- Department of Biological Sciences, UST Laboratories for Vaccine Science, Molecular Biology, and Biotechnology, Research Center for the Natural and Applied Sciences, University of Santo Tomas, Manila, Philippines
Vaccines that are delivered orally have several advantages over their counterparts that are administered via injection. Despite the advantages of oral delivery, however, approved oral vaccines are currently limited either to diseases that affect the gastrointestinal tract or to pathogens that have a crucial life cycle stage in the gut. Moreover, all of the approved oral vaccines for these diseases involve live-attenuated or inactivated pathogens. This mini-review summarizes the potential and challenges of yeast oral vaccine delivery systems for animal and human infectious diseases. These delivery systems utilize whole yeast recombinant cells that are consumed orally to transport candidate antigens to the immune system of the gut. This review begins with a discussion of the challenges associated with oral administration of vaccines and the distinct benefits offered by whole yeast delivery systems over other delivery systems. It then surveys the emerging yeast oral vaccines that have been developed over the past decade to combat animal and human diseases. In recent years, several candidate vaccines have emerged that can elicit the necessary immune response to provide significant protection against challenge by pathogen. They serve as proof of principle to show that yeast oral vaccines hold much promise.
Introduction
Vaccines that are delivered orally have several advantages over their counterparts that are administered via injection (Wang and Coppel, 2008; Alotaibi et al., 2021). Physiologically, oral delivery of vaccines can stimulate both the humoral and cellular immune response at both systemic and mucosal sites, which, in principle, should establish broader and longer-lasting protection (Russell and Mestecky, 2022). They are also non-invasive, convenient, and can be self-administered. These are characteristics of a vaccine that can help increase immunization coverage of a population during a pandemic: It is striking that a recent study of 15,000+ adults in the United Kingdom revealed that injection fears can explain 10% of COVID-19 vaccine hesitancy (Freeman et al., 2021). These are also characteristics that would benefit developing, i.e., low- and middle-income, countries (LMICs), which often have poor healthcare systems that lack healthcare workers (Levine, 2010).
Despite the advantages of oral delivery, approved oral vaccines are currently limited to diseases that affect the gastrointestinal tract (GIT) or to pathogens that have a crucial life cycle stage in the GIT (Vela Ramirez et al., 2017). Moreover, all of the approved oral vaccines for these disease involve live-attenuated or inactivated pathogens. However, there are a variety of delivery systems that are being developed for oral delivery of vaccines including particle-based, lipid-based, adenoviral-based, and bacterial-based vectors (Coffey et al., 2021). Many of these emerging oral delivery systems utilize subunit vaccines involving components of the pathogen rather than whole-viral or whole-cell formulations.
This mini-review summarizes the potential and challenges of yeast oral vaccine delivery systems for human and animal infectious diseases. These delivery systems utilize whole yeast recombinant cells that are consumed orally to transport candidate antigens to the immune system of the GIT. The review begins with a discussion of the challenges associated with oral administration of vaccines and the distinct benefits offered by whole yeast delivery systems, particularly the commonly used budding yeast, Saccharomyces cerevisiae, over other delivery systems that rely on particles, lipid vesicles, viruses, or bacterial cells. It then surveys the emerging oral vaccines that have been developed over the past decade using yeast to counter animal and human diseases. In the past few years, several candidate vaccines have emerged that can elicit the necessary immune response to provide significant protection against challenge by pathogen. However, there is a need for studies involving adjuvants and nutritional supplements to try to optimize the use of yeast oral vaccines, especially if they are to move to human clinical trials and beyond.
The inherent advantages of yeast oral vaccines
There are four fundamental biochemical and physiological challenges that need to be overcome by any efficacious oral vaccine targeting the small intestine, which is the target region of the mammalian GIT for all currently approved oral vaccines and for all the emerging yeast candidate delivery systems. First, a candidate oral vaccine must survive exposure to low pH, bile salts, and digestive enzymes including peptidases and other proteolytic enzymes, as it traverses the gut. Many candidate immunogens such as antigenic proteins and peptides are highly susceptible to degradation and denaturation in this environment (Renukuntla et al., 2013; Yang et al., 2022). Second, once it reaches the gut, the candidate vaccine has a time limit for absorption that is determined by the relatively brief residence time (3–4 h) in the small intestine (Tyagi et al., 2018; Vinarov et al., 2021). If the immunogen is not absorbed in time, then it cannot be presented to the immune system.
Third, upon absorption, the oral vaccine must deliver the immunogen across the intestinal mucosa, which is structured to prevent the unwanted uptake of pathogens and macromolecules, so that the antigen can interact with the gut-associated lymphoid tissue (GALT) and be presented to the immune system. Finally, an oral vaccine must overcome the complex mechanisms of immune tolerance that allow human beings to live alongside commensal organisms and the benign environmental antigens found in food (Tordesillas and Berin, 2018; Foong and Santos, 2022; Zhao and Maynard, 2022). It is likely that this final challenge explains the higher doses of antigen (sometimes up to 100-fold) required for immune stimulation by oral vaccines as compared to their injected parenteral counterparts (Pavot et al., 2012).
