- 1School of Food and Pharmaceutical Engineering, Guizhou Institute of Technology, Guiyang, China
- 2Center of Excellence in Fungal Research, Mae Fah Luang University, Chiang Rai, Thailand
- 3School of Science, Mae Fah Luang University, Chiang Rai, Thailand
- 4University of Nicosia School of Veterinary Medicine, Nicosia, Cyprus
- 5Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang, China
- 6College of Agriculture and Biological Sciences, Dali University, Dali, China
Entomopathogenic fungi comprise an ecologically important group of specialized pathogens infecting other fungi, invertebrates, and plants. These fungi are species-rich with high diversity and broad distribution worldwide. The majority of entomopathogenic fungi belong to clavicipitoids, which consist of the hypocrealean families, Clavicipitaceae, Cordycipitaceae, Ophiocordycipitaceae, and Polycephalomycetaceae. The latter is a newly established entomopathogenic family that recently separated from the family Ophiocordycipitaceae to accommodate the genera, Perennicordyceps, Pleurocordyceps, and Polycephalomyces. In recent years, Polycephalomycetaceae has been enriched with parasitic and hyperparasitic fungi. With 16 species spread across China, Ecuador, Japan, and Thailand, Pleurocordyceps is the most speciose genus in the family. In this study, we expand the number of taxa in the genus by introducing four new Pleurocordyceps species from China, namely, P. clavisynnema, P. multisynnema, P. neoagarica, and P. sanduensis. We provide detailed descriptions and illustrations and infer genus-level phylogenies based on a combined 6-loci gene sequence dataset comprising the internal transcribed spacer gene region (ITS), small subunit ribosomal RNA gene region (SSU), large subunit rRNA gene region (LSU), translation elongation factor 1-alpha gene region (TEF-1α), RNA polymerase II largest subunit gene region (RPB1), and RNA polymerase II second largest subunit (RPB2). This study contributes to knowledge with regard to the diversity of Pleurocordyceps specifically and entomopathogenic Hypocreales more broadly.
Introduction
Insect pathogenic fungi, also known as entomopathogenic fungi, comprise a group of over 2,000 species spanning 90 genera (Saltamachia and Araujo, 2020). The phylogenetic diversity of entomopathogenic fungi is notable, with the majority belonging to Hypocreales, the largest group of plant and insect pathogens in Sordariomycetes (Sung et al., 2007; Maharachchikumbura et al., 2016; Wijayawardene et al., 2018). Within Hypocreales, the families Clavicipitaceae, Cordycipitaceae, Ophiocordycipitaceae, and Polycephalomycetaceae are collectively known as the clavicipitoid fungi and contain the majority of known insect pathogens (Hyde et al., 2020; Wei et al., 2020; Wijayawardene et al., 2020; Huang et al., 2021; Xiao et al., 2023). Some species are well known in the fields of agriculture and related industries, including Beauveria bassiana (biological control agent), Cordyceps militaris (medicinal), Metarhizium anisopliae (biological control agent), and Ophiocordyceps sinensis (medicinal) (Zimmermann, 2007; Acuña Jiménez et al., 2015; Li et al., 2020; Eiamthaworn et al., 2022). Thus, entomopathogenic fungi have gained the attention of researchers as a crucial fungal resource (Fernández-Grandon et al., 2020; Sharma et al., 2020; Sobczak et al., 2020; Zha et al., 2021).
The taxonomy of entomopathogenic fungi has undergone substantial changes since the advent of the molecular era (Tasanathai et al., 2016; Dong et al., 2022). Chaverri et al. (2005) initiated this molecular exploration by providing LSU, TEF, and RPB1 data for Polycephalomyces formosus and Polycephalomyces ramosopulvinatus (current name: Pleurocordyceps ramosopulvinata). Ban et al. (2009) used a 504-base-pair LSU fragment, but it fell short in resolving deep fungal nodes (Kepler et al., 2013). Different loci were selected for the analysis of novel species, with Wang et al. (2014) using a 4-loci (SSU, LSU, TEF, and RPB1), Wang et al. (2015b) using a 5-loci (SSU, LSU, TEF, RPB1, and RPB2), and Wang et al. (2015a) and Xiao et al. (2018) utilizing a 6-loci (ITS, SSU, LSU, TEF, and RPB1, and RPB2). The phylogenetic placement of Polycephalomyces or the segregation of new genera from Polycephalomyces was analyzed using both 5-loci (SSU, LSU, TEF, RPB1, and RPB2) and 6-loci (ITS, SSU, LSU, TEF, RPB1, and RPB2) (Kepler et al., 2013; Matočec et al., 2014; Wang et al., 2021). Building on this molecular groundwork, Xiao et al. (2023) established a new family, Polycephalomycetaceae, accommodating three genera (Perennicordyceps, Pleurocordyceps, and Polycephalomyces) and comprising 28 species using 6 loci (ITS, SSU, LSU, TEF, RPB1, and RPB2).
Over the past decade, a multitude of new species have been described in the family Polycephalomycetaceae, including those documented by Kepler et al. (2012), Wang et al. (2015a,b), and Yang et al. (2020), contributing to a deeper understanding of its classification. Recent studies by Wei et al. (2022) and Xiao et al. (2023) have introduced additional new species, sparking renewed interest in the taxonomy of the family. The sexual morph of Polycephalomycetaceae is distinguished by producing superficial or immersed ascomata with a stipe, three layers of peridium, narrowly cylindrical asci, multiseptate ascospores, and short cylindrical part spores (Matočec et al., 2014; Wang et al., 2021; Xiao et al., 2023). Its asexual morphs have congregated mycelia on the surface of the host, light-colored synnemata with stipules, divergent conidiophores, and one or both types of phialides and conidia (Matočec et al., 2014; Wang et al., 2021; Xiao et al., 2023). Most species in Polycephalomycetaceae are found in tropical and subtropical regions, with fewer taxa found in temperate regions (Van Vooren and Audibert, 2005; Wang et al., 2012, 2015a; Matočec et al., 2014; Xiao et al., 2018, 2023). A high diversity of polycephalomycetous fungi has been found in China and Japan (Kobayasi, 1939, 1941; Kobayasi and Shimizu, 1982; Chen et al., 1984; Wang et al., 2012, 2014, 2015a,b, 2021; Kepler et al., 2013; Quandt et al., 2014; Yang et al., 2020; Xiao et al., 2023).
With 16 species, Pleurocordyceps is the most speciose genus in the family Polycephalomycetaceae (Wang et al., 2021; Xiao et al., 2023). Pleurocordyceps was established by Wang et al. (2021) with the type species, P. sinensis, which was found on Ophiocordyceps sinensis (Chen et al., 1984). Pleurocordyceps is distinguished from closely related genera by its lateral fertile pulvinate stromata near the tip of the sexual morph and its two types of phialides and conidia in the asexual morph (Wang et al., 2021; Xiao et al., 2023). Wang et al. (2021) provided a key to the 10 accepted Pleurocordyceps species (Wang et al., 2021; Xiao et al., 2023). The insect host orders associated with Pleurocordyceps sp. comprise Coleoptera, Hymenoptera, Hemiptera, Lepidoptera, Orthoptera, and Homoptera (Kobayasi, 1939; Kobayasi and Shimizu, 1982; Bischoff et al., 2003; Ban et al., 2009; Wang et al., 2012, 2015a,b; Crous et al., 2017; Xiao et al., 2018; Poinar and Vega, 2020). In addition to parasitizing insects, most species in the genus are also parasites of fungi (Kobayasi, 1941; Seifert, 1985; Bischoff et al., 2003; Ban et al., 2009; Wang et al., 2015a; Xiao et al., 2023). In recent years, Ophiocordyceps sp. has been frequently reported as the host of Polycephalomyces-like species (Sun et al., 2019; Xiao et al., 2023). Specifically, Pleurocordyceps agarica, P. aurantiacus, P. lianzhouensis, P. sinensis, and P. yunnanensis are parasites on Ophiocordyceps sp. and insects (Chen et al., 1984; Wang et al., 2012, 2015a,b, 2021; Xiao et al., 2018). In general, Pleurocordyceps spp. exhibit significant potential for producing a diverse range of secondary metabolites. For instance, Pleurocordyceps nipponicus and P. phaothaiensis contain natural antioxidant, antibacterial, antitumorigenic, anti-inflammatory, and antimicrobial compounds (Sangdee et al., 2017; Somsila et al., 2018; Sonyot et al., 2020). Gokhale et al. (2020) reported that the secondary metabolites of P. sinensis have antibacterial potential. However, there are noticeable gaps in critical areas, such as chemistry, industry, and ecology of Pleurocordyceps species. Thus, there is a compelling need for further research to explore the wide array of capabilities and applications within Pleurocordyceps.
In China, there are records of nine Pleurocordyceps species, along with more than 200 taxa of clavicipitoid fungi that have been found in the country (Wang et al., 2012, 2014, 2015a,b; Liang et al., 2016; Yang et al., 2020; Xiao et al., 2023). In this study, we introduce four new species of Pleurocordyceps, namely, P. clavisynnema, P. multisynnema, P. neoagarica, and P. sanduensis. We provide a detailed morphological description along with phylogenetic analyses using a combined 6-loci gene region (ITS, SSU, LSU, tef-1α, rpb1, and rpb2).
