
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 05 September 2023
Sec. Microbe and Virus Interactions with Plants
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1269567
This article is part of the Research TopicBacterial Wilt: Pathogenic Mechanism, Disease Control, Bacteria-Plant and Bacteria-Environmental Microorganism InteractionsView all 6 articles
Ralstonia solanacearum, the causal agent of bacterial wilt, is a devastating plant pathogenic bacterium that infects more than 450 plant species. Until now, there has been no efficient control strategy against bacterial wilt. In this study, we screened a library of 100 plant-derived compounds for their antibacterial activity against R. solanacearum. Twelve compounds, including harmine, harmine hydrochloride, citral, vanillin, and vincamine, suppressed bacterial growth of R. solanacearum in liquid medium with an inhibition rate higher than 50%. Further focus on harmine revealed that the minimum inhibitory concentration of this compound is 120 mg/L. Treatment with 120 mg/L of harmine for 1 and 2 h killed more than 90% of bacteria. Harmine treatment suppressed the expression of the virulence-associated gene xpsR. Harmine also significantly inhibited biofilm formation by R. solanacearum at concentrations ranging from 20 mg/L to 60 mg/L. Furthermore, application of harmine effectively reduced bacterial wilt disease development in both tobacco and tomato plants. Collectively, our results demonstrate the great potential of plant-derived compounds as antibacterial agents against R. solanacearum, providing alternative ways for the efficient control of bacterial wilt.
Ralstonia solanacearum, the causal agent of bacterial wilt, is a devastating soil-borne bacterium capable of causing wilting disease in plants (Álvarez et al., 2010). Being a species complex, R. solanacearum has a wide host range and can infect over 450 plant species (Ahmed et al., 2022). Under favorable conditions, the bacterium can survive for extended periods in soil, plant debris, and freshwater (Álvarez et al., 2010; Caldwell et al., 2017). Upon sensing plant signals, R. solanacearum exhibits chemotaxis, moving toward the plant roots using its flagella, and then infects the plant through wounds or natural openings. Once inside the roots, R. solanacearum rapidly multiplies in the xylem and secretes large amounts of extracellular polysaccharides that clog the plant’s water and nutrient-conducting xylem vessels when the bacterial population is well established (Tran et al., 2016; She et al., 2017). This ultimately leads to wilting of the entire plant, known as bacterial wilt. Due to its significance academic impact and devastating economic losses, R. solanacearum is recognized as the second most important bacterial pathogen (Mansfield et al., 2012).
Control measures for bacterial wilt are crucial to minimize its devastating impact on plant growth (Hayward, 1991). Currently, agricultural practices mainly include methods such as soil disinfection, grafting, crop rotation, resistance breeding, and the use of chemical or biological agents to control bacterial wilt (Ahmed et al., 2022). Soil disinfection techniques, such as solarization and fumigation, aim to reduce the pathogen population in the soil (Ahmed et al., 2022). Crop rotation involves alternating susceptible and resistant crops to interrupt the pathogen’s life cycle (Fan et al., 2022). Breeding programs focus on developing resistant varieties through conventional breeding or genetic engineering (Genin and Denny, 2012; Safni et al., 2014). While certain control measures have exhibited a degree of effectiveness, they frequently do not meet the anticipated outcomes. Although the use of molecular markers to identify resistance genes in hosts of R. solanacearum has greatly facilitated the breeding of resistant varieties, challenges remain in stabilizing the inheritance of resistance genes and determining the impact of resistant varieties on yield (Qian et al., 2013; Nelson et al., 2018; Abebe et al., 2020). Chemical pesticides can effectively eradicate R. solanacearum; nevertheless, they not only induce environmental pollution but also foster the development of pesticide resistance within the bacterium (Nicolopoulou-Stamati et al., 2016). Therefore, it is crucial to develop safe, environmentally friendly, efficient, and economically viable strategies for controlling bacterial wilt.
Natural products refer to bioactive secondary metabolites isolated from animals, plants, microorganisms, and marine organisms. They offer advantages such as low cost, high safety, and effectiveness (Shen and Hao, 2020). In recent years, many natural products have not only been applied in the prevention and treatment of bacterial plant diseases but also serve as lead compounds for biopesticides (Zhang et al., 2018). Plant-derived compounds, specifically New 8-O-4’ Neolignans isolated from the whole plant of Clematis lasiandra Maxim, have been found to inhibit various plant pathogens, including R. solanacearum (Hao et al., 2020). Coumarins, secondary metabolites derived from plants, have also been discovered to significantly inhibit R. solanacearum, with enhanced activity through hydroxylation at the C-6, C-7, and C-8 positions of the coumarin structure (Yang et al., 2021b).
Harmine, also known as telepathine, is a natural alkaloid found in several plants, including Peganum harmala L. and Banisteriopsis caapi (Callaway et al., 1999). It has gained considerable attention due to its potential therapeutic effects and wide-ranging pharmacological activities. Research suggests that harmine exhibits a range of effects on the central nervous system (dos Santos and Hallak, 2017). Harmine has been found to act as a reversible inhibitor of monoamine oxidase-A (MAO-A), an enzyme involved in the breakdown of neurotransmitters such as serotonin, norepinephrine, and dopamine (Dakic et al., 2016; dos Santos and Hallak, 2017). By inhibiting MAO-A, harmine can increase the levels of these neurotransmitters, leading to mood enhancement and potential antidepressant effects (Dakic et al., 2016). Moreover, harmine has been investigated for its impact on cognitive function. Studies have indicated its potential as a neuroprotective agent, with the ability to enhance memory and improve learning abilities (Zhang et al., 2020). Additionally, harmine has been explored for its anti-inflammatory, antioxidant, and anticancer properties, showing promising results in various preclinical studies. However, the effect of harmine on plant pathogenic bacteria, such as R. solanacearum, remains unknown.
In this study, we identified harmine as a promising antibacterial agent against R. solanacearum. We found that harmine has a minimum inhibitory concentration of 120 mg/L. Biofilm formation of R. solanacearum was also significantly suppressed by different concentrations of harmine. Application of harmine effectively delayed bacterial wilt disease development in both tobacco and tomato plants.
The R. solanacearum strain CQPS-1, which was originally isolated from diseased tobacco plants in Pengshui, Chongqing, was used in this study (Liu et al., 2017). R. solanacearum was grown in B liquid medium (1% peptone, 0.1% tryptone, 0.1% yeast extract, 2.5% glucose) or B solid medium supplemented with 1.5% agar at 28°C.
All plant-derived compounds (HPLC >98%) used in this study were purchased from Targetmol (Shanghai, China). Compounds were dissolved in dimethyl sulfoxide (DMSO) to a final concentration of 10 mM or 100 mM and stored at-20°C. Ten minutes before use, the frozen compound solutions were thawed at room temperature and added to B or B agar medium to designed concentrations. The same volume of DMSO was used as solvent control.