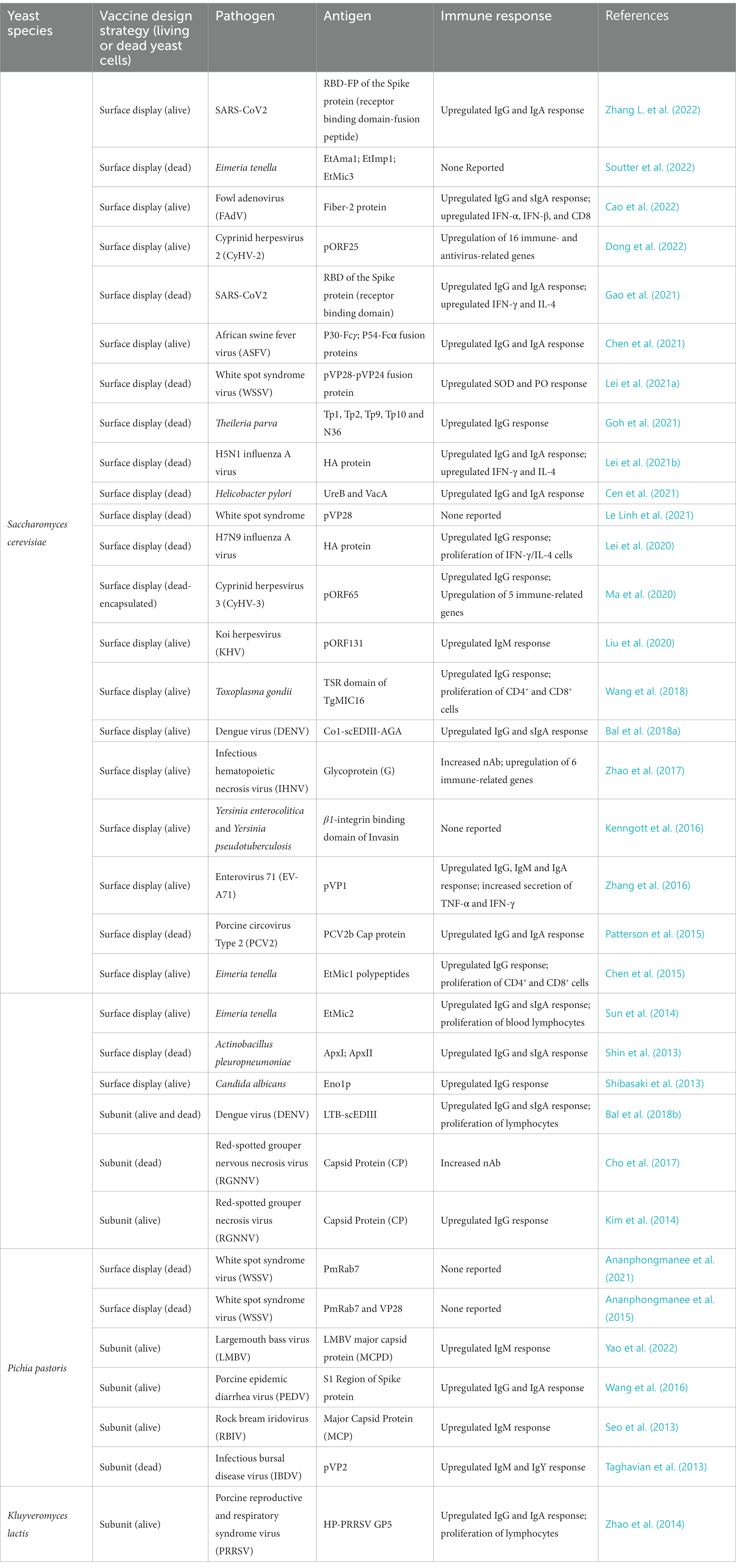
Yeast delivery platforms have at least three inherent advantages over other oral platforms that rely on particle, lipid vesicle, and viral or bacterial systems. We will focus here on the budding yeast, Saccharomyces cerevisiae, which is the most popular yeast being developed into oral vaccines at this time (Table 1). Two cousins of this budding yeast, Pichia pastoris and Kluyveromyces lactis, are also being tested as oral vaccines in a handful of animal models (Seo et al., 2013; Zhao et al., 2014; Wang et al., 2016; Embregts et al., 2019; Ananphongmanee et al., 2021), but much less is known about the interactions of these yeasts with the mammalian digestive and immune systems.
First, S. cerevisiae has been used in the fermentation of food and drink for 1,000 of years, with GRAS (“generally regarded as safe”) status in the food industry. Probiotic strains of both S. cerevisiae and its conspecific relative, Saccharomyces boulardii, have been tested in numerous human clinical trials and have been found to be safe for human consumption (McFarland, 2010; Lam et al., 2019; Pais et al., 2020). Significantly, when they are consumed, these yeasts are known to survive transit through the harsh environment of the gut: When S. boulardii was given to healthy volunteers as a probiotic at therapeutic doses (1–2 × 1010/d), colonic levels were 2 × 108/g stool (Klein et al., 1993). One possible reason for this is that S. boulardii cells, and to a lesser extent, S. cerevisiae, are resistant to low pH and bile acid (Fietto et al., 2004; Edwards-Ingram et al., 2007; Cascio et al., 2013; Hudson et al., 2014). As such, these yeasts are prime candidates for a safe and efficacious vaccine delivery system through the harsh environment of the gut.
Second, these yeasts have a relatively long residence time in the human gut: For instance, the probiotic budding yeast, S. boulardii, takes 3–5 days to be cleared after oral administration is discontinued (Blehaut et al., 1989; Klein et al., 1993; Elmer et al., 1999). Recall that most food items usually takes just hours to transit the GIT. One paper has reported that orally delivered S. cerevisiae are engulfed by dendritic cells, and that the GFP gene packaged in the yeast is released in the mammalian cytosol where it is expressed (Kiflmariam et al., 2013). Another study has shown that recombinant S. cerevisiae cells bioencapsulated in Artemia could deliver intact antigen to the hindgut of carp larvae and that the antigen could be detected in the mucosal layer of the intestine (Ma et al., 2020). Together, this data suggests that a yeast oral vaccine and its cargo immunogen would have enough time to be absorbed and processed in the gut.
