
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 06 July 2023
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1183598
This article is part of the Research Topic Interactions between Bioactive Food Ingredients and Intestinal Microbiota, volume II View all 16 articles
Obesity has been regarded as one of the major health problems worldwide. Studies demonstrated that ursolic acid (UA) can significantly ameliorate the progress of obesity. However, whether the effect of UA on obesity depends on the regulation of gut microbiota and metabolism is uncertain. To investigate the regulatory role of UA in obese mice from the perspective of intestinal microbiome and metabolomics analyses, an obese mice model was established with a high-fat diet, and the effect of UA on obesity was evaluated. The alterations of gut microbiota and metabolism related to obesity were evaluated by bioinformatic analysis. The results of the gut microbiota analysis showed that UA intervention could shift the Firmicutes to Bacteroidetes ratio at the phylum level and increase in the genera of Lactobacillus, Bacteroides, and Akkermansia. Additionally, metabolomics analysis showed that the beneficial influence of UA on obesity partly depended on amino acid metabolism. The current study demonstrated the roles of UA in the anti-obesity process, which depends in part on alterations in the gut microbiota and metabolism. Therefore, our findings highlight the potential therapeutic effect of UA on the improvement of diet-induced obesity in humans.
Obesity the third most prevalent isepidemiological factor affecting human health after smoking and AIDS. It is also intimately connected to several chronic illnesses, including cancer, type 2 diabetes, renal disease, liver disease, and heart disease, all of which are detrimental to human health (Medanić and Pucarin-Cvetković, 2012; Pan et al., 2018; Ojulari et al., 2019). Therefore, the search for effective solutions to improve obesity is extremely important. There are several preventive or therapeutic strategies for combating obesity, including lifestyle changes, such as diet and exercise, drug treatment, and surgery. However, these approaches can be less satisfactory in the long term, especially drug therapies, which may have serious adverse effects that lead to their successive withdrawal in recent years (Khera et al., 2016; Clément et al., 2018). Therefore, a more effective method should be explored to improve obesity.
The gut microbiota refers to a complex ecosystem in the human digestive tract that includes bacteria, fungi, viruses, archaea, and protists. It could participate in the regulation of multiple physiological functions that maintain the metabolic homeostasis of the host (Qu et al., 2018; Li et al., 2020; Sun et al., 2022). Gut dysbiosis has various adverse effects on the host and may result in metabolic diseases, especially in the obesity condition, which has been revealed by growing evidence over the last two decades. In mice, Candida parapsilosis expansion in the gut has been shown to contribute to the promotion of obesity, mainly in terms of higher body weight, accumulation of fungal lipase, and alteration of serum biochemical indicators (Sun S. et al., 2021). Yu et al. (2022) found that the onset of diabetes type 2 mellitus in obese mice was impeded by gut microbiota disturbance, and glucose decomposition may be the primary mechanism by which the jejunal bacteria control the host metabolism. Meanwhile, Wang et al. (2019) demonstrated the numerous metabolic benefits of orally administering live Parabacteroides distasonis in high-fat diet (HFD)-induced obese mice, mainly via controlling weight gain, lowering hyperglycemia and hyperlipidemia, and improving hepatic steatosis through the production of succinate and secondary bile acids. According to the findings, effective regulation of host metabolism by intestinal flora is crucial to the development of obesity.
Plant-derived biologically active products have various biological functions in the regulation of a wide spectrum of diseases (Diarra et al., 2016; de Freitas Junior and de Almeida, 2017; Sehrawat et al., 2017). Ursolic acid (UA) is commonly found in fruits and vegetables. According to reports, UA controls a variety of biological functions, including the modulation of several signaling pathways that may stop the onset of chronic diseases (Seo et al., 2018). Recently, the relation of UA to obesity has been documented extensively. Tang et al. (2022) examined the anti-obesity impact of UA in obese rats induced by high fat and streptozotocin via altering insulin and c-Jun N-terminal kinase signaling pathways. Sun A. et al. (2021) demonstrated the crucial role of UA in improving the obesity and metabolic status of obese mice via increasing irisin production, promoting white adipose tissue beigeing, and lowering weight. Moreover, UA has been shown to have potential therapeutic utility in obesity and diabetes via preventing insulin resistance and hyperinsulinemia and by reversing hyperinsulinemia induced by obesity in rats fed with a HFD (González-Garibay et al., 2020). However, the function of UA on the metabolism and gut microbiota during obesity is largely unexplored, so more studies are needed to ascertain whether UA can affect host metabolic pathways.
The objective of this study was to identify the interrelationship among UA, gut microbiota, and metabolites in obese mice induced by diet. 16S rRNA sequencing was conducted to analyze the gut microbiota in mice receiving a HFD, and a metabolomics analysis was used to investigate the mechanism of UA in obesity from the perspective of microbiome changes. This study provides a useful exploration of the potential mechanisms of UA in the regulation of obesity though microbiomics and metabolomics analysis and further demonstrated the beneficial effect of UA on obesity improvement.
In the current research, C57BL/6J male mice ordered form SiPeiFu Biotechnology Co., Ltd (Beijing, China). Then 5-week-old mice were firstly housed under a 12 h light/dark cycle with unrestricted access to food and water, the temperature and humidity of the feeding environment were controlled at 24 ± 1°C and 55 ± 5%, respectively. After 1 week adaptive feeding, the mice were separated equally into three groups for treatment (8 mice per group): (1) control group (fed with the normal diet), (2) HFD group (fed with the HFD), (3) HFD + UA group (fed with the HFD and given a moderate dose of UA, 100 mg/kg/d). The UA was purchased from Xi’an Ruilin Biotechnology Co., Ltd. (Xi’an, China). The normal-fat diet (10 kcal% fat, 20 kcal% protein, and 70 kcal% carbohydrate, catalog no. D12450B) and the HFD (60 kcal% fat, 20 kcal% protein, and 20 kcal% carbohydrate, catalog no. D12492) were Research Diets purchased from Xiaoshuyoutai Biotechnology Co., Ltd. (Beijing, China). During the experiment, the body weight of the mice was record once per week. The treatment were continued for 8 weeks.
Fresh fecal samples were obtained after an 8-week treatment and stored at −80°C for the gut microbiota and metabolite analyses. After the last treatment administration, mice were allowed with free access to water but no food for 12 h, then were anesthetized via intraperitoneal injection of 1.25% tribromoethanol and the blood samples from the eye orbit were collected. The liver, epididymal white adipose tissue (eWAT), perirenal white adipose tissue (pWAT), and mesenteric fat were stripped and weighed immediately at 4°C after mice were sacrificed, and then maintained at −80°C for the experiment. The collected blood was then centrifuged at 3,000 rpm for 15 min to obtain the serum. After being frozen at −80°C, the serum biochemical indexes were tested using assay kits (Jiancheng, China), including total cholesterol (TC), total triglyceride (TG), high-density lipoprotein cholesterol (HDL-C), and low-density lipoprotein cholesterol (LDL-C). The following formula was used to calculate the index:
After being treated in 4% formaldehyde solution for paraffin sections, partial liver samples were cut into 5-μm-thick slices for hematoxylin-eosin staining. An optical microscope was used to take pictures of the dyed liver sections (Olympus, Tokyo, Japan).
We extracted genomic DNA from fecal samples using the DNA Extraction Kit (Nobleryder, China) following the manufacturer’s instructions. The 16S rRNA gene’s hypervariable region V3–4 was amplified through PCR using a specific primer. Wekemon Biotechnology Co., Ltd. (Guangzhou, China) carried out the sequencing using the NovaSeq 6000 platform. Quantitative Insights Into Microbial Ecology (QIIME, version 2.0) software was used to obtain quality filter raw sequences. Paired-end reads were assembled using FLASH. Following the detection of chimeras, the remaining high-quality sequences were clustered into operational taxonomic units (OTUs) at 97% sequence identity by UCLUST. A representative sequence was selected from each OTU using default parameters. OTU taxonomic classification was then conducted by BLAST searching the representative sequences set against the Green Genes Database. Based on the emergence of OTUs between groups a Venn diagram was generated using the R package to visualize the shared and unique OTUs between groups. The core-diversity plugin within the open-source QIIME 2 platform was used to calculate the alpha and beta diversity. The specific gut microbiota was analyzed through linear discriminant analysis (LDA) and effect size (LEfSe) analyses. A non-parametric factorial Kruskal–Wallis sum-rank test was used for LEfSe to determine the differences in abundance between groups and used LDA to assess the effect size of each feature, and the threshold on the logarithmic score of LDA analysis was set to 2.0. Spearman’s analysis was used to determine the relationship between microbial communities and serum biochemical parameters based on the relative abundance of microbial species at various taxon levels. All settings were set to default, unless otherwise stated.
