
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 16 June 2023
Sec. Extreme Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1152985
This article is part of the Research Topic Extremozymes: Characteristics, Structure, Protein Engineering and Applications View all 8 articles
 Yalpi Karthik1
Yalpi Karthik1 Manjula Ishwara Kalyani1*
Manjula Ishwara Kalyani1* Srinivasa Krishnappa2
Srinivasa Krishnappa2 Krishnaveni Ramakrishna3
Krishnaveni Ramakrishna3 Samy M. Sayed4
Samy M. Sayed4 Ohud Muslat Aharthy5
Ohud Muslat Aharthy5 Seham Sater Alhelaify5
Seham Sater Alhelaify5 Muntazir Mushtaq6*
Muntazir Mushtaq6*Streptomyces is a group of microbes known for antibiotic production and has contributed to more than 70% of present commercially available antibiotics. These antibiotics are important in the management, protection, and treatment of chronic illnesses. In the present study, the isolated S. tauricus strain from mangrove soil in Mangalore, India (GenBank accession number: MW785875) was subjected for differential cultural characterization, phenotype involving brown pigmentation, filamentous mycelia, and ash-colored spore production was observed using field emission scanning electron microscopy (FESEM) analysis revealing filamentous mycelia possessing a straight spore chain. Spores were visualized as elongated, rod-shaped, smooth surfaces with curved edges. After optimized growth conditions for S. tauricus on starch-casein agar medium, the GC/MS analysis of S. tauricus intracellular extract detected bioactive compounds reported for pharmacological applications. Analyzed using the NIST library, most of the bioactive compounds identified in intracellular extract had molecular weights of less than 1 kDa. On the PC3 cell line, the Sephadex G-10 partially purified eluted peak protein fraction demonstrated significant anticancer activity. The LCMS analysis revealed the presence of Tryprostatin B, Fumonisin B1, Microcystin LR, and Surfactin C with molecular weights below 1 kDa. This study found that small molecular weight microbial compounds are more effective in a variety of biological applications.
Mangrove habitat possesses unique environmental conditions characterized by high tidal forces, hypersaline conditions, substantial changes in temperature, and ideal flora and fauna diversity (Karthik et al., 2020). These vulnerable conditions are helpful for microbes in adjusting themselves to any extreme environment. This habitat will serve as a prominent repository for the isolation of biologically important compounds (Alongi, 2015). Actinomyces are Gram-positive filamentous bacteria that differ in color and produce spores when mature (Chater, 2013), and share the characteristics of bacteria and fungi. The genetic and environmental adaptability of the Actinomyces group supports the production of valuable and biologically important compounds. Among the Actinomyces, the genus Streptomyces shares more than 70 percent of the active compounds with the pharmaceutical industry for the treatment of various ailments (Chater, 2013; Karthik et al., 2023).
These Streptomyces are broadly used in the control, prevention, and treatment of diseases through the production of bioactive compounds. They are widely used as antibiotics, anti-cancer, anti-tumor, anthelmintic, anti-oxidant, anti-diabetic, immunosuppressors, neuritogenic, anti-inflammatory, anti-angiogenic, anti-algal, anti-fungal, anti-parasitic, anti-malarial, anti-viral, anti-bacterial and many more biological applications (Nishioka and Katayama, 1978; Renner et al., 1999; Fernebro, 2011; Janardhan et al., 2014; Desouky et al., 2015; Abd-Elnaby et al., 2016).
The S. lividans is involved in post-translational modification by acylation at lysine residue of active site for production of CoA ligases and many organic acids (Burckhardt et al., 2020). The cyclic antimicrobial peptide receptor protein and novel lipopeptide daptomycin are produced by S. roseosporus (Wu et al., 2021). These studies made us to search alternative sources of antimicrobial peptides from different mangrove sources. One such prominent strain was described in detail in our previous study on isolation procedure as well as the microscopic and macroscopic characters identified as S. tauricus with the GenBank accession number MW785875 (Karthik and Kalyani, 2022). The intracellular protein, extraction, estimation, and purification, along with their potential antimicrobial activity, were observed against test pathogens, S. typhimurium, S. aureus, P. aeruginosa, B. cereus, and P. vulgaris cultures. The protein was characterized through LCMS and SDS PAGE techniques, and small peptides were detected (Karthik and Kalyani, 2022).
In the present study, the optimization of suitable growth media for S. tauricus and its micromorphology were analyzed using FESEM (field emission scanning electron microscopy). The isolation of an intracellular extract of S. tauricus was characterized through GCMS and LCMS. This GC-MS analysis showed the maximum number of small bioactive compounds having efficient biological activities, whereas the LCMS characterization detected Tryprostatin B, FumonisinB1, Microcystin LR, and Surfactin C having a low molecular weight and exhibiting an effective anticancer activity against a prostate cancer cell line compared with standard cisplatin.
The soil sample was collected from mangroves in the Dakshina Kannada region of Mangalore. The Ashok Nagar, Tannira Bavi (ANTB) sample collection site is located at 12°54′16.5″N and 74°49′09.4″E. At the site of the collection, the soil had a sandy texture and was black, and the environmental factors of pH 7.2 and temperature of 21°C were documented. In sterile containers, the samples were taken to the Molecular Research Laboratory (MRL), Division of Microbiology, Jnana Kaveri, Mangalore University, India. The soil samples were pre-heated for 2 h at 60°C before serial dilution and isolation to prevent bacterial and fungal growth (Mohan et al., 2013; Sridevi et al., 2015; Sapkota et al., 2020).
One gram of soil sample was serially diluted seven folds and aliquots of 100 μL of each dilution were spread out on SCN plates in triplicates (starch: 10 g, casein: 0.30 g, KNO3: 2 g, MgSO4.7H2O: 0.050 g, K2HPO4: 2 g, NaCl: 2 g, CaCO3: 0.20 g, FeSO4: 0.010 g in 1 L of dH2O, pH 7.2) and incubated at 30°C ± 2°C for 7 days. The plates were screened for Actinomyces colonies and single colonies were selected, sub-cultured on SCN media (Karthik et al., 2020; Wang et al., 2020). The culture characteristics were visualized and recorded such as color of aerial and substrate mycelia, and diffusible pigments production.
