- 1Department of Microbiology, School of Basic Sciences, Islamic Azad University Science and Research Branch, Tehran, Iran
- 2Nervous System Stem Cells Research Center, Semnan University of Medical Sciences, Semnan, Iran
- 3Cellular and Molecular Research Center, Qom University of Medical Sciences, Qom, Iran
- 4Department of Medical Microbiology, Faculty of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran
- 5Student Research Committee, USERN Office, Lorestan University of Medical Sciences, Khorramabad, Iran
- 6Department of Microbiology, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran
- 7Department of Immunology, Faculty of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 8Department of Medical Microbiology, School of Medicine, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
- 9Department of Medical Microbiology, School of Medicine, Iran University of Medical Sciences, Tehran, Iran
- 10Department of Medical Microbiology, Faculty of Medicine, Shahed University, Tehran, Iran
Mesenchymal stromal cells, commonly referred to as MSCs, are a type of multipotent stem cells that are typically extracted from adipose tissue and bone marrow. In the field of tissue engineering and regenerative medicine, MSCs and their exosomes have emerged as revolutionary tools. Researchers are now devoting greater attention to MSCs because of their ability to generate skin cells like fibroblasts and keratinocytes, as well as their distinctive potential to decrease inflammation and emit pro-angiogenic molecules at the site of wounds. More recent investigations revealed that MSCs can exert numerous direct and indirect antimicrobial effects that are immunologically mediated. Collectively, these antimicrobial properties can remove bacterial infections when the MSCs are delivered in a therapeutic setting. Regardless of the positive therapeutic potential of MSCs for a multitude of conditions, transplanted MSC cell retention continues to be a major challenge. Since MSCs are typically administered into naturally hypoxic tissues, understanding the impact of hypoxia on the functioning of MSCs is crucial. Hypoxia has been postulated to be among the factors determining the differentiation of MSCs, resulting in the production of inflammatory cytokines throughout the process of tissue regeneration and wound repair. This has opened new horizons in developing MSC-based systems as a potent therapeutic tool in oxygen-deprived regions, including anaerobic wound infection sites. This review sheds light on the role of hypoxia-MSCs in the treatment of anaerobic bacterial wound infection in terms of both their regenerative and antimicrobial activities.
Introduction
Mesenchymal stem cells (MSCs) are described as stromal cells that possess multipotent, immune-regulatory, and regenerative characteristics. MSCs may originate from bone marrow (BM), adipose tissue, skeletal muscle, dental pulp, and amniotic fluid (Xu et al., 2019; Mahjoor et al., 2021; Nowak-Stępniowska et al., 2022). As a result of the possible therapeutic qualities they offer, MSCs have lately attracted a lot of interest and are increasingly being employed as a therapeutic option for a broad variety of inflammatory immune system disorders (Mahjoor et al., 2023; Wang et al., 2023). In particular, they have emerged as a valuable approach for promoting the healing process of skin wounds (Pulido-Escribano et al., 2023). MSCs have the potential to transform into skin substitutes, thereby acting as a viable alternative to dermal fibroblasts in the process of epidermis generation and skin wound healing. They are capable of differentiating into several cell types in injured areas, including dermal fibroblasts, keratinocytes, and endothelial cells (Hermann et al., 2023; Mahmoudvand et al., 2023; Nilforoushzadeh et al., 2023). Apart from their differential ability, MSCs have additional characteristics, such as being easily harvested and showing minimal immunogenicity. These characteristics, together with their inevitable involvement in the physiology of wound repair, present MSCs as a safe and practical therapeutic method. At the site of injury, MSCs promote the migration of cutaneous cells, angiogenesis, and re-epithelialization, as well as the establishment of granulation tissue, all of which facilitate the process of wound healing (Azari et al., 2022; Bian et al., 2022). The latest evidence confirmed that MSCs can hinder the growth of microorganisms. To provide their profound antibacterial properties, these cells take advantage of both direct and indirect signaling pathways. Directly by constitutively secreted factors and Indirect pathways form by activating the host’s innate immune cells. MSCs can also produce antimicrobial peptides (Leroux et al., 2010). AMPs annihilate bacteria directly by disrupting the integrity of their membrane, or alternatively by inducing the release of proinflammatory cytokines (Leroux et al., 2010; Mahlapuu et al., 2016). A variety of AMPs are released by MSCs, including the cathelicidin peptide LL-37 (Krasnodembskaya et al., 2010), hepcidin (Alcayaga-Miranda et al., 2015), β-defensin 2, and lipocalin 2 (Gupta et al., 2012). These AMPs are regarded as a vital regulator of the capacity of MSCs administered therapeutically to eliminate bacterial infections. MSCs can directly influence the immunological properties of neutrophils and macrophages by secreting PGE2 (Vasandan et al., 2016) IL-6, IL-8, and IFN-β, among other factors (Maqbool et al., 2011). Following the exposure to MSC-secreted factors, macrophages acquire an enhanced capacity of phagocytosis, mediated in part by NADPH oxidase activation (Rabani et al., 2018). Neutrophils exposed to MSC conditioned medium are resistant to apoptosis and exhibit a propagated ability of migration (Raffaghello et al., 2008). Studies in animal models of infection have shown that MSCs can increase monocyte recruitment and decrease excessive neutrophil influx as well as neutrophil elastase generation, particularly in mouse models of pulmonary Pseudomonas aeruginosa infection and cystic fibrosis (Sutton et al., 2017). Indirect mechanisms involve the recruitment of immune cells and stimulation of macrophages (Chauhan et al., 2023). Macrophages as major parts of the immune system, are implicated in bacterial autophagy and tissue repair (Wang et al., 2023). Under different circumstances, they can develop the anti-inflammatory M2 or the pro-inflammatory M1 phenotypes. Evidence points to the induction of the M2 phenotype by activated allogeneic murine MSCs in infected tissues, while untreated infected areas possess an M1 dominant population. The ability of MSCs to induce the M1 macrophage phenotype is implicated in their anti-bacterial properties. M2 macrophages are assumed to ameliorate the process of wound healing, confirmed by the improved physical and histological appearance of the group that received activated MSCs compared to the other groups (Johnson et al., 2017). MSCs may also propagate alveolar macrophage phagocytosis as shown in a recent in-vivo study (Morrison et al., 2017). In a similar way, MSCs promote the recruitment of neutrophils and increase their inflammatory responses in the early stages of bacterial challenge (Brandau et al., 2010). MSCs have emerged as appealing mediators in the treatment of wound infections as a result of these features (Mirshekar et al., 2023).
Notwithstanding the therapeutic advantages of MSCs for wound healing, the cell retention of MSCs after transplantation continues to be exceedingly challenging. MSCs naturally inhabit BM, which has a hypoxic habitat. In addition, the therapeutic delivery of MSCs is often performed in tissues that are hypoxic under normal circumstances. Therefore, several investigations on in-vitro cell cultures and subsequent therapeutic applications proposed the cultivation of MSCs under hypoxic conditions (1–10% oxygen; Beegle et al., 2015; Ejtehadifar et al., 2015). Hypoxia is an important factor in the coordination of cell functions, notably controlling the generation of stem cells (Li et al., 2021). Hypoxia-inducing factors (HIFs), which are highly expressed in the presence of diminished oxygen levels, exert different effects on cellular contexts by influencing diverse components of cell biology. Akin to other cell types, activation of HIF-1 elicits a multifaceted response in MSCs within their microenvironment, including alterations in growth, proliferation, differentiation, and gene expression patterns. These effects are mediated by a network of signaling pathways, including Notch and Oct4 (Keith and Simon, 2007; Ejtehadifar et al., 2015).
The normal regenerative process of stem cells is crucial for the replacement of compromised or aging cells with differentiated cells, assisting the proper functioning of various body tissues. Nevertheless, the complete capabilities of these cells have yet to be thoroughly investigated. Several experiments have been conducted to enhance the efficacy of MSCs in promoting therapeutic benefits. Various techniques, including the implementation of hypoxic environments and the isolation of exosomes, were explored. According to a study, MSCs may exhibit resistance to oxygen limitation. Furthermore, hypoxia has the potential to trigger a multitude of stress and survival signaling pathways in MSCs. The research conducted revealed that subjecting MSCs to a hypoxic setting can trigger pathways associated with cellular survival, such as glucose and glutamine metabolism pathways, as well as pathways related to differentiation, growth, and migration (Leroux et al., 2010; Ahmed et al., 2016; Antebi et al., 2018; Lin et al., 2021; Yusoff et al., 2022).
