
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Microbiol. , 02 March 2023
Sec. Microbial Symbioses
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1110474
This article is part of the Research Topic Diversity of Beetles and Associated Microorganisms View all 22 articles
Introduction: Ambrosia beetles maintain strict associations with specific lineages of fungi. However, anthropogenic introductions of ambrosia beetles into new ecosystems can result in the lateral transfer of their symbionts to other ambrosia beetles. The ability of a Florida endemic ambrosia beetle, Xyleborus bispinatus, to feed and establish persistent associations with two of its known symbionts (Raffaelea subfusca and Raffaelea arxii) and two other fungi (Harringtonia lauricola and Fusarium sp. nov.), which are primary symbionts of invasive ambrosia beetles, was investigated.
Methods: The stability of these mutualisms and their effect on the beetle’s fitness were monitored over five consecutive generations. Surface-disinfested pupae with non-developed mycangia were reared separately on one of the four fungal symbionts. Non-treated beetles (i.e., lab colony) with previously colonized mycangia were used as a control group.
Results: Xyleborus bispinatus could exchange its fungal symbionts, survive, and reproduce on different fungal diets, including known fungal associates and phylogenetically distant fungi, which are plant pathogens and primary symbionts of other invasive ambrosia beetles. These changes in fungal diets resulted in persistent mutualisms, and some symbionts even increased the beetle’s reproduction. Females that developed on Fusarium sp. nov. had a significantly greater number of female offspring than non-treated beetles. Females that fed solely on Harringtonia or Raffaelea symbionts produced fewer female offspring.
Discussion: Even though some ambrosia beetles like X. bispinatus can partner with different ambrosia fungi, their symbiosis under natural conditions is modulated by their mycangium and possibly other environmental factors. However, exposure to symbionts of invasive beetles can result in stable partnerships with these fungi and affect the population dynamics of ambrosia beetles and their symbionts.
Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) live in obligate nutritional relationships with ambrosia fungi (Farrell et al., 2001; Mueller et al., 2005; Biedermann and Vega, 2020). In this mutualistic relationship, the fungi serve as food sources for the beetles, whereas the fungi rely on the beetles for protection, dispersal, and maintenance (Biedermann and Taborsky, 2011; Hulcr and Stelinski, 2017; Nuotclà et al., 2019). Some ambrosia beetles are associated with a single dominant mutualistic fungus, while others are associated with multiple fungal symbionts (Batra, 1963; Gebhardt et al., 2004; Harrington et al., 2010; Kostovcik et al., 2015; Menocal et al., 2017, 2018a; Cruz et al., 2018, 2019, 2021; Saucedo-Carabez et al., 2018; Carrillo et al., 2019). Females in the Xylosandrus and Euwallacea species-complex are commonly associated with Ambrosiella (Microascales: Ceratocystidaceae) and Fusarium (Hypocreales: Nectriaceae) species, respectively, whereas females in the genera Xyleborinus and Xyleborus are strictly associated with Raffaelea species (Ophiostomatales: Ophiostomateacea), suggesting that ambrosia beetles exhibit fidelity toward specific groups of fungal symbionts (Biedermann et al., 2009, 2013; Kasson et al., 2013; Bateman et al., 2015; Mayers et al., 2015, 2020a,b; O’Donnell et al., 2015; Biedermann, 2020).
A few studies have investigated ambrosia beetle symbiont exchange or transition to specific fungi. Carrillo et al. (2014) reported for the first time the lateral transfer of nutritional symbionts between native and exotic ambrosia beetles. Skelton et al. (2019) documented that Xylosandrus compactus Eichhoff can exchange nutritional fungal symbionts among closely related Ambrosiella species, but a shift to more phylogenetically distant fungal species is less likely to occur. In a different study, Carrillo et al. (2020a) demonstrated that various shot hole borers (Euwallacea spp.) can survive and reproduce using each other’s Fusarium fungal symbionts. These studies suggest that transition to other nutritional fungal symbionts could occur more commonly among congeneric ambrosia beetle species that share closely related fungi and in species with relatively small mycangia [pre-oral (Xyleborus spp.) vs. mesonotal mycangia (Xylosandrus spp.)] (Joseph and Keyhani, 2021; Mayers et al., 2022).
Acquisition of new fungal symbionts may occur naturally when one ambrosia beetle species interacts with the fungi inside the gallery system of another species or, when mature females that search for new hosts, encounter fungi present in the environment (Kendra et al., 2011; Carrillo et al., 2014; Rassati et al., 2019). However, only the first mechanism is expected to involve nutritional fungal symbionts. Nutritional ambrosia fungi are not directly transmitted from mother to offspring. Larvae grow feeding on symbiotic fungi, but their gut contents are emptied during the pupal stage. Then, naïve adults acquire their symbionts via feeding (Xyleborus and Euwallacea) or by close contact with the gallery walls (Xylosandrus). This period of independent growth is a critical point for the acquisition of new nutritional fungal symbiont (s) (Six, 2012).
Some invasive ambrosia beetles partner with plant pathogens that can seriously damage forest and agricultural systems (Hanula et al., 2008; Hulcr and Dunn, 2011; Six, 2012; Ploetz et al., 2013; Hughes et al., 2017; Hulcr and Stelinski, 2017). Two new diseases currently threaten avocado production in South Florida. Laurel wilt (LW) and Fusarium dieback (FD) are caused by Harringtonia lauricola (T. C. Harr, Fraedrich & Aghayeva) Z. W. de Beer & M. Procter and Fusarium sp. nov., primary symbionts of Xyleborus glabratus Eichhoff and Euwallacea perbrevis Schedl, respectively (Harrington and Fraedrich, 2010; Eskalen et al., 2013; Smith et al., 2019; de Beer et al., 2022). Although X. glabratus is rarely detected in avocado orchards, other native and exotic ambrosia beetles in these orchards have acquired H. lauricola via lateral transfer (Carrillo et al., 2014; Kendra et al., 2020; Cloonan et al., 2022). The LW pathogen is now associated with the mycangial communities of several Xyleborus, Ambrosiodmus, Xyleborinus, and Xylosandrus species (Carrillo et al., 2014; Ploetz et al., 2017; Cruz et al., 2021). Unlike H. lauricola and its secondary vectors, the FD pathogen (s) are only vectored by Euwallacea spp. However, experiments evaluating the interaction between Fusarium spp. and other Scolytinae species outside the genus Euwallacea are lacking.
Florida avocado orchards house a diverse community of ambrosia beetles that often breed in trees affected by either LW or FD (Carrillo et al., 2012, 2016; Kendra et al., 2017, 2020; Menocal et al., 2018b, 2022; Owens et al., 2019a,b; Rivera et al., 2020; Cloonan et al., 2022). One of these species, Xyleborus bispinatus Eichhoff, is a Florida native beetle that has shown plasticity in its fungal symbiosis (Cruz et al., 2018). This species has become relevant due to its frequent partnership with H. lauricola, and its ability to transmit the pathogen to healthy avocado trees (Carrillo et al., 2014). However, the persistence of this association, the possible additional association with FD pathogen (s), and the effect of these pathogens on the beetle’s fitness are still understudied. To gain insights into this novel partnership, the symbionts of X. bispinatus were removed and the symbiosis was re-established with selected ambrosia fungi species. The specific objectives of this study were to: (1) determine whether X. bispinatus can feed and reproduce on H. lauricola, Fusarium sp. nov., and its original nutritional symbionts R. subfusca and R. arxii, (2) determine how stable these associations could be over several generations, and (3) determine the effect that each fungal symbiont has on offspring production.
Xyleborus bispinatus adult females were collected in-flight at dusk from an avocado orchard (N25° 35′42” W80° 28′ 21″) (Kendra et al., 2012). Briefly, a white cotton fabric (3 × 3 m) was placed on the ground, and ethanol lures (ultra-high release; Contech Enterprises, Inc., Victoria, BC, Canada) were hung from a tripod at ~1 m above the fabric. Beetles landing on the fabric were collected and placed into plastic containers with moist tissue paper for transport to the laboratory. Then, all females were surface disinfested in 70% ethanol for 10 s to reduce contaminants that could jeopardize laboratory rearing. More active and vigorous females were introduced individually into rearing tubes filled with sterile avocado sawdust artificial media to start new colonies (Menocal et al., 2017, 2018a). Rearing tubes were stored in a walk-in rearing room (25 ± 1°C, 70% RH) for 24 days, allowing the new progeny to reach the pupal stage (Cruz et al., 2018). Then, colonies were dissected under sterile conditions in a laminar flow hood by systematically cutting the rearing media plug into small pieces. Gallery tunnels were opened carefully by removing the medium around them. All male and female pupae from a single colony were gently removed and placed into a sterilized Petri dish. Pupae were individually transferred to Eppendorf tubes to be surface-disinfested by a sequence of washes with phosphate-buffered saline (PBS) + 0.1% Tween, 2% sodium hypochlorite, 70% ethanol, and sterile water for 10, 5, 10, and 10 s, respectively. After the washes, presumably aposymbiotic pupae from each colony were regrouped and allowed to dry before being exposed to selected fungi.
