
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 10 July 2023
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1163269
This article is part of the Research TopicThe Origin and Establishment Process of Gut Microbiota in Early LifeView all 18 articles
 Yu Liu1,2
Yu Liu1,2 Jingmei Ma1,2
Jingmei Ma1,2 Baoli Zhu3
Baoli Zhu3 Fei Liu3
Fei Liu3 Shengtang Qin1,2
Shengtang Qin1,2 Na Lv3
Na Lv3 Ye Feng1,2
Ye Feng1,2 Shuxian Wang1,2
Shuxian Wang1,2 Huixia Yang1,2*
Huixia Yang1,2*The establishment of human gut microbiota in early life is closely associated with both short- and long-term infant health. Delivery mode and feeding pattern are two important determinants of infant gut microbiota. In this longitudinal cohort study, we examined the interplay between the delivery mode and feeding pattern on the dynamics of infant gut microbiota from 6 weeks to 6 months post-delivery in 139 infants. We also assessed the relationship between infant respiratory infection susceptibility and gut microbial changes associated with delivery mode and feeding pattern. At 6 weeks postpartum, the composition and structure of gut microbiota of cesarean section-delivered (CSD) infants differed from those of vaginally delivered (VD) infants, with decreased Bacteroides and Escherichia-Shigella and increased Klebsiella, Veillonella, and Enterococcus. At 6 months postpartum, these delivery mode-induced microbial shifts were restored by exclusive breastfeeding, resulting in similar gut microbial profiles between VD and CSD infants who were exclusively breastfed (P = 0.57) and more variable gut microbial profiles between VD and CSD infants who were mixed fed (P < 0.001). We identified that the VD-associated genera were enriched in healthy infants, while the CSD-associated genera were enriched in infants who suffered from respiratory infections. Our findings indicate that exclusive breastfeeding may play a health-promoting role by reducing infant respiratory infection susceptibility through the restoration of gut microbiota perturbations caused by cesarean section.
The gut microbiota is regarded as a complex and dynamic organ that interacts with the host metabolic pathways, immune responses, and developmental processes, influencing long-term host health (Tamburini et al., 2016; Robertson et al., 2019; Brodin, 2022). During the first years of life, the human gut microbiota develops toward an adult-like community by 2–3 years old, exhibiting a highly stage-specific progression (Stewart et al., 2018). The maturation of gut microbiota is shaped by numerous perinatal factors, including delivery mode (Reyman et al., 2019; Shao et al., 2019; Song et al., 2021), feeding pattern (Stewart et al., 2018; Fehr et al., 2020), antibiotic exposure (Bokulich et al., 2016), and gestational age (Fouhy et al., 2019). Recent longitudinal cohort studies have proven that delivery mode is a major determinant of gut microbiota during the first weeks of life, with cesarean section delivery (CSD) disrupting the natural transmission of gut microbiota from mothers to offspring (Shao et al., 2019). Specifically, the enrichment of Bifidobacterium, Escherichia, Bacteroides, and Parabacteroides in vaginally delivered (VD) infants can promote human milk oligosaccharide (HMO) utilization (Wang et al., 2019) and immune stimulation in early life (Jakobsson et al., 2014; Wampach et al., 2018). In contrast, the gut microbiota of infants delivered by cesarean section is dominated by Enterococcus, Staphylococcus, Streptococcus, and Klebsiella, which are associated with the hospital environment (Lax et al., 2017).
A growing body of evidence shows that cesarean section delivery is associated with adverse effects on infant and child immune development (Pattaroni et al., 2018), resulting in higher rates of asthma (Roduit et al., 2009; Tang et al., 2021) and respiratory infections (RIs) as morbidities (Bosch et al., 2016; Baumfeld et al., 2018; Reyman et al., 2019). A reduction in health-promoting Bifidobacterium and an enrichment in potentially pathogenic Enterococcus and Klebsiella in cesarean section-delivered infants have been linked with more RIs during the first year of life (Reyman et al., 2019), indicating a mediating role of gut microbial changes in the cesarean delivery-induced disease susceptibility.
The effect of the delivery mode gradually diminishes by 6 months of life (Hill et al., 2017) or earlier (Reyman et al., 2019), indicating that the gut microbiota could recover from the state associated with cesarean section delivery. Our previous study demonstrated that shifts in infant gut microbiota associated with cesarean section delivery were alleviated by exclusive breastfeeding (EBF) in the first several weeks of life, suggesting an interactive impact on feeding pattern and the delivery mode of gut microbiota (Liu et al., 2019). Additionally, prolonged breastfeeding duration influenced the gut microbiota of CSD infants but not VD infants at 24 weeks of age, indicating that CSD infants may benefit from breastmilk by obtaining specific bacteria, initially lacking due to delivery mode (Hill et al., 2017). The gut microbiota of exclusively breastfed and formula-fed infants remain distinct (Backhed et al., 2015b), even when specific components are added to the formula to promote breastfed-like microbial communities (Baumann-Dudenhoeffer et al., 2018), demonstrating that the nutritional and immune benefits of breastfeeding were indispensable (Gopalakrishna and Hand, 2020; Gridneva et al., 2021; Donald et al., 2022).
However, few studies have explored the relationship between infant respiratory health and gut microbiota shifts associated with delivery mode and feeding pattern. In this prospective cohort study containing 139 infants, we investigated the health-promoting role of exclusive breastfeeding on infant gut microbiota shifts induced by cesarean section, which were associated with reduced respiratory infection susceptibility during the first months of life.
Written informed consent was obtained from the legal guardian for the publication of any potentially identifiable data included in this article.
This ongoing prospective cohort study has been conducted at Peking University First Hospital since October 2017, which recruited 139 infants at 6 weeks postpartum, and 72.7% (101/139) of them completed the 6-month follow-up. This study was approved by the Institutional Ethics Committee of Peking University First Hospital (V2.0/201504.20), and all the participants or legal guardians provided written informed consent.
Prenatal and perinatal information was obtained from electronic medical records and questionnaire surveys (Supplementary Table 1), including maternal age, gravidity, parity, pre-pregnancy body mass index (BMI), delivery mode, gestational age, infant sex, birth weight, feeding patterns, and the occurrence of respiratory infection (RI) events during the first 6 months of age. Considering the confounding effect of delivery mode, we also recorded the type (labored or elective) and cause of cesarean section. EBF is defined as feeding with breast milk exclusively after birth. MF is defined as feeding with a mixture of varying proportions of breast milk and formula milk. RI events are defined as the occurrence of the following mother-reported symptoms: pneumonia, bronchitis, or fever (>38°C) accompanied by snuffling, sneezing, coughing, or wheezing.
