
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 17 February 2023
Sec. Infectious Agents and Disease
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1118000
This article is part of the Research Topic Advances and Challenges in the Detection and Treatment of Pathogenic Microorganisms in Infectious Disease Control View all 25 articles
 Fu-Shun Yen1
Fu-Shun Yen1 James Cheng-Chung Wei2,3,4
James Cheng-Chung Wei2,3,4 Yu-Tung Hung5,6
Yu-Tung Hung5,6 Chung Y. Hsu7
Chung Y. Hsu7 Chii-Min Hwu8,9*
Chii-Min Hwu8,9* Chih-Cheng Hsu10,11,12,13*
Chih-Cheng Hsu10,11,12,13*Introduction: We conducted this study to compare the risk of pneumonia between thiazolidinedione (TZD) use and nonuse in persons with type 2 diabetes (T2D).
Methods: We identified 46,763 propensity-score matched TZD users and nonusers from Taiwan’s National Health Insurance Research Database between January 1, 2000, and December 31, 2017. The Cox proportional hazards models were used for comparing the risk of morbidity and mortality associated with pneumonias.
Results: Compared with the nonuse of TZDs, the adjusted hazard ratios (95% CI) for TZD use in hospitalization for all-cause pneumonia, bacterial pneumonia, invasive mechanical ventilation, and death due to pneumonia were 0.92 (0.88–0.95), 0.95 (0.91–0.99), 0.80 (0.77–0.83), and 0.73 (0.64–0.82), respectively. The subgroup analysis revealed that pioglitazone, not rosiglitazone, was associated with a significantly lower risk of hospitalization for all-cause pneumonia [0.85 (0.82–0.89)]. Longer cumulative duration and higher cumulative dose of pioglitazone were associated with further lower adjusted hazard ratios in these outcomes compared to no-use of TZDs.
Discussion: This cohort study demonstrated that TZD use was associated with significantly lower risks of hospitalization for pneumonia, invasive mechanical ventilation, and death due to pneumonia in patients with T2D. Higher cumulative duration and dose of pioglitazone were associated with a further lower risk of outcomes.
The Institute for Health Metrics and Evaluation showed that cases of lower respiratory tract infections worldwide increased from 414.3 million to 488.9 million between 1990 and 2019s (The Institute for Health Metrics and Evaluation (IHME), Global Health data exchange, GBD results tool, 2019). Due to the potential impact of accumulated hyperglycemia and oxidative stress, persons with diabetes showed reduced lung function and impaired neutrophil capability (Kornum et al., 2008; Gan, 2013). Studies have shown that persons with diabetes have a 1.2-to 2.6-fold higher risk of pneumonia than those without diabetes (Kornum et al., 2008; Harding et al., 2020). Recently, the incidence of macrovascular and microvascular complications in persons with type 2 diabetes (T2D) has decreased in many countries, possibly attributable to the aggressive control of blood pressure, lipids, and glucose levels. However, the occurrence of pneumonia is still on the rise (Wang et al., 2019; Pearson-Stuttard et al., 2022). The Taiwan Diabetes Atlas reported that the risks of hospitalization and mortality from pneumonia significantly increased from 2005 to 2014 in persons with T2D (Li et al., 2019; Wang et al., 2019). However, the diabetes guidelines for pneumonia management are limited (American Diabetes Association, 2021).
Peroxisome proliferator-activated receptors (PPARs) belong to a large superfamily of nuclear hormone receptors for retinoid, glucocorticoid, and thyroid hormones. PPARs are ligand-activated transcription factors crucial for regulating glucose homeostasis, adipocyte proliferation, atherosclerosis, cell cycle control, and inflammation (Zingarelli and Cook, 2005). Thiazolidinediones (TZDs) are the synthetic ligands of PPARs. Studies have demonstrated that in addition to improving insulin resistance, TZDs have anti-inflammatory and immunomodulatory properties. Preclinical studies have shown that TZDs can decrease neutrophil recruitment, downregulate inflammatory cytokines, and attenuate inflammation in acute lung injury (Zingarelli and Cook, 2005; Grommes et al., 2012). Thus, TZDs can influence the development or progression of pneumonia. One meta-analysis of 13 randomized clinical trials revealed that TZDs could moderately increase the risk of pneumonia in patients with T2D (Singh et al., 2011). However, pneumonias were adverse events of these trials, and most trials had low event rates. Without individual patient data, the pooled results may be worrying. Therefore, we conducted this nationwide cohort study to compare the risk of pneumonia between TZD users and nonusers to assess the impact of TZDs on pneumonia development or progression in persons with T2D.
The Bureau of National Health Insurance implemented Taiwan’s National Health Insurance (NHI) program in 1995. The NHI program is a compulsory insurance system. The government and customers pay most of the premium, and the public only pays a small percentage. Approximately 99% of Taiwan’s 23 million persons joined the NHI program in 2000 (Cheng, 2003). All personal information of the insured, including sex, age, area of residence, insurance premium, diagnoses, medical procedures, and prescriptions, are recorded in the NHI Research Database (NHIRD). The diagnosis was based on the International Classification of Diseases, Ninth and Tenth Revision, Clinical Modification (ICD-9/10-CM). The NHIRD linked to the National Death Registry to verify mortality information. This study was approved by the Research Ethics Committee of China Medical University and Hospital [CMUH110-REC1-038 (CR-1)]. The identifiable information of the participants and caregivers was scrambled and encrypted before release to protect individual privacy. Informed consent was waived by the Research Ethics Committee.
