- 1State Key Laboratory of Functions and Applications of Medicinal Plants & Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou Medical University, Guiyang, China
- 2The High Efficacy Application of Natural Medicinal Resources Engineering Center of Guizhou Province (The Key Laboratory of Optimal Utilization of Natural Medicine Resources), School of Pharmaceutical Sciences, Guizhou Medical University, Guiyang, China
- 3Center for Yunnan Plateau Biological Resources Protection and Utilization, College of Biological Resource and Food Engineering, Qujing Normal University, Qujing, China
- 4Section of Microbiology, Institute for Research and Development in Health and Social Care, Battaramulla, Sri Lanka
- 5Engineering and Research Center for Southwest Bio-Pharmaceutical Resources of National Education Ministry of China, Guizhou University, Guiyang, China
- 6Research Center of Microbial Diversity and Sustainable Utilization, Chiang Mai University, Chiang Mai, Thailand
- 7Department of Biology, Faculty of Science, Chiang Mai University, Chiang Mai, Thailand
- 8Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, School of Basic Medical Sciences, Guizhou Medical University, Guiyang, China
During an investigation of Diatrypaceae from southern China, 10 xylariales-like taxa have been collected. Morphological and multi-gene analyses confirmed that these taxa reside in Diatrypaceae and represent eight novel taxa and two new records belonging to six genera (viz., Allocryptovalsa, Diatrype, Diatrypella, Paraeutypella, Peroneutypa, and Vasilyeva gen. nov.). Vasilyeva gen. nov. was proposed to accommodate Vasilyeva cinnamomi sp. nov. Among the other collections, seven new species were introduced (viz., Diatrype camelliae-japonicae sp. nov., Diatrype rubi sp. nov., Diatrypella guiyangensis sp. nov., Diatrypella fatsiae-japonicae sp. nov., Paraeutypella subguizhouensis sp. nov., Peroneutypa hainanensis sp. nov., and Peroneutypa qianensis sp. nov.), while two were reported as new records from China (Allocryptovalsa rabenhorstii and Diatrype enteroxantha). For Diatrypaceae, the traditional taxonomic approach based on morphology may not be applicable.
Introduction
The family Diatrypaceae was erected by Nitschke (1870) to accommodate five genera viz., Calosphaeria Tul. & C. Tul., Diatrype Fr., Diatrypella (Ces. & De Not.) De Not., Quaternaria Tul. & C. Tul., and Scoptria Nitschke. The members of Diatrypaceae thrive in both aquatic and terrestrial habitats (Chlebicki, 1986; Glawe and Jacobs, 1987; Carmarán and Romero, 1992; Carmarán et al., 2006; Trouillas et al., 2010a; de Almeida et al., 2016), with different life modes, such as saprobes, pathogens, and endophytes, on economic crops and forest trees with a worldwide distribution (Vasilyeva and Ma, 2014; Dayarathne et al., 2016; Mayorquin et al., 2016; Senwanna et al., 2017; Hyde et al., 2020a; Konta et al., 2020). Phytopathogenic diatrypaceous taxa have been reported as causal agents of cankers, dieback, and grapevine trunk diseases (Glawe and Rogers, 1984; Rappaz, 1987; Trouillas and Gubler, 2004; Lardner et al., 2005; Luque et al., 2006; Catal et al., 2007), such as Cryptosphaeria populina (Pers.) Sacc., Cryptosphaeria pullmanensis Glawe, Eutypa leptoplaca (Durieu & Mont.) Rappaz, and Eutypella parasitica R.W. Davidson & R.C. Lorenz.
Kirk et al. (2008) accepted 13 genera in this family. Subsequently, Allocryptovalsa Senwanna et al., Allodiatrype Konta & K.D. Hyde, Diatrypasimilis Jian L. Zhou & Kohlm., Halodiatrype Dayar. & K.D. Hyde, Halocryptosphaeria Dayarath et al., Halocryptovalsa Dayar. & K.D. Hyde, Monosporascus Pollack & Uecker, Neoeutypella M. Raza et al., and Pedumispora K.D. Hyde & E.B.G. Jones were introduced as members of Diatrypaceae (Abdel-Wahab et al., 2014; Klaysuban et al., 2014; Maharachchikumbura et al., 2015; Dayarathne et al., 2016, 2020a,b; Senwanna et al., 2017; Phookamsak et al., 2019; Konta et al., 2020). In a recent study, Hyde et al. (2020b) and Wijayawardene et al. (2020) accepted 20 genera in Diatrypaceae. A total of 23 genera including five genera that lacks sequences data were accepted into the family by Zhu et al. (2021). Currently, 26 genera were included in Diatrypaceae, such as Allocryptovalsa Senwanna et al., Allodiatrype Konta & K.D. Hyde, Anthostoma Nitschke., Cryptosphaeria Ces. & De Not., Cryptovalsa Ces. & De Not., Diatrypasimilis Jian L. Zhou & Kohlm., Diatrype Fr., Diatrypella (Ces. & De Not.) De Not., Dothideovalsa Speg., Echinomyces Rappaz, Endoxylina Romell, Eutypa Tul. & C. Tul., Eutypella (Nitschke) Sacc., Halocryptosphaeria Dayarath., Devadatha, V.V. Sarma & K.D. Hyde, Halocryptovalsa Dayar. & K.D. Hyde, Halodiatrype Dayar. & K.D. Hyde, Leptoperidia Rappaz, Libertella Desm., Monosporascus Pollack & Uecker, Neoeutypella M. Raza, Q.J. Shang, Phookamsak & L. Cai, Paraeutypella L.S. Dissan., J.C. Kang, Wijayaw. & K.D. Hyde, Pedumispora K.D. Hyde & E.B.G. Jones, Peroneutypa Berl., Pseudodiatrype S.H. Long & Q.R. Li, Quaternaria Tul. & C. Tul., and Rostronitschkia Fitzp. (Hyde et al., 2020b; Konta et al., 2020; Dissanayake et al., 2021; Long et al., 2021; Samarakoon et al., 2022).
Diatrypaceae has been referred to as allantosporous taxa, which possess allantoid ascospores. Early classification systems of Diatrypaceae were mainly based on stromatal features including the degree of stromatal development, structure of perithecial necks, and type of host tissue (Fries, 1823; Glawe and Jacobs, 1987; Rappaz, 1987). Vasilyeva (1986) regarded that the morphology of the stromata causes significant confusion within Diatrypaceae.
A total of seven diatrypaceous species were known from the northeastern provinces of China before 2000 (Tai, 1979; Teng, 1996). Subsequent studies by Vasilyeva and Stephenson (2009) who carried out investigations in northeastern China, introduced nine species of pyrenomycetous fungi from China, including Cryptosphaeria exornata, C. venusta, and Diatrype macounii. In total, 15 species of Diatrype, Diatrypella, Eutypa, and Eutypella were documented by Vasilyeva from Heilongjiang province (Vasilyeva, 2011). A total of 13 species of Diatrype and Cryptosphaeria were collected from Heilongjiang and Jilin provinces by Vasilyeva and Ma (2014). Ma et al. (2016) reported Cryptosphaeria pullmanensis as the pathogens of a canker disease of willow and poplar in Xinjiang (Paraeutypella and a new species Diatrypella longiasca were reported from Guizhou by Dissanayake et al., 2021). In total, three new species (Allodiatrype trigemina, Diatrype betulaceicola, and Diatrype larissae) were reported based on morphological and molecular characteristics (Peng et al., 2021; Yang et al., 2022). Zhu et al. (2021) introduced nine novel species (viz. Allocryptovalsa castaneae, A. castaneicola, Diatrype betulae, D. castaneicola, D. quercicola, Diatrypella betulae, D. betulicola, D. hubeiensis, and D. shennongensis), a known species of Diatrypella favacea and a new host of Eutypella citricola, and asserted the high diversity of Diatrypaceae in China. Long et al. (2021) made a new contribution to Diatrypaceae from karst areas in China and figured out that the number of ascospores per ascus is not a good diagnostic feature at the genus level.
During the investigation of Xylariales from south China, 20 samples belonging to 12 species of Diatrypaceae were collected. Based on morpho-molecular analyses, a new genus (viz. Vasilyeva), eight new species, and two new country records are reported in this study.
Materials and methods
Collection, morphology, and isolation
During the rainy seasons of 2020–2021, 20 samples of Diatrypaceae on dead woods and barks were collected from south China (Guizhou, Hainan, and Yunnan Provinces). The samples were stored in paper bags and taken back to the lab for examination. Macro-morphological characteristics were examined and photographed using a camera fixed to the Olympus SZ61 stereo microscope (Olympus Corporation, Japan). Microscopic examinations were carried out using a Nikon Ni compound microscope (Nikon Corporation, Japan), and photographs were taken using a Canon 550 camera. More than 30 asci and ascospores were measured with Tarosoft (R) Image Frame Work (v.0.9.7). Graphic plates were arranged with Adobe Photoshop v. CS6.
Single-spore isolation was obtained following the method of Chomnunti et al. (2014). The ascospores were picked into a small amount of sterile water, mixed well, and smeared on a potato dextrose agar (PDA). After 12 h, the germination of ascospores was observed using a stereomicroscope, and the germinated ascospores were transferred to a new PDA plate in a sterile environment. The specimens were deposited at the Herbarium of Cryptogams, Herbarium of Kunming Institute of Botany, Chinese Academy of Sciences (KUN-HKAS), Yunnan province, China, and Herbarium of Guizhou Medical University (GMB), Guizhou Province, China. The cultures were deposited at the Guizhou Medical University Culture Collection (GMBC). Nomenclatural novelties were deposited in the MycoBank (Crous et al., 2004).
DNA extraction, PCR amplification, and sequencing
Colonies were grown on PDA for ~1 week until the hyphae covered the plate. Mycelium was scraped off using a sterile scalpel for DNA extraction. Total DNA was extracted from fresh mycelia using the BIOMIGA Fungus Genomic DNA Extraction Kit following its instruction. The segments of the internal transcribed spacer (ITS) region, large-subunit (LSU) ribosomal RNA, β-tubulin (tub2), and RNA polymerase II subunit (rpb2) genes were amplified separately by primer pairs ITS4/ITS5, LR0R/LR5, and T1/T22 (T1/Bt2b and Bt2a/Bt2b), and RPB2-5f/RPB2-7Cr, respectively (Vilgalys and Hester, 1990; White et al., 1990; Glass and Donaldson, 1995; O'Donnell and Cigelnik, 1997). The PCR amplification conditions were performed following the study of Long et al. (2021). PCR products were checked with the gel electrophoresis method and sent to Sangon Biotech (Shanghai) Co., Ltd. for sequencing. All new sequences were uploaded on GenBank (https://www.ncbi.nlm.nih.gov/).
Sequence alignment and phylogenetic analyses
Sequences for alignment were downloaded from GenBank and are presented in Table 1. The sequences mainly referred to recent articles, such as Zhu et al. (2021) and Long et al. (2021). The dataset of combined ITS and β-tubulin gene alignments was aligned using MAFFT (http://mafft.cbrc.jp/alignment/server/index.html) (Katoh and Standley, 2013). Multi-gene sequence alignment was assembled using BioEdit 7.2.6.1. Phylip file for RAxML analyses and Nexus file for Bayesian analyses were obtained on the phylogeny website tools ALTER (http://sing.ei.uvigo.es/ALTER/) (Glez-Peña et al., 2010).
Maximum likelihood (ML) analyses were carried out on the CIPRES Science Gateway v.3.3 (http://www.phylo.org/portal2; Miller et al., 2010), using RAxML v.8.2.8 as of the ‘RAxML-HPC BlackBox' tool (Stamatakis and Ott, 2008). GTRGAMMA + I model was selected. The best-scoring tree was selected with a final ML optimization likelihood value of −20426.053370. Branch support (BS) for ML analyses was calculated by 1,000 bootstrap replicates.
The best-fit evolution model for each dataset for Bayesian inference (BI) was calculated with MrModeltest 2.3. The GTR+I+G model of DNA substitution and a gamma distribution rate variation across sites were selected for the construction of a Bayesian phylogenetic tree (Ronquist and Huelsenbeck, 2003). Posterior probabilities (PPs) (Rannala and Yang, 1996) were determined by Markov Chain Monte Carlo sampling (MCMC) (Ronquist and Huelsenbeck, 2003). A total of six simultaneous Markov chains were run from random starting trees for 1.2 million generations, and trees were sampled every 1,000 generations. The first 25% of generations were discarded as burn-in. The remaining trees were used to calculate the posterior probabilities in the majority rule consensus tree. Phylogenetic trees were visualized with FigTree v.1.4.4 and annotated by software of Microsoft Office PowerPoint and Adobe Photoshop v. CS6.
Results
Phylogenetic analyses
The topologies of RAxML and BYPP analyses were similar to overall tree topologies and did not differ significantly. The dataset consists of 171 taxa for representative strains of species in Diatrypaceae, including outgroup taxa with 1,451 characteristics and gaps (ITS: 1–487 and β-tubulin: 488–1451). The RAxML analyses resulted in a best-scoring likelihood tree which is shown in Figure 1.
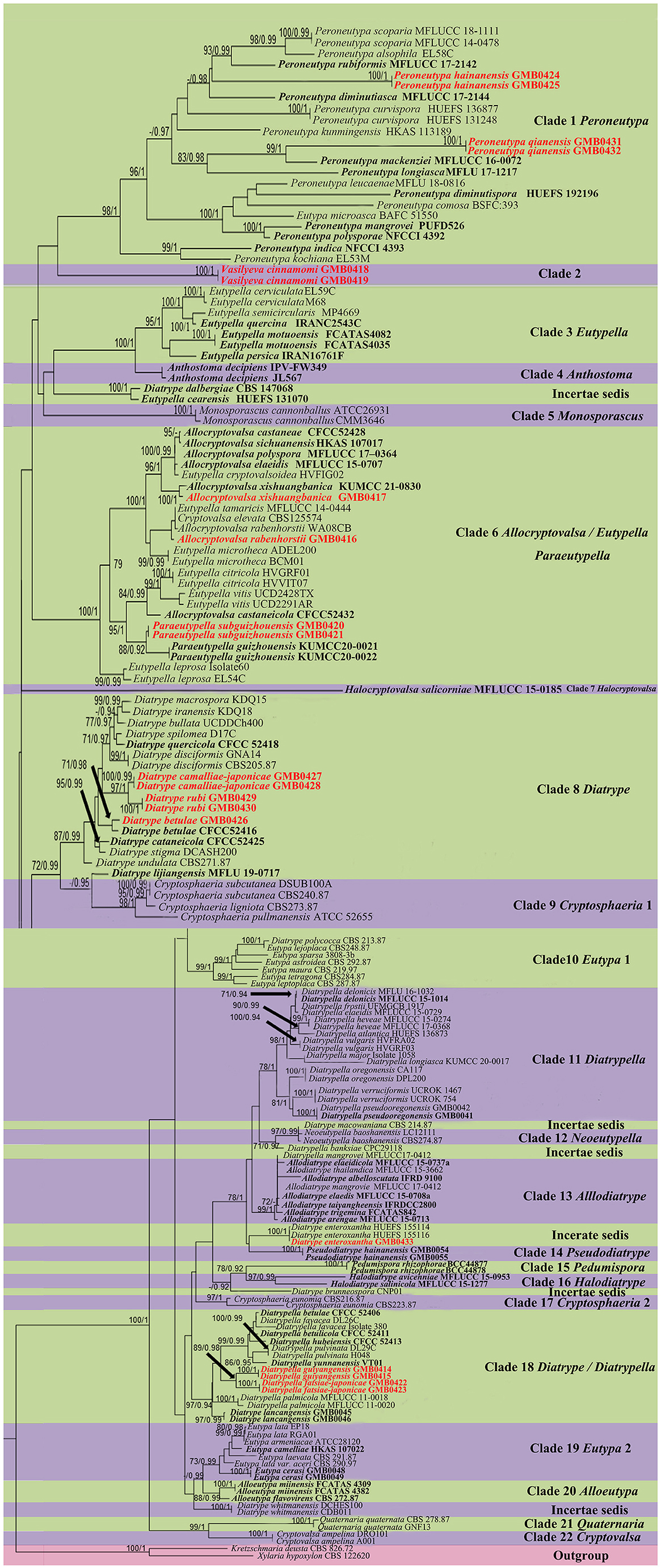
Figure 1. Phylogram generated from maximum likelihood (RAxML) analyses, based on ITS-β-tubulin matrix. ML bootstrap supports (≥70%) and Bayesian posterior probability (≥0.90) are indicated as ML/BYPP. The tree is rooted to Kretzschmaria deusta (CBS 826.72) and Xylaria hypoxylon (CBS 122620). Ex-type strains are in black bold. Newly generated strains are in red bold.
