
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 27 July 2023
Sec. Infectious Agents and Disease
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1229950
This article is part of the Research TopicZoonotic Diseases: Epidemiology, Multi-omics, and Host-pathogen interactionsView all 25 articles
 Abid Ali1*
Abid Ali1* Muhammad Kashif Obaid1
Muhammad Kashif Obaid1 Mashal M. Almutairi2
Mashal M. Almutairi2 Abdulaziz Alouffi3
Abdulaziz Alouffi3 Muhammad Numan1
Muhammad Numan1 Shafi Ullah1
Shafi Ullah1 Gauhar Rehman1
Gauhar Rehman1 Zia Ul Islam4
Zia Ul Islam4 Sher Bahadar Khan5
Sher Bahadar Khan5 Tetsuya Tanaka6*
Tetsuya Tanaka6*Tick-borne Coxiella spp. are emerging in novel regions infecting different hosts, but information regarding their occurrence is limited. The purpose of this study was the molecular screening of Coxiella spp. in various ticks infesting goats, sheep, camels, cattle, wild mice, and domestic fowls (Gallus gallus domesticus) in various districts of Khyber Pakhtunkhwa, Pakistan. Morphologically identified tick species were confirmed by obtaining their cox1 sequences and were molecularly screened for Coxiella spp. by sequencing GroEL fragments. Almost 345 out of 678 (50.9%) hosts were infested by nine tick species. Regarding the age groups, the hosts having an age >3 years were highly infested (192/345, 55.6%), while gender-wise infestation was higher in female hosts (237/345, 68.7%). In collected ticks, the nymphs were outnumbered (613/1,119, 54.8%), followed by adult females (293/1,119, 26.2%) and males (213/1,119, 19.7%). A total of 227 ticks were processed for molecular identification and detection of Coxiella spp. The obtained cox1 sequences of nine tick species such as Hyalomma dromedarii, Hyalomma anatolicum, Haemaphysalis cornupunctata, Haemaphysalis bispinosa, Haemaphysalis danieli, Haemaphysalis montgomeryi, Rhipicephalus haemaphysaloides, Rhipicephalus microplus, and Argas persicus showed maximum identities between 99.6% and 100% with the same species and in the phylogenetic tree, clustered to the corresponding species. All the tick species except Ha. danieli and R. microplus were found positive for Coxiella spp. (40/227, 17.6%), including Coxiella burnetii (15/40, 6.7%), Coxiella endosymbionts (14/40, 6.3%), and different Coxiella spp. (11/40, 4.9%). By the BLAST results, the GroEL fragments of Coxiella spp. showed maximum identity to C. burnetii, Coxiella endosymbionts, and Coxiella sp., and phylogenetically clustered to the corresponding species. This is the first comprehensive report regarding the genetic characterization of Coxiella spp. in Pakistan's ticks infesting domestic and wild hosts. Proper surveillance and management measures should be undertaken to avoid health risks.
Ticks are hematophagous ectoparasites, actively contributing to transmitting infectious agents to wild and domestic animals and humans (De la Fuente et al., 2017). Numerous ticks act as distinguished vectors and reservoirs for various pathogens, including bacteria causing rickettsiosis, anaplasmosis, Lyme disease, viruses such as Powassan, and protozoan agents such as Theileria spp. and Babesia spp. (De la Fuente et al., 2017; Karim et al., 2017; Rochlin and Toledo, 2020; Ali et al., 2021). Aside from transmitting various infectious agents, ticks are hosts to many endosymbionts and a diversified microbiome (Špitalská et al., 2018).
Among the bacterial genus Coxiella having one pathogenic species, Coxiella burnetii is a Gram-negative obligate intracellular bacterium distributed worldwide except in New Zealand and French Polynesia (Musso et al., 2014; Eldin et al., 2017). Common reservoirs of C. burnetii are domestic mammals, including cattle, sheep, goats, and camels as well as reptiles, birds, and ticks (Anderson et al., 2013; Abdel-Moein and Hamza, 2017), and have the potential to cause query (Q) fever (Musso et al., 2014). The Q-fever was reported for the first time in 1935 from Australia as an outbreak of febrile illness with flu-like symptoms (Derrick, 1937), and its causative agent was initially named Rickettsia burnetii, but later on renamed as C. burnetii (Philip, 1948). Coxiella spp. have been isolated from almost 40 tick species, and hence considered as its tick-borne transmission to animals and humans (Eklund et al., 1947; Beaman and Hung, 1989; Duron et al., 2014). Tick species, including Hyalomma dromedarii, Hyalomma anatolicum, Hyalomma scupense, Rhipicephalus microplus, and Rhipicephalus annulatus, may serve as vector reservoirs for the transmission of C. burnetii in Pakistan (Karim et al., 2017). Coxiella-like endosymbionts were also found in various tick species, and an obligatory mutualism between this bacteria and host ticks has been proven (Smith et al., 2015).
Ticks can transmit Coxiella spp. both transovarially and transstadially to their offspring. Infected ticks excrete enormous amounts of Coxiella spp. in their feces, contaminating the skin of host animals and playing a significant role in the spread of Coxiella infection (Cong et al., 2015; Seo et al., 2016). Coxiella burnetii may resist harsh environmental factors, for instance, dry and hot weather, desiccation, and other antiseptics. As it may affect the productive and reproductive abilities, humans and animals could face long-term infection risks (Ullah et al., 2019). Various techniques have been effectively followed for the surveillance of C. burnetii infection. Still, ELISA is considered an effective technique for its serological diagnosis. In combination with sequencing, PCR is believed to be the best technique for the molecular identification and genetic characterization of C. burnetii (Niemczuk et al., 2014; Bontje et al., 2016).
Coxiella spp. have been detected in different tick species, animals, humans, and soil samples in Asia that have been reported in various studies. Coxiella burnetii is the causative agent of Q-fever, one of the ignored zoonoses in developing countries, including Pakistan. To the best of our knowledge, approximately 24 studies from 1955 to 2022 regarding this infection have been reported in Pakistan, and Q-fever in humans, goats, sheep, cattle, buffaloes, as well as rodents has been serologically documented (Ahmed, 1987; Ullah et al., 2019; Ali et al., 2022a; Hussain et al., 2022). The C. burnetii is also considered a soil-borne pathogen as its isolation has been confirmed from soil samples (Shabbir et al., 2015). Due to the information dearth regarding numerous tick-borne pathogens (TBPs) that infect ruminants and other animals in Pakistan, substantial research is required to investigate the genetic composition of various TBPs, specifically Coxiella spp. Hence, this study aimed to molecularly characterize different tick species infesting domestic and wild animals and screen out the associated Coxiella spp. in Pakistan and summarize the association of Coxiella spp. with ticks infesting various hosts in Asia.
The Advance Studies and Research Board (ASRB: Dir/A&R/AWKUM/2022/9396) of the Department of Zoology, Abdul Wali Khan University Mardan, Pakistan, approved prior consent for this study. Additionally, permission was taken from the owners of the animals to observe hosts and ticks collection. All the rules regarding animal welfare regulations were followed while handling the animals.
Different districts including Lakki Marwat (32.5993° N, 70.9160° E), Mansehra (34.3271° N, 73.1992° E), Bajaur (34.7522° N, 71.5162° E), Dir Upper (35.3274° N, 72.0907° E), Dir Lower (34.8364° N, 71.8964° E), Abbottabad (34.1534° N, 73.2215° E), Buner (34.459129° N, 72.557252° E), Charsadda (34.161297° N, 71.755377° E), Chitral (35.727064° N, 71.759794° E), and Nowshera (33.998608° N, 71.999144° E) of Khyber Pakhtunkhwa were selected for the current study. These study locations have desertic plains, arid plains, arid hilly, humid plains, and hilly areas with variations in their climatic conditions, altitude, and seasons (winter, spring, summer, and autumn). The summer season is comparatively hot and longer in district Lakki Marwat than in other districts; however, snowfall occurs in winter in the districts of Chitral, Mansehra, Buner, Bajaur, Dir Upper, Dir Lower, and Abbottabad (climate-data.org; accessed on 20 February 2023). Goats, sheep, camels, and cattle are the livestock of the region that are intended for producing dairy products and transportation. These transhumant animals move from one place to another within the district for food and natural pastures. Their diet and resources depend upon climatic conditions that remarkably vary spatiotemporally. The Global Positioning System was used for the geographical coordinates of the districts as mentioned above and designed the study map through ArcGIS v 10.3.1 (Figure 1).
The herds of goats, sheep, camels, and cattle were visited for tick collection from September 2021 to August 2022. Moreover, wild mice captured by local farmers on agricultural land and domestic fowls (Gallus gallus domesticus) were also examined for tick specimens. Tick specimens were collected manually from different hosts in the study districts. The collected ticks were morphologically identified under a stereomicroscope (SZ61, Olympus, Japan) using available standard morphological keys (Hoogstraal and Kaiser, 1959; Hoogstraal and Varma, 1962; Hoogstraal and Trapido, 1966; Dhanda and Kulkarni, 1969; Kohls et al., 1970; Cerný and Hoogstraal, 1977; Apanaskevich, 2003; Apanaskevich et al., 2008; Ahmad et al., 2022; Ali et al., 2022b). The identified tick species were categorized according to species, gender, and nymph stage or adult stage, and then preserved in 100% ethanol at room temperature before further analyses.