A total of 139 infants were collected at 6 weeks, with 101 infants longitudinally collected at 6 months. Fresh stool samples were self-collected at home, according to the standardized protocol, as described in a previous study (Liu et al., 2019). All stool samples were frozen at −80°C within 2 h. DNA was extracted with the QIAamp PowerFecal DNA Kit (Qiagen, Hilden, Germany), following the manufacturer's protocols. The V3-V4 region of the 16S rRNA gene was amplified by polymerase chain reaction (PCR) with 341 forward primers (5′ CCTACGGGNBGCASCAG) and 805 reverse primers (5′ GACTACNVGGGTATCTAATCC). PCR was performed in a 25-μl volume with 1 U of HiFi HotStart DNA Polymerase (KK2502, Kapa Biosystems, Cape Town, South Africa), 12.5 ng of template DNA, and 5 μM of forward and reverse primers with the following amplification program: initial denaturation at 95°C for 3 min; 25 cycles of denaturation at 95°C for 30 s, annealing at 55°C for 30 s, and extension at 72°C for 30 s; and final extension at 72°C for 5 min and hold at 4°C. AMPure XP beads (A63882, Beckman) were used to purify the 16S V3-V4 amplicons away from the free primers and primer dimers.
For Illumina sequencing adapter attachment, PCR was performed for a second time with Illumina sequencing primers under the same conditions as the first time, only with seven cycles and an annealing step increased to 1 min. DNA libraries were quantified with a NanoDrop 2000 system and purified with AMPure XP beads before sequencing on the Illumina HiSeq 2500 platform. Fast Length Adjustment of Short Reads (FLASH) was used to merge paired-end reads from sequencing (Magoc and Salzberg, 2011). Low-quality reads were filtered with the FASTQ quality filter (-p 90 -q 25 -Q33) using the FASTX Toolkit 0.0.14, and chimeric reads were removed by USEARCH 64-bit v8.0.1517. The number of reads for each sample was normalized based on the smallest sample size by random subtraction.
The 16s rRNA sequencing data were processed by Quantitative Insights Into Microbial Ecology (QIIME). Operational taxonomic units (OTUs) were aligned by the UCLUST algorithm with 97% identity and taxonomically classified using the SILVA 16S rRNA database v128. The bacterial compositions were visualized with a heatmap, which was generated via Seaborn, a Python data visualization library. Alpha diversity was evaluated by Shannon and Simpson indexes. Beta diversity was calculated based on weighted UniFrac and Bray–Curtis distance matrices and visualized by principal coordinates analysis (PCoA) of weighted UniFrac and Bray–Curtis distance matrices, in 1,000 permutations, and statistical comparisons of groups were calculated by multivariate permutational analysis of variance (PERMANOVA) methods using the Adonis function in the R package “vegan.” Metric variables are shown as the mean ± SD or median (interquartile range) and compared with Student's t-test or Mann–Whitney U-test, according to the normality of the data distribution. The Chi-squared and Fisher's exact tests were used to compare the proportions of analyses. The significance was set at a P-value of < 0.05. GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA) was used for statistical and graphical preparation.
In this longitudinal study, stool samples were collected from each individual at ~6 weeks postpartum (6W, n = 139) and 6 months postpartum (6M, n = 101), and some of the individuals had been included in our previous study (Liu et al., 2019). Among 139 infants, 93 (66.9%) infants were born by vaginal delivery, and 46 infants were born by cesarean section delivery. Among 101 infants, 71 (70.1%) infants were born by vaginal delivery, and 30 infants were born by cesarean section delivery. Basic characteristics at 6W postpartum and 6M postpartum stratified by delivery mode, are shown in Table 1. Clinical variables were similar between the VD and CSD groups, with the exception of gestational age (P < 0.001 at 6W; P = 0.03 at 6M), which was intrinsically related to the delivery mode. Notably, VD infants were more likely to receive exclusive breastfeeding; this difference persisted to 6M, although significance was not reached. The occurrence rate of RI over the first 6 months was significantly higher in CSD infants than in VD infants (P = 0.02).
In total, 38,955,382 bacterial reads were identified in 240 fecal samples, which were annotated into 654 OTUs distributed over 8 bacterial phyla. Firmicutes (43.9%) was the most abundant phylum, followed by Actinobacteria (21.9%), Proteobacteria (21.2%), and Bacteroidetes (12.6%).
We first conducted a chronological comparison of infant gut microbial composition and community structure between 6 weeks and 6 months of age. No significant difference was found in alpha diversity between the 6W and 6M groups, according to either the Shannon index (Figure 1A, P = 0.41) or the Simpson index (Figure 1B, P = 0.43). Beta diversity was assessed using Bray–Curtis distance matrices, and infant gut microbiota structure remained markedly distinct between 6W and 6M (Figure 1C, Adonis, P < 0.001, R2 = 0.058). The gut microbiota of infants converged toward a tighter cluster at 6M compared to 6W. This observation was further consolidated by a comparison of the weighted UniFrac within-group distance (Figure 1D, P < 0.0001), suggesting that infants shared a more homogeneous microbial community with aged.
