- 1National Navel Orange Engineering Research Center, Gannan Normal University, Ganzhou, China
- 2Key Laboratory of South China Agricultural Plant Molecular Analysis and Genetic Improvement, Guangdong Provincial Key Laboratory of Applied Botany, South China Botanical Garden, Chinese Academy of Sciences, Guangzhou, China
- 3Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, China
Gannan navel orange is a famous brand in China but the isolation of its endophytic fungi was rarely reported. In this study, a total of 54 strains of endophytic fungi were successfully isolated from the pulp, peel, twig, and leaf of Gannan navel orange; they were successfully identified to belong to 17 species of 12 genera. All these strains were fermented using potato-dextrose agar (PDA) medium, and their secondary metabolites were then extracted with ethyl acetate (EtOAc). The antibacterial assays of Escherichia coli (E. coli), methicillin-resistant Staphylococcus aureus (MRSA), and Xanthomonas citri subsp. citri (Xcc) were also performed for the EtOAc extracts of these strains. As a result, the extracts of both Geotrichum sp. (gc-1-127-30) and Diaporthe biconispora (gc-1-128-79) demonstrated significant antibacterial activities against Xcc, and the MIC value for the extract of Colletotrichum gloeosporioides against MRSA was low to 62.5 μg/mL. Moreover, the chemical components of the extracts of Colletotrichum sp., Diaporthe biconispora, and Annulohypoxylon atroroseum were primarily investigated, and they successfully led to the isolation of 24 compounds involving a new botryane sesquiterpene. Among the isolated products, compound 2 showed significant inhibitory activities toward SA, MRSA, E. coli, and Xcc with MIC values of 12.5, 3.1, 125, and 12.5 μg/mL, respectively. This study revealed that the endophytic fungi of Gannan navel orange showed high potency to produce secondary metabolites with significant antibacterial effects.
1. Introduction
China is one of the most important countries for oranges production with an amount of 7.6 million tons in 2022 (USDA, 2022). Gannan, located in the south of Jiangxi province, is a critical cultivation area for orange in China. The Newhall navel orange (Citrus sinensis Osbeck cv. Newhall) produced in Gannan, namely Gannan navel orange, is honored as a national geographic indication product in China (Chen et al., 2009; Zhang et al., 2022b).
Plant endophytes have been disclosed to be an extremely important resource for the discovery of new microorganisms (Cabral et al., 1999). In recent years, numerous studies had disclosed that a huge number of endophytic fungi were present in plants, constructing a mutually beneficial symbiotic relationship with their hosts (Collinge et al., 2022). Moreover, most of them showed the capability to help the host to tolerate the external environments (Manzur et al., 2022), promote the growth of plants (Abdalla and Matasyoh, 2014), and biosynthesize some bioactive ingredients to protect the host plants (Mattoo and Nonzom, 2021). Although a large number of studies have been conducted to illustrate the interaction between endophytes and host plants, systematic investigations on the Gannan navel orange and its endophytes are still rare.
Bacterial infection is becoming to be one of the most intractable infectious diseases because of its emerging resistance to the existing antibiotics. Nowadays, the pathogenic bacterium resistant to widely used antibiotics is considered as one of the most serious threats to global public health, which is responsible for serious infections due to high rates of morbidity and mortality in clinical treatment. Among the frequently encountered bacteria, pathogenic Escherichia coli usually causes severe diarrhea worldwide. There are nearly 1.7 billion cases of diarrheal disease reported every year (Yang S. C. et al., 2017), and 9.4% of them are caused by diarrheagenic E. coli (Indhuprakash et al., 2021), which has resulted in about 760,000 death of children every year from 2011 to 2015 (Chowdhury et al., 2015). Methicillin-resistant Staphylococcus aureus (MRSA) is well known as a predominant nosocomial pathogenic bacterium, which represents a crucial crisis in public health systems and poses a serious threat to human life, especially in developed countries (Rebecca, 2008). Therefore, the discovery of novel therapeutic agents that are effective against pathogenic bacteria is of great interest to both medicinal scientists and the pharmaceutical industry to maintain public health in future.
The endophytic fungus is now serving as a promisingly strategic natural bioresource for the discovery of novel natural products with interesting chemical structures and versatile biological activities (Fan et al., 2022). In particular, it was well-respected as a treasure house for developing novel antibacterial agents (Cruz et al., 2020). However, the endophytic fungus of Gannan navel oranges has been rarely investigated. In one of our previous studies, we had isolated and identified an endophytic fungus Aspergillus aculeatus GC-09 from Gannan navel orange, the antibacterial screening disclosed that its crude EtOAc extract showed significant antifungal activity against Penicillium italicum with a MIC of 31.3 μg/mL, which was comparable to the positive control prochloraz (Zhang et al., 2022a). Except that, there were no reports on the endophytic fungi of Gannan navel oranges.
In order to recognize the abundance and distribution of endophytic fungus species of Gannan navel oranges and illustrate their antibacterial potential as a particular interest, a comprehensive investigation on endophytic fungi from Gannan navel orange was performed in the present study. As a result, 54 strains of endophytic fungi were successfully isolated from the healthy pulp, peel, twig, and leaf of Newhall navel orange for the first time. The molecular identification through the ITS rDNA sequences of the isolated endophytic fungi successfully distinguished them to be 17 species of 12 genera. Moreover, the antimicrobial screening against MRSA, E. coli, and X. citri subsp. citri (Xcc) was carried out, revealing that a few fermentation extracts of the isolated endophytic fungi showed significant antibacterial activity. To the best of our knowledge, this study is the first systematic report on the biodiversity, phylogeny, and antibacterial activity of endophytic fungi associated with Gannan navel orange.
2. Materials and methods
2.1. Plant material and pathogens
Healthy and symptomless Gannan navel oranges (Citrus sinensis Osbeck cv. Newhall) were collected from Gannan Normal University Navel Orange Resource Nursery, Ganzhou County, Jiangxi Province, China, in May 2020. E. coli (ATCC 12435), SA (CMCC 26003), and MRSA (JCSC 3063) were obtained from Guangdong Microbiology Culture Center (Guangzhou, China), and X. citri subsp. citri was obtained from the China-USA Citrus Huanglongbing Joint Laboratory.
2.2. Isolation and purification of fungal endophytes
The isolation of endophytic fungi from collected plant parts was according to a standard procedure established previously (Li et al., 2020). The pulp, peel, twig, and leaf of Gannan navel orange were used to isolate the corresponding endophytic fungi. The samples were washed with sterile water and dried with sterile filter paper. Then, the cleared tissues were cut into small pieces using a sterile blade and surface-sterilized by sequential immersion in 75% ethanol for 1 min, 1.3 mol/L sodium hypochlorite (3–5% available chlorine) for 30 s, and they were rinsed three times with sterile water between each step simultaneously. These tissue pieces were placed on potato dextrose agar (PDA) plates with 0.4 μg/mL ampicillin and kanamycin to isolate the fungal endophytes. The tissue pieces were observed for mycelia growth. Morphologically distinct isolates were similarly sub-cultured several times to purify and obtain pure isolates. As soon as the fungal mycelium grows out of the tissue slice, the mycelium is transferred to fresh PDA plates for purification and culture. The pure isolates were numbered and kept in a storage tube at −20°C for further study.
2.3. ITS sequence processing and molecular identification
The identification of endophytic fungi was based on molecular and morphological analysis. The molecular identification was carried out using the internal transcript spacer regions (ITS1 and ITS4) and the intervening 5.8S rRNA region sequencing. Polymerase chain reaction (PCR) was performed to amplify the ITS region of the fungal isolates using the universal ITS primers, ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′) and ITS4 (5′-TCCTCCGCTTATTGATATGC-3′). The PCR reaction mixture (20 μL) contained 1 μL template (100 ng/μL purified DNA sample), 10 μM of each primer, 0.5 μL 2 × Taq PCR Master Mix, and 8 μL ddH2O. PCR conditions performed were as follows: initial denaturation at 95°C for 3 min, followed by 35 cycles of 94°C for 40 s, 52°C for 50 s, and 72°C for 1 min as well as a final extension at 72°C for 10 mins. The amplified PCR products were checked on 1% agarose gel and then sent to Sangon Biotech (Shanghai) Co., Ltd for sequencing. All obtained fungal ITS sequences have been deposited in GenBank and analyzed using BLAST search in the National Center of Biotechnology Information (NCBI) database to compare the sequence homology with closely related organisms.
The ITS sequences were aligned to determine the identity of the corresponding fungal ITS sequences by using BLAST to search from the GenBank database. The endophytic fungus was identified by the percentage identity, and the species level of the targeted endophytic fungus was successfully distinguished with a sequencing identity of more than 99%, whereas the genus level was assigned if sequence identity was between 95 and 99%. Notably, when the sequence identity was determined ≤ 95%, the related endophytic fungus could be only identified at the family or ordinal level by referring to the suggestion of Landeweert et al. (2003).
The sequencing results were analyzed in the SeqMan module of DNAStar 5.0 software for peak plot analysis (Burland, 1999), the sequence fragments were proofread and saved in fasta format and compared with known sequences in Gene bank using BioEdit v7.1 (Hall, 1999, 2011). Then, the sequences were cut and edited, and a matrix was constructed based on the aforementioned results. The sequence output was saved in fas format for phylogenetic tree construction. Bayesian inference was performed by MrBayes v3.1.2 (Ronquist and Huelsenbeck, 2003) with the Markov Chain Monte Carlo algorithm and treated the random initial trees as BI settings. Four chains were run, generating 1,000,000 generations per chain. Sampling was conducted every 1,000 generations, and the first 2,500 trees were discarded as burn-in. The remaining trees were used to construct the majority-rule consensus trees. ClustalW Multiple was initially used to perform alignments by BioEdit. Bootstrap (Felsenstein, 1985) values of internal nodes were obtained with 1,000 replicates. Bayesian inference (BI) was operated with the best-fit model (K80+ G) which was selected based on the AIC criterion using MrModeltest 2.0. Calophoma aquilegiicola, Didymella glomerata, Leptosphaeria microscopica, and Paraboeremia litseae were selected as outgroup species.
