strain R31 genome had annotated 274 proteins, including 132 metalloproteases and 107 serine proteases. Three M36 metalloproteases were identified in the R31 genome that may contribute to the extracellular digestion of proteins during predatory behavior (Dong et al., 2023). Proteins of the M23 family were endopeptidases that cleaved bacterial cell wall peptidoglycan by degrading the peptide bonds of cross-linked peptides (Odintsov et al., 2004). Proteases and peptidases, such as the M36 metalloprotease MepA secreted by M. xanthus strain DK1622, may promote the predation of prey by degrading proteins of prey cells (Berleman et al., 2014).
2.5 Formaldehyde dismutase
When Pseudomonas aeruginosa was preyed on, it secreted toxic formaldehyde to resist predation. Fomaldehyde can be converted into formate and methanol by formaldehyde detoxifying enzymes, such as formaldehyde dismutase (Fdm), produced by P. aeruginosa to protect itself (Willsey et al., 2016). It was shown that myxobacteria could produce formaldehyde dismutase. Therefore, myxobacteria had the ability to prey on P. aeruginosa by converting toxic formaldehyde secreted by P. aeruginosa into non-toxic substances (Sutton et al., 2019). Myxobacteria may also be able to prey on similar plant pathogens by a similar way.
3 Small molecule compounds
The biological control activity of myxobacteria against pathogen depends on not only enzymes, but also some small molecule compounds. These compounds include isooctanol, di-isobutyl phthalate, myxovirescin, cystobactamid derivatives, hyalodione, argyrin derivatives, Methyl (2R)-2-azido-3-hydroxyl-2-methylpropanoate, and N-(3-Amino-2-hydroxypropyl)-N-meth-ylsulfuric diamide, etc. Some small molecule compounds produced by myxobacteria to control plant pathogens are shown in Figure 1 and Table 1.
3.1 Isooctanol
Strain EGB exhibited superior biological control activity against F. oxysporum. F. oxysporum is a ubiquitous soil-borne plant pathogen that can cause vascular wilt in a variety of crops (Pietro et al., 2003). A total of 32 volatile compounds produced by strain EGB were identified, and isooctanol had the highest antifungal activity. The mycelia of F. oxysporum treated with isooctanol showed severely shrinkage and collapse. The hyphae of F. oxysporum treated by isooctanol, the transcript levels of many genes related to the cell wall integrity (CWI) pathway and redox reactions were significantly increased by 15-to 40-fold. The transcription levels of chitin synthase (FOXG_12345, FOXG_10443 and FOXG_04179), chitinase (FOXG_19879 and FOXG_17332), endo-1,3 (4)-β-glucanase (FOXG_22849, FOXG_10637 and FOXG_03928) were upregulated after the mycelia of F. oxysporum were treated with isooctanol. The transcription levels of genes related to components corresponding to cell wall integrity (FOXG_09228), programmed cell death control protein (FOXG_03587) and cell division control protein (FOXG_00362) increased, slowing down the growth and division rate of cells, and activating cell apoptosis. Isooctanol destroyed the cell wall and cell membrane of F. oxysporum, causing intracellular reactive oxygen species (ROS) to accumulate, leading to apoptosis and cell death. A dose of only 3.75 μL/plate of isooctanol was sufficient to inhibit F. oxysporum (Ye et al., 2020a).
3.2 Diisobutyl phthalate
M. fulvus strain ST/P/71 had obvious antibacterial activity against B. subtilis, and the extract from the strain ST/P/71 mainly showed inhibition activity against B. subtilis. During seperating by Reverse Phase High Performance Liquid Chromatography (RP-HPLC), two pure compounds were eluted at RT 54.24 (Ra2) and RT 71.27 (Ra3). Ra2 was identified as di-isobutyl phthalate. This substance showed biofilm formation inhibitory activity against B. subtilis, with an MBIC50 of 2.703 μg/mL (Sharma et al., 2021). Di-isobutyl phthalate had biofilm formation inhibitory activity against B. subtilis, so it could have similar functions to plant pathogenic bacteria. Therefore, diisobutyl phthalate may have great potential in the prevention and control of plant pathogenic bacteria.