The isolated S. tauricus strain was subjected to FESEM analysis at different objective distances, spore structure (1 and 2 μm) mycelial structure (10 and 20 μm) to visualize the complete micromorphological structures. The sequencing and identification of S. tauricus were reported in our previous work (Karthik and Kalyani, 2022).
The five distinct media were used to analyze the phenotypic characteristics of potential Actinomyces isolates. The media that were used are malt extract agar (MEA) malt extract: 30.0 g, peptone (5.0 g), and agar (15 g). Sucrose peptone agar (SPA) was prepared using sucrose (20.0 g), peptone (5.0 g), K2HPO4 (0.50 g), MgSO4.7H2O (0.25 g), and agar 15.0 g. The glucose asparagine agar (GAA) was prepared using glucose (10 g), asparagine (0.50 g), K2HPO4 (0.50 g), and agar (15.0 g). Nutrient agar (NA) was prepared using peptone: 5.0 g, sodium chloride: 5.0 g beef extract: 3.0 g. The yeast extract agar (YEA) was prepared using yeast extract (3.0 g), peptone (5.0 g), and agar (15.0 g). These different media were used for growth studies of potential Actinomyces isolates.
Streptomyces tauricus was grown in SCN broth for 7–10 days at 30°C ± 2°C with constant agitation at 100 rpm. The separated cultivated biomass cells were centrifuged at 7,000 rpm for 8 min, twice washed in phosphate-buffered saline devoid of Mg2+ and Ca2+, and then centrifuged one more time. The cells were then suspended in 10 mL of ice-cold acetone, allowed to stand on ice for 5 min, then centrifuged for 8 min at 7,000 rpm. Following the removal of any remaining acetone with a stream of nitrogen, the intracellular extract was incubated with 1.0 mL of 1% SDS for 2 min. Spectrometric (LC-MS and GC-MS) methods were used to characterize this intracellular extract, and it was analyzed using NIST library.
The GC–MS studies of the intracellular extract of S. tauricus were performed with a Perkin Elmer Gas Chromatograph Clarus 680 and a Perkin Elmer Mass Spectrometer Clarus SQ 8C. The equipment has a Perkin Elmer Elite-5MS standard column with dimensions of 30 m long × 0.250 mm inner diameter × 1 micron (60°C–350°C). With a split ratio of 10:1, the injected volume of 2 μL was completely run for 26.6 min. The carrier gas used is helium, with a flow rate of 2 mL/min. The source temperature was operated at 230°C, and the inlet temperature was operated at 250°C. The oven temperature was initially set to 80°C with a 2.0 min hold time; ramp 1 was 10.0/min to 150°C with a 1.0 min hold time; and ramp 2 was 15.0/min to 250°C with a 10.0 min hold time. Comparing them to those in the NIST computer library allowed the components to be identified, which were connected to the GC-MS apparatus, and the findings were reported.
The peak fraction obtained from Sephadex G-10 was analyzed and subjected to LC-MS; Model Synapt G2 a method of analytical chemistry was used that combine liquid chromatography’s physical separation capabilities with other methods. With mobile phase A: 0.1% Formic acid in water and mobile phase B: 0.1% formic acid acetonitrile with the mass analysis capabilities of mass spectrometry (MS) a vacuum degasser, binary pump, and well-plate auto sampler were included in an Agilent 1,100 LC system. The BEH C18, 50 mm × 1.0 mm, 1.7 μm C18 column was used (Waters United States) compared to the NIST computer library.
The in vitro studies were conducted using prostate cancer cells that were grown in T-25 flasks. Trypsinized PC3 cells were aspirated into a 5 mL centrifuge tube, which were centrifuged for 300 rpm and the cell pellet were separated. The cell count was adjusted in DMEM-HG media to produce a 200 μL suspension with approximately 10,000 cells. Using an ESCO model CLM170B-8-UV CO2 incubator, 200 μL of the cell suspension was put into each well of the 96-well microtiter plate. The plate was then incubated at 37°C with 5% CO2 for 24 h. The used medium was aspirated 24 h later. The standard medication cisplatin and several test drug concentrations totaling 200 μL each was introduced to the corresponding wells. The plates were then incubated for 24 h at 37 degrees Celsius with 5% CO2. The drug-containing medium was aspirated when the plate was taken out of the incubator. The plate was incubated at 37°C with 5% CO2 for 3 h after 200 μL of media containing 10% and 0.5 mg/mL of MTT reagent were added to each well. Without disrupting the crystals that had grown, the culture medium was removed. The produced formazan was then solubilized by adding 100 μL of solubilization solution (DMSO) and gently shaking the plate in a gyrator shaker. The absorbance was measured using a microplate reader of the Multiskan sky ELISA spectrophotometer at a wavelength of 570 nm and also at 630 nm (Karthik et al., 2023).
The Ashok Nagar, Tannir Baavi mangrove region in Mangalore, India, served as a suitable source for isolating 20 distinct Actinomyces cultures. Among these 20 distinct Actinomyces cultures, the S. tauricus (GenBank accession number MW785875) was isolated, and their biological activities were reported in our previous work (Karthik and Kalyani, 2022). In continuation to previous work, the S. tauricus strain was initially examined for morphological characteristics after FESEM analysis. GCMS was also used to characterize biologically important chemical components found in the intracellular extract of the S. tauricus strain, and LCMS was used to characterize a partially purified protein sample, which revealed a significant number of bioactive compounds.
On starch casein nitrate agar medium, the cultural characteristics of the mangrove-adapted S. tauricus strain showed white filamentous mycelia and at maturity, ash-colored spores. Brown pigmentation was observed to be produced on SCNA media. Gram staining was found to be positive after further microscopic examination.
The isolate when further subjected to FESEM microscopic studies revealed the mycelial morphological characteristics of the genus Streptomyces. Further, filamentous mycelia possessed a straight spore chain. Spores were visualized as elongated, rod-shaped, smooth surfaces with curved edges, as shown in Figure 1. When grown on different Actinomyces-specific media, S. tauricus displayed distinct phenotypic characteristics as shown in Table 1.