The preserved ticks were washed with 70% ethanol, followed by their immersion in distilled water for 10 min to eliminate the external contamination, and subsequently dried on a sterile filter paper. A subset of 227 (137 N, 49 F, and 41 M) ticks including 65 Ha. cornupunctata (41 N, 14 F, and 10 M), 28 Ha. montgomeryi (17 N, 6 F, and 5 M), 28 Hy. anatolicum (17 N, 6 F, and 5 M), 27 A. persicus (17 N, 6 F, and 4 M), 23 R. haemaphysaloides (15 N, 5 F, and 3 M), 19 R. microplus (9 N, 5 F, and 5 M), 19 Ha. bispinosa (11 N, 4 F, and 4 M), 12 Hy. dromedarii (7 N, 1 F, and 4 M), and 6 Ha. danieli (3 N, 2 F, and 1 M) were randomly selected and used individually for DNA extraction. Each stage of the morphologically identified tick species was individually crushed using sterile scissors to extract genomic DNA through the standard protocol of the phenol–chloroform method (Sambrook et al., 1989).
The whole extracted genomic DNA of each morphologically identified tick species (each stage) was individually used to amplify cox1 fragments by utilizing species-specific primers in a conventional PCR (Table 1). Nested PCR was performed to amplify the GroEL fragment of Coxiella spp. (GE-96G, BIOER, Hangzhou, China). In nested PCR, two pairs of primers were used for the said purpose (Table 1). PCR reaction mixtures were performed in 25 μL, comprised of 1 μL of each primer at a concentration of 10 pmol/μL (first pair of primers in case of GroEL), 8.5 μL PCR water, 2 μL (100 ng/μL) genomic DNA, and 12.5 μL DreamTaq MasterMix (2×) (Thermo Fisher Scientific, Inc., Waltham, MA, USA). However, 2 μL of PCR product from the first PCR amplified reaction was used instead of genomic DNA in the second PCR run (in the case of GroEL) along with the second pair of primers at the same concentration. In each PCR reaction, PCR water was taken as a negative control, while Hyalomma scupense and Rickettsia massiliae DNA were taken as a positive control for ticks and Coxiella, respectively. The amplified products were loaded in 2% agarose gel to observe the expected band through the Gel Documentation System (BioDoc-It™ Imaging Systems, UVP, LLC, Upland, CA, USA). PCR amplified products were purified via GeneClean II Kit (Qbiogene, Il-lkirch, France) following the manufacturer's protocol. The amplified amplicons were sequenced bidirectionally through the Sanger-based sequencing method (Macrogen, Inc., Seoul, South Korea).
The chromatograms of all the obtained sequences were manually observed and trimmed for purification purposes to remove the contaminated and poor reading regions through SeqMan V. 5 (DNASTAR, Inc., Madison, WI, USA). Final trimmed sequences were subjected to Basic Local Alignment Search Tool (BLAST) (Altschul et al., 1990) at National Center for Biotechnology Information to get the high identity sequences in FASTA format. ClustalW multiple alignments (Thompson et al., 1994) were used to align all the downloaded sequences along with the obtained and selected outgroup sequences in BioEdit Sequence Alignment Editor V.7.0.5 (Raleigh, NC, USA) (Hall et al., 2011). The phylogenetic trees based on partial fragments of cox1 and GroEL were constructed in MEGA-X (Molecular Evolutionary Genetics Analysis) (Kumar et al., 2018) through the neighbor-joining method (Tamura-Nei model) and the Maximum Parsimony method (Tamura-Nei model) (Tamura and Nei, 1993) with support of 1000 bootstrapping replicons, respectively. The coding fragments (cox1 and GroEL) were aligned using MUSCLE (Edgar, 2004).
The literature search was conducted using databases such as PubMed, Google Scholar, and Web of Sciences, to overview the published studies regarding the detection of C. burnetii in different ticks, animals, humans, or soil in Asia. The keywords used for the search were as follows: tick(s), small ruminant(s), livestock, C. burnetii, Coxiellosis, and Q-fever. Combinations of the aforementioned various keywords were used to retrieve full-text research articles, review articles, short communications, and conference papers. Reference lists of retrieved articles were screened to identify relevant articles (accessed on 16 April 2023) (Table 2).
The highest number of observed hosts (374/678, 55.2%) were included in a group having age > 3 years, followed by various hosts (192/678, 28.3%) having age 1–3 years, and the lowest numbers of observed hosts (112/678, 16.5%) belonging to the age group having age <1 year. Different hosts having age >3 years were highly infested (192/345, 55.6%), while animals having age ≤1 year were least infested (62/345, 18.0%). The examined and infested female hosts were more predominant in number (486/678; 71.7%, 237/345; 68.7%) than male hosts (192/678; 28.3%, 108/345; 31.3%). The highest number of infested hosts was recorded in summer (June–August) (146/345, 42.3%), followed by spring (March–May) (99/345, 28.7%), autumn (September–November) (65/345, 18.8%), and winter (December–February) (35/345, 10.4%), respectively (Table 3).
A total of 678 different hosts such as goats, sheep, camels, cattle, wild mice, and domestic fowls were examined for tick collection in the selected localities, of which other hosts (number = 345/678, 50.9%), including goats (78/149, 52.3%), sheep (75/136, 55.1%), camels (36/93, 38.7%), cattle (69/129, 53.5%), wild mice (15/48, 31.2%), and domestic fowls (72/123, 58.5%) were found tick infested. The highest prevalence of infested hosts was recorded in district Lakki Marwat (44/351, 12.5%), followed by Charsadda (36/351, 10.3%), Nowshera, Buner, Mansehra, and Abbottabad (35/351, 9.7%), Chitral (34/351, 9.7%), Dir Upper and Dir Lower (33/351, 9.4%), while least infestation rate was recorded in district Bajaur (31/351, 8.8%) (Table 4).
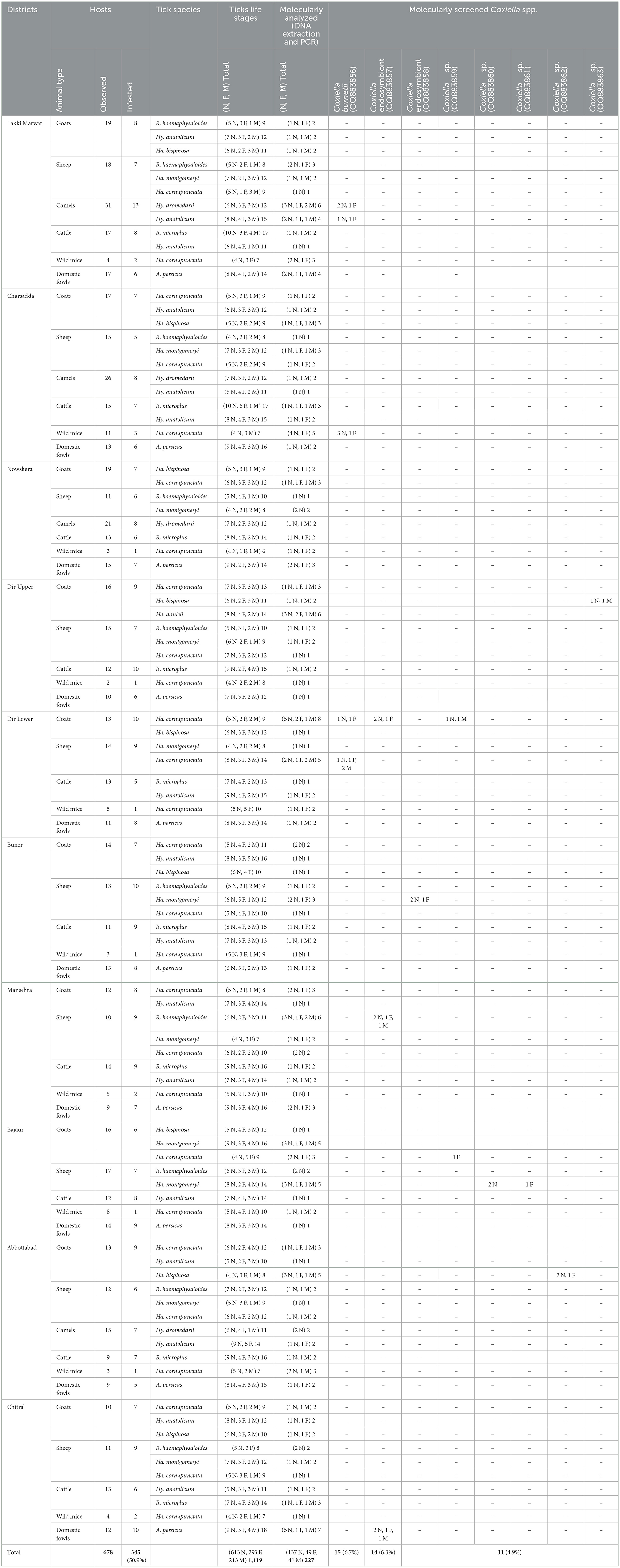
Table 4. Occurrence of ticks and molecular detection of Coxiella spp. in different districts of Khyber Pakhtunkhwa.