Materials and methods
Sample collection, isolation, and morphological studies
Fresh specimens, comprising a total of eight, were collected from soil in Anhui and Guizhou provinces, China. The samples were transported in plastic boxes to the laboratory, and pertinent metadata (location, longitude, and latitude) were recorded. The fruiting bodies were examined using a stereomicroscope (SMZ 745 and SMZ 800N, Nikon, Tokyo, Japan) and free-hand sections were obtained for analysis. Micromorphological features such as synnemata, conidiophores, phialides, and conidia were captured using a Nikon DS-Ri2 digital camera connected to a Nikon ECLIPSE microscope (Tokyo, Japan). The strains were obtained from fresh tissue by removing a small piece of mycelium from the host, which was then transferred with a sterile needle onto PDA plates and incubated at 25°C. The pure culture was stored in the Guizhou Culture Collection, China (GZCC). The specimens were deposited at the Guizhou Institute of Technology Herbarium (Herb. GZLG). The guidelines of the Facesoffungi database (https://www.indexfungorum.org) were followed to obtain Index Fungorum numbers, as outlined by Jayasiri et al. (2015). The morphological structures were measured using Tarosoft (R) v.0.9.7 Image Frame Work, and the photographic plates were processed using Adobe Photoshop CC 2022 (Adobe Systems, USA).
DNA extraction, PCR amplification, and sequencing
Total DNA was extracted from fruiting bodies and cultures using the Fungal DNA MiniKit (Biotech, USA), following the manufacturer's instructions. Internal transcribed spacer gene region (ITS), small subunit ribosomal RNA gene region (SSU), large subunit rRNA gene region (LSU), RNA polymerase II largest subunit gene region (rpb1), RNA polymerase II second largest subunit (rpb2), and translation elongation factor 1-alpha gene region (tef-1α) gene amplifications were performed using the ITS5/ITS4, NS1/NS4, LR0R/LR5, CRPB1A/RPB1Cr, fRPB2-5F/fRPB2-7Cr, and 983F/2218R primers, respectively (Vilgalys and Hester, 1990; White et al., 1990; Hopple and Vilgalys, 1999; Castlebury et al., 2004; Sung et al., 2007). Previous studies have demonstrated that the use of these six genetic loci optimally resolves the phylogenetic placement of the species Pleurocordyceps (Xiao et al., 2018, 2023; Wang et al., 2021; Wei et al., 2022). The nuclear gene amplification reactions followed the protocol outlined by Yang et al. (2021). PCR products were sent to Tsingke Biotechnology for sequencing (Chongqing, China). All newly generated sequences were uploaded to GenBank, and accession numbers were assigned (Table 1).
Phylogenetic analyses
Using SeqMan, all newly generated sequences were assembled (Clewley, 1995). The reference taxa for phylogenetic analyses were obtained based on the BLAST search results (https://blast.ncbi.nlm.nih.gov/Blast.cgi) against the non-redundant protein sequence database (NRDB) using default parameters and previously published datasets (Table 1). Individual sequences were aligned using MAFFT v.7 (https://mafft.cbrc.jp/alignment/server/) and trimmed with Trimal v 1.4 (Capella-Gutiérrez et al., 2009; Katoh and Standley, 2013). Alignment was manually adjusted using BioEdit where needed (Hall, 1999). Maximum likelihood (ML) and Bayesian inference (BI) were used to infer phylogenies from a combined six-genetic marker dataset. Outgroup taxa were chosen as Perennicordyceps cuboidea (NBRC 101740) and Pe. cuboidea (NBRC 103836) (Schoch et al., 2012).
The ML phylogeny was inferred using IQ-TREE 2 with partitioned models and 1,000 exhaustive bootstrap replications (Minh et al., 2020). The model of evolution for each locus was chosen by the built-in ModelFinder tool (Kalyaanamoorthy et al., 2017). The BI analysis was conducted using MCMC sampling and MrBayes version 3.1.2 (Ronquist et al., 2012). The sampling was performed with six simultaneous Markov chains for 1,850,000 generations based on the standard deviation of split frequencies being < 0.01, with trees being sampled every 1,000 generations. The initial 25% of trees were considered as the burn-in phase and were discarded. The posterior probability (PP) was calculated using the remaining trees (Dissanayake et al., 2020). FigTree v.1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) was used to visualize the ML tree. Based on the guidelines provided by Chethana et al. (2021), Jayawardena et al. (2021), and Maharachchikumbura et al. (2021), novel species descriptions were created.
Results
Phylogenetic analyses
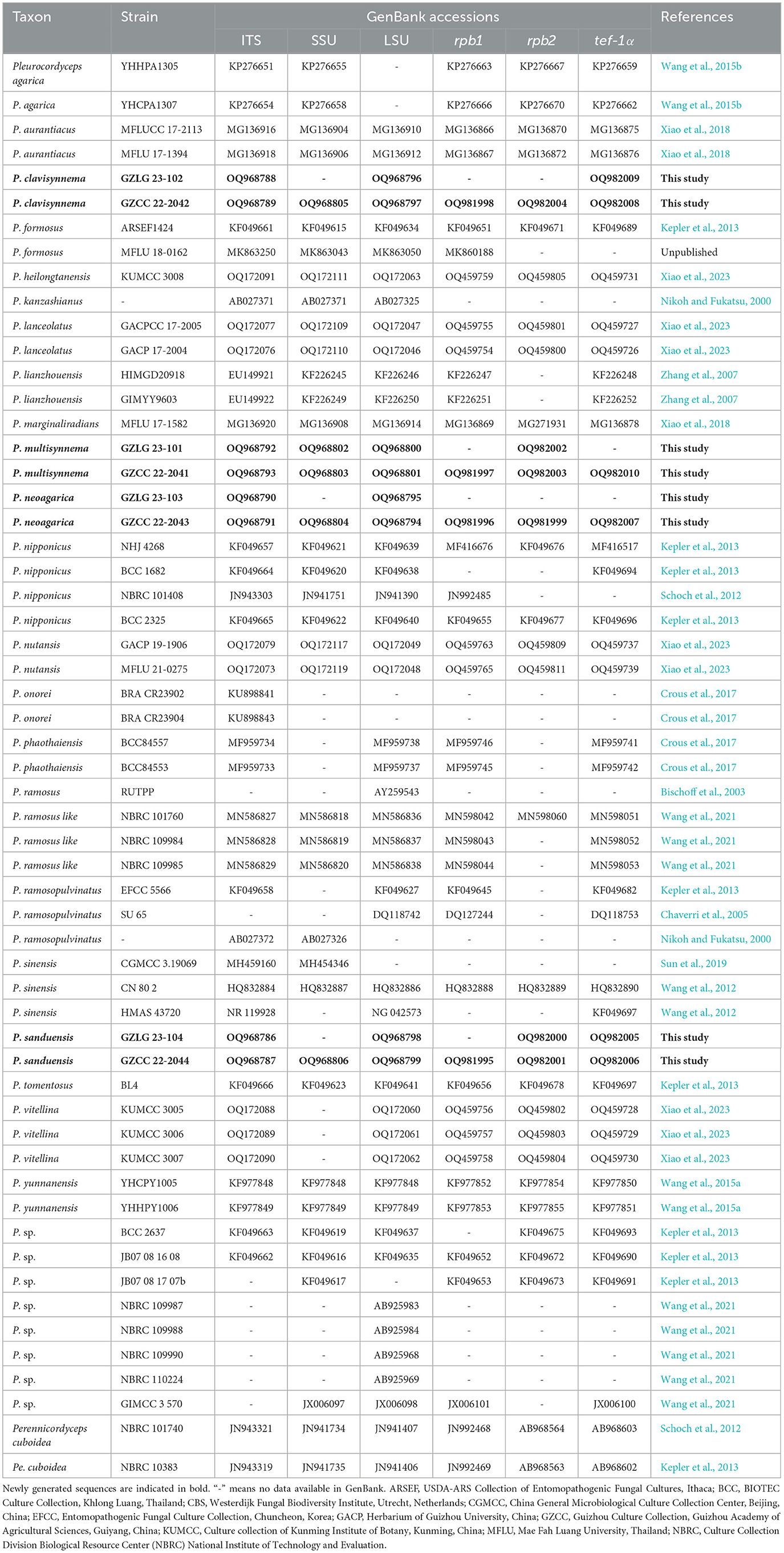
Sequences from 58 taxa representing 24 species of the family Polycephalomycetaceae were obtained from GenBank. The alignment contained 4,791 characteristics, representing 58 taxa. LSU: 847 bp, ITS: 531 bp, SSU: 943 bp, tef-1α: 844 bp, rpb1: 680 bp, and rpb2: 946 bp sequence data, including gaps, were combined in the final alignment. Outgroup taxa included Perennicordyceps cuboidea (NBRC 101740) and Perennicordyceps cuboidea (NBRC 103836). The topologies of ML and BI analyses were nearly congruent. Figure 1 displays that the maximum likelihood bootstrap (MLBS) is higher than 75%. The collections were determined as four new species, namely, Pleurocordyceps clavisynnema, P. multisynnema, P. neoagarica, and P. sanduensis. The phylogenetic placement of the new species is described in detail in the notes section below.
