
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Microbiol., 16 March 2023
Sec. Microbiotechnology
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1134078
This article is part of the Research TopicMicrobiology of Radioactive EnvironmentsView all 6 articles
 Miguel A. Ruiz-Fresneda*
Miguel A. Ruiz-Fresneda* Marcos F. Martinez-Moreno
Marcos F. Martinez-Moreno Cristina Povedano-Priego
Cristina Povedano-Priego Mar Morales-Hidalgo
Mar Morales-Hidalgo Fadwa Jroundi
Fadwa Jroundi Mohamed L. Merroun
Mohamed L. MerrounTo date, the increasing production of radioactive waste due to the extensive use of nuclear power is becoming a global environmental concern for society. For this reason, many countries have been considering the use of deep geological repositories (DGRs) for the safe disposal of this waste in the near future. Several DGR designs have been chemically, physically, and geologically well characterized. However, less is known about the influence of microbial processes for the safety of these disposal systems. The existence of microorganisms in many materials selected for their use as barriers for DGRs, including clay, cementitious materials, or crystalline rocks (e.g., granites), has previously been reported. The role that microbial processes could play in the metal corrosion of canisters containing radioactive waste, the transformation of clay minerals, gas production, and the mobility of the radionuclides characteristic of such residues is well known. Among the radionuclides present in radioactive waste, selenium (Se), uranium (U), and curium (Cm) are of great interest. Se and Cm are common components of the spent nuclear fuel residues, mainly as 79Se isotope (half-life 3.27 × 105 years), 247Cm (half-life: 1.6 × 107 years) and 248Cm (half-life: 3.5 × 106 years) isotopes, respectively. This review presents an up-to-date overview about how microbes occurring in the surroundings of a DGR may influence their safety, with a particular focus on the radionuclide-microbial interactions. Consequently, this paper will provide an exhaustive understanding about the influence of microorganisms in the safety of planned radioactive waste repositories, which in turn might improve their implementation and efficiency.
The risk associated to the generation and storage of radioactive waste produced by the activities of nuclear power plants is an environmental problem that must be seriously considered. It is well known that these kinds of residues contain radionuclide contaminated materials, which must be contained and managed for a long period of time until their radio toxicity decreases to natural levels. For this purpose, deep geological repositories (DGRs) have been proposed by many countries as the most immediate and safest option for their disposal. This multi-barrier system is based on the encapsulation of the radioactive waste in containers of steel, copper, titanium and nickel-based materials, which will be placed underground at depths of 500–1,000 m (IAEA, 2018; Hall et al., 2021). In addition, the containers will be surrounded by engineered (bentonite clay, cementitious materials, etc.) and natural (host rocks) barriers for their mechanical, hydraulic, and thermal protection. Indeed, clay formations will play a crucial role in many DGR designs as a host rock and engineered barriers will be used in many countries, including France, Spain, Belgium, and Switzerland. Specifically in Spain, bentonite clay from the El Cortijo de Archidona (Almería, Spain) has been selected as a reference material for the engineered barriers due to its well-characterized physical and geochemical properties (Villar et al., 2006).
A wide distribution of microorganisms has been previously reported in many materials selected to be used as barriers in the DGRs, including bentonite formations (Mijnendonckx et al., 2021; Burzan et al., 2022). Many studies have evidenced the role that many microbial processes may play in the corrosion of metal containers, on the clay mineral transformation, gas production, and the mobility of radionuclides present in radioactive waste (Beaton et al., 2019; Lopez-Fernandez et al., 2020; Hall et al., 2021). It is clear that microorganisms could threaten the safety of DGR systems. Several microbial mechanisms such as biotransformation, biosorption, biomineralization, and bioaccumulation are thought to be involved, probably affecting the radionuclide migration behavior throughout the repository. Among the radionuclides present in radioactive waste, selenium (Se), curium (Cm), and uranium (U) stand out as being of great interest. Selenium is a common component of the spent nuclear fuel present mainly as 79Se isotope (half-life 3.27 × 105 years; Hassan et al., 2021). Although other Se isotopes such as 75Se (half-life 120 days) or 80Se (stable isotope) can be found in radioactive residues, only 79Se present in high-level radioactive waste is radiotoxic enough (betta particle emitter) to compromise long-term security (Abdalla et al., 2020). This element can exist in nature in different oxidation states: +VI, +IV, 0, and –II. Selenite (SeIV) and selenate (SeVI) are the most soluble and toxic forms, while elemental Se (Se0) and selenide (Se-II) are very insoluble. Cm is also a highly radiotoxic element present in nuclear spent fuel, mainly as 247Cm and 248Cm isotopes (Kooyman et al., 2018). The excellent luminescence properties of this element, as a representative of trivalent actinides (AnIII), are suitable for the study of their chemical speciation at environmentally relevant concentrations. In the same way, europium (Eu), an inactive analog of AnIII, also provides excellent luminescence properties. This inactivity makes Eu a suitable element for speciation studies of AnIII. Uranium is the main component of the spent nuclear fuel from nuclear plants constituting approximately 95% of their composition (Kumari et al., 2020). Several radioactive isotopes of uranium, such as 238U, 235U, and 234U, are known to have long half-lives (e.g., 4.47 × 109 years for 238U; Mitchell et al., 2013).
This review focuses on a fundamental understanding of the microbial processes including container corrosion, gas production, clay mineral transformation, and direct interaction with radionuclides, in the safety of DGRs. Therefore, it provides useful information in predicting the microbial impact on the performance of the radioactive waste repositories. Furthermore, knowledge about these underlying mechanisms could beessential for the development of effective and accurate bioremediation strategies.
Radioactivity is defined as the process by which certain chemical elements emit radiation because of the spontaneous decay of their atomic nuclei. This phenomenon is characteristic of unstable isotopes, which tend to directly or progressively decay in more stable isotopes, by producing the emission of high energy radiation (Porcelli, 2018). Radioactive isotopes, also known as radionuclides, can emit 3 different types of ionizing radiation: alpha (α), beta (β), and gamma (γ). Electromagnetic waves produced by unstable nuclei are known as gamma radiation. This is the highest penetrating ionizing radiation due to the lack of mass and charge and consequently, the most dangerous to living beings. Only very thick and electron dense materials can retain them. Radioactive contamination of natural habitats may cause detrimental effects on living organisms, depending on several factors, including radiation type, received dose, affected tissue, etc. In human beings, the main radioactivity effect is based on water radiolysis, which triggers the formation of hazardous free radicals due to the ionizing action of the emitted radiation. Free radicals could lead to structural changes in biomolecules, preventing the successful fulfilling of biological functions. For example, irrevocable changes in the chemical structure of DNA would result in different kinds of tumors.
Radioactive isotopes exist naturally in soils, rocks [such as Radon-222 (222Rn)] and in the atmosphere (Kónya and Nagy, 2018; Porcelli, 2018). However, radioactivity is not unique to natural isotopes. Artificially produced radionuclides are much more abundant in the environment. These radioisotopes are mainly due to man-made activities, such as in medical applications in hospitals, research activities, and the nuclear power industry. One of the major global environmental risks associated to the use of these radionuclides derives from nuclear reactor accidents and nuclear waste management (Zhang et al., 2019). The large-scale release of radionuclides to the environment from nuclear activities may cause serious social and environmental problems to society (Prăvălie and Bandoc, 2018). Since 1950, approximately 20 nuclear accidents have occurred in the world (Burns, 2012; Lelieveld et al., 2012), being that of Chernobyl on 26 April 1986, undoubtedly the biggest nuclear disaster ever known. To date, this catastrophic event has contributed to the highest release of radioactive material to the environment (Burns, 2012; UNSCEAR, 2013). Cesium-137 (137Cs) and Iodine-131 (131I) are examples of some of the most dangerous radionuclides emitted during that episode. Not all radionuclides have a deep impact on our lives, but some of them are especially hazardous due to their half-life, type of radiation emitted, concentration, etc. The study of trivalent actinides (AnIII) such as curium (247Cm, 248Cm) or americium (242Am), other actinides such as neptunium (237Np) or uranium (235U), and some significant radioisotopes of selenium (Se), is extremely important due to their high radiotoxicity (Hamed et al., 2017; Kooyman et al., 2018). Among all the AnIII, curium (Cm) is one of the most studied. Cm is a very toxic radionuclide due to the α-activity of some isotopes, such as 247Cm and 248Cm, characteristic of spent nuclear fuel (Gorietti et al., 2017; Kooyman et al., 2018). Many of the studies focused on the study of Cm use europium (Eu) as an inactive analogous of AnIII. This inactivity, together with its excellent luminescent properties, makes Eu an exceptional element for the study of AnIII (Ansoborlo et al., 2007). Most of the Se radioisotopes have a short half-life ranging from 20 s, in the case of 77mSe, to 120 days, in the case of 75Se. The only one of special interest due to its high radioactivity and hazard to the environment and the long-term safety of future DGR, is 79Se (Atwood, 2010). The radioisotope 79Se presents a half-life of about 3.27 × 105 years and is mainly produced in the nuclear industry through fission reactions of 235U and other radionuclides such as 239Pu. As an artificial radionuclide, the only possible source of 79Se in nature would be nuclear accidents or the release of nuclear waste to the environment.
The high global energy demand is considerably increasing nowadays, mainly due to demographic and industrial growth. Almost the total global energy is still supplied by non-renewable sources such as fossil fuels and resources. This fact makes the development of renewable energies or new alternatives such as the nuclear power crucial. Despite the danger of nuclear reactors present, this low-carbon technology could be a great opportunity to minimize the consequences of global warming, one of the toughest environmental problems facing human society today (IAEA, 2015). Obviously, simultaneous strategies based on the establishment of renewable energies are crucial for this purpose. Despite the above mentioned comments, there is a continuous controversy about the use of nuclear energy due to the associated risk involved, such as environmental pollution and the generation and management of radioactive waste. The proper and safe storage of the nuclear residues produced implies a real threat to the environment because a completely safe solution is still non-existent. The IAEA, which is officially in charge of radioactive waste management, classify this in six different types (IAEA, 2009a):
• Exempt waste (EW): contains very low concentration of radionuclides, resulting in their exclusion from the regulatory control of radiation protection.
• Very short-lived waste (VSLW): contains very short half-life radionuclides that must be stored until the end of their radioactivity. It is mainly produced in hospitals and research centers.
• Very low-level waste (VLLW): waste containing a radioisotope concentration slightly higher than EW, which can be disposed in near surface landfill type facilities with limited regulatory control. These residues usually include natural radionuclides from soil, rocks, or the mining industry.
• Low level waste (LLW): includes limited amounts of long-lived radioisotopes, which requires isolation for periods of up to a few hundred years in near surface disposal.
• Intermediate level waste (ILW): waste including long-lived radionuclides in significant quantities to be stored in higher security levels than those provided by near surface facilities. Therefore, this type of residue requires to be isolated at greater depths ranging between tens and hundreds of meters.
• High level waste (HLW): contains large amounts of long-lived radionuclides and requires a greater degree of containment and isolation than ILW to guarantee long-term security. This type of residue is mainly composed of nuclear waste, which is the most hazardous one because of its radioactivity, which may persist for up to 1,000 years (Horvath and Rachlew, 2016). HLW generates significant quantities of heat as a result of the atomic nuclei decay of radionuclides. For this reason, heat dissipation is an important issue to be considered during the design of a repository for this kind of waste. Nowadays, the production of HLW is extremely alarming, since it is estimated that each nuclear reactor (approximately 440 operational worldwide) produces around 30 tons per year (Gerstner, 2009; IAEA, 2009b; Rosa et al., 2010; Alwaeli and Mannheim, 2022).
The high generation rate of radioactive waste is a major environmental concern that needs to be adequately solved as soon as possible. Reprocessing spent nuclear waste is an example to partially solve this problem. According to the IAEA, more than 5,000 tons are reprocessed every year (IAEA, 2009b; Handrlica, 2019). However, it seems evident that this procedure is not enough to reduce the total volume of radioactive waste in the world. In the last few years, the global scientific and political consensus has determined the isolation of radioactive waste in deep geological repositories (DGRs) as being the safest option for its long-term management (NEA, 2020a).
The deep geological repository (DGR) concept was introduced in 1995 by the Nuclear Energy Agency (NEA) with the aim to safely isolate radioactive waste for long-term periods (NEA, 2020b). The DGR system is based on the disposal of radioactive waste at a depth of around 1,000 m underground (Figure 1). Multi-barrier systems composed of natural (soil, host rock, etc.) and artificial barriers such as metal canisters and filling/sealing materials are planned to properly isolate the residues (Ma et al., 2018). Clay, crystalline rock and salt deposits have been studied and selected as the best options for natural barriers due to their excellent physico-chemical properties (Ahonen et al., 2016). France, Belgium, and Switzerland have chosen clay formations as the preferred host rock option for their repositories, while crystalline rock (granite) has been selected by other countries such as Finland, Sweden, Canada, and the Czech Republic (Ahonen et al., 2016). However, the EEUU is considering salt deposits for their geological repository emplacements. Regarding the artificially engineered barriers, copper, steel, and concrete are planned to be used as canister materials, while cementitious materials and bentonite clay formations seem to have the best sealing capabilities. It is worth noting that all these materials would act as a protective shield against radiation (Abrahamsen et al., 2015). In these systems, an initial period with oxic conditions is expected mainly for the introduction of oxygen accumulated during the construction of the disposal facility. However, after the closure of the repository, anoxic and reducing conditions will prevail because of the low oxygen levels underground, the presence of specific electron donors and acceptors, and the dilution of reduced minerals (Duro et al., 2014). For all the above-mentioned reasons, DGR systems provide a stable environment, making them the best solution for the long-term isolation of long-lived radionuclides characterizing HLW.
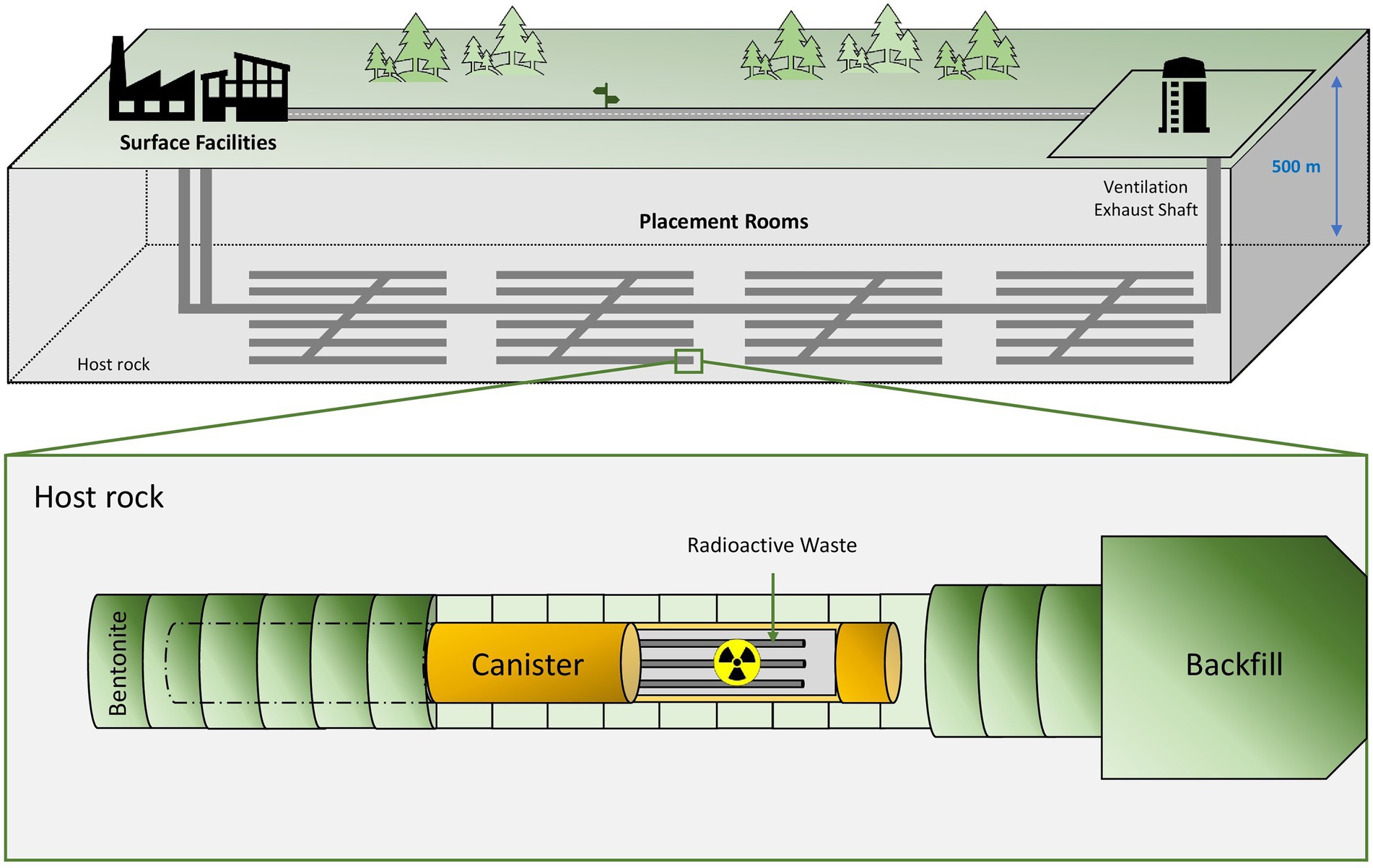
Figure 1. Diagram of the deep geological repository system according to the Nuclear Waste Management Organization (NWMO).
Nowadays, several countries are planning or have already started the construction of their own DGRs (Nuclear Waste Management Organization, 2022). For example, The Netherlands, Ukraine, or Spain are currently investigating the type of geology to be used (crystalline rocks, clays or salt deposits). The United States and South Korea governments have agreed to build a DGR facility, but they are still in the early planning stages. The Nuclear Waste Management Organization (NWMO) of Canada is currently studying about the crystalline and sedimentary site selection both in the province of Ontario. Similarly, the Nuclear Waste Management Organization of Japan (NUMO) has an active program for the site selection. Further, China has announced the pre-selection of a national plan for the storage of radioactive waste in granite geological areas in the Gansu province. Switzerland has also already selected the location of its DGR (north of Zürich), but still expects to submit a license application by 2026. France, one of the biggest nuclear energy producers, is designing through the Agence Nationale pour la gestión des Déchets Radioactifs (ANDRA), a DGR called Cigéo over a clay rock emplacement to the east of the country. License applications are still being prepared for Cigéo and its construction is programmed to begin as early as 2023. Similarly, the Swedish government has recently granted permission to the SKB company to build a deep disposal approximately around the mid-2020s with final disposal structure expected to be finished around 2030. The most advanced country in this respect is Finland, where the Onkalo Facility is expected to begin operating in 2025. The Onkalo DGR will be the first in the world to start the final disposal of spent nuclear fuel. The objective is to store HLW and ILW using a system based on cementitious materials for both containers and sealing materials.
