
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 21 March 2023
Sec. Evolutionary and Genomic Microbiology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1140127
This article is part of the Research Topic Insights on Fungal Diversity of Ascomycetes and Basidiomycetes: Taxonomy and Interaction with Their Host View all 24 articles
Heterophyllidiae, one of the main subgenus of Russula (Russulaceae, Russulales), is both ecologically and economically important. Although many studies have focused on subgenus Heterophyllidiae in China, the diversity, taxonomy, and molecular phylogeny still remained incompletely understood. In the present study, two new species, R. discoidea and R. niveopicta, and two known taxa, R. xanthovirens and R. subatropurpurea, were described based on morphology and molecular phylogenetic analyses of ITS and 28S DNA sequences with new collections of subgenus Heterophyllidiae from southern China. Both morphological and phylogenetic analyses consistently confirmed that R. niveopicta and R. xanthovirens belong to the subsect. Virescentinae, R. discoidea and R. subatropurpurea come under subsect. Heterophyllae, and R. prasina is synonymized with R. xanthovirens.
The genus Russula Pers. was established by Persoon (1796). Recently, the genus has been divided into eight subgenera: Archaeae Buyck and V. Hofst., Brevipedum Buyck and V. Hofst., Compactae (Fr.) Bon, Crassotunicatae Buyck and V. Hofst., Glutinosae Buyck and X. H. Wang, Heterophyllidiae Romagn., Malodorae Buyck and V. Hofst., and Russula Pers. (Buyck et al., 2018, 2020). Among them, subg. Heterophyllidiae is characterized by medium to large basidiomata, adnate lamellae, rare or no lamellulae, a mild to strongly acrid taste, white or cream spore prints, an inamyloid or partly amyloid suprahilar spot on the spores, absence of primordial hyphae, a suprapellis comprising mainly inflated hyphal extremities, and mycorrhizal properties (Knudsen and Borgen, 1982; Romagnesi, 1987; Buyck et al., 1996, 2018), which has received much attention. The subgenus includes six sections: Aureotactineae R. Heim, Heterophyllae Fr., Ilicinae Romagn., Indolentinae Melzer and Zvára, Ingratae Quel., and Virescentinae (Singer) Sarnari, and two subsections: Cyanoxanthinae Singer and Substriatinae X. H. Wang and Buyck (Persoon, 1796; Buyck et al., 2018, 2020).
In previous studies, about 161 species within subg. Heterophyllidiae were revealed around the world (Ying, 1983; Li et al., 2013, 2015, 2018, 2019, 2021; Chen et al., 2014, 2019, 2021a,b,c,d; Zhao et al., 2015; Zhang et al., 2017; Li and Deng, 2018; Song et al., 2018a,b, 2020; Wang et al., 2019; Yuan et al., 2019; Ghosh et al., 2020; Wisitrassameewong et al., 2020, 2022; Vera et al., 2021; Altaf et al., 2022; Han et al., 2022; Song, 2022). Moreover, the edibility and poisonousness of the subgenus have also been noted, e.g., edible species, R. maguanensis J. Wang, X. H. Wang, Buyck and T. Bau, R. substriata J. Wang, X. H. Wang, Buyck and T. Bau, R. vesca Fr., and R. viridirubrolimbata J. Z. Ying; and poisonous mushroom R. senecis S. Imai (Mao, 2006; Chen et al., 2014; Tolgor et al., 2014; Wang, 2019; Wu et al., 2019).
In China, 38 species of subg. Heterophyllidiae have also been described/reported, which greatly enriched the species diversity of this subgenus (Ying, 1983; Chou and Wang, 2005; Li et al., 2013, 2015, 2018, 2019, 2021; Chen et al., 2014, 2019, 2021a,b,c,d; Zhao et al., 2015; Zhang et al., 2017; Li and Deng, 2018; Song et al., 2018a,b, 2020; Wang et al., 2019; Yuan et al., 2019; Han et al., 2022; Song, 2022). Even so, the diversity and taxonomy still remained incompletely understood in the country. In the present study, with new collections of subg. Heterophyllidiae made from southern China, two new species were described, and the information of two known taxa was updated based on the morphological and molecular phylogenetic analyses, aiming to contribute to the knowledge of this subgenus.
Specimens were photographed under daylight in the field, and their macroscopic characteristics were measured and recorded based on fresh basidiomata. Specimens were dried at 50°C–60°C and then deposited in the Fungal Herbarium of Hainan Medical University (FHMU) (Index Herbariorum), Haikou City, Hainan Province, China. Color codes follow Kornerup and Wanscher (1981). The description templates and terminology of the micromorphological characters referred to Adamčík et al. (2019). The pileipellis section taken from the pileus between the center and margin, and the stipitipellis from the middle part along the longitudinal axis of the stipe were also observed (Zeng et al., 2013). Estimates of spore ornamentation density from scanning electron microscopy pictures follow Adamčík and Marhold (2000). The hymenial cystidia density estimates refer to Buyck (1991). The pileipellis ortho- or metachromatic reactions were examined in Cresyl Blue after Buyck (1989). Sulfovanillin (SV) was used to observe color changes in cystidia contents (Caboň et al., 2017). Observations and measurements of microscopic features were made in 1% Congo Red, 5% potassium hydroxide (KOH), or Melzer’s reagent. The size of the basidiospore was measured with the exclusion of ornamentation and apiculus. The basidiospores were examined using a TM4000Plus or Zeiss Sigma 300 scanning electron microscope (SEM). All the microscopic structures were drawn by free hand. The number of measured basidiospores is given as n/m/p, where “n” represents the total number of basidiospores measured from “m” basidiomata of “p” collections. Dimensions of basidiospores are presented as (a–)b–e–c(−d), where the range “b–c” represents a minimum of 90% of the measured values (5th to 95th percentile), and extreme values (a and d), whenever present (a < 5th percentile, d > 95th percentile), are in parentheses, and “e” refers to the average length/width of basidiospores. “Q” refers to the length/width ratio of basidiospores; “Qm” refers to the average “Q” of basidiospores and is given with standard deviation.
Total genomic DNA was extracted from collections dried with silica gel using the Plant Genomic DNA Kit (CWBIO, Beijing, China) according to the manufacturer’s instructions. Primer pairs used for amplification were as follows: nuc 28S rDNA D1-D2 domains (28S) with LR0R/LR5 (Vilgalys and Hester, 1990; James et al., 2006), nuc rDNA region encompassing the internal transcribed spacers 1 and 2, along with the 5.8S rDNA (ITS) with ITS5/ITS4 (White et al., 1990), and EF1-F/EF1-R (Mikheyev et al., 2006) were used for the translation elongation factor 1-α gene (TEF1). PCR reactions were performed for 4 min of initial denaturation at 95°C, followed by 35 cycles of denaturation at 94°C for 30 s, annealing at the appropriate temperature (52°C for 28S and ITS; 53°C for TEF1) for 30 s, extension at 72°C for 120 s, and a final extension at 72°C for 7 min. Amplified PCR products were purified using the DNA Purification Kit (TIANGEN, Beijing, China) according to the manufacturer’s instructions and then directly sequenced using a BigDye terminator v3.1 kit and an ABI 3730xl DNA Analyzer (Guangzhou Branch of BGI, China) with the same primers used for PCR amplification. DNA sequences were compiled with BioEdit v7.0.9 (Hall, 1999) and then deposited in GenBank (Table 1).
A total of 28 DNA sequences (10 28S, 10 ITS, and 8 TEF1) from 12 collections were newly generated. Edited sequences were deposited in GenBank; the GenBank accession numbers of 28S and ITS are listed in Table 1, and eight TEF1s are presented here [N.K. Zeng3025 (FHMU1986): OP830898; N.K. Zeng3041 (FHMU2002): OP830899; N.K. Zeng4898 (FHMU4841): OP830900; N.K. Zeng4910 (FHMU4854): OP830901; N.K. Zeng5034 (FHMU4812): OP830902; N.K. Zeng4764 (FHMU5454): OP830903; N.K. Zeng4895 (FHMU4847): OP830904; and N.K. Zeng4968 (FHMU5535): OP830905]. For the concatenated dataset, 28S and ITS sequences from new collections were aligned with sequences from related taxa of subg. Heterophyllidiae (Table 1). Russula maguanensis and R. substriata were chosen as out-group referred from Chen et al. (2021a,b). Sequences were aligned using MUSCLE (Edgar, 2004) separately to test for phylogenetic conflict. Then, the sequences of the two genes were concatenated using Phyutility v2.2 for further analyses (Smith and Dunn, 2008).
Maximum likelihood (ML) and Bayesian inference (BI) were employed for phylogenetic analysis. ML analysis was conducted with the program RAxML 7.2.6 (Stamatakis, 2006) running 1,000 replicates combined with an ML search. Bayesian analysis with MrBayes 3.1 (Huelsenbeck and Ronquist, 2005) implementing the Markov Chain Monte Carlo (MCMC) technique and parameters predetermined with MrModeltes 2.3 (Nylander, 2004) was performed. The best-fit likelihood models for 28S and ITS were GTR + I + G and GTR + I + G, respectively. Bayesian analysis was repeated for 3.5 million generations and sampled every 100. Trees sampled from the first 25% of the generations were discarded as burn-in, and Bayesian posterior probabilities (PP) were then calculated for a majority consensus tree of the retained Bayesian trees. At the end of the run, the average deviation of split frequencies was 0.008640.
The two-locus dataset (28S + ITS) consisted of 107 taxa and 1,601 nucleotide sites, and the alignment was submitted to TreeBASE (S30038). The topologies generated from ML and BI analyses were identical, though statistical support for some branches showed slight differences. The ML phylogram with branch lengths inferred from the 28S and ITS dataset is shown in Figure 1.
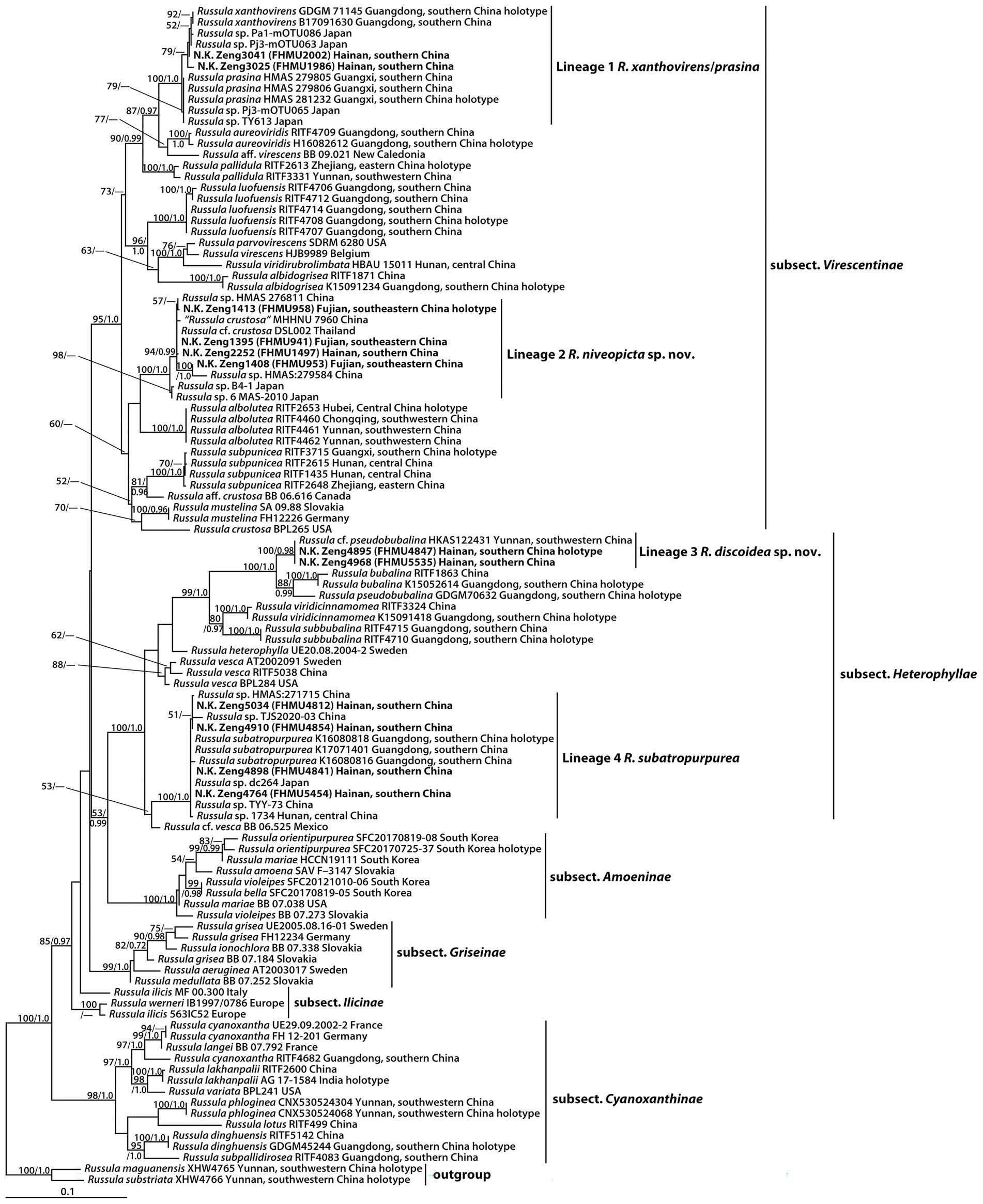
Figure 1. Phylogram of Russula subg. Heterophyllidiae inferred from a two-locus (rDNA 28S and ITS) dataset using RAxML. BS ≥ 50% and PP ≥ 0.95 are indicated above or below the branches as RAxML BS/PP.