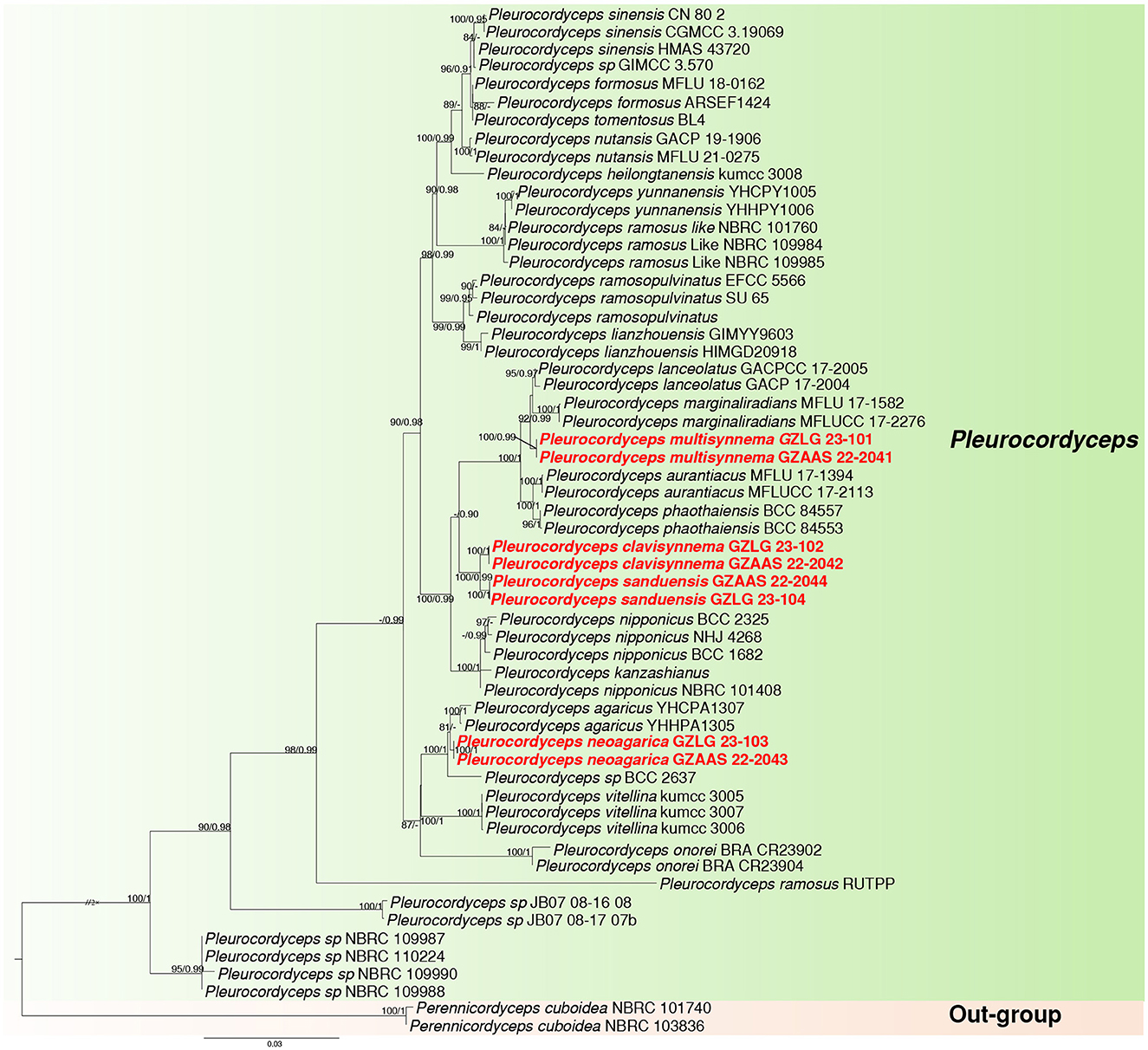
Figure 1. Maximum likelihood phylogenetic tree of 58 taxa and 4,791 sites combining LSU, SSU, ITS, tef-1α, rpb1, and rpb2 sequence data. MLBS higher than 75% and PP >0.90 are denoted near the nodes as MLBS/PP, and the newly generated sequences are in red bold font. The genus clade Pleurocordyceps is highlighted in green, while the outgroup is marked with a light orange background.
Taxonomy
Pleurocordyceps clavisynnema Y. P. Xiao and Y. Yang sp. nov (Figure 2).
Index Fungorum number: IF900449; Faceoffungi number: FoF 14158
Etymology: Name referring to clavate synnemata.
Holotype: GZLG 23-102
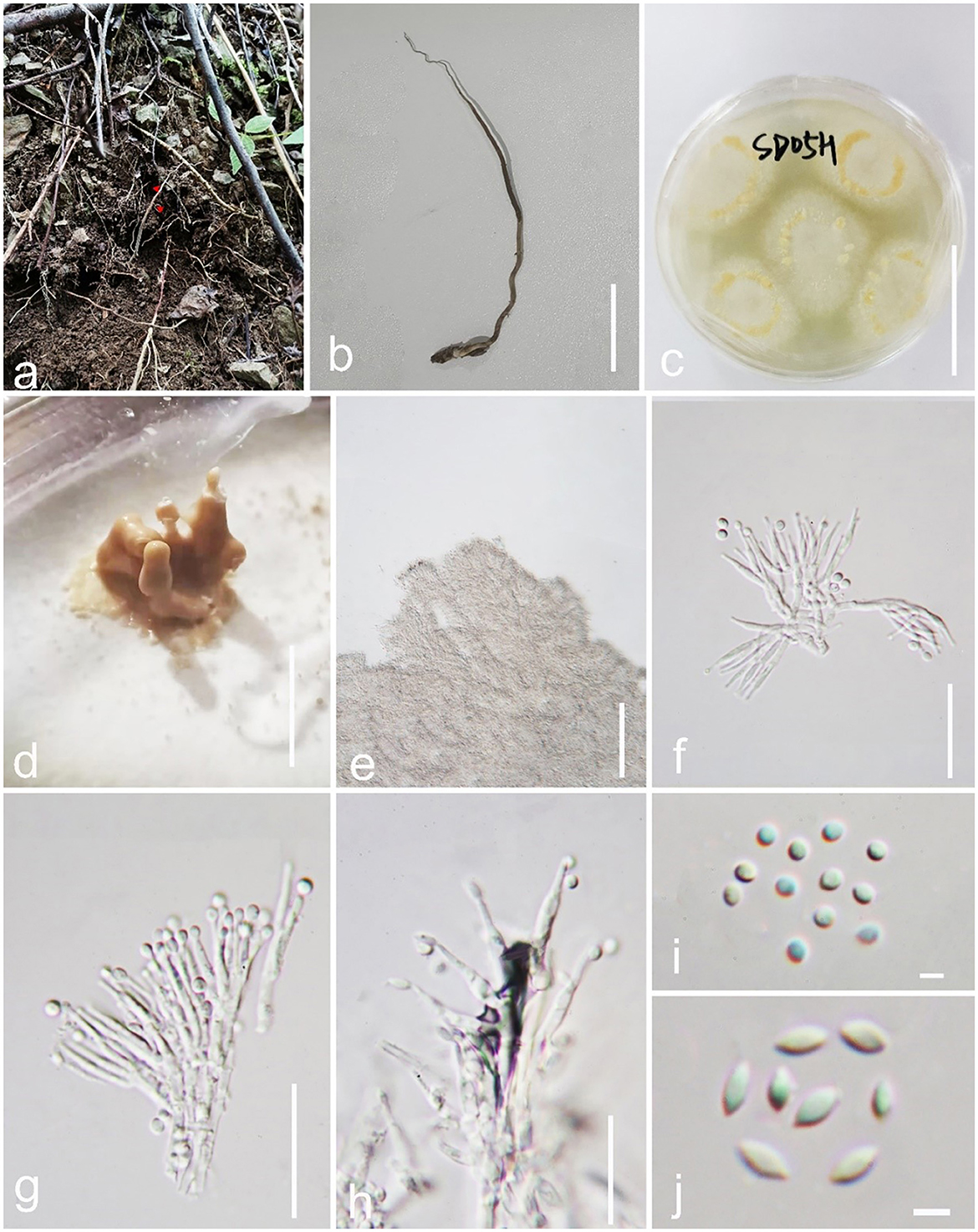
Figure 2. Pleurocordyceps clavisynnema (GZLG 23-102, Holotype). (a, b) Host: Ophiocordyceps neogryllotalpae (c) Upper side of the colony. (d) Synnemata on the culture. (e) Conidiophores. (f, g) α-phialides. (h) β-phialides. (i) α-conidia. (J) β-conidia. Scale bars: (b, c) 3 cm, (d) 0.5 cm, (e) 100 μm, (f–h) 20 μm, (i, j) 3 μm.
Parasitism on Ophiocordyceps neogryllotalpae (Ophiocordycipitaceae, Hypocreales). Sexual morph: Not observed. Asexual morph: Hyphomycetous. Culture characteristics: Colonies on PDA fast-growing, derived from tissue isolation, reaching 3 cm wide in 2 weeks at 25°C, white, and obverse brown. Synnemata emerging after 20 days, clavate or with a mucronate apex, solitary, unbranched, and 2–5 mm long. Fertile head 0.6–2.3 mm wide, yellowish to yellow, emerging on the middle part of the synnemata or on the top, with conidial masses on the surface. Conidial masses brown, slimy. Conidiophore 21–39 μm long ( = 20 μm, n = 40), 2–6 phialides in one. Phialides has two types α-phialides 8.3–14.5 × 0.9–1.7 μm ( = 11.4 × 1.3 μm, n = 40) smooth, hyaline, solitary. β-phialides 12.3–21.6 × 0.8–1.8 μm ( =16.95 × 1.3 μm, n = 40), smooth, hyaline, solitary. α-conidia 1.7–2.6 μm (= 2.15 μm, n = 50) wide, globose, 1-celled, smooth-walled; β-conidia 3.1–4.1 × 1.6–2.2 μm ( = 3.6 × 1.9 μm, n = 50), hyaline, fusiform, 1-celled, smooth.
