- 1Engineering Research Center of Chuanxibei RHS Construction at Mianyang Teachers’College of Sichuan Province, Mianyang Teachers’ College, Mianyang, China
- 2School of Resources and Environmental Engineering, Mianyang Teachers’ College, Mianyang, China
- 3Key Laboratory of Mountain Surface Processes and Ecological Regulation, Institute of Mountain Hazards and Environment, Chinese Academy of Sciences, Chengdu, China
- 4Northwest Sichuan Geological Team, Sichuan Provincial Bureau of Geology and Mineral Resources Exploration and Development, Mianyang, China
In this study, we investigated the soil physicochemical parameters and responses of rhizospheric fungal communities of Hippophae rhamnoides to Mn stress under different sexual competition patterns. The results showed that competition significantly affects soil physicochemical properties, enzyme activity, and rhizosphere-associated fungal community structures. Under Mn stress, soils with intersexual competition had higher levels of N supply than those with the intrasexual competition. Moreover, fungal communities under intersexual interaction were more positive to Mn stress than intrasexual interaction. Under intrasexual competition, female plants had higher total phosphorus content, neutral phosphatase activity, and relative abundance of symbiotic fungi in soils to obtain phosphorus nutrients to alleviate Mn stress. In contrast, male plants had relatively stable fungal communities in soils. In the intersexual competition, rhizosphere fungal diversity and relative abundance of saprophytic fungi in male plants were significantly higher than in female plants under Mn stress. In addition, female plants showed greater plasticity in the response of rhizosphere microorganisms to their neighbors of different sexes. The microbial composition in soils of female plants varied more than male plants between intrasexual and intersexual competition. These results indicated that sex-specific competition and neighbor effects regulate the microbial community structure and function of dioecious plants under heavy metal stress, which might affect nutrient cycling and phytoremediation potential in heavy metal-contaminated soils.
1. Introduction
Industrialization and urbanization have led to ecological degradation and dramatic increases in heavy metals at regional and global scales due to anthropogenic and environmental factors (Zhong et al., 2016). Manganese (Mn) is a kind of heavy metal most widely used in industry and is also an essential trace element for plant growth and reproduction (Yao et al., 2012). Adequate amounts of Mn play an essential role in plant photosynthesis, activation of enzyme-catalyzed reactions, and maintenance of healthy cell organelle structure (Rayen et al., 2010; Bashir et al., 2013). The concentration of Mn required for normal plant growth and development is 20–40 mg/kg dry weight (Rayen et al., 2010). Mn toxicity symptoms usually appear when plants accumulate Mn above 150 mg/kg dry weight such as reduced photosynthetic efficiency, oxidative stress, damage to cellular ultrastructure, interference with the uptake of other nutrients, reduced biomass, and even death (Miyata et al., 2007; Rayen et al., 2010, 2012; Xie et al., 2015; Santos et al., 2017). Mn not only affects plant growth and development but also leads to further deterioration of environmental quality and even endangers human health by the food chain (Li C. et al., 2019).
Dioecious plant is an essential component of terrestrial ecosystems and plays an important role in maintaining species diversity and ecological stability (Hultine et al., 2016). The latest statistics show about 15,600 species of dioecious plants worldwide, belonging to about 175 families and 987 genera (Renner, 2014). Many morphological, physiological, and ecological differences have been observed in dioecious plants under environmental stresses, including drought, temperature, light, salinity, nutrient deficiencies, and heavy metal stresses (Chen M. et al., 2016, 2021; Liu et al., 2020, 2022a,c). Sexual differences in reproductive investment in plants may lead to sexual dimorphism in dioecious plants. Female plants in dioecious plants usually invest more in reproduction and less in growth than male plants (Juvany and Munné-Bosch, 2015; Li L. et al., 2019). Some literature reported that male plants of dioecious plants may grow better and exhibit higher tolerance in stressful environments than female plants due to differences in reproductive investment and ecophysiological responses (Lei et al., 2017; Melnikova et al., 2017; Liu et al., 2021a). The female plants of Populus yunnanensis displayed higher levels of ROS and weaker effective protection against excess zinc (Zn) conditions as compared to that of male plants (Jiang et al., 2013). Under Mn stress, male Populus cathayana plants were more resistant and tolerant than female plants (Chen et al., 2013). Liu et al. (2020) showed that female P. cathayana plants exhibited higher Cd uptake and root crown translocation capacity, while male plants showed more robust antioxidant capacity. Xia et al. (2020) showed that male P. cathayana had higher tolerance than female plants under low phosphorus nutrient stress by enhancing symbiosis with mycorrhizal fungi. Under the salt stress, the addition of ammonium and nitrate nitrogen promoted male P. cathayana efflux Na+ ions through the roots, and fewer Na+ ions were transferred to the shoot, which makes male plants have a higher salt tolerance than female plants (Liu et al., 2022b).
Competition and facilitation profoundly affect plant growth and environmental adaptation (Liancourt et al., 2005). Similarly, sexual competition patterns also significantly affected the stress tolerance of male and female plants under adversity. Populus deltoides females in intersexual competition grew faster than male plants, while males showed higher osmoregulatory capacity and antioxidant activity under salt stress (Li et al., 2016). Under herbivore stress, female P. cathayana in intersexual competition have better herbivore resistance than male plants, accumulating more secondary metabolites through the leaves (He et al., 2021). Under the intersexual competition between male and female plants, the high N growth stimulating effect was more significant for female P. cathayana; in contrast, male plants adapted more to intrasexual competition under low N (Chen et al., 2015). Compared with the intersexual competition, female plants in intrasexual competition accumulated more Cd and exhibited more injury under Cd stress (Chen J. et al., 2016). Pb stress and intrasexual competition had a more significant negative impact on growth and physiological parameters in female plants than in male plants (Chen et al., 2017). Most of the previous studies of dioecious plants have focused on the aboveground physiological response under stress, whether single-sex or sexual competition studies, while the response of rhizosphere microbial communities has been greatly neglected, especially under heavy metal stress.
Hippophae rhamnoides L. is a fast-growing dioecious woody plant widely distributed in northwestern China, characterized by drought tolerance and resistance to wind and sand, which plays a vital role in maintaining ecological stability. Studies have shown that H. rhamnoides can be used as an anti-pollution tree species for revegetation in heavy metal-contaminated mining areas (Yakun et al., 2016). However, the sex-specific response of H. rhamnoides to heavy metal stress in sexual competition patterns has mainly been neglected, especially regarding rhizospheric microbial community structure. Our study aims to address the following two questions: 1. Do sexual competition patterns and Mn stress affect rhizospheric soil physicochemical properties and enzymatic activities of H. rhamnoides? 2. How do sexual competition patterns and Mn stress affect rhizospheric fungal community composition and diversity?
2. Materials and methods
2.1. Plant materials and experimental design
Male and female seedlings of H. rhamnoides were collected from the Seedling cultivation base in Fuxin, Liaoning Province. The experiment was completely randomized with three factorial sex, Mn, and competition combinations. Two sexes (females and males), two Mn regimes (0, 4,000 mg Mn2+ kg−1 dry soil), and three competition treatments (female × female, FF; female × male, FM; male × male, MM) were used in the experiment. Corresponding intrasexual competition treatments are denoted as F/FF for females and M/MM for males, and intersexual competition treatments as F/FM for females and M/FM for males. The experiment was conducted in the Taiyi Xianshan Botanical Garden at Mianyang, Sichuan Province, China (31°27′ N, 104°49′E). On April 3, 2021, 160 healthy annual seedlings (80 females and 80 males) with relatively consistent growth were selected for planting. For interactions, two plants (two females, two males, or a female and a male) were cultivated 20 cm apart from each other in a plastic pot (external diameter 52 cm and height 35 cm) filled with 6.6 kg of homogenized soil of the same origin. Soil samples were air-dried and sieved through a 2 mm sieve. All pots were arranged randomly, and each treatment was replicated 10 times. After 8 weeks of growth, the plants were subjected to an Mn treatment for 12 weeks. The Mn treatments started on June 8, 2021, and the plants were harvested on September 11, 2021. In the Mn treatment, 100 mL of 486 mmol L−1 MnCl2·4H2O was evenly added to the pots every day during the first 10 days. The final Mn level reached 4,000 mg Mn2+ kg−1 dry soil, while the control plants were irrigated with equal quantities of deionized water (Chen J. et al., 2016). Throughout the experiment, the temperature range was 24–30°C during the daytime, 14–19°C during the nighttime, and 70–80% relative air humidity.
2.2. Soil sample collection and determination
The roots of H. rhamnoides were completely excavated, and the bulk soil of the roots was gently shaken off. Then the rhizosphere soil (soil closely attached to the surface of the root system about 2 mm) was collected in sterile bags with a sterile brush, frozen in liquid nitrogen and stored in an ultra-low temperature refrigerator at −80°C for rhizosphere soil microbial DNA extraction. The shaken off soil around the roots was also collected and divided into two parts, one stored at room temperature (25°C) for air-drying and the other stored at −20°C in the refrigerator for the determination of soil enzymatic activity.
Soil organic matter (SOM) content was determined by potassium dichromate oxidation - ferrous sulfate titration (Nelson and Sommers, 1996). Total nitrogen (TN) was determined by the Kjeldahl nitrogen method (Bremner, 1960). Ammonium (NH4+-N) and (NO3−-N) nitrate nitrogen contents were determined by 2 mol L−1 potassium chloride extraction-colorimetric method (Bao, 2000). Total phosphorus (TP) and available phosphorus (AP) content were determined by molybdenum blue colorimetric method (Olsen and Sommers, 1982). Determination of soil pH (the ratio of soil to water is 1:2.5) by an Acidimeter (PHS-3C; LEICI, Shanghai). Soil Mn content was determined by flame atomic absorption method. Soil enzyme activity was measured using the method described by Guan (1986). In brief, sucrase (SC) was determined by the 3,5 - dinitrosalicylic acid colorimetry method, and the sucrase activity was expressed as the mass (mg) of glucose released from 1 g of soil after 1 day; urease (UE) was determined by the phenol-sodium hypochlorite colorimetric method, and the urease activity was expressed as the mass (mg) of NH3−-N released from 1 g of soil after 1 day; neutral phosphatase (NP) was determined by disodium benzene phosphate colorimetric method, and the phosphatase activity was expressed as the mass (mg) of phenol released from 1 g of soil after 1 day; protease (PT) was determined by the ninhydrin colorimetric method, and the phosphatase activity was expressed as the mass (mg) of glycine released from 1 g of soil after 1 day. Soil enzymes were measured in fresh soil and converted to enzyme activity units per gram of dry soil by water content.
