- Department of Ecology, Jinan University, Guangzhou, China
Feeding effects are crucial for evaluating the capacity of zooplankton to regulate phytoplankton populations within freshwater ecosystems. To examine the impact of the bloom-forming cyanobacteria Raphidiopsis raciborskii, which occurs in tropical and subtropical freshwaters, on the growth of zooplankton Daphnia in relation to toxins, filament length and fatty acid content, we fed D. magna with R. raciborskii only (cylindrospermopsin (CYN)-producing and non-CYN-producing, as the negative controls), Chlorella pyrenoidosa only (as the positive control) and a mixed diet containing R. raciborskii (CYN-producing and non-CYN-producing) and C. pyrenoidosa. Consequently, our findings revealed that the toxic effect of CYN-producing R. raciborskii strains on Daphnia was mitigated by the coexistence of C. pyrenoidosa containing stearidonic acid (SDA, C18:4 ω3) in mixed diets. This was evident in the elevated survival rate compared that from diets containing only R. raciborskii and a significantly higher reproduction and population intrinsic increase rate compared to diets consisting of only R. raciborskii or C. pyrenoidos. Additionally, a strong positive correlation was observed between arachidonic acid (ARA, 20:4ω6) and the population intrinsic increase rate of Daphnia; notably, R. raciborskii strains were found to be rich in the ω6 polyunsaturated fatty acid ARA. These outcomes reinforce the crucial role of polyunsaturated fatty acids in predicting the population increase of crustacean zooplankton, which has long been neglected. Furthermore, our results underscore the potential effectiveness of zooplankton, particularly in temperate lakes, in controlling CYN-producing R. raciborskii populations.
Introduction
The outbreak of cyanobacterial blooms, a symptom of eutrophication in water bodies, disrupts the balance of aquatic ecosystems. Certain harmful cyanobacteria have the ability to produce hepatotoxins or neurotoxins, as well as other unknown toxic compounds, which have a serious negative impact on the safety of aquatic organisms and human health (Dittmann and Wiegand, 2006; Buratti et al., 2017). Raphidiopsis raciborskii (previously known as Cylindrospermopsis raciborskii), recognized as one of the most successful bloom-forming cyanobacteria in freshwater, has been described as a tropical species. However, its recent expansion into temperate regions has made it a cosmopolitan species in freshwater systems around the world (Antunes et al., 2015; Wu et al., 2022). Notably, R. raciborskii can produce diverse cyanotoxins, including cynlindrospermopsin (CYN) and saxitoxin. Exposure to CYN may result in severe cytotoxicity, genotoxicity, and reproductive toxicity, posing a serious risk to the health of both humans and animals (Buratti et al., 2017).
Zooplankton, encompassing a vital group of primary consumers, play an important role as effective grazers of phytoplankton; nevertheless, they are assumed to be negatively affected by cyanobacterial metabolites (toxicity hypothesis) (Codd, 2000; Ger et al., 2014; Lyu et al., 2016a,b). Initially, this toxicity only referred to microcystins, and currently, the focus has shifted toward other cyanotoxins (Wilson et al., 2006; Schwarzenberger, 2022). Among these, CYN is the most commonly reported compound produced by R. raciborskii (Rzymski and Poniedziałek, 2014). Feeding zooplankton with a CYN-producing R. raciborskii led to higher mortality and lower growth in D. magna juveniles compared to feeding them a non-CYN-producing strain (Nogueira et al., 2004, 2006). Intriguingly, a CYN-producing strain did not exhibit lethal toxicity toward three Daphnia species (Hawkins and Lampert, 1989). Due to limited studies, the effects of CYN-producing R. raciborskii on Daphnia remain elusive (Schwarzenberger, 2022). Toxic effects seem to be strain specific, and different Daphnia species display different sensitivities to cyanotoxin exposure (Wilson et al., 2006; Ferrão-Filho et al., 2008; Costa et al., 2013). Additionally, it is noteworthy that zooplankton possess the ability to gradually develop desensitization to toxins through a series of adaptive mechanisms when coexisting with cyanobacteria (Kirk and Gilbert, 1992; Ka et al., 2012; Lyu et al., 2016a), minimizing adverse effects arising from toxin exposure.
The reduced feeding activity of Daphnia when fed R. raciborskii has been attributed to potential mechanical interference caused by long filaments impeding the feeding apparatus of grazers (Gliwicz and Lampert, 1990; DeMott et al., 2001; Bednarska et al., 2014), hence leading to negative effects on the growth and reproduction of Daphnia. However, Rangel et al. (2016) argued that toxicity may override morphology regarding the effects of toxic R. raciborskii on zooplankton. Some laboratory experiments even demonstrated that the filament length of R. raciborskii did not have a distinct influence on the clearance rates of D. magna (Panosso and Lürling, 2010). D. galeata actually benefits from the presence of filaments in the food suspension (Abrusán, 2004). Furthermore, by synthesizing data from 66 published laboratory studies, representing 597 experimental comparisons, Wilson et al. (2006) revealed that filamentous cyanobacteria were indeed found to be notably better food sources for grazers than single-celled cyanobacteria across all the studies. Thus, feeding inhibition by filaments may not hold the same level of significance as previously described.
Apart from filament length or toxins, the poor food quality offered by cyanobacteria may also exert adverse effects on the growth and reproduction of zooplankton. iTRAQ-Based proteomic profiling indicated that when exposing to microcystin-producing and microcystin-free Microcystis aeruginosa, Daphnia showed 94 and 117 differentially expressed proteins respectively, all of which correspond to changes in metabolism necessary to adjust the body growth rate of Daphnia (Lyu et al., 2016b). Food quality, including various essential elements and biochemicals, may constrain consumer performance by specifically affecting physiological processes and thus disrupt energy flow in aquatic food webs (Becker and Boersma, 2005; Ruiz et al., 2021). The essential biochemicals cyanobacteria lack but are vital for consumers include polyunsaturated fatty acids (PUFAs), especially eicosapentaenoic acid (EPA, C20: 5ω3, Gulati and DeMott, 1997), or alternative resources, such as an effective EPA enhancing fatty acid, namely, stearidonic acid (SDA, C18:4 ω3, Lenihan-Geels et al., 2013; Abonyi et al., 2023). These PUFAs play a crucial role in maintaining membrane structure and function and serve as precursors for bioactive compounds in both vertebrates and invertebrates, and their de novo synthesis is very scarce (Kainz et al., 2009; Twining et al., 2021). In addition, these ω3 fatty acids have been reported to attenuate the toxic effects of various oxidative stresses in mammals (Haimeur et al., 2012; Sakai et al., 2017). The deficiency of EPA in diets restricts the growth of zooplankton (Müller-Navarra, 1995a; Brett et al., 2009; Tang et al., 2019), impacting the performance of crustacean grazers within aquatic ecosystems according to lake investigations and experimental studies (Müller-Navarra et al., 2000; Tang et al., 2023). More importantly, the lack of dietary supply of both EPA and SDA can dramatically affect the reproduction of Daphnia due to the high investment of EPA in eggs (Wacker and Martin-Creuzburg, 2007; Kainz et al., 2009). Consequently, cyanobacteria can hardly support the somatic growth and reproduction of zooplankton even in the absence of toxins (Lampert, 1987). To date, no research has revealed the fatty acid profile of R. raciborskii. However, we assume that R. raciborskii strains, being cyanobacteria, also lack EPA or SDA.