Finally, budding yeast is already known to stimulate the immune system, making it a natural vaccine adjuvant. Saccharomyces cerevisiae cells injected subcutaneously induce a robust immunological response in mammals and are avidly taken up by dendritic cells and macrophages via phagocytosis (Stubbs et al., 2001; Heintel et al., 2003; Bernstein et al., 2008; Howland et al., 2008; Wansley et al., 2008; Liu et al., 2011). This leads to priming of MHC class I- and class II-restricted, antigen-specific T-cell responses (Stubbs et al., 2001; Bernstein et al., 2008). In contrast, although S. boulardii induces a systemic humoral immune response in mice when administered orally, this response is small in magnitude and not directed against S. boulardii itself (Hudson et al., 2016). Nonetheless, it is significant that S. cerevisiae, administered subcutaneously, and S. boulardii, administered orally, have been able to function as adjuvants to enhance the mammalian immune response to vaccines (Grover et al., 2016; Silveira et al., 2017). This suggests that budding yeast may be an ideal oral delivery system, not only because they can efficiently deliver the antigen through the gut and to the immune system, but also because they can inherently stimulate the innate immune system as a stalwart adjuvant as well.
Emerging yeast oral vaccines for infectious diseases
Currently, there are two basic design strategies for yeast oral vaccine delivery systems that are being deployed against animal and human infectious diseases. The first involves the cell-surface display of peptide or protein antigens, while the second involves the expression and possible secretion of the same.
First, several research teams have used cell-surface display technology to create numerous yeast oral vaccine delivery systems. Cell-surface display directs the expression of target peptides or proteins to the cell surface of a diverse range of cell types through the connection of a protein of interest fused to an anchor protein (Teymennet-Ramírez et al., 2022). Of the 34 papers published in the last 10 years describing a candidate yeast oral vaccine, 26 of them have used cell-surface display to coat the yeast cell with a candidate immunogen (Table 1). These coated yeast cells were then administered orally to animals.
Candidate yeast oral vaccines with cell surface display are being developed against a diverse array of pathogens, including White Spot Syndrome Virus, H5N1 Influenza A Virus, African Swine Fever Virus, and SARS-CoV2, among others, that infect a wide range of animal organisms including shrimp, fish, fowl, swine, and human beings (Table 1). Most of these yeast oral vaccines use the a-agglutinin Aga1p-Aga2p Yeast Surface Display (YSD) system in S. cerevisiae (Mei et al., 2017; Zhang C. et al., 2022). Here, the heterologous protein of interest, in our case, the candidate antigen/immunogen, is expressed as a fusion to the Aga2p protein, which in vivo is linked to cell-wall covalently-associated Aga1p through two disulfide bonds. A second system uses the alpha-agglutinin Sag1p to anchor the heterologous protein of interest directly to the cell wall (Zhang C. et al., 2022).
The majority of emerging yeast oral vaccines with cell surface display target animal viruses (Table 1). Recently, however, two novel candidate vaccines have been developed against SARS-CoV2 (Gao et al., 2021; Zhang L. et al., 2022). Both candidate COVID-19 vaccines used the receptor binding domain (RBD) of the SARS-CoV2 Spike protein as a candidate immunogen. In one case, the RBD domain was also fused to adjacent the fusion peptide (FP) domain (Zhang L. et al., 2022). Both candidate vaccines were able to elicit robust IgG and IgA responses against the SARS-CoV2 RBD domain. Gao et al. also showed that their candidate vaccine was able to trigger robust cellular immune responses: T-cells proliferated with increased IFN-𝛾 and IL-4 expression. There was also an increase in Th1 bias in memory lymphocytes (Gao et al., 2021). Strikingly, these heightened immune responses were noted even though two distinct vaccination schedules were used suggesting that the precise schedule may not be critical. For one study, mice were orally administered the yeast vaccine on days 1 and 2 for prime immunization and then again on days 14 and 15 for boost immunization (Gao et al., 2021). In contrast, the second SARS-CoV2 study reported that their mice received their oral vaccines on day 1 for prime immunization and then again on days 5, 10, and 21, for boost immunization (Zhang L. et al., 2022). Neither of these studies reported the levels of neutralizing antibodies against SARS-CoV2, and neither vaccine was the subject to a direct challenge animal trial. Both of these will have to be assessed if these vaccine candidates are to proceed to human clinical trials.
Returning now to the other yeast oral vaccines using surface display technology to target non-human animal pathogens, most of the candidate vaccines were able to elicit a strong immune response akin to the responses observed with their SARS-CoV2 counterparts described above. However, many of them were also able to protect vaccinated animals against direct challenge from their target pathogen. These include candidate yeast oral vaccines against red-spotted grouper nervous necrosis virus in convict groupers (Cho et al., 2017); Helicobacter pylori in mice (Cen et al., 2021); cyprinid herpesvirus-3 in the common carp (Ma et al., 2020); avian H5N1 influenza virus in chickens (Lei et al., 2021b); and white spot syndrome virus in shrimp (Ananphongmanee et al., 2021), and among others. This growing list of successful animal vaccines suggests that cell-surface display is an effective technology for creating effective yeast oral vaccines.
Next, other research groups are generating subunit yeast oral vaccines where antigenic peptides or proteins are expressed and retained within the budding yeast cell, or are expressed and secreted into the environment. Two emerging yeast oral vaccines of this kind that target animal disease have been recently described.
Recombinant whole budding yeast expressing the capsid protein (CP) of the red-spotted grouper necrosis virus (RGNNV) that were orally administered to mice provoked significantly higher levels of anti-RGNNV IgG antibodies as compared with mice given purified capsid protein (Kim et al., 2014). Moreover, this yeast oral vaccine was able to elicit neutralizing activity against RGNNV while the purified antigen could not. Significantly, when given to fish in a freeze-dried form after disruption, the same CP vaccine was able to reduce mortality in groupers in response to direct challenge with RGNNV (Cho et al., 2017). Neither of these studies included adjuvants to try to enhance the immunogenicity of the antigen. In contrast, another research team fused the E. coli heat-labile toxin protein B-subunit (LTB) to the consensus dengue envelope domain III (scEDIII) antigen to create a yeast oral vaccine against dengue virus (Bal et al., 2018b). Both living whole cell (WC) and dead cell-free extracts (CFE) of this oral vaccine were able to stimulate a systemic humoral immune response as well as a mucosal immune response. The team also reported neutralizing activity against DENV-1 and DENV-2, two representative serotypes that cause severe dengue infection, though only the CFE formulation was able to trigger nAbs against the latter serotype. Notably, neither of these subunit vaccines tried to target their antigens directly to the GALT of gut. They simply transported their antigens to the intestines. Nonetheless, there is a report that suggests that the strategy of targeting the candidate antigen to the immune system using peptides that direct the localization of proteins to the GALT is a sound one that could enhance the systemic immune response (Bagherpour et al., 2018).