3.3 Myxovirescin
One of the 18 metabolites of M. xanthus strain DK1622 is polyketide myxovirescin (antibiotic TA), which had antibacterial activity. Myxovirescin played an important role in killing Escherichia coli, including lysis and subsequent predation (Xiao et al., 2011). The antibiotic TA was produced and named after M. vanthits strain TA(ATCC31046) (Rosenberg et al., 1982). The antibiotic TA could inhibit the incorporation of diamibopimelic acid and uridine diphosphate-N-acetylglucosamine into E. coli cell wall, and antibiotic TA interfered with the polymerizaton of the lipid-disacchar-pentapeptide (Rosenberg et al., 1982; Paitan et al., 1999). Myxobacteria encode a variety of substances to attack prey cells, with antibiotics serving as a front line weapon. Antibiotics can act as small molecule weapons to penetrate and kill or neutralize the metabolism of prey. The prevention and control principle of myxovirescin against bacterial pathogens has been relatively clear. This substance can be applied to research on prevention and control of plant pathogenic bacteria.
3.4 Cystobactamid derivatives
Myxobacteria produce specialized metabolites when preying, the production of specialized metabolites and lytic proteins of myxobacteria is related to their predation (Akbar and Stevens, 2021). Müller et al. found that the target compound inhibiting P. aeruginosa and other bacterial pathogens had a UV absorption spectrum similar to that of cystobactamides. Cystobacamids are aromatic oligoamides that exert their natural antibacterial properties by inhibition of bacterial gyrases (Solga et al., 2024). The improved orthogonally functionalized methoxyaspartate of cystobactamides could expand the synthesis of new cystobactamides. At present, four new types of cystobactamides 919-1 (1), 919-2 (2), 920-1 (3), 920-2 (4) and cystobactamides 861-2 (5) had been successfully synthesized and measured. Moeller et al. (2019) compared the antibacterial properties of this class of substanaces, the cyano derivative of cystobactamide 861-2(5) had antimicrobial activity against Gram-negative bacteria and its activity was higher than that of any natural cystobactamide tested so far.
Culture isolation of C. coralline strain M23 yielded coralmycin A (1), B (2) and another derivative, cystobactin 919-2. Coralmycin A had the strongest antibacterial activity against Gram-negative bacteria and had a wide antibacterial spectrum. Coralmycin A and B were cystobactamid derivatives (Kim et al., 2016). Seven new coralmycin derivatives and three known compounds were also isolated from the another culture of strain M23. The coralmycin derivatives are C (1), D (2), E (3), F (4), G (5), H (6), and I (7), the known compounds are cystobactamide 891-2(8), 905-2(9), and 507(10). The compounds had DNA gyrase inhibitory activity and antibacterial activity. The β-methoxyasparagine structure of coralmycin may affect prey ingestion (Kim et al., 2019). Research on the structure of cystobactamides can be used to develop new structural substances with more antibacterial activity. Therefore, this type of substances may also have great potential in the prevention and control of Gram-negative plant pathogenic bacteria.
3.5 Hyalodione
Hyalodione isolated from the extract of the Hyalangium minutum strain NOCB-2T had antibacterial activity against P. aeruginosa. Hyalodione is a novel S-methyl cyclohexadiene-dione, which belongs to the class qinone (Herrmann et al., 2017). Hyaladione had broad antibacterial and antifungal activity. The tested strains included P. aeruginosa, Rhodotorula glutarum and Staphylococcus aureus (Okanya et al., 2012). Research on how hyalodione control bacteria and fungi need to be continued. Hyalodione also has great potential in controlling plant pathogenic bacteria and fungi.
3.6 Argyrin derivatives
The culture medium of the strains of Archangium gephyra contained a group of cyclic peptides composed of naturally produced octapeptides, which exhibited strong antibiotic effects against P. aeruginosa (Nickeleit et al., 2008; Stauch et al., 2010; Wieland et al., 2022). Among them, argyrin participates in the non-ribosomal peptide synthetase pathway, combining with elongation factor G (EF-G) as its target (Pogorevc et al., 2019). Argyrins inhibit protein synthesis by interfering with EF-G binding to the ribosome (Wieland et al., 2022). Several derivatives of argyrin A could be obtained by modifying the methoxytry ptophan residue. The methoxy group of this residue was crucial for its antibacterial activity and its activity will be lost if the position is replaced by other substituents (Siebert et al., 2019). Argyrin has antibacterial activity against bacteria, so there is great practical value to study its antibacterial activity against plant pathogenic bacteria.
