- 1College of Animal Science, Guizhou University, Guiyang, China
- 2Guizhou Grassland Technology Extending Station, Guiyang, China
- 3Key Laboratory of Animal Genetics, Breeding and Reproduction in the Plateau Mountainous Region, Ministry of Education, Guizhou University, Guiyang, China
Silage can be contaminated with mycotoxins and accidental fungi after aerobic exposure. The study assessed the effects of bunker silos (BS), round bales (RB), and silage bags (SB) on the nutritional characteristics, fermentation quality, aerobic stability, mycotoxin levels and microbial communities of whole-plant corn silage (WPCS). After 90 days of fermentation, silages were opened and sampled at 0, 1, 3, 5, 7, and 9 days of exposure. SB group conserved higher lactic acid and dry matter contents and a lower pH value than other groups after 9 days of exposure (p < 0.05). The SB group showed the longest aerobic stability (202 h) among all silages (p < 0.05). The concentrations of aflatoxin B1, trichothecenes and fumonisin B1 were significantly lower in SB after 9 days of exposure (p < 0.05). Acetobacter became the dominant bacteria in BS and RB groups after 5 days of exposure. However, Lactobacillus still dominated the bacterial community in SB group. Acetobacter was positively correlated with pH, acetic acid content, and ammonia-N content (p < 0.05). Lactobacillus was positively correlated with Kazachstania and Candida abundances (p < 0.01) but negatively correlated with Fusarium abundance (p < 0.05). Considering the feed value and food safety of silage in the feeding process, silage bags are recommended for WPCS according to the observed nutritional quality, fermentation index and mycotoxin content.
1. Introduction
Food safety has received increasing international attention in public regulation, private supply chain coordination, and international trade in recent years (Unnevehr, 2015). The World Health Organization (WHO) estimated that there are approximately 600 million illnesses and 420,000 deaths worldwide caused by 31 foodborne hazards annually (World Health Organization, 2015). Livestock products such as meat, milk, and eggs represent a considerable source of animal protein for human food (Cai et al., 2021). However, these products are easily spoiled by many factors, such as zoonotic diseases, mycotoxins and undesired microorganisms. To prevent the negative effects associated with livestock products, it is suggested that feed safety should be considered a prerequisite in developing a farm-to-fork food safety program for animal source foods (FAO, 2019). Silage plays an important role in the global agricultural and agri-food industries, ensuring a constantly nutritious supply for ruminants, especially whole-plant corn silage (WPCS), which is used in ruminant feeding as an important source of a high energy content and digestible fiber (Khan et al., 2015; Gheller et al., 2021). However, silage can be contaminated with biological and chemical hazards from inherent mycotoxins and accidental fungi when there is a lack of standardized manufacturing and processing, storage or transport (Ghilardelli et al., 2022). This contamination is worse during the aerobic exposure period than during other stages. Da Silva et al. (2015) reported that increases in temperature and pH could lead to silage decay significantly after silage was exposed to air. Several studies have shown that ensiled forage, particularly the superficial silage in bunker silos (BS), usually accumulates mycotoxins such as aflatoxin B1 (AFB1), trichothecenes (T-2), fumonisin B1 (FB1), zearalenone (ZEN), deoxynivalenol (DON), and many other fungal secondary metabolites under aerobic exposure conditions (Ogunade et al., 2018). It was also found that intake of mycotoxins destroys the structure and function of the animal intestinal tract, damages the immune and antioxidant systems, causes disorders of intestinal metabolism, and eventually impairs the health of ruminants (Chen et al., 2022). Furthermore, there is a potential possibility that mycotoxins would accumulate in livestock products such as meat, milk, eggs, and blood products. Intake of livestock products with mycotoxins causes destructive effects on humans including altered genome expression and kidney diseases, diminished reproductive system activity, the intestinal tract disruption, and the development of cancer-causing cells in the body (Luo et al., 2018).
Bunker silos (BS), round bales (RB) and silage bags (SB) are becoming common ways to store silage. A lack of scientific ensiling management usually results in poor chemical composition and excessive butyric acid contents (Liu et al., 2019). Feeding WPCS from BS has the advantage of being more efficient than feeding WPCS from RB and SB. The plastic consumption per gram of crop in BS was calculated to be more than 5 times lower than that in SB (Randby et al., 2020). However, there have been opposite conclusions considering the nutritional quality and feeding value of silages. Muck et al. (2015) found that silage quality was worse in BS than in SB and tower silo. Randby and Bakken (2021) also reported a similar phenomenon in which the sum of dry matter (DM) lost by crop respiration, effluent runoff, anaerobic fermentation, aerobic deterioration and gaseous losses was significantly higher in BS than in RB.
However, most previous studies have focused on comparing different ensiling methods on the commercial value of WPCS during fermentation, ignoring its quality and safety to livestock in the process of utilization with air exposure. Hence, the objectives of this study were to investigate the effect of BS, RB and SB on the chemical characteristics, fermentation quality, aerobic stability, microbial community, and mycotoxin contents of WPCS during an aerobic exposure period (0, 1, 3, 5, 7, and 9 days). Furthermore, the results will be helpful to determine an optimal ensiling method to limit silage degradation and to reduce the impact of poor silage quality on ruminants and human health.
2. Materials and methods
2.1. Silage preparation
Row whole-plant corn (Qingfeng 4) was obtained from a commercial plant base located in Liupanshui City, Guizhou Province (104.83 °E, 26.60 °N) on September 30, 2020 during the dough stage. The plant material was chopped to a theoretical cut length of 1–2 cm by a precision chopper. After mixing thoroughly, the chopped whole-plant corn was divided into three parts for: (i) ensiling in BS (7 m × 24 m with three 3.2 m high walls, and a capacity of 280 tones fresh crop weight), (ii) ensiling in RB (bales were immediately wrapped with 8 layers of 0.75 m wide and 0.025 mm thick black plastic film, dimensions: 0.8 m diameter × 0.6 m length, with a maximal capacity of 190 kg fresh crop weight), and (iii) ensiling in SB (dimensions: 0.5 m wide × 0.6 m height × 1.0 m length, and with a maximal capacity of 200 kg fresh crop weight). After 90 days of fermentation, silages were opened for 9 days of aerobic exposure. The density of the BS ranged from 496 to 504 kg/m3, while that of the RB and SB groups ranged from 592 to 604 kg/m3 and 644 to 655 kg/m3, respectively. The ambient temperature ranged from 0 to 7.5°C. Samples (about 1 kg each) from the middle of silages were taken at the same six time points (0, 1, 3, 5, 7, and 9 days of aerobic exposure) during the unloading of the BS, RB and SB, respectively. For BS, samples were collected from 3 silos as three replicates at 6 different sampling times. For RB and SB, totally 36 silos were taken for ensiling, all silos were opened and samples were collected from 3 silos of each treatment at one sampling time. In total, the study comprised 3 BS, 18 RB, and 18 SB. The samples (3 treatments × 6 time points × 3 replicates) were used to determine the chemical composition, fermentation quality, aerobic stability, microbial community and mycotoxin contents. One part of silage was sampled in 50 mL cryogenic vials and stored in a −80°C freezer for microbial community analysis, and another part was stored in a −20°C freezer for chemical composition, fermentation quality, and mycotoxin content analysis.
2.2. Chemical, fermentation and aerobic stability analyses
20 g of silage samples were combined with 180 mL of distilled water and stored in a 4°C refrigerator for 1 day, then four layers of cheese cloth were used to filter the silage, for the determination of the fermentation profile. The pH was measured using a pH meter (PHSJ-3F, CANY, Shanghai, China). Concentrations of lactic acid, acetic acid, propionic acid and butyric acid were determined by high-performance liquid chromatography, according to the methods described by Wu et al. (2022). The ammonia-N content was measured using phenol-hypochlorite colorimetry (Broderick and Kang, 1980).
WPCS was dried at 65°C for 48 h in a dry oven to measure the dry matter (DM) content. Then grounded into powder for total nitrogen, crude protein (CP), water-soluble carbohydrate (WSC), neutral detergent fiber (NDF) and acid detergent fiber (ADF) analyses. The CP content was calculated via the total nitrogen content multiplied by 6.25, and the total nitrogen was analyzed by a Kjeldahl nitrogen analyzer (Kjeltec 8400 Analyzer; Foss, Sweden). According to the methods described by Turula et al. (2010), WSC was determined by colorimetric after-reaction with anthrone reagent. The NDF and ADF were measured based on Van Soest procedures, a heat stable alpha amylase was used in the NDF procedure, the results were expressed on a DM basis including residual ash. Aerobic stability was measured after 90 days of ensiling. About 3 kg of silages from each silo were taken and mixed thoroughly, then put into separate new plastic silos (capacity 10 L) without compaction and uncovered. The geometric-center of the silage masses and the ambient temperature were recorded with sensors every 2 h. The aerobic stability was determined based on the time when the temperature of silage exposed to air exceeded the ambient temperature by 2°C (Ranjit and Kung, 2000).
2.3. Analyses of the microbial community
WPCS (20 g) was homogenized with distilled water (180 mL), and subsequently filtered through two layers of medical gauze and centrifuged for 5 min at 8,000 g/min to collect microorganism cells. The total genomic DNA was extracted via the HiPure Soil Kit (QIAGEN, Inc., Venlo, Netherlands), then the purity, concentration, and integrity of the isolated DNA samples were determined by agarose gel electrophoresis. Thereafter, the 16S rRNA V5–V7 regions of genomic DNA was amplified via Pyrobest DNA Polymerase (TaKaRa, DR500A) with the universal primers of 799F (AACMGGATTAGATACCCKG) and 1193R (ACGTCATCCCCACCTTCC). ITS genes of regions (ITS1_other) were amplified using the specific primers ITS1-F (CTTGGTCATTTAGAGGAAGTAA) and ITS2 (GCTGCGTTCTTCATCGATGC) with Barcode. AMPure XP Beads (Beckman Coulter, Indianapolis, IN, United States) was adopted for the purification of the polymerase chain reaction (PCR) products, and the ABI StepOnePlus Real-Time PCR System (Life Technologies, United States) was used for quantification (Toju et al., 2012). Following the generate sequencing libraries according to the manufacturer’s instructions, the library quality was performed on the Illumina HiSeq 2500 platform by Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China). After high-throughput sequencing and the filtration of chimera and low-quality sequences, the bioinformatics analyses of the microbial community were mainly performed using the QIIME (Version 2.15.3) and R software (Version 4.0.0).
