- 1College of Traditional Chinese Medicine, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, China
- 2Research Base of Traditional Chinese Medicine Syndrome, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, China
- 3Key Laboratory of Traditional Chinese Medicine Health Status Identification, Fuzhou, Fujian, China
Background: Studies showed that development of gut microbial dysbiosis has a close association with type 2 diabetes (T2D). It is not yet clear if there is a causal relationship between gut microbiota and T2D.
Methods: The data collected from the published genome-wide association studies (GWASs) on gut microbiota and T2D were analyzed. Two-sample Mendelian randomization (MR) analyses were performed to identify causal relationship between bacterial taxa and T2D. Significant bacterial taxa were further analyzed. To confirm the findings’ robustness, we performed sensitivity, heterogeneity, and pleiotropy analyses. A reverse MR analysis was also performed to check for potential reverse causation.
Results: By combining the findings of all the MR steps, we identified six causal bacterial taxa, namely, Lachnoclostridium, Oscillospira, Roseburia, Ruminococcaceae UCG003, Ruminococcaceae UCG010 and Streptococcus. The risk of T2D might be positively associated with a high relative abundance of Lachnoclostridium, Roseburia and Streptococcus but negatively associated with Oscillospira, Ruminococcaceae UCG003 and Ruminococcaceae UCG010. The results of MR analyses revealed that there were causal relationships between the six different genera and T2D. And the reverse MR analysis did not reveal any evidence of a reverse causality.
Conclusion: This study implied that Lachnoclostridium, Roseburia and Streptococcus might have anti-protective effect on T2D, whereas Oscillospira, Ruminococcaceae UCG003 and Ruminococcaceae UCG010 genera might have protective effect on T2D. Our study revealed that there was a causal relationship between specific gut microbiota genera and T2D.
Introduction
Type 2 diabetes (T2D) is typically diagnosed in older individuals or middle-aged people. It is a metabolic disorder that can be caused by either beta cell dysfunction or insulin resistance (Taylor, 2013). The evidence supporting the increasing prevalence of T2D has shown a steady rise over the past few decades (Lastra et al., 2014). The rapid emergence and prevalence of diabetes has attracted global attention (Jaacks et al., 2016). In 2021, International Diabetes Federation released that the global diabetes prevalence rate among 20-79-year-old people is expected to increase to 12.2% in 2045, and most of them will be T2D (Sun et al., 2022).
The development of T2D can be triggered by various factors, such as unhealthy lifestyle and genetic factors. It has been theorized that the composition of gut microbiome plays a role in pathogenic mechanism of T2D (Cani, 2018; Hsieh et al., 2018; Zaky et al., 2021; Liu et al., 2022). Recently, great interest has been attracted by the gut microbiota which have been demonstrated in studies regulating host physiological activities. Studies have shown that gut microbiome were important in maintaining homeostasis (Geva-Zatorsky et al., 2017) and the establishment of immune system (Shi et al., 2017). Gut microbiota have important roles in the pathological process for various diseases by affecting the cell differentiation (Jubair et al., 2018) and cytokine release(Chappert, 2014), as well as regulating drug absorption and metabolism (Scher et al., 2020). Gut microbiota in individuals with T2D differs from non-diabetic ones (Larsen et al., 2010; Kashtanova et al., 2018; Takagi et al., 2020), the development of gut microbial dysbiosis has known to be a clinical manifestation of T2D (Forslund et al., 2015; Wu et al., 2017). But the results of various studies differ from one another. Larsen’s study indicated that the proportion of Firmicutes was significantly higher in healthy individuals compared with the diabetic, Bacteroidetes was somewhat enriched in the diabetic group, which results in a lower Firmicutes/Bacteroidetes ratio (Larsen et al., 2010). Nevertheless, Sedighi et al. found that an increased level of Firmicutes and decreased level of Bacteroidetes in T2D individuals, which means a higher Firmicutes/Bacteroidetes ratio in diabetic group (Sedighi et al., 2017). Whilst the specific microbiome features identified as responsible for the appearance of T2D have differed among studies (Doumatey et al., 2020; Kitten et al., 2021), Kootte’s study showed that insulin sensitivity increased in obese metabolic syndrome participants who received fecal microbiota transplantation (FMT) from lean donors (Kootte et al., 2017). As a valid treatment for patients with T2D, FMT introduced (transplanted) gut microbiota acquired from the faeces of healthy donors into the patient’s gastrointestinal tract (Antushevich, 2020). This is also a good application of gut microbiota in the treatment of T2D. The most important intention for research is to provide guidance for microbiota-orientated interventions treating T2D clinically.
Although the correlation between the gut microbiome and T2D is widely acknowledged, the exact causal relationship remains unclear. Elucidation of the causal relationships could be helpful for deepening comprehending of the responsibility of gut microbiota for the pathogenesis of T2D. As a statistical method, Mendelian randomization (MR) is usually used to clarify causal relationship between exposures to outcomes. MR uses single-nucleotide polymorphisms (SNPs) which strongly related to exposure as instrumental variables (IVs), and the relationship between the exposure and outcome could be evaluated (Emdin et al., 2017). The results of large-scale genome-wide association studies (GWASs) of the gut microbiota and diseases have led to the widespread use of MR analysis in various research fields (Bonder et al., 2016; Goodrich et al., 2016). Unlike traditional observational studies, MR assists researchers in identifying the causal relationship between the exposure and outcome directly, and interference of reverse causation and confounding factors can be avoided (Zheng et al., 2017). Our research performed a two-sample MR analysis to explore relationship between gut microbiota and T2D.
Materials and methods
Ethics statement
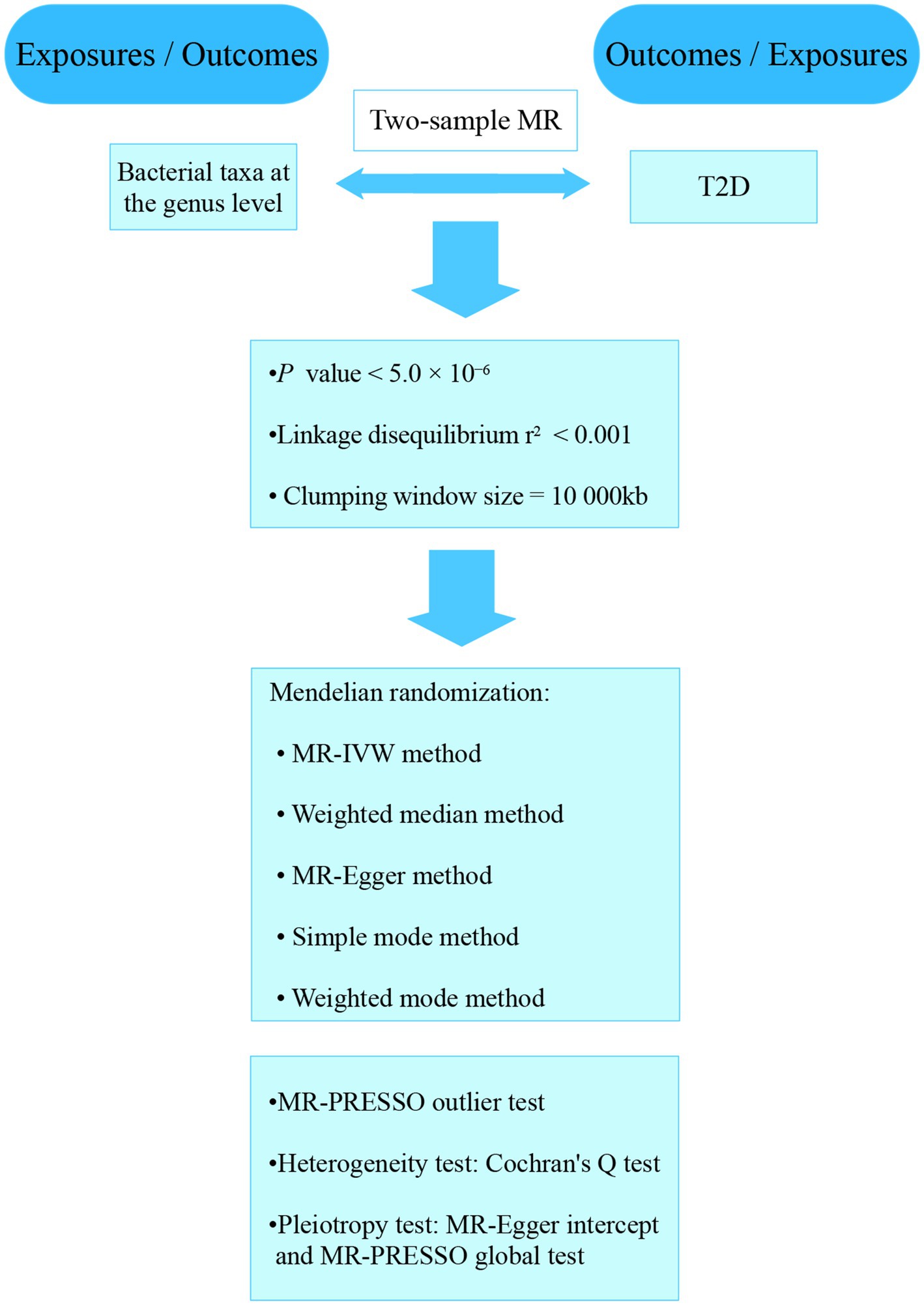
Our analyses used the summary statistics of publicly available GWASs. No new data was collected, and the study was conducted without new ethical approval. The whole process that we studied was presented in a flowchart in Figure 1. The gut microbiota was the exposure, and T2D was outcome. A reverse MR analysis was also performed to examine the effects of T2D on the gut microbiota.