3.7 Methyl (2R)-2-azido-3-hydroxyl-2-methylpropanoate and N-(3-amino-2-hydroxypropyl)-N-meth-ylsulfuric diamide
The predation of bacteria and fungi by predatory myxobacteria has been mostly studied, but the predation of oomycetes has received little attention. P. infestans is the pathogen that causes potato late blight, which is one of the most serious diseases of potato (Rix et al., 2002). Wu collected myxobacteria isolated from soil samples in central Inner Mongolia. Eighty-three percent of the myxobacterial strains were resistant to P. infestans, among which the strains of Myxococcus and Coralococcus accounted for a higher proportion. The strains with the most significant antibacterial activity were M. xanthus B25-I-1, M. fulvus B25-I-3 and M. stipitatus X6-II-1. Strain B25-I-1 exhibited antagonistic activity against a variety of fungi and bacteria, and its active substances reduced the content of soluble proteins and the activity of protective enzymes (PPO, POD, PAL, and SOD) in P. infestans, increased oxidative damage and cell membrane permeability. It had a strong inhibitory effect on the hyphae, asexual reproduction and sexual reproduction of P. infestans (Wu, 2018). The active substance of strain B25-I-3 showed a strong inhibitory effect on the growth of P. infestans, inhibited the growth of mycelium and asexual reproduction, and reduced the infection ability of pathogens (Wu, 2018; Wu Z. et al., 2021; Wu Z. H. et al., 2021). After the fermentation products of B25-I-1 and B25-I-3 were separated, it was found that the components that had antagonistic effects on P. infestans contained Methyl(2R)-2-azido-3-hydroxyl-2-methylpropanoate and N-(3-Amino-2-hydroxypropyl)-N-meth-ylsulfuricdiamide.
3.8 Some unknown substances
P. infestans causes devastating diseases by invading the leaves, stems and tubers of potato plants (Berleman and Kirby, 2009). M. xanthus YR-7 isolated from soil samples in Bayannur area of Inner Mongolia had significant resistance to P. infestans. The growth inhibition rate of strain YR-7 against P. infestans hyphae was as high as 96.67%. The fermentation product of YR-7 was tested in isolated leaves. The experimental results proved that the active substance against P. infestans is a non-protein substance (Ren et al., 2016). About 72% of the myxobacteria isolated from soil samples in Ordos and Wuhai areas of Inner Mongolia had varying degrees of antagonistic effects on the growth of P. infestans. The ones with stronger ability to inhibit oomycetes were C. exiguous E10, M. fallax E11 and C. coralloides E12. The diameters of the inhibition zones were 26 mm, 24 mm and 24 mm (Ding, 2017; Wu, 2018). About 78.75% of the myxobacteria isolated from soil samples in Alxa area of Inner Mongolia had varying degrees of activity against P. infestans. The resistance of Myxococcus fulvus AL-24 and Anqiococcu AL-10 was outstanding. The fermentation products of strain AL-24 and strain AL-10 had good infection prevention activity and weak infection treatment activity on detached potato leaves (Zhao, 2018). Myxobacteria isolated from Indian soil samples, the extracts of Coralococcus parvum S104 and S145 showed broad-spectrum antibacterial activity. The water extract (WE) and DMSO extract (DE) of GNDU172 showed obvious activity against B. subtilis , and the DE of strain S213 and strain S223 showed similar activity. The DE of S223 has activity against Pseudomonas syringae and B. cereus (Kumar et al., 2017). However, all of these active substances are unknow and need to be seperated and identified in the future.
4 Discussion
Plant pathogen is a major threat to crops worldwide, not only reducing crop yields but also causing significant damage to crop quality. In order to control or avoid excessive economic losses caused by plant pathogens, synthetic agrochemicals are often used in agricultural production as the most common method to control plant pathogens and improve crop yield quality. However, excessive use can cause adverse effects on the environment and human health.