Microorganisms are widely described for their ability to colonize almost every single habitat on earth, and DGRs are no exception. The high diversity of microorganisms in geological formations selected for their optimal use in the disposal of radioactive waste such as clay, granite, and saline deposits, have been reported (Bachran et al., 2018; Lopez-Fernandez et al., 2018a, 2021). Many studies not only about microbial diversity, but also the activity and influence of biochemical processes on the safety of these systems are currently on-going. In those countries where clay has been chosen for their respective DGRs, there have been recent studies. This is the case of Opalinus clay in Switzerland, Boom clay in Belgium, Cavallo-Oxfordian in France, and bentonite clay in Spain (Mijnendonckx et al., 2021; Povedano-Priego et al., 2021; Burzan et al., 2022; Shrestha et al., 2022). Bentonite clay formations have been described as a reference material for their use as artificial barriers in DGRs due to their excellent mineralogic, physic-chemical, mechanistic, geochemical, and hydraulic properties (Villar et al., 2006). Specifically, they present low permeability (decrease in groundwater filtrations), mechanical support (provide stability against container weight), high ion exchange capacity (radionuclide retention), high plasticity, swelling capacity (self-sealing of cracks), thermal conductivity, and optimal compaction properties (García-Romero et al., 2019). The results obtained by Lopez-Fernandez et al. (2014, 2015, 2018b) revealed the high microbial diversity in bentonite formations from the El Cortijo de Archidona (Almería, Spain). Most of the species identified correspond to facultative and obligate aerobic microorganisms. Some of them have been previously described for their ability to interact with radionuclides and heavy metals characteristic of radioactive waste. This is the case of bacterial genera such as Acidovorax, Variovorax, Pseudomonas, Stenotrophomonas, and Ralstonia (Choudhary and Sar, 2009; Hedrich et al., 2011; Hupert-Kocurek et al., 2013; Gerber et al., 2016; Ruiz Fresneda et al., 2018; Ruiz-Fresneda et al., 2020a). Indeed, the bentonite-isolate bacterium Stenotrophomonas bentonitica, exhibits a great versatility and capacity to interact with different elements such as SeIV, CmIII, or UVI through different microbial mechanisms (Lopez-Fernandez et al., 2014; Ruiz Fresneda et al., 2018, 2019, 2020b). Opalinus clay from the Mont Terri rock laboratory (Canton Jura, Switzerland) also shows the presence of relevant microbial activity. Although there are limited conditions for microbial survival in Opalinus clay, a rich and diverse group of microorganisms seem to be ubiquitous. This suggests that many microorganisms could have been introduced mainly as a result of anthropogenic activities including construction, operational activities and experimental installations and also due to natural geological processes such as water infiltration, landslides, fissures, etc. Specifically, sulfate-reducing bacteria (Desulfosporosinus, Desulfotomaculum, Desulfocapsa), nitrate- and nitrite-reducing bacteria (Pseudomonas, Thiobacillus, Acidovorax) and other bacterial genera (Pleomorphomonas, Peptococcaceae, Sphingomonas) have been found (Leupin et al., 2017). Another clay type that has been investigated in depth is Boom clay, considered as a potential host formation in Belgium. Mijnendonckx et al. (2019, 2022) detected the presence of methanogenic Archaea, such as Methanobacterium alcaliphilum and Methanomassiliicoccus luminyensis, nitrate-reducing bacteria (Acidovorax, Simplicispira, Hydrogenophaga), or sulfate reducing bacteria (Pseudomonas), among others.
To summarize, most certainly the DGR systems will not be sterile environments. Not only will indigenous microorganisms from soils, barriers and different materials be present, but also allochthonous microbes introduced accidentally during the construction of the repositories. Although the conditions will not be the best for microbial growth, it should be emphasized that the presence of nutrients, electron donors and acceptors, carbon and nitrogen sources will help the development and metabolic activity of the microorganisms. Even in the presence of ionizing radiation, some microorganisms from sediments have been described to be able to tolerate dose rates representative of gamma radiation emitted from radioactive waste (Brown et al., 2015). Not only microorganisms, but also components of these sediments such as FeIII, NO3-, or SO42- can remain active. Anyway, some microorganisms can immobilize radioactive isotopes through passive processes (biosorption, bioaccumulation, etc.), even if they do not survive radiation (see section 4.4).
Sulfate-reducing, nitrate-reducing, methanogens, iron-reducing, metal-resistant, and other microorganisms will potentially affect the environment and hence the safety of the DGRs through different processes including gas production, metal corrosion, modification of the redox conditions, mineral clay transformation, and radionuclide interaction. For all the above-mentioned reasons, microorganisms must be considered when evaluating the security level of DGRs.
Bacteria can adhere to the surface of different inorganic matrices through the production of extracellular polymeric substances (EPSs) promoting bio-corrosion, known as microbially influenced corrosion (MIC) of materials such as steel, copper, concrete, etc. (Kip and Van Veen, 2015). MIC could be mediated by the direct or indirect action of electrochemical reactions conducted by microorganisms (Videla and Herrera, 2009). Within the concept of the DGRs, the oxygen will be gradually consumed after their closure, so an anoxic environment will be predominant (Rashwan et al., 2022). Under these DGR conditions, sulfate-reducing bacteria (SRB) could obtain energy through the reduction of sulfates or other oxidized inorganic sulfur compounds, resulting in sulfide production (H2S). This is one of the most important biogenic corrosive compounds in the DGR environment and it accelerates the corrosion rate of metal canisters (Bagnoud et al., 2016a).
The nature of the metal canister to be used depends on the DGR concept of each country. Copper (e.g., Canada, Finland, and Sweden) and steel-based materials (e.g., Belgium and the Czech Republic) are the predominant options for the canisters. Regarding steel-based material, Enning and Garrelfs (2014) reported the SRB contribution to the corrosion of this material through two main mechanisms: electrical microbially influenced corrosion (EMIC), and chemical microbially induced corrosion (CMIC; Figure 2). EMIC is a direct mechanism where SRB are involved in the speed up of abiotic corrosion. In this process, specially adapted SRB pull out electrons from elemental iron leading to the disposition by means of electro-conductive iron sulfides and releasing excess electron acceptors such as H2 (Figure 2A). On the other hand, CMIC is the indirect mechanism that results from the sulfidogenic degradation of organic matter under oxygen-free environments resulting in the production of biogenic and corrosive sulfide which reacts with the metallic iron (Figures 2B,C). Furthermore, the biogenic formed hydrogen sulfide can cause sulfide stress cracking of the iron (Figure 2D). FeS may, temporarily, act as a protective action against iron corrosion as it is strongly adhered to the metal surface through the direct reaction of the dissolved sulfide with metallic iron (Newman et al., 1992; Sun and Nešic, 2007). Several studies have reported MIC on steel-based materials by the activity of SRB. Xu et al. (2008) detected the presence of kansite (Fe9S8) and pitting corrosion in stainless steel in the presence of Desulfovibrio sp. and Leptothrix sp. El Hajj et al. (2010) found mackinawite (FeSx) on P235H steel mediated by the activity of extremophiles and spore-forming SRB such as Desulfosporosinus sp., Desulfotomaculum sp., and Thermosubterraneum sp. The generation of biogenic mackinawite was also reported by Černoušek et al. (2019) when carbon steel MIC with Desulfomicrobium sp. and Desulvibrio spp. was studied. It should be noted that, although this review focuses on the SRB group, other bacterial communities can promote the steel-based material corrosion processes. Shrestha et al. (2021) highlighted the key role of nitrate-reducing bacteria (NRB), such as Methyloversatilis, Pseudomonas and Brevundimonas, related to carbon steel corrosion since they could be involved in the formation of corrosion compounds such as magenetite, mackinawite [FeS(1 − x)], akageneite [Fe3+O(OH, Cl)], and rozenite [Fe2+SO4·4(H2O)]. All these authors concluded that the biofilm formation promotes and accelerates the corrosion.
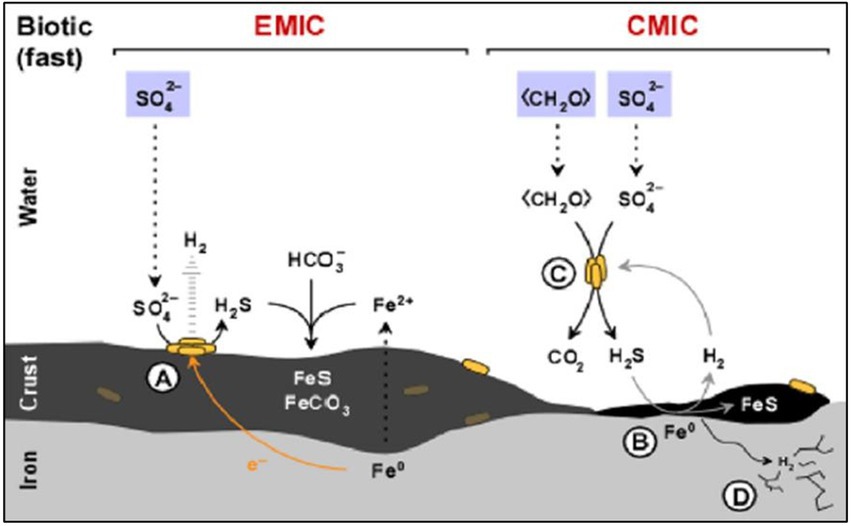
Figure 2. Diagram of the influence of sulfate-reducing bacteria (SRB) activity in the corrosion of iron. (A) Electrical microbially influenced corrosion (EMIC) mechanism. (B) Chemical microbially influenced corrosion (CMIC) mechanism. (C) Overall representation of CMIC. (D) Sulfide stress cracking. Modified from Enning and Garrelfs (2014).
As in the case of steel-based materials, sulfide is the main corrosive agent of copper materials. According to Dou et al. (2020), H2S produced by SRB cause uniform corrosion accompanied by pitting corrosion of Cu (high concentration of H2S) or intergranular corrosion of this metal (low levels of H2S). In this study, the dissimilatory reduction of sulfate mediated by SRB produced HS- from the secreted H2S. This metabolite can be decomposed to H+ and S2- or when combined with H+ forms H2S. The HS- diffuse to Cu surface and reacts with it resulting in Cu2S (Figure 3). Chen et al. (2011) described the different phases of the anaerobic corrosion of Cu starting with the formation of a chemisorbed CuI surface state acting as a precursor to forming a film. In the presence of SH- in the medium, it can bind to Cu producing the chemisorbed species of Cu (SH)ads. SH- can then react with the Cu surface and the adsorbed species leads to the beginning of the formation of a Cu2S film with partial protective capacity. In presence of Cl-, it can react with the surface intermediate Cu (SH)ads forming a chloride complex (CuCl2-). This compound can lead to Cu+ transport through the pores to the Cu2S/solution interface and react with SH- to form Cu2S. Several studies have shown the presence of chalcocite (Cu2S; Pedersen, 2010; Chen et al., 2014; Kurmakova et al., 2019) and surface pits (Chen et al., 2014) on the surface of oxygen-free copper under anaerobic conditions mediated by SRB.
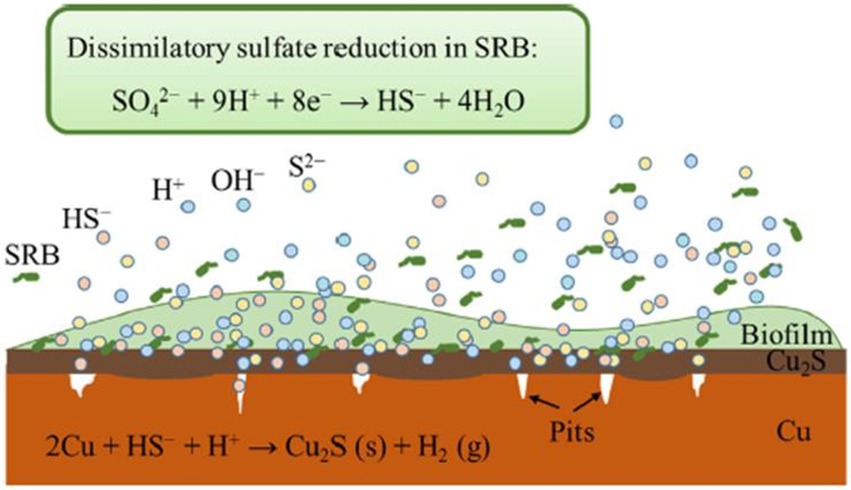
Figure 3. Schematic representation of copper corrosion mediated by sulfate-reducing bacteria (SRB) activity. Obtained from Dou et al. (2020).
As we have seen, the MIC rate of each material mainly depends on the presence of the sulfide produced by the activity of anaerobic microorganisms such as SRB. In a DGR, the corrosion of metal canisters will probably depend on the sulfide concentration at the boundary between the groundwater and the compacted bentonite (Bengtsson and Pedersen, 2016). Moreover, MIC may not only affect the release of radionuclides from canister, but could also be involved in gas production within the DGR system, which will be discussed in the next section.
As mentioned before, a wide variety of organic and inorganic compounds present in the DGR environment will provide nutrients, electron donors and acceptors, carbon, nitrogen, and sulfur sources which would enhance microbial activity. Not only some physicochemical processes such as corrosion of metal canisters or the radiolysis of water, but also microbial metabolism may contribute to the build-up of a gas phase in DGR environments (Guo and Fall, 2021). Gas accumulation derived from microorganisms is mainly produced in form of hydrogen (H2), methane (CH4), and carbon dioxide (CO2), and can lead to an increased pressure within the repositories, compromising the integrity of the clay as barriers (Bagnoud et al., 2016b).
H2 generation is one of the main gaseous sources within DGRs and may be conducted by the anoxic corrosion of the metal canisters, radiolysis of water, and diverse microbial activities (Beaton et al., 2019; Hall et al., 2021). Although the production of this gaseous form can increase the pressure level, some microorganisms can use it as a source of energy for microbial growth by means of methanogenesis or sulfate reduction (Grigoryan et al., 2021; Hall et al., 2021). CH4 can be produced through microbial methanogenesis through reactions between H2 or H2O and inorganic carbons (CO2 and CO) or acetate fermentation (CH3COOH; Eqs. 1, 2; Chen et al., 2019; Abramova et al., 2023). Methanogenesis is assumed to decrease gas pressure in DGR surroundings by consuming both CO2 and H2 (Eq. 1; Leupin et al., 2017; Beaton et al., 2019). However, the production of both CH4 and CO2 derived from aceticlastic methanogenesis (Eq. 2) could play the opposite role.
Recently, metagenomic and metaproteomic analyses on the microbial community present in Opalinus clay under DGR simulated conditions, have been performed to determine the role of microbial metabolic pathways on the safety of DGRs. These analyses indicate the high contribution of the bacterial genus Pseudomonas, as an SRB, in the oxidation of H2 coupled with sulfate (SO42-) reduction (Eqs. 3, 4; Bagnoud et al., 2016a; Smart et al., 2017). SRB from the family Peptococcaceae also appear to be involved in the formation of H2S as a result of the respiration of sulfates and CO2 from the oxidation of acetates and other organic compounds (Eq. 5; Bagnoud et al., 2016a). Finally, the CO2 produced as a result of some of the biochemical reactions mentioned above can dissolve in pore water, precipitated through its interaction with cementitious materials or diffused in a gaseous form, depending on the area where it is produced within the storage facilities (Bagnoud et al., 2016a).
As commented in previous sections, the presence of methanogenic and sulfate-reducing microbes has been detected in several clay types that will be used in DGR systems. For this reason, it is crucial to investigate in depth the influence of their metabolism on the safety of deep disposal. As different gases are consumed and produced during all these mechanisms, it is important to quantify the in-situ rates of microbial H2 oxidation and SO42- reduction to determine the total net production of gases. The calculations of Bagnoud et al. (2016c) concluded that sulfate-reducing microorganisms from Opalinus clay could be beneficial for the safety of the geological disposal of nuclear waste as indicated by high hydrogen and sulfate consumption rates, which would reduce the gas pressure build-up. However, it is not easy to estimate the overall role of microbes in terms of security since very different chemical, biological, and physical processes may be involved. For instance, the removal of sulfate gases by SRB may induce steel corrosion of the containers through the production of H2S or sulfides (S2-) as we discussed in section 4.1.
To sum up, microorganisms could both positively and negatively influence the safety of DGRs as far as the utilization and production of gases is concerned. However, it is extremely difficult to determine their overall impact due to the complexity of the biogeochemical processes that will be involved. In addition, the final role of microorganisms in terms of gas production will depend partly on the microbial community structure present in the natural and artificial barriers surrounding the canisters. Further research on microbial metabolism and gas formation is crucial to evaluate the safety conditions of DGRs.
One of the materials selected as an artificial filling and sealing barrier by current DGR models is bentonite, a volcanic origin phyllosilicate. This type of clay is mainly composed of smectites, highlighting montmorillonite as the principal mineral phase. Montmorillonite is characterized by a layered structure consisting of each one of 3 sheets in a 2:1 ratio, i.e., two tetrahedral silica sheets bordered in between them by an octahedral aluminum sheet (T-O-T structure). These layers have a negative charge balanced by cation exchange, providing the bentonite with most of its physico-chemical properties (Abdullahi and Audu, 2017; García-Romero et al., 2019). Bentonite from multiple locations has been widely studied as a buffer material for DGRs (MX80 from United States, FEBEX from Spain, FoCa from France and GMZ from China, among others; Xu et al., 2019).
Both abiotic (temperature, radiation, etc.) and biotic factors could compromise the stability of the bentonite barrier and therefore jeopardize the safety of the system. During the first phases of the repository, high temperatures are expected to be reached due to the heat generated by the decay of the stored radionuclides (Tripathy et al., 2017). The heat would diffuse throughout the repository reaching up to 100°C–200°C in the bentonite buffer with a maximum temperature gradient of up to 24°C, over a thickness of about 35 cm (Hökmark and Fälth, 2003; Faybishenko et al., 2017; Zheng et al., 2017). Smits et al. (2013) supported that an increase in temperature means an increase in thermal conductivity, probably due to an additional heat transfer as latent heat. However, this temperature effect decreases with high bentonite dry density. High temperatures are also a matter of great concern as they could lead to an abiotic smectite illitization process. Mills et al. (2022) discussed a possible mechanism of illitization at 200°C that would occur through a layer-by-layer transformation. The individual phyllosilicate layers are enriched in charge due to the substitution of Si by Al in the tetrahedral sheet, the substitution of Al by Mg in the octahedral sheet and an interlayer cation exchange of K+ by Na+ resulting in the formation of mixed illite/smectite layers. This phenomenon results in chemical alterations which could affect bentonite properties such as swelling and plasticity capacities (Zheng et al., 2017). In addition to temperature, bentonite will inevitably be exposed to certain doses of ionizing radiation, mainly gamma (from Cs-137). Many studies have focused on characterizing the effect of γ-radiation on this clay and reported only negligible effects on the alterations of its physical and chemical properties (Pushkareva et al., 2002; Plötze et al., 2003; Huang and Chen, 2004). However, Holmboe et al. (2011) tested the effect of this radiation on the ability of MX80 bentonite to retain radionuclides such as Cs+ and Co2+. Only Co2+ sorption was significantly affected by γ-radiation, which decreased in the irradiated samples. This result could indicate that this kind of irradiation would have altered surface characteristics thus decreasing its ability to bind this radionuclide. However, some authors have reported the stability of bentonite mineralogy when treated with different solutions (e.g., distilled water, sodium nitrate, glycerol-2-phosphate, and uranyl nitrate) after 6 months of aerobic incubation (Povedano-Priego et al., 2019). XRD semi-quantitative estimation showed the same composition of smectite, quartz, phyllosilicates, and plagioclases. Smectite represented the dominant mineral phase with 91%. Similar results were obtained for bentonite microcosms incubated for 6 months but under anoxic conditions, which indicates the stability of bentonite and no illitization process under different short-term incubation (Povedano-Priego et al., 2022).