2.4. Mycotoxin analyses
Mycotoxins were extracted simultaneously from 2 g of a dried silage sample using a 40 mL centrifuge tube with 20 mL of an acetonitrile: water solution (80:20 v/v). After the samples were horizontally shaken for 40 min, 1.0 g of NaCl and 2.0 g of anhydrous magnesium sulfate was added to obtain phase separation. After shaking for another 1 min and centrifugation at 6,000 g/min for 5 min, the upper acetonitrile phase was recovered. The mixture was evaporated to dryness under a nitrogen steam at 40°C and re-dissolved in methanol: formate in water solution (10:90, v/v), then the final extract was filtered through a filter (Millipore Corporation, Bedford, United States; HV 0.45 μm). A 20 μL sample was injected into the HPLC with MS/MS system according to the methods described by Gallo et al. (2010).
2.5. Statistical analysis
Results are reported as the mean and the standard error of the mean (SEM). The data related to fermentation quality, chemical composition, mycotoxin levels and alpha diversity were subjected to two-way analysis of variance. Aerobic stability of WPCS after ensiling were subjected to one-way analysis of variance. When there were significant differences (p < 0.05), the group means were further compared with Duncan’s multiple range tests. The statistical analyses were performed using SPSS 26.0 (SPSS, Chicago, IL, United States). Alpha diversity metrics (Chao1, Shannon and Good’s coverage) were calculated with QIIME software (Version 2.15.3). Sample ordination based on the beta diversity was examined using the principal coordinate’s analysis (PCoA). The relative abundances of microbial communities at the phylum and genus levels were also analyzed. The resultant correlation matrix was analyzed by “corrplot” in R language (method = Spearman).
3. Results
3.1. Silage characteristics and aerobic stability of WPCS during aerobic exposure
The chemical composition of the WPCS was shown in Table 1. The content of DM first increased and then decreased significantly over time (p < 0.05), and the highest DM content was observed at 3–5 days. The content of DM in each group was in the order of SB > RB > BS at any stage of aerobic exposure. The WSC content decreased after 9 days of aerobic exposure (p < 0.05), and the WSC content in the RB and BS groups was lower than that in the SB group. The CP content of the three groups showed a similar decreasing trend with increasing aerobic exposure duration. Compared to the RB and BS groups, the SB group retained the highest CP content after 9 days of aerobic exposure. There were significant increases in NDF and ADF contents during the exposure period in all groups (p < 0.05). The BS group had higher NDF and ADF contents than the other groups after 5 days of aerobic exposure. It was also found that the aerobic exposure days, ensiling methods, and their interaction significantly affected the contents of DM, CP, NDF, and ADF in the WPCS (p < 0.001).
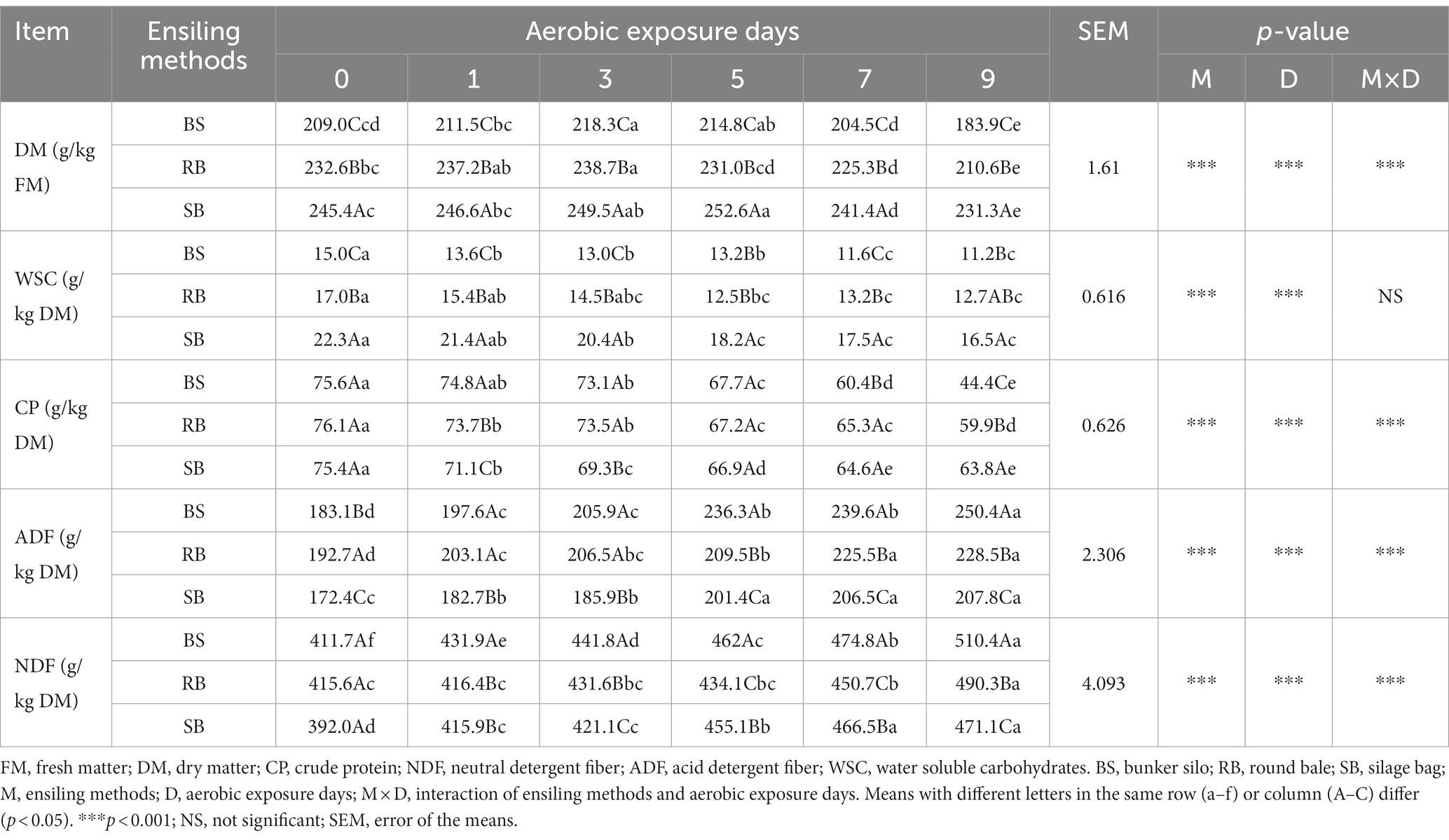
Table 1. Effect of different ensiling methods on chemical compositions of WPCS during aerobic exposure.
As shown in Table 2, the pH of the WPCS was significantly affected by the prolonged aerobic exposure duration (p < 0.001). The pH value of SB group ranged from 3.78 to 4.01 from d 0 to d 9, while BS and RB group had increased over 4.20 at d 3 and d 5, respectively. Although the contents of LA decreased with prolonged aerobic exposure times, the SB group had a higher concentration than the other groups. Lower PA and AA contents were also found in the SB group (p < 0.05), and PA was not detected until day 7. Furthermore, BA was not detected in the RB and SB groups. The content of ammonia-N in all groups were increased after 9 days of aerobic exposure (p < 0.05). The lowest ammonia-N content was found in the SB group on all days of aerobic exposure.
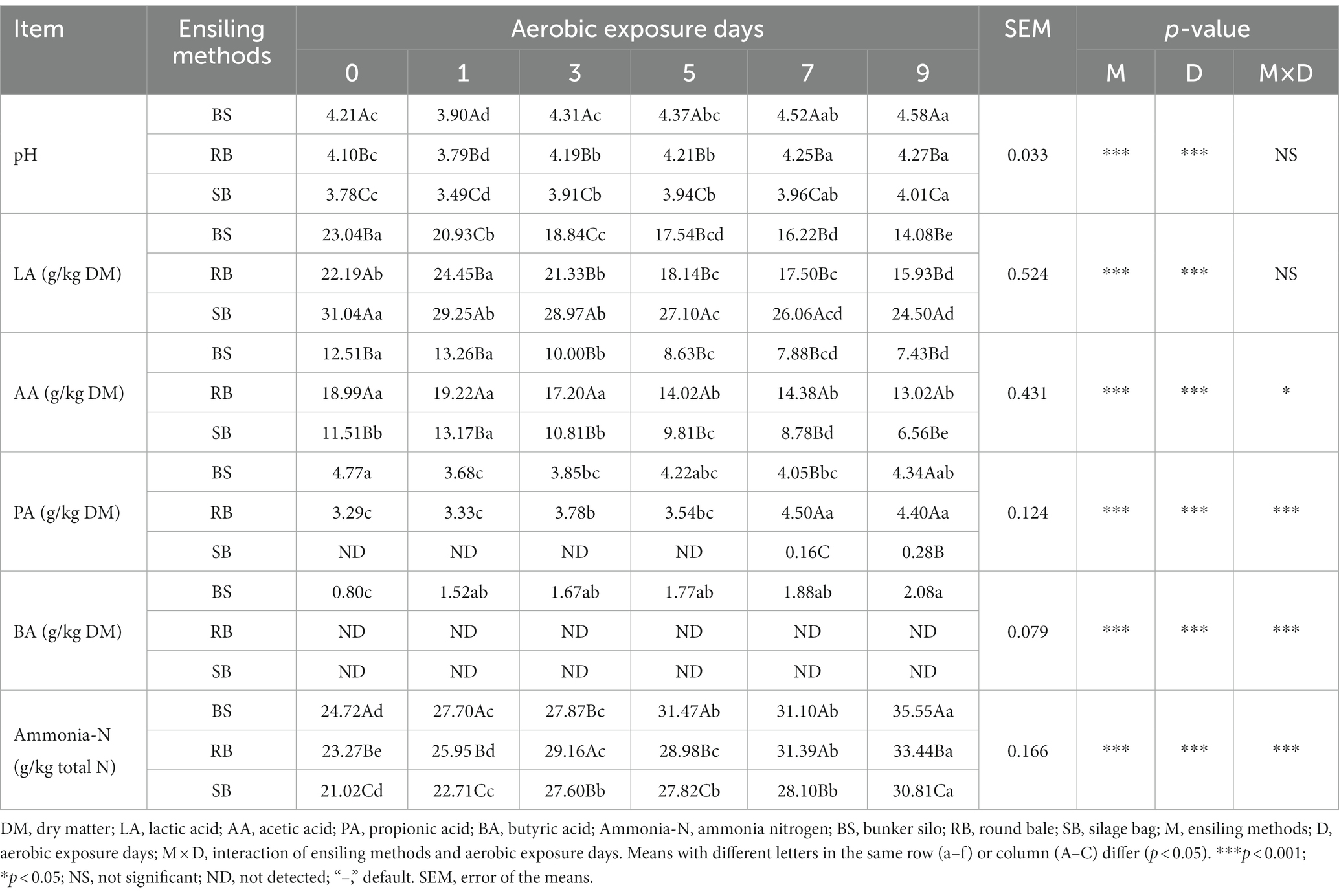
Table 2. Effect of different ensiling methods on fermentation quality of WPCS during aerobic exposure.