Myxobacteria feed on bacteria and fungi in the soil and obtain nutrients by preying on other microorganisms. The cell wall of plant pathogens protects cells from external invasion and is a key barrier between predatory myxobacteria and prey cells (Berleman and Kirby, 2009). In order to break through the cell wall barrier, Myxobacteria have evolved targeted preying methods. One method is to degrade the cell wall or increase the permeability of the cell membrane through antibacterial metabolites and cell wall degrading enzymes. The other one is a targeted, contactdependent killing mechanism through the Tad-like system and protein secretion system (Thiery et al., 2022). The Tad-like system mediates the contact-dependent killing of myxobacteria on prey cells (Seef et al., 2021).
The general method for myxobacteria to control plant pathogenic bacteria is the first method mentioned above. During the interaction between myxobacteria and prey cells, antibiotics, lytic enzymes, hydrolases, etc. involved in the process. This review summarizes the prevention and control principles of myxobacteria in plant pathogenic fungi, oomycetes, and bacterial disasters (Table 1). Myxobacteria secrete enzymes that can degrade cell wall components. Myxobacteria produce small molecule compounds that inhibit the normal growth and proliferation of pathogens. The enzymes secreted by myxobacteria are mainly chitinase, β-1,6-glucanase and β-1,3-glucanase, etc, which increase intracellular osmotic pressure by degrading the cell wall of prey cells, promoting cell lysis and death. Some small molecule compounds produced by myxobacteria affect the normal growth and reproduction of prey cells or changing their permeability, thereby inducing apoptosis of prey cells.
In the process of preventing and controlling plant diseases, it is possible to use myxobacteria to develop a certain BCAs that inhibits pathogens efficiently, which will be helpful to reduce the cost increase and environmental pollution caused by excessive use of pesticides, and maximize economic and environmental benefits.
In addition to preventing and controlling plant diseases in agriculture, myxobacteria can also be used to increase production, storage, and transportation of agricultural and sideline products. For example, myxobacteria-mediated paper impregnated with silver nanoparticles (AgNPs) is used in fruit packaging to extend the shelf life to 15 days (Bhople et al., 2016).
At present, there are only relevant reports on the prevention and control of plant diseases by myxobacteria on fungi, oomycetes and bacteria. There are no reports on the prevention and control of plant virus damage. The principle of prevention and treatment of animal viruses by myxobacteria is to inhibit the activity of RNA-dependent RNA polymerase complex, ion channel inhibitor, and signal pathway inhibition. Myxobacteria are also very likely to inhibit RNA synthesis or inhibit signaling pathways in the prevention and control of plant virus damage. Therefore, myxobacteria also have great research value in the prevention and control of plant virus damage.
Author contributions
LZ: Conceptualization, Investigation, Software, Writing – original draft. LB: Methodology, Writing – review & editing. SL: Data curation, Formal analysis, Writing – original draft. YL: Resources, Writing – review & editing. HL: Project administration, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (no. 32260696) and the Science and Technology Plan Project of Inner Mongolia Autonomous Region (no. 2021GG0079).
Acknowledgments