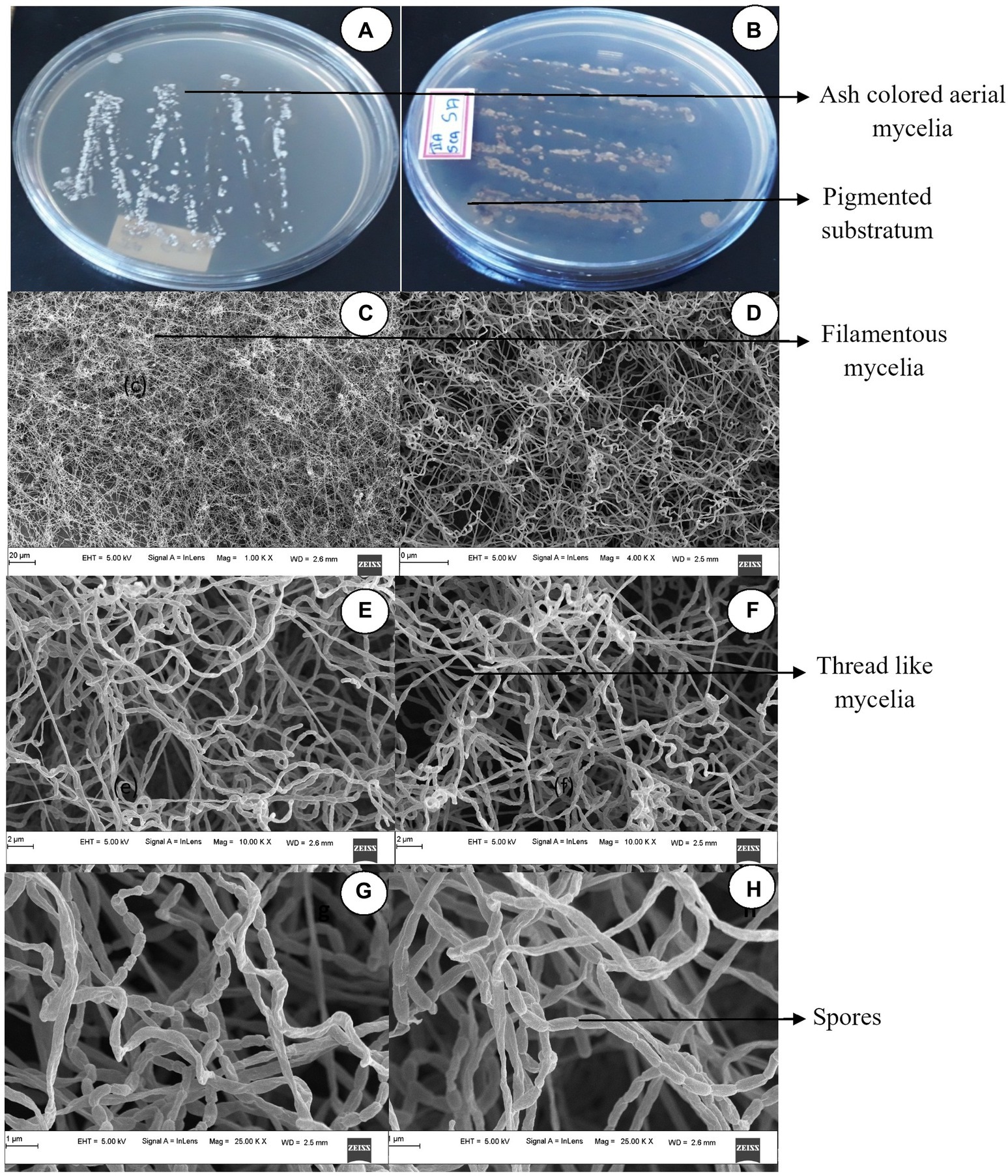
Figure 1. Cultural characteristics of Streptomyces tauricus. (A) Front view. (B) Rear view. (C,D) Mycelia observations under FESEM. (E,F) Mycelia along with spore analysis under FESEM (G,H) Spore structure analysis using FESEM.
Excellent growth was achieved on starch casein nitrate (SCN) agar, whereas moderate growth was observed on sucrose-peptone agar, yeast extract agar, and nutrient agar media. Moderate growth was seen on malt extract agar. No growth was seen on glucose-leucine agar. Yeast malt extract agar as the best growth medium for S. tauricus in another study (Rather et al., 2017).
In our previous report, when S. tauricus was subjected to the simple and rapid disruption method according to Bhaduri, it yielded significant intracellular extract (Bhaduri and Demchick, 1983).
The GCMS studies depicted the presence of 98 bioactive compounds present in the intracellular extract of S. tauricus and the elution profile as shown in Supplementary Figure S1. GC–MS analysis revealed the most likely compounds, such as octanoic acid and methyl ester with 70% probability, 2-pentanone, 4-hydroxy-methyl with 87.4% probability, 4-(benzoylmethyl)-6-methyl-2H-1 with 68.2% probability, and 4-benzoxazin-3-one with 68.2% probability, as shown in Figure 2. All the compounds detected through GC-MS showed a low molecular weight below 1 kDa with various pharmacological applications. The majority of bioactive compounds have shown antimicrobial, enzyme inhibitors, activators, antioxidants, anti-inflammatory, anti-cancer, agrochemical, insecticide, anti-obese, and many other applications as listed in Supplementary Table S1.