In sum, though not as common as their cell-surface display counterparts, the few subunit yeast oral vaccines that have been described suggest that they too can elicit the necessary immune response to provide significant protection against challenge by pathogen. It should be interesting to do a head-to-head comparison between two yeast oral vaccines, one surface display and the other a subunit vaccine, that deliver the same antigen to the gut. Which strategy is the more efficacious one?
Finally, a brief comment on whether or not a yeast oral vaccine should be inactivated prior to oral administration. The animal studies described in this review include trials that have tested both living and dead yeast as oral vaccines (Table 1). Moreover, as I noted above, a direct comparison between living WC and dead CFE revealed that both can stimulate the humoral and mucosal immune responses (Bal et al., 2018b), probably because antigens remain intact after inactivation (Arnold et al., 2012). In toto, these studies suggest that both living and dead yeast are able to deliver antigen through the stomach in to the gut. In my view, however, one of the advantages of a yeast oral vaccine built upon an FDA approved probiotic is that it can be administered to human subjects alive. The yeast cells that survive transit through the harsh environment of the stomach would be able to continue to grow and to synthesize antigen de novo that would trigger the immune system in the small intestine.
Future directions
In recent years, several candidate yeast oral vaccines have emerged that have provided significant protection against challenge by pathogen. They serve as proof of principle that confirms that this emerging vaccine technology has much promise. In my view, what is most striking about the technology at this time is that there appear to be numerous ways to arrive at an efficacious yeast oral vaccine. Whether you choose to display your antigen or to express it, both strategies work. Whether you use living or dead yeast to orally administer the antigen, both strategies work. Whether you prime and boost just twice or multiple times, both strategies work.
In light of these realities, we should focus now on increasing the efficacy of all of these yeast oral vaccines, regardless of their particular design or immunization schedule. Two specific proposals come to mind. One is to explore the role of adjuvants. Only one published study has linked a known adjuvant—the E. coli heat-labile toxin protein B-subunit (LTB)—to a candidate antigen (Bal et al., 2018b). We need to determine if other adjuvants, alone or together, can boost the efficacy of yeast oral vaccines. Another is to determine if known nutritional supplements can increase the viability of the yeast delivery system and thus the efficacy of the vaccine. For example, S-Adenosyl-L-Methionine and trehalose are known to protect budding yeast cells from acid-induced cell death (Malakar et al., 2008; Cascio et al., 2013; Eun Moon et al., 2020). Can they be used as oral supplements to increase efficacy of yeast oral vaccines? Moreover, since the Ras/PKA signal transduction pathway has been implicated in the regulation of yeast cell death in an acidic environment (Lastauskiene et al., 2014), can modulating this pathway enhance yeast oral vaccine efficiency? These questions and others like them can contribute to a research program that can advance these candidate yeast oral vaccines to human clinical trials and beyond.
Finally, there is the question of regulating these yeast oral vaccines as genetically modified organisms (GMO). Legislation regulating GMO throughout the world is complex and diverse. However, there have been recent calls to simplify them so that advanced therapy medicinal products (ATMPs), such as gene therapies and vaccines that consist of or contain GMOs, can be brought to clinical trials (Kauffmann et al., 2019; Beattie, 2021).
Author contributions
The author confirms being the sole contributor of this work and has approved it for publication.
Funding
NA received a grant from the Department of Science and Technology of the Republic of the Philippines. However, the grant does not include any funds for payment of open access fees.
Acknowledgments
NA thanks Gabriel Mendoza for his assistance in identifying studies of interest for this review. NA’s laboratory was supported by a research grant from the Philippine Council for Health Research and Development (PCHRD) of the Department of Science and Technology (DOST), and the Balik Scientist Program of the Republic of the Philippines.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alotaibi, B. S., Buabeid, M., Ibrahim, N. A., Kharaba, Z. J., Ijaz, M., and Murtaza, G. (2021). Recent strategies driving oral biologic administration. Expert Rev. Vaccines 20, 1587–1601. doi: 10.1080/14760584.2021.1990044