The authors appreciate the support of the National Natural Science Foundation of China and the Science and Technology Program of Inner Mongolia Autonomous region and the help of College of Life Sciences of Inner Mongolia Agricultural University and other members in the lab.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1294854/full#supplementary-material
References
Akbar, S., and Stevens, D. C. (2021). Functional genomics study of Pseudomonas putida to determine traits associated with avoidance of a myxobacterial predator. Sci. Rep. 11:16445. doi: 10.1038/s41598-021-96046-8
Arguelles-Arias, A., Ongena, M., Halimi, B., Lara, Y., Brans, A., Joris, B., et al. (2009). Bacillus amyloliquefaciens GA1 as a source of potent antibiotics and other secondary metabolites for biocontrol of plant pathogens. Microb. Cell Factories 8:63. doi: 10.1186/1475-2859-8-63
Bader, C. D., Panter, F., Garcia, R., Tchesnokov, E. P., Haid, S., Walt, C., et al. (2022). Sandacrabins - structurally unique antiviral RNA polymerase inhibitors from a rare myxobacterium. Chem. Eur. J. 28:e202104484. doi: 10.1002/chem.202104484
Berleman, J. E., Allen, S., Danielewicz, M. A., Remis, J. P., Gorur, A., Cunha, J., et al. (2014). The lethal cargo of Myxococcus xanthus outer membrane vesicles. Front. Microbiol. 5:474. doi: 10.3389/fmicb.2014.00474
Berleman, J. E., and Kirby, J. R. (2009). Deciphering the hunting strategy of a bacterial wolfpack. FEMS Microbiol. Rev. 33, 942–957. doi: 10.1111/j.1574-6976.2009.00185.x
Bhople, S., Gaikwad, S., Deshmukh, S., Bonde, S., Gade, A., Sen, S., et al. (2016). Myxobacteria-mediated synthesis of silver nanoparticles and their impregnation in wrapping paper used for enhancing shelf life of apples. IET Nanobiotechnol. 10, 389–394. doi: 10.1049/iet-nbt.2015.0111
Bowman, S. M., and Free, S. J. (2006). The structure and synthesis of the fungal cell wall. Bio Essays 28, 799–808. doi: 10.1002/bies.20441
Davies, J., Spiegelman, G. B., and Yim, G. (2006). The world of subinhibitory antibiotic concentrations. Curr. Opin. Microbiol. 9, 445–453. doi: 10.1016/j.mib.2006.08.006
Ding, Y. (2017). Isolation and Identification of Myxobacteria from the Ordos Plateau Area and Preliminary Analysis of Their Antagonistic Activity Against Phytophthora infestans. Master, Innebr Mongolia Agricultural University. Available online at: https://wap.cnki.net/touch/web/Dissertation/Article/10129-1017211855.nh.html (Accessed January 23, 2024).
Dong, H., Gao, R., Dong, Y., Yao, Q., and Zhu, H. (2023). Whole-genome sequencing of a biocontrol Myxococcus xanthus R31 isolate and comparative genomic analysis. Gene 863:147286. doi: 10.1016/j.gene.2023.147286
Gemperlein, K., Zaburannyi, N., Garcia, R., La Clair, J., and Müller, R. (2018). Metabolic and biosynthetic diversity in marine myxobacteria. Mar. Drugs 16:314. doi: 10.3390/md16090314
Herrmann, J., Fayad, A. A., and Müller, R. (2017). Natural products from myxobacteria: novel metabolites and bioactivities. Nat. Prod. Rep. 34, 135–160. doi: 10.1039/c6np00106h
Iizuka, T., Jojima, Y., Fudou, R., Hiraishi, A., Ahn, J.-W., and Yamanaka, S. (2003a). Plesiocystis pacifica gen. nov., sp. nov., a marine myxobacterium that contains dihydrogenated menaquinone, isolated from the Pacific coasts of Japan. Int. J. Syst. Evol. Microbiol. 53, 189–195. doi: 10.1099/ijs.0.02418-0
Iizuka, T., Jojima, Y., Fudou, R., Tokura, M., Hiraishi, A., and Yamanaka, S. (2003b). Enhygromyxa salina gen. nov., sp. nov., a slightly halophilic myxobacterium isolated from the coastal areas of Japan. Syst. Appl. Microbiol. 26, 189–196. doi: 10.1078/072320203322346038