2.4. Preparation of EtOAc extracts of different endophytic fungi
Submerged cultivation of the endophytic fungi was carried out to produce secondary metabolites. The strain of the agar with the fungal colony was added to a conical flask (500 mL) with 250 mL potato dextrose broth (PDB) at 28°C for 7 days under constant shaking (150 rpm). After cultivation, the secondary metabolites of each endophyte were extracted by ultrasonic with EtOAc.
2.5. Isolation of secondary metabolites
The endophytic fungi Colletotrichum sp., Diaporthe biconispora, and Annulohypoxylon atroroseum were cultured on an autoclaved rice solid medium (5 × 3 L Erlenmeyer flasks, each containing 300 g of grains and 360 mL of distilled water) for 30 days at 28°C. After cultivation, the mycelia and rice solid medium were extracted three times with EtOAc crude extract were obtained. We obtained the main compounds of these two strains that using silica gel chromatography, reversed-phase C18 column chromatography, and Sephadex LH-20 column chromatography were isolated.
2.6. Antibacterial activity
The EtOAc extracts of each endophytic fungi were evaluated for their antibacterial activity. MIC values were determined by the methodology of micro broth dilution in Mueller-Hinton broth (MHB) medium according to CLSI guidelines, the positive control was kanamycin, vancomycin, and CuSO4. Briefly, 180 μL bacteriological solution (1.25 × 106 CFU/mL) with resazurin as an indicator was added into each well of the first row in the 96-well plate, whereas 100 μL bacteriological solution (1.25 × 106 CFU/mL) with resazurin as indicator was added to the other wells in the 96-well plate. A total of 20 μL tested extracts in DMSO solution with a concentration of 10 mg/mL were added to 180 μL bacterial liquid. Then, the method of double dilution was adopted in the 96-well plates. The change of the indicator in the 96-well plates was observed, and the lowest concentration of the drug preventing visible growth of the pathogen was taken as the MIC value (Wang et al., 2022). Similarly, the monomer compounds were also tested.
3. Results
3.1. Diversity of cultural endophytic fungi from Gannan navel orange
In this study, 54 strains of endophytic fungi were successfully isolated from different tissues of Gannan navel orange, which included the pulp, peel, twig, and leaf. Among these endophytic fungi strains, 2 strains (3.70%) were isolated from pulp, 44 strains (81.48%) were purified from the peel, 5 strains (9.26%) originated from twig, and 3 strains (9.26%) were isolated from leaf (Supplementary Table S1). The results indicated that there were abundant endophytic fungi presenting in the Gannan navel orange. Moreover, this study also suggested that the peel of Gannan navel orange might be the most suitable host place for the endophytic fungi.
3.2. ITS identification of the endophytic fungi of Gannan navel orange
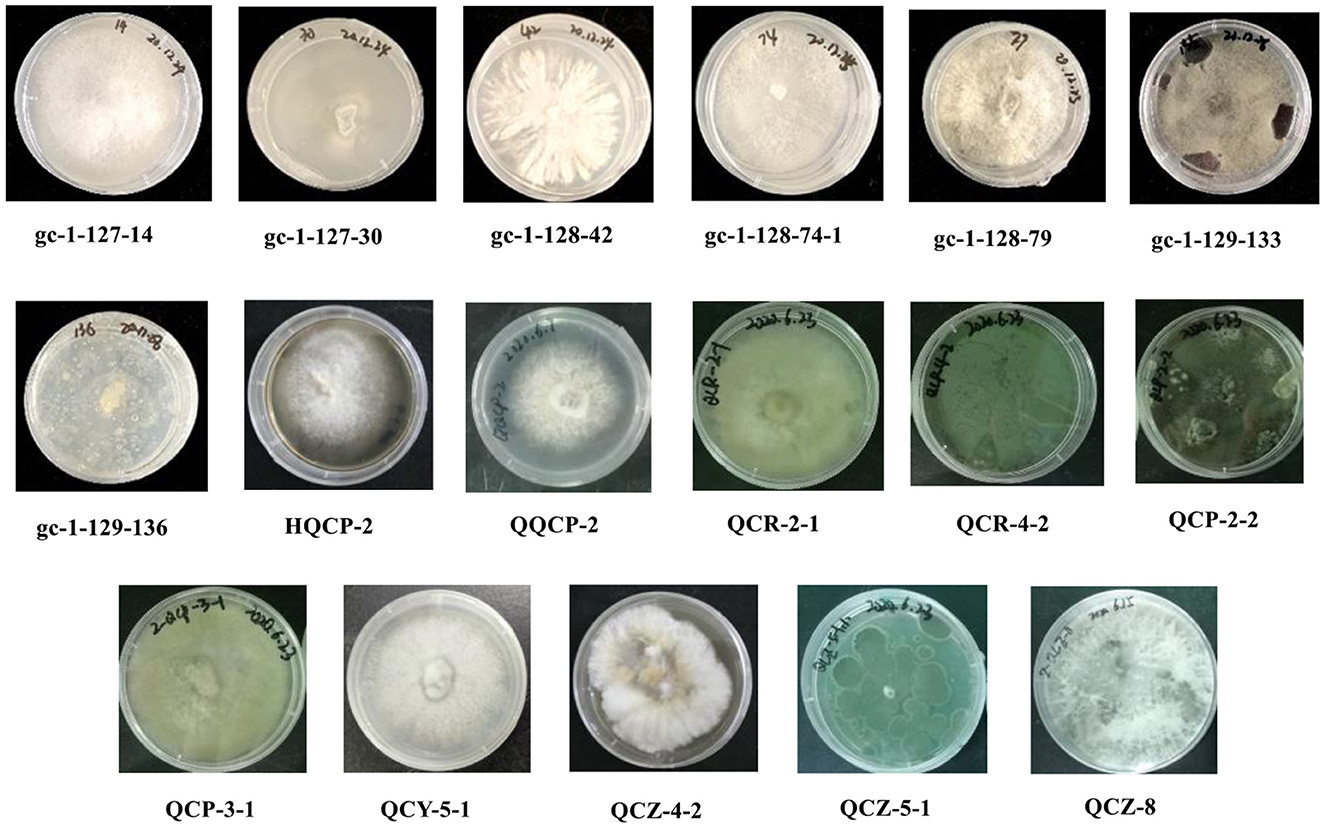
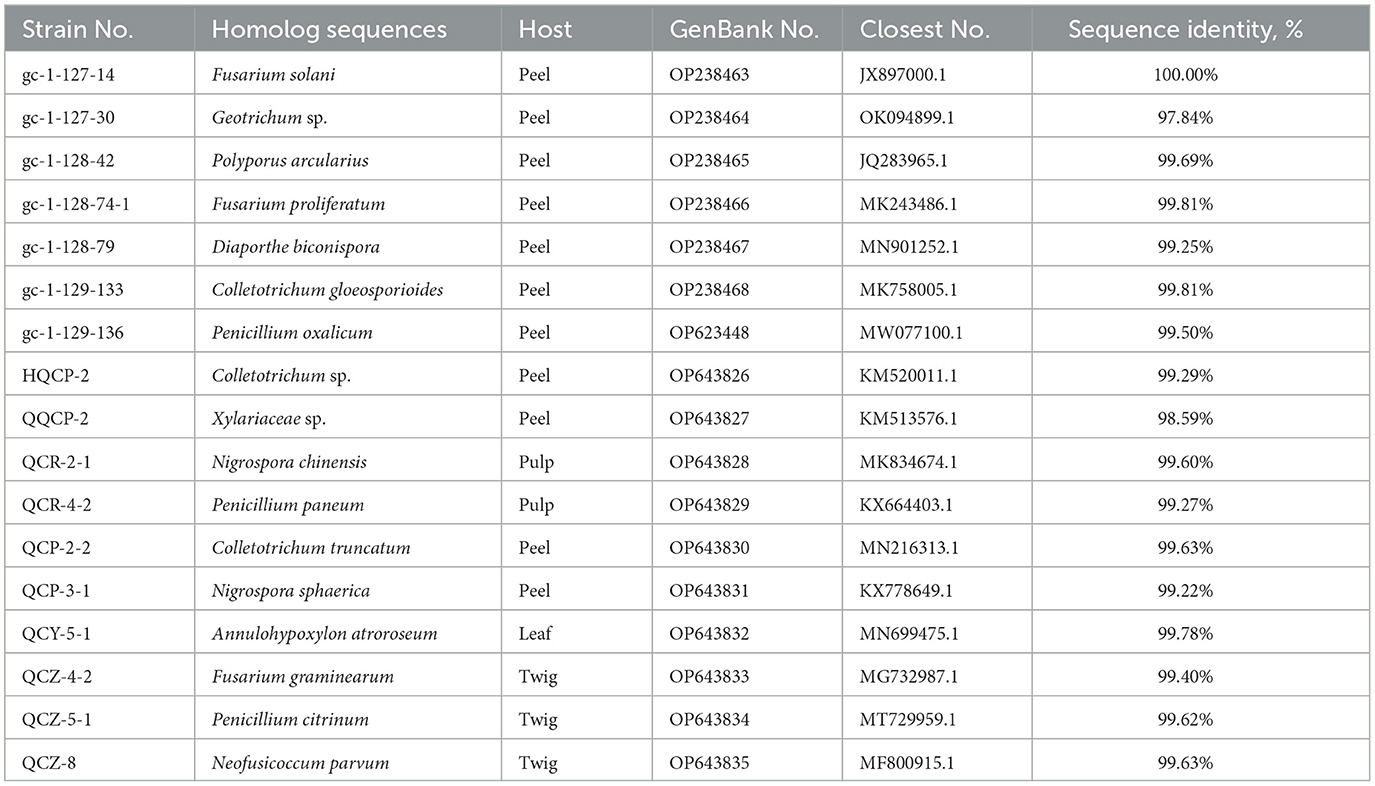
Based on the inspection and comparison of the fungal morphological characteristics and phenotypes appearance on the PDA culture medium for the 203 purified strains of endophytic fungi, 54 strains showing different morphological features were selected for the molecular biology identification via the sequence analysis of the rDNA ITS (internal transcribed spacer) region. From the results, all of the endophytic fungi strains isolated from Gannan navel orange could be assigned to 17 representative morphotypes, as shown in Figure 1. Subsequently, ITS rDNA sequences of each morphotype were generated and submitted to the NCBI database to obtain the corresponding sequence numbers (Table 1). Moreover, a phylogenetic tree of the endophytic fungi of Gannan navel orange was successfully constructed as depicted in Figure 2.