However, the present review focus mainly on biotic factors related to the presence of microorganisms in the different barriers. One of the main concerns related to bentonite buffer is the ability of microorganisms to interact with the minerals causing weathering, dissolution and a second mineral formation (Meleshyn, 2014). In particular, smectite biotransformation into illite through Fe (III) bio-reduction is one of the most alarming processes. Pedersen (2013) reported that both the hydrogen sulfide produced by SRBs (Masurat et al., 2010), and Fe (II) generated by iron-reducing bacteria (IRB) could lead to a possible illitization process that would alter the properties of the smectite. Furthermore, Kim et al., 2019 studied the ability of the strain Shewanella oneidensis MR-1 to induce smectite dissolution by Fe (III) bio-reduction processes. Numerous studies reviewed by Dong et al. (2009), demonstrated the bioreduction of Fe (III) by microorganisms in clay minerals. The iron bioreduction rate was related to different factors, including total Fe content in the bentonite, particle size, amount of microorganisms present, pH, etc. (Lopez-Fernandez et al., 2021).
Radionuclides cannot be entirely removed, but they can be transformed to lower toxicity forms. Microorganisms are known for their capacity to do so, largely due to their high tolerance to toxic elements and other stressful conditions including radiation, desiccation, and the presence of oxidative agents (Brim et al., 2000). A wide variety of bacteria have been previously described to efficiently resist toxic elements such as uranium, nickel, copper, cadmium, selenium, cesium, strontium, etc., including those belonging to the genera Bacillus, Pseudomonas, or Stenotrophomonas (Fakhar et al., 2022; Ruiz-Fresneda et al., 2023).
Microbial activity can indirectly influence solubility and hence the mobilization of radionuclides by the alteration of the geochemical conditions within the DGRs (pH, oxidation–reduction potential, etc.). Such modifications can lead to changes in the oxidation state of some radionuclides, affecting their solubility and mobility through the repositories. However, microorganisms can directly influence the mobility of radionuclides and other elements through different processes such as intracellular accumulation, biotransformation (redox reactions) biosorption, or biomineralization (Shukla et al., 2017; Lopez-Fernandez et al., 2020; Ruiz-Fresneda et al., 2020a; Martinez-Rodriguez et al., 2022; Figure 4).
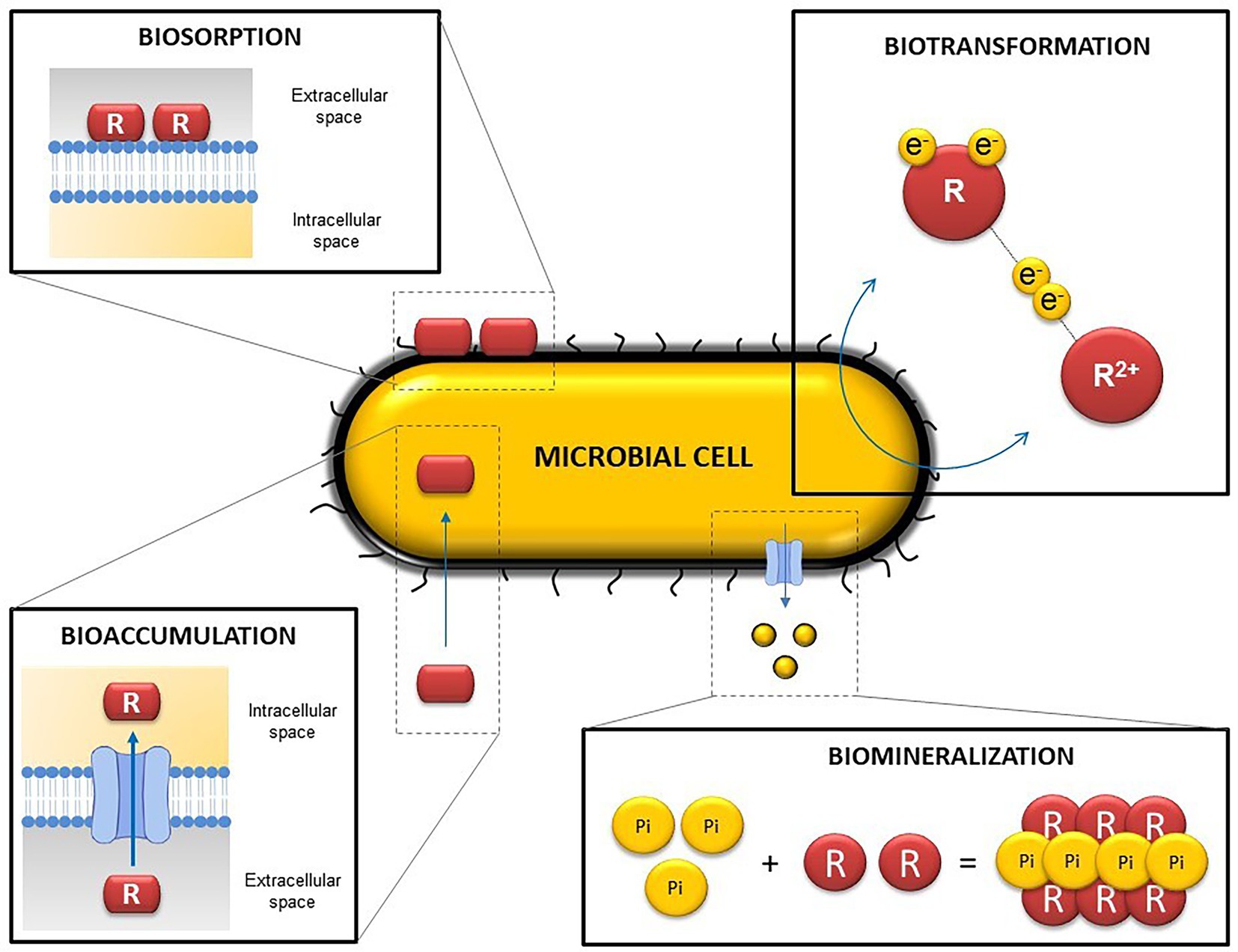
Figure 4. Representative diagram illustrating the main microbial mechanisms involved in radionuclide and heavy metal mobilization. R, radionuclide/ligand.
Biotransformation occurs when a specific element changes its oxidation state as a result of microbial activity through redox reactions and can lead to changes in its solubility (Francis and Nancharaiah, 2015). The bioreduction of soluble and mobile oxidized forms can trigger their immobilization (Rui et al., 2013). Enzymatic reduction of radionuclides and other toxic elements by bacteria have been widely reported in recent years. Several bacterial species are able to anaerobically reduce the soluble form UVI, to the insoluble UIV, by using UVI as the final electron acceptor (Kolhe et al., 2018). Bacterial genera such as Geobacter, Desulfovibrio, y Shewanella are remarkable bacterial genera in uranium reduction (Cologgi et al., 2014; Stylo et al., 2015; Grouzdev et al., 2018). The bioreduction of oxidized forms of other elements have also been described. SeVI and SeIV can be reduced to less mobile forms (Se0 and Se-II), among others, by the genera Bacillus and Stenotrophomonas (Tan et al., 2016; Lampis et al., 2017; Kora, 2018; Ruiz-Fresneda et al., 2020a). Volatilization is another biotransformation process considered as a rising green process because of it generally implies the production of non-toxic compounds through reduction and methylation reactions.
Biosorption can be defined as the binding of positively charged metal ions to negatively charged cell surface components through physico-chemical interactions (Gadd, 2004; Prakash et al., 2013). Specifically, it can occur by electrostatic interactions, ionic exchange, or complexation and chelation processes (Yang et al., 2015; Diep et al., 2018). For this reason, biosorption is considered a passive process and can even take place with cellular fragments (cell membranes, cell walls, etc.) and dead microbial biomass (Fomina and Gadd, 2014; Ayangbenro and Babalola, 2017). Biosorption can act directly between metal cations and anionic functional groups from cell walls, or indirectly with extracellular polymeric substances (EPS), S-layer proteins, and the bacterial capsule (Merroun et al., 2005; Dobrowolski et al., 2017).
According to Tabak et al. (2005), bioaccumulation consists of accumulating a specific substance or element inside an organism. In contrast to biosorption, intracellular accumulation is usually considered a metabolically active process, in which microorganisms introduce radionuclides or other elements into the intracellular space by using a transport system or altering the permeability of the membrane (Diep et al., 2018). However, some authors indicate that bioaccumulation is closely related to biosorption, since it requires fast interactions with anions from components of the cell surface before being introduced into the intracellular space (Tišáková et al., 2013). For this reason, other authors have considered that intracellular accumulation can occur through both passive sorption (metabolism-independent) and active uptake (metabolism-dependent). Once the radionuclide is inside, it can be sequestered by proteins, packaged in lipid membranes, vacuoles, or different biological storage systems (Mishra and Malik, 2013).
Bioprecipitation is based on the precipitation of elements with ligands (phosphates, sulfides, carbonates, hydroxides, etc.) released by microorganisms resulting in the production of insoluble and immobile complexes (Shukla et al., 2017; Sreedevi et al., 2022). Some bacterial species from the genera Sphingomonas, Bacillus and Stenotrophomonas have the capacity to release inorganic phosphates (Pi) through phosphatase activity, which precipitates with UVI and produce insoluble uranium phosphates (Chandwadkar et al., 2018; Zhang et al., 2018; Sánchez-Castro et al., 2020; Zhong et al., 2021). When the complex formed is a mineral this bioprecipitation process is called biomineralization (Kolhe et al., 2018). It is important to note that the metals or radionuclides precipitated as a result of this process do not present any change in their oxidation states, unlike other processes formerly described, such as biotransformation.
Tolerance of microbes to radiation must be considered of particular interest in microorganism-radionuclide interaction. It is well known that radiation can both directly and indirectly affect bacterial biomolecules, including nucleic acids, proteins, lipids, and carbohydrates. For instance, ionizing particles produced as a consequence of radioactivity can damage the DNA structure, disrupting the functionality of the DNA (Close et al., 2013; Jung et al., 2017). Ionizing radiation can also indirectly damage the microbial DNA and other molecules by production of reactive oxygen species through the radiolysis of water, as discussed in Section 2. To neutralize radiotoxicity, microorganisms have developed defense mechanisms, such as the production of regulatory secondary metabolites, DNA repairing machineries, anti-oxidative systems, etc. (Gabani and Singh, 2013). This is the case of ionizing radiation-resistant bacteria such as Deinococcus radiodurans, which tolerate high radiation doses (12 kGy), Kineococcus radiotolerans (2 kGy), or Rubrobacter radiotolerans (1 kGy), among others (Jung et al., 2017). However, microorganisms do not implicitly need to tolerate high radiation doses to play a positive role in DGR safety. As mentioned before, radionuclide biosorption is an electro-chemical passive process, in which the cells do not have to be viable to successfully interact with the radionuclides. Even when the viability is almost non-existent in a bacterial population, their metabolism could remain active under stress situations. Indeed, some studies have revealed that less than 2% of the cells of the bentonite isolate S. bentonitica are viable under DGR simulated conditions (anaerobiosis, SeIV stress, etc.) after 6 days’ incubation, but more than 50% remain metabolically active and interact with SeIV through its biotransformation to Se0 (Ruiz-Fresneda et al., 2019). In addition, radiation will be strongly attenuated and absorbed in the proximity of the DGRs overtime and the help of insulating materials and multi-barrier systems. Nevertheless, when we do not specifically know when the interaction may occur (whether the radiation is active or not), we must study as many conditions as possible in all the interaction cases.
Despite heavy metal-bacteria interaction having been studied in depth during the last decades, not many studies have been reported in relation to the possible role of these interactions influencing the safety of DGRs in the case of a radionuclide escape. A lot of effort has been put into very interesting studies which have been published in recent years with the aim of elucidating as far as possible how microbial processes can affect the mobility of elements of interest present in radioactive waste. These studies will allow us to evaluate how microorganisms can positively or negatively affect the safety of future DGRs. In this review, we have focused on Se, as one of the critical radionuclides of radioactive waste, and some representatives of trivalent actinides, such as Cm and their inactive analogs (trivalent lanthanides such as Eu).
Microorganisms can interact with Se mainly through biochemical biotransformation processes. There are many which are capable of transforming different species of Se by means of reduction–oxidation (redox) reactions, methylation, and demethylation processes (Eswayah et al., 2016). The microbial reduction of oxidized and soluble forms of Se (SeVI and SeIV) to insoluble Se0 has been previously described by bacteria of the genera Bacillus, Shewanella, Comamonas, Stenotrophomonas, Azospirillum, and Pseudomonas, among others (Figure 5; Kora, 2018; Vogel et al., 2018; Baggio et al., 2021; Staicu et al., 2022; Ruiz-Fresneda et al., 2023). Currently, the number of known SeVI reducing strains is lower than that of SeIV reducing ones. The mechanism of reduction of SeVI varies among the microorganisms studied to date. Some bacterial species such as Thauera selenatis and Seleniivibrio woodruffii are able to respire SeVI when using it as an electron acceptor (Rauschenbach et al., 2013; Mohapatra et al., 2022). On the other hand, some enzymes such as selenate reductase have shown their ability to reduce SeVI in strains such as Comamonas testosteroni S44 (Tan et al., 2018). The reduction of SeIV can also be carried out through different enzymatic mechanisms. Nitrite reductase, sulfite reductase, fumarate reductase and selenite reductase seem to be involved in this process (Song et al., 2017; Wang et al., 2018, 2022). Furthermore, compound-mediated reactions with thiol groups (-SH) have also been studied. The participation of glutathione (GSH) in the reduction of SeIV has been investigated with special interest. Kessi and Hanselmann (2004) found that GSH and the enzyme glutathione reductase (GR) are involved in the reduction of SeIV to Se0 in Rhodospirillum rubrum through a series of reactions. Recently, the studies of Wang et al. (2022) conducted with the strain Proteus penneri LAB-1 support this mechanism by suggesting that the gluthathione pathway plays a critical role in the SeIV reduction process carried out by this bacterium.
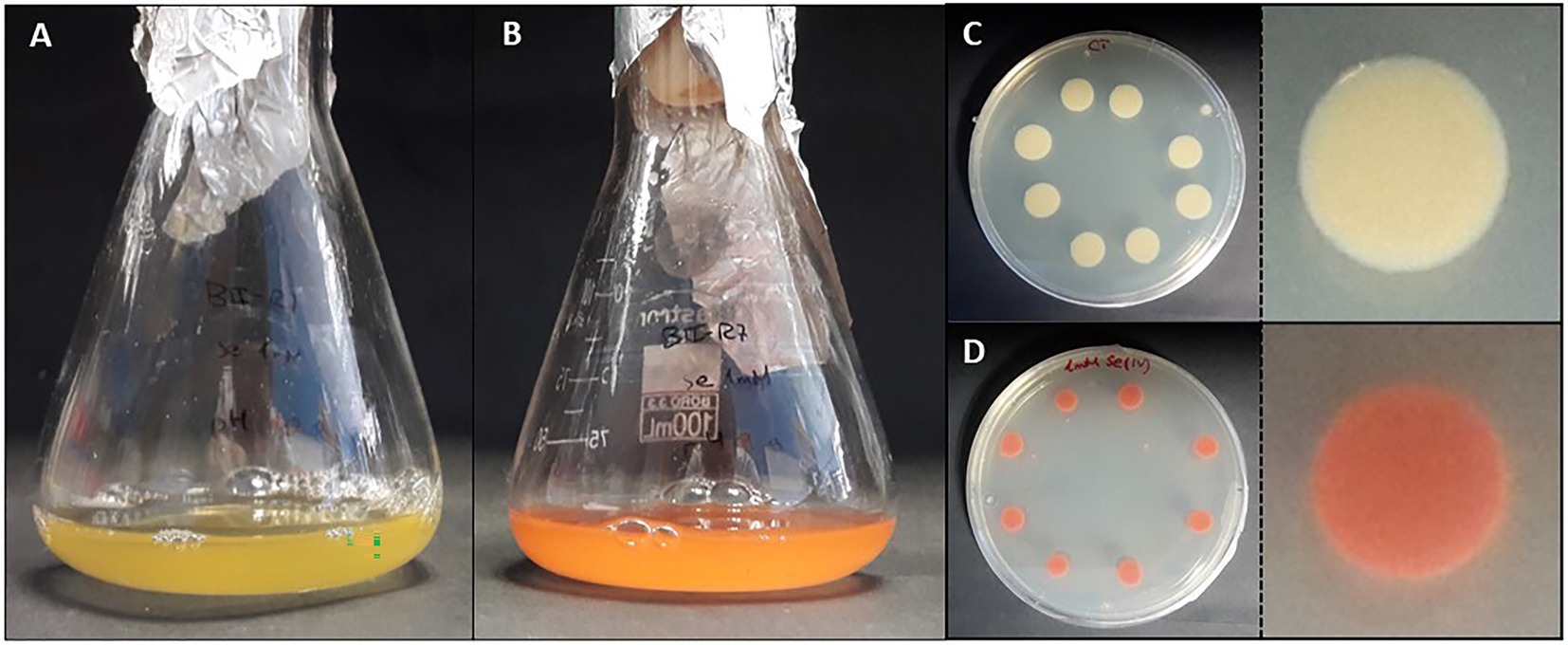
Figure 5. Colorful transition of liquid (A,B) and solid (C,D) cultures of the bacterium Stenotrophomonas bentonitica to red Se 0 when SeIV is added (personal archive of Dr. Merroun).
In most cases, the reduced Se0 products are accumulated in the form of selenium nanoparticles (SeNPs), known for their use in numerous medical and industrial applications (Zambonino et al., 2021). These nanoparticles are characterized by different physico-chemical properties (morphology, size, structure, etc.), that could affect their solubility and mobility in the environment (Jain et al., 2017). For example, Bacillus subtiliis BSN313, Bacillus mycoides SeITE01, and Stenotrophomonas maltophilia SeITE02 are able to produce Se spheres with an amorphous nature at the nanoscale (Bulgarini et al., 2021; Ullah et al., 2021). The studies by Benko et al. (2012) demonstrated the lower toxicity of Se nanospheres compared to the oxidized forms (SeVI and SeIV). However, there is some controversy in this respect since different studies have indicated completely the opposite (Li et al., 2008). The formation of Se nanowires by microorganisms present in anaerobic granular sludge has also been proved by Jain et al. (2017). These authors have described the lower colloidal stability and mobility of these nanostructures compared to biologically produced nanospheres. On the other hand, the crystallinity of some Se nanostructures also seems to affect their immobilization by increasing their settleability (Lenz et al., 2009).
Some studies have revealed the presence of organic matter layers, composed mainly of proteins and polysaccharides surrounding the biologically produced Se nanostructures (Dobias et al., 2011; Kamnev et al., 2017; Bulgarini et al., 2021). The physico-chemical properties, and therefore the mobility of Se nanostructures can be largely affected by the presence of these associated organic layers (Jain et al., 2017). In addition, some authors have also suggested the role of proteins in the synthesis and transformation of SeNPs, as well as in controlling their size (Dobias et al., 2011). According to Ruiz-Fresneda et al. (2020a), proteins produced by the bacterium S. bentonitica maybe involved in the transformation of amorphous nanospheres to crystalline trigonal Se nanofibers (Figure 6). Although the specific transformation mechanism is still unknown, what seems clear is the direct role of the cells and their proteins during the process.