To obtain the supernatant for LC-MS analysis, fecal samples (100 mg) were mixed with extract solution (methanol:water = 4:5, v:v) and centrifuged. A Vanquish UHPLC System from Wekemon Biotechnology Co., Ltd. (Guangzhou, China) was used to perform the LC separation. Orthogonal partial least-squares discriminant analysis (OPLS-DA) was used to compare metabolites in different groups. The variable influence on projection value together with the p-value obtained using the two-tailed t-test (VIP value > 1.0 and p-value < 0.05) were used to select the potential biomarkers. The Human Metabolome Database1 was selected to compare the typical MS/MS fragments. The MetaboAnalyst website2 based on the Kyoto Encyclopedia of Genes and Genomes (KEGG) database3 was used for pathway enrichment analyses.
All data were analyzed using one-way ANOVA in SPSS 24.0 (IBM), and Prism 8.0.2 software (GraphPad Software, La Jolla, CA, USA) was used to create all graphics. Spearman correlation was used to examine the correlation between significant microbial communities and metabolism; p-value < 0.05 indicated significance.
In this study, we investigated the effects of UA on obese mice by constructing an obesity model as shown in Figure 1A. According to Figure 1B, the body weight of mice in the HFD group increased rapidly relative to that of those in the control group over the course of 8 weeks. Nevertheless, UA supplementation decreased this increment (p < 0.05). In addition, the HFD could significantly increase the ratio of fat to body weight and visceral fat in contrast to the control, and the UA treatment also reduced the elevated levels but still remained above the normal level (p < 0.05) (Figures 1C, D).
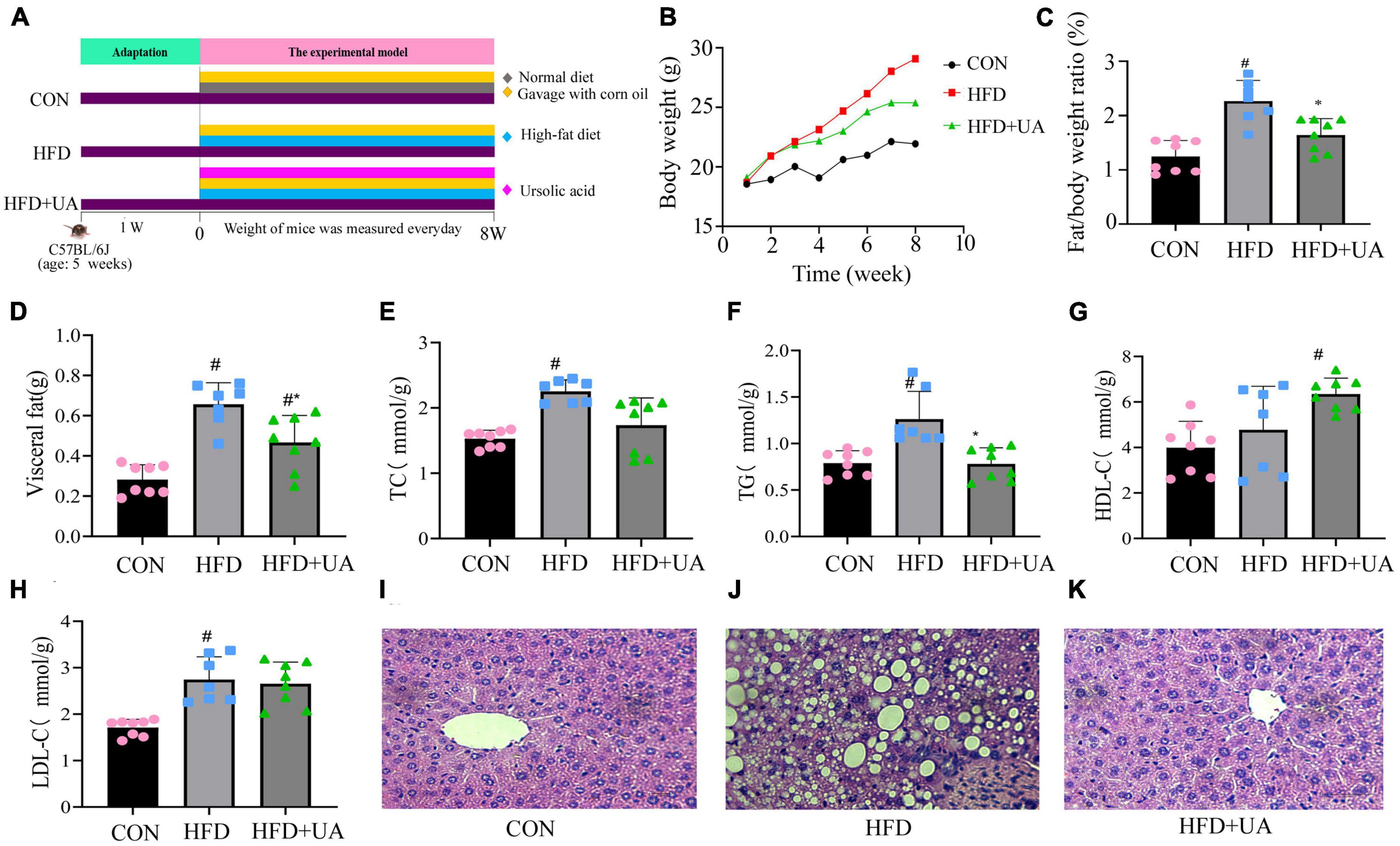
Figure 1. Effect of ursolic acid administration on physiological, serological, and liver histopathological changes. (A) Experimental design (B) Body weight growth; (C) ratio of fat to body weight; (D) visceral fat; (E–H) Changes in serum biochemical indexes in each group of mice; (I–K) hemotoxin-eosin staining of liver tissue sections of mice in different groups (#P < 0.05 compared to control group, *p < 0.05 relative to the high-fat diet group).
Additionally, the levels of TC, TG, HDL-C, and LDL-C were measured to ascertain the potential contribution of UA to the reduction of lipid buildup in mice (Figures 1E–H). The HFD-fed mice had higher TC, TG, and LDL-C levels in serum after 8 weeks of feeding relative to the control group (p < 0.05), and these increases were lowered by UA administration, but still remained above normal levels. Interestingly, HDL-C presented high levels of expression in the HFD group relative to those in the control group and remained significantly higher after UA intervention.
Hematoxylin-eosin staining of liver samples was used to assess the triacylglycerol accumulation. The result showed that following the 8-week treatment period, the HFD-fed mice had substantial lipid droplet buildup relative to those in the control group. This accumulation was greatly alleviated after UA intervention (Figures 1I–K), suggesting that UA can successfully limit the buildup of liver lipids and slow the onset of fatty liver in HFD-group mice.
For the 16S rRNA sequencing results included Sparsity curve analysis and OTUs Venn diagram were shown in Supplementary Figures 1, 2. Alpha diversity analysis was used to evaluate the diversity of the microbiota community in each sample, which was presented by diversity indices including Shannon, Ace, and Chao1. The Shannon index reflects the richness and evenness of a community, whereas the Ace and Chao1 indices were positively correlated with community richness (Figures 2A–C). In contrast to those in the control group, the Ace and Chao1 indexes in the HFD group were considerably lower (p < 0.05) and greatly increased after UA supplementation. According to the Shannon index, less diversity was present in the HFD group, and there was no improvement as a result of UA supplementation.