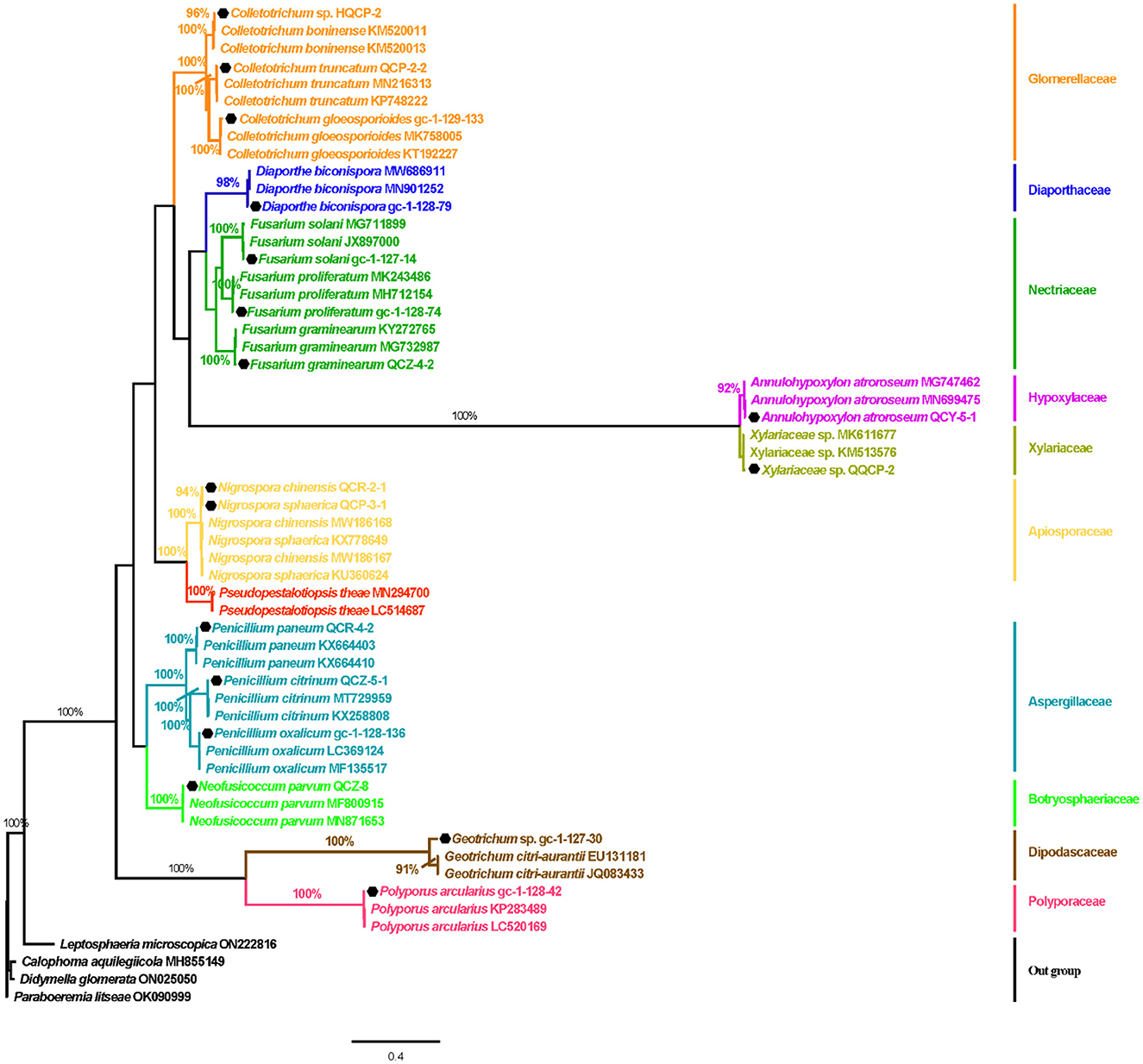
Figure 2. Bayes tree of the Gannan navel orange endophytic fungi based on the ITS sequences. Numbers on branches are support values PPBI. PPBI ≥90%, PPBI = 100%. The scale bar represents 0.4 genetic variation per nucleotide sequence. Leptosphaeria microscopica (ON222816), Calophoma aquilegiicola (MH855149), Didymella glomerata (ON025050), and Paraboeremia litseae (OK090999) were used as outgroup species.
Moreover, the GenBank accession numbers, homolog sequences, sequence identities, and closest accession numbers of the identified endophytic fungal isolates were systematically cataloged and shown in Table 1. Therefore, according to the results of morphological and molecular identification, 17 representative morphotypes were successfully identified belonging to 12 genera. Moreover, it is worth noting that 14 endophytic fungi from the 17 ones were identified as species (Figure 1). Furthermore, all the isolated endophytic fungi were identified to be 2 phyla (Ascomycota and Basidiomycota), 5 classes (Sordariomycetes, Saccharomycetes, Agaricomycetes, Eurotiomycetes, and Dothideomycetes), 9 orders (Hypocreales, Saccharomycetales, Polyporales, Diaporthales, Glomerellales, Eurotiales, Glomerellales, Xylariales, and Botryosphaeriales), 10 families (Nectriaceae, Dipodascaceae, Polyporaceae, Diaporthaceae, Glomerellaceae, Aspergillaceae, Xylariaceae, Apiosporaceae, Hypoxylaceae, and Botryosphaeriaceae), 12 genera, and 17 species in total.
3.3. Antibacterial screening of the EtOAc extracts from endophytic fungi cultures
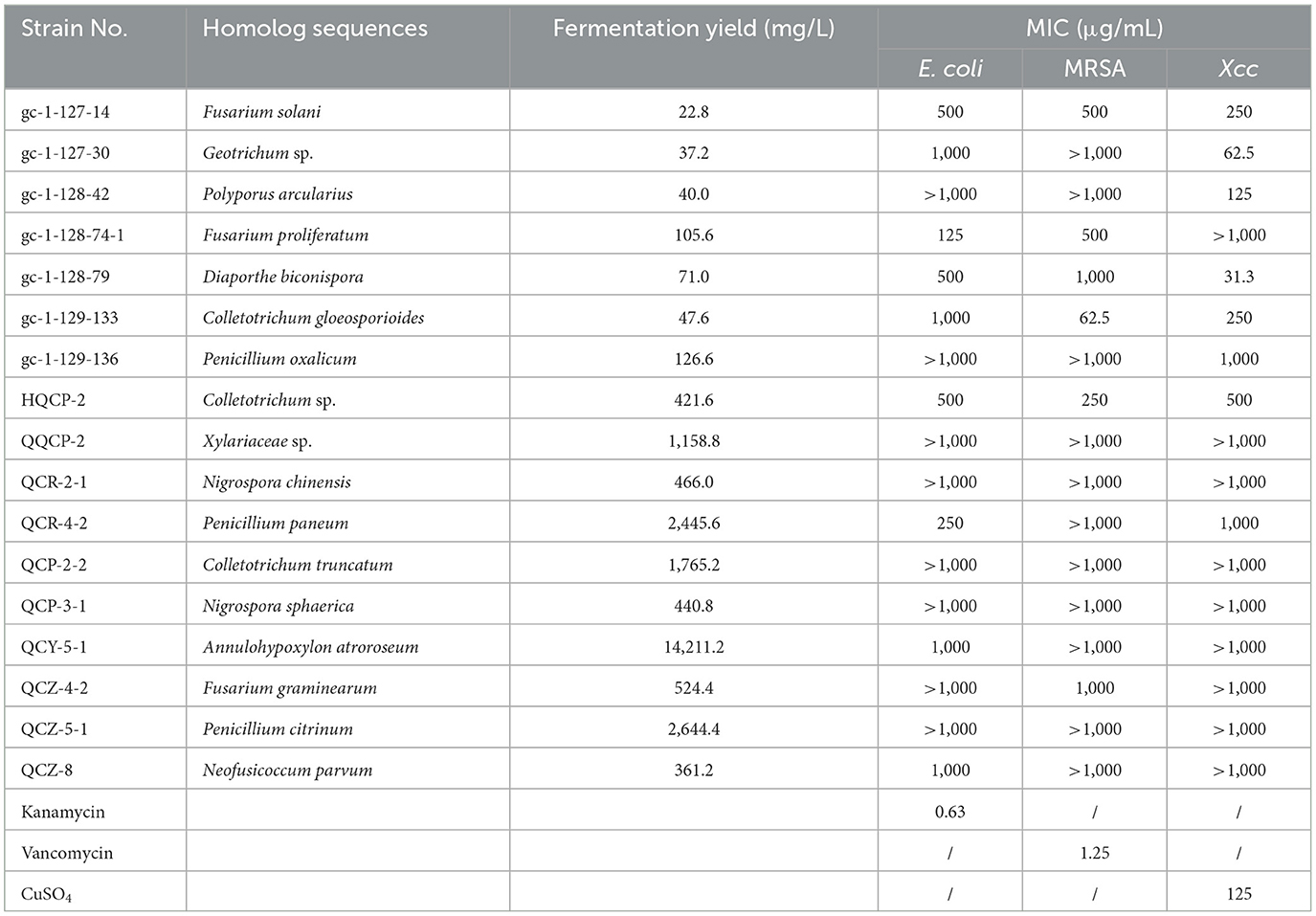
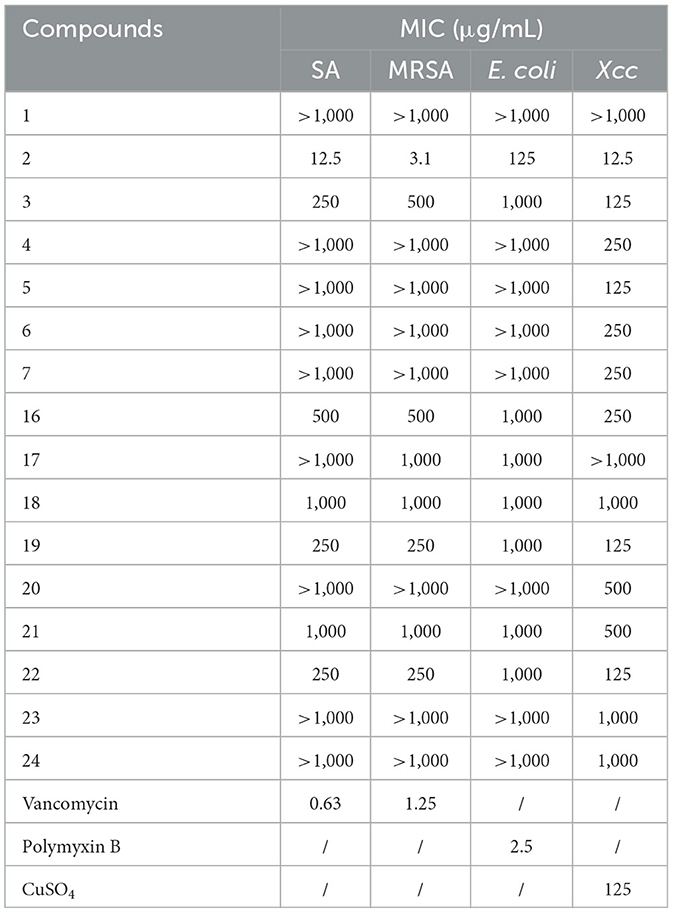
With the fermentation products of the endophytic fungi from Gannan navel orange in hand, the antimicrobial screening for the discovery of potential strain-producing biologically interesting secondary metabolites was then conducted. The antimicrobial results of the tested extracts are depicted in Table 2. The results revealed that the EtOAc extracts of 8 endophytic fungi exhibited obvious inhibitory activities against Gram-negative bacterium E. coil, whereas the other 9 EtOAc extracts showed no noticeable antimicrobial activities. Notably, the aforementioned 8 antibacterial strains belonged to 7 genera of Fusarium, Geotrichum, Fusarium, Diaporthe, Colletotrichum, Penicillium, Annulohypoxylon, and Neofusicoccum.