2.3. Fungal microbiome analysis
Soil microbial DNA was extracted from 0.5 g frozen soil samples using the Powerful Soil DNA Isolation Kit (MO BIO Laboratories, Carlsbad, CA, United States), following the kit instructions. The purity and integrity of DNA were detected by 1% agarose gel electrophoresis and Nanodrop (Nanodrop 2000, Thermo Fisher Scientific). The primers used for fungal ITS rRNA gene amplifications were ITS4 (5′- TCCTCCGCTTATTGATATGC-3′) and gITS7F (5’-GTGARTCATCGARTCTTTG-3′; Ihrmark et al., 2012). The PCR amplification reaction system and conditions were based on the previous study (Li et al., 2014). After PCR reactions, quality control, and purification processes, a library was constructed. All PCR products were sequenced on the Illumina NovaSeq platform (Illumina Inc., CA, United States) by Chengdu Institute of Biology, CAS. The raw data obtained by sequencing were spliced, quality filtered, and chimeras were removed to obtain effective data (Caporaso et al., 2010; Edgar et al., 2011). Based on the 97% sequence similarity level, all effective data were assigned to Operational Taxonomic Units (OTUs) using UPARSE pipeline (Edgar, 2013). Classification of fungal taxa was done using UNITE version 8.0 as a reference database (Kõljalg et al., 2005). FUNGuild functional annotation was used for predicting the ecological functions of fungal communities (Nguyen et al., 2016). The sequencing data were submitted to NCBI (BioProject accession number: PRJNA903369).
2.4. Statistical analyses
Statistical analysis was performed using the SPSS software package (version 25.0). Before ANOVAs, all data were checked for normality and the homogeneity of variances. Tukey’s test of one-way ANOVA analysis was used to determine the individual differences between the mean values. Independent-sample t-test was used to determine the significant differences between the control group and the Mn treatment group, and the significant differences were P < 0.05.
Based on the OTUs information, alpha diversity indices, including Observed species Chao1, richness, Shannon index, and Simpson index, were calculated with Qiime v1.9.0. Beta diversity was calculated based on Bray-Curtis distance metrics. Principal coordinates analysis (PCoA) was used to visualize the distribution of rhizosphere fungal communities in different treatment groups based on Bray-Curtis distance using the “Vegan” package in R. The PERMANOVA test was used to assess the percentage of variation explained by the Heavy metal, competition, sex, and their interactions on the rhizospheric fungal communities along with its statistical significance using the “Vegan” package. The linear discriminant analysis (LDA) effect size was performed to identify the significantly abundant taxa (phylum to genera) of fungi in different treatments, irrespective of sexual competition patterns (Class: Mn treatment; Subclass: sexual competition pattern), as well as in different sexual competition patterns irrespective of Mn treatment (Class: sexual competition patterns; Subclass: Mn treatment). Based on the FunGuild database, fungal functional groups were classified into symbiotroph, pathotroph, and saprotroph. Then, the relative abundance of the three functional groups was calculated, and their differences among the different treatment groups were analyzed by Tukey’s test and independent sample t-test. Pearson correlation analyses were performed to show relationships between soil environmental factors, fungal diversity, and fungal guilds. Prior to redundancy analysis (RDA), we used the ggvegan package to conduct a variance inflation factor (VIF) analysis for 12 soil environmental factors and then 10 soil environmental factors were used in RDA, TN, and Mn were omitted. The vegan and ggplot2 packages were used for RDA results to examine the correlation between soil environmental factors and fungal community changes.
3. Results
3.1. Soil physicochemical properties and enzyme activity
Under excess Mn conditions, the TP content of FF soil treatment was significantly 21 and 16% higher than MM and FM treatment, respectively, but there was no significant difference in the control group (Table 1). In addition, the TN content of FM soil treatment under excess Mn conditions was significantly 17% higher than FF, while in the control group, there was no significant difference between FM and FF, and the soil TN content of MM was the lowest. In control treatments, the soil in FF treatment showed 32 and 2% higher SOM content and pH than MM, respectively, while 20% lower the NH4+-N content than MM. Excess Mn treatment decreased the SOM content in soils from FF interactive pattern and NH4+-N content in the MM interactive pattern by 31 and 29%, respectively. In addition, the TN, AP, and Mn contents of all interaction patterns were significantly higher in the excess Mn treatment group than in the control group, and Mn content increased by 180, 213, 164%, in FF, MM, and FM, respectively (Table 1).
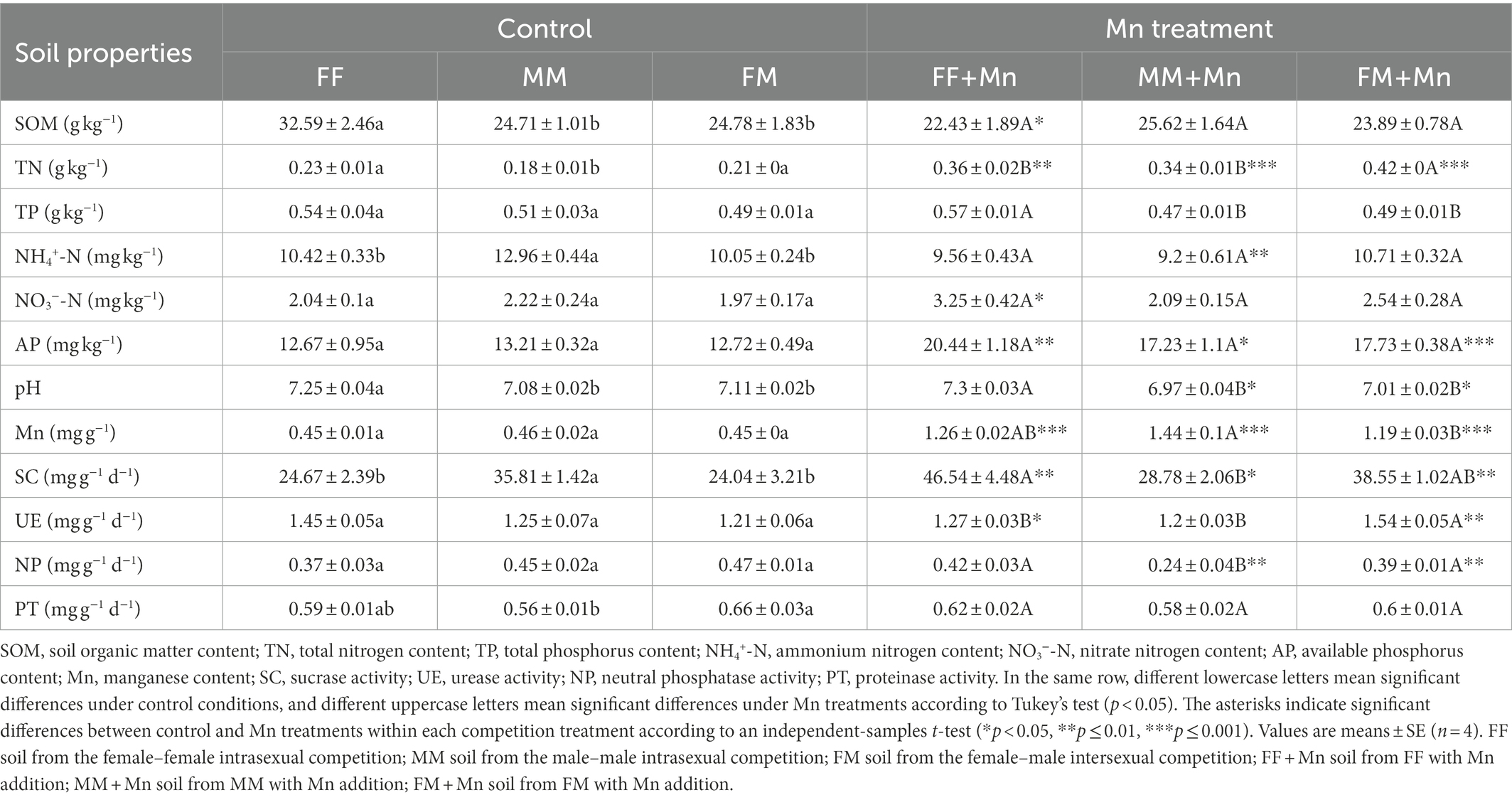
Table 1. Soil physical and chemical properties and enzyme activities under different competition and Mn treatments.
In Mn treatment group, the SC activity of FF was 62% higher than that of MM, while in the control group, it was 10% lower than that of MM (Table 1). In addition, the UE activity of FM was 21 and 28% higher than that of FF and MM under Mn treatment, respectively, and the NP activity of MM competition patterns was lowest under excess Mn conditions. However, both above enzymes in different interactive patterns were not significantly different in the control group. Moreover, UE activity increased by 27% under FM interactive pattern when excess Mn was used. In contrast, UE activity decreased by 12% in FF but remained stable in MM treatment. On the other hand, the PT activity of FM was significantly 18% higher than MM treatment in the control group; however, there was no significant difference in the Mn treatment group.
3.2. Composition of rhizosphere fungal community
No significant difference in the α-diversity of rhizospheric soil was observed in the control treatment (Table 2). The α-diversity of fungal communities was compared in rhizospheric soil from females and males under different competition patterns and excess Mn treatments (Table 2). There were no significant differences in Chao1 and Observed species in intra- and intersexual competition, except that in M/FM + Mn was significantly higher than M/MM + Mn treatment. In addition, the Shannon index and Simpson index were higher in M/FM + Mn than F/FM + Mn treatment, while there was no significant difference in intrasexual competition patterns. Compared with the control group, excessive Mn treatment significantly increased the Chao1 index of M/FM.
