
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 12 January 2024
Sec. Food Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1289490
According to the Chinese encyclopedia “Ben Cao Gang Mu” (AD 1552–1578), Caprifoliaceae and Scutellaria baicalensis Georgi are used in traditional Chinese medicine to clear heat, detoxify, and treat wind-heat colds, upper respiratory tract infections, and pneumonia. However, the mechanism and the effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on intestinal health remain unclear. From the perspective of intestinal microbes, this study assessed the antioxidant, anti-inflammatory, and intestinal protective properties of Caprifoliaceae and Scutellaria baicalensis Georgi. Mice received diets with or without Caprifoliaceae and Scutellaria baicalensis Georgi extractive (BCA) for 2 weeks in this study. The results showed that BCA increased body weight gain, feed intake, and catalase (CAT) content in the mice but reduced γ-glutamyl transpeptidase (γ-GT) content in the serum (p < 0.05). BCA improved the Sobs, Chao, and Ace indices, as well as the number of Campylobacterota, Patercibacteria, and Desulfobacterota in the colon microbiota, while it decreased the Firmicutes phylum (p < 0.05). At the genus level, BCA increased Candidatus_Saccharimonas, Helicobacter, unclassified_f_Lachnospiraceae, Alistipes, norank_f_norank_o_Clostridia_vadinBB60_group, norank_f_Ruminococcaceae, unclassified_f_Ruminococcaceae, etc. abundance (p < 0.05), but it significantly decreased Lactobacillus and Lachnospiraceae_UCG_001 abundance (p < 0.05). Moreover, BCA improved the concentration of acetic acid, butyric acid, propionic acid, valeric acid, and isovaleric acid and diminished the concentration of isobutyric acid (p < 0.05). Correlation analysis shows that the changes in short-chain fatty acids and antioxidant and inflammatory indices in the serum were significantly correlated with the BCA-enriched microbiota. This study supplemented a database for the application of Caprifoliaceae and Scutellaria baicalensis Georgi in clinical and animal production.
Traditional Chinese medicine Caprifoliaceae and Scutellaria baicalensis Georgi, important natural active substances, have been widely used around the world (Zhao et al., 2019; Li et al., 2020). Pharmacological research has shown the antibacterial, anti-inflammatory, antiviral, antioxidant, and liver protection activities of Caprifoliaceae (Shi et al., 2016). One extractive for Caprifoliaceae is chlorogenic acid, a phenylpropanoid compound that has extensive biological activities including antibacterial, antiviral, and antioxidant (Miao and Xiang, 2020). Scutellaria baicalensis Georgi is effective in the treatment of respiratory infections, gastroenteritis, and diarrhea (Zhao et al., 2016). The main extractive of Scutellaria baicalensis Georgi is baicalin, a class of flavonoids that has been reported to have anti-inflammatory and immune-regulatory activities (Wang et al., 2022). The mixture of baicalin and chlorogenic acid could synergistically deliver a stronger antioxidant and anti-inflammatory effect.
The intestinal microbiota is critical for regulating host nutrition, metabolism, intestinal microbiota balance, and immune function (Maynard et al., 2012). In vivo, the intestinal microbiota widely participates in the metabolism of traditional Chinese medicine. After oral administration, baicalin or other traditional Chinese medicines are degraded by the intestinal microbiota and produce aglycones that have pharmacological effects (Huang et al., 2019). The intestinal microbiota takes part in herb metabolism by producing various enzymes and then changing the trend of medicines' action (Huang et al., 2018). Therefore, the research on the effects of Chinese herbal medicines on the intestinal microbiota to understand their mechanism of treating disease in vivo is a visionary effort.
Baicalin and chlorogenic acid are hardly absorbed in the small intestine and are stuck in the intestine for a long period of time. As a result, they are mostly broken down by microbiota and susceptible to the influence of microbiota. In this study, 16S rRNA gene sequencing analysis was used to investigate the effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and its metabolites. The purpose of this study was to provide a database for the application of BCA in clinical and animal production.
The compound extract of Caprifoliaceae and Scutellaria baicalensis Georgi was supplied by Beijing Center Biology Co., Ltd, of which the core components are baicalin and chlorogenic acid, and the ratio was 1:10. The purity of baicalin and chlorogenic acid was 85% and 65%, respectively. The plant name has been checked with “World Flora Online” (www.worldfloraonline.org).
In total, 24 Institute of Cancer Research (ICR) mice (21-day-old) were purchased from the School of Medicine, Peking University (Beijing, China) and randomly assigned into a control group (CON) and a compound extract of Caprifoliaceae and Scutellaria baicalensis Georgi group (BCA). The mice in the CON group were given regular food, while the mice in the BCA group were given food with 500 mg/kg of BCA for 2 weeks. The mice were allowed to eat and drink freely during the experiment.
Blood was collected by removing the eyeball and then centrifuged for 10 min at 3,000 rpm under 4°C to separate the serum. In post-trial, the mice were euthanized using the cervical dislocation method, and the jejunum, colon, and colonic chyme were excised for processing. The experimental protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Institute of Animal Science at the Chinese Academy of Agricultural Sciences (IAS2020-85).
The jejunum and colon tissue samples were prepared on tissue slides according to the procedure reported earlier (Tang et al., 2022). The tissues were embedded in paraffin blocks, cut into 4-μm slices, and stained with hematoxylin and eosin (H&E). Then, the slices were dried and sealed for follow-up observation and analysis.
The activities of γ-glutamyl transpeptidase (γ-GT, cat. no. C017-2-1), catalase (CAT, cat. no. A007-2-1), malondialdehyde (MDA, cat. no. A003-1-2), total antioxidant capacity (T-AOC, cat. no. A015-2-1), and diamine oxidase (DAO, cat. no. A088-1-1) were performed via appropriate assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) using the builder's standard method. In addition, interleukin-1β (IL-1β, cat. no. H002-1-2), interleukin-6 (IL-6, cat. no. H007-1-1), and tumor necrosis factor-α (TNF-α, cat. no. H052-1-1) levels in the serum were tested via the enzyme-linked immunosorbent assay (ELISA) (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer's instructions.