Moreover, the antibacterial activities against the Gram-positive bacterium MRSA and plant pathogenic bacterial Xcc were also conducted for the 17 EtOAc extracts of the isolated endophytic fungi. As a result, 6 EtOAc extracts of endophytic fungi exhibited significant inhibitory activities against MRSA, involving Fusarium solani, Fusarium proliferatum, Diaporthe biconispora, Colletotrichum gloeosporioides, Colletotrichum sp., and Fusarium graminearum. Notably, among them, Colletotrichum gloeosporioides (gc-1-129-133) demonstrated the highest antibacterial activity with a MIC value of 62.5 μg/mL, which was closely comparable to that of the clinical drug vancomycin (positive control, MIC = 1.3 μg/mL), showing significant potential for the discovery of novel antibacterial drugs. Similarly, some EtOAc extracts of the endophytic fungi exhibited considerable inhibitory activities against the plant pathogenic fungus Xcc, which were Fusarium solani, Geotrichum sp., Polyporus arcularius, Diaporthe biconispora, Colletotrichum gloeosporioides, Penicillium oxalicum, Colletotrichum sp., and Penicillium paneum. Interestingly, both the EtOAc extracts of Geotrichum sp. (gc-1-127-30) and Diaporthe biconispora (gc-1-128-79) demonstrated very potent antibacterial activity against Xcc with MIC values as low as 62.5 and 31.3 μg/mL, respectively, strongly suggesting that these two endophytic fungi might be severed as promising natural bioresources for the discovery of novel antibacterial agents.
Taken together, the endophytic fungus Fusarium solani showed the best antibacterial activities against all of the tested bacteria involving E. coli (MIC = 500 μg/mL), MRSA (MIC = 500 μg/mL), and Xcc (MIC = 250 μg/mL), which were much more potent than other sixteen endophytic fungi from Gannan navel orange (Table 2). Notably, the attractable results also came from the endophytic fungus Fusarium solani, which showed comprehensive antibacterial activities against all of the Gram-negative bacterium (E. coli), Gram-positive bacterium (MRSA), and pathogenic fungus (Xcc), suggesting that the EtOAc extract of the endophytic fungus Fusarium solani might be developed as promising biocontrol agent with broad-spectrum antibacterial effect.
3.4. Isolation of the EtOAc extracts
Based on the antibacterial effects of all EtOAc extracts tested, three endophytic fungi Colletotrichum sp., D. biconispora, and A. atroroseum were selected for the discovery of antibacterial novel secondary metabolites by column chromatography method.
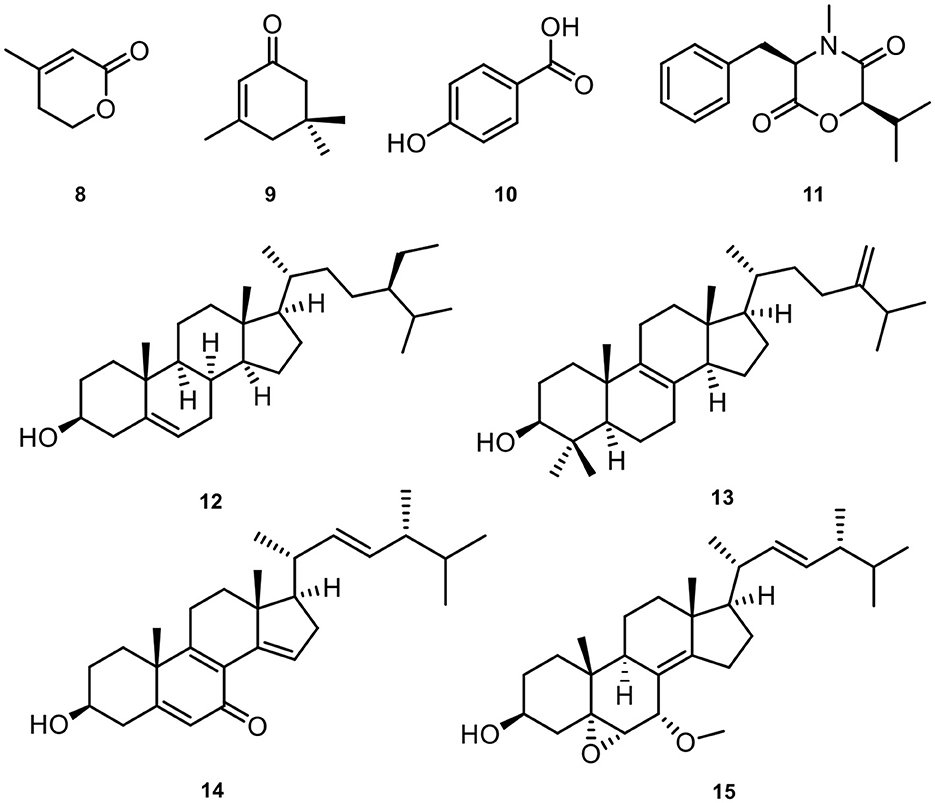
As a result, a new botryane sesquiterpene colletene A (1) and six known compounds 3,4-dihydroxybenzyl alcohol (2) (Du et al., 2011), 4β-acetoxy-9β,l0β,15α-trihydroxyprobotrydial (3) (Collado et al., 1995), O-methyldihydrobotrydial (4) (Kimura et al., 1988), botry-[1(10),5(9)-2H]-dien-[15-2H]-ol (5) (Daoubi et al., 2006), ergosta-4,6,8(14),22-tetraen-3-one (6) (Durán-Patrón et al., 2001), and 4β-acetoxyprobotryane-9β,15α-diol (7) (Wei et al., 2004) were successfully isolated from Colletotrichum sp. (Figure 3).