Gut microbiota sample
The summary statistics data collected from a large-scale GWAS meta-analysis which analyze the gut microbial taxa of 18,340 participants from various ethnic groups (Kurilshikov et al., 2021). The twenty cohort studies included single ancestry samples from various regions, such as European (n = 13,266), Middle Eastern (n = 481), Latin American (n = 1,097), East Asian (n = 811), African American (n = 114). The data collected from four cohorts included multiple ancestries (n = 2,571). The researchers analyzed the microbial composition using seven different extraction methods and three different rRNA regions. All the datasets were rarefied to around 10,000 reads per sample to be obligated to sequencing depth differences. Only taxa that belonged to over 10% of all the samples were included in this study to examine the effects of host genetic variation on gut bacterial species. The researchers analyzed 211 taxa. The total included 131 genera, 35 families and 20 orders, as well as 16 classes, 9 phyla.
T2D sample
GWAS summary statistics for T2D were gathered from a large sample meta-analysis from the publicly available GWAS analyses. The study investigated T2D cases (62,892) and controls (596,424), which included over 16 million genetic variants by combining three GWAS datasets of European (Xue et al., 2018).
Selection of IVs
The total bacterial taxa included 211 species at five taxonomic levels. We analyzed with genus level which is the smallest and most specific of above illustrated levels with the current 16S rRNA gene sequencing technology. We analyzed 131 taxa and excluded 12 unknown group taxa. Then, we included 119 bacterial taxa in the subsequent MR analyses.
To select potential IVs, we first included SNPs those associated with gut bacterial taxa at the genome-wide significance threshold p < 5.0 × 10−6. Eligible IVs must meet the followed quality control steps. First, the linkage disequilibrium (LD) threshold for clumping was set to r2 < 0.001 and the window size for clumping 10,000 kb to minimize the effects of LD on the results. Second, we selected only exposures those had at least three independent genetic instruments. Third, we excluded palindromic SNPs with middle allele frequency. We calculated the F statistic based on the formula: F = (β/SE)2.
Statistical analysis
Effects of gut microbiota on T2D were estimated through a multi-test MR analysis. For the bacterial genera, various tests were performed included the random-effects inverse variance weighted (IVW) (Burgess et al., 2013), MR-Egger regression and weighted median. The evaluation of the heterogeneity related to each bacterial genus was conducted by the Cochrane’s Q statistics. The random-effects IVW test was used to make sure a conservative but robust estimate when heterogeneity existed (p < 0.05). The weighted median test