
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 14 September 2023
Sec. Virology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1260812
This article is part of the Research TopicTransmission and Infection of ArbovirusesView all 14 articles
 Devojit Kumar Sarma1*†
Devojit Kumar Sarma1*† Lokendra Rathod1†
Lokendra Rathod1† Sweta Mishra1†
Sweta Mishra1† Deepanker Das1
Deepanker Das1 Ankita Agarwal2
Ankita Agarwal2 Gaurav Sharma1
Gaurav Sharma1 Tanim Arpit Singh3
Tanim Arpit Singh3 Manoj Kumawat1
Manoj Kumawat1 Samradhi Singh1
Samradhi Singh1 Vinod Verma4
Vinod Verma4 Manoj Kumar1
Manoj Kumar1 Swasti Shubham1
Swasti Shubham1 Rajnarayan R. Tiwari1
Rajnarayan R. Tiwari1 Anil Prakash1
Anil Prakash1Introduction: Dengue fever is hyperendemic in several Southeast and South Asian countries, including India, with all four serotypes (DENV 1–4) circulating at different periods and in different locations. Sustainable and improved virological and entomological surveillance is the only tool to prevent dengue and other vector-borne diseases.
Objectives: The present study has been carried out to detect and characterize the circulating dengue virus (DENV) in field-collected Aedes mosquitoes in Bhopal, Central India.
Methods: Aedes mosquitoes were collected from 29 localities within Bhopal city during October 2020 to September 2022. DENV infection was assessed in the individual head and thorax regions of Aedes mosquitoes using reverse transcriptase PCR. Positive samples were sequenced, and the circulating serotypes and genotypes were determined using phylogenetic analysis.
Results: DENV RNA was detected in 7 Aedes aegypti and 1 Aedes albopictus, with infection rates of 0.59 and 0.14%, respectively. Phylogenetic analysis revealed all the isolates belonged to DENV serotype 2 and distinctly clustered with the non-Indian lineage (cosmopolitan genotype 4a), which was not recorded from the study area earlier. The time to most common recent ancestor (TMRCA) of these sequences was 7.4 years old, with the highest posterior density (HPD) of 3.5–12.2 years, indicating that this new lineage emerged during the year 2014. This is the first report on the DENV incrimination in both Ae. aegypti and Ae. albopictus mosquitoes collected from Bhopal, Central India.
Conclusion: The observed emergence of the non-Indian lineage of DENV-2 in Bhopal, which again is a first report from the area, coincides with the gradual increase in DENV cases in Bhopal since 2014. This study emphasizes the importance of DENV surveillance and risk assessment in this strategically important part of the country to decipher its outbreak and severe disease-causing potential.
Dengue fever is caused by the dengue virus (DENV), a single-stranded positive-sense RNA virus of the Flaviviridae family. It is the most recognized arbovirus in the world, transmitted by infected female mosquitoes, especially Aedes aegypti and Ae. albopictus (Hayes and Gubler, 1992). Dengue infections have increased exponentially in the majority of the tropics and subtropics over the past three decades. Currently, one third of the world’s population is susceptible to DENV infection. The WHO estimates that the global burden of dengue infection has increased about 10.3-fold in the last two decades (World Health Organization, 2023). This condition is primarily linked to uncontrolled urbanization, climate change, poor water supply and sewage management, rapid movement of people, animals, and trade via air and sea routes, and unsustainable vector control programmes (Wilder-Smith and Gubler, 2008; Gubler, 2011; Bhatia et al., 2022; Romanello et al., 2022).
Dengue infection is caused by four antigenically different serotypes: DENV-1, DENV-2, DENV-3, and DENV-4 (Gubler, 2002) and the disease manifests itself in a range of ways, from asymptomatic infection and mild febrile illness (dengue fever) to more severe forms including dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS). The most severe clinical manifestation, dengue shock syndrome (DSS), is characterized by coagulation abnormalities, hemorrhage, plasma leakage, and organ failure (Bhatt et al., 2021). The present dengue case classification, according to World Health Organization, comprises symptomatic patients with and without warning signals, as well as severe dengue (World Health Organization, 2009). Based on the genetic make-up of DENV each serotype has been subdivided into 4–5 genotypes (Weaver and Vasilakis, 2009). Furthermore, depending on their phylogenetic classification, each genotype has a range of lineages (Shrivastava et al., 2015). Infection with one serotype results in lifetime immunity against homologous serotypes, while there is no or limited immunity against infection to heterologous dengue serotypes. This secondary infection, which leads to enhanced dengue severity, is primarily caused by antibody-dependent enhancement (ADE), a mechanism mediated mostly by immunoglobulin G (IgG) (Teo et al., 2023). Additionally, certain lineages and genotypes have been associated with severe forms of dengue fever. These genetic variants with modest variations are also responsible for major outbreaks due to rapid transmission in both humans and mosquitoes (Messer et al., 2003). Hence, regular surveillance is necessary for the control and management of dengue.
Recent anthropogenic and climate-related changes have resulted in accelerated transmission of DENV, causing frequent dengue epidemics (Sarma et al., 2022). Previous studies suggested that several Southeast Asian countries demonstrated dengue hyperendemicity that was 18-fold higher than the Americas (Murray et al., 2013). Currently, almost all Indian states are under constant threat of dengue transmission. A nationwide dengue sero-survey during 2017–2018 showed that nearly half of the Indian population (48.7; 95% CI 43.5–54.0) was sero-positive for DENV (Murhekar et al., 2019). Since 2000, the north Indian states, including New Delhi, Rajasthan, Uttar Pradesh, Madhya Pradesh, Haryana, and Punjab, have reported dengue outbreaks at regular intervals (Mishra et al., 2015). Similarly, the western (Maharashtra), eastern (Odisha), and southern states (Kerala, Andhra Pradesh, and Telangana) also reported massive dengue outbreaks along with chikungunya (Anoop et al., 2010; Cecilia et al., 2011; Shrivastava et al., 2015). In view of this, there is an increasing demand for a safe and effective DENV vaccine that elicits immunity against all the four serotypes. At the moment, at least seven DENV vaccines are at various stages of clinical trials or pre-clinical investigations. Three of these, Dengvaxia® (CYT-TDV), Qdenga® (TAK-003) and TV003 (NIAD/Butantan/Merck) have shown promising results in clinical trials (Torres-Flores et al., 2022; Angelin et al., 2023). Dengvaxia®, manufactured by Sanofi Pasteur, has been licensed in 20 countries and approved for use in individuals aged 6–45 years with laboratory confirmed previous dengue infection and living in endemic countries (Thomas and Yoon, 2019; Torres-Flores et al., 2022). However, shortcomings such as vaccine administration limited to dengue immune individuals only, non-availability in non-endemic countries and safety concerns in vaccine recipients who were dengue non-immune at the time of vaccine administration (Hadinegoro et al., 2015) in the case of Dengvaxia® and less protection against DENV-3 (Rivera et al., 2022), as well as a lack of data for elderly individuals (Angelin et al., 2023) in the case of Qdenga®, have posed significant challenges to the widespread and effective control of dengue fever through vaccination efforts. Until an effective vaccine becomes available, vector control will continue to be the primary approach for managing dengue transmission. Furthermore, systematic molecular monitoring of circulating DENV serotypes in mosquitoes and humans, as well as early detection of any serotype or genotype shift in a geographic area, would aid in the prediction of imminent DENV epidemics and devise targeted public health strategies for dengue control.
