- Department of Gastroenterology, The Third Xiangya Hospital of Central South University, Changsha, Hunan, China
Background: The increasing antibiotic resistance is the main issue causing Helicobacter pylori (H. pylori) eradication failure. As a nutritional supplement, Egg Yolk Antibody (Ig Y) provides a new approach for H. pylori infection rescue therapy.
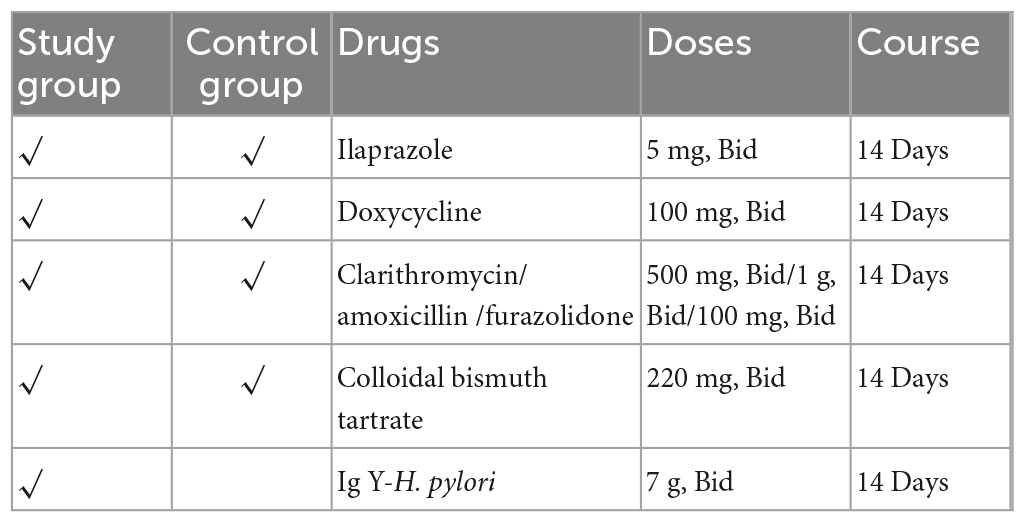
Methods: In this randomized, controlled study, 100 H. pylori-positive patients with previous H. pylori eradication treatment were included. All individuals received standard bismuth-containing quadruple therapy twice daily (5 mg ilaprazole, 100 mg doxycycline, 500 mg clarithromycin or 1 g amoxicillin or 100 mg furazolidone, and 220 mg colloidal bismuth tartrate) for 14 days and were randomized to receive either twice daily 7 g Ig Y-H. pylori treatment (study group) or not (control group). 4 weeks after the end of treatment, urea breath tests were used to assess the H. pylori eradication rate. All participants scored by the Global Overall Symptom scale (GOS) and recorded adverse events during the trial.
Results: The H. pylori eradication rates were 84.0% (95% CI 73.5–94.5%) vs. 80.0% (95% CI 68.5–91.5%) in the study and control groups at intention-to-treat (ITT) analysis and 85.7% (95% CI 75.6–95.9%) vs. 80.0% (95% CI 68.5–91.5%) at per-protocol (PP) analysis, respectively. The number of over 80% symptom relief after treatment in the two groups was 27 (60%) and 12 (29.2%) (p < 0.05), and the incidences of adverse events were 4 (8%) and 6 (12%), respectively.
Conclusion: Both groups achieved satisfactory eradication efficiency in H. pylori rescue therapy and Ig Y-H. pylori effectively alleviates the symptoms with good compliance and fewer adverse effects.
Introduction
Helicobacter pylori (H. pylori) was recognized as a class I carcinogen by the World Health Organization in 1994 and ranked as a high-priority bacterium in the list of the deadliest superbugs threatening human health in 2017 (Dang and Graham, 2017; Mommersteeg et al., 2018; Tacconelli et al., 2018). Although the overall global prevalence of H. pylori infection is declining, the consequences should not be underestimated (Ansari and Yamaoka, 2022; Wang et al., 2022).
Increased antimicrobial resistance in H. pylori contributes to the declined efficiency of previous standard eradication, especially in rescue treatments (Han et al., 2022). Available studies have shown that the eradication rate of standard triple therapy is already below 80% (Savoldi et al., 2018). Other improved regimens, including bismuth-containing quadruple therapy, concomitant therapy, and sequential therapy, to some extent help to enhance eradicating effectiveness. However, about 3 to 24% of patients still failed at first-line therapy (Lin and Hsu, 2018). Clearly, more appropriate regimens are required for rescue therapies (Peitz et al., 2002). Most guidelines recommend low-resistance antibiotic regimens, such as levofloxacin-based therapy, as a second-line treatment for H. pylori infection. Still, some clinical studies noted that these regimens failed to demonstrate satisfactory eradication rates and adverse effects (Bilardi et al., 2004; Gisbert et al., 2007). Hence, in the context of high global antibiotic resistance, it is essential to explore novel and remedial approaches to reduce multiple treatments failure of H. pylori.