The screening of bioactive antibacterial compounds against R. solanacearum was carried out in 1.5 mL tubes. Briefly, 282 μL of B liquid medium was added to individual tube, followed by adding 15 μL of OD600 = 0.01 bacterial suspension. Then 3 μL of 10 mM compound solution was added to the culture mixture to reach the final concentration of 100 μM. The same volume of DMSO was used as solvent control. The culture mixture was incubated in a shaker with 180 rpm at 28°C. The OD600 was measured 24 h post-incubation. Three technical replicates were used for each treatment. The inhibitory rate was calculated by the following formula, inhibitory rate (%) = (OD600 of DMSO treated samples − OD600 of compound treated samples) / OD600 of DMSO treated samples × 100%.
The growth curve of R. solanacearum was determined as follows. Briefly, 25 μL of OD600 = 0.1 bacterial suspension was added into 5 mL of B liquid medium, followed by adding different amounts of harmine to reach the final concentration of 20 mg/L, 40 mg/L, 60 mg/L, 80 mg/L or 100 mg/L, respectively. The same volume of DMSO was used as solvent control. The culture mixture was incubated in a shaker with 180 rpm at 28°C. The OD600 was measured every 2 h post-incubation. Three technical replicates were used for each treatment.
The minimum inhibitory concentration (MIC) was determined using the dilution method with a series of harmine concentrations ranging from 20 mg/L to 120 mg/L. The OD600 of the R. solanacearum suspension was adjusted to 0.01 with sterile water. Next, 15 μL of the OD600-adjusted bacterial suspension was added into 285 μL of liquid B medium containing different concentrations of harmine, ranging from 20 mg/L to 120 mg/L. The inoculated culture was then incubated at 28°C for 24 h. The MIC was defined as the lowest concentration at which no visible growth of R. solanacearum was observed at 48 h post-incubation.
The inhibitory effect of harmine on the growth of R. solanacearum in solid medium was evaluated as follows. Harmine was added into B solid medium to reach final concentrations ranging from 40 mg/L to 340 mg/L. Then, 50 μL of OD600 = 0.0001 bacterial suspension were plated on B solid medium supplemented with different concentrations of harmine. The bacterial growth was observed at 2 days post-incubation.
The overnight-cultured R. solanacearum suspension was centrifuged and re-suspended in sterile water. The OD600 was adjusted to 0.1. Then, the bacterial suspension was divided into 15 mL centrifuge tubes, with 3 mL in each tube. Harmine was added to each tube to generate final concentrations of 60, 120, 180, or 240 mg/L. The control group was treated with an equal amount of DMSO. The harmine-treated bacterial suspension was then incubated at 28°C. At 1 h or 2 h post-incubation, 100 μL of the incubated suspension was sampled and diluted to create 10−1, 10−2, 10−3, 10−4, and 10−5 dilutions. Three drops of 5 μL of each diluted bacterial suspension were then placed on B agar plates. The B agar medium was incubated at 28°C for 48 h. Live bacteria were counted, and the sterilizing rate was calculated.
Biofilm formation was analyzed as previously described with some modifications (Wu et al., 2015). Briefly, 10 μL of R. solanacearum suspension (OD600 = 0.1) was added to 190 μL of liquid medium B in a 96-well polystyrene microplate. Harmine was then added to each well to reach final concentrations of 20 mg/L, 40 mg/L, or 60 mg/L. DMSO was used as the negative control. The 96-well plate was placed in an incubator at 28°C for static cultivation for 24 h. After incubation, the culture medium was gently aspirated using a micropipette, followed by gentle washes with 200 μL of sterile water to remove the medium. Next, 220 μL of 0.1% crystal violet was added and the plate was stained at room temperature for 30 min. After staining, the crystal violet was aspirated, and the wells were washed twice with 200 μL of sterile water to remove excess dye. The plate was air-dried at room temperature for 30 min, and then 200 μL of 95% ethanol was added to dissolve the crystal violet adsorbed on the biofilm. Finally, the 96-well plate was placed in a microplate reader to measure the absorbance at 530 nm.
The effect of harmine on the expression of virulence-related genes of R. solanacearum was tested using quantitative real-time PCR (qPCR), following the procedure previously described with minor modifications (Wu et al., 2015). Fifty microliters of R. solanacearum suspension (OD600 = 1.0) was added to 5 mL of liquid medium, and harmine was added to reach a final concentration of 80 mg/L. The mixture was then incubated at 28°C for 6 h. The bacterial cells were harvested by centrifugation, and the total RNA of R. solanacearum was extracted using the Unizol Total RNA Extraction Reagent, following the manufacturer’s instructions (Genesand). Subsequently, cDNA was synthesized with the HiScript II 1st Strand cDNA Synthesis Kit, following the protocol provided by the company (Vazyme). For qPCR, 20 μL reactions (BioRad, CFX96) were prepared, each containing 1 pmol of forward and reverse primers, 2 μL of 1 to 10 diluted cDNA, and 4 μL of ChamQ Universal SYBR qPCR Master Mix (Vazyme). The primers used were the same as previously described (Han et al., 2021). Relative gene expression was calculated using the 2-∆∆CT method.
The naturalistic soil soak assay was used to evaluate the effectiveness of harmine in controlling tobacco and tomato bacterial wilt. Briefly, 4-week-old tobacco and tomato plants were first inoculated with 10 mL of R. solanacearum suspension (OD600 = 0.1). One hour later, the seedlings were treated with 10 mL of harmine at a concentration of 150 mg/L. An equal volume of diluted DMSO was used as the negative control. The inoculated plants were then placed in a growth chamber at 28°C with a 14/10 h light/dark photoperiod. The symptoms were scored daily using a disease index scale ranging from 0 to 4 (0, no symptoms appeared, 1, 1 to 25% of leaves wilted, 2, 26 to 50% of leaves wilted; 3, 51 to 75% of leaves wilted; 4, 76 to 100% of leaves wilted). The control efficiency was calculated with the following formula: Control efficiency % = (Disease index of DMSO treated seedlings – Disease index of harmine treated seedlings) / Disease index of DMSO treated seedlings × 100%.
The data were analyzed with either Excel or GraphPad Prism using Student’s t-test (*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001).
To identify new antibacterial agents against R. solanacearum, we tested the impact of 100 plant-derived compounds on its growth in liquid medium at a concentration of 100 μM. To quantify the antibacterial activity of each compound, we used the inhibitory rate, which refers to the percentage reduction in bacterial growth (the OD600 value) as a result of the treatment with a specific compound. Twelve compounds, namely harmine, harmine hydrochloride, citral, vanillin, vincamine, anisodamine, decitabine, higenamine hydrochloride, solanesol, alpha-Boswellic acid, and trimethoxystilbene, exhibited a very strong inhibitory effect on the growth of R. solanacearum, with an inhibitory rate higher than 50% (Table 1). Among these bioactive compounds, harmine showed the most potent inhibitory effect. The average OD600 of the DMSO-treated sample, which served as the solvent control, was 1.19, while the average OD600 of the harmine-treated sample was 0.25 (Table 1), indicating a significant suppression of R. solanacearum growth by harmine in liquid medium. Some other compounds, such as eburnalritardo and chlorogenic acid, also exhibited inhibitory effects on the growth of R. solanacearum, though the inhibitory rate was not as strong as that of the 12 compounds mentioned above (Table 1). Collectively, the screening identified several plant-derived compounds as novel antibacterial agents against R. solanacearum.
