- 1College of Food and Bioengineering, Henan University of Science and Technology, Luoyang, Henan, China
- 2School of Food Science and Technology, Dalian Polytechnic University, Dalian, Liaoning, China
- 3Heilongjiang Feihe Dairy Co. Ltd., Beijing, China
Staphylococcus aureus is a common foodborne pathogen which can form biofilms to help them resist to antimicrobials. It brings great harm to human health. Punicalagin has good antimicrobial activities against S. aureus, but its effect on biofilm formation has not been clearly illustrated. The aim of this study was to explore the antibiofilm effects of punicalagin against S. aureus. Results showed that punicalagin did not significantly interfere with the growth of S. aureus at the concentrations of 1/64 MIC to 1/16 MIC. The biomass and metabolic activity of biofilms were significantly reduced when exposed to sub-inhibitory concentrations of punicalagin. The number of viable cells in the biofilms was also decreased after punicalagin treatment. Scanning electron microscopy and confocal laser scanning microscopy images confirmed that punicalagin damaged the structure of biofilms. The antibiofilm mechanism was partly due to the modification of the cell surface which led to the reduction of cell surface hydrophobicity. These findings suggest that punicalagin has the potential to be developed as an alternative to control S. aureus biofilms.
Introduction
Most bacteria adhere to a surface in the form of biofilm in nature. In comparison with planktonic bacteria, biofilm bacteria have stronger resistance to disinfectants, ultraviolet rays, heavy metals, antibiotics, acids, alkalis and salts owing to genetic and metabolic adaptations (Phuong et al., 2017). Biofilm also brings great potential safety hazards to food industry. Some pathogenic bacteria can colonize, grow and form biofilm on food surface, food processing machinery surface and pipeline, food processing environment and packaging materials, which often lead to pipeline corrosion or product pollution, thus bringing huge economic losses. Staphylococcus aureus is a common pathogen which can form biofilm and bring serious safety risks to medical and food fields. There is an urgent need to search for safe and effective antimicrobials for the treatment of the bacteria.
In recent years, many researchers have turned their attention to natural products, especially plant-derived active substances (Bouarab Chibane et al., 2019). Punicalagin, the main component of pomegranate peel polyphenols, is a high molecular weight (1084.72) water-soluble compound (Aqil et al., 2012). It is known for various biological properties, including antioxidant, anti-inflammatory, anti-cancer and immunomodulatory activities (Benchagra et al., 2021; Berdowska et al., 2021; Čolić et al., 2022; Xu et al., 2022). It has also been reported to possess antimicrobial activities against several pathogenic bacteria such as S. aureus, Salmonella, and Vibrio parahaemolyticus (Li et al., 2014; Mun et al., 2016; Liu et al., 2022). Our previous study has shown that punicalagin exhibited a good bacteriostatic effect on S. aureus with the minimum inhibitory concentration (MIC) of 0.25 mg/ml (Xu et al., 2017). However, the effect of punicalagin on the biofilm formation of S. aureus and its action mechanism is unclear. Therefore, this study was aimed to investigate the antibiofilm effects of punicalagin against S. aureus. It provides a theoretical and experimental basis for the development and utilization of punicalagin and the control of S. aureus biofilm.
Materials and methods
Bacterial strains and cultural conditions
Staphylococcus aureus ATCC 29213, obtained from the American Type Culture Collection, was stored in tryptone soybean broth (TSB; Land Bridge Technology Co. Ltd., Beijing, China) containing 25% glycerol at −80°C. It was taken out from the refrigerator and inoculated on tryptone soybean agar (TSA; Land Bridge Technology Co. Ltd., Beijing, China) plate before each experiment. Single colony of the strain was picked out, inoculated into TSB, and cultured at 37°C for about 12 h. The absorbance of the bacterial suspension was measured by a spectrophotometer (Smart Spec™ plus, Bio-Rad Laboratories, Hercules, CA) to obtain the optical density about 0.5 at 600 nm.
Growth curves
The growth curves of S. aureus were detected when treated with or without punicalagin using the Bioscreen C automated microbiology growth curve analysis system (Labsystems, Helsinki, Finland) as described by Zheng et al. (2020). Punicalagin (≥98%, CAS 65995–63-3, Must Bio-Technology Co. Ltd., Chengdu, China) was added into the wells to obtain the final concentrations of 0 (control), 1/64, 1/32, 1/16 and 1/8 MIC, respectively. The above bacterial suspension was inoculated at a ratio of 1% containing about 106 colony-forming units (CFU)/mL. Sterile TSB containing corresponding concentrations of punicalagin was taken as negative control. The microplate was incubated statically at 37°C and the absorbance at 600 nm wavelength was measured in 1 h intervals for 24 h.
Biofilm formation assay
The effect of punicalagin on biofilm biomass was conducted by crystal violet staining method referred to Fan et al. (2022b). Briefly, S. aureus suspensions exposure to punicalagin at 0 (control), 1/64, 1/32 and 1/16 MIC were incubated in a 96-well microplate at 37°C for 24 h. After removing the planktonic cells, wells were washed with sterile phosphate buffered saline (PBS) for three times and fixed with methanol for 15 min. Then crystal violet solution (1%) was added to stain biofilms and the wells were rinsed thrice with distilled water, followed by the addition of acetic acid (33%, vol/vol). After shaking at low speed for 5 min, the absorbance at 570 nm was measured using a microplate reader (model 680; Bio-Rad). The relative biofilm formation was calculated by the ODtreatment normalized with ODcontrol.
Biofilm metabolic activity assay
The effect of punicalagin on biofilm metabolism was examined by the method of Jadhav et al. (2013). The prepared bacterial suspensions containing different concentrations of punicalagin (0, 1/64, 1/32 and 1/16 MIC) were separately added to a 96-well plate and incubated at 37°C for 24 h. Then the bacterial suspensions were removed. The plate was gently rinsed three times with PBS. A total of 250 μl of 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyltetrazolium bromide (MTT; Beijing Solarbio Science and Technology Co., Ltd., Beijing, China) solution at the concentration of 0.5 mg/ml was added to each well, and incubated at 37°C for 3 h. The insoluble purple formazan was further dissolved in dimethyl sulfoxide (DMSO) and the absorbance was measured at a wavelength of 570 nm using a microplate spectrophotometer (model 680; Bio-Rad).