Egg Yolk Antibody (Ig Y), also known as yolk immunoglobulin Y, is a particular immunoglobulin produced by specific pathogens-activated avian B lymphocytes. It is transferred from serum to yolk, then used to treat relevant pathogens by passive immunization. It is worth pointing out that Ig Y is a normal human dietary component, which is safe and free of drug resistance (Müller et al., 2015). The Food and Drug Administration (FDA) has classified yolk antibodies in the “generally recognized as safe” category. Previous studies indicated that Ig Y was considered as a potential antibiotic alternative for oral, respiratory and gastrointestinal infections (Rahman et al., 2013; Abbas et al., 2019; El-Kafrawy et al., 2023). Furthermore, Ig Y is structurally similar to mammalian immunoglobulin G (IgG) but does not bind human Fc receptors or complement system (Fink et al., 2017; Zhang et al., 2021). Compared to the traditional method of obtaining antibodies from animal serum, stable Ig Y can be extracted in large quantities from egg yolk, a non-invasive preparation process that is more economical and ethical (Mine and Kovacs-Nolan, 2002; Kovacs-Nolan and Mine, 2012). Attallah et al. (2009) found that yolk antibodies (IgY-Hp58) obtained from H. pylori-stimulated immunization effectively inhibited H. pylori infection and alleviated gastritis in BALB/c mice. Another clinical study showed that oral anti-Hp mIgY for 2 weeks reduces urea breath test values and inhibits H. pylori activity (Guo et al., 2021). Currently, Ig Y is used as an oral passive immunotherapy for preventing and controlling gastric and oral infections, with active applications in treating viral diarrhea and other gastrointestinal diseases. However, most existing Ig Y-H. pylori-related studies have been conducted in animal studies (Nomura et al., 2005; Yang et al., 2012; Hong et al., 2018), and fewer clinical studies. Thus, this single-center, randomized, controlled study aimed to find novel, safe and effective approaches for patients who have failed H. pylori eradication therapy, and to identify the feasibility of Ig Y-H. pylori as a supplementary treatment to rescue therapy.
Materials and methods
Study design and patients
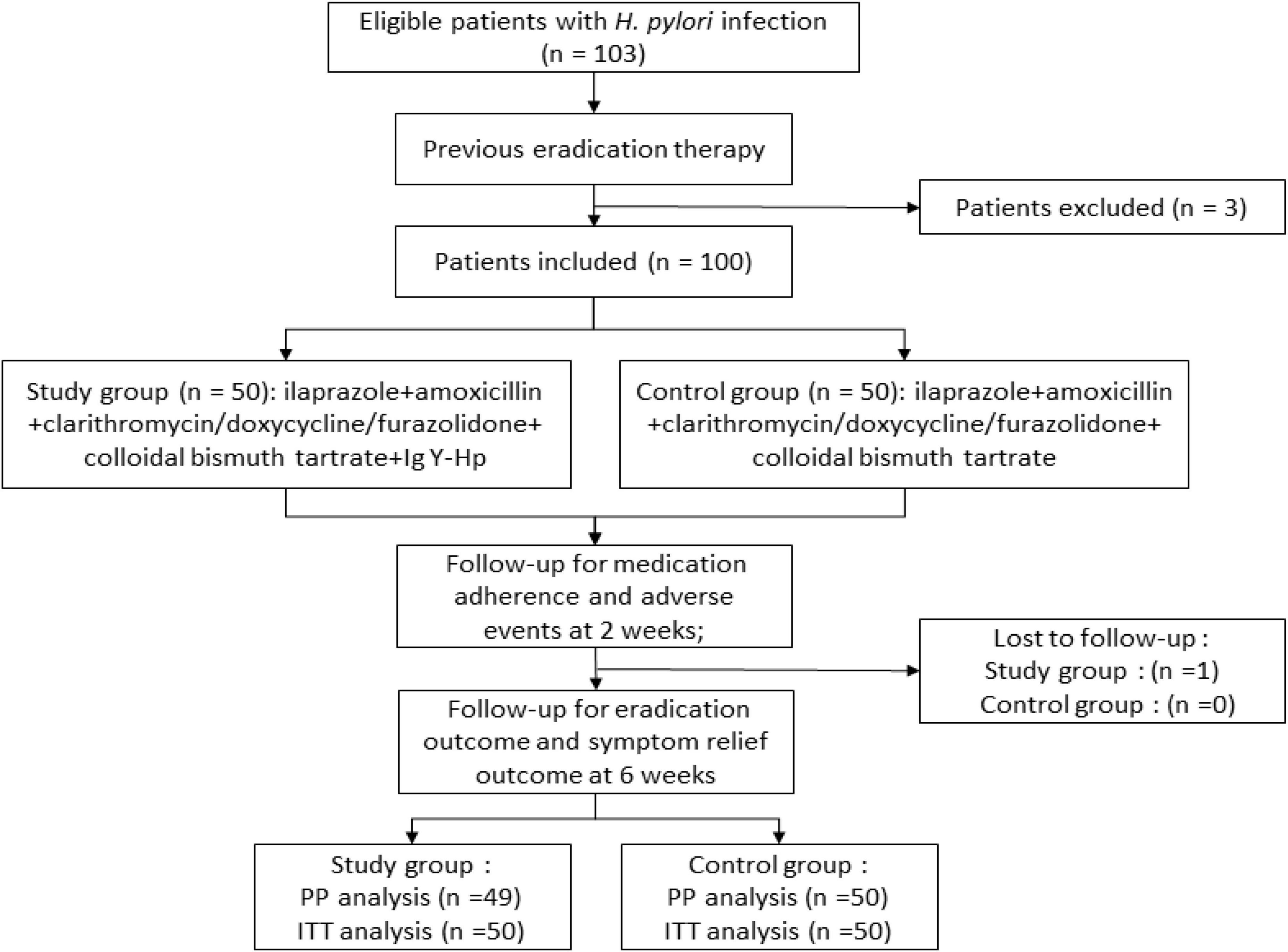
This single-center, prospective, randomized controlled trial was conducted in The Third Xiangya Hospital of Central South University between April 2022 and December 2022. Subjects were randomly assigned to study or control group based on randomized tables generated by random number generators. The study was approved by the committee of central research institution (Third Xiangya Hospital of Central South University) and obtained the informed consent from the participants.
Adult H. pylori-infected subjects who had taken complete standard eradication therapies but failed were recruited. H. pylori infection was determined by 13C/14C urea breath test (UBT) or/and rapid urease test, or/and histological testing of gastric mucosa. None of the participants were taking H2 receptor antagonists or proton pump inhibitors (PPIs) within the past 2 weeks, or antibiotics, bismuth or any other medications that might affect H. pylori activity within the past 4 weeks. Exclusion criteria were as follows: (1) allergy to Ig Y product components or drugs used in this study; (2) recent history of gastrointestinal hemorrhage, obstruction, perforation, tumor, and other gastrointestinal severe diseases; (3) other out gastrointestinal severe diseases; (4) mental illness, psychological disorders that cannot be expressed commonly; (5) pregnancy or lactation.