The different ensiling methods significantly (p < 0.001) influenced the aerobic stability of the WPCS (Figure 1). The BS group spoiled within 174 h, RB group was stable for 182 h, which was (p > 0.05) longer than BS group (8 h). The SB group showed the strongest aerobic stability (202 h) among all silage.
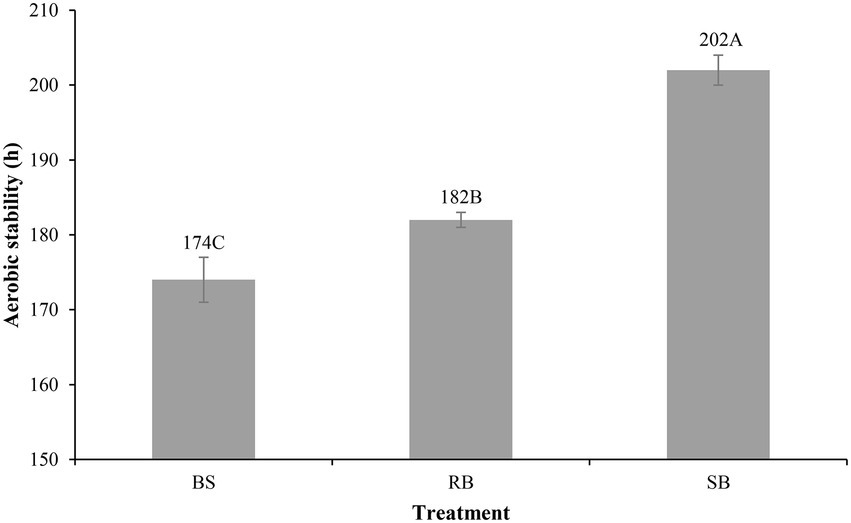
Figure 1. Effect of different ensiling methods on the aerobic stability (h) of WPCS after 90 days of ensiling (SEM = 1.247, p < 0.001). Vertical bars are the standard errors of the means, bars with different letters differ (p < 0.05). Treatment: BS, Bunker silo; RB, Round bale; SB, Silage bag.
3.2. Mycotoxin levels in WPCS during aerobic exposure
The results of the mycotoxin levels are summarized in Table 3. The AFB1, ZEN, T-2, DON and FB1 concentrations showed a significant interaction between ensiling methods and aerobic exposure days (p < 0.001). Overall, the concentrations of mycotoxins continually increased with increasing aerobic exposure (p < 0.05), and the BS group had higher concentrations than the RB and SB groups after 9 days of exposure (p < 0.05). AFB1, T-2 and FB1 concentrations were significantly lower in the SB group than in the other groups (p < 0.05), while the contamination levels of ZEN and DON did not show significant differences between the RB and SB groups after 9 days of oxygen exposure (p > 0.05).
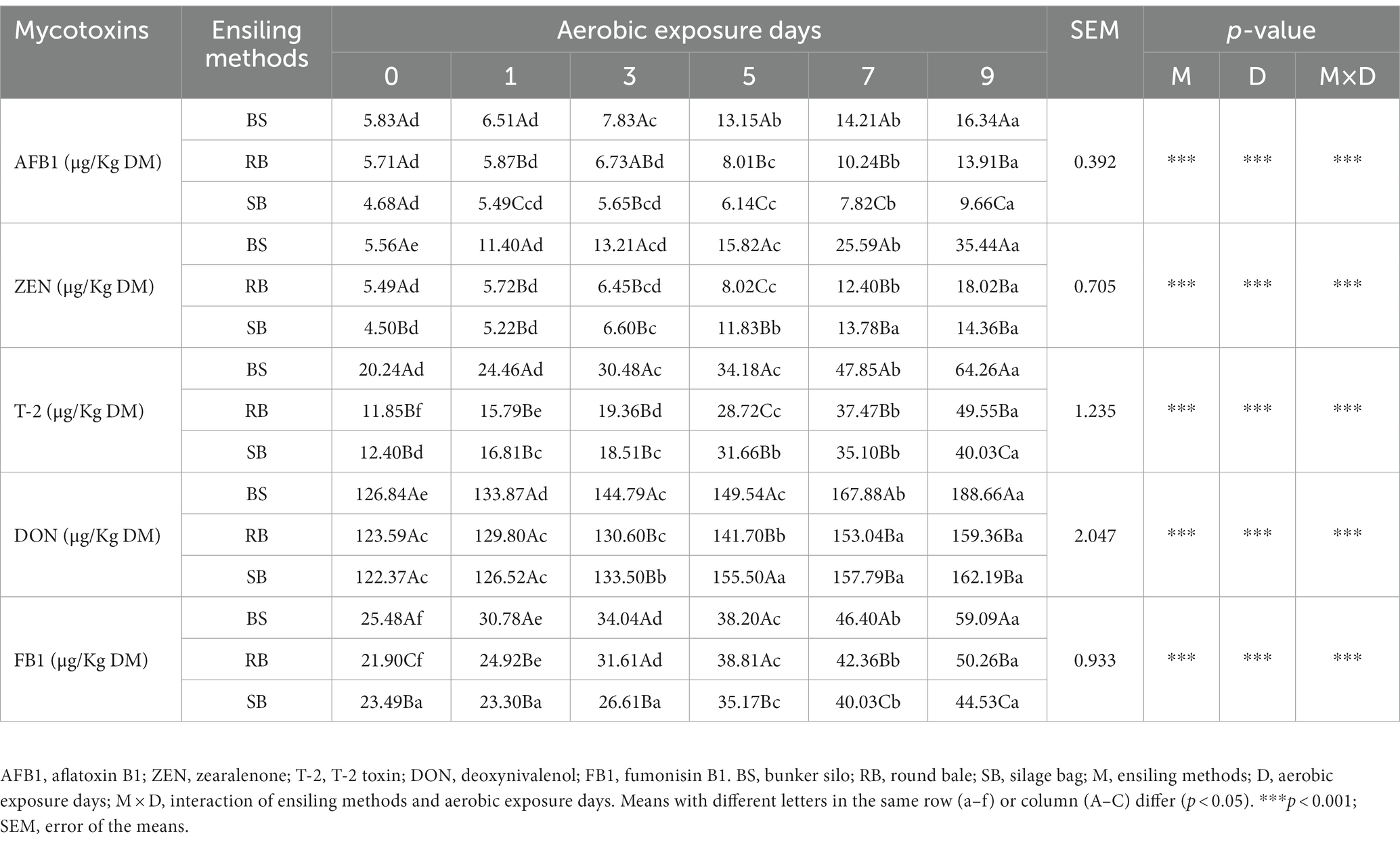
Table 3. Effect of different ensiling methods on mycotoxins concentration of WPCS during aerobic exposure.
3.3. Microbial community diversity in the WPCS during aerobic exposure
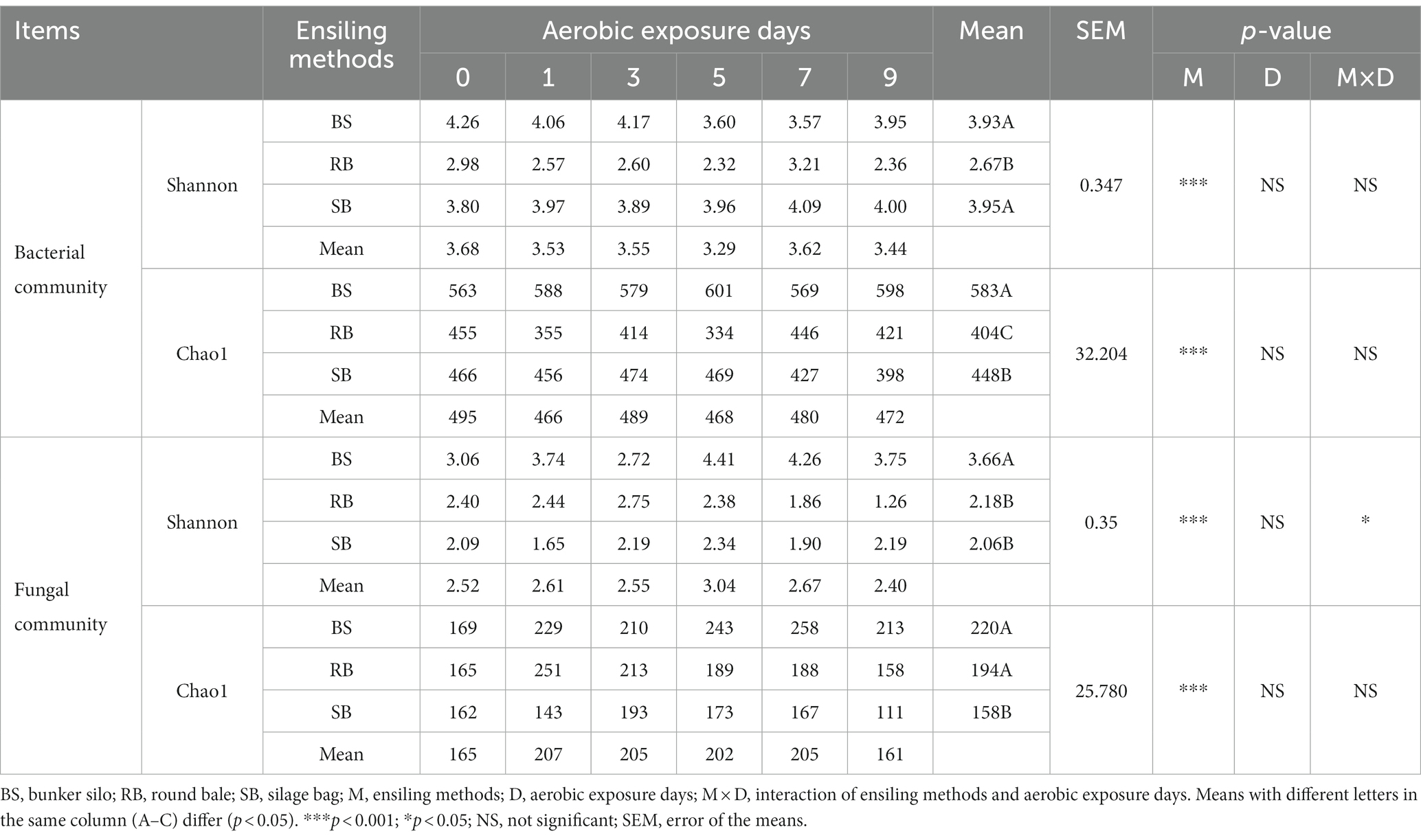
The Good’s coverage values were greater than 99%, indicating that the sampling depth adequately captured most of the bacterial and fungal communities. The alpha diversities of the WPCS were evaluated through Shannon and Chao1 indexes (Table 4). The ensiling methods significantly affected the alpha diversities of the microbial community (p < 0.001). The RB group had lower Shannon and Chao1 indexes than the BS and SB groups in the bacterial community (p < 0.05). However, the SB groups maintained lower Shannon and Chao1 indexes in the fungal community (p < 0.05).