The phylogenetic tree contains 22 clades within Diatrypaceae. Peroneutypa hainanensis and Peroneutypa qianensis cluster with Peroneutypa species in Clade 1, Peroneutypa hainanensis formed a distinct branch basal to Peroneutypa alsophila, P. rubiformis, and P. scoparia, and P. qianensis was sister to P. mackenziei with the high bootstrap support (99/1). Vasilyeva formed a separate branch sister to Peroneutypa with low bootstrap support (42/0.84). In clade 6, Paraeutypella subguizhouensis formed a sister clade to Paraeutypella guizhouensis with moderate bootstrap and PP support, respectively (88/0.92). This clade is not well-resolved and comprises three genera viz. Allocryptovalsa, Eutypella, and Paraeutypella. In clade 8, Diatrype camelliae-japonicae and D. rubi formed a distinct branch in clade 8 and clustered with Diatrype s. str., and D. betulae (GMB0426) formed a sister clade with ex-type strain Diatrype betulae CFCC52416 with high bootstrap support (71/0.98). Diatrype camelliae-japonicae and D. rubi were introduced as two new species. Diatrype betulae (GMB0426) was introduced with the sexual morph. Diatrypella guiyangensis and D. fatsiae-japonicae formed a separate branch in clade 18, which is an unsolved clade that contains Diatrype and Diatrypella.
Taxonomy
A total of 12 taxa of Diatrypaceae were collected from southern China, including one new genus, eight new species, two new records for China, and two known species.
Allocryptovalsa Senwanna, Phookamsak & K.D. Hyde, Mycosphere 8(10): 1839 (2017).
MycoBank No: MB 553857.
Notes: The genus Allocryptovalsa was introduced by Senwanna et al. (2017) and was typified with A. polyspora C. Senwanna et al. This genus was characterized by present or absent stromata mostly in the bark, asci clavate to spindle-shape, long pedicellate, polysporous asci, and allantoid to sub-allantoid ascospores (Senwanna et al., 2017). The asexual morph was not determined. In this study, we report a new record of Allocryptovalsa rabenhorstii and re-describe a known species of Allocryptovalsa xishuangbanica from China.
Allocryptovalsa rabenhorstii (Nitschke) C. Senwanna, Phookamsak & K.D. Hyde, Mycosphere 8(10): 1841 (2017) (Figure 2).
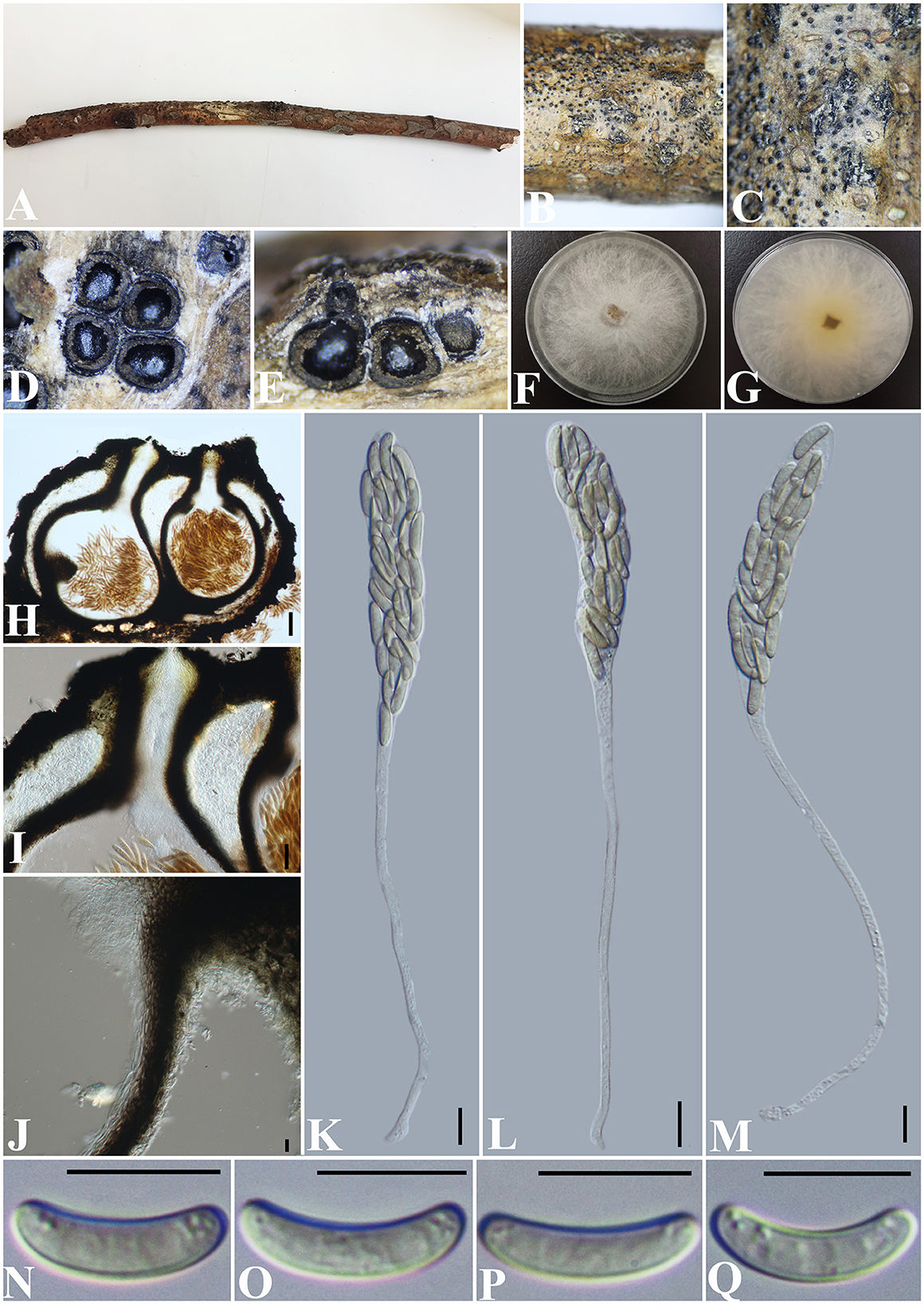
Figure 2. Allocryptovalsa rabenhorstii (GMB0416). (A) Material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F, G) Culture on PDA. (H) Section through the ascostroma. (I) Ostiolar canal. (J) Peridium. (K–M) Asci. (N–Q) Ascospores. Scale bars: (H) = 100 μm; (I) = 50 μm; (J–Q) = 10 μm.
Basionym: Valsa rabenhorstii Nitschke, Pyrenomyc. Germ. 1: 158.
MycoBank No: MB 553864.
Material examined: China, Guizhou Province, Qianxinan Buyi Miao Autonomous Prefecture, Anlong County (25°5′53.44″N, 105°26′33.64″E) on branches of an unidentified plant, 24 September 2021, Altitude: 833 m, S. H. Long & Q. R. Li., ALX4-2 (GMB0416, new record from China), living culture GMBC0416.
Saprobic on a dead twig of an unidentified plant. Sexual morph: Stromata solitary to gregarious, 1–4 loculate, immersed to semi-immersed, becoming raised to erumpent through the bark. Perithecia 380–550 μm diameter, 625–800 μm high, globose to subglobose, dark brown to black, ostiolate, papillate, perithecial, dark brown to black, gregarious or solitary, immersed to semi-immersed in the substrate. Ostioles opening separately, papillate, central. Peridium 35–50 μm wide, composed of two types of layers of cells, the outer layer comprising several layers of thick-walled, dark brown to black textura angularis cells, the inner layer comprising 3–5 layers of thin-walled, hyaline textura angularis cells. Asci 170– 230 × 11.5–20 μm ( = 202.8 × 15.4 μm, n = 30), polysporous, unitunicate, thin-walled, clavate, long pedicellate, apically rounded. Ascospores 12.5–17.5 × 3–4 μm ( = 14.8 × 3.4 μm, n = 30), crowded, pale yellowish to pale brown at maturity, oblong to allantoid, aseptate, slightly curved, smooth-walled, with small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light yellow, dense, but thinning toward the edge, margin rough, white from above, reverse white at margin, light yellow at the center, no pigmentation, and no sporulation produced on the PDA medium.
Notes: In morphology, our new collection of Allocryptovalsa rabenhorstii (GMB0416) resembles Allocryptovalsa s.str. Sequences generated from the cultures of Allocryptovalsa rabenhorstii (GMB0416) are similar to Allocryptovalsa rabenhorstii WA08CB (ITS: 99.1%, 3/434 gaps; BT: 99.0%, 0/200 gaps). Allocryptovalsa rabenhorstii has been previously reported from Australia and Iran (Trouillas et al., 2011; Mehrabi et al., 2016), and this is the first report of Allocryptovalsa rabenhorstii from China.
Allocryptovalsa xishuangbanica Maharachch. & Wanas., Life 12(5, no. 635): 9 (2022) (Figure 3).

Figure 3. Allocryptovalsa xishuangbanica (GMB0417). (A) Material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Cultures on PDA. (G) Section through the ascostroma. (H) Ostiolar canal. (I) Peridium. (J–L) Asci. (M–P) Ascospores. Scale bars: (G) = 50 μm; (H–P) = 10 μm.
MycoBank No: MB 843438.
Material examined: China, Guizhou Province, Anshun City, Pingba District (26°20'36.23“N, 106°19'20.68”E) on branches of Bombax ceiba Linnaeus, 12 December 2021, Altitude: 1220 m, S. H. Long & Q. R. Li., PB200 (GMB0417, first report from Guizhou Province, China), living culture GMBC0417.
Saprobic on the surface of Bombax ceiba branches. Sexual morph: Stromata 1.5–4.5 cm long and 0.3–0.5 cm broad ( = 2.6 × 0.4 cm, n = 30), ~0.4 mm high, well-developed, erumpent through the bark, irregular in shape, widely effused, flat, margin diffuse, surface dark brown to black, with punctiform ostioles scattered at the surface. Regions between perithecia necks are occupied by white pseudoparenchymatous entostromatic tissue. Endostroma consists of an outer layer of black, small, dense, and thin parenchymal cells and an inner layer of white, large, and loose parenchymal cells. Perithecia 200–324 μm high, 346–477 μm diameter (. =250 × 408 μm, n = 10), immersed in stromata, globose to subglobose with ostiole, the tissue between perithecia is white. Ostioles opening separately, papillate, central. Peridium 30–50 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 81.5–142 × 5–11 μm ( = 120.7 × 9.0 μm, n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded to truncate apex, apical rings inamyloid. Ascospores 8–12 × 1.8–3 μm ( = 10 × 2.4 μm, n = 30), overlapping, allantoid, slightly curved, subhyaline, smooth, aseptate, usually with small guttules at ends. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became luteous, dense, but thinning toward the edge, margin rough, white from above, reverse white to luteous, no pigmentation, and no sporulation produced on the PDA medium.
Notes: Figure 1 shows that our new collection (GMB0417) belongs to the genus Allocryptovalsa. Morphologically, GMB0417 closely resembles Allocryptovalsa xishuangbanica (HKAS122936, holotype), such as immersed or semi-immersed stromata, but GMB0417 has longer asci (81.5–142 × 5–11 μm vs. 60–80 × 7–10 μm) and slightly longer ascospores (8–12 × 1.8–3 μm vs. 7–10.5 × 1.8–2.6 μm) (Maharachchikumbura et al., 2022). The ITS sequence of Allocryptovalsa xishuangbanica GMB0417 is similar to the ITS sequence of A. xishuangbanica (HKAS122936) (99.2%, 0/476 gaps). Based on the molecular data, we identified it as Allocryptovalsa xishuangbanica. This species was originally introduced from the Yunnan province, China, but this is the first report from the Guizhou province, China.
Diatrype Fr.
MycoBank No: MB 1504.
Notes: The genus Diatrype was introduced by Fries (1849) with Diatrype disciformis as the generic type. The genus is characterized by stromata widely effuse or verrucose, flat or slightly convex, with discoid or sulcate ostioles at the surface, 8-spored and long-stalked asci, and hyaline or brownish, allantoid ascospores. The asexual morph of Diatrype is reported as libertella-like and dumortieria-like (Kirk et al., 2008; Maharachchikumbura et al., 2015; Senanayake et al., 2015). In this study, we introduce two new species (viz., Diatrype camelliae-japonicae and Diatrype rubi) while reporting a new record of Diatrype enteroxantha and a known species of Diatrype betulae from China.
Diatrype betulae H.Y. Zhu & X.L. Fan, Frontiers in Microbiology 12(no. 646262): 8 (2021) (Figure 4).
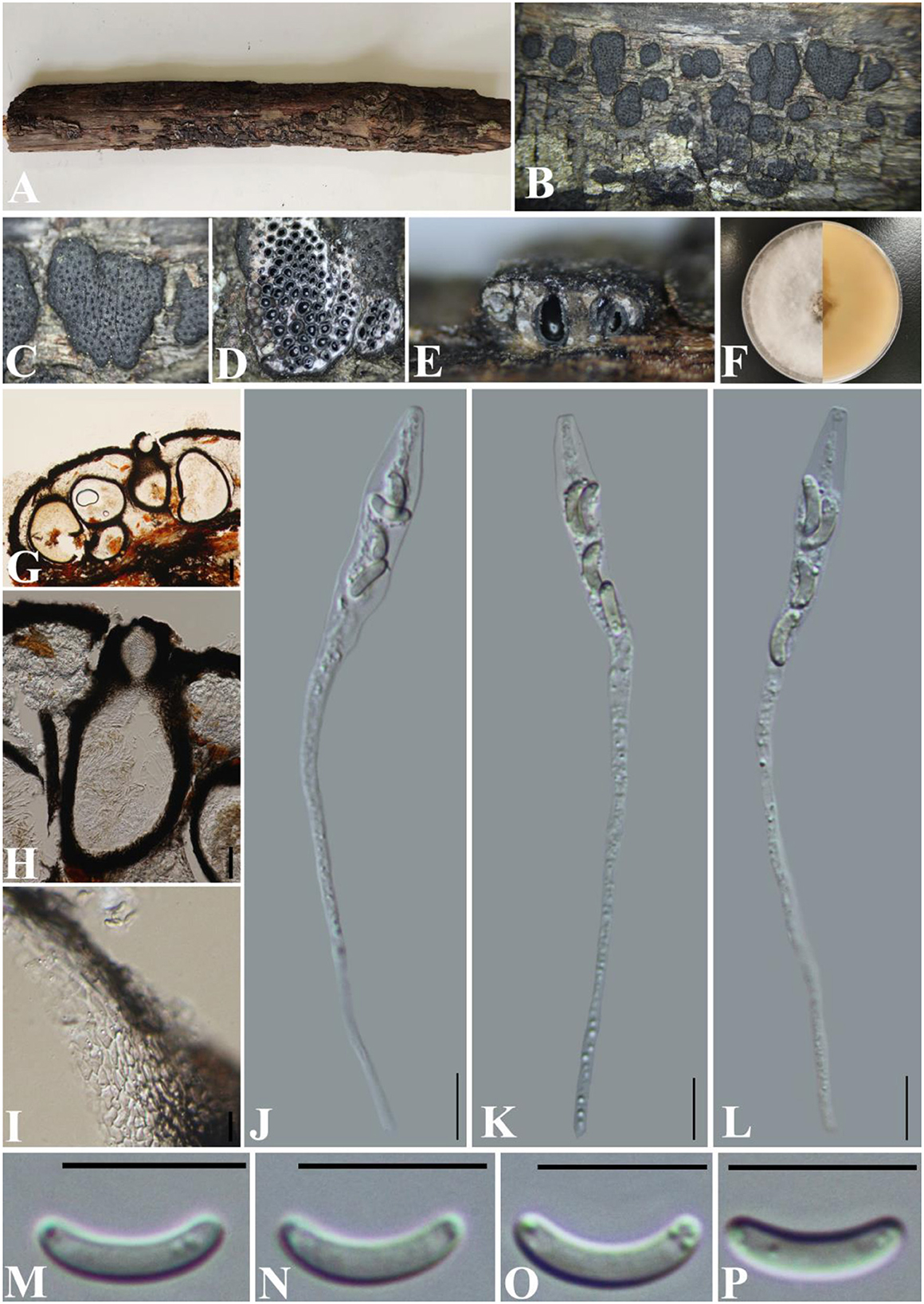
Figure 4. Diatyrpe betulae (GMB0426). (A) Material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Culture on PDA. (G) Section through the ascostroma. (H) Ostiolar canal. (I) Peridium. (J–L) Asci. (M–P) Ascospores. Scale bars: (G) = 50 μm; (H–P) = 10 μm.