can also provide consistent estimates if more than 50% of the weights come from valid IVs (Bowden et al., 2016). The MR-Egger regression allows for the presence of pleiotropy in more than 50% of IVs (Bowden et al., 2015). Further analyses were conducted on bacterial genera that were identified to be significant in the previous steps. With MR-Egger regression, we detected horizontal pleiotropy. Based on the “leave-one-out” method, we conducted sensitivity analysis (Dong et al., 2023).
Reverse MR analysis
We carried out reverse MR analysis to examine the effect of T2D on the identified bacterial genus. SNPs related to T2D were used as IVs. The results of the various statistical analyses were generated using R (version 4.2.2). The “TwoSampleMR” package was applied to perform IVW, weighted median, and MR-Egger regression. Through the “MRPRESSO” package, we conducted MR-PRESSO test.
Results
Selection of IVs
According to the selection criteria of IVs, 482 SNPs associated with 97 genera were selected as IVs. F statistics for all SNPs were greater than 10, which suggested there were no weak IVs (Supplementary Table S1).
Causal effects of gut microbiota on T2D
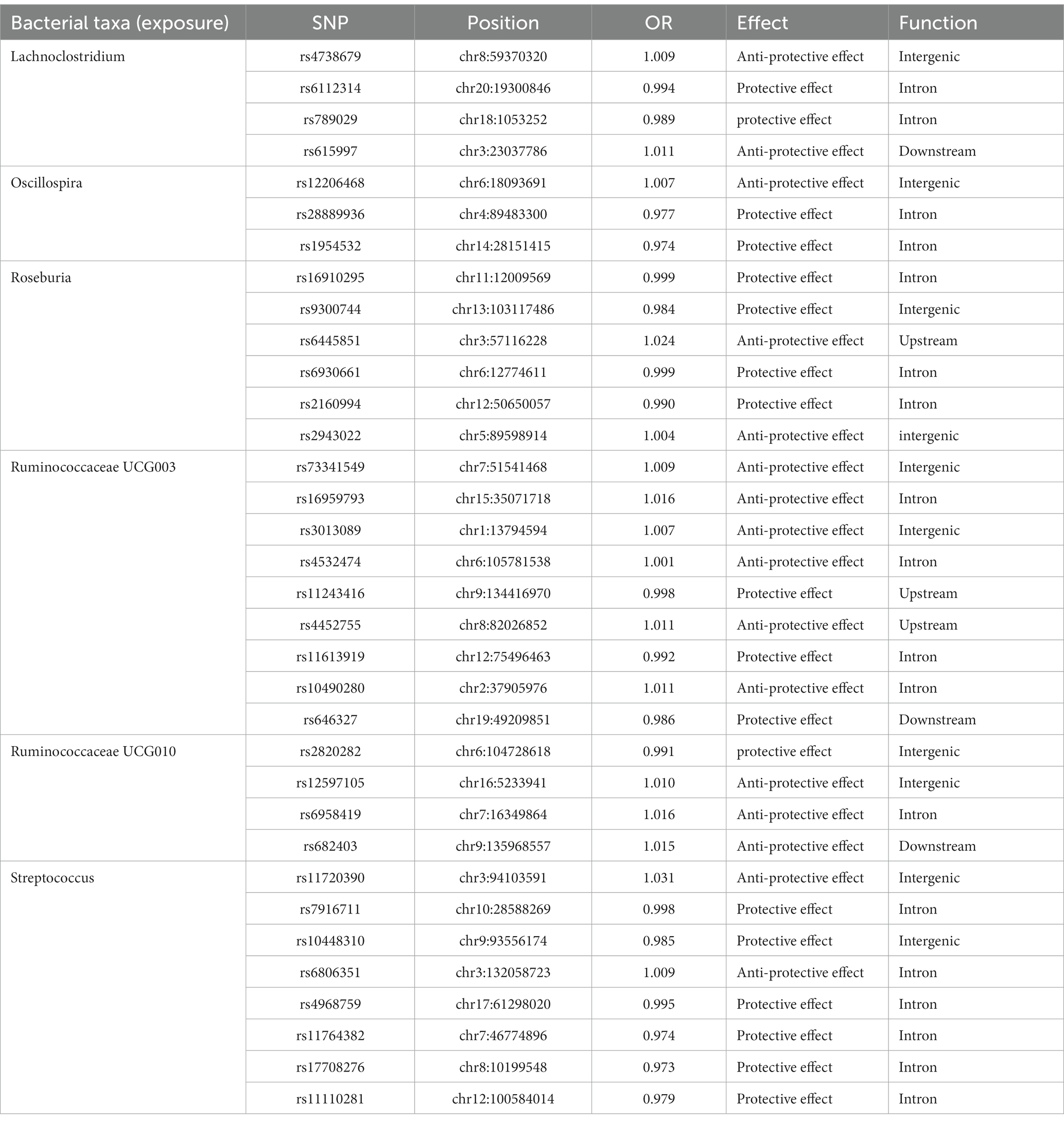
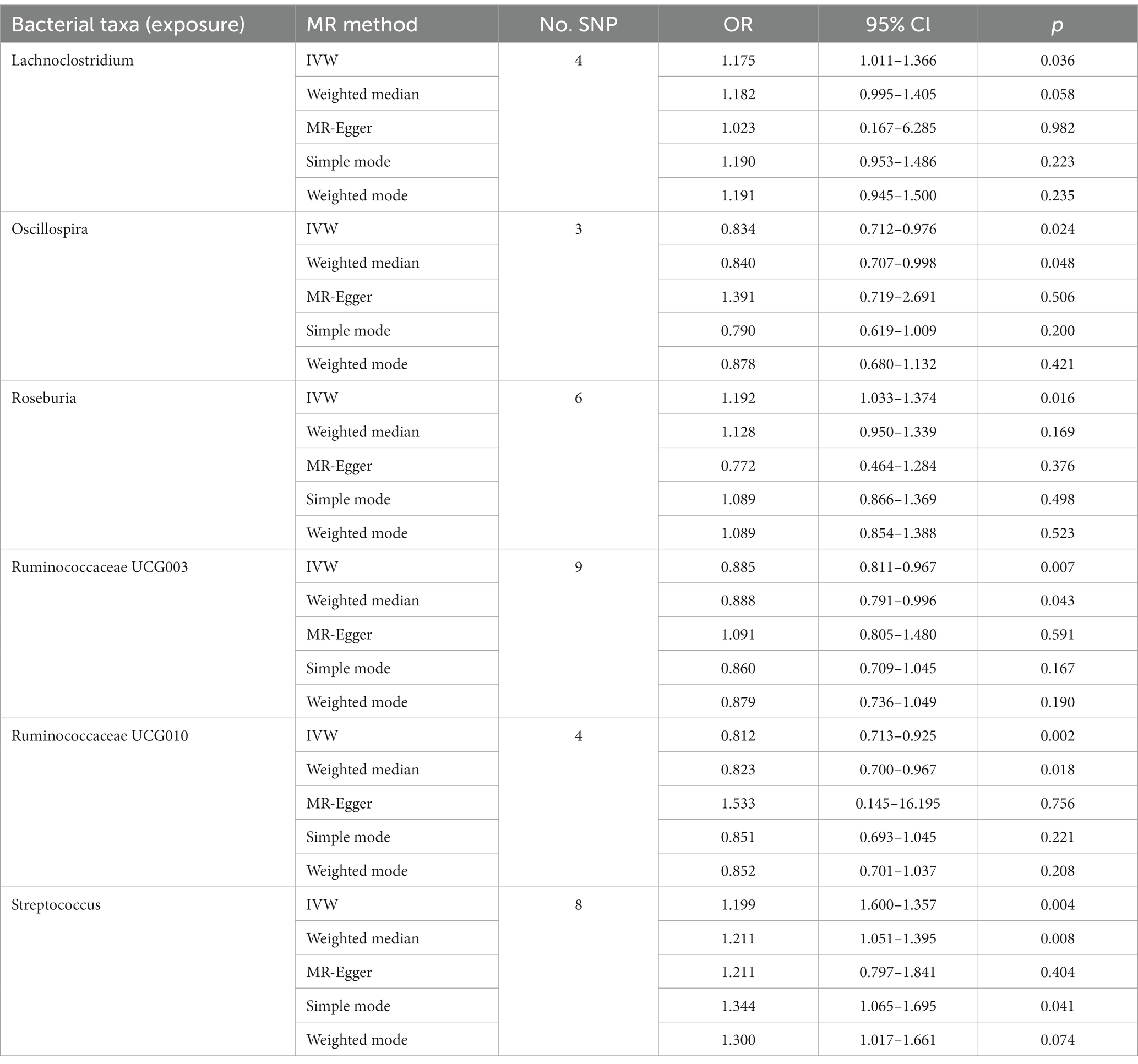
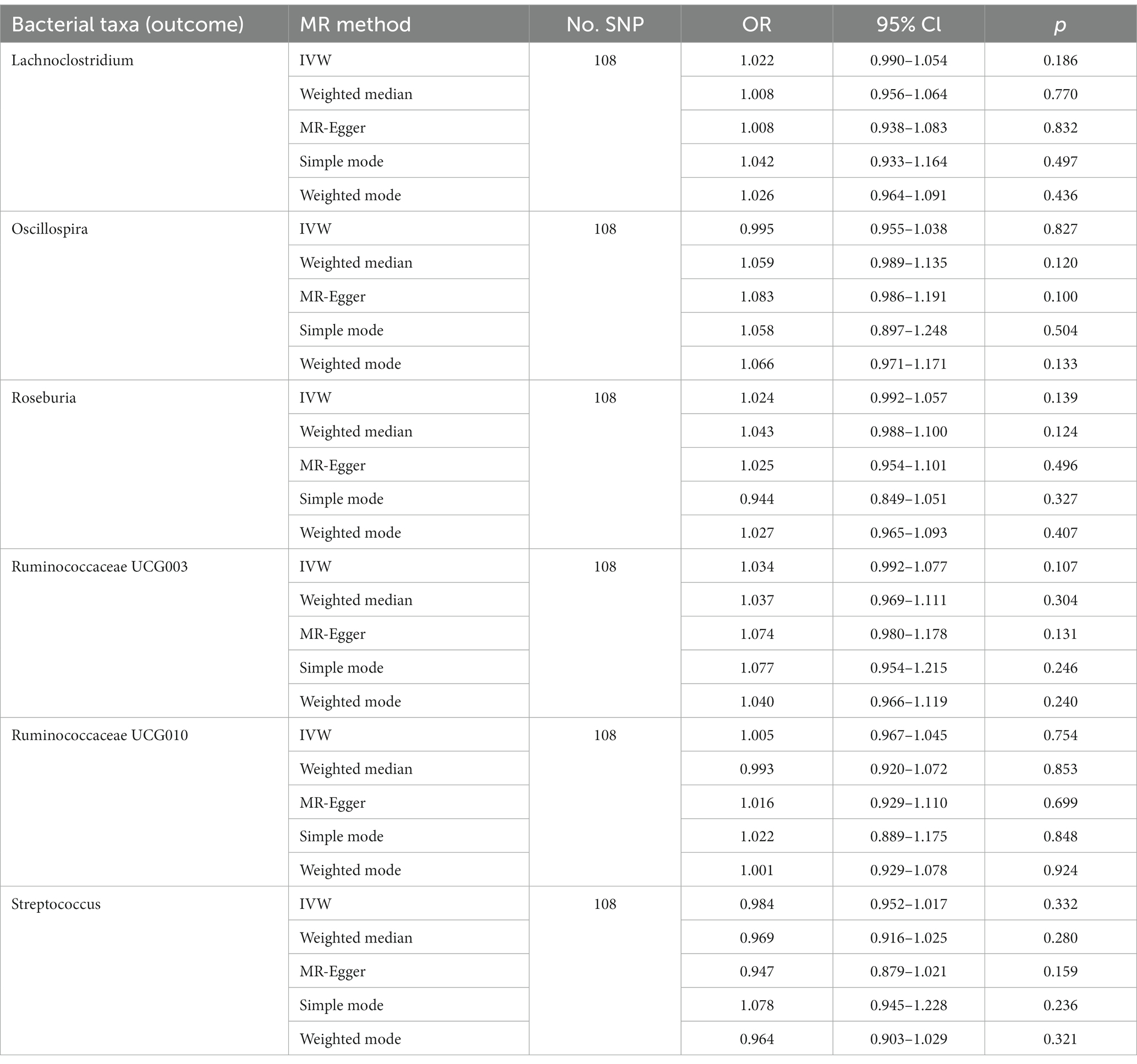
Of all the 97 genera, six significant bacterial genera were selected for further MR analyses. Furthermore, four independent SNPs were associated with Lachnoclostridium and Oscillospira, respectively. Five independent SNPs were associated with Ruminococcaceae UCG010, eight SNPs were associated with Roseburia and Streptococcus. Nine SNPs were associated with Ruminococcaceae UCG003 (Supplementary Table S2). SNP detailed message (Position, OR, Effect, Function) of significant genera in MR analyses were shown in Table 1.
The genetically predicted relative abundances of six genera were associated with T2D. Indeed, high genetically predicted levels of Oscillospira (OR: 0.834, 95% CI: 0.712–0.976), Ruminococcaceae UCG003 (OR: 0.885, 95% CI: 0.811–0.967) and Ruminococcaceae UCG010 (OR: 0.812, 95% CI: 0.713–0.925) were negatively associated with the risk of T2D. Nevertheless, Lachnoclostridium (OR: 1.175, 95% CI: 1.011–1.366), Roseburia (OR: 1.192, 95% CI: 1.033–1.374) and Streptococcus (OR: 1.199, 95% CI: 1.600–1.357) were positively associated with the risk of T2D. The weighted median method also supported parts of the results. The genetically predicted Streptococcus level was positively associated with the risk of T2D (OR: 1.211, 95% CI: 1.051–1.395). Meanwhile, Oscillospira (OR: 0.840, 95% CI: 0.707–0.998), Ruminococcaceae UCG003 (OR: 0.888, 95% CI: 0.791–0.996) and Ruminococcaceae UCG010 (OR: 0.823, 95% CI: 0.700–0.967) might have protective effect on T2D (Tables 2, 3). The underlying mechanisms of significant genera on T2D were shown in Figure 2.
