
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
MINI REVIEW article
Front. Microbiol. , 10 February 2023
Sec. Microorganisms in Vertebrate Digestive Systems
Volume 14 - 2023 | https://doi.org/10.3389/fmicb.2023.1108661
This article is part of the Research Topic Reviews in the Impact of Gut Microbiota in Health and Disease View all 16 articles
Rosacea is a chronic inflammatory cutaneous disorder of uncertain etiology that mainly affects the centrofacial region, including cheeks, nose, chin, forehead, and eyes. The pathogenesis of rosacea remains unclear because it involves several complex factors. Additionally, the potential treatment methods need to be explored. We reviewed the common bacterial species in the skin microbiota and gut microbiota of rosacea patients such as Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, Cutibacterium acnes, and Helicobacter pylori and identified their role in the pathogenesis. Besides, we summarized the influence factors such as temperature and age on rosacea patients. We also systematically reviewed the commonly used clinical treatment methods, including antibiotics, probiotics. as well as their treatment mechanism and application precautions.
Rosacea is a chronic inflammatory cutaneous disorder of uncertain etiology that mainly affects the centrofacial region, including cheeks, nose, chin, forehead, and eyes. There are four subtypes of rosacea, which are erythematotelangiectatic rosacea, papulopustular rosacea, phymatous rosacea, and ocular rosacea (Wilkin et al., 2002). However, these subtypes can progress from one type to another, so the current clinical recommendation is to classify rosacea according to clinical presentation, as patients with rosacea can have different clinical signs and symptoms. The newest research has classified rosacea symptoms into recurrent flushes or transient erythema, persistent erythema, morphological changes, papules, pustules, and telangiectasia (van Zuuren et al., 2021). The pathogenesis of rosacea involves several complex factors. Not only genetic factors but also environmental factors have been linked to rosacea. There are several flare triggers in patients with rosacea, including temperature changes, heat, cold, exercise, ultraviolet radiation, spicy food, and alcohol (Buddenkotte and Steinhoff, 2018). These factors can make patients more susceptible to skin disorders because they alter the skin’s epidermal barrier function or disrupt immune function (Park et al., 2021). Rosacea is associated with many systemic complications such as gastrointestinal disease, cardiovascular disease, neurological disease, psychiatric disease, and autoimmune disease, but the exact pathogenesis of rosacea remains unclear (Holmes et al., 2018; Figure 1). The classification of rosacea is shown in Figure 1.
In the pathogenesis of rosacea, there has been extensive discussion on the skin microbiota and its related inflammatory effects. Many different communities of microorganisms have been studied in the skin, formed by hundreds of microbial species occupying different environmental niches in the skin (Xu and Li, 2019). The skin microbiota is essential for regulating inflammation and immune responses. The epidermis, dermis, and deeper subcutaneous tissue together form a physical and chemical barrier against external pathogens (Chen et al., 2021). Temporary non-specific immune cells and highly specific long-acting immune components constitute the skin immune barrier (Chaplin, 2010). The bacteria, fungi, viruses, and arthropods that live on the human skin together make up the human skin microbiome, all of which have been found to play a role in regulating immune responses. Some of these can cross the skin barrier and interact with deeper cells. If the skin microbiome is disturbed by internal or external factors, it can interfere with the function of the immune barrier to maintain homeostasis. Microorganisms in the skin not only trigger the release of certain antimicrobial peptides, but also regulate components of the complement system, and aggravate skin inflammation by accumulating neutrophils and producing interleukins (Park and Lee, 2018). However, the skin is not only affected by its own microorganisms, because recent studies have suggested that the skin can be affected by the gastrointestinal microbiome. The most frequently mentioned comorbidity is gastrointestinal disease among all kinds of rosacea. It has been gradually recognized that commensal microbes may play a significant part in the development of certain cutaneous disorders, and it is also believed that a weakened external barrier to pathogens leads to dysregulation of the skin microecology (Lam et al., 2022). Therefore, in this review, we summarize reports about the association between rosacea and the skin microbiota and gastrointestinal microbiota and provide an overall picture of the impact of rosacea treatment on the skin and gut microbiota.
Like most organ systems, the microbiota within the skin is indispensable for promoting efficient immune function. Researchers have identified several microbes as potential contributors to the development of rosacea; these are Demodex folliculorum, Staphylococcus epidermidis, Bacillus oleronius, and Cutibacterium acnes (Holmes, 2013).
Demodex folliculorum are microscopic mites which are usually found at the base of the eyelashes. The adult mites are cigar-shaped with four legs to grasp cylindrical structures like eyelashes. Demodex infection can cause activation of the immune system, inflammation, and follicular changes that may lead to disease (Fromstein et al., 2018).
Staphylococcus epidermidis is a Gram-positive biofilm-producing symbiotic bacteria and is the most important member of coagulase-negative staphylococci, widely present on human skin and mucosa, S. epidermidis is one of the most abundant colonizers on human skin. It could attach to foreign objects and form biofilms, which contributes to its ability to cause infectious disease (Yuan et al., 2020).
The Bacillus genus is a group of Gram-positive rod-shaped bacteria that can produce endospores under adverse conditions, making them widespread in nature. Bacillus species include some pathogens of clinical interest, bacterial contaminants in food, and some are used as industrial organisms to produce various enzymes (Owusu-Darko et al., 2017).
Cutibacterium acnes is a lipophilic anaerobic Gram-positive bacterium belonging to the Cutibacterium spp. family. It is a part of the skin commensal flora and is generally found in hair follicles and sebaceous glands, and can also exist in the oral mucosa, nose, urogenital tract, and large intestine (Achermann et al., 2014).