MycoBank No: MB 837784.
Material examined: China, Yunnan Province, Chuxiong Yi Autonomous Prefecture, Chuxiong city, Zixi Mountain (25°1′15.13″N, 107°23′48.44″E) on branches of an unidentified plant, 2 August 2021, Altitude: 2314 m, S. H. Long & Q. R. Li., ZXS04 (GMB0426, first report of sexual morph), living culture GMBC0426.
Saprobic on the surface of dead wood. Sexual morph: Stromata 1.4–3.3 mm diameter, ~0.5–0.7 mm thick, erumpent through the bark, extending into a black area, aggregated, circular to irregular in shape, flat, margin diffused, surface dark brown to black, with punctiform ostioles scattered on the surface, with tissues soft, white between perithecia. Entostroma dark with embedded perithecia in one layer. Perithecia 370–580 μm high, 200–270 μm broad ( = 415.5 × 248.0 μm, n = 10), semi-immersed in stromata, globose to subglobose, glabrous, with a short neck. Ostioles opening separately, papillate, central. Peridium 25–40 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 77–122 × 5.5–8.5 μm ( = 106 × 6.8 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded, apical rings inamyloid. Ascospores 8.5–12 × 1.5–2.5 μm ( = 10.1 × 1.7 μm, n = 30), overlapping, allantoid, curved, hyaline, smooth, aseptate, usually with small guttules. Asexual morph: See Zhu et al. (2021).
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light brown, dense, but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to sepia and at the center, no pigmentation, and no sporulation produced on the PDA medium.
Notes: Diatrype betulae (CFCC 52416, ex-type) was introduced by Zhu et al. (2021) only based on the asexual morph. In the phylogenetic analyses, our new collection (GMB0426) formed a sister clade with Diatrype betulae CFCC 52416 with moderate bootstrap and PP support, respectively (71/0.98). ITS sequence of GMB0426 is similar to that generated from Diatrype betulae (CFCC 52416, ex-type) (ITS: 99.6%, 0/479 gaps). Based on the phylogenetic analyses and megablast, we conclude GMB0426 is representing the sexual morph of Diatrype betulae, and this is the first time reporting its sexual morph.
Diatrype camelliae-japonicae S. H. Long & Q. R. Li. sp. nov. (Figure 5).
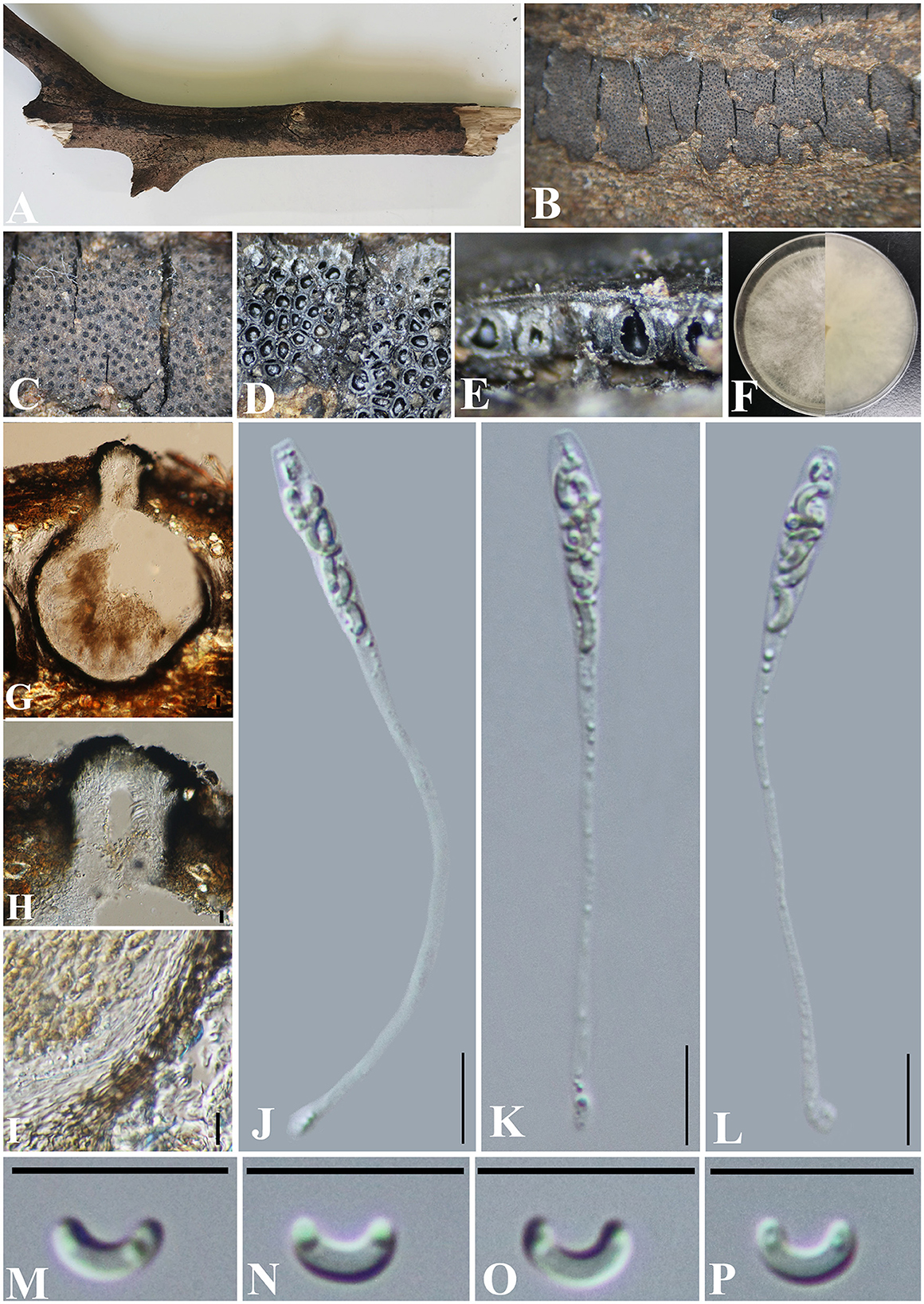
Figure 5. Diatrype camelliae-japonicae (GMB0427, holotype). (A) Type material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Culture on PDA. (G) Section through the ascostroma. (H) Ostiolar canal. (I) Peridium. (J–L) Asci. (M–P) Ascospores. Scale bars: (G) = 50 μm; (H–P) = 10 μm.
MycoBank No: MB 846768.
Etymology: Refers to its host, Camellia japonica L.
Material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Duyun City, Doupeng Mountain (26°21′49.23″N, 107°22′36.25″E) on branches of Camellia japonica L., 7 July 2021, Altitude: 1105 m, S. H. Long & Q. R. Li., DPS20 (GMB0427, holotype), ex-type GMBC0427; ibid (KUN-HKAS 126458, isotype).
Saprobic on branches of Camellia japonica. Sexual morph: Stromata 0.2–6 cm long and 0.4–1 cm board, ~0.5 mm thick, erumpent through the bark, extending into a black area, aggregated, irregular in shape, widely effused, flat, margin diffused, surface dark brown to black, with punctiform ostioles scattered at the surface, with tissues soft, white between perithecia. Entostroma dark with embedded perithecia in one layer. Perithecia 230–380 μm high, 170–220 μm broad ( = 315.5 × 198.0 μm, n = 10), semi-immersed in the stroma, globose to subglobose, glabrous, with cylindrical neck. Ostioles opening separately, papillate or apapillate, central. Peridium 25–40 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 74–107 × 3.8–5.5 μm ( = 85.5 × 4.7 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded, apical rings inamyloid. Ascospores 5.0–7.6 × 1.2–2.8 μm ( = 6.6 × 1.4 μm, n = 30), overlapping, allantoid, curved, hyaline, smooth, aseptate, usually with small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light brown, dense, but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to sepia and at the center, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Duyun City, Doupeng Mountain (26°21′30.19″N, 107°22′9.55″E) on branches of an unidentified plant, 7 July 2021, Altitude: 1292 m, S. H. Long & Q. R. Li., DPS183 (GMB0428, paratype, ex-paratype GMBC0428).
Notes: In the phylogenetic analyses, Diatrype camelliae-japonicae formed a distinct clade in Diatrype. Morphologically, the stromata of Diatrype camelliae-japonicae are similar to D. stigma, D. undulata, D. hypoxyloides, D. playstoma, and D. subundulata (Vasilyeva and Ma, 2014). However, the ascospores of GMB0027 are shorter than those of D. playstoma (7–9 × 1–1.3 μm) and D. subundulata (7–9 × 1.7–1.9 μm) and wider than those of D. undulata (5–7 × 0.9–1.3 μm) and D. hypoxyloides (4–6 μm long, very thin) (Vasilyeva and Ma, 2014). Moreover, the ascospores of D. camelliae-japonicae are hyaline while D. subundulata and D. undulate have yellowish ascospores (Vasilyeva and Ma, 2014). Diatrype camelliae-japonicae can be distinguished from D. stigma since the ascospores of the former are moderately curved, while those of the latter are straight (Vasilyeva and Ma, 2014).
Diatrype rubi S. H. Long & Q. R. Li. sp. nov. (Figure 6).
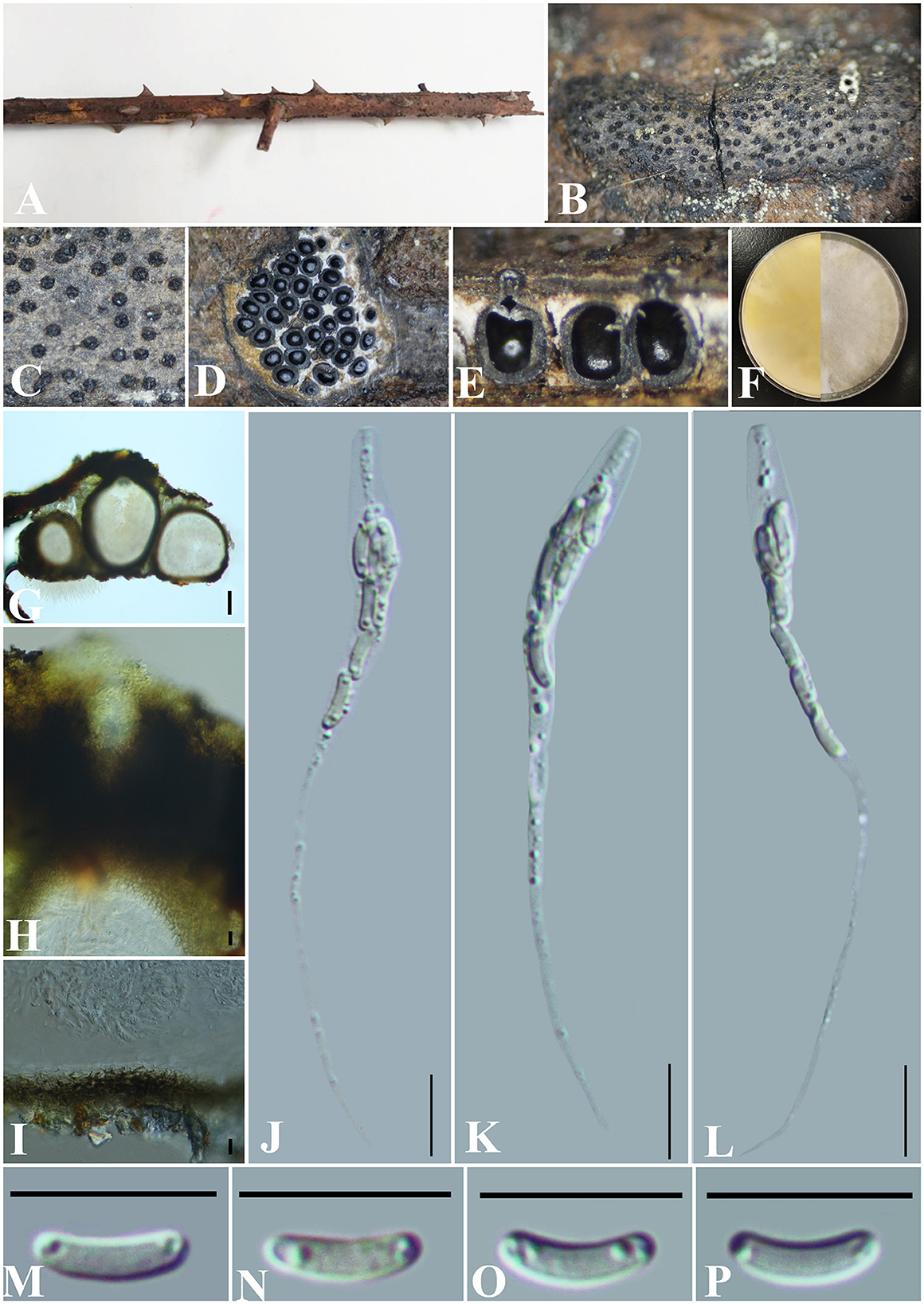
Figure 6. Diatrype rubi (GMB0429, holotype). (A) Type material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Culture on PDA. (G) Section through the ascostroma. (H) Ostiolar canal. (I) Peridium. (J–L) Asci. (M–P) Ascospores. Scale bars: (G) = 50 μm; (H–P) = 10 μm.
MycoBank No: MB 846769.
Etymology: Refers to its host, Rubus corchorifolius L. f.
Material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Dushan County, Jingshin Valley Scenic Area (25°82′49.23″N, 107°54′36.25″E) on branches of Rubus corchorifolius L. f., 18 November 2021, Altitude: 1001 m, S. H. Long & Q. R. Li., JXG3 (GMB0429, holotype), ex-type GMBC0429; ibid (KUN-HKAS 126459, isotype).
Saprobic on the branch surface of Rubus corchorifolius. Sexual morph: Stromata 0.2–0.7 cm long and 0.15–0.4 cm broad ( = 0.4 × 0.25 mm, n = 30), ~0.5 mm thick, semi-immersed through host bark, irregular in shape, widely effused, margin diffused, surface dark brown, with punctiform ostioles scattered at the surface, with tissues soft, white between perithecia. Entostroma dark with embedded perithecia in one layer. Perithecia semi-immersed in the stroma, globose to subglobose, glabrous, with cylindrical neck, brevicollous or longicollous, 287–500 μm high, 200–294 μm broad ( = 369.5 × 245.5 μm, n = 10), ovoid, obovoid to oblong, monostichous, aterrimus. Ostioles opening separately, papillate or apapillate, central. Peridium 20–30 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 73–97 × 4–6 μm ( = 79 × 5.2 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded, apical rings inamyloid. Ascospores 6.5–8 × 1.5–2 μm ( = 6.9 × 1.5 μm, n = 30), overlapping, allantoid, straight to slightly curved, hyaline, smooth, aseptate, usually with small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light yellow, dense but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to sepia and at the center, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Dushan County, Jingshin Valley Scenic Area (25°82′70.33″N, 107°54′31.23″E) on branches of thorns, 18 November 2021, Altitude: 1,001 m, S. H. Long & Q. R. Li., JXG11 (GMB0430, paratype, ex-paratype GMBC0430).