To analyze the distribution and structure of bacterial and fungal communities in WPCS samples at different aerobic times, principal coordinates analysis (PCoA) based on Bray–Curtis distance at the OTU level was conducted (Figure 2). Good separation and differences in microbial communities were observed between different ensiling methods, and the microbial community of SB group showed less variation during aerobic exposure stages.
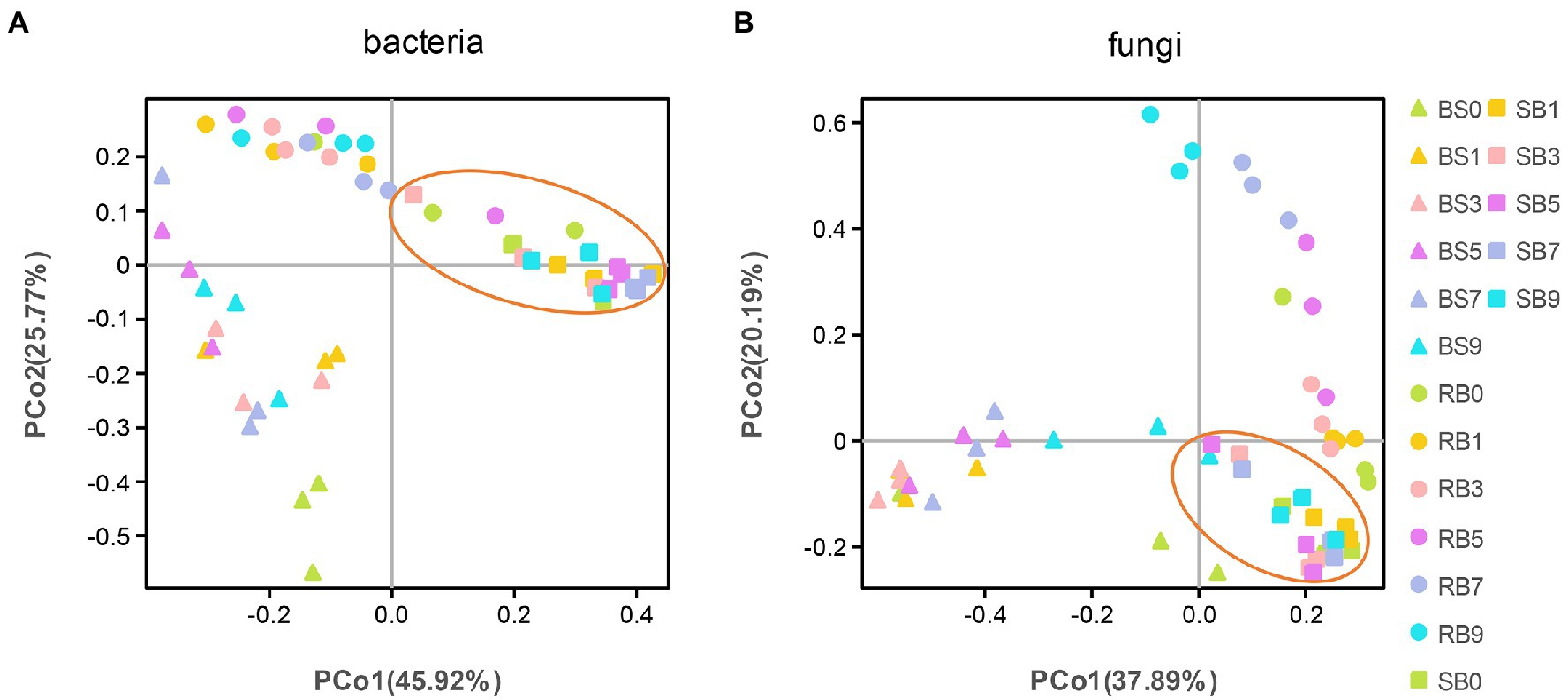
Figure 2. (A, B) Principal Coordinate Analysis (PCoA) of the bacterial and fungal community of the WPCS during different aerobic period. BS, Bunker silo; RB, Round bale; SB, silage bag. 0, 1, 3, 5, 7, 9: 0, 1, 3, 5, 7, 9 days of aerobic exposure, respectively.
3.4. Bacterial community dynamics in the WPCS during aerobic exposure
Alterations in the bacterial community at the phylum level after aerobic exposure are presented in Figure 3A. Overall, Proteobacteria and Firmicutes were the dominant phyla in all of the samples, covering more than 80% of the total sequences observed. The abundance of Proteobacteria increased dramatically in the BS and RB groups within 1 day of aerobic exposure, and Proteobacteria then became the most abundant phylum with prolonged exposure days. However, Firmicutes remained the most abundant phylum in the SB group despite exposure to the air.
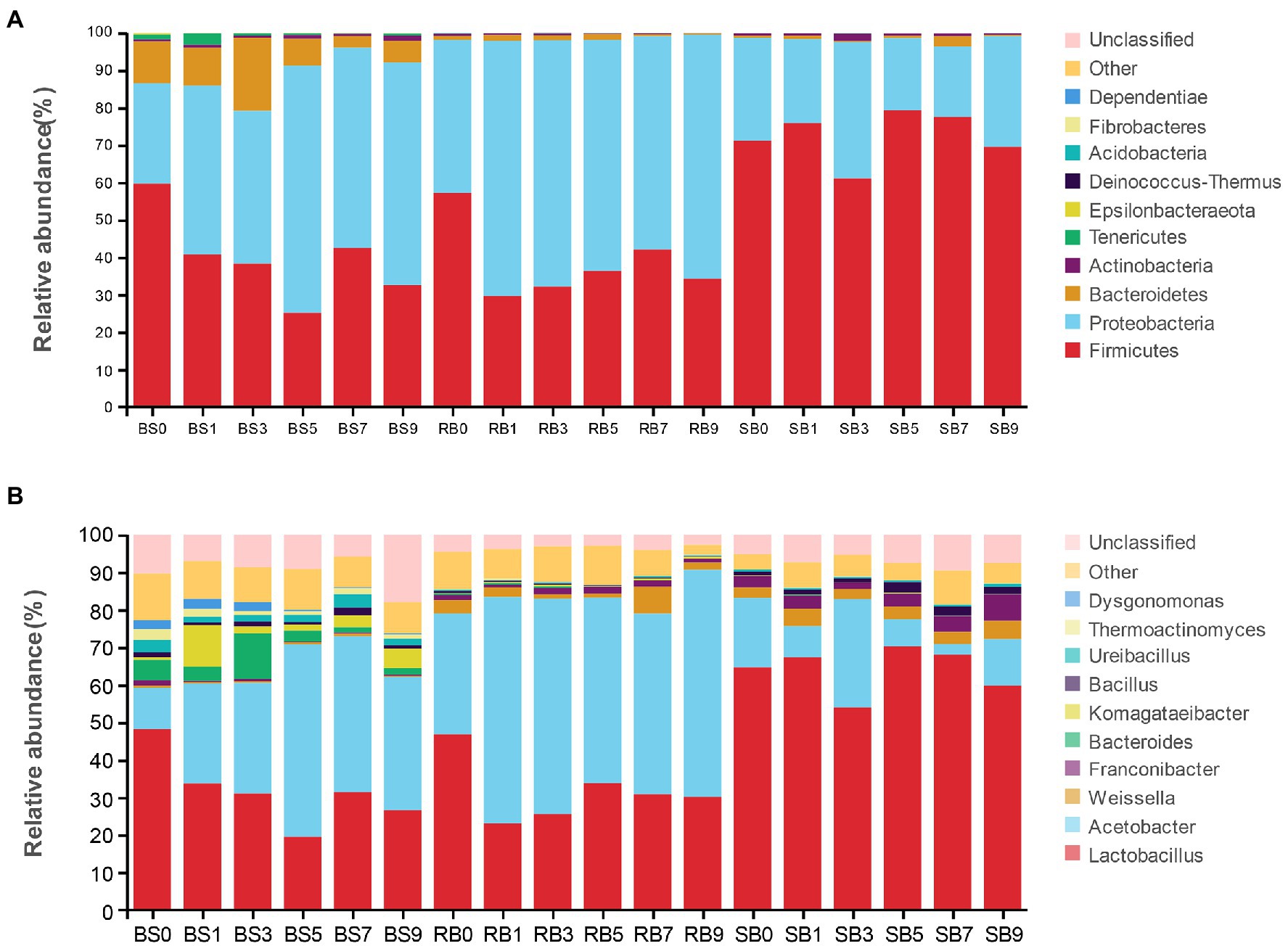
Figure 3. The dynamic bacterial community of the WPCS during aerobic exposure. The bacterial communities were shown at the phylum level (A) and the genus level (B). BS, Bunker silo; RB, Round bale; SB, silage bag. 0, 1, 3, 5, 7, 9: 0, 1, 3, 5, 7, 9 days of aerobic exposure, respectively.