Sensitivity analyses
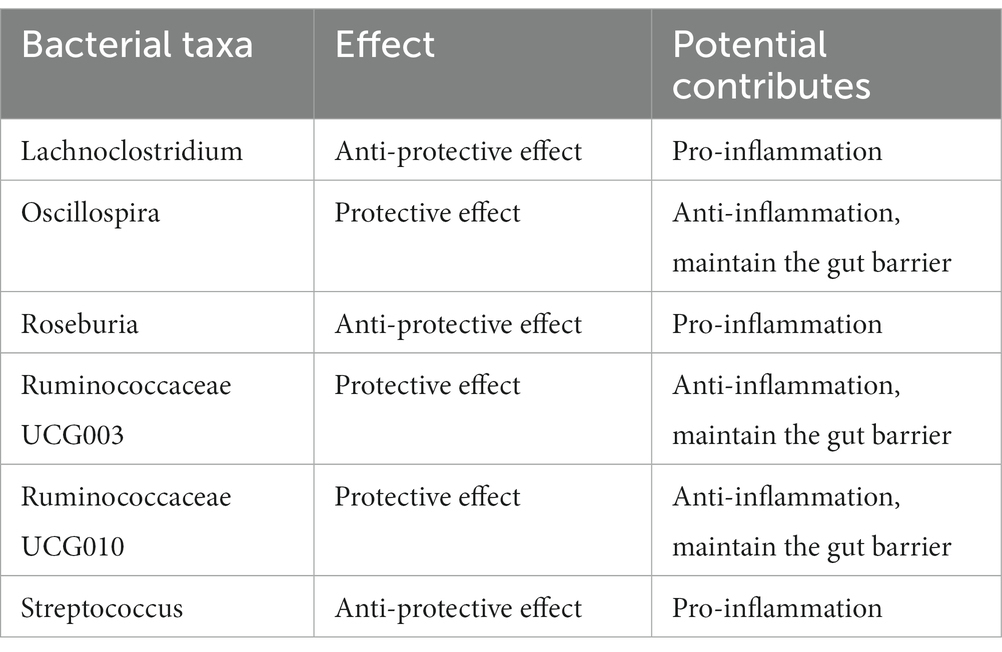
There was no heterogeneity (A genetic trait can be caused by changes in multiple different genetic materials) within the IVs of all the six genera (Supplementary Table S3). The MR-Egger regression intercepts indicated no horizontal pleiotropy (p > 0.05) (Supplementary Table S4). The scatter plots illustrated that Oscillospira, Ruminococcaceae UCG003 and Ruminococcaceae UCG010 genera might have protective effect on T2D, whereas Lachnoclostridium, Roseburia and Streptococcus genera might have anti-protective effect on T2D. The weights of MR analysis methods described in the scatter plots are listed in order, the IVW method, the MR-Egger, weighted median, weighted mode, and simple mode. The lines moving upward from left to right were found to be positive indicators of the relationship between the genus and T2D, while those going down from left to right were protective genera (Figure 3). There were no potential outliers of the IVs of all six genera for T2D in “leave-one-out” analysis, suggesting that all the identified causal associations were not influenced by single IV (Figure 4). Reverse MR results analysis revealed that T2D had no causal impact on all six genera (Table 4).
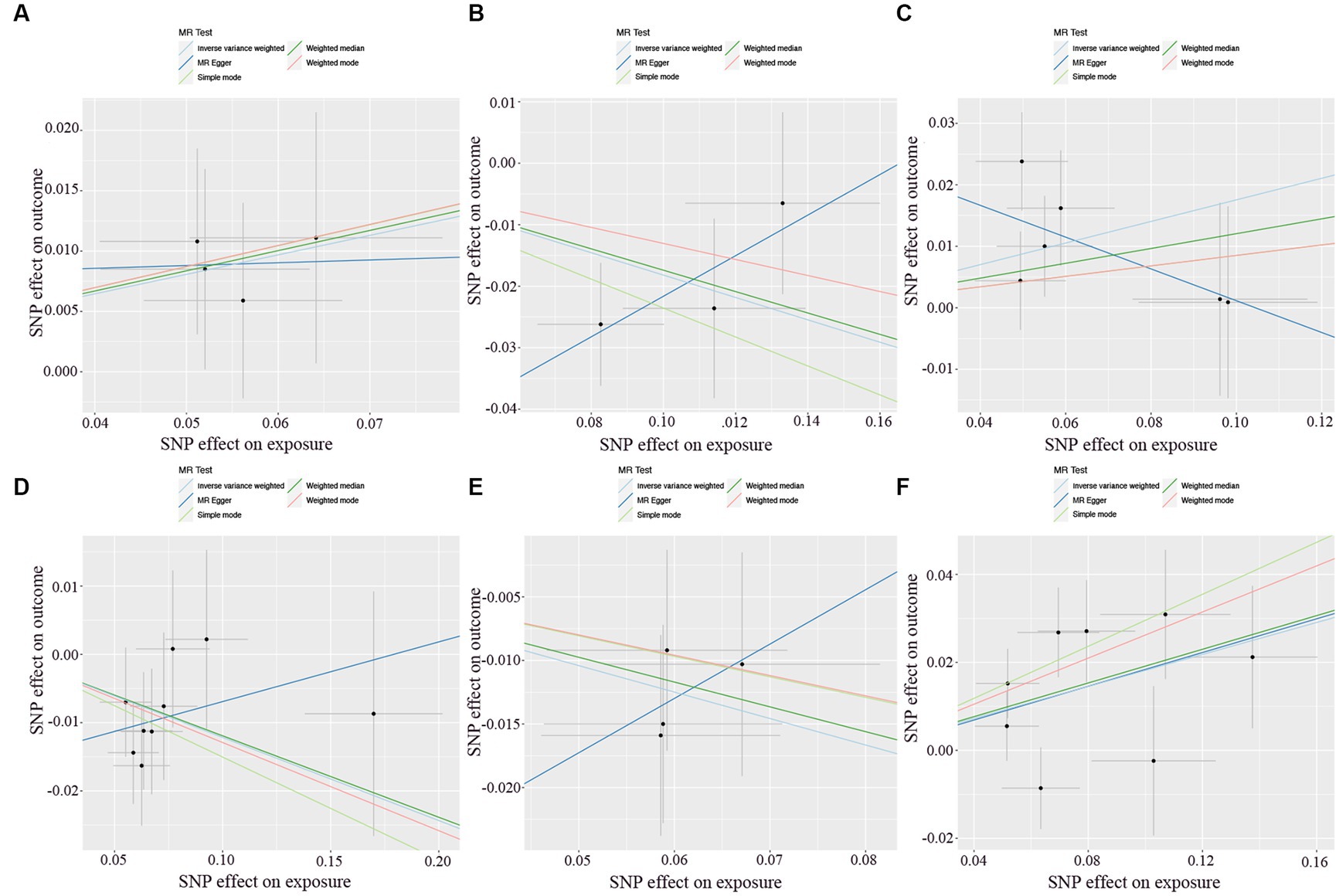
Figure 3. Scatter plots of each genus associated with the risk of type 2 diabetes. (A) Lachnoclostridium. (B) Oscillospira. (C) Roseburia. (D) Ruminococcaceae UCG003. (E) Ruminococcaceae UCG010. (F) Streptococcus.