Notes: Phylogenetic analyses show that Diatrype rubi has a close relationship with D. camelliae-japonicae (Figure 1). Morphologically, the stromata of D. rubi is similar to D. stigma, D. undulata, D. hypoxyloides, D. playstoma, and D. subundulata, but the ascospores of D. rubi are wider than those of D. playstoma (7–9 × 1–1.3 μm) (Vasilyeva and Ma, 2014). The ascospores of D. undulata (5–7 × 0.9–1.3 μm) and D. hypoxyloides (4–6 long, very thin) are narrower than those of D. rubi (Vasilyeva and Ma, 2014). The ascospores of D. rubi are hyaline while D. subundulata and D. undulate have yellowish ascospores (Vasilyeva and Ma, 2014). In addition, Diatrype rubi can be distinguished from D. stigma by its longer asci (73–97 × 4–6 μm vs. 25–30 × 5–7 μm) (Vasilyeva and Ma, 2014) and from D. camelliae-japonicae by the size of ascospores (6.5–8 × 1.5–2 μm vs. 5.0–7.6 × 1.2–2.8). Moreover, the ascospores of D. rubi are straight to slightly curved, and the ascospores of D. camelliae-japonicae are slightly curved. Here, we introduce Diatrype rubi based on both morpho-molecular analyses.
Diatrype enteroxantha (Sacc.) Berl., Icon. fung. (Abellini) 3(3-4): 93 (1902) (Figure 7).
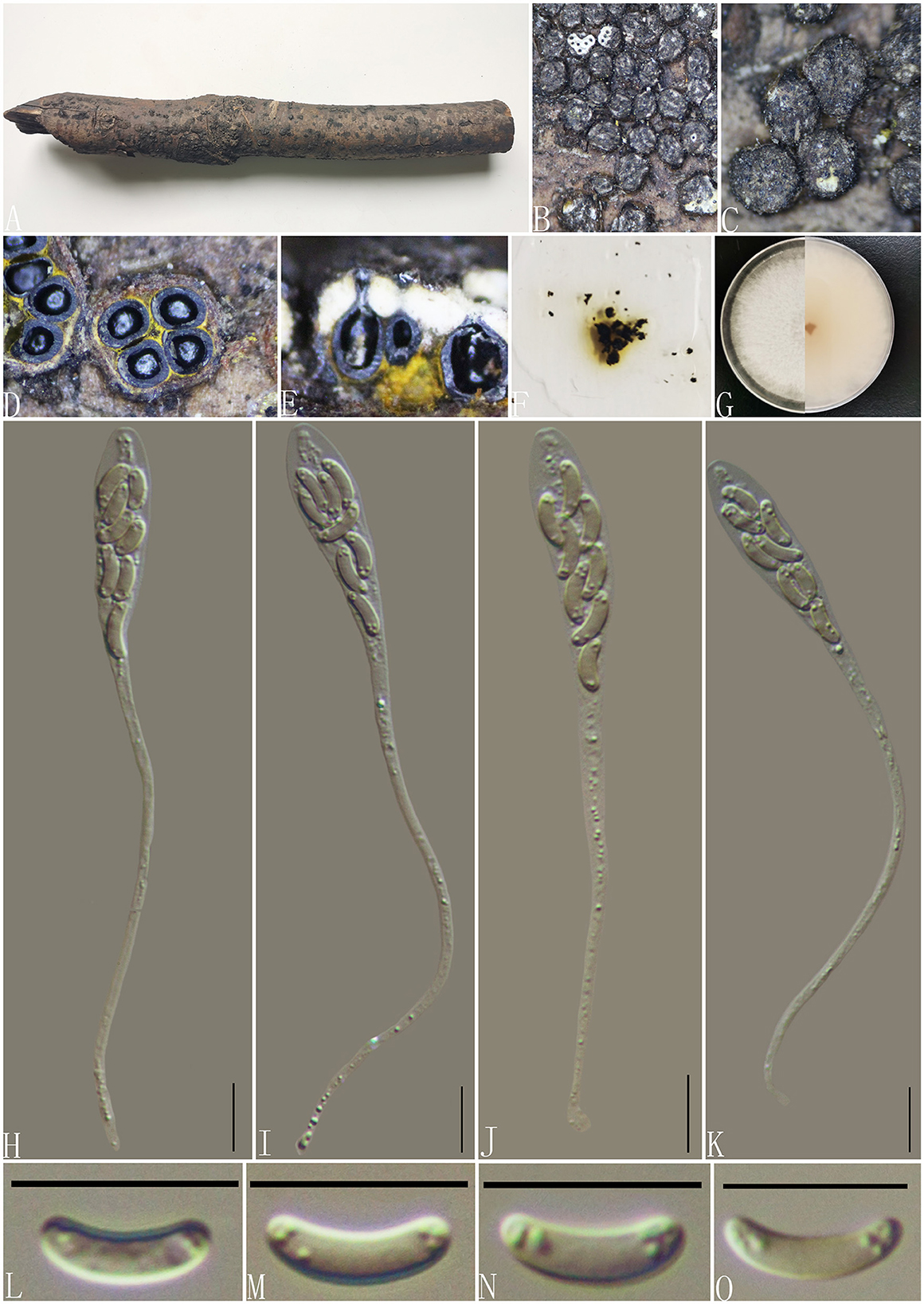
Figure 7. Diatrype enteroxantha (GMB0433). (A) Material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Pigments in KOH. (G) Culture on PDA. (H–K) Asci. (L–O) Ascospores. Scale bars: (H–O) = 10 μm.
MycoBank No: MB 454899.
Material examined: China, Guizhou Province, Guiyang City, Huaxi Wetland Park (26°11′33.23″N, 106°54′10.11″E) on branches of an unidentified plant, 7 October 2020, Altitude: 1,140 m, S. H. Long & Q. R. Li., HX10 (GMB0433, new record from China), living culture GMBC0433.
Saprobic on the surface of dead wood. Sexual morph: Stromata 0.9–2.55 mm in diameter, 0.6–1 mm high, erumpent through the bark, irregular to circular in shape, solitary to gregarious, and surface dark brown to black. Entostroma is composed of two parts; the base region was bases occupied by thin, powdery, yellow tissue, and the entostromatic region between perithecial necks occupied by thick, white tissue. Perithecia 520–640 μm high, 230–260 μm broad ( = 315.5 × 198 μm, n = 10), globose to subglobose, glabrous, with cylindrical neck. Ostioles opening separately, papillate or apapillate, central. Peridium 30–40 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 94–133 × 7–9.5 μm ( = 117.2 × 8.4 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, apically rounded to truncate, apical rings inamyloid. Ascospores 7–10.5 × 1.5–2.5 μm ( = 8.5 × 2 μm, n = 30), overlapping, allantoid, slightly curved, hyaline, smooth, aseptate, usually with small guttules. Asexual morph: Not formed.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light brown, dense, but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to sepia and at the center, no pigmentation, and no sporulation produced on the PDA medium.
Notes: In the phylogenetic analyses, GMB0433 clusters with the strains Diatrype enteroxantha HUEFS 155114 and HUEFS 155116 with a high support value (100/1) (Figure 1). Morphologically, GMB0433 is consistent with the descriptions of the holotype of D. enteroxantha (Rappaz, 1987). Sequences of GMB0433 are similar to Diatrype enteroxantha (HUEFS 155116) (ITS: 99.4%, 3/501 gaps). Diatrype enteroxantha has been reported in Argentina, Brazil, Guyana, and South Africa (Doidge, 1941; Rappaz, 1987; de Almeida et al., 2016), and this is the first report from Asia and China.
Diatrypella (Ces. & De Not.) De Not.
MycoBank No: MB 1505.
Note: The genus Diatrypella was introduced by Cesati and De Notaris (1863) and was typified with Diatrypella verruciformis (Ehrh.) Nitschke. This genus was characterized by pustule-like stromata erumpent through the host surface, polysporous asci and allantoid ascospores, and libertella-like asexual morphs (Senanayake et al., 2015; Hyde et al., 2017; Shang et al., 2017). In this study, we introduced two new species of Diatrypella (viz., Diatrypella fatsiae-japonica, Diatrypella guiyangensis).
Diatrypella fatsiae-japonica S. H. Long & Q. R. Li. sp. nov. (Figure 8).
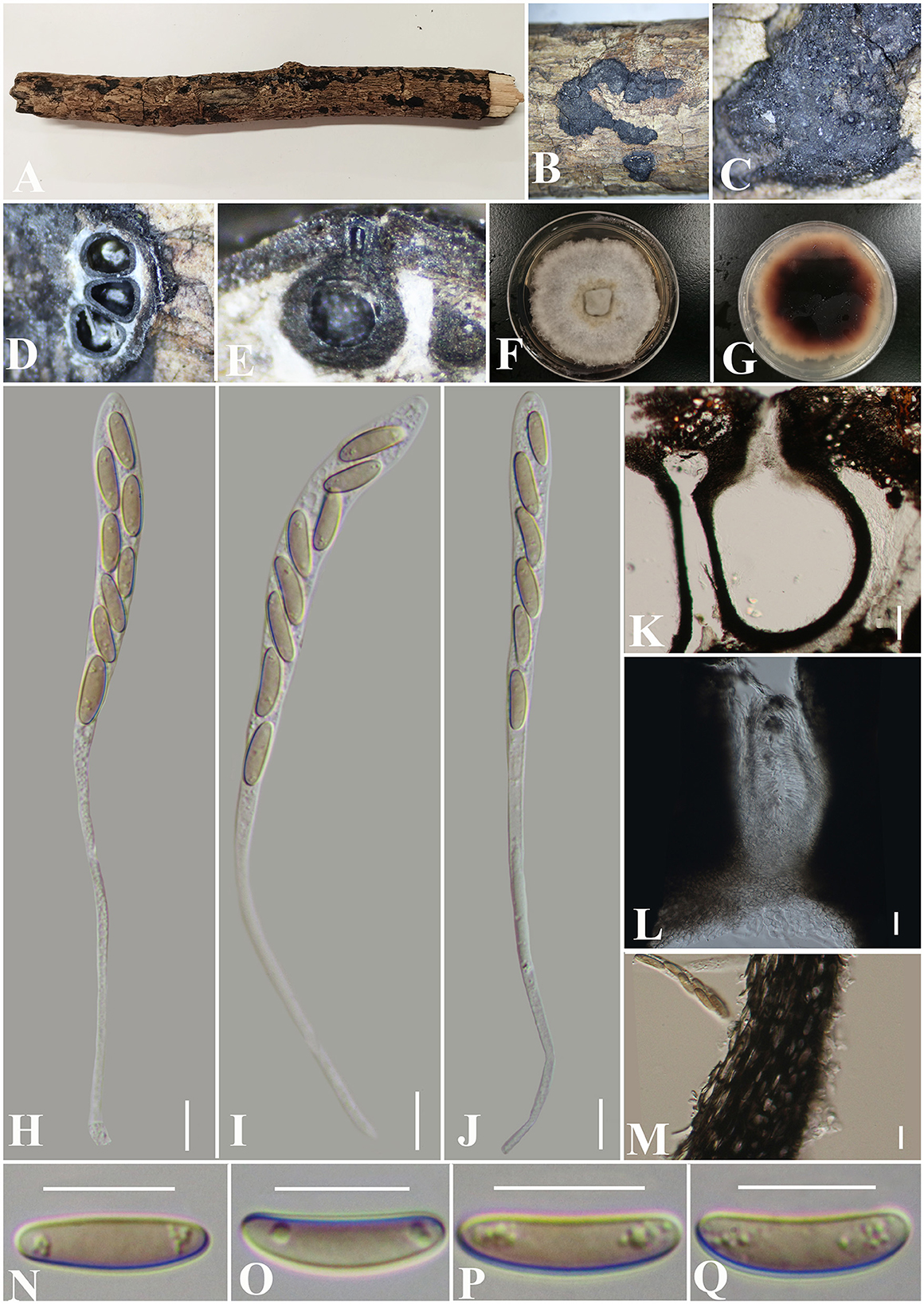
Figure 8. Diatrypella fatsiae-japonicae (GMB0422, holotype). (A) Type material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F, G) Cultures on PDA. (H–J) Asci. (K) Section through the ascostroma. (L) Ostiolar canal. (M) Peridium. (N–Q) Ascospores. Scale bars: (H–J, L–Q) = 10 μm; (K) = 50 μm.
MycoBank No: MB 846767.
Etymology: Refers to the host of Fatsia japonica (Thunb.) Decne.
Material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Lan Ding Mountain (25°28′58.27″N, 107°53′53.70″E) on branches of Fatsia japonica (Thunb.) Decne. et Planch., 12 June 2021, Altitude: 545 m, S. H. Long & Q. R. Li., LDS61 (GMB0422, holotype), ex-type GMBC0422; ibid (KUN-HKAS 126460, isotype).
Saprobic on the surface of dead branches of Fatsia japonica. Sexual morph: Stromata 0.4–0.7 cm long and 0.4–0.6 cm broad ( = 0.6 × 0.4 mm, n = 30), ~0.6 mm thick, well-developed, erumpent through the bark, irregular in shape, effused, sometimes patch-like, pustulate, rugose, visible as black, solitary to gregarious, numerous ascomata immersed in one stroma. Endostroma consists of an outer layer of black, small, dense, thin parenchymal cells and an inner layer of white, large, loose parenchymal cells. Perithecia 285–557.5 μm high, 223.5–320 μm diameter ( = 510.5 × 259.7 μm, n = 10), semi-immersed in the stroma, globose to subglobose with a long cylindrical neck in the stroma. Ostioles opening separately, papillate, central. Peridium 25–40 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 150.5–186 × 8–10 μm ( = 165.6 × 8.8 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded, apical rings inamyloid. Ascospores 10–17.5 × 3–4.5 μm ( = 13 × 3.9 μm, n = 30), overlapping, ellipsoid to allantoid, straight or slightly curved, light olivaceous, smooth, aseptate, usually with small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became light brown, dense, but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to sepia and at the center, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Lan Ding Mountain (25°28′31.28″N, 107°53′13.38″E) on branches of an unidentified plant, 12 June 2021, Altitude: 833 m, S. H. Long & Q. R. Li., LDS107 (GMB0423, paratype, ex-paratype GMBC0423).
Notes: Figure 1 shows that the GMB0422 is located in the unsolved clade which contains Diatrype and Diatrypella. However, the ascospores of GMB0422 are longer than those of Diatrypella favacea (10–17.5 vs. 6–8 μm) (Vasilyeva and Stephenson, 2005). Diatrypella guiyangensis, Diatrype lancangensis, and Diatrype palmicola have 8-spored asci; however, the ascospores of GMB0422 are wider than those of Diatrype langcangensis (10–17.5 × 3–4.5 μm vs. 11–18.5 × 2–4 μm) (Long et al., 2021) and larger than those of Diatrype palmicola (10–17.5 × 3–4.5 μm vs. 7–8 × 1.5–2 μm) (Liu et al., 2015), and the ascospores of GMB0422 are light olivaceous which are different from brown to dark brown in Diatrype lancangensis and hyaline in Diatrypella guiyangensis (Long et al., 2021).
Diatrypella guiyangensis S. H. Long & Q. R. Li. sp. nov. (Figure 9).