As shown in Figure 3B, the relative abundances of the bacterial community were further analyzed at the genus level. Lactobacillus and Acetobacter were the most dominant genera in all treatments. The abundance of the genus Lactobacillus in the BS group decreased significantly with increasing exposure time, while the relative abundance of Acetobacter increased. The relative abundance of Acetobacter in the RB group increased to 60.41%, which was significantly higher than that in the other groups after 9 days of aerobic exposure. Furthermore, the abundance of Lactobacillus decreased dramatically on the first day of aerobic exposure, and then kept a stable relative abundance in the RB group. However, Lactobacillus was still the most abundant bacteria in the SB group during aerobic exposure, and its relative abundance remained above 53.93%. Conspicuous changes were observed that the relative abundance of Weissella in the BS group was lower than that in the other groups, while the relative abundance of Bacillus in the SB group increased after 9 days of exposure, and was significantly higher than that in the other groups.
3.5. Fungal community dynamics in WPCS during aerobic exposure
Changes in the fungal community at the phylum level during aerobic exposure are shown in Figure 4A. The phyla Ascomycota, Basidiomycota, Anthophyta, Mortierellomycota, and Mucoromycota were identified in the WPCS. Overall, the predominant ITS detected sequence belonged to the Ascomycota phylum and was followed by lower Basidiomycota, together covering more than 88% of the total sequences observed. Ascomycota was the most abundant phylum in all groups and was also the dominant phylum in the WPCS during the aerobic exposure process.
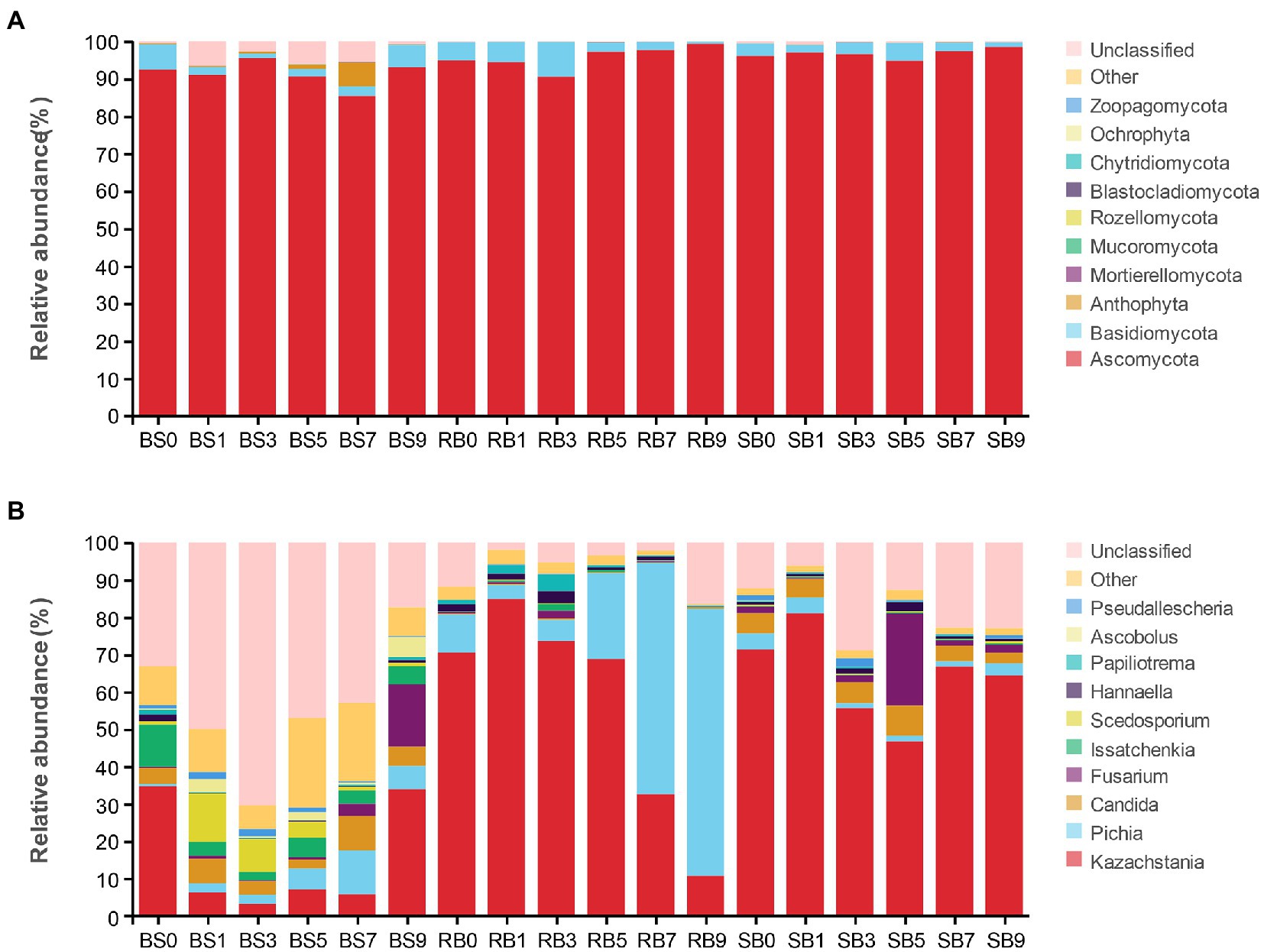
Figure 4. The dynamic fungal community of the WPCS during aerobic exposure. The fungal communities were shown at the phylum level (A) and the genus level (B). BS, Bunker silo; RB, Round bale; SB, silage bag. 0, 1, 3, 5, 7, 9: 0, 1, 3, 5, 7, 9 days of aerobic exposure, respectively.
As shown in Figure 4B, at the genus level, Kazachstania and Pichia were the dominant genera in the RB group. The abundance of Kazachstania in the RB group increased dramatically after 1 day of exposure and then continuously decreased until 9 days. Conversely, the abundance of Pichia first decreased within 1 day of aerobic exposure, and then continuously increased until day 9. Pichia became the dominant fungus instead of Kazachstania after 7 days of exposure. However, neither Kazachstania nor Pichia showed a remarkable dominance in fungal communities in the BS group from days 1–7. Fusarium increased significantly, ranging from 0.64 to 16.72%, from days 5–9 in the BS group. In addition to BS and RB groups, Kazachstania was the most abundant fungus in the SB group, followed by Candida, Pichia and Fusarium during the whole aerobic exposure. Moreover, the relative abundance of Kazachstania was significantly higher in the SB group than in the other groups from days 7–9. The relative abundance of Pichia was observed to be lower in the SB group than in the other groups after aerobic exposure.
3.6. Correlation of the bacterial community with fermentation characteristics and the fungal community
Spearman’s correlation between the bacterial community and fermentation is shown in Figure 5A. Specifically, the pH value was negatively correlated with the relative abundances of Lactobacillus and Weissella (p < 0.001) and positively correlated with the Acetobacter abundance (p < 0.01). The LA concentration was significantly positively associated with the abundances of Lactobacillus and Weissella (p < 0.001). The AA content was positively correlated with Acetobacter and Exiguobacterium abundances (p < 0.05) but negatively correlated with Bacillus and Ureibacillus abundances (p < 0.001). The contents of PA and BA were both negatively associated with Lactobacillus, Weissella and Franconibater abundances (p < 0.001) and positively correlated with Bacteroides, Thermoactinomyces and Dysgonomonas abundances (p < 0.001). Finally, the ammonia-N concentration was negatively correlated with Lactobacillus abundances (p < 0.001) but positively correlated with Acetobacter abundances (p < 0.01).
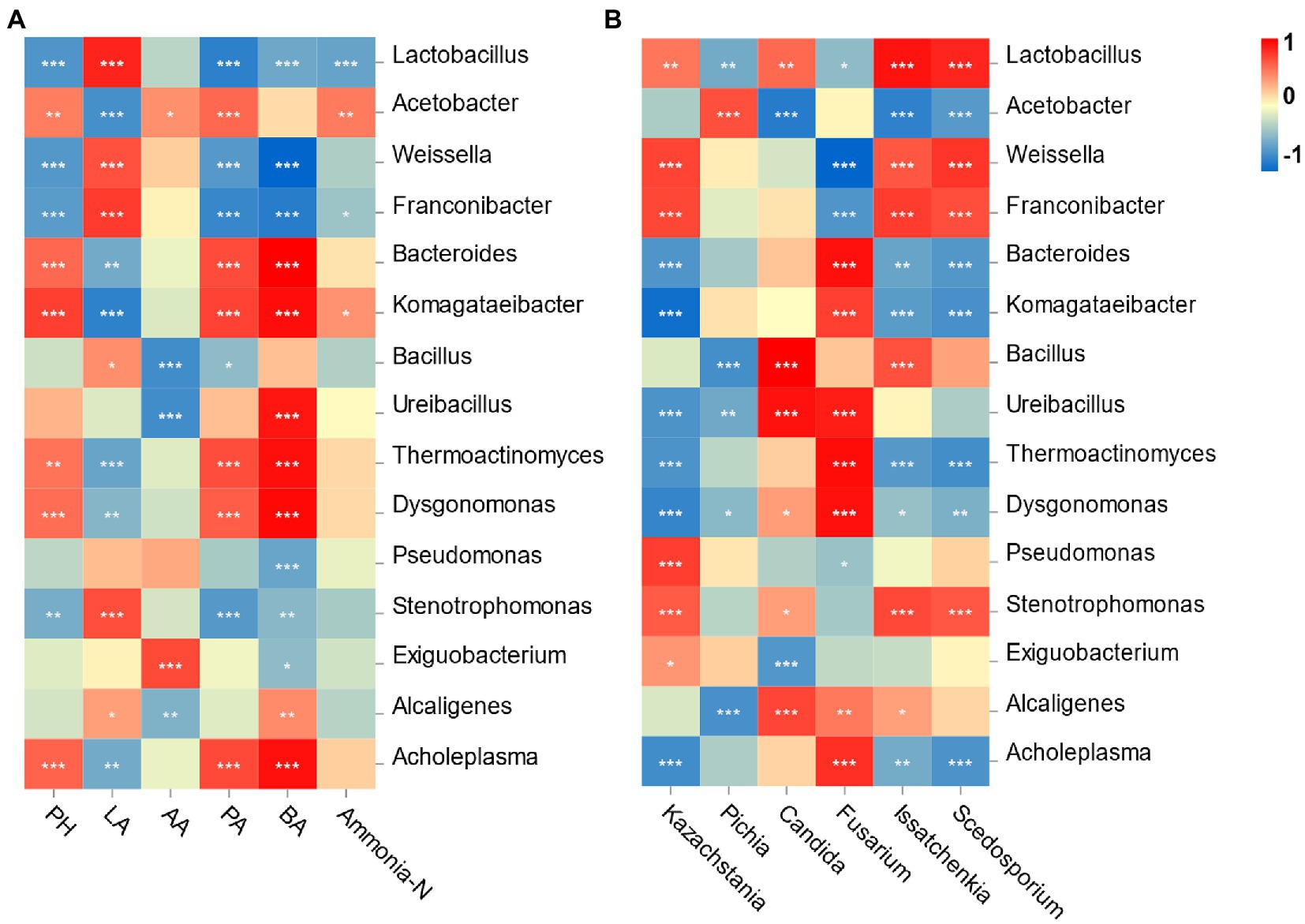
Figure 5. Correlation analysis of the bacterial genus with fermentation characteristic and the top 6 fungi genus. (A) X and Y axis are fermentation factors and bacterial genus, respectively; (B) X and Y axis are genera of fungi and bacteria, respectively. Red squares represent positive correlation (0 < r < 1), whereas blue squares represent negative correlation (−1 < r < 0). The value of p < 0.05 is marked with “*,” p < 0.01 is marked with “**,” p < 0.001 is marked with “***.”
