
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 31 July 2023
Sec. Terrestrial Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1198808
This article is part of the Research TopicSoil Microbiome Community and Functional Succession Mechanism Driven by Different Factors in Agricultural EcologyView all 43 articles
Introduction: Bacterial communities are important for soil functions, but the effect of clomazone on network complexity, composition, and stability is not well studied.
Method: In this study, two agricultural soils were used to test the impact of clomazone on bacterial communities, and the two soils were treated with three concentrations of clomazone (0, 0.8, 8, and 80 mg kg1) in an incubator.
Results and discussion: Bacterial network nodes, links, and average degrees were all decreased by 9–384, 648–829, and 0.703–2.429, respectively. Based on keystone nodes, the topological roles of the nodes were also influenced by clomazone. Bacterial network composition was also impacted based on the analysis of similarity (ANOSIM) and network dissimilarity. Compared with control and clomazone treatments in both soils, the ANOSIM between control and all clomazone treatments was higher than 0.6, network dissimilarities were 0.97–0.98, shared nodes were 131–260, and shared links were 12–100. The bacterial network stability was decreased by clomazone, with decreased robustness by 0.01–0.016 and increased vulnerability by 0.00023–0.00147 in both soils. There were fewer bacterial network modules preserved after clomazone treatment, and the bacterial network community functions were also impacted in both soils. Based on these results, soil bacterial species connections, modularization, and network stability were significantly impacted by clomazone.
The soil microorganismal community is an interconnected unity through various and complicated relationships, such as mutualism, commensalism, parasitism, neutral predation, competition, and amensalism (Faust and Raes, 2012; Coyte et al., 2015). For all these processes, soil microorganisms perform functions by maintaining material, energy, and information exchange (Montoya et al., 2006; Glaze et al., 2022) and are fundamental to organic-matter degradation, pollution control, agricultural production, groundwater quality keeping, nitrogen cycling, and greenhouse gas regulation (Falkowski et al., 2008; Li et al., 2021). Therefore, intermicrobial connections are vital for maintaining homeostasis in soil processes. Network analysis has been used to characterize the complex ecological relationships among microbial species, and network nodes and links are used to represent species and their relationships, respectively (Przulj and Malod-Dognin, 2016). Therefore, networks are useful for examining species relationships and ecosystem processes (Berry and Widder, 2014).
Soil microbial community networks are threatened by many challenges such as global warming (Yuan et al., 2021; Zhu et al., 2022), soil erosion (Qiu et al., 2021), and terrestrial pollution (Du et al., 2021). Network composition is important for the stability of soil processes. Among the factors that may perturb the integrity of soil microbial networks, pesticide application has become a substantial threat yet is a standard practice in modern agriculture. Indeed, previous studies have shown that pesticides have a direct impact on microbial communities and their functions (Lerner et al., 2020; Li et al., 2020; Liu et al., 2020; Qiao et al., 2020; Yang, 2021). For example, researchers have analyzed the effect of pesticides on soil microbial network topological indices (Gao et al., 2018). However, studies related to node persistence, microbial network composition, and stability were limited. It is well known that soil microbial composition is the base of soil ecology function. Therefore, the analysis of changes in network composition in response to pesticides has important implications on soil community functions.
Clomazone {2-[(2-chlorophenyl) methyl]-4,4-dimethyl-1,2-oxazolidin-3-one} is an isoxazolidinone compound commonly used as a selective herbicide for many crops, and it has a half-life of >195 days in the field (PPDB1). Previous reports showed that clomazone can influence soil microbial communities (Du et al., 2018), indicating that the network structure can be altered, yet no study has been carried out concerning whether clomazone can affect microbial networks. To address this issue, we carried out a microcosmic experiment indoors over a period of 3 months. In this study, network complexity, dissimilarity, network stability, and preserved modules were used to evaluate the impact of clomazone on bacterial network composition and stability. In addition, the correlations between functions and the network community were also analyzed to ascertain whether the functions were changed. These indices will reflect the impact of clomazone on bacterial network.
There were two soils from the Jiansanjiang reclamation area (JSJ) and the Langfang research base of the Chinese Academy of Agricultural Sciences (LF). According to soil particle diameter, the soil form JSJ was identified as silty clay, and the soil form LF was identified as silty loam. The silty loam had 18 g organic matter kg−1, 74.9 mg available P kg−1, 289.8 mg available K kg−1, and a pH of 7.07; the silty clay had 25.8 g organic matter kg−1, 51.7 mg available P kg−1, 289.8 mg available K kg−1, and a pH of 7.24. The soils were sieved with 2-mm mesh and preincubated for 2 weeks (Trabue et al., 2006). The concentration transfer of this study was based on soil depth of 10 cm with a bulk density of 1.5 g cm−3 (GB/T31270.1-2014, 2014). The purity of clomazone is 98.4% and purchased from Beijing Qinchengyixin Technology Development Co., Ltd. (Beijing, China). Three clomazone treatments were prepared in brown bottles: 0.8 mg kg−1 (active ingredients per soil dry weight; L), 8 mg kg−1 (a.i./dw; M), and 80 mg kg−1 (a.i./dw; H). The L level represents recommended application rate in the field; the M level represents excessive use of clomazone in the field, and the H level represents extremely polluted soil (e.g., soil near a pesticide factory). In addition, a control treatment was also needed. These treatments were all prepared in triplicate. Soil moisture was adjusted daily by deionized water to 50% of the maximum water-holding capacity. The soil samples were kept for 90 days in an artificial climate box, and the temperature was maintained at 25°C. The samples were taken on days 7, 15, 30, 60, and 90 and kept in a refrigerator at -80°C.