To further determine the impact of harmine on inhibiting R. solanacearum growth, we tested the inhibitory effect of different concentrations of harmine, ranging from 20 mg/L to 100 mg/L. The growth curve from 12 h to 24 h indicated that all tested concentrations had a strong inhibitory effect on the growth of R. solanacearum (Figure 1A). The inhibitory rate reached about 90% at 100 mg/L and decreased with the reduction of harmine concentration. At 24 h post-treatment, the inhibitory rate decreased to about 50% at 20 mg/L (Figures 1A,B). The growth of R. solanacearum was completely suppressed when the liquid medium was supplemented with 120 mg/L of harmine (Figure 1B). Therefore, this concentration was defined as the MIC of harmine against R. solanacearum. We further confirmed the inhibitory effect of harmine against R. solanacearum using the solid dilution method. The result showed that supplementation of harmine into the agar plate significantly suppressed the growth of R. solanacearum at 120 mg/L or higher (Figure 1C).
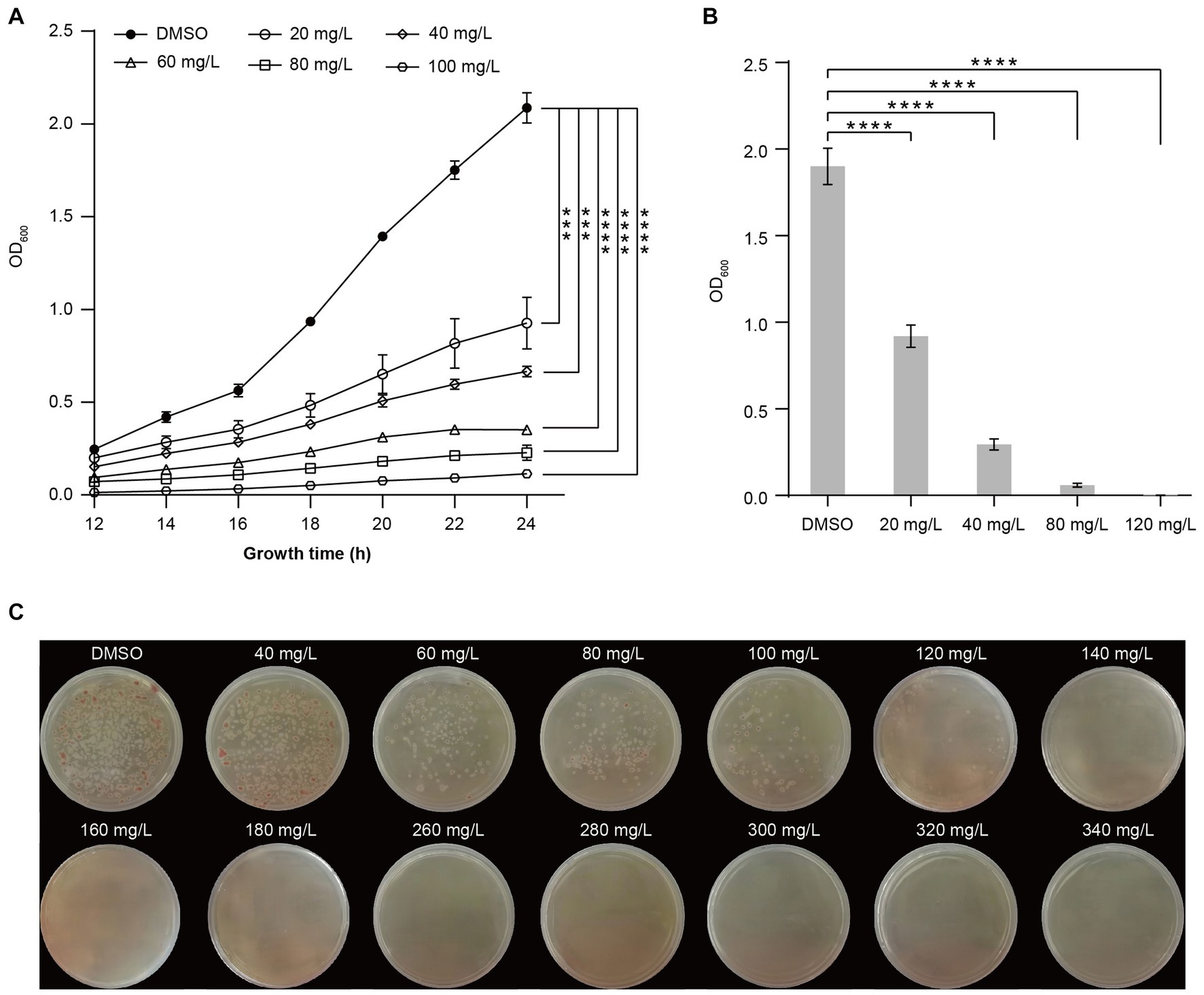
Figure 1. Harmine inhibits the growth of R. solanacearum in a concentration-dependent manner. (A) The growth curve of R. solanacearum in liquid medium supplemented with DMSO or different concentrations of harmine. The OD600 was measured every 2 h starting from 12 h post-incubation. (B) The inhibitory effect of different concentrations of harmine on R. solanacearum growth at 24 h post-incubation. Data are presented as the mean ± SD (n = 3). ***p < 0.001, ****p < 0.0001 (Student’s t-test). (C) Inhibition of R. solanacearum growth on B agar plate supplemented with different concentrations of harmine. B agar plate was supplemented with 0.005% triphenyl tetrazolium chloride (TTC). Pictures were taken 48 h post-incubation.
To determine if harmine exhibits bactericidal activity, we treated R. solanacearum suspension with different concentrations of harmine, using DMSO as the negative control. At 1 h and 2 h post-treatment, bacterial suspensions were diluted and plated on agar plates for live bacteria counting. Treatment with 60 mg/L of harmine resulted in the death of 85 and 90% of bacteria at 1 h and 2 h, respectively (Figure 2). When 180 mg/L of harmine was applied, 96 and 98% of bacteria were killed at 1 h and 2 h, respectively (Figure 2). Furthermore, at 2 h post-treatment with 240 mg/L of harmine, 99.3% of bacteria were killed (Figure 2). These results strongly suggest that harmine possesses bactericidal activity against R. solanacearum.