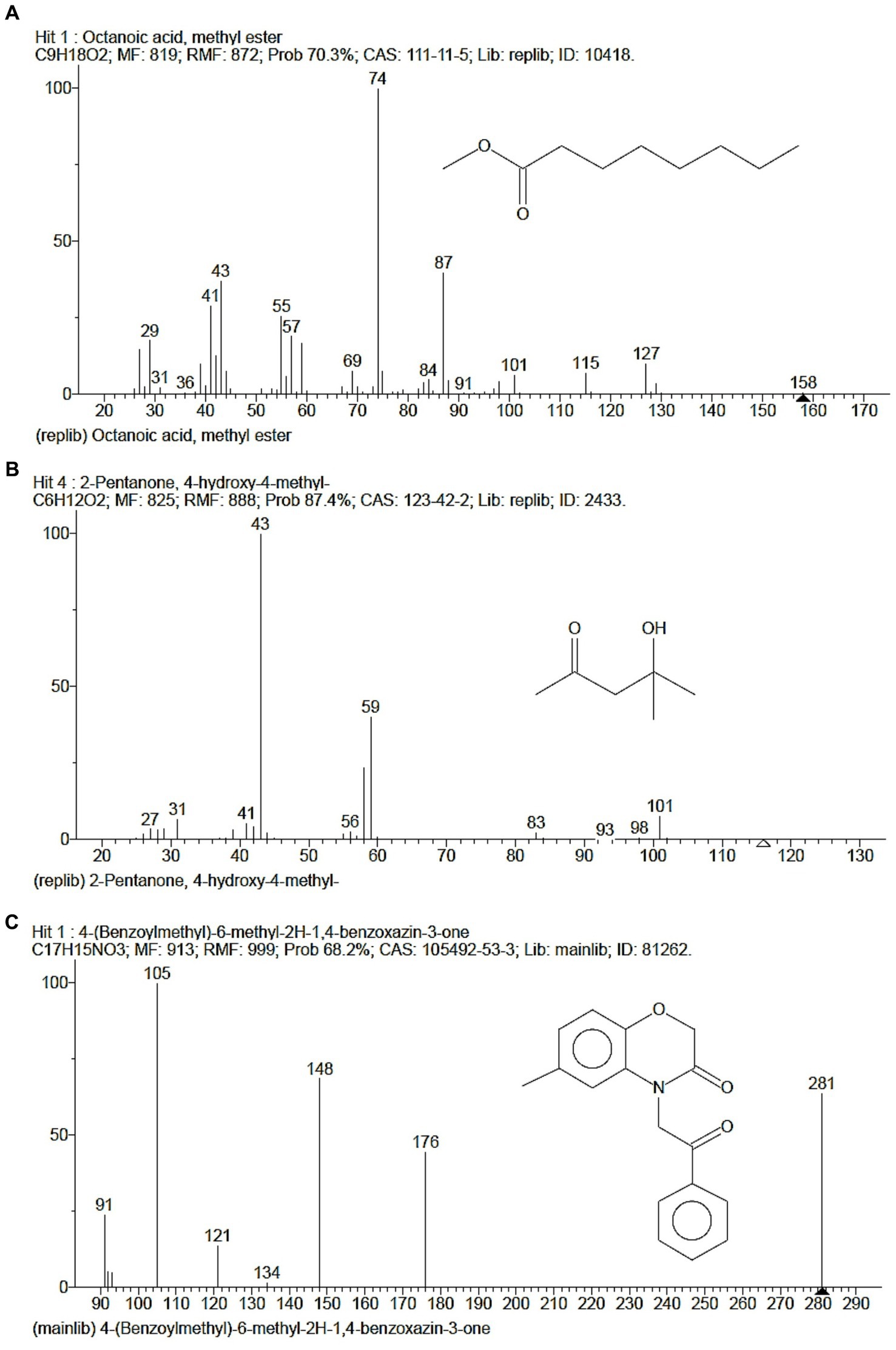
Figure 2. GC-MS depicted highest probable compounds. (A) Octanoic acid, methyl ester having 70% probability. (B) 2-Pentanone, 4-hydroxy-methyl having 87.4% probability. (C) 4-(Benzoylmethyl)-6-methyl-2H-1, 4-benzoxazin-3-one having 68.2% probability.
The eluted peak fraction protein of S. tauricus showed significant activity for different protein concentrations, such as 87% cell viability for 50 μg of protein, 79% cell viability for 100 μg, 71% cell viability for 150 μg, and 62% cell viability for 200 μg against prostate cancer PC3 cell line comparison with standard drug cisplatin at 5 μg showed 53% cell viability, p > 0.05 statistically non-significant and error graph as shown in Figure 3.

Figure 3. (A) Anticancer activity of Streptomyces tauricus strain protein. MTT assay performed by using prostate cancer PC3 cell line. (B) Untreated cells of PC3 cell line. (C) Standard cisplatin at 5 μg/mL. (D) 37.351% Anticancer activity of S. tauricus protein at 200 μg/mL.
A similar study reported that Actinomycin-D, a bioactive compound, was isolated from S. tauricus, characterized through LCMS, and showed effective antiproliferative activity against HeLa, PC3, THP-1, and Caco-2 cell lines (Rather et al., 2017). Another study reported that S. variabilis is able to produce bioactive compounds and showed increases in adipogenesis and glyceroneogenesis, exhibits anticancer and cytotoxic activity, and has purging properties in autologous bone marrow transplantation. It also alters the differentiation of human liposarcoma (Kesavan et al., 2014). Antimicrobial metabolites were also successfully isolated from Actinomyces grown on starch, casein, and nitrate agar in a marine salt pan (Ballav et al., 2015).
The Sephadex G-10 eluted peak protein fraction was further subjected to LCMS analysis. The LCMS analysis and elution profile, as shown in Supplementary Figure S2, revealed the detection of pharmacologically applicable bioactive peptide compounds. The elution peak from LCMS analysis and detection through the NIST computer library resulted in the identification of Tryprostatin B, fumonisin B1, Microcystin LR, and Surfactin C. Figure 4 depicts an LCMS analysis and comparison with the NIST library of Tryprostatin B with a 351 Da molecular weight structure and mass confirmation. The mass confirmation and structure of fumonisin B1 with a molecular weight of 721 Da are shown in Figure 5. Figure 6 depicts the structure and mass confirmation of Microcystin LR, which has a molecular weight of 994 Da. Figure 7 depicts Surfactin C with a molecular weight of 1,035 Da, structure, and mass confirmation. These bioactive molecules are well-known for their effective activity in various biological applications.