Microbial DNA in colonic chyme was obtained using the E.Z.N.A.® soil DNA kit (Omega Bio-Tek, Norcross, GA, United States), according to the builder's standard method. The V3-V4 hypervariable regions of the microbial 16S rRNA gene were amplified with PCR using primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) via the ABI Gene Amp® 9700 PCR thermocycler (ABI, CA, United States). Metagenomic sequencing was executed according to the Illumina platform by Miseq PE300. Raw reads were deposited into the NCBI Sequence Read Archive database (SRA: PRJNA765910). Raw sequences were treated and filtered into chimeric sequences by the Majorbio I-Sanger Cloud Platform (www.i-sanger.com, Majorbio, Shanghai, China). The Majorbio I-Sanger Cloud Platform (www.i-sanger.com, Majorbio, Shanghai, China) was used to perform unweighted principal coordinate analysis (PCoA), beta-diversity analysis, and alpha-diversity analysis using default values.
Colon chyme samples were used to test short-chain fatty acids (SCFAs) concentrations using a gas chromatography-mass spectrometer (GC-MS), as described by Wan et al. (2022). Colon chyme samples of 0.1 g were weighed, suspended in 1 ml of ddH2O, homogenized, and centrifuged (10,000 rpm, 10 min, 4°C). The supernatant was obtained, and 25% of metaphosphoric acid was added. Then, the supernatant was filtered and analyzed using an Agilent 6890 gas chromatography (Agilent Technologies, Inc., Palo Alto, CA, United States) system.
Quantitative data are expressed as the mean ± standard deviation (SD). The SAS 9.4 software (SAS 9.4, Institute, Cary, NC, United States) was used for all the analyses. Comparisons of means between the BCA and CON groups were performed using an unpaired t-test. Significance was established at p ≤ 0.05.
BCA significantly increased body weight gain and feed intake of the mice (Figure 1A, p < 0.05), but tended to reduce water intake (p = 0.0669). Intestinal morphology was intact both in the control and BCA groups, and villi and crypts were neatly arranged (Figures 1B, C).
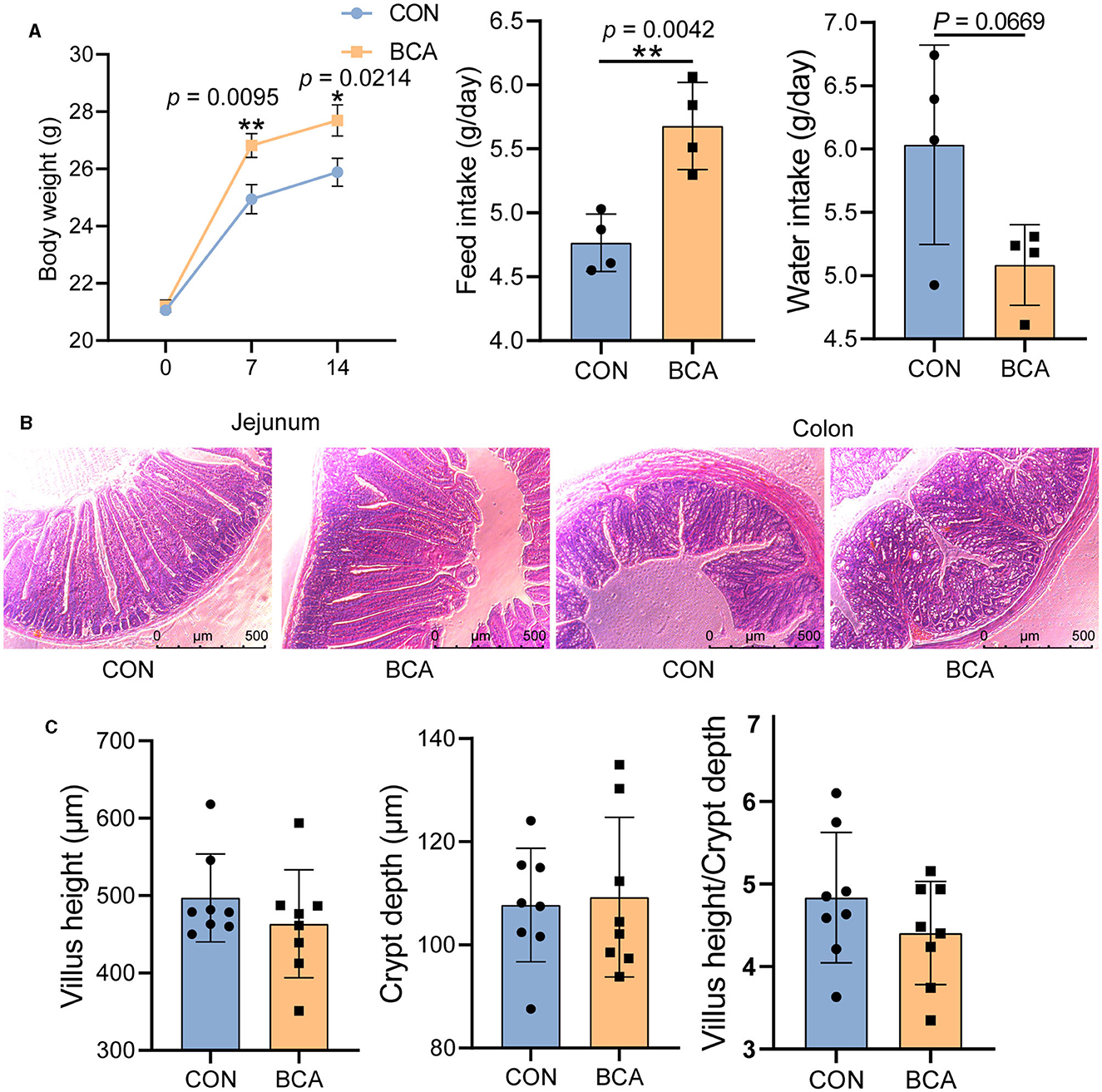
Figure 1. Growth performance and intestinal morphology. (A) Body weight (n = 12), feed intake, and water intake; (B) staining profiles by H&E of the jejunum and colon (scale bars: 500 μm); and (C) villus height, crypt depth, and villus height/crypt depth of jejunum, n = 8. Data are expressed as mean with SD. The intergroup difference test was performed using an unpaired t-test. *means p < 0.05; **means p < 0.01.