Interventions
The eligible subjects were randomly divided into the study or control group based on a computerized random sequence. The control group was treated with bismuth-containing quadruple therapy (5 mg ilaprazole, 100 mg doxycycline, 500 mg clarithromycin or 1 g amoxicillin or 100 mg furazolidone, and 220 mg colloidal bismuth tartrate) twice daily for 14 days. The study group received an additional Ig Y-H. pylori (7 g, twice daily) for 14 days. We avoided reusing patients’ previous antibiotics empirically. Detailed medications were administered as shown in the Table 1.
The entire study design is shown in Figure 1. Before receiving treatment, all subjects received face-to-face training and were given written cards to record drugs use, adverse effects, and clinical symptoms. Satisfactory adherence was defined as taking more than 80% of the total medication.
Follow-up and assessment of study endpoints
Subjects were followed up three times after being screened and enrolled (day 0). The 1st visit after 2 weeks of medication (day 14 ± 2), the 2nd visit after 4 weeks of medication (day 28 ± 2), and the review after 6 weeks of medication, along with the last 3rd visit (day 42 ± 2). During H. pylori eradicating treatment, patients took their medication followed by prescription and counted the remaining at the end of the quantity. Patients were encouraged to contact the investigator in case of discomfort or doubt to gain good compliance and safety.
Outcomes
The primary outcome of the trial was the H. pylori eradication rate (number of successful eradications/number of eradications), which was diagnostic by 13/14C-UBT. One of the secondary outcomes was symptoms remission, where subjects’ gastrointestinal symptoms were scored by the 7-point Global Overall Symptom scale (GOS) before and after treatment, respectively, and scored as cured (≥80% decrease in score after treatment), remission (0–80% decrease in score after treatment), and ineffective (no reduction in score or worsening of symptoms after treatment) (Veldhuyzen van Zanten et al., 2006). The other secondary outcome was the incidence of adverse events.
Statistical analysis
The data were analyzed using SPSS.26.0 software. Categorical variables were described by frequencies and proportions (%), and continuous variables were described by mean and standard deviation (SD), unless otherwise stated. The H. pylori eradication rates were calculated by per-protocol (PP) and intention-to-treat (ITT) analysis, expressed as percentages, and assessed with 95% confidence intervals. Significance of categorical data was assessed using the chi-square test or Fisher’s exact test, while continuous variables were tested using the t-test. p < 0.05 represents statistical significance.
Results
Characteristics of the patients
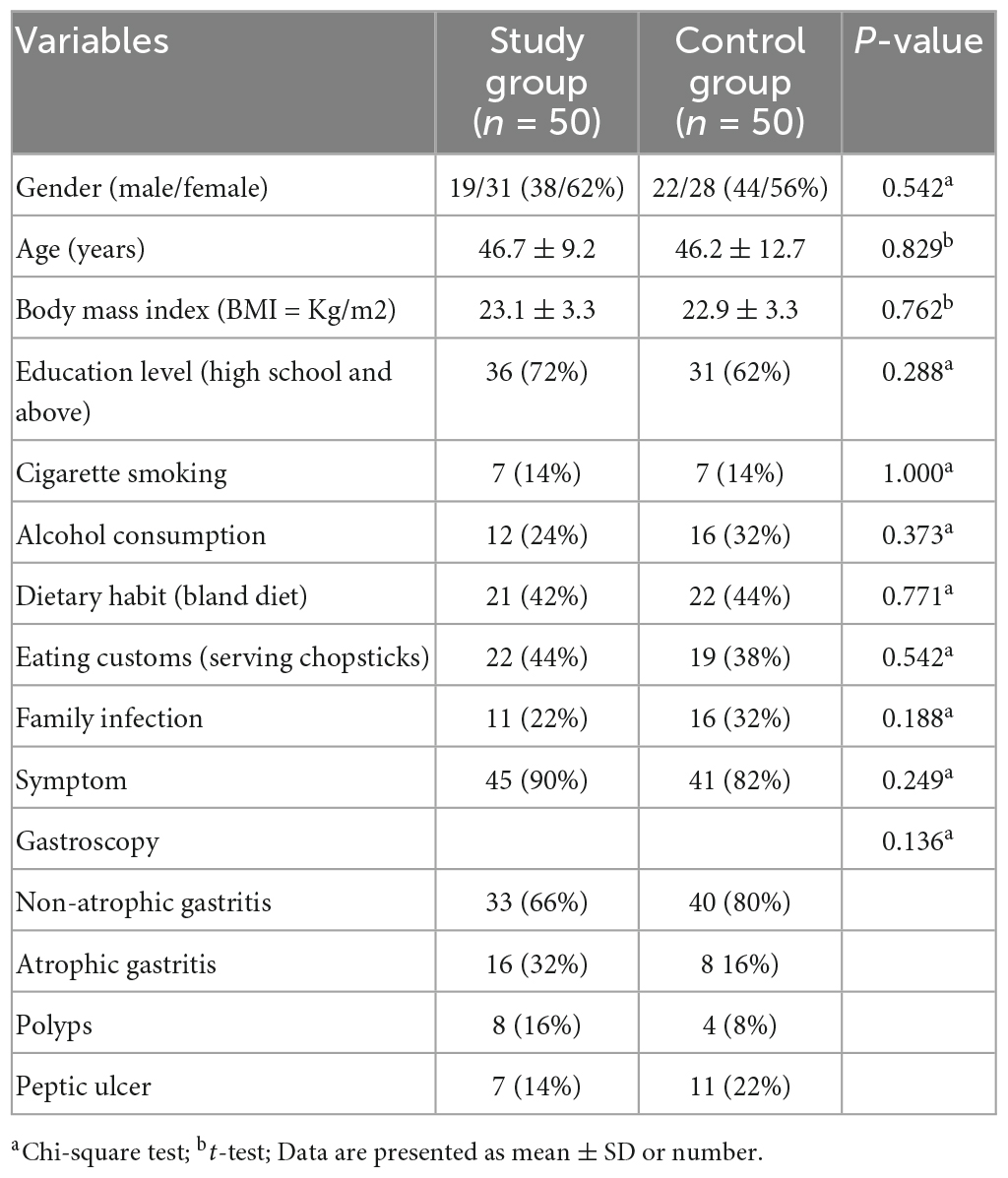
A total of 103 patients were screened for eligibility and 100 patients (3 were excluded for refusing to participate) were randomly divided into study group (n = 50) and control group (n = 50). Patient characteristics were similar between the two groups (p > 0.05) (Table 2). Among all participants, 49 and 50 patients completed the entire trial and follow-up, respectively.