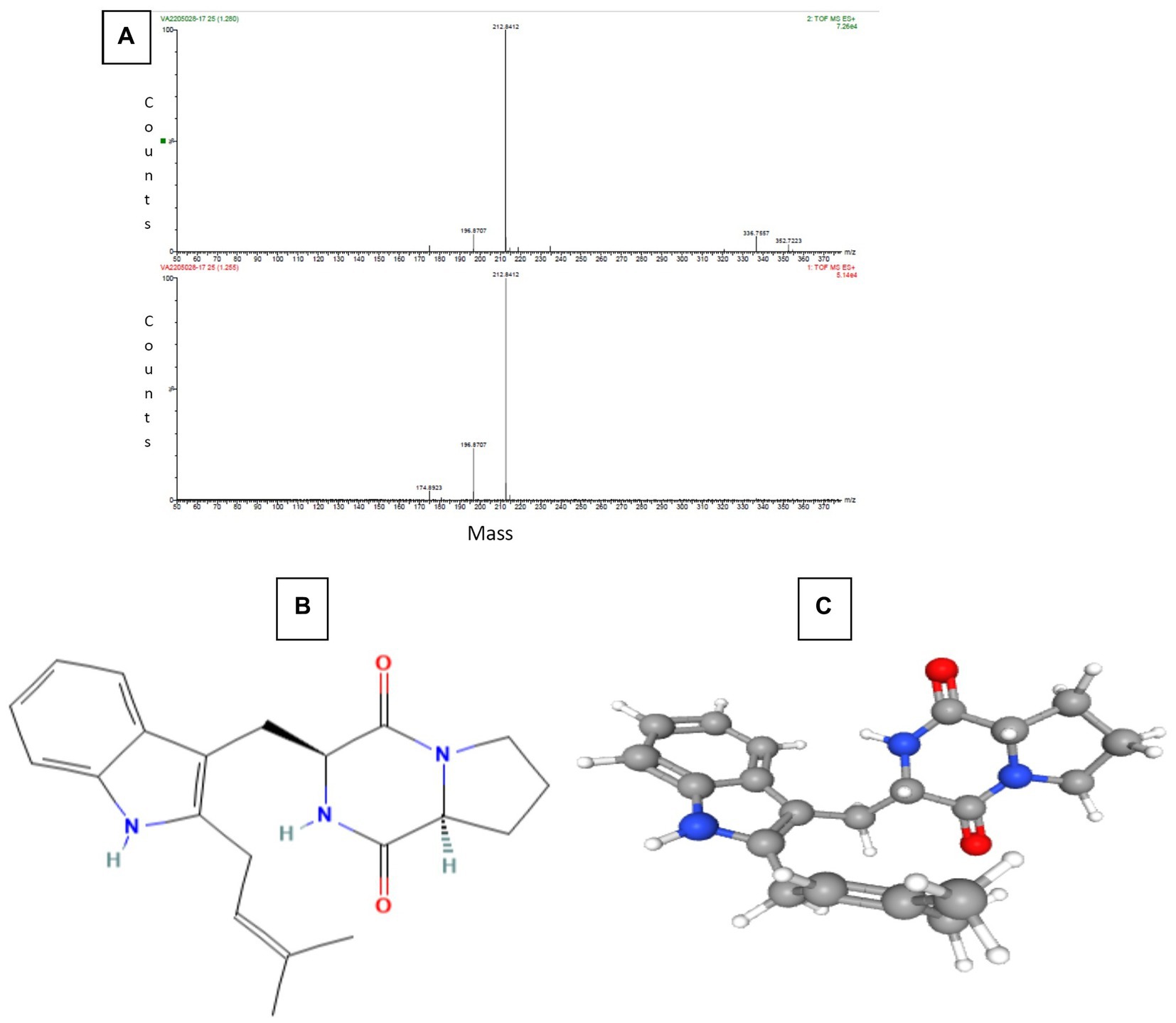
Figure 4. LC-MS analysis. (A) Mass confirmation of Tryprostatin B. (B) 2D structure of Tryptostatin B. (C) 3D structure of Tryptostatin B.
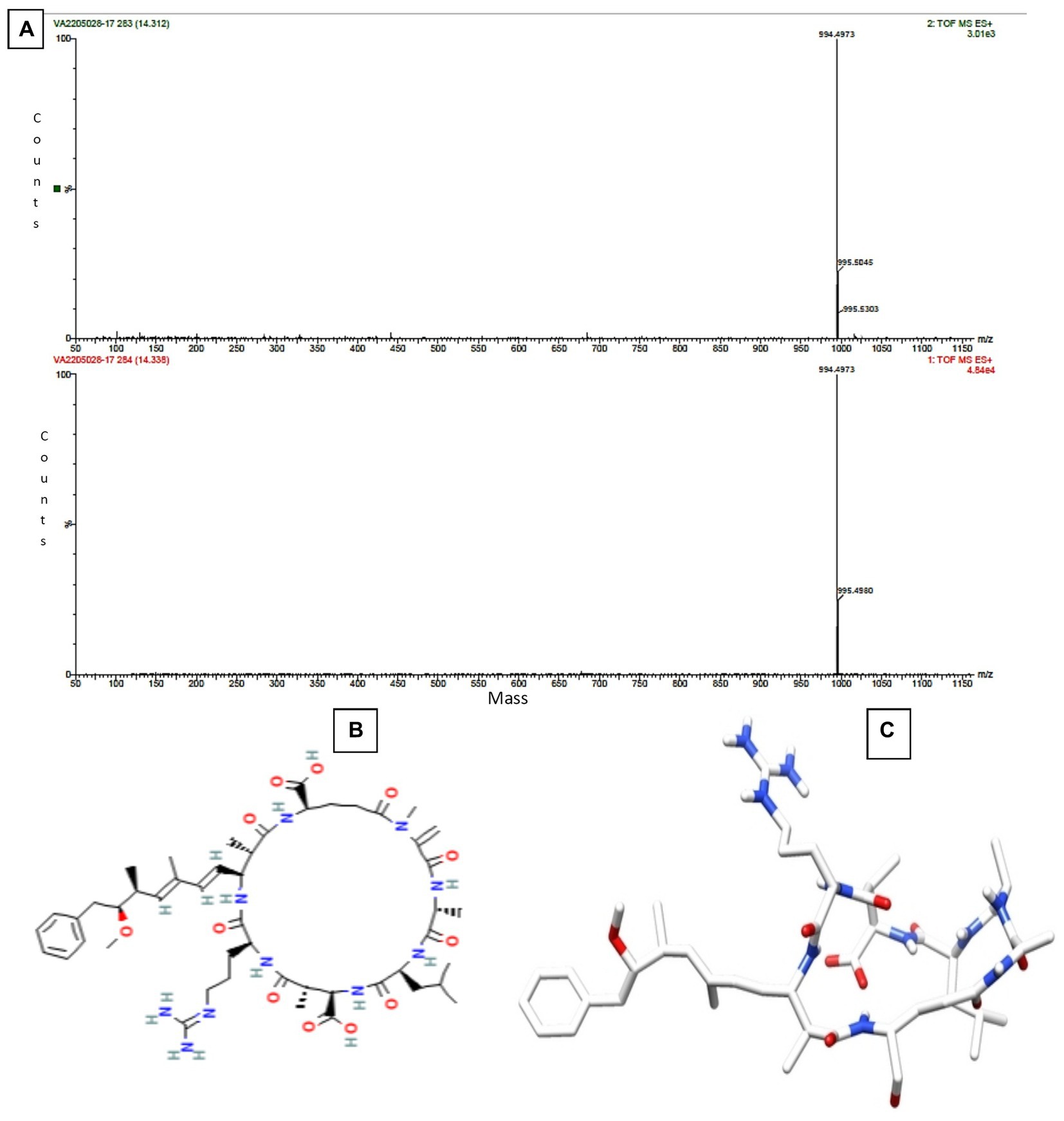
Figure 6. LC-MS analysis. (A) Mass confirmation of Microcystin LR. (B) 2D structure of Microcystin LR. (C) 3D structure of Microcystin LR.