Despite the shortage of EPA or SDA, R. raciborskii strains could provide essential nutritional components necessary for zooplankton. These components encompass carbohydrates, proteins, and common saturated and unsaturated fatty acids. It is noted that feeding Daphnia with R. raciborskii cells only is rarely seen in nature. Concurrently, other phytoplankton species of considerable nutritional value coexist with R. raciborskii, even during Raphidiopsis blooms (Soares et al., 2009; Chislock et al., 2014; Frau et al., 2018). Given the relatively low EPA need in zooplankton (Müller-Navarra et al., 2000; Becker and Boersma, 2005; Tang et al., 2019). Wenzel et al. (2021) proposed that food sources, such as bacteria, which may not fully meet grazers’ dietary needs, could still confer nutritional benefits if other complementary food components are available in sufficient quantities to compensate for any biochemical deficiencies. Interestingly, even lower-quality food such as vascular plants can be utilized by zooplankton when simultaneously provided with algal food (Taipale et al., 2016; Tang et al., 2021). Considering the role of ω3 fatty acids in detoxication, we hypothesized that the performance of zooplankton feeding R. raciborskii would be dramatically improved by the concurrent feeding of good-quality algae. Reis et al. (2023) reported that the fitness of these small-bodied cladocerans feeding on R. raciborskii was improved when the supply of nutritious food increased from 10 to 50% in proportion. In natural lakes, the co-occurrence of R. raciborskii bloom and filter-feeding zooplankton is commonly seen as previously reported (Bouvy et al., 2001; Leonard and Paerl, 2005; Soares et al., 2009; Gao et al., 2022).
To test our hypothesis, we conducted a feeding experiment to compare the dietary effect of R. raciborskii only (CYN- and non-CYN-producing strains), Chlorella pyrenoidosa only and a mixed diet of R. raciborskii and C. pyrenoidosa on the growth, reproduction and population dynamics of Daphnia magna. Additionally, we analyzed the fatty acid profile of these different dietary algal strains to uncover the role of specific fatty acids in influencing the growth and reproduction of Daphnia. Our findings revealed that the mixed diet led to a higher survival rate of Daphnia compared to the R. raciborskii only diet and an even higher population intrinsic growth rate compared to the C. pyrenoidos only diet. The presence of C. pyrenoidosa appears to diminish the toxic effect of CYN-producing R. raciborskii strains. In addition, the strongly positive relationship between ω6 PUFA and the population intrinsic increase rate of Daphnia, as well as the rich content of ω6 PUFA ARA in R. raciborskii, indicates that R. raciborskii might be beneficial for the population increase of Daphnia as a nutritional supplement. Elevated zooplankton populations to phytoplankton ratios normally indicate a more robust capacity for phytoplankton control (Søndergaard et al., 2008; Jeppesen et al., 2012). Thus, our results point toward the potential for employing a top-down biomanipulation approach to control R. raciborskii blooms, particularly in temperate areas.
Materials and methods
Experimental algae and animals
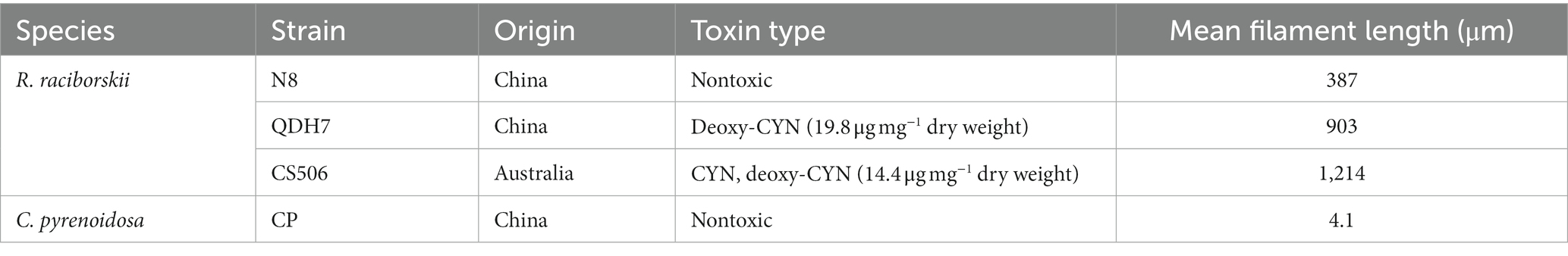
The green algae Chlorella pyrenoidosa was obtained from the Institute of Hydrobiology, Jinan University. Two CYN-producing strains (R. raciborskii CS506 and QDH7) and one non-CYN-producing strain (R. raciborskii N8) were used in the experiments. R. raciborskii CS506 was obtained from the Australian National Algae Culture Collection (ANACC) and can produce CYN and deoxy-CYN (Willis et al., 2015). Strain QDH7 mainly produced deoxy-CYN, which was identified by LC–MS/MS analysis (Lu et al., 2020). R. raciborskii N8 is a nontoxic strain due to the absence of the CYN biosynthesis gene cluster in its whole genome (Chen et al., 2022). All four strains were grown on BG11 medium at 28°C at a light intensity of 60 μmol m−2 s−1 in a 12:12 h light/dark cycle. Under these conditions, C. pyrenoidosa grew as single-cell populations with an average diameter of 4.1 μm. Filaments of R. raciborskii N8, QDH7 and CS506 had average lengths of 387 μm, 902 μm and 1,214 μm, respectively (Table 1).
The cladoceran D. magna was maintained at 20°C and fed with the green algae C. pyrenoidosa in 1-L glass jars. Water from Liuxihe Reservoir in Guangzhu city was used to prepare all media after sequential filtration through a 1.2 and 0.45-μm filter. The filtrate was stored statically at 25°C for 2 days before use. Neonates (<24 h old) were randomly chosen from parthenogenetically reproducing females for the life history experiments.
Feeding experiments
Daphnia magna was fed three different kinds of diets: C. pyrenoidosa only, 1:1 mixtures of C. pyrenoidosa with either R. raciborskii N8, QDH7 or CS506, and R. raciborskii (N8, QDH7 or CS506) only. All diets had a fixed total food concentration of 2 mg C L−1. The food carbon concentrations were determined using OD (optical extinction) values according to the regression curve we built for OD and carbon concentration for different algae at 682 nm.
Before the life history experiments, neonates (<24 h old) originating from the same broods were collected from beakers and starved for 4 h to empty their guts. Thirty neonates were randomly selected for each treatment. Each neonate was transferred into 50 mL of food suspension and incubated under the same conditions as the stock D. magna cultures. Each selected neonate was transferred daily to clean beakers with freshly prepared food suspensions. Any observed offspring were removed immediately after their presence was recorded. The experiments lasted for 15 days.
The body lengths of zooplankton were measured using a stereo binocular microscope, and the biomass was calculated based on the body length measurement. Somatic growth rates at the juvenile stage (g) were determined by assessing the increase in biomass from Day 1 (M1) to Day 7 (M7) within the experimental period (t = 6 d) using the Equation g = (ln M7 – ln M1)/t. In addition, the survival rate and the daily number of offspring produced were also recorded.
The intrinsic rates of increase r (d−1) in the population of D. magna were calculated using Euler’s formula as follows:
where x is the age or time interval (day), lx is the proportion of individuals surviving to age x, and mx represents the number of offspring produced per surviving female at age x.