Counting of viable bacteria in biofilms
The number of viable bacteria in biofilms was counted as previously reported with some modifications (Amalaradjou and Venkitanarayanan, 2011). Briefly, the bacterial suspensions containing different concentrations of punicalagin (0, 1/64, 1/32 and 1/16 MIC) were inoculated into a 24-well polystyrene plate at 2 ml per well and cultured at 37°C for 24 h. Then the wells were carefully washed three times with sterile PBS, followed by the addition of another 2 ml of PBS. The biofilms were wiped off and mixed thoroughly. After a series of 10-fold dilution, the suspensions were spread on TSA, and cultured at 37°C overnight before the colonies were counted.
Field-emission scanning electron microscopy observation
FESEM was performed as described by Li et al. (2021) with some modifications. The bacterial suspensions containing punicalagin at different concentrations (0, 1/64, 1/32 and 1/16 MIC) were added to a 12-well plate pre-placed with sterile glass sheets. After incubated at 37°C for 24 h, the glass sheets were rinsed gently with 2 ml of PBS for three times to wash off the planktonic bacteria. Then they were placed in 2.5% glutaraldehyde solution (prepared in PBS) at 4°C for 5 h, followed by fixation with 1% osmic acid solution for 5 h. Samples were washed with PBS and dehydrated with different concentrations of ethanol solution (30, 50, 70, 80, 90 and 100%). After naturally air dried in a fume hood overnight, the samples were immobilized on a support, and sprayed with Au-Pd under vacuum. Finally, the morphology of the biofilm was observed and photographed by a scanning electron microscope (S-4800, Hitachi, Tokyo, Japan).
Confocal laser scanning microscopy observation
CLSM was carried out according to Fan et al. (2022a) with minor changes. As described above, S. aureus biofilm was formed on glass sheets with or without the treatment of punicalagin (0, 1/64, 1/32 and 1/16 MIC) at 37°C for 24 h. After gently rinsed three times with PBS, the glass sheets were transferred to a new 12-well plate. A total of 1 ml of SYTO 9 and propidium iodide (PI) fluorescent dye mixture was added to the wells, followed by the incubation at room temperature in the dark for 15 min. The biofilms were observed under a confocal laser scanning fluorescence microscope (A1; Nikon, Tokyo, Japan). Live bacteria with intact cell membranes emitted green fluorescence, while dead or damaged bacteria emitted red fluorescence.
Determination of bacterial surface hydrophobicity
The effect of punicalagin on the cell surface hydrophobicity of S. aureus was assessed by the method of microbial adhesion to hydrocarbons (MATH) as reported previously (Tang et al., 2020). In brief, overnight S. aureus culture was collected, washed, and resuspended in PBS. Punicalagin was added to reach the concentrations of 0, 1/64, 1/32 and 1/16 MIC. The absorbance of each sample was detected at 600 nm. Then, 1 ml of hexadecane was added to 2 ml of the bacterial suspension and mixed thoroughly for 1 min. The absorbance of aqueous phase was detected again after incubated for 15 min at 37°C. The hydrophobicity was calculated as follow.
Where Aa is the initial absorbance at 600 nm, and Ab is the absorption value after the treatment of punicalagin. Control groups were those without punicalagin treatment.
Statistical analysis
Mean values and standard deviations were obtained from three replicate experiments. Statistical analysis was performed by the analysis of variance (ANOVA) with SPSS 20.0 software. Tukey’s multiple range test was used to calculate the significant differences (p < 0.05) between the control and treatment groups.
Results
Effect of punicalagin on bacterial growth
The effect of punicalagin on the growth of S. aureus was shown in Figure 1. After about 2 h of lag, the strains of control groups quickly entered the logarithmic growth phase and reached a stationary phase within 8 h. The growth of S. aureus was obviously inhibited by punicalagin at 1/8 MIC compared to the control. However, punicalagin has minor effect on the growth of S. aureus at 1/64 MIC to 1/16 MIC. Therefore, the concentrations of punicalagin from 1/64 MIC to 1/16 MIC were considered as sub-inhibitory concentrations (SICs) against S. aureus which were chosen for the following experiments.
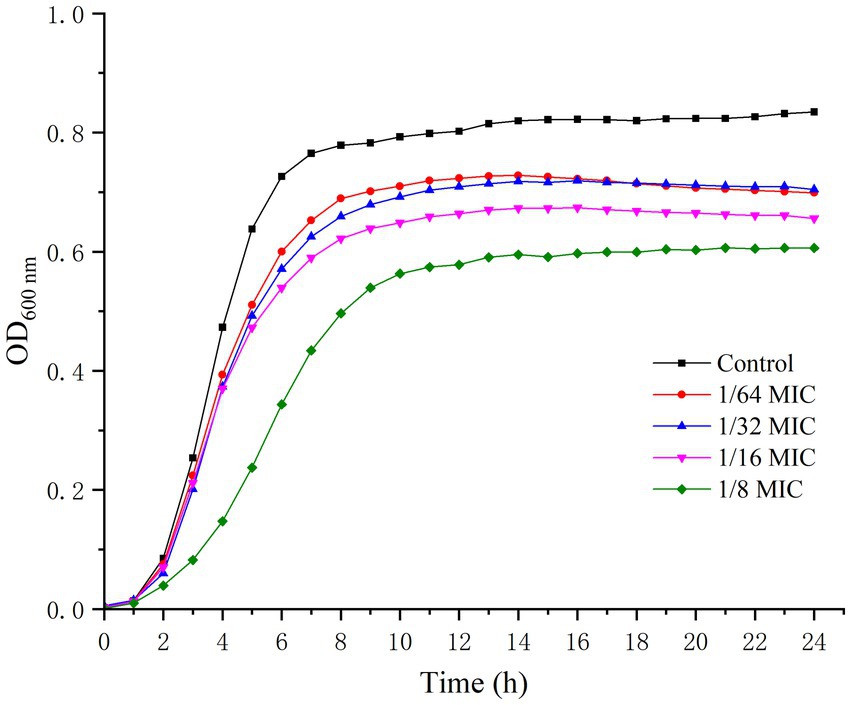
Figure 1. Growth curves of S. aureus in the absence or presence of punicalagin (0, 1/64 MIC, 1/32 MIC, 1/16 MIC and 1/8 MIC).