Material examined: China, Guizhou Province, Qiannan Buyi and Miao Autonomous Prefecture, Sandu Shui Autonomous County. Parasitic on Ophiocordyceps neogryllotalpae (Ophiocordycipitaceae, Hypocreales), in the soil, 10 April 2022, Yu Yang, SD05H (GZLG 23-102, holotype; ex-type living culture, GZCC 22-2042).
Notes: Pleurocordyceps sanduensis is the closest match to our new sample of P. clavisynnema. This is also confirmed by phylogenetic analyses, whereby the two are sister taxa with maximum statistical support (100% ML/1.00 PP; Figure 1). Base pair differences between P. clavisynnema and P. sanduensis are 23/824 in tef-1α, 8/1130 in SSU, 2/678 in rpb1, and 3/1050 in rpb2. Morphologically, P. clavisynnema differs from P. sanduensis by having longer synnemata, larger conidiophore, smaller phialides, and shorter conidia. Hence, this study introduces Pleurocordyceps clavisynnema as a new species based on morphological and phylogenetic analyses.
Pleurocordyceps multisynnema Y. Yang and Y. P. Xiao sp. nov (Figure 3).
Index Fungorum number: IF900451; Faceoffungi number: FoF 14160
Etymology: Name referring to the multiple synnemata of the host and culture.
Holotype: GZLG 23-101
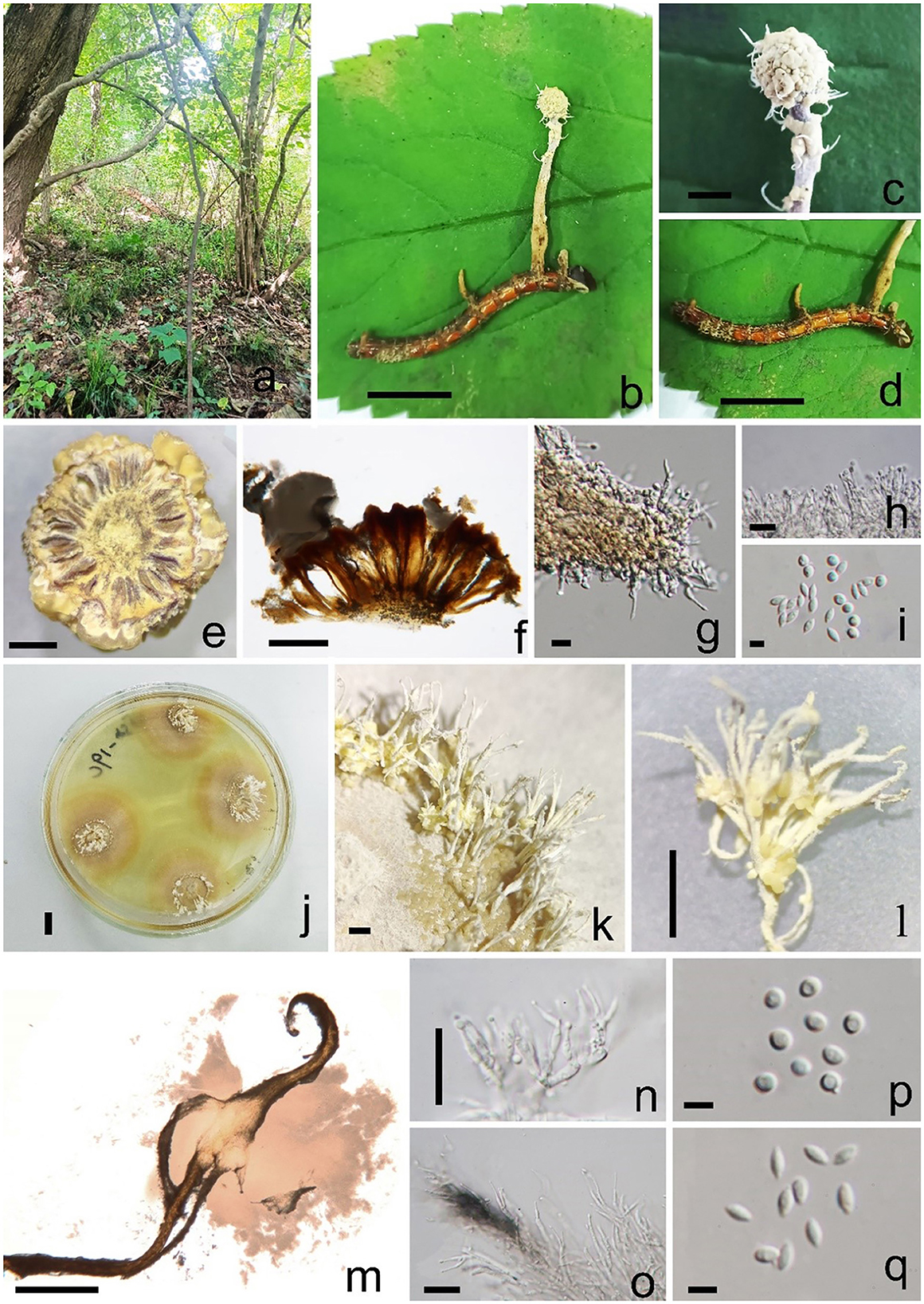
Figure 3. Pleurocordyceps multisynnema (GZLG 23-101, holotype) (a) Habitat. (b) Overview of Pleurocordyceps multisynnema. (c) Synnemata on the host. (d) Host of Paraisaria sp. (e, f) Section of host. (g) β-phialides. (h) α-phialides. (i) α-conidia and β-conidia. (j) Culture from above on PDA medium. (k–m) Synnemata on the culture. (n) α-phialides. (o) β-phialides. (p) α-conidia. (q) β-conidia. Scale bars: (b, d, j) 1 cm, (c) 0.2 cm, (e) 300 μm, (f) 200 μm, (g, h) 10 μm, (i, p, q) 3 μm, (k, l) 0.5 cm, (m) 500 μm, (n, o) 20 μm.
Sexual morph: absent. Asexual morph: Synnemata generating from the fertile head of the host, single, light yellow, cylindrical, without a fertile head, stipitate, usually unbranched. Conidial mass yellowish, covered the surfaces of the host. α-phialides 9–15 × 1.1–2.2 μm ( = 12 × 1.65 μm, n = 40), solitary, narrow lanceolate, from the synnema. β-phialides 19.8–25.9 × 1.7–2.6 μm ( = 22.85 × 2.15 μm, n = 40), directly from hyphae, solitary, narrow lanceolate, suddenly tapering from the bottom to the apex. Conidia one-celled, hyaline, smooth, two types. α-conidia 2.1–2.5 μm ( = 2.3 μm, n = 50), spherical, one-celled, smooth. β-conidia 2.9–3.8 × 1.3–2.2 μm ( = 3.7 × 1.9 μm, n = 50), fusiform, one-celled, smooth.
Colonies on PDA medium slow-growing, isolated from the tissue of synnemata, circular, attaining 3 cm in 35 days at 25°C, dry yellow. Synnemata arising the margin of the colony after 30 days, without a fertile head, solitary or two- or three-branched, 2–6 × 0.9–1.8 mm ( = 4 × 1.35 mm, n = 30), with several radiating ring-like distributions. Conidial masses pale yellow to yellow, covered the surface of the colony or generated from the middle part of the synnemata with hyaline to white yellow slime. Conidiophore 2–4 phialides in one. α-phialides 9–13.4 × 0.9–1.3 μm ( = 11.2 × 1.1 μm, n = 40) unbranched, hyaline, smooth. β-phialides 12.8–20.9 × 1.9–2.8 μm ( = 16.85 × 2.35 μm, n = 40), solitary, generating from hyphae laterally, hyaline, smooth. α-conidia 1.7–2.5 μm wide ( = 2.1 μm, n = 50), globose, one-celled, smooth-walled; β-conidia 2.6–3.5 × 1.3–2.2 μm ( = 3.05 × 1.75 μm, n = 50) hyaline, 1-celled, fusiform, smooth-walled.
Material examined: China, Anhui Province, Chuzhou City, parasitic on Paraisaria sp., on leaf litter, 25 August 2021, Yu Yang, HFS19a (GZLG 23-101, holotype; ex-type living culture, GZCC 22-2041).
Notes: Pleurocordyceps multisynnema has a high support value (100% ML/1 PP) and is sister to P. lanceolatus and P. marginaliradians in the phylogenetic tree (Figure 1). Comparing the ITS, LSU, SSU, tef-1α, rpb1, and rpb2 sequences of P. multisynnema and P. lanceolatus revealed 97.89% (12 bp differences), 99.28% (5 bp differences), 99.27% (6 bp differences), 99.77% (2 bp differences), 98.38% (11 bp differences), and 98.97% (10 bp differences) sequence similarities, respectively. Pleurocordyceps multisynnema differs from P. lanceolatus in that it is parasitic on Paraisaria species and produces conidia that range from coiled to thread-like but lack fertile heads (Xiao et al., 2023). Pleurocordyceps multisynnema differs from P. marginaliradians in distinct hosts (Paraisaria sp. vs. Cossidae larva), shorter phialides, and conidia (Xiao et al., 2018). As a result, Pleurocordyceps multisynnema is described as a new species of Pleurocordyceps.