The phylogeny indicated that our new collections of subg. Heterophyllidiae were grouped into four independent lineages (1–4) (Figure 1). Lineage 1, with strong statistical support (BS = 100%, PP = 1.0), included the holotype (GDGM 71145) of R. xanthovirens Y. Song and L.H. Qiu, the holotype (HMAS 281232) of R. prasina G.J. Li and R.L. Zhao, one specimen (B17091630) identified as R. xanthovirens, two collections (HMAS 279805 and HMAS 279806) identified as R. prasina, four unidentified Russula collections (Pa1-mOTU086, Pj3-mOTU063, Pj3-mOTU065, and TY613), and two new collections (FHMU1986 and FHMU2002); lineage 2, with high statistical support (BS = 100%, PP = 1.0), was comprised of four new specimens (FHMU958, FHMU941, FHMU1497, and FHMU953), five unidentified Russula collections (HMAS276811, HMAS279584, B4-1, 6 MAS-2010, and DSL002), and one specimen (MHHNU 7960) labeled as R. crustosa Peck; lineage 3, with strong statistical support (BS = 100%, PP = 0.98), included two new collections (FHMU4847 and FHMU5535) and one specimen (HKAS122431) labeled as R. cf. pseudobubalina J.W. Li and L.H. Qiu; lineage 4, with strong statistical support (BS = 100%, PP = 1.0), was comprised of the holotype (K16080818) of R. subatropurpurea J.W. Li and L.H. Qiu, two specimens (K17071401 and K16080816) identified as R. subatropurpurea, five unidentified Russula specimens (HMAS:271715, TJS2020-03, dc264, TYY-73, and 1734), and four new specimens (FHMU4812, FHMU4841, FHMU4854, and FHMU5454) (Figure 1).
Russula discoidea N.K. Zeng, Y.X. Han, and Zhi Q. Liang, sp. nov.
Figures 2A,B, 3A,B, 4, 5.

Figure 2. Basidiomata of Russula subg. Heterophyllidiae species. (A,B) Russula discoidea (FHMU4847, holotype); (C,D) Russula niveopicta (FHMU958, holotype); (E–H) R. subatropurpurea (E,H) FHMU5454; (F) FHMU4812; (G) FHMU4841; (I–L) R. xanthovirens (I,K) FHMU2002; (J,L) FHMU1986; scale bars = 1 cm; photographs: N. K. Zeng.
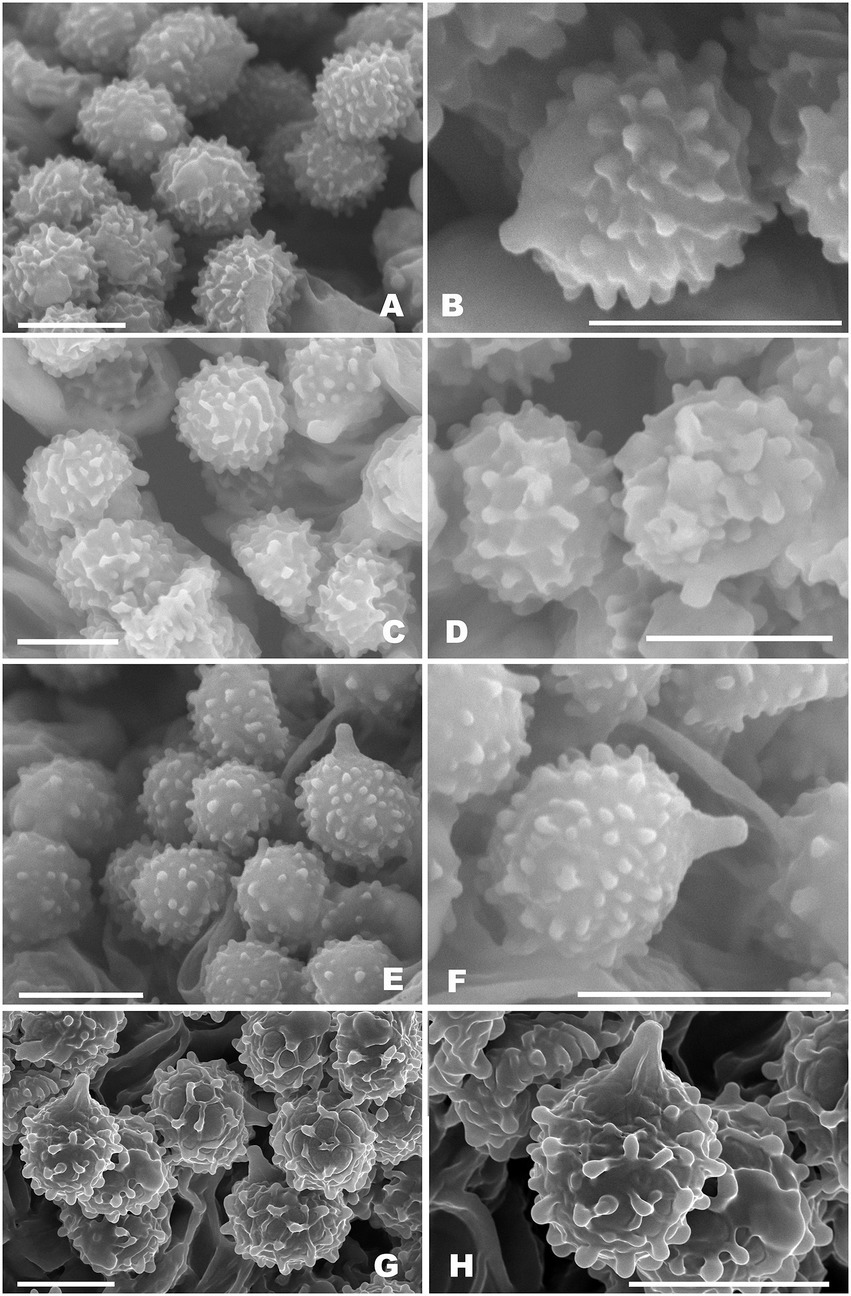
Figure 3. Basidiospores of Russula subg. Heterophyllidiae species from herbarium materials under SEM. (A,B) Russula discoidea (FHMU4847, holotype); (C,D) Russula niveopicta (FHMU958, holotype); (E,F) R. subatropurpurea (FHMU5454); (G,H) R. xanthovirens (FHMU1986); scale bars = 5 μm; photographs: Y. X. Han.
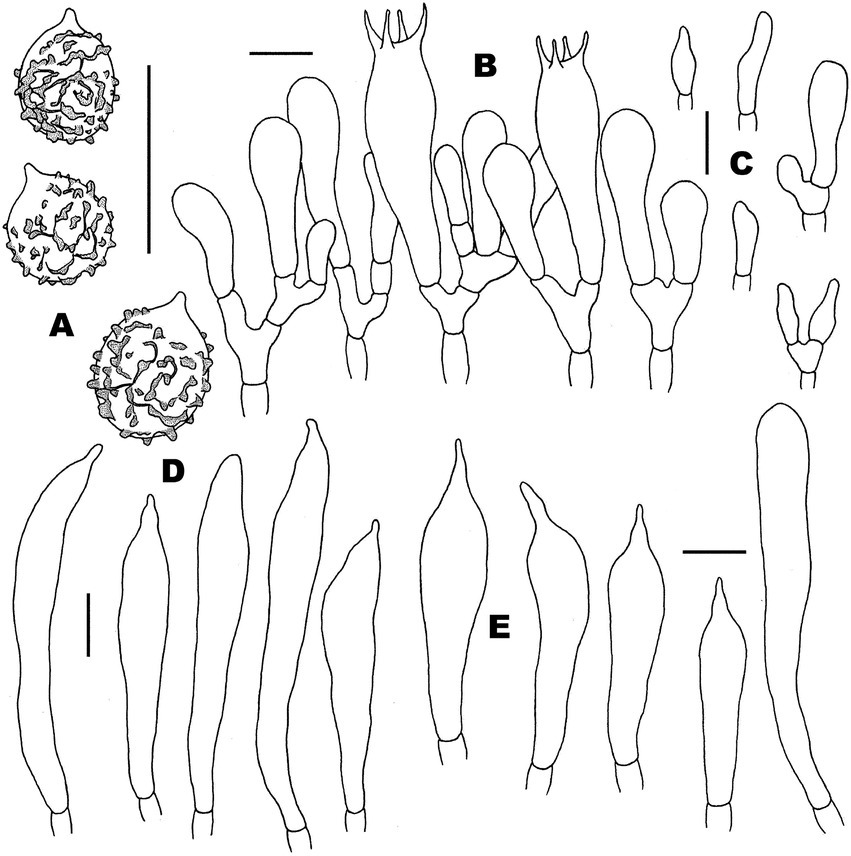
Figure 4. Microscopic features of Russula discoidea (FHMU4847, holotype). (A) Basidiospores. (B) Basidia and basidiola. (C) Marginal cells. (D) Pleurocystidia. (E) Cheilocystidia. Scale bars = 10 μm. Drawings by Y. X. Han.