We identified participants who were newly diagnosed with T2D between January 1, 2000, and December 31, 2017, and followed them until December 31, 2018. The diagnosis of T2D was based on ICD codings (ICD-9-CM codes: 250, except 250.1x; ICD-10-CM: E11) for at least 2 outpatient visits or one hospitalization. The algorithm for using ICD codes to define T2D was validated by a study in Taiwan with an accuracy of 74.6% (Lin et al., 2005). Participants were excluded (Supplementary Figure 1) under the following conditions: (The Institute for Health Metrics and Evaluation (IHME), Global Health data exchange, GBD results tool, 2019) age, below 20 or above 80 years; (Gan, 2013) missing age or sex information; (Kornum et al., 2008) diagnosis of type 1 diabetes (Supplementary Table 1), heart failure, or hepatic failure; (Harding et al., 2020) diagnosis of T2D established before January 1, 2000, to exclude prevalent cases.
We defined the first date of TZD use as the index date. Participants who never received TZD treatment served as controls. We recorded the same period from the diagnosis of T2D to the use of TZDs as the index date for the control cases. Some related variables, checked and matched between TZD users and nonusers, were as follows: age (20–40, 41–60, 60–79 years), sex, obesity, smoking status; comorbidities, including alcohol-related disorders, hypertension, dyslipidemia, coronary artery disease (CAD), stroke, peripheral arterial occlusive disease (PAOD), chronic kidney disease (CKD), pneumonia, chronic obstructive pulmonary disease (COPD), liver cirrhosis, psychosis, depression, diagnosed within 1 year before the index date; medications, including oral antidiabetic drugs (OAD), insulin, statin, aspirin, corticosteroid, and immunosuppressants, used during the follow-up period. We calculated the Charlson Comorbidity Index (CCI), Diabetes Complication Severity Index (DCSI) score (Meduru et al., 2007; Young et al., 2008), and the number of oral antidiabetic drugs to evaluate the severity of T2D.
The observed main outcomes of this study were hospitalization for all-cause pneumonia, hospitalization for bacterial pneumonia, invasive mechanical ventilation (IMV) use, and death due to pneumonia. One study in Taiwan validated the algorithm of using ICD codes to define pneumonia, with a sensitivity of 92.3–94.7% (Su et al., 2014). We calculated the events, person-years, and incidence rates for these outcomes during the follow-up period. We compared the cumulative incidences of the main outcomes between TZD users and nonusers.
We used propensity-score matching to optimize the relevant covariates between TZD users and nonusers (D’Agostino, 1998). The propensity score for each participant was estimated using non-parsimonious multivariable logistic regression, with TZD use as the dependent variable. We included 35 clinically related covariates as independent variables (Table 1). The nearest-neighbor algorithm was adopted to construct matched pairs, assuming the standardized mean difference (SMD) value <0.1 to be a negligible difference between the study and comparison cohorts.
We used crude and multivariable-adjusted Cox proportional hazards models to compare outcomes between TZD users and nonusers. The results were presented as hazard ratios (HRs) and 95% confidence intervals (CIs) for TZD users compared with nonusers. This study is based on the intention-to-treat hypothesis. To calculate the observed risks, we censored the participants until the date of respective outcomes, death, or at the end of follow-up on December 31, 2018, whichever came first. The Kaplan–Meier method and log-rank tests were used to compare the cumulative incidences of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia during the follow-up time between TZD users and nonusers. We compared the risk of hospitalization for all-cause pneumonia among different subgroups of age, sex, comorbidities, medications (rosiglitazone, pioglitazone, and others) for clinical applicability of results. We also assessed the cumulative duration (<153, 153–549, ≧550 days) and dose (<2,940, 2,940–10,009, ≧10,110 mg) of pioglitazone for the risks of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia compared with no-use of TZDs to explore the dose relationship. We performed a stratified analysis to see the effect of TZD vs. non-TZD in the risk of all-cause pneumonia stratified by the subgroups of metformin use vs. no-use, SU use vs. no-use, DPP-4 inhibitor use-vs. no-use trying to determine whether other hypoglycemic agents have effect on pneumonia risk; stratified by patient’s resident areas of the Northern, Central, Southern, and Eastern Taiwan trying to see whether the different environmental exposures have different effect on pneumonia risk.
A two-tailed value of p <0.05 was considered significant. SAS (version 9.4; SAS Institute, Cary, NC, United States) was used for statistical analysis.
From January 1, 2000, to December 31, 2017, we identified 338,361 participants with newly diagnosed T2D. Of these, 52,147 were TZD users, and 159,103 were nonusers (Supplementary Figure 1). After excluding unsuitable participants, 1: 1 propensity-score matching was used to construct 46,763 pairs of TZD users and nonusers. In the matched cohorts (Table 1), 46.34% of the participants were female; the mean (SD) age was 58.81 (11.46) years. The mean follow-up time for TZD users and nonusers was 7.80 (4.65) years and 5.21 (3.79) years, respectively.
In the matched cohorts (Table 2), 7,753 (16.57%) TZD users and 5,220 (11.16%) nonusers were hospitalized for all-cause pneumonia during the follow-up time (incidence rate: 21.79 vs. 21.82 per 1,000 person-years). In the multivariable model, TZD users showed a significantly lower risk of hospitalization for all-cause pneumonia than nonusers (aHR = 0.92, 95% CI = 0.88–0.95). Compared with nonusers, TZD users also showed significantly lower risks of hospitalization for bacterial pneumonia (aHR 0.95, 95%CI 0.91–0.99), IMV (aHR 0.80, 95% CI 0.77–0.83), and death due to pneumonia (aHR 0.73, 95% CI 0.64–0.82).