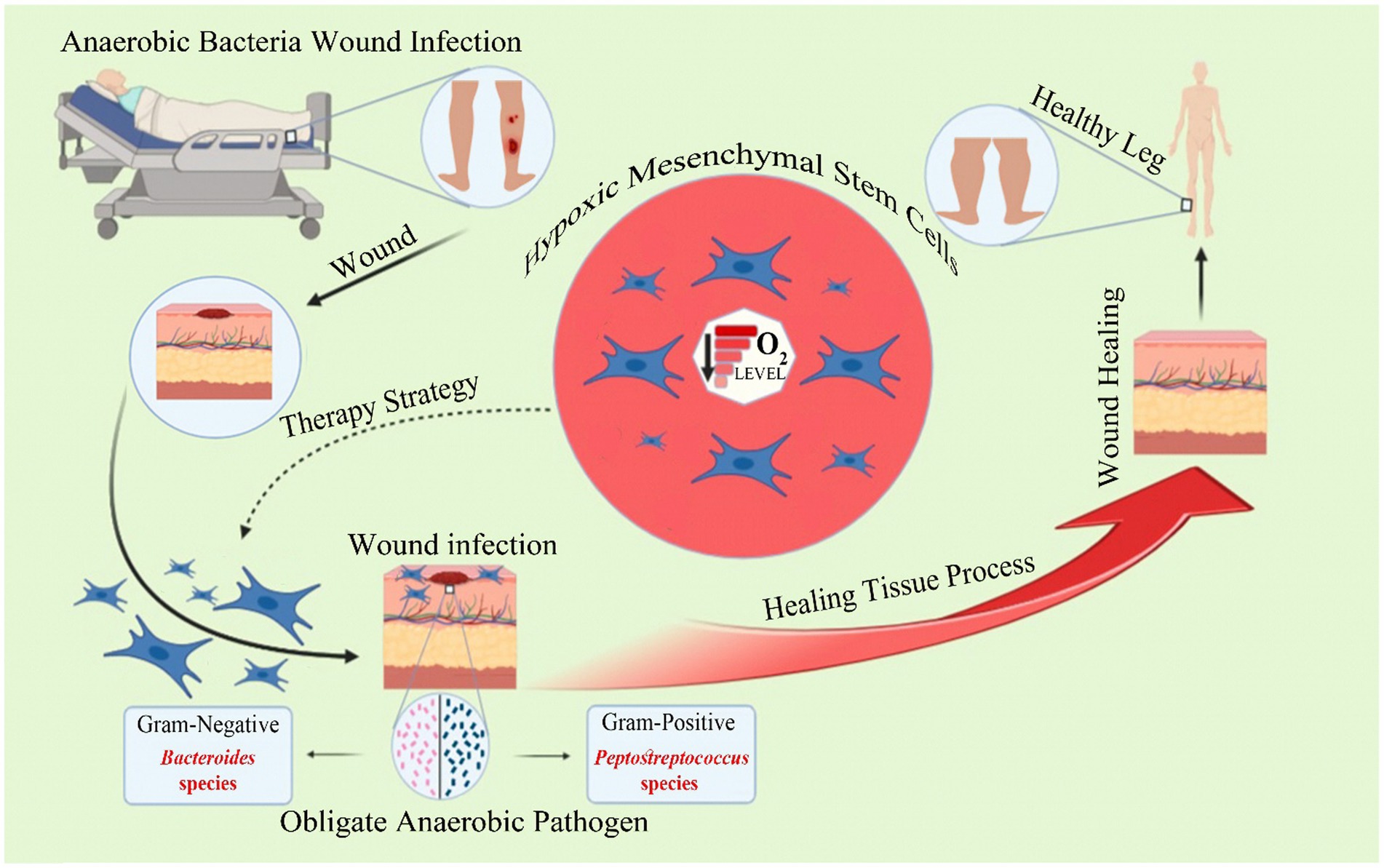
This paper presents a review of the utilization of hypoxia-MSCs (hi-MSCs) for the treatment of anaerobic bacterial wound infections, focusing on both regenerative and antimicrobial mechanisms.
Anaerobic bacteria
Anaerobic microorganisms are the primary constituents of the indigenous bacterial flora of human mucous membranes and skin and are commonly implicated in endogenous microbial infections (SaiKiran et al., 2022). Anaerobes are challenging to isolate and frequently go unnoticed due to their fastidious nature. Anaerobic microorganisms are frequently detected in mixed infections of both aerobic and anaerobic nature. After the establishment of anaerobic species, there appears to be a phagocytosis obstruction to prevent the decomposition of coexisting microorganisms. Moreover, the transfer of nutrients from one bacterium tends to support the development and spread of another (Negut et al., 2018; Eberly et al., 2022). Infections caused by anaerobic bacteria can arise in sterile areas of the body and pose a significant risk to an individual’s health and well-being (Tjampakasari et al., 2022). Anaerobic infections might appear across many anatomical regions of the body, including but not limited to the central nervous system, oral cavity, chest, abdomen, pelvis, soft tissues, and cutaneous tissue (Brook, 2016).
In regards to wound infections, the existing literature primarily concentrates on facultative or aerobic organisms that are linked to the wound bacterial flora. In contrast, there is limited research that has examined the involvement of anaerobes in chronic wounds (Cheong et al., 2022). The cause of this phenomenon could be attributed to the fact that in the majority of research studies, wounds have been observed over a limited duration, during which anaerobic microorganisms tend to exhibit a slower rate of proliferation compared to their aerobic counterparts (Verbanic et al., 2020). The part played by anaerobic bacteria in wound infection sites is intricate and differs from that of aerobic pathogens. Prolonged surface bacterial colonization may result in venous insufficiency and subsequent colonization of deep tissue layers by anaerobic microorganisms. The probable cause of this condition is the lower oxygen levels in the underlying tissue, which creates a favorable environment for the proliferation of both facultative and obligate anaerobic microorganisms (Finegold, 1993).
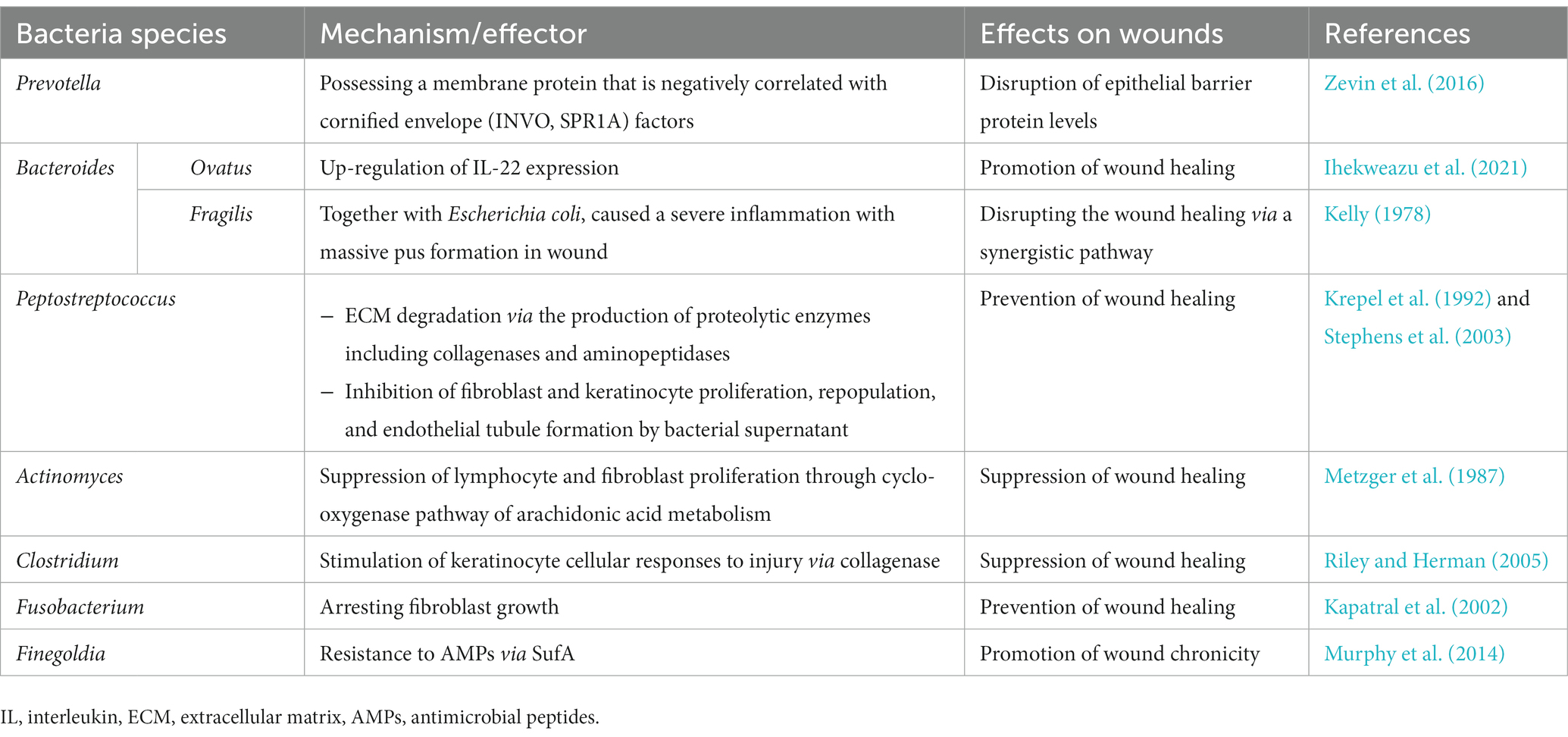
The predominant anaerobic bacterial species identified in chronic wounds are Actinomyces species, Bacteroides species, and Clostridium species. In addition, Peptostreptococcus species, Fusobacterium species, Finegoldia species, Prevotella species, and Porphyromonas species can contribute to wound infection. These organisms usually emerge on the 15th day of the infection process and subsequently exhibit a decline in frequency as time progresses (Nahid et al., 2021). Although particular anaerobic bacteria have been acknowledged for their positive contribution to wound repair, various species have been found to impede the wound-healing process. This is primarily exerted by the production of proteolytic enzymes, stimulation of immune factor release, or suppression of fibroblast and keratinocyte growth (Lindsay et al., 2017; Luqman and Götz, 2021). Table 1 lists anaerobic bacteria that have been found to affect the wound-healing process.