Soil microbial DNA was extracted using a PowerSoil Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA), according to the instructions, and the DNA quality was evaluated using an ND-1000 spectrophotometer (NanoDrop Technologies). 16S rRNA gene was amplified by the primer sets of 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) (Yu et al., 2005). Microbial DNA was amplified in 50 μl reactions per sample, and each PCR solution contained 100–300 ng of DNA template, 1.5 μl of each 10 μM primer, 5 μl of 2 mM dNTPs, 1 μl of KOD-Plus-Neo enzyme (Toyobo, Shanghai, China), 5 μl of 10 × PCR Buffer for KOD-Plus-Neo, 3 μl of 25 mM MgSO4, and water to 50 μl. Reaction procedures were as follows: an initial step was 94°C and kept for 2 min, followed by 35 cycles of 98°C for 10 s, 62°C for 30 s, and 68°C for 30 s, and the final extension temperature was 68°C for 10 min. Negative-control reactions were also needed. PCR products were analyzed by 1.5% agarose gel electrophoresis and purified with a PCR Purification Kit from QIAGEN (Hilden, Germany). Purified PCR products were sequenced using Illumina equipment (Santiago, CA, USA). Amplicon sequencing data were processed through the USEARCH pipeline (Edgar, 2010, 2013), and clean data were clustered into operational taxonomic units (OTUs) with 97% similarity.
All networks were established on the basis of Pearson's correlations and performed on Cytoscape (Faust and Raes, 2016). The correlation coefficient was set as 0.9. The network topological indices were also based on Cytoscape. For each network, the topological roles of each node were classified and calculated by nodes' within-module connectivity (Zi) and among-module connectivity (Pi) (Guimerà and Nunes Amaral, 2005). An adopted criterion in previous studies (Olesen et al., 2007; Zhou et al., 2011; Shi et al., 2016; Yuan et al., 2021) was used in this study to identify module hubs (Zi ≥ 2.5, Pi < 0.62), connectors (Zi < 2.5, Pi ≥ 0.62), and network hubs (Zi ≥ 2.5, Pi ≥ 0.62). Module hubs referred to nodes that were highly connected to other members in a module, connectors referred to nodes that linked different modules, and network hubs were nodes that were both a module hub and a connector. These nodes were referred to as keystone nodes. Other nodes were classified as peripherals (Banerjee et al., 2019; Röttjers and Faust, 2019).
In a network, the node composition in one network module differs from that in other modules. However, some modules may preserve with some shared nodes after environmental change (Deng et al., 2012). Fisher's exact test is used to evaluate an association of two categorical variables (Warner, 2013) and has been used to identify the preserved modules in previous studies (Horvath, 2011; Langfelder et al., 2011; Deng et al., 2012; Dong et al., 2021). This method has also been used to evaluate preserved modules between control and clomazone treatments in two soils. There were four categories for the nodes within the two networks during the evaluation of preserved modules. In the first case, members were included in two modules; in the second case, members were included in one module of the pair; in the third case, members were included in the other module of the pair; in the fourth case, members were not included in these two modules. To determine whether nodes in the two modules were independent or exclusive, the observed frequency of the four categories was placed into four cells of a contingency table for one-sided exact testing. Each p-value from the exact tests was adjusted through the Bonferroni procedure within each network. Through Fisher's exact test, two modules from different networks that both included a significant part of the same nodes were considered as preserved modules.
Network stability could evaluate ecological system stability to disturbance (Thébault and Fontaine, 2010), and it was usually evaluated by network robustness and vulnerability (Wu et al., 2021; Yuan et al., 2021). Robustness and vulnerability were used to evaluate network stability (Wu et al., 2021; Yuan et al., 2021). Robustness is defined as the remaining proportion of species after random removal in the network (Montesinos-Navarro et al., 2017). In this study, every 0.05% of nodes was randomly removed to simulate random species removal. Vulnerability is calculated as V = max [(E – Ei)/E], in which E is the global efficiency and Ei is the global efficiency after removing node i and its entire links (Deng et al., 2012). Global efficiency is calculated as E = ∑j≠i[1/d(i,j)]/n(n – 1), in which d(i,j) is the number of edges in the shortest path of node i to j (Deng et al., 2012).
The metabolic function of each sample was predicted by tax4fun based on 16S rRNA gene data (Aßhauer et al., 2015). There was one cellular process, three genetic information processing, and 11 metabolism categories that have been used to analyze the correlation with the bacterial community using the Mantel test (Duan et al., 2020). The cellular process was cell growth and death (CGD); the genetic information processing categories were folding, sorting, and degradation (FSD), replication and repair (RR), and translation; and the metabolism categories were carbohydrate metabolism (CM), lipid metabolism (LM), amino acid metabolism (AAM), metabolism of cofactors and vitamins (MCV), xenobiotic biodegradation and metabolism (XBM), biosynthesis of other secondary metabolites (BOSM), energy metabolism (EM), metabolism of terpenoids and polyketides (MTP), metabolism of other amino acids (MOAA), nucleotide metabolism (NM), and glycan biosynthesis and metabolism (GBM). Some functions were fundamental for ecological balance, for example, XBM is important for chemical pollution cleaning (Thelusmond et al., 2019).
Analysis of similarity (ANOSIM) has been used to evaluate differences in bacterial community structure based on Bray–Curtis distance (Oksanen et al., 2012). Network dissimilarity is an effective tool to evaluate networks' differences, and it is based on network nodes and edges (Poisot et al., 2012; Mo et al., 2021). Shared nodes and edges of two networks are used to evaluate coexisting elements of different networks. Correlation coefficient “r” has been used to evaluate the correlation of functions to the bacterial community; Fisher's least significant difference test has been used to evaluate significant differences, and a 5% level was set (p < 0.05).