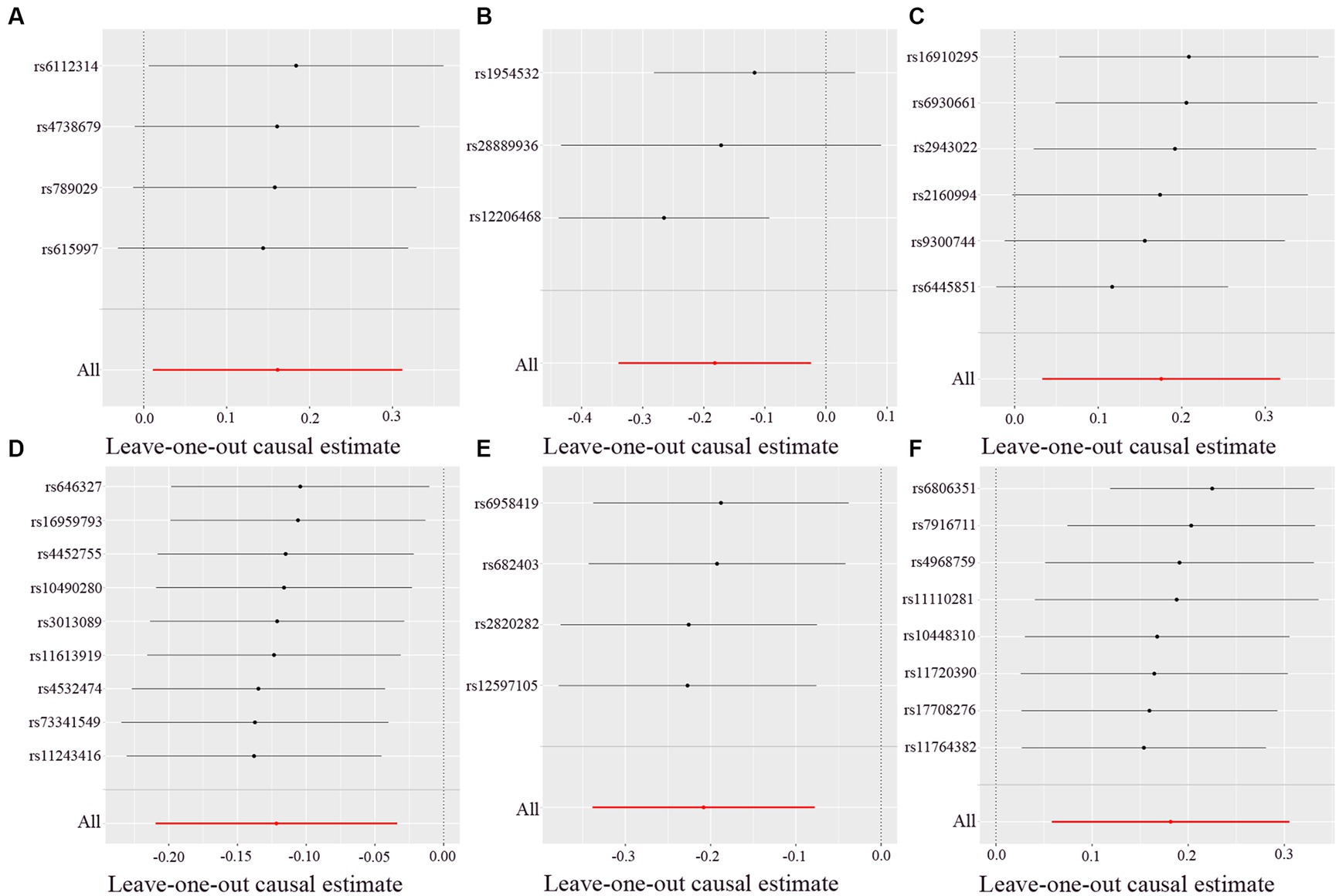
Figure 4. Leave-one-out analysis of each genus associated with type 2 diabetes. (A) Lachnoclostridium. (B) Oscillospira. (C) Roseburia. (D) Ruminococcaceae UCG003. (E) Ruminococcaceae UCG010. (F) Streptococcus. Red lines represent estimations from the IVW test.
Discussion
Using two-sample MR methods, the causal relationship between the gut microbiota and T2D was clarified. By combining evidence from MR and sensitivity analyses, we identified that the bacterial genera Lachnoclostridium, Oscillospira, Roseburia, Ruminococcaceae UCG003, Ruminococcaceae UCG010 and Streptococcus were causally associated with T2D. Among all six genera, Lachnoclostridium, Roseburia and Streptococcus were positively associated with the risk of T2D, they might have anti-protective effect on T2D. Oscillospira, Ruminococcaceae UCG003 and Ruminococcaceae UCG010 were negatively associated with the risk of T2D, in other words, the three genera might have protective effect on T2D.
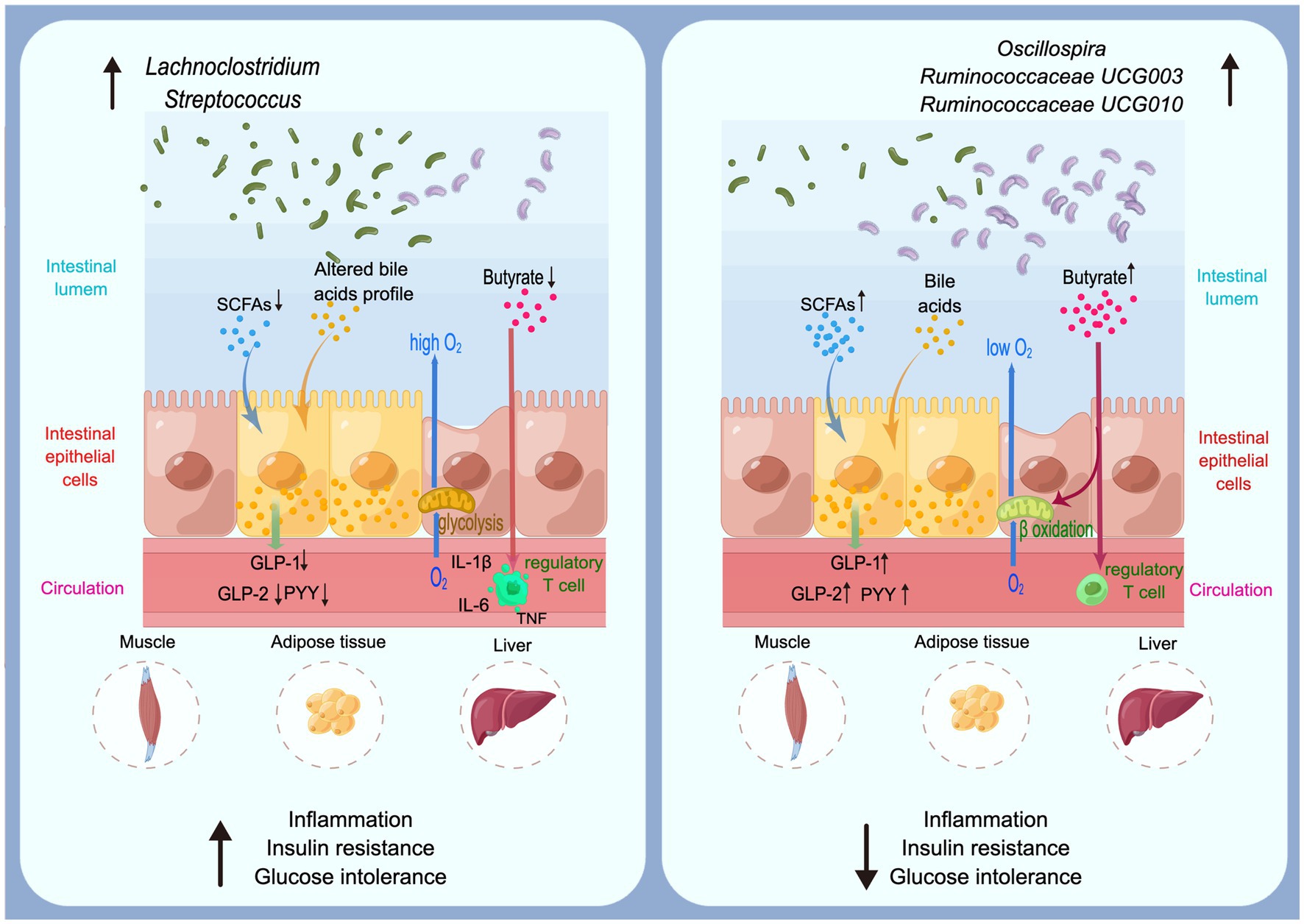
The pathogeneses of T2D are controversial and only partly understood, in which gut microbiota might be included in several underlying mechanisms (Figure 2). Short-chain fatty acids (SCFAs) and bile acids (BAs) could activate several key receptors expressed by enteroendocrine cells, thus increasing the secretion of key gut peptides including glucagon-like peptide (GLP)-1, GLP-2, and peptide YY (PYY). GLP-1, GLP-2, and PYY lower glucose concentrations (Meier, 2012), intestinal permeability (Cani et al., 2009), cytokine and amylase release (Vona-Davis and McFadden, 2007), thereby improving metabolic disorders and inflammation during diabetes. Butyrate has been described in numerous studies which provide essential energy for proliferating of colonic cells and maintain the gut barrier (Martin-Gallausiaux et al., 2021; Stoeva et al., 2021; Tang et al., 2022). Indeed, butyrate contributes to control of the anaerobic condition in the colon which is a key requirement for the remain of anaerobic bacteria in the close vicinity of the epithelium through activating the β-oxidation in the mitochondria (Rardin et al., 2013). A reduce of butyrate-producing bacteria might active immune cells including T cells, which secrete pro-inflammatory cytokines including IL-1β, IL-6 and TNF (Castoldi et al., 2015; Li et al., 2021). Through these underlying mechanisms, gut microbiota is associated with T2D development by regulate numerous metabolic pathways in the gut and at distance such as in the muscles, the adipose tissue, and the liver.