H. pylori eradication rates
In the ITT analysis, the eradication rates were 84% (95% CI 73.5–94.5%) in study group and 80% (95% CI 68.5–91.5%) in control group and 85.7% (95% CI 75.6–95.9%) and 80% (95% CI 68.5–91.5%) in the PP analysis (Figure 2A and Supplementary Table 3). Further subgroup analysis was performed according to repeated treatment times. The data revealed that in the population that once failed before, the eradication rates in the two groups were 93.5 and 83.9%, while in ≥2 eradication attempts, 72.2 and 73.7%, respectively (Figures 2C, D and Supplementary Table 4).
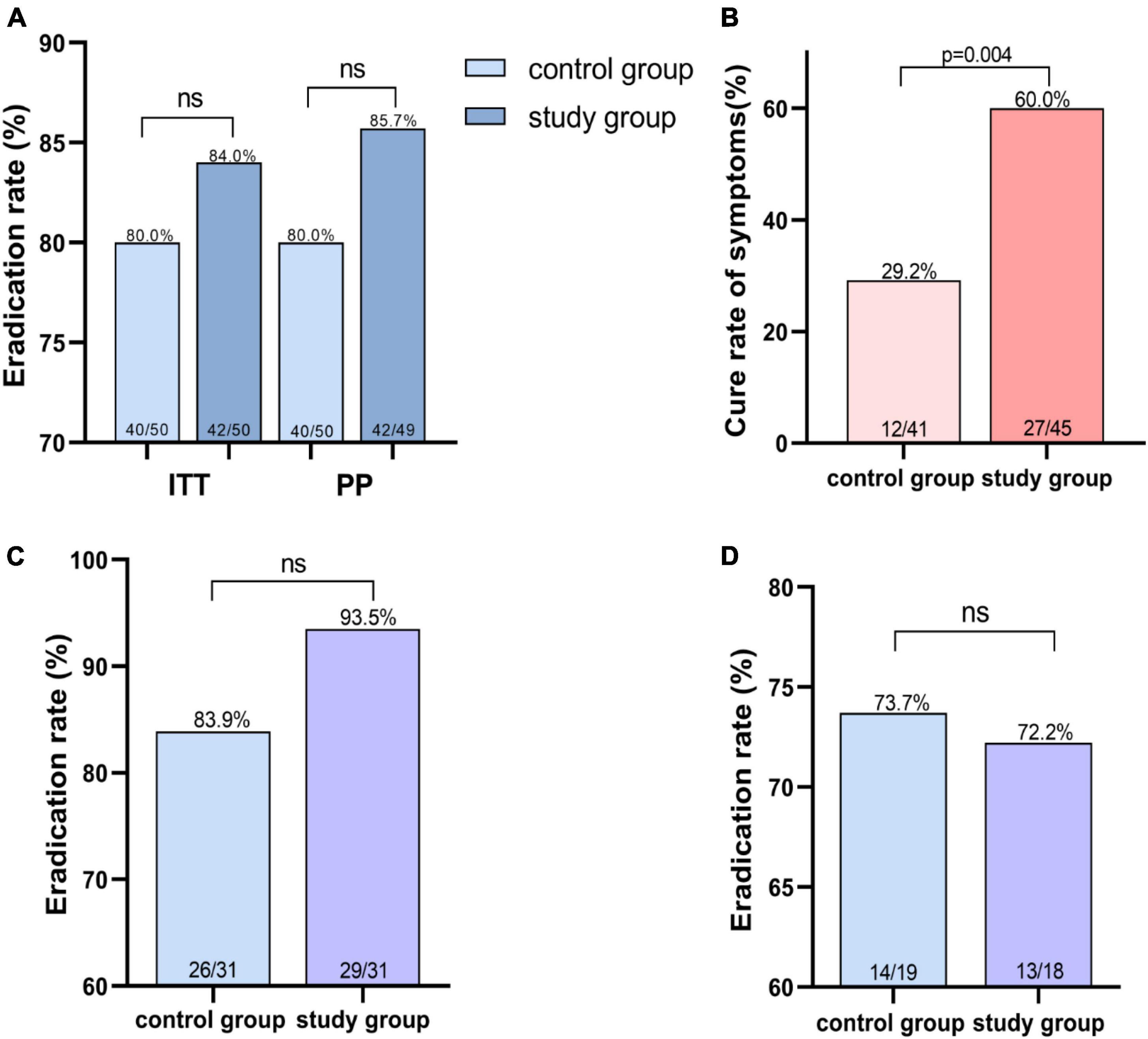
Figure 2. Helicobacter pylori eradication rate and symptom cure rate. (A) Eradication rate of H. pylori infection in different treatment groups. (B) Higher symptom cure rates in study group using Ig Y-H. pylori. (C) Eradication rate of second-line therapy in different treatment groups. (D) Eradication rates for the third or more treatments in the different treatment groups. ns indicates not significantly different.