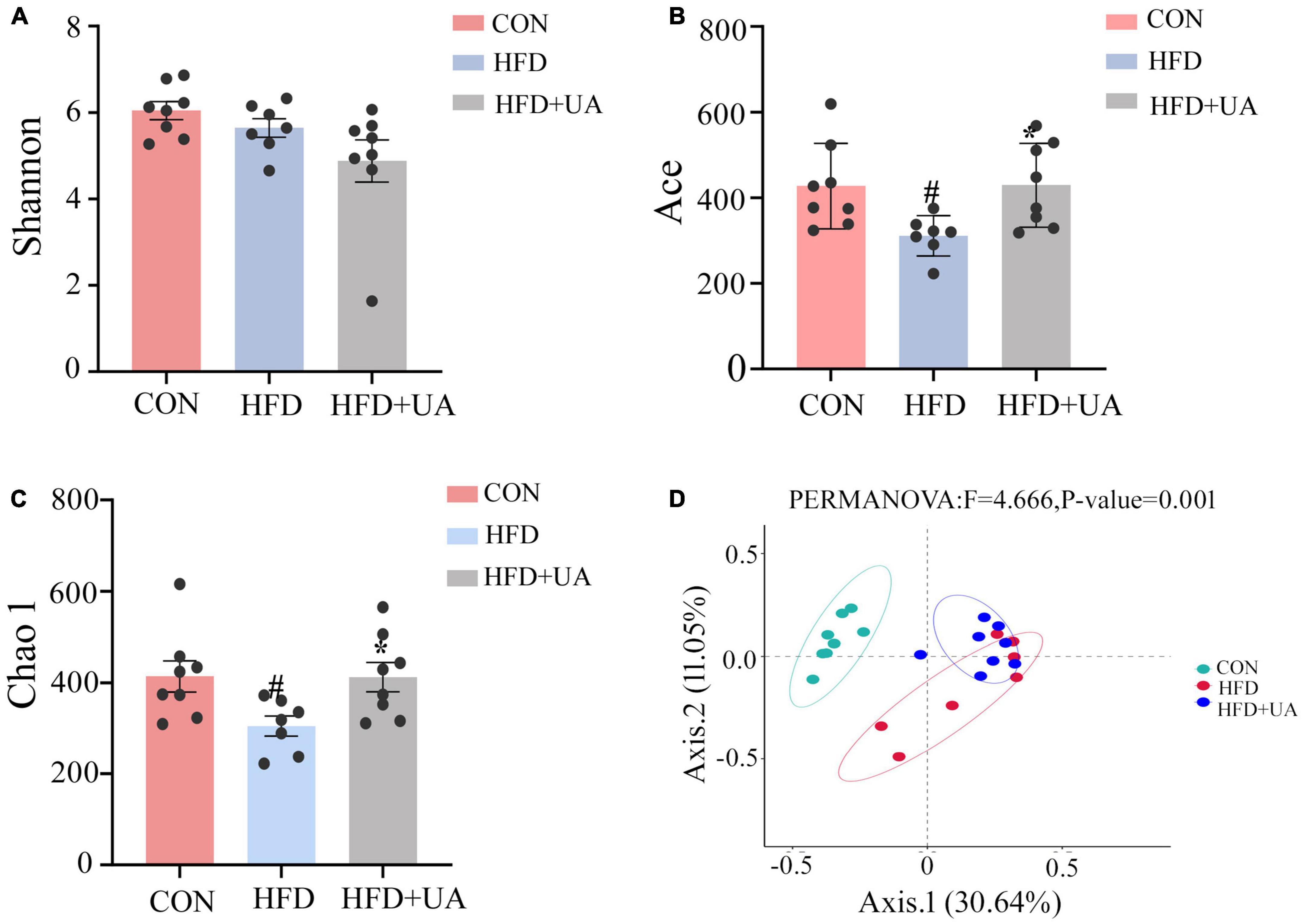
Figure 2. Analysis of alpha and beta diversity of intestinal flora in three groups of mice: (A) Shannon index; (B) Ace index; (C) Chao1 index; (D) Principal coordinates analysis (PCoA) (#p < 0.05 compared to control group, *p < 0.05 relative to the high-fat diet group).
Beta diversity analysis in mice was explained using the Principal Coordinates Analysis (PCoA). According to Figure 2D, there was a clear division in the microbiota community among the control and HFD groups, whereas in the HFD and UA treatment groups, the structure of the microbiota community was closer, suggesting that UA and HFD may influence the structure of the gut microbiota.
The relative bacterial abundance of the different groups was evaluated to identify specific taxa in the UA treatment group. According to Figure 3A, Firmicutes and Bacteroidetes were the dominant phyla in all groups. HFD treatment significantly changed the gut microbiota composition and structure compared with control group. For example, Firmicutes and Proteobacteria increased significantly, which was accompanied with decreased Bacteroidetes and Verrucomicrobia. Notably, the Firmicutes to Bacteroidetes (F/B) ratio was 0.93 in the control group, and increased rapidly to 2.82 after the HFD treatment, but the changes were reversed after UA supplementation, resulting in a lower F/B ratio of 2.22, which was accompanied by an increase in Verrucomicrobia.
At the genus level, the relative abundance of Akkermansia, Allobaculum, and Lactobacillus decreased and that of Oscillospira, Ruminococcus, Parabacteroides, and Erysipelotrichaceae-Clostridium increased in the HFD group (Figure 3B). However, UA supplementation increased the abundance of Lactobacillus, Bacteroides, and Akkermansia, which is inconsistent with the results from the HFD group.
By using LEfSe to look for variations in the abundance of bacterial taxa between three groups, the features of microbiota in the mice were examined. As presented in Figures 4A, B, 10 significant discriminative features were uncovered in the HFD group and eight were revealed in the UA treatment groups. The indicator microorganisms in the HFD group were identified as belonging to Clostridia, Clostridiales, Lachnospiraceae, Micrococcaceae, Aerococcaceae, Holdemania, Facklamia, Ruminococcus, Pseudoramibacter-Eubacterium, and Clostridium; likewise, Rhodobacterales, Methylobacteriaceae, Rhodobacteraceae, Methylobacterium, Coprobacillus, Clostridium, Serratia, and Clostridium were the dominant microbes in the UA treatment group (linear discriminant analysis score > 2, Figure 4A).
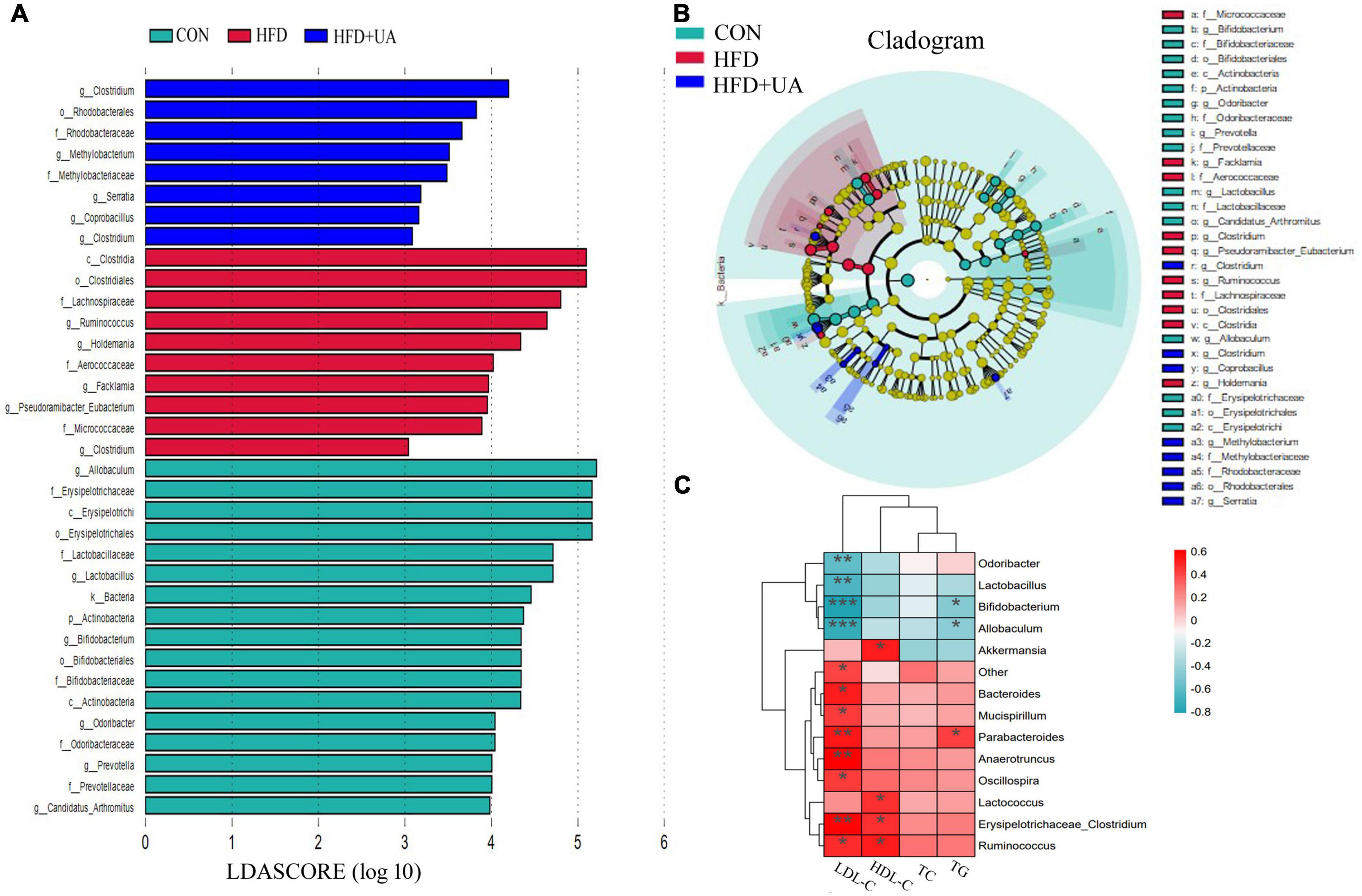
Figure 4. (A) Bacterial populations with a linear discriminant analysis score > 2 are displayed. (B) Taxonomy cladogram from linear discriminant analysis effect size analysis. (C) Heatmap depicting the correlation between major microbial communities and obese-related indicators. *p < 0.05; **p < 0.01; ***p < 0.001.