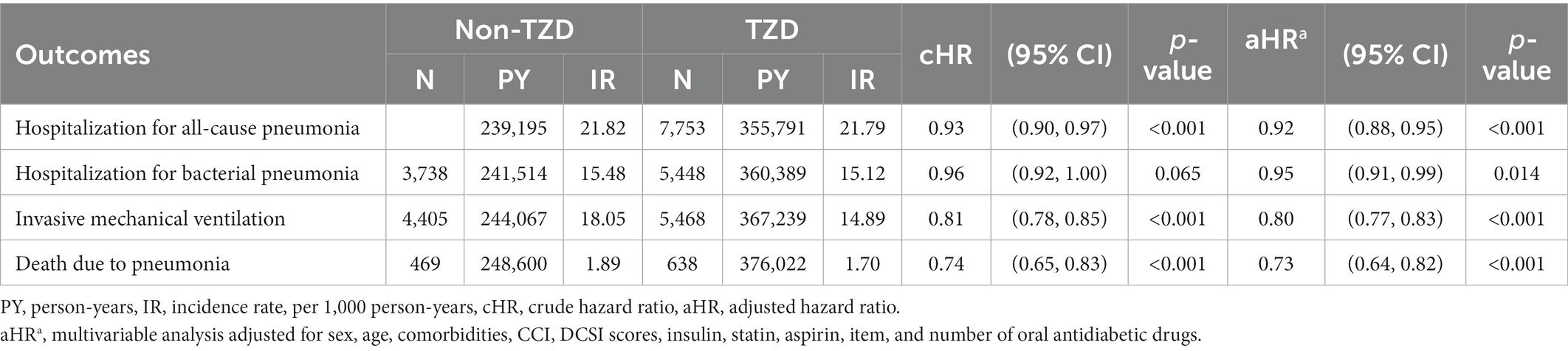
Table 2. Incidence rate and hazard ratio of main outcomes between TZD use and no-use in patients with T2D.
The Kaplan–Meier analysis showed that the cumulative incidences of hospitalization for all-cause pneumonia, IMV use, and death due to pneumonia were significantly lower in TZD users than nonusers (Log-rank test value of p<0.001). However, the cumulative incidence of hospitalization for bacterial pneumonia was non-significantly lower in TZD users than in nonusers (Log-rank test value of p = 0.066) (Supplementary Figure 2).
We assessed the variables associated with the risk of hospitalization for all-cause pneumonia and found a significantly lower risk among participants using pioglitazone and statin. However, males, older age, participants with alcohol-related disorders, chronic kidney disease, COPD, depression, higher numbers of oral antidiabetic drugs, insulin, and aspirin use had a significantly higher risk of hospitalization for all-cause pneumonia (Table 3).
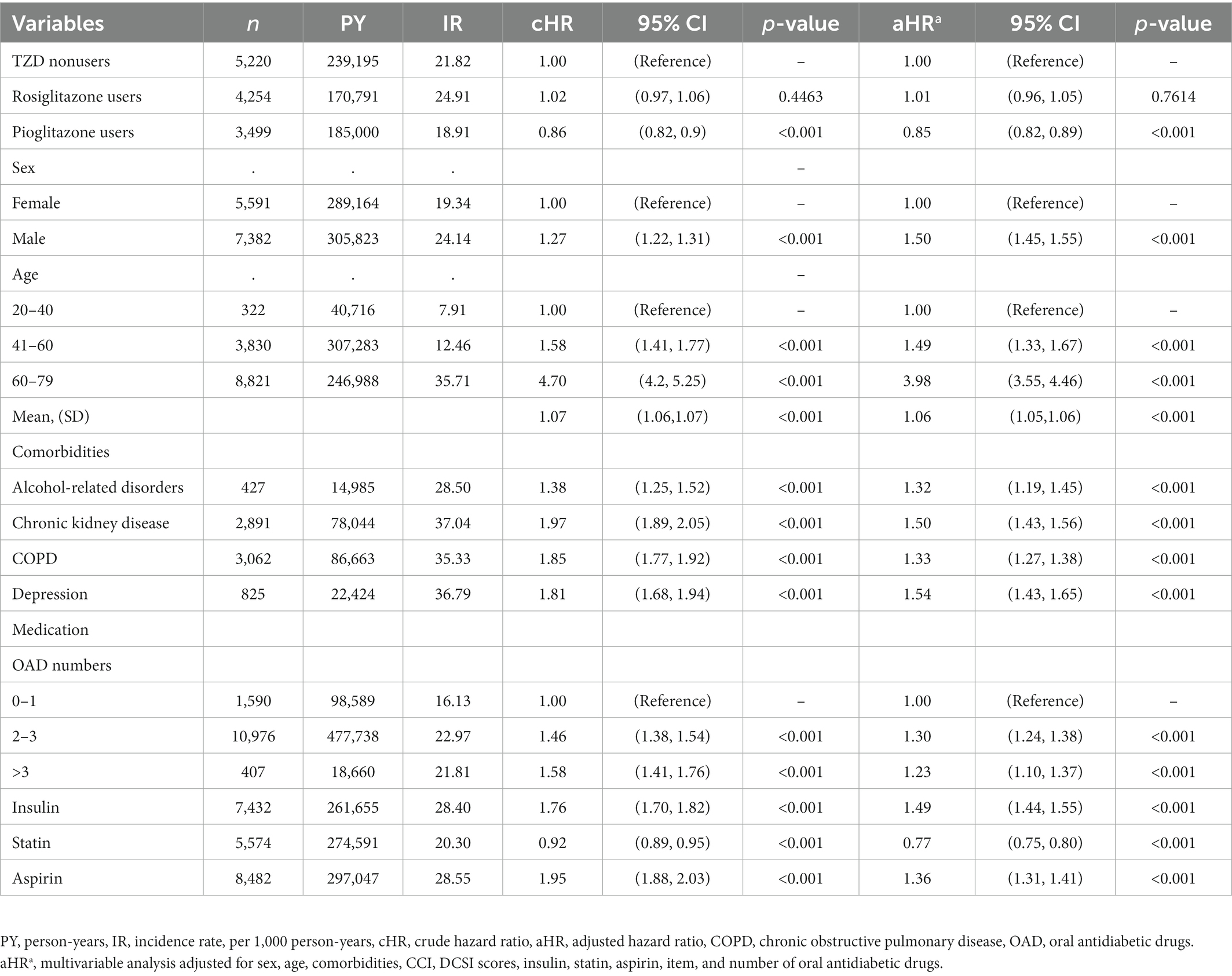
Table 3. Risk of hospitalization for all-cause pneumonia in patients with T2D stratified by variables.