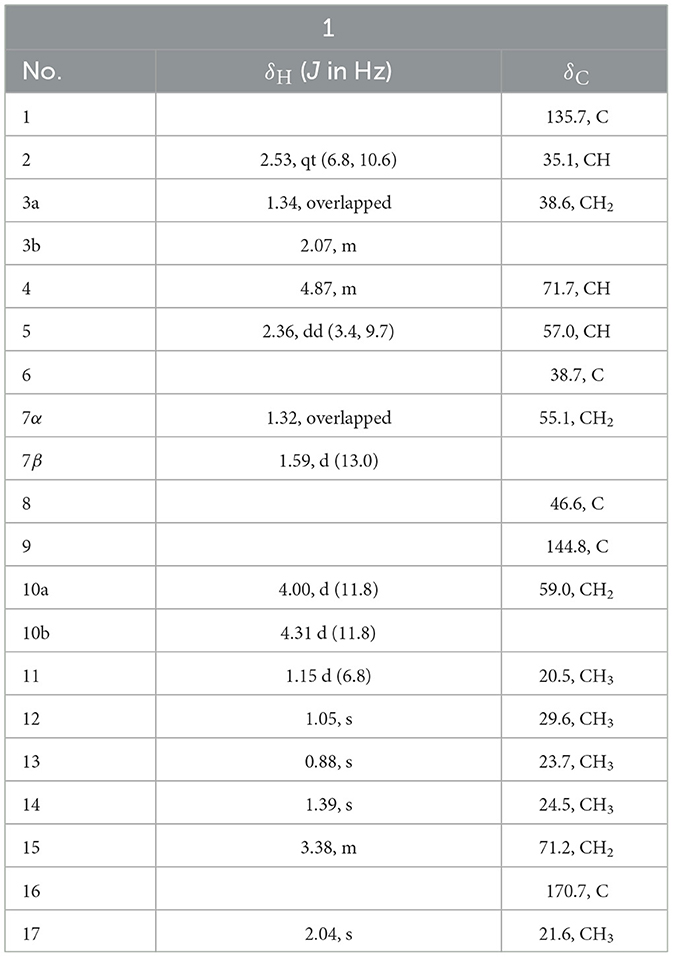
Colletene A (1) was obtained as a colorless oil with the chemical molecular formula of C17H28O4, which was successfully established on the basis of (+)-HRESIMS m/z 319.1887 [M + Na]+ (calcd for C17H28NaO4, 319.1880), implying four degrees of hydrogen deficiency. The 1H NMR data (Table 3) showed typical signals for five methyl groups [δH 0.88 (3H, s, H-12), 1.05 (3H, s, H-13), 1.15 (3H, d, H-11), 1.39 (3H, s, H-14), and 2.04 (3H, s, H-17)]. The 13C NMR and HSQC spectra of 1 revealed the presence of 17 carbon resonances, including five methyls (δC 20.5, 21.6, 23.7, 24.5, and 29.6), four methylenes involving two oxygen carbons (δC 38.6, 55.1, 59.0, and 71.2), three methines involving an oxygen carbon (δC 35.1, 57.0, and 71.7), and five non-protonated carbons (two quaternary carbons at δC 38.7 and 46.6, two olefinic carbons at δC 135.7 and 144.8, a carbonyl carbon at δC 170.7). These results strongly suggested that compound 1 might be a sesquiterpene derivative.
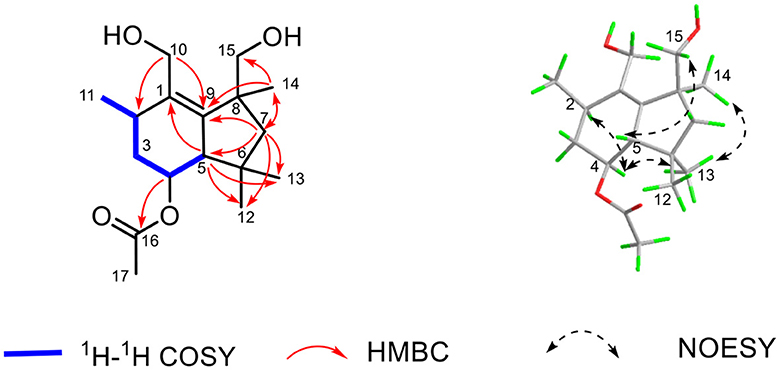
The established functional groups chemo-logically accounted for two degrees of hydrogen deficiency. Therefore, the remaining two degrees of hydrogen deficiency necessitated that compound 1 should possess a bicyclic ring system. In order to construct the bicyclic skeleton of compound 1, the 2D NMR spectra involving both the HMBC and 1H-1H COSY (Figure 4) spectra were performed and elucidated. First, the 1H-1H COSY correlations of H3-11/H-2/H2-3/H-4/H-5 informatively suggested the presence of one independent spin system. The key HMBC correlations from H2-10 to C-2 and C-9, H3-14 to C-7, C-9, and C-15, H-5 to C-1, C-12, and C-13, H2-7 to C-5, C-9, C-12, C-13, C-14, and C-15 could readily conclude the existence of rings A-B, which formatted as a 6/5 fused bicyclic ring system. The aforementioned result also coincides with its hydrogen deficiency. Moreover, the obvious HMBC correlation from H-4 to C-16 further confirmed that the carbonyl group should be attached at the C-4 position. Therefore, the planar structure of 1 was completely established, which suggested that compound 1 should be a new botryane sesquiterpene.
The relative configuration of 1 was determined by the careful analysis of its NOESY spectrum (Figure 4). The key NOESY signals between H-4 to H-2 and H3-13 as well as H3-13 to H3-14 informatively suggested that these protons were on the same side and assigned as α-orientation. Then, the NOESY correlations of H-5 to H2-15 demonstrated that these protons were β-orientated. Notably, the absolute configuration of 1 could not be determined because of its weak Cotton effects. Therefore, the chemical structure of 1 was finally established as depicted in Figure 4, and named as colletene A.
Meanwhile, eight known compounds were obtained from Diaporthe biconispora, including 3-methyl-2-penten-5-olide (8) (Shimomura et al., 2008), isophorone (9) (Tarantilis and Polissiou, 1997), p-hydroxy benzoic acid (10) (Boonyaketgoson et al., 2015), lateritin (11) (Hasumi et al., 1993), β-sitosterol (12) (Prakash and Chaturvedula, 2012), 4,4-dimethyl-5α-ergosta-8,24(28)-dien-3β-ol (13) (Fattorusso et al., 1992), 3β-hydroxyl-(22E, 24R)-ergosta-5,8,14,22-tetraen-7-one (14) (Wang et al., 2008), 22E-7α-methoxy-5α,6α-epoxyergosta-8(14),22-dien-3β-ol (15) (Gao et al., 2010). The chemical structures of the isolated natural products from D. biconispora are given in Figure 5.
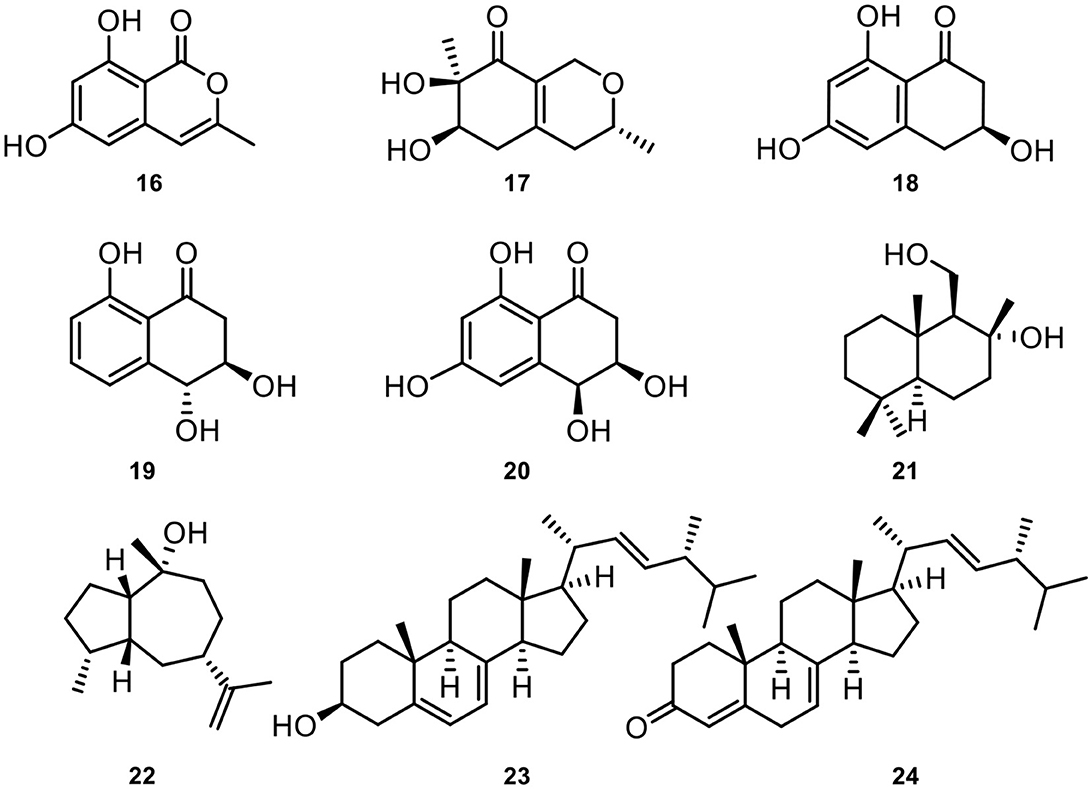
In addition, nine known compounds involving 6,8-dihydroxy-3-methylisocoumarin (16) (Feng et al., 2020), xylariphilone (17) (Arunpanichlert et al., 2016), scytalone (18) (Yang et al., 2020), trans-3,4-dihydro-3,4,8-trihydroxynaphthalen-1(2H)-one (19) (Couché et al., 2009), cis-4-hydroxyscytalone (20) (Couché et al., 2009), 8-hydroxyl-drimanol (21) (Li et al., 2018), pogostol (22) (Amand et al., 2012), ergosterol (23) (Li et al., 2014), (22E,24R)-ergosta-4,7,22-trien-3-one (24) (Zhang et al., 2009) from Annulohypoxylon atroroseum. The chemical structures of these isolated natural products could be seen in Figure 6.
