
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol., 17 April 2023
Sec. Microbial Symbioses
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1130695
This article is part of the Research TopicRising Stars in Microbial Symbioses: 2022View all 8 articles
 Krystal Salas1
Krystal Salas1 Ana M. Cabello1,2
Ana M. Cabello1,2 Kendra A. Turk-Kubo1
Kendra A. Turk-Kubo1 Jonathan P. Zehr1*
Jonathan P. Zehr1* Francisco M. Cornejo-Castillo1,3*
Francisco M. Cornejo-Castillo1,3*The multiple symbiotic partnerships between closely related species of the haptophyte algae Braarudosphaera bigelowii and the nitrogen-fixing cyanobacteria Candidatus Atelocyanobacterium thalassa (UCYN-A) contribute importantly to the nitrogen and carbon cycles in vast areas of the ocean. The diversity of the eukaryotic 18S rDNA phylogenetic gene marker has helped to identify some of these symbiotic haptophyte species, yet we still lack a genetic marker to assess its diversity at a finer scale. One of such genes is the ammonium transporter (amt) gene, which encodes the protein that might be involved in the uptake of ammonium from UCYN-A in these symbiotic haptophytes. Here, we designed three specific PCR primer sets targeting the amt gene of the haptophyte species (A1-Host) symbiotic with the open ocean UCYN-A1 sublineage, and tested them in samples collected from open ocean and near-shore environments. Regardless of the primer pair used at Station ALOHA, which is where UCYN-A1 is the pre-dominant UCYN-A sublineage, the most abundant amt amplicon sequence variant (ASV) was taxonomically classified as A1-Host. In addition, two out of the three PCR primer sets revealed the existence of closely-related divergent haptophyte amt ASVs (>95% nucleotide identity). These divergent amt ASVs had higher relative abundances than the haptophyte typically associated with UCYN-A1 in the Bering Sea, or co-occurred with the previously identified A1-Host in the Coral Sea, suggesting the presence of new diversity of closely-related A1-Hosts in polar and temperate waters. Therefore, our study reveals an overlooked diversity of haptophytes species with distinct biogeographic distributions partnering with UCYN-A, and provides new primers that will help to gain new knowledge of the UCYN-A/haptophyte symbiosis.
Biological dinitrogen (N2) fixation, i.e., the reduction of dissolved N2 gas into ammonia by N2-fixing prokaryotic microorganisms (diazotrophs), supplies new nitrogen (N) and thus supports primary production in the surface ocean (Zehr and Capone, 2020). In the ocean, some of these diazotrophs are symbiotic with phytoplankton species, which is the case for the group of unicellular N2-fixing symbiotic cyanobacteria known as Candidatus Atelocyanobacterium thalassa (hereafter UCYN-A). UCYN-A live in symbiosis with the unicellular haptophyte alga Braarudosphaera bigelowii and close relative species (Thompson et al., 2012; Hagino et al., 2013), being both symbiotic partners relevant contributors to marine N2 and carbon fixation, respectively, (Montoya et al., 2004; Martínez-Pérez et al., 2016; Zehr et al., 2016).
The diversity of the UCYN-A/haptophyte symbiosis has been explored using both phylogenetic and functional gene markers, which results have yielded differences in the number of phylotypes. For instance, while the 16S rDNA phylogenetic marker has resolved up to 3 distinct UCYN-A sublineages (UCYN-A1, −A2, and −A3) (Thompson et al., 2012; Hagino et al., 2013; Cornejo-Castillo et al., 2019), the diversity of the nifH gene, typically used for exploring the diversity of N2-fixers, has served to define up to 6–7 distinct UCYN-A sublineages with different biogeographical distributions (Farnelid et al., 2016; Turk-Kubo et al., 2017). However, regarding the diversity of the haptophyte species symbiotic with UCYN-A, only 2 different species have been identified based on the sequence diversity of the 18S rRNA phylogenetic marker (Thompson et al., 2012; Hagino et al., 2013; Cornejo-Castillo et al., 2019). Therefore, we currently lack a functional gene marker that could provide a finer phylogenetic resolution for these symbiotic haptophytes similar to the nifH gene marker in UCYN-A.
Not only is ammonium considered to be the preferred form of N for phytoplankton uptake (Glibert et al., 2016), but it has also been suggested to be one of the forms of N that these symbiotic haptophytes receive from UCYN-A (Sarkar et al., 2021). Ammonium addition experiments performed on natural populations of haptophyte species harboring UCYN-A1 or UCYN-A2 symbionts showed that these symbiotic haptophytes do not take up enough external ammonium to meet their nitrogen requirements, while UCYN-A kept providing fixed N to their haptophyte partners (Mills et al., 2020), further suggesting that UCYN-A is the main source of fixed N for these haptophytes. The protein involved in the uptake of ammonium is encoded by the ammonium transporter (amt) gene and, in metatranscriptomic data generated by the TARA Oceans project, this gene has been reported to be transcribed in the south Atlantic in, at least, the haptophyte species harboring the UCYN-A1 sublineage (Vorobev et al., 2020), altogether indicating that the amt gene might be relevant for the uptake of fixed N from UCYN-A in these haptophytes.