Correlation analysis was performed to illustrate the relationship between bacteria and fungi at the genus level (Figure 5B). Lactobacillus and Weissella were positively correlated with Kazachstania (p < 0.01) but negatively correlated with Fusarium (p < 0.05). Candida was positively associated with Lactobacillus (p < 0.01) but negatively associated with genus Acetobacter (p < 0.001). Pichia was positively correlated with the genera Acetobacter (p < 0.001) but negatively correlated with Lactobacillus (p < 0.01).
4. Discussion
4.1. Effect of different ensiling methods on the fermentation quality, chemical composition and aerobic stability of WPCS after aerobic exposure
Aerobic deterioration is a process that causes nutrient degradation (De Melo et al., 2023). The DM content increased first after aerobic exposure. This was caused by the water and volatile organic compounds in silage volatilized after exposed to the external environment (Merkevičiūte-Venslovė et al., 2023). As expect, the contents of DM, WSC and CP decreased after 9 days of aerobic exposure in this study. DM loss was achieved through the activity of yeasts and molds consuming residual WSC and LA during aerobic exposure (Muck et al., 1991). The higher contents of DM, WSC and LA in the SB group indicated the nutrients were well stored. Limited air exposure due to strong compaction, may illustrate the phenomenon of reduced nutrient degradation in the SB group. Conversely, the contents of ADF and NDF increased during aerobic exposure, which was indirectly caused by the loss of WSC in the WPCS (Randby et al., 2020).
Fermentation characteristics illustrate the fermentation quality of silages (Hao et al., 2022). The pH value of silage is a basic indicator to evaluate microbial activity, and well-fermented WPCS should not exceed a pH of 4.2 (Kung et al., 2018). During 1 days of aerobic exposure, silage was in an unstable state due to the fierce competition between LAB and yeasts. The previous study demonstrated that various organic acids were produced to compete with yeasts and improve the aerobic stability during aerobic exposure (Hu et al., 2020). Hence, the pH value was decreased at 1 day of aerobic exposure. After exposed to the air, the environment changed to aerobic conditions, and oxygen activated the lactate-assimilating yeasts in the WPCS. These yeasts consumed the LA concentration and increased pH value during aerobic exposure (Kung et al., 2003). However, the SB group did not exceed the pH value of 4.2 after 9 days of exposure. This result could be due to the higher LA content and relative abundance of lactic acid bacteria (LAB) observed in SB group than in BS and RB groups (Figure 3B). The higher density of SB may also reduce the entry of oxygen (Tian et al., 2020). Moreover, BA was not observed in the RB and SB groups. The presence of BA may be caused by the metabolic activity of harmful microorganisms, accompanied by substantial DM losses in silages. The contents of ammonia-N in all groups increased with prolonged aerobic exposure. This result is probably due to protein hydrolysis, which is typically caused by extensive protease (Wang et al., 2022). Higher contents of ammonia-N and lower CP were observed in the BS group than in the other groups, illustrating that amino acid deamination was relatively active in BS silages. Overall, with higher contents of LA and lower pH value and PA and ammonia-N contents, the SB group was considered to have lower fermentation degradation after aerobic exposure than the RB and BS groups. In the study, the better aerobic stability of SB group (202 h) was consistent with its lower pH value and higher silage density among all groups. The greater silage density can enhance the aerobic stability of corn silage (Gallo et al., 2018), and the low pH can also improve the aerobic stability to inhibit spoilage microorganisms (Ranjit and Kung, 2000).
4.2. Effect of different ensiling methods on mycotoxins levels in WPSC after aerobic exposure
Aflatoxins (AFs) are produced by several species of Aspergillus section Flavi (Sweeney and Dobson, 1998). AFB1 is considered the most toxic and carcinogenic AF, since it is highly toxic and causes carcinogenic and mutagenic effects in mammals. In the present study, the contents of AFB1 increased with prolonged exposure times, which was similar to the phenomenon reported by Ferrero et al. (2019). Moreover, lower AFB1 contamination was observed in SB than in RB and BS groups. Proper silage management and well-preserved WPCS are essential to inhibit massive concentrations of AFB1. In addition, a higher abundance of LAB (Figure 3B) may also be considered to play a crucial role to limit the accumulation of mycotoxins in SB (Lahtinen et al., 2004).
Fusarium toxins are known to be frequently found in corn and animal feed globally (Vaičiulienė et al., 2022). T-2, DON, FB1 and ZEN are secondary metabolites primarily produced by several members of Fusarium (Ogunade et al., 2018). The accumulation of oxygen contents allows for spoilage and toxigenic microorganisms to grow continually with prolonged aerobic exposure (Driehuis et al., 2018). Therefore, the concentrations of mycotoxins produced by undesirable microorganisms increased significantly in all groups. Higher Fusarium toxin contamination was observed in the BS group than the other groups after 9 days of exposure. This may be caused by the higher relative abundance of Fusarium observed in the BS group (Figure 3B). Furthermore, the bunker was prepared on bare ground and thus was highly exposed to the environment (Alonso et al., 2013). A larger exposed area from the ground may cause aerobic microorganism survival and increase the content of mycotoxins in BS silage. Niderkorn et al. (2006) reported that LAB could detoxify Fusarium mycotoxins. Thus, the higher abundance of Lactobacillus in the SB group than in the other groups may result in reduced accumulation of Fusarium mycotoxins. Overall, the contents of AFB1 T-2, ZEN, FB1 and DON in all groups did not exceed the guidance level of adult ruminants after 9 days of exposure (Commission of the European Community, 2006). The silages were opened for exposure from Dec. to Jan., and ambient temperature was detected from 0 to 7.5°C. High temperatures are known to promote aggressive fungal growth (Ogunade et al., 2018). Hence, the lower temperature in the current study may have limited the synthesis of mycotoxins.
4.3. Dynamic changes in microbial communities during aerobic exposure
The quality of natural silage depends on the complex composition of the microflora, and the composition of the bacterial community in silage is different at various stages (Yang et al., 2019; Ran et al., 2022). The dominant bacteria at the phylum level were Firmicutes and Proteobacteria in the WPCS during aerobic exposure (Hu et al., 2018), which was supported by the present study. Proteobacteria became the dominant phylum instead of Firmicutes in the BS and RB groups after 1 day of exposure. This result indicated that the WPCS involved a notable shift in the bacterial community from Firmicutes to Proteobacteria after the environment changed from anaerobic to aerobic (Liu et al., 2019). At the genus level, Acetobacter showed a predominance of the BS and RB groups during oxygen exposure. Acetobacter are often regarded as an initiation of aerobic deterioration, which causes silage deterioration and further limits Lactobacillus proliferation (Guan et al., 2018). A high abundance of Acetobacter is usually found in bunker silages (Wang et al., 2014), which was observed in the current study. However, Lactobacillus still dominated the structure of the bacterial community in the SB group, although the abundance of Lactobacillus decreased after 9 days of oxygen exposure. Lactobacillus is well known as a LAB in maize silages and has been reported by many studies (Guan et al., 2018; Hu et al., 2018; Huang et al., 2021). Lactobacillus could inhibit fungal growth, and prevent them from becoming dominant microorganisms in the early stage of aerobic exposure (Guan et al., 2020). In addition, Lactobacillus has an excellent ability to remove mycotoxins (Hathout and Aly, 2014). A higher relative abundance of Bacillus was also detected in the SB group during aerobic exposure. Some Bacillus species can produce bacteriocin to inhibit the growth of some undesirable microbes (Lara et al., 2016). Moreover, these bacteria have also been found to be effective in the removal of mycotoxins from liquid medium (Hathout and Aly, 2014). Therefore, the lower mycotoxin level and undesirable microbes in the SB group than in the other groups may be caused by the higher relative abundances of Bacillus and Lactobacillus.
Due to the response to oxygen exposure, various fungi began to compete with the dominance of the microbial community in silages. The majority of fungi in corn silage belonged to Ascomycota, followed by Basidiomycota (May et al., 2001). At the genus level, Kazachstania, Pichia and Candida were the top 3 most abundant fungi in the WPCS (Xu et al., 2019). Kazachstania, Pichia, and Candida belong to the Saccharomycetes, regarded as yeasts frequently through culture-based methods (Wang et al., 2020). It is well known that Pichia species are often considered the major initiators of silage aerobic deterioration. The predominant fungal genus shifted to Pichia in the RB group after aerobic exposure, illustrating that the composition of the fungal community was significantly changed during oxygen exposure. Kazachstania is usually observed as the dominate genus in WPCS after aerobic exposure and is associated with the deterioration of silage (Dolci et al., 2011; Xu et al., 2019). Candida can assimilate LA and promote WPCS deterioration (Pahlow et al., 2003). To respond to oxygen exposure, yeasts became active and utilized various organic acids, resulting in a continual increase in pH value. Thus, the proliferation of massive spoilage microorganisms in silages was promoted, leading to silage deterioration and mycotoxin accumulation (McAllister et al., 1995). It is also worth noting that Fusarium appeared in silages during aerobic exposure. This result may also cause mycotoxin accumulation, as shown in Table 3.