There were eight networks that have been established in Figure 1A. In all networks, the node comprised mostly of eight phyla: Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Gemmatimonadetes, Planctomycetes, Proteobacteria, and Verrucomicrobia. In the JSJ soil, the node percentages in clomazone treatments were increased in Acidobacteria, Proteobacteria (except for H treatment), and Verrucomicrobia (except for L treatment) but decreased in Actinobacteria, Gemmatimonadetes, Bacteroidetes (except for L treatment), Chloroflexi (except for M treatment), and Planctomycetes (except for M treatment) (Table 1). In the LF soil, Acidobacteria (except for H treatment), Chloroflexi (except for L treatment), Planctomycetes, and Verrucomicrobia were increased, while others were decreased (except for Actinobacteria in H treatment and Proteobacteria in M treatment) (Table 1).
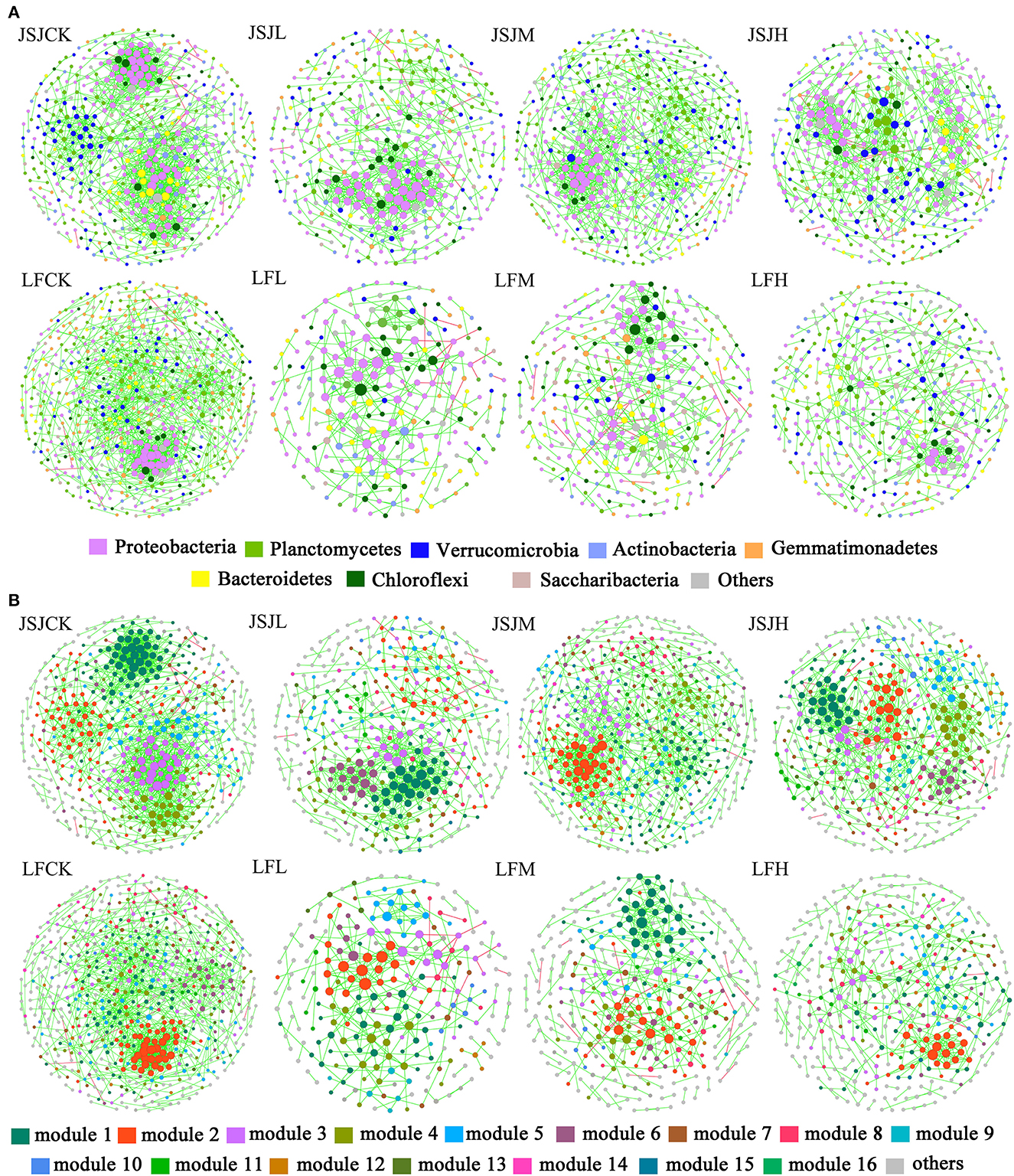
Figure 1. Visualization of bacterial networks for each treatment in the two soils. (A) The color of the nodes represents different phyla. (B) The color of the nodes represents different modules.