Ananphongmanee, V., Lertpreedakorn, N., Taengchaiyaphum, S., Charoenrat, T., Sritunyalucksana, K., and Boonchird, C. (2021). Shrimp protected from a virus by feed containing yeast with a surface-displayed viral binding protein. J. Biotechnol. 342, 45–53. doi: 10.1016/j.jbiotec.2021.09.014
Ananphongmanee, V., Srisala, J., Sritunyalucksana, K., and Boonchird, C. (2015). Yeast surface display of two proteins previously shown to be protective against white spot syndrome virus (WSSV) in shrimp. PLoS One 10, 1–14. doi: 10.1371/journal.pone.0128764
Arnold, M., Durairaj, V., Mundt, E., Schulze, K., Breunig, K. D., and Behrens, S. E. (2012). Protective vaccination against infectious bursal disease virus with whole recombinant Kluyveromyces lactis yeast expressing the viral VP2 subunit. PLoS One 7:e42870. doi: 10.1371/JOURNAL.PONE.0042870
Bagherpour, G., Ghasemi, H., Zand, B., Zarei, N., Roohvand, F., Ardakani, E. M., et al. (2018). Oral Administration of Recombinant Saccharomyces boulardii expressing ovalbumin-CPE fusion protein induces antibody response in mice. Front. Microbiol. 9:723. doi: 10.3389/fmicb.2018.00723
Bal, J., Jung, H. Y., Nguyen, L. N., Park, J., Jang, Y. S., and Kim, D. H. (2018a). Evaluation of cell-surface displayed synthetic consensus dengue EDIII cells as a potent oral vaccine candidate 11 medical and health sciences 1107 immunology. Microb. Cell Fact. 17, 1–13. doi: 10.1186/s12934-018-0994-8
Bal, J., Luong, N. N., Park, J., Song, K. D., Jang, Y. S., and Kim, D. H. (2018b). Comparative immunogenicity of preparations of yeast-derived dengue oral vaccine candidate. Microb. Cell Fact. 17, 24–14. doi: 10.1186/s12934-018-0876-0
Beattie, S. (2021). Call for more effective regulation of clinical trials with advanced therapy medicinal products consisting of or containing genetically modified organisms in the European Union. Hum. Gene Ther. 32, 997–1003. doi: 10.1089/HUM.2021.058
Bernstein, M. B., Chakraborty, M., Wansley, E. K., Guo, Z., Franzusoff, A., Mostböck, S., et al. (2008). Recombinant Saccharomyces cerevisiae (yeast-CEA) as a potent activator of murine dendritic cells. Vaccine 26, 509–521. doi: 10.1016/J.VACCINE.2007.11.033
Blehaut, H., Massot, J., Elmer, G. W., and Levy, R. H. (1989). Disposition kinetics of saccharomyces boulardii in man and rat. Biopharm. Drug Dispos. 10, 353–364. doi: 10.1002/BDD.2510100403
Cao, H., Hua, D., Zhang, H., Zhang, H., Liu, N., Feng, Z., et al. (2022). Oral immunization of recombinant Saccharomyces cerevisiae expressing fiber-2 of fowl adenovirus serotype 4 induces protective immunity against homologous infection. Vet. Microbiol. 271:109490. doi: 10.1016/j.vetmic.2022.109490
Cascio, V., Gittings, D., Merloni, K., Hurton, M., Laprade, D., and Austriaco, N. (2013). S-Adenosyl-L-methionine protects the probiotic yeast, saccharomyces boulardii, from acid-induced cell death. BMC Microbiol. 13, 1–11. doi: 10.1186/1471-2180-13-35/FIGURES/6
Cen, Q., Gao, T., Ren, Y., Lu, X., and Lei, H. (2021). Immune evaluation of a Saccharomyces cerevisiae-based oral vaccine against helicobacter pylori in mice. Helicobacter 26:e12772. doi: 10.1111/hel.12772
Chen, C., Hua, D., Shi, J., Tan, Z., Zhu, M., Tan, K., et al. (2021). Porcine immunoglobulin fc fused P30/P54 protein of African swine fever virus displaying on surface of S. cerevisiae elicit strong antibody production in swine. Virol. Sin. 36, 207–219. doi: 10.1007/s12250-020-00278-3
Chen, P., Lv, J., Zhang, J., Sun, H., Chen, Z., Li, H., et al. (2015). Evaluation of immune protective efficacies of Eimeria tenella EtMic1 polypeptides with different domain recombination displayed on yeast surface. Exp. Parasitol. 155, 1–7. doi: 10.1016/j.exppara.2015.04.020
Cho, S. Y., Kim, H. J., Lan, N. T., Han, H. J., Lee, D. C., Hwang, J. Y., et al. (2017). Oral vaccination through voluntary consumption of the convict grouper Epinephelus septemfasciatus with yeast producing the capsid protein of red-spotted grouper nervous necrosis virus. Vet. Microbiol. 204, 159–164. doi: 10.1016/j.vetmic.2017.04.022
Coffey, J. W., Gaiha, G. D., and Traverso, G. (2021). Oral biologic delivery: advances toward Oral subunit, DNA, and mRNA vaccines and the potential for mass vaccination during pandemics. Annu. Rev. Pharmacol. Toxicol. 61, 517–540. doi: 10.1146/annurev-pharmtox-030320-092348
Dong, Z. R., Mu, Q. J., Kong, W. G., Qin, D. C., Zhou, Y., Wang, X. Y., et al. (2022). Gut mucosal immune responses and protective efficacy of oral yeast cyprinid herpesvirus 2 (CyHV-2) vaccine in Carassius auratus gibelio. Front. Immunol. 13, 1–15. doi: 10.3389/fimmu.2022.932722
Edwards-Ingram, L., Gitsham, P., Burton, N., Warhurst, G., Clarke, I., Hoyle, D., et al. (2007). Genotypic and physiological characterization of saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 73, 2458–2467. doi: 10.1128/AEM.02201-06
Elmer, G. W., McFarland, L. V., Surawicz, C. M., Danko, L., and Greenberg, R. N. (1999). Behaviour of saccharomyces boulardii in recurrent Clostridium difficile disease patients. Aliment. Pharmacol. Ther. 13, 1663–1668. doi: 10.1046/J.1365-2036.1999.00666.X