Harringtonia lauricola, R. subfusca, and R. arxii isolates recovered from X. bispinatus and a Fusarium sp. isolate recovered from E. perbrevis were used in the experiment (Table 1). Spore solutions of each fungus were obtained by gently scraping two-week-old monosporic cultures with a sterile plastic rod (Fisher Scientific Catalog No. 23600896) and resuspending them in sterile water to a final concentration of 1 × 106 conidia/mL. The spore solutions (100 μl) were individually plated on 47-mm Petri dishes containing the same avocado sawdust media used to rear the beetles. These cultures were incubated at 25°C for 10 days in complete darkness until the avocado sawdust media was colonized entirely. Then, groups of one male and ten female aposymbiotic pupae from the same colony were transferred to the Petri dishes with the fungal cultures and maintained in complete darkness at room temperature (25°C). Several holes, 0.5 cm deep, were made on the surface of the artificial substrate to promote boring by emerging adults. Adults remained in the plates for 7–10 days to complete sclerotization, fungal acquisition, and mating. Afterward, 20 active, vigorous, and presumably fertilized females, exposed to a given fungal species were placed individually in rearing tubes with sterile avocado sawdust media to establish new colonies. Non-altered X. bispinatus colonies (i.e., with non-modified symbionts) containing R. arxii, R. subfusca, R. subalba, and numerous yeasts, but not H. lauricola (Cruz et al., 2018) were used as the control treatment. All colonies were dissected 40 days later to extract 20 new fully sclerotized females to start new colonies. This procedure was repeated until completing five generations. The number of female and male offspring was recorded for each colony of each generation.
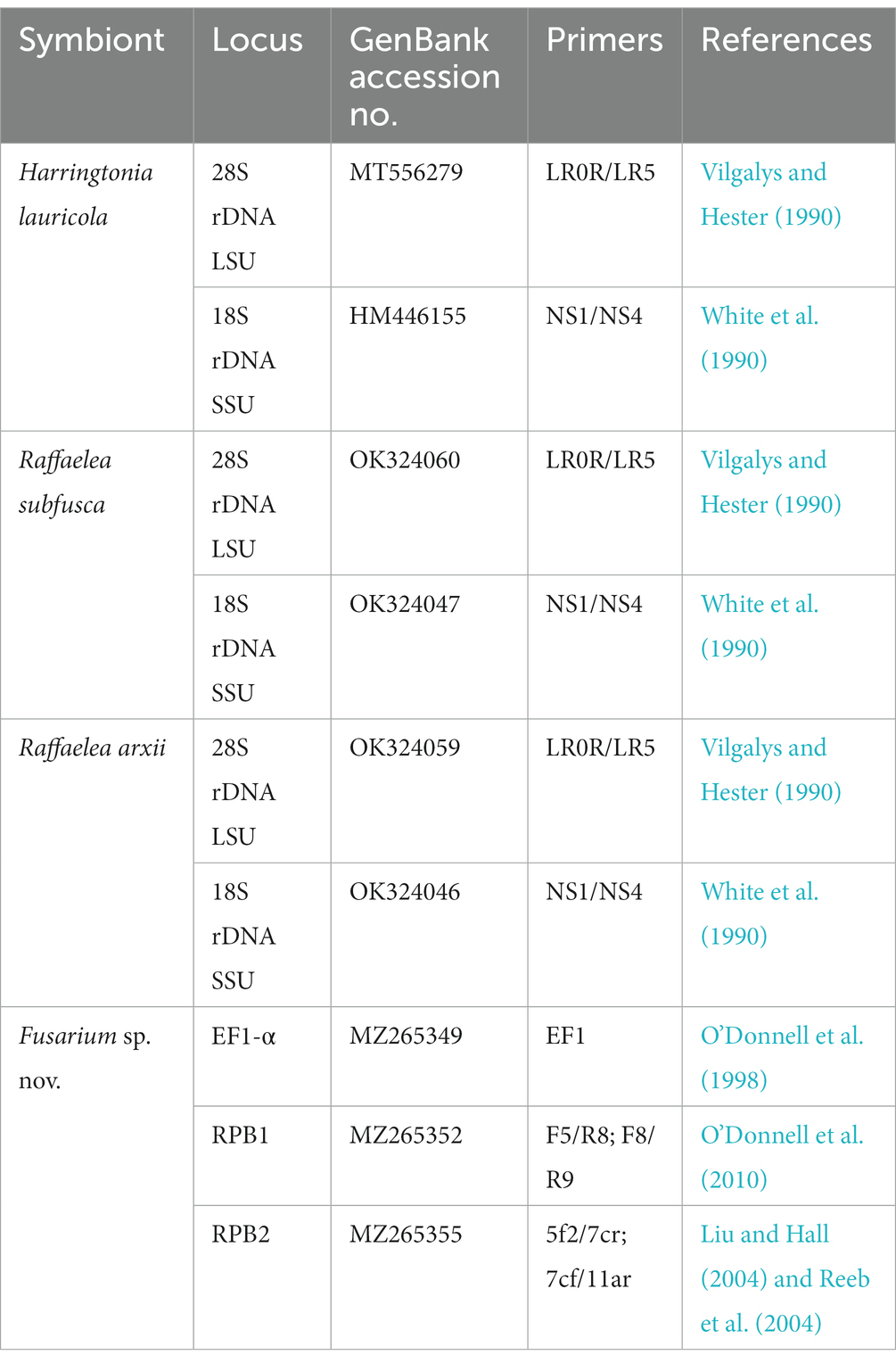
Table 1. GenBank accession numbers and primers used for identification of four fungal symbionts used as diets by Xyleborus bispinatus in this study.
One female offspring from each colony and each generation was assayed for fungal presence. Briefly, females were surface disinfested in 70% ethanol, agitated for 10 s, and subsequently rinsed in sterile deionized water. Then, females were individually macerated in 500 μl of sterile water. Aliquots of 100 μl of the macerate were plated on cycloheximide-streptomycin-malt agar (CSMA), a selective medium for Ophiostomatales (Harrington et al., 2010), and potato dextrose agar amended with 0.1 g/l streptomycin (PDA+). Plates were incubated for 5–7 days at 25°C in complete darkness. The number of colony-forming units (CFUs) was recorded for each colony in each generation. Plates exhibiting contaminants such as Aspergillus spp., Penicillium spp., and/or more than one fungal phenotype were discarded along with its corresponding rearing tube. Plates displaying phenotypically identical CFUs were subcultured to obtain four to six single-spore cultures. DNA was extracted from the mycelium of monosporic isolates following a modified Cetyltrimethylammonium Bromide (CTAB) protocol (Doyle and Doyle, 1987). Fungal symbionts were identified using the nuclear ribosomal large subunit (28S), the nuclear ribosomal small subunit (18S), translation elongation factor 1-alpha (EF1α), RNA polymerase largest subunit (RPB1), and RNA polymerase second largest subunit (RPB2). Primers are listed in Table 1. In addition, the identity of H. lauricola was confirmed with two diagnostic microsatellite markers, CHK and IFW (Dreaden et al., 2014). PCR products were purified using ExoSAP-IT (Affymetrix, CA, USA) following the manufacturer’s guidelines. Sequencing of the PCR products was carried out by Eurofins genomics (Louisville, KY).
Data were analyzed using SAS v. 9.4 (PROC GLIMMIX, SAS Institute 2010, Cary, NC, USA). Separate one-way analyses of variance (ANOVA) per fungal symbiont were conducted to detect differences in the number of CFUs and female offspring among generations. Number of males were too few for statistical analysis. A negative binomial distribution was used when analyzing CFU data due to overdispersion in the dataset, and a Poisson distribution was used for female offspring production. Due to the non-normality of the data set and variance heterogeneity, separate Kruskal-Wallis tests were used to detect differences in female offspring production between each fungal symbiont against non-treated beetles in each generation. To determine which fungal symbiont supported more X. bispinatus progeny, female offspring were compared among fungal treatments through an analysis of variance (ANOVA) with a Poisson distribution. Finally, any significant differences were identified by multiple post-hoc comparisons (Tukey’s HSD test) at p < 0.05. All figures were generated using JMP Pro 14.
The mean number of H. lauricola propagules recovered from X. bispinatus was relatively constant among generations (F = 0.4752; df = 4, 28; p = 0.7218) (Figure 1A). Females fed solely on H. lauricola increased reproduction (i.e., female offspring) in the first three generations and declined drastically in the last two (F = 9.4419; df = 4, 28; p < 0.0001) (Figure 1B). Switching to a H. lauricola diet resulted in significantly fewer female offspring than that recorded from control females with unaltered symbiosis throughout the five generations (F1: X2 = 9.3272, p = 0.0023; F2: X2 = 11.2295, p = 0.0008; F3: X2 = 7.4715, p = 0.0063; F4: X2 = 14.4687, p = 0.0023; F5: X2 = 12.4670, p = 0.0004) (Figure 1B). Notably, no individuals fed exclusively on H. lauricola survived after the fifth generation.