Effect of punicalagin on biofilm formation
As shown in Figure 2, the relative biofilm formation by S. aureus on polystyrene microplate was markedly decreased after punicalagin treatment. The biomass of the biofilm was decreased to 42.0% at the presence of punicalagin at 1/64 MIC compared to the control. And a higher reduction to 8.1% occurred as the concentration of punicalagin increased to 1/32 MIC. There was no significant difference between the 1/32 MIC and 1/16 MIC groups. This result indicated that punicalagin prevented biofilm formation effectively at SICs.
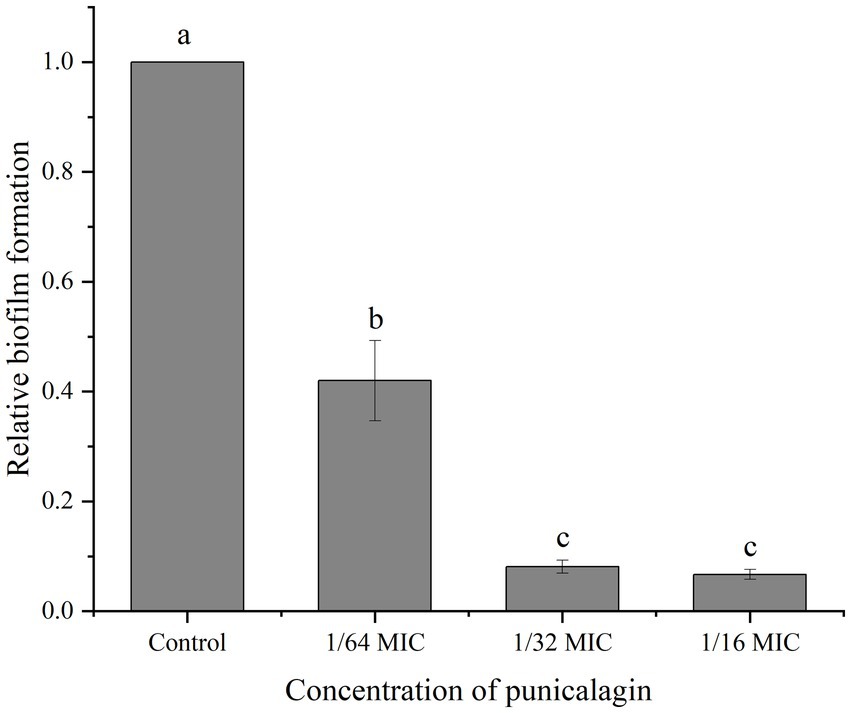
Figure 2. Inhibition of punicalagin (0, 1/64 MIC, 1/32 MIC, and 1/16 MIC) on S. aureus biofilm formation by crystal violet staining assay. Each bar indicates means ± the standard deviation. Different letters represent a significant difference compared to the control (p < 0.05).
Effect of punicalagin on metabolic activity of biofilms
MTT staining method reflects the metabolism of live bacteria in the biofilm and the result was depicted in Figure 3. At 1/64 MIC, punicalagin has a significant inhibitory effect on the metabolism of bacteria in the biofilm. The inhibitory effect increased with the increase of the concentration of punicalagin. At the highest concentration tested (1/16 MIC), the optical density was reduced by 97.3% in comparison with the control.
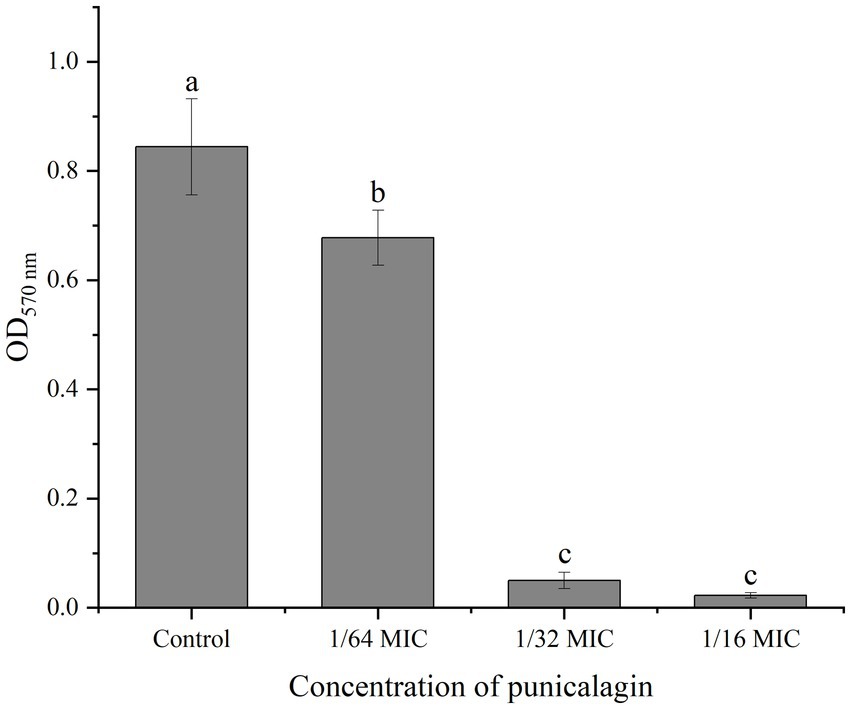
Figure 3. The effect of punicalagin on biofilm metabolic activity by MTT staining assay. Each bar indicates means ± the standard deviation. Different letters represent a significant difference compared to the control (p < 0.05).