Altogether, 1,119 ticks including nymphs (613/1,119, 54.8%), adult females (293/1,119, 26.2%), and males (213/1,119, 19.0%) were collected from infested hosts and were categorized into four genera (Haemaphysalis, Hyalomma, Rhipicephalus, and Argas). The highest number of collected tick species was Ha. cornupunctata (258/1,119, 23.1%), followed by Hy. anatolicum (209/1,119, 18.7%), A. persicus (146/1,119, 13.0%), R. microplus (137/1,119, 12.2%), Ha. montgomeryi (119/1,119, 10.6%), R. haemaphysaloides (97/1,119, 8.7%), Ha. bispinosa (92/1,119, 8.2%), Hy. dromedarii (47/1,119, 4.2%), and Ha. danieli (14/1,119, 1.2%). Genomic DNA was extracted from 227 morphologically identified ticks (137 N, 49 F, and 41 M), and all identified tick species were molecularly confirmed via sequencing of the cox1 partial fragment (Table 4).
Extracted DNA of 227 (20.3%) was used to amplify the fragments of GroEL of Coxiella spp. A total of 40/227 (17.6%) ticks were found positive for Coxiella spp., including C. burnetii (15, 6.7%), Coxiella endosymbionts (14, 6.3%), and Coxiella sp. (11, 4.9%). Coxiella burnetii was detected in ticks collected from camels (Hy. dromedarii and Hy. anatolicum) in the district of Lakki Marwat, wild mice (Ha. cornupunctata) in Charsadda, and goats and sheep (Ha. cornupunctata) in Dir Lower. Coxiella endosymbionts were detected in ticks collected from goats (Ha. cornupunctata) in district Dir Lower sheep (Ha. montgomeryi and R. haemaphysaloides) in Buner and Mansehra, and domestic fowls (A. persicus) in Chitral. Coxiella spp. were detected in ticks collected from goats (Ha. bispinosa) in the district of Dir Upper, from goats (Ha. cornupunctata) in Dir Lower, from goats and sheep (Ha. cornupunctata and Ha. montgomeryi) in Bajaur, and from goats (Ha. bispinosa) in Abbottabad (Table 4).
All amplified PCR products were separately sequenced. The identical sequences were considered as a single consensus sequence. Trimmed and purified sequences of cox1 fragments were obtained from nine tick species, including Ha. cornupunctata, Ha. bispinosa, Ha. danieli, Ha. montgomeryi, Hy. anatolicum, Hy. dromedarii, R. haemaphysaloides, R. microplus, and A. persicus. The BLAST results showed that the cox1 fragments of the nine tick species were 99.6–100% identical to the corresponding species. Additionally, these sequences were phylogenetically clustered to the corresponding species reported from Pakistan, China, India, Bangladesh, Kazakhstan, Kenya, and Iran (Figure 2).
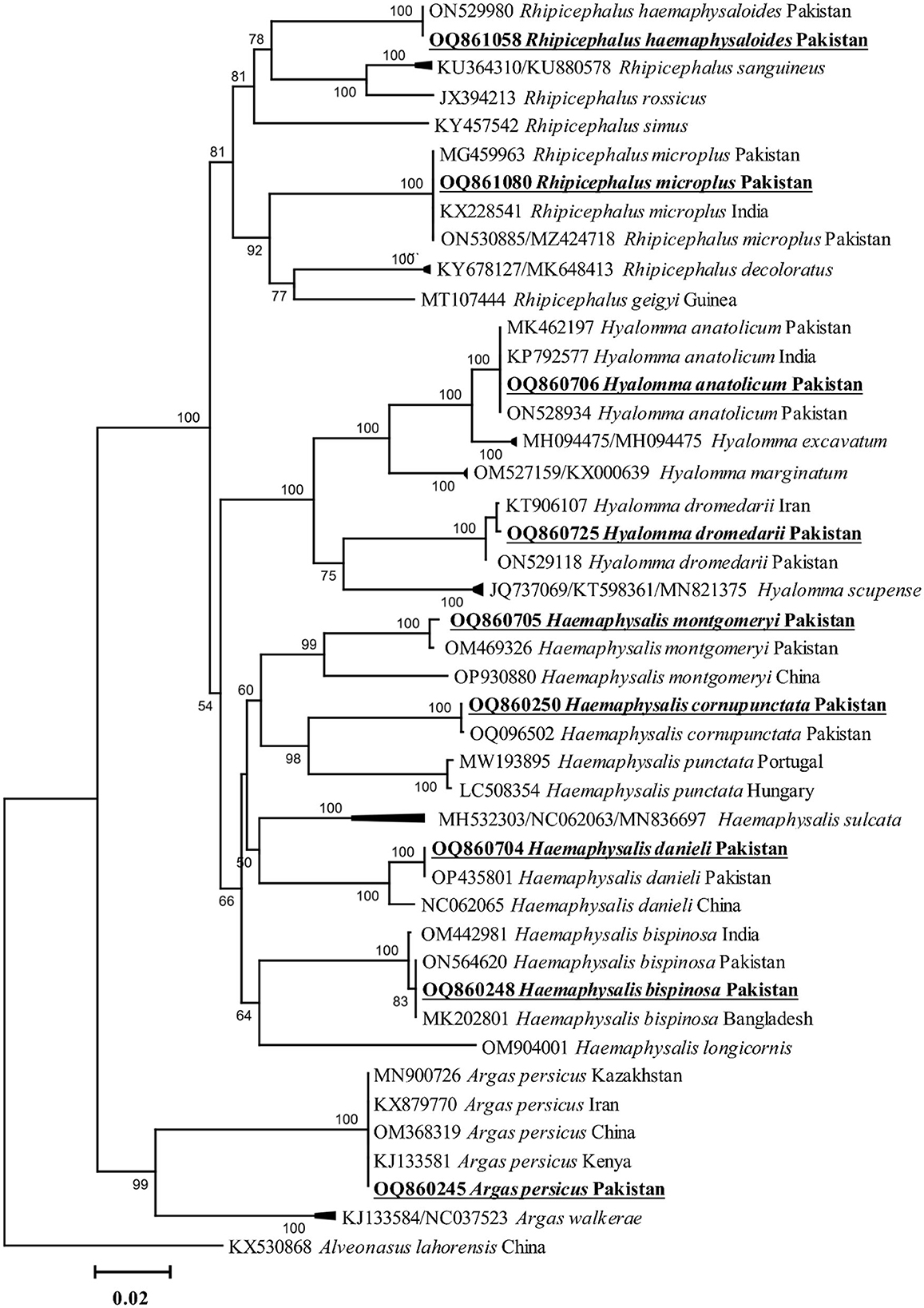
Figure 2. Phylogenetic tree based on cox1 fragments of tick species. The sequence of Alveonasus lahorensis (KX530868) was used as an outgroup. The levels of bootstrap support (≥60%) for phylogenetic groupings are given at each node. The obtained sequences are represented with bold and underlined fonts.
Based on the GroEL fragment, eight GroEL fragments of Coxiella spp. were detected in the aforementioned tick species except for Ha. danieli and R. microplus. By the BLAST results, the obtained GroEL fragment of Coxiella sp. (detected in Ha. cornupunctata, Hy. dromedarii, and Hy. anatolicum) showed maximum identity (99.8–100%) with the C. burnetii and phylogenetically clustered with the corresponding species reported from Slovakia (MG860513), China (ON455116), Russia (EF627450), USA (CP040059), and Thailand (MZ327921).
The obtained GroEL fragment of Coxiella spp. (detected in Ha. cornupunctata, A. persicus, and R. haemaphysaloides) showed 100% identity with the Coxiella endosymbiont reported from China (MZ367034 and MZ367036). Another GroEL fragment of Coxiella sp. (detected in Ha. montgomeryi) showed 99.8% maximum identity with the Coxiella endosymbiont reported from France (KP985488) and China (KP985490). Both partial fragments clustered with the corresponding Coxiella endosymbiont in the phylogenetic tree.
The BLAST results of the obtained GroEL fragments of Coxiella sp. OQ883859 (detected in Ha. cornupunctata) showed 88–90% maximum identity with Coxiella endosymbiont reported from the United Kingdom (KP985492), Coxiella sp. OQ883860 (detected in Ha. montgomeryi) showed 90.23% identity with Coxiella sp. reported from France (KP985502 and KP985500), Tunisia (KP985456), and Algeria (KP985472). The Coxiella sp. OQ883861 (detected in Ha. montgomeryi) showed 95.4% identity with Coxiella sp. reported from Chile (KJ459055) and Spain (MW287611), while Coxiella sp. OQ883862 (detected in Ha. bispinosa) showed 93.9% identity with Coxiella sp. reported from China (OK625731 and OK625732), and Coxiella sp. OQ883863 (detected in Ha. bispinosa) showed 91.3% identity with Coxiella sp. reported from China (OK625731 and OK625732). In the phylogenetic tree, all the obtained aforementioned Coxiella sp. sequences were clustered to the corresponding Coxiella sp. sequences (Figure 3).