As shown in Figure 4C, Spearman’s correlation analysis revealed that obesity-related indicators were strongly correlated with the microbial communities. Parabacteroides, Anaerotruncus, and Erysipelotrichaceae_Clostridium were significantly positively associated with LDL-C, and Bacteroides, Mucispirillum, Oscillospira, and Ruminococcus were positively correlated with LDL-C. Meanwhile, Odoribacter, Lactobacillus, Bifidobacterium, and Allobaculum showed a significant negative correlation with LDL-C. HDL-C was positively correlated with Akkermansia, Lactococcus, Erysipelotrichaceae_Clostridium, and Ruminococcus; Parabacteroides also showed a positive correlation with TG, but Bifidobacterium and Allobaculum were negatively correlated with TG.
To examine the impact of UA on obesity from the viewpoint of host metabolism, the metabolic profiles of mice in the HFD and UA treatment groups were analyzed using non-targeted metabolomics. As shown in Figure 5A, OPLS-DA was conducted to analyze the effect of UA on classification, and the results showed a clear distribution for each group, as the R2Y and Q2 values of the OPLS-DA score analysis were 0.976 and 0.766, respectively, demonstrating a stable and precise prediction from the existing models. Moreover, the intercepts of R2 and Q2 were + 0.777 and −0.501, showing that the statistical models are valid and not overfit (Figure 5B).
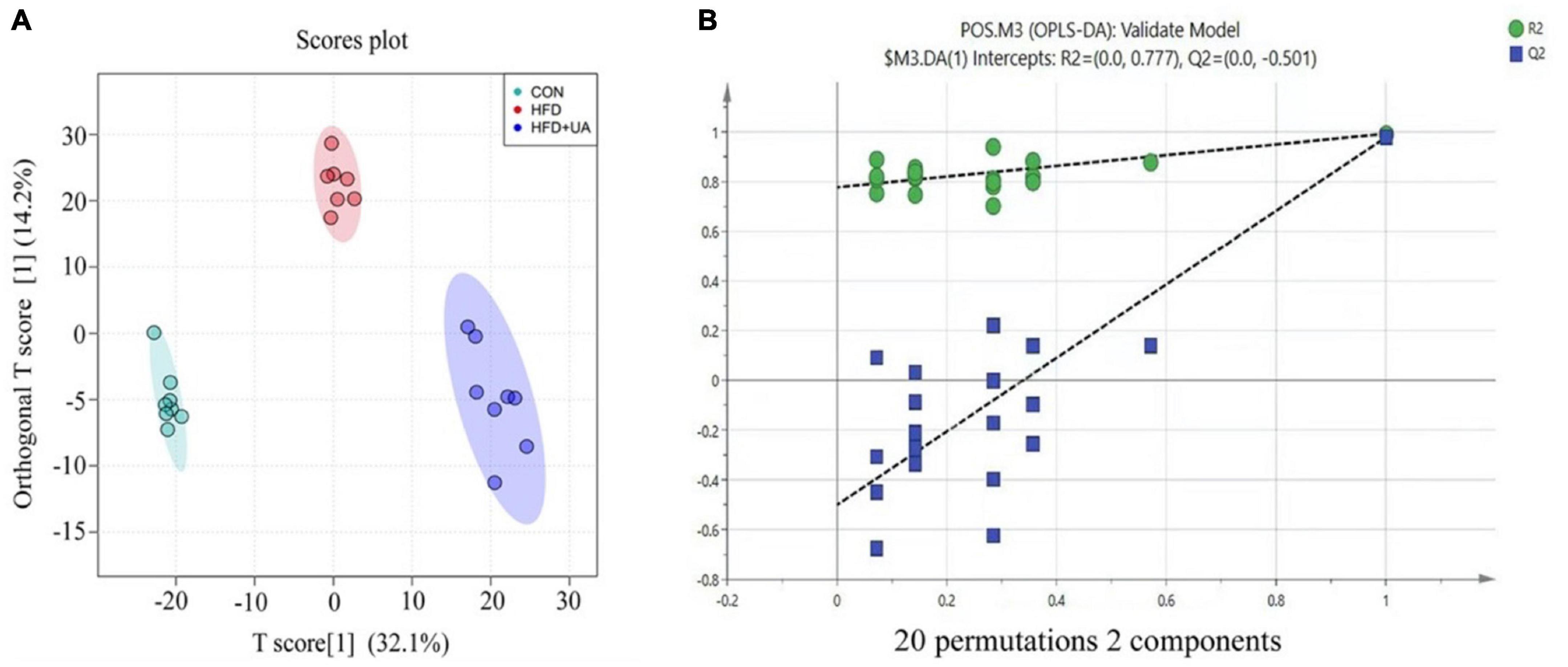
Figure 5. (A) Score plots of fecal samples derived from the LC-MS metabolite profiles. Samples from control (green circles), HFD (red circles) and HFD + UA (purple circles) groups. (B) Permutation tests were conducted with 20 random permutations in the OPLS-DA model.
Metabolite changes in the feces of mice are presented in Figure 6, showing that the HFD led to 490 metabolite changes (>1-fold, p < 0.05) relative to the control group, with 258 increasing and 232 decreasing (Figure 6A). Moreover, the intervention with UA led to 38 metabolite changes (>1-fold, p < 0.05) relative to the HFD group, with 18 increasing and 20 decreasing (Figure 6B). These findings indicted that the HFD and UA intervention caused the changes in metabolites in the feces of mice.