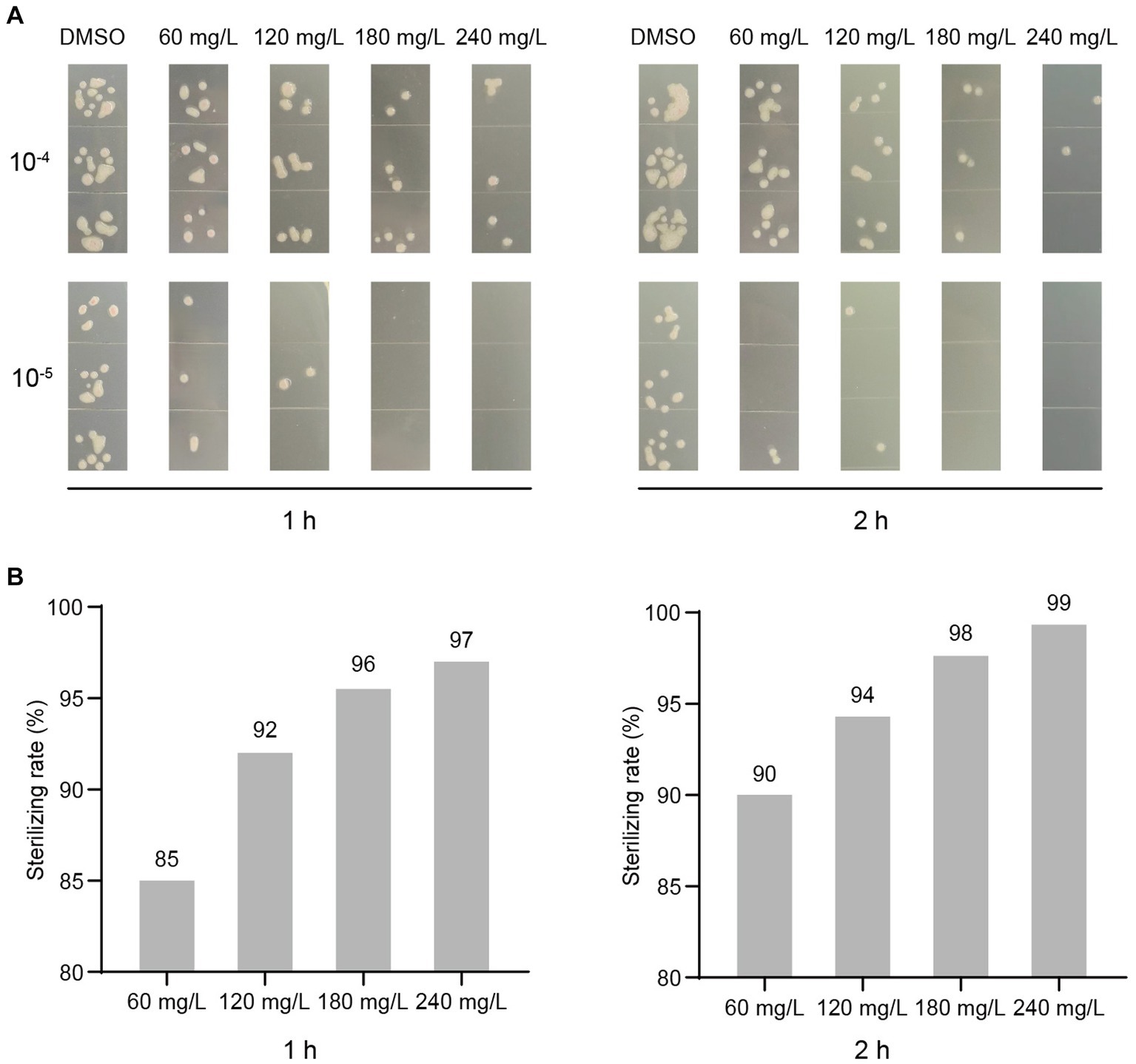
Figure 2. Harmine exhibits bactericidal activity against R. solanacearum. The OD600 of the R. solanacearum suspension was adjusted to 0.1. DMSO and different concentrations of harmine were added to the R. solanacearum suspension. The harmine-treated bacterial suspension was then incubated at 28°C. At 1 h and 2 h post-treatment, the bacterial suspension was diluted, plated on agar medium, and further incubated at 28°C for 2 days. (A) The live bacteria that grew on agar plates from dilutions of 10−4 and 10−5 were shown. (B) The live bacteria from dilutions of 10−4 were counted, and the sterilizing rates were calculated, with DMSO-treated samples as the background.
To evaluate the effect of harmine on biofilm formation by R. solanacearum, we utilized the standard polyvinyl chloride (PVC) assay to quantify biofilm in the absence or presence of harmine, with concentrations ranging from 20 mg/L to 60 mg/L. Compared to the DMSO treatment, supplementation with harmine significantly reduced biofilm formation by R. solanacearum. When 20 mg/L and 60 mg/L of harmine were applied, the biofilm formation was reduced by 59 and 72%, respectively (Figure 3). Notably, the reduction in biofilm formation was similar when different concentrations (20 mg/L, 40 mg/L, and 60 mg/L) of harmine were applied (Figure 3), despite that statistical significance was also detected between 20 mg/L and 40 mg/L treatment.
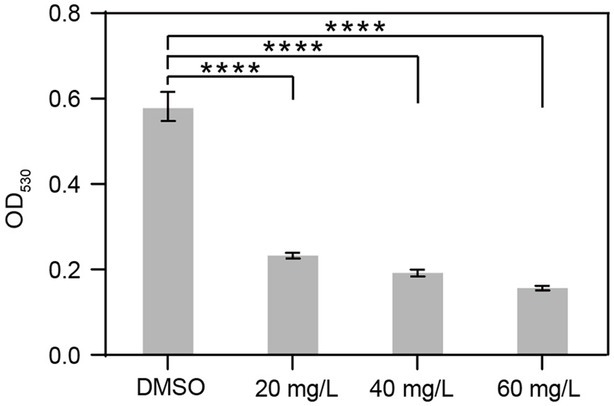
Figure 3. Harmine reduces biofilm formation by R. solanacearum. R. solanacearum was grown in liquid medium supplemented with different concentrations of harmine for 24 h and then subjected to biofilm quantification. Data are presented as the mean ± SD (n = 3). ****p < 0.0001 (Student’s t-test).
To gain insights into the preliminary mechanism by which harmine inhibits R. solanacearum, we employed qPCR to quantify the expression of several virulence-related genes in the presence and absence of harmine. The analyzed genes included espE and xpsR, involved in extracellular polysaccharide synthesis, as well as hrpG and popA, associated with the type III secretion system, and CheW and CheA, linked to chemotaxis (Han et al., 2021). The results revealed that harmine significantly suppressed the expression of xpsR. Furthermore, harmine treatment led to a reduction in espE expression, although the difference between DMSO and harmine treatment was not statistically significant. However, the expression of all other tested genes remained unchanged upon harmine treatment (Figure 4). These findings suggest that harmine specifically inhibits genes related to extracellular polysaccharide synthesis.