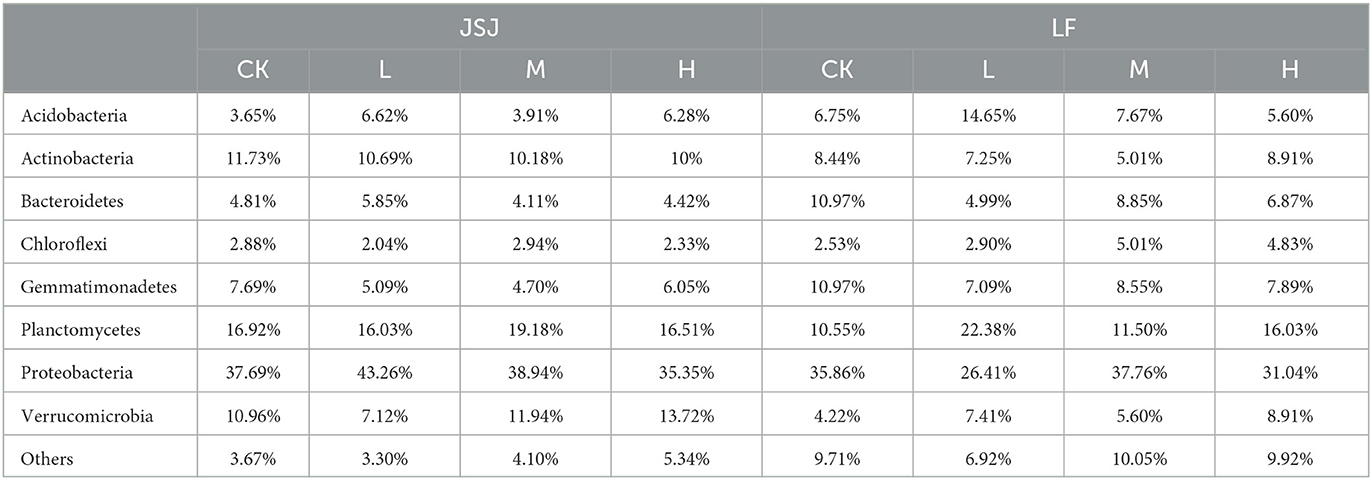
Table 1. Percentages of the predominant phyla in CK (control), L (0.8 mg kg−1), M (8 mg kg−1), and H (80 mg kg−1) treatments in the JSJ and LF soils.
The network's topological indices are shown in Table 2. Compared with the network of the JSJ control soil, network size (total nodes), the number of links, and the degree of clomazone treatments were decreased by 9–127, 648–829, and 2.249–2.429, respectively. While in the LF soil, network size (total nodes), the number of links, and the degree of clomazone treatments were decreased by 228–384, 667–753, and 0.703–1.371, respectively.
The influenced network could result in changing the roles of the networked members. Based on the criteria of node classification, keystone nodes were 473, 360, 466, and 392 for control, L, M, and H in the JSJ soil, respectively; network keystone nodes were 549, 360, 466, and 466 for control, L, M, and H in the LF soil, respectively (Figure 2). The shared keystone nodes were 173, 231, and 165 for the comparison of control and L, control and M, and control and H in the JSJ soil. The shared keystone nodes were 86, 119, and 119 for the comparison of control and L, control and M, and control and H in the LF soil (Figure 3).
ANOSIM is for the comparison of control and clomazone treatments. They were 0.617–0.879 in the JSJ soil and 0.985–0.997 in the LF soil (Table 3). The network dissimilarities between control and clomazone treatments were 0.954–0.983 in the JSJ soil and 0.979–0.990 in the LF soil (Table 3). These results revealed that the composition of each network was significantly impacted by clomazone in both soils. In addition, the shared nodes between control and clomazone treatments were used to evaluate the effects of clomazone in the JSJ soil and LF soil. Compared with the nodes in the JSJ soil control, the shared nodes were 196, 260, and 194 for L, M, and H treatments, respectively (Table 3). For the LF soil, there were 131, 151, and 171 shared nodes for L, M, and H treatments, compared with the nodes in the control treatments (Table 3). Compared with the links in the JSJ soil control, the shared links were 71, 100, and 36 for L, M, and H treatments, respectively (Table 3). For the LF soil, there were 17, 25, and 12 shared nodes for L, M, and H treatments, compared with the nodes in the control treatments (Table 3).
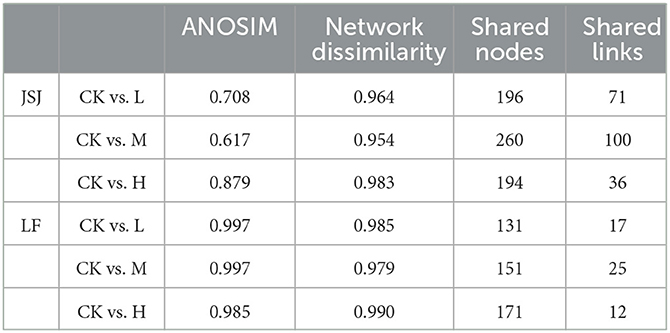
Table 3. ANOSIM, network dissimilarity, shared nodes, and links of the networked communities between control and clomazone treatments.
Based on random species loss, the robustness was decreased by 0.014–0.016 in clomazone treatments in the JSJ soil; for the LF soil, it was decreased by 0.01–0.013 in clomazone treatments (Figure 4). For vulnerability in the JSJ soil, it was increased by 0.00079–0.00147 in clomazone treatments; in the LF soil, it was decreased by 0.00023–0.00044 for clomazone treatments.
The influenced networks suggested that clomazone could alter network organization. The big modules (i.e., ≥5 nodes) were used to analyze preserved modules based on Fisher's exact test. In total, there were 25 preserved module pairs in the two soils (Table 4), and most of the preserved module individuals belong to the phyla of Proteobacteria, Acidobacteria, and Planctomycetes (Figures 1A, B). Specifically, in the JSJ soil, there were five module pairs accounted for 0.39% of total module pairs between CK and L, seven module pairs accounted for 0.55% of total module pairs between CK and M, and six module pairs accounted for 0.58% of total module pairs between CK and H (Table 4). In the LF soil, there were two module pairs accounted for 0.13% of total module pairs between CK and L, three module pairs accounted for 0.18% of total module pairs between CK and M, and two module pairs accounted for 0.09% of total module pairs between CK and H (Table 4).