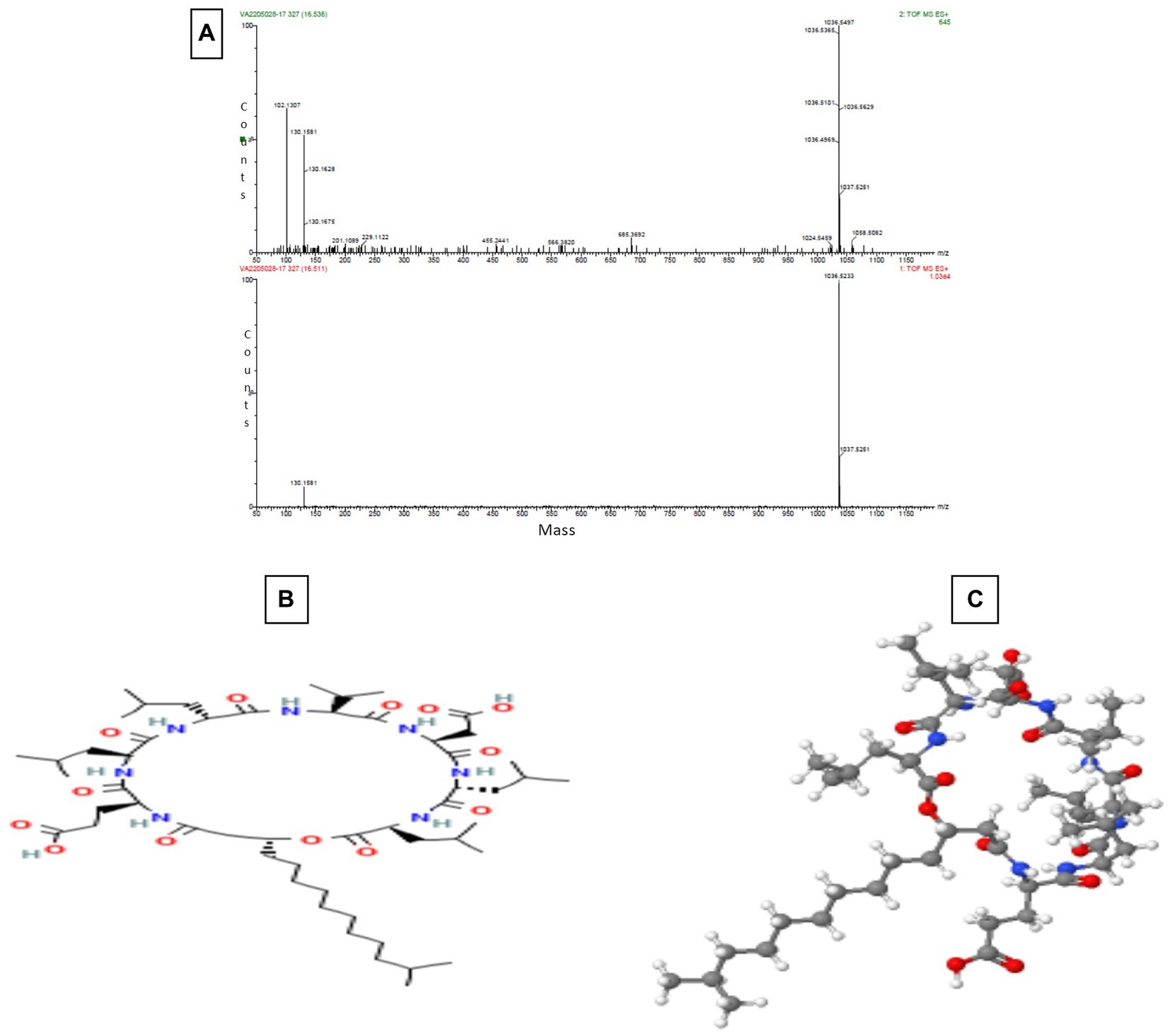
Figure 7. LC-MS Analysis (A) Mass confirmation of Surfactin C (B) 2D structure of Surfactin C (C) 3D structure of Surfactin C.
Tryprostatin B is an active peptide with varied biological properties, including anticancer, anti-mitotic, and potential in vitro and in vivo anticancer action. Brevianamide F and tryprostatin B are both cyclic dipeptides. Tryprostatin B differs from Brevianamide F by having a prenyl group added to the second position of the indole ring (Yamakawa et al., 2010; Huisman et al., 2019). Deoxy brevianamide E, also known as tryprostatin B, is a crucial step in the biosynthesis of the anti-mitotic pathway (Cytotoxic activity). Studies in biochemistry and genetics have revealed the roles of biosynthetic genes and isolated significant intermediates and shunt products from these processes (Pan et al., 2014).