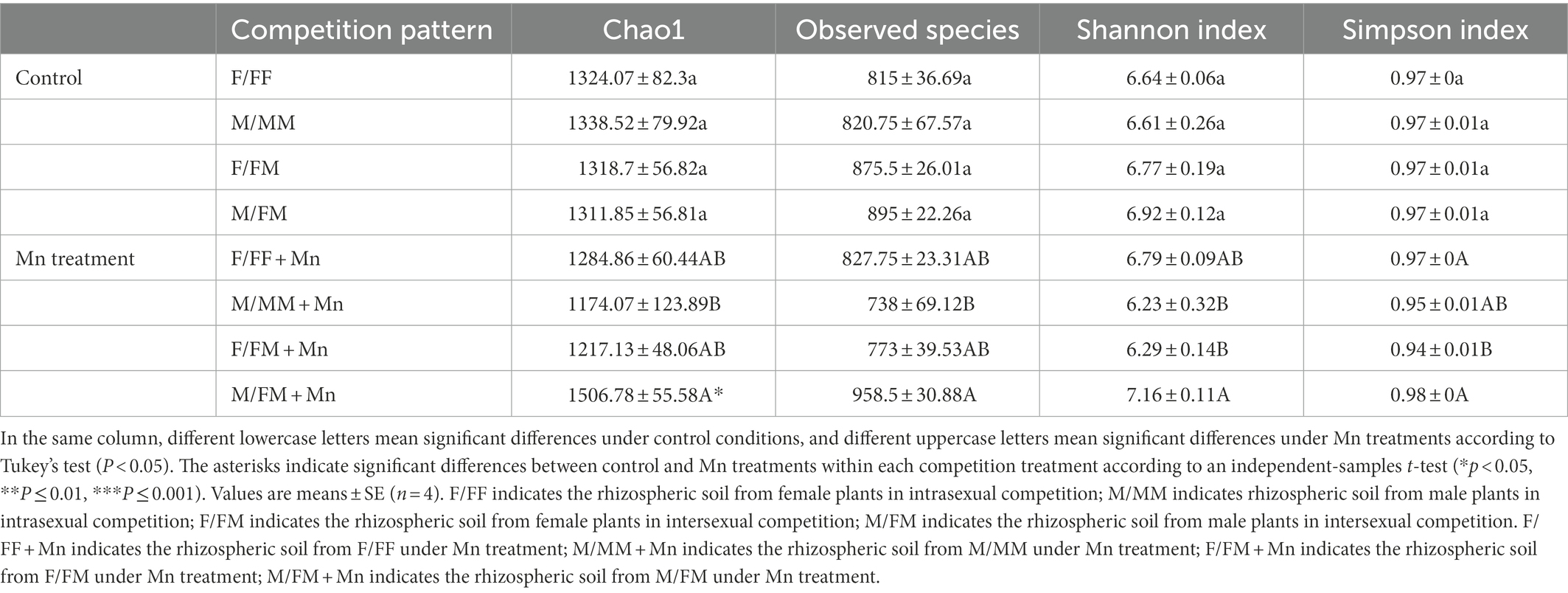
Table 2. Alpha diversity of fungi from the rhizospheric soil of Hippophae rhamnoides under different sexual competition patterns and Mn treatments.
As shown in Figure 1A, sexual competition patterns affected fungal community structure, and the composition of fungal communities was generally separated according to heavy metal and competition. Permutational multivariate analysis of variance (PERMANOVA) demonstrated that heavy metal was the largest source of variation (20.23%, p < 0.001; Figure 1B). The competitions were the second largest source of variation (11.95%, p < 0.001; Figure 1B).
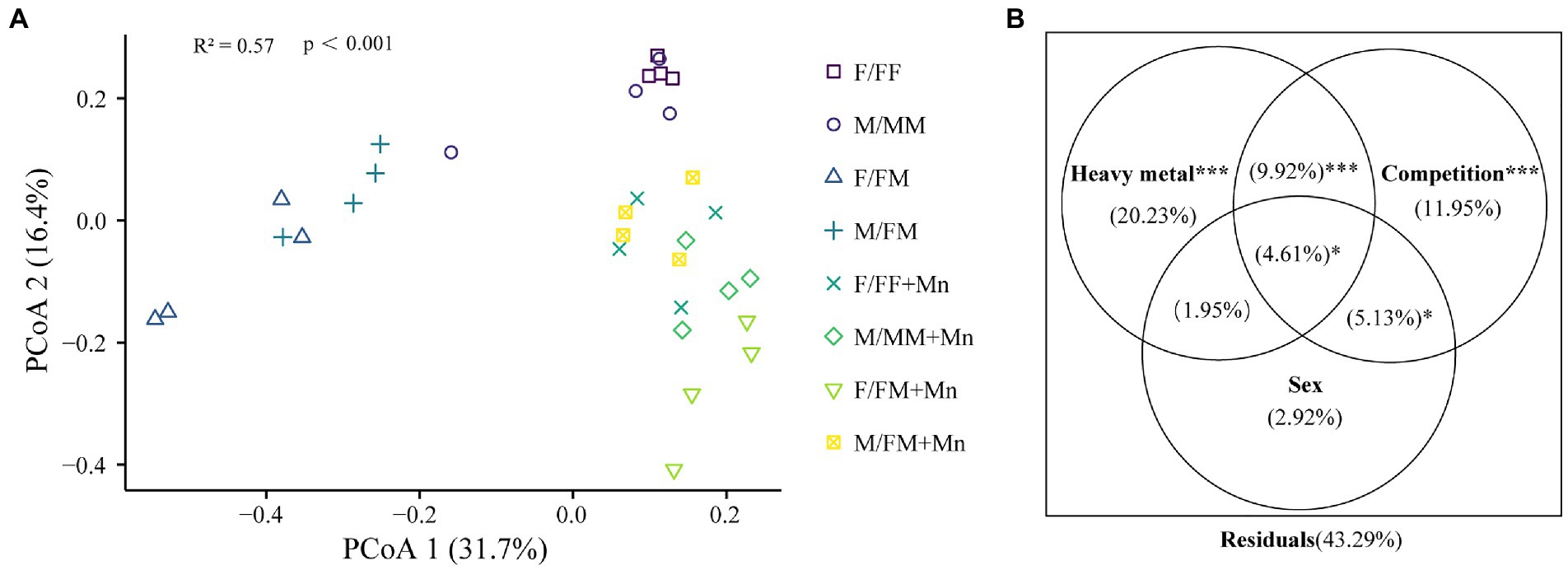
Figure 1. Principal coordinates analysis (PCoA) (A) and PERMANOVA results (B) based on Bray-Curtis distance metrics. Different colors or shapes represent different sample groups under different sexual competition patterns and Mn treatment; treatment codes are the same as in Table 2. Heavy metal: control, Mn treatment; Competition: intrasexual competition, intersexual competition; Sex: F, M. *0.01 < p ≤ 0.05; **0.001 < p ≤ 0.01; ***p ≤ 0.001.
The taxonomic composition of fungal communities at the phylum level (relative abundance >1%) and genus level (top 10 relative abundance) is shown in Figures 2A,B. Ascomycota (68–90%) was an absolute dominant phylum in all treatments. Excess Mn reduced the Ascomycota phylum’s relative abundance in female plants’ rhizosphere in both inter- and intrasexual competition (Figure 2C). Basidiomycota, Rozellomycota, and Mortierellomycota were the dominant phyla with relative abundance greater than 1%. Excess Mn reduced the relative abundance of Mortierellomycota under intersexual competition. At the same time, the contrary was true for the Rozellomycota phylum, and females were significantly higher than males (Figure 2C). In addition, the relative abundance of Rozellomycota phylum was higher under excess Mn treatment than under control conditions in male form intrasexual competition. The abundances of the top 10 genera were in the following order: Zopfiella, Cercophora, Podospora, Pseudeurotium, Mycothermus, Clonostachys, Humicola, Psathyrella, Mortierella, Chaetomium (Figure 2B). Almost all fungal genera in intersexual competition differed between excess Mn treatment and control conditions, except Zopfiella, Psathyrella, and Chaetomium, while excess Mn treatment had no significant effect on all fungal genera in intrasexual competition (Figure 2D).