Demodex mites are associated with the presence of other microbiota in the skin. Firmicutes, Actinobacteria, and Proteobacteria were the most represented phyla in these Demodex related microbiota. Studies comparing rosacea patients with healthy standardized skin surface biopsies to study Demodex-associated microbiota, reported that Proteobacteria and Firmicutes were more abundant at the phylum level, whereas actinobacteria were less abundant (Murillo et al., 2014). By analyzing the microbial β-diversity, the researchers found that the patient-to-sample cluster was less pronounced, while the treatment-to-sample cluster was least pronounced. Staphylococcus, Cutibacterium, Pseudomonas, Corynebacterium, Acinetobacter, and Snodgrasella were the main bacterial groups at the genus level in untreated rosacea patients (Tutka et al., 2020). Keratomyces acnes (Rainer et al., 2020) and S. epidermidis (Woo et al., 2020b) are the most diverse bacteria on the skin of patients with rosacea.
When focused on the species level, S. epidermidis was the most common bacterial species, followed by Stenotrophomonas rootophilus, C. acnes, and Corynebacterium tuberculostearicum (Woo et al., 2020b). Previous studies had revealed diversity in the microbiota among different subtypes of rosacea. The phylum profile in papulopustular rosacea microbial communities was significantly different from erythematotelangiectatic rosacea. Actinomycetes accounted for only about one tenth of all clones in the papulopustular rosacea community, while most clones were found in erythematotelangiectatic rosacea. On the other hand, the proportions of Proteobacteria and Firmicutes in papulopustular rosacea communities were increased compared with erythematotelangiectatic rosacea (Murillo et al., 2014).
Many studies have shown that the innate immune system is aberrantly activated by some skin microorganisms through Toll-like receptor 2 (TLR 2). After TLR 2 expression, antimicrobial peptides can be abnormally produced, and the expression and activity of serine kallikrein were also increased (Picardo and Ottaviani, 2014). Furthermore, TLR 2 can elicit erythema, telangiectasia, and infammation via expression of cytokines, chemokines, proteases, and pro-angiogenic factors (van Zuuren et al., 2021). Moreover, rosacea skin evidently showed increased cathelicidin expression, which was expressed by leukocytes as well as epithelial cells, compared to normal skin. This can lead to several unwanted downstream effects such as leukocyte chemotaxis, vasodilatation, angiogenesis, and extracellular matrix deposition (Weiss and Katta, 2017). At the same time, these effects may eventually lead to the development of a long-lasting non-infectious skin condition. C. acnes may play a role in protecting healthy skin (Barnard et al., 2020). It could prevent other microorganism from colonizing the skin because it breaks down sebum into free fatty acids (Marples et al., 1971).
The skin microbiome is a variable phenomenon, that alters with age, sex, environmental factors, and the use of cosmetics and antibiotics. There are differences in the pathogenesis of papules and pustules between acne and rosacea, which have been shown to be caused by age affecting the skin microbiome. Some studies have suggested that the severity of rosacea increases with age (Woo et al., 2020b). Under different temperature conditions, members of the normal skin microbiota that do not normally cause disease, such as S. epidermidis, can replicate at different rates and can also secrete more virulence factors (Dahl et al., 2004). Staphylococcus epidermidis strains isolated from the skin of rosacea patients were found to produce more protein at 37°C than at 30°C. Research has suggested that sudden changes in temperature can lead to worsening rosacea symptoms. The increased mobility and survival of Demodex mites at higher temperatures may explain that heat contributes to the worsening of rosacea (He et al., 2018). Bacteria behave differently at varying temperatures and produce different bacterial products. Skin temperature is likely to influence the activity of other skin microbiota, such as aerobic bacteria, anaerobic bacteria, and Demodex mites.
The human gut, like the skin, is home to countless microbes. Intestinal bacterial species such as Lactobacillus, Escherichia coli, Bifidobacterium, and Streptococcus thermophilus help to maintain human health, while others are more likely to cause disease, such as Clostridium difficile, Campylobacter, Enterococcus faecalis, and Helicobacter pylori.
Probiotics are living beneficial microbial species, but one way for a host to provide useful substrates for probiotic bacteria is the consumption of prebiotics, for example, foodstuffs or supplements containing certain saccharides (fructose, glucose, galactose, inulin, lactulose, sorbitol, or xylitol), These compounds can affect the intestinal microbiota and improve the environment of the skin, by increasing the number of beneficial gut microbes (Szántó et al., 2019).
Helicobacter pylori colonizes the human stomach and duodenum and is a microaerophilic Gram-negative bacterial species (Zeng et al., 2015). It can lead to a lifelong infection that is difficult to eradicate and may infect more than half of the human population worldwide. Helicobacter pylori can produce cytotoxins and cause gastric mucosal inflammation by proliferating and producing nitric oxide. It can alter physiological processes such as vasodilation, inflammation, and immune regulation (Mahmud et al., 2022). Rosacea is also associated with H. pylori seropositivity (Holmes, 2013). One mechanism for this theoretical association has been suggested to be that H. pylori can cause skin inflammation and flushing by the activity of cytotoxins and gastrin (Holmes, 2013), while other mechanisms have also been proposed. An autoimmune mechanism involving cross-reactive antibodies has also been hypothesized. This is based on systemic effects due to increased mucosal permeability to digestive tract antigens, or impaired vascular integrity (Wedi and Kapp, 2002). Helicobacter pylori infection has been found to be a risk factor for rosacea, but the association between them is weak. However, researchers reported there was a strong association between a positive C13-urea breath test and rosacea, and the C13-urea breath test is accepted as high diagnostic value for H. pylori infection (Jørgensen et al., 2017). This may be due to differences in the way H. pylori was diagnosed in the past. Besides, various strains of H. pylori have different virulence factors, which might lead to the divergence in the reported results (Woo et al., 2020a). Studies have also linked rosacea to overgrowth of various bacteria in the small intestine (Woo et al., 2020b).