All the isolated compounds were evaluated for antibacterial activities against SA, MRSA, E. coli, and Xcc. Notably, the phenolic compound 2 obtained from Colletotrichum sp. showed significant inhibitory effects toward all the tested bacterial strains of SA, MRSA, E. coli, and Xcc with MIC values of 12.5, 3.1, 125, and 12.5 μg/mL, respectively (Table 4). Satisfactorily, these results are consistent with our previous screening experiment for the EtOAc crude extract of Colletotrichum sp. Moreover, compounds 19 and 22 isolated from A. atroroseum also showed weak antibacterial activities against SA, MRSA, and Xcc.
4. Discussion
The fruits of Gannan navel orange contain abundant biologically meaningful chemical components with significant health benefits such as flavonoids (Tripoli et al., 2007), polyphenols (Hu et al., 2021), vitamins (Liu et al., 2010), essential oils (Bermejo et al., 2011), carotenoids (Yang C. et al., 2017), limonenes (Eldahshan and Halim, 2016), and other beneficial chemicals (Cai and Rui, 2013), which shared significantly pharmaceutical and nutritional bio-functions such as anti-cancer (Raghavan and Gurunathan, 2021), anti-oxidation (Zhu et al., 2018), antibacterial (Kawaguchi et al., 2004), lowering cholesterol level (Chau et al., 2004), and other related biological effects. Moreover, its peel has been evidenced to show very potent antimicrobial and anticancer activities (Yang C. et al., 2017; Guo et al., 2018), and it was widely used as healthy food supplants.
The significant health benefits and potent biological activities of Gannan navel orange suggested that its endophytic fungi might also be interesting. In the present study, a total of 54 endophytic fungi were isolated from Gannan navel orange fruits. Meanwhile, we also tested the antibacterial activity of the EtOAc extracts of most of the isolated endophytic fungi against Gram-negative bacterium E. coli, MRSA, and Xcc as three representative pathogenic bacteria for the first time. Therefore, this study will provide some valuable information for the wide utility of endophytic fungi of Gannan navel oranges and facilitate the development of the Gannan navel oranges industry as well.
In this study, we have successfully isolated and identified 17 strains of endophytic fungi from the Gannan navel orange (C. sinensis Osbeck cv. Newhall). To the best of our knowledge, this is the first comprehensive investigation on the isolation and identification of endophytic fungi from healthy Gannan navel orange tissues. As shown in Table 1, most of the endophytic fungi such as Fusarium solani (Kurt et al., 2020), Geotrichum sp. (Zhao et al., 2020), Fusarium proliferatum (Hyun et al., 2000), Diaporthe biconispora (Huang et al., 2015), Colletotrichum gloeosporioides (Ramos et al., 2016), Penicillium oxalicum (Kaur et al., 2020), Xylariaceae sp. (Ho et al., 2012), Colletotrichum truncatum (Cheng et al., 2013), Penicillium citrinum (Sadeghi et al., 2019), and Neofusicoccum parvum (Bezerra et al., 2021) have been previously discovered in the genus of Citrus as pathogenic fungi. Notably, the Polyporus arcularius, Penicillium paneum, Nigrospora chinensis, Nigrospora sphaerica, Annulohypoxylon atroroseum, and Fusarium graminearum were discovered from the family of Citrus for the first time, and they might be served as characteristic fungi of Gannan navel orange, although more evidences should be provided to support this statement in future study.
Moreover, this study has not only first investigated the endophytic fungi of Gannan navel orange, but also provided some valuable information for biologists to explore the potent relationship between the endophytic fungi and their host plants. The investigation of its rarely studied endophytic fungi might broaden the microcosmic knowledge and illustrate their pharmaceutical potency. Therefore, this study greatly enriched the species diversity of endophytic fungi of Gannan navel orange, and it laid a good foundation for the subsequent research on the endophytic resource of Gannan navel orange.
Endophytic fungi have been extensively investigated due to their remarkable ability for the production of a variety of secondary metabolites with pronounced pharmaceutical activity and interesting chemical structures (Newman and Cragg, 2020). In addition, endophytic fungi usually play important roles in the protection of host plants and produce different bioactive natural products under different certain conditions (Wang et al., 2023), which makes them much more interesting for natural product discovery and plant-microbial interaction (Schulz et al., 2002). In this study, we tested the inhibitory effect of secondary metabolites of endophytic fungi of navel orange against three different pathogens, and many of them showed considerable antimicrobial effects. The results strongly suggested that the endophytic fungal types of Gannan navel orange are rich and diverse. Moreover, this study also provides scientific data for the exploitation of endophytic fungal resources of Gannan navel orange. Metabolites from endophytic fungi have great potential for the discovery of pharmaceutical agents, and the present study provided important information for the discovery of antibacterial natural compounds from the endophytic fungi of Gannan navel orange.
In particular, many countries have encountered huge economic losses because of the severity of CBC caused by Xcc. The traditional treatment of the disease with copper-based chemical sprays and streptomycin has led to the emergence of resistant Xanthomonas strains (Martins et al., 2020). The development of alternative biocontrol agents has now become a major challenge for scientists. In the antibacterial activity screening, the crude extracts of Colletotrichum sp., D. biconispora have better inhibitory effects against Xcc. Therefore, we have performed the chemical composition investigation and an anti-Xcc assay of individual compounds isolated from Colletotrichum sp. and D. biconispora. The active compound 2 might be responsible for the antibacterial effects of the EtOAc extracts. The current results suggested that it might be a prospective and promising way to discover anti-Xcc pesticides from Citrus plants itself based on the protective effect of endophytic fungi on the host plant.
Furthermore, we also selected A. atroroseum for the chemical study, which successfully resulted in the isolation of nine compounds involving isocoumarin, azaphilones, tetralones, and steroids, but none of these compounds showed significant antibacterial activity against SA, MRSA, and E. coli. More bioassay-guided isolation strategies should be performed in our future study to identify highly potent antibacterial compounds from the isolated endophytes. Moreover, the biological evaluations of Gannan navel orange endophytic fungi in this study were limited by the experimental platforms, other biological activities such as the antiviral, anti-inflammatory, antifungal, and various enzyme inhibitory effects should be carried out to fully illustrate the pharmaceutical potential of endophytic fungi of Gannan navel orange in future.
5. Summary
In conclusion, the identification of endophytic fungi from Gannan navel orange, the antibacterial screening of their crude fermentation products, and the isolation of bioactive compounds from the EtOAc extracts of two bioactive endophytic fungi were carried out in the present study. A total of 54 strains belonging to 17 species of 12 genera were successfully isolated from Gannan navel orange for the first time. The EtOAc extract of Fusarium proliferatum (gc-1-128-74-1) exhibited excellent antibacterial activity against E. coli, and the EtOAc extracts of both Geotrichum sp. (gc-1-127-30) and Diaporthe biconispora (gc-1-128-79) demonstrated significant antibacterial activity against Xcc. In addition, The MIC value of the EtOAc extract of Colletotrichum gloeosporioides against MRSA was comparable to the positive control vancomycin. Furthermore, 24 compounds involving a new compound were isolated from the EtOAc extracts of Colletotrichum sp., D. biconispora, and A. atroroseum. In addition, compound 2 showed significant inhibitory activities toward SA, MRSA, E. coli, and Xcc with MIC values of 12.5, 3.1, 125, and 12.5 μg/mL. The present results indicated that the endophytic fungi of Gannan navel orange were abundant and had great potential to produce antibacterial compounds against E. coli, SA, MRSA, and Xcc deserving more attention in future studies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
Author contributions
HW conducted the experiment and collected the experimental data. ZL guided the molecular experimental and phylogenetic analysis. YC and KQ evaluated the activities of the extracts. FD and QX revised the manuscript. HL performed the experiments of compounds isolation. JZ and HT conceived and designed the experiments. All authors contributed to the article and approved the submitted version.
Funding
Financial support for this research was provided by the National Natural Science Foundation of China (82173711), Youth Innovation Promotion Association of CAS (2020342), and the Foundation of Key Laboratory of Plant Resources Conservation and Sustainable Utilization, South China Botanical Garden, Chinese Academy of Sciences.