We investigated the association between the cumulative duration of pioglitazone use and the risks of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia (Table 4). A longer cumulative duration of pioglitazone use was associated with further lower risks of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia compared with no-use of TZDs. The value of ps for the trend were all significant (Table 4).
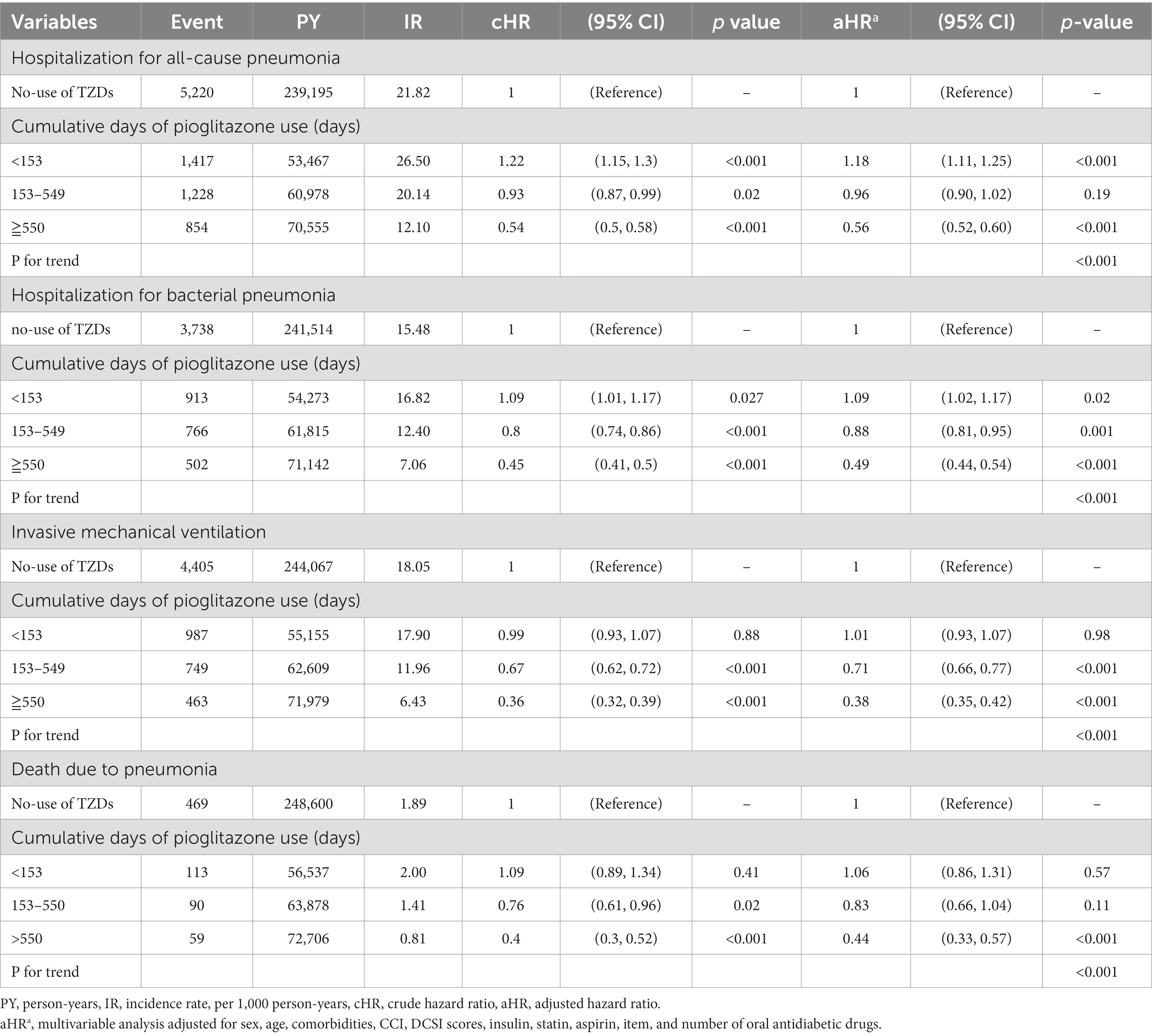
Table 4. Hazard ratios and 95% confidence intervals for outcomes with the cumulative duration of pioglitazone use.
We also observed an association between the cumulative dose of pioglitazone and the risks of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia (Table 4). The higher cumulative dose of pioglitazone use was associated with further lower risks of hospitalization for all-cause pneumonia, bacterial pneumonia, IMV, and death due to pneumonia compared with no-use of TZDs; the value of ps for the trend were all significant (Table 5).