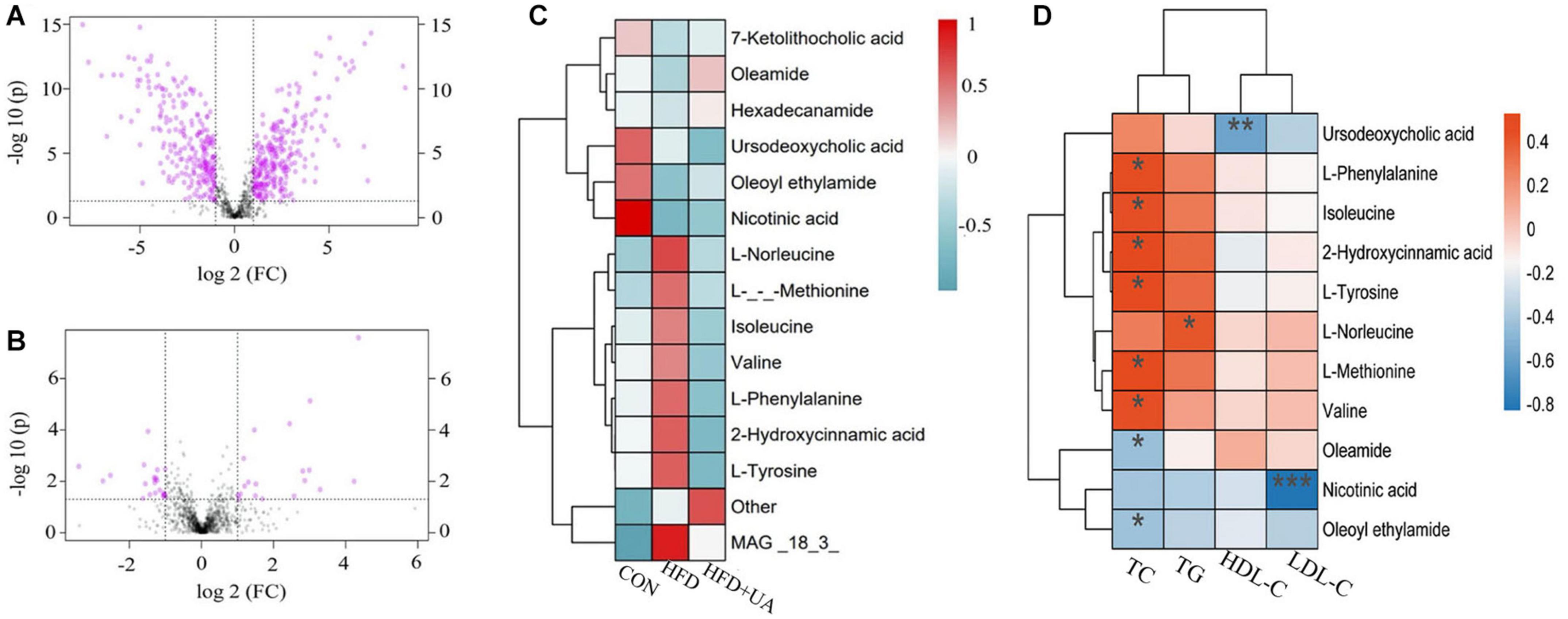
Figure 6. Metabolites in mice feces between groups. (A) Univariate statistical analysis of metabolites in fecal samples in the control and HFD groups (multiple > 1 and p < 0.05). (B) Univariate statistical analysis of metabolites in fecal samples in the HFD and UA treatment groups (multiple > 1 and p < 0.05). (C) Cluster analysis of the difference in metabolite expression among different groups using hierarchical clustering heatmap. (D) Heatmap depicting the correlation between differential metabolites and obese-related indicators. *p < 0.05; **p < 0.01; ***p < 0.001.
A hierarchical clustering heatmap was used to display the relative level of metabolites between three groups. We found that there were eight up-regulated metabolites, mainly including L-norleucine, L-methionine, isoleucine, valine, L-phenylalanine, 2-hydroxycinnamic acid, and L-tyrosine, in mice fed with the HFD relative to those in the control group, and the level decreased after UA intervention. Moreover, there were six down-regulated differential metabolites, 7-ketolithocholic acid, oleamide, hexadecanamide, ursodeoxycholic acid, oleoyl ethylamide, and nicotinic acid in mice fed with the HFD, and the level increased after UA intervention except for that of ursodeoxycholic acid (Figure 6C). Interestingly, correlation analysis of the differential metabolite expression and obese-related indicators using a hierarchical clustering heatmap revealed that the amino acids negatively correlated with UA levels were all positively correlated with TC except for L-norleucine, which was positively correlated with TG. Meanwhile, oleamide and oleoyl ethylamide was negatively correlated with TC, and nicotinic acid showed a significant negative correlation with LDL-C. Notably, these three amino acids also have been showed a positive correlation with UA (Figure 6D). These findings indicated that UA may influence the expression levels of obese-related indicators by regulating amino acid metabolism.
To further explore the changes in metabolites after UA intervention, the KEGG database was used for pathway enrichment analysis to identify the relevant pathways and functions of differential metabolites between the HFD and UA treatment groups. According to Figure 7A, the KEGG analysis showed 39 annotated pathways, and the most representative pathways were steroid hormone biosynthesis, phenylalanine, tyrosine and tryptophan biosynthesis, ubiquinone and other terpenoid-quinone biosynthesis, and vitamin B6 metabolism.
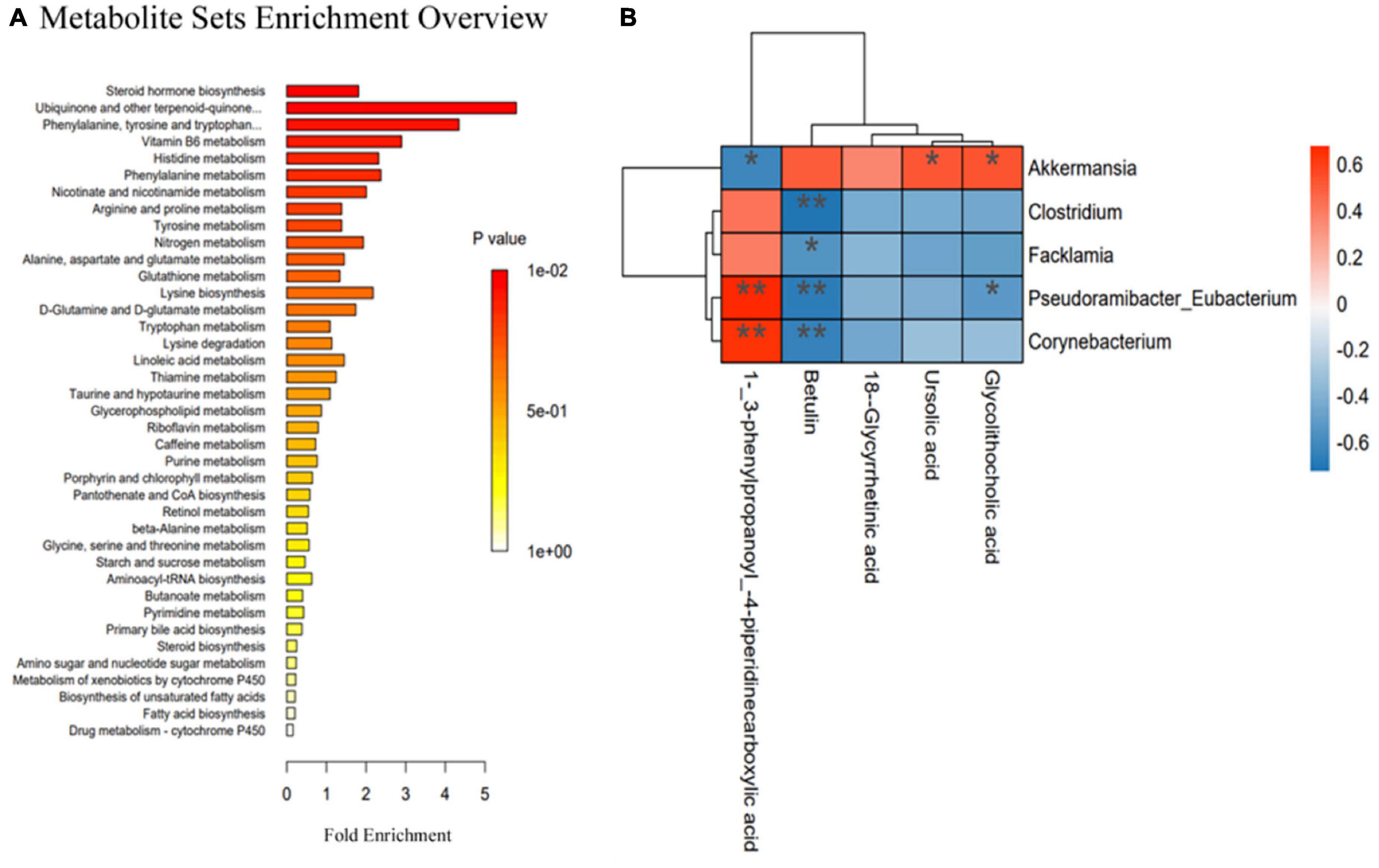
Figure 7. (A) Relevant pathways and functions of differential metabolites in Kyoto Encyclopedia of Genes and Genomes analysis; (B) Spearman’s correlation heatmap reveals the relationship between the gut microbiota and metabolites. *p < 0.05; **p < 0.01.