An intriguing issue is whether the alterations in bacterial network composition as a result of clomazone treatment caused alterations in microbial community functions and their associated ecosystem processes. We used the Mantel test to address this issue, and the relationships are shown in Figure 5. In the JSJ soil, bacterial network community correlated with MOAA and MTP in the control treatment (r ≥ 0.4); in clomazone treatments, the correlations of MOAA (only in L treatment), BOSM, RR, MTP, and CM (only in H treatment) with the bacterial community are higher than 0.4. In the LF soil, bacterial network community correlated with BOSM, CM, AAM, and LM in the control treatment (r ≥ 0.4); in L treatment, it correlated with EM, RR, and MOAA; in M treatment, it correlated with EM; in H treatment, it correlated with translation, RR, MCV, MOAA, and FSD (r ≥ 0.4).
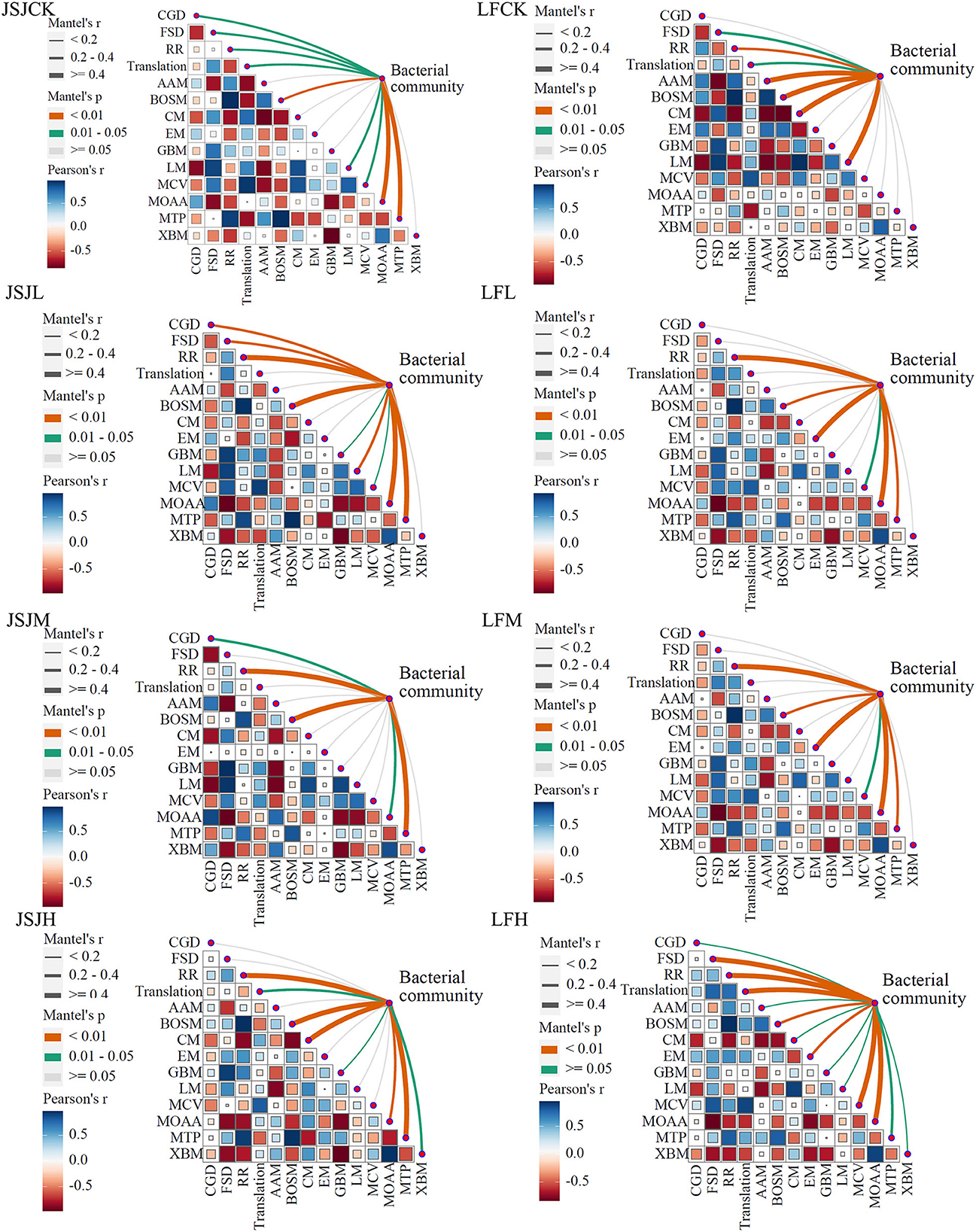
Figure 5. Relationships between bacterial network communities and functions for each treatment in the two soils.
Soil microbiome is an ecological system that is important in material cycling and nutrient maintaining. In an ecological system, there are complicated relationships. The microbial network has gradually been used to evaluate these complicated relationships (Ze et al., 2013; Przulj and Malod-Dognin, 2016; Mo et al., 2021). In this study, the influenced network complexities suggested that clomazone significantly altered bacterial network composition and relationships. The amount of total nodes in clomazone treatment networks indicated that the disconnected bacterial species were increased by clomazone. The significantly decreased links and average degrees also indicated that the connections of the network species were decreased. The most possible reason, due to some bacterial species, could use clomazone as a carbon resource and increase their abundance, and some bacterial species have been inhibited by clomazone or other bacterial species. Different effects of bacterial species' abundance were the reasons for the impacted bacterial connections. Therefore, that is the reason of impacted bacterial network topological indices, stability, and organization. In the study by Zhang et al. (2021), thiamethoxam also decreased bacterial network nodes, links, and average degrees. These influences by clomazone also induced the topological role of the network nodes to change.