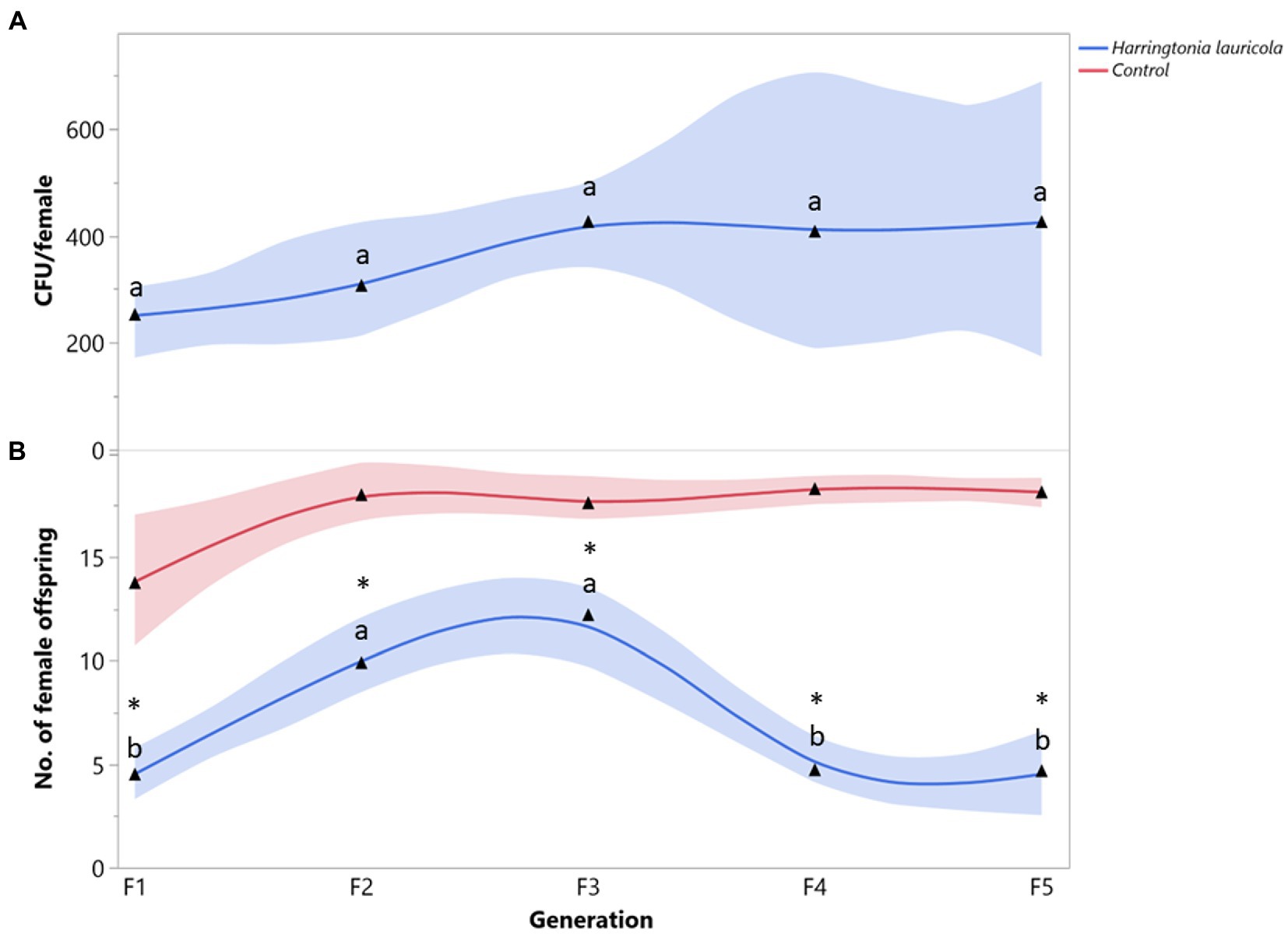
Figure 1. Xyleborus bispinatus performance with a single mutualistic fungus (Harringtonia lauricola). (A) Mean number of CFUs (▲) per female offspring per generation and (B) Mean number of female offspring (▲) produced by single female founder per generation. Same letters within the same color are not significantly different (Tukey HSD, α = 0.05). Asterisks show significant differences in female offspring between fungal symbiont and control within generation (Kruskall-Wallis, α = 0.05).
The number of R. subfusca propagules was not significantly different among generations (F = 0.8447; df = 4, 63; p = 0.6969) (Figure 2A). Females fed on R. subfusca diet produced a similar number of female offspring in the first three generations, but reproduction decreased in the last two (F = 6.2366; df = 4, 63; p = 0.0003) (Figure 2B). Females exposed to a R. subfusca diet produced an F1 with a similar number of females to the unaltered control (X2 = 2.5452, p = 0.1106). However, reproduction in subsequent generations was less than in the control (F2: X2 = 18.7822, p < 0.0001; F3: X2 = 18.9397, p < 0.0001; F4: X2 = 21.5985, p < 0.0001; F5: X2 = 25.1267, p < 0.0001) (Figure 2B).
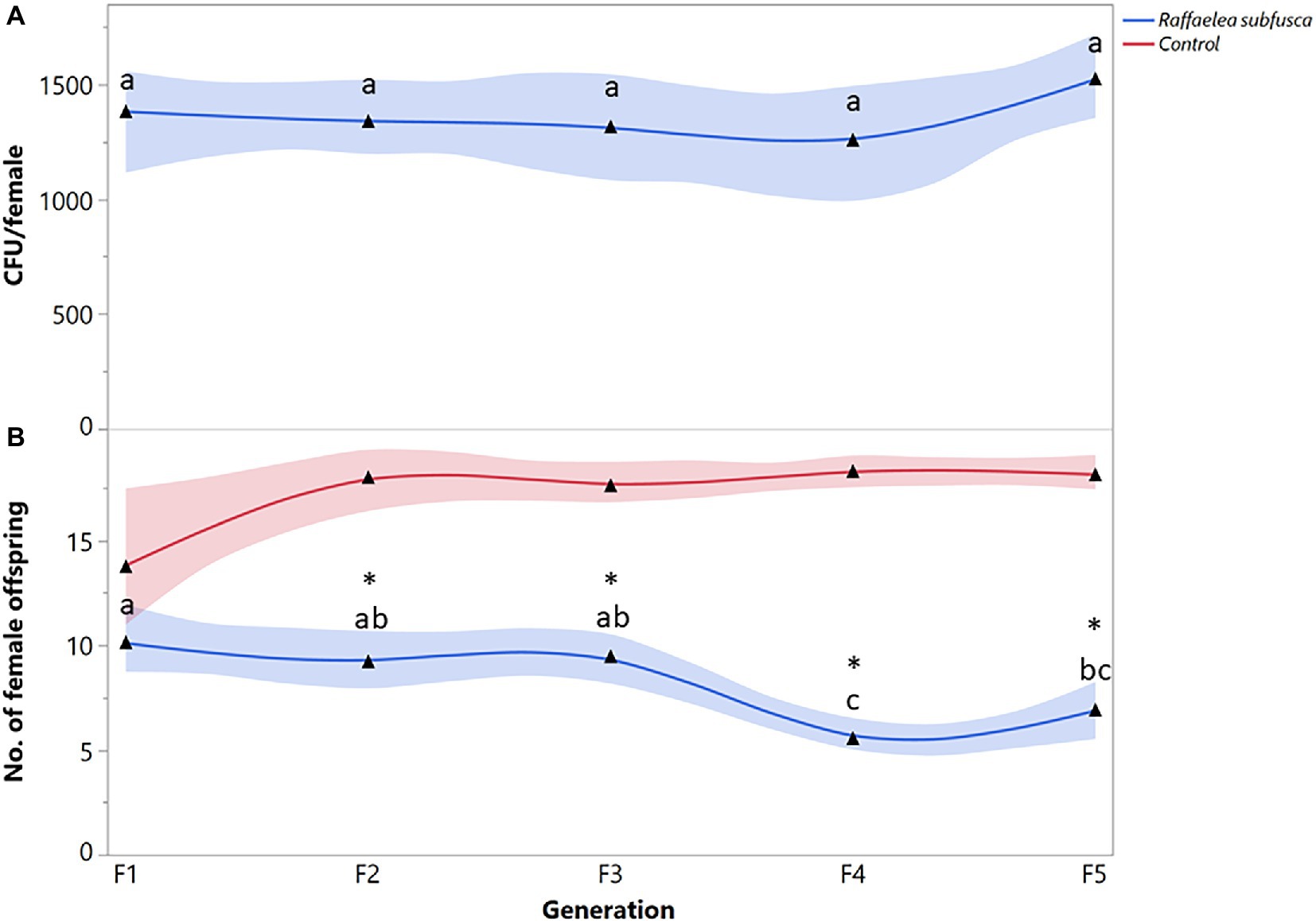
Figure 2. Xyleborus bispinatus performance with a single mutualistic fungus (Raffaelea subfusca). (A) Mean number of CFUs (▲) recovered from single female offspring per generation and (B) Mean number of female offspring (▲) produced by single female founder per generation. Same letters within the same color are not significantly different (Tukey HSD, α = 0.05). Asterisks show significant differences in female offspring between fungal symbiont and control within generation (Kruskall-Wallis, α = 0.05).