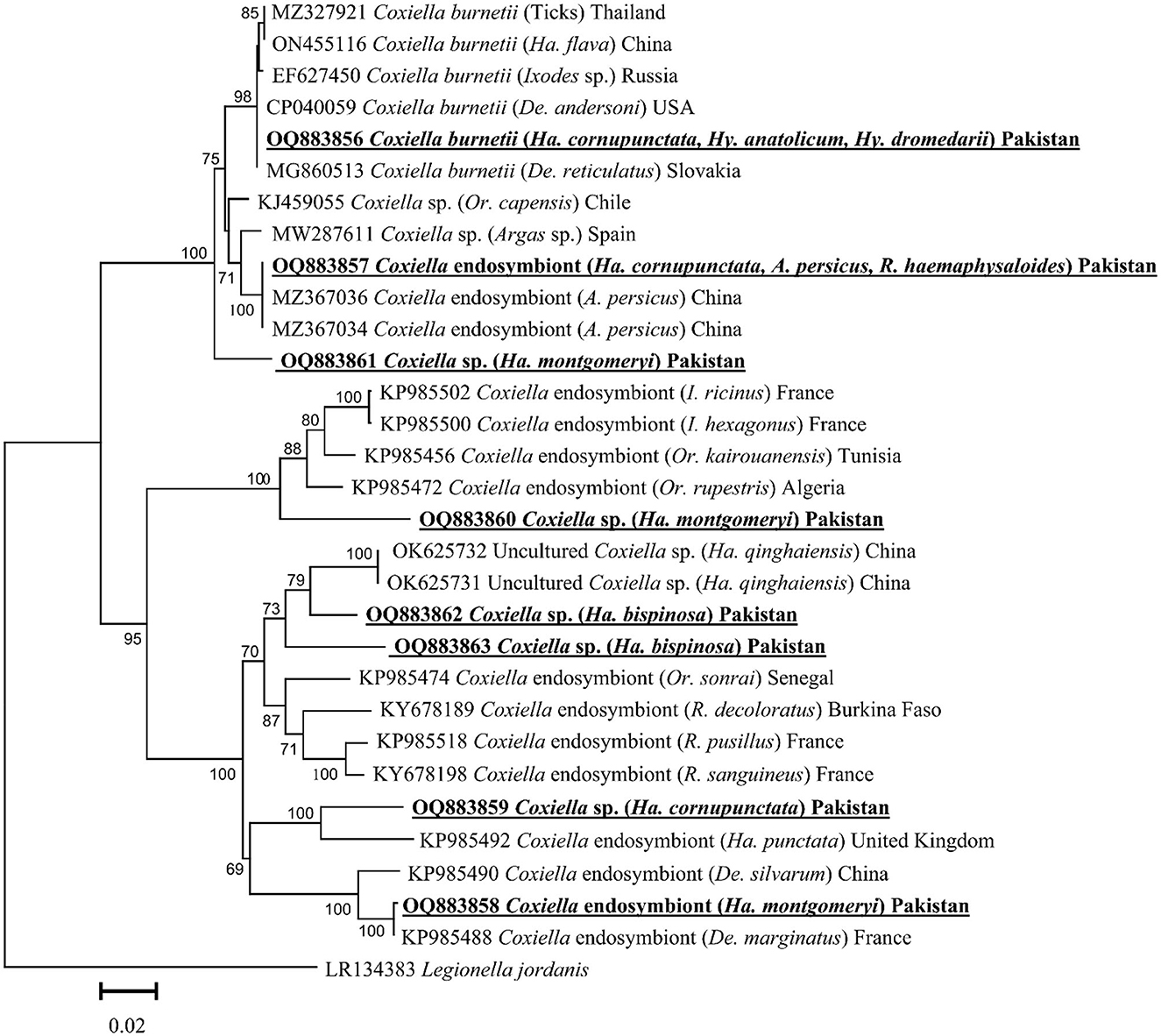
Figure 3. Phylogenetic tree based on GroEL fragments of Coxiella spp. detected in tick species. The sequence of Legionella jordanis (LR134383) was used as an outgroup. The levels of bootstrap support (≥ 60%) for phylogenetic groupings are given at each node. The obtained sequences are represented with bold and underlined fonts.
The obtained cox1 partial fragments of ticks were submitted to GenBank under accession numbers: OQ860250 (Ha. cornupunctata), OQ860248 (Ha. bispinosa), OQ860704 (Ha. danieli), OQ860705 (Ha. montgomeryi), OQ860706 (Hy. anatolicum), OQ860725 (Hy. dromedarii), OQ861058 (R. haemaphysaloides), OQ861080 (R. microplus), and OQ860245 (A. persicus). The obtained GroEL partial fragments of Coxiella spp. were submitted to GenBank under accession numbers: OQ883856 (C. burnetii), OQ883857 (Coxiella endosymbiont), OQ883858 (Coxiella endosymbiont), OQ883859 (Coxiella sp.), OQ883860 (Coxiella sp.), OQ883861 (Coxiella sp.), OQ883862 (Coxiella sp.).
Various ticks and their associated pathogens ideally propagate in Pakistan's humid and variable climatic conditions (Karim et al., 2017; Ali et al., 2019, 2020, 2021; Obaid et al., 2023). Microbiota belonging to different bacterial genera have been detected in different tick species in Pakistan (Karim et al., 2017; Ali et al., 2021; Alam et al., 2022; Khan Z. et al., 2022; Khan S. M. et al., 2023; Numan et al., 2022). Some serological surveys of Q-fever in small ruminants, large ruminants, rodents, and humans have been reported from Pakistan (Ahmed, 1987; Ali et al., 2022a). There is limited information available regarding the molecular characterization of C. burnetii and thus it remains an ignored zoonotic disease in the country. The association of Coxiella spp. with different ticks infesting various hosts has been reviewed globally (Guatteo et al., 2011); therefore, in this study, we summarized this association of Coxiella spp. with different ticks in Asia. Nine tick species including Ha. cornupunctata, Ha. bispinosa, Ha. montgomeryi, Ha. danieli, Hy. anatolicum, Hy. dromedarii, R. haemaphysaloides, R. microplus, and A. persicus infesting goats, sheep, camels, cattle, wild mice, and domestic fowls were genetically characterized. In addition, this is the first report regarding the molecular detection and phylogenetic positioning of Coxiella spp. associated with ticks in Pakistan. Overall, C. burnetii, two Coxiella endosymbionts, and five undetermined Coxiella sp. were genetically characterized based on GroEL fragments in various tick species.
Environmental factors such as humidity and temperature mainly affect the distribution of ticks, TBDs, and their zoonotic threats to human and animal health (Léger et al., 2013). Since the current study area's existing environmental and climatic conditions are favorable for tick infestation and propagation of various pathogens (Aiman et al., 2022; Ali et al., 2023), many ticks were collected during this survey. Contrary to previous studies, Ha. cornupunctata tick was more prevalent than other tick species such as R. microplus and Hy. anatolicum in Pakistan (Karim et al., 2017; Ali et al., 2019, 2021; Khan Z. et al., 2022). It may be due to examining different hosts, such as goats, sheep, and wild mice attributing a closed association with this species.
The age of the host is a significant factor to tick infestation. According to previous reports, a high tick burden was recorded on adult hosts compared with young ones (Ali et al., 2021; Kamran et al., 2021; Khan Z. et al., 2022). Large body surfaces and free grazing practices of adult animals make them more vulnerable due to high tick infestation. In contrast, the robust immune system, less grazing, and low body surface of the younger hosts contribute to less tick infestation (Swai et al., 2005). Female hosts were highly tick infested compared with the male hosts, which is consistent with previous findings (Ullah et al., 2023). Higher levels of progesterone and prolactin hormones in females make them susceptible to tick infestation (Anderson et al., 2013; Ahmed et al., 2023). The higher levels of progesterone and prolactin hormones may increase the susceptibility of females to tick's infections (Lloyd, 1983; Ahmed et al., 2023). Additionally, in the current study, ticks were predominantly reported in summer (June–August) compared with other seasons because the warm and humid climatic conditions in the region provide a suitable environment for the development of all stages of ticks (Ali et al., 2019, 2021). The comparatively wide host range noted for different Haemaphysalis, Hyalomma, and Rhipicephalus ticks may be due to frequent practices such as putting various hosts in the same shelter and over-crowded livestock and concurrent grazing in the survey area.
Major consequences have been revealed in the epidemiology of Q-fever upon the molecular detection of C. burnetii DNA in ticks collected from the environment, domestic and wild animals (Yessinou et al., 2022). It has been observed that ticks may transmit the Q-fever agent and pollute the environment as well as the host's body in Pakistan (Ullah et al., 2019). The association between different ticks and C. burnetii and its transstadially and transovarially transmission has been reported, suggesting the Q-fever transmission from infected to healthy animals through blood meal (Gong et al., 2020). In this study, molecular detection of Coxiella spp. varied in the aforementioned seven tick species collected from various hosts. A high prevalence of Q-fever in camels in this study may be attributable to the camels' vulnerability regarding C. burnetii infection or camel tick competence as a reservoir for this pathogen (Gumi et al., 2013). Common reservoirs for C. burnetii are small ruminants that may excrete a diverse number of these bacteria in their birth byproducts (placenta). Coxiella spp. were highly detected in ticks collected from small ruminants (goats and sheep), and these findings agreed with the previous serosurvey conducted in Pakistan (Ullah et al., 2019). Coxiella sp. detected in A. persicus ticks collected from domestic fowls suggest that different soft ticks may also be investigated as host reservoirs for various undetermined Coxiella spp., as reported in other studies (Trinachartvanit et al., 2018).