Mesenchymal stem cells
The BM stroma exhibits a structured arrangement of diverse cell types, encompassing stem cells, endothelial cells, adipocytes, fibroblasts, and osteocytes, among others. The two distinct categories of BM stem cells are hematopoietic stem cells and MSCs. Mesenchymal cells can preserve, repair, and restore impaired tissues (Xu et al., 2019). MSCs possess a unique capacity for self-renewing and differentiation. These cells might be extracted from various sources such as the umbilical cord, BM, adipose tissue, endometrial polyps, and menstrual blood (Ding et al., 2011).
MSCs are characterized by the presence of specific markers, including a cluster of differentiation (Vusirikala et al., 2022)73, CD90, and CD105, whereas they lack proteins such as CD11b, CD14, CD34, CD45, and CD79a (Andrzejewska et al., 2019). MSCs are capable of generating an abundance of cytokines, including those that facilitate the maintenance of hematopoietic stem cells in their silent phase or promote their self-renewal, such as oncostatin (OSM), stem cell factor (SCF), leukemia inhibitory factor (LIF; Ratcliffe, 2013), stromal cell-derived factor1 (SDF-1), transforming growth factor beta (TGF-β), bone morphogenetic protein4 (BMP-4), and Fms related receptor tyrosine kinase3 (FLT-3). Besides, MSCs release interleukins (IL)-1, 1L-6, IL-7, IL-8, IL-11, IL-12, IL-14, IL-15 (Dazzi et al., 2006).
MSCs generate an extensive list of growth factors, chemokines, and hormones that are involved in various biological processes such as blood vessel formation, immune regulation, and anti-apoptotic functions. MSCs have been observed to augment the process of tissue reconstruction via paracrine mechanisms after their transplantation. The protein known as versican plays an integral part in the mechanism of repair. The utilization of MSCs conditioned medium in the cultivation of monocytes can enhance the synthesis of versican protein and may serve as an acceptable substitute for the transplantation of MSCs (Brennan et al., 2020).
What is a hypoxia condition?
Adenosine 5’triphosphate is synthesized by mammalian cells using the utilization of oxygen and nutrients. The significance of oxygen in numerous biochemical reactions necessitates the preservation the maintenance of oxygen balance. In the presence of hypoxia, cells initiate multiple subsequent cascades, including autophagy, cell stress pathways (e.g., endoplasmic reticulum stress), and energy metabolic pathways [e.g., mTOR complex1 (mTORC1) and HIF-1]. The aforementioned pathways are responsible for the maintenance of homeostasis during periods of hypoxic stress (Ratcliffe, 2013; Nakazawa et al., 2016; Tirpe et al., 2019; Lee et al., 2020). HIFs are a group of heterodimeric factors, comprising HIF-1, HIF-2, and HIF-3 (Tirpe et al., 2019). The mentioned factors consist of a stable β-subunit and an oxygen-sensitive α-subunit. Under normoxic conditions, the separation of the heterodimer results in the hydroxylation of proline residues (proline-402 and proline-564) in the α subunit by prolyl hydroxylases (PHDs; Eales et al., 2016; Dabral et al., 2019), then HIF-1α is ubiquitinated by von-Hippel-Lindau protein (pVHL) enzyme and degraded by the proteasome (Tirpe et al., 2019).
HIFs regulate the transcriptional activation of a multitude of genes that participate in biological processes, including but not confined to cellular growth and proliferation, programmed cell death, cellular metabolism, glycolysis, bacterial infection, and immune system response, as well as tumor formation and spread (Luo et al., 2022).
The normal oxygen concentration in arterial blood is approximately 12%, while its concentration in tissues is 3%. Embryonic stem cells are known to exist under hypoxic conditions, from the point of implantation to fetal growth. In general, during the initial stages of pregnancy, the level of oxygen concentration on the surface of the uterus is in proximity to 2%. Following the development of the placenta, there is a notable elevation in the concentration of oxygen to approximately 8%. Adult stem cells also reside in hypoxic environments in their natural setting. The available evidence indicates that hematopoietic stem cells and BM-derived MSCs (BM-MSCs) coexist within a shared environment (Abdollahi et al., 2011).
Mesenchymal cells can exhibit varying responses to differing concentrations of oxygen, contingent upon their specific microenvironment. For example, the oxygen pressure levels in BM range from 1 to 7%, while in umbilical cord blood and amniotic fluid, they range from 1.5 to 8%. Adipose tissue, on the other hand, exhibits oxygen pressure levels of 10–15%. Under normoxic conditions, it has been predicted that a greater quantity of free radicals is generated, thereby causing interference with the operation of mesenchymal cells. The colony-forming capacity and proliferation rate of MSCs display an augmentation under hypoxic conditions with low oxygen pressure ranging from 1 to 5% (Samal et al., 2021).
Effects of MSCs on the homeostasis phase of wound healing
Many research projects present evidence that MSCs can boost the coagulation process. It has been revealed that a correlation exists between procoagulant activities and the expression of tissue factors (Mansori et al., 2020), a transmembrane protein that upregulates the production of the thrombin-antithrombin complex (Rangasami et al., 2021). It is noteworthy that extended culture of MSCs results in a boosted expression of TF on the surface of these cells. This may potentially heighten the likelihood of thrombosis (Silachev et al., 2019).
Besides TFs, phosphatidylserine is a pro-coagulation factor that facilitates the synthesis of the thrombin-activating complex by translocating from the inner to the outer layer of the cell membrane. It may be postulated that extracellular vesicles (EVs) obtained from an MSCs-conditioned medium may harbor TF and/or external phosphatidylserine, thereby exerting an influence on blood hemostasis (Silachev et al., 2019). Moreover, the expression of Annexin V on the cellular membrane conduces to the buildup of phosphatidylserine and enhances the process of coagulation (Chance et al., 2019).
MSCs amend the inflammatory phase
MSCs present at the site of injury determine the immune response of macrophages, neutrophils, and lymphocytes through the secretion of various factors and cytokines. Among these are chemokines (C-C motif) ligand 2 (CCL-2), vascular endothelial growth factor (VEGF), LIF, IL-10, and hepatocyte growth factor (HGF), all of which possess immunosuppressive and regenerative properties. As a result, MSCs enhance the immune system’s defenses against potential infections in wounded tissues (Zhang et al., 2010; Hoang et al., 2020; Silva et al., 2020; Camões et al., 2022).
Besides, MSCs can induce a shift in macrophage polarization from a pro-inflammatory M1 state to an anti-inflammatory M2 state (Ulivi et al., 2014). MSCs have been noted to impede the generation of inflammatory agents from M1 macrophage, including tumor necrosis factor (TNF)-α, while concurrently elevating the levels of TGF-β1 from myofibroblasts (Jiang et al., 2013).
Likewise, MSCs regulate the equilibrium of T helper (Th)1-Th2 cytokines, inducing the synthesis of anti-inflammatory cytokines, among them IL4, while reducing the secretion of the pro-inflammatory interferon-gamma (IFN-γ; Zanone et al., 2010). Finally, MSCs exhibit a suppressive impact on the activity and cytotoxicity of natural killer (NK) cells. The inquiry into the subject reveals that the presence of MSCs results in major alterations in both ligands and receptors that have been proven to facilitate NK cell interactions, along with a reduction in the number of NK cells (Najar et al., 2019).
Effects of MSCs on the proliferative phase
During the third stage of wound healing, fibroblasts and myofibroblasts play a pivotal role. At this stage, epithelial cells undergo proliferation and restoration, and collagen and other ECM proteins are synthesized. MSCs have been noticed to encourage the growth, migration, and secretion of fibroblasts primarily through the action of platelet-derived growth factor BB (Liu et al., 2022). The utilization of EVs derived from MSCs in a mouse skin burn model resulted in an augmentation of epithelial cell proliferation, thereby promoting wound healing (Zhang et al., 2015; Yates et al., 2017).
MSCs enhance tissue repair by regulating the release of effector T-cell cytokines and switching macrophage polarization to an anti-inflammatory M2 state (Di et al., 2017). In-vivo, the expression of CK19, PCNA, and collagen I was all boosted by MSCs. In-vitro, exosomes derived from human umbilical cord MSCs triggered the growth of skin cells while protecting them from undergoing apoptosis after heat stress. Exosomes have been confirmed to be enriched in Wnt4, which raises β-catenin nuclear translocation and functioning, thereby promoting skin cell proliferation and migration. This effect could be inhibited by the β-catenin inhibitor ICG001 (Zhang et al., 2015). By manufacturing pro-angiogenic substances, especially VEGFs, epidermal growth factor, C-X-C Motif Chemokine Ligand 12 and HIF-1, MSCs can contribute to the angiogenesis process (Yang et al., 2005; Zhang et al., 2006; Li et al., 2008; Guillamat-Prats, 2021).