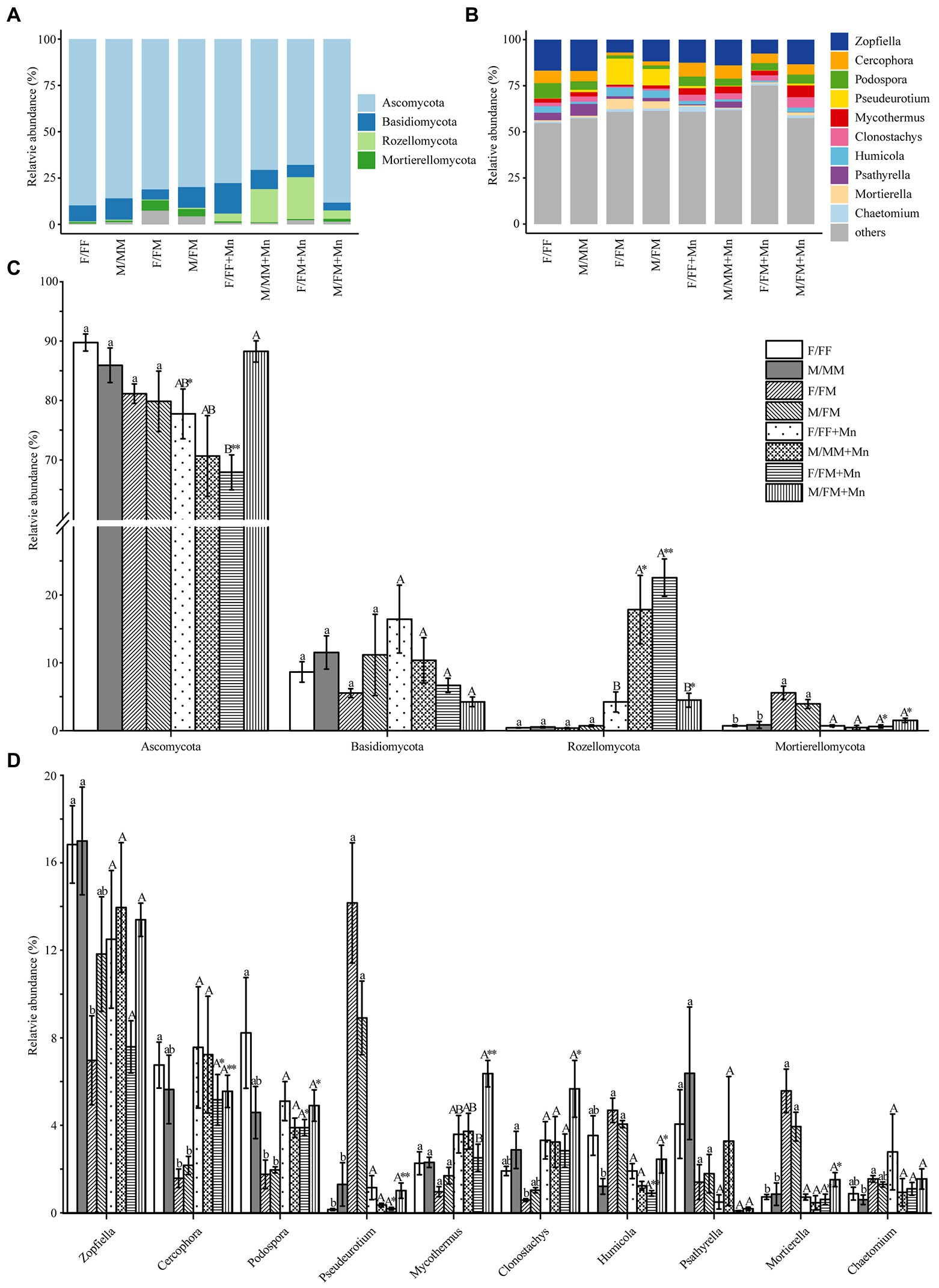
Figure 2. Relative abundances of dominant fungal phyla (A,C) and genera (B,D) under different sexual competition patterns and Mn treatment. Different lowercase letters mean significant differences under control conditions, and different uppercase letters mean significant differences under Mn treatments, according to Tukey’s test (p < 0.05). The asterisks indicate significant differences between control and Mn treatments within each competition treatment according to an independent-samples t-test (*p < 0.05, **p ≤ 0.01, ***p ≤ 0.001). Values are means ± SE (n = 4). Treatment codes are the same as in Table 2.
The linear discriminant analysis (LDA) effect size analysis (LEfSe) was performed to compare the fungal composition from phyla to genera between Mn treatments, as well as between sexual competition patterns (Figure 3). We found that fungal compositions showed significant differences among sexual competition patterns and Mn treatments. The family Rhytismataceae was enriched in plants without excess Mn, while the phylum Basidiomycota, the class Agaricomycetes, the order GS11, the family Cucurbitariaceae, the genus Chrysosporium were predominant under excess Mn (Figure 3A). Irrespective of the Mn treatment, the phylum Mortierellomycota, the classes Mortierellomycetes, Agaricomycetes, Pezizomycetes and Eurotiomycetes, the order Mortierellales, the families Cucurbitariaceae and Mortierellaceae, the genera Humicola, Zopfiella, Staphylotrichum and Microdochium were abundant in the rhizosphere soil of F/FF, whereas the orders Pleosporales and Microascales, the families Trichocomaceae and Orbiliaceae, the genera Talaromyces and Mycothermus were more abundant in the rhizosphere soil of M/MM under intrasexual interaction (Figure 3B). In addition, the order Chaetothyriales, the genera Pezicula and Oidiodendron were dominant in F/FM under intersexual interactions, while the family Dermateaceae, the genus Natantispora were more abundant in M/FM (Figure 3B).
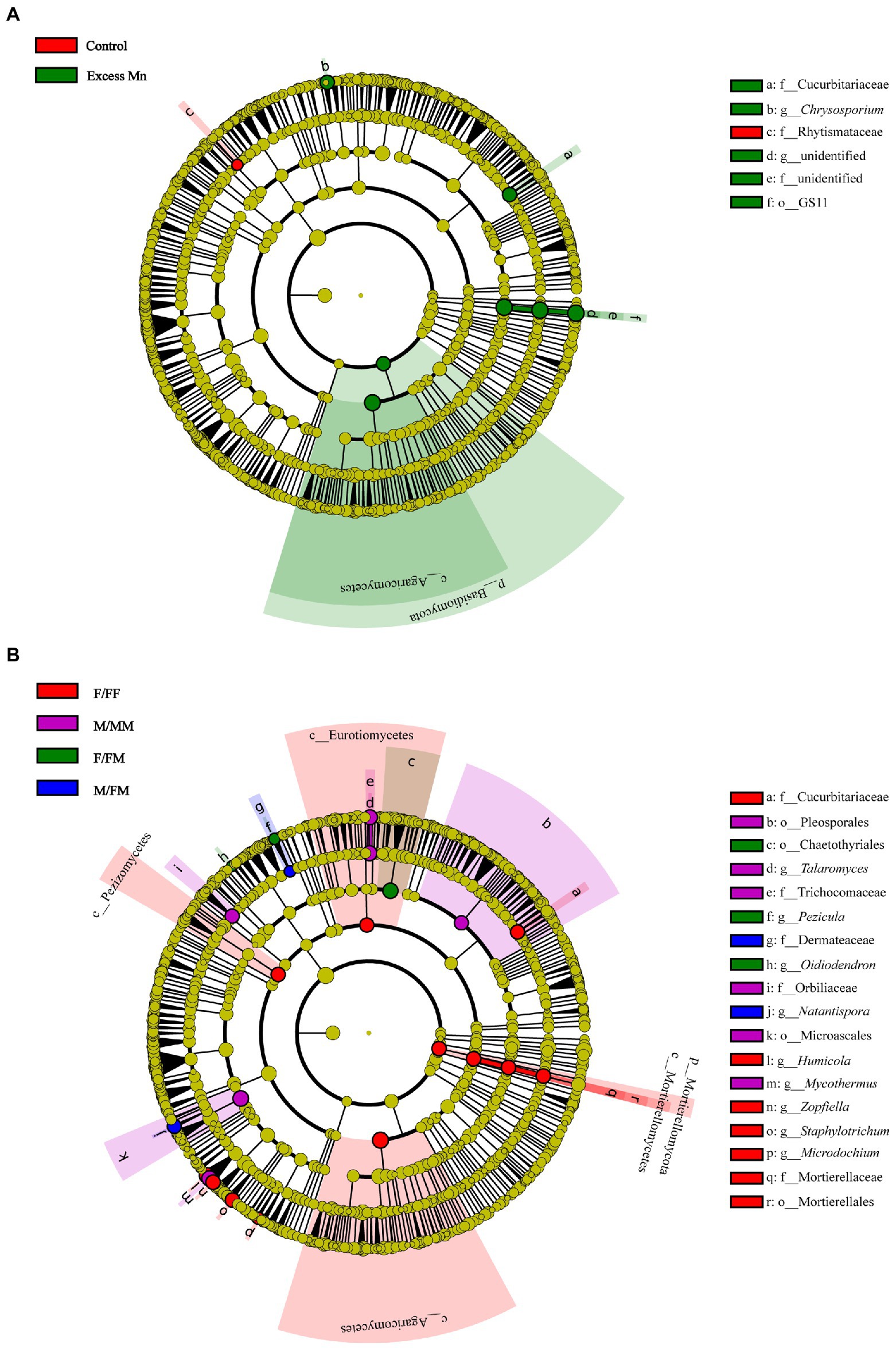
Figure 3. Fungal taxa with different abundance changes between control and Mn treatment, irrespective of sexual competition patterns (A), and between sexual competition patterns, irrespective of Mn treatment as detected by the linear discriminant analysis effect size (LEfSe) analysis (B). Only taxa with LDA over 5.5 are shown. The node color indicates taxa enriched under different treatment and interaction patterns.
3.3. Fungal function guilds
In the excess Mn treatment group, the relative abundance of symbiotroph in F/FF was significantly higher than M/MM, while there was no significant difference in intersexual competition (Figure 4A). The relative abundance of pathotroph in F/FF and M/FM was significantly higher in the excess Mn treatments group than in the control group (Figure 4B). In addition, excessive Mn treatment significantly reduced the relative abundance of saprotrophs in F/FM compared to the control and was significantly lower than M/MM and M/FM in the excess Mn treatment group (Figure 4C).

Figure 4. Relative abundances of symbiotrophs (A), pathotrophs (B), and saprotrophs (C) under different sexual competition patterns and Mn treatments. The asterisks indicate significant differences between control and Mn treatments within each competition treatment according to an independent-samples t-test (*P < 0.05, **0.001 < P ≤ 0.01). Values are means ± SE (n = 4). Treatment codes and statistical significance codes are the same as in Table 2.
3.4. Associations between soil environmental factors and rhizosphere fungal communities
The Pearson correlation heatmap showed that soil neutral phosphatase activities were significantly positively correlated with Observed species, Shannon index, Simpson index, and the relative abundance of symbiotrophs. The soil pH and TP content were significantly positively correlated with the Simpson index and symbiotrophs, respectively. In addition, the relative abundance of saprotroph was negatively correlated with many soil factors, including AP, Mn, NO3−-N, TN, and soil sucrase activities (Figure 5).