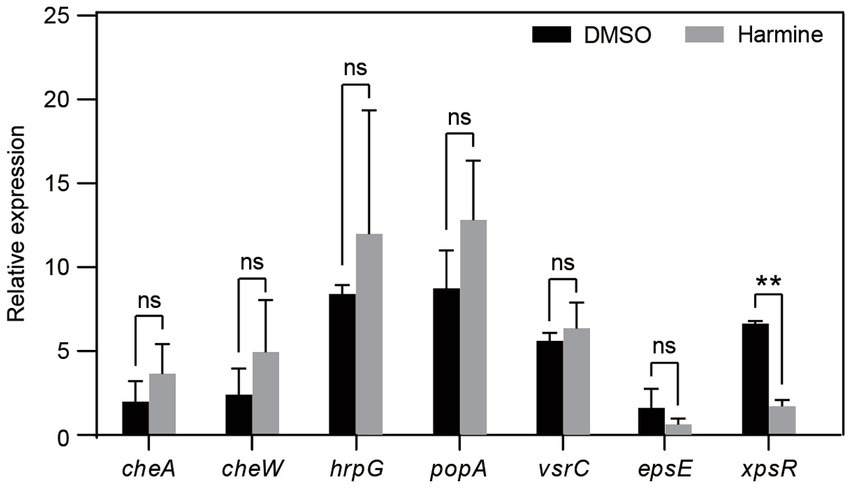
Figure 4. Harmine inhibits the expression of the virulence gene xpsR. R. solanacearum grown in liquid medium was treated with 80 mg/L harmine for 6 h. Total RNA was extracted and used for qPCR analysis. The expression of candidate genes was normalized to the reference gene serC. Data are presented as the mean ± SD (n = 3). ns, not significant; **p < 0.05 (Student’s t-test).
Having established that harmine exhibits strong antibacterial activity against R. solanacearum, reduces its biofilm formation and suppresses xpsR expression, we aimed to investigate whether harmine could delay or reduce the development of bacterial wilt disease. Given that R. solanacearum infects a wide range of host plants, including Solanaceae crops, we initially examined its effect on tobacco disease development. We first inoculated 4-week-old tobacco seedlings with R. solanacearum, followed by treating the seedlings with either DMSO or 150 mg/L. Compared to the DMSO treatment, the 150 mg/L harmine treatment significantly reduced the bacterial wilt disease development in tobacco seedlings (Figure 5A). Next, we assessed the effect of harmine on tomato bacterial wilt development. Similar to tobacco, treatment with 150 mg/L of harmine also resulted in a significant reduction in disease progression in tomato plants (Figure 5B). We further evaluated the control efficiency of harmine against bacterial wilt in both tobacco and tomato. Harmine treatment led to 25 and 50% control efficiency in tobacco and tomato, respectively, at 8 days post-inoculation (Figures 5C,D). These results demonstrate that harmine has the potential to be an effective antibacterial agent for the control of bacterial wilt caused by R. solanacearum.
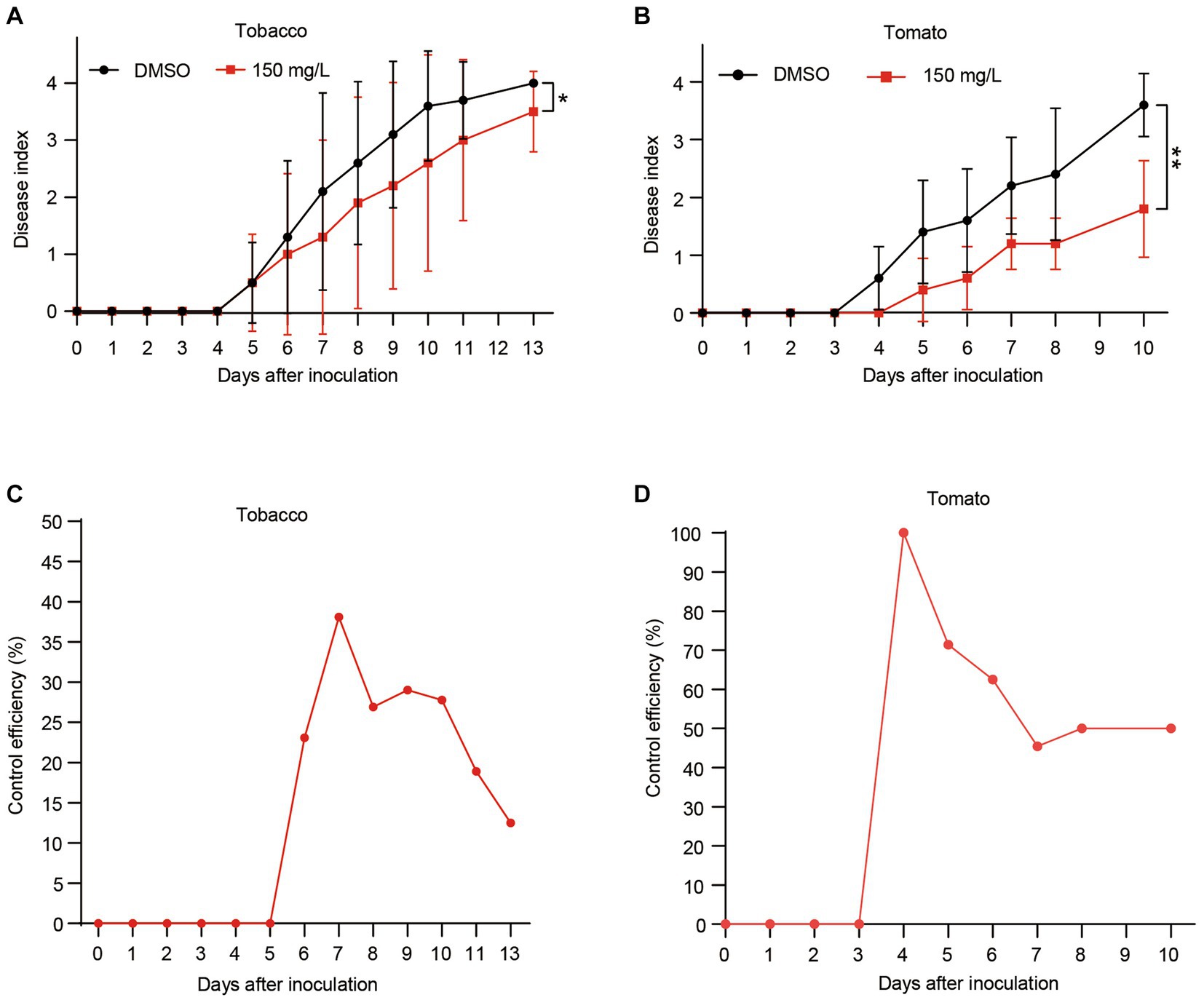
Figure 5. Harmine reduces bacterial wilt disease development in both tobacco and tomato. Four-week-old tobacco (A) and tomato (B) seedlings were inoculated with 10 mL of bacterial suspension at OD600 of 0.1, followed by treating the seedlings with either DMSO or 150 mg/L of harmine. Disease symptoms were recorded daily using a disease index scale ranging from 0 to 4 (0, healthy; 1, 1 to 25% of leaves wilted; 2, 26 to 50% of leaves wilted; 3, 51 to 75% of leaves wilted; 4, 76 to 100% of leaves wilted). Each point represents the mean disease index of 12 plants. *p < 0.05, **p < 0.01 (Student’s t-test). (C,D) The control efficiency of harmine on tobacco bacterial wilt (C) and tomato bacterial wilt (D). The control efficiency was calculated with the following formula: Control efficiency % = (Disease index of DMSO treated seedlings – Disease index of harmine treated seedlings) / Disease index of DMSO treated seedlings × 100.