The enzyme ceramide synthase, also known as sphingosine N-acyltransferase, which acylates sphingoid bases, is inhibited by fumonisin B1. This prevents the production of ceramide in two different ways. It prevents the production of new sphinganine, fatty acyl-CoA, and sphingosine, which are formed when ceramidase breaks down ceramide (Merrill et al., 2001). The cytotoxic and growth-inhibitory properties of sphinganine and sphingosine are present. These sphingoid bases also trigger apoptosis. Increased apoptosis appears to be crucial for the harmful effects, including the formation of tumors (Zentai et al., 2019).
In freshwater lakes and rivers throughout the world, Microcystis aeruginosa is the primary producer of microcystins (MCs), the most pervasive cyanobacterial toxins. The seven-membered peptide ring of MCs, a complicated class of monocyclic heptapeptides with considerable hepatotoxicity, is made up of two protein amino acids and five non protein amino acids. Due to the different proportions of the two variable protein amino acids, over 100 MC variants have been discovered thus far and named according to the variable amino acids that complete their structures. MC-LR, MC-RR, MC-YR, and MC-LA (L, leucine; R, arginine; Y, tyrosine; and A, alanine) are the most prevalent and abundant MCs, with MC-LR being the most researched and hazardous of the four. Hepatotoxins called microcystins strongly bind to serine/threonine protein phosphatases (PPs), enzymes that can remove phosphate from proteins in a variety of metabolic pathways (Li et al., 2017; Wu et al., 2019).
Numerous Bacillus strains produce the highly effective bio-surfactant lipo-peptide Surfactin C, which has hemolytic, antiviral, anticancer, and antibacterial properties. This anionic cyclic lipopeptide is composed of a heptapeptide linked to a-hydroxy fatty acid. Surfactin permeabilizes and perturbs target cells as a result of integrating into the phospholipid bilayer due to its amphipathic nature. As a result of the rise in antibiotic resistance as well as several noteworthy Surfactin activities, it is thought to be a potential chemical for treating a variety of health-related problems (Seydlová and Svobodová, 2008).
The present study is illustrative for exploring untapped mangrove habitats in the Mangalore region of Karnataka. The S. tauricus spores were visualized as elongated, rod-shaped, smooth surfaces with curved edges. The intracellular extract was purified and characterized using GCMS and LCMS analysis reveals the peptides having molecular weight of <1 kDa (Tryprostatin B, Fumonisin B1, Microcystin LR, and Surfactin C) is an efficient microbe to produce bioactive peptides for both antimicrobial and anticancer activity. Hence, this study supports and proves that the genus Streptomyces is an effective microbial group for the isolation source of bioactive peptides to treat multidrug-resistant pathogens, anticancer activity on prostate cancer cell lines, and various other related ailments.
The original contributions presented in the study are publicly available. This data can be found here: GenBank repository, accession number MW785875.
YK and MK contributed to the design of the experiments. YK performed the experiments. MK, SK, and KR guided in performing the experiments. YK and MM analyzed the results and wrote the manuscript. YK, MK, SK, KR, SS, SA, OA, and MM wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the Science and Engineering Research Board, DST, Govt. of India, and Vision Group of Science and Technology Govt. of Karnataka by providing financial and equipment grants.
Our sincere thanks to Mangalore University, India for providing infrastructure facilities. The authors would like to thank Deanship of Scientific research, Taif University, Taif, Saudi Arabia for supporting this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1152985/full#supplementary-material
Abd-Elnaby, H., Abo-Elala, G., Abdel-Raouf, U., Abd-elwahab, A., and Hamed, M. (2016). Antibacterial and anticancer activity of marine Streptomyces parvus: optimization and application. Biotechnol Biotechnol Equipment 30, 180–191. doi: 10.1080/13102818.2015.1086280
Alongi, D. M. (2015). The impact of climate change on mangrove forests. Curr Clim Change Rep 1, 30–39. doi: 10.1007/s40641-015-0002-x
Ballav, S., Kerkar, S., Thomas, S., and Augustine, N. (2015). Halophilic and halotolerant actinomycetes from a marine saltern of Goa, India producing anti-bacterial metabolites. J. Biosci. Bioeng. 119, 323–330. doi: 10.1016/j.jbiosc.2014.08.017
Bhaduri, S., and Demchick, P. H. (1983). Simple and rapid method for disruption of bacteria for protein studies. Appl. Environ. Microbiol. 46, 941–943. doi: 10.1128/aem.46.4.941-943.1983
Burckhardt, R. M., Van Drisse, C. M., Tucker, A. C., and Escalante-Semerena, J. C. (2020). New AMP-forming acid:CoA ligases from Streptomyces lividans, some of which are posttranslationally regulated by reversible lysine acetylation. Mol. Microbiol. 113, 253–269. doi: 10.1111/mmi.14414
Chater, K. F. (2013). “Streptomyces” in Brenner’s encyclopedia of genetics. 2nd Edn. ed. J. Parker (Cambridge, Massachusetts, United States: Elsevier), 565–567.