In the current study, phylogenetic analysis via cox1 fragments of nine different tick species revealed a close evolutionary relationship with the same species reported from Pakistan, China, India, Bangladesh, and Iran, and these findings were supported by previous studies (Ahmad et al., 2022; Alam et al., 2022; Ali et al., 2022a; Khan S. M. et al., 2023). Phylogenetic analysis of Coxiella spp., detected in different tick species, showed close association with their respective species reported from the same or different tick species and humans. This association of Coxiella spp. may be due to the close interaction of infested animals with humans, which enhances zoonotic infections such as Q-fever in humans. So-far neglected surveillance of Coxiella spp. in the region demands immediate attention to its pathogenic consequences.
Coxiella spp. were molecularly detected in ticks infesting goats, sheep, camels, cattle, wild mice, and domestic fowls and were confirmed through sequencing for the first time in Pakistan. Further research is essential to investigate any potential health risks due to these agents. The veterinarian livestock holders and farm workers lack knowledge regarding the epidemiology of Q-fever and its causative agents in Pakistan. Livestock holders should be adequately educated regarding Q-fever prevention and management practices because the occurrence of this agent can lead to long-term environmental contamination, which is a potential threat to animals and humans. Consequently, effective measures associated with Q-fever must be implemented, including limiting contact between herds, quarantining newly purchased animals, and using disinfectants that can reduce the spread of infection and possible transmission to humans.
The Advance Studies and Research Board (ASRB: Dir/A&R/AWKUM/2022/9396) of the Department of Zoology, Abdul Wali Khan University Mardan, Pakistan, approved prior consent for this study. Additionally, permission was taken from the owners of the animals to observe hosts and ticks collection. All the rules regarding animal welfare regulations were followed while handling the animals.
The datasets presented in the study have been deposited to the NCBI GenBank repository, accession numbers OQ860250 (Ha. cornupunctata), OQ860248 (Ha. bispinosa), OQ860704 (Ha. danieli), OQ860705 (Ha. montgomeryi), OQ860706 (Hy. anatolicum), OQ860725 (Hy. dromedarii), OQ861058 (R. haemaphysaloides), OQ861080 (R. microplus), OQ860245 (A. persicus), OQ883856 (C. burnetii), OQ883857 (Coxiella endosymbiont), OQ883858 (Coxiella endosymbiont), OQ883859 (Coxiella sp.), OQ883860 (Coxiella sp.), OQ883861 (Coxiella sp.), and OQ883862 (Coxiella sp.).
The animal study was reviewed and approved by the Advance Studies and Research Board (ASRB: Dir/A&R/AWKUM/2022/9396) of the Department of Zoology, Abdul Wali Khan University Mardan, Pakistan, approved prior consent for this study. Written informed consent was obtained from the owners for the participation of their animals in this study.
AAli designed the study. AAli, MMA, TT, SBK, and AAlo carried out the experiments of the study. AAli, MN, ZUI, GR, MKO, SBK, and SU collected the tick samples and performed the experiments. AAli, MKO, and MN performed the phylogenetic and statistical analyses. All authors have read and agreed to the published version of the manuscript.
The authors acknowledge the financial support provided by the Higher Education Commission of Pakistan and the Pakistan Science Foundation. The researchers support project number (RSP2023R494), King Saud University, Riyadh, Saudi Arabia. This work was supported by JSPS KAKENHI Grant Numbers JP20KK0154 and JP22H02522, JSPS Bilateral Program (Grant number JPJSBP120239937).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Moein, K. A., and Hamza, D. A. (2017). The burden of Coxiella burnetii among aborted dairy animals in Egypt and its public health implications. Acta Trop. 166, 92–95. doi: 10.1016/j.actatropica.2016.11.011
Ahmad, I., Ullah, S., Alouffi, A., Almutairi, M. M., Khan, M., Numan, M., et al. (2022). Description of male, redescription of female, host record, and phylogenetic position of Haemaphysalis danieli. Pathogens. 11, 1495. doi: 10.3390/pathogens11121495
Ahmed, I. P. (1987). A serological investigation of Q fever in Pakistan. J. Pak. Med. Assoc. 37, 126–129.
Ahmed, M., Ibrahim, A., and Mahamed, S. (2023). Study on prevalence of one humped camel mange and its associated risk factors in selected kebeles of Kebribeyah District of Fafan Zone, Somali Regional State, Ethiopia. Res. Squ. [Preprint]. doi: 10.21203/rs.3.rs-2619682/v1
Aiman, O., Ullah, S., Chitimia-Dobler, L., Nijhof, A. M., and Ali, A. (2022). First report of Nosomma monstrosum ticks infesting Asian water buffaloes (Bubalus bubalis) in Pakistan. Ticks Tick-Borne Dis. 13, 101899. doi: 10.1016/j.ttbdis.2022.101899
Akbarian, Z., Ziay, G., Schauwers, W., Noormal, B., Saeed, I., Qanee, A. H., et al. (2015). Brucellosis and Coxiella burnetii infection in householders and their animals in secure villages in Herat province, Afghanistan: a cross-sectional study. PLoS Negl. Trop. Dis. 9, e0004112. doi: 10.1371/journal.pntd.0004112
Alam, S., Khan, M., Alouffi, A., Almutairi, M. M., Ullah, S., Numan, M., et al. (2022). Spatio-temporal patterns of ticks and molecular survey of Anaplasma marginale, with notes on their phylogeny. Microorganisms. 10, 1663. doi: 10.3390/microorganisms10081663
Al-Graibawi, M. A., Yousif, A. A., Gharban, H. A., and Zinsstag, J. (2021). First serodetection and molecular phylogenetic documentation of Coxiella burnetii isolates from female camels in Wasit governorate, Iraq. Iraqi J. Vet. Sci. 35, 47–52. doi: 10.33899/ijvs.2021.130888.1890
Ali, A., Khan, M. A., Zahid, H., Yaseen, P. M., Qayash Khan, M., Nawab, J., et al. (2019). Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 10, 793. doi: 10.3389/fphys.2019.00793
Ali, A., Mulenga, A., and Vaz, I. S. Jr. (2020). Tick and tick-borne pathogens: molecular and immune targets for control strategies. Front. Physiol. 11, 744. doi: 10.3389/fphys.2020.00744
Ali, A., Numan, M., Ullah, S., Khan, M., and Kamran, K. (2023). Genetic characterization of Haemaphysalis (Rhipistoma) indica and Haemaphysalis (Segalia) montgomeryi ticks (Ixodoidea: Ixodidae). Ticks Tick Borne Dis. 14, 102105. doi: 10.1016/j.ttbdis.2022.102105
Ali, A., Shehla, S., Zahid, H., Ullah, F., Zeb, I., Ahmed, H., et al. (2022a). Molecular survey and spatial distribution of Rickettsia spp. in ticks infesting free-ranging wild animals in Pakistan (2017–2021). Pathogens. 11, 162. doi: 10.3390/pathogens11020162
Ali, A., Zahid, H., Zeb, I., Tufail, M., Khan, S., Haroon, M., et al. (2021). Risk factors associated with tick infestations on equids in Khyber Pakhtunkhwa, Pakistan, with notes on Rickettsia massiliae detection. Parasit. Vectors. 14, 1–12. doi: 10.1186/s13071-021-04836-w
Ali, S., Saeed, U., Rizwan, M., El-Adawy, H., Mertens-Scholz, K., and Neubauer, H. (2022b). Serological prevalence of and risk factors for Coxiella burnetii infection in women of Punjab Province, Pakistan. Int. J. Environ. Res. Public Health. 19, 4576. doi: 10.3390/ijerph19084576
Al-Kindi, N., Al-Yaaqoubi, M., Al-Rashdi, Y., Al-Rashdi, A., Al-Ajmi, A., and Al-Maani, A. (2022). The first confirmed pediatric chronic osteomyelitis due to Coxiella burnetii in Oman. Oman Med. J. 37, e449. doi: 10.5001/omj.2023.03
Almogren, A., Shakoor, Z., Hasanato, R., and Adam, M. H. (2013). Q fever: a neglected zoonosis in Saudi Arabia. Ann. Saudi Med. 33, 464–468. doi: 10.5144/0256-4947.2013.464
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Amitai, Z., Bromberg, M., Bernstein, M., Raveh, D., Keysary, A., David, D., et al. (2010). A large Q fever outbreak in an urban school in central Israel. Clin. Infect. Dis. 50, 1433–1438. doi: 10.1086/652442
Anderson, K., Ezenwa, V. O., and Jolles, A. E. (2013). Tick infestation patterns in free ranging African buffalo (Syncercus caffer): effects of host innate immunity and niche segregation among tick species. Int. J. Parasitol. Parasites Wildl. 2, 1–9. doi: 10.1016/j.ijppaw.2012.11.002
Apanaskevich, D. A. (2003). Differentiation of closely related species Hyalomma anatolicum and H. excavatum (Acari: Ixodidae) based on a study of all life cycle stages, throughout entire geographical range. Parazitologiia. 37, 259–280.