The number of R. arxii propagules recovered from X. bispinatus varied significantly among generations (F = 5.2992; df = 4, 65; p = 0.0254). Raffaelea arxii CFUs were equally constant in the first three generations but increased in the last two (Figure 3A). Xyleborus bispinatus females transitioned to a R. arxii diet produced similar number of female offspring during five generations (F = 0.7125; df = 4, 65; p = 0.5864) (Figure 3B). The F1 female offspring of R. arxii fed females was similar to the control with unaltered symbiosis (X2 = 2.6894, p = 0.1010), but reproduction in subsequent generations was significantly less than in the control (F2: X2 = 12.2685, p = 0.0005; F3: X2 = 11.5424, p = 0.0007; F4: X2 = 19.3982, p < 0.0001; F5: X2 = 24.8068, p < 0.0001) (Figure 3B).

Figure 3. Xyleborus bispinatus performance with a single mutualistic fungus (Raffaelea arxii). (A) Mean number of CFUs (▲) recovered from single female offspring per generation and (B) Mean number of female offspring (▲) produced by single female founder per generation. Same letters within the same color are not significantly different (Tukey HSD, α = 0.05). Asterisks show significant differences in female offspring between fungal symbiont and control within generation (Kruskall-Wallis, α = 0.05).
Finally, the number of Fusarium sp. nov. propagules recovered from X. bispinatus varied significantly among generations (F = 4.2888; df = 4, 95; p = 0.0261). CFUs of Fusarium sp. nov. increased from the first to the second generation but decreased in the third generation remaining similar throughout the subsequent generations (Figure 4A). Xyleborus bispinatus females that fed on Fusarium sp. nov. diet produced a higher number of female offspring in the last four generations than in the first one (F = 13.5657; df = 4, 95; p < 0.0001) (Figure 4B). In the first generation, Fusarium sp. nov. fed females produced similar number of female offspring to the control, but reproduction increased in subsequent generations, significantly more than in the control (Figure 4B).
Figure 4. Xyleborus bispinatus performance with a single mutualistic fungus (Fusarium sp. nov.). (A) Mean number of CFUs (▲) recovered from single female offspring per generation and (B) Mean number of female offspring (▲) produced by single female founder per generation. Same letters within the same color are not significantly different (Tukey HSD, α = 0.05). Asterisks show significant differences in female offspring between fungal symbiont and control within generation (Kruskall-Wallis, α = 0.05).
The number of female offspring (F = 4.78; df = 4, 311; p < 0.0001) varied among fungal treatments (Figure 5). Females fed on a Fusarium sp. nov. diet produced the highest number of female offspring (Figure 5). Non-treated beetles (i.e., control) produced fewer individuals than those fed on Fusarium sp. nov., but significantly more than beetles reared on H. lauricola and the two Raffaelea species. There were no significant differences in the number of female offspring among H. lauricola and the Raffaelea treatments (Figure 5). The number of males ranged between 1 or 2 males per colony and did not vary among treatments.
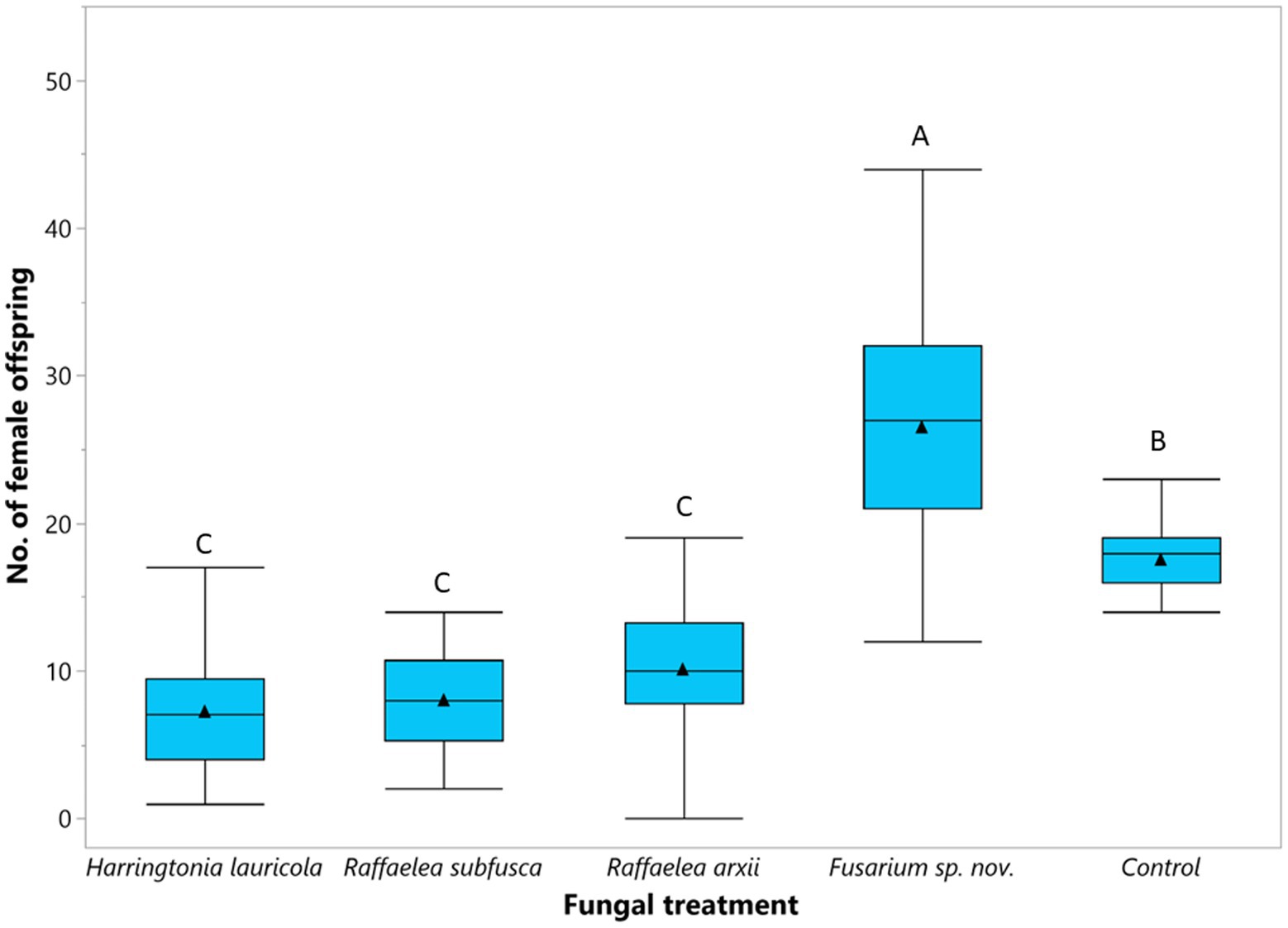
Figure 5. Comparison of the mean number of female offspring (▲) produced by single female founder adapted to single fungal symbiont. Box plots topped with the same letter are not significantly different (Tukey HSD, α = 0.05).
Laboratory reared X. bispinatus exhibits flexibility in its symbiotic associations and can establish stable populations feeding on different fungal diets, including known fungal associates and phylogenetically distant fungi, which are primary symbionts of other ambrosia beetle genera. These changes in fungal diets resulted in persistent mutualistic associations, and some symbionts even increased the beetle’s reproduction. However, ambrosia beetles exhibit high fidelity in their symbiotic relationships with specific groups of fungi under natural conditions. The mechanisms that drive this fidelity have been a matter of active and recent research. In some bark and ambrosia beetles, symbiont fidelity is thought to be mediated by the mycangium (Bracewell and Six, 2015; Mayers et al., 2015, 2020a,b, 2022; Li et al., 2017; Skelton et al., 2019). For instance, Ambrosiella fungi thrive in the mycangium of Xylosandrus beetles, whereas distantly unrelated fungi are suppressed. The mycangium of X. bispinatus does not appear to provide this type of selectivity, allowing mutualistic interactions with distant unrelated fungi. Xyleborus spp. have small pre-oral mycangia (Li et al., 2018; Spahr et al., 2020; Mayers et al., 2020a), which are presumed to be less selective or more permissive than larger, more complex mycangia (Mayers et al., 2015, 2020a; Hulcr and Stelinski, 2017; Joseph and Keyhani, 2021). We infer that species with less mycangial selectivity such as X. bispinatus are more likely to establish mutualisms with new ambrosia fungi. Interactions with other organisms inhabiting beetle galleries can also play an important role in the fidelity of ambrosia symbiosis. Grubbs et al. (2020) and Nuotclà et al. (2021) suggested that bacteria may selectively exclude non-mutualistic fungi from the galleries and mycangia. In addition, the mycangia may provide an environment and specific nutrients that favor the growth of particular nutritional fungi (e.g., biological screening). The host tree physiology may also affect symbiotic fungi and, thus, their association with a given beetle. Some ambrosia beetles target stressed trees producing ethanol while others do not. Ethanol-enriched wood favors the growth of particular nutritional symbionts while suppressing others. This suggests that ethanol content in wood may influence the symbiont composition and thus the likelihood of novel symbiont acquisition (Ranger et al., 2018; Cavaletto et al., 2021, 2022; Lehenberger et al., 2021b). However, such selective effects of substrate were not present in our artificial medium and may influence the effects of particular fungi on beetle fitness in vivo.