Effects of MSCs on the remodeling phase
The remodeling process is the final stage of wound healing, during which MSCs help coordinate any last-minute changes in the ECM, blood vessels, and resident cells. In particular, MSCs can accelerate wound healing through the secretion of factors related to cell proliferation and differentiation, angiogenic mechanisms, immune suppression, and anti-apoptotic factors (Willer et al., 2022). During this phase, the tissue is subject to regeneration and the collagen fibers undergo organization. MSCs play an active part in the process of matrix remodeling by releasing matrix metalloproteinases (MMPs) to promote the deposition of matrix and secreting tissue inhibitors of metalloproteinases to prevent the deposition of ECM proteins. Evidence confirms that inflammatory cytokines, namely TNF-α, IL-1β, and TGF-β1, propagate MSCs to synthesize MMPs, which in turn triggers the chemotactic migration of MSCs across the extracellular matrix.
It has been found that IL-1β can induce the expression of matrix metalloproteinase-3 (MMP-3) in BM-MSCs. IL-1β can trigger MMP-3 expression via ERK1/2, JNK, MAPK p38, and Akt signaling cascades, thereby promoting the migration of MSCs. Stromal cell-derived factor 1 (SDF-1) expressed by MSCs is attributed to the homing ability of MSCs towards the ischemia-induced deteriorated heart muscle tissue. TGF-β1, monocyte chemotactic protein (MCP)-1, TNF-α, and ILs are also believed to boost the migration of MSCs to the injured tissues (Chang et al., 2021).
MSCs are also recognized to emit growth factors and cytokines, including HGF, IL-10, and adrenomedullin, which possess anti-fibrotic traits and aid in the healing of wounds without scarring. HGF and prostaglandin (Arron et al., 2021) E2 generated by MSCs at the site of injury, hinder fibroblast differentiation and help MSCs evade the epithelial-mesenchymal switch. Moreover, classical growth factors and cytokines, such as VEGF, CNTF, GDNF, TGF-β, IL-1β, IL-6, and IL-8, act as paracrine control molecules secreted to extracellular vesicles or exosomes. Recent evidence also insinuates the role of signaling by microRNAs in MSC-derived exosomes (Hofer and Tuan, 2016; Lan et al., 2017).
MSCs and healing of anaerobic bacterial wound infection in hypoxia condition
Since they were initially identified by Friedenstein et al. (1968) as plastic adherent cells capable of differentiating into different cell lines, MSCs have been broadly investigated for their regenerative characteristics. MSCs exhibit noteworthy regenerative capabilities attributed to their inherent ability to self-renew and differentiate into various tissue types. Recently, there has been a lot of focus on the impact of MSCs on the process of wound repair (Liu et al., 2021). As previously stated, the healing process of skin wounds is intricate and involves various phases, namely homeostasis, inflammation, proliferation, and remodeling. MSCs are known to participate in all phases of the wound-healing process, thereby exerting therapeutic benefits (Mahmoudvand et al., 2023).
On top of that, MSCs have demonstrated robust antimicrobial characteristics via both direct and indirect mechanisms (Alcayaga-Miranda et al., 2017).
AMPs are effective in eliminating microbes by disrupting membrane integrity, preventing binding to DNA, and disrupting protein synthesis. AMPs secreted from MSCs can act on fungi, yeasts, and viruses (Mirshekar et al., 2023).
Moreover, MSCs-derived exosomes, which possess antibacterial characteristics, expedite the healing process of diabetic foot ulcers. The exosomes are comprised of biologically active molecules (e.g., nucleic acids, growth factors, and proteins), as well as inactive substances (e.g., antibiotics; Raghav et al., 2021).
Collectively, they alleviate bacterial removal by enhancing the migratory and phagocytic capabilities of neutrophils, which is achieved through the upregulation of IL-6, IL-8, and granulocyte-macrophage colony-stimulating factor levels. These molecules ultimately contribute to the elimination of the infection and promote tissue regeneration, as evidenced in the process of wound healing (Joel et al., 2019). Studies conducted in-vivo regarding the antimicrobial properties of MSCs have demonstrated that their transplantation in mice results in the suppression of inflammatory response and the facilitation of bacterial elimination.
The defensin family of AMPs consists of alpha-defensins, β-defensins, and θ-defensins. Defensins are highly implicated in innate and adaptive immunity against microbial and viral pathogens and also contribute to wound healing by upregulating the expression of cytokines and chemokines, producing histamine, and boosting antibody responses. β-Defensins, hBD-1, hBD-2, and hBD-3 are the main functional peptides in humans expressed by many epithelial cells, granulocytes, and MSCs. To date, only one cathelicidin (CAMP) gene has been detected in mice and humans. This gene expresses a protein known as CRAMP, which exhibits a wide spectrum of antimicrobial and anticancer activities as well as chemotactic and antiangiogenic features. It can be detected in several cell types and plays a central part in mucosal defense (Sung et al., 2016).
MSCs also play a crucial role in regulating the immune response and combatting pathogenic microorganisms. This is achieved via the production of AMPs that specifically target a range of microorganisms including yeasts, fungi, bacteria, and viruses. MSCs are known to produce several noteworthy AMPs, including LL-37, β-defensin-2, cathelicidin, hepcidin, and lipocalin-2. These peptides are involved in the processes of regeneration, control of proliferation, and migration of MSCs (Gupta et al., 2012; Silva-Carvalho et al., 2022). Similarly, IL-17 and indoleamine-2,3-dioxygenase (Pulido-Escribano et al., 2023) are overexpressed in MSCs. IDO exhibits potent antimicrobial activity against a broad spectrum of bacteria (e.g., S. aureus, S. epidermidis, Group B streptococci, and E. faecium), viral pathogens (Cytomegalovirus, Herpes simplex virus), and parasitic infections (Toxoplasma gondii; Alcayaga-Miranda et al., 2017). The results of an experiment suggested that MSCs can enhance the anti-microbial capacity of equine keratocytes by promoting the expression of AMPs through the secretion of CCL2 (Marx et al., 2021).
The functions of MSCs could potentially be impacted by various environmental factors, including hypoxic conditions. One potential approach to improve the survival of MSCs is to subject them to hypoxic conditions (1–4% oxygen) for a period of 24–48 h before their implantation, which may represent a significant and feasible strategy. MSCs exhibit an up-regulation of HIF-1α in response to pre-exposure to hypoxic conditions (Palomäki et al., 2013). Under the influence of hypoxia, MSCs employ HIF-1α to trigger the AKT signaling cascade, thereby augmenting their growth and survival (Lee et al., 2017). Data from experiments imply that hypoxia preconditioning amplifies the paracrine capacities of MSCs in the context of vascular renewal (Han et al., 2020; Yusoff et al., 2022). The expression of various angiogenic factors, such as VEGF and HGF, is enhanced under hypoxic conditions of 1% oxygen during in-vitro culture (Ishiuchi et al., 2020). Hypoxic preconditioning has the potential for amplifying additional traits in MSCs, which may include heightened immunosuppressive properties (Roemeling-van Rhijn et al., 2013), or the synthesis of regenerative growth factors (Chang et al., 2013). Chen et al. (2014) established that under hypoxic conditions, BM-MSCs exhibited elevated expression and secretion levels of basic fibroblast growth factor (bFGF), VEGF-A, IL-6, and IL-8. In addition, the utilization of hypoxic BM-MSCs-derived conditioned medium (hypoCM) in comparison to normoxic BM-MSCs-derived conditioned medium (norCM) resulted in a noteworthy increase in the proliferation of keratinocytes, fibroblasts, and endothelial cells. It also facilitated the migration of keratinocytes, fibroblasts, endothelial cells, and monocytes, along with the production of tubular structures by endothelial cells. The findings of this study indicate that the application of topical hypoCM resulted in a notable acceleration of cutaneous wound contraction in Balb/c nude mice, which is in agreement with the in-vitro results. In contrast, the application of norCM or the vesicle control did not produce a similar effect. In-vivo, the subjects subjected to hypoCM exhibited a noticeable rise in cell proliferation, neovascularization, and recruitment of inflammatory macrophages. Additionally, a notable decrease in collagen I and collagen III was observed in this group. Similarly, Jun et al. (2014) the study demonstrated that hypoxia had a dual effect on amniotic fluid MSCs (AF-MSCs), as it not only stimulated their proliferation but additionally preserved their inherent features, including surface marker expression and differentiation abilities. It is worth noting that AF-MSCs released a higher number of paracrine factors, specifically VEGF and TGF-β1, into AF-MSCs-hypoCM as compared to AF-MSCs-norCM. The potential of AF-MSCs-hypoCM to improve wound repair has been attributed to its ability to stimulate the production of hypoxia-induced paracrine factors by activating the TGF-β/SMAD2 and PI3K/AKT pathways. The available evidence suggests that hi-MSCs possess augmented capabilities for the process of wound repair (Han et al., 2020).