BCA significantly reduced the γ-GT content in the serum (Figure 2A, p < 0.05) but increased CAT content (p < 0.05). In addition, BCA significantly reduced DAO content (p < 0.05) and tended to reduce the TNF-α content in the serum (Figure 2B, p = 0.0609).
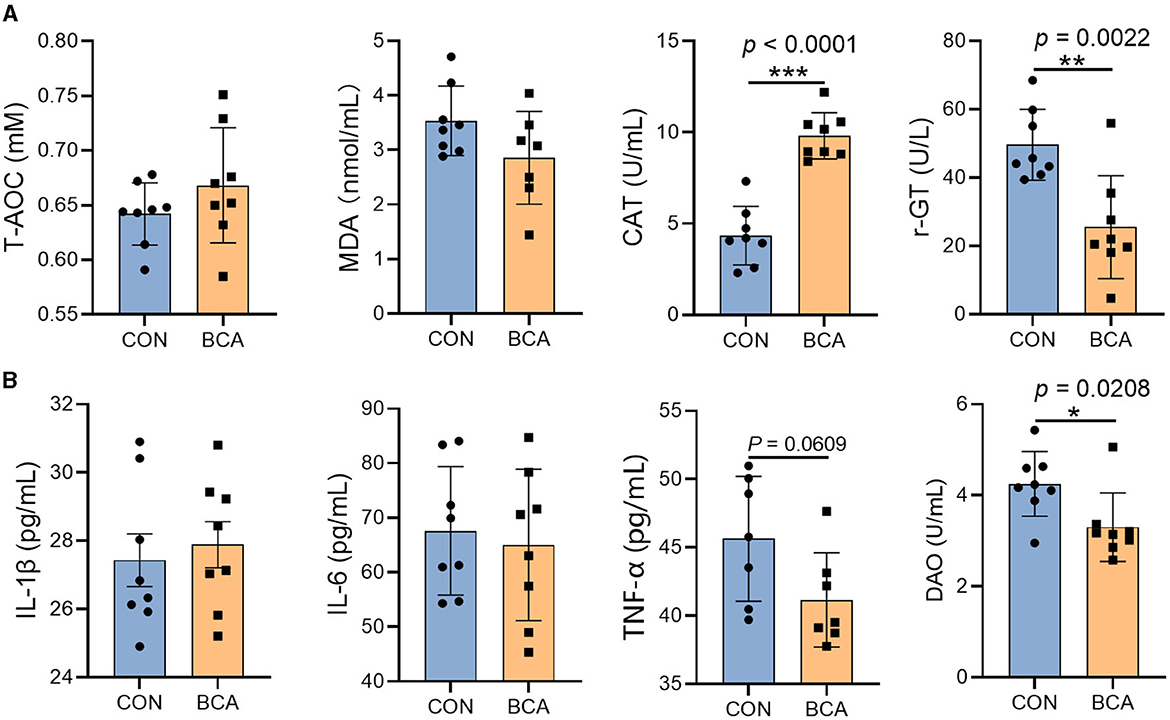
Figure 2. Serum biochemical parameters and inflammatory cytokines. (A) Antioxidant enzymes (T-AOC, MDA, CAT, and γ-GT) and (B) inflammatory cytokines (IL-1β, IL-6, and TNF-α) and DAO in the serum. Data are expressed as mean with SD. The intergroup difference test was performed using an unpaired t-test (n = 8). *means p < 0.05; **means p < 0.01; ***means p < 0.001.
Figure 3 shows the effect of BCA on the diversity of the colonic microbiota. A PCoA analysis revealed that there was a clear cluster of the microbial community at the OTU level between the BCA and CON groups (Figure 3A). For alpha diversity, BCA significantly improved the sobs, ace, and chao indices (p < 0.01, Figure 3B).
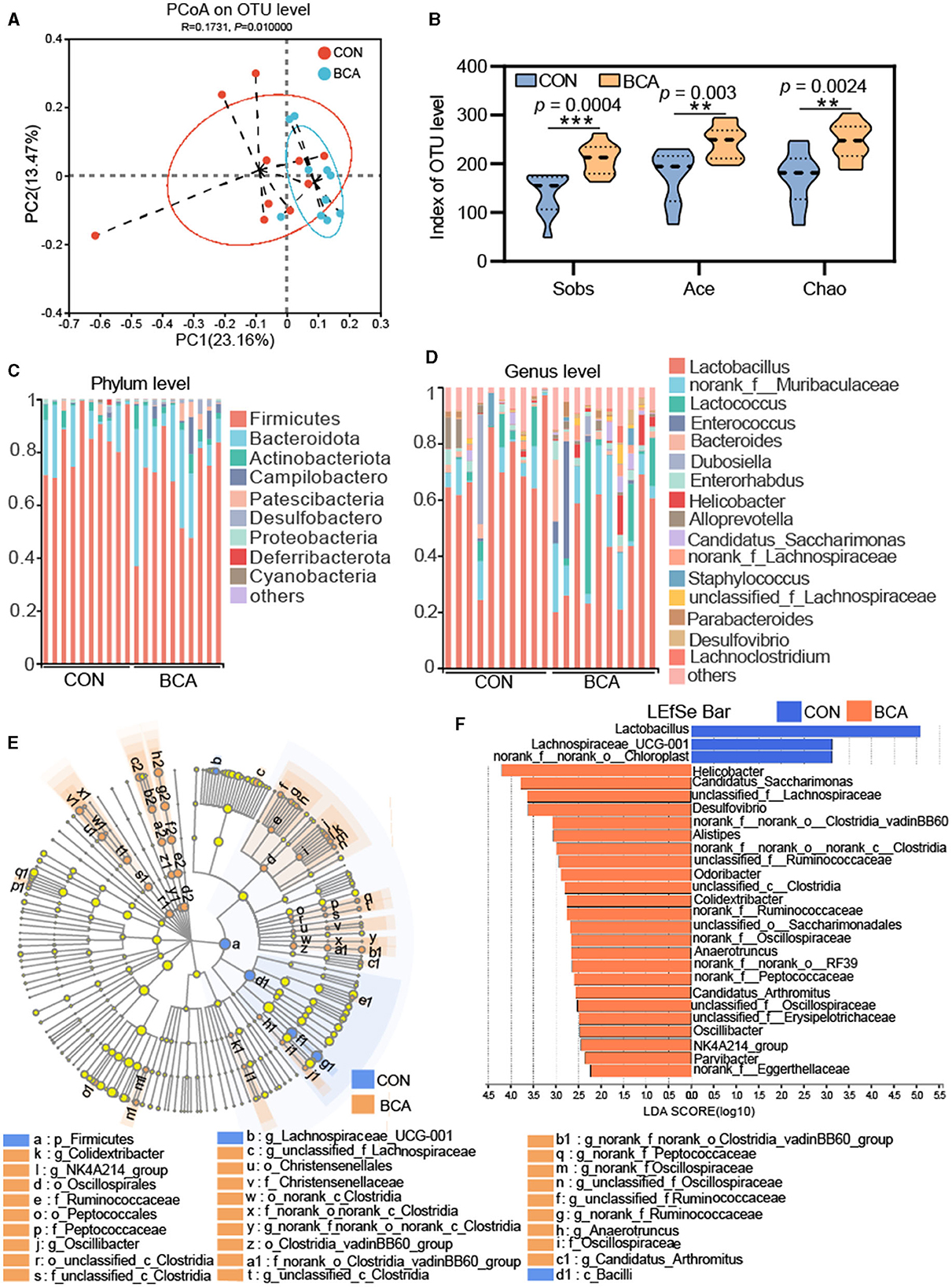
Figure 3. Microbiota analysis in colon chyme (n = 10). (A) PCoA analysis based on unweighted UniFrac. (B) Alpha-diversity (Sobs, Chao, and Ace indices) of the microbiota. Data are expressed as mean with SD. The intergroup difference test was performed using an unpaired t-test. **means p < 0.01; ***means p < 0.001. (C) Relative abundance of the microbiome at phylum level and (D) genus level. Less than 1% abundance of phyla or genera was merged into others. (E) LEfSe analysis at the genus level and the threshold of the LDA score was 2.0. Blue represents the genus enriched by CON, and orange represents the genus enriched by BCA. (F) Lefse bar in genus levels in CON vs. BCA.