Some ambrosia beetles can use one or more closely related fungi (Skelton et al., 2019; Carrillo et al., 2020a) as nutritional food sources. Raffaelea species are common partners of Xyleborus species (Harrington et al., 2010; Campbell et al., 2016; Cruz et al., 2018, 2019; Saucedo-Carabez et al., 2018); therefore, the survival and reproduction of X. bispinatus on R. subfusca and R. arxii diets was expected. By contrast, X. bispinatus reared on H. lauricola declined progressively and stopped reproducing after five generations. This result suggests that H. lauricola was a suboptimal food source for X. bispinatus, which contrasts with previous reports indicating that this fungus enables high fecundity rates and significant brood production in this beetle (Saucedo et al., 2017). Our results also suggest that X. bispinatus benefits from feeding upon multiple ambrosia fungi. Colonies fed singly on H. lauricola or Raffaelea spp. had fewer female offspring than colonies from the control treatment (Figures 1B, 2B, 3B). In agreement with these results, a previous study demonstrated that wild X. bispinatus increased reproduction when H. lauricola was added to their diet (Menocal et al., 2018a). Altogether, the available information suggests that X. bispinatus benefits from the new partnership with H. lauricola, but it is unlikely to use it as its primary symbiont.
Mutualisms between Euwallacea beetles and Fusarium spp. are well recognized (Freeman et al., 2012, 2016; Carrillo et al., 2016, 2020b; O’Donnell et al., 2016; Kendra et al., 2022), but associations between Fusarium spp. and other ambrosia beetles are presumed to be incidental and non-mutualistic (Biedermann et al., 2013; Kostovcik et al., 2015; Bateman et al., 2016; Biedermann, 2020). In our study, X. bispinatus engaged in a persistent mutualism with Fusarium sp. nov. [currently >20 generations – data not shown]. This fungus sustained significantly more female offspring than any other single fungus or the unmanipulated colonies with several fungal symbionts (Figure 5). This result is noteworthy because Fusarium spp. belongs to a distant unrelated taxonomic order (Hypocreales) than most other ambrosia fungi (Ophiostomatales). Interestingly, Fusarium solani (Norris and Baker, 1967, 1968) and Fusarium sp. (AF-9) (Kasson et al., 2013) were reported as mycangial fungal partners of Xyleborus ferrugineus Fabricius. Moreover, X. ferrugineus and X. bispinatus were considered synonyms until Atkinson et al. (2013) recognized them as separate species. These results and our findings suggest that both beetles may exhibit mutualisms with Fusarium spp. and that these symbiotic relationships could be more common than previously presumed.
Our results suggest that exotic ambrosia beetles can induce changes in resident ambrosia beetle communities and their symbionts. In their native range, ambrosia beetles are rooted in a network of interactions with native symbiotic fungi, including endophytes, plant pathogens, and mutualists (Simmons et al., 2016; Carrillo et al., 2019). In invaded areas, ambrosia beetles face novel environments, new host opportunities, and potentially novel symbionts (Rassati et al., 2019). In some cases, invasive beetles may not survive in-or adapt to-a new environment (e.g., X. glabratus in avocado) but their fungi (e.g., H. lauricola) can establish novel partnerships with resident ambrosia beetles (Carrillo et al., 2014; Ploetz et al., 2017; Cruz et al., 2021). The symbionts’ ecology could play a major role in developing novel mutualisms. Most ambrosia beetle symbionts only grow in the xylem-sapwood in close proximity to the beetle galleries and inside the beetle mycangia. Unlike most Raffaelea species, H. lauricola displays systemic growth in its lauraceous hosts (Lynch et al., 2012; Ploetz et al., 2012; Campbell et al., 2017). This could be a significant contributing factor for the lateral transfer of this fungus to multiple ambrosia beetles in areas of recent invasion in the USA. For ambrosia fungi with localized growth, sympatric beetle breeding and natal gallery overlapping may be required for fungal exchange. Under these circumstances, competitive fungi displaying fast growth and the ability to nourish ambrosia beetles can outcompete other symbionts and engage in new partnerships. In our experiment, Fusarium sp. nov. appeared to colonize the rearing substrate faster than other fungi. Fusarium sp. nov. may be more nutritious or better at translocating elements from the rearing plug to its fruiting bodies. Fungal mutualists translocate essential elements such as calcium (Ca), nitrogen (N), phosphorous (P), potassium (K), magnesium (Mg), manganese (Mn), and sulfur (S), through their hyphae from the sapwood to fruiting structures in the galleries (Six and Elser, 2019; Lehenberger et al., 2021a). These elements are essential for fungal and beetle growth (Filipiak et al., 2016). A better understanding of the nutritional quality and growth patterns of ambrosia fungi may help identify species with invasive potential and predict novel ambrosia symbioses.
In conclusion, species such as X. bispinatus with fungus-feeding plasticity are likely to establish new symbiotic relationships with exotic ambrosia fungi like H. lauricola. However, our study was conducted under simplified laboratory conditions. Interactions with multiple abiotic (e.g., temperature, relative humidity) and biotic (plant-secondary compounds and other microbial players) factors under natural conditions may lead to other fitness effects and more complex associations. The ecology of the fungal symbionts may also play an important role in novel ambrosia symbiosis. Further experiments on ambrosia beetle symbiosis may help identify patterns and characteristics that make some species more likely to become invasive.
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors. The data (i.e., fungal sequences) presented in the study were uploaded to the NCBI GenBank (Home - Nucleotide - NCBI (https://www.ncbi.nlm.nih.gov) database and are publicly available (Accession numbers are included in Table 1).
OM, LC, and DC conceived and designed the study. OM and LC performed the experiments and collected the data. OM analyzed the data. PK and MB contributed with reagents/materials/analysis tools. OM wrote the initial draft of the manuscript. LC and DC provided feedback and comments on previous versions of the manuscript. PK and DC reviewed and edited the final version of the manuscript. All authors contributed to the article and approved the submitted version.
This research was funded by NIFA grant 2015–51181-24257 to Daniel Carrillo and by a Non-Assistance Cooperative Agreement between the USDA-ARS and the University of Florida (number: 58-6038–8-004).