Acknowledgments
The authors would like to thank Dr. Shuo Duan for providing us with Xanthomonas citri subsp. citri as an experimental strain, and the undergraduate students in our lab, Lu Yang, Shaomin Yu, Lingfeng Liu, Xiangyang He, Huiting Yan, Chun Xu, and Zhikun Xu for their contributions to the fermentation and activity experiments of the strains.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1172629/full#supplementary-material
References
Abdalla, M. A., and Matasyoh, J. C. (2014). Endophytes as producers of peptides: an overview about the recently discovered peptides from endophytic microbes. Nat. Prod. Bioprospect. 4, 257–270. doi: 10.1007/s13659-014-0038-y
Amand, S., Langenfeld, A., Blond, A., Dupont, J., Nay, B., and Prado, S. (2012). Guaiane sesquiterpenes from Biscogniauxia nummularia featuring potent antigerminative activity. J. Nat. Prod. 75, 798–801. doi: 10.1021/np2009913
Arunpanichlert, J., Rukachaisirikul, V., Phongpaichit, S., Supaphon, O., and Sakayaroj, J. (2016). Xylariphilone: a new azaphilone derivative from the seagrass-derived fungus Xylariales sp. PSU-ES163. Nat. Prod. Res. 30, 46–51. doi: 10.1080/14786419.2015.1032282
Bermejo, A., Llosa, M. J., and Cano, A. (2011). Analysis of bioactive compounds in seven citrus cultivars. Food. Sci. Technol. Int. 17, 55–62. doi: 10.1177/1082013210368556
Bezerra, J. D. P., Crous, P. W., Aiello, D., Gullino, M. L., Polizzi, G., and Guarnaccia, V. (2021). Genetic diversity and pathogenicity of Botryosphaeriaceae species associated with symptomatic citrus plants in Europe. Plants (Basel) 10, 492. doi: 10.3390/plants10030492
Boonyaketgoson, S., Trisuwan, K., Bussaban, B., Rukachaisirikul, V., and Phongpaichit, S. (2015). Isochromanone derivatives from the endophytic fungus Fusarium sp. PDB51F5. Tetrahedron Lett. 56, 5076–5078. doi: 10.1016/j.tetlet.2015.07.048
Burland, T. G. (1999). “DNASTAR's lasergene sequence analysis software,” in Bioinformatics Methods and Protocols, eds. S. Misener and S.A. Krawetz. (Totowa, NJ: Humana Press) 71–91. doi: 10.1385/1-59259-192-2:71
Cabral, D., Cafaro, M. J., Saidman, B., Lugo, M., Reddy, P. V., and White, J. F. (1999). Evidence supporting the occurrence of a new species of endophyte in some South American grasses. Mycologia 91, 315–325. doi: 10.1080/00275514.1999.12061021
Cai, D. J., and Rui, Y. K. (2013). Determination of trace elements and rare earth elements in Gannan navel orange fruit by ICP-MS. Asian J. Chem. 25, 579–580. doi: 10.14233/ajchem.2013.13085
Chau, C. F., Huang, Y. L., and Lin, C. Y. (2004). Investigation of the cholesterol-lowering action of insoluble fibre derived from the peel of Citrus sinensis L. cv. Liucheng. Food Chem. 87, 361–366. doi: 10.1016/j.foodchem.2003.12.006
Chen, C. X., Zheng, J. H., Xie, J. Z., Xie, X. T., and Mao, R. Q. (2009). Pest management based on petroleum spray oil in navel orange orchard in Ganzhou, South China. J. Pet. Sci. 82, 155–162. doi: 10.1007/s10340-008-0234-9
Cheng, B. P., Huang, Y. H., Song, X. B., Peng, A. T., Ling, J. F., and Chen, X. (2013). First report of Colletotrichum siamense causing leaf drop and fruit spot of Citrus reticulata Blanco cv. Shiyue Ju in China. Plant Dis. 97, 1508. doi: 10.1094/PDIS-04-13-0352-PDN
Chowdhury, F., Khan, I. A., Patel, S., Siddiq, A. U., Saha, N. C., Khan, A. I., et al. (2015). Diarrheal illness and healthcare seeking behavior among a population at high risk for Diarrhea in Dhaka, Bangladesh. PLoS ONE 10, e0130105. doi: 10.1371/journal.pone.0130105
Collado, I. G., Hernández-Galán, R., Durán-Patrón, R., and Cantoral, J. M. (1995). Metabolites from a shake culture of Botrytis cinerea. Phytochemistry 38, 647–650. doi: 10.1016/0031-9422(94)00690-U
Collinge, D. B., Jensen, B., and Jørgensen, H. J. L. (2022). Fungal endophytes in plants and their relationship to plant disease. Curr. Opin. Microbiol. 69, 102177. doi: 10.1016/j.mib.2022.102177
Couché, E., Fkyerat, A., and Tabacchi, R. (2009). Stereoselective synthesis ofcis-andtrans-3,4-Dihydro-3,4,8-trihydroxynaphthalen-1(2H)-one. Helv. Chim. Acta. 92, 903–917. doi: 10.1002/hlca.200800380
Cruz, J. S., da Silva, C. A., and Hamerski, L. (2020). Natural products from endophytic fungi associated with Rubiaceae species. J. Fungi 6, 128. doi: 10.3390/jof6030128
Daoubi, M., Durán-Patrón, R., Hernández-Galán, R., Benharref, A., Hanson, J. R., and Collado, I. G. (2006). The role of botrydienediol in the biodegradation of the sesquiterpenoid phytotoxin botrydial by Botrytis cinerea. Tetrahedron 62, 8256–8261. doi: 10.1016/j.tet.2006.06.064
Du, X., Zhao, B., Zheng, Z., Xu, Q., and Su, W. (2011). Study on the isolation identification and antitumor activity of a phenol derivative from mangrove fungus BYY-1. J. Jimei Univ. 16, 424–428. doi: 10.19715/j.jmuzr.2011.06.005
Durán-Patrón, R., Colmenares, A. J., Hernández-Galán, R., and Collado, I. G. (2001). Some key metabolic intermediates in the biosynthesis of botrydial and related compounds. Tetrahedron 57, 1929–1933. doi: 10.1016/S0040-4020(01)00016-3
Eldahshan, O. A., and Halim, A. F. (2016). Comparison of the composition and antimicrobial activities of the essential oils of green branches and leaves of Egyptian navel orange (Citrus sinensis (L.) Osbeck var. malesy). Chem. Biodivers. 13, 681–685. doi: 10.1002/cbdv.201500139
Fan, Y. Q., Ma, Z. H., Zhang, Y., Wang, Y. F., Ding, Y. S., Wang, C., et al. (2022). Sulfur-containing compounds from endophytic fungi: sources, structures and bioactivities. J. Fungi 8, 628. doi: 10.3390/jof8060628
Fattorusso, E., Giovannitti, B., Lanzotti, V., Magno, S., and Violante, U. (1992). 4,4-dimethyl-5α-ergosta-8,24(28)-dien-3β-ol from the fungus Marasmius oreades. Steroids 57, 119–121. doi: 10.1016/0039-128X(92)90069-L
Felsenstein, J. (1985). Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39, 783–791. doi: 10.2307/2408678
Feng, L. X., Zhang, B. Y., Zhu, H. J., Pan, L., and Cao, F. (2020). Bioactive metabolites from Talaromyces purpureogenus, an endophytic fungus from Panax notoginseng. Chem. Nat. Comp. 56, 974–976. doi: 10.1007/s10600-020-03206-9
Gao, H., Hong, K., Chen, G. D., Wang, C. X., Tang, J. S., Yu, Y., et al. (2010). New oxidized sterols from Aspergillus awamori and the endo-boat conformation adopted by the cyclohexene oxide system. Magn. Reson. Chem. 48, 38–43. doi: 10.1002/mrc.2536
Guo, Q., Liu, K., Deng, W., Zhong, B., Yang, W., and Chun, J. (2018). Chemical composition and antimicrobial activity of Gannan navel orange (Citrus sinensis Osbeck cv. Newhall) peel essential oils. Food Sci. Nutr. 6, 1431–1437. doi: 10.1002/fsn3.688
Hall, T. (2011). BioEdit: An important software for molecular biology. Gerf. Bull. Biosci. 2, 60–61.
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41, 95–98.