Pleurocordyceps neoagarica Y. Yang and Y. P. Xiao sp. nov (Figure 4).
Index Fungorum number: IF900450; Faceoffungi number: FoF 14159
Etymology: Name referring to the similar species, Pleurocordyceps agarica.
Holotype: GZLG 23-103
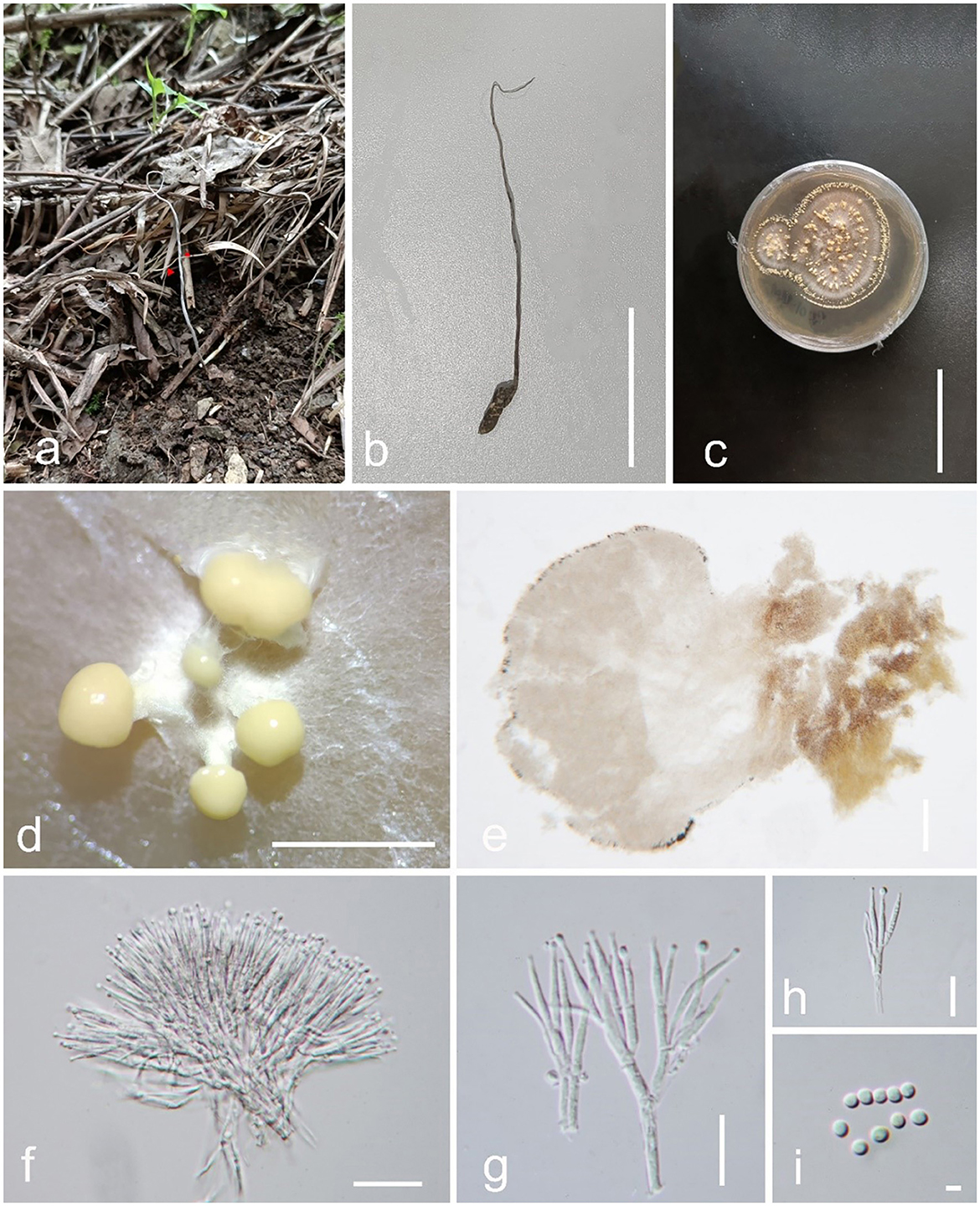
Figure 4. Pleurocordyceps neoagarica (GZLG 23-103, Holotype). (a, b) Host: Ophiocordyceps neogryllotalpae (c) Upper side of the colony. (d, e) Synnemata on the culture. (f) Conidiophores. (g, h) Phialides. (i) Conidia. Scale bars: (b, c) 5 cm, (d) 3 mm, (e) 200 μm, (f) 20 μm, (g, h) 10 μm, (i) 2 μm.
Parasitism on Ophiocordyceps neogryllotalpae (Ophiocordycipitaceae, Hypocreales). Sexual morph: Not observed. Asexual morph: Hyphomycetous. Culture characteristics: Colonies on PDA quickly grown, isolated from the tissue, reaching 5 cm wide in 25 days at 25°C, white, reverse brown. Synnemata appearing after 15 days, 0.5–3 mm long, solitary, non-branched, displaying several ring-like distributions. Fertile head 1.2–2.3 mm wide, globose, pale yellow, producing from the top of the synnemata. Conidial masses covered the surface of synnemata or the top of synnemata, white yellow, slimy. Conidiophore 42–63 μm long ( = 52.5 μm, n = 40), 2–4 phialides in one. Phialides 11.6–17.4 × 1.1–1.9 μm ( = 14.5 × 1.5 μm, n = 50), one type, narrowly slim lanceolate, cylindrical at the base, 6–13 μm long, tapered into a long neck, 1.2–3.1 μm long, hyaline, smooth. Conidia 2.1–2.9 μm ( = 2.5 μm, n = 50), arising from the apex of phialides, globose, 1-celled, hyaline.
Material examined: China, Guizhou Province, Qiannan Buyi and Miao Autonomous Prefecture, Sandu Shui Autonomous County. Parasitic on Ophiocordyceps neogryllotalpae (Ophiocordycipitaceae, Hypocreales), in the soil, 10 April 2022, Yu Yang, SD10H (GZLG 23-103, holotype; ex-type living culture, GZCC 22-2043).
Notes: Pleurocordyceps neoagarica (Host: Ophiocordyceps neogryllotalpae) differs from P. agarica (Host: Ophiocordyceps barnesii) morphologically due to its distinct host, longer synnemata and conidiophore, and shorter phialides (Wang et al., 2015b). P. neoagarica produces only one type of phialides and conidia, whereas P. agarica produces two. In the phylogenetic tree, the new collections (GZLG 23-103) shared a sister relationship with Pleurocordyceps agarica (Figure 1). The type of strain of P. neoagarica differs from P. agarica by 4 bp in ITS, 7 bp in SSU, 4 bp in rpb1, and 14 bp in rpb2 (Wang et al., 2015b). Given the significant morphological differences between these two taxa and their distinct phylogenetic placement, we conclude that they are separate species.
Pleurocordyceps sanduensis Y. P. Xiao and Y. Yang sp. nov (Figure 5).
Index Fungorum number: IF900447; Faceoffungi number: FoF 14157
Etymology: Name referring to the locality Sandu County.