In a study by Diniz et al. (2016), the utilization of an alginate hydrogel-based delivery system for gingival MSCs exhibited notable antimicrobial efficacy against Aggregatibacter actinomycetemcomitans, a type of gram-negative anaerobic bacteria. The antimicrobial activity was dose-dependent, with the highest antimicrobial efficacy being observed at a concentration of 0.50 mg/mL, while concurrently preserving cellular viability. On the contrary, data point to the reciprocal effect of bacterial pathogens on the activities of MSCs. Recent research has shown that the management of the microbiome in wounds can facilitate wound healing (Tang et al., 2023). The analysis of the impact of cell balance between anaerobic bacteria and probiotics on the regenerative properties of MSCs has shown that the simulation of the equilibrium between oral pathogenic bacteria and probiotics using an extract of Limosilactobacillus reuteri and Porphyromonas gingivalis bacteria can result in bone differentiation and migration of MSCs in controlled laboratory settings. The present study revealed that the combination of L. reuteri and P. gingivalis has the potential to induce the wound-healing cascade via the activation of MSCs (Osakabe et al., 2017; Han et al., 2020). The findings indicate that co-culturing MSCs with anaerobic pathogens in an anaerobic environment provokes the induction of cytokine secretion in the former by the latter, specifically in the anaerobic bacterium F. nucleatum (Kriebel et al., 2013). After being co-cultured with F. nucleatum, BM-MSCs were found to have the greatest level of IL-8 production, as shown by the results of Biedermann and colleagues. In general, it seems reasonable to depend on hiMSCs in the treatment of anaerobic bacterial wound infection (Han et al., 2020).
Conclusion
Briefly, MSCs are key cells that mainly originate from BM and adipose tissue. These cells have qualities that are essential for the body, such as the capability of self-regeneration and differentiation. MSCs generate a variety of cytokines, including those that maintain hematopoietic stem cells in the latent phase or drive their self-renewal, such as SCF, OSM, SDF-1, LIF, and BMP-4. TGF-β, which in this manner plays a substantial part in the repair of injuries.
In addition to playing a role in all phases of wound healing, MSCs exhibit strong antimicrobial features. These qualities are manifested in MSCs’ ability to stimulate host innate immune cells and produce AMPs. Because of this, MSCs have shown to be a reliable tool in the fight against bacterial wound infections. MSCs have been proven to be resistant to bacterial infection in anoxic areas because they normally live in a hypoxic microenvironment in the BM. This highlights their potential applicability as an exciting tool in the field of regenerative medicine in areas of the human body that are in contact with anaerobic bacteria. MSCs produced under hypoxia were emphasized in a wide variety of papers for in-vitro cell cultures and subsequent therapeutic applications. According to the findings of these investigations, MSCs and anaerobic bacteria have a symbiotic interaction. Delivery systems based on MSCs possess antibacterial characteristics that are effective against anaerobic bacteria. On the other hand, anaerobic bacteria, namely F. nucleatum, can cause MSCs to secrete cytokines. When taken together, these results introduce MSCs as a potentially useful tool for wound healing. Nevertheless, despite these advancements, the application of MSCs as a useful instrument in the battle against anaerobic bacterial infections is still in its infancy stage. Subsequent measures needed to be taken to obtain a risk-free MSCs-based strategy for the treatment of wound infections. In this case, verifying the batch release of MSCs-based systems by in-vitro assays and assessing their biodistribution and potential adverse effects in pre-clinical studies are of great importance.
Author contributions
EA and MK: wrote the manuscript. GM, ER, MM, and AT: edited the manuscript and designed figures of the manuscript. HA: design and supervision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdollahi, H., Harris, L. J., Zhang, P., McIlhenny, S., Srinivas, V., Tulenko, T., et al. (2011). The role of hypoxia in stem cell differentiation and therapeutics. J. Surg. Res. 165, 112–117. doi: 10.1016/j.jss.2009.09.057
Ahmed, N. E.-M. B., Murakami, M., Kaneko, S., and Nakashima, M. (2016). The effects of hypoxia on the stemness properties of human dental pulp stem cells (DPSCs). Sci. Rep. 6:35476. doi: 10.1038/srep35476
Alcayaga-Miranda, F., Cuenca, J., and Khoury, M. (2017). Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front. Immunol. 8:339. doi: 10.3389/fimmu.2017.00339
Alcayaga-Miranda, F., Cuenca, J., Martin, A., Contreras, L., Figueroa, F. E., Khoury, M., et al. (2015). Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther 6:199. doi: 10.1186/s13287-015-0192-0
Andrzejewska, A., Lukomska, B., and Janowski, M. (2019). Concise review: mesenchymal stem cells: from roots to boost. Stem Cells 37, 855–864. doi: 10.1002/stem.3016
Antebi, B., Rodriguez, L. A., Walker, K. P., Asher, A. M., Kamucheka, R. M., Alvarado, L., et al. (2018). Short-term physiological hypoxia potentiates the therapeutic function of mesenchymal stem cells. Stem Cell Res. Ther. 9:265. doi: 10.1186/s13287-018-1007-x
Arron, M. N., Greijdanus, N. G., Ten Broek, R. P., Dekker, J. W. T., van Workum, F., van Goor, H., et al. (2021). Trends in risk factors of anastomotic leakage after colorectal cancer surgery (2011–2019): a Dutch population-based study. Colorectal Dis 23, 3251–3261. doi: 10.1111/codi.15911
Azari, Z., Nazarnezhad, S., Webster, T. J., Hoseini, S. J., Brouki Milan, P., Baino, F., et al. (2022). Stem cell-mediated angiogenesis in skin tissue engineering and wound healing. Wound Repair Regen. 30, 421–435. doi: 10.1111/wrr.13033
Beegle, J., Lakatos, K., Kalomoiris, S., Stewart, H., Isseroff, R. R., Nolta, J. A., et al. (2015). Hypoxic preconditioning of mesenchymal stromal cells induces metabolic changes, enhances survival, and promotes cell retention in vivo. Stem Cells 33, 1818–1828. doi: 10.1002/stem.1976
Bian, D., Wu, Y., Song, G., Azizi, R., and Zamani, A. (2022). The application of mesenchymal stromal cells (MSCs) and their derivative exosome in skin wound healing: a comprehensive review. Stem Cell Res Ther 13:24. doi: 10.1186/s13287-021-02697-9
Brandau, S., Jakob, M., Hemeda, H., Bruderek, K., Janeschik, S., Bootz, F., et al. (2010). Tissue-resident mesenchymal stem cells attract peripheral blood neutrophils and enhance their inflammatory activity in response to microbial challenge. J. Leukoc. Biol. 88, 1005–1015. doi: 10.1189/jlb.0410207
Brennan, M. Á., Layrolle, P., and Mooney, D. J. (2020). Biomaterials functionalized with MSC secreted extracellular vesicles and soluble factors for tissue regeneration. Adv. Funct. Mater. 30:1909125. doi: 10.1002/adfm.201909125
Brook, I. (2016). Spectrum and treatment of anaerobic infections. J. Infect. Chemother. 22, 1–13. doi: 10.1016/j.jiac.2015.10.010
Camões, S. P., Bulut, O., Yazar, V., Gaspar, M. M., Simões, S., Ferreira, R., et al. (2022). 3D-MSCs A151 ODN-loaded exosomes are immunomodulatory and reveal a proteomic cargo that sustains wound resolution. J. Adv. Res. 41, 113–128. doi: 10.1016/j.jare.2022.01.013
Chance, T. C., Rathbone, C. R., Kamucheka, R. M., Peltier, G. C., Cap, A. P., and Bynum, J. A. (2019). The effects of cell type and culture condition on the procoagulant activity of human mesenchymal stromal cell-derived extracellular vesicles. J. Trauma Acute Care Surg. 87, S74–S82. doi: 10.1097/TA.0000000000002225
Chang, C. P., Chio, C. C., Cheong, C. U., Chao, C. M., Cheng, B. C., and Lin, M. T. (2013). Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin. Sci. (Lond.) 124, 165–176. doi: 10.1042/CS20120226
Chang, C. H., Lin, Y. L., Tyan, Y. S., Chiu, Y. H., Liang, Y. H., Chen, C. P., et al. (2021). Interleukin-1β-induced matrix metalloproteinase-3 via ERK1/2 pathway to promote mesenchymal stem cell migration. PLoS One 16:e0252163. doi: 10.1371/journal.pone.0252163