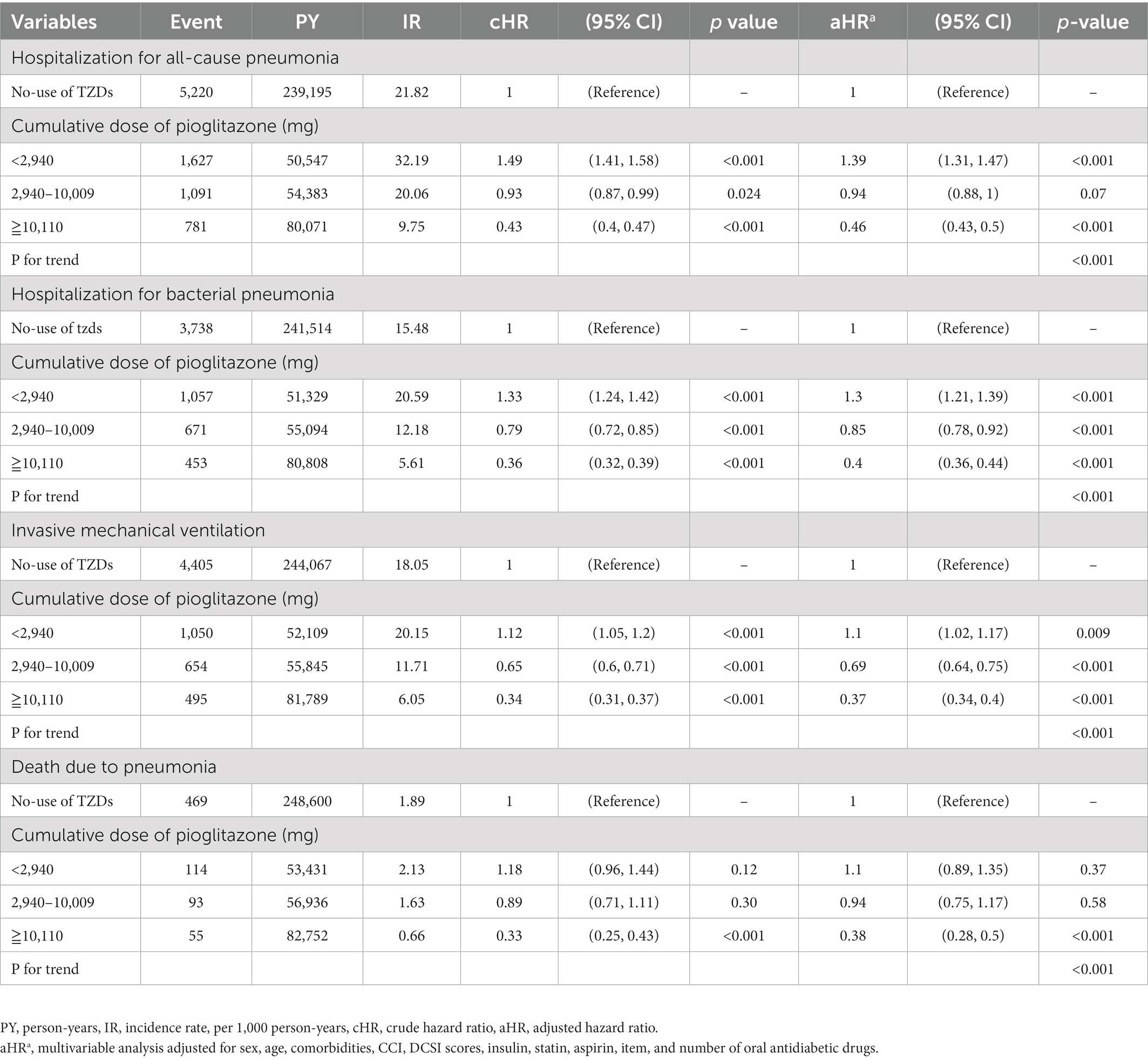
Table 5. Hazard ratios and 95% confidence intervals for outcomes with the cumulative dose of pioglitazone use.
The stratified analysis of different 4 regions of Taiwan on all-cause pneumonia risk between TZD users vs. nonusers seems to be consistent (Supplementary Table 2). But among other hypoglycemic agents, combined use of DPP-4 inhibitor and TZD seems to have a higher risk of hospitalization for all-cause pneumonia (Supplementary Table 2).
This study demonstrated that TZD use was associated with significantly lower risks of hospitalization for pneumonia, IMV, and death due to pneumonia than TZD no-use in persons with T2D. The subgroup analysis revealed that the reduced risk of pneumonia by TZDs could be due to pioglitazone use. The dose–response analysis showed that longer cumulative duration and a higher cumulative dose of pioglitazone were associated with further lower risks of these outcomes.
Studies have shown that patients with diabetes have a higher risk of pneumonia than those without diabetes (Kornum et al., 2008; Harding et al., 2020). Patients with suboptimal glycemic control showed a higher risk of pneumonia (Kornum et al., 2008). Our study also revealed that older people, persons with comorbidities, using more oral antidiabetic drugs, insulin, and aspirin had a higher risk of pneumonia. However, patients using TZDs, especially pioglitazone and statin, had a lower risk of hospitalization for all-cause pneumonia. Reports show that statin use is associated with a lower risk of pneumonia due to its potential anti-inflammatory effect (Macedo et al., 2014). However, a systemic review and meta-analysis by Sigh et al. revealed that TZD use was associated with a modestly elevated risk of pneumonia [relative risk (RR) 1.4(1.08–1.82)] (Singh et al., 2011). Gorricho et al. conducted a nested case–control study comparing the use of oral antidiabetic drugs and the risk of community-acquired pneumonia. They showed that TZDs combined with other antidiabetic drugs were associated with an increased risk of pneumonia compared to metformin plus sulfonylureas (Gorricho et al., 2017). The different results obtained from the three studies could be due to differences in the methodology and the study population. Moreover, Shih et al. conducted a case–control study and showed that TZD use was associated with a modest reduction of sepsis risk compared to TZD no-use in persons with T2D (Shih et al., 2015). To our knowledge, our research is the first study designed to compare the risk of pneumonia between TZD users and nonusers and suggest that TZD use may attenuate the risk of hospitalization for all-cause [aHR 0.92 (0.88, 0.95)] and bacterial pneumonia [aHR 0.95 (0.91, 0.99)]. This result may not be affected by the different resident environment of Taiwan. Bu if the patient is combined use of DPP-4 inhibitor and TZD seems to make the TZD lose their protective effect against all-cause pneumonia for reasons that are unclear. This study also showed that pioglitazone, not rosiglitazone, could reduce the risk of pneumonia. Rosiglitazone is a PPARγ agonist, but pioglitazone has both α and γ effects. Each TZD has different patterns of effects on the regulation of gene transcription (Kung and Henry, 2012). Previous studies have shown that the impact of pioglitazone and rosiglitazone on cardiovascular diseases was different (Kung and Henry, 2012). Preclinical studies have also found that the effect of pioglitazone and rosiglitazone on inflammation may be dissimilar (Zingarelli and Cook, 2005; Singh et al., 2011). More research is needed to determine any difference in the effectiveness of pioglitazone and rosiglitazone in the risk of pneumonia.