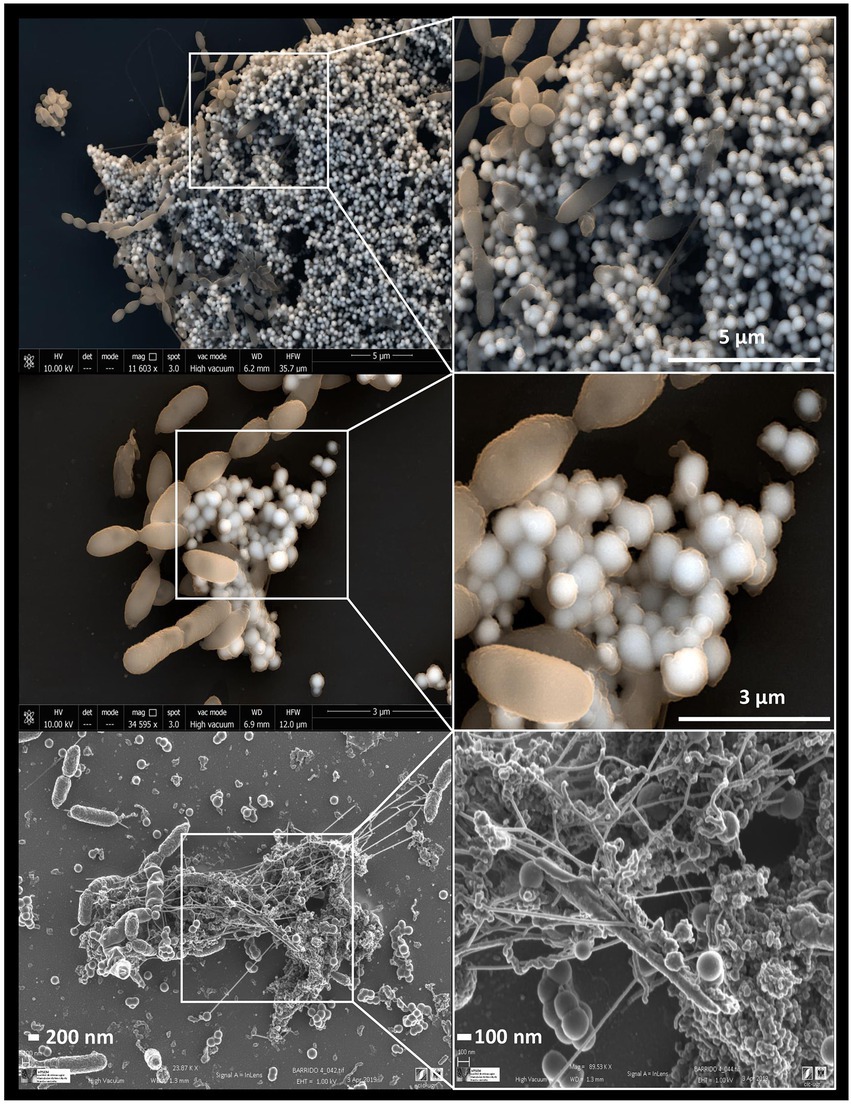
Figure 6. Electron microscopy images showing Se0 nanostructures produced by the bacterium Stenotrophomonas bentonitica when supplemented with SeIV (personal archive of Dr. Merroun; growth conditions detailed in Ruiz Fresneda et al., 2018, 2019, 2020a).
In the same way as for Se0, methylated selenide compounds are basically insoluble and poorly bioavailable for living beings (Favorito et al., 2021). For this reason, Se methylation is considered one of the most important transformation processes related to bioremediation. It is a detoxification mechanism for microorganisms since volatile Se methylated compounds are notably less toxic than the oxidized forms (Davis et al., 2013; Favorito et al., 2021). The production of methylated selenides has been proved in different bacterial species such as Methylococcus capsulatus, Methylosinus trichosporium OB3b, or Pseudomonas tolaasii (Eswayah et al., 2017; Liu et al., 2021). These bacterial strains can produce volatile compounds such as dimethyl selenide (DMSe, CH3SeCH3), dimethyl diselenide (DMDSe, CH3SeSeCH3), and dimethyl selenenyl sulfide (DMSeS, CH3SeSCH3). To date, several mechanisms have been proposed for the biomethylation of Se. Chasteen and Bentley (2003) suggested that Se0 is reduced to the selenide form (H-Se-X), which is subsequently methylated to CH3SeCH3 and CH3SeH. Other mechanisms similarly propose the formation of CH3SeCH3 from SeIV through numerous reduction and methylation reactions (Challenger, 1945). Finally, not many studies have reported the oxidation of Se0 and organic Se by microorganisms present in soils. Very recently, Luo et al., 2022 discovered four bacterial strains (Dyella sp. LX-1 and LX-66, and Rhodanobacter sp. LX-99 and LX-100) capable of oxidizing Se-II and Se0 to SeIV. However, unlike reduction processes, the low oxidation rate of Se reduced forms indicates that this process should not be considered as relevant for the environment (Losi and Frankenberger, 1998; Eswayah et al., 2016). Similarly, demethylation processes of Se compounds are not usually considered due to the low rates at which these reactions occur (Eswayah et al., 2016).
On the basis of all the comments mentioned above, it is clear that indigenous microorganisms from natural and engineered barriers in a DGR or are introduced during the disposal construction and could play a direct effect on radionuclide mobilization. For example, the bacterial species S. bentonitica, isolated from bentonite clay, has been recently described to efficiently reduce SeVI and SeIV to Se0 nanostructures and methylated Se (Pinel-Cabello et al., 2021a; Ruiz-Fresneda et al., 2023). The physico-chemical characterization of these Se reduction products suggests that this bacterium plays an important role in Se immobilization in the context of DGRs. Recently, Povedano-Priego et al. (2023) have described the SeIV reduction to Se0, producing precipitates of different shapes (from nanospheres to complex nanostructures) in bentonite samples treated with SeIV. This is the first time that this process has been shown within a ternary system (bentonite, indigenous microorganisms, and Se). Several bacteria have been found enriched in SeIV-treated bentonite such as Pseudomonas, Stenotrophomonas, Desulfosporosinus, among others, potentially involved in Se reduction (Povedano-Priego et al., 2023).
Different interaction mechanisms such as biosorption, biomineralization/bioprecipitation and intracellular accumulation have been described as affecting the mobility of representatives of trivalent actinides such as Cm, and their inactive analogs including Eu.
Biosorption is one of the main processes involved in the case of Cm and Eu. The cell surfaces of many bacteria have a high density of functional groups that can serve as a binding site of certain components and metal cations (Hufton et al., 2021). Specifically, the carboxyl groups present in the peptidoglycan layer of the cell wall of gram-positive bacteria seem to act as the main binding sites for actinides (Barkleit et al., 2009). Moreover, carboxyl, phosphoryl, and hydroxyl groups present on lipopolysaccharides (LPS) of the outer membrane, characteristic of gram-negative bacteria, can also act as a ligand for actinides such as U, Cm, and Np (Moll et al., 2021). Different bacterial species such as Pseudomonas fluorescens and Sporomusa sp. MT-2.99 have been described for the efficient biosorption of CmIII and EuIII to their cell surfaces (Moll et al., 2013, 2014). Pseudomonas fluorescence was isolated from groundwater from the Äspö Hard Rock Laboratory tunnel, a unique research facility testing the safety of final repositories for nuclear fuel, while Sporomusa sp. MT-2.99 is indigenous from Mont Terri Opalinus clay. In the case of Sporomusa sp. MT-2.99, the speciation and structure of the surface complexes formed with CmIII and EuIII was determined by Time Resolved Laser-Induced Fluorescence Spectroscopy (TRLFS). The results show that carboxyl and phosphoryl groups are responsible for EuIII and CmIII binding and the cell surface of this bacterium (Moll et al., 2014). Recent new research has demonstrated the role of proteins from the plasma membrane fractions of L. sphaericus in CmIII complexation (Moll et al., 2021).
Not only have bacterial cells been analyzed using this technique, but there have also been recent studies about the yeast Rhodotorula mucilaginosa BII-R8, isolated from selected Spanish bentonite, which has shown its ability to retain both EuIII and CmIII at the cell surface EuIII (Lopez-Fernandez et al., 2018c; Figure 7). In the same way, carboxyl and phosphoryl groups present in the cell envelopes of R. mucilaginosa BII-R8 seem to be involved in the interaction of these two elements according to the results obtained by TRLFS. This study described for the first time the ability of a yeast to efficiently interact with CmIII and EuIII. Archaea can also interact with CmIII and EuIII as indicated by studies on Halobacterium noricense DSM15987T (Bader et al., 2019), where phosphate and carboxylic groups from the cell surface and released by the cells most probably act as binding sites. H. noricense DSM15987T was isolated from rock salt, one of the natural barriers being considered for radioactive waste disposal.
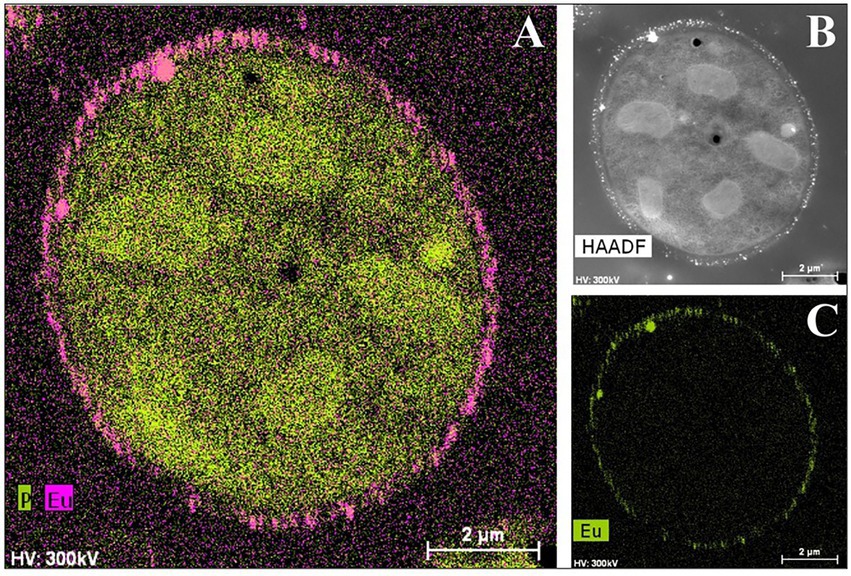
Figure 7. HAADF-STEM micrographs of a thin section and EDX element distribution maps showing the Eu biosorption to the cell surface of Rhodotorula mucilaginosa BII-R8. A: P and Eu elemental mapping image; B: HAADF image; C: Eu elemental mapping image (Lopez-Fernandez et al., 2018c).
Bioaccumulation and biomineralization/bioprecipitation have also played an important role in the microbial interaction with CmIII/EuIII. The bacterial species Thermus scotoductus SA-01 accumulates EuIII precipitates both intracellularly and extracellularly and tolerate higher concentrations than those usually found in nature (up to 1 mM; Maleke et al., 2019). Analysis of these samples by various spectroscopic techniques suggest the presence of extracellular accumulation may be due to a biomineralization process from carbonates produced by the reaction of CO2, from respiration, with OH– radicals. They have also demonstrated the possible role of carboxyl, carbonyl, and phosphate groups in the biosorption of EuIII according to the data obtained by ATR-FTIR. Specifically, they suggest the formation of EuIII carbonates at surface level as indicated by XPS analyses. These results show how several mechanisms of interaction can simultaneously take place in a cell when it is exposed to a stressful condition. This is also the case of S. bentonitica, a bentonite-isolated bacterium which has recently been reported to interact with EuIII and CmIII through biosorption and bioaccumulation mechanisms, and possibly biomineralization (Ruiz-Fresneda et al., 2020b). The ability of this bacterium to additionally interact with Se, as mentioned before, suggests it as a potential positive candidate for the immobilization of radionuclides in the context of DGRs.
To sum up, microbial biosorption and bioaccumulation processes may play an important role in the immobilization of CmIII, EuIII, and other toxic elements if microbial biomass binds to inert supports through the formation of biofilms (Gadd, 2009). Biomineralization may also lead to the immobilization of these elements due to the formation of insoluble precipitates (Shukla et al., 2017). For all these reasons, the study of microbial interactions with actinides (CmIII) and analogous elements (EuIII) has been acquiring a special interest in recent years, both for the evaluation of the safety of radioactive waste repositories, and for the bioremediation of contaminated environments.
Uranium will be the dominant and the most critical radionuclide in the DGR system. Interactions between bentonite microbial communities and such radionuclide could be an imperative solution for the retention and/or immobilization, avoiding a release of UVI in the environment and allow a safe and long-term storage of the radioactive waste.
The mobility and solubility of U in natural systems are governed by its oxidation state as well as its chemical speciation, which in turn is influenced by abiotic and biotic processes. U has two major oxidation states, UVI and UIV. The oxidized form possesses an elevated number of positive charges that results in its solubility, mobility, and therefore toxicity to the microbial cells, while UIV is insoluble and less toxic under anoxic conditions. The chemical speciation of UVI is highly pH-dependent, in the sense that under alkaline conditions, such as those of bentonite, soluble uranyl ions it may form complexes with carbonate groups (Waite et al., 1994; Kushwaha et al., 2018), which are potentially mobile in presence of water. Under acidic conditions these oxidized forms would be absorbed on the mineral phases, complexed by organic matter or precipitated as autunite [Ca(UO2)2(PO4)2], one of the insoluble uranyl phosphate minerals (Kolhe et al., 2018). In addition to chemical toxicity, U also displays a radiological (mainly from 235U and 238U isotopes) hazard, although its radiotoxicity is reported to be low. In contrast to other metals, it does not have any biological functions in living organisms. However, it has long been known to cause lung, renal, and hepatic damage in humans (Priest, 2001), as it may provoke oxidative stress in lung epithelial cells, by the loss of glutathione (an effective antioxidant) and superoxide dismutase enzyme (Periyakaruppan et al., 2007). In microorganisms, many effects of U toxicity are recognized including the loss of cell viability and activity, distortion of cell surfaces, oxidative damage, as well as the suspension of DNA replication and transcriptional and translational processes (Park and Jiao, 2014; Sepulveda-Medina et al., 2015). However, as mentioned above, in order to resist metal and radionuclide toxicity, microbes have developed a variety of mechanisms that allow potential immobilization and bioremediation. Microorganisms that interact with U are reported to be a good remediation strategy for decreasing the solubility and mobility of this toxic element (Sánchez-Castro et al., 2020). They can affect its speciation and migration in different environments, including DGRs (Povedano-Priego et al., 2019, 2022). Among the mechanisms adopted by many microorganisms to tolerate and survive in uranium contaminated sites, biosorption, bioaccumulation, biomineralization, and biotransformation (through oxidation–reduction reactions) constitute the most relevant in the in situ and ex situ bioremediation techniques. However, the two microbial processes that have been most investigated so far, and therefore gained more attention and confidence, are the enzymatically catalyzed reduction of UVI to UIV and the bioprecipitation of the oxidized form of U with inorganic phosphates as ligands.
Uranium (as oxidized form UVI) has shown to be highly susceptible to enzymatic reduction by microorganisms, which can occur in the cytoplasm, periplasm, at the outer membrane or extracellularly (You et al., 2021). They could be involved in a direct enzymatic reduction of soluble UVI to insoluble forms, while biologically mediated indirect reduction using electrons obtained in the oxidation–reduction reactions of iron or sulfate has also been reported to be important in the immobilization of UVI (Roh et al., 2015). Uranium-reducing microorganisms are ubiquitous in the environment. Several species of prokaryotes have been reported to be involved in uranium reduction. Iron-reducing species like Geobacter uraniireducens, G. daltonii, and Shewanella oneidensis and sulfate-reducing bacteria (SRB) mainly Desulfovibrio, Desulfotomaculum, and Desulfobacterium have been described as using uranium as an electron donor in addition to iron and sulfate (Akob et al., 2012). The first ones can conserve energy for their anaerobic growth through the reduction of uranium, while the SRB are unable to conserve such energy for their growth. Desulfovibrio has been found in different uranium-contaminated sites such as sub-surface sediments (Castañeda-Carrión et al., 2010; Newsome et al., 2014), and water (Parihar et al., 2013; Jroundi et al., 2020). Povedano-Priego et al. (2022) identified Desulfovibrio in the bacterial community of uranium-treated bentonite under anoxic conditions in presence of glycerol-2-phosphate (G2P). Significant presence of Desulfovibrio in these conditions indicates that a potential U-tolerance mechanism maybe active with the mediation of G2P. The capacity of members of this genus to use glycerol as electron donor has been reported (Ben Ali Gam et al., 2018). In addition, Desulfovibrio has been shown to use cytochrome c3 (cysA) functioning as UVI reductase in combination with a hydrogenase as a physiological electron donor, although additional pathways from organic electron donors (such as glycerol) to UVI that can bypass the cytochrome, have been suggested to occur (Payne et al., 2002). Several iron reducers (e.g., Clostridium, Geobacter) can reduce uranium by different mechanisms mediated by enzymes and cytochromes. Clones related with Clostridium species have been detected in the bacterial community of uranium-contaminated sediment from an inactive uranium mine (Midnite mine, eastern Washington), producing the reduction of UVI (Suzuki et al., 2003). The main mechanism involved is an enzymatic reduction of UVI mediated by hydrogenases (Gao and Francis, 2013). Other cytochromes have been found to be imperative to induce uranium reduction in Geobacter as well as in other bacteria such as S. oneidensis. In addition to the periplasmic c7-type cytochrome PpcA, an important intermediate electron carrier (in the absence of hydrogen), GscA (Geobacter sub-surface c-type cytochrome A), MacA (diheme c-type cytochrome peroxidase), MtrC (also known as OmcB; outer membrane c-type cytochrome) and OmcZ (outer-surface c-type cytochrome) has shown to be essential for the reduction of uranium (Yun et al., 2016; Rogiers et al., 2022). Bacterial pili also seem to play a role as an electron conductor between the cells and the electron acceptors. The reduced UIV appears mainly localized in the periplasm and outside of the cells, indicating the involvement of outer membrane-bound enzymes in the reduction process (Majumder and Wall, 2017). It has also been suggested that uranium reduction maybe linked to iron metabolism, since siderophores have been shown to form stable complexes with several metals and radionuclides, including uranium (Gallois et al., 2022). Siderophores and a large number of proteins associated to iron uptake systems, such as ABC-transport type proteins in the Chernobyl isolate Microbacterium oleivorans A9 or a transcriptional regulator of the Fur family in Desulfotomaculum reducens MI-1, are upregulated as an iron starvation response to uranium stress (Junier et al., 2011; Gallois et al., 2022).