This study investigated the phylogenetic relationship of DENV-2 serotypes prevalent in central India (Bhopal, Madhya Pradesh) and reported the emergence of a new lineage (Genotype 4a, cosmopolitan non-Indian lineage) in naturally infected, wild-caught Ae. aegypti and Ae. albopictus mosquitoes. This study highlights the urgent need of DENV surveillance and clinical characterization of dengue fever in this strategically located part of the country.
The study was conducted in Bhopal, the capital city of Madhya Pradesh in central India. House-frequenting adult Aedes mosquitoes were collected from October 2020 to September 2022 during morning and evening hours from 29 different locations in Bhopal (Figure 1). Mosquitoes were collected using mechanical aspirators and transported to the laboratory, and then identified to species level using standard taxonomic keys (Tyagi et al., 2015). Each female individual of Ae. aegypti and Ae. albopictus was bisected into the head, thorax, and abdomen and stored separately in 1.5 mL micro-centrifuge tubes filled with 50 μl of RNAlater™ stabilization solution (Sigma-Aldrich, Cat. # R0901-100ML) at −20°C till further processing. As some of the adult mosquitoes were damaged during transportation, their head and thorax could not be bisected and hence were not processed further. Aedes immatures were also collected from the 29 localities in different larval habitats. The collected larvae were transferred to the lab and reared up to the 4th instar. A total of 10 larvae were kept separately in 1.5-ml micro-centrifuge tubes filled with 100 μl of RNAlater™ stabilization solution and stored at −20°C till further processing. The remaining larvae were reared up to adult stage, identified, and stored at 4°C.
RNA was extracted from individual mosquito’s head-thorax region and from larvae pool (10 larvae/pool) using the Qiagen Viral RNA Mini Kit (Qiagen, cat. # 52906), according to the manufacturer’s instructions. DENV infection in the extracted RNA of individual mosquitoes, as well as larval pool was assessed by one-step reverse transcription-PCR (Thermo Fisher Scientific, Cat. # 12594025) targeting the capsid-pre-membrane (C-prM) gene region with primers and methods as described (Lanciotti et al., 1992). The 511-bp PCR product of the DENV-positive individuals was gel purified, sequenced in both directions, and used to identify and characterize circulating serotypes and genotypes.
The DENV sequences generated in this study were aligned with representative DENV C-prM sequences from India and other countries (Supplementary Table 1) using the MUSCLE programme, and a maximum likelihood phylogenetic tree with 1000 bootstrap replicates was reconstructed using the MEGA10 software (Kumar et al., 2018) to identify the serotypes and genotypes of the DENV isolates. These sequences have been submitted to GenBank with the accession numbers OQ842497-OQ842504. The genetic distance within and between the genotypes was calculated using MEGA10 software. A maximum clade credibility (MCC) tree was used to determine the time to the most recent common ancestor (TMRCA) using DENV-2 genotype 4a and 4b sequences, with sylvatic sequences as the outgroup, using the BEAST 2.5 package (Bouckaert et al., 2019). Based on the lowest BIC (Bayesian Information Criterion) scores, Kimura 2 parameter with discrete gamma distribution (K2 + G) model, assessed through MEGA10 software, was used as the best fit model for Bayesian Markov Chain Monte Carlo (MCMC) analysis. Relaxed uncorrelated lognormal molecular clock model was used and an effective population size of >200 was ensured by running MCMC chains for 4E08 generations with a sampling frequency of 10000 and burn-in of 1000. Tracer v1.7.2, TreeAnnotator v1.10.4 and FigTree v1.4.4 was used to analyze the output and view and annotate the MCC tree.
The present study was approved by the Institutional Ethics Committee of the ICMR-National Institute for Research in Environmental Health, Bhopal (NIREH/BPL/IEC/2018-19/3130, dated March 18, 2019).
A total of 2,371 adult female Aedes mosquitoes (1,498 Ae. aegypti and 873 Ae. albopictus) were collected. Of these, 1,890 mosquitoes (1,186 Ae. aegypti and 704 Ae. albopictus) were tested for DENV infection. Similarly, 370 Aedes larvae were tested for DENV infection in 37 pools (32 pools for Ae. aegypti and 5 pools for Ae. albopictus). Based on the RT-PCR amplification of C-PrM gene region (Supplementary Figure 1), DENV RNA was detected in seven Ae. aegypti and one Ae. albopictus individuals. Overall DENV infection rates were 0.59 and 0.14% in Ae. aegypti and Ae. albopictus, respectively. Area wise S_15 (Nizamuddin colony area) recorded the highest DENV infection rate (5.26%), while S_11 (Kotra Sultanabad area) recorded the lowest DENV infection rate (1.25%) for Ae. aegypti mosquitoes. Ae. albopictus was incriminated only in S_25 (Shakti Nagar) with an infection rate of 0.8% (Table 1). No DENV infection was detected in larval pools.
Phylogenetic analysis revealed that all eight DENV C-prM sequences were belonged to serotype 2 (Figure 2). Analysis of the representative serotype 2 C-prM sequences from India and other countries unambiguously clustered these 8 sequences with the non-Indian lineage of DENV-2 (Cosmopolitan genotype 4a) (Figures 3A, B). These sequences are substantially divergent from the other genotypes, with nucleotide divergence ranging from 7.4 to 22.2% (Supplementary Figure 2). The maximum clade credibility tree indicated a mean TMRCA of 7.4 years (95% HPD: 3.5–12.2) for the sequences in the present study, suggesting that this lineage was introduced in 2014 (95% HPD: 2009.8–2018.5) (Figure 4). Other isolates collected in Bhopal in 2016 (GenBank accession nos. MH051272-MH051275) grouped into two distinct clades within the most prevalent cosmopolitan 4b genotype, with identical TMRCA (2014, 95% HPD: 2011–2016), implying that both lineages of the DENV-2 cosmopolitan genotype circulated in Bhopal at the same time. When compared to DENV-2 reference strain KM204118 and other DENV-2 sequences, this lineage showed four significant amino acid alterations, namely E19A, M104I, L108M, and D143N (Supplementary Table 2). A statistically significant positive selection was observed in the DENV capsid protein at amino acid position 19 by different methods (MEME: p-value 0.05; FEL: p-value 0.09; and FUBER: probability 0.962).