Apanaskevich, D. A., Schuster, A. L., and Horak, I. G. (2008). The genus Hyalomma: VII. Redescription of all parasitic stages of H. (Euhyalomma) dromedarii and H. (E.) schulzei (Acari: Ixodidae). J. Med. Entomol. 45, 817–831. doi: 10.1093/jmedent/45.5.817
Barigye, R., Hassan, N. A. D., Abdalla-Alfaki, I. M., Al Alawi, H. K. K., Aldhaheri, A. M. B., Ghanim, F. M., et al. (2022). Pilot serosurvey of Coxiella burnetii in domesticated small ruminants in the United Arab Emirates. Trop. Anim. Health Prod. 54, 1–6. doi: 10.1007/s11250-022-03150-6
Beaman, M. H., and Hung, J. (1989). Pericarditis associated with tick-borne Q fever. Intern. Med. 19, 254–256. doi: 10.1111/j.1445-5994.1989.tb00258.x
Bontje, D. M., Backer, J. A., Hogerwerf, L., Roest, H. I. J., and Van Roermund, H. J. W. (2016). Analysis of Q fever in Dutch dairy goat herds and assessment of control measures by means of a transmission model. Prev. Vet. Med. 123, 71–89. doi: 10.1016/j.prevetmed.2015.11.004
Burns, R. J., Douangngeun, B., Theppangna, W., Khounsy, S., Mukaka, M., Selleck, P. W., et al. (2018). Serosurveillance of Coxiellosis (Q-fever) and Brucellosis in goats in selected provinces of Lao People's Democratic Republic. PLoS Negl. Trop. Dis. 12, e0006411. doi: 10.1371/journal.pntd.0006411
Cerný, V., and Hoogstraal, H. (1977). Haemaphysalis (Allophysalis) danieli, sp. n. (Ixodoidea: Ixodidae), female and tentatively associated immature stages from high mountains of northern Pakistan and Afghanistan. J. Parasitol. 567-574. doi: 10.2307/3280022
Chakrabartty, A., Nahar, A., Rahman, M. S., Rahman, A. K. M. A., Sarker, A. S., Hasan, M. M., et al. (2021). Sero-molecular investigation of Coxiella burnetii infection in domestic ruminants and humans and associated risk factors based on “One Health” approach in Bangladesh. J. Vet. Med. OH Res, 3, 93–117. doi: 10.36111/jvmohr.2021.3(1).0027
Chan, J. F., Tse, H., To, K. K., Li, I. W., Tang, B. S., Cheng, V. C., et al. (2010). Q fever: underdiagnosed in Hong Kong. Hong Kong Med. J. 16, 56–58.
Chang, C. C., Lin, P. S., Hou, M. Y., Lin, C. C., Hung, M. N., Wu, T. M., et al. (2010). Identification of risk factors of Coxiella burnetii (Q fever) infection in veterinary-associated populations in southern Taiwan. Zoonoses Public Health. 57, e95–e101. doi: 10.1111/j.1863-2378.2009.01290.x
Chou, C. H., Yeh, T. M., Lu, Y. P., Shih, W. L., Chang, C. D., Chien, C. H., et al. (2014). Prevalence of zoonotic pathogens by molecular detection in stray dogs in central Taiwan. Thai J. Vet. Med. 44, 363–375.
Cong, W., Meng, Q. F., Shan, X. F., Sun, W. W., Kang, Y. H., Chen, L., Wang, W. L., and Qian, A. D. (2015). Coxiella burnetii (Q fever) infection in farmed ruminants in three northeastern provinces and Inner Mongolia autonomous region, China. Vector Borne Zoonotic Dis. 15, 512–514. doi: 10.1089/vbz.2015.1789
Dabaja, M. F., Greco, G., Blanda, V., Tempesta, M., Bayan, A., Torina, A., et al. (2020). Multispacer sequence typing of Coxiella burnetii from milk and hard tick samples from ruminant farms in Lebanon. Vet. Ital. 56, 289–296. doi: 10.12834/VetIt.1799.13290.1
Dabaja, M. F., Greco, G., Villari, S., Bayan, A., Vesco, G., Gargano, V., et al. (2018). The first serological study of Q fever in humans in Lebanon. Vector Borne Zoonotic Dis. 18, 138–143. doi: 10.1089/vbz.2016.2102
De la Fuente, J., Antunes, S., Bonnet, S., Cabezas-Cruz, A., Domingos, A. G., Estrada-Peña, A., et al. (2017). Tick-pathogen interactions and vector competence: identification of molecular drivers for tick-borne diseases. Front. Cell. Infect. Microbiol. 7, 114. doi: 10.3389/fcimb.2017.00114
Derrick, E. H. (1937). “Q” fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Med. J. Aust. 2, 43743. doi: 10.5694/j.1326-5377.1937.tb43743.x
Dhanda, V., and Kulkarni, S. M. (1969). Immature stages of Haemaphysalis cornupunctata Hoogstraal and Varma, 1962 (Acarina: Ixodidae) with new host and locality records, and notes on its ecology. Orient. Insects. 3, 15–21. doi: 10.1080/00305316.1969.10433891
Doung-Ngern, P., Chuxnum, T., Pangjai, D., Opaschaitat, P., Kittiwan, N., Rodtian, P., et al. (2017). Seroprevalence of Coxiella burnetii antibodies among ruminants and occupationally exposed people in Thailand, 2012–2013. Am. J. Trop. Med. Hyg. 96, 786. doi: 10.4269/ajtmh.16-0336
Duron, O., Jourdain, E., and McCoy, K. D. (2014). Diversity and global distribution of the Coxiella intracellular bacterium in seabird ticks. Ticks tick-borne Dis. 5, 557–563. doi: 10.1016/j.ttbdis.2014.04.003
Edgar, R. C. (2004). MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 5, 113. doi: 10.1186/1471-2105-5-113
Eklund, C. M., Parker, R. R., Lackman, D. B., Ecklund, C. M., and Lockman, D. B. (1947). A case of Q fever probably contracted by exposure to ticks in nature. Public Health Rep. 1896–1970, 1413–1416. doi: 10.2307/4586287
El Tigani-Asil, E. T. A., Blanda, V., Abdelwahab, G. E., Hammadi, Z. M. A., Habeeba, S., Khalafalla, A. I., et al. (2021). Molecular investigation on tick-borne hemoparasites and Coxiella burnetii in dromedary camels (Camelus dromedarius) in Al Dhafra Region of Abu Dhabi, UAE. Animals. 11, 666. doi: 10.3390/ani11030666
Eldin, C., Mélenotte, C., Mediannikov, O., Ghigo, E., Million, M., Edouard, S., et al. (2017). From Q fever to Coxiella burnetii infection: a paradigm change. Clin. Microbiol. Rev. 30, 115–190. doi: 10.1128/CMR.00045-16
El-Mahallawy, H. S., Kelly, P., Zhang, J., Yang, Y., Wei, L., Tian, L., et al. (2016). Serological and molecular evidence of Coxiella burnetii in samples from humans and animals in China. Ann. Agric. Environ. Med. 23, 1196859. doi: 10.5604/12321966.1196859
Esmaeili, S., Mohabati Mobarez, A., Khalili, M., Mostafavi, E., and Moradnejad, P. (2019). Genetic evidence of Coxiella burnetii infection in acute febrile illnesses in Iran. Plos Neg. Trop. Dis. 13, e0007181. doi: 10.1371/journal.pntd.0007181
Esson, C., Skerratt, L. F., Berger, L., Malmsten, J., Strand, T., Lundkvist, Å., et al. (2019). Health and zoonotic infections of snow leopards Panthera unica in the South Gobi Desert of Mongolia. Infect. Ecol. Epidemiol. 9, 1604063. doi: 10.1080/20008686.2019.1604063
Folmer, O., Hoeh, W. R., Black, M., and Vrijenhoek, R. C. (1994). Conserved primers for PCR amplification of mitochondrial DNA from different invertebrate phyla. Mol. Marine Biol. Biotechnol. 3, 294–299.
Gharban, H. A., and Yousif, A. A. (2020). Serological and molecular phylogenetic detection of Coxiella burnetii in lactating cows, Iraq. Iraqi J.Vet.Med. 44, 42–50. doi: 10.30539/ijvm.v44i(E0).1020
Gong, X. Q., Xiao, X., Liu, J. W., Han, H. J., Qin, X. R., Lei, S. C., et al. (2020). Occurrence and genotyping of Coxiella burnetii in hedgehogs in China. Vector Borne Zoonotic Dis. 20, 580–585. doi: 10.1089/vbz.2019.2589
Guatteo, R., Seegers, H., Taurel, A. F., Joly, A., and Beaudeau, F. (2011). Prevalence of Coxiella burnetii infection in domestic ruminants: a critical review. Vet. Microbiol. 149, 1–16. doi: 10.1016/j.vetmic.2010.10.007
Gumi, B., Firdessa, R., Yamuah, L., Sori, T., Tolosa, T., Aseffa, A., et al. (2013). Seroprevalence of brucellosis and Q-fever in southeast Ethiopian pastoral livestock. J. Vet. Sci. Med. Diagn. 2, 109. doi: 10.4172/2325-9590.1000109
Hall, T., Biosciences, I., and Carlsbad, C. J. (2011). BioEdit: an important software for molecular biology. GERF Bull Biosci. 2, 60–61.