Spearman’s correlation analysis was used to reveal the functional correlation of the key microbial phylotypes to the altered fecal metabolites. As shown in Figure 7B, UA and glycolithocholic acid both had a positive correlation with Akkermansia, but glycolithocholic acid had a negative correlation with Pseudoramibacter-Eubacterium; 1-(3-phenylpropanoy)-4-piperidinecarboxylic acid was positively correlated with Pseudoramibacter-Eubacterium and Corynebacterium but was negatively associated with Akkermansia; betulin had a negative correlation with Clostridium, Facklamia, Pseudoramibacter-Eubacterium, and Corynebacterium.
Obesity is regarded as a worldwide public health problem, significantly affecting the health of people in all age groups, and the major factor causing obesity is a high-fat diet (Hariri and Thibault, 2010). UA has multiple biological functions and plays a positive regulatory role in metabolic diseases, especially diabetes and obesity (González-Garibay et al., 2020), but few studies have focused on its impacts on the gut microbiota and metabolites in the disease process. In the present study, we established an obesity model by feeding mice a HFD with or without UA supplement for 8 weeks to explore the effects of UA on the gut microbiota and metabolites in the obesity process.
We found that several serum biochemical indicators closely related to obesity increased in the HFD group as documented by previous research (Kim et al., 2018; Guo et al., 2020), and the level was rapidly reduced by UA intervention. A previous study demonstrated that UA could decreased the lipid accumulation in the adipose tissues and liver. Meanwhile, the TG and LDL concentrations also decreased while the HDL concentration was increased significantly in mice administrated by UA, which is consistent with our results (Jia et al., 2015). Additionally, The similar results were also reported in other studies that UA treatment significantly decreased the concentration of TG (Li et al., 2014; Chu et al., 2015). Evidence from an animal study indicated that the elevated levels of TC, TG, and LDL-C in serum could improve atherosclerosis and associated cardiovascular disease (Sathyapalan et al., 2018). Moreover, recent research showed that TC, TG, LDL-C and HDL-C could be used as a specific lipid biomarkers to assess the risk of cardiovascular disease among different population groups (Georgoulis et al., 2022). Based on these results, we proposed that UA could regulate lipid metabolism and reduce the risk of atherosclerosis as well as related cardiovascular disease induced by obesity.
To explore the effect of UA on the composition of the intestinal flora of obese mice, we analyzed the gut microbiota structure of mice in the HFD and UA treatment groups. It was found that UA intervention shifted the Firmicutes to Bacteroidetes (F/B) ratio in fecal samples of the HFD-induced mice at the phylum level. Recent research demonstrated that Firmicutes has more efficient sugar metabolism relative to Bacteroidetes, which promotes energy absorption and weight gain that contribute to obesity (Bai et al., 2019; Lu et al., 2021; Li F. et al., 2022). Thus, a lower F/B ratio may reduce obesity through energy metabolism, and a similar effect of the F/B ratio on obesity in mice has been further demonstrated in a study that focused on the function of Rosa roxburghii Tratt fruit vinegar in the prevention of obesity (Li J. et al., 2022).
The crucial roles of the gut microbiota in obtaining energy from the diet and influencing the pathophysiology of obesity by modulating lipid or glucose metabolism have been demonstrated (Wang and Jia, 2016). In particular, Akkermansia muciniphila was shown to participate in the regulation of obesity and could serve as an indicator to evaluate the body’s metabolic status, especially in terms of glucose or lipid metabolism homeostasis (Dao et al., 2016; Wu et al., 2020). Moreover, A. muciniphila has a beneficial impact on liver disorders, systemic inflammation, and gut disruption caused by obesity (Xu et al., 2020). Our research found that UA had a positive correlation with Akkermansia, and supplementation with UA could significantly increase the abundance of Akkermansia relative to that of mice in the HFD group. These findings suggested that the function of UA in the improvement of obesity, at least in part, depends on increasing the abundance of Akkermansia, which is beneficial for the steady state of the host’s basal metabolism. In addition, recent research has demonstrated that Lactobacillus, as a beneficial bacteria in gut, could alleviate the process of HFD-induced obesity by regulating the intestinal flora (Cai et al., 2020; Hussain et al., 2020; Liu et al., 2022). Our results showed that the abundance of Lactobacillus increased after UA intervention, so we further speculated that the anti-obesity action of UA in mice was realized though increasing the commensal gut beneficial microbes and improving the gut barrier integrity.
Obesity can lead to various degrees of metabolic imbalance, particularly in terms of lipid and carbohydrate metabolism, but amino acid metabolism is also affected. Altered amino acid profiles in the obesity condition are often accompanied by concomitant changes in the resistance and secretion of insulin (Simonson et al., 2020). In particular, the branched-chain amino acids (BCAAs), including leucine, isoleucine, and valine, play vital roles in the pathogenesis of metabolic disorders observed in obesity, and increased BCAA circulating levels are related to insulin resistance (Ridaura et al., 2013; Lynch and Adams, 2014; Pedersen et al., 2016). Mice fed a diet with a lower level of BCAA could quickly lose weight and fat mass before achieving a normal weight, and glucoregulatory control is improved (Cummings et al., 2018). Likewise, ginsenoside Rb1 has been demonstrated to ameliorate glycemic disorder in obese mice partly by decreasing the level of BCAAs, especially isoleucine and leucine (Yang et al., 2021). This provides additional support for our results. Our results showed that UA supplementation could decrease the level of amino acids like BCAAs, tyrosine and others relative to the HFD group, indicating a positive effect of UA on obesity. Furthermore, previous reports showed that BCAAs are partly produced and metabolized by the intestinal microbiome, and obese patients exhibit an imbalanced intestinal microbiome with increased BCAA synthesis and decreased BCAA catabolism (Gill et al., 2006; Liu et al., 2017). Thus, we speculated that the beneficial influence of UA on obesity partly depends on amino acid metabolism and the intestinal microbiome and their interaction.
Notably, besides the BCAAs, obesity risk was also associated with aromatic amino acids, especially tryptophan, which influences host metabolism and has become another potential obesity treatment (Aron-Wisnewsky et al., 2021). As described recently, moderate tryptophan restriction can cause loss of appetite and thermogenesis, whereas severe tryptophan restriction causes hypophagia and weight loss (Zapata et al., 2018). Together with tryptophan itself, metabolites generated from it, including indole, serotonin, and kynurenine, can also contribute to obesity (Konopelski et al., 2019; Pan et al., 2019; Simonson et al., 2020; Aron-Wisnewsky et al., 2021). Additionally, increasing evidence has shown that phenylalanine and tyrosine were also related to the increased incidence of obesity (Libert et al., 2018; Bai et al., 2020; Cheng et al., 2021). Our metabolic analysis showed that the different metabolites could participate in phenylalanine, tyrosine, and tryptophan biosynthesis, further implying that the positive effects of UA on obesity partly depend on its effect on amino acid metabolism.
In summary, the effects of UA on the microbiome structure and fecal metabolome of obese mice induced by a HFD were investigated. These results indicated that UA intervention not only changed the microbiota composition but also altered host metabolism, in particular that of amino acid metabolism. Our findings could further our understanding of the anti-obesity effects of UA and its potential as a treatment for obesity.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession numbers can be found in the NCBI database under accession number: PRJNA960690 (https://www.ncbi.nlm.nih.gov/sra/PRJNA960690), Metabolomics raw data has been uploaded in Metabolights database under accession number: MTBLS7741 (https://www.ebi.ac.uk/metabolights/editor/study/MTBLS7741).
The animal study was reviewed and approved by the Ethics Committee of Baotou Medical College.
CT: investigation, writing–original draft, visualization, and formal analysis. JL: methodology, validation, investigation, and writing–review and editing. LG: conceptualization, resources, supervision, and project administration. YB: investigation, writing–review and editing, and funding acquisition. LS: investigation and supervision. KL: data curation and visualization. MS: supervision. All authors contributed to the article and approved the submitted version.
This study was supported by the Medical and Health Science and Technology Plan Project of Inner Mongolia Autonomous Region Health Commission (202201368); the Natural Science Foundation of Inner Mongolia (2021LHMS08017); the Young Scientific and Technological Talents Inner Mongolia Universities (NJYT22119); the Baotou Medical College Doctoral Research Start-up-fund Project (BSJJ201801); Heilongjiang Provincial Colleges and Universities Basic Scientific Research Projects, China (2022-KYYWF-0649); and Jiamusi University Young Innovative Talent Training Support Program Project, China (JMSUQP2022014).