As a soil-borne plant disease, the control of bacterial wilt is extremely challenging, despite that some alternative strategies have been used to control this disease (Jiang et al., 2017). The use of chemical pesticides is the major way for the control of bacterial wilt. Chemical pesticides, while effective in controlling plant diseases in agriculture, can have significant side effects on the environment, human health, and non-target organisms (Tudi et al., 2021; Mansoor and Shad, 2022). Furthermore, pesticide resistance can develop in target pests over time, rendering the chemicals less effective and leading to a need for stronger and more toxic formulations (Ishii, 2006; Shao et al., 2021; Mansoor and Shad, 2022). As a result, there is a growing need to adopt more sustainable and environmentally friendly alternatives to minimize the side effects associated with chemical pesticides. Plant-derived compounds offer several advantages, such as environmentally friendly and lower toxicity to humans, over chemical pesticides, making them an attractive alternative for plant disease control (Sparks and Bryant, 2022). Plant-derived compounds have the potential to revolutionize disease management, providing a greener and more sustainable approach to protect crops while minimizing the negative impacts associated with chemical pesticides. Previous studies have identified several plant-derived compounds, such as alkyl gallates, Lansiumamide B, 7-methoxycoumarin, hydroxycoumarins, coumarins, that show strong inhibitory effect on the growth of R. solanacearum (Ooshiro et al., 2011; Li et al., 2014; Yang et al., 2016, 2021a; Han et al., 2021). Some plant-derived compounds are able to suppress the virulence factors (e.g., type III secretion system and biofilm formation) of R. solanacearum and thus reduce its pathogenicity on host plants (Yang et al., 2017; Han et al., 2021). However, these studies report the inhibitory effect of single compound on the growth of R. solanacearum. Large-scale screening of antibacterial activity of plant-derived compounds against R. solanacearum has rarely been reported.
In this study, we conducted a screening of 100 plant-derived compounds to assess their antibacterial activity against R. solanacearum. Remarkably, 12 compounds demonstrated strong inhibitory effects on the growth of R. solanacearum, with an inhibitory rate exceeding 50% (Table 1). This indicates that a considerable proportion of plant-derived compounds possess potent antibacterial activity against this bacterium. Among the tested compounds, harmine exhibited the most remarkable antibacterial activity compared to the other 11 compounds, with a MIC of 120 mg/L. It is worth noting that this MIC value is slightly higher than the one observed for daphnetin (Yang et al., 2016). Their study assessed the inhibitory effect of daphnetin against R. solanacearum using the solid dilution method for 12 h. In contrast, we evaluated the MIC of harmine at 24 h post-treatment. This disparity in testing duration may account for the relatively higher MIC observed for harmine in our study.
Biofilm formation plays a crucial role in bacterial virulence and infection (O’Loughlin et al., 2013). In R. solanacearum, biofilms facilitate its ability to infect plants and enhance its colonization in plant roots and stems (Yao and Allen, 2007). Thus, the normal formation of biofilms is essential for the successful infection and proliferation of R. solanacearum. Many natural products derived from plants have shown potential in inhibiting the pathogenicity of R. solanacearum by restricting biofilm formation (Wu et al., 2015; Yang et al., 2017; Han et al., 2021). This study found that harmine can effectively inhibit the formation of biofilms by R. solanacearum. In previous research, the minimum concentration required to inhibit biofilm formation generally correlated with the MIC of the compound. However, in our study, we observed that the lowest concentration of harmine needed to inhibit biofilm formation was significantly lower than the MIC, indicating that low concentrations of harmine are still able to suppress biofilm formation by R. solanacearum.
The accumulation of extracellular polysaccharides is a key trigger for wilting symptom development in plants infected with R. solanacearum (Saile et al., 1997). This accumulation facilitates the rapid wilting of infected plants (Genin and Denny, 2012; Lowe-Power et al., 2018). Studies have shown that ginger extract can inhibit the growth of R. solanacearum and significantly reduce the production of extracellular polysaccharides by the bacterium (Zhang et al., 2022). After treating R. solanacearum with harmine in vitro, we observed a significant decrease in the expression of the key transcription factor xpsR, which regulates the biosynthesis of extracellular polysaccharides (Huang et al., 1995). Previous research involving the plant growth regulator silicon demonstrated its ability to inhibit the expression of the xpsR gene and the secretion of extracellular polysaccharides in R. solanacearum, thereby reducing its pathogenicity (Wang et al., 2022). This suggests that harmine reduces the pathogenicity of R. solanacearum by inhibiting the expression of the xpsR gene. Notably, the expression of epsE, a downstream gene involved in extracellular polysaccharide biosynthesis, did not show a significant suppression by harmine, although a decrease in expression was observed. This could be attributed to the fact that xpsR regulates the expression of multiple genes, and its inhibitory effect on a single gene may be attenuated.
Our study found that harmine did not inhibit the expression of several other virulence-related genes tested, such as those associated with the type III secretion system or chemotaxis. We speculate that this discrepancy may be due to the nutrient-rich medium used in our study, as R. solanacearum may only express type III secretion system associated genes in nutrient-poor minimal medium (Genin et al., 1992; Zhang et al., 2011). Interestingly, harmine was recently shown to inhibit the type III secretion system of Salmonella enterica serovar Typhimurium (Shi et al., 2022). S. enterica serovar Typhimurium is an animal pathogenic bacterium. Given the differences of the type III secretion system proteins between animal and plant pathogenic pathogens, it is conceivable that harmine targets the type III secretion system of specific pathogens instead of a broad range of pathogens. Therefore, harmine may specifically target certain pathways, such as xpsR, rather than affecting multiple aspects of R. solanacearum’s virulence.
In summary, this study identified several plant-derived compounds as potent antibacterial agents against R. solanacearum, with harmine exhibiting the strongest activity, with a MIC of 120 mg/L. Harmine significantly inhibited R. solanacearum biofilm formation and downregulated the expression of certain virulence-related genes. Moreover, harmine effectively suppressed the development of tobacco and tomato bacterial wilt disease. These findings suggest that plant-derived compounds, like harmine, hold tremendous potential for controlling the devastating bacterial wilt disease caused by R. solanacearum.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
HX: Writing – original draft, Investigation. YH: Writing – original draft, Investigation. RW: Investigation, Writing – review & editing. XT: Investigation, Writing – review & editing. JC: Investigation, Writing – review & editing. S-xL: Conceptualization, Writing – review & editing. LJ: Conceptualization, Funding acquisition, Writing – original draft. DW: Conceptualization, Funding acquisition, Writing – original draft.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Natural Science Foundation of Chongqing (CSTB2022NSCQ-MSX0813), the Natural Science Foundation of Hunan (2021JJ40058 and 2023JJ60473), the Young Elite Scientists Sponsorship Program by CAST (grant no. 2021QNRC001), the National Natural Science Foundation of China (82104055), the China Postdoctoral Science Foundation (2020 M671163), and the Hunan Provincial Department of Education Science Research Project (22B0396).