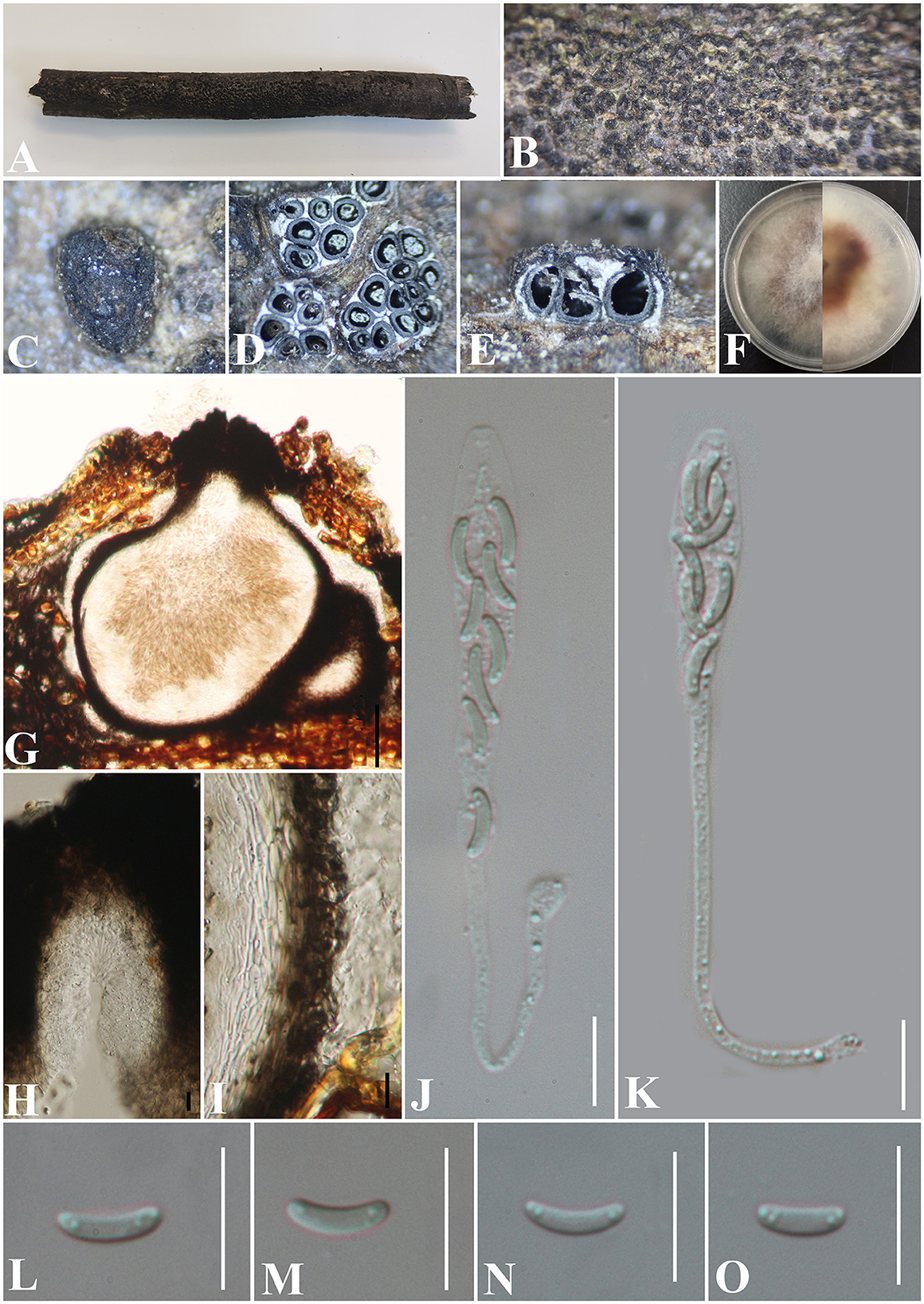
Figure 9. Diatrypella guiyangensis (GMB0414, holotype). (A) Type material. (B, C) Close-up of stromata. (D) Transverse section through stromata. (E) Vertical section through stromata. (F) Cultures on PDA. (G) Section through the ascostroma. (H) Ostiolar canal. (I) Peridium. (J, K) Asci. (L–O) Ascospores. Scale bars: (G) = 50 μm; (H–O) = 10 μm.
MycoBank No: MB 846766.
Etymology: Refers to the collection area of type specimens, Guiyang city.
Material examined: China, Guizhou Province, Guiyang City, Guiyang Medical University (26°22′31.28“N, 106°38′18.38″E) on branches of an unidentified plant, 1 August 2020, Altitude: 1128 m, S. H. Long & Q. R. Li., 2020G24 (GMB0414, holotype), ex-type GMBC0414; ibid (KUN-HKAS 126457, isotype).
Saprobic on the bark of an unidentified plant branch. Sexual morph: Stromata erumpent through the bark, extending into a black area, postulate to irregular in shape, rugose, gregarious, 3–10 ascomata immersed in one stroma, 0.9–1.3 mm diameter ( = 1.1 × 1.2 mm, n = 30), ~0.6 mm high. Endostroma consists of an outer layer of black, small, dense, thin parenchymal cells and an inner layer of white, large, loose parenchymal cells. Perithecia 530–640 μm high, 250–425 μm diameter ( = 563.8 × 336.2 μm, n = 10), embedded in bark, globose to subglobose with cylindrical neck. Ostioles opening separately, papillate, central. Peridium 40–60 μm thick, dark brown to hyaline with textura angularis cell layers. Asci (71) 86.5–126.5 × 4.6–8 μm ( = 99 × 6.7 μm, n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded to truncate apex, apical rings inamyloid. Ascospores 6.5–8 × 1–2 μm ( = 7.3 × 1.5 μm, n = 30), overlapping, allantoid, slightly curved, subhyaline, smooth, aseptate, usually with two small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became mauve, dense, but thinning toward the edge, margin rough, white from above, reverse white at the margin, mauve to luteous at the center, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Guiyang City, Guiyang Medical University (26°22′73.90″N, 106°39′10.88″E) on branches of an unidentified plant, 8 August 2022, Altitude: 1147 m, S. H. Long & Q. R. Li., 2020G53 (GMB0415, paratype, ex-paratype GMBC0415).
Notes: In Figure 1, GMB0414 was closely related to species of Diatrype and Diatrypella, but Diatrypella favacea has polysporous asci, whereas GMB0414 has only eight ascospores (Croxall, 1950; Glawe and Rogers, 1984). Moreover, the asci of GMB0414 are longer than those of Diatrypella favacea (86.5–126.5 × 4.6–8 μm vs. 70–90 × 8–12 μm) (Vasilyeva and Stephenson, 2005). Diatrypella pulvinata was introduced as an asexual fungus on a branch of Quercus garryana (Zhu et al., 2021). Diatrype lancangensis and Diatrype palmicola have 8-spored asci, but the stromata of both species are flat, whereas the stromata of GMB0414 are verrucose to conical, and the ascospores of GMB0414 are smaller than those of Diatrype langcangensis (6.5–8 × 1–2 μm vs. 11–18.5 × 2–4 μm), and the asci are larger than those of Diatrype palmicola (86.5–126.5 × 4.6–8 μm vs. 70–110 × 7–9 μm) (Liu et al., 2015).
Paraeutypella L.S. Dissan., J.C. Kang, Wijayaw. & K.D. Hyde, Biodiversity Data Journal 9: e63864, 11 (2021).
MycoBank No: MB 557954.
Note: Paraeutypella was introduced by Dissanayake et al. (2021) and was typified by P. guizhouensis L.S. Dissan., J.C. Kang & K.D. Hyde. The genus shows eutypella-like morphology (Dissanayake et al., 2021), having immersed stromata with elongated ostiolar neck, 8-spored, clavate to cylindrical clavate or spindle-shaped asci, allantoid ascospores. The asexual morph was reported as coelomycetous (Vasilyeva and Stephenson, 2006). In this study, we introduce a new species of Paraeutypella from China.
Paraeutypella subguizhouensis S. H. Long & Q. R. Li. sp. nov. (Figure 10).
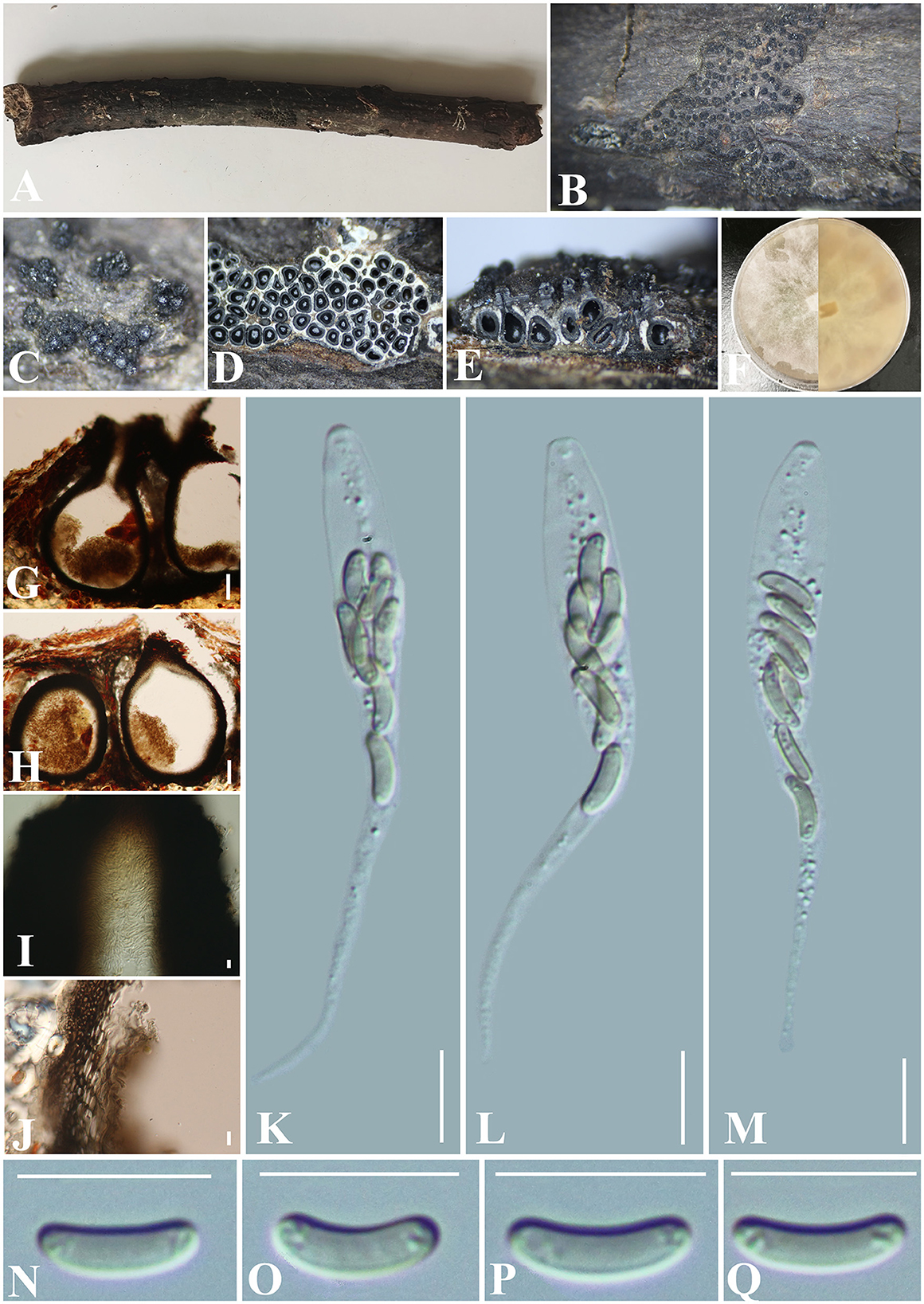
Figure 10. Paraeutypella subguizhouensis (GMB0420, holotype). (A) Type material. (B, C) Close-up of stromata. (D) Transverse section trough stromata. (E) Vertical section through stromata. (F) Cultures on PDA. (G, H) Sections through the stromata. (I) Ostiolar canal. (J) Peridium. (K–M) Asci. (N–Q) Ascospores. Scale bars: (G, H) = 100 μm; (I–Q) = 10 μm.
MycoBank No: MB 846772.
Etymology: Morphologically similar to Paraetypella guizhouensis.
Material examined: China, Guizhou Province, Guiyang City, Guiyang Forest Park (26°32′52.79″N, 106°45′10.31″E) on branches of an unidentified plant, 22 June 2021, Altitude: 1165 m, S. H. Long & Q. R. Li., GYSLGY22 (GMB0420, holotype), ex-type GMBC0420; ibid (KUN-HKAS 126462, isotype).
Saprobic on the surface of dead wood. Sexual morph: Stromata poorly developed, immersed in bark, aggregated, circular to irregular in shape, 0.4–1.5 cm long and 0.3–1 cm broad ( = 0.9 × 0.5 cm, n = 30), ~1 mm thick, numerous ascomata immersed in one stroma showing clustered beaks. Endostroma consists of an outer layer of black, small, dense, thin parenchymal cells and an inner layer of white, large, loose parenchymal cells. Perithecia 720–860 μm high, 280–335 μm diameter ( = 807.2 × 308.3 μm, n = 10), semi-immersed in the stroma, globose to subglobose with a long cylindrical neck (350–410 μm) in and out of the bark. Ostioles opening separately from the top of the neck, papillate, central. Peridium 50–80 μm thick, dark brown to hyaline with textura angularis cell layers. Asci 61–90 × 6–7.5 μm ( = 79 × 7.2 μm n = 30), 8-spored, unitunicate, long-cylindrical, with long stipe, rounded to truncate apex, apical rings inamyloid. Ascospores 7.5–10.5 × 1.5–2.5 μm ( = 8.8 × 2.2 μm, n = 30), overlapping, allantoid, slightly curved, subhyaline, smooth, aseptate, usually with small guttules. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became mauve, dense but thinning toward the edge, margin rough, white from above, reverse mauve to luteous, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Guiyang City, Guiyang Forest Park (26°32′77.35”N, 106°44′19.92″E) on branches of an unidentified plant, 23 June 2022, Altitude: 1165 m, S. H. Long & Q. R. Li., GYSLGY51 (GMB0421, paratype, ex-paratype GMBC0421).
Notes: In stromatal morphology, Paraeutypella subguizhouensis (GMB0420) resembles the species of Paraeutypella (Dissanayake et al., 2021). In our phylogenetic analyses, GMB0420 was accommodated in Paraeutypella s. str. (Figure 1). Paraeutypella psedoguizhouensis can differ from other species of Paraeutypella in having more than 25 ascomata in one stroma, however, species of Prareutypella only have 4–25 ascomata immersed in one stroma (Dissanayake et al., 2021). Moreover, GMB0420 differs from Paraetypella guizhouensis in having a shorter ostiolar neck (350–410 μm vs. 400–418 μm) (Dissanayake et al., 2021), from Paraetypella vitis in having longer asci (61–90 × 6–7.5 μm vs. 40–46 × 6–8 μm) and smaller ascospores (7.5–10.5 × 1.5–2.5 μm vs. 9.6–12 × 2–2.4 μm) (Glawe and Jacobs, 1987), and from Paraeytypella citricola by having smaller ascospores (7.5–10.5 × 1.5–2.5 μm vs. 10–12 × 2–3 μm) (Trouillas et al., 2011). The phylogenetic position of Allocryptovalsa castaneicola is consistent with the previous article (Zhu et al., 2021), and it was introduced as a species of Allocryptovalsa since it has polyspored asci. Paraeutypella subguizhouensis differs from Allocryptovalsa castaneicola in having shorter ascospores (7.5–10.5 × 1.5–2.5 μm vs. 22–25 × 5–6 μm) (Zhu et al., 2021). Here, we temporarily classify it as Paraeutypella until the classification of Diatrypaceae is clearer at the genus level.
Peroneutypa Berl., Icon. fung. (Abellini) 3(3-4): 80 (1902).
MycoBank No: MB 3834.
Notes: Peroneutypa was introduced by Berlese (1902) for having valsoid stroma with long prominent necks, sessile to long stalks, small, clavate asci with truncated apices, and allantoid ascospores (Saccardo and Saccardo, 1905; Carmarán et al., 2006, 2014). Rappaz (1987) proposed P. bellula (Desm.) Berl. as the type species of Peroneutypa. The asexual morph of this genus is not reported so far.
Peroeutypa hainanensis S. H. Long & Q. R. Li. sp. nov. (Figure 11).