4.4. Correlation analysis of the bacterial community with fermentation characteristics and the fungal community
Silage quality was influenced by the bacterial community through a series of metabolites. The Lactobacillus abundance had a positive correlation with the concentration of LA (Huang et al., 2021), and Weissella and Lactobacillus abundance showed a negative correlation with the pH value (Yang et al., 2019). The results of these studies were supported by the current research. Acetobacter can consume ethanol to produce AA after exposure to air (Nanda et al., 2001). This can be proven by the content of AA being positively correlated with Acetobacter, and higher contents of AA were found in the RB group. The positive correlation between Acetobacter and ammonia-N contents illustrated that the existence of Acetobacter probably caused the degradation of protein in this study.
It is also important to explore the relationship between bacteria and fungi to understand the decomposition of WPCS. Yeasts play a vital role in facilitating the symbiosis of various microorganisms (Alvarez-Martin et al., 2008). After aerobic exposure, there was a synergistic effect between yeasts and LAB (Roostita and Fleet, 1996). Kazachstania and Candida can metabolize organic acids, and metabolites associated with nutrients help LAB grow. In addition, LAB could also promote a suitable environment for Kazachstania and Candida to multiply after aerobic exposure (Wang et al., 2020). The accumulation of Fusarium-derived mycotoxins is caused by the metabolic activity of toxigenic strains of Fusarium genus, and AFB 1 is mainly produced by toxigenic Aspergillus species (Kalúzová et al., 2022). However, the direct relationship between the Aspergillus and AFB 1 in WPCS was not found. Similarly, the previous study also reported that no correlations were observed between fungal DNA and mycotoxin contents (Vandicke et al., 2021). A negative correlation of Fusarium with Lactobacillus and Weissella was found in this study. It is known that Fusarium cannot tolerate a low pH environment (Cheli et al., 2013). Therefore, the higher relative abundance of Lactobacillus and lower pH in the SB group than in the other groups may have inhibited the proliferation of Fusarium and further limited the accumulation of Fusarium mycotoxins.
5. Conclusion
This study analyzed the effects of different ensiling methods on nutritional characteristics, fermentation quality, aerobic stability, mycotoxin levels and microbial communities in whole-plant corn silage after aerobic exposure. The WPCS in SB group deteriorated later than other groups, and the losses of DM, CP and WSC contents in SB silage were also less serious. A considerably lower proportion of mycotoxins was observed in SB than in BS groups after 9 days of aerobic exposure, indicating the higher safety of SB to ruminants. The fungal community was affected by different ensiling methods. After 9 days of aerobic exposure, Pichia was the predominant fungal genus in the RB group, while Kazachstania was the most abundant fungus in the SB group. Although exposed to air, Lactobacillus still dominated the bacterial community of SB and reduced fermentation degradation, as shown by a higher level of LA and a lower level of BA, ammonia-N and pH value. Therefore, with a lower pH value and the dominance of Lactobacillus in SB, the proliferation of toxic microorganisms was limited.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
G-hX, JH, and M-zZ designed the experiment. G-hX, YH, and C-rW performed the experiment, analysis, and writing. FY, H-yY, CC, and JH performed the editing and revision. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by Scientific Research Cultivation Project of Guizhou University [(2020)17], Major Special Project of Science and Technology of Guizhou Province [(2020)3009-2], Science and Technology Support project of Guizhou Province [(2021)-043], and Guizhou Talent Base of Grassland Ecological Animal Husbandry (RCJD2018-13).
Acknowledgments
We are grateful for the sequencing platform and bioinformation analysis of Gene Denovo Biotechnology Co., Ltd. (Guangzhou, China).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alonso, V. A., Pereyra, C. M., Keller, L. A. M., Dalcero, A. M., Rosa, C. A. R., Chiacchiera, S. M., et al. (2013). Fungi and mycotoxins in silage: an overview. J. Appl. Microbiol. 115, 637–643. doi: 10.1111/jam.12178
Alvarez-Martin, P., Florez, A. B., Hernandez-Barranco, A., and Mayo, B. (2008). Interaction between dairy yeasts and lactic acid bacteria strains during milk fermentation. Food Control 19, 62–70. doi: 10.1016/j.foodcont.2007.02.003
Broderick, G. A., and Kang, J. H. (1980). Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63, 64–75. doi: 10.3168/jds.S0022-0302(80)82888-8
Cai, Y., Tang, R., Tian, L., and Chang, S. X. (2021). Environmental impacts of livestock excreta under increasing livestock production and management considerations: implications for developing countries. Curr. Opin. Environ. Sci. Health. 24:100300. doi: 10.1016/j.coesh.2021.100300
Cheli, F., Campagnoli, A., and Dell'Orto, V. (2013). Fungal populations and mycotoxins in silages: from occurrence to analysis. Anim. Feed Sci. Technol. 183, 1–16. doi: 10.1016/j.anifeedsci.2013.01.013
Chen, H., He, L., Liu, T., and Yang, L. (2022). Research progress of effects of mycotoxin on intestinal function and its mechanism. Chin. J. Anim. Nutr. 34, 1–11. doi: 10.3969/j.issn.1006-267x.2022.02.012
Commission of the European Community . (2006). On the presence of deoxynivalenol, zearalenone, ochratoxin a, T-2 and HT-2 and fumonisins in products intended for animal feeding. Available at: https://eur-lex.europa.eu/eli/reco/2006/576/oj/ (accessed August 26, 2006).
Da Silva, T. C., Smith, M. L., Barnard, A. M., and Kong, L. (2015). The effect of a chemical additive on the fermentation and aerobic stability of high-moisture corn. J. Dairy Sci. 98, 8904–8912. doi: 10.3168/jds.2015-9640
De Melo, N. N., Carvalho-Estrada, P. D. A., Tavares, Q. G., Pereira, L. D. M., Delai Vigne, G. L., Camargo Rezende, D. M. L., et al. (2023). The effects of short-time delayed sealing on fermentation, aerobic stability and chemical composition on maize silages. Agronomy 13:223. doi: 10.3390/agronomy13010223
Dolci, P., Tabacco, E., Cocolin, L., and Borreani, G. (2011). Microbial dynamics during aerobic exposure of corn silage stored under oxygen barrier or polyethylene films. Appl. Environ. Microbiol. 77, 7499–7507. doi: 10.1128/AEM.05050-11
Driehuis, F., Wilkinson, J. M., Jiang, Y., Ogunade, I., and Adesogan, A. T. (2018). Silage review: animal and human health risks from silage. J. Dairy Sci. 101, 4093–4110. doi: 10.3168/jds.2017-13836
FAO (2019). Why is feed safety important? Available at: http://www.fao.org/feed-safety/background/why-feed-safety/en/
Ferrero, F., Prencipe, S., Spadaro, D., Gullino, M. L., Cavallarin, L., Piano, S., et al. (2019). Increase in aflatoxins due to aspergillus section flavi multiplication during the aerobic deterioration of corn silage treated with different bacteria inocula. J. Dairy Sci. 102, 1176–1193. doi: 10.3168/jds.2018-15468
Gallo, A., Bernardes, T. F., Copani, G., Fortunati, P., Giuberti, G., Bruschi, S., et al. (2018). Effect of inoculation with Lactobacillus buchneri lb1819 and Lactococcus lactis o224 on fermentation and mycotoxin production in maize silage compacted at different densities. Anim. Feed Sci. Technol. 246, 36–45. doi: 10.1016/j.anifeedsci.2018.09.009
Gallo, A., Masoero, F., Bertuzzi, T., Piva, G., and Pietri, A. (2010). Effect of the inclusion of adsorbents on aflatoxin b1 quantification in animal feedstuffs. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 27, 54–63. doi: 10.1080/02652030903207219
Gheller, L. S., Ghizzi, L. G., Takiya, C. S., Grigoletto, N. T. S., Silva, T. B. P., Marques, J. A., et al. (2021). Different organic acid preparations on fermentation and microbiological profile, chemical composition, and aerobic stability of whole-plant corn silage. Anim. Feed Sci. Technol. 281:115083. doi: 10.1016/j.anifeedsci.2021.115083
Ghilardelli, F., Barbato, M., and Gallo, A. A. (2022). Preliminary study to classify corn silage for high or low mycotoxin contamination by using near infrared spectroscopy. Toxins 14:323. doi: 10.3390/toxins14050323
Guan, H., Shuai, Y., Ran, Q. F., Yan, Y. H., Wang, X., Li, D. D., et al. (2020). The microbiome and metabolome of napier grass silages prepared with screened lactic acid bacteria during ensiling and aerobic exposure. Anim. Feed Sci. Technol. 269:114673. doi: 10.1016/j.anifeedsci.2020.114673
Guan, H., Yan, Y. H., Li, X. L., Li, X. M., Shuai, Y., Feng, G. Y., et al. (2018). Microbial communities and natural fermentation of corn silages prepared with farm bunker-silo in Southwest China. Bioresour. Technol. 265, 282–290. doi: 10.1016/j.biortech.2018.06.018
Hao, J., Sun, W. T., Wu, C. R., Zhang, M. Z., Xia, G. H., Zheng, Y. L., et al. (2022). Fermentation quality, bacterial community, and aerobic stability of perennial recut Broussonetia papyrifera silage with different additives and wilting time. Fermentation 8:226. doi: 10.3390/fermentation8060262
Hathout, A. S., and Aly, S. E. (2014). Biological detoxification of mycotoxins: a review. Ann. Microbiol. 64, 905–919. doi: 10.1007/s13213-014-0899-7
Hu, Z. F., Chang, J., Yu, J. H., Li, S. G., and Niu, H. X. (2018). Diversity of bacterial community during ensiling and subsequent exposure to air in whole-plant maize silage. Asian Australas J. Anim. Sci. 31, 1464–1473. doi: 10.5713/ajas.17.0860