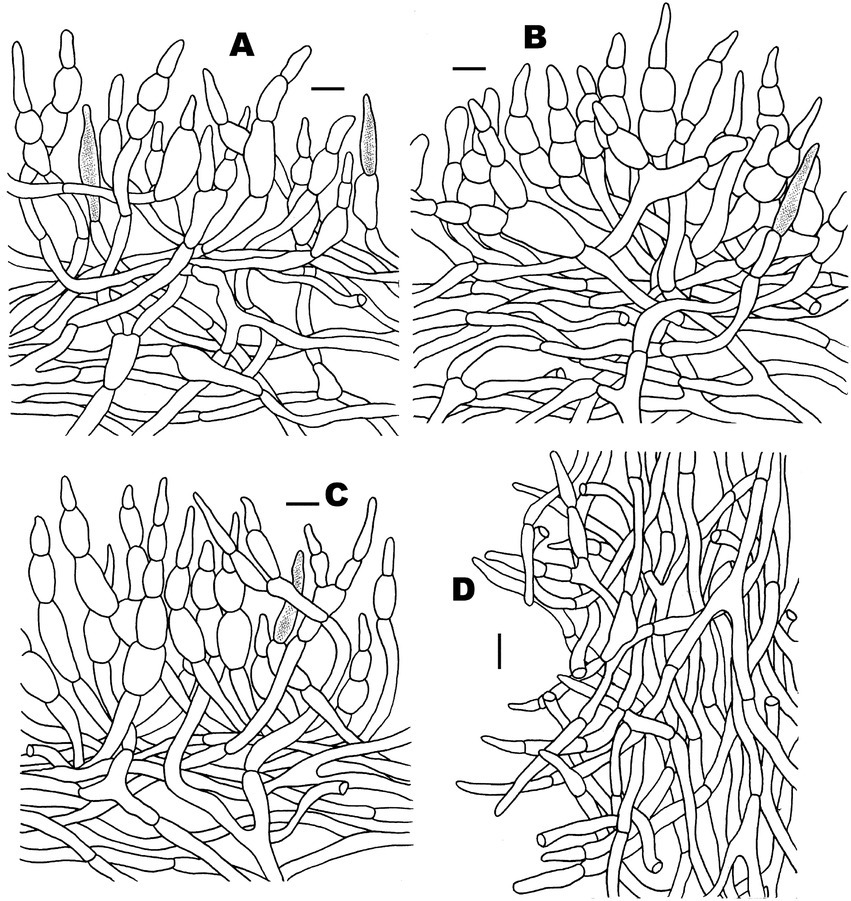
Figure 5. Microscopic features of Russula discoidea (FHMU4847, holotype). (A) Pileipellis at pileus center. (B) Pileipellis at the middle part between the center and margin of the pileus. (C) Pileipellis at pileus margin. (D) Stipitipellis. Scale bars = 10 μm. Drawings by Y. X. Han.
MycoBank: MB846471.
Diagnosis: Differs from closest species of R. subg. Heterophyllidiae by a cinnamon buff pileus, occasionally forked lamellae, basidiospores with small crests and ridges (0.3–0.7 μm high) forming an incomplete reticulum, cystidia slightly becoming yellowish brown in SV, and it is associated with fagaceous trees.
Etymology: Latin “discoidea” refers to the discoid pileus.
Holotype: CHINA. Hainan Province: Wanning County, Bofangling, elev. 80 m, 29 August 2020, N.K. Zeng4895 (FHMU4847).
Basidiomata medium-sized. Pileus 5.6–6.8 cm in diameter, convex to applanate, center slightly depressed, margin occasionally cracked; surface dry, cinnamon buff (7A2), margin with radial tuberculate-striate; context 4.5–7 mm thick at the center of the pileus, white (3A1), unchanging in color when injured. Hymenophore lamellate adnate; lamellae 3.5–4 mm in height, occasionally forked, white (3A1), unchanging in color when injured; lamellulae common, concolorous with lamellae. Stipe 3.6–4.5 × 1.1 cm, central, subcylindric to cylindric; surface dry, white (3A1) to cinnamon buff (7B4). Odor indistinct. Spore print not obtained.
Basidiospores (excluding ornamentation) [40/2/2] 5–6.1–7(−7.5) × 4–5–6(−6.5) μm, Q = 1.0–1.5(−1.75), Qm = 1.21 ± 0.15, globose to ellipsoid, ornamentation composed of relatively small, dense (8–10 in a 3 μm diameter circle), amyloid, subcylindrical warts, 0.3–0.7 μm high, isolated or rarely fused (0–3 fusions in the circle), small crests and ridges forming an incomplete reticulum, connected by occasional line connections (1–3 in the circle); suprahilar spot inamyloid. Basidia 26.5–35–38.5 × 9–10.5–11 μm, hyaline in KOH, thin- to slightly thick-walled (0.4–0.5 μm), clavate to subcylindrical, four-spored; sterigmata 4–6 μm, slightly tortuous, sometimes straight; basidiola cylindric, then narrowly clavate, ca. 4–8.5 μm wide. Pleurocystidia numerous, ca. 1,800/mm2, 46.5–57–66.5 × 5.5–7–9(−10.5) μm, narrowly clavate to subcylindrical, apex often obtuse or acute, sometimes moniliform, occasionally with 2–6 μm long appendage, thin- to slightly thick-walled (0.4–0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Cheilocystidia 36–41–57(−63.5) × 7.5–9–10.5 μm, fusiform to subcylindrical, apex obtuse or mucronate, sometimes with 5–9 μm long appendage, slightly thick-walled (up to 0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Lamellae edges fertile. Marginal cells (11–)12–15.5–19 × (3.5–)4–5–6.5 μm, clavate or subcylindrical, usually shorter than basidioles, thin- to slightly thick-walled (up to 0.4 μm). Lamellar trama mainly composed of spherocytes measuring up to 38 μm in diameter, hyaline in KOH, slightly thick-walled (up to 1 μm). Pileipellis orthochromatic in Cresyl Blue, sharply delimited from the underlying context, 100–180 μm thick, two-layered, weakly gelatinized; composed of suprapellis (75–100 μm thick) and subpellis (30–80 μm thick). Suprapellis composed of erect to suberect hyphae 4–11 μm in diameter, thin-walled (up to 0.4 μm). Subpellis composed of horizontally oriented, 3–10 μm wide intricate hyphae. Acid-resistant incrustations absent. Hyphal terminations near the pileus margin sometimes branched, not flexuous, thin-walled (up to 0.4 μm); terminal cells 10–17.5–22 × 3.5–4–4.5 μm, narrowly subcylindrical or tapering upward; subterminal cells often subcylindrical to slightly inflated, occasionally branched. Hyphal terminations on the middle part between the center and margin of pileus sometimes branched and not flexuous; terminal cells 10–16.5–25(−40) × (3.5–)4.5–6–7 μm, attenuate subcylindrical; subterminal cells often subcylindrial to slightly inflated, occasionally branched. Hyphal terminations near the pileus center sometimes branched and not flexuous; terminal cells (8–)11.5–17–21 × 3.5–4–5.5(−6) μm, narrowly subcylindrical or tapering upward; subterminal cells often subcylindrial to slightly inflated, occasionally branched. Pileal trama composed of hyphae up to 30 μm in diameter, slightly thick-walled (up to 1 μm), hyaline in KOH. Pileocystidia near the pileus margin one-celled, 25–27.5–31 × 6–7–7.5 μm, cylindrical to clavate, apex usually obtuse, contents granulose, yellow in Congo Red slightly becoming yellowish brown in SV. Pileocystidia near the pileus center cylindrical to clavate, one-celled, 25–29–34.5 × 5–5.5–6 μm, contents granulose, yellow in Congo Red slightly becoming yellowish brown in SV. Cystidioid hyphae in subpellis and context, contents granulose. Stipitipellis a cutis, composed of hyphae thin- to slightly thick-walled (up to 0.4 μm), 3–7 μm wide, hyaline in KOH; terminal cells 9–38 × 3.5–5.5 μm, subcylindrical, or subclavate. Stipe trama mainly composed of spherocytes measuring up to 32 μm in diameter, hyaline in KOH, thick-walled (1–1.5 μm). Clamp connections are absent in all tissues.
Habitat: Solitary on the ground in forests dominated by fagaceous trees.
Known distribution: Southern China (Hainan Province).
Additional specimen examined: CHINA. Hainan Province: Changjiang County, Bawangling National Nature Reserve, elev. 650 m, 3 September 2020, N.K. Zeng4968 (FHMU5535).
Notes: Phylogenetically, our new species R. discoidea is closely related to R. bubalina J.W. Li and L.H. Qiu and R. pseudobubalina J.W. Li and L.H. Qiu (Figure 1). However, R. bubalina, originally described in Guangdong Province of southern China, has a smaller basidioma (pileus 3.5–5.4 cm in diameter), basidiospores with ornamentations composed of subcylindrical warts and not forming reticulum (Li et al., 2019); R. pseudobubalina, also described from Guangdong Province of southern China, has a smaller basidioma (pileus 3.1–4.6 cm in diameter), an absence of forked lamellae, basidiospores with ornamentations composed of subcylindrical warts, not forming a reticulum, and uninflated subterminal cells in the pileipellis (Li et al., 2019). Moreover, sequence comparison of the newly generated ITS sequences via BLAST showed that the new species R. discoidea was most closely related to a collection labeled as R. cf. pseudobubalina (HKAS122431) (99.04% similarity) from China, a specimen also labeled as R. cf. pseudobubalina (DSL001) (96.41%) from Thailand, a collection labeled as R. sp. (YM25) (95.48%) from Japan, a material labeled as R. sp. (YM220) (95.20%) from Japan, and a collection labeled as R. sp. (YM4589) (95.20%) from Japan.
Morphologically, R. discoidea may be confused with R. subbubalina B. Chen and J.F. Liang, a recently described species from Guangdong Province of southern China. However, R. subbubalina has a larger basidioma (pileus 5–10 cm in diameter), a dark salmon pileus with rusty spots when young and pruina in some parts, the striation on pileus is inconspicuous, pleurocystidia, cheilocystidia, and pileocystidia near the pileus margin turning reddish black in SV, and pileocystidia near the pileus center turning reddish in SV (Chen et al., 2021a).
Russula niveopicta N.K. Zeng, Y.X. Han and Zhi Q. Liang, sp. nov.
Figures 2C,D, 3C,D, 6, 7.
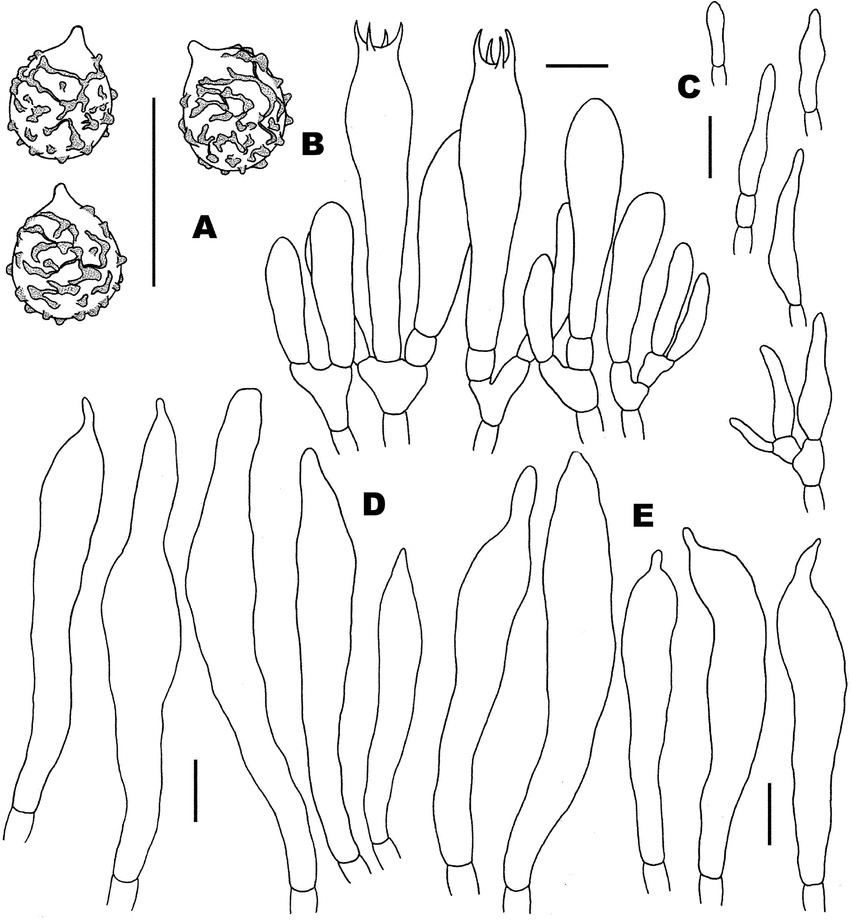
Figure 6. Microscopic features of Russula niveopicta (FHMU958, holotype). (A) Basidiospores. (B) Basidia and basidiola. (C) Marginal cells. (D) Pleurocystidia. (E) Cheilocystidia. Scale bars = 10 μm. Drawings by Y. X. Han.