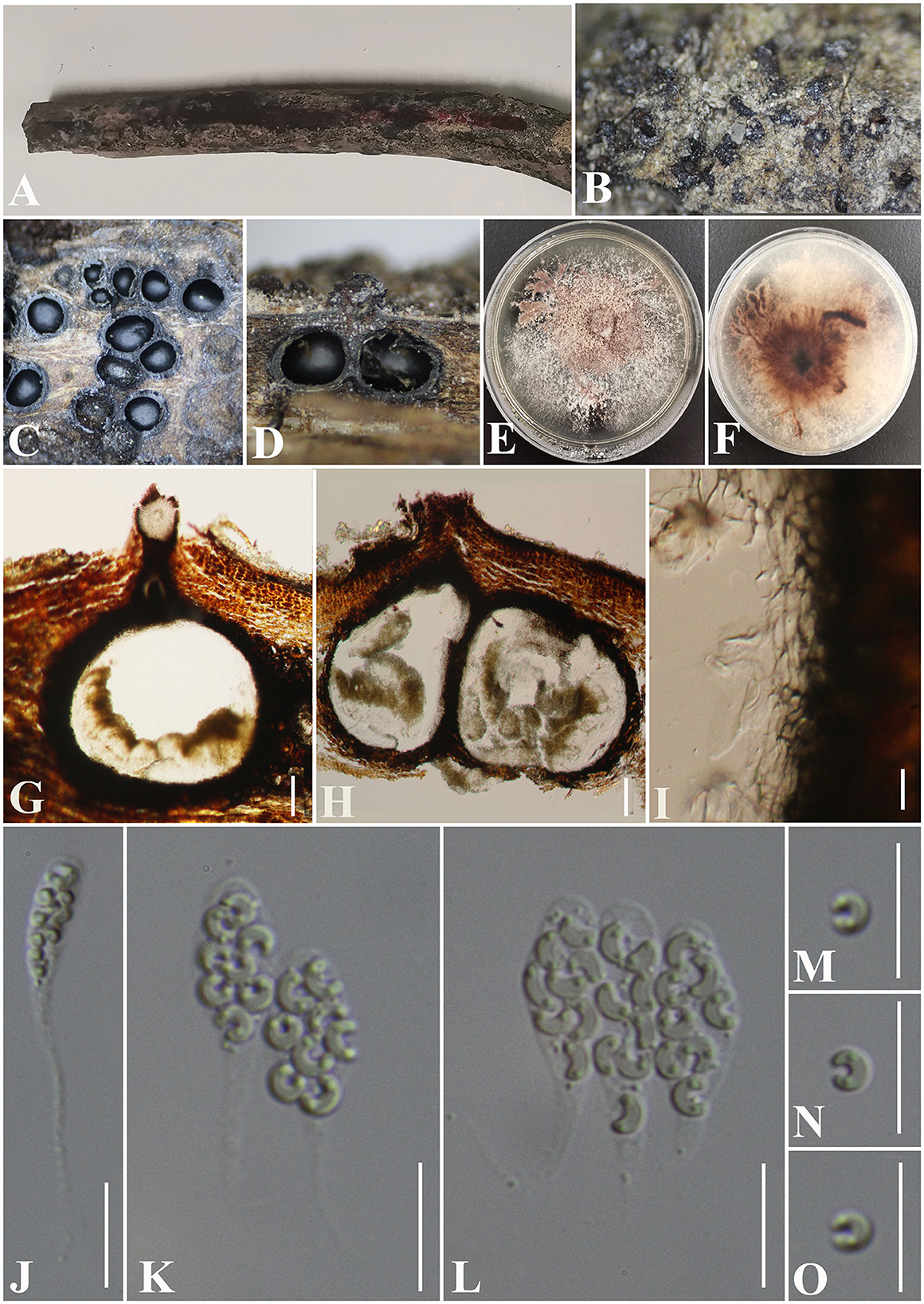
Figure 11. Peroeutypa hainanensis (GMB0424, holotype). (A) Type material. (B). Close-up of stromata. (C) Transverse section through stromata. (D) Vertical section through stromata. (E, F) Culture on PDA. (G, H) Sections through the ascostroma. (I) Peridium. (J–L) Asci. (M–O) Ascospores Bar: (G, H) =100 μm; (I–O) = 10 μm.
MycoBank No: MB 846770.
Etymology: Refers to the collection area, Hainan Province.
Material examined: China, Hainan Province, Wenchang City, Tongguling Nature Reserve (19°39'16.23“N, 111°1'38.68”E) on branches of an unidentified plant, 12 November 2021. Altitude: 67 m, S. H. Long & Q. R. Li., TGL4 (GMB0424, holotype), ex-type GMBC0424; ibid (KUN-HKAS 126463, isotype).
Saprobic on dead branches of an unidentified plant. Sexual morph: Stromata 0.4–0.7 mm diameter × 0.1–0.3 mm long, non-sulcate, poorly developed, solitary to gregarious, immersed, ostiolar canals raised to erumpent the surface of stromata, dark brown to black, 1–7 perithecia immersed in one stroma. Perithecia 350–600 μm high × 130–300 μm diameter ( = 375 × 202 μm, n = 10), immersed, globose to subglobose, brown to black, ostiolate. Ostiolar canal 105–420 μm high, 80–120 μm diameter ( = 265 × 100 μm, n = 25), cylindrical, sulcate, at the apex curved, periphysate. Peridium 45–65 μm wide, composed of two layers, outer section dark brown to black, thick-walled cells, arranged in textura globulosa to textura angularis, inner part comprising hyaline textura angularis cells. Asci 28.5–40 × 3.5–6.5 μm ( = 33.5 × 5.5 μm, n = 30), 8-spored, unitunicate, clavate, with long stipitate, apically rounded to truncate, apical rings inamyloid. Ascospores 5.0–7.3 × 1–2 μm ( = 6 × 1.5 μm n = 30), overlapping, allantoid, strongly curved, subhyaline, with small guttules at ends. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA after 24 h. Colonies white when young, became pale brown circular to irregular, medium dense, flat or effuse, slightly raised, fluffy to powder, margin rough, white at the margin and light brown at the center from below, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Hainan Province, Wenchang City, Tongguling Nature Reserve (19°39′38.81″N, 111°0′50.82″E) on branches of an unidentified plant, 12 November 2021. Altitude: 73 m, S. H. Long & Q. R. Li., TGL53 (GMB0425, paratype, ex-paratype GMBC0425).
Notes: Figure 1 shows that Peroeutypa hainanensis clustered with species of Peroneutypa. Morphologically, the ascospores of P. obesa and P. curvispora are strongly curved, but the ascospores of Peroeutypa hainanensis (5.0–7.3 × 1–2 μm) are longer than those of P. curvispora (3.0–4.5 × 1–1.5 μm) (Carmarán et al., 2006; Shang et al., 2018). The spiny or bristly appearance of the stromata surface of P. obesa can be distinguished from P. hainanensis, and the stromata of GMB0424 are smaller than those of P. obesa (10–15 mm diameter × 7–10 m long) (Rappaz, 1987). Based on both molecular and phylogenetic analyses, here, we introduce new species, Peroeutypa hainanensis.
Peroneutypa qianensis S. H. Long & Q. R. Li. sp. nov. (Figure 12).

Figure 12. Peroneutypa qianensis (GMB0431, holotype). (A) Type material. (B) Close-up of stromata. (C) Transverse section through stromata. (D) Vertical section through stromata. (E) Section through the ascostroma. (F) Peridium. (G–I) Asci. (J–L) Ascospores. Scale bars: (E) = 100 μm; (F–L) = 10 μm.
MycoBank No: MB846771.
Etymology: Refers to the location of the type specimen, Qian is the abbreviation of Guizhou Province in Chinese.
Material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Maolan National Nature Reserve (25°18′2.76″N, 108°4′29.48″E) on branches of an unidentified plant, 7 July 2021. Altitude: 545 m, S. H. Long & Q. R. Li., MLB62 (GMB0431, holotype), ex-type GMBC0431; ibid (KUN-HKAS 126464, isotype).
Saprobic on dead branches of an unidentified plant. Sexual morph: Stromata poorly developed, 1.5–2 mm wide, solitary to gregarious, immersed; ostiolar canals raised to erumpent the surface of stromata, dark brown to black, non-sulcate. Perithecia 320–540 μm high × 175–290 μm diameter ( = 375 × 202 μm, n = 10), immersed, globose to subglobose, brown to black, ostiolate. Ostiolar canal 105–420 μm high, 80–120 μm diameter ( = 265 × 100 μm, n = 25), cylindrical, sulcate, at the apex curved, periphysate. Peridium 45–65 μm wide, composed of two layers, outer section dark brown to black, thick-walled cells, arranged in textura globulosa to textura angularis, inner part comprising hyaline textura angularis cells. Asci 16.5–20.5 × 4–6 μm ( = 18.4 × 5 μm, n = 30), 8-spored, unitunicate, clavate, sessile, apically rounded to truncate, apical rings inamyloid. Ascospores 4.5–6.3 × 1.5–0.3 μm ( = 5.6 × 1.8 μm n = 30), overlapping, allantoid, straight to slightly curved, subhyaline, with small guttules at ends. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA after 24 h. Colonies white when young, became pale brown circular to irregular, medium dense, flat or effuse, slightly raised, fluffy to powder, margin rough, white at the margin and light brown at the center from below, no pigmentation, and no sporulation produced on the PDA medium.
Additional material examined: China, Guizhou Province, Qiannan Buyi Miao Autonomous Prefecture, Maolan National Nature Reserve (25°17′52.14″N, 108°4′27.01″E) on branches of an unidentified plant, 7 July 2021. Altitude: 651 m, MLB150 (GMB0432, paratype, ex-paratype, GMBC0432).
Note: Our phylogenetic analyses (Figure 1) show that Peroneutypa qianensis resides as the sister clade to P. mackenziei, with high bootstrap and PP values (99/1). Morphologically, P. qianensis is similar to P. mackenziei (MFLU 16-1441, holotype), in that both of them have the clavate, sessile ascospores (Shang et al., 2017). However, the ascospores of P. mackenziei are narrower than those of the new specimen GMB0431 (4.5–6.5 × 1–2 μm vs. 4.5–6.3 × 1.5–3 μm) (Shang et al., 2017). Combining morphological and molecular data, we introduce GMB0431 as a new species of Peroneutypa.
Vasilyeva S. H. Long, Wijayaw. & Q. R. Li. gen. nov.
MycoBank No: MB846773.
Etymology: We dedicate this genus to L.N. Vasilyeva, an excellent taxonomist who extensively worked on Diatrypacaea research in China.
Saprobic on an unidentified wood. Sexual morph: Stromata poorly developed, immersed in the host tissue, showing a long beak higher than the wood surface and a long channel immersed, the beak in the air covered with the long setae. Perithecia with a long beak, scattered or in rows, circular to oblate. Ostioles apparent on the surface of the substrate, higher than the surface of the wood, emerging on the surface separately. Asci 8-spored, unitunicate, clavate to long-cylindrical, with long stipe, apically rounded, apical rings inamyloid. Ascospores overlapping, allantoid, straight or slightly curved, subhyaline to hyaline, with oil droplets at ends. Asexual morph: Undetermined.
Type species: Vasilyeva cinnamomi S. H. Long, Wijayaw. & Q. R. Li.
Notes: The genus Vasilyeva is introduced to accommodate the new collection made from Hainan, China. Figure 1 shows that the new collection formed a distinct branch which is sister to Peroneutypa. Morphologically, Vasilyeva has stromata covered with long setae, long stipe asci, allantoid, and straight or slightly curved ascospores. The perithecia of Vasilyeva cinnamomi are immersed in the stromata with a long beak which includes a part higher than the surface of wood covered with long setae and a long channel immersed, and the ostioles emerging on the surface separately. It is different from all genera in Diatrypaceae. Based on morphological and phylogenetic analyses, Vasilyeva was proposed as a new genus.
Vasilyeva cinnamomi S. H. Long, Wijayaw. & Q. R. Li sp. nov. (Figure 13).
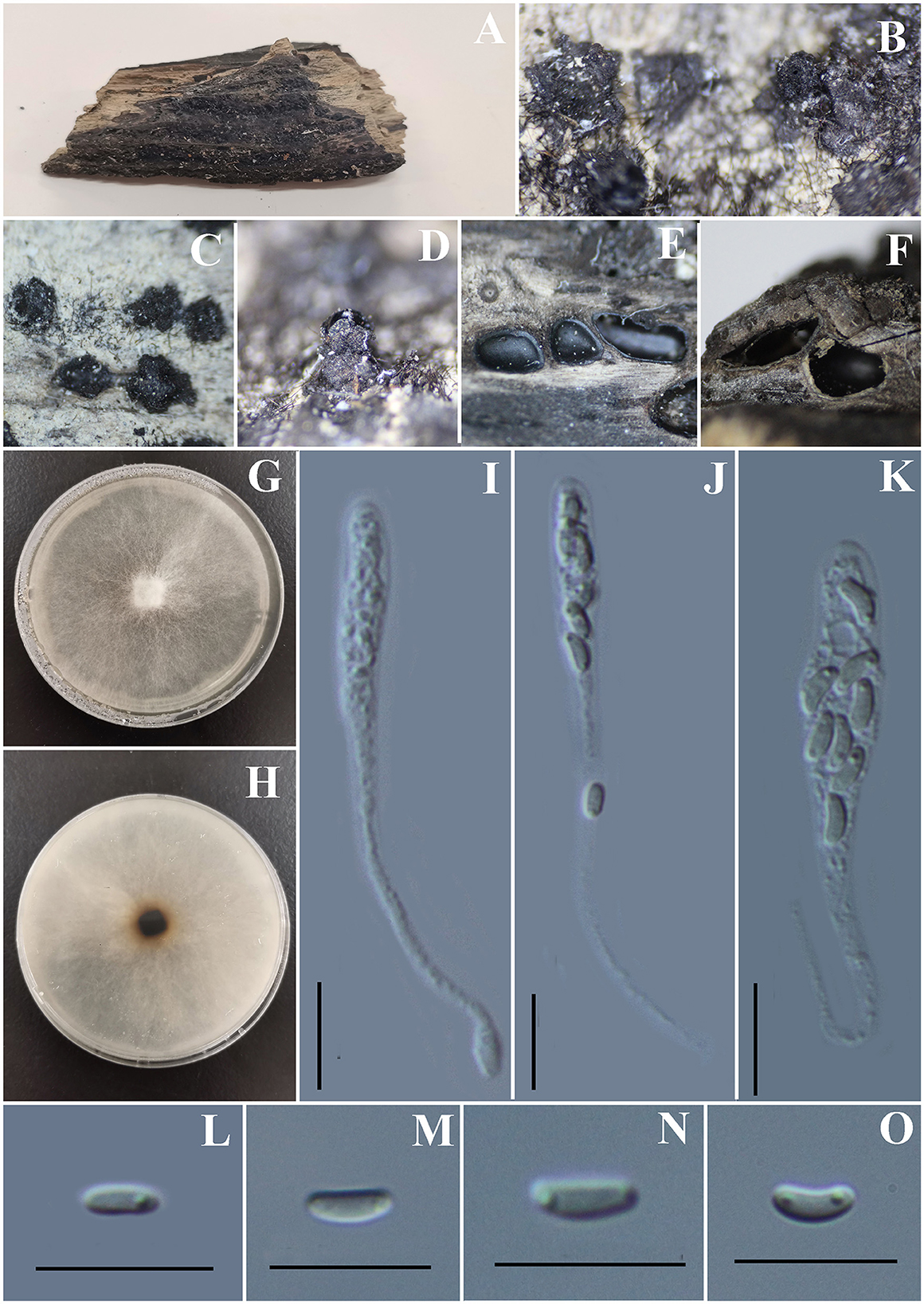
Figure 13. Vasilyeva cinnamomi (GMB0418, holotype). (A) Type material. (B–D) Close-up of stromata. (E) Transverse section through stromata. (F) Vertical section through stromata. (G, H) Culture on PDA. (I–K) Asci. (L–O) Ascospores. Scale bars: (C–F) = 1 mm; (I–O) = 10 μm.
MycoBank No: 846774.
Etymology: Refers to its host, Cinnamomum cinnamomi (L.) Presl.