Fatty acid analysis and CYN measurement
The fatty acids within the cells of C. pyrenoidosa and three R. raciborskii strains were extracted and esterified according to the method outlined by Wiltshire et al. (2000). Quantitative analysis was carried out using a gas chromatography–mass spectrometer (GC–MS) with a specific temperature configuration. The cultures of the four strains were collected by centrifugation and then subjected to freeze-drying. Approximately 20 mg of dry biomass was utilized to extract total lipids three times with dichloromethane/methanol (2:1, v/v), and the pooled cell-free extracts were evaporated to dryness. Subsequently, the extracted samples were transesterified with 3 mol L−1 methanolic HCl (60°C, 15 min). Fatty acids were analyzed with a Finnigan TRACE GC–MS equipped with a flame ionization detector and a DB-23 column (60 m × 0.32 mm). The fatty acid methyl esters (FAME) were quantified by comparison with standard Supelco 37 Component FAME mix or an internal standard (C12:0 methyl esters).
Total CYN concentrations in R. raciborskii QDH7 and CS506 cells were measured before the feeding experiments. Prior to CYN measurement, R. raciborskii cells were lysed by ultrasonic treatment, and insoluble cell debris was removed by centrifugation. The supernatant was then analyzed with a Cylindrospermopsin Plate Kit (Beacon Analytical Systems Inc., USA) in accordance with the manufacturer’s specifications.
Data analysis
To assess and compare the fatty acid (FA) content across different diet treatments, a one-way analysis of variance (ANOVA) was employed. Significant differences among treatments were then evaluated using the least significant difference (LSD) multiple comparison test. One-way ANOVA followed by the LSD test was also performed to identify differences in the growth and reproduction of D. magna between different diets. Regression analyses were performed to determine the relationship between population intrinsic increase rates and PUFA content in diets. An SPSS 22.0 statistical package was used for all statistical analyses. Before the statistical analysis, data were checked using a normal probability plot of the residuals and Levene’s test of homogeneity of variances for compliance of ANOVA assumptions and logarithmic transformation, if necessary. Detailed information on the statistics is presented in the Supplementary Table S1.
Results
Fatty acid composition
The green algae C. pyrenoidosa was characterized by high amounts of C16:0 and C18:0 saturated fatty acids, very small amounts of monounsaturated fatty acids and considerable amounts of PUFAs, mainly including C18:2ω6 (linoleic acid, LA), C18:3ω3 (linolenic acid, ALA) and C18:4ω3 (stearidonic acid, SDA) (Supplementary Table S1). All R. raciborskii strains lacked SDA but exhibited significantly higher levels of C20:4ω6 (arachidonic acid, ARA) than C. pyrenoidosa (Figure 1). Detailed information on the fatty acid profile of the algae is presented in the Supplementary Table S2.

Figure 1. The main PUFA concentrations (μg/mg C) of C. pyrenoidosa (CP) and three strains of R. raciborskii (N8, QDH7 and CS506). Error bars indicate 1SD. The “*” above the bars are significantly different from CP, as revealed by the independent-samples t test (*p < 0.05; **p < 0.01).
Dietary treatment effects on the performance of Daphnia individuals
In the case of pure R. raciborskii dietary treatments, all D. magna exposed to 100% QDH7 and 100% CS506 strains experienced toxicity, resulting in mortality within 9 days. When C. pyrenoidosa was added to the diets with two toxic R. raciborskii strains, the survival rate was greatly elevated to above 60% (Figure 2A).
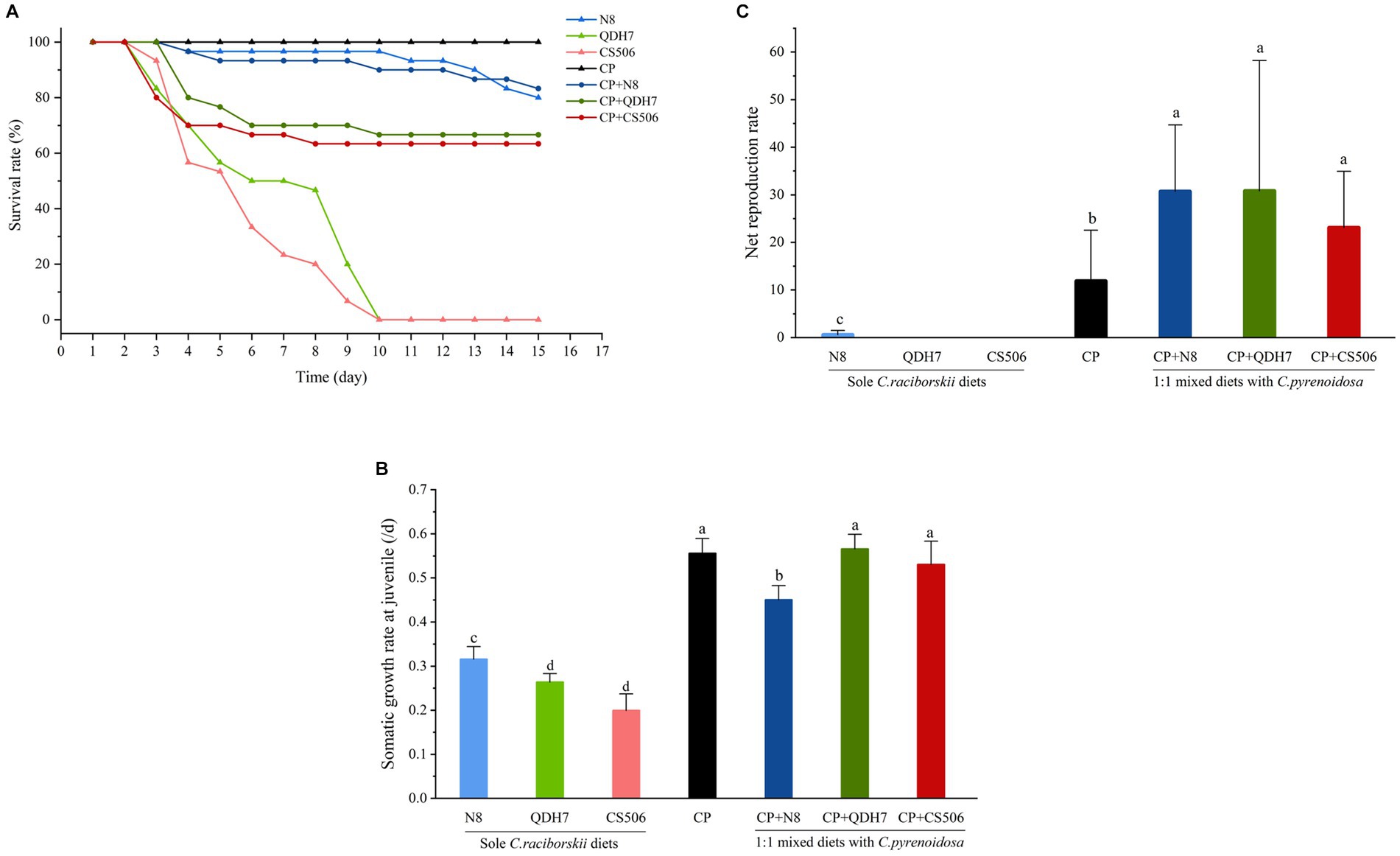
Figure 2. Individual performance of D. magna fed with pure diets of C. pyrenoidosa, three strains of R. raciborskii and 1:1 mixed diets with C. pyrenoidosa and R. raciborskii strains including survival rate (A) somatic growth rate at juvenile (B) and net reproduction rate if reproduced after juvenile (C). Different letters indicate significant differences (p < 0.05) using the LSD multiple comparison test.