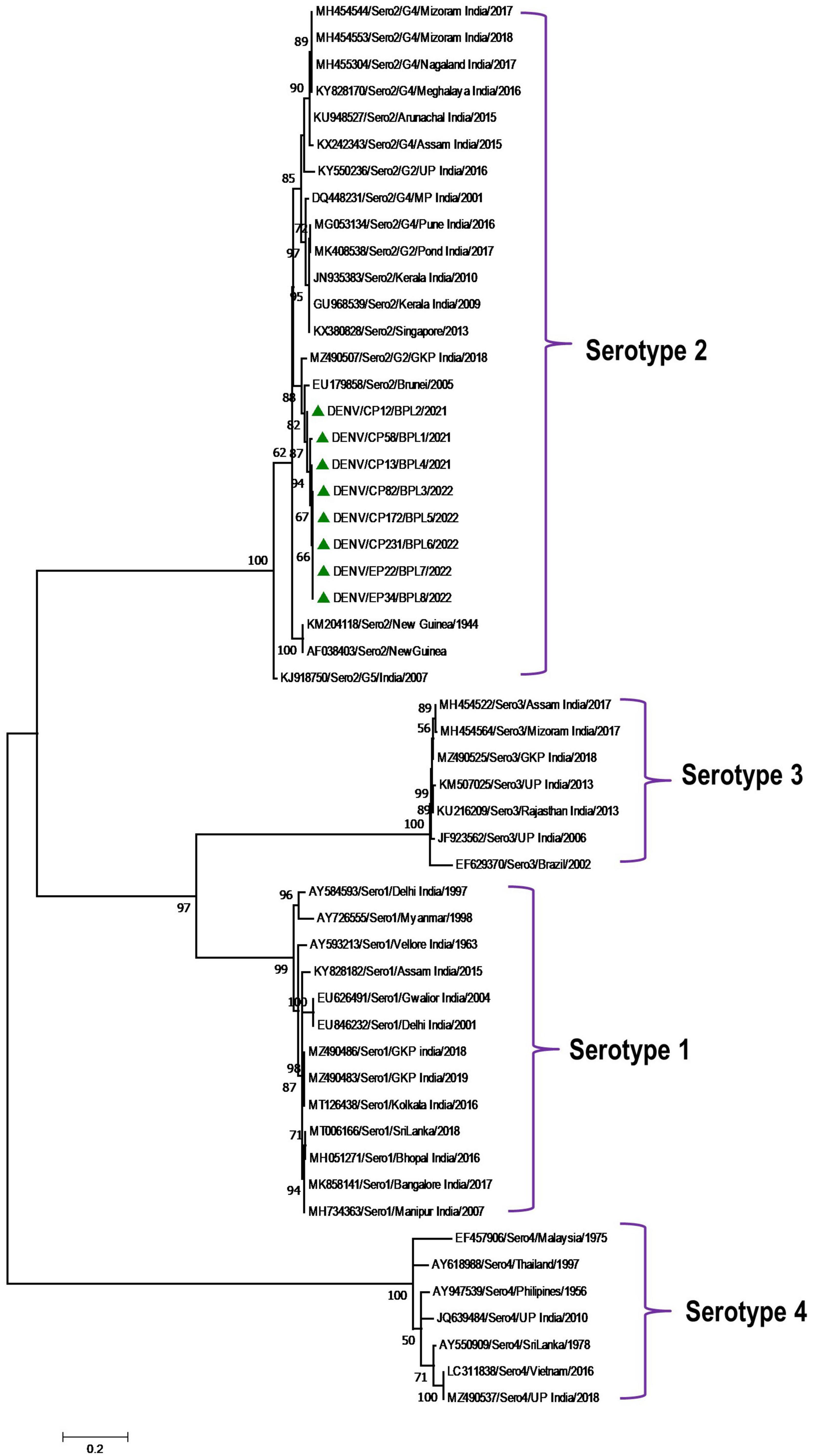
Figure 2. Serotype placement and phylogenetic relationship of DENV isolates using C-prM sequences from the present study and other sequences from India and other countries. The sequences with green colored triangle shape indicates sequence generated in this study. Scale bar indicates number of nucleotide substitutions per site.
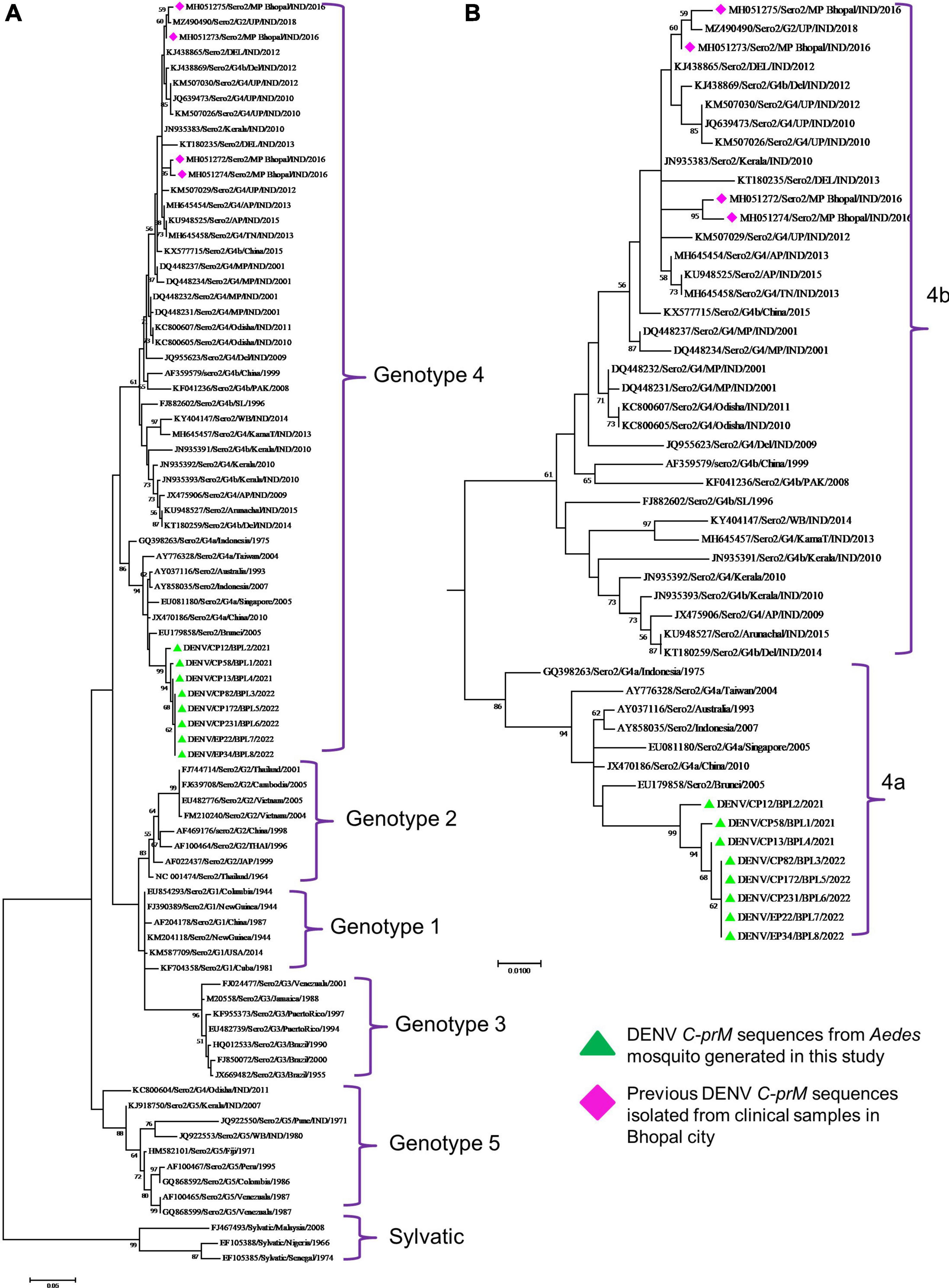
Figure 3. (A) Genotypic placement and phylogenetic relationship of DENV-2 isolates using C-prM sequences from the present study and from other sequences form India and other countries, (B) Phylogenetic tree of DENV-2 corresponding to cosmopolitan genotype (4ab) Sequences. The sequences with green colored triangle shape indicates sequence generated in this study, the sequences with red colored diamond shape indicates previous DENV-2 sequences isolated from clinical samples in Bhopal city. Scale bar indicates number of nucleotide substitutions per site.