Microorganism-mediated biomineralization of uranium is a widely used mechanism, which is applied for the remediation of radionuclide contaminated sites. Large numbers of microorganisms are known for their capacity to biomineralize uranium as metautunite-like precipitates using phytase, phosphatases, or complexed with microbe-associated ligands, such as phosphate, carbonate, or hydroxide functional groups (Lin et al., 2023). Thus, U bioprecipitation results frequently from an enzymatic process. Microbial phosphatases are involved in the U biomineralization, since these enzymes release inorganic phosphates (Pi) by hydrolysing organic phosphate substrates, which interact with the radionuclide and precipitate in the form of an insoluble phosphate mineral (Figure 8). A plethora of bacteria isolated from radionuclide and metal contaminated sites have demonstrated uranium precipitation owing their acid or alkaline phosphatase activities. As examples of these, Gram-positive bacteria Bacillus sphaericus JG-7B (Merroun et al., 2011), Paenibacillus sp. JG-TB8 (facultative anaerobic bacterium; Reitz et al., 2014), and Microbacterium sp. A9 (Theodorakopoulos et al., 2015) and many Gram-negative members of Alphaproteobacteria, Deltaproteobacteria, and Gammaproteobacteria classes (Kolhe et al., 2018; Pinel-Cabello et al., 2021b) should be mentioned. In most of these cases, U bioprecipitation occurs in the form of a stable and insoluble meta-autunite mineral (uranyl phosphate mineral phase). On the contrary, Beazley et al. (2007) isolated an Arthrobacter sp., from sub-surface soils at the US Oak Ridge field Research Centre, as a negative phosphatase bacterium, which was unable to precipitate uranium. Different phosphatase enzymes were identified as responsible for the uranium biomineralization that includes PhoY, the alkaline phosphatase PhoK, and the acid phosphatase PhoN in Caulobacter crescentus, in Sphingomonas, and Serratia sp., respectively (Nilgiriwala et al., 2008; Paterson-Beedle et al., 2012; Yung and Jiao, 2014). Biomineralization by polyphosphates has also been proven to occur in many uranium contaminated sites. Polyphosphates (phosphate polymers) can also be degraded as an alternative to obtain phosphates that precipitate with the metal intracellularly (Acharya et al., 2017). For example, in Pseudomonas aeruginosa, an over-expression of the polyphosphate kinase (ppk) gene has been reported to result in the release of phosphates, which precipitate in the form of uranyl phosphate minerals in the cell membranes (Renninger et al., 2004). Pseudomonas has been identified in several bentonite samples (Lopez-Fernandez et al., 2015; Povedano-Priego et al., 2021), including uranium-treated microcosms in the presence of G2P (Povedano-Priego et al., 2022). G2P as an organic phosphate source enrich Pseudomonas since these bacteria have been described to possess gene encoding phosphatases that may have the G2P as a substrate to release inorganic phosphate (Liu et al., 2016; Sarikhani et al., 2019). Other environmental microorganisms such as Bacillus, Rhanella, Arthrobacter and Cellulomonas have shown their capacity to immobilize U as biogenic uranyl phosphate minerals owing to their polyphosphate metabolism or organophosphate hydrolase activity (Martinez et al., 2007; Sivaswamy et al., 2011). Povedano-Priego et al. (2019) reported the phosphatase activity in Amycolatopsis ruanii, a significantly abundant genus in G2P-uranium treated bentonites. The highest concentrations of Pi in solution were observed when Amycolatopsis was treated with G2P. However, electron-dense precipitates were found with, and without the G2P amendment (Figures 9A,B). These precipitates were composed of phosphorus and uranium (Figures 9C,D). Thus, the biomineralization of uranium has also been observed without the G2P amendment (Figure 9A). Uranium phosphates have been identified extracellularly and at the cell-wall level with and without G2P (Figures 9A,B), while intracellular needle-like fibril precipitates were only detected in presence of G2P (Figure 9B). These results confirm the capacity of these bacteria to biomineralize uranium, which had been enhanced by the G2P amendment through phosphatase activity. In the study of Martinez-Rodriguez et al. (2022) Microbacterium sp. Be9 strain was used, previously isolated from U-mill tailings and determined that the U-biomineralization process was dependent on the type of phosphate source. This is relevant for the bioremediation of uranium since the solubilization of orthophosphates derived from waste products containing P-compounds could occur (Martinez-Rodriguez et al., 2022). However, high concentrations of uranium could produce a harmful effect on bacterial activity. To avoid this negative effect, Sánchez-Castro et al. (2021) embedded bacterial cells of Stenotrophomonas sp. Br8 in an alginate matrix to protect the cells from hazardous agents and enhanced the immobilization rate. This methodology could be applied to bioremediate U-contaminated mining water.
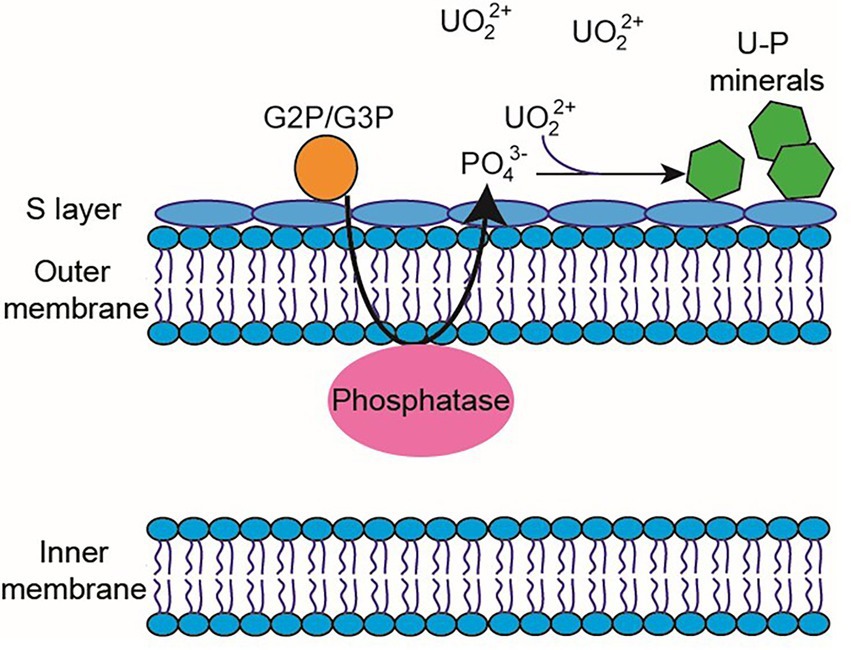
Figure 8. Diagram of the periplasmic phosphatase-mediated biomineralization process. Phosphatase releases inorganic phosphate from an organic phosphate source (e.g., G2P/G3P) to interact with UVI and produce the precipitation of uranium phosphates.
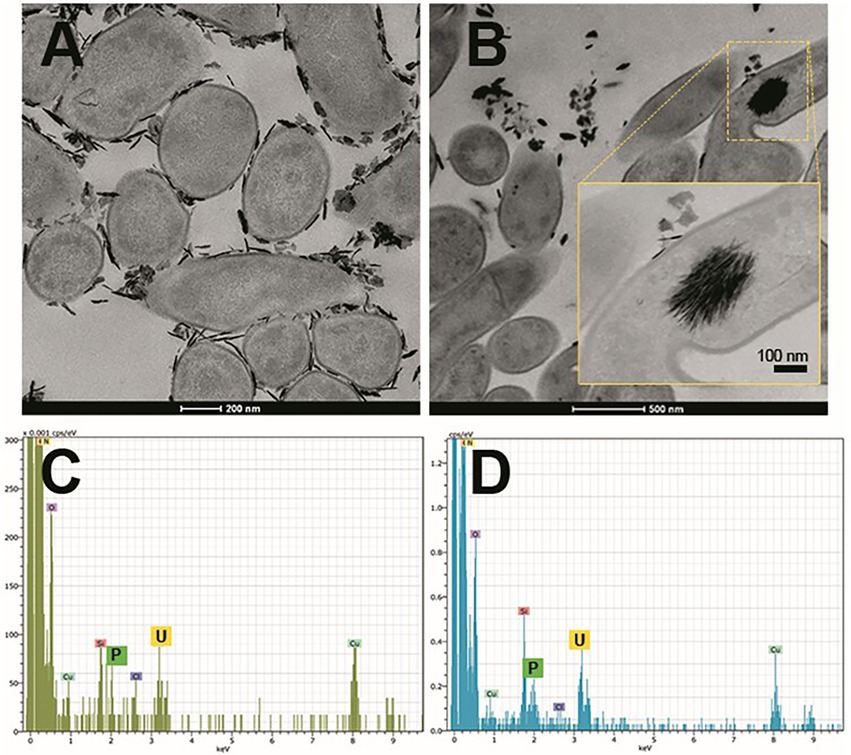
Figure 9. Scanning transmission electron microscopy (STEM) micrographs of thin sections of Amycolatopsis ruanii/bacterial consortium (Bradyrhizobium-Rhizobium and Pseudomonas) cells treated with uranium (A), and uranium + glycerol-2-phosphate (B). Extracellular and intracellular precipitates of uranium phosphates and their corresponding EDX spectra with peaks of U and P (C,D) respectively. Personal archive of Dr. Merroun; growth conditions detailed in Povedano-Priego et al. (2019).
Nonetheless, U-phosphate biomineralization is affected by many environmental factors including temperature, carbonate, pH, and ammonium. In alkaline conditions, for example, carbonate impacts U-phosphate biomineralization due to the higher affinity of CO32− with UO22+ than PO43−. Additionally, NH4+ can interact with UO22+ as well as with PO43− promoting the precipitation of uranium. A handful of studies have profiled the influence of uranium on the microbial communities of several ecosystems, which is known to induce changes in functional processes as well as in the taxonomic composition (Suriya et al., 2017; Sutcliffe et al., 2017; Sánchez-Castro et al., 2020). The microbial communities in different U-treated bentonites have been largely studied by using multiple state-of-the-art techniques (Lopez-Fernandez et al. 2018b; Povedano-Priego et al., 2019). Anoxic conditions will prevail in such systems and the presence of strict and facultative anaerobes is well established (Povedano-Priego et al., 2022).
This review highlights the potential role of microorganisms related to the safety of DGRs. As previously mentioned, this type of disposal is based on a multi-barrier system in which the waste will be encapsulated in corrosion-resistant metal canisters, surrounded by filling and sealing materials, which will be placed at depths of between 500–1,000 m in a stable geological formation. Microorganisms are able to colonize almost every single habitat on Earth, and the barriers of the DGRs are no exception. Even though microorganisms could be passively introduced through human manipulation of the different materials during the construction of the DGR, natural microbial communities in the different barriers may be the most important source of microbial activity within the repository. It is worth noting the importance of microorganisms naturally occurring in the filling and sealing materials (e.g., bentonite clay) that will be in direct contact with the metal canisters and, therefore, close to the radioactive waste. For this reason, the different bentonites (e.g., Opalinus, Boom and Spanish clay) have been extensively studied at microbiological level in order to understand the different processes in which microorganisms may be involved.
As we have tried to show in this review, microorganisms could influence the stability and security of the DGRs at different levels. Certain types of microbial communities (e.g., SRB) can cause the alteration of metal canisters (e.g., steel-based materials and copper) through direct or indirect processes, also known as microbially influenced corrosion (MIC). The canister corrosion, in the worst-case scenario, could suffer small pits or fissures, leaving the residue exposed to the next engineered barrier (the filling and sealing material). Moreover, MIC could be involved in the gas production in the DGR environment. MIC, radiolysis of water and microbial metabolism could promote a rise in the gas phase in the DGRs and produce an increase in pressure which would affect the integrity of the clay barrier. However, the metabolism of microorganisms could have a double effect related to gases in the repositories. The production of certain gases due to the metabolism of some bacterial groups may be used by other groups for their own metabolism, thus minimizing the possible pressure produced, positively affecting the safety of the DGR.
Additionally, microbial activity can indirectly influence solubility and hence the mobilization of radionuclides by the alteration of the geochemical conditions within the DGRs. These changes may result in changes in their oxidation state, affecting their solubility and mobility through the different barriers. Nonetheless, microorganisms could be involved in the mobility of these radionuclides. As mentioned before, several mechanisms such as biotransformation, biosorption, bioaccumulation, and biomineralization, may be involved in the interaction microbe-radionuclides. The result of these interactions produces a positive effect since certain microorganisms are able to immobilize elements such as Se, Eu, Cm, and U, making them unavailable to other organisms or, preventing their diffusion through the subsequent engineered barriers and then through to the environment.
Notwithstanding, the purpose of this review is to give an overview of the effect that microorganisms could have on the stability and safety of future DGRs. It will be necessary to consider different factors that could affect the bacterial communities present in the bentonite after the definitive closure of a DGR. One of these parameters is the temperature evolution throughout the confinement period. This temperature will initially increase due to the heat resulting from the decay of the HLW, and then slowly decrease until reaching environmental geosphere conditions over a period of 100,000 years (McMurry et al., 2003). Most models suggest a maximum temperature of 80°C–100°C reached within the first 100 years (McMurry et al., 2003; Butov et al., 2017; Leong et al., 2021). Moreover, another aspect to consider is the compaction density of the bentonite. The compaction has been shown to affect SRB viability demonstrating that the higher the density is, the lower the viability of this bacterial group (Bengtsson and Pedersen, 2017). In addition to these determinant factors, others must be considered such as the evolution to an anaerobic environment due to the gradual consumption of oxygen by the organisms, the slow diffusion of pore water through the bentonite and the low concentration of organic matter, among others. All these factors make the DGR system a harsh environment for certain groups of microorganisms preventing their growth and even their survival. Otherwise, many bacteria could remain in a dormant state until growth conditions became favorable again, such as size decrease, spore formation or cell desiccation (O'Sullivan et al., 2015). For this reason, it is crucial to investigate the microbial influence and the conditions that affect the bacterial communities within the DGR environment, in order to ensure the safety of the repository facilities throughout their service life.
MAR-F and MLM planned the work. MAR-F wrote the first draft of the manuscript, which was revised by MLM. MFM-M, CP-P, MM-H, and FJ assisted writing some sections of the manuscript. The final version was edited and revised by MAR-F, MFM-M, CP-P, MM-H, FJ, and MLM. All authors contributed to the article and approved the submitted version.
This work is funded by FEDER/Junta de Andalucía-Consejería de Transformación Económica, Industria, Conocimiento, y Universidades/Proyecto (B-RNM-250-UGR20) and grant RTI2018-101548-B-I00 of MCIN/AEI/10.13039/501100011033/ FEDER “Una manera de hacer Europa. B.RNM.250.UGR20 financiado por la Consejería de Universidad, Investigación e Innovación de la Junta de Andalucía y por FEDER.”
The authors acknowledge the assistance of Maria del Mar Abad Ortega, Isabel Sánchez Almazo, and Concepción Hernández Castillo (Centro de Instrumentación Científica, University of Granada, Spain) for their help with microscopy measurements and sample preparation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdalla, Z. A. Y., Ismail, M. Y. A., Njoroge, E. G., Hlatshwayo, T. T., Wendler, E., and Malherbe, J. B. (2020). Migration behaviour of selenium implanted into polycrystalline 3C–SiC. Vacuum 175:109235. doi: 10.1016/j.vacuum.2020.109235
Abdullahi, S. L., and Audu, A. A. (2017). Comparative analysis on chemical composition of bentonite clays obtained from Ashaka and tango deposits in Gombe state, Nigeria. ChemSearch J. 8, 35–40.