Desouky, S. E., Shojima, A., Singh, R. P., Matsufuji, T., Igarashi, Y., Suzuki, T., et al. (2015). Cyclodepsipeptides produced by actinomycetes inhibit cyclic-peptide-mediated quorum sensing in gram-positive bacteria. FEMS Microbiol. Lett. 362:fnv109. doi: 10.1093/femsle/fnv109
Fernebro, J. (2011). Fighting bacterial infections-future treatment options. Drug Resist. Updat. 14, 125–139. doi: 10.1016/j.drup.2011.02.001
Huisman, M., Rahaman, M., Asad, S., Oehm, S., Novin, S., Rheingold, A. L., et al. (2019). Total synthesis of Tryprostatin B: synthesis and asymmetric phase-transfer-catalyzed reaction of Prenylated Gramine salt. Org. Lett. 21, 134–137. doi: 10.1021/acs.orglett.8b03593
Janardhan, A., Kumar, A. P., Viswanath, B., Saigopal, D. V., and Narasimha, G. (2014). Production of bioactive compounds by actinomycetes and their antioxidant properties. Biotechnol. Res. Int. 2014:217030. doi: 10.1155/2014/217030
Karthik, Y., Ishwara Kalyani, M., Krishnappa, S., Devappa, R., Anjali Goud, C., Ramakrishna, K., et al. (2023). Antiproliferative activity of antimicrobial peptides and bioactive compounds from the mangrove Glutamicibacter mysorens. Front. Microbiol. 14:826. doi: 10.3389/fmicb.2023.1096826
Karthik, Y., and Kalyani, M. I. (2022). Occurrence of Streptomyces tauricus in mangrove soil of Mangalore region in Dakshina Kannada as a source for antimicrobial peptide. J. Basic Microbiol. 63, 389–403. doi: 10.1002/jobm.202200108
Karthik, Y., Kalyani, M. I., Ishwar, M., et al. (2020). Cytotoxic and antimicrobial activities of microbial proteins from mangrove soil actinomycetes of Mangalore, Dakshina Kannada. Biomedicine 40, 59–67. doi: 10.51248/.v40i1.104
Kesavan, S. S. S., Bavanilatha, M., Vijyalakshmi, R., and Hemalatha, S. (2014). Analysis of bioactive constituents from a new Streptomyces Variabilis strain SU5 by gas chromatography-mass spectrometry. International. J. Pharm. Pharm. Sci. 6, 224–226.
Li, J., Li, R., and Li, J. (2017). Current research scenario for microcystins biodegradation – a review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 595, 615–632. doi: 10.1016/j.scitotenv.2017.03.285
Merrill, A. H., Sullards, M. C., Wang, E., Voss, K. A., and Riley, R. T. (2001). Sphingolipid metabolism: roles in signal transduction and disruption by Fumonisins. Environ. Health Perspect. 109, 283–289. doi: 10.2307/3435020
Mohan, Y., Sirisha, B., Haritha, R., and Ramana, T. (2013). Selective screening, isolation and characterization of antimicrobial agents from marine actinomycetes. Int J Pharm Pharm Sci 5, 443–449.
Nishioka, K., and Katayama, I. (1978). Angiogenic activity in culture supernatant of antigen-stimulated lymph node cells. J. Pathol. 126, 63–69. doi: 10.1002/path.1711260202
Pan, L.-L., Yang, Y., and Merz, K. M. (2014). Origin of product selectivity in a Prenyl transfer reaction from the same intermediate: exploration of multiple Ftm PT1-catalyzed Prenyl transfer pathways. Biochemistry 53, 6126–6138. doi: 10.1021/bi500747z
Rather, S. A., Shah, A. M., Ali, S. A., Dar, R. A., Rah, B., Ali, A., et al. (2017). Isolation and characterization of Streptomyces tauricus from Thajiwas glacier—a new source of actinomycin-D. Med. Chem. Res. 26, 1897–1902. doi: 10.1007/s00044-017-1842-9
Renner, M. K., Shen, Y.-C., Cheng, X.-C., Jensen, P. R., Frankmoelle, W., Kauffman, C. A., et al. (1999). Cyclomarins a−C, new Antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.). J. Am. Chem. Soc. 121, 11273–11276. doi: 10.1021/ja992482o
Sapkota, A., Thapa, A., Budhathoki, A., Sainju, M., Shrestha, P., and Aryal, S. (2020). Isolation, characterization, and screening of antimicrobial-producing Actinomycetes from soil samples. Int J Microbiol 2020, 1–7. doi: 10.1155/2020/2716584
Seydlová, G., and Svobodová, J. (2008). Review of surfactin chemical properties and the potential biomedical applications. Cent. Eur. J. Med 3, 123–133. doi: 10.2478/s11536-008-0002-5
Sridevi, V., Priyadarshini, P. S., and Gautam, Y. A. (2015). Selection of marine actinomycetes with bioactive potential isolated from sediments of bay of Bengal and characterization of promising isolate, ABT-103. Microbiol Res J Int, 10, 1–9. doi: 10.9734/BMRJ/2015/20132
Wang, C., Lu, Y., and Cao, S. (2020). Antimicrobial compounds from marine actinomycetes. Arch. Pharm. Res. 43, 677–704. doi: 10.1007/s12272-020-01251-0
Wu, J., Chen, D., Wu, J., Chu, X., Yang, Y., Fang, L., et al. (2021). Comparative transcriptome analysis demonstrates the positive effect of the cyclic AMP receptor protein Crp on Daptomycin biosynthesis in Streptomyces roseosporus. Front. Bioeng. Biotechnol. 9:e618029. doi: 10.3389/fbioe.2021.618029
Wu, J., Wang, X., Wang, Q., Lou, Z., Li, S., Zhu, Y., et al. (2019). Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes (II). Chem. Soc. Rev. 48, 1004–1076. doi: 10.1039/C8CS00457A
Yamakawa, T., Ideue, E., Shimokawa, J., and Fukuyama, T. (2010). Total synthesis of Tryprostatins A and B. Angew. Chem. Int. Ed. Engl. 49, 9262–9265. doi: 10.1002/anie.201004963
Keywords: anticancer, chromatography, FESEM, mangroves, Streptomyces tauricus
Citation: Karthik Y, Kalyani MI, Krishnappa S, Ramakrishna K, Sayed SM, Aharthy OM, Alhelaify SS and Mushtaq M (2023) Promising bioactive metabolites of mangrove inhabitant Streptomyces tauricus and prostate cancer PC3 cell inhibition by antimicrobial peptides. Front. Microbiol. 14:1152985. doi: 10.3389/fmicb.2023.1152985
Received: 28 January 2023; Accepted: 22 May 2023;
Published: 16 June 2023.
Edited by:
Mohammad Ali Amoozegar, University of Tehran, IranReviewed by:
Kamal A. Qureshi, Qassim University, Saudi ArabiaCopyright © 2023 Karthik, Kalyani, Krishnappa, Ramakrishna, Sayed, Aharthy, Alhelaify and Mushtaq. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manjula Ishwara Kalyani, bWFuanVnYW5lc2g3MTc2QGdtYWlsLmNvbQ==; Muntazir Mushtaq, bXVudGF6aXJodWRhQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.