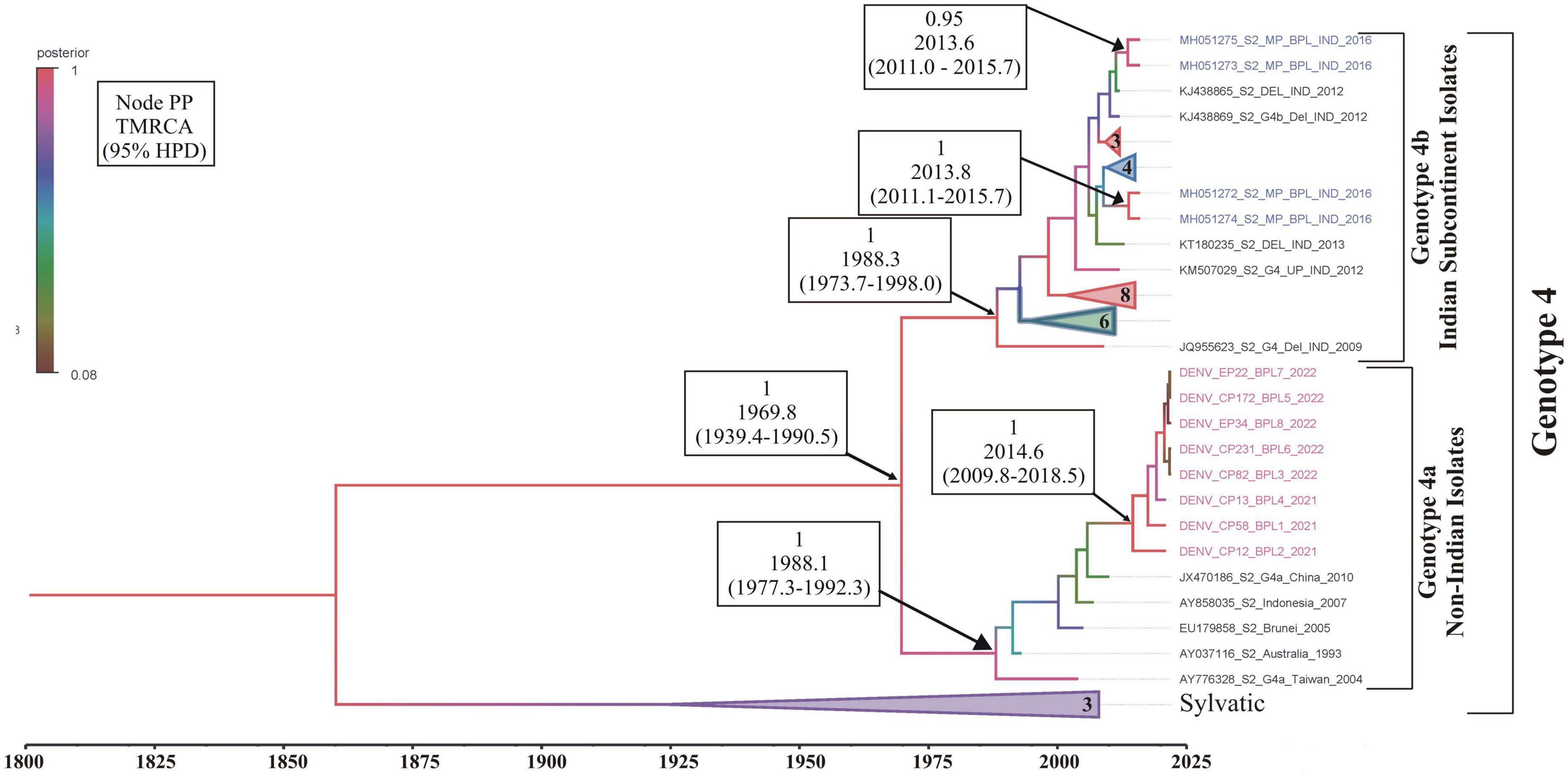
Figure 4. Maximum clade credibility (MCC) phylogenetic tree of DENV-2 corresponding to cosmopolitan genotype (4a,b) generated by Bayesian inference method in BEAST calculated using C-prM gene sequences. Sequences in pink represents DENV-2 sequences generated in this study, while sequences in blue are the previous sequences generated from the same study area (Bhopal). All horizontal branch lengths are drawn to a scale of years. PP, posterior probability; TMRCA, the mean estimated time to the most recent common ancestor; 95% HPD, 95% highest posterior density interval.
Dengue is continuing to be the most serious arboviral infection in various Southeast and South Asian nations, including India. This is due to severe environmental changes, rapid urbanization, and increased human long-distance travel, which have resulted in the spread of Aedes species in previously unexplored regions (Hussain and Dhiman, 2022). The first virological dengue outbreak in India was reported in 1963 from Kolkata (East India) (Chatterjee et al., 1965). Both Ae. aegypti and Ae. albopictus have been identified as potential dengue vectors in different parts of the country, with varied incidences of all four known serotypes (Table 2). Central India, on the other hand, recorded its first dengue outbreak in 1966 in the Jabalpur district of Madhya Pradesh, with DENV-3 as the causative agent. Subsequently, other districts of Madhya Pradesh, such as Sagar (1966), Sarguja (1997), Gwalior (2002), and Bhopal and Indore (2009), also observed dengue outbreaks with DENV 1, 2, and 3 as causative agents (Sehgal et al., 1967; Mahadev et al., 1997; Parida et al., 2002; Kalpana et al., 2010). According to Mahadev et al. (1997), Ae. aegypti was the primary cause of the outbreaks in Madhya Pradesh.