Microbial composition is shown in Figures 3C, D. Firmicutes, Actinobacteriota, Patescibacteria, and Bacteroidota were abundant in both CON and BCA groups. Campylobactero and Desulfobactero were important components for the BCA group but not for the CON group. At the genus level, the top six abundance genera in the CON group were Lactobacillus, norank_f__Muribaculaceae, Lactococcus, Dubosiella, Enterorhabdus, and Alloprevotella. The top six abundance genera in the BCA group were Lactobacillus, norank_f__Muribaculaceae, Lactococcus, Enterococcus, Bacteroides, and Helicobacter. Linear discriminate analysis effect size (LEfSe) was used to identify the specific taxa from phylum to genus level, and the threshold of the linear discriminant analysis (LDA) score was 2.0. Firmicutes and Bacilli were identified in the CON group. A total of 6, 7, or 15 microbiotas were identified in the BCA group at the order, family, and genus levels, respectively. The LEfSe bar shows the identified genus in the CON and BCA groups. The top five abundant genera of identified taxa in the BCA group were Helicobacter, Candidatus_Saccharimonas, unclassified_f_Lachnospiraceae, Desulfovibrio, and norank_f_norank_o_Clostridia_vadinBB60_group.
As shown in Figure 4A, 92% of the identified genera were improved in the BCA group (p < 0.05), among which the top 10 abundant genera were Candidatus_Saccharimonas, Helicobacter, Desulfovibrio, unclassified_f_Lachnospiraceae, Alistipes, norank_f_norank_o_Clostridia_vadinBB60_group, Odoribacter, norank_f_Ruminococcaceae, unclassified_f_Ruminococcaceae, and Colidexinbater. In contrast, Lactobacillus and Lachnospiraceae_UCG_001 were significantly reduced in the BCA group (p < 0.05). More than 65% of altered microbiotas belong to the Firmicutes phylum, and 82% of them belong to the Clostridia class. At the phylum level, BCA significantly reduced Firmicutes abundance but increased Campylobacterota, Patercibacteria, and Desulfobacterota abundance.