Chauhan, A., Agarwal, S., Masih, M., and Gautam, P. K. (2023). The multifunction role of tumor-associated mesenchymal stem cells and their interaction with immune cells in breast cancer. Immunol. Investig. 52, 856–878. doi: 10.1080/08820139.2023.2249025
Chen, L., Xu, Y., Zhao, J., Zhang, Z., Yang, R., Xie, J., et al. (2014). Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 9:e96161. doi: 10.1371/journal.pone.0096161
Cheong, J. A., Irvine, J. M., Roesemann, S., Nora, A., Morgan, C. E., Daniele, C., et al. (2022). Ankle brachial indices and anaerobes: is peripheral arterial disease associated with anaerobic bacteria in diabetic foot ulcers? Ther. Adv. Endocrinol. Metab. 13:20420188221118747. doi: 10.1177/20420188221118747
Dabral, S., Muecke, C., Valasarajan, C., Schmoranzer, M., Wietelmann, A., Semenza, G. L., et al. (2019). A RASSF1A-HIF1α loop drives Warburg effect in cancer and pulmonary hypertension. Nat. Commun. 10:2130. doi: 10.1038/s41467-019-10044-z
Dazzi, F., Ramasamy, R., Glennie, S., Jones, S. P., and Roberts, I. (2006). The role of mesenchymal stem cells in haemopoiesis. Blood Rev. 20, 161–171. doi: 10.1016/j.blre.2005.11.002
Di, G., Du, X., Qi, X., Zhao, X., Duan, H., Li, S., et al. (2017). Mesenchymal stem cells promote diabetic corneal epithelial wound healing through TSG-6-dependent stem cell activation and macrophage switch. Invest. Ophthalmol. Vis. Sci. 58, 4344–4354. doi: 10.1167/iovs.17-21506
Ding, D.-C., Shyu, W.-C., and Lin, S.-Z. (2011). Mesenchymal stem cells. Cell Transplant. 20, 5–14. doi: 10.3727/096368910X
Diniz, I. M., Chen, C., Ansari, S., Zadeh, H. H., Moshaverinia, M., Chee, D., et al. (2016). Gingival mesenchymal stem cell (GMSC) delivery system based on RGD-coupled alginate hydrogel with antimicrobial properties: a novel treatment modality for Peri-Implantitis. J. Prosthodont. 25, 105–115. doi: 10.1111/jopr.12316
Eales, K. L., Hollinshead, K. E. R., and Tennant, D. A. (2016). Hypoxia and metabolic adaptation of cancer cells. Oncogenesis 5, e190–e. doi: 10.1038/oncsis.2015.50
Eberly, A. R., Elvert, J. L., and Schuetz, A. N. (2022). Best practices for the pre-analytic phase of anaerobic bacteriology. Clin. Microbiol. Newsl. 44, 63–71. doi: 10.1016/j.clinmicnews.2022.04.001
Ejtehadifar, M., Shamsasenjan, K., Movassaghpour, A., Akbarzadehlaleh, P., Dehdilani, N., Abbasi, P., et al. (2015). The effect of hypoxia on mesenchymal stem cell biology. Adv Pharm Bull. 5, 141–149. doi: 10.15171/apb.2015.021
Finegold, S. M. (1993). Host factors predisposing to anaerobic infections. FEMS Immunol. Med. Microbiol. 6, 159–163. doi: 10.1111/j.1574-695X.1993.tb00319.x
Friedenstein, A. J., Petrakova, K. V., Kurolesova, A. I., and Frolova, G. P. (1968). Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247. doi: 10.1097/00007890-196803000-00009
Guillamat-Prats, R. (2021). The role of MSC in wound healing, scarring and regeneration. Cells 10:1729. doi: 10.3390/cells10071729
Gupta, N., Krasnodembskaya, A., Kapetanaki, M., Mouded, M., Tan, X., Serikov, V., et al. (2012). Mesenchymal stem cells enhance survival and bacterial clearance in murine Escherichia coli pneumonia. Thorax 67, 533–539. doi: 10.1136/thoraxjnl-2011-201176
Han, N., Jia, L., Guo, L., Su, Y., Luo, Z., Du, J., et al. (2020). Balanced oral pathogenic bacteria and probiotics promoted wound healing via maintaining mesenchymal stem cell homeostasis. Stem Cell Res. Ther. 11:61. doi: 10.1186/s13287-020-1569-2
Hermann, M., Peddi, A., Gerhards, A., Schmid, R., Schmitz, D., Arkudas, A., et al. (2023). Secretome of adipose-derived stem cells cultured in platelet lysate improves migration and viability of keratinocytes. Int. J. Mol. Sci. 24:3522. doi: 10.3390/ijms24043522
Hoang, D. H., Nguyen, T. D., Nguyen, H.-P., Nguyen, X.-H., Do, P. T. X., Dang, V. D., et al. (2020). Differential wound healing capacity of mesenchymal stem cell-derived exosomes originated from bone marrow, adipose tissue and umbilical cord under serum- and Xeno-free condition. Front. Mol. Biosci. :7:119. doi: 10.3389/fmolb.2020.00119
Hofer, H. R., and Tuan, R. S. (2016). Secreted trophic factors of mesenchymal stem cells support neurovascular and musculoskeletal therapies. Stem Cell Res Ther 7:131. doi: 10.1186/s13287-016-0394-0
Ihekweazu, F. D., Engevik, M. A., Ruan, W., Shi, Z., Fultz, R., Engevik, K. A., et al. (2021). Bacteroides ovatus promotes IL-22 production and reduces Trinitrobenzene sulfonic acid–driven colonic inflammation. Am. J. Pathol. 191, 704–719. doi: 10.1016/j.ajpath.2021.01.009
Ishiuchi, N., Nakashima, A., Doi, S., Yoshida, K., Maeda, S., Kanai, R., et al. (2020). Hypoxia-preconditioned mesenchymal stem cells prevent renal fibrosis and inflammation in ischemia-reperfusion rats. Stem Cell Res Ther 11:130. doi: 10.1186/s13287-020-01642-6
Jiang, D., Qi, Y., Walker, N. G., Sindrilaru, A., Hainzl, A., Wlaschek, M., et al. (2013). The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials 34, 2501–2515. doi: 10.1016/j.biomaterials.2012.12.014
Joel, M. D. M., Yuan, J., Wang, J., Yan, Y., Qian, H., Zhang, X., et al. (2019). MSC: immunoregulatory effects, roles on neutrophils and evolving clinical potentials. Am. J. Transl. Res. 11, 3890–3904.
Johnson, V., Webb, T., Norman, A., Coy, J., Kurihara, J., Regan, D., et al. (2017). Activated mesenchymal stem cells interact with antibiotics and host innate immune responses to control chronic bacterial infections. Sci. Rep. 7:9575. doi: 10.1038/s41598-017-08311-4
Jun, E. K., Zhang, Q., Yoon, B. S., Moon, J. H., Lee, G., Park, G., et al. (2014). Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int. J. Mol. Sci. 15, 605–628. doi: 10.3390/ijms15010605
Kapatral, V., Anderson, I., Ivanova, N., Reznik, G., Los, T., Lykidis, A., et al. (2002). Genome sequence and analysis of the Oral bacterium Fusobacterium nucleatum strain ATCC 25586. J. Bacteriol. 184, 2005–2018. doi: 10.1128/JB.184.7.2005-2018.2002
Keith, B., and Simon, M. C. (2007). Hypoxia-inducible factors, stem cells, and Cancer. Cells 129, 465–472. doi: 10.1016/j.cell.2007.04.019
Kelly, M. J. (1978). The quantitative and histological demonstration of pathogenic synergy between Escherichia Coli and Bacteroides Fragilis in Guinea-pig wounds. J. Med. Microbiol. 11, 513–523. doi: 10.1099/00222615-11-4-513
Krasnodembskaya, A., Song, Y., Fang, X., Gupta, N., Serikov, V., Lee, J.-W., et al. (2010). Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28, 2229–2238. doi: 10.1002/stem.544
Krepel, C., Gohr, C., Walker, A., Farmer, S., and Edmiston, C. (1992). Enzymatically active Peptostreptococcus magnus: association with site of infection. J. Clin. Microbiol. 30, 2330–2334. doi: 10.1128/jcm.30.9.2330-2334.1992
Kriebel, K., Biedermann, A., Kreikemeyer, B., and Lang, H. (2013). Anaerobic co-culture of mesenchymal stem cells and anaerobic pathogens – a new in vitro model system. PLoS One 8:e78226. doi: 10.1371/journal.pone.0078226
Lan, Y. W., Theng, S. M., Huang, T. T., Choo, K. B., Chen, C. M., Kuo, H. P., et al. (2017). Oncostatin M-preconditioned mesenchymal stem cells alleviate bleomycin-induced pulmonary fibrosis through paracrine effects of the hepatocyte growth factor. Stem Cells Transl. Med. 6, 1006–1017. doi: 10.5966/sctm.2016-0054
Lee, P., Chandel, N. S., and Simon, M. C. (2020). Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat. Rev. Mol. Cell Biol. 21, 268–283. doi: 10.1038/s41580-020-0227-y
Lee, J. H., Yoon, Y. M., and Lee, S. H. (2017). Hypoxic preconditioning promotes the bioactivities of mesenchymal stem cells via the HIF-1α-GRP78-Akt Axis. Int. J. Mol. Sci. 18:1320. doi: 10.3390/ijms18061320
Leroux, L., Descamps, B., Tojais, N. F., Séguy, B., Oses, P., Moreau, C., et al. (2010). Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle Fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol. Ther. 18, 1545–1552. doi: 10.1038/mt.2010.108
Li, G., Liu, J., Guan, Y., and Ji, X. (2021). The role of hypoxia in stem cell regulation of the central nervous system: from embryonic development to adult proliferation. CNS Neurosci. Ther. 27, 1446–1457. doi: 10.1111/cns.13754