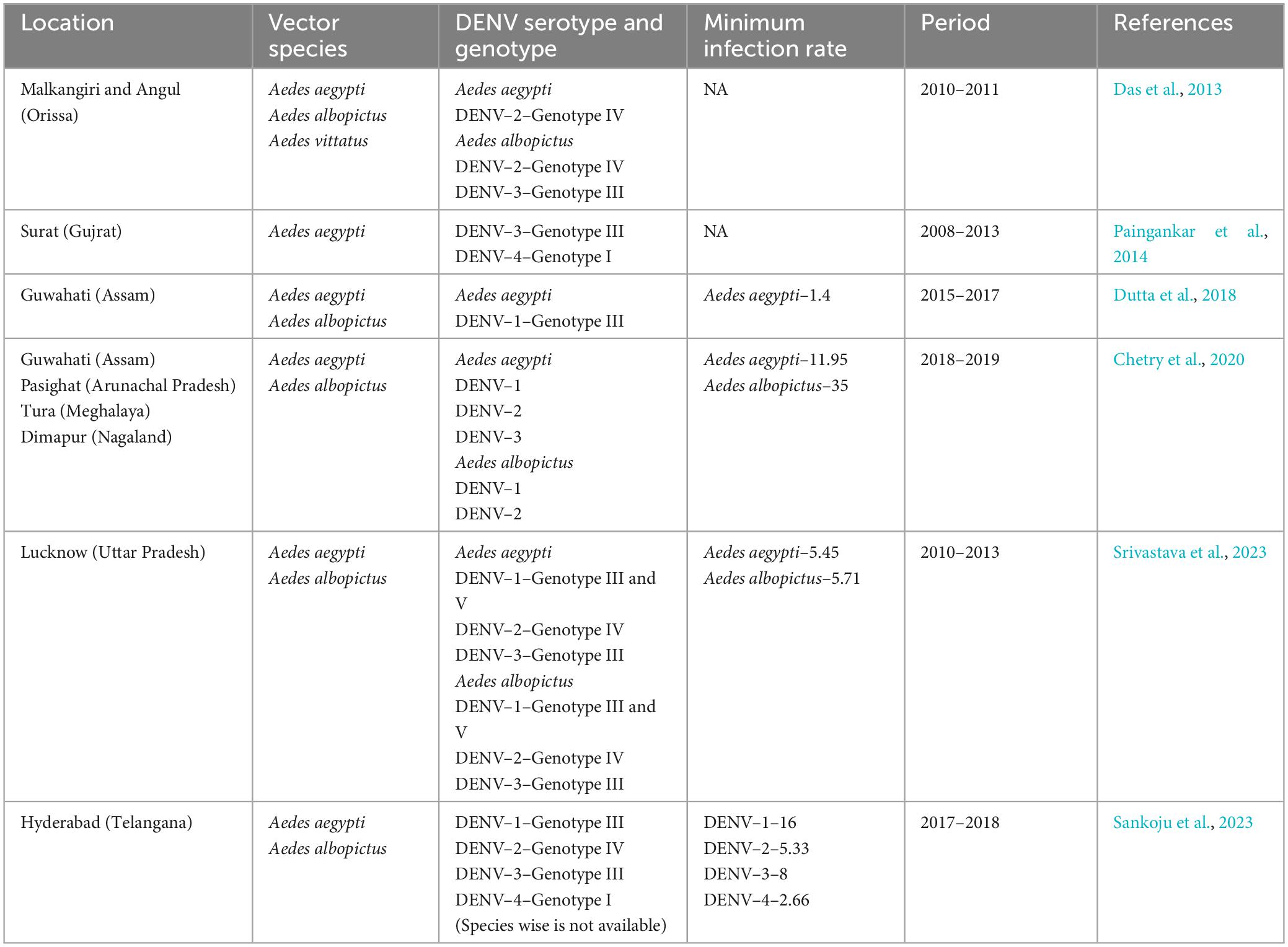
Table 2. Summary of prevalent DENV serotypes and genotypes detected in Aedes mosquitoes across the India.
All four serotypes of DENV have been linked to several outbreaks in India in the past (Guo et al., 2017), however, the majority of these outbreaks were associated with DENV-2 (Singh et al., 1999; Dash et al., 2006; Agarwal et al., 2023). The first DHF outbreak caused by DENV-2 occurred in New Delhi in 1996 (Dar et al., 1999). Since 2012, DENV infections in India have steadily increased, with active circulation of the DENV-2 serotype in different regions with varying prevalence (Sankari et al., 2012; Afreen et al., 2016; Murhekar et al., 2019; Alagarasu et al., 2021). However, the prevalence and distribution of DENV-2 in Aedes mosquitoes from Bhopal (Central India) remain unknown. The present study reports for the first time the circulation of DENV-2 serotype and its prevalence in both Ae. aegypti and Ae. albopictus collected from 29 different localities of Bhopal city. It has been observed that most of the DENV incriminated sites were in the South and South-western part of the city (Figure 1), which were also known to be the hot-spot area of dengue transmission in Bhopal city (Sarma et al., 2022). This finding further confirms the entomological risk for dengue transmission in these areas.
Dengue virus infection in wild-caught Aedes mosquitoes was assessed, and circulating DENV serotypes were characterized by sequence comparison and phylogenetic analysis in Bhopal city during the study. This is crucial because molecular characterization of these viruses aids in the identification of molecular subtypes or genotypes and the introduction of any new lineages. Previously, various regions of the dengue genome have been used for molecular phylogenetic analysis, but many studies have reported the C-prM gene as a tool in dengue virus genotyping (Murugesan et al., 2020; Titir et al., 2021). We retrieved previously reported C-prM gene sequences from the NCBI GenBank database for phylogenetic analysis.
It is evident that cosmopolitan genotypes of the DENV-2 virus are circulating across India (Table 1). However, when analyzing the phylogenetic relationship of DENV sequences generated in this study alongside other global sequences, it became evident that they clustered with non-Indian isolates, indicating the establishment of a novel lineage, lineage 4a, of DENV serotype 2 (Figure 3) in Aedes mosquitoes collected from Central India (Bhopal, Madhya Pradesh). Additionally, the MCC tree indicated that this lineage (4a) was introduced in Bhopal around 2013–2014. Previously, the introduction of a new genotype or lineage was proposed as the root cause of the transmission of a severe strain of dengue sickness in other parts of the world, such as the Americas (Rico-Hesse et al., 1997; Messer et al., 2003). It is pertinent to note that Bhopal experienced severe outbreaks of DENV in 2014 and 2016 (Agarwal et al., 2019), and since then the number of dengue cases has been increasing steadily in Bhopal (Sarma et al., 2022). This might be possible because lineages’ extinction and inevitable incursion were connected to a virus’s transmission bottleneck. For example, in Thailand, increased viral transmission by a mosquito vector was also recently connected to lineage replacement (Lambrechts et al., 2012). Similarly, Shrivastava et al. (2015) also reported the new lineage of DENV-3 (genotype 4) in eastern India, which was implicated in the dengue outbreak during the year 2011. However, in view of the scarcity of molecular data on the circulating serotypes/genotypes during that time, pinpointing the lineage that possibly triggered those outbreaks is difficult. The MCC tree shows both the lineages (4a and 4b of the cosmopolitan genotype) were circulating in Bhopal, perhaps with different degrees of dominance. The same has been evidenced by a recent hospital based study in the same area, where out of total 154 RT-PCR positive dengue cases, majority (66 nos.) of them belonged to DENV-2 serotype and of these 66 DENV isolates, 13 were sequenced of which 12 were found to belonged to genotype 4a during 2019 and 2021. It was also observed that the new lineage of DENV-2 was also associated with a longer duration of hospitalization (>10 ± 3 days) and hemorrhagic manifestations indicating its role in severity if disease in the studied area (Agarwal et al., 2023). Higher evolutionary and increased transmission rates of one DENV genotype may outnumber the others in the community, resulting in a lineage shift. This shift may be due to several factors, including changes in the mosquito vector population, host immunity, environmental conditions, and international travel (Shrivastava et al., 2015). Bhopal, a central Indian city, is a favorite destination for international tourists and received 316,195 international travelers during 2014 (India Tourism Statistics, 2014). Although most of the incident dengue cases are of local origin, there are some immigrated cases also, which, along with the international travelers, might facilitates the introduction of this new lineage to the study area.