Here, we investigated the utility of the ammonium transporter (amt) gene as a meaningful marker to explore the diversity of the haptophyte species symbiotic with the UCYN-A1 sublineage at a fine-phylogenetic resolution. We designed three sets of PCR primer pairs to detect the amt gene of the haptophyte species (hereafter, A1-Host) that live in symbiosis with the nitrogen-fixing cyanobacterial sublineage UCYN-A1, which is considered to be the most abundant and widespread UCYN-A sublineage in open ocean waters (Cabello et al., 2016; Martínez-Pérez et al., 2016; Turk-Kubo et al., 2017). We tested these new PCR primer sets in environmental samples collected from a variety of locations, including both open ocean and near-shore sites (Table 1), and analyzed the amplified amt gene sequences. Our study offers a useful set of primers to detect these symbiotic haptophytes in environmental samples, and provides new insights on its biodiversity and biogeography.
The three new sets of amt PCR primer pairs designed here were tested in environmental DNA samples collected from diverse marine regions. Information about the geographic region and coordinates of the collected samples as well as the volume of seawater filtered, the size fraction collected, and whether the sample was pre-filtered for each sample is summarized in Table 1.
In order to design specific primers for the detection of the A1-Host amt gene, we first collected and analyzed the diversity of closely related amt gene sequences together with an A1-Host amt gene sequence (MATOU-v1_26947674) that had been identified as such based on co-abundant gene groups that included gene sequences from both UCYN-A1 and its haptophyte host (for further details see Vorobev et al., 2020). We performed a default blastn search on NCBI (nr/nt database; October 2019) using the A1-Host amt gene sequence (MATOU-v1_26947674) as query and collected amt gene sequences from different species (Table 2). In addition, we also included amt gene sequences from a study that explored the genetic structure of genes involved in the metabolism of N in phytoplankton species, including ammonium transporter genes (Bhadury et al., 2011).
We used the amt gene sequence diversity to identify unique regions within the A1-Host amt gene sequence. Briefly, amt gene sequences were aligned using the multiple sequence alignment (MSA) tool in Geneious Software (11.0.5). Regions of the amt sequence with sufficient mismatches across species to ensure specificity of amplification of the primers toward A1-Host were carefully selected and three different primer pairs targeting different regions of the A1-Host amt gene sequence were designed (Table 3). In addition, to further assure the specificity in silico of the newly designed primers, we ran the ‘Primer BLAST’ tool through NCBI.
DNA extractions were performed using a protocol based on the DNeasy Plant Mini kit (Qiagen) with slight modifications following Moisander et al. (2008). DNA extracts were stored at −80°C. The following PCR program was used: initial denaturation at 95°C for 3 min, followed by 25 cycles of denaturation at 95°C for 30 s, annealing at 58°C for 30 s and elongation at 72°C for 30 s. All PCR reactions (25 μL) contained Platinum™ Taq DNA polymerase (6 units; Invitrogen, Carlsbad, CA, USA), 1X PCR Buffer (-MgCl2), 4 mM MgCl2, 400 μM dNTP mix, 0.5 μM of each primer (forward and reverse) (Table 3), and 1 μL of template DNA. PCR products were visualized by electrophoresis on 1% agarose gel using 1X TBE buffer diluted with milliQ and 100BP Gene Ladder (Thermo Scientific GeneRuler 100 bp Gene Ladder).
The PCR amplification used the newly designed primers (Table 2) with 5′ common sequence linkers CS-F (5′-ACA CTG ACG ACA TGG TTC TAC A-3′) in the forward primers and CS-R (5′-TAC GGT AGC AGA GAC TTG GTC T-3′) in the reverse primers (Moonsamy et al., 2013). Reaction conditions and thermocycling parameters are described in the previous section. Library preparation and amplicon sequencing using Illumina MiSeq technology was carried out at the DNA Services Facility at the University of Chicago, Illinois.1 A total of 1,365,483 raw paired-end reads were obtained, ranging from 27,278 up to 118,887 read counts per sample (integrating two technical replicates per sample) for a total of 19 samples (Supplementary Table 1).
The dada2 pipeline was used for processing and analyzing the amplicon data of the Illumina sequences following the protocol published in https://benjjneb.github.io/dada2/index.html (Callahan et al., 2016; Supplementary Table 1). In order to determine amplicon sequence variants (ASVs) closely related to the A1-Host amt gene, those ASVs accounting for >0.5% of the total sequences were aligned with the A1-Host amt gene using the MSA Tool in Geneious Software (11.0.5). Sequences sharing at least 95% identity at the nucleotide level with the A1-Host amt gene were classified as A1-Host closely related ASVs since this percentage has been previously defined as a convenient threshold to co-assemble single-amplified protist genomes that belonged to the same species (Latorre et al., 2021).