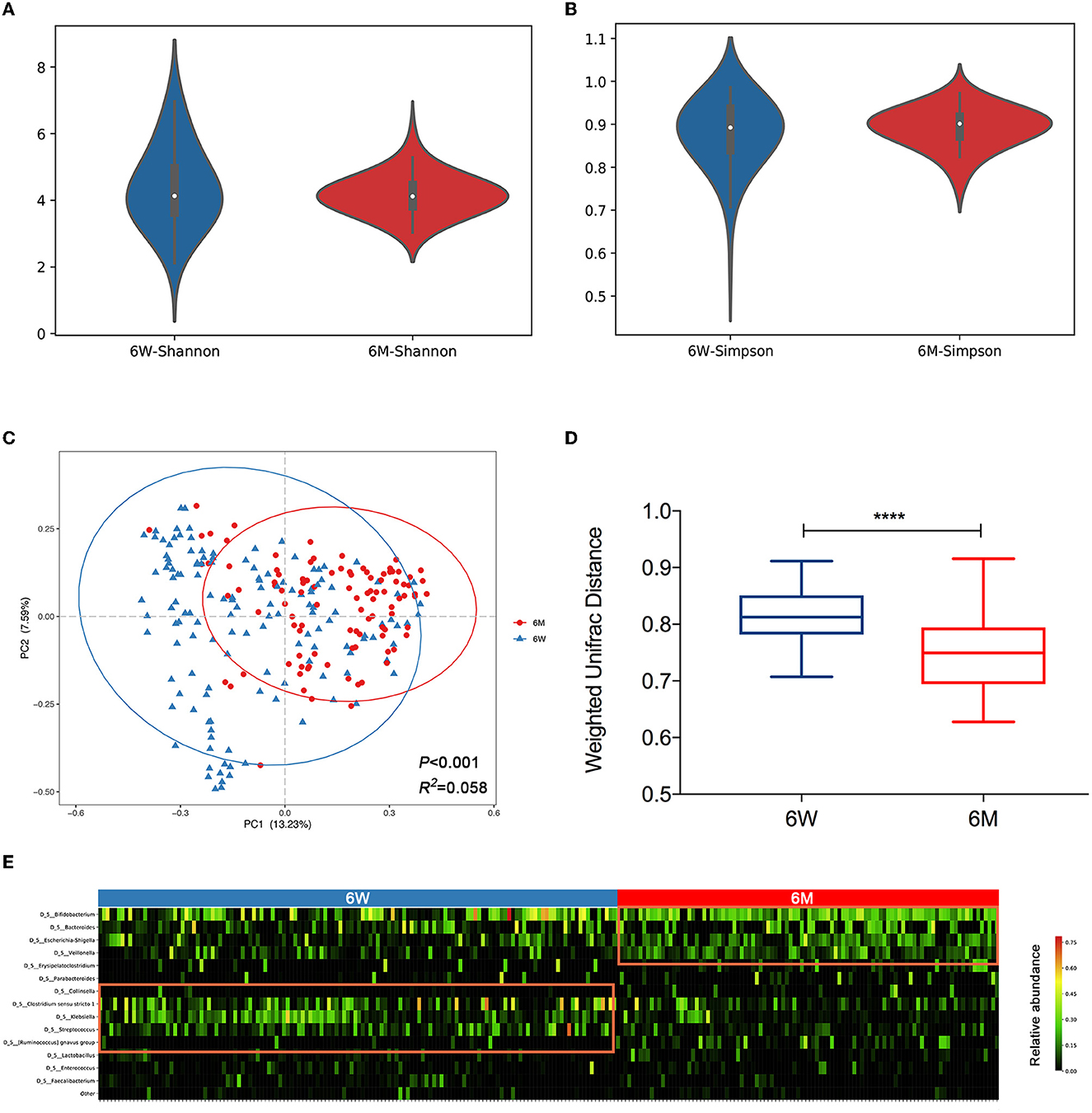
Figure 1. The distinct composition and community structure of gut microbiota in 6-week-old and 6-month-old infants. Alpha diversity is shown as the Shannon index (A) and Simpson index (B). (C) Principal coordinates analysis (PCoA) based on Bray–Curtis distance is shown along the first two principal coordinate (PC) axes with Adonis p-value and effect sizes (R2). (D) Comparison of the weighted UniFrac within-group distance of infants. The shorter distance indicated greater similarity in microbial community composition. Boxplots were shown as medians with range. (E) A heatmap of the top 15 bacterial OTUs (average relative abundance >1%) clustered according to age. Values range from low (black) to high (red). A significant difference was determined by Student's t-test (****p < 0.0001). 6W, 6-weeks-old infants; 6M, 6-months-old infants.
A heatmap of the top 15 genera based on an average relative abundance of >1% was used to identify age-associated patterns in infant gut microbiota. The heatmap revealed that Clostridium sensu stricto1, Klebsiella, and Streptococcus (highlighted on the left side of the heatmap), which are associated with the hospital environment (Lax et al., 2017), are present at higher relative abundances at 6W (P < 0.05, P < 0.001, and P < 0.001, respectively). In contrast, Bifidobacterium, Bacteroides, Escherichia-Shigella, and Veillonella (highlighted on the right side of the heatmap) were enriched at 6M (P < 0.05, P = 0.11, P < 0.001, and P < 0.001, respectively, Figure 1E).
We further investigated the impact of delivery mode on the infant gut microbiota, stratified by age. At 6W, a significant difference was found in the gut microbial community structure between VD and CSD infants (Figure 2A, Adonis, P = 0.037, R2 = 0.012). However, this difference dissipated at 6M (Figure 2B, Adonis, P = 0.215, R2 = 0.012). These results are consistent with previous studies (Stewart et al., 2018; Wampach et al., 2018; Reyman et al., 2019; Shao et al., 2019), demonstrating that delivery mode is a major determinant of gut microbiota in early life but with a gradually diminished effect throughout infancy.
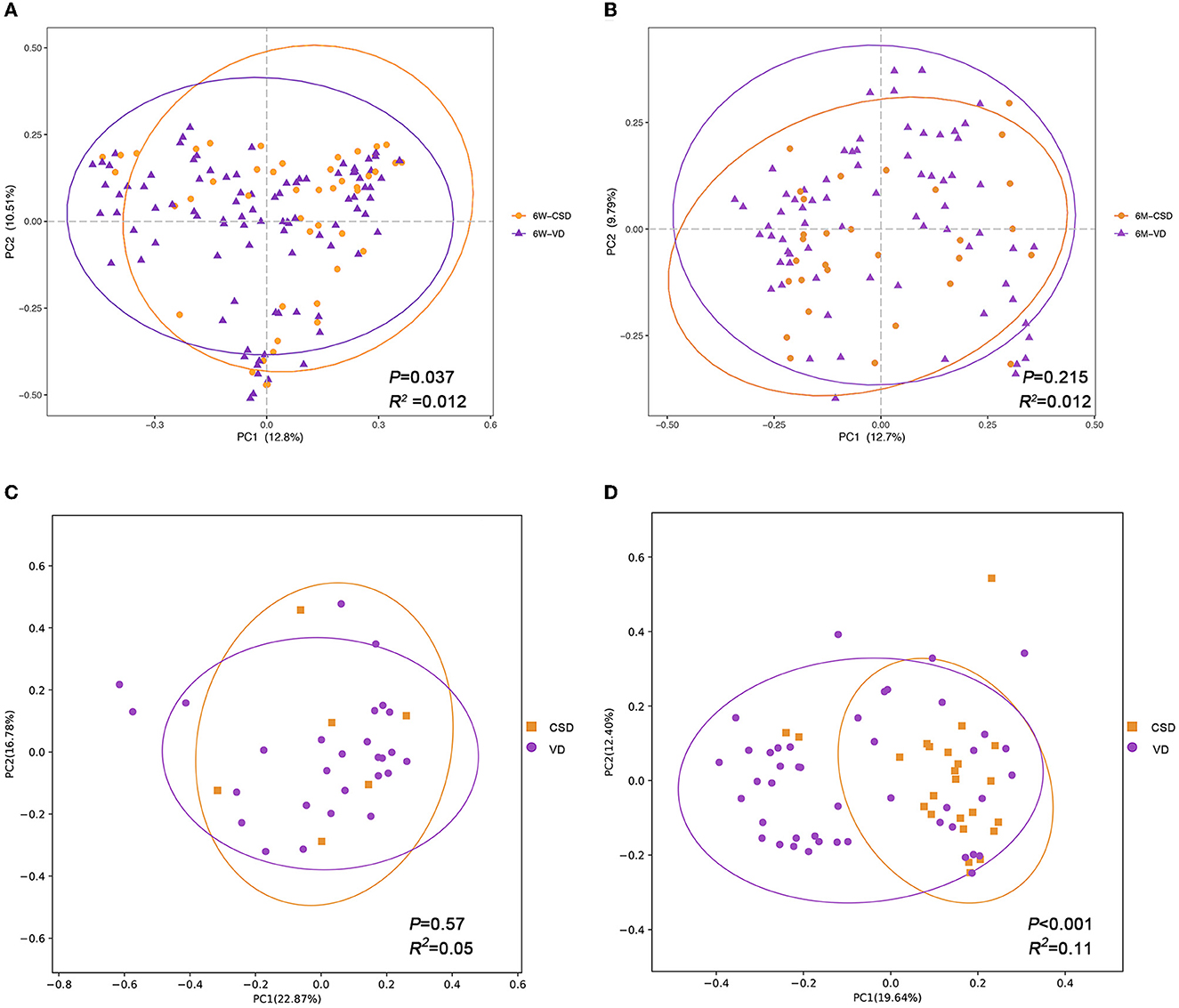
Figure 2. The impact of delivery mode on infant gut microbiota is gradually diminished and influenced by exclusive breastfeeding. Principal coordinates analysis (PCoA) plots of infant samples stratified according to the delivery mode, based on Bray–Curtis distance, are shown along the first two principal coordinate (PC) axes with Adonis p-value and effect sizes (R2). (A) Infants aged 6 weeks. (B) Infants aged 6 months. (C) Infants who were exclusively breastfed during 6 months. (D) Infants who were mixed fed during 6 months. VD, vaginally delivered; CSD, cesarean section delivered.