Aedes mosquitoes have the ability to transmit the virus to their offspring, which is identified as vertical transmission. This phenomenon is important to understand as it may contribute to the existence of viruses in the environment in the absence of susceptible hosts. This phenomenon may also be linked to disease endemism, implying that vertical transmission plays an important role in the establishment of endemicity (Hull et al., 1984). The presence and significance of this transmission in Aedes have been reported in previous studies from different regions of the world (Martins et al., 2012; Buckner et al., 2013; Wijesinghe et al., 2021). Within India, natural vertical transmission of DENV was observed in dengue hyper-endemic areas such as Rajasthan (Angel and Joshi, 2008; Bina et al., 2008), Tamil Nadu (Arunachalam et al., 2008), New Delhi (Bina et al., 2008), and Kerala (Thenmozhi et al., 2007). Although no DENV infection was observed in the larvae of Ae. aegypti and Ae. albopictus in the present study, which may be due to low to mid endemicity of the disease in the studied area and low number of larvae processed, the presence of natural vertical transmission of DENV in the studied area couldn’t be ruled out. More molecular surveillance with large numbers larvae is required to confirm the natural vertical transmission of DENV in field collected Aedes mosquitoes in this part of India.
The present study, for the first time, confirms the prevalence of DENV serotype 2 (genotype 4) in field collected Aedes mosquitoes from Bhopal city, Central India. The phylogenetic placement of the DENV sequences with non-Indian lineages indicates the circulation of a new lineage (4a) of cosmopolitan genotype. This lineage transition of DENV-2 is a major cause of concern and requires routine surveillance of viral circulation throughout endemic and non-endemic areas for a better understanding of transmission dynamics and effective control and management of the dengue burden in this central part of India.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
DS: Conceptualization, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Writing—original draft. LR: Data curation, Formal Analysis, Investigation, Methodology, Writing—review and editing. SM: Data curation, Formal Analysis, Investigation, Methodology, Writing—review and editing. DD: Investigation, Methodology, Visualization, Writing—review and editing. AA: Formal Analysis, Investigation, Methodology, Visualization, Writing—review and editing. GS: Formal Analysis, Methodology, Writing—original draft. TS: Formal Analysis, Methodology, Writing—review and editing. MKw: Visualization, Writing—review and editing. SaS: Investigation, Writing—review and editing. VV: Visualization, Writing—review and editing. MK: Investigation, Methodology, Writing—review and editing. SwS: Visualization, Writing—review and editing. RT: Supervision, Writing—review and editing. AP: Conceptualization, Methodology, Supervision, Writing—review and editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article. This study was funded by Indian Council of Medical Council, New Delhi (https://main.icmr.nic.in/) to DS [award number 6/9-7 (208)/2019-ECD-II].
We sincerely thank the District Malaria Officer, Bhopal district and Dr. N. Banerjee for their encouragement to carry out this study. We are grateful to Mr. Kamlesh Mewada and Mr. Mahendra Damle for their assistance in mosquito collection. We also thank Dr. P. K. Mishra, Scientist-F and Head, Molecular Biology Division, ICMR-National Institute for Research in Environmental Health, Bhopal for his critical reviews and suggestion which helped to improve the manuscript substantially.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1260812/full#supplementary-material
Afreen, N., Naqvi, I. H., Broor, S., Ahmed, A., Kazim, S. N., Dohare, R., et al. (2016). Evolutionary analysis of dengue serotype 2 viruses using phylogenetic and Bayesian methods from New Delhi, India. PLoS Negl. Trop. Dis. 10:e0004511. doi: 10.1371/journal.pntd.0004511
Agarwal, A., Ganvir, R., Kale, D., Chaurasia, D., and Kapoor, G. (2023). Continued dominance of dengue virus serotype 2 during the recent Central India outbreaks (2019-2021) with evidence of genetic divergence. Pathog. Glob. Health doi: 10.1080/20477724.2023.2246712 [Epub ahead of print].
Agarwal, A., Gupta, S., Chincholkar, T., Singh, V., Umare, I. K., Ansari, K., et al. (2019). Co-circulation of dengue virus serotypes in Central India: Evidence of prolonged viremia in DENV-2. Infect. Genet. Evol. 70, 72–79. doi: 10.1016/j.meegid.2019.02.024
Alagarasu, K., Patil, J. A., Kakade, M. B., More, A. M., Yogesh, B., Newase, P., et al. (2021). Serotype and genotype diversity of dengue viruses circulating in India: a multi-centre retrospective study involving the Virus Research Diagnostic Laboratory Network in 2018. Int. J. Infect. Dis. 111, 242–252. doi: 10.1016/j.ijid.2021.08.045
Angel, B., and Joshi, V. (2008). Distribution and seasonality of vertically transmitted dengue viruses in Aedes mosquitoes in arid and semi-arid areas of Rajasthan, India. J. Vector Borne Dis. 45:56.
Angelin, M., Sjölin, J., Kahn, F., Hedberg, A. L., Rosdahl, A., Skorup, P., et al. (2023). Qdenga® -A promising dengue fever vaccine; can it be recommended to non-immune travelers? Travel Med. Infect. Dis. 2023:102598. doi: 10.1016/j.tmaid.2023.102598
Anoop, M., Issac, A., Mathew, T., Philip, S., Kareem, N. A., Unnikrishnan, R., et al. (2010). Genetic characterization of dengue virus serotypes causing concurrent infection in an outbreak in Ernakulam, Kerala, South India. Indian J. Exp. Biol. 48, 849–857.
Arunachalam, N., Tewari, S. C., Thenmozhi, V., Rajendran, R., Paramasivan, R., Manavalan, R., et al. (2008). Natural vertical transmission of dengue viruses by Aedes aegypti in Chennai, Tamil Nadu, India. Indian J. Med. Res. 127, 395–407.
Bhatia, S., Bansal, D., Patil, S., Pandya, S., Ilyas, Q. M., and Imran, S. (2022). A Retrospective Study of Climate Change Affecting Dengue: Evidences, Challenges and Future Directions. Front. Public Health 10:884645. doi: 10.3389/fpubh.2022.884645
Bhatt, P., Sabeena, S. P., Varma, M., and Arunkumar, G. (2021). Current understanding of the pathogenesis of dengue virus infection. Curr. Microbiol 78, 17–32. doi: 10.1007/s00284-020-02284-w
Bina, P. D., Katyal, R., Abhay, S., Raina, V. K., Saxena, V. K., and Lal, S. (2008). Natural vertical transmission of dengue virus in peak summer collections of Aedes aegypti (Diptera: Culicidae) from urban areas of Jaipur (Rajasthan) and Delhi. J. Commun. Dis. 40, 155–157.