The authors thank James Colee (UF-IFAS – Statistical Consulting Unit) for his help during the statistical analysis. Their special thanks go to Jiri Hulcr (School of Forest, Fisheries, and Geomatics Sciences, University of Florida) and Kirsten Pelz-Stelinski (Citrus Research and Education Center, University of Florida) for suggestions to improve the manuscript. The authors are extremely grateful to Rita E. Duncan, Armando Padilla, and José Alegría for their technical assistance in experimental set up and data collection.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Atkinson, T. H., Carrillo, D., Duncan, R. E., and Peña, J. E. (2013). Occurrence of Xyleborus bispinatus (Coleoptera: Curculionidae: Scolytinae) Eichhoff in southern Florida. Zootaxa 3669, 96–100. doi: 10.11646/zootaxa.3669.1.10
Bateman, C., Kendra, P. E., Rabaglia, R., and Hulcr, J. (2015). Fungal symbionts in three exotic ambrosia beetles, Xylosandrus amputatus, Xyleborinus andrewesi, and Dryoxylon onoharaense (Coleoptera: Curculionidae: Scolytinae: Xyleborini) in Florida. Symbiosis 66, 141–148. doi: 10.1007/s13199-015-0353-z
Bateman, C., Sigut, M., Skelton, J., Smith, K. E., and Hulcr, J. (2016). Fungal associates of the Xylosandrus compactus (Coleoptera: Curculionidae, Scolytinae) are spatially segregated on the insect body. Environ. Entomol. 45, 883–890. doi: 10.1093/ee/nvw070
Batra, L. R. (1963). Ecology of ambrosia fungi and their dissemination by beetles. Trans. Kans. Acad. Sci. 66, 213–236. doi: 10.2307/3626562
Biedermann, P. H. W. (2020). Cooperative breeding in the ambrosia beetle Xyleborus affinis and management of its fungal symbionts. Front. Ecol. Evol. 8:518954. doi: 10.3389/fevo.2020.518954
Biedermann, P. H. W., Klepzig, K. D., and Taborsky, M. (2009). Fungus cultivation by ambrosia beetles: behavior and laboratory breeding success in three Xyleborine species. Environ. Entomol. 38, 1096–1105. doi: 10.1603/022.038.0417
Biedermann, P. H. W., Klepzig, K. D., Taborsky, M., and Six, D. L. (2013). Abundance and dynamics of filamentous fungi in the complex ambrosia gardens of the primitively eusocial beetle Xyleborinus saxesenii Ratzeburg (Coleoptera: Curculionidae, Scolytinae). FEMS Microbiol. Ecol. 83, 711–723. doi: 10.1111/1574-6941.12026
Biedermann, P. H. W., and Taborsky, M. (2011). Larval helpers and age polyethism in ambrosia beetles. Proc. Natl. Acad. Sci. U. S. A. 108, 17064–17069. doi: 10.1073/pnas.1107758108
Biedermann, P. H. W., and Vega, F. E. (2020). Ecology and evolution of insect-fungus mutualisms. Annu. Rev. Entomol. 65, 431–455. doi: 10.1146/annurev-ento-011019-024910
Bracewell, R. R., and Six, D. L. (2015). Experimental evidence of bark beetle adaptation to a fungal symbiont. Ecol. Evol. 5, 5109–5119. doi: 10.1002/ece3.1772
Campbell, A. S., Ploetz, R. C., Dreaden, T. J., Kendra, P. E., and Montgomery, W. S. (2016). Geographic variation in mycangial communities of Xyleborus glabratus. Mycologia 108, 657–667. doi: 10.3852/15-133
Campbell, A. S., Ploetz, R. C., and Smith, J. A. (2017). Comparing avocado, swamp bay, and camphortree as hosts of Raffaelea lauricola using a green fluorescent protein (GFP)-labeled strain of the pathogen. Phytopathology 107, 70–74. doi: 10.1094/PHYTO-02-16-0072-R
Carrillo, D., Cruz, L. F., Kendra, P. E., Narvaez, T. I., Montgomery, W. S., Monterroso, A., et al. (2016). Distribution, pest status and fungal associates of Euwallacea nr. fornicatus in Florida avocado groves. Insects. 7:55. doi: 10.3390/insects7040055
Carrillo, J. D., Dodge, C., Stouthamer, R., and Eskalen, A. (2020a). Fungal symbionts of the polyphagous and Kuroshio shot hole borers (Coleoptera: Scolytinae, Euwallacea spp.) in California can support both ambrosia beetle systems on artificial media. Symbiosis 80, 155–168. doi: 10.1007/s13199-019-00652-0
Carrillo, D., Duncan, R. E., and Peña, J. E. (2012). Ambrosia beetles (Coleoptera: Curculionidae: Scolytinae) that breed in avocado wood in Florida. Fla. Entomol. 95, 573–579. doi: 10.1653/024.095.0306
Carrillo, D., Duncan, R. E., Ploetz, J. N., Campbell, A. F., Ploetz, R. C., and Peña, J. E. (2014). Lateral transfer of a phytopathogenic symbiont among native and exotic ambrosia beetles. Plant Pathol. 63, 54–62. doi: 10.1111/ppa.12073
Carrillo, J. D., Mayorquin, J. S., Stajich, J. E., and Eskalen, A. (2020b). Probe-based multiplex real-time PCR as diagnostic tool to distinguish distinct fungal symbionts associated with Euwallacea kuroshio and Euwallacea whitfordiodendrus in California. Plant Dis. 104, 227–238. doi: 10.1094/PDIS-01-19-0201-RE
Carrillo, J. D., Rugman-Jones, P. F., Husein, D., Stajich, J. E., Kasson, M. T., Carrillo, D., et al. (2019). Members of the Euwallacea fornicatus species complex exhibit promiscuous mutualism with ambrosia fungi in Taiwan. Fungal Genet. Biol. 133:103269. doi: 10.1016/j.fgb.2019.103269
Cavaletto, G., Faccoli, M., Ranger, C. M., and Rassati, D. (2021). Ambrosia beetle response to ethanol concentration and host tree species. J. Appl. Entomol. 145, 800–809. doi: 10.1111/jen.12895
Cavaletto, G., Ranger, C. M., Reding, M. E., and Rassati, D. (2022). Species-specific effects of ethanol concentration on host colonization by four common species of ambrosia beetles. J. Pest. Sci., 1–11. doi: 10.1007/s10340-022-01537-w
Cloonan, K. R., Montgomery, W. S., Narvaez, T. I., Carrillo, D., and Kendra, P. E. (2022). Community of bark and ambrosia beetles (Coleoptera: Curculionidae: Scolytinae and Platypodinae) in agricultural and forest ecosystems with laurel wilt. Insects 13:971. doi: 10.3390/insects13110971
Cruz, L. F., Menocal, O., Kendra, P. E., and Carrillo, D. (2021). Phoretic and internal transport of Raffaelea lauricola by different species of ambrosia beetle associated with avocado trees. Symbiosis 84, 151–161. doi: 10.1007/s13199-021-00776-2
Cruz, L. F., Menocal, O., Mantilla, J., Ibarra-Juarez, L. A., and Carrillo, D. (2019). Xyleborus volvulus (Coleoptera: Curculionidae): biology and fungal associates. Appl. Environ. Microbiol. 85, e01190–e01119. doi: 10.1128/AEM.01190-19
Cruz, L. F., Rocio, S., Duran, L., Menocal, O., Garcia-Avila, C. J., and Carrillo, D. (2018). Developmental biology of Xyleborus bispinatus (Coleoptera: Curculionidae) reared on an artificial medium and fungal cultivation of symbiotic fungi in the beetle’s galleries. Fungal Ecol. 35, 116–126. doi: 10.1016/j.funeco.2018.07.007
de Beer, Z. W., Procter, M., Wingfield, M. J., Marincowitz, S., and Duong, T. A. (2022). Generic boundaries in the Ophiostomatales reconsidered and revised. Stud. Mycol. 101, 57–120. doi: 10.3114/sim.2022.101.02
Doyle, J. J., and Doyle, J. L. (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull. 19, 11–15.
Dreaden, T. J., Davis, J. M., Harmon, C. L., Ploetz, R. C., Palmateer, A. J., Soltis, P. S., et al. (2014). Development of multilocus PCR assays for Raffaelea lauricola, causal agent of laurel wilt disease. Plant Dis. 98, 379–383. doi: 10.1094/PDIS-07-13-0772-RE
Eskalen, A., Stouthamer, R., Lynch, S. C., Rugman-Jones, P. F., Twizeyimana, M., Gonzalez, A., et al. (2013). Host range of Fusarium dieback and its ambrosia beetle (Coleoptera: Scolytinae) vector in southern California. Plant Dis. 97, 938–951. doi: 10.1094/PDIS-11-12-1026-RE
Farrell, B. D., Sequeira, A. S., O’Meara, B. C., Normark, B. B., Chung, J. H., and Jordal, B. H. (2001). The evolution of agriculture in beetles (Curculionidae: Scolytinae and Platypodinae). Evolution 55, 2011–2027. doi: 10.1111/j.0014-3820.2001.tb01318.x
Filipiak, M., Sobczyk, L., and Weiner, J. (2016). Fungal transformation of tree stumps into a suitable resource for xylophagous beetles via changes in elemental ratios. Insects 7:13. doi: 10.3390/insects7020013
Freeman, S., Protasov, A., Sharon, M., Mohotti, K., Eliyahu, M., Okon-Levy, N., et al. (2012). Obligate feed requirement of Fusarium sp. nov., an avocado wilting agent, by the ambrosia beetle Euwallacea aff. fornicata. Symbiosis 58, 245–251. doi: 10.1007/s13199-013-0222-6