The impacted network indices suggested that bacterial network composition has been changed. The results of ANOSIM, network dissimilarity, and shared nodes confirmed this inference. ANOSIM has been used to evaluate the difference between different network communities (Yuan et al., 2021). In the study by Yuan, they used ANOSIM to analyze whether the network composition was changed by climate warming (Yuan et al., 2021). Network dissimilarity has first been published by Poisot et al. (2012) and has also been used by other researchers to evaluate network dissimilarity (Mo et al., 2021; Liao et al., 2023). For example, Liao et al. (2023) analyzed the difference in marine medaka gut and gill microbial networks by network dissimilarity; in the study by Mo et al. (2021), they used shared node and edges and network dissimilarity to evaluate the difference in microeukaryotic plankton network in different salinity in the subtropical urban reservoir. These results suggested that ANOSIM and network dissimilarity are effective in evaluating bacterial network composition dissimilarity.
The impacted network indices and composition suggested that the bacterial network stability of the soils has been impacted. Network robustness and vulnerability were always used to evaluate network stability (Wu et al., 2021). In this study, the decreased network robustness and increased vulnerability suggested that bacterial network stability was decreased by clomazone. It also suggested that the resistance of the bacterial network to disturbance was decreased, and more species will lose from the connected network in clomazone-treated soils. The connection and cooperation of bacterial species will be more fragile after clomazone treatment. The decreased edges of networks of all clomazone treatments suggested that decreased edges should be responsible for decreased network stability (Yuan et al., 2021). Microbial network stability is important in ecosystem function (Coyte et al., 2015; Pan et al., 2023). The profile of network modules and functions proved this suggestion.
Normally, most species in the network will cluster as modules and the species exert their functions through modules (Segal et al., 2003; Banerjee et al., 2018). This demonstrates that preserved network modules will preserve some functions (Yuan et al., 2021). There were more shared nodes and links between control and clomazone treatments in the JSJ soil which suggested that more modules will preserve in the JSJ soil. This suggestion has been proven in the results of the preserved modules in the two soils. However, there were fewer modules preserved after clomazone treatment. These suggested that the functions of bacterial network have been changed. The relationships between network community and functions further proved this indication. In both soils, the correlation of network community with functions suggested that the functions of the bacterial community have been changed. In the JSJ soil, the function diversity of the bacterial network community was increased by clomazone with more functions correlated with the network community. In addition, MTP was significantly correlated with the bacterial community in all treatments in the JSJ soil which suggested that the bacterial network community function of MTP was stable in facing clomazone. Soil bacterial functions are sensitive to pesticides and have been improved by another study (Han et al., 2022). In the study by Han et al. (2022), boscalid significantly impacted N cycling genes.
In this study, we used network complexities, composition, keystone node, and stability to analyze the impact of clomazone on soil bacterial networks. The results indicated that clomazone decreased bacterial network nodes, links, and average degrees. Clomazone impacted the bacterial network composition, and the topological role of the nodes was also impacted according to keystone nodes. The decreased robustness and increased vulnerability suggested that network stability was increased by clomazone. Preserved modules and the correlation of bacterial network community to soil bacterial functions manifest that the functions of the bacterial network community have been changed. Overall, the soil bacterial network has been significantly changed by clomazone.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: NCBI - SAMN08721528-SAMN08721647.
HH and PD conceived and wrote this manuscript. HH, PD, and JH performed the bioinformatics analyses. ZZ, XZ, and WF revised this manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by the National Natural Science Foundation of China (Grant Number 31901918) and the State Key Laboratory for Managing Biotic and Chemical Threats to the Quality and Safety of Agroproducts (Grant Number 2010DS700124-KF2008).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aßhauer, K. P., Wemheuer, B., Daniel, R., and Meinicke, P. (2015). Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data Tax4Fun: predicting functional profiles from metagenomic 16S rRNA data. Bioinformatics 31, 2882–2884. doi: 10.1093/bioinformatics/btv287
Banerjee, S., Schlaeppi, K., and van der Heijden, M. G. A. (2018). Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 16, 567–576. doi: 10.1038/s41579-018-0024-1