Considering the interplay between breastfeeding and delivery mode in relation to the infant gut microbiota (Hill et al., 2017; Liu et al., 2019), we hypothesized that the impact of delivery mode would be influenced by feeding patterns. To test this hypothesis, we conducted a stratified analysis based on samples collected at 6M separately (n = 101). The 101 infants were divided into two groups according to feeding patterns: exclusive breastfeeding (n = 33) and mixed feeding (n = 68) groups. As expected, no significant difference in the gut microbiota structure was observed between VD (n = 27) and CSD (n = 6) infants in the breastfeeding groups (Figure 2C, Adonis, P = 0.57, R2 = 0.05). However, in the mixed feeding groups, a stronger significant difference in gut microbiota structure was observed between VD (n = 44) and CSD (n = 24) infants, with a higher R2 value of 0.11 and a P-value of <0.001 (Figure 2D). This suggested that breastfeeding may alleviate the disturbance of gut microbiota in CSD infants.
Next, we aimed to identify specific taxa responsible for this feeding pattern-dependent change. The average relative abundances of the top 10 genera were compared between VD and CSD infants via a cross-sectional analysis. At 6W, all VD infants (n = 93) were enriched in Bacteroides and Escherichia-Shigella (Figure 3A, P < 0.01 and P < 0.05, respectively), while CSD infants (n = 46) were depleted of these two commensal genera and enriched in Klebsiella, Veillonella, and Enterococcus (Figure 3A, P < 0.05 for all). These findings are in agreement with recent observations in other cohort studies (Backhed et al., 2015a; Reyman et al., 2019; Shao et al., 2019). At 6M, the relative abundances of the top 10 genera were comparable between VD and CSD infants in the breastfeeding groups (Figure 3B), whereas the relative abundances of the aforementioned discriminative taxa remained different between VD and CSD infants in the mixed-feeding groups, with a lower relative abundance of Bacteroides (Figure 3C, P < 0.01) and a higher abundance of Klebsiella, Veillonella, and Streptococcus in CSD infants (P < 0.05 for all), while the relative abundances of Escherichia-Shigella were comparable between VD and CSD infants (P = 0.6).
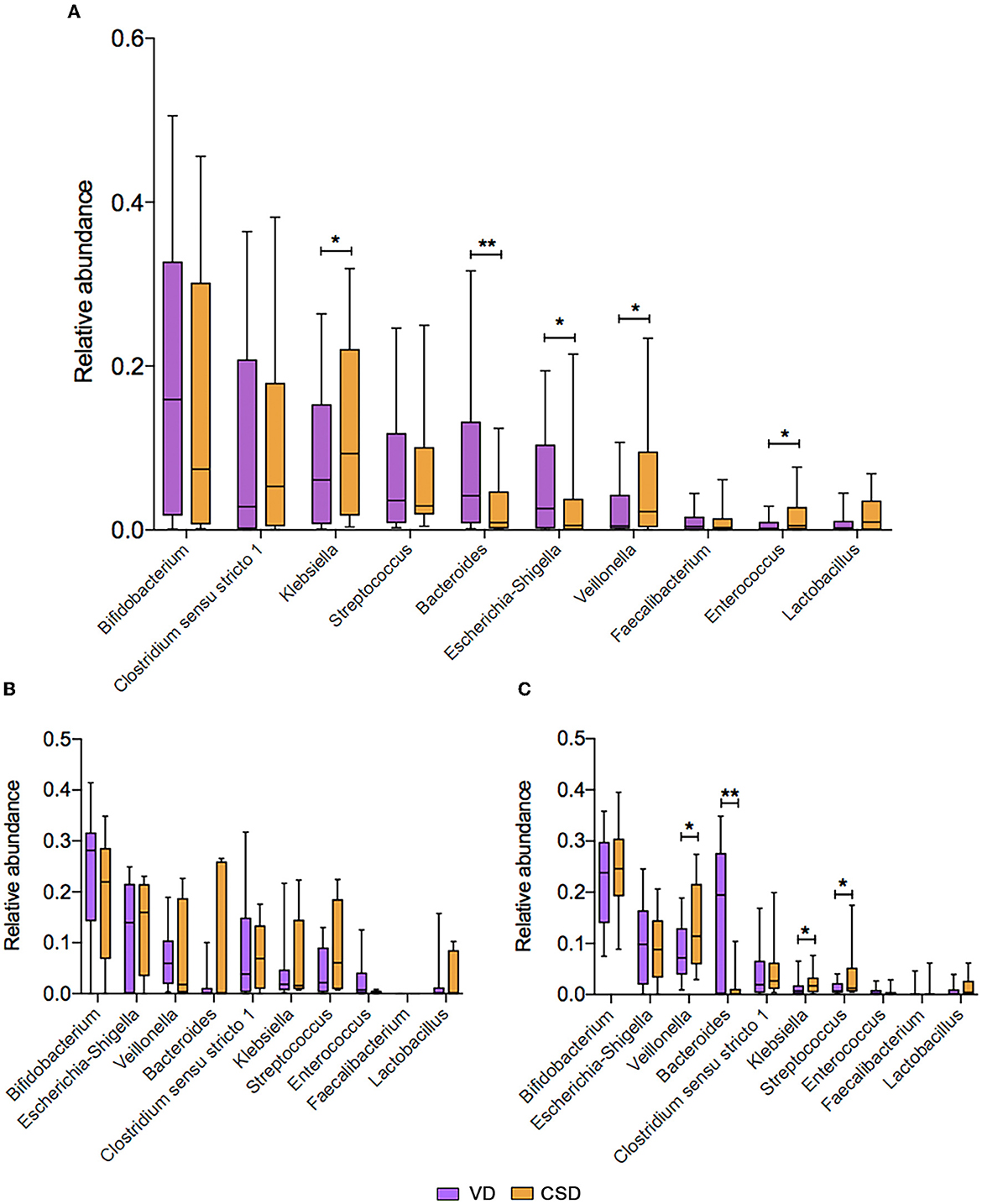
Figure 3. The mean relative abundance of the top 10 genera associated with the delivery mode. (A) The comparison of mean relative abundance of the top 10 genera between VD and CSD infants at P6W. The comparison of mean relative abundance of the top 10 genera between VD and CSD infants who were exclusively breastfed (B) and mixed fed (C) at P6M. A significant difference was determined by Student's t-test or the Mann–Whitney U-test (*p < 0.05; **p < 0.01). VD, vaginally delivered; CSD, cesarean section delivered.
Since previous studies have reported an association between delivery mode and susceptibility to respiratory diseases (Baumfeld et al., 2018), which might be mediated by gut microbiota composition (Reyman et al., 2019), we further investigated the relationship between delivery mode and infant RI status, according to parental self-reporting over the first 6 months. Indeed, the incidence of RI was significantly higher in CSD infants (9/71) than in VD infants (10/30, 12.7 vs. 33.3%, P = 0.02), prompting our hypothesis