Symptom relief
The severity of the following symptoms–upper abdominal pain, bloating (or postprandial fullness), upper abdominal discomfort, belching, heartburn, nausea, acid reflux, and early satiety–were assessed in the subjects using the Global Overall Symptom scale (Veldhuyzen van Zanten et al., 2006), and the relevant clinical symptoms were explained as shown in the Supplementary Tables 1, 2. And 45 people (study group) and 41 people (control group) showed gastrointestinal symptoms, such as abdominal pain and bloating and epigastric discomfort, and so on. The number of patients with cured, relieved and ineffective symptoms in the two groups after treatment were 27, 17, 1 (study group) and 12, 27, 12 (control group), respectively. The use of Ig Y can better ameliorate the patient’s symptoms (Figure 2B and Supplementary Table 5).
Adverse events
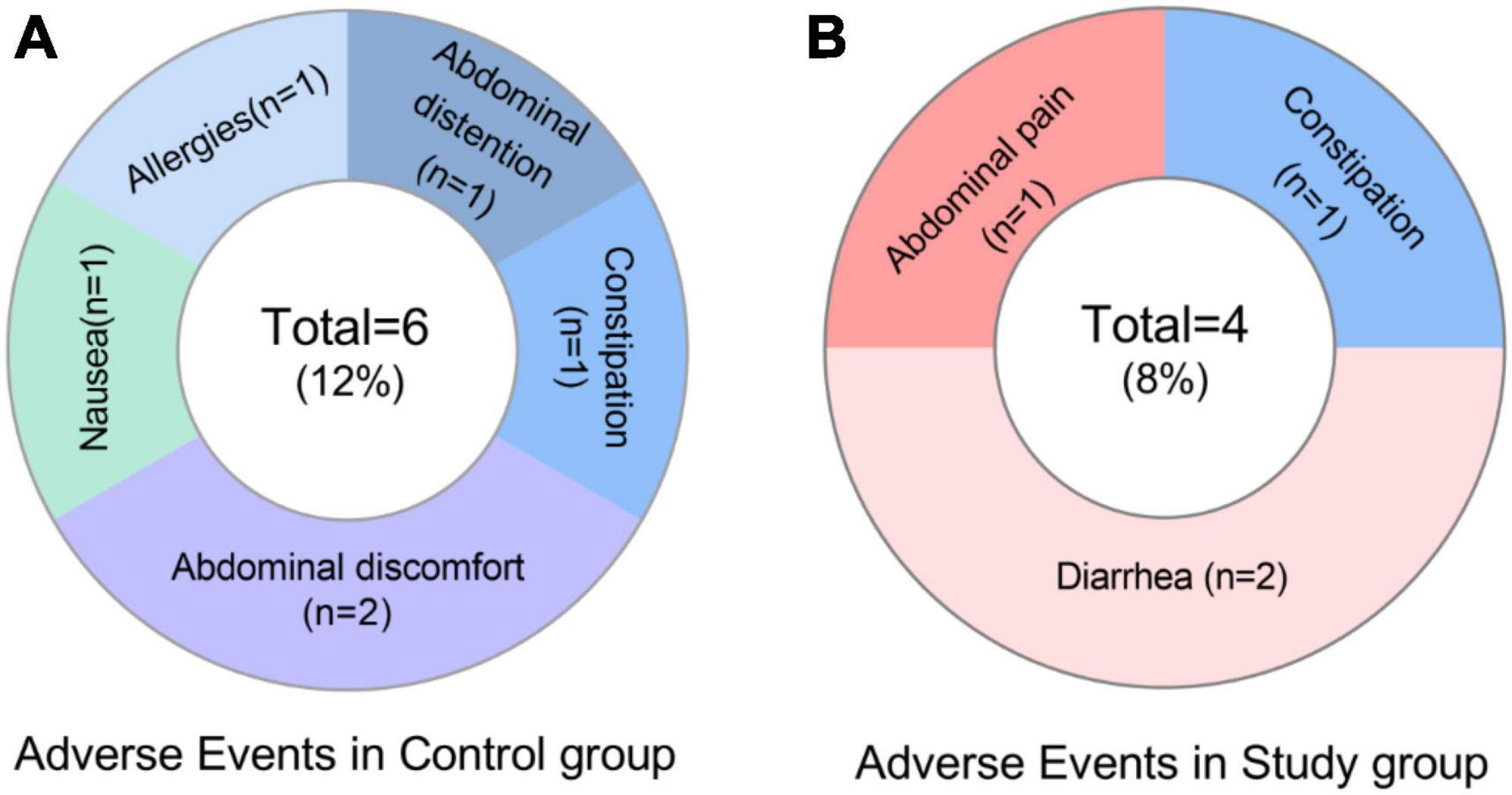
The incidence of AE was 8% (4/49) and 12% (6/50) in the study and control groups, respectively. Major manifestations were abdominal pain, diarrhea, constipation, allergy (rash), bloating, abdominal discomfort and nausea (Figure 3 and Supplementary Table 6). Side effects are mainly mild or moderate and recovered after discontinuation of the drugs.
Discussion
This study is the first randomized controlled clinical trial to investigate the effect of Ig Y-H. pylori as an adjuvant therapy in H. pylori rescue eradication treatments. Our results showed that Ig Y-H. pylori supplementation to the quadruple therapy significantly increased symptoms relief.
Both groups achieved good eradication rate of H. pylori. However, the eradication rate of the combination of Ig Y-H. pylori were superior to the quadruple regimen alone, using ITT (84 vs. 80%) and PP analysis (85.7 vs. 80%). After classification according to the number of eradications, we found that Ig Y- H. pylori increased eradication rates in second-line therapy (93.5 vs. 83.9%), but in patients with refractory H. pylori infection (at least 2 failed eradication), the two groups had similar eradication rates (72.2 vs. 73.7%, Figure 2 and Supplementary Table 4). Although there was no statistical difference in eradication rates between the two regimens, this may be related to the small sample size. In addition, Ig Y treated study group significantly relieved gastrointestinal symptoms (p < 0.05) and fewer adverse events. These results suggest that the combination Ig Y-H. pylori regimen can achieve high eradication efficiency and further deliver better symptom relief benefits, improving patients’ quality of life. Hence, it is a better rescue therapy option for the H. pylori eradication failure population.