Bouckaert, R., Vaughan, T. G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., et al. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 15:e1006650. doi: 10.1371/journal.pcbi.1006650
Buckner, E. A., Alto, B. W., and Lounibos, L. P. (2013). Vertical transmission of Key West dengue-1 virus by Aedes aegypti and Aedes albopictus (Diptera: Culicidae) mosquitoes from Florida. J. Med. Entomol. 50, 1291–1297. doi: 10.1603/me13047
Cecilia, D., Kakade, M. B., Bhagat, A. B., Vallentyne, J., Singh, A., Patil, J. A., et al. (2011). Detection of dengue-4 virus in Pune, Western India after an absence of 30 years-its association with two severe cases. Virol. J. 8, 1–4. doi: 10.1186/1743-422X-8-46
Chatterjee, S. N., Chakravarti, S. K., Mitra, A. C., and Sarkar, J. K. (1965). Virological investigation of cases with neurological complications during the outbreak of haemorrhagic fever in Calcutta. J. Indian Med. Assoc. 45, 314–316.
Chetry, S., Patgiri, S. J., Bhattacharyya, D. R., Dutta, P., and Kumar, N. P. (2020). Incrimination of Aedes aegypti and Aedes albopictus as vectors of dengue virus serotypes 1, 2 and 3 from four states of Northeast India. Access Microbiol. 2:101.
Dar, L., Broor, S., Sengupta, S., Xess, I., and Seth, P. (1999). The first major outbreak of dengue hemorrhagic fever in Delhi, India. Emerg. Infect. Dis. 5:589. doi: 10.3201/eid0504.990427
Das, B., Das, M., Dwibedi, B., Kar, S. K., and Hazra, R. K. (2013). Molecular investigations of dengue virus during outbreaks in Orissa state, Eastern India from 2010 to 2011. Infect. Genet. Evol. 16, 401–410. doi: 10.1016/j.meegid.2013.03.016
Dash, P. K., Parida, M. M., Saxena, P., Abhyankar, A., Singh, C. P., Tewari, K. N., et al. (2006). Reemergence of dengue virus type-3 (subtype-III) in India: implications for increased incidence of DHF & DSS. Virol. J. 3, 1–10. doi: 10.1186/1743-422X-3-55
Dutta, P., Khan, S. A., Chetry, S., and Abdul, M. (2018). Incrimination of Aedes aegypti for dengue virus serotype-1 in Assam, Northeast India. J. Vector Borne Dis. 55, 330–333. doi: 10.4103/0972-9062.256572
Gubler, D. J. (2002). The global emergence/resurgence of arboviral diseases as public health problems. Arch. Med. Res. 33, 330–342. doi: 10.1016/s0188-4409(02)00378-8
Gubler, D. J. (2011). Dengue, urbanization and globalization: the unholy trinity of the 21st century. Trop. Med. Health 39, S3–S11. doi: 10.2149/tmh.2011-S05
Guo, C., Zhou, Z., Wen, Z., Liu, Y., Zeng, C., Xiao, D., et al. (2017). Global epidemiology of dengue outbreaks in 1990–2015: a systematic review and meta-analysis. Front. Cell. Infect. 7:317. doi: 10.3389/fcimb.2017.00317
Hadinegoro, S. R., Arredondo-García, J. L., Capeding, M. R., Deseda, C., Chotpitayasunondh, T., Dietze, R., et al. (2015). Efficacy and long-term safety of a dengue vaccine in regions of endemic disease. N. Eng. J. Med. 373, 1195–1206. doi: 10.1056/NEJMoa1506223
Hayes, E. B., and Gubler, D. J. (1992). Dengue and dengue hemorrhagic fever. Pediatr. Infect Dis. J. 11, 311–317. doi: 10.1097/00006454-199204000-00010
Hull, B., Tikasingh, E., de Souza, M., and Martinez, R. (1984). Natural transovarial transmission of dengue 4 virus in Aedes aegypti in Trinidad. Am. J. Trop. Med. Hyg. 33, 1248–1250. doi: 10.4269/ajtmh.1984.33.1248
Hussain, S. S. A., and Dhiman, R. C. (2022). Distribution expansion of dengue vectors and climate change in India. Geohealth 6:e2021GH000477. doi: 10.1029/2021GH000477
India Tourism Statistics (2014). Ministry of Tourism, Government of India. Lucknow: India Tourism Statistics.
Kalpana, B., Kumar, S. P., Mohalia, M. M., and Dhariwal, A. C. (2010). A study on dengue outbreak during 2009 in Bhopal and Indore districts of Madhya Pradesh, India. J. Commun. Dis. 42, 273–279.
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 35:1547. doi: 10.1093/molbev/msy096
Lambrechts, L., Fansiri, T., Pongsiri, A., Thaisomboonsuk, B., Klungthong, C., Richardson, J. H., et al. (2012). Dengue-1 virus clade replacement in Thailand associated with enhanced mosquito transmission. Virol. J. 86, 1853–1861. doi: 10.1128/jvi.06458-11
Lanciotti, R. S., Calisher, C. H., Gubler, D. J., Chang, G. J., and Vorndam, A. V. (1992). Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 30, 545–551. doi: 10.1128/jcm.30.3.545-551.1992
Mahadev, P. V., Prasad, S. R., Ilkal, M. A., Mavale, M. S., Bedekar, S. S., and Banerjee, K. (1997). Activity of dengue-2 virus and prevalence of Aedes aegypti in the Chirimiri colliery area, Madhya Pradesh, India. Southeast Asian J. Trop. Med. Public Health 28, 126–137.
Martins, V. E. P., Alencar, C. H., Kamimura, M. T., de Carvalho Araujo, F. M., De Simone, S. G., Dutra, R. F., et al. (2012). Occurrence of natural vertical transmission of dengue-2 and dengue-3 viruses in Aedes aegypti and Aedes albopictus in Fortaleza. Ceará, Brazil. PLoS One 7:e41386. doi: 10.1371/journal.pone.0041386
Messer, W. B., Gubler, D. J., Harris, E., Sivananthan, K., and De Silva, A. M. (2003). Emergence and global spread of a dengue serotype 3, subtype III virus. Emerg. Infect. Dis. 9:800. doi: 10.3201/eid0907.030038
Mishra, G., Jain, A., Prakash, O., Prakash, S., Kumar, R., Garg, R. K., et al. (2015). Molecular characterization of dengue viruses circulating during 2009–2012 in Uttar Pradesh, India. J. Med. Virol. 87, 68–75. doi: 10.1002/jmv.23981
Murhekar, M. V., Kamaraj, P., Kumar, M. S., Khan, S. A., Allam, R. R., Barde, P., et al. (2019). Burden of dengue infection in India, 2017: a cross-sectional population based serosurvey. Lancet Glob. Health. 7, 1065–1073. doi: 10.1016/S2214-109X(19)30250-5
Murray, N. E. A., Quam, M. B., and Wilder-Smith, A. (2013). Epidemiology of dengue: past, present and future prospects. Clin. Epidemiol. 5, 299–309. doi: 10.2147/CLEP.S34440
Murugesan, A., Aridoss, D., Senthilkumar, S., Sivathanu, L., Sekar, R., Shankar, E. M., et al. (2020). Molecular Diversity of Dengue Virus Serotypes 1–4 during an Outbreak of Acute Dengue Virus Infection in Theni, India. Indian J. Med. Microbiol. 38, 401–408. doi: 10.4103/ijmm.IJMM_20_89
Paingankar, M. S., Gokhale, M. D., Vaishnav, K. G., and Shah, P. S. (2014). Monitoring of dengue and chikungunya viruses in field-caught Aedes aegypti (Diptera: Culicidae) in Surat city, India. Curr. Sci. 106, 1559–1567.