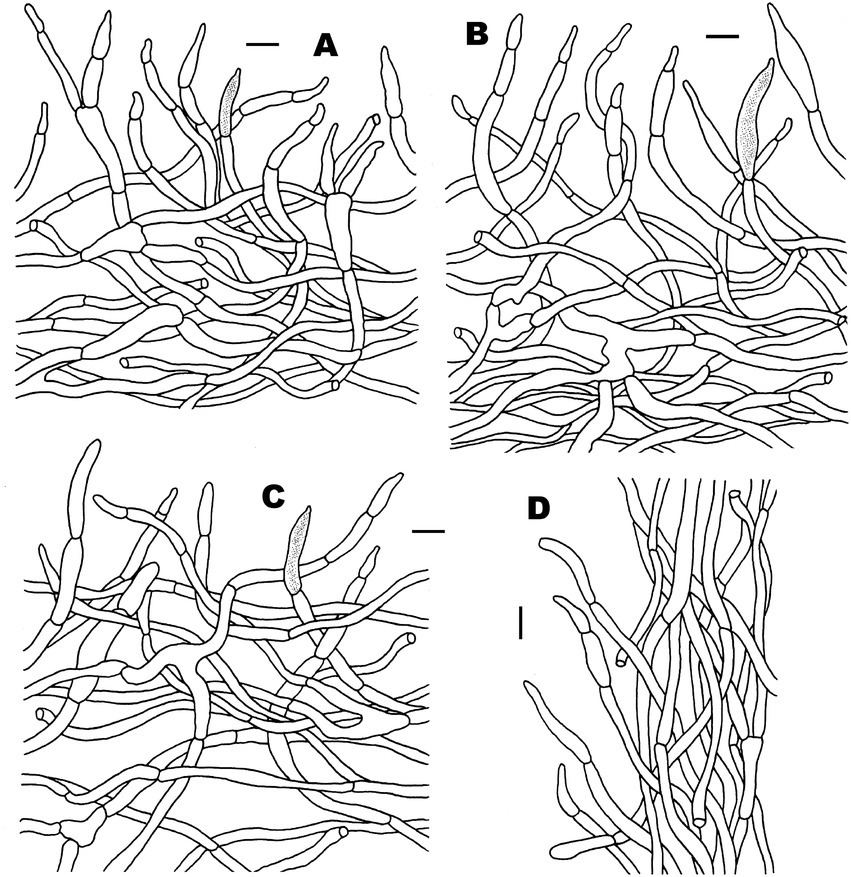
Figure 7. Microscopic features of Russula niveopicta (FHMU958, holotype). (A) Pileipellis at pileus center. (B) Pileipellis at the middle part between the center and margin of the pileus. (C) Pileipellis at pileus margin. (D) Stipitipellis. Scale bars = 10 μm. Drawings by Y. X. Han.
MycoBank: MB846472.
Diagnosis: Differs from closest species of R. subg. Heterophyllidiae by a white pileus with white tuberculate-striate margin, forked lamellae, a white stipe, basidiospores with small crests and ridges (0.4–0.7 μm) forming an incomplete reticulum, cystidia slightly becoming yellowish brown in SV, and it is associated with fagaceous trees.
Etymology: Latin “niveopicta” refers to the pileus with the white tuberculate-striate margin.
Holotype: CHINA. Fujian Province: Zhangping County, Xinqiao Town, Chengkou Village, elev. 350 m, 13 August 2013, N.K. Zeng1413 (FHMU958).
Basidiomata small- to medium-sized. Pileus 3.5–5.5 cm diameter, convex to applanate, center slightly depressed, margin occasionally cracked; surface dry, white (2A1), margin with white radial tuberculate-striate; context 3–5 mm thick at the center of the pileus, white (3A1), unchanging in color when injured. Hymenophore lamellate, adnate; lamellae 2–5 mm in height, occasionally forked, white (3A1), unchanging in color when injured; occasionally with lamellulae, concolorous with lamellae. Stipe 3–4.5 × 0.8–1.3 cm, central, subcylindric to cylindric, hollow; surface white (3A1), with finely longitudinally white veins. Odor indistinct. Spore print not obtained.
Basidiospores (excluding ornamentation) [100/5/4] 5–6.2–7(−8) × 4.5–5.3–6(−6.5) μm, Q = 1–1.3(−1.4), Qm = 1.16 ± 0.10, globose to broadly ellipsoid, ornamentation composed of relatively small, moderately distant to dense (6–8 in a 3 μm diameter circle) amyloid, subcylindrical warts, 0.4–0.7 μm high, isolated or rarely fused (0–2 fusions in the circle), small crests and ridges forming an incomplete reticulum, connected by occasional line connections [(0–)1–3 in the circle]; suprahilar spot inamyloid. Basidia (38–)40–49.5–53 × 9–10.5–11.5(−12) μm, hyaline in KOH, slightly thick-walled (0.5 μm), clavate, four-spored; sterigmata 4–5 μm, slightly tortuous, sometimes straight; basidiola cylindric, then narrowly clavate, ca. 4.5–11 μm wide. Pleurocystidia numerous, ca. 2,600/mm2, (45.5–)66–73.5–81 × 7–10–11.5(−12.5) μm, clavate to subcylindrical, apex often mucronate, sometimes moniliform, occasionally with 2–5 μm long appendage, slightly thick-walled (up to 0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Cheilocystidia 46–55.5–65(−69.5) × 7.5–9–10.5 μm, clavate to subcylindrical, apex obtuse or mucronate, sometimes with 3–9 μm long appendage, slightly thick-walled (up to 0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Lamellae edges fertile. Marginal cells (10–)16.5–20–25 × 4–4.5–5 μm, clavate or subcylindrical, usually shorter than basidioles, thin-walled (up to 0.4 μm). Lamellar trama mainly composed of spherocytes measuring up to 38 μm in diameter, hyaline in KOH, slightly thick-walled (up to 1 μm). Pileipellis orthochromatic in Cresyl Blue, sharply delimited from the underlying context, 190–270 μm thick, two-layered, weakly gelatinized; composed of suprapellis (70–100 μm thick) and subpellis (125–180 μm thick). Suprapellis composed of erect to suberect hyphae 3–8 μm in diameter, slightly thick-walled (up to 0.5 μm). Subpellis composed of horizontally oriented, 3.5–9 μm wide intricate hyphae. Acid-resistant incrustations absent. Hyphal terminations near the pileus margin not flexuous, slightly thick-walled (up to 0.5 μm); terminal cells (12–)15–20.5–31 × 3.5–4–5 μm, narrowly subcylindrical; subterminal cells often wider, unbranched. Hyphal terminations on the middle part between the center and margin of pileus unbranched and not flexuous; terminal cells 16–22.5–27.5(−32) × (3–)3.5–4–5.5 μm, subcylindrical; subterminal cells often wider, unbranched. Hyphal terminations near the pileus center branched and not flexuous; terminal cells (8–)15–17.5–21(−22) × 4–5–5.5 μm, mainly clavate, occasionally subcylindrical; subterminal cells subcylindrical, sometimes branched. Pileal trama composed of hyphae up to 38 μm in diameter, slightly thick-walled (up to 1 μm), hyaline in KOH. Pileocystidia near the pileus margin one-celled, 28–35.5–42 × 4.5–5–5.5 μm, cylindrical to clavate, apex usually mucronate, contents granulose, yellow in Congo Red, slightly becoming yellowish brown in SV. Pileocystidia near the pileus center cylindrical to clavate, one-celled, 21–38.5–47 × 5–6–6.5(−7) μm, contents granulose, yellow in Congo Red, slightly becoming yellowish brown in SV. Cystidioid hyphae in subpellis and context, contents granulose. Stipitipellis a cutis, composed of hyphae thin-walled (up to 0.4 μm), 3–8 μm wide, hyaline in KOH; terminal cells 16–32 × 3.5–5.5 μm, subcylindrical or subclavate. Stipe trama mainly composed of spherocytes measuring up to 40.5 μm in diameter, hyaline in KOH, slightly thick-walled (up to 1 μm). Clamp connections are absent in all tissues.
Habitat: Gregarious or solitary on the ground in forests dominated by trees of Castanopsis (D. Don) Spach.
Known distribution: Southern and southeastern China (Hainan and Fujian Provinces).
Additional specimens examined: CHINA. Fujian Province: Zhangping County, Xinqiao Town, Chengkou Village, elev. 350 m, 9 August 2013, N.K. Zeng1395 (FHMU941); same location, 13 August 2013, N.K. Zeng1408 (FHMU953); Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 700 m, 30 July 2015, N.K. Zeng2252 (FHMU1497).
Notes: In China, our new species R. niveopicta was misidentified as R. crustosa (Figure 1), originally described in North America. However, R. crustosa has a yellowish brown pileus with defined patches, basidiospores with warty ornamentations, not forming a reticulum (Peck, 1886).
Morphologically, R. niveopicta may be confused with four species: R. albidogrisea J.W. Li and L.H. Qiu, R. alboareolata Hongo, R. albolutea B. Chen and J.F. Liang, and R. pallidula Bin Chen and J. F. Liang. However, the Chinese species R. albidogrisea, originally described in Guangdong Province of southern China, has basidiospores with lower ornamentations composed of conical to hemispherical wart (up to 0.4 μm high), forming an almost complete reticulum, and pleurocystidia, cheilocystidia, and pileocystidia unchanged in SV (Das et al., 2017). Russula alboareolata, originally described from Japan, has equal lamellae, inflated subterminal cells, and basidiospores with ornamentations tend to be almost a complete reticulum (Hongo, 1979); moreover, the molecular phylogeny based on the 28S dataset indicated that R. niveopicta is genetically distant from two collections of R. alboareolata from Japan (data not shown). Russula albolutea, originally described from the Hubei Province of central China, possesses a larger basidioma (pileus 5–7.5 cm in diameter), pleurocystidia, and cheilocystidia turning mauve in SV, and pileocystidia turning reddish in SV (Chen et al., 2021b). Russula pallidula, originally described from Zhejiang Province of eastern China, is distinct in its basidiospores with lower ornamentations composed of bluntly conical wart (up to 0.35 μm high), forming a partial reticulum, pleurocystidia dark gray in SV, and inflated subterminal cells in pileipellis (Chen et al., 2019).
Sequence comparison of the newly generated ITS sequences via BLAST showed that the new species R. niveopicta was most closely related to a collection labeled as R. sp. (HMAS:279584) (99.79%) from China, a specimen labeled as R. sp. (HMAS 276811) (99.68%) from China, a material misidentified as R. crustosa (MHHNU 7960) (99.38%) from China, a collection labeled as R. cf. crustosa (DSL002) (99.38%) from Thailand, and a specimen labeled as R. sp. (MAS-2010) (98.61%) from Japan.
Russula subatropurpurea J.W. Li and L.H. Qiu, Phytotaxa 392 (4): 272, 2019.
Figures 2E–H, 3E,F, 8, 9.