A recent concept called the gut-skin axis has been proposed to explain the pathogenesis of many chronic inflammatory disorders, which proposes that skin homeostasis and allostasis are influenced by gastrointestinal health, through a complicated interplay between the immune system, metabolic system, and nervous systems (O'Neill et al., 2016). The gut microbiome has a bidirectional regulatory effect on host immunity, which is considered the primary regulator of the gut-skin axis (Forbes et al., 2016). Disturbances in the gut microbiome could affect the equilibrium of the immune system.
Some studies have analyzed the composition of the gut microbiota and found that there are significant differences between rosacea patients and control groups (Nam et al., 2018). There is ongoing debate about the effect of digestive diseases on rosacea. In rosacea patients’ intestinal bacterial overgrowth, irritable bowel syndrome and chronic inflammatory bowel disease may be more common (Daou et al., 2021). One study found that altered levels of the mammalian synthetic AMP pheromone, plantaricin A could also play a part in rosacea (Nakatsuji and Gallo, 2012).
A complicated link between the alimentary tract, brain and skin has been recognized because patients have been found to improve their skin conditions after oral consumption of probiotics or prebiotics, but researchers have yet to thoroughly investigate the link (Tan-Lim et al., 2021). Changes in gastrointestinal microecology are often accompanied by the diagnosis of psychological disorders such as depression and anxiety. It is known that various neurotransmitters or neuropeptides can be induced by psychological stressors (Salem et al., 2018). This may increase intestinal permeability and therefore lead to enteric and systemic inflammation.
The activation of the plasma kallikrein–kinin system could also be influenced by intestinal bacteria (Kendall, 2004). Researchers have reported the increased stimulation of the plasma kallikrein–kinin system in patients with intestinal inflammation and rosacea (Parodi et al., 1980).
Treatment for rosacea usually involves education, including avoiding ultraviolet light exposure, extreme temperatures, diet and alcohol. In addition, skin-irritating cosmetics should be avoided and daily use of sunscreen is recommended because ultraviolet exposure can cause severe effects on the skin. Studies have suggested that the signs and symptoms of rosacea should be treated based on the patient phenotype. For individual major symptoms such as transient and persistent erythema, inflammatory papules or pustules, telangiectasia, or lumps, a first-line treatment followed by a general skin-care regimen should be recommended. Several first-line treatments are listed as follow. Transient erythema: α-adrenergics (topical) and beta blockers (oral). Persistent erythema: brimonidine (topical), IPL and PDL. Inflammatory papules/pustules: azelaic acid (topical), ivermectin (topical), doxycycline (oral) and metronidazole (topical). Telangiectasia: electrodessication, IPL, and lasers. Phyma: doxycycline (oral) and Isotretinoin (oral). If there are multiple symptoms in a single patient, a variety of drugs could be used simultaneously to treat them. If treatment is unsatisfactory within a certain period, another treatment, or the addition of another first-line drug is recommended. The type of treatment and the patient’s preference determine whether to continue treatment (Schaller et al., 2017).
Facial erythema can be treated with topical β-blockers or 2-epinephrine agonists, while oral β-blockers have also been shown to be effective (Logger et al., 2020). In severe infections which oral antibiotics have failed to improve, or which relapse after discontinuation of antibiotics, oral low-dose isotretinoin therapy could be effective. Research has suggested that bacteria sensitive to antibiotics may directly or indirectly cause papules and pustules (Dahl et al., 2004). Antibiotic treatment makes the disease less severe and increases the amount of Weissella confusa, a potentially beneficial microbe (Ferček et al., 2021). Studies have found that when rosacea is treated with topical or systemic antibiotics, papules and pustules tend to disappear rapidly. Papules and pustules also disappear rapidly when patients are treated with a range of chemically different antibiotics. Treatment can include erythromycin, clindamycin, ampicillin, metronidazole, clarithromycin, and any of the sulfonamides. The apparent disappearance of papules and pustules in patients treated with chemically different antibiotics suggests that bacteria do play a role in the pathogenesis (Dahl et al., 2004). In patients with rosacea, abnormalities in the hair follicles or the microenvironment of the skin surface can lead to worsening disease (Dahl et al., 2004). Coagulase-negative staphylococci produce and secrete proteins in the skin or follicles of patients with rosacea, which may lead to increased inflammation and to papules, pustules and dermatitis.
Many dermatologists treat rosacea patients with papules and pustules with topical or systemic antibiotics. Systemic antibiotics must be used continuously in patients with numerous papules and pustules. The anti-inflammatory activity of systemic antibiotics can lead to the disappearance of papules and pustules in rosacea patients.
Tetracycline has several mechanisms of action, such as antibacterial activity, regulation of innate immunity, inhibition of proinflammatory mediators and protease enzymes, etc. However, it is unclear which is the most relevant mechanism for the eliminatiopapules or pustules. Current studies suggest that an imbalance in the intestinal microbiota can lead to inflammatory skin diseases. Because intestinal bacteria may lead to disturbed immune responses, the use of oral metronidazole treatment can improve both inflammatory enteritis and rosacea symptoms (Vera et al., 2018).