Li, L., Zhang, Y., Li, Y., Yu, B., Xu, Y., Zhao, S., et al. (2008). Mesenchymal stem cell transplantation attenuates cardiac fibrosis associated with isoproterenol-induced global heart failure. Transpl. Int. 21, 1181–1189. doi: 10.1111/j.1432-2277.2008.00742.x
Lin, J., Wang, X., Wang, X., Wang, S., Shen, R., Yang, Y., et al. (2021). Hypoxia increases the expression of stem cell markers in human osteosarcoma cells. Oncol. Lett. 21:217. doi: 10.3892/ol.2021.12478
Lindsay, S., Oates, A., and Bourdillon, K. (2017). The detrimental impact of extracellular bacterial proteases on wound healing. Int. Wound J. 14, 1237–1247. doi: 10.1111/iwj.12790
Liu, C., Wang, C., Yang, F., Lu, Y., Du, P., Hu, K., et al. (2022). The conditioned medium from mesenchymal stromal cells pretreated with proinflammatory cytokines promote fibroblasts migration and activation. PLoS One 17:e0265049. doi: 10.1371/journal.pone.0265049
Liu, J., Yan, Z., Yang, F., Huang, Y., Yu, Y., Zhou, L., et al. (2021). Exosomes derived from human umbilical cord mesenchymal stem cells accelerate cutaneous wound healing by enhancing angiogenesis through delivering Angiopoietin-2. Stem Cell Rev. Rep. 17, 305–317. doi: 10.1007/s12015-020-09992-7
Luo, Z., Tian, M., Yang, G., Tan, Q., Chen, Y., Li, G., et al. (2022). Hypoxia signaling in human health and diseases: implications and prospects for therapeutics. Signal Transduct. Target. Ther. 7:218. doi: 10.1038/s41392-022-01080-1
Luqman, A., and Götz, F. (2021). The ambivalent role of skin microbiota and adrenaline in wound healing and the interplay between them. Int. J. Mol. Sci. 22:4996. doi: 10.3390/ijms22094996
Mahjoor, M., Afkhami, H., Mollaei, M., Nasr, A., Shahriary, S., and Khorrami, S. J. L. S. (2021). MicroRNA-30c delivered by bone marrow-mesenchymal stem cells induced apoptosis and diminished cell invasion in U-251 glioblastoma cell line. Life Sci. 279:119643. doi: 10.1016/j.lfs.2021.119643
Mahjoor, M., Afkhami, H., Najafi, M., Nasr, A., and Khorrami, S. (2023). The role of microRNA-30c in targeting interleukin 6, as an inflammatory cytokine, in the mesenchymal stem cell: a therapeutic approach in colorectal cancer. J Cancer Res Clin Oncol 149, 3149–3160. doi: 10.1007/s00432-022-04123-w
Mahlapuu, M., Håkansson, J., Ringstad, L., and Björn, C. (2016). Antimicrobial peptides: an emerging category of therapeutic agents. Front Cell Infect Microbiol 6:194. doi: 10.3389/fcimb.2016.00194
Mahmoudvand, G., Karimi Rouzbahani, A., Razavi, Z. S., Mahjoor, M., and Afkhami, H. (2023). Mesenchymal stem cell therapy for non-healing diabetic foot ulcer infection: new insight. Front Bioeng Biotechnol 11:1158484. doi: 10.3389/fbioe.2023.1158484
Mansori, K., Moradi, Y., Naderpour, S., Rashti, R., Moghaddam, A. B., Saed, L., et al. (2020). Helicobacter pylori infection as a risk factor for diabetes: a meta-analysis of case-control studies. BMC Gastroenterol 20:77. doi: 10.1186/s12876-020-01223-0
Maqbool, M., Vidyadaran, S., George, E., and Ramasamy, R. (2011). Human mesenchymal stem cells protect neutrophils from serum-deprived cell death. Cell Biol Int 35, 1247–1251. doi: 10.1042/CBI20110070
Marx, C., Gardner, S., Harman, R. M., Wagner, B., and Van de Walle, G. R. (2021). Mesenchymal stromal cell-secreted CCL2 promotes antibacterial defense mechanisms through increased antimicrobial peptide expression in keratinocytes. Stem Cells Transl. Med. 10, 1666–1679. doi: 10.1002/sctm.21-0058
Metzger, Z., Hoffeld, J. T., Charon, J., and Mergenhagen, S. E. (1987). Suppression of lymphocyte and fibroblast proliferation by Actinomyces viscosus-activated murine macrophages. J. Periodontal Res. 22, 456–460. doi: 10.1111/j.1600-0765.1987.tb02055.x
Mirshekar, M., Afkhami, H., Razavi, S., Masjedian Jazi, F., Darban-Sarokhalil, D., Ohadi, E., et al. (2023). Potential antibacterial activity and healing effect of topical administration of bone marrow and adipose mesenchymal stem cells encapsulated in collagen-fibrin hydrogel scaffold on full-thickness burn wound infection caused by Pseudomonas aeruginosa. Burns. 49, 1237–1486. doi: 10.1016/j.burns.2023.01.005
Morrison, T. J., Jackson, M. V., Cunningham, E. K., Kissenpfennig, A., McAuley, D. F., O’Kane, C. M., et al. (2017). Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196, 1275–1286. doi: 10.1164/rccm.201701-0170OC
Murphy, E. C., Mohanty, T., and Frick, I. M. (2014). FAF and SufA: proteins of Finegoldia magna that modulate the antibacterial activity of histones. J. Innate Immun. 6, 394–404. doi: 10.1159/000356432
Nahid, M. A., Griffin, J. M., Lustik, M. B., Hayes, J. J., Fong, K. S. K., Horseman, T. S., et al. (2021). A longitudinal evaluation of the bacterial pathogens colonizing chronic non-healing wound sites at a United States military treatment facility in the pacific region. Infect. Drug Resist. 14, 1–10. doi: 10.2147/IDR.S260708
Najar, M., Fayyad-Kazan, M., Merimi, M., Meuleman, N., Bron, D., Fayyad-Kazan, H., et al. (2019). Reciprocal immuno-biological alterations occur during the co-culture of natural killer cells and adipose tissue-derived mesenchymal stromal cells. Cytotechnology 71, 375–388. doi: 10.1007/s10616-019-00294-6
Nakazawa, M. S., Keith, B., and Simon, M. C. (2016). Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673. doi: 10.1038/nrc.2016.84
Negut, I., Grumezescu, V., and Grumezescu, A. M. (2018). Treatment strategies for infected wounds. Molecules 23:2392. doi: 10.3390/molecules23092392
Nilforoushzadeh, M. A., Raoofi, A., Afzali, H., Gholami, O., Zare, S., Nasiry, D., et al. (2023). Promotion of cutaneous diabetic wound healing by subcutaneous administration of Wharton's jelly mesenchymal stem cells derived from umbilical cord. Arch. Dermatol. Res. 315, 147–159. doi: 10.1007/s00403-022-02326-2
Nowak-Stępniowska, A., Osuchowska, P. N., Fiedorowicz, H., and Trafny, E. A. (2022). Insight in hypoxia-mimetic agents as potential tools for mesenchymal stem cell priming in regenerative medicine. Stem Cells Int. 2022, 1–24. doi: 10.1155/2022/8775591
Osakabe, L., Utsumi, A., Saito, B., Okamatsu, Y., Kinouchi, H., Nakamaki, T., et al. (2017). Influence of Oral anaerobic Bacteria on hematopoietic stem cell transplantation patients: Oral mucositis and general condition. Transplant. Proc. 49, 2176–2182. doi: 10.1016/j.transproceed.2017.09.012
Palomäki, S., Pietilä, M., Laitinen, S., Pesälä, J., Sormunen, R., Lehenkari, P., et al. (2013). HIF-1α is upregulated in human mesenchymal stem cells. Stem Cells 31, 1902–1909. doi: 10.1002/stem.1435
Pulido-Escribano, V., Torrecillas-Baena, B., Dorado, G., Gálvez-Moreno, M. Á., Camacho-Cardenosa, M., and Casado-Díaz, A. (2023). Combination of biomaterials and extracellular vesicles from mesenchymal stem-cells: new therapeutic strategies for skin-wound healing. Appl. Sci. 13:2702. doi: 10.3390/app13042702
Rabani, R., Volchuk, A., Jerkic, M., Ormesher, L., Garces-Ramirez, L., Canton, J., et al. (2018). Mesenchymal stem cells enhance NOX2-dependent reactive oxygen species production and bacterial killing in macrophages during sepsis. Eur Respir J 51:1702021. doi: 10.1183/13993003.02021-2017
Raffaghello, L., Bianchi, G., Bertolotto, M., Montecucco, F., Busca, A., Dallegri, F., et al. (2008). Human mesenchymal stem cells inhibit neutrophil apoptosis: a model for neutrophil preservation in the bone marrow niche. Stem Cells 26, 151–162. doi: 10.1634/stemcells.2007-0416
Raghav, A., Tripathi, P., Mishra, B. K., Jeong, G.-B., Banday, S., Gautam, K. A., et al. (2021). Mesenchymal stromal cell-derived tailored exosomes treat Bacteria-associated diabetes foot ulcers: a customized approach from bench to bed. Front. Microbiol. 12:712588. doi: 10.3389/fmicb.2021.712588
Rangasami, V. K., Nawale, G., Asawa, K., Kadekar, S., Samanta, S., Nilsson, B., et al. (2021). Pluronic micelle-mediated tissue factor silencing enhances Hemocompatibility, Stemness, differentiation potential, and paracrine signaling of mesenchymal stem cells. Biomacromolecules 22, 1980–1989. doi: 10.1021/acs.biomac.1c00070
Ratcliffe, P. J. (2013). Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J. Physiol. 591, 2027–2042. doi: 10.1113/jphysiol.2013.251470
Riley, K. N., and Herman, I. M. (2005). Collagenase promotes the cellular responses to injury and wound healing in vivo. J Burns Wounds 4:e8.