Compared with the diet comprising only C. pyrenoidosa, the growth rates of D. magna fed only R. raciborskii were significantly lower (Figure 2B). D. magna exhibited better growth when fed pure diets of non-CYN-producing N8 compared to the CYN-producing strains (QDH7 and CS506). In terms of inhibiting D. magna growth, no significant difference was observed between the aforementioned two toxic strains. Interestingly, R. raciborskii supplemented with C. pyrenoidosa enhanced the growth of D. magna. Daphnia fed mixed diets displayed significantly better growth than those exclusively fed R. raciborskii, similar to the positive control group that was fed C. pyrenoidosa.
In the pure R. raciborskii dietary treatments, all D. magna exposed to 100% QDH7 and 100% CS506 strains did not reproduce. The D. magna on the pure diets of the non-CYN-producing N8 strain was able to reproduce successfully but showed the lowest net reproduction value. When all Daphnia were fed mixed diets, they exhibited robust net reproduction rates comparable to the positive control group (Figure 2C). The maximum net reproduction (net value) was observed in the 50% QDH7 treatment.
Intrinsic population increase rate of Daphnia and its relation with dietary PUFA supply
The intrinsic rate of population increase of Daphnia feeding mixed diets was found to be significantly higher than that of the positive control group feeding C. pyrenoidosa (Figure 3A). Among all the mixed dietary treatments, the maximal population intrinsic increase rate (mean value) was found in the 50% QDH7 treatment, and the lowest was found in the 50% N8 treatment. Since D. magna exposed to 100% QDH7 and 100% CS506 strains showed no reproduction, their population intrinsic increase rate could not be calculated. Regression analyses revealed a very strong correlation between the ARA content in diets and the population intrinsic increase rates of Daphnia in all dietary treatments containing SDA (Figure 3B).
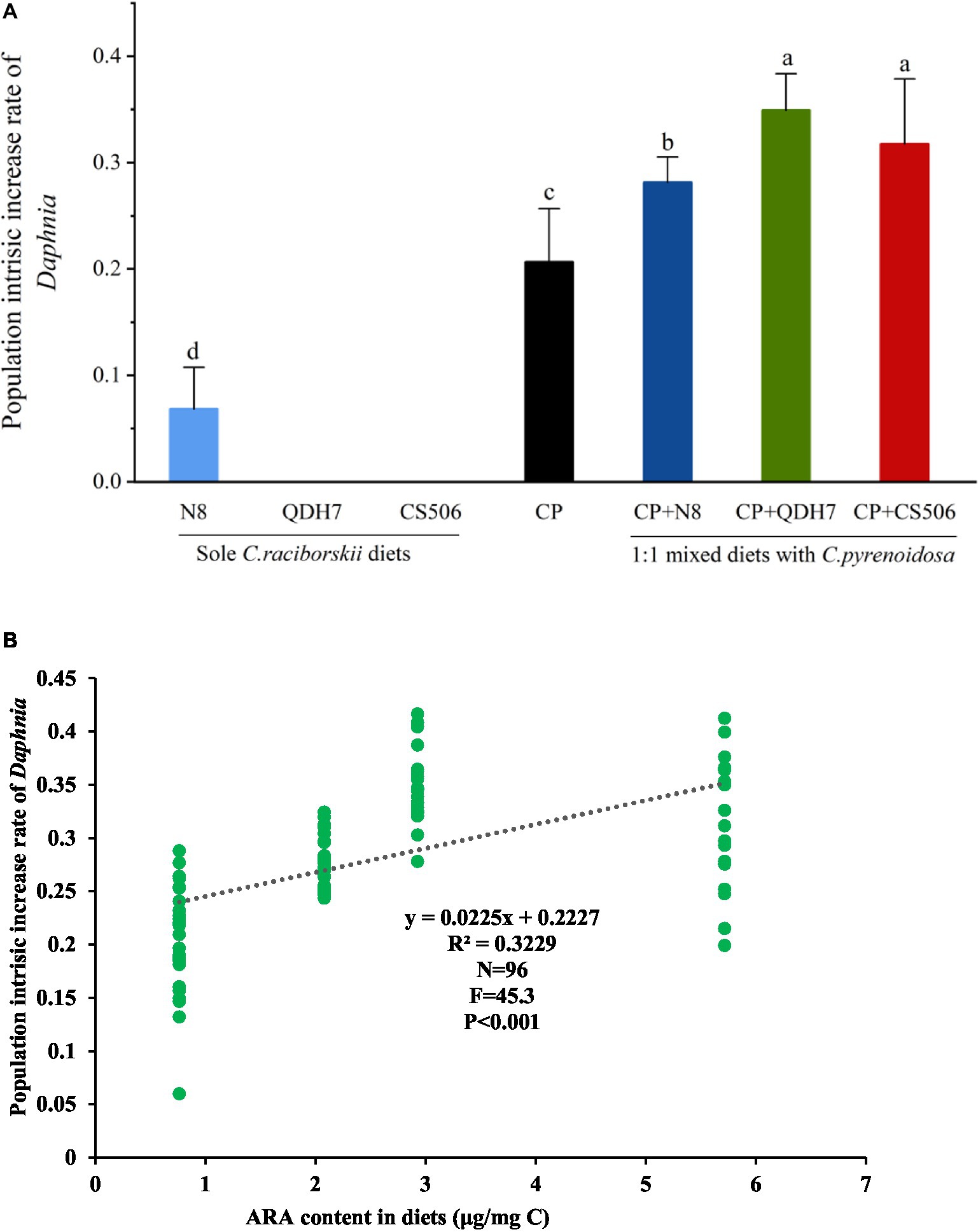
Figure 3. The intrinsic rate of population increase of D. magna fed with pure diets of C. pyrenoidosa, three strains of R. raciborskii and 1:1 mixed diets with C. pyrenoidosa and R. raciborskii strains if they reproduced (A) and its relation with dietary supply of arachidonic acid (ARA, 20:4 ω6) within stearidonic acid (SDA, 18:4 ω3)-containing diets (B). Different letters indicate significant differences (p < 0.05) using the LSD multiple comparison test.
Discussion
By setting up feeding experiments to compare the dietary impact of different food sources on the growth and reproduction of Daphnia, we found that the highest intrinsic population growth rate was observed in Daphnia fed a mixed diet, followed by those on a diet of C. pyrenoidosa, then non-CYN-producing R. raciborskii, and finally, the lowest growth rate was seen in Daphnia fed with CYN-producing R. raciborskii. Thus, a mitigation of the negative effect of CYN-producing and non-CYN-producing R. raciborskii on D. magna by C. pyrenoidosa as diet supplements was observed, inconsistent with our hypothesis.
In our study, the ability to produce CYN had a notable impact on the growth of Daphnia. Among the treatments involving a sole diet, Daphnia fed on two toxic strains exhibited significantly reduced growth and reproduction rates compared to those fed on nontoxic strain N8 or C. pyrenoidosa, consistent with the observation made by Nogueira et al. (2004, 2006), underscoring the detrimental effect of CYN toxins. Strikingly, the negative effects of the toxins produced by both toxic R. raciborskii strains on Daphnia were mitigated when C. pyrenoidosa was concurrently provided as part of their diets, particularly in terms of the intrinsic population increase rate.