Both minocycline and doxycycline were found to treat rosacea with similar results. Minocycline is a broad-spectrum antibiotic used to treat skin infections caused by many bacteria. The most common non-cutaneous adverse event in the treatment of rosacea with minocycline was viral upper respiratory tract infection, while the most common cutaneous adverse event was pruritus (Martins et al., 2021). Studies found that the skin microbiome α-diversity of rosacea patients treated with oral doxycycline was basically the same before and after systemic antibiotic treatment (Woo et al., 2020b). After treatment of rosacea with doxycycline for six weeks, there was a significant increase in the abundance of a bacterium called Weissella confusa. Between rosacea subjects and healthy controls, the researchers found that gut microbiome α-diversity was basically the same (Nam et al., 2018). When it came to the diversity of gut microbiota samples, their results were also the same. In one recent study, treatment with doxycycline significantly reduced the severity of rosacea and the number of inflammatory papules or pustules. Doxycycline (40 mg orally) was as effective as minocycline (100 mg orally) and there was no difference in the rate of adverse events (van Zuuren et al., 2019). Delayed release doxycycline 40 mg MR was as effective as 100 mg, with fewer side effects (Del Rosso et al., 2008). Several reports have used sub-antimicrobial doses of doxycycline hyclate 20 mg (SDD). One study used 20 mg of SDD twice daily for eight weeks to treat 50 patients with various stages of rosacea. On average, the inflammatory lesions were reduced by 80% to 100% and the erythema was reduced by 50% (Bikowski, 2003).
Some studies have shown that 0.75% metronidazole gel can be used as a first-line topical treatment for the treatment of rosacea. Researchers used 0.75% metronidazole gel twice a day for 12 weeks in the treatment of rosacea and found that inflammatory lesions and erythema were significantly improved, by 79% for papules and 94% for pustules (Miyachi et al., 2022). Reactive oxygen species and oxidative stress are closely associated with a range of skin conditions. Topical metronidazole can both reduce the production of reactive oxygen species and exert its efficacy in rosacea related diseases through anti-inflammatory and immunomodulatory pathways.
Topical 1% ivermectin can effectively reduce Demodex mite density and had a significant effect on rosacea (Ebbelaar et al., 2018). It could also be observed under reflectance confocal microscopy that Demodex follicularis would undergo morphological changes through the action of ivermectin, such as “phantom mites.” Mite density decreased significantly after treatment and clinical improvement. Topical permethrin, benzyl benzoate and crotamine have also been shown to affect Demodex populations (Forton and De Maertelaer, 2020). Studies have been conducted to treat rosacea with 1% ivermectin cream once daily. Of 910 participants who received ivermectin, 615 showed improvement, with a post-treatment improvement rate of 68% (van Zuuren et al., 2019). Benzyl benzoate and crotamiton have also been shown to be effective.
The long-term use of broad-spectrum antibiotics can lead to the emergence of resistant strains, more adverse events and compliance problems. Sarecycline is a novel tetracycline derivative with narrow spectrum activity targeting Gram-positive bacteria, especially Bacillus acnes (Bunick et al., 2021). In a 12-week study of 72 subjects who received oral administration of sarecycline once daily according to body weight, the results showed that sarecycline was effective in treating papules and pustules in adults with rosacea, with an efficacy of 80% (Rosso et al., 2021).
Although rosacea can be treated with effective oral or topical antibiotics, sulfur compounds can change the facial microbiota (van Zuuren et al., 2015) and there is no conclusive evidence that these changes in the skin microbiota are effective in treating the disease. The effects of antibiotic treatment on the gut microbiota are both short-term and long-term. Although antibiotic treatment may be effective in the short term, most skin diseases are associated with long-term disturbances in the microbiota, so this treatment strategy may not be optimal (de Gunzburg et al., 2018).
Some studies have found that topical application of probiotics could directly affect the skin microbiota and immune response (Yu et al., 2020). The effect of topical probiotics on various skin conditions has not been fully explored. Topical and oral probiotics have both been shown to be effective in treating some local diseases. Besides, a combination of topical and oral probiotic treatment may be the most effective (Knackstedt et al., 2020). In general, treatment with probiotics may improve the skin barrier function, reduce inflammation, and reduce the dysregulation of the skin microbiome by restoring a healthy balance of cytokines. For example, TLR2 may be upregulated in rosacea and could be a possible target for probiotics (Tripathi et al., 2019). Besides, oral probiotics can regulate the intestinal microfora and indirectly affect cutaneous conditions (Yu et al., 2020). The consumption of Bifidobacteria and Lactobacillus to affect the gut can also be used to treat certain cutaneous conditions (Hacini-Rachinel et al., 2009). Bacillus subtilis produces spores to colonize the gastrointestinal tract and alter the mucosal barrier microbiome, thereby eradicating H. pylori to reduce rosacea symptoms and associated gastrointestinal problems (Pinchuk et al., 2001). The microorganisms in the intestinal microbiome and skin microbiota described in this review are shown in Table 1.