Material examined: China, Hainan Province, Wuzhishan City Wuzhishan Nature Reserve (18°54'21.47“N, 109°40'57.99”E) on wood chips of Cinnamomum cinnamomi (L.) Presl, 15 November 2021. Altitude: 795 m, S. H. Long & Q. R. Li., WZS28 (GMB0418, holotype), ex-type GMBC0418; ibid (KUN-HKAS 126465, isotype).
Saprobic on dead wood chips of Cinnamomum cinnamomi. Sexual morph: Stromata poorly developed, immersed in the host tissue, showing a black beak on the wood surface. Perithecia 0.8–1.3 mm high × 1.3 – 2.3 mm diameter ( = 0.9 × 1.8 mm, n = 10) (the length of the beak is not included), with a long beak [partly in the wood (0.6–0.8 mm high) and partly on the surface of the wood (0.5–0.9 mm) covered with the long setae], scattered or in rows, circular to oblate. Ostioles apparent on the surface of the substrate, higher than the surface of wood, emerging on the surface separately. Asci 58 – 77.5 × 4 – 7 μm ( = 66.7 × 5.1 μm, n = 30), 8-spored, unitunicate, clavate to long-cylindrical, with long stipe, apically rounded, apical rings inamyloid. Ascospores 4.0 – 6.0 × 1.5 – 2.5 μm ( = 4.7 × 1.9 μm n = 30), overlapping, allantoid, straight or slightly curved, subhyaline to hyaline, with small guttules at ends. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h. Colonies on PDA, white when young, became brown, dense, but thinning toward the edge, margin rough, white from above, reverse white to brown, no pigmentation, and no sporulation produced on the PDA medium.
Additional Material examined: China, Hainan Province, Wuzhishan City Wuzhishan Nature Reserve (18°54′70.43″N, 109°41′10.59″E) on branches of an unidentified plant, 15 November 2021. Altitude: 833 m, S. H. Long & Q. R. Li., WZS90 (GMB0419, paratype, ex-paratype GMBC0419).
Notes: Vasilyeva cinnamomi is a morphologically and phylogenetically distinct species from other known species in Diatrypaceae. A peculiar feature of Vasilyeva cinnamomi is the ostioles appearing separately on the surface and the perithecia which are immersed in the stromata with a long beak are higher than the surface of the wood.
Discussion
Diatrypaceae species have a cosmopolitan distribution and often inhabit the deadwood and bark of many plant species. However, the generic concepts of Diatrypaceae have been unstable; thus, many species were transferred from one genus to another (Phookamsak et al., 2019; Konta et al., 2020).
In this study, one new genus and eight new species were described based on phylogenetic analyses and morphological characteristics. The new genus Vasilyeva differs from other genera in its perithecia which have two parts, the lower part is immersed in the stromata, and the higher part has a long beak and is higher than the surface of the wood. Diatrype camelliae-japonicae, Diatrype rubi, Diatrypella guiyangensis, Diatrypella fatsiae-japonicae, Peroneutypa hainanensis, Peroneutypa qianensis, and Paraeutypella subguizhouensis have been introduced as novel taxa from various substrates in Guizhou and Hainan provinces, China.
In addition, Allocryptovalsa rabenhorstii and Diatrype enteroxantha have been reported from China for the first time. Two known species of Allocryptovalsa xishuangbanica and Diatrype betulae were described and illustrated, of which Allocryptovalsa xishuangbanica was the first reported from Guizhou province from China. Based on the phylogenetic analyses and megablast, we conclude GMB0426 is representing the sexual morph of Diatrype betulae, and this is the first time reporting its sexual morph.
Our phylogenetic analyses show that the division of genera is confusing which is consistent with the previous studies (Acero et al., 2004; Trouillas et al., 2011; Mehrabi et al., 2015, 2016; de Almeida et al., 2016; Shang et al., 2017; Dissanayake et al., 2021; Long et al., 2021; Zhu et al., 2021; Ma et al., 2023). Compared to the number of Diatrypaceae species, the available sequences in NCBI are relatively fewer. Most species in Diatrypaceae are lacking DNA sequences. Moreover, several genera (e.g., Dothideovalsa, Echinomyces, Endoxylina, and Rostronitschkia) still have no available sequences. The current molecular phylogenetic study of Diatrypaceae only uses ITS and β-tubulin gene sequences, which do not distinguish this family well, and we believe that the sequences of the large subunit (LSU) ribosomal RNA gene and RNA polymerase II second largest subunit (RPB2) gene sequences should be added in future studies for a more accurate phylogenetic analysis of this family.
In our investigation, we found that the molecular data did not correlate well with morphological characteristics, and two materials with 99% similarity differed significantly in morphology. The morphological comparison shows that there is little morphological difference between genera, and the traditional morphological characteristics such as the number of ascospores per ascus and the morphology of stromata do not distinguish well among genera. Long et al. (2021) stated that there are eight ascospores or polysporous in each ascus in different species of the same genus. The number of ascospores in an ascus can no longer be regarded as the main feature of the genus of Diatrypaceae, although this feature has been widely used in the establishment of the genus (Glawe and Rogers, 1984; Vasilyeva and Stephenson, 2005; Konta et al., 2020). Vasilyeva (1986) proposed that the morphology of stromata was influenced by the host, environments, and some other factors, and there were limitations in the use of substratum morphology as a basis for the identification which is consistent with our research. The stromata of Neoeutypella, Allodiatrype, Diatrype, Diatrypella, Allocryptovalsa, Cryptovalsa, Eutypella, and Paraeutypella is similar. Therefore, we consider that the morphological characteristics of the stromata may not be used as a basis for the identification of Diatrypaceae. The authority of the number of ascospores in the ascus as the important feature of identification at the species level is also challenging to some extent. These morphological taxonomic features, which were considered to be very important in the early stage, constitute the main taxonomic basis of the current genera of Diatrypaceae (Tiffany and Gilman, 1965; Glawe and Rogers, 1984; Rappaz, 1987; Vasilyeva and Stephenson, 2005; Senanayake et al., 2015; Senwanna et al., 2017). However, more and more molecular data show that the classification of these genera is unresolved and inconsistent with their morphology (Konta et al., 2020; Long et al., 2021; Zhu et al., 2021; Ma et al., 2023). We must admit that the main DNA sequences currently used for the systematics of Diatrypaceae only include ITS and BT, which is not so sufficient. Does the systematics of Diatrypaceae need to be started from scratch? We do not have a clear answer yet. However, we believe that Diatrypaceae needs to be revised at the genus level, based on type materials, newly collected specimens, more DNA sequences, more suitable morphological features, and other features in the future.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
Y-QK and XZ conceived and designed the experiments. H-MH, Y-PW, Q-ZW, and YL performed the experiment. Q-RL and S-HL analyzed the data and wrote the manuscript. NW, J-CK, and JK provided some materials and polished the language. Y-QK and X-CS revised and approved the final version of the manuscript. All authors contributed extensively to the study presented in the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32000009, 31960005, and 32170019), Talent Base Project of Guizhou Province, China (RCJD2018-22), Ministry of Education Project (07150120711), the Fund of the Science and Technology Foundation of Guizhou Province ([2020]1Y059), Guizhou Province Ordinary Colleges and Universities Youth Science and Technology Talent Growth Project ([2021]154), 111 Project (D20009), and Talent Base Project of Guizhou Province, China (FCJD2018-22).
Acknowledgments
The authors would like to thank the State Key Laboratory of Functions and Applications of Medicinal Plants, Guizhou Medical University, Science and Technology Department of Guizhou Province. NW would like to thank the High-Level Talent Recruitment Plan of Yunnan Province (High-End Foreign Experts Program). JK would like to thank Chiang Mai University for partial support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Wahab, M. A., Hodhod, M. S., Bahkali, A. H. A., and Jones, E. B. G. (2014). Marine fungi of Saudi Arabia. Bot. Mar. 57, 323–335. doi: 10.1515/bot-2014-0010
Acero, F. J., González, V., Sánchez-Ballesteros, J., Rubio, V., Checa, J., Bills, G. F., et al. (2004). Molecular phylogenetic studies on the Diatrypaceae based on rDNA-ITS sequences. Mycologia 96, 249–259. doi: 10.1080/15572536.2005.11832975
Arhipova, N., Gaitnieks, T., Donis, J., Stenlid, J., and Vasaitis, R. (2012). Heart-rot and associated fungi in Alnus glutinosa stands in Latvia. Scand. J.For. Res. 27, 327–336. doi: 10.1080/02827581.2012.670727
Carmarán, C. C., Robles, C. A., D'Jonsiles, M. F., Ceriani, E., Español, E., López, S., et al. (2014). Clave para la identifificación de especies de la familia Diatrypaceae (Xylariales, Ascomycota). Lilloa Fundación Miguel Lillo Tucumán Argentina 51, 20–32.
Carmarán, C. C., and Romero, A. I. (1992). Problemas taxonómicos en el orden Diatrypales. Contribución a su esclarecimiento I. Boletín Soc. Argentina Botánica. 28, 139–150.
Carmarán, C. C., Romero, A. I., and Giussani, L. M. (2006). An approach towards a new phylogenetic classification in Diatrypaceae. Fungal Divers. 23, 67–87. doi: 10.1111/j.1439-0507.2006.01312.x
Catal, M., Jordan, S. A., Butterworth, S. C., and Schilder, A. M. C. (2007). Detection of Eutypa lata and Eutypella vitis in grapevine by nested multiplex polymerase chain reaction. Phytopathology 97, 737–747. doi: 10.1094/PHYTO-97-6-0737
Cesati, V., and De Notaris, G. (1863). Schema di classificazione degli sferiacei italici aschigeri : piuò meno appartenenti al genere Sphaeria nell'antico significato attribuitogli da Persoon. CommentarioSoc. Crittogamol. Ital. 1, 177–240.
Chlebicki, A. (1986). Variability in diatrypella favacea in Poland. Trans. Br. Mycol. Soc. 86, 441–449. doi: 10.1016/S0007-1536(86)80187-5
Chomnunti, P., Hongsanan, S., Hudson, B. A., Tian, Q., Peršoh, D., Dhami, M. K., et al. (2014). The sooty moulds. Fungal Divers. 66, 1–36. doi: 10.1007/s13225-014-0278-5
Crous, P. W., Gams, W., Stalpers, J. A., Robert, V., and Stegehuis, G. (2004). MycoBank: an online initiative to launch mycology into the 21st century. Stud. Mycol. 50, 19–22. doi: 10.1023/B:MYCO.0000012225.79969.29
Crous, P. W., Wingfifield, M. J., Burgess, T. I., St. Hardy, J., Crane, C., Barrett, S., et al. (2016). Fungal planet description sheets: 469–557. Persoonia 37, 218–403. doi: 10.3767/003158516X694499
Croxall, H. E. (1950). Studies on British pyrenomycetes III. The British species of the genus Diatrypella Cesati and de Notaris. Trans. Br. Mycol. Soc. 33, 45–72. doi: 10.1016/S0007-1536(50)80047-5
Dayarathne, M. C., Jones, E. B. G., Maharachchikumbura, S. S. N., Devadatha, B., Sarma, V. V., Khongphinitbunjong, K., et al. (2020a). Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11, 1–188. doi: 10.5943/mycosphere/11/1/1
Dayarathne, M. C., Phookamsak, R., Hyde, K. D., Manawasinghe, I. S., Toanun, C., and Jones, E. B. G. (2016). Halodiatrype, a novel diatrypaceous genus from mangroves with H. salinicola and H. avicenniae spp. nov. Mycosphere 7, 612–627. doi: 10.5943/mycosphere/7/5/7
Dayarathne, M. C., Wanasinghe, D. N., Devadatha, B., Abeywickrama, P., Gareth, J. E. B., Chomnunti, P., et al. (2020b). Modern taxonomic approaches to identifying diatrypaceous fungi from marine habitats, with a novel genus Halocryptovalsa Dayarathne and K.D. Hyde, gen. nov. Cryptogam. Mycol. 41, 21–67. doi: 10.5252/cryptogamie-mycologie2020v41a3
de Almeida, D. A. C., Gusmão, L. F. P., and Miller, A. N. (2016). Taxonomy and molecular phylogeny of Diatrypaceae (Ascomycota, Xylariales) species from the Brazilian semi-arid region, including four new species. Mycol. Prog. 15, 1–27. doi: 10.1007/s11557-016-1194-8
Dissanayake, L. S., Wijayawardene, N. N., Dayarathne, M. C., Samarakoon, M. C., Dai, D. Q., Hyde, K. D., et al. (2021). Paraeutypella guizhouensis gen. et sp. nov. and Diatrypella longiasca sp. nov. (Diatrypaceae) from China. Biodiver. Data J. 9, e63864. doi: 10.3897/BDJ.9.e63864
Glass, N. L., and Donaldson, G. C. (1995). Development of primer sets designed for use with the PCR to amplify conserved genes from flamentous ascomycetes. Appl. Environ. Microbiol. 61, 1323–1330. doi: 10.1002/bit.260460112
Glawe, A., and Jacobs, K. A. (1987). Taxonomic notes on Eutypella vitis, Cryptosphaeria populina, and Diatrype stigma. Mycologia 79, 135–139. doi: 10.1080/00275514.1987.12025379
Glawe, D. A., and Rogers, J. D. (1984). Diatrypaceae in the Pacific Northwest. Mycotaxon 20: 401–460.