Based on the fatty acid profiles of dietary algae, we further proposed that the differences in Daphnia performance across various dietary treatments can be attributed to variations in the fatty acid contents. One particularly crucial fatty acid, EPA, has been shown to limit Daphnia growth and reproduction both in laboratory settings and in natural environments, and it also plays a predictive role in carbon transfer between primary producers and consumers (Müller-Navarra, 1995b; Müller-Navarra et al., 2000). Both SDA and ALA can be converted to EPA by consumers (Kainz et al., 2009). Due to the low conversion rate of ALA to EPA, researchers have noted that supplying SDA may increase EPA levels more effectively than ALA supplementation by bypassing a rate-limiting step (Lenihan-Geels et al., 2013; Abonyi et al., 2023). The R. raciborskii strains lack both the crucial ω3 PUFA EPA and the alternative EPA precursor SDA. This deficiency in essential fatty acids contributes to the poor food quality of R. raciborskii and significantly limited the population increase of Daphnia in our study. Even when fed its non-CNY-producing strain, Daphnia showed considerably lower growth and reproduction rates than those fed C. pyrenoidosa. It was evident that food containing SDA (including mixed diets and C. pyrenoidosa only diet) significantly supported growth and reproduction when compared to food lacking SDA (such as different strains of R. raciborskii as only diets).
More importantly, it is worth noting that oxidative stress, induced by the rapid increase in the production of reactive oxygen species (ROS), represents a pivotal mechanism underlying CYN toxicity (Poniedzialek et al., 2015). EPA has been documented to attenuate oxidative stress-induced DNA damage and elevate glutathione peroxidase activity in mammals (Haimeur et al., 2012; Sakai et al., 2017). Moreover, increased levels of glutathione peroxidase have been demonstrated to participate in the detoxication of cyanotoxins, including CYN, in Daphnia (Nogueira et al., 2004; Lindsay et al., 2006; Schwarzenberger, 2022). Hence, it is plausible that the SDA derived from the supplemented C. pyrenoidosa enhanced the EPA content in zooplankton, eventually mitigating the adverse effects of toxic R. raciborskii strains on Daphnia magna. The mitigation of more dietary chlorophyte addition on the performance of Daphnia fed toxic R. raciborskii strains could also be demonstrated by data from Reis et al. (2023) for growth and reproduction, as well as Panosso and Lürling (2010) for feeding rate, supporting our view.
Unlike Microcystis (Ahlgren et al., 1992), R. raciborskii strains in our study were rich in ω6 PUFA ARA. Although most ecologists pay more attention to ω3 PUFA (Twining et al., 2021), inadequate availability of ω6 PUFA ARA can also constrain the fitness of Daphnia (Ilić et al., 2019), especially when EPA or EPA-enhancing fatty acid SDA is already present in their diet. ARA serves as a precursor for tissue hormones such as prostaglandin and related eicosanoids, which play critical roles in mediating reproduction (Heckmann et al., 2008; Stanley and Kim, 2019). Previously, a ω3/ω6 ratio ranging from 2.6 to 4.0 was reported for wild filtering cladoceran species (Persson and Vrede, 2006). In our study, the observed positive effects of adding R. raciborskii on reproduction and population increase might be attributed to nutritional supplements of ω6 PUFA. This speculation could be further demonstrated by the positive correlation between ARA content in the food and the intrinsic population increase rate of Daphnia when fed diets containing SDA. Other nutritional components, for example, proteins, may also work here, but we did not determine all the nutritional profiles and focused on essential fatty acids.
The observed beneficial effects of our CYN-producing and non-CYN-producing R. raciborskii strain in addition to C. pyrenoidosa as diets on the population increase of D. magna, however, were different from what Reis et al. (2023) observed. They reported that R. raciborskii constrains the fitness of Daphnia when this strain was added to chlorophyte as diets, by using different R. raciborskii strains (saxitoxin-producing strain), different Daphnia species (D. laveis and D. gessneri) and different chloryphyte species (Monoraphidium capricornutum and Ankistrodesmus stiptatus). A possible explanation is that algae may exhibit strain differences in their fatty acid profiles (Dunstan et al., 1993). And the fatty acid profile would eventually affected the growth of Daphnia if their needs for essential fatty acids were not met (Gulati and Demott, 1997). In addition, different Daphnia species, or even clones, may respond differently to different diets according to their different sensitivities to different or the same toxins (Ferrão-Filho et al., 2008; Costa et al., 2013) and nutritional requirements (Ferrão-Filho et al., 2019).
The poor manageability of filamentous R. raciborskii previously caused a reduction in both the growth rate and fecundity of Daphnia (Bednarska et al., 2014). In our feeding experiments, we observed that the trichomes of strain CS506 were three times longer than those of the N8 strain. Surprisingly, the length of R. raciborskii filaments did not appear to have a pronounced impact on Daphnia fitness in our study. This finding aligns with the work of Panosso and Lürling (2010), who demonstrated that longer R. raciborskii filaments may not necessarily cause stronger feeding inhibition than shorter ones for large-bodied D. magna (2–3 mm) within the range they tested. Increasing feeding inhibition in larger body-sized animals exposed to filamentous cyanobacteria were reported (Demott et al., 2001), but the conclusion was not generally-accepted for cladocerans due to their species-specific or clone-specific sensitivities when exposed to cyanobacteria (Bednarska et al., 2014). Relatively high feeding rates of R. raciborskii were also reported in daphnids of different body sizes, e.g., 1.1 mm D. longispina and 1.4 mm D. pulicaria, 1.6 mm D. laevis and 2.5 mm D. similis (Ferrão-Filho et al., 2017; Sikora and Dawidowicz, 2017; Ferrão-Filho et al., 2020), despite feeding inhibition being previously observed in 0.6–1.3 mm D. galeata, 1.2 mm D. cucullata, 1.9 mm D. hyalina, and 2.3 mm D. pulicaria (Schoenberg and Carlson, 1984; Gliwicz and Lampert, 1990). Notably, the D. magna clone used in our study might be less sensitive to clogging, as previously described (Soares et al., 2009).
Daphnia magna is a typical filter-feeding water flea and has long been used as a model for food quality and aquatic ecotoxicity studies. Most aquatic filter-feeders including cladocerans and rotifers, shared necessary requirements for polyunsaturated fatty acids such as SDA, EPA and ARA etc. (Schälicke et al., 2019; Thomas et al., 2022). Thus, our results lend further support to the idea that crustacean zooplankton may have the potential to control CYN-producing R. raciborskii populations in temperate lakes, as previously proposed (Gao et al., 2022), but with a particular emphasis on the nutritional perspective. Compared to temperate lakes, weak top-down effects were recorded due to the presence of fish species that spawn multiple times per year, resulting in an abundance of young-of-the-year fish all year around that prey on the large-bodied zooplankton in tropical and subtropical lakes (Liu et al., 2018; He et al., 2022), decreasing the control of large-bodied zooplankton on large-bodied zooplankton. Ferrão-Filho et al. (2020) showed the control of small-bodied zooplankton by saxitoxin-producing R. raciborskii in a mesocosm study; however, they suggested that high nutrient recycling other than the grazing effect by fish might weaken zooplankton’s control on cyanobacteria in trophic areas. Taken together, these findings may partially explain why R. raciborskii is more prevalent in tropical and subtropical areas than in temperate areas.