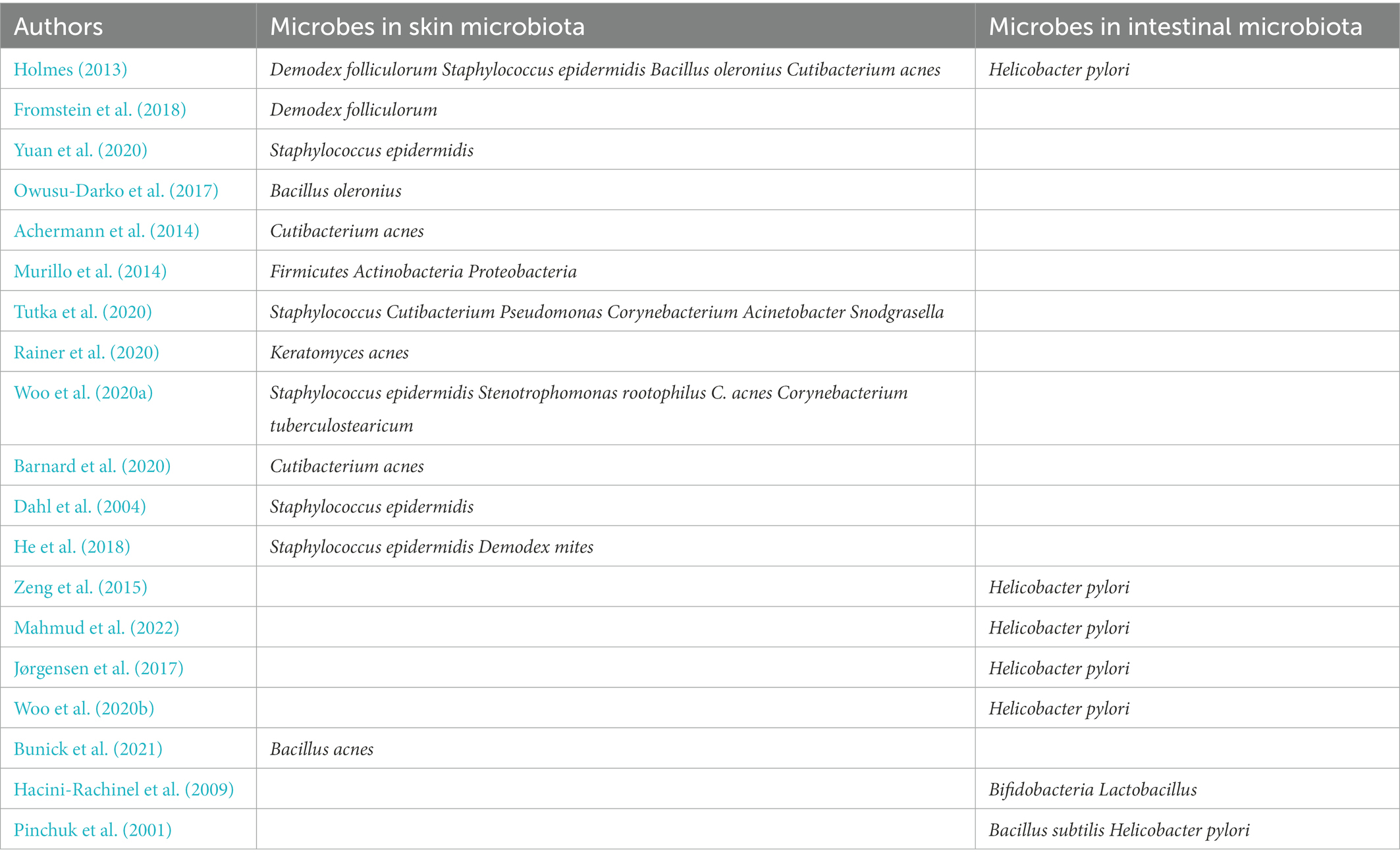
Table 1. Some microorganisms closely related to rosacea in intestinal microbiome and skin microbiota.
Human skin provides a suitable environment for the growth of both beneficial and pathogenic bacteria. It has been shown that rosacea is associated with disturbances in the microbiome of the skin and gut. Therefore, treating rosacea with antibiotics or microbiome modulation has been an attractive approach to disease management. Most dermatologists treat rosacea patients with papules and pustules with topical or systemic antibiotics. Thus, research on changes in the skin and gut microbiota in rosacea patients could contribute to a better understanding of the development and prognosis of the disease.
The role of the gut microbiota in the pathogenesis of rosacea should be further explored. In future studies, the relative abundance of microbial distribution at the strain level will need to be analyzed and different DNA sequencing techniques will need to be used to confirm the various findings. In addition, the clinical complications of rosacea often occur and the pathogenesis and treatment of complications still needs to be further explored, to better manage this disease.
WZ contributed to data acquisition, analysis, data interpretation, and manuscript drafting. XW contributed to data acquisition, analysis, supervised the review, and revised the manuscript for intellectual content. MH critically edited the article for content and presentation. All authors contributed to the article and approved the submitted version.
National Natural Science Foundation of China (81903226). MH was supported by US NIH Grants R01AI050875 and R21AI121700.