In order to detect and amplify the A1-Host amt gene from environmental samples, three primer pairs (AMT-555F/AMT-705R, AMT-557F/AMT-715R, and AMT-330F/AMT-456R) targeting different regions of the A1-Host amt gene sequence were designed (Table 3). Briefly, after aligning the A1-Host amt gene sequence with closely related amt sequences, we detected three conserved nucleotide regions with enough mismatches that would potentially ensure specificity of the primers toward the A1-Host amt gene (Supplementary Figures 1–3). The primer pair AMT-555F/AMT-705R showed at least 3 mismatches (2 and 1 with AMT-555F and AMT-705R, respectively) with non-A1-Host amt gene sequences (Supplementary Figure 1). Similarly, the primer pair AMT-557F/AMT-715R showed at least 3 mismatches (2 and 1 with AMT-557F and AMT-715R, respectively) with non-A1-Host amt gene sequences (Supplementary Figure 2). Primers AMT-330F/AMT-456R showed at least 6 mismatches (2 and 4 with AMT-330F and AMT-456R, respectively) with non-A1-Host amt gene sequences (Supplementary Figure 3).
To test the performance of the primers for amplifying the A1-Host amt gene sequence, PCR amplifications using each of the three different amt PCR primer sets were carried out using environmental samples collected from open ocean [North Pacific, Coral Sea (South Pacific), Arctic Ocean (Bering Sea and Beaufort Sea)] and near-shore locations [Tosa Bay (Japan), Santa Cruz (CA, USA) and San Diego (CA, USA)] (Table 1 and Supplementary Table 1). These samples were chosen because they had previously been shown to contain different UCYN-A sublineages based on nifH gene sequencing and/or Catalyzed Reporter Deposition Fluorescence In Situ Hybridization (CARD-FISH) (Hagino et al., 2013; Harding et al., 2018; Henke et al., 2018; Cornejo-Castillo et al., 2019; Cabello et al., 2020; Gradoville et al., 2020; Turk-Kubo et al., 2021). PCR products showed that primers AMT-557F/AMT-715R amplified DNA fragments of the expected size in all samples except the Santa Cruz Wharf (Supplementary Figure 4). Likewise, primers AMT-555F/AMT-705R showed amplification in the North Pacific and Arctic Ocean samples, although dim PCR bands could also be observed in the Coral Sea and Tosa Bay (Japan) (Supplementary Figure 4). Finally, the AMT-330F/AMT-456R primer pair showed a more restricted amplification, only exhibiting PCR bands in the North Pacific and Coral Sea samples (Supplementary Figure 4).
Illumina sequencing of the PCR products showed the diversity of amt gene sequences captured by each amt PCR primer set. In total, the primer pairs AMT-555F/AMT-705R and AMT-557F/AMT-715R recovered a higher number of amplicon sequence variants (ASVs) across samples than the AMT-330F/AMT-456R primer pair (Figure 1). In particular, the primer pair AMT-555F/AMT-705R generated 596 ASVs, and similarly, the primer pair AMT-557F/AMT-715R amplified 733 ASVs. ASVs captured by these primer pairs and accounting for greater than 0.5% of the total reads, represented 23 ASVs and 78% of the total reads for the primer pair AMT-555F/AMT-705R, and 12 ASVs and 86% of the reads for the primer pair AMT-557F/AMT-715R (Figure 1). The most abundant ASV, i.e., the one containing the highest number of reads, for both the AMT-555F/AMT-705R [ASV-1(555F/705R)] and the AMT-557F/AMT-715R [ASV-1(557F/715R)] primer pairs shared 100% nucleotide identity with the A1-host amt gene (Figure 2). ASV-1(555F/705R) and ASV-1(557F/715R) were mainly found in the North Pacific and Coral Sea, and also at the SIO Pier (San Diego) but at much lower relative abundance (Figure 2). Other ASVs showing high percentage of similarity with the A1-Host amt gene were recovered with the AMT-555F/AMT-705R primer pair, specifically ASV-5(555F/705R), ASV-9(555F/705R), ASV-15(555F/705R), and ASV-19(555F/705R) shared 96.5, 99.1, 94.7, and 95.6% nucleotide identity with the A1-Host amt gene, respectively, (Figure 2). Interestingly, these ASVs had a different distribution than the ASV-1(555F/705R), for instance, ASV-9(555F/705R), found preferentially in the Coral Sea, or ASV-19(555F/705R), in the Bering Sea (Figure 2).