Hoogstraal, H., and Kaiser, M. (1959). Observations on Egyptian Hyalomma ticks (Ixodoidea, Ixodidae). 5. Biological notes and differences in identity of H. anatolicum and its subspecies anatolicum Koch and excavatum Koch among Russian and other workers. Identity of H. lusitanicum Koch. Ann. Entomol. Soc. Am. 52, 3 243–261. doi: 10.1093/aesa/52.3.243
Hoogstraal, H., and Trapido, H. (1966). Redescription of the type materials of Haemaphysalis (Kaiseriana) bispinosa Neumann (India), H. (K.) neumanni Dönitz (Japan), H. (K.) lagrangei Larrousse (Vietnam), and H. (K.) yeni Toumanoff (Vietnam) (Ixodoidea, Ixodidae). J. Parasitol. 52, 1188–1198. doi: 10.2307/3276366
Hoogstraal, H., and Varma, M. G. R. (1962). Haemaphysalis cornupunctata sp. n. and H. kashmirensis sp. n. from Kashmir, with Notes on H. sundrai Sharif and H. sewelli Sharif of India and Pakistan (Ixodoidea, Ixodidae). J. Parasitol. 48, 185–194. doi: 10.2307/3275561
Hussain, S., Saqib, M., El-Adawy, H., Hussain, M. H., Jamil, T., Sajid, M. S., et al. (2022). Seroprevalence and molecular evidence of Coxiella burnetii in dromedary camels of Pakistan. Front. Vet. Sci. 9, 908479. doi: 10.3389/fvets.2022.908479
Jalboush, N., and Alzuheir, I. (2017). Serosurvey of Q fever in active reproductive rams in northern Palestine. Iraqi J. Vet. Sci. 31, 87–90. doi: 10.33899/ijvs.2017.145602
Kamran, K., Ali, A., Villagra, C., Siddiqui, S., Alouffi, A. S., and Iqbal, A. (2021). A cross-sectional study of hard ticks (acari: ixodidae) on horse farms to assess the risk factors associated with tick-borne diseases. Zoonoses Public Health. 68, 247–262. doi: 10.1111/zph.12809
Karim, S., Budachetri, K., Mukherjee, N., Williams, J., Kausar, A., Hassan, M. J., et al. (2017). A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 11, e0005681. doi: 10.1371/journal.pntd.0005681
Kato, K., Arashima, Y., Asai, S., Furuya, Y., Yoshida, Y., Murakami, M., et al. (1998). Detection of Coxiella burnetii specific DNA in blood samples from Japanese patients with chronic nonspecific symptoms by nested polymerase chain reaction. FEMS Immunol. Med. Microbiol. 21, 139–144. doi: 10.1111/j.1574-695X.1998.tb01159.x
Khademi, P., Ownagh, A., Ataei, B., Kazemnia, A., Enferadi, A., Khalili, M., et al. (2020). Prevalence of C. burnetii DNA in sheep and goats milk in the northwest of Iran. Int. J. Food Microbiol. 331, 108716. doi: 10.1016/j.ijfoodmicro.2020.108716
Khan, S. M., Khan, M., Alouffi, A., Almutairi, M. M., Numan, M., Ullah, S., et al. (2023). Phylogenetic position of Haemaphysalis kashmirensis and Haemaphysalis cornupunctata, with Notes on Rickettsia spp. Genes. 14, 360. doi: 10.3390/genes14020360
Khan, Z., Shehla, S., Alouffi, A., Kashif Obaid, M., Zeb Khan, A., Almutairi, M. M., et al. (2022). Molecular survey and genetic characterization of Anaplasma marginale in ticks collected from livestock hosts in Pakistan. Animals. 12, 1708. doi: 10.3390/ani12131708
Khor, C. S., Mohd-Rahim, N. F., Hassan, H., Chandren, J. R., Nore, S. S., Johari, J., et al. (2018). Seroprevalence of Q fever among the indigenous people (Orang Asli) of Peninsular Malaysia. Vector Borne Zoonotic Dis. 18, 131–137. doi: 10.1089/vbz.2017.2153
Kilic, S., Yilmaz, G. R., Komiya, T., Kurtoglu, Y., and Karakoc, E. A. (2008). Prevalence of Coxiella burnetii antibodies in blood donors in Ankara, Central Anatolia, Turkey. New Microbiol. 31, 527–534
Kohls, G. M., Hoogstraal, H., Clifford, C. M., and Kaiser, M. N. (1970). The subgenus Persicargas (Ixodoidea, Argasidae, Argas). 9. Redescription and New World records of Argas (P.) persicus (Oken), and resurrection, redescription, and records of A. (P.) radiatus Railliet, A. (P.) sanchezi Dugès, and A. (P.) miniatus Koch, New World ticks misidentified as A. (P.) persicus. Ann. Entomol. Soc. Am. 63, 590–606. doi: 10.1093/aesa/63.2.590
Kumar, S., Stecher, G., Li, M., Knyaz, C., and Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol., 35, 1547. doi: 10.1093/molbev/msy096
Lafi, S. Q., Talafha, A. Q., Abu-Dalbouh, M. A., Hailat, R. S., and Khalifeh, M. S. (2020). Seroprevalence and associated risk factors of Coxiella burnetii (Q fever) in goats and sheep in northern Jordan. Trop. Anim. Health Prod. 52, 1553–1559. doi: 10.1007/s11250-019-02153-0
Lee, S. H., Lee, J. H., Park, S., Lee, H. K., Do Hwang, S., Jeong, H. W., et al. (2020). Isolation of Coxiella burnetii in patients with nonspecific febrile illness in South Korea. BMC Infect. Dis. 20, 1–5. doi: 10.1186/s12879-020-05130-3
Léger, E., Vourc'h, G., Vial, L., Chevillon, C., and McCoy, K. D. (2013). Changing distributions of ticks: causes and consequences. Exp. Appl. Acarol. 59, 219–244. doi: 10.1007/s10493-012-9615-0
Leski, T. A., Malanoski, A. P., Gregory, M. J., Lin, B., and Stenger, D. A. (2011). Application of a broad-range resequencing array for detection of pathogens in desert dust samples from Kuwait and Iraq. Appl. Environ. Microbiol. 77, 4285–4292. doi: 10.1128/AEM.00021-11
Lloyd, S. (1983). Effect of pregnancy and lactation upon infection. Vet. Immunol. Immunopathol. 4, 153–176. doi: 10.1016/0165-2427(83)90057-0
Mohabati Mobarez, A., Khalili, M., Mostafavi, E., and Esmaeili, S. (2021). Molecular detection of Coxiella burnetii infection in aborted samples of domestic ruminants in Iran. PLoS ONE. 16, e0250116. doi: 10.1371/journal.pone.0250116
Mohammed, O. B., Jarelnabi, A. A., Aljumaah, R. S., Alshaikh, M. A., Bakhiet, A. O., Omer, S. A., et al. (2014). Coxiella burnetii, the causative agent of Q fever in Saudi Arabia: molecular detection from camel and other domestic livestock. Asian Pac. J. Trop. Med. 7, 715–719. doi: 10.1016/S1995-7645(14)60122-X
Mumcuoglu, K. Y., Arslan-Akveran, G., Aydogdu, S., Karasartova, D., Kosar, N., Gureser, A. S., et al. (2022). Pathogens in ticks collected in Israel: I. Bacteria and protozoa in Hyalomma aegyptium and Hyalomma dromedarii collected from tortoises and camels. Ticks Tick-Borne Dis. 13, 101866. doi: 10.1016/j.ttbdis.2021.101866
Musso, D., Broult, J., Parola, P., Raoult, D., and Fournier, P. E. (2014). Absence of antibodies to Rickettsia spp., Bartonella spp., Ehrlichia spp. and Coxiella burnetii in Tahiti, French Polynesia. BMC Infect. Dis. 14, 1–4. doi: 10.1186/1471-2334-14-255
Ni, J., Lin, H., Xu, X., Ren, Q., Aizezi, M., Luo, J., et al. (2020). Coxiella burnetii is widespread in ticks (Ixodidae) in the Xinjiang areas of China. BMC Vet. Res. 16, 1–9. doi: 10.1186/s12917-020-02538-6
Niemczuk, K., Szymańska-Czerwińska, M., Smietanka, K., and Bocian, Ł. (2014). Comparison of diagnostic potential of serological, molecular and cell culture methods for detection of Q fever in ruminants. Vet. Microbiol. 171, 147–152. doi: 10.1016/j.vetmic.2014.03.015
Numan, M., Islam, N., Adnan, M., Zaman Sa, fi, S., Chitimia-Dob ler, L., Labruna, M. B., et al. (2022). First genetic report of Ixodes kashmiricus and associated Rickettsia sp. Parasi. Vectors. 15, 1–12. doi: 10.1186/s13071-022-05509-y
Nurkunasegran, M., Kho, K., Koh, F., Tan, P., Nizam, Q., Ong, B., et al. (2017). Molecular detection of Coxiella burnetii from farm animals and ticks in Malaysia. Trop. Biomed. 34, 675–680.
Obaid, M. K., Almutairi, M. M., Alouf, A., Sa, S. Z., Tanaka, T., and Ali, A. (2023). Assessment of cypermethrin and amitraz resistance and molecular profiling of voltage-gated sodium channel and octopamine tyramine genes of Rhipicephalus microplus. Front. Cell. Infect. Microbiol. 13, 1176013. doi: 10.3389/fcimb.2023.1176013
Obaidat, M. M., Malania, L., Imnadze, P., Roess, A. A., Salman, A. E. B., and Arner, R. J. (2019). Seroprevalence and risk factors for Coxiella burnetii in Jordan. Am. J. Trop. Med. Hyg. 101, 40. doi: 10.4269/ajtmh.19-0049
Ozgen, E. K., Kilicoglu, Y., Yanmaz, B., Ozmen, M., Ulucan, M., Bagatir, P. S., et al. (2022). Molecular epidemiology of Coxiella burnetii detected in humans and domestic ruminants in Turkey. Vet. Microbiol. 273, 109519. doi: 10.1016/j.vetmic.2022.109519
Panth, Y., Shrestha, S., and Bastola, R. (2017). Demonstration of circulating antibodies of Coxiella burnetii in dairy cattle of Rupandehi district, Nepal. Int. J. Inno. Res. Multi. Field. 3, 46–49.