We thank each author for their sincere effort in this research and the North Medicine and Functional Food Characteristic Subject Project in Heilongjiang Providence for the help provided.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1183598/full#supplementary-material
HFD, high-fat diet; TC, total cholesterol; TG, total triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LEfSe, linear discriminant analysis effect size; OPLS-DA, orthogonal partial least-squares discriminant analysis; UA, ursolic acid; BCAA, branched-chain amino acids; KEGG, Kyoto Encyclopedia of Genes and Genomes; PCoA, principal coordinates analysis.
Aron-Wisnewsky, J., Warmbrunn, M. V., Nieuwdorp, M., and Clément, K. (2021). Metabolism and metabolic disorders and the microbiome: The intestinal microbiota associated with obesity, lipid metabolism, and metabolic health-pathophysiology and therapeutic strategies. Gastroenterology 160, 573–599. doi: 10.1053/j.gastro.2020.10.057
Bai, L., Gao, M., Cheng, X., Kang, G., Cao, X., and Huang, H. (2020). Engineered butyrate-producing bacteria prevents high fat diet-induced obesity in mice. Microb. Cell Fact. 19:94. doi: 10.1186/s12934-020-01350-z
Bai, Y. F., Wang, S. W., Wang, X. X., Weng, Y. Y., Fan, X. Y., Sheng, H., et al. (2019). The flavonoid-rich Quzhou Fructus Aurantii extract modulates gut microbiota and prevents obesity in high-fat diet-fed mice. Nutr. Diabetes 9:30. doi: 10.1038/s41387-019-0097-6
Cai, H., Wen, Z., Li, X., Meng, K., and Yang, P. (2020). Lactobacillus plantarum FRT10 alleviated high-fat diet-induced obesity in mice through regulating the PPARα signal pathway and gut microbiota. Appl. Microbiol. Biotechnol. 104, 5959–5972. doi: 10.1007/s00253-020-10620-0
Cheng, D., Zhao, X., Yang, S., Cui, H., and Wang, G. (2021). Metabolomic signature between metabolically healthy overweight/obese and metabolically unhealthy overweight/obese: A systematic review. Diabetes Metab. Syndr. Obes. 14, 991–1010. doi: 10.2147/DMSO.S294894
Chu, X., He, X., Shi, Z., Li, C., Guo, F., Li, S., et al. (2015). Ursolic acid increases energy expenditure through enhancing free fatty acid uptake and β-oxidation via an UCP3/AMPK-dependent pathway in skeletal muscle. Mol. Nutr. Food Res. 59, 1491–1503. doi: 10.1002/mnfr.201400670
Clément, K., Biebermann, H., Farooqi, I. S., Van der Ploeg, L., Wolters, B., Poitou, C., et al. (2018). MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 24, 551–555. doi: 10.1038/s41591-018-0015-9
Cummings, N. E., Williams, E. M., Kasza, I., Konon, E. N., Schaid, M. D., Schmidt, B. A., et al. (2018). Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. (Lond.) 596, 623–645. doi: 10.1113/JP275075
Dao, M. C., Everard, A., Aron-Wisnewsky, J., Sokolovska, N., Prifti, E., Verger, E. O., et al. (2016). Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 65, 426–436. doi: 10.1136/gutjnl-2014-308778
de Freitas Junior, L. M., and de Almeida, E. B. Jr. (2017). Medicinal plants for the treatment of obesity: Ethnopharmacological approach and chemical and biological studies. Am. J. Transl. Res. 9, 2050–2064.
Diarra, M., El Ouahabi, H., Bouxid, H., Boujraf, S., Khabbal, Y., and Ajdi, F. (2016). Medicinal plants in type 2 diabetes: Therapeutic and economical aspects. Int. J. Prev. Med. 7:56. doi: 10.4103/2008-7802.178370
Georgoulis, M., Chrysohoou, C., Georgousopoulou, E., Damigou, E., Skoumas, I., Pitsavos, C., et al. (2022). Long-term prognostic value of LDL-C, HDL-C, lp(a) and TG levels on cardiovascular disease incidence, by body weight status, dietary habits and lipid-lowering treatment: The ATTICA epidemiological cohort study (2002-2012). Lipids Health Dis. 21:141. doi: 10.1186/s12944-022-01747-2
Gill, S. R., Pop, M., Deboy, R. T., Eckburg, P. B., Turnbaugh, P. J., Samuel, B. S., et al. (2006). Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359. doi: 10.1126/science.1124234
González-Garibay, A. S., López-Vázquez, A., García-Bañuelos, J., Sánchez-Enríquez, S., Sandoval-Rodríguez, A. S., Bueno-Topete, M. R., et al. (2020). Effect of ursolic acid on insulin resistance and Hyperinsulinemia in rats with diet-induced obesity: Role of adipokines expression. J. Med. Food 23, 297–304. doi: 10.1089/jmf.2019.0154
Guo, W. L., Guo, J. B., Liu, B. Y., Lu, J. Q., Chen, M., Liu, B., et al. (2020). Ganoderic acid A from Ganoderma lucidum ameliorates lipid metabolism and alters gut microbiota composition in hyperlipidemic mice fed a high-fat diet. Food Funct. 11, 6818–6833. doi: 10.1039/d0fo00436g
Hariri, N., and Thibault, L. (2010). High-fat diet-induced obesity in animal models. Nutr. Res. Rev. 23, 270–299. doi: 10.1017/S0954422410000168
Hussain, A., Kwon, M. H., Kim, H. K., Lee, H. S., Cho, J. S., and Lee, Y. I. (2020). Anti-obesity effect of Lactobacillus plantarum LB818 is associated with regulation of gut microbiota in high-fat diet-fed obese mice. J. Med. Food 23, 750–759. doi: 10.1089/jmf.2019.4627
Jia, Y., Kim, S., Kim, J., Kim, B., Wu, C., Lee, J. H., et al. (2015). Ursolic acid improves lipid and glucose metabolism in high-fat-fed C57BL/6J mice by activating peroxisome proliferator-activated receptor alpha and hepatic autophagy. Mol. Nutr. Food Res. 59, 344–354. doi: 10.1002/mnfr.201400399
Khera, R., Murad, M. H., Chandar, A. K., Dulai, P. S., Wang, Z., Prokop, L. J., et al. (2016). Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and metaanalysis. JAMA 315, 2424–2434. doi: 10.1001/jama.2016.7602
Kim, H. M., Kim, Y., Lee, E. S., Huh, J. H., and Chung, C. H. (2018). Caffeic acid ameliorates hepatic steatosis and reduces ER stress in high fat diet-induced obese mice by regulating autophagy. Nutrition 55–56, 63–70. doi: 10.1016/j.nut.2018.03.010
Konopelski, P., Konop, M., Gawrys-Kopczynska, M., Podsadni, P., Szczepanska, A., and Ufnal, M. (2019). Indole-3-propionic acid, a tryptophan-derived bacterial metabolite, reduces weight gain in rats. Nutrients 11:591. doi: 10.3390/nu11030591
Li, S., Liao, X., Meng, F., Wang, Y., Sun, Z., Guo, F., et al. (2014). Therapeutic role of ursolic acid on ameliorating hepatic steatosis and improving metabolic disorders in high-fat diet-induced non-alcoholic fatty liver disease rats. PLoS One 29:e86724. doi: 10.1371/journal.pone.0086724
Li, F., Yang, S., Zhang, L., Qiao, L., Wang, L., He, S., et al. (2022). Comparative metagenomics analysis reveals how the diet shapes the gut microbiota in several small mammals. Ecol. Evol. 12:e8470. doi: 10.1002/ece3.8470
Li, H., Fang, Q., Nie, Q., Hu, J., Yang, C., Huang, T., et al. (2020). Hypoglycemic and hypolipidemic mechanism of tea polysaccharides on type 2 diabetic rats via gut microbiota and metabolism alteration. J. Agric. Food Chem. 68, 10015–10028. doi: 10.1021/acs.jafc.0c01968