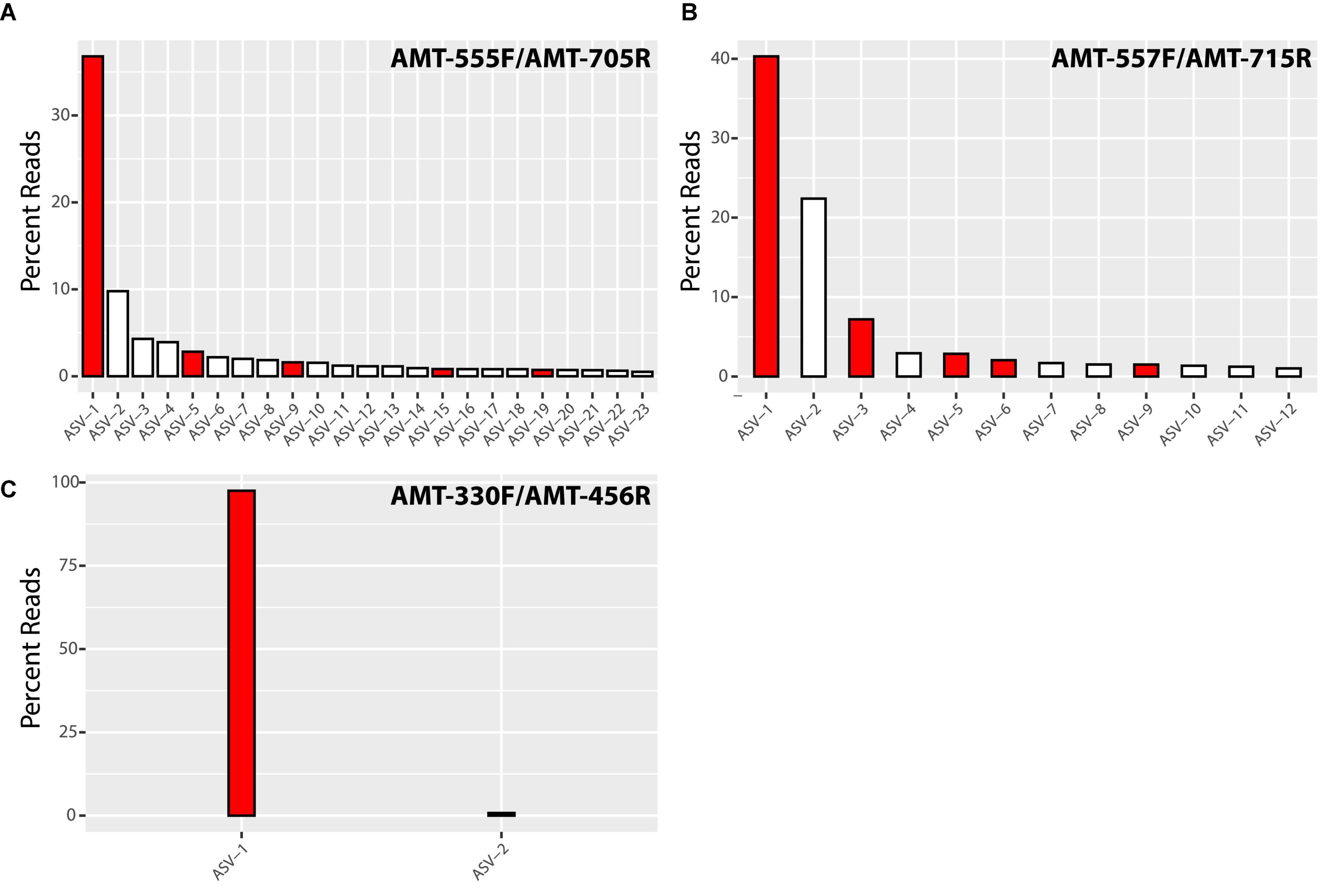
Figure 1. Bar chart of the percentage of total reads represented by significant amplicon sequence variants (ASVs) amplified by AMT-555F/AMT-705R (A), AMT-557F/AMT-715R (B) and AMT-330F/AMT-456R (C) primer pairs. We have defined significant ASVs as those accounting for more than 0.5% of total reads. Red bars represent ASVs closely related (>95% nucleotide identity) to the A1-Host amt gene, while white bars indicate non-A1-Host related ASVs.
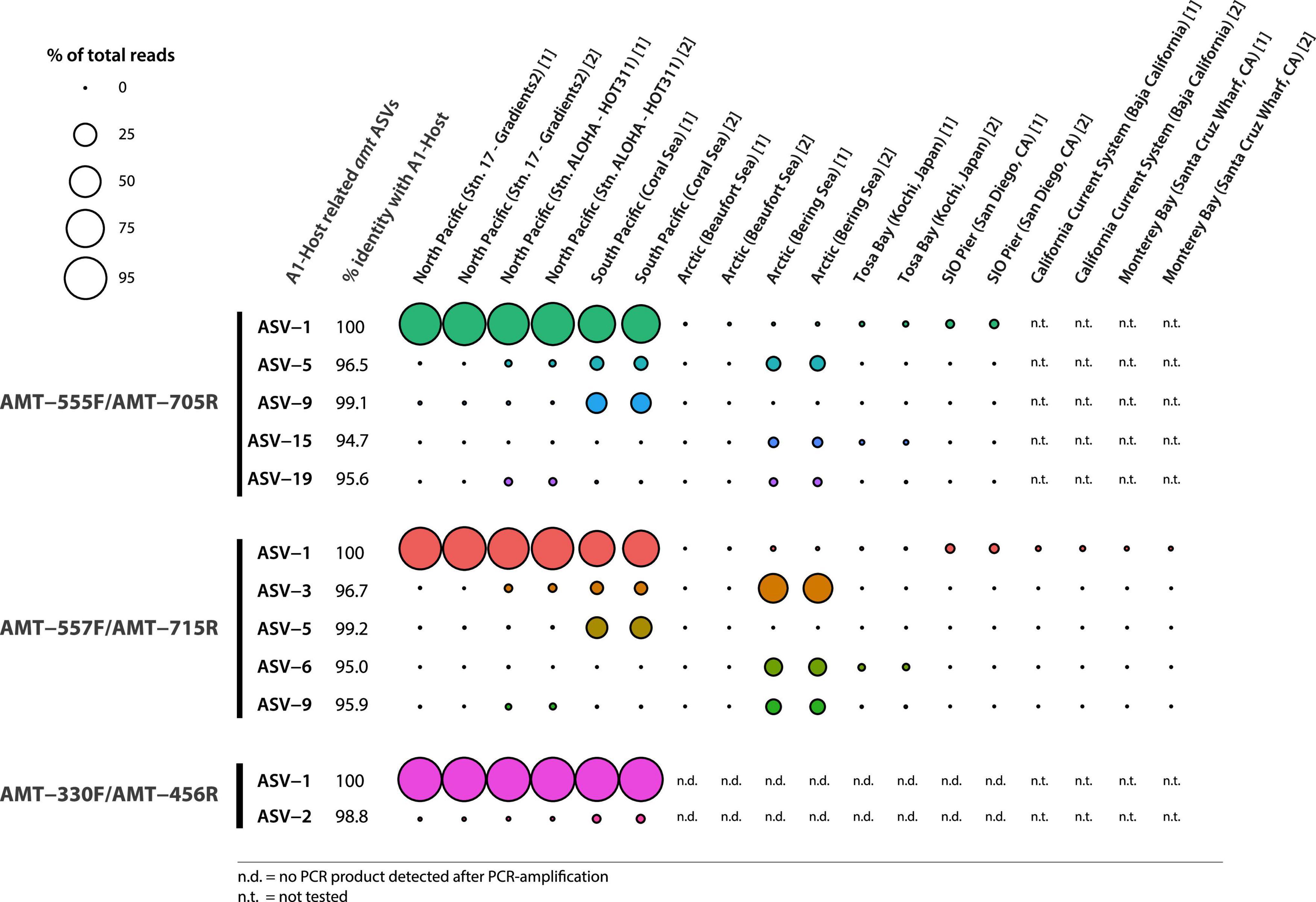
Figure 2. Bubble chart representing the relative abundance of ASVs closely related to the A1-Host amt gene (red bars in Figure 1) across samples (technical replicates are indicated with numbers between brackets after the name of the samples). ASVs amplified by different primer pairs are indicated. Bubble size is determined by percent reads represented by every ASV within the same sample. Closely related ASVs and the percent identity shared with the A1-Host amt gene are shown as well as the % of reads captured in each of the tested samples.
The primer pair AMT-557F/AMT-715R captured mostly the same diversity as the primer pair AMT-555F/AMT-705R (Figure 2). Therefore, the primer pair AMT-557F/AMT-715R, in addition to ASV-1(557F/715R), also captured ASVs that were closely related to the A1-Host amt gene. In particular, ASV-3(557F/715R), ASV-5(557F/715R), ASV-6(557F/715R), and ASV-9(557F/715R) shared 96.7, 99.2, 95.0, and 95.9% nucleotide identity with the A1-Host amt gene, respectively, (Figure 2). As shown before, these ASVs were also closely related to the A1-Host, and yet showed a different distribution than ASV-1(557F/715R). For instance, ASV-3(557F/715R), ASV-6(557F/715R), and ASV-9(557F/715R) were relatively more abundant than ASV-1(557F/715R) in the Bering Sea, and ASV-3(557F/715R) also was present in the Coral Sea and North Pacific with lower relative abundance when compared with ASV-1(557F/715R) (Figure 2).