The authors would like to thank members of DW laboratory for helpful discussions and technical support on the project.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abebe, A. M., Choi, J., Kim, Y., Oh, C. S., Yeam, I., Nou, I. S., et al. (2020). Development of diagnostic molecular markers for marker-assisted breeding against bacterial wilt in tomato. Breed. Sci. 70, 462–473. doi: 10.1270/jsbbs.20027
Ahmed, W., Yang, J., Tan, Y., Munir, S., Liu, Q., Zhang, J., et al. (2022). Ralstonia solanacearum, a deadly pathogen: revisiting the bacterial wilt biocontrol practices in tobacco and other Solanaceae. Rhizosphere 21:100479. doi: 10.1016/j.rhisph.2022.100479
Álvarez, B., Biosca, E. G., and López, M. M. (2010). “On the life of Ralstonia solanacearum, a destructive bacterial plant pathogen,” in Current research, technology and education topics in applied microbiology and microbial. ed. A. Mendez-Vilas (Badajoz: Formatex), 267–279.
Caldwell, D., Kim, B.-S., and Iyer-Pascuzzi, A. S. (2017). Ralstonia solanacearum differentially colonizes roots of resistant and susceptible tomato plants. Phytopathology 107, 528–536. doi: 10.1094/PHYTO-09-16-0353-R
Callaway, J. C., McKenna, D. J., Grob, C. S., Brito, G. S., Raymon, L. P., Poland, R. E., et al. (1999). Pharmacokinetics of Hoasca alkaloids in healthy humans. J. Ethnopharmacol. 65, 243–256. doi: 10.1016/S0378-8741(98)00168-8
Dakic, V., de Moraes Maciel, R., Drummond, H., Nascimento, J. M., Trindade, P., and Rehen, S. K. (2016). Harmine stimulates proliferation of human neural progenitors. PeerJ 4:e2727. doi: 10.7717/peerj.2727
dos Santos, R. G., and Hallak, J. E. C. (2017). Effects of the natural β-carboline alkaloid harmine, a main constituent of ayahuasca, in memory and in the hippocampus: a systematic literature review of preclinical studies. J. Psychoactive Drugs 49, 1–10. doi: 10.1080/02791072.2016.1260189
Fan, P., Lai, C., Yang, J., Hong, S., Yang, Y., Wang, Q., et al. (2022). Crop rotation suppresses soil-borne fusarium wilt of banana and alters microbial communities. Arch. Agron. Soil Sci. 68, 447–459. doi: 10.1080/03650340.2020.1839058
Genin, S., and Denny, T. P. (2012). Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 50, 67–89. doi: 10.1146/annurev-phyto-081211-173000
Genin, S., Gough, C. L., Zischek, C., and Boucher, C. A. (1992). Evidence that the hrpB gene encodes a positive regulator of pathogenicity genes from Pseudomonas solanacearum. Mol. Microbiol. 6, 3065–3076. doi: 10.1111/j.1365-2958.1992.tb01764.x
Han, S., Yang, L., Wang, Y., Ran, Y., Li, S., and Ding, W. (2021). Preliminary studies on the antibacterial mechanism of a new plant-derived compound, 7-methoxycoumarin, against Ralstonia solanacearum. Front. Microbiol. 12:697911. doi: 10.3389/fmicb.2021.697911
Hao, N., Han, L., Li, Y., Li, J., Tian, X., Kong, D., et al. (2020). New 8-O-4′ neolignans and their antibacterial activity from the whole plants of Clematis lasiandra. ACS Omega 5, 19661–19666. doi: 10.1021/acsomega.0c02339
Hayward, A. C. (1991). Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 29, 65–87. doi: 10.1146/annurev.py.29.090191.000433
Huang, J., Carney, B. F., Denny, T. P., Weissinger, A. K., and Schell, M. A. (1995). A complex network regulates expression of eps and other virulence genes of Pseudomonas solanacearum. J. Bacteriol. 177, 1259–1267. doi: 10.1128/jb.177.5.1259-1267.1995
Ishii, H. (2006). Impact of fungicide resistance in plant pathogens on crop disease control and agricultural environment. Japan Agric. Res. Q. 40, 205–211. doi: 10.6090/jarq.40.205
Jiang, G., Wei, Z., Xu, J., Chen, H., Zhang, Y., She, X., et al. (2017). Bacterial wilt in China: history, current status, and future perspectives. Front. Plant Sci. 8:1549. doi: 10.3389/fpls.2017.01549
Li, L., Feng, X., Tang, M., Hao, W., Han, Y., Zhang, G., et al. (2014). Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 169, 522–526. doi: 10.1016/j.micres.2013.12.003
Liu, Y., Tang, Y., Qin, X., Yang, L., Jiang, G., Li, S., et al. (2017). Genome sequencing of Ralstonia solanacearum CQPS-1, a phylotype I strain collected from a highland area with continuous cropping of tobacco. Front. Microbiol. 8:974. doi: 10.3389/fmicb.2017.00974
Lowe-Power, T. M., Khokhani, D., and Allen, C. (2018). How Ralstonia solanacearum exploits and thrives in the flowing plant xylem environment. Trends Microbiol. 26, 929–942. doi: 10.1016/j.tim.2018.06.002
Mansfield, J., Genin, S., Magori, S., Citovsky, V., Sriariyanum, M., Ronald, P., et al. (2012). Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 13, 614–629. doi: 10.1111/j.1364-3703.2012.00804.x
Mansoor, M. M., and Shad, S. A. (2022). Risk assessment of cyromazine and methoxyfenozide resistance suggests higher additive genetic but lower environmental variation supporting quick resistance development in non-target Chrysoperla carnea (Stephens). Environ. Monit. Assess. 194:66. doi: 10.1007/s10661-021-09735-2
Nelson, R., Wiesner-Hanks, T., Wisser, R., and Balint-Kurti, P. (2018). Navigating complexity to breed disease-resistant crops. Nat. Rev. Genet. 19, 21–33. doi: 10.1038/nrg.2017.82
Nicolopoulou-Stamati, P., Maipas, S., Kotampasi, C., Stamatis, P., and Hens, L. (2016). Chemical pesticides and human health: the urgent need for a new concept in agriculture. Front. Public Health 4:148. doi: 10.3389/fpubh.2016.00148
O’Loughlin, C. T., Miller, L. C., Siryaporn, A., Drescher, K., Semmelhack, M. F., and Bassler, B. L. (2013). A quorum-sensing inhibitor blocks Pseudomonas aeruginosa virulence and biofilm formation. Proc. Natl. Acad. Sci. U. S. A. 110, 17981–17986. doi: 10.1073/pnas.1316981110