Banerjee, S., Schlaeppi, K., and van der Heijden, M. G. A. (2019). Reply to ‘can we predict microbial keystones?'. Nat. Rev. Microbiol. 17, 194–194. doi: 10.1038/s41579-018-0133-x
Berry, D., and Widder, S. (2014). Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 5:219. doi: 10.3389/fmicb.2014.00219
Coyte, K. Z., Schluter, J., and Foster, K. R. (2015). The ecology of the microbiome: networks, competition, and stability. Science . 350, 663–666. doi: 10.1126/science.aad2602
Deng, Y., Jiang, Y. -H., Yang, Y., He, Z., Luo, F., and Zhou, J. (2012). Molecular ecological network analyses. BMC Bioinformatics 13:113. doi: 10.1186/1471-2105-13-113
Dong, S., Song, C., Qi, B., Jiang, X., Liu, L., and Xu, Y. (2021). Strongly preserved modules between cancer tissue and cell line contribute to drug resistance analysis across multiple cancer types. Genomics 113, 1026–1036. doi: 10.1016/j.ygeno.2021.02.015
Du, P., He, H., Wu, X., Xu, J., Dong, F., Liu, X., et al. (2021). Mesosulfuron-methyl influenced biodegradability potential and N transformation of soil. J. Hazard. Mater. 416:125770. doi: 10.1016/j.jhazmat.2021.125770
Du, P., Wu, X., Xu, J., Dong, F., Liu, X., Zhang, Y., et al. (2018). Clomazone influence soil microbial community and soil nitrogen cycling. Sci. Total Environ. 644, 475–485. doi: 10.1016/j.scitotenv.2018.06.214
Duan, X. Z., Sun, J. T., Wang, L. T., Shu, X. H., Guo, Y., Keiichiro, M., et al. (2020). Recent infection by Wolbachia alters microbial communities in wild Laodelphax striatellus populations. Microbiome 8, 104. doi: 10.1186/s40168-020-00878-x
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–8. doi: 10.1038/nmeth.2604
Falkowski, P. G., Fenchel, T., and Delong, E. F. (2008). The microbial engines that drive earth's biogeochemical cycles. Science. 320, 1034–1039. doi: 10.1126/science.1153213
Faust, K., and Raes, J. (2012). Microbial interactions: from networks to models. Nat. Rev. Microbiol. 10, 538–550. doi: 10.1038/nrmicro2832
Faust, K., and Raes, J. (2016). CoNet app: inference of biological association networks using Cytoscape. F1000Res 5, 1519–1519. doi: 10.12688/f1000research.9050.2
Gao, W., Wu, H., Siddiqui, M. K., and Baig, A. Q. (2018). Study of biological networks using graph theory. Saudi J. Biol. Sci. 25, 1212–1219. doi: 10.1016/j.sjbs.2017.11.022
GB/T31270.1-2014 (2014). Test Guidelines on Environmental Safety Assessment for Chemical Pesticides-Part 16: Soil Microorganism Toxicity Test.
Glaze, T. D., Erler, D. V., and Siljanen, H. M. P. (2022). Microbially facilitated nitrogen cycling in tropical corals. ISME J. 16, 68–77. doi: 10.1038/s41396-021-01038-1
Guimerà, R., and Nunes Amaral, L. A. (2005). Functional cartography of complex metabolic networks. Nature 433, 895–900. doi: 10.1038/nature03288
Han, L., Xu, M., Kong, X., Liu, X., Wang, Q., Chen, G., et al. (2022). Deciphering the diversity, composition, function, and network complexity of the soil microbial community after repeated exposure to a fungicide boscalid. Environ. Pollut. 312:120060. doi: 10.1016/j.envpol.2022.120060
Horvath, S. (2011). Evaluating Whether a Module is Preserved in Another Network. Weighted Network Analysis: Applications in Genomics and Systems Biology (New York, NY: Springer New York), 207–247.
Langfelder, P., Luo, R., Oldham, M. C., and Horvath, S. (2011). Is my network module preserved and reproducible? PLoS Comput. Biol. 7:e1001057. doi: 10.1371/journal.pcbi.1001057
Lerner, H., Öztürk, B., Dohrmann, A. B., Thomas, J., Marchal, K., De Mot, R., et al. (2020). Culture-independent analysis of linuron-mineralizing microbiota and functions in on-farm biopurification systems via DNA-stable isotope probing: comparison with enrichment culture. Environ. Sci. Technol. 54, 9387–9397. doi: 10.1021/acs.est.0c02124
Li, D., Ni, H., Jiao, S., Lu, Y., Zhou, J., Sun, B., et al. (2021). Coexistence patterns of soil methanogens are closely tied to methane generation and community assembly in rice paddies. Microbiome 9, 20. doi: 10.1186/s40168-020-00978-8
Li, M., Rong, L., Zhou, S., Xiao, X., Wu, L., Fan, Y., et al. (2020). Dissipation of sulfonamides in soil emphasizing taxonomy and function of microbiomes by metagenomic analysis. J. Agric. Food Chem. 68, 13594–13607. doi: 10.1021/acs.jafc.0c04496
Liao, X., Zhao, P., Hou, L., Adyari, B., Xu, E. G., Huang, Q., et al. (2023). Network analysis reveals significant joint effects of microplastics and tetracycline on the gut than the gill microbiome of marine medaka. J. Hazard. Mater. 442:129996. doi: 10.1016/j.jhazmat.2022.129996
Liu, J., Song, Y., Tang, M., Lu, Q., and Zhong, G. (2020). Enhanced dissipation of xenobiotic agrochemicals harnessing soil microbiome in the tillage-reduced rice-dominated agroecosystem. J. Hazard. Mater. 398:122954. doi: 10.1016/j.jhazmat.2020.122954
Mo, Y., Peng, F., Gao, X., Xiao, P., Logares, R., Jeppesen, E., et al. (2021). Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 9, 128. doi: 10.1186/s40168-021-01079-w
Montesinos-Navarro, A., Hiraldo, F., Tella, J. L., and Blanco, G. (2017). Network structure embracing mutualism-antagonism continuums increases community robustness. Nat. Ecol. Evol. 1, 1661–1669. doi: 10.1038/s41559-017-0320-6