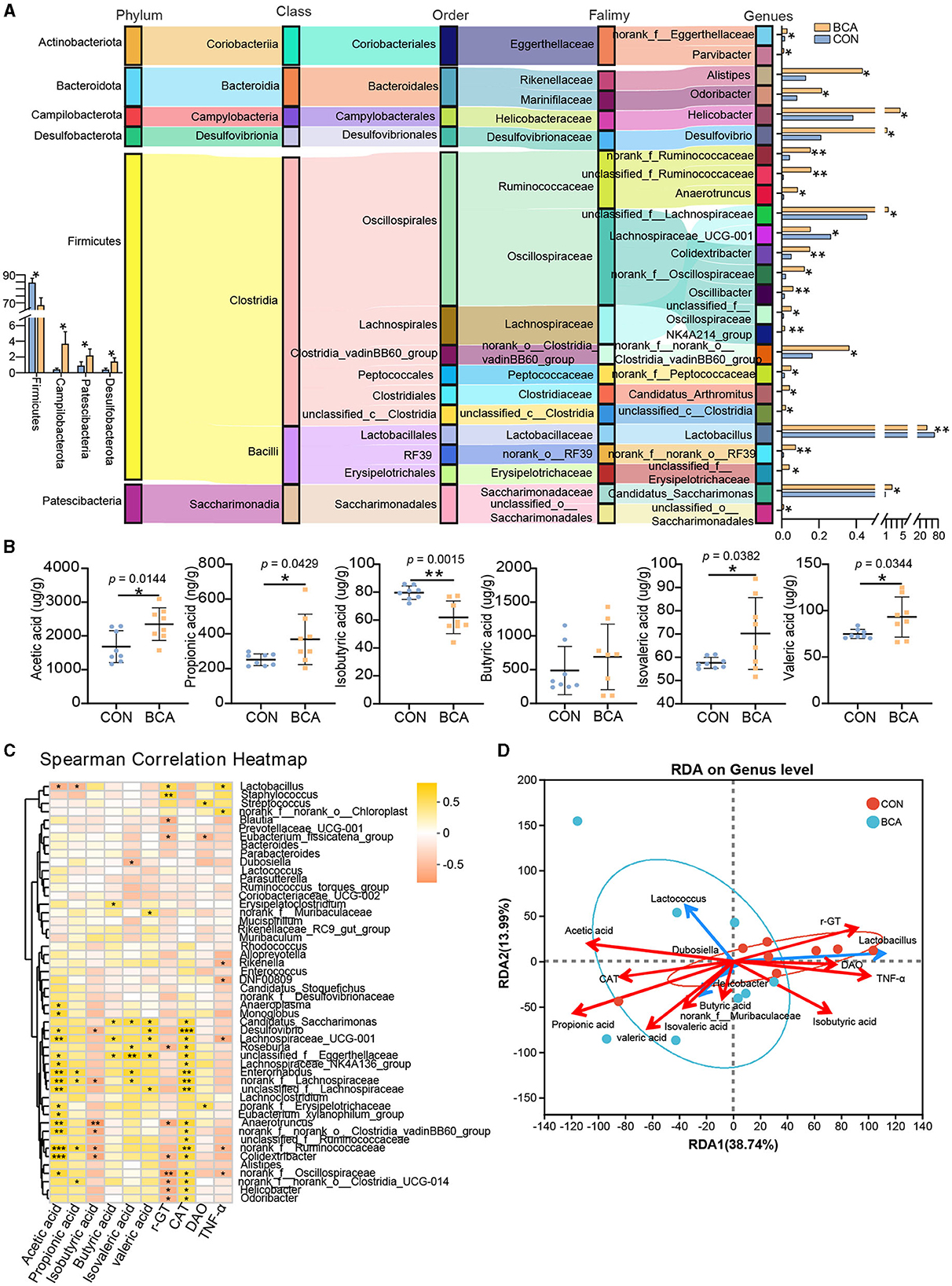
Figure 4. Differential genera and short-chain fatty acid analysis. (A) Taxonomy of differentially abundant genera in the BCA vs. CON group from phylum to genus level. (B) Short-chain fatty acid concentration of colon chyme (n = 8). Data are expressed as mean with SD. The intergroup difference test was performed using an unpaired t-test. (C) Correlation analysis between SCFAs or serum index and the top 50% genera was performed using Spearman. (D) Redundancy analysis (RDA) of the colonic microbiota composition at the genus level relative to colonic SCFAs and serum index. The top five microbes are shown. *means p < 0.05, **means p < 0.01, ***means p < 0.001.
SCFAs quantification in colon chyme from CON and BCA groups was measured and depicted in Figure 4B. BCA treatment did improve the concentration of acetic acid, butyric acid, propionic acid, valeric acid, and isovaleric acid and diminish the concentration of isobutyric acid (p < 0.05).
The correlation heatmap revealed the correlation conducted using Spearman correlation analysis between SCFAs or serum index and microbiotas in Figure 4C. The acetic acid level and CAT content in the serum had a positive correlation with the abundance of Lachnospiraceae_UCG_001, Desulfovibrio, Colidexinbater, norank_f_Ruminococcaceae, unclassified_f_Eggerthellaceae, Lachnospiraceae_NK4A136_group, unclassified_f_Lachnospiraceae, norank_f_Lachnospiraceae, Enterorhabdus, Monoglobus, Anaerotruncus, norank_f_norank_o_Clostridia_vadinBB60_group, Eubacterium_xylanophilum_group, norank_f_Erysipelotrichaceae, norank_Oscillospiraceae, and Anaeropiasma, but a negative correlation with the abundance of Lactobacillus. The concentration of propionic acid had a positive correlation with the abundance of norank_f_Ruminococcaceae, norank_f_Lachnospiraceae, Enterorhabdus, and norank_f_norank_o_Clostridia_UCG_014, but a negative correlation with the abundance of Lactobacillus. The concentration of isobutyric acid was a negative correlation with the abundance of Desulfovibrio, Colidexinbater, norank_f_Ruminococcaceae, norank_f_Lachnospiraceae, Anaeropiasma, and norank_f_ norank_o_Clostridia_vadinBB60_group. The concentration of butyric acid, isovaleric acid, and valeric acid had a positive correlation with Lachnospiraceae_UCG_001, Desulfovibrio, Candidatus_Saccharimonas, unclassified_f_Eggerthellaceae, etc. γ-GT content in the serum had a negative correlation with norank_Oscillospiraceae, Alistipes, Helicobacter, etc. To further explore the association among bacteria, SCFAs, or serum index, RDA analysis was completed. The top five bacteria are shown in Figure 4D. The results showed that the BCA group was separated from those in the control group. Genera (Lactococcus, Helicobacter, Dubosiella, and norank_f_Muribaculaceae) enriched in the BCA group were positively correlated with acetic acid, propionic acid, butyric acid, isovaleric acid, valeric acid, and CAT. In contrast, genera (Lactobacillus) enriched in the CON group were positively correlated with isobutyric acid, γ-GT, DAO, and TNF-α.