Li, J., Zhang, J., Zhang, Y., Shi, Y., Feng, D., Zuo, Y., et al. (2022). Effect and correlation of Rosa roxburghii tratt fruit vinegar on obesity, dyslipidemia and intestinal microbiota disorder in high-fat diet mice. Foods 11:4108. doi: 10.3390/foods11244108
Libert, D. M., Nowacki, A. S., and Natowicz, M. R. (2018). Metabolomic analysis of obesity, metabolic syndrome, and type 2 diabetes: Amino acid and acylcarnitine levels change along a spectrum of metabolic wellness. PeerJ 6:e5410. doi: 10.7717/peerj.5410
Liu, R., Hong, J., Xu, X., Feng, Q., Zhang, D., Gu, Y., et al. (2017). Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868. doi: 10.1038/nm.4358
Liu, Z., Zhou, X., Wang, W., Gu, L., Hu, C., Sun, H., et al. (2022). Lactobacillus paracasei 24 attenuates lipid accumulation in high-fat diet-induced obese mice by regulating the gut microbiota. J. Agric. Food Chem. 70, 4631–4643. doi: 10.1021/acs.jafc.1c07884
Lu, X., Jing, Y., Li, Y., Zhang, N., Zhang, W., and Cao, Y. (2021). The differential modulatory effects of Eurotium cristatum on the gut microbiota of obese dogs and mice are associated with improvements in metabolic disturbances. Food Funct. 12, 12812–12825. doi: 10.1039/d1fo02886c
Lynch, C. J., and Adams, S. H. (2014). Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736. doi: 10.1038/nrendo.2014.171
Medanić, D., and Pucarin-Cvetković, J. (2012). Pretilost–javnozdravstveni problem i izazov [Obesity–a public health problem and challenge]. Acta Med. Croatica 66, 347–355. Croatian
Ojulari, O. V., Lee, S. G., and Nam, J. O. (2019). Beneficial effects of natural bioactive compounds from Hibiscus sabdariffa L. on obesity. Molecules 24:210. doi: 10.3390/molecules24010210
Pan, H., Fu, C., Huang, L., Jiang, Y., Deng, X., Guo, J., et al. (2018). Anti-obesity effect of chitosan oligosaccharide capsules (COSCs) in obese rats by ameliorating leptin resistance and adipogenesis. Mar. Drugs 16:198. doi: 10.3390/md16060198
Pan, Q., Liu, Q., Wan, R., Kalavagunta, P. K., Liu, L., Lv, W., et al. (2019). Selective inhibition of intestinal 5-HT improves neurobehavioral abnormalities caused by high-fat diet mice. Metab. Brain Dis. 34, 747–761. doi: 10.1007/s11011-019-0392-x
Pedersen, H. K., Gudmundsdottir, V., Nielsen, H. B., Hyotylainen, T., Nielsen, T., Jensen, B. A., et al. (2016). Human gut microbes impact host serum metabolome and insulin sensitivity. Nature 535, 376–381. doi: 10.1038/nature18646
Qu, W., Nie, C., Zhao, J., Ou, X., Zhang, Y., Yang, S., et al. (2018). Microbiome-metabolomics analysis of the impacts of long-term dietary advanced-glycation-end-product consumption on C57BL/6 mouse fecal microbiota and metabolites. J. Agric. Food Chem. 66, 8864–8875. doi: 10.1021/acs.jafc.8b01466
Ridaura, V. K., Faith, J. J., Rey, F. E., Cheng, J., Duncan, A. E., Kau, A. L., et al. (2013). Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science 341:1241214. doi: 10.1126/science.1241214
Sathyapalan, T., Aye, M., Rigby, A. S., Thatcher, N. J., Dargham, S. R., Kilpatrick, E. S., et al. (2018). Soy isoflavones improve cardiovascular disease risk markers in women during the early menopause. Nutr. Metab. Cardiovasc. Dis. 28, 691–697. doi: 10.1016/j.numecd.2018.03.007
Sehrawat, A., Roy, R., Pore, S. K., Hahm, E. R., Samanta, S. K., Singh, K. B., et al. (2017). Mitochondrial dysfunction in cancer chemoprevention by phytochemicals from dietary and medicinal plants. Semin. Cancer Biol. 47, 147–153. doi: 10.1016/j.semcancer.2016.11.009
Seo, D. Y., Lee, S. R., Heo, J. W., No, M. H., Rhee, B. D., Ko, K. S., et al. (2018). Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 22, 235–248. doi: 10.4196/kjpp.2018.22.3.235
Simonson, M., Boirie, Y., and Guillet, C. (2020). Protein, amino acids and obesity treatment. Rev. Endocr. Metab. Disord. 21, 341–353. doi: 10.1007/s11154-020-09574-5
Sun, A., Hu, X., Chen, H., Ma, Y., Yan, X., Peng, D., et al. (2021). Ursolic acid induces white adipose tissue beiging in high-fat-diet obese male mice. Food Funct. 12, 6490–6501. doi: 10.1039/d1fo00924a
Sun, S., Sun, L., Wang, K., Qiao, S., Zhao, X., Hu, X., et al. (2021). The gut commensal fungus, Candida parapsilosis, promotes high fat-diet induced obesity in mice. Commun. Biol. 4:1220. doi: 10.1038/s42003-021-02753-3
Sun, X., Wen, J., Guan, B., Li, J., Luo, J., Li, J., et al. (2022). Folic acid and zinc improve hyperuricemia by altering the gut microbiota of rats with high-purine diet-induced hyperuricemia. Front. Microbiol. 29:907952. doi: 10.3389/fmicb.2022.907952
Tang, S., Fang, C., Liu, Y., Tang, L., and Xu, Y. (2022). Anti-obesity and anti-diabetic Effect of ursolic acid against streptozotocin/high fat induced obese in diabetic rats. J. Oleo Sci. 71, 289–300. doi: 10.5650/jos.ess21258
Wang, J., and Jia, H. (2016). Metagenome-wide association studies: Fine-mining the microbiome. Nat. Rev. Microbiol. 14, 508–522. doi: 10.1038/nrmicro.2016.83
Wang, K., Liao, M., Zhou, N., Bao, L., Ma, K., Zheng, Z., et al. (2019). Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep. 26, 222–235.e5. doi: 10.1016/j.celrep.2018.12.028
Wu, F., Guo, X., Zhang, M., Ou, Z., Wu, D., Deng, L., et al. (2020). An Akkermansia muciniphila subtype alleviates high-fat diet-induced metabolic disorders and inhibits the neurodegenerative process in mice. Anaerobe 61:102138. doi: 10.1016/j.anaerobe.2019.102138
Xu, Y., Wang, N., Tan, H. Y., Li, S., Zhang, C., and Feng, Y. (2020). Function of Akkermansia muciniphila in obesity: Interactions with lipid metabolism, immune response and gut systems. Front. Microbiol. 11:219. doi: 10.3389/fmicb.2020.00219
Yang, X., Dong, B., An, L., Zhang, Q., Chen, Y., Wang, H., et al. (2021). Ginsenoside Rb1 ameliorates glycemic disorder in mice with high fat diet-induced obesity via regulating gut microbiota and amino acid metabolism. Front. Pharmacol. 12:756491. doi: 10.3389/fphar.2021.756491
Yu, Z., Yu, X. F., Kerem, G., and Ren, P. G. (2022). Perturbation on gut microbiota impedes the onset of obesity in high fat diet-induced mice. Front. Endocrinol. (Lausanne) 13:795371. doi: 10.3389/fendo.2022.795371
Keywords: ursolic acid, obesity, gut microbiota, metabolism, high-fat diet, metabolomics
Citation: Tian C, Li J, Bao Y, Gao L, Song L, Li K and Sun M (2023) Ursolic acid ameliorates obesity of mice fed with high-fat diet via alteration of gut microbiota and amino acid metabolism. Front. Microbiol. 14:1183598. doi: 10.3389/fmicb.2023.1183598
Received: 14 March 2023; Accepted: 12 June 2023;
Published: 06 July 2023.
Edited by:
Xiaodong Xia, Dalian Polytechnic University, ChinaReviewed by:
Sidharth Prasad Mishra, University of South Florida, United StatesCopyright © 2023 Tian, Li, Bao, Gao, Song, Li and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Bao, YnlfMTk3N0AxNjMuY29t; Long Gao, MTU4MDQ3MTczNjJAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.