In contrast, the primer pair AMT-330F/AMT-456R amplified a much lower diversity of amt gene sequences, capturing a total of 103 ASVs. More than 98% of the total reads clustered in a single ASV [ASV-1(330F/456R)] that shared 100% nucleotide identity with the A1-Host amt gene (Figure 2). ASV-1(330F/456R) was the dominant variant in the North Pacific and the Coral Sea samples (Figure 2). The only one additional ASV with a relative abundance above 0.5% was ASV-2(330F/456R), and shared a very high percentage of nucleotide identity (98.8%) with the A1-Host amt gene. ASV-2(330F/456R) accounted for 0.8% of total reads and was mainly found in the Coral Sea, and also at lower relative abundance in the North Pacific samples (Figure 2). It is worth noticing that using the AMT-330F/AMT-456R primer pair, no PCR products were amplified in samples from the Arctic Ocean, Tosa Bay (Japan) or SIO Pier (San Diego, CA) (Supplementary Figure 4).
In this study, we designed and tested new PCR primers to detect and explore the diversity of amt gene sequences of the haptophytes species symbiotic with the UCYN-A1 sublineage (A1-Host). The three PCR primer sets showed preferential amplification toward the A1-Host amt gene (ASV-1), in particular in samples where UCYN-A1 is the UCYN-A dominating sublineage, such as in the North Pacific and Coral Sea (Turk-Kubo et al., 2017; Henke et al., 2018; Cornejo-Castillo et al., 2019; Gradoville et al., 2020; Figure 2). The primer pair AMT-330F/AMT-456R showed the highest specificity to amplify the A1-Host amt gene, which was further confirmed by the lack of PCR amplification product in samples collected at the locations typically associated with UCYN-A2, such as San Diego (SIO Pier), Baja California, Tosa Bay (Japan), and Santa Cruz (Santa Cruz Municipal Wharf) (Hagino et al., 2013; Bombar et al., 2014; Thompson et al., 2014; Turk-Kubo et al., 2017; Cornejo-Castillo et al., 2019; Cabello et al., 2020), thus suggesting that this primer set does not amplify the amt gene of haptophytes partnering with the UCYN-A2 sublineage (Thompson et al., 2014).