MH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc., Cleveland, OH; Hologenix Inc. Santa Monica, CA; Vielight, Toronto, Canada; JOOVV Inc., Minneapolis-St. Paul MN; Sunlighten, Kansas City, MO; Consulting; USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany; Klox Asia, Guangzhou, China. Stockholding: Niraxx Light Therapeutics, Inc., Irvine CA; JelikaLite Corp, New York NY.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achermann, Y., Goldstein, E. J., Coenye, T., and Shirtliff, M. E. (2014). Propionibacterium acnes: from commensal to opportunistic biofilm-associated implant pathogen. Clin. Microbiol. Rev. 27, 419–440. doi: 10.1128/CMR.00092-13
Barnard, E., Shi, B., Kang, D., Craft, N., and Li, H. (2020). The balance of metagenomic elements shapes the skin microbiome in acne and health. Sci Rep. 10:6037. doi: 10.1038/srep39491
Bikowski, J. B. (2003). Subantimicrobial dose doxycycline for acne and rosacea. Skinmed 2, 234–245. doi: 10.1111/j.1540-9740.2003.03014.x
Buddenkotte, J., and Steinhoff, M. (2018). Recent advances in understanding and managing rosacea. F1000Res 7:F1000 faculty Rev-1885. doi: 10.12688/f1000research.16537.1
Bunick, C. G., Keri, J., Tanaka, S. K., Furey, N., Damiani, G., Johnson, J. L., et al. (2021). Antibacterial mechanisms and efficacy of Sarecycline in animal models of infection and inflammation. Antibiotics (Basel). 10:439. doi: 10.3390/antibiotics10040439
Chaplin, D. D. (2010). Overview of the immune response. J. Allergy Clin. Immunol. 125, S3–S23. doi: 10.1016/j.jaci.2009.12.980
Chen, P., He, G., Qian, J., Zhan, Y., and Xiao, R. (2021). Potential role of the skin microbiota in inflammatory skin diseases. J. Cosmet. Dermatol. 20, 400–409. doi: 10.1111/jocd.13538
Dahl, M. V., Ross, A. J., and Schlievert, P. M. (2004). Temperature regulates bacterial protein production: possible role in rosacea. J. Am. Acad. Dermatol. 50, 266–272. doi: 10.1016/j.jaad.2003.05.005
Daou, H., Paradiso, M., Hennessy, K., and Seminario-Vidal, L. (2021). Rosacea and the microbiome: a systematic review. Dermatol Ther (Heidelb). 11, 1–12. doi: 10.1007/s13555-020-00460-1
de Gunzburg, J., Ghozlane, A., Ducher, A., le Chatelier, E., Duval, X., Ruppé, E., et al. (2018). Protection of the human gut microbiome from antibiotics. J. Infect. Dis. 217, 628–636. doi: 10.1093/infdis/jix604
Del Rosso, J. Q., Schlessinger, J., and Werschler, P. (2008). Comparison of anti-inflammatory dose doxycycline versus doxycycline 100 mg in the treatment of rosacea. J. Drugs Dermatol. 7, 573–576.
Ebbelaar, C. C. F., Venema, A. W., and Van Dijk, M. R. (2018). Topical Ivermectin in the treatment of Papulopustular rosacea: a systematic review of evidence and clinical guideline recommendations. Dermatol. Ther (Heidelb). 8, 379–387. doi: 10.1007/s13555-018-0249-y
Ferček, I., Lugović-Mihić, L., Tambić-Andrašević, A., Ćesić, D., Grginić, A. G., Bešlić, I., et al. (2021). Features of the skin microbiota in common inflammatory skin diseases. Life (Basel) 11:962. doi: 10.3390/life11090962
Forbes, J. D., Van Domselaar, G., and Bernstein, C. N. (2016). The gut microbiota in immune-mediated inflammatory diseases. Front. Microbiol. 7:1081. doi: 10.3389/fmicb.2016.01081
Forton, F. M. N., and De Maertelaer, V. (2020). Treatment of rosacea and demodicosis with benzyl benzoate: effects of different doses on Demodex density and clinical symptoms. J. Eur. Acad. Dermatol. Venereol. 34, 365–369. doi: 10.1111/jdv.15938
Fromstein, S. R., Harthan, J. S., Patel, J., and Opitz, D. L. (2018). Demodex blepharitis: clinical perspectives. Clin. Optom (Auckl). 10, 57–63. doi: 10.2147/OPTO.S142708
Hacini-Rachinel, F., Gheit, H., Le Luduec, J. B., Dif, F., Nancey, S., and Kaiserlian, D. (2009). Oral probiotic control skin inflammation by acting on both effector and regulatory T cells. PLoS One 4:e4903. doi: 10.1371/journal.pone.0004903
He, A., Grandhi, R., and Kwatra, S. G. (2018). Rosacea and rate of temperature change: examining real-time data from 2004 to 2016. Ann. Dermatol. 30, 739–741. doi: 10.5021/ad.2018.30.6.739
Holmes, A. D. (2013). Potential role of microorganisms in the pathogenesis of rosacea. J. Am. Acad. Dermatol. 69, 1025–1032. doi: 10.1016/j.jaad.2013.08.006
Holmes, A. D., Spoendlin, J., Chien, A. L., Baldwin, H., and Chang, A. L. S. (2018). Evidence-based update on rosacea comorbidities and their common physiologic pathways. J. Am. Acad. Dermatol. 78, 156–166. doi: 10.1016/j.jaad.2017.07.055
Jørgensen, A. R., Egeberg, A., Gideonsson, R., Weinstock, L. B., Thyssen, E. P., and Thyssen, J. P. (2017). Rosacea is associated with helicobacter pylori: a systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 31, 2010–2015. doi: 10.1111/jdv.14352