Caprifoliaceae and Scutellaria baicalensis Georgi have high medicinal value and are widely used in traditional Chinese medicine. Chlorogenic acid, the main component of Caprifoliaceae, and baicalin, the main component of Scutellaria baicalensis Georgi, have also received extensive attention. In the field of animal production, baicalin has been reported to improve the performance of pigs and chickens and improve intestinal diseases (Yin et al., 2021). Chlorogenic acid has been reported to have antibacterial, antiviral, and microbiota-regulation effects (Kim et al., 2020). In this study, BCA promoted the body weight of the mice. This may be related to the improvement of the feed intake, intestinal health, and immunity of mice after BCA treatment. In a previous study, we found that baicalin regulates appetite-related genes and promotes feed intake in mice (Zhang et al., 2023). Chlorogenic acid supplementation upregulates anti-inflammatory cytokines and antioxidant enzymes and improves DSS-induced colitis through the Nrf2 signaling pathway (Wan et al., 2021). DAO levels in the serum generally remain low, which increased when the intestinal barrier was compromised (Lu et al., 2022). In this study, lower DAO levels in the serum after BCA treatment may indicate enhanced epithelial integrity of the intestinal barrier (Yang et al., 2019). In addition, the intestinal microbiota has been extensively reported to be closely related to host intestinal digestion and absorption (Khudhair et al., 2020). BCA supplementation in this study increased the colonization of Firmicutes, especially Clostridia and other probiotics. BCA-enriched bacteria Ruminococcaceae have been reported to reduce intestinal permeability and are involved in food digestion and carbohydrate metabolism (Xi et al., 2021). Lactococcus, enriched in the BCA group, has been widely reported to promote animal growth and secrete antimicrobial peptides and organic acids to inhibit the growth of pathogenic bacteria and maintain intestinal homeostasis (Feng et al., 2019; Kakade et al., 2022; Werum et al., 2022). Lactococcus also regulated host immunity by secreting exopolysaccharides and was considered a probiotic due to its ability to inhibit the growth of pathogenic bacteria (Sun et al., 2020; Pan et al., 2022).
Baicalin and chlorogenic acid play an antioxidant role in the phenolic hydroxyl structure, which effectively removes hydroxyl radicals and superoxide anions generated in the free radical reaction by reacting easily with free radicals (Xia et al., 2021). In the present research, BCA significantly improved the antioxidant function and reduced inflammation in mice, including reducing the content of γ-GT and TNF-α and improving the content of CAT in the serum. In addition to the special structure and direct effects of chlorogenic acid and baicalin, the anti-inflammatory functions of BCA may also be related to the intestinal microbiota, which has been widely reported (Negatu et al., 2020; Shen et al., 2022). The correlation and RDA analyses showed that antioxidant and inflammatory indicators were significantly correlated with BCA-enriched intestinal microbiota in this study. Some of these microbiotas may be associated with anti-inflammatory and antioxidant functions. Lachnospiraceae_NK4A136_group shows anti-inflammatory properties, promoting the repair of the intestinal mucosa and relieving colitis (Zhong et al., 2021). norank_f_Muribaculaceae could improve intestinal mucositis in mice (Wang et al., 2016). Above all, BCA supplementation improved antioxidant and anti-inflammatory function in mice. We speculate that the antioxidant and anti-inflammatory functions of BCA are partly attributed to the functional components baicalin and chlorogenic acid and whether the synergistic effects of the two are more worthy of further investigation.
SCFAs have been widely shown to maintain intestinal balance and actively participate in immune regulation, cell proliferation, and host metabolism (Akhtar et al., 2022; Li et al., 2022). Acetic acid could be taken up and utilized by many tissues, which was the main way for tissues to use carbohydrates that cannot be absorbed by the small intestine. Butyric acid, the most important energy source for the colon and cecum, was absorbed by the epithelial cells. In this study, BCA treatment significantly increased the contents of acetic acid, isovaleric acid, propionic acid, and valeric acid in the colonic chyme of mice. Higher concentrations of SCFAs inhibit pathogenic bacteria growth by reducing intestinal pH (He et al., 2019). After BCA intervention, SCFA-producing bacteria were abundant in the colon, which could promote microbial fermentation to produce more SCFAs and other metabolites. These metabolites could participate in intestinal development, immune regulation, digestion, and absorption by the body. This was confirmed by the correlation analysis between SCFA and the intestinal microbiota. Interestingly, most of the microbes associated with SCFAs and CAT belong to Firmicutes, especially Clostridia. Alistipes could produce indoles, acetic acid, and propionic acid and promote broilers' growth performance, which was considered to be potentially beneficial (Parker et al., 2020). norank_Oscillospiraceae, a butyric acid producer, could use gluconates (Leth et al., 2022). unclassified_f_Ruminococcaceae and norank_f__Ruminococcaceae belong to the Ruminococcaceae family and are the cause of SCFAs from carbohydrate or oligosaccharide degradation (Cheng et al., 2018). In addition, Ruminococcaceae was also a potential probiotic that could improve immunity and the intestinal environment (Gu et al., 2022).
In summary, BCA regulates the gut microbiota (increases Firmicutes, especially Clostridia), improves antioxidant function, and promotes feed intake and growth performance in mice. These results reveal the potential value of BCA in intestine protection and antioxidant function. However, whether the antioxidant function of BCA is achieved by gut microbes and the efficacy of these microbes in antioxidant and intestine protection remains to be further explored.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The animal study was approved by Institutional Animal Care and Use Committee of the Institute of Animal Science at the Chinese Academy of Agricultural Sciences. The study was conducted in accordance with the local legislation and institutional requirements.
SZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing—original draft, Writing—review & editing. HL: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Writing—review & editing. XC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Software, Writing—review & editing. ST: Conceptualization, Data curation, Investigation, Writing—review & editing. RZ: Conceptualization, Data curation, Investigation, Methodology, Writing—review & editing. LC: Data curation, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Writing—review & editing. HZ: Funding acquisition, Project administration, Resources, Supervision, Validation, Visualization, Writing—review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the National Natural Science Foundation of China (32102582), the Zhejiang Province Traditional Chinese Medicine Science and Technology Project (2022ZB270), the Agricultural Science and Technology Innovation Program (CAAS-ZDRW202006-02, ASTIPIAS07), the Central Public-Interest Scientific Institution Basal Research Fund (No. 2021-YWF-ZYSQ-01), and the State Key Laboratory of Animal Nutrition (2004DA125184G2102).