The American Gastroenterological Association (AGA) clinical guidelines state that patients with a history of eradication failure should be treated cautiously and avoid repeat antibiotic therapy (Chey et al., 2017). The Maastricht VI/Florence consensus report recommends that fluoroquinolone-containing triple therapy regimens be considered for rescue therapy following failure of standard triple therapy, non-bismuth quadruple therapy, or bismuth quadruple therapy (Malfertheiner et al., 2022). However, some clinical studies indicate that fluoroquinolone-containing triple therapy have failed to achieve satisfactory eradication (Bilardi et al., 2004; Gisbert et al., 2007). A randomized controlled study reported 22 and 11.5% overall adverse events in a quadruple regimen containing levofloxacin with bismuth and a standard levofloxacin triple regimen for remedial treatment, respectively, with the latter having an eradication rate of only 69.2% (Hsu et al., 2017). Similarly, botanical components or probiotic adjuvant therapy for H. pylori can reduce drug side effects and improve adherence (Yang et al., 2021; Guo et al., 2022; Viazis et al., 2022). Other studies showed that individualized and precise treatment based on drug sensitivity testing is another better rescue therapy choice, but the high cost of gastroscopy and drug sensitivity testing brings higher economic costs and invasive injury, even as some studies point to no significant difference between eradication rates guided by drug sensitivity testing and empirical treatment (Gomollón et al., 2000; Chen et al., 2019; Gingold-Belfer et al., 2021). For the above reasons, empirical treatment tends to dominate. Our study found that Ig Y-H. pylori improved the eradication rates of resistant strains after repeated treatment with antibiotic and had better clinical symptom relief and lower rates of adverse events.
Based on the characteristic of safety and no-resistance, Ig Y is more suitable for infants and children, immunodeficient patients (Rahman et al., 2013). In a randomized double-blind placebo-controlled trial in Japan, Takeuchi et al. (2016) used Ig Y to treat candida albicans infections in the elderly. Sarker et al. (2001) also reported encouraging results for Ig Y in alleviating diarrhea caused by rotavirus infection in children. The mechanisms for its safe and effective characteristic of Yolk antibodies may come from (a) specifically binding to antigenic epitopes of the pathogen and adsorbing the pathogen, (b) inhibiting the spread and multiplication of the pathogen, (c) enhancing the recognition and clearance of immune cells, and (d) neutralizing virulence factor (Rahman et al., 2013). Ig Y interferes with the colonization of H. pylori, promotes H. pylori excretion from the gastric mucosa, causes bacterial aggregation, and improves the recognition and phagocytosis efficiency of immune cells. In addition, Ig Y specifically recognizes and binds to virulence factors produced by H. pylori through antigen-antibody interactions, avoiding gastric mucosal damage and associated inflammatory responses (Nomura et al., 2005; Chalghoumi et al., 2009; Lee et al., 2021). As a candidate for passive immunization against pathogens, Ig Y is safer and more economical than antibiotics with high specificity and applicability.
There were some limitations to this study, firstly, it was a single-center clinical trial that requires further research in expanded population. Secondly, there was a bias in the subjective perceptions of patient during clinical symptom assessment. We used the Global Overall Symptom scale, a simple and validated scale (Heading et al., 2016), to record subjects’ pre- and post-treatment symptom scores using a 7-point scale. Finally, due to the experimental conditions, we did not use antibiotic susceptibility test or genotype resistance test to determine strain resistance. These limitations need to be further validated in a large sample, randomized controlled double-blind trial.
Conclusion
In conclusion, for patients with repeated H. pylori eradication, Ig Y-H. pylori combined with bismuth-based quadruple therapy showed comparable efficacy to bismuth-based quadruple therapy with better symptomatic relief and fewer adverse effects. It can be recommended for the eradication of H. pylori infection.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of Third Xiangya Hospital of Central South University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
CS, LJ, and XCX prepared the first draft of the manuscript. JC and HL wrote the study protocol. ZWF and LJH were investigators.
Funding
This work was supported by the National Natural Science Foundation of China (No. 81570509), Changsha Municipal Natural Science Foundation Project (kq2202118), and Hunan Provincial Innovation Foundation for Postgraduate (No. QL20220061).
Acknowledgments
We would like to thank Beijing Yucheng Health Technology Co., Ltd. for providing free Ig Y-H. pylori. We also thank the Committee of Central Research Institution (Third Xiangya Hospital of Central South University) for their guidance on this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1150129/full#supplementary-material
References
Abbas, A. T., El-Kafrawy, S. A., Sohrab, S. S., and Azhar, E. I. A. (2019). IgY antibodies for the immunoprophylaxis and therapy of respiratory infections. Hum. Vaccin. Immunother. 15, 264–275.
Ansari, S., and Yamaoka, Y. (2022). Helicobacter pylori infection, its laboratory diagnosis, and antimicrobial resistance: A perspective of clinical relevance. Clin. Microbiol. Rev. 35:e0025821. doi: 10.1128/cmr.00258-21
Attallah, A. M., Abbas, A. T., Ismail, H., Abdel-Raouf, M., and El-Dosoky, I. (2009). Efficacy of passive immunization with IgY antibodies to a 58-kDa H. pylori antigen on severe gastritis in BALB/c mouse model. J. Immunoassay Immunochem. 30, 359–377. doi: 10.1080/15321810903187922
Bilardi, C., Dulbecco, P., Zentilin, P., Reglioni, S., Iiritano, E., Parodi, A., et al. (2004). A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: A controlled trial. Clin. Gastroenterol. Hepatol. 2, 997–1002. doi: 10.1016/s1542-3565(04)00458-6
Chalghoumi, R., Théwis, A., Beckers, Y., Marcq, C., Portetelle, D., and Schneider, Y. J. (2009). Adhesion and growth inhibitory effect of chicken egg yolk antibody (IgY) on Salmonella enterica serovars Enteritidis and Typhimurium in vitro. Foodborne Pathog. Dis. 6, 593–604. doi: 10.1089/fpd.2008.0258
Chen, Q., Long, X., Ji, Y., Liang, X., Li, D., Gao, H., et al. (2019). Randomised controlled trial: Susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment. Pharmacol. Ther. 49, 1385–1394. doi: 10.1111/apt.15273
Chey, W. D., Leontiadis, G. I., Howden, C. W., and Moss, S. F. (2017). ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 112, 212–239.