The AMT-555F/AMT-705R and the AMT-557F/AMT-715R primer sets both presented a similar amplification pattern across locations and provided a higher number of ASVs than the primer pair AMT-330F/AMT-456R, revealing new amt genetic diversity (Figure 2). The A1-Host amt gene (ASV-1) was detected at the SIO Pier (San Diego, CA), although at very low relative abundances using the AMT-555F/AMT-705R and the AMT-557F/AMT-715R primer pairs, but not with the AMT-330F/AMT-456R primer pair. The detection of the A1-Host amt gene is consistent with previous studies showing the presence of the UCYN-A1 sublineage in low abundance in UCYN-A2-dominated coastal regions (Turk-Kubo et al., 2017; Cornejo-Castillo et al., 2019). However, the lack of recovery of the ASV-1 in these coastal areas when using the AMT-330F/AMT-456R primer pair could reflect slightly divergent amt sequences of A1-Host ecotypes adapted to different environments (oligotrophic subtropical versus near-shore areas). Similarly, although the UCYN-A1 sublineage has been previously detected in the Arctic (Harding et al., 2018; Shiozaki et al., 2018), the most abundant A1-Host related ASV amplified with the primer pairs AMT-555F/AMT-705R [ASV-1(555F/705R)] and AMT-557F/AMT-715R [ASV-1(557F/715R)] exhibited little to no amplification in those same Arctic samples.
The second most abundant amt host-related ASV detected with the primers AMT-555F/AMT-705R [ASV-5(555F/705R)] and AMT-557F/AMT-715R [ASV-3(557F/715R)], showed amplification not only in Arctic samples but also in the Coral Sea and Pacific Ocean, suggesting the presence of a divergent A1-Host that might be in symbiosis with a closely related (but different) UCYN-A1 sublineage in those areas. Also, both primer pairs AMT-555F/705R and AMT-557F/AMT-715R amplified ASVs that share ca. 95% of nucleotide identity with the A1-Host in the North and South Pacific regions, where UCYN-A3 has been previously reported (Thompson et al., 2014; Henke et al., 2018; Cornejo-Castillo et al., 2019), which suggests that some of the amplified ASVs might correspond with the amt gene of the haptophyte species partnering with the UCYN-A3 sublineage. Additionally, the biogeographic distribution of ASV-5(555F/705R), ASV-9(555F/705R), ASV-3(557F/715R), and ASV-5(557F/715R) was limited to the Coral Sea, where a genetic variant of the UCYN-A2 sublineage (oligo43) has been previously found (Turk-Kubo et al., 2017). Due to their genetic closeness, we hypothesize that some of these amplified ASVs might represent new amt gene diversity of haptophytes partnering divergent UCYN-A sublineages, although further investigations will be needed to verify this hypothesis.
Although all the non-target sequences (i.e., sequences from species a priori not associated symbiotically with UCYN-A) that were considered during the design of the amt primers presented mismatches with the primer sequences (Supplementary Figures 1–3), these might be not enough to prevent the amplification of some of them. For instance, when considering all samples together, the primers AMT-555F/AMT-705R amplified non-A1-Host ASVs that accounted for 35% of the total relative abundance (Figure 1). However, this percentage comes mainly from samples where the A1-Host was absent (or in a very low relative abundance) such as the Arctic samples (Figure 2), suggesting that the amplification of non-target sequences could be more important in samples where the A1-Host and/or its close-relative species are not present. Similarly, when using the primer pair AMT-557F/AMT-715R, non-A1-Host ASVs accounted for 32% of the total relative abundance (Figure 1) in samples where the A1-Host and/or its close-relative species were mainly absent, such as in the Santa Cruz Wharf, Baja California, Beaufort Sea, and Tosa Bay (Japan) (Figure 2). These are locations where UCYN-A2 dominates over UCYN-A1 (Cabello et al., 2020), which supports the hypothesis that both AMT-555F/AMT-705R and AMT-557F/AMT-715R primer pairs might capture non-target amt diversity when the A1-Host is in low abundance (or absent). Finally, either the lack of PCR amplification (Supplementary Figure 3) or the absence of dominant ASVs related with (but not identical to) the A1-Host amt gene in locations where UCYN-A2 dominates over UCYN-A1 suggests that none of the three newly designed primer pairs would amplify the amt gene of the UCYN-A2 host.
Our study offers new PCR primers for the detection of the amt gene of haptophytes species symbiotic with UCYN-A1, key members of the C and N cycles in the open ocean. The biogeography of the amt gene sequence variants shown in this study were in agreement with previous findings based on the UCYN-A1 nifH marker gene and CARD-FISH research, thus validating the use of these novel amt-targeted PCR primers as a proxy to explore the distribution of the UCYN-A1/haptophyte symbiosis. Furthermore, these new primers uncovered new variants of the amt gene that might represent an overlooked diversity of haptophytes harboring UCYN-A symbionts with distinct biogeographical distributions that needs to be further explored in future studies.
The sequencing data presented in this study are deposited in the NCBI repository, under the accession number: PRJNA946153 (https://www.ncbi.nlm.nih.gov/sra/PRJNA946153).