Abrahamsen, L., Arnold, T., Brinkmann, H., Leys, N., Merroun, M., Mijnendonckx, K., et al. (2015). A review of anthropogenic organic wastes and their degradation behaviour, Microbiology In Nuclear waste Disposal (MIND Project). Available at: https://igdtp.eu/wpcontent/uploads/2017/10/MIND-2015-12-D1.1-ReviewOfAnthropogenicOrganicWastes.pdf
Abramova, E., Popova, N., Artemiev, G., Boldyrev, K., Kazakov, K., Kryuchkov, D., et al. (2023). Biological factors affecting the evolution of safety barrier materials in the Yeniseisky deep geological repository. Eng. Geol. 312:106931. doi: 10.1016/j.enggeo.2022.106931
Acharya, C., Chandwadkar, P., and Nayak, C. (2017). Unusual versatility of the filamentous, Diazotrophic cyanobacterium Anabaena torulosa revealed for its survival during prolonged uranium exposure. Appl. Environ. Microbiol. 83, e03356–e03316. doi: 10.1128/AEM.03356-16
Ahonen, L., Pedersen, K., Small, J., Mijnendonckx, K., Weetjens, E., and Wouters, K. (2016). Year one evaluation report, microbiology in nuclear waste disposal (MIND project). Available at: https://mind15.eu/wp-content/uploads/2015/11/MIND-Deliverable-3.1-Y1-Evaluation.pdf
Akob, D., Lee, S., Sheth, M., Küsel, K., Watson, D., Palumbo, A. V., et al. (2012). Gene expression correlates with process rates quantified for sulfate- and Fe(III)-reducing bacteria in UVI-contaminated sediments. Front. Microbiol. 3:280. doi: 10.3389/fmicb.2012.00280
Alwaeli, M., and Mannheim, V. (2022). Investigation into the current state of nuclear energy and nuclear waste management—a state-of-the-art review. Energies 15:4275. doi: 10.3390/en15124275
Ansoborlo, E., Bion, L., Doizi, D., Moulin, C., Lourenco, V., Madic, C., et al. (2007). Current and future radionuclide speciation studies in biological media. Radiat. Prot. Dosimetry 127, 97–102. doi: 10.1093/rpd/ncm258
Ayangbenro, A. S., and Babalola, O. O. (2017). A new strategy for heavy metal polluted environments: a review of microbial biosorbents. Int. J. Environ. Res. Public Health 14:94. doi: 10.3390/ijerph14010094
Bachran, M., Kluge, S., Lopez-Fernandez, M., and Cherkouk, A. (2018). Microbial diversity in an arid, naturally saline environment. Microb. Ecol. 78, 494–505. doi: 10.1007/s00248-018-1301-2
Bader, M., Moll, H., Steudtner, R., Lösch, H., Drobot, B., Stumpf, T., et al. (2019). Association of Eu(III) and cm(III) onto an extremely halophilic archaeon. Environ. Sci. Pollut. Res. 26, 9352–9364. doi: 10.1007/s11356-019-04165-7
Baggio, G., Groves, R. A., Chignola, R., Piacenza, E., Presentato, A., Lewis, I. A., et al. (2021). Untargeted metabolomics investigation on selenite reduction to elemental selenium by bacillus mycoides SeITE01. Front. Microbiol. 12:711000. doi: 10.3389/fmicb.2021.711000
Bagnoud, A., Chourey, K., Hettich, R. L., de Bruijn, I., Andersson, A. F., Leupin, O. X., et al. (2016a). Reconstructing a hydrogen-driven microbial metabolic network in Opalinus clay rock. Nat. Commun. 7:12770. doi: 10.1038/ncomms12770
Bagnoud, A., de Bruijn, I., Andersson, A. F., Diomidis, N., Leupin, O. X., Schwyn, B., et al. (2016b). A minimalistic microbial food web in an excavated deep subsurface clay rock. FEMS Microbiol. Ecol. 92:fiv138. doi: 10.1093/femsec/fiv138
Bagnoud, A., Leupin, O., Schwyn, B., and Bernier-Latmani, R. (2016c). Rates of microbial hydrogen oxidation and sulfate reduction in Opalinus clay rock. Appl. Geochem. 72, 42–50. doi: 10.1016/J.APGEOCHEM.2016.06.011
Barkleit, A., Moll, H., and Bernhard, G. (2009). Complexation of uranium(VI) with peptidoglycan. Dalton Trans. 27, 5379–5385. doi: 10.1039/b818702a
Beaton, D., Pelletier, P., and Goulet, R. R. (2019). Microbial degradation of cellulosic material and gas generation: implications for the management of low-and intermediate-level radioactive waste. Front. Microbiol. 10, 1–13. doi: 10.3389/fmicb.2019.00204
Beazley, M. J., Martinez, R. J., Sobecky, P. A., Webb, S. M., and Taillefert, M. (2007). Uranium biomineralization as a result of bacterial phosphatase activity: insights from bacterial isolates from a contaminated subsurface. Environ. Sci. Technol. 41, 5701–5707. doi: 10.1021/es070567g
Ben Ali Gam, Z., Thioye, A., Cayol, J.-L., Joseph, M., Fauque, G., and Labat, M. (2018). Characterization of Desulfovibrio salinus sp. nov., a slightly halophilic sulfate-reducing bacterium isolated from a saline lake in Tunisia. Int. J. Syst. Evol. Microbiol. 68, 715–720. doi: 10.1099/ijsem.0.002567
Bengtsson, A., and Pedersen, K. (2016). Microbial sulfate-reducing activity overload pressure and density in water saturated boom clay. Appl. Clay Sci. 132-133, 542–551. doi: 10.1016/j.clay.2016.08.002
Bengtsson, A., and Pedersen, K. (2017). Microbial sulphide-producing activity in water saturated Wyoming MX-80, Asha and Calcigel bentonites at wet densities from 1500 to 2000 kg m− 3. Appl. Clay Sci. 137, 203–212. doi: 10.1016/j.clay.2016.12.024
Benko, I., Nagy, G., Tanczos, B., Ungvari, E., Sztrik, A., Eszenyi, P., et al. (2012). Subacute toxicity of nano-selenium compared to other selenium species in mice. Environ. Toxicol. Chem. 31, 2812–2820. doi: 10.1002/etc.1995
Brim, H., McFarlan, S. C., Fredrickson, J. K., Minton, K. W., Zhai, M., Wackett, L. P., et al. (2000). Engineering Deinococcus radiodurans for metal remediation in radioactive mixed waste environments. Nat. Biotechnol. 18, 85–90. doi: 10.1038/71986
Brown, A. R., Boothman, C., Pimblott, S. M., and Lloyd, J. R. (2015). The impact of gamma radiation on sediment microbial processes. Appl. Environ. Microbiol. 81, 4014–4025. doi: 10.1128/AEM.00590-15
Bulgarini, A., Lampis, S., Turner, R. J., and Vallini, G. (2021). Biomolecular composition of capping layer and stability of biogenic selenium nanoparticles synthesized by five bacterial species. J. Microbial. Biotechnol. 14, 198–212. doi: 10.1111/1751-7915.13666
Burns, P. C. (2012). Nuclear Fuel in a Reactor Accident. Science 335, 1184–1189. doi: 10.1126/science.1211285
Burzan, N., Murad Lima, R., Frutschi, M., Janowczyk, A., Reddy, B., Rance, A., et al. (2022). Growth and persistence of an aerobic microbial Community in Wyoming Bentonite MX-80 despite anoxic in situ conditions. Front. Microbiol. 13:858324. doi: 10.3389/fmicb.2022.858324
Butov, R. A., Drobyshevsky, N. I., Moiseenko, E. V., and Tokarev, Y. N. (2017). 3D numerical modelling of the thermal state of deep geological nuclear waste repositories. J. Phys 899:052002. doi: 10.1088/1742-6596/899/5/052002
Castañeda-Carrión, I. N., Sheik, C. S., and Krumholz, L. R. (2010). Desulfovibrio africanus subsp. uniflagellum subsp. nov., a sulfate-reducing bacterium from a uranium-contaminated subsurface aquifer. Int. J. Syst. Evol. Microbiol. 60, 880–886. doi: 10.1099/ijs.0.006668-0
Černoušek, T., Shrestha, R., Kovářová, H., Špánek, R., Ševců, A., Sihelská, K., et al. (2019). Microbially influenced corrosion of carbon steel in the presence of anaerobic sulfate-reducing bacteria. Corros. Engineer. Sci. Technol. 55, 127–137. doi: 10.1080/1478422X.2019.170064
Chandwadkar, P., Misra, H. S., and Acharya, C. (2018). Uranium biomineralization induced by a metal tolerant: Serratia strain under acid, alkaline and irradiated conditions. Metallomics 10, 1078–1088. doi: 10.1039/C8MT00061A
Chasteen, T. G., and Bentley, R. (2003). Biomethylation of selenium and tellurium: microorganisms and plants. Chem. Rev. 103, 1–26. doi: 10.1021/cr010210+
Chen, X., Ottosen, L. D. M., and Kofoed, M. V. W. (2019). How low can You go: methane production of Methanobacterium congolense at low CO2 concentrations. Front. Bioeng. Biotechnol. 7:34. doi: 10.3389/fbioe.2019.00034
Chen, J., Qin, Z., and Shoesmith, D. W. (2011). Long-term corrosion of copper in a dilute anaerobic sulfide solution. Electrochim. Acta 56, 7854–7861. doi: 10.1016/j.electacta.2011.04.086
Chen, S., Wang, P., and Zhang, D. (2014). Corrosion behaviour of copper under biofilm of sulfate-reducing bacteria. Corros. Sci. 87, 407–415. doi: 10.1016/j.corsci.2014.07.001
Choudhary, S., and Sar, P. (2009). Characterization of a metal resistant pseudomonas sp. isolated from uranium mine for its potential in heavy metal (Ni2+, Co2+, Cu2+, and Cd2+) sequestration. Bioresour. Technol. 100, 2482–2492. doi: 10.1016/j.biortech.2008.12.015
Close, D. M., Nelson, W. H., and Bernhard, W. A. (2013). DNA damage by the direct effect of ionizing radiation: products produced by two sequential one-electron oxidations. J. Phys. Chem. A 117, 12608–12615. doi: 10.1021/jp4084844
Cologgi, D. L., Speers, A. M., Bullard, B. A., Kelly, S. D., and Reguera, G. (2014). Enhanced uranium immobilization and reduction by Geobacter sulfurreducens biofilms. Appl. Environ. Microbiol. 80, 6638–6646. doi: 10.1128/AEM.02289-14
Davis, T. Z., Stegelmeier, B. L., Welch, K. D., Pfister, J. A., Panter, K. E., and Hall, J. O. (2013). Comparative oral dose toxicokinetics of selenium compounds commonly found in selenium accumulator plants. J. Anim. Sci. 91, 4501–4509. doi: 10.2527/jas.2012-6101
Diep, P., Mahadevan, R., and Yakunin, A. F. (2018). Heavy metal removal by bioaccumulation using genetically engineered microorganisms. Front. Bioeng. Biotechnol. 6:157. doi: 10.3389/fbioe.2018.00157
Dobias, J., Suvorova, E. I., and Bernier-Latmani, R. (2011). Role of proteins in controlling selenium nanoparticle size. Nanotechnology 22:195605. doi: 10.1088/0957-4484/22/19/195605
Dobrowolski, R., Szcześ, A., Czemierska, M., and Jarosz-Wikołazka, A. (2017). Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour. Technol. 225, 113–120. doi: 10.1016/j.biortech.2016.11.040
Dong, H., Jaisi, D. P., Kim, J., and Zhang, G. (2009). Microbe-clay mineral interactions. Am. Mineral. 94, 1505–1519. doi: 10.2138/am.2009.3246
Dou, W., Pu, Y., Han, X., Song, Y., Chen, S., and Gu, T. (2020). Corrosion of cu by a sulfate reducing bacterium in anaerobic vials with different headspace volumes. Bioelectrochemistry 133:107478. doi: 10.1016/j.bioelechem.2020.107478
Duro, L., Domènech, C., Grivé, M., Roman-Ross, G., Bruno, J., and Källström, K. (2014). Assessment of the evolution of the redox conditions in a low and intermediate level nuclear waste repository (SFR1, Sweden). Appl. Geochem. 49, 192–205. doi: 10.1016/j.apgeochem.2014.04.015
El Hajj, H., Abdelouas, A., Grambow, B., Martin, C., and Dion, M. (2010). Microbial corrosion of P235GH steel under geological conditions. Phys. Chem. Earth 35, 248–253. doi: 10.1016/j.pce.2010.04.007
Enning, D., and Garrelfs, J. (2014). Corrosion of iron by sulfate-reducing bacteria: new views of an old problem. Appl. Environ. Microbiol. 80, 1226–1236. doi: 10.1128/AEM.02848-13
Eswayah, A. S., Smith, T. J., and Gardiner, P. H. E. (2016). Microbial transformations of selenium species of relevance to bioremediation. Appl. Environ. Microbiol. 82, 4848–4859. doi: 10.1128/AEM.00877-16
Eswayah, A. S., Smith, T. J., Scheinost, A. C., Hondow, N., and Gardiner, P. H. E. (2017). Microbial transformations of selenite by methane-oxidizing bacteria. Appl. Microbiol. Biotechnol. 101, 6713–6724. doi: 10.1007/s00253-017-8380-8
Fakhar, A., Gul, B., Gurmani, A. R., Khan, S. M., Ali, S., Sultan, T., et al. (2022). Heavy metal remediation and resistance mechanism of Aeromonas, bacillus, and pseudomonas: a review. Crit. Rev. Environ. Sci. Technol. 52, 1868–1914. doi: 10.1080/10643389.2020.1863112
Favorito, J. E., Grossl, P. R., Davis, T. Z., Eick, M. J., and Hankes, N. (2021). Soil-plant-animal relationships and geochemistry of selenium in the Western phosphate resource area (United States): a review. Chemosphere 266:128959. doi: 10.1016/j.chemosphere.2020.128959
Faybishenko, B., Birkholzer, J., Sassani, D., and Swift, P. (2017). In: International approaches for deep geological disposal of nuclear waste: geological challenges in radioactive waste isolation-5th worldwide review. LBNL-1006984-2016.
Fomina, M., and Gadd, G. M. (2014). Biosorption: current perspectives on concept, definition and application. Bioresour. Technol. 160, 3–14. doi: 10.1016/j.biortech.2013.12.102
Francis, A. J., and Nancharaiah, Y. V. (2015). “9-in situ and ex situ bioremediation of radionuclide-contaminated soils at nuclear and norm sites” in Woodhead publishing series in energy: Environmental remediation and restoration of contaminated nuclear and norm sites. ed. L. van Velzen (Sawston, United Kingdom: Woodhead) 185–236. doi: 10.1016/B978-1-78242-231-0.00009-0
Gabani, P., and Singh, O. V. (2013). Radiation-resistant extremophiles and their potential in biotechnology and therapeutics. Appl. Microbiol. Biotechnol. 97, 993–1004. doi: 10.1007/s00253-012-4642-7
Gadd, G. M. (2004). Microbial influence on metal mobility and application for bioremediation. Geoderma 122, 109–119. doi: 10.1016/j.geoderma.2004.01.002
Gadd, G. M. (2009). Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J. Chem. Technol. Biotechnol. 84, 13–28. doi: 10.1002/jctb.1999
Gallois, N., Alpha-Bazin, B., Bremond, N., Ortet, P., Barakat, M., Piette, L., et al. (2022). Discovery and characterization of UipA, a uranium- and iron-binding PepSY protein involved in uranium tolerance by soil bacteria. ISME J. 16, 705–716. doi: 10.1038/s41396-021-01113-7
Gao, W., and Francis, A. J. (2013). Fermentation and hydrogen metabolism affect uranium reduction by clostridia. ISRN Biotechnol. 2013, 1–11. doi: 10.5402/2013/657160
García-Romero, E., María Manchado, E., Suárez, M., and García-Rivas, J. (2019). Spanish bentonites: a review and new data on their geology, mineralogy, and crystal chemistry. Fortschr. Mineral. 9:696. doi: 10.3390/min9110696
Gerber, U., Zirnstein, I., Krawczyk-Bärsch, E., Lünsdorf, H., Arnold, T., and Merroun, M. L. (2016). Combined use of flow cytometry and microscopy to study the interactions between the gram-negative betaproteobacterium Acidovorax facilis and uranium(VI). J. Hazard. Mater. 317, 127–134. doi: 10.1016/j.jhazmat.2016.05.062
Gorietti, D., Giardina, I., Arginelli, D., and Battisti, P. (2017). Determination of plutonium, americium and curium isotopes in radioactive metal wastes deriving from nuclear decommissioning. J. Radioanal. Nucl. Chem. 314, 1785–1792. doi: 10.1007/s10967-017-5553-y
Grigoryan, A. A., Jalique, D. R., Stroes-Gascoyne, S., Wolfaardt, G. M., Keech, P. G., and Korber, D. R. (2021). Prediction of bacterial functional diversity in clay microcosms. Heliyon 7:e08131. doi: 10.1016/j.heliyon.2021.e08131
Grouzdev, D. S., Safonov, A., Babich, T. L., Tourova, T. P., Krutkina, M. S., and Nazina, T. N. (2018). Draft genome sequence of a dissimilatory U(VI)-reducing bacterium, Shewanella xiamenensis strain DCB2-1, isolated from nitrate- and radionuclide-contaminated groundwater in Russia. Genome Announc. 6, e00555–e00518. doi: 10.1128/genomeA.00555-18
Guo, G., and Fall, M. (2021). Advances in modelling of hydro-mechanical processes in gas migration within saturated bentonite: a state-of-art review. Eng. Geol. 287:106123. doi: 10.1016/j.enggeo.2021.106123
Hall, D. S., Behazin, M., Jeffrey Binns, W., and Keech, P. G. (2021). An evaluation of corrosion processes affecting copper-coated nuclear waste containers in a deep geological repository. Prog. Mater. Sci. 118:100766. doi: 10.1016/j.pmatsci.2020.100766
Hamed, M. M., Holiel, M., and El-Aryan, Y. F. (2017). Removal of selenium and iodine radionuclides from waste solutions using synthetic inorganic ion exchanger. J. Mol. Liq. 242, 722–731. doi: 10.1016/j.molliq.2017.07.035
Handrlica, J. (2019). Reprocessing of nuclear fuel: certain legal issues arising from this unique technology. Int. J. Legal Res. 9, 150–161.
Hassan, R. S., Abass, M. R., Eid, M. A., and Abdel-Galil, E. A. (2021). Sorption of some radionuclides from liquid waste solutions using anionic clay hydrotalcite sorbent. Appl. Radiat. Isot. 178:109985. doi: 10.1016/j.apradiso.2021.109985
Hedrich, S., Schlömann, M., and Barrie Johnson, D. (2011). The iron-oxidizing proteobacteria. Microbiology 157, 1551–1564. doi: 10.1099/mic.0.045344-0
Hökmark, H., and Fälth, B. (2003). Thermal dimensioning of the deep repository. Influence of canister spacing, canister power, rock thermal properties and nearfield design on the maximum canister surface temperature. SKB Technical Report TR-03-09, Sweden.
Holmboe, M., Norrfors, K. K., Jonsson, M., and Wold, S. (2011). Effect of γ-radiation on radionuclide retention in compacted bentonite. Radiat. Phys. Chem. 80, 1371–1377. doi: 10.1016/j.radphyschem.2011.08.004
Horvath, A., and Rachlew, E. (2016). Nuclear power in the 21st century: challenges and possibilities. Ambio 45, 38–49. doi: 10.1007/s13280-015-0732-y
Huang, W. H., and Chen, W. C. (2004). Swelling behavior of a potential buffer material under simulated near field environment. J. Nucl. Sci. Technol. 41, 1271–1279. doi: 10.1080/18811248.2004.9726356
Hufton, J., Harding, J., Smith, T., and Romero-González, M. E. (2021). The importance of the bacterial cell wall in uranium(VI) biosorption. Phys. Chem. Chem. Phys. 23, 1566–1576. doi: 10.1039/D0CP04067C
Hupert-Kocurek, K., Saczyńska, A., and Piotrowska-Seget, Z. (2013). Cadmium increases catechol 2,3-dioxygenase activity in Variovorax sp. 12S, a metal-tolerant and phenol-degrading strain. Antonie Van Leeuwenhoek 104, 845–853. doi: 10.1007/s10482-013-9997-y
IAEA. (2009a). Classification of radioactive waste. General safety guide [internet]. Vienna, International Atomic Energy Agency. IAEA safety standards series, no. GSG-1 STI/PUB/1419. Available at: https://www-pub.iaea.org/mtcd/publications/pdf/pub1419_web.pdf
IAEA. (2009b). Status and trends of nuclear technologies: Report of the international project on innovative nuclear reactors and fuel cycles (INPRO). Vienna, International Atomic Energy Agency. Available at: https://www-pub.iaea.org/MTCD/Publications/PDF/TE_1622_Web.pdf
IAEA. (2015). Climate change and nuclear power 2015. Vienna, International Atomic Energy Agency. Available at: https://www-pub.iaea.org/MTCD/Publications/PDF/CCANP2015Web-78834554.pdf
IAEA. (2018). Status and trends in spent fuel and radioactive waste management. Vienna, International Atomic Energy Agency. Nuclear Energy Series No. NW-T-1.14, 74. Available at: https://www-pub.iaea.org/MTCD/Publications/PDF/PUB1963_web.pdf
Jain, R., Jordan, N., Tsushima, S., Hübner, R., Weiss, S., and Lens, P. N. L. (2017). Shape change of biogenic elemental selenium nanomaterials from nanospheres to nanorods decreases their colloidal stability. Environ. Sci. Nano 4, 1054–1063. doi: 10.1039/c7en00145b
Jroundi, F., Descostes, M., Povedano-Priego, C., Sánchez-Castro, I., Suvannagan, V., Grizard, P., et al. (2020). Profiling native aquifer bacteria in a uranium roll-front deposit and their role in biogeochemical cycle dynamics: insights regarding in situ recovery mining. Sci. Total Environ. 721:137758. doi: 10.1016/j.scitotenv.2020.137758
Jung, K. W., Lim, S., and Bahn, Y. S. (2017). Microbial radiation-resistance mechanisms. J. Microbiol. 55, 499–507. doi: 10.1007/s12275-017-7242-5
Junier, P., Vecchia, E. D., and Bernier-Latmani, R. (2011). The response of Desulfotomaculum reducens MI-1 to UVI exposure: a transcriptomic study. Geomicrobiol J. 28, 483–496. doi: 10.1080/01490451.2010.512031
Kamnev, A. A., Mamchenkova, P., Dyatlova, Y. A., and Tugarova, A. V. (2017). FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J. Mol. Struct. 1140, 106–112. doi: 10.1016/j.molstruc.2016.12.003
Kessi, J., and Hanselmann, K. W. (2004). Similarities between the abiotic reduction of selenite with glutathione and the dissimilatory reaction mediated by Rhodospirillum rubrum and Escherichia coli. J. Biol. Chem. 279, 50662–50669. doi: 10.1074/jbc.M405887200
Kim, J., Dong, H., Yang, K., Park, H., Elliott, W. C., Spivack, A., et al. (2019). Naturally occurring, microbially induced smectite-to-illite reaction. Geology 47, 535–539. doi: 10.1130/G46122.1
Kip, N., and Van Veen, J. A. (2015). The dual role of microbes in corrosion. Int. Soc. Microb. Ecol. J. 9, 542–551. doi: 10.1038/ismej.2014.169
Kolhe, N., Zinjarde, S., and Acharya, C. (2018). Responses exhibited by various microbial groups relevant to uranium exposure. Biotechnol. Adv. 36, 1828–1846. doi: 10.1016/j.biotechadv.2018.07.002
Kónya, J., and Nagy, N. M. (2018). “Environmental radioactivity” in Nuclear and radiochemistry. eds. J. Kónya and N. M. Nagy. 2nd ed (Netherlands: Elsevier), 399–419.