Hasumi, K., Shinohara, C., Iwanaga, T., and Endo, A. (1993). Lateritin, a new inhibitor of acyl-CoA:cholesterol acyltransferase produced by Gibberella lateritium IFO 7188. J. Antibiot. 46, 1782–1787. doi: 10.7164/antibiotics.46.1782
Ho, M., Chung, W., Huang, H., Chung, W., and Chung, W. (2012). Identification of endophytic fungi of medicinal herbs of Lauraceae and Rutaceae with antimicrobial property. Taiwania 57, 229–241. doi: 10.6165/tai.2012.57(3).229
Hu, M. Y., Zhang, L., Ruan, Z., Han, P. H., and Yu, Y. (2021). The regulatory effects of citrus peel powder on liver metabolites and gut flora in mice with non-alcoholic fatty liver disease (NAFLD). Foods 10, 3022. doi: 10.3390/foods10123022
Huang, F., Udayanga, D., Wang, X., Hou, X., Mei, X., Fu, Y., et al. (2015). Endophytic Diaporthe associated with Citrus: a phylogenetic reassessment with seven new species from China. Fungal Biol. 119, 331–347. doi: 10.1016/j.funbio.2015.02.006
Hyun, J. W., Lee, S. C., Kim, D. H., Ko, S. W., and Kim, K. S. (2000). Fusarium fruit rot of citrus in Jeju Island. Mycobiology 28, 158–162. doi: 10.1080/12298093.2000.12015743
Indhuprakash, S. T., Karthikeyan, M., Gopal, G., Ambi, S. V., Sekaran, S., Palaniappan, B., et al. (2021). Antibody therapy against antibiotic-resistant diarrheagenic Escherichia coli: a systematic review. Immunotherapy 13, 1305–1320. doi: 10.2217/imt-2021-0079
Kaur, R., Kaur, J., Kaur, M., Kalotra, V., Chadha, P., and Kaur, A. (2020). An endophytic Penicillium oxalicum isolated from Citrus limon possesses antioxidant and genoprotective potential. J. Appl. Microbiol. 128, 1400–1413. doi: 10.1111/jam.14553
Kawaguchi, K., Kikuchi, S., Hasunuma, R., Maruyama, H., Ryll, R., and Kumazawa, Y. (2004). Suppression of infection-induced endotoxin shock in mice by a Citrus flavanone naringin. Planta Med. 70, 17–22. doi: 10.1055/s-2004-815449
Kimura, Y., Fujioka, H., Nakajima, H., Hamasaki, T., and Isogai, A. (1988). Isolation and structures of O-methyldihydrobotrydial and deacetyl-O-methyldihydrobotrydialone produced by Botrytis squamosa. Agric. Biol. Chem. 52, 1845–1847. doi: 10.1271/bbb1961.52.1845
Kurt, S., Uysal, A., Soylu, E. M., Kara, M., and Soylu, S. (2020). Characterization and pathogenicity of Fusarium solani associated with dry root rot of citrus in the eastern Mediterranean region of Turkey. J. Gen. Plant Pathol. 86, 326–332. doi: 10.1007/s10327-020-00922-6
Landeweert, R., Leeflang, P., Kuyper, T. W., Hoffland, E., Rosling, A., and Wernars (2003). Molecular identification of ectomycorrhizal mycelium in soil horizons. Appl. Environ. Microbiol. 69, 327–333. doi: 10.1128/AEM.69.1.327-333.2003
Li, D., Zhang, S., Song, Z., Li, W., Zhu, F., Zhang, J., et al. (2018). Synthesis and bio-inspired optimization of drimenal: discovery of chiral drimane fused oxazinones as promising antifungal and antibacterial candidates. Eur. J. Med. Chem. 143, 558–567. doi: 10.1016/j.ejmech.2017.11.051
Li, G. R., Cao, B. H., Liu, W., Ren, R. H., Feng, J., and Lv, D. J. (2020). Isolation and identification of endophytic fungi in Kernels of Coix lachrymal-jobi L. Cultivars. Curr. Microbiol. 77, 1448–1456. doi: 10.1007/s00284-020-01950-3
Li, W., Zhou, W., Lee, D.-S., Shim, S. H., Kim, Y.-C., and Kim, Y. H. (2014). Hericirine, a novel anti-inflammatory alkaloid from Hericium erinaceum. Tetrahedron Lett. 55, 4086–4090. doi: 10.1016/j.tetlet.2014.05.117
Liu, Y. D., Sun, X. D., Zhou, J. M., Zhang, H. L., and Yang, C. (2010). Linear and nonlinear multivariate regressions for determination sugar content of intact Gannan navel orange by Vis–NIR diffuse reflectance spectroscopy. Math. Comput. Modell. 51, 1438–1443. doi: 10.1016/j.mcm.2009.10.003
Manzur, M. E., Garello, F. A., Omacini, M., Schnyder, H., Sutka, M. R., and Garcia-Parisi, P. A. (2022). Endophytic fungi and drought tolerance: ecophysiological adjustment in shoot and root of an annual mesophytic host grass. Funct. Plant Biol. 49, 272–282. doi: 10.1071/FP21238
Martins, P. M. M., de Oliveira Andrade, M., Benedetti, C. E., and de Souza, A. A. (2020). Xanthomonas citri subsp. citri: host interaction and control strategies. Trop. Plant Pathol. 45, 213–236. doi: 10.1007/s40858-020-00376-3
Mattoo, A. J., and Nonzom, S. (2021). Endophytic fungi: understanding complex cross-talks. Symbiosis 83, 237–264. doi: 10.1007/s13199-020-00744-2
Newman, D. J., and Cragg, G. M. (2020). Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 83, 770–803. doi: 10.1021/acs.jnatprod.9b01285
Prakash, I., and Chaturvedula, V. S. P. (2012). Isolation of stigmasterol and β-Sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharmaceut. J. 1, 239–242. doi: 10.3329/icpj.v1i9.11613
Raghavan, S., and Gurunathan, J. (2021). Citrus species – a golden treasure box of metabolites that is beneficial against disorders. J. Herb. Med. 28, 100438. doi: 10.1016/j.hermed.2021.100438
Ramos, A. P., Talhinhas, P., Sreenivasaprasad, S., and Oliveira, H. (2016). Characterization of Colletotrichum gloeosporioides, as the main causal agent of citrus anthracnose, and C. karstii as species preferentially associated with lemon twig dieback in Portugal. Phytoparasitica 44, 549–561. doi: 10.1007/s12600-016-0537-y
Rebecca, G. (2008). The risks of pigging out on antibiotics. Science 321, 1294. doi: 10.1126/science.321.5894.1294a
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Sadeghi, F., Samsampour, D., Seyahooei, M. A., Bagheri, A., and Soltani, J. (2019). Diversity and spatiotemporal distribution of fungal endophytes associated with Citrus reticulata cv. Siyahoo. Curr. Microbiol. 76, 279–289. doi: 10.1007/s00284-019-01632-9
Schulz, B., Boyle, C., Draeger, S., Römmert, A.-K., and Krohn, K. (2002). Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol. Res. 106, 996–1004. doi: 10.1017/S0953756202006342
Shimomura, H., Sashida, Y., Mimaki, Y., Adachi, T., and Yoshinari, K. (2008). A new mevalonolactone glucoside derivative from the bark of Prunus buergeriana. Chem. Pharm. Bull. 37, 829–830. doi: 10.1248/cpb.37.829
Tarantilis, P. A., and Polissiou, M. G. (1997). Isolation and identification of the aroma components from saffron (Crocus sativus). J. Agric. Food. Chem. 45, 459–462. doi: 10.1021/jf960105e
Tripoli, E., Guardia, M. L., Giammanco, S., Majo, D. D., and Giammanco, M. (2007). Citrus flavonoids: molecular structure, biological activity and nutritional properties: a review. Food Chem. 104, 466–479. doi: 10.1016/j.foodchem.2006.11.054
Wang, F. Z., Fang, Y. C., Zhang, M., Lin, A. Q., Zhu, T. J., Gu, Q. Q., et al. (2008). Six new ergosterols from the marine-derived fungus Rhizopus sp. Steroids 73, 19–26. doi: 10.1016/j.steroids.2007.08.008
Wang, M. M., Huo, L. Q., Liu, H. X., Zhao, L. Y., Xu, Z. F., Tan, H. B., et al. (2022). Thujasutchins N and O, two new compounds from the stems and roots of Thuja sutchuenensis. Nat. Prod. Res. 36, 2356–2362. doi: 10.1080/14786419.2020.1836627
Wang, Z. C., Wang, L., Pan, Y. P., Zheng, X. X., Liang, X. N., Sheng, L. L., et al. (2023). Research advances on endophytic fungi and their bioactive metabolites. Bioproc. Biosyst. Eng. 46, 165–170. doi: 10.1007/s00449-022-02840-7
Wei, H., Itoh, T., Kinoshita, M., Nakai, Y., Kurotaki, M., and Kobayashi, M. (2004). Cytotoxic sesterterpenes, 6-epi-ophiobolin G and 6-epi-ophiobolin N, from marine derived fungus Emericella variecolor GF10. Tetrahedron 60, 6015–6019. doi: 10.1016/j.tet.2004.05.021
Yang, C., Chen, H., Chen, H. L., Zhong, B. L., Luo, X. Z., and Chun, J. (2017). Antioxidant and anticancer activities of essential oil from Gannan navel orange peel. Molecules 22, 1–10. doi: 10.3390/molecules22081391
Yang, N. N., Ma, Q. Y., Kong, F. D., Xie, Q. Y., Dai, H. F., Zhou, L. M., et al. (2020). Napthrene compounds from mycelial fermentation products of Marasmius berteroi. Molecules 25, 3898. doi: 10.3390/molecules25173898
Yang, S. C., Lin, C. H., Aljuffali, I. A., and Fang, J. Y. (2017). Current pathogenic Escherichia coli foodborne outbreak cases and therapy development. Arch. Microbiol. 199, 811–825. doi: 10.1007/s00203-017-1393-y
Zhang, J., He, L., Guo, C., Liu, Z. Y., Kaliaperumal, K., Zhong, B., et al. (2022a). Evaluation of Aspergillus aculeatus GC-09 for the biological control of citrus blue mold caused by Penicillium italicum. Fungal Biol. 126, 201–212. doi: 10.1016/j.funbio.2021.12.006
Zhang, J., Zhang, J. Y., Shan, Y. X., Guo, C., He, L., Zhang, L. Y., et al. (2022b). Effect of harvest time on the chemical composition and antioxidant capacity of Gannan navel orange (Citrus sinensis L. Osbeck ‘Newhall') juice. J. Integr. Agric. 21, 261–272. doi: 10.1016/S2095-3119(20)63395-0
Zhang, W., Draeger, S., Schulz, B., and Krohn, K. (2009). Ring B aromatic steroids from an endophytic fungus, Colletotrichum sp. Nat. Prod. Commun. 4, 1934578X0900401101. doi: 10.1177/1934578X0900401101
Zhao, J., Zhang, D. Y., Wang, Z., Tian, Z. H., Yang, F., Lu, X. J., et al. (2020). Genome sequencing and transcriptome analysis of Geotrichum citri-aurantii on citrus reveal the potential pathogenic- and guazatine-resistance related genes. Genomics 112, 4063–4071. doi: 10.1016/j.ygeno.2020.07.013
Keywords: endophytic fungus, Gannan navel orange, antibacterial activity, fungal diversity, secondary metabolites
Citation: Wang H, Liu Z, Duan F, Chen Y, Qiu K, Xiong Q, Lin H, Zhang J and Tan H (2023) Isolation, identification, and antibacterial evaluation of endophytic fungi from Gannan navel orange. Front. Microbiol. 14:1172629. doi: 10.3389/fmicb.2023.1172629
Received: 23 February 2023; Accepted: 23 May 2023;
Published: 15 June 2023.
Edited by:
Yongbo Xue, Sun Yat-sen University, ChinaReviewed by:
Fei He, Southern Medical University, ChinaTao Feng, South-Central University for Nationalities, China
Copyright © 2023 Wang, Liu, Duan, Chen, Qiu, Xiong, Lin, Zhang and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Zhang, YnJpNzE1MjcxNTJAb3V0bG9vay5jb20=; Haibo Tan, dGFuaGFpYm9Ac2NiZy5hYy5jbg==
†These authors have contributed equally to this work and share first authorship
 Huan Wang1,2†
Huan Wang1,2† Jun Zhang
Jun Zhang Haibo Tan
Haibo Tan