that delivery mode-induced gut microbial changes are associated with the RI status. Consequently, all infants at 6 months postpartum (n = 101) were divided into two groups based on the occurrence of at least one RI event: the RI group (n = 19) and the non-RI group (n = 82). The relative abundances of the five delivery mode-associated bacterial taxa (Bacteroides, Escherichia-Shigella, Klebsiella, Veillonella, and Enterococcus) were compared between the RI and non-RI groups. As expected, the VD-associated Escherichia-Shigella was more abundant in the non-RI group (Figure 4, P < 0.0001), whereas CSD-associated Klebsiella was enriched in the RI group (P < 0.001). Additionally, Bacteroides, enriched in VD infants, was more abundant in the non-RI group (P = 0.32), while Veillonella and Enterococcus, enriched in CSD infants, were more abundant in the RI group (P = 0.15, P = 0.78, respectively), although the difference did not reach the statistical significance.
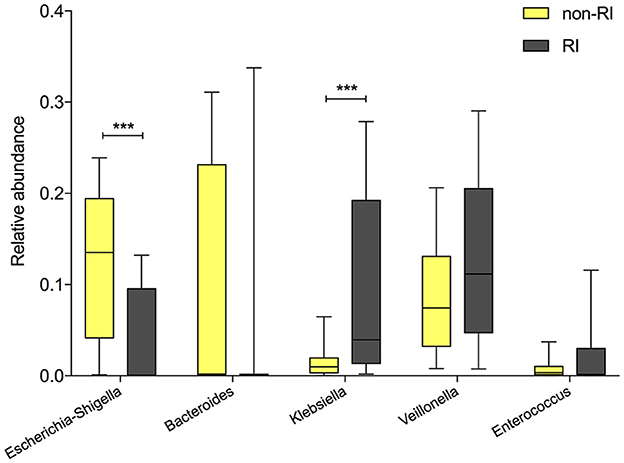
Figure 4. The comparison of the relative abundance of delivery mode-associated bacterial taxa between the RI and non-RI groups. A significant difference was determined by Student's t-test or the Mann–Whitney U-test (***p < 0.001). RI, respiratory infection.
Considering the mediating role of the feeding pattern on the relationship between delivery mode and gut microbiota, we further categorized infants (n = 101) into four groups based on the combination of delivery mode and feeding pattern: vaginally delivered and exclusively breastfed (VB, n = 27), vaginally delivered and mixed fed (VM, n = 44), cesarean delivered and exclusively breastfed (CB, n = 6), and cesarean delivered and mixed fed (CM, n = 24). We defined the VB group as the healthy reference group and found that the incidence of RIs of the CB group (1/6, 16.7%) was similar to that of the VB group (5/27, 18.5%), which was much lower than that of the CM group (9/24, 36%). Although the difference did not reach statistical significance (P = 0.26), this result indicated that exclusive breastfeeding may play a health-promoting role by rectifying the gut microbial composition induced by the cesarean section delivery.
Gut microbiota establishment in early life is essential to host health and disease susceptibility, which is greatly influenced by two perinatal factors, delivery mode and feeding pattern. However, the knowledge of the relationship between infant health and gut microbiota shifts associated with delivery mode and feeding pattern is limited. In this longitudinal study of 139 infants, we observed that the perturbations of infant gut microbiota caused by cesarean section were associated with higher risks of respiratory infection in the first months of life. These delivery mode-induced microbiota shifts were alleviated by exclusive breastfeeding, resulting in reduced respiratory infection susceptibility. Our study indicates a health-promoting role of exclusive breastfeeding on infants through restoring the delivery mode-induced gut microbial perturbations.