Conclusion
In summary, Chlorella pyrenoidosa relieved the negative effect of cylindrospermopsin-producing and non-cylindrospermopsin-producing Raphidiopsis raciborskii on D. magna in our study. The findings underscore the potential effectiveness of zooplankton, particularly in temperate lakes, in controlling CYN-producing R. raciborskii populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LL: Data curation, Writing – original draft, Writing – review & editing. SL: Data curation, Writing – review & editing. WL: Methodology, Writing – review & editing. YL: Investigation, Writing – review & editing. HZ: Formal analysis, Writing – review & editing. YT: Conceptualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported financially by National Natural Science Foundation of China (Nos. 32371616 and 32371615), and Natural Science Foundation of Guangdong Province (No. 2023A1515012361).
Acknowledgments
We are grateful for the work of numerous participants who collected and analyzed samples during the experimental period.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1292277/full#supplementary-material
References
Abonyi, A., Rasconi, S., Ptacnik, R., Pilecky, M., and Kainz, M. J. (2023). Chytrids enhance Daphnia fitness by selectively retained chytrid-synthesised stearidonic acid and conversion of short-chain to long-chain polyunsaturated fatty acids. Freshw. Biol. 68, 77–90. doi: 10.1111/FWB.14010
Abrusán, G. (2004). Filamentous cyanobacteria, temperature and Daphnia growth: the role of fluid mechanics. Oecologia 141, 395–401. doi: 10.1007/s00442-004-1660-x
Ahlgren, G., Gustafsson, I. B., and Boberg, M. (1992). Fatty acid content and chemical composition of freshwater microalgae 1. J. Phycol. 28, 37–50. doi: 10.1111/j.0022-3646.1992.00037.x
Antunes, J. T., Leão, P. N., and Vasconcelos, V. M. (2015). Cylindrospermopsis raciborskii: review of the distribution, phylogeography, and ecophysiology of a global invasive species. Front. Microbiol. 6:473. doi: 10.3389/fmicb.2015.00473
Becker, C., and Boersma, M. (2005). Differential effects of phosphorus and fatty acids on Daphnia magna growth and reproduction. Limnol. Oceanogr. 50, 388–397. doi: 10.2307/3597910
Bednarska, A., Pietrzak, B., and Pijanowska, J. (2014). Effect of poor manageability and low nutritional value of cyanobacteria on Daphnia magna life history performance. J. Plankton Res. 36, 838–847. doi: 10.1093/plankt/fbu009
Bouvy, M., Pagano, M., and Troussellier, M. (2001). Effects of a cyanobacterial bloom (Cylindrospermopsis raciborskii) on bacteria and zooplankton communities in Ingazeira reservoir (Northeast Brazil). Aquat. Microb. Ecol. 25, 215–227. doi: 10.3354/ame025215
Brett, M. T., Kainz, M. J., Taipale, S. J., and Seshan, H. (2009). Phytoplankton, not allochthonous carbon, sustains herbivorous zooplankton production. Proc. Natl. Acad. Sci. 106, 21197–21201. doi: 10.1073/pnas.0904129106
Buratti, F. M., Manganelli, M., Vichi, S., Stefanelli, M., Scardala, S., Testai, E., et al. (2017). Cyanotoxins: producing organisms, occurrence, toxicity, mechanism of action and human health toxicological risk evaluation. Arch. Toxicol. 91, 1049–1130. doi: 10.1007/s00204-016-1913-6
Chen, Z. J., Ruan, Z. X., Cheng, N., Xiao, L. J., Peng, L., Han, B.-P., et al. (2022). Whole-genome sequencing and phosphorus uptake and transport pathway comparative analysis of Cylindrospermopsis raciborskii N8. Acta Hydrobiol. Sin. 46, 1130–1141. doi: 10.7541/2022.2021.0197
Chislock, M. F., Sharp, K. L., and Wilson, A. E. (2014). Cylindrospermopsis raciborskii dominates under very low and high nitrogen-to-phosphorus ratios. Water Res. 49, 207–214. doi: 10.1016/j.watres.2013.11.022
Codd, G. A. (2000). Cyanobacterial toxins, the perception of water quality, and the prioritisation of eutrophication control. Ecol. Eng. 16, 51–60. doi: 10.1016/S0925-8574(00)00089-6
Costa, S. M., Ferrão-Filho, A. S., and Azevedo, S. M. F. O. (2013). Effects of saxitoxin- and non-saxitoxin-producing strains of the cyanobacterium Cylindrospermopsis raciborskii on the fitness of temperate and tropical cladocerans. Harmful Algae 28, 55–63. doi: 10.1016/j.hal.2013.05.017
DeMott, W. R., Gulati, R. D., and Donk, E. V. (2001). Effects of dietary phosphorus deficiency on the abundance, phosphorus balance, and growth of Daphnia cucullata in three hypereutrophic Dutch lakes. Limnol. Oceanogr. 46, 1871–1880. doi: 10.2307/3069058
Dittmann, E., and Wiegand, C. (2006). Cyanobacterial toxins–occurrence, biosynthesis and impact on human affairs. Mol. Nutr. Food Res. 50, 7–17. doi: 10.1002/mnfr.200500162
Dunstan, G. A., Volkman, J. K., Barrett, S. M., and Garland, C. D. (1993). Changes in the lipid composition and maximisation of the polyunsaturated fatty acid content of three microalgae grown in mass culture. J. Appl. Phycol. 5, 71–83. doi: 10.1007/BF02182424
Ferrão-Filho, A. S., Abreu, S. S. D., Oliveira, T., Magalhães, V. F., Pflugmacher, S., and Silva, E. M. (2017). Single and combined effects of microcystin and saxitoxin producing cyanobacteria on the fitness and antioxidant defenses of cladocerans. Environ. Toxicol. Chem. 36, 2689–2697. doi: 10.1002/etc.3819
Ferrão-Filho, A. D. S., da Costa, S. M., Ribeiro, M. G. L., and Azevedo, S. M. (2008). Effects of a saxitoxin-producer strain of Cylindrospermopsis raciborskii (cyanobacteria) on the swimming movements of cladocerans. Environ. Toxicol. Int. J. 23, 161–168. doi: 10.1002/tox.20320
Ferrão-Filho, A. D. S., Dias, T. M., Pereira, U. J., Dos Santos, J. A. A., and Kozlowsky-Suzuki, B. (2019). Nutritional and toxicity constraints of phytoplankton from a Brazilian reservoir to the fitness of cladoceran species. Environ. Sci. Pollut. Res. 26, 12881–12893. doi: 10.1007/s11356-019-04851-6
Ferrão-Filho, A. S., Pereira, U. J., Vilar, M. C., de Magalhães, L., and Marinho, M. M. (2020). Can small-bodied Daphnia control Raphidiopsis raciborskii in eutrophic tropical lakes? A mesocosm experiment. Environ. Sci. Pollut. Res. 27, 35459–35473. doi: 10.1007/s11356-020-09737-6
Frau, D., de Tezanos Pinto, P., and Mayora, G. (2018). Are cyanobacteria total, specific and trait abundance regulated by the same environmental variables? Ann. Limnol. - Int. J. Lim. 54:3. doi: 10.1051/limn/2017030