HL was employed by Beijing Centre Biology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1289490/full#supplementary-material
Akhtar, M., Chen, Y., Ma, Z., Zhang, X., Shi, D., Khan, J. A., et al. (2022). Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation, Anim. Nutr. 8, 350–360. doi: 10.1016/j.aninu.2021.11.005
Cheng, C., Wei, H., Xu, C., Xie, X., Jiang, S., Peng, J., et al. (2018). Maternal soluble fiber diet during pregnancy changes the intestinal microbiota, improves growth performance, and reduces intestinal permeability in piglets, Appl. Environ. Microbiol. 84, 18. doi: 10.1128/AEM.01047-18
Feng, J., Chang, X., Zhang, Y., Yan, X., Zhang, J., Nie, G., et al. (2019). Effects of Lactococcus lactis from Cyprinus carpio L. as probiotics on growth performance, innate immune response and disease resistance against Aeromonas hydrophila. Fish Shellfish Immunol. 93, 73–81. doi: 10.1016/j.fsi.2019.07.028
Gu, X., Sim, J. X. Y., Lee, W. L., Cui, L., Chan, Y. F. Z., Chang, E. D., et al. (2022). Gut Ruminococcaceae levels at baseline correlate with risk of antibiotic-associated diarrhea. iScience 25, 103644. doi: 10.1016/j.isci.2021.103644
He, T., Zhu, Y. H., Yu, J., Xia, B., Liu, X., Yang, G. Y., et al. (2019). Lactobacillus johnsonii L531 reduces pathogen load and helps maintain short-chain fatty acid levels in the intestines of pigs challenged with Salmonella enterica Infantis. Vet. Microbiol. 230, 187–194. doi: 10.1016/j.vetmic.2019.02.003
Huang, T., Liu, Y., and Zhang, C. (2019). Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review, Eur J Drug Metab Pharmacokinet 44, 159–168. doi: 10.1007/s13318-018-0509-3
Huang, W., Long, C., and Lam, E. (2018). Roles of plant-associated microbiota in traditional herbal medicine, Trends Plant Sci. 23, 559–562. doi: 10.1016/j.tplants.2018.05.003
Kakade, A., Salama, E. S., Usman, M., Arif, M., Feng, P., and Li, X. (2022). Dietary application of Lactococcus lactis alleviates toxicity and regulates gut microbiota in Cyprinus carpio on exposure to heavy metals mixture. Fish Shellfish Immunol. 120, 190–201. doi: 10.1016/j.fsi.2021.11.038
Khudhair, Z., Alhallaf, R., Eichenberger, R. M., Whan, J., Kupz, A., Field, M., et al. (2020). Gastrointestinal helminth infection improves insulin sensitivity, decreases systemic inflammation, and alters the composition of gut microbiota in distinct mouse models of type 2 diabetes. Front. Endocrinol. (Lausanne) 11, 606530. doi: 10.3389/fendo.2020.606530
Kim, H., Park, J., Kang, H., Yun, S. P., Lee, Y. S., Lee, Y. I., et al. (2020). Activation of the Akt1-CREB pathway promotes RNF146 expression to inhibit PARP1-mediated neuronal death, Sci. Signal 13, aax7119. doi: 10.1126/scisignal.aax7119
Leth, M. L., Pichler, M. J., and Abou Hachem, M. (2022). Butyrate-producing colonic clostridia: picky glycan utilization specialists. Essays Biochem. 67, 415–428 doi: 10.1042/EBC20220125
Li, Y., Li, W., Fu, C., Song, Y., and Fu, Q. (2020). Lonicerae japonicae flos and Lonicerae flos: a systematic review of ethnopharmacology, phytochemistry and pharmacology, Phytochem. Rev. 19, 1–61. doi: 10.1007/s11101-019-09655-7
Li, Y., Xia, D., Chen, J., Zhang, X., Wang, H., Huang, L., et al. (2022). Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: roles of gut microbiota and short-chain fatty acid, Poult. Sci. 101, 101742. doi: 10.1016/j.psj.2022.101742
Lu, S., Xu, S., Chen, L., Deng, Y., and Feng, J. (2022). Periplaneta americana extract pretreatment alleviates oxidative stress and inflammation and increases the abundance of gut akkermansia muciniphila in diquat-induced mice, Antioxidants (Basel) 11, 1806. doi: 10.3390/antiox11091806
Maynard, C. L., Elson, C. O., Hatton, R. D., and Weaver, C. T. (2012). Reciprocal interactions of the intestinal microbiota and immune system. Nature 489, 231–241. doi: 10.1038/nature11551
Miao, M., and Xiang, L. (2020). Pharmacological action and potential targets of chlorogenic acid, Adv. Pharmacol. 87, 71–88. doi: 10.1016/bs.apha.2019.12.002
Negatu, D. A., Gengenbacher, M., Dartois, V., and Dick, T. (2020). Indole propionic acid, an unusual antibiotic produced by the gut microbiota, with anti-inflammatory and antioxidant properties, Front. Microbiol. 11, 575586. doi: 10.3389/fmicb.2020.575586
Pan, L., Wang, Q., Qu, L., Liang, L., Han, Y., Wang, X., et al. (2022). Pilot-scale production of exopolysaccharide from Leuconostoc pseudomesenteroides XG5 and its application in set yogurt. J. Dairy Sci. 105, 1072–1083. doi: 10.3168/jds.2021-20997
Parker, B. J., Wearsch, P. A., Veloo, A. C. M., and Rodriguez-Palacios, A. (2020). The genus alistipes: gut bacteria with emerging implications to inflammation, cancer, mental health. Front. Immunol. 11, 906. doi: 10.3389/fimmu.2020.00906
Shen, C., Luo, Z., Ma, S., Yu, C., Gao, Q., Zhang, M., et al. (2022). Microbe-derived antioxidants reduce lipopolysaccharide-induced inflammatory responses by activating the Nrf2 pathway to inhibit the ROS/NLRP3/IL-1β signaling pathway, Int. J. Mol. Sci. 23, 2477. doi: 10.3390/ijms232012477