Kaushal, G., and Singh, S. P. (2020). Comparative genome analysis provides shreds ofmolecular evidence for reclassification of LeuconostocmesenteroidesMTCC 10508 as a strain of Leu. suionicum. Genomics 112, 4023–4031. doi: 10.1016/j.ygeno.2020.06.040
Kim, B.-M., Minh, N. V., Choi, H.-Y., and Kim, W.-G. (2019). Coralmycin derivatives with potent anti-gram negative activity produced by the myxobacteria Corallococcus coralloides M23. Molecules 24:1390. doi: 10.3390/molecules24071390
Kim, Y. J., Kim, H.-J., Kim, G.-W., Cho, K., Takahashi, S., Koshino, H., et al. (2016). Isolation of Coralmycins a and B, potent anti-gram negative compounds from the Myxobacteria Corallococcus coralloides M23. J. Nat. Prod. 79, 2223–2228. doi: 10.1021/acs.jnatprod.6b00294
Kumar, S., Yadav, A. K., Chambel, P., and Kaur, R. (2017). Molecular and functional characterization of myxobacteria isolated from soil in India. 3 Biotech 7:112. doi: 10.1007/s13205-017-0722-9
Li, Z., Xia, C., Wang, Y., Li, X., Qiao, Y., Li, C., et al. (2019a). Identification of an endo-chitinase from Corallococcus sp. EGB and evaluation of its antifungal properties. Int. J. Biol. Macromol. 132, 1235–1243. doi: 10.1016/j.ijbiomac.2019.04.056
Li, Z., Ye, X., Liu, M., Xia, C., Zhang, L., Luo, X., et al. (2019b). A novel outer membrane ß-1,6-glucanase is deployed in the predation of fungi by myxobacteria. ISME J. 13, 2223–2235. doi: 10.1038/s41396-019-0424-x
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. doi: 10.1111/j.1364-3703.2012.00804.x
Moeller, M., Norris, M. D., Planke, T., Cirnski, K., Herrmann, J., Müller, R., et al. (2019). Scalable syntheses of methoxyaspartate and preparation of the antibiotic cystobactamid 861-2 and highly potent derivatives. Organ. Lett. 21, 8369–8372. doi: 10.1021/acs.orglett.9b03143
Nickeleit, I., Zender, S., Sasse, F., Geffers, R., Brandes, G., Sörensen, I., et al. (2008). Argyrin A reveals a critical role for the tumor suppressor protein p27kip1 in mediating antitumor activities in response to proteasome inhibition. Cancer Cell 14, 23–35. doi: 10.1016/j.ccr.2008.05.016
Odintsov, S. G., Sabala, I., Marcyjaniak, M., and Bochtler, M. (2004). Latent LytMat 1.3Å resolution. J. Mol. Biol. 335, 775–785. doi: 10.1016/j.jmb.2003.11.009
Okanya, P. W., Mohr, K. I., Gerth, K., Steinmetz, H., Huch, V., Jansen, R., et al. (2012). Hyaladione, an S-methyl Cyclohexadiene-dione from Hyalangium minutum. J. Nat. Prod. 75, 768–770. doi: 10.1021/np200776v
Oren, A., and Garrity, G. M. (2021). Valid publication of the[[Inline Image]] names of forty-two phyla of prokaryotes. Int. J. Syst. Evol. Microbiol. 71:4. doi: 10.1099/ijsem.0.005056
Paitan, Y., Orr, E., Ron, E. Z., and Rosenberg, E. (1999). A nonessential signal peptidase II (Lsp) of Myxococcus xanthus might be involved in biosynthesis of the polyketide antibiotic TA. J. Bacteriol. 181, 5644–5651. doi: 10.1128/JB.181.18.5644-5651.1999
Pietro, A. D., Madrid, M. P., Caracuel, Z., Delgado-Jarana, J., and Roncero, M. I. G. (2003). Fusarium oxysporum: exploring the molecular arsenal of a vascular wilt fungus. Mol. Plant Pathol. 4, 315–325. doi: 10.1046/j.1364-3703.2003.00180.x
Pogorevc, D., Tang, Y., Hoffmann, M., Zipf, G., Bernauer, H. S., Popoff, A., et al. (2019). Biosynthesis and heterologous production of argyrins. ACS Synth. Biol. 8, 1121–1133. doi: 10.1021/acssynbio.9b00023
Ren, X. (2016). Isolation and Identification of Myxobacteria from Soil in Bayannaoer Area and Preliminary Study on their Antibiotic Activities Against Phytophthora Infestans Master, Inner Mongolia Agricultural University. Available at: https://cdmd.cnki.com.cn/Article/CDMD-10129-1016249574.htm