Gao, X., Wang, W., Ndayishimiye, J. C., Govaert, L., Chen, H., Jeppesen, E., et al. (2022). Invasive and toxic cyanobacteria regulate allochthonous resource use and community niche width of reservoir zooplankton. Freshw. Biol. 67, 1344–1356. doi: 10.1111/FWB.13921
Ger, K. A., Hansson, L. A., and Lürling, M. (2014). Understanding cyanobacteria-zooplankton interactions in a more eutrophic world. Freshw. Biol. 59, 1783–1798. doi: 10.1111/fwb.12393
Gliwicz, Z. M., and Lampert, W. (1990). Food thresholds in Daphnia species in the absence and presence of blue-green filaments. Ecology 71, 691–702. doi: 10.2307/1940323
Gulati, R., and Demott, W. (1997). The role of food quality for zooplankton: remarks on the state-of-the-art, perspectives and priorities. Freshw. Biol. 38, 753–768. doi: 10.1046/j.1365-2427.1997.00275.x
Haimeur, A., Ulmann, L., Mimouni, V., Guéno, F., Pineau-Vincent, F., Meskini, N., et al. (2012). The role of Odontella aurita, a marine diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 11, 1–13. doi: 10.1186/1476-511X-11-147
Hawkins, P. R., and Lampert, W. (1989). The effect of Daphnia body size on fifiltering rate inhibition in the presence of a fifilamentous cyanobacterium. Limnol. Oceanogr. 34, 1084–1089. doi: 10.2307/2837197
He, H., Qian, T., Shen, R., Yu, J., Li, K., Liu, Z., et al. (2022). Piscivore stocking significantly suppresses small fish but does not facilitate a clear-water state in subtropical shallow mesocosms: a biomanipulation experiment. Sci. Total Environ. 842:156967. doi: 10.1016/J.SCITOTENV.2022.156967
Heckmann, L. H., Sibly, R. M., Timmermans, M. J., and Callaghan, A. (2008). Outlining eicosanoid biosynthesis in the crustacean Daphnia. Front. Zool. 5, 11–19. doi: 10.1186/1742-9994-5-11
Ilić, M., Werner, C., and Fink, P. (2019). Equal relevance of omega-3 and omega-6 polyunsaturated fatty acids for the fitness of Daphnia spp. Limnol. Oceanogr. 64, 2512–2525. doi: 10.1002/lno.11201
Jeppesen, E., Søndergaard, M., Lauridsen, T. L., Davidson, T. A., Liu, Z., Mazzeo, N., et al. (2012). Biomanipulation as a restoration tool to combat eutrophication: recent advances and future challenges. Adv. Ecol. Res. 47, 411–488. doi: 10.1016/B978-0-12-398315-2.00006-5
Ka, S., Mendoza-Vera, J. M., Bouvy, M., Champalbert, G., N’Gom-Kâ, R., and Pagano, M. (2012). Can tropical freshwater zooplankton graze efficiently on cyanobacteria? Hydrobiologia 679, 119–138. doi: 10.1007/s10750-011-0860-8
Kainz, M., Brett, M. T., and Arts, M. T. (2009). Lipids in aquatic ecosystems. Springer-Verlag: New York.
Kirk, K. L., and Gilbert, J. J. (1992). Variation in herbivore response to chemical defenses -zooplankton foraging on toxic cyanobacteria. Ecology 73, 2208–2217. doi: 10.2307/1941468
Lampert, W. (1987). Laboratory studies on zooplankton-cyanobacteria interactions. N. Z. J. Mar. Freshw. Res. 21, 483–490. doi: 10.1080/00288330.1987.9516244
Lenihan-Geels, G., Bishop, K. S., and Ferguson, L. R. (2013). Alternative sources of omega-3 fats: can we find a sustainable substitute for fish? Nutrients 5, 1301–1315. doi: 10.3390/nu5041301
Leonard, J. A., and Paerl, H. W. (2005). Zooplankton community structure, microzooplankton grazing impact, and seston energy content in the St. Johns river system, Florida as influenced by the toxic cyanobacterium Cylindrospermopsis raciborskii. Hydrobiologia 537, 89–97. doi: 10.1007/s10750-004-2483-9
Lindsay, J., Metcalf, J. S., and Codd, G. A. (2006). Protection against the toxicity of microcystin-LR and cylindrospermopsin in Artemia salina and Daphnia spp. by pre-treatment with cyanobacterial lipopolysaccharide (LPS). Toxicon 48, 995–1001. doi: 10.1016/j.toxicon.2006.07.036
Liu, Z., Hu, J., Zhong, P., Zhang, X., Ning, J., Larsen, S. E., et al. (2018). Successful restoration of a tropical shallow eutrophic lake: strong bottom-up but weak top-down effects recorded. Water Res. 146, 88–97. doi: 10.1016/j.watres.2018.09.007
Lu, Y., Lei, M., Ye, J., Lei, L. M., and Han, B.-P. (2020). Intraspecific variation of morphological traits and toxin-producing capacity and phylogenetic analysis for Cylindrospermopsis raciborskii from Qiandenghu Lake, Guangdong Province. J. Lake Sci. 32, 144–153. doi: 10.18307/2020.0114
Lyu, K., Guan, H., Wu, C., Wang, X., Wilson, A. E., and Yang, Z. (2016a). Maternal consumption of non-toxic Microcystis, by Daphnia magna induces tolerance to toxic Microcystis in offspring. Freshw. Biol. 61, 219–228. doi: 10.1111/fwb.12695
Lyu, K., Meng, Q., Zhu, X., Dai, D., Zhang, L., Huang, Y., et al. (2016b). Changes in iTRAQ-based proteomic profiling of the cladoceran Daphnia magna exposed to microcystin-producing and microcystin-free Microcystis aeruginosa. Environ. Sci. Technol. 50, 4798–4807. doi: 10.1021/acs.est.6b00101
Müller-Navarra, D. C. (1995a). Evidence that a highly unsaturated fatty acid limits Daphnia growth in nature. Arch. Hydrobiol. 132, 297–307. doi: 10.1127/ARCHIV-HYDROBIOL/132/1995/297
Müller-Navarra, D. C. (1995b). Biochemical versus mineral limitation in Daphnia. Limnol. Oceanogr. 40, 1209–1214. doi: 10.2307/2838677
Müller-Navarra, D. C., Brett, M. T., Liston, A. M., and Goldman, C. R. (2000). A highly unsaturated fatty acid predicts carbon transfer between primary producers and consumers. Nature 403, 74–77. doi: 10.1038/47469
Nogueira, I. C., Lobo-da-Cunha, A., and Vasconcelos, V. M. (2006). Effects of Cylindrospermopsis raciborskii and Aphanizomenon ovalisporum (cyanobacteria) ingestion on Daphnia magna midgut and associated diverticula epithelium. Aquat. Toxicol. 80, 194–203. doi: 10.1016/j.aquatox.2006.08.008
Nogueira, I. C., Saker, M. L., Pflugmacher, S., Wiegand, C., and Vasconcelos, V. M. (2004). Toxicity of the cyanobacterium Cylindrospermopsis raciborskii to Daphnia magna. Environ. Toxicol. Int. J. 19, 453–459. doi: 10.1002/tox.20050
Panosso, R., and Lürling, M. (2010). Daphnia magna feeding on Cylindrospermopsis raciborskii: the role of food composition, filament length and body size. J. Plankton Res. 32, 1393–1404. doi: 10.1093/plankt/fbq057
Persson, J., and Vrede, T. (2006). Polyunsaturated fatty acids in zooplankton: variation due to taxonomy and trophic position. Freshw. Biol. 51, 887–900. doi: 10.1111/j.1365-2427.2006.01540.x
Poniedzialek, B., Rzymski, P., and Karczewski, J. (2015). The role of the enzymatic antioxidant system in cylindrospermopsin-induced toxicity in human lymphocytes. Toxicol. In Vitro 29, 926–932. doi: 10.1016/j.tiv.2015.03.023