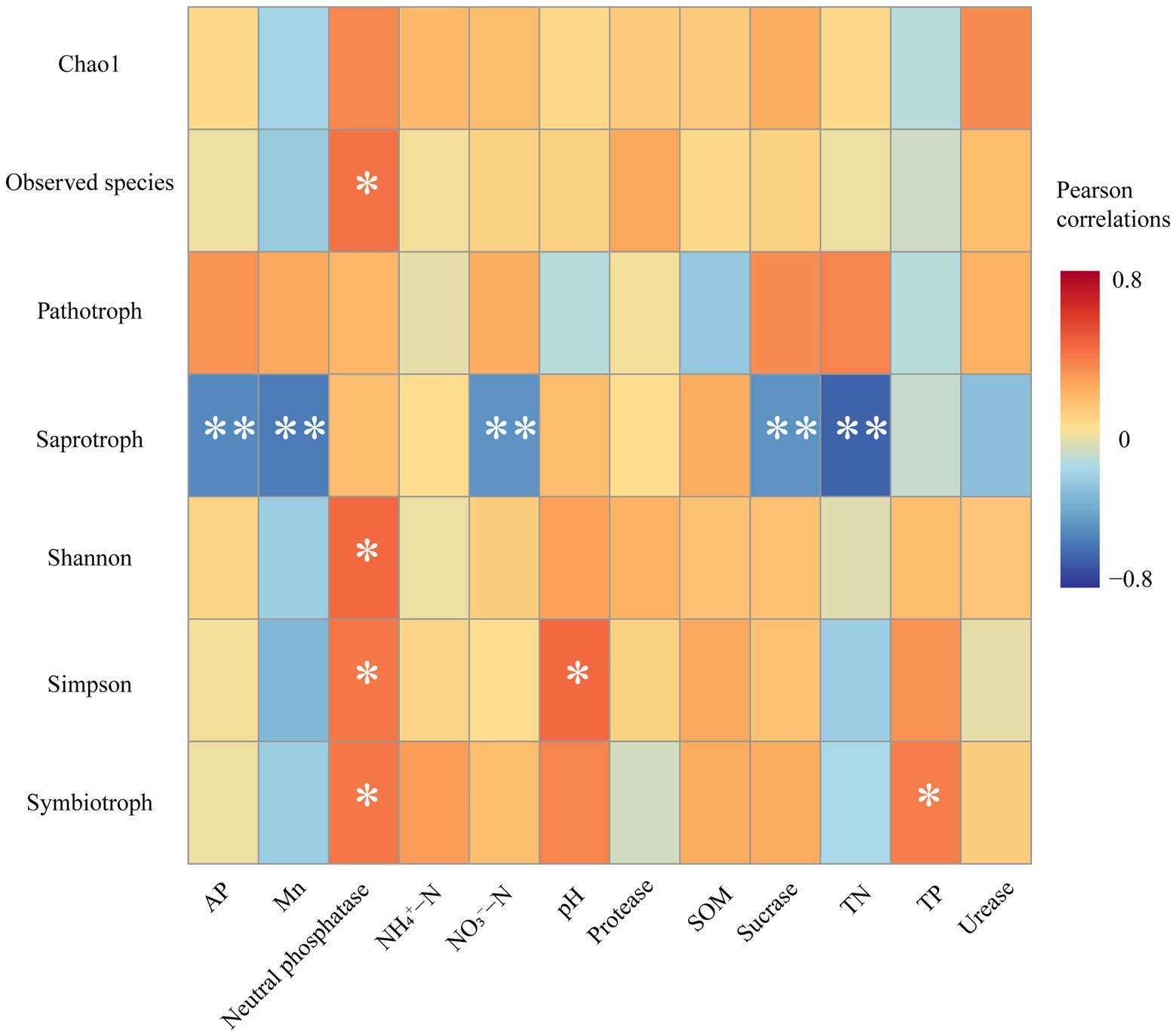
Figure 5. Pearson correlations between soil environmental factors, fungal diversity, and fungal functional guilds. Significances are marked as ***p ≤ 0.001; **p ≤ 0.01; *p ≤ 0.05.
Collectively, the abiotic factors in Redundancy analysis (RDA) explained 70.18% of the variation in the relative abundances of fungal families. The first ordination axis RDA1 explained 52.77% and the second axis RDA2 explained 17.41% of the changes in the fungal communities (Figure 6). The results showed that SOM affected Psathyrellaceae, Microascaceaea, and Chaetomiaceae fungal communities. AP and sucrase affected Nectriaceae and Bionectriaceae fungal communities. Moreover, soil neutral phosphatase and Protease activities affected Pseudeurotiaceae, Mortierellaceae, and Bolbitiaceae fungal communities.
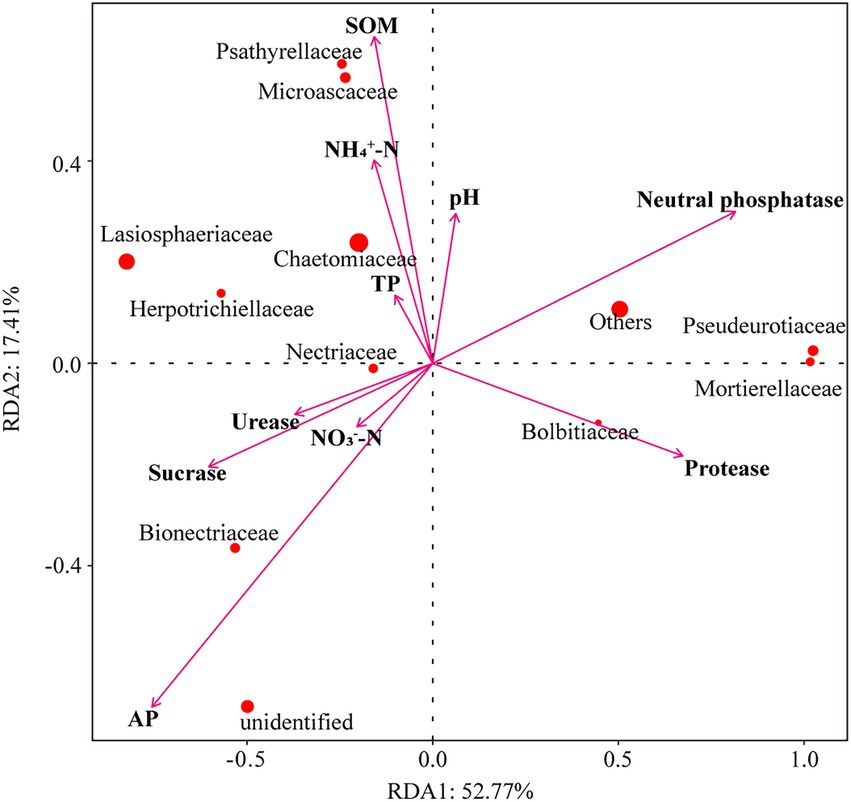
Figure 6. Redundancy analysis (RDA) of fungal families constrained by soil environmental factors across all experimental units. The arrows refer to soil environmental factors. The red circle represents fungal families, and the circle size represents relative abundance.
4. Discussion
4.1. Effects of sexual competition patterns and Mn stress on soil physicochemical properties and enzyme activities
Positive interspecific interactions among plants can improve soil conditions and promote plant nutrient uptake to enhance environmental tolerance (Shi et al., 2021). Previous studies have reported sex differences in nutrient element composition of male and female plant organs under different environments and competition patterns (Chen et al., 2014, 2017; Song et al., 2019). In this study, sexual competition patterns and Mn stress affected soil nutrients and enzyme activities. Under conditions of Mn stress and intrasexual competition, females had higher TP content, sucrase, and neutral phosphatase activity than males. It was also found that soil neutral phosphatase and TP content were significantly and positively correlated with Symbiotic fungi (Figure 5). When the supply of phosphorus in soil is insufficient, the symbiotic fungi dominated by mycorrhizal fungi accelerate the mineralization of organic phosphorus by secreting soil phosphatase to produce inorganic phosphorus for plants to absorb (Oehl et al., 2010). In addition, the AP content of the FF group was significantly higher by 61.3% after Mn addition compared to the control. The above results indicate that intra-female sexual competition effectively promotes the mineralization process of soil phosphorus, which can provide more phosphorus nutrients to plants. Interestingly, under Mn stress, the combined male and female treatments possessed the highest TN content and urease activity, indicating a higher level of nitrogen availability. It is possible that male and female plants in intersexual competition require more nitrogen nutrients to sustain plant growth. Plant competition can alter the availability and transformation of essential nutrients, which may affect plant responses to abiotic stresses and nutrient cycling in local ecosystems (Chen et al., 2017).
Previous studies have shown that soil enzymes play a vital role in soil ecological processes and can be used as effective indicators of the ecological impact of heavy metal contamination in soil (Šmejkalová et al., 2003; Paz-Ferreiro and Fu, 2013; Tang et al., 2019). In our study, females showed lower sucrase activity than males in intrasexual interaction under control treatments, while the opposite was true under Mn stress (Table 1). On the one hand, this may be related to the differences in resource utilization patterns between males and females (Juvany and Munné-Bosch, 2015). On the other hand, heavy metal stress may change the intensity of sex-specific competition (Chen et al., 2017).
4.2. Effects of sexual competition patterns and Mn stress on the structure and function of fungal communities
Soil microbes can spread horizontally through their surroundings, such as neighboring plants (Meyer et al., 2022). Rhizosphere and rhizoplane are important interfaces for microbial diffusion (Xiong et al., 2021). Heavy metals can also affect rhizosphere-driven microbial community structure (Hou et al., 2017). In addition, sex-specific interactions of dioecious plants have been shown to affect the composition of soil microorganisms (Xia et al., 2022). Our study further confirms this finding. We found that sexual competition patterns regulated the response of α-diversity of male and female H. rhamnoides rhizosphere fungi to Mn stress. In the control group, there were no significant differences in rhizosphere fungal diversity and richness in different sexual competition patterns, but significant differences were shown in Mn stress group. The fungal community richness of M/FM was significantly higher than that of M/MM under Mn stress. In addition, the diversity of the M/FM fungal community was significantly higher than that of F/FM under Mn stress. The root metabolites of M/FM under Mn stress may provide a broader niche for specific microbes adapted to particular substrates, and this puts its rhizosphere fungal community diversity and richness at a high level in the sexual competition (Xia et al., 2022). Soil physicochemical properties can affect fungal α- diversity (Berg and Smalla, 2009; Constancias et al., 2015). In this study, soil pH was significantly and positively correlated with the fungal Simpson index, which was similar to the studies of Yang et al. (2019) as well as Wang et al. (2020), and there were also studies showing that soil fungal community composition was highly significantly and positively correlated with soil pH (Shi et al., 2020). Elevated pH can reduce the availability of heavy metals and then mitigate damage to heavy metal-intolerant fungi, which may increase soil fungal diversity and change community structure. Compared to the control, Mn stress did not cause significant changes in rhizosphere fungal diversity and richness in male and female H. rhamnoides, except for Chao1 of M/FM. However, the fungal community structure was strongly affected by Mn treatment and competition and their interaction (Table 2), which probably involved root nutrients and Mn bioavailability (Liu et al., 2021b).