In recent decades, the rates of cesarean section have steadily increased worldwide, reaching 32.4% in the US and 34.9% in China (Li et al., 2017). This rise has been linked to a higher risk of metabolic and immune disorders in CSD offspring in the short and long terms (Huh et al., 2012; Sevelsted et al., 2015). Although the exact mechanism remains poorly understood, increasing evidence has demonstrated that gut microbiota might mediate the association between cesarean section delivery and susceptibility to obesity (Tun et al., 2018), diabetes mellitus (Andersen et al., 2020; Chavarro et al., 2020), asthma (Stokholm et al., 2020), respiratory tract infections (Reyman et al., 2019), and chronic immune issues (Wampach et al., 2018). Consistent with these findings, we observed a higher incidence of RIs during the first 6 months of life in CSD infants compared with VD infants. This outcome was related to low Escherichia-Shigella and high Klebsiella profiles in the intestine caused by cesarean section delivery. In addition to these two bacterial taxa, a decreased relative abundance of Bacteroides and the enrichment of Veillonella and Enterococcus were observed in our CSD infants, which aligns with previous studies (Reyman et al., 2019; Shao et al., 2019).
The lack of specific bacterial taxa in CSD infants has been shown to disturb the maturation of the infant intestine and immune systems (Jakobsson et al., 2014; Tamburini et al., 2016). For example, the low Bacteroides profile in CSD infants, considering a signature of disturbed gut microbiota development in early life (Shao et al., 2019), is related to a reduction in lipopolysaccharide (LPS) exposure, thus decreasing the stimulation of primary human immune cells and exerting a long-term impact on immune-mediated diseases (Jenmalm, 2011; Jakobsson et al., 2014; Wampach et al., 2018). Klebsiella, regarded as an opportunistic pathogen, is a common cause of hospital infection, and an increased ratio of Klebsiella/Bifidobacterium in early life is related to the latter development of pediatric allergies (Low et al., 2017). Combining our observation, the enrichment of Klebsiella may be involved not only in the risk of immunological disorders but also in the risk of infectious diseases in CSD offspring.
Consistent with previous studies (Hill et al., 2017; Reyman et al., 2019; Shao et al., 2019), we found that the effect of delivery mode on microbial profiles gradually decreased with infant age. Unique to our study, we further determined whether this dynamic trajectory was affected by feeding patterns, which was prompted by our previous observation that microbial alterations caused by cesarean section delivery could be rectified by EBF in a single cross-sectional analysis at 6 weeks of life (Liu et al., 2019). As expected, the dynamic effect of delivery mode on infant gut microbiota was dependent on the feeding pattern, demonstrating that the microbial compositional disturbance associated with cesarean section delivery could be corrected by EBF through increasing or decreasing specific bacterial taxa but not by MF. Taking Klebsiella as an example, the difference in relative abundance between VD and CSD infants at 6 weeks was rectified by EBF lasting for 6 months of life but exacerbated by MF.
A recent systematic review assessed that the relative abundances of particular gut-commensal bacteria genera were associated with childhood respiratory infection (Alcazar et al., 2022), suggesting that gut microbiota might be a determinant of childhood respiratory disease. In this study, we further compared the incidence of RIs among VB, CB, and CM infants, taking VB infants as a healthy reference. Although no significant difference was found, we observed a trend toward a higher incidence of RIs in CM infants than in CB infants. In terms of gut microbiota, our results provide evidence to support the previous viewpoint that prolonged EBF reduces the risk of infectious diseases in infancy (Duijts et al., 2010; Brodin, 2022), especially in CSD infants. However, the proportion and duration of EBF were significantly lower in CSD infants (Vestermark et al., 1991; Dewey et al., 2003).
Breast milk and its components exert an important influence on the nature of early-life immune responses during microbial colonization. Although artificial oligosaccharides are added to infant formula milk to mimic the composition of human breast milk, a significant difference in gut microbial composition is still observed between EBF and MF infants (Azad et al., 2013; Stewart et al., 2018). Presumably, IgA antibodies and maternal antibodies in breast milk help tip the balance toward tolerance and immune-microbe mutualism while providing passive immunity to defend against invasive bacteria (Brodin, 2022). In addition to the different components between breast milk and formula milk, the breast milk microbiota also plays an important role (Le Doare et al., 2018). The abundance of specific microbial strains of Bifidobacterium, Lactobacillus, Enterococcus, and Staphylococcus species in the infant's intestine increased with the proportion of daily breast milk intake in a dose-dependent manner (Pannaraj et al., 2017), strongly indicating the transfer of microbes from breast milk to the infant intestine (Martin et al., 2012). Furthermore, HMOs in breast milk are metabolized by microbiota, leading to the production of metabolites such as indole-3-lactic acid, which likely mediates some of the beneficial effects of breast milk early in life (Henrick et al., 2021). Taken together, these results highlight the importance of EBF, especially for CSD infants, and provide a potential reference for optimizing formula milk by adding specific bacterial taxa, resulting in a gut microbial profile resembling that of EBF infants.
The strengths of our study included the longitudinal sampling, which enabled us to assess the dynamic effect of delivery mode on infant gut microbiota. In addition, a mixed feeding regimen was included in our study, which was often introduced when mothers return to work or prepare to wean in China, while most previous studies usually focused on the comparison of gut microbiota in EBF and exclusively formula-fed infants (Azad et al., 2013; Gomez-Llorente et al., 2013; Bergstrom et al., 2014). Finally, we highlighted the association between infant clinical consequences and delivery mode-induced gut microbial perturbations. However, our study has some limitations. The incidence of respiratory infection was determined by the self-report of the mother, which may be biased by factitious factors. To investigate the relationship between health outcomes and delivery mode, detailed information about clinical phenotype was lacking.
In conclusion, we assessed the dynamic impact of delivery mode on infant gut microbiota and attenuated it in a feeding pattern-dependent manner during the first months of life. The perturbation of infant gut microbiota caused by cesarean section was restored by exclusive breastfeeding, resulting in reduced respiratory infection susceptibility in the first years of life. Our study suggests a health-promoting role of exclusive breastfeeding on infants by restoring the delivery mode-induced gut microbial perturbations.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary material.
The studies involving human participants were reviewed and approved by Peking University First Hospital (V2.0/201504.20). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
YL, JM, and HY conceived the study design. YL, SQ, YF, and SW were responsible for the recruitment and collection of samples. NL, FL, and BZ were responsible for the laboratory assays. YL performed the data analysis and completed the initial manuscript. HY revised the manuscript. All authors have read and approved the final version of the manuscript.
This research was supported by the National Key Research and Development Program of China (No. 2021YFC2700700).