Holotype: GZLG 23-104
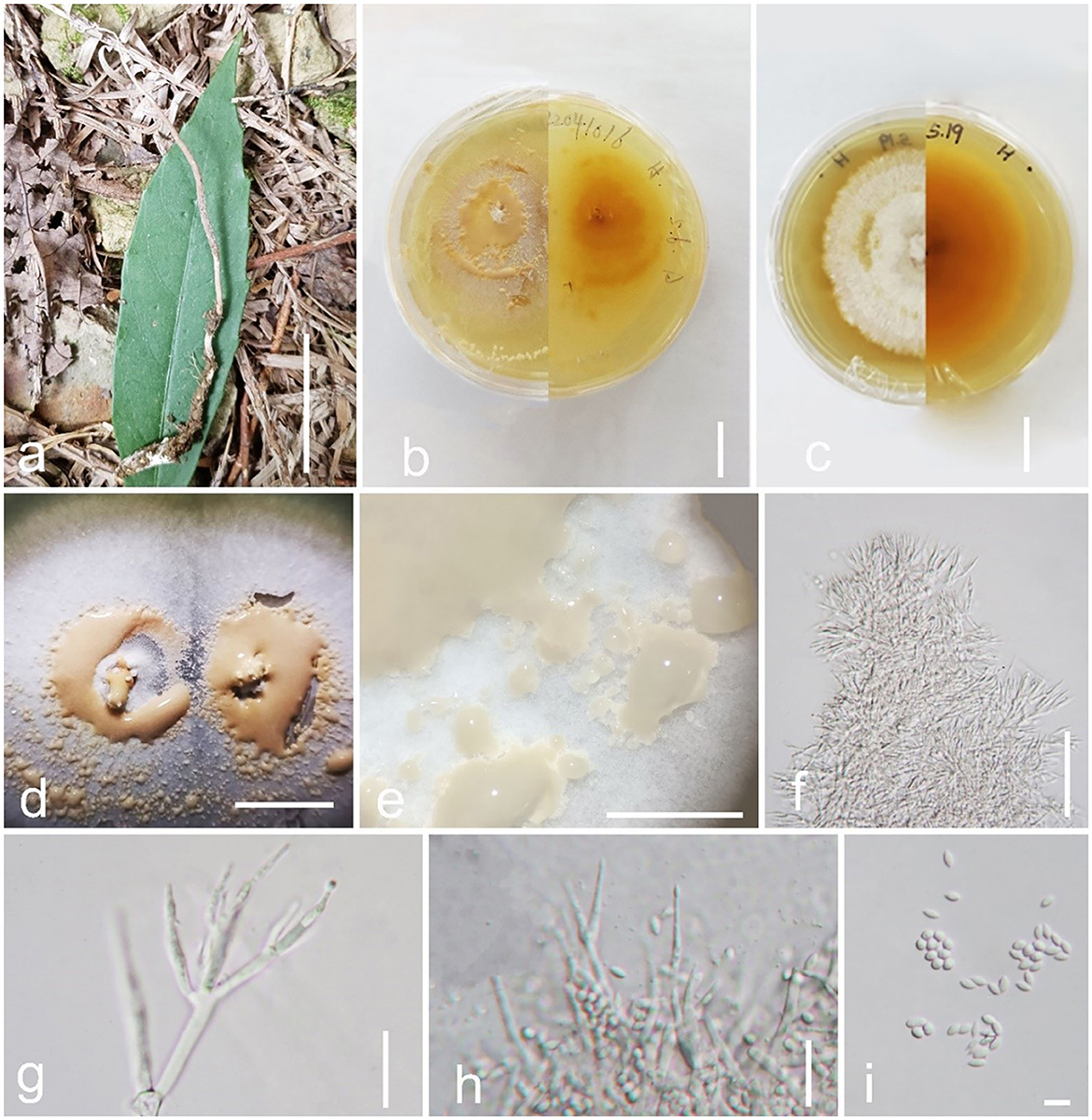
Figure 5. Pleurocordyceps sanduensis (GZLG 23-104, Holotype). (a) Host: Ophiocordyceps neogryllotalpae (b, c) Upper and back side of the colony. (d, e) Conidial masses on the culture. (f) Conidiophores. (g) α-phialides. (h) β-phialides (I) α-conidia and β-conidia. Scale bars: (a) 5 cm; (b–d) 1 cm, (e) 0.5 cm, (f) 50 μm, (g) = 10 μm, (h) 20 μm, (i) 5 μm.
Parasite on Ophiocordyceps neogryllotalpae (Ophiocordycipitaceae, Hypocreales). Sexual morph: Not observed. Asexual morph: Hyphomycetous. Culture characteristics: Colonies on PDA fast-growing, obtained from tissue, reaching 5 cm wide in 20 days at 25°C, white, reverse yellow to brown, presenting multiple radiating ring-like distributions. Synnemata emerging after 25 days, solitary, unbranched, 0.1–0.5 mm long, distribution at the edge, with small or without a fertile head. Conidial masses covered the surface of the colony, pale yellow when young, later change to brown color, slime. Conidiophore 12–23 μm long ( = 17.5 μm, n = 30), multiple phialides in one. Phialides exist in α-phialides and β-phialides. α-phialides 9.5–18.7 × 0.8–2.1 μm ( = 14.1 × 1.45 μm, n = 40), smooth, hyaline, solitary. β-phialides 19–33.4 × 0.9–1.8 μm ( = 26.2 × 1.35 μm, n = 40), smooth, hyaline, solitary. α-conidia 2.1–3.1 μm ( = 2.6 μm, n = 50) wide, globose, unicellular, smooth-walled; β-conidia 3.3–5.5 × 1.5–2.1 μm ( = 4.4 × 1.8 μm, n = 50) fusiform, unicellular, hyaline, smooth-walled.
Material examined: China, Guizhou Province, Qiannan Buyi and Miao Autonomous Prefecture, Sandu Shui Autonomous County. Parasitic on Ophiocordyceps neogryllotalpae associated with the larva of Gryllotalpa species, in soil, collected on 10 April 2022, Xingcan Peng, SD16 (GZLG 23-104, holotype; ex-type living culture, GZCC 22-2044).
Notes: Pleurocordyceps sanduensis (holotype: GZLG 23-104) is sister to P. clavisynnema (holotype: GZLG 23-102) with maximum statistical support (100% ML/1.00 PP) (Figure 1). Pleurocordyceps sanduensis is isolated from the same host as P. clavisynnema. However, the two are distinct in terms of both morphology and phylogeny. Base pair differences between P. clavisynnema and P. sanduensis are 23/824 in tef-1α, 8/1130 in SSU, 2/678 in rpb1, and 3/1050 in rpb2. Morphologically, Pleurocordyceps sanduensis differs from P. clavisynnema in shorter synnemata, smaller conidiophore, larger phialides, and longer conidia. Hence, this study introduces P. clavisynnema as a new species based on morphological and phylogenetic analyses.
Discussion
Herein, we describe four new species of Pleurocordyceps (P. clavisynnema, P. multisynnema, P. neoagarica, and P. sanduensis) using a combination of morphology and phylogeny. The newly established species group distinctly form independent clades in the phylogenetic tree (Figure 1). Morphologically, three of the new species (P. clavisynnema, P. multisynnema, and P. sanduensis) are similar to P. aurantiacus, P. agarica, P. heilongtanensis, P. lanceolatus, P. marginaliradians, P. nutansis, P. sinensis, P. vitellina, and P. yunnanensis in that they have two types of phialides and conidia. However, the hosts on which P. clavisynnema, P. multisynnema, and P. sanduensis parasitize differ from those of other species of Pleurocordyceps (Wang et al., 2012, 2015a,b; Xiao et al., 2018, 2023). Meanwhile, P. neoagarica is similar to P. lianzhouensis and P. parvicapitata in that it has one type of phialides and conidia (Wang et al., 2014; Xiao et al., 2023). However, P. neoagarica differs from P. lianzhouensis and P. parvicapitata as it parasitizes different hosts and produces longer phialides and smaller conidia (Wang et al., 2014; Xiao et al., 2023).
The discovery of the new species of Pleurocordyceps adds to the diversity of the genus and the associated family. Several Pleurocordyceps taxa have been found in China, indicating a high diversity of these organisms in the country. Pleurocordyceps species display variable host specialization (Wang et al., 2012; Xiao et al., 2023). A few are host-specific. Herein, Pleurocordyceps clavisynnema, P. neoagarica, and P. sanduensis were isolated from the same host, Ophiocordyceps neogryllotalpae. This is similar to the previous results, whereby P. nutansis and P. yunnanensis are parasitic on the same fungus, Ophiocordyceps nutans (Wang et al., 2015a; Xiao et al., 2023). Most Pleurocordyceps taxa are not host-specific, and multiple species have been documented in the same host (Bischoff et al., 2003; Wang et al., 2012, 2015a,b; Matočec et al., 2014; Crous et al., 2017; Xiao et al., 2018). Members of the genus parasitize insects and fungi, several species of which have broad geographic distributions possibly reflecting the diversity of Pleurocordyceps habitats. Future studies should focus on collecting additional Polycephalomycetaceae taxa to not only uncover the full extent of diversity of this family but also understand their distribution in relation to their hosts.
Data availability statement
The data presented in the study are deposited in the Guizhou Institute of Technology herbarium, accession number GZLG 23-102, GZCC 22-2042, GZLG 23-101, GZCC 22-2041, GZLG 23-103, GZCC 22-2043, GZLG 23-104, and GZCC 22-2044.
Author contributions
Y-PX: Writing – original draft. YY: Writing – original draft. RJ: Writing – review & editing. EG: Writing – review & editing. X-CP: Formal analysis, Writing – review & editing. Z-LL: Writing – review & editing. Y-ZL: Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Guizhou Provincial Key Technology R&D Program [grant no. Qian Ke He Zhi Cheng (2021) Generally 200], Guizhou Province high-level talent innovation and entrepreneurship merit funding project (no. 202104), and Youth Science and Technology Talent Development Project from Guizhou Provincial Department of Education (QJHKYZ[2022]345).
Acknowledgments
YY would like to thank the Mushroom Research Foundation, Chiang Rai, Thailand for supporting this research. The authors also thank Dr. Shaun Pennycook (Landcare Research Manaaki Whenua, New Zealand) for advising on the fungal names.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Acuña Jiménez, M., García Gutiérrez, C., Rosas García, N. M., López Meyer, M., and Saínz Hernández, J. C. (2015). Formulation of Metarhizium anisopliae (Metschnikoff) Sorokin with biodegradable polymers and their virulence against Heliothis virescens (Fabricius). Rev. Int. Contam. Ambie. 31, 219–226.
Ban, S., Sakane, T., Toyama, K., and Nakagiri, A. (2009). Teleomorph-anamorph relationships and reclassification of Cordyceps cuboidea and its allied species. Mycoscience 50, 261–272. doi: 10.1007/S10267-008-0480-Y
Bischoff, J. F., Sullivan, R. F., Struwe, L., Hywel-Jones, N. L., and White, J. F. (2003). Resurrection of Blistum tomentosum and its exclusion from Polycephalomyces (Hyphomycetes, Deuteromycota) based on 28S rDNA sequence data. Mycotaxon 86, 433–444.