Bacterial genera Oscillospira, Ruminococcaceae UCG003 and Ruminococcaceae UCG010 are all members of family Ruminococcaceae, phylum Firmicutes. Gut microbiota affected the major human diseases, such as obesity and diabetes, through metabolic and immune signals that enter the circulation (Holmes et al., 2012). Gut microbiota involving genera from family Ruminococcaceae can affect the production of SCFAs and the conversion of primary BAs to secondary BAs (Koh et al., 2016). SCFAs are important metabolites in maintaining intestinal homeostasis among which butyrate has been investigated most extensively (Parada Venegas et al., 2019). Gut microbiota could reshape the host metabolic, signaling pathways and intestinal barrier functions, which are related to the insulin resistance in T2D (Sharma and Tripathi, 2019). As a member of family Ruminococcaceae, Oscillospira is a genus capable of producing SCFAs such as butyrate. Studies have shown that the variation in Oscillospira abundance has a obviously favorable influence on human health (Konikoff and Gophna, 2016) especially for metabolic diseases (Konikoff and Gophna, 2016; Gophna et al., 2017) and it has been referred to as a potential next-generation probiotic (Yang et al., 2021). Through over 6.2 years of follow-up for 2,731 participants without T2D initially, Miao et al. found that compared with 276 diabetics, Oscillospira were enriched in participants without T2D (Miao et al., 2020). Shen et al. investigated the effect of L-arabinose for preventing or treating T2D, and they found that L-arabinose reversed the decrease in the relative abundance of Oscillospira in T2D rats as well as aggravation symptoms of diabetes mellitus (Shen et al., 2021). These results are evidence of the protective effect of Oscillospira on T2D. Butyrate-producing bacteria produce butyrate by fermenting carbohydrate. Accumulating evidences have shown that the butyrate can interact with various physiological function, such as glucose homeostasis and appetite (Canfora et al., 2015). Butyrate has important functions for host health (McMurdie et al., 2022); studies have illustrated that butyrate-producing bacteria can protect against various types of metabolic diseases (Qin et al., 2012; Valles-Colomer et al., 2019; Stoeva et al., 2021; McMurdie et al., 2022). Depletion in butyrate-producing taxa has been linked to the development of T2D (Qin et al., 2012). Although Roseburia is a major genus of butyrate-producing bacteria in the intestinal tract of animals and humans, we found it might be risk factor for T2D. Roseburia is also a member of phylum Firmicutes, the various species of Roseburia that are all known to produce SCFAs (Tamanai-Shacoori et al., 2017). Some works observed that Roseburia exerts beneficial effects on human health through producing metabolites to prevent intestinal inflammation and maintain energy homeostasis (Tamanai-Shacoori et al., 2017; Song et al., 2021). A metagenome-wide association study(MGWAS) discovered that patients with T2D showed a decreasing trend of R. intestinalis (Qin et al., 2012), which was different from our study.
Ruminococcaceae UCG003 and Ruminococcaceae UCG010 are two bacterial genera in the same family. Recent studies discovered that Ruminococcaceae UCG003 is one of the main genera that plays a positive role in chronic insomnia and cardiometabolic diseases (Jiang et al., 2022), as well as depressive symptoms (Radjabzadeh et al., 2022). Huang et al. (2022) characterized the change in the gut microbiota composition after metabolic surgery in patients with diabetes. They found that the abundance of the Ruminococcaceae UCG003 group increased after metabolic surgery (Huang et al., 2022), implying that the abundance of Ruminococcaceae UCG003 was negatively associated with diabetes. In another study (Gao et al., 2020), levels of Ruminococcaceae UCG003 abundance were observed significantly reduced in women with hyperglycemia in pregnancy. Our findings also support the result that Ruminococcaceae UCG003 has protective effect against diabetes. Chen et al. (2021) performed a microbiome-wide study with a large population to examine the role of gut microbiome composition in insulin resistance as well as T2D. They found 12 groups of bacteria were related to T2D, which included Ruminococcaceae UCG010, and a high abundance of Ruminococcaceae UCG010 was negatively associated with the risk of T2D, suggesting its protective effects against T2D. Differ from the above study, Esquivel-Hernández et al. observed that Ruminococcaceae UCG010 is one of the genera which driving the progress of T2D in a Mexican cohort (Esquivel-Hernández et al., 2023).
A case–control study conducted by Allin et al. (2018) analyzed the gut microbiota between normal and anormal glucose regulation individuals, and they found that the abundance of various bacterial genera and over 30 operational taxonomic units (OTUs) were differentially between the two groups. In their findings, the abundance of Streptococcus increased in adults with prediabetes. Studies have shown that Streptococcus was closely related to various inflammation (Takahashi et al., 2011; Ge and Sun, 2014; Zbinden et al., 2015), which might be involved in the pathogeneses of T2D. Our findings also supported their results that increasing of Streptococcus abundance is a mark of progression to diabetes. Wang et al. (2020) observed the antiobesity effects of the natural polyphenol resveratrol (RSV) and found that RSV treatment significantly changed the composition of gut microbiome in mice, which showed an enrichment of six genera including Lachnoclostridium. Next, a high-fat diet treated mice were transplanted of the six RSV-microbiota, then the mice weight gain decreased, insulin sensitivity increased, and intestinal barrier function improved. Tettamanzi et al. (2021) conducted a 3-week randomized controlled crossover feeding trial to investigate the intervention mechanism of two types of dietaries including different components but isocaloric on 20 obese women with insulin-resistance. The study revealed that the genus Lachnoclostridium might be negatively impact to glucose metabolism, and thus, T2D. Lachnoclostridium, as a member of Lachnospiraceae, are also implied in BAs transformation (Thomas et al., 2008). Dysregulation of BAs homeostasis might associate with the progress of T2D (Chávez-Talavera et al., 2017; McGlone and Bloom, 2019). Our finding consistent with the result of Tettamanzi’s study, which suggested Lachnoclostridium was found to be positively correlated with T2D (Tettamanzi et al., 2021). Differ from our findings, Wang’s study showed that Lachnoclostridium had a positive effect on increasing insulin sensitivity (Wang et al., 2020).
Our study found a causal relationship between six genera of gut microbiota and T2D. Nevertheless, T2D is a multi-factorial disease that can be influenced by environment, gender, lifestyle, diet, aging, epigenetics, and genetics (Scott et al., 2007; Ling and Groop, 2009; NCD Risk Factor Collaboration, 2016). Previous studies have shown that the role of genera Roseburia, Rumnococcaceae UCG010, and Lachnoclostridium in T2D are controversial, and different studies have yielded different results. Our findings provide reference for dispute resolution. A change in an individual bacterium cannot account for the incidence of T2D, specific genus may have a different type of effect in specific biological context. Two large-scale metagenome analyses explored the structural features of gut microbiota in China and Europe healthy people and T2D patients, which illustrated that large differences still exist in metagenomic clusters of cohorts in both area (Qin et al., 2012; Karlsson et al., 2013). It shows that the susceptibility for T2D would not be affected by single bacteria. This situation maybe an explanation for the inconsistency of our results with some other findings.
Our study has several limitations. First, participants in the two GWASs were primarily of European descent, therefore the applicability of our results need further investigation. Second, current research methods of microbiota GWASs limit the scope of our study for further specialized level. The specificity and accuracy of current results can be increased by using advanced analyzing method, then the generalizability of our results and the accuracy of our study might be improved. Related work needs to be done to identify associations between T2D and gut microbiome by combining the evidence from cohort studies, clinical trials, and functional studies, such an investigation is conducive for exploring the pathogenesis of diabetes.
Conclusion
Our study found that there could be causal implications for T2D from the presence of six genera in the gut microbiome. Oscillospira, Ruminococcaceae UCG003, and Ruminococcaceae UCG010 were identified to be negatively associated with the risk of T2D. Lachnoclostridium, Roseburia, and Streptococcus might have anti-protective effect on T2D.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
HL designed the study, collected, and analyzed the data and wrote the manuscript. CL edited the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Joint Funds of the National Natural Science Foundation of China (No. U1705286).