Kooyman, T., Buiron, L., and Rimpault, G. (2018). A comparison of curium, neptunium and americium transmutation feasibility. Ann. Nucl. Energy 112, 748–758. doi: 10.1016/j.anucene.2017.09.041
Kora, A. J. (2018). Bacillus cereus, selenite-reducing bacterium from contaminated lake of an industrial area: a renewable nanofactory for the synthesis of selenium nanoparticles. Bioresourc. Bioprocess. 5:30. doi: 10.1186/s40643-018-0217-5
Kumari, I., Kumar, B. V. R., and Khanna, A. (2020). A review on UREX processes for nuclear spent fuel reprocessing. Nucl. Eng. Des. 358:110410. doi: 10.1016/j.nucengdes.2019.110410
Kurmakova, I., Kupchyk, O., Bondar, O., Demchenko, N., and Vorobyova, V. (2019). Corrosion of copper in a medium of bacteria sulfate reduction proceeding. J. Chem. Technol. Metallurgy 54, 416–422.
Kushwaha, S., Marcus, A. K., and Rittmann, B. E. (2018). pH-dependent speciation and hydrogen (H2) control U(VI) respiration by Desulfovibrio vulgaris. Biotechnol. Bioeng. 115, 1465–1474. doi: 10.1002/bit.26579
Lampis, S., Zonaro, E., Bertolini, C., Cecconi, D., Monti, F., Micaroni, M., et al. (2017). Selenite biotransformation and detoxification by Stenotrophomonas maltophilia SeITE02: novel clues on the route to bacterial biogenesis of selenium nanoparticles. J. Hazard. Mater. 324, 3–14. doi: 10.1016/j.jhazmat.2016.02.035
Lelieveld, J., Kunkel, D., and Lawrence, M. G. (2012). Global risk of radioactive fallout after major nuclear reactor accidents. Atmos. Chem. Phys. 12, 4245–4258. doi: 10.5194/acp-12-4245-2012
Lenz, M., Van Aelst, A. C., Smit, M., Corvini, P. F. X., and Lens, P. N. L. (2009). Biological production of selenium nanoparticles from waste waters. Adv. Mat. Res. 71–73, 721–724. doi: 10.4028/www.scientific.net/AMR.71-73.721
Leong, J., Ponnambalam, K., Binns, J., and Elkamel, A. (2021). Thermally constrained conceptual deep geological repository design under spacing and placing uncertainties. Appl. Sci. 11:11874. doi: 10.3390/app112411874
Leupin, O. X., Bernier-Latmani, R., Bagnoud, A., Moors, H., Leys, N., Wouters, K., et al. (2017). Fifteen years of microbiological investigation in Opalinus clay at the Mont Terri rock laboratory (Switzerland). Swiss J. Geosci. 110, 343–354. doi: 10.1007/s00015-016-0255-y
Li, H., Zhang, J., Wang, T., Luo, W., Zhou, Q., and Jiang, G. (2008). Elemental selenium particles at nano-size (Nano-se) are more toxic to Medaka (Oryzias latipes) as a consequence of hyper-accumulation of selenium: a comparison with sodium selenite. Aquat. Toxicol. 89, 251–256. doi: 10.1016/j.aquatox.2008.07.008
Lin, H., Zhou, M., Li, B., and Dong, Y. (2023). Mechanisms, application advances and future perspectives of microbial-induced heavy metal precipitation: a review. Int. Biodeter. Biodegr. 178:105544. doi: 10.1016/j.ibiod.2022.105544
Liu, Y., Hedwig, S., Schäffer, A., Lenz, M., and Martinez, M. (2021). Sulfur amino acid status controls selenium methylation in pseudomonas tolaasii: identification of a novel metabolite from promiscuous enzyme reactions. Appl. Environ. Microbiol. 87, e00104–e00121. doi: 10.1128/AEM.00104-21
Liu, X., Long, D., You, H., Yang, D., Zhou, S., Zhang, S., et al. (2016). Phosphatidylcholine affects the secretion of the alkaline phosphatase PhoA in pseudomonas strains. Microbiol. Res. 192, 21–29. doi: 10.1016/j.micres.2016.02.001
Lopez-Fernandez, M., Jroundi, F., Ruiz-Fresneda, M. A., and Merroun, M. L. (2020). Microbial interaction with and tolerance of radionuclides: underlying mechanisms and biotechnological applications. J. Microbial. Biotechnol. 14, 810–828. doi: 10.1111/1751-7915.13718
Lopez-Fernandez, M., Cherkouk, A., Vilchez-Vargas, R., Jauregui, R., Pieper, D., Boon, N., et al. (2015). Bacterial diversity in bentonites, engineered barrier for deep geological disposal of radioactive wastes. Microb. Ecol. 70, 922–935. doi: 10.1007/s00248-015-0630-7
Lopez-Fernandez, M., Fernández-Sanfrancisco, O., Moreno-García, A., Martín-Sánchez, I., Sánchez-Castro, I., and Merroun, M. L. (2014). Microbial communities in bentonite formations and their interactions with uranium. Appl. Geochem. 49, 77–86. doi: 10.1016/j.apgeochem.2014.06.022
Lopez-Fernandez, M., Matschiavelli, N., and Merroun, M. L. (2021). Bentonite geomicrobiology. Microbiol. Nuclear Waste Disposal 2021, 137–155. doi: 10.1016/B978-0-12-818695-4.00007-1
Lopez-Fernandez, M., Simone, D., Wu, X., Soler, L., Nilsson, E., Holmfeldt, K., et al. (2018a). Metatranscriptomes reveal that all three domains of life are active but are dominated by bacteria in the Fennoscandian crystalline granitic continental deep biosphere. MBio 9, e01792–e01718. doi: 10.1128/mBio.01792-18
Lopez-Fernandez, M., Vilchez-Vargas, R., Jroundi, F., Boon, N., Pieper, D., and Merroun, M. L. (2018b). Microbial community changes induced by uranyl nitrate in bentonite clay microcosms. Appl. Clay Sci. 160, 206–216. doi: 10.1016/j.clay.2017.12.034
Lopez-Fernandez, M., Moll, H., and Merroun, M. L. (2018c). Reversible pH-dependent curium(III) biosorption by the bentonite yeast isolate Rhodotorula mucilaginosa BII-R8. J. Hazard. Mater. 370, 156–163. doi: 10.1016/j.jhazmat.2018.06.054
Losi, M. E., and Frankenberger, W. T. Jr. (1998). Microbial oxidation and solubilization of precipitated elemental selenium in soil. J. Environ. Qual. 27, 836–843. doi: 10.2134/jeq1998.00472425002700040018x
Luo, X., Wang, Y., Lan, Y., An, L., Wang, G., Li, M., et al. (2022). Microbial oxidation of organic and elemental selenium to selenite. Sci. Total Environ. 833:155203. doi: 10.1016/j.scitotenv.2022.155203
Ma, B., Charlet, L., Fernandez-Martinez, A., Kang, M., and Madé, B. (2018). Review of the retention mechanisms of redox-sensitive radionuclides in multi-barrier systems. Appl. Geochem. 100, 414–431. doi: 10.1016/j.apgeochem.2018.12.001
Majumder, E. L.-W., and Wall, J. D. (2017). Uranium bio-transformations: chemical or biological processes? Open J. Inorgan. Chem. 7, 28–60. doi: 10.4236/ojic.2017.72003
Maleke, M., Valverde, A., Vermeulen, J. G., Cason, E., Gomez-Arias, A., Moloantoa, K., et al. (2019). Biomineralization and bioaccumulation of europium by a thermophilic metal resistant bacterium. Front. Microbiol. 10, 1–10. doi: 10.3389/fmicb.2019.00081
Meleshyn, A. (2014). Microbial processes relevant for the long-term performance of high-level radioactive waste repositories in clays. Geological Society. (London: Special Publications) 400, 179–194. doi: 10.1144/SP400.6
Martinez, R. J., Beazley, M. J., Taillefert, M., Arakaki, A. K., Skolnick, J., and Sobecky, P. A. (2007). Aerobic uranium VI bioprecipitation by metal-resistant bacteria isolated from radionuclide- and metal-contaminated subsurface soils. Environ. Microbiol. 9, 3122–3133. doi: 10.1111/j.1462-2920.2007.01422.x
Martinez-Rodriguez, P., Sanchez-Castro, I., Ojeda, J. J., Abad, M. M., Descostes, M., and Merroun, M. L. (2022). Effect of different phosphate sources on uranium biomineralization by the microbacterium sp. Be9 strain: a multidisciplinary approach study. Front. Microbiol. 13:1092184. doi: 10.3389/fmicb.2022.1092184
Masurat, P., Eriksson, S., and Pedersen, K. (2010). Evidence of indigenous Sulphate-reducing bacteria in commercial Wyoming bentonite MX-80. Appl. Clay Sci. 47, 51–57. doi: 10.1016/j.clay.2008.07.002
McMurry, J., Dixon, D. A., Garroni, J. D., Ikeda, B. M., Stroes-Gascoyne, S., Baumgartner, P., et al. (2003). Evolution of a Canadian deep geologic repository: Base scenario, report 06819, 01200-100092. Ontario Power Generation, Nuclear Waste Management Division. Toronto Canada.
Merroun, M. L., Nedelkova, M., Ojeda, J. J., Reitz, T., Fernández, M. L., Arias, J. M., et al. (2011). Bio-precipitation of uranium by two bacterial isolates recovered from extreme environments as estimated by potentiometric titration, TEM and X-ray absorption spectroscopic analyses. J. Hazard. Mater. 197, 1–10. doi: 10.1016/j.jhazmat.2011.09.049
Merroun, M. L., Raff, J., Rossberg, A., Hennig, C., Reich, T., and Selenska-Pobell, S. (2005). Complexation of uranium by cells and S-layer sheets of Bacillus sphaericus JG-A12. Appl. Environ. Microbiol. 71, 5532–5543. doi: 10.1128/AEM.71.9.5532-5543.2005
Mijnendonckx, K., Bleyen, N., van Gompel, A., Coninx, I., and Leys, N. (2022). pH and microbial community determine the denitrifying activity in the presence of nitrate-containing radioactive waste. Front. Microbiol. 13:968220. doi: 10.3389/fmicb.2022.968220
Mijnendonckx, K., Miroslav, H., Wang, L., Jacops, E., Provoost, A., Mysara, M., et al. (2019). An active microbial community in boom clay pore water collected from piezometers impedes validating predictive modelling of ongoing geochemical processes. Appl. Geochem. 106, 149–160. doi: 10.1016/j.apgeochem.2019.05.009
Mijnendonckx, K., Monsieurs, P., Černá, K., Hlaváčková, V., Steinová, J., Burzan, N., et al. (2021). Chapter 4: molecular techniques for understanding microbial abundance and activity in clay barriers used for geodisposal. In: J. L. Lloyd and A. Cherkouk (Eds.) The microbiology of nuclear waste disposal, (Amsterdam, The Netherlands: Elsevier) 71–96. doi: 10.1016/B978-0-12-818695-4.00004-6
Mills, M. M., Sanchez, A. C., Boisvert, L., Payne, C. B., Ho, T. A., and Wang, Y. (2022). Understanding smectite to illite transformation at elevated (> 100° C) temperature: effects of liquid/solid ratio, interlayer cation, solution chemistry and reaction time. Chem. Geol. 615:121214. doi: 10.1016/j.chemgeo.2022.121214
Mishra, A., and Malik, A. (2013). Recent advances in microbial metal bioaccumulation. Crit. Rev. Environ. Sci. Technol. 43, 1162–1222. doi: 10.1080/10934529.2011.627044
Mitchell, N., Pérez-Sánchez, D., and Thorne, M. C. (2013). A review of the behaviour of U-238 series radionuclides in soils and plants. J. Radiol. Prot. 33:R17. doi: 10.1088/0952-4746/33/2/R17
Mohapatra, D. P., Robinson, K. A., Huang, F., Kirpalani, D., and Loewen, M. C. (2022). Insights into increasing Selenate reductase enzyme activity in the presence of nitrogen-doped graphite electrodes for selenium effluent treatment. Water 14:931. doi: 10.3390/w14060931
Moll, H., Barkleit, A., Frost, L., and Raff, J. (2021). Curium(III) speciation in the presence of microbial cell wall components. Ecotoxicol. Environ. Saf. 227:112887. doi: 10.1016/j.ecoenv.2021.112887
Moll, H., Lütke, L., Bachvarova, V., Cherkouk, A., Selenska-Pobell, S., and Bernhard, G. (2014). Interactions of the Mont Terri Opalinus clay isolate Sporomusa sp. MT-2.99 with curium(III) and europium(III). Geomicrobiol J. 31, 682–696. doi: 10.1080/01490451.2014.889975
Moll, H., Lütke, L., Barkleit, A., and Bernhard, G. (2013). Curium(III) speciation studies with cells of a groundwater strain of Pseudomonas fluorescens. Geomicrobiol J. 30, 337–346. doi: 10.1080/01490451.2012.688927
NEA. (2020a). Management and disposal of high-level radioactive waste: Global Progress and solutions, OECD Publishing, Paris Available at: https://www.oecd-nea.org/jcms/pl_32567/management-and-disposal-of-high-level-radioactive-waste-global-progress-and-solutions
NEA. (2020b). The environmental and ethical basis of geological disposal of Long-lived radioactive wastes, OECD Publishing, Paris. Available at: https://oecd-nea.org/jcms/pl_34631/the-environmental-and-ethical-basis-of-geological-disposal-of-long-lived-radioactive-wastes
Newman, R. C., Rumash, K., and Webster, B. J. (1992). The effect of pre-corrosion on the corrosion rate of steel in neutral solutions containing sulphide: relevance to microbially influenced corrosion. Corros. Sci. 33, 1877–1884. doi: 10.1016/0010-938X(92)90190-E
Newsome, L., Morris, K., Trivedi, D., Atherton, N., and Lloyd, J. R. (2014). Microbial reduction of uraniumVI in sediments of different lithologies collected from Sellafield. Appl. Geochem. 51, 55–64. doi: 10.1016/j.apgeochem.2014.09.008
Nilgiriwala, K. S., Alahari, A., Rao, A. S., and Apte, S. K. (2008). Cloning and overexpression of alkaline phosphatase PhoK from Sphingomonas sp. strain BSAR-1 for bioprecipitation of uranium from alkaline solutions. Appl. Environ. Microbiol. 74, 5516–5523. doi: 10.1128/AEM.00107-08
Nuclear Waste Management Organization. (2022). Programs around the world for managing used nuclear fuel. Toronto: nwmo; [updated April 2022]. Available at: https://www.nwmo.ca/~/media/Site/Files/PDFs/2022/05/31/12/28/Programs-around-the-world-backgrounder-2022.ashx?la=en
O'Sullivan, L. A., Roussel, E. G., Weightman, A. J., Webster, G., Hubert, C. R., Bell, E., et al. (2015). Survival of Desulfotomaculum spores from estuarine sediments after serial autoclaving and high-temperature exposure. ISME J. 9, 922–933. doi: 10.1038/ismej.2014.190
Parihar, L., Johal, J. K., and Singh, V. (2013). Bioremediation of uranium in contaminated water samples of Bathinda, Punjab by Desulfovibrio genus. J. Soil Sci. Environ. Manage. 4, 1–5. doi: 10.5897/JSSEM12.058
Park, D. M., and Jiao, Y. (2014). Modulation of medium pH by Caulobacter crescentus facilitates recovery from uranium-induced growth arrest. Appl. Environ. Microbiol. 80, 5680–5688. doi: 10.1128/AEM.01294-14
Paterson-Beedle, M., Jeong, B. C., Lee, C. H., Jee, K. Y., Kim, W. H., Renshaw, J. C., et al. (2012). Radiotolerance of phosphatases of a Serratia sp.: potential for the use of this organism in the biomineralization of wastes containing radionuclides. Biotechnol. Bioeng. 109, 1937–1946. doi: 10.1002/bit.24467
Payne, R. B., Gentry, D. M., Rapp-Giles, B. J., Casalot, L., and Wall, J. D. (2002). Uranium reduction by Desulfovibrio desulfuricans strain G20 and a cytochrome c3 mutant. Appl. Environ. Microbiol. 68, 3129–3132. doi: 10.1128/AEM.68.6.3129-3132.2002
Pedersen, K. (2010). Analysis of copper corrosion in compacted bentonite clay as a function of clay density and growth conditions for sulfate-reducing bacteria. J. Appl. Microbiol. 108, 1094–1104. doi: 10.1111/j.1365-2672.2009.04629.x
Pedersen, K. (2013). Metabolic activity of subterranean microbial communities in deep granitic groundwater supplemented with methane and H2. ISME J. 7, 839–849. doi: 10.1038/ismej.2012.144
Periyakaruppan, A., Kumar, F., Sarkar, S., Sharma, C. S., and Ramesh, G. T. (2007). Uranium induces oxidative stress in lung epithelial cells. Arch. Toxicol. 81, 389–395. doi: 10.1007/s00204-006-0167-0
Pinel-Cabello, M., Chapon, V., Ruiz-Fresneda, M. A., Alpha-Bazin, B., Berthomieu, C., Armengaud, J., et al. (2021a). Delineation of cellular stages and identification of key proteins for reduction and biotransformation of se(IV) by Stenotrophomonas bentonitica BII-R7. J. Hazard. Mater. 418:126150. doi: 10.1016/j.jhazmat.2021.126150
Pinel-Cabello, M., Jroundi, F., López-Fernández, M., Geffers, R., Jarek, M., Jauregui, R., et al. (2021b). Multisystem combined uranium resistance mechanisms and bioremediation potential of Stenotrophomonas bentonitica BII-R7: transcriptomics and microscopic study. J. Hazard. Mater. 403:123858. doi: 10.1016/j.jhazmat.2020.123858
Plötze, M., Kahr, G., and Stengele, R. H. (2003). Alteration of clay minerals — gamma-irradiation effects on physico chemical properties. Appl. Clay Sci. 23, 195–202. doi: 10.1016/S0169-1317(03)00103-0
Porcelli, D. (2018). “Radioactivity” in Encyclopedia of geochemistry: A comprehensive reference source on the chemistry of the earth. ed. W. M. White (Cham: Springer International Publishing), 1295–1298.