Diabetes may reduce lung function and pulmonary diffusion capacity due to microangiopathic changes in the lungs (Pitocco et al., 2012). Animal studies have demonstrated that pioglitazone can attenuate endotoxin-induced acute lung injury and pulmonary edema (Grommes et al., 2012). Kim et al. have shown that insulin sensitizers (metformin or TZDs) were independently associated with improvements in forced vital capacity (FVC) in persons with T2D and COPD (Kim et al., 2010). Our study demonstrated that TZDs were significantly associated with a lower risk of invasive mechanical ventilation than non-TZDs in persons with T2D [aHR 0.80 (0.77, 0.83)]. More studies are needed to explore the effect of TZDs on respiratory function, pulmonary microangiopathy, and inflammation.
Although the availability of excellent antibiotics has resulted in a significant reduction in mortality from pneumonia, the reduction in mortality within 7 days of the onset of pneumonia is not prominent. This finding may be due to the inability of antibiotics to rapidly reduce inflammatory events in the lungs (Corrales-Medina and Musher, 2011). Pneumonia may also be an important factor in accelerating premature death in persons with T2D and multimorbidity (Fine et al., 1996; Li et al., 2019; Pearson-Stuttard et al., 2022). Notably, this study showed that TZDs were significantly associated with a lower risk of death due to pneumonia [aHR 0.73 (0.64, 0.82)]. This finding may be attributable to the reduced risk of hospitalization for pneumonia and IMV support by TZD use. This study also showed that TZDs were more effective in protecting against hospitalization for all-cause pneumonia, IMV use, and death due to pneumonia than against hospitalization for bacterial pneumonia (Table 2), which may indicate that the anti-inflammatory effect of TZDs on protection against pneumonia may be greater than their antibacterial effect.
The possible grounds for TZDs to decrease the development and progression of pneumonia in persons with T2D are as follows: (The Institute for Health Metrics and Evaluation (IHME), Global Health data exchange, GBD results tool, 2019) pharmacological activation of PPARγ by TZDs can inhibit proinflammatory gene expression and reduce the production of C-reactive protein (CRP), tumor necrosis factor (TNF)-α, interleukin (IL)- 1β, IL-6, inducible nitric oxide synthase (iNOS), inducible cyclooxygenase (COX)-2, matrix metalloproteinase (MMP)-9, macrophage chemoattractant protein (MCP)-1, and plasminogen activator inhibitor (PAI)-1 (Zingarelli and Cook, 2005; Hanefeld et al., 2007; Gan, 2013). The induction of heat shock proteins by PPARγ ligands may alter the activation of nuclear factor-kB (NF-kB) and regulate inflammation (Zingarelli and Cook, 2005; Kornum et al., 2008). TZDs may augment CD36 expression, tether apoptotic cells to macrophages to promote efferocytosis, and evoke an anti-inflammatory response with the resolution of tissue injury (Zingarelli and Cook, 2005; Lea et al., 2014; Harding et al., 2020). Studies also showed that TZDs could increase IL-10 levels, enhance neutrophil recruitment to the infection foci, raise fibroblast growth factor (FGF) 21 levels, and improve survival in animals with sepsis (Trevelin et al., 2017; Pearson-Stuttard et al., 2022). PPARγ may play a role in the differentiation of naive T cells to effector T cells and improve adaptive immunity (Daynes and Jones, 2002; Wang et al., 2019). Preclinical studies demonstrated that TZDs could have direct antibacterial activity (Stegenga et al., 2009; Masadeh et al., 2011; Li et al., 2019). In animal models of lung injury, TZDs decreased pulmonary edema, fibrosis, inflammation, and mortality (Belvisi and Mitchell, 2009; Grommes et al., 2012).
This study has some limitations. First, this NHI dataset lacks complete information on dietary patterns, smoking habits, alcohol drinking, nutritional state, physical activity, vaccination status, and family history. It does not contain data on glucose, hemoglobin A1C, biochemical and microbiological tests, immune condition, pulmonary function tests, and imaging studies, which prevents a better understanding of the patient’s health status and the severity of diabetes. However, we matched the demographic information on sex and age to achieve a balance between the study and control groups. We also matched the items and number of oral antidiabetic drugs, insulin use, and DCSI scores to balance the severity of diabetes and increase the comparability between the study and comparison groups. Second, we had information on prescriptions, but patient compliance with medications was unknown. We could not obtain clues to the doctor’s preference for prescribing and the patient’s choice of medications from this database. Third, almost all the participants in this study were Chinese, and hence, the results may not be generalizable to other races. Fourth, cohort studies are usually associated with few unknown or unobserved confounding factors; therefore, randomized controlled trials are needed to verify our results. Finally, because TZDs have the concern of heart failure risk, if we want to use TZDs to reduce the risk of hospitalization for pneumonia, we must pay close attention to patients for signs and symptoms of heart failure to avoid unexpected harm to patients.
Persons with diabetes are more likely to contract and die from pneumonia than those without diabetes. Although the incidence of vascular complications has decreased, the occurrence of pneumonia is still rising. In addition to recommending persons with diabetes to receive influenza and pneumococcal vaccinations, perhaps TZD use may be an option to attenuate the morbidity and mortality of pneumonia. Additional studies are warranted to clarify all the effects of TZDs potentially linked to pneumonia.