Vellend (2016) ecological community theory suggests that microbial community assembly is influenced by four evolutionary processes: dispersal, selection, diversification, and ecological drift. Selection effects due to the influence of biotic or abiotic factors can lead to changes in the abundance of microorganisms within the community (Cordovez et al., 2019; Fitzpatrick et al., 2020). In our study, Ascomycota was the absolute dominant phylum in all treatment groups. Ascomycota includes many saprophytic and parasitic fungi, mainly saprophytic fungi in soil, and can secrete a variety of cellulose and hemicellulolytic enzymes (Schoch et al., 2009; Baldrian et al., 2011). Mn stress significantly reduced the relative abundance of Ascomycota in female H. rhamnoides under intersexual and intrasexual competition (Figure 2C). At the same time, the relative abundance of saprophytic fungi was significantly lower in F/FM than in M/FM under Mn stress (Figure 4C). Therefore, we speculated that Mn stress inhibited carbon source metabolism of fungi in the rhizosphere soil of female H. rhamnoides. This inhibitory effect on females under intersexual competition was stronger than under intrasexual competition. In addition, the relative abundance of saprophytic fungi was higher in M/FM than in F/FM under Mn stress, which made M/FM more capable of organic matter decomposition and promoted above-ground plant growth. We also found that Agaricomycetes were enriched in the excess Mn treatments and F/FF groups (Figure 3). In contrast, most Agaricomycetes belonged to arbuscular mycorrhizal fungi and could provide essential nutrients and promote plant growth (Hibbett, 2006) Female H. rhamnoides from the intrasexual competition will strengthen the association with symbiotic fungi such as Agaricomycetes to resist Mn stress by forming mycorrhizal structures in the roots, which can enhance P acquisition capacity to promote growth. Meanwhile, the relative abundance of F/FF rhizosphere Symbiotrops was significantly higher than M/MM under Mn stress (Figure 4A), which may make females from intrasexual competition more tolerant to Mn than males from the intrasexual competition by absorbing more phosphorus nutrition.
Previous studies have shown that neighboring plants have a greater impact on the performance of female plants, and the effect may be positive or negative (Graff et al., 2018). In our study, we found that F/FF and F/FM showed differential changes in the relative abundance of Zopfiella, Cercophora, Podospora, Pseudeurotium, and Mortierella under control conditions (Figure 2D). Zopfiella can control plant diseases by secreting antifungal compounds that inhibit the growth of plant pathogens (Huang et al., 2015), Cercophora can produce indole acetic acid and has the ability to dissolve and mineralize elemental phosphorus (Miranda et al., 2020), Pseudeurotium was a saprophytic fungus (Schadt and Rosling, 2015), the relative abundance of these three fungi in F/FF was higher than that in F/FM. However, the relative abundance of Podospora and Mortierella was significantly higher in F/FM than in F/FF. These two fungal taxa have important ecological functions in lignocellulose degradation and litter decomposition (Ellegaard-Jensen et al., 2013; Xie et al., 2014). Our results suggest that the rhizosphere microbes of H. rhamnoides females showed more plasticity in response to different sex neighbors. In contrast, the rhizosphere microbes of males are less sensitive to the sexual identity of neighbors. In addition, studies have shown that female plants from intra- and intersexual competition can distinguish the sexual identity of neighbors and change the investment models between growth and chemical defense (He et al., 2021; Zhang et al., 2021). We also found specific fungal colonization in the rhizosphere of H. rhamnoides under different sex competition patterns (Figure 3), which may result from the regulation of rhizosphere microorganisms by sexual competition patterns.
Furthermore, we found that male and female H. rhamnoides in the intersexual competition pattern were more positive to Mn stress than the intrasexual competition pattern in the rhizosphere microbial response (Figure 2D). Mn stress significantly increased the relative abundance of Cercophora, Podospora, Mycothermus and Clonostachys in the rhizosphere of intersexual competition of H. rhamnoides, which would enhance the cellulose degradation ability and disease resistance of H. rhamnoides (Che et al., 2002; Aquino et al., 2003; Cota et al., 2009). In addition, the relative abundance of Pseudeurotium, Humicola and Mortierella decreased, which may affect the accumulation of organic carbon and phosphorus mineralization (Ferrari et al., 2011; Clemmensen et al., 2015). However, H. rhamnoides in intrasexual competition was not affected by Mn stress. In the intersexual competition patterns, the mixed growth of male and female H. rhamnoides with different functions creates different rhizosphere microenvironments. Mixed sex planting increases microbial diversity in female and male rhizosphere (Xia et al., 2022). Previous studies have shown that resource complementarity and niche differentiation are fundamental mechanisms for improving ecosystem functioning (Loreau and Hector, 2001; Yu et al., 2022). We hypothesize that females and males in the intersexual competition patterns can create a more heterogeneous soil environment through resource complementarity or ecological niche differentiation to recruit more microbes at the rhizosphere than in intrasexual competition patterns. In addition, soil microbes are key drivers of changes in plant community structure and plant–plant interactions (Hodge and Fitter, 2013). This sex-specific adaptation and biochemical plasticity in dioecious plants are expected to lead to local adaptation and potential sex segregation (Charlesworth, 2002; Xia et al., 2022).
In summary, we believe that in the future production practice process, for male H. rhamnoides, intersexual competition is better than intrasexual competition; for female H. rhamnoides, intrasexual competition is better than intersexual competition.
5. Conclusion
The present study showed that Mn stress and sex competition strongly affected soil physicochemical properties, enzyme activity, and rhizosphere fungal abundance and diversity. Under Mn stress, there were significant differences in soil physicochemical properties and enzyme activities between male and female H. rhamnoides under different sex competition patterns, resulting in different nutrient availability. For example, F/FF in intrasexual competition patterns had a stronger soil phosphorus mineralization capacity and phosphorus supply level than M/MM. In contrast, intersexual competition FM soils had a higher nitrogen supply level under Mn stress. In addition, competition patterns and Mn treatment altered the structure of rhizosphere fungal communities of H. rhamnoides. In intersexual interaction, M/FM rhizosphere fungal diversity was significantly higher than F/FM under Mn stress. Females in intrasexual competition patterns can alleviate Mn stress by recruiting symbiotic fungi such as Agaricomycetes to obtain more P in symbiosis; in contrast, males in intrasexual competition patterns have a more stable microbial community to face Mn stress. In addition, females showed greater plasticity in the response of rhizosphere microorganisms to their neighbors of different sexes, and the rhizosphere microorganism of male and female H. rhamnoides to Mn Stress under intersexual interaction is more positive than intrasexual interaction. This study provides a new perspective for the remediation of heavy metal contaminated soil by H. rhamnoides, while more attention should be paid to the effect of sex interactions on dioecious plants in phytoremediation.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
YL is responsible to experiment conduct, data collection and analysis, and manuscript writing. JC is responsible to complete experimental design and final manuscript. LF, XS, and HC are responsible to some data collect and analysis. YH, BD, RL, and CC are responsible to some data analysis. All authors have reviewed and approved the submission of our manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31500505), Sichuan Provincial Science and Technology Department Project (Nos. 2021YJ0293, 23NSFSC1968, 23NSFSC3100), and Postgraduate Innovation Fund Project (Nos. CX202204, CX202209).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aquino, A. C. M. M., Jorge, J. A., Terenzi, H. F., and Polizeli, M. L. T. M. (2003). Studies on a thermostable α-amylase from the thermophilic fungus Scytalidium thermophilum. Appl. Microbiol. Biotechnol. 61, 323–328. doi: 10.1007/s00253-003-1290-y
Baldrian, P., Voriskova, J., Dobiasova, P., Merhautova, V., Lisa, L., and Valaskova, V. (2011). Production of extracellular enzymes and degradation of biopolymers by saprotrophic microfungi from the upper layers of forest soil. Plant Soil 338, 111–125. doi: 10.1007/s11104-010-0324-3
Bashir, K., Takahashi, R., Nakanishi, H., and Nishizawa, N. (2013). The road to micronutrient biofortification of rice: Progress and prospects. Front. Plant Sci. 4:15. doi: 10.3389/fpls.2013.00015
Berg, G., and Smalla, K. (2009). Plant species and soil type cooperatively shape the structure and function of microbial communities in the rhizosphere. FEMS Microbiol. Ecol. 68, 1–13. doi: 10.1111/j.1574-6941.2009.00654.x
Bremner, J. M. (1960). Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 55, 11–33. doi: 10.1017/s0021859600021572
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Charlesworth, D. (2002). Plant sex determination and sex chromosomes. Heredity 88, 94–101. doi: 10.1038/sj.hdy.6800016
Che, Y., Gloer, J. B., Koster, B., and Malloch, D. (2002). Decipinin a and Decipienolides a and b: New bioactive metabolites from the coprophilous fungus Podospora decipiens. J. Nat. Prod. 65, 916–919. doi: 10.1021/np010575p