We thank all the participants for their support and cooperation in our study. We also thank all the researchers, clinicians, and technicians for their participation and technical support in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1163269/full#supplementary-material
Alcazar, C. G., Paes, V. M., Shao, Y., Oesser, C., Miltz, A., Lawley, T. D., et al. (2022). The association between early-life gut microbiota and childhood respiratory diseases: a systematic review. Lancet Microbe 3, e867–e880. doi: 10.1016/S2666-5247(22)00184-7
Andersen, V., Moller, S., Jensen, P. B., Moller, F. T., and Green, A. (2020). Caesarean delivery and risk of chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, coeliac disease, and diabetes mellitus): a population based registry study of 2,699,479 births in Denmark during 1973-2016. Clin. Epidemiol. 12, 287–293. doi: 10.2147/CLEP.S229056
Azad, M. B., Konya, T., Maughan, H., Guttman, D. S., Field, C. J., Chari, R. S., et al. (2013). Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ 185, 385–394. doi: 10.1503/cmaj.121189
Backhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015a). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 690–703. doi: 10.1016/j.chom.2015.04.004
Backhed, F., Roswall, J., Peng, Y., Feng, Q., Jia, H., Kovatcheva-Datchary, P., et al. (2015b). Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host Microbe 17, 852. doi: 10.1016/j.chom.2015.05.012
Baumann-Dudenhoeffer, A. M., D'souza, A. W., Tarr, P. I., Warner, B. B., and Dantas, G. (2018). Infant diet and maternal gestational weight gain predict early metabolic maturation of gut microbiomes. Nat. Med. 24, 1822–1829. doi: 10.1038/s41591-018-0216-2
Baumfeld, Y., Walfisch, A., Wainstock, T., Segal, I., Sergienko, R., Landau, D., et al. (2018). Elective cesarean delivery at term and the long-term risk for respiratory morbidity of the offspring. Eur. J. Pediatr. 177, 1653–1659. doi: 10.1007/s00431-018-3225-8
Bergstrom, A., Skov, T. H., Bahl, M. I., Roager, H. M., Christensen, L. B., Ejlerskov, K. T., et al. (2014). Establishment of intestinal microbiota during early life: a longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 80, 2889–2900. doi: 10.1128/AEM.00342-14
Bokulich, N. A., Chung, J., Battaglia, T., Henderson, N., Jay, M., Li, H., et al. (2016). Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 8, 343ra382. doi: 10.1126/scitranslmed.aad7121
Bosch, A., Levin, E., Van Houten, M. A., Hasrat, R., Kalkman, G., Biesbroek, G., et al. (2016). Development of upper respiratory tract microbiota in infancy is affected by mode of delivery. EBioMedicine 9, 336–345. doi: 10.1016/j.ebiom.2016.05.031
Brodin, P. (2022). Immune-microbe interactions early in life: a determinant of health and disease long term. Science 376, 945–950. doi: 10.1126/science.abk2189
Chavarro, J. E., Martin-Calvo, N., Yuan, C., Arvizu, M., Rich-Edwards, J. W., Michels, K. B., et al. (2020). Association of birth by cesarean delivery with obesity and type 2 diabetes among adult women. JAMA Netw. Open 3, e202605. doi: 10.1001/jamanetworkopen.2020.2605
Dewey, K. G., Nommsen-Rivers, L. A., Heinig, M. J., and Cohen, R. J. (2003). Risk factors for suboptimal infant breastfeeding behavior, delayed onset of lactation, and excess neonatal weight loss. Pediatrics 112, 607–619. doi: 10.1542/peds.112.3.607
Donald, K., Petersen, C., Turvey, S. E., Finlay, B. B., and Azad, M. B. (2022). Secretory IgA: linking microbes, maternal health, and infant health through human milk. Cell Host Microbe 30, 650–659. doi: 10.1016/j.chom.2022.02.005
Duijts, L., Jaddoe, V. W., Hofman, A., and Moll, H. A. (2010). Prolonged and exclusive breastfeeding reduces the risk of infectious diseases in infancy. Pediatrics 126, e18–25. doi: 10.1542/peds.2008-3256
Fehr, K., Moossavi, S., Sbihi, H., Boutin, R. C. T., Bode, L., Robertson, B., et al. (2020). Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers' milk and the infant gut: the CHILD cohort study. Cell Host Microbe 28, 285–297 e284. doi: 10.1016/j.chom.2020.06.009
Fouhy, F., Watkins, C., Hill, C. J., O'shea, C. A., Nagle, B., Dempsey, E. M., et al. (2019). Perinatal factors affect the gut microbiota up to four years after birth. Nat. Commun. 10, 1517. doi: 10.1038/s41467-019-09252-4
Gomez-Llorente, C., Plaza-Diaz, J., Aguilera, M., Munoz-Quezada, S., Bermudez-Brito, M., Peso-Echarri, P., et al. (2013). Three main factors define changes in fecal microbiota associated with feeding modality in infants. J. Pediatr. Gastroenterol. Nutr. 57, 461–466. doi: 10.1097/MPG.0b013e31829d519a
Gopalakrishna, K. P., and Hand, T. W. (2020). Influence of maternal milk on the neonatal intestinal microbiome. Nutrients 12. doi: 10.3390/nu12030823
Gridneva, Z., Lai, C. T., Rea, A., Tie, W. J., Ward, L. C., Murray, K., et al. (2021). Human milk immunomodulatory proteins are related to development of infant body composition during the first year of lactation. Pediatr. Res. 89, 911–921. doi: 10.1038/s41390-020-0961-z
Henrick, B. M., Rodriguez, L., Lakshmikanth, T., Pou, C., Henckel, E., Arzoomand, A., et al. (2021). Bifidobacteria-mediated immune system imprinting early in life. Cell 184, 3884–3898 e3811. doi: 10.1016/j.cell.2021.05.030
Hill, C. J., Lynch, D. B., Murphy, K., Ulaszewska, M., Jeffery, I. B., O'shea, C. A., et al. (2017). Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 5, 4. doi: 10.1186/s40168-016-0213-y
Huh, S. Y., Rifas-Shiman, S. L., Zera, C. A., Edwards, J. W., Oken, E., Weiss, S. T., et al. (2012). Delivery by caesarean section and risk of obesity in preschool age children: a prospective cohort study. Arch. Dis. Child. 97, 610–616. doi: 10.1136/archdischild-2011-301141
Jakobsson, H. E., Abrahamsson, T. R., Jenmalm, M. C., Harris, K., Quince, C., Jernberg, C., et al. (2014). Decreased gut microbiota diversity, delayed Bacteroidetes colonisation and reduced Th1 responses in infants delivered by caesarean section. Gut 63, 559–566. doi: 10.1136/gutjnl-2012-303249