Number of viable bacteria in biofilm
Figure 4 shows the number of viable bacteria in biofilm. It can be observed that the biofilm-associated population of S. aureus was reduced by the treatment of punicalagin and higher concentrations of punicalagin lead to more reduction in viable bacteria. Specifically, the viable cell counts were decreased by 0.94 and 1.75 log CFU/mL in comparison with the control after incubated with punicalagin at the 1/32 MIC and 1/16 MIC, respectively.
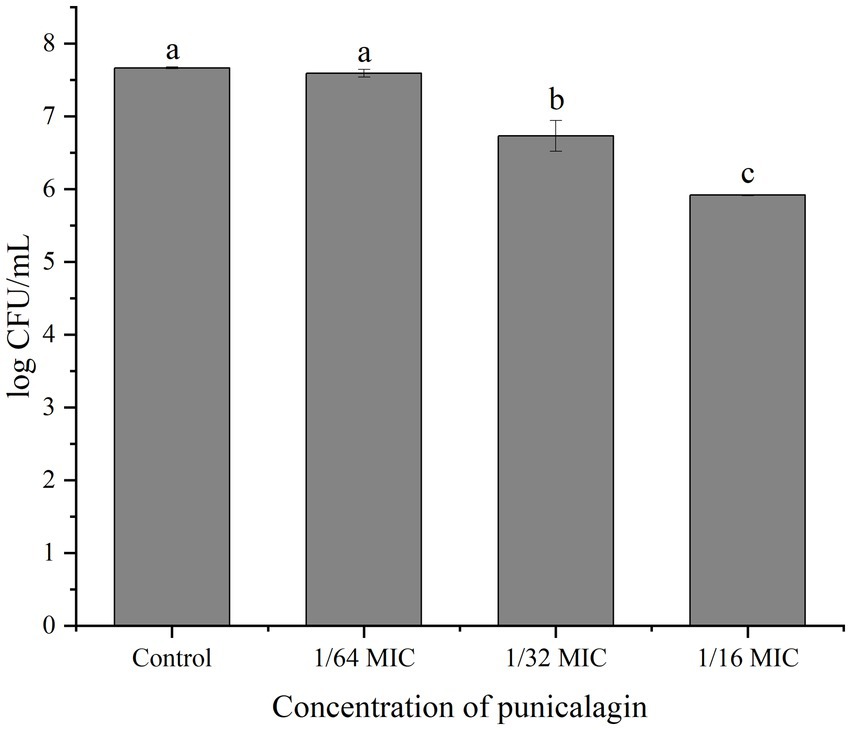
Figure 4. Cell enumeration of biofilms after exposed to various concentrations of punicalagin. Error bars represent standard deviations from triplicate analyzes of each sample. Different letters represent a significant difference compared to the control (p < 0.05).
Effect of punicalagin on biofilm of Staphylococcus aureus under FESEM
FESEM was used to observe the effect of punicalagin on the structure of biofilm. The results are shown in Figure 5. In Figure 5A, the cells are tightly adhered to each other on the surface of the glass slide and stacked on top of each other, suggesting the strong biofilm formation ability of the strain. The cells in Figures 5B–D are under the action of SICs of punicalagin. The adhesion between the cells became looser and the distribution became sparser than the control. This experiment visually illustrates the inhibitory effect of punicalagin on the biofilm formation of S. aureus. It also can be clearly seen that the surface of the control cells was smooth, while the surface of the punicalagin-treated cells was rough, indicating that punicalagin disrupted the cell surface morphology.