Parida, M. M., Dash, P. K., Upadhyay, C., Saxena, P. J. A., and Jana, A. M. (2002). Serological & virological investigation of an outbreak of. Indian J. Med. Res. 116, 248–254.
Rico-Hesse, R., Harrison, L. M., Salas, R. A., Tovar, D., Nisalak, A., and Ramos, C. (1997). Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 230, 244–251. doi: 10.1006/viro.1997.8504
Rivera, L., Biswal, S., Sáez-Llorens, X., Reynales, H., López-Medina, E., Borja-Tabora, C., et al. (2022). Three-year efficacy and safety of Takeda’s dengue vaccine candidate (TAK-003). Clin. Infect. Dis. 75, 107–117. doi: 10.1093/cid/ciab864
Romanello, M., Di Napoli, C., Drummond, P., Green, C., Kennard, H., Lampard, P., et al. (2022). The 2022 report of the Lancet Countdown on health and climate change: health at the mercy of fossil fuels. Lancet 400, 1619–1654. doi: 10.1016/S0140-6736(22)01540-9
Sankari, T., Hoti, S. L., Singh, T. B., and Shanmugavel, J. (2012). Outbreak of dengue virus serotype-2 (DENV-2) of Cambodian origin in Manipur. India-Association with meteorological factors. Indian J. Med. Res. 136:649.
Sankoju, P., Ravinuthala, V. S. U., Mopuri, R., Mutheneni, S. R., and Addlagatta, A. (2023). Genomic characterization and evolutionary analysis of dengue virus from Aedes mosquitoes in Telangana, India. J. Vector Borne Dis. 60, 179–186. doi: 10.4103/0972-9062.364766
Sarma, D. K., Kumar, M., Balabaskaran Nina, P., Balasubramani, K., Pramanik, M., Kutum, R., et al. (2022). An assessment of remotely sensed environmental variables on Dengue epidemiology in Central India. PLoS Negl. Trop. Dis. 16:e0010859. doi: 10.1371/journal.pntd.0010859
Sehgal, P. N., Kalra, N. L., Pattanayak, S., Wattal, B. L., and Shrivastav, J. B. (1967). A study of an outbreak of dengue epidemic in Jabalpur, Madhya Pradesh. Bull. Indian Soc. Malaria Other Commun. Dis. 4, 91–108.
Shrivastava, A., Soni, M., Shrivastava, S., Sharma, S., Dash, P. K., Gopalan, N., et al. (2015). Lineage shift of dengue virus in Eastern India: an increased implication for DHF/DSS. Epidemiol. Infect. 143, 599–1605.
Singh, U. B., Maitra, A., Broor, S., Rai, A., Pasha, S. T., and Seth, P. (1999). Partial nucleotide sequencing and molecular evolution of epidemic causing dengue 2 strains. J. Infect. Dis. 180, 959–965. doi: 10.1086/315043
Srivastava, N. N., Maan, H. S., Dhole, T. N., Singh, J., Sharma, S., Pandey, S. N., et al. (2023). Dengue Virus Serotypes Circulating among Aedes Mosquitoes in the Lucknow District of North India: Molecular Identification and Characterization. J. Pure Appl. Microbiol. 17, 1141–1153.
Teo, A., Tan, H. D., Loy, T., Chia, P. Y., and Chua, C. L. L. (2023). Understanding antibody-dependent enhancement in dengue: Are afucosylated IgG1s a concern? PLoS Pathog. 19:e1011223. doi: 10.1371/journal.ppat.1011223
Thenmozhi, V., Hiriyan, J. G., Tewari, S. C., Samuel, P. P., Paramasivan, R., Rajendran, R., et al. (2007). Natural vertical transmission of dengue virus in Aedes albopictus (Diptera: Culicidae) in Kerala, a southern Indian state. Jpn. J. Infect. Dis. 60:245.
Thomas, S. J., and Yoon, I. K. (2019). A review of Dengvaxia®: development to deployment. Hum. Vaccines Immunother. 15, 2295–2314. doi: 10.1080/21645515.2019.1658503
Titir, S. R., Paul, S. K., Ahmed, S., Haque, N., Nasreen, S. A., Hossain, K. S., et al. (2021). Nationwide distribution of dengue virus type 3 (Denv-3) genotype I and emergence of denv-3 genotype III during the 2019 outbreak in Bangladesh. Trop. Med. Infect. Dis. 6:58. doi: 10.3390/tropicalmed6020058
Torres-Flores, J. M., Reyes-Sandoval, A., and Salazar, M. I. (2022). Dengue vaccines: An update. BioDrugs 36, 325–336. doi: 10.1007/s40259-022-00531-z
Tyagi, B. K., Munirathinam, A., and Venkatesh, A. (2015). A catalogue of Indian mosquitoes. Int. J. Mosq. Res. 2, 50–97.
Weaver, S. C., and Vasilakis, N. (2009). Molecular evolution of dengue viruses: contributions of phylogenetics to understanding the history and epidemiology of the preeminent arboviral disease. Infect. Genet. Evol. 9, 523–540.
Wijesinghe, C., Gunatilake, J., Kusumawathie, P. H. D., Sirisena, P. D. N. N., Daulagala, S. W. P. L., and Iqbal, B. N. (2021). Circulating dengue virus serotypes and vertical transmission in Aedes larvae during outbreak and inter-outbreak seasons in a high dengue risk area of Sri Lanka. Parasit. Vectors. 14, 1–11. doi: 10.1186/s13071-021-05114-5
Wilder-Smith, A., and Gubler, D. J. (2008). Geographic expansion of dengue: the impact of international travel. Med. Clin. North Am. 92, 1377–1390. doi: 10.1016/j.mcna.2008.07.002
World Health Organization (2009). Special Programme for Research, Training in Tropical Diseases, World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. Geneva: World Health Organization.
Keywords: dengue virus, Aedes mosquito, surveillance, epidemiology, India font: Italic, complex script font: Italic considering the growing threat
Citation: Sarma DK, Rathod L, Mishra S, Das D, Agarwal A, Sharma G, Singh TA, Kumawat M, Singh S, Verma V, Kumar M, Shubham S, Tiwari RR and Prakash A (2023) Molecular surveillance of dengue virus in field-collected Aedes mosquitoes from Bhopal, central India: evidence of circulation of a new lineage of serotype 2. Front. Microbiol. 14:1260812. doi: 10.3389/fmicb.2023.1260812
Received: 18 July 2023; Accepted: 31 August 2023;
Published: 14 September 2023.
Edited by:
James Weger-Lucarelli, Virginia Tech, United StatesReviewed by:
Ambuj Shrivastava, Defence Research and Development Establishment (DRDE), IndiaCopyright © 2023 Sarma, Rathod, Mishra, Das, Agarwal, Sharma, Singh, Kumawat, Singh, Verma, Kumar, Shubham, Tiwari and Prakash. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Devojit Kumar Sarma, ZGtiaW90ZWtAZ21haWwuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.