Dang, B. N., and Graham, D. Y. (2017). Helicobacter pylori infection and antibiotic resistance: A WHO high priority? Nat. Rev. Gastroenterol. Hepatol. 14, 383–384. doi: 10.1038/nrgastro.2017.57
El-Kafrawy, S. A., Abbas, A. T., Oelkrug, C., Tahoon, M., Ezzat, S., Zumla, A., et al. (2023). IgY antibodies: The promising potential to overcome antibiotic resistance. Front. Immunol. 14:1065353. doi: 10.3389/fimmu.2023.1065353
Fink, A. L., Williams, K. L., Harris, E., Alvine, T. D., Henderson, T., Schiltz, J., et al. (2017). Dengue virus specific IgY provides protection following lethal dengue virus challenge and is neutralizing in the absence of inducing antibody dependent enhancement. PLoS Negl. Trop. Dis. 11:e0005721. doi: 10.1371/journal.pntd.0005721
Gingold-Belfer, R., Niv, Y., Schmilovitz-Weiss, H., Levi, Z., and Boltin, D. (2021). Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 36, 2649–2658. doi: 10.1111/jgh.15575
Gisbert, J. P., Gisbert, J. L., Marcos, S., Moreno-Otero, R., and Pajares, J. M. (2007). Levofloxacin- vs. ranitidine bismuth citrate-containing therapy after H. pylori treatment failure. Helicobacter 12, 68–73. doi: 10.1111/j.1523-5378.2007.00472.x
Gomollón, F., Sicilia, B., Ducóns, J. A., Sierra, E., Revillo, M. J., and Ferrero, M. (2000). Third line treatment for Helicobacter pylori: A prospective, culture-guided study in peptic ulcer patients. Aliment. Pharmacol. Ther. 14, 1335–1338. doi: 10.1046/j.1365-2036.2000.00833.x
Guo, K., Wang, L., Mahe, J., Li, L., Jiao, S., Wang, H., et al. (2022). Effect of aqueous extract of seed of broccoli on inflammatory cytokines and Helicobacter pylori infection: A randomized, double-blind, controlled trial in patients without atrophic gastritis. Inflammopharmacology 30, 1659–1668. doi: 10.1007/s10787-022-01030-x
Guo, R., Wu, S., Guan, L., Xie, Y., Yang, X., Li, Z., et al. (2021). Dietary multivalent anti-Helicobacter pylori immunoglobulin Y significantly increase the H. pylori eradication and improve the clinical symptoms in patients. Helicobacter 26:e12843. doi: 10.1111/hel.12843
Han, Z., Li, Y., Kong, Q., Liu, J., Wang, J., Wan, M., et al. (2022). Efficacy of bismuth for antibiotic-resistant Helicobacter pylori strains eradication: A systematic review and meta-analysis. Helicobacter 27:e12930. doi: 10.1111/hel.12930
Heading, R. C., Thomas, E. C., Sandy, P., Smith, G., Fass, R., and Hungin, P. S. (2016). Discrepancies between upper GI symptoms described by those who have them and their identification by conventional medical terminology: A survey of sufferers in four countries. Eur. J. Gastroenterol. Hepatol. 28, 455–462. doi: 10.1097/MEG.0000000000000565
Hong, K. S., Ki, M. R., Ullah, H. M. A., Lee, E. J., Kim, Y. D., Chung, M. J., et al. (2018). Preventive effect of anti-VacA egg yolk immunoglobulin (IgY) on Helicobacter pylori-infected mice. Vaccine 36, 371–380. doi: 10.1016/j.vaccine.2017.11.082
Hsu, P. I., Tsai, F. W., Kao, S. S., Hsu, W. H., Cheng, J. S., Peng, N. J., et al. (2017). Ten-day quadruple therapy comprising proton pump inhibitor, bismuth, tetracycline, and levofloxacin is more effective than standard levofloxacin triple therapy in the second-line treatment of Helicobacter pylori infection: A randomized controlled trial. Am. J. Gastroenterol. 112, 1374–1381. doi: 10.1038/ajg.2017.195
Kovacs-Nolan, J., and Mine, Y. (2012). Egg yolk antibodies for passive immunity. Annu. Rev. Food Sci. Technol. 3, 163–182.
Lee, L., Samardzic, K., Wallach, M., Frumkin, L. R., and Mochly-Rosen, D. (2021). Immunoglobulin Y for potential diagnostic and therapeutic applications in infectious diseases. Front. Immunol. 12:696003. doi: 10.3389/fimmu.2021.696003
Lin, T. F., and Hsu, P. I. (2018). Second-line rescue treatment of Helicobacter pylori infection: Where are we now? World J. Gastroenterol. 24, 4548–4553. doi: 10.3748/wjg.v24.i40.4548
Malfertheiner, P., Megraud, F., Rokkas, T., Gisbert, J. P., Liou, J. M., Schulz, C., et al. (2022). Management of Helicobacter pylori infection: The maastricht VI/Florence consensus report. Gut doi: 10.1136/gutjnl-2022-327745 [Epub ahead of print].