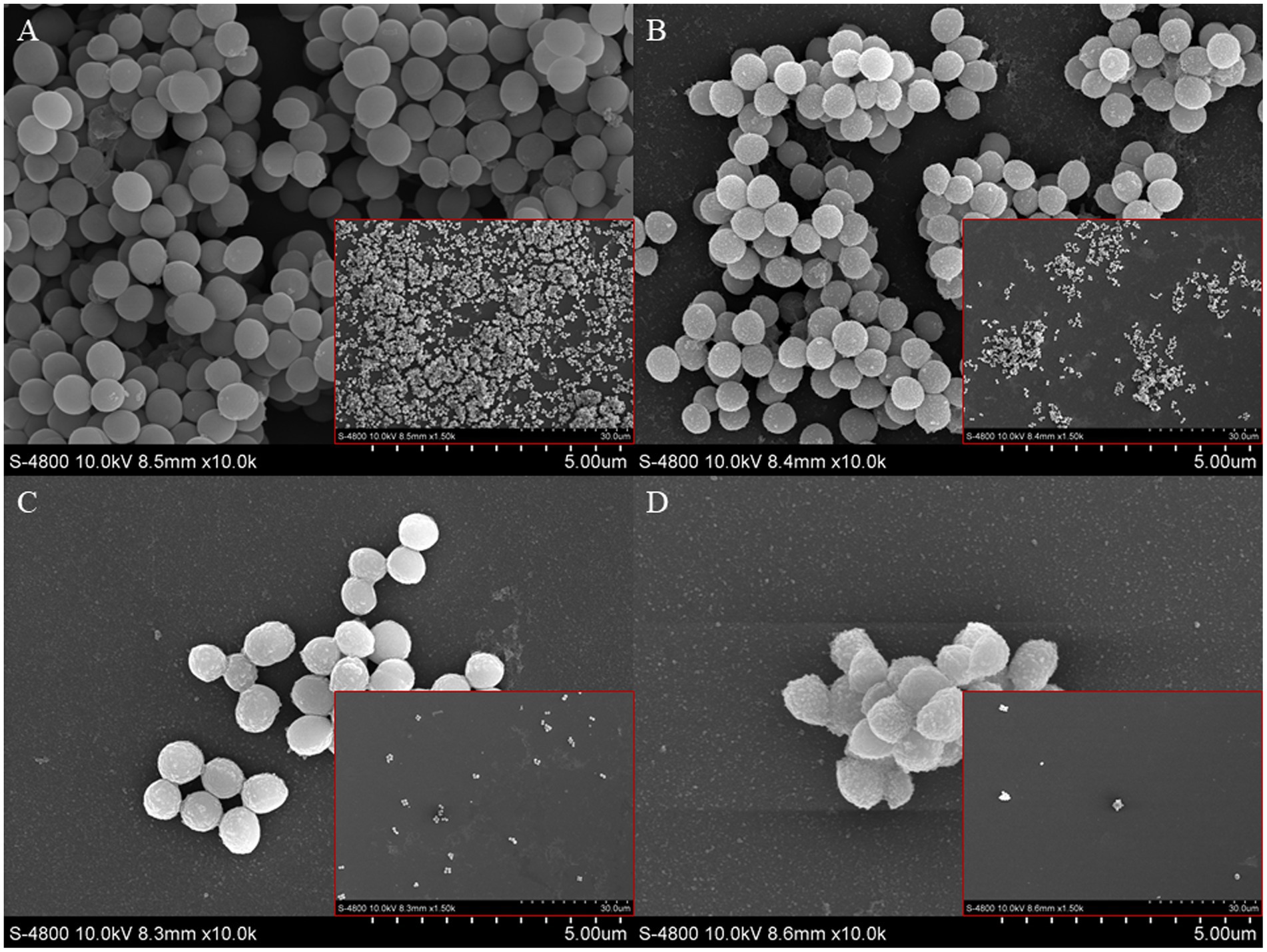
Figure 5. Scanning electron microscopic images of S. aureus biofilm in the presence of punicalagin at concentrations of 0 (A), 1/64 MIC (B), 1/32 MIC (C) and 1/16 MIC (D). Biofilm images in small red boxes are at 10,000 × magnification, and the large images are 1,500 × magnified.
Effect of punicalagin on biofilm of Staphylococcus aureus by CLSM
The inhibitory effect of punicalagin on the biofilm formation of S. aureus was also observed by CLSM. As can be seen from Figure 6, the fluorescence intensity of the control group was significantly higher than that of the experimental group after the action of punicalagin. A large number of S. aureus aggregates can be seen in the control group. After the treatment of punicalagin, the ability of S. aureus to form biofilm was significantly reduced. The amount of biofilm formation decreased as the concentration of punicalagin increased. The proportion of viable bacteria in all groups accounted for the majority (green). Only a few bacteria emitted red fluorescence, suggesting impaired cell membranes.
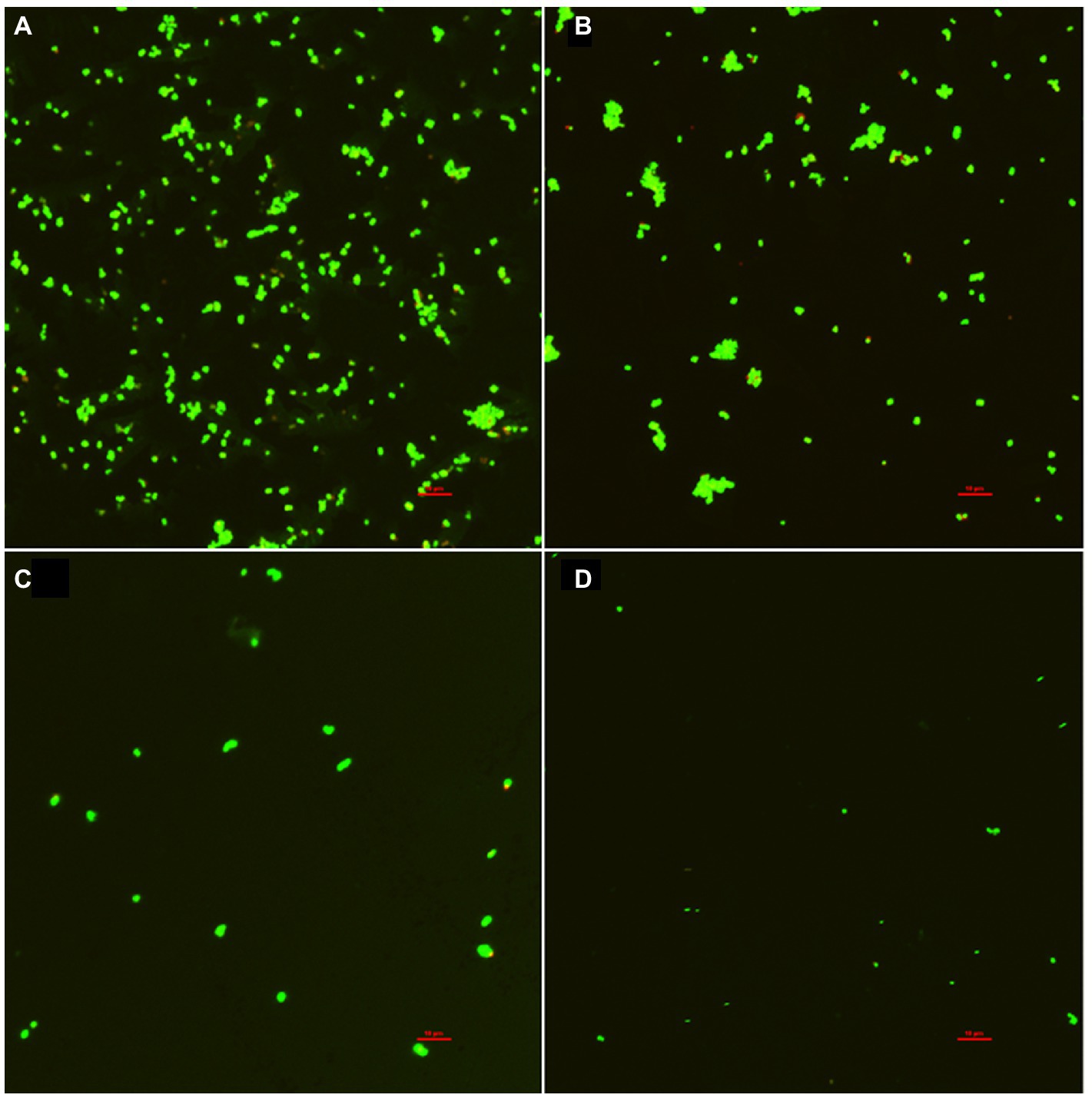
Figure 6. Confocal laser scanning microscopic images of S. aureus biofilm in the presence of punicalagin at concentrations of 0 (A), 1/64 MIC (B), 1/32 MIC (C) and 1/16 MIC (D). Scale bar =10 μm.