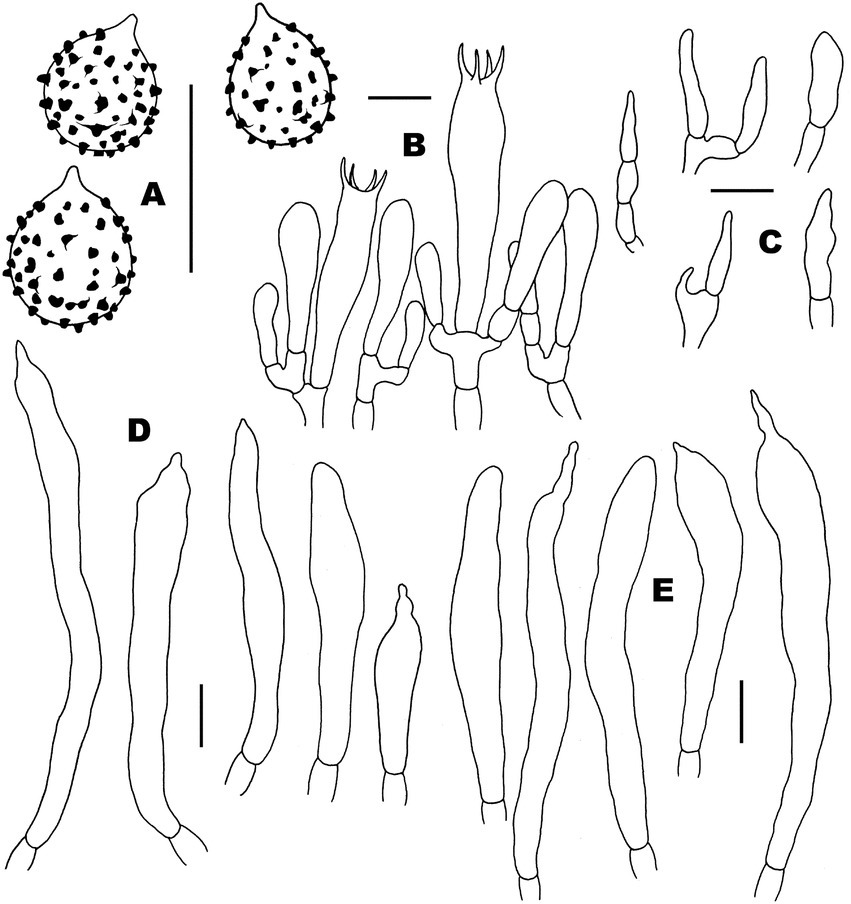
Figure 8. Microscopic features of Russula subatropurpurea (FHMU5454). (A) Basidiospores. (B) Basidia and basidiola. (C) Marginal cells. (D) Pleurocystidia. (E) Cheilocystidia. Scale bars = 10 μm. Drawings by Y. X.Han.
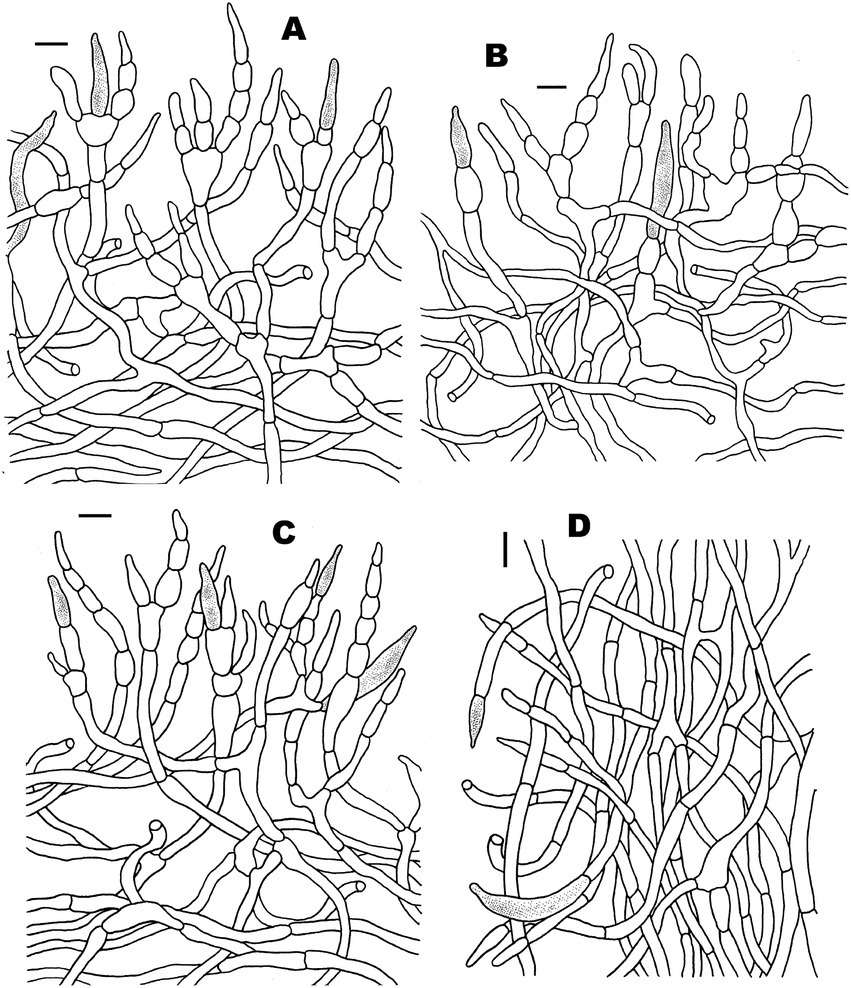
Figure 9. Microscopic features of Russula subatropurpurea (FHMU5454). (A) Pileipellis at pileus center. (B) Pileipellis at the middle part between the center and margin of the pileus. (C) Pileipellis at pileus margin. (D) Stipitipellis. Scale bars = 10 μm. Drawings by Y. X. Han.
Basidiomata small- to medium-sized. Pileus 4–6.5 cm in diameter, hemispherical at first, then applanate, center slightly depressed, margin occasionally cracked; surface dry, purplish brown (8F2), yellowish brown (2B3) to pale yellow (1A3) on pileus center, margin with radial tuberculate-striate; context 4–8 mm thick at the center of the pileus, white (2A1), unchanging in color when injured. Hymenophore lamellate, adnate; lamellae 2.5–5 mm in height, crowded, often forked, white (2A1), unchanging in color when injured; lamellulae absence. Stipe 2.8–5.3 × 0.9–1.5 cm, central, subcylindrical to cylindrical, slightly narrow toward base; surface white (4A1). Odor indistinct. Spore print not obtained.
Basidiospores (excluding ornamentation) [80/7/4] 5–6.1–7(−8) × 4–5.2–6(−6.5) μm, Q = 1–1.4(−1.5), Qm = 1.18 ± 0.11, globose to ellipsoid, ornamentation composed of relatively small, dense [(8–)9–13 in a 3 μm diameter circle], amyloid, subcylindrical warts, 0.3–0.5 μm high, isolated or occasionally fused (0–2 fusions in the circle), without line connections, never forming a reticulum; suprahilar spot inamyloid. Basidia (20–)24.5–28.5–32(−40) × (5–)5.5–7–8.5(−9) μm, hyaline in KOH, thin-walled (up to 0.4 μm), clavate to subcylindrical, four-spored; sterigmata 3–9 μm, slightly tortuous, sometimes straight; basidiola clavate, ca. 4–7 μm wide. Pleurocystidia numerous 2,400/mm2, (30–)48–60–80 × 5.5–7–9 μm, clavate to slender fusiform, most with mucronate to moniliformous, occasionally with 2.5–5 μm long appendage, slightly thick-walled (up to 0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Cheilocystidia 50–69–76 × (5.5–)6–7.5–8 μm, narrowly clavate to slender subcylindrical, apex obtuse or mucronate, sometimes with 3–9 μm long appendage, slightly thick-walled (up to 0.5 μm); contents granulose, yellowish in Congo Red, slightly becoming yellowish brown in SV. Lamellae edges fertile. Marginal cells (11–)11.5–15–17 × 3–4.5–5(−6) μm, clavate or subcylindrical, usually shorter than basidiola, and thin-walled (up to 0.4 μm). Lamellar trama mainly composed of spherocytes measuring up to 31 μm in diameter, hyaline in KOH, slightly thick-walled (up to 1 μm). Pileipellis orthochromatic in Cresyl Blue, sharply delimited from the underlying context, 270–350 μm thick, two-layered, weakly gelatinized; composed of suprapellis (125–170 μm thick) and subpellis (150–200 μm thick). Suprapellis composed of erect to suberect hyphae 2.5–9 μm in diameter, slightly thick-walled (up to 0.4 μm). Subpellis composed of horizontally oriented, 3–8 μm wide intricate hyphae. Acid-resistant incrustations absent. Hyphal terminations near the pileus margin sometimes branched, not flexuous, slightly thick-walled (up to 0.4 μm); terminal cells (7–)8–15–20 × 2.5–3–5 μm, mainly attenuate acicular to subcylindrial; subterminal cells often wider and slightly inflated, and branched. Hyphal terminations on the middle part between the center and margin of pileus less flexuous, sometimes branched, terminal cells (8–)12.5–18–22 × 3.5–4–5.5 μm, mainly clavate, occasionally attenuate, subcylindrical to acicular; subterminal cells often wider and slightly inflated, occasionally branched. Hyphal terminations near the pileus center not flexuous; terminal cells 7–12.5–20 × 4–4.5–5(−5.5) μm, attenuate subcylindrical to acicular; subterminal cells often wider and slightly inflated, sometimes branched. Pileal trama is made up of hyphae up to 41 μm in diameter, slightly thick-walled (up to 1 μm), hyaline to pale yellowish in KOH. Pileocystidia near the pileus margin always one-celled, (16–)17.5–26–37 × 4.5–6–9.5 μm, cylindrical to fusiform, apex occasionally obtuse or usually mucronate, contents yellow in Congo Red, slightly becoming yellowish brown in SV. Pileocystidia near the pileus center narrower cylindrical to clavate, one-celled, 22–34–45 × 4.5–5.5–6 μm, contents granulose, yellow in Congo Red, slightly becoming yellowish brown in SV. Cystidioid hyphae in subpellis and context, contents granulose. Stipitipellis a cutis composed of interwoven hyphae thin-walled (up to 0.4 μm), 3–7 μm wide, hyaline in KOH; terminal cells 10–22 × 3–4.5 μm, subcylindrical or subclavate. Stipe trama mainly composed of spherocytes measuring up to 32 μm in diameter, hyaline to pale yellowish in KOH, slightly thick-walled (up to 1 μm). Clamp connections are absent in all tissues.
Habitat: Gregarious or solitary on the ground in forests dominated by fagaceous trees.
Known distribution: Southern China (Guangdong and Hainan Provinces).
Specimens examined: CHINA. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 650 m, 14 August 2020, N.K. Zeng4764 (FHMU5454); same location, 4 September 2020, N.K. Zeng5034 (FHMU4812); Wanning County, Bofangling, elev. 80 m, 29 August 2020, N.K. Zeng4898 (FHMU4841); same location and date, N.K. Zeng4910 (FHMU4854).
Notes: Russula subatropurpurea was originally described in the Guangdong Province of southern China (Li et al., 2019). In the present study, it was also found to distribute in Hainan Province, tropical China. The species was redescribed according to our new specimens, which is characterized by a purplish brown, yellowish brown to pale yellow pileus, forking lamellae, an absence of lamellulae, basidiospores usually with subcylindrical isolated warts (0.3–0.5 μm), never forming a reticulum, long pleurocystidia and cheilocystidia slightly becoming yellowish brown in SV, and it is associated with fagaceous trees. Moreover, we noted that the pileus color and the striate on the pileus margin were described as “whole pileus purplish brown,” and “absent,” respectively (Li et al., 2019), whereas the pileus of our collections is pale yellow on the center, and the striate on the pileal margin is present.
Russula xanthovirens Y. Song and L.H. Qiu, Cryptogamie, Mycologie 39 (1): 135, 2018.
Figures 2I–L, 3G,H, 10, 11.