Jenmalm, M. C. (2011). Childhood immune maturation and allergy development: regulation by maternal immunity and microbial exposure. Am. J. Reprod. Immunol. 66(Suppl. 1), 75–80. doi: 10.1111/j.1600-0897.2011.01036.x
Lax, S., Sangwan, N., Smith, D., Larsen, P., Handley, K. M., Richardson, M., et al. (2017). Bacterial colonization and succession in a newly opened hospital. Sci. Transl. Med. 9, eaah6500. doi: 10.1126/scitranslmed.aah6500
Le Doare, K., Holder, B., Bassett, A., and Pannaraj, P. S. (2018). Mother's milk: a purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 9, 361. doi: 10.3389/fimmu.2018.00361
Li, H. T., Luo, S., Trasande, L., Hellerstein, S., Kang, C., Li, J. X., et al. (2017). Geographic variations and temporal trends in cesarean delivery rates in China, 2008-2014. JAMA 317, 69–76. doi: 10.1001/jama.2016.18663
Liu, Y., Qin, S., Song, Y., Feng, Y., Lv, N., Xue, Y., et al. (2019). The perturbation of infant gut microbiota caused by cesarean delivery is partially restored by exclusive breastfeeding. Front. Microbiol. 10, 598. doi: 10.3389/fmicb.2019.00598
Low, J. S. Y., Soh, S. E., Lee, Y. K., Kwek, K. Y. C., Holbrook, J. D., Van Der Beek, E. M., et al. (2017). Ratio of Klebsiella/Bifidobacterium in early life correlates with later development of paediatric allergy. Benef. Microbes 8, 681–695. doi: 10.3920/BM2017.0020
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Martin, V., Maldonado-Barragan, A., Moles, L., Rodriguez-Banos, M., Campo, R. D., Fernandez, L., et al. (2012). Sharing of bacterial strains between breast milk and infant feces. J. Hum. Lact. 28, 36–44. doi: 10.1177/0890334411424729
Pannaraj, P. S., Li, F., Cerini, C., Bender, J. M., Yang, S., Rollie, A., et al. (2017). Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatr. 171, 647–654. doi: 10.1001/jamapediatrics.2017.0378
Pattaroni, C., Watzenboeck, M. L., Schneidegger, S., Kieser, S., Wong, N. C., Bernasconi, E., et al. (2018). Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 24, 857–865 e854. doi: 10.1016/j.chom.2018.10.019
Reyman, M., Van Houten, M. A., Van Baarle, D., Bosch, A., Man, W. H., Chu, M., et al. (2019). Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat. Commun. 10, 4997. doi: 10.1038/s41467-019-13014-7
Robertson, R. C., Manges, A. R., Finlay, B. B., and Prendergast, A. J. (2019). The human microbiome and child growth - first 1000 days and beyond. Trends Microbiol. 27, 131–147. doi: 10.1016/j.tim.2018.09.008
Roduit, C., Scholtens, S., De Jongste, J. C., Wijga, A. H., Gerritsen, J., Postma, D. S., et al. (2009). Asthma at 8 years of age in children born by caesarean section. Thorax 64, 107–113. doi: 10.1136/thx.2008.100875
Sevelsted, A., Stokholm, J., Bonnelykke, K., and Bisgaard, H. (2015). Cesarean section and chronic immune disorders. Pediatrics 135, e92–98. doi: 10.1542/peds.2014-0596
Shao, Y., Forster, S. C., Tsaliki, E., Vervier, K., Strang, A., Simpson, N., et al. (2019). Stunted microbiota and opportunistic pathogen colonization in caesarean-section birth. Nature 574, 117–121. doi: 10.1038/s41586-019-1560-1
Song, S. J., Wang, J., Martino, C., Jiang, L., Thompson, W. K., Shenhav, L., et al. (2021). Naturalization of the microbiota developmental trajectory of Cesarean-born neonates after vaginal seeding. Medicine 2, 951–964 e955. doi: 10.1016/j.medj.2021.05.003
Stewart, C. J., Ajami, N. J., O'brien, J. L., Hutchinson, D. S., Smith, D. P., Wong, M. C., et al. (2018). Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 562, 583–588. doi: 10.1038/s41586-018-0617-x
Stokholm, J., Thorsen, J., Blaser, M. J., Rasmussen, M. A., Hjelmso, M., Shah, S., et al. (2020). Delivery mode and gut microbial changes correlate with an increased risk of childhood asthma. Sci. Transl. Med. 12, eaax9929. doi: 10.1126/scitranslmed.aax9929
Tamburini, S., Shen, N., Wu, H. C., and Clemente, J. C. (2016). The microbiome in early life: implications for health outcomes. Nat. Med. 22, 713–722. doi: 10.1038/nm.4142
Tang, H. H. F., Lang, A., Teo, S. M., Judd, L. M., Gangnon, R., Evans, M. D., et al. (2021). Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 147, 1683–1691. doi: 10.1016/j.jaci.2020.10.009
Tun, H. M., Bridgman, S. L., Chari, R., Field, C. J., Guttman, D. S., Becker, A. B., et al. (2018). Roles of birth mode and infant gut microbiota in intergenerational transmission of overweight and obesity from mother to offspring. JAMA Pediatr. 172, 368–377. doi: 10.1001/jamapediatrics.2017.5535
Vestermark, V., Hogdall, C. K., Birch, M., Plenov, G., and Toftager-Larsen, K. (1991). Influence of the mode of delivery on initiation of breast-feeding. Eur. J. Obstet. Gynecol. Reprod. Biol. 38, 33–38. doi: 10.1016/0028-2243(91)90204-X
Wampach, L., Heintz-Buschart, A., Fritz, J. V., Ramiro-Garcia, J., Habier, J., Herold, M., et al. (2018). Birth mode is associated with earliest strain-conferred gut microbiome functions and immunostimulatory potential. Nat. Commun. 9, 5091. doi: 10.1038/s41467-018-07631-x
Keywords: gut microbiota, early life, delivery mode, exclusive breastfeeding, disease susceptibility
Citation: Liu Y, Ma J, Zhu B, Liu F, Qin S, Lv N, Feng Y, Wang S and Yang H (2023) A health-promoting role of exclusive breastfeeding on infants through restoring delivery mode-induced gut microbiota perturbations. Front. Microbiol. 14:1163269. doi: 10.3389/fmicb.2023.1163269
Received: 10 February 2023; Accepted: 15 June 2023;
Published: 10 July 2023.
Edited by:
Franck Carbonero, Washington State University Health Sciences Spokane, United StatesReviewed by:
Richard Agans, Air Force Research Laboratory, United StatesCopyright © 2023 Liu, Ma, Zhu, Liu, Qin, Lv, Feng, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huixia Yang, eWFuZ2h1aXhpYUBiam11LmVkdS5jbg==; eWh4a3R6MjAxOEAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.