Povedano-Priego, C., Jroundi, F., Lopez-Fernandez, M., Morales-Hidalgo, M., Martin-Sánchez, I., Huertas, F. J., et al. (2022). Impact of anoxic conditions, uraniumVI and organic phosphate substrate on the biogeochemical potential of the indigenous bacterial community of bentonite. Appl. Clay Sci. 216:106331. doi: 10.1016/j.clay.2021.106331
Povedano-Priego, C., Jroundi, F., Lopez-Fernandez, M., Sánchez-Castro, I., Martin-Sánchez, I., Huertas, F. J., et al. (2019). Shifts in bentonite bacterial community and mineralogy in response to uranium and glycerol-2-phosphate exposure. Sci. Total Environ. 692, 219–232. doi: 10.1016/j.scitotenv.2019.07.228
Povedano-Priego, C., Jroundi, F., Lopez-Fernandez, M., Shrestha, R., Spanek, R., Martín-Sánchez, I., et al. (2021). Deciphering indigenous bacteria in compacted bentonite through a novel and efficient DNA extraction method: insights into biogeochemical processes within the deep geological disposal of nuclear waste concept. J. Hazard. Mater. 408:124600. doi: 10.1016/j.jhazmat.2020.124600
Povedano-Priego, C., Jroundi, F., Solari, P. L., Guerra-Tschuschke, I., Abad-Ortega, M. D. M., Link, A., et al. (2023). Unlocking the bentonite microbial diversity and its implications in selenium bioreduction and biotransformation: advances in deep geological repositories. J. Hazard. Mater. 445:130557. doi: 10.1016/j.jhazmat.2022.130557
Prakash, D., Gabani, P., Chandel, A. K., Ronen, Z., and Singh, O. (2013). Bioremediation: a genuine technology to remediate radionuclides from the environment. J. Microbial. Biotechnol. 6, 349–360. doi: 10.1111/1751-7915.12059
Prăvălie, R., and Bandoc, G. (2018). Nuclear energy: between global electricity demand, worldwide decarbonisation imperativeness, and planetary environmental implications. J. Environ. Manage. 209, 81–92. doi: 10.1016/j.jenvman.2017.12.043
Priest, N. D. (2001). Toxicity of depleted uranium. Lancet 357, 244–246. doi: 10.1016/S0140-6736(00)03605-9
Pushkareva, R., Kalinichenko, E., Lytovchenko, A., Pushkarev, A., Kadochnikov, V., and Plastynina, M. (2002). Irradiation effect on physico-chemical properties of clay minerals. Appl. Clay Sci. 21, 117–123. doi: 10.1016/S0169-1317(01)00097-7
Rashwan, T. L., Asad, M. A., Molnar, I. L., Behazin, M., Keech, P. G., and Krol, M. M. (2022). Exploring the governing transport mechanisms of corrosive agents in a Canadian deep geological repository. Sci. Total Environ. 828:153944. doi: 10.1016/j.scitotenv.2022.153944
Rauschenbach, I., Posternak, V., Cantarella, P., McConnell, J., Starovoytov, V., and Häggblom, M. M. (2013). Seleniivibrio woodruffii gen. Nov., sp. nov., a selenate- and arsenate-respiring bacterium in the Deferribacteraceae. Int. J. Syst. Evol. Microbiol. 63, 3659–3665. doi: 10.1099/ijs.0.043547-0
Reitz, T., Rossberg, A., Barkleit, A., Selenska-Pobell, S., and Merroun, M. L. (2014). Decrease of UVI immobilization capability of the facultative anaerobic strain Paenibacillus sp. JG-TB8 under anoxic conditions due to strongly reduced phosphatase activity. PLoS One 9:e102447. doi: 10.1371/journal.pone.0102447
Renninger, N., Knopp, R., Nitsche, H., Clark, D. S., and Keasling, J. D. (2004). Uranyl precipitation by Pseudomonas aeruginosa via controlled polyphosphate metabolism. Appl. Environ. Microbiol. 70, 7404–7412. doi: 10.1128/AEM.70.12.7404-7412.2004
Rogiers, T., Houdt, R. V., Williamson, A., Leys, N., Boon, N., and Mijnendonckx, K. (2022). Molecular mechanisms underlying bacterial uranium resistance. Front. Microbiol. 13:822197. doi: 10.3389/fmicb.2022.822197
Roh, C., Kang, C. K., and Lloyd, J. R. (2015). Microbial bioremediation processes for radioactive waste. Korean J. Chem. Eng. 32, 1720–1726. doi: 10.1007/s11814-015-0128-5
Rosa, E. A., Tuler, S. P., Fischhoff, B., Webler, T., Friedman, S. M., Sclove, R. E., et al. (2010). Nuclear waste: knowledge waste? Science 329, 762–763. doi: 10.1126/science.1193205
Rui, X., Kwon, M. J., O’Loughlin, E. J., Dunham-Cheatham, S., Fein, J. B., Bunker, B., et al. (2013). Bioreduction of hydrogen uranyl phosphate: mechanisms and U(IV) products. Environ. Sci. Technol. 47, 5668–5678. doi: 10.1021/es305258p
Ruiz Fresneda, M. A., Delgado Martín, J., Gómez Bolívar, J., Fernández Cantos, M., Bosch-Estévez, G., Martínez Moreno, M. F., et al. (2018). Green synthesis and biotransformation of amorphous se nanospheres to trigonal 1D se nanostructures: impact on se mobility within the concept of radioactive waste disposal. Environ. Sci. Nano 5, 2103–2116. doi: 10.1039/c8en00221e
Ruiz-Fresneda, M. A., Eswayah, A. S., Romero-González, M., Gardiner, P. H. E., Solari, P. L., and Merroun, M. L. (2020a). Chemical and structural characterization of SeIV biotransformations by Stenotrophomonas bentonitica into Se0 nanostructures and volatiles se species. Environ. Sci. Nano 7, 2140–2155. doi: 10.1039/D0EN00507J
Ruiz-Fresneda, M. A., Fernández-Cantos, M., Gómez-Bolívar, J., Eswayah, A. S., Gardiner, P. H. E., Pinel-Cabello, M., et al. (2023). Combined bioreduction and volatilization of SeVI by Stenotrophomonas bentonitica: formation of trigonal selenium nanorods and methylated species. Sci. Total Environ. 858:160030. doi: 10.1016/j.scitotenv.2022.160030
Ruiz-Fresneda, M. A., Gomez-Bolivar, J., Delgado-Martin, J., del Mar Abad-Ortega, M., Guerra-Tschuschke, I., and Merroun, M. L. (2019). The bioreduction of selenite under anaerobic and alkaline conditions analogous to those expected for a deep geological repository system. Molecules 24:3868. doi: 10.3390/molecules24213868
Ruiz-Fresneda, M. A., Lopez-Fernandez, M., Martinez-Moreno, M. F., Cherkouk, A., Ju-Nam, Y., Ojeda, J. J., et al. (2020b). Molecular binding of EuIII/CmIIIby Stenotrophomonas bentonitica and its impact on the safety of future Geodisposal of radioactive waste. Environ. Sci. Technol. 54, 15180–15190. doi: 10.1021/acs.est.0c02418
Sánchez-Castro, I., Martínez-Rodríguez, P., Abad, M. M., Descostes, M., and Merroun, M. L. (2021). Uranium removal from complex mining waters by alginate beads doped with cells of Stenotrophomonas sp. Br8: novel perspectives for metal bioremediation. J. Environ. Manage. 296:113411. doi: 10.1016/j.jenvman.2021.113411
Sánchez-Castro, I., Martínez-Rodríguez, P., Jroundi, F., Solari, P. L., Descostes, M., and Merroun, M. L. (2020). High-efficient microbial immobilization of solved UVI by the Stenotrophomonas strain Br8. Water Res. 183:116110. doi: 10.1016/j.watres.2020.116110
Sarikhani, M. R., Malboobi, M., Aliasgharzad, N., and Greiner, R. (2019). Identification of two novel bacterial phosphatase-encoding genes in pseudomonas putida strain P13. J. Appl. Microbiol. 127, 1113–1124. doi: 10.1111/jam.14376
Sepulveda-Medina, P., Katsenovich, Y., Musaramthota, V., Lee, M., Lee, B., Dua, R., et al. (2015). The effect of uranium on bacterial viability and cell surface morphology using atomic force microscopy in the presence of bicarbonate ions. Res. Microbiol. 166, 419–427. doi: 10.1016/j.resmic.2015.03.003
Shrestha, R., Cerna, K., Spanek, R., Bartak, D., Cernousek, T., and Sevcu, A. (2022). The effect of low-pH concrete on microbial community development in bentonite suspensions as a model for microbial activity prediction in future nuclear waste repository. Sci. Total Environ. 808:151861. doi: 10.1016/j.scitotenv.2021.151861
Shrestha, R., Černoušek, T., Stoulil, J., Kovářová, H., Sihelská, K., Špánek, R., et al. (2021). Anaerobic microbial corrosion of carbon steel under conditions relevant for deep geological repository of nuclear waste. Sci. Total Environ. 800:149539. doi: 10.1016/j.scitotenv.2021.149539
Shukla, A., Parmar, P., and Saraf, M. (2017). Radiation, radionuclides and bacteria: An in-perspective review. J. Environ. Radioact. 180, 27–35. doi: 10.1016/j.jenvrad.2017.09.013
Sivaswamy, V., Boyanov, M. I., Peyton, B. M., Viamajala, S., Gerlach, R., Apel, W. A., et al. (2011). Multiple mechanisms of uranium immobilization by Cellulomonas sp. strain ES6. Biotechnol. Bioeng. 108, 264–276. doi: 10.1002/bit.22956
Smart, N. R., Reddy, B., Rance, A. P., Nixon, D. J., Frutschi, M., Bernier-Latmani, R., et al. (2017). The anaerobic corrosion of carbon steel in compacted bentonite exposed to natural Opalinus clay porewater containing native microbial populations. Corros. Engineer. Sci. Technol. 52, 101–112. doi: 10.1080/1478422X.2017.1315233
Smits, K. M., Sakaki, T., Howington, S. E., Peters, J. F., and Illangasekare, T. H. (2013). Temperature dependence of thermal properties of sands across a wide range of temperatures (30-70°C). Vadose Zone J. 138, 2256–2265. doi: 10.2136/vzj2012.0033
Song, D., Li, X., Cheng, Y., Xiao, X., Lu, Z., Wang, Y., et al. (2017). Aerobic biogenesis of selenium nanoparticles by Enterobacter cloacae Z0206 as a consequence of fumarate reductase mediated selenite reduction. Sci. Rep. 7:3239. doi: 10.1038/s41598-017-03558-3
Sreedevi, P. R., Suresh, K., and Jiang, G. (2022). Bacterial bioremediation of heavy metals in wastewater: a review of processes and applications. J. Water Process Engineer. 48:102884. doi: 10.1016/j.jwpe.2022.102884
Staicu, L. C., Wójtowicz, P. J., Molnár, Z., Ruiz-Agudo, E., Gallego, J. L. R., Baragaño, D., et al. (2022). Interplay between arsenic and selenium biomineralization in Shewanella sp. O23S. Environ. Pollut. 306:119451. doi: 10.1016/j.envpol.2022.119451
Stylo, M., Neubert, N., Roebbert, Y., Weyer, S., and Bernier-Latmani, R. (2015). Mechanism of uranium reduction and immobilization in Desulfovibrio vulgaris biofilms. Environ. Sci. Technol. 49, 10553–10561. doi: 10.1021/acs.est.5b01769
Sun, W., and Nešic, S. (2007). A mechanistic model of H2S corrosion of mild steel. In: Paper presented at the CORROSION 2007, Nashville, Tennessee, march 2007.
Suriya, J., Chandra Shekar, M., Nathani, N. M., Suganya, T., Bharathiraja, S., and Krishnan, M. (2017). Assessment of bacterial community composition in response to uranium levels in sediment samples of sacred Cauvery River. Appl. Microbiol. Biotechnol. 101, 831–841. doi: 10.1007/s00253-016-7945-2
Sutcliffe, B., Chariton, A. A., Harford, A. J., Hose, G. C., Greenfield, P., Elbourne, L. D. H., et al. (2017). Effects of uranium concentration on microbial community structure and functional potential. Environ. Microbiol. 19, 3323–3341. doi: 10.1111/1462-2920.13839
Suzuki, Y., Kelly, S. D., Kemner, K. M., and Banfield, J. F. (2003). Microbial populations stimulated for hexavalent uranium reduction in uranium mine sediment. Appl. Microbiol. Biotechnol. 69, 1337–1346. doi: 10.1128/AEM.69.3.1337-1346.2003
Tabak, H. H., Lens, P., van Hullebusch, E. D., and Dejonghe, W. (2005). Developments in bioremediation of soils and sediments polluted with metals and radionuclides-1. Microbial processes and mechanisms affecting bioremediation of metal contamination and influencing metal toxicity and transport. Rev. Environ. Sci. Biotechnol. 4, 115–156. doi: 10.1007/s11157-005-2169-4
Tan, Y., Wang, Y., Wang, Y., Xu, D., Huang, Y., Wang, D., et al. (2018). Novel mechanisms of selenate and selenite reduction in the obligate aerobic bacterium Comamonas testosteroni S44. J. Hazard. Mater. 359, 129–138. doi: 10.1016/j.jhazmat.2018.07.014
Tan, Y., Yao, R., Wang, R., Wang, D., Wang, G., and Zheng, S. (2016). Reduction of selenite to se(0) nanoparticles by filamentous bacterium Streptomyces sp. ES2-5 isolated from a selenium mining soil. Microb. Cell Fact. 15:157. doi: 10.1186/s12934-016-0554-z
Theodorakopoulos, N., Chapon, V., Coppin, F., Floriani, M., Vercouter, T., Sergeant, C., et al. (2015). Use of combined microscopic and spectroscopic techniques to reveal interactions between uranium and microbacterium sp. A9, a strain isolated from the Chernobyl exclusion zone. J. Hazard. Mater. 285, 285–293. doi: 10.1016/j.jhazmat.2014.12.018
Tišáková, L., Pipíška, M., Godány, A., Horník, M., Vidová, B., and Augustín, J. (2013). Bioaccumulation of 137Cs and 60Co by bacteria isolated from spent nuclear fuel pools. J. Radioanal. Nucl. Chem. 295, 737–748. doi: 10.1007/s10967-012-1932-6
Tripathy, S., Thomas, H. R., and Stratos, P. (2017). Response of compacted bentonites to thermal and thermo-hydraulic loadings at high temperatures. Geosciences 7:53. doi: 10.3390/geosciences7030053
Ullah, A., Yin, X., Wang, F., Xu, B., Mirani, Z. A., Xu, B., et al. (2021). Biosynthesis of selenium nanoparticles (via Bacillus subtilis BSN313), and their isolation, characterization, and bioactivities. Molecules 26:5559. doi: 10.3390/molecules26185559
UNSCEAR. (2013), Sources, effects and risks of ionizing radiation: report to the general assembly with scientific annexes. UNSCEAR 2013 report volume I. New York, United Nations, 2014. Available at: http://www.unscear.org/unscear/en/publications/2013_1.html
Videla, H. A., and Herrera, L. K. (2009). Understanding microbial inhibition of corrosion. A comprehensive overview. Int. Biodeter. Biodegr. 63, 896–900. doi: 10.1016/j.ibiod.2009.02.002
Villar, M. V., Pérez del Villar, L., Martín, P. L., Pelayo, M., Fernández, A. M., Garralon, A., et al. (2006). The study of Spanish clays for their use as sealing materials in nuclear waste repositories: 20 years of progress. J. Iber. Geol. 32, 15–36.
Vogel, M., Fischer, S., Maffert, A., Hübner, R., Scheinost, A. C., Franzen, C., et al. (2018). Biotransformation and detoxification of selenite by microbial biogenesis of selenium-sulfur nanoparticles. J. Hazard. Mater. 344, 749–757. doi: 10.1016/j.jhazmat.2017.10.034
Waite, T. D., Davis, J. A., Payne, T. E., Waychunas, G. A., and Xu, N. (1994). UraniumVI adsorption to ferrihydrite: application of a surface complexation model. Geochim. Cosmochim. Acta 58, 5465–5478. doi: 10.1016/0016-7037(94)90243-7
Wang, M., Jiang, D., and Huang, X. (2022). Selenium nanoparticle rapidly synthesized by a novel highly selenite-tolerant strain Proteus penneri LAB-1. iScience 25:104904. doi: 10.1016/j.isci.2022.104904
Wang, Y., Shu, X., Zhou, Q., Fan, T., Wang, T., Chen, X., et al. (2018). Selenite reduction and the biogenesis of selenium nanoparticles by Alcaligenes faecalis se03 isolated from the gut of Monochamus alternatus (Coleoptera: Cerambycidae). Int. J. Mol. Sci. 19:2799. doi: 10.3390/ijms19092799
Xu, Y., Zeng, Z., and Lv, H. (2019). Temperature dependence of apparent thermal conductivity of compacted bentonites as buffer material for high-level radioactive waste repository. Appl. Clay Sci. 174, 10–14. doi: 10.1016/j.clay.2019.03.017
Xu, C., Zhang, Y., Cheng, G., and Zhu, W. (2008). Pitting corrosion behavior of 316L stainless steel in the media of sulfate-reducing and iron-oxidizing bacteria. Mater Charact 59, 245–255. doi: 10.1016/j.matchar.2007.01.001
Yang, T., Chen, M. L., and Wang, J. H. (2015). Genetic and chemical modification of cells for selective separation and analysis of heavy metals of biological or environmental significance. Trends Anal. Chem. 66, 90–102. doi: 10.1016/j.trac.2014.11.016
You, W., Peng, W., Tian, Z., and Zheng, M. (2021). Uranium bioremediation with UVI-reducing bacteria. Sci. Total Environ. 798:149107. doi: 10.1016/j.scitotenv.2021.149107
Yun, J., Malvankar, N. S., Ueki, T., and Lovley, D. R. (2016). Functional environmental proteomics: elucidating the role of a c-type cytochrome abundant during uranium bioremediation. ISME J. 10, 310–320. doi: 10.1038/ismej.2015.113
Yung, M. C., and Jiao, Y. (2014). Biomineralization of uranium by PhoY phosphatase activity aids cell survival in Caulobacter crescentus. Appl. Environ. Microbiol. 80, 4795–4804. doi: 10.1128/AEM.01050-14
Zambonino, M. C., Quizhpe, E. M., Jaramillo, F. E., Rahman, A., Santiago Vispo, N., Jeffryes, C., et al. (2021). Green synthesis of selenium and tellurium nanoparticles: current trends, biological properties and biomedical applications. Int. J. Mol. Sci. 22:989. doi: 10.3390/ijms22030989
Zhang, X., Gu, P., and Liu, Y. (2019). Decontamination of radioactive wastewater: state of the art and challenges forward. Chemosphere 215, 543–553. doi: 10.1016/j.chemosphere.2018.10.029
Zhang, J., Song, H., Chen, Z., Liu, S., Wei, Y., Huang, J., et al. (2018). Biomineralization mechanism of U(VI) induced by Bacillus cereus 12-2: the role of functional groups and enzymes. Chemosphere 206, 682–692. doi: 10.1016/j.chemosphere.2018.04.181
Zheng, L., Rutqvist, J., Xu, H., and Birkholzer, J. T. (2017). Couple THMC models for bentonite in an argillite repository for nuclear waste: Illitization and its effect on swelling stress under high temperature. Eng. Geol. 230, 118–129. doi: 10.1016/j.enggeo.2017.10.002
Keywords: radioactivity, waste, deep geological repository, microorganisms, perspectives
Citation: Ruiz-Fresneda MA, Martinez-Moreno MF, Povedano-Priego C, Morales-Hidalgo M, Jroundi F and Merroun ML (2023) Impact of microbial processes on the safety of deep geological repositories for radioactive waste. Front. Microbiol. 14:1134078. doi: 10.3389/fmicb.2023.1134078
Received: 29 December 2022; Accepted: 27 February 2023;
Published: 16 March 2023.
Edited by:
Sudhir K. Shukla, Bhabha Atomic Research Centre (BARC), IndiaReviewed by:
Atif Khan, Bhabha Atomic Research Centre (BARC), IndiaCopyright © 2023 Ruiz-Fresneda, Martinez-Moreno, Povedano-Priego, Morales-Hidalgo, Jroundi and Merroun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miguel A. Ruiz-Fresneda, bWFmcmVzQHVnci5lcw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.