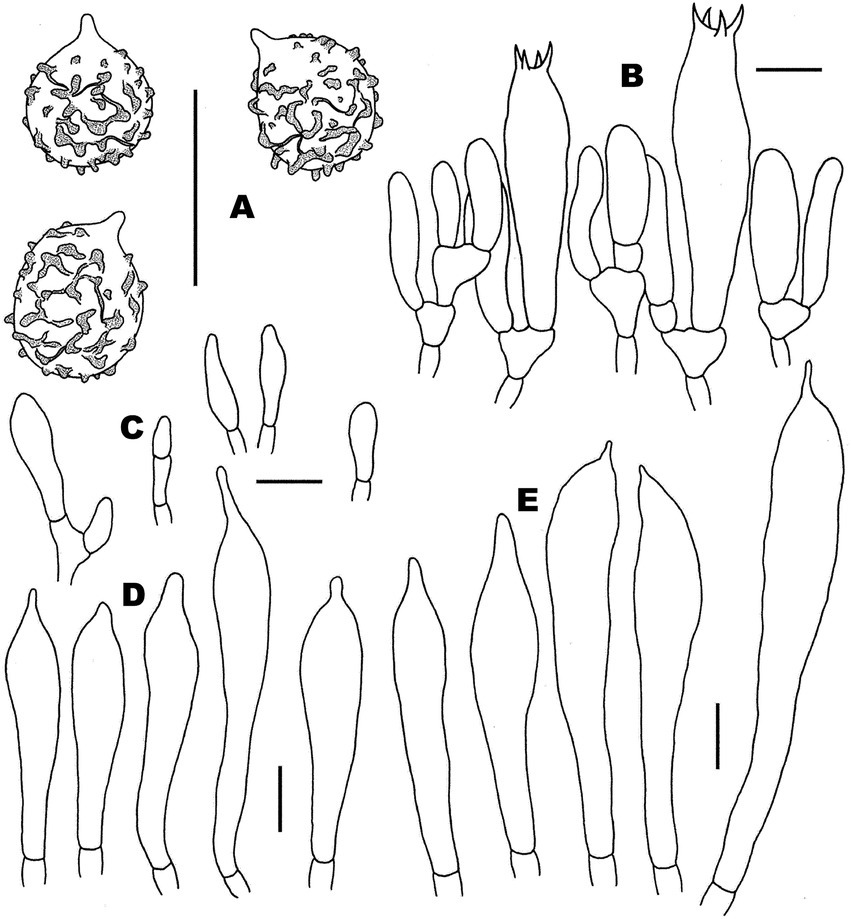
Figure 10. Microscopic features of Russula xanthovirens (FHMU1986). (A) Basidiospores. (B) Basidia and basidiola. (C) Marginal cells. (D) Pleurocystidia. (E) Cheilocystidia. Scale bars = 10 μm. Drawings by Y. X. Han.
Figure 11. Microscopic features of Russula xanthovirens (FHMU1986). (A) Pileipellis at pileus center. (B) Pileipellis at the middle part between the center and margin of the pileus. (C) Pileipellis at pileus margin. (D) Stipitipellis. Scale bars = 10 μm. Drawings by Y. X. Han.
Synonym: Russula prasina G.J. Li and R.L. Zhao, Fungal Diversity 96: 215, 2019.
Basidiomata medium-sized. Pileus 6–7 cm in diameter, hemispherical at first, then applanate, center slightly depressed, cracked with age; surface dry, smooth, pale greenish (27A4) to dark greenish (27C6), with a pale yellowish center (3A3), margin with radial tuberculate-striate; context about 5 mm thick at the center of the pileus, white (2A1), unchanging in color when injured. Hymenophore lamellate, adnate; lamellae about 5 mm in height, crowded, often forked, white (2A1), unchanging in color when injured, lamellulae rare. Stipe 4.5–6.5 × 1–1.7 cm, central, subcylindrical to cylindrical; surface white (4A1), with striae. Odor indistinct. Spore print not obtained.
Basidiospores (excluding ornamentation) [40/2/2] 6–6.5–7 × 5–5.8–6.5 μm, Q = 1–1.3(−1.4), Qm = 1.11 ± 0.11, globose to broadly ellipsoid, ornamentation composed of relatively small, moderately distant to dense [(6–)7–8 in a 3 μm diameter circle] amyloid subcylindrical warts, 0.3–0.8 μm high, isolated or occasionally fused (0–2 fusions in the circle); small crests and ridges forming an incomplete reticulum, connected by occasional line connections [(0–)1–3 in the circle]; suprahilar spot inamyloid. Basidia (35–)39–42.5–45 × 10–10.5–11 μm, clavate to subcylindrical, hyaline in KOH, slightly thick-walled (up to 0.6 μm), clavate, four-spored; sterigmata 3–5 μm, slightly tortuous, sometimes straight; basidiola clavate, ca. 4.5–8 μm wide. Pleurocystidia moderately numerous, 1,100/mm2, (38–)41–52.5–62.5 × 8–8.5–9 μm, subcylindrical to fusoid, apically often obtuse or acute, occasionally with 3–8 μm long appendage, slightly thick-walled (up to 0.4 μm); contents granulose, yellowish in Congo Red, negative in SV. Cheilocystidia (47.5–)50–59–63.5(−88.5) × (8.5–)9.5–10–11.5 μm, clavate to fusoid, apex obtuse or mucronate, sometimes with 3–6 μm long appendage, slightly thick-walled (up to 0.4 μm); contents granulose, yellowish in Congo Red, negative in SV. Lamellae edges fertile. Marginal cells (6–)12–15–20 × 4–4.5–6(−6.5) μm, clavate or subcylindrical, usually shorter than basidiola, slightly thick-walled (up to 0.4 μm). Lamellar trama mainly composed of spherocytes measuring up to 38 μm in diameter, hyaline in KOH, slightly thick-walled (up to 1 μm). Pileipellis orthochromatic in Cresyl Blue, sharply delimited from the underlying context, 190–300 μm thick, two-layered, gelatinized; composed of suprapellis (110–170 μm thick) and subpellis (90–130 μm thick). Suprapellis composed of erect to suberect hyphae 3–10 μm in diameter, thin-walled (up to 0.4 μm). Subpellis composed of horizontally oriented, 2.5–9 μm wide intricate hyphae. Acid-resistant incrustations absent. Hyphal terminations near the pileus margin unbranched, not flexuous, thin-walled (up to 0.4 μm); terminal cells (9–)12–15.5–17 × 3.5–5–7 μm, subcylindrical to subulate; subterminal cells often wider, ellipsoid to globose. Hyphal terminations on the middle part between the center and margin of pileus not flexuous and unbranched, terminal cells (8–)18–21.5–28 × (3–)4–5–5.5 μm, subcylindrical to subulate; subterminal cells often wider, ellipsoid to globose. Hyphal terminations near the pileus center not flexuous; terminal cells 8–10.5–15 × 5–5.5–6.5(−7) μm, subcylindrical, apically obtuse; subterminal cells often wider, ellipsoid to subcylindrical, rarely branched. Pileal trama made up of hyphae up to 34.5 μm in diameter, thick-walled (up to 1 μm), hyaline to pale yellowish in KOH. Pileocystidia near the pileus margin one-celled, (22–)36–54.5–63 × 4–5–5.5 μm, cylindrical to clavate, apex occasionally obtuse or usually mucronate, contents yellow in Congo Red, unchanging in SV. Pileocystidia near the pileus center cylindrical to clavate, one-celled, (25–)30.5–36–40 × 4–4.5–5 μm, contents granulose, yellow in Congo Red, unchanging in SV. Cystidioid hyphae in subpellis and context, contents granulose. Stipitipellis a cutis composed of hyphae slightly thick-walled (up to 0.4 μm), 3–9 μm wide, hyaline in KOH; terminal cells 13–21 × 3.5–5 μm, subcylindrical or subclavate. Stipe trama mainly composed of spherocytes measuring up to 54 μm in diameter, hyaline to pale yellowish in KOH, slightly thick-walled (up to 1 μm). Clamp connections are absent in all tissues.
Habitat: Solitary on the ground in forests dominated by fagaceous trees.
Known distribution: Southern China (Guangdong and Hainan Provinces).
Specimens examined: CHINA. Hainan Province: Yinggeling of Hainan Tropical Rainforest National Park, elev. 650 m, 28 May 2017, N.K. Zeng3025 (FHMU1986); same location, 29 May 2017, N.K. Zeng3041 (FHMU2002).
Notes: Russula xanthovirens was originally described in the Guangdong Province of southern China (Song et al., 2018b); then, it was also reported from the Hainan Province, tropical China (Zeng and Jiang, 2020). The species was redescribed according to our new specimens, which is characterized by a greenish pileus, forking lamellae with rare lamellulae, basidiospores usually with small crests and ridges (0.3–0.8 μm), forming an incomplete reticulum, cystidia negative in SV, a two layers pileipellis, suprapellis with inflated subterminal cells, and it is associated with fagaceous trees.
The phylogenetic analyses showed that the holotype of R. xanthovirens and the holotype of R. prasina were in the same species-level lineage (Figure 1); moreover, there are no essential morphological differences between the two taxa (Song et al., 2018b; Hyde et al., 2019). We, therefore, treat R. prasina as a synonym of R. xanthovirens.
High species diversity of subg. Heterophyllidiae in China was revealed in previous/present studies, and 38 taxa of the subgenus have been described/reported in the country (Table 2). These taxa are members of sect. Ingratae (Quél.) Maire, subsect. Cyanoxanthinae Singer, subsect. Griseinae Jul. Schäff., subsect. Heterophyllae (Fr.) Jul. Schäff., subsect. Substriatinae X.H. Wang and Buyck, and subsect. Virescentinae Singer, respectively (Table 2). The combination of morphological features and phylogenetic analyses indicated that our new species R. niveopicta is a member of the subsect. Virescentinae, whereas R. discoidea belongs to the subsect. Heterophyllae (Figure 1). It is worth noting that R. vesca Fr., originally described in Europe, was reported to be distributed in China (Song, 2022); however, the Chinese collections identified as R. vesca are somewhat distant from European R. vesca in phylogenies (Figure 1; Song, 2022). The occurrence of R. vesca in China should be further defined in the future.
In China, most species of subg. Heterophyllidiae distribute in subtropical and tropical areas, only few taxa, namely R. atroaeruginea G.J. Li, Q. Zhao and H.A. Wen, R. nigrovirens Q. Zhao, Yang K. Li, and J. F. Liang, and R. pseudopectinatoides G. J. Li and H. A. Wen, occur in temperate areas (Li et al., 2013, 2015; Zhao et al., 2015). The geographical distribution pattern indicates that the subtropical–tropical region is the current species diversity center of subg. Heterophyllidiae in China.
Morphological characteristics used to define species of subg. Heterophyllidiae have been extensively discussed in previous studies (Chou and Wang, 2005; Li et al., 2013, 2015, 2018, 2019, 2021; Chen et al., 2014, 2019, 2021a,b,c,d; Zhao et al., 2015; Zhang et al., 2017; Li and Deng, 2018; Song et al., 2018a,b, 2020; Wang et al., 2019; Yuan et al., 2019; Han et al., 2022; Song, 2022). Ecological preference, also a useful feature to delimitate species, receives little attention. In the present study, our two new species R. discoidea and R. niveopicta are both associated with trees of Fagaceae Dumort. In addition to Fagaceae, we also noted that species of subg. Heterophyllidiae are associated with many other trees including Betulaceae Gray, Dipterocarpaceae Blume, Ericaceae Juss., Orchidaceae Juss., Pinaceae Spreng. ex F. Rudolphi, Rosaceae Juss., and Sterculiaceae (Candolle) Bartling (Das et al., 2013; Dutta et al., 2015; Zhao et al., 2015; Crous et al., 2017; Chen et al., 2021b). In China, together with our two new species, the vast majority of species of the subgenus such as R. albolutea, R. clavulus, R. fusiformata, R. lotus, R. luofuensis, R. subbubalina, R. subpunctipes, and R. viridirubrolimbata are associated with trees of Fagaceae (Ying, 1983; Li and Deng, 2018; Song et al., 2020; Chen et al., 2021a,b,d; Song, 2022); a great number of species including R. atroaeruginea, R. indocatillus, R. multilamellula, R. pseudopectinatoides, R. straminella, R. subpectinatoides, and R. succinea are associated with trees of Pinaceae (Li et al., 2013, 2015, 2021; Chen et al., 2021d); R. hainanensis is associated with trees of Dipterocarpaceae (Han et al., 2022); some species, e.g., R. indocatillus A. Ghosh, K. Das, and R. P. Bhatt, can be associated with both trees of Fagaceae and Pinaceae (Ghosh et al., 2020; Li et al., 2021). In addition, we also noted that R. subpunicea was reported to grow under trees of Betulaceae and Fagaceae (Chen et al., 2021b), and R. nigrovirens was found under trees of Ericaceae, Pinaceae, and Rosaceae (Zhao et al., 2015).