Ooshiro, A., Kaji, M., Katoh, Y., Kawaide, H., and Natsume, M. (2011). Antibacterial activity of alkyl gallates and related compounds against Ralstonia solanacearum. J. Pestic. Sci. 36, 240–242. doi: 10.1584/jpestics.G10-84
Qian, Y. L., Wang, X. S., Wang, D. Z., Zhang, L. N., Zu, C. L., Gao, Z. L., et al. (2013). The detection of QTLs controlling bacterial wilt resistance in tobacco (N. tabacum L.). Euphytica 192, 259–266. doi: 10.1007/s10681-012-0846-2
Safni, I., Cleenwerck, I., De Vos, P., Fegan, M., Sly, L., and Kappler, U. (2014). Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacerum. Int. J. Syst. Evol. Microbiol 64, 3087–3103. doi: 10.1099/ijs.0.066712-0
Saile, E., McGarvey, J. A., Schell, M. A., and Denny, T. P. (1997). Role of extracellular polysaccharide and endoglucanase in root invasion and colonization of tomato plants by Ralstonia solanacearum. Phytopathology 87, 1264–1271. doi: 10.1094/PHYTO.1997.87.12.1264
Shao, W., Zhao, Y., and Ma, Z. (2021). Advances in understanding fungicide resistance in Botrytis cinerea in China. Phytopathology 111, 455–463. doi: 10.1094/PHYTO-07-20-0313-IA
She, X., Yu, L., Lan, G., Tang, Y., and He, Z. (2017). Identification and genetic characterization of Ralstonia solanacearum species complex isolates from cucurbita maxima in China. Front. Plant Sci. 8:1794. doi: 10.3389/fpls.2017.01794
Shen, Y., and Hao, X. (2020). Natural product sciences: an integrative approach to the innovations of plant natural products. Sci. China Life Sci. 63, 1634–1650. doi: 10.1007/s11427-020-1799-y
Shi, Y., Chen, X., Shu, J., Liu, Y., Zhang, Y., Lv, Q., et al. (2022). Harmine, an inhibitor of the type III secretion system of Salmonella enterica serovar typhimurium. Front. Cell. Infect. Microbiol. 12, 1–10. doi: 10.3389/fcimb.2022.967149
Sparks, T. C., and Bryant, R. J. (2022). Impact of natural products on discovery of, and innovation in, crop protection compounds. Pest Manag. Sci. 78, 399–408. doi: 10.1002/ps.6653
Tran, T. M., Macintyre, A., Khokhani, D., Hawes, M., and Allen, C. (2016). Extracellular DNases of Ralstonia solanacearum modulate biofilms and facilitate bacterial wilt virulence. Environ. Microbiol. 18, 4103–4117. doi: 10.1111/1462-2920.13446
Tudi, M., Ruan, H. D., Wang, L., Lyu, J., Sadler, R., Connell, D., et al. (2021). Agriculture development, pesticide application and its impact on the environment. Int. J. Environ. Res. Public Health 18, 1–24. doi: 10.3390/ijerph18031112
Wang, L., Gao, Y., Jiang, N., Yan, J., Lin, W., and Cai, K. (2022). Silicon controls bacterial wilt disease in tomato plants and inhibits the virulence-related gene expression of Ralstonia solanacearum. Int. J. Mol. Sci. 23:6965. doi: 10.3390/ijms23136965
Wu, D., Ding, W., Zhang, Y., Liu, X., and Yang, L. (2015). Oleanolic acid induces the type III secretion system of Ralstonia solanacearum. Front. Microbiol. 6:1466. doi: 10.3389/fmicb.2015.01466
Yang, L., Ding, W., Xu, Y., Wu, D., Li, S., Chen, J., et al. (2016). New insights into the antibacterial activity of hydroxycoumarins against Ralstonia solanacearum. Molecules 21, 1–13. doi: 10.3390/molecules21040468
Yang, L., Li, S., Qin, X., Jiang, G., Chen, J., Li, B., et al. (2017). Exposure to Umbelliferone reduces Ralstonia solanacearum biofilm formation, transcription of type III secretion system regulators and effectors and virulence on tobacco. Front. Microbiol. 8:1234. doi: 10.3389/fmicb.2017.01234
Yang, L., Wang, Y., He, X., Xiao, Q., Han, S., Jia, Z., et al. (2021a). Discovery of a novel plant-derived agent against Ralstonia solanacearum by targeting the bacterial division protein FtsZ. Pestic. Biochem. Physiol. 177:104892. doi: 10.1016/j.pestbp.2021.104892
Yang, L., Wei, Z., Li, S., Xiao, R., Xu, Q., Ran, Y., et al. (2021b). Plant secondary metabolite, daphnetin reduces extracellular polysaccharides production and virulence factors of Ralstonia solanacearum. Pestic. Biochem. Physiol. 179:104948. doi: 10.1016/j.pestbp.2021.104948
Yao, J., and Allen, C. (2007). The plant pathogen Ralstonia solanacearum needs aerotaxis for normal biofilm formation and interactions with its tomato host. J. Bacteriol. 189, 6415–6424. doi: 10.1128/JB.00398-07
Zhang, Y., Kiba, A., Hikichi, Y., and Ohnishi, K. (2011). prhKLM genes of Ralstonia solanacearum encode novel activators of hrp regulon and are required for pathogenesis in tomato. FEMS Microbiol. Lett. 317, 75–82. doi: 10.1111/j.1574-6968.2011.02213.x
Zhang, L., Li, D., and Yu, S. (2020). Pharmacological effects of harmine and its derivatives: a review. Arch. Pharm. Res. 43, 1259–1275. doi: 10.1007/s12272-020-01283-6
Zhang, L., Qin, M., Yin, J., Liu, X., Zhou, J., Zhu, Y., et al. (2022). Antibacterial activity and mechanism of ginger extract against Ralstonia solanacearum. J. Appl. Microbiol. 133, 2642–2654. doi: 10.1111/jam.15733
Keywords: Ralstonia solanacearum , bacterial wilt, harmine, plant-derived compound, disease control
Citation: Xia H, Huang Y, Wu R, Tang X, Cai J, Li S-x, Jiang L and Wu D (2023) A screening identifies harmine as a novel antibacterial compound against Ralstonia solanacearum. Front. Microbiol. 14:1269567. doi: 10.3389/fmicb.2023.1269567
Received: 30 July 2023; Accepted: 22 August 2023;
Published: 05 September 2023.
Edited by:
Peng Li, Hainan Normal University, ChinaCopyright © 2023 Xia, Huang, Wu, Tang, Cai, Li, Jiang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dousheng Wu, ZG91c2hlbmcud3VAaG51LmVkdS5jbg==; Lin Jiang, NjcwMDYxNDU4QHFxLmNvbQ==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.