Kendall, S. N. (2004). Remission of rosacea induced by reduction of gut transit time. Clin. Exp. Dermatol. 29, 297–299. doi: 10.1111/j.1365-2230.2004.01461.x
Knackstedt, R., Knackstedt, T., and Gatherwright, J. (2020). The role of topical probiotics in skin conditions: a systematic review of animal and human studies and implications for future therapies. Exp. Dermatol. 29, 15–21. doi: 10.1111/exd.14032
Lam, M., Hu, A., Fleming, P., and Lynde, C. W. (2022). The impact of acne treatment on skin bacterial microbiota: a systematic review. J. Cutan. Med. Surg. 26, 93–97. doi: 10.1177/12034754211037994
Logger, J. G. M., Olydam, J. I., and Driessen, R. J. B. (2020). Use of beta-blockers for rosacea-associated facial erythema and flushing: a systematic review and update on proposed mode of action. J. Am. Acad. Dermatol. 83, 1088–1097. doi: 10.1016/j.jaad.2020.04.129
Mahmud, M. R., Akter, S., Tamanna, S. K., Mazumder, L., Esti, I. Z., Banerjee, S., et al. (2022). Impact of gut microbiome on skin health: gut-skin axis observed through the lenses of therapeutics and skin diseases. Gut Microbes 14:2096995. doi: 10.1080/19490976.2022.2096995
Marples, R. R., Downing, D. T., and Kligman, A. M. (1971). Control of free fatty acids in human surface lipids by Corynebacterium acnes. J. Invest. Dermatol. 56, 127–131. doi: 10.1111/1523-1747.ep12260695
Martins, A. M., Marto, J. M., Johnson, J. L., and Graber, E. M. (2021). A review of systemic minocycline side effects and topical minocycline as a safer alternative for treating acne and rosacea. Antibiotics (Basel) 10:757. doi: 10.3390/antibiotics10070757
Miyachi, Y., Yamasaki, K., Fujita, T., and Fujii, C. (2022). Metronidazole gel (0.75%) in Japanese patients with rosacea: a randomized, vehicle-controlled, phase 3 study. J. Dermatol. 49, 330–340. doi: 10.1111/1346-8138.16254
Murillo, N., Aubert, J., and Raoult, D. (2014). Microbiota of Demodex mites from rosacea patients and controls. Microb. Pathog. 71–72, 37–40. doi: 10.1016/j.micpath.2014.04.002
Nakatsuji, T., and Gallo, R. L. (2012). Antimicrobial peptides: old molecules with new ideas. J. Invest. Dermatol. 132, 887–895. doi: 10.1038/jid.2011.387
Nam, J. H., Yun, Y., Kim, H. S., Kim, H. N., Jung, H. J., Chang, Y., et al. (2018). Rosacea and its association with enteral microbiota in Korean females. Exp. Dermatol. 27, 37–42. doi: 10.1111/exd.13398
O'Neill, C. A., Monteleone, G., McLaughlin, J. T., and Paus, R. (2016). The gut-skin axis in health and disease: a paradigm with therapeutic implications. BioEssays 38, 1167–1176. doi: 10.1002/bies.201600008
Owusu-Darko, R., Allam, M., Mtshali, S., Ismail, A., and Buys, E. M. (2017). Draft genome sequence of bacillus oleronius DSM 9356 isolated from the termite Reticulitermes santonensis. Genom Data. 12, 76–78. doi: 10.1016/j.gdata.2017.03.005
Park, D. H., Kim, J. W., Park, H. J., and Hahm, D. H. (2021). Comparative analysis of the microbiome across the gut-skin axis in atopic dermatitis. Int. J. Mol. Sci. 22:4228. doi: 10.3390/ijms22084228
Park, Y. J., and Lee, H. K. (2018). The role of skin and Orogenital microbiota in protective immunity and chronic immune-mediated inflammatory disease. Front. Immunol. 8:1955. doi: 10.3389/fimmu.2017.01955
Parodi, A., Guarrera, M., and Rebora, A. (1980). Flushing in rosacea: an experimental approach. Arch. Dermatol. Res. 269, 269–273. doi: 10.1007/BF00406420
Picardo, M., and Ottaviani, M. (2014). Skin microbiome and skin disease: the example of rosacea. J. Clin. Gastroenterol. 48, S85–S86. doi: 10.1097/MCG.0000000000000241
Pinchuk, I. V., Bressollier, P., Verneuil, B., Fenet, B., Sorokulova, I. B., Mégraud, F., et al. (2001). In vitro anti-helicobacter pylori activity of the probiotic strain Bacillus subtilis 3 is due to secretion of antibiotics. Antimicrob. Agents Chemother. 45, 3156–3161. doi: 10.1128/AAC.45.11.3156-3161.2001
Rainer, B. M., Thompson, K. G., Antonescu, C., Florea, L., Mongodin, E. F., Bui, J., et al. (2020). Characterization and analysis of the skin microbiota in rosacea: a case-control study. Am. J. Clin. Dermatol. 21, 139–147. doi: 10.1007/s40257-019-00471-5
Rosso, J. Q., Draelos, Z. D., Effron, C., and Kircik, L. H. (2021). Oral Sarecycline for treatment of Papulopustular rosacea: results of a pilot study of effectiveness and safety. J. Drugs Dermatol. 20, 426–431. doi: 10.36849/JDD.2021.5923
Salem, I., Ramser, A., Isham, N., and Ghannoum, M. A. (2018). The gut microbiome as a major regulator of the gut-skin axis. Front. Microbiol. 9:1459. doi: 10.3389/fmicb.2018.01459