Chen, J., Dong, T., Duan, B., Korpelainen, H., Niinemets, Ü., and Li, C. (2015). Sexual competition and N supply interactively affect the dimorphism and competiveness of opposite sexes in Populus cathayana. Plant Cell Environ. 38, 1285–1298. doi: 10.1111/pce.12477
Chen, J., Duan, B., Wang, M., Korpelainen, H., and Li, C. (2014). Intra- and inter-sexual competition of Populus cathayana under different watering regimes. Funct. Ecol. 28, 124–136. doi: 10.1111/1365-2435.12180
Chen, J., Duan, B., Xu, G., Korpelainen, H., Niinemets, Ü., and Li, C. (2016). Sexual competition affects biomass partitioning, carbon–nutrient balance, cd allocation and ultrastructure of Populus cathayana females and males exposed to cd stress. Tree Physiol. 36, 1353–1368. doi: 10.1093/treephys/tpw054
Chen, J., Han, Q., Duan, B., Korpelainen, H., and Li, C. (2017). Sex-specific competition differently regulates ecophysiological responses and phytoremediation of Populus cathayana under Pb stress. Plant Soil 421, 203–218. doi: 10.1007/s11104-017-3450-3
Chen, M., Huang, Y., Liu, G., Qin, F., Yang, S., and Xu, X. (2016). Effects of enhanced UV-B radiation on morphology, physiology, biomass, leaf anatomy and ultrastructure in male and female mulberry (Morus alba) saplings. Environ. Exp. Bot. 129, 85–93. doi: 10.1016/j.envexpbot.2016.03.006
Chen, J., Liu, Q., Yu, L., Korpelainen, H., Niinemets, Ü., and Li, C. (2021). Elevated temperature and CO2 interactively modulate sexual competition and ecophysiological responses of dioecious Populus cathayana. For. Ecol. Manag. 481:118747. doi: 10.1016/j.foreco.2020.118747
Chen, F. G., Zhang, S., Zhu, G., Korpelainen, H., and Li, C. (2013). Populus cathayana males are less affected than females by excess manganese: comparative proteomic and physiological analyses. Proteomics 13, 2424–2437. doi: 10.1002/pmic.201200365
Clemmensen, K. E., Finlay, R. D., Dahlberg, A., Stenlid, J., Wardle, D. A., and Lindahl, B. D. (2015). Carbon sequestration is related to mycorrhizal fungal community shifts during long-term succession in boreal forests. New Phytol. 205, 1525–1536. doi: 10.1111/nph.13208
Constancias, F., Terrat, S., Saby, N. P. A., Horrigue, W., Villerd, J., Guillemin, J. P., et al. (2015). Mapping and determinism of soil microbial community distribution across an agricultural landscape. Microbiol. Open 4, 505–517. doi: 10.1002/mbo3.255
Cordovez, V., Dini-Andreote, F., Carrión, V. J., and Raaijmakers, J. M. (2019). Ecology and evolution of plant microbiomes. Annu. Rev. Microbiol. 73, 69–88. doi: 10.1146/annurev-micro-090817-062524
Cota, L. V., Maffia, L. A., Mizubuti, E. S. G., and Macedo, P. E. F. (2009). Biological control by Clonostachys rosea as a key component in the integrated management of strawberry gray mold. Biol. Control 50, 222–230. doi: 10.1016/j.biocontrol.2009.04.017
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Ellegaard-Jensen, L., Aamand, J., Kragelund, B. B., Johnsen, A. H., and Rosendahl, S. (2013). Strains of the soil fungus Mortierella show different degradation potentials for the phenylurea herbicide diuron. Biodegradation 24, 765–774. doi: 10.1007/s10532-013-9624-7
Ferrari, B., Zhang, C., and van Dorst, J. (2011). Recovering greater fungal diversity from pristine and diesel fuel contaminated sub-Antarctic soil through cultivation using both a high and a low nutrient media approach. Front. Microbiol. 2:217. doi: 10.3389/fmicb.2011.00217
Fitzpatrick, C. R., Salas-González, I., Conway, J. M., Finkel, O. M., Gilbert, S., Russ, D., et al. (2020). The plant microbiome: from ecology to reductionism and beyond. Annu. Rev. Microbiol. 74, 81–100. doi: 10.1146/annurev-micro-022620-014327
Graff, P., Aguiar, M. R., and Almeida, R. J. (2018). Females engage in stronger relationships: positive and negative effects of shrubs are more intense for Poa ligularis females than for males. Ann. Bot. 122, 435–443. doi: 10.1093/aob/mcy085
He, Y., Zhu, Z., Guo, Q., and Xia, Z. (2021). Sex-specific interactions affect foliar defense compound accumulation and resistance to herbivores in Populus cathayana. Sci. Total Environ. 774:145819. doi: 10.1016/j.scitotenv.2021.145819
Hibbett, D. S. (2006). A phylogenetic overview of the Agaricomycotina. Mycologia 98, 917–925. doi: 10.3852/mycologia.98.6.917
Hodge, A., and Fitter, A. (2013). Microbial mediation of plant competition and community structure. Funct. Ecol. 27, 865–875. doi: 10.2307/23480995
Hou, D. D., Wang, K., Liu, T., Wang, H. X., Lin, Z., Qian, J., et al. (2017). Unique rhizosphere micro-characteristics facilitate phytoextraction of multiple metals in soil by the hyperaccumulating plant sedum alfredii. Environ. Sci. Technol. 51, 5675–5684. doi: 10.1021/acs.est.6b06531
Huang, X. Q., Liu, L. L., Wen, T., Zhu, R., Zhang, J. B., and Cai, Z. C. (2015). Illumina MiSeq investigations on the changes of microbial community in the Fusarium oxysporum f.sp cubense infected soil during and after reductive soil disinfestation. Microbiol. Res. 181, 33–42. doi: 10.1016/j.micres.2015.08.004
Hultine, K. R., Grady, K. C., Wood, T. E., Shuster, S. M., Stella, J. C., and Whitham, T. G. (2016). Climate change perils for dioecious plant species. Nature Plants 2:16109. doi: 10.1038/nplants.2016.109
Ihrmark, K., Bodeker, I. T. M., Cruz-Martinez, K., Friberg, H., Kubartova, A., Schenck, J., et al. (2012). New primers to amplify the fungal ITS2 region - evaluation by 454-sequencing of artificial and natural communities. FEMS Microbiol. Ecol. 82, 666–677. doi: 10.1111/j.1574-6941.2012.01437.x
Jiang, H., Korpelainen, H., and Li, C. (2013). Populus yunnanensis males adopt more efficient protective strategies than females to cope with excess zinc and acid rain. Chemosphere 91, 1213–1220. doi: 10.1016/j.chemosphere.2013.01.041
Juvany, M., and Munné-Bosch, S. (2015). Sex-related differences in stress tolerance in dioecious plants: a critical appraisal in a physiological context. J. Exp. Bot. 66, 6083–6092. doi: 10.1093/jxb/erv343
Kõljalg, U., Larsson, K.-H., Abarenkov, K., Nilsson, R. H., Alexander, I. J., Eberhardt, U., et al. (2005). UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi. New Phytol. 166, 1063–1068. doi: 10.1111/j.1469-8137.2005.01376.x
Lei, Y., Yonglei, J., Chen, K., Duan, B., Zhang, S., Korpelainen, H., et al. (2017). Reproductive investments driven by sex and altitude in sympatric Populus and Salix trees. Tree Physiol. 37, 1503–1514. doi: 10.1093/treephys/tpx075
Li, L., Barrett, S., Song, Z., and Chen, J. (2019). Sex-specific plasticity of reproductive allocation in response to water depth in a clonal, dioecious macrophyte. Am. J. Bot. 106, 42–50. doi: 10.1002/ajb2.1218
Li, Y., Duan, B., Chen, J., Korpelainen, H., Niinemets, Ü., and Li, C. (2016). Males exhibit competitive advantages over females of Populus deltoides under salinity stress. Tree Physiol. 36, 1573–1584. doi: 10.1093/treephys/tpw070
Li, X., Rui, J., Mao, Y., Yannarell, A., and Mackie, R. (2014). Dynamics of the bacterial community structure in the rhizosphere of a maize cultivar. Soil Biol. Biochem. 68, 392–401. doi: 10.1016/j.soilbio.2013.10.017
Li, C., Zhou, K., Qin, W., Tian, C., Qi, M., Yan, X., et al. (2019). A review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil Sediment Contam. Int. J. 28, 380–394. doi: 10.1080/15320383.2019.1592108
Liancourt, P., Callaway, R., and Michalet, R. (2005). Stress tolerance and competitive-response ability determine the outcome of biotic interactions. Ecology 86, 1611–1618. doi: 10.1890/04-1398
Liu, M., Korpelainen, H., and Li, C. (2021a). Sexual differences and sex ratios of dioecious plants under stressful environments. J. Plant Ecol. 14, 920–933. doi: 10.1093/jpe/rtab038
Liu, M., Liu, X., Kang, J., Korpelainen, H., and Li, C. (2020). Are males and females of Populus cathayana differentially sensitive to cd stress? J. Hazard. Mater. 393:122411. doi: 10.1016/j.jhazmat.2020.122411
Liu, M., Liu, X., Zhao, Y., Korpelainen, H., and Li, C. (2022a). Sex-specific nitrogen allocation tradeoffs in the leaves of Populus cathayana cuttings under salt and drought stress. Plant Physiol. Biochem. 172, 101–110. doi: 10.1016/j.plaphy.2022.01.009
Liu, M., Wang, Y., Liu, X., Korpelainen, H., and Li, C. (2021b). Intra- and intersexual interactions shape microbial community dynamics in the rhizosphere of Populus cathayana females and males exposed to excess Zn. J. Hazard. Mater. 402:123783. doi: 10.1016/j.jhazmat.2020.123783
Liu, M., Zhao, Y., Liu, X., Korpelainen, H., and Li, C. (2022b). Ammonium and nitrate affect sexually different responses to salt stress in Populus cathayana. Physiol. Plant. 174:e13626. doi: 10.1111/ppl.13626
Liu, M., Zhao, Y., Wang, Y., Korpelainen, H., and Li, C. (2022c). Stem xylem traits and wood formation affect sex-specific responses to drought and re-watering in Populus cathayana. Tree Physiol. 42, 1350–1363. doi: 10.1093/treephys/tpac011
Loreau, M., and Hector, A. (2001). Partitioning selection and complementarity in biodiversity experiments. Nature 412, 72–76. doi: 10.1038/35083573
Melnikova, N. V., Borkhert, E. V., Snezhkina, A. V., Kudryavtseva, A. V., and Dmitriev, A. A. (2017). Sex-specific response to stress in Populus. Front. Plant Sci. 8:1827. doi: 10.3389/fpls.2017.01827
Meyer, K. M., Porch, R., Muscettola, I. E., Vasconcelos, A. L. S., Sherman, J. K., Metcalf, C. J. E., et al. (2022). Plant neighborhood shapes diversity and reduces interspecific variation of the phyllosphere microbiome. ISME J. 16, 1376–1387. doi: 10.1038/s41396-021-01184-6
Miranda, V., Scervino, J. M., Barros, J., Rodriguez, M. A., and Fracchia, S. (2020). Physiological characterization of coprophilous fungal isolates that behave as plant root associates. Soil Res. 58, 748–758. doi: 10.1071/SR20141
Miyata, N., Tani, Y., Sakata, M., and Iwahori, K. (2007). Microbial manganese oxide formation and interaction with toxic metal ions. J. Biosci. Bioeng. 104, 1–8. doi: 10.1263/jbb.104.1
Nelson, D. W., and Sommers, L. E. (1996). “Total carbon, organic carbon, and organic matter” in Methods of soil analysis part 3: Chemical methods. ed. D. L. Sparks (Madison: Soil Science Society of America), 961–1010.