Capella-Gutiérrez, S., Silla-Martínez, J. M., and Gabaldón, T. (2009). TrimAl: a tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 25, 1972–1973. doi: 10.1093/bioinformatics/btp348
Castlebury, L. A., Rossman, A. Y., Sung, G. H., Hyten, A. S., and Spatafora, J. W. (2004). Multigene phylogeny reveals new lineage for Stachybotrys chartarum, the indoor air fungus. Mycol Res. 108, 864–872. doi: 10.1017/S0953756204000607
Chaverri, P., Bischoff, J. F., Evans, H. C., and Hodge, K. T. (2005). Regiocrella, a new entomopathogenic genus with a pycnidial anamorph and its phylogenetic placement in the Clavicipitaceae. Mycologia 97, 1225–1237. doi: 10.1080/15572536.2006.11832732
Chen, Q. T., Xiao, S. R., and Shi, Z. Y. (1984). Paecilomyces sinensis sp. nov. and its connection with Cordyceps sinensis. Acta Mycol. Sin. 12, 24–28.
Chethana, K. W. T., Manawasinghe, I. S., Hurdeal, V. G., Bhunjun, C. S., Appadoo, M. A., Gentekaki, E., et al. (2021). What are fungal species and how to delineate them? Fungal Divers. 109, 1–25. doi: 10.1007/s13225-021-00483-9
Clewley, J. P. (1995). Macintosh sequence analysis software. DNAStar's LaserGene. Mol. Biotechnol. 3, 221–224. doi: 10.1007/BF02789332
Crous, P. W., Wingfield, M. J., Burgess, T. I., Carnegie, A. J., Hardy, G., et al. (2017). Fungal Planet description sheets: 625-715. Persoonia 39, 270–467. doi: 10.3767/persoonia.2017.39.11
Dissanayake, A. J., Bhunjun, C. S., Maharachchikumbura, S. S. N., and Liu, J. K. (2020). Applied aspects of methods to infer phylogenetic relationships amongst fungi. Mycosphere 11, 2652–2676. doi: 10.5943/mycosphere/11/1/18
Dong, Q. Y., Wang, Y., Wang, Z. Q., Tang, D. X., Zhao, Z. Y., et al. (2022). Morphology and phylogeny reveal five novel species in the genus Cordyceps (Cordycipitaceae, Hypocreales) from Yunnan, China. Front. Microbiol. 13, 846909. doi: 10.3389/fmicb.2022.846909
Eiamthaworn, K., Kaewkod, T., Bovonsombut, S., and Tragoolpua, Y. (2022). Efficacy of Cordyceps militaris extracts against some skin pathogenic bacteria and antioxidant activity. J. Fungi 8, 327. doi: 10.3390/jof8040327
Fernández-Grandon, G. M., Harte, S. J., Ewany, J., Bray, D., and Stevenson, P. C. (2020). Additive effect of botanical insecticide and entomopathogenic fungi on pest mortality and the behavioral response of its natural enemy. Plants 9, 173. doi: 10.3390/plants9020173
Gokhale, M., Raj, D., and Deshpande, I. (2020). Antibacterial and antioxidant activity of biomolecules extracted from Paecilomyces sinensis – an endophyte of Oroxylum indicum (L.) Vent. J. Mycol. Plant Pathol. 50, 57–66.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic. Acids. Symp. Ser. 41, 95–98.
Hopple, J. S., and Vilgalys, R. (1999). Phylogenetic relationships in the mushroom genus Coprinus and dark-spored allies based on sequence data from the nuclear gene coding for the large ribosomal subunit RNA: divergent domains, outgroups, and monophyly. Mol. Phylogenetic. Evol. 13, 1–19. doi: 10.1006/mpev.1999.0634
Huang, S. K., Hyde, K. D., Maharachchikumbura, S. S. N., McKenzie, E. H. C., and Wen, T. C. (2021). Taxonomic studies of Coronophorales and Niessliaceae (Hypocreomycetidae). Mycosphere 12, 875–992. doi: 10.5943/mycosphere/12/1/9
Hyde, K. D., Norphanphoun, C., Maharachchikumbura, S. S. N., Bhat, D. J., Jones, E. B. G., Bundhun, D., et al. (2020). Refined families of Sordariomycetes. Mycosphere 11, 305–1059. doi: 10.5943/mycosphere/11/1/7
Jayasiri, S. C., Hyde, K. D., Ariyawansa, H. A., Bhat, J., Buyck, B., Cai, L., et al. (2015). The faces of fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers. 74, 3–18. doi: 10.1007/s13225-015-0351-8
Jayawardena, R. S., Hyde, K. D., de Farias, A. R. G., Bhunjun, C. S., Ferdinandez, H. S., Manamgoda, D. S., et al. (2021). What is a species in fungal plant pathogens? Fungal Divers. 109, 239–266. doi: 10.1007/s13225-021-00484-8
Kalyaanamoorthy, S., Minh, B. Q., Wong, T. K. F., von Haeseler, A., and Jermiin, L. S. (2017). ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589. doi: 10.1038/nmeth.4285
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. E30, 772–780. doi: 10.1093/molbev/mst010
Kepler, R. M., Ban, S., Nakagiri, A., Bischoff, J., Hywel-Jones, N., Owensby, C. A., et al. (2013). The phylogenetic placement of hypocrealean insect pathogens in the genus Polycephalomyces: an application of one fungus one name. Fungal Biol. 117, 611–622. doi: 10.1016/j.funbio.2013.06.002
Kepler, R. M., Sung, G. H., Harada, Y., Tanaka, K., Tanaka, E., Hosoya, T., et al. (2012). Host jumping onto close relatives and across kingdoms by Tyrannicordyceps (Clavicipitaceae) gen. nov. and Ustilaginoidea (Clavicipitaceae). Am. J. Bot. 99, 552–561. doi: 10.3732/ajb.1100124
Kobayasi, Y. (1939). On the genus Cordyceps and its allies on cicadae from Japan. Bullet. Biogeograph. Soc. Japan 9, 145–176.
Kobayasi, Y., and Shimizu, D. (1982). Cordyceps species from Japan. 4. Bull. Natl. Sci. Mus. 8, 79–91.
Li, L. Q., Song, A. X., Yin, J. Y., Siu, K. C., Wong, W. T, and Wu, J.Y. (2020). Antiinflammation activity of exopolysaccharidesproduced by amedicinal fungus Cordycep sinensis Cs-HK1in cell andanimal models. Int. J. Biol.Macromol. 149, 1042–1050. doi: 10.1016/j.ijbiomac.2020.02.022
Liang, Z. Q., Chen, W. H., Liang, J. D., Han, Y. F., and Zou, X. (2016). Phenotypic polymorphism of the synnematous entomogenous fungi in an ant nest of Ponera I. Mycosystema 35, 906–917. doi: 10.13346/j.mycosystema.150069
Maharachchikumbura, S. S. N., Chen, Y., Ariyawansa, H. A., Hyde, K. D., Haelewaters, D., Perera, R. H., et al. (2021). Integrative approaches for species delimitation in Ascomycota. Fungal Diver. 109, 155–179. doi: 10.1007/s13225-021-00486-6
Maharachchikumbura, S. S. N., Hyde, K. D., Jones, E. B. G., McKenzie, E. H. C., Bhat, J. D., Dayarathne, M. C., et al. (2016). Families of Sordariomycetes. Fungal Diver. 79, 1–317. doi: 10.1007/s13225-016-0369-6
Matočec, N., Kušan, I., and Ozimec, R. (2014). The genus Polycephalomyces (Hypocreales) in the frame of monitoring veternica cave (Croatia) with a new segregate genus Perennicordyceps. Ascomycete 5, 125–133.
Minh, B. Q., Schmidt, H. A., Chernomor, O., Schrempf, D., Woodhams, M. D., Von Haeseler, A., et al. (2020). IQ-TREE 2: new models and efficient methodsfor phylogenetic inference in the genomic era. Mol. Biol. E 37, 1530–1534. doi: 10.1093/molbev/msaa015
Nikoh, N., and Fukatsu, T. (2000). Interkingdom host jumping underground: phylogenetic analysis of entomoparasitic fungi of the genus cordyceps. Mol. Biol. Evol. 17, 629–638. doi: 10.1093/oxfordjournals.molbev.a026341
Poinar, G., and Vega, F. E. (2020). Entomopathogenic fungi (Hypocreales: Ophiocordycipitaceae) infecting bark lice (Psocoptera) in Dominican and Baltic amber. Mycology 11, 71–77. doi: 10.1080/21501203.2019.1706657
Quandt, C. A., Kepler, R. M., Gams, W., Araújo, J. P., Ban, S., Evans, H. C., et al. (2014). Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5, 121–134. doi: 10.5598/imafungus.2014.05.01.12
Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D. L., Darling, A., Höhna, S., et al. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inferenceand model choice across a large model space. Syst. Biol. 61, 539–542. doi: 10.1093/sysbio/sys029
Saltamachia, S. J., and Araujo, J. P. M. (2020). Ophiocordyceps desmidiospora, a basal lineage within the “Zombie-ant fungi” clade. Mycologia 112, 1171–1183. doi: 10.1080/00275514.2020.1732147
Sangdee, A., Sangdee, K., Seephonkai, P., Jaihan, P., and Kanyaphum, T. (2017). Colony characteristics, nucleoside analog profiles, and genetic variations of medicinal fungus Polycephalomyces nipponicus (Ascomycetes) isolates from northeast Thailand. Int. J. Med. Mushrooms 19, 445–455. doi: 10.1615/IntJMedMushrooms.v19.i5.60
Schoch, C. L., Seifert, K. A., Huhndorf, S., Robert, V., Spouge, J. L., Levesque, C. A., et al. (2012). Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. U.S.A. 109, 6241–6246. doi: 10.1073/pnas.1117018109
Seifert, K. A. (1985). A Monograph of Stilbella and some allied Hyphomycetes. Stud. Mycol. 27, 1–235.