Glez-Peña, D., Gómez-Blanco, D., Reboiro-Jato, M., Fdez-Riverola, F., and David, P. (2010). ALTER: programoriented conversion of DNA and protein alignments. Nucleic Acids Res. 38, 14–18. doi: 10.1093/nar/gkq321
Grassi, E., Belen Pildain, M., Levin, L., and Carmaran, C. (2014). Studies in Diatrypaceae: the new species Eutypa microasca and investigation of ligninolytic enzyme production. Sydowia 66, 99–114. doi: 10.12905/0380.sydowia66(1)2014-0099
Hyde, K. D., Dong, Y., Phookamsak, R., Jeewon, R., Jeewon, R., Bhat, D. J., et al. (2020a). Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 100,5–277. doi: 10.1007/s13225-020-00439-5
Hyde, K. D., Norphanphoun, C., Abreu, V. P., Bazzicalupo, A., Chethana, K. W. T., Clericuzio, M., et al. (2017). Fungal diversity notes 603–708: taxonomic andphylogenetic notes on genera and species. Fungal Divers. 87, 1–235. doi: 10.1007/s13225-017-0391-3
Hyde, K. D., Norphanphoun, C., Maharachchikumbura, S. S. N., Bhat, D. J., Jones, E. B. G., Bundhun, D., et al. (2020b). Refifined families of Sordariomycetes. Mycosphere 11, 305–1059. doi: 10.5943/mycosphere/11/1/7
Hyde, K. D., Tennakoon, D. S., Jeewon, R., Bhat, D. J., Maharachchikumbura, S. S. N., Rossi, W., et al. (2019). Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 96, 1–242. doi: 10.1007/s13225-019-00429-2
Jurc, D., Ogris, N., Slippers, B., and Stenlid, J. (2006). First report of Eutypella canker of Acer pseudoplatanus in Europe. Plant Pathol. 55, 577–577. doi: 10.1111/j.1365-3059.2006.01426.x
Katoh, K., and Standley, D. M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780. doi: 10.1093/molbev/mst010
Kirk, P. M., Cannon, P. F., Minter, D. W., and Stalper, J. A. (2008). Ainsworth and Bisby's Dictionary of the Fungi, 10th Edn. London: CAB International. doi: 10.1079/9780851998268.0000
Klaysuban, A., Sakayaroj, J., and Jones, E. G. (2014). An additional marine fungal lineage in the Diatrypaceae, Xylariales: Pedumispora rhizophorae. Bot. Mar. 57, 413–420. doi: 10.1515/bot-2014-0017
Konta, S., Maharachchikumbura, S. S. N., Senanayake, I. C., Mckenzie, E. H. C., Stadler, M., Boonmee, S., et al. (2020). A new genus Allodiatrype, fifive new species and a new host record of diatrypaceous fungi from palms (Arecaceae). Mycosphere 11, 239–268. doi: 10.5943/mycosphere/11/1/4
Lardner, R., Stummer, B. E., Sosnowski, M. R., and Scott, E. S. (2005). Molecular identifification and detection of Eutypa lata in grapevine. Mycol. Res. 109, 799–808. doi: 10.1017/S0953756205002893
Li, G. J., Hyde, K. D., Zhao, R. L., Hongsanan, S., Abdel-Aziz, F. A., Abdel-Wahab, M. A., et al. (2016). Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers. 78, 1–237. doi: 10.1007/s13225-016-0366-9
Li, X. H., Wu, H. X., Li, J. C., Song, J. Y., Wang, Q., Promputtha, I., et al. (2022). Two new species of Allodiatrype from monsoon evergreen broad-leaved forest in Pu'er, Yunnan, China. Chiang Mai J. Sci. 49, 565–580. doi: 10.12982/CMJS.2022.047
Liu, J. K., Hyde, K. D., Jones, E. B. G., Ariyawansa, H. A., Bhat, D. J., Boonmee, S., et al. (2015). Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers. 72, 1–197. doi: 10.1007/s13225-015-0324-y
Long, S. H., Liu, L. L., Pi, Y. H., Wu, Y. P., Lin, Y., Zhang, X., et al. (2021). New contributions to Diatrypaceae from karst areas in China. MycoKeys 83, 1–37. doi: 10.3897/mycokeys.83.68926
Luque, J., Garcia-Figueres, F., Legorburu, F. J., Muruamendiaraz, A., Armengol, J., and Trouillas, F. P. (2012). Species of Diatrypaceae associated with grapevine trunk diseases in Eastern Spain. Phytopathol. Mediterr. 51, 528–540. doi: 10.14601/Phytopathol_Mediterr-9953
Luque, J., Sierra, D., Torres, E., and Garcia, F. (2006). Cryptovalsa ampelina on grapevines in NE Spain: identifification and pathogenicity. Phytopathol. Mediterr. 45, 101–109. doi: 10.1520/STP37480S
Lynch, S. C., Eskalen, A., Zambino, P. J., Mayorquin, J. S., and Wang, D. H. (2013). Identifcation and pathogenicity of Botryosphaeriaceae species associated with coast live oak (Quercus agrifolia) decline in southern California. Mycologia 105, 125–140. doi: 10.3852/12-047
Ma, H. X., Yang, Z. E., Song, Z. K., Qu, Z, Li, Y., and Zhu, A. H. (2023). Taxonomic and phylogenetic contributions to Diatrypaceae from southeastern Tibet in China. Front. Microbiol. 14:1073548. doi: 10.3389/fmicb.2023.1073548
Ma, R., Zhu, Y. F., Fan, X. L., and Tian, C. M. (2016). Canker disease of willow and poplar caused by Cryptosphaeria pullmanensis recorded in China. For. Pathol. 46, 327–335. doi: 10.1111/efp.12261
Maharachchikumbura, S. S. N., Hyde, K. D., Jones, E. B. G., and McKenzie, E. H. C. (2015). Towards a natural classifification and backbone tree for Sordariomycetes. Fungal Divers. 72, 199–301. doi: 10.1007/s13225-015-0331-z
Maharachchikumbura, S. S. N., Wanasinghe, D. N., Elgorban, A. M., Al-Rejaie, S. S., Kazerooni, E. A., and Cheewangkoon, R. (2022). Brunneosporopsis yunnanensis gen. et sp. nov. and Allocryptovalsa xishuangbanica sp. nov., new terrestrial Sordariomycetes from Southwest China. Life 12, 635. doi: 10.3390/life12050635
Mayorquin, J. S., Wang, D. H., Twizeyimana, M., and Eskalen, A. (2016). Identifification, distribution, and pathogenicity of Diatrypaceae and Botryosphaeriaceae associated with Citrus branch canker in the Southern California desert. Plant Dis. 100, 2402–2413. doi: 10.1094/PDIS-03-16-0362-RE
Mehrabi, M., Bita, A., and Roghayeh, H. (2019). Two new species of Eutypella and a new combination in the genus Peroneutypa (Diatrypaceae). Mycol. Prog. 18, 1057–1069. doi: 10.1007/s11557-019-01503-4
Mehrabi, M., Hemmati, R., Vasilyeva, L. N., and Trouillas, F. P. (2015). A new species and a new record of Diatrypaceae from Iran. Mycosphere 6, 60–68. doi: 10.5943/mycosphere/6/1/7
Mehrabi, M., Hemmati, R., Vasilyeva, L. N., and Trouillas, F. P. (2016). Diatrypella macrospora sp. nov. and new records of diatrypaceous fungi from Iran. Phytotaxa 252, 43–55. doi: 10.11646/phytotaxa.252.1.4
Miller, M. A., Pfeiffer, W., and Schwartz, T. (2010). “Creating the CIPRES Science Gateway for inference of large phylogenetic trees,” in Proceedings of the Gateway Computing Environments Workshop (GCE) (New Orleans, LA), 1–8. doi: 10.1109/GCE.2010.5676129
O'Donnell, K., and Cigelnik, E. (1997). Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7, 103–116. doi: 10.1006/mpev.1996.0376
Paolinelli-Alfonso, M., Serrrano Gomez, C., and Hernandez-Martinez, R. (2015). Occurrence of Eutypella microtheca in grapevine cankers in Mexico. Phytopathol. Mediterr. 54, 86–93. doi: 10.14601/Phytopathol_Mediterr-14998
Peng, M. K., Zhang, B., Qu, Z., Li, Y., and Ma, H. X. (2021). New record genus and a new species of Allodiatrype from China based on morphological and molecular characters. Phytotaxa 500, 275–284. doi: 10.11646/phytotaxa.500.4.3
Peršoh, D., Melcher, M., Graf, K., Fournier, J., Stadler, M., and Rmbold, G. (2009). Molecular and morphological evidence for the delimitation of Xylaria hypoxylon. Mycologia 101, 256–268. doi: 10.3852/08-108
Phookamsak, R., Hyde, K. D., Jeewon, R., Bhat, D. J., Jones, E. B. G., Maharachchikumbura, S. S. N., et al. (2019). Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 95, 1–273. doi: 10.1007/s13225-019-00421-w
Phukhamsakda, C., Nilsson, R. H., Bhunjun, C. S., et al. (2022). The numbers of fungi: contributions from traditional taxonomic studies and challenges of metabarcoding. Fungal Divers. 114, 327–386. doi: 10.1007/s13225-022-00502-3
Rannala, B., and Yang, Z. (1996). Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J. Mol. Evol. 43, 304–311. doi: 10.1007/BF02338839
Rappaz, F. (1987). Taxonomie et nomenclatijre des Diatrypaceae à asques octospores. Mycol. Helv. 2, 285–648.
Rolshausen, P. E., Mahoney, N. E., Molyneux, R. J., and Gubler, W. D. (2006). A reassessment of the species concept in Eutypa lata, the causal agent of Eutypa dieback of grapevine. Phytopathology 96, 369–377. doi: 10.1094/PHYTO-96-0369
Ronquist, F., and Huelsenbeck, J. P. (2003). MrBayes 3: bayesian phylogenetic inference under mixed models. Bioinformatics 19, 1572–1574. doi: 10.1093/bioinformatics/btg180
Samarakoon, M. C., Hyde, K. D., Maharachchikumbura, S. S. N., et al. (2022). Taxonomy, phylogeny, molecular dating and ancestral state reconstruction of Xylariomycetidae (Sordariomycetes). Fungal Divers. 112, 1–88. doi: 10.1007/s13225-021-00495-5
Senanayake, I. C., Maharachchikumbura, S. S. N., Hyde, K. D., Bhat, D. J., Jones, E. B. G., McKenzie, E. H. C., et al. (2015). Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers. 73, 73–144. doi: 10.1007/s13225-015-0340-y
Senwanna, C., Phookamsak, R., Doilom, M., Hyde, K. D., and Cheewangkoon, R. (2017). Novel taxa of Diatrypaceae from Para rubber (Hevea brasiliensis) in northern Thailand; introducing a novel genus Allocryptovalsa. Mycosphere 8, 1835–1855. doi: 10.5943/mycosphere/8/10/9
Shang, Q. J., Hyde, K. D., Jeewon, R., Khan, S., Promputtha, I., and Phookamsak, R. (2018). Morphomolecular characterization of Peroneutypa (Diatrypaceae, Xylariales) with two novel species from Thailand. Phytotaxa 356, 1–18. doi: 10.11646/phytotaxa.356.1.1
Shang, Q. J., Hyde, K. D., Phookamsak, R., Doilom, M., Bhat, D. J., Maharachchikumbura, S. S. N., et al. (2017). Diatrypella tectonae, and Peroneutypa mackenziei, spp. nov. (diatrypaceae) from northern Thailand. Mycol. Progress 16, 463–476. doi: 10.1007/s11557-017-1294-0
Stamatakis, A., and Ott, M. (2008). Efficient computation of the phylogenetic likelihood function on multi-gene alignments and multi-core architectures. Philos. Trans. R. Soc. B Biol. Sci. 363, 3977–3984. doi: 10.1098/rstb.2008.0163
Thambugala, K. M., Daranagama, D. A., Phillips, A. J. L., et al. (2017). Microfungi on Tamarix. Fungal Divers. 82, 239–306. doi: 10.1007/s13225-016-0371-z
Thiyagaraja, V., Senanayake, I. C., Wanasinghe, D. N., Karunarathna, S. C., Worthy, F. R., and To-Anun, C. (2019). Phylogenetic and morphological appraisal of Diatrype lijiangensis sp. nov. (Diatrypaceae, Xylariales) from China. Asian J. Mycol. 2, 198–208. doi: 10.5943/ajom/2/1/10
Tiffany, L. H., and Gilman, J. C. (1965). Iowa ascomycetes 4: Diatrypaceae. Iowa State J. Sci. 40, 121–161.
Trouillas, F. P., and Gubler, W. D. (2004). Identifification and characterization of Eutypa leptoplaca, a new pathogen of grapevine in Northern California. Mycol. Res. 108, 1195–1204. doi: 10.1017/S0953756204000863
Trouillas, F. P., and Gubler, W. D. (2010). Host range, biological variation, and phylogenetic diversity of Eutypa lata in California. Phytopathology 100, 1048–1056. doi: 10.1094/PHYTO-02-10-0040
Trouillas, F. P., Hand, F. P., Inderbitzin, P., and Gubler, W. D. (2015). The genus Cryptosphaeria in the western United States: taxonomy, multilocus phylogeny and a new species, C. multicontinentalis. Mycologia 107, 1304–1313. doi: 10.3852/15-115
Trouillas, F. P., Pitt, W. M., Sosnowski, M. R., Huang, R., Peduto, F., Loschiavo, A., et al. (2011). Taxonomy and DNA phylogeny of Diatrypaceae associated with Vitis vinifera and other woody plants in Australia. Fungal Divers. 49, 203–223. doi: 10.1007/s13225-011-0094-0
Trouillas, F. P., Sosnowski, M. R., and Gubler, W. D. (2010a). Two new species of Diatrypaceae from coastal wattle in Coorong National Park, South Australia. Mycosphere 1, 183–188.
Trouillas, F. P., Urbez-Torres, J. R., and Gubler, W. D. (2010b). Diversity of diatrypaceous fungi associated with grapevine canker diseases in California. Mycologia 102, 319–336. doi: 10.3852/08-185
Úrbez-Torres, J. R., Adams, P., Kamas, J., and Gubler, W. D. (2009). Identification, incidence, and pathogenicity of fungal species associated with grapevine dieback in Texas. Am. J. Enol. Vitic. 60, 497–507. doi: 10.5344/ajev.2009.60.4.497
Úrbez-Torres, J. R., Peduto, F., Striegler, R. K., Urrearomero, K. E., Rupe, J. C., Cartwright, R. D., et al. (2012). Characterization of fungal pathogens associated with grapevine trunk diseases in Arkansas and Missouri. Fungal Divers. 52, 169–189. doi: 10.1007/s13225-011-0110-4
U'ren, J. M., Miadlikowska, J., Zimmerman, N. B., Ltzoni, F., Stajich, J. E., and Arnold, A. E. (2016). Contributions of North American endophytes to the phylogeny, ecology, and taxonomy of Xylariaceae (Sordariomycetes, Ascomycota). Mol. Phylogenet. Evol. 98, 210–232. doi: 10.1016/j.ympev.2016.02.010
Vasilyeva, L. N. (2011). “Ascomycetes: Sordariomycetidae, Dothideomycetidae, Erysiphomycetidae, Rhytismomycetidae,” in Fungi of Ussuri River Valley, eds Y. Li and Z, M. Azbukina (Beijing: Science Press), 1–32.
Vasilyeva, L. N., and Ma, H. (2014). Diatrypaceous fungi in north-eastern China. 1. Cryptosphaeria and Diatrype. Phytotaxa 186, 261–270. doi: 10.11646/phytotaxa.186.5.3
Vasilyeva, L. N., and Stephenson, S. L. (2005). Pyrenomycetes of the Great Smoky Mountains National Park. II. Cryptovalsa Ces. et De Not. and Diatrypaceae (Ces. et De Not.) Nitschke (Diatrypaceae). Fungal Divers. 19, 189–200.
Vasilyeva, L. N., and Stephenson, S. L. (2006). Pyrenomycetes of the Great Smoky Mountains National Park. III. Cryptosphaeria, Eutypa and Eutypella (Diatrypaceae). Fungal Divers. 22, 243–254. doi: 10.1111/j.1439-0507.2006.01249.x
Vasilyeva, L. N., and Stephenson, S. L. (2009). The genus Diatrype (Ascomycota, Diatrypaceae) in Arkansas and Texas (USA). Mycotaxon 107, 307–313. doi: 10.5248/107.307
Vieira, M. L. A., Hughes, A. F. S., Gil, V. B., Vaz, A. B. M., Alves, T. M. A., Zani, C. L., et al. (2011). Diversity and antimicrobial activities of the fungal endophyte community associated with the traditional Brazilian medicinal plant Solanum cernuum Vell. (Solanaceae). Can. J. Microbiol. 58, 54–56. doi: 10.1139/w11-105
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Vu, D., Groenewald, M., de Vries, M., Gehrmann, T., Stielow, B., Eberhardt, U., et al. (2019). Large-scale generation and analyses of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 92, 135–154. doi: 10.1016/j.simyco.2018.05.001
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. Guide Methods Appl. 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wijayawardene, N. N., Hyde, K. D., Al-Ani, L. K. T., Tedersoo, L., Haelewaters, D., Rajeshkumar, K. C., et al. (2020). Outline of fungi and fungus-like taxa. Mycosphere 11, 1060–1456. doi: 10.5943/mycosphere/11/1/8
Yang, Z., Zhang, B., Qu, Z., Song, Z., Pan, X., Zhao, C., et al. (2022). Two new species of Diatrype (Xylariales, Ascomycota) with polysporous asci from China. Diversity 14, 149. doi: 10.3390/d14020149
Keywords: 8 new taxa, phylogeny, saprobe, taxonomy, Xylariales
Citation: Li Q-R, Long S-H, Lin Y, Wu Y-P, Wu Q-Z, Hu H-M, Shen X-C, Zhang X, Wijayawardene NN, Kang J-C, Kumla J and Kang Y-Q (2023) Diversity, morphology, and molecular phylogeny of Diatrypaceae from southern China. Front. Microbiol. 14:1140190. doi: 10.3389/fmicb.2023.1140190
Received: 08 January 2023; Accepted: 07 March 2023;
Published: 06 April 2023.
Edited by:
Francesco Dal Grande, University of Padua, ItalyReviewed by:
Fuming Shi, Hebei University, ChinaMark Seasat Calabon, University of the Philippines Visayas, Philippines
Copyright © 2023 Li, Long, Lin, Wu, Wu, Hu, Shen, Zhang, Wijayawardene, Kang, Kumla and Kang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ying-Qian Kang, am95Y2VrYW5ndG9reW9AZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Qi-Rui Li
Qi-Rui Li Si-Han Long
Si-Han Long Yan Lin2
Yan Lin2 Nalin Nilusha Wijayawardene
Nalin Nilusha Wijayawardene Ji-Chuan Kang
Ji-Chuan Kang Jaturong Kumla
Jaturong Kumla