Embregts, C. W. E., Reyes-Lopez, F., Pall, A. C., Stratmann, A., Tort, L., Lorenzen, N., et al. (2019). Pichia pastoris yeast as a vehicle for oral vaccination of larval and adult teleosts. Fish Shellfish Immunol. 85, 52–60. doi: 10.1016/j.fsi.2018.07.033
Eun Moon, J., Heo, W., Hoon Lee, S., Hee Lee, S., Gu Lee, H., Hyup Lee, J., et al. (2020). Trehalose protects the probiotic yeast saccharomyces boulardii against oxidative stress-induced cell death. J. Microbiol. Biotechnol. 30, 54–61. doi: 10.4014/jmb.1906.06041
Fietto, J. L. R., Araújo, R. S., Valadão, F. N., Fietto, L. G., Brandão, R. L., Neves, M. J., et al. (2004). Molecular and physiological comparisons between Saccharomyces cerevisiae and saccharomyces boulardii. Can. J. Microbiol. 50, 615–621. doi: 10.1139/W04-050
Foong, R.-X., and Santos, A. F. (2022). Oral tolerance induction-opportunities and mechanisms. Foods 11:3386. doi: 10.3390/FOODS11213386
Freeman, D., Lambe, S., Yu, L. M., Freeman, J., Chadwick, A., Vaccari, C., et al. (2021). Injection fears and COVID-19 vaccine hesitancy. Psychol. Med. 53, 1185–1195. doi: 10.1017/S0033291721002609
Gao, T., Ren, Y., Li, S., Lu, X., and Lei, H. (2021). Immune response induced by oral administration with a Saccharomyces cerevisiae-based SARS-CoV-2 vaccine in mice. Microb. Cell Fact. 20:95. doi: 10.1186/s12934-021-01584-5
Goh, S., Kolakowski, J., Holder, A., Pfuhl, M., Ngugi, D., Ballingall, K., et al. (2021). Development of a potential yeast-based vaccine platform for Theileria parva infection in cattle. Front. Immunol. 12, 1–18. doi: 10.3389/fimmu.2021.674484
Grover, A., McLean, J. L., Troudt, J. L. M., Foster, C., Izzo, L., Creissen, E., et al. (2016). Heat killed Saccharomyces cerevisiae as an adjuvant for the induction of vaccine-mediated immunity against infection with mycobacterium tuberculosis. Vaccine 34, 2798–2805. doi: 10.1016/J.VACCINE.2016.04.052
Heintel, T., Breinig, F., Schmitt, M. J., and Meyerhans, A. (2003). Extensive MHC class I-restricted CD8 T lymphocyte responses against various yeast genera in humans. FEMS Immunol. Med. Microbiol. 39, 279–286. doi: 10.1016/S0928-8244(03)00294-3
Howland, S. W., Tsuji, T., Gnjatic, S., Ritter, G., Old, L. J., and Wittrup, K. D. (2008). Inducing efficient cross-priming using antigen-coated yeast particles. J. Immunother. 31, 607–619. doi: 10.1097/CJI.0B013E318181C87F
Hudson, L. E., Fasken, M. B., McDermott, C. D., McBride, S. M., Kuiper, E. G., Guiliano, D. B., et al. (2014). Functional heterologous protein expression by genetically engineered probiotic yeast Saccharomyces boulardii. PLoS One 9, 1–12. doi: 10.1371/journal.pone.0112660
Hudson, L. E., McDermott, C. D., Stewart, T. P., Hudson, W. H., Rios, D., Fasken, M. B., et al. (2016). Characterization of the probiotic yeast saccharomyces boulardii in the healthy mucosal immune system. PLoS One 11, 1–21. doi: 10.1371/journal.pone.0153351
Kauffmann, F., Van Damme, P., Leroux-Roels, G., Vandermeulen, C., Berthels, N., Beuneu, C., et al. (2019). Clinical trials with GMO-containing vaccines in Europe: status and regulatory framework. Vaccine 37, 6144–6153. doi: 10.1016/J.VACCINE.2019.08.018
Kenngott, E. E., Kiefer, R., Schneider-Daum, N., Hamann, A., Schneider, M., Schmitt, M. J., et al. (2016). Surface-modified yeast cells: a novel eukaryotic carrier for oral application. J. Control. Release 224, 1–7. doi: 10.1016/j.jconrel.2015.12.054
Kiflmariam, M. G., Yang, H., and Zhang, Z. (2013). Gene delivery to dendritic cells by orally administered recombinant Saccharomyces cerevisiae in mice. Vaccine 31, 1360–1363. doi: 10.1016/J.VACCINE.2012.11.048
Kim, H. J., Lee, J. Y., Kang, H. A., Lee, Y., Park, E. J., and Kim, H. J. (2014). Oral immunization with whole yeast producing viral capsid antigen provokes a stronger humoral immune response than purified viral capsid antigen. Lett. Appl. Microbiol. 58, 285–291. doi: 10.1111/lam.12188
Klein, S. M., Elmer, G. W., McFarland, L. V., Surawicz, C. M., and Levy, R. H. (1993). Recovery and elimination of the biotherapeutic agent, saccharomyces boulardii, in healthy human volunteers. Pharm. Res. 10, 1615–1619. doi: 10.1023/A:1018924820333
Lam, S., Zuo, T., Ho, M., Chan, F. K. L., Chan, P. K. S., and Ng, S. C. (2019). Review article: fungal alterations in inflammatory bowel diseases. Aliment. Pharmacol. Ther. 50, 1159–1171. doi: 10.1111/APT.15523
Lastauskiene, E., Zinkevičiene, A., and Čitavičius, D. (2014). Ras/PKA signal transduction pathway participates in the regulation of Saccharomyces cerevisiae cell apoptosis in an acidic environment. Biotechnol. Appl. Biochem. 61, 3–10. doi: 10.1002/BAB.1183
Le Linh, H., Thu, N. P. A., Dung, T. T. X., Van Hau, N., Nghia, N. H., and Thao, D. T. P. (2021). Yeast cell surface displaying VP28 antigen and its potential application for shrimp farming. Appl. Microbiol. Biotechnol. 105, 6345–6354. doi: 10.1007/S00253-021-11493-7/METRICS