Shi, Z., Liu, Z., Liu, C., Wu, M., Su, H., Ma, X., et al. (2016). Spectrum-effect relationships between chemical fingerprints and antibacterial effects of lonicerae japonicae flos and lonicerae flos base on UPLC and microcalorimetry. Front. Pharmacol. 7, 12. doi: 10.3389/fphar.2016.00012
Sun, M., Wang, Q., Zhang, M., Zhang, G., Wu, T., Liu, R., et al. (2020). Leuconostoc pseudomesenteroides improves microbiota dysbiosis and liver metabolism imbalance and ameliorates the correlation between dihydroceramide and strains of Firmicutes and Proteobacteria in high fat diet obese mice, Food Funct. 11, 6855–6865. doi: 10.1039/D0FO01009J
Tang, S., Xie, J., Fang, W., Wen, X., Yin, C., Meng, Q., et al. (2022). Chronic heat stress induces the disorder of gut transport and immune function associated with endoplasmic reticulum stress in growing pigs. Animal Nutr. 11, 228–241. doi: 10.1016/j.aninu.2022.08.008
Wan, F., Cai, X., Wang, M., Chen, L., Zhong, R., Liu, L., et al. (2021). Chlorogenic acid supplementation alleviates dextran sulfate sodium (DSS)-induced colitis via inhibiting inflammatory responses and oxidative stress, improving gut barrier integrity and Nrf-2/HO-1 pathway. J. Funct. Food., 87, 104808. doi: 10.1016/j.jff.2021.104808
Wan, F., Wang, M., Zhong, R., Chen, L., Han, H., Liu, L., et al. (2022). Supplementation with chinese medicinal plant extracts from Lonicera hypoglauca and Scutellaria baicalensis mitigates colonic inflammation by regulating oxidative stress and gut microbiota in a colitis mouse model, Front. Cell. Infect. Microbiol. 11, 798052. doi: 10.3389/fcimb.2021.798052
Wang, X., Fan, F., and Cao, Q. (2016). Modified Pulsatilla decoction attenuates oxazolone-induced colitis in mice through suppression of inflammation and epithelial barrier disruption. Mol. Med. Rep. 14, 1173–1179. doi: 10.3892/mmr.2016.5358
Wang, X., Xie, L., Long, J., Liu, K., Lu, J., Liang, Y., et al. (2022). effect of baicalin on inflammatory bowel disease: A review, J Ethnopharmacol 283, 114749. doi: 10.1016/j.jep.2021.114749
Werum, V., Ehrmann, M., Vogel, R., and Hilgarth, M. (2022). Comparative genome analysis, predicted lifestyle and antimicrobial strategies of Lactococcus carnosus and Lactococcus paracarnosus isolated from meat, Microbiol. Res. 258, 126982. doi: 10.1016/j.micres.2022.126982
Xi, L., Song, Y., Han, J., and Qin, X. (2021). Microbiome analysis reveals the significant changes in gut microbiota of diarrheic Baer's Pochards (Aythya baeri), Microb. Pathog. 157, 105015. doi: 10.1016/j.micpath.2021.105015
Xia, G. H., Li, X. H., Zhang, Z., and Jiang, Y. H. (2021). Effects of fermentation treatments on Polygonatum odoratum flavones' antioxidant activities. Saudi J. Biol. Sci. 28, 5011–5016. doi: 10.1016/j.sjbs.2021.01.026
Yang, F., Wei, J. D., Lu, Y. F., Sun, Y. L., Wang, Q., Zhang, R. L., et al. (2019). Galacto-oligosaccharides modulate gut microbiota dysbiosis and intestinal permeability in rats with alcohol withdrawal syndrome. J. Funct. Food. 60, 103423. doi: 10.1016/j.jff.2019.103423
Yin, B., Li, W., Qin, H., Yun, J., and Sun, X. (2021). The use of chinese skullcap (Scutellaria baicalensis) and its extracts for sustainable animal production. Animals (Basel) 11, 39. doi: 10.3390/ani11041039
Zhang, S., Lv, H., Zhong, R., Tang, S., Han, H., Cai, X. F., et al. (2023). Baicalin promotes appetite by regulating gut microbiome and immunity? J. Funct. Food. 105, 105557. doi: 10.1016/j.jff.2023.105557
Zhao, Q., Chen, X. Y., and Martin, C. (2016). Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants, Sci. Bull. (Beijing) 61, 1391–1398. doi: 10.1007/s11434-016-1136-5
Zhao, T., Tang, H., Xie, L., Zheng, Y., Ma, Z., Sun, Q., et al. (2019). Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol. 71, 1353–1369. doi: 10.1111/jphp.13129
Zhong, Y. B., Kang, Z. P., Wang, M. X., Long, J., Wang, H. Y., Huang, J. Q., et al. (2021). Curcumin ameliorated dextran sulfate sodium-induced colitis via regulating the homeostasis of DCs and Treg and improving the composition of the gut microbiota. J. Funct. Food. 86, 104716. doi: 10.1016/j.jff.2021.104716
Keywords: Scutellaria baicalensis Georgi, Caprifoliaceae, gut microbe, antioxidant, inflammatory
Citation: Zhang S, Lv H, Cai X, Tang S, Zhong R, Chen L and Zhang H (2024) Effects of the compound extracts of Caprifoliaceae and Scutellaria baicalensis Georgi on the intestinal microbiota and antioxidant function. Front. Microbiol. 14:1289490. doi: 10.3389/fmicb.2023.1289490
Received: 06 September 2023; Accepted: 22 December 2023;
Published: 12 January 2024.
Edited by:
Samanta Thomas-Valdés, Universidad de Valparaiso, ChileReviewed by:
Tongxing Song, Huazhong Agricultural University, ChinaCopyright © 2024 Zhang, Lv, Cai, Tang, Zhong, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruqing Zhong, emhvbmdydXFpbmdAY2Fhcy5jbg==; Xueying Cai, Y2FpeHloc0AxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.