Patra, G., Ghosh, S., Priyanka, E.fimova, M. A., Sahara, A., Al-Awsi, G. R. L., Polley, S., et al. (2020). Molecular detection of Coxiella burnetii and Borrelia burgdorferi in ticks infesting goats in North-Eastern States of India. Int. J. Acarol. 46, 431–438. doi: 10.1080/01647954.2020.1805000
Philip, C. B. (1948). Comments on the Name of the Q fever organism. Public Health Rep. 63, 402. doi: 10.2307/4586402
Psaroulaki, A., Hadjichristodoulou, C., Loukaides, F., Soteriades, E., Konstantinidis, A., Papastergiou, P., et al. (2006). Epidemiological study of Q fever in humans, ruminant animals, and ticks in Cyprus using a geographical information system. Eur. J. Clin. Microbiol. Infect. Dis. 25, 576–586. doi: 10.1007/s10096-006-0170-7
Rahman, M., Alam, M., Islam, M., Bhuiyan, A. K., and Rahman, A. K. M. (2016). Serological and molecular evidence of Q fever in domestic ruminants in Bangladesh. Vet. Med. Int. 2016, 9098416. doi: 10.1155/2016/9098416
Rini, E. P., Sasaki, M., Astuti, D., Juniantito, V., Wibawan, I. W. T., Sawa, H., et al. (2022). First molecular detection of Coxiella burnetii in beef cattle in West Java, Indonesia. Jpn. J. Infect. Dis. 75, 83–85. doi: 10.7883/yoken.JJID.2020.769
Rochlin, I., and Toledo, A. (2020). Emerging tick-borne pathogens of public health importance: a mini-review. J. Med. Microbiol. 69, 781. doi: 10.1099/jmm.0.001206
Royal, J., Riddle, M. S., Mohareb, E., Monteville, M. R., Porter, C. K., and Faix, D. J. (2013). Seroepidemiologic survey for Coxiella burnetii among US military personnel deployed to Southwest and Central Asia in 2005. Am. J. Trop. Med. Hyg. 89, 991. doi: 10.4269/ajtmh.12-0174
Sahu, R., Kale, S. B., Vergis, J., Dhaka, P., Kumar, M., Choudhary, M., et al. (2018). Apparent prevalence and risk factors associated with occurrence of Coxiella burnetii infection in goats and humans in Chhattisgarh and Odisha, India. Comp. Immunol. Microbiol. Infect. Dis. 60, 46–51. doi: 10.1016/j.cimid.2018.08.004
Sambrook, J., Fritsch, E. F., and Maniatis, T. E. (1989). Molecular Cloning: A Laboratory Manual (No. Ed. 2). Cold Spring Harbor Laboratory Press, New York, NY, United States.
Selmi, R., Said, M. B., Mamlouk, A., Yahia, H. B., and Messadi, L. (2019). Molecular detection and genetic characterization of the potentially pathogenic Coxiella burnetii and the endosymbiotic Candidatus Midichloria mitochondrii in ticks infesting camels (Camelus dromedarius) from Tunisia. Microb. Pathog. 136, 103655. doi: 10.1016/j.micpath.2019.103655
Seo, M. G., Lee, S. H., VanBik, D., Ouh, I. O., Yun, S. H., Choi, E., et al. (2016). Detection and genotyping of Coxiella burnetii and Coxiella-like bacteria in horses in South Korea. PLoS ONE. 11, e0156710. doi: 10.1371/journal.pone.0156710
Shabbir, M. Z., Jamil, T., Ali, A. A., Ahmad, A., Naeem, M., Chaudhary, M. H., et al. (2015). Prevalence and distribution of soil-borne zoonotic pathogens in Lahore district of Pakistan. Front. Microbiol. 6, 917. doi: 10.3389/fmicb.2015.00917
Siengsanan-Lamont, J., Kong, L., Heng, T., Khoeun, S., Tum, S., Selleck, P. W., et al. (2023). Risk mapping using serologic surveillance for selected One Health and transboundary diseases in Cambodian goats. PLoS Negl. Trop. Dis. 17, e0011244. doi: 10.1371/journal.pntd.0011244
Smith, T. A., Driscoll, T., Gillespie, J. J., and Raghavan, R. (2015). A Coxiella-like endosymbiont is a potential vitamin source for the Lone Star tick. Genome Biol. Evol. 7, 831–838. doi: 10.1093/gbe/evv016
Špitalská, E., Sparagano, O., Stanko, M., Schwarzová, K., Špitalský, Z., Škultéty, L., et al. (2018). Diversity of Coxiella-like and Francisella-like endosymbionts, and Rickettsia spp., Coxiella burnetii as pathogens in the tick populations of Slovakia, Central Europe. Ticks Tick Borne Dis. 9, 1207–1211. doi: 10.1016/j.ttbdis.2018.05.002
Sultankulova, K. T., Shynybekova, G. O., Issabek, A. U., Mukhami, N. N., Melisbek, A. M., Chervyakova, O. V., et al. (2022). The Prevalence of Pathogens among Ticks Collected from Livestock in Kazakhstan. Pathogens. 11, 1206. doi: 10.3390/pathogens11101206
Swai, E. S., Mbise, A. N., Kessy, V., Kaaya, E., Sanka, P., and Loomu, P. M. (2005). Farm constraints, cattle disease perception and tick management practices in pastoral Maasai community-Ngorongoro, Tanzania. Livest. Res. Rural. Dev. 17, 17–20. Available online at: https://www.lrrd.cipav.org.co/lrrd17/2/swai17017.htm
Tamura, K., and Nei, M. (1993). Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol. Biol. Evol. 10, 512–526.
Tarasevič, I. V., Plotnikova, L. F., Fetisova, N. F., Makarova, V. A., Jablonskaja, V. A., Reháček, J., et al. (1976). Rickettsioses studies: 1. natural foci of rickettsioses in the Armenian Soviet Socialist Republic. Bull. World Health Organ. 53, 25.
Thi Vinh An, D., Thi Viet Ha, B., Xuan Co, D., Minh Tam, V., Thi Diem Tuyet, L., and Van Truong, V. (2022). The first case of Coxiella burnetii infection detected through bone marrow biopsy in Vietnam. Clin. Pathol. 15, 397. doi: 10.1177/2632010X221096397
Thompson, J. D., Higgins, D. G., and Gibson, T. J. (1994). CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22, 4673–4680. doi: 10.1093/nar/22.22.4673
Trinachartvanit, W., Maneewong, S., Kaenkan, W., Usananan, P., Baimai, V., and Ahantarig, A. (2018). Coxiella-like bacteria in fowl ticks from Thailand. Parasit. Vectors. 11, 1–6. doi: 10.1186/s13071-018-3259-9
Tshokey, T., Stenos, J., Durrheim, D. N., Eastwood, K., Nguyen, C., Vincent, G., et al. (2018). Rickettsial infections and Q fever amongst febrile patients in Bhutan. Trop. Med. Infect. Dis. 3, 12. doi: 10.3390/tropicalmed3010012
Tshokey, T., Stenos, J., Tenzin, T., Drukpa, K., Gurung, R. B., and Graves, S. R. (2019). Serological evidence of Rickettsia, Orientia, and Coxiella in domestic animals from Bhutan: preliminary findings. Vector Borne Zoonotic Dis. 19, 95–101. doi: 10.1089/vbz.2018.2336
Ullah, Q., El-Adawy, H., Jamil, T., Jamil, H., Qureshi, Z. I., Saqib, M., et al. (2019). Serological and molecular investigation of Coxiella burnetii in small ruminants and ticks in Punjab, Pakistan. Int. J. Environ. Res. Public Health. 16, 4271. doi: 10.3390/ijerph16214271
Ullah, S., Alouffi, A., Almutairi, M. M., Islam, N., Rehman, G., Islam, Z. U., et al. (2023). First report of Rickettsia conorii in Hyalomma kumari ticks. Animals. 13, 1488. doi: 10.3390/ani13091488
Keywords: ticks, cox1, Coxiella burnetii, GroEL, domestic and wild animals, Pakistan
Citation: Ali A, Obaid MK, Almutairi MM, Alouffi A, Numan M, Ullah S, Rehman G, Islam ZU, Khan SB and Tanaka T (2023) Molecular detection of Coxiella spp. in ticks (Ixodidae and Argasidae) infesting domestic and wild animals: with notes on the epidemiology of tick-borne Coxiella burnetii in Asia. Front. Microbiol. 14:1229950. doi: 10.3389/fmicb.2023.1229950
Received: 30 May 2023; Accepted: 03 July 2023;
Published: 27 July 2023.
Edited by:
Hong Yin, Chinese Academy of Agricultural Sciences (CAAS), ChinaReviewed by:
Rosa Estela Quiroz Castañeda, Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias (INIFAP), MexicoCopyright © 2023 Ali, Obaid, Almutairi, Alouffi, Numan, Ullah, Rehman, Islam, Khan and Tanaka. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abid Ali, dW9wX2FsaUB5YWhvby5jb20=; Tetsuya Tanaka, azYxOTk0MzFAa2FkYWkuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.