Roemeling-van Rhijn, M., Mensah, F. K., Korevaar, S. S., Leijs, M. J., van Osch, G. J., Ijzermans, J. N., et al. (2013). Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front. Immunol. 4:203. doi: 10.3389/fimmu.2013.00203
SaiKiran, K., Biswal, D., Agrawal, S. K., Batra, P., Sagar, T., Choudhary, S., et al. (2022). Anaerobes in cardiac infections: a decade experience from the tertiary care center. Indian J. Med. Microbiol. 40, 274–278. doi: 10.1016/j.ijmmb.2021.12.013
Samal, J. R. K., Rangasami, V. K., Samanta, S., Varghese, O. P., and Oommen, O. P. (2021). Discrepancies on the role of oxygen gradient and culture condition on mesenchymal stem cell fate. Adv. Healthc. Mater. 10:e2002058. doi: 10.1002/adhm.202002058
Silachev, D., Goryunov, K., Shpilyuk, M., Beznoschenko, O., Morozova, N., Kraevaya, E., et al. (2019). Effect of MSCs and MSC-derived extracellular vesicles on human blood coagulation. Cells 8:258. doi: 10.3390/cells8030258
Silva, D. N., Santos, G. C., Orge, I. D., Sampaio, G. A., Silveira, B. M., Paredes, B. D., et al. (2020). Leukemia inhibitory factor (LIF) overexpression increases angiogenic potential of mouse derived bone marrow mesenchymal stem/stromal cells. Cytotherapy 22:S104. doi: 10.1016/j.jcyt.2020.03.187
Silva-Carvalho, A. É., Cardoso, M. H., Alencar-Silva, T., Bogéa, G. M. R., Carvalho, J. L., Franco, O. L., et al. (2022). Dissecting the relationship between antimicrobial peptides and mesenchymal stem cells. Pharmacol. Ther. 233:108021. doi: 10.1016/j.pharmthera.2021.108021
Stephens, P., Wall, I. B., Wilson, M., Hill, K. E., Davies, C. E., Hill, C. M., et al. (2003). Anaerobic cocci populating the deep tissues of chronic wounds impair cellular wound healing responses in vitro. Br. J. Dermatol. 148, 456–466. doi: 10.1046/j.1365-2133.2003.05232.x
Sung, D. K., Chang, Y. S., Sung, S. I., Yoo, H. S., Ahn, S. Y., and Park, W. S. (2016). Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell. Microbiol. 18, 424–436. doi: 10.1111/cmi.12522
Sutton, M. T., Fletcher, D., Episalla, N., Auster, L., Kaur, S., Gwin, M. C., et al. (2017). Mesenchymal stem cell soluble mediators and cystic fibrosis. J Stem Cell Res Ther 7:400. doi: 10.4172/2157-7633.1000400
Tang, Q., Xue, N., Ding, X., Tsai, K. H. Y., Hew, J. J., Jiang, R., et al. (2023). Role of wound microbiome, strategies of microbiota delivery system and clinical management. Adv. Drug Deliv. Rev. 192:114671. doi: 10.1016/j.addr.2022.114671
Tirpe, A. A., Gulei, D., Ciortea, S. M., Crivii, C., and Berindan-Neagoe, I. (2019). Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 20:6140. doi: 10.3390/ijms20246140
Tjampakasari, C. R., Prasetyo, D. S., Ningsih, I., and Kiranasari, A. (2022). Distribution of anaerobic bacteria and their sensitivity pattern to several antibiotics at the clinical microbiology laboratory of school of medicine, universitas Indonesia, Jakarta in 2019–2020. Iran. J. Microbiol. 14:24. doi: 10.18502/ijm.v14i1.8797
Ulivi, V., Tasso, R., Cancedda, R., and Descalzi, F. (2014). Mesenchymal stem cell paracrine activity is modulated by platelet lysate: induction of an inflammatory response and secretion of factors maintaining macrophages in a proinflammatory phenotype. Stem Cells Dev. 23, 1858–1869. doi: 10.1089/scd.2013.0567
Vasandan, A. B., Jahnavi, S., Shashank, C., Prasad, P., Kumar, A., and Prasanna, S. (2016). Human mesenchymal stem cells program macrophage plasticity by altering their metabolic status via a PGE2-dependent mechanism. Sci Rep 6:38308. doi: 10.1038/srep38308
Verbanic, S., Shen, Y., Lee, J., Deacon, J. M., and Chen, I. A. (2020). Microbial predictors of healing and short-term effect of debridement on the microbiome of chronic wounds. NPJ Biofilms Microbiomes 6:21. doi: 10.1038/s41522-020-0130-5
Vusirikala, A., Charles, H., Balasegaram, S., Macdonald, N., Kumar, D., Barker-Burnside, C., et al. (2022). Epidemiology of early monkeypox virus transmission in sexual networks of gay and bisexual men, England, 2022. Emerg Infect Dis 28, 2082–2086. doi: 10.3201/eid2810.220960
Wang, C.-H., Chen, C.-Y., Wang, K.-H., Kao, A.-P., Chen, Y.-J., Lin, P.-H., et al. (2023). Comparing the therapeutic mechanism and immune response of human and mouse mesenchymal stem cells in immunocompetent mice with acute liver failure. Stem Cells Transl. Med. 12, 39–53. doi: 10.1093/stcltm/szac084
Wang, E.-j., Wu, M.-Y., Ren, Z.-Y., Zheng, Y., Ye, R. D., Tan, C. S. H., et al. (2023). Targeting macrophage autophagy for inflammation resolution and tissue repair in inflammatory bowel disease. Burns Trauma. 11:tkad004. doi: 10.1093/burnst/tkad004
Willer, H., Spohn, G., Morgenroth, K., Thielemann, C., Elvers-Hornung, S., Bugert, P., et al. (2022). Pooled human bone marrow-derived mesenchymal stromal cells with defined trophic factors cargo promote dermal wound healing in diabetic rats by improved vascularization and dynamic recruitment of M2-like macrophages. Front. Immunol. 13:976511. doi: 10.3389/fimmu.2022.976511
Xu, W., Xu, R., Li, Z., Wang, Y., and Hu, R. (2019). Hypoxia changes chemotaxis behaviour of mesenchymal stem cells via HIF-1α signalling. J. Cell. Mol. Med. 23, 1899–1907. doi: 10.1111/jcmm.14091
Yang, J., Dai, C., and Liu, Y. (2005). A novel mechanism by which hepatocyte growth factor blocks tubular epithelial to mesenchymal transition. J. Am. Soc. Nephrol. 16, 68–78. doi: 10.1681/ASN.2003090795
Yates, C. C., Rodrigues, M., Nuschke, A., Johnson, Z. I., Whaley, D., Stolz, D., et al. (2017). Multipotent stromal cells/mesenchymal stem cells and fibroblasts combine to minimize skin hypertrophic scarring. Stem Cell Res. Ther. 8:193. doi: 10.1186/s13287-017-0644-9
Yusoff, F. M., Nakashima, A., Kawano, K.-i., Kajikawa, M., Kishimoto, S., Maruhashi, T., et al. (2022). Implantation of hypoxia-induced mesenchymal stem cell advances therapeutic angiogenesis. Stem Cells Int. 2022, 1–14. doi: 10.1155/2022/6795274
Zanone, M. M., Favaro, E., Miceli, I., Grassi, G., Camussi, E., Caorsi, C., et al. (2010). Human mesenchymal stem cells modulate cellular immune response to islet antigen glutamic acid decarboxylase in type 1 diabetes. J. Clin. Endocrinol. Metab. 95, 3788–3797. doi: 10.1210/jc.2009-2350
Zevin, A. S., Xie, I. Y., Birse, K., Arnold, K., Romas, L., Westmacott, G., et al. (2016). Microbiome composition and function drives wound-healing impairment in the female genital tract. PLoS Pathog. 12:e1005889. doi: 10.1371/journal.ppat.1005889
Zhang, Q.-Z., Su, W.-R., Shi, S.-H., Wilder-Smith, P., Xiang, A. P., Wong, A., et al. (2010). Human gingiva-derived mesenchymal stem cells elicit polarization of M2 macrophages and enhance cutaneous wound healing. Stem Cells 28, 1856–1868. doi: 10.1002/stem.503
Zhang, A., Wang, M.-H., Dong, Z., and Yang, T. (2006). Prostaglandin E2 is a potent inhibitor of epithelial-to-mesenchymal transition: interaction with hepatocyte growth factor. Am. J. Physiol. Renal Physiol. 291, F1323–F1331. doi: 10.1152/ajprenal.00480.2005
Keywords: mesenchymal stem cell, hypoxia, wound healing, anaerobic bacteria, wound infection
Citation: Andalib E, Kashfi M, Mahmoudvand G, Rezaei E, Mahjoor M, Torki A and Afkhami H (2023) Application of hypoxia-mesenchymal stem cells in treatment of anaerobic bacterial wound infection: wound healing and infection recovery. Front. Microbiol. 14:1251956. doi: 10.3389/fmicb.2023.1251956
Edited by:
Amr H. Hashem, Al-Azhar University, EgyptReviewed by:
Mikaeel Young, Baylor University, United StatesEmoke Pall, University of Agricultural Sciences and Veterinary Medicine of Cluj-Napoca, Romania
Copyright © 2023 Andalib, Kashfi, Mahmoudvand, Rezaei, Mahjoor, Torki and Afkhami. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hamed Afkhami, aGFtZWRhZmtoYW1pNzBAZ21haWwuY29t
†These authors share first authorship
 Elahe Andalib
Elahe Andalib Mojtaba Kashfi2,3,4†
Mojtaba Kashfi2,3,4† Golnaz Mahmoudvand
Golnaz Mahmoudvand Hamed Afkhami
Hamed Afkhami