Freeman, S., Sharon, M., Dori-Bachash, M., Maymon, M., Belausov, E., Maoz, Y., et al. (2016). Symbiotic association of three fungal species throughout the life cycle of the ambrosia beetle Euwallacea nr. fornicatus. Symbiosis 68, 115–128. doi: 10.1007/s13199-015-0356-9
Gebhardt, H., Begerow, D., and Oberwinkler, F. (2004). Identification of ambrosia fungus of Xyleborus monographus and X. dryographus (Coleoptera: Curculionidae, Scolytinae). Mycol. Prog. 3, 95–102. doi: 10.1007/s11557-006-0080-1
Grubbs, K. J., Surup, F., Biedermann, P. H. W., McDonald, B. R., Klassen, J. L., Carlson, C. M., et al. (2020). Cycloheximide-producing Streptomyces associated with Xyleborinus saxesenii and Xyleborus affinis fungus-farming ambrosia beetles. Front. Microbiol. 11:562140. doi: 10.3389/fmicb.2020.562140
Hanula, J. L., Mayfield, A. E. III, Fraedrich, S. W., and Rabaglia, R. J. (2008). Biology and host associations of the redbay ambrosia beetle (Coleoptera: Curculionidae: Scolytinae), exotic vector of laurel wilt killing redbay trees in the southeastern United States. J. Econ. Entomol. 101, 1276–1286. doi: 10.1093/jee/101.4.1276
Harrington, T. C., Aghayeva, D. N., and Fraedrich, S. W. (2010). New combinations in Raffaelea, Ambrosiella, and Hyalorhinocladiella, and four new species from the redbay ambrosia beetle, Xyleborus glabratus. Mycotaxon 111, 337–361. doi: 10.5248/111.337
Harrington, T. C., and Fraedrich, S. W. (2010). Quantification of propagules of the laurel wilt fungus and other mycangial fungi from the redbay ambrosia beetles, Xyleborus glabratus. Phytopathology 100, 1118–1123. doi: 10.1094/PHYTO-01-10-0032
Hughes, M. A., Riggins, J. J., Koch, F. H., Cognato, A. I., Anderson, C., Formby, J. P., et al. (2017). No rest for the laurels: symbiotic invaders cause unprecedented damage to the southern USA forest. Biol. Invasions 19, 2143–2157. doi: 10.1007/s10530-017-1427-z
Hulcr, J., and Dunn, R. R. (2011). The sudden emergence of pathogenicity in insect-fungus symbioses threatens naïve forest ecosystems. Proc. R. Soc. B 278, 2866–2873. doi: 10.1098/rspb.2011.1130
Hulcr, J., and Stelinski, L. L. (2017). The ambrosia symbiosis: from evolutionary ecology to practical management. Annu. Rev. Entomol. 62, 285–303. doi: 10.1146/annurev-ento-031616-035105
Joseph, R., and Keyhani, N. O. (2021). Fungal mutualisms and pathosystems: life and death in the ambrosia beetle mycangia. Appl. Microbiol. Biotechnol. 105, 3393–3410. doi: 10.1007/s00253-021-11268-0
Kasson, M. T., O’Donnell, K., Rooney, A. P., Sink, S., Ploetz, R. C., Ploetz, J. N., et al. (2013). An inordinate fondness for Fusarium: phylogenetic diversity of fusaria cultivated by ambrosia beetles in the genus Euwallacea on avocado and other plant hosts. Fungal Genet. Biol. 56, 147–157. doi: 10.1016/j.fgb.2013.04.004
Kendra, P. E., Montgomery, W. S., Narvaez, T. I., and Carrillo, D. (2020). Comparison of trap designs for detection of Euwallacea nr. fornicatus and other Scolytinae (Coleoptera: Curculionidae) that vector fungal pathogens of avocado trees in Florida. J. Econ. Entomol. 113, 980–987. doi: 10.1093/jee/toz311
Kendra, P. E., Montgomery, W. S., Sanchez, J. S., Deyrup, M. A., Niogret, J., and Epsky, N. D. (2012). Method for collection of live redbay ambrosia beetles, Xyleborus glabratus (Coleoptera: Curculionidae: Scolytinae). Fla. Entomol. 95, 513–516. doi: 10.1653/024.095.0244
Kendra, P. E., Owens, D., Montgomery, W. S., Narvaez, T. I., Bauchan, G. R., Schnell, E. Q., et al. (2017). α-Copaene is an attractant, synergistic with quercivorol, for improved detection of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae). PLoS One 12:e0179416. doi: 10.1371/journal.pone.0179416
Kendra, P. E., Sanchez, J. S., Montgomery, W. S., Okins, K. E., Niogret, J., Peña, J. E., et al. (2011). Diversity of Scolytinae (Coleoptera: Curculionidae) attracted to avocado, lychee, and essential oil lures. Fla. Entomol. 94, 123–130. doi: 10.1653/024.094.0201
Kendra, P. E., Tabanca, N., Cruz, L. F., Menocal, O., Schnell, E. Q., and Carrillo, D. (2022). Volatile emissions and relative attraction of the fungal symbionts of tea shot hole borer (Coleoptera: Curculionidae). Biomol. 12:97. doi: 10.3390/biom12010097
Kostovcik, M., Bateman, C. C., Kolarik, M., Stelinski, L. L., Jordal, B. H., and Hulcr, J. (2015). The ambrosia symbiosis is specific in some species and promiscuous in others: evidence from community pyrosequencing. ISME J. 9, 126–138. doi: 10.1038/ismej.2014.115
Lehenberger, M., Benkert, M., and Biedermann, P. H. W. (2021b). Ethanol-enriched substrate facilitates ambrosia beetle fungi, but inhibits their pathogens and fungal symbionts of bark beetles. Front. Microbiol. 11:590111. doi: 10.3389/fmicb.2020.590111
Lehenberger, M., Foh, N., Göttlein, A., Six, D., and Biedermann, P. H. W. (2021a). Nutrient-poor breeding substrates of ambrosia beetles are enriched with biological important elements. Front. Microbiol. 12:664542. doi: 10.3389/fmicb.2021.664542
Li, Y., Bateman, C. C., Skelton, J., Jusino, M. A., Nolen, Z. J., Simmons, D. R., et al. (2017). Wood decay fungus Flavodon ambrosius (Basidiomycota: Polyporales) is widely farmed by two genera of ambrosia beetles. Fungal Biol. 121, 984–989. doi: 10.1016/j.funbio.2017.08.004
Li, Y., Huang, Y.-T., Kasson, M. T., Macias, A. M., Skelton, J., Carlson, P. S., et al. (2018). Specific and promiscuous ophiostomatalean fungi associated with Platypodinae ambrosia beetles in the southeastern United States. Fungal Ecol. 35, 42–50. doi: 10.1016/j.funeco.2018.06.006
Liu, Y. J., and Hall, B. D. (2004). Body plan evolution of ascomycetes, as inferred from an RNA polymerase II phylogeny. Proc. Natl. Acad. Sci. U. S. A. 101, 4507–4512. doi: 10.1073/pnas.0400938101
Lynch, S., Ploetz, R., Held, B., and Blanchette, R. (2012). Histological and anatomical responses in avocado, Persea americana, induced by the vascular wilt pathogen, Raffaelea lauricola. Botany 90, 627–635. doi: 10.1139/b2012-015
Mayers, C. G., Harrington, T. C., and Biedermann, P. H. W. (2022). “Mycangia define the diverse ambrosia beetle–fungus symbioses” in The convergent evolution of agriculture in humans and insects. eds. T. R. Schultz, P. N. Peregrine, and R. Gawne (Cambridge, MA: MIT Press), 1–38.
Mayers, C. G., Harrington, T. C., Masuya, H., Jordal, B. H., McNew, D. L., Shih, H.-H., et al. (2020a). Patterns of coevolution between ambrosia beetles mycangia and the Ceratocystidaceae, with five new fungal genera and seven new species. Persoonia 44, 41–66. doi: 10.3767/persoonia.2020.44.02
Mayers, C. G., Harrington, T. C., McNew, D. L., Roeper, R. A., Biedermann, P. H. W., Masuya, H., et al. (2020b). Four mycangium types and four genera of ambrosia fungi suggest a complex history of fungus farming in the ambrosia beetle tribe Xyletorini. Mycologia 112, 1104–1137. doi: 10.1080/00275514.2020.1755209
Mayers, C. G., McNew, D. L., Harrington, T. C., Roeper, R. A., Fraedrich, S. W., Biedermann, P. H. W., et al. (2015). Three genera in the Ceratocystidaceae are the respective symbionts of three independent lineages of ambrosia beetles with large, complex mycangia. Fungal Biol. 119, 1075–1092. doi: 10.1016/j.funbio.2015.08.002
Menocal, O., Cruz, L. F., Kendra, P. E., Crane, J. H., Cooperband, M. F., Ploetz, R. C., et al. (2018a). Xyleborus bispinatus reared on artificial media in the presence or absence of the laurel wilt pathogen (Raffaelea lauricola). Insects 9:30. doi: 10.3390/insects9010030
Menocal, O., Cruz, L. F., Kendra, P. E., Crane, J. H., Ploetz, R. C., and Carrillo, D. (2017). Rearing Xyleborus volvulus (Coleoptera: Curculionidae) on media containing sawdust from avocado or silkbay, with or without Raffaelea lauricola (Ophiostomatales: Ophiostomataceae). Environ. Entomol. 46, 1275–1283. doi: 10.1093/ee/nvx151
Menocal, O., Kendra, P. E., Montgomery, W. S., Crane, J. H., and Carrillo, D. (2018b). Vertical distribution and daily flight periodicity of ambrosia beetles (Coleoptera: Curculionidae) in Florida avocado orchards affected by laurel wilt. J. Econ. Entomol. 111, 1190–1196. doi: 10.1093/jee/toy044