Recent phylogenetic studies have provided new insights into the phylogeny and geography of subg. Heterophyllidiae (Song et al., 2018b; Li et al., 2019; Chen et al., 2021a,b). Our phylogeny based on two-locus DNA sequences (28S + ITS) with 12 new specimens from southern China has contributed to new knowledge of subg. Heterophyllidiae. The phylogenetic analyses indicated that there are several clades having taxa from both sides of the Pacific, and allied species from China and North America are obvious (Figure 1). For example, Chinese R. subpunicea is closely related to one collection labeled as R. aff. crustosa from North America; one specimen identified as R. parvovirescens Buyck, D. Mitch., and Parrent from North America is affiliated with one material of R.viridirubrolimbata J.Z. Ying from China (Figure 1). The present study did not identify disjunct populations of the same purported taxon in the two regions (Figure 1). Similar scenarios have been documented for many other macrofungi (Halling, 2001; Zeng et al., 2013, 2016, 2017; Zhang et al., 2022a).
Biogeographic connections between China and Europe have been discussed in other macrofungi such as Phylloporus Quél., Cantharellus Adans. ex Fr., and Craterellus Pers. (Zeng et al., 2013; Wu et al., 2022; Zhang et al., 2022a,b). The geography of subg. Heterophyllidiae between the two regions was also noted, for example, one specimen identified as R. virescens (Schaeff.) Fr. from Europe is closely related to Chinese R. viridirubrolimbata (Figure 1). In addition, one Chinese material labeled as R. cyanoxantha (Schaeff.) Fr. is affiliated with European collections identified as R. cyanoxantha or R. langei Bon (Figure 1). The populations of the same species of subg. Heterophyllidiae between the two regions will be defined in the future.
The affinities of subg. Heterophyllidiae species between China and Southeast/South Asia are evident. For example, R. lakhanpalii A. Ghosh, K. Das, and R.P. Bhatt occurs in both China and India, and our new species R. niveopicta was shared between China and Thailand (Figure 1). Moreover, we also noted that R. xanthovirens and R. subatropurpurea are distributed in both China and Japan (Figure 1).
The recognition of several sections in this subgenus for which already available names include Ingratae, Heterophyllae, and Virescentinae. Probably subsect. Cyanoxanthinae and Substriatinae also merit upgrading (Buyck et al., 2018).
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Z-QL and N-KZ contributed to the conceptualization, wrote, reviewed, and edited the manuscript, and supervised the data. Y-XH performed the methodology, wrote the original draft preparation, and carried out the formal analysis. N-KZ carried out the project administration and funding acquisition. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (No. 32160001), the Natural Science Foundation of Hainan Medical University (No. JBGS202112), and the Hainan Institute of National Park.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adamčík, S., Looney, B., Caboň, M., Jančovičová, S., Adamčíková, K., Avis, P. G., et al. (2019). The quest for a globally comprehensible Russula language. Fungal Divers. 99, 369–449. doi: 10.1007/s13225-019-00437-2
Adamčík, S., and Marhold, K. (2000). Taxonomy of the Russula xerampelina group. I. Morphometric study of the Russula xerampelina group in Slovakia. Mycotaxon 76, 463–480.
Altaf, U., Verma, K., Ghosh, A., Mehmood, T., and Sharma, Y. P. (2022). A new species of genus Russula subsect. Ilicinae (Russulaceae) from Kashmir Himalaya based on morphology and molecular phylogeny. Nord. J. Bot. 2022, 1–8. doi: 10.1111/njb.03452
Bi, Z. S., and Li, T. H. (1986). A preliminary note on Russula species from Guangdong, with a new species and a new variety. Guihaia 6, 193–199.
Buyck, B. (1989). Valeur taxonomique du bleu de cre’syl pour le genre Russula. Bulletin de la Société Mycologique de France 105, 1–6.
Buyck, B. (1991). The study of microscopic features in Russula 2. Sterile cells of the hymenium. Russulales News 1, 62–85.
Buyck, B., Hofstetter, V., Eberhardt, U., Verbeken, A., and Kauff, F. (2008). Walking the thin line between Russula and Lactarius: the dilemma of Russula subsect. Ochricompactae. Fungal Divers. 28, 15–40.
Buyck, B., Thoen, D., and Watling, R. (1996). Ectomycorrhizal fungi of the Guinea-Congo region. P. Roy. Soc. Edinb. A. 104, 313–333. doi: 10.1017/S0269727000006175
Buyck, B., Wang, X. H., Adamčíková, K., Caboň, M., Jančovičová, S., Hofstetter, V., et al. (2020). One step closer to unravelling the origin of Russula: subgenus Glutinosae subg. Nov. Mycosphere 11, 285–304. doi: 10.5943/mycosphere/11/1/6
Buyck, B., Zoller, S., and Hofstetter, V. (2018). Walking the thin line… ten years later: the dilemma of above- versus below-ground features to support phylogenies in the Russulaceae (Basidiomycota). Fungal Divers. 89, 267–292. doi: 10.1007/s13225-018-0397-5
Caboň, M., Eberhardt, U., Looney, B., Hampe, F., Kolařík, M., Jančovičová, S., et al. (2017). New insights in Russula subsect. Rubrinae: phylogeny and the quest for synapomorphic characters. Mycol. Prog. 16, 877–892. doi: 10.1007/s11557-017-1322-0
Chen, B., Jiang, X. M., Song, J., Liang, J. F., Wang, S. K., and Lu, J. K. (2019). Morphological and phylogenetic analyses reveal the new species Russula pallidula from China. Sydowia 71, 1–10. doi: 10.12905/0380.sydowia71-2019-0001
Chen, B., Song, J., Chen, Y. L., Zhang, J. H., and Liang, J. F. (2021a). Morphological and phylogenetic evidence for two new species of Russula subg. Heterophyllidia from Guangdong Province of China. MycoKeys 82, 139–157. doi: 10.3897/mycokeys.82.64913
Chen, B., Song, J., Liang, J. F., and Li, Y. K. (2021b). Two new species of Russula subsect. Virescentinae from southern China. Mycol. Prog. 20, 993–1005. doi: 10.21203/rs.3.rs-168367/v1
Chen, B., Song, J., Wang, Q., and Liang, J. F. (2021c). Russula indocatillus, a new record species in China. Chin. J. Trop. Crops 42, 2542–2548. doi: 10.3969/j.issn.1000-2561.2021.09.014
Chen, B., Song, J., Zhang, J. H., and Liang, J. F. (2021d). Morphology and molecular phylogeny reveal two new species in Russula sect. Ingratae from China. Phytotaxa 525, 109–123. doi: 10.11646/phytotaxa.525.2.2
Chen, Z. H., Zhang, P., and Zhang, Z. G. (2014). Investigation and analysis of 102 mushroom poisoning cases in southern China from 1994 to 2012. Fungal Divers. 64, 123–131. doi: 10.1007/s13225-013-0260-7
Chou, W. N., and Wang, Y. Z. (2005). Nine species of Russula (Basidiomycotina) new to Taiwan. Taiwania 50, 93–100. doi: 10.6165/tai.2005.50(2).93
Crous, P. W., Wingfield, M. J., Burgess, T. I., Hardy, G. E., Barber, P. A., Alvarado, P., et al. (2017). Fungal planet description sheets: 558–624. Persoonia 38, 240–384. doi: 10.3767/003158517X698941
Das, K., Atri, N. S., and Buyck, B. (2013). Three new species of Russula (Russulales) from India. Mycosphere 4, 722–732. doi: 10.5943/mycosphere/4/4/9
Das, K., Ghosh, A., Chakraborty, D., Li, J. W., Qiu, L. H., Baghela, A., et al. (2017). Fungal biodiversity profiles 31–40. Cryptogam. Mycol. 38, 353–406. doi: 10.7872/crym/v38.iss3.2017.353
De Crop, E., Nuytinck, J., Van de Putte, K., Wisitrassameewong, K., Hackel, J., Stubbe, D., et al. (2017). A multi-gene phylogeny of Lactifluus (Basidiomycota, Russulales) translated into a new infrageneric classification of the genus. Persoonia 38, 58–80. doi: 10.3767/003158517X693255
Deng, C. Y., Shi, L. Y., Wang, J., Xiang, Z., Li, S. M., Li, G. J., et al. (2020). Species diversity of Russula virescens complex qingtoujun in southern China. Mycosystema 39, 1–23. doi: 10.13346/j.mycosystema.200145
Dutta, A. K., Paloi, S., Pradhan, P., and Acharya, K. (2015). A new species of Russula (Russulaceae) from India based on morphological and molecular (ITS sequence) data. Turk. J. Bot. 39, 850–856. doi: 10.3906/bot-1407-1
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32, 1792–1797. doi: 10.1093/nar/gkh340
Ghosh, A., Das, K., Bhatt, R. P., and Hembrom, M. E. (2020). Two new species of genus Russula from Western Himalaya with morphological details and phylogenetic estimations. Nova Hedwigia 111, 115–130. doi: 10.1127/nova_hedwigia/2020/0588
Hall, T. A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analyses program for windows 95/98/NT. Nucleic Acids Symp. Ser. 734, 95–98. doi: 10.1021/bk-1999-0734.ch008
Halling, R. E. (2001). Ectomycorrhizae: co-evolution, significance, and biogeography. Ann. Mo. Bot. Gard. 88, 5–13. doi: 10.2307/2666128
Han, Y. X., Liang, Z. Q., Jiang, S., and Zeng, N. K. (2022). Russula hainanensis (Russulaceae, Russulales), a new species from tropical China. Phytotaxa 552, 35–050. doi: 10.11646/phytotaxa.552.1.3
Huang, J., Nara, K., Zong, K., Wang, J., Xue, S. G., Peng, K. J., et al. (2014). Ectomycorrhizal fungal communities associated with Masson pine (Pinus massoniana) and white oak (Quercus fabri) in a manganese mining region in Hunan Province, China. Fungal Ecol. 9, 1–10. doi: 10.1016/j.funeco.2014.01.001
Huelsenbeck, J. P., and Ronquist, F. (2005). “Bayesian analysis of molecular evolution using MrBayes” in Statistical methods in molecular evolution. ed. R. Nielsen (New York, NY: Springer), 183–226.
Hyde, K. D., Tennakoon, D. S., Jeewon, R., Bhat, D. J., Maharachchikumbura, S. S. N., Rossi, W., et al. (2019). Fungal diversity notes 1036–1150: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers. 96, 1–242. doi: 10.1007/s13225-020-00439-5
Ishikawa, A., Uehara, I., and Tanaka, M. (2020). Ectomycorrhizal fungal communities in the boundary between secondary broad-leaved forests and Japanese cypress plantations. J. Forest. Res. 25, 397–404. doi: 10.1080/13416979.2020.1816614
James, T. Y., Kauf, F., Schoch, C., Matheny, P. B., Hofstetter, V., Cox, C., et al. (2006). Reconstructing the early evolution of the fungi using a six gene phylogeny. Nature 443, 818–822. doi: 10.1038/nature05110
Kaewgrajang, T., Kaewjunsri, S., Jannual, N., and Nipitwattanaphon, M. (2020). Morphology and molecular identification of some Lactarius and Russula species. Genomics Genet. 13, 44–58. doi: 10.14456/gag.2020.6
Knudsen, H., and Borgen, T. (1982). Russulaceae in Greenland. In: Arctic and alpine mycology 1. University of Washington Press, Seattle and London 1–559.
Kornerup, A., and Wanscher, J. H. (1981). Taschenlexikon der Farben, 3rd Edn. Muster-Schmidt Verlag, Göttingen, 1–242.