The data analyzed in this study is subject to the following licenses/restrictions: Data of this study are available from the National Health Insurance Research Database (NHIRD) published by Taiwan National Health Insurance (NHI) Administration. The data utilized in this study cannot be made available in the paper, the supplemental files, or in a public repository due to the “Personal Information Protection Act” executed by Taiwan government starting from 2012. Requests for data can be sent as a formal proposal to the NHIRD Office (https://dep.mohw.gov.tw/DOS/cp-2516-3591-113.html) or by email to c3RzdW5nQG1vaHcuZ292LnR3. Requests to access these datasets should be directed to c3RzdW5nQG1vaHcuZ292LnR3
The studies involving human participants were reviewed and approved by Research Ethics Committee of China Medical University and Hospital. The ethics committee waived the requirement of written informed consent for participation.
F-SY: study concept, design, and drafting the manuscript, critical revision of the manuscript for important intellectual content, and study supervision. JW: critical revision of the manuscript for important intellectual content, technical and material support, and study supervision. Y-TH: data acquisition, analysis, interpretation, and statistical analysis. CH: data acquisition, interpretation, funding acquisition, technical or material support, and critical revision of the manuscript for important intellectual content. C-MH: study concept and design, data acquisition, analysis, interpretation, drafting the manuscript, critical revision of the manuscript for important intellectual content, statistical analysis, funding acquisition, technical or material support, and study supervision. C-CH: analysis and interpretation of the data, drafting the manuscript, critical revision of the manuscript for important intellectual content, and study supervision. All authors contributed to the article and approved the submitted version.
This study is supported in part by the Ministry of Science and Technology (MOHW109-TDU-B-212-114004) and China Medical University Hospital (DMR-111-105). This work received grant support from Taipei Veterans General Hospital (V101C-156, V108C-172, V109C-189). These funding agencies had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation. No organization provided funds to assist with the preparation of this paper, and data analysis was not performed by employees of funders or any author who received funding. The funders had no role in study design, data collection, data analysis, the decision to publish, or manuscript preparation. This study received no additional external funding.
We are grateful to the Health Data Science Center of China Medical University Hospital for providing administrative, technical, and funding support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1118000/full#supplementary-material
American Diabetes Association (2021). Comprehensive medical evaluation and assessment of comorbidities: standards of medical care in diabetes-2021. Diabetes Care 44, S40–S52. doi: 10.2337/dc21-S004
Belvisi, M. G., and Mitchell, J. A. (2009). Targeting PPAR receptors in the airway for the treatment of inflammatory lung disease. Br. J. Pharmacol. 158, 994–1003. doi: 10.1111/j.14765381.2009.00373.x
Cheng, T. M. (2003). Taiwan’s new national health insurance program: genesis and experience so far. Health Aff (Millwood) 22, 61–76. doi: 10.1377/hlthaff.22.3.61
Corrales-Medina, V. F., and Musher, D. M. (2011). Immunomodulatory agents in the treatment of community-acquired pneumonia: a systematic review. J. Infect. 63, 187–199. doi: 10.1016/j.jinf.2011.06.009
D’Agostino, R. B. (1998). Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat. Med. 17, 2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b
Daynes, R. A., and Jones, D. C. (2002). Emerging roles of PPARs in inflammation and immunity. Nat. Rev. Immunol. 2, 748–759. doi: 10.1038/nri912
Fine, M. J., Smith, M. A., Carson, C. A., Mutha, S. S., Sankey, S. S., Weissfeld, L. A., et al. (1996). Prognosis and outcomes of patients with community-acquired pneumonia. A meta-analysis. JAMA 275, 134–141. doi: 10.1001/jama.275.2.134
Gan, Y. H. (2013). Host susceptibility factors to bacterial infections in type 2 diabetes. PLoS Pathog. 9:e1003794. doi: 10.1371/journal.ppat.1003794
Gorricho, J., Garjón, J., Alonso, A., Celaya, M. C., Saiz, L. C., Erviti, J., et al. (2017). Use of oral antidiabetic agents and risk of community-acquired pneumonia: a nested case-control study. Br. J. Clin. Pharmacol. 83, 2034–2044. doi: 10.1111/bcp.13288
Grommes, J., Mörgelin, M., and Soehnlein, O. (2012). Pioglitazone attenuates endotoxin-induced acute lung injury by reducing neutrophil recruitment. Eur. Respir. J. 40, 416–423. doi: 10.1183/09031936.00091011
Hanefeld, M., Marx, N., Pfützner, A., Baurecht, W., Lübben, G., Karagiannis, E., et al. (2007). Anti-inflammatory effects of pioglitazone and/or simvastatin in high cardiovascular risk patients with elevated high sensitivity C-reactive protein: the PIOSTAT study. J. Am. Coll. Cardiol. 49, 290–297. doi: 10.1016/j.jacc.2006.08.054
Harding, J. L., Benoit, S. R., Gregg, E. W., Pavkov, M. E., and Perreault, L. (2020). Trends in rates of infections requiring hospitalisation among adults with versus without diabetes in the US, 2000-2015. Diabetes Care 43, 106–116. doi: 10.2337/dc19-0653
Kim, H. J., Lee, J. Y., Jung, H. S., Kim, D. K., Lee, S. M., Yim, J. J., et al. (2010). The impact of insulin sensitisers on lung function in patients with chronic obstructive pulmonary disease and diabetes. Int. J. Tuberc. Lung Dis. 14, 362–367.