Acknowledgments
The authors appreciated all the genetics consortiums for making the GWAS summary data publicly available.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1184734/full#supplementary-material
References
Allin, K. H., the IMI-DIRECT consortium, Tremaroli, V., Caesar, R., Jensen, B. A. H., Damgaard, M. T. F., et al. (2018). Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 61, 810–820. doi: 10.1007/s00125-018-4550-1
Antushevich, H. (2020). Fecal microbiota transplantation in disease therapy. Clin. Chim. Acta 503, 90–98. doi: 10.1016/j.cca.2019.12.010
Bonder, M. J., Kurilshikov, A., Tigchelaar, E. F., Mujagic, Z., Imhann, F., Vila, A. V., et al. (2016). The effect of host genetics on the gut microbiome. Nat. Genet. 48, 1407–1412. doi: 10.1038/ng.3663
Bowden, J., Davey Smith, G., and Burgess, S. (2015). Mendelian randomization with invalid instruments: effect estimation and bias detection through egger regression. Int. J. Epidemiol. 44, 512–525. doi: 10.1093/ije/dyv080
Bowden, J., Davey Smith, G., Haycock, P. C., and Burgess, S. (2016). Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 40, 304–314. doi: 10.1002/gepi.21965
Burgess, S., Butterworth, A., and Thompson, S. G. (2013). Mendelian randomization analysis with multiple genetic variants using summarized data. Genet. Epidemiol. 37, 658–665. doi: 10.1002/gepi.21758
Canfora, E. E., Jocken, J. W., and Blaak, E. E. (2015). Short-chain fatty acids in control of body weight and insulin sensitivity. Nat. Rev. Endocrinol. 11, 577–591. doi: 10.1038/nrendo.2015.128
Cani, P. D. (2018). Human gut microbiome: hopes, threats and promises. Gut 67, 1716–1725. doi: 10.1136/gutjnl-2018-316723
Cani, P. D., Possemiers, S., van de Wiele, T., Guiot, Y., Everard, A., Rottier, O., et al. (2009). Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 58, 1091–1103. doi: 10.1136/gut.2008.165886
Castoldi, A., Naffah de Souza, C., Câmara, N. O., and Moraes-Vieira, P. M. (2015). The macrophage switch in obesity development. Front. Immunol. 6:637. doi: 10.3389/fimmu.2015.00637
Chappert, P. (2014). Role of SFB in autoimmune arthritis: an example of regulation of autoreactive T cell sensitivity in the gut. Gut Microbes 5, 259–264. doi: 10.4161/gmic.28134
Chávez-Talavera, O., Tailleux, A., Lefebvre, P., and Staels, B. (2017). Bile acid control of metabolism and inflammation in obesity, type 2 diabetes, dyslipidemia, and nonalcoholic fatty liver disease. Gastroenterology 152, 1679–94.e3. doi: 10.1053/j.gastro.2017.01.055
Chen, Z., Radjabzadeh, D., Chen, L., Kurilshikov, A., Kavousi, M., Ahmadizar, F., et al. (2021). Association of Insulin Resistance and Type 2 diabetes with Gut microbial diversity: a microbiome-wide analysis from population studies. JAMA Netw. Open 4:e2118811. doi: 10.1001/jamanetworkopen.2021.18811
Dong, Y., Zou, Z., Deng, P., Fan, X., and Li, C. (2023). Circulating metabolites and depression: a bidirectional Mendelian randomization. Front. Neurosci. 17:1146613. doi: 10.3389/fnins.2023.1146613
Doumatey, A. P., Adeyemo, A., Zhou, J., Lei, L., Adebamowo, S. N., Adebamowo, C., et al. (2020). Gut microbiome profiles are associated with type 2 diabetes in urban Africans. Front. Cell. Infect. Microbiol. 10:63. doi: 10.3389/fcimb.2020.00063
Emdin, C. A., Khera, A. V., and Kathiresan, S. (2017). Mendelian randomization. JAMA 318, 1925–1926. doi: 10.1001/jama.2017.17219
Esquivel-Hernández, D. A., Martínez-López, Y. E., Sánchez-Castañeda, J. P., Neri-Rosario, D., Padrón-Manrique, C., Giron-Villalobos, D., et al. (2023). A network perspective on the ecology of gut microbiota and progression of type 2 diabetes: linkages to keystone taxa in a Mexican cohort. Front. Endocrinol. (Lausanne). 14:1128767. doi: 10.3389/fendo.2023.1128767
Forslund, K., MetaHIT consortium, Hildebrand, F., Nielsen, T., Falony, G., le Chatelier, E., et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature 528, 262–266. doi: 10.1038/nature15766
Gao, B., Zhong, M., Shen, Q., Wu, Y., Cao, M., Ju, S., et al. (2020). Gut microbiota in early pregnancy among women with Hyperglycaemia vs Normal blood glucose. BMC Pregnancy Childbirth 20:284. doi: 10.1186/s12884-020-02961-5
Ge, R., and Sun, X. (2014). Iron acquisition and regulation systems in Streptococcus species. Metallomics 6, 996–1003. doi: 10.1039/c4mt00011k
Geva-Zatorsky, N., Sefik, E., Kua, L., Pasman, L., Tan, T. G., Ortiz-Lopez, A., et al. (2017). Mining the human Gut microbiota for immunomodulatory organisms. Cells 168, 928–43.e11. doi: 10.1016/j.cell.2017.01.022
Goodrich, J. K., Davenport, E. R., Beaumont, M., Jackson, M. A., Knight, R., Ober, C., et al. (2016). Genetic determinants of the Gut microbiome in UK twins. Cell Host Microbe 19, 731–743. doi: 10.1016/j.chom.2016.04.017
Gophna, U., Konikoff, T., and Nielsen, H. B. (2017). Oscillospira and related bacteria - from metagenomic species to metabolic features. Environ. Microbiol. 19, 835–841. doi: 10.1111/1462-2920.13658
Holmes, E., Li, J. V., Marchesi, J. R., and Nicholson, J. K. (2012). Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell Metab. 16, 559–564. doi: 10.1016/j.cmet.2012.10.007
Hsieh, M. C., Tsai, W. H., Jheng, Y. P., Su, S. L., Wang, S. Y., Lin, C. C., et al. (2018). The beneficial effects of Lactobacillus reuteri ADR-1 or ADR-3 consumption on type 2 diabetes mellitus: a randomized, double-blinded, placebo-controlled trial. Sci. Rep. 8:16791. doi: 10.1038/s41598-018-35014-1
Huang, H. H., Lin, T. L., Lee, W. J., Chen, S. C., Lai, W. F., Lu, C. C., et al. (2022). Impact of metabolic surgery on Gut microbiota and sera Metabolomic patterns among patients with diabetes. Int. J. Mol. Sci. 23:7797. doi: 10.3390/ijms23147797
Jaacks, L. M., Siegel, K. R., Gujral, U. P., and Narayan, K. M. (2016). Type 2 diabetes: a 21st century epidemic. Best Pract. Res. Clin. Endocrinol. Metab. 30, 331–343. doi: 10.1016/j.beem.2016.05.003
Jiang, Z., Zhuo, L. B., He, Y., Fu, Y., Shen, L., Xu, F., et al. (2022). The gut microbiota-bile acid axis links the positive association between chronic insomnia and cardiometabolic diseases. Nat. Commun. 13:3002. doi: 10.1038/s41467-022-30712-x
Jubair, W. K., Hendrickson, J. D., Severs, E. L., Schulz, H. M., Adhikari, S., Ir, D., et al. (2018). Modulation of inflammatory arthritis in mice by Gut microbiota through mucosal inflammation and autoantibody generation. Arthritis Rheumatol. 70, 1220–1233. doi: 10.1002/art.40490
Karlsson, F. H., Tremaroli, V., Nookaew, I., Bergström, G., Behre, C. J., Fagerberg, B., et al. (2013). Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 498, 99–103. doi: 10.1038/nature12198
Kashtanova, D. A., Tkacheva, O. N., Doudinskaya, E. N., Strazhesko, I. D., Kotovskaya, Y. V., Popenko, A. S., et al. (2018). Gut microbiota in patients with different metabolic statuses: Moscow study. Microorganisms 6:98. doi: 10.3390/microorganisms6040098
Kitten, A. K., Ryan, L., Lee, G. C., Flores, B. E., and Reveles, K. R. (2021). Gut microbiome differences among Mexican Americans with and without type 2 diabetes mellitus. PLoS One 16:e0251245. doi: 10.1371/journal.pone.0251245
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary Fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cells 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Konikoff, T., and Gophna, U. (2016). Oscillospira: a central, enigmatic component of the human Gut microbiota. Trends Microbiol. 24, 523–524. doi: 10.1016/j.tim.2016.02.015
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra, A. V., Udayappan, S. D., et al. (2017). Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 26, 611–9.e6. doi: 10.1016/j.cmet.2017.09.008
Kurilshikov, A., Medina-Gomez, C., Bacigalupe, R., Radjabzadeh, D., Wang, J., Demirkan, A., et al. (2021). Large-scale association analyses identify host factors influencing human gut microbiome composition. Nat. Genet. 53, 156–165. doi: 10.1038/s41588-020-00763-1
Larsen, N., Vogensen, F. K., van den Berg, F. W., Nielsen, D. S., Andreasen, A. S., Pedersen, B. K., et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One 5:e9085. doi: 10.1371/journal.pone.0009085
Lastra, G., Syed, S., Kurukulasuriya, L. R., Manrique, C., and Sowers, J. R. (2014). Type 2 diabetes mellitus and hypertension: an update. Endocrinol. Metab. Clin. N. Am. 43, 103–122. doi: 10.1016/j.ecl.2013.09.005