Li, F., and Deng, Q. L. (2018). Three new species of Russula from South China. Mycol. Prog. 17, 1305–1321. doi: 10.1007/s11557-018-1447-9
Li, G. J., Li, S. M., Buyck, B., Zhao, S. Y., Xie, X. J., Shi, L. Y., et al. (2021). Three new Russula species in sect. Ingratae (Russulales, Basidiomycota) from southern China. MycoKeys 84, 103–139. doi: 10.3897/mycokeys.84.68750
Li, G. J., Zhang, C. L., Lin, F. C., and Zhao, R. L. (2018). Hypogeous gasteroid Lactarius sulphosmus sp. nov. and agaricoid Russula vinosobrunneola sp. nov. (Russulaceae) from China. Mycosphere 9, 838–858. doi: 10.5943/mycosphere/9/4/9
Li, G. J., Zhao, D., Li, S. F., and Wen, H. A. (2015). Russula chiui and R. pseudopectinatoides, two new species from southwestern China supported by morphological and molecular evidence. Mycol. Prog. 14, 1–14. doi: 10.1007/s11557-015-1054-y
Li, G. J., Zhao, Q., Zhao, D., Yue, S. F., Li, S. F., Wen, H. A., et al. (2013). Russula atroaeruginea and R. sichuanensis spp. nov. from Southwest China. Mycotaxon 124, 173–188. doi: 10.5248/124.173
Li, J. W., Zheng, J. F., Song, Y., Yuan, F., and Qiu, L. H. (2019). Three novel species of Russula from southern China based on morphological and molecular evidence. Phytotaxa 392, 264–276. doi: 10.11646/phytotaxa.392.4.2
Looney, B. P., Ryberg, M., Hampe, F., Sánchez-García, M., and Matheny, P. B. (2016). Into and out of the tropics: global diversification patterns in a hyperdiverse clade of ectomycorrhizal fungi. Mol. Ecol. 25, 630–647. doi: 10.1111/mec.13506
Mao, X. L. (2006). Poisonous mushrooms and their toxins in China. Mycosystema 22, 1013–1021. doi: 10.1016/S1872-2075(06)60070-8
Mikheyev, A. S., Mueller, U. G., and Abbot, P. (2006). Cryptic sex and many-Toone coevolution in the fungus-growing ant symbiosis. Proc. Natl. Acad. Sci. U. S. A. 103, 10702–10706. doi: 10.1073/pnas.0601441103
Miller, S. L., and Buyck, B. (2002). Molecular phylogeny of the genus Russula in Europe with a comparison of modern infrageneric classifications. Mycol. Res. 106, 259–276. doi: 10.1017/S0953756202005610
Miyamoto, Y., Terashima, Y., and Nara, K. (2018). Temperature niche position and breadth of ectomycorrhizal fungi: reduced diversity under warming predicted by a nested community structure. Glob. Chang. Biol. 24, 5724–5737. doi: 10.1111/gcb.14446
Motomura, H., Selosse, M. A., Martos, F., Kagawa, A., and Yukawa, T. (2010). Mycoheterotrophy evolved from mixotrophic ancestors: evidence in cymbidium (Orchidaceae). Ann. Bot. 106, 573–581. doi: 10.1093/aob/mcq156
Murata, M., and Nara, K. (2017). Ectomycorrhizal fungal communities at different soil depths in a forest dominated by endangered Pseudotsuga japonica. J. Japan For. Soc. 99, 195–201. doi: 10.4005/jjfs.99.195
Nylander, J. A. A. (2004). MrModeltest 2.3. Program distributed by the author. Uppsala University: Evolutionary Biology Center.
Park, M. S., Fong, J. J., Lee, H., Oh, S. Y., Jung, P. E., Min, Y. J., et al. (2013). Delimitation of Russula subgenus Amoenula in Korea using three molecular markers. Mycobiology 41, 191–201. doi: 10.5941/MYCO.2013.41.4.191
Persoon, C. H. (1796). Seu Descriptiones tam novorum quan notabilium fungorum. Observ. mycol. Lipsiae 221
Romagnesi, H. (1987). Status et noms nou veaux pour les taxa infragénériques dans le genere Russula. Documentation Mycologique 18, 39–40.
Smith, S. A., and Dunn, C. W. (2008). Phyutility: a phyloinformatics tool for trees, alignments and molecular data. Bioinformation 24, 715–716. doi: 10.1093/bioinformatics/btm619
Song, Y. (2022). Species of Russula subgenus Heterophyllidiae (Russulaceae, Basidiomycota) from Dinghushan biosphere reserve. Eur. J. Taxon. 826, 1–32. doi: 10.5852/ejt.2022.826.1831
Song, Y., Buyck, B., Li, J. W., Yuan, F., Zhang, Z. W., and Qiu, L. H. (2018a). Two novel and a forgotten Russula species in sect. Ingratae (Russulales) from Dinghushan biosphere reserve in southern China. Cryptogam. Mycol. 39, 341–357. doi: 10.7872/crym/v39.iss3.2018.341
Song, J., Chen, B., Liang, J. F., Li, H. J., Wang, S. K., and Lu, J. K. (2020). Morphology and phylogeny reveal Russula subpunctipes sp. nov., from southern China. Phytotaxa 459, 16–24. doi: 10.11646/phytotaxa.459.1.2
Song, Y., Li, J. W., Buyck, B., Zheng, J. F., and Qiu, L. H. (2018b). Russula verrucospora sp. nov. and R. xanthovirens sp. nov., two novel species of Russula (Russulaceae) from southern China. Cryptogam. Mycol. 39, 129–142. doi: 10.7872/crym/v39.iss1.2018.129
Song, J., Liang, J. F., Mehrabi-Koushki, M., Krisai-Greilhuber, I., Ali, B., Bhat, V. K., et al. (2019). Fungal systematics and evolution: FUSE 5. Sydowia 71, 141–245. doi: 10.12905/0380.sydowia71-2019-0141
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22, 2688–2690. doi: 10.1093/bioinformatics/btl446
Tolgor, B., Bao, H. Y., and Li, Y. (2014). A revised checklist of poisonous mushrooms in China. Mycosystema 33, 517–548. doi: 10.13346/j.mycosystema.130256
Vera, M., Adamčík, S., Adamčíková, K., Hampe, F., Caboň, M., Manz, C., et al. (2021). Morphological and genetic diversification of Russula floriformis, sp. nov., along the isthmus of Panama. Mycologia 113, 807–827. doi: 10.1080/00275514.2021.1897377
Vilgalys, R., and Hester, M. (1990). Rapid genetic identifcation and mapping of enzymatically amplifed ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
Wang, J. (2019). Taxonomy of edible species of Russula in Yunnan Jilin Agricultural University, Changchun. 1–120.
Wang, J., Buyck, B., Wang, X. H., and Tolgor, B. (2019). Visiting Russula (Russulaceae, Russulales) with samples from southwestern China finds one new subsection of R. subg. Heterophyllidia with two new species. Mycol. Prog. 18, 771–784. doi: 10.1007/s11557-019-01487-1
Wang, R., Herrera, M., Xu, W. J., Zhang, P., Moreno, J. P., Colinas, C., et al. (2022). Ethnomycological study on wild mushrooms in Pu’er prefecture, Southwest Yunnan, China. J. Ethnobiol. Ethnomed. 18, 1–24. doi: 10.1186/s13002-022-00551-7
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). Amplifcation and direct sequencing of fungal ribosomal RNA genes for phylogenies. PCR Protocols 18, 315–322. doi: 10.1016/B978-0-12-372180-8.50042-1
Wisitrassameewong, K., Manz, C., Hampe, F., Looney, B. P., Boonpratuang, T., Verbeken, A., et al. (2022). Two new Russula species (fungi) from dry dipterocarp forest in Thailand suggest niche specialization to this habitat type. Sci. Rep. 12, 2826–2815. doi: 10.1038/s41598-022-06836-x
Wisitrassameewong, K., Park, M. S., Lee, H., Ghosh, A., Das, K., Buyck, B., et al. (2020). Taxonomic revision of Russula subsection Amoeninae from South Korea. MycoKeys 75, 1–29. doi: 10.3897/mycokeys.75.53673
Wu, F., Man, X. W., Tohtirjap, A., and Dai, Y. C. (2022). A comparison of polypore funga and species composition in forest ecosystems of China, North America, and Europe. For. Ecosyst. 9:100051. doi: 10.1016/j.fecs.2022.100051
Wu, F., Zhou, L. W., Yang, Z. L., Tolgor, B., Li, T. H., and Dai, Y. C. (2019). Resource diversity of Chinese macrofungi: edible, medicinal and poisonous species. Fungal Divers. 98, 1–76. doi: 10.1007/s13225-019-00432-7
Yamato, M., Kosaka, R., Masui, Y., Goda, Y., Shirasaka, S., Maruyama, A., et al. (2021). Mycorrhizal associates of Cephalanthera falcata (Orchidaceae) in a habitat with giant individuals. Ecol. Res. 36, 177–188. doi: 10.1111/1440-1703.12187
Ying, J. Z. (1983). A study on Russula viridi-rubrolimbata sp. nov. and its related species of subsection Virescentinae. Mycosystema 2, 34–37.
Yuan, F., Song, Y., Buyck, B., Li, J. W., and Qiu, L. H. (2019). Russula viridicinnamomea F. Yuan & Y. Song, sp. nov. and R. pseudocatillus F. Yuan & Y. Song, sp. nov., two new species from southern China. Cryptogam. Mycol. 40, 45–56. doi: 10.5252/cryptogamie-mycologie2019v40a4
Zeng, N. K., and Jiang, S. (2020). Atlas of macrofungi from Yinggeling of Hainan, China. Nan Hai Publish Company, Haikou.
Zeng, N. K., Liang, Z. Q., Tang, L. P., Li, Y. C., and Yang, Z. L. (2017). The genus Pulveroboletus (Boletaceae, Boletales) in China. Mycologia 109, 422–442. doi: 10.1080/00275514.2017.1331689
Zeng, N. K., Liang, Z. Q., Wu, G., Li, Y. C., Yang, Z. L., and Liang, Z. Q. (2016). The genus Retiboletus in China. Mycologia 108, 363–380. doi: 10.3852/15-072
Zeng, N. K., Tang, L. P., Li, Y. C., Tolgor, B., Zhu, X. T., Zhao, Q., et al. (2013). The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Divers. 58, 73–101. doi: 10.1007/s13225-012-0184-7
Zhang, J. B., Li, J. W., Li, F., and Qiu, L. H. (2017). Russula dinghuensis sp. nov. and R. subpallidirosea sp.nov., two new species from southern China supported by morphological and molecular evidence. Cryptogam. Mycol. 38, 191–203. doi: 10.7872/crym/v38.iss2.2017.191
Zhang, Y. Z., Lin, W. F., Buyck, B., Liang, Z. Q., Su, M. S., Chen, Z. H., et al. (2022a). Morphological and phylogenetic evidences reveal four new species of Cantharellus subgenus Cantharellus (Hydnaceae, Cantharellales) from China. Front. Microbiol. 13:900329. doi: 10.3389/fmicb.2022.900329
Zhang, Y. Z., Zhang, P., Buyck, B., Tang, L. P., Liang, Z. Q., Su, M. S., et al. (2022b). A contribution to knowledge of Craterellus (Hydnaceae, Cantharellales) in China: three new taxa and amended descriptions of two previous species. Front. Microbiol. 13:906296. doi: 10.3389/fmicb.2022.906296
Keywords: ectomycorrhizal fungi, molecular phylogeny, morphology, new taxa, taxonomy
Citation: Han Y-X, Liang Z-Q and Zeng N-K (2023) Notes on four species of Russula subgenus Heterophyllidiae (Russulaceae, Russulales) from southern China. Front. Microbiol. 14:1140127. doi: 10.3389/fmicb.2023.1140127
Received: 08 January 2023; Accepted: 23 February 2023;
Published: 21 March 2023.
Edited by:
Yong-Zhong Lu, Guizhou Institute of Technology, ChinaReviewed by:
Fang Wu, Beijing Forestry University, ChinaCopyright © 2023 Han, Liang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Qun Liang, bGl6aHF1MTk4MEAxMjYuY29t; Nian-Kai Zeng, bmlhbmthaXpAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.