FC-C and JZ conceptualized the study. KS designed the PCR primers with advice from FC-C and tested the primers in environmental samples with the help of AC and KT-K. KS and FC-C analyzed the sequencing data and prepared the manuscript with contributions of all co-authors. All authors contributed to the article and approved the submitted version.
FC-C has received the support of the European Union’s Horizon 2020 research programme through a Marie Skłodowska-Curie global fellowship (UCYN2PLAST; grant 749380), a fellowship (grant 105090) from “La Caixa” Foundation (ID 100010434) and from the European Union’s Horizon 2020 Research and Innovation Program under the Marie Skłodowska-Curie actions (grant 847648), the Spanish Ministry of Science and Innovation through a “Ramón y Cajal” fellowship (RYC2021-032949-I), and acknowledges funding from the Spanish government through the “Severo Ochoa Centre of Excellence” accreditation (CEX2019-000928-S). JZ and KT-K were partially supported by Simons Foundation grants (Life Sciences #545171, #824082 and Simons SCOPE #724220).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1130695/full#supplementary-material
Bhadury, P., Song, B., and Ward, B. B. (2011). Intron features of key functional genes mediating nitrogen metabolism in marine phytoplankton. Mar. Genomics 4, 207–213. doi: 10.1016/j.margen.2011.06.002
Bombar, D., Heller, P., Sanchez-Baracaldo, P., Carter, B. J., and Zehr, J. P. (2014). Comparative genomics reveals surprising divergence of two closely related strains of uncultivated UCYN-A cyanobacteria. ISME J. 8, 2530–2542. doi: 10.1038/ismej.2014.167
Cabello, A. M., Cornejo-Castillo, F. M., Raho, N., Blasco, D., Vidal, M., Audic, S., et al. (2016). Global distribution and vertical patterns of a prymnesiophyte–cyanobacteria obligate symbiosis. ISME J. 10, 693–706. doi: 10.1038/ismej.2015.147
Cabello, A. M., Turk-Kubo, K. A., Hayashi, K., Jacobs, L., Kudela, R. M., and Zehr, J. P. (2020). Unexpected presence of the nitrogen-fixing symbiotic cyanobacterium UCYN-A in Monterey Bay, California. J. Phycol. 56, 1521–1533. doi: 10.1111/jpy.13045-20-043
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Cornejo-Castillo, F. M., Muñoz-Marín, M. D. C., Turk-Kubo, K. A., Royo-Llonch, M., Farnelid, H., Acinas, S. G., et al. (2019). UCYN-A3, a newly characterized open ocean sublineage of the symbiotic N2-fixing cyanobacterium Candidatus Atelocyanobacterium thalassa. Environ. Microbiol. 21, 111–124. doi: 10.1111/1462-2920.14429
Farnelid, H., Turk-Kubo, K., del Carmen Muñoz-Marín, M., and Zehr, J. P. (2016). New insights into the ecology of the globally significant uncultured nitrogen-fixing symbiont UCYN-A. Aquat. Microb. Ecol. 77, 125–138. doi: 10.3354/ame01794
Glibert, P. M., Wilkerson, F. P., Dugdale, R. C., Raven, J. A., Dupont, C. L., Leavitt, P. R., et al. (2016). Pluses and minuses of ammonium and nitrate uptake and assimilation by phytoplankton and implications for productivity and community composition, with emphasis on nitrogen-enriched conditions. Limnol. Oceanogr. 61, 165–197. doi: 10.1002/lno.10203
Gradoville, M. R., Farnelid, H., White, A. E., Turk-Kubo, K. A., Stewart, B., Ribalet, F., et al. (2020). Latitudinal constraints on the abundance and activity of the cyanobacterium UCYN-A and other marine diazotrophs in the North Pacific. Limnol. Oceanogr. 65, 1858–1875. doi: 10.1002/lno.11423
Hagino, K., Onuma, R., Kawachi, M., and Horiguchi, T. (2013). Discovery of an endosymbiotic nitrogen-fixing cyanobacterium UCYN-A in Braarudosphaera bigelowii (Prymnesiophyceae). PLoS One 8:e81749. doi: 10.1371/journal.pone.0081749
Harding, K., Turk-Kubo, K. A., Sipler, R. E., Mills, M. M., Bronk, D. A., and Zehr, J. P. (2018). Symbiotic unicellular cyanobacteria fix nitrogen in the Arctic Ocean. Proc. Natl. Acad. Sci. U.S.A. 115, 13371–13375. doi: 10.1073/pnas.1813658115
Henke, B. A., Turk-Kubo, K. A., Bonnet, S., and Zehr, J. P. (2018). Distributions and abundances of sublineages of the N2-fixing cyanobacterium Candidatus Atelocyanobacterium thalassa (UCYN-A) in the new caledonian coral lagoon. Front. Microbiol. 9:554. doi: 10.3389/fmicb.2018.00554
Latorre, F., Deutschmann, I. M., Labarre, A., Obiol, A., Krabberød, A. K., Pelletier, E., et al. (2021). Niche adaptation promoted the evolutionary diversification of tiny ocean predators. Proc. Natl. Acad. Sci. U.S.A. 118:e2020955118. doi: 10.1073/pnas.2020955118