Menocal, O., Kendra, P. E., Padilla, A., Chagas, P. C., Chagas, E. A., Crane, J. H., et al. (2022). Influence of canopy cover and meteorological factors on the abundance of bark and ambrosia beetles (Coleoptera: Curculionidae) in avocado orchards affected by laurel wilt. Agronomy 12:547. doi: 10.3390/agronomy12030547
Mueller, U. G., Gerardo, N. M., Aanen, D. K., Six, D. L., and Schultz, T. R. (2005). The evolution of agriculture in insects. Annu. Rev. Ecol. Evol. Syst. 36, 563–595. doi: 10.1146/annurev.ecolsys.36.102003.152626
Norris, D. M., and Baker, J. M. (1967). Symbiosis: effects of a mutualistic fungus upon the growth and reproduction of Xyleborus ferrugineus. Science 156, 1120–1122. doi: 10.1126/science.156.3778.1120
Norris, D. M., and Baker, J. M. (1968). A minimal nutritional substrate required by Fusarium solani to fulfill its mutualistic relationship with Xyleborus ferrugineus. Ann. Entomol. Soc. Am. 61, 1473–1475. doi: 10.1093/aesa/61.6.1473
Nuotclà, J. A., Biedermann, P. H. W., and Taborsky, M. (2019). Pathogen defence is a potential driver of social evolution in ambrosia beetles. Proc. R. Soc. B 286:20192332. doi: 10.1098/rspb.2019.2332
Nuotclà, J. A., Diehl, J. M. C., and Taborsky, M. (2021). Habitat quality determines dispersal decisions and fitness in a beetle – fungus mutualism. Front. Ecol. Evol. 9:602672. doi: 10.3389/fevo.2021.602672
O’Donnell, K., Kistler, H. C., Cigelnik, E., and Ploetz, R. C. (1998). Multiple evolutionary origins of the fungus causing Panama disease of banana: concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. U. S. A. 95, 2044–2049. doi: 10.1073/pnas.95.5.2044
O’Donnell, K., Libeskind-Hadas, R., Hulcr, J., Bateman, C., Kasson, M. T., Ploetz, R. C., et al. (2016). Invasive Asian Fusarium–Euwallacea ambrosia beetle mutualists pose a serious threat to forests, urban landscapes and the avocado industry. Phytoparasitica 44, 435–442. doi: 10.1007/s12600-016-0543-0
O’Donnell, K., Sink, S., Libeskind-Hadas, R., Hulcr, J., Kasson, M. T., Ploetz, R. C., et al. (2015). Discordant phylogenies suggest repeated host shifts in the Fusarium-Euwallacea ambrosia beetle mutualism. Fungal Gen Biol. 82, 277–290. doi: 10.1016/j.fgb.2014.10.014
O’Donnell, K., Sutton, D. A., Rinaldi, M. G., Sarver, B. A., Balajee, S. A., Schroers, H.-J., et al. (2010). Internet-accessible DNA sequence database for identifying Fusaria from human and animal infections. J. Clin. Microbiol. 48, 3708–3718. doi: 10.1128/JCM.00989-10
Owens, D., Kendra, P. E., Tabanca, N., Narvaez, T. I., Montgomery, W. S., Schnell, E. Q., et al. (2019a). Quantitative analysis of contents and volatile emissions from α-copaene and quercivorol lures, and longevity for attraction of Euwallacea nr. fornicatus in Florida. J. Pest. Sci. 92, 237–252. doi: 10.1007/s10340-018-0960-6
Owens, D., Seo, M., Montgomery, W. S., Rivera, M. J., Stelinski, L. L., and Kendra, P. E. (2019b). Dispersal behavior of Euwallacea nr. fornicatus (Coleoptera: Curculionidae: Scolytinae) in avocado groves and estimation of lure sampling range. Agr Forest Entomol. 21, 199–208. doi: 10.1111/afe.12321
Ploetz, R. C., Hulcr, J., Wingfield, M. J., and de Beer, Z. W. (2013). Destructive tree diseases associated with ambrosia and bark beetles: Black swan events in tree pathology? Plant Dis. 97, 856–872. doi: 10.1094/PDIS-01-13-0056-FE
Ploetz, R. C., Konkol, J. L., Narvaez, T., Duncan, R. E., Saucedo, R. J., Campbell, A., et al. (2017). Presence and prevalence of Raffaelea lauricola, cause of laurel wilt, in different species of ambrosia beetle in Florida, USA. J. Econ. Entomol. 110, 347–354. doi: 10.1093/jee/tow292
Ploetz, R. C., Perez-Martinez, J. M., Smith, J. A., Hughes, M., Dreaden, T. J., Inch, S. A., et al. (2012). Responses of avocado to laurel wilt, caused by Raffaelea lauricola. Plant Pathol. 61, 801–808. doi: 10.1111/j.1365-3059.2011.02564.x
Ranger, C. M., Biedermann, P. H. W., Phuntumart, V., Beligala, G. U., Ghosh, S., Palmquist, D. E., et al. (2018). Symbiont selection via alcohol benefits fungus farming by ambrosia beetles. Proc. Natl. Acad. Sci. U. S. A. 115, 4447–4452. doi: 10.1073/pnas.1716852115
Rassati, D., Marini, L., and Malacrino, A. (2019). Acquisition of fungi from the environment modifies ambrosia beetle mycobiome during invasion. PeerJ 7:e8103. doi: 10.7717/peerj.8103
Reeb, V., Lutzoni, F., and Roux, C. (2004). Contribution of RPB2 to multilocus phylogentic studies of the euascomycetes (Pezizomycotina, fungi) with special emphasis on the lichen-forming Acarosporaceae and evolution of polyspory. Mol. Phylogenet. Evol. 32, 1036–1060. doi: 10.1016/j.ympev.2004.04.012
Rivera, M. J., Martini, X., Conover, D., Mafra-Neto, A., Carrillo, D., and Stelinski, L. L. (2020). Evaluation of semiochemical based push-pull strategy for population suppression off ambrosia beetle vectors of laurel wilt disease in avocado. Sci. Rep. 10:2670. doi: 10.1038/s41598-020-59569-0
Saucedo, J., Ploetz, R., Konkol, J., Angel, M., Mantilla, J., Menocal, O., et al. (2017). Nutritional symbionts of a putative vector, Xyleborus bispinatus, of the laurel wilt pathogen of avocado, Raffaelea lauricola. Symbiosis 75, 29–38. doi: 10.1007/s13199-017-0514-3
Saucedo-Carabez, J. R., Ploetz, R. C., Konkol, J. L., Carrillo, D., and Gazis, R. (2018). Partnerships between ambrosia beetles and fungi: lineage-specific promiscuity among vectors of the laurel wilt pathogen, Raffaelea lauricola. Microb Ecol. 76, 925–940. doi: 10.1007/s00248-018-1188-y
Simmons, D. R., de Beer, Z. W., Huang, Y.-T., Bateman, C., Campbell, A. S., Dreaden, T. J., et al. (2016). New Raffaelea species (Ophiostomatales) from the USA and Taiwan associated with ambrosia beetles and plant hosts. IMA Fungus. 7, 265–273. doi: 10.5598/imafungus.2016.07.02.06
Six, D. L. (2012). Ecological and evolutionary determinants of bark beetles-fungus symbioses. Insects 3, 339–366. doi: 10.3390/insects3010339
Six, D. L., and Elser, J. J. (2019). Extreme ecological stoichiometry of a bark beetle-fungus mutualism. Ecol. Entomol. 44, 543–551. doi: 10.1111/een.12731
Skelton, J., Johnson, A. J., Jusino, M. A., Bateman, C. C., Li, Y., and Hulcr, J. (2019). A selective fungal transport organ (mycangium) maintains coarse phylogenetic congruence between fungus-farming ambrosia beetles and their symbionts. Proc. R. Soc. B 286:20182127. doi: 10.1098/rspb.2018.2127
Smith, S. M., Gomez, D. E., Beaver, R. A., Hulcr, J., and Cognato, A. I. (2019). Reassessment of the species in the Euwallacea fornicatus (Coleoptera: Curculionidae: Scolytinae) complex after the rediscovery of the “lost” type specimen. Insects 10:261. doi: 10.3390/insects10090261
Spahr, E., Kasson, M. T., and Kijimoto, T. (2020). Micro-computed tomography permits enhanced visualization of mycangia across development and between sexes in Euwallacea ambrosia beetles. PLoS One 15:e0236653. doi: 10.1371/journal.pone.0236653
Vilgalys, R., and Hester, M. (1990). Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 172, 4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990
White, T. J., Bruns, T., Lee, S., and Taylor, J. (1990). “Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics” in PCR protocols: A guide to methods and application. eds. M. A. Innis, D. H. Gelfand, J. J. Sninsky, and T. J. White (San Diego, CA: Academic Press), 315–322.
Keywords: ambrosia beetles, flexible symbiosis, fungal partners, Fusarium, Harringtonia lauricola, laurel wilt, mutualism, Raffaelea lauricola
Citation: Menocal O, Cruz LF, Kendra PE, Berto M and Carrillo D (2023) Flexibility in the ambrosia symbiosis of Xyleborus bispinatus. Front. Microbiol. 14:1110474. doi: 10.3389/fmicb.2023.1110474
Received: 28 November 2022; Accepted: 06 February 2023;
Published: 02 March 2023.
Edited by:
Peter H. W. Biedermann, University of Freiburg, GermanyReviewed by:
Kier Klepzig, University of Georgia, United StatesCopyright © 2023 Menocal, Cruz, Kendra, Berto and Carrillo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Octavio Menocal, b21lbm9jYWwxOEBnbWFpbC5jb20=; Daniel Carrillo, ZGFuY2FyQHVmbC5lZHU=
†Present address: Octavio Menocal, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville, FL, United States
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.