Lei, H., Li, S., Lu, X., and Ren, Y. (2021a). Oral administration of Saccharomyces cerevisiae displaying VP28-VP24 confers protection against white spot syndrome virus in shrimp. Virus Res. 302:198467. doi: 10.1016/j.virusres.2021.198467
Lei, H., Lu, X., Li, S., and Ren, Y. (2021b). High immune efficacy against different avian influenza H5N1 viruses due to oral administration of a Saccharomyces cerevisiae-based vaccine in chickens. Sci. Rep. 11:8977. doi: 10.1038/s41598-021-88413-2
Lei, H., Xie, B., Gao, T., Cen, Q., and Ren, Y. (2020). Yeast display platform technology to prepare oral vaccine against lethal H7N9 virus challenge in mice. Microb. Cell Fact. 19:53. doi: 10.1186/s12934-020-01316-1
Levine, M. M. (2010). Developing countries: lessons from a live cholera vaccine. BMC Biol. 8, 2–11. doi: 10.1186/1741-7007-8-129
Liu, M., Clemons, K. V., Bigos, M., Medovarska, I., Brummer, E., and Stevens, D. A. (2011). Immune responses induced by heat killed Saccharomyces cerevisiae: a vaccine against fungal infection. Vaccine 29, 1745–1753. doi: 10.1016/J.VACCINE.2010.12.119
Liu, Z., Wu, J., Ma, Y., Hao, L., Liang, Z., Ma, J., et al. (2020). Protective immunity against CyHV-3 infection via different prime-boost vaccination regimens using CyHV-3 ORF131-based DNA/protein subunit vaccines in carp Cyprinus carpio var. Jian. Fish Shellfish Immunol. 98, 342–353. doi: 10.1016/j.fsi.2020.01.034
Ma, Y., Liu, Z., Hao, L., Wu, J., Qin, B., Liang, Z., et al. (2020). Oral vaccination using Artemia coated with recombinant Saccharomyces cerevisiae expressing cyprinid herpesvirus-3 envelope antigen induces protective immunity in common carp (Cyprinus carpio var. Jian) larvae. Res. Vet. Sci. 130, 184–192. doi: 10.1016/j.rvsc.2020.03.013
Malakar, D., Dey, A., Basu, A., and Ghosh, A. K. (2008). Antiapoptotic role of S-adenosyl-l-methionine against hydrochloric acid induced cell death in Saccharomyces cerevisiae. Biochim. Biophys. Acta 1780, 937–947. doi: 10.1016/J.BBAGEN.2008.03.014
McFarland, L. V. (2010). Systematic review and meta-analysis of saccharomyces boulardii in adult patients. World J. Gastroenterol. 16, 2202–2222. doi: 10.3748/WJG.V16.I18.2202
Mei, M., Zhou, Y., Peng, W., Yu, C., Ma, L., Zhang, G., et al. (2017). Application of modified yeast surface display technologies for non-antibody protein engineering. Microbiol. Res. 196, 118–128. doi: 10.1016/J.MICRES.2016.12.002
Pais, P., Almeida, V., Yılmaz, M., and Teixeira, M. C. (2020). Saccharomyces boulardii: what makes it tick as successful probiotic? J. Fungi 6, 1–15. doi: 10.3390/jof6020078
Patterson, R., Eley, T., Browne, C., Martineau, H. M., and Werling, D. (2015). Oral application of freeze-dried yeast particles expressing the PCV2b cap protein on their surface induce protection to subsequent PCV2b challenge in vivo. Vaccine 33, 6199–6205. doi: 10.1016/j.vaccine.2015.10.003
Pavot, V., Rochereau, N., Genin, C., Verrier, B., and Paul, S. (2012). New insights in mucosal vaccine development. Vaccine 30, 142–154. doi: 10.1016/J.VACCINE.2011.11.003
Renukuntla, J., Vadlapudi, A. D., Patel, A., Boddu, S. H. S., and Mitra, A. K. (2013). Approaches for enhancing oral bioavailability of peptides and proteins. Int. J. Pharm. 447, 75–93. doi: 10.1016/J.IJPHARM.2013.02.030
Russell, M. W., and Mestecky, J. (2022). Mucosal immunity: the missing link in comprehending SARS-CoV-2 infection and transmission. Front. Immunol. 13:957107. doi: 10.3389/FIMMU.2022.957107
Seo, J. Y., Chung, H. J., and Kim, T. J. (2013). Codon-optimized expression of fish iridovirus capsid protein in yeast and its application as an oral vaccine candidate. J. Fish Dis. 36, 763–768. doi: 10.1111/JFD.12037
Shibasaki, S., Aoki, W., Nomura, T., Miyoshi, A., Tafuku, S., Sewaki, T., et al. (2013). An oral vaccine against candidiasis generated by a yeast molecular display system. Pathog. Dis. 69, 262–268. doi: 10.1111/2049-632X.12068
Shin, M. K., Kang, M. L., Jung, M. H., Cha, S. B., Lee, W. J., Kim, J. M., et al. (2013). Induction of protective immune responses against challenge of Actinobacillus pleuropneumoniae by oral administration with Saccharomyces cerevisiae expressing Apx toxins in pigs. Vet. Immunol. Immunopathol. 151, 132–139. doi: 10.1016/j.vetimm.2012.11.003
Silveira, M. M., Conceição, F. R., Mendonça, M., Garcia Moreira, G. M. S., Da Cunha, C. E. P., Conrad, N. L., et al. (2017). Saccharomyces boulardii improves humoral immune response to DNA vaccines against leptospirosis. J. Med. Microbiol. 66, 184–190. doi: 10.1099/JMM.0.000414
Soutter, F., Werling, D., Nolan, M., Küster, T., Attree, E., Marugán-Hernández, V., et al. (2022). A novel whole yeast-based subunit Oral vaccine against Eimeria tenella in chickens. Front. Immunol. 13, 1–13. doi: 10.3389/fimmu.2022.809711
Stubbs, A. C., Martin, K. S., Coeshott, C., Skaates, S. V., Kuritzkes, D. R., Bellgrau, D., et al. (2001). Whole recombinant yeast vaccine activates dendritic cells and elicits protective cell-mediated immunity. Nat. Med. 7, 625–629. doi: 10.1038/87974