Sharma, A., Srivastava, A., Shukla, A. K., Srivastava, K., Srivastava, A. K., and Saxena, A. K. (2020). “Entomopathogenic fungi: a potential source for biological control of insect pests,” in Phytobiomes: Current Insights and Future Vistas, eds M. Solanki, P. Kashyap, and B. Kumari (Singapore: Springer), 225–250.
Sobczak, J. F., Arruda, I. D. P., Fonseca, E. O., Rabelo, P. J. Q., de Sousa Nóbrega, F. A., Pires, J. C., et al. (2020). Manipulation of wasp (Hymenoptera: Vespidae) behavior by the entomopathogenic fungus Ophiocordyceps humbertii in the Atlantic forest in Ceará, Brazil1. Entomol. News 129, 98–104. doi: 10.3157/021.129.0115
Somsila, P., Sakee, U., Srifa, A., and Kanchanarach, W. (2018). Antioxidant and antimicrobial activities of Polycephalomyces nipponicus. J. Pure. Appl. Microbiol 12, 567–576. doi: 10.22207/JPAM.12.2.15
Sonyot, W., Lamlertthon, S., Luangsa-Ard, J. J., Mongkolsamrit, S., Usuwanthim, K., Ingkaninan, K., et al. (2020). In vitro antibacterial and anti-inflammatory effects of novel insect fungus polycephalomyces phaothaiensis extract and its constituents against Propionibacterium acnes. Antibiotics 9, 274. doi: 10.3390/antibiotics9050274
Sun, J. Z., Liu, X. Z., McKenzie, E. H., Jeewon, R., Liu, J. K., Zhang, X. L., et al. (2019). Fungicolous fungi: terminology, diversity, distribution, evolution, and species checklist. Fungal Div. 95, 337–430. doi: 10.1007/s13225-019-00422-9
Sung, G. H., Hywel-Jones, N., Sung, J. M., Luangsa-Ard, J. J., Shrestha, B., Spatafora, J., et al. (2007). Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol. 57, 5–59. doi: 10.3114/sim.2007.57.01
Tasanathai, K., Thanakitpipattana, D., Noisripoom, W., Khonsanit, A., Kumsao, J., Luangsa-Ard, J., et al. (2016). Two new Cordyceps species from a community forest in Thailand. Mycol. Prog. 15, 28. doi: 10.1007/s11557-016-1170-3
Van Vooren, N., and Audibert, C. (2005). Révision du complexe ≪Cordyceps sphecocephala≫. 1re partie: les guêpes végétales. Bull. Mens. Soc. Linn. Lyon 74, 221–254. doi: 10.3406/linly.2005.13604
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, L., Li, H. H., Chen, Y. Q., Zhang, W. M., and Qu, L. H. (2014). Polycephalomyces lianzhouensis sp nov., a new species, co-occurs with Ophiocordyceps crinalis. Mycol. Prog. 13, 1089–1096. doi: 10.1007/s11557-014-0996-9
Wang, W. J., Wang, X. L., Li, Y., Xiao, S. R., Kepler, R., Yao, Y. J., et al. (2012). Molecular and morphological studies of Paecilomyces sinensis reveal a new clade in clavicipitaceous fungi and its new systematic position. System. Biodivers. 10, 1–12. doi: 10.1080/14772000.2012.690784
Wang, Y. B., Yu, H., Dai, Y. D., Chen, Z. H., Zeng, W. B., Liang, Z. Q., et al. (2015a). Polycephalomyces yunnanensis (Hypocreales), a new species of Polycephalomyces parasitizing Ophiocordyceps nutans and stink bugs (hemipteran adults). Phytotaxa 208, 34–44. doi: 10.11646/phytotaxa.208.1.3
Wang, Y. B., Yu, H., Dai, Y. D., Wu, C. K., Zeng, W. B., Yuan, F., et al. (2015b). Polycephalomyces agarica, a new hyperparasite of Ophiocordyceps sp. infecting melolonthid larvae in southwestern China. Mycol. Prog. 14, 70. doi: 10.1007/s11557-015-1090-7
Wang, Y. H., Ban, S., Wang, W. J., Li, Y., Wang, K., Kirk, P. M., et al. (2021). Pleurocordyceps gen. nov. for a clade of fungi previously included in Polycephalomyces based on molecular phylogeny and morphology. J. Syst. E 59, 1065–1080. doi: 10.1111/jse.12705
Wei, D. P., Gentekaki, E., Wanasinghe, D. N., Tang, S. M., and Hyde, K. D. (2022). Diversity, molecular dating and ancestral characters state reconstruction of entomopathogenic fungi in Hypocreales. Mycosphere 13, 281–351. doi: 10.5943/mycosphere/si/1f/8
Wei, D. P., Wanasinghe, D. N., Xu, J. C., To-Anun, C., Mortimer, P. E., Hyde, K. D., et al. (2020). Three novel entomopathogenic fungi from China and Thailand. Front. Microbiol. 11, 608991. doi: 10.3389/fmicb.2020.608991
White, T. J., Bruns, T., Lee, S., and Taylor, J. W. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wijayawardene, N. N., Hyde, K. D., Al-Ani, L. K. T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K. C., et al. (2020). Outline of fungi and fungus-like taxa. Mycosphere 11, 1160–1456. doi: 10.5943/mycosphere/11/1/8
Wijayawardene, N. N., Hyde, K. D., Lumbsch, H. T., Liu, J. K., Maharachchikumbura, S. S. N., Ekanayaka, A. H., et al. (2018). Outline of Ascomycota: 2017. Fungal Diver. 88, 167–263. doi: 10.1007/s13225-018-0394-8
Xiao, Y. P., Wen, T. C., Hongsanan, S., Jeewon, R., Luangsa-Ard, J. J., et al. (2018). Multigene phylogenetics of Polycephalomyces (Ophiocordycipitaceae, Hypocreales), with two new species from Thailand. Sci. Rep. 8, 18087. doi: 10.1038/s41598-018-36792-4
Xiao, Y. P., Wen, T. C., Hongsanan, S., Jeewon, R., Luangsa-Ard, J. J., Brooks, S., et al. (2023). Polycephalomycetaceae, a new family of clavicipitoid fungi segregates from Ophiocordycipitaceae. Fungal Diver. 120, 1–76. doi: 10.1007/s13225-023-00517-4
Yang, J. I., Stadler, M., Chuang, W. Y., Wu, S., and Ariyawansa, H. A. (2020). In vitro inferred interactions of selected entomopathogenic fungi from Taiwan and eggs of Meloidogyne graminicola. Mycol. Prog. 19, 97–109. doi: 10.1007/s11557-019-01546-7
Yang, Y., Xiao, Y., Yu, G., Wen, T., Deng, C., Meng, J., et al. (2021). Ophiocordyceps aphrophoridarum sp. nov., a new entomopathogenic species from Guizhou, China. Biodivers. Data J. 9, e66115. doi: 10.3897/BDJ.9.e66115
Zha, L. S., Kryukov, V. Y., Ding, J. H., Jeewon, R., and Chomnunti, P. (2021). Novel taxa and species diversity of Cordyceps sensu lato (Hypocreales, Ascomycota) developing on wireworms (Elateroidea and Tenebrionoidea, Coleoptera). MycoKeys 78, 79–117. doi: 10.3897/mycokeys.78.61836
Zhang, W. M., Wang, L., Tao, M. H., Chen, Y. Q., and Qu, L. H. (2007). Two species of Cordyceps simultaneously parasitic on a larva of Lepidoptera. Mycosystema 26, 7–21.
Keywords: entomopathogenic fungi, morphology, Polycephalomycetaceae, phylogeny, taxonomy
Citation: Xiao Y-P, Yang Y, Jayawardena RS, Gentekaki E, Peng X-C, Luo Z-L and Lu Y-Z (2024) Four novel Pleurocordyceps (Polycephalomycetaceae) species from China. Front. Microbiol. 14:1256967. doi: 10.3389/fmicb.2023.1256967
Received: 12 July 2023; Accepted: 08 December 2023;
Published: 10 January 2024.
Edited by:
Zhou Shi, Gladstone Institutes, United StatesReviewed by:
Samantha Chandranath Karunarathna, Qujing Normal University, ChinaYang Liu, Dana–Farber Cancer Institute, United States
Copyright © 2024 Xiao, Yang, Jayawardena, Gentekaki, Peng, Luo and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong-Zhong Lu, eXpsdUBnaXQuZWR1LmNu
†These authors have contributed equally to this work
 Yuan-Pin Xiao
Yuan-Pin Xiao Yu Yang
Yu Yang Ruvishika S. Jayawardena2,3
Ruvishika S. Jayawardena2,3 Eleni Gentekaki
Eleni Gentekaki Zong-Long Luo
Zong-Long Luo