Rangel, L. M., Ger, K. A., Silva, L. H. S., Soares, M. C. S., Faassen, E. J., and Lürling, M. (2016). Toxicity overrides morphology on Cylindrospermopsis raciborskii grazing resistance to the Calanoid copepod Eudiaptomus gracilis. Microb. Ecol. 71, 835–844. doi: 10.1007/s00248-016-0734-8
Reis, G. C. D., de Carvalho, G. H. A., Vilar, M. C. P., Azevedo, S. M. F. D. O. E., and Ferrão-Filho, A. D. S. (2023). Saxitoxin-producing Raphidiopsis raciborskii (Cyanobacteria) constrains Daphnia fitness and feeding rate despite high nutritious food availability. Toxics 11:693. doi: 10.3390/toxics11080693
Ruiz, T., Koussoroplis, A. M., Danger, M., Aguer, J. P., Morel-Desrosiers, N., and Bec, A. (2021). Quantifying the energetic cost of food quality constraints on resting metabolism to integrate nutritional and metabolic ecology. Ecol. Lett. 24, 2339–2349. doi: 10.1111/ele.13855
Rzymski, P., and Poniedziałek, B. (2014). In search of environmental role of cylindrospermopsin: a review on global distribution and ecology of its producers. Water Res. 66, 320–337. doi: 10.1016/j.watres.2014.08.029
Sakai, C., Ishida, M., Ohba, H., Yamashita, H., Uchida, H., Yoshizumi, M., et al. (2017). Fish oil omega-3 polyunsaturated fatty acids attenuate oxidative stress-induced DNA damage in vascular endothelial cells. PLoS One 12:e0187934. doi: 10.1371/journal.pone.0187934
Schälicke, S., Sobisch, L. Y., Martin-Creuzburg, D., and Wacker, A. (2019). Food quantity-quality co-limitation: interactive effects of dietary carbon and essential lipid supply on population growth of a freshwater rotifer. Freshw. Biol. 64, 903–912. doi: 10.1111/fwb.13272
Schoenberg, S. A., and Carlson, R. E. (1984). Direct and indirect effects of zooplankton grazing on phytoplankton in a hypereutrophic lake. Oikos 42, 291–302. doi: 10.2307/3544397
Schwarzenberger, A. (2022). Negative effects of cyanotoxins and adaptative responses of Daphnia. Toxins 14:770. doi: 10.3390/TOXINS14110770
Sikora, A., and Dawidowicz, P. (2017). Breakage of cyanobacterial filaments by small- and large-sized Daphnia: are there any temperaturedependent differences? Hydrobiologia 798, 119–126. doi: 10.1007/s10750-015-2436-5
Soares, M. C. S., Rocha, M. I. D. A., Marinho, M. M., Azevedo, S. M., Branco, C. W., and Huszar, V. L. (2009). Changes in species composition during annual cyanobacterial dominance in a tropical reservoir: physical factors, nutrients and grazing effects. Aquat. Microb. Ecol. 57, 137–149. doi: 10.3354/ame01336
Søndergaard, M., Liboriussen, L., Pedersen, A. R., and Jeppesen, E. (2008). Lake restoration by fish removal: short-and long-term effects in 36 Danish lakes. Ecosystems 11, 1291–1305. doi: 10.1007/s10021-008-9193-5
Stanley, D., and Kim, Y. (2019). Insect prostaglandins and other eicosanoids: from molecular to physiological actions. Adv. Insect Physiol. 56, 283–343. doi: 10.1016/bs.aiip.2019.01.003
Taipale, S. J., Galloway, A. W., Aalto, S. L., Kahilainen, K. K., Strandberg, U., and Kankaala, P. (2016). Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Sci. Rep. 6:30897. doi: 10.1038/srep30897
Tang, Y., Su, L., Xu, R., Wang, S., Su, Y., Liu, Z., et al. (2023). Response of zooplankton to inputs of terrestrial dissolved organic matter: food quality constraints induced by microbes. Limnol. Oceanogr. 68, 709–722. doi: 10.1002/lno.12304
Tang, Y., Yang, X., Xu, R., Zhang, X., Liu, Z., Zhang, Y., et al. (2019). Heterotrophic microbes upgrade food value of a terrestrial carbon resource for Daphnia magna. Limnol. Oceanogr. 64, 474–482. doi: 10.1002/lno.11052
Tang, Y., Zhou, D., Su, L., Liu, Z., Zhang, X., and Dumont, H. J. (2021). Vallisneria natans detritus supports Daphnia magna somatic growth and reproduction under addition of periphyton. Aquat. Ecol. 55, 579–588. doi: 10.1007/S10452-021-09846-5
Thomas, P. K., Kunze, C., Van de Waal, D. B., Hillebrand, H., and Striebel, M. (2022). Elemental and biochemical nutrient limitation of zooplankton: a meta-analysis. Ecol. Lett. 25, 2776–2792. doi: 10.1111/ele.14125
Twining, C. W., Bernhardt, J. R., Derry, A. M., Hudson, C. M., Ishikawa, A., Kabeya, N., et al. (2021). The evolutionary ecology of fatty-acid variation: implications for consumer adaptation and diversification. Ecol. Lett. 24, 1709–1731. doi: 10.1111/ELE.13771
Wacker, A., and Martin-Creuzburg, D. (2007). Allocation of essential lipids in Daphnia magna during exposure to poor food quality. Funct. Ecol. 21, 738–747. doi: 10.1111/j.1365-2435.2007.01274.x
Wenzel, A., Vrede, T., Jansson, M., and Bergström, A. K. (2021). Daphnia performance on diets containing different combinations of high-quality algae, heterotrophic bacteria, and allochthonous particulate organic matter. Freshw. Biol. 66, 157–168. doi: 10.1111/fwb.13626
Willis, A., Adams, M. P., Chuang, A. W., Orr, P. T., O’Brien, K. R., and Burford, M. A. (2015). Constitutive toxin production under various nitrogen and phosphorus regimes of three ecotypes of Cylindrospermopsis raciborskii ((Wołoszyńska) Seenayya et Subba Raju). Harmful Algae 47, 27–34. doi: 10.1016/j.hal.2015.05.011
Wilson, A. E., Sarnelle, O., and Tillmanns, A. R. (2006). Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnol. Oceanogr. 51, 1915–1924. doi: 10.4319/lo.2006.51.4.1915
Wiltshire, K. H., Boersma, M., Möller, A., and Buhtz, H. (2000). Extraction of pigments and fatty acids from the green alga Scenedesmus obliquus (Chlorophyceae). Aquat. Ecol. 34, 119–126. doi: 10.1023/A:1009911418606
Keywords: Raphidiopsis raciborskii , zooplankton, essential fatty acids, feeding experiments, nutritional supplements
Citation: Lei L, Lai S, Liu W, Li Y, Zhang H and Tang Y (2023) Chlorella pyrenoidosa mitigated the negative effect of cylindrospermopsin-producing and non-cylindrospermopsin-producing Raphidiopsis raciborskii on Daphnia magna as a dietary supplement. Front. Microbiol. 14:1292277. doi: 10.3389/fmicb.2023.1292277
Edited by:
Da Huo, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Mauro Vilar, Federal University of Rio de Janeiro, BrazilYunfei Sun, Nanjing Normal University, China
Copyright © 2023 Lei, Lai, Liu, Li, Zhang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yali Tang, eWFsaXRhbmdAam51LmVkdS5jbg==
 Lamei Lei
Lamei Lei Shuyan Lai
Shuyan Lai Yali Tang
Yali Tang