Kornum, J. B., Thomsen, R. W., Riis, A., Lervang, H. H., Schønheyder, H. C., and Sørensen, H. T. (2008). Diabetes, glycemic control, and risk of hospitalisation with pneumonia: a population-based case-control study. Diabetes Care 31, 1541–1545. doi: 10.2337/dc08-0138
Kung, J., and Henry, R. R. (2012). Thiazolidinedione safety. Expert Opin. Drug Saf. 11, 565–579. doi: 10.1517/14740338.2012.691963
Lea, S., Plumb, J., Metcalfe, H., Spicer, D., Woodman, P., Fox, J. C., et al. (2014). The effect of peroxisome proliferator-activated receptor-γ ligands on in vitro and in vivo models of COPD. Eur. Respir. J. 43, 409–420. doi: 10.1183/09031936.00187812
Li, H. Y., Wu, Y. L., Tu, S. T., Hwu, C. M., Liu, J. S., and Chuang, L. M. (2019). Trends of mortality in diabetic patients in Taiwan: a nationwide survey in 2005-2014. J. Formos. Med. Assoc. 118, S83–S89. doi: 10.1016/j.jfma.2019.07.008
Lin, C. C., Lai, M. S., Syu, C. Y., Chang, S. Y., and Teng, F. Y. (2005). Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J. Formos. Med. Assoc. 104, 157–163.
Macedo, A. F., Taylor, F. C., Casas, J. P., Adler, A., Prieto-Merino, D., and Ebrahim, S. (2014). Unintended effects of statins from observational studies in the general population: systematic review and meta-analysis. BMC Med. 12:51. doi: 10.1186/1741-7015-12-51
Masadeh, M. M., Mhaidat, N. M., Al-Azzam, S. I., and Alzoubi, K. H. (2011). Investigation of the antibacterial activity of pioglitazone. Drug Des. Devel. Ther. 5, 421–425. doi: 10.2147/DDDT.S24126
Meduru, P., Helmer, D., Rajan, M., Tseng, C. L., Pogach, L., and Sambamoorthi, U. (2007). Chronic illness with complexity: implications for performance measurement of optimal glycemic control. J. Gen. Intern. Med. 22, 408–418. doi: 10.1007/s11606-007-0310-5
Pearson-Stuttard, J., Cheng, Y. J., Bennett, J., Vamos, E. P., Zhou, B., Valabhji, J., et al. (2022). Trends in leading causes of hospitalisation of adults with diabetes in England from 2003 to 2018: an epidemiological analysis of linked primary care records. Lancet Diabetes Endocrinol. 10, 46–57. doi: 10.1016/S2213-8587(21)00288-6
Pitocco, D., Fuso, L., Conte, E. G., Zaccardi, F., Condoluci, C., Scavone, G., et al. (2012). The diabetic lung--a new target organ? Rev. Diabet. Stud. 9, 23–35. doi: 10.1900/RDS.2012.9.23
Shih, C. J., Wu, Y. L., Chao, P. W., Kuo, S. C., Yang, C. Y., Li, S. Y., et al. (2015). Association between use of oral anti-diabetic drugs and the risk of sepsis: a nested case-control study. Sci. Rep. 5:15260. doi: 10.1038/srep15260
Singh, S., Loke, Y. K., and Furberg, C. D. (2011). Long-term use of thiazolidinediones and the associated risk of pneumonia or lower respiratory tract infection: systematic review and meta-analysis. Thorax 66, 383–388. doi: 10.1136/thx.2010.152777
Stegenga, M. E., Florquin, S., de Vos, A. F., and van der Poll, T. (2009). The thiazolidinedione ciglitazone reduces bacterial outgrowth and early inflammation during Streptococcus pneumoniae pneumonia in mice. Crit. Care Med. 37, 614–618. doi: 10.1097/CCM.0b013e31819599b6
Su, V. Y., Liu, C. J., Wang, H. K., Wu, L. A., Chang, S. C., Perng, D. W., et al. (2014). Sleep apnea and risk of pneumonia: a nationwide population-based study. CMAJ 186, 415–421. doi: 10.1503/cmaj.131547
The Institute for Health Metrics and Evaluation (IHME), Global Health data exchange, GBD results tool (2019). Available at: https://ghdx.healthdata.org/gbd-results-tool (accessed April 8, 2022).
Trevelin, S. C., Carlos, D., Beretta, M., da Silva, J. S., and Cunha, F. Q. (2017). Diabetes mellitus and sepsis: a challenging association. Shock 47, 276–287. doi: 10.1097/SHK.0000000000000778
Wang, J. S., Wu, Y. L., Shin, S. J., Tien, K. J., Chin, M. C., and Hwu, C. M. (2019). Hospitalization in patients with type 2 diabetes mellitus in Taiwan: a nationwide population-based observational study. J. Formos. Med. Assoc. 118, S90–S95. doi: 10.1016/j.jfma.2019.06.017
Young, B. A., Lin, E., Von Korff, M., Simon, G., Ciechanowski, P., Ludman, E. J., et al. (2008). Diabetes complications severity index and risk of mortality, hospitalization, and health care utilization. Am. J. Manag. Care 14, 15–23.
Keywords: all-cause pneumonia, bacterial pneumonia, death, invasive mechanical ventilation, pioglitazone
Citation: Yen F-S, Wei JC-C, Hung Y-T, Hsu CY, Hwu C-M and Hsu C-C (2023) Thiazolidinediones lower the risk of pneumonia in patients with type 2 diabetes. Front. Microbiol. 14:1118000. doi: 10.3389/fmicb.2023.1118000
Received: 11 December 2022; Accepted: 31 January 2023;
Published: 17 February 2023.
Edited by:
Jun Feng, Shanghai Municipal Center for Disease Control and Prevention (SCDC), ChinaReviewed by:
Shih-Yi Lin, Taichung Veterans General Hospital, TaiwanCopyright © 2023 Yen, Wei, Hung, Hsu, Hwu and Hsu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chii-Min Hwu, ✉ Y2hod3VAdmdodHBlLmdvdi50dw==; Chih-Cheng Hsu, ✉ Y2NoQG5ocmkuZWR1LnR3
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.