Sun, H., Wang, L., Wang, T., Zhang, J., Liu, Q., Chen, P., et al. (2014). Display of Eimeria tenella EtMic2 protein on the surface of Saccharomyces cerevisiae as a potential oral vaccine against chicken coccidiosis. Vaccine 32, 1869–1876. doi: 10.1016/j.vaccine.2014.01.068
Taghavian, O., Spiegel, H., Hauck, R., Hafez, H. M., Fischer, R., and Schillberg, S. (2013). Protective oral vaccination against infectious bursal disease virus using the major viral antigenic protein VP2 produced in Pichia pastoris. PLoS One 8:e83210. doi: 10.1371/journal.pone.0083210
Teymennet-Ramírez, K. V., Martínez-Morales, F., and Trejo-Hernández, M. R. (2022). Yeast surface display system: strategies for improvement and biotechnological applications. Front. Bioeng. Biotechnol. 9:1451. doi: 10.3389/FBIOE.2021.794742/BIBTEX
Tordesillas, L., and Berin, M. C. (2018). Mechanisms of Oral tolerance. Clin Rev Allergy Immunol 55, 107–117. doi: 10.1007/S12016-018-8680-5
Tyagi, P., Pechenov, S., and Anand Subramony, J. (2018). Oral peptide delivery: translational challenges due to physiological effects. J. Control. Release 287, 167–176. doi: 10.1016/J.JCONREL.2018.08.032
Vela Ramirez, J. E., Sharpe, L. A., and Peppas, N. A. (2017). Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 114, 116–131. doi: 10.1016/j.addr.2017.04.008
Vinarov, Z., Abdallah, M., Agundez, J. A. G., Allegaert, K., Basit, A. W., Braeckmans, M., et al. (2021). Impact of gastrointestinal tract variability on oral drug absorption and pharmacokinetics: an UNGAP review. Eur. J. Pharm. Sci. 162:105812. doi: 10.1016/J.EJPS.2021.105812
Wang, L., and Coppel, R. L. (2008). Oral vaccine delivery: can it protect against non-mucosal pathogens? Expert rev. Vaccine 7, 729–738. doi: 10.1586/14760584.7.6.729
Wang, X., Wang, Z., Xu, H., Xiang, B., Dang, R., and Yang, Z. (2016). Orally administrated whole yeast vaccine against porcine epidemic diarrhea virus induced high levels of IgA response in mice and piglets. Viral Immunol. 29, 526–531. doi: 10.1089/VIM.2016.0067
Wang, L. J., Xiao, T., Xu, C., Li, J., Liu, G., Zhen Yin, K., et al. (2018). Protective immune response against toxoplasma gondii elicited by a novel yeast-based vaccine with microneme protein 16. Vaccine 36, 3943–3948. doi: 10.1016/j.vaccine.2018.05.072
Wansley, E. K., Chakraborty, M., Hance, K. W., Bernstein, M. B., Boehm, A. L., Guo, Z., et al. (2008). Vaccination with a recombinant Saccharomyces cerevisiae expressing aTumor antigen breaks ImmuneTolerance and ElicitsTherapeutic antitumor responses. Clin. Cancer Res. 14, 4316–4325. doi: 10.1158/1078-0432.CCR-08-0393
Yang, Y., Zhou, R., Wang, Y., Zhang, Y., Yu, J., and Gu, Z. (2022). Recent advances in Oral and transdermal protein delivery systems. Angew. Chem. Int. Ed. Engl. 62:e202214795. doi: 10.1002/ANIE.202214795
Yao, J. Y., Zhang, C. S., Yuan, X. M., Huang, L., Hu, D. Y., Yu, Z., et al. (2022). Oral vaccination with recombinant Pichia pastoris expressing Iridovirus major capsid protein elicits protective immunity in largemouth bass (Micropterus salmoides). Front. Immunol. 13, 1–13. doi: 10.3389/fimmu.2022.852300
Zhang, C., Chen, H., Zhu, Y., Zhang, Y., Li, X., and Wang, F. (2022). Saccharomyces cerevisiae cell surface display technology: strategies for improvement and applications. Front. Bioeng. Biotechnol. 10, 1–15. doi: 10.3389/fbioe.2022.1056804
Zhang, C., Wang, Y., Ma, S., Li, L., Chen, L., Yan, H., et al. (2016). Human Enterovirus 71 protein displayed on the surface of Saccharomyces cerevisiae as an Oral vaccine. Viral Immunol. 29, 288–295. doi: 10.1089/VIM.2015.0110
Zhang, L., Yao, L., Guo, Y., Li, X., Ma, L., Sun, R., et al. (2022). Oral SARS-CoV-2 spike protein recombinant yeast candidate prompts specific antibody and gut microbiota reconstruction in mice. Front. Microbiol. 13, 1–11. doi: 10.3389/fmicb.2022.792532
Zhao, Q., and Maynard, C. L. (2022). Mucus, commensals, and the immune system. Gut Microbes 14:2041342. doi: 10.1080/19490976.2022.2041342
Zhao, H., Wang, Y., Ma, Z., Wang, Y., and Feng, W. H. (2014). Recombinant Kluyveromyces lactis expressing highly pathogenic porcine reproductive and respiratory syndrome virus GP5 elicits mucosal and cell-mediated immune responses in mice. J. Vet. Sci. 15, 199–208. doi: 10.4142/JVS.2014.15.2.199
Keywords: yeast, oral vaccine, Saccharomyces boulardii, Saccharomyces cerevisiae, vaccine, vaccine adjuvant and delivery system
Citation: Austriaco N (2023) Yeast oral vaccines against infectious diseases. Front. Microbiol. 14:1150412. doi: 10.3389/fmicb.2023.1150412
Edited by:
Julio Villena, CONICET Centro de Referencia para Lactobacilos (CERELA), ArgentinaReviewed by:
Dirk Werling, Royal Veterinary College (RVC), United KingdomFernando Roger Esquivel-Guadarrama, Autonomous University of the State of Morelos, Mexico
Copyright © 2023 Austriaco. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nicanor Austriaco, bmF1c3RyaWFjb0B1c3QuZWR1LnBo
 Nicanor Austriaco
Nicanor Austriaco