Mine, Y., and Kovacs-Nolan, J. (2002). Chicken egg yolk antibodies as therapeutics in enteric infectious disease: A review. J. Med. Food 5, 159–169. doi: 10.1089/10966200260398198
Mommersteeg, M. C., Yu, J., Peppelenbosch, M. P., and Fuhler, G. M. (2018). Genetic host factors in Helicobacter pylori-induced carcinogenesis: Emerging new paradigms. Biochim. Biophys. Acta Rev. Cancer 1869, 42–52. doi: 10.1016/j.bbcan.2017.11.003
Müller, S., Schubert, A., Zajac, J., Dyck, T., and Oelkrug, C. (2015). IgY antibodies in human nutrition for disease prevention. Nutr. J. 14:109.
Nomura, S., Suzuki, H., Masaoka, T., Kurabayashi, K., Ishii, H., Kitajima, M., et al. (2005). Effect of dietary anti-urease immunoglobulin Y on Helicobacter pylori infection in Mongolian gerbils. Helicobacter 10, 43–52. doi: 10.1111/j.1523-5378.2005.00290.x
Peitz, U., Sulliga, M., Wolle, K., Leodolter, A., Von Arnim, U., Kahl, S., et al. (2002). High rate of post-therapeutic resistance after failure of macrolide-nitroimidazole triple therapy to cure Helicobacter pylori infection: Impact of two second-line therapies in a randomized study. Aliment. Pharmacol. Ther. 16, 315–324. doi: 10.1046/j.1365-2036.2002.01173.x
Rahman, S., Van Nguyen, S., Icatlo, F. C. Jr., Umeda, K., and Kodama, Y. (2013). Oral passive IgY-based immunotherapeutics: A novel solution for prevention and treatment of alimentary tract diseases. Hum. Vaccin. Immunother. 9, 1039–1048. doi: 10.4161/hv.23383
Sarker, S. A., Casswall, T. H., Juneja, L. R., Hoq, E., Hossain, I., Fuchs, G. J., et al. (2001). Randomized, placebo-controlled, clinical trial of hyperimmunized chicken egg yolk immunoglobulin in children with rotavirus diarrhea. J. Pediatr. Gastroenterol. Nutr. 32, 19–25. doi: 10.1097/00005176-200101000-00009
Savoldi, A., Carrara, E., Graham, D. Y., Conti, M., and Tacconelli, E. (2018). Prevalence of Antibiotic Resistance in Helicobacter pylori: A systematic review and meta-analysis in world health organization regions. Gastroenterology 155, 1372–1382.e17. doi: 10.1053/j.gastro.2018.07.007
Tacconelli, E., Carrara, E., Savoldi, A., Harbarth, S., Mendelson, M., Monnet, D. L., et al. (2018). Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327. doi: 10.1016/S1473-3099(17)30753-3
Takeuchi, S., Motohashi, J., Kimori, H., Nakagawa, Y., and Tsurumoto, A. (2016). Effects of oral moisturising gel containing egg yolk antibodies against Candida albicans in older people. Gerodontology 33, 128–134. doi: 10.1111/ger.12139
Veldhuyzen van Zanten, S. J., Chiba, N., Armstrong, D., Barkun, A. N., Thomson, A. B., Mann, V., et al. (2006). Validation of a 7-point global overall symptom scale to measure the severity of dyspepsia symptoms in clinical trials. Aliment. Pharmacol. Ther. 23, 521–529. doi: 10.1111/j.1365-2036.2006.02774.x
Viazis, N., Argyriou, K., Kotzampassi, K., Christodoulou, D. K., Apostolopoulos, P., Georgopoulos, S. D., et al. (2022). A four-probiotics regimen combined with a standard Helicobacter pylori-eradication treatment reduces side effects and increases eradication rates. Nutrients 14:632. doi: 10.3390/nu14030632
Wang, Z., Han, W., Xue, F., Zhao, Y., Wu, P., Chen, Y., et al. (2022). Nationwide gastric cancer prevention in China, 2021-2035: A decision analysis on effect, affordability and cost-effectiveness optimisation. Gut 71, 2391–2400. doi: 10.1136/gutjnl-2021-325948
Yang, C., Liang, L., Lv, P., Liu, L., Wang, S., Wang, Z., et al. (2021). Effects of non-viable Lactobacillus reuteri combining with 14-day standard triple therapy on Helicobacter pylori eradication: A randomized double-blind placebo-controlled trial. Helicobacter 26:e12856. doi: 10.1111/hel.12856
Yang, Y. H., Park, D., Yang, G., Lee, S. H., Bae, D. K., Kyung, J., et al. (2012). Anti-Helicobacter pylori effects of IgY from egg york of immunized hens. Lab. Anim. Res. 28, 55–60. doi: 10.5625/lar.2012.28.1.55
Keywords: Helicobacter pylori, egg yolk antibody (Ig Y), Ig Y-H. pylori, rescue therapy, bismuth-based quadruple therapy
Citation: Cheng S, Li H, Luo J, Chi J, Zhao W, Lin J and Xu C (2023) Egg yolk antibody combined with bismuth-based quadruple therapy in Helicobacter pylori infection rescue treatment: a single-center, randomized, controlled study. Front. Microbiol. 14:1150129. doi: 10.3389/fmicb.2023.1150129
Received: 02 February 2023; Accepted: 03 April 2023;
Published: 16 May 2023.
Edited by:
Nagendran Tharmalingam, Rhode Island Hospital, United StatesReviewed by:
Sasikala Muthusamy, Academia Sinica, TaiwanAditya Yashwant Sarode, Columbia University, United States
Copyright © 2023 Cheng, Li, Luo, Chi, Zhao, Lin and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Canxia Xu, eHVjYW54aWEyMDAwQDE2My5jb20=
†These authors share first authorship
 Sha Cheng
Sha Cheng Huan Li
Huan Li Ju Luo
Ju Luo Jingshu Chi
Jingshu Chi Wenfang Zhao
Wenfang Zhao Jiahui Lin
Jiahui Lin Canxia Xu
Canxia Xu