Rix, U., Fischer, C., Remsing, L. L., and Rohr, J. (2002). Modification of post-PKS tailoring steps through combinatorial biosynthesis. Nat. Prod. Rep. 19, 542–580. doi: 10.1039/b103920m
Rosenberg, E., Fytlovitch, S., Carmeli, S., and Kashman, Y. (1982). Chemical properties of Myxococcus xanthus antibiotic TA. J. Antibiot. 35, 788–793. doi: 10.7164/antibiotics.35.788
Ryosuke, F., Yasuko, J., Takashi, I., and Yamanaka, S. (2002). Haliangium ochraceum gen. nov., sp. nov. and Haliangium tepidum sp. nov.: novel moderately halophilic myxobacteria isolated from coastal saline environments. J. Gen. Appl. Microbiol. 48, 109–116. doi: 10.2323/jgam.48.109
Saggu, S. K., Nath, A., and Kumar, S. (2023). Myxobacteria: biology and bioactive secondary metabolites. Res. Microbiol. 174:104079. doi: 10.1016/j.resmic.2023.104079
Seef, S., Herrou, J., de Boissier, P., My, L., Brasseur, G., Robert, D., et al. (2021). A Tad-like apparatus is required for contact-dependent prey killing in predatory social bacteria. eLife 10:e72409. doi: 10.7554/eLife.72409
Selitrennikoff, C. P. (2001). Antifungal Proteins. Appl. Environ. Microbiol. 67, 2883–2894. doi: 10.1128/aem.67.7.2883-2894.2001
Sharma, A., Kumar, A., Babu, V., Ali, A., and Katoch, M. (2021). Myxobacteria from animal dung pellets collected from northwestern Himalayas: a new source of di-isobutyl phthalate. J. Basic Microbiol. 62, 162–173. doi: 10.1002/jobm.202100518
Shehata, A. N., Abd El Aty, A. A., Darwish, D. A., Abdel Wahab, W. A., and Mostafa, F. A. (2018). Purification, physicochemical and thermodynamic studies of antifungal chitinase with production of bioactive chitosan-oligosaccharide from newly isolated aspergillus griseoaurantiacus KX010988. Int. J. Biol. Macromol. 107, 990–999. doi: 10.1016/j.ijbiomac.2017.09.071
Shen, E., Wang, X., Lu, Z., Zhou, F., Ma, W., Cui, Z., et al. (2023). Overexpression of a beta-1, 6-glucanase gene GluM in transgenic rice confers high resistance to rice blast, sheath blight and false smut. Pest Manag. Sci. 79, 2152–2162. doi: 10.1002/ps.7394
Siebert, D. C. B., Sommer, R., Pogorevc, D., Hoffmann, M., Wenzel, S. C., Müller, R., et al. (2019). Chemical synthesis of tripeptide thioesters for the biotechnological incorporation into the myxobacterial secondary metabolite argyrin via mutasynthesis. Beilstein J. Org. Chem. 15, 2922–2929. doi: 10.3762/bjoc.15.286
Solga, D., Wieske, L. H. E., Wilcox, S., Zeilinger, C., Jansen-Olliges, L., Cirnski, K., et al. (2024). Is simultaneous binding toDNA and gyrase important for the antibacterial activity of cystobactamids? Chemistry 13:e202303796. doi: 10.1002/chem.202303796
Stauch, B., Simon, B., Basile, T., Schneider, G., Malek, N. P., Kalesse, M., et al. (2010). Elucidation of the structure and intermolecular interactions of a reversible cyclicpeptide inhibitor of the proteasome by NMR spectroscopy and molecular modeling. Angew. Chem. Int. Ed. 49, 3934–3938. doi: 10.1002/anie.201000140
Sutton, D., Livingstone, P. G., Furness, E., Swain, M. T., and Whitworth, D. E. (2019). Genome-wide identification of myxobacterial predation genes and demonstration of formaldehyde secretion as a potentially predation-resistant trait of Pseudomonas aeruginosa. Front. Microbiol. 10:2650. doi: 10.3389/fmicb.2019.02650
Talbot, N. J. (2003). On the trail of a cereal killer: exploring the biology of Magnaporthe grisea. Annu. Rev. Microbiol. 57, 177–202. doi: 10.1146/annurev.micro.57.030502.090957
Thiery, S., Turowski, P., Berleman, J. E., and Kaimer, C. (2022). The predatory soil bacterium Myxococcus xanthus combines a Tad- and an atypical type 3-like protein secretion system to kill bacterial cells. Cell Rep. 40:111340. doi: 10.1016/j.celrep.2022.111340