Effect of punicalagin on Staphylococcus aureus surface hydrophobicity
The result of bacterial surface hydrophobicity was shown in Figure 7. No significant difference was seen on the hydrophobic rates between the control and 1/64 MIC groups with the result of 70.9 and 69.6%, respectively. But the hydrophobic rates were significantly reduced to 55.5 and 37.2% after treated with 1/32 MIC and 1/16 MIC of punicalagin.
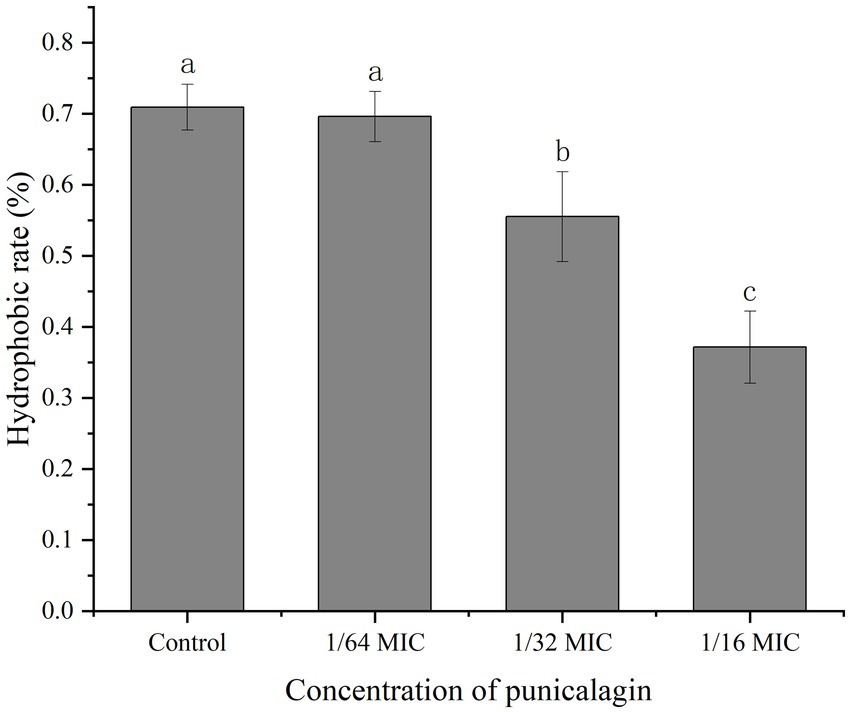
Figure 7. The effect of punicalagin on cell surface hydrophobicity of S. aureus. Error bars represent standard deviations from triplicate analyzes of each sample. Different letters represent a significant difference in comparison with the control (p < 0.05).
Discussion
In this study, the antibiofilm activity of punicalagin against S. aureus was investigated. Punicalagin at SICs could significantly inhibit the production of biofilm biomass. Many antibiofilm agents have been reported to possess similar activities against S. aureus. Shikimic acid was confirmed to reduce the biomass of the biofilm dose-dependently at its sub-MICs (Bai et al., 2019). Thymol exhibited a concentration-dependent antibiofilm activity with maximum biofilm inhibition of 88% at 100 μg/ml without affecting growth (Valliammai et al., 2020). The metabolic activity of S. aureus biofilm was significantly reduced when treated with punicalagin from 1/64 MIC to 1/16 MIC. There are some other compounds that inhibited the biofilm formation of S. aureus coupled with the reduction in cellular metabolic activity of biofilm. Kannappan et al. (2017) reported the inhibitory effect on the biofilm formation and metabolic activity of S. aureus by Vetiveria zizanioides root extract. Parai et al. (2020) found reserpine stopped the metabolic activity of 50.6% bacterial cells at 1/2 MIC. Punicalagin also induced a reduction of the number of bacteria in the biofilm. But this result was not exactly the same as the biofilm biomass and metabolic activity due to the difference of experimental principles and methods.
Microscopic visualization of punicalagin induced alterations on biofilm architecture of S. aureus was made through FESEM analyzes. The biofilm without punicalagin treatment was observed to adhere on the surface of glass slide and form thick aggregates, while the biofilms exposed to SICs of punicalagin gradually decreased. This result was in accordance with previous reports that many natural compounds were able to destroy the structure of biofilm. For instance, Gu et al. (2022) observed the biofilm of S. aureus USA300 on slides decreased as the concentration of geraniol increased. The morphological changes of S. aureus biofilm treated with punicalagin was also confirmed by CLSM. The fluorescent images were well correlated with the measured antibiofilm effects. It was reported that kaempferol dose-dependently inhibited the biofilm formation of S. aureus as observed by the fluorescence microscopy (Ming et al., 2017). Similarly, a reduction in the thickness of biofilm formation was noticed in myrtenol treated samples compared to the control (Selvaraj et al., 2019).