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., et al. (2016). FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/j.funeco.2015.06.006
Oehl, F., Laczko, E., Bogenrieder, A., Stahr, K., Bösch, R., Van der Heijden, M., et al. (2010). Soil type and land use intensity determine the composition of arbuscular mycorrhizal fungal communities. Soil Biol. Biochem. 42, 724–738. doi: 10.1016/j.soilbio.2010.01.006
Olsen, S. R., and Sommers, L. E. (1982). “Phosphorus: phosphorus soluble in sodium bicarbonate” in Methods of soil analysis, part 2—Chemical and microbiological properties. eds. A. L. Page, R. H. Miller, and D. R. Keeney. 2nd Edn. (Madison: Soil Science Society of America), 403–430.
Paz-Ferreiro, J., and Fu, S. (2013). Biological indices for soil quality evaluation: perspectives and limitations. Land Degrad. Dev. 27, 14–25. doi: 10.1002/ldr.2262
Rayen, M., Reyes-Díaz, M., Alberdi, M., Ivanov, A., Król, M., and Huner, N. (2012). Excess manganese differentially inhibits photosystem I versus II in Arabidopsis. J. Exp. Bot. 64, 343–354. doi: 10.1093/jxb/ers339
Rayen, M., Reyes-Díaz, M., Ivanov, A., Mora, M. L., and Alberdi, M. (2010). Manganese as essential and toxic element for plants: transport, accumulation and resistance mechanisms. J. Soil Sci. Plant Nutr. 10, 470–481. doi: 10.4067/S0718-95162010000200008
Renner, S. S. (2014). The relative and absolute frequencies of angiosperm sexual systems: Dioecy, monoecy, gynodioecy, and an updated online database. Am. J. Bot. 101, 1588–1596. doi: 10.3732/ajb.1400196
Santos, E., Santini, J., Paixao, A., Furlani Junior, E., Lavres, J., Campos, M., et al. (2017). Physiological highlights of manganese toxicity symptoms in soybean plants: Mn toxicity responses. Plant Physiol. Biochem. 113, 6–19. doi: 10.1016/j.plaphy.2017.01.022
Schadt, C. W., and Rosling, A. (2015). Comment on “global diversity and geography of soil fungi”. Science 348:1438. doi: 10.1126/science.aaa4269
Schoch, C., Sung, G.-H., Lopez-Giraldez, F., Townsend, J., Miadlikowska, J., Valerie, H., et al. (2009). The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst. Biol. 58, 224–239. doi: 10.1093/sysbio/syp020
Shi, Y., Delgado-Baquerizo, M., Li, Y. T., Yang, Y. F., Zhu, Y. G., Penuelas, J., et al. (2020). Abundance of kinless hubs within soil microbial networks are associated with high functional potential in agricultural ecosystems. Environ. Int. 142:105869. doi: 10.1016/j.envint.2020.105869
Shi, X., Zhao, X.-h., Ren, J., Dong, J., Zhang, H., Dong, Q., et al. (2021). Influence of Peanut, sorghum, and soil salinity on microbial community composition in interspecific interaction zone. Front. Microbiol. 12:678250. doi: 10.3389/fmicb.2021.678250
Šmejkalová, M., Mikanová, O., and Boruvka, L. (2003). Effects of heavy metal concentrations on biological activity of soil micro-organisms. Plant Soil Environ. 49, 321–326. doi: 10.17221/4131-PSE
Song, H., Cai, Z., Liao, J., Tang, D., and Zhang, S. (2019). Sexually differential gene expressions in poplar roots in response to nitrogen deficiency. Tree Physiol. 39, 1614–1629. doi: 10.1093/treephys/tpz057
Tang, J., Ren, L., Zhou, Y., Gao, J., Luo, L., Yang, Y., et al. (2019). Diagnosis of soil contamination using microbiological indices: a review on heavy metal pollution. J. Environ. Manag. 242, 121–130. doi: 10.1016/j.jenvman.2019.04.061
Vellend, M. (2016). The Theory of Ecological Communities (MPB-57). William Street Princeton, New Jersey, United States: Princeton University Press.
Wang, X. L., Ma, K., Fu, Y. Z., Wang, Z. Q., and An, Y. Y. (2020). Effects of no-tillage, mulching, and organic fertilization on soil fungal community composition and diversity. Chin. J. Appl. Ecol. 31, 890–898. doi: 10.13287/j.1001-9332.202003.039
Xia, Z., He, Y., Korpelainen, H., Niinemets, Ü., and Li, C. (2022). Sex-specific interactions shape root phenolics and rhizosphere microbial communities in Populus cathayana. For. Ecol. Manag. 504:119857. doi: 10.1016/j.foreco.2021.119857
Xia, Z., He, Y., Yu, L., Lv, R., Korpelainen, H., and Li, C. (2020). Sex-specific strategies of phosphorus (P) acquisition in Populus cathayana as affected by soil P availability and distribution. New Phytol. 225, 782–792. doi: 10.1111/nph.16170
Xie, N., Chapeland-Leclerc, F., Silar, P., and Ruprich-Robert, G. (2014). Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina. Environ. Microbiol. 16, 141–161. doi: 10.1111/1462-2920.12253
Xie, Q., Li, Z., Yang, L., Lv, J., and Jobe, T. (2015). A newly identified passive Hyperaccumulator Eucalyptus grandis × E. urophylla under manganese stress. PLoS One 10:e0136606. doi: 10.1371/journal.pone.0136606
Xiong, C., Zhu, Y. G., Wang, J. T., Singh, B., Han, L. L., Shen, J. P., et al. (2021). Host selection shapes crop microbiome assembly and network complexity. New Phytol. 229, 1091–1104. doi: 10.1111/nph.16890
Yakun, S., Xingmin, M., Kairong, L., and Shao, H. (2016). Soil characterization and differential patterns of heavy metal accumulation in woody plants grown in coal gangue wastelands in Shaanxi, China. Environ. Sci. Pollut. Res. Int. 23, 13489–13497. doi: 10.1007/s11356-016-6432-8
Yang, L., Sui, X., Wei, D., Cui, F., Zhu, D., and Ni, H. (2019). Fungal diversity in the brown coniferous forest soils of Daxing’anling mountains, Northeast China. Chin. J. Appl. Ecol. 30, 3411–3418. doi: 10.13287/j.1001-9332.2019110.030
Yao, Y., Xu, G., Mou, D., Wang, J., and Ma, J. (2012). Subcellular Mn compartation, anatomic and biochemical changes of two grape varieties in response to excess manganese. Chemosphere 89, 150–157. doi: 10.1016/j.chemosphere.2012.05.030
Yu, Q., Barrett, S., Wang, X., Li, Z., Wang, H., Li, D., et al. (2022). Sexual dimorphism, temporal niche differentiation and evidence for the Jack sprat effect in an annual dioecious plant. J. Syst. Evol. 60, 1078–1091. doi: 10.1111/jse.12753
Zhang, C. Y., Zhu, J., Liu, G., Huang, Y. Y., Huang, G. Q., and Xu, X. (2021). The sexual dimorphism displayed by the roots of mulberry (Morus alba) saplings depends on the sex of the neighboring plants. J. Plant Ecol. 14, 1037–1046. doi: 10.1093/jpe/rtab043
Zhong, C., Yang, Z., Jiang, W., Hu, B., Hou, Q., Yu, T., et al. (2016). Ecological geochemical assessment and source identification of trace elements in atmospheric deposition of an emerging industrial area: Beibu gulf economic zone. Sci. Total Environ. 573, 1519–1526. doi: 10.1016/j.scitotenv.2016.08.057
Keywords: dioecious plants, sexual competition, Mn stress, fungal community, rhizosphere
Citation: Lin Y, Fang L, Chen H, Sun X, He Y, Duan B, Li R, Cao C and Chen J (2023) Sex-specific competition differently regulates the response of the rhizosphere fungal community of Hippophae rhamnoides–A dioecious plant, under Mn stress. Front. Microbiol. 14:1102904. doi: 10.3389/fmicb.2023.1102904
Edited by:
Kanika Khanna, Guru Nanak Dev University, IndiaReviewed by:
Nitika Kapoor, Hans Raj Mahila Maha Vidyalaya (HRMMV), IndiaGeetika Sirhindi, Punjabi University, India
Copyright © 2023 Lin, Fang, Chen, Sun, He, Duan, Li, Cao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Juan Chen, ✉ Y2owNDE2OThAMTYzLmNvbQ==
 Yuhu Lin
Yuhu Lin Ling Fang1,2
Ling Fang1,2 Juan Chen
Juan Chen