Waite, D. W., Chuvochina, M., Pelikan, C., Parks, D. H., Yilmaz, P., Wagner, M., et al. (2020). Proposal to reclassify the proteobacterial classes Deltaproteobacteria and Oligoflexia, and the phylum Thermodesulfobacteria into four phyla reflecting major functional capabilities. Int. J. Syst. Evol. Microbiol. 70, 5972–6016. doi: 10.1099/ijsem.0.004213
Weissman, K. J., and Müller, R. (2010). Myxobacterial secondary metabolites: bioactivities and modes-of-action. Nat. Prod. Rep. 27, 1276–1295. doi: 10.1039/c001260m
Wieland, M., Holm, M., Rundlet, E. J., Morici, M., Koller, T. O., Maviza, T. P., et al. (2022). The cyclic octapeptide antibiotic argyrin B inhibits translation by trapping EFG on the ribosome during translocation. Proc. Natl. Acad. Sci. U.S.A. 119:e2114214119. doi: 10.1073/pnas.2114214119
Willsey, G. G., Wargo, M. J., and O’Toole, G. A. (2016). Sarcosine catabolism in Pseudomonas aeruginosa is transcriptionally regulated by SouR. J. Bacteriol. 198, 301–310. doi: 10.1128/jb.00739-15
Wu, Z. (2018). Isolation of Myxobacteria from the Central Region of Inner Mongolia and their Activity and Components Against Potato Late Blight Pathogen Doctor, Inner Mongolia Agricultural University. Available at: https://cdmd.cnki.com.cn/Article/CDMD-10129-1018881788.htm
Wu, Z., Cui, H., Sun, Z., and Liu, H. (2021). Biocontrol mechanism of Myxococcus xanthus B25-I-1 against Phytophthora infestans. Pestic. Biochem. Physiol. 175:104832. doi: 10.1016/j.pestbp.2021.104832
Wu, Z. H., Ma, Q., Sun, Z. N., Cui, H. C., and Liu, H. R. (2021). Biocontrol mechanism of Myxococcus fulvus B25-I-3 against Phytophthora infestans and its control efficiency on potato late blight. Folia Microbiol. 66, 555–567. doi: 10.1007/s12223-021-00865-1
Xiao, Y., Wei, X., Ebright, R., and Wall, D. (2011). Antibiotic production by Myxobacteria plays a role in predation. J. Bacteriol. 193, 4626–4633. doi: 10.1128/jb.05052-11
Ye, X., Chen, Y., Ma, S., Yuan, T., Wu, Y., Li, Y., et al. (2020a). Biocidal effects of volatile organic compounds produced by the myxobacterium Corrallococcus sp. EGB against fungal phytopathogens. Food Microbiology 91:103502. doi: 10.1016/j.fm.2020.103502
Ye, X., Li, Z., Luo, X., Wang, W., Li, Y., Li, R., et al. (2020b). A predatory myxobacterium controls cucumber fusarium wilt by regulating the soil microbial community. Microbiome 8:49. doi: 10.1186/s40168-020-00824-x
Zhang, L., Dong, C., Wang, J., Liu, M., Wang, J., Hu, J., et al. (2023). Predation of oomycetes by myxobacteria via a specialized CAZyme system arising from adaptive evolution. ISME J. 17, 1089–1103. doi: 10.1038/s41396-023-01423-y
Zhao, P. (2018). Isolation and Identification of Myxobacteria in Alashan Area and Preliminary Study on their Antibiotic Activity Against Phytophthora infestans Master, Inner Mongolia Agricultural University. Available at: https://cdmd.cnki.com.cn/Article/CDMD-10129-1018882358.htm
Keywords: myxobacteria, plant diseases, biological control, carbohydrate-active enzymes, small molecule compounds
Citation: Zhang L, Bao L, Li S, Liu Y and Liu H (2024) Active substances of myxobacteria against plant diseases and their action mechanisms. Front. Microbiol. 14:1294854. doi: 10.3389/fmicb.2023.1294854
Edited by:
Li Zhoukun, Nanjing Agricultural University, ChinaReviewed by:
Yuqiang Zhao, Jiangsu Province and Chinese Academy of Sciences, ChinaOrlando Borras-Hidalgo, Qilu University of Technology, China
Copyright © 2024 Zhang, Bao, Li, Liu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huirong Liu, aHVpcm9uZ19saXVAaW1hdS5lZHUuY24=
 Lele Zhang
Lele Zhang Liangliang Bao2
Liangliang Bao2 Huirong Liu
Huirong Liu