Adhesion is the initial and key step of biofilm formation. Bacterial adhesive ability is closely relevant to cell surface hydrophobicity (Zhu et al., 2022). Generally, the higher hydrophobicity, the stronger adhesive ability. The ability of bacterial hydrophobicity was decreased by the treatment of punicalagin, which resulted in the decrease of cell attachment, and eventually interfered with biofilm formation. Wen et al. (2021) reported the significant dose-related reduction in cell surface hydrophobicity of S. aureus with increasing concentrations of naringenin. Wang et al. (2017) found that hydrophobic rates of S. aureus decreased to 32.1 and 28.1% after exposed to MIC and MBC level of Dodartia orientalis L. essential oil, respectively. However, the mechanism of S. aureus biofilm formation is complex (Peng et al., 2023). The effect of punicalagin on the expression of genes critical for biofilm formation needs to be determined in the future.
Besides, how to apply punicalagin to food industry is another unaddressed issue. Tayel et al. (2012) added the ethanol extract of pomegranate peel into the meat steaks for decontamination. Andrade et al. (2023) incorporated pomegranate peel extract (85.84 mg/g punicalagin, 6.67 mg/g ellagic acid) into polylactic acid-based packaging film to extend the shelf life of beef meat. More studies about its activity in the food system are necessitated before application.
Conclusion
In conclusion, our study demonstrated that punicalagin exhibited good antibiofilm capacity against S. aureus in vitro. It reduced the biomass, metabolic activity and the number of microcolonies in the biofilm and impaired biofilm structure at SICs in a dose-dependent manner. Antibiofilm effect of punicalagin could be partly explained by the change of bacteria surface hydrophobicity. Based on these findings, punicalagin may have the potential to be developed as an antibiofilm agent against S. aureus.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
XX conceived and designed the experiments. YX and WG performed the experiments. JC and QX analyzed the data. DL, PL, and JX contributed to reagents, materials, and analysis tools. YX wrote the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Doctor Scientific Research Start-up Fund of Henan University of Science and Technology (13480067).
Conflict of interest
QX was employed by Heilongjiang Feihe Dairy Co. Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amalaradjou, M. A. R., and Venkitanarayanan, K. (2011). Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Prot. 74, 200–208. doi: 10.4315/0362-028x.Jfp-10-296
Andrade, M. A., Rodrigues, P. V., Barros, C., Cruz, V., Machado, A. V., Barbosa, C. H., et al. (2023). Extending high fatty foods shelf-life protecting from lipid oxidation and microbiological contamination: an approach using active packaging with pomegranate extract. Coatings 13:93. doi: 10.3390/coatings13010093
Aqil, F., Munagala, R., Vadhanam, M. V., Kausar, N., Jeyabalan, J., Schultz, D. J., et al. (2012). Anti-proliferative activity and protection against oxidative DNA damage by punicalagin isolated from pomegranate husk. Food Res. Int. 49, 345–353. doi: 10.1016/j.foodres.2012.07.059
Bai, J., Zhong, K., Wu, Y., Elena, G., and Gao, H. (2019). Antibiofilm activity of shikimic acid against Staphylococcus aureus. Food Control 95, 327–333. doi: 10.1016/j.foodcont.2018.08.020
Benchagra, L., Berrougui, H., Islam, M. O., Ramchoun, M., Boulbaroud, S., Hajjaji, A., et al. (2021). Antioxidant effect of Moroccan pomegranate (Punica granatum L. Sefri variety) extracts rich in punicalagin against the oxidative stress process. Foods 10:2219. doi: 10.3390/foods10092219
Berdowska, I., Matusiewicz, M., and Fecka, I. (2021). Punicalagin in cancer prevention-via signaling pathways targeting. Nutrients 13:2733. doi: 10.3390/nu13082733
Bouarab Chibane, L., Degraeve, P., Ferhout, H., Bouajila, J., and Oulahal, N. (2019). Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 99, 1457–1474. doi: 10.1002/jsfa.9357
Čolić, M., Mihajlović, D., Bekić, M., Marković, M., Dragišić, B., Tomić, S., et al. (2022). Immunomodulatory activity of punicalagin, punicalin, and ellagic acid differs from the effect of pomegranate peel extract. Molecules 27:7871. doi: 10.3390/molecules27227871
Fan, Q., He, Q., Zhang, T., Song, W., Sheng, Q., Yuan, Y., et al. (2022a). Antibiofilm potential of lactobionic acid against salmonella Typhimurium. LWT 162:113461. doi: 10.1016/j.lwt.2022.113461
Fan, Q., Yuan, Y., Zhang, T., Song, W., Sheng, Q., and Yue, T. (2022b). Inhibitory effects of lactobionic acid on Vibrio parahaemolyticus planktonic cells and biofilms. Food Microbiol. 103:103963. doi: 10.1016/j.fm.2021.103963
Gu, K., Ouyang, P., Hong, Y., Dai, Y., Tang, T., He, C., et al. (2022). Geraniol inhibits biofilm formation of methicillin-resistant Staphylococcus aureus and increase the therapeutic effect of vancomycin in vivo. Front. Microbiol. 13, 1–12. doi: 10.3389/fmicb.2022.960728
Jadhav, S., Shah, R., Bhave, M., and Palombo, E. A. (2013). Inhibitory activity of yarrow essential oil on listeria planktonic cells and biofilms. Food Control 29, 125–130. doi: 10.1016/j.foodcont.2012.05.071