Hu, Z., Niu, H., Tong, Q., Chang, J., Yu, J., Li, S., et al. (2020). The microbiota dynamics of alfalfa silage during ensiling and after air exposure, and the metabolomics after air exposure are effected by Lactobacillus casei and cellulase addition. Front. Microbiol. 11:519121. doi: 10.3389/fmicb.2020.519121
Huang, Y., Liang, L., Dai, S., Wu, C., Chen, C., and Hao, J. (2021). Effect of different regions and ensiling periods on fermentation quality and the bacterial community of whole-plant maize silage. Front. Microbiol. 12:743695. doi: 10.3389/fmicb.2021.743695
Kalúzová, M., Kačániová, M., Bíro, D., Šimko, M., Gálik, B., Rolinec, M., et al. (2022). The change in microbial diversity and mycotoxins concentration in corn silage after addition of silage additives. Diversity 14:592. doi: 10.3390/d14080592
Khan, N. A., Yu, P. Q., Ali, M., Cone, J. W., and Hendriks, W. H. (2015). Nutritive value of maize silage in relation to dairy cow performance and milk quality. J. Sci. Food Agric. 95, 238–252. doi: 10.1002/jsfa.6703
Kung, L. Jr., Taylor, C. C., Lynch, M. P., and Neylon, J. M. (2003). The effect of treating alfalfa with Lactobacillus buchneri 40788 on silage fermentation, aerobic stability, and nutritive value for lactating dairy cows. J. Dairy Sci. 86, 336–343. doi: 10.3168/jds.S0022-0302(03)73611-X
Kung, L., Shaver, R. D., Grant, R. J., and Schmidt, R. J. (2018). Silage review: interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 101, 4020–4033. doi: 10.3168/jds.2017-13909
Lahtinen, S. J., Haskard, C. A., Ouwehand, A. C., Salminen, S. J., and Ahokas, J. T. (2004). Binding of aflatoxin b1 to cell wall components of Lactobacillus rhamnosus strain gg. Food Addit. Contam. 21, 158–164. doi: 10.1080/02652030310001639521
Lara, E. C., Basso, F. C., de Assis, F. B., Souza, F. A., Berchielli, T. T., and Reis, R. A. (2016). Changes in the nutritive value and aerobic stability of corn silages inoculated with bacillus subtilis alone or combined with Lactobacillus plantarum. Anim. Prod. Sci. 56, 1867–1874. doi: 10.1071/AN14686
Liu, B. Y., Huan, H. L., Gu, H. R., Xu, N. X., Shen, Q., and Ding, C. L. (2019). Dynamics of a microbial community during ensiling and upon aerobic exposure in lactic acid bacteria inoculation-treated and untreated barley silages. Bioresour. Technol. 273, 212–219. doi: 10.1016/j.biortech.2018.10.041
Luo, Y., Liu, X. J., and Li, J. K. (2018). Updating techniques on controlling mycotoxins - a review. Food Control 89, 123–132. doi: 10.1016/j.foodcont.2018.01.016
May, L. A., Smiley, B., and Schmidt, M. G. (2001). Comparative denaturing gradient gel electrophoresis analysis of fungal communities associated with whole plant corn silage. Can. J. Microbiol. 47, 829–841. doi: 10.1139/cjm-47-9-829
McAllister, T. A., Selinger, L. B., Mcmahon, L. R., Bae, H. D., Lysyk, T. J., and Oosting, J. (1995). Intake, digestibility and aerobic stability of barley silage inoculated with mixtures of Lactobacillus plantarum and Enterococcus faecium. Can. J. Anim. Sci. 75, 425–432. doi: 10.4141/cjas95-062
Merkevičiūte-Venslovė, L., Venslovas, E., Mankevičienė, A., Šlepetienė, A., and Cesevičienė, J. (2023). Effect of Ustilago maydis on the nutritive value and aerobic deterioration of maize silage. Agronomy 13:111. doi: 10.3390/agronomy13010111
Muck, R. E., Brink, G. E., and Broderick, G. A. (2015). Effects of silo type on ensiling alfalfa. Appl. Eng. Agric. 16, 479–486. doi: 10.13031/aea.31.10994
Muck, R. E., Pitt, R. E., and Leibensperger, R. Y. (1991). A model of aerobic fungal growth in silage. Grass Forage Sci. 46, 283–299. doi: 10.1111/j.1365-2494.1991.tb02234.x
Nanda, K., Taniguchi, M., Ujike, S., Ishihara, N., Mori, H., Ono, H., et al. (2001). Characterization of acetic acid bacteria in traditional acetic acid fermentation of rice vinegar (komesu) and unpolished rice vinegar (kurosu) produced in Japan. Appl. Environ. Microbiol. 67, 986–990. doi: 10.1128/AEM.67.2.986-990.2001
Niderkorn, V., Boudra, H., and Morgavi, D. P. (2006). Binding of fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Microbiol. 101, 849–856. doi: 10.1111/j.1365-2672.2006.02958.x
Ogunade, I. M., Martinez-Tuppia, C., Queiroz, O. C. M., Jiang, Y., Drouin, P., Wu, F., et al. (2018). Silage review: mycotoxins in silage: occurrence, effects, prevention, and mitigation. J. Dairy Sci. 101, 4034–4059. doi: 10.3168/jds.2017-13788
Pahlow, G., Muck, R. E., Driehuis, F., Elferink, S. J. W. H. O., and Spoelstra, S. F. (2003). “Microbiology of ensiling” in Silage science and technology. eds. D. R. Buxton, R. E. Muck, and J. H. Harrison (Madison, WI: American Society of Agronomy), 31–93.
Ran, Q., Guan, H., Li, H., He, W., Zhu, R., Zhang, L., et al. (2022). Effect of formic acid and inoculants on microbial community and fermentation profile of wilted or un-wilted Italian ryegrass silages during ensiling and aerobic exposure. Fermentation 8:755. doi: 10.3390/fermentation8120755
Randby, A. T., and Bakken, A. K. (2021). Bunkers or round bales: Losses and silage quality with or without acid treatment of low dry matter grass crops. Anim. Feed Sci. Technol. 275:114868. doi: 10.1016/j.anifeedsci.2021.114868
Randby, A. T., Halvorsen, H. N., and Bakken, A. K. (2020). Losses and grass silage quality in bunker silos compacted by tractor versus wheel loader. Anim. Feed Sci. Technol. 266:114523. doi: 10.1016/j.anifeedsci.2020.114523
Ranjit, N. K., and Kung, L. (2000). The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 83, 526–535. doi: 10.3168/jds.S0022-0302(00)74912-5
Roostita, R., and Fleet, G. H. (1996). The occurrence and growth of yeasts in camembert and blue-veined cheeses. Int. J. Food Microbiol. 28, 393–404. doi: 10.1016/0168-1605(95)00018-6
Sweeney, M. J., and Dobson, A. D. (1998). Mycotoxin production by aspergillus, fusarium and penicillium species. Int. J. Food Microbiol. 43, 141–158. doi: 10.1016/S0168-1605(98)00112-3
Tian, J. P., Xu, N. X., Liu, B. Y., Huan, H. L., Gu, H. R., Dong, C. F., et al. (2020). Interaction effect of silo density and additives on the fermentation quality, microbial counts, chemical composition and in vitro degradability of rice straw silage. Bioresour. Technol. 297:122412. doi: 10.1016/j.biortech.2019.122412
Toju, H., Tanabe, A. S., Yamamoto, S., and Sato, H. (2012). High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples. PLoS One 7:e40863. doi: 10.1371/journal.pone.0040863
Turula, V. E., Gore, T., Singh, S., and Arumugham, R. G. (2010). Automation of the anthrone assay for carbohydrate concentration determinations. Anal. Chem. 82, 1786–1792. doi: 10.1021/ac902664x
Unnevehr, L. (2015). Food safety in developing countries: Moving beyond exports. Glob Food Secur Agr. 4, 24–29. doi: 10.1016/j.gfs.2014.12.001
Vaičiulienė, G., Bakutis, B., Jovaišienė, J., Falkauskas, R., Gerulis, G., Bartkienė, E., et al. (2022). Effects of ethanol extracts of origanum vulgare and thymus vulgaris on the mycotoxin concentrations and the hygienic quality of maize (Zea mays L.) silage. Toxins 14:298. doi: 10.3390/toxins14050298
Vandicke, J., Visschere, K. D., Ameye, M., Croubels, S., Saeger, S. D., Audenaert, K., et al. (2021). Multi-mycotoxin contamination of maize silages in Flanders, Belgium: monitoring mycotoxin levels from seed to feed. Toxins 13:202. doi: 10.3390/toxins13030202
Wang, C., Han, H. Y., Gu, X. Y., Yu, Z., and Nishino, N. (2014). A survey of fermentation products and bacterial communities in corn silage produced in a bunker silo in China. Anim. Sci. J. 85, 32–36. doi: 10.1111/asj.12076
Wang, W., Tan, Z., Gu, L., Ma, H., Wang, Z., Wang, L., et al. (2022). Variation of microbial community and fermentation wuality in corn silage treated with lactic acid bacteria and artemisia argyi during aerobic exposure. Toxins 14:349. doi: 10.3390/toxins14050349
Wang, T. W., Teng, K. L., Cao, Y. H., Shi, W. X., Xuan, Z. Y., Zhou, J. H., et al. (2020). Effects of Lactobacillus hilgardii 60ts-2, with or without homofermentative Lactobacillus plantarum b90, on the aerobic stability, fermentation quality and microbial community dynamics in sugarcane top silage. Bioresour. Technol. 312:123600. doi: 10.1016/j.biortech.2020.123600
World Health Organization (2015). WHO estimates of the global burden of foodborne diseases: Foodborne disease burden epidemiology reference group 2007–2015. Available at: https://www.who.int/publications/i/item/9789241565165/ (accessed Dcember 1, 2015).
Wu, C., Sun, W., Huang, Y., Dai, S., Peng, C., Zheng, Y., et al. (2022). Effects of different additives on the bacterial community and fermentation mode of whole-plant paper mulberry silage. Front. Microbiol. 13:904193. doi: 10.3389/fmicb.2022.904193
Xu, S. W., Yang, J. L., Qi, M., Smiley, B., Rutherford, W., Wang, Y. X., et al. (2019). Impact of Saccharomyces cerevisiae and Lactobacillus buchneri on microbial communities during ensiling and aerobic spoilage of corn silage. J. Anim. Sci. 97, 1273–1285. doi: 10.1093/jas/skz021
Keywords: whole-plant corn silage, aerobic exposure, microbial community, mycotoxins, fermentation
Citation: Xia G-h, Huang Y, Wu C-r, Zhang M-z, Yin H-y, Yang F, Chen C and Hao J (2023) Characterization of mycotoxins and microbial community in whole-plant corn ensiled in different silo types during aerobic exposure. Front. Microbiol. 14:1136022. doi: 10.3389/fmicb.2023.1136022
Edited by:
Siran Wang, Nanjing Agricultural University, ChinaReviewed by:
Gentu Ge, Inner Mongolia Agricultural University, ChinaShuai Du, Zhejiang University, China
Copyright © 2023 Xia, Huang, Wu, Zhang, Yin, Yang, Chen and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Hao, amhhb0BnenUuZWR1LmNu
 Guang-hao Xia1
Guang-hao Xia1 Yuan Huang
Yuan Huang Chang-rong Wu
Chang-rong Wu Jun Hao
Jun Hao