Schaller, M., Almeida, L. M., Bewley, A., Cribier, B., Dlova, N. C., Kautz, G., et al. (2017). Rosacea treatment update: recommendations from the global ROSacea COnsensus (ROSCO) panel. Br. J. Dermatol. 176, 465–471. doi: 10.1111/bjd.15173
Szántó, M., Dózsa, A., Antal, D., Szabó, K., Kemény, L., and Bai, P. (2019). Targeting the gut-skin axis-probiotics as new tools for skin disorder management? Exp. Dermatol. 28, 1210–1218. doi: 10.1111/exd.14016
Tan-Lim, C. S. C., Esteban-Ipac, N. A. R., Recto, M. S. T., Castor, M. A. R., Casis-Hao, R. J., and Nano, A. L. M. (2021). Comparative effectiveness of probiotic strains on the prevention of pediatric atopic dermatitis: a systematic review and network meta-analysis. Pediatr. Allergy Immunol. 32, 1255–1270. doi: 10.1111/pai.13514
Tripathi, R., Mazmudar, R. S., Ezaldein, H. H., Bordeaux, J. S., and Scott, J. F. (2019). Prison malpractice litigation involving dermatologists: a cross-sectional analysis of dermatologic medical malpractice cases involving incarcerated patients during 1970-2018. J. Am. Acad. Dermatol. 81, 1019–1021. doi: 10.1016/j.jaad.2019.02.035
Tutka, K., Żychowska, M., and Reich, A. (2020). Diversity and composition of the skin, blood and gut microbiome in rosacea-a systematic review of the literature. Microorganisms 8:1756. doi: 10.3390/microorganisms8111756
van Zuuren, E. J., Arents, B. W. M., van der Linden, M. M. D., Vermeulen, S., Fedorowicz, Z., and Tan, J. (2021). Rosacea: new concepts in classification and treatment. Am. J. Clin. Dermatol. 22, 457–465. doi: 10.1007/s40257-021-00595-7
van Zuuren, E. J., Fedorowicz, Z., Carter, B., van der Linden, M. M., and Charland, L. (2015). Interventions for rosacea. Cochrane Database Syst. Rev. 2015:CD003262. doi: 10.1002/14651858.CD003262.pub5
van Zuuren, E. J., Fedorowicz, Z., Tan, J., van der Linden, M. M. D., Arents, B. W. M., Carter, B., et al. (2019). Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br. J. Dermatol. 181, 65–79. doi: 10.1111/bjd.17590
Vera, N., Patel, N. U., and Seminario-Vidal, L. (2018). Rosacea comorbidities. Dermatol. Clin. 36, 115–122. doi: 10.1016/j.det.2017.11.006
Wedi, B., and Kapp, A. (2002). Helicobacter pylori infection in skin diseases: a critical appraisal. Am. J. Clin. Dermatol. 3, 273–282. doi: 10.2165/00128071-200203040-00005
Weiss, E., and Katta, R. (2017). Diet and rosacea: the role of dietary change in the management of rosacea. Dermatol. Pract. Concept. 7, 31–37. doi: 10.5826/dpc.0704a08
Wilkin, J., Dahl, M., Detmar, M., Drake, L., Feinstein, A., Odom, R., et al. (2002). Standard classification of rosacea: report of the National Rosacea Society expert committee on the classification and staging of rosacea. J. Am. Acad. Dermatol. 46, 584–587. doi: 10.1067/mjd.2002.120625
Woo, Y. R., Han, Y. J., Kim, H. S., Cho, S. H., and Lee, J. D. (2020a). Updates on the risk of neuropsychiatric and gastrointestinal comorbidities in rosacea and its possible relationship with the gut-brain-skin Axis. Int. J. Mol. Sci. 21:8427. doi: 10.3390/ijms21228427
Woo, Y. R., Lee, S. H., Cho, S. H., Lee, J. D., and Kim, H. S. (2020b). Characterization and analysis of the skin microbiota in rosacea: impact of systemic antibiotics. J. Clin. Med. 9:185. doi: 10.3390/jcm9010185
Xu, H., and Li, H. (2019). Acne; the skin microbiome; and antibiotic treatment. Am. J. Clin. Dermatol. 20, 335–344. doi: 10.1007/s40257-018-00417-3
Yu, Y., Dunaway, S., Champer, J., Kim, J., and Alikhan, A. (2020). Changing our microbiome: probiotics in dermatology. Br. J. Dermatol. 182, 39–46. doi: 10.1111/bjd.18088
Yuan, C., Ma, Y., Wang, Y., Wang, X., Qian, C., Hocquet, D., et al. (2020). Rosacea is associated with conjoined interactions between physical barrier of the skin and microorganisms: a pilot study. J. Clin. Lab. Anal. 34:e23363. doi: 10.1002/jcla.23363
Zeng, M., Mao, X. H., Li, J. X., Tong, W. D., Wang, B., Zhang, Y. J., et al. (2015). Efficacy; safety; and immunogenicity of an oral recombinant helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1457–1464. doi: 10.1016/S0140-6736(15)60310-5
Keywords: rosacea, skin microbiota, gastrointestinal microbiome, influence factors, treatment
Citation: Zhu W, Hamblin MR and Wen X (2023) Role of the skin microbiota and intestinal microbiome in rosacea. Front. Microbiol. 14:1108661. doi: 10.3389/fmicb.2023.1108661
Received: 01 December 2022; Accepted: 09 January 2023;
Published: 10 February 2023.
Edited by:
Junling Shi, Northwestern Polytechnical University, ChinaReviewed by:
Mara Mihai, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2023 Zhu, Hamblin and Wen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang Wen, ✉ eGlhbmd3ZW5fd2N1bXNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.