Kannappan, A., Gowrishankar, S., Srinivasan, R., Pandian, S. K., and Ravi, A. V. (2017). Antibiofilm activity of Vetiveria zizanioides root extract against methicillin-resistant Staphylococcus aureus. Microb. Pathog. 110, 313–324. doi: 10.1016/j.micpath.2017.07.016
Li, J., Li, S., Li, H., Guo, X., Guo, D., Yang, Y., et al. (2021). Antibiofilm activity of shikonin against listeria monocytogenes and inhibition of key virulence factors. Food Control 120:107558. doi: 10.1016/j.foodcont.2020.107558
Li, G., Yan, C., Xu, Y., Feng, Y., Wu, Q., Lv, X., et al. (2014). Punicalagin inhibits salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 80, 6204–6211. doi: 10.1128/aem.01458-14
Liu, H., Zhu, W., Cao, Y., Gao, J., Jin, T., Qin, N., et al. (2022). Punicalagin inhibits biofilm formation and virulence gene expression of Vibrio parahaemolyticus. Food Control 139:109045. doi: 10.1016/j.foodcont.2022.109045
Ming, D., Wang, D., Cao, F., Xiang, H., Mu, D., Cao, J., et al. (2017). Kaempferol inhibits the primary attachment phase of biofilm formation in Staphylococcus aureus. Front. Microbiol. 8:2263. doi: 10.3389/fmicb.2017.02263
Mun, S.-H., Kong, R., Seo, Y.-S., Zhou, T., Kang, O.-H., Shin, D.-W., et al. (2016). Subinhibitory concentrations of punicalagin reduces expression of virulence-related exoproteins by Staphylococcus aureus. FEMS Microbiol. Lett. 363, 1–6. doi: 10.1093/femsle/fnw253
Parai, D., Banerjee, M., Dey, P., and Mukherjee, S. K. (2020). Reserpine attenuates biofilm formation and virulence of Staphylococcus aureus. Microb. Pathog. 138:103790. doi: 10.1016/j.micpath.2019.103790
Peng, Q., Tang, X., Dong, W., Sun, N., and Yuan, W. (2023). A review of biofilm formation of Staphylococcus aureus and its regulation mechanism. Antibiotics 12:12. doi: 10.3390/antibiotics12010012
Phuong, N. T. M., van Quang, N., Mai, T. T., Anh, N. V., Kuhakarn, C., Reutrakul, V., et al. (2017). Antibiofilm activity of α-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. J. Trop. Med. 10, 1154–1160. doi: 10.1016/j.apjtm.2017.10.022
Selvaraj, A., Jayasree, T., Valliammai, A., and Pandian, S. K. (2019). Myrtenol attenuates MRSA biofilm and virulence by suppressing sarA expression dynamism. Front. Microbiol. 10:2027. doi: 10.3389/fmicb.2019.02027
Tang, C., Chen, J., Zhang, L., Zhang, R., Zhang, S., Ye, S., et al. (2020). Exploring the antibacterial mechanism of essential oils by membrane permeability, apoptosis and biofilm formation combination with proteomics analysis against methicillin-resistant Staphylococcus aureus. Int. J. Med. Microbiol. 310:151435. doi: 10.1016/j.ijmm.2020.151435
Tayel, A. A., El-Tras, W. F., Moussa, S. H., and El-Sabbagh, S. M. (2012). Surface decontamination and quality enhancement in meat steaks using plant extracts as natural biopreservatives. Foodborne Pathog. Dis. 9, 755–761. doi: 10.1089/fpd.2012.1203
Valliammai, A., Selvaraj, A., Yuvashree, U., Aravindraja, C., and Karutha Pandian, S. (2020). sarA-dependent antibiofilm activity of thymol enhances the antibacterial efficacy of rifampicin against Staphylococcus aureus. Front. Microbiol. 11:1744. doi: 10.3389/fmicb.2020.01744
Wang, F., Wei, F., Song, C., Jiang, B., Tian, S., Yi, J., et al. (2017). Dodartia orientalis L. essential oil exerts antibacterial activity by mechanisms of disrupting cell structure and resisting biofilm. Ind. Crop. Prod. 109, 358–366. doi: 10.1016/j.indcrop.2017.08.058
Wen, Q., Wang, R., Zhao, S., Chen, B., and Zeng, X. A. (2021). Inhibition of biofilm formation of foodborne Staphylococcus aureus by the citrus flavonoid naringenin. Foods 10:2614. doi: 10.3390/foods10112614
Xu, J., Cao, K., Liu, X., Zhao, L., Feng, Z., and Liu, J. (2022). Punicalagin regulates signaling pathways in inflammation-associated chronic diseases. Antioxidants 11:29. doi: 10.3390/antiox11010029
Xu, Y., Shi, C., Wu, Q., Zheng, Z., Liu, P., Li, G., et al. (2017). Antimicrobial activity of punicalagin against Staphylococcus aureus and its effect on biofilm formation. Foodborne Pathog. Dis. 14, 282–287. doi: 10.1089/fpd.2016.2226
Zheng, X., Guo, J., Rao, H., Guo, D., Huang, Y., Xu, Y., et al. (2020). Antibacterial and antibiofilm activity of coenzyme Q0 against Vibrio parahaemolyticus. Food Control 109:106955. doi: 10.1016/j.foodcont.2019.106955
Keywords: punicalagin, Staphylococcus aureus, antibiofilm, microscopy, hydrophobicity
Citation: Xu Y, Guo W, Luo D, Li P, Xiang J, Chen J, Xia X and Xie Q (2023) Antibiofilm effects of punicalagin against Staphylococcus aureus in vitro. Front. Microbiol. 14:1175912. doi: 10.3389/fmicb.2023.1175912
Edited by:
Peng Fei, Nanyang Institute of Technology, ChinaReviewed by:
Panxue Wang, Shaanxi University of Science and Technology, ChinaQinnan Yang, University of Nebraska-Lincoln, United States
Copyright © 2023 Xu, Guo, Luo, Li, Xiang, Chen, Xia and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaodong Xia, Zm9vZHNjaXhpYW9kb25nQGRscHUuZWR1LmNu; Qinggang Xie, eGllcWluZ2dhbmdAZmVpaGUuY29t
 Yunfeng Xu
Yunfeng Xu Weiping Guo
Weiping Guo Denglin Luo
Denglin Luo Peiyan Li1
Peiyan Li1 Xiaodong Xia
Xiaodong Xia