Martínez-Pérez, C., Mohr, W., Löscher, C. R., Dekaezemacker, J., Littmann, S., Yilmaz, P., et al. (2016). The small unicellular diazotrophic symbiont, UCYN-A, is a key player in the marine nitrogen cycle. Nat. Microbiol. 1:16163. doi: 10.1038/nmicrobiol.2016.163
Mills, M. M., Turk-Kubo, K. A., van Dijken, G. L., Henke, B. A., Harding, K., Wilson, S. T., et al. (2020). Unusual marine cyanobacteria/haptophyte symbiosis relies on N2 fixation even in N-rich environments. ISME J. 14, 2395–2406. doi: 10.1038/s41396-020-0691-6
Moisander, P. H., Beinart, R. A., Voss, M., and Zehr, J. P. (2008). Diversity and abundance of diazotrophic microorganisms in the South China Sea during intermonsoon. ISME J. 2, 954–967. doi: 10.1038/ismej.2008.51
Montoya, J. P., Holl, C. M., Zehr, J. P., Hansen, A., Villareal, T. A., and Capone, D. G. (2004). High rates of N2 fixation by unicellular diazotrophs in the oligotrophic Pacific Ocean. Nature 430, 1027–1031. doi: 10.1038/nature02824
Moonsamy, P. V., Williams, T., Bonella, P., Holcomb, C. L., Höglund, B. N., Hillman, G., et al. (2013). High throughput HLA genotyping using 454 sequencing and the fluidigm access array™ system for simplified amplicon library preparation. Tissue Antigens 81, 141–149. doi: 10.1111/tan.12071
Sarkar, D., Landa, M., Bandyopadhyay, A., Pakrasi, H. B., Zehr, J. P., and Maranas, C. D. (2021). Elucidation of trophic interactions in an unusual single-cell nitrogen-fixing symbiosis using metabolic modeling. PLoS Comput. Biol. 17:e1008983. doi: 10.1371/journal.pcbi.1008983
Shiozaki, T., Fujiwara, A., Ijichi, M., Harada, N., Nishino, S., Nishi, S., et al. (2018). Diazotroph community structure and the role of nitrogen fixation in the nitrogen cycle in the Chukchi Sea (western Arctic Ocean). Limnol. Oceanogr. 63, 2191–2205. doi: 10.1002/lno.10933
Thompson, A., Carter, B. J., Turk-Kubo, K., Malfatti, F., Azam, F., and Zehr, J. P. (2014). Genetic diversity of the unicellular nitrogen-fixing cyanobacteria UCYN-A and its prymnesiophyte host. Environ. Microbiol. 16, 3238–3249. doi: 10.1111/1462-2920.12490
Thompson, A. W., Foster, R. A., Krupke, A., Carter, B. J., Musat, N., Vaulot, D., et al. (2012). Unicellular cyanobacterium symbiotic with a single-celled eukaryotic alga. Science 337, 1546–1550. doi: 10.1126/science.1222700
Turk-Kubo, K. A., Farnelid, H. M., Shilova, I. N., Henke, B., and Zehr, J. P. (2017). Distinct ecological niches of marine symbiotic N2-fixing cyanobacterium Candidatus Atelocyanobacterium thalassa sublineages. J. Phycol. 53, 451–461. doi: 10.1111/jpy.12505
Turk-Kubo, K. A., Mills, M. M., Arrigo, K. R., van Dijken, G., Henke, B. A., Stewart, B., et al. (2021). UCYN-A/haptophyte symbioses dominate N2 fixation in the Southern California Current System. ISME Commun. 1:42. doi: 10.1038/s43705-021-00039-7
Vorobev, A., Dupouy, M., Carradec, Q., Delmont, T. O., Annamalé, A., Wincker, P., et al. (2020). Transcriptome reconstruction and functional analysis of eukaryotic marine plankton communities via high-throughput metagenomics and metatranscriptomics. Genome Res. 30, 647–659. doi: 10.1101/gr.253070.119
Zehr, J. P., and Capone, D. G. (2020). Changing perspectives in marine nitrogen fixation. Science 368:eaay9514. doi: 10.1126/science.aay9514
Keywords: UCYN-A/haptophyte N2-fixing symbiosis, haptophyte biogeography, Braarudosphaera bigelowii, PCR primer design, ammonium transporter (amt) gene diversity
Citation: Salas K, Cabello AM, Turk-Kubo KA, Zehr JP and Cornejo-Castillo FM (2023) Primer design for the amplification of the ammonium transporter genes from the uncultured haptophyte algal species symbiotic with the marine nitrogen-fixing cyanobacterium UCYN-A1. Front. Microbiol. 14:1130695. doi: 10.3389/fmicb.2023.1130695
Received: 23 December 2022; Accepted: 21 March 2023;
Published: 17 April 2023.
Edited by:
Francesca Turroni, University of Parma, ItalyReviewed by:
Shan-Hua Yang, National Taiwan University, TaiwanCopyright © 2023 Salas, Cabello, Turk-Kubo, Zehr and Cornejo-Castillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jonathan P. Zehr, emVocmpAdWNzYy5lZHU=; Francisco M. Cornejo-Castillo, Zm1jb3JuZWpvQGljbS5jc2ljLmVz
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.