Montoya, J. M., Pimm, S. L., and Sole, R. V. (2006). Ecological networks and their fragility. Nature 442, 259–264. doi: 10.1038/nature04927
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O'Hara, R. B., et al. (2012). vegan: Community Ecology Package. Available online at: http://CRAN.R-project.org/package=vegan
Olesen, J. M., Bascompte, J., Dupont, Y. L., and Jordano, P. (2007). The modularity of pollination networks. Proc. Nat. Acad. Sci. 104, 19891–19896. doi: 10.1073/pnas.0706375104
Pan, C., Yu, W., Sun, C., Guo, J., Yu, Y., and Li, X. (2023). Saprotrophic fungi buffer the adverse effects of soil acidification on the soil nutrient supply ability of Chinese fir (Cunninghamia lanceolata) plantations. Eur. J. Soil Biol. 114:103462. doi: 10.1016/j.ejsobi.2022.103462
Poisot, T., Canard, E., Mouillot, D., Mouquet, N., and Gravel, D. (2012). The dissimilarity of species interaction networks. Ecol. Lett. 15, 1353–1361. doi: 10.1111/ele.12002
Przulj, N., and Malod-Dognin, N. (2016). Network analytics in the age of big data. Science. 353, 123–124. doi: 10.1126/science.aah3449
Qiao, W., Puentes Jácome, L. A., Tang, X., Lomheim, L., Yang, M. I., Gaspard, S., et al. (2020). Microbial communities associated with sustained anaerobic reductive dechlorination of alpha-, beta-, gamma-, and delta-hexachlorocyclohexane isomers to monochlorobenzene and benzene. Environ. Sci. Technol. 54, 255–265. doi: 10.1021/acs.est.9b05558
Qiu, L., Zhang, Q., Zhu, H., Reich, P. B., Banerjee, S., van der Heijden, M. G. A., et al. (2021). Erosion reduces soil microbial diversity, network complexity and multifunctionality. Isme .J 15, 2474–2489. doi: 10.1038/s41396-021-00913-1
Röttjers, L., and Faust, K. (2019). Can we predict keystones? Nat. Rev. Microbiol. 17, 193. doi: 10.1038/s41579-018-0132-y
Segal, E., Shapira, M., Regev, A., Pe'er, D., Botstein, D., Koller, D., et al. (2003). Module networks: identifying regulatory modules and their condition specific regulators from gene expression data. Nat. Genet. 34, 166–176. doi: 10.1038/ng1165
Shi, S., Nuccio, E. E., Shi, Z. J., He, Z., Zhou, J., and Firestone, M. K. (2016). The interconnected rhizosphere: high network complexity dominates rhizosphere assemblages. Ecol. Lett. 19, 926–936. doi: 10.1111/ele.12630
Thébault, E., and Fontaine, C. (2010). Stability of ecological communities and the architecture of mutualistic and trophic networks. Science. 329, 853–856. doi: 10.1126/science.1188321
Thelusmond, J. -R., Strathmann, T. J., and Cupples, A. M. (2019). Carbamazepine, triclocarban and triclosan biodegradation and the phylotypes and functional genes associated with xenobiotic degradation in four agricultural soils. Sci. Total Environ. 20, 1138–1149. doi: 10.1016/j.scitotenv.2018.12.145
Trabue, S. L., Palmquist, D. E., Lydick, T. M., and Singles, S. K. (2006). Effects of soil storage on the microbial community and degradation of metsulfuron-methyl. J. Agric. Food Chem. 54, 142–151. doi: 10.1021/jf0512048
Warner, P. (2013). Testing association with Fisher's exact test. J. Fam. Plann. Reprod. Health Care 39, 281–284. doi: 10.1136/jfprhc-2013-100747
Wu, M. H., Chen, S. Y., Chen, J. W., Xue, K., Chen, S. L., Wang, X. M., et al. (2021). Reduced microbial stability in the active layer is associated with carbon loss under alpine permafrost degradation. Proc. Natl. Acad. Sci. USA. 118:e2025321118. doi: 10.1073/pnas.2025321118
Yang, X. (2021). Loss of microbial diversity does not decrease gamma-HCH degradation but increases methanogenesis in flooded paddy soil. Soil Biol. Biochem. 156:108210. doi: 10.1016/j.soilbio.2021.108210
Yu, Y., Lee, C., Kim, J., and Hwang, S. (2005). Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 89, 670–679. doi: 10.1002/bit.20347
Yuan, M. M., Guo, X., Wu, L., Zhang, Y., Xiao, N., Ning, D., et al. (2021). Climate warming enhances microbial network complexity and stability. Nat. Clim. Chang. 11, 343–U100. doi: 10.1038/s41558-021-00989-9
Ze, X., Le Mougen, F., Duncan, S. H., Louis, P., and Flint, H. J. (2013). Some are more equal than others: the role of “keystone” species in the degradation of recalcitrant substrates. Gut Microbes 4, 236–240. doi: 10.4161/gmic.23998
Zhang, H., Zhang, Z., Song, J., Mei, J., Fang, H., and Gui, W. (2021). Reduced bacterial network complexity in agricultural soils after application of the neonicotinoid insecticide thiamethoxam. Environ. Pollut. 274:116540. doi: 10.1016/j.envpol.2021.116540
Zhou, J., Deng, Y., Luo, F., He, Z., and Yang, Y. (2011). Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. mBio 2:e00122-11. doi: 10.1128/mBio.00122-11
Keywords: clomazone, bacterial network, network composition, stability, dissimilarity
Citation: He H, Huang J, Zhao Z, Feng W, Zheng X and Du P (2023) Impact of clomazone on bacterial communities in two soils. Front. Microbiol. 14:1198808. doi: 10.3389/fmicb.2023.1198808
Received: 02 April 2023; Accepted: 11 July 2023;
Published: 31 July 2023.
Edited by:
Bin Huang, Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Junwei Zhao, Northeast Agricultural University, ChinaCopyright © 2023 He, Huang, Zhao, Feng, Zheng and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoke Zheng, emhlbmd4ay4yMDA2QDE2My5jb20=; Pengqiang Du, ZHVwZW5ncUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.