Li, G., Lin, J., Zhang, C., Gao, H., Lu, H., Gao, X., et al. (2021). Microbiota metabolite butyrate constrains neutrophil functions and ameliorates mucosal inflammation in inflammatory bowel disease. Gut Microbes 13:1968257. doi: 10.1080/19490976.2021.1968257
Ling, C., and Groop, L. (2009). Epigenetics: a molecular link between environmental factors and type 2 diabetes. Diabetes 58, 2718–2725. doi: 10.2337/db09-1003
Liu, L., Zhang, J., Cheng, Y., Zhu, M., Xiao, Z., Ruan, G., et al. (2022). Gut microbiota: a new target for T2DM prevention and treatment. Front. Endocrinol. (Lausanne) 13:958218. doi: 10.3389/fendo.2022.958218
Martin-Gallausiaux, C., Marinelli, L., Blottière, H. M., Larraufie, P., and Lapaque, N. (2021). SCFA: mechanisms and functional importance in the gut. Proc. Nutr. Soc. 80, 37–49. doi: 10.1017/S0029665120006916
McGlone, E. R., and Bloom, S. R. (2019). Bile acids and the metabolic syndrome. Ann. Clin. Biochem. 56, 326–337. doi: 10.1177/0004563218817798
McMurdie, P. J., Stoeva, M. K., Justice, N., Nemchek, M., Sieber, C. M. K., Tyagi, S., et al. (2022). Increased circulating butyrate and ursodeoxycholate during probiotic intervention in humans with type 2 diabetes. BMC Microbiol. 22:19. doi: 10.1186/s12866-021-02415-8
Meier, J. J. (2012). GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 8, 728–742. doi: 10.1038/nrendo.2012.140
Miao, Z., Lin, J. S., Mao, Y., Chen, G. D., Zeng, F. F., Dong, H. L., et al. (2020). Erythrocyte n-6 polyunsaturated fatty acids, Gut microbiota, and incident type 2 diabetes: a prospective cohort study. Diabetes Care 43, 2435–2443. doi: 10.2337/dc20-0631
NCD Risk Factor Collaboration (2016). Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 387, 1513–1530. doi: 10.1016/S0140-6736(16)00618-8
Parada Venegas, D., de la Fuente, M. K., Landskron, G., González, M. J., Quera, R., Dijkstra, G., et al. (2019). Short chain fatty acids (SCFAs)-mediated Gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 10:277. doi: 10.3389/fimmu.2019.00277
Qin, J., Li, Y., Cai, Z., Li, S., Zhu, J., Zhang, F., et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60. doi: 10.1038/nature11450
Radjabzadeh, D., Bosch, J. A., Uitterlinden, A. G., Zwinderman, A. H., Ikram, M. A., van Meurs, J. B. J., et al. (2022). Gut microbiome-wide association study of depressive symptoms. Nat. Commun. 13:7128. doi: 10.1038/s41467-022-34502-3
Rardin, M. J., He, W., Nishida, Y., Newman, J. C., Carrico, C., Danielson, S. R., et al. (2013). SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933. doi: 10.1016/j.cmet.2013.11.013
Scher, J. U., Nayak, R. R., Ubeda, C., Turnbaugh, P. J., and Abramson, S. B. (2020). Pharmacomicrobiomics in inflammatory arthritis: gut microbiome as modulator of therapeutic response. Nat. Rev. Rheumatol. 16, 282–292. doi: 10.1038/s41584-020-0395-3
Scott, L. J., Mohlke, K. L., Bonnycastle, L. L., Willer, C. J., Li, Y., Duren, W. L., et al. (2007). A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316, 1341–1345. doi: 10.1126/science.1142382
Sedighi, M., Razavi, S., Navab-Moghadam, F., Khamseh, M. E., Alaei-Shahmiri, F., Mehrtash, A., et al. (2017). Comparison of gut microbiota in adult patients with type 2 diabetes and healthy individuals. Microb. Pathog. 111, 362–369. doi: 10.1016/j.micpath.2017.08.038
Sharma, S., and Tripathi, P. (2019). Gut microbiome and type 2 diabetes: where we are and where to go? J. Nutr. Biochem. 63, 101–108. doi: 10.1016/j.jnutbio.2018.10.003
Shen, D., Lu, Y., Tian, S., Ma, S., Sun, J., Hu, Q., et al. (2021). Effects of L-arabinose by hypoglycemic and modulating gut microbiome in a high-fat diet- and streptozotocin-induced mouse model of type 2 diabetes mellitus. J. Food Biochem. 45:e13991. doi: 10.1111/jfbc.13991
Shi, N., Li, N., Duan, X., and Niu, H. (2017). Interaction between the gut microbiome and mucosal immune system. Mil. Med. Res. 4:14. doi: 10.1186/s40779-017-0122-9
Song, L., He, M., Sun, Q., Wang, Y., Zhang, J., Fang, Y., et al. (2021). Roseburia hominis increases intestinal melatonin level by activating p-CREB-AANAT pathway. Nutrients 14:117. doi: 10.3390/nu14010117
Stoeva, M. K., Garcia-So, J., Justice, N., Myers, J., Tyagi, S., Nemchek, M., et al. (2021). Butyrate-producing human gut symbiont, clostridium butyricum, and its role in health and disease. Gut Microbes 13, 1–28. doi: 10.1080/19490976.2021.1907272
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2022). IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183:109119. doi: 10.1016/j.diabres.2021.109119
Takagi, T., Naito, Y., Kashiwagi, S., Uchiyama, K., Mizushima, K., Kamada, K., et al. (2020). Changes in the Gut microbiota are associated with hypertension, hyperlipidemia, and type 2 diabetes mellitus in Japanese subjects. Nutrients 12:2996. doi: 10.3390/nu12102996
Takahashi, T., Ubukata, K., and Watanabe, H. (2011). Invasive infection caused by Streptococcus dysgalactiae subsp. equisimilis: characteristics of strains and clinical features. J. Infect. Chemother. 17, 1–10. doi: 10.1007/s10156-010-0084-2
Tamanai-Shacoori, Z., Smida, I., Bousarghin, L., Loreal, O., Meuric, V., Fong, S. B., et al. (2017). Roseburiaspp.: a marker of health? Future Microbiol. 12, 157–170. doi: 10.2217/fmb-2016-0130
Tang, G., du, Y., Guan, H., Jia, J., Zhu, N., Shi, Y., et al. (2022). Butyrate ameliorates skeletal muscle atrophy in diabetic nephropathy by enhancing gut barrier function and FFA2-mediated PI3K/Akt/mTOR signals. Br. J. Pharmacol. 179, 159–178. doi: 10.1111/bph.15693
Taylor, R. (2013). Type 2 diabetes: etiology and reversibility. Diabetes Care 36, 1047–1055. doi: 10.2337/dc12-1805
Tettamanzi, F., Bagnardi, V., Louca, P., Nogal, A., Monti, G. S., Mambrini, S. P., et al. (2021). A high protein diet is more effective in improving insulin resistance and glycemic variability compared to a Mediterranean diet-a cross-over controlled inpatient dietary study. Nutrients 13:4380. doi: 10.3390/nu13124380
Thomas, C., Pellicciari, R., Pruzanski, M., Auwerx, J., and Schoonjans, K. (2008). Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 7, 678–693. doi: 10.1038/nrd2619
Valles-Colomer, M., Falony, G., Darzi, Y., Tigchelaar, E. F., Wang, J., Tito, R. Y., et al. (2019). The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 4, 623–632. doi: 10.1038/s41564-018-0337-x
Vona-Davis, L., and McFadden, D. W. (2007). PYY and the pancreas: inhibition of tumor growth and inflammation. Peptides 28, 334–338. doi: 10.1016/j.peptides.2006.07.033
Wang, P., Li, D., Ke, W., Liang, D., Hu, X., and Chen, F. (2020). Resveratrol-induced gut microbiota reduces obesity in high-fat diet-fed mice. Int. J. Obes. 44, 213–225. doi: 10.1038/s41366-019-0332-1
Wu, H., Esteve, E., Tremaroli, V., Khan, M. T., Caesar, R., Mannerås-Holm, L., et al. (2017). Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858. doi: 10.1038/nm.4345
Xue, A., Wu, Y., Zhu, Z., Zhang, F., Kemper, K. E., Zheng, Z., et al. (2018). Genome-wide association analyses identify 143 risk variants and putative regulatory mechanisms for type 2 diabetes. Nat. Commun. 9:2941. doi: 10.1038/s41467-018-04951-w
Yang, J., Li, Y., Wen, Z., Liu, W., Meng, L., and Huang, H. (2021). Oscillospira - a candidate for the next-generation probiotics. Gut Microbes 13:1987783. doi: 10.1080/19490976.2021.1987783
Zaky, A., Glastras, S. J., Wong, M. Y. W., Pollock, C. A., and Saad, S. (2021). The role of the Gut microbiome in diabetes and obesity-related kidney disease. Int. J. Mol. Sci. 22:9641. doi: 10.3390/ijms22179641
Zbinden, A., Bostanci, N., and Belibasakis, G. N. (2015). The novel species Streptococcus tigurinus and its association with oral infection. Virulence 6, 177–182. doi: 10.4161/21505594.2014.970472
Keywords: causal relationship, gut microbiota, genome-wide association study, Mendelian randomization, type 2 diabetes
Citation: Li H and Li C (2023) Causal relationship between gut microbiota and type 2 diabetes: a two-sample Mendelian randomization study. Front. Microbiol. 14:1184734. doi: 10.3389/fmicb.2023.1184734
Edited by:
Junling Shi, Northwestern Polytechnical University, ChinaReviewed by:
Kaijian Hou, Shantou University, ChinaRobert Cody Sharp, University of Florida, United States
Copyright © 2023 Li and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Candong Li, Zmp6eWxjZEAxMjYuY29t
 Hanjing Li
Hanjing Li Candong Li1,2,3*
Candong Li1,2,3*