
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 15 February 2023
Sec. Microbial Physiology and Metabolism
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1112584
 Yunfei Cai1†
Yunfei Cai1† Xue Chen1†
Xue Chen1† Peixuan Li1
Peixuan Li1 Weiheng Ren1
Weiheng Ren1 Qiang Zhang1
Qiang Zhang1 Yiwen Wang1
Yiwen Wang1 Yina Jiang1
Yina Jiang1 Pinkuan Zhu1
Pinkuan Zhu1 Hideyoshi Toyoda2
Hideyoshi Toyoda2 Ling Xu1*
Ling Xu1*Adenylate cyclase (AC) regulates growth, reproduction, and pathogenicity in many fungi by synthesizing cyclic adenosine monophosphate (cAMP) and activating downstream protein kinase A (PKA). Botrytis cinerea is a typical necrotrophic plant-pathogenic fungus. It shows a typical photomorphogenic phenotype of conidiation under light and sclerotia formation under dark; both are important reproduction structures for the dispersal and stress resistance of the fungus. The report of B. cinerea adenylate cyclase (BAC) mutation showed it affects the production of conidia and sclerotia. However, the regulatory mechanisms of the cAMP signaling pathways in photomorphogenesis have not been clarified. In this study, the S1407 site was proven to be an important conserved residue in the PP2C domain which poses a remarkable impact on the phosphorylation levels and enzyme activity of the BAC and the overall phosphorylation status of total proteins. The point mutation bacS1407P, complementation bacP1407S, phosphomimetic mutation bacS1407D, and phosphodeficient mutation bacS1407A strains were used for comparison with the light receptor white-collar mutant Δbcwcl1 to elucidate the relationship between the cAMP signaling pathway and the light response. The comparison of photomorphogenesis and pathogenicity phenotype, evaluation of circadian clock components, and expression analysis of light response transcription factor genes Bcltf1, Bcltf2, and Bcltf3 showed that the cAMP signaling pathway could stabilize the circadian rhythm that is associated with pathogenicity, conidiation, and sclerotium production. Collectively, this reveals that the conserved S1407 residue of BAC is a vital phosphorylation site to regulate the cAMP signaling pathway and affects the photomorphogenesis, circadian rhythm, and pathogenicity of B. cinerea.
Adenylate cyclase (AC) is an enzyme that converts ATP into cAMP, and the product cAMP is an important second messenger in eukaryotes. By combining with cAMP, PKA is activated to regulate downstream substrates in response to external environmental signals. Studies on AC, represented by human beings, show that a large number of hormone receptors activated are related to the synthesis of second messenger cAMP (cAMP) by membrane-bound adenylate cyclase. The cAMP signal transduction mediates a wide range of cellular responses and participates in the regulation of cardiac contraction, insulin secretion, and neurotransmitter release (Gloerich and Bos, 2010). In recent years, the most detailed three-dimensional structure diagram of AC has been obtained by analyzing the AC-G protein dimer using freeze electron microscopy, which explains the regulation and self-regulation mechanism of cAMP synthesis (Korkhov and Qi, 2019). In addition, the study on plant growth hormone receptor TIR1/AFBs shows that they have not only E3 ubiquitin ligase activity but also adenylate cyclase activity, and AC activity is crucial for auxin-induced transcriptional regulation. This study proves that the auxin receptor TIR1/AFBs has AC activity which is crucial to its receptor function (Qi et al., 2022). In fungi, the cAMP signaling pathway mediated by AC also attracted extensive research activities due to its association with fungal metabolism, development, reproduction, and pathogenicity (Li et al., 2007).
In our early molecular genetics study with B. cinerea, a spontaneously variant mutant strain with defects in pathogenicity, conidiation, and sclerotium reproduction was characterized to be caused by a single nucleotide mutation at the S1407 site of adenylate cyclase (BAC) (Chen et al., 2020), in the PP2C (protein phosphatase, family 2C) domain, which is a negative regulatory domain of protein kinase cascade through dephosphorylation. Besides, BAC contains a Gα binding domain that can bind with upstream G protein; a RAS interacting domain; an LRR domain that can participate in a variety of biological processes; and a catalytic domain of adenosine cyclase type III that catalyzes the transformation of ATP into cAMP (Bassler et al., 2018).
In fungi, studies on AC show that different external signals, like peptidoglycan (PGN), CO2, pH, and temperature, can stimulate the activity of AC via different domains (Xu et al., 2008). Besides, some studies have shown that some kinds of phosphokinase can phosphorylate AC. In animals, the research of frog erythrocytes shows that the 12-O-tetradecanoyl phorbol-13-acetate (TPA), a phorbol ester that activates protein kinase C (PKC), could induce PKC to phosphorylation of the catalytic unit of AC, and that may be involved in the phorbol ester-induced enhancement of AC activity (Yoshimasa et al., 1987). The research of mice shows that the A-kinase-anchoring protein79/150 (AKAPS 79/150) could cluster PKA to phosphorylation AC5 to negative cAMP pathway, and the AKAP79 also could interact with AC6 and AC9 to regulate their activity (Efendiev et al., 2010). In Saccharomyces cerevisiae adenylate cyclase Cyr1, Snf1/AMPK could phosphorylate Cyr1 to inhibit its synthesis of cAMP ability and lower PKA activity under low glucose conditions (Nicastro et al., 2015). The above studies show that the phosphorylation level of AC will affect its ability to synthesize cAMP.
Studies on adenylate cyclase of Schizosaccharomyces pombe Cyr1 showed that the Gsα domain is the binding site of subunit Gpa2 (Ivey and Hoffman, 2005). In Candida albicans, Gpa2 and Gpr1 protein-coupled receptors participate in the response of external amino acid and glucose signals (Maidan et al., 2005a,b), the mutant strains of these two genes showed the defective phenotype of mycelial growth on a solid medium depending on the cAMP signal pathway (Ivey and Hoffman, 2005). In addition, the yeast-double hybridization proved the interaction between Ras1 and the RAS domain, and the mutation of conserved residues in the RAS domain would block the interaction between Ras1 and Cyr1 to affect the cAMP signal pathway (Fang and Wang, 2006). In S. cerevisiae, Sgt1 has been shown to affect cAMP signal transduction through direct interaction with the LRR domain of Cyr1 (Dubacq et al., 2002). Moreover, the muramyl dipeptides, a component of peptidoglycan, could activate cAMP synthesis by binding with the LRR domain of Cyr1 (Xu et al., 2008). The above research shows that different external signals regulate the cAMP signal pathway via regulatory domains to activate AC domain enzymes. However, the functions and effects of the PP2C domain of AC have rarely been reported.
Botrytis cinerea is a necrotrophic fungus that is widely distributed worldwide and can infect more than 1,400 plants (Elad et al., 2016). The diseases caused by B. cinerea can occur in both pre and postharvest crops, and huge economic losses are ascribed to this pathogen every year (Dean et al., 2012). B. cinerea can form sclerotia and conidia under different light conditions (Schumacher, 2017). Sclerotia can be formed under continuous dark conditions (DD), while the conidia can form under continuous light (LL) and alternation of light and dark (LD) conditions, and microconidia can be formed under continuous dark and low-temperature conditions (Faretra et al., 1988). Therefore, B. cinerea can adjust its survival strategy by forming different biological structures to adapt to the external environment in the presence of different light conditions (Hua et al., 2018).
The regulation of growth, pathogenicity, and development is a manifestation of the self-regulation of B. cinerea in response to external signals (Hua et al., 2018). Conidia and sclerotia reproduction are photomorphogenesis phenotypes of B. cinerea (Canessa et al., 2013; Schumacher et al., 2014; Cohrs et al., 2016; Brandhoff et al., 2017), and changes in light signals during the day induce differences in pathogenicity at different time points in a day (Hevia et al., 2015). The oscillation of the circadian clock components, B. cinerea frequency gene 1 (Bcfrq1), plays an important role in pathogenesis. In the interaction system between B. cinerea and Arabidopsis thaliana, the circadian clock of B. cinerea is more important for pathogenicity at different time points during the pathogenic process (Hevia et al., 2015). The main pathogenic strategy of B. cinerea involves spreading and transmitting diseases via the conidia generated under light conditions (Schumacher et al., 2014; Fillinger and Elad, 2016). Therefore, the generation and pathogenesis of conidia have been the focus for controlling B. cinerea.
The light-responsive transcription factor (LTF) gene is crucial for responding to different light conditions to regulate conidia and sclerotia reproduction (Schumacher, 2017). The light receptor Bcwcl1 and three LTF genes, Bcltf1, Bcltf2, and Bcltf3, are the most important components involved in conidia and sclerotia reproduction. The white-collar complex (WCC) can regulate the expression of these genes (Schumacher et al., 2014; Cohrs et al., 2016; Brandhoff et al., 2017). However, as a light receptor and core component of the circadian clock, BcWCL1 does not regulate the expression pattern of light transcription factors under natural light conditions.
Both light and cAMP signals are involved in the formation of conidia and sclerotia in B. cinerea. However, the relationship between the B. cinerea adenylate cyclase (BAC)–mediated cAMP signaling pathway and the formation of conidia and sclerotia and the circadian clock still needs to be explored. We previously reported that the growth rate, conidia, sclerotia production, and pathogenicity are seriously defective in association with the BAC S1407 site mutation (Chen et al., 2020). In this study, the analysis of the S1407 site of BAC demonstrated that the phosphorylation level of BAC could significantly affect some protein phosphorylation levels, B. cinerea pathogenicity at different times of the day, and photomorphogenesis. Furthermore, the circadian clock core components Bcfrq1, Bcwcl1, and the three LTF genes, Bcltf1, Bcltf2, and Bcltf3, were detected, and the effects of the AC-mediated cAMP pathway on the circadian clock, conidia, and sclerotia reproduction were evaluated.
The DNA and protein sequences from B. cinerea B05.10 were downloaded from the Ensembl Fungi Botrytis database (Amselem et al., 2011; Staats and van Kan, 2012; van Kan et al., 2017).1 Other fungal sequences were from the database of the National Centre for Biotechnology (NCBI).2 The amino acid sequences of the putative orthologues genes were analyzed using the BlastP algorithm (Altschul et al., 1990) at the NCBI. The functional protein domains of BAC protein were predicted using Interpro and SMART.3,4 Additionally, the phosphorylation site of BAC was predicted using Netphos-3.1.5 The analysis of WCC binding sites was conducted according to the previous reports (Froehlich et al., 2003; Wang et al., 2015; Baek et al., 2019).
Supplementary Table S1 lists the wild-type and mutant strains of B. cinerea used in the present study. The wild type of B. cinerea strain B05.10 (Büttner et al., 1994), the BAC single mutant strain bacS1407P, and the complemented strain bacP1407S (Chen et al., 2020) were used in the present experiments. The bacS1407A and bacS1407D strains were generated in this work by introducing homologous recombination fragments into the B05.10 strain according to the previous method (Schumacher et al., 2012).
All strains of B. cinerea were ordinarily cultivated on solid potato dextrose agar (PDA) or complete medium (CM), the PDA medium for strain subculture, and the CM medium were used to avoid unknown nutrients (Pontecorvo et al., 1953). The strains were grown at 23°C using Percival incubators, which were equipped with cool white light fluorescent tubes (light intensity up to 100 μM/m2/s; wavelength 400–720 nm) at 12-h intervals of light and dark (12:12-h LD).
The growth rate of the mycelial colony was determined by measuring the increase of the colony diameter per day for each strain growing on the solid medium in a Petri dish. To determine the yield of conidia, the colonies cultured for 14 days were washed twice with 5 ml of water to harvest the conidia suspension, which was subsequently filtered by four layers of gauze to remove the mycelial debris. The conidia suspended in the final filtrate (10 ml) were counted under a hemocytometer.
The PP2C and AC domain-coding sequences of BAC and BACS1407P were isolated from the wild-type and mutant strains and inserted into pET28a+ (Supplementary Figure S4) to produce the BAC-His6 PP2C-AC and BACS1407P-His6 PP2C-AC vectors, respectively. These vectors were transformed into Escherichia coli BL21 cells by a heat-shock method. Target proteins were produced by the addition of 1.5 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 30°C. The BAC PP2C-AC domain and BACS1407P PP2C-AC domain were individually linked to six histamines (His6) and expressed as fusion proteins; eventually, the BAC-His6 PP2C-AC domain and BACS1407P-His6 PP2C-AC domain fusion proteins were produced and then purified using the Ni-NTA 6FF Sefinose (TM) Resin Kit (Shenggong, Shanghai, China).
For the gel retardation assay, the purified protein was run in SDS-PAGE or mixed with Phos-tag (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) to examine the phosphorylation levels of different proteins following the reported method (Gou et al., 2015). In this assay, the phosphate group on the protein binds the Phos-tag with the manganese ion in the gel. Eventually, the relative mass of the protein becomes larger, and therefore, the mobility in the gel becomes slower. The variance of the position of the protein is representative of the phosphorylation levels between different proteins. The fusion proteins were specified by binding with anti-His6 and secondary antibody goat anti-mouse IgG HRP (AB-M-M100, GOOD HERE, Hangzhou, China) according to the manufacturer’s instructions. This binding was detected by the Western Blot test reagent dye solution (34,580, Thermo Scientific™, Shanghai, China), where the second antibody was bound to HRP, and the substrate of ECL produced chemiluminescence after being catalyzed by HRP.
For the total protein phosphorylation level test, the mycelia growth on cellophane-covered CM medium for 3 days under light or dark conditions, and the protein extraction buffer (Yang et al., 2013) was used to extract mycelia protein. The anti-phosphoserine antibody (ab9332, Abcam, United Kingdom) was used to analyze the total phosphorylation level.
The adenylyl cyclase activity assay was performed as described previously with some modifications (Korkhov and Qi, 2019). The reaction mixtures contained 50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.1% digitonin, 5 mM MnCl2, 10 nM to 100 μM total ATP (Shenggong, Shanghai, China), and 0.01 mg/ml purified BAC. The reactions were started by adding ATP to the protein. The reactions were incubated for 10–30 min at 23°C and were terminated by treatment at 85°C for 10 s. The cAMP content was measured using a highly sensitive ELISA kit (Biosamite, Shanghai, China) according to the manufacturer’s instructions.
Mutants of B. cinerea were constructed using protoplasts by the method reported previously (Schumacher et al., 2012). The mutant construction strategy is shown in Figure 1A and Supplementary Figure S4. The bacS1407A and bacS1407D homologous recombination fragments were constructed by PCR-amplifying their coding regions from chromosomal DNAs using 5′- and 3′-corresponding primers and recombining them with a NAT (nourseothricin resistance cassette) of the pNAN-OGG. These products were transferred to the protoplasts of B. cinerea to generate the mutants mentioned above. The pNAN-OGG was used as a template to amplify the NAT fragments. The recombinant sequences were detected by PCR amplification with specific primer sets, as shown in Figure 1A; Supplementary Figure S4. Supplementary Table S2 shows the sequences of these primers.
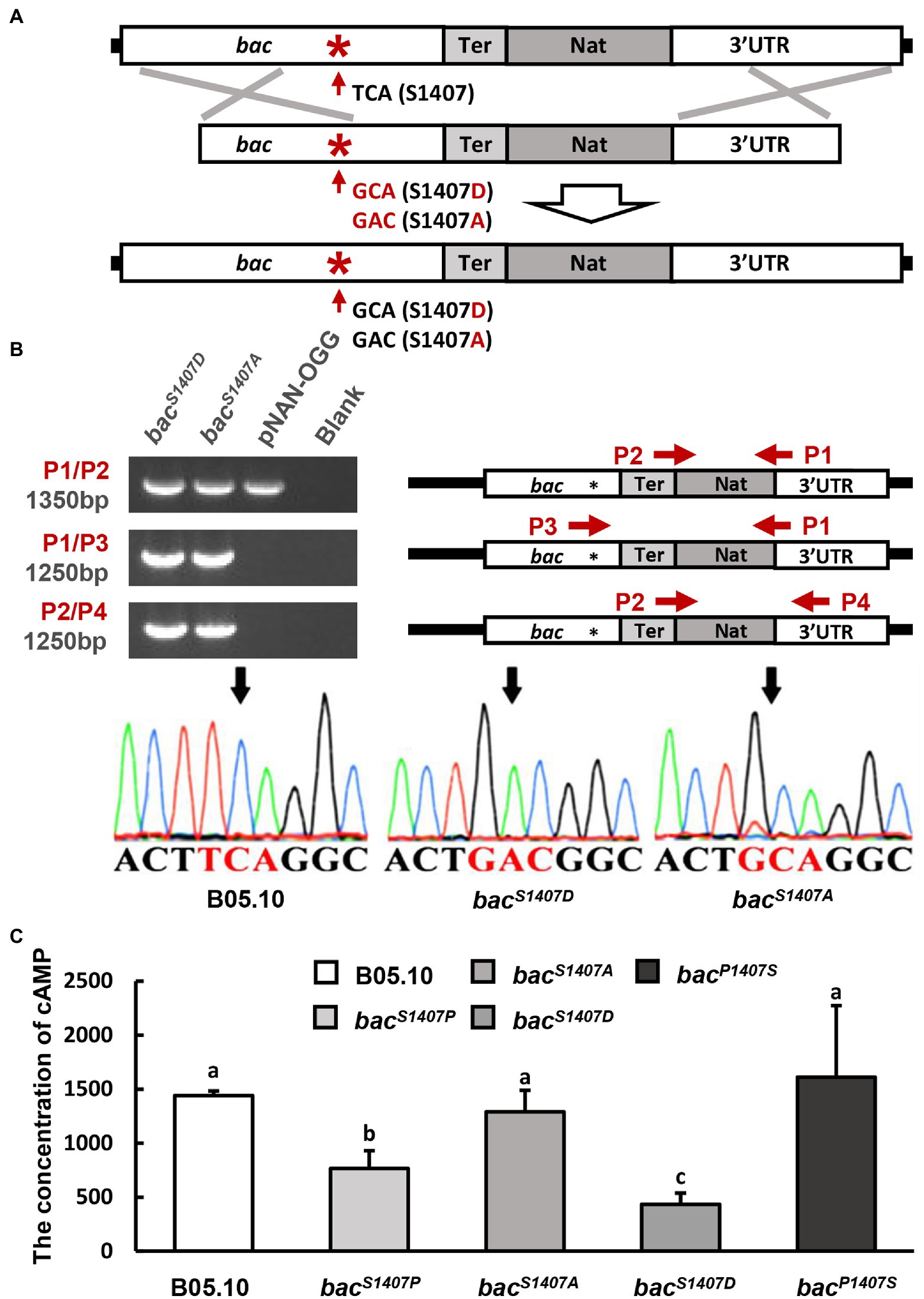
Figure 1. (A) Schematic representation of the in situ point mutation strategy. (B) PCR amplification of target sequences (bacS1407D and bacS1407A) by different primer sets (right), and electrophoretic identification of the target sequences (left). (C) Intracellular levels of cAMP in the wild type (B05.10), mutant strain (bacS1407P, bacS1407A, bacS1407D, and bacP1407S) observed after incubation on CM for 3 days under conditions involving light. The different letters on the columns indicate significant differences at p < 0.05. The bars present mean values ± SD (n = 3).
The pathogenicity assay was performed as described previously (Chen et al., 2020). The healthy mature fruits of grape (Vitis vinifera), apple (Malus pumila Mill), tomato fruit (Solanum lycopersicum), 4-week-old tomato leaves, and 2-week-old A. thaliana leaves were used in the inoculation assay. Prior to inoculation, the fruits were rinsed in sterile water, submerged in 70% alcohol for the 30 s, and soaked in sterile water for 2 min. The 10 μl droplets of conidial suspensions (1 × 106/ml GB) were dropped onto the inoculation site of the fruit and leaves surface. The inoculated fruits and leaves were placed in a box to keep 90% relative humidity. The lesion area of the inoculation site has been measured via Image J software.
The banding pattern denotes the alternated, ring-shaped grayish and white regions formed on radially growing mycelial colonies in the presence and absence of light illumination, respectively (Canessa et al., 2013). In order to observe the banding pattern, all strains were incubated for 7 days under LD conditions on the solid CM medium supplemented with 0.02% SDS, which reduces the mycelial growth to approximately 50% of radial growth on the SDS-free medium. This SDS treatment was conducted to make the banding pattern more distinct.
The banding patterns of different strains were further analyzed using a race tube method. The race tube was a transparent cylindrical tube (inner diameter, 9 mm) with air-permeable plugs at both ends. A solid medium was laid inside the tube and placed horizontally, and mycelia clumps of the test strains were separately placed at one end of the tube and incubated at 23°C to examine the change in the banding pattern under different conditions.
To observe the effect of the cAMP signal pathway on the banding pattern, an inhibitor of the cAMP decomposing enzyme PDE, 3-isobutyl-1-methylxanthine (IBMX) (Agarwal et al., 2010) was dissolved in the CM medium with Dimethyl sulfoxide (DMSO) to final concentration 10 μM in solid CM medium and used for the culture of the strains at 23°C under LD condition for a week.
For isolating RNAs from the strains (B05.10, Δbcwcl1, Δbcwcl1-com, and bacS1407P), they were grown on cellophane membrane-covered PDA plates under the alternation of 12 h-light and 12 h-dark conditions. Samples were harvested in a temperature-controlled (23°C) darkroom equipped with low-intensity red-safety lights. The collected mycelial samples were immediately frozen in liquid nitrogen and subsequently kept at −80°C until further usage.
To test protein–protein interactions in yeast cells, the CDS of BcpkaR, Bcwcl1, and Bcfrq1 were cloned to pGBKT7 and pGADT7 vectors and then transferred to yeast strain AH109, which has a tryptophan (Trp) and leucine (Leu) deficiency for screening the transformants. Namely, the cells transformed with the constructed pGADT7 and pGBDT7 plasmids were able to grow on the medium lacking His, Ade, Trp, and Leu. In this experiment, the colonies from SD/−Trp/−Leu (-LW) plates were transferred to SD/-Trp//-Leu/-His//-Ade/3-amino-1,2,4-triazole (3-AT) (-LWH/) plates to confirm protein–protein interactions under 28°C for 1 week.
To test the binding activity of transcription factors on the promoters of target genes, the CDS of Bcwcl1 was cloned to pB42AD, and the promoters of Bcfrq1, Bcltf1, Bcltf2, and Bcltf3 were cloned to pLacZ vector and then transferred to yeast strain EGY48. The yeast was cultured at 30°C until it grew monoclonal at SD/−Trp/-Ura plates. Moreover, the colonies from SD/−Trp/-Ura plates were transferred to SD/−Trp/-Ura plates containing X-gal for culture at 30°C for 2–3 days to confirm the binding activities of transcription factors to promoters, and the combined yeast colonies that can be transcriptionally activated will turn blue.
For isolating RNAs from the strains, the cellophane membrane-covered PDA plates were used to culture strains. The collected mycelial samples were immediately frozen in liquid nitrogen and subsequently kept at −80°C until further usage. Frozen mycelia were ground to powder in liquid nitrogen, and total RNA was isolated using TRIzol reagent (Invitrogen) as described by Canessa et al. (2013). Total RNA quantity and quality were verified using NanoDrop (Thermo Scientific). All experiments were replicated three times. The expression of different genes in different strains at different times under LD conditions was compared with the expression of 3 h Bcfrq1 in wild type (B05.10) according to the method described previously (Hevia et al., 2015).
Statistical data were expressed as means ± standard errors (SE) from three repetitions. Bar charts represent mean values with standard deviations, using Tukey’s honestly significant (HSD) test to examine if differences between groups of samples were significant at a p-value of <0.01.
Our previous study demonstrated that mutation at the S1407 residue in BAC of B. cinerea caused obvious defects in growth, development, and pathogenicity (Chen et al., 2020). Here, we used NetPhos-3.1 to predict that the BAC S1407 site was located in the type 2C serine/threonine phosphatases (PP2C) domain of BAC (Figure 2A). The present prediction analysis showed that S1407 was a phosphorylation site of BAC (Supplementary Figure S1). Simultaneously, a comparison of the protein sequences of AC among different fungi by sequence alignment showed that the S1407 was a highly conserved residue (Figure 2B).

Figure 2. (A) Botrytis cinerea adenylate cyclase (BAC) domains analyzed using InterPro and SMART. (B) Conservation analysis of the S1407 locus of BAC. (C) The phosphorylation of BAC-His6 and BACS1407P-His6 was detected by a mobility shift on a Phos-tag SDS–PAGE gel. Red arrows represent different positions of migration (migration direction from bottom to top). (D) Concentration curve of cAMP synthesized by BAC-His6 PP2C and AC domains and BACS1407P-His6 PP2C and AC domains under different concentrations of ATP.
To verify the possibility of S1407 as a phosphorylation site, we expressed the BAC-His6 PP2C-AC domains (subsequently referred to as BAC) and BACS1407P-His6 PP2C-AC domains (subsequently referred to as BACS1407P) using a prokaryotic expression system. Western blot analysis after the protein phosphorylation level assay showed that the position of the BAC band was higher than that of BACS1407P in SDS-PAGE with Phos-tag; however, the bands were parallel in SDS-PAGE results (Figure 2C). These results indicate that mutations at the S1407 site in the PP2C domain of BAC affected the overall phosphorylation levels of BAC. By adding ATP substrates with different concentration gradients, the change curves of cAMP synthesized by BAC and BACS1407P were plotted (Figure 2D). The results showed that mutation at the S1407 site of BAC affected the ability of the BAC to convert ATP to cAMP. Taken together, the aforementioned results demonstrate that mutation of the S1407 site in the PP2C domain altered the phosphorylation level of BAC and affected its ability to convert ATP into cAMP.
Replacing the serine residue with alanine can cause this site to dephosphorylate continuously, while mutation to aspartic acid leads to continuous phosphorylation (Kamada et al., 2010). BAC S1407 phosphomimetic strain bacS1407D, and BAC S1407 phosphodeficient strain bacS1407A, were obtained by homologous recombination (Figures 1A,B). The intracellular cAMP contents of bacS1407P and bacS1407D were lower than those of the wild type, bacS1407A, and bacP1407S (Figure 1C). This is consistent with the results observed with the phosphorylation levels of BAC. In addition, the growth rates of the wild-type (B05.10) and mutant strains (bacS1407P, bacS1407D, bacP1407S) were observed in CM medium supplemented with or without exogenous cAMP. The growth rate of bacS1407P and bacS1407D was slightly restored by exogenous cAMP (Supplementary Figure S2). After 7 days of culture, the colony morphology of bacS1407P and bacS1407D was recovered to some extent (Supplementary Figure S2). These results indicate that the S1407 site of BAC is a phosphorylation site, and the phosphorylation level of this site can affect the BAC activity of cAMP synthesis and mycelial growth.
PP2C domain is a domain that can remove the phosphates from substrate protein. The mutation of the S1407 site of the PP2C domain of BAC affects the phosphorylation of BAC and the ability of BAC to synthesize cAMP. It may also affect the phosphorylation level of substrate protein of the PP2C domain. We detected the total protein phosphorylation level of strains B05.10, bacS1407P, bacS1407A, and bacS1407D (Figure 3). The results showed that the total protein phosphorylation level of bacS1407P and bacS1407D was significantly higher than that of wild type, and the phosphorylation levels of some protein band increased significantly in bacS1407P and bacS1407D strains under light and dark conditions (Figure 3), which indicated that the PP2C domain of BAC might have the ability to remove the substrate protein phosphorylation level.
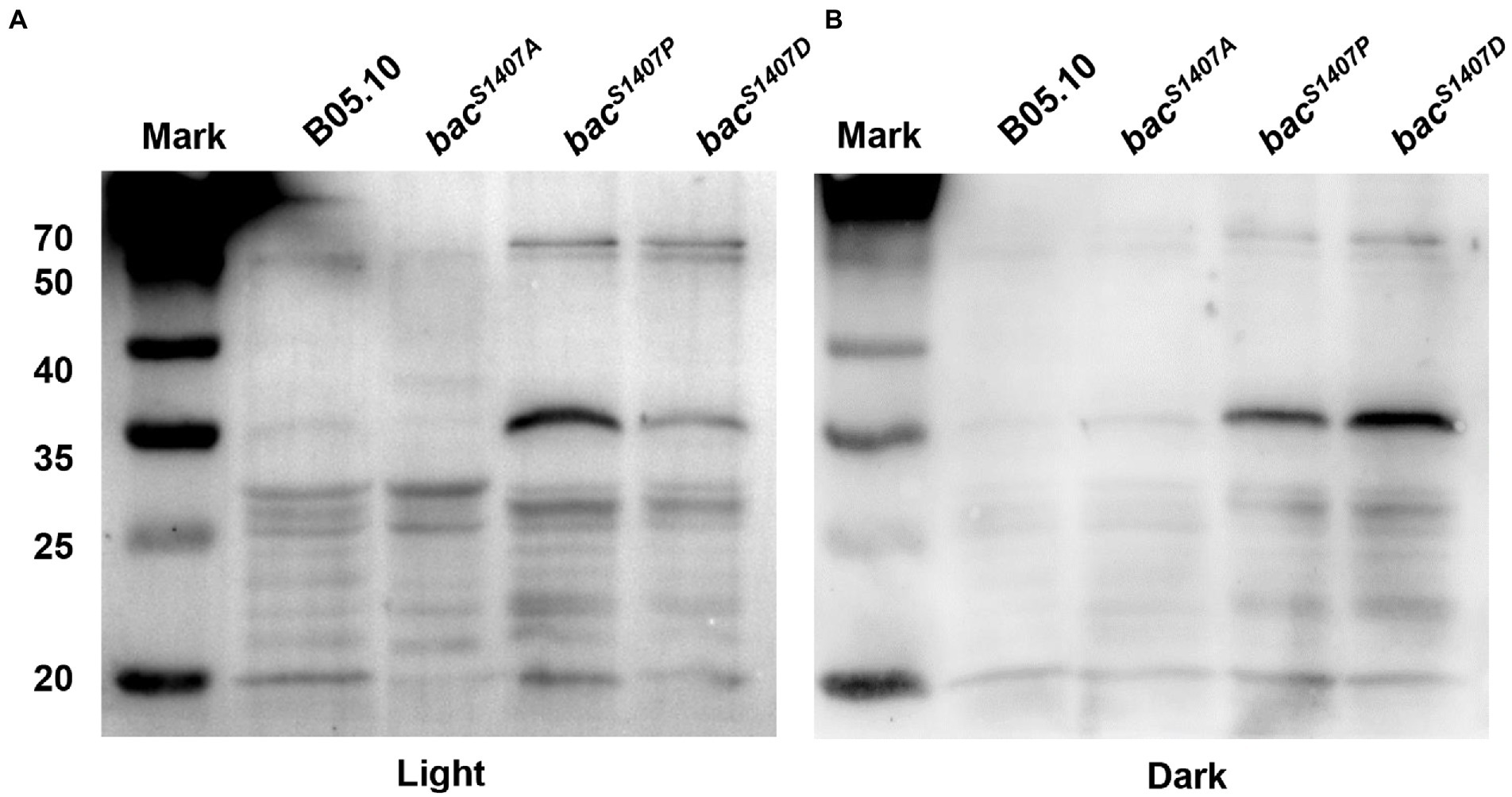
Figure 3. Differences in phosphorylation levels of total proteins of mycelia of wild-type (B05.10) and mutation strains (bacS1407P, bacS1407A, and bacS1407D) growing for 3 days on CM under light (A) and dark (B) conditions.
Due to mutation at the S1407 residue, the phosphorylation level and function of BAC are greatly affected. The growth rate of bacS1407D was significantly lower than that of the wild type and even lower than that of bacS1407P. In addition, bacS1407D mutant continuously produced conidia under LD conditions (Figures 4A–D). The pathogenicity and response to light of bacS1407D were weaker than those of bacS1407P, while the pathogenicity, conidiation, and sclerotia production and light responses of the bacS1407A mutant were similar to those of the wild type (Figures 4E,F). The above results prove that the phosphorylation level of BAC can regulate the production of conidia and sclerotia. The light signaling pathway is also involved in regulating the development of conidia and sclerotia. We compared the conidia-producing phenotype of the Δbcwcl1 mutant with the BAC S1407 site mutant strains and found that the yield of conidia increased in Δbcwcl1 but decreased in bacS1407P and bacS1407D (Supplementary Figure S2C). The cAMP signaling pathway of B. cinerea may be involved in the regulation of photomorphogenesis, which is crucial for the formation of conidia and sclerotia of B. cinerea.
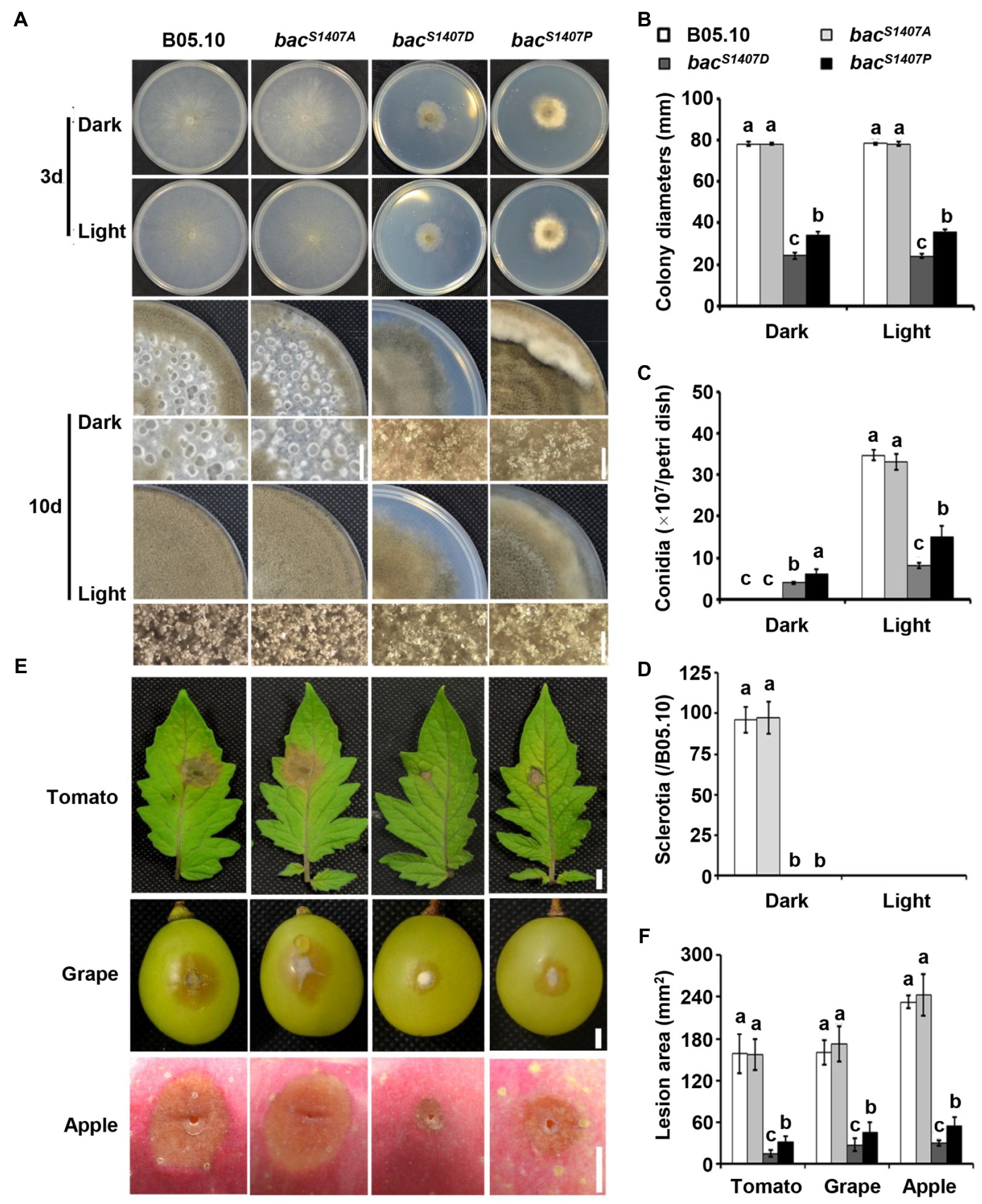
Figure 4. Growth and pathogenic phenotype analysis of different strains. (A) Wild-type (B05.10) and mutant strains (bacS1407P, bacS1407A, bacS1407D, and bacP1407S) were incubated onto solid CM medium for 3 or 10 days under continuous white light (LL) and dark (DD) conditions. (B–D) Statistical analyses of the colony diameters, conidia, and sclerotia of (A). (E) Wild-type (B05.10) and mutant strains (bacS1407P, bacS1407A, bacS1407D, and bacP1407S) were incubated onto grape (72 h), tomato leaf (48 h), and apple surfaces (96 h). (F) Statistical analysis of the lesion area of (E). Different letters indicate significant differences at p < 0.01. The bars present mean values ± SD (n = 3).
We further explored the effects of varied site mutations of BAC S1407 on growth rate under different light conditions. The colony appearances of different strains grown on CM medium under Blue light (BL), Red light (RL), DD, and LL conditions are shown (Figure 5A). The growth rates of bacS1407P and bacS1407D were significantly lower than those of the wild type under BL, RL, LL, and DD (Figure 5B). Interestingly, the growth rates of bacS1407P and bacS1407D under BL and LL conditions were higher than those of RL and DD (Figure 5B), suggesting that cAMP participates in mediating the response to light in B. cinerea.
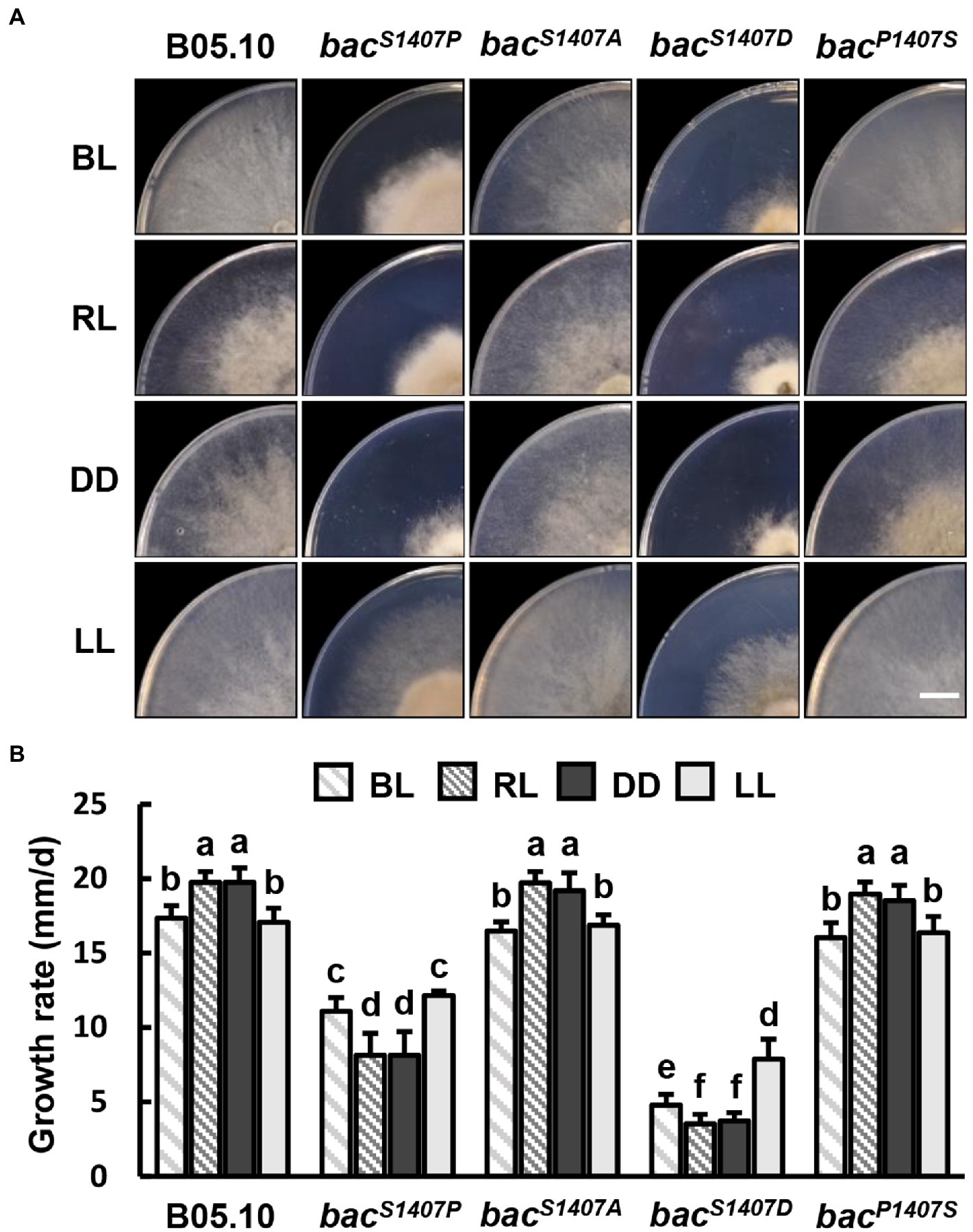
Figure 5. (A) Colony appearance of different strains under blue light (BL), red light (RL), continuous dark (DD), and continuous light (LL) conditions. Wild-type (B05.10) and mutant strains (bacS1407P, bacS1407A, bacS1407D, and bacP1407S) were incubated onto the solid CM medium for 3 days under BL, RL, DD, and LL conditions. The white bar represents 10 mm. (B) The colony growth rate of test strains under the same conditions as those depicted in (A). Different letters indicate significant differences at p < 0.05. The bars present mean values ± SDs (n = 3).
The conidia of B. cinerea are important propagules for infection and transmission, and their formation is regulated by light (Williamson et al., 2007). A study on the interaction between B. cinerea and A. thaliana showed that the pathogenicity of B. cinerea was significantly higher at dusk than at dawn, and this phenomenon is regulated by the circadian clock of B. cinerea (Hevia et al., 2015). In the bacS1407P strain, the yield of conidia was significantly affected, and the ability to produce sclerotia was lost. The conidia of wild type (B05.10) and BAC with S1407 site mutations (bacS1407P, bacS1407A, bacP1407S) were inoculated on A. thaliana leaves (Figures 6A–C) and tomato fruits (Figures 6D–F). The pathogenicity of wild-type, bacS1407A, and bacP1407S conidia was significantly different at dawn and dusk (Figures 6A,D). However, the differences in pathogenicity between bacS1407P and bacS1407D at dawn and dusk were significantly greater than those of the wild type (Figures 6B,C,E,F). These results indicate that the BAC-mediated cAMP signaling pathway is involved in the regulation of differences in the pathogenicity of B. cinerea between dawn and dusk.
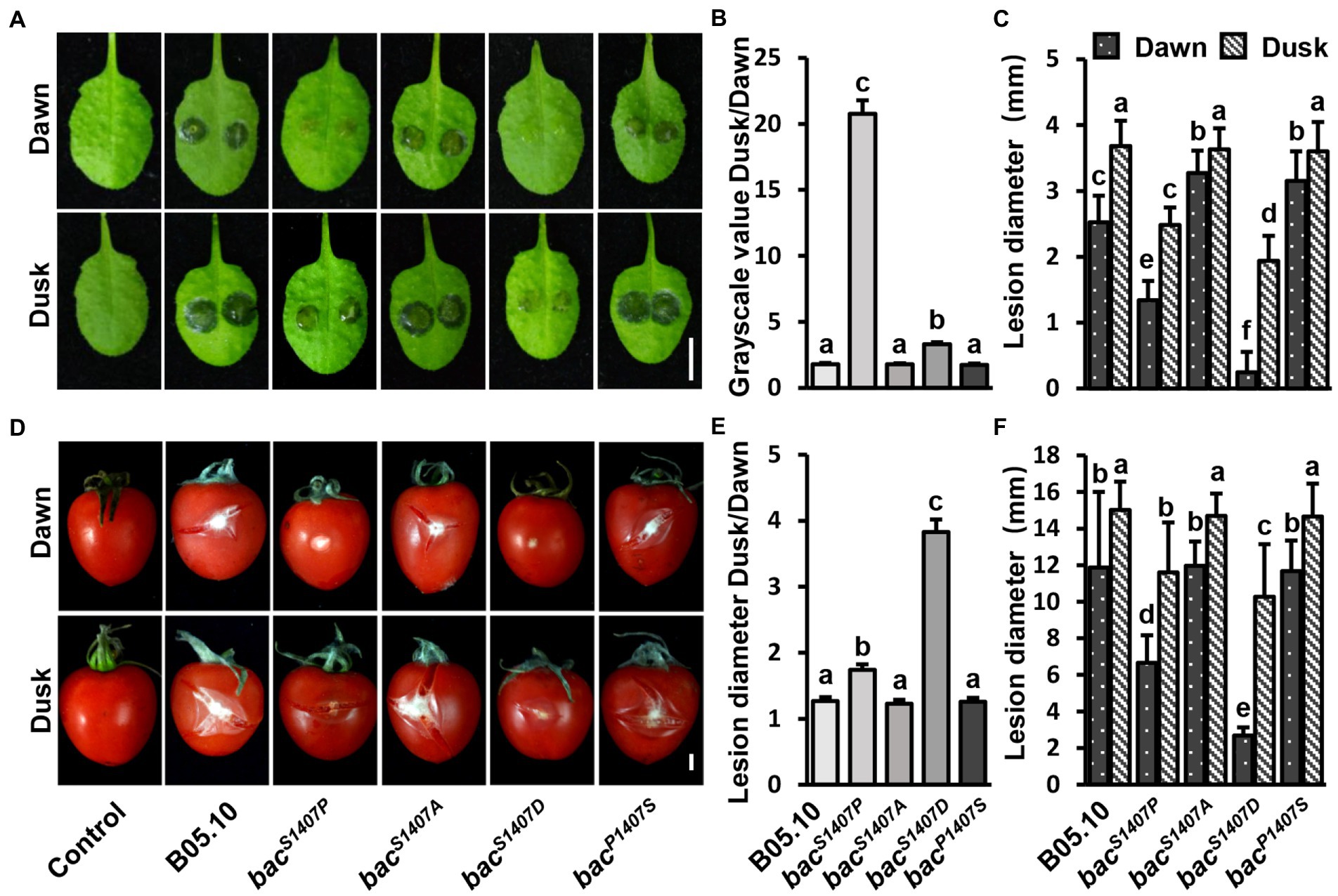
Figure 6. Comparison of lesion areas at dawn and dusk during the interaction of B. cinerea and A. thaliana leaves (A) and B. cinerea and tomato fruits (D). The conidia were cultured under LD conditions. PDB was used to suspend the conidia to 1 × 106 conidia/μl, the suspension was then placed in the dark for 24 h after inoculation. The white bar represents 5 mm. (B,E) Statistical analysis of contrast of gray values of the lesions recorded at dusk and dawn in (A,D). (C,F) Statistical analysis of lesion diameter in (A,D). Different letters indicate significant differences at p < 0.05. The bars present mean values ± SDs (n = 3).
The light signaling pathway is one of the most common environmental factors that regulate the circadian rhythm of fungi (Hevia et al., 2016). Under alternating light and dark conditions, fungi form a weak ring which is due to different growth states and is recognized as the growth rhythm. This growth rhythm is more apparent if SDS is added to the medium because the mycelium growth rate is reduced (Canessa et al., 2013). We compared the differences in circadian rhythms between the wild type (B05.10) and mutant (bacS1407P, bacS1407D, bacS1407A, and Δbcwcl1) under LD conditions (Figure 7A). The photoreceptor BcWCL1 is sensitive to light and is an important component of the circadian clock. Therefore, Δbcwcl1 showed no apparent growth rhythm on the CM supplemented with SDS. The bacS1407A showed no significant changes compared with the wild type, and the growth of bacS1407D was seriously affected. Hence, it was difficult to observe the rhythm phenotype. However, the growth rhythm of bacS1407P was more significant than that of wild-type B05.10 (Figure 7A). This is consistent with the results of circadian rhythm observed in different PDA and CM media without SDS (Supplementary Figure S3). These results indicate that the BAC-mediated cAMP signaling pathway is involved in the operation of the circadian clock.
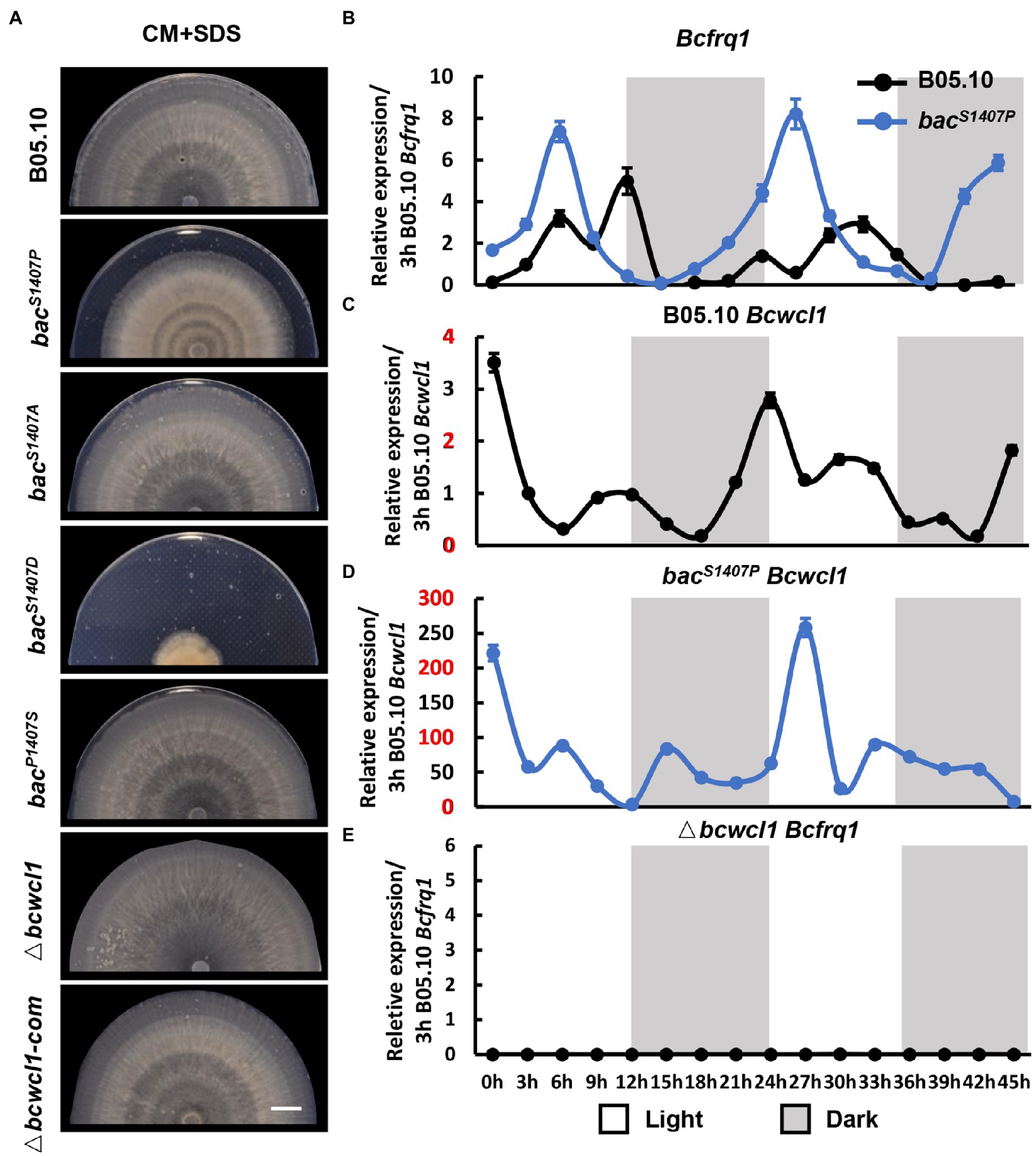
Figure 7. (A) The banding pattern of the mycelial colony produced by the wild type (B05.10) and mutant (bacS1407P, bacS1407A, bacS1407D, bacP1407S, Δbcwcl1, and Δbcwcl1-com) strains on SDS-containing CM medium for 7 days under LD conditions. SDS-containing CM medium was used to reduce mycelial growth to approximately 50% of ordinary radial growth to obtain a clearer pattern. The white bar represents 10 mm. The expression of circadian clock components Bcfrq1 in wild-type B05.10, bacS1407P (B) and Δbcwcl1 (E), and Bcwcl1 in the wild type (C,D). The expression of B05.10 Bcfrq1 at 3 h (B,E) and B05.10 Bcwcl1 at 3 h (C,D) was used as a ruler to compare the expression changes of genes at different time points under LD conditions for 2 days. Error bars are standard deviations (n = 3).
The 45 h-expression curve of the circadian clock core components Bcwcl1 and Bcfrq1 in wild type and bacS1407P under conditions with light–dark alternation was verified at this point (Figures 7B–E). Transcript levels of Bcfrq1 and Bcwcl1 showed rhythmic changes in the wild type (Figures 6B,C). Compared with that in bacS1407P, the expression of Bcfrq1 and Bcwcl1 was significantly higher, and the expression of Bcwcl1 increased significantly in bacS1407P (Figure 7D). The rhythm of Bcfrq1 in bacS1407P was faster than the wild type, and the expression peak was reduced (Figure 7B). The expression of Bcfrq1 in Δbcwcl1 was significantly decreased without rhythm, which was consistent with its rhythmic phenotype (Figure 7A). This result is consistent with the phenotype of the mutant strains observed at the S1407 site of BAC, with elevated BAC phosphorylation levels contributing to the more significant difference in pathogenicity between dawn and dusk (Figure 6). These results further demonstrate that BAC is involved in the regulation of rhythmic growth and pathogenicity.
Phosphodiesterases (PDEs), which can decompose cAMP, are inhibited by isobutylmethylxanthine (IBMX) for the disruption of the cAMP signaling pathway (Agarwal et al., 2010). The inhibitor IBMX was added to the CM medium in the race tube to verify the role of the BAC-mediated cAMP-PKA signaling pathway in the circadian clock. In the race tube containing CM medium supplemented with IBMX, the banding pattern of bacS1407P disappeared under LD conditions (Figure 8A). Owing to the lack of operation of the circadian clock, the surface of the mycelial colony of all strains became flattered in comparison to that observed with the IBMX-free CM medium under LD conditions (Supplementary Figure S3B). These results verify the regulatory effect of cAMP on the circadian clock.
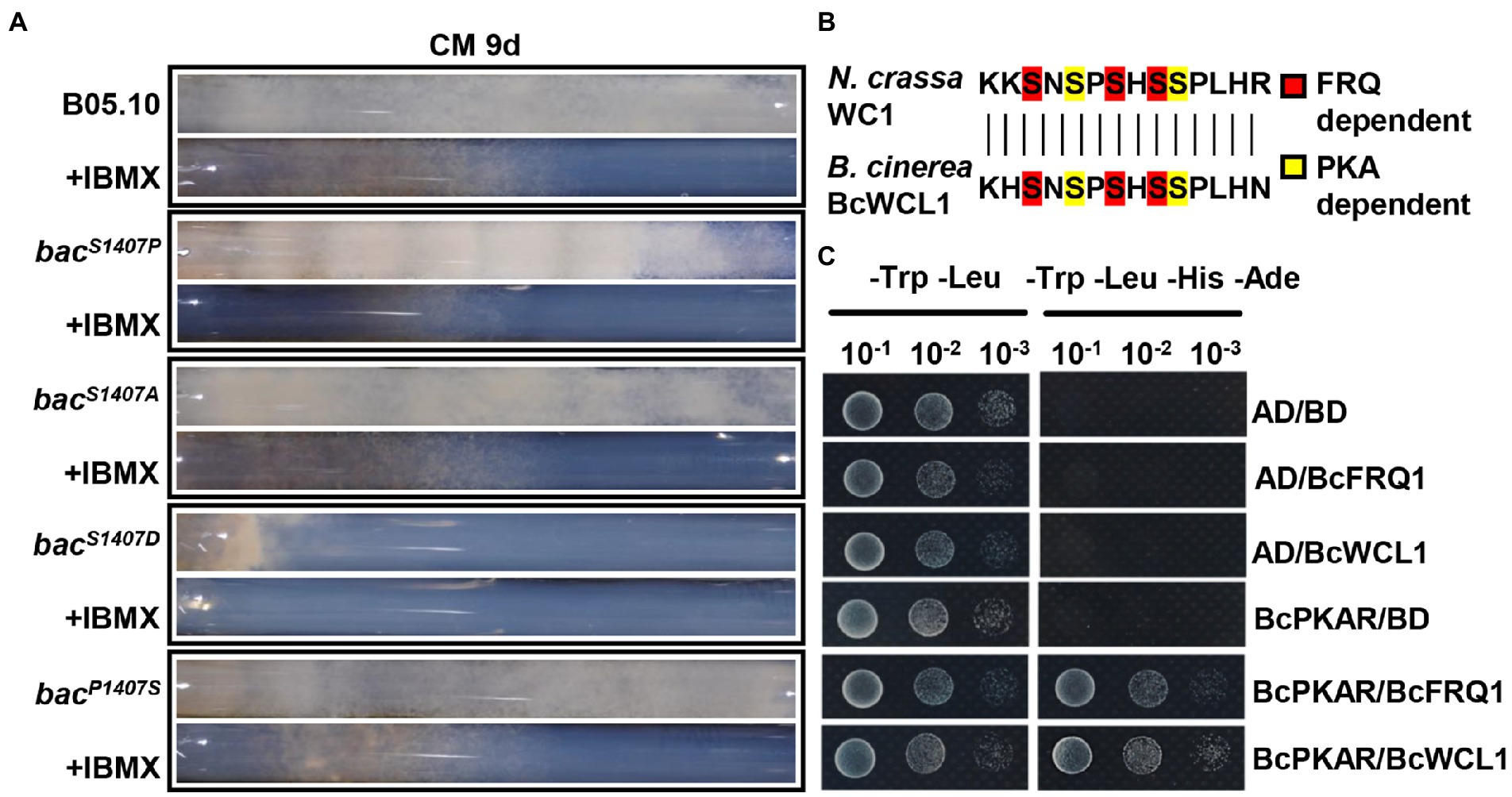
Figure 8. (A) Race tube assay for the same strains grown on CM media with or without IBMX at 9 days under LD conditions along with the circadian rhythms after incubation. (B) Comparison of amino acid sequences in phosphorylation sites between N. crassa WC1 and B. cinerea BcWCL. (C) Yeast two-hybrid analysis of BcPKAR, BcFRQ1, and BcWCL1. In this assay, yeast cells were used at various densities (10−1 to 10−3 conidia/μl dilution). AD and BD represent empty plasmids.
Studies in N. crassa have shown that PKA can phosphorylate FRQ1 and WC1 (Huang et al., 2007), and nucleotide sequence comparison has shown that the phosphorylation sites of WCL1 are largely conserved in comparison to those in WC1 (Huang et al., 2007; Figure 8B). The cAMP-PKA signaling pathway regulatory subunit BcPKAR can be activated by cAMP to transfer the signal (Liñeiro et al., 2016). The present yeast two-hybrid (Y2H) assay system analysis indicated that BcPKAR interacted with BcWCL1 and BcFRQ1 (Figure 8C). These results suggest that B. cinerea may have a similar regulatory mechanism as N. crassa.
The phenotypes of BAC S1407 mutations indicate that BAC is crucial to the photomorphogenesis of B. cinerea and that Bcltf1, Bcltf2, and Bcltf3 are the key transcription regulators downstream of WCC that regulate conidia production under light and sclerotia under dark conditions in B. cinerea (Schumacher et al., 2014; Cohrs et al., 2016; Brandhoff et al., 2017). The changes in the expression of Bcltf1, Bcltf2, and Bcltf3 in wild type and bacS1407P observed under LD conditions showed that these three genes were significantly different from those in the wild type (Figure 9). In the wild type, compared with the expression curves of core components of the circadian clock, namely, Bcfrq1 and Bcwcl1 (Figures 9B,C), the expression curves of Bcltf1 and Bcltf2 showed that they were more remarkably controlled by the circadian clock (Figures 9A,D). However, Bcltf3 was less sensitive to the clock in the wild type, which was more relevant to light regulation (Figure 9H). The expression oscillation amplitude of Bcltf1 increased (Figure 9B), and the expression oscillation of Bcltf3 was significantly reduced (Figure 9I) in bacS1407P, indicating that the BAC-mediated cAMP signaling pathway has a regulatory effect on it. Unlike the predicted results, the oscillation of Bcltf2 expression was remarkably reduced, and the rhythm disappeared in bacS1407P compared to that in B05.10 (Figure 9E). This also explains why the production of conidia sharply declined due to the remarkable reduction in Bcltf2 expression, which was influenced by the cAMP signaling pathway in bacS1407P.
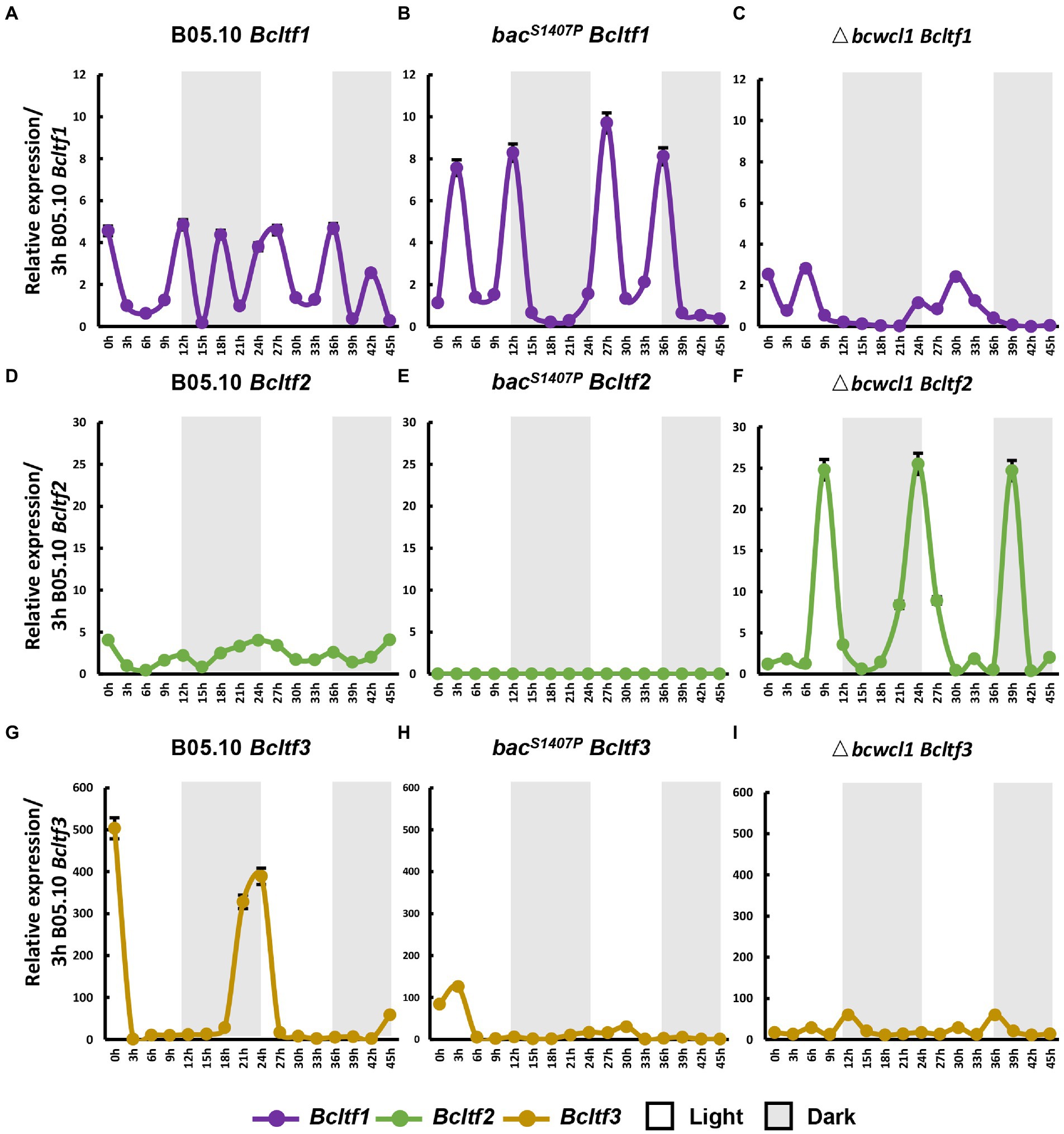
Figure 9. The conidia and sclerotia reproduction-related genes, Bcltf1, Bcltf2, and Bcltf3 in the wild type (B05.10) (A,D,G), bacS1407P (B,E,H), and Δbcwcl1 (C,F,I) at different time points under LD conditions. The expression of B05.10 Bcltf1 (A–C), B05.10 Bcltf2 (D–F), and B05.10 Bcltf3 (G–I) recorded after 3 h was used as a ruler to compare the changes in the expression of genes at different time points under LD conditions for 2 days. Error bars represent standard deviations (n = 3).
In bacS1407P, the expression curve of Bcwcl1 is significantly higher than that of wild type (Figure 7D), while Bcfrq1 in Δbcwcl1 is almost not expressed (Figure 7E), and the expression of LTF in bacS1407P and Δbcwcl1 is significantly changed compared with that of wild type (Figures 9C,F,J), which indicates that BcWCL1, the core component of the circadian clock, can regulate LTF, but its regulatory mechanism has not been reported.
The zinc finger domain of WCC is highly conserved, and the amino acid sequence for DNA binding has been identified in this domain (Wang et al., 2015). Based on the WCC consensus-binding site reported by Baek et al. (2019), the reported WCC downstream gene (Bcfrq1, Bcltf1, Bcltf2, and Bcltf3) promoter regions were analyzed. Bcfrq1 had three putative binding sites on the promoter, whereas Bcltf1 and Bcltf2 possessed one putative site (Figure 10A). In contrast, Bcltf3 did not carry a WCC consensus-binding site (Figure 10A). These results indicate that Bcltf3 is indirectly regulated by WCC. The yeast one-hybrid assay was used to verify the transcriptional regulation of Bcfrq1, Bcltf1, Bcltf2, and Bcltf3 in BcWCL1 (Figure 10B). Consistent with the promoter binding site prediction of Bcfrq1, Bcltf1, Bcltf2, and Bcltf3 in BcWCL1, the yeast one-hybrid assay proved that BcWCL1 could regulate the promoters of Bcfrq1, Bcltf1, and Bcltf2, but not that of Bcltf3 (Figure 10B). In conclusion, the BAC mediated cAMP signaling pathway can regulate the expression of Bcwcl1 and LTF to change the survival strategy of B. cinerea in the natural environment.
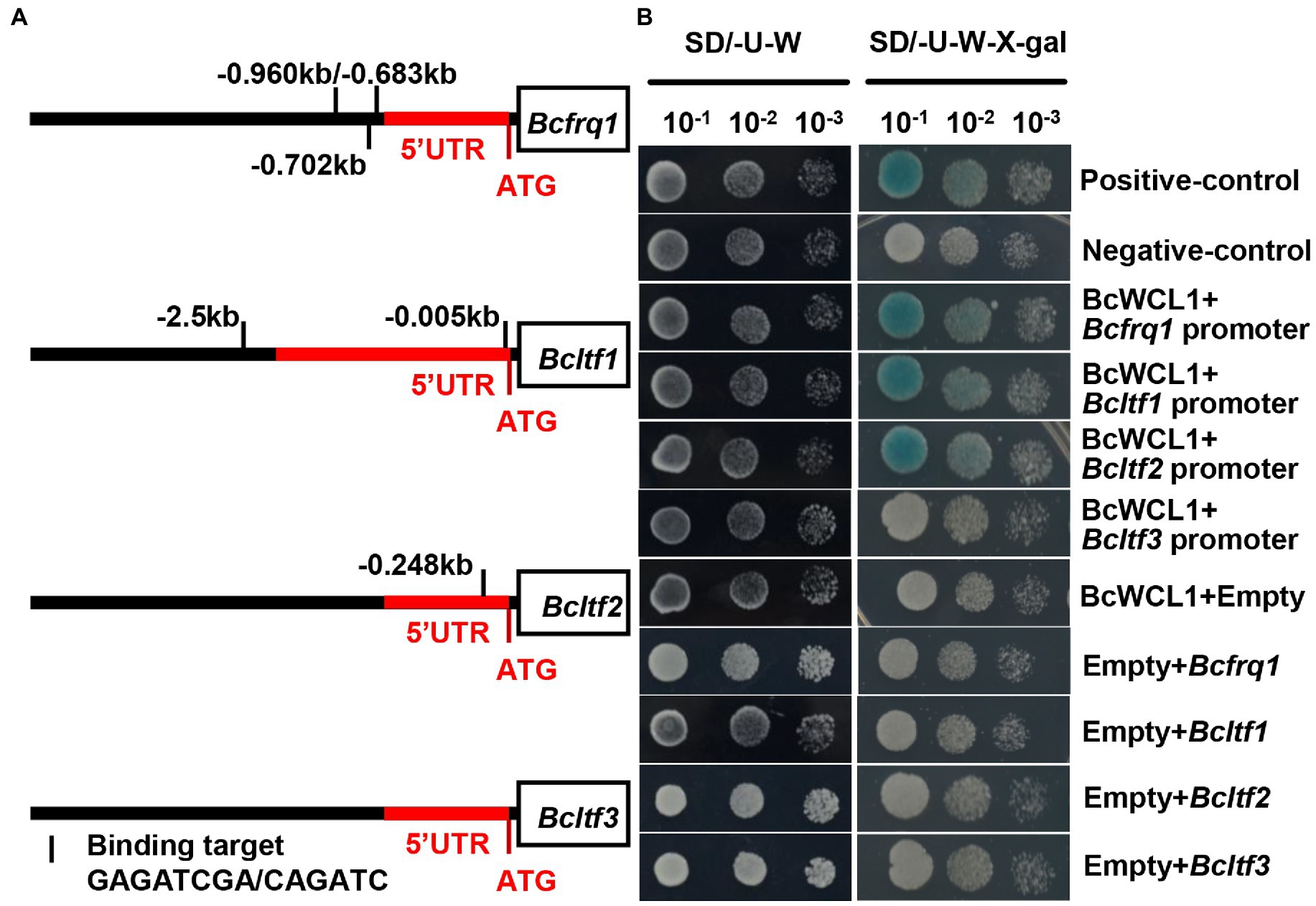
Figure 10. (A) WCC binding site analysis of the Bcfrq1, Bcltf1, Bcltf2, and Bcltf3 promoter regions. The red and black regions represent the gene 5’UTR and the genome region before the 5’UTR, respectively. (B) Promoter binding evaluation of BcWCL1 and Bcfrq1, Bcltf1, Bcltf2, and Bcltf3 by Yeast one-hybrid analysis.
Adenylate cyclase (ACs) in fungi regulate various physiological events during their life cycles. In fact, in Magnaporthe grisea (Gushchin et al., 2017), N. crassa (Héctor et al., 1974), Aspergillus flavus (Yang et al., 2016), Colletotrichum higginsianum (Zhu et al., 2017), Beauveria bassiana (Wang et al., 2014), and some Fusarium species (Li et al., 2018), AC mutations affect mycelial growth, conidia development, and pathogenicity. In addition, the AC mutant sac1 of Sclerotinia sclerotiorum showed a defect in sclerotia development, and the AC mutant acyA of A. flavus affected sclerotia formation (Klimpel et al., 2002; Lafon et al., 2006). AC mutations that mediate a defect in the production of conidia and sclerotia are conserved in many fungi and affect the regulation of the cAMP signaling pathway. Klimpel et al. (2002) first reported that BAC mutations affect vegetative growth, sporulation, sclerotium production, and pathogenicity. Schumacher et al. (2008) reported that the cAMP-PKA signal pathway is involved in mediating other component functions, and deletion of the PKA regulatory subunit BcPKAR leads to the loss of PKA activity. The ΔbcpkaR mutations have a similar photo-morphological defect phenotype as bac, which only produces conidia but does not produce sclerotia. Our study showed that the cAMP signaling pathway mediated by AC could stabilize the operation of the circadian clock. Eventually, it ensures that B. cinerea can grow hyphae and produce conidia or sclerotia under appropriate light conditions.
Other domains of fungal adenylate cyclase similar to BAC have been reported, but the functions of the PP2C domain in AC have not been sufficiently verified in fungi. The protein family with PP2C as the main domain in fungi is called the PTC family. Studies on the members of this family in S. cerevisiae and C. albicans have shown that the PTC family is widely involved in the phosphorylation level regulation of different kinases in the fungal MAPK signal pathway (Ariño et al., 2011). Different from S. cerevisiae, BcPtc3, but not BcPtc1, negatively regulates phosphorylation of BcSak1 (the homolog of S. cerevisiae Hog1) in B. cinerea, and both BcPTC1 and BcPTC3 could rescue the yeast PTC1 deletion mutations growth defects phenotype under various stress conditions (Yang et al., 2013). Our study showed that the S1407 site is located in the PP2C domain and is a very important phosphorylation site; the mutation from serine to proline and aspartate at this site can directly affect the phosphorylation level of BAC and the function of cAMP synthesis, whereas the mutation from serine to alanine at this site does not affect the function of BAC, it indicates that the PP2C domain plays a very important role in BAC functions.
Studies on the PP2C family in fungi show that they are widely involved in the regulation of the phosphorylation level of the MAPK signaling pathway in fungi (Ariño et al., 2011). In addition, Bcptc1 and Bcptc3 of the PP2C family were proven to be involved in regulating the HOG-MAPK signaling pathway in B. cinerea (Yang et al., 2013). In our study, compared with the wild type, the mutation of the S1407 site of the PP2C domain of BAC will increase the phosphorylation level of the total protein and will not be affected by light, and the phosphorylation level of some protein bands increase significantly. Otherwise, the exogenous addition of cAMP did not completely restore the bacS1407P phenotype (Supplementary Figure S3). The PP2C domain of AC was considered to be involved in substrate dephosphorylation, in addition to the synthesis of cAMP. The functions of the PP2C domain in AC should be further explored in future studies.
It was previously revealed that the results of the interaction between B. cinerea and A. thaliana varied with the time of day and that the circadian clock of fungi was necessary for maximum pathogenicity at dusk (Hevia et al., 2015). In this study, the bacS1407P and bacS1407D mutations in BAC increased the differences in the pathogenicity of B. cinerea between dawn and dusk, indicating that the cAMP signaling pathway can also affect the circadian rhythm-regulated pathogenesis oscillation in the fungal pathogen. In nature, the alternation of light and dark is an environmental change experienced by most organisms. Research on the changes in the pathogenicity of plant-pathogenic fungi will help us better prevent and control them.
Compared with the wild type, mutation at the S1407 site of BAC significantly enhanced the circadian rhythm of bacS1407P, indicating that the cAMP signaling pathway can stabilize the circadian clock component expression. Moreover, compared with the B05.10, two Bcfrq1 expression peaks in the light phase under LD conditions and the early expression peak of Bcfrq1 in bacS1407P under LD conditions were affected, which may imply that BAC-mediated cAMP production participates in the early light response. In addition to these downstream genes, the genes upstream of AC and the external environmental signals that can regulate AC are of great significance for the B. cinerea study.
Among the light-responsive transcription factors, Bcltf1, Bcltf2, and Bcltf3 are the three most important LTFs for regulating the development of conidia and sclerotia. The white-collar complex (WCC) can regulate the expression of these genes (Schumacher et al., 2014; Cohrs et al., 2016; Brandhoff et al., 2017). In this study, the expression curve of Bcltf2 in bacS1407P and Δbcwcl1 under LD conditions might explain why conidia production in bacS1407P was significantly reduced, while that in Δbcwcl1 was significantly increased. Compared with the expression curves of Bcltf1 and Bcltf2 in wild type and Δbcwcl1 under LD conditions, Bcltf3 was more tightly regulated by light via the WCC than that observed with the circadian clock. The analysis of the promoters of BcWCL1 and LTF in the yeast single-hybrid assay verified this finding. This indicates that the mutation at the S1407 site of BAC mediates an increase in BAC phosphorylation levels and the loss of cAMP synthesis ability, ultimately regulating the photomorphogenesis of B. cinerea by affecting the expression mode of light transcription factors.
In summary, we identified BAC through the synthesis of cAMP to affect circadian clock component expression stability, and downstream genes, such as Bcltf1, Bcltf2, and Bcltf3, were regulated by circadian clock components to form conidia or sclerotium in response to changes in the light environment (Figure 11). This indicates that the cAMP signal, which is mediated by BAC, is very important for adjusting the survival strategy of B. cinerea.
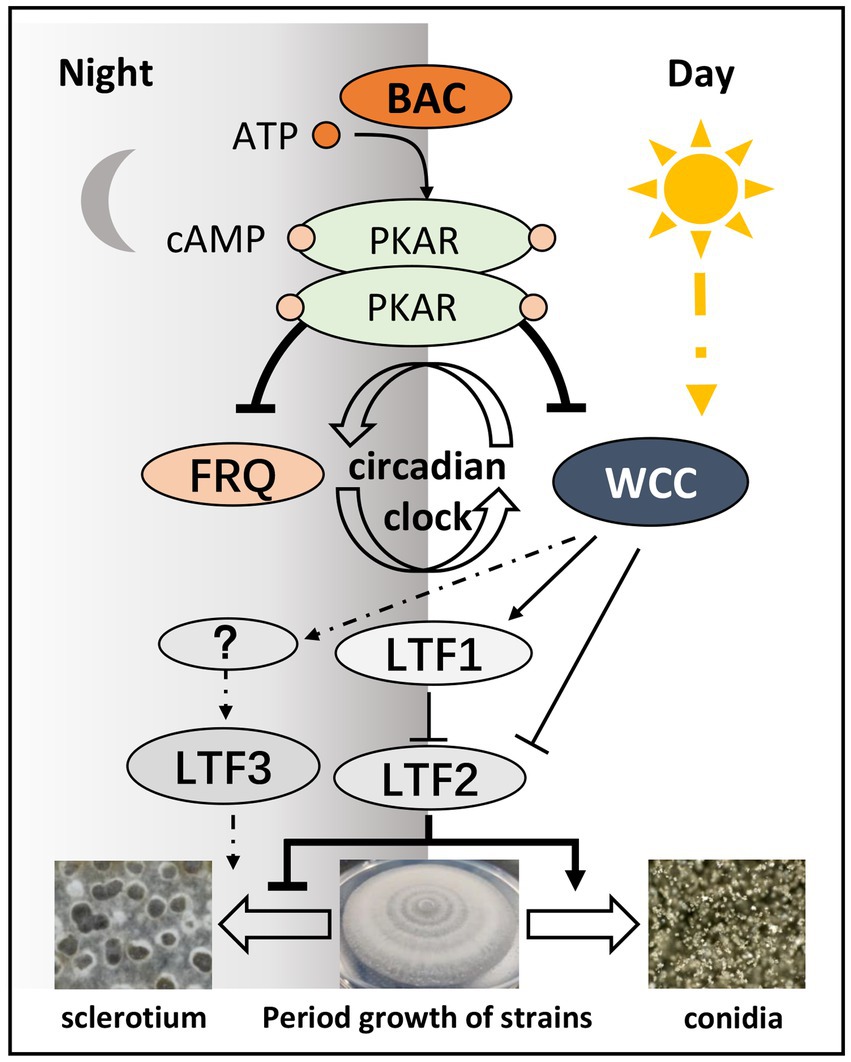
Figure 11. The conidiation and sclerotia formation in B. cinerea are regulated by the cAMP signaling pathway.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
YC and LX conceived the study. YC, XC, PL, WR, QZ, and YW performed the experiments. YC, YJ, PZ, HT, and LX analyzed the data and interpreted the results. YC, HT, and LX took the lead in writing the manuscript. All authors read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (grant nos. 31972121 and 32061133006).
We thank Yanzhang Wang from the Science of Plant Physiology and Ecology and Danial Hassani from East China Normal University School of life science for critically reading the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1112584/full#supplementary-material
1. ^http://fungi.ensembl.org/Botrytis_cinerea/Info/Index
2. ^https://www.ncbi.nlm.nih.gov/
3. ^https://www.ebi.ac.uk/interpro/
4. ^http://smart.embl-heidelberg.de/
5. ^https://services.healthtech.dtu.dk/service.php?NetPhos-3.1
Agarwal, C., Schultz, D. J., and Perlin, M. H. (2010). Two phosphodiesterases from Ustilago maydis share structural and biochemical properties with non-fungal phosphodiesterases. Front. Microbiol. 1:127. doi: 10.3389/fmicb.2010.00127
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., and Lipman, D. J. (1990). Basic local alignment search tool. J. Mol. Biol. 215, 403–410. doi: 10.1016/S0022-2836(05)80360-2
Amselem, J., Cuomo, C. A., van Kan, J. A. L., Viaud, M., Benito, E. P., Couloux, A., et al. (2011). Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 7:e1002230. doi: 10.1371/journal.pgen.1002230
Ariño, J., Casamayor, A., and González, A. (2011). Type 2C protein phosphatases in fungi. Eukaryot. Cell 10, 21–33. doi: 10.1128/EC.00249-10
Baek, M., Virgilio, S., Lamb, T. M., Ibarra, O., Andrade, J. M., Gonçalves, R. D., et al. (2019). Circadian clock regulation of the glycogen synthase (gsn) gene by WCC is critical for rhythmic glycogen metabolism in Neurospora crassa. Proc. Natl. Acad. Sci. U. S. A. 116, 10435–10440. doi: 10.1073/pnas.1815360116
Bassler, J., Schultz, J. E., and Lupas, A. N. (2018). Adenylate cyclases: receivers, transducers, and generators of signals. Cell. Signal. 46, 135–144. doi: 10.1016/j.cellsig.2018.03.002
Brandhoff, B., Simon, A., Dornieden, A., and Schumacher, J. (2017). Regulation of conidiation in Botrytis cinerea involves the light-responsive transcriptional regulators BcLTF3 and BcREG1. Curr. Genet. 63, 931–949. doi: 10.1007/s00294-017-0692-9
Büttner, P., Koch, F., Voigt, K., Quidde, T., Risch, S., Blaich, R., et al. (1994). Variations in ploidy among isolates of Botrytis cinerea: implications for genetic and molecular analyses. Curr. Genet. 25, 445–450. doi: 10.1007/BF00351784
Canessa, P., Schumacher, J., Hevia, M. A., Tudzynski, P., and Larrondo, L. F. (2013). Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: characterization of the white collar complex. PLoS One 8:12. doi: 10.1371/journal.pone.0084223
Chen, X., Zhang, X., Zhu, P., Wang, Y., Na, Y., Guo, H., et al. (2020). A single nucleotide mutation in adenylate cyclase affects vegetative growth, sclerotial formation and virulence of Botrytis cinerea. Int. J. Mol. Sci. 21:2912. doi: 10.3390/ijms21082912
Cohrs, K. C., Simon, A., Viaud, M., and Schumacher, J. (2016). Light governs asexual differentiation in the grey mould fungus Botrytis cinerea via the putative transcription factor BcLTF2. Environ. Microbiol. 18, 4068–4086. doi: 10.1111/1462-2920.13431
Dean, R., van Kan, J. A. L., Pretorius, Z. A., Hammond-Kosack, K. E., Di Pietro, A., Spanu, P. D., et al. (2012). The top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 13, 414–430. doi: 10.1111/j.1364-3703.2011.00783.x
Dubacq, C., Guerois, R., Courbeyrette, R., Kitagawa, K., and Mann, C. (2002). Sgt1p contributes to cyclic AMP pathway activity and physically interacts with the adenylyl cyclase Cyr1p/Cdc35p in budding yeast. Eukaryot. Cell 1, 568–582. doi: 10.1128/EC.1.4.568-582.2002
Efendiev, R., Samelson, B. K., Nguyen, B. T., Phatarpekar, P. V., Baameur, F., Scott, J. D., et al. (2010). AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and-6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J. Biol. Chem. 285, 14450–14458. doi: 10.1074/jbc.M110.109769
Elad, Y., Pertot, I., Cotes Prado, A. M., and Stewart, A. (2016). “Plant hosts of Botrytis spp.” in Botrytis – The Fungus, the Pathogen and Its Management in Agricultural Systems. eds. S. Fillinger and Y. Elad (Cham: Springer International Publishing), 413–486.
Fang, H.-M., and Wang, Y. (2006). RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol. Microbiol. 61, 484–496. doi: 10.1111/j.1365-2958.2006.05248.x
Faretra, F., Antonacci, E., and Pollastro, S. (1988). Sexual behaviour and mating system of Botryotinia fuckeliana, Teleomorph of Botrytis cinerea. Microbiology 134, 2543–2550. doi: 10.1099/00221287-134-9-2543
Fillinger, S., and Elad, Y. (2016). Botrytis – The Fungus, the Pathogen and Its Management in Agricultural Systems. Cham: Springer International Publishing.
Froehlich, A. C., Loros, J. J., and Dunlap, J. C. (2003). Rhythmic binding of a WHITE COLLAR-containing complex to the frequency promoter is inhibited by FREQUENCY. Proc. Natl. Acad. Sci. U. S. A. 100, 5914–5919. doi: 10.1073/pnas.1030057100
Gloerich, M., and Bos, J. L. (2010). Epac: defining a new mechanism for cAMP action. Annu. Rev. Pharmacol. Toxicol. 50, 355–375. doi: 10.1146/annurev.pharmtox.010909.105714
Gou, J., Li, K., Wu, K., Wang, X., Lin, H., Cantu, D., et al. (2015). Wheat stripe rust resistance protein WKS1 reduces the ability of the thylakoid-associated ascorbate peroxidase to detoxify reactive oxygen species. Plant Cell 27, 1755–1770. doi: 10.1105/tpc.114.134296
Gushchin, I. Y., Melnikov, I. I., Polovinkin, V., Ishchenko, A., Yuzhakova, A., Buslaev, P., et al. (2017). Mechanism of transmembrane signaling by sensor histidine kinases. Science 356:1043. doi: 10.1126/science.aah6345
Héctor, F. T., Mirtha, M. F., and Héctor, N. T. (1974). A Neurospora crassa morphological mutant showing reduced adenylate cyclase activity. Biochem. Biophys. Res. Commun. 58, 990–996. doi: 10.1016/s0006-291x(74)80241-x
Hevia, M. A., Canessa, P., and Larrondo, L. F. (2016). Circadian clocks and the regulation of virulence in fungi: getting up to speed. Semin. Cell Dev. Biol. 57, 147–155. doi: 10.1016/j.semcdb.2016.03.021
Hevia, M. A., Canessa, P., Müller-Esparza, H., and Larrondo, L. F. (2015). A circadian oscillator in the fungus Botrytis cinerea regulates virulence when infecting Arabidopsis thaliana. Proc. Natl. Acad. Sci. U. S. A. 112, 8744–8749. doi: 10.1073/pnas.1508432112
Hua, L., Yong, C., Zhanquan, Z., Boqiang, L., Guozheng, Q., and Shiping, T. (2018). Pathogenic mechanisms and control strategies of Botrytis cinerea causing post-harvest decay in fruits and vegetables. Food Qual. Saf. 2, 111–119. doi: 10.1093/fqsafe/fyy016
Huang, G., Chen, S., Li, S., Cha, J., Long, C., Li, L., et al. (2007). Protein kinase A and casein kinases mediate sequential phosphorylation events in the circadian negative feedback loop. Genes Dev. 21, 3283–3295. doi: 10.1101/gad.1610207
Ivey, F. D., and Hoffman, C. S. (2005). Direct activation of fission yeast adenylate cyclase by the Gpa2 Galpha of the glucose signaling pathway. Proc. Natl. Acad. Sci. U. S. A. 102, 6108–6113. doi: 10.1073/pnas.0502270102
Kamada, Y., Yoshino, K., Kondo, C., Kawamata, T., Oshiro, N., Yonezawa, K., et al. (2010). Tor directly controls the Atg1 kinase complex to regulate autophagy. Mol. Cell. Biol. 30, 1049–1058. doi: 10.1128/MCB.01344-09
Klimpel, A., Gronover, C. S., Willianmson, B., Stewart, J. A., and Tudzynski, B. (2002). The adenylate cyclase (BAC) in Botrytis cinerea is required for full pathogenicity. Mol. Plant Pathol. 3, 439–450. doi: 10.1046/j.1364-3703.2002.00137.x
Korkhov, V. M., and Qi, C. (2019). The structure of a membrane adenylyl cyclase bound to an activated stimulatory G protein. Science 364, 389–394. doi: 10.1126/science.aav0778
Lafon, A., Han, K.-H., Seo, J.-A., Yu, J.-H., and d'Enfert, C. (2006). G-protein and cAMP-mediated signaling in aspergilli: a genomic perspective. Fungal Genet. Biol. 43, 490–502. doi: 10.1016/j.fgb.2006.02.001
Li, L., Wright, S. J., Krystofova, S., Park, G., and Borkovich, K. A. (2007). Heterotrimeric G protein signaling in filamentous fungi. Annu. Rev. Microbiol. 61, 423–452. doi: 10.1146/annurev.micro.61.080706.093432
Li, C., Zhang, Y., Wang, H., Chen, L., Zhang, J., Sun, M., et al. (2018). The PKR regulatory subunit of protein kinase a (PKA) is involved in the regulation of growth, sexual and asexual development, and pathogenesis in Fusarium graminearum. Mol. Plant Pathol. 19, 909–921. doi: 10.1111/mpp.12576
Liñeiro, E., Chiva, C., Cantoral, J. M., Sabido, E., and Fernández-Acero, F. J. (2016). Phosphoproteome analysis of B. cinerea in response to different plant-based elicitors. J. Proteome 139, 84–94. doi: 10.1016/j.jprot.2016.03.019
Maidan, M. M., Rop, L., de Serneels, J., Exler, S., Rupp, S., Tournu, H., et al. (2005a). The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase a pathway to induce morphogenesis in Candida albicans. Mol. Biol. Cell 16, 1971–1986. doi: 10.1091/mbc.e04-09-0780
Maidan, M. M., Thevelein, J. M., and van Dijck, P. (2005b). Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-protein-coupled receptor Gpr1. Biochem. Soc. Trans. 33, 291–293. doi: 10.1042/BST0330291
Nicastro, R., Tripodi, F., Gaggini, M., Castoldi, A., Reghellin, V., Nonnis, S., et al. (2015). Snf1 phosphorylates adenylate cyclase and negatively regulates protein kinase A-dependent transcription in Saccharomyces cerevisiae. J. Biol. Chem. 290, 24715–24726. doi: 10.1074/jbc.M115.658005
Pontecorvo, G., Roper, J. A., Chemmons, L. M., Macdonald, K. D., and Bufton, A. (1953). The genetics of Aspergillus nidulans. Adv. Genet. 38, 96–238. doi: 10.1016/S0007-1536(55)80017-4
Qi, L., Kwiatkowski, M., Chen, H., Hoermayer, L., Sinclair, S., Zou, M., et al. (2022). Adenylate cyclase activity of TIR1/AFB auxin receptors in plants. Nature 611, 133–138. doi: 10.1038/s41586-022-05369-7
Schumacher, J. (2017). How light affects the life of Botrytis. Fungal Genet. Biol. 106, 26–41. doi: 10.1016/j.fgb.2017.06.002
Schumacher, J., Kokkelink, L., Huesmann, C., Jimenez-Teja, D., Collado, I. G., Barakat, R., et al. (2008). The cAMP-dependent signaling pathway and its role in conidial germination, growth, and virulence of the gray mold Botrytis cinerea. Mol. Plant Microbe. Interact 21, 1443–1459. doi: 10.1094/MPMI-21-11-1443
Schumacher, J., Pradier, J.-M., Simon, A., Traeger, S., Moraga, J., Collado, I. G., et al. (2012). Natural variation in the VELVET gene bcvel1 affects virulence and light-dependent differentiation in Botrytis cinerea. PLoS One 7:840. doi: 10.1371/journal.pone.0047840
Schumacher, J., Simon, A., Cohrs, K. C., Viaud, M., and Tudzynski, P. (2014). The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLoS Genet. 10:e1004040. doi: 10.1371/journal.pgen.1004040
Staats, M., and van Kan, J. A. L. (2012). Genome update of Botrytis cinerea strains B05.10 and T4. Eukaryot. Cell 11, 1413–1414. doi: 10.1128/EC.00164-12
van Kan, J. A., Stassen, J. H. M., Mosbach, A., van der Lee, T. A. J., Faino, L., Farmer, A. D., et al. (2017). A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 18, 75–89. doi: 10.1111/mpp.12384
Wang, B., Zhou, X., Loros, J. J., and Dunlap, J. C. (2015). Alternative use of DNA binding domains by the Neurospora white collar complex dictates circadian regulation and light responses. Mol. Cell. Biol. 36, 781–793. doi: 10.1128/MCB.00841-15
Wang, J., Zhou, G., Ying, S.-H., and Feng, M.-G. (2014). Adenylate cyclase orthologues in two filamentous entomopathogens contribute differentially to growth, conidiation, pathogenicity, and multistress responses. Fungal Biol. 118, 422–431. doi: 10.1016/j.funbio.2014.03.001
Williamson, B., Tudzynski, B., Tudzynski, P., and van Kan, J. A. L. (2007). Botrytis cinerea: the cause of grey mould disease. Mol. Plant Pathol. 8, 561–580. doi: 10.1111/j.1364-3703.2007.00417.x
Xu, X.-L., Lee, R. T. H., Fang, H.-M., Wang, Y.-M., Li, R., Zou, H., et al. (2008). Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe 4, 28–39. doi: 10.1016/j.chom.2008.05.014
Yang, Q., Jiang, J., Mayr, C., Hahn, M., and Ma, Z. (2013). Involvement of two type 2C protein phosphatases BcPtc1 and BcPtc3 in the regulation of multiple stress tolerance and virulence of Botrytis cinerea. Environ. Microbiol. 15, 2696–2711. doi: 10.1111/1462-2920.12126
Yang, K., Qin, Q., Liu, Y., Zhang, L., Liang, L., Lan, H., et al. (2016). Adenylate Cyclase AcyA regulates development, aflatoxin biosynthesis and fungal virulence in Aspergillus flavus. Front. Cell. Infect. Microbiol. 6:190. doi: 10.3389/fcimb.2016.00190
Yoshimasa, T., Sibley, D. R., Bouvier, M., Lefkowitz, R. J., and Caron, M. G. (1987). Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature 327, 67–70. doi: 10.1038/327067a0
Keywords: Botrytis cinerea, adenylate cyclase, cAMP, circadian clock, photomorphogenesis, pathogenicity
Citation: Cai Y, Chen X, Li P, Ren W, Zhang Q, Wang Y, Jiang Y, Zhu P, Toyoda H and Xu L (2023) Phosphorylation status of a conserved residue in the adenylate cyclase of Botrytis cinerea is involved in regulating photomorphogenesis, circadian rhythm, and pathogenicity. Front. Microbiol. 14:1112584. doi: 10.3389/fmicb.2023.1112584
Received: 30 November 2022; Accepted: 20 January 2023;
Published: 15 February 2023.
Edited by:
Hari S. Misra, Bhabha Atomic Research Centre, IndiaCopyright © 2023 Cai, Chen, Li, Ren, Zhang, Wang, Jiang, Zhu, Toyoda